User login
Wastewater can signal upswing in flu, RSV
according to new research reported at an annual scientific meeting on infectious diseases.
The analysis of wastewater in Calgary (Alta.) found a “positive correlation” between positivity rates for these three viruses in wastewater and weekly laboratory-confirmed clinical cases and test positivity rates, study investigator Kristine Du, with Cumming School of Medicine, University of Calgary, told this news organization.
Wastewater monitoring of viral activity has become an established tool for COVID-19 pandemic monitoring, providing a leading indicator to cases and hospitalizations. However, less is known about its potential for monitoring endemic respiratory viruses.
The new study shows that wastewater-based surveillance is a “robust and adaptable” tool for community-level surveillance of seasonal respiratory viruses – “one that can complement health care clinical testing because it’s independent from testing biases, and we can actually correlate our cases very well with it,” Ms. Du said during a preconference media briefing.
Tracking community trends
For the study, Ms. Du and colleagues assessed the occurrence of influenza A, influenza B, and RSV RNA in all three wastewater treatment plants in Calgary between March 2022 and April 2023 and its correlation with clinical disease.
They found that viral signals in Calgary’s wastewater for influenza A and B and RSV correlated significantly with weekly confirmed clinical cases in Calgary residents.
Influenza A peaked in Calgary’s wastewater between November and December 2022; influenza B peaked between February and April 2023; and RSV between November 2022 and February 2023.
“Wastewater gives us unbiased, objective, and comprehensive data. It can be used in addition to other testing for assessing the community burden that disease may have, and it is complementary to clinical testing,” Ms. Du said.
Their team, Ms. Du said, is continuing to proactively monitor wastewater for influenza and RSV, as well as other agents of “pandemic potential to make sure we know what could affect humans – and make sure everyone is aware of that.”
Commenting on the research, briefing moderator Belinda Ostrowsky, MD, MPH, Albert Einstein College of Medicine, New York, said, “Wastewater surveillance illustrates how understanding community levels of viral trends can identify hotspots, inform local public health decision-making, and prepare clinicians and hospitals for potential outreach. This topic is particularly timely as we head into the flu and RSV season.”
The study had no commercial funding. Ms. Du and Dr. Ostrowsky report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new research reported at an annual scientific meeting on infectious diseases.
The analysis of wastewater in Calgary (Alta.) found a “positive correlation” between positivity rates for these three viruses in wastewater and weekly laboratory-confirmed clinical cases and test positivity rates, study investigator Kristine Du, with Cumming School of Medicine, University of Calgary, told this news organization.
Wastewater monitoring of viral activity has become an established tool for COVID-19 pandemic monitoring, providing a leading indicator to cases and hospitalizations. However, less is known about its potential for monitoring endemic respiratory viruses.
The new study shows that wastewater-based surveillance is a “robust and adaptable” tool for community-level surveillance of seasonal respiratory viruses – “one that can complement health care clinical testing because it’s independent from testing biases, and we can actually correlate our cases very well with it,” Ms. Du said during a preconference media briefing.
Tracking community trends
For the study, Ms. Du and colleagues assessed the occurrence of influenza A, influenza B, and RSV RNA in all three wastewater treatment plants in Calgary between March 2022 and April 2023 and its correlation with clinical disease.
They found that viral signals in Calgary’s wastewater for influenza A and B and RSV correlated significantly with weekly confirmed clinical cases in Calgary residents.
Influenza A peaked in Calgary’s wastewater between November and December 2022; influenza B peaked between February and April 2023; and RSV between November 2022 and February 2023.
“Wastewater gives us unbiased, objective, and comprehensive data. It can be used in addition to other testing for assessing the community burden that disease may have, and it is complementary to clinical testing,” Ms. Du said.
Their team, Ms. Du said, is continuing to proactively monitor wastewater for influenza and RSV, as well as other agents of “pandemic potential to make sure we know what could affect humans – and make sure everyone is aware of that.”
Commenting on the research, briefing moderator Belinda Ostrowsky, MD, MPH, Albert Einstein College of Medicine, New York, said, “Wastewater surveillance illustrates how understanding community levels of viral trends can identify hotspots, inform local public health decision-making, and prepare clinicians and hospitals for potential outreach. This topic is particularly timely as we head into the flu and RSV season.”
The study had no commercial funding. Ms. Du and Dr. Ostrowsky report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new research reported at an annual scientific meeting on infectious diseases.
The analysis of wastewater in Calgary (Alta.) found a “positive correlation” between positivity rates for these three viruses in wastewater and weekly laboratory-confirmed clinical cases and test positivity rates, study investigator Kristine Du, with Cumming School of Medicine, University of Calgary, told this news organization.
Wastewater monitoring of viral activity has become an established tool for COVID-19 pandemic monitoring, providing a leading indicator to cases and hospitalizations. However, less is known about its potential for monitoring endemic respiratory viruses.
The new study shows that wastewater-based surveillance is a “robust and adaptable” tool for community-level surveillance of seasonal respiratory viruses – “one that can complement health care clinical testing because it’s independent from testing biases, and we can actually correlate our cases very well with it,” Ms. Du said during a preconference media briefing.
Tracking community trends
For the study, Ms. Du and colleagues assessed the occurrence of influenza A, influenza B, and RSV RNA in all three wastewater treatment plants in Calgary between March 2022 and April 2023 and its correlation with clinical disease.
They found that viral signals in Calgary’s wastewater for influenza A and B and RSV correlated significantly with weekly confirmed clinical cases in Calgary residents.
Influenza A peaked in Calgary’s wastewater between November and December 2022; influenza B peaked between February and April 2023; and RSV between November 2022 and February 2023.
“Wastewater gives us unbiased, objective, and comprehensive data. It can be used in addition to other testing for assessing the community burden that disease may have, and it is complementary to clinical testing,” Ms. Du said.
Their team, Ms. Du said, is continuing to proactively monitor wastewater for influenza and RSV, as well as other agents of “pandemic potential to make sure we know what could affect humans – and make sure everyone is aware of that.”
Commenting on the research, briefing moderator Belinda Ostrowsky, MD, MPH, Albert Einstein College of Medicine, New York, said, “Wastewater surveillance illustrates how understanding community levels of viral trends can identify hotspots, inform local public health decision-making, and prepare clinicians and hospitals for potential outreach. This topic is particularly timely as we head into the flu and RSV season.”
The study had no commercial funding. Ms. Du and Dr. Ostrowsky report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2023
CHEST launches sepsis resources in partnership with the CDC
Earlier this year, CHEST released new clinical resources on sepsis and antibiotic stewardship developed by the Sepsis Resources Steering Committee with grant support from the US Centers for Disease Control and Prevention (CDC).
The resources – including infographics, videos, podcasts, and research commentaries – aim to help clinicians increase their knowledge of sepsis prevention and treatment, especially when considering the use of antibiotics.
According to CHEST Past President, Steven Q. Simpson, MD, FCCP, who serves as Chair of the Sepsis Resources Steering Committee, sepsis is the number one cause of death in U.S. hospitals . It’s also the most expensive condition treated in those hospitals.
“Perhaps the single most important tool we have to fight sepsis is our array of antimicrobial therapies, including antibacterial, antifungal, and antiviral agents,” Dr. Simpson said. “It is vital that we use the antibiotics we have wisely and preserve them for future use.”
He pointed to the apparent tension between the need to administer broad-spectrum antimicrobials quickly to patients with sepsis and the need to limit the use of broad-spectrum agents as much as possible. But these concepts aren’t at odds with each another, he said. They’re allies in the sepsis war.
CHEST’s new resources can help clinicians practice good antimicrobial stewardship as they balance these needs. Included in the collection is a two-part video discussion exploring conservative and aggressive approaches to antibiotic use in suspected sepsis. A series of podcasts delves into complex sepsis cases, and easy-reference infographics outline key components of an antimicrobial stewardship program, rapid diagnostics for infectious diseases in the ICU, and sepsis mimics.
Steering committee members were chosen from CHEST’s membership for their clinical expertise in sepsis, infectious diseases, and antimicrobial stewardship. The committee selected topics based on current practice and knowledge gaps where education is most needed.
Working with the CDC increases CHEST’s impact in this area. Much of the care of patients with sepsis happens before they reach the ICU. The CDC’s broad reach with general and specialty medical audiences allows CHEST to share these resources with a wide array of clinicians who practice inside and outside of the ICU.
“Cooperation with the CDC gives us an opportunity to spread CHEST’s knowledge and expertise to a much broader audience, making the CDC a powerful partner and allowing us to serve the nation and beyond in a way that we cannot do by ourselves,” Dr. Simpson said.
Access the full collection of sepsis resources at chestnet.org/topic-collections/sepsis.
Earlier this year, CHEST released new clinical resources on sepsis and antibiotic stewardship developed by the Sepsis Resources Steering Committee with grant support from the US Centers for Disease Control and Prevention (CDC).
The resources – including infographics, videos, podcasts, and research commentaries – aim to help clinicians increase their knowledge of sepsis prevention and treatment, especially when considering the use of antibiotics.
According to CHEST Past President, Steven Q. Simpson, MD, FCCP, who serves as Chair of the Sepsis Resources Steering Committee, sepsis is the number one cause of death in U.S. hospitals . It’s also the most expensive condition treated in those hospitals.
“Perhaps the single most important tool we have to fight sepsis is our array of antimicrobial therapies, including antibacterial, antifungal, and antiviral agents,” Dr. Simpson said. “It is vital that we use the antibiotics we have wisely and preserve them for future use.”
He pointed to the apparent tension between the need to administer broad-spectrum antimicrobials quickly to patients with sepsis and the need to limit the use of broad-spectrum agents as much as possible. But these concepts aren’t at odds with each another, he said. They’re allies in the sepsis war.
CHEST’s new resources can help clinicians practice good antimicrobial stewardship as they balance these needs. Included in the collection is a two-part video discussion exploring conservative and aggressive approaches to antibiotic use in suspected sepsis. A series of podcasts delves into complex sepsis cases, and easy-reference infographics outline key components of an antimicrobial stewardship program, rapid diagnostics for infectious diseases in the ICU, and sepsis mimics.
Steering committee members were chosen from CHEST’s membership for their clinical expertise in sepsis, infectious diseases, and antimicrobial stewardship. The committee selected topics based on current practice and knowledge gaps where education is most needed.
Working with the CDC increases CHEST’s impact in this area. Much of the care of patients with sepsis happens before they reach the ICU. The CDC’s broad reach with general and specialty medical audiences allows CHEST to share these resources with a wide array of clinicians who practice inside and outside of the ICU.
“Cooperation with the CDC gives us an opportunity to spread CHEST’s knowledge and expertise to a much broader audience, making the CDC a powerful partner and allowing us to serve the nation and beyond in a way that we cannot do by ourselves,” Dr. Simpson said.
Access the full collection of sepsis resources at chestnet.org/topic-collections/sepsis.
Earlier this year, CHEST released new clinical resources on sepsis and antibiotic stewardship developed by the Sepsis Resources Steering Committee with grant support from the US Centers for Disease Control and Prevention (CDC).
The resources – including infographics, videos, podcasts, and research commentaries – aim to help clinicians increase their knowledge of sepsis prevention and treatment, especially when considering the use of antibiotics.
According to CHEST Past President, Steven Q. Simpson, MD, FCCP, who serves as Chair of the Sepsis Resources Steering Committee, sepsis is the number one cause of death in U.S. hospitals . It’s also the most expensive condition treated in those hospitals.
“Perhaps the single most important tool we have to fight sepsis is our array of antimicrobial therapies, including antibacterial, antifungal, and antiviral agents,” Dr. Simpson said. “It is vital that we use the antibiotics we have wisely and preserve them for future use.”
He pointed to the apparent tension between the need to administer broad-spectrum antimicrobials quickly to patients with sepsis and the need to limit the use of broad-spectrum agents as much as possible. But these concepts aren’t at odds with each another, he said. They’re allies in the sepsis war.
CHEST’s new resources can help clinicians practice good antimicrobial stewardship as they balance these needs. Included in the collection is a two-part video discussion exploring conservative and aggressive approaches to antibiotic use in suspected sepsis. A series of podcasts delves into complex sepsis cases, and easy-reference infographics outline key components of an antimicrobial stewardship program, rapid diagnostics for infectious diseases in the ICU, and sepsis mimics.
Steering committee members were chosen from CHEST’s membership for their clinical expertise in sepsis, infectious diseases, and antimicrobial stewardship. The committee selected topics based on current practice and knowledge gaps where education is most needed.
Working with the CDC increases CHEST’s impact in this area. Much of the care of patients with sepsis happens before they reach the ICU. The CDC’s broad reach with general and specialty medical audiences allows CHEST to share these resources with a wide array of clinicians who practice inside and outside of the ICU.
“Cooperation with the CDC gives us an opportunity to spread CHEST’s knowledge and expertise to a much broader audience, making the CDC a powerful partner and allowing us to serve the nation and beyond in a way that we cannot do by ourselves,” Dr. Simpson said.
Access the full collection of sepsis resources at chestnet.org/topic-collections/sepsis.
Nonhealing Ulcer in a Patient With Crohn Disease
The Diagnosis: Mycobacterium abscessus Infection
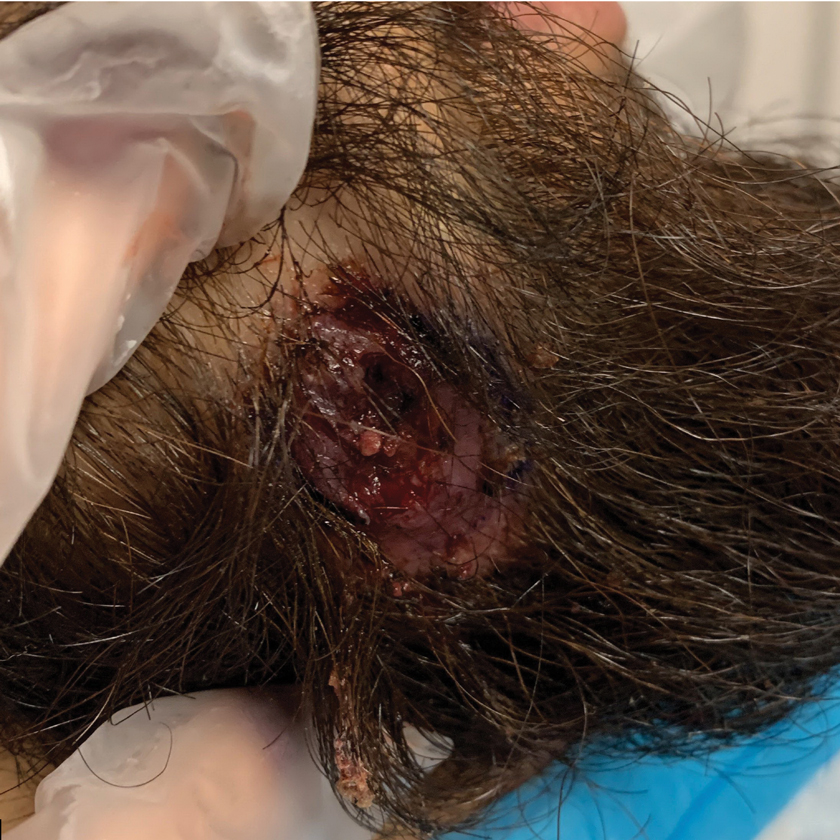
Upon further testing, cultures were positive for Mycobacterium abscessus. Our patient was referred to infectious disease for co-management, and his treatment plan consisted of intravenous amikacin 885 mg 3 times weekly, intravenous imipenem 1 g twice daily, azithromycin 500 mg/d, and omadacycline 150 mg/d for at least 3 months. Magnetic resonance imaging findings were consistent with a combination of cellulitis and osteomyelitis, and our patient was referred to plastic surgery for debridement. He subsequently was lost to follow-up.
Mycobacterium abscessus is classified as both a nontuberculous and rapidly growing mycobacterium. Mycobacterium abscessus recently has emerged as a pathogen of increasing public health concern, especially due to its high rate of antibiotic resistance.1-5 It is highly prevalent in the environment, and infection has been reported from a wide variety of environmental sources.6-8 Immunocompromised individuals, such as our patient, undergoing anti–tumor necrosis factor therapy are at increased risk for infection from all Mycobacterium species.9-11 Recognizing these infections quickly is a priority for patient care, as M abscessus can lead to disseminated infection and high mortality rates.1
Histopathology of M abscessus consists of granulomatous inflammation with mixed granulomas12; however, these findings are not always appreciable, and staining does not always reveal visible organisms. In our patient, histopathology revealed patchy plasmalymphocytic infiltrates of the dermis and subcutaneous tissue, which are signs of generalized inflammation (Figure). Therefore, cultures positive for M abscessus are the gold standard for diagnosis and established the diagnosis in this case.
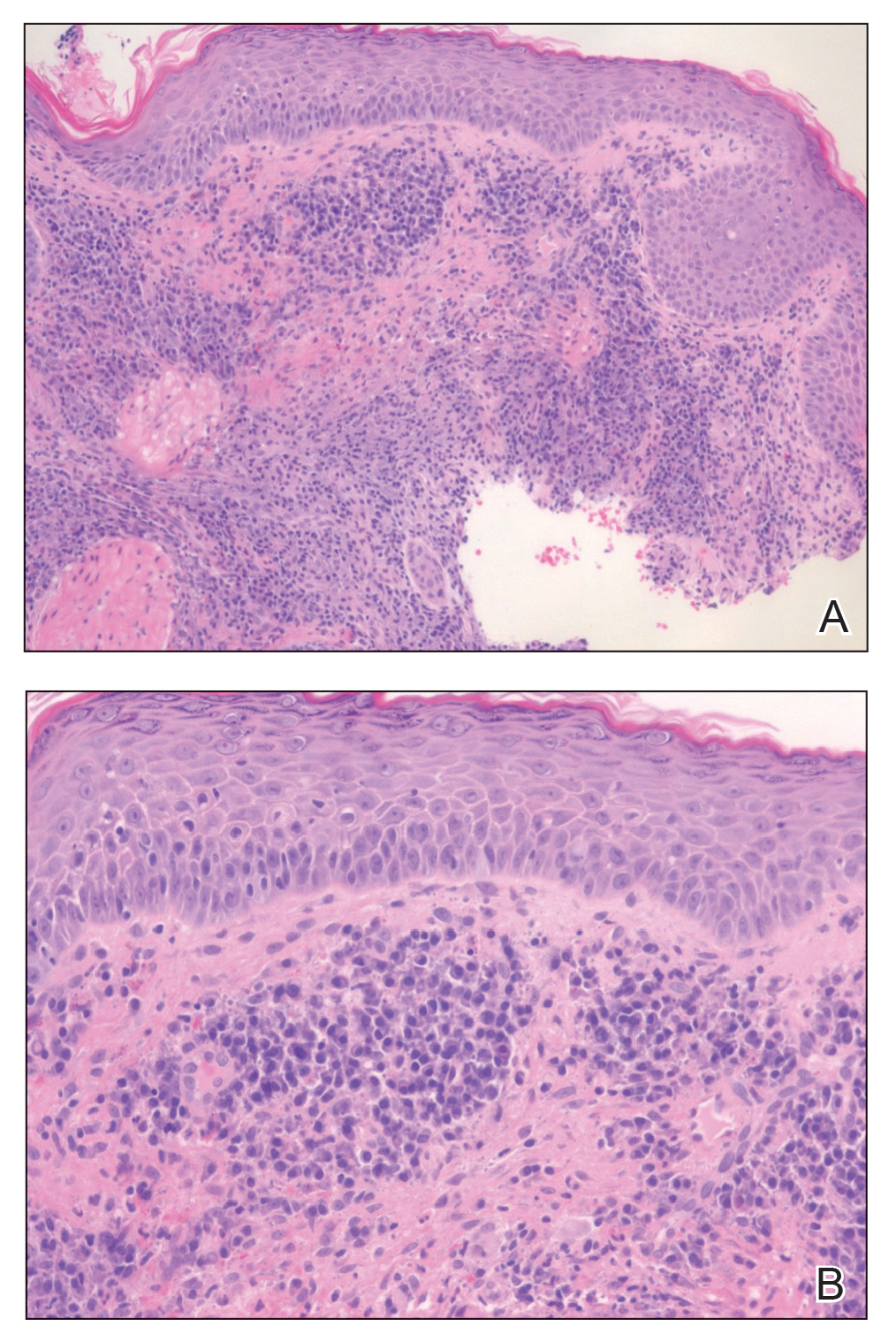
The differential diagnoses for our patient’s ulceration included squamous cell carcinoma, pyoderma gangrenosum, aseptic abscess ulcer, and pyodermatitispyostomatitis vegetans. Immunosuppressive therapy is a risk factor for squamous cell carcinoma13,14; however, ulcerated squamous cell carcinoma typically presents with prominent everted edges with a necrotic tumor base.15 Biopsy reveals cells with abundant eosinophilic cytoplasm, large nuclei, and variable keratin pearls.16 Pyoderma gangrenosum is an inflammatory skin condition associated with Crohn disease and often is a diagnosis of exclusion characterized by neutrophilic infiltrates on biopsy.17-19 Aseptic abscess ulcers are characterized by neutrophil-filled lesions that respond to corticosteroids but not antibiotics.20 Pyodermatitis-pyostomatitis vegetans is a rare skin manifestation of inflammatory bowel disease associated with a pustular eruption of the skin and/or mouth. Histopathology reveals pustules within or below the epidermis with many eosinophils or neutrophils. Granulomas do not occur as in M abscessus.21
Treatment of M abscessus infection requires the coadministration of several antibiotics across multiple classes to ensure complete disease resolution. High rates of antibiotic resistance are characterized by at least partial resistance to almost every antibiotic; clarithromycin has near-complete efficacy, but resistant strains have started to emerge. Amikacin and cefoxitin are other antibiotics that have reported a resistance rate of less than 50%, but they are only effective 90% and 70% of the time, respectively.1,22 The antibiotic omadacycline, which is approved by the US Food and Drug Administration to treat acute bacterial skin and soft-tissue infections, also may have utility in treating M abscessus infections.23,24 Finally, phage therapy may offer a potential mode of treatment for this bacterium and was used to treat pulmonary infection in a patient with cystic fibrosis.25 Despite these newer innovations, the current standard of care involves clarithromycin or azithromycin in combination with a parenteral antibiotic such as cefoxitin, amikacin, or imipenem for at least 4 months.1
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Strnad L, Winthrop KL. Treatment of Mycobacterium abscessus complex. Semin Respir Crit Care Med. 2018;39:362-376.
- Cardenas DD, Yasmin T, Ahmed S. A rare insidious case of skin and soft tissue infection due to Mycobacterium abscessus: a case report. Cureus. 2022;14:E25725.
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-577.
- Dickison P, Howard V, O’Kane G, et al. Mycobacterium abscessus infection following penetrations through wetsuits. Australas J Dermatol. 2019;60:57-59.
- Choi H, Kim YI, Na CH, et al. Mycobacterium abscessus skin infection associated with shaving activity in a 75-year-old man. Ann Geriatr Med Res. 2018;22:204.
- Costa-Silva M, Cesar A, Gomes NP, et al. Mycobacterium abscessus infection in a spa worker. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:159-161.
- Besada E. Rapid growing mycobacteria and TNF-α blockers: case report of a fatal lung infection with Mycobacterium abscessus. Clin Exp Rheumatol. 2011;29:705-707.
- Mufti AH, Toye BW, Mckendry RR, et al. Mycobacterium abscessus infection after use of tumor necrosis factor α inhibitor therapy: case report and review of infectious complications associated with tumor necrosis factor α inhibitor use. Diagn Microbiol Infect Dis. 2005;53:233-238.
- Lee SK, Kim SY, Kim EY, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013;191:565-571.
- Rodríguez G, Ortegón M, Camargo D, et al. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997;137:214-218.
- Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
- Walker HS, Hardwicke J. Non-melanoma skin cancer. Surgery (Oxford). 2022;40:39-45.
- Browse NL. The skin. In: Browse NL, ed. An Introduction to the Symptoms and Signs of Surgical Disease. 3rd ed. London Arnold Publications; 2001:66-69.
- Weedon D. Squamous cell carcinoma. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010;691-700.
- Powell F, Schroeter A, Su W, et al. Pyoderma gangrenosum: a review of 86 patients. QJM Int J Med. 1985;55:173-186.
- Brunsting LA, Goeckerman WH, O’Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol. 1982;118:743-768.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86:145. doi:10.1097/md.0b013e18064f9f3
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36:67-78.
- Duah M, Beshay M. Omadacycline in first-line combination therapy for pulmonary Mycobacterium abscessus infection: a case series. Int J Infect Dis. 2022;122:953-956.
- Minhas R, Sharma S, Kundu S. Utilizing the promise of omadacycline in a resistant, non-tubercular mycobacterial pulmonary infection. Cureus. 2019;11:E5112.
- Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25:730-733.
The Diagnosis: Mycobacterium abscessus Infection
Upon further testing, cultures were positive for Mycobacterium abscessus. Our patient was referred to infectious disease for co-management, and his treatment plan consisted of intravenous amikacin 885 mg 3 times weekly, intravenous imipenem 1 g twice daily, azithromycin 500 mg/d, and omadacycline 150 mg/d for at least 3 months. Magnetic resonance imaging findings were consistent with a combination of cellulitis and osteomyelitis, and our patient was referred to plastic surgery for debridement. He subsequently was lost to follow-up.
Mycobacterium abscessus is classified as both a nontuberculous and rapidly growing mycobacterium. Mycobacterium abscessus recently has emerged as a pathogen of increasing public health concern, especially due to its high rate of antibiotic resistance.1-5 It is highly prevalent in the environment, and infection has been reported from a wide variety of environmental sources.6-8 Immunocompromised individuals, such as our patient, undergoing anti–tumor necrosis factor therapy are at increased risk for infection from all Mycobacterium species.9-11 Recognizing these infections quickly is a priority for patient care, as M abscessus can lead to disseminated infection and high mortality rates.1
Histopathology of M abscessus consists of granulomatous inflammation with mixed granulomas12; however, these findings are not always appreciable, and staining does not always reveal visible organisms. In our patient, histopathology revealed patchy plasmalymphocytic infiltrates of the dermis and subcutaneous tissue, which are signs of generalized inflammation (Figure). Therefore, cultures positive for M abscessus are the gold standard for diagnosis and established the diagnosis in this case.

The differential diagnoses for our patient’s ulceration included squamous cell carcinoma, pyoderma gangrenosum, aseptic abscess ulcer, and pyodermatitispyostomatitis vegetans. Immunosuppressive therapy is a risk factor for squamous cell carcinoma13,14; however, ulcerated squamous cell carcinoma typically presents with prominent everted edges with a necrotic tumor base.15 Biopsy reveals cells with abundant eosinophilic cytoplasm, large nuclei, and variable keratin pearls.16 Pyoderma gangrenosum is an inflammatory skin condition associated with Crohn disease and often is a diagnosis of exclusion characterized by neutrophilic infiltrates on biopsy.17-19 Aseptic abscess ulcers are characterized by neutrophil-filled lesions that respond to corticosteroids but not antibiotics.20 Pyodermatitis-pyostomatitis vegetans is a rare skin manifestation of inflammatory bowel disease associated with a pustular eruption of the skin and/or mouth. Histopathology reveals pustules within or below the epidermis with many eosinophils or neutrophils. Granulomas do not occur as in M abscessus.21
Treatment of M abscessus infection requires the coadministration of several antibiotics across multiple classes to ensure complete disease resolution. High rates of antibiotic resistance are characterized by at least partial resistance to almost every antibiotic; clarithromycin has near-complete efficacy, but resistant strains have started to emerge. Amikacin and cefoxitin are other antibiotics that have reported a resistance rate of less than 50%, but they are only effective 90% and 70% of the time, respectively.1,22 The antibiotic omadacycline, which is approved by the US Food and Drug Administration to treat acute bacterial skin and soft-tissue infections, also may have utility in treating M abscessus infections.23,24 Finally, phage therapy may offer a potential mode of treatment for this bacterium and was used to treat pulmonary infection in a patient with cystic fibrosis.25 Despite these newer innovations, the current standard of care involves clarithromycin or azithromycin in combination with a parenteral antibiotic such as cefoxitin, amikacin, or imipenem for at least 4 months.1
The Diagnosis: Mycobacterium abscessus Infection
Upon further testing, cultures were positive for Mycobacterium abscessus. Our patient was referred to infectious disease for co-management, and his treatment plan consisted of intravenous amikacin 885 mg 3 times weekly, intravenous imipenem 1 g twice daily, azithromycin 500 mg/d, and omadacycline 150 mg/d for at least 3 months. Magnetic resonance imaging findings were consistent with a combination of cellulitis and osteomyelitis, and our patient was referred to plastic surgery for debridement. He subsequently was lost to follow-up.
Mycobacterium abscessus is classified as both a nontuberculous and rapidly growing mycobacterium. Mycobacterium abscessus recently has emerged as a pathogen of increasing public health concern, especially due to its high rate of antibiotic resistance.1-5 It is highly prevalent in the environment, and infection has been reported from a wide variety of environmental sources.6-8 Immunocompromised individuals, such as our patient, undergoing anti–tumor necrosis factor therapy are at increased risk for infection from all Mycobacterium species.9-11 Recognizing these infections quickly is a priority for patient care, as M abscessus can lead to disseminated infection and high mortality rates.1
Histopathology of M abscessus consists of granulomatous inflammation with mixed granulomas12; however, these findings are not always appreciable, and staining does not always reveal visible organisms. In our patient, histopathology revealed patchy plasmalymphocytic infiltrates of the dermis and subcutaneous tissue, which are signs of generalized inflammation (Figure). Therefore, cultures positive for M abscessus are the gold standard for diagnosis and established the diagnosis in this case.

The differential diagnoses for our patient’s ulceration included squamous cell carcinoma, pyoderma gangrenosum, aseptic abscess ulcer, and pyodermatitispyostomatitis vegetans. Immunosuppressive therapy is a risk factor for squamous cell carcinoma13,14; however, ulcerated squamous cell carcinoma typically presents with prominent everted edges with a necrotic tumor base.15 Biopsy reveals cells with abundant eosinophilic cytoplasm, large nuclei, and variable keratin pearls.16 Pyoderma gangrenosum is an inflammatory skin condition associated with Crohn disease and often is a diagnosis of exclusion characterized by neutrophilic infiltrates on biopsy.17-19 Aseptic abscess ulcers are characterized by neutrophil-filled lesions that respond to corticosteroids but not antibiotics.20 Pyodermatitis-pyostomatitis vegetans is a rare skin manifestation of inflammatory bowel disease associated with a pustular eruption of the skin and/or mouth. Histopathology reveals pustules within or below the epidermis with many eosinophils or neutrophils. Granulomas do not occur as in M abscessus.21
Treatment of M abscessus infection requires the coadministration of several antibiotics across multiple classes to ensure complete disease resolution. High rates of antibiotic resistance are characterized by at least partial resistance to almost every antibiotic; clarithromycin has near-complete efficacy, but resistant strains have started to emerge. Amikacin and cefoxitin are other antibiotics that have reported a resistance rate of less than 50%, but they are only effective 90% and 70% of the time, respectively.1,22 The antibiotic omadacycline, which is approved by the US Food and Drug Administration to treat acute bacterial skin and soft-tissue infections, also may have utility in treating M abscessus infections.23,24 Finally, phage therapy may offer a potential mode of treatment for this bacterium and was used to treat pulmonary infection in a patient with cystic fibrosis.25 Despite these newer innovations, the current standard of care involves clarithromycin or azithromycin in combination with a parenteral antibiotic such as cefoxitin, amikacin, or imipenem for at least 4 months.1
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Strnad L, Winthrop KL. Treatment of Mycobacterium abscessus complex. Semin Respir Crit Care Med. 2018;39:362-376.
- Cardenas DD, Yasmin T, Ahmed S. A rare insidious case of skin and soft tissue infection due to Mycobacterium abscessus: a case report. Cureus. 2022;14:E25725.
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-577.
- Dickison P, Howard V, O’Kane G, et al. Mycobacterium abscessus infection following penetrations through wetsuits. Australas J Dermatol. 2019;60:57-59.
- Choi H, Kim YI, Na CH, et al. Mycobacterium abscessus skin infection associated with shaving activity in a 75-year-old man. Ann Geriatr Med Res. 2018;22:204.
- Costa-Silva M, Cesar A, Gomes NP, et al. Mycobacterium abscessus infection in a spa worker. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:159-161.
- Besada E. Rapid growing mycobacteria and TNF-α blockers: case report of a fatal lung infection with Mycobacterium abscessus. Clin Exp Rheumatol. 2011;29:705-707.
- Mufti AH, Toye BW, Mckendry RR, et al. Mycobacterium abscessus infection after use of tumor necrosis factor α inhibitor therapy: case report and review of infectious complications associated with tumor necrosis factor α inhibitor use. Diagn Microbiol Infect Dis. 2005;53:233-238.
- Lee SK, Kim SY, Kim EY, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013;191:565-571.
- Rodríguez G, Ortegón M, Camargo D, et al. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997;137:214-218.
- Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
- Walker HS, Hardwicke J. Non-melanoma skin cancer. Surgery (Oxford). 2022;40:39-45.
- Browse NL. The skin. In: Browse NL, ed. An Introduction to the Symptoms and Signs of Surgical Disease. 3rd ed. London Arnold Publications; 2001:66-69.
- Weedon D. Squamous cell carcinoma. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010;691-700.
- Powell F, Schroeter A, Su W, et al. Pyoderma gangrenosum: a review of 86 patients. QJM Int J Med. 1985;55:173-186.
- Brunsting LA, Goeckerman WH, O’Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol. 1982;118:743-768.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86:145. doi:10.1097/md.0b013e18064f9f3
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36:67-78.
- Duah M, Beshay M. Omadacycline in first-line combination therapy for pulmonary Mycobacterium abscessus infection: a case series. Int J Infect Dis. 2022;122:953-956.
- Minhas R, Sharma S, Kundu S. Utilizing the promise of omadacycline in a resistant, non-tubercular mycobacterial pulmonary infection. Cureus. 2019;11:E5112.
- Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25:730-733.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Strnad L, Winthrop KL. Treatment of Mycobacterium abscessus complex. Semin Respir Crit Care Med. 2018;39:362-376.
- Cardenas DD, Yasmin T, Ahmed S. A rare insidious case of skin and soft tissue infection due to Mycobacterium abscessus: a case report. Cureus. 2022;14:E25725.
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-577.
- Dickison P, Howard V, O’Kane G, et al. Mycobacterium abscessus infection following penetrations through wetsuits. Australas J Dermatol. 2019;60:57-59.
- Choi H, Kim YI, Na CH, et al. Mycobacterium abscessus skin infection associated with shaving activity in a 75-year-old man. Ann Geriatr Med Res. 2018;22:204.
- Costa-Silva M, Cesar A, Gomes NP, et al. Mycobacterium abscessus infection in a spa worker. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:159-161.
- Besada E. Rapid growing mycobacteria and TNF-α blockers: case report of a fatal lung infection with Mycobacterium abscessus. Clin Exp Rheumatol. 2011;29:705-707.
- Mufti AH, Toye BW, Mckendry RR, et al. Mycobacterium abscessus infection after use of tumor necrosis factor α inhibitor therapy: case report and review of infectious complications associated with tumor necrosis factor α inhibitor use. Diagn Microbiol Infect Dis. 2005;53:233-238.
- Lee SK, Kim SY, Kim EY, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung. 2013;191:565-571.
- Rodríguez G, Ortegón M, Camargo D, et al. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997;137:214-218.
- Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161-168.
- Walker HS, Hardwicke J. Non-melanoma skin cancer. Surgery (Oxford). 2022;40:39-45.
- Browse NL. The skin. In: Browse NL, ed. An Introduction to the Symptoms and Signs of Surgical Disease. 3rd ed. London Arnold Publications; 2001:66-69.
- Weedon D. Squamous cell carcinoma. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010;691-700.
- Powell F, Schroeter A, Su W, et al. Pyoderma gangrenosum: a review of 86 patients. QJM Int J Med. 1985;55:173-186.
- Brunsting LA, Goeckerman WH, O’Leary PA. Pyoderma (ecthyma) gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol. 1982;118:743-768.
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466.
- André MFJ, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86:145. doi:10.1097/md.0b013e18064f9f3
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Kasperbauer SH, De Groote MA. The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36:67-78.
- Duah M, Beshay M. Omadacycline in first-line combination therapy for pulmonary Mycobacterium abscessus infection: a case series. Int J Infect Dis. 2022;122:953-956.
- Minhas R, Sharma S, Kundu S. Utilizing the promise of omadacycline in a resistant, non-tubercular mycobacterial pulmonary infection. Cureus. 2019;11:E5112.
- Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25:730-733.
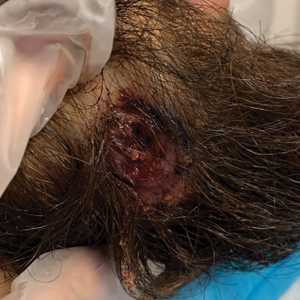
A 24-year-old man presented to our dermatology clinic with a painful lesion on the right buccal cheek of 4 months’ duration that had not changed in size or appearance. He had a history of Crohn disease that was being treated with 6-mercaptopurine and infliximab. He underwent jaw surgery 7 years prior for correction of an underbite, followed by subsequent surgery to remove the hardware 1 year after the initial procedure. He experienced recurring skin abscesses following the initial jaw surgery roughly once a year that were treated with bedside incision and drainage procedures in the emergency department followed by trimethoprim-sulfamethoxazole with complete resolution; however, treatment with mupirocin ointment 2%, trimethoprim-sulfamethoxazole, and azithromycin did not provide symptomatic relief or resolution for the current lesion. Physical examination revealed a 4-cm ulceration with actively draining serosanguineous discharge. Two punch biopsies were performed; 48-hour bacterial and fungal cultures, as well as Giemsa, acid-fast bacilli, and periodic acid–Schiff staining were negative.
Young women rate top sources for STI self-testing
, based on surveys from 92 individuals.
Direct-to-consumer (DTC) sexually transmitted infection (STI) screening methods involve the use of self-collected samples outside of a clinical setting, and may help reach women who avoid screening or lack access to clinical care, wrote Stacey B. Griner, PhD, of the University of North Texas Health Science Center, Fort Worth, and colleagues.
However, data on the methods used to promote DTC to the young female population are limited, and the goal of the current study was to identify preferred sources and communication channels for DTC STI information in this population, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from 92 women aged 18-24 years at a single university who participated in an online survey. Of these, 24 also participated in in-depth interviews. The mean age of the participants was 20.0 years, and all reported being sexually active in the past year. Approximately two-thirds (68.5%) were White, 24% were Hispanic, 13% were Black or African American; 63.0% overall were heterosexual.
Participants received a description of DTC methods and were asked whether they were interested in receiving more information, and if so, what were their preferred sources for receiving the information. Potential sources included health care providers, friends, family members, partners, the Internet, college resources, classes, and other, and participants were asked to rank these choices in order of preference.
More than half of the participants identified health care providers as their preferred source of information (56.5%), followed by trusted websites (25%), and university-based resources or friends (6.5% for both).
Overall, participants who underwent STI screening in the past 12 months ranked college resources higher than those who had not undergone screening.
Race played a significant role in ranking partners and family members as resources. Compared with Black participants, White participants and those who were biracial/multiracial/another race ranked partners as a significantly more preferred source, but the differences between White and biracial/multiracial/another race were not significant. White participants and Black participants were similar in ranking family as a preferred information source, but White participants, compared with biracial/multiracial/other participants, ranked family as a significantly more preferred source.
Differences in rankings were similar across sexual orientations.
In-depth interviews were conducted on the college campus prior to the COVID-19 pandemic. The mean age of the interview participants was 19.5 years, and most were non-Hispanic White. Sexual orientation was varied, with 50% identifying as heterosexual and 50% identifying as a sexual minority.
In the interviews, health care providers were seen as influential for considering DTC methods, with gynecologists, other specialists, and more experienced physicians deemed the most trustworthy. Interviewees noted social media sites as a way to provide information and raise awareness of DTC methods, such as through the advertisements feature on Instagram. They also identified university orientation as a way to reach students and provide information about DTC options in the context of other health-related orientation topics such as sexual consent and alcohol use.
Many interviewees also mentioned friends as a resource for discussing sex, sexuality, and STI screening, and said they would be accepting of information, knowledge, and emotional support when learning about DTC from friends.
The findings were limited by several factors, including the cross-sectional design, use of data from a single campus setting, and the overrepresentation of White women, and more studies are needed to identify differences by region and campus type that might guide interventions, the researchers noted. The study also was limited by “the lack of specificity of what participants considered to be credible Internet information sources,” they said.
However, the results suggest that using health care providers, trusted websites, and established college resources as dissemination channels may help increase the awareness and use of DTC methods for STI screening in young women, they concluded.
The study was supported in part by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS/STD Prevention at Indiana University and by the University of South Florida College of Public Health. The researchers had no financial conflicts to disclose.
, based on surveys from 92 individuals.
Direct-to-consumer (DTC) sexually transmitted infection (STI) screening methods involve the use of self-collected samples outside of a clinical setting, and may help reach women who avoid screening or lack access to clinical care, wrote Stacey B. Griner, PhD, of the University of North Texas Health Science Center, Fort Worth, and colleagues.
However, data on the methods used to promote DTC to the young female population are limited, and the goal of the current study was to identify preferred sources and communication channels for DTC STI information in this population, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from 92 women aged 18-24 years at a single university who participated in an online survey. Of these, 24 also participated in in-depth interviews. The mean age of the participants was 20.0 years, and all reported being sexually active in the past year. Approximately two-thirds (68.5%) were White, 24% were Hispanic, 13% were Black or African American; 63.0% overall were heterosexual.
Participants received a description of DTC methods and were asked whether they were interested in receiving more information, and if so, what were their preferred sources for receiving the information. Potential sources included health care providers, friends, family members, partners, the Internet, college resources, classes, and other, and participants were asked to rank these choices in order of preference.
More than half of the participants identified health care providers as their preferred source of information (56.5%), followed by trusted websites (25%), and university-based resources or friends (6.5% for both).
Overall, participants who underwent STI screening in the past 12 months ranked college resources higher than those who had not undergone screening.
Race played a significant role in ranking partners and family members as resources. Compared with Black participants, White participants and those who were biracial/multiracial/another race ranked partners as a significantly more preferred source, but the differences between White and biracial/multiracial/another race were not significant. White participants and Black participants were similar in ranking family as a preferred information source, but White participants, compared with biracial/multiracial/other participants, ranked family as a significantly more preferred source.
Differences in rankings were similar across sexual orientations.
In-depth interviews were conducted on the college campus prior to the COVID-19 pandemic. The mean age of the interview participants was 19.5 years, and most were non-Hispanic White. Sexual orientation was varied, with 50% identifying as heterosexual and 50% identifying as a sexual minority.
In the interviews, health care providers were seen as influential for considering DTC methods, with gynecologists, other specialists, and more experienced physicians deemed the most trustworthy. Interviewees noted social media sites as a way to provide information and raise awareness of DTC methods, such as through the advertisements feature on Instagram. They also identified university orientation as a way to reach students and provide information about DTC options in the context of other health-related orientation topics such as sexual consent and alcohol use.
Many interviewees also mentioned friends as a resource for discussing sex, sexuality, and STI screening, and said they would be accepting of information, knowledge, and emotional support when learning about DTC from friends.
The findings were limited by several factors, including the cross-sectional design, use of data from a single campus setting, and the overrepresentation of White women, and more studies are needed to identify differences by region and campus type that might guide interventions, the researchers noted. The study also was limited by “the lack of specificity of what participants considered to be credible Internet information sources,” they said.
However, the results suggest that using health care providers, trusted websites, and established college resources as dissemination channels may help increase the awareness and use of DTC methods for STI screening in young women, they concluded.
The study was supported in part by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS/STD Prevention at Indiana University and by the University of South Florida College of Public Health. The researchers had no financial conflicts to disclose.
, based on surveys from 92 individuals.
Direct-to-consumer (DTC) sexually transmitted infection (STI) screening methods involve the use of self-collected samples outside of a clinical setting, and may help reach women who avoid screening or lack access to clinical care, wrote Stacey B. Griner, PhD, of the University of North Texas Health Science Center, Fort Worth, and colleagues.
However, data on the methods used to promote DTC to the young female population are limited, and the goal of the current study was to identify preferred sources and communication channels for DTC STI information in this population, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from 92 women aged 18-24 years at a single university who participated in an online survey. Of these, 24 also participated in in-depth interviews. The mean age of the participants was 20.0 years, and all reported being sexually active in the past year. Approximately two-thirds (68.5%) were White, 24% were Hispanic, 13% were Black or African American; 63.0% overall were heterosexual.
Participants received a description of DTC methods and were asked whether they were interested in receiving more information, and if so, what were their preferred sources for receiving the information. Potential sources included health care providers, friends, family members, partners, the Internet, college resources, classes, and other, and participants were asked to rank these choices in order of preference.
More than half of the participants identified health care providers as their preferred source of information (56.5%), followed by trusted websites (25%), and university-based resources or friends (6.5% for both).
Overall, participants who underwent STI screening in the past 12 months ranked college resources higher than those who had not undergone screening.
Race played a significant role in ranking partners and family members as resources. Compared with Black participants, White participants and those who were biracial/multiracial/another race ranked partners as a significantly more preferred source, but the differences between White and biracial/multiracial/another race were not significant. White participants and Black participants were similar in ranking family as a preferred information source, but White participants, compared with biracial/multiracial/other participants, ranked family as a significantly more preferred source.
Differences in rankings were similar across sexual orientations.
In-depth interviews were conducted on the college campus prior to the COVID-19 pandemic. The mean age of the interview participants was 19.5 years, and most were non-Hispanic White. Sexual orientation was varied, with 50% identifying as heterosexual and 50% identifying as a sexual minority.
In the interviews, health care providers were seen as influential for considering DTC methods, with gynecologists, other specialists, and more experienced physicians deemed the most trustworthy. Interviewees noted social media sites as a way to provide information and raise awareness of DTC methods, such as through the advertisements feature on Instagram. They also identified university orientation as a way to reach students and provide information about DTC options in the context of other health-related orientation topics such as sexual consent and alcohol use.
Many interviewees also mentioned friends as a resource for discussing sex, sexuality, and STI screening, and said they would be accepting of information, knowledge, and emotional support when learning about DTC from friends.
The findings were limited by several factors, including the cross-sectional design, use of data from a single campus setting, and the overrepresentation of White women, and more studies are needed to identify differences by region and campus type that might guide interventions, the researchers noted. The study also was limited by “the lack of specificity of what participants considered to be credible Internet information sources,” they said.
However, the results suggest that using health care providers, trusted websites, and established college resources as dissemination channels may help increase the awareness and use of DTC methods for STI screening in young women, they concluded.
The study was supported in part by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS/STD Prevention at Indiana University and by the University of South Florida College of Public Health. The researchers had no financial conflicts to disclose.
FROM SEXUALLY TRANSMITTED DISEASES
Paxlovid tied to benefits in high-risk patients with COVID
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
More evidence shows COVID-19’s link to risk for autoimmune disease
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Research from South Korea provides additional evidence for the connection between COVID-19 and an increased risk for autoimmune conditions post infection.
METHODOLOGY:
- In this retrospective study, researchers identified 354,527 individuals diagnosed with COVID-19 via polymerase chain reaction (PCR) testing from Oct. 8, 2020, to Dec. 31, 2021.
- Researchers compared the COVID-19 group with 6,134,940 healthy individuals who had no evidence of COVID-19 to quantify the risk for autoimmune and autoinflammatory connective tissue disorders.
- Patients were followed until diagnosis, death, or end of study period (Dec. 31, 2021).
TAKEAWAY:
- Risks for alopecia areata, alopecia totalis, antineutrophil cytoplasmic antibody–associated vasculitis, Crohn’s disease, and sarcoidosis were higher in the COVID-19 group.
- Patients with more severe COVID-19 (admitted to the ICU) were at greater risk for many autoimmune conditions, including alopecia totalis, psoriasis, vitiligo, and vasculitis.
IN PRACTICE:
“Our results emphasize the need to focus on managing not only the acute stages of COVID-19 itself but also autoimmune diseases as complications of COVID-19,” the authors wrote.
SOURCE:
Sung Ha Lim, MD, of Yonsei University, Wonju, South Korea, was the first author of the study, published in JAMA Network Open.
LIMITATIONS:
The study was retrospective and was composed almost exclusively of individuals from a single ethnicity. The study could have included individuals with COVID-19 in the control group who did not undergo PCR testing. The analysis did not include detailed information on each patient, including genetic information, that could have contributed to autoimmune disease risk.
DISCLOSURES:
The study was supported by a fund from the research program of the Korea Medical Institute and by grants from the Korea Health Industry Development Institute, the Korean Ministry of Health & Welfare, and the National Research Foundation of Korea. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Respiratory infections, asthma rise before type 2 diabetes
HAMBURG, GERMANY – , shows a longitudinal study looking at comorbidities both 25 years before and 25 years after a type 2 diabetes diagnosis.
About 40% of people had respiratory tract infections at the time of diagnosis with type 2 diabetes, compared with 4% who were not diagnosed. Likewise, ear, nose, and throat infections were present in 20% of people at type 2 diabetes diagnosis, compared with around 2% who were not diagnosed. A similar pattern was seen with asthma.
Taken together, the data suggest that subacute inflammation manifesting in asthma as well as the onset of asthma or an acute infection may be a precursor to a type 2 diabetes diagnosis.
“We have also found that in the years prior to diagnosis, there are associations with infections and inflammatory disorders to a much greater degree than in those people who do not get a diabetes diagnosis but who have very similar demographics,” Adrian Heald, MD, study lead and diabetes consultant from Salford (England) Royal Hospital, said in an interview.
Five years prior to diagnosis, respiratory tract infections were documented in around 23% of patients who were later diagnosed with type 2 diabetes versus 2.5% in those not diagnosed, and a similar pattern was seen for ear, nose, and throat infections and asthma. The findings suggest that patients reporting infections, in addition to other known risk factors for type 2 diabetes, might benefit from diabetes tests and early interventions, if needed.
“These novel insights offer a fascinating and fresh perspective on the onset and natural progression to type 2 diabetes and beyond, suggesting an early phase of inflammation-related disease activity long before any clinical diagnosis of type 2 diabetes is made.”
Dr. Heald points out that clinicians may intervene to stave off progression to a type 2 diabetes diagnosis in at risk patients. “At this point, an intervention could relate to lifestyle changes and involve highlighting to the patient that the morbidity they have already accumulated is suggestive of diabetes risk,” he said, adding that, “they may have dyslipidemia, hypertension, and most often excess weight so annual checks of their HbA1c, weight management, and blood pressure would need checking,” he explained.
Moderator Coen Stehouwer, MD, professor of internal medicine at Maastricht University, the Netherlands, commented, “Before clinical diagnosis of type 2 diabetes there is often a lengthy period of undiagnosed disease and before that, prediabetes, because glucose can be abnormal up to 10 years prior to clinical diagnosis.”
But he added that, “It’s not entirely clear whether the rise seen before clinical diagnosis in this study correlates with undiagnosed diabetes or prediabetes or even if it precedes type 2 diabetes – it might be because inflammation is a common origin for type 2 diabetes and various comorbidities. This might explain how they go together.”
Longitudinal study 25 years before and 25 years after type 2 diagnosis
Dr. Heald presented the findings at a session on inflammation in diabetes at the annual meeting of the European Association for the Study of Diabetes. The work was also published in Diabetes Therapy.
The researchers wanted to investigate the pattern of comorbidities in the years and decades prior to a diagnosis of type 2 diabetes as well as after: “With the database we used, called DARE [Diabetes Alliance for Research in England], we are able to explore phenomena longitudinally going right back to the beginning of their digital health records, looking at phenotypes over time.”
By mapping significant health issues in people who went on to develop type 2 diabetes alongside those that did not, Dr. Heald managed to develop a continuum spanning 25 years prior and 25 years after diagnosis of type 2 diabetes. The researchers also examined relationships between sociodemographic factors and longitudinal health outcomes of relevance to cardiac conditions and lower respiratory tract infections. His talk in Hamburg primarily addressed clinical phenotypes before the point of diagnosis.
Data were drawn from 1,932 people with (1,196) and without (736) type 2 diabetes. Participants in both groups were aged 66-67 years, 43%-46% were women, age at diagnosis was 50-52 years, and participants lived in Greater Manchester, United Kingdom.
In the years leading up to type 2 diagnosis, individuals consistently exhibited a considerable increase in several clinical phenotypes, reported Dr. Heald. Of note, he added, “immediately prior to type 2 diagnosis, there was a significantly greater proportion of hypertension at 35%, respiratory tract infection at 34%, heart disease at 17%, ear, nose, and throat infection at 19%, and asthma at 12%. And by comparison, the corresponding disease trajectory in matched controls was much less dramatic.”
“There is a huge difference in people who went on to receive a diagnosis of type 2 diabetes and those who did not, and not just what we’d expect – so hypertension for example or manifestations of renal disease, but importantly inflammatory disorders are more common,” he emphasized.
In addition, a larger signal for ischemic heart disease was seen just before type 2 diabetes diagnosis.
These data suggest that longitudinal clinical histories prior to a diagnosis of type 2 diabetes might offer new information, both genetic and nongenetic, about development of type 2 diabetes in relation to comorbidities.
After type 2 diabetes diagnosis, the proportion of people exhibiting coronary artery disease, hypertension, chronic kidney disease, retinopathy, and infections climbed rapidly before plateauing, reported Dr. Heald. “We also know that individuals with coronary artery disease are more highly represented in socially disadvantaged groups, and this is borne out in the data at 25 years prior and after type 2 diagnosis.”
Dr. Heald has received speaker fees or contributed to advisory boards from Lilly, AstraZeneca, Janssen, Bristol-Myers Squibb, Besins, Bayer, Sanofi, and Recordati. Research grants from Novo Nordisk, Pfizer, and Besins. Professor Stehouwer has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – , shows a longitudinal study looking at comorbidities both 25 years before and 25 years after a type 2 diabetes diagnosis.
About 40% of people had respiratory tract infections at the time of diagnosis with type 2 diabetes, compared with 4% who were not diagnosed. Likewise, ear, nose, and throat infections were present in 20% of people at type 2 diabetes diagnosis, compared with around 2% who were not diagnosed. A similar pattern was seen with asthma.
Taken together, the data suggest that subacute inflammation manifesting in asthma as well as the onset of asthma or an acute infection may be a precursor to a type 2 diabetes diagnosis.
“We have also found that in the years prior to diagnosis, there are associations with infections and inflammatory disorders to a much greater degree than in those people who do not get a diabetes diagnosis but who have very similar demographics,” Adrian Heald, MD, study lead and diabetes consultant from Salford (England) Royal Hospital, said in an interview.
Five years prior to diagnosis, respiratory tract infections were documented in around 23% of patients who were later diagnosed with type 2 diabetes versus 2.5% in those not diagnosed, and a similar pattern was seen for ear, nose, and throat infections and asthma. The findings suggest that patients reporting infections, in addition to other known risk factors for type 2 diabetes, might benefit from diabetes tests and early interventions, if needed.
“These novel insights offer a fascinating and fresh perspective on the onset and natural progression to type 2 diabetes and beyond, suggesting an early phase of inflammation-related disease activity long before any clinical diagnosis of type 2 diabetes is made.”
Dr. Heald points out that clinicians may intervene to stave off progression to a type 2 diabetes diagnosis in at risk patients. “At this point, an intervention could relate to lifestyle changes and involve highlighting to the patient that the morbidity they have already accumulated is suggestive of diabetes risk,” he said, adding that, “they may have dyslipidemia, hypertension, and most often excess weight so annual checks of their HbA1c, weight management, and blood pressure would need checking,” he explained.
Moderator Coen Stehouwer, MD, professor of internal medicine at Maastricht University, the Netherlands, commented, “Before clinical diagnosis of type 2 diabetes there is often a lengthy period of undiagnosed disease and before that, prediabetes, because glucose can be abnormal up to 10 years prior to clinical diagnosis.”
But he added that, “It’s not entirely clear whether the rise seen before clinical diagnosis in this study correlates with undiagnosed diabetes or prediabetes or even if it precedes type 2 diabetes – it might be because inflammation is a common origin for type 2 diabetes and various comorbidities. This might explain how they go together.”
Longitudinal study 25 years before and 25 years after type 2 diagnosis
Dr. Heald presented the findings at a session on inflammation in diabetes at the annual meeting of the European Association for the Study of Diabetes. The work was also published in Diabetes Therapy.
The researchers wanted to investigate the pattern of comorbidities in the years and decades prior to a diagnosis of type 2 diabetes as well as after: “With the database we used, called DARE [Diabetes Alliance for Research in England], we are able to explore phenomena longitudinally going right back to the beginning of their digital health records, looking at phenotypes over time.”
By mapping significant health issues in people who went on to develop type 2 diabetes alongside those that did not, Dr. Heald managed to develop a continuum spanning 25 years prior and 25 years after diagnosis of type 2 diabetes. The researchers also examined relationships between sociodemographic factors and longitudinal health outcomes of relevance to cardiac conditions and lower respiratory tract infections. His talk in Hamburg primarily addressed clinical phenotypes before the point of diagnosis.
Data were drawn from 1,932 people with (1,196) and without (736) type 2 diabetes. Participants in both groups were aged 66-67 years, 43%-46% were women, age at diagnosis was 50-52 years, and participants lived in Greater Manchester, United Kingdom.
In the years leading up to type 2 diagnosis, individuals consistently exhibited a considerable increase in several clinical phenotypes, reported Dr. Heald. Of note, he added, “immediately prior to type 2 diagnosis, there was a significantly greater proportion of hypertension at 35%, respiratory tract infection at 34%, heart disease at 17%, ear, nose, and throat infection at 19%, and asthma at 12%. And by comparison, the corresponding disease trajectory in matched controls was much less dramatic.”
“There is a huge difference in people who went on to receive a diagnosis of type 2 diabetes and those who did not, and not just what we’d expect – so hypertension for example or manifestations of renal disease, but importantly inflammatory disorders are more common,” he emphasized.
In addition, a larger signal for ischemic heart disease was seen just before type 2 diabetes diagnosis.
These data suggest that longitudinal clinical histories prior to a diagnosis of type 2 diabetes might offer new information, both genetic and nongenetic, about development of type 2 diabetes in relation to comorbidities.
After type 2 diabetes diagnosis, the proportion of people exhibiting coronary artery disease, hypertension, chronic kidney disease, retinopathy, and infections climbed rapidly before plateauing, reported Dr. Heald. “We also know that individuals with coronary artery disease are more highly represented in socially disadvantaged groups, and this is borne out in the data at 25 years prior and after type 2 diagnosis.”
Dr. Heald has received speaker fees or contributed to advisory boards from Lilly, AstraZeneca, Janssen, Bristol-Myers Squibb, Besins, Bayer, Sanofi, and Recordati. Research grants from Novo Nordisk, Pfizer, and Besins. Professor Stehouwer has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – , shows a longitudinal study looking at comorbidities both 25 years before and 25 years after a type 2 diabetes diagnosis.
About 40% of people had respiratory tract infections at the time of diagnosis with type 2 diabetes, compared with 4% who were not diagnosed. Likewise, ear, nose, and throat infections were present in 20% of people at type 2 diabetes diagnosis, compared with around 2% who were not diagnosed. A similar pattern was seen with asthma.
Taken together, the data suggest that subacute inflammation manifesting in asthma as well as the onset of asthma or an acute infection may be a precursor to a type 2 diabetes diagnosis.
“We have also found that in the years prior to diagnosis, there are associations with infections and inflammatory disorders to a much greater degree than in those people who do not get a diabetes diagnosis but who have very similar demographics,” Adrian Heald, MD, study lead and diabetes consultant from Salford (England) Royal Hospital, said in an interview.
Five years prior to diagnosis, respiratory tract infections were documented in around 23% of patients who were later diagnosed with type 2 diabetes versus 2.5% in those not diagnosed, and a similar pattern was seen for ear, nose, and throat infections and asthma. The findings suggest that patients reporting infections, in addition to other known risk factors for type 2 diabetes, might benefit from diabetes tests and early interventions, if needed.
“These novel insights offer a fascinating and fresh perspective on the onset and natural progression to type 2 diabetes and beyond, suggesting an early phase of inflammation-related disease activity long before any clinical diagnosis of type 2 diabetes is made.”
Dr. Heald points out that clinicians may intervene to stave off progression to a type 2 diabetes diagnosis in at risk patients. “At this point, an intervention could relate to lifestyle changes and involve highlighting to the patient that the morbidity they have already accumulated is suggestive of diabetes risk,” he said, adding that, “they may have dyslipidemia, hypertension, and most often excess weight so annual checks of their HbA1c, weight management, and blood pressure would need checking,” he explained.
Moderator Coen Stehouwer, MD, professor of internal medicine at Maastricht University, the Netherlands, commented, “Before clinical diagnosis of type 2 diabetes there is often a lengthy period of undiagnosed disease and before that, prediabetes, because glucose can be abnormal up to 10 years prior to clinical diagnosis.”
But he added that, “It’s not entirely clear whether the rise seen before clinical diagnosis in this study correlates with undiagnosed diabetes or prediabetes or even if it precedes type 2 diabetes – it might be because inflammation is a common origin for type 2 diabetes and various comorbidities. This might explain how they go together.”
Longitudinal study 25 years before and 25 years after type 2 diagnosis
Dr. Heald presented the findings at a session on inflammation in diabetes at the annual meeting of the European Association for the Study of Diabetes. The work was also published in Diabetes Therapy.
The researchers wanted to investigate the pattern of comorbidities in the years and decades prior to a diagnosis of type 2 diabetes as well as after: “With the database we used, called DARE [Diabetes Alliance for Research in England], we are able to explore phenomena longitudinally going right back to the beginning of their digital health records, looking at phenotypes over time.”
By mapping significant health issues in people who went on to develop type 2 diabetes alongside those that did not, Dr. Heald managed to develop a continuum spanning 25 years prior and 25 years after diagnosis of type 2 diabetes. The researchers also examined relationships between sociodemographic factors and longitudinal health outcomes of relevance to cardiac conditions and lower respiratory tract infections. His talk in Hamburg primarily addressed clinical phenotypes before the point of diagnosis.
Data were drawn from 1,932 people with (1,196) and without (736) type 2 diabetes. Participants in both groups were aged 66-67 years, 43%-46% were women, age at diagnosis was 50-52 years, and participants lived in Greater Manchester, United Kingdom.
In the years leading up to type 2 diagnosis, individuals consistently exhibited a considerable increase in several clinical phenotypes, reported Dr. Heald. Of note, he added, “immediately prior to type 2 diagnosis, there was a significantly greater proportion of hypertension at 35%, respiratory tract infection at 34%, heart disease at 17%, ear, nose, and throat infection at 19%, and asthma at 12%. And by comparison, the corresponding disease trajectory in matched controls was much less dramatic.”
“There is a huge difference in people who went on to receive a diagnosis of type 2 diabetes and those who did not, and not just what we’d expect – so hypertension for example or manifestations of renal disease, but importantly inflammatory disorders are more common,” he emphasized.
In addition, a larger signal for ischemic heart disease was seen just before type 2 diabetes diagnosis.
These data suggest that longitudinal clinical histories prior to a diagnosis of type 2 diabetes might offer new information, both genetic and nongenetic, about development of type 2 diabetes in relation to comorbidities.
After type 2 diabetes diagnosis, the proportion of people exhibiting coronary artery disease, hypertension, chronic kidney disease, retinopathy, and infections climbed rapidly before plateauing, reported Dr. Heald. “We also know that individuals with coronary artery disease are more highly represented in socially disadvantaged groups, and this is borne out in the data at 25 years prior and after type 2 diagnosis.”
Dr. Heald has received speaker fees or contributed to advisory boards from Lilly, AstraZeneca, Janssen, Bristol-Myers Squibb, Besins, Bayer, Sanofi, and Recordati. Research grants from Novo Nordisk, Pfizer, and Besins. Professor Stehouwer has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
AT EASD 2023
Updated guidance from USPSTF on PrEP for HIV prevention
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.
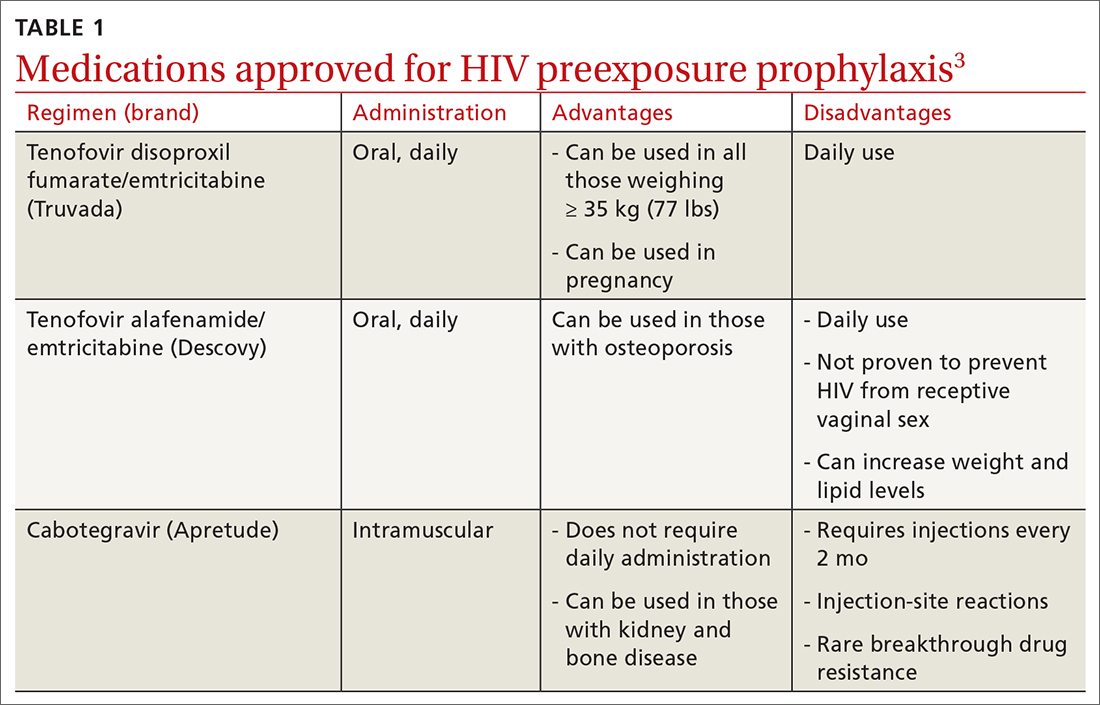
Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.

Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.

Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
Severity score predicts mortality in pulmonary tuberculosis
, based on data from approximately 400 individuals.
Although a mortality risk-prediction score could improve treatment for pulmonary tuberculosis patients, such a score has not been previously reported, wrote Takeshi Osawa, MD, of Fukujuji Hospital, Tokyo, and colleagues.
In a study published in the journal CHEST, the researchers used 252 patients from a previous perspective study of newly diagnosed pulmonary tuberculosis as the development cohort, and recruited 165 additional patients between March 2021 and September 2022.
The primary endpoint was all-cause in-hospital mortality. Based on data from the development group, the researchers found that age 65 years and older and age 80 years and older, hypoxemia, activities of daily living, bilateral pulmonary lesions, lymphocyte count of less than 720 microliters, serum albumin less than 2.86 mg/dL, C-reactive protein (CRP) 3.97 mg/dL or higher, and procalcitonin (PCT) 0.130 ng/mL or higher were predictors of all-cause in hospital mortality.
The researchers used this information to create the disease severity score, known as the AHL score. The AHL included three clinical parameters: activity in daily living (semi-dependent, 1 point; totally dependent, 2 points); hypoxemia (1 point) and lymphocytes (< 720 /mcL, 1 point).
The scoring systems for the three parameters were, respectively, 1 point for semi-dependent and 2 points totally dependent (for activity in daily living), 1 point for presence of hypoxemia, and 1 point for lymphocytes less than 720 per microliter. The researchers stratified the scores into levels of low, intermediate, and high risk, with scores of 0, 1-2, and 3-4, respectively.
All-cause in hospital mortality occurred in 39 (15.5%) and 17 (10.3%) of patients in the developmental and validation cohorts, respectively.
The AHL score effectively predicted mortality, dividing patients into three groups of 1.3% low-risk, 8.9% intermediate risk, and 39.3% high-risk in the validation cohort, with a Harrell’s c-statistic of 0.842.
The corresponding numbers for the development cohort were 0, 13.5%, and 55.8%, with a c-statistic of 0.902.
The findings were limited by several factors, including the lack of data from “smear-negative” patients who were treated as outpatients, and more research is needed to determine the applicability of the AHL score in an outpatient population, the researchers noted. Other limitations included the lack of data on long-term mortality in surviving patients who were discharged, and the reliance on assessments that can be performed only in clinical settings in developed countries, they said.
However, the results support the feasibility of the AHL score in clinical settings to accurately predict mortality in patients with pulmonary TB, and may help optimize treatments for this population, they concluded.
The study received no outside funding. All authors disclosed nonfinancial support in the form of measuring reagents from Fujifilm Wako Pure Chemical Corporation during the study but had no relevant financial conflicts to disclose.
, based on data from approximately 400 individuals.
Although a mortality risk-prediction score could improve treatment for pulmonary tuberculosis patients, such a score has not been previously reported, wrote Takeshi Osawa, MD, of Fukujuji Hospital, Tokyo, and colleagues.
In a study published in the journal CHEST, the researchers used 252 patients from a previous perspective study of newly diagnosed pulmonary tuberculosis as the development cohort, and recruited 165 additional patients between March 2021 and September 2022.
The primary endpoint was all-cause in-hospital mortality. Based on data from the development group, the researchers found that age 65 years and older and age 80 years and older, hypoxemia, activities of daily living, bilateral pulmonary lesions, lymphocyte count of less than 720 microliters, serum albumin less than 2.86 mg/dL, C-reactive protein (CRP) 3.97 mg/dL or higher, and procalcitonin (PCT) 0.130 ng/mL or higher were predictors of all-cause in hospital mortality.
The researchers used this information to create the disease severity score, known as the AHL score. The AHL included three clinical parameters: activity in daily living (semi-dependent, 1 point; totally dependent, 2 points); hypoxemia (1 point) and lymphocytes (< 720 /mcL, 1 point).
The scoring systems for the three parameters were, respectively, 1 point for semi-dependent and 2 points totally dependent (for activity in daily living), 1 point for presence of hypoxemia, and 1 point for lymphocytes less than 720 per microliter. The researchers stratified the scores into levels of low, intermediate, and high risk, with scores of 0, 1-2, and 3-4, respectively.
All-cause in hospital mortality occurred in 39 (15.5%) and 17 (10.3%) of patients in the developmental and validation cohorts, respectively.
The AHL score effectively predicted mortality, dividing patients into three groups of 1.3% low-risk, 8.9% intermediate risk, and 39.3% high-risk in the validation cohort, with a Harrell’s c-statistic of 0.842.
The corresponding numbers for the development cohort were 0, 13.5%, and 55.8%, with a c-statistic of 0.902.
The findings were limited by several factors, including the lack of data from “smear-negative” patients who were treated as outpatients, and more research is needed to determine the applicability of the AHL score in an outpatient population, the researchers noted. Other limitations included the lack of data on long-term mortality in surviving patients who were discharged, and the reliance on assessments that can be performed only in clinical settings in developed countries, they said.
However, the results support the feasibility of the AHL score in clinical settings to accurately predict mortality in patients with pulmonary TB, and may help optimize treatments for this population, they concluded.
The study received no outside funding. All authors disclosed nonfinancial support in the form of measuring reagents from Fujifilm Wako Pure Chemical Corporation during the study but had no relevant financial conflicts to disclose.
, based on data from approximately 400 individuals.
Although a mortality risk-prediction score could improve treatment for pulmonary tuberculosis patients, such a score has not been previously reported, wrote Takeshi Osawa, MD, of Fukujuji Hospital, Tokyo, and colleagues.
In a study published in the journal CHEST, the researchers used 252 patients from a previous perspective study of newly diagnosed pulmonary tuberculosis as the development cohort, and recruited 165 additional patients between March 2021 and September 2022.
The primary endpoint was all-cause in-hospital mortality. Based on data from the development group, the researchers found that age 65 years and older and age 80 years and older, hypoxemia, activities of daily living, bilateral pulmonary lesions, lymphocyte count of less than 720 microliters, serum albumin less than 2.86 mg/dL, C-reactive protein (CRP) 3.97 mg/dL or higher, and procalcitonin (PCT) 0.130 ng/mL or higher were predictors of all-cause in hospital mortality.
The researchers used this information to create the disease severity score, known as the AHL score. The AHL included three clinical parameters: activity in daily living (semi-dependent, 1 point; totally dependent, 2 points); hypoxemia (1 point) and lymphocytes (< 720 /mcL, 1 point).
The scoring systems for the three parameters were, respectively, 1 point for semi-dependent and 2 points totally dependent (for activity in daily living), 1 point for presence of hypoxemia, and 1 point for lymphocytes less than 720 per microliter. The researchers stratified the scores into levels of low, intermediate, and high risk, with scores of 0, 1-2, and 3-4, respectively.
All-cause in hospital mortality occurred in 39 (15.5%) and 17 (10.3%) of patients in the developmental and validation cohorts, respectively.
The AHL score effectively predicted mortality, dividing patients into three groups of 1.3% low-risk, 8.9% intermediate risk, and 39.3% high-risk in the validation cohort, with a Harrell’s c-statistic of 0.842.
The corresponding numbers for the development cohort were 0, 13.5%, and 55.8%, with a c-statistic of 0.902.
The findings were limited by several factors, including the lack of data from “smear-negative” patients who were treated as outpatients, and more research is needed to determine the applicability of the AHL score in an outpatient population, the researchers noted. Other limitations included the lack of data on long-term mortality in surviving patients who were discharged, and the reliance on assessments that can be performed only in clinical settings in developed countries, they said.
However, the results support the feasibility of the AHL score in clinical settings to accurately predict mortality in patients with pulmonary TB, and may help optimize treatments for this population, they concluded.
The study received no outside funding. All authors disclosed nonfinancial support in the form of measuring reagents from Fujifilm Wako Pure Chemical Corporation during the study but had no relevant financial conflicts to disclose.
FROM THE JOURNAL CHEST
Pulmonary aspergillosis predicts poor outcomes in critically ill flu patients
Critically ill influenza patients with associated pulmonary aspergillosis were more than twice as likely to die in intensive care than those without the added infection, based on data from a meta-analysis of more than 1,700 individuals.
Reports of influenza-associated pulmonary aspergillosis (IAPA) are rising in critically ill patients, but data on risk factors, clinical features, and outcomes are limited, Lawrence Y. Lu, MD, of The Prince Charles Hospital, Brisbane, Australia, and colleagues wrote. In addition, diagnosis of IAPA can be challenging, and many clinicians report low awareness of the condition.
In a study published in the journal Chest, the researchers reviewed data from 10 observational studies including 1,720 critically ill influenza patients aged 16 years and older; of these, 331 had IAPA, for a prevalence of 19.2%. The primary outcomes were all-cause mortality in the hospital and in the ICU. Secondary outcomes included ICU length of stay, hospital length of stay, and the need for supportive care (invasive and noninvasive mechanical ventilation, renal replacement therapy, pressor support, and extracorporeal membranous oxygenation).
Overall, mortality among flu patients in the ICU was significantly higher for those with IAPA than those without IAPA (45.0% vs. 23.8%, respectively), as was all-cause mortality (46.4% vs. 26.2%, respectively; odds ratio, 2.6 and P < .001 for both ICU and all-cause mortality).
Factors significantly associated with an increased risk for IAPA included organ transplant (OR, 4.8), hematogenous malignancy (OR, 2.5), being immunocompromised in some way (OR, 2.2), and prolonged corticosteroid use prior to hospital admission (OR, 2.4).
IAPA also was associated with more severe disease, a higher rate of complications, longer ICU stays, and a greater need for organ supports, the researchers noted. Clinical features not significantly more common in patients with IAPA included fever, hemoptysis, and acute respiratory distress syndrome.
The findings were limited by several factors including the retrospective design of the included studies and inability to control for all potential confounders, the researchers noted. Other limitations included the variations in study design, variability of practice patterns across locations, and inclusion of data mainly from countries of high socioeconomic status.
“Given the apparent waning of the COVID-19 pandemic and re-emergence of influenza, our analysis also revealed other gaps in the current literature, including the need to validate newer diagnostic methods and to develop a system to measure severity of IAPA,” the researchers added.
However, the current study results reflect IAPA prevalence from previous studies, and support the need to have a lower threshold for IAPA testing and initiation of antifungal treatment, even with limited data for clinical guidance, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Critically ill influenza patients with associated pulmonary aspergillosis were more than twice as likely to die in intensive care than those without the added infection, based on data from a meta-analysis of more than 1,700 individuals.
Reports of influenza-associated pulmonary aspergillosis (IAPA) are rising in critically ill patients, but data on risk factors, clinical features, and outcomes are limited, Lawrence Y. Lu, MD, of The Prince Charles Hospital, Brisbane, Australia, and colleagues wrote. In addition, diagnosis of IAPA can be challenging, and many clinicians report low awareness of the condition.
In a study published in the journal Chest, the researchers reviewed data from 10 observational studies including 1,720 critically ill influenza patients aged 16 years and older; of these, 331 had IAPA, for a prevalence of 19.2%. The primary outcomes were all-cause mortality in the hospital and in the ICU. Secondary outcomes included ICU length of stay, hospital length of stay, and the need for supportive care (invasive and noninvasive mechanical ventilation, renal replacement therapy, pressor support, and extracorporeal membranous oxygenation).
Overall, mortality among flu patients in the ICU was significantly higher for those with IAPA than those without IAPA (45.0% vs. 23.8%, respectively), as was all-cause mortality (46.4% vs. 26.2%, respectively; odds ratio, 2.6 and P < .001 for both ICU and all-cause mortality).
Factors significantly associated with an increased risk for IAPA included organ transplant (OR, 4.8), hematogenous malignancy (OR, 2.5), being immunocompromised in some way (OR, 2.2), and prolonged corticosteroid use prior to hospital admission (OR, 2.4).
IAPA also was associated with more severe disease, a higher rate of complications, longer ICU stays, and a greater need for organ supports, the researchers noted. Clinical features not significantly more common in patients with IAPA included fever, hemoptysis, and acute respiratory distress syndrome.
The findings were limited by several factors including the retrospective design of the included studies and inability to control for all potential confounders, the researchers noted. Other limitations included the variations in study design, variability of practice patterns across locations, and inclusion of data mainly from countries of high socioeconomic status.
“Given the apparent waning of the COVID-19 pandemic and re-emergence of influenza, our analysis also revealed other gaps in the current literature, including the need to validate newer diagnostic methods and to develop a system to measure severity of IAPA,” the researchers added.
However, the current study results reflect IAPA prevalence from previous studies, and support the need to have a lower threshold for IAPA testing and initiation of antifungal treatment, even with limited data for clinical guidance, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Critically ill influenza patients with associated pulmonary aspergillosis were more than twice as likely to die in intensive care than those without the added infection, based on data from a meta-analysis of more than 1,700 individuals.
Reports of influenza-associated pulmonary aspergillosis (IAPA) are rising in critically ill patients, but data on risk factors, clinical features, and outcomes are limited, Lawrence Y. Lu, MD, of The Prince Charles Hospital, Brisbane, Australia, and colleagues wrote. In addition, diagnosis of IAPA can be challenging, and many clinicians report low awareness of the condition.
In a study published in the journal Chest, the researchers reviewed data from 10 observational studies including 1,720 critically ill influenza patients aged 16 years and older; of these, 331 had IAPA, for a prevalence of 19.2%. The primary outcomes were all-cause mortality in the hospital and in the ICU. Secondary outcomes included ICU length of stay, hospital length of stay, and the need for supportive care (invasive and noninvasive mechanical ventilation, renal replacement therapy, pressor support, and extracorporeal membranous oxygenation).
Overall, mortality among flu patients in the ICU was significantly higher for those with IAPA than those without IAPA (45.0% vs. 23.8%, respectively), as was all-cause mortality (46.4% vs. 26.2%, respectively; odds ratio, 2.6 and P < .001 for both ICU and all-cause mortality).
Factors significantly associated with an increased risk for IAPA included organ transplant (OR, 4.8), hematogenous malignancy (OR, 2.5), being immunocompromised in some way (OR, 2.2), and prolonged corticosteroid use prior to hospital admission (OR, 2.4).
IAPA also was associated with more severe disease, a higher rate of complications, longer ICU stays, and a greater need for organ supports, the researchers noted. Clinical features not significantly more common in patients with IAPA included fever, hemoptysis, and acute respiratory distress syndrome.
The findings were limited by several factors including the retrospective design of the included studies and inability to control for all potential confounders, the researchers noted. Other limitations included the variations in study design, variability of practice patterns across locations, and inclusion of data mainly from countries of high socioeconomic status.
“Given the apparent waning of the COVID-19 pandemic and re-emergence of influenza, our analysis also revealed other gaps in the current literature, including the need to validate newer diagnostic methods and to develop a system to measure severity of IAPA,” the researchers added.
However, the current study results reflect IAPA prevalence from previous studies, and support the need to have a lower threshold for IAPA testing and initiation of antifungal treatment, even with limited data for clinical guidance, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL CHEST