User login
Chlorophyll water can trigger pseudoporphyria, expert warns
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
AT PDA 2022
Why it’s important for dermatologists to learn about JAK inhibitors
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
PORTLAND, ORE. – according to Andrew Blauvelt, MD, MBA.
“In dermatology, you need to know about JAK inhibitors, and you need to know how to use them,” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the annual meeting of the Pacific Dermatologic Association. “Making the choice, ‘I’m not going to use those drugs because of safety concerns,’ may be okay in 2022, but we are going to be getting a lot more indications for these drugs. So instead of avoiding JAK inhibitors, I would say try to learn [about] them, understand them, and get your messaging out on safety.”
It’s difficult to imagine a clinician-researcher who has more experience with the use of biologics and JAK inhibitors in AD than Dr. Blauvelt, who has been the international investigator on several important trials of treatments that include dupilumab, tralokinumab, abrocitinib, and upadacitinib for AD such as CHRONOS, ECZTEND, JADE REGIMEN, and HEADS UP. At the meeting, he discussed his clinical approach to selecting systemic agents for AD and shared prescribing tips. He began by noting that the approval of dupilumab for moderate to severe AD in 2017 ushered in a new era of treating the disease systemically.
“When it was approved, experts went right to dupilumab if they could, and avoided the use of cyclosporine or methotrexate,” said Dr. Blauvelt, who is also an elected member of the American Society for Clinical Investigation and the International Eczema Council. “I still think that dupilumab is a great agent to start with. We’ve had a bit of difficulty improving upon it.”
Following dupilumab’s approval, three other systemic options became available for patients with moderate to severe AD: the human IgG4 monoclonal antibody tralokinumab that binds to interleukin-13, which is administered subcutaneously; and, more recently, the oral JAK inhibitors abrocitinib and upadacitinib, approved in January for moderate to severe AD.
“I’m a big fan of JAK inhibitors because I think they offer things that biologic and topical therapies can’t offer,” Dr. Blauvelt said. “Patients like the pills versus shots. They also like the speed; JAK inhibitors work faster than dupilumab and tralokinumab. So, if you have a patient with bad AD who wants to get better quickly, that would be a reason to choose a JAK inhibitor over a biologic if you can.”
When Dr. Blauvelt has asked AD clinical trial participants if they’d rather be treated with a biologic agent or with a JAK inhibitor, about half choose one over the other.
“Patients who shy away from the safety issues would choose the biologic trial while the ones who wanted the fast relief would choose the JAK trial,” he said. “But if you present both options and the patients prefer a pill, I think the JAK inhibitors do better with a rapid control of inflammation as well as pruritus – the latter within 2 days of taking the pills.”
When counseling patients initiating a JAK inhibitor, Dr. Blauvelt mentioned three advantages, compared with biologics: the pill formulation, the rapidity of response in pruritus control, and better efficacy. “The downside is the safety,” he said. “Safety is the elephant in the room for the JAK inhibitors.”
The risks listed in the boxed warning in the labeling for JAK inhibitors include: an increased risk of serious bacterial, fungal, and opportunistic infections such as TB; a higher rate of all-cause mortality, including cardiovascular death; a higher rate of MACE (major adverse cardiovascular events, defined as cardiovascular death, MI, and stroke); the potential for malignancy, including lymphoma; and the potential for thrombosis, including an increased incidence of pulmonary embolism (PE).
“Risk of thrombosis seems to be a class effect for all JAK inhibitors,” Dr. Blauvelt said. “As far as I know, it’s idiosyncratic. For nearly all the DVT [deep vein thrombosis] cases that have been reported, patients had baseline risk factors for DVT and PE, which are obesity, smoking, and use of oral contraceptives.”
Dr. Blauvelt pointed out that the boxed warning related to mortality, malignancies, and MACE stemmed from a long-term trial of the JAK inhibitor tofacitinib in RA patients. “Those patients had to be at least 50 years old, 75% of them were on concomitant methotrexate and/or prednisone, and they had to have at least one cardiac risk factor to get into the trial,” he said.
“I’m not saying those things can’t happen in dermatology patients, but if you look at the safety data of JAK inhibitors in the AD studies and in the alopecia areata studies, we are seeing a few cases of these things here and there, but not major signals,” he said. To date, “they look safer in dermatologic diseases compared to tofacitinib in RA data in older populations.”
He emphasized the importance of discussing each of the risks in the boxed warning with patients who are candidates for JAK inhibitor therapy.
Dr. Blauvelt likened the lab monitoring required for JAK inhibitors to that required for methotrexate. This means ordering at baseline, a CBC with differential, a chem-20, a lipid panel, and a QuantiFERON-TB Gold test. The JAK inhibitor labels do not include information on the frequency of monitoring, “but I have a distinct opinion on this because of my blood test monitoring experience in the trials for many years,” he said.
“I think it’s good to do follow-up testing at 1 month, then every 3 months in the first year. In my experience, the people who drop blood cell counts or increase their lipids tend to do it in the first year.”
After 1 year of treatment, he continued, follow-up testing once every 6 months is reasonable. “If CPK [creatine phosphokinase] goes up, I don’t worry about it; it’s not clinically relevant. There is no recommendation for CPK monitoring, so if you’re getting that on your chem-20, I’d say don’t worry about it.”
Dr. Blauvelt reported that he is an investigator and a scientific adviser for several pharmaceutical companies developing treatments for AD, including companies that are evaluating or marketing JAK inhibitors for AD, including AbbVie, Incyte, and Pfizer, as well as dupilumab’s joint developers Sanofi and Regeneron.
AT PDA 2022
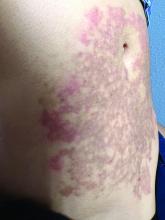
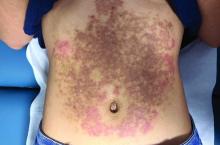
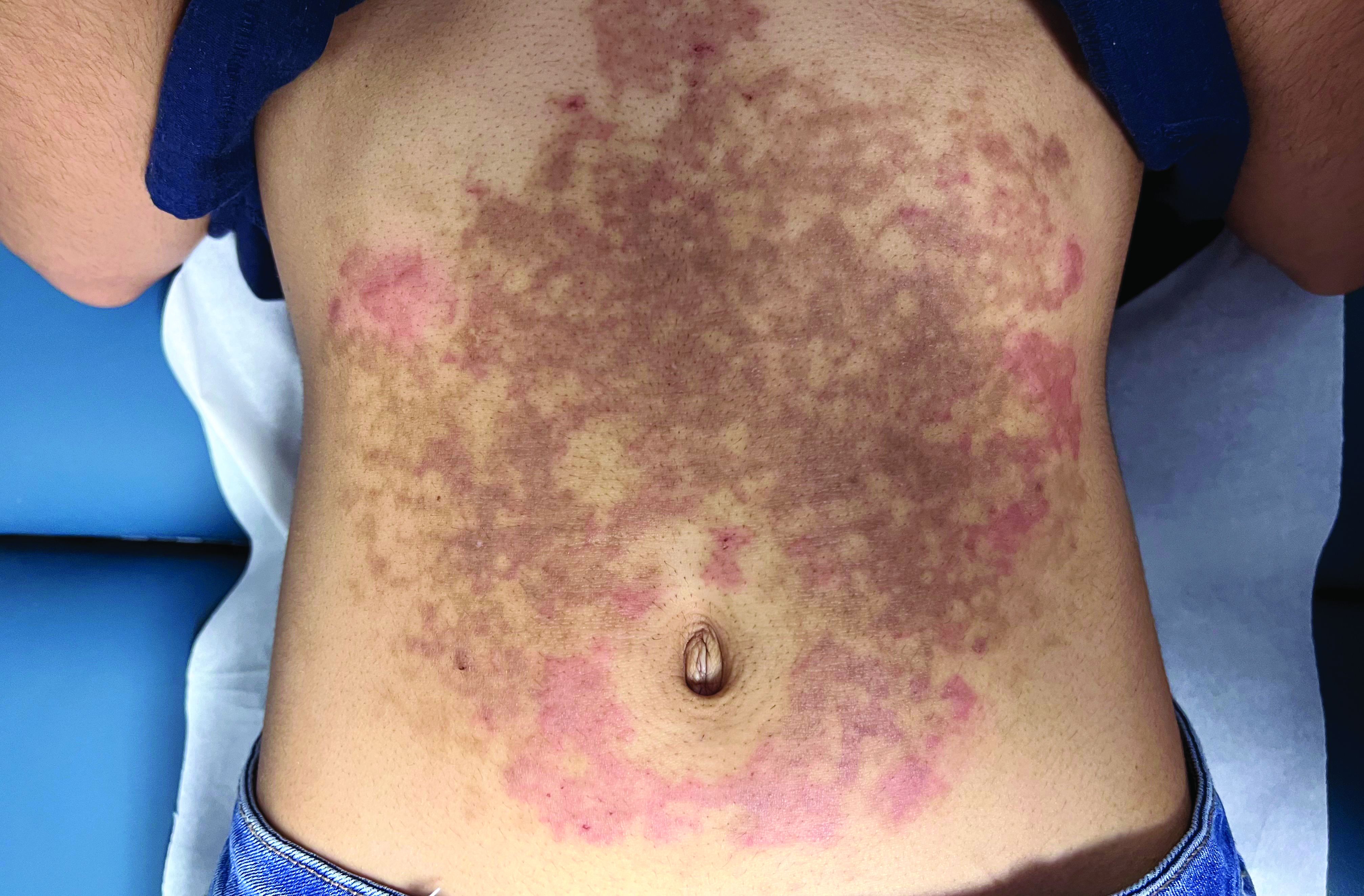
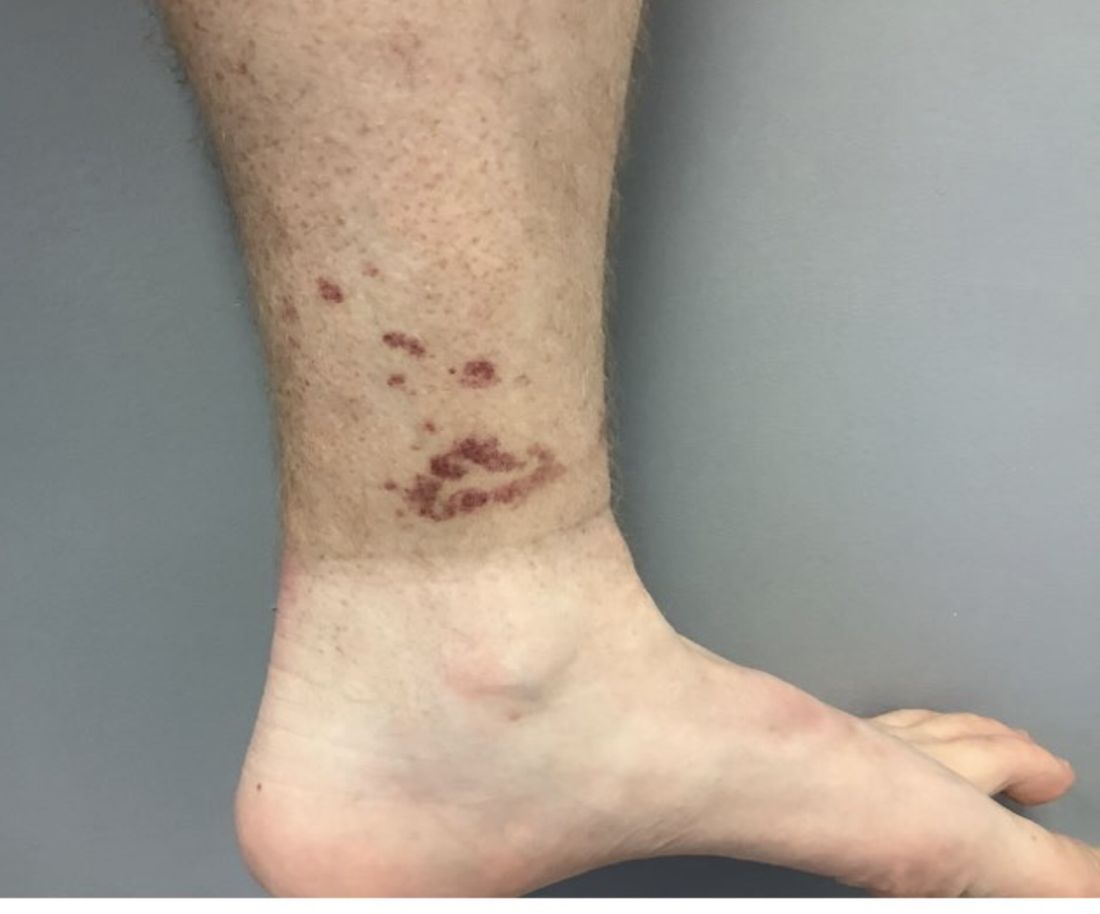
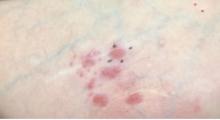
A White female presented with pruritic, reticulated, erythematous plaques on the abdomen
It is characterized by pruritic, erythematous papules, papulovesicles, and vesicles that appear in a reticular pattern, most commonly on the trunk. The lesions are typically followed by postinflammatory hyperpigmentation (PIH).
Although PP has been described in people of all races, ages, and sexes, it is predominantly observed in Japan, often in female young adults. Triggers may include a ketogenic diet, diabetes mellitus, and pregnancy. Friction and contact allergic reactions to chrome or nickel have been proposed as exogenous trigger factors. Individual cases of Sjögren’s syndrome, Helicobacter pylori infections, and adult Still syndrome have also been associated with recurrent eruptions.
The diagnosis of PP is made both clinically and by biopsy. The histological features vary according to the stage of the disease. In early-stage disease, superficial and perivascular infiltration of neutrophils are prominent. Later stages are characterized by spongiosis and necrotic keratinocytes.
The first-line therapy for prurigo pigmentosa is oral minocycline. However, for some patients, doxycycline, macrolide antibiotics, or dapsone may be indicated. Adding carbohydrates to a keto diet may be helpful. In this patient, a punch biopsy was performed, which revealed an interface dermatitis with eosinophils and neutrophils, consistent with prurigo pigmentosa. The cause of her PP remains idiopathic. She was treated with 100 mg doxycycline twice a day, which resulted in a resolution of active lesions. The patient did have postinflammatory hyperpigmentation.
This case and photo were submitted by Brooke Resh Sateesh, MD, of San Diego Family Dermatology, San Diego, California, and Mina Zulal, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany. Dr. Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Beutler et al. Am J Clin Dermatol. 2015 Dec;16(6):533-43.
2. Kim et al. J Dermatol. 2012 Nov;39(11):891-7.
3. Mufti et al. JAAD Int. 2021 Apr 10;3:79-87.
It is characterized by pruritic, erythematous papules, papulovesicles, and vesicles that appear in a reticular pattern, most commonly on the trunk. The lesions are typically followed by postinflammatory hyperpigmentation (PIH).
Although PP has been described in people of all races, ages, and sexes, it is predominantly observed in Japan, often in female young adults. Triggers may include a ketogenic diet, diabetes mellitus, and pregnancy. Friction and contact allergic reactions to chrome or nickel have been proposed as exogenous trigger factors. Individual cases of Sjögren’s syndrome, Helicobacter pylori infections, and adult Still syndrome have also been associated with recurrent eruptions.
The diagnosis of PP is made both clinically and by biopsy. The histological features vary according to the stage of the disease. In early-stage disease, superficial and perivascular infiltration of neutrophils are prominent. Later stages are characterized by spongiosis and necrotic keratinocytes.
The first-line therapy for prurigo pigmentosa is oral minocycline. However, for some patients, doxycycline, macrolide antibiotics, or dapsone may be indicated. Adding carbohydrates to a keto diet may be helpful. In this patient, a punch biopsy was performed, which revealed an interface dermatitis with eosinophils and neutrophils, consistent with prurigo pigmentosa. The cause of her PP remains idiopathic. She was treated with 100 mg doxycycline twice a day, which resulted in a resolution of active lesions. The patient did have postinflammatory hyperpigmentation.
This case and photo were submitted by Brooke Resh Sateesh, MD, of San Diego Family Dermatology, San Diego, California, and Mina Zulal, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany. Dr. Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Beutler et al. Am J Clin Dermatol. 2015 Dec;16(6):533-43.
2. Kim et al. J Dermatol. 2012 Nov;39(11):891-7.
3. Mufti et al. JAAD Int. 2021 Apr 10;3:79-87.
It is characterized by pruritic, erythematous papules, papulovesicles, and vesicles that appear in a reticular pattern, most commonly on the trunk. The lesions are typically followed by postinflammatory hyperpigmentation (PIH).
Although PP has been described in people of all races, ages, and sexes, it is predominantly observed in Japan, often in female young adults. Triggers may include a ketogenic diet, diabetes mellitus, and pregnancy. Friction and contact allergic reactions to chrome or nickel have been proposed as exogenous trigger factors. Individual cases of Sjögren’s syndrome, Helicobacter pylori infections, and adult Still syndrome have also been associated with recurrent eruptions.
The diagnosis of PP is made both clinically and by biopsy. The histological features vary according to the stage of the disease. In early-stage disease, superficial and perivascular infiltration of neutrophils are prominent. Later stages are characterized by spongiosis and necrotic keratinocytes.
The first-line therapy for prurigo pigmentosa is oral minocycline. However, for some patients, doxycycline, macrolide antibiotics, or dapsone may be indicated. Adding carbohydrates to a keto diet may be helpful. In this patient, a punch biopsy was performed, which revealed an interface dermatitis with eosinophils and neutrophils, consistent with prurigo pigmentosa. The cause of her PP remains idiopathic. She was treated with 100 mg doxycycline twice a day, which resulted in a resolution of active lesions. The patient did have postinflammatory hyperpigmentation.
This case and photo were submitted by Brooke Resh Sateesh, MD, of San Diego Family Dermatology, San Diego, California, and Mina Zulal, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany. Dr. Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Beutler et al. Am J Clin Dermatol. 2015 Dec;16(6):533-43.
2. Kim et al. J Dermatol. 2012 Nov;39(11):891-7.
3. Mufti et al. JAAD Int. 2021 Apr 10;3:79-87.
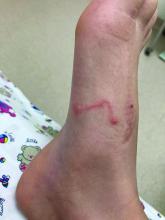
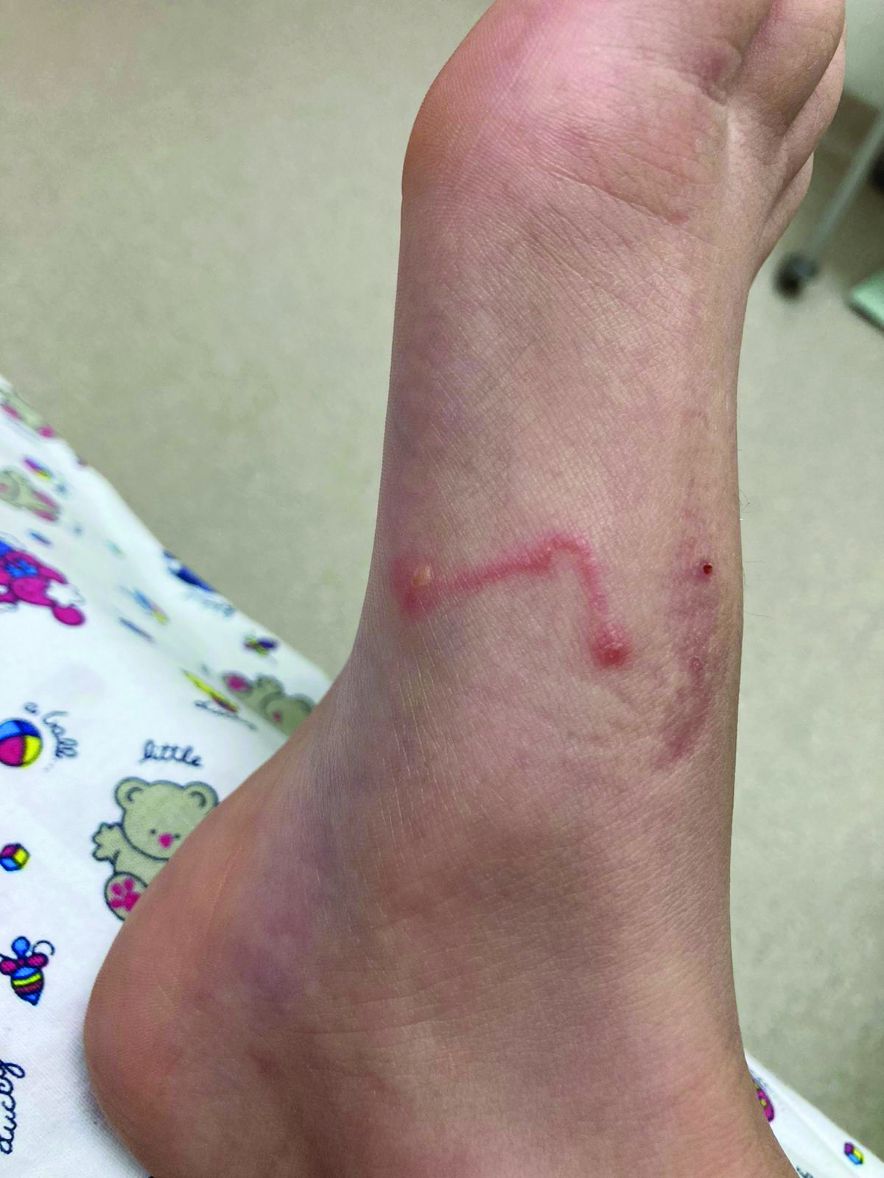
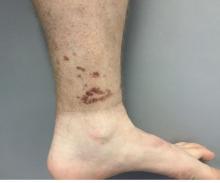
A 9-year-old girl was evaluated for a week-long history of rash on the feet
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
A complete body examination failed to reveal any other lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. She was diagnosed with cutaneous larva migrans (CLM) given the clinical appearance of the lesions and the recent travel history.
CLM is a zoonotic infection caused by several hookworms such as Ancylostoma braziliense, Ancylostoma caninum, and Uncinaria stenocephala, as well as human hookworms such as Ancylostoma duodenale and Necator americanus. The hookworms can be present in contaminated soils and sandy beaches on the coastal regions of South America, the Caribbean, the Southeastern United States, Southeast Asia, and Africa.1-5
It is a common disease in the tourist population visiting tropical countries because of exposure to the hookworms in the soil without use of proper foot protection.
The clinical features are of an erythematous linear serpiginous plaque that is pruritic and can progress from millimeters to centimeters in size within a few days to weeks. Vesicles and multiple tracks can also be seen. The most common locations are the feet, buttocks, and thighs.
The larvae in the soil come from eggs excreted in the feces of infected cats and dogs. The infection is caused by direct contact of the larvae with the stratum corneum of the skin creating a burrow and an inflammatory response that will cause erythema, edema, track formation, and pruritus.
Diagnosis is made clinically. Rarely, a skin biopsy is warranted. The differential diagnosis includes tinea pedis, granuloma annulare, larva currens, contact dermatitis, and herpes zoster.
Tinea pedis is a fungal infection of the skin of the feet, commonly localized on the web spaces. The risk factors are a hot and humid environment, prolonged wear of occlusive footwear, excess sweating, and prolonged exposure to water.6 Diagnosis is confirmed by microscopic evaluation of skin scrapings with potassium hydroxide or a fungal culture. The infection is treated with topical antifungal creams and, in severe cases, systemic antifungals. Granuloma annulare is a benign chronic skin condition that presents with annular-shaped lesions. Its etiology is unknown. The lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The feature distinguishing granuloma annulare from other annular lesions is its absence of scale.
Allergic contact dermatitis is caused by skin exposure to an allergen and a secondary inflammatory response to this material on the skin causing inflammation, vesiculation, and pruritus. Lesions are treated with topical corticosteroids and avoidance of the allergen.
Herpes zoster is caused by a viral infection of the latent varicella-zoster virus. Its reactivation causes the presence of vesicles with an erythematous base that have a dermatomal distribution. The lesions are usually tender. Treatment is recommended to be started within 72 hours of the eruption with antivirals such as acyclovir or valacyclovir.
Cutaneous larva currens is caused by the cutaneous infection with Strongyloides stercoralis. In comparison with CLM, the lesions progress faster, at up to a centimeter within hours.
CLM is usually self-limited. If the patient has multiple lesions or more severe disease, oral albendazole or ivermectin can be prescribed. Other treatments, though not preferred, include freezing and topical thiabendazole solutions.
As our patient had several lesions, oral ivermectin was chosen as treatment and the lesions cleared within a week. Also, she was recommended to always wear shoes when walking on the beach.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Valderrama is a pediatric dermatologist at Fundación Cardioinfantil, Bogota, Colombia.
References
1. Feldmeier H and Schuster A. Eur J Clin Microbiol Infect Dis. 2012 Jun;31(6):915-8.
2. Jacobson CC and Abel EA. J Am Acad Dermatol. 2007 Jun;56(6):1026-43.
3. Kincaid L et al. Travel Med Infect Dis. 2015 Sep-Oct;13(5):382-7.
4. Gill N et al. Adv Skin Wound Care. 2020 Jul;33(7):356-9.
5. Rodenas-Herranz T et al. Dermatol Ther. 2020 May;33(3):e13316.
6. Pramod K et al. In: StatPearls [Internet]. Treasure Island (Fla): StatPearls Publishing; 2022 Jan.
Her mother reported recent travel to a beachside city in Colombia. A review of systems was negative. She was not taking any other medications or vitamin supplements. There were no pets at home and no other affected family members. Physical exam was notable for an erythematous curvilinear plaque on the feet and a small vesicle.
Does hidradenitis suppurativa worsen during pregnancy?
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
AT PDA 2022
FDA acts against sales of unapproved mole and skin tag products on Amazon, other sites
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
A healthy White male presented with a rash consisting of erythematous to purpuric macules
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. .” A form of EIV has been described in the literature as “Disney dermatitis.” It is often seen in healthy adults after a long day of walking at the parks. Other forms of exercise, such as jogging, hiking, or swimming, may also cause the condition.
Clinically, EIV affects the lower legs and presents as purpuric macules. Edema may be present. Lesions may be asymptomatic or may present with pruritus or burning. Diagnosis is often made clinically. Skin biopsies for H&E and DIF (direct immunofluorescence) can help distinguish the type of vasculitis that is present. Laboratory tests may be needed to exclude other causes of vasculitis. Episodes may be recurrent.
Henoch-Schönlein purpura (HSP), also called anaphylactoid purpura, is a subtype of small-vessel vasculitis where IgA immunoglobulin is deposited in the vessel walls. It is the most common form of vasculitis is children (usually ages 4-8). In addition to skin, organs such as joints, kidneys, and intestines can be involved. Schamberg’s disease, or capillaritis, is also called pigmented purpura. In this benign condition, leakage from capillaries results in erythematous to brown patches on the lower extremities. A true vasculitis is not seen. The brown discoloration is due to hemosiderin deposition. Cryoglobulinemia is a rare condition in which abnormal immunoglobulin complexes deposit in tissues and vessels. Leukocytoclastic vasculitis is present in small vessels. Palpable purpura and livedo may be seen clinically, and systemic symptoms may be present.
Treatment of EIV is largely supportive as lesions will resolve on their own over 3-4 weeks. Postinflammatory hyperpigmentation may result. Temporary cessation of exercise and compression stockings can help speed up the resolution of lesions. Systemic medications used in the treatment of severe vasculitis, such as systemic steroids, dapsone, and colchicine, are not needed in EIV.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. .” A form of EIV has been described in the literature as “Disney dermatitis.” It is often seen in healthy adults after a long day of walking at the parks. Other forms of exercise, such as jogging, hiking, or swimming, may also cause the condition.
Clinically, EIV affects the lower legs and presents as purpuric macules. Edema may be present. Lesions may be asymptomatic or may present with pruritus or burning. Diagnosis is often made clinically. Skin biopsies for H&E and DIF (direct immunofluorescence) can help distinguish the type of vasculitis that is present. Laboratory tests may be needed to exclude other causes of vasculitis. Episodes may be recurrent.
Henoch-Schönlein purpura (HSP), also called anaphylactoid purpura, is a subtype of small-vessel vasculitis where IgA immunoglobulin is deposited in the vessel walls. It is the most common form of vasculitis is children (usually ages 4-8). In addition to skin, organs such as joints, kidneys, and intestines can be involved. Schamberg’s disease, or capillaritis, is also called pigmented purpura. In this benign condition, leakage from capillaries results in erythematous to brown patches on the lower extremities. A true vasculitis is not seen. The brown discoloration is due to hemosiderin deposition. Cryoglobulinemia is a rare condition in which abnormal immunoglobulin complexes deposit in tissues and vessels. Leukocytoclastic vasculitis is present in small vessels. Palpable purpura and livedo may be seen clinically, and systemic symptoms may be present.
Treatment of EIV is largely supportive as lesions will resolve on their own over 3-4 weeks. Postinflammatory hyperpigmentation may result. Temporary cessation of exercise and compression stockings can help speed up the resolution of lesions. Systemic medications used in the treatment of severe vasculitis, such as systemic steroids, dapsone, and colchicine, are not needed in EIV.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. .” A form of EIV has been described in the literature as “Disney dermatitis.” It is often seen in healthy adults after a long day of walking at the parks. Other forms of exercise, such as jogging, hiking, or swimming, may also cause the condition.
Clinically, EIV affects the lower legs and presents as purpuric macules. Edema may be present. Lesions may be asymptomatic or may present with pruritus or burning. Diagnosis is often made clinically. Skin biopsies for H&E and DIF (direct immunofluorescence) can help distinguish the type of vasculitis that is present. Laboratory tests may be needed to exclude other causes of vasculitis. Episodes may be recurrent.
Henoch-Schönlein purpura (HSP), also called anaphylactoid purpura, is a subtype of small-vessel vasculitis where IgA immunoglobulin is deposited in the vessel walls. It is the most common form of vasculitis is children (usually ages 4-8). In addition to skin, organs such as joints, kidneys, and intestines can be involved. Schamberg’s disease, or capillaritis, is also called pigmented purpura. In this benign condition, leakage from capillaries results in erythematous to brown patches on the lower extremities. A true vasculitis is not seen. The brown discoloration is due to hemosiderin deposition. Cryoglobulinemia is a rare condition in which abnormal immunoglobulin complexes deposit in tissues and vessels. Leukocytoclastic vasculitis is present in small vessels. Palpable purpura and livedo may be seen clinically, and systemic symptoms may be present.
Treatment of EIV is largely supportive as lesions will resolve on their own over 3-4 weeks. Postinflammatory hyperpigmentation may result. Temporary cessation of exercise and compression stockings can help speed up the resolution of lesions. Systemic medications used in the treatment of severe vasculitis, such as systemic steroids, dapsone, and colchicine, are not needed in EIV.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Can dietary tweaks improve some skin diseases?
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
A 64-year-old woman presents with a history of asymptomatic erythematous grouped papules on the right breast
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
Nevus of Ota: Does the 1064-nm Q-switched Nd:YAG laser work in Black patients?
SAN DIEGO – Using a , results from a small single-center study showed.
Nevus of Ota is a benign melanocytic lesion that presents as a unilateral blue-gray to blue-brown facial patch favoring the distribution of the first two branches of the trigeminal nerve. Among Asians, the prevalence of the condition among Asians is estimated to be between 0.03% and 1.113%, while the prevalence among Blacks population is estimated to be between 0.01% and 0.016%, Shelby L. Kubicki, MD, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery.
“Most existing literature describes the characteristics and treatment of Nevus of Ota based on Asian patients with skin types I-IV,” said Dr. Kubicki, a third-year dermatology resident at the University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston. “Special considerations are required when treating [Fitzpatrick skin types] V-VI, which is why it’s important to characterize these patients, to make sure they’re well represented in the literature.”
In what she said is the largest reported case series of its kind, Dr. Kubicki and colleagues identified eight Fitzpatrick skin type V or VI patients who underwent laser treatment for Nevus of Ota from 2016-2021. All were treated with the 1,064-nm Q‐switched Nd:YAG and on average, received 5.4 treatments at 2-10 month intervals. Fluence ranged from 1.8 to 2.4 J/cm2, and total pulse count ranged from 536.8 to 831.1. Two of these patients were additionally treated with 1,550-nm nonablative fractional resurfacing with a mean of six treatments. Primary outcomes were based on improvement of before and after clinical photographs by three independent board-certified dermatologists, who used a 5-point visual analogue scale for grading.
The mean age of patients was 30.4 years and ranged from 9 months to 45 years. Six were females and two were males, two had Fitzpatrick skin type V, and six had Fitzpatrick skin type VI. Of the eight patients, six had blue-gray lesions, one patient had a dark brown lesion, and one patient had “a hybrid lesion that had blue-gray and brown discoloration,” Dr. Kubicki said.
After grading of the clinical photographs, patients demonstrated a mean improvement of 51%-75% at follow-up 5-56 weeks after treatment (a mean of 16.9 weeks). No long-term adverse events were encountered in either group, but three patients developed mild guttate hypopigmentation following laser treatment.
“Lesional color may contribute to outcome, and patients should be educated about the risk of guttate hypopigmentation,” Dr. Kubicki said. “More studies are needed to determine the optimal device and treatment settings in this population.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that, while the study results impressed her, she speculated that the patients may require more treatments in the future. “What to look out for is the risk of rebound,” Dr. Onwudiwe said. “Because Nevus of Ota is a hamartomatous lesion, it’s very hard to treat, and sometimes it will come back. It will be nice to see how long this treatment can last. If you can use a combination therapy and have ... cases where you’re only needing a touch-up every so often, that’s still a win.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, D.C., said that he wondered what histologic analysis following treatment might show, and if a biopsy after treatment would show “if we really got rid of the nevus, or if we are just cosmetically improving the appearance temporarily.”
Neither Dr. Kubicki nor Dr. Onwudiwe reported having financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure. He has also received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
SAN DIEGO – Using a , results from a small single-center study showed.
Nevus of Ota is a benign melanocytic lesion that presents as a unilateral blue-gray to blue-brown facial patch favoring the distribution of the first two branches of the trigeminal nerve. Among Asians, the prevalence of the condition among Asians is estimated to be between 0.03% and 1.113%, while the prevalence among Blacks population is estimated to be between 0.01% and 0.016%, Shelby L. Kubicki, MD, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery.
“Most existing literature describes the characteristics and treatment of Nevus of Ota based on Asian patients with skin types I-IV,” said Dr. Kubicki, a third-year dermatology resident at the University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston. “Special considerations are required when treating [Fitzpatrick skin types] V-VI, which is why it’s important to characterize these patients, to make sure they’re well represented in the literature.”
In what she said is the largest reported case series of its kind, Dr. Kubicki and colleagues identified eight Fitzpatrick skin type V or VI patients who underwent laser treatment for Nevus of Ota from 2016-2021. All were treated with the 1,064-nm Q‐switched Nd:YAG and on average, received 5.4 treatments at 2-10 month intervals. Fluence ranged from 1.8 to 2.4 J/cm2, and total pulse count ranged from 536.8 to 831.1. Two of these patients were additionally treated with 1,550-nm nonablative fractional resurfacing with a mean of six treatments. Primary outcomes were based on improvement of before and after clinical photographs by three independent board-certified dermatologists, who used a 5-point visual analogue scale for grading.
The mean age of patients was 30.4 years and ranged from 9 months to 45 years. Six were females and two were males, two had Fitzpatrick skin type V, and six had Fitzpatrick skin type VI. Of the eight patients, six had blue-gray lesions, one patient had a dark brown lesion, and one patient had “a hybrid lesion that had blue-gray and brown discoloration,” Dr. Kubicki said.
After grading of the clinical photographs, patients demonstrated a mean improvement of 51%-75% at follow-up 5-56 weeks after treatment (a mean of 16.9 weeks). No long-term adverse events were encountered in either group, but three patients developed mild guttate hypopigmentation following laser treatment.
“Lesional color may contribute to outcome, and patients should be educated about the risk of guttate hypopigmentation,” Dr. Kubicki said. “More studies are needed to determine the optimal device and treatment settings in this population.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that, while the study results impressed her, she speculated that the patients may require more treatments in the future. “What to look out for is the risk of rebound,” Dr. Onwudiwe said. “Because Nevus of Ota is a hamartomatous lesion, it’s very hard to treat, and sometimes it will come back. It will be nice to see how long this treatment can last. If you can use a combination therapy and have ... cases where you’re only needing a touch-up every so often, that’s still a win.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, D.C., said that he wondered what histologic analysis following treatment might show, and if a biopsy after treatment would show “if we really got rid of the nevus, or if we are just cosmetically improving the appearance temporarily.”
Neither Dr. Kubicki nor Dr. Onwudiwe reported having financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure. He has also received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
SAN DIEGO – Using a , results from a small single-center study showed.
Nevus of Ota is a benign melanocytic lesion that presents as a unilateral blue-gray to blue-brown facial patch favoring the distribution of the first two branches of the trigeminal nerve. Among Asians, the prevalence of the condition among Asians is estimated to be between 0.03% and 1.113%, while the prevalence among Blacks population is estimated to be between 0.01% and 0.016%, Shelby L. Kubicki, MD, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery.
“Most existing literature describes the characteristics and treatment of Nevus of Ota based on Asian patients with skin types I-IV,” said Dr. Kubicki, a third-year dermatology resident at the University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston. “Special considerations are required when treating [Fitzpatrick skin types] V-VI, which is why it’s important to characterize these patients, to make sure they’re well represented in the literature.”
In what she said is the largest reported case series of its kind, Dr. Kubicki and colleagues identified eight Fitzpatrick skin type V or VI patients who underwent laser treatment for Nevus of Ota from 2016-2021. All were treated with the 1,064-nm Q‐switched Nd:YAG and on average, received 5.4 treatments at 2-10 month intervals. Fluence ranged from 1.8 to 2.4 J/cm2, and total pulse count ranged from 536.8 to 831.1. Two of these patients were additionally treated with 1,550-nm nonablative fractional resurfacing with a mean of six treatments. Primary outcomes were based on improvement of before and after clinical photographs by three independent board-certified dermatologists, who used a 5-point visual analogue scale for grading.
The mean age of patients was 30.4 years and ranged from 9 months to 45 years. Six were females and two were males, two had Fitzpatrick skin type V, and six had Fitzpatrick skin type VI. Of the eight patients, six had blue-gray lesions, one patient had a dark brown lesion, and one patient had “a hybrid lesion that had blue-gray and brown discoloration,” Dr. Kubicki said.
After grading of the clinical photographs, patients demonstrated a mean improvement of 51%-75% at follow-up 5-56 weeks after treatment (a mean of 16.9 weeks). No long-term adverse events were encountered in either group, but three patients developed mild guttate hypopigmentation following laser treatment.
“Lesional color may contribute to outcome, and patients should be educated about the risk of guttate hypopigmentation,” Dr. Kubicki said. “More studies are needed to determine the optimal device and treatment settings in this population.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that, while the study results impressed her, she speculated that the patients may require more treatments in the future. “What to look out for is the risk of rebound,” Dr. Onwudiwe said. “Because Nevus of Ota is a hamartomatous lesion, it’s very hard to treat, and sometimes it will come back. It will be nice to see how long this treatment can last. If you can use a combination therapy and have ... cases where you’re only needing a touch-up every so often, that’s still a win.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, D.C., said that he wondered what histologic analysis following treatment might show, and if a biopsy after treatment would show “if we really got rid of the nevus, or if we are just cosmetically improving the appearance temporarily.”
Neither Dr. Kubicki nor Dr. Onwudiwe reported having financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure. He has also received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
AT ASLMS 2022