User login
Medicaid coverage of HPV vaccine in adults: Implications in dermatology
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
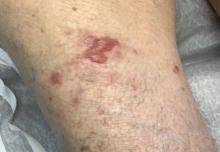
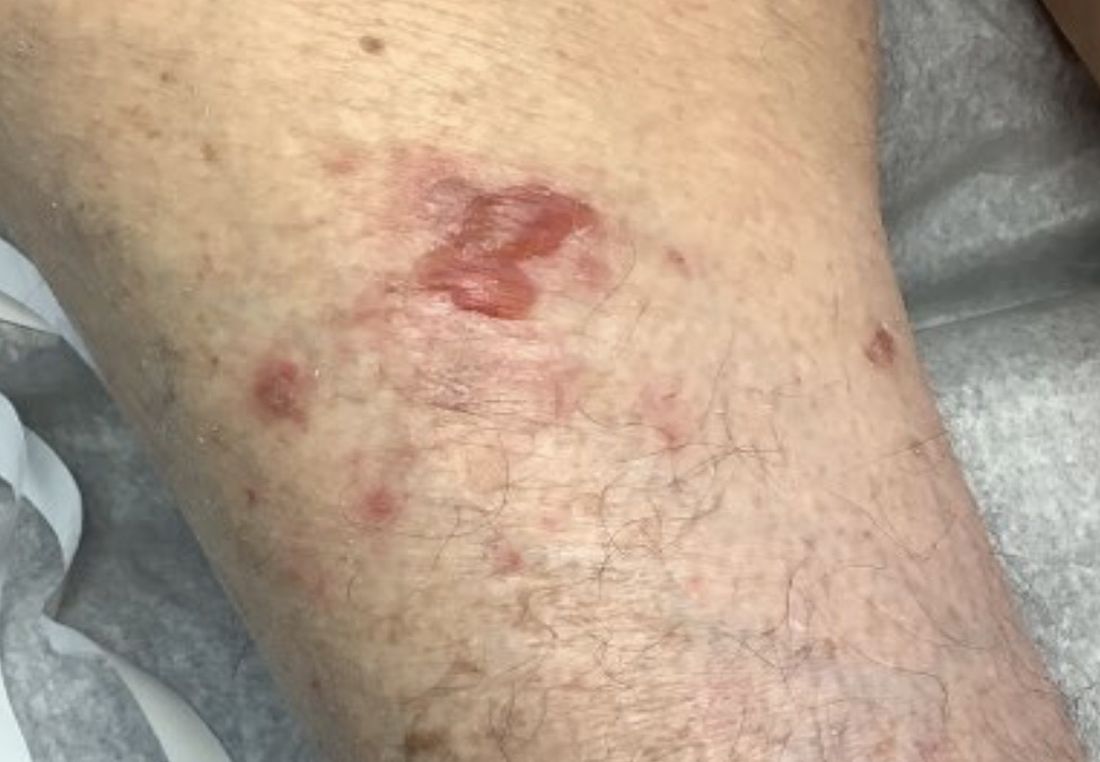
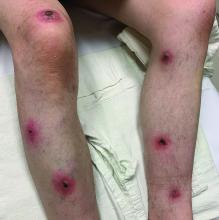
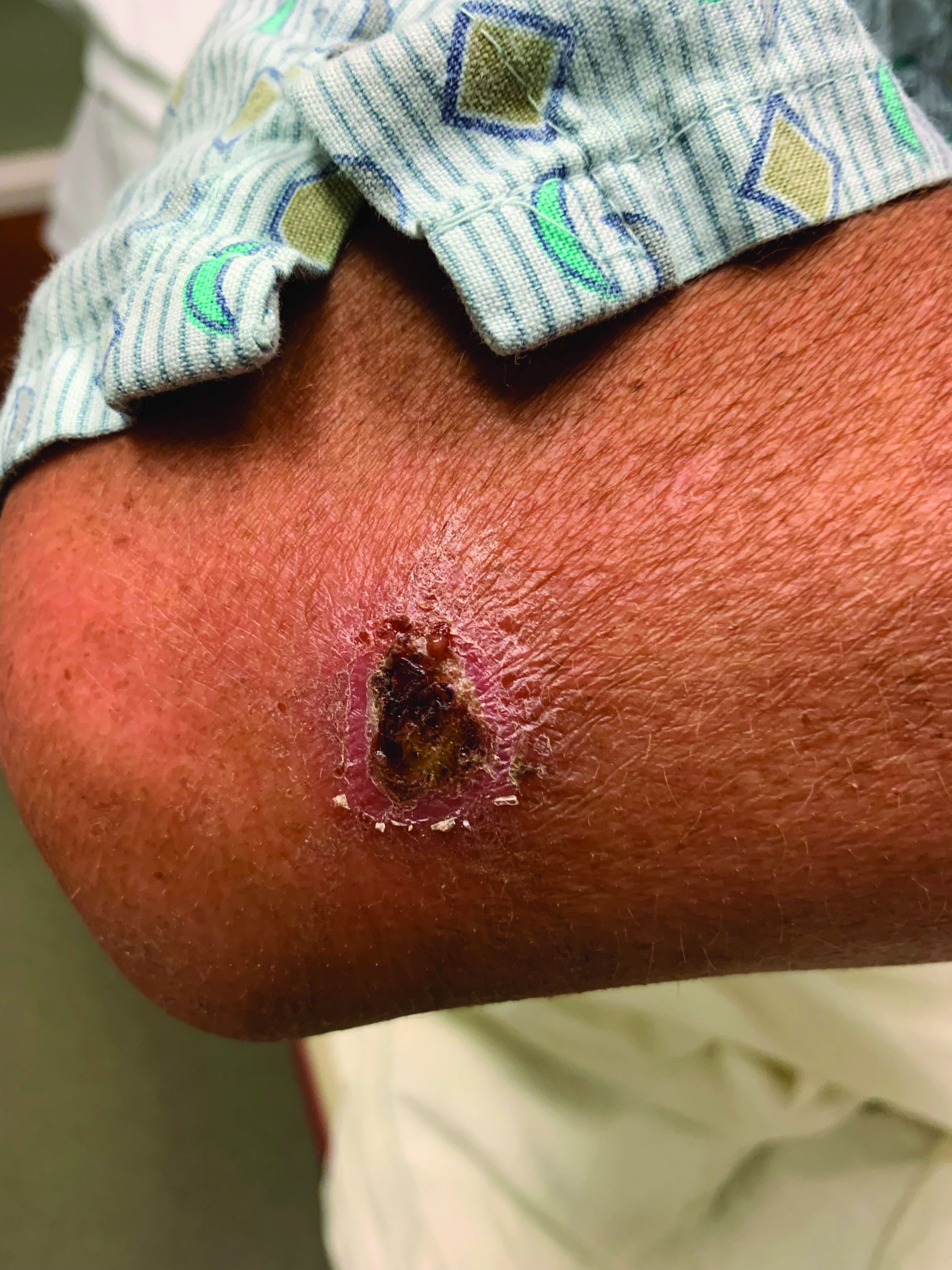
A 95-year-old White male with hypertension presented with itchy patches and bullae on the trunk and extremities
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
Novel stepwise method found to benefit patients with severe rhinophyma
DENVER –
Rhinophyma occurs primarily in the sixth and seventh decades of life and is marked by facial hypertrophy that leads to tumor-like growth, inflammation, fibrosis, and loss of the cosmetic nasal subunits. “When it becomes severe it leads to a degree of embarrassment as well,” one of the study authors, Patricia Richey, MD, said during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery. “We found that our method has been efficacious, but most often, and more importantly, leads to an improvement in the patient’s quality of life.”
To date, clinicians have used fully ablative lasers to treat varying degrees of rhinophyma but at a cost of prolonged healing time and higher rates of scarring and pigment or textural changes. However, not all dermatologists use full-field ablative lasers in their practices.
“Fractionated ablative lasers have been used in the past for mild to moderate rhinophyma, but they cannot ablate to 100% density, which would be necessary to debulk the marked hypertrophy present in our patients,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “That’s why we added a surgical component.”
She and colleague Mathew M. Avram, MD, JD, developed a three-step method for treating severe rhinophyma that they performed on three elderly patients. Step 1 is the surgical debulk. Following infiltration of local anesthesia, a razor blade or 15-blade is used to excise the most prominent lobules of hypertrophied sebaceous tissue down to the fibrofatty layer of the nose as a partial thickness excision that does not reach the level of the perichondrium or cartilage. “Hemostasis is achieved with electrocoagulation and application of petrolatum ointment, followed by a pressure dressing,” Dr. Richey said. “The location of the debulk varies by patient.”
Step 2 involves fractionated ablative laser treatment 4 weeks later with either the CO2 or erbium:YAG (Er:YAG) 2,940-nm laser. According to Dr. Richey, the typical setting for the fractionated CO2 is a fluence of 70mJ/cm2 and a high density, performing six out of four passes with 60 seconds between each pass, “though these settings may vary based on the patient presentation,” she said.
The treatment level ranges from 5 (14% density) to 10 (70% density, for the most severe cases). Meanwhile, a representative setting for the ablative fractionated Er:YAG 2,940-nm laser is 250 mcm, no coagulation, 5.5% density, and one pass. “If a second surgical debulk is performed on the same day as ablative laser treatment, the sites of shave removal are typically avoided with the laser,” she said. If a certain portion of the nose has recently healed following surgical debulk 4 weeks prior, they may perform only two passes in this region.
In an interview, Dr. Avram, who directs the MGH Dermatology Laser and Cosmetic Center, characterized the staged method as providing “transformative change to severe, cosmetically disfiguring rhinophyma. The ablative fractional laser provides more fine-tuned contouring.”
The three patients studied had an average of three to four monthly treatments. “There is typically a great deal of improvement by the second treatment,” Dr. Richey said. Add-on treatments may include low voltage electrodessication at 1.8 watts for patients with well-demarcated papules of sebaceous hyperplasia, and a vascular laser such as the pulsed dye laser if telangiectasias are present.
One limitation of the stepwise method, she said, is that the surgical debulk typically results in a scar, “but it’s rarely noticeable if carefully performed, likely due to fractionated ablative use during the scar remodeling period. It’s important to set expectations with your patient at the initial consult. We always discuss treatment goals and that while we aim achieve the most desirable outcome possible, we’re never going to get them back to having a completely normal nose. They’re always going to have some mild or moderate rhinophymatous changes present.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on these results, characterized the stepwise method as promising. “Though more treatments are required, the easier recovery, safe outcomes in the case presented and excellent cosmetic result made it an interesting alternative when fully ablative resurfacing is daunting, either for patients or physicians involved,” he said in an interview.
The researchers reported having no relevant disclosures. Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory board for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
DENVER –
Rhinophyma occurs primarily in the sixth and seventh decades of life and is marked by facial hypertrophy that leads to tumor-like growth, inflammation, fibrosis, and loss of the cosmetic nasal subunits. “When it becomes severe it leads to a degree of embarrassment as well,” one of the study authors, Patricia Richey, MD, said during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery. “We found that our method has been efficacious, but most often, and more importantly, leads to an improvement in the patient’s quality of life.”
To date, clinicians have used fully ablative lasers to treat varying degrees of rhinophyma but at a cost of prolonged healing time and higher rates of scarring and pigment or textural changes. However, not all dermatologists use full-field ablative lasers in their practices.
“Fractionated ablative lasers have been used in the past for mild to moderate rhinophyma, but they cannot ablate to 100% density, which would be necessary to debulk the marked hypertrophy present in our patients,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “That’s why we added a surgical component.”
She and colleague Mathew M. Avram, MD, JD, developed a three-step method for treating severe rhinophyma that they performed on three elderly patients. Step 1 is the surgical debulk. Following infiltration of local anesthesia, a razor blade or 15-blade is used to excise the most prominent lobules of hypertrophied sebaceous tissue down to the fibrofatty layer of the nose as a partial thickness excision that does not reach the level of the perichondrium or cartilage. “Hemostasis is achieved with electrocoagulation and application of petrolatum ointment, followed by a pressure dressing,” Dr. Richey said. “The location of the debulk varies by patient.”
Step 2 involves fractionated ablative laser treatment 4 weeks later with either the CO2 or erbium:YAG (Er:YAG) 2,940-nm laser. According to Dr. Richey, the typical setting for the fractionated CO2 is a fluence of 70mJ/cm2 and a high density, performing six out of four passes with 60 seconds between each pass, “though these settings may vary based on the patient presentation,” she said.
The treatment level ranges from 5 (14% density) to 10 (70% density, for the most severe cases). Meanwhile, a representative setting for the ablative fractionated Er:YAG 2,940-nm laser is 250 mcm, no coagulation, 5.5% density, and one pass. “If a second surgical debulk is performed on the same day as ablative laser treatment, the sites of shave removal are typically avoided with the laser,” she said. If a certain portion of the nose has recently healed following surgical debulk 4 weeks prior, they may perform only two passes in this region.
In an interview, Dr. Avram, who directs the MGH Dermatology Laser and Cosmetic Center, characterized the staged method as providing “transformative change to severe, cosmetically disfiguring rhinophyma. The ablative fractional laser provides more fine-tuned contouring.”
The three patients studied had an average of three to four monthly treatments. “There is typically a great deal of improvement by the second treatment,” Dr. Richey said. Add-on treatments may include low voltage electrodessication at 1.8 watts for patients with well-demarcated papules of sebaceous hyperplasia, and a vascular laser such as the pulsed dye laser if telangiectasias are present.
One limitation of the stepwise method, she said, is that the surgical debulk typically results in a scar, “but it’s rarely noticeable if carefully performed, likely due to fractionated ablative use during the scar remodeling period. It’s important to set expectations with your patient at the initial consult. We always discuss treatment goals and that while we aim achieve the most desirable outcome possible, we’re never going to get them back to having a completely normal nose. They’re always going to have some mild or moderate rhinophymatous changes present.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on these results, characterized the stepwise method as promising. “Though more treatments are required, the easier recovery, safe outcomes in the case presented and excellent cosmetic result made it an interesting alternative when fully ablative resurfacing is daunting, either for patients or physicians involved,” he said in an interview.
The researchers reported having no relevant disclosures. Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory board for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
DENVER –
Rhinophyma occurs primarily in the sixth and seventh decades of life and is marked by facial hypertrophy that leads to tumor-like growth, inflammation, fibrosis, and loss of the cosmetic nasal subunits. “When it becomes severe it leads to a degree of embarrassment as well,” one of the study authors, Patricia Richey, MD, said during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery. “We found that our method has been efficacious, but most often, and more importantly, leads to an improvement in the patient’s quality of life.”
To date, clinicians have used fully ablative lasers to treat varying degrees of rhinophyma but at a cost of prolonged healing time and higher rates of scarring and pigment or textural changes. However, not all dermatologists use full-field ablative lasers in their practices.
“Fractionated ablative lasers have been used in the past for mild to moderate rhinophyma, but they cannot ablate to 100% density, which would be necessary to debulk the marked hypertrophy present in our patients,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “That’s why we added a surgical component.”
She and colleague Mathew M. Avram, MD, JD, developed a three-step method for treating severe rhinophyma that they performed on three elderly patients. Step 1 is the surgical debulk. Following infiltration of local anesthesia, a razor blade or 15-blade is used to excise the most prominent lobules of hypertrophied sebaceous tissue down to the fibrofatty layer of the nose as a partial thickness excision that does not reach the level of the perichondrium or cartilage. “Hemostasis is achieved with electrocoagulation and application of petrolatum ointment, followed by a pressure dressing,” Dr. Richey said. “The location of the debulk varies by patient.”
Step 2 involves fractionated ablative laser treatment 4 weeks later with either the CO2 or erbium:YAG (Er:YAG) 2,940-nm laser. According to Dr. Richey, the typical setting for the fractionated CO2 is a fluence of 70mJ/cm2 and a high density, performing six out of four passes with 60 seconds between each pass, “though these settings may vary based on the patient presentation,” she said.
The treatment level ranges from 5 (14% density) to 10 (70% density, for the most severe cases). Meanwhile, a representative setting for the ablative fractionated Er:YAG 2,940-nm laser is 250 mcm, no coagulation, 5.5% density, and one pass. “If a second surgical debulk is performed on the same day as ablative laser treatment, the sites of shave removal are typically avoided with the laser,” she said. If a certain portion of the nose has recently healed following surgical debulk 4 weeks prior, they may perform only two passes in this region.
In an interview, Dr. Avram, who directs the MGH Dermatology Laser and Cosmetic Center, characterized the staged method as providing “transformative change to severe, cosmetically disfiguring rhinophyma. The ablative fractional laser provides more fine-tuned contouring.”
The three patients studied had an average of three to four monthly treatments. “There is typically a great deal of improvement by the second treatment,” Dr. Richey said. Add-on treatments may include low voltage electrodessication at 1.8 watts for patients with well-demarcated papules of sebaceous hyperplasia, and a vascular laser such as the pulsed dye laser if telangiectasias are present.
One limitation of the stepwise method, she said, is that the surgical debulk typically results in a scar, “but it’s rarely noticeable if carefully performed, likely due to fractionated ablative use during the scar remodeling period. It’s important to set expectations with your patient at the initial consult. We always discuss treatment goals and that while we aim achieve the most desirable outcome possible, we’re never going to get them back to having a completely normal nose. They’re always going to have some mild or moderate rhinophymatous changes present.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on these results, characterized the stepwise method as promising. “Though more treatments are required, the easier recovery, safe outcomes in the case presented and excellent cosmetic result made it an interesting alternative when fully ablative resurfacing is daunting, either for patients or physicians involved,” he said in an interview.
The researchers reported having no relevant disclosures. Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory board for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
AT ASDS 2022
Combination of energy-based treatments found to improve Becker’s nevi
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
Denver – out to 40 weeks, results of a small retrospective case series demonstrated.
During an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery, presenting author Shelby L. Kubicki, MD, said that NAFR and LHR target the clinically bothersome Becker’s nevi features of hyperpigmentation and hypertrichosis via different mechanisms. “NAFR creates microcolumns of thermal injury in the skin, which improves hyperpigmentation,” explained Dr. Kubicki, a 3rd-year dermatology resident at University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston.
“LHR targets follicular melanocytes, which are located more deeply in the dermis,” she said. “This improves hypertrichosis and likely prevents recurrence of hyperpigmentation by targeting these melanocytes that are not reached by NAFR.”
Dr. Kubicki and her colleagues retrospectively reviewed 12 patients with Becker’s nevus who underwent a mean of 5.3 NAFR treatments at a single dermatology practice at intervals that ranged between 1 and 4 months. The long-pulsed 755-nm alexandrite laser was used for study participants with skin types I-III, while the long-pulsed 1,064-nm Nd: YAG laser was used for those with skin types IV-VI. Ten of the 12 patients underwent concomitant LHR with one of the two devices and three independent physicians used a 5-point visual analog scale (VAS) to rate clinical photographs. All patients completed a strict pre- and postoperative regimen with either 4% hydroquinone or topical 3% tranexamic acid and broad-spectrum sunscreen and postoperative treatment with a midpotency topical corticosteroid for 3 days.
The study is the largest known case series of therapy combining 1,550-nm NAFR and LHR for Becker’s nevus patients with skin types III-VI.
After comparing VAS scores at baseline and follow-up, physicians rated the cosmetic appearance of Becker’s nevus as improving by a range of 51%-75%. Two patients did not undergo LHR: one male patient with Becker’s nevus in his beard region, for whom LHR was undesirable, and a second patient with atrichotic Becker’s nevus. These two patients demonstrated improvements in VAS scores of 26%-50% and 76%-99%, respectively.
No long-term adverse events were observed during follow-up, which ranged from 6 to 40 weeks. “We do want more long-term follow-up,” Dr. Kubicki said, noting that there are more data on some patients to extend the follow-up.
She and her coinvestigators concluded that the results show that treatment with a combination of NAFR and LHR safely addresses both hyperpigmentation and hypertrichosis in Becker’s nevi. “In addition, LHR likely prevents recurrence of hyperpigmentation by targeting follicular melanocytes,” she said. “In our study, we did have one patient experience recurrence of a Becker’s nevus during follow-up, but [the rest] did not, which we considered a success.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on the study, characterized Becker’s nevus as a difficult-to-treat condition that is made even more difficult to treat in skin types III-VI.
“Combining laser hair removal using appropriate wavelengths with 1,550-nm nonablative fractional resurfacing yielded good clinical results with few recurrences,” he said in an interview with this news organization. “Though it was a small series, it definitely is an interesting option for practicing dermatologists who encounter patients interested in improving the appearance of a Becker’s nevus.”
The researchers reported having no relevant disclosures.
Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory boards for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
AT ASDS 2022
How do patients with chronic urticaria fare during pregnancy?
In addition, the rates of preterm births and medical problems of newborns in patients with CU are similar to those of the normal population and not linked to treatment used during pregnancy.
Those are the key findings from an analysis of new data from PREG-CU, an international, multicenter study of the Urticaria Centers of Reference and Excellence (UCARE) network. Results from the first PREG-CU analysis published in 2021 found that CU improved in about half of patients with CU during pregnancy. “However, two in five patients reported acute exacerbations of CU especially at the beginning and end of pregnancy,” investigators led by Emek Kocatürk, MD, of the department of dermatology and UCARE at Koç University School of Medicine, Istanbul, wrote in the new study, recently published in the Journal of the European Academy of Dermatology and Venereology.
“In addition, 1 in 10 pregnant CU patients required urticaria emergency care and 1 of 6 had angioedema during pregnancy,” they said. Risk factors for worsening CU during pregnancy, they added, were “mild disease and no angioedema before pregnancy, not taking treatment before pregnancy, chronic inducible urticaria, CU worsening during a previous pregnancy, stress as a driver of exacerbations, and treatment during pregnancy.”
Analysis involved 288 pregnant women
To optimize treatment of CU during pregnancy and to better understand how treatment affects pregnancy outcomes, the researchers analyzed 288 pregnancies in 288 women with CU from 13 countries and 21 centers worldwide. Their mean age at pregnancy was 32.1 years, and their mean duration of CU was 84.9 months. Prior to pregnancy, 35.7% of patients rated the severity of their CU symptoms as mild, 34.2% rated it as moderate, and 29.7% rated it as severe.
The researchers found that during pregnancy, 60% of patients used urticaria medication, including standard-dose second-generation H1-antihistamines (35.1%), first-generation H1-antihistamines (7.6%), high-dose second-generation H1-antihistamines (5.6%), and omalizumab (5.6%). The preterm birth rate was 10.2%, which was similar between patients who did and did not receive treatment during pregnancy (11.6% vs. 8.7%, respectively; P = .59).
On multivariate logistic regression, two predictors for preterm birth emerged: giving birth to twins (a 13.3-fold increased risk; P = .016) and emergency referrals for CU (a 4.3-fold increased risk; P =.016). The cesarean delivery rate was 51.3%, and more than 90% of newborns were healthy at birth. There was no link between any patient or disease characteristics or treatments and medical problems at birth.
In other findings, 78.8% of women with CU breastfed their babies. Of the 58 patients who did not breastfeed, 20.7% indicated severe urticaria/angioedema and/or taking medications as the main reason for not breastfeeding.
“Most CU patients use treatment during pregnancy and such treatments, especially second generation H1 antihistamines, seem to be safe during pregnancy regardless of the trimester,” the researchers concluded. “Outcomes of pregnancy in patients with CU were similar compared to the general population and not linked to treatment used during pregnancy. Notably, emergency referral for CU was an independent risk factor for preterm birth,” and the high cesarean delivery rate was “probably linked to comorbidities associated with the disease,” they added. “Overall, these findings suggest that patients should continue their treatments using an individualized dose to provide optimal symptom control.”
International guidelines
The authors noted that international guidelines for the management of urticaria published in 2022 suggest that modern second-generation H1-antihistamines should be used for pregnant patients, preferably loratadine with a possible extrapolation to desloratadine, cetirizine, or levocetirizine.
“Similarly, in this population, we found that cetirizine and loratadine were the most commonly used antihistamines, followed by levocetirizine and fexofenadine,” Dr. Kocatürk and colleagues wrote.
“Guidelines also suggest that the use of first-generation H1-antihistamines should be avoided given their sedative effects; but if these are to be given, it would be wise to know that use of first-generation H1-antihistamines immediately before parturition could cause respiratory depression and other adverse effects in the neonate,” they added, noting that chlorpheniramine and diphenhydramine are the first-generation H1-antihistamines with the greatest evidence of safety in pregnancy.
They acknowledged certain limitations of the analysis, including its retrospective design and the fact that there were no data on low birth weight, small for gestational age, or miscarriage rates. In addition, disease activity or severity during pregnancy and after birth were not monitored.
Asked to comment on these results, Raj Chovatiya, MD, PhD, who directs the center for eczema and itch in the department of dermatology at Northwestern University, Chicago, noted that despite a higher prevalence of CU among females compared with males, very little is known about how the condition is managed during pregnancy. “This retrospective study shows that most patients continue to utilize CU treatment during pregnancy (primarily second-generation antihistamines), with similar birth outcomes as the general population,” he said. “Interestingly, cesarean rates were higher among mothers with CU, and emergency CU referral was a risk factor for preterm birth. While additional prospective studies are needed, these results suggest that CU patients should be carefully managed, particularly during pregnancy, when treatment should be optimized.”
Dr. Kocatürk reported having received personal fees from Novartis, Ibrahim Etem-Menarini, and Sanofi, outside the submitted work. Many coauthors reported having numerous financial disclosures. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
In addition, the rates of preterm births and medical problems of newborns in patients with CU are similar to those of the normal population and not linked to treatment used during pregnancy.
Those are the key findings from an analysis of new data from PREG-CU, an international, multicenter study of the Urticaria Centers of Reference and Excellence (UCARE) network. Results from the first PREG-CU analysis published in 2021 found that CU improved in about half of patients with CU during pregnancy. “However, two in five patients reported acute exacerbations of CU especially at the beginning and end of pregnancy,” investigators led by Emek Kocatürk, MD, of the department of dermatology and UCARE at Koç University School of Medicine, Istanbul, wrote in the new study, recently published in the Journal of the European Academy of Dermatology and Venereology.
“In addition, 1 in 10 pregnant CU patients required urticaria emergency care and 1 of 6 had angioedema during pregnancy,” they said. Risk factors for worsening CU during pregnancy, they added, were “mild disease and no angioedema before pregnancy, not taking treatment before pregnancy, chronic inducible urticaria, CU worsening during a previous pregnancy, stress as a driver of exacerbations, and treatment during pregnancy.”
Analysis involved 288 pregnant women
To optimize treatment of CU during pregnancy and to better understand how treatment affects pregnancy outcomes, the researchers analyzed 288 pregnancies in 288 women with CU from 13 countries and 21 centers worldwide. Their mean age at pregnancy was 32.1 years, and their mean duration of CU was 84.9 months. Prior to pregnancy, 35.7% of patients rated the severity of their CU symptoms as mild, 34.2% rated it as moderate, and 29.7% rated it as severe.
The researchers found that during pregnancy, 60% of patients used urticaria medication, including standard-dose second-generation H1-antihistamines (35.1%), first-generation H1-antihistamines (7.6%), high-dose second-generation H1-antihistamines (5.6%), and omalizumab (5.6%). The preterm birth rate was 10.2%, which was similar between patients who did and did not receive treatment during pregnancy (11.6% vs. 8.7%, respectively; P = .59).
On multivariate logistic regression, two predictors for preterm birth emerged: giving birth to twins (a 13.3-fold increased risk; P = .016) and emergency referrals for CU (a 4.3-fold increased risk; P =.016). The cesarean delivery rate was 51.3%, and more than 90% of newborns were healthy at birth. There was no link between any patient or disease characteristics or treatments and medical problems at birth.
In other findings, 78.8% of women with CU breastfed their babies. Of the 58 patients who did not breastfeed, 20.7% indicated severe urticaria/angioedema and/or taking medications as the main reason for not breastfeeding.
“Most CU patients use treatment during pregnancy and such treatments, especially second generation H1 antihistamines, seem to be safe during pregnancy regardless of the trimester,” the researchers concluded. “Outcomes of pregnancy in patients with CU were similar compared to the general population and not linked to treatment used during pregnancy. Notably, emergency referral for CU was an independent risk factor for preterm birth,” and the high cesarean delivery rate was “probably linked to comorbidities associated with the disease,” they added. “Overall, these findings suggest that patients should continue their treatments using an individualized dose to provide optimal symptom control.”
International guidelines
The authors noted that international guidelines for the management of urticaria published in 2022 suggest that modern second-generation H1-antihistamines should be used for pregnant patients, preferably loratadine with a possible extrapolation to desloratadine, cetirizine, or levocetirizine.
“Similarly, in this population, we found that cetirizine and loratadine were the most commonly used antihistamines, followed by levocetirizine and fexofenadine,” Dr. Kocatürk and colleagues wrote.
“Guidelines also suggest that the use of first-generation H1-antihistamines should be avoided given their sedative effects; but if these are to be given, it would be wise to know that use of first-generation H1-antihistamines immediately before parturition could cause respiratory depression and other adverse effects in the neonate,” they added, noting that chlorpheniramine and diphenhydramine are the first-generation H1-antihistamines with the greatest evidence of safety in pregnancy.
They acknowledged certain limitations of the analysis, including its retrospective design and the fact that there were no data on low birth weight, small for gestational age, or miscarriage rates. In addition, disease activity or severity during pregnancy and after birth were not monitored.
Asked to comment on these results, Raj Chovatiya, MD, PhD, who directs the center for eczema and itch in the department of dermatology at Northwestern University, Chicago, noted that despite a higher prevalence of CU among females compared with males, very little is known about how the condition is managed during pregnancy. “This retrospective study shows that most patients continue to utilize CU treatment during pregnancy (primarily second-generation antihistamines), with similar birth outcomes as the general population,” he said. “Interestingly, cesarean rates were higher among mothers with CU, and emergency CU referral was a risk factor for preterm birth. While additional prospective studies are needed, these results suggest that CU patients should be carefully managed, particularly during pregnancy, when treatment should be optimized.”
Dr. Kocatürk reported having received personal fees from Novartis, Ibrahim Etem-Menarini, and Sanofi, outside the submitted work. Many coauthors reported having numerous financial disclosures. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
In addition, the rates of preterm births and medical problems of newborns in patients with CU are similar to those of the normal population and not linked to treatment used during pregnancy.
Those are the key findings from an analysis of new data from PREG-CU, an international, multicenter study of the Urticaria Centers of Reference and Excellence (UCARE) network. Results from the first PREG-CU analysis published in 2021 found that CU improved in about half of patients with CU during pregnancy. “However, two in five patients reported acute exacerbations of CU especially at the beginning and end of pregnancy,” investigators led by Emek Kocatürk, MD, of the department of dermatology and UCARE at Koç University School of Medicine, Istanbul, wrote in the new study, recently published in the Journal of the European Academy of Dermatology and Venereology.
“In addition, 1 in 10 pregnant CU patients required urticaria emergency care and 1 of 6 had angioedema during pregnancy,” they said. Risk factors for worsening CU during pregnancy, they added, were “mild disease and no angioedema before pregnancy, not taking treatment before pregnancy, chronic inducible urticaria, CU worsening during a previous pregnancy, stress as a driver of exacerbations, and treatment during pregnancy.”
Analysis involved 288 pregnant women
To optimize treatment of CU during pregnancy and to better understand how treatment affects pregnancy outcomes, the researchers analyzed 288 pregnancies in 288 women with CU from 13 countries and 21 centers worldwide. Their mean age at pregnancy was 32.1 years, and their mean duration of CU was 84.9 months. Prior to pregnancy, 35.7% of patients rated the severity of their CU symptoms as mild, 34.2% rated it as moderate, and 29.7% rated it as severe.
The researchers found that during pregnancy, 60% of patients used urticaria medication, including standard-dose second-generation H1-antihistamines (35.1%), first-generation H1-antihistamines (7.6%), high-dose second-generation H1-antihistamines (5.6%), and omalizumab (5.6%). The preterm birth rate was 10.2%, which was similar between patients who did and did not receive treatment during pregnancy (11.6% vs. 8.7%, respectively; P = .59).
On multivariate logistic regression, two predictors for preterm birth emerged: giving birth to twins (a 13.3-fold increased risk; P = .016) and emergency referrals for CU (a 4.3-fold increased risk; P =.016). The cesarean delivery rate was 51.3%, and more than 90% of newborns were healthy at birth. There was no link between any patient or disease characteristics or treatments and medical problems at birth.
In other findings, 78.8% of women with CU breastfed their babies. Of the 58 patients who did not breastfeed, 20.7% indicated severe urticaria/angioedema and/or taking medications as the main reason for not breastfeeding.
“Most CU patients use treatment during pregnancy and such treatments, especially second generation H1 antihistamines, seem to be safe during pregnancy regardless of the trimester,” the researchers concluded. “Outcomes of pregnancy in patients with CU were similar compared to the general population and not linked to treatment used during pregnancy. Notably, emergency referral for CU was an independent risk factor for preterm birth,” and the high cesarean delivery rate was “probably linked to comorbidities associated with the disease,” they added. “Overall, these findings suggest that patients should continue their treatments using an individualized dose to provide optimal symptom control.”
International guidelines
The authors noted that international guidelines for the management of urticaria published in 2022 suggest that modern second-generation H1-antihistamines should be used for pregnant patients, preferably loratadine with a possible extrapolation to desloratadine, cetirizine, or levocetirizine.
“Similarly, in this population, we found that cetirizine and loratadine were the most commonly used antihistamines, followed by levocetirizine and fexofenadine,” Dr. Kocatürk and colleagues wrote.
“Guidelines also suggest that the use of first-generation H1-antihistamines should be avoided given their sedative effects; but if these are to be given, it would be wise to know that use of first-generation H1-antihistamines immediately before parturition could cause respiratory depression and other adverse effects in the neonate,” they added, noting that chlorpheniramine and diphenhydramine are the first-generation H1-antihistamines with the greatest evidence of safety in pregnancy.
They acknowledged certain limitations of the analysis, including its retrospective design and the fact that there were no data on low birth weight, small for gestational age, or miscarriage rates. In addition, disease activity or severity during pregnancy and after birth were not monitored.
Asked to comment on these results, Raj Chovatiya, MD, PhD, who directs the center for eczema and itch in the department of dermatology at Northwestern University, Chicago, noted that despite a higher prevalence of CU among females compared with males, very little is known about how the condition is managed during pregnancy. “This retrospective study shows that most patients continue to utilize CU treatment during pregnancy (primarily second-generation antihistamines), with similar birth outcomes as the general population,” he said. “Interestingly, cesarean rates were higher among mothers with CU, and emergency CU referral was a risk factor for preterm birth. While additional prospective studies are needed, these results suggest that CU patients should be carefully managed, particularly during pregnancy, when treatment should be optimized.”
Dr. Kocatürk reported having received personal fees from Novartis, Ibrahim Etem-Menarini, and Sanofi, outside the submitted work. Many coauthors reported having numerous financial disclosures. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
FROM JEADV
FDA approves dupilumab for treatment of prurigo nodularis
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
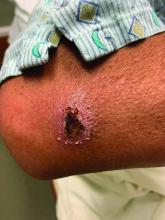
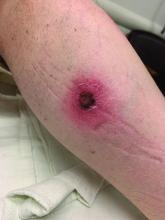
A White male presented with a 1-month history of recurrent, widespread, painful sores
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Hope shines bright for hidradenitis suppurativa treatments
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
in separate trials.
Around 40%-50% of patients exhibited a clinical response to these agents at 16 weeks, a leading HS expert reported at the annual congress of the European Academy of Dermatology and Venereology.
Time in the spotlight for HS
Research into HS is “an incredibly active field at this moment,” said Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, and president and chief executive officer of Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston.
It’s “been great for advancing our understanding of the biology and the treatments that we will be able to use,” she said.
During the late-breaking sessions at the annual EADV Congress, Dr. Kimball presented data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety and tolerability of the interleukin (IL) 17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS.
“This is only the second phase 3 program we have ever seen in HS and the first one since 2016,” Dr. Kimball said of the trials. It’s also the largest trial program in HS conducted to date, she added, “so it really is a milestone.”
The last big development was when adalimumab, a tumor necrosis factor (TNF) blocker, gained regulatory approval for HS in 2016, observed Neil Patel, PhD, MRCP, who leads the HS service at Imperial College Healthcare NHS Trust in London.
“Adalimumab has been very helpful for many patients, but not all patients respond, and others may respond initially but then the treatment starts to fail after a year or 2,” Dr. Patel said in an interview with this news organization.
“There is definitely a huge need for alternative medication for this condition, which still has a lack of effective treatment options,” added Dr. Patel, who was not involved in either of the studies.
“One major upside for secukinumab is that its safety profile is generally very good and familiarity in the dermatologic community is already well established,” Christopher Sayed, MD, said in a separate interview.
“This will make most providers very comfortable offering it as a potential treatment option sooner rather than later given that its efficacy has now been demonstrated in phase 3 trials,” added Dr. Sayed, associate professor of dermatology at the University of North Carolina at Chapel Hill.
Two identically designed trials
Altogether, SUNSHINE and SUNRISE enrolled just over 1,000 patients at 219 sites in 33 countries. Both trials were identical in their design: A 4-week run-in phase before a randomized, double-blind treatment phase that tested two dosing regimens of secukinumab (300 mg administered subcutaneously) every 2 or 4 weeks vs. placebo for 16 weeks. The trial continued after this time, with patients in the placebo arm re–randomly assigned to treatment with one of the two secukinumab regimens out to a year.
The primary endpoint was the percentage of patients achieving a Hidradenitis Suppurativa Clinical Response (HiSCR) after 16 weeks of treatment, with key secondary endpoints, which were abscess and inflammatory nodule (AN) count, occurrence of flares, and at least a 30% reduction in Patient’s Global Assessment of Skin Pain assessed using a numeric rating scale (NPRS30).
Secukinumab superior to placebo
The HiSCR is defined as at least a 50% decrease in AN count with no increase in the number of abscesses or in the number of draining fistulas relative to baseline. This was achieved by about 42%-45% of patients who received secukinumab every 2 weeks, about 42%-46% of those who received secukinumab every 4 weeks, and about 31%-33% of those on placebo in both studies.
Of note, fewer patients treated with secukinumab (about 15%-20% among those treated every 2 weeks, and about 15% to 23% among those treated every 4 weeks) than those on placebo (27%-29%) experienced flares, defined as at least a 25% increase in AN count and at least a two-point increase relative to baseline values.
Improvement in HS pain can be a difficult parameter to meet, Dr. Kimball noted. “Pain is such an important feature of this disease as it so debilitating for the patients.” More than one-third (almost 36%-39%) of patients given secukinumab vs. just over a quarter (26.9%) given placebo achieved at least a 30% reduction in NPRS30 ratings, she reported. The difference between active and placebo treatment was significant only when secukinumab was given every 2 weeks, however.
“The placebo rates that we see in these studies are exactly parallel to what we saw in other studies, and other disease states when we had a 50% bar of improvement,” Dr. Kimball said when questioned about these results.
“HS is a highly variable disease; it’s maybe not so much the placebo rate or the scoring system used but maybe the 50% bar set for improvement is too low. It’s likely, as data start to mature and a 75% HiSCR can be calculated, that the placebo rates will drop,” she said.
There were no surprises when it came to the safety of secukinumab, being an old player in a new game, she noted. It was “well tolerated” and tolerability was “consistent with the known safety profile,” Dr. Kimball said, “so we expect it to be a new, safe, and effective add to our armamentarium in treating this disease.”
This research involves “basically borrowing drugs from other areas and trying them in HS to see what effect they may have,” Dr. Patel said, noting that drugs such as adalimumab and secukinumab already had a proven track record in other diseases, such as psoriasis. “These early data for secukinumab definitely are very exciting, but we would need to see real-life results” in patients with HS who are not enrolled in trials to see the benefits, he added.
‘Tipping point’ for HS research
“I think we will look back on this meeting and realize that it was an incredibly important tipping point for the treatment of this incredibly debilitating disease,” Dr. Kimball said.
Elsewhere at the meeting, she had presented findings from a phase 2a study that pitted three different kinase inhibitors with different modes of action against each other and compared them with placebo.
The three agents evaluated are an IL-1 receptor–associated kinase 4 inhibitor known as PF-06650833, a tyrosine kinase 2 (TYK2) JAK1 inhibitor brepocitinib, and the TYK2 inhibitor PF-06826647.
“This technique has been used in oncology,” Dr. Kimball said, noting that the ability to test multiple drugs at the same time “means we can really much more efficiently test two different things at the same time, and also put fewer patients at risk for potential problems if drugs don’t work.”
Positive signs for brepocitinib, not the other kinases tested
The results showed that though brepocitinib worked in HS, the other two novel compounds did not appear to have beneficial effects. Just over half (52%) of the 52 patients treated with brepocitinib achieved an HiSCR at 16 weeks, compared with around one-third of those given placebo, PF-06650833, or PF-06826647.
A similar benefit was seen in terms of reduction in flares for brepocitinib but not the other agents, although there was no difference between them all in terms of NPRS30 pain reduction.
“We’ve been able to test three different modalities. This tells us some things about the pathophysiology for HS, which is a very profoundly intensive inflammatory process,” which, Dr. Kimball said, “may require multiple modalities of action to get it under control.” In addition, these “general modalities seem to safe and well tolerated,” she added.
Take-homes for practice and future research
“While it is disappointing that two of the drugs tested did not clearly demonstrate efficacy, it is very possible that these mechanisms of action may be successful targets in the future as new dosing strategies and drugs targeting these pathways are developed,” Dr. Sayed said.
A case in point, he added, was that “adalimumab did not meet treatment endpoints at a dose of 40 mg every other week, but clearly has made a major impact at 40 mg weekly.”
The bottom line is that “both secukinumab and beprocitinib demonstrated efficacy over placebo and are likely to be helpful for a significant number of patients with HS,” Dr. Sayed said. “Hopefully, we’ll see head-to-head trials and more data regarding proportions of patients with deeper responses using criteria such as HiSCR75 and HiSCR90.”
Moreover, “having a larger number of drugs with a range of mechanisms of action is extraordinarily helpful given how difficult the disease can be to manage. We will hopefully continue to see creative approaches and further successes in the current wave of phase 1, 2, and 3 trials that are already underway.”
The SUNSHINE and SUNRISE studies were funded by Novartis Pharma AG, Basel, Switzerland. The phase 2A study Dr. Kimball presented was sponsored by Pfizer.
Dr. Kimball disclosed ties to both Novartis and Pfizer and acts as a consultant and investigator to AbbVie, Bristol-Myers Squibb, Janssen, Eli Lilly, Novartis, and UCB. She is an investigator for Incyte and AnaptysBio; acts as a consultant to Bayer, Boehringer Ingelheim, Ventyz, Moonlake, Lily, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen, and serves on the Board of Directors for Almirall.
Dr. Patel had no conflicts of interest to disclose. Dr. Sayed is the director of the HS Foundation, a nonprofit organization, and has acted as an adviser or consultant to, speaker for, and received research funding from multiple drug companies including AbbVie, ChemoCentryx, Incyte, InflaRx, Novartis, and UCB.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Roflumilast foam effectively eases seborrheic dermatitis
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Dupilumab offers ‘clinically meaningful’ improvements in prurigo nodularis
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS