User login
No falls, fractures, or bone density benefits from vitamin D supplements
There is little justification for the use of vitamin D supplementation for the prevention of fractures or falls or for increasing bone density, according to the authors of a meta-analysis that found no benefits from supplementation.
A systematic review and meta-analysis, published in the Oct. 4 edition of Lancet Diabetes & Endocrinology, examined 81 randomized controlled trials – involving 53,537 participants – of the effects of vitamin D supplementation on fractures, falls, or bone mineral density.
In the pooled analyses, researchers found that vitamin D supplementation did not reduce total fracture, hip fracture, or falls, even in trials in which participants took doses greater than 800 IU/day. Their results were similar when researchers compared high doses and low doses in their trials.
Similarly, vitamin D supplementation was not associated with any clinically relevant improvements in bone mineral density at any site; lumbar spine, total hip, femoral neck, forearm, or total body.
Even a post hoc analysis of randomized, controlled trials that compared daily high doses with daily low doses, as well as trials that compared intermittent high doses with intermittent low doses found no significant interactions for any outcome.
The paper also explored whether baseline vitamin D levels might influence outcomes. Eighteen trials in the analysis reported the results of subgroup analyses using baseline serum 25-hydroxyvitamin D (25[OH]D); three found no effects of vitamin D supplements in different subgroups of baseline, five studies found no effects of subgroups or interaction with baseline serum 25[OH]D, and one found mixed effects with respect to falls.
The outcomes for bone mineral density, as related to baseline serum 25[OH]D, were slightly more mixed. One trial found a positive effect of vitamin D supplements a bone mineral density for different subgroups of baseline serum, five trials reported mixed effects, and eight trials found no effects.
“The strengths of the current analyses are that they are comprehensive, include all available data from a large number of new trials, and concomitantly assess the major clinical and surrogate endpoints for musculoskeletal health,” wrote Mark J. Bolland, MD, of the department of medicine at the University of Auckland (New Zealand), and his coauthors. “Therefore, there is little justification for the use of vitamin D supplements to maintain or improve musculoskeletal health, and clinical guidelines should reflect these findings.”
They also conducted trial sequential analyses, which is a type of cumulative meta-analysis. For each outcome, they set a relative risk reduction threshold, then progressively reduced that threshold until the optimum sample size for that threshold exceeded the actual sample size.
“The trial sequential analyses are important because they provide estimates about the reliability of current evidence and the likelihood of future trials to change current conclusions,” the authors wrote.
Using this approach, they once again found clear evidence that vitamin D supplementation did not reduce fractures or falls for any measure of relative risk reduction. For hip fracture, the trial sequential analysis even found some uncertainty as to whether vitamin D supplementation might increase the risk of hip fractures.
Given the results of the trial sequential analyses, the authors argued that further similar trials were unlikely to alter their conclusion.
“If a large future trial has markedly different results to the current trials, adding its results will substantially increase the heterogeneity of the trial results, which in turn will reduce the weighting the new large trial receives in the pooled analyses,” they wrote. “Thus, adding a positive result from a large randomized, controlled trial will have only a small effect on the pooled result and is unlikely to alter the conclusions of these meta-analyses.”
They also noted that some of the studies had methodological limitations, and smaller studies of shorter duration tended to have “inflated” effect sizes, such that “the results of small, short-duration studies should be interpreted very cautiously, since they might not be replicated in larger, longer studies.”
The study was funded by the Health Research Council of New Zealand. Two authors declared grants from the Health Research Council during the study, one author is a shareholder in a company that provides bone mineral density measurements, and one reported grants from the Scottish Government Health and Social Care Directorates during the study.
SOURCE: Bolland M et al. Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587(18)30265-1.
While there have been more than a dozen meta-analyses exploring the effect of vitamin D supplements on fractures, falls, and bone mineral density, this latest one incorporates a large amount of new research information. It also comes at a time when vitamin D often is touted as a cure-all, both in research and on social media.
One of the unanswered questions is that the majority of the daily treatment groups in the studies involved doses less than 1,000 IU per day, so serum 25-hydroxyvitamin D (25[OH]D) concentrations may not have reached the range of interest.
There are still likely to be questions about the extraskeletal benefits of vitamin D supplementation, which may be answered by large randomized, controlled trials currently underway that are expected to report in the next few years.
J. Chris Gallagher, MD, is a professor at the Creighton University Medical Center, Omaha. These comments are taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587[18]30269-9). No conflicts of interest were declared.
While there have been more than a dozen meta-analyses exploring the effect of vitamin D supplements on fractures, falls, and bone mineral density, this latest one incorporates a large amount of new research information. It also comes at a time when vitamin D often is touted as a cure-all, both in research and on social media.
One of the unanswered questions is that the majority of the daily treatment groups in the studies involved doses less than 1,000 IU per day, so serum 25-hydroxyvitamin D (25[OH]D) concentrations may not have reached the range of interest.
There are still likely to be questions about the extraskeletal benefits of vitamin D supplementation, which may be answered by large randomized, controlled trials currently underway that are expected to report in the next few years.
J. Chris Gallagher, MD, is a professor at the Creighton University Medical Center, Omaha. These comments are taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587[18]30269-9). No conflicts of interest were declared.
While there have been more than a dozen meta-analyses exploring the effect of vitamin D supplements on fractures, falls, and bone mineral density, this latest one incorporates a large amount of new research information. It also comes at a time when vitamin D often is touted as a cure-all, both in research and on social media.
One of the unanswered questions is that the majority of the daily treatment groups in the studies involved doses less than 1,000 IU per day, so serum 25-hydroxyvitamin D (25[OH]D) concentrations may not have reached the range of interest.
There are still likely to be questions about the extraskeletal benefits of vitamin D supplementation, which may be answered by large randomized, controlled trials currently underway that are expected to report in the next few years.
J. Chris Gallagher, MD, is a professor at the Creighton University Medical Center, Omaha. These comments are taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587[18]30269-9). No conflicts of interest were declared.
There is little justification for the use of vitamin D supplementation for the prevention of fractures or falls or for increasing bone density, according to the authors of a meta-analysis that found no benefits from supplementation.
A systematic review and meta-analysis, published in the Oct. 4 edition of Lancet Diabetes & Endocrinology, examined 81 randomized controlled trials – involving 53,537 participants – of the effects of vitamin D supplementation on fractures, falls, or bone mineral density.
In the pooled analyses, researchers found that vitamin D supplementation did not reduce total fracture, hip fracture, or falls, even in trials in which participants took doses greater than 800 IU/day. Their results were similar when researchers compared high doses and low doses in their trials.
Similarly, vitamin D supplementation was not associated with any clinically relevant improvements in bone mineral density at any site; lumbar spine, total hip, femoral neck, forearm, or total body.
Even a post hoc analysis of randomized, controlled trials that compared daily high doses with daily low doses, as well as trials that compared intermittent high doses with intermittent low doses found no significant interactions for any outcome.
The paper also explored whether baseline vitamin D levels might influence outcomes. Eighteen trials in the analysis reported the results of subgroup analyses using baseline serum 25-hydroxyvitamin D (25[OH]D); three found no effects of vitamin D supplements in different subgroups of baseline, five studies found no effects of subgroups or interaction with baseline serum 25[OH]D, and one found mixed effects with respect to falls.
The outcomes for bone mineral density, as related to baseline serum 25[OH]D, were slightly more mixed. One trial found a positive effect of vitamin D supplements a bone mineral density for different subgroups of baseline serum, five trials reported mixed effects, and eight trials found no effects.
“The strengths of the current analyses are that they are comprehensive, include all available data from a large number of new trials, and concomitantly assess the major clinical and surrogate endpoints for musculoskeletal health,” wrote Mark J. Bolland, MD, of the department of medicine at the University of Auckland (New Zealand), and his coauthors. “Therefore, there is little justification for the use of vitamin D supplements to maintain or improve musculoskeletal health, and clinical guidelines should reflect these findings.”
They also conducted trial sequential analyses, which is a type of cumulative meta-analysis. For each outcome, they set a relative risk reduction threshold, then progressively reduced that threshold until the optimum sample size for that threshold exceeded the actual sample size.
“The trial sequential analyses are important because they provide estimates about the reliability of current evidence and the likelihood of future trials to change current conclusions,” the authors wrote.
Using this approach, they once again found clear evidence that vitamin D supplementation did not reduce fractures or falls for any measure of relative risk reduction. For hip fracture, the trial sequential analysis even found some uncertainty as to whether vitamin D supplementation might increase the risk of hip fractures.
Given the results of the trial sequential analyses, the authors argued that further similar trials were unlikely to alter their conclusion.
“If a large future trial has markedly different results to the current trials, adding its results will substantially increase the heterogeneity of the trial results, which in turn will reduce the weighting the new large trial receives in the pooled analyses,” they wrote. “Thus, adding a positive result from a large randomized, controlled trial will have only a small effect on the pooled result and is unlikely to alter the conclusions of these meta-analyses.”
They also noted that some of the studies had methodological limitations, and smaller studies of shorter duration tended to have “inflated” effect sizes, such that “the results of small, short-duration studies should be interpreted very cautiously, since they might not be replicated in larger, longer studies.”
The study was funded by the Health Research Council of New Zealand. Two authors declared grants from the Health Research Council during the study, one author is a shareholder in a company that provides bone mineral density measurements, and one reported grants from the Scottish Government Health and Social Care Directorates during the study.
SOURCE: Bolland M et al. Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587(18)30265-1.
There is little justification for the use of vitamin D supplementation for the prevention of fractures or falls or for increasing bone density, according to the authors of a meta-analysis that found no benefits from supplementation.
A systematic review and meta-analysis, published in the Oct. 4 edition of Lancet Diabetes & Endocrinology, examined 81 randomized controlled trials – involving 53,537 participants – of the effects of vitamin D supplementation on fractures, falls, or bone mineral density.
In the pooled analyses, researchers found that vitamin D supplementation did not reduce total fracture, hip fracture, or falls, even in trials in which participants took doses greater than 800 IU/day. Their results were similar when researchers compared high doses and low doses in their trials.
Similarly, vitamin D supplementation was not associated with any clinically relevant improvements in bone mineral density at any site; lumbar spine, total hip, femoral neck, forearm, or total body.
Even a post hoc analysis of randomized, controlled trials that compared daily high doses with daily low doses, as well as trials that compared intermittent high doses with intermittent low doses found no significant interactions for any outcome.
The paper also explored whether baseline vitamin D levels might influence outcomes. Eighteen trials in the analysis reported the results of subgroup analyses using baseline serum 25-hydroxyvitamin D (25[OH]D); three found no effects of vitamin D supplements in different subgroups of baseline, five studies found no effects of subgroups or interaction with baseline serum 25[OH]D, and one found mixed effects with respect to falls.
The outcomes for bone mineral density, as related to baseline serum 25[OH]D, were slightly more mixed. One trial found a positive effect of vitamin D supplements a bone mineral density for different subgroups of baseline serum, five trials reported mixed effects, and eight trials found no effects.
“The strengths of the current analyses are that they are comprehensive, include all available data from a large number of new trials, and concomitantly assess the major clinical and surrogate endpoints for musculoskeletal health,” wrote Mark J. Bolland, MD, of the department of medicine at the University of Auckland (New Zealand), and his coauthors. “Therefore, there is little justification for the use of vitamin D supplements to maintain or improve musculoskeletal health, and clinical guidelines should reflect these findings.”
They also conducted trial sequential analyses, which is a type of cumulative meta-analysis. For each outcome, they set a relative risk reduction threshold, then progressively reduced that threshold until the optimum sample size for that threshold exceeded the actual sample size.
“The trial sequential analyses are important because they provide estimates about the reliability of current evidence and the likelihood of future trials to change current conclusions,” the authors wrote.
Using this approach, they once again found clear evidence that vitamin D supplementation did not reduce fractures or falls for any measure of relative risk reduction. For hip fracture, the trial sequential analysis even found some uncertainty as to whether vitamin D supplementation might increase the risk of hip fractures.
Given the results of the trial sequential analyses, the authors argued that further similar trials were unlikely to alter their conclusion.
“If a large future trial has markedly different results to the current trials, adding its results will substantially increase the heterogeneity of the trial results, which in turn will reduce the weighting the new large trial receives in the pooled analyses,” they wrote. “Thus, adding a positive result from a large randomized, controlled trial will have only a small effect on the pooled result and is unlikely to alter the conclusions of these meta-analyses.”
They also noted that some of the studies had methodological limitations, and smaller studies of shorter duration tended to have “inflated” effect sizes, such that “the results of small, short-duration studies should be interpreted very cautiously, since they might not be replicated in larger, longer studies.”
The study was funded by the Health Research Council of New Zealand. Two authors declared grants from the Health Research Council during the study, one author is a shareholder in a company that provides bone mineral density measurements, and one reported grants from the Scottish Government Health and Social Care Directorates during the study.
SOURCE: Bolland M et al. Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587(18)30265-1.
FROM LANCET DIABETES & ENDOCRINOLOGY
Key clinical point: Vitamin D does not reduce the risk of falls or fractures or to improve bone mineral density.
Major finding: Researchers found that vitamin D supplementation did not reduce total fracture, hip fracture, or falls, even in trials in which participants took doses greater than 800 IU/day.
Study details: Systematic review, meta-analysis, and trial sequential analysis of 81 randomized controlled trials of vitamin D supplementation.
Disclosures: The study was funded by the Health Research Council of New Zealand. Two authors declared grants from the Health Research Council during the study, one author is a shareholder in a company that provides bone mineral density measurements, and one reported grants from the Scottish Government Health and Social Care Directorates during the study.
Source: Bolland M et al. Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587(18)30265-1.
Zoledronate reduces fracture risk in elderly women with osteopenia
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
REPORTING FROM ASBMR
Key clinical point: Vertebral and nonvertebral fracture risk was significantly lower in osteopenic women who received zoledronate, compared with those who received a placebo.
Major finding: Fragility fractures occurred in 122 women in a zoledronate group and 190 women in a placebo group. The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
Study details: A 6-year randomized, double-blind trial of 2,000 women aged 65 years and older with osteopenia.
Disclosures: The study was supported in part by grants from the Health Research Council of New Zealand; Novartis provided the medication. Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
Source: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
Guidelines released for perimenopausal depression
and affect women with no previous symptoms of depression, according to recent guidelines on perimenopausal depression copublished in the Journal of Women’s Health and Menopause.
“Epidemiologic findings, animal data, and clinical observations have shed some light into plausible mechanistic hypotheses on why some, but not all, women may be particularly sensitive to changes in the hormonal milieu experienced premenstrually, during the postpartum period or during the menopause transition,” Pauline M. Maki, PhD, past president of the North American Menopause Society (NAMS) and professor of psychiatry and psychology at the University of Illinois at Chicago, and her colleagues wrote. “The notion of a menopause-associated depression, however, has been the focus of clinical and scientific debate for years. The lack of consensus on this issue has also led to a lack of clarity in how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.”
The guidelines were developed on behalf of the NAMS Board of Trustees and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Dr. Maki and her colleagues convened an 11-person expert panel on perimenopausal depression, which looked at the effects of factors such as epidemiology; clinical presentation; antidepressants; hormone therapy; and other therapies such as exercise, natural health products, and psychotherapy.
Most women who experience perimenopausal depression have previously undergone a major depressive episode (MDE), while major depressive disorder (MDD) onset at midlife is less common. However, even among women with no previous history of depression, the risk of perimenopausal depression – both depressive symptoms and MDE – is elevated for women at midlife. Studies suggest that 45%-68% of perimenopausal women have elevated depression symptoms.
Dr. Maki and her associates cited studies that showed women who underwent surgical menopause in the form of hysterectomy with and without oophorectomy and women with ovarian insufficiency also showed an elevated rate of depression.
Other risk factors for perimenopausal depression included sociodemographic (black race, financial difficulties) and psychosocial factors (adverse life events, low social support), anxiety, and menopausal symptoms such as interrupted sleep and vasomotor symptoms. Risk factors for MDD include use of antidepressants, premenstrual depressive symptoms, anxiety, menopausal sleep disturbance, sociodemographic factors such as high body mass index and black race, and psychosocial factors such as social isolation and upsetting life events.
Depressive symptoms in perimenopause present as classic depressive symptoms but may also be in combination with perimenopausal symptoms such as changes in weight, cognitive shifts, night sweats, hot flashes, and sexual and sleep disturbances. In addition, the stressors of life for women in midlife can further complicate depressive symptoms.
“Many women face a series of stressors including, but not exclusive to, caring for aging parents, death of parents, medical illness in self and family, adjusting to emotional and physical sequelae of surgical menopause and other health issues that are common to this stage of life, children leaving the home, and changes in marital status. With the onset of childbirth at an increasingly later age, women are often faced with the dual responsibility of raising young children amid caring for aging parents while navigating their careers and ensuing challenges,” Dr. Maki and her colleagues wrote. “These multiple demands are often faced without supports in place to identify or address the ensuing distress placed on a woman during this stage.”
Assessment and diagnosis should include factoring all these symptoms in and disentangling menopausal and psychiatric symptoms, evaluating women with past MDEs and MDD for a mood disorder, and use of differential diagnosis for psychiatric symptoms.
There is no menopause-specific mood disorder scale, Dr. Maki and her associates emphasized, but the Patient Health Questionnaire-9 can be used to categorize mood disorder diagnoses. There are “validated menopause symptom and health-related quality of life scales [that] include mood items” such as the Menopause Rating Scale, and the Menopause-Specific Quality of Life Scale.
Frontline treatment of MDE with traditional therapies such as antidepressants, cognitive behavioral therapy, and other psychotherapies is appropriate, while previous antidepressant trial and responses should be followed to find the best efficacy and tolerability for a women with a history of MDD. There is data on some SSRIs and serotonin norepinephrine reuptake inhibitors suggesting efficacy and tolerability at usual doses. Of note, Dr. Maki and her colleagues found estrogen therapy has some evidence for use as an antidepressant, but most studies on hormone therapy examined unopposed estrogen instead of estrogen plus progestogen, which has limited data.
The authors recommended exercise as a complement to psychotherapy and pharmacotherapies for perimenopausal women with depression, but said there is no available evidence to recommend “botanical or complementary/alternative approaches for treating depression related to the perimenopause.”
Several authors have reported honoraria, research support, consulting fees, and grants from numerous pharmaceutical companies, the National Pregnancy Registry for Atypical Antipsychotics; the Brain & Behavior Research Foundation; the Ontario Brain Institute; and the Ontario Ministry of Technology, Innovation, and Science. Six of the authors reported no relevant conflicts of interest.
SOURCE: Maki PM et al. J Womens Health. 2018 Sep 5. doi: 10.1089/jwh.2018.27099.mensocrec.
I think the authors of this paper did a beautiful job summarizing a decade or more of very good observational research and even some randomized, controlled trials on a complex topic. This paper is really important because it takes a large body of evidence on the topic and pulls it together in a coherent way by asking specific questions and then looking to the literature to address those questions. The team of 11 experts in the field – led by Dr. Maki, who is a past president of the North American Menopause Society and began this paper as her presidential project – deserves a lot of credit for doing a beautiful job addressing some important questions with the research that is already available.
There are many clinical implications in these guidelines for any provider who cares for women in their 40s and 50s, whether they are gynecologists, family physicians, internists, psychiatrists, or psychologists. These health care practitioners need to be aware that this is a high-risk period for both depressive symptoms and major depression. The authors reported about one-third of premenopausal women complain of depressive symptoms, and yet, in those women experiencing perimenopause, that percentage is between 45% and 68%. Health care practitioners caring for women in this age group need to be aware of, and looking for, these symptoms so they can identify them, address them, let women know that they’re common at this time, and help them get appropriate treatment.
The authors also looked at the literature on the impact of the menopausal transition on sleep and how that can affect depressive symptoms and major depression; it is important for health care providers to think about sleep disruption in women at this age. The domino hypothesis, the theory that hot flashes can lead to sleep disruption that then leads to depressive symptoms of the menopause transition, was examined in a literature review. The authors found some of the literature shows that these symptoms are separate from hot flashes.
Menopausal transition and the association with symptoms of depression is not only looking at hormonal fluctuations but also recognizing this is a time of extraordinary psychosocial and physical change for women. They may have responsibilities for their partners and children as well as for aging parents. They may have their own health problems and the health problems of their partner to handle. Career changes may be happening at this time. This is a very complex psychosocial time in women’s lives that may be complicated by other health issues occurring at the same time.
Jan Leslie Shifren, MD , is director of the Midlife Women’s Health Center in the department of obstetrics and gynecology at Massachusetts General Hospital, Boston. She also is an Ob.Gyn. News editorial board member. Dr. Shifren reported no relevant conflicts of interest.
I think the authors of this paper did a beautiful job summarizing a decade or more of very good observational research and even some randomized, controlled trials on a complex topic. This paper is really important because it takes a large body of evidence on the topic and pulls it together in a coherent way by asking specific questions and then looking to the literature to address those questions. The team of 11 experts in the field – led by Dr. Maki, who is a past president of the North American Menopause Society and began this paper as her presidential project – deserves a lot of credit for doing a beautiful job addressing some important questions with the research that is already available.
There are many clinical implications in these guidelines for any provider who cares for women in their 40s and 50s, whether they are gynecologists, family physicians, internists, psychiatrists, or psychologists. These health care practitioners need to be aware that this is a high-risk period for both depressive symptoms and major depression. The authors reported about one-third of premenopausal women complain of depressive symptoms, and yet, in those women experiencing perimenopause, that percentage is between 45% and 68%. Health care practitioners caring for women in this age group need to be aware of, and looking for, these symptoms so they can identify them, address them, let women know that they’re common at this time, and help them get appropriate treatment.
The authors also looked at the literature on the impact of the menopausal transition on sleep and how that can affect depressive symptoms and major depression; it is important for health care providers to think about sleep disruption in women at this age. The domino hypothesis, the theory that hot flashes can lead to sleep disruption that then leads to depressive symptoms of the menopause transition, was examined in a literature review. The authors found some of the literature shows that these symptoms are separate from hot flashes.
Menopausal transition and the association with symptoms of depression is not only looking at hormonal fluctuations but also recognizing this is a time of extraordinary psychosocial and physical change for women. They may have responsibilities for their partners and children as well as for aging parents. They may have their own health problems and the health problems of their partner to handle. Career changes may be happening at this time. This is a very complex psychosocial time in women’s lives that may be complicated by other health issues occurring at the same time.
Jan Leslie Shifren, MD , is director of the Midlife Women’s Health Center in the department of obstetrics and gynecology at Massachusetts General Hospital, Boston. She also is an Ob.Gyn. News editorial board member. Dr. Shifren reported no relevant conflicts of interest.
I think the authors of this paper did a beautiful job summarizing a decade or more of very good observational research and even some randomized, controlled trials on a complex topic. This paper is really important because it takes a large body of evidence on the topic and pulls it together in a coherent way by asking specific questions and then looking to the literature to address those questions. The team of 11 experts in the field – led by Dr. Maki, who is a past president of the North American Menopause Society and began this paper as her presidential project – deserves a lot of credit for doing a beautiful job addressing some important questions with the research that is already available.
There are many clinical implications in these guidelines for any provider who cares for women in their 40s and 50s, whether they are gynecologists, family physicians, internists, psychiatrists, or psychologists. These health care practitioners need to be aware that this is a high-risk period for both depressive symptoms and major depression. The authors reported about one-third of premenopausal women complain of depressive symptoms, and yet, in those women experiencing perimenopause, that percentage is between 45% and 68%. Health care practitioners caring for women in this age group need to be aware of, and looking for, these symptoms so they can identify them, address them, let women know that they’re common at this time, and help them get appropriate treatment.
The authors also looked at the literature on the impact of the menopausal transition on sleep and how that can affect depressive symptoms and major depression; it is important for health care providers to think about sleep disruption in women at this age. The domino hypothesis, the theory that hot flashes can lead to sleep disruption that then leads to depressive symptoms of the menopause transition, was examined in a literature review. The authors found some of the literature shows that these symptoms are separate from hot flashes.
Menopausal transition and the association with symptoms of depression is not only looking at hormonal fluctuations but also recognizing this is a time of extraordinary psychosocial and physical change for women. They may have responsibilities for their partners and children as well as for aging parents. They may have their own health problems and the health problems of their partner to handle. Career changes may be happening at this time. This is a very complex psychosocial time in women’s lives that may be complicated by other health issues occurring at the same time.
Jan Leslie Shifren, MD , is director of the Midlife Women’s Health Center in the department of obstetrics and gynecology at Massachusetts General Hospital, Boston. She also is an Ob.Gyn. News editorial board member. Dr. Shifren reported no relevant conflicts of interest.
and affect women with no previous symptoms of depression, according to recent guidelines on perimenopausal depression copublished in the Journal of Women’s Health and Menopause.
“Epidemiologic findings, animal data, and clinical observations have shed some light into plausible mechanistic hypotheses on why some, but not all, women may be particularly sensitive to changes in the hormonal milieu experienced premenstrually, during the postpartum period or during the menopause transition,” Pauline M. Maki, PhD, past president of the North American Menopause Society (NAMS) and professor of psychiatry and psychology at the University of Illinois at Chicago, and her colleagues wrote. “The notion of a menopause-associated depression, however, has been the focus of clinical and scientific debate for years. The lack of consensus on this issue has also led to a lack of clarity in how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.”
The guidelines were developed on behalf of the NAMS Board of Trustees and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Dr. Maki and her colleagues convened an 11-person expert panel on perimenopausal depression, which looked at the effects of factors such as epidemiology; clinical presentation; antidepressants; hormone therapy; and other therapies such as exercise, natural health products, and psychotherapy.
Most women who experience perimenopausal depression have previously undergone a major depressive episode (MDE), while major depressive disorder (MDD) onset at midlife is less common. However, even among women with no previous history of depression, the risk of perimenopausal depression – both depressive symptoms and MDE – is elevated for women at midlife. Studies suggest that 45%-68% of perimenopausal women have elevated depression symptoms.
Dr. Maki and her associates cited studies that showed women who underwent surgical menopause in the form of hysterectomy with and without oophorectomy and women with ovarian insufficiency also showed an elevated rate of depression.
Other risk factors for perimenopausal depression included sociodemographic (black race, financial difficulties) and psychosocial factors (adverse life events, low social support), anxiety, and menopausal symptoms such as interrupted sleep and vasomotor symptoms. Risk factors for MDD include use of antidepressants, premenstrual depressive symptoms, anxiety, menopausal sleep disturbance, sociodemographic factors such as high body mass index and black race, and psychosocial factors such as social isolation and upsetting life events.
Depressive symptoms in perimenopause present as classic depressive symptoms but may also be in combination with perimenopausal symptoms such as changes in weight, cognitive shifts, night sweats, hot flashes, and sexual and sleep disturbances. In addition, the stressors of life for women in midlife can further complicate depressive symptoms.
“Many women face a series of stressors including, but not exclusive to, caring for aging parents, death of parents, medical illness in self and family, adjusting to emotional and physical sequelae of surgical menopause and other health issues that are common to this stage of life, children leaving the home, and changes in marital status. With the onset of childbirth at an increasingly later age, women are often faced with the dual responsibility of raising young children amid caring for aging parents while navigating their careers and ensuing challenges,” Dr. Maki and her colleagues wrote. “These multiple demands are often faced without supports in place to identify or address the ensuing distress placed on a woman during this stage.”
Assessment and diagnosis should include factoring all these symptoms in and disentangling menopausal and psychiatric symptoms, evaluating women with past MDEs and MDD for a mood disorder, and use of differential diagnosis for psychiatric symptoms.
There is no menopause-specific mood disorder scale, Dr. Maki and her associates emphasized, but the Patient Health Questionnaire-9 can be used to categorize mood disorder diagnoses. There are “validated menopause symptom and health-related quality of life scales [that] include mood items” such as the Menopause Rating Scale, and the Menopause-Specific Quality of Life Scale.
Frontline treatment of MDE with traditional therapies such as antidepressants, cognitive behavioral therapy, and other psychotherapies is appropriate, while previous antidepressant trial and responses should be followed to find the best efficacy and tolerability for a women with a history of MDD. There is data on some SSRIs and serotonin norepinephrine reuptake inhibitors suggesting efficacy and tolerability at usual doses. Of note, Dr. Maki and her colleagues found estrogen therapy has some evidence for use as an antidepressant, but most studies on hormone therapy examined unopposed estrogen instead of estrogen plus progestogen, which has limited data.
The authors recommended exercise as a complement to psychotherapy and pharmacotherapies for perimenopausal women with depression, but said there is no available evidence to recommend “botanical or complementary/alternative approaches for treating depression related to the perimenopause.”
Several authors have reported honoraria, research support, consulting fees, and grants from numerous pharmaceutical companies, the National Pregnancy Registry for Atypical Antipsychotics; the Brain & Behavior Research Foundation; the Ontario Brain Institute; and the Ontario Ministry of Technology, Innovation, and Science. Six of the authors reported no relevant conflicts of interest.
SOURCE: Maki PM et al. J Womens Health. 2018 Sep 5. doi: 10.1089/jwh.2018.27099.mensocrec.
and affect women with no previous symptoms of depression, according to recent guidelines on perimenopausal depression copublished in the Journal of Women’s Health and Menopause.
“Epidemiologic findings, animal data, and clinical observations have shed some light into plausible mechanistic hypotheses on why some, but not all, women may be particularly sensitive to changes in the hormonal milieu experienced premenstrually, during the postpartum period or during the menopause transition,” Pauline M. Maki, PhD, past president of the North American Menopause Society (NAMS) and professor of psychiatry and psychology at the University of Illinois at Chicago, and her colleagues wrote. “The notion of a menopause-associated depression, however, has been the focus of clinical and scientific debate for years. The lack of consensus on this issue has also led to a lack of clarity in how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.”
The guidelines were developed on behalf of the NAMS Board of Trustees and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Dr. Maki and her colleagues convened an 11-person expert panel on perimenopausal depression, which looked at the effects of factors such as epidemiology; clinical presentation; antidepressants; hormone therapy; and other therapies such as exercise, natural health products, and psychotherapy.
Most women who experience perimenopausal depression have previously undergone a major depressive episode (MDE), while major depressive disorder (MDD) onset at midlife is less common. However, even among women with no previous history of depression, the risk of perimenopausal depression – both depressive symptoms and MDE – is elevated for women at midlife. Studies suggest that 45%-68% of perimenopausal women have elevated depression symptoms.
Dr. Maki and her associates cited studies that showed women who underwent surgical menopause in the form of hysterectomy with and without oophorectomy and women with ovarian insufficiency also showed an elevated rate of depression.
Other risk factors for perimenopausal depression included sociodemographic (black race, financial difficulties) and psychosocial factors (adverse life events, low social support), anxiety, and menopausal symptoms such as interrupted sleep and vasomotor symptoms. Risk factors for MDD include use of antidepressants, premenstrual depressive symptoms, anxiety, menopausal sleep disturbance, sociodemographic factors such as high body mass index and black race, and psychosocial factors such as social isolation and upsetting life events.
Depressive symptoms in perimenopause present as classic depressive symptoms but may also be in combination with perimenopausal symptoms such as changes in weight, cognitive shifts, night sweats, hot flashes, and sexual and sleep disturbances. In addition, the stressors of life for women in midlife can further complicate depressive symptoms.
“Many women face a series of stressors including, but not exclusive to, caring for aging parents, death of parents, medical illness in self and family, adjusting to emotional and physical sequelae of surgical menopause and other health issues that are common to this stage of life, children leaving the home, and changes in marital status. With the onset of childbirth at an increasingly later age, women are often faced with the dual responsibility of raising young children amid caring for aging parents while navigating their careers and ensuing challenges,” Dr. Maki and her colleagues wrote. “These multiple demands are often faced without supports in place to identify or address the ensuing distress placed on a woman during this stage.”
Assessment and diagnosis should include factoring all these symptoms in and disentangling menopausal and psychiatric symptoms, evaluating women with past MDEs and MDD for a mood disorder, and use of differential diagnosis for psychiatric symptoms.
There is no menopause-specific mood disorder scale, Dr. Maki and her associates emphasized, but the Patient Health Questionnaire-9 can be used to categorize mood disorder diagnoses. There are “validated menopause symptom and health-related quality of life scales [that] include mood items” such as the Menopause Rating Scale, and the Menopause-Specific Quality of Life Scale.
Frontline treatment of MDE with traditional therapies such as antidepressants, cognitive behavioral therapy, and other psychotherapies is appropriate, while previous antidepressant trial and responses should be followed to find the best efficacy and tolerability for a women with a history of MDD. There is data on some SSRIs and serotonin norepinephrine reuptake inhibitors suggesting efficacy and tolerability at usual doses. Of note, Dr. Maki and her colleagues found estrogen therapy has some evidence for use as an antidepressant, but most studies on hormone therapy examined unopposed estrogen instead of estrogen plus progestogen, which has limited data.
The authors recommended exercise as a complement to psychotherapy and pharmacotherapies for perimenopausal women with depression, but said there is no available evidence to recommend “botanical or complementary/alternative approaches for treating depression related to the perimenopause.”
Several authors have reported honoraria, research support, consulting fees, and grants from numerous pharmaceutical companies, the National Pregnancy Registry for Atypical Antipsychotics; the Brain & Behavior Research Foundation; the Ontario Brain Institute; and the Ontario Ministry of Technology, Innovation, and Science. Six of the authors reported no relevant conflicts of interest.
SOURCE: Maki PM et al. J Womens Health. 2018 Sep 5. doi: 10.1089/jwh.2018.27099.mensocrec.
FROM THE JOURNAL OF WOMEN’S HEALTH
What works best for genitourinary syndrome of menopause: vaginal estrogen, vaginal laser, or combined laser and estrogen therapy?
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
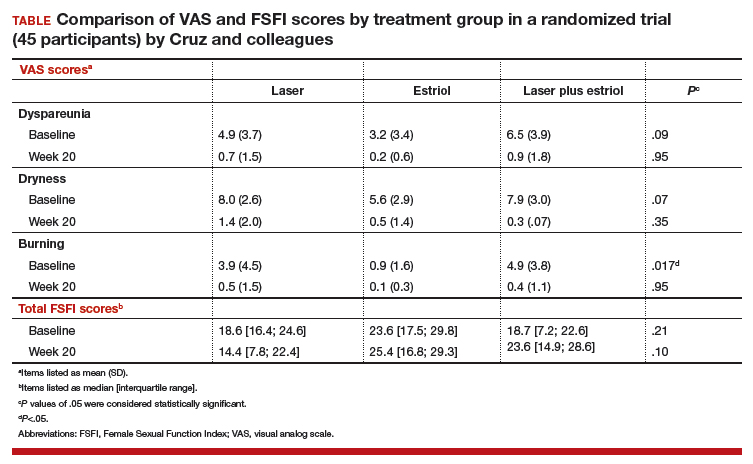
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
Migraine and menopause: Longitudinal study shows what to expect
SAN FRANCISCO – What can women with migraine expect during the menopausal transition?
About 60% will experience a change in their headache pattern. And for 60% of that group, it’s a change for the worse, Yu-Chen Cheng, MD, reported at the annual meeting of the American Headache Society.
She presented a retrospective longitudinal study of 60 women with a preexisting history of migraine who were followed through the menopausal transition. All had long-term medical records available, including brain imaging results and hormonal laboratory data.
The impetus for the study was the fact that even though three-quarters of America’s estimated 38 million migraineurs are women, all of whom will eventually undergo menopause, the question of what happens to them headache-wise as they go through this process of permanent cessation of ovarian function has received little research attention.
“This longitudinal study addresses the pattern of change of migraine during menopausal transition, an important but underestimated and undermanaged issue. We need more awareness of this. We hope in the future that physicians can pay more attention to this and provide better treatment for our patients with impaired quality of life,” said Dr. Cheng, a neurologist and postdoctoral fellow at Massachusetts General Hospital and Harvard Medical School, Boston.
Of the 35 women who experienced a change in their migraine attacks in association with menopause, the change occurred perimenopausally or postmenopausally – that is, after the final menstrual period – in 84% of cases. Premenopausal change in migraine in women who hadn’t yet missed a menstrual period in the past 12 months was a less frequent event.
No significant demographic differences existed between the 35 women with migraine change during the menopausal transition and the 25 women whose headache pattern remained stable. However, there were significant differences between the two groups in terms of the change over time in serum estradiol and follicle-stimulating hormone (FSH) levels. The median estradiol level in women whose migraine pattern remained stable went from 29 pg/mL premenopausally to 16.5 pg/mL post menopause, a statistically nonsignificant difference. In contrast, the median estradiol in women who experienced a change in migraine pattern dropped from 52.6 pg/mL premenopausally to 22.5 pg/mL post menopause, which was a significant difference.
Similarly, the pre- to postmenopause change in median FSH from 38.6 to 62.8 IU/L in the stable migraine group didn’t attain statistical significance, while the bigger shift in the migraine change group – from 13.5 IU/L premenopausally to 62.2 IU/L post menopause, was statistically significant.
“So we can say there’s a greater hormonal change in the migraine change group for women in the menopausal transition,” the neurologist said. “This suggests the possibility that a significant steep decline in estradiol level may stimulate migraine change.”
Brain imaging findings in the two groups were similar: Nearly two-thirds of women in both groups had normal brain MRI results, while the rest had nonspecific findings.
Several female headache specialists in the audience rose to thank Dr. Cheng for shining new light on a major understudied issue with far-reaching quality-of-life implications. Could hormone replacement therapy possibly prevent worsening of migraine attacks in association with menopause? she was asked.
Dr. Cheng noted that hormone replacement therapy was used by about two-thirds of women whose migraines remained stable and a similar proportion of those whose headaches changed. But the study wasn’t designed or sized to examine any possible migraine-preventive effect of hormone therapy. That would properly be addressed in a large prospective study. Anecdotally, however, it has been her clinical impression as well as that of some of her fellow neurologists at Massachusetts General that hormone replacement therapy does seem to protect against worsening migraine attacks in menopause, she added.
Dr. Cheng reported having no financial conflicts regarding her National Institutes of Health–funded study.
SOURCE: Cheng Y-C and Maleki N. Headache. 2018;58:71. Abstract OR16.
SAN FRANCISCO – What can women with migraine expect during the menopausal transition?
About 60% will experience a change in their headache pattern. And for 60% of that group, it’s a change for the worse, Yu-Chen Cheng, MD, reported at the annual meeting of the American Headache Society.
She presented a retrospective longitudinal study of 60 women with a preexisting history of migraine who were followed through the menopausal transition. All had long-term medical records available, including brain imaging results and hormonal laboratory data.
The impetus for the study was the fact that even though three-quarters of America’s estimated 38 million migraineurs are women, all of whom will eventually undergo menopause, the question of what happens to them headache-wise as they go through this process of permanent cessation of ovarian function has received little research attention.
“This longitudinal study addresses the pattern of change of migraine during menopausal transition, an important but underestimated and undermanaged issue. We need more awareness of this. We hope in the future that physicians can pay more attention to this and provide better treatment for our patients with impaired quality of life,” said Dr. Cheng, a neurologist and postdoctoral fellow at Massachusetts General Hospital and Harvard Medical School, Boston.
Of the 35 women who experienced a change in their migraine attacks in association with menopause, the change occurred perimenopausally or postmenopausally – that is, after the final menstrual period – in 84% of cases. Premenopausal change in migraine in women who hadn’t yet missed a menstrual period in the past 12 months was a less frequent event.
No significant demographic differences existed between the 35 women with migraine change during the menopausal transition and the 25 women whose headache pattern remained stable. However, there were significant differences between the two groups in terms of the change over time in serum estradiol and follicle-stimulating hormone (FSH) levels. The median estradiol level in women whose migraine pattern remained stable went from 29 pg/mL premenopausally to 16.5 pg/mL post menopause, a statistically nonsignificant difference. In contrast, the median estradiol in women who experienced a change in migraine pattern dropped from 52.6 pg/mL premenopausally to 22.5 pg/mL post menopause, which was a significant difference.
Similarly, the pre- to postmenopause change in median FSH from 38.6 to 62.8 IU/L in the stable migraine group didn’t attain statistical significance, while the bigger shift in the migraine change group – from 13.5 IU/L premenopausally to 62.2 IU/L post menopause, was statistically significant.
“So we can say there’s a greater hormonal change in the migraine change group for women in the menopausal transition,” the neurologist said. “This suggests the possibility that a significant steep decline in estradiol level may stimulate migraine change.”
Brain imaging findings in the two groups were similar: Nearly two-thirds of women in both groups had normal brain MRI results, while the rest had nonspecific findings.
Several female headache specialists in the audience rose to thank Dr. Cheng for shining new light on a major understudied issue with far-reaching quality-of-life implications. Could hormone replacement therapy possibly prevent worsening of migraine attacks in association with menopause? she was asked.
Dr. Cheng noted that hormone replacement therapy was used by about two-thirds of women whose migraines remained stable and a similar proportion of those whose headaches changed. But the study wasn’t designed or sized to examine any possible migraine-preventive effect of hormone therapy. That would properly be addressed in a large prospective study. Anecdotally, however, it has been her clinical impression as well as that of some of her fellow neurologists at Massachusetts General that hormone replacement therapy does seem to protect against worsening migraine attacks in menopause, she added.
Dr. Cheng reported having no financial conflicts regarding her National Institutes of Health–funded study.
SOURCE: Cheng Y-C and Maleki N. Headache. 2018;58:71. Abstract OR16.
SAN FRANCISCO – What can women with migraine expect during the menopausal transition?
About 60% will experience a change in their headache pattern. And for 60% of that group, it’s a change for the worse, Yu-Chen Cheng, MD, reported at the annual meeting of the American Headache Society.
She presented a retrospective longitudinal study of 60 women with a preexisting history of migraine who were followed through the menopausal transition. All had long-term medical records available, including brain imaging results and hormonal laboratory data.
The impetus for the study was the fact that even though three-quarters of America’s estimated 38 million migraineurs are women, all of whom will eventually undergo menopause, the question of what happens to them headache-wise as they go through this process of permanent cessation of ovarian function has received little research attention.
“This longitudinal study addresses the pattern of change of migraine during menopausal transition, an important but underestimated and undermanaged issue. We need more awareness of this. We hope in the future that physicians can pay more attention to this and provide better treatment for our patients with impaired quality of life,” said Dr. Cheng, a neurologist and postdoctoral fellow at Massachusetts General Hospital and Harvard Medical School, Boston.
Of the 35 women who experienced a change in their migraine attacks in association with menopause, the change occurred perimenopausally or postmenopausally – that is, after the final menstrual period – in 84% of cases. Premenopausal change in migraine in women who hadn’t yet missed a menstrual period in the past 12 months was a less frequent event.
No significant demographic differences existed between the 35 women with migraine change during the menopausal transition and the 25 women whose headache pattern remained stable. However, there were significant differences between the two groups in terms of the change over time in serum estradiol and follicle-stimulating hormone (FSH) levels. The median estradiol level in women whose migraine pattern remained stable went from 29 pg/mL premenopausally to 16.5 pg/mL post menopause, a statistically nonsignificant difference. In contrast, the median estradiol in women who experienced a change in migraine pattern dropped from 52.6 pg/mL premenopausally to 22.5 pg/mL post menopause, which was a significant difference.
Similarly, the pre- to postmenopause change in median FSH from 38.6 to 62.8 IU/L in the stable migraine group didn’t attain statistical significance, while the bigger shift in the migraine change group – from 13.5 IU/L premenopausally to 62.2 IU/L post menopause, was statistically significant.
“So we can say there’s a greater hormonal change in the migraine change group for women in the menopausal transition,” the neurologist said. “This suggests the possibility that a significant steep decline in estradiol level may stimulate migraine change.”
Brain imaging findings in the two groups were similar: Nearly two-thirds of women in both groups had normal brain MRI results, while the rest had nonspecific findings.
Several female headache specialists in the audience rose to thank Dr. Cheng for shining new light on a major understudied issue with far-reaching quality-of-life implications. Could hormone replacement therapy possibly prevent worsening of migraine attacks in association with menopause? she was asked.
Dr. Cheng noted that hormone replacement therapy was used by about two-thirds of women whose migraines remained stable and a similar proportion of those whose headaches changed. But the study wasn’t designed or sized to examine any possible migraine-preventive effect of hormone therapy. That would properly be addressed in a large prospective study. Anecdotally, however, it has been her clinical impression as well as that of some of her fellow neurologists at Massachusetts General that hormone replacement therapy does seem to protect against worsening migraine attacks in menopause, she added.
Dr. Cheng reported having no financial conflicts regarding her National Institutes of Health–funded study.
SOURCE: Cheng Y-C and Maleki N. Headache. 2018;58:71. Abstract OR16.
REPORTING FROM THE AHS ANNUAL MEETING
Key clinical point: For migraineurs, the menopausal transition is a time of change in headache pattern, often for the worse.
Major finding: Sixty percent of migraineurs experienced a change in headache pattern during the menopausal transition, and for 60% of them it involved worsening migraine intensity and/or frequency.
Study details: This retrospective longitudinal study followed 60 women with migraine before and through the menopausal transition.
Disclosures: The presenter reported having no financial conflicts regarding her National Institutes of Health–funded study.
Source: Cheng Y-C and Maleki N. Headache. 2018;58:71. Abstract OR16.
Postmenopausal estrogen use down since 2006
according to a commercial database with prescription claims for more than 12 million women.
The prevalence of prescriptions for noncontraceptive oral estrogen dropped from 83 per 1,000 women in 2007 to 42 per 1,000 in 2015 for women aged 50 years and older, Joel L. Weissfeld, MD, MPH, of the Office of Surveillance and Epidemiology at the Center for Drug Evaluation and Research at the Food and Drug Administration, and his associates reported based on data from IQVIA Real World Data Adjudicated Claims – U.S. Database.
Prescriptions for vaginal forms (creams, rings, and inserts) rose from 27 per 1,000 women in 2006 to 42 per 1,000 in 2011 but declined to 35 per 1,000 by 2017, the investigators reported in Menopause.
For women aged 50-54 years, use of vaginal rings and inserts was steady for 2 years and then dropped every year after 2009, with prevalence lower in 2017 than in 2006. Vaginal ring/insert use increased for the first 2 years among those aged 55-59 years and for the first 5 years for those aged 60-64 years, but then each group started a fairly rapid and ongoing decline that had 2017 levels below those in 2006. Among women aged 65 years and older, however, prevalence of ring/insert use rose for most of the study period, and the declines left prevalences for 2015 above those for 2006, Dr. Weissfeld and his associates wrote.
The data source couldn’t provide reasons for use of vaginal rings or inserts, but the researchers noted that it’s possible that use for vasomotor symptoms “predominated in younger (aged 50-59 years) women closer in age to the onset of menopause. We presume that use for [vulvar and vaginal atrophy] predominated in women 60-65 years of age and older, women who are more distant in age from onset of menopause.”
Trends for use of transdermal patches also varied by age group but with smaller levels of difference. Use among women aged 50-54 years and 55-59 years fluctuated but showed no overall change. Those aged 60-64 years had a gradual decline over the study period, those aged 70-74 years had an initial increase in 2007 and then a decline, and those aged 75 years or older had an increase that lasted until 2011 before use started to fall, they wrote.
SOURCE: Weissfeld JL et al. Menopause. 2018;25(6):611-14.
according to a commercial database with prescription claims for more than 12 million women.
The prevalence of prescriptions for noncontraceptive oral estrogen dropped from 83 per 1,000 women in 2007 to 42 per 1,000 in 2015 for women aged 50 years and older, Joel L. Weissfeld, MD, MPH, of the Office of Surveillance and Epidemiology at the Center for Drug Evaluation and Research at the Food and Drug Administration, and his associates reported based on data from IQVIA Real World Data Adjudicated Claims – U.S. Database.
Prescriptions for vaginal forms (creams, rings, and inserts) rose from 27 per 1,000 women in 2006 to 42 per 1,000 in 2011 but declined to 35 per 1,000 by 2017, the investigators reported in Menopause.
For women aged 50-54 years, use of vaginal rings and inserts was steady for 2 years and then dropped every year after 2009, with prevalence lower in 2017 than in 2006. Vaginal ring/insert use increased for the first 2 years among those aged 55-59 years and for the first 5 years for those aged 60-64 years, but then each group started a fairly rapid and ongoing decline that had 2017 levels below those in 2006. Among women aged 65 years and older, however, prevalence of ring/insert use rose for most of the study period, and the declines left prevalences for 2015 above those for 2006, Dr. Weissfeld and his associates wrote.
The data source couldn’t provide reasons for use of vaginal rings or inserts, but the researchers noted that it’s possible that use for vasomotor symptoms “predominated in younger (aged 50-59 years) women closer in age to the onset of menopause. We presume that use for [vulvar and vaginal atrophy] predominated in women 60-65 years of age and older, women who are more distant in age from onset of menopause.”
Trends for use of transdermal patches also varied by age group but with smaller levels of difference. Use among women aged 50-54 years and 55-59 years fluctuated but showed no overall change. Those aged 60-64 years had a gradual decline over the study period, those aged 70-74 years had an initial increase in 2007 and then a decline, and those aged 75 years or older had an increase that lasted until 2011 before use started to fall, they wrote.
SOURCE: Weissfeld JL et al. Menopause. 2018;25(6):611-14.
according to a commercial database with prescription claims for more than 12 million women.
The prevalence of prescriptions for noncontraceptive oral estrogen dropped from 83 per 1,000 women in 2007 to 42 per 1,000 in 2015 for women aged 50 years and older, Joel L. Weissfeld, MD, MPH, of the Office of Surveillance and Epidemiology at the Center for Drug Evaluation and Research at the Food and Drug Administration, and his associates reported based on data from IQVIA Real World Data Adjudicated Claims – U.S. Database.
Prescriptions for vaginal forms (creams, rings, and inserts) rose from 27 per 1,000 women in 2006 to 42 per 1,000 in 2011 but declined to 35 per 1,000 by 2017, the investigators reported in Menopause.
For women aged 50-54 years, use of vaginal rings and inserts was steady for 2 years and then dropped every year after 2009, with prevalence lower in 2017 than in 2006. Vaginal ring/insert use increased for the first 2 years among those aged 55-59 years and for the first 5 years for those aged 60-64 years, but then each group started a fairly rapid and ongoing decline that had 2017 levels below those in 2006. Among women aged 65 years and older, however, prevalence of ring/insert use rose for most of the study period, and the declines left prevalences for 2015 above those for 2006, Dr. Weissfeld and his associates wrote.
The data source couldn’t provide reasons for use of vaginal rings or inserts, but the researchers noted that it’s possible that use for vasomotor symptoms “predominated in younger (aged 50-59 years) women closer in age to the onset of menopause. We presume that use for [vulvar and vaginal atrophy] predominated in women 60-65 years of age and older, women who are more distant in age from onset of menopause.”
Trends for use of transdermal patches also varied by age group but with smaller levels of difference. Use among women aged 50-54 years and 55-59 years fluctuated but showed no overall change. Those aged 60-64 years had a gradual decline over the study period, those aged 70-74 years had an initial increase in 2007 and then a decline, and those aged 75 years or older had an increase that lasted until 2011 before use started to fall, they wrote.
SOURCE: Weissfeld JL et al. Menopause. 2018;25(6):611-14.
FROM MENOPAUSE
A combination hormone capsule for vasomotor symptoms
A capsule containing a combination of 17-beta-estradiol and progesterone significantly improved vasomotor symptoms in menopausal women without causing a single case of endometrial hyperplasia.
The results of the 12-week REPLENISH study suggested that this preparation effectively treats vasomotor symptoms and could be a safe alternative to the popular, but unstudied, compounded bioidentical hormones that millions of women turned to after the Women’s Health Initiative study cast doubt on the safety of hormone therapy, Rogerio A. Lobo, MD, and his colleagues wrote in Obstetrics and Gynecology.
“17-beta-estradiol–progesterone may represent a new option, using natural hormones, for postmenopausal women, including the estimated millions currently using inadequately studied, non–FDA approved, compounded [hormone therapy],” wrote Dr. Lobo of Columbia University, New York.
REPLENISH randomized 1,845 postmenopausal women (mean age 55 years) to placebo or one of four active, daily, oral estradiol-progesterone doses (1 mg/100 mg, 0.5 mg/100 mg, 0.5 mg/50 mg, or 0.25 mg/50 mg). The primary safety outcome was endometrial hyperplasia. There were two primary efficacy endpoints: mean changes in frequency and severity of moderate to severe vasomotor symptoms from baseline at weeks 4 and 12.
There were no cases of endometrial hyperplasia with any estradiol-progesterone dose, nor were there any endometrial cancers. The rates of endometrial proliferation and endometrial polyps were low (about 3% each).
The frequency of vasomotor symptoms decreased significantly, compared with placebo, in all active groups. The severity of vasomotor symptoms also decreased significantly and in a dose-dependent manner. Onset of action was similarly dose-dependent, with the 1 mg/100 mg group experiencing a clinically meaningful benefit by week 3 and the 0.5 mg/50 mg group by week 6.
Adverse events were mild-moderate and included breast tenderness, headache, nausea, pelvic pain, vaginal bleeding, and vaginal discharge. Serious adverse events included acute pancreatitis, deep vein thrombosis (in a woman with prior left femoral popliteal bypass surgery and a family history of deep vein thrombosis), chronic obstructive pulmonary disease, infective cholecystitis, and breast cancer.
TherapeuticsMD sponsored the study; Dr. Lobo has received research grants from TherapeuticsMD and has served as a consultant for the company and several others. Some coauthors report additional research support from and consulting with TherapeuticsMD and other companies, and three coauthors are stock-holding employees of TherapeuticsMD.
SOURCE: Lobo RA et al. Obstet Gynecol. 2018 Jan;132(1):161-70.
A capsule containing a combination of 17-beta-estradiol and progesterone significantly improved vasomotor symptoms in menopausal women without causing a single case of endometrial hyperplasia.
The results of the 12-week REPLENISH study suggested that this preparation effectively treats vasomotor symptoms and could be a safe alternative to the popular, but unstudied, compounded bioidentical hormones that millions of women turned to after the Women’s Health Initiative study cast doubt on the safety of hormone therapy, Rogerio A. Lobo, MD, and his colleagues wrote in Obstetrics and Gynecology.
“17-beta-estradiol–progesterone may represent a new option, using natural hormones, for postmenopausal women, including the estimated millions currently using inadequately studied, non–FDA approved, compounded [hormone therapy],” wrote Dr. Lobo of Columbia University, New York.
REPLENISH randomized 1,845 postmenopausal women (mean age 55 years) to placebo or one of four active, daily, oral estradiol-progesterone doses (1 mg/100 mg, 0.5 mg/100 mg, 0.5 mg/50 mg, or 0.25 mg/50 mg). The primary safety outcome was endometrial hyperplasia. There were two primary efficacy endpoints: mean changes in frequency and severity of moderate to severe vasomotor symptoms from baseline at weeks 4 and 12.
There were no cases of endometrial hyperplasia with any estradiol-progesterone dose, nor were there any endometrial cancers. The rates of endometrial proliferation and endometrial polyps were low (about 3% each).
The frequency of vasomotor symptoms decreased significantly, compared with placebo, in all active groups. The severity of vasomotor symptoms also decreased significantly and in a dose-dependent manner. Onset of action was similarly dose-dependent, with the 1 mg/100 mg group experiencing a clinically meaningful benefit by week 3 and the 0.5 mg/50 mg group by week 6.
Adverse events were mild-moderate and included breast tenderness, headache, nausea, pelvic pain, vaginal bleeding, and vaginal discharge. Serious adverse events included acute pancreatitis, deep vein thrombosis (in a woman with prior left femoral popliteal bypass surgery and a family history of deep vein thrombosis), chronic obstructive pulmonary disease, infective cholecystitis, and breast cancer.
TherapeuticsMD sponsored the study; Dr. Lobo has received research grants from TherapeuticsMD and has served as a consultant for the company and several others. Some coauthors report additional research support from and consulting with TherapeuticsMD and other companies, and three coauthors are stock-holding employees of TherapeuticsMD.
SOURCE: Lobo RA et al. Obstet Gynecol. 2018 Jan;132(1):161-70.
A capsule containing a combination of 17-beta-estradiol and progesterone significantly improved vasomotor symptoms in menopausal women without causing a single case of endometrial hyperplasia.
The results of the 12-week REPLENISH study suggested that this preparation effectively treats vasomotor symptoms and could be a safe alternative to the popular, but unstudied, compounded bioidentical hormones that millions of women turned to after the Women’s Health Initiative study cast doubt on the safety of hormone therapy, Rogerio A. Lobo, MD, and his colleagues wrote in Obstetrics and Gynecology.
“17-beta-estradiol–progesterone may represent a new option, using natural hormones, for postmenopausal women, including the estimated millions currently using inadequately studied, non–FDA approved, compounded [hormone therapy],” wrote Dr. Lobo of Columbia University, New York.
REPLENISH randomized 1,845 postmenopausal women (mean age 55 years) to placebo or one of four active, daily, oral estradiol-progesterone doses (1 mg/100 mg, 0.5 mg/100 mg, 0.5 mg/50 mg, or 0.25 mg/50 mg). The primary safety outcome was endometrial hyperplasia. There were two primary efficacy endpoints: mean changes in frequency and severity of moderate to severe vasomotor symptoms from baseline at weeks 4 and 12.
There were no cases of endometrial hyperplasia with any estradiol-progesterone dose, nor were there any endometrial cancers. The rates of endometrial proliferation and endometrial polyps were low (about 3% each).
The frequency of vasomotor symptoms decreased significantly, compared with placebo, in all active groups. The severity of vasomotor symptoms also decreased significantly and in a dose-dependent manner. Onset of action was similarly dose-dependent, with the 1 mg/100 mg group experiencing a clinically meaningful benefit by week 3 and the 0.5 mg/50 mg group by week 6.
Adverse events were mild-moderate and included breast tenderness, headache, nausea, pelvic pain, vaginal bleeding, and vaginal discharge. Serious adverse events included acute pancreatitis, deep vein thrombosis (in a woman with prior left femoral popliteal bypass surgery and a family history of deep vein thrombosis), chronic obstructive pulmonary disease, infective cholecystitis, and breast cancer.
TherapeuticsMD sponsored the study; Dr. Lobo has received research grants from TherapeuticsMD and has served as a consultant for the company and several others. Some coauthors report additional research support from and consulting with TherapeuticsMD and other companies, and three coauthors are stock-holding employees of TherapeuticsMD.
SOURCE: Lobo RA et al. Obstet Gynecol. 2018 Jan;132(1):161-70.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: A 17-beta-estradiol–progesterone capsule significantly decreased the frequency and severity of vasomotor symptoms.
Major finding: The compound reduced vasomotor symptoms, with no cases of endometrial hyperplasia.
Study details: The study randomized 1,845 women to placebo or one of four active hormone doses.
Disclosures: TherapeuticsMD sponsored the study; Dr. Lobo is a consultant for the company.
Source: Lobo RA et al. Obstet Gynecol. 2018 Jan;132:161-70.
High testosterone in postmenopausal women may add CVD risk
In postmenopausal women, a higher level of testosterone in comparison to estrogen may increase the cardiovascular disease risk later in life, results of a recent analysis suggest.
A higher ratio of testosterone to estradiol was associated with the development of cardiovascular disease, coronary heart disease, and heart failure in postmenopausal women, according to results from an analysis based on 2,834 postmenopausal women in MESA (Multi-Ethnic Study of Atherosclerosis).
In addition, total testosterone levels were associated with increased cardiovascular disease and coronary heart disease, while estradiol is associated with a reduced risk of coronary heart disease and heart failure with reduced ejection fraction, investigators reported in the Journal of the American College of Cardiology.
Without any interventional studies as guidance, it’s not clear what the “best strategy” would be to modify sex hormone levels and reduce cardiovascular disease risk, wrote investigator Di Zhao, PhD, of the department of epidemiology at Johns Hopkins University Bloomberg School of Public Health, Baltimore, and her study coauthors.
“Nonetheless, a more androgenic sex hormone profile may identify a woman at higher risk for cardiovascular disease who may benefit from other risk-reducing strategies,” Dr. Zhao and her colleagues wrote in their report.
The postmenopausal women included in this analysis all had baseline measurements of testosterone, estradiol, dehydroepiandrosterone, and sex hormone–binding globulin levels between 2000 and 2002, according to the report.
After more than 12 years of follow-up, investigators found that a higher total testosterone to estradiol ratio was independently associated with an increased risk of incident cardiovascular disease (hazard ratio, 1.19; 95% confidence interval, 1.02-1.40), coronary heart disease (HR, 1.45; 95% CI, 1.19-1.78), and heart failure (HR, 1.31; 95% CI, 1.01-1.70).
Investigators also reported a statistically significant association between total testosterone levels and risk of cardiovascular disease and coronary heart disease but not risk of heart failure.
In a subgroup analysis of postmenopausal women with heart failure, investigators found a significant positive association with the testosterone to estradiol ratio (HR, 1.65; 95% CI, 1.07-2.54) and inverse associations for estradiol (HR, 0.60; 95% CI, 0.39-0.93) and for dehydroepiandrosterone (HR, 0.59; 95% CI, 0.44-0.78).
On their own, estradiol levels had no association with cardiovascular disease events overall, but they were associated with lower coronary heart disease risk, according to investigators. Estradiol also was associated with a lower heart failure risk, though the trend did not reach statistical significance.
The study was partially funded by the American Heart Association’s Go Red for Women Research Network. Study authors reported disclosures related to Cordex Systems, Siemens Diagnostics, and other entities.
SOURCE: Zhao D et al. J Am Coll Cardiol. 2018 Jun 5; 71:2555-66.
This study offers new insights into the relationships between endogenous hormones and how they influence cardiovascular event risk, according to Virginia M. Miller, PhD, and Rekha Mankad, MD.
Previous observational studies have established that decreases in endogenous estrogen led to increases in cardiovascular event risk and incidence, Dr. Miller and Dr. Mankad said in an editorial referencing the study.
What’s less clear is the role of testosterone in cardiovascular risk and event incidence, both by itself and in relation to other endogenous hormones.
“Few studies have gone beyond singular associations of hormone levels with cardiovascular events,” the editorial authors wrote in the Journal of the American College of Cardiology.
The study by Zhao and her colleagues is unique in part because their analysis considers the ratio of testosterone to estradiol. In particular, they found that a higher testosterone to estradiol level was associated with a higher incidence of cardiovascular events overall and of coronary heart disease.
What was unexpected, according to Dr. Miller and Dr. Mankad, was a U-shaped association with incident heart failure. In subgroup analysis, investigators found a positive association between testosterone to estradiol ratio and heart failure with reduced ejection fraction but not with heart failure with preserved ejection fraction.
“By addressing a set of defined incident events, this study provides new information needed to develop mechanistic hypotheses of causal relationships of hormones with specific aspects of cardiac function,” the editorial authors wrote.
Dr. Miller and Dr. Mankad are both with the women’s health research center and department of cardiovascular disease at the Women’s Heart Clinic at the Mayo Clinic, Rochester, Minn. These comments are derived from their editorial in the Journal of the American College of Cardiology. Dr. Mankad had no disclosures, while Dr. Miller reported support from a National Institutes of Health grant.
This study offers new insights into the relationships between endogenous hormones and how they influence cardiovascular event risk, according to Virginia M. Miller, PhD, and Rekha Mankad, MD.
Previous observational studies have established that decreases in endogenous estrogen led to increases in cardiovascular event risk and incidence, Dr. Miller and Dr. Mankad said in an editorial referencing the study.
What’s less clear is the role of testosterone in cardiovascular risk and event incidence, both by itself and in relation to other endogenous hormones.
“Few studies have gone beyond singular associations of hormone levels with cardiovascular events,” the editorial authors wrote in the Journal of the American College of Cardiology.
The study by Zhao and her colleagues is unique in part because their analysis considers the ratio of testosterone to estradiol. In particular, they found that a higher testosterone to estradiol level was associated with a higher incidence of cardiovascular events overall and of coronary heart disease.
What was unexpected, according to Dr. Miller and Dr. Mankad, was a U-shaped association with incident heart failure. In subgroup analysis, investigators found a positive association between testosterone to estradiol ratio and heart failure with reduced ejection fraction but not with heart failure with preserved ejection fraction.
“By addressing a set of defined incident events, this study provides new information needed to develop mechanistic hypotheses of causal relationships of hormones with specific aspects of cardiac function,” the editorial authors wrote.
Dr. Miller and Dr. Mankad are both with the women’s health research center and department of cardiovascular disease at the Women’s Heart Clinic at the Mayo Clinic, Rochester, Minn. These comments are derived from their editorial in the Journal of the American College of Cardiology. Dr. Mankad had no disclosures, while Dr. Miller reported support from a National Institutes of Health grant.
This study offers new insights into the relationships between endogenous hormones and how they influence cardiovascular event risk, according to Virginia M. Miller, PhD, and Rekha Mankad, MD.
Previous observational studies have established that decreases in endogenous estrogen led to increases in cardiovascular event risk and incidence, Dr. Miller and Dr. Mankad said in an editorial referencing the study.
What’s less clear is the role of testosterone in cardiovascular risk and event incidence, both by itself and in relation to other endogenous hormones.
“Few studies have gone beyond singular associations of hormone levels with cardiovascular events,” the editorial authors wrote in the Journal of the American College of Cardiology.
The study by Zhao and her colleagues is unique in part because their analysis considers the ratio of testosterone to estradiol. In particular, they found that a higher testosterone to estradiol level was associated with a higher incidence of cardiovascular events overall and of coronary heart disease.
What was unexpected, according to Dr. Miller and Dr. Mankad, was a U-shaped association with incident heart failure. In subgroup analysis, investigators found a positive association between testosterone to estradiol ratio and heart failure with reduced ejection fraction but not with heart failure with preserved ejection fraction.
“By addressing a set of defined incident events, this study provides new information needed to develop mechanistic hypotheses of causal relationships of hormones with specific aspects of cardiac function,” the editorial authors wrote.
Dr. Miller and Dr. Mankad are both with the women’s health research center and department of cardiovascular disease at the Women’s Heart Clinic at the Mayo Clinic, Rochester, Minn. These comments are derived from their editorial in the Journal of the American College of Cardiology. Dr. Mankad had no disclosures, while Dr. Miller reported support from a National Institutes of Health grant.
In postmenopausal women, a higher level of testosterone in comparison to estrogen may increase the cardiovascular disease risk later in life, results of a recent analysis suggest.
A higher ratio of testosterone to estradiol was associated with the development of cardiovascular disease, coronary heart disease, and heart failure in postmenopausal women, according to results from an analysis based on 2,834 postmenopausal women in MESA (Multi-Ethnic Study of Atherosclerosis).
In addition, total testosterone levels were associated with increased cardiovascular disease and coronary heart disease, while estradiol is associated with a reduced risk of coronary heart disease and heart failure with reduced ejection fraction, investigators reported in the Journal of the American College of Cardiology.
Without any interventional studies as guidance, it’s not clear what the “best strategy” would be to modify sex hormone levels and reduce cardiovascular disease risk, wrote investigator Di Zhao, PhD, of the department of epidemiology at Johns Hopkins University Bloomberg School of Public Health, Baltimore, and her study coauthors.
“Nonetheless, a more androgenic sex hormone profile may identify a woman at higher risk for cardiovascular disease who may benefit from other risk-reducing strategies,” Dr. Zhao and her colleagues wrote in their report.
The postmenopausal women included in this analysis all had baseline measurements of testosterone, estradiol, dehydroepiandrosterone, and sex hormone–binding globulin levels between 2000 and 2002, according to the report.
After more than 12 years of follow-up, investigators found that a higher total testosterone to estradiol ratio was independently associated with an increased risk of incident cardiovascular disease (hazard ratio, 1.19; 95% confidence interval, 1.02-1.40), coronary heart disease (HR, 1.45; 95% CI, 1.19-1.78), and heart failure (HR, 1.31; 95% CI, 1.01-1.70).
Investigators also reported a statistically significant association between total testosterone levels and risk of cardiovascular disease and coronary heart disease but not risk of heart failure.
In a subgroup analysis of postmenopausal women with heart failure, investigators found a significant positive association with the testosterone to estradiol ratio (HR, 1.65; 95% CI, 1.07-2.54) and inverse associations for estradiol (HR, 0.60; 95% CI, 0.39-0.93) and for dehydroepiandrosterone (HR, 0.59; 95% CI, 0.44-0.78).
On their own, estradiol levels had no association with cardiovascular disease events overall, but they were associated with lower coronary heart disease risk, according to investigators. Estradiol also was associated with a lower heart failure risk, though the trend did not reach statistical significance.
The study was partially funded by the American Heart Association’s Go Red for Women Research Network. Study authors reported disclosures related to Cordex Systems, Siemens Diagnostics, and other entities.
SOURCE: Zhao D et al. J Am Coll Cardiol. 2018 Jun 5; 71:2555-66.
In postmenopausal women, a higher level of testosterone in comparison to estrogen may increase the cardiovascular disease risk later in life, results of a recent analysis suggest.
A higher ratio of testosterone to estradiol was associated with the development of cardiovascular disease, coronary heart disease, and heart failure in postmenopausal women, according to results from an analysis based on 2,834 postmenopausal women in MESA (Multi-Ethnic Study of Atherosclerosis).
In addition, total testosterone levels were associated with increased cardiovascular disease and coronary heart disease, while estradiol is associated with a reduced risk of coronary heart disease and heart failure with reduced ejection fraction, investigators reported in the Journal of the American College of Cardiology.
Without any interventional studies as guidance, it’s not clear what the “best strategy” would be to modify sex hormone levels and reduce cardiovascular disease risk, wrote investigator Di Zhao, PhD, of the department of epidemiology at Johns Hopkins University Bloomberg School of Public Health, Baltimore, and her study coauthors.
“Nonetheless, a more androgenic sex hormone profile may identify a woman at higher risk for cardiovascular disease who may benefit from other risk-reducing strategies,” Dr. Zhao and her colleagues wrote in their report.
The postmenopausal women included in this analysis all had baseline measurements of testosterone, estradiol, dehydroepiandrosterone, and sex hormone–binding globulin levels between 2000 and 2002, according to the report.
After more than 12 years of follow-up, investigators found that a higher total testosterone to estradiol ratio was independently associated with an increased risk of incident cardiovascular disease (hazard ratio, 1.19; 95% confidence interval, 1.02-1.40), coronary heart disease (HR, 1.45; 95% CI, 1.19-1.78), and heart failure (HR, 1.31; 95% CI, 1.01-1.70).
Investigators also reported a statistically significant association between total testosterone levels and risk of cardiovascular disease and coronary heart disease but not risk of heart failure.
In a subgroup analysis of postmenopausal women with heart failure, investigators found a significant positive association with the testosterone to estradiol ratio (HR, 1.65; 95% CI, 1.07-2.54) and inverse associations for estradiol (HR, 0.60; 95% CI, 0.39-0.93) and for dehydroepiandrosterone (HR, 0.59; 95% CI, 0.44-0.78).
On their own, estradiol levels had no association with cardiovascular disease events overall, but they were associated with lower coronary heart disease risk, according to investigators. Estradiol also was associated with a lower heart failure risk, though the trend did not reach statistical significance.
The study was partially funded by the American Heart Association’s Go Red for Women Research Network. Study authors reported disclosures related to Cordex Systems, Siemens Diagnostics, and other entities.
SOURCE: Zhao D et al. J Am Coll Cardiol. 2018 Jun 5; 71:2555-66.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Levels of testosterone and estradiol after menopause correlated with increased cardiovascular risks later in life.
Major finding: A higher total testosterone to estradiol ratio was independently associated with increased risk of incident cardiovascular disease (hazard ratio, 1.19; 95% confidence interval, 1.02-1.40).
Study details: Analysis including 2,834 postmenopausal women in the MESA (Multi-Ethnic Study of Atherosclerosis) study.
Disclosures: Partial funding came from the American Heart Association’s Go Red for Women Research Network. Study authors reported disclosures related to Cordex Systems, Siemens Diagnostics, and other entities.
Source: Zhao D et al. J Am Coll Cardiol. 2018 Jun 5; 71:2555-66.
2018 Update on menopause
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.