User login
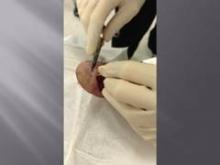
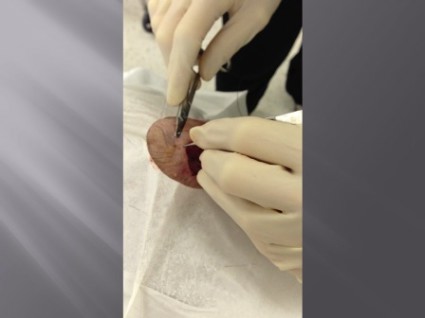
Pulley stitch: A go-to for defects under tension
EDITOR'S NOTE: August 26, 2013: This article has been amended since it was first published to make it clear that Dr. Kelley Pagliai Redbord's description of the pulley stitch procedure was taken directly from an article published by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg.2011;37:1503-5). In her presentation, Dr. Redbord credited Dr. Yag-Howard and her article. However, this credit and attribution to Dr. Yag-Howard was not included in the article published.
WASHINGTON – The pulley stitch "is my go-to stitch for defects under tension," said Dr. Kelley Pagliai Redbord.
The pulley stitch allows for considerable reduction in the surface area of a large defect that can’t be closed by side-to-side stitches alone, making it an excellent choice for use on the scalp and legs, Dr. Redbord said at the Atlantic Dermatological Conference.
"When the tension across the wound is decreased, buried dermal sutures can be placed more easily and accurately," she said. "I use it a lot as an intraoperative tissue expander."
Dr. Redbord said that her description of the pulley stitch was taken from an article by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg. 2011; 37:1503-5).*
The pulley stitch can serve as a temporary suture that can be left in place or removed, said Dr. Redbord, a dermatologist in group practice in Rockville, Md.
The technique follows a far-near-near-far pattern, starting the stitch 8 mm from the wound edge (far), then bringing it to the opposite side just 4 mm from the wound edge (near). Dr. Redbord then reenters the stitch 4 mm from the wound edge on the initial side (near), and makes another pass to the opposite side 8 mm from the wound edge (far).
Multiple passes through the tissue create resistance that keeps the suture from slipping. "The loops of the stitch are placed at an oblique angle so that the inner and outer loops are offset and do not override each other," she noted. This technique minimizes potential skin damage from pressure necrosis caused by overriding loop sutures. The pulley stitch has a 2:1 mechanical advantage over an interrupted suture, and the additional friction of a second loop prevents the knot from slipping.
A modification of the pulley stitch is to loop the suture through an external loop on the opposite side of the incision, and pull across. "This new loop functions as a pulley and directs the tension away from the other strands," she said.
Another stitch with excellent eversion, in which the pulley stitch plays a key role, is the subcutaneous inverted cross mattress stitch (SICM). The SCIM is entirely subcutaneous, and combines the buried vertical mattress stitch and the buried pulley stitch.
The SCIM "uses the buried vertical mattress’s ability to evert wound edges and combines it with the pulley stitch’s ability to decrease tension at the wound edge," she said.
The four-step process is as follows:
• 1. Insert the needle into the dermis 3-5 mm lateral to the wound edge. Advance the needle into the upper reticular dermis, and then curve down to exit through the lower reticular dermis.
• 2. Insert the needle into the opposite edge of the wound at the lower reticular dermis and advance into the upper reticular dermis, then curve down and exit intradermally.
• 3. Insert the needle across the defect using an intradermal approach 1-2 mm lateral to the initial needle insertion point. Then, create a second buried vertical mattress stitch.
• 4. Pull the two stitches to close, which "creates a pulley effect with minimal recoil, and tie off," Dr. Redbord said.
"The pulley system locks the wound edges so that a knot can be tied without slipping," she added.
Dr. Redbord said she had no relevant financial disclosures.
EDITOR'S NOTE: August 26, 2013: This article has been amended since it was first published to make it clear that Dr. Kelley Pagliai Redbord's description of the pulley stitch procedure was taken directly from an article published by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg.2011;37:1503-5). In her presentation, Dr. Redbord credited Dr. Yag-Howard and her article. However, this credit and attribution to Dr. Yag-Howard was not included in the article published.
WASHINGTON – The pulley stitch "is my go-to stitch for defects under tension," said Dr. Kelley Pagliai Redbord.
The pulley stitch allows for considerable reduction in the surface area of a large defect that can’t be closed by side-to-side stitches alone, making it an excellent choice for use on the scalp and legs, Dr. Redbord said at the Atlantic Dermatological Conference.
"When the tension across the wound is decreased, buried dermal sutures can be placed more easily and accurately," she said. "I use it a lot as an intraoperative tissue expander."
Dr. Redbord said that her description of the pulley stitch was taken from an article by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg. 2011; 37:1503-5).*
The pulley stitch can serve as a temporary suture that can be left in place or removed, said Dr. Redbord, a dermatologist in group practice in Rockville, Md.
The technique follows a far-near-near-far pattern, starting the stitch 8 mm from the wound edge (far), then bringing it to the opposite side just 4 mm from the wound edge (near). Dr. Redbord then reenters the stitch 4 mm from the wound edge on the initial side (near), and makes another pass to the opposite side 8 mm from the wound edge (far).
Multiple passes through the tissue create resistance that keeps the suture from slipping. "The loops of the stitch are placed at an oblique angle so that the inner and outer loops are offset and do not override each other," she noted. This technique minimizes potential skin damage from pressure necrosis caused by overriding loop sutures. The pulley stitch has a 2:1 mechanical advantage over an interrupted suture, and the additional friction of a second loop prevents the knot from slipping.
A modification of the pulley stitch is to loop the suture through an external loop on the opposite side of the incision, and pull across. "This new loop functions as a pulley and directs the tension away from the other strands," she said.
Another stitch with excellent eversion, in which the pulley stitch plays a key role, is the subcutaneous inverted cross mattress stitch (SICM). The SCIM is entirely subcutaneous, and combines the buried vertical mattress stitch and the buried pulley stitch.
The SCIM "uses the buried vertical mattress’s ability to evert wound edges and combines it with the pulley stitch’s ability to decrease tension at the wound edge," she said.
The four-step process is as follows:
• 1. Insert the needle into the dermis 3-5 mm lateral to the wound edge. Advance the needle into the upper reticular dermis, and then curve down to exit through the lower reticular dermis.
• 2. Insert the needle into the opposite edge of the wound at the lower reticular dermis and advance into the upper reticular dermis, then curve down and exit intradermally.
• 3. Insert the needle across the defect using an intradermal approach 1-2 mm lateral to the initial needle insertion point. Then, create a second buried vertical mattress stitch.
• 4. Pull the two stitches to close, which "creates a pulley effect with minimal recoil, and tie off," Dr. Redbord said.
"The pulley system locks the wound edges so that a knot can be tied without slipping," she added.
Dr. Redbord said she had no relevant financial disclosures.
EDITOR'S NOTE: August 26, 2013: This article has been amended since it was first published to make it clear that Dr. Kelley Pagliai Redbord's description of the pulley stitch procedure was taken directly from an article published by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg.2011;37:1503-5). In her presentation, Dr. Redbord credited Dr. Yag-Howard and her article. However, this credit and attribution to Dr. Yag-Howard was not included in the article published.
WASHINGTON – The pulley stitch "is my go-to stitch for defects under tension," said Dr. Kelley Pagliai Redbord.
The pulley stitch allows for considerable reduction in the surface area of a large defect that can’t be closed by side-to-side stitches alone, making it an excellent choice for use on the scalp and legs, Dr. Redbord said at the Atlantic Dermatological Conference.
"When the tension across the wound is decreased, buried dermal sutures can be placed more easily and accurately," she said. "I use it a lot as an intraoperative tissue expander."
Dr. Redbord said that her description of the pulley stitch was taken from an article by Dr. Cyndi Yag-Howard in Dermatologic Surgery (Dermatol. Surg. 2011; 37:1503-5).*
The pulley stitch can serve as a temporary suture that can be left in place or removed, said Dr. Redbord, a dermatologist in group practice in Rockville, Md.
The technique follows a far-near-near-far pattern, starting the stitch 8 mm from the wound edge (far), then bringing it to the opposite side just 4 mm from the wound edge (near). Dr. Redbord then reenters the stitch 4 mm from the wound edge on the initial side (near), and makes another pass to the opposite side 8 mm from the wound edge (far).
Multiple passes through the tissue create resistance that keeps the suture from slipping. "The loops of the stitch are placed at an oblique angle so that the inner and outer loops are offset and do not override each other," she noted. This technique minimizes potential skin damage from pressure necrosis caused by overriding loop sutures. The pulley stitch has a 2:1 mechanical advantage over an interrupted suture, and the additional friction of a second loop prevents the knot from slipping.
A modification of the pulley stitch is to loop the suture through an external loop on the opposite side of the incision, and pull across. "This new loop functions as a pulley and directs the tension away from the other strands," she said.
Another stitch with excellent eversion, in which the pulley stitch plays a key role, is the subcutaneous inverted cross mattress stitch (SICM). The SCIM is entirely subcutaneous, and combines the buried vertical mattress stitch and the buried pulley stitch.
The SCIM "uses the buried vertical mattress’s ability to evert wound edges and combines it with the pulley stitch’s ability to decrease tension at the wound edge," she said.
The four-step process is as follows:
• 1. Insert the needle into the dermis 3-5 mm lateral to the wound edge. Advance the needle into the upper reticular dermis, and then curve down to exit through the lower reticular dermis.
• 2. Insert the needle into the opposite edge of the wound at the lower reticular dermis and advance into the upper reticular dermis, then curve down and exit intradermally.
• 3. Insert the needle across the defect using an intradermal approach 1-2 mm lateral to the initial needle insertion point. Then, create a second buried vertical mattress stitch.
• 4. Pull the two stitches to close, which "creates a pulley effect with minimal recoil, and tie off," Dr. Redbord said.
"The pulley system locks the wound edges so that a knot can be tied without slipping," she added.
Dr. Redbord said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ATLANTIC DERMATOLOGICAL CONFERENCE
Major finding: Key numerical finding (e.g., number needed to treat to prevent one death/event; number lived or died as result of intervention). Maximum 10 words/1 sentence.
Data source: Include type of study (e.g., randomized, placebo controlled trial; retrospective case-control study). Include number in the study.
Disclosures: Sponsor of study, funding source, relevant disclosures. If author has no relevant disclosures, "Dr. X reported having no financial disclosures." If necessary, "Meeting Y did not require reports of financial disclosures." Check meeting website because many list disclosures. Written in sentence form.
Sequential laser therapy clears basal cell carcinomas
BOSTON – Sequential application of pulsed dye and Nd:YAG lasers is safe and effective for treating small basal cell carcinomas, based on data from a prospective study of patients with nodular and superficial BCC subtypes on the trunk and extremities.
The findings were presented at the annual meeting of the American Society for Laser Medicine and Surgery.
In a study of 10 patients with BCC, 7 of the 12 lesions treated with pulsed dye laser followed by Nd:YAG laser showed completed clinical and histologic clearance, said Dr. H. Ray Jalian of Massachusetts General Hospital in Boston.
"Targeting the microvasculature of BCC offers a promising new treatment approach. These tumors have large caliber feeding vessels; oftentimes these vessels are larger than the surrounding stroma," he said.
Data from a previous study (Lasers Surg. Med. 2009;41:417-42) showed a 92% regression rate of BCC lesions smaller than 1.5 cm treated with a 595 nm pulsed dye laser, Dr. Jalian noted.
The rationale for the sequential laser therapy is that pulsed dye laser energy is well absorbed by hemoglobin, which generates methemoglobin that in turn absorbs 1064 nm Nd:YAG energy, allowing the energy to penetrate to deep vessels.
"We hypothesized that targeting the vasculature of basal cells at two levels may be able to selectively destroy deeper vessels and perhaps achieve a higher cure rate," he said.
The investigators conducted a prospective study with 10 patients who had a total of 13 BCC of nodular and superficial subtypes on the trunk and extremities (1 patient with a single lesion was not available for follow-up).
The treated lesions were less than 2 cm with clearly visible margins that would be suitable for treatment with standard surgical excision. Patients with scars or infections in the area to be treated were excluded, as were those who were immunocompromised or pregnant.
The participants underwent four laser treatments 2-4 weeks apart with a 585-nm PDL set for a 7-mm spot size, 8-J/cm2, 2-ms pulse duration, followed by a 1064-nm Nd:YAG laser set with a 7-mm spot, 40-J/cm2, 15-ms pulse duration.
A total of 7 of the 12 lesions available for follow-up were completely cleared on both clinical and histologic evaluation. Of the eight tumors under 1 cm in size, six were completely cleared by sequential laser therapy,
Of the four patients with 5 lesions with residual disease after four laser sessions, three were on anticoagulation therapy with aspirin, and one with warfarin.
"We did see a clearance of the nodular component in most cases, but there was persistent residual superficial BCC in these patients," Dr. Jalian said.
Anticoagulation may hamper the laser effect by reducing laser-induced vascular injury, he noted.
Treatment-related side effects included erythema, scarring, and hyperpigmentation. Erythema and scarring decreased from the first treatment to the last follow-up visit, while hyperpigmentation increased slightly from the first to the third treatment, and then plateaued.
Biopsy scars improved with sequential treatments, Dr. Jalian noted.
Possible explanations for the lower success rate treating BCC compared to previous studies include the use of a slightly lower wavelength laser (585 vs. 595), and lower energy settings (8 J/cm2 for 2 ms, vs. 15 J/cm2 for 3 ms), and by mix of histologic subtypes, said Dr. Jalian.
"Superficial subtypes present in residual lesions suggest there may be a different vascular pattern in these lesions."
The findings also suggest that anticoagulation therapy may need to be suspended before treatment with pulsed dye, Nd:YAG, and other vascular-specific lasers, he added.
The study was internally supported. Dr. Jalian reported having no financial disclosures.
BOSTON – Sequential application of pulsed dye and Nd:YAG lasers is safe and effective for treating small basal cell carcinomas, based on data from a prospective study of patients with nodular and superficial BCC subtypes on the trunk and extremities.
The findings were presented at the annual meeting of the American Society for Laser Medicine and Surgery.
In a study of 10 patients with BCC, 7 of the 12 lesions treated with pulsed dye laser followed by Nd:YAG laser showed completed clinical and histologic clearance, said Dr. H. Ray Jalian of Massachusetts General Hospital in Boston.
"Targeting the microvasculature of BCC offers a promising new treatment approach. These tumors have large caliber feeding vessels; oftentimes these vessels are larger than the surrounding stroma," he said.
Data from a previous study (Lasers Surg. Med. 2009;41:417-42) showed a 92% regression rate of BCC lesions smaller than 1.5 cm treated with a 595 nm pulsed dye laser, Dr. Jalian noted.
The rationale for the sequential laser therapy is that pulsed dye laser energy is well absorbed by hemoglobin, which generates methemoglobin that in turn absorbs 1064 nm Nd:YAG energy, allowing the energy to penetrate to deep vessels.
"We hypothesized that targeting the vasculature of basal cells at two levels may be able to selectively destroy deeper vessels and perhaps achieve a higher cure rate," he said.
The investigators conducted a prospective study with 10 patients who had a total of 13 BCC of nodular and superficial subtypes on the trunk and extremities (1 patient with a single lesion was not available for follow-up).
The treated lesions were less than 2 cm with clearly visible margins that would be suitable for treatment with standard surgical excision. Patients with scars or infections in the area to be treated were excluded, as were those who were immunocompromised or pregnant.
The participants underwent four laser treatments 2-4 weeks apart with a 585-nm PDL set for a 7-mm spot size, 8-J/cm2, 2-ms pulse duration, followed by a 1064-nm Nd:YAG laser set with a 7-mm spot, 40-J/cm2, 15-ms pulse duration.
A total of 7 of the 12 lesions available for follow-up were completely cleared on both clinical and histologic evaluation. Of the eight tumors under 1 cm in size, six were completely cleared by sequential laser therapy,
Of the four patients with 5 lesions with residual disease after four laser sessions, three were on anticoagulation therapy with aspirin, and one with warfarin.
"We did see a clearance of the nodular component in most cases, but there was persistent residual superficial BCC in these patients," Dr. Jalian said.
Anticoagulation may hamper the laser effect by reducing laser-induced vascular injury, he noted.
Treatment-related side effects included erythema, scarring, and hyperpigmentation. Erythema and scarring decreased from the first treatment to the last follow-up visit, while hyperpigmentation increased slightly from the first to the third treatment, and then plateaued.
Biopsy scars improved with sequential treatments, Dr. Jalian noted.
Possible explanations for the lower success rate treating BCC compared to previous studies include the use of a slightly lower wavelength laser (585 vs. 595), and lower energy settings (8 J/cm2 for 2 ms, vs. 15 J/cm2 for 3 ms), and by mix of histologic subtypes, said Dr. Jalian.
"Superficial subtypes present in residual lesions suggest there may be a different vascular pattern in these lesions."
The findings also suggest that anticoagulation therapy may need to be suspended before treatment with pulsed dye, Nd:YAG, and other vascular-specific lasers, he added.
The study was internally supported. Dr. Jalian reported having no financial disclosures.
BOSTON – Sequential application of pulsed dye and Nd:YAG lasers is safe and effective for treating small basal cell carcinomas, based on data from a prospective study of patients with nodular and superficial BCC subtypes on the trunk and extremities.
The findings were presented at the annual meeting of the American Society for Laser Medicine and Surgery.
In a study of 10 patients with BCC, 7 of the 12 lesions treated with pulsed dye laser followed by Nd:YAG laser showed completed clinical and histologic clearance, said Dr. H. Ray Jalian of Massachusetts General Hospital in Boston.
"Targeting the microvasculature of BCC offers a promising new treatment approach. These tumors have large caliber feeding vessels; oftentimes these vessels are larger than the surrounding stroma," he said.
Data from a previous study (Lasers Surg. Med. 2009;41:417-42) showed a 92% regression rate of BCC lesions smaller than 1.5 cm treated with a 595 nm pulsed dye laser, Dr. Jalian noted.
The rationale for the sequential laser therapy is that pulsed dye laser energy is well absorbed by hemoglobin, which generates methemoglobin that in turn absorbs 1064 nm Nd:YAG energy, allowing the energy to penetrate to deep vessels.
"We hypothesized that targeting the vasculature of basal cells at two levels may be able to selectively destroy deeper vessels and perhaps achieve a higher cure rate," he said.
The investigators conducted a prospective study with 10 patients who had a total of 13 BCC of nodular and superficial subtypes on the trunk and extremities (1 patient with a single lesion was not available for follow-up).
The treated lesions were less than 2 cm with clearly visible margins that would be suitable for treatment with standard surgical excision. Patients with scars or infections in the area to be treated were excluded, as were those who were immunocompromised or pregnant.
The participants underwent four laser treatments 2-4 weeks apart with a 585-nm PDL set for a 7-mm spot size, 8-J/cm2, 2-ms pulse duration, followed by a 1064-nm Nd:YAG laser set with a 7-mm spot, 40-J/cm2, 15-ms pulse duration.
A total of 7 of the 12 lesions available for follow-up were completely cleared on both clinical and histologic evaluation. Of the eight tumors under 1 cm in size, six were completely cleared by sequential laser therapy,
Of the four patients with 5 lesions with residual disease after four laser sessions, three were on anticoagulation therapy with aspirin, and one with warfarin.
"We did see a clearance of the nodular component in most cases, but there was persistent residual superficial BCC in these patients," Dr. Jalian said.
Anticoagulation may hamper the laser effect by reducing laser-induced vascular injury, he noted.
Treatment-related side effects included erythema, scarring, and hyperpigmentation. Erythema and scarring decreased from the first treatment to the last follow-up visit, while hyperpigmentation increased slightly from the first to the third treatment, and then plateaued.
Biopsy scars improved with sequential treatments, Dr. Jalian noted.
Possible explanations for the lower success rate treating BCC compared to previous studies include the use of a slightly lower wavelength laser (585 vs. 595), and lower energy settings (8 J/cm2 for 2 ms, vs. 15 J/cm2 for 3 ms), and by mix of histologic subtypes, said Dr. Jalian.
"Superficial subtypes present in residual lesions suggest there may be a different vascular pattern in these lesions."
The findings also suggest that anticoagulation therapy may need to be suspended before treatment with pulsed dye, Nd:YAG, and other vascular-specific lasers, he added.
The study was internally supported. Dr. Jalian reported having no financial disclosures.
AT LASER 2013
Major finding: Seven of 12 basal cell carcinomas treated with sequential lasers showed complete clinical and histologic clearance.
Data source: Prospective case series of 10 patients with 13 BCC lesions.
Disclosures: The study was internally supported. Dr. Jalian reported having no financial disclosures.
'Slow Mohs' advised for lentigo maligna
MAUI, HAWAII – "Slow Mohs" has gained near-universal acceptance among skin cancer specialists as a definitive surgical technique for complete removal of lentigo maligna melanoma while simultaneously sparing normal tissue, according to Dr. Ellen Marmur of Mount Sinai School of Medicine, New York.
The big advantage that slow Mohs has over standard wide local excision with 0.5- to 1-cm margins is a 5-year cure rate approaching 100%. In contrast, standard excision has a recurrence rate of up to 20%, she said at the Hawaii Dermatology Seminar, sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Slow Mohs is a modified form of Mohs micrographic surgery. The surgery compares with conventional Mohs: It is staged, margin-controlled excision. But in slow Mohs, rush permanent sections are sent off to the pathologist rather than the frozen sections integral to conventional Mohs.
Dr. Marmur relies upon slow Mohs, with "bread-loafing" of the central tumor by the pathologist, because the permanent sections better preserve the tumor’s microscopic features. Interpreting atypical melanocytes in frozen sections can be quite a challenge. However, she added, some Mohs surgeons have found that using rapid immunostains also markedly improves the sensitivity and specificity of frozen sections in lentigo maligna surgery.
Slow Mohs takes place over the course of days, she said. "Basically, you do your Wood’s lamp to define the lesion diameter, you measure out your margins, you excise the tumor, pack the area with a bandage, and send the patient home. You rush your pathology, and you don’t do any reconstruction until you get the margins clear."
A pathology report that comes back stating narrow margins are present is "a heart stopper," she added.
"You have the option of observing the area if the margin is clear but the tumor was close to the margin. That’s a good approach for an elderly patient or when the lentigo maligna was in a cosmetically important area."
Lentigo maligna melanoma accounts for 4% of all cases of melanoma. It typically arises on sun-damaged skin in individuals in their 70s or older. Common sites include the malar area, forehead, nose, and temple. The differential diagnosis includes seborrheic keratosis, pigmented actinic keratosis, and pigmented nevus.
Lentigo maligna becomes lentigo maligna melanoma when malignant melanoma cells invade the dermis and deeper appendages. Roughly 5% of lentigo malignas eventually progress to invasive melanoma, according to Dr. Marmur. Typically, a lentigo maligna undergoes extended gradual horizontal growth before beginning a vertical growth phase.
"It spreads like an oil slick for many years," Dr. Marmur said at the seminar.
Established treatment modalities for patients who aren’t surgical candidates include cryotherapy, radiotherapy, and topical imiquimod 5%. All have disadvantages, including high 5-year recurrence rates.
Dr. Marmur noted that a newer nonsurgical therapy drawing considerable interest involves off-label use of topical combination therapy with imiquimod and tazarotene gel. The concept is to use the topical retinoid to disrupt the stratum corneum in order to enhance imiquimod penetration, thereby achieving a greater inflammatory response than possible with imiquimod alone.
Initial data have been published by researchers at the University of Utah, Salt Lake City. They randomized 90 patients with 91 lentigo malignas to imiquimod 5% cream applied 5 days per week for 3 months or to the imiquimod regimen plus tazarotene 0.1% gel on the other 2 days per week. After 3 months of topical therapy, patients underwent conservative staged excision with frozen section analysis with Melan A immunostaining to confirm negative margins.
Of those treated with dual topical therapy for 3 months, 29 of 37 lesions (78%) had complete responses with no residual lentigo maligna at the time of staged excision. So did 27 of 42 (64%) treated with imiquimod alone (Arch. Dermatol. 2012;148:592-6).
The modest difference in outcome was not significant (P = .17). Nevertheless, the Utah investigators wrote that topical pretreatment appears to reduce surgical defect sizes, an important consideration in lentigo maligna because the lesions are often large and located on cosmetically sensitive facial sites. At the patient’s first visit, the researchers saucerize the entire tumor to remove all visible evidence of lentigo maligna and nip in the bud any invasive element that might be present. One month later, after the wound has healed by secondary intention, the patient begins topical imiquimod therapy 5 days per week. If no inflammation is observed, tazarotene gel is added on the other 2 days per week. After 3 months of topical therapy, the patient goes off treatment for 2 months so the inflammatory response can subside. Then a staged excision is performed with 2-mm margins around the perimeter of the original tumor outline.
"This is an interesting thought, but it’s still not considered standard of care," Dr. Marmur commented in describing the Utah research.
Session chair Dr. Allan C. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center in New York, said he and his colleagues, like the Utah group, are using a lot of topical imiquimod as adjunctive therapy for lentigo maligna.
"Because we’re a referral center, we see all those patients whose melanomas unfortunately do recur at the edge of large surgeries. We see a lot of patients whose local surgeons have chased around their faces for a certain amount of time. So I think the general concept of the cure rates of surgery is a bit overstated. I’m not trying to sell an off-label use of a drug, but I think sometimes imiquimod can be very helpful," Dr. Halpern said.
He offered a clinical pearl in diagnosing lentigo maligna: Consider a broad shave biopsy of a suspicious lesion rather than a deeper, smaller-diameter biopsy.
"Sampling errors are a real problem with lentigo maligna. We learned early on that if you take a large lentigo maligna and do multiple biopsies of the same lesion and send them to what we think are our very expert pathologists, on average, we get two or three back that were melanoma and two or three back that were pigmented actinic keratoses. The sampling error tends to be more in the horizontal than in the depth for these very early lesions," according to Dr. Halpern.
Dr. Marmur and Dr. Halpern reported having no relevant financial interests. SDEF and this news organization are owned by the same parent company.
MAUI, HAWAII – "Slow Mohs" has gained near-universal acceptance among skin cancer specialists as a definitive surgical technique for complete removal of lentigo maligna melanoma while simultaneously sparing normal tissue, according to Dr. Ellen Marmur of Mount Sinai School of Medicine, New York.
The big advantage that slow Mohs has over standard wide local excision with 0.5- to 1-cm margins is a 5-year cure rate approaching 100%. In contrast, standard excision has a recurrence rate of up to 20%, she said at the Hawaii Dermatology Seminar, sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Slow Mohs is a modified form of Mohs micrographic surgery. The surgery compares with conventional Mohs: It is staged, margin-controlled excision. But in slow Mohs, rush permanent sections are sent off to the pathologist rather than the frozen sections integral to conventional Mohs.
Dr. Marmur relies upon slow Mohs, with "bread-loafing" of the central tumor by the pathologist, because the permanent sections better preserve the tumor’s microscopic features. Interpreting atypical melanocytes in frozen sections can be quite a challenge. However, she added, some Mohs surgeons have found that using rapid immunostains also markedly improves the sensitivity and specificity of frozen sections in lentigo maligna surgery.
Slow Mohs takes place over the course of days, she said. "Basically, you do your Wood’s lamp to define the lesion diameter, you measure out your margins, you excise the tumor, pack the area with a bandage, and send the patient home. You rush your pathology, and you don’t do any reconstruction until you get the margins clear."
A pathology report that comes back stating narrow margins are present is "a heart stopper," she added.
"You have the option of observing the area if the margin is clear but the tumor was close to the margin. That’s a good approach for an elderly patient or when the lentigo maligna was in a cosmetically important area."
Lentigo maligna melanoma accounts for 4% of all cases of melanoma. It typically arises on sun-damaged skin in individuals in their 70s or older. Common sites include the malar area, forehead, nose, and temple. The differential diagnosis includes seborrheic keratosis, pigmented actinic keratosis, and pigmented nevus.
Lentigo maligna becomes lentigo maligna melanoma when malignant melanoma cells invade the dermis and deeper appendages. Roughly 5% of lentigo malignas eventually progress to invasive melanoma, according to Dr. Marmur. Typically, a lentigo maligna undergoes extended gradual horizontal growth before beginning a vertical growth phase.
"It spreads like an oil slick for many years," Dr. Marmur said at the seminar.
Established treatment modalities for patients who aren’t surgical candidates include cryotherapy, radiotherapy, and topical imiquimod 5%. All have disadvantages, including high 5-year recurrence rates.
Dr. Marmur noted that a newer nonsurgical therapy drawing considerable interest involves off-label use of topical combination therapy with imiquimod and tazarotene gel. The concept is to use the topical retinoid to disrupt the stratum corneum in order to enhance imiquimod penetration, thereby achieving a greater inflammatory response than possible with imiquimod alone.
Initial data have been published by researchers at the University of Utah, Salt Lake City. They randomized 90 patients with 91 lentigo malignas to imiquimod 5% cream applied 5 days per week for 3 months or to the imiquimod regimen plus tazarotene 0.1% gel on the other 2 days per week. After 3 months of topical therapy, patients underwent conservative staged excision with frozen section analysis with Melan A immunostaining to confirm negative margins.
Of those treated with dual topical therapy for 3 months, 29 of 37 lesions (78%) had complete responses with no residual lentigo maligna at the time of staged excision. So did 27 of 42 (64%) treated with imiquimod alone (Arch. Dermatol. 2012;148:592-6).
The modest difference in outcome was not significant (P = .17). Nevertheless, the Utah investigators wrote that topical pretreatment appears to reduce surgical defect sizes, an important consideration in lentigo maligna because the lesions are often large and located on cosmetically sensitive facial sites. At the patient’s first visit, the researchers saucerize the entire tumor to remove all visible evidence of lentigo maligna and nip in the bud any invasive element that might be present. One month later, after the wound has healed by secondary intention, the patient begins topical imiquimod therapy 5 days per week. If no inflammation is observed, tazarotene gel is added on the other 2 days per week. After 3 months of topical therapy, the patient goes off treatment for 2 months so the inflammatory response can subside. Then a staged excision is performed with 2-mm margins around the perimeter of the original tumor outline.
"This is an interesting thought, but it’s still not considered standard of care," Dr. Marmur commented in describing the Utah research.
Session chair Dr. Allan C. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center in New York, said he and his colleagues, like the Utah group, are using a lot of topical imiquimod as adjunctive therapy for lentigo maligna.
"Because we’re a referral center, we see all those patients whose melanomas unfortunately do recur at the edge of large surgeries. We see a lot of patients whose local surgeons have chased around their faces for a certain amount of time. So I think the general concept of the cure rates of surgery is a bit overstated. I’m not trying to sell an off-label use of a drug, but I think sometimes imiquimod can be very helpful," Dr. Halpern said.
He offered a clinical pearl in diagnosing lentigo maligna: Consider a broad shave biopsy of a suspicious lesion rather than a deeper, smaller-diameter biopsy.
"Sampling errors are a real problem with lentigo maligna. We learned early on that if you take a large lentigo maligna and do multiple biopsies of the same lesion and send them to what we think are our very expert pathologists, on average, we get two or three back that were melanoma and two or three back that were pigmented actinic keratoses. The sampling error tends to be more in the horizontal than in the depth for these very early lesions," according to Dr. Halpern.
Dr. Marmur and Dr. Halpern reported having no relevant financial interests. SDEF and this news organization are owned by the same parent company.
MAUI, HAWAII – "Slow Mohs" has gained near-universal acceptance among skin cancer specialists as a definitive surgical technique for complete removal of lentigo maligna melanoma while simultaneously sparing normal tissue, according to Dr. Ellen Marmur of Mount Sinai School of Medicine, New York.
The big advantage that slow Mohs has over standard wide local excision with 0.5- to 1-cm margins is a 5-year cure rate approaching 100%. In contrast, standard excision has a recurrence rate of up to 20%, she said at the Hawaii Dermatology Seminar, sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Slow Mohs is a modified form of Mohs micrographic surgery. The surgery compares with conventional Mohs: It is staged, margin-controlled excision. But in slow Mohs, rush permanent sections are sent off to the pathologist rather than the frozen sections integral to conventional Mohs.
Dr. Marmur relies upon slow Mohs, with "bread-loafing" of the central tumor by the pathologist, because the permanent sections better preserve the tumor’s microscopic features. Interpreting atypical melanocytes in frozen sections can be quite a challenge. However, she added, some Mohs surgeons have found that using rapid immunostains also markedly improves the sensitivity and specificity of frozen sections in lentigo maligna surgery.
Slow Mohs takes place over the course of days, she said. "Basically, you do your Wood’s lamp to define the lesion diameter, you measure out your margins, you excise the tumor, pack the area with a bandage, and send the patient home. You rush your pathology, and you don’t do any reconstruction until you get the margins clear."
A pathology report that comes back stating narrow margins are present is "a heart stopper," she added.
"You have the option of observing the area if the margin is clear but the tumor was close to the margin. That’s a good approach for an elderly patient or when the lentigo maligna was in a cosmetically important area."
Lentigo maligna melanoma accounts for 4% of all cases of melanoma. It typically arises on sun-damaged skin in individuals in their 70s or older. Common sites include the malar area, forehead, nose, and temple. The differential diagnosis includes seborrheic keratosis, pigmented actinic keratosis, and pigmented nevus.
Lentigo maligna becomes lentigo maligna melanoma when malignant melanoma cells invade the dermis and deeper appendages. Roughly 5% of lentigo malignas eventually progress to invasive melanoma, according to Dr. Marmur. Typically, a lentigo maligna undergoes extended gradual horizontal growth before beginning a vertical growth phase.
"It spreads like an oil slick for many years," Dr. Marmur said at the seminar.
Established treatment modalities for patients who aren’t surgical candidates include cryotherapy, radiotherapy, and topical imiquimod 5%. All have disadvantages, including high 5-year recurrence rates.
Dr. Marmur noted that a newer nonsurgical therapy drawing considerable interest involves off-label use of topical combination therapy with imiquimod and tazarotene gel. The concept is to use the topical retinoid to disrupt the stratum corneum in order to enhance imiquimod penetration, thereby achieving a greater inflammatory response than possible with imiquimod alone.
Initial data have been published by researchers at the University of Utah, Salt Lake City. They randomized 90 patients with 91 lentigo malignas to imiquimod 5% cream applied 5 days per week for 3 months or to the imiquimod regimen plus tazarotene 0.1% gel on the other 2 days per week. After 3 months of topical therapy, patients underwent conservative staged excision with frozen section analysis with Melan A immunostaining to confirm negative margins.
Of those treated with dual topical therapy for 3 months, 29 of 37 lesions (78%) had complete responses with no residual lentigo maligna at the time of staged excision. So did 27 of 42 (64%) treated with imiquimod alone (Arch. Dermatol. 2012;148:592-6).
The modest difference in outcome was not significant (P = .17). Nevertheless, the Utah investigators wrote that topical pretreatment appears to reduce surgical defect sizes, an important consideration in lentigo maligna because the lesions are often large and located on cosmetically sensitive facial sites. At the patient’s first visit, the researchers saucerize the entire tumor to remove all visible evidence of lentigo maligna and nip in the bud any invasive element that might be present. One month later, after the wound has healed by secondary intention, the patient begins topical imiquimod therapy 5 days per week. If no inflammation is observed, tazarotene gel is added on the other 2 days per week. After 3 months of topical therapy, the patient goes off treatment for 2 months so the inflammatory response can subside. Then a staged excision is performed with 2-mm margins around the perimeter of the original tumor outline.
"This is an interesting thought, but it’s still not considered standard of care," Dr. Marmur commented in describing the Utah research.
Session chair Dr. Allan C. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center in New York, said he and his colleagues, like the Utah group, are using a lot of topical imiquimod as adjunctive therapy for lentigo maligna.
"Because we’re a referral center, we see all those patients whose melanomas unfortunately do recur at the edge of large surgeries. We see a lot of patients whose local surgeons have chased around their faces for a certain amount of time. So I think the general concept of the cure rates of surgery is a bit overstated. I’m not trying to sell an off-label use of a drug, but I think sometimes imiquimod can be very helpful," Dr. Halpern said.
He offered a clinical pearl in diagnosing lentigo maligna: Consider a broad shave biopsy of a suspicious lesion rather than a deeper, smaller-diameter biopsy.
"Sampling errors are a real problem with lentigo maligna. We learned early on that if you take a large lentigo maligna and do multiple biopsies of the same lesion and send them to what we think are our very expert pathologists, on average, we get two or three back that were melanoma and two or three back that were pigmented actinic keratoses. The sampling error tends to be more in the horizontal than in the depth for these very early lesions," according to Dr. Halpern.
Dr. Marmur and Dr. Halpern reported having no relevant financial interests. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
FDA advisory panel nixes approval of drug-device for liver metastases
SILVER SPRING, MD. – The risks outweigh any possible benefits of treatment with a drug-device combination that delivers melphalan directly to the livers of patients with liver metastases from ocular melanoma, a Food and Drug Administration advisory panel concluded in a 16-0 vote.
At a meeting of the FDA’s Oncologic Drugs Advisory Committee, several panelists said that the treatment was promising but should remain investigational, given its marked toxicity and lack of effect on overall survival.
The Melblez Kit is a combination of melphalan and the Delcath Hepatic Delivery System, which includes two catheters and an extracorporeal hemofiltration component. The catheter is used to administer high doses of the chemotherapy drug directly to the liver via the hepatic artery, and the hemofiltration component lowers the drug level before the blood is returned to the systemic circulation, according to the manufacturer, Delcath Systems. Patients are hospitalized for about 4 days for the procedure, which takes about 3 hours and is performed at 4-week intervals under general anesthesia.
There are no FDA-approved treatments for patients with unresectable metastatic ocular melanoma to the liver, which is the indication under FDA review.
Treatment with the kit was associated with antitumor activity, but it also was associated with fatal and life-threatening adverse reactions. There was a trend towards a detrimental effect on survival, and the risk evaluation and mitigation strategy (REMS) proposed by the company to address those risks "will not improve the observed benefit-risk profile," Dr. Geoffrey Kim, a medical officer in the FDA’s office of hematology and oncology products, told the panel.
In an open-label, randomized multicenter phase-III study conducted between 2006 and 2010 in the United States, the device was used to treat 44 patients. Their outcomes were compared with those of 49 matched patients given the best alternative care (BAC). All patients had surgically unresectable hepatic-dominant metastatic ocular or cutaneous melanoma (89% had ocular melanoma, and almost half were treated at the National Cancer Institute). Subjects were treated until their hepatic disease progressed. The dose administered with the Melblez Kit was 3.0 mg/kg for a median of three treatment cycles and a median of 120 days; best alternative care included systemic chemotherapy in 49% of patients and intrahepatic chemotherapy in 22%.
The primary end point, median hepatic progression-free survival (hPFS) was 7 months among those on the device, compared with 1.6 months among those on BAC, a statistically significant difference that represented a 61% reduction in risk (hazard ratio, 0.39), according to the company. The median overall PFS was 4.8 months among those treated with the device, compared with 1.6 months among those on BAC, also a statistically significant difference.
Overall survival was comparable: 9.8 months in the device-treated group and 9.9 months in those on best alternative care. Further, almost 80% of patients in the Melblez Kit arm had a serious adverse event and almost 70% had a grade-4 adverse event. With best alternative care, the rate of serious adverse events was 16% and the rate of grade-4 events was 2%. No patients on best alternative care died because of an adverse event. Three patients treated with the drug-device died from adverse events.
The adverse reactions in a combined population of 121 patients in the phase-III and phase-II studies and in 28 patients in the BAC arm who crossed over to treatment with the device included toxic deaths in 7% (including cases of hepatic failure, streptococcal sepsis, and GI hemorrhage), cerebral infarction in 4%, MI in 2%, and grade-4 bone marrow suppression with a median time to recovery of more than 1 week in more than 70%. About half had to be rehospitalized for an adverse event.
For a cancer treatment, this safety profile "is unprecedented, in terms of the toxicity," Dr. Richard Pazdur, director of the FDA’s office of hematology and oncology products, remarked.
After the vote, panel member Dr. Louis Diehl, professor of medicine at Duke University, Durham, N.C., said that progression-free survival is valued in studies as an indicator of improved quality of life and it can be an early marker of increased survival. "Unfortunately, this treatment has an increase in morbidity and an increase in mortality and I can’t see from the survival curve that it will ever translate into an improvement in survival."
Delcath did not issue a response after the panel’s vote.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting, although a panelist may occasionally be given a waiver. At this meeting, two panelists who had expertise in the topic were given waivers (one panelist is the principal investigator in a study of a competing device and the other works at a medical center where a study of a competing device is being conducted).
About 2,000 cases of ocular melanoma are diagnosed annually in the United States and about 50% metastasize, most often to the liver, according to Delcath. In Europe and Australia, the company markets the device for a broad range of liver metastases, not just those caused by ocular melanoma, according to the company.
SILVER SPRING, MD. – The risks outweigh any possible benefits of treatment with a drug-device combination that delivers melphalan directly to the livers of patients with liver metastases from ocular melanoma, a Food and Drug Administration advisory panel concluded in a 16-0 vote.
At a meeting of the FDA’s Oncologic Drugs Advisory Committee, several panelists said that the treatment was promising but should remain investigational, given its marked toxicity and lack of effect on overall survival.
The Melblez Kit is a combination of melphalan and the Delcath Hepatic Delivery System, which includes two catheters and an extracorporeal hemofiltration component. The catheter is used to administer high doses of the chemotherapy drug directly to the liver via the hepatic artery, and the hemofiltration component lowers the drug level before the blood is returned to the systemic circulation, according to the manufacturer, Delcath Systems. Patients are hospitalized for about 4 days for the procedure, which takes about 3 hours and is performed at 4-week intervals under general anesthesia.
There are no FDA-approved treatments for patients with unresectable metastatic ocular melanoma to the liver, which is the indication under FDA review.
Treatment with the kit was associated with antitumor activity, but it also was associated with fatal and life-threatening adverse reactions. There was a trend towards a detrimental effect on survival, and the risk evaluation and mitigation strategy (REMS) proposed by the company to address those risks "will not improve the observed benefit-risk profile," Dr. Geoffrey Kim, a medical officer in the FDA’s office of hematology and oncology products, told the panel.
In an open-label, randomized multicenter phase-III study conducted between 2006 and 2010 in the United States, the device was used to treat 44 patients. Their outcomes were compared with those of 49 matched patients given the best alternative care (BAC). All patients had surgically unresectable hepatic-dominant metastatic ocular or cutaneous melanoma (89% had ocular melanoma, and almost half were treated at the National Cancer Institute). Subjects were treated until their hepatic disease progressed. The dose administered with the Melblez Kit was 3.0 mg/kg for a median of three treatment cycles and a median of 120 days; best alternative care included systemic chemotherapy in 49% of patients and intrahepatic chemotherapy in 22%.
The primary end point, median hepatic progression-free survival (hPFS) was 7 months among those on the device, compared with 1.6 months among those on BAC, a statistically significant difference that represented a 61% reduction in risk (hazard ratio, 0.39), according to the company. The median overall PFS was 4.8 months among those treated with the device, compared with 1.6 months among those on BAC, also a statistically significant difference.
Overall survival was comparable: 9.8 months in the device-treated group and 9.9 months in those on best alternative care. Further, almost 80% of patients in the Melblez Kit arm had a serious adverse event and almost 70% had a grade-4 adverse event. With best alternative care, the rate of serious adverse events was 16% and the rate of grade-4 events was 2%. No patients on best alternative care died because of an adverse event. Three patients treated with the drug-device died from adverse events.
The adverse reactions in a combined population of 121 patients in the phase-III and phase-II studies and in 28 patients in the BAC arm who crossed over to treatment with the device included toxic deaths in 7% (including cases of hepatic failure, streptococcal sepsis, and GI hemorrhage), cerebral infarction in 4%, MI in 2%, and grade-4 bone marrow suppression with a median time to recovery of more than 1 week in more than 70%. About half had to be rehospitalized for an adverse event.
For a cancer treatment, this safety profile "is unprecedented, in terms of the toxicity," Dr. Richard Pazdur, director of the FDA’s office of hematology and oncology products, remarked.
After the vote, panel member Dr. Louis Diehl, professor of medicine at Duke University, Durham, N.C., said that progression-free survival is valued in studies as an indicator of improved quality of life and it can be an early marker of increased survival. "Unfortunately, this treatment has an increase in morbidity and an increase in mortality and I can’t see from the survival curve that it will ever translate into an improvement in survival."
Delcath did not issue a response after the panel’s vote.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting, although a panelist may occasionally be given a waiver. At this meeting, two panelists who had expertise in the topic were given waivers (one panelist is the principal investigator in a study of a competing device and the other works at a medical center where a study of a competing device is being conducted).
About 2,000 cases of ocular melanoma are diagnosed annually in the United States and about 50% metastasize, most often to the liver, according to Delcath. In Europe and Australia, the company markets the device for a broad range of liver metastases, not just those caused by ocular melanoma, according to the company.
SILVER SPRING, MD. – The risks outweigh any possible benefits of treatment with a drug-device combination that delivers melphalan directly to the livers of patients with liver metastases from ocular melanoma, a Food and Drug Administration advisory panel concluded in a 16-0 vote.
At a meeting of the FDA’s Oncologic Drugs Advisory Committee, several panelists said that the treatment was promising but should remain investigational, given its marked toxicity and lack of effect on overall survival.
The Melblez Kit is a combination of melphalan and the Delcath Hepatic Delivery System, which includes two catheters and an extracorporeal hemofiltration component. The catheter is used to administer high doses of the chemotherapy drug directly to the liver via the hepatic artery, and the hemofiltration component lowers the drug level before the blood is returned to the systemic circulation, according to the manufacturer, Delcath Systems. Patients are hospitalized for about 4 days for the procedure, which takes about 3 hours and is performed at 4-week intervals under general anesthesia.
There are no FDA-approved treatments for patients with unresectable metastatic ocular melanoma to the liver, which is the indication under FDA review.
Treatment with the kit was associated with antitumor activity, but it also was associated with fatal and life-threatening adverse reactions. There was a trend towards a detrimental effect on survival, and the risk evaluation and mitigation strategy (REMS) proposed by the company to address those risks "will not improve the observed benefit-risk profile," Dr. Geoffrey Kim, a medical officer in the FDA’s office of hematology and oncology products, told the panel.
In an open-label, randomized multicenter phase-III study conducted between 2006 and 2010 in the United States, the device was used to treat 44 patients. Their outcomes were compared with those of 49 matched patients given the best alternative care (BAC). All patients had surgically unresectable hepatic-dominant metastatic ocular or cutaneous melanoma (89% had ocular melanoma, and almost half were treated at the National Cancer Institute). Subjects were treated until their hepatic disease progressed. The dose administered with the Melblez Kit was 3.0 mg/kg for a median of three treatment cycles and a median of 120 days; best alternative care included systemic chemotherapy in 49% of patients and intrahepatic chemotherapy in 22%.
The primary end point, median hepatic progression-free survival (hPFS) was 7 months among those on the device, compared with 1.6 months among those on BAC, a statistically significant difference that represented a 61% reduction in risk (hazard ratio, 0.39), according to the company. The median overall PFS was 4.8 months among those treated with the device, compared with 1.6 months among those on BAC, also a statistically significant difference.
Overall survival was comparable: 9.8 months in the device-treated group and 9.9 months in those on best alternative care. Further, almost 80% of patients in the Melblez Kit arm had a serious adverse event and almost 70% had a grade-4 adverse event. With best alternative care, the rate of serious adverse events was 16% and the rate of grade-4 events was 2%. No patients on best alternative care died because of an adverse event. Three patients treated with the drug-device died from adverse events.
The adverse reactions in a combined population of 121 patients in the phase-III and phase-II studies and in 28 patients in the BAC arm who crossed over to treatment with the device included toxic deaths in 7% (including cases of hepatic failure, streptococcal sepsis, and GI hemorrhage), cerebral infarction in 4%, MI in 2%, and grade-4 bone marrow suppression with a median time to recovery of more than 1 week in more than 70%. About half had to be rehospitalized for an adverse event.
For a cancer treatment, this safety profile "is unprecedented, in terms of the toxicity," Dr. Richard Pazdur, director of the FDA’s office of hematology and oncology products, remarked.
After the vote, panel member Dr. Louis Diehl, professor of medicine at Duke University, Durham, N.C., said that progression-free survival is valued in studies as an indicator of improved quality of life and it can be an early marker of increased survival. "Unfortunately, this treatment has an increase in morbidity and an increase in mortality and I can’t see from the survival curve that it will ever translate into an improvement in survival."
Delcath did not issue a response after the panel’s vote.
The FDA usually follows the recommendations of its advisory panels. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting, although a panelist may occasionally be given a waiver. At this meeting, two panelists who had expertise in the topic were given waivers (one panelist is the principal investigator in a study of a competing device and the other works at a medical center where a study of a competing device is being conducted).
About 2,000 cases of ocular melanoma are diagnosed annually in the United States and about 50% metastasize, most often to the liver, according to Delcath. In Europe and Australia, the company markets the device for a broad range of liver metastases, not just those caused by ocular melanoma, according to the company.
AT AN FDA ADVISORY PANEL MEETING
FDA: Tanning lamps should warn against skin cancer
Indoor tanning beds should carry warnings against their use in people under age 18 years and should advise users to be screened regularly for skin cancer, according to a proposal announced by the Food and Drug Administration on May 6.
The agency seeks to reclassify the ultraviolet lamps used in tanning beds, upgrading them to class II (moderate risk) from class I (low risk) and to rename them "sunlamps." As class I devices, these lamps are currently deemed to be at the same risk level as adhesive bandages and tongue depressors.
Under the proposal, manufacturers would be required to display "a prominent visible label on the tanning bed itself," warning against use in people under age 18 years, Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said during a briefing held to announce the proposal. Manufacturers also would have to add labels contraindicating the use of sunlamps in people with certain skin lesions.
Information advising regular skin cancer screenings would be added to materials such as brochures, catalogues, and consumer websites, he added.
"We believe that our proposal will allow for safer, more reliable sunlamps and better arm consumers with the critical information they need," Dr. Shuren said.
In 2010, an FDA advisory panel unanimously recommended that these devices be switched to at least class II.
The proposed reclassification does not prohibit the use of sunlamp products in minors.
Manufacturers are currently not required to submit applications to market these devices, but if they are reclassified as class II devices, a "premarket notification" application will be required and "manufacturers would have to show that their products have met certain performance testing requirements, address certain product design characteristics and provide comprehensive labeling that presents consumers with clear information on the risks of use," according to the FDA statement announcing the proposal.
Clinical trials would not be required, but manufacturers would be required test the performance of timers and alarms and ensure that sunlamps provide the correct amount of energy to prevent burns. Reports of burns associated with these products indicate that this testing is not being done properly now, Dr. Shuren said.
According to the American Academy of Dermatology, the risk of melanoma increases by 75% among people exposed to ultraviolet radiation from indoor tanning products, and the risk increases with increased use.
During the briefing, Dr. Mary Maloney, chair of the academy’s regulatory policy committee, said that an estimated 2.3 million teens use indoor tanning facilities every year, and that melanoma is the most common form of cancer in adults aged 25-29 years and the second most common form of invasive cancer among people aged 15-29 years. In a 2011 youth risk behavior survey, 13% of all high school students said that they had used indoor tanning, and by 12th grade, 32% of girls had reported using a tanning bed, according to the Centers for Disease Control and Prevention.
Dr. Maloney also referred to evidence that young people are given misinformation about the risks of indoor tanning, citing a study by Washington University in St. Louis, which found that 43% of indoor tanning facilities in Missouri denied there were any risks associated with indoor tanning and that two-thirds allowed minors aged 10-12 years to use tanning devices, sometimes without parental consent.
The FDA will accept comments on the proposed order at www.regulations.gov for 90 days from publication in the Federal Register.
Indoor tanning beds should carry warnings against their use in people under age 18 years and should advise users to be screened regularly for skin cancer, according to a proposal announced by the Food and Drug Administration on May 6.
The agency seeks to reclassify the ultraviolet lamps used in tanning beds, upgrading them to class II (moderate risk) from class I (low risk) and to rename them "sunlamps." As class I devices, these lamps are currently deemed to be at the same risk level as adhesive bandages and tongue depressors.
Under the proposal, manufacturers would be required to display "a prominent visible label on the tanning bed itself," warning against use in people under age 18 years, Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said during a briefing held to announce the proposal. Manufacturers also would have to add labels contraindicating the use of sunlamps in people with certain skin lesions.
Information advising regular skin cancer screenings would be added to materials such as brochures, catalogues, and consumer websites, he added.
"We believe that our proposal will allow for safer, more reliable sunlamps and better arm consumers with the critical information they need," Dr. Shuren said.
In 2010, an FDA advisory panel unanimously recommended that these devices be switched to at least class II.
The proposed reclassification does not prohibit the use of sunlamp products in minors.
Manufacturers are currently not required to submit applications to market these devices, but if they are reclassified as class II devices, a "premarket notification" application will be required and "manufacturers would have to show that their products have met certain performance testing requirements, address certain product design characteristics and provide comprehensive labeling that presents consumers with clear information on the risks of use," according to the FDA statement announcing the proposal.
Clinical trials would not be required, but manufacturers would be required test the performance of timers and alarms and ensure that sunlamps provide the correct amount of energy to prevent burns. Reports of burns associated with these products indicate that this testing is not being done properly now, Dr. Shuren said.
According to the American Academy of Dermatology, the risk of melanoma increases by 75% among people exposed to ultraviolet radiation from indoor tanning products, and the risk increases with increased use.
During the briefing, Dr. Mary Maloney, chair of the academy’s regulatory policy committee, said that an estimated 2.3 million teens use indoor tanning facilities every year, and that melanoma is the most common form of cancer in adults aged 25-29 years and the second most common form of invasive cancer among people aged 15-29 years. In a 2011 youth risk behavior survey, 13% of all high school students said that they had used indoor tanning, and by 12th grade, 32% of girls had reported using a tanning bed, according to the Centers for Disease Control and Prevention.
Dr. Maloney also referred to evidence that young people are given misinformation about the risks of indoor tanning, citing a study by Washington University in St. Louis, which found that 43% of indoor tanning facilities in Missouri denied there were any risks associated with indoor tanning and that two-thirds allowed minors aged 10-12 years to use tanning devices, sometimes without parental consent.
The FDA will accept comments on the proposed order at www.regulations.gov for 90 days from publication in the Federal Register.
Indoor tanning beds should carry warnings against their use in people under age 18 years and should advise users to be screened regularly for skin cancer, according to a proposal announced by the Food and Drug Administration on May 6.
The agency seeks to reclassify the ultraviolet lamps used in tanning beds, upgrading them to class II (moderate risk) from class I (low risk) and to rename them "sunlamps." As class I devices, these lamps are currently deemed to be at the same risk level as adhesive bandages and tongue depressors.
Under the proposal, manufacturers would be required to display "a prominent visible label on the tanning bed itself," warning against use in people under age 18 years, Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said during a briefing held to announce the proposal. Manufacturers also would have to add labels contraindicating the use of sunlamps in people with certain skin lesions.
Information advising regular skin cancer screenings would be added to materials such as brochures, catalogues, and consumer websites, he added.
"We believe that our proposal will allow for safer, more reliable sunlamps and better arm consumers with the critical information they need," Dr. Shuren said.
In 2010, an FDA advisory panel unanimously recommended that these devices be switched to at least class II.
The proposed reclassification does not prohibit the use of sunlamp products in minors.
Manufacturers are currently not required to submit applications to market these devices, but if they are reclassified as class II devices, a "premarket notification" application will be required and "manufacturers would have to show that their products have met certain performance testing requirements, address certain product design characteristics and provide comprehensive labeling that presents consumers with clear information on the risks of use," according to the FDA statement announcing the proposal.
Clinical trials would not be required, but manufacturers would be required test the performance of timers and alarms and ensure that sunlamps provide the correct amount of energy to prevent burns. Reports of burns associated with these products indicate that this testing is not being done properly now, Dr. Shuren said.
According to the American Academy of Dermatology, the risk of melanoma increases by 75% among people exposed to ultraviolet radiation from indoor tanning products, and the risk increases with increased use.
During the briefing, Dr. Mary Maloney, chair of the academy’s regulatory policy committee, said that an estimated 2.3 million teens use indoor tanning facilities every year, and that melanoma is the most common form of cancer in adults aged 25-29 years and the second most common form of invasive cancer among people aged 15-29 years. In a 2011 youth risk behavior survey, 13% of all high school students said that they had used indoor tanning, and by 12th grade, 32% of girls had reported using a tanning bed, according to the Centers for Disease Control and Prevention.
Dr. Maloney also referred to evidence that young people are given misinformation about the risks of indoor tanning, citing a study by Washington University in St. Louis, which found that 43% of indoor tanning facilities in Missouri denied there were any risks associated with indoor tanning and that two-thirds allowed minors aged 10-12 years to use tanning devices, sometimes without parental consent.
The FDA will accept comments on the proposed order at www.regulations.gov for 90 days from publication in the Federal Register.
Therapeutic combos make inroads in advanced melanoma
WAILEA, HAWAII – "The past 2 years have been a really exciting time for those of us who have spent the last several decades" in the field of melanoma, said Dr. Allan C. Halpern, chief of the dermatology service at Memorial Sloan Kettering Cancer Center, New York.
"We are in a whole new place with a very promising future for turning stage IV melanoma into maybe a chronic disease for many patients, instead of a death sentence. For some patients, we’re already seeing what may be cures," he said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
The greatest enthusiasm in the field now involves combining a pathway-targeted agent, such as vemurafenib, with an immunologic checkpoint blocker, such as ipilimumab. The vemurafenib knocks down 60%-70% of metastatic melanomas temporarily and the ipilimumab promotes durable responses.
But there’s a formidable economic obstacle to this approach: The strongest drug combinations often put big pharmaceutical companies in the uncomfortable position of having to cooperate with their competitors in expensive research projects. "A lot of the drug companies, to their credit, are finding ways to make it work," Dr. Halpern said.
Dr. Halpern detailed the therapeutic history that has revolutionized the treatment of metastatic melanoma.
Prior to 2011 there were only two Food and Drug Administration–approved therapies for metastatic melanoma, dacarbazine and high-dose interleukin II. Both were unimpressive. The therapeutic dry spell has ended, he said. "There are for the first time in melanoma, instead of no hopeful drugs, a slew of hopeful drugs."
Targeted therapeutic approaches, the result of laboratory insights into the molecular pathways to melanoma and the key genetic mutations involved, led to the development of vemurafenib, a selective, first-in-class BRAF inhibitor approved in 2011.
"Vemurafenib is an astounding drug. When you give it to a BRAF-mutated cell, it essentially turns off the cell’s metabolic activity." When given to patients whose tumors test positive for the BRAF mutation, "it’s dramatically effective in 60%-70%." But the response does not persist. "After about 6-18 months, the tumor develops resistance to the drug. It’s like somebody hit a switch to turn the tumor back on. The tumor comes roaring back, in the same places for the most part," Dr. Halpern said.
As a result of this limited success, ongoing clinical trials are aimed at determining whether dual pathway blockade using combination therapy will provide more durable responses. Trials are underway with the oral BRAF inhibitor dabrafenib plus the oral MEK 1/2 pathway inhibitor trametinib. Other dual pathway combinations are also under study in melanoma.
The prospects are even more promising, according to Dr. Halpern, for immunologic checkpoint blockade, which is based upon the concept that some cancers progress because the immune system turns off prematurely and stops battling the malignancy. Ipilimumab is one such agent. An anti-CTLA-4 antibody, ipilimumab enhances T-cell activation and proliferation and has earned FDA approval as single-agent therapy in advanced melanoma.
Tumors often don’t begin to shrink until after 3-4 months, but the response is impressively durable in the roughly 30% of patients who respond to immunologic checkpoint blockade.
"These people look like they might be cured," said Dr. Halpern.
Another important immunologic checkpoint molecule is PD-1. The anti-PD-1 agent known as MDX-1106 appears to be nearly as effective as ipilimumab, but with less toxicity. The early impression from ongoing clinical trials is that dual immunologic checkpoint blockade using anti-CTLA-4 therapy along with an anti-PD-1 drug provides synergistic anti-tumor activity.
Dr. Halpern reported serving as a consultant to Canfield Scientific, DermTech, SciBase, Quintiles, and Lucid.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – "The past 2 years have been a really exciting time for those of us who have spent the last several decades" in the field of melanoma, said Dr. Allan C. Halpern, chief of the dermatology service at Memorial Sloan Kettering Cancer Center, New York.
"We are in a whole new place with a very promising future for turning stage IV melanoma into maybe a chronic disease for many patients, instead of a death sentence. For some patients, we’re already seeing what may be cures," he said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
The greatest enthusiasm in the field now involves combining a pathway-targeted agent, such as vemurafenib, with an immunologic checkpoint blocker, such as ipilimumab. The vemurafenib knocks down 60%-70% of metastatic melanomas temporarily and the ipilimumab promotes durable responses.
But there’s a formidable economic obstacle to this approach: The strongest drug combinations often put big pharmaceutical companies in the uncomfortable position of having to cooperate with their competitors in expensive research projects. "A lot of the drug companies, to their credit, are finding ways to make it work," Dr. Halpern said.
Dr. Halpern detailed the therapeutic history that has revolutionized the treatment of metastatic melanoma.
Prior to 2011 there were only two Food and Drug Administration–approved therapies for metastatic melanoma, dacarbazine and high-dose interleukin II. Both were unimpressive. The therapeutic dry spell has ended, he said. "There are for the first time in melanoma, instead of no hopeful drugs, a slew of hopeful drugs."
Targeted therapeutic approaches, the result of laboratory insights into the molecular pathways to melanoma and the key genetic mutations involved, led to the development of vemurafenib, a selective, first-in-class BRAF inhibitor approved in 2011.
"Vemurafenib is an astounding drug. When you give it to a BRAF-mutated cell, it essentially turns off the cell’s metabolic activity." When given to patients whose tumors test positive for the BRAF mutation, "it’s dramatically effective in 60%-70%." But the response does not persist. "After about 6-18 months, the tumor develops resistance to the drug. It’s like somebody hit a switch to turn the tumor back on. The tumor comes roaring back, in the same places for the most part," Dr. Halpern said.
As a result of this limited success, ongoing clinical trials are aimed at determining whether dual pathway blockade using combination therapy will provide more durable responses. Trials are underway with the oral BRAF inhibitor dabrafenib plus the oral MEK 1/2 pathway inhibitor trametinib. Other dual pathway combinations are also under study in melanoma.
The prospects are even more promising, according to Dr. Halpern, for immunologic checkpoint blockade, which is based upon the concept that some cancers progress because the immune system turns off prematurely and stops battling the malignancy. Ipilimumab is one such agent. An anti-CTLA-4 antibody, ipilimumab enhances T-cell activation and proliferation and has earned FDA approval as single-agent therapy in advanced melanoma.
Tumors often don’t begin to shrink until after 3-4 months, but the response is impressively durable in the roughly 30% of patients who respond to immunologic checkpoint blockade.
"These people look like they might be cured," said Dr. Halpern.
Another important immunologic checkpoint molecule is PD-1. The anti-PD-1 agent known as MDX-1106 appears to be nearly as effective as ipilimumab, but with less toxicity. The early impression from ongoing clinical trials is that dual immunologic checkpoint blockade using anti-CTLA-4 therapy along with an anti-PD-1 drug provides synergistic anti-tumor activity.
Dr. Halpern reported serving as a consultant to Canfield Scientific, DermTech, SciBase, Quintiles, and Lucid.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – "The past 2 years have been a really exciting time for those of us who have spent the last several decades" in the field of melanoma, said Dr. Allan C. Halpern, chief of the dermatology service at Memorial Sloan Kettering Cancer Center, New York.
"We are in a whole new place with a very promising future for turning stage IV melanoma into maybe a chronic disease for many patients, instead of a death sentence. For some patients, we’re already seeing what may be cures," he said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
The greatest enthusiasm in the field now involves combining a pathway-targeted agent, such as vemurafenib, with an immunologic checkpoint blocker, such as ipilimumab. The vemurafenib knocks down 60%-70% of metastatic melanomas temporarily and the ipilimumab promotes durable responses.
But there’s a formidable economic obstacle to this approach: The strongest drug combinations often put big pharmaceutical companies in the uncomfortable position of having to cooperate with their competitors in expensive research projects. "A lot of the drug companies, to their credit, are finding ways to make it work," Dr. Halpern said.
Dr. Halpern detailed the therapeutic history that has revolutionized the treatment of metastatic melanoma.
Prior to 2011 there were only two Food and Drug Administration–approved therapies for metastatic melanoma, dacarbazine and high-dose interleukin II. Both were unimpressive. The therapeutic dry spell has ended, he said. "There are for the first time in melanoma, instead of no hopeful drugs, a slew of hopeful drugs."
Targeted therapeutic approaches, the result of laboratory insights into the molecular pathways to melanoma and the key genetic mutations involved, led to the development of vemurafenib, a selective, first-in-class BRAF inhibitor approved in 2011.
"Vemurafenib is an astounding drug. When you give it to a BRAF-mutated cell, it essentially turns off the cell’s metabolic activity." When given to patients whose tumors test positive for the BRAF mutation, "it’s dramatically effective in 60%-70%." But the response does not persist. "After about 6-18 months, the tumor develops resistance to the drug. It’s like somebody hit a switch to turn the tumor back on. The tumor comes roaring back, in the same places for the most part," Dr. Halpern said.
As a result of this limited success, ongoing clinical trials are aimed at determining whether dual pathway blockade using combination therapy will provide more durable responses. Trials are underway with the oral BRAF inhibitor dabrafenib plus the oral MEK 1/2 pathway inhibitor trametinib. Other dual pathway combinations are also under study in melanoma.
The prospects are even more promising, according to Dr. Halpern, for immunologic checkpoint blockade, which is based upon the concept that some cancers progress because the immune system turns off prematurely and stops battling the malignancy. Ipilimumab is one such agent. An anti-CTLA-4 antibody, ipilimumab enhances T-cell activation and proliferation and has earned FDA approval as single-agent therapy in advanced melanoma.
Tumors often don’t begin to shrink until after 3-4 months, but the response is impressively durable in the roughly 30% of patients who respond to immunologic checkpoint blockade.
"These people look like they might be cured," said Dr. Halpern.
Another important immunologic checkpoint molecule is PD-1. The anti-PD-1 agent known as MDX-1106 appears to be nearly as effective as ipilimumab, but with less toxicity. The early impression from ongoing clinical trials is that dual immunologic checkpoint blockade using anti-CTLA-4 therapy along with an anti-PD-1 drug provides synergistic anti-tumor activity.
Dr. Halpern reported serving as a consultant to Canfield Scientific, DermTech, SciBase, Quintiles, and Lucid.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dermoscopy characterized as patient trust builder
MAUI, HAWAII – Dermatologists in the know view dermoscopy as a powerful tool to increase diagnostic accuracy in evaluating pigmented lesions; less well appreciated is dermoscopy’s value in building patient trust in the physician, according to Dr. Steven Q. Wang.
"With the dermoscope, people feel like you’re providing a much more detailed exam," observed Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan-Kettering Cancer Center’s Basking Ridge, N.J., campus.
"We are a tertiary referral center. We have lots of patients come in who are high risk, with a personal or family history of melanoma and numerous nevi. I always ask why they have transferred their care. The common answer I hear is they feel their dermatologist was not giving them a detailed examination," he said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wang said he makes a point of giving every patient a full-body clinical examination, including looking between the toes. And he views every single lesion with his dermoscope, up close and personal, scope to skin.
"The dermoscope makes it easier to spot the outlier lesions, the ugly ducklings. And we’re all so busy in the office, running from room to room – dermoscopy helps me slow down my mind and really look," he explained.
It doesn’t take all that long, either. In a classic multicenter study, 1,328 patients with one or more melanocytic or nonmelanocytic skin lesions were randomized to receive a complete skin examination with or without dermoscopy. The median time for a complete skin examination alone was 70 seconds; with dermoscopy it rose to 142 seconds (Arch. Dermatol. 2008;144:509-13).
"You double the time required, but it’s still only a little over 2 minutes. Yet you’ve changed the patient’s perception," Dr. Wang said.
A digital dermoscope is basically a handheld microscope that permits detailed visualization of structures in the deep epidermis and superficial dermis not visible to the naked eye. There’s a learning curve involved. Dermatologists who pick up a dermoscope and try to use it without formal training have worse diagnostic accuracy than with clinical examination, while experienced dermoscopists have significantly greater diagnostic accuracy than can be achieved with clinical exam alone (Lancet Oncol. 2002;3:159-65).
Dr. Wang said that because patients have more trust in their dermatologist when they feel they are receiving a thorough skin examination including dermoscopy, they are more likely to be adherent to scheduled follow-up evaluations. And that, in turn, spells improved long-term outcomes in patients at elevated risk for melanoma.
This point was already brought home forcefully for him nearly a decade ago, he said, when he and his coinvestigators reported their experience with long-term follow-up of 258 patients at high risk for melanoma. The monitoring strategy consisted of annual total body photography, total skin examination, and dermoscopy. The cumulative 10-year incidence of melanoma was 14% in the 160 patients with classic atypical mole syndrome and 10% in the other 98 high-risk patients. Impressively, all of the melanomas were either in situ or less than 1 mm thick. There were no metastases and no melanoma-related deaths (J. Am. Acad. Dermatol. 2004;50:15-20).
Although dermoscopy is used primarily in examining pigmented skin lesions, it has other applications. For example, in performing Mohs surgery for basal cell carcinomas, Dr. Wang has found dermoscopy to be of assistance in a couple of ways: In patients with ill-defined tumor borders on clinical examination, dermoscopy can define the tumor borders presurgically, thereby reducing the number of Mohs surgical stages required; and when a patient returns for Mohs surgery 6 weeks after skin biopsy and the biopsy site has healed so completely it can’t be found with the naked eye, the dermoscope can identify the site by visualizing subtle scars and telangiectasias.
In general dermatology, Dr. Wang said he turns to dermoscopy as an aid in diagnosing connective tissue diseases, including dermatomyositis, scleroderma, and lupus. He applies ultrasound gel to the proximal nail fold and examines the site using the dermoscope. A finding of dilated blood vessels stands out as a helpful diagnostic clue.
In addition, Dr. Wang said he has utilized the dermoscope in diagnosing scabies by spotting the mites and their trails, in detecting the telltale Wickham striae of lichen planus, and in diagnosing other dermatologic disorders.
Dr. Wang reported having no relevant financial conflicts.
SDEF and this news organization are owned by the same parent company.
MAUI, HAWAII – Dermatologists in the know view dermoscopy as a powerful tool to increase diagnostic accuracy in evaluating pigmented lesions; less well appreciated is dermoscopy’s value in building patient trust in the physician, according to Dr. Steven Q. Wang.
"With the dermoscope, people feel like you’re providing a much more detailed exam," observed Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan-Kettering Cancer Center’s Basking Ridge, N.J., campus.
"We are a tertiary referral center. We have lots of patients come in who are high risk, with a personal or family history of melanoma and numerous nevi. I always ask why they have transferred their care. The common answer I hear is they feel their dermatologist was not giving them a detailed examination," he said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wang said he makes a point of giving every patient a full-body clinical examination, including looking between the toes. And he views every single lesion with his dermoscope, up close and personal, scope to skin.
"The dermoscope makes it easier to spot the outlier lesions, the ugly ducklings. And we’re all so busy in the office, running from room to room – dermoscopy helps me slow down my mind and really look," he explained.
It doesn’t take all that long, either. In a classic multicenter study, 1,328 patients with one or more melanocytic or nonmelanocytic skin lesions were randomized to receive a complete skin examination with or without dermoscopy. The median time for a complete skin examination alone was 70 seconds; with dermoscopy it rose to 142 seconds (Arch. Dermatol. 2008;144:509-13).
"You double the time required, but it’s still only a little over 2 minutes. Yet you’ve changed the patient’s perception," Dr. Wang said.
A digital dermoscope is basically a handheld microscope that permits detailed visualization of structures in the deep epidermis and superficial dermis not visible to the naked eye. There’s a learning curve involved. Dermatologists who pick up a dermoscope and try to use it without formal training have worse diagnostic accuracy than with clinical examination, while experienced dermoscopists have significantly greater diagnostic accuracy than can be achieved with clinical exam alone (Lancet Oncol. 2002;3:159-65).
Dr. Wang said that because patients have more trust in their dermatologist when they feel they are receiving a thorough skin examination including dermoscopy, they are more likely to be adherent to scheduled follow-up evaluations. And that, in turn, spells improved long-term outcomes in patients at elevated risk for melanoma.
This point was already brought home forcefully for him nearly a decade ago, he said, when he and his coinvestigators reported their experience with long-term follow-up of 258 patients at high risk for melanoma. The monitoring strategy consisted of annual total body photography, total skin examination, and dermoscopy. The cumulative 10-year incidence of melanoma was 14% in the 160 patients with classic atypical mole syndrome and 10% in the other 98 high-risk patients. Impressively, all of the melanomas were either in situ or less than 1 mm thick. There were no metastases and no melanoma-related deaths (J. Am. Acad. Dermatol. 2004;50:15-20).
Although dermoscopy is used primarily in examining pigmented skin lesions, it has other applications. For example, in performing Mohs surgery for basal cell carcinomas, Dr. Wang has found dermoscopy to be of assistance in a couple of ways: In patients with ill-defined tumor borders on clinical examination, dermoscopy can define the tumor borders presurgically, thereby reducing the number of Mohs surgical stages required; and when a patient returns for Mohs surgery 6 weeks after skin biopsy and the biopsy site has healed so completely it can’t be found with the naked eye, the dermoscope can identify the site by visualizing subtle scars and telangiectasias.
In general dermatology, Dr. Wang said he turns to dermoscopy as an aid in diagnosing connective tissue diseases, including dermatomyositis, scleroderma, and lupus. He applies ultrasound gel to the proximal nail fold and examines the site using the dermoscope. A finding of dilated blood vessels stands out as a helpful diagnostic clue.
In addition, Dr. Wang said he has utilized the dermoscope in diagnosing scabies by spotting the mites and their trails, in detecting the telltale Wickham striae of lichen planus, and in diagnosing other dermatologic disorders.
Dr. Wang reported having no relevant financial conflicts.
SDEF and this news organization are owned by the same parent company.
MAUI, HAWAII – Dermatologists in the know view dermoscopy as a powerful tool to increase diagnostic accuracy in evaluating pigmented lesions; less well appreciated is dermoscopy’s value in building patient trust in the physician, according to Dr. Steven Q. Wang.
"With the dermoscope, people feel like you’re providing a much more detailed exam," observed Dr. Wang, director of dermatologic surgery and dermatology at Memorial Sloan-Kettering Cancer Center’s Basking Ridge, N.J., campus.
"We are a tertiary referral center. We have lots of patients come in who are high risk, with a personal or family history of melanoma and numerous nevi. I always ask why they have transferred their care. The common answer I hear is they feel their dermatologist was not giving them a detailed examination," he said at the Hawaii Dermatology Seminar sponsored by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wang said he makes a point of giving every patient a full-body clinical examination, including looking between the toes. And he views every single lesion with his dermoscope, up close and personal, scope to skin.
"The dermoscope makes it easier to spot the outlier lesions, the ugly ducklings. And we’re all so busy in the office, running from room to room – dermoscopy helps me slow down my mind and really look," he explained.
It doesn’t take all that long, either. In a classic multicenter study, 1,328 patients with one or more melanocytic or nonmelanocytic skin lesions were randomized to receive a complete skin examination with or without dermoscopy. The median time for a complete skin examination alone was 70 seconds; with dermoscopy it rose to 142 seconds (Arch. Dermatol. 2008;144:509-13).
"You double the time required, but it’s still only a little over 2 minutes. Yet you’ve changed the patient’s perception," Dr. Wang said.
A digital dermoscope is basically a handheld microscope that permits detailed visualization of structures in the deep epidermis and superficial dermis not visible to the naked eye. There’s a learning curve involved. Dermatologists who pick up a dermoscope and try to use it without formal training have worse diagnostic accuracy than with clinical examination, while experienced dermoscopists have significantly greater diagnostic accuracy than can be achieved with clinical exam alone (Lancet Oncol. 2002;3:159-65).
Dr. Wang said that because patients have more trust in their dermatologist when they feel they are receiving a thorough skin examination including dermoscopy, they are more likely to be adherent to scheduled follow-up evaluations. And that, in turn, spells improved long-term outcomes in patients at elevated risk for melanoma.
This point was already brought home forcefully for him nearly a decade ago, he said, when he and his coinvestigators reported their experience with long-term follow-up of 258 patients at high risk for melanoma. The monitoring strategy consisted of annual total body photography, total skin examination, and dermoscopy. The cumulative 10-year incidence of melanoma was 14% in the 160 patients with classic atypical mole syndrome and 10% in the other 98 high-risk patients. Impressively, all of the melanomas were either in situ or less than 1 mm thick. There were no metastases and no melanoma-related deaths (J. Am. Acad. Dermatol. 2004;50:15-20).
Although dermoscopy is used primarily in examining pigmented skin lesions, it has other applications. For example, in performing Mohs surgery for basal cell carcinomas, Dr. Wang has found dermoscopy to be of assistance in a couple of ways: In patients with ill-defined tumor borders on clinical examination, dermoscopy can define the tumor borders presurgically, thereby reducing the number of Mohs surgical stages required; and when a patient returns for Mohs surgery 6 weeks after skin biopsy and the biopsy site has healed so completely it can’t be found with the naked eye, the dermoscope can identify the site by visualizing subtle scars and telangiectasias.
In general dermatology, Dr. Wang said he turns to dermoscopy as an aid in diagnosing connective tissue diseases, including dermatomyositis, scleroderma, and lupus. He applies ultrasound gel to the proximal nail fold and examines the site using the dermoscope. A finding of dilated blood vessels stands out as a helpful diagnostic clue.
In addition, Dr. Wang said he has utilized the dermoscope in diagnosing scabies by spotting the mites and their trails, in detecting the telltale Wickham striae of lichen planus, and in diagnosing other dermatologic disorders.
Dr. Wang reported having no relevant financial conflicts.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Melanoma increases in adolescents
Since the 1970s, the incidence of melanoma has been rising about 2% per year in adolescents, the same as it has in adults, according to an epidemiologic study published online April 16 in Pediatrics.
The reasons for this increase are not yet clear. Individual-level studies rather than population-level studies are needed to find the explanation for this trend, said Jeannette R. Wong of the division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md., and her associates.
Recent studies have documented the increase in adult melanoma cases and "illuminated likely contributing factors," but none have assessed childhood and adolescent melanoma, the researchers noted. They analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database for nine U.S. geographic regions from 1973 through 2009. They identified all first melanomas diagnosed among patients aged 19 years and younger.
A total of 1,317 cases of melanoma were identified during the study period. Because few of the malignancies developed in nonwhite patients or in patients of unknown race/ethnicity, only the 1,230 cases that developed in white patients were included in the analysis.
The overall incidence of melanoma rose by an average of 2% per year for both boys and girls (Pediatrics 2013 April 16 [doi: 10.1542/peds.2012-2520]).
Melanoma was nearly twice as common in girls compared to boys overall (61% vs. 39%) with similar percentages within each age group (0-9 years, 10-14 years, and 15-19 years).
The incidence increased with age. The majority of melanomas – 77% – were diagnosed in adolescents aged 15-19 years. Only 8% of melanomas were diagnosed in children aged 9 years and younger, and 15% were diagnosed in those aged 10-14 years.
The incidence of localized melanoma was much higher (77%) than that of regional (13%), distant (2%), or unstaged disease (8%).
The most frequent melanoma sites in girls were the lower limbs and hips, on which melanomas increased by a significant annual percentage change of 3% over the study period. Among boys, melanomas were most common on the skin of the face and trunk, with annual percentage increase of 5% over the study period.
UVB exposure did not appear to be the primary factor contributing to the increase in melanoma, the researchers noted. In fact, melanoma rates were slightly higher in geographic areas that had low UVB exposure (such as Connecticut and Washington state) than in areas with high UVB exposure (such as Hawaii and California). "However, all significantly increasing trends for melanoma over our study period occurred in sun-exposed areas of the body," they said.
This finding suggests that tanning facilities may instead be a major source of the increase in incidence, because there are many more such facilities in low-UV regions, the researchers said.
Increased use of tanning facilities also may explain why the rate of melanoma is higher in girls than in boys, since girls are much more likely than boys to use such facilities, they added.
It is also possible that heightened awareness of melanoma in recent years has improved detection rates in the pediatric population, the researchers said.
These data are consistent with those of previous studies that have reported increasing rates of melanoma in the pediatric populations of Australia, Sweden, and England.
Although this study included more than 30 years of data on melanoma incidence, it was limited in that it did not include individual-level data on outdoor UV exposure, use of tanning facilities, or familial factors related to melanoma risk, the researchers said.
Since the 1970s, the incidence of melanoma has been rising about 2% per year in adolescents, the same as it has in adults, according to an epidemiologic study published online April 16 in Pediatrics.
The reasons for this increase are not yet clear. Individual-level studies rather than population-level studies are needed to find the explanation for this trend, said Jeannette R. Wong of the division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md., and her associates.
Recent studies have documented the increase in adult melanoma cases and "illuminated likely contributing factors," but none have assessed childhood and adolescent melanoma, the researchers noted. They analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database for nine U.S. geographic regions from 1973 through 2009. They identified all first melanomas diagnosed among patients aged 19 years and younger.
A total of 1,317 cases of melanoma were identified during the study period. Because few of the malignancies developed in nonwhite patients or in patients of unknown race/ethnicity, only the 1,230 cases that developed in white patients were included in the analysis.
The overall incidence of melanoma rose by an average of 2% per year for both boys and girls (Pediatrics 2013 April 16 [doi: 10.1542/peds.2012-2520]).
Melanoma was nearly twice as common in girls compared to boys overall (61% vs. 39%) with similar percentages within each age group (0-9 years, 10-14 years, and 15-19 years).
The incidence increased with age. The majority of melanomas – 77% – were diagnosed in adolescents aged 15-19 years. Only 8% of melanomas were diagnosed in children aged 9 years and younger, and 15% were diagnosed in those aged 10-14 years.
The incidence of localized melanoma was much higher (77%) than that of regional (13%), distant (2%), or unstaged disease (8%).
The most frequent melanoma sites in girls were the lower limbs and hips, on which melanomas increased by a significant annual percentage change of 3% over the study period. Among boys, melanomas were most common on the skin of the face and trunk, with annual percentage increase of 5% over the study period.
UVB exposure did not appear to be the primary factor contributing to the increase in melanoma, the researchers noted. In fact, melanoma rates were slightly higher in geographic areas that had low UVB exposure (such as Connecticut and Washington state) than in areas with high UVB exposure (such as Hawaii and California). "However, all significantly increasing trends for melanoma over our study period occurred in sun-exposed areas of the body," they said.
This finding suggests that tanning facilities may instead be a major source of the increase in incidence, because there are many more such facilities in low-UV regions, the researchers said.
Increased use of tanning facilities also may explain why the rate of melanoma is higher in girls than in boys, since girls are much more likely than boys to use such facilities, they added.
It is also possible that heightened awareness of melanoma in recent years has improved detection rates in the pediatric population, the researchers said.
These data are consistent with those of previous studies that have reported increasing rates of melanoma in the pediatric populations of Australia, Sweden, and England.
Although this study included more than 30 years of data on melanoma incidence, it was limited in that it did not include individual-level data on outdoor UV exposure, use of tanning facilities, or familial factors related to melanoma risk, the researchers said.
Since the 1970s, the incidence of melanoma has been rising about 2% per year in adolescents, the same as it has in adults, according to an epidemiologic study published online April 16 in Pediatrics.
The reasons for this increase are not yet clear. Individual-level studies rather than population-level studies are needed to find the explanation for this trend, said Jeannette R. Wong of the division of cancer epidemiology and genetics at the National Cancer Institute, Rockville, Md., and her associates.
Recent studies have documented the increase in adult melanoma cases and "illuminated likely contributing factors," but none have assessed childhood and adolescent melanoma, the researchers noted. They analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database for nine U.S. geographic regions from 1973 through 2009. They identified all first melanomas diagnosed among patients aged 19 years and younger.
A total of 1,317 cases of melanoma were identified during the study period. Because few of the malignancies developed in nonwhite patients or in patients of unknown race/ethnicity, only the 1,230 cases that developed in white patients were included in the analysis.
The overall incidence of melanoma rose by an average of 2% per year for both boys and girls (Pediatrics 2013 April 16 [doi: 10.1542/peds.2012-2520]).
Melanoma was nearly twice as common in girls compared to boys overall (61% vs. 39%) with similar percentages within each age group (0-9 years, 10-14 years, and 15-19 years).
The incidence increased with age. The majority of melanomas – 77% – were diagnosed in adolescents aged 15-19 years. Only 8% of melanomas were diagnosed in children aged 9 years and younger, and 15% were diagnosed in those aged 10-14 years.
The incidence of localized melanoma was much higher (77%) than that of regional (13%), distant (2%), or unstaged disease (8%).
The most frequent melanoma sites in girls were the lower limbs and hips, on which melanomas increased by a significant annual percentage change of 3% over the study period. Among boys, melanomas were most common on the skin of the face and trunk, with annual percentage increase of 5% over the study period.
UVB exposure did not appear to be the primary factor contributing to the increase in melanoma, the researchers noted. In fact, melanoma rates were slightly higher in geographic areas that had low UVB exposure (such as Connecticut and Washington state) than in areas with high UVB exposure (such as Hawaii and California). "However, all significantly increasing trends for melanoma over our study period occurred in sun-exposed areas of the body," they said.
This finding suggests that tanning facilities may instead be a major source of the increase in incidence, because there are many more such facilities in low-UV regions, the researchers said.
Increased use of tanning facilities also may explain why the rate of melanoma is higher in girls than in boys, since girls are much more likely than boys to use such facilities, they added.
It is also possible that heightened awareness of melanoma in recent years has improved detection rates in the pediatric population, the researchers said.
These data are consistent with those of previous studies that have reported increasing rates of melanoma in the pediatric populations of Australia, Sweden, and England.
Although this study included more than 30 years of data on melanoma incidence, it was limited in that it did not include individual-level data on outdoor UV exposure, use of tanning facilities, or familial factors related to melanoma risk, the researchers said.
FROM PEDIATRICS
Major finding: The incidence of melanoma has increased by an average of 2% per year for both boys and girls in the past 30 years.
Data source: An analysis from the SEER database of 1,317 cases of melanoma arising in patients younger than 19 years from the period 1973-2009.
Disclosures: This study was supported in part by the National Institutes of Health and the National Cancer Institute. No financial conflicts of interest were reported.
Are dermatologists falling behind the technology curve?
WAILEA, MAUI – Dermatology is arguably the least technologically advanced specialty in medicine, but forces are at work to remedy that situation, according to Dr. Allan C. Halpern.
"We are this astoundingly visual specialty. Everything we do is captured in images. And there has been this astounding imaging revolution occurring all around us," said Dr. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center, New York.
"Yet, not only are we not driving the bus, most of us aren’t even sitting in one of the seats on the bus," Dr. Halpern said.
"Everywhere in medicine there has been application of technologies. We [skin cancer specialists] really are the slowest adopters outside of the laser/cosmetic space," Dr. Halpern noted. "If we don’t figure out how to leverage all these technologies in our astounding information age, we will get left behind in the dirt," he cautioned at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Halpern is a key figure in a major collaborative effort to help bring dermatology into the 21st century by upgrading the state of melanoma detection. The International Skin Imaging Collaboration Melanoma Project is an ambitious new effort coordinated by Memorial Sloan-Kettering Cancer Center. Key collaborators include the National Institutes of Health cancer bioinformatics working group, the International Dermoscopy Society, the International Society for Digital Imaging of the Skin, and International Business Machines (IBM).
For the ISIC Melanoma Project, IBM intends to apply the same effort used in training its computer, Watson, to become the champion of the game show Jeopardy. But this time the challenge will be to improve methods of analyzing clinical and dermoscopic digital skin lesion images to create powerful new decision support and automated diagnostic systems, Dr. Halpern said.
The consortium already has commitments from academic institutions and industry to provide hundreds of thousands of images of melanomas and benign skin lesions accurately annotated for pathology and clinical diagnosis. This archive will be in the public domain, and will be available for teaching purposes, teledermatology, and for commercial development of automated diagnostic systems. An inventor who, for example, comes up with what he thinks is a superior smartphone app for melanoma detection can test its accuracy on 50,000 validated images, Dr. Halpern explained.
Dr. Halpern said he remains bullish about the future for smartphone melanoma apps, despite the much-publicized disappointing diagnostic accuracy of four such apps in a study published earlier this year (JAMA Dermatol. doi:10.1001/jamadermatol.2013.2382).
"That study made it to the front page of the Wall Street Journal. But the fact of the matter is it’s not surprising that the apps now are not all that accurate. Most of them are garage startups where somebody got their hands on images by going to their local dermatologist and getting a convenient sample of images," Dr. Halpern said.
By contrast, the ISIC Melanoma Project collaborative will make it possible for innovators to develop their apps and other diagnostic tools using vast sums of imaging data. Five years from now, phone apps for melanoma detection are going to be enormously improved, Dr. Halpern predicted.
"Why are these apps so important? Well, who finds most melanomas? It’s not us," he said. "If we’re not screening the public for melanoma, then how can we be negative about anything that makes the public better at finding their own melanomas?" he added.
"You hear doctors saying, ‘This is dangerous, people are going to use these things and they’re not going to go see their doctor.’ Well, right now there’s a good chance they’re not seeing their doctor anyway. And if these apps are making [individuals] dramatically more aware and more comfortable in checking their skin and coming in for a visit, that’s a good thing," Dr. Halpern asserted.
The data show that physicians detect about 15% of melanomas, while the other 85% are detected by the patients themselves, and there is strong demand for tools to help them do even better, Dr. Halpern added.
"The melanomas that many of us saw coming into our practices 30 years ago can’t be compared to the overwhelming majority of the melanomas we’re seeing in our practices today brought to our attention by patients. They’re getting much better at early detection," Dr. Halpern said.
Another major goal for the melanoma project is to develop dermatologic digital imaging standards and standardized terminology for industry in terms of camera quality, resolution, techniques, image encryption and compression, and other basic issues.
"Imagine going to get a chest x-ray in different parts of the world if every technician had their own way of doing it and there were no standards in the machines. That’s the current state of dermatologic imaging," Dr. Halpern noted.
One useful technology for melanoma is serial total body photography to detect dynamically changing and therefore suspicious skin lesions.
"You’d want it if you were the patient. But it’s a logistical nightmare to set it up," Dr. Halpern observed.
Conventional total body photography is two-dimensional. Dr. Halpern and his colleagues are working with industry to develop a 3-D total body imaging system. The prototype takes 1 millisecond to acquire a 360-degree color total-body representation. Although such technology is not practical for the typical office practice, it may be a boon for patient care in high-volume skin cancer centers.
American dermatologists as a whole are about a decade behind their European colleagues in embracing dermoscopy as a tool for enhanced assessment of concerning lesions, Dr. Halpern noted.
"We’re still not as good at it as many of them, because they started on the learning curve earlier. But we are rapidly catching up," he added.
The next major advance in melanoma detection that improves upon dermoscopy will require subsurface imaging that provides cellular detail. Optical coherence tomography and high-resolution ultrasound are among the technologies under development, Dr. Halpern said.
He said he believes that reflectance confocal microscopy holds the greatest promise. "Now there’s a handheld device that runs off of a laptop [VivaNet by Lucid Inc.]. There are clinics in Europe using it routinely. It may or may not play out in clinical practice here," he said.
At present, the only Food and Drug Administration–approved device for melanoma detection is MelaFind, the multispectral imaging system marketed by Mela Sciences. Other diagnostic systems the pipeline for melanoma include the use of electrical impedance spectroscopy and the noninvasive genomic detection of melanoma via RNA analysis of a scraping of stratum corneum off of the lesion.
Dr. Halpern reported serving as a consultant to Canfield Scientific, DermTech, SciBase, Quintiles, and Lucid.
SDEF and this news organization are owned by the same parent company.
WAILEA, MAUI – Dermatology is arguably the least technologically advanced specialty in medicine, but forces are at work to remedy that situation, according to Dr. Allan C. Halpern.
"We are this astoundingly visual specialty. Everything we do is captured in images. And there has been this astounding imaging revolution occurring all around us," said Dr. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center, New York.
"Yet, not only are we not driving the bus, most of us aren’t even sitting in one of the seats on the bus," Dr. Halpern said.
"Everywhere in medicine there has been application of technologies. We [skin cancer specialists] really are the slowest adopters outside of the laser/cosmetic space," Dr. Halpern noted. "If we don’t figure out how to leverage all these technologies in our astounding information age, we will get left behind in the dirt," he cautioned at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Halpern is a key figure in a major collaborative effort to help bring dermatology into the 21st century by upgrading the state of melanoma detection. The International Skin Imaging Collaboration Melanoma Project is an ambitious new effort coordinated by Memorial Sloan-Kettering Cancer Center. Key collaborators include the National Institutes of Health cancer bioinformatics working group, the International Dermoscopy Society, the International Society for Digital Imaging of the Skin, and International Business Machines (IBM).
For the ISIC Melanoma Project, IBM intends to apply the same effort used in training its computer, Watson, to become the champion of the game show Jeopardy. But this time the challenge will be to improve methods of analyzing clinical and dermoscopic digital skin lesion images to create powerful new decision support and automated diagnostic systems, Dr. Halpern said.
The consortium already has commitments from academic institutions and industry to provide hundreds of thousands of images of melanomas and benign skin lesions accurately annotated for pathology and clinical diagnosis. This archive will be in the public domain, and will be available for teaching purposes, teledermatology, and for commercial development of automated diagnostic systems. An inventor who, for example, comes up with what he thinks is a superior smartphone app for melanoma detection can test its accuracy on 50,000 validated images, Dr. Halpern explained.
Dr. Halpern said he remains bullish about the future for smartphone melanoma apps, despite the much-publicized disappointing diagnostic accuracy of four such apps in a study published earlier this year (JAMA Dermatol. doi:10.1001/jamadermatol.2013.2382).
"That study made it to the front page of the Wall Street Journal. But the fact of the matter is it’s not surprising that the apps now are not all that accurate. Most of them are garage startups where somebody got their hands on images by going to their local dermatologist and getting a convenient sample of images," Dr. Halpern said.
By contrast, the ISIC Melanoma Project collaborative will make it possible for innovators to develop their apps and other diagnostic tools using vast sums of imaging data. Five years from now, phone apps for melanoma detection are going to be enormously improved, Dr. Halpern predicted.
"Why are these apps so important? Well, who finds most melanomas? It’s not us," he said. "If we’re not screening the public for melanoma, then how can we be negative about anything that makes the public better at finding their own melanomas?" he added.
"You hear doctors saying, ‘This is dangerous, people are going to use these things and they’re not going to go see their doctor.’ Well, right now there’s a good chance they’re not seeing their doctor anyway. And if these apps are making [individuals] dramatically more aware and more comfortable in checking their skin and coming in for a visit, that’s a good thing," Dr. Halpern asserted.
The data show that physicians detect about 15% of melanomas, while the other 85% are detected by the patients themselves, and there is strong demand for tools to help them do even better, Dr. Halpern added.
"The melanomas that many of us saw coming into our practices 30 years ago can’t be compared to the overwhelming majority of the melanomas we’re seeing in our practices today brought to our attention by patients. They’re getting much better at early detection," Dr. Halpern said.
Another major goal for the melanoma project is to develop dermatologic digital imaging standards and standardized terminology for industry in terms of camera quality, resolution, techniques, image encryption and compression, and other basic issues.
"Imagine going to get a chest x-ray in different parts of the world if every technician had their own way of doing it and there were no standards in the machines. That’s the current state of dermatologic imaging," Dr. Halpern noted.
One useful technology for melanoma is serial total body photography to detect dynamically changing and therefore suspicious skin lesions.
"You’d want it if you were the patient. But it’s a logistical nightmare to set it up," Dr. Halpern observed.
Conventional total body photography is two-dimensional. Dr. Halpern and his colleagues are working with industry to develop a 3-D total body imaging system. The prototype takes 1 millisecond to acquire a 360-degree color total-body representation. Although such technology is not practical for the typical office practice, it may be a boon for patient care in high-volume skin cancer centers.
American dermatologists as a whole are about a decade behind their European colleagues in embracing dermoscopy as a tool for enhanced assessment of concerning lesions, Dr. Halpern noted.
"We’re still not as good at it as many of them, because they started on the learning curve earlier. But we are rapidly catching up," he added.
The next major advance in melanoma detection that improves upon dermoscopy will require subsurface imaging that provides cellular detail. Optical coherence tomography and high-resolution ultrasound are among the technologies under development, Dr. Halpern said.
He said he believes that reflectance confocal microscopy holds the greatest promise. "Now there’s a handheld device that runs off of a laptop [VivaNet by Lucid Inc.]. There are clinics in Europe using it routinely. It may or may not play out in clinical practice here," he said.
At present, the only Food and Drug Administration–approved device for melanoma detection is MelaFind, the multispectral imaging system marketed by Mela Sciences. Other diagnostic systems the pipeline for melanoma include the use of electrical impedance spectroscopy and the noninvasive genomic detection of melanoma via RNA analysis of a scraping of stratum corneum off of the lesion.
Dr. Halpern reported serving as a consultant to Canfield Scientific, DermTech, SciBase, Quintiles, and Lucid.
SDEF and this news organization are owned by the same parent company.
WAILEA, MAUI – Dermatology is arguably the least technologically advanced specialty in medicine, but forces are at work to remedy that situation, according to Dr. Allan C. Halpern.
"We are this astoundingly visual specialty. Everything we do is captured in images. And there has been this astounding imaging revolution occurring all around us," said Dr. Halpern, chief of the dermatology service at Memorial Sloan-Kettering Cancer Center, New York.
"Yet, not only are we not driving the bus, most of us aren’t even sitting in one of the seats on the bus," Dr. Halpern said.
"Everywhere in medicine there has been application of technologies. We [skin cancer specialists] really are the slowest adopters outside of the laser/cosmetic space," Dr. Halpern noted. "If we don’t figure out how to leverage all these technologies in our astounding information age, we will get left behind in the dirt," he cautioned at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Halpern is a key figure in a major collaborative effort to help bring dermatology into the 21st century by upgrading the state of melanoma detection. The International Skin Imaging Collaboration Melanoma Project is an ambitious new effort coordinated by Memorial Sloan-Kettering Cancer Center. Key collaborators include the National Institutes of Health cancer bioinformatics working group, the International Dermoscopy Society, the International Society for Digital Imaging of the Skin, and International Business Machines (IBM).
For the ISIC Melanoma Project, IBM intends to apply the same effort used in training its computer, Watson, to become the champion of the game show Jeopardy. But this time the challenge will be to improve methods of analyzing clinical and dermoscopic digital skin lesion images to create powerful new decision support and automated diagnostic systems, Dr. Halpern said.
The consortium already has commitments from academic institutions and industry to provide hundreds of thousands of images of melanomas and benign skin lesions accurately annotated for pathology and clinical diagnosis. This archive will be in the public domain, and will be available for teaching purposes, teledermatology, and for commercial development of automated diagnostic systems. An inventor who, for example, comes up with what he thinks is a superior smartphone app for melanoma detection can test its accuracy on 50,000 validated images, Dr. Halpern explained.
Dr. Halpern said he remains bullish about the future for smartphone melanoma apps, despite the much-publicized disappointing diagnostic accuracy of four such apps in a study published earlier this year (JAMA Dermatol. doi:10.1001/jamadermatol.2013.2382).
"That study made it to the front page of the Wall Street Journal. But the fact of the matter is it’s not surprising that the apps now are not all that accurate. Most of them are garage startups where somebody got their hands on images by going to their local dermatologist and getting a convenient sample of images," Dr. Halpern said.
By contrast, the ISIC Melanoma Project collaborative will make it possible for innovators to develop their apps and other diagnostic tools using vast sums of imaging data. Five years from now, phone apps for melanoma detection are going to be enormously improved, Dr. Halpern predicted.
"Why are these apps so important? Well, who finds most melanomas? It’s not us," he said. "If we’re not screening the public for melanoma, then how can we be negative about anything that makes the public better at finding their own melanomas?" he added.
"You hear doctors saying, ‘This is dangerous, people are going to use these things and they’re not going to go see their doctor.’ Well, right now there’s a good chance they’re not seeing their doctor anyway. And if these apps are making [individuals] dramatically more aware and more comfortable in checking their skin and coming in for a visit, that’s a good thing," Dr. Halpern asserted.
The data show that physicians detect about 15% of melanomas, while the other 85% are detected by the patients themselves, and there is strong demand for tools to help them do even better, Dr. Halpern added.
"The melanomas that many of us saw coming into our practices 30 years ago can’t be compared to the overwhelming majority of the melanomas we’re seeing in our practices today brought to our attention by patients. They’re getting much better at early detection," Dr. Halpern said.
Another major goal for the melanoma project is to develop dermatologic digital imaging standards and standardized terminology for industry in terms of camera quality, resolution, techniques, image encryption and compression, and other basic issues.
"Imagine going to get a chest x-ray in different parts of the world if every technician had their own way of doing it and there were no standards in the machines. That’s the current state of dermatologic imaging," Dr. Halpern noted.
One useful technology for melanoma is serial total body photography to detect dynamically changing and therefore suspicious skin lesions.
"You’d want it if you were the patient. But it’s a logistical nightmare to set it up," Dr. Halpern observed.
Conventional total body photography is two-dimensional. Dr. Halpern and his colleagues are working with industry to develop a 3-D total body imaging system. The prototype takes 1 millisecond to acquire a 360-degree color total-body representation. Although such technology is not practical for the typical office practice, it may be a boon for patient care in high-volume skin cancer centers.
American dermatologists as a whole are about a decade behind their European colleagues in embracing dermoscopy as a tool for enhanced assessment of concerning lesions, Dr. Halpern noted.
"We’re still not as good at it as many of them, because they started on the learning curve earlier. But we are rapidly catching up," he added.
The next major advance in melanoma detection that improves upon dermoscopy will require subsurface imaging that provides cellular detail. Optical coherence tomography and high-resolution ultrasound are among the technologies under development, Dr. Halpern said.
He said he believes that reflectance confocal microscopy holds the greatest promise. "Now there’s a handheld device that runs off of a laptop [VivaNet by Lucid Inc.]. There are clinics in Europe using it routinely. It may or may not play out in clinical practice here," he said.
At present, the only Food and Drug Administration–approved device for melanoma detection is MelaFind, the multispectral imaging system marketed by Mela Sciences. Other diagnostic systems the pipeline for melanoma include the use of electrical impedance spectroscopy and the noninvasive genomic detection of melanoma via RNA analysis of a scraping of stratum corneum off of the lesion.
Dr. Halpern reported serving as a consultant to Canfield Scientific, DermTech, SciBase, Quintiles, and Lucid.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
One in four melanoma patients shun sunscreen
WASHINGTON – While melanoma survivors appear to be more aware of sun safety than does the general public, more than a quarter do not regularly use sunscreen.
"We know that melanoma is a malignancy prevalent in our population, and we know that for many people with melanoma, sun exposure is a major risk factor for recurrence; and sun protection may reduce their chances of getting melanoma again," Dr. Anees B. Chagpar said at the annual meeting of the American Association for Cancer Research. "Although we found that melanoma survivors did better than the general public at protecting their skin from the sun, we also found that more than a quarter of melanoma survivors never wear sunscreen. That blew my mind."
A few survivors – about 2% – even frequent tanning salons, said Dr. Chagpar, an associate professor of surgery at Yale University, New Haven, Conn.
Dr. Chagpar and her colleagues based their findings on data extracted from the 2010 National Health Interview Survey. They focused on self-reported history of melanoma, sun protection practices, and indoor tanning.
The 2010 survey included information on 27,120 adults; 171 had a history of melanoma. Of the adults included in the survey, most (55%) were men; 10% were younger than 40 years.
Compared with the general population, melanoma survivors demonstrated an overall increased rate of sun awareness. Significantly more melanoma survivors reported that they always stay in the shade, compared with the general population (16% vs. 10%, respectively). They were significantly more likely to always wear a baseball cap or visor (31% vs. 18%), a wide-brimmed hat (20% vs. 6%), and a long-sleeved shirt (12% vs. 5%) when going outside on a warm, sunny day for more than 1 hour. They were significantly more likely to report always using sunscreen (32% vs. 17%).
However, Dr. Chagpar said, a good proportion of melanoma survivors are not adequately protecting themselves from sun exposure. About 15% reported rarely or never staying in the shade, and 27% reported never wearing sunscreen.
"The bright spot in this story is that melanoma survivors are more likely to use sunscreen than nonmelanoma survivors," she said in an interview. "But when over a quarter of melanoma survivors admit that they never use sunscreen, we have considerable work to do in educating people about the importance of sun protective behaviors."
And while melanoma survivors overall were significantly less likely to use indoor tanning devices, 2% still reported having done so in the past 12 moths.
"It is distressing to know that melanoma survivors continue to tan," she said. "We have to do a better job in educating our patients about the risks of UV irradiation and the risk of developing melanoma – particularly in those who have survived the disease once already."
Dr. Chagpar had no financial disclosures.
WASHINGTON – While melanoma survivors appear to be more aware of sun safety than does the general public, more than a quarter do not regularly use sunscreen.
"We know that melanoma is a malignancy prevalent in our population, and we know that for many people with melanoma, sun exposure is a major risk factor for recurrence; and sun protection may reduce their chances of getting melanoma again," Dr. Anees B. Chagpar said at the annual meeting of the American Association for Cancer Research. "Although we found that melanoma survivors did better than the general public at protecting their skin from the sun, we also found that more than a quarter of melanoma survivors never wear sunscreen. That blew my mind."
A few survivors – about 2% – even frequent tanning salons, said Dr. Chagpar, an associate professor of surgery at Yale University, New Haven, Conn.
Dr. Chagpar and her colleagues based their findings on data extracted from the 2010 National Health Interview Survey. They focused on self-reported history of melanoma, sun protection practices, and indoor tanning.
The 2010 survey included information on 27,120 adults; 171 had a history of melanoma. Of the adults included in the survey, most (55%) were men; 10% were younger than 40 years.
Compared with the general population, melanoma survivors demonstrated an overall increased rate of sun awareness. Significantly more melanoma survivors reported that they always stay in the shade, compared with the general population (16% vs. 10%, respectively). They were significantly more likely to always wear a baseball cap or visor (31% vs. 18%), a wide-brimmed hat (20% vs. 6%), and a long-sleeved shirt (12% vs. 5%) when going outside on a warm, sunny day for more than 1 hour. They were significantly more likely to report always using sunscreen (32% vs. 17%).
However, Dr. Chagpar said, a good proportion of melanoma survivors are not adequately protecting themselves from sun exposure. About 15% reported rarely or never staying in the shade, and 27% reported never wearing sunscreen.
"The bright spot in this story is that melanoma survivors are more likely to use sunscreen than nonmelanoma survivors," she said in an interview. "But when over a quarter of melanoma survivors admit that they never use sunscreen, we have considerable work to do in educating people about the importance of sun protective behaviors."
And while melanoma survivors overall were significantly less likely to use indoor tanning devices, 2% still reported having done so in the past 12 moths.
"It is distressing to know that melanoma survivors continue to tan," she said. "We have to do a better job in educating our patients about the risks of UV irradiation and the risk of developing melanoma – particularly in those who have survived the disease once already."
Dr. Chagpar had no financial disclosures.
WASHINGTON – While melanoma survivors appear to be more aware of sun safety than does the general public, more than a quarter do not regularly use sunscreen.
"We know that melanoma is a malignancy prevalent in our population, and we know that for many people with melanoma, sun exposure is a major risk factor for recurrence; and sun protection may reduce their chances of getting melanoma again," Dr. Anees B. Chagpar said at the annual meeting of the American Association for Cancer Research. "Although we found that melanoma survivors did better than the general public at protecting their skin from the sun, we also found that more than a quarter of melanoma survivors never wear sunscreen. That blew my mind."
A few survivors – about 2% – even frequent tanning salons, said Dr. Chagpar, an associate professor of surgery at Yale University, New Haven, Conn.
Dr. Chagpar and her colleagues based their findings on data extracted from the 2010 National Health Interview Survey. They focused on self-reported history of melanoma, sun protection practices, and indoor tanning.
The 2010 survey included information on 27,120 adults; 171 had a history of melanoma. Of the adults included in the survey, most (55%) were men; 10% were younger than 40 years.
Compared with the general population, melanoma survivors demonstrated an overall increased rate of sun awareness. Significantly more melanoma survivors reported that they always stay in the shade, compared with the general population (16% vs. 10%, respectively). They were significantly more likely to always wear a baseball cap or visor (31% vs. 18%), a wide-brimmed hat (20% vs. 6%), and a long-sleeved shirt (12% vs. 5%) when going outside on a warm, sunny day for more than 1 hour. They were significantly more likely to report always using sunscreen (32% vs. 17%).
However, Dr. Chagpar said, a good proportion of melanoma survivors are not adequately protecting themselves from sun exposure. About 15% reported rarely or never staying in the shade, and 27% reported never wearing sunscreen.
"The bright spot in this story is that melanoma survivors are more likely to use sunscreen than nonmelanoma survivors," she said in an interview. "But when over a quarter of melanoma survivors admit that they never use sunscreen, we have considerable work to do in educating people about the importance of sun protective behaviors."
And while melanoma survivors overall were significantly less likely to use indoor tanning devices, 2% still reported having done so in the past 12 moths.
"It is distressing to know that melanoma survivors continue to tan," she said. "We have to do a better job in educating our patients about the risks of UV irradiation and the risk of developing melanoma – particularly in those who have survived the disease once already."
Dr. Chagpar had no financial disclosures.
FROM THE AACR ANNUAL MEETING
Major finding: About 27% of melanoma survivors reported never wearing sunscreen, and 2% reported using a tanning bed.
Data source: A database review comparing sun safety behaviors between 171 melanoma survivors and almost 27,000 people who never had the cancer.
Disclosures: Dr. Chagpar had no financial disclosures.