User login
New test edges closer to rapid, accurate ID of active TB
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
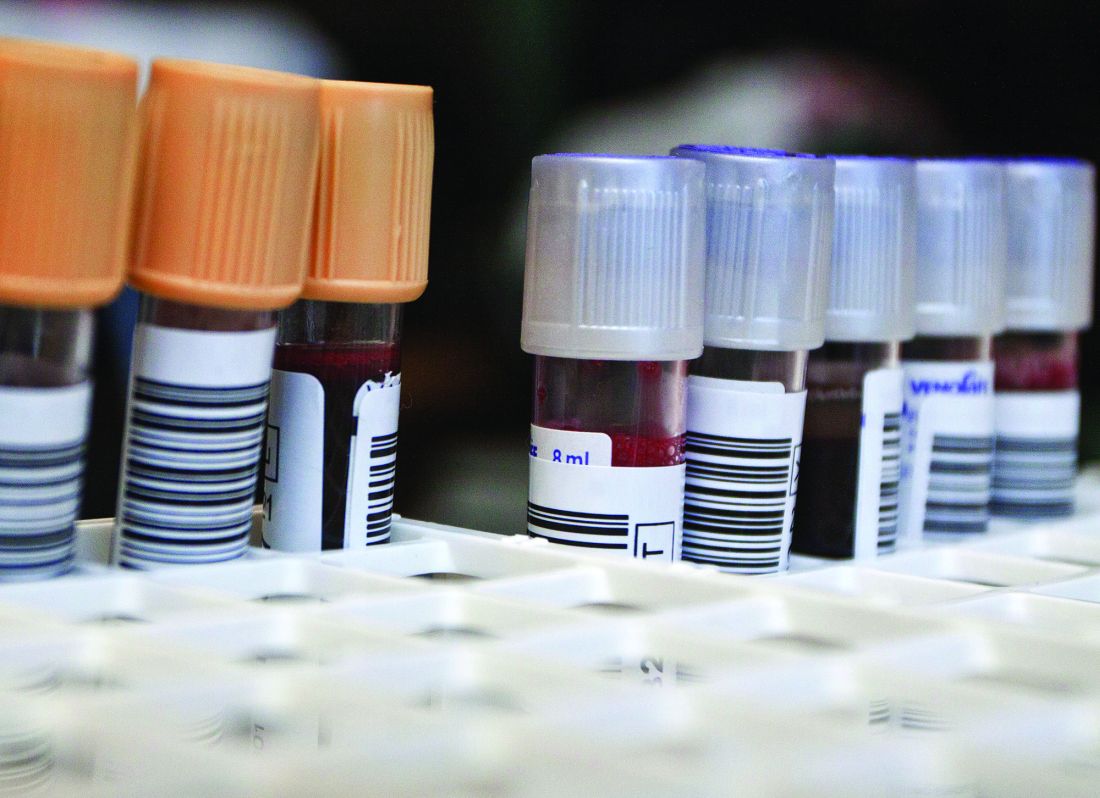
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection.
Major finding: The assay had a sensitivity of 86%.
Study details: A machine learning and validation study involving patients with chronic cough from multiple countries.
Disclosures: The Bill and Melinda Gates Foundation funded the study. The investigators reported relationships with Quanterix Corporation and FIND.
Source: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
Rare mixed HCV genotypes found in men who have sex with men
A low percentage of mixed genotypes of hepatitis C virus (HCV) was found in a small study of recently infected HIV+ and HIV– men who have sex with men (MSM) according to a report by Thuy Nguyen, PhD, of the University of North Carolina, Chapel Hill, and colleagues published in the International Journal of Antimicrobial Agents.
The researchers assessed 58 HCV-infected individuals with a median age of 38.5 years, 50 of whom were HIV positive and 18 of whom were HIV negative. Most of the patients were MSM (85.3%), with the rest of unknown sexual orientation. HCV genotyping by Sanger found types GT1a, GT4d, GT3a, and GT2k infection in 47.1%, 41.2%, 8.8%, and 2.9% of the individuals.
After eliminating suspected contaminations, three patients (4.4%) were found with mixed GT infections All three patients were infected with HCV for the first time; two-thirds were coinfected with HIV. The mixed GTs comprised only GT4d and GT1a at different ratios. Mixed infections are potentially problematic when using direct-acting antiviral therapy without broad-spectrum activity, according to the researchers. In this case, however, all HCV patients achieved treatment success.
“From a public health perspective, the MSM population engaging in high-risk behaviors still requires special attention in terms of mixed infections compared with the general HCV-infected population with a regular monitoring of anti-HCV treatment response, particularly when pangenotypic treatment is not used,” the researchers concluded.
The study was funded by the French government; the authors reported having no conflicts.
SOURCE: Nguyen T et al. Int J Antimicrobial Agents. 2019. 54[4]:523-7.
A low percentage of mixed genotypes of hepatitis C virus (HCV) was found in a small study of recently infected HIV+ and HIV– men who have sex with men (MSM) according to a report by Thuy Nguyen, PhD, of the University of North Carolina, Chapel Hill, and colleagues published in the International Journal of Antimicrobial Agents.
The researchers assessed 58 HCV-infected individuals with a median age of 38.5 years, 50 of whom were HIV positive and 18 of whom were HIV negative. Most of the patients were MSM (85.3%), with the rest of unknown sexual orientation. HCV genotyping by Sanger found types GT1a, GT4d, GT3a, and GT2k infection in 47.1%, 41.2%, 8.8%, and 2.9% of the individuals.
After eliminating suspected contaminations, three patients (4.4%) were found with mixed GT infections All three patients were infected with HCV for the first time; two-thirds were coinfected with HIV. The mixed GTs comprised only GT4d and GT1a at different ratios. Mixed infections are potentially problematic when using direct-acting antiviral therapy without broad-spectrum activity, according to the researchers. In this case, however, all HCV patients achieved treatment success.
“From a public health perspective, the MSM population engaging in high-risk behaviors still requires special attention in terms of mixed infections compared with the general HCV-infected population with a regular monitoring of anti-HCV treatment response, particularly when pangenotypic treatment is not used,” the researchers concluded.
The study was funded by the French government; the authors reported having no conflicts.
SOURCE: Nguyen T et al. Int J Antimicrobial Agents. 2019. 54[4]:523-7.
A low percentage of mixed genotypes of hepatitis C virus (HCV) was found in a small study of recently infected HIV+ and HIV– men who have sex with men (MSM) according to a report by Thuy Nguyen, PhD, of the University of North Carolina, Chapel Hill, and colleagues published in the International Journal of Antimicrobial Agents.
The researchers assessed 58 HCV-infected individuals with a median age of 38.5 years, 50 of whom were HIV positive and 18 of whom were HIV negative. Most of the patients were MSM (85.3%), with the rest of unknown sexual orientation. HCV genotyping by Sanger found types GT1a, GT4d, GT3a, and GT2k infection in 47.1%, 41.2%, 8.8%, and 2.9% of the individuals.
After eliminating suspected contaminations, three patients (4.4%) were found with mixed GT infections All three patients were infected with HCV for the first time; two-thirds were coinfected with HIV. The mixed GTs comprised only GT4d and GT1a at different ratios. Mixed infections are potentially problematic when using direct-acting antiviral therapy without broad-spectrum activity, according to the researchers. In this case, however, all HCV patients achieved treatment success.
“From a public health perspective, the MSM population engaging in high-risk behaviors still requires special attention in terms of mixed infections compared with the general HCV-infected population with a regular monitoring of anti-HCV treatment response, particularly when pangenotypic treatment is not used,” the researchers concluded.
The study was funded by the French government; the authors reported having no conflicts.
SOURCE: Nguyen T et al. Int J Antimicrobial Agents. 2019. 54[4]:523-7.
FROM THE INTERNATIONAL JOURNAL OF ANTIMICROBIAL AGENTS
Vitamin C–based regimens in sepsis plausible, need more data, expert says
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
EXPERT ANALYSIS FROM CHEST 2019
In-hospital flu shot reduced readmissions in pneumonia patients
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
REPORTING FROM CHEST 2019
Pulmonary Hemorrhage as the Initial Presentation of AIDS-Related Kaposi Sarcoma
To the Editor:
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
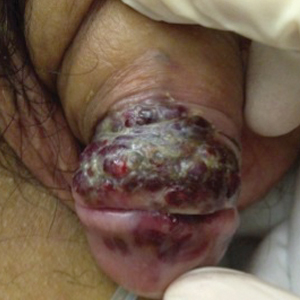
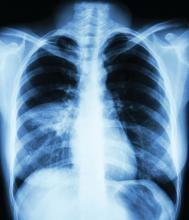
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.

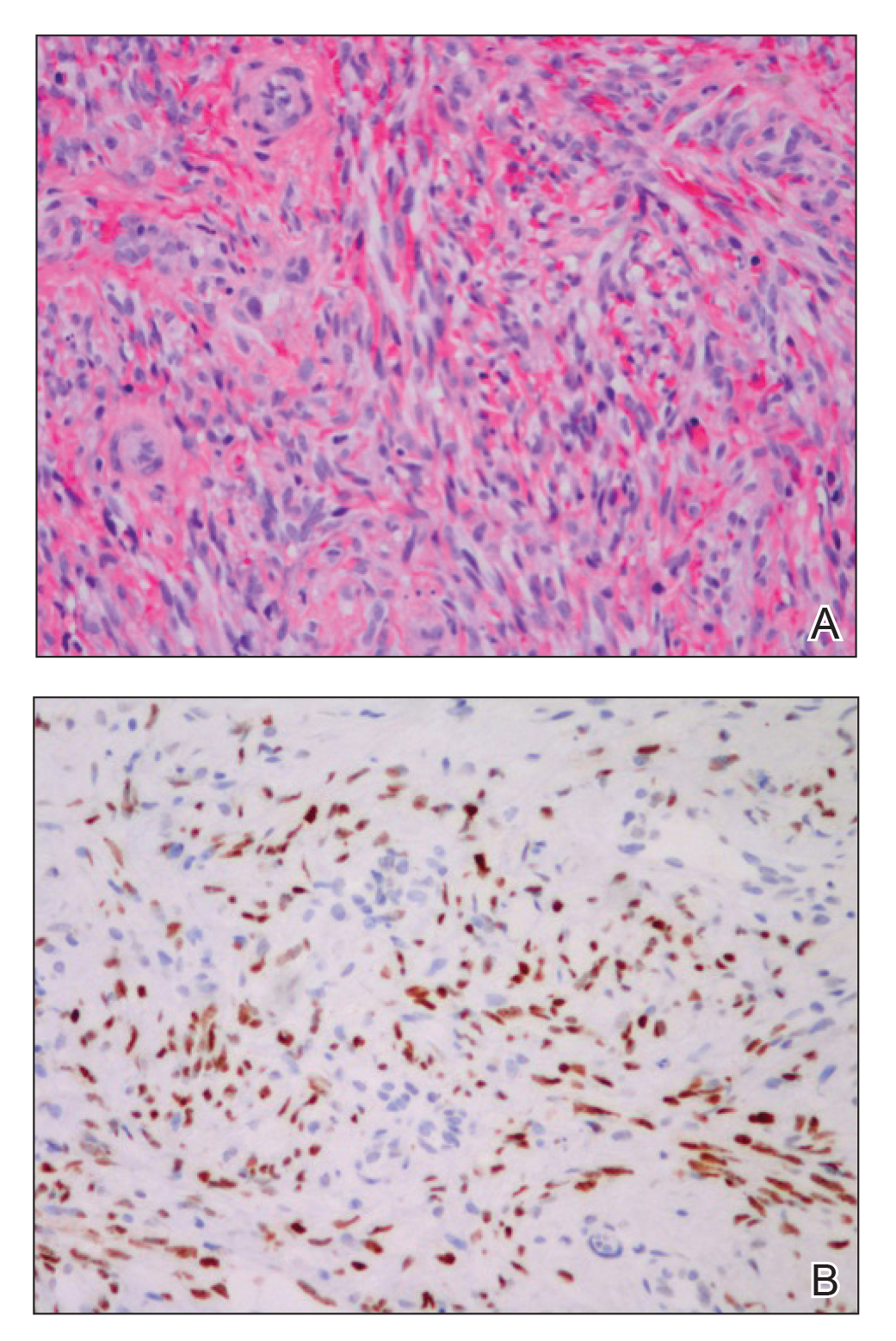
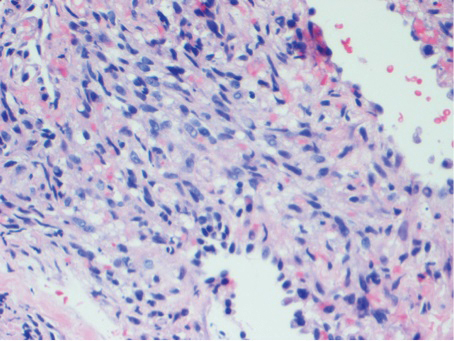
Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.
This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
To the Editor:
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.

Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.


This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
To the Editor:
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.

Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.


This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
Practice Points
- Visceral Kaposi sarcoma (KS) should be considered in patients with unexplained systemic symptoms in the setting of poorly controlled human immunodeficiency virus (HIV).
- If cutaneous KS is diagnosed in an HIV patient, a detailed history and physical examination should be undertaken to evaluate for signs of systemic disease.
Crusted Demodicosis in an Immunocompetent Patient
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
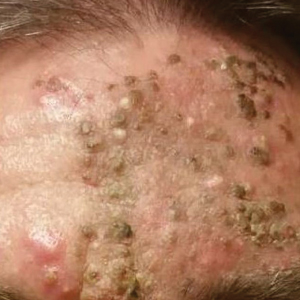
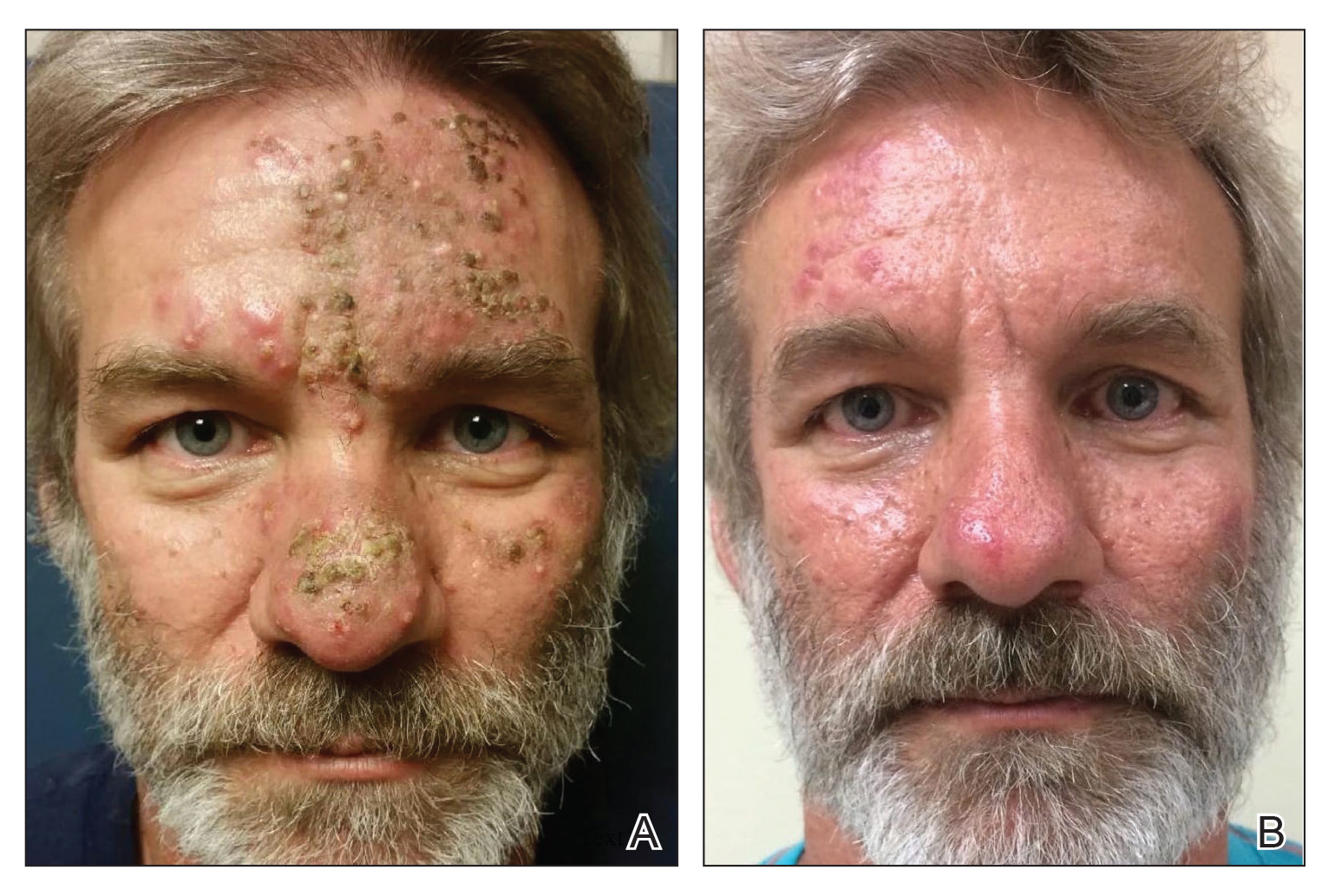
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).
The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
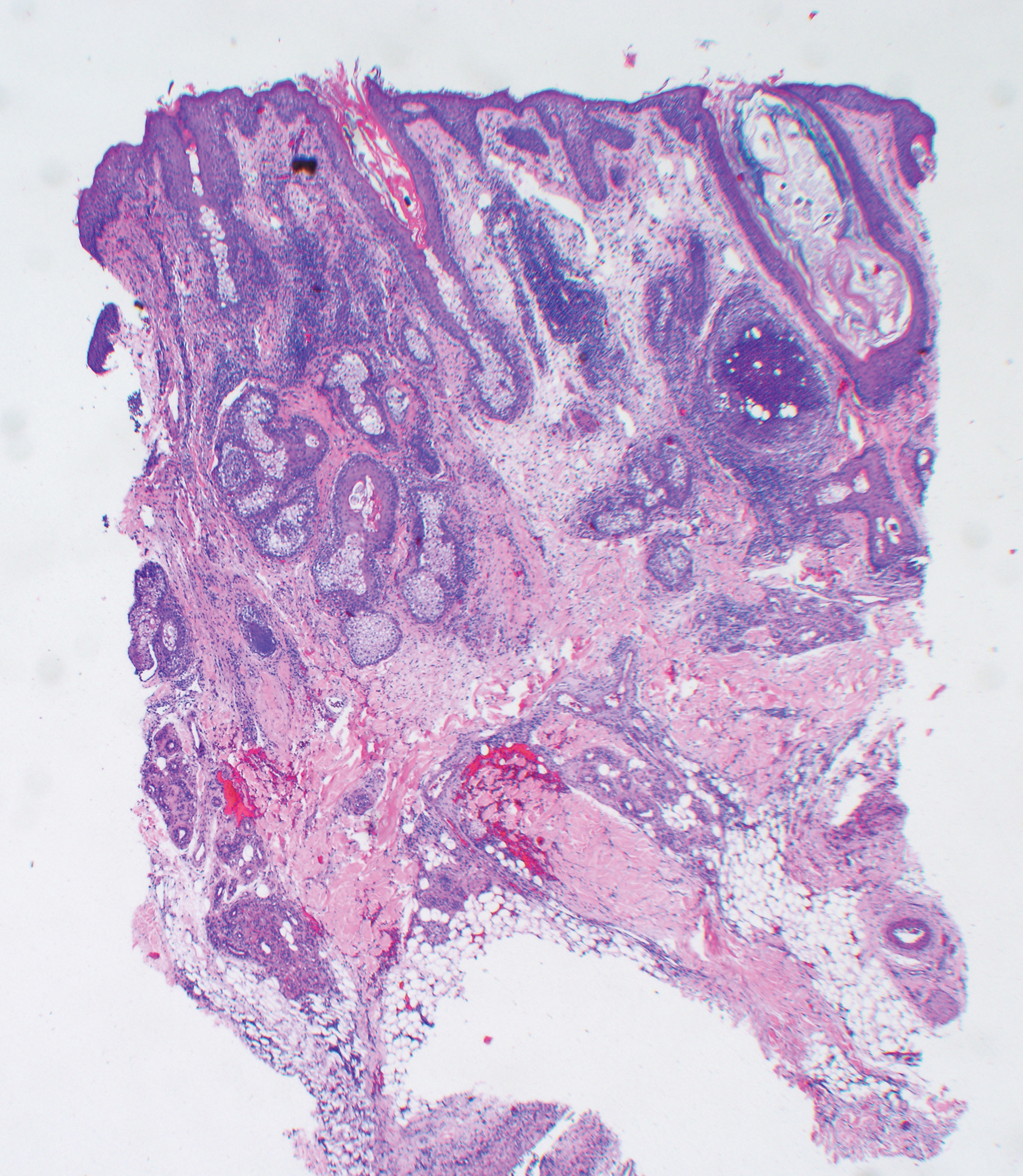
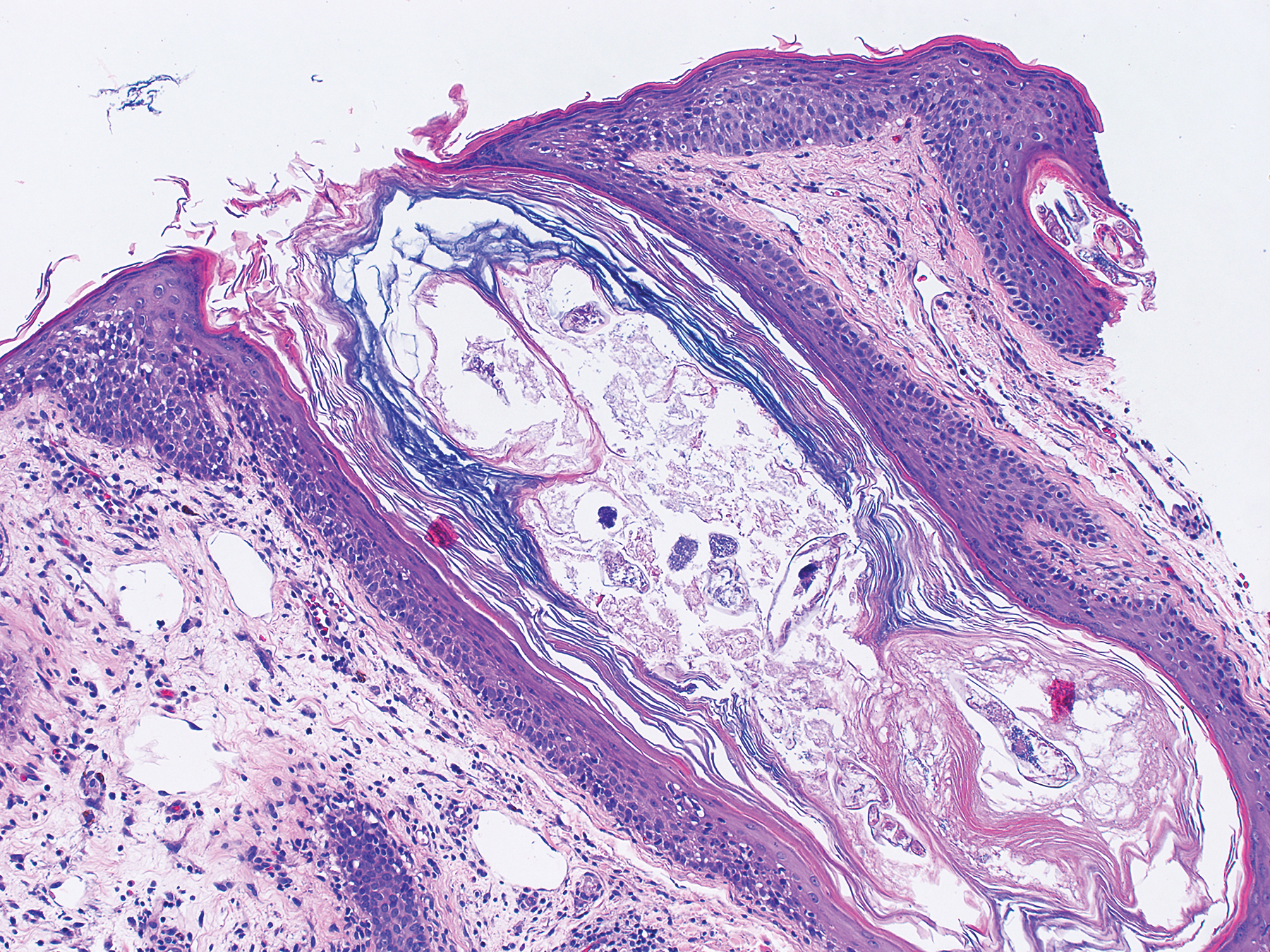
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.
Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).

The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.


Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).

The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.


Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
Practice Points
- The Demodex mite, believed to be a commensal species in humans, has the ability to shift to a pathologic form in immunocompromised patients.
- Demodicosis can manifest in a variety of forms including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis.
Flu vaccine: Larger impact on influenza burden than you thought?
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
One monoclonal dose gives preterm neonates season-long RSV protection
WASHINGTON – A single dose of a novel monoclonal antibody against a respiratory syncytial virus surface protein safely protected preterm infants against severe infections for 150 days during their first winter season in a randomized trial with more than 1,400 children.
One intramuscular injection of nirsevimab (also known as MEDI8897) administered to infants born at 29-35 weeks’ gestation at the start of the local respiratory syncytial virus (RSV) season (November in the Northern hemisphere) led to a 70% relative reduction in the rate of medically attended lower respiratory tract infections with RSV during the subsequent 150 days, compared with placebo, the study’s primary efficacy outcome, M. Pamela Griffin, MD, said at an annual scientific meeting on infectious diseases.
In a secondary efficacy measure, the rate of hospitalizations for RSV-caused lower respiratory tract infections, a single injection of nirsevimab dropped the incidence by 78%, relative to placebo. Both effects were statistically significant. The rate of total adverse events and serious adverse events was similar in the two treatment arms, reported Dr. Griffin, a clinical development lead with AstraZeneca.
These positive results for a single intramuscular injection of nirsevimab are the first findings from a series of studies aimed at getting the monoclonal antibody onto the U.S. market as a superior alternative to palivizumab (Synagis), which acts in a similar way to block RSV infection (albeit by targeting a different viral surface protein) but which requires administration every 30 days. This need for serial dosing of palivizumab in children younger than 1 year old for complete seasonal protection against RSV is probably a reason why the American Academy of Pediatrics, as well as other medical societies, have targeted using palivizumab only on certain types of high-risk infants: those born before 29 weeks’ gestational age, with chronic lung disease of prematurity, or with hemodynamically significant congenital heart disease (Pediatrics. 2014 Aug;134[2]:415-20). “It’s not feasible for most infants to come for five treatments during RSV season,” Dr. Griffin noted. A tweak in the structure of nirsevimab gives it a much longer blood half-life than palivizumab and allows a single dose to maintain efficacy for 5 months, the duration of RSV season.
“The big advantage of nirsevimab is one dose instead of five,” she said in an interview.
The study randomized 969 preterm infants to nirsevimab and 484 to placebo when the children averaged 3 months old and 4.5 kg. The incidence of the primary endpoint was 2.6% in the nirsevimab-treated infants and 9.5% in those who received placebo. The incidence of hospitalizations associated with an RSV lower respiratory tract infection was 0.8% in the nirsevimab group and 4.1% on placebo. Nirsevimab was equally effective regardless of RSV subtype, infant age, or sex. The rate of hypersensitivity reactions was low, less than 1%, and similar in the two treatment arms, as was the rate of detection of antidrug antibody, 3.8% with placebo and 5.6% with nirsevimab.
Two other large trials are underway to document the performance of nirsevimab in other types of infants. One study is examining the drug’s performance compared with placebo in term infants with a gestational age of at least 36 weeks, while another is comparing nirsevimab against a five-dose regimen of palivizumab in high-risk infants who are recommended to receive palivizumab by local medical societies. In the United States, this would be infants born at less than 29 weeks’ gestation, and those with either hemodynamically significant congenital heart disease or chronic lung disease of prematurity. In these studies, the researchers also will assess the cost effectiveness of nirsevimab relative to the costs for medical care needed by infants who receive comparator treatments, Dr. Griffin said.
The study was funded by AstraZeneca, the company developing nirsevimab. Dr. Griffin is an employee of and shareholder in AstraZeneca.
SOURCE: ClinicalTrials.gov identifier: NCT02878330.
WASHINGTON – A single dose of a novel monoclonal antibody against a respiratory syncytial virus surface protein safely protected preterm infants against severe infections for 150 days during their first winter season in a randomized trial with more than 1,400 children.
One intramuscular injection of nirsevimab (also known as MEDI8897) administered to infants born at 29-35 weeks’ gestation at the start of the local respiratory syncytial virus (RSV) season (November in the Northern hemisphere) led to a 70% relative reduction in the rate of medically attended lower respiratory tract infections with RSV during the subsequent 150 days, compared with placebo, the study’s primary efficacy outcome, M. Pamela Griffin, MD, said at an annual scientific meeting on infectious diseases.
In a secondary efficacy measure, the rate of hospitalizations for RSV-caused lower respiratory tract infections, a single injection of nirsevimab dropped the incidence by 78%, relative to placebo. Both effects were statistically significant. The rate of total adverse events and serious adverse events was similar in the two treatment arms, reported Dr. Griffin, a clinical development lead with AstraZeneca.
These positive results for a single intramuscular injection of nirsevimab are the first findings from a series of studies aimed at getting the monoclonal antibody onto the U.S. market as a superior alternative to palivizumab (Synagis), which acts in a similar way to block RSV infection (albeit by targeting a different viral surface protein) but which requires administration every 30 days. This need for serial dosing of palivizumab in children younger than 1 year old for complete seasonal protection against RSV is probably a reason why the American Academy of Pediatrics, as well as other medical societies, have targeted using palivizumab only on certain types of high-risk infants: those born before 29 weeks’ gestational age, with chronic lung disease of prematurity, or with hemodynamically significant congenital heart disease (Pediatrics. 2014 Aug;134[2]:415-20). “It’s not feasible for most infants to come for five treatments during RSV season,” Dr. Griffin noted. A tweak in the structure of nirsevimab gives it a much longer blood half-life than palivizumab and allows a single dose to maintain efficacy for 5 months, the duration of RSV season.
“The big advantage of nirsevimab is one dose instead of five,” she said in an interview.
The study randomized 969 preterm infants to nirsevimab and 484 to placebo when the children averaged 3 months old and 4.5 kg. The incidence of the primary endpoint was 2.6% in the nirsevimab-treated infants and 9.5% in those who received placebo. The incidence of hospitalizations associated with an RSV lower respiratory tract infection was 0.8% in the nirsevimab group and 4.1% on placebo. Nirsevimab was equally effective regardless of RSV subtype, infant age, or sex. The rate of hypersensitivity reactions was low, less than 1%, and similar in the two treatment arms, as was the rate of detection of antidrug antibody, 3.8% with placebo and 5.6% with nirsevimab.
Two other large trials are underway to document the performance of nirsevimab in other types of infants. One study is examining the drug’s performance compared with placebo in term infants with a gestational age of at least 36 weeks, while another is comparing nirsevimab against a five-dose regimen of palivizumab in high-risk infants who are recommended to receive palivizumab by local medical societies. In the United States, this would be infants born at less than 29 weeks’ gestation, and those with either hemodynamically significant congenital heart disease or chronic lung disease of prematurity. In these studies, the researchers also will assess the cost effectiveness of nirsevimab relative to the costs for medical care needed by infants who receive comparator treatments, Dr. Griffin said.
The study was funded by AstraZeneca, the company developing nirsevimab. Dr. Griffin is an employee of and shareholder in AstraZeneca.
SOURCE: ClinicalTrials.gov identifier: NCT02878330.
WASHINGTON – A single dose of a novel monoclonal antibody against a respiratory syncytial virus surface protein safely protected preterm infants against severe infections for 150 days during their first winter season in a randomized trial with more than 1,400 children.
One intramuscular injection of nirsevimab (also known as MEDI8897) administered to infants born at 29-35 weeks’ gestation at the start of the local respiratory syncytial virus (RSV) season (November in the Northern hemisphere) led to a 70% relative reduction in the rate of medically attended lower respiratory tract infections with RSV during the subsequent 150 days, compared with placebo, the study’s primary efficacy outcome, M. Pamela Griffin, MD, said at an annual scientific meeting on infectious diseases.
In a secondary efficacy measure, the rate of hospitalizations for RSV-caused lower respiratory tract infections, a single injection of nirsevimab dropped the incidence by 78%, relative to placebo. Both effects were statistically significant. The rate of total adverse events and serious adverse events was similar in the two treatment arms, reported Dr. Griffin, a clinical development lead with AstraZeneca.
These positive results for a single intramuscular injection of nirsevimab are the first findings from a series of studies aimed at getting the monoclonal antibody onto the U.S. market as a superior alternative to palivizumab (Synagis), which acts in a similar way to block RSV infection (albeit by targeting a different viral surface protein) but which requires administration every 30 days. This need for serial dosing of palivizumab in children younger than 1 year old for complete seasonal protection against RSV is probably a reason why the American Academy of Pediatrics, as well as other medical societies, have targeted using palivizumab only on certain types of high-risk infants: those born before 29 weeks’ gestational age, with chronic lung disease of prematurity, or with hemodynamically significant congenital heart disease (Pediatrics. 2014 Aug;134[2]:415-20). “It’s not feasible for most infants to come for five treatments during RSV season,” Dr. Griffin noted. A tweak in the structure of nirsevimab gives it a much longer blood half-life than palivizumab and allows a single dose to maintain efficacy for 5 months, the duration of RSV season.
“The big advantage of nirsevimab is one dose instead of five,” she said in an interview.
The study randomized 969 preterm infants to nirsevimab and 484 to placebo when the children averaged 3 months old and 4.5 kg. The incidence of the primary endpoint was 2.6% in the nirsevimab-treated infants and 9.5% in those who received placebo. The incidence of hospitalizations associated with an RSV lower respiratory tract infection was 0.8% in the nirsevimab group and 4.1% on placebo. Nirsevimab was equally effective regardless of RSV subtype, infant age, or sex. The rate of hypersensitivity reactions was low, less than 1%, and similar in the two treatment arms, as was the rate of detection of antidrug antibody, 3.8% with placebo and 5.6% with nirsevimab.
Two other large trials are underway to document the performance of nirsevimab in other types of infants. One study is examining the drug’s performance compared with placebo in term infants with a gestational age of at least 36 weeks, while another is comparing nirsevimab against a five-dose regimen of palivizumab in high-risk infants who are recommended to receive palivizumab by local medical societies. In the United States, this would be infants born at less than 29 weeks’ gestation, and those with either hemodynamically significant congenital heart disease or chronic lung disease of prematurity. In these studies, the researchers also will assess the cost effectiveness of nirsevimab relative to the costs for medical care needed by infants who receive comparator treatments, Dr. Griffin said.
The study was funded by AstraZeneca, the company developing nirsevimab. Dr. Griffin is an employee of and shareholder in AstraZeneca.
SOURCE: ClinicalTrials.gov identifier: NCT02878330.
REPORTING FROM ID WEEK 2019
Guidelines updated for treating community-acquired pneumonia
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
“Ever since we wrote the first CAP [community-acquired pneumonia] guidelines in 1993, we’ve heard good and bad things, and I agree with both,” Michael S. Niederman, MD, FCCP, said in a presentation at IDWeek 2019. “For good or for bad, [guidelines] are a standard against which care can be evaluated.” He discussed how, as guidelines have become more evidence based, they have often become “more wishy washy,” that when the evidence is weak, the recommendation is weak, and the guidelines merely advise doctors: “You figure it out.”
However, he pointed out that, since CAP guidelines were developed, there have been overall improvements in patient care and antibiotic stewardship. But he saw several weaknesses in the new guidelines, including the fact that they did not update minor criteria for determining severe CAP from the 2007 guidelines, despite several studies indicating that there were other criteria to consider. In addition, the updated guidelines held a negative view of the use of serum procalcitonin to guide site-of-care decisions, which Dr. Niederman argued went against an analysis of the Etiology of Pneumonia in the Community (EPIC) study (CHEST. 2016; 150[4]:819-28) and other studies that showed its utility. He referred to his own editorial, in which he discussed the subject extensively (Lancet Resp Med. 2016;4[12]:956).
“Similarly, to me, the macrolide issue is not resolved,” he added, citing several studies that, in contrast to the guideline recommendations, used outpatient macrolide monotherapy to good results, and one study showed that “there was a much better patient outcome for patients who got macrolide monotherapy than for those who got quinolones” (Resp Med. 2012;106[3]:451-8).
Dr. Niederman is clinical director of the division of pulmonary and critical care medicine at New York Presbyterian Hospital/Weill Cornell Medical Center, and professor of clinical medicine at Weill Cornell Medical College, New York. He disclosed that he is a consultant for and has received grants from a variety of pharmaceutical companies, including Bayer and Merck.
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
An update to the 2007 guidelines on the treatment of community-acquired pneumonia (CAP) was published by two medical societies, based upon the work of a multidisciplinary panel that “conducted pragmatic systematic reviews of the relevant research and applied Grading of Recommendations, Assessment, Development, and Evaluation methodology for clinical recommendations.”
The panel addressed 16 questions in the areas including diagnostic testing, determination of site of care, selection of initial empiric antibiotic therapy, and subsequent management decisions. Some of their recommendations remained unchanged from the 2007 guideline, but others were updated based upon more-recent clinical trials and epidemiological studies, according to Joshua P. Metlay, MD, of Massachusetts General Hospital, Boston, and colleagues on behalf of the Infectious Diseases Society of America and the American Thoracic Society.
Among the key recommendations differing from the previous guidelines, the 2019 guidelines include the following:
- Sputum and blood culture samples are recommended in patients with severe disease, as well as in all inpatients empirically treated for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa.
- Macrolide monotherapy is only conditionally recommended for outpatients based on resistance levels.
- Procalcitonin assessment, not covered in the 2007 guidelines, is not recommended in order to determine initial antibiotic therapy.
- Corticosteroid use, not covered in the 2007 guidelines, is not recommended, though it may be considered in patients with refractory septic shock.
- The use of health care–associated pneumonia (HCAP) as a category should be dropped, with a switch to an emphasis on local epidemiology and validated risk factors to determine the need for MRSA or P. aeruginosa treatment.
- Standard empiric therapy for severe CAP should be beta-lactam/macrolide and beta-lactam/fluoroquinolone combinations, but with stronger evidence in favor of the beta-lactam/macrolide combination.
The updated guidelines also include a number of other recommendations, such as those dealing with the management of patients with comorbidities, and were published in the American Journal of Respiratory and Critical Care Medicine.
“A difference between this guideline and previous ones is that we have significantly increased the proportion of patients in whom we recommend routinely obtaining respiratory tract samples for microbiologic studies. This decision is largely based on a desire to correct the overuse of anti-MRSA and antipseudomonal therapy that has occurred since the introduction of the HCAP classification (which we recommend abandoning) rather than high-quality evidence,” the authors stated in their conclusions. They added that they “expect our move against endorsing monotherapy with macrolides, which is based on population resistance data rather than high-quality clinical studies, will generate future outcomes studies comparing different treatment strategies.”
Many of the authors reported relationships with a variety of pharmaceutical companies; full disclosures are detailed at the end of the guideline publication.
SOURCE: Metlay JP et al. Am J Respir Crit Med. 2019;200(7):e45-67.
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Amoxicillin/clavulanate emerges as best antibiotic for childhood bronchiectasis
MADRID – A placebo-controlled trial has confirmed that amoxicillin/clavulanate is beneficial for resolution of acute exacerbations in nonsevere bronchiectasis while also demonstrating a greater relative effect than azithromycin, based on data presented at the annual congress of the European Respiratory Society.
“We now have robust data with which to support our guidelines,” reported Vikas Goyal, MD, of the Children’s Health Clinical Unit, University of Queensland, Brisbane, Australia.
The study addresses a knowledge gap. Antibiotics are already recommended by many guidelines for treatment of acute exacerbations in children with bronchiectasis, but Dr. Goyal said that no controlled trials have ever been performed in this age group to confirm superiority to placebo.
In this multicenter study, called BEST-1, 197 children with bronchiectasis were randomized at the start of an exacerbation to placebo, 45 mg/kg per day of amoxicillin/clavulanate, or 5 mg/kg per day of azithromycin. To maintain blinding, patients in the active treatment groups received a dummy for the opposite antibiotic while patients on placebo received dummies for both active agents.
For the primary outcome, 65% of children randomized to amoxicillin/clavulanate had resolution of their exacerbation by day 14 versus 61% of those randomized to azithromycin and 43% of those randomized to placebo. On the basis of relative risk for reaching this end point, the outcome was superior to placebo for amoxicillin/clavulanate (RR, 1.5; P = .015).
Although the relative risk for azithromycin (RR, 1.4; P = .042) was only slightly lower, it did not reach a prespecified level of significance set at P = .025. Dr. Goyal did report that the resolution rate at 14 days in the placebo group was “higher than expected.”
In this trial, 53% of the 154 children who were tested for respiratory viruses with nasal swabs on day 1 of the exacerbation were found to have respiratory viruses. Of these viruses, rhinovirus was the most common, according to Dr. Goyal, whose data were published just prior to his presentation (Lancet Respir Med. 2019;7:791-801).
The median durations of the exacerbations were 7 days, 8 days, and 10 days for those treated with amoxicillin/clavulanate, azithromycin, and placebo, respectively. The difference between amoxicillin/clavulanate and placebo, but not that between azithromycin and placebo, reached statistical significance, Dr. Goyal said.
There were no between group differences in the time to next exacerbation.
In discussing limitations of this study, Dr. Goyal pointed out that the optimal doses of amoxicillin/clavulanate or azithromycin have never been established for the treatment of exacerbations in children with bronchiectasis. He noted that some infectious disease specialists have advocated higher doses of both than those employed in this trial, but dose-ranging studies have never been conducted in this age group.
In this study, adverse events were less common on azithromycin than amoxicillin/clavulanate (21% vs. 30%), but none were severe, according to Dr. Goyal. He said treatment with azithromycin was associated with increased macrolide-resistant bacteria.
On the basis of these data, Dr. Goyal concluded that amoxicillin/clavulanate should remain, as already specified in some guidelines, the standard first-line therapy for nonsevere exacerbations in nonhospitalized children with bronchiectasis. He recommended reserving azithromycin as an alternative therapy.
Dr. Goyal reports no potential conflicts of interest.
SOURCE: Goyal V et al. Lancet Respir Med. 2019;7:791-801.
MADRID – A placebo-controlled trial has confirmed that amoxicillin/clavulanate is beneficial for resolution of acute exacerbations in nonsevere bronchiectasis while also demonstrating a greater relative effect than azithromycin, based on data presented at the annual congress of the European Respiratory Society.
“We now have robust data with which to support our guidelines,” reported Vikas Goyal, MD, of the Children’s Health Clinical Unit, University of Queensland, Brisbane, Australia.
The study addresses a knowledge gap. Antibiotics are already recommended by many guidelines for treatment of acute exacerbations in children with bronchiectasis, but Dr. Goyal said that no controlled trials have ever been performed in this age group to confirm superiority to placebo.
In this multicenter study, called BEST-1, 197 children with bronchiectasis were randomized at the start of an exacerbation to placebo, 45 mg/kg per day of amoxicillin/clavulanate, or 5 mg/kg per day of azithromycin. To maintain blinding, patients in the active treatment groups received a dummy for the opposite antibiotic while patients on placebo received dummies for both active agents.
For the primary outcome, 65% of children randomized to amoxicillin/clavulanate had resolution of their exacerbation by day 14 versus 61% of those randomized to azithromycin and 43% of those randomized to placebo. On the basis of relative risk for reaching this end point, the outcome was superior to placebo for amoxicillin/clavulanate (RR, 1.5; P = .015).
Although the relative risk for azithromycin (RR, 1.4; P = .042) was only slightly lower, it did not reach a prespecified level of significance set at P = .025. Dr. Goyal did report that the resolution rate at 14 days in the placebo group was “higher than expected.”
In this trial, 53% of the 154 children who were tested for respiratory viruses with nasal swabs on day 1 of the exacerbation were found to have respiratory viruses. Of these viruses, rhinovirus was the most common, according to Dr. Goyal, whose data were published just prior to his presentation (Lancet Respir Med. 2019;7:791-801).
The median durations of the exacerbations were 7 days, 8 days, and 10 days for those treated with amoxicillin/clavulanate, azithromycin, and placebo, respectively. The difference between amoxicillin/clavulanate and placebo, but not that between azithromycin and placebo, reached statistical significance, Dr. Goyal said.
There were no between group differences in the time to next exacerbation.
In discussing limitations of this study, Dr. Goyal pointed out that the optimal doses of amoxicillin/clavulanate or azithromycin have never been established for the treatment of exacerbations in children with bronchiectasis. He noted that some infectious disease specialists have advocated higher doses of both than those employed in this trial, but dose-ranging studies have never been conducted in this age group.
In this study, adverse events were less common on azithromycin than amoxicillin/clavulanate (21% vs. 30%), but none were severe, according to Dr. Goyal. He said treatment with azithromycin was associated with increased macrolide-resistant bacteria.
On the basis of these data, Dr. Goyal concluded that amoxicillin/clavulanate should remain, as already specified in some guidelines, the standard first-line therapy for nonsevere exacerbations in nonhospitalized children with bronchiectasis. He recommended reserving azithromycin as an alternative therapy.
Dr. Goyal reports no potential conflicts of interest.
SOURCE: Goyal V et al. Lancet Respir Med. 2019;7:791-801.
MADRID – A placebo-controlled trial has confirmed that amoxicillin/clavulanate is beneficial for resolution of acute exacerbations in nonsevere bronchiectasis while also demonstrating a greater relative effect than azithromycin, based on data presented at the annual congress of the European Respiratory Society.
“We now have robust data with which to support our guidelines,” reported Vikas Goyal, MD, of the Children’s Health Clinical Unit, University of Queensland, Brisbane, Australia.
The study addresses a knowledge gap. Antibiotics are already recommended by many guidelines for treatment of acute exacerbations in children with bronchiectasis, but Dr. Goyal said that no controlled trials have ever been performed in this age group to confirm superiority to placebo.
In this multicenter study, called BEST-1, 197 children with bronchiectasis were randomized at the start of an exacerbation to placebo, 45 mg/kg per day of amoxicillin/clavulanate, or 5 mg/kg per day of azithromycin. To maintain blinding, patients in the active treatment groups received a dummy for the opposite antibiotic while patients on placebo received dummies for both active agents.
For the primary outcome, 65% of children randomized to amoxicillin/clavulanate had resolution of their exacerbation by day 14 versus 61% of those randomized to azithromycin and 43% of those randomized to placebo. On the basis of relative risk for reaching this end point, the outcome was superior to placebo for amoxicillin/clavulanate (RR, 1.5; P = .015).
Although the relative risk for azithromycin (RR, 1.4; P = .042) was only slightly lower, it did not reach a prespecified level of significance set at P = .025. Dr. Goyal did report that the resolution rate at 14 days in the placebo group was “higher than expected.”
In this trial, 53% of the 154 children who were tested for respiratory viruses with nasal swabs on day 1 of the exacerbation were found to have respiratory viruses. Of these viruses, rhinovirus was the most common, according to Dr. Goyal, whose data were published just prior to his presentation (Lancet Respir Med. 2019;7:791-801).
The median durations of the exacerbations were 7 days, 8 days, and 10 days for those treated with amoxicillin/clavulanate, azithromycin, and placebo, respectively. The difference between amoxicillin/clavulanate and placebo, but not that between azithromycin and placebo, reached statistical significance, Dr. Goyal said.
There were no between group differences in the time to next exacerbation.
In discussing limitations of this study, Dr. Goyal pointed out that the optimal doses of amoxicillin/clavulanate or azithromycin have never been established for the treatment of exacerbations in children with bronchiectasis. He noted that some infectious disease specialists have advocated higher doses of both than those employed in this trial, but dose-ranging studies have never been conducted in this age group.
In this study, adverse events were less common on azithromycin than amoxicillin/clavulanate (21% vs. 30%), but none were severe, according to Dr. Goyal. He said treatment with azithromycin was associated with increased macrolide-resistant bacteria.
On the basis of these data, Dr. Goyal concluded that amoxicillin/clavulanate should remain, as already specified in some guidelines, the standard first-line therapy for nonsevere exacerbations in nonhospitalized children with bronchiectasis. He recommended reserving azithromycin as an alternative therapy.
Dr. Goyal reports no potential conflicts of interest.
SOURCE: Goyal V et al. Lancet Respir Med. 2019;7:791-801.
REPORTING FROM ERS 2019