User login
Click for Credit: Long-term antibiotics & stroke, CHD; Postvaccination seizures; more
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
Here are 5 articles from the November issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Poor response to statins hikes risk of cardiovascular events
To take the posttest, go to: https://bit.ly/2MVHlDR
Expires April 17, 2020
2. Postvaccination febrile seizures are no more severe than other febrile seizures
To take the posttest, go to: https://bit.ly/2VUJzaE
Expires April 19, 2020
3. Hydroxychloroquine adherence in SLE: worse than you thought
To take the posttest, go to: https://bit.ly/2oT00Z9
Expires April 22, 2020
4. Long-term antibiotic use may heighten stroke, CHD risk
To take the posttest, go to: https://bit.ly/2OUUVu5
Expires April 28, 2020
5. Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
To take the posttest, go to: https://bit.ly/2Msgqkb
Expires May 1, 2020
STI update: Testing, treatment, and emerging threats
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
KEY POINTS
- Screen for gonorrhea and chlamydia annually—and more frequently for those at highest risk—in sexually active women age 25 and younger and in men who have sex with men, who should also be screened at the same time for human immunodeficiency virus (HIV) and syphilis.
- Test for Trichomonas vaginalis in women who have symptoms suggesting it, and routinely screen for this pathogen in women who are HIV-positive.
- Nucleic acid amplification is the preferred test for gonorrhea, chlamydia, trichomoniasis, and M genitalium infection; the use of urine specimens is acceptable.
- Consider M genitalium if therapy for gonorrhea and chlamydia fails or tests for those diseases are negative.
- Single-dose antibiotic therapy is preferred for chlamydia and uncomplicated gonorrhea. It is also available for trichomoniasis, although metronidazole 500 mg twice a day for 7 days has a higher cure rate.
Appropriate laboratory testing in Lyme disease
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
Lyme disease is a complex multisystem bacterial infection affecting the skin, joints, heart, and nervous system. The full spectrum of disease was first recognized and the disease was named in the 1970s during an outbreak of arthritis in children in the town of Lyme, Connecticut.1
This review describes the epidemiology and pathogenesis of Lyme disease, the advantages and disadvantages of current diagnostic methods, and diagnostic algorithms.
THE MOST COMMON TICK-BORNE INFECTION IN NORTH AMERICA
Lyme disease is the most common tick-borne infection in North America.2,3 In the United States, more than 30,000 cases are reported annually. In fact, in 2017, the number of cases was about 42,000, a 16% increase from the previous year, according to the US Centers for Disease Control and Prevention (CDC).
Infected nymphs account for most cases.
The infection is caused by Borrelia burgdorferi, a particularly arthritogenic spirochete transmitted by Ixodes scapularis (the black-legged deer tick, (Figure 1) and Ixodes pacificus (the Western black-legged tick). Although the infection can occur at any time of the year, its peak incidence is in May to late September, coinciding with increased outdoor recreational activity in areas where ticks live.3,4 The typical tick habitat consists of deciduous woodland with sufficient humidity provided by a good layer of decaying vegetation. However, people can contract Lyme disease in their own backyard.3
Most cases of Lyme disease are seen in the northeastern United States, mainly in suburban and rural areas.2,3 Other areas affected include the midwestern states of Minnesota, Wisconsin, and Michigan, as well as northern California.4 Fourteen states and the District of Columbia report a high average incidence (> 10 cases per 100,000 persons) (Table 1).2
FIRST COMES IgM, THEN IgG
The pathogenesis and the different stages of infection should inform laboratory testing in Lyme disease.
It is estimated that only 5% of infected ticks that bite people actually transmit their spirochetes to the human host.5 However, once infected, the patient’s innate immune system mounts a response that results in the classic erythema migrans rash at the bite site. A rash develops in only about 85% of patients who are infected and can appear at any time between 3 and 30 days, but most commonly after 7 days. Hence, a rash occurring within the first few hours of tick contact is not erythema migrans and does not indicate infection, but rather an early reaction to tick salivary antigens.5
Antibody levels remain below the detection limits of currently available serologic tests in the first 7 days after exposure. Immunoglobulin M (IgM) antibody titers peak between 8 and 14 days after tick contact, but IgM antibodies may never develop if the patient is started on early appropriate antimicrobial therapy.5
If the infection is not treated, the spirochete may disseminate through the blood from the bite site to different tissues.3 Both cell-mediated and antibody-mediated immunity swing into action to kill the spirochetes at this stage. The IgM antibody response occurs in 1 to 2 weeks, followed by a robust IgG response in 2 to 4 weeks.6
Because IgM can also cross-react with antigens other than those associated with B burgdorferi, the IgM test is less specific than the IgG test for Lyme disease.
Once a patient is exposed and mounts an antibody-mediated response to the spirochete, the antibody profile may persist for months to years, even after successful antibiotic treatment and cure of the disease.5
Despite the immune system’s robust series of defenses, untreated B burgdorferi infection can persist, as the organism has a bag of tricks to evade destruction. It can decrease its expression of specific immunogenic surface-exposed proteins, change its antigenic properties through recombination, and bind to the patient’s extracellular matrix proteins to facilitate further dissemination.3
Certain host-genetic factors also play a role in the pathogenesis of Lyme disease, such as the HLA-DR4 allele, which has been associated with antibiotic-refractory Lyme-related arthritis.3
LYME DISEASE EVOLVES THROUGH STAGES
Lyme disease evolves through stages broadly classified as early and late infection, with significant variability in its presentation.7
Early infection
Early disease is further subdivided into “localized” infection (stage 1), characterized by a single erythema migrans lesion and local lymphadenopathy, and “disseminated” infection (stage 2), associated with multiple erythema migrans lesions distant from the bite site, facial nerve palsy, radiculoneuritis, meningitis, carditis, or migratory arthritis or arthralgia.8
Highly specific physical findings include erythema migrans, cranial nerve palsy, high-grade or progressive conduction block, and recurrent migratory polyarthritis. Less specific symptoms and signs of Lyme disease include arthralgia, myalgia, neck stiffness, palpitations, and myocarditis.5
Erythema migrans lesions are evident in at least 85% of patients with early disease.9 If they are not apparent on physical examination, they may be located at hidden sites and may be atypical in appearance or transient.5
If treatment is not started in the initial stage of the disease, 60% of infected patients may develop disseminated infection.5 Progressive, untreated infection can manifest with Lyme arthritis and neuroborreliosis.7
Noncutaneous manifestations are less common now than in the past due to increased awareness of the disease and early initiation of treatment.10
Late infection
Manifestations of late (stage 3) infection include oligoarthritis (affecting any joint but often the knee) and neuroborreliosis. Clinical signs and symptoms of Lyme disease may take months to resolve even after appropriate antimicrobial therapy is completed. This should not be interpreted as ongoing, persistent infection, but as related to host immune-mediated activity.5
INTERPRET LABORATORY RESULTS BASED ON PRETEST PROBABILITY
The usefulness of a laboratory test depends on the individual patient’s pretest probability of infection, which in turn depends on the patient’s epidemiologic risk of exposure and clinical features of Lyme disease. Patients with a high pretest probability—eg, a history of a tick bite followed by the classic erythema migrans rash—do not need testing and can start antimicrobial therapy right away.11
Serologic tests are the gold standard
Prompt diagnosis is important, as early Lyme disease is easily treatable without any future sequelae.11
Tests for Lyme disease can be divided into direct methods, which detect the spirochete itself by culture or by polymerase chain reaction (PCR), and indirect methods, which detect antibodies (Table 2). Direct tests lack sensitivity for Lyme disease; hence, serologic tests remain the gold standard. Currently recommended is a standard 2-tier testing strategy using an enzyme-linked immunosorbent assay (ELISA) followed by Western blot for confirmation.
DIRECT METHODS
Culture lacks sensitivity
A number of factors limit the sensitivity of direct culture for diagnosing Lyme disease. B burgdorferi does not grow easily in culture, requiring special media, low temperatures, and long periods of incubation. Only a relatively few spirochetes are present in human tissues and body fluids to begin with, and bacterial counts are further reduced with duration and dissemination of infection.5 All of these limit the possibility of detecting this organism.
Polymerase chain reaction may help in some situations
Molecular assays are not part of the standard evaluation and should be used only in conjunction with serologic testing.7 These tests have high specificity but lack consistent sensitivity.
That said, PCR testing may be useful:
- In early infection, before antibody responses develop
- In reinfection, when serologic tests are not reliable because the antibodies persist for many years after an infection in many patients
- In endemic areas where serologic testing has high false-positive rates due to high baseline population seropositivity for anti-Borrelia antibodies caused by subclinical infection.3
PCR assays that target plasmid-borne genes encoding outer surface proteins A and C (OspA and OspC) and VisE (variable major protein-like sequence, expressed) are more sensitive than those that detect chromosomal 16s ribosomal ribonucleic acid (rRNA) genes, as plasmid-rich “blebs” are shed in larger concentrations than chromosomal DNA during active infection.7 However, these plasmid-contained genes persist in body tissues and fluids even after the infection is cleared, and their detection may not necessarily correlate with ongoing disease.8 Detection of chromosomal 16s rRNA genes is a better predictor of true organism viability.
The sensitivity of PCR for borrelial DNA depends on the type of sample. If a skin biopsy sample is taken of the leading edge of an erythema migrans lesion, the sensitivity is 69% and the specificity is 100%. In patients with Lyme arthritis, PCR of the synovial fluid has a sensitivity of up to 80%. However, the sensitivity of PCR of the cerebrospinal fluid of patients with neurologic manifestations of Lyme disease is only 19%.7 PCR of other clinical samples, including blood and urine, is not recommended, as spirochetes are primarily confined to tissues, and very few are present in these body fluids.3,12
The disadvantage of PCR is that a positive result does not always mean active infection, as the DNA of the dead microbe persists for several months even after successful treatment.8
INDIRECT METHODS
Enzyme-linked immunosorbent assay
ELISAs detect anti-Borrelia antibodies. Early-generation ELISAs, still used in many laboratories, use whole-cell extracts of B burgdorferi. Examples are the Vidas Lyme screen (Biomérieux, biomerieux-usa.com) and the Wampole B burgdorferi IgG/M EIA II assay (Alere, www.alere.com). Newer ELISAs use recombinant proteins.13
Three major targets for ELISA antibodies are flagellin (Fla), outer surface protein C (OspC), and VisE, especially the invariable region 6 (IR6). Among these, VisE-IR6 is the most conserved region in B burgdorferi.
Early-generation assays have a sensitivity of 89% and specificity of 72%.11 However, the patient’s serum may have antibodies that cross-react with unrelated bacterial antigens, leading to false-positive results (Table 3). Whole-cell sonicate assays are not recommended as an independent test and must be confirmed with Western blot testing when assay results are indeterminate or positive.11
Newer-generation ELISAs detect antibodies targeting recombinant proteins of VisE, especially a synthetic peptide C6, within IR6.13 VisE-IR6 is the most conserved region of the B burgdorferi complex, and its detection is a highly specific finding, supporting the diagnosis of Lyme disease. Antibodies against VisE-IR6 antigen are the earliest to develop.5 An example of a newer-generation serologic test is the VisE C6 Lyme EIA kit, approved as a first-tier test by the US Food and Drug Administration in 2001. This test has a specificity of 99%,14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%).15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier testing during early infection (sensitivity 29%–74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in early disseminated infection).6
During early infection, older and newer ELISAs are less sensitive because of the limited number of antigens expressed at this stage.13 All patients suspected of having early Lyme disease who are seronegative at initial testing should have follow-up testing to look for seroconversion.13
Western blot
Western blot (immunoblot) testing identifies IgM and IgG antibodies against specific B burgdorferi antigens. It is considered positive if it detects at least 2 of a possible 3 specific IgM bands in the first 4 weeks of disease or at least 5 of 10 specific IgG bands after 4 weeks of disease (Table 4 and Figure 2).16
The nature of the bands indicates the duration of infection: Western blot bands against 23-kD OspC and 41-kD FlaB are seen in early localized infection, whereas bands against all 3 B burgdorferi proteins will be seen after several weeks of disease.17 The IgM result should be interpreted carefully, as only 2 bands are required for the test to be positive, and IgM binds to antigen less specifically than IgG.12
Interpreting the IgM Western blot test: The ‘1-month rule’
If clinical symptoms and signs of Lyme disease have been present for more than 1 month, IgM reactivity alone should not be used to support the diagnosis, in view of the likelihood of a false-positive test result in this situation.18 This is called the “1-month rule” in the diagnosis of Lyme disease.13
In early localized infection, Western blot is only half as sensitive as ELISA testing. Since the overall sensitivity of a 2-step algorithm is equal to that of its least sensitive component, 2-tiered testing is not useful in early disease.13
Although currently considered the most specific test for confirmation of Lyme disease, Western blot has limitations. It is technically and interpretively complex and is thus not universally available.13 The blots are scored by visual examination, compromising the reproducibility of the test, although densitometric blot analysis techniques and automated scanning and scoring attempt to address some of these limitations.13 Like the ELISA, Western blot can have false-positive results in healthy individuals without tick exposure, as nonspecific IgM immunoblots develop faint bands. This is because of cross-reaction between B burgdorferi antigens and antigens from other microorganisms. Around 50% of healthy adults show low-level serum IgG reactivity against the FlaB antigen, leading to false-positive results as well. In cases in which the Western blot result is indeterminate, other etiologies must be considered.
False-positive IgM Western blots are a significant problem. In a 5-year retrospective study done at 63 US Air Force healthcare facilities, 113 (53.3%) of 212 IgM Western blots were falsely positive.19 A false-positive test was defined as one that failed to meet seropositivity (a first-tier test omitted or negative, > 30 days of symptoms with negative IgG blot), lack of exposure including residing in areas without documented tick habitats, patients having atypical or no symptoms, and negative serology within 30 days of a positive test.
In a similar study done in a highly endemic area, 50 (27.5%) of 182 patients had a false-positive test.20 Physicians need to be careful when interpreting IgM Western blots. It is always important to consider locale, epidemiology, and symptoms when interpreting the test.
Limitations of serologic tests for Lyme disease
Currently available serologic tests have inherent limitations:
- Antibodies against B burgdorferi take at least 1 week to develop
- The background rate of seropositivity in endemic areas can be up to 4%, affecting the utility of a positive test result
- Serologic tests cannot be used as tests of cure because antibodies can persist for months to years even after appropriate antimicrobial therapy and cure of disease; thus, a positive serologic result could represent active infection or remote exposure21
- Antibodies can cross-react with related bacteria, including other borrelial or treponemal spirochetes
- False-positive serologic test results can also occur in association with other medical conditions such as polyclonal gammopathies and systemic lupus erythematosus.12
RECOMMENDATIONS FOR TESTING
Standard 2-tier testing
The CDC released recommendations for diagnosing Lyme disease after a second national conference of serologic diagnosis of Lyme disease in October 1994.18 The 2-tiered testing method, involving a sensitive ELISA followed by the Western blot to confirm positive and indeterminate ELISA results, was suggested as the gold standard for diagnosis (Figure 3). Of note, negative ELISA results do not require further testing.11
The sensitivity of 2-tiered testing depends on the stage of the disease. Unfortunately, this method has a wide range of sensitivity (17% to 78%) in stage 1 disease. In the same stage, the sensitivity increases from 14.1% in patients with a single erythema migrans lesion and early localized infection to 65.4% in those with multiple lesions. The algorithm has excellent sensitivity in late stage 3 infection (96% to 100%).5
A 2-step ELISA algorithm
A 2-step ELISA algorithm (without the Western blot) that includes the whole-cell sonicate assay followed by the VisE C6 peptide assay actually showed higher sensitivity and comparable specificity compared with 2-tiered testing in early localized disease (sensitivity 61%–74% vs 29%–48%, respectively; specificity 99.5% for both methods).22 This higher sensitivity was even more pronounced in early disseminated infection (sensitivity 100% vs 40%, respectively). By late infection, the sensitivities of both testing strategies reached 100%. Compared with the Western blot, the 2-step ELISA algorithm was simpler to execute in a reproducible fashion.5
The Infectious Diseases Society of America is revising its current guidelines, with an update expected late this year, which may shift the recommendation from 2-tiered testing to the 2-step ELISA algorithm.
Multiplex testing
To overcome the intrinsic problems of protein-based assays, a multiplexed, array-based assay for the diagnosis of tick-borne infections called Tick-Borne Disease Serochip (TBD-Serochip) was established using recombinant antigens that identify key immunodominant epitopes.8 More studies are needed to establish the validity and usefulness of these tests in clinical practice.
Who should not be tested?
The American College of Physicians6 recommends against testing in patients:
- Presenting with nonspecific symptoms (eg, headache, myalgia, fatigue, arthralgia) without objective signs of Lyme disease
- With low pretest probability of infection based on epidemiologic exposures and clinical features
- Living in Lyme-endemic areas with no history of tick exposure6
- Presenting less than 1 week after tick exposure5
- Seeking a test of cure for treated Lyme disease.
DIAGNOSIS IN SPECIAL SITUATIONS
Early Lyme disease
The classic erythema migrans lesion on physical examination of a patient with suspected Lyme disease is diagnostic and does not require laboratory confirmation.10
In ambiguous cases, 2-tiered testing of a serum sample during the acute presentation and again 4 to 6 weeks later can be useful. In patients who remain seronegative on paired serum samples despite symptoms lasting longer than 6 weeks and no antibiotic treatment in the interim, the diagnosis of Lyme disease is unlikely, and another diagnosis should be sought.3
Antimicrobial therapy may block the serologic response; hence, negative serologic testing in patients started on empiric antibiotics should not rule out Lyme disease.6
PCR or bacterial culture testing is not recommended in the evaluation of suspected early Lyme disease.
Central nervous system Lyme disease
Central nervous system Lyme disease is diagnosed by 2-tiered testing using peripheral blood samples because all patients with this infectious manifestation should have mounted an adequate IgG response in the blood.11
B cells migrate to and proliferate inside the central nervous system, leading to intrathecal production of anti-Borrelia antibodies. An index of cerebrospinal fluid to serum antibody greater than 1 is thus also indicative of neuroborreliosis.12 Thus, performing lumbar puncture to detect intrathecal production of antibodies may support the diagnosis of central nervous system Lyme disease; however, it is not necessary.11
Antibodies persist in the central nervous system for many years after appropriate antimicrobial treatment.
Lyme arthritis
Articular involvement in Lyme disease is characterized by a robust humoral response such that a negative IgG serologic test virtually rules out Lyme arthritis.23 PCR testing of synovial fluid for borrelial DNA has a sensitivity of 80% but may become falsely negative after 1 to 2 months of antibiotic treatment.24,25 In an algorithm suggested by Puius et al,23 PCR testing of synovial fluid should be done in patients who have minimal to no response after 2 months of appropriate oral antimicrobial therapy to determine whether intravenous antibiotics are merited.
Table 5 summarizes the tests of choice in different clinical stages of infection.
Acknowledgment: The authors would like to acknowledge Anita Modi, MD, and Ceena N. Jacob, MD, for reviewing the manuscript and providing valuable suggestions, and Belinda Yen-Lieberman, PhD, for contributing pictures of the Western blot test results.
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
- Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20(1):7–17. doi:10.1002/art.1780200102
- Centers for Disease Control and Prevention (CDC). Lyme disease: recent surveillance data. https://www.cdc.gov/lyme/datasurveillance/recent-surveillance-data.html. Accessed August 12, 2019.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015; 29(2):269–280. doi:10.1016/j.idc.2015.02.004
- Schriefer ME. Lyme disease diagnosis: serology. Clin Lab Med 2015; 35(4):797–814. doi:10.1016/j.cll.2015.08.001
- Hu LT. Lyme disease. Ann Intern Med 2016; 164(9):ITC65–ITC80. doi:10.7326/AITC201605030
- Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med 2015; 35(4):815–825. doi:10.1016/j.cll.2015.07.005
- Dumler JS. Molecular diagnosis of Lyme disease: review and meta-analysis. Mol Diagn 2001; 6(1):1–11. doi:10.1054/modi.2001.21898
- Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med 2005; 142(9):751–755. doi:10.7326/0003-4819-142-9-200505030-00011
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089–1134. doi:10.1086/508667
- Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med 1997; 127(12):1106–1108. doi:10.7326/0003-4819-127-12-199712150-00010
- Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Continuum (Minneap Minn) 2012; 18(6 Infectious Disease):1338–1350. doi:10.1212/01.CON.0000423850.24900.3a
- Branda JA, Body BA, Boyle J, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis 2018; 66(7):1133–1139. doi:10.1093/cid/cix943
- Immunetics. Immunetics® C6 Lyme ELISA™ Kit. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/CF-E601-096A-C6-Pkg-Insrt.pdf. Accessed August 12, 2019.
- Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 2014; 15(1):34–48. doi:10.1038/nrg3575
- Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44(31):590–591. pmid:7623762
- Steere AC, Mchugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47(2):188–195. doi:10.1086/589242
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 1995; 274(12):937. pmid:7674514
- Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect 2019. doi:10.1016/j.cmi.2019.02.020. Epub ahead of print.
- Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18(12):1236–1240. doi:10.1111/j.1469-0691.2011.03749.x
- Hilton E, DeVoti J, Benach JL, et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am J Med 1999; 106(4):404–409. doi:10.1016/s0002-9343(99)00046-7
- Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53(6):541–547. doi:10.1093/cid/cir464
- Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 2008; 22(2):289–300. doi:10.1016/j.idc.2007.12.014
- Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med 1994; 330(4):229–234. doi:10.1056/NEJM199401273300401
- Liebling MR, Nishio MJ, Rodriguez A, Sigal LH, Jin T, Louie JS. The polymerase chain reaction for the detection of Borrelia burgdorferi in human body fluids. Arthritis Rheum 1993; 36(5):665–975. doi:10.1002/art.1780360514
KEY POINTS
- Lyme disease, the most common tick-borne infection in North America, is a complex multisystem bacterial disease caused by Borrelia burgdorferi.
- Lyme disease preferably affects the skin, joints, and nervous system and presents with typical and atypical features. Certain clinical features are diagnostic. Its diagnosis is mainly clinical and epidemiologic and, when doubtful, is supported by serologic testing.
- Standard 2-tiered testing is the diagnostic testing method of choice—enzyme-linked immunoassay followed by Western blot. Interpretation of the bands depends on the duration of infection.
- When interpreting the test results, be aware of false-positives and the reasons for them.
A sepsis death linked to fecal microbiota transplantation
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Fecal microbiota transplantation could have therapeutic utility in a range of conditions in which primary dysbiosis is suspected, but this study shows the procedure may carry risks that only become apparent after treatment. Improved screening of donors and fecal material could reduce the risks of infections by known agents. However, new pathogens may not be recognized until after they have been transplanted into a new host.
The benefits and risks of fecal microbiota transplantation must be balanced, but up to now the complications have been infrequent and the benefits have clearly outweighed the risks.
Martin J. Blaser, MD, is from Rutgers University in New Brunswick, N.J. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMe1913807). Dr. Blaser declared personal fees and stock options from the medical sector unrelated to the work.
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
Two cases of bacteremia have been described in two patients who received fecal microbiota transplants from the same donor.
Writing in the New England Journal of Medicine, researchers reported the two case studies of extended-spectrum beta-lactamase (ESBL)–producing Escherichia coli bacteremia, one of which ended in the death of the patient. These cases were previously announced by the Food and Drug Administration in a June 2019 safety alert.
Zachariah DeFilipp, MD, from Massachusetts General Hospital at Harvard Medical School, Boston, and coauthors wrote that fecal microbiota transplantation is rarely associated with complications. Placebo-controlled trials and a systematic review have found similar rates of complications in immunocompromised and immunocompetent recipients. Only four cases of gram-negative bacteremia previously have been reported, and in three of these, there was a plausible alternative explanation for the bacteremia.
In this paper, both patients received fecal microbiota transplantation via frozen oral capsules containing donor stool. These capsules were prepared prior to the implementation of screening for ESBL-producing organisms at the institution, and were not retrospectively tested since this expanded donor screening.
The first patient was a 69-year-old man with liver cirrhosis attributed to hepatitis C infection who was enrolled in a trial of fecal microbiota transplantation via oral capsules to treat hepatic encephalopathy. The first sign of the adverse event was a fever and cough, which developed 17 days after the final dose of 15 capsules. He was treated for pneumonia but failed to improve after 2 days, at which time gram-negative rods were discovered in blood cultures taken at the initial presentation.
After admission and further treatment, blood cultures were found to have ESBL-producing E. coli, and after further treatment, the patient was clinically stable. A stool sample taken after treatment was negative for ESBL-producing E. coli.
The second case study was a 73-year-old man with therapy-related myelodysplastic syndrome who was undergoing allogeneic hematopoietic stem cell transplantation and was receiving fecal microbiota transplantation via oral capsule as part of a phase 2 trial.
Eight days after the last dose of oral capsules, and 5 days after the stem-cell infusion, the man developed a fever, chills, febrile neutropenia and showed altered mental status. He was treated with cefepime but developed hypoxia and labored breathing later that evening, which prompted clinicians to intubate and begin mechanical ventilation.
His blood culture results showed gram-negative rods, and meropenem was added to his antibiotic regimen. However, the patient’s condition worsened, and he died of severe sepsis 2 days later with blood cultures confirmed as positive for ESBL-producing E. coli.
A follow-up investigation revealed that both patients received stool from the same donor. Each lot of three capsules from that donor was found to contain ESBL-producing E. coli with a resistance pattern similar to that seen in the two recipients.
Twenty-two patients had received capsules from this donor. Researchers contacted all the recipients and offered them stool screening for ESBL-producing E. coli. Twelve underwent testing, which found that five had samples that grew on ESBL-producing E. coli–selective medium.
The remaining seven patients who had follow-up testing were receiving treatment for recurrent or refractory Clostridioides difficile infection, and four of these grew samples on the selective medium.
“When FMT is successful, the recipient’s metagenomic burden of antimicrobial resistance genes mimics that of the donor,” the authors wrote. “Although we cannot conclusively attribute positive screening results for ESBL-producing organisms in other asymptomatic recipients to FMT, the rates of positive tests are, in our opinion, unexpectedly high and probably represent transmission through FMT.”
The authors said the donor had no risk factors for carriage of multidrug-resistant organism and had previously donated fecal material before the introduction of routine screening for ESBL-producing organisms.
However, they noted that both patients had risk factors for bacteremia, namely advanced cirrhosis and allogeneic hematopoietic stem cell transplantation and they also received oral antibiotics around the time of the fecal microbiota transplantation.
“Despite the infectious complications reported here, the benefits of FMT should be balanced with the associated risks when considering treatment options for patients with recurrent or refractory C. difficile infection,” the authors wrote. “Ongoing assessment of the risks and benefit of FMT research is needed, as are continuing efforts to improve donor screening to limit transmission of microorganisms that could lead to adverse infectious events.”
The American Gastroenterological Association FMT National Registry is a critical effort to track short- and long-term patient outcomes and potential risks associated with FMT. The registry's goal is to track 4,000 patients for 10 years. If you perform FMT, please contribute to this important initiative. Learn more at www.gastro.org/FMTRegistry.
The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two were attached to a diagnostics company involved in the study.
SOURCE: DeFilipp Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
* This story was updated on Oct. 31, 2019.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Two cases of bacteremia – one fatal – have been linked to a fecal microbiota transplant.
Major finding: Two patients developed bacteremia after receiving a fecal microbiota transplant from the same donor.
Study details: Case studies.
Disclosures: The study was supported by a grant from the American College of Gastroenterology. Three authors declared personal fees and grants from the medical sector outside the submitted work, and two authors were attached to a diagnostics company involved in the study.
Source: DeFillip Z et al. N Engl J Med. 2019 Oct 30. doi: 10.1056/NEJMoa1910437.
Pink Polycyclic Ulcerations on the Lower Back and Buttocks
The Diagnosis: Herpes Simplex Virus
A skin biopsy was sent for tissue culture and was negative for mycobacterial, bacterial, and fungal growth. Histopathologic examination showed ballooning degeneration of keratinocytes with herpetic cytopathic effect consistent with herpetic ulceration (Figure). A swab of the lesion on the buttock was sent for human herpesvirus (HHV) and varicella-zoster virus nucleic acid testing, which was positive for HHV-2. She was started on oral valacyclovir 1000 mg twice daily for 10 days and then was continued on chronic suppression with 500 mg once daily. The patient's ulcerations healed slowly over the following few weeks.
Human herpesvirus 2 is the most common cause of genital ulcer disease and may present as chronic and recurrent ulcers in immunocompromised patients.1 It usually is spread by sexual contact. Primary infection typically occurs in the cells of the dermis and epidermis. Two weeks after the primary infection, extragenital lesions can occur in the lumbosacral area on the buttocks, fingers, groin, or thighs, as seen in our patient,2 which is a direct result of viral shedding and spread. Reactivation of HHV from the ganglia can occur with or without symptoms. Common locations for viral shedding in women are the cervix, vulva, and perianal areas.3 Patients should be counseled to avoid sexual contact during recurrences.
Cancer patients have a particularly increased risk for developing HHV-2 due to their limited cell-mediated immunity and exposure to immunosuppressive drugs.4 Moreover, approximately 5% of immunocompromised patients develop resistance to antiviral therapy.5 Although this phenomenon was not observed in our patient, identification of novel strategies to treat these new groups of patients will be essential.
The differential diagnosis includes perianal candidiasis, which is classified by erythematous plaques with satellite vesicles and pustules. Contact dermatitis is common in the buttock area and usually secondary to ingredients in cleansing wipes and topical treatments. It is defined by a well-demarcated, symmetric rash, which is more eczematous in nature. Cutaneous T-cell lymphoma was high in our differential given the patient's history of the disease. There are many variants, and tumor-stage disease may result in ulceration of the skin. Cutaneous T-cell lymphoma is differentiated by histology with immunophenotyping in conjunction with the clinical picture. Epstein-Barr virus (EBV) may cause genital ulcerations, which can be diagnosed with a positive EBV serology and detection of EBV by a polymerase chain reaction swab of the ulceration.
- Schiffer JT, Corey L. New concepts in understanding genital herpes. Curr Infect Dis Rep. 2009;11:457-464.
- Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex. Proc (Bayl Univ Med Cent). 2016;29:48-49.
- Tata S, Johnston C, Huang ML, et al. Overlapping reactivations of HSV-2 in the genital and perianal mucosa. J Infect Dis. 2010;201:499-504.
- Tang IT, Shepp DH. Herpes simplex virus infection in cancer patients: prevention and treatment. Oncology (Williston Park). 1992;6:101-106.
- Jiang YC, Feng H, Lin YC, et al. New strategies against drug resistance to herpes simplex virus. Int J Oral Sci. 2016;8:1-6.
The Diagnosis: Herpes Simplex Virus
A skin biopsy was sent for tissue culture and was negative for mycobacterial, bacterial, and fungal growth. Histopathologic examination showed ballooning degeneration of keratinocytes with herpetic cytopathic effect consistent with herpetic ulceration (Figure). A swab of the lesion on the buttock was sent for human herpesvirus (HHV) and varicella-zoster virus nucleic acid testing, which was positive for HHV-2. She was started on oral valacyclovir 1000 mg twice daily for 10 days and then was continued on chronic suppression with 500 mg once daily. The patient's ulcerations healed slowly over the following few weeks.
Human herpesvirus 2 is the most common cause of genital ulcer disease and may present as chronic and recurrent ulcers in immunocompromised patients.1 It usually is spread by sexual contact. Primary infection typically occurs in the cells of the dermis and epidermis. Two weeks after the primary infection, extragenital lesions can occur in the lumbosacral area on the buttocks, fingers, groin, or thighs, as seen in our patient,2 which is a direct result of viral shedding and spread. Reactivation of HHV from the ganglia can occur with or without symptoms. Common locations for viral shedding in women are the cervix, vulva, and perianal areas.3 Patients should be counseled to avoid sexual contact during recurrences.
Cancer patients have a particularly increased risk for developing HHV-2 due to their limited cell-mediated immunity and exposure to immunosuppressive drugs.4 Moreover, approximately 5% of immunocompromised patients develop resistance to antiviral therapy.5 Although this phenomenon was not observed in our patient, identification of novel strategies to treat these new groups of patients will be essential.
The differential diagnosis includes perianal candidiasis, which is classified by erythematous plaques with satellite vesicles and pustules. Contact dermatitis is common in the buttock area and usually secondary to ingredients in cleansing wipes and topical treatments. It is defined by a well-demarcated, symmetric rash, which is more eczematous in nature. Cutaneous T-cell lymphoma was high in our differential given the patient's history of the disease. There are many variants, and tumor-stage disease may result in ulceration of the skin. Cutaneous T-cell lymphoma is differentiated by histology with immunophenotyping in conjunction with the clinical picture. Epstein-Barr virus (EBV) may cause genital ulcerations, which can be diagnosed with a positive EBV serology and detection of EBV by a polymerase chain reaction swab of the ulceration.
The Diagnosis: Herpes Simplex Virus
A skin biopsy was sent for tissue culture and was negative for mycobacterial, bacterial, and fungal growth. Histopathologic examination showed ballooning degeneration of keratinocytes with herpetic cytopathic effect consistent with herpetic ulceration (Figure). A swab of the lesion on the buttock was sent for human herpesvirus (HHV) and varicella-zoster virus nucleic acid testing, which was positive for HHV-2. She was started on oral valacyclovir 1000 mg twice daily for 10 days and then was continued on chronic suppression with 500 mg once daily. The patient's ulcerations healed slowly over the following few weeks.
Human herpesvirus 2 is the most common cause of genital ulcer disease and may present as chronic and recurrent ulcers in immunocompromised patients.1 It usually is spread by sexual contact. Primary infection typically occurs in the cells of the dermis and epidermis. Two weeks after the primary infection, extragenital lesions can occur in the lumbosacral area on the buttocks, fingers, groin, or thighs, as seen in our patient,2 which is a direct result of viral shedding and spread. Reactivation of HHV from the ganglia can occur with or without symptoms. Common locations for viral shedding in women are the cervix, vulva, and perianal areas.3 Patients should be counseled to avoid sexual contact during recurrences.
Cancer patients have a particularly increased risk for developing HHV-2 due to their limited cell-mediated immunity and exposure to immunosuppressive drugs.4 Moreover, approximately 5% of immunocompromised patients develop resistance to antiviral therapy.5 Although this phenomenon was not observed in our patient, identification of novel strategies to treat these new groups of patients will be essential.
The differential diagnosis includes perianal candidiasis, which is classified by erythematous plaques with satellite vesicles and pustules. Contact dermatitis is common in the buttock area and usually secondary to ingredients in cleansing wipes and topical treatments. It is defined by a well-demarcated, symmetric rash, which is more eczematous in nature. Cutaneous T-cell lymphoma was high in our differential given the patient's history of the disease. There are many variants, and tumor-stage disease may result in ulceration of the skin. Cutaneous T-cell lymphoma is differentiated by histology with immunophenotyping in conjunction with the clinical picture. Epstein-Barr virus (EBV) may cause genital ulcerations, which can be diagnosed with a positive EBV serology and detection of EBV by a polymerase chain reaction swab of the ulceration.
- Schiffer JT, Corey L. New concepts in understanding genital herpes. Curr Infect Dis Rep. 2009;11:457-464.
- Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex. Proc (Bayl Univ Med Cent). 2016;29:48-49.
- Tata S, Johnston C, Huang ML, et al. Overlapping reactivations of HSV-2 in the genital and perianal mucosa. J Infect Dis. 2010;201:499-504.
- Tang IT, Shepp DH. Herpes simplex virus infection in cancer patients: prevention and treatment. Oncology (Williston Park). 1992;6:101-106.
- Jiang YC, Feng H, Lin YC, et al. New strategies against drug resistance to herpes simplex virus. Int J Oral Sci. 2016;8:1-6.
- Schiffer JT, Corey L. New concepts in understanding genital herpes. Curr Infect Dis Rep. 2009;11:457-464.
- Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex. Proc (Bayl Univ Med Cent). 2016;29:48-49.
- Tata S, Johnston C, Huang ML, et al. Overlapping reactivations of HSV-2 in the genital and perianal mucosa. J Infect Dis. 2010;201:499-504.
- Tang IT, Shepp DH. Herpes simplex virus infection in cancer patients: prevention and treatment. Oncology (Williston Park). 1992;6:101-106.
- Jiang YC, Feng H, Lin YC, et al. New strategies against drug resistance to herpes simplex virus. Int J Oral Sci. 2016;8:1-6.
A 32-year-old woman with stage IV cutaneous T-cell lymphoma was admitted with pancytopenia and septic shock secondary to methicillin-susceptible Staphylococcus aureus bacteremia. Dermatology was consulted regarding sacral ulcerations. The lesions were asymptomatic and had been slowly enlarging over the course of 1 month. Physical examination revealed well-demarcated, pink, polycyclic ulcerations on the lower back and buttocks extending onto the perineum. There was no pain or tingling associated with the ulcerations. She denied a history of cold sore lesions on the lips or genitals. A skin biopsy was sent for tissue culture and histopathologic examination.
Flu vaccine cuts infection severity in kids and adults
WASHINGTON –
During recent U.S. flu seasons, children and adults who contracted influenza despite vaccination had significantly fewer severe infections and infection complications, compared with unimmunized people, according to two separate reports from CDC researchers presented at an annual scientific meeting on infectious diseases.
One of the reports tracked the impact of flu vaccine in children using data that the CDC collected at seven medical centers that participated in the agency’s New Vaccine Surveillance Network, which provided information on children aged 6 months to 17 years who were hospitalized for an acute respiratory illness, including more than 1,700 children during the 2016-2017 flu season and more than 1,900 during the 2017-2018 season. Roughly 10% of these children tested positive for influenza, and the subsequent analysis focused on these cases and compared incidence rates among children who had been vaccinated during the index season and those who had remained unvaccinated.
Combined data from both seasons showed that vaccinated children were 50% less likely to have been hospitalized for an acute influenza infection, compared with unvaccinated kids, a pattern consistently seen both in children aged 6 months to 8 years and in those aged 9-17 years. The pattern of vaccine effectiveness also held regardless of which flu strain caused the infections, reported Angela P. Campbell, MD, a CDC medical officer.
“We saw a nice benefit from vaccination, both in previously healthy children and in those with an underlying medical condition,” a finding that adds to existing evidence of vaccine effectiveness, Dr. Campbell said in a video interview. The results confirmed that flu vaccination does not just prevent infections but also cuts the rate of more severe infections that lead to hospitalization, she explained.
Another CDC study looked at data collected by the agency’s Influenza Hospitalization Surveillance Network from adults at least 18 years old who were hospitalized for a laboratory-confirmed influenza infection during five flu seasons, 2013-2014 through 2017-18. The data, which came from more than 250 acute-care hospitals in 13 states, included more than 43,000 people hospitalized for an identified influenza strain and with a known vaccination history who were not institutionalized and had not received any antiviral treatment.
After propensity-weighted adjustment to create better parity between the vaccinated and unvaccinated patients, the results showed that people 18-64 years old with vaccination had statistically significant decreases in mortality of a relative 36%, need for mechanical ventilation of 34%, pneumonia of 20%, and need for ICU admission of a relative 19%, as well as an 18% drop in average ICU length of stay, Shikha Garg, MD, said at the meeting. The propensity-weighted analysis of data from people at least 65 years old showed statistically significant relative reductions linked with vaccination: 46% reduction in the need for mechanical ventilation, 28% reduction in ICU admissions, and 9% reduction in hospitalized length of stay.
Further analysis of these outcomes by the strains that caused these influenza infections showed that the statistically significant benefits from vaccination were seen only in patients infected with an H1N1 strain. Statistically significant effects on these severe outcomes were not apparent among people infected with the H3N2 or B strains, said Dr. Garg, a medical epidemiologist at the CDC.
“All adults should receive an annual flu vaccination as it can improve outcomes among those who develop influenza despite vaccination,” she concluded.
Results from a third CDC study reported at the meeting examined the importance of two vaccine doses (administered at least 4 weeks apart) given to children aged 6 months to 8 years for the first season they receive flu vaccination, which is the immunization approach for flu recommended by the CDC. The findings from a total of more than 7,500 children immunized during the 2014-2018 seasons showed a clear increment in vaccine protection among kids who received two doses during their first season vaccinated, especially in children who were 2 years old or younger. In that age group, administration of two doses produced vaccine effectiveness of 53% versus a 23% vaccine effectiveness after a single vaccine dose, reported Jessie Chung, a CDC epidemiologist.
WASHINGTON –
During recent U.S. flu seasons, children and adults who contracted influenza despite vaccination had significantly fewer severe infections and infection complications, compared with unimmunized people, according to two separate reports from CDC researchers presented at an annual scientific meeting on infectious diseases.
One of the reports tracked the impact of flu vaccine in children using data that the CDC collected at seven medical centers that participated in the agency’s New Vaccine Surveillance Network, which provided information on children aged 6 months to 17 years who were hospitalized for an acute respiratory illness, including more than 1,700 children during the 2016-2017 flu season and more than 1,900 during the 2017-2018 season. Roughly 10% of these children tested positive for influenza, and the subsequent analysis focused on these cases and compared incidence rates among children who had been vaccinated during the index season and those who had remained unvaccinated.
Combined data from both seasons showed that vaccinated children were 50% less likely to have been hospitalized for an acute influenza infection, compared with unvaccinated kids, a pattern consistently seen both in children aged 6 months to 8 years and in those aged 9-17 years. The pattern of vaccine effectiveness also held regardless of which flu strain caused the infections, reported Angela P. Campbell, MD, a CDC medical officer.
“We saw a nice benefit from vaccination, both in previously healthy children and in those with an underlying medical condition,” a finding that adds to existing evidence of vaccine effectiveness, Dr. Campbell said in a video interview. The results confirmed that flu vaccination does not just prevent infections but also cuts the rate of more severe infections that lead to hospitalization, she explained.
Another CDC study looked at data collected by the agency’s Influenza Hospitalization Surveillance Network from adults at least 18 years old who were hospitalized for a laboratory-confirmed influenza infection during five flu seasons, 2013-2014 through 2017-18. The data, which came from more than 250 acute-care hospitals in 13 states, included more than 43,000 people hospitalized for an identified influenza strain and with a known vaccination history who were not institutionalized and had not received any antiviral treatment.
After propensity-weighted adjustment to create better parity between the vaccinated and unvaccinated patients, the results showed that people 18-64 years old with vaccination had statistically significant decreases in mortality of a relative 36%, need for mechanical ventilation of 34%, pneumonia of 20%, and need for ICU admission of a relative 19%, as well as an 18% drop in average ICU length of stay, Shikha Garg, MD, said at the meeting. The propensity-weighted analysis of data from people at least 65 years old showed statistically significant relative reductions linked with vaccination: 46% reduction in the need for mechanical ventilation, 28% reduction in ICU admissions, and 9% reduction in hospitalized length of stay.
Further analysis of these outcomes by the strains that caused these influenza infections showed that the statistically significant benefits from vaccination were seen only in patients infected with an H1N1 strain. Statistically significant effects on these severe outcomes were not apparent among people infected with the H3N2 or B strains, said Dr. Garg, a medical epidemiologist at the CDC.
“All adults should receive an annual flu vaccination as it can improve outcomes among those who develop influenza despite vaccination,” she concluded.
Results from a third CDC study reported at the meeting examined the importance of two vaccine doses (administered at least 4 weeks apart) given to children aged 6 months to 8 years for the first season they receive flu vaccination, which is the immunization approach for flu recommended by the CDC. The findings from a total of more than 7,500 children immunized during the 2014-2018 seasons showed a clear increment in vaccine protection among kids who received two doses during their first season vaccinated, especially in children who were 2 years old or younger. In that age group, administration of two doses produced vaccine effectiveness of 53% versus a 23% vaccine effectiveness after a single vaccine dose, reported Jessie Chung, a CDC epidemiologist.
WASHINGTON –
During recent U.S. flu seasons, children and adults who contracted influenza despite vaccination had significantly fewer severe infections and infection complications, compared with unimmunized people, according to two separate reports from CDC researchers presented at an annual scientific meeting on infectious diseases.
One of the reports tracked the impact of flu vaccine in children using data that the CDC collected at seven medical centers that participated in the agency’s New Vaccine Surveillance Network, which provided information on children aged 6 months to 17 years who were hospitalized for an acute respiratory illness, including more than 1,700 children during the 2016-2017 flu season and more than 1,900 during the 2017-2018 season. Roughly 10% of these children tested positive for influenza, and the subsequent analysis focused on these cases and compared incidence rates among children who had been vaccinated during the index season and those who had remained unvaccinated.
Combined data from both seasons showed that vaccinated children were 50% less likely to have been hospitalized for an acute influenza infection, compared with unvaccinated kids, a pattern consistently seen both in children aged 6 months to 8 years and in those aged 9-17 years. The pattern of vaccine effectiveness also held regardless of which flu strain caused the infections, reported Angela P. Campbell, MD, a CDC medical officer.
“We saw a nice benefit from vaccination, both in previously healthy children and in those with an underlying medical condition,” a finding that adds to existing evidence of vaccine effectiveness, Dr. Campbell said in a video interview. The results confirmed that flu vaccination does not just prevent infections but also cuts the rate of more severe infections that lead to hospitalization, she explained.
Another CDC study looked at data collected by the agency’s Influenza Hospitalization Surveillance Network from adults at least 18 years old who were hospitalized for a laboratory-confirmed influenza infection during five flu seasons, 2013-2014 through 2017-18. The data, which came from more than 250 acute-care hospitals in 13 states, included more than 43,000 people hospitalized for an identified influenza strain and with a known vaccination history who were not institutionalized and had not received any antiviral treatment.
After propensity-weighted adjustment to create better parity between the vaccinated and unvaccinated patients, the results showed that people 18-64 years old with vaccination had statistically significant decreases in mortality of a relative 36%, need for mechanical ventilation of 34%, pneumonia of 20%, and need for ICU admission of a relative 19%, as well as an 18% drop in average ICU length of stay, Shikha Garg, MD, said at the meeting. The propensity-weighted analysis of data from people at least 65 years old showed statistically significant relative reductions linked with vaccination: 46% reduction in the need for mechanical ventilation, 28% reduction in ICU admissions, and 9% reduction in hospitalized length of stay.
Further analysis of these outcomes by the strains that caused these influenza infections showed that the statistically significant benefits from vaccination were seen only in patients infected with an H1N1 strain. Statistically significant effects on these severe outcomes were not apparent among people infected with the H3N2 or B strains, said Dr. Garg, a medical epidemiologist at the CDC.
“All adults should receive an annual flu vaccination as it can improve outcomes among those who develop influenza despite vaccination,” she concluded.
Results from a third CDC study reported at the meeting examined the importance of two vaccine doses (administered at least 4 weeks apart) given to children aged 6 months to 8 years for the first season they receive flu vaccination, which is the immunization approach for flu recommended by the CDC. The findings from a total of more than 7,500 children immunized during the 2014-2018 seasons showed a clear increment in vaccine protection among kids who received two doses during their first season vaccinated, especially in children who were 2 years old or younger. In that age group, administration of two doses produced vaccine effectiveness of 53% versus a 23% vaccine effectiveness after a single vaccine dose, reported Jessie Chung, a CDC epidemiologist.
REPORTING FROM ID WEEK 2019
Stay Informed About Informed Consent
On May 24, 2011, a 53-year-old woman presented to a Wisconsin hospital emergency department (ED) with complaints of severe abdominal pain, a rapid heartbeat, and a fever of 101.3°F. During her 9-hour visit, she was treated by a PA and his supervising physician. She was seen by the physician for a total of 6 minutes; the rest of her care was provided by the PA. The patient was discharged around midnight with instructions to contact her gynecologist in the morning for management of uterine fibroids. At the time of discharge, her temperature was 102.9°F.
The following day, May 25, the patient collapsed in her home and was transported to another hospital. She was treated for septic shock from a group A streptococcus infection. Although the infection was halted, the patient sustained ischemic damage to her extremities and a month later required amputation of her 4 limbs.The plaintiff claimed that the supervising physician was negligent in failing to diagnose the strep A infection, which, left undetected, led to septic shock. She also alleged that the PA should have recognized the potential for her condition’s severity to quickly escalate. She maintained that the supervising physician should have been more involved in her case because of its complexity.
Plaintiff’s counsel also argued that the PA should have provided “alternative medical diagnoses,” which would have prompted consideration of other treatment options. The plaintiff contended that under Wisconsin’s informed consent law, both the PA and the physician failed to disclose enough information about her condition and failed to inform her of any choices for treatment.
The defense argued that the plaintiff received proper treatment based on the information available to the providers at the time.
VERDICT
The jury found for the plaintiff and apportioned 65% liability to the physician and 35% liability to the PA. A total of $25,342,096 was awarded to the plaintiff.
COMMENTARY
This is a huge verdict. Cases involving group A strep or necrotizing fasciitis frequently give rise to large medical malpractice verdicts, because everything about them is difficult to defend: Although there is typically trivial to no trauma involved, the wounds from these infections provide explicit images of damage, intraoperatively and postoperatively. Vasopressors required for hemodynamic support or sepsis itself frequently result in limb ischemia, gangrene, and amputation. In this case, the plaintiff, as a quadruple amputee, was a sympathetic and impressive courtroom presence—the personal toll was evident to anyone in the room.
Two providers—a PA and a physician—saw the patient. We are told only that she complained of severe abdominal pain, rapid heartbeat, and fever, which increased at some point during her ED stay. We aren’t given specifics on the rest of the patient’s vital signs or examination details. However, we can infer that the exam and lab findings were not impressive, because they weren’t mentioned in the case report. But as a result of the failure to catch the group A strep infection, the plaintiff suffered what one judge hearing the case described as a harrowing and unimaginable ordeal: the life-changing amputation of 4 limbs.1 While the jury did not find the PA or physician negligent, they still found the clinicians liable and awarded a staggering verdict.
Continue to: How could this happen?
How could this happen? The answer is the theory of recovery: The jury found that the physician and the PA failed to provide the patient with informed consent in the form of “alternative medical diagnoses.”2 The plaintiff’s attorney argued that the patient was never told a life-threatening bacterial infection was one possible diagnosis and claimed that if she had known, the patient would have pursued other treatment.
As in many malpractice cases, the plaintiff alleged failure to diagnose and failure to provide informed consent. Depending on state law, there are 3 standards for informed consent: subjective patient, reasonable patient, and reasonable physician.3 About half of the states have a physician-focused standard, while the other half have a patient-focused standard.3
Under the subjective patient standard, we would ask, “What would this patient need to know and understand to make an informed decision?”4 The subjective standard requires the clinician to essentially “get in the head” of a specific patient to determine what he or she would want to know when making a medical decision. This standard is problematic because it requires the clinician to have an intimate familiarity with the patient’s belief system and medical decision-making process—a daunting requirement for many clinicians, particularly in the absence of a longstanding clinician-patient relationship, as is the case in most emergency settings. Thankfully, the subjective patient standard is not followed by most states that have a patient-focused standard.
Under the objective reasonable patient standard, we would ask “What would the average patient need to know to be an informed participant in the decision?”4 One could argue that this standard more adequately allows the patient to be an active participant in shared decision-making. However, the drawback is that what is “reasonable” often falls on a spectrum, which would require the clinician to gauge the volume and type of information a patient cohort would want to have when making a medical decision. Under this standard, the plaintiff must prove that the clinician omitted information that a reasonable patient would want to know. Therefore, these standards are more friendly to the plaintiff, whereas the reasonable physician standard is more defendant friendly.
To meet the standard of care under a reasonable physician standard, information must be provided to the patient that a “reasonably prudent practitioner in the same field of practice or specialty” would provide to a patient.5 For a plaintiff to successfully sue under this standard, the plaintiff’s expert must testify that a reasonably prudent physician would have disclosed the omitted information.6 The reasonable physician standard is obviously better for malpractice defendants.
Continue to: While reasonable clinicians...
While reasonable clinicians can disagree (as can reasonable patients), clinicians are more likely to be closer in opinion. Clinicians are a smaller group whose opinions are underpinned by similar education, training, and experience. By contrast, among the general population, beliefs held by one hypothetical “reasonable person” are much less settled, and in some cases, wildly divergent from another’s. For example, vaccine skepticism would probably be considered unreasonable in the majority of jury pools but absolutely reasonable in some. The large size of the general population, coupled with opinions untethered to any definable discipline, make the reasonable patient standard hard to predict.
Additionally, the reasonable physician standard forces the plaintiff to prove his or her case by producing an expert witness (clinician) to specifically testify that the standard of care required the defendant clinician to disclose certain specific information, and that disclosure was lacking. That is an important requirement. Under patient-focused standards, the plaintiff doesn’t need a medical expert on this point and can simply argue to the jury that a reasonable patient would require an exhaustive discussion of each possibility in the differential diagnosis. Therefore, I would argue that the reasonable physician standard is more predictable and workable and should be followed.
At the time of this case, Wisconsin’s informed consent law was based on the reasonable patient standard. As a result of this case, Wisconsin lawmakers changed the law to a “reasonable physician standard,” which states “any physician who treats a patient shall inform the patient about the availability of reasonable alternate medical modes of treatment and about the benefits and risks of these treatments.”7 However, the law stipulates that this duty to inform does not require disclosure of (among others):
- Detailed technical information that in all probability a patient would not understand
- Risks apparent or known to the patient
- Extremely remote possibilities that might falsely or detrimentally alarm the patient
- Information about alternate medical modes of treatment for any condition the physician has not included in his or her diagnosis at the time the physician informs the patient.7
Finally, this case involved an extremely high verdict of more than $25 million. It may surprise you to learn that many states have caps for medical malpractice awards for noneconomic damages, such as pain and suffering. If you’re having a holiday dinner with friends or family members who are plaintiff’s attorneys and you’re itching for a good argument, skip current politics and go all-in: How about liability caps, Uncle Jim? Get ready for a lively debate.
Of the $25 million verdict, $16.5 million was awarded for pain and suffering—the jury was obviously shocked by the extent of the life-changing nature of the plaintiff’s injuries. At the time of this case, Wisconsin had a cap of $750,000 for noneconomic damages.8 However, plaintiffs may challenge state constitutionality of these caps when they feel they have the right case, which the plaintiff and her attorney felt they did. Two lower courts found the state cap unconstitutional and gave the plaintiff the full award. But the state Supreme Court later reversed that decision, upholding the cap.1 The court decided that the legislature had a rational basis for making the law and changes to it should occur through the legislature, not the courts. The dissenting justices argued that there was no rational basis for the $750,000 cap, because there was no evidence that clinicians would flee the state fearing malpractice liability, or practice more defensive medicine, or suffer runaway malpractice insurance premiums without the cap. As a result of this case, the cap was upheld, and there was a “lively debate” on this issue at the highest levels of government.
Continue to: IN SUM
IN SUM
Become familiar with your state’s informed consent laws. Involve patients in decision-making, and convey information related to reasonable treatment options and risks. Document all of these discussions. Lastly, state-level political discussions on issues of tort reform, caps, and malpractice matters are ongoing—so take n
1. Mayo v Wisconsin Injured Patients & Families Compensation Fund. WI 78 (2018).
2. Spivak C. Jury awards Milwaukee woman $25.3 million in medical malpractice case. Milwaukee Journal Sentinel. July 7, 2014.
3. Moore GP, Matlock AG, Kiley JL, et al. Emergency physicians: beware of the consent standard of care. Clin Pract Cases Emerg Med. 2018; 2(2):109-111.
4. Gossman W, Thornton I, Hipskind JE. Informed Consent. StatPearls. www.ncbi.nlm.nih.gov/books/NBK430827/. Updated July 10, 2019. Accessed October 25, 2019.
5. King JS, Moulton BW. Rethinking informed consent: the case for shared medical decision-making. Am J Law Med. 2006;32:429-501.
6. Tashman v Gibbs, 556 SE 2d 772 (263 Va 2002).
7. Wis Stat subchapter 2, §448.30.
8. Wis Stat §893.55.
On May 24, 2011, a 53-year-old woman presented to a Wisconsin hospital emergency department (ED) with complaints of severe abdominal pain, a rapid heartbeat, and a fever of 101.3°F. During her 9-hour visit, she was treated by a PA and his supervising physician. She was seen by the physician for a total of 6 minutes; the rest of her care was provided by the PA. The patient was discharged around midnight with instructions to contact her gynecologist in the morning for management of uterine fibroids. At the time of discharge, her temperature was 102.9°F.
The following day, May 25, the patient collapsed in her home and was transported to another hospital. She was treated for septic shock from a group A streptococcus infection. Although the infection was halted, the patient sustained ischemic damage to her extremities and a month later required amputation of her 4 limbs.The plaintiff claimed that the supervising physician was negligent in failing to diagnose the strep A infection, which, left undetected, led to septic shock. She also alleged that the PA should have recognized the potential for her condition’s severity to quickly escalate. She maintained that the supervising physician should have been more involved in her case because of its complexity.
Plaintiff’s counsel also argued that the PA should have provided “alternative medical diagnoses,” which would have prompted consideration of other treatment options. The plaintiff contended that under Wisconsin’s informed consent law, both the PA and the physician failed to disclose enough information about her condition and failed to inform her of any choices for treatment.
The defense argued that the plaintiff received proper treatment based on the information available to the providers at the time.
VERDICT
The jury found for the plaintiff and apportioned 65% liability to the physician and 35% liability to the PA. A total of $25,342,096 was awarded to the plaintiff.
COMMENTARY
This is a huge verdict. Cases involving group A strep or necrotizing fasciitis frequently give rise to large medical malpractice verdicts, because everything about them is difficult to defend: Although there is typically trivial to no trauma involved, the wounds from these infections provide explicit images of damage, intraoperatively and postoperatively. Vasopressors required for hemodynamic support or sepsis itself frequently result in limb ischemia, gangrene, and amputation. In this case, the plaintiff, as a quadruple amputee, was a sympathetic and impressive courtroom presence—the personal toll was evident to anyone in the room.
Two providers—a PA and a physician—saw the patient. We are told only that she complained of severe abdominal pain, rapid heartbeat, and fever, which increased at some point during her ED stay. We aren’t given specifics on the rest of the patient’s vital signs or examination details. However, we can infer that the exam and lab findings were not impressive, because they weren’t mentioned in the case report. But as a result of the failure to catch the group A strep infection, the plaintiff suffered what one judge hearing the case described as a harrowing and unimaginable ordeal: the life-changing amputation of 4 limbs.1 While the jury did not find the PA or physician negligent, they still found the clinicians liable and awarded a staggering verdict.
Continue to: How could this happen?
How could this happen? The answer is the theory of recovery: The jury found that the physician and the PA failed to provide the patient with informed consent in the form of “alternative medical diagnoses.”2 The plaintiff’s attorney argued that the patient was never told a life-threatening bacterial infection was one possible diagnosis and claimed that if she had known, the patient would have pursued other treatment.
As in many malpractice cases, the plaintiff alleged failure to diagnose and failure to provide informed consent. Depending on state law, there are 3 standards for informed consent: subjective patient, reasonable patient, and reasonable physician.3 About half of the states have a physician-focused standard, while the other half have a patient-focused standard.3
Under the subjective patient standard, we would ask, “What would this patient need to know and understand to make an informed decision?”4 The subjective standard requires the clinician to essentially “get in the head” of a specific patient to determine what he or she would want to know when making a medical decision. This standard is problematic because it requires the clinician to have an intimate familiarity with the patient’s belief system and medical decision-making process—a daunting requirement for many clinicians, particularly in the absence of a longstanding clinician-patient relationship, as is the case in most emergency settings. Thankfully, the subjective patient standard is not followed by most states that have a patient-focused standard.
Under the objective reasonable patient standard, we would ask “What would the average patient need to know to be an informed participant in the decision?”4 One could argue that this standard more adequately allows the patient to be an active participant in shared decision-making. However, the drawback is that what is “reasonable” often falls on a spectrum, which would require the clinician to gauge the volume and type of information a patient cohort would want to have when making a medical decision. Under this standard, the plaintiff must prove that the clinician omitted information that a reasonable patient would want to know. Therefore, these standards are more friendly to the plaintiff, whereas the reasonable physician standard is more defendant friendly.
To meet the standard of care under a reasonable physician standard, information must be provided to the patient that a “reasonably prudent practitioner in the same field of practice or specialty” would provide to a patient.5 For a plaintiff to successfully sue under this standard, the plaintiff’s expert must testify that a reasonably prudent physician would have disclosed the omitted information.6 The reasonable physician standard is obviously better for malpractice defendants.
Continue to: While reasonable clinicians...
While reasonable clinicians can disagree (as can reasonable patients), clinicians are more likely to be closer in opinion. Clinicians are a smaller group whose opinions are underpinned by similar education, training, and experience. By contrast, among the general population, beliefs held by one hypothetical “reasonable person” are much less settled, and in some cases, wildly divergent from another’s. For example, vaccine skepticism would probably be considered unreasonable in the majority of jury pools but absolutely reasonable in some. The large size of the general population, coupled with opinions untethered to any definable discipline, make the reasonable patient standard hard to predict.
Additionally, the reasonable physician standard forces the plaintiff to prove his or her case by producing an expert witness (clinician) to specifically testify that the standard of care required the defendant clinician to disclose certain specific information, and that disclosure was lacking. That is an important requirement. Under patient-focused standards, the plaintiff doesn’t need a medical expert on this point and can simply argue to the jury that a reasonable patient would require an exhaustive discussion of each possibility in the differential diagnosis. Therefore, I would argue that the reasonable physician standard is more predictable and workable and should be followed.
At the time of this case, Wisconsin’s informed consent law was based on the reasonable patient standard. As a result of this case, Wisconsin lawmakers changed the law to a “reasonable physician standard,” which states “any physician who treats a patient shall inform the patient about the availability of reasonable alternate medical modes of treatment and about the benefits and risks of these treatments.”7 However, the law stipulates that this duty to inform does not require disclosure of (among others):
- Detailed technical information that in all probability a patient would not understand
- Risks apparent or known to the patient
- Extremely remote possibilities that might falsely or detrimentally alarm the patient
- Information about alternate medical modes of treatment for any condition the physician has not included in his or her diagnosis at the time the physician informs the patient.7
Finally, this case involved an extremely high verdict of more than $25 million. It may surprise you to learn that many states have caps for medical malpractice awards for noneconomic damages, such as pain and suffering. If you’re having a holiday dinner with friends or family members who are plaintiff’s attorneys and you’re itching for a good argument, skip current politics and go all-in: How about liability caps, Uncle Jim? Get ready for a lively debate.
Of the $25 million verdict, $16.5 million was awarded for pain and suffering—the jury was obviously shocked by the extent of the life-changing nature of the plaintiff’s injuries. At the time of this case, Wisconsin had a cap of $750,000 for noneconomic damages.8 However, plaintiffs may challenge state constitutionality of these caps when they feel they have the right case, which the plaintiff and her attorney felt they did. Two lower courts found the state cap unconstitutional and gave the plaintiff the full award. But the state Supreme Court later reversed that decision, upholding the cap.1 The court decided that the legislature had a rational basis for making the law and changes to it should occur through the legislature, not the courts. The dissenting justices argued that there was no rational basis for the $750,000 cap, because there was no evidence that clinicians would flee the state fearing malpractice liability, or practice more defensive medicine, or suffer runaway malpractice insurance premiums without the cap. As a result of this case, the cap was upheld, and there was a “lively debate” on this issue at the highest levels of government.
Continue to: IN SUM
IN SUM
Become familiar with your state’s informed consent laws. Involve patients in decision-making, and convey information related to reasonable treatment options and risks. Document all of these discussions. Lastly, state-level political discussions on issues of tort reform, caps, and malpractice matters are ongoing—so take n
On May 24, 2011, a 53-year-old woman presented to a Wisconsin hospital emergency department (ED) with complaints of severe abdominal pain, a rapid heartbeat, and a fever of 101.3°F. During her 9-hour visit, she was treated by a PA and his supervising physician. She was seen by the physician for a total of 6 minutes; the rest of her care was provided by the PA. The patient was discharged around midnight with instructions to contact her gynecologist in the morning for management of uterine fibroids. At the time of discharge, her temperature was 102.9°F.
The following day, May 25, the patient collapsed in her home and was transported to another hospital. She was treated for septic shock from a group A streptococcus infection. Although the infection was halted, the patient sustained ischemic damage to her extremities and a month later required amputation of her 4 limbs.The plaintiff claimed that the supervising physician was negligent in failing to diagnose the strep A infection, which, left undetected, led to septic shock. She also alleged that the PA should have recognized the potential for her condition’s severity to quickly escalate. She maintained that the supervising physician should have been more involved in her case because of its complexity.
Plaintiff’s counsel also argued that the PA should have provided “alternative medical diagnoses,” which would have prompted consideration of other treatment options. The plaintiff contended that under Wisconsin’s informed consent law, both the PA and the physician failed to disclose enough information about her condition and failed to inform her of any choices for treatment.
The defense argued that the plaintiff received proper treatment based on the information available to the providers at the time.
VERDICT
The jury found for the plaintiff and apportioned 65% liability to the physician and 35% liability to the PA. A total of $25,342,096 was awarded to the plaintiff.
COMMENTARY
This is a huge verdict. Cases involving group A strep or necrotizing fasciitis frequently give rise to large medical malpractice verdicts, because everything about them is difficult to defend: Although there is typically trivial to no trauma involved, the wounds from these infections provide explicit images of damage, intraoperatively and postoperatively. Vasopressors required for hemodynamic support or sepsis itself frequently result in limb ischemia, gangrene, and amputation. In this case, the plaintiff, as a quadruple amputee, was a sympathetic and impressive courtroom presence—the personal toll was evident to anyone in the room.
Two providers—a PA and a physician—saw the patient. We are told only that she complained of severe abdominal pain, rapid heartbeat, and fever, which increased at some point during her ED stay. We aren’t given specifics on the rest of the patient’s vital signs or examination details. However, we can infer that the exam and lab findings were not impressive, because they weren’t mentioned in the case report. But as a result of the failure to catch the group A strep infection, the plaintiff suffered what one judge hearing the case described as a harrowing and unimaginable ordeal: the life-changing amputation of 4 limbs.1 While the jury did not find the PA or physician negligent, they still found the clinicians liable and awarded a staggering verdict.
Continue to: How could this happen?
How could this happen? The answer is the theory of recovery: The jury found that the physician and the PA failed to provide the patient with informed consent in the form of “alternative medical diagnoses.”2 The plaintiff’s attorney argued that the patient was never told a life-threatening bacterial infection was one possible diagnosis and claimed that if she had known, the patient would have pursued other treatment.
As in many malpractice cases, the plaintiff alleged failure to diagnose and failure to provide informed consent. Depending on state law, there are 3 standards for informed consent: subjective patient, reasonable patient, and reasonable physician.3 About half of the states have a physician-focused standard, while the other half have a patient-focused standard.3
Under the subjective patient standard, we would ask, “What would this patient need to know and understand to make an informed decision?”4 The subjective standard requires the clinician to essentially “get in the head” of a specific patient to determine what he or she would want to know when making a medical decision. This standard is problematic because it requires the clinician to have an intimate familiarity with the patient’s belief system and medical decision-making process—a daunting requirement for many clinicians, particularly in the absence of a longstanding clinician-patient relationship, as is the case in most emergency settings. Thankfully, the subjective patient standard is not followed by most states that have a patient-focused standard.
Under the objective reasonable patient standard, we would ask “What would the average patient need to know to be an informed participant in the decision?”4 One could argue that this standard more adequately allows the patient to be an active participant in shared decision-making. However, the drawback is that what is “reasonable” often falls on a spectrum, which would require the clinician to gauge the volume and type of information a patient cohort would want to have when making a medical decision. Under this standard, the plaintiff must prove that the clinician omitted information that a reasonable patient would want to know. Therefore, these standards are more friendly to the plaintiff, whereas the reasonable physician standard is more defendant friendly.
To meet the standard of care under a reasonable physician standard, information must be provided to the patient that a “reasonably prudent practitioner in the same field of practice or specialty” would provide to a patient.5 For a plaintiff to successfully sue under this standard, the plaintiff’s expert must testify that a reasonably prudent physician would have disclosed the omitted information.6 The reasonable physician standard is obviously better for malpractice defendants.
Continue to: While reasonable clinicians...
While reasonable clinicians can disagree (as can reasonable patients), clinicians are more likely to be closer in opinion. Clinicians are a smaller group whose opinions are underpinned by similar education, training, and experience. By contrast, among the general population, beliefs held by one hypothetical “reasonable person” are much less settled, and in some cases, wildly divergent from another’s. For example, vaccine skepticism would probably be considered unreasonable in the majority of jury pools but absolutely reasonable in some. The large size of the general population, coupled with opinions untethered to any definable discipline, make the reasonable patient standard hard to predict.
Additionally, the reasonable physician standard forces the plaintiff to prove his or her case by producing an expert witness (clinician) to specifically testify that the standard of care required the defendant clinician to disclose certain specific information, and that disclosure was lacking. That is an important requirement. Under patient-focused standards, the plaintiff doesn’t need a medical expert on this point and can simply argue to the jury that a reasonable patient would require an exhaustive discussion of each possibility in the differential diagnosis. Therefore, I would argue that the reasonable physician standard is more predictable and workable and should be followed.
At the time of this case, Wisconsin’s informed consent law was based on the reasonable patient standard. As a result of this case, Wisconsin lawmakers changed the law to a “reasonable physician standard,” which states “any physician who treats a patient shall inform the patient about the availability of reasonable alternate medical modes of treatment and about the benefits and risks of these treatments.”7 However, the law stipulates that this duty to inform does not require disclosure of (among others):
- Detailed technical information that in all probability a patient would not understand
- Risks apparent or known to the patient
- Extremely remote possibilities that might falsely or detrimentally alarm the patient
- Information about alternate medical modes of treatment for any condition the physician has not included in his or her diagnosis at the time the physician informs the patient.7
Finally, this case involved an extremely high verdict of more than $25 million. It may surprise you to learn that many states have caps for medical malpractice awards for noneconomic damages, such as pain and suffering. If you’re having a holiday dinner with friends or family members who are plaintiff’s attorneys and you’re itching for a good argument, skip current politics and go all-in: How about liability caps, Uncle Jim? Get ready for a lively debate.
Of the $25 million verdict, $16.5 million was awarded for pain and suffering—the jury was obviously shocked by the extent of the life-changing nature of the plaintiff’s injuries. At the time of this case, Wisconsin had a cap of $750,000 for noneconomic damages.8 However, plaintiffs may challenge state constitutionality of these caps when they feel they have the right case, which the plaintiff and her attorney felt they did. Two lower courts found the state cap unconstitutional and gave the plaintiff the full award. But the state Supreme Court later reversed that decision, upholding the cap.1 The court decided that the legislature had a rational basis for making the law and changes to it should occur through the legislature, not the courts. The dissenting justices argued that there was no rational basis for the $750,000 cap, because there was no evidence that clinicians would flee the state fearing malpractice liability, or practice more defensive medicine, or suffer runaway malpractice insurance premiums without the cap. As a result of this case, the cap was upheld, and there was a “lively debate” on this issue at the highest levels of government.
Continue to: IN SUM
IN SUM
Become familiar with your state’s informed consent laws. Involve patients in decision-making, and convey information related to reasonable treatment options and risks. Document all of these discussions. Lastly, state-level political discussions on issues of tort reform, caps, and malpractice matters are ongoing—so take n
1. Mayo v Wisconsin Injured Patients & Families Compensation Fund. WI 78 (2018).
2. Spivak C. Jury awards Milwaukee woman $25.3 million in medical malpractice case. Milwaukee Journal Sentinel. July 7, 2014.
3. Moore GP, Matlock AG, Kiley JL, et al. Emergency physicians: beware of the consent standard of care. Clin Pract Cases Emerg Med. 2018; 2(2):109-111.
4. Gossman W, Thornton I, Hipskind JE. Informed Consent. StatPearls. www.ncbi.nlm.nih.gov/books/NBK430827/. Updated July 10, 2019. Accessed October 25, 2019.
5. King JS, Moulton BW. Rethinking informed consent: the case for shared medical decision-making. Am J Law Med. 2006;32:429-501.
6. Tashman v Gibbs, 556 SE 2d 772 (263 Va 2002).
7. Wis Stat subchapter 2, §448.30.
8. Wis Stat §893.55.
1. Mayo v Wisconsin Injured Patients & Families Compensation Fund. WI 78 (2018).
2. Spivak C. Jury awards Milwaukee woman $25.3 million in medical malpractice case. Milwaukee Journal Sentinel. July 7, 2014.
3. Moore GP, Matlock AG, Kiley JL, et al. Emergency physicians: beware of the consent standard of care. Clin Pract Cases Emerg Med. 2018; 2(2):109-111.
4. Gossman W, Thornton I, Hipskind JE. Informed Consent. StatPearls. www.ncbi.nlm.nih.gov/books/NBK430827/. Updated July 10, 2019. Accessed October 25, 2019.
5. King JS, Moulton BW. Rethinking informed consent: the case for shared medical decision-making. Am J Law Med. 2006;32:429-501.
6. Tashman v Gibbs, 556 SE 2d 772 (263 Va 2002).
7. Wis Stat subchapter 2, §448.30.
8. Wis Stat §893.55.
No infection increase seen with biologics in older psoriasis patients
MADRID – Psoriasis patients aged 65 years and older are at more than twice the risk of serious bacterial and opportunistic infections, compared with younger patients, but that risk is not further elevated by being on biologic agents, Joseph F. Merola, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a large, The study implications, he said, are clear: When moderate to severe psoriasis warrants consideration of highly effective biologic therapies, that therapeutic option shouldn’t be taken off the table on the basis of a mistaken belief that biologics pose a greater infection risk just because the affected patient is over age 65 years.
“We really think that older patients should be offered treatments at the same level of disease control as all the rest of our psoriasis patients, in the context of shared decision making,” said Dr. Merola, a dermatologist and rheumatologist who is the director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston.
The study utilized longitudinal claims data from a very large U.S. database covering the years 2003-2017. Among the 185 million covered lives were 1.1 million individuals with psoriasis, including 150,000 aged 65 years or older. After excluding older psoriasis patients with comorbid cancer or autoimmune disease, the investigators were left with 11,218 older psoriasis patients initiating systemic therapy for the first time and therefore eligible for propensity score matching using a highly accurate proprietary platform. The final study population consisted of 2,795 older psoriasis patients newly initiating biologic therapy, 2,795 others newly initiating nonbiologic systemic agents, and 2,529 seniors starting phototherapy. The matching was based upon factors including age, sex, prior infections, comorbid psoriatic arthritis, diabetes, and obesity.
The primary study endpoint was the rate of serious bacterial or opportunistic infections requiring hospitalization during the first 6 months of treatment. The bottom line: The rates were closely similar across all three groups, with the most common serious infections being pneumonia and cellulitis.
In contrast, among a population of 115,047 senior psoriasis patients who never used systemic therapy, the risk of serious infection was 12.2 events per 1,000 patients over 6 months, compared with 5.3 events in 120,174 matched controls without psoriasis. That translates to a 2.24-fold increased risk.
One audience member commented that a limitation of the study was that all biologics were lumped together. He would expect that the tumor necrosis factor inhibitors, for example, would be associated with a significantly higher serious infection risk than biologics with other targets.
Dr. Merola conceded the point, adding that the investigators are trying to reanalyze the data in a more granular way to address that shortcoming. Other study limitations included an inability to access the specific doses of systemic treatments used or to stratify patients by disease severity.
Another audience member noted that dermatologists often reassure surgeons that there’s no increased risk of infection associated with psoriasis when in fact there is increased risk in older psoriasis patients, according to these new data.
“We’re not trying to send a message to surgeons to withhold a knee transplant because of a psoriasis plaque over the knee,” Dr. Merola replied. “I think we’ve all been there; we’ve all fought that battle.” Based on the data, he said, he would advise that “our patients who need to be on systemics should remain appropriately on systemics as we see fit.”
The study was entirely funded by Brigham and Women’s Hospital. Dr. Merola reported serving as a consultant to and/or recipient of research grants from nearly two dozen pharmaceutical companies.
MADRID – Psoriasis patients aged 65 years and older are at more than twice the risk of serious bacterial and opportunistic infections, compared with younger patients, but that risk is not further elevated by being on biologic agents, Joseph F. Merola, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a large, The study implications, he said, are clear: When moderate to severe psoriasis warrants consideration of highly effective biologic therapies, that therapeutic option shouldn’t be taken off the table on the basis of a mistaken belief that biologics pose a greater infection risk just because the affected patient is over age 65 years.
“We really think that older patients should be offered treatments at the same level of disease control as all the rest of our psoriasis patients, in the context of shared decision making,” said Dr. Merola, a dermatologist and rheumatologist who is the director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston.
The study utilized longitudinal claims data from a very large U.S. database covering the years 2003-2017. Among the 185 million covered lives were 1.1 million individuals with psoriasis, including 150,000 aged 65 years or older. After excluding older psoriasis patients with comorbid cancer or autoimmune disease, the investigators were left with 11,218 older psoriasis patients initiating systemic therapy for the first time and therefore eligible for propensity score matching using a highly accurate proprietary platform. The final study population consisted of 2,795 older psoriasis patients newly initiating biologic therapy, 2,795 others newly initiating nonbiologic systemic agents, and 2,529 seniors starting phototherapy. The matching was based upon factors including age, sex, prior infections, comorbid psoriatic arthritis, diabetes, and obesity.
The primary study endpoint was the rate of serious bacterial or opportunistic infections requiring hospitalization during the first 6 months of treatment. The bottom line: The rates were closely similar across all three groups, with the most common serious infections being pneumonia and cellulitis.
In contrast, among a population of 115,047 senior psoriasis patients who never used systemic therapy, the risk of serious infection was 12.2 events per 1,000 patients over 6 months, compared with 5.3 events in 120,174 matched controls without psoriasis. That translates to a 2.24-fold increased risk.
One audience member commented that a limitation of the study was that all biologics were lumped together. He would expect that the tumor necrosis factor inhibitors, for example, would be associated with a significantly higher serious infection risk than biologics with other targets.
Dr. Merola conceded the point, adding that the investigators are trying to reanalyze the data in a more granular way to address that shortcoming. Other study limitations included an inability to access the specific doses of systemic treatments used or to stratify patients by disease severity.
Another audience member noted that dermatologists often reassure surgeons that there’s no increased risk of infection associated with psoriasis when in fact there is increased risk in older psoriasis patients, according to these new data.
“We’re not trying to send a message to surgeons to withhold a knee transplant because of a psoriasis plaque over the knee,” Dr. Merola replied. “I think we’ve all been there; we’ve all fought that battle.” Based on the data, he said, he would advise that “our patients who need to be on systemics should remain appropriately on systemics as we see fit.”
The study was entirely funded by Brigham and Women’s Hospital. Dr. Merola reported serving as a consultant to and/or recipient of research grants from nearly two dozen pharmaceutical companies.
MADRID – Psoriasis patients aged 65 years and older are at more than twice the risk of serious bacterial and opportunistic infections, compared with younger patients, but that risk is not further elevated by being on biologic agents, Joseph F. Merola, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
He presented a large, The study implications, he said, are clear: When moderate to severe psoriasis warrants consideration of highly effective biologic therapies, that therapeutic option shouldn’t be taken off the table on the basis of a mistaken belief that biologics pose a greater infection risk just because the affected patient is over age 65 years.
“We really think that older patients should be offered treatments at the same level of disease control as all the rest of our psoriasis patients, in the context of shared decision making,” said Dr. Merola, a dermatologist and rheumatologist who is the director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston.
The study utilized longitudinal claims data from a very large U.S. database covering the years 2003-2017. Among the 185 million covered lives were 1.1 million individuals with psoriasis, including 150,000 aged 65 years or older. After excluding older psoriasis patients with comorbid cancer or autoimmune disease, the investigators were left with 11,218 older psoriasis patients initiating systemic therapy for the first time and therefore eligible for propensity score matching using a highly accurate proprietary platform. The final study population consisted of 2,795 older psoriasis patients newly initiating biologic therapy, 2,795 others newly initiating nonbiologic systemic agents, and 2,529 seniors starting phototherapy. The matching was based upon factors including age, sex, prior infections, comorbid psoriatic arthritis, diabetes, and obesity.
The primary study endpoint was the rate of serious bacterial or opportunistic infections requiring hospitalization during the first 6 months of treatment. The bottom line: The rates were closely similar across all three groups, with the most common serious infections being pneumonia and cellulitis.
In contrast, among a population of 115,047 senior psoriasis patients who never used systemic therapy, the risk of serious infection was 12.2 events per 1,000 patients over 6 months, compared with 5.3 events in 120,174 matched controls without psoriasis. That translates to a 2.24-fold increased risk.
One audience member commented that a limitation of the study was that all biologics were lumped together. He would expect that the tumor necrosis factor inhibitors, for example, would be associated with a significantly higher serious infection risk than biologics with other targets.
Dr. Merola conceded the point, adding that the investigators are trying to reanalyze the data in a more granular way to address that shortcoming. Other study limitations included an inability to access the specific doses of systemic treatments used or to stratify patients by disease severity.
Another audience member noted that dermatologists often reassure surgeons that there’s no increased risk of infection associated with psoriasis when in fact there is increased risk in older psoriasis patients, according to these new data.
“We’re not trying to send a message to surgeons to withhold a knee transplant because of a psoriasis plaque over the knee,” Dr. Merola replied. “I think we’ve all been there; we’ve all fought that battle.” Based on the data, he said, he would advise that “our patients who need to be on systemics should remain appropriately on systemics as we see fit.”
The study was entirely funded by Brigham and Women’s Hospital. Dr. Merola reported serving as a consultant to and/or recipient of research grants from nearly two dozen pharmaceutical companies.
REPORTING FROM EADV 2019
Birth year linked to influenza-subtype susceptibility
WASHINGTON – People may differ in their susceptibility to different influenza subtypes based in part on the year when they were born and the flu strains that circulated during their birth year, according to infection patterns during a recent U.S. flu season.
“Our findings may indicate protection against H1 [influenza] viruses in age groups with early exposure to H1N1pdm09 during the 2009 pandemic or to older, antigenically similar H1N1 viruses,” Shikha Garg, MD, said at an annual scientific meeting on infectious diseases. If results from further studies confirm this relationship it could have implications for flu vaccine effectiveness in various age groups and influence the composition of flu vaccines based on the ages of the people who will receive them, said Dr. Garg, a medical epidemiologist with the Centers for Disease Control and Prevention in Atlanta.
The analysis she reported using data collected by the CDC’s Influenza Hospitalization Surveillance Network on 18,699 people hospitalized for influenza infection during the 2018-2019 season, Oct. 1, 2018–April 30, 2019. The database provides a representative sampling of patients hospitalized for influenza at more than 250 acute care hospitals in 13 states. During the season studied, both the H1N1 and H3N2 subtypes circulated and caused similar cumulative rates of infections, with H1N1 causing about 32 confirmed cases per 100,000 people and H3N2 causing about 29 cases/100,000.
But a more granular analysis that divided the hospitalized patients by their birth year showed an excess of H1N1 infections in two demographic slices: those born during 2010-2019 (corresponding to children 0-9 years old), in whom H1N1 accounted for roughly 60% of cases; and also in those born during 1948-1995 (people aged 24-70 years old) in whom H1N1 caused roughly 70% or more of all infections in some for some birth-year groups in this demographic range. In contrast, infection with the circulating H3N2 strain in the 2018-2019 season dominated among those born during 1996-2009 (people aged 10-23), as well as in those born in 1947 or earlier (those who were at least 71 years old). Some age groups within those born in 1996-2009 had H3N2 infection rates that made up 70% or more of all flu infections, and among nonagenarians well over three-quarters of flu infection were by the H3N2 subtype.
Dr. Garg also showed a similar pattern of predominant flu subtype by age using U.S. influenza hospitalization data for the 2017-2018 season, as well as for all types of 2018-2019 U.S. influenza infections that underwent strain typing including outpatients as well as in patients. All of these findings support the hypothesis and extend the data published earlier this year by Dr. Garg and several of her CDC colleagues that described a pattern of “antigen imprinting” that appeared caused by influenza exposure during the first year of life (J Infect Dis. 2019 Sep 1;220[5]:820-9). However, more data are needed to better assess time trends for children who were first exposed to H1N1 influenza during the 2009 pandemic, Dr. Garg said.
[email protected]
SOURCE: Garg S. ID Week 2019, Abstract LB19.
WASHINGTON – People may differ in their susceptibility to different influenza subtypes based in part on the year when they were born and the flu strains that circulated during their birth year, according to infection patterns during a recent U.S. flu season.
“Our findings may indicate protection against H1 [influenza] viruses in age groups with early exposure to H1N1pdm09 during the 2009 pandemic or to older, antigenically similar H1N1 viruses,” Shikha Garg, MD, said at an annual scientific meeting on infectious diseases. If results from further studies confirm this relationship it could have implications for flu vaccine effectiveness in various age groups and influence the composition of flu vaccines based on the ages of the people who will receive them, said Dr. Garg, a medical epidemiologist with the Centers for Disease Control and Prevention in Atlanta.
The analysis she reported using data collected by the CDC’s Influenza Hospitalization Surveillance Network on 18,699 people hospitalized for influenza infection during the 2018-2019 season, Oct. 1, 2018–April 30, 2019. The database provides a representative sampling of patients hospitalized for influenza at more than 250 acute care hospitals in 13 states. During the season studied, both the H1N1 and H3N2 subtypes circulated and caused similar cumulative rates of infections, with H1N1 causing about 32 confirmed cases per 100,000 people and H3N2 causing about 29 cases/100,000.
But a more granular analysis that divided the hospitalized patients by their birth year showed an excess of H1N1 infections in two demographic slices: those born during 2010-2019 (corresponding to children 0-9 years old), in whom H1N1 accounted for roughly 60% of cases; and also in those born during 1948-1995 (people aged 24-70 years old) in whom H1N1 caused roughly 70% or more of all infections in some for some birth-year groups in this demographic range. In contrast, infection with the circulating H3N2 strain in the 2018-2019 season dominated among those born during 1996-2009 (people aged 10-23), as well as in those born in 1947 or earlier (those who were at least 71 years old). Some age groups within those born in 1996-2009 had H3N2 infection rates that made up 70% or more of all flu infections, and among nonagenarians well over three-quarters of flu infection were by the H3N2 subtype.
Dr. Garg also showed a similar pattern of predominant flu subtype by age using U.S. influenza hospitalization data for the 2017-2018 season, as well as for all types of 2018-2019 U.S. influenza infections that underwent strain typing including outpatients as well as in patients. All of these findings support the hypothesis and extend the data published earlier this year by Dr. Garg and several of her CDC colleagues that described a pattern of “antigen imprinting” that appeared caused by influenza exposure during the first year of life (J Infect Dis. 2019 Sep 1;220[5]:820-9). However, more data are needed to better assess time trends for children who were first exposed to H1N1 influenza during the 2009 pandemic, Dr. Garg said.
[email protected]
SOURCE: Garg S. ID Week 2019, Abstract LB19.
WASHINGTON – People may differ in their susceptibility to different influenza subtypes based in part on the year when they were born and the flu strains that circulated during their birth year, according to infection patterns during a recent U.S. flu season.
“Our findings may indicate protection against H1 [influenza] viruses in age groups with early exposure to H1N1pdm09 during the 2009 pandemic or to older, antigenically similar H1N1 viruses,” Shikha Garg, MD, said at an annual scientific meeting on infectious diseases. If results from further studies confirm this relationship it could have implications for flu vaccine effectiveness in various age groups and influence the composition of flu vaccines based on the ages of the people who will receive them, said Dr. Garg, a medical epidemiologist with the Centers for Disease Control and Prevention in Atlanta.
The analysis she reported using data collected by the CDC’s Influenza Hospitalization Surveillance Network on 18,699 people hospitalized for influenza infection during the 2018-2019 season, Oct. 1, 2018–April 30, 2019. The database provides a representative sampling of patients hospitalized for influenza at more than 250 acute care hospitals in 13 states. During the season studied, both the H1N1 and H3N2 subtypes circulated and caused similar cumulative rates of infections, with H1N1 causing about 32 confirmed cases per 100,000 people and H3N2 causing about 29 cases/100,000.
But a more granular analysis that divided the hospitalized patients by their birth year showed an excess of H1N1 infections in two demographic slices: those born during 2010-2019 (corresponding to children 0-9 years old), in whom H1N1 accounted for roughly 60% of cases; and also in those born during 1948-1995 (people aged 24-70 years old) in whom H1N1 caused roughly 70% or more of all infections in some for some birth-year groups in this demographic range. In contrast, infection with the circulating H3N2 strain in the 2018-2019 season dominated among those born during 1996-2009 (people aged 10-23), as well as in those born in 1947 or earlier (those who were at least 71 years old). Some age groups within those born in 1996-2009 had H3N2 infection rates that made up 70% or more of all flu infections, and among nonagenarians well over three-quarters of flu infection were by the H3N2 subtype.
Dr. Garg also showed a similar pattern of predominant flu subtype by age using U.S. influenza hospitalization data for the 2017-2018 season, as well as for all types of 2018-2019 U.S. influenza infections that underwent strain typing including outpatients as well as in patients. All of these findings support the hypothesis and extend the data published earlier this year by Dr. Garg and several of her CDC colleagues that described a pattern of “antigen imprinting” that appeared caused by influenza exposure during the first year of life (J Infect Dis. 2019 Sep 1;220[5]:820-9). However, more data are needed to better assess time trends for children who were first exposed to H1N1 influenza during the 2009 pandemic, Dr. Garg said.
[email protected]
SOURCE: Garg S. ID Week 2019, Abstract LB19.
REPORTING FROM ID WEEK 2019
NIH seeks gene-based cures for HIV, sickle cell disease
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.

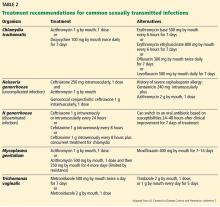




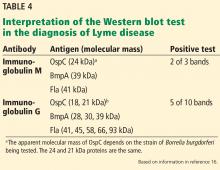
![Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto. Positive Western blot test (Borrelia B31 ViraStripe [Viramed Diagnostics]) in a patient who presented with rash and arthritis. This test uses purified specific antigens of strain B31 of Borrelia burgdorferi sensu stricto.](https://cdn.mdedge.com/files/s3fs-public/styles/medium/public/756fig2.jpg?itok=fMD7ruHJ)