User login
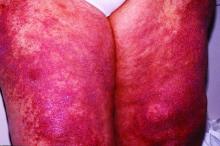
Clinical signs differ between children and adults with vasculitis
Researchers have found a link between age of diagnosis and various clinical characteristics and outcomes in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV).
The findings, presented at the annual meeting of the American College of Rheumatology, may have implications for research and treatment, especially in children.
AAV is a group of conditions characterized by the development of autoantibodies to the neutrophil proteins proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA).
The rare autoimmune condition can cause systemic inflammation and damage, sometimes permanent, to small- and medium-sized arteries. Clinical presentations vary and can include several organs, including skin, stomach, intestines, lung, and kidney, as well as airways in ear, nose, and throat.
Data limited on child vs. adult characteristics
AAV can be diagnosed in any decade of life, but clinical characteristics and outcomes often differ between children and adults, and data are limited. Studies often exclude children.
Lead author Jessica Bloom, MD, MSCS, a pediatric rheumatologist and assistant professor of pediatrics at Children’s Hospital Colorado, Aurora, and colleagues performed an analysis of patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) who were enrolled in the Vasculitis Clinical Research Consortium Longitudinal Studies from 2013 to 2021.
Patients with eosinophilic GPA (EGPA) were analyzed separately. Children and young adults with EGPA were combined because of the small sample size (n = 87).
The groups were sorted by the age they were diagnosed: under 18 years old, 18-40, 40-65, and older than 65.
More than 1,000 patients included
Dr. Bloom’s team analyzed data from 1,020 patients: 61 diagnosed as children, 240 as young adults, 560 as middle-aged adults, and 159 diagnosed as older adults. At all ages, about nine out of 10 patients were White.
They found 852 (84%) had GPA and 165 (16%) had MPA. The analysis also showed 893 (92%) of patients with ANCA results were ANCA positive: 637 (65%) with PR3-ANCA, 247 (25%) with MPO-ANCA, and 9 (1%) with both.
Differences between age groups included:
- Children experienced more subglottic stenosis and alveolar hemorrhage than adults with the condition.
- About half of patients diagnosed in childhood received both cyclophosphamide and rituximab. That rate decreased with increasing age of diagnosis to as low as 14% for those diagnosed in older adulthood.
- More females than males in all age groups were diagnosed with AAV, but the difference was most pronounced when diagnosed in childhood, and female predominance declined as age increased.
- Older adults experienced more neurologic disease and less musculoskeletal and sinus involvement.
Additionally, for those diagnosed after age 65, after adjusting for disease length and whether they were taking cyclophosphamide and/or rituximab, the Vasculitis Damage Index (VDI) and ANCA Vasculitis Index of Damage (AVID) scores were higher than for those diagnosed in childhood.
“However, these differences are no longer significant when medication toxicity and comorbidity-related items are excluded. Thus, differences in the VDI and AVID scores are driven by non–disease-specific damage,” Dr. Bloom said.
Bringing children into the clinical discussion
Dr. Bloom said in an interview that
For example, the findings that children have more subglottic stenosis and alveolar hemorrhage than adults “may warrant more aggressive therapy,” she said. Children also have different growth and psychosocial risk factors during their disease course and may live longer with the disease than those in older age groups.
“Our study helps to point out these differences and bring children into the discussion,” Dr. Bloom said. “It also recognizes that damage scores used in studies and care may not adequately assess disease across the lifespan, as they are largely influenced by items not specific to the disease but rather medication toxicity and comorbidities, such as osteoporosis, cataracts, and malignancy.”
Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at Hospital for Special Surgery, New York, said in an interview that the work highlights interesting information about the fact that disease features are skewed differently in children – “in particular the higher likelihood of upper airway [subglottic] disease, and potentially severe lower airway disease [alveolar hemorrhage].”
However, from a practical standpoint, Dr. Spiera said, “I am not sure that this will change our clinical approach to different patients, but the differences in disease features and even the sex differences in terms of who are afflicted with GPA [more often children and more likely to be female] may offer insights into disease pathogenesis.”
Dr. Bloom received funding from the Vasculitis Clinical Research Consortium and Vasculitis Foundation to conduct this work as a VCRC-VF fellow. Several coauthors reported various conflicts of interest with pharmaceutical companies. Dr. Spiera declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have found a link between age of diagnosis and various clinical characteristics and outcomes in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV).
The findings, presented at the annual meeting of the American College of Rheumatology, may have implications for research and treatment, especially in children.
AAV is a group of conditions characterized by the development of autoantibodies to the neutrophil proteins proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA).
The rare autoimmune condition can cause systemic inflammation and damage, sometimes permanent, to small- and medium-sized arteries. Clinical presentations vary and can include several organs, including skin, stomach, intestines, lung, and kidney, as well as airways in ear, nose, and throat.
Data limited on child vs. adult characteristics
AAV can be diagnosed in any decade of life, but clinical characteristics and outcomes often differ between children and adults, and data are limited. Studies often exclude children.
Lead author Jessica Bloom, MD, MSCS, a pediatric rheumatologist and assistant professor of pediatrics at Children’s Hospital Colorado, Aurora, and colleagues performed an analysis of patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) who were enrolled in the Vasculitis Clinical Research Consortium Longitudinal Studies from 2013 to 2021.
Patients with eosinophilic GPA (EGPA) were analyzed separately. Children and young adults with EGPA were combined because of the small sample size (n = 87).
The groups were sorted by the age they were diagnosed: under 18 years old, 18-40, 40-65, and older than 65.
More than 1,000 patients included
Dr. Bloom’s team analyzed data from 1,020 patients: 61 diagnosed as children, 240 as young adults, 560 as middle-aged adults, and 159 diagnosed as older adults. At all ages, about nine out of 10 patients were White.
They found 852 (84%) had GPA and 165 (16%) had MPA. The analysis also showed 893 (92%) of patients with ANCA results were ANCA positive: 637 (65%) with PR3-ANCA, 247 (25%) with MPO-ANCA, and 9 (1%) with both.
Differences between age groups included:
- Children experienced more subglottic stenosis and alveolar hemorrhage than adults with the condition.
- About half of patients diagnosed in childhood received both cyclophosphamide and rituximab. That rate decreased with increasing age of diagnosis to as low as 14% for those diagnosed in older adulthood.
- More females than males in all age groups were diagnosed with AAV, but the difference was most pronounced when diagnosed in childhood, and female predominance declined as age increased.
- Older adults experienced more neurologic disease and less musculoskeletal and sinus involvement.
Additionally, for those diagnosed after age 65, after adjusting for disease length and whether they were taking cyclophosphamide and/or rituximab, the Vasculitis Damage Index (VDI) and ANCA Vasculitis Index of Damage (AVID) scores were higher than for those diagnosed in childhood.
“However, these differences are no longer significant when medication toxicity and comorbidity-related items are excluded. Thus, differences in the VDI and AVID scores are driven by non–disease-specific damage,” Dr. Bloom said.
Bringing children into the clinical discussion
Dr. Bloom said in an interview that
For example, the findings that children have more subglottic stenosis and alveolar hemorrhage than adults “may warrant more aggressive therapy,” she said. Children also have different growth and psychosocial risk factors during their disease course and may live longer with the disease than those in older age groups.
“Our study helps to point out these differences and bring children into the discussion,” Dr. Bloom said. “It also recognizes that damage scores used in studies and care may not adequately assess disease across the lifespan, as they are largely influenced by items not specific to the disease but rather medication toxicity and comorbidities, such as osteoporosis, cataracts, and malignancy.”
Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at Hospital for Special Surgery, New York, said in an interview that the work highlights interesting information about the fact that disease features are skewed differently in children – “in particular the higher likelihood of upper airway [subglottic] disease, and potentially severe lower airway disease [alveolar hemorrhage].”
However, from a practical standpoint, Dr. Spiera said, “I am not sure that this will change our clinical approach to different patients, but the differences in disease features and even the sex differences in terms of who are afflicted with GPA [more often children and more likely to be female] may offer insights into disease pathogenesis.”
Dr. Bloom received funding from the Vasculitis Clinical Research Consortium and Vasculitis Foundation to conduct this work as a VCRC-VF fellow. Several coauthors reported various conflicts of interest with pharmaceutical companies. Dr. Spiera declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have found a link between age of diagnosis and various clinical characteristics and outcomes in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV).
The findings, presented at the annual meeting of the American College of Rheumatology, may have implications for research and treatment, especially in children.
AAV is a group of conditions characterized by the development of autoantibodies to the neutrophil proteins proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA).
The rare autoimmune condition can cause systemic inflammation and damage, sometimes permanent, to small- and medium-sized arteries. Clinical presentations vary and can include several organs, including skin, stomach, intestines, lung, and kidney, as well as airways in ear, nose, and throat.
Data limited on child vs. adult characteristics
AAV can be diagnosed in any decade of life, but clinical characteristics and outcomes often differ between children and adults, and data are limited. Studies often exclude children.
Lead author Jessica Bloom, MD, MSCS, a pediatric rheumatologist and assistant professor of pediatrics at Children’s Hospital Colorado, Aurora, and colleagues performed an analysis of patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) who were enrolled in the Vasculitis Clinical Research Consortium Longitudinal Studies from 2013 to 2021.
Patients with eosinophilic GPA (EGPA) were analyzed separately. Children and young adults with EGPA were combined because of the small sample size (n = 87).
The groups were sorted by the age they were diagnosed: under 18 years old, 18-40, 40-65, and older than 65.
More than 1,000 patients included
Dr. Bloom’s team analyzed data from 1,020 patients: 61 diagnosed as children, 240 as young adults, 560 as middle-aged adults, and 159 diagnosed as older adults. At all ages, about nine out of 10 patients were White.
They found 852 (84%) had GPA and 165 (16%) had MPA. The analysis also showed 893 (92%) of patients with ANCA results were ANCA positive: 637 (65%) with PR3-ANCA, 247 (25%) with MPO-ANCA, and 9 (1%) with both.
Differences between age groups included:
- Children experienced more subglottic stenosis and alveolar hemorrhage than adults with the condition.
- About half of patients diagnosed in childhood received both cyclophosphamide and rituximab. That rate decreased with increasing age of diagnosis to as low as 14% for those diagnosed in older adulthood.
- More females than males in all age groups were diagnosed with AAV, but the difference was most pronounced when diagnosed in childhood, and female predominance declined as age increased.
- Older adults experienced more neurologic disease and less musculoskeletal and sinus involvement.
Additionally, for those diagnosed after age 65, after adjusting for disease length and whether they were taking cyclophosphamide and/or rituximab, the Vasculitis Damage Index (VDI) and ANCA Vasculitis Index of Damage (AVID) scores were higher than for those diagnosed in childhood.
“However, these differences are no longer significant when medication toxicity and comorbidity-related items are excluded. Thus, differences in the VDI and AVID scores are driven by non–disease-specific damage,” Dr. Bloom said.
Bringing children into the clinical discussion
Dr. Bloom said in an interview that
For example, the findings that children have more subglottic stenosis and alveolar hemorrhage than adults “may warrant more aggressive therapy,” she said. Children also have different growth and psychosocial risk factors during their disease course and may live longer with the disease than those in older age groups.
“Our study helps to point out these differences and bring children into the discussion,” Dr. Bloom said. “It also recognizes that damage scores used in studies and care may not adequately assess disease across the lifespan, as they are largely influenced by items not specific to the disease but rather medication toxicity and comorbidities, such as osteoporosis, cataracts, and malignancy.”
Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at Hospital for Special Surgery, New York, said in an interview that the work highlights interesting information about the fact that disease features are skewed differently in children – “in particular the higher likelihood of upper airway [subglottic] disease, and potentially severe lower airway disease [alveolar hemorrhage].”
However, from a practical standpoint, Dr. Spiera said, “I am not sure that this will change our clinical approach to different patients, but the differences in disease features and even the sex differences in terms of who are afflicted with GPA [more often children and more likely to be female] may offer insights into disease pathogenesis.”
Dr. Bloom received funding from the Vasculitis Clinical Research Consortium and Vasculitis Foundation to conduct this work as a VCRC-VF fellow. Several coauthors reported various conflicts of interest with pharmaceutical companies. Dr. Spiera declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACR 2022
First recommendations for cancer screening in myositis issued
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
Rheumatic diseases and assisted reproductive technology: Things to consider
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
The field of “reproductive rheumatology” has received growing attention in recent years as we learn more about how autoimmune rheumatic diseases and their treatment affect women of reproductive age. In 2020, the American College of Rheumatology published a comprehensive guideline that includes recommendations and supporting evidence for managing issues related to reproductive health in patients with rheumatic diseases and has since launched an ongoing Reproductive Health Initiative, with the goal of translating established guidelines into practice through various education and awareness campaigns. One area addressed by the guideline that comes up commonly in practice but receives less attention and research is the use of assisted reproductive technology (ART) in patients with rheumatic diseases.
Literature is conflicting regarding whether patients with autoimmune rheumatic diseases are inherently at increased risk for infertility, defined as failure to achieve a clinical pregnancy after 12 months or more of regular unprotected intercourse, or subfertility, defined as a delay in conception. Regardless, several factors indirectly contribute to a disproportionate risk for infertility or subfertility in this patient population, including active inflammatory disease, reduced ovarian reserve, and medications.
Patients with subfertility or infertility who desire pregnancy may pursue ovulation induction with timed intercourse or intrauterine insemination, in vitro fertilization (IVF)/intracytoplasmic sperm injection with either embryo transfer, or gestational surrogacy. Those who require treatment with cyclophosphamide or who plan to defer pregnancy for whatever reason can opt for oocyte cryopreservation (colloquially known as “egg freezing”). For IVF and oocyte cryopreservation, controlled ovarian stimulation is typically the first step (except in unstimulated, or “natural cycle,” IVF).
Various protocols are used for ovarian stimulation and ovulation induction, the nuances of which are beyond the scope of this article. In general, ovarian stimulation involves gonadotropin therapy (follicle-stimulating hormone and/or human menopausal gonadotropin) administered via scheduled subcutaneous injections to stimulate follicular growth, as well as gonadotropin-releasing hormone (GnRH) agonists or antagonists to suppress luteinizing hormone, preventing ovulation. Adjunctive oral therapy (clomiphene citrate or letrozole, an aromatase inhibitor) may be used as well. The patient has frequent lab monitoring of hormone levels and transvaginal ultrasounds to measure follicle number and size and, when the timing is right, receives an “ovulation trigger” – either human chorionic gonadotropin or GnRH agonist, depending on the protocol. At this point, transvaginal ultrasound–guided egg retrieval is done under sedation. Recovered oocytes are then either frozen for later use or fertilized in the lab for embryo transfer. Lastly, exogenous hormones are often used: estrogen to support frozen embryo transfers and progesterone for so-called luteal phase support.
ART is not contraindicated in patients with autoimmune rheumatic diseases, but there may be additional factors to consider, particularly for those with systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and antiphospholipid antibodies (aPL) without clinical APS.
Ovarian stimulation elevates estrogen levels to varying degrees depending on the patient and the medications used. In all cases, though, peak levels are significantly lower than levels reached during pregnancy. It is well established that elevated estrogen – whether from hormone therapies or pregnancy – significantly increases thrombotic risk, even in healthy people. High-risk patients should receive low-molecular-weight heparin – a prophylactic dose for patients with either positive aPL without clinical APS (including those with SLE) or with obstetric APS, and a therapeutic dose for those with thrombotic APS – during ART procedures.
In patients with SLE, another concern is that increased estrogen will cause disease flare. One case series published in 2017 reported 37 patients with SLE and/or APS who underwent 97 IVF cycles, of which 8% were complicated by flare or thrombotic events. Notably, half of these complications occurred in patients who stopped prescribed therapies (immunomodulatory therapy in two patients with SLE, anticoagulation in two patients with APS) after failure to conceive. In a separate study from 2000 including 19 patients with SLE, APS, or high-titer aPL who underwent 68 IVF cycles, 19% of cycles in patients with SLE were complicated by flare, and no thrombotic events occurred in the cohort. The authors concluded that ovulation induction does not exacerbate SLE or APS. In these studies, the overall pregnancy rates were felt to be consistent with those achieved by the general population through IVF. Although obstetric complications, such as preeclampsia and preterm delivery, were reported in about half of the pregnancies described, these are known to occur more frequently in those with SLE and APS, especially when active disease or other risk factors are present. There are no large-scale, controlled studies evaluating ART outcomes in patients with autoimmune rheumatic diseases to date.
Finally, ovarian hyperstimulation syndrome (OHSS) is an increasingly rare but severe complication of ovarian stimulation. OHSS is characterized by capillary leak, fluid overload, and cytokine release syndrome and can lead to thromboembolic events. Comorbidities like hypertension and renal failure, which can go along with autoimmune rheumatic diseases, are risk factors for OHSS. The use of human chorionic gonadotropin to trigger ovulation is also associated with an increased risk for OHSS, so a GnRH agonist trigger may be preferable.
The ACR guideline recommends that individuals with any of these underlying conditions undergo ART only in expert centers. The ovarian stimulation protocol needs to be tailored to the individual patient to minimize risk and optimize outcomes. The overall goal when managing patients with autoimmune rheumatic diseases during ART is to establish and maintain disease control with pregnancy-compatible medications (when pregnancy is the goal). With adequate planning, appropriate treatment, and collaboration between obstetricians and rheumatologists, individuals with autoimmune rheumatic diseases can safely pursue ART and go on to have successful pregnancies.
Dr. Siegel is a 2022-2023 UCB Women’s Health rheumatology fellow in the rheumatology reproductive health program of the Barbara Volcker Center for Women and Rheumatic Diseases at Hospital for Special Surgery/Weill Cornell Medicine, New York. Her clinical and research focus is on reproductive health issues in individuals with rheumatic disease. Dr. Chan is an assistant professor at Weill Cornell Medical College and an attending physician at Hospital for Special Surgery and Memorial Sloan Kettering Cancer Center in New York. Before moving to New York City, she spent 7 years in private practice in Rhode Island and was a columnist for a monthly rheumatology publication, writing about the challenges of starting life as a full-fledged rheumatologist in a private practice. Follow Dr Chan on Twitter. Dr. Siegel and Dr. Chan disclosed no relevant financial relationships.
A version of this article – an editorial collaboration between Medscape and the Hospital for Special Surgery – first appeared on Medscape.com.
Metabolites may distinguish severe subtypes of PAH
, based on data from approximately 1,500 individuals.
The overall prognosis and therapeutic response for patients with pulmonary arterial hypertension associated with systemic sclerosis (SSc-PAH) tends to be worse than for patients with other types of PAH, such as idiopathic pulmonary arterial hypertension (IPAH), but the impact of different metabolite profiles among subtypes of disease has not been explored, wrote Mona Alotaibi, MD, of the University of California, San Diego, and colleagues.
“Recently, metabolic dysregulation has been proposed as a key mechanism by which IPAH and SSc-PAH differ and could control such disparities,” they noted. Clarifying the molecular mechanisms of SSc-PAH could inform management and treatment, they added.
In a study published in the journal Chest, the researchers sought to identify a bioactive lipid signature unique to SSc-PAH. They identified 400 patients with SSc-PAH and 1,082 with IPAH. An additional 100 patients with scleroderma but no PH and 44 patients with scleroderma who had PH were included for external validation. The mean ages of the patients with IPAH and SSc-PAH in the discovery and validation cohorts ranged from approximately 51 to 65 years; more than 75% of patients across the groups were women.
The researchers tested more than 700 bioactive lipid metabolites using liquid chromatography/mass spectrometry. They found five metabolites that distinguished SSc-PAH and IPAH that were significantly associated with markers of disease severity: 17-beta estradiol, novel Eic, nervonic acid, fatty acid esters of hydroxy fatty acids, and prostaglandin F2 alpha (PGF 2 alpha).
The biomarkers were increased in SSc-PAH patients compared to patients with SSC alone, which suggests that the biomarkers are related to PAH and not to scleroderma alone, the researchers noted.
In particular, nervonic acid was associated with worse functional capacity, in SSc-PAH patients, as were higher levels of 17-beta estradiol and prostaglandin F2 alpha. Also, 17-beta estradiol was associated with lower cardiac impairment (CI) and stroke volume index (SVI) in SSc-PAH patients, but higher SVI in IPAH patients. PGF 2 alpha was associated with lower CI and SVI and higher pulmonary vascular resistance in SSc-PAH and IPAH combined.
The study findings were limited by several factors including the inability to adjust for all potential confounders between IPAH and SSc-PAH, and the fact that a clear causal relationship could not be determined, the researchers noted. Inadequate statistical power to analyze SSc-PAH data was another limitation, and studies with detailed scleroderma phenotypes are needed to validate the results, they said.
However, the current study provides insight on the metabolic differences in SSc-PAH and the potential impact on disease pathology that may inform diagnosis, prognosis, and treatment strategies for SSc-PAH patients, they concluded.
The study was supported by the National Institutes of Health. Several individual investigators received support from organizations including the American Heart Association and the Chest Foundation, and from companies including Livanova, Equillium, Corvus, Bayer, and Actelion, but the authors had no relevant financial conflicts to disclose.
, based on data from approximately 1,500 individuals.
The overall prognosis and therapeutic response for patients with pulmonary arterial hypertension associated with systemic sclerosis (SSc-PAH) tends to be worse than for patients with other types of PAH, such as idiopathic pulmonary arterial hypertension (IPAH), but the impact of different metabolite profiles among subtypes of disease has not been explored, wrote Mona Alotaibi, MD, of the University of California, San Diego, and colleagues.
“Recently, metabolic dysregulation has been proposed as a key mechanism by which IPAH and SSc-PAH differ and could control such disparities,” they noted. Clarifying the molecular mechanisms of SSc-PAH could inform management and treatment, they added.
In a study published in the journal Chest, the researchers sought to identify a bioactive lipid signature unique to SSc-PAH. They identified 400 patients with SSc-PAH and 1,082 with IPAH. An additional 100 patients with scleroderma but no PH and 44 patients with scleroderma who had PH were included for external validation. The mean ages of the patients with IPAH and SSc-PAH in the discovery and validation cohorts ranged from approximately 51 to 65 years; more than 75% of patients across the groups were women.
The researchers tested more than 700 bioactive lipid metabolites using liquid chromatography/mass spectrometry. They found five metabolites that distinguished SSc-PAH and IPAH that were significantly associated with markers of disease severity: 17-beta estradiol, novel Eic, nervonic acid, fatty acid esters of hydroxy fatty acids, and prostaglandin F2 alpha (PGF 2 alpha).
The biomarkers were increased in SSc-PAH patients compared to patients with SSC alone, which suggests that the biomarkers are related to PAH and not to scleroderma alone, the researchers noted.
In particular, nervonic acid was associated with worse functional capacity, in SSc-PAH patients, as were higher levels of 17-beta estradiol and prostaglandin F2 alpha. Also, 17-beta estradiol was associated with lower cardiac impairment (CI) and stroke volume index (SVI) in SSc-PAH patients, but higher SVI in IPAH patients. PGF 2 alpha was associated with lower CI and SVI and higher pulmonary vascular resistance in SSc-PAH and IPAH combined.
The study findings were limited by several factors including the inability to adjust for all potential confounders between IPAH and SSc-PAH, and the fact that a clear causal relationship could not be determined, the researchers noted. Inadequate statistical power to analyze SSc-PAH data was another limitation, and studies with detailed scleroderma phenotypes are needed to validate the results, they said.
However, the current study provides insight on the metabolic differences in SSc-PAH and the potential impact on disease pathology that may inform diagnosis, prognosis, and treatment strategies for SSc-PAH patients, they concluded.
The study was supported by the National Institutes of Health. Several individual investigators received support from organizations including the American Heart Association and the Chest Foundation, and from companies including Livanova, Equillium, Corvus, Bayer, and Actelion, but the authors had no relevant financial conflicts to disclose.
, based on data from approximately 1,500 individuals.
The overall prognosis and therapeutic response for patients with pulmonary arterial hypertension associated with systemic sclerosis (SSc-PAH) tends to be worse than for patients with other types of PAH, such as idiopathic pulmonary arterial hypertension (IPAH), but the impact of different metabolite profiles among subtypes of disease has not been explored, wrote Mona Alotaibi, MD, of the University of California, San Diego, and colleagues.
“Recently, metabolic dysregulation has been proposed as a key mechanism by which IPAH and SSc-PAH differ and could control such disparities,” they noted. Clarifying the molecular mechanisms of SSc-PAH could inform management and treatment, they added.
In a study published in the journal Chest, the researchers sought to identify a bioactive lipid signature unique to SSc-PAH. They identified 400 patients with SSc-PAH and 1,082 with IPAH. An additional 100 patients with scleroderma but no PH and 44 patients with scleroderma who had PH were included for external validation. The mean ages of the patients with IPAH and SSc-PAH in the discovery and validation cohorts ranged from approximately 51 to 65 years; more than 75% of patients across the groups were women.
The researchers tested more than 700 bioactive lipid metabolites using liquid chromatography/mass spectrometry. They found five metabolites that distinguished SSc-PAH and IPAH that were significantly associated with markers of disease severity: 17-beta estradiol, novel Eic, nervonic acid, fatty acid esters of hydroxy fatty acids, and prostaglandin F2 alpha (PGF 2 alpha).
The biomarkers were increased in SSc-PAH patients compared to patients with SSC alone, which suggests that the biomarkers are related to PAH and not to scleroderma alone, the researchers noted.
In particular, nervonic acid was associated with worse functional capacity, in SSc-PAH patients, as were higher levels of 17-beta estradiol and prostaglandin F2 alpha. Also, 17-beta estradiol was associated with lower cardiac impairment (CI) and stroke volume index (SVI) in SSc-PAH patients, but higher SVI in IPAH patients. PGF 2 alpha was associated with lower CI and SVI and higher pulmonary vascular resistance in SSc-PAH and IPAH combined.
The study findings were limited by several factors including the inability to adjust for all potential confounders between IPAH and SSc-PAH, and the fact that a clear causal relationship could not be determined, the researchers noted. Inadequate statistical power to analyze SSc-PAH data was another limitation, and studies with detailed scleroderma phenotypes are needed to validate the results, they said.
However, the current study provides insight on the metabolic differences in SSc-PAH and the potential impact on disease pathology that may inform diagnosis, prognosis, and treatment strategies for SSc-PAH patients, they concluded.
The study was supported by the National Institutes of Health. Several individual investigators received support from organizations including the American Heart Association and the Chest Foundation, and from companies including Livanova, Equillium, Corvus, Bayer, and Actelion, but the authors had no relevant financial conflicts to disclose.
FROM CHEST
IVIG proves effective for dermatomyositis in phase 3 trial
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Evusheld PrEP may protect immunocompromised patients from severe COVID-19
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
FROM RMD OPEN
Breakthrough COVID studies lend support to use of new boosters in immunosuppressed patients
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
Hormones’ impact described in transgender rheumatology patients
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
Cell-killing cancer therapy treats lupus successfully
In a first-of-its-kind clinical trial, researchers in Germany used a cancer-killing cell therapy to successfully treat lupus in a small number of patients.
Their study, published online in Nature Medicine, included five patients with systemic lupus erythematosus (SLE). All of the patients were treated with chimeric antigen receptor (CAR) T-cell therapy, a treatment regularly used to kill cancer cells. Researchers harvested the patients’ immune cells and engineered them to destroy dysfunctional cells when infused back into the body.
The five patients – all of whom had an aggressive form of the autoimmune disease – underwent a single infusion of the experimental treatment. All five patients were able to stop their standard treatments for as long as 17 months following the therapy, the study found. The patients also stopped experiencing severe symptoms such as lung inflammation, fibrosis of the heart valves, arthritis, and fatigue. The patients have not relapsed.
“Our data reveal unexpected insights for a role of CAR T cells in nonmalignant diseases that could provide new opportunities for the treatment of autoimmune disease,” the study authors wrote.
Lupus is a chronic inflammatory disease in which the immune system attacks the body’s own cells. Both antibody-producing B and T cells in individuals with lupus become overactive, which can lead to a flare of symptoms that range from mild pain and fatigue to life-threatening inflammation and tissue damage. They are often treated with medications that deplete their B cells or change the way they function to help wipe out infected cells.
The approach used by the study researchers is similar to monoclonal antibody therapies that destroy dysfunctional B cells, such as rituximab (Rituxan and biosimilars) and obinutuzumab (Gazyva), according to Michael Belmont, MD, codirector of New York University’s Lupus Center and medical director of Bellevue Hospital Lupus Clinic, also in New York.
“Previously, this has been accomplished with monoclonal antibodies that target surface markers on B cells and results in their removal,” said Dr. Belmont, who was not connected to the study. “The report describes a novel approach that harnesses a patient’s own T cells, another type of white blood cell, to eliminate that patient’s own B cells.”
Preclinical studies involving mice previously showed that CAR T-cell therapy could help to reset the immune system. However, this latest study also found that patients did not need to continue any of their previous therapies, even after they regained their B cells about 4 months after the therapy.
“A deep depletion of CD19+ B cells and plasmablasts in the tissues could trigger an immune reset in SLE that could allow the cessation of immunosuppressive treatment,” said Mehrnaz Hojjati, MD, a rheumatologist and director of rheumatology operations at Loma Linda (Calif.) University Health. Dr. Hojjati was not affiliated with the study.
While the single-treatment therapy is promising, transfused T cells do carry risks. Some of the patients in the study experienced fever and muscle pain following the procedure, the authors noted. Dr. Belmont said more serious risks for this kind of therapy may include organ injury.
“This treatment can [also] increase incidence, for example, of pneumonia or shingles,” he said.
The study authors initially documented their work in a correspondence published in August 2021 in the New England Journal of Medicine. At that time, they reported that a 20-year-old woman with a severe refractory SLE went into remission following the treatment.
The five patients in the current study – four women and one man – were aged 18-24 years. All of the patients had previously been treated with several immunosuppressive medications, the study authors noted.
“This is an exciting approach, but many more patients need to be treated to really understand the efficacy and safety,” Dr. Belmont said.
Experts, including Dr. Belmont, also said the procedure is also costly and requires access to labs that can engineer a patient’s own T cells after they’ve been donated.
“The entire process must maintain sterility to avoid contamination, which would be harmful when reinfused into the patient,” he said.
According to Arthur Kavanaugh, MD, professor of medicine at UC San Diego Health, this form of therapy may be an option for severe refractory patients who have not responded well to other more established therapies.
“[It’s] exciting data, but very intense and so not likely to be something for an average patient in the near future,” said Dr. Kavanaugh, who was not affiliated with the study.
The study authors say they intend to create a larger trial to further explore which type of patient may benefit the most from this treatment, and for how long.
The study was supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union, and the Innovative Medicines Initiative–funded project, Rheuma Tolerance for Cure. The study received no commercial funding, and the authors said they had no competing interests related to the study. None of the experts interviewed reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first-of-its-kind clinical trial, researchers in Germany used a cancer-killing cell therapy to successfully treat lupus in a small number of patients.
Their study, published online in Nature Medicine, included five patients with systemic lupus erythematosus (SLE). All of the patients were treated with chimeric antigen receptor (CAR) T-cell therapy, a treatment regularly used to kill cancer cells. Researchers harvested the patients’ immune cells and engineered them to destroy dysfunctional cells when infused back into the body.
The five patients – all of whom had an aggressive form of the autoimmune disease – underwent a single infusion of the experimental treatment. All five patients were able to stop their standard treatments for as long as 17 months following the therapy, the study found. The patients also stopped experiencing severe symptoms such as lung inflammation, fibrosis of the heart valves, arthritis, and fatigue. The patients have not relapsed.
“Our data reveal unexpected insights for a role of CAR T cells in nonmalignant diseases that could provide new opportunities for the treatment of autoimmune disease,” the study authors wrote.
Lupus is a chronic inflammatory disease in which the immune system attacks the body’s own cells. Both antibody-producing B and T cells in individuals with lupus become overactive, which can lead to a flare of symptoms that range from mild pain and fatigue to life-threatening inflammation and tissue damage. They are often treated with medications that deplete their B cells or change the way they function to help wipe out infected cells.
The approach used by the study researchers is similar to monoclonal antibody therapies that destroy dysfunctional B cells, such as rituximab (Rituxan and biosimilars) and obinutuzumab (Gazyva), according to Michael Belmont, MD, codirector of New York University’s Lupus Center and medical director of Bellevue Hospital Lupus Clinic, also in New York.
“Previously, this has been accomplished with monoclonal antibodies that target surface markers on B cells and results in their removal,” said Dr. Belmont, who was not connected to the study. “The report describes a novel approach that harnesses a patient’s own T cells, another type of white blood cell, to eliminate that patient’s own B cells.”
Preclinical studies involving mice previously showed that CAR T-cell therapy could help to reset the immune system. However, this latest study also found that patients did not need to continue any of their previous therapies, even after they regained their B cells about 4 months after the therapy.
“A deep depletion of CD19+ B cells and plasmablasts in the tissues could trigger an immune reset in SLE that could allow the cessation of immunosuppressive treatment,” said Mehrnaz Hojjati, MD, a rheumatologist and director of rheumatology operations at Loma Linda (Calif.) University Health. Dr. Hojjati was not affiliated with the study.
While the single-treatment therapy is promising, transfused T cells do carry risks. Some of the patients in the study experienced fever and muscle pain following the procedure, the authors noted. Dr. Belmont said more serious risks for this kind of therapy may include organ injury.
“This treatment can [also] increase incidence, for example, of pneumonia or shingles,” he said.
The study authors initially documented their work in a correspondence published in August 2021 in the New England Journal of Medicine. At that time, they reported that a 20-year-old woman with a severe refractory SLE went into remission following the treatment.
The five patients in the current study – four women and one man – were aged 18-24 years. All of the patients had previously been treated with several immunosuppressive medications, the study authors noted.
“This is an exciting approach, but many more patients need to be treated to really understand the efficacy and safety,” Dr. Belmont said.
Experts, including Dr. Belmont, also said the procedure is also costly and requires access to labs that can engineer a patient’s own T cells after they’ve been donated.
“The entire process must maintain sterility to avoid contamination, which would be harmful when reinfused into the patient,” he said.
According to Arthur Kavanaugh, MD, professor of medicine at UC San Diego Health, this form of therapy may be an option for severe refractory patients who have not responded well to other more established therapies.
“[It’s] exciting data, but very intense and so not likely to be something for an average patient in the near future,” said Dr. Kavanaugh, who was not affiliated with the study.
The study authors say they intend to create a larger trial to further explore which type of patient may benefit the most from this treatment, and for how long.
The study was supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union, and the Innovative Medicines Initiative–funded project, Rheuma Tolerance for Cure. The study received no commercial funding, and the authors said they had no competing interests related to the study. None of the experts interviewed reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first-of-its-kind clinical trial, researchers in Germany used a cancer-killing cell therapy to successfully treat lupus in a small number of patients.
Their study, published online in Nature Medicine, included five patients with systemic lupus erythematosus (SLE). All of the patients were treated with chimeric antigen receptor (CAR) T-cell therapy, a treatment regularly used to kill cancer cells. Researchers harvested the patients’ immune cells and engineered them to destroy dysfunctional cells when infused back into the body.
The five patients – all of whom had an aggressive form of the autoimmune disease – underwent a single infusion of the experimental treatment. All five patients were able to stop their standard treatments for as long as 17 months following the therapy, the study found. The patients also stopped experiencing severe symptoms such as lung inflammation, fibrosis of the heart valves, arthritis, and fatigue. The patients have not relapsed.
“Our data reveal unexpected insights for a role of CAR T cells in nonmalignant diseases that could provide new opportunities for the treatment of autoimmune disease,” the study authors wrote.
Lupus is a chronic inflammatory disease in which the immune system attacks the body’s own cells. Both antibody-producing B and T cells in individuals with lupus become overactive, which can lead to a flare of symptoms that range from mild pain and fatigue to life-threatening inflammation and tissue damage. They are often treated with medications that deplete their B cells or change the way they function to help wipe out infected cells.
The approach used by the study researchers is similar to monoclonal antibody therapies that destroy dysfunctional B cells, such as rituximab (Rituxan and biosimilars) and obinutuzumab (Gazyva), according to Michael Belmont, MD, codirector of New York University’s Lupus Center and medical director of Bellevue Hospital Lupus Clinic, also in New York.
“Previously, this has been accomplished with monoclonal antibodies that target surface markers on B cells and results in their removal,” said Dr. Belmont, who was not connected to the study. “The report describes a novel approach that harnesses a patient’s own T cells, another type of white blood cell, to eliminate that patient’s own B cells.”
Preclinical studies involving mice previously showed that CAR T-cell therapy could help to reset the immune system. However, this latest study also found that patients did not need to continue any of their previous therapies, even after they regained their B cells about 4 months after the therapy.
“A deep depletion of CD19+ B cells and plasmablasts in the tissues could trigger an immune reset in SLE that could allow the cessation of immunosuppressive treatment,” said Mehrnaz Hojjati, MD, a rheumatologist and director of rheumatology operations at Loma Linda (Calif.) University Health. Dr. Hojjati was not affiliated with the study.
While the single-treatment therapy is promising, transfused T cells do carry risks. Some of the patients in the study experienced fever and muscle pain following the procedure, the authors noted. Dr. Belmont said more serious risks for this kind of therapy may include organ injury.
“This treatment can [also] increase incidence, for example, of pneumonia or shingles,” he said.
The study authors initially documented their work in a correspondence published in August 2021 in the New England Journal of Medicine. At that time, they reported that a 20-year-old woman with a severe refractory SLE went into remission following the treatment.
The five patients in the current study – four women and one man – were aged 18-24 years. All of the patients had previously been treated with several immunosuppressive medications, the study authors noted.
“This is an exciting approach, but many more patients need to be treated to really understand the efficacy and safety,” Dr. Belmont said.
Experts, including Dr. Belmont, also said the procedure is also costly and requires access to labs that can engineer a patient’s own T cells after they’ve been donated.
“The entire process must maintain sterility to avoid contamination, which would be harmful when reinfused into the patient,” he said.
According to Arthur Kavanaugh, MD, professor of medicine at UC San Diego Health, this form of therapy may be an option for severe refractory patients who have not responded well to other more established therapies.
“[It’s] exciting data, but very intense and so not likely to be something for an average patient in the near future,” said Dr. Kavanaugh, who was not affiliated with the study.
The study authors say they intend to create a larger trial to further explore which type of patient may benefit the most from this treatment, and for how long.
The study was supported by the German Research Foundation, the German Federal Ministry of Education and Research, the European Union, and the Innovative Medicines Initiative–funded project, Rheuma Tolerance for Cure. The study received no commercial funding, and the authors said they had no competing interests related to the study. None of the experts interviewed reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Autoimmune diseases linked to spike in post-MI events
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
, in a large propensity-matched analysis.
At a median of 2 years after their MI, Medicare beneficiaries with an IMID had adjusted risks that were:
- 15% higher for all-cause death (hazard ratio, 1.15);
- 12% higher for heart failure (HR, 1.12);
- 8% higher for recurrent MI (HR, 1.08); and
- 6% higher risk for coronary reintervention (HR, 1.06; P < .05 for all).
In addition, interventions known to improve outcomes in this context, such as coronary revascularization, were less common in patients with IMID.
“This could be because they usually are sicker and have more risk factors when they present, like kidney disease, so maybe they’re not eligible for the therapy. But by itself, it was surprising they’re not offered these interventions as common[ly] as people who don’t have the disease,” Amgad Mentias, MD, a clinical cardiologist at the Cleveland Clinic, said in an interview.
The study was published Sept. 14 in the Journal of the American Heart Association, with Dr. Mentias as senior author and Heba Wassif, MD, MPH, also with Cleveland Clinic, as first author.
IMIDs, such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel disease, are known to be associated with significantly higher cardiovascular disease (CVD) risk due to a greater prevalence of traditional CVD risk factors and chronic systemic inflammation.
Certain disease-modifying agents may also increase patients’ cardiovascular risk. This has been a long-simmering issue for the arthritis and ulcerative colitis drug tofacitinib (Xeljanz, Xeljanz XR), resulting in an updated boxed warning in 2021.
Many of these patients also have joint disease, pain, and fatigue, which can limit physical activity, Dr. Mentias said. “So these small nuances of how to manage these patients, or balance between controlling the inflammation but also improv[ing] cardiac risk factors, is not an easy task.”
Evidence regarding post-MI events has been inconsistent and limited to smaller single-center studies, he said. After propensity-score matching, the present study included 59,820 patients with and 178,547 patients without rheumatic IMIDs followed for a maximum of 6 years.
They were drawn from a cohort of 1.6 million persons aged 65 or older in the Medicare Provider Analysis and Review (MedPAR) file who had been admitted for an MI between 2014 and 2019. Of these, 60,072 had a prior history of rheumatic IMIDs, most commonly rheumatoid arthritis (77.8%), followed by systemic lupus erythematosus (12.2%), psoriasis (5.1%), systemic sclerosis (2.8%), and myositis/dermatomyositis (1.8%).
Patients with an IMID were more often women; had a higher prevalence of valve disease, pulmonary hypertension, hypothyroidism, and anemia; and were more likely to present with non–ST-segment MI (NSTEMI).
Rates of coronary angiography (46.1% vs. 51.5%), percutaneous coronary intervention (31.6% vs. 33.6%), and coronary artery bypass grafting (7.7% vs. 10.7%) were significantly lower in patients with IMIDs who had NSTEMI, compared with patients without an IMID who had NSTEMI. Rates of these interventions were also lower in patients with IMIDs who presented with STEMI versus their peers without an IMID, at 78.2% vs. 80.7%, 70.2% vs. 71.5%, and 4.9% vs. 6.4%, respectively.
Dr. Mentias pointed out that the emerging subspecialty of cardiorheumatology is gaining traction, especially at large hospitals and academic centers, but that less than one-third of persons in the United States with an IMID are likely to be under the care of such specialists.
“It’s important before developing an MI to try and control the different risk factors and improve the risk profile for these patients as much as possible by both specialties, and then, after an unfortunate event like MI happens, it’s important to make sure we offer therapies and treatments that are known to improve outcomes,” he said.
Commenting for this article, Jon Tyler Giles, MD, a clinical researcher who focuses on cardiovascular diseases in rheumatology at Columbia University Vagelos College of Physicians and Surgeons, New York, said that “at least for rheumatoid arthritis, this is something that we already knew. People with rheumatic arthritis, when they have a heart attack, are less likely to get the standard kind of treatments and have worse outcomes. This is a little larger sample, but it’s not a surprise, not a surprise at all.”
He noted that the study could have answered questions regarding potential drivers, but “they didn’t dig down into any of the factors that were associated with the poorer outcomes in the patients with rheumatoid arthritis and lupus and scleroderma.”
Indeed, the investigators acknowledge that the study lacked information on coronary anatomy, duration and severity of the autoimmune disease, imaging data, and medications such as anti-inflammatory or immune-targeted therapies.
Dr. Giles highlighted several factors that can contribute to a poorer post-MI prognosis in patients with rheumatic diseases; these include frailty, being more hypercoaguable, increased rates of myocardial dysfunction and other heart and blood vessel diseases, and chronic treatment with steroids and nonsteroidal anti-inflammatory drugs that often interferes with anticoagulation after a MI or when putting in a stent. “So, there’s lot of moving parts, and not one single thing that is likely the answer.”
In addition, he said, “there’s always going to be a portion of these patients who, despite doing the best that we can with treatment, are going to have very severe disease. That may or may not be the subset of patients that did the worst, but likely they’re overrepresented in those patients.”
Finally, the inability to move the needle may lie with the lack of evidence-based screening and management guidelines for cardiovascular disease in any rheumatic disease, Dr. Giles observed. “There’s no guideline for us to use to decide who needs screening over and above what’s recommended for the general population, and then, even if you do screen, what do you do other than what you would normally?”
Rheumatologists are often reluctant to take up the cardiovascular screening side of things because visits are short, and a lot of that time is spent trying to manage the inflammatory components of a patient’s disease, he said. There’s also a barrier in getting some patients to add a cardiologist to the mix of physicians they already see, especially if they don’t have any symptoms.
“If someone has had an event, it’s a lot easier for people to be convinced to go see the cardiologist, obviously, but prior to having an event, the preventative side of things is something that often gets missed or goes to the wayside,” Dr. Giles said.
The study was partly funded by philanthropic gifts by the Haslam family, Bailey family, and Khouri family to the Cleveland Clinic for coauthor Dr. Milind Desai’s research. Dr. Desai is a consultant for Medtronic and Bristol Myers Squibb and serves on an executive steering committee of a BMS-sponsored trial. The remaining authors report having no relevant disclosures. Dr. Giles is a consultant on drug cardiovascular safety for Pfizer, AbbVie, and Eli Lilly.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION