User login
Office hysteroscopic evaluation of postmenopausal bleeding
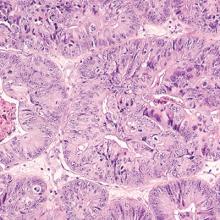
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.
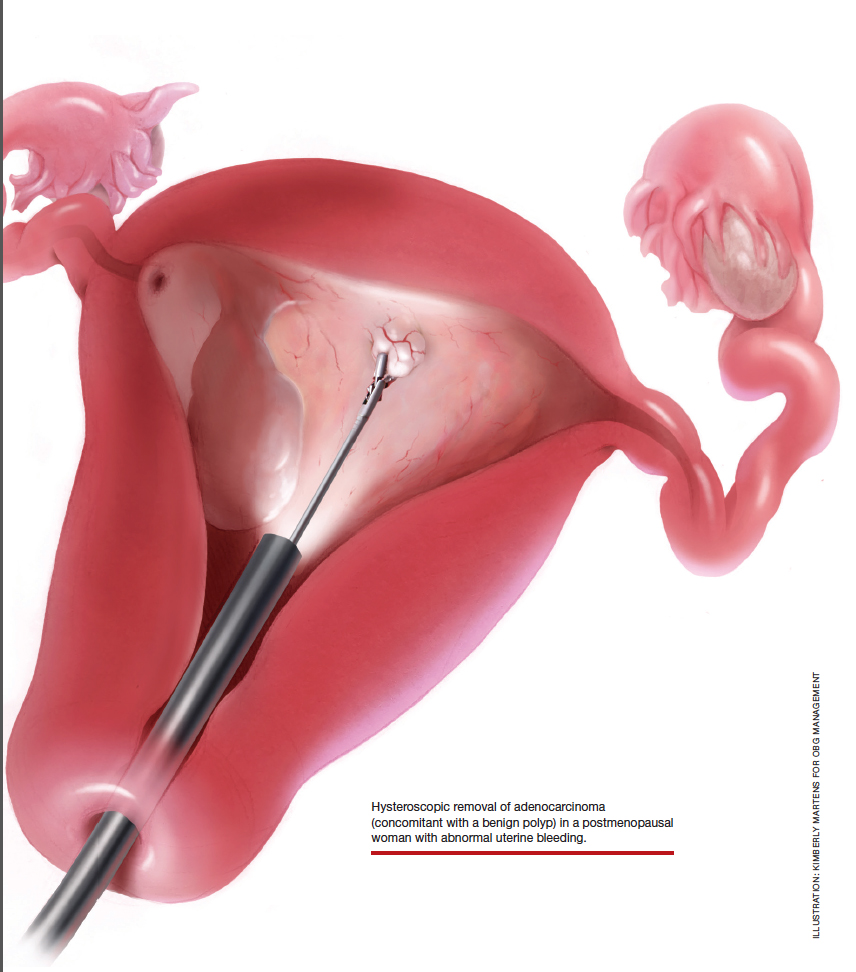
Postmenopausal bleeding: Its risk for cancer
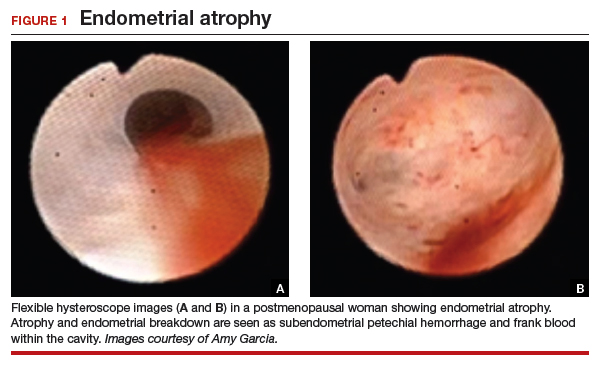
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.
Evaluation of postmenopausal bleeding
Transvaginal ultrasound
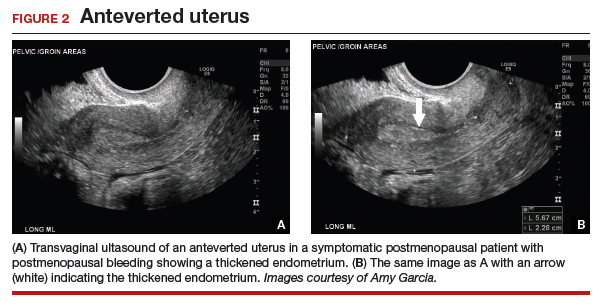
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.
Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
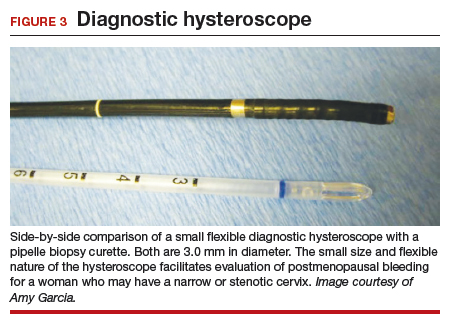
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).
Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
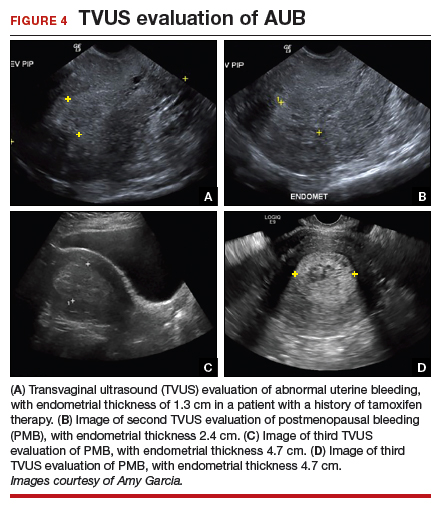
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.
At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
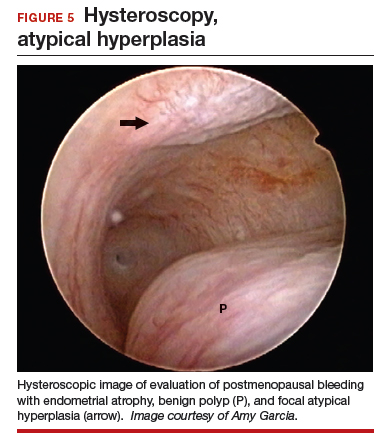
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3

This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

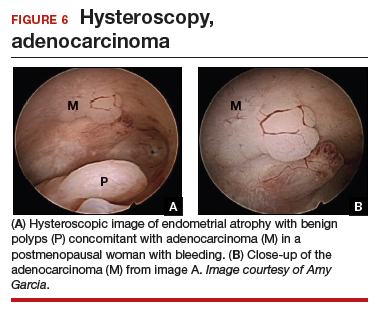
At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B

This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Transdermal estradiol may modulate the relationship between sleep, cognition
LOS ANGELES – Estrogen therapy may have scored another goal in its comeback game, as a 7-year prospective study shows that a transdermal formulation preserves some measures of cognitive function and brain architecture in postmenopausal women.
In addition to performing better on subjective tests of memory, women using the estrogen patch experienced less cortical atrophy and were less likely to show amyloid on brain imaging. The observations were moderately associated with the improved sleep these women reported, Burcu Zeydan, MD, said at the Alzheimer’s Association International Conference.
“By 7 years, among the cognitive domains studied ... [less brain and cognitive change] correlated with lower global sleep score, meaning better sleep quality in the estradiol group,” said Dr. Zeydan, assistant professor of radiology at the Mayo Clinic in Rochester, Minn. “We previously found that preservation of dorsolateral prefrontal cortex over 7 years was associated with lower cortical beta-amyloid deposition on PET only in the estradiol group, pointing out the potential role of estrogen receptors in modulating this relationship.”
Dysregulated sleep is more common among women than men, particularly as menopause approaches and estrogen levels fluctuate, then decline, Dr. Zeydan said.
Dr. Zeydan reported the sleep substudy of KEEPS (the Kronos Early Estrogen Prevention Study), a randomized, double-blind, placebo-controlled, multisite trial that compared oral conjugated equine estrogen with transdermal estradiol. A control group received oral placebo and a placebo patch.*
Brain architecture was similar between the placebo and transdermal groups, but it was actually worse in some measures in the oral-estrogen group, compared with the placebo group. Women taking oral estrogen had more white matter hyperintensities, greater ventricle enlargement, and more cortical thinning. Those differences resolved after they stopped taking the oral formulation, bringing them into line with the transdermal and placebo groups.
The investigation also found that the transdermal group showed lower cerebral amyloid binding on PET scans relative to both placebo and oral estrogen.
“The relative preservation of dorsolateral prefrontal cortical volume in the [transdermal estradiol] group over 7 years indicates that hormone therapy may have long-term effects on the brain,” the team concluded. They noted that the original KEEPS study didn’t find any cognitive correlation with these changes.
The subanalysis looked at 69 women of the KEEPS cohort who had been followed for the full 7 years (4 years on treatment and 3 years off treatment). They were randomized to oral placebo and a placebo patch,* oral conjugated equine estrogen (0.45 mg/day), or transdermal estradiol (50 mcg/day). Participants in the active treatment groups received oral micronized progesterone 12 days each month. All had complete data on cognitive testing and brain imaging. Sleep quality was measured by the Pittsburgh Sleep Quality Index. Dr. Zeydan compared cognition and brain architecture findings in relation to the sleep score; lower scores mean better sleep.
The women were aged 42-58 years at baseline, and within 36 months from menopause. They had no history of menopausal hormone therapy or cardiovascular disease.
The investigators were particularly interested in how estrogen might have modulated the disturbed sleep patterns that often accompany perimenopause and early menopause, and whether the observed brain and cognitive changes tracked with sleep quality.
“During this time, 40% to 60% of women report problems sleeping, and estrogen decline seems to play an important role in sleep disturbances during this phase,” Dr. Zeydan said. “Although poor sleep quality is common in recently menopausal women, sleep quality improves with hormone therapy, as was previously demonstrated in KEEPS hormone therapy trial in recently menopausal women.”
By year 7, the cohort’s mean age was 61 years. The majority had at least some college education. The percentage who carried an apolipoprotein E epsilon-4 allele varied by group, with 15% positivity in the oral group, 48% in the transdermal group, and 16% in the placebo group.
Cognitive function was estimated with a global cognitive measure and four cognitive domain scores: verbal learning and memory, auditory attention and working memory, visual attention and executive function, and mental flexibility.
Higher attention and executive function scores were moderately correlated with a lower sleep score in the transdermal group (r = –0.54, a significant difference compared with the oral formulation). Lower sleep scores also showed a moderate correlation with preserved cortical volume of the dorsolateral prefrontal region (r = –0.47, also significantly different from the oral group).
Lower brain amyloid also positively correlated with better sleep. The correlation between sleep and global amyloid burden in the transdermal group was also moderate (r = 0.45), while the correlation in the oral group was significantly weaker (r = 0.18).
“We can say that sleep quality and transdermal estradiol during early postmenopausal years somehow interact to influence beta-amyloid deposition, preservation of dorsolateral prefrontal cortex volume, and attention and executive function,” Dr. Zeydan said.
Dr. Zeydan had no financial disclosures.
*Correction, 8/7/2019: An earlier version of this story did not make clear that participants in the control group received oral placebo and a placebo patch.
LOS ANGELES – Estrogen therapy may have scored another goal in its comeback game, as a 7-year prospective study shows that a transdermal formulation preserves some measures of cognitive function and brain architecture in postmenopausal women.
In addition to performing better on subjective tests of memory, women using the estrogen patch experienced less cortical atrophy and were less likely to show amyloid on brain imaging. The observations were moderately associated with the improved sleep these women reported, Burcu Zeydan, MD, said at the Alzheimer’s Association International Conference.
“By 7 years, among the cognitive domains studied ... [less brain and cognitive change] correlated with lower global sleep score, meaning better sleep quality in the estradiol group,” said Dr. Zeydan, assistant professor of radiology at the Mayo Clinic in Rochester, Minn. “We previously found that preservation of dorsolateral prefrontal cortex over 7 years was associated with lower cortical beta-amyloid deposition on PET only in the estradiol group, pointing out the potential role of estrogen receptors in modulating this relationship.”
Dysregulated sleep is more common among women than men, particularly as menopause approaches and estrogen levels fluctuate, then decline, Dr. Zeydan said.
Dr. Zeydan reported the sleep substudy of KEEPS (the Kronos Early Estrogen Prevention Study), a randomized, double-blind, placebo-controlled, multisite trial that compared oral conjugated equine estrogen with transdermal estradiol. A control group received oral placebo and a placebo patch.*
Brain architecture was similar between the placebo and transdermal groups, but it was actually worse in some measures in the oral-estrogen group, compared with the placebo group. Women taking oral estrogen had more white matter hyperintensities, greater ventricle enlargement, and more cortical thinning. Those differences resolved after they stopped taking the oral formulation, bringing them into line with the transdermal and placebo groups.
The investigation also found that the transdermal group showed lower cerebral amyloid binding on PET scans relative to both placebo and oral estrogen.
“The relative preservation of dorsolateral prefrontal cortical volume in the [transdermal estradiol] group over 7 years indicates that hormone therapy may have long-term effects on the brain,” the team concluded. They noted that the original KEEPS study didn’t find any cognitive correlation with these changes.
The subanalysis looked at 69 women of the KEEPS cohort who had been followed for the full 7 years (4 years on treatment and 3 years off treatment). They were randomized to oral placebo and a placebo patch,* oral conjugated equine estrogen (0.45 mg/day), or transdermal estradiol (50 mcg/day). Participants in the active treatment groups received oral micronized progesterone 12 days each month. All had complete data on cognitive testing and brain imaging. Sleep quality was measured by the Pittsburgh Sleep Quality Index. Dr. Zeydan compared cognition and brain architecture findings in relation to the sleep score; lower scores mean better sleep.
The women were aged 42-58 years at baseline, and within 36 months from menopause. They had no history of menopausal hormone therapy or cardiovascular disease.
The investigators were particularly interested in how estrogen might have modulated the disturbed sleep patterns that often accompany perimenopause and early menopause, and whether the observed brain and cognitive changes tracked with sleep quality.
“During this time, 40% to 60% of women report problems sleeping, and estrogen decline seems to play an important role in sleep disturbances during this phase,” Dr. Zeydan said. “Although poor sleep quality is common in recently menopausal women, sleep quality improves with hormone therapy, as was previously demonstrated in KEEPS hormone therapy trial in recently menopausal women.”
By year 7, the cohort’s mean age was 61 years. The majority had at least some college education. The percentage who carried an apolipoprotein E epsilon-4 allele varied by group, with 15% positivity in the oral group, 48% in the transdermal group, and 16% in the placebo group.
Cognitive function was estimated with a global cognitive measure and four cognitive domain scores: verbal learning and memory, auditory attention and working memory, visual attention and executive function, and mental flexibility.
Higher attention and executive function scores were moderately correlated with a lower sleep score in the transdermal group (r = –0.54, a significant difference compared with the oral formulation). Lower sleep scores also showed a moderate correlation with preserved cortical volume of the dorsolateral prefrontal region (r = –0.47, also significantly different from the oral group).
Lower brain amyloid also positively correlated with better sleep. The correlation between sleep and global amyloid burden in the transdermal group was also moderate (r = 0.45), while the correlation in the oral group was significantly weaker (r = 0.18).
“We can say that sleep quality and transdermal estradiol during early postmenopausal years somehow interact to influence beta-amyloid deposition, preservation of dorsolateral prefrontal cortex volume, and attention and executive function,” Dr. Zeydan said.
Dr. Zeydan had no financial disclosures.
*Correction, 8/7/2019: An earlier version of this story did not make clear that participants in the control group received oral placebo and a placebo patch.
LOS ANGELES – Estrogen therapy may have scored another goal in its comeback game, as a 7-year prospective study shows that a transdermal formulation preserves some measures of cognitive function and brain architecture in postmenopausal women.
In addition to performing better on subjective tests of memory, women using the estrogen patch experienced less cortical atrophy and were less likely to show amyloid on brain imaging. The observations were moderately associated with the improved sleep these women reported, Burcu Zeydan, MD, said at the Alzheimer’s Association International Conference.
“By 7 years, among the cognitive domains studied ... [less brain and cognitive change] correlated with lower global sleep score, meaning better sleep quality in the estradiol group,” said Dr. Zeydan, assistant professor of radiology at the Mayo Clinic in Rochester, Minn. “We previously found that preservation of dorsolateral prefrontal cortex over 7 years was associated with lower cortical beta-amyloid deposition on PET only in the estradiol group, pointing out the potential role of estrogen receptors in modulating this relationship.”
Dysregulated sleep is more common among women than men, particularly as menopause approaches and estrogen levels fluctuate, then decline, Dr. Zeydan said.
Dr. Zeydan reported the sleep substudy of KEEPS (the Kronos Early Estrogen Prevention Study), a randomized, double-blind, placebo-controlled, multisite trial that compared oral conjugated equine estrogen with transdermal estradiol. A control group received oral placebo and a placebo patch.*
Brain architecture was similar between the placebo and transdermal groups, but it was actually worse in some measures in the oral-estrogen group, compared with the placebo group. Women taking oral estrogen had more white matter hyperintensities, greater ventricle enlargement, and more cortical thinning. Those differences resolved after they stopped taking the oral formulation, bringing them into line with the transdermal and placebo groups.
The investigation also found that the transdermal group showed lower cerebral amyloid binding on PET scans relative to both placebo and oral estrogen.
“The relative preservation of dorsolateral prefrontal cortical volume in the [transdermal estradiol] group over 7 years indicates that hormone therapy may have long-term effects on the brain,” the team concluded. They noted that the original KEEPS study didn’t find any cognitive correlation with these changes.
The subanalysis looked at 69 women of the KEEPS cohort who had been followed for the full 7 years (4 years on treatment and 3 years off treatment). They were randomized to oral placebo and a placebo patch,* oral conjugated equine estrogen (0.45 mg/day), or transdermal estradiol (50 mcg/day). Participants in the active treatment groups received oral micronized progesterone 12 days each month. All had complete data on cognitive testing and brain imaging. Sleep quality was measured by the Pittsburgh Sleep Quality Index. Dr. Zeydan compared cognition and brain architecture findings in relation to the sleep score; lower scores mean better sleep.
The women were aged 42-58 years at baseline, and within 36 months from menopause. They had no history of menopausal hormone therapy or cardiovascular disease.
The investigators were particularly interested in how estrogen might have modulated the disturbed sleep patterns that often accompany perimenopause and early menopause, and whether the observed brain and cognitive changes tracked with sleep quality.
“During this time, 40% to 60% of women report problems sleeping, and estrogen decline seems to play an important role in sleep disturbances during this phase,” Dr. Zeydan said. “Although poor sleep quality is common in recently menopausal women, sleep quality improves with hormone therapy, as was previously demonstrated in KEEPS hormone therapy trial in recently menopausal women.”
By year 7, the cohort’s mean age was 61 years. The majority had at least some college education. The percentage who carried an apolipoprotein E epsilon-4 allele varied by group, with 15% positivity in the oral group, 48% in the transdermal group, and 16% in the placebo group.
Cognitive function was estimated with a global cognitive measure and four cognitive domain scores: verbal learning and memory, auditory attention and working memory, visual attention and executive function, and mental flexibility.
Higher attention and executive function scores were moderately correlated with a lower sleep score in the transdermal group (r = –0.54, a significant difference compared with the oral formulation). Lower sleep scores also showed a moderate correlation with preserved cortical volume of the dorsolateral prefrontal region (r = –0.47, also significantly different from the oral group).
Lower brain amyloid also positively correlated with better sleep. The correlation between sleep and global amyloid burden in the transdermal group was also moderate (r = 0.45), while the correlation in the oral group was significantly weaker (r = 0.18).
“We can say that sleep quality and transdermal estradiol during early postmenopausal years somehow interact to influence beta-amyloid deposition, preservation of dorsolateral prefrontal cortex volume, and attention and executive function,” Dr. Zeydan said.
Dr. Zeydan had no financial disclosures.
*Correction, 8/7/2019: An earlier version of this story did not make clear that participants in the control group received oral placebo and a placebo patch.
REPORTING FROM AAIC 2019
CVD risk upped in postmenopausal breast cancer survivors
according to a new study of nearly 300 women.
Previous studies have shown that cardiovascular risk is greater among postmenopausal women treated for breast cancer compared with those without cancer, but specific risk factors have not been well studied, wrote Daniel de Araujo Brito Buttros, MD, of Paulista State University, Sao Paulo, Brazil, and colleagues.
In a study published in Menopause, the researchers evaluated several CVD risk factors in 96 postmenopausal women with breast cancer and 192 women without breast cancer, including metabolic syndrome, subclinical atherosclerosis, and heat shock proteins (HSP) 60 and 70.
Overall, breast cancer patients had significantly higher HSP60 levels and lower HSP70 levels than those of their cancer-free peers. These two proteins have an antagonistic relationship in cardiovascular disease, with HSP60 considered a risk factor for CVD, and HSP70 considered a protective factor. Average HSP60 levels for the breast cancer and control groups were 35 ng/mL and 10.8 ng/mL, respectively; average HSP70 levels were 0.5 ng/mL and 1.3 ng/mL, respectively.
Both diabetes and metabolic syndrome were significantly more common among breast cancer patients vs. controls (19.8% vs. 6.8% and 54.2% vs. 30.7%, respectively). Carotid artery plaque also was more common in breast cancer patients vs. controls (19.8% vs. 9.4%, respectively, P = 0.013).
In addition, systolic and diastolic blood pressure levels were significantly higher among the breast cancer patients, as were triglycerides and glucose.
The findings were limited by several factors including the cross-sectional design that could not prove a causal relationship between CVD risk and breast cancer, the researchers noted.
However, the results demonstrate the increased CVD risk for breast cancer patients, and “[therefore], women diagnosed with breast cancer might receive multidisciplinary care, including cardiology consultation at the time of breast cancer diagnosis and also during oncologic follow-up visits,” they said.
“Heart disease appears more commonly in women treated for breast cancer because of the toxicities of chemotherapy, radiation therapy, and use of aromatase inhibitors, which lower estrogen. Heart-healthy lifestyle modifications will decrease both the risk of recurrent breast cancer and the risk of developing heart disease,” JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said in a statement. “Women should schedule a cardiology consultation when breast cancer is diagnosed and continue with ongoing follow-up after cancer treatments are completed,” she emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Buttros DAB et al. Menopause. 2019. doi: 10.1097/GME.0000000000001348.
according to a new study of nearly 300 women.
Previous studies have shown that cardiovascular risk is greater among postmenopausal women treated for breast cancer compared with those without cancer, but specific risk factors have not been well studied, wrote Daniel de Araujo Brito Buttros, MD, of Paulista State University, Sao Paulo, Brazil, and colleagues.
In a study published in Menopause, the researchers evaluated several CVD risk factors in 96 postmenopausal women with breast cancer and 192 women without breast cancer, including metabolic syndrome, subclinical atherosclerosis, and heat shock proteins (HSP) 60 and 70.
Overall, breast cancer patients had significantly higher HSP60 levels and lower HSP70 levels than those of their cancer-free peers. These two proteins have an antagonistic relationship in cardiovascular disease, with HSP60 considered a risk factor for CVD, and HSP70 considered a protective factor. Average HSP60 levels for the breast cancer and control groups were 35 ng/mL and 10.8 ng/mL, respectively; average HSP70 levels were 0.5 ng/mL and 1.3 ng/mL, respectively.
Both diabetes and metabolic syndrome were significantly more common among breast cancer patients vs. controls (19.8% vs. 6.8% and 54.2% vs. 30.7%, respectively). Carotid artery plaque also was more common in breast cancer patients vs. controls (19.8% vs. 9.4%, respectively, P = 0.013).
In addition, systolic and diastolic blood pressure levels were significantly higher among the breast cancer patients, as were triglycerides and glucose.
The findings were limited by several factors including the cross-sectional design that could not prove a causal relationship between CVD risk and breast cancer, the researchers noted.
However, the results demonstrate the increased CVD risk for breast cancer patients, and “[therefore], women diagnosed with breast cancer might receive multidisciplinary care, including cardiology consultation at the time of breast cancer diagnosis and also during oncologic follow-up visits,” they said.
“Heart disease appears more commonly in women treated for breast cancer because of the toxicities of chemotherapy, radiation therapy, and use of aromatase inhibitors, which lower estrogen. Heart-healthy lifestyle modifications will decrease both the risk of recurrent breast cancer and the risk of developing heart disease,” JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said in a statement. “Women should schedule a cardiology consultation when breast cancer is diagnosed and continue with ongoing follow-up after cancer treatments are completed,” she emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Buttros DAB et al. Menopause. 2019. doi: 10.1097/GME.0000000000001348.
according to a new study of nearly 300 women.
Previous studies have shown that cardiovascular risk is greater among postmenopausal women treated for breast cancer compared with those without cancer, but specific risk factors have not been well studied, wrote Daniel de Araujo Brito Buttros, MD, of Paulista State University, Sao Paulo, Brazil, and colleagues.
In a study published in Menopause, the researchers evaluated several CVD risk factors in 96 postmenopausal women with breast cancer and 192 women without breast cancer, including metabolic syndrome, subclinical atherosclerosis, and heat shock proteins (HSP) 60 and 70.
Overall, breast cancer patients had significantly higher HSP60 levels and lower HSP70 levels than those of their cancer-free peers. These two proteins have an antagonistic relationship in cardiovascular disease, with HSP60 considered a risk factor for CVD, and HSP70 considered a protective factor. Average HSP60 levels for the breast cancer and control groups were 35 ng/mL and 10.8 ng/mL, respectively; average HSP70 levels were 0.5 ng/mL and 1.3 ng/mL, respectively.
Both diabetes and metabolic syndrome were significantly more common among breast cancer patients vs. controls (19.8% vs. 6.8% and 54.2% vs. 30.7%, respectively). Carotid artery plaque also was more common in breast cancer patients vs. controls (19.8% vs. 9.4%, respectively, P = 0.013).
In addition, systolic and diastolic blood pressure levels were significantly higher among the breast cancer patients, as were triglycerides and glucose.
The findings were limited by several factors including the cross-sectional design that could not prove a causal relationship between CVD risk and breast cancer, the researchers noted.
However, the results demonstrate the increased CVD risk for breast cancer patients, and “[therefore], women diagnosed with breast cancer might receive multidisciplinary care, including cardiology consultation at the time of breast cancer diagnosis and also during oncologic follow-up visits,” they said.
“Heart disease appears more commonly in women treated for breast cancer because of the toxicities of chemotherapy, radiation therapy, and use of aromatase inhibitors, which lower estrogen. Heart-healthy lifestyle modifications will decrease both the risk of recurrent breast cancer and the risk of developing heart disease,” JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said in a statement. “Women should schedule a cardiology consultation when breast cancer is diagnosed and continue with ongoing follow-up after cancer treatments are completed,” she emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Buttros DAB et al. Menopause. 2019. doi: 10.1097/GME.0000000000001348.
FROM MENOPAUSE
An app to help women and clinicians manage menopausal symptoms
In North America, women experience menopause (the permanent cessation of menstruation due to loss of ovarian activity) at a median age of 51 years. They may experience symptoms of perimenopause, or the menopause transition, for several years before menstruation ceases. Menopausal symptoms include vasomotor symptoms, such as hot flushes, and vaginal symptoms, such as vaginal dryness and pain during intercourse.1
Women may have questions about treating menopausal symptoms, maintaining their health, and preventing such age-related diseases as osteoporosis and cardiovascular disease. The decision to treat menopausal symptoms is challenging for women as well as their clinicians given that recommendations have changed over the past few years.
A free app with multiple features. The North American Menopause Society (NAMS) has developed a no-cost mobile health application called MenoPro for menopausal symptom management based on the organization’s 2017 recommendations.2 The app has 2 modes: one for clinicians and one for women/patients to support shared decision making.
For clinicians, the app helps identify which patients with menopausal symptoms are candidates for pharmacologic treatment and the options for optimal therapy. The app also can be used to calculate a 10-year cardiovascular disease (heart disease and stroke) risk assessment. In addition, it contains links to a breast cancer risk assessment as well as an osteoporosis/bone fracture risk assessment tool (FRAX model calculator). Finally, MenoPro includes NAMS’s educational materials and information pages on lifestyle modifications to reduce hot flushes, contraindications and cautions to hormone therapy, pros and cons of hormonal versus nonhormonal options, a comparison of oral (pills) and transdermal (patches, gels, sprays) therapies, treatment options for vaginal dryness and pain with sexual activities, and direct links to tables with the various formulations and doses of medications.
The TABLE details the features of the MenoPro app based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).3 I hope that the app described here will assist you in caring for women in the menopausal transition.

1. American College of Obstetricians and Gynecologists. Practice bulletin no. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
2. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2018;25:13621387.
3. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
In North America, women experience menopause (the permanent cessation of menstruation due to loss of ovarian activity) at a median age of 51 years. They may experience symptoms of perimenopause, or the menopause transition, for several years before menstruation ceases. Menopausal symptoms include vasomotor symptoms, such as hot flushes, and vaginal symptoms, such as vaginal dryness and pain during intercourse.1
Women may have questions about treating menopausal symptoms, maintaining their health, and preventing such age-related diseases as osteoporosis and cardiovascular disease. The decision to treat menopausal symptoms is challenging for women as well as their clinicians given that recommendations have changed over the past few years.
A free app with multiple features. The North American Menopause Society (NAMS) has developed a no-cost mobile health application called MenoPro for menopausal symptom management based on the organization’s 2017 recommendations.2 The app has 2 modes: one for clinicians and one for women/patients to support shared decision making.
For clinicians, the app helps identify which patients with menopausal symptoms are candidates for pharmacologic treatment and the options for optimal therapy. The app also can be used to calculate a 10-year cardiovascular disease (heart disease and stroke) risk assessment. In addition, it contains links to a breast cancer risk assessment as well as an osteoporosis/bone fracture risk assessment tool (FRAX model calculator). Finally, MenoPro includes NAMS’s educational materials and information pages on lifestyle modifications to reduce hot flushes, contraindications and cautions to hormone therapy, pros and cons of hormonal versus nonhormonal options, a comparison of oral (pills) and transdermal (patches, gels, sprays) therapies, treatment options for vaginal dryness and pain with sexual activities, and direct links to tables with the various formulations and doses of medications.
The TABLE details the features of the MenoPro app based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).3 I hope that the app described here will assist you in caring for women in the menopausal transition.

In North America, women experience menopause (the permanent cessation of menstruation due to loss of ovarian activity) at a median age of 51 years. They may experience symptoms of perimenopause, or the menopause transition, for several years before menstruation ceases. Menopausal symptoms include vasomotor symptoms, such as hot flushes, and vaginal symptoms, such as vaginal dryness and pain during intercourse.1
Women may have questions about treating menopausal symptoms, maintaining their health, and preventing such age-related diseases as osteoporosis and cardiovascular disease. The decision to treat menopausal symptoms is challenging for women as well as their clinicians given that recommendations have changed over the past few years.
A free app with multiple features. The North American Menopause Society (NAMS) has developed a no-cost mobile health application called MenoPro for menopausal symptom management based on the organization’s 2017 recommendations.2 The app has 2 modes: one for clinicians and one for women/patients to support shared decision making.
For clinicians, the app helps identify which patients with menopausal symptoms are candidates for pharmacologic treatment and the options for optimal therapy. The app also can be used to calculate a 10-year cardiovascular disease (heart disease and stroke) risk assessment. In addition, it contains links to a breast cancer risk assessment as well as an osteoporosis/bone fracture risk assessment tool (FRAX model calculator). Finally, MenoPro includes NAMS’s educational materials and information pages on lifestyle modifications to reduce hot flushes, contraindications and cautions to hormone therapy, pros and cons of hormonal versus nonhormonal options, a comparison of oral (pills) and transdermal (patches, gels, sprays) therapies, treatment options for vaginal dryness and pain with sexual activities, and direct links to tables with the various formulations and doses of medications.
The TABLE details the features of the MenoPro app based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).3 I hope that the app described here will assist you in caring for women in the menopausal transition.

1. American College of Obstetricians and Gynecologists. Practice bulletin no. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
2. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2018;25:13621387.
3. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
1. American College of Obstetricians and Gynecologists. Practice bulletin no. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
2. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2018;25:13621387.
3. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
2019 Update on menopause
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
What to do when a patient presents with breast pain
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.
Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.
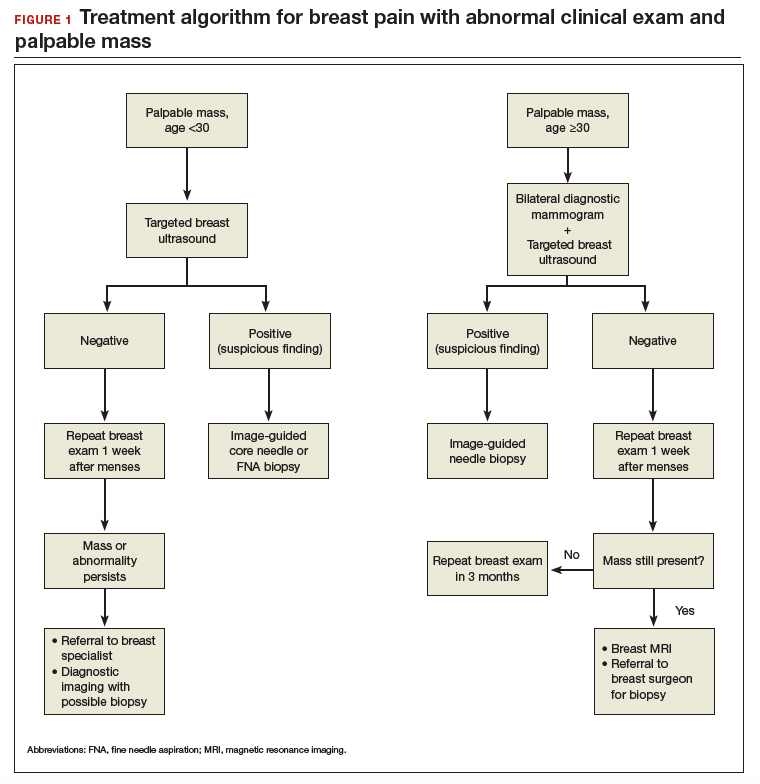
Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...
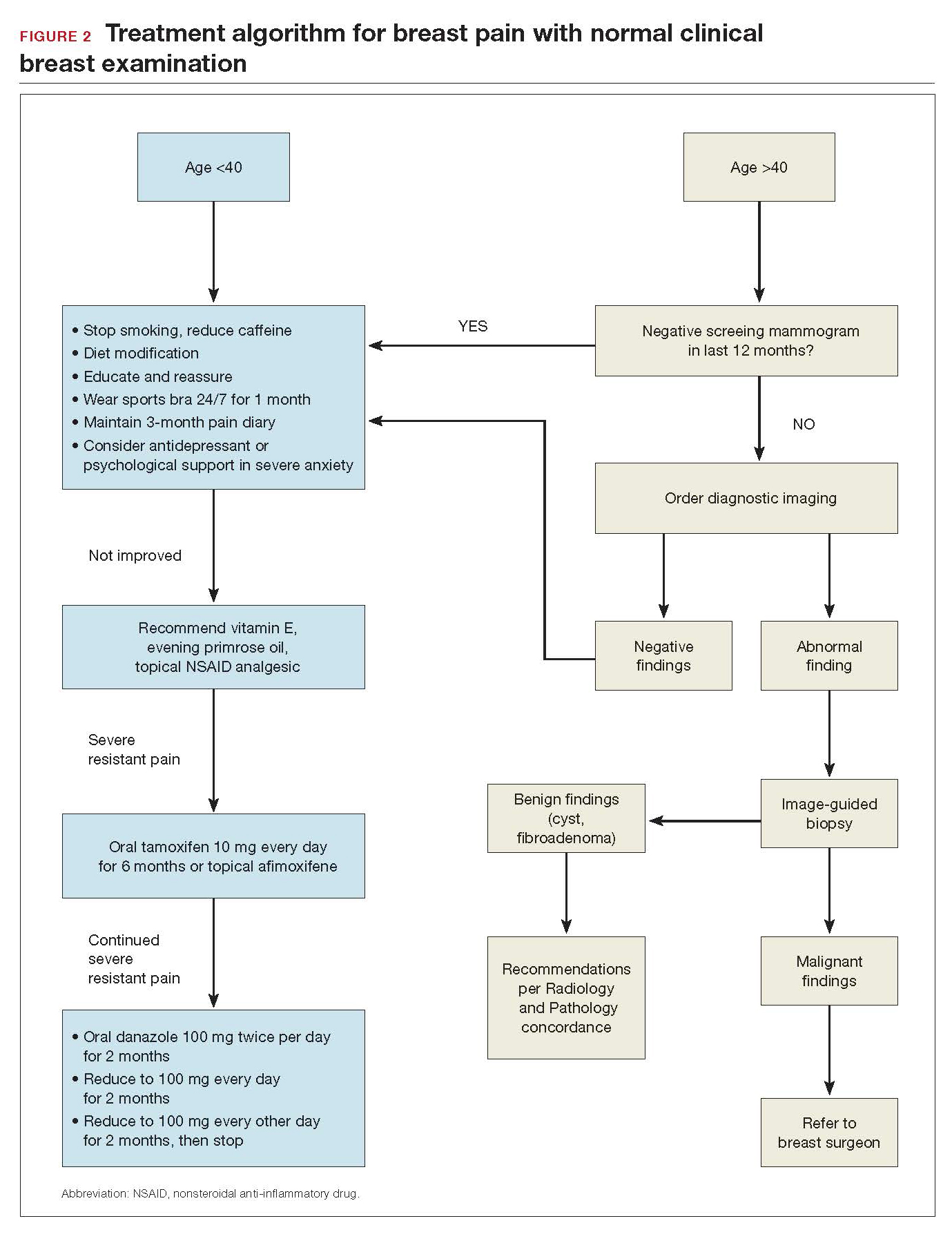
In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.

Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.

Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...

In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.

Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.

Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...

In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
Online counseling clarifies treatment options for menopausal women
NASHVILLE, TENN. – a post-intervention survey showed.
Of 36 women who completed the counseling, 72% were able to express a clear preference for a particular treatment. Additionally, 90% to 100% of those who completed the final survey said the various components of the counseling–such as an educational brochure or a telephone call from a research nurse–made them feel more prepared to speak with their provider about treatment options, Sandra Dayaratna, MD, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Among 18 women with vasomotor symptoms, 6 (33%) preferred non-hormone treatment, 7 (39%) preferred hormone treatment, and 5 (28%) were unsure; among 18 with genitourinary symptoms of menopause, 6 (33%) preferred non-prescription treatment, 7 (39%) preferred topical hormone therapy, and 5 (28%) were unsure, Dr. Dayaratna, division director and clinical associate professor at Thomas Jefferson University Hospital and Sidney Kimmel Medical College, Philadelphia, found.
Of women who were not being treated for vasomotor symptoms, 5 (56%) expressed a clear treatment preference after counseling, whereas 8 (80%) of those not receiving treatment for genitourinary symptoms expressed a clear preference. Among 7 women receiving systemic hormone therapy for vasomotor symptoms, 86% preferred this treatment after counseling, and 3 women (50%) receiving topical hormone therapy preferred topical treatment after counseling.
The study included women aged 36-50 years from diverse educational and racial backgrounds who were referred to the counseling program after reporting menopausal symptoms, completing a baseline survey, and providing consent. The counseling, which was adapted from a tool developed at Thomas Jefferson University Hospital for the assessment of patients with colon and cancer or lung cancer, involved an educational brochure that was mailed to participants. It also offered access to an online tool that provided information about systemic hormone therapy, non-hormone prescription therapy, topical hormone treatment, and non-prescription treatment for vasomotor and genitourinary symptoms of menopause.
A research nurse contacted participants by phone and used the online program to review the brochure, clarify treatment preference, and produce a summary of the results.
In an interview, Dr. Dayaratna noted that the concept of “shared decision making” is often misunderstood to mean that patients are provided with information about options and then they make a choice.
Actually, this study demonstrates that shared decision making really involves a “values clarification” component, she explained. In this study, counseling that incorporates this type of shared decision making helped women feel more prepared to speak with their providers about treatment and thus may add value to care of menopausal women, she concluded.
“This is relevant because we know that over 50% of patients within 90 minutes of leaving their doctor’s office have forgotten what they were told, and over 50% do not comply with their treatment prescriptions,” she said. “So if patients can do this process ahead of time, when they come to speak to their physician about their symptoms and selection of medication, it’s a more effective and efficient conversation.”
Further research should evaluate impact on the subsequent office visit, she added.
This study was funded by an educational grant from Pfizer, Inc. and by the National Cancer Institute. Dr. Dayaratna reported having no disclosures.
SOURCE: Dayaratna S et al., ACOG 2019: Abstract 21M.
NASHVILLE, TENN. – a post-intervention survey showed.
Of 36 women who completed the counseling, 72% were able to express a clear preference for a particular treatment. Additionally, 90% to 100% of those who completed the final survey said the various components of the counseling–such as an educational brochure or a telephone call from a research nurse–made them feel more prepared to speak with their provider about treatment options, Sandra Dayaratna, MD, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Among 18 women with vasomotor symptoms, 6 (33%) preferred non-hormone treatment, 7 (39%) preferred hormone treatment, and 5 (28%) were unsure; among 18 with genitourinary symptoms of menopause, 6 (33%) preferred non-prescription treatment, 7 (39%) preferred topical hormone therapy, and 5 (28%) were unsure, Dr. Dayaratna, division director and clinical associate professor at Thomas Jefferson University Hospital and Sidney Kimmel Medical College, Philadelphia, found.
Of women who were not being treated for vasomotor symptoms, 5 (56%) expressed a clear treatment preference after counseling, whereas 8 (80%) of those not receiving treatment for genitourinary symptoms expressed a clear preference. Among 7 women receiving systemic hormone therapy for vasomotor symptoms, 86% preferred this treatment after counseling, and 3 women (50%) receiving topical hormone therapy preferred topical treatment after counseling.
The study included women aged 36-50 years from diverse educational and racial backgrounds who were referred to the counseling program after reporting menopausal symptoms, completing a baseline survey, and providing consent. The counseling, which was adapted from a tool developed at Thomas Jefferson University Hospital for the assessment of patients with colon and cancer or lung cancer, involved an educational brochure that was mailed to participants. It also offered access to an online tool that provided information about systemic hormone therapy, non-hormone prescription therapy, topical hormone treatment, and non-prescription treatment for vasomotor and genitourinary symptoms of menopause.
A research nurse contacted participants by phone and used the online program to review the brochure, clarify treatment preference, and produce a summary of the results.
In an interview, Dr. Dayaratna noted that the concept of “shared decision making” is often misunderstood to mean that patients are provided with information about options and then they make a choice.
Actually, this study demonstrates that shared decision making really involves a “values clarification” component, she explained. In this study, counseling that incorporates this type of shared decision making helped women feel more prepared to speak with their providers about treatment and thus may add value to care of menopausal women, she concluded.
“This is relevant because we know that over 50% of patients within 90 minutes of leaving their doctor’s office have forgotten what they were told, and over 50% do not comply with their treatment prescriptions,” she said. “So if patients can do this process ahead of time, when they come to speak to their physician about their symptoms and selection of medication, it’s a more effective and efficient conversation.”
Further research should evaluate impact on the subsequent office visit, she added.
This study was funded by an educational grant from Pfizer, Inc. and by the National Cancer Institute. Dr. Dayaratna reported having no disclosures.
SOURCE: Dayaratna S et al., ACOG 2019: Abstract 21M.
NASHVILLE, TENN. – a post-intervention survey showed.
Of 36 women who completed the counseling, 72% were able to express a clear preference for a particular treatment. Additionally, 90% to 100% of those who completed the final survey said the various components of the counseling–such as an educational brochure or a telephone call from a research nurse–made them feel more prepared to speak with their provider about treatment options, Sandra Dayaratna, MD, reported during a poster session at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Among 18 women with vasomotor symptoms, 6 (33%) preferred non-hormone treatment, 7 (39%) preferred hormone treatment, and 5 (28%) were unsure; among 18 with genitourinary symptoms of menopause, 6 (33%) preferred non-prescription treatment, 7 (39%) preferred topical hormone therapy, and 5 (28%) were unsure, Dr. Dayaratna, division director and clinical associate professor at Thomas Jefferson University Hospital and Sidney Kimmel Medical College, Philadelphia, found.
Of women who were not being treated for vasomotor symptoms, 5 (56%) expressed a clear treatment preference after counseling, whereas 8 (80%) of those not receiving treatment for genitourinary symptoms expressed a clear preference. Among 7 women receiving systemic hormone therapy for vasomotor symptoms, 86% preferred this treatment after counseling, and 3 women (50%) receiving topical hormone therapy preferred topical treatment after counseling.
The study included women aged 36-50 years from diverse educational and racial backgrounds who were referred to the counseling program after reporting menopausal symptoms, completing a baseline survey, and providing consent. The counseling, which was adapted from a tool developed at Thomas Jefferson University Hospital for the assessment of patients with colon and cancer or lung cancer, involved an educational brochure that was mailed to participants. It also offered access to an online tool that provided information about systemic hormone therapy, non-hormone prescription therapy, topical hormone treatment, and non-prescription treatment for vasomotor and genitourinary symptoms of menopause.
A research nurse contacted participants by phone and used the online program to review the brochure, clarify treatment preference, and produce a summary of the results.
In an interview, Dr. Dayaratna noted that the concept of “shared decision making” is often misunderstood to mean that patients are provided with information about options and then they make a choice.
Actually, this study demonstrates that shared decision making really involves a “values clarification” component, she explained. In this study, counseling that incorporates this type of shared decision making helped women feel more prepared to speak with their providers about treatment and thus may add value to care of menopausal women, she concluded.
“This is relevant because we know that over 50% of patients within 90 minutes of leaving their doctor’s office have forgotten what they were told, and over 50% do not comply with their treatment prescriptions,” she said. “So if patients can do this process ahead of time, when they come to speak to their physician about their symptoms and selection of medication, it’s a more effective and efficient conversation.”
Further research should evaluate impact on the subsequent office visit, she added.
This study was funded by an educational grant from Pfizer, Inc. and by the National Cancer Institute. Dr. Dayaratna reported having no disclosures.
SOURCE: Dayaratna S et al., ACOG 2019: Abstract 21M.
REPORTING FROM ACOG 2019
Discuss compounded bioidentical hormones and cancer risk
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at [email protected].
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
Romosozumab gets FDA approval for treating osteoporosis
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
“These are women who have a history of osteoporotic fracture or multiple risk factors or have failed other treatments for osteoporosis,” according to a news release from the agency.
The monthly treatment of two injections (given one after the other at one visit) mainly works by increasing new bone formation, but these effects wane after 12 doses. If patients still need osteoporosis therapy after that maximum of 12 doses, it’s recommended they are put on treatments that reduce bone breakdown. Romosozumab-aqqg is “a monoclonal antibody that blocks the effects of the protein sclerostin,” according to the news release.
The treatment’s efficacy and safety was evaluated in two clinical trials of more than 11,000 women with postmenopausal osteoporosis. In one trial, women received 12 months of either romosozumab-aqqg or placebo. The treatment arm had a 73% lower risk of vertebral fracture than did the placebo arm, and this benefit was maintained over a second year when both groups were switched to denosumab, another osteoporosis therapy. In the second trial, one group received romosozumab-aqqg for 1 year and then a year of alendronate, and the other group received 2 years of alendronate, another osteoporosis therapy, according to the news release. In this trial, the romosozumab-aqqg arm had 50% less risk of vertebral fractures than did the alendronate-only arm, as well as reduced risk of nonvertebral fractures.
Romosozumab-aqqg was associated with higher risks of cardiovascular death, heart attack, and stroke in the alendronate trial, so the treatment comes with a boxed warning regarding those risks and recommends that the drug not be used in patients who have had a heart attack or stroke within the previous year, according to the news release. Common side effects include joint pain and headache, as well as injection-site reactions.
What is the association of menopausal HT use and risk of Alzheimer disease?
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
EXPERT COMMENTARY
Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ. 2019;364:1665.
Alzheimer disease represents the most common cause of dementia. Although sex hormones may play a role in the etiology of AD in women, studies addressing the impact of menopausal HT on risk of AD have conflicting findings.
Finnish researchers Savolainen-Peltonen and colleagues aimed to compare postmenopausal HT use in women with and without AD. They used national drug and population registries to identify patients with AD, control women without a diagnosis of AD, and data on postmenopausal HT use.
Details of the study
In Finland, reimbursement for treatment related to AD requires cognitive testing, brain imaging, and a statement from a specialist physician. Using national records, the study investigators identified 84,739 women with a diagnosis of AD during the years 1999–2013 and the same number of control women (without AD) during the same period. A national drug reimbursement registry was used to identify HT use from the year 1994.
Findings. Women diagnosed with AD were more likely to have been current or former users of systemic HT than controls (18.6% vs 17.0%, P<.001). The odds ratios (ORs) for AD were 1.09 for the estradiol-only group and 1.17 for the estrogen-progestin group (P<.05 for both comparisons).
Initiation of HT prior to age 60 was less common among AD cases than controls (P = .006). As a continuous variable, age was not a determinant for disease risk in estradiol-only users (OR, 1.0), estrogen-progestin users (OR, 1.0), or any HT use (OR, 1.0).
The exclusive use of vaginal estrogen therapy was not associated with an elevated risk of AD (OR, 0.99).
Study strengths and limitations
This study on the association between HT and AD included a very large number of participants from a national population registry, and the use of HT was objectively determined from a controlled registry (not self-reported). In addition, AD was accurately diagnosed and differentiated from other forms of dementia.
Limitations of the study include the lack of baseline demographic data for AD risk factors for both HT users and controls. Further, an increased risk of AD may have been a cause for HT use and not a consequence, given that initial cognitive impairments may occur 7 to 8 years prior to AD diagnosis and the possibility exists that such women may have sought help for cognitive symptoms from HT. In addition, the lack of brain imaging or neurologic examination to exclude AD might also account for undiagnosed disease in controls. The authors noted that they were unable to compare the use of oral and transdermal HT preparations or the use of cyclic and continuous estrogen-progestin therapy.
Alzheimer disease is more prevalent in women, and women are more likely to be caregivers for individuals with AD than men, making AD an issue of particular concern to midlife and older women. Current guidance from The North American Menopause Society and other organizations does not recommend use of systemic HT to prevent AD.1 As Savolainen-Peltonen and colleagues note in their observational study, the small risk increases for AD with use of HT are subject to bias. Editorialists agree with this concern and point out that a conclusive large randomized trial assessing HT's impact on AD is unlikely to be performed.2 I agree with the editorialists that the findings of this Finnish study should not change current practice. For recently menopausal women who have bothersome vasomotor symptoms and no contraindications, I will continue to counsel that initiating systemic HT is appropriate.
ANDREW M. KAUNITZ, MD
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Maki PM, Girard LM, Manson JE. Menopausal hormone therapy and cognition. BMJ. 2019;364:1877.