User login
Counterphobia and Poor Sun Protection Practices in First-Degree Relatives of Melanoma Patients
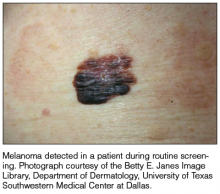
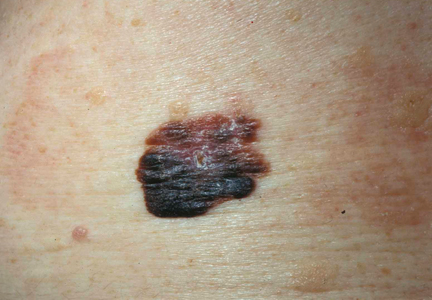
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.
Aggressive secondary squamous carcinoma appeared during BRAF inhibitor targeted therapy
A woman undergoing BRAF inhibitor targeted therapy for advanced melanoma has presented with invasive spindle cell squamous carcinoma masquerading as a secondary cutaneous squamous cell carcinoma, highlighting the importance of histologic evaluation of these lesions.
"Secondary cutaneous squamous cell carcinomas (cSCCs) are adverse effects of BRAF inhibitor targeted therapy for advanced melanoma," wrote Dr. Daniel N. Cohen and his associates online Feb. 26 in JAMA Dermatology.
The most commonly seen histologic type of secondary cutaneous squamous cell carcinomas is keratoacanthoma-like cSCC (cSCC-KA), which is thought to have a low risk of metastasis or recurrence, said lead author Dr. Cohen of the Vanderbilt University Medical Center, Nashville, Tenn.
In this case report, however, a woman in her 50s with BRAF-mutant metastatic melanoma developed more than 100 new cutaneous squamous proliferations across her face, trunk, and extremities within 4 weeks of starting treatment with the BRAF inhibitor dabrafenib as part of a clinical trial, with some lesions appearing to be a more aggressive type upon analysis.
The lesions began as acrochordons on her face and extremities, as well as new nevi on her torso and axilla. She also developed fever, chills, and fatigue and had enlarging, tender, and bleeding lesions on her trunk and extremities.
Seven large, tender, and indurated lesions were removed using a deep scoop shave biopsy, revealing a biphasic malignant growth pattern (JAMA Dermatology 2014 Feb 26 [doi:10.1001/jamadermatol.2013.7784]).
"The superficial portion demonstrated conventional cSCC-KA features of hyperkeratosis, epidermal acanthosis, and central core of glassy eosinophilic keratin with pseudopapillomatosis and a base with focal invasive lobules of cytologically atypical keratinocytes, consistent with previously reported cSCC-KA" the study authors reported.
However, they added, "in stark contrast to prior reports, the deep aspects of 6 of 7 lesions showed invasive spindled and epithelioid cells with monomorphic elongated nuclei with condensed chromatin and mitoses consistent with spindle cell squamous carcinoma, an aggressive subtype of squamous cell carcinoma."
The cells were strongly immunoreactive for cytokeratin CK5/6 and CK903, and both the squamous and spindle components were vimentin reactive and showed increased proliferation index. In contrast to the usual pattern of a spindle cell melanoma, the spindle tumor cells were also MART-1 and S100 negative.
The authors said this was the first known report of invasive spindle cell squamous carcinoma that mimicked keratoacanthoma-like secondary SCC appearing during BRAF inhibitor therapy and suggested that the discovery has implications for management of secondary squamous cell carcinoma.
"Because the clinical appearance of cSCC-KA and the spindle cell squamous carcinomas in our patient are indistinguishable, histologic evaluation of the entire lesion (via saucerization biopsy or incisional biopsy) is vital to prevent inadequate treatment of a deeply invasive process with a probable higher malignant potential," Dr. Cohen and his associates wrote.
The patient stopped BRAF inhibitor therapy and had no recurrence or new development of cutaneous secondary SCCs. A single later recurrence of melanoma was resected but she remains disease free.
One author declared a consultancy with Bristol-Myers Squibb and Genentech, as well as grant support from Genentech. There were no other financial disclosures reported.
A woman undergoing BRAF inhibitor targeted therapy for advanced melanoma has presented with invasive spindle cell squamous carcinoma masquerading as a secondary cutaneous squamous cell carcinoma, highlighting the importance of histologic evaluation of these lesions.
"Secondary cutaneous squamous cell carcinomas (cSCCs) are adverse effects of BRAF inhibitor targeted therapy for advanced melanoma," wrote Dr. Daniel N. Cohen and his associates online Feb. 26 in JAMA Dermatology.
The most commonly seen histologic type of secondary cutaneous squamous cell carcinomas is keratoacanthoma-like cSCC (cSCC-KA), which is thought to have a low risk of metastasis or recurrence, said lead author Dr. Cohen of the Vanderbilt University Medical Center, Nashville, Tenn.
In this case report, however, a woman in her 50s with BRAF-mutant metastatic melanoma developed more than 100 new cutaneous squamous proliferations across her face, trunk, and extremities within 4 weeks of starting treatment with the BRAF inhibitor dabrafenib as part of a clinical trial, with some lesions appearing to be a more aggressive type upon analysis.
The lesions began as acrochordons on her face and extremities, as well as new nevi on her torso and axilla. She also developed fever, chills, and fatigue and had enlarging, tender, and bleeding lesions on her trunk and extremities.
Seven large, tender, and indurated lesions were removed using a deep scoop shave biopsy, revealing a biphasic malignant growth pattern (JAMA Dermatology 2014 Feb 26 [doi:10.1001/jamadermatol.2013.7784]).
"The superficial portion demonstrated conventional cSCC-KA features of hyperkeratosis, epidermal acanthosis, and central core of glassy eosinophilic keratin with pseudopapillomatosis and a base with focal invasive lobules of cytologically atypical keratinocytes, consistent with previously reported cSCC-KA" the study authors reported.
However, they added, "in stark contrast to prior reports, the deep aspects of 6 of 7 lesions showed invasive spindled and epithelioid cells with monomorphic elongated nuclei with condensed chromatin and mitoses consistent with spindle cell squamous carcinoma, an aggressive subtype of squamous cell carcinoma."
The cells were strongly immunoreactive for cytokeratin CK5/6 and CK903, and both the squamous and spindle components were vimentin reactive and showed increased proliferation index. In contrast to the usual pattern of a spindle cell melanoma, the spindle tumor cells were also MART-1 and S100 negative.
The authors said this was the first known report of invasive spindle cell squamous carcinoma that mimicked keratoacanthoma-like secondary SCC appearing during BRAF inhibitor therapy and suggested that the discovery has implications for management of secondary squamous cell carcinoma.
"Because the clinical appearance of cSCC-KA and the spindle cell squamous carcinomas in our patient are indistinguishable, histologic evaluation of the entire lesion (via saucerization biopsy or incisional biopsy) is vital to prevent inadequate treatment of a deeply invasive process with a probable higher malignant potential," Dr. Cohen and his associates wrote.
The patient stopped BRAF inhibitor therapy and had no recurrence or new development of cutaneous secondary SCCs. A single later recurrence of melanoma was resected but she remains disease free.
One author declared a consultancy with Bristol-Myers Squibb and Genentech, as well as grant support from Genentech. There were no other financial disclosures reported.
A woman undergoing BRAF inhibitor targeted therapy for advanced melanoma has presented with invasive spindle cell squamous carcinoma masquerading as a secondary cutaneous squamous cell carcinoma, highlighting the importance of histologic evaluation of these lesions.
"Secondary cutaneous squamous cell carcinomas (cSCCs) are adverse effects of BRAF inhibitor targeted therapy for advanced melanoma," wrote Dr. Daniel N. Cohen and his associates online Feb. 26 in JAMA Dermatology.
The most commonly seen histologic type of secondary cutaneous squamous cell carcinomas is keratoacanthoma-like cSCC (cSCC-KA), which is thought to have a low risk of metastasis or recurrence, said lead author Dr. Cohen of the Vanderbilt University Medical Center, Nashville, Tenn.
In this case report, however, a woman in her 50s with BRAF-mutant metastatic melanoma developed more than 100 new cutaneous squamous proliferations across her face, trunk, and extremities within 4 weeks of starting treatment with the BRAF inhibitor dabrafenib as part of a clinical trial, with some lesions appearing to be a more aggressive type upon analysis.
The lesions began as acrochordons on her face and extremities, as well as new nevi on her torso and axilla. She also developed fever, chills, and fatigue and had enlarging, tender, and bleeding lesions on her trunk and extremities.
Seven large, tender, and indurated lesions were removed using a deep scoop shave biopsy, revealing a biphasic malignant growth pattern (JAMA Dermatology 2014 Feb 26 [doi:10.1001/jamadermatol.2013.7784]).
"The superficial portion demonstrated conventional cSCC-KA features of hyperkeratosis, epidermal acanthosis, and central core of glassy eosinophilic keratin with pseudopapillomatosis and a base with focal invasive lobules of cytologically atypical keratinocytes, consistent with previously reported cSCC-KA" the study authors reported.
However, they added, "in stark contrast to prior reports, the deep aspects of 6 of 7 lesions showed invasive spindled and epithelioid cells with monomorphic elongated nuclei with condensed chromatin and mitoses consistent with spindle cell squamous carcinoma, an aggressive subtype of squamous cell carcinoma."
The cells were strongly immunoreactive for cytokeratin CK5/6 and CK903, and both the squamous and spindle components were vimentin reactive and showed increased proliferation index. In contrast to the usual pattern of a spindle cell melanoma, the spindle tumor cells were also MART-1 and S100 negative.
The authors said this was the first known report of invasive spindle cell squamous carcinoma that mimicked keratoacanthoma-like secondary SCC appearing during BRAF inhibitor therapy and suggested that the discovery has implications for management of secondary squamous cell carcinoma.
"Because the clinical appearance of cSCC-KA and the spindle cell squamous carcinomas in our patient are indistinguishable, histologic evaluation of the entire lesion (via saucerization biopsy or incisional biopsy) is vital to prevent inadequate treatment of a deeply invasive process with a probable higher malignant potential," Dr. Cohen and his associates wrote.
The patient stopped BRAF inhibitor therapy and had no recurrence or new development of cutaneous secondary SCCs. A single later recurrence of melanoma was resected but she remains disease free.
One author declared a consultancy with Bristol-Myers Squibb and Genentech, as well as grant support from Genentech. There were no other financial disclosures reported.
FROM JAMA DERMATOLOGY
Major finding: A woman undergoing BRAF inhibitor targeted therapy for advanced melanoma has presented with invasive spindle cell squamous carcinoma masquerading as a secondary cutaneous squamous cell carcinoma.
Data source: Case report.
Disclosures: One author declared a consultancy with Bristol-Myers Squibb and Genentech, as well as grant support from Genentech. There were no other financial disclosures reported.
Dabrafenib in advanced melanoma with BRAF V600E mutation
Dabrafenib was recently approved by the US Food and Drug Administration for treatment of unresectable or metastatic melanoma with BRAF V600E mutations as detected by an FDA-approved test. The THxID BRAF assay, for detection of BRAF V600E mutations was concurrently approved. Dabrafenib is not indicated for the treatment of patients with wild-type BRAF melanoma, because of the potential risk of tumor promotion. About 50% of melanomas have an activating mutation in the BRAF gene, with about 80%-90% of those having a V600E mutation, and 10%-20% having a V600K mutation.
Click on the PDF icon at the top of this introduction to read the full article.
Dabrafenib was recently approved by the US Food and Drug Administration for treatment of unresectable or metastatic melanoma with BRAF V600E mutations as detected by an FDA-approved test. The THxID BRAF assay, for detection of BRAF V600E mutations was concurrently approved. Dabrafenib is not indicated for the treatment of patients with wild-type BRAF melanoma, because of the potential risk of tumor promotion. About 50% of melanomas have an activating mutation in the BRAF gene, with about 80%-90% of those having a V600E mutation, and 10%-20% having a V600K mutation.
Click on the PDF icon at the top of this introduction to read the full article.
Dabrafenib was recently approved by the US Food and Drug Administration for treatment of unresectable or metastatic melanoma with BRAF V600E mutations as detected by an FDA-approved test. The THxID BRAF assay, for detection of BRAF V600E mutations was concurrently approved. Dabrafenib is not indicated for the treatment of patients with wild-type BRAF melanoma, because of the potential risk of tumor promotion. About 50% of melanomas have an activating mutation in the BRAF gene, with about 80%-90% of those having a V600E mutation, and 10%-20% having a V600K mutation.
Click on the PDF icon at the top of this introduction to read the full article.
Video intervention inspires men to undergo skin exams
Video interventions on the importance of undergoing clinical skin examinations may increase melanoma discovery rates, according to the results of a randomized clinical trial published online Feb. 19 in JAMA Dermatology.
A total of 870 Australian men aged 50 and older were randomly assigned to receive either a video plus written educational materials (436 men in the intervention group) or only the educational materials (434 men in the control group). Six months later, 35.3% of the intervention group patients reported that they had received a whole-body clinical skin examination from a physician during the interim, compared with 27.2% of the control group, the investigators reported (JAMA Dermatol. 2014 Feb. 19 [doi:10.1001/jamadermatol.2013.9313]).
The video emphasized the seriousness of a melanoma diagnosis, explained risk factors for the disease and stressed the increased risk for men over age 50, modeled a whole-body self-examination, and showed a melanoma surgeon encouraging people to do their own skin exams and to request them from their physicians. The video also demonstrated a clinical skin exam being performed by a physician, and featured a well-known athlete, as well as melanoma survivors, who encouraged men to be screened for skin cancer, according to Monika Janda, Ph.D., of the School of Public Health and Biomedical Innovation, Queensland University of Technology, Brisbane, and her associates.
Overall, 34.1% of the intervention group underwent surgical excision or biopsy of at least 1 skin lesion, compared with 27.1% of the control group. Of the 130 lesions for which pathology reports were available, 2 were melanomas, 29 were squamous cell carcinomas, 38 were basal cell carcinomas, 17 were solar keratoses, 9 were benign nevi, 3 were dysplatic nevi, and 32 were other lesions. Significantly more skin cancers were detected in the intervention group (60%) than in the control group (40%), suggesting that the video intervention may lead to earlier detection of melanoma and other skin malignancies, Dr. Janda and her associates concluded.
This study was funded by the Australian National Health and Medical Research Committee. No financial conflicts of interest were reported.
Video interventions on the importance of undergoing clinical skin examinations may increase melanoma discovery rates, according to the results of a randomized clinical trial published online Feb. 19 in JAMA Dermatology.
A total of 870 Australian men aged 50 and older were randomly assigned to receive either a video plus written educational materials (436 men in the intervention group) or only the educational materials (434 men in the control group). Six months later, 35.3% of the intervention group patients reported that they had received a whole-body clinical skin examination from a physician during the interim, compared with 27.2% of the control group, the investigators reported (JAMA Dermatol. 2014 Feb. 19 [doi:10.1001/jamadermatol.2013.9313]).
The video emphasized the seriousness of a melanoma diagnosis, explained risk factors for the disease and stressed the increased risk for men over age 50, modeled a whole-body self-examination, and showed a melanoma surgeon encouraging people to do their own skin exams and to request them from their physicians. The video also demonstrated a clinical skin exam being performed by a physician, and featured a well-known athlete, as well as melanoma survivors, who encouraged men to be screened for skin cancer, according to Monika Janda, Ph.D., of the School of Public Health and Biomedical Innovation, Queensland University of Technology, Brisbane, and her associates.
Overall, 34.1% of the intervention group underwent surgical excision or biopsy of at least 1 skin lesion, compared with 27.1% of the control group. Of the 130 lesions for which pathology reports were available, 2 were melanomas, 29 were squamous cell carcinomas, 38 were basal cell carcinomas, 17 were solar keratoses, 9 were benign nevi, 3 were dysplatic nevi, and 32 were other lesions. Significantly more skin cancers were detected in the intervention group (60%) than in the control group (40%), suggesting that the video intervention may lead to earlier detection of melanoma and other skin malignancies, Dr. Janda and her associates concluded.
This study was funded by the Australian National Health and Medical Research Committee. No financial conflicts of interest were reported.
Video interventions on the importance of undergoing clinical skin examinations may increase melanoma discovery rates, according to the results of a randomized clinical trial published online Feb. 19 in JAMA Dermatology.
A total of 870 Australian men aged 50 and older were randomly assigned to receive either a video plus written educational materials (436 men in the intervention group) or only the educational materials (434 men in the control group). Six months later, 35.3% of the intervention group patients reported that they had received a whole-body clinical skin examination from a physician during the interim, compared with 27.2% of the control group, the investigators reported (JAMA Dermatol. 2014 Feb. 19 [doi:10.1001/jamadermatol.2013.9313]).
The video emphasized the seriousness of a melanoma diagnosis, explained risk factors for the disease and stressed the increased risk for men over age 50, modeled a whole-body self-examination, and showed a melanoma surgeon encouraging people to do their own skin exams and to request them from their physicians. The video also demonstrated a clinical skin exam being performed by a physician, and featured a well-known athlete, as well as melanoma survivors, who encouraged men to be screened for skin cancer, according to Monika Janda, Ph.D., of the School of Public Health and Biomedical Innovation, Queensland University of Technology, Brisbane, and her associates.
Overall, 34.1% of the intervention group underwent surgical excision or biopsy of at least 1 skin lesion, compared with 27.1% of the control group. Of the 130 lesions for which pathology reports were available, 2 were melanomas, 29 were squamous cell carcinomas, 38 were basal cell carcinomas, 17 were solar keratoses, 9 were benign nevi, 3 were dysplatic nevi, and 32 were other lesions. Significantly more skin cancers were detected in the intervention group (60%) than in the control group (40%), suggesting that the video intervention may lead to earlier detection of melanoma and other skin malignancies, Dr. Janda and her associates concluded.
This study was funded by the Australian National Health and Medical Research Committee. No financial conflicts of interest were reported.
FROM JAMA DERMATOLOGY
Major Finding: After 6 months, 35.3% of the intervention group patients reported that they had received a whole-body clinical skin examination from a physician, compared with 27.2% of the control group.
Data Source: A randomized clinical trial of Australian men aged 50 and older who were given a video intervention promoting skin examination plus written education materials, or just the written educational materials.
Disclosures: This study was funded by the Australian National Health and Medical Research Committee. No financial conflicts of interest were reported.
Ingenol mebutate improves outcomes in actinic keratosis
Sequential topical field-directed therapy with ingenol mebutate gel for 3 days following cryosurgery significantly improves the outcomes in patients with actinic keratosis, a phase III randomized study showed.
Enrolled patients had four to eight clinically typical lesions on the face or scalp and received cryosurgery to all visible lesions followed by once-daily treatment of ingenol mebutate 0.015% gel or vehicle gel for 3 consecutive days at home, after 3 weeks of healing.
At 11 weeks, patients who received treatment with ingenol mebutate gel (n = 167) postsurgery, saw higher rates of complete clearance of lesions, compared with patients receiving the vehicle alone after surgery (n = 162) (60.5% vs. 49.4%; P=.04), reported Dr. Stephen K. Tyring and his associates this month (J. Drugs Dermatol. 2014;13:154-60).
In longer-term follow-up at 12 months, ingenol mebutate gel continued to show significantly improved complete clearance rates, compared with vehicle gel (30.5% vs. 18.5%; P = .01), Dr. Tyring reported at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
The mean percentage reduction of lesions at 12 months was significantly higher with ingenol mebutate for all lesions (59.5% vs 44.4%, P=0.004) and 38.9% of patients receiving ingenol mebutate saw emergence of new lesions on the treatment area between baseline lesions, compared with 51.9% of patients in the vehicle group (P=0.01), he said.
The complete clearance at 11 weeks (60.5% vs 49.4%; P=0.04) with a relative complete actinic keratosis clearance ratio of 1.22 (1.01-1.49) increased to 1.67 (1.12-2.50) at 12 months in the treatment group vs vehicle, following cryosurgery, noted Dr. Tyring of University of Texas Health Science Center at Houston.
The most frequently reported adverse events in the treatment group were application site discomfort and pruritus. Over the 12-month follow-up, 16 patients in the ingenol mebutate group reported one or more adverse events, as did 6 in the vehicle group. These events, however, were minor and well tolerated, said Dr. Tyring.
Ingenol mebutate gel was approved by the Food and Drug Administration in 2012 to treat actinic keratosis. Its advantage over other topical field agents such as 5-fluorouracil, imiquimod, and diclofenac is its short treatment period of 2-3 days, depending on the treatment area. This reduces the likelihood of patients discontinuing because of inflammation, burning, or other adverse events, Dr. Tyring noted.
Ingenol mebutate significantly enhanced the efficacy of cryosurgery, a difference that comes "both from enhancing the effect of cryosurgery with ingenol mebutate on the visible baseline lesions, and from a field treatment effect of ingenol mebutate on subclinical lesions not visible at baseline," Dr. Tyring said.
Dr. Tyring disclosed financial relationships with Astellas, Epiphany, Catalyst, GlaxoSmithKline, Novartis, 3M, VaxGen, Merck, BMS, Amgen, Biogen, Genentech, Corixa, Abbott, Graceway, Leo, and Warner Chilcott. SDEF and this news organization are owned by the same parent company.
Sequential topical field-directed therapy with ingenol mebutate gel for 3 days following cryosurgery significantly improves the outcomes in patients with actinic keratosis, a phase III randomized study showed.
Enrolled patients had four to eight clinically typical lesions on the face or scalp and received cryosurgery to all visible lesions followed by once-daily treatment of ingenol mebutate 0.015% gel or vehicle gel for 3 consecutive days at home, after 3 weeks of healing.
At 11 weeks, patients who received treatment with ingenol mebutate gel (n = 167) postsurgery, saw higher rates of complete clearance of lesions, compared with patients receiving the vehicle alone after surgery (n = 162) (60.5% vs. 49.4%; P=.04), reported Dr. Stephen K. Tyring and his associates this month (J. Drugs Dermatol. 2014;13:154-60).
In longer-term follow-up at 12 months, ingenol mebutate gel continued to show significantly improved complete clearance rates, compared with vehicle gel (30.5% vs. 18.5%; P = .01), Dr. Tyring reported at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
The mean percentage reduction of lesions at 12 months was significantly higher with ingenol mebutate for all lesions (59.5% vs 44.4%, P=0.004) and 38.9% of patients receiving ingenol mebutate saw emergence of new lesions on the treatment area between baseline lesions, compared with 51.9% of patients in the vehicle group (P=0.01), he said.
The complete clearance at 11 weeks (60.5% vs 49.4%; P=0.04) with a relative complete actinic keratosis clearance ratio of 1.22 (1.01-1.49) increased to 1.67 (1.12-2.50) at 12 months in the treatment group vs vehicle, following cryosurgery, noted Dr. Tyring of University of Texas Health Science Center at Houston.
The most frequently reported adverse events in the treatment group were application site discomfort and pruritus. Over the 12-month follow-up, 16 patients in the ingenol mebutate group reported one or more adverse events, as did 6 in the vehicle group. These events, however, were minor and well tolerated, said Dr. Tyring.
Ingenol mebutate gel was approved by the Food and Drug Administration in 2012 to treat actinic keratosis. Its advantage over other topical field agents such as 5-fluorouracil, imiquimod, and diclofenac is its short treatment period of 2-3 days, depending on the treatment area. This reduces the likelihood of patients discontinuing because of inflammation, burning, or other adverse events, Dr. Tyring noted.
Ingenol mebutate significantly enhanced the efficacy of cryosurgery, a difference that comes "both from enhancing the effect of cryosurgery with ingenol mebutate on the visible baseline lesions, and from a field treatment effect of ingenol mebutate on subclinical lesions not visible at baseline," Dr. Tyring said.
Dr. Tyring disclosed financial relationships with Astellas, Epiphany, Catalyst, GlaxoSmithKline, Novartis, 3M, VaxGen, Merck, BMS, Amgen, Biogen, Genentech, Corixa, Abbott, Graceway, Leo, and Warner Chilcott. SDEF and this news organization are owned by the same parent company.
Sequential topical field-directed therapy with ingenol mebutate gel for 3 days following cryosurgery significantly improves the outcomes in patients with actinic keratosis, a phase III randomized study showed.
Enrolled patients had four to eight clinically typical lesions on the face or scalp and received cryosurgery to all visible lesions followed by once-daily treatment of ingenol mebutate 0.015% gel or vehicle gel for 3 consecutive days at home, after 3 weeks of healing.
At 11 weeks, patients who received treatment with ingenol mebutate gel (n = 167) postsurgery, saw higher rates of complete clearance of lesions, compared with patients receiving the vehicle alone after surgery (n = 162) (60.5% vs. 49.4%; P=.04), reported Dr. Stephen K. Tyring and his associates this month (J. Drugs Dermatol. 2014;13:154-60).
In longer-term follow-up at 12 months, ingenol mebutate gel continued to show significantly improved complete clearance rates, compared with vehicle gel (30.5% vs. 18.5%; P = .01), Dr. Tyring reported at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
The mean percentage reduction of lesions at 12 months was significantly higher with ingenol mebutate for all lesions (59.5% vs 44.4%, P=0.004) and 38.9% of patients receiving ingenol mebutate saw emergence of new lesions on the treatment area between baseline lesions, compared with 51.9% of patients in the vehicle group (P=0.01), he said.
The complete clearance at 11 weeks (60.5% vs 49.4%; P=0.04) with a relative complete actinic keratosis clearance ratio of 1.22 (1.01-1.49) increased to 1.67 (1.12-2.50) at 12 months in the treatment group vs vehicle, following cryosurgery, noted Dr. Tyring of University of Texas Health Science Center at Houston.
The most frequently reported adverse events in the treatment group were application site discomfort and pruritus. Over the 12-month follow-up, 16 patients in the ingenol mebutate group reported one or more adverse events, as did 6 in the vehicle group. These events, however, were minor and well tolerated, said Dr. Tyring.
Ingenol mebutate gel was approved by the Food and Drug Administration in 2012 to treat actinic keratosis. Its advantage over other topical field agents such as 5-fluorouracil, imiquimod, and diclofenac is its short treatment period of 2-3 days, depending on the treatment area. This reduces the likelihood of patients discontinuing because of inflammation, burning, or other adverse events, Dr. Tyring noted.
Ingenol mebutate significantly enhanced the efficacy of cryosurgery, a difference that comes "both from enhancing the effect of cryosurgery with ingenol mebutate on the visible baseline lesions, and from a field treatment effect of ingenol mebutate on subclinical lesions not visible at baseline," Dr. Tyring said.
Dr. Tyring disclosed financial relationships with Astellas, Epiphany, Catalyst, GlaxoSmithKline, Novartis, 3M, VaxGen, Merck, BMS, Amgen, Biogen, Genentech, Corixa, Abbott, Graceway, Leo, and Warner Chilcott. SDEF and this news organization are owned by the same parent company.
FROM SDEF HAWAII DERMATOLOGY SEMINAR
Major finding: Ingenol mebutate gel following cryosurgery significantly improved complete clearance rates, compared with vehicle gel at 12 months (30.5% vs. 18.5%; P =.01). Mean percentage reduction of AK lesions at 12 months was significantly higher with ingenol mebutate for all lesions than with vehicle (59.5% vs. 44.4%; P = .004)
Data source: Phase III multicenter, randomized, double-blind study of 329 patients with actinic keratosis on the face or scalp.
Disclosures: Dr. Tyring disclosed financial relationships with Astellas, Epiphany, Catalyst, GlaxoSmithKline, Novartis, 3M, VaxGen, Merck, BMS, Amgen, Biogen, Genentech, Corixa, Abbott, Graceway, Leo, and Warner Chilcott.
Youth tanning curbed in states with restrictions
States with stronger laws to restrict tanning bed access for minors reported significantly lower instances of tanning among teens than states without restrictions, according to a large-scale review published by the American Journal of Public Health.
Overall, female minors in states with tanning access laws used the beds 30% less than teens in states without any restrictions. The laws include warning signs on indoor tanning devices, limited advertising, and mandatory protective eyewear.
"State indoor tanning laws, especially age restrictions, may be effective in reducing indoor tanning among our nation’s youth," wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention and his colleagues.
Six states (California, Illinois, Nevada, Oregon, Texas, and Vermont), currently restrict indoor tanning among minors younger than 18 years, the researchers noted.
In addition, data from states with youth access laws – laws that require parental permission or place an outright ban on teen use of tanning beds – indicated that female students use tanning beds 42% less than those in states without the laws (Am. J. Public Health 2014 [doi: 10.2105/AJPH.2013.301850]).
The researchers used nationally representative data from 31,835 adolescents from 2009 and 2011 collected as part of the national Youth Risk Behavior Surveys of U.S. high school students in grades 9-12. They conducted a multivariable logistic regression analysis to examine the associations between the indoor tanning laws of different states and the reports of indoor tanning among high school students in those states. The survey found that 23.4 % of the young women surveyed said they used indoor tanning, and 6.5% of young men admitted to using the devices.
The researchers had no financial conflicts to disclose.
States with stronger laws to restrict tanning bed access for minors reported significantly lower instances of tanning among teens than states without restrictions, according to a large-scale review published by the American Journal of Public Health.
Overall, female minors in states with tanning access laws used the beds 30% less than teens in states without any restrictions. The laws include warning signs on indoor tanning devices, limited advertising, and mandatory protective eyewear.
"State indoor tanning laws, especially age restrictions, may be effective in reducing indoor tanning among our nation’s youth," wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention and his colleagues.
Six states (California, Illinois, Nevada, Oregon, Texas, and Vermont), currently restrict indoor tanning among minors younger than 18 years, the researchers noted.
In addition, data from states with youth access laws – laws that require parental permission or place an outright ban on teen use of tanning beds – indicated that female students use tanning beds 42% less than those in states without the laws (Am. J. Public Health 2014 [doi: 10.2105/AJPH.2013.301850]).
The researchers used nationally representative data from 31,835 adolescents from 2009 and 2011 collected as part of the national Youth Risk Behavior Surveys of U.S. high school students in grades 9-12. They conducted a multivariable logistic regression analysis to examine the associations between the indoor tanning laws of different states and the reports of indoor tanning among high school students in those states. The survey found that 23.4 % of the young women surveyed said they used indoor tanning, and 6.5% of young men admitted to using the devices.
The researchers had no financial conflicts to disclose.
States with stronger laws to restrict tanning bed access for minors reported significantly lower instances of tanning among teens than states without restrictions, according to a large-scale review published by the American Journal of Public Health.
Overall, female minors in states with tanning access laws used the beds 30% less than teens in states without any restrictions. The laws include warning signs on indoor tanning devices, limited advertising, and mandatory protective eyewear.
"State indoor tanning laws, especially age restrictions, may be effective in reducing indoor tanning among our nation’s youth," wrote Gery P. Guy Jr., Ph.D., of the Centers for Disease Control and Prevention and his colleagues.
Six states (California, Illinois, Nevada, Oregon, Texas, and Vermont), currently restrict indoor tanning among minors younger than 18 years, the researchers noted.
In addition, data from states with youth access laws – laws that require parental permission or place an outright ban on teen use of tanning beds – indicated that female students use tanning beds 42% less than those in states without the laws (Am. J. Public Health 2014 [doi: 10.2105/AJPH.2013.301850]).
The researchers used nationally representative data from 31,835 adolescents from 2009 and 2011 collected as part of the national Youth Risk Behavior Surveys of U.S. high school students in grades 9-12. They conducted a multivariable logistic regression analysis to examine the associations between the indoor tanning laws of different states and the reports of indoor tanning among high school students in those states. The survey found that 23.4 % of the young women surveyed said they used indoor tanning, and 6.5% of young men admitted to using the devices.
The researchers had no financial conflicts to disclose.
FROM THE AMERICAN JOURNAL OF PUBLIC HEALTH
Major finding: Youth access laws reduce indoor tanning use by 42% in teenage girls.
Data source: Data review performed by the CDC based on National Youth Risk Behavior Surveys from 2009 and 2011.
Final results validate sentinel-node biopsy for melanoma
Sentinel-node biopsy clearly benefits patients with intermediate-thickness or thick primary melanomas who are found to have nodal involvement and undergo immediate lymphadenectomy, tripling their disease-free survival and doubling their melanoma-specific survival and distant disease-free survival, according to a report published online Feb. 12 in the New England Journal of Medicine.
These long-term findings, the final results of 20 years of investigation in MSLT-I (Multicenter Selective Lymphadenectomy Trial), "clearly validate the use of sentinel-node biopsy" in this patient population. Among patients found to have no nodal involvement, "the procedure provides accurate and important staging information," while among those found to have nodal involvement, it enhances regional disease control and substantially improves survival, said Dr. Donald L. Morton of the John Wayne Cancer Institute at Saint John’s Health Center, Santa Monica, Calif., and his associates.
Dr. Morton passed away shortly before publication of this final MSLT-I report.
The MSLT-I, which began in 1994, was intended to determine whether sentinel-node biopsy – a new procedure at the time that was developed by Dr. Morton – could accurately identify clinically occult metastases. More important, since only about 20% of such patients are found to have metastases, the MSLT-I was to determine whether it was worthwhile to subject all patients to the procedure. In other words, did sentinel-node biopsy with immediate lymphadenectomy of involved nodes yield better outcomes than did watchful waiting with delayed lymphadenectomy, performed only when nodal recurrence became evident during observation?
This final report involved 1,560 patients with localized cutaneous primary melanomas: 1,270 with intermediate-thickness (1.20-3.50 mm) lesions and 290 with thick (greater than 3.50 mm) lesions. A total of 943 patients were randomly assigned to undergo sentinel-node biopsy plus immediate lymphadenectomy if metastases were detected in sentinel nodes, and 617 were randomly assigned to close observation with delayed lymphadenectomy if nodal metastases developed during observation.
All the study participants were monitored periodically throughout follow-up using clinical examination, blood testing for biomarkers of melanoma, chest radiography, PET scanning, CT scanning, and/or nodal ultrasonography.
The primary end point, melanoma-specific survival across the entire study cohort, was not significantly different between patients who underwent sentinel-node biopsy (81.4%) and those who had observation only (78.3%). This is not surprising as only the 20% of patients who actually had nodal metastases could potentially derive a survival benefit from the procedure.
Latent-subgroup statistical methods were used to assess only this 20% of patients with nodal metastases. In this, the most relevant, data analysis, disease-free survival was increased by a factor of 3.2, distant disease-free survival was increased by a factor of 2.1, and melanoma-specific survival was increased by a factor of 2.0 for patients who underwent sentinel-node biopsy compared with those who had observation only, Dr. Morton and his associates wrote (N. Engl. J. Med. 2014 Feb 12 [doi:10.1056/NEJMoa1310460]).
Among patients with intermediate-thickness melanomas, the estimated treatment effect on disease-free survival was 1.17 (P less than .001), and the estimated effect on distant disease-free survival was 0.73 (P = .04). For melanoma-specific survival, the estimated treatment effect was 0.68 (P = .05).
"Our long-term results confirm that sentinel-node biopsy correctly determines the pathologic status of the nodal basin in 96% of cases and is the most powerful prognostic indicator," they noted.
The data also indicate that "essentially all metastases detected by sentinel-node biopsy eventually would have become clinically evident if [they had] not [been] removed," the investigators added.
The MSLT-I was supported by the National Cancer Institute and the Australian and New Zealand Melanoma Trials Group. Dr. Morton reported no financial conflicts of interest; his associates reported ties to GlaxoSmithKline, Roche, Provectus, Merck, Myriad Genetics, and Melanoma Diagnostics.
The final data from this landmark trial "corroborate the profound prognostic significance of micrometastasis identified by sentinel-node biopsy," said Dr. Charles M. Balch and Dr. Jeffrey E. Gershenwald.
The finding that early lymphadenectomy improved melanoma-specific survival by 44% implies that delaying lymphadenectomy until there was clinical evidence of metastasis allowed an opportunity for further dissemination of regional disease. And the fact that the primary end point (improved melanoma-specific survival across the entire cohort of patients with intermediate thickness melanomas) of MSLT-I was not achieved "doesn’t detract from the clinical importance of regional lymph-node staging," they said.
Dr. Balch is at the University of Texas Southwestern Medical Center, Dallas; he reported ties to Merck and Amgen. Dr. Gershenwald is in the department of surgical oncology at the melanoma and skin center at the University of Texas M.D. Anderson Cancer Center, Houston.; he reported ties to Navidea, GlaxoSmithKline, UpToDate, and Mercator Therapeutics. These remarks were taken from their editorial accompanying Dr. Morton’s report (N. Engl. J. Med. 2014 Feb. 12 [doi:10.1056e1313690]).
The final data from this landmark trial "corroborate the profound prognostic significance of micrometastasis identified by sentinel-node biopsy," said Dr. Charles M. Balch and Dr. Jeffrey E. Gershenwald.
The finding that early lymphadenectomy improved melanoma-specific survival by 44% implies that delaying lymphadenectomy until there was clinical evidence of metastasis allowed an opportunity for further dissemination of regional disease. And the fact that the primary end point (improved melanoma-specific survival across the entire cohort of patients with intermediate thickness melanomas) of MSLT-I was not achieved "doesn’t detract from the clinical importance of regional lymph-node staging," they said.
Dr. Balch is at the University of Texas Southwestern Medical Center, Dallas; he reported ties to Merck and Amgen. Dr. Gershenwald is in the department of surgical oncology at the melanoma and skin center at the University of Texas M.D. Anderson Cancer Center, Houston.; he reported ties to Navidea, GlaxoSmithKline, UpToDate, and Mercator Therapeutics. These remarks were taken from their editorial accompanying Dr. Morton’s report (N. Engl. J. Med. 2014 Feb. 12 [doi:10.1056e1313690]).
The final data from this landmark trial "corroborate the profound prognostic significance of micrometastasis identified by sentinel-node biopsy," said Dr. Charles M. Balch and Dr. Jeffrey E. Gershenwald.
The finding that early lymphadenectomy improved melanoma-specific survival by 44% implies that delaying lymphadenectomy until there was clinical evidence of metastasis allowed an opportunity for further dissemination of regional disease. And the fact that the primary end point (improved melanoma-specific survival across the entire cohort of patients with intermediate thickness melanomas) of MSLT-I was not achieved "doesn’t detract from the clinical importance of regional lymph-node staging," they said.
Dr. Balch is at the University of Texas Southwestern Medical Center, Dallas; he reported ties to Merck and Amgen. Dr. Gershenwald is in the department of surgical oncology at the melanoma and skin center at the University of Texas M.D. Anderson Cancer Center, Houston.; he reported ties to Navidea, GlaxoSmithKline, UpToDate, and Mercator Therapeutics. These remarks were taken from their editorial accompanying Dr. Morton’s report (N. Engl. J. Med. 2014 Feb. 12 [doi:10.1056e1313690]).
Sentinel-node biopsy clearly benefits patients with intermediate-thickness or thick primary melanomas who are found to have nodal involvement and undergo immediate lymphadenectomy, tripling their disease-free survival and doubling their melanoma-specific survival and distant disease-free survival, according to a report published online Feb. 12 in the New England Journal of Medicine.
These long-term findings, the final results of 20 years of investigation in MSLT-I (Multicenter Selective Lymphadenectomy Trial), "clearly validate the use of sentinel-node biopsy" in this patient population. Among patients found to have no nodal involvement, "the procedure provides accurate and important staging information," while among those found to have nodal involvement, it enhances regional disease control and substantially improves survival, said Dr. Donald L. Morton of the John Wayne Cancer Institute at Saint John’s Health Center, Santa Monica, Calif., and his associates.
Dr. Morton passed away shortly before publication of this final MSLT-I report.
The MSLT-I, which began in 1994, was intended to determine whether sentinel-node biopsy – a new procedure at the time that was developed by Dr. Morton – could accurately identify clinically occult metastases. More important, since only about 20% of such patients are found to have metastases, the MSLT-I was to determine whether it was worthwhile to subject all patients to the procedure. In other words, did sentinel-node biopsy with immediate lymphadenectomy of involved nodes yield better outcomes than did watchful waiting with delayed lymphadenectomy, performed only when nodal recurrence became evident during observation?
This final report involved 1,560 patients with localized cutaneous primary melanomas: 1,270 with intermediate-thickness (1.20-3.50 mm) lesions and 290 with thick (greater than 3.50 mm) lesions. A total of 943 patients were randomly assigned to undergo sentinel-node biopsy plus immediate lymphadenectomy if metastases were detected in sentinel nodes, and 617 were randomly assigned to close observation with delayed lymphadenectomy if nodal metastases developed during observation.
All the study participants were monitored periodically throughout follow-up using clinical examination, blood testing for biomarkers of melanoma, chest radiography, PET scanning, CT scanning, and/or nodal ultrasonography.
The primary end point, melanoma-specific survival across the entire study cohort, was not significantly different between patients who underwent sentinel-node biopsy (81.4%) and those who had observation only (78.3%). This is not surprising as only the 20% of patients who actually had nodal metastases could potentially derive a survival benefit from the procedure.
Latent-subgroup statistical methods were used to assess only this 20% of patients with nodal metastases. In this, the most relevant, data analysis, disease-free survival was increased by a factor of 3.2, distant disease-free survival was increased by a factor of 2.1, and melanoma-specific survival was increased by a factor of 2.0 for patients who underwent sentinel-node biopsy compared with those who had observation only, Dr. Morton and his associates wrote (N. Engl. J. Med. 2014 Feb 12 [doi:10.1056/NEJMoa1310460]).
Among patients with intermediate-thickness melanomas, the estimated treatment effect on disease-free survival was 1.17 (P less than .001), and the estimated effect on distant disease-free survival was 0.73 (P = .04). For melanoma-specific survival, the estimated treatment effect was 0.68 (P = .05).
"Our long-term results confirm that sentinel-node biopsy correctly determines the pathologic status of the nodal basin in 96% of cases and is the most powerful prognostic indicator," they noted.
The data also indicate that "essentially all metastases detected by sentinel-node biopsy eventually would have become clinically evident if [they had] not [been] removed," the investigators added.
The MSLT-I was supported by the National Cancer Institute and the Australian and New Zealand Melanoma Trials Group. Dr. Morton reported no financial conflicts of interest; his associates reported ties to GlaxoSmithKline, Roche, Provectus, Merck, Myriad Genetics, and Melanoma Diagnostics.
Sentinel-node biopsy clearly benefits patients with intermediate-thickness or thick primary melanomas who are found to have nodal involvement and undergo immediate lymphadenectomy, tripling their disease-free survival and doubling their melanoma-specific survival and distant disease-free survival, according to a report published online Feb. 12 in the New England Journal of Medicine.
These long-term findings, the final results of 20 years of investigation in MSLT-I (Multicenter Selective Lymphadenectomy Trial), "clearly validate the use of sentinel-node biopsy" in this patient population. Among patients found to have no nodal involvement, "the procedure provides accurate and important staging information," while among those found to have nodal involvement, it enhances regional disease control and substantially improves survival, said Dr. Donald L. Morton of the John Wayne Cancer Institute at Saint John’s Health Center, Santa Monica, Calif., and his associates.
Dr. Morton passed away shortly before publication of this final MSLT-I report.
The MSLT-I, which began in 1994, was intended to determine whether sentinel-node biopsy – a new procedure at the time that was developed by Dr. Morton – could accurately identify clinically occult metastases. More important, since only about 20% of such patients are found to have metastases, the MSLT-I was to determine whether it was worthwhile to subject all patients to the procedure. In other words, did sentinel-node biopsy with immediate lymphadenectomy of involved nodes yield better outcomes than did watchful waiting with delayed lymphadenectomy, performed only when nodal recurrence became evident during observation?
This final report involved 1,560 patients with localized cutaneous primary melanomas: 1,270 with intermediate-thickness (1.20-3.50 mm) lesions and 290 with thick (greater than 3.50 mm) lesions. A total of 943 patients were randomly assigned to undergo sentinel-node biopsy plus immediate lymphadenectomy if metastases were detected in sentinel nodes, and 617 were randomly assigned to close observation with delayed lymphadenectomy if nodal metastases developed during observation.
All the study participants were monitored periodically throughout follow-up using clinical examination, blood testing for biomarkers of melanoma, chest radiography, PET scanning, CT scanning, and/or nodal ultrasonography.
The primary end point, melanoma-specific survival across the entire study cohort, was not significantly different between patients who underwent sentinel-node biopsy (81.4%) and those who had observation only (78.3%). This is not surprising as only the 20% of patients who actually had nodal metastases could potentially derive a survival benefit from the procedure.
Latent-subgroup statistical methods were used to assess only this 20% of patients with nodal metastases. In this, the most relevant, data analysis, disease-free survival was increased by a factor of 3.2, distant disease-free survival was increased by a factor of 2.1, and melanoma-specific survival was increased by a factor of 2.0 for patients who underwent sentinel-node biopsy compared with those who had observation only, Dr. Morton and his associates wrote (N. Engl. J. Med. 2014 Feb 12 [doi:10.1056/NEJMoa1310460]).
Among patients with intermediate-thickness melanomas, the estimated treatment effect on disease-free survival was 1.17 (P less than .001), and the estimated effect on distant disease-free survival was 0.73 (P = .04). For melanoma-specific survival, the estimated treatment effect was 0.68 (P = .05).
"Our long-term results confirm that sentinel-node biopsy correctly determines the pathologic status of the nodal basin in 96% of cases and is the most powerful prognostic indicator," they noted.
The data also indicate that "essentially all metastases detected by sentinel-node biopsy eventually would have become clinically evident if [they had] not [been] removed," the investigators added.
The MSLT-I was supported by the National Cancer Institute and the Australian and New Zealand Melanoma Trials Group. Dr. Morton reported no financial conflicts of interest; his associates reported ties to GlaxoSmithKline, Roche, Provectus, Merck, Myriad Genetics, and Melanoma Diagnostics.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: Among patients with nodal metastases, disease-free survival was increased by a factor of 3.2, distant-disease-free survival was increased by a factor of 2.1, and melanoma-specific survival was increased by a factor of 2.0 for those who underwent sentinel-node biopsy, compared with those who had observation only.
Data source: The 20-year international randomized MSLT-I study involving 1,560 patients with intermediate-thickness or thick localized cutaneous melanomas comparing SNB with nodal observation.
Disclosures: MSLT-I was supported by the National Cancer Institute and the Australian and New Zealand Melanoma Trials Group. Dr. Morton reported no financial conflicts of interest; his associates reported ties to GlaxoSmithKline, Roche, Provectus, Merck, Myriad Genetics, and Melanoma Diagnostics.
Cosmetic Corner: Dermatologists Weigh in on Sunless Tanners
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top sunless tanners. Consideration must be given to:
La Roche-Posay Laboratoire Dermatologique
Recommended by Marta I. Rendon, MD, Boca Raton, Florida
Johnson & Johnson Consumer Companies, Inc
“For daily use Aveeno Continuous Radiance Moisturizing Lotion is excellent.”—Patricia K. Farris, Metairie, Lousiana
Clinique Laboratories, LLC
“Clinique Self Sun is my favorite as it provides a gradual tanning. Always explain to your patient that she needs to exfoliate the skin before applying.”—Antonella Tosti, MD, Miami, Florida
Kao USA Inc.
“I am a fan of Jergens sunless tanner. It is gradual, inexpensive, and easy to use. I always remind my patients that sunless tanners do not product from UV damage. You still need SPF on top of your sunless tan. But, a gradual tanner is a good and safe way to minimize minor imperfections like spider veins.”—Elizabeth K. Hale, MD, New York, New York
Clarins
“For a quick bronzing product, I like Clarins Self Tanning Instant Gel. It is easy to apply and gives you a great glow.”—Patricia K. Farris, MD, Metairie, Louisiana
PZ Cussons Beauty LLP
“There are many different products offered and shades. The tan produced is even and natural looking.”—Anthony M. Rossi, MD, New York, New York
Kiehl's
Recommended by Gary Goldenberg, MD, New York, New York
Iredale Mineral Cosmetics, Ltd
“Jane Iredale Tantasia is my favorite sunless tanner. It has a pleasant citrus scent, isn’t greasy or streaky, can be used on the face without causing acne, and gives a natural golden glow.”—Wm. Philip Werschler, MD, Seattle, Washington
Cutis invites readers to send us their recommendations. OTC antifungals, OTC hair restorations, and antiperspirants will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top sunless tanners. Consideration must be given to:
La Roche-Posay Laboratoire Dermatologique
Recommended by Marta I. Rendon, MD, Boca Raton, Florida
Johnson & Johnson Consumer Companies, Inc
“For daily use Aveeno Continuous Radiance Moisturizing Lotion is excellent.”—Patricia K. Farris, Metairie, Lousiana
Clinique Laboratories, LLC
“Clinique Self Sun is my favorite as it provides a gradual tanning. Always explain to your patient that she needs to exfoliate the skin before applying.”—Antonella Tosti, MD, Miami, Florida
Kao USA Inc.
“I am a fan of Jergens sunless tanner. It is gradual, inexpensive, and easy to use. I always remind my patients that sunless tanners do not product from UV damage. You still need SPF on top of your sunless tan. But, a gradual tanner is a good and safe way to minimize minor imperfections like spider veins.”—Elizabeth K. Hale, MD, New York, New York
Clarins
“For a quick bronzing product, I like Clarins Self Tanning Instant Gel. It is easy to apply and gives you a great glow.”—Patricia K. Farris, MD, Metairie, Louisiana
PZ Cussons Beauty LLP
“There are many different products offered and shades. The tan produced is even and natural looking.”—Anthony M. Rossi, MD, New York, New York
Kiehl's
Recommended by Gary Goldenberg, MD, New York, New York
Iredale Mineral Cosmetics, Ltd
“Jane Iredale Tantasia is my favorite sunless tanner. It has a pleasant citrus scent, isn’t greasy or streaky, can be used on the face without causing acne, and gives a natural golden glow.”—Wm. Philip Werschler, MD, Seattle, Washington
Cutis invites readers to send us their recommendations. OTC antifungals, OTC hair restorations, and antiperspirants will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top sunless tanners. Consideration must be given to:
La Roche-Posay Laboratoire Dermatologique
Recommended by Marta I. Rendon, MD, Boca Raton, Florida
Johnson & Johnson Consumer Companies, Inc
“For daily use Aveeno Continuous Radiance Moisturizing Lotion is excellent.”—Patricia K. Farris, Metairie, Lousiana
Clinique Laboratories, LLC
“Clinique Self Sun is my favorite as it provides a gradual tanning. Always explain to your patient that she needs to exfoliate the skin before applying.”—Antonella Tosti, MD, Miami, Florida
Kao USA Inc.
“I am a fan of Jergens sunless tanner. It is gradual, inexpensive, and easy to use. I always remind my patients that sunless tanners do not product from UV damage. You still need SPF on top of your sunless tan. But, a gradual tanner is a good and safe way to minimize minor imperfections like spider veins.”—Elizabeth K. Hale, MD, New York, New York
Clarins
“For a quick bronzing product, I like Clarins Self Tanning Instant Gel. It is easy to apply and gives you a great glow.”—Patricia K. Farris, MD, Metairie, Louisiana
PZ Cussons Beauty LLP
“There are many different products offered and shades. The tan produced is even and natural looking.”—Anthony M. Rossi, MD, New York, New York
Kiehl's
Recommended by Gary Goldenberg, MD, New York, New York
Iredale Mineral Cosmetics, Ltd
“Jane Iredale Tantasia is my favorite sunless tanner. It has a pleasant citrus scent, isn’t greasy or streaky, can be used on the face without causing acne, and gives a natural golden glow.”—Wm. Philip Werschler, MD, Seattle, Washington
Cutis invites readers to send us their recommendations. OTC antifungals, OTC hair restorations, and antiperspirants will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Product News: 02 2014
Current options and future directions in the systemic treatment of metastatic melanoma
Systemic treatment options for metastatic melanoma have historically been limited, with conventional cytotoxic chemotherapies demonstrating only modest benefit. Recent advances, however, have dramatically changed the treatment landscape and can be considered in 2 general categories: immunotherapeutic approaches that enhance antitumor immunity, and targeted therapeutic approaches that block oncogenic driver mutations. Immunotherapy with antibodies that block cytotoxic T-lymphocyte antigen 4 and programmed death-1 receptor can result in durable responses in a subset of patients. These treatments may be considered for patients irrespective of their mutational status, and ongoing research continues to investigate biomarkers associated with clinical outcomes. Side effects of these agents result from immune-mediated reactions involving various organ sites and can include: diarrhea, rash, hepatitis, and endocrinopathies.
Click on the PDF icon at the top of this introduction to read the full article.
Systemic treatment options for metastatic melanoma have historically been limited, with conventional cytotoxic chemotherapies demonstrating only modest benefit. Recent advances, however, have dramatically changed the treatment landscape and can be considered in 2 general categories: immunotherapeutic approaches that enhance antitumor immunity, and targeted therapeutic approaches that block oncogenic driver mutations. Immunotherapy with antibodies that block cytotoxic T-lymphocyte antigen 4 and programmed death-1 receptor can result in durable responses in a subset of patients. These treatments may be considered for patients irrespective of their mutational status, and ongoing research continues to investigate biomarkers associated with clinical outcomes. Side effects of these agents result from immune-mediated reactions involving various organ sites and can include: diarrhea, rash, hepatitis, and endocrinopathies.
Click on the PDF icon at the top of this introduction to read the full article.
Systemic treatment options for metastatic melanoma have historically been limited, with conventional cytotoxic chemotherapies demonstrating only modest benefit. Recent advances, however, have dramatically changed the treatment landscape and can be considered in 2 general categories: immunotherapeutic approaches that enhance antitumor immunity, and targeted therapeutic approaches that block oncogenic driver mutations. Immunotherapy with antibodies that block cytotoxic T-lymphocyte antigen 4 and programmed death-1 receptor can result in durable responses in a subset of patients. These treatments may be considered for patients irrespective of their mutational status, and ongoing research continues to investigate biomarkers associated with clinical outcomes. Side effects of these agents result from immune-mediated reactions involving various organ sites and can include: diarrhea, rash, hepatitis, and endocrinopathies.
Click on the PDF icon at the top of this introduction to read the full article.