User login
45-year-old woman • fever and chills • diffuse abdominal pain • shortness of breath • Dx?
THE CASE
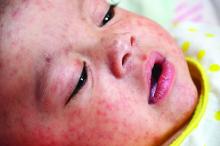
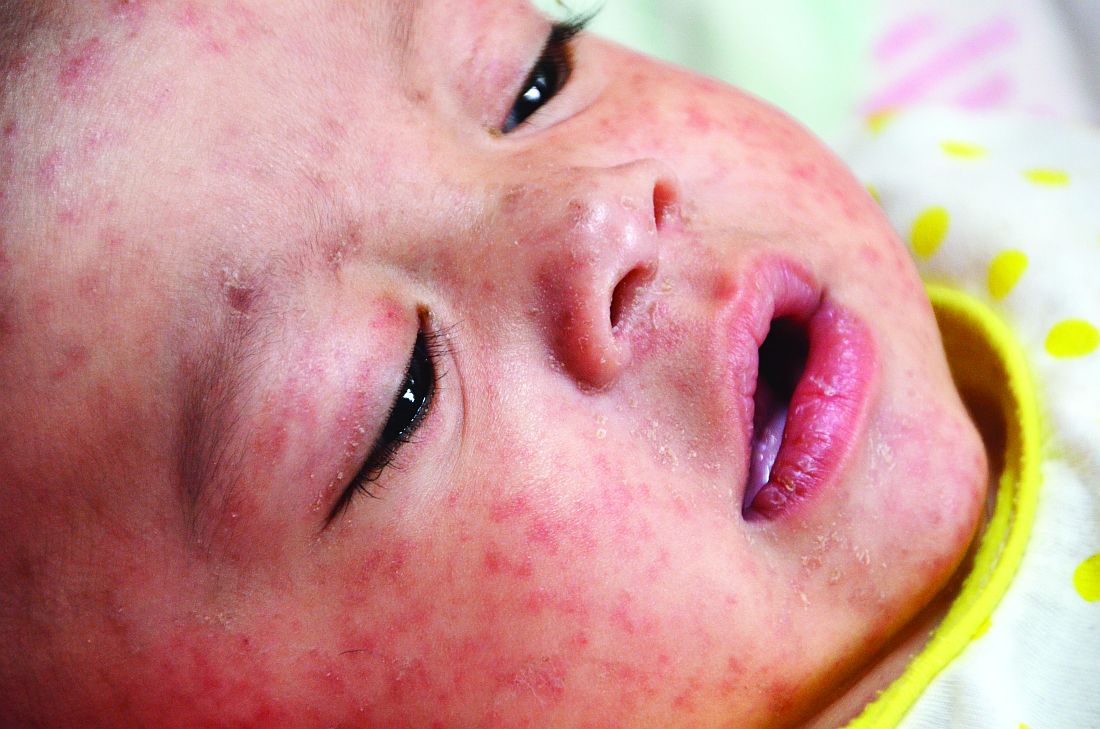
A 45-year-old white woman presented to our emergency department (ED) with a 3-day history of fever, chills, diffuse abdominal pain, severe headache, and shortness of breath.
The patient’s medical and surgical history was notable for acromegaly secondary to pituitary microadenoma, pituitary resection, and complete thyroidectomy 4 years earlier. Her medications included lanreotide, levothyroxine, gabapentin, alprazolam, and zolpidem. She had no history of cardiac disease, diabetes mellitus, immunodeficiency, or injection drug use. Three months prior to presenting to the ED, she underwent an outpatient gynecologic procedure for insertion of a levonorgestrel-releasing intrauterine device (IUD) for menorrhagia.
In the ED, the patient had a fever (101.5°F) and an elevated white blood cell count of 13,600/mm3 (reference range, 4,000–10,000/mm3). Cardiac auscultation revealed a regular heart rate and rhythm, with normal S1 and S2 sounds without murmur. Electrocardiogram documented normal sinus rhythm with no abnormalities. The physical examination revealed a diffusely tender lower abdomen without rebound or guarding. A pelvic examination was not conducted, and there was no collection of a vaginal swab sample to test for gonorrhea, chlamydia, or group B Streptococcus (GBS). Further workups for infection, including urinalysis, lumbar puncture, and chest x-ray, all yielded normal results.
Shortly after she was discharged from the ED, the patient was called to return to the hospital after blood cultures grew GBS; she was admitted for treatment.
THE DIAGNOSIS
A diagnosis of sepsis secondary to GBS bacteremia was made. However, the source of the GBS bacteremia and the patient’s abdominal symptoms remained unclear. Further workup included computed tomography (CT) of the abdomen, pelvis, and head, and magnetic resonance imaging of the brain; all imaging revealed no acute findings. Blood work (chem-7 panel, complete blood count, human immunodeficiency virus testing) was unremarkable except for an elevated level of C-reactive protein of 90 mg/L (reference range, 0–10 mg/L).
Radiography confirmed that the IUD was in the correct intrauterine position. However, transesophageal echocardiography (TEE) showed vegetations on the mitral and aortic valves, with preserved cardiac function. A diagnosis of GBS endocarditis was made, and infectious disease specialists were consulted. Because the patient had an anaphylactic allergy to penicillin, she was treated with intravenous vancomycin for 4 weeks. One month later, she had the IUD removed because of persistent abdominal pain.
DISCUSSION
Although the source of GBS bacteremia and endocarditis in our patient remained nondefinitive, the recent insertion of the IUD continued to be the suspected source and leading diagnosis.
Continue to: Other sources of GBS bacteremia...
Other sources of GBS bacteremia were unlikely based on the examination and imaging results. The patient’s abdominal exam was benign, and no intra-abdominal abscess was detected on CT. Although Streptococcus viridans, S bovis, and enterococcus are far more common pathogens for infective endocarditis,1 there was no evidence of dental caries, gastrointestinal pathology, or urinary tract infection to suggest misidentification of bacteria.
Theoretically, GBS bacteremia after a gynecologic procedure is possible since GBS frequently colonizes the vagina.2 However, most reports document transient rather than persistent bacteremia and/or endocarditis.3,4
IUD insertion as a cause of bacteremia. The medical literature offers scant evidence of endocarditis or severe GBS bacteremia related to IUD insertion. Of 124 gynecology-related reports of infective endocarditis between 1946 and 1986, only 3 were associated with IUDs.5 All 3 women had underlying cardiac disease, and 2 of the 3 had identifiable pelvic infections.5
Among 12 case reports of endocarditis related to gynecologic procedures from 1985 to 2003, therapeutic abortion was the most common antecedent event, and no cases were related to IUD insertion.2 Compared with cases reported before 1985, in these cases most patients (64%) did not have underlying valvular disease, and most had a subacute course with low mortality but high morbidity (8 of 11 patients had clinically significant emboli).2 The study authors also mentioned a case of endocarditis following a Pap smear test, suggesting that minimally invasive procedures may result in infective endocarditis.2
THE TAKEAWAY
Our patient presented with fever, fatigue, and abdominal pain in the setting of recent IUD insertion. She was found to have GBS bacteremia with endocarditis based on TEE and positive blood culture growth. Her clinical situation was suspicious for a gynecologic source of bacteremia.
Continue to: There is no definitive way...
There is no definitive way to confirm that IUD insertion 3 months prior caused the GBS bacteremia. However, this case illustrates that it is important to consider a usually benign gynecologic procedure as the source of clinically significant persistent bacteremia.
Evidence is insufficient to recommend prophylactic antibiotic use prior to a gynecologic procedure, and it is not recommended by current practice guidelines of the American College of Obstetricians and Gynecologists or the European Society of Cardiology.6,7
This patient case raises our suspicion for IUD-related bacteremia as an adverse reaction in healthy women with recent IUD insertion who present with fever and diffuse abdominal pain without apparent signs of a pelvic infection. Prompt antibiotic treatment is necessary to prevent significant morbidity and mortality.
CORRESPONDENCE
Lauren Cowen, MD, 777 South Clinton Avenue, Rochester, NY 14620; [email protected]
1. Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486.
2. Crespo A, Retter AS, Lorber B. Group B streptococcal endocarditis in obstetric and gynecologic practice. Infect Dis Obstet Gynecol. 2003;11:109-115.
3. Murray S, Hickey JB, Houang E. Significant bacteremia associated with replacement of intrauterine contraceptive device. Am J Obstet Gynecol. 1987;156:698-700.
4. Everett ED, Reller LB, Droegemueller W, et al. Absence of bacteremia after insertion or removal of intrauterine devices. Obstet Gynecol. 1976;47:207-209.
5. Seaworth BJ, Durack DT. Infective endocarditis in obstetric and gynecologic practice. Am J Obstet Gynecol. 1986;154:180-188.
6. ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
7. Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2015;36:3075-3128.
THE CASE
A 45-year-old white woman presented to our emergency department (ED) with a 3-day history of fever, chills, diffuse abdominal pain, severe headache, and shortness of breath.
The patient’s medical and surgical history was notable for acromegaly secondary to pituitary microadenoma, pituitary resection, and complete thyroidectomy 4 years earlier. Her medications included lanreotide, levothyroxine, gabapentin, alprazolam, and zolpidem. She had no history of cardiac disease, diabetes mellitus, immunodeficiency, or injection drug use. Three months prior to presenting to the ED, she underwent an outpatient gynecologic procedure for insertion of a levonorgestrel-releasing intrauterine device (IUD) for menorrhagia.
In the ED, the patient had a fever (101.5°F) and an elevated white blood cell count of 13,600/mm3 (reference range, 4,000–10,000/mm3). Cardiac auscultation revealed a regular heart rate and rhythm, with normal S1 and S2 sounds without murmur. Electrocardiogram documented normal sinus rhythm with no abnormalities. The physical examination revealed a diffusely tender lower abdomen without rebound or guarding. A pelvic examination was not conducted, and there was no collection of a vaginal swab sample to test for gonorrhea, chlamydia, or group B Streptococcus (GBS). Further workups for infection, including urinalysis, lumbar puncture, and chest x-ray, all yielded normal results.
Shortly after she was discharged from the ED, the patient was called to return to the hospital after blood cultures grew GBS; she was admitted for treatment.
THE DIAGNOSIS
A diagnosis of sepsis secondary to GBS bacteremia was made. However, the source of the GBS bacteremia and the patient’s abdominal symptoms remained unclear. Further workup included computed tomography (CT) of the abdomen, pelvis, and head, and magnetic resonance imaging of the brain; all imaging revealed no acute findings. Blood work (chem-7 panel, complete blood count, human immunodeficiency virus testing) was unremarkable except for an elevated level of C-reactive protein of 90 mg/L (reference range, 0–10 mg/L).
Radiography confirmed that the IUD was in the correct intrauterine position. However, transesophageal echocardiography (TEE) showed vegetations on the mitral and aortic valves, with preserved cardiac function. A diagnosis of GBS endocarditis was made, and infectious disease specialists were consulted. Because the patient had an anaphylactic allergy to penicillin, she was treated with intravenous vancomycin for 4 weeks. One month later, she had the IUD removed because of persistent abdominal pain.
DISCUSSION
Although the source of GBS bacteremia and endocarditis in our patient remained nondefinitive, the recent insertion of the IUD continued to be the suspected source and leading diagnosis.
Continue to: Other sources of GBS bacteremia...
Other sources of GBS bacteremia were unlikely based on the examination and imaging results. The patient’s abdominal exam was benign, and no intra-abdominal abscess was detected on CT. Although Streptococcus viridans, S bovis, and enterococcus are far more common pathogens for infective endocarditis,1 there was no evidence of dental caries, gastrointestinal pathology, or urinary tract infection to suggest misidentification of bacteria.
Theoretically, GBS bacteremia after a gynecologic procedure is possible since GBS frequently colonizes the vagina.2 However, most reports document transient rather than persistent bacteremia and/or endocarditis.3,4
IUD insertion as a cause of bacteremia. The medical literature offers scant evidence of endocarditis or severe GBS bacteremia related to IUD insertion. Of 124 gynecology-related reports of infective endocarditis between 1946 and 1986, only 3 were associated with IUDs.5 All 3 women had underlying cardiac disease, and 2 of the 3 had identifiable pelvic infections.5
Among 12 case reports of endocarditis related to gynecologic procedures from 1985 to 2003, therapeutic abortion was the most common antecedent event, and no cases were related to IUD insertion.2 Compared with cases reported before 1985, in these cases most patients (64%) did not have underlying valvular disease, and most had a subacute course with low mortality but high morbidity (8 of 11 patients had clinically significant emboli).2 The study authors also mentioned a case of endocarditis following a Pap smear test, suggesting that minimally invasive procedures may result in infective endocarditis.2
THE TAKEAWAY
Our patient presented with fever, fatigue, and abdominal pain in the setting of recent IUD insertion. She was found to have GBS bacteremia with endocarditis based on TEE and positive blood culture growth. Her clinical situation was suspicious for a gynecologic source of bacteremia.
Continue to: There is no definitive way...
There is no definitive way to confirm that IUD insertion 3 months prior caused the GBS bacteremia. However, this case illustrates that it is important to consider a usually benign gynecologic procedure as the source of clinically significant persistent bacteremia.
Evidence is insufficient to recommend prophylactic antibiotic use prior to a gynecologic procedure, and it is not recommended by current practice guidelines of the American College of Obstetricians and Gynecologists or the European Society of Cardiology.6,7
This patient case raises our suspicion for IUD-related bacteremia as an adverse reaction in healthy women with recent IUD insertion who present with fever and diffuse abdominal pain without apparent signs of a pelvic infection. Prompt antibiotic treatment is necessary to prevent significant morbidity and mortality.
CORRESPONDENCE
Lauren Cowen, MD, 777 South Clinton Avenue, Rochester, NY 14620; [email protected]
THE CASE
A 45-year-old white woman presented to our emergency department (ED) with a 3-day history of fever, chills, diffuse abdominal pain, severe headache, and shortness of breath.
The patient’s medical and surgical history was notable for acromegaly secondary to pituitary microadenoma, pituitary resection, and complete thyroidectomy 4 years earlier. Her medications included lanreotide, levothyroxine, gabapentin, alprazolam, and zolpidem. She had no history of cardiac disease, diabetes mellitus, immunodeficiency, or injection drug use. Three months prior to presenting to the ED, she underwent an outpatient gynecologic procedure for insertion of a levonorgestrel-releasing intrauterine device (IUD) for menorrhagia.
In the ED, the patient had a fever (101.5°F) and an elevated white blood cell count of 13,600/mm3 (reference range, 4,000–10,000/mm3). Cardiac auscultation revealed a regular heart rate and rhythm, with normal S1 and S2 sounds without murmur. Electrocardiogram documented normal sinus rhythm with no abnormalities. The physical examination revealed a diffusely tender lower abdomen without rebound or guarding. A pelvic examination was not conducted, and there was no collection of a vaginal swab sample to test for gonorrhea, chlamydia, or group B Streptococcus (GBS). Further workups for infection, including urinalysis, lumbar puncture, and chest x-ray, all yielded normal results.
Shortly after she was discharged from the ED, the patient was called to return to the hospital after blood cultures grew GBS; she was admitted for treatment.
THE DIAGNOSIS
A diagnosis of sepsis secondary to GBS bacteremia was made. However, the source of the GBS bacteremia and the patient’s abdominal symptoms remained unclear. Further workup included computed tomography (CT) of the abdomen, pelvis, and head, and magnetic resonance imaging of the brain; all imaging revealed no acute findings. Blood work (chem-7 panel, complete blood count, human immunodeficiency virus testing) was unremarkable except for an elevated level of C-reactive protein of 90 mg/L (reference range, 0–10 mg/L).
Radiography confirmed that the IUD was in the correct intrauterine position. However, transesophageal echocardiography (TEE) showed vegetations on the mitral and aortic valves, with preserved cardiac function. A diagnosis of GBS endocarditis was made, and infectious disease specialists were consulted. Because the patient had an anaphylactic allergy to penicillin, she was treated with intravenous vancomycin for 4 weeks. One month later, she had the IUD removed because of persistent abdominal pain.
DISCUSSION
Although the source of GBS bacteremia and endocarditis in our patient remained nondefinitive, the recent insertion of the IUD continued to be the suspected source and leading diagnosis.
Continue to: Other sources of GBS bacteremia...
Other sources of GBS bacteremia were unlikely based on the examination and imaging results. The patient’s abdominal exam was benign, and no intra-abdominal abscess was detected on CT. Although Streptococcus viridans, S bovis, and enterococcus are far more common pathogens for infective endocarditis,1 there was no evidence of dental caries, gastrointestinal pathology, or urinary tract infection to suggest misidentification of bacteria.
Theoretically, GBS bacteremia after a gynecologic procedure is possible since GBS frequently colonizes the vagina.2 However, most reports document transient rather than persistent bacteremia and/or endocarditis.3,4
IUD insertion as a cause of bacteremia. The medical literature offers scant evidence of endocarditis or severe GBS bacteremia related to IUD insertion. Of 124 gynecology-related reports of infective endocarditis between 1946 and 1986, only 3 were associated with IUDs.5 All 3 women had underlying cardiac disease, and 2 of the 3 had identifiable pelvic infections.5
Among 12 case reports of endocarditis related to gynecologic procedures from 1985 to 2003, therapeutic abortion was the most common antecedent event, and no cases were related to IUD insertion.2 Compared with cases reported before 1985, in these cases most patients (64%) did not have underlying valvular disease, and most had a subacute course with low mortality but high morbidity (8 of 11 patients had clinically significant emboli).2 The study authors also mentioned a case of endocarditis following a Pap smear test, suggesting that minimally invasive procedures may result in infective endocarditis.2
THE TAKEAWAY
Our patient presented with fever, fatigue, and abdominal pain in the setting of recent IUD insertion. She was found to have GBS bacteremia with endocarditis based on TEE and positive blood culture growth. Her clinical situation was suspicious for a gynecologic source of bacteremia.
Continue to: There is no definitive way...
There is no definitive way to confirm that IUD insertion 3 months prior caused the GBS bacteremia. However, this case illustrates that it is important to consider a usually benign gynecologic procedure as the source of clinically significant persistent bacteremia.
Evidence is insufficient to recommend prophylactic antibiotic use prior to a gynecologic procedure, and it is not recommended by current practice guidelines of the American College of Obstetricians and Gynecologists or the European Society of Cardiology.6,7
This patient case raises our suspicion for IUD-related bacteremia as an adverse reaction in healthy women with recent IUD insertion who present with fever and diffuse abdominal pain without apparent signs of a pelvic infection. Prompt antibiotic treatment is necessary to prevent significant morbidity and mortality.
CORRESPONDENCE
Lauren Cowen, MD, 777 South Clinton Avenue, Rochester, NY 14620; [email protected]
1. Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486.
2. Crespo A, Retter AS, Lorber B. Group B streptococcal endocarditis in obstetric and gynecologic practice. Infect Dis Obstet Gynecol. 2003;11:109-115.
3. Murray S, Hickey JB, Houang E. Significant bacteremia associated with replacement of intrauterine contraceptive device. Am J Obstet Gynecol. 1987;156:698-700.
4. Everett ED, Reller LB, Droegemueller W, et al. Absence of bacteremia after insertion or removal of intrauterine devices. Obstet Gynecol. 1976;47:207-209.
5. Seaworth BJ, Durack DT. Infective endocarditis in obstetric and gynecologic practice. Am J Obstet Gynecol. 1986;154:180-188.
6. ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
7. Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2015;36:3075-3128.
1. Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435-1486.
2. Crespo A, Retter AS, Lorber B. Group B streptococcal endocarditis in obstetric and gynecologic practice. Infect Dis Obstet Gynecol. 2003;11:109-115.
3. Murray S, Hickey JB, Houang E. Significant bacteremia associated with replacement of intrauterine contraceptive device. Am J Obstet Gynecol. 1987;156:698-700.
4. Everett ED, Reller LB, Droegemueller W, et al. Absence of bacteremia after insertion or removal of intrauterine devices. Obstet Gynecol. 1976;47:207-209.
5. Seaworth BJ, Durack DT. Infective endocarditis in obstetric and gynecologic practice. Am J Obstet Gynecol. 1986;154:180-188.
6. ACOG Committee on Practice Bulletins–Gynecology. Practice bulletin no. 186: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
7. Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2015;36:3075-3128.
Poll: Clostridium difficile
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
[polldaddy:10452484]
Click on page 2 below to find out what the correct answer is...
The correct answer is a.) 1 to 2
To learn more, see this month's PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
[polldaddy:10452484]
Click on page 2 below to find out what the correct answer is...
The correct answer is a.) 1 to 2
To learn more, see this month's PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
[polldaddy:10452484]
Click on page 2 below to find out what the correct answer is...
The correct answer is a.) 1 to 2
To learn more, see this month's PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
Measles infection linked to impaired ‘immune memory’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science.
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 the children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
The study did find increases in measles virus–specific antigens in children both after measles infection and MMR vaccination. However the authors did not detect any changes in total IgG, IgA, or IgM levels.
Dr. Mina and associates wrote.
They also noted that controls who received the MMR vaccine showed a marked increase in overall antibody repertoire.
In a separate investigation reported in Science Immunology, Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles to determine if B-cell impairment can lead to measles-associated immunosuppression. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IgVH-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B-cell frequencies in measles patients recovered to levels before infection, they had significant changes in IgVH-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IgVH-J genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IgVH genes, Dr. Petrova and associates reported.
The study by Mina et al. was supported by grants from various U.S., European, and Finnish foundations and national organizations. Some of the coauthors had relationships with biotechnology and pharmaceutical companies, and three reported a patent holding related to technology used in the study. The study by Petrova et al. was funded by grants to the investigators from various Indonesian and German organizations and the Wellcome Trust. The authors reported no conflicts of interest.
SOURCES: Mina M et al. Science. 2019 Nov 1;366:599-606; Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125.
As a result of reduced vaccination, after decades of decline, the number of worldwide cases of measles has increased by nearly 300% since 2018. Epidemiologic evidence has associated measles infections with increases in morbidity and mortality for as long as 5 years after the infection and suggests that, in the prevaccine era, measles virus may have been associated with up to 50% of all childhood deaths, mostly because of nonmeasles infections. Measles replication in immune cells has been hypothesized to impair immune memory, potentially causing what some scientists call “immunological amnesia.”A measles virus receptor, called CD150/ SLAMF1, is highly expressed on memory T, B, and plasma cells. Measles virus gains entry to these immune memory cells using that receptor and kills the cells.
The scientists stated that it could take months or years to return the immune repertoire back to baseline. During the rebuilding process, children would be at increased risk for infectious diseases they had previously experienced.
In a second outstanding paper, Petrova et al. in Science Immunology studied B cells before and after measles infection, and identified two immunologic consequences: The naive B-cell pool was depleted, leading to a return to immunologic immaturity, and the memory B-cell pool was depleted, resulting in compromised immune memory to previously encountered pathogens.
Thus, the link between measles infections and increased susceptibility to other infections and increased deaths from nonmeasles infectious diseases in the aftermath of measles has been revealed. This information adds new data to share with parents who consider refusing measles vaccination. The risks are far greater than getting measles.
Michael E. Pichichero, MD, is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He was asked to comment on the articles. Dr. Pichichero has no conflicts to declare.
As a result of reduced vaccination, after decades of decline, the number of worldwide cases of measles has increased by nearly 300% since 2018. Epidemiologic evidence has associated measles infections with increases in morbidity and mortality for as long as 5 years after the infection and suggests that, in the prevaccine era, measles virus may have been associated with up to 50% of all childhood deaths, mostly because of nonmeasles infections. Measles replication in immune cells has been hypothesized to impair immune memory, potentially causing what some scientists call “immunological amnesia.”A measles virus receptor, called CD150/ SLAMF1, is highly expressed on memory T, B, and plasma cells. Measles virus gains entry to these immune memory cells using that receptor and kills the cells.
The scientists stated that it could take months or years to return the immune repertoire back to baseline. During the rebuilding process, children would be at increased risk for infectious diseases they had previously experienced.
In a second outstanding paper, Petrova et al. in Science Immunology studied B cells before and after measles infection, and identified two immunologic consequences: The naive B-cell pool was depleted, leading to a return to immunologic immaturity, and the memory B-cell pool was depleted, resulting in compromised immune memory to previously encountered pathogens.
Thus, the link between measles infections and increased susceptibility to other infections and increased deaths from nonmeasles infectious diseases in the aftermath of measles has been revealed. This information adds new data to share with parents who consider refusing measles vaccination. The risks are far greater than getting measles.
Michael E. Pichichero, MD, is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He was asked to comment on the articles. Dr. Pichichero has no conflicts to declare.
As a result of reduced vaccination, after decades of decline, the number of worldwide cases of measles has increased by nearly 300% since 2018. Epidemiologic evidence has associated measles infections with increases in morbidity and mortality for as long as 5 years after the infection and suggests that, in the prevaccine era, measles virus may have been associated with up to 50% of all childhood deaths, mostly because of nonmeasles infections. Measles replication in immune cells has been hypothesized to impair immune memory, potentially causing what some scientists call “immunological amnesia.”A measles virus receptor, called CD150/ SLAMF1, is highly expressed on memory T, B, and plasma cells. Measles virus gains entry to these immune memory cells using that receptor and kills the cells.
The scientists stated that it could take months or years to return the immune repertoire back to baseline. During the rebuilding process, children would be at increased risk for infectious diseases they had previously experienced.
In a second outstanding paper, Petrova et al. in Science Immunology studied B cells before and after measles infection, and identified two immunologic consequences: The naive B-cell pool was depleted, leading to a return to immunologic immaturity, and the memory B-cell pool was depleted, resulting in compromised immune memory to previously encountered pathogens.
Thus, the link between measles infections and increased susceptibility to other infections and increased deaths from nonmeasles infectious diseases in the aftermath of measles has been revealed. This information adds new data to share with parents who consider refusing measles vaccination. The risks are far greater than getting measles.
Michael E. Pichichero, MD, is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He was asked to comment on the articles. Dr. Pichichero has no conflicts to declare.
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science.
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 the children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
The study did find increases in measles virus–specific antigens in children both after measles infection and MMR vaccination. However the authors did not detect any changes in total IgG, IgA, or IgM levels.
Dr. Mina and associates wrote.
They also noted that controls who received the MMR vaccine showed a marked increase in overall antibody repertoire.
In a separate investigation reported in Science Immunology, Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles to determine if B-cell impairment can lead to measles-associated immunosuppression. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IgVH-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B-cell frequencies in measles patients recovered to levels before infection, they had significant changes in IgVH-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IgVH-J genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IgVH genes, Dr. Petrova and associates reported.
The study by Mina et al. was supported by grants from various U.S., European, and Finnish foundations and national organizations. Some of the coauthors had relationships with biotechnology and pharmaceutical companies, and three reported a patent holding related to technology used in the study. The study by Petrova et al. was funded by grants to the investigators from various Indonesian and German organizations and the Wellcome Trust. The authors reported no conflicts of interest.
SOURCES: Mina M et al. Science. 2019 Nov 1;366:599-606; Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125.
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science.
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 the children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
The study did find increases in measles virus–specific antigens in children both after measles infection and MMR vaccination. However the authors did not detect any changes in total IgG, IgA, or IgM levels.
Dr. Mina and associates wrote.
They also noted that controls who received the MMR vaccine showed a marked increase in overall antibody repertoire.
In a separate investigation reported in Science Immunology, Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles to determine if B-cell impairment can lead to measles-associated immunosuppression. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IgVH-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B-cell frequencies in measles patients recovered to levels before infection, they had significant changes in IgVH-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IgVH-J genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IgVH genes, Dr. Petrova and associates reported.
The study by Mina et al. was supported by grants from various U.S., European, and Finnish foundations and national organizations. Some of the coauthors had relationships with biotechnology and pharmaceutical companies, and three reported a patent holding related to technology used in the study. The study by Petrova et al. was funded by grants to the investigators from various Indonesian and German organizations and the Wellcome Trust. The authors reported no conflicts of interest.
SOURCES: Mina M et al. Science. 2019 Nov 1;366:599-606; Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125.
FROM SCIENCE
Oral antibiotics as effective as IV for stable endocarditis patients
Background: Patients with left-sided infective endocarditis often are treated with prolonged courses of intravenous (IV) antibiotics. The safety of switching from IV to oral antibiotics is unknown.
Study design: Randomized, multicenter, noninferiority study.
Setting: Cardiac centers in Denmark during July 2011–August 2017.
Synopsis: The study enrolled 400 patients with left-sided infective endocarditis and positive blood cultures from Streptococcus, Enterococcus, Staphylococcus aureus, or coagulase-negative staph (non–methicillin-resistant Staphylococcus aureus), without evidence of valvular abscess. Following at least 7 days (for those who required surgical intervention) or 10 days (for those who did not require surgical intervention) of IV antibiotics, patients with ongoing fever, leukocytosis, elevated C-reactive protein, or concurrent infections were excluded from the study. Patients were randomized to receive continued IV antibiotic treatment or switch to oral antibiotic treatment. The IV treatment group received a median of 19 additional days of therapy, compared with 17 days in the oral group. The primary composite outcome of death, unplanned cardiac surgery, embolic event, and relapse of bacteremia occurred in 12.1% in the IV therapy group and 9% in the oral therapy group (difference of 3.1%; 95% confidence interval, –3.4 to 9.6; P = .40), meeting the studies prespecified noninferiority criteria. Poor representation of women, obese patients, and patients who use IV drugs may limit the study’s generalizability. An accompanying editorial advocated for additional research before widespread change to current treatment recommendations are made.
Bottom line: For patients with left-sided infective endocarditis who have been stabilized on IV antibiotic treatment, transitioning to an oral antibiotic regimen may be a noninferior approach.
Citation: Iverson K et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Eng J Med. 2019 Jan 31;380(5):415-24.
Dr. Phillips is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine at Harvard Medical School.
Background: Patients with left-sided infective endocarditis often are treated with prolonged courses of intravenous (IV) antibiotics. The safety of switching from IV to oral antibiotics is unknown.
Study design: Randomized, multicenter, noninferiority study.
Setting: Cardiac centers in Denmark during July 2011–August 2017.
Synopsis: The study enrolled 400 patients with left-sided infective endocarditis and positive blood cultures from Streptococcus, Enterococcus, Staphylococcus aureus, or coagulase-negative staph (non–methicillin-resistant Staphylococcus aureus), without evidence of valvular abscess. Following at least 7 days (for those who required surgical intervention) or 10 days (for those who did not require surgical intervention) of IV antibiotics, patients with ongoing fever, leukocytosis, elevated C-reactive protein, or concurrent infections were excluded from the study. Patients were randomized to receive continued IV antibiotic treatment or switch to oral antibiotic treatment. The IV treatment group received a median of 19 additional days of therapy, compared with 17 days in the oral group. The primary composite outcome of death, unplanned cardiac surgery, embolic event, and relapse of bacteremia occurred in 12.1% in the IV therapy group and 9% in the oral therapy group (difference of 3.1%; 95% confidence interval, –3.4 to 9.6; P = .40), meeting the studies prespecified noninferiority criteria. Poor representation of women, obese patients, and patients who use IV drugs may limit the study’s generalizability. An accompanying editorial advocated for additional research before widespread change to current treatment recommendations are made.
Bottom line: For patients with left-sided infective endocarditis who have been stabilized on IV antibiotic treatment, transitioning to an oral antibiotic regimen may be a noninferior approach.
Citation: Iverson K et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Eng J Med. 2019 Jan 31;380(5):415-24.
Dr. Phillips is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine at Harvard Medical School.
Background: Patients with left-sided infective endocarditis often are treated with prolonged courses of intravenous (IV) antibiotics. The safety of switching from IV to oral antibiotics is unknown.
Study design: Randomized, multicenter, noninferiority study.
Setting: Cardiac centers in Denmark during July 2011–August 2017.
Synopsis: The study enrolled 400 patients with left-sided infective endocarditis and positive blood cultures from Streptococcus, Enterococcus, Staphylococcus aureus, or coagulase-negative staph (non–methicillin-resistant Staphylococcus aureus), without evidence of valvular abscess. Following at least 7 days (for those who required surgical intervention) or 10 days (for those who did not require surgical intervention) of IV antibiotics, patients with ongoing fever, leukocytosis, elevated C-reactive protein, or concurrent infections were excluded from the study. Patients were randomized to receive continued IV antibiotic treatment or switch to oral antibiotic treatment. The IV treatment group received a median of 19 additional days of therapy, compared with 17 days in the oral group. The primary composite outcome of death, unplanned cardiac surgery, embolic event, and relapse of bacteremia occurred in 12.1% in the IV therapy group and 9% in the oral therapy group (difference of 3.1%; 95% confidence interval, –3.4 to 9.6; P = .40), meeting the studies prespecified noninferiority criteria. Poor representation of women, obese patients, and patients who use IV drugs may limit the study’s generalizability. An accompanying editorial advocated for additional research before widespread change to current treatment recommendations are made.
Bottom line: For patients with left-sided infective endocarditis who have been stabilized on IV antibiotic treatment, transitioning to an oral antibiotic regimen may be a noninferior approach.
Citation: Iverson K et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Eng J Med. 2019 Jan 31;380(5):415-24.
Dr. Phillips is a hospitalist at Beth Israel Deaconess Medical Center and instructor in medicine at Harvard Medical School.
HCV testing/awareness successful as part of HIV integrated care
(PWID), according to researchers reporting on a multisite randomized trial of nearly 12,000 HIV-infected individuals in India.
HCV antibody prevalence at these sites ranged from 7.2%-76.6%. Across six integrated care centers (ICCs), 5,263 clients underwent HCV testing, of whom 2,278 were newly diagnosed. At evaluation, PWID in ICC clusters were nearly four times more likely to report being tested for HCV than those in usual care clusters (adjusted prevalence ratio [aPR]: 3.69), according to the report by Sunil Suhas Solomon, MD, of Johns Hopkins University School of Medicine, Baltimore, and colleagues.
PWID in ICC clusters were also seven times more likely to be aware of their HCV status (aPR: 7.11; 95% confidence interval: 1.14, 44.3) and significantly more likely to initiate treatment, (aPR: 9.86; 95% CI: 1.52, 63.8), than individuals in usual care, the authors stated in their report published online ahead of press in the Journal of Hepatology.
“These data provide among the first empirical support of the benefits of integrating HCV testing with HIV prevention and treatment services for PWID. Over a short duration, we observed significant impact on community-level HCV testing and awareness of HCV status among PWID. While additional strategies might be required to improve population awareness levels, integration of HCV testing with HIV programs for PWID particularly given the high burden of HIV/HCV coinfection represents a critical first step,” the researchers concluded.
The study was funded by the National Institutes of Health and the Elton John AIDS Foundation. The authors reported that they had no relevant disclosures.
SOURCE: Solomon, SS et al. J Hepatol. 2019. doi.org/10.1016/j.jhep.2019.09.022.
(PWID), according to researchers reporting on a multisite randomized trial of nearly 12,000 HIV-infected individuals in India.
HCV antibody prevalence at these sites ranged from 7.2%-76.6%. Across six integrated care centers (ICCs), 5,263 clients underwent HCV testing, of whom 2,278 were newly diagnosed. At evaluation, PWID in ICC clusters were nearly four times more likely to report being tested for HCV than those in usual care clusters (adjusted prevalence ratio [aPR]: 3.69), according to the report by Sunil Suhas Solomon, MD, of Johns Hopkins University School of Medicine, Baltimore, and colleagues.
PWID in ICC clusters were also seven times more likely to be aware of their HCV status (aPR: 7.11; 95% confidence interval: 1.14, 44.3) and significantly more likely to initiate treatment, (aPR: 9.86; 95% CI: 1.52, 63.8), than individuals in usual care, the authors stated in their report published online ahead of press in the Journal of Hepatology.
“These data provide among the first empirical support of the benefits of integrating HCV testing with HIV prevention and treatment services for PWID. Over a short duration, we observed significant impact on community-level HCV testing and awareness of HCV status among PWID. While additional strategies might be required to improve population awareness levels, integration of HCV testing with HIV programs for PWID particularly given the high burden of HIV/HCV coinfection represents a critical first step,” the researchers concluded.
The study was funded by the National Institutes of Health and the Elton John AIDS Foundation. The authors reported that they had no relevant disclosures.
SOURCE: Solomon, SS et al. J Hepatol. 2019. doi.org/10.1016/j.jhep.2019.09.022.
(PWID), according to researchers reporting on a multisite randomized trial of nearly 12,000 HIV-infected individuals in India.
HCV antibody prevalence at these sites ranged from 7.2%-76.6%. Across six integrated care centers (ICCs), 5,263 clients underwent HCV testing, of whom 2,278 were newly diagnosed. At evaluation, PWID in ICC clusters were nearly four times more likely to report being tested for HCV than those in usual care clusters (adjusted prevalence ratio [aPR]: 3.69), according to the report by Sunil Suhas Solomon, MD, of Johns Hopkins University School of Medicine, Baltimore, and colleagues.
PWID in ICC clusters were also seven times more likely to be aware of their HCV status (aPR: 7.11; 95% confidence interval: 1.14, 44.3) and significantly more likely to initiate treatment, (aPR: 9.86; 95% CI: 1.52, 63.8), than individuals in usual care, the authors stated in their report published online ahead of press in the Journal of Hepatology.
“These data provide among the first empirical support of the benefits of integrating HCV testing with HIV prevention and treatment services for PWID. Over a short duration, we observed significant impact on community-level HCV testing and awareness of HCV status among PWID. While additional strategies might be required to improve population awareness levels, integration of HCV testing with HIV programs for PWID particularly given the high burden of HIV/HCV coinfection represents a critical first step,” the researchers concluded.
The study was funded by the National Institutes of Health and the Elton John AIDS Foundation. The authors reported that they had no relevant disclosures.
SOURCE: Solomon, SS et al. J Hepatol. 2019. doi.org/10.1016/j.jhep.2019.09.022.
FROM THE JOURNAL OF HEPATOLOGY
Clinicians ask FDA for continued ‘discretion’ to do fecal transplants
Attendees at a public meeting on Nov. 4 gave the US Food and Drug Administration conflicting views on whether the agency should continue to allow a relatively loose regulatory environment for fecal microbiota transplants (FMT) – debating the limits of “enforcement discretion” the FDA now has in place.
The question is especially relevant as use of the procedure is growing, while safety data are not being rigorously collected in all cases. The death of an immunocompromised FMT patient earlier in 2018 from an invasive bacterial infection caused by drug-resistant Escherichia coli, as reported by Medscape Medical News, is seen by some as an example of the consequences of a loose policy.
Still, the American Gastroenterological Association (AGA) presented new, unpublished follow-up data at the meeting that showed that the majority of FMT patients in a national registry had no adverse events.
Some companies developing FMT-based products argued at the meeting that the agency should impose stricter requirements, while stool banks and clinicians offering the therapy outside of clinical trials said that the current policy – in place since 2013 – in which the FDA has exercised “enforcement discretion,” should be allowed to continue.
“Enforcement discretion has been successful in enabling and overcoming key barriers to access to treatment,” said Majdi Osman, MD, clinical program director at OpenBiome, a nonprofit stool bank based in Cambridge, Mass. Dr. Osman said that 98% of the U.S. population now lives within a 2-hour drive of an FMT provider.
Amanda Kabage, a researcher and donor program coordinator for the Microbiota Therapeutics program at the University of Minnesota in Minneapolis, and herself a former recipient of FMT, said she was in favor of continuing the FDA policy.
“If enforcement discretion were to go away, patients far sicker than I was will not have access. They’ll get sicker and they will die,” Ms. Kabage said.
But, she added, the FDA had missed an opportunity by not insisting on collecting outcomes and safety data. Minnesota has established a patient registry to do just that, and physicians cannot administer FMT unless they agree to participate, she said. In response, FDA panelists noted that the agency cannot mandate data collection under an enforcement policy.
Lee Jones, founder and chief executive officer of Rebiotix/Ferring, a biotech company focused on the development of microbiome-based therapeutics, argued for tighter restrictions, however, claiming that increased access – and the FDA policy – had led to a fourfold decrease in enrollment since the company began study of its lead FMT product, RBX2660, in 2013.
“We’re dealing with an orphan indication and the patients were hard to come by to begin with,” she said at the meeting. “Enforcement discretion has slowed our clinical development and delayed patient access to FDA-approved therapies by over 2 years.”
An investigator at the University of Texas Health Science Center at Houston, Herbert DuPont, MD, who has administered FMT and is conducting a trial for Rebiotix, said his center wanted the FDA policy to continue “allowing multiple groups to perform FMT for recurrent [Clostridium difficile], because of the incredible public health need.”
But, he added, “We’re very concerned about industry and ability to do clinical trials.”
Those trials are important, Dr. DuPont said. “I think we have to address very actively how industry can move these products through,” he said, “because all of us want to remove the F from FMT,” by isolating the necessary elements of the process while not having the risk sometimes associated with human stool.
Policy slow to evolve
“I’m frustrated that it’s taken over 6 years and three draft guidances to get us this far,” Christian John Lillis, executive director of the Peggy Lillis Foundation – a group dedicated to creating awareness about the dangers of C. difficile – said at the meeting.
Mr. Lillis said that probably several thousand deaths had been prevented through increased FMT access, but that it was time to create a concrete policy that advanced the therapy.
The FDA guidance issued in 2013 allowed physicians to provide FMT for recurrent or refractory C. difficile infection without filing an investigational new drug (IND) application.
Clinicians must obtain informed consent that includes a discussion of the risks, and a statement that FMT is investigational. In March 2016, the agency issued revised draft guidance that it was aiming to require stool banks to apply for INDs, as reported by Medscape Medical News.
OpenBiome has flourished under the current policy. It has provided more than 50,000 treatments to 1,200 hospitals and clinics, and has provided FMT for 49 clinical trials and for 16 single patients who received INDs, Dr. Osman said.
But requiring INDs for all centers is a bad idea, he said. “IND requirements are insurmountable for most health centers,” Dr. Osman said, noting that most of the FMT material OpenBiome produces is sent to community-based physicians.
“These requirements would likely mean restrictions in access for stool bank–provided FMT and potentially pushing patients to physician-directed FMT or discouraging physicians from using FMT at all,” he said.
Stacy Kahn, MD, FMT director at Boston Children’s Hospital in Massachusetts, said that having ready access from a stool bank was crucial.
“Universal donor FMT is much easier, much faster and much more cost effective than what we can do as clinicians,” she said.
New safety and efficacy data
One unpublished study showed that 75% of patients treated since 2011 had a sustained cure, noted Colleen Kelly, MD, a Brown University professor of medicine and principal investigator for the National Institutes of Health–funded national FMT registry (although the data in this study were not from the FMT registry).
The study, which was a collaboration between the Alpert Medical School of Brown University, Brigham and Women’s Hospital, and Indiana University School of Medicine, attempted follow-up on 533 patients; 208 were successfully contacted, and an additional 55 had died, none due to FMT.
Dr. Kelly also presented data from the FMT National Registry showing that at 1 month posttransplant, two (1%) of 253 patients had an infection possibly related to FMT; one with Bacteroides fragilis and one with enteropathogenic E. coli. Seven hospitalizations were deemed related or possibly related to FMT, including two recurrences of C. difficile.
At 6 months posttransplant, 8 (5%) of 152 patients had a serious infection, and 23 patients reported a diagnosis of a new condition, primarily diarrhea-predominant irritable bowel syndrome, which is common post FMT, said Dr. Kelly, who presented the data on behalf of AGA, which administers the registry.
The AGA supports a continuation of the enforcement discretion as a means to maintain patient access where the evidence supports the use of FMT, but the group does not back use of FMT outside medical supervision, Dr. Kelly said.
This article originally appeared on Medscape. For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Attendees at a public meeting on Nov. 4 gave the US Food and Drug Administration conflicting views on whether the agency should continue to allow a relatively loose regulatory environment for fecal microbiota transplants (FMT) – debating the limits of “enforcement discretion” the FDA now has in place.
The question is especially relevant as use of the procedure is growing, while safety data are not being rigorously collected in all cases. The death of an immunocompromised FMT patient earlier in 2018 from an invasive bacterial infection caused by drug-resistant Escherichia coli, as reported by Medscape Medical News, is seen by some as an example of the consequences of a loose policy.
Still, the American Gastroenterological Association (AGA) presented new, unpublished follow-up data at the meeting that showed that the majority of FMT patients in a national registry had no adverse events.
Some companies developing FMT-based products argued at the meeting that the agency should impose stricter requirements, while stool banks and clinicians offering the therapy outside of clinical trials said that the current policy – in place since 2013 – in which the FDA has exercised “enforcement discretion,” should be allowed to continue.
“Enforcement discretion has been successful in enabling and overcoming key barriers to access to treatment,” said Majdi Osman, MD, clinical program director at OpenBiome, a nonprofit stool bank based in Cambridge, Mass. Dr. Osman said that 98% of the U.S. population now lives within a 2-hour drive of an FMT provider.
Amanda Kabage, a researcher and donor program coordinator for the Microbiota Therapeutics program at the University of Minnesota in Minneapolis, and herself a former recipient of FMT, said she was in favor of continuing the FDA policy.
“If enforcement discretion were to go away, patients far sicker than I was will not have access. They’ll get sicker and they will die,” Ms. Kabage said.
But, she added, the FDA had missed an opportunity by not insisting on collecting outcomes and safety data. Minnesota has established a patient registry to do just that, and physicians cannot administer FMT unless they agree to participate, she said. In response, FDA panelists noted that the agency cannot mandate data collection under an enforcement policy.
Lee Jones, founder and chief executive officer of Rebiotix/Ferring, a biotech company focused on the development of microbiome-based therapeutics, argued for tighter restrictions, however, claiming that increased access – and the FDA policy – had led to a fourfold decrease in enrollment since the company began study of its lead FMT product, RBX2660, in 2013.
“We’re dealing with an orphan indication and the patients were hard to come by to begin with,” she said at the meeting. “Enforcement discretion has slowed our clinical development and delayed patient access to FDA-approved therapies by over 2 years.”
An investigator at the University of Texas Health Science Center at Houston, Herbert DuPont, MD, who has administered FMT and is conducting a trial for Rebiotix, said his center wanted the FDA policy to continue “allowing multiple groups to perform FMT for recurrent [Clostridium difficile], because of the incredible public health need.”
But, he added, “We’re very concerned about industry and ability to do clinical trials.”
Those trials are important, Dr. DuPont said. “I think we have to address very actively how industry can move these products through,” he said, “because all of us want to remove the F from FMT,” by isolating the necessary elements of the process while not having the risk sometimes associated with human stool.
Policy slow to evolve
“I’m frustrated that it’s taken over 6 years and three draft guidances to get us this far,” Christian John Lillis, executive director of the Peggy Lillis Foundation – a group dedicated to creating awareness about the dangers of C. difficile – said at the meeting.
Mr. Lillis said that probably several thousand deaths had been prevented through increased FMT access, but that it was time to create a concrete policy that advanced the therapy.
The FDA guidance issued in 2013 allowed physicians to provide FMT for recurrent or refractory C. difficile infection without filing an investigational new drug (IND) application.
Clinicians must obtain informed consent that includes a discussion of the risks, and a statement that FMT is investigational. In March 2016, the agency issued revised draft guidance that it was aiming to require stool banks to apply for INDs, as reported by Medscape Medical News.
OpenBiome has flourished under the current policy. It has provided more than 50,000 treatments to 1,200 hospitals and clinics, and has provided FMT for 49 clinical trials and for 16 single patients who received INDs, Dr. Osman said.
But requiring INDs for all centers is a bad idea, he said. “IND requirements are insurmountable for most health centers,” Dr. Osman said, noting that most of the FMT material OpenBiome produces is sent to community-based physicians.
“These requirements would likely mean restrictions in access for stool bank–provided FMT and potentially pushing patients to physician-directed FMT or discouraging physicians from using FMT at all,” he said.
Stacy Kahn, MD, FMT director at Boston Children’s Hospital in Massachusetts, said that having ready access from a stool bank was crucial.
“Universal donor FMT is much easier, much faster and much more cost effective than what we can do as clinicians,” she said.
New safety and efficacy data
One unpublished study showed that 75% of patients treated since 2011 had a sustained cure, noted Colleen Kelly, MD, a Brown University professor of medicine and principal investigator for the National Institutes of Health–funded national FMT registry (although the data in this study were not from the FMT registry).
The study, which was a collaboration between the Alpert Medical School of Brown University, Brigham and Women’s Hospital, and Indiana University School of Medicine, attempted follow-up on 533 patients; 208 were successfully contacted, and an additional 55 had died, none due to FMT.
Dr. Kelly also presented data from the FMT National Registry showing that at 1 month posttransplant, two (1%) of 253 patients had an infection possibly related to FMT; one with Bacteroides fragilis and one with enteropathogenic E. coli. Seven hospitalizations were deemed related or possibly related to FMT, including two recurrences of C. difficile.
At 6 months posttransplant, 8 (5%) of 152 patients had a serious infection, and 23 patients reported a diagnosis of a new condition, primarily diarrhea-predominant irritable bowel syndrome, which is common post FMT, said Dr. Kelly, who presented the data on behalf of AGA, which administers the registry.
The AGA supports a continuation of the enforcement discretion as a means to maintain patient access where the evidence supports the use of FMT, but the group does not back use of FMT outside medical supervision, Dr. Kelly said.
This article originally appeared on Medscape. For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Attendees at a public meeting on Nov. 4 gave the US Food and Drug Administration conflicting views on whether the agency should continue to allow a relatively loose regulatory environment for fecal microbiota transplants (FMT) – debating the limits of “enforcement discretion” the FDA now has in place.
The question is especially relevant as use of the procedure is growing, while safety data are not being rigorously collected in all cases. The death of an immunocompromised FMT patient earlier in 2018 from an invasive bacterial infection caused by drug-resistant Escherichia coli, as reported by Medscape Medical News, is seen by some as an example of the consequences of a loose policy.
Still, the American Gastroenterological Association (AGA) presented new, unpublished follow-up data at the meeting that showed that the majority of FMT patients in a national registry had no adverse events.
Some companies developing FMT-based products argued at the meeting that the agency should impose stricter requirements, while stool banks and clinicians offering the therapy outside of clinical trials said that the current policy – in place since 2013 – in which the FDA has exercised “enforcement discretion,” should be allowed to continue.
“Enforcement discretion has been successful in enabling and overcoming key barriers to access to treatment,” said Majdi Osman, MD, clinical program director at OpenBiome, a nonprofit stool bank based in Cambridge, Mass. Dr. Osman said that 98% of the U.S. population now lives within a 2-hour drive of an FMT provider.
Amanda Kabage, a researcher and donor program coordinator for the Microbiota Therapeutics program at the University of Minnesota in Minneapolis, and herself a former recipient of FMT, said she was in favor of continuing the FDA policy.
“If enforcement discretion were to go away, patients far sicker than I was will not have access. They’ll get sicker and they will die,” Ms. Kabage said.
But, she added, the FDA had missed an opportunity by not insisting on collecting outcomes and safety data. Minnesota has established a patient registry to do just that, and physicians cannot administer FMT unless they agree to participate, she said. In response, FDA panelists noted that the agency cannot mandate data collection under an enforcement policy.
Lee Jones, founder and chief executive officer of Rebiotix/Ferring, a biotech company focused on the development of microbiome-based therapeutics, argued for tighter restrictions, however, claiming that increased access – and the FDA policy – had led to a fourfold decrease in enrollment since the company began study of its lead FMT product, RBX2660, in 2013.
“We’re dealing with an orphan indication and the patients were hard to come by to begin with,” she said at the meeting. “Enforcement discretion has slowed our clinical development and delayed patient access to FDA-approved therapies by over 2 years.”
An investigator at the University of Texas Health Science Center at Houston, Herbert DuPont, MD, who has administered FMT and is conducting a trial for Rebiotix, said his center wanted the FDA policy to continue “allowing multiple groups to perform FMT for recurrent [Clostridium difficile], because of the incredible public health need.”
But, he added, “We’re very concerned about industry and ability to do clinical trials.”
Those trials are important, Dr. DuPont said. “I think we have to address very actively how industry can move these products through,” he said, “because all of us want to remove the F from FMT,” by isolating the necessary elements of the process while not having the risk sometimes associated with human stool.
Policy slow to evolve
“I’m frustrated that it’s taken over 6 years and three draft guidances to get us this far,” Christian John Lillis, executive director of the Peggy Lillis Foundation – a group dedicated to creating awareness about the dangers of C. difficile – said at the meeting.
Mr. Lillis said that probably several thousand deaths had been prevented through increased FMT access, but that it was time to create a concrete policy that advanced the therapy.
The FDA guidance issued in 2013 allowed physicians to provide FMT for recurrent or refractory C. difficile infection without filing an investigational new drug (IND) application.
Clinicians must obtain informed consent that includes a discussion of the risks, and a statement that FMT is investigational. In March 2016, the agency issued revised draft guidance that it was aiming to require stool banks to apply for INDs, as reported by Medscape Medical News.
OpenBiome has flourished under the current policy. It has provided more than 50,000 treatments to 1,200 hospitals and clinics, and has provided FMT for 49 clinical trials and for 16 single patients who received INDs, Dr. Osman said.
But requiring INDs for all centers is a bad idea, he said. “IND requirements are insurmountable for most health centers,” Dr. Osman said, noting that most of the FMT material OpenBiome produces is sent to community-based physicians.
“These requirements would likely mean restrictions in access for stool bank–provided FMT and potentially pushing patients to physician-directed FMT or discouraging physicians from using FMT at all,” he said.
Stacy Kahn, MD, FMT director at Boston Children’s Hospital in Massachusetts, said that having ready access from a stool bank was crucial.
“Universal donor FMT is much easier, much faster and much more cost effective than what we can do as clinicians,” she said.
New safety and efficacy data
One unpublished study showed that 75% of patients treated since 2011 had a sustained cure, noted Colleen Kelly, MD, a Brown University professor of medicine and principal investigator for the National Institutes of Health–funded national FMT registry (although the data in this study were not from the FMT registry).
The study, which was a collaboration between the Alpert Medical School of Brown University, Brigham and Women’s Hospital, and Indiana University School of Medicine, attempted follow-up on 533 patients; 208 were successfully contacted, and an additional 55 had died, none due to FMT.
Dr. Kelly also presented data from the FMT National Registry showing that at 1 month posttransplant, two (1%) of 253 patients had an infection possibly related to FMT; one with Bacteroides fragilis and one with enteropathogenic E. coli. Seven hospitalizations were deemed related or possibly related to FMT, including two recurrences of C. difficile.
At 6 months posttransplant, 8 (5%) of 152 patients had a serious infection, and 23 patients reported a diagnosis of a new condition, primarily diarrhea-predominant irritable bowel syndrome, which is common post FMT, said Dr. Kelly, who presented the data on behalf of AGA, which administers the registry.
The AGA supports a continuation of the enforcement discretion as a means to maintain patient access where the evidence supports the use of FMT, but the group does not back use of FMT outside medical supervision, Dr. Kelly said.
This article originally appeared on Medscape. For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Previously healthy patients hospitalized for sepsis show increased mortality
WASHINGTON – Although severe, community-acquired sepsis in previously healthy U.S. adults is relatively uncommon, it occurs often enough to strike about 40,000 people annually, and when previously healthy people are hospitalized for severe sepsis, their rate of in-hospital mortality was double the rate in people with one or more comorbidities who have severe, community-acquired sepsis, based on a review of almost 7 million Americans hospitalized for sepsis.
The findings “underscore the importance of improving public awareness of sepsis and emphasizing early sepsis recognition and treatment in all patients,” including those without comorbidities, Chanu Rhee, MD, said at an annual scientific meeting on infectious diseases. He hypothesized that the increased sepsis mortality among previously healthy patients may have stemmed from factors such as delayed sepsis recognition resulting in hospitalization at a more advanced stage and less aggressive management.
In addition, “the findings provide context for high-profile reports about sepsis death in previously healthy people,” said Dr. Rhee, an infectious diseases and critical care physician at Brigham and Women’s Hospital in Boston. Dr. Rhee and associates found that, among patients hospitalized with what the researchers defined as “community-acquired” sepsis, 3% were judged previously healthy by having no identified major or minor comorbidity or pregnancy at the time of hospitalization, a percentage that – while small – still translates into roughly 40,000 such cases annually in the United States. That helps explain why every so often a headline appears about a famous person who died suddenly and unexpectedly from sepsis, he noted.
The study used data collected on hospitalized U.S. patients in the Cerner Health Facts, HCA Healthcare, and Institute for Health Metrics and Evaluation databases, which included about 6.7 million people total including 337,983 identified as having community-acquired sepsis, defined as patients who met the criteria for adult sepsis advanced by the Centers for Disease Control and Prevention within 2 days of their hospital admission. The researchers looked further into the hospital records of these patients and divided them into patients with one or more major comorbidities (96% of the cohort), patients who were pregnant or had a “minor” comorbidity such as a lipid disorder, benign neoplasm, or obesity (1% of the study group), or those with no chronic comorbidity (3%; the subgroup the researchers deemed previously healthy).
In a multivariate analysis that adjusted for patients’ age, sex, race, infection site, and illness severity at the time of hospital admission the researchers found that the rate of in-hospital death among the previously healthy patients was exactly twice the rate of those who had at least one major chronic comorbidity, Dr. Rhee reported. Differences in the treatment received by the previously-healthy patients or in their medical status compared with patients with a major comorbidity suggested that the previously health patients were sicker. They had a higher rate of mechanical ventilation, 30%, compared with about 18% for those with a comorbidity; a higher rate of acute kidney injury, about 43% in those previously healthy and 28% in those with a comorbidity; and a higher percentage had an elevated lactate level, about 41% among the previously healthy patients and about 22% among those with a comorbidity.
SOURCE: Alrawashdeh M et al. Open Forum Infect Dis. 2019 Oct 23;6. Abstract 891.
WASHINGTON – Although severe, community-acquired sepsis in previously healthy U.S. adults is relatively uncommon, it occurs often enough to strike about 40,000 people annually, and when previously healthy people are hospitalized for severe sepsis, their rate of in-hospital mortality was double the rate in people with one or more comorbidities who have severe, community-acquired sepsis, based on a review of almost 7 million Americans hospitalized for sepsis.
The findings “underscore the importance of improving public awareness of sepsis and emphasizing early sepsis recognition and treatment in all patients,” including those without comorbidities, Chanu Rhee, MD, said at an annual scientific meeting on infectious diseases. He hypothesized that the increased sepsis mortality among previously healthy patients may have stemmed from factors such as delayed sepsis recognition resulting in hospitalization at a more advanced stage and less aggressive management.
In addition, “the findings provide context for high-profile reports about sepsis death in previously healthy people,” said Dr. Rhee, an infectious diseases and critical care physician at Brigham and Women’s Hospital in Boston. Dr. Rhee and associates found that, among patients hospitalized with what the researchers defined as “community-acquired” sepsis, 3% were judged previously healthy by having no identified major or minor comorbidity or pregnancy at the time of hospitalization, a percentage that – while small – still translates into roughly 40,000 such cases annually in the United States. That helps explain why every so often a headline appears about a famous person who died suddenly and unexpectedly from sepsis, he noted.
The study used data collected on hospitalized U.S. patients in the Cerner Health Facts, HCA Healthcare, and Institute for Health Metrics and Evaluation databases, which included about 6.7 million people total including 337,983 identified as having community-acquired sepsis, defined as patients who met the criteria for adult sepsis advanced by the Centers for Disease Control and Prevention within 2 days of their hospital admission. The researchers looked further into the hospital records of these patients and divided them into patients with one or more major comorbidities (96% of the cohort), patients who were pregnant or had a “minor” comorbidity such as a lipid disorder, benign neoplasm, or obesity (1% of the study group), or those with no chronic comorbidity (3%; the subgroup the researchers deemed previously healthy).
In a multivariate analysis that adjusted for patients’ age, sex, race, infection site, and illness severity at the time of hospital admission the researchers found that the rate of in-hospital death among the previously healthy patients was exactly twice the rate of those who had at least one major chronic comorbidity, Dr. Rhee reported. Differences in the treatment received by the previously-healthy patients or in their medical status compared with patients with a major comorbidity suggested that the previously health patients were sicker. They had a higher rate of mechanical ventilation, 30%, compared with about 18% for those with a comorbidity; a higher rate of acute kidney injury, about 43% in those previously healthy and 28% in those with a comorbidity; and a higher percentage had an elevated lactate level, about 41% among the previously healthy patients and about 22% among those with a comorbidity.
SOURCE: Alrawashdeh M et al. Open Forum Infect Dis. 2019 Oct 23;6. Abstract 891.
WASHINGTON – Although severe, community-acquired sepsis in previously healthy U.S. adults is relatively uncommon, it occurs often enough to strike about 40,000 people annually, and when previously healthy people are hospitalized for severe sepsis, their rate of in-hospital mortality was double the rate in people with one or more comorbidities who have severe, community-acquired sepsis, based on a review of almost 7 million Americans hospitalized for sepsis.
The findings “underscore the importance of improving public awareness of sepsis and emphasizing early sepsis recognition and treatment in all patients,” including those without comorbidities, Chanu Rhee, MD, said at an annual scientific meeting on infectious diseases. He hypothesized that the increased sepsis mortality among previously healthy patients may have stemmed from factors such as delayed sepsis recognition resulting in hospitalization at a more advanced stage and less aggressive management.
In addition, “the findings provide context for high-profile reports about sepsis death in previously healthy people,” said Dr. Rhee, an infectious diseases and critical care physician at Brigham and Women’s Hospital in Boston. Dr. Rhee and associates found that, among patients hospitalized with what the researchers defined as “community-acquired” sepsis, 3% were judged previously healthy by having no identified major or minor comorbidity or pregnancy at the time of hospitalization, a percentage that – while small – still translates into roughly 40,000 such cases annually in the United States. That helps explain why every so often a headline appears about a famous person who died suddenly and unexpectedly from sepsis, he noted.
The study used data collected on hospitalized U.S. patients in the Cerner Health Facts, HCA Healthcare, and Institute for Health Metrics and Evaluation databases, which included about 6.7 million people total including 337,983 identified as having community-acquired sepsis, defined as patients who met the criteria for adult sepsis advanced by the Centers for Disease Control and Prevention within 2 days of their hospital admission. The researchers looked further into the hospital records of these patients and divided them into patients with one or more major comorbidities (96% of the cohort), patients who were pregnant or had a “minor” comorbidity such as a lipid disorder, benign neoplasm, or obesity (1% of the study group), or those with no chronic comorbidity (3%; the subgroup the researchers deemed previously healthy).
In a multivariate analysis that adjusted for patients’ age, sex, race, infection site, and illness severity at the time of hospital admission the researchers found that the rate of in-hospital death among the previously healthy patients was exactly twice the rate of those who had at least one major chronic comorbidity, Dr. Rhee reported. Differences in the treatment received by the previously-healthy patients or in their medical status compared with patients with a major comorbidity suggested that the previously health patients were sicker. They had a higher rate of mechanical ventilation, 30%, compared with about 18% for those with a comorbidity; a higher rate of acute kidney injury, about 43% in those previously healthy and 28% in those with a comorbidity; and a higher percentage had an elevated lactate level, about 41% among the previously healthy patients and about 22% among those with a comorbidity.
SOURCE: Alrawashdeh M et al. Open Forum Infect Dis. 2019 Oct 23;6. Abstract 891.
REPORTING FROM ID WEEK 2019
Do Probiotics Reduce C diff Risk in Hospitalized Patients?
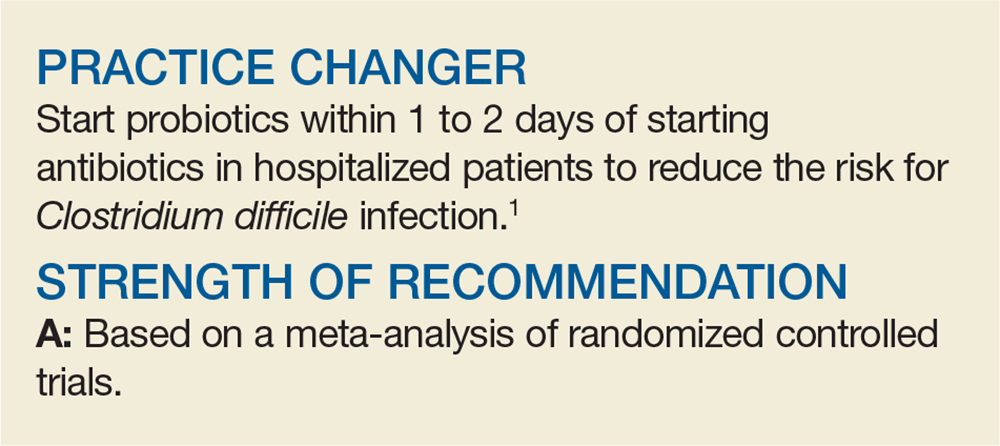
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.

A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).

A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
Requests for crowd diagnoses of STDs common on social media
Requests for crowd diagnosis of sexually transmitted diseases were frequent on a social media website, new research found.
The social media website Reddit, which currently has 330 million monthly active users, is home to more than 230 health-related subreddits, including r/STD, a forum that allows users to publicly share “stories, concerns, and questions” about “anything and everything STD related,” Alicia L. Nobles, PhD, of the department of medicine at the University of California, San Diego, and associates wrote in a research letter published in JAMA.
Dr. Noble and associates conducted an analysis of all posts published to r/STD from the subreddit’s inception during November 2010–February 2019, a total of 16,979 posts. Three coauthors independently coded each post, recording whether or not a post requested a crowd diagnosis, and if so, whether that request was made to obtain a second opinion after a visit to a health care professional.
About 58% of posts requested a crowd diagnosis, 31% of which included an image of the physical signs. One-fifth of the requests for a crowd diagnosis were seeking a second opinion after a previous diagnosis by a health care professional. Nearly 90% of all crowd-diagnosis requests received at least one reply (mean responses, 1.7), with a median response time of 3.04 hours. About 80% of requests were answered in less than 1 day.
While crowd diagnoses do seem to be popular and have the benefits of anonymity, rapid response, and multiple opinions, the accuracy of crowd diagnoses is unknown given the limited information responders operate with and the potential lack of responder medical training, the study authors noted. Misdiagnosis could allow further disease transmission, and third parties viewing posts could incorrectly self-diagnose their own condition.
“Health care professionals could partner with social media outlets to promote the potential benefits of crowd diagnosis while suppressing potential harms, for example by having trained professionals respond to posts to better diagnose and make referrals to health care centers,” Dr. Nobles and associates concluded.
One coauthor reported receiving personal fees from Bloomberg and Good Analytics, and another reported receiving grants from the National Institutes of Health; no other disclosures were reported.
SOURCE: Nobles AL et al. JAMA. 2019 Nov 5;322(17):1712-3.
Requests for crowd diagnosis of sexually transmitted diseases were frequent on a social media website, new research found.
The social media website Reddit, which currently has 330 million monthly active users, is home to more than 230 health-related subreddits, including r/STD, a forum that allows users to publicly share “stories, concerns, and questions” about “anything and everything STD related,” Alicia L. Nobles, PhD, of the department of medicine at the University of California, San Diego, and associates wrote in a research letter published in JAMA.
Dr. Noble and associates conducted an analysis of all posts published to r/STD from the subreddit’s inception during November 2010–February 2019, a total of 16,979 posts. Three coauthors independently coded each post, recording whether or not a post requested a crowd diagnosis, and if so, whether that request was made to obtain a second opinion after a visit to a health care professional.
About 58% of posts requested a crowd diagnosis, 31% of which included an image of the physical signs. One-fifth of the requests for a crowd diagnosis were seeking a second opinion after a previous diagnosis by a health care professional. Nearly 90% of all crowd-diagnosis requests received at least one reply (mean responses, 1.7), with a median response time of 3.04 hours. About 80% of requests were answered in less than 1 day.
While crowd diagnoses do seem to be popular and have the benefits of anonymity, rapid response, and multiple opinions, the accuracy of crowd diagnoses is unknown given the limited information responders operate with and the potential lack of responder medical training, the study authors noted. Misdiagnosis could allow further disease transmission, and third parties viewing posts could incorrectly self-diagnose their own condition.
“Health care professionals could partner with social media outlets to promote the potential benefits of crowd diagnosis while suppressing potential harms, for example by having trained professionals respond to posts to better diagnose and make referrals to health care centers,” Dr. Nobles and associates concluded.
One coauthor reported receiving personal fees from Bloomberg and Good Analytics, and another reported receiving grants from the National Institutes of Health; no other disclosures were reported.
SOURCE: Nobles AL et al. JAMA. 2019 Nov 5;322(17):1712-3.
Requests for crowd diagnosis of sexually transmitted diseases were frequent on a social media website, new research found.
The social media website Reddit, which currently has 330 million monthly active users, is home to more than 230 health-related subreddits, including r/STD, a forum that allows users to publicly share “stories, concerns, and questions” about “anything and everything STD related,” Alicia L. Nobles, PhD, of the department of medicine at the University of California, San Diego, and associates wrote in a research letter published in JAMA.
Dr. Noble and associates conducted an analysis of all posts published to r/STD from the subreddit’s inception during November 2010–February 2019, a total of 16,979 posts. Three coauthors independently coded each post, recording whether or not a post requested a crowd diagnosis, and if so, whether that request was made to obtain a second opinion after a visit to a health care professional.
About 58% of posts requested a crowd diagnosis, 31% of which included an image of the physical signs. One-fifth of the requests for a crowd diagnosis were seeking a second opinion after a previous diagnosis by a health care professional. Nearly 90% of all crowd-diagnosis requests received at least one reply (mean responses, 1.7), with a median response time of 3.04 hours. About 80% of requests were answered in less than 1 day.
While crowd diagnoses do seem to be popular and have the benefits of anonymity, rapid response, and multiple opinions, the accuracy of crowd diagnoses is unknown given the limited information responders operate with and the potential lack of responder medical training, the study authors noted. Misdiagnosis could allow further disease transmission, and third parties viewing posts could incorrectly self-diagnose their own condition.
“Health care professionals could partner with social media outlets to promote the potential benefits of crowd diagnosis while suppressing potential harms, for example by having trained professionals respond to posts to better diagnose and make referrals to health care centers,” Dr. Nobles and associates concluded.
One coauthor reported receiving personal fees from Bloomberg and Good Analytics, and another reported receiving grants from the National Institutes of Health; no other disclosures were reported.
SOURCE: Nobles AL et al. JAMA. 2019 Nov 5;322(17):1712-3.
FROM JAMA
Key clinical point: Crowd-diagnosis requests of STDs are popular on a social media–based health forum.
Major finding: Nearly 60% of r/STD posts were a request for diagnosis, 87% of which received a reply (mean responses, 1.7; mean response time, 3.0 hours).
Study details: A review of 16,979 posts on the subreddit r/STD.
Disclosures: One coauthor reported receiving personal fees from Bloomberg and Good Analytics, and another reported receiving grants from the National Institutes of Health; no other disclosures were reported.Source: Nobles AL et al. JAMA. 2019 Nov 5;322(17):1712-3.
Seborrhea Herpeticum: Cutaneous Herpes Simplex Virus Infection Within Infantile Seborrheic Dermatitis
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.
Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.

Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.

Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
Practice Points
- Cutaneous herpes simplex virus may present in a seborrheic distribution within infantile seborrheic dermatitis, suggesting underlying dysfunction secondary to seborrheic dermatitis.
- Treatment of seborrhea herpeticum involves antiviral therapy to treat the secondary viral infection and gentle skin care precautions for the primary condition.