User login
Ezetimibe-statin combo lowers liver fat in open-label trial
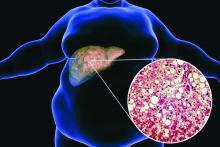
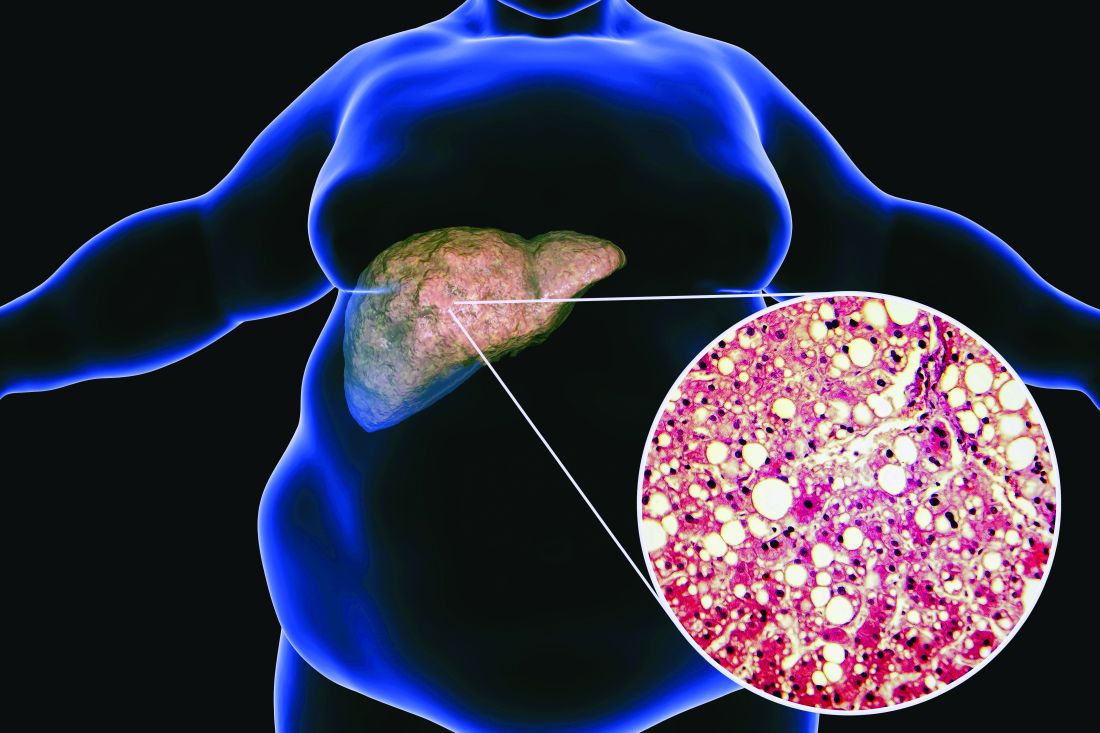
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
FROM EASD 2022
Aspirin primary prevention benefit in those with raised Lp(a)?
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.
Aspirin may be of specific benefit for the primary prevention of cardiovascular disease in individuals with raised Lp(a) levels, a new study has suggested.
The study analyzed data from the ASPREE (ASPirin in Reducing Events in the Elderly) trial, which randomized 19,000 individuals aged 70 years or older without a history of cardiovascular disease to aspirin (100 mg/day) or placebo. While the main results, reported previously, showed no net benefit of aspirin in the overall population, the current analysis suggests there may be a benefit in individuals with raised Lp(a) levels.
The current analysis was published online in the Journal of the American College of Cardiology.
“Our study provides evidence that aspirin may specifically benefit older individuals with genotypes for elevated plasma Lp(a) in the setting of high-risk primary prevention of cardiovascular events and that overall benefit may outweigh harm related to major bleeding,” the authors, led by Paul Lacaze, PhD, Monash University, Melbourne, conclude.
They also point out that similar observations have been previously seen in another large aspirin primary prevention study conducted in younger women, the Women’s Health Study, and the current analysis provides validation of those findings.
“Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of cardiovascular events,” the researchers say.
They acknowledge that these results would be strengthened by the use of directly measured plasma Lp(a) levels, in addition to Lp(a) genotypes.
But they add: “Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated cardiovascular risk.”
Dr. Lacaze and colleagues explain that elevated plasma Lp(a) levels confer up to fourfold increased risk of cardiovascular disease, with around 20%-30% of the general population affected. Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacologic therapies targeting this lipoprotein. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated cardiovascular disease, it will be many years before these candidates are assessed for primary prevention.
For the current study, researchers analyzed data from 12,815 ASPREE participants who had undergone genotyping and compared outcomes with aspirin versus placebo in those with and without genotypes associated with elevated Lp(a) levels.
Results showed that individuals with elevated Lp(a)-associated genotypes, defined in two different ways, showed a reduction in ischemic events with aspirin versus placebo, and this benefit was not outweighed by an increased bleeding risk.
Specifically, in the placebo group, individuals who carried the rs3798220-C allele, which is known to be associated with raised Lp(a) levels, making up 3.2% of the genotyped population in the study, had an almost twofold increased risk of major adverse cardiovascular events than those not carrying this genotype. However, the risk was attenuated in the aspirin group, with carriers of the rs3798220-C allele actually having a lower rate of cardiovascular events than noncarriers.
In addition, rs3798220-C carrier status was not significantly associated with increased risk of clinically significant bleeding events in the aspirin group.
Similar results were seen with the second way of identifying patients with a high risk of elevated Lp(a) levels using a 43-variant genetic risk score (LPA-GRS).
In the whole study population, aspirin reduced major adverse cardiovascular events by 1.7 events per 1,000 person-years and increased clinically significant bleeding events by 1.7 events per 1,000 person-years, suggesting parity between overall benefit versus harm.
However, in the rs3798220-C subgroup, aspirin reduced major adverse cardiovascular events by 11.4 events per 1,000 person-years (a more than sixfold higher magnitude of cardiovascular disease risk reduction than in the overall cohort), with a bleeding risk of 3.3 events per 1,000 person-years, the researchers report.
“Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years,” they state.
In the highest LPA-GRS quintile, aspirin reduced major adverse cardiovascular events by 3.3 events per 1,000 person-years (approximately twofold higher magnitude of risk reduction, compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort). This shifted the benefit versus harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
Similar findings in the Women’s Health Study
Dr. Lacaze and colleagues point out that similar results have also been seen in another large aspirin primary prevention study – the Women’s Health Study (WHS).
The WHS compared aspirin 100 mg every other day with placebo in initially healthy younger women. Previously reported results showed that women carrying the rs3798220-C variant, associated with highly elevated Lp(a) levels, had a twofold higher risk of cardiovascular events than noncarrier women in the placebo group, but this risk was reduced in the aspirin group. And there was no increased risk of bleeding in women with elevated Lp(a).
“These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a),” Dr. Lacaze and colleagues note.
“In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women),” they add.
They say this validation of the WHS result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) – those carrying the rs3798220-C allele – may benefit from low-dose aspirin for the primary prevention of cardiovascular events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk.
But they point out that rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population, while the polygenic LPA-GRS explains about 60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals at increased risk.
The researchers note, however, that it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin.
“If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20%-30% of individuals with elevated Lp(a), the potential utility of aspirin for the primary prevention of cardiovascular events would increase substantially,” they say.
‘Very high clinical relevance’
In an accompanying editorial, Ana Devesa, MD, Borja Ibanez, MD, PhD, and Valentin Fuster, MD, PhD, The National Center for Cardiovascular Research, Madrid, say that: “[Dr.] Lacaze et al. are to be congratulated for a study of very high clinical relevance that represents a first indication for primary prevention for patients at high cardiovascular risk.”
They explain that the pathogenic mechanism of Lp(a) is believed to be a combination of prothrombotic and proatherogenic effects, and the current findings support the hypothesis that the prothrombotic mechanism of Lp(a) is mediated by platelet aggregation.
This would explain the occurrence of thrombotic events in the presence of atherosclerosis in that elevated Lp(a) levels may induce platelet adhesion and aggregation to the activated atherosclerotic plaque, thus enhancing the atherothrombotic process. Moreover, activated platelets release several mediators that result in cell adhesion and attraction of chemokines and proinflammatory cytokines, driving an inflammatory response and mediating atherosclerosis progression, they add.
The editorialists highlight the limitations of the study already acknowledged by the authors: The analysis used genotypes rather than elevated Lp(a) levels and included only those of European ancestry, meaning the results are difficult to extrapolate to other populations.
“The next steps in clinical practice should be defined, and there are still questions to be answered,” they conclude. “Will every patient benefit from antithrombotic therapies? Should all patients who have elevated Lp(a) levels be treated with aspirin?”
The ASPREE Biobank is supported by grants from the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne, National Institutes of Health, National Health and Medical Research Council of Australia, and the Victorian Cancer Agency. Dr. Lacaze is supported by a National Heart Foundation Future Leader Fellowship.
A version of this article first appeared on Medscape.com.
Coffee linked to reduced cardiovascular disease and mortality risk
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Emphasis on weight loss in new type 2 diabetes guidance
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Uncontrolled BP linked to one-third of ED visits for CVD
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Add PCSK9 inhibitor to high-intensity statin at primary PCI, proposes sham-controlled EPIC-STEMI
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Eat more dairy, less red meat to prevent type 2 diabetes
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Whole grains may improve survival in people with type 2 diabetes
STOCKHOLM – Higher consumption of whole grains, fish, fiber, and omega-3 polyunsaturated fatty acids reduces deaths from all causes in people with type 2 diabetes, show new data.
Results from the systematic review and meta-analysis were presented at the annual meeting of the European Association for the Study of Diabetes by lead author Janett Barbaresko, PhD, a researcher from the German Diabetes Center in Düsseldorf.
Adding just one serving (around 20 g/day) of whole grains from foods such as brown bread, brown rice, or breakfast cereals was associated with about a 16% reduction in all-cause mortality, and each portion of fish consumed per week was associated with a 5% lower risk of all-cause mortality. In addition, eating 5 g/day of fiber was associated with a 14% reduction in all-cause mortality, and 0.1 g/day of omega-3 polyunsaturated fatty acids with a 13% reduction.
Diet also has role in improving survival in those with type 2 diabetes
Dr. Barbaresko explained that most dietary recommendations for people with type 2 diabetes are not evidence based or are derived from studies of the general population, and that the degree to which different components of diet are associated with all-cause mortality, or indeed the prevention of morbidity and mortality, remains unknown.
By way of example, she noted the American Diabetes Association 2022 guidelines for the prevention and management of diabetes complications advises limited intake of saturated and trans fatty acids, higher intake of polyunsaturated fatty acids, and following the Mediterranean or DASH (Dietary Approaches to Stop Hypertension) diets.
“Our findings show that dietary factors not only play a role in the prevention of type 2 diabetes, but also seem to be relevant for improving survival in people with diagnosed diabetes,” she said, adding that, “in particular, we found some key aspects of a healthy diet such as higher intakes of whole grains, fiber, fish, and omega-3 polyunsaturated fatty acids may improve survival of individuals with type 2 diabetes.”
She noted that individuals with type 2 diabetes are known to be more prone to circulatory diseases, dementia, cancer, and bone fractures, and that lifestyle modifications, including diet – with or without medications – underpin most management strategies.
“For the first time, we have provided a summary of all published studies on any dietary factor in association to all-cause mortality in individuals with type 2 diabetes,” said Dr. Barbaresko. “Moreover, the certainty of evidence has been evaluated for the first time.”
Matthias Schulze, MD, head of the German Institute of Human Nutrition, Berlin, moderated the session.
The new work “summarizes the available evidence, providing important dietary advice for patients with diabetes, for example, recommending whole grains,” he remarked. “However, the study also points to gaps in knowledge, so for many diet factors, we have either no or few studies, or study quality considered to be low, which calls for more research to fill the gap.”
High versus low intake of various dietary factors
The researchers performed meta-analyses based on published studies of all-cause mortality in individuals with type 2 diabetes aged 18 years and over, as associated with dietary patterns, macronutrients (carbohydrates, protein, fat), micronutrients (vitamins and minerals), secondary plant compounds (for example, polyphenols), and supplements.
Studies were conducted mainly in the United States and Europe with a mean follow-up of 10 years. Low and high intake were compared, and a dose-response relationship between different dietary factors and all-cause mortality was explored to generate summary risk ratios. The researchers also explored how the certainty of evidence was determined.
Decreased mortality from any cause was found for a higher intake of fish (SRR per serving/week, 0.95; over six studies); whole grain (SRR per 20 g/day, 0.84; two studies); fiber (SRR per 5 g/day, 0.86; three studies), and omega-3 polyunsaturated fatty acids (SRR per 0.1 g/day, 0.87; two studies).
A low certainty of evidence was found for an inverse association between all-cause mortality and vegetable consumption (SRR per 100 g/day, 0.88; two studies) and plant protein intake (SRR per 10 g/day, 0.91; three studies).
Eggs were associated with an increased risk of all-cause mortality (SRR per 10 g/day, 1.05; seven studies), as was dietary cholesterol (SRR per 300 mg/day, 1.19; two studies).
Regarding other dietary patterns, including the Mediterranean diet and low-carbohydrate diet, either no association was found and/or the evidence was very uncertain. Likewise, evidence was uncertain for foods including nuts, dairy, meat, sugar and sweets; macronutrients, including carbohydrates; and micronutrients, such as caffeine and vitamin D.
“With the Mediterranean diet, we saw an inverse association [with all-cause mortality] comparing high adherence with low adherence to the Mediterranean diet, but the certainty of evidence was very low, indicating a really uncertain meta-evidence,” remarked Dr. Barbaresko.
She concluded that a greater number of studies is needed to investigate the association of dietary factors with all-cause mortality in type 2 diabetes to strengthen the evidence for several other dietary factors. She also cautioned that meta-analyses are affected by unmeasured and residual confounding.
Dr. Barbaresko and Dr. Schulze reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Higher consumption of whole grains, fish, fiber, and omega-3 polyunsaturated fatty acids reduces deaths from all causes in people with type 2 diabetes, show new data.
Results from the systematic review and meta-analysis were presented at the annual meeting of the European Association for the Study of Diabetes by lead author Janett Barbaresko, PhD, a researcher from the German Diabetes Center in Düsseldorf.
Adding just one serving (around 20 g/day) of whole grains from foods such as brown bread, brown rice, or breakfast cereals was associated with about a 16% reduction in all-cause mortality, and each portion of fish consumed per week was associated with a 5% lower risk of all-cause mortality. In addition, eating 5 g/day of fiber was associated with a 14% reduction in all-cause mortality, and 0.1 g/day of omega-3 polyunsaturated fatty acids with a 13% reduction.
Diet also has role in improving survival in those with type 2 diabetes
Dr. Barbaresko explained that most dietary recommendations for people with type 2 diabetes are not evidence based or are derived from studies of the general population, and that the degree to which different components of diet are associated with all-cause mortality, or indeed the prevention of morbidity and mortality, remains unknown.
By way of example, she noted the American Diabetes Association 2022 guidelines for the prevention and management of diabetes complications advises limited intake of saturated and trans fatty acids, higher intake of polyunsaturated fatty acids, and following the Mediterranean or DASH (Dietary Approaches to Stop Hypertension) diets.
“Our findings show that dietary factors not only play a role in the prevention of type 2 diabetes, but also seem to be relevant for improving survival in people with diagnosed diabetes,” she said, adding that, “in particular, we found some key aspects of a healthy diet such as higher intakes of whole grains, fiber, fish, and omega-3 polyunsaturated fatty acids may improve survival of individuals with type 2 diabetes.”
She noted that individuals with type 2 diabetes are known to be more prone to circulatory diseases, dementia, cancer, and bone fractures, and that lifestyle modifications, including diet – with or without medications – underpin most management strategies.
“For the first time, we have provided a summary of all published studies on any dietary factor in association to all-cause mortality in individuals with type 2 diabetes,” said Dr. Barbaresko. “Moreover, the certainty of evidence has been evaluated for the first time.”
Matthias Schulze, MD, head of the German Institute of Human Nutrition, Berlin, moderated the session.
The new work “summarizes the available evidence, providing important dietary advice for patients with diabetes, for example, recommending whole grains,” he remarked. “However, the study also points to gaps in knowledge, so for many diet factors, we have either no or few studies, or study quality considered to be low, which calls for more research to fill the gap.”
High versus low intake of various dietary factors
The researchers performed meta-analyses based on published studies of all-cause mortality in individuals with type 2 diabetes aged 18 years and over, as associated with dietary patterns, macronutrients (carbohydrates, protein, fat), micronutrients (vitamins and minerals), secondary plant compounds (for example, polyphenols), and supplements.
Studies were conducted mainly in the United States and Europe with a mean follow-up of 10 years. Low and high intake were compared, and a dose-response relationship between different dietary factors and all-cause mortality was explored to generate summary risk ratios. The researchers also explored how the certainty of evidence was determined.
Decreased mortality from any cause was found for a higher intake of fish (SRR per serving/week, 0.95; over six studies); whole grain (SRR per 20 g/day, 0.84; two studies); fiber (SRR per 5 g/day, 0.86; three studies), and omega-3 polyunsaturated fatty acids (SRR per 0.1 g/day, 0.87; two studies).
A low certainty of evidence was found for an inverse association between all-cause mortality and vegetable consumption (SRR per 100 g/day, 0.88; two studies) and plant protein intake (SRR per 10 g/day, 0.91; three studies).
Eggs were associated with an increased risk of all-cause mortality (SRR per 10 g/day, 1.05; seven studies), as was dietary cholesterol (SRR per 300 mg/day, 1.19; two studies).
Regarding other dietary patterns, including the Mediterranean diet and low-carbohydrate diet, either no association was found and/or the evidence was very uncertain. Likewise, evidence was uncertain for foods including nuts, dairy, meat, sugar and sweets; macronutrients, including carbohydrates; and micronutrients, such as caffeine and vitamin D.
“With the Mediterranean diet, we saw an inverse association [with all-cause mortality] comparing high adherence with low adherence to the Mediterranean diet, but the certainty of evidence was very low, indicating a really uncertain meta-evidence,” remarked Dr. Barbaresko.
She concluded that a greater number of studies is needed to investigate the association of dietary factors with all-cause mortality in type 2 diabetes to strengthen the evidence for several other dietary factors. She also cautioned that meta-analyses are affected by unmeasured and residual confounding.
Dr. Barbaresko and Dr. Schulze reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Higher consumption of whole grains, fish, fiber, and omega-3 polyunsaturated fatty acids reduces deaths from all causes in people with type 2 diabetes, show new data.
Results from the systematic review and meta-analysis were presented at the annual meeting of the European Association for the Study of Diabetes by lead author Janett Barbaresko, PhD, a researcher from the German Diabetes Center in Düsseldorf.
Adding just one serving (around 20 g/day) of whole grains from foods such as brown bread, brown rice, or breakfast cereals was associated with about a 16% reduction in all-cause mortality, and each portion of fish consumed per week was associated with a 5% lower risk of all-cause mortality. In addition, eating 5 g/day of fiber was associated with a 14% reduction in all-cause mortality, and 0.1 g/day of omega-3 polyunsaturated fatty acids with a 13% reduction.
Diet also has role in improving survival in those with type 2 diabetes
Dr. Barbaresko explained that most dietary recommendations for people with type 2 diabetes are not evidence based or are derived from studies of the general population, and that the degree to which different components of diet are associated with all-cause mortality, or indeed the prevention of morbidity and mortality, remains unknown.
By way of example, she noted the American Diabetes Association 2022 guidelines for the prevention and management of diabetes complications advises limited intake of saturated and trans fatty acids, higher intake of polyunsaturated fatty acids, and following the Mediterranean or DASH (Dietary Approaches to Stop Hypertension) diets.
“Our findings show that dietary factors not only play a role in the prevention of type 2 diabetes, but also seem to be relevant for improving survival in people with diagnosed diabetes,” she said, adding that, “in particular, we found some key aspects of a healthy diet such as higher intakes of whole grains, fiber, fish, and omega-3 polyunsaturated fatty acids may improve survival of individuals with type 2 diabetes.”
She noted that individuals with type 2 diabetes are known to be more prone to circulatory diseases, dementia, cancer, and bone fractures, and that lifestyle modifications, including diet – with or without medications – underpin most management strategies.
“For the first time, we have provided a summary of all published studies on any dietary factor in association to all-cause mortality in individuals with type 2 diabetes,” said Dr. Barbaresko. “Moreover, the certainty of evidence has been evaluated for the first time.”
Matthias Schulze, MD, head of the German Institute of Human Nutrition, Berlin, moderated the session.
The new work “summarizes the available evidence, providing important dietary advice for patients with diabetes, for example, recommending whole grains,” he remarked. “However, the study also points to gaps in knowledge, so for many diet factors, we have either no or few studies, or study quality considered to be low, which calls for more research to fill the gap.”
High versus low intake of various dietary factors
The researchers performed meta-analyses based on published studies of all-cause mortality in individuals with type 2 diabetes aged 18 years and over, as associated with dietary patterns, macronutrients (carbohydrates, protein, fat), micronutrients (vitamins and minerals), secondary plant compounds (for example, polyphenols), and supplements.
Studies were conducted mainly in the United States and Europe with a mean follow-up of 10 years. Low and high intake were compared, and a dose-response relationship between different dietary factors and all-cause mortality was explored to generate summary risk ratios. The researchers also explored how the certainty of evidence was determined.
Decreased mortality from any cause was found for a higher intake of fish (SRR per serving/week, 0.95; over six studies); whole grain (SRR per 20 g/day, 0.84; two studies); fiber (SRR per 5 g/day, 0.86; three studies), and omega-3 polyunsaturated fatty acids (SRR per 0.1 g/day, 0.87; two studies).
A low certainty of evidence was found for an inverse association between all-cause mortality and vegetable consumption (SRR per 100 g/day, 0.88; two studies) and plant protein intake (SRR per 10 g/day, 0.91; three studies).
Eggs were associated with an increased risk of all-cause mortality (SRR per 10 g/day, 1.05; seven studies), as was dietary cholesterol (SRR per 300 mg/day, 1.19; two studies).
Regarding other dietary patterns, including the Mediterranean diet and low-carbohydrate diet, either no association was found and/or the evidence was very uncertain. Likewise, evidence was uncertain for foods including nuts, dairy, meat, sugar and sweets; macronutrients, including carbohydrates; and micronutrients, such as caffeine and vitamin D.
“With the Mediterranean diet, we saw an inverse association [with all-cause mortality] comparing high adherence with low adherence to the Mediterranean diet, but the certainty of evidence was very low, indicating a really uncertain meta-evidence,” remarked Dr. Barbaresko.
She concluded that a greater number of studies is needed to investigate the association of dietary factors with all-cause mortality in type 2 diabetes to strengthen the evidence for several other dietary factors. She also cautioned that meta-analyses are affected by unmeasured and residual confounding.
Dr. Barbaresko and Dr. Schulze reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Do lipid labs need to be fasting?
When I worked as a scribe prior to starting medical school, it was commonplace for patients to have fasting labs. I always felt terrible for the patients we saw late in the afternoon that had somehow fasted all day. For many other patients, there was the challenge of finding a time when they could return to have fasting labs drawn.
However, I have still observed instances when patients need to have fasting labs. We can look at an example case to better understand when and why patients do and do not need to fast prior to having their lipids checked.
Case
A 57-year-old woman presents for an annual wellness visit. She has been healthy this past year with no new concerns. Her blood pressure has been well controlled, and she continues on a statin for hyperlipidemia. She is due for annual labs. She ate breakfast this morning. Which of the following do you recommend?
A. Obtain lipids with her other blood work now.
B. Have her return tomorrow to obtain fasting labs.
In this situation, A is the correct answer. The patient is due for routine screening labs and there are no current indications that fasting labs are necessary.
Studies of fasting vs. nonfasting lipids
Sidhu and Naugler performed a cross-sectional analysis comparing lipid values at fasting intervals of 1 hour to 16 hours.1 They found the mean total cholesterol and HDL cholesterol values differed by greater than 2%. For LDL cholesterol, the values differed by less than 10% and triglycerides values differed by less than 20%. With this information, the researchers concluded fasting for routine lipids is generally unnecessary.
Mora and colleagues performed a post hoc prospective follow-up of a randomized control
trial to assess if nonfasting lipid measurements could cause misclassification of cardiovascular risk assessment.2 Based on 8,270 participants, coronary events associated with fasting vs. nonfasting lipid values were similar when adjusted hazard ratios were compared. They also found an agreement of 94.8% when classifying participants into ASCVD risk categories for fasting and nonfasting lipid values. These outcomes led them to support the use of nonfasting lipid labs for routine cardiovascular risk assessment.
Rahman and colleagues performed a systematic review and found the use of nonfasting lipid values can reliably determine statin management in most situations.3 Circumstances where fasting labs should be used are if patients have a genetic dyslipidemia, if patients have severe hypertriglyceridemia (greater than 500 mg/dL), and if patients have pancreatitis. Triglyceride values fluctuate the most between the fasting and nonfasting state as seen above from Sidhu and Naugler. This could impact triglyceride disorder management and the accuracy of LDL cholesterol estimation (calculated by the Friedewald equation: LDL cholesterol = total cholesterol – HDL cholesterol – triglycerides/5 in mg/dL).3
Benefits of nonfasting lipid labs
There are many benefits of nonfasting labs. For the patients, they do not have to come to their appointments hungry, we can reduce the risk of hypoglycemia for those with diabetes, and they do not have to come back at a later date if they ate something earlier in the day.
For the lab, we can improve efficiency and decrease early morning congestion when patients typically come in for fasting labs.
Lastly, for the provider, nonfasting labs can improve workflow and help decrease the number of patients lost to follow-up who were unable to complete fasting labs the same day as their appointment.
Summary
Patients do not need to fast prior to having lipid levels drawn for routine screening. Fasting labs should be considered for patients who have a genetic dyslipidemia or if there is concern for hypertriglyceridemia.
Per the ACC/AHA guidelines, nonfasting lipids can be used to assess ASCVD risk and to establish a baseline LDL cholesterol in adults 20 years and older. If a patient has nonfasting triglycerides greater than 400 mg/dL, repeat fasting lipids should be drawn to assess fasting triglycerides and to establish a baseline LDL cholesterol.4
Ms. Ervin is a fourth-year medical student at the University of Washington, Seattle. She has no conflicts to disclose. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, and he serves as third-year medical student clerkship director at the university. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Rahman F et al. Curr Atheroscler Rep. 2018;20(3):14. Published 2018 Feb 17.
2. Mora S et al. JAMA Intern Med. 2019;179(7):898-905.
3. Sidhu D and Naugler C. Arch Intern Med. 2012;172(22):1707-10.
4. Hoover LE. Am Fam Physician. 2019 May 1;99(9):589-91.
When I worked as a scribe prior to starting medical school, it was commonplace for patients to have fasting labs. I always felt terrible for the patients we saw late in the afternoon that had somehow fasted all day. For many other patients, there was the challenge of finding a time when they could return to have fasting labs drawn.
However, I have still observed instances when patients need to have fasting labs. We can look at an example case to better understand when and why patients do and do not need to fast prior to having their lipids checked.
Case
A 57-year-old woman presents for an annual wellness visit. She has been healthy this past year with no new concerns. Her blood pressure has been well controlled, and she continues on a statin for hyperlipidemia. She is due for annual labs. She ate breakfast this morning. Which of the following do you recommend?
A. Obtain lipids with her other blood work now.
B. Have her return tomorrow to obtain fasting labs.
In this situation, A is the correct answer. The patient is due for routine screening labs and there are no current indications that fasting labs are necessary.
Studies of fasting vs. nonfasting lipids
Sidhu and Naugler performed a cross-sectional analysis comparing lipid values at fasting intervals of 1 hour to 16 hours.1 They found the mean total cholesterol and HDL cholesterol values differed by greater than 2%. For LDL cholesterol, the values differed by less than 10% and triglycerides values differed by less than 20%. With this information, the researchers concluded fasting for routine lipids is generally unnecessary.
Mora and colleagues performed a post hoc prospective follow-up of a randomized control
trial to assess if nonfasting lipid measurements could cause misclassification of cardiovascular risk assessment.2 Based on 8,270 participants, coronary events associated with fasting vs. nonfasting lipid values were similar when adjusted hazard ratios were compared. They also found an agreement of 94.8% when classifying participants into ASCVD risk categories for fasting and nonfasting lipid values. These outcomes led them to support the use of nonfasting lipid labs for routine cardiovascular risk assessment.
Rahman and colleagues performed a systematic review and found the use of nonfasting lipid values can reliably determine statin management in most situations.3 Circumstances where fasting labs should be used are if patients have a genetic dyslipidemia, if patients have severe hypertriglyceridemia (greater than 500 mg/dL), and if patients have pancreatitis. Triglyceride values fluctuate the most between the fasting and nonfasting state as seen above from Sidhu and Naugler. This could impact triglyceride disorder management and the accuracy of LDL cholesterol estimation (calculated by the Friedewald equation: LDL cholesterol = total cholesterol – HDL cholesterol – triglycerides/5 in mg/dL).3
Benefits of nonfasting lipid labs
There are many benefits of nonfasting labs. For the patients, they do not have to come to their appointments hungry, we can reduce the risk of hypoglycemia for those with diabetes, and they do not have to come back at a later date if they ate something earlier in the day.
For the lab, we can improve efficiency and decrease early morning congestion when patients typically come in for fasting labs.
Lastly, for the provider, nonfasting labs can improve workflow and help decrease the number of patients lost to follow-up who were unable to complete fasting labs the same day as their appointment.
Summary
Patients do not need to fast prior to having lipid levels drawn for routine screening. Fasting labs should be considered for patients who have a genetic dyslipidemia or if there is concern for hypertriglyceridemia.
Per the ACC/AHA guidelines, nonfasting lipids can be used to assess ASCVD risk and to establish a baseline LDL cholesterol in adults 20 years and older. If a patient has nonfasting triglycerides greater than 400 mg/dL, repeat fasting lipids should be drawn to assess fasting triglycerides and to establish a baseline LDL cholesterol.4
Ms. Ervin is a fourth-year medical student at the University of Washington, Seattle. She has no conflicts to disclose. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, and he serves as third-year medical student clerkship director at the university. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Rahman F et al. Curr Atheroscler Rep. 2018;20(3):14. Published 2018 Feb 17.
2. Mora S et al. JAMA Intern Med. 2019;179(7):898-905.
3. Sidhu D and Naugler C. Arch Intern Med. 2012;172(22):1707-10.
4. Hoover LE. Am Fam Physician. 2019 May 1;99(9):589-91.
When I worked as a scribe prior to starting medical school, it was commonplace for patients to have fasting labs. I always felt terrible for the patients we saw late in the afternoon that had somehow fasted all day. For many other patients, there was the challenge of finding a time when they could return to have fasting labs drawn.
However, I have still observed instances when patients need to have fasting labs. We can look at an example case to better understand when and why patients do and do not need to fast prior to having their lipids checked.
Case
A 57-year-old woman presents for an annual wellness visit. She has been healthy this past year with no new concerns. Her blood pressure has been well controlled, and she continues on a statin for hyperlipidemia. She is due for annual labs. She ate breakfast this morning. Which of the following do you recommend?
A. Obtain lipids with her other blood work now.
B. Have her return tomorrow to obtain fasting labs.
In this situation, A is the correct answer. The patient is due for routine screening labs and there are no current indications that fasting labs are necessary.
Studies of fasting vs. nonfasting lipids
Sidhu and Naugler performed a cross-sectional analysis comparing lipid values at fasting intervals of 1 hour to 16 hours.1 They found the mean total cholesterol and HDL cholesterol values differed by greater than 2%. For LDL cholesterol, the values differed by less than 10% and triglycerides values differed by less than 20%. With this information, the researchers concluded fasting for routine lipids is generally unnecessary.
Mora and colleagues performed a post hoc prospective follow-up of a randomized control
trial to assess if nonfasting lipid measurements could cause misclassification of cardiovascular risk assessment.2 Based on 8,270 participants, coronary events associated with fasting vs. nonfasting lipid values were similar when adjusted hazard ratios were compared. They also found an agreement of 94.8% when classifying participants into ASCVD risk categories for fasting and nonfasting lipid values. These outcomes led them to support the use of nonfasting lipid labs for routine cardiovascular risk assessment.
Rahman and colleagues performed a systematic review and found the use of nonfasting lipid values can reliably determine statin management in most situations.3 Circumstances where fasting labs should be used are if patients have a genetic dyslipidemia, if patients have severe hypertriglyceridemia (greater than 500 mg/dL), and if patients have pancreatitis. Triglyceride values fluctuate the most between the fasting and nonfasting state as seen above from Sidhu and Naugler. This could impact triglyceride disorder management and the accuracy of LDL cholesterol estimation (calculated by the Friedewald equation: LDL cholesterol = total cholesterol – HDL cholesterol – triglycerides/5 in mg/dL).3
Benefits of nonfasting lipid labs
There are many benefits of nonfasting labs. For the patients, they do not have to come to their appointments hungry, we can reduce the risk of hypoglycemia for those with diabetes, and they do not have to come back at a later date if they ate something earlier in the day.
For the lab, we can improve efficiency and decrease early morning congestion when patients typically come in for fasting labs.
Lastly, for the provider, nonfasting labs can improve workflow and help decrease the number of patients lost to follow-up who were unable to complete fasting labs the same day as their appointment.
Summary
Patients do not need to fast prior to having lipid levels drawn for routine screening. Fasting labs should be considered for patients who have a genetic dyslipidemia or if there is concern for hypertriglyceridemia.
Per the ACC/AHA guidelines, nonfasting lipids can be used to assess ASCVD risk and to establish a baseline LDL cholesterol in adults 20 years and older. If a patient has nonfasting triglycerides greater than 400 mg/dL, repeat fasting lipids should be drawn to assess fasting triglycerides and to establish a baseline LDL cholesterol.4
Ms. Ervin is a fourth-year medical student at the University of Washington, Seattle. She has no conflicts to disclose. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, and he serves as third-year medical student clerkship director at the university. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Rahman F et al. Curr Atheroscler Rep. 2018;20(3):14. Published 2018 Feb 17.
2. Mora S et al. JAMA Intern Med. 2019;179(7):898-905.
3. Sidhu D and Naugler C. Arch Intern Med. 2012;172(22):1707-10.
4. Hoover LE. Am Fam Physician. 2019 May 1;99(9):589-91.
Waist-hip ratio beats BMI for predicting obesity’s mortality risk
STOCKHOLM – New evidence continues to show that alternative measures of adiposity than body mass index, such as waist-to-hip ratio, work better for predicting the risk a person with overweight or obesity faces from their excess weight.
A direct comparison of waist-to-hip ratio (WHR), body mass index (BMI), and fat mass index (FMI) in a total of more than 380,000 United Kingdom residents included in the UK Biobank showed that WHR had the strongest and most consistent relationship to all-cause death, compared with the other two measures, indicating that clinicians should pay more attention to adiposity distribution than they do to BMI when prioritizing obesity interventions, Irfan Khan said at the annual meeting of the European Association for the Study of Diabetes.
Although it’s likely “way too early” to fully replace BMI as a measure of adiposity, because it is so established in guidelines and in practice, it is now time to “use WHR as an adjunct to BMI” suggested Mr. Khan in an interview.
“A lot of work still needs to be done to translate WHR into practice, but I think it’s getting closer,” said Mr. Khan, a medical student at McMaster University, Hamilton, Ont., who performed his analyses in collaboration with a research team based primarily at McMaster.
Moving away from BMI-centric obesity
“This is a timely topic, because guidelines for treating people with obesity have depended so much on BMI. We want to go from a BMI-centric view to a view of obesity that depends more on disease burden,” commented Matthias Blüher, MD, professor of molecular endocrinology and head of the Obesity Outpatient Clinic for Adults at the University of Leipzig (Germany).
For example, the 2016 obesity management guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology called for a “complications-centric” approach to assessing and intervening in people with obesity rather than a “BMI-centric” approach.
But Dr. Blüher went a step further in an interview, adding that “waist-to-hip ratio is now outdated,” with adjusted measures of WHR such as waist-to-height ratio “considered a better proxy for all-cause death.” He also gave high marks to the Edmonton Obesity Staging System, which independently added to BMI as well as to a diagnosis of metabolic syndrome for predicting mortality in a sample from the U.S. National Health and Nutrition Examination Survey (NHANES). The Edmonton System also surpassed BMI for disease-severity staging using data from more than 23,000 Canadians with a BMI that denoted obesity.
1 standard deviation increase in WHR linked with a 41% increased mortality
The study reported by Mr. Khan used both epidemiologic and Mendelian randomization analyses on data collected from more than 380,000 U.K. residents included in the UK Biobank database to examine the statistical associations between BMI, FMI, and WHR and all-cause death. This showed that while BMI and FMI both had significant, independent associations with all-cause mortality, with hazard ratios of 1.14 for each 1 standard deviation increase in BMI and of 1.17 for each standard deviation increase in FMI, the link was a stronger 1.41 per standard deviation increase in WHR, he said.
Another analysis that divided the entire UK Biobank study cohort into 20 roughly similar subgroups by their BMI showed that WHR had the most consistent association across the BMI spectrum.
Further analyses showed that WHR also strongly and significantly linked with cardiovascular disease death and with other causes of death that were not cardiovascular, cancer-related, or associated with respiratory diseases. And the WHR link to all-cause mortality was strongest in men, and much less robust in women, likely because visceral adiposity is much more common among men, even compared with the postmenopausal women who predominate in the UK Biobank cohort.
One more feature of WHR that makes it an attractive metric is its relative ease of measurement, about as easy as BMI, Mr. Khan said.
The study received no commercial funding, and Mr. Khan had no disclosures. Dr. Blüher has been a consultant to or speaker on behalf of Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, and Sanofi.
STOCKHOLM – New evidence continues to show that alternative measures of adiposity than body mass index, such as waist-to-hip ratio, work better for predicting the risk a person with overweight or obesity faces from their excess weight.
A direct comparison of waist-to-hip ratio (WHR), body mass index (BMI), and fat mass index (FMI) in a total of more than 380,000 United Kingdom residents included in the UK Biobank showed that WHR had the strongest and most consistent relationship to all-cause death, compared with the other two measures, indicating that clinicians should pay more attention to adiposity distribution than they do to BMI when prioritizing obesity interventions, Irfan Khan said at the annual meeting of the European Association for the Study of Diabetes.
Although it’s likely “way too early” to fully replace BMI as a measure of adiposity, because it is so established in guidelines and in practice, it is now time to “use WHR as an adjunct to BMI” suggested Mr. Khan in an interview.
“A lot of work still needs to be done to translate WHR into practice, but I think it’s getting closer,” said Mr. Khan, a medical student at McMaster University, Hamilton, Ont., who performed his analyses in collaboration with a research team based primarily at McMaster.
Moving away from BMI-centric obesity
“This is a timely topic, because guidelines for treating people with obesity have depended so much on BMI. We want to go from a BMI-centric view to a view of obesity that depends more on disease burden,” commented Matthias Blüher, MD, professor of molecular endocrinology and head of the Obesity Outpatient Clinic for Adults at the University of Leipzig (Germany).
For example, the 2016 obesity management guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology called for a “complications-centric” approach to assessing and intervening in people with obesity rather than a “BMI-centric” approach.
But Dr. Blüher went a step further in an interview, adding that “waist-to-hip ratio is now outdated,” with adjusted measures of WHR such as waist-to-height ratio “considered a better proxy for all-cause death.” He also gave high marks to the Edmonton Obesity Staging System, which independently added to BMI as well as to a diagnosis of metabolic syndrome for predicting mortality in a sample from the U.S. National Health and Nutrition Examination Survey (NHANES). The Edmonton System also surpassed BMI for disease-severity staging using data from more than 23,000 Canadians with a BMI that denoted obesity.
1 standard deviation increase in WHR linked with a 41% increased mortality
The study reported by Mr. Khan used both epidemiologic and Mendelian randomization analyses on data collected from more than 380,000 U.K. residents included in the UK Biobank database to examine the statistical associations between BMI, FMI, and WHR and all-cause death. This showed that while BMI and FMI both had significant, independent associations with all-cause mortality, with hazard ratios of 1.14 for each 1 standard deviation increase in BMI and of 1.17 for each standard deviation increase in FMI, the link was a stronger 1.41 per standard deviation increase in WHR, he said.
Another analysis that divided the entire UK Biobank study cohort into 20 roughly similar subgroups by their BMI showed that WHR had the most consistent association across the BMI spectrum.
Further analyses showed that WHR also strongly and significantly linked with cardiovascular disease death and with other causes of death that were not cardiovascular, cancer-related, or associated with respiratory diseases. And the WHR link to all-cause mortality was strongest in men, and much less robust in women, likely because visceral adiposity is much more common among men, even compared with the postmenopausal women who predominate in the UK Biobank cohort.
One more feature of WHR that makes it an attractive metric is its relative ease of measurement, about as easy as BMI, Mr. Khan said.
The study received no commercial funding, and Mr. Khan had no disclosures. Dr. Blüher has been a consultant to or speaker on behalf of Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, and Sanofi.
STOCKHOLM – New evidence continues to show that alternative measures of adiposity than body mass index, such as waist-to-hip ratio, work better for predicting the risk a person with overweight or obesity faces from their excess weight.
A direct comparison of waist-to-hip ratio (WHR), body mass index (BMI), and fat mass index (FMI) in a total of more than 380,000 United Kingdom residents included in the UK Biobank showed that WHR had the strongest and most consistent relationship to all-cause death, compared with the other two measures, indicating that clinicians should pay more attention to adiposity distribution than they do to BMI when prioritizing obesity interventions, Irfan Khan said at the annual meeting of the European Association for the Study of Diabetes.
Although it’s likely “way too early” to fully replace BMI as a measure of adiposity, because it is so established in guidelines and in practice, it is now time to “use WHR as an adjunct to BMI” suggested Mr. Khan in an interview.
“A lot of work still needs to be done to translate WHR into practice, but I think it’s getting closer,” said Mr. Khan, a medical student at McMaster University, Hamilton, Ont., who performed his analyses in collaboration with a research team based primarily at McMaster.
Moving away from BMI-centric obesity
“This is a timely topic, because guidelines for treating people with obesity have depended so much on BMI. We want to go from a BMI-centric view to a view of obesity that depends more on disease burden,” commented Matthias Blüher, MD, professor of molecular endocrinology and head of the Obesity Outpatient Clinic for Adults at the University of Leipzig (Germany).
For example, the 2016 obesity management guidelines from the American Association of Clinical Endocrinologists and the American College of Endocrinology called for a “complications-centric” approach to assessing and intervening in people with obesity rather than a “BMI-centric” approach.
But Dr. Blüher went a step further in an interview, adding that “waist-to-hip ratio is now outdated,” with adjusted measures of WHR such as waist-to-height ratio “considered a better proxy for all-cause death.” He also gave high marks to the Edmonton Obesity Staging System, which independently added to BMI as well as to a diagnosis of metabolic syndrome for predicting mortality in a sample from the U.S. National Health and Nutrition Examination Survey (NHANES). The Edmonton System also surpassed BMI for disease-severity staging using data from more than 23,000 Canadians with a BMI that denoted obesity.
1 standard deviation increase in WHR linked with a 41% increased mortality
The study reported by Mr. Khan used both epidemiologic and Mendelian randomization analyses on data collected from more than 380,000 U.K. residents included in the UK Biobank database to examine the statistical associations between BMI, FMI, and WHR and all-cause death. This showed that while BMI and FMI both had significant, independent associations with all-cause mortality, with hazard ratios of 1.14 for each 1 standard deviation increase in BMI and of 1.17 for each standard deviation increase in FMI, the link was a stronger 1.41 per standard deviation increase in WHR, he said.
Another analysis that divided the entire UK Biobank study cohort into 20 roughly similar subgroups by their BMI showed that WHR had the most consistent association across the BMI spectrum.
Further analyses showed that WHR also strongly and significantly linked with cardiovascular disease death and with other causes of death that were not cardiovascular, cancer-related, or associated with respiratory diseases. And the WHR link to all-cause mortality was strongest in men, and much less robust in women, likely because visceral adiposity is much more common among men, even compared with the postmenopausal women who predominate in the UK Biobank cohort.
One more feature of WHR that makes it an attractive metric is its relative ease of measurement, about as easy as BMI, Mr. Khan said.
The study received no commercial funding, and Mr. Khan had no disclosures. Dr. Blüher has been a consultant to or speaker on behalf of Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, and Sanofi.
AT EASD 2022