User login
CAR T-Cell Therapy: Promising Treatments in Development for DLBCL
There have been several recent developments in the treatment of B-cell lymphoma; however, one of the most significant advances has been the development of chimeric antigen receptor (CAR) T-cell therapy. CAR T-cell therapy is a type of personalized immunotherapy that can help cure some people with aggressive non-Hodgkin lymphoma (NHL), including diffuse large B-cell lymphoma (DLBCL), the most common form of aggressive NHL. CAR T-cell therapy has revolutionized the treatment of hematologic malignancies over the past 5 years, with impressive response rates and durable remissions for patients who previously had no viable options. This strategy is highly effective in patients with relapsed/refractory DLBCL, as well as mantle cell lymphoma, follicular lymphoma, acute lymphoblastic leukemia (ALL), and multiple myeloma, as evidenced by recent regulatory approvals.
In 2021, the FDA also approved lisocabtagene maraleucel (liso-cel), a new CAR T-cell therapy for the treatment of adults with relapsed or refractory (nonresponsive) large B-cell lymphoma (LBCL) have been treated with at least 2 prior lines of therapy. These products have design differences, including differences in the costimulatory domain, mechanism of gene/transgene delivery, ability for cryopreservation, and need for T-cell selection.
The CAR T-cell therapy axi-cel demonstrated superior results in the ZUMA-7 clinical trial, which compared CAR T-cell therapy directly to traditional chemotherapy with intended autologous stem cell transplant (ASCT). About 55% of patients were still alive 4 years after receiving axi-cel, compared with 46% of those who initially received the standard treatment for relapsed disease. Based on these results, axi-cel is now the preferred treatment for people whose DLBCL has recurred with 12 months of front-line treatment or who are resistant to standard initial treatment.
Additionally, the BELINDA trial was a randomized phase 3 trial that compared CAR T-cell therapy with liso-cel with second-line chemotherapy with planned ASCT. Like ZUMA-7, this study also demonstrated an improvement in progression-free survival (PFS) compared to standard treatment. As such, CAR T-cell therapy represents the new standard of care for second-line treatment in appropriate patients with refractory or early relapsing LBCL.
There have been several other recent studies on the use of CAR T-cell therapy for B-cell lymphoma. One study, published in Blood Advances (2023), found that receiving a greater number of therapies prior to CAR T-cell therapy is associated with poorer outcomes in patients with aggressive relapsed/refractory B-cell NHL. The study, which included 514 patients from 13 centers treated with CAR-T for aggressive B-cell NHL between 2015 and 2021, found that a greater number of lines of therapy before CAR-T apheresis and bridging therapy were predictive of inferior PFS and overall survival.
Another study compared 2 CD19-targeting CAR T-cell treatments, axi-cel and tisa-cel, with ASCT in the second line setting for LBCL. The study found that axi-cel was superior to ASCT, with longer median event-free survival and a higher response rate. However, tisa-cel was not found to be superior to ASCT. Further studies will be needed to definitively characterize the relative benefits of CAR-T cell therapies and standard second-line treatments for different subgroups of patients with LBCL.
An increasing number of effective targeted agents for DLBCL, including novel monoclonal antibodies (tafasitamab) and antibody-drug conjugates (polatuzumab vedotin and loncastuximab teserine), are being used in earlier lines of therapy. Additionally, 2 anti-CD20 bispecific antibodies (epcoritamab and glofitamab) have gained approval for relapsed/refractory DLBCL due to high response rates. Future studies will be needed to determine if treatment with these agents can produce durable remissions like that of CAR-T cell therapy.
There have been several recent developments in the treatment of B-cell lymphoma; however, one of the most significant advances has been the development of chimeric antigen receptor (CAR) T-cell therapy. CAR T-cell therapy is a type of personalized immunotherapy that can help cure some people with aggressive non-Hodgkin lymphoma (NHL), including diffuse large B-cell lymphoma (DLBCL), the most common form of aggressive NHL. CAR T-cell therapy has revolutionized the treatment of hematologic malignancies over the past 5 years, with impressive response rates and durable remissions for patients who previously had no viable options. This strategy is highly effective in patients with relapsed/refractory DLBCL, as well as mantle cell lymphoma, follicular lymphoma, acute lymphoblastic leukemia (ALL), and multiple myeloma, as evidenced by recent regulatory approvals.
In 2021, the FDA also approved lisocabtagene maraleucel (liso-cel), a new CAR T-cell therapy for the treatment of adults with relapsed or refractory (nonresponsive) large B-cell lymphoma (LBCL) have been treated with at least 2 prior lines of therapy. These products have design differences, including differences in the costimulatory domain, mechanism of gene/transgene delivery, ability for cryopreservation, and need for T-cell selection.
The CAR T-cell therapy axi-cel demonstrated superior results in the ZUMA-7 clinical trial, which compared CAR T-cell therapy directly to traditional chemotherapy with intended autologous stem cell transplant (ASCT). About 55% of patients were still alive 4 years after receiving axi-cel, compared with 46% of those who initially received the standard treatment for relapsed disease. Based on these results, axi-cel is now the preferred treatment for people whose DLBCL has recurred with 12 months of front-line treatment or who are resistant to standard initial treatment.
Additionally, the BELINDA trial was a randomized phase 3 trial that compared CAR T-cell therapy with liso-cel with second-line chemotherapy with planned ASCT. Like ZUMA-7, this study also demonstrated an improvement in progression-free survival (PFS) compared to standard treatment. As such, CAR T-cell therapy represents the new standard of care for second-line treatment in appropriate patients with refractory or early relapsing LBCL.
There have been several other recent studies on the use of CAR T-cell therapy for B-cell lymphoma. One study, published in Blood Advances (2023), found that receiving a greater number of therapies prior to CAR T-cell therapy is associated with poorer outcomes in patients with aggressive relapsed/refractory B-cell NHL. The study, which included 514 patients from 13 centers treated with CAR-T for aggressive B-cell NHL between 2015 and 2021, found that a greater number of lines of therapy before CAR-T apheresis and bridging therapy were predictive of inferior PFS and overall survival.
Another study compared 2 CD19-targeting CAR T-cell treatments, axi-cel and tisa-cel, with ASCT in the second line setting for LBCL. The study found that axi-cel was superior to ASCT, with longer median event-free survival and a higher response rate. However, tisa-cel was not found to be superior to ASCT. Further studies will be needed to definitively characterize the relative benefits of CAR-T cell therapies and standard second-line treatments for different subgroups of patients with LBCL.
An increasing number of effective targeted agents for DLBCL, including novel monoclonal antibodies (tafasitamab) and antibody-drug conjugates (polatuzumab vedotin and loncastuximab teserine), are being used in earlier lines of therapy. Additionally, 2 anti-CD20 bispecific antibodies (epcoritamab and glofitamab) have gained approval for relapsed/refractory DLBCL due to high response rates. Future studies will be needed to determine if treatment with these agents can produce durable remissions like that of CAR-T cell therapy.
There have been several recent developments in the treatment of B-cell lymphoma; however, one of the most significant advances has been the development of chimeric antigen receptor (CAR) T-cell therapy. CAR T-cell therapy is a type of personalized immunotherapy that can help cure some people with aggressive non-Hodgkin lymphoma (NHL), including diffuse large B-cell lymphoma (DLBCL), the most common form of aggressive NHL. CAR T-cell therapy has revolutionized the treatment of hematologic malignancies over the past 5 years, with impressive response rates and durable remissions for patients who previously had no viable options. This strategy is highly effective in patients with relapsed/refractory DLBCL, as well as mantle cell lymphoma, follicular lymphoma, acute lymphoblastic leukemia (ALL), and multiple myeloma, as evidenced by recent regulatory approvals.
In 2021, the FDA also approved lisocabtagene maraleucel (liso-cel), a new CAR T-cell therapy for the treatment of adults with relapsed or refractory (nonresponsive) large B-cell lymphoma (LBCL) have been treated with at least 2 prior lines of therapy. These products have design differences, including differences in the costimulatory domain, mechanism of gene/transgene delivery, ability for cryopreservation, and need for T-cell selection.
The CAR T-cell therapy axi-cel demonstrated superior results in the ZUMA-7 clinical trial, which compared CAR T-cell therapy directly to traditional chemotherapy with intended autologous stem cell transplant (ASCT). About 55% of patients were still alive 4 years after receiving axi-cel, compared with 46% of those who initially received the standard treatment for relapsed disease. Based on these results, axi-cel is now the preferred treatment for people whose DLBCL has recurred with 12 months of front-line treatment or who are resistant to standard initial treatment.
Additionally, the BELINDA trial was a randomized phase 3 trial that compared CAR T-cell therapy with liso-cel with second-line chemotherapy with planned ASCT. Like ZUMA-7, this study also demonstrated an improvement in progression-free survival (PFS) compared to standard treatment. As such, CAR T-cell therapy represents the new standard of care for second-line treatment in appropriate patients with refractory or early relapsing LBCL.
There have been several other recent studies on the use of CAR T-cell therapy for B-cell lymphoma. One study, published in Blood Advances (2023), found that receiving a greater number of therapies prior to CAR T-cell therapy is associated with poorer outcomes in patients with aggressive relapsed/refractory B-cell NHL. The study, which included 514 patients from 13 centers treated with CAR-T for aggressive B-cell NHL between 2015 and 2021, found that a greater number of lines of therapy before CAR-T apheresis and bridging therapy were predictive of inferior PFS and overall survival.
Another study compared 2 CD19-targeting CAR T-cell treatments, axi-cel and tisa-cel, with ASCT in the second line setting for LBCL. The study found that axi-cel was superior to ASCT, with longer median event-free survival and a higher response rate. However, tisa-cel was not found to be superior to ASCT. Further studies will be needed to definitively characterize the relative benefits of CAR-T cell therapies and standard second-line treatments for different subgroups of patients with LBCL.
An increasing number of effective targeted agents for DLBCL, including novel monoclonal antibodies (tafasitamab) and antibody-drug conjugates (polatuzumab vedotin and loncastuximab teserine), are being used in earlier lines of therapy. Additionally, 2 anti-CD20 bispecific antibodies (epcoritamab and glofitamab) have gained approval for relapsed/refractory DLBCL due to high response rates. Future studies will be needed to determine if treatment with these agents can produce durable remissions like that of CAR-T cell therapy.
What’s New in Diffuse Large B-cell Lymphoma?
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.
Lymphoma specialist to lead MD Anderson’s cancer medicine division
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
“My research uncovered a series of physicians who served as ‘clinical champions’ and dramatically sped the process of drug development,” Dr. Flowers recalled in an interview. “This early career research inspired me to become the type of clinical champion that I uncovered.”
Over his career, hematologist-oncologist Dr. Flowers has developed lifesaving therapies for lymphoma, which has transformed into a highly treatable and even curable disease. He’s listed as a coauthor of hundreds of peer-reviewed cancer studies, reports, and medical society guidelines. And he’s revealed stark disparities in blood cancer care: His research shows that non-White patients suffer from worse outcomes, regardless of factors like income and insurance coverage.
The University of Texas MD Anderson Cancer Center, Houston, recently named physician-scientist Dr. Flowers as division head of cancer medicine, a position he’s held on an interim basis. As of Sept. 1, he will permanently oversee 300 faculty and more than 2,000 staff members.
A running start in Seattle
For Dr. Flowers, track and field is a sport that runs in the family. His grandfather was a top runner in both high school and college, and both Dr. Flowers and his brother ran competitively in Seattle, where they grew up. But Dr. Flowers chose a career in oncology, earning a medical degree at Stanford and master’s degrees at both Stanford and the University of Washington, Seattle.
The late Kenneth Melmon, MD, a groundbreaking pharmacologist, was a major influence. “He was one of the first people that I met when I began as an undergraduate at Stanford. We grew to be long-standing friends, and he demonstrated what outstanding mentorship looks like. In our research collaboration, we investigated the work of Dr. Gertrude Elion and Dr. George Hitchings involving the translation of pharmacological data from cellular and animal models to clinically useful drugs including 6-mercaptopurine, allopurinol, azathioprine, acyclovir, and zidovudine.”
The late Oliver Press, MD, a blood cancer specialist, inspired Dr. Flower’s interest in lymphoma. “I began work with him during an internship at the University of Washington. Ollie was a great inspiration and a key leader in the development of innovative therapies for lymphoma. He embodied the role of a clinical champion translating work in radioimmunotherapy to new therapeutics for patients with lymphomas. Working with him ultimately led me to pursue a career in hematology and oncology with a focus on the care for patients with lymphomas.”
Career blooms as lymphoma care advances
Dr. Flowers went on to Emory University, Atlanta, where he served as scientific director of the Research Informatics Shared Resource and a faculty member in the department of biomedical informatics. “I applied my training in informatics and my clinical expertise to support active grants from the Burroughs Wellcome Fund for Innovation in Regulatory Science and from the National Cancer Institute to develop informatics tools for pathology image analysis and prognostic modeling.”
For 13 years, he also served the Winship Cancer Institute as director of the Emory Healthcare lymphoma program (where his patients included Kansas City Chiefs football star Eric Berry), and for 4 years as scientific director of research informatics. Meanwhile, Dr. Flowers helped develop national practice guidelines for the American Society of Clinical Oncology, the American Cancer Society, and the American College of Radiology. He also chaired the ASCO guideline on management of febrile neutropenia.
In 2019, MD Anderson hired Dr. Flowers as chair of the department of lymphoma/myeloma. A year later, he was appointed division head ad interim for cancer medicine.
“Chris is a unique leader who expertly combines mentorship, sponsorship, and bidirectional open, honest communication,” said Sairah Ahmed, MD, associate professor of lymphoma at MD Anderson. “He doesn’t just empower his team to reach their goals. He also inspires those around him to turn vision into reality.”
As Dr. Flowers noted, many patients with lymphoma are now able to recover and live normal lives. He himself played a direct role himself in boosting lifespans.
“I have been fortunate to play a role in the development of several treatments that have led to advances in first-line therapy for patients with aggressive lymphomas. I partnered with others at MD Anderson, including Dr. Sattva Neelapu and Dr. Jason Westin, who have developed novel therapies like chimeric antigen receptor T-cell therapy for patients with relapse lymphomas,” he said. “Leaders in the field at MD Anderson like Dr. Michael Wang have developed new oral treatments for patients with rare lymphoma subtypes like mantle cell lymphoma. Other colleagues such as Dr. Nathan Fowler and Dr. Loretta Nastoupil have focused on the care for patients with indolent lymphomas and developed less-toxic therapies that are now in common use.”
Exposing the disparities in blood cancer care
Dr. Flowers, who’s African American, has also been a leader in health disparity research. In 2016, for example, he was coauthor of a study into non-Hodgkin’s lymphoma that revealed that Blacks in the United States have dramatically lower survival rates than Whites. The 10-year survival rate for Black women with chronic lymphocytic leukemia was just 47%, for example, compared with 66% for White females. “Although incidence rates of lymphoid neoplasms are generally higher among Whites, Black men tend to have poorer survival,” Dr. Flowers and colleagues wrote.
In a 2021 report for the ASCO Educational Book, Dr. Flowers and hematologist-oncologist Demetria Smith-Graziani, MD, now with Emory University, explored disparities across blood cancers and barriers to minority enrollment in clinical trials. “Some approaches that clinicians can apply to address these disparities include increasing systems-level awareness, improving access to care, and reducing biases in clinical setting,” the authors wrote.
Luis Malpica Castillo, MD, assistant professor of lymphoma at MD Anderson Cancer Center, lauded the work of Dr. Flowers in expanding opportunities for minority patients with the disease.
“During the past years, Dr. Flowers’ work has not only had a positive impact on the Texan community, but minority populations living with cancer in the United States and abroad,” he said. “Currently, we are implementing cancer care networks aimed to increase diversity in clinical trials by enrolling a larger number of Hispanic and African American patients, who otherwise may not have benefited from novel therapies. The ultimate goal is to provide high-quality care to all patients living with cancer.”
In addition to his research work, Dr. Flowers is an advocate for diversity within the hematology community. He’s a founding member and former chair of the American Society of Hematology’s Committee on Diversity, Equity and Inclusion (formerly the Committee on Promoting Diversity), and he helped develop the society’s Minority Recruitment Initiative.
What’s next for Dr. Flowers? For one, he plans to continue working as a mentor; he received the ASH Mentor Award in honor of his service in 2022. “I am strongly committed to increasing the number of tenure-track investigators trained in clinical and translational cancer research and to promote their career development.”
And he looks forward to helping develop MD Anderson’s recently announced $2.5 billion hospital in Austin. “This will extend the exceptional care that we provide as the No. 1 cancer center in the United States,” he said. “It will also create new opportunities for research and collaboration with experts at UT Austin.”
When he’s not in clinic, Dr. Flowers embraces his lifelong love of speeding through life on his own two feet. He’s even inspired his children to share his passion. “I run most days of the week,” he said. “Running provides a great opportunity to think and process new research ideas, work through leadership challenges, and sometimes just to relax and let go of the day.”
MCL: Pathophysiology and Epidemiology
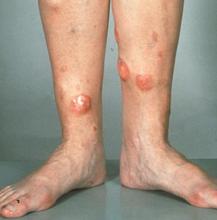
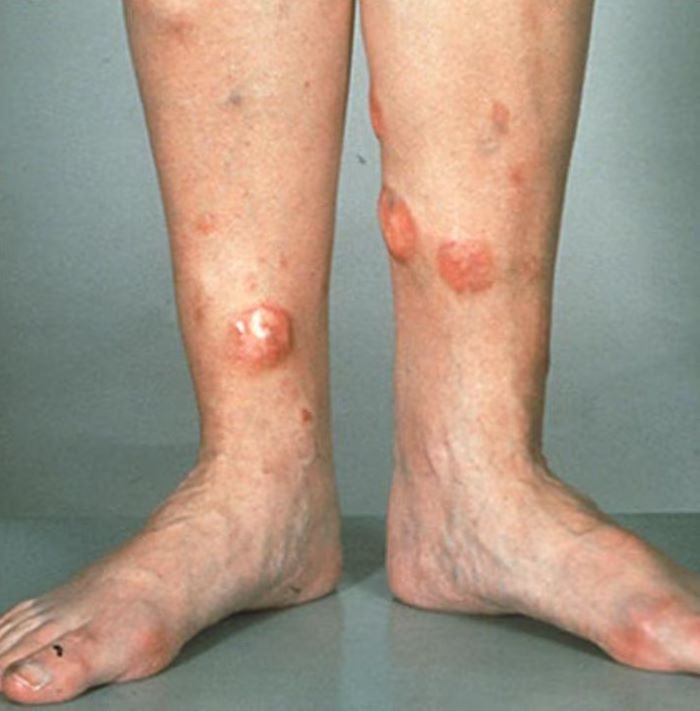
Red nodules on legs
Given the patient's diagnosis of stage IV MCL, the presentation of diffuse skin lesions, and the histopathologic and immunophenotyping results of those lesions, this patient is diagnosed with secondary cutaneous MCL. The hematologist-oncologist discusses the findings with the patient and presents potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all NHLs are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. Skin manifestations are not as common as other extranodal manifestations. Primary cutaneous MCL occurs in up to 6% of patients with MCL; secondary cutaneous involvement is slightly more common, occurring in 17% of patients with MCL. Secondary cutaneous MCL usually presents in late-stage disease. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, that are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test also help diagnose MCL. Secondary cutaneous MCL is diagnosed on the basis of an MCL diagnosis along with diffuse infiltration of the skin, with multiple erythematous papules and nodules coalescing to form plaques; skin biopsy and immunohistopathology showing monotonous proliferation of small- to medium-sized lymphoid cells with scant cytoplasm; irregular cleaved nuclei with coarse chromatin; and inconspicuous nucleoli as well as a spared papillary dermis.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options, and associated adverse events as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, which were once used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grants from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's diagnosis of stage IV MCL, the presentation of diffuse skin lesions, and the histopathologic and immunophenotyping results of those lesions, this patient is diagnosed with secondary cutaneous MCL. The hematologist-oncologist discusses the findings with the patient and presents potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all NHLs are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. Skin manifestations are not as common as other extranodal manifestations. Primary cutaneous MCL occurs in up to 6% of patients with MCL; secondary cutaneous involvement is slightly more common, occurring in 17% of patients with MCL. Secondary cutaneous MCL usually presents in late-stage disease. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, that are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test also help diagnose MCL. Secondary cutaneous MCL is diagnosed on the basis of an MCL diagnosis along with diffuse infiltration of the skin, with multiple erythematous papules and nodules coalescing to form plaques; skin biopsy and immunohistopathology showing monotonous proliferation of small- to medium-sized lymphoid cells with scant cytoplasm; irregular cleaved nuclei with coarse chromatin; and inconspicuous nucleoli as well as a spared papillary dermis.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options, and associated adverse events as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, which were once used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grants from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's diagnosis of stage IV MCL, the presentation of diffuse skin lesions, and the histopathologic and immunophenotyping results of those lesions, this patient is diagnosed with secondary cutaneous MCL. The hematologist-oncologist discusses the findings with the patient and presents potential next steps and treatment options.
MCL is a type of B-cell neoplasm that, with advancements in the understanding of non-Hodgkin lymphoma (NHL) in the past 30 years, has been defined as its own clinicopathologic entity by the Revised European-American Lymphoma and World Health Organization classifications. Up to 10% of all NHLs are MCL. Clinical presentation includes advanced disease with B symptoms (eg, night sweats, fever, weight loss), generalized lymphadenopathy, abdominal distention associated with hepatosplenomegaly, and fatigue. Skin manifestations are not as common as other extranodal manifestations. Primary cutaneous MCL occurs in up to 6% of patients with MCL; secondary cutaneous involvement is slightly more common, occurring in 17% of patients with MCL. Secondary cutaneous MCL usually presents in late-stage disease. Men are more likely to present with MCL than are women by a ratio of 3:1. Median age at presentation is 67 years.
Diagnosing MCL is a multipronged approach. Physical examination may reveal lymphadenopathy and hepatosplenomegaly. Lymph node biopsy and aspiration with immunophenotyping in MCL reveals monoclonal B cells expressing surface immunoglobulin (Ig), IgM, or IgD, that are characteristically CD5+ and pan B-cell antigen–positive (eg, CD19, CD20, CD22) but lack expression of CD10 and CD23 and overexpress cyclin D1. Bone marrow aspirate/biopsy are used more for staging than for diagnosis. Blood studies, including anemia and cytopenias secondary to bone marrow infiltration (with up to 40% of cases showing lymphocytosis > 4000/μL), abnormal liver function tests, and a negative Coombs test also help diagnose MCL. Secondary cutaneous MCL is diagnosed on the basis of an MCL diagnosis along with diffuse infiltration of the skin, with multiple erythematous papules and nodules coalescing to form plaques; skin biopsy and immunohistopathology showing monotonous proliferation of small- to medium-sized lymphoid cells with scant cytoplasm; irregular cleaved nuclei with coarse chromatin; and inconspicuous nucleoli as well as a spared papillary dermis.
Pathogenesis of MCL involves disordered lymphoproliferation in a subset of naive pregerminal center cells in primary follicles or in the mantle region of secondary follicles. Most cases are linked with translocation of chromosome 14 and 11, which induces overexpression of protein cyclin D1. Viral infection (Epstein-Barr virus, HIV, human T-lymphotropic virus type 1, human herpes virus 6), environmental factors, and primary and secondary immunodeficiency are also associated with the development of NHL.
Patient education should include detailed information about clinical trials, available treatment options, and associated adverse events as well as psychosocial and nutrition counseling.
Chemoimmunotherapy is standard initial treatment for MCL, but relapse is expected. Chemotherapy-free regimens with biologic targets, which were once used in second-line treatment, have increasingly become an important first-line treatment given their efficacy in the relapsed/refractory setting. Chimeric antigen receptor T-cell therapy is also a second-line treatment option. In patients with MCL and a TP53 mutation, clinical trial participation is encouraged because of poor prognosis.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grants from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 72-year-old man presents to his hematologist-oncologist with red ulcerative nodules on both legs. Six months before, the patient was diagnosed with stage IV mantle cell lymphoma (MCL) and began chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Initial patient reports at diagnosis were abdominal distention, generalized lymphadenopathy, night sweats, and fatigue; he received a referral to hematology-oncology after his complete blood count with differential revealed anemia and cytopenias. Additional blood studies showed lymphocytosis > 4000/μL, elevated lactate dehydrogenase levels, abnormal liver function tests, and a negative result on the Coombs test. Ultrasound of the abdomen revealed hepatosplenomegaly and abdominal lymphadenopathy. The hematologist-oncologist ordered a lymph node biopsy and aspiration. Immunophenotyping showed CD5 and CD20 expression but a lack of CD23 and CD10 expression; cyclin D1 was overexpressed. Bone marrow biopsy revealed hypercellular marrow spaces showing infiltration by sheets of atypical lymphoid cells.
Because the patient presents with red ulcerative nodules on both legs, the hematologist-oncologist orders a skin biopsy of the lesions. Histopathologic evaluation shows monotonous proliferation of small- to medium-sized lymphoid cells with scant cytoplasm, irregular cleaved nuclei with coarse chromatin, and inconspicuous nucleoli as well as a spared papillary dermis. Immunophenotyping shows CD5 and CD20 expression but a lack of CD23 and CD10 expression; cyclin D1 is overexpressed.
ESMO helps hematologists assess new cancer drugs
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
It consists of 11 2- to 3-page forms with checklists to grade treatment trials on the extent to which they meet efficacy and safety thresholds. Each of the 11 forms covers a specific trial scenario, such as a randomized controlled trial with curative intent or a trial of a therapy that is not likely to be curative with a primary endpoint of overall survival.
Treatments with curative intent are graded A, B, or C, while treatments in the noncurative setting are graded on a descending scale from 5 to 1. Scores of A and B in the curative setting and 5 and 4 in the noncurative setting represent substantial benefit.
On the form for RCTs with curative intent, for instance, a survival improvement of 5% or more garners an A but an improvement of less than 3% gets a C. Scores are also annotated for serious acute and/or persistent toxicity if present.
The tool, dubbed the ESMO-MCBS:H (European Society for Medical Oncology Magnitude of Clinical Benefit Scale: Hematology), is explained in an article published in Annals of Oncology. The evaluation forms are available online.
The idea behind the work is to help health care professionals and others to more “accurately assess the value of and prioritise therapies for patients with blood cancers. For clinicians, ESMO-MCBS:H will aid in their clinical decision-making and in the development of evidence-based practice and guidelines,” ESMO said in a press release.
To develop ESMO-MCBS:H, the group tailored its tool for evaluating solid tumor therapies, the ESMO-MCBS, to account for the sometimes different endpoints used in hematologic malignancy trials and the very indolent nature of some blood cancers, such as follicular lymphoma, which hampers development of mature data.
Specific changes include adding a new evaluation form to grade single-arm trials with curative intent, such as those used for CAR-T-cell therapies; incorporating molecular surrogate endpoints used in CML trials; and adding a way to grade outcomes for indolent cancers, among others.
The development process included applying the solid tumor tool to 80 blood cancer studies to identify shortcomings and improve its applicability. The final tool was field tested with 51 international experts from EHA and ESMO who largely agreed on the reasonableness of the trial scores.
ESMO said it expects ESMO-MCBS:H will be useful. The solid tumor tool, first published in 2015, is used by the World Health Organization to screen medications for its essential medicines list as well as by ESMO to generate guidelines and oncology centers across Europe to help with resource allocation decisions.
FROM ANNALS OF ONCOLOGY
Targeted Therapies in Younger and Older Patients With Mantle Cell Lymphoma
Background
Of the approximately 80,000 individuals diagnosed annually in the United States with a non-Hodgkin lymphoma (NHL), MCL accounts for an estimated 5%.1,2 At the time of diagnosis, most of these patients have advanced disease. The diagnosis of MCL is made based on characteristic immunophenotype and the presence of (11;14)(q13;q32) translocation resulting in overexpression of cyclin D1.3,4 Long-term survival has been observed in a small proportion of patients with MCL, but this disease is generally considered incurable.5
Except for the approximately 10% of patients with MCL who present with asymptomatic indolent disease, for whom a watch-and-wait approach is generally used,6 there are 2 types of treatment strategies. One is applied to people who are fit and relatively young. In these cases, intensive chemotherapy with or without ASCT has been the dominant approach. In patients who are poor candidates for the toxicities associated with aggressive treatment, less intensive approaches are applied. These strategies include not only better-tolerated combinations of cytotoxic chemotherapies, but also various combinations that involve immunomodulators or small molecule enzyme inhibitors. Although less toxic, these regimens are active, often achieving a complete response (CR) and an extended progression-free survival (PFS).3
These 2 pathways of MCL treatment are reflected in guidelines from the National Comprehensive Cancer Network (NCCN), which describe separate first-line algorithms for stage I and stage II non-bulky disease and stage II bulky and advanced stage disease.7 For stage II bulky or advanced stage disease, separate pathways are described for indolent, TP53-mutated, and TP53 wild-type MCL and are further divided into pathways for those who are candidates for ASCT and those who are not.
Currently, “chemotherapy-free” therapies, a term that is sometimes used to identify drug combinations with modest or no cytotoxic effects, though inaccurate, are not preferred for first-line therapy in any group in the NCCN guidelines. However, immunomodulators, such as lenalidomide and targeted therapies, such as Bruton tyrosine kinase inhibitors (BTKis) are being actively tested in the front-line setting with promising results. Practical approaches to the application of these agents are described in trials presented or published in the last year, including TRIANGLE and SHINE.10,11
Rethinking Front-Line MCL Therapy in the Young and Fit
Case Study
A 52-year-old man with a history of smoking presented with shortness of breath and general fatigue. The medical history included no major chronic diseases. The patient, who was referred after a routine examination, reported a recent decrease in body weight of unknown cause. Enlargement of inguinal, axillary, and submaxillary lymph nodes on examination along with laboratory abnormalities, such as anemia, and elevated lymphoid cells in the peripheral blood, raised suspicion of a lymphoproliferative disorder. A diagnosis of MCL was reached based on characteristic lymphoid cell morphology and immunotyping positive for CCND1 on lymph node biopsy. Ki-67 was 50% with wild-type TP53 on next-generation sequencing. The disease was characterized as stage III with intermediate risk MIPI (Mantle Cell Lymphoma International Prognostic Index).
For this presentation, one NCCN-guideline recommendation is a cytarabine-containing intensive chemotherapy regimen with rituximab followed by ASCT with maintenance rituximab in patients who are fit for transplant,7 but the recent data from the multicenter open-label TRIANGLE study has challenged this paradigm.10
In TRIANGLE, 870 treatment-naïve patients younger than age 65 (median age 57 years) were randomized to 1 of 3 study arms.10 In the control arm, patients received the standard-of-care induction with intensive chemoimmunotherapy (CIT) with ASCT consolidation (CIT + ASCT). In 1 of 2 experimental arms, patients received CIT + ibrutinib followed by ASCT consolidation and 2 years of ibrutinib maintenance (CIT + I + ASCT). In the other experimental arm, patients received CIT + ibrutinib followed by 2 years of ibrutinib maintenance with ASCT omitted (CIT + I). Rituximab maintenance as a single dose administered every 2 months for up to 3 years was permitted in all arms.
Most (87%) of the patients in TRIANGLE had stage IV disease and most (85%) had low- or intermediate-risk MIPI. The primary endpoint was failure-free survival (FFS). Rates of FFS at 3 years were 72% for the CIT+ ASCT arm, 88% for the CIT + I + ASCT arm, and 86% for the CIT + I arm. Overall survival (OS) at 3 years, during which time the trial was amended to permit rituximab maintenance in all 3 study arms, numerically favored ibrutinib arms (92% for CIT + I and 91% for CIT + I + ASCT), over chemotherapy alone (86% for CIT + ASCT).
The TRIANGLE trial does not yet establish a new standard for the types of patients enrolled, but it does show clearly that the use of ibrutinib with CIT was not inferior to the standard intensive approach integrating ASCT, and most types of adverse events occurred with less frequency in the ibrutinib-only arm.
There are numerous questions to pose and a broader understanding of applicability to be gained as more follow-up of this study and other studies utilizing targeted therapies, including other BTK inhibitors, provide mor data. Of particular interest is whether the presence of minimal residual disease (MRD) and the prognostic implications of MRD are affected by the use of a BTKi and/or ASCT. The E4151 and E4181 clinical trials may collectively provide greater insight here.12,13
Rethinking Front-Line MCL Therapy in Older Patients
Case Study
A 74-year-old man with a history of cardiovascular disease, including a prior ST-elevated myocardial infarction, presents with nonspecific symptoms, including night sweats, intermittent fevers, and fatigue. Despite his symptoms, he continues to work 3 days per week and participates in a weekly game of doubles tennis. Axillary swelling leads him to seek medical attention. Imaging demonstrates diffuse lymphadenopathy. An axillary lymph node biopsy confirms a diagnosis of MC with FISH (fluorescence in situ hybridization) positive for t(11;14). He is of intermediate risk on MIPI scoring.
Due to his age and concurrent heart disease, he is not a candidate for aggressive chemotherapy and ASCT. Less aggressive therapies including bendamustine plus rituximab (BR),14,15 lenalidomide plus rituximab (RR),8 and rituximab, bendamustine, and cytarabine (R-BAC) are discussed with this patient.16
Based on STiL data and BRIGHT studies, BR has become a widely used regimen.14,15 However, attempts are being made to improve upon the BR backbone with the addition of BTK inhibitors.11 In SHINE, BR plus ibrutinib further improved PFS relative to BR alone. SHINE was a 2-arm study, which was restricted to patients 65 years of age or older (median age 71 years); 523 previously untreated patients with good performance status and acceptable organ function were randomized to BR or BR plus ibrutinib. Most patients had intermediate- (~48%) or high- (~34%) risk MIPI. More than 90% had advanced-stage disease. Of patients in whom the TP53 mutation status was established, only about 10% were positive.
In the arm receiving BR alone, the median PFS was 52.9 months. With the addition of 560 mg once-daily ibrutinib to 6 cycles of BR followed by maintenance rituximab and continued ibrutinib, the median PFS, which was the primary endpoint, climbed to a median of 80.6 months. BR plus ibrutini was associated with a 41% reduction in the hazard ratio (HR) for progression or death (HR 0.75; P=.01). When stratified by risk factors, the advantage of BR plus ibrutinib was particularly pronounced in patients with intermediate-risk, (although not high-risk) nonmutated TP53, and less bulky disease.11
There was no significant effect of the addition of ibrutinib on OS at the last analysis, but the longer PFS was achieved with only a modest increase in adverse events (AEs). For AEs of grade 3 or higher, the AE rates for BR plus ibrutinib and BR alone were 81.5% and 77.3%, respectively. Rates of cytopenias, including grade 3 or higher, were similar in the 2 arms. Rash and gastrointestinal AEs, such as diarrhea, nausea, and abdominal pain, occurred more frequently among patients who received ibrutinib.11
Without an OS advantage, the SHINE trial does not establish a new standard of care, particularly given that it was voluntarily revoked from the market for the treatment of MCL. However, results are likely to accelerate interest in evaluating other targeted therapies, in combination with other relatively well-tolerated treatments. In patients with MCL unfit for ASCT, there is interest in pursuing other BTK inhibitors, particularly with ibrutinib being revoked as an indication for MCL. including the newer noncovalent pirtobrutinib, which was recently approved for MCL in the relapsed/refractory setting,17 and bispecific T-cell engagers (BiTEs) such as glofitamab.18
Rethinking Front-Line in TP53-Mutated MCL
Case Study
A previously healthy 62-year-old woman who presents with rapidly progressing lymphadenopathy and constitutional symptoms is diagnosed with MCL that has multiple adverse features. She has a Ki-67 level higher than 30%, a TP53 mutation, and blastoid morphology.19
The NCCN guidelines strongly recommend a clinical trial for patients with a TP53 mutation.7 Despite various high-intensity combinations to control disease in these patients, the 2017 pooled analysis demonstrated that most patients with TP53 mutations have a poor or no response to chemotherapy with a high side effect burden.19 In particular, such patients derive little benefit from high-intensity chemotherapy using ASCT.19
Nonetheless, for TP53-mutated MCL, several regimens have demonstrated activity. Most of these have used highly targeted therapies that offer the potential for low relative rates of toxicity. Two “chemotherapy-free” combinations involving venetoclax, the CD20-targeted obinutuzumab, and BTK inhibitors have completed phase 2 trials with promising results.20,21 In a study evaluating the BOVen regimen (the second-generation BTK inhibitor zanubrutinib, obinutuzumab, and venetoclax) as time-limited therapy in TP53-mutated patients, 89% of patients achieved MRD at 26 months of follow-up.20
Several novel therapies being tested in the relapsed/refractory setting have generated interest for evaluation in front-line clinical studies. These strategies include the BiTE glofitamab,18 the antibody-drug conjugate zilovertamab vedotin,22 and the chimeric antigen receptor (CAR) T-cell therapy brexucabtagene autoleucel (brexu-cel).23 Brexu-cel is already approved in relapsed/refractory MCL.23 Given the poor response to available treatments seen in patients with TP53 mutations, these novel therapies have the potential to improve outcomes in this population of high unmet need.
Summary
Durable remissions of MCL can be achieved with aggressive combinations of chemotherapy, but recent studies suggest a momentum away from cytotoxic drugs toward therapies with more targeted effects. In at least some patient populations, these therapies can rival the degree and duration of disease control achieved with less well-tolerated treatment. If ongoing trials corroborate the long-term efficacy and safety of these approaches, these therapies may represent an important evolution in MCL management.
- Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol. 2016;34(11):1256-1269. doi:10.1200/JCO.2015.63.5904
- Fu S, Wang M, Lairson DR, Li R, Zhao B, Du XL. Trends and variations in mantle cell lymphoma incidence from 1995 to 2013: a comparative study between Texas and National SEER areas. Oncotarget. 2017;8(68):112516-112529. doi:10.18632/oncotarget.22367
- Armitage JO, Longo DL. Mantle-cell lymphoma. N Engl J Med. 2022;386(26): 2495-2506. doi:10.1056/NEJMra2202672
- Schieber M, Gordon LI, Karmali R. Current overview and treatment of mantle cell lymphoma. F1000Res. 2018;7:F1000 Faculty Rev-1136. doi:10.12688/f1000research.14122.1
- Pu JJ, Savani M, Huang N, Epner EM. Mantle cell lymphoma management trends and novel agents: where are we going? Ther Adv Hematol. 2022;13:20406207221080743. doi:10.1177/20406207221080743
- Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol. 2019;94(6):710-725. doi:10.1002/ajh.25487
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: B cell lymphomas. Version 2.2023. Updated February 8, 2023. Accessed March 4, 2023. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
- Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood. 2018;132(19):2016-2025. doi:10.1182/blood-2018-07-859769
- Jain P, Zhao S, Lee HJ, et al. Ibrutinib with rituximab in first-line treatment of older patients with mantle cell lymphoma. J Clin Oncol. 2022;40(2):202-212. doi:10.1200/JCO.21.01797
- Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized TRIANGLE trial by the European MCL Network. Blood. 2022;140(suppl 1):1-3. doi.org/10.1182/blood-2022-163018
- Wang ML, Jurczak W, Jerkeman M, et al. Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med. 2022;386(26):2482-2494. doi:10.1056/NEJMoa2201817
- Rituximab with or without stem cell transplant in treating patients with minimal residual disease-negative mantle cell lymphoma in first complete remission. Clinicaltrials.gov. Updated January 4, 2023. Accessed March 4, 2023. https://clinicaltrials.gov/ct2/show/results/NCT03267433
- A comparison of three chemotherapy regimens for the treatment of patients with newly diagnosed mantle cell lymphoma. Clinicaltrials.gov. Updated January 25, 2023. Accessed March 4, 2023. https://www.clinicaltrials.gov/ct2/show/results/NCT04115631
- Rummel MJ, Niederle N, Maschmeyer G, et al; for the Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210. doi:10.1016/S0140-6736(12)61763-2
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944-2952. doi:10.1182/blood-2013-11-531327
- Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017;4(1):e15-e23. doi:10.1016/S2352-3026(16)30185-5
- US Food and Drug Administration. FDA grants accelerated approval to pirtobrutinib for relapsed or refractory mantle cell lymphoma [press release]. Published January 27, 2023. Accessed March 4, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pirtobrutinib-relapsed-or-refractory-mantle-cell-lymphoma
- Phillips TJ, Dickenson M, Morschhauser F, et al. Glofitamab monotherapy induces high complete response rates in patients with heavily pretreated relapsed or refractory mantle cell lymphoma. Blood. 2022;140 (suppl 1):178-180. doi.org/10.1182/blood-2022-157777
- Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903-1910. doi:10.1182/blood-2017-04-779736
- Kumar A, Soumerai JD, Abramson JS, et al. Preliminary safety and efficacy from a multicenter, investigator-initiated phase II study in untreated TP53 mutant mantle cell lymphoma with zanubrutinib, obinutuzumab, and venetoclax (BOVen). Blood. 2021;138(suppl 1):3540. doi.org/10.1182/blood-2021-151831
- Le Gouill S, Morschhauser F, Chiron D, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood. 2021;137(7):877-887. doi:10.1182/blood.2020008727
- Lee HJ, Choi MY, Siddiqi T, et al. Phase 1/2 trial of zilovertamab and ibrutinib in mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL), and marginal zone lymphoma (MZL). Blood. 2022;140(suppl 1):566-568. doi.org/10.1182/blood-2022-167153
- Wang Y, Jain P, Locke FL, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in standard-of-care practice: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2023:JCO2201797. doi:10.1200/JCO.22.01797
Background
Of the approximately 80,000 individuals diagnosed annually in the United States with a non-Hodgkin lymphoma (NHL), MCL accounts for an estimated 5%.1,2 At the time of diagnosis, most of these patients have advanced disease. The diagnosis of MCL is made based on characteristic immunophenotype and the presence of (11;14)(q13;q32) translocation resulting in overexpression of cyclin D1.3,4 Long-term survival has been observed in a small proportion of patients with MCL, but this disease is generally considered incurable.5
Except for the approximately 10% of patients with MCL who present with asymptomatic indolent disease, for whom a watch-and-wait approach is generally used,6 there are 2 types of treatment strategies. One is applied to people who are fit and relatively young. In these cases, intensive chemotherapy with or without ASCT has been the dominant approach. In patients who are poor candidates for the toxicities associated with aggressive treatment, less intensive approaches are applied. These strategies include not only better-tolerated combinations of cytotoxic chemotherapies, but also various combinations that involve immunomodulators or small molecule enzyme inhibitors. Although less toxic, these regimens are active, often achieving a complete response (CR) and an extended progression-free survival (PFS).3
These 2 pathways of MCL treatment are reflected in guidelines from the National Comprehensive Cancer Network (NCCN), which describe separate first-line algorithms for stage I and stage II non-bulky disease and stage II bulky and advanced stage disease.7 For stage II bulky or advanced stage disease, separate pathways are described for indolent, TP53-mutated, and TP53 wild-type MCL and are further divided into pathways for those who are candidates for ASCT and those who are not.
Currently, “chemotherapy-free” therapies, a term that is sometimes used to identify drug combinations with modest or no cytotoxic effects, though inaccurate, are not preferred for first-line therapy in any group in the NCCN guidelines. However, immunomodulators, such as lenalidomide and targeted therapies, such as Bruton tyrosine kinase inhibitors (BTKis) are being actively tested in the front-line setting with promising results. Practical approaches to the application of these agents are described in trials presented or published in the last year, including TRIANGLE and SHINE.10,11
Rethinking Front-Line MCL Therapy in the Young and Fit
Case Study
A 52-year-old man with a history of smoking presented with shortness of breath and general fatigue. The medical history included no major chronic diseases. The patient, who was referred after a routine examination, reported a recent decrease in body weight of unknown cause. Enlargement of inguinal, axillary, and submaxillary lymph nodes on examination along with laboratory abnormalities, such as anemia, and elevated lymphoid cells in the peripheral blood, raised suspicion of a lymphoproliferative disorder. A diagnosis of MCL was reached based on characteristic lymphoid cell morphology and immunotyping positive for CCND1 on lymph node biopsy. Ki-67 was 50% with wild-type TP53 on next-generation sequencing. The disease was characterized as stage III with intermediate risk MIPI (Mantle Cell Lymphoma International Prognostic Index).
For this presentation, one NCCN-guideline recommendation is a cytarabine-containing intensive chemotherapy regimen with rituximab followed by ASCT with maintenance rituximab in patients who are fit for transplant,7 but the recent data from the multicenter open-label TRIANGLE study has challenged this paradigm.10
In TRIANGLE, 870 treatment-naïve patients younger than age 65 (median age 57 years) were randomized to 1 of 3 study arms.10 In the control arm, patients received the standard-of-care induction with intensive chemoimmunotherapy (CIT) with ASCT consolidation (CIT + ASCT). In 1 of 2 experimental arms, patients received CIT + ibrutinib followed by ASCT consolidation and 2 years of ibrutinib maintenance (CIT + I + ASCT). In the other experimental arm, patients received CIT + ibrutinib followed by 2 years of ibrutinib maintenance with ASCT omitted (CIT + I). Rituximab maintenance as a single dose administered every 2 months for up to 3 years was permitted in all arms.
Most (87%) of the patients in TRIANGLE had stage IV disease and most (85%) had low- or intermediate-risk MIPI. The primary endpoint was failure-free survival (FFS). Rates of FFS at 3 years were 72% for the CIT+ ASCT arm, 88% for the CIT + I + ASCT arm, and 86% for the CIT + I arm. Overall survival (OS) at 3 years, during which time the trial was amended to permit rituximab maintenance in all 3 study arms, numerically favored ibrutinib arms (92% for CIT + I and 91% for CIT + I + ASCT), over chemotherapy alone (86% for CIT + ASCT).
The TRIANGLE trial does not yet establish a new standard for the types of patients enrolled, but it does show clearly that the use of ibrutinib with CIT was not inferior to the standard intensive approach integrating ASCT, and most types of adverse events occurred with less frequency in the ibrutinib-only arm.
There are numerous questions to pose and a broader understanding of applicability to be gained as more follow-up of this study and other studies utilizing targeted therapies, including other BTK inhibitors, provide mor data. Of particular interest is whether the presence of minimal residual disease (MRD) and the prognostic implications of MRD are affected by the use of a BTKi and/or ASCT. The E4151 and E4181 clinical trials may collectively provide greater insight here.12,13
Rethinking Front-Line MCL Therapy in Older Patients
Case Study
A 74-year-old man with a history of cardiovascular disease, including a prior ST-elevated myocardial infarction, presents with nonspecific symptoms, including night sweats, intermittent fevers, and fatigue. Despite his symptoms, he continues to work 3 days per week and participates in a weekly game of doubles tennis. Axillary swelling leads him to seek medical attention. Imaging demonstrates diffuse lymphadenopathy. An axillary lymph node biopsy confirms a diagnosis of MC with FISH (fluorescence in situ hybridization) positive for t(11;14). He is of intermediate risk on MIPI scoring.
Due to his age and concurrent heart disease, he is not a candidate for aggressive chemotherapy and ASCT. Less aggressive therapies including bendamustine plus rituximab (BR),14,15 lenalidomide plus rituximab (RR),8 and rituximab, bendamustine, and cytarabine (R-BAC) are discussed with this patient.16
Based on STiL data and BRIGHT studies, BR has become a widely used regimen.14,15 However, attempts are being made to improve upon the BR backbone with the addition of BTK inhibitors.11 In SHINE, BR plus ibrutinib further improved PFS relative to BR alone. SHINE was a 2-arm study, which was restricted to patients 65 years of age or older (median age 71 years); 523 previously untreated patients with good performance status and acceptable organ function were randomized to BR or BR plus ibrutinib. Most patients had intermediate- (~48%) or high- (~34%) risk MIPI. More than 90% had advanced-stage disease. Of patients in whom the TP53 mutation status was established, only about 10% were positive.
In the arm receiving BR alone, the median PFS was 52.9 months. With the addition of 560 mg once-daily ibrutinib to 6 cycles of BR followed by maintenance rituximab and continued ibrutinib, the median PFS, which was the primary endpoint, climbed to a median of 80.6 months. BR plus ibrutini was associated with a 41% reduction in the hazard ratio (HR) for progression or death (HR 0.75; P=.01). When stratified by risk factors, the advantage of BR plus ibrutinib was particularly pronounced in patients with intermediate-risk, (although not high-risk) nonmutated TP53, and less bulky disease.11
There was no significant effect of the addition of ibrutinib on OS at the last analysis, but the longer PFS was achieved with only a modest increase in adverse events (AEs). For AEs of grade 3 or higher, the AE rates for BR plus ibrutinib and BR alone were 81.5% and 77.3%, respectively. Rates of cytopenias, including grade 3 or higher, were similar in the 2 arms. Rash and gastrointestinal AEs, such as diarrhea, nausea, and abdominal pain, occurred more frequently among patients who received ibrutinib.11
Without an OS advantage, the SHINE trial does not establish a new standard of care, particularly given that it was voluntarily revoked from the market for the treatment of MCL. However, results are likely to accelerate interest in evaluating other targeted therapies, in combination with other relatively well-tolerated treatments. In patients with MCL unfit for ASCT, there is interest in pursuing other BTK inhibitors, particularly with ibrutinib being revoked as an indication for MCL. including the newer noncovalent pirtobrutinib, which was recently approved for MCL in the relapsed/refractory setting,17 and bispecific T-cell engagers (BiTEs) such as glofitamab.18
Rethinking Front-Line in TP53-Mutated MCL
Case Study
A previously healthy 62-year-old woman who presents with rapidly progressing lymphadenopathy and constitutional symptoms is diagnosed with MCL that has multiple adverse features. She has a Ki-67 level higher than 30%, a TP53 mutation, and blastoid morphology.19
The NCCN guidelines strongly recommend a clinical trial for patients with a TP53 mutation.7 Despite various high-intensity combinations to control disease in these patients, the 2017 pooled analysis demonstrated that most patients with TP53 mutations have a poor or no response to chemotherapy with a high side effect burden.19 In particular, such patients derive little benefit from high-intensity chemotherapy using ASCT.19
Nonetheless, for TP53-mutated MCL, several regimens have demonstrated activity. Most of these have used highly targeted therapies that offer the potential for low relative rates of toxicity. Two “chemotherapy-free” combinations involving venetoclax, the CD20-targeted obinutuzumab, and BTK inhibitors have completed phase 2 trials with promising results.20,21 In a study evaluating the BOVen regimen (the second-generation BTK inhibitor zanubrutinib, obinutuzumab, and venetoclax) as time-limited therapy in TP53-mutated patients, 89% of patients achieved MRD at 26 months of follow-up.20
Several novel therapies being tested in the relapsed/refractory setting have generated interest for evaluation in front-line clinical studies. These strategies include the BiTE glofitamab,18 the antibody-drug conjugate zilovertamab vedotin,22 and the chimeric antigen receptor (CAR) T-cell therapy brexucabtagene autoleucel (brexu-cel).23 Brexu-cel is already approved in relapsed/refractory MCL.23 Given the poor response to available treatments seen in patients with TP53 mutations, these novel therapies have the potential to improve outcomes in this population of high unmet need.
Summary
Durable remissions of MCL can be achieved with aggressive combinations of chemotherapy, but recent studies suggest a momentum away from cytotoxic drugs toward therapies with more targeted effects. In at least some patient populations, these therapies can rival the degree and duration of disease control achieved with less well-tolerated treatment. If ongoing trials corroborate the long-term efficacy and safety of these approaches, these therapies may represent an important evolution in MCL management.
Background
Of the approximately 80,000 individuals diagnosed annually in the United States with a non-Hodgkin lymphoma (NHL), MCL accounts for an estimated 5%.1,2 At the time of diagnosis, most of these patients have advanced disease. The diagnosis of MCL is made based on characteristic immunophenotype and the presence of (11;14)(q13;q32) translocation resulting in overexpression of cyclin D1.3,4 Long-term survival has been observed in a small proportion of patients with MCL, but this disease is generally considered incurable.5
Except for the approximately 10% of patients with MCL who present with asymptomatic indolent disease, for whom a watch-and-wait approach is generally used,6 there are 2 types of treatment strategies. One is applied to people who are fit and relatively young. In these cases, intensive chemotherapy with or without ASCT has been the dominant approach. In patients who are poor candidates for the toxicities associated with aggressive treatment, less intensive approaches are applied. These strategies include not only better-tolerated combinations of cytotoxic chemotherapies, but also various combinations that involve immunomodulators or small molecule enzyme inhibitors. Although less toxic, these regimens are active, often achieving a complete response (CR) and an extended progression-free survival (PFS).3
These 2 pathways of MCL treatment are reflected in guidelines from the National Comprehensive Cancer Network (NCCN), which describe separate first-line algorithms for stage I and stage II non-bulky disease and stage II bulky and advanced stage disease.7 For stage II bulky or advanced stage disease, separate pathways are described for indolent, TP53-mutated, and TP53 wild-type MCL and are further divided into pathways for those who are candidates for ASCT and those who are not.
Currently, “chemotherapy-free” therapies, a term that is sometimes used to identify drug combinations with modest or no cytotoxic effects, though inaccurate, are not preferred for first-line therapy in any group in the NCCN guidelines. However, immunomodulators, such as lenalidomide and targeted therapies, such as Bruton tyrosine kinase inhibitors (BTKis) are being actively tested in the front-line setting with promising results. Practical approaches to the application of these agents are described in trials presented or published in the last year, including TRIANGLE and SHINE.10,11
Rethinking Front-Line MCL Therapy in the Young and Fit
Case Study
A 52-year-old man with a history of smoking presented with shortness of breath and general fatigue. The medical history included no major chronic diseases. The patient, who was referred after a routine examination, reported a recent decrease in body weight of unknown cause. Enlargement of inguinal, axillary, and submaxillary lymph nodes on examination along with laboratory abnormalities, such as anemia, and elevated lymphoid cells in the peripheral blood, raised suspicion of a lymphoproliferative disorder. A diagnosis of MCL was reached based on characteristic lymphoid cell morphology and immunotyping positive for CCND1 on lymph node biopsy. Ki-67 was 50% with wild-type TP53 on next-generation sequencing. The disease was characterized as stage III with intermediate risk MIPI (Mantle Cell Lymphoma International Prognostic Index).
For this presentation, one NCCN-guideline recommendation is a cytarabine-containing intensive chemotherapy regimen with rituximab followed by ASCT with maintenance rituximab in patients who are fit for transplant,7 but the recent data from the multicenter open-label TRIANGLE study has challenged this paradigm.10
In TRIANGLE, 870 treatment-naïve patients younger than age 65 (median age 57 years) were randomized to 1 of 3 study arms.10 In the control arm, patients received the standard-of-care induction with intensive chemoimmunotherapy (CIT) with ASCT consolidation (CIT + ASCT). In 1 of 2 experimental arms, patients received CIT + ibrutinib followed by ASCT consolidation and 2 years of ibrutinib maintenance (CIT + I + ASCT). In the other experimental arm, patients received CIT + ibrutinib followed by 2 years of ibrutinib maintenance with ASCT omitted (CIT + I). Rituximab maintenance as a single dose administered every 2 months for up to 3 years was permitted in all arms.
Most (87%) of the patients in TRIANGLE had stage IV disease and most (85%) had low- or intermediate-risk MIPI. The primary endpoint was failure-free survival (FFS). Rates of FFS at 3 years were 72% for the CIT+ ASCT arm, 88% for the CIT + I + ASCT arm, and 86% for the CIT + I arm. Overall survival (OS) at 3 years, during which time the trial was amended to permit rituximab maintenance in all 3 study arms, numerically favored ibrutinib arms (92% for CIT + I and 91% for CIT + I + ASCT), over chemotherapy alone (86% for CIT + ASCT).
The TRIANGLE trial does not yet establish a new standard for the types of patients enrolled, but it does show clearly that the use of ibrutinib with CIT was not inferior to the standard intensive approach integrating ASCT, and most types of adverse events occurred with less frequency in the ibrutinib-only arm.
There are numerous questions to pose and a broader understanding of applicability to be gained as more follow-up of this study and other studies utilizing targeted therapies, including other BTK inhibitors, provide mor data. Of particular interest is whether the presence of minimal residual disease (MRD) and the prognostic implications of MRD are affected by the use of a BTKi and/or ASCT. The E4151 and E4181 clinical trials may collectively provide greater insight here.12,13
Rethinking Front-Line MCL Therapy in Older Patients
Case Study
A 74-year-old man with a history of cardiovascular disease, including a prior ST-elevated myocardial infarction, presents with nonspecific symptoms, including night sweats, intermittent fevers, and fatigue. Despite his symptoms, he continues to work 3 days per week and participates in a weekly game of doubles tennis. Axillary swelling leads him to seek medical attention. Imaging demonstrates diffuse lymphadenopathy. An axillary lymph node biopsy confirms a diagnosis of MC with FISH (fluorescence in situ hybridization) positive for t(11;14). He is of intermediate risk on MIPI scoring.
Due to his age and concurrent heart disease, he is not a candidate for aggressive chemotherapy and ASCT. Less aggressive therapies including bendamustine plus rituximab (BR),14,15 lenalidomide plus rituximab (RR),8 and rituximab, bendamustine, and cytarabine (R-BAC) are discussed with this patient.16
Based on STiL data and BRIGHT studies, BR has become a widely used regimen.14,15 However, attempts are being made to improve upon the BR backbone with the addition of BTK inhibitors.11 In SHINE, BR plus ibrutinib further improved PFS relative to BR alone. SHINE was a 2-arm study, which was restricted to patients 65 years of age or older (median age 71 years); 523 previously untreated patients with good performance status and acceptable organ function were randomized to BR or BR plus ibrutinib. Most patients had intermediate- (~48%) or high- (~34%) risk MIPI. More than 90% had advanced-stage disease. Of patients in whom the TP53 mutation status was established, only about 10% were positive.
In the arm receiving BR alone, the median PFS was 52.9 months. With the addition of 560 mg once-daily ibrutinib to 6 cycles of BR followed by maintenance rituximab and continued ibrutinib, the median PFS, which was the primary endpoint, climbed to a median of 80.6 months. BR plus ibrutini was associated with a 41% reduction in the hazard ratio (HR) for progression or death (HR 0.75; P=.01). When stratified by risk factors, the advantage of BR plus ibrutinib was particularly pronounced in patients with intermediate-risk, (although not high-risk) nonmutated TP53, and less bulky disease.11
There was no significant effect of the addition of ibrutinib on OS at the last analysis, but the longer PFS was achieved with only a modest increase in adverse events (AEs). For AEs of grade 3 or higher, the AE rates for BR plus ibrutinib and BR alone were 81.5% and 77.3%, respectively. Rates of cytopenias, including grade 3 or higher, were similar in the 2 arms. Rash and gastrointestinal AEs, such as diarrhea, nausea, and abdominal pain, occurred more frequently among patients who received ibrutinib.11
Without an OS advantage, the SHINE trial does not establish a new standard of care, particularly given that it was voluntarily revoked from the market for the treatment of MCL. However, results are likely to accelerate interest in evaluating other targeted therapies, in combination with other relatively well-tolerated treatments. In patients with MCL unfit for ASCT, there is interest in pursuing other BTK inhibitors, particularly with ibrutinib being revoked as an indication for MCL. including the newer noncovalent pirtobrutinib, which was recently approved for MCL in the relapsed/refractory setting,17 and bispecific T-cell engagers (BiTEs) such as glofitamab.18
Rethinking Front-Line in TP53-Mutated MCL
Case Study
A previously healthy 62-year-old woman who presents with rapidly progressing lymphadenopathy and constitutional symptoms is diagnosed with MCL that has multiple adverse features. She has a Ki-67 level higher than 30%, a TP53 mutation, and blastoid morphology.19
The NCCN guidelines strongly recommend a clinical trial for patients with a TP53 mutation.7 Despite various high-intensity combinations to control disease in these patients, the 2017 pooled analysis demonstrated that most patients with TP53 mutations have a poor or no response to chemotherapy with a high side effect burden.19 In particular, such patients derive little benefit from high-intensity chemotherapy using ASCT.19
Nonetheless, for TP53-mutated MCL, several regimens have demonstrated activity. Most of these have used highly targeted therapies that offer the potential for low relative rates of toxicity. Two “chemotherapy-free” combinations involving venetoclax, the CD20-targeted obinutuzumab, and BTK inhibitors have completed phase 2 trials with promising results.20,21 In a study evaluating the BOVen regimen (the second-generation BTK inhibitor zanubrutinib, obinutuzumab, and venetoclax) as time-limited therapy in TP53-mutated patients, 89% of patients achieved MRD at 26 months of follow-up.20
Several novel therapies being tested in the relapsed/refractory setting have generated interest for evaluation in front-line clinical studies. These strategies include the BiTE glofitamab,18 the antibody-drug conjugate zilovertamab vedotin,22 and the chimeric antigen receptor (CAR) T-cell therapy brexucabtagene autoleucel (brexu-cel).23 Brexu-cel is already approved in relapsed/refractory MCL.23 Given the poor response to available treatments seen in patients with TP53 mutations, these novel therapies have the potential to improve outcomes in this population of high unmet need.
Summary
Durable remissions of MCL can be achieved with aggressive combinations of chemotherapy, but recent studies suggest a momentum away from cytotoxic drugs toward therapies with more targeted effects. In at least some patient populations, these therapies can rival the degree and duration of disease control achieved with less well-tolerated treatment. If ongoing trials corroborate the long-term efficacy and safety of these approaches, these therapies may represent an important evolution in MCL management.
- Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol. 2016;34(11):1256-1269. doi:10.1200/JCO.2015.63.5904
- Fu S, Wang M, Lairson DR, Li R, Zhao B, Du XL. Trends and variations in mantle cell lymphoma incidence from 1995 to 2013: a comparative study between Texas and National SEER areas. Oncotarget. 2017;8(68):112516-112529. doi:10.18632/oncotarget.22367
- Armitage JO, Longo DL. Mantle-cell lymphoma. N Engl J Med. 2022;386(26): 2495-2506. doi:10.1056/NEJMra2202672
- Schieber M, Gordon LI, Karmali R. Current overview and treatment of mantle cell lymphoma. F1000Res. 2018;7:F1000 Faculty Rev-1136. doi:10.12688/f1000research.14122.1
- Pu JJ, Savani M, Huang N, Epner EM. Mantle cell lymphoma management trends and novel agents: where are we going? Ther Adv Hematol. 2022;13:20406207221080743. doi:10.1177/20406207221080743
- Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol. 2019;94(6):710-725. doi:10.1002/ajh.25487
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: B cell lymphomas. Version 2.2023. Updated February 8, 2023. Accessed March 4, 2023. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
- Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood. 2018;132(19):2016-2025. doi:10.1182/blood-2018-07-859769
- Jain P, Zhao S, Lee HJ, et al. Ibrutinib with rituximab in first-line treatment of older patients with mantle cell lymphoma. J Clin Oncol. 2022;40(2):202-212. doi:10.1200/JCO.21.01797
- Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized TRIANGLE trial by the European MCL Network. Blood. 2022;140(suppl 1):1-3. doi.org/10.1182/blood-2022-163018
- Wang ML, Jurczak W, Jerkeman M, et al. Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med. 2022;386(26):2482-2494. doi:10.1056/NEJMoa2201817
- Rituximab with or without stem cell transplant in treating patients with minimal residual disease-negative mantle cell lymphoma in first complete remission. Clinicaltrials.gov. Updated January 4, 2023. Accessed March 4, 2023. https://clinicaltrials.gov/ct2/show/results/NCT03267433
- A comparison of three chemotherapy regimens for the treatment of patients with newly diagnosed mantle cell lymphoma. Clinicaltrials.gov. Updated January 25, 2023. Accessed March 4, 2023. https://www.clinicaltrials.gov/ct2/show/results/NCT04115631
- Rummel MJ, Niederle N, Maschmeyer G, et al; for the Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210. doi:10.1016/S0140-6736(12)61763-2
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944-2952. doi:10.1182/blood-2013-11-531327
- Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017;4(1):e15-e23. doi:10.1016/S2352-3026(16)30185-5
- US Food and Drug Administration. FDA grants accelerated approval to pirtobrutinib for relapsed or refractory mantle cell lymphoma [press release]. Published January 27, 2023. Accessed March 4, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pirtobrutinib-relapsed-or-refractory-mantle-cell-lymphoma
- Phillips TJ, Dickenson M, Morschhauser F, et al. Glofitamab monotherapy induces high complete response rates in patients with heavily pretreated relapsed or refractory mantle cell lymphoma. Blood. 2022;140 (suppl 1):178-180. doi.org/10.1182/blood-2022-157777
- Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903-1910. doi:10.1182/blood-2017-04-779736
- Kumar A, Soumerai JD, Abramson JS, et al. Preliminary safety and efficacy from a multicenter, investigator-initiated phase II study in untreated TP53 mutant mantle cell lymphoma with zanubrutinib, obinutuzumab, and venetoclax (BOVen). Blood. 2021;138(suppl 1):3540. doi.org/10.1182/blood-2021-151831
- Le Gouill S, Morschhauser F, Chiron D, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood. 2021;137(7):877-887. doi:10.1182/blood.2020008727
- Lee HJ, Choi MY, Siddiqi T, et al. Phase 1/2 trial of zilovertamab and ibrutinib in mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL), and marginal zone lymphoma (MZL). Blood. 2022;140(suppl 1):566-568. doi.org/10.1182/blood-2022-167153
- Wang Y, Jain P, Locke FL, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in standard-of-care practice: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2023:JCO2201797. doi:10.1200/JCO.22.01797
- Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol. 2016;34(11):1256-1269. doi:10.1200/JCO.2015.63.5904
- Fu S, Wang M, Lairson DR, Li R, Zhao B, Du XL. Trends and variations in mantle cell lymphoma incidence from 1995 to 2013: a comparative study between Texas and National SEER areas. Oncotarget. 2017;8(68):112516-112529. doi:10.18632/oncotarget.22367
- Armitage JO, Longo DL. Mantle-cell lymphoma. N Engl J Med. 2022;386(26): 2495-2506. doi:10.1056/NEJMra2202672
- Schieber M, Gordon LI, Karmali R. Current overview and treatment of mantle cell lymphoma. F1000Res. 2018;7:F1000 Faculty Rev-1136. doi:10.12688/f1000research.14122.1
- Pu JJ, Savani M, Huang N, Epner EM. Mantle cell lymphoma management trends and novel agents: where are we going? Ther Adv Hematol. 2022;13:20406207221080743. doi:10.1177/20406207221080743
- Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol. 2019;94(6):710-725. doi:10.1002/ajh.25487
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: B cell lymphomas. Version 2.2023. Updated February 8, 2023. Accessed March 4, 2023. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
- Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood. 2018;132(19):2016-2025. doi:10.1182/blood-2018-07-859769
- Jain P, Zhao S, Lee HJ, et al. Ibrutinib with rituximab in first-line treatment of older patients with mantle cell lymphoma. J Clin Oncol. 2022;40(2):202-212. doi:10.1200/JCO.21.01797
- Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized TRIANGLE trial by the European MCL Network. Blood. 2022;140(suppl 1):1-3. doi.org/10.1182/blood-2022-163018
- Wang ML, Jurczak W, Jerkeman M, et al. Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med. 2022;386(26):2482-2494. doi:10.1056/NEJMoa2201817
- Rituximab with or without stem cell transplant in treating patients with minimal residual disease-negative mantle cell lymphoma in first complete remission. Clinicaltrials.gov. Updated January 4, 2023. Accessed March 4, 2023. https://clinicaltrials.gov/ct2/show/results/NCT03267433
- A comparison of three chemotherapy regimens for the treatment of patients with newly diagnosed mantle cell lymphoma. Clinicaltrials.gov. Updated January 25, 2023. Accessed March 4, 2023. https://www.clinicaltrials.gov/ct2/show/results/NCT04115631
- Rummel MJ, Niederle N, Maschmeyer G, et al; for the Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210. doi:10.1016/S0140-6736(12)61763-2
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944-2952. doi:10.1182/blood-2013-11-531327
- Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017;4(1):e15-e23. doi:10.1016/S2352-3026(16)30185-5
- US Food and Drug Administration. FDA grants accelerated approval to pirtobrutinib for relapsed or refractory mantle cell lymphoma [press release]. Published January 27, 2023. Accessed March 4, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pirtobrutinib-relapsed-or-refractory-mantle-cell-lymphoma
- Phillips TJ, Dickenson M, Morschhauser F, et al. Glofitamab monotherapy induces high complete response rates in patients with heavily pretreated relapsed or refractory mantle cell lymphoma. Blood. 2022;140 (suppl 1):178-180. doi.org/10.1182/blood-2022-157777
- Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903-1910. doi:10.1182/blood-2017-04-779736
- Kumar A, Soumerai JD, Abramson JS, et al. Preliminary safety and efficacy from a multicenter, investigator-initiated phase II study in untreated TP53 mutant mantle cell lymphoma with zanubrutinib, obinutuzumab, and venetoclax (BOVen). Blood. 2021;138(suppl 1):3540. doi.org/10.1182/blood-2021-151831
- Le Gouill S, Morschhauser F, Chiron D, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood. 2021;137(7):877-887. doi:10.1182/blood.2020008727
- Lee HJ, Choi MY, Siddiqi T, et al. Phase 1/2 trial of zilovertamab and ibrutinib in mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL), and marginal zone lymphoma (MZL). Blood. 2022;140(suppl 1):566-568. doi.org/10.1182/blood-2022-167153
- Wang Y, Jain P, Locke FL, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in standard-of-care practice: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2023:JCO2201797. doi:10.1200/JCO.22.01797
NORD: Making Progress Through Collaboration
While people living with rare cancers continue to face daunting obstacles, progress is being made, and there are reasons to hope for a better future. Advances in genomic testing and precision medicine provide increasing evidence that rare cancers can be more efficiently and effectively diagnosed and treated. Genomic tests examine tumor DNA to identify mutations that are unique to an individual’s cancer. This genetic information enables a more precise diagnosis and targeted treatment approach. Jim Palma, Co-Lead of the NORD Rare Cancer Coalition, said “There is promise for rare cancer patients due to increased legislative efforts to cover the costs of genomic testing coupled by an increase in FDA approvals for targeted and tissue agnostic therapies.”
In 2019, the National Cancer Institute established MyPART, a vast pediatric and adult rare tumor network that aims to bolster patient involvement in research and develop effective therapies through tumor sample collection, shared data, shared samples, new methods to test treatments, and new trial designs. In 2022, MyPART welcomed NORD’s Rare Cancer Coalition as an advocacy partner.
Meanwhile, advocacy organizations are giving rare cancer a rising voice. NORD’s Rare Cancer Coalition unites rare cancer patient advocacy organizations and helps them drive progress together. The coalition promotes research and awareness through its annual Rare Cancer Day (September 30) campaign. Additionally, NORD has produced over 22 continuing medical education modules on rare cancers in collaboration with PlatformQ Health, providing updates on new therapies and treatment approaches. NORD also offers rare disease reports and educational videos on rare cancers, sessions inclusive of rare cancer topics at the annual NORD Summit, and a quarterly e-newsletter, “Caring for Rare” for healthcare professionals. Please visit us at rarediseases.org to access these resources.
Much work on rare cancers remains to be done, but the progress over recent years points to better outcomes moving forward. We are grateful for the work you do and your dedication to your patients, including those with rare cancers and other rare conditions. We hope you will find the information in this special issue useful for your clinical practice.
– Katie Kowalski, MPH
Associate Director of Education
National Organization for Rare Disorders
- About Rare Cancers. National Cancer Institute. Posted February 27, 2019. Accessed April 28, 2023. http://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/about-rare-cancers
- Gatta G, Capocaccia R, Botta L, et al. Burden and centralized treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017,18(8):1022–1039. doi:10.1016/S1470-2045(17)30445-X
While people living with rare cancers continue to face daunting obstacles, progress is being made, and there are reasons to hope for a better future. Advances in genomic testing and precision medicine provide increasing evidence that rare cancers can be more efficiently and effectively diagnosed and treated. Genomic tests examine tumor DNA to identify mutations that are unique to an individual’s cancer. This genetic information enables a more precise diagnosis and targeted treatment approach. Jim Palma, Co-Lead of the NORD Rare Cancer Coalition, said “There is promise for rare cancer patients due to increased legislative efforts to cover the costs of genomic testing coupled by an increase in FDA approvals for targeted and tissue agnostic therapies.”
In 2019, the National Cancer Institute established MyPART, a vast pediatric and adult rare tumor network that aims to bolster patient involvement in research and develop effective therapies through tumor sample collection, shared data, shared samples, new methods to test treatments, and new trial designs. In 2022, MyPART welcomed NORD’s Rare Cancer Coalition as an advocacy partner.
Meanwhile, advocacy organizations are giving rare cancer a rising voice. NORD’s Rare Cancer Coalition unites rare cancer patient advocacy organizations and helps them drive progress together. The coalition promotes research and awareness through its annual Rare Cancer Day (September 30) campaign. Additionally, NORD has produced over 22 continuing medical education modules on rare cancers in collaboration with PlatformQ Health, providing updates on new therapies and treatment approaches. NORD also offers rare disease reports and educational videos on rare cancers, sessions inclusive of rare cancer topics at the annual NORD Summit, and a quarterly e-newsletter, “Caring for Rare” for healthcare professionals. Please visit us at rarediseases.org to access these resources.
Much work on rare cancers remains to be done, but the progress over recent years points to better outcomes moving forward. We are grateful for the work you do and your dedication to your patients, including those with rare cancers and other rare conditions. We hope you will find the information in this special issue useful for your clinical practice.
– Katie Kowalski, MPH
Associate Director of Education
National Organization for Rare Disorders
While people living with rare cancers continue to face daunting obstacles, progress is being made, and there are reasons to hope for a better future. Advances in genomic testing and precision medicine provide increasing evidence that rare cancers can be more efficiently and effectively diagnosed and treated. Genomic tests examine tumor DNA to identify mutations that are unique to an individual’s cancer. This genetic information enables a more precise diagnosis and targeted treatment approach. Jim Palma, Co-Lead of the NORD Rare Cancer Coalition, said “There is promise for rare cancer patients due to increased legislative efforts to cover the costs of genomic testing coupled by an increase in FDA approvals for targeted and tissue agnostic therapies.”
In 2019, the National Cancer Institute established MyPART, a vast pediatric and adult rare tumor network that aims to bolster patient involvement in research and develop effective therapies through tumor sample collection, shared data, shared samples, new methods to test treatments, and new trial designs. In 2022, MyPART welcomed NORD’s Rare Cancer Coalition as an advocacy partner.
Meanwhile, advocacy organizations are giving rare cancer a rising voice. NORD’s Rare Cancer Coalition unites rare cancer patient advocacy organizations and helps them drive progress together. The coalition promotes research and awareness through its annual Rare Cancer Day (September 30) campaign. Additionally, NORD has produced over 22 continuing medical education modules on rare cancers in collaboration with PlatformQ Health, providing updates on new therapies and treatment approaches. NORD also offers rare disease reports and educational videos on rare cancers, sessions inclusive of rare cancer topics at the annual NORD Summit, and a quarterly e-newsletter, “Caring for Rare” for healthcare professionals. Please visit us at rarediseases.org to access these resources.
Much work on rare cancers remains to be done, but the progress over recent years points to better outcomes moving forward. We are grateful for the work you do and your dedication to your patients, including those with rare cancers and other rare conditions. We hope you will find the information in this special issue useful for your clinical practice.
– Katie Kowalski, MPH
Associate Director of Education
National Organization for Rare Disorders
- About Rare Cancers. National Cancer Institute. Posted February 27, 2019. Accessed April 28, 2023. http://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/about-rare-cancers
- Gatta G, Capocaccia R, Botta L, et al. Burden and centralized treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017,18(8):1022–1039. doi:10.1016/S1470-2045(17)30445-X
- About Rare Cancers. National Cancer Institute. Posted February 27, 2019. Accessed April 28, 2023. http://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/about-rare-cancers
- Gatta G, Capocaccia R, Botta L, et al. Burden and centralized treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017,18(8):1022–1039. doi:10.1016/S1470-2045(17)30445-X
2023 Rare Diseases Report: Cancers
This edition of Rare Diseases Report: Cancers highlights the latest breakthroughs and remaining unmet needs in the management of rare cancers. In addition to celebrating the great progress that has been made in recent years, we also discuss new challenges, such as how the healthcare system can prepare to manage the growing number of rare cancer survivors who are living longer due to improvements in disease management.
INTRODUCTION
NORD: Making Progress Through Collaboration
By Katie Kowalski, MPH
IN THIS ISSUE
The Complex Challenge of Survival After HPV-Associated Oropharyngeal Cancer
By Vlad C. Sandulache, MD, PhD
Progress in Ovarian Cancer: Discovery of Fallopian Tube Involvement
By Ronny Drapkin, MD, PhD
An Evolving Understanding of Adenosquamous Carcinoma of the Lung
By Rajwanth Veluswamy, MD, MSCR
Gastrointestinal Stromal Tumor: Reflecting on 2 Decades of Clinical Advancements
By Jason K. Sicklick, MD, FACS
Progress in Treating Testicular Cancer
By Liang Cheng, MD
Strategies to Improve Long-Term Outcomes in Younger Patients with Hodgkin Lymphoma
By Ann LaCasce, MD, MMSc
Targeted Therapies in Younger and Older Patients with Mantle Cell Lymphoma
By Reem Karmali, MD, MS
Advances in Management of Relapsed/Refractory Hairy Cell Leukemia
By Robert J. Kreitman, MD
Treatment Needs of Older Adults With Newly Diagnosed Acute Myeloid Leukemia
By Harry Erba, MD, PhD
Progress in Management of Advanced Acute Lymphocytic Leukemia in Children
By Susan Colace, MD, MSCI
This edition of Rare Diseases Report: Cancers highlights the latest breakthroughs and remaining unmet needs in the management of rare cancers. In addition to celebrating the great progress that has been made in recent years, we also discuss new challenges, such as how the healthcare system can prepare to manage the growing number of rare cancer survivors who are living longer due to improvements in disease management.
INTRODUCTION
NORD: Making Progress Through Collaboration
By Katie Kowalski, MPH
IN THIS ISSUE
The Complex Challenge of Survival After HPV-Associated Oropharyngeal Cancer
By Vlad C. Sandulache, MD, PhD
Progress in Ovarian Cancer: Discovery of Fallopian Tube Involvement
By Ronny Drapkin, MD, PhD
An Evolving Understanding of Adenosquamous Carcinoma of the Lung
By Rajwanth Veluswamy, MD, MSCR
Gastrointestinal Stromal Tumor: Reflecting on 2 Decades of Clinical Advancements
By Jason K. Sicklick, MD, FACS
Progress in Treating Testicular Cancer
By Liang Cheng, MD
Strategies to Improve Long-Term Outcomes in Younger Patients with Hodgkin Lymphoma
By Ann LaCasce, MD, MMSc
Targeted Therapies in Younger and Older Patients with Mantle Cell Lymphoma
By Reem Karmali, MD, MS
Advances in Management of Relapsed/Refractory Hairy Cell Leukemia
By Robert J. Kreitman, MD
Treatment Needs of Older Adults With Newly Diagnosed Acute Myeloid Leukemia
By Harry Erba, MD, PhD
Progress in Management of Advanced Acute Lymphocytic Leukemia in Children
By Susan Colace, MD, MSCI
This edition of Rare Diseases Report: Cancers highlights the latest breakthroughs and remaining unmet needs in the management of rare cancers. In addition to celebrating the great progress that has been made in recent years, we also discuss new challenges, such as how the healthcare system can prepare to manage the growing number of rare cancer survivors who are living longer due to improvements in disease management.
INTRODUCTION
NORD: Making Progress Through Collaboration
By Katie Kowalski, MPH
IN THIS ISSUE
The Complex Challenge of Survival After HPV-Associated Oropharyngeal Cancer
By Vlad C. Sandulache, MD, PhD
Progress in Ovarian Cancer: Discovery of Fallopian Tube Involvement
By Ronny Drapkin, MD, PhD
An Evolving Understanding of Adenosquamous Carcinoma of the Lung
By Rajwanth Veluswamy, MD, MSCR
Gastrointestinal Stromal Tumor: Reflecting on 2 Decades of Clinical Advancements
By Jason K. Sicklick, MD, FACS
Progress in Treating Testicular Cancer
By Liang Cheng, MD
Strategies to Improve Long-Term Outcomes in Younger Patients with Hodgkin Lymphoma
By Ann LaCasce, MD, MMSc
Targeted Therapies in Younger and Older Patients with Mantle Cell Lymphoma
By Reem Karmali, MD, MS
Advances in Management of Relapsed/Refractory Hairy Cell Leukemia
By Robert J. Kreitman, MD
Treatment Needs of Older Adults With Newly Diagnosed Acute Myeloid Leukemia
By Harry Erba, MD, PhD
Progress in Management of Advanced Acute Lymphocytic Leukemia in Children
By Susan Colace, MD, MSCI
Multiprong strategy makes clinical trials less White
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ASCO 2023