User login
Tech encourages HIV prevention among women
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
Access to technology, particularly cellphones, is tied to a higher awareness of pre-exposure prophylaxis (PrEP) in women, according to survey results presented at the Association of Nurses in AIDS Care 2023 Annual Meeting.
Those with limited access to technology, older women, and women who had been incarcerated were also less likely to be aware of their medication options.
Researchers collected responses from 206 women in New York and Philadelphia by computer survey. The women were HIV negative and eligible to receive medication but were not currently taking any.
Most participants were Black (61%) or Hispanic (24%), and the average age of participants was 39 years. Nearly 60% of the group reported they were not aware of PrEP.
Younger women, Hispanic women, women who had not been incarcerated, and women with access to technology were most likely to be aware that they could take medication to prevent HIV.
“Women who utilized their cell phones for activities such as texting, emailing, watching videos, playing games, downloading apps, and accessing social media were more likely to be aware of PrEP,” point out the researchers led by Su Kyung Kim, PhD, WHNP-BC, an assistant professor at Thomas Jefferson University, Philadelphia.
These findings could help direct efforts to increase awareness among women where uptake has remained low, the researchers report. “Mobile technologies, in particular, offer a nimble, customizable, and accessible way to reach this target population and increase awareness of PrEP.”
A version of this article first appeared on Medscape.com.
Upper respiratory infections: Viral testing in primary care
It’s upper respiratory infection (URI) season. The following is a clinical scenario drawn from my own practice. I’ll tell you what I plan to do, but I’m most interested in crowdsourcing a response from all of you to collectively determine best practice. So please answer the polling questions and contribute your thoughts in the comments, whether you agree or disagree with me.
The patient
The patient is a 69-year-old woman with a 3-day history of cough, nasal congestion, malaise, tactile fever, and poor appetite. She has no sick contacts. She denies dyspnea, presyncope, and chest pain. She has tried guaifenesin and ibuprofen for her symptoms, which helped a little.
She is up to date on immunizations, including four doses of COVID-19 vaccine and the influenza vaccine, which she received 2 months ago.
The patient has a history of heart failure with reduced ejection fraction, coronary artery disease, hypertension, chronic kidney disease stage 3aA2, obesity, and osteoarthritis. Current medications include atorvastatin, losartan, metoprolol, and aspirin.
Her weight is stable at 212 lb, and her vital signs today are:
- Temperature: 37.5° C
- Pulse: 60 beats/min
- Blood pressure: 150/88 mm Hg
- Respiration rate: 14 breaths/min
- SpO2: 93% on room air
What information is most critical before deciding on management?
Your peers chose:
- The patient’s history of viral URIs
14%
- Whether her cough is productive and the color of the sputum
38%
- How well this season’s flu vaccine matches circulating influenza viruses
8%
- Local epidemiology of major viral pathogens (e.g., SARS-CoV-2, influenza, RSV)
40%
Dr. Vega’s take
To provide the best care for our patients when they are threatened with multiple viral upper respiratory pathogens, it is imperative that clinicians have some idea regarding the epidemiology of viral infections, with as much local data as possible. This knowledge will help direct appropriate testing and treatment.
Modern viral molecular testing platforms are highly accurate, but they are not infallible. Small flaws in specificity and sensitivity of testing are magnified when community viral circulation is low. In a U.K. study conducted during a period of low COVID-19 prevalence, the positive predictive value of reverse-transcriptase polymerase chain reaction (RT-PCR) testing was just 16%. Although the negative predictive value was much higher, the false-positive rate of testing was still 0.5%. The authors of the study describe important potential consequences of false-positive results, such as being temporarily removed from an organ transplant list and unnecessary contact tracing.
Testing and treatment
Your county public health department maintains a website describing local activity of SARS-CoV-2 and influenza. Both viruses are in heavy circulation now.
What is the next best step in this patient’s management?
Your peers chose:
- Treat empirically with ritonavir-boosted nirmatrelvir
7%
- Treat empirically with oseltamivir or baloxavir
14%
- Perform lab-based multiplex RT-PCR testing and wait to treat on the basis of results
34%
- Perform rapid nucleic acid amplification testing (NAAT) and treat on the basis of results
45%
Every practice has different resources and should use the best means available to treat patients. Ideally, this patient would undergo rapid NAAT with results available within 30 minutes. Test results will help guide not only treatment decisions but also infection-control measures.
The Infectious Diseases Society of America has provided updates for testing for URIs since the onset of the COVID-19 pandemic. Both laboratory-based and point-of-care rapid NAATs are recommended for testing. Rapid NAATs have been demonstrated to have a sensitivity of 96% and specificity of 100% in the detection of SARS-CoV-2. Obviously, they also offer a highly efficient means to make treatment and isolation decisions.
There are multiple platforms for molecular testing available. Laboratory-based platforms can test for dozens of potential pathogens, including bacteria. Rapid NAATs often have the ability to test for SARS-CoV-2, influenza, and respiratory syncytial virus (RSV). This functionality is important, because these infections generally are difficult to discriminate on the basis of clinical information alone.
The IDSA clearly recognizes the challenges of trying to manage cases of URI. For example, they state that testing of the anterior nares (AN) or oropharynx (OP) is acceptable, even though testing from the nasopharynx offers increased sensitivity. However, testing at the AN/OP allows for patient self-collection of samples, which is also recommended as an option by the IDSA. In an analysis of six cohort studies, the pooled sensitivity of patient-collected nasopharyngeal samples from the AN/OP was 88%, whereas the respective value for samples taken by health care providers was 95%.
The U.S. Centers for Disease Control and Prevention also provides recommendations for the management of patients with acute upper respiratory illness. Patients who are sick enough to be hospitalized should be tested at least for SARS-CoV-2 and influenza using molecular assays. Outpatients should be tested for SARS-CoV-2 with either molecular or antigen testing, and influenza testing should be offered if the findings will change decisions regarding treatment or isolation. Practically speaking, the recommendations for influenza testing mean that most individuals should be tested, including patients at high risk for complications of influenza and those who might have exposure to individuals at high risk.
Treatment of COVID-19 should only be provided in cases of a positive test within 5 days of symptom onset. However, clinicians may treat patients with anti-influenza medications presumptively if test results are not immediately available and the patient has worsening symptoms or is in a group at high risk for complications.
What are some of the challenges that you have faced during the COVID-19 pandemic regarding the management of patients with acute URIs? What have you found in terms of solutions, and where do gaps in quality of care persist? Please add your comments. I will review and circle back with a response. Thank you!
A version of this article first appeared on Medscape.com.
It’s upper respiratory infection (URI) season. The following is a clinical scenario drawn from my own practice. I’ll tell you what I plan to do, but I’m most interested in crowdsourcing a response from all of you to collectively determine best practice. So please answer the polling questions and contribute your thoughts in the comments, whether you agree or disagree with me.
The patient
The patient is a 69-year-old woman with a 3-day history of cough, nasal congestion, malaise, tactile fever, and poor appetite. She has no sick contacts. She denies dyspnea, presyncope, and chest pain. She has tried guaifenesin and ibuprofen for her symptoms, which helped a little.
She is up to date on immunizations, including four doses of COVID-19 vaccine and the influenza vaccine, which she received 2 months ago.
The patient has a history of heart failure with reduced ejection fraction, coronary artery disease, hypertension, chronic kidney disease stage 3aA2, obesity, and osteoarthritis. Current medications include atorvastatin, losartan, metoprolol, and aspirin.
Her weight is stable at 212 lb, and her vital signs today are:
- Temperature: 37.5° C
- Pulse: 60 beats/min
- Blood pressure: 150/88 mm Hg
- Respiration rate: 14 breaths/min
- SpO2: 93% on room air
What information is most critical before deciding on management?
Your peers chose:
- The patient’s history of viral URIs
14%
- Whether her cough is productive and the color of the sputum
38%
- How well this season’s flu vaccine matches circulating influenza viruses
8%
- Local epidemiology of major viral pathogens (e.g., SARS-CoV-2, influenza, RSV)
40%
Dr. Vega’s take
To provide the best care for our patients when they are threatened with multiple viral upper respiratory pathogens, it is imperative that clinicians have some idea regarding the epidemiology of viral infections, with as much local data as possible. This knowledge will help direct appropriate testing and treatment.
Modern viral molecular testing platforms are highly accurate, but they are not infallible. Small flaws in specificity and sensitivity of testing are magnified when community viral circulation is low. In a U.K. study conducted during a period of low COVID-19 prevalence, the positive predictive value of reverse-transcriptase polymerase chain reaction (RT-PCR) testing was just 16%. Although the negative predictive value was much higher, the false-positive rate of testing was still 0.5%. The authors of the study describe important potential consequences of false-positive results, such as being temporarily removed from an organ transplant list and unnecessary contact tracing.
Testing and treatment
Your county public health department maintains a website describing local activity of SARS-CoV-2 and influenza. Both viruses are in heavy circulation now.
What is the next best step in this patient’s management?
Your peers chose:
- Treat empirically with ritonavir-boosted nirmatrelvir
7%
- Treat empirically with oseltamivir or baloxavir
14%
- Perform lab-based multiplex RT-PCR testing and wait to treat on the basis of results
34%
- Perform rapid nucleic acid amplification testing (NAAT) and treat on the basis of results
45%
Every practice has different resources and should use the best means available to treat patients. Ideally, this patient would undergo rapid NAAT with results available within 30 minutes. Test results will help guide not only treatment decisions but also infection-control measures.
The Infectious Diseases Society of America has provided updates for testing for URIs since the onset of the COVID-19 pandemic. Both laboratory-based and point-of-care rapid NAATs are recommended for testing. Rapid NAATs have been demonstrated to have a sensitivity of 96% and specificity of 100% in the detection of SARS-CoV-2. Obviously, they also offer a highly efficient means to make treatment and isolation decisions.
There are multiple platforms for molecular testing available. Laboratory-based platforms can test for dozens of potential pathogens, including bacteria. Rapid NAATs often have the ability to test for SARS-CoV-2, influenza, and respiratory syncytial virus (RSV). This functionality is important, because these infections generally are difficult to discriminate on the basis of clinical information alone.
The IDSA clearly recognizes the challenges of trying to manage cases of URI. For example, they state that testing of the anterior nares (AN) or oropharynx (OP) is acceptable, even though testing from the nasopharynx offers increased sensitivity. However, testing at the AN/OP allows for patient self-collection of samples, which is also recommended as an option by the IDSA. In an analysis of six cohort studies, the pooled sensitivity of patient-collected nasopharyngeal samples from the AN/OP was 88%, whereas the respective value for samples taken by health care providers was 95%.
The U.S. Centers for Disease Control and Prevention also provides recommendations for the management of patients with acute upper respiratory illness. Patients who are sick enough to be hospitalized should be tested at least for SARS-CoV-2 and influenza using molecular assays. Outpatients should be tested for SARS-CoV-2 with either molecular or antigen testing, and influenza testing should be offered if the findings will change decisions regarding treatment or isolation. Practically speaking, the recommendations for influenza testing mean that most individuals should be tested, including patients at high risk for complications of influenza and those who might have exposure to individuals at high risk.
Treatment of COVID-19 should only be provided in cases of a positive test within 5 days of symptom onset. However, clinicians may treat patients with anti-influenza medications presumptively if test results are not immediately available and the patient has worsening symptoms or is in a group at high risk for complications.
What are some of the challenges that you have faced during the COVID-19 pandemic regarding the management of patients with acute URIs? What have you found in terms of solutions, and where do gaps in quality of care persist? Please add your comments. I will review and circle back with a response. Thank you!
A version of this article first appeared on Medscape.com.
It’s upper respiratory infection (URI) season. The following is a clinical scenario drawn from my own practice. I’ll tell you what I plan to do, but I’m most interested in crowdsourcing a response from all of you to collectively determine best practice. So please answer the polling questions and contribute your thoughts in the comments, whether you agree or disagree with me.
The patient
The patient is a 69-year-old woman with a 3-day history of cough, nasal congestion, malaise, tactile fever, and poor appetite. She has no sick contacts. She denies dyspnea, presyncope, and chest pain. She has tried guaifenesin and ibuprofen for her symptoms, which helped a little.
She is up to date on immunizations, including four doses of COVID-19 vaccine and the influenza vaccine, which she received 2 months ago.
The patient has a history of heart failure with reduced ejection fraction, coronary artery disease, hypertension, chronic kidney disease stage 3aA2, obesity, and osteoarthritis. Current medications include atorvastatin, losartan, metoprolol, and aspirin.
Her weight is stable at 212 lb, and her vital signs today are:
- Temperature: 37.5° C
- Pulse: 60 beats/min
- Blood pressure: 150/88 mm Hg
- Respiration rate: 14 breaths/min
- SpO2: 93% on room air
What information is most critical before deciding on management?
Your peers chose:
- The patient’s history of viral URIs
14%
- Whether her cough is productive and the color of the sputum
38%
- How well this season’s flu vaccine matches circulating influenza viruses
8%
- Local epidemiology of major viral pathogens (e.g., SARS-CoV-2, influenza, RSV)
40%
Dr. Vega’s take
To provide the best care for our patients when they are threatened with multiple viral upper respiratory pathogens, it is imperative that clinicians have some idea regarding the epidemiology of viral infections, with as much local data as possible. This knowledge will help direct appropriate testing and treatment.
Modern viral molecular testing platforms are highly accurate, but they are not infallible. Small flaws in specificity and sensitivity of testing are magnified when community viral circulation is low. In a U.K. study conducted during a period of low COVID-19 prevalence, the positive predictive value of reverse-transcriptase polymerase chain reaction (RT-PCR) testing was just 16%. Although the negative predictive value was much higher, the false-positive rate of testing was still 0.5%. The authors of the study describe important potential consequences of false-positive results, such as being temporarily removed from an organ transplant list and unnecessary contact tracing.
Testing and treatment
Your county public health department maintains a website describing local activity of SARS-CoV-2 and influenza. Both viruses are in heavy circulation now.
What is the next best step in this patient’s management?
Your peers chose:
- Treat empirically with ritonavir-boosted nirmatrelvir
7%
- Treat empirically with oseltamivir or baloxavir
14%
- Perform lab-based multiplex RT-PCR testing and wait to treat on the basis of results
34%
- Perform rapid nucleic acid amplification testing (NAAT) and treat on the basis of results
45%
Every practice has different resources and should use the best means available to treat patients. Ideally, this patient would undergo rapid NAAT with results available within 30 minutes. Test results will help guide not only treatment decisions but also infection-control measures.
The Infectious Diseases Society of America has provided updates for testing for URIs since the onset of the COVID-19 pandemic. Both laboratory-based and point-of-care rapid NAATs are recommended for testing. Rapid NAATs have been demonstrated to have a sensitivity of 96% and specificity of 100% in the detection of SARS-CoV-2. Obviously, they also offer a highly efficient means to make treatment and isolation decisions.
There are multiple platforms for molecular testing available. Laboratory-based platforms can test for dozens of potential pathogens, including bacteria. Rapid NAATs often have the ability to test for SARS-CoV-2, influenza, and respiratory syncytial virus (RSV). This functionality is important, because these infections generally are difficult to discriminate on the basis of clinical information alone.
The IDSA clearly recognizes the challenges of trying to manage cases of URI. For example, they state that testing of the anterior nares (AN) or oropharynx (OP) is acceptable, even though testing from the nasopharynx offers increased sensitivity. However, testing at the AN/OP allows for patient self-collection of samples, which is also recommended as an option by the IDSA. In an analysis of six cohort studies, the pooled sensitivity of patient-collected nasopharyngeal samples from the AN/OP was 88%, whereas the respective value for samples taken by health care providers was 95%.
The U.S. Centers for Disease Control and Prevention also provides recommendations for the management of patients with acute upper respiratory illness. Patients who are sick enough to be hospitalized should be tested at least for SARS-CoV-2 and influenza using molecular assays. Outpatients should be tested for SARS-CoV-2 with either molecular or antigen testing, and influenza testing should be offered if the findings will change decisions regarding treatment or isolation. Practically speaking, the recommendations for influenza testing mean that most individuals should be tested, including patients at high risk for complications of influenza and those who might have exposure to individuals at high risk.
Treatment of COVID-19 should only be provided in cases of a positive test within 5 days of symptom onset. However, clinicians may treat patients with anti-influenza medications presumptively if test results are not immediately available and the patient has worsening symptoms or is in a group at high risk for complications.
What are some of the challenges that you have faced during the COVID-19 pandemic regarding the management of patients with acute URIs? What have you found in terms of solutions, and where do gaps in quality of care persist? Please add your comments. I will review and circle back with a response. Thank you!
A version of this article first appeared on Medscape.com.
Vaccination status doesn’t impact infectivity timeline in kids
TOPLINE:
according to a new study. The findings indicate that return-to-school policies for infected children may not need to differ on the basis of vaccine or booster status.
METHODOLOGY:
- The study looked at 76 children, both vaccinated and unvaccinated, aged 7-18 years who had tested positive for COVID-19.
- Researchers performed nasal swabs every other day for 10 days, sending the swab to a lab to be tested for cytopathic effect (CPE), or cell death, an indicator of infectivity.
- They took pictures of the lab cultures to look for signs of CPE starting at 6 days after the test, which corresponds to the 2nd day after testing positive.
- If CPE characteristics were present in at least 30% of images, children were considered infectious.
TAKEAWAY:
- By day 3, half of study participants were noninfectious, independent of whether they had been vaccinated.
- By day 5, less than 25% of children were infectious, regardless of vaccination status.
- Among vaccinated children, the duration of infectivity was similar for children who received a booster and for those who had not.
- The authors state that these results are consistent with those of a study in adults with the Omicron variant, which found no association between vaccination status and infectivity duration.
IN PRACTICE:
“Our findings suggest that current policies requiring isolation for 5 days after a positive test might be appropriate, as the majority of children were not infectious by day 5. Additionally, return-to-school policies may not need to discriminate by vaccine or booster status,” the authors wrote.
SOURCE:
The study was led by Neeraj Sood, PhD, of the University of Southern California in Los Angeles, and was published in JAMA Pediatrics.
LIMITATIONS:
The sample size was small, and the authors identified the potential for nonresponse bias. The research did not include data from children who didn’t receive a test. CPE is the standard for estimating infectivity, but it can still carry inaccuracies.
DISCLOSURES:
The authors report no disclosures. The study was funded by RF Catalytic Capital.
A version of this article first appeared on Medscape.com.
TOPLINE:
according to a new study. The findings indicate that return-to-school policies for infected children may not need to differ on the basis of vaccine or booster status.
METHODOLOGY:
- The study looked at 76 children, both vaccinated and unvaccinated, aged 7-18 years who had tested positive for COVID-19.
- Researchers performed nasal swabs every other day for 10 days, sending the swab to a lab to be tested for cytopathic effect (CPE), or cell death, an indicator of infectivity.
- They took pictures of the lab cultures to look for signs of CPE starting at 6 days after the test, which corresponds to the 2nd day after testing positive.
- If CPE characteristics were present in at least 30% of images, children were considered infectious.
TAKEAWAY:
- By day 3, half of study participants were noninfectious, independent of whether they had been vaccinated.
- By day 5, less than 25% of children were infectious, regardless of vaccination status.
- Among vaccinated children, the duration of infectivity was similar for children who received a booster and for those who had not.
- The authors state that these results are consistent with those of a study in adults with the Omicron variant, which found no association between vaccination status and infectivity duration.
IN PRACTICE:
“Our findings suggest that current policies requiring isolation for 5 days after a positive test might be appropriate, as the majority of children were not infectious by day 5. Additionally, return-to-school policies may not need to discriminate by vaccine or booster status,” the authors wrote.
SOURCE:
The study was led by Neeraj Sood, PhD, of the University of Southern California in Los Angeles, and was published in JAMA Pediatrics.
LIMITATIONS:
The sample size was small, and the authors identified the potential for nonresponse bias. The research did not include data from children who didn’t receive a test. CPE is the standard for estimating infectivity, but it can still carry inaccuracies.
DISCLOSURES:
The authors report no disclosures. The study was funded by RF Catalytic Capital.
A version of this article first appeared on Medscape.com.
TOPLINE:
according to a new study. The findings indicate that return-to-school policies for infected children may not need to differ on the basis of vaccine or booster status.
METHODOLOGY:
- The study looked at 76 children, both vaccinated and unvaccinated, aged 7-18 years who had tested positive for COVID-19.
- Researchers performed nasal swabs every other day for 10 days, sending the swab to a lab to be tested for cytopathic effect (CPE), or cell death, an indicator of infectivity.
- They took pictures of the lab cultures to look for signs of CPE starting at 6 days after the test, which corresponds to the 2nd day after testing positive.
- If CPE characteristics were present in at least 30% of images, children were considered infectious.
TAKEAWAY:
- By day 3, half of study participants were noninfectious, independent of whether they had been vaccinated.
- By day 5, less than 25% of children were infectious, regardless of vaccination status.
- Among vaccinated children, the duration of infectivity was similar for children who received a booster and for those who had not.
- The authors state that these results are consistent with those of a study in adults with the Omicron variant, which found no association between vaccination status and infectivity duration.
IN PRACTICE:
“Our findings suggest that current policies requiring isolation for 5 days after a positive test might be appropriate, as the majority of children were not infectious by day 5. Additionally, return-to-school policies may not need to discriminate by vaccine or booster status,” the authors wrote.
SOURCE:
The study was led by Neeraj Sood, PhD, of the University of Southern California in Los Angeles, and was published in JAMA Pediatrics.
LIMITATIONS:
The sample size was small, and the authors identified the potential for nonresponse bias. The research did not include data from children who didn’t receive a test. CPE is the standard for estimating infectivity, but it can still carry inaccuracies.
DISCLOSURES:
The authors report no disclosures. The study was funded by RF Catalytic Capital.
A version of this article first appeared on Medscape.com.
New meningococcal vaccine wins FDA approval
The new formulation called Penbraya is manufactured by Pfizer and combines the components from two existing meningococcal vaccines, Trumenba the group B vaccine and Nimenrix groups A, C, W-135, and Y conjugate vaccine.
This is the first pentavalent vaccine for meningococcal disease and is approved for use in people aged 10-25.
“Today marks an important step forward in the prevention of meningococcal disease in the U.S.,” Annaliesa Anderson, PhD, head of vaccine research and development at Pfizer, said in a news release. “In a single vaccine, Penbraya has the potential to protect more adolescents and young adults from this severe and unpredictable disease by providing the broadest meningococcal coverage in the fewest shots.”
One shot, five common types
“Incomplete protection against invasive meningococcal disease,” is common, added Jana Shaw, MD, MPH, a pediatric infectious diseases specialist from Upstate Golisano Children’s Hospital in Syracuse, N.Y. Reducing the number of shots is important because streamlining the vaccination process should help increase the number of young people who get fully vaccinated against meningococcal disease.
Rates are low in the United States, according to the Centers for Disease Control and Prevention, and in 2021 there were around 210 cases reported. But a statewide outbreak has been going on in Virginia since June 2022, with 29 confirmed cases and 6 deaths.
The FDA’s decision is based on the positive results from phase 2 and phase 3 trials, including a randomized, active-controlled and observer-blinded phase 3 trial assessing the safety, tolerability, and immunogenicity of the pentavalent vaccine candidate, compared with currently licensed meningococcal vaccines. The phase 3 trial evaluated more than 2,400 patients from the United States and Europe.
The CDC Advisory Committee on Immunization Practices is meeting on Oct. 25 to discuss recommendations for the appropriate use of Penbraya in young people.
A version of this article first appeared on Medscape.com.
The new formulation called Penbraya is manufactured by Pfizer and combines the components from two existing meningococcal vaccines, Trumenba the group B vaccine and Nimenrix groups A, C, W-135, and Y conjugate vaccine.
This is the first pentavalent vaccine for meningococcal disease and is approved for use in people aged 10-25.
“Today marks an important step forward in the prevention of meningococcal disease in the U.S.,” Annaliesa Anderson, PhD, head of vaccine research and development at Pfizer, said in a news release. “In a single vaccine, Penbraya has the potential to protect more adolescents and young adults from this severe and unpredictable disease by providing the broadest meningococcal coverage in the fewest shots.”
One shot, five common types
“Incomplete protection against invasive meningococcal disease,” is common, added Jana Shaw, MD, MPH, a pediatric infectious diseases specialist from Upstate Golisano Children’s Hospital in Syracuse, N.Y. Reducing the number of shots is important because streamlining the vaccination process should help increase the number of young people who get fully vaccinated against meningococcal disease.
Rates are low in the United States, according to the Centers for Disease Control and Prevention, and in 2021 there were around 210 cases reported. But a statewide outbreak has been going on in Virginia since June 2022, with 29 confirmed cases and 6 deaths.
The FDA’s decision is based on the positive results from phase 2 and phase 3 trials, including a randomized, active-controlled and observer-blinded phase 3 trial assessing the safety, tolerability, and immunogenicity of the pentavalent vaccine candidate, compared with currently licensed meningococcal vaccines. The phase 3 trial evaluated more than 2,400 patients from the United States and Europe.
The CDC Advisory Committee on Immunization Practices is meeting on Oct. 25 to discuss recommendations for the appropriate use of Penbraya in young people.
A version of this article first appeared on Medscape.com.
The new formulation called Penbraya is manufactured by Pfizer and combines the components from two existing meningococcal vaccines, Trumenba the group B vaccine and Nimenrix groups A, C, W-135, and Y conjugate vaccine.
This is the first pentavalent vaccine for meningococcal disease and is approved for use in people aged 10-25.
“Today marks an important step forward in the prevention of meningococcal disease in the U.S.,” Annaliesa Anderson, PhD, head of vaccine research and development at Pfizer, said in a news release. “In a single vaccine, Penbraya has the potential to protect more adolescents and young adults from this severe and unpredictable disease by providing the broadest meningococcal coverage in the fewest shots.”
One shot, five common types
“Incomplete protection against invasive meningococcal disease,” is common, added Jana Shaw, MD, MPH, a pediatric infectious diseases specialist from Upstate Golisano Children’s Hospital in Syracuse, N.Y. Reducing the number of shots is important because streamlining the vaccination process should help increase the number of young people who get fully vaccinated against meningococcal disease.
Rates are low in the United States, according to the Centers for Disease Control and Prevention, and in 2021 there were around 210 cases reported. But a statewide outbreak has been going on in Virginia since June 2022, with 29 confirmed cases and 6 deaths.
The FDA’s decision is based on the positive results from phase 2 and phase 3 trials, including a randomized, active-controlled and observer-blinded phase 3 trial assessing the safety, tolerability, and immunogenicity of the pentavalent vaccine candidate, compared with currently licensed meningococcal vaccines. The phase 3 trial evaluated more than 2,400 patients from the United States and Europe.
The CDC Advisory Committee on Immunization Practices is meeting on Oct. 25 to discuss recommendations for the appropriate use of Penbraya in young people.
A version of this article first appeared on Medscape.com.
Sputum microbiome may augur treatment success in NTM-PD
HONOLULU – The diversity of species in the sputum of patients undergoing therapy for nontuberculosis mycobacterial pulmonary disease (NTM-PD) could be a marker for treatment efficacy, authors of a small prospective study suggest.
Among 14 patients treated for NTM-PD, 7 of whom had treatment-refractory disease and 7 of whom had microbiological cures after antibiotic therapy, the diversity of the microbiome in sputum was greater for those patients who were cured, indicating that , said Noeul Kang, MD, PhD, from Samsung Medical Center in Seoul, South Korea.
“What we found was that in NTM-PD patients, the sputum of the patients who remained in long-time stabilization without recurrence exhibited higher microbiome diversity than that of treatment-refractory patients, and several genera were identified in the samples of the cured group. We hope to do more research on this, and we are planning to compare the patients who have never been treated with those who respond to treatment,” she said at the annual meeting of the American College of Chest Physicians (CHEST).
NTM-PD on the rise
The incidence and prevalence of NTM-PD in both South Korea and the United States have been rising steadily since 2007, with the highest incidence occurring among those 65 and older.
“NTM-PD is becoming a global burden,” Dr. Kang said.
Across the world the most commonly occurring organisms in NTM-PD patients are Mycobacterium avium complex (MAC), with other mycobacteria species varying in frequency by region.
Outcomes of treatment differ according to the etiologic organism, with M. avium complex infections being successfully treated in about 60% of patients, compared with 70% of patients’ infections with the M. abscessus massiliense, and 30%-40% of infections yielding to antibiotics in patients with M. abscessus abscessus, Dr. Kang said.
To compare the characteristics of the sputum microbiota of NTM-PD patients based on their treatment outcomes, Dr. Kang and colleagues looked at sputum from all patients with NTM-PD who agreed to provide samples at their center from 2018 through 2022.
After excluding those who did not receive antibiotics, those who were on treatment but did not have refractory disease, and those who were lost to follow-up or whose samples did not pass quality control, they identified seven patients who had microbiological cures, and seven whose disease remained refractory to treatment.
They defined culture conversion at three or more consecutive negative sputum cultures after treatment, collected at least 4 weeks apart, and microbiological cures at maintenance of multiple consecutive negative cultures without any positive cultures of the causative species from respiratory samples.
Infections were deemed to be refractory if there were sustained positive cultures from respiratory samples of causative NTM species after at least 1 year of antibiotic therapy.
Diversity analysis
Samples from 8 of the 14 participants had M. abscessus-PD, with the proportion higher among those who had a sustained microbiological cure (71.4% vs. 42.9%).
At baseline, patients with refractory disease were found to have significantly lower alpha diversity, a measure of microbial diversity within a single sample, compared with those whose infections were cured (P = .025).
In addition, samples at 6-month follow-up from those with baseline refractory infections had differences in the species level of beta-diversity (that is, differences among samples), compared with both baseline and follow-up samples from the cured group (P = .022 and .024, respectively).
The investigators also used linear discriminant analysis to look at taxonomic biomarkers, and observed that several species were more abundant in samples from the microbiological cure group than from the refractory disease group (P < .05) These species included organisms in the Streptococcus pneumoniae group, Prevotella melaninogenica, and Haemophilus parahaemolyticus group.
Promising start
A pulmonologist who was not involved in the study commented in an interview that, although the findings need further study, the microbiome of sputum samples has the potential for predictive value.
“I think this will be clinically useful, actually, if we’re able to identify and diagnose patients with MAC disease and then we identify their sputum microbiome, it might give us an idea whether these patients are more sensitive or refractory to treatment,” said Muhammad U. Khawar, MD, from the University of Cincinnati.
Dr. Khawar moderated the session where Dr. Kang reported her data.
The investigators did not report a funding source. Dr. Kang and Dr. Khawar reported that they had no relevant disclosures.
HONOLULU – The diversity of species in the sputum of patients undergoing therapy for nontuberculosis mycobacterial pulmonary disease (NTM-PD) could be a marker for treatment efficacy, authors of a small prospective study suggest.
Among 14 patients treated for NTM-PD, 7 of whom had treatment-refractory disease and 7 of whom had microbiological cures after antibiotic therapy, the diversity of the microbiome in sputum was greater for those patients who were cured, indicating that , said Noeul Kang, MD, PhD, from Samsung Medical Center in Seoul, South Korea.
“What we found was that in NTM-PD patients, the sputum of the patients who remained in long-time stabilization without recurrence exhibited higher microbiome diversity than that of treatment-refractory patients, and several genera were identified in the samples of the cured group. We hope to do more research on this, and we are planning to compare the patients who have never been treated with those who respond to treatment,” she said at the annual meeting of the American College of Chest Physicians (CHEST).
NTM-PD on the rise
The incidence and prevalence of NTM-PD in both South Korea and the United States have been rising steadily since 2007, with the highest incidence occurring among those 65 and older.
“NTM-PD is becoming a global burden,” Dr. Kang said.
Across the world the most commonly occurring organisms in NTM-PD patients are Mycobacterium avium complex (MAC), with other mycobacteria species varying in frequency by region.
Outcomes of treatment differ according to the etiologic organism, with M. avium complex infections being successfully treated in about 60% of patients, compared with 70% of patients’ infections with the M. abscessus massiliense, and 30%-40% of infections yielding to antibiotics in patients with M. abscessus abscessus, Dr. Kang said.
To compare the characteristics of the sputum microbiota of NTM-PD patients based on their treatment outcomes, Dr. Kang and colleagues looked at sputum from all patients with NTM-PD who agreed to provide samples at their center from 2018 through 2022.
After excluding those who did not receive antibiotics, those who were on treatment but did not have refractory disease, and those who were lost to follow-up or whose samples did not pass quality control, they identified seven patients who had microbiological cures, and seven whose disease remained refractory to treatment.
They defined culture conversion at three or more consecutive negative sputum cultures after treatment, collected at least 4 weeks apart, and microbiological cures at maintenance of multiple consecutive negative cultures without any positive cultures of the causative species from respiratory samples.
Infections were deemed to be refractory if there were sustained positive cultures from respiratory samples of causative NTM species after at least 1 year of antibiotic therapy.
Diversity analysis
Samples from 8 of the 14 participants had M. abscessus-PD, with the proportion higher among those who had a sustained microbiological cure (71.4% vs. 42.9%).
At baseline, patients with refractory disease were found to have significantly lower alpha diversity, a measure of microbial diversity within a single sample, compared with those whose infections were cured (P = .025).
In addition, samples at 6-month follow-up from those with baseline refractory infections had differences in the species level of beta-diversity (that is, differences among samples), compared with both baseline and follow-up samples from the cured group (P = .022 and .024, respectively).
The investigators also used linear discriminant analysis to look at taxonomic biomarkers, and observed that several species were more abundant in samples from the microbiological cure group than from the refractory disease group (P < .05) These species included organisms in the Streptococcus pneumoniae group, Prevotella melaninogenica, and Haemophilus parahaemolyticus group.
Promising start
A pulmonologist who was not involved in the study commented in an interview that, although the findings need further study, the microbiome of sputum samples has the potential for predictive value.
“I think this will be clinically useful, actually, if we’re able to identify and diagnose patients with MAC disease and then we identify their sputum microbiome, it might give us an idea whether these patients are more sensitive or refractory to treatment,” said Muhammad U. Khawar, MD, from the University of Cincinnati.
Dr. Khawar moderated the session where Dr. Kang reported her data.
The investigators did not report a funding source. Dr. Kang and Dr. Khawar reported that they had no relevant disclosures.
HONOLULU – The diversity of species in the sputum of patients undergoing therapy for nontuberculosis mycobacterial pulmonary disease (NTM-PD) could be a marker for treatment efficacy, authors of a small prospective study suggest.
Among 14 patients treated for NTM-PD, 7 of whom had treatment-refractory disease and 7 of whom had microbiological cures after antibiotic therapy, the diversity of the microbiome in sputum was greater for those patients who were cured, indicating that , said Noeul Kang, MD, PhD, from Samsung Medical Center in Seoul, South Korea.
“What we found was that in NTM-PD patients, the sputum of the patients who remained in long-time stabilization without recurrence exhibited higher microbiome diversity than that of treatment-refractory patients, and several genera were identified in the samples of the cured group. We hope to do more research on this, and we are planning to compare the patients who have never been treated with those who respond to treatment,” she said at the annual meeting of the American College of Chest Physicians (CHEST).
NTM-PD on the rise
The incidence and prevalence of NTM-PD in both South Korea and the United States have been rising steadily since 2007, with the highest incidence occurring among those 65 and older.
“NTM-PD is becoming a global burden,” Dr. Kang said.
Across the world the most commonly occurring organisms in NTM-PD patients are Mycobacterium avium complex (MAC), with other mycobacteria species varying in frequency by region.
Outcomes of treatment differ according to the etiologic organism, with M. avium complex infections being successfully treated in about 60% of patients, compared with 70% of patients’ infections with the M. abscessus massiliense, and 30%-40% of infections yielding to antibiotics in patients with M. abscessus abscessus, Dr. Kang said.
To compare the characteristics of the sputum microbiota of NTM-PD patients based on their treatment outcomes, Dr. Kang and colleagues looked at sputum from all patients with NTM-PD who agreed to provide samples at their center from 2018 through 2022.
After excluding those who did not receive antibiotics, those who were on treatment but did not have refractory disease, and those who were lost to follow-up or whose samples did not pass quality control, they identified seven patients who had microbiological cures, and seven whose disease remained refractory to treatment.
They defined culture conversion at three or more consecutive negative sputum cultures after treatment, collected at least 4 weeks apart, and microbiological cures at maintenance of multiple consecutive negative cultures without any positive cultures of the causative species from respiratory samples.
Infections were deemed to be refractory if there were sustained positive cultures from respiratory samples of causative NTM species after at least 1 year of antibiotic therapy.
Diversity analysis
Samples from 8 of the 14 participants had M. abscessus-PD, with the proportion higher among those who had a sustained microbiological cure (71.4% vs. 42.9%).
At baseline, patients with refractory disease were found to have significantly lower alpha diversity, a measure of microbial diversity within a single sample, compared with those whose infections were cured (P = .025).
In addition, samples at 6-month follow-up from those with baseline refractory infections had differences in the species level of beta-diversity (that is, differences among samples), compared with both baseline and follow-up samples from the cured group (P = .022 and .024, respectively).
The investigators also used linear discriminant analysis to look at taxonomic biomarkers, and observed that several species were more abundant in samples from the microbiological cure group than from the refractory disease group (P < .05) These species included organisms in the Streptococcus pneumoniae group, Prevotella melaninogenica, and Haemophilus parahaemolyticus group.
Promising start
A pulmonologist who was not involved in the study commented in an interview that, although the findings need further study, the microbiome of sputum samples has the potential for predictive value.
“I think this will be clinically useful, actually, if we’re able to identify and diagnose patients with MAC disease and then we identify their sputum microbiome, it might give us an idea whether these patients are more sensitive or refractory to treatment,” said Muhammad U. Khawar, MD, from the University of Cincinnati.
Dr. Khawar moderated the session where Dr. Kang reported her data.
The investigators did not report a funding source. Dr. Kang and Dr. Khawar reported that they had no relevant disclosures.
AT CHEST 2023
AI chatbot ‘hallucinates’ faulty medical intelligence
Artificial intelligence (AI) models are typically a year out of date and have this “charming problem of hallucinating made-up data and saying it with all the certainty of an attending on rounds,” Isaac Kohane, MD, PhD, Harvard Medical School, Boston, told a packed audience at plenary at an annual scientific meeting on infectious diseases.
Dr. Kohane, chair of the department of biomedical informatics, says the future intersection between AI and health care is “muddy.”
Echoing questions about the accuracy of new AI tools, researchers at the meeting presented the results of their new test of ChatGPT.
To test the accuracy of ChatGPT’s version 3.5, the researchers asked it if there are any boxed warnings on the U.S. Food and Drug Administration’s label for common antibiotics, and if so, what they are.
ChatGPT provided correct answers about FDA boxed warnings for only 12 of the 41 antibiotics queried – a matching rate of just 29%.
For the other 29 antibiotics, ChatGPT either “incorrectly reported that there was an FDA boxed warning when there was not, or inaccurately or incorrectly reported the boxed warning,” Rebecca Linfield, MD, infectious diseases fellow, Stanford (Calif.) University, said in an interview.
Uncritical AI use risky
Nine of the 41 antibiotics included in the query have boxed warnings. And ChatGPT correctly identified all nine, but only three were the matching adverse event (33%). For the 32 antibiotics without an FDA boxed warning, ChatGPT correctly reported that 28% (9 of 32) do not have a boxed warning.
For example, ChatGPT stated that the antibiotic fidaxomicin has a boxed warning for increased risk for Clostridioides difficile, “but it is the first-line antibiotic used to treat C. difficile,” Dr. Linfield pointed out.
ChatGPT also reported that cefepime increased the risk for death in those with pneumonia and fabricated a study supporting that assertion. “However, cefepime is a first-line drug for those with hospital-acquired pneumonia,” Dr. Linfield explained.
“I can imagine a worried family member finding this through ChatGPT, and needing to have extensive reassurances from the patient’s physicians about why this antibiotic was chosen,” she said.
ChatGPT also incorrectly stated that aztreonam has a boxed warning for increased mortality.
“The risk is that both physicians and the public uncritically use ChatGPT as an easily accessible, readable source of clinically validated information, when these large language models are meant to generate fluid text, and not necessarily accurate information,” Dr. Linfield told this news organization.
Dr. Linfield said that the next step is to compare the ChatGPT 3.5 used in this analysis with ChatGPT 4, as well as with Google’s Med-PaLM 2 after it is released to the public.
Advancing fast
At plenary, Dr. Kohane pointed out that AI is a quick learner and improvements in tools are coming fast.
As an example, just 3 years ago, the best AI tool could score about as well as the worst student taking the medical boards, he told the audience. “Three years later, the leading large language models are scoring better than 90% of all the candidates. What’s it going to be doing next year?” he asked.
“I don’t know,” Dr. Kohane said, “but it will be better than this year.” AI will “transform health care.”
A version of this article first appeared on Medscape.com.
Artificial intelligence (AI) models are typically a year out of date and have this “charming problem of hallucinating made-up data and saying it with all the certainty of an attending on rounds,” Isaac Kohane, MD, PhD, Harvard Medical School, Boston, told a packed audience at plenary at an annual scientific meeting on infectious diseases.
Dr. Kohane, chair of the department of biomedical informatics, says the future intersection between AI and health care is “muddy.”
Echoing questions about the accuracy of new AI tools, researchers at the meeting presented the results of their new test of ChatGPT.
To test the accuracy of ChatGPT’s version 3.5, the researchers asked it if there are any boxed warnings on the U.S. Food and Drug Administration’s label for common antibiotics, and if so, what they are.
ChatGPT provided correct answers about FDA boxed warnings for only 12 of the 41 antibiotics queried – a matching rate of just 29%.
For the other 29 antibiotics, ChatGPT either “incorrectly reported that there was an FDA boxed warning when there was not, or inaccurately or incorrectly reported the boxed warning,” Rebecca Linfield, MD, infectious diseases fellow, Stanford (Calif.) University, said in an interview.
Uncritical AI use risky
Nine of the 41 antibiotics included in the query have boxed warnings. And ChatGPT correctly identified all nine, but only three were the matching adverse event (33%). For the 32 antibiotics without an FDA boxed warning, ChatGPT correctly reported that 28% (9 of 32) do not have a boxed warning.
For example, ChatGPT stated that the antibiotic fidaxomicin has a boxed warning for increased risk for Clostridioides difficile, “but it is the first-line antibiotic used to treat C. difficile,” Dr. Linfield pointed out.
ChatGPT also reported that cefepime increased the risk for death in those with pneumonia and fabricated a study supporting that assertion. “However, cefepime is a first-line drug for those with hospital-acquired pneumonia,” Dr. Linfield explained.
“I can imagine a worried family member finding this through ChatGPT, and needing to have extensive reassurances from the patient’s physicians about why this antibiotic was chosen,” she said.
ChatGPT also incorrectly stated that aztreonam has a boxed warning for increased mortality.
“The risk is that both physicians and the public uncritically use ChatGPT as an easily accessible, readable source of clinically validated information, when these large language models are meant to generate fluid text, and not necessarily accurate information,” Dr. Linfield told this news organization.
Dr. Linfield said that the next step is to compare the ChatGPT 3.5 used in this analysis with ChatGPT 4, as well as with Google’s Med-PaLM 2 after it is released to the public.
Advancing fast
At plenary, Dr. Kohane pointed out that AI is a quick learner and improvements in tools are coming fast.
As an example, just 3 years ago, the best AI tool could score about as well as the worst student taking the medical boards, he told the audience. “Three years later, the leading large language models are scoring better than 90% of all the candidates. What’s it going to be doing next year?” he asked.
“I don’t know,” Dr. Kohane said, “but it will be better than this year.” AI will “transform health care.”
A version of this article first appeared on Medscape.com.
Artificial intelligence (AI) models are typically a year out of date and have this “charming problem of hallucinating made-up data and saying it with all the certainty of an attending on rounds,” Isaac Kohane, MD, PhD, Harvard Medical School, Boston, told a packed audience at plenary at an annual scientific meeting on infectious diseases.
Dr. Kohane, chair of the department of biomedical informatics, says the future intersection between AI and health care is “muddy.”
Echoing questions about the accuracy of new AI tools, researchers at the meeting presented the results of their new test of ChatGPT.
To test the accuracy of ChatGPT’s version 3.5, the researchers asked it if there are any boxed warnings on the U.S. Food and Drug Administration’s label for common antibiotics, and if so, what they are.
ChatGPT provided correct answers about FDA boxed warnings for only 12 of the 41 antibiotics queried – a matching rate of just 29%.
For the other 29 antibiotics, ChatGPT either “incorrectly reported that there was an FDA boxed warning when there was not, or inaccurately or incorrectly reported the boxed warning,” Rebecca Linfield, MD, infectious diseases fellow, Stanford (Calif.) University, said in an interview.
Uncritical AI use risky
Nine of the 41 antibiotics included in the query have boxed warnings. And ChatGPT correctly identified all nine, but only three were the matching adverse event (33%). For the 32 antibiotics without an FDA boxed warning, ChatGPT correctly reported that 28% (9 of 32) do not have a boxed warning.
For example, ChatGPT stated that the antibiotic fidaxomicin has a boxed warning for increased risk for Clostridioides difficile, “but it is the first-line antibiotic used to treat C. difficile,” Dr. Linfield pointed out.
ChatGPT also reported that cefepime increased the risk for death in those with pneumonia and fabricated a study supporting that assertion. “However, cefepime is a first-line drug for those with hospital-acquired pneumonia,” Dr. Linfield explained.
“I can imagine a worried family member finding this through ChatGPT, and needing to have extensive reassurances from the patient’s physicians about why this antibiotic was chosen,” she said.
ChatGPT also incorrectly stated that aztreonam has a boxed warning for increased mortality.
“The risk is that both physicians and the public uncritically use ChatGPT as an easily accessible, readable source of clinically validated information, when these large language models are meant to generate fluid text, and not necessarily accurate information,” Dr. Linfield told this news organization.
Dr. Linfield said that the next step is to compare the ChatGPT 3.5 used in this analysis with ChatGPT 4, as well as with Google’s Med-PaLM 2 after it is released to the public.
Advancing fast
At plenary, Dr. Kohane pointed out that AI is a quick learner and improvements in tools are coming fast.
As an example, just 3 years ago, the best AI tool could score about as well as the worst student taking the medical boards, he told the audience. “Three years later, the leading large language models are scoring better than 90% of all the candidates. What’s it going to be doing next year?” he asked.
“I don’t know,” Dr. Kohane said, “but it will be better than this year.” AI will “transform health care.”
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2023
What’s Eating You? Phlebotomine Sandflies and Leishmania Parasites
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4 Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
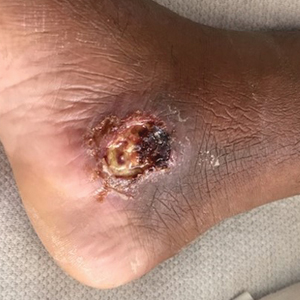
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.
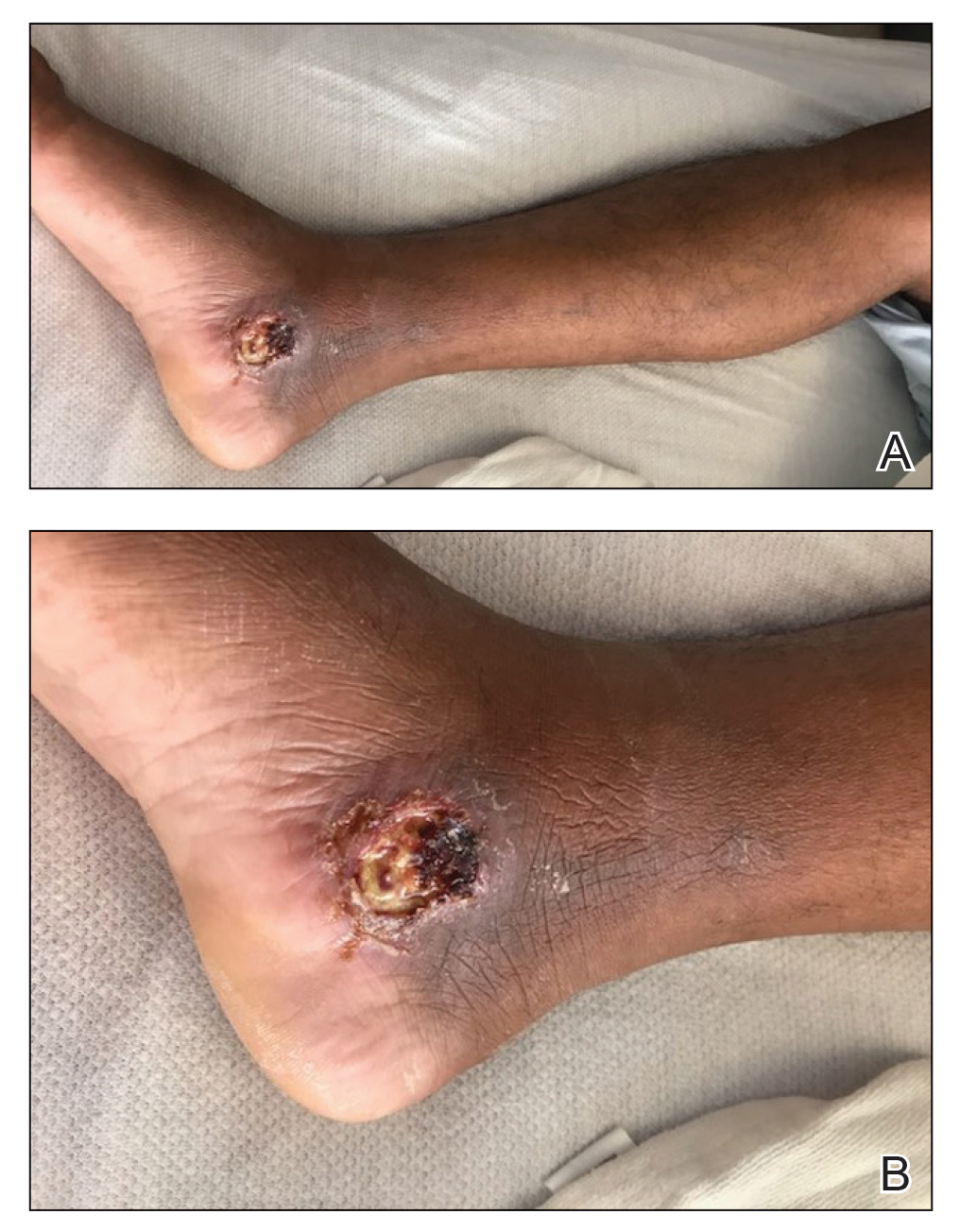
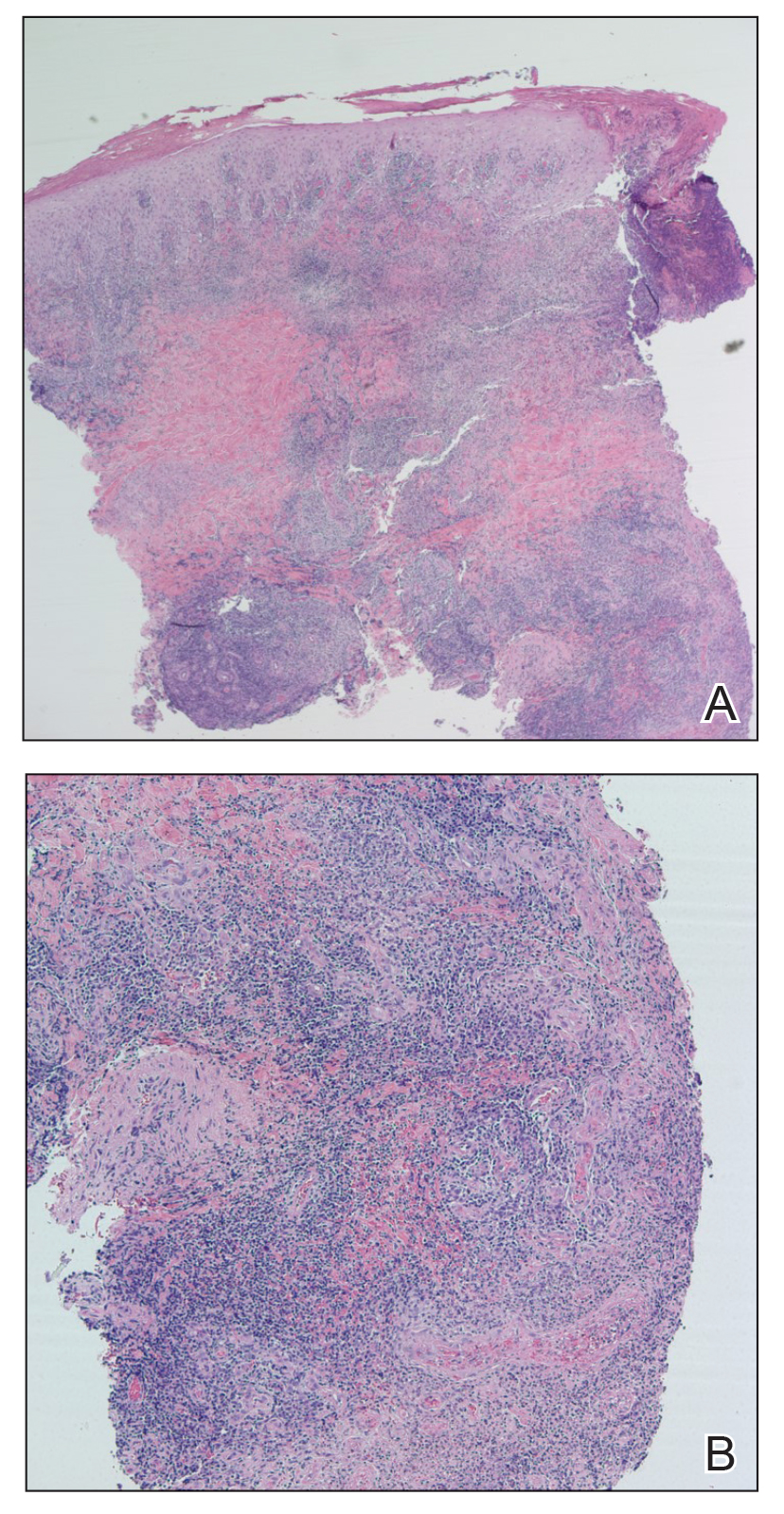
Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.
Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4 Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.

Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.

Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4 Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.

Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.

Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
Practice Points
- The Phlebotomus and Lutzomyia genera of sandflies are vectors of Leishmania parasites, which can result in an array of clinical findings associated with leishmaniasis.
- Treatment options for leishmaniasis differ based on whether the infection is considered uncomplicated or complicated, which depends on the species of Leishmania; the number, size, and location of the lesion(s); and host immune status.
- All US practitioners should be aware of this pathogen, especially with regard to patients who have a history of travel to other countries. Health care professionals in states such as Texas and Oklahoma should be especially cognizant because these constitute one of the few areas in the United States where locally acquired cases of leishmaniasis have been reported.
Iododerma Simulating Cryptococcal Infection
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
Practice Points
- Halogenodermas are rare cutaneous reactions to excess exposure to or ingestion of halogen-containing drugs or substances such as bromine, iodine (iododerma), fluorine, and rarely lithium.
- The clinical presentation of a halogenoderma varies; the most characteristic manifestation is a vegetative or exudative plaque with a peripheral rim of pustules.
- Histologically, lesions of a halogenoderma are characterized by pseudoepitheliomatous hyperplasia associated with numerous intraepidermal microabscesses overlying a dense mixed inflammatory infiltrate of neutrophils, plasma cells, eosinophils, histiocytes, and scattered multinucleated giant cells.
- Rarely, the dermal infiltrate of a halogenoderma contains abundant acellular bodies surrounded by capsulelike vacuolated spaces mimicking Cryptococcus neoformans.
Antibiotics ‘like gold’ for some, driving inappropriate use
Personal beliefs and health care system barriers contribute to inappropriate antibiotic use by patients, report researchers presenting results at an annual scientific meeting on infectious diseases.
Nonprescription antibiotic use includes accessing medication left over from a prior prescribed course, obtained from social networks, and purchased over-the-counter in other countries or illegally in stores and markets in the United States.
Overuse and misuse of antibiotics contributes to a growing threat of antimicrobial resistance, and it is tough to say how common it is, Lindsey A. Laytner, PhD, MPH, with Baylor College of Medicine, Houston, pointed out in her presentation.
“This is an understudied area. We don’t routinely collect these data, so we don’t actually know what the true prevalence is. The factors that contribute to this unsafe practice in the U.S. are also underexplored,” Dr. Laytner said.
To investigate, the researchers conducted in-depth interviews with 86 adults (median age, 49 years; 62% women) to identify patients’ motivations to use antibiotics without a prescription. All of them answered “yes” when asked in a previous survey whether they would use antibiotics without contacting a doctor, nurse, dentist, or clinic.
Dr. Laytner said several prominent themes emerged.
Nearly all interviewees reported nonprescription antibiotic use for symptoms that mostly do not warrant antibiotics. These included symptoms of COVID-19, influenza, and the common cold, as well as for pain management, allergies, and even wounds.
Ineffectively treating symptoms
Many felt they “knew their body, knew what they had, and knew how to treat themselves” without a health care provider, Dr. Laytner said.
They also felt the over-the-counter medicines “don’t always work and that antibiotics are like gold or this cure-all and because they are difficult to get a prescription for, they should be kept on hand,” she explained.
A variety of health care system barriers also contribute to inappropriate antibiotic use, including long wait times to schedule appointments and to see the doctor while at their appointments; high costs for clinic visits and prescriptions; and transportation issues.
Many patients opted to use nonprescription antibiotics out of “convenience,” Laytner added.
She explains that the findings could help inform community-level education efforts on inappropriate use of antibiotics and help shape policies to promote antibiotic stewardship.
Access to care, education
Commenting on the study, Emily Sydnor Spivak, MD, associate professor of medicine at University of Utah, Salt Lake City, said she “wasn’t totally surprised by the results, but found it very interesting how there was a theme of autonomy, or ‘I know my body,’ that seemed to drive patients to get antibiotics for relief of symptoms.”
said Dr. Spivak, who is also medical director of antimicrobial stewardship programs at University of Utah Health and VA Salt Lake City Health Care System.
“Given the lack of access to health care as a reason some patients use nonprescription antibiotics, we need to think about access to the health care system and process changes and policy changes to allow better access. Without better access or interaction with the health care system, we can’t educate patients,” Dr. Spivak said.
The study had no commercial funding. Dr. Laytner and Dr. Spivak report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Personal beliefs and health care system barriers contribute to inappropriate antibiotic use by patients, report researchers presenting results at an annual scientific meeting on infectious diseases.
Nonprescription antibiotic use includes accessing medication left over from a prior prescribed course, obtained from social networks, and purchased over-the-counter in other countries or illegally in stores and markets in the United States.
Overuse and misuse of antibiotics contributes to a growing threat of antimicrobial resistance, and it is tough to say how common it is, Lindsey A. Laytner, PhD, MPH, with Baylor College of Medicine, Houston, pointed out in her presentation.
“This is an understudied area. We don’t routinely collect these data, so we don’t actually know what the true prevalence is. The factors that contribute to this unsafe practice in the U.S. are also underexplored,” Dr. Laytner said.
To investigate, the researchers conducted in-depth interviews with 86 adults (median age, 49 years; 62% women) to identify patients’ motivations to use antibiotics without a prescription. All of them answered “yes” when asked in a previous survey whether they would use antibiotics without contacting a doctor, nurse, dentist, or clinic.
Dr. Laytner said several prominent themes emerged.
Nearly all interviewees reported nonprescription antibiotic use for symptoms that mostly do not warrant antibiotics. These included symptoms of COVID-19, influenza, and the common cold, as well as for pain management, allergies, and even wounds.
Ineffectively treating symptoms
Many felt they “knew their body, knew what they had, and knew how to treat themselves” without a health care provider, Dr. Laytner said.
They also felt the over-the-counter medicines “don’t always work and that antibiotics are like gold or this cure-all and because they are difficult to get a prescription for, they should be kept on hand,” she explained.
A variety of health care system barriers also contribute to inappropriate antibiotic use, including long wait times to schedule appointments and to see the doctor while at their appointments; high costs for clinic visits and prescriptions; and transportation issues.
Many patients opted to use nonprescription antibiotics out of “convenience,” Laytner added.
She explains that the findings could help inform community-level education efforts on inappropriate use of antibiotics and help shape policies to promote antibiotic stewardship.
Access to care, education
Commenting on the study, Emily Sydnor Spivak, MD, associate professor of medicine at University of Utah, Salt Lake City, said she “wasn’t totally surprised by the results, but found it very interesting how there was a theme of autonomy, or ‘I know my body,’ that seemed to drive patients to get antibiotics for relief of symptoms.”
said Dr. Spivak, who is also medical director of antimicrobial stewardship programs at University of Utah Health and VA Salt Lake City Health Care System.
“Given the lack of access to health care as a reason some patients use nonprescription antibiotics, we need to think about access to the health care system and process changes and policy changes to allow better access. Without better access or interaction with the health care system, we can’t educate patients,” Dr. Spivak said.
The study had no commercial funding. Dr. Laytner and Dr. Spivak report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Personal beliefs and health care system barriers contribute to inappropriate antibiotic use by patients, report researchers presenting results at an annual scientific meeting on infectious diseases.
Nonprescription antibiotic use includes accessing medication left over from a prior prescribed course, obtained from social networks, and purchased over-the-counter in other countries or illegally in stores and markets in the United States.
Overuse and misuse of antibiotics contributes to a growing threat of antimicrobial resistance, and it is tough to say how common it is, Lindsey A. Laytner, PhD, MPH, with Baylor College of Medicine, Houston, pointed out in her presentation.
“This is an understudied area. We don’t routinely collect these data, so we don’t actually know what the true prevalence is. The factors that contribute to this unsafe practice in the U.S. are also underexplored,” Dr. Laytner said.
To investigate, the researchers conducted in-depth interviews with 86 adults (median age, 49 years; 62% women) to identify patients’ motivations to use antibiotics without a prescription. All of them answered “yes” when asked in a previous survey whether they would use antibiotics without contacting a doctor, nurse, dentist, or clinic.
Dr. Laytner said several prominent themes emerged.
Nearly all interviewees reported nonprescription antibiotic use for symptoms that mostly do not warrant antibiotics. These included symptoms of COVID-19, influenza, and the common cold, as well as for pain management, allergies, and even wounds.
Ineffectively treating symptoms
Many felt they “knew their body, knew what they had, and knew how to treat themselves” without a health care provider, Dr. Laytner said.
They also felt the over-the-counter medicines “don’t always work and that antibiotics are like gold or this cure-all and because they are difficult to get a prescription for, they should be kept on hand,” she explained.
A variety of health care system barriers also contribute to inappropriate antibiotic use, including long wait times to schedule appointments and to see the doctor while at their appointments; high costs for clinic visits and prescriptions; and transportation issues.
Many patients opted to use nonprescription antibiotics out of “convenience,” Laytner added.
She explains that the findings could help inform community-level education efforts on inappropriate use of antibiotics and help shape policies to promote antibiotic stewardship.
Access to care, education
Commenting on the study, Emily Sydnor Spivak, MD, associate professor of medicine at University of Utah, Salt Lake City, said she “wasn’t totally surprised by the results, but found it very interesting how there was a theme of autonomy, or ‘I know my body,’ that seemed to drive patients to get antibiotics for relief of symptoms.”
said Dr. Spivak, who is also medical director of antimicrobial stewardship programs at University of Utah Health and VA Salt Lake City Health Care System.
“Given the lack of access to health care as a reason some patients use nonprescription antibiotics, we need to think about access to the health care system and process changes and policy changes to allow better access. Without better access or interaction with the health care system, we can’t educate patients,” Dr. Spivak said.
The study had no commercial funding. Dr. Laytner and Dr. Spivak report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
IDWEEK 2023
New RSV vaccine will cut hospitalizations, study shows
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2023