User login
Researchers identify a cause of L-DOPA–induced dyskinesia in Parkinson’s disease
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
FROM Science Advances
When mania isn’t what it seems
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).
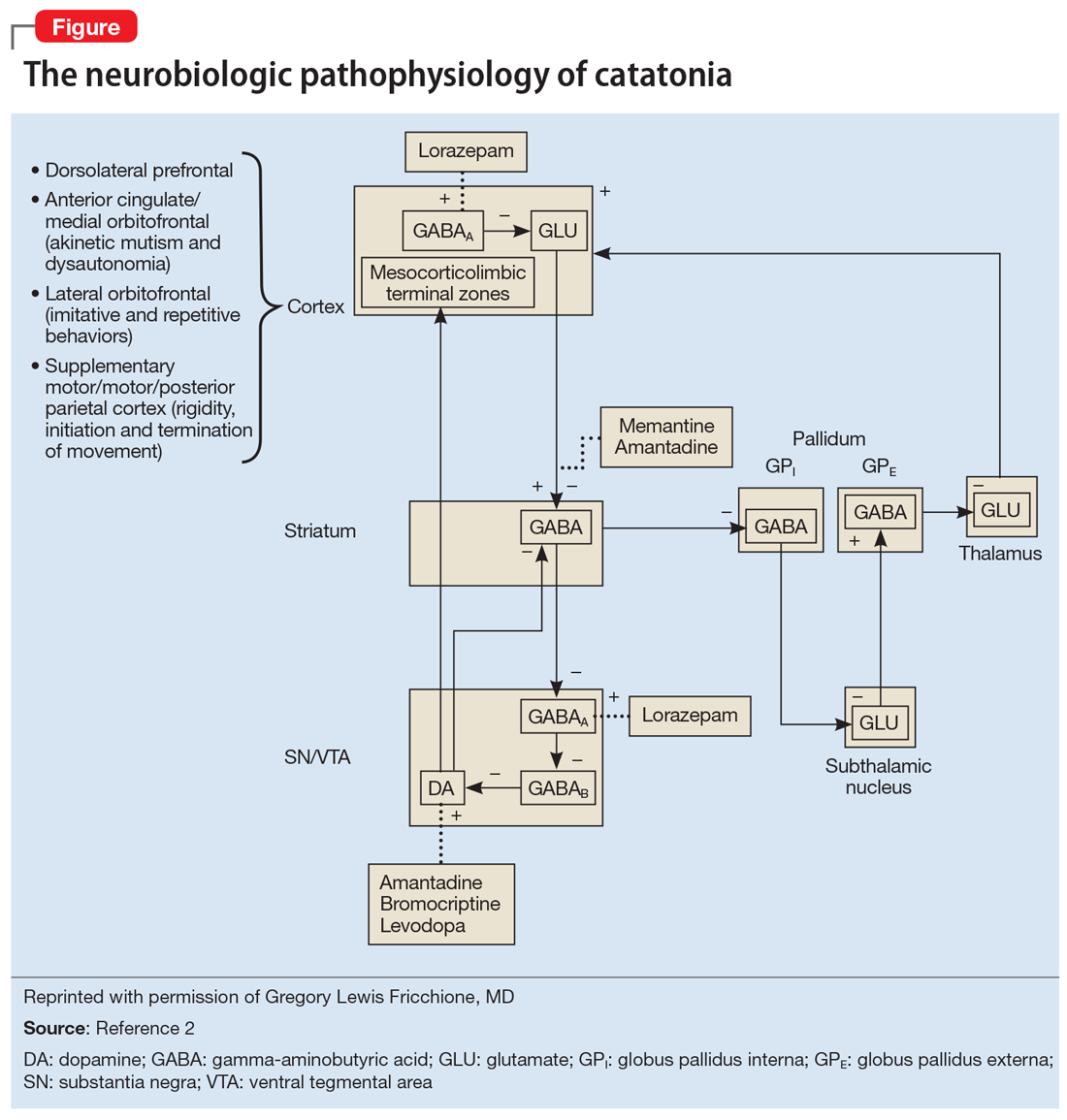
Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).

Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).

Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
Targeting gut bacteria may improve levodopa uptake
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
FROM GMFH 2020
Expert says progress in gut-brain research requires an open mind
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
FROM GMFH 2020
As costs for neurologic drugs rise, adherence to therapy drops
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
For their study, published online Feb. 19 in Neurology, Brian C. Callaghan, MD, of the University of Michigan, Ann Arbor, and colleagues looked at claims records from a large national private insurer to identify new cases of dementia, Parkinson’s disease, and neuropathy between 2001 and 2016, along with pharmacy records following diagnoses.
The researchers identified more than 52,000 patients with neuropathy on gabapentinoids and another 5,000 treated with serotonin-norepinephrine reuptake inhibitors for the same. They also identified some 20,000 patients with dementia taking cholinesterase inhibitors, and 3,000 with Parkinson’s disease taking dopamine agonists. Dr. Callaghan and colleagues compared patient adherence over 6 months for pairs of drugs in the same class with similar or equal efficacy, but with different costs to the patient.
Such cost differences can be stark: The researchers noted that the average 2016 out-of-pocket cost for 30 days of pregabalin, a drug used in the treatment of peripheral neuropathy, was $65.70, compared with $8.40 for gabapentin. With two common dementia drugs the difference was even more pronounced: $79.30 for rivastigmine compared with $3.10 for donepezil, both cholinesterase inhibitors with similar efficacy and tolerability.
Dr. Callaghan and colleagues found that such cost differences bore significantly on patient adherence. An increase of $50 in patient costs was seen decreasing adherence by 9% for neuropathy patients on gabapentinoids (adjusted incidence rate ratio [IRR] 0.91, 0.89-0.93) and by 12% for dementia patients on cholinesterase inhibitors (adjusted IRR 0.88, 0.86-0.91, P less than .05 for both). Similar price-linked decreases were seen for neuropathy patients on SNRIs and Parkinson’s patients on dopamine agonists, but the differences did not reach statistical significance.
Black, Asian, and Hispanic patients saw greater drops in adherence than did white patients associated with the same out-of-pocket cost differences, leading the researchers to note that special care should be taken in prescribing decisions for these populations.
“When choosing among medications with differential [out-of-pocket] costs, prescribing the medication with lower [out-of-pocket] expense will likely improve medication adherence while reducing overall costs,” Dr. Callaghan and colleagues wrote in their analysis. “For example, prescribing gabapentin or venlafaxine to patients with newly diagnosed neuropathy is likely to lead to higher adherence compared with pregabalin or duloxetine, and therefore, there is a higher likelihood of relief from neuropathic pain.” The researchers noted that while combination pills and extended-release formulations may be marketed as a way to increase adherence, the higher out-of-pocket costs of such medicines could offset any adherence benefit.
Dr. Callaghan and his colleagues described as strengths of their study its large sample and statistical approach that “allowed us to best estimate the causal relationship between [out-of-pocket] costs and medication adherence by limiting selection bias, residual confounding, and the confounding inherent to medication choice.” Nonadherence – patients who never filled a prescription after diagnosis – was not captured in the study.
The American Academy of Neurology funded the study. Two of its authors reported financial conflicts of interest in the form of compensation from pharmaceutical or device companies. Its lead author, Dr. Callaghan, reported funding for a device maker and performing medical legal consultations.
SOURCE: Reynolds EL et al. Neurology. 2020 Feb 19. doi/10.1212/WNL.0000000000009039.
FROM NEUROLOGY
Palliative care improves QoL for patients with Parkinson’s disease and related disorders
, according to results from a randomized clinical trial in JAMA Neurology.
The benefits of palliative care even extended to patients’ caregivers, who also appeared to benefit from outpatient palliative care at the 12-month mark, according to lead author Benzi M. Kluger, MD, of the department of neurology, University of Colorado at Denver, Aurora, and colleagues.
Between November 2015 and September 2017, Dr. Kluger and colleagues included 210 patients into the trial from three participating academic tertiary care centers who had “moderate to high palliative care needs” as assessed by the Palliative Care Needs Assessment Tool, which the researchers said are “common reasons for referral” and “reflect a desire to meet patient-centered needs rather than disease-centered markers.” Patients were primarily non-Hispanic white men with a mean age of about 70 years. The researchers also included 175 caregivers in the trial, most of whom were women, spouses to the patients, and in their caregiver role for over 5.5 years.
Patients with PDRD were randomized to receive standard care – usual care through their primary care physician and a neurologist – or “integrated outpatient palliative care,” from a team consisting of a palliative neurologist, nurse, social worker, chaplain, and board-certified palliative medicine physician. The goal of palliative care was addressing “nonmotor symptoms, goals of care, anticipatory guidance, difficult emotions, and caregiver support,” which patients received every 3 months through an in-person outpatient visit or telemedicine.
Quality of life for patients was measured through the Quality of Life in Alzheimer’s Disease (QoL-AD) scale, and caregiver burden was assessed with the Zarit Burden Interview (ZBI-12). The researchers also measured symptom burden and other QoL measures using the Edmonton Symptom Assessment Scale–Revised for Parkinson’s Disease, Parkinson’s Disease Questionnaire, Hospital Anxiety and Depression Scale, Prolonged Grief Disorder questionnaire, and Functional Assessment of Chronic Illness Therapy–Spiritual Well-Being.
Overall, 87 of 105 (82.1%) of patients in the palliative care group went to all their outpatient palliative care visits, and 19 of 106 (17.9%) patients received palliative care through telemedicine. Patients in the palliative care group also attended more neurology visits (4.66 visits) than those in the standard care (3.16 visits) group.
Quality of life significantly improved for patients in the palliative care group, compared with patients receiving standard care only at 6 months (0.66 vs. –0.84; between-group difference, 1.87; 95% confidence interval, 0.47-3.27; P = .009) and at 12 months (0.68 vs. –0.42; between-group difference, 1.36; 95% CI, −0.01 to 2.73; P = .05). These results remained significant at 6 months and 12 months after researchers used multiple imputation to fill in missing data. While there was no significant difference in caregiver burden between groups at 6 months, there was a statistically significant difference at 12 months favoring the palliative care group (between-group difference, −2.60; 95% CI, −4.58 to −0.61; P = .01).
Patients receiving palliative care also had better nonmotor symptom burden, motor symptom severity, and were more likely to complete advance directives, compared with patients receiving standard care alone. “We hypothesize that motor improvements may have reflected an unanticipated benefit of our palliative care team’s general goal of encouraging activities that promoted joy, meaning, and connection,” Dr. Kluger and colleagues said. Researchers also noted that the intervention patients with greater need for palliative care tended to benefit more than patients with patients with lower palliative care needs.
“Because the palliative care intervention is time-intensive and resource-intensive, future studies should optimize triage tools and consider alternative models of care delivery, such as telemedicine or care navigators, to provide key aspects of the intervention at lower cost,” they said.
In a related editorial, Bastiaan R. Bloem, MD, PhD, from the Center of Expertise for Parkinson & Movement Disorders, at Radboud University Medical Center, in the Netherlands, and colleagues acknowledged that the study by Kluger et al. is “timely and practical” because it introduces a system for outpatient palliative care for patients with PDRD at a time when there is “growing awareness that palliative care may also benefit persons with neurodegenerative diseases like Parkinson’s disease.”
The study is also important because it highlights that patients at varying stages of disease, including mild disease, may benefit from an integrated outpatient palliative model, which is not usually considered when determining candidates for palliative care in other scenarios, such as in patients with cancer. Future studies are warranted to assess how palliative care models can be implemented in different disease states and health care settings, they said.
“These new studies should continue to highlight the fact that palliative care is not about terminal diseases and dying,” Dr. Bloem and colleagues concluded. “As Kluger and colleagues indicate in their important clinical trial, palliative care is about how to live well.”
Six authors reported receiving a grant from the Patient-Centered Outcomes Research Institute, which was the funding source for the study. Two authors reported receiving grants from the University Hospital Foundation during the study. One author reported receiving grants from Allergan and Merz Pharma and is a consultant for GE Pharmaceuticals and Sunovion Pharmaceuticals; another reported receiving grants from the Archstone Foundation, the California Health Care Foundation, the Cambia Health Foundation, the Gordon and Betty Moore Foundation, the National Institute of Nursing Research, the Stupski Foundation, and the UniHealth Foundation. Dr. Bloem and a colleague reported their institution received a center of excellence grant from the Parkinson’s Foundation.
SOURCE: Kluger B et al. JAMA Neurol. doi: 10.1001/jamaneurol.2019.4992.
, according to results from a randomized clinical trial in JAMA Neurology.
The benefits of palliative care even extended to patients’ caregivers, who also appeared to benefit from outpatient palliative care at the 12-month mark, according to lead author Benzi M. Kluger, MD, of the department of neurology, University of Colorado at Denver, Aurora, and colleagues.
Between November 2015 and September 2017, Dr. Kluger and colleagues included 210 patients into the trial from three participating academic tertiary care centers who had “moderate to high palliative care needs” as assessed by the Palliative Care Needs Assessment Tool, which the researchers said are “common reasons for referral” and “reflect a desire to meet patient-centered needs rather than disease-centered markers.” Patients were primarily non-Hispanic white men with a mean age of about 70 years. The researchers also included 175 caregivers in the trial, most of whom were women, spouses to the patients, and in their caregiver role for over 5.5 years.
Patients with PDRD were randomized to receive standard care – usual care through their primary care physician and a neurologist – or “integrated outpatient palliative care,” from a team consisting of a palliative neurologist, nurse, social worker, chaplain, and board-certified palliative medicine physician. The goal of palliative care was addressing “nonmotor symptoms, goals of care, anticipatory guidance, difficult emotions, and caregiver support,” which patients received every 3 months through an in-person outpatient visit or telemedicine.
Quality of life for patients was measured through the Quality of Life in Alzheimer’s Disease (QoL-AD) scale, and caregiver burden was assessed with the Zarit Burden Interview (ZBI-12). The researchers also measured symptom burden and other QoL measures using the Edmonton Symptom Assessment Scale–Revised for Parkinson’s Disease, Parkinson’s Disease Questionnaire, Hospital Anxiety and Depression Scale, Prolonged Grief Disorder questionnaire, and Functional Assessment of Chronic Illness Therapy–Spiritual Well-Being.
Overall, 87 of 105 (82.1%) of patients in the palliative care group went to all their outpatient palliative care visits, and 19 of 106 (17.9%) patients received palliative care through telemedicine. Patients in the palliative care group also attended more neurology visits (4.66 visits) than those in the standard care (3.16 visits) group.
Quality of life significantly improved for patients in the palliative care group, compared with patients receiving standard care only at 6 months (0.66 vs. –0.84; between-group difference, 1.87; 95% confidence interval, 0.47-3.27; P = .009) and at 12 months (0.68 vs. –0.42; between-group difference, 1.36; 95% CI, −0.01 to 2.73; P = .05). These results remained significant at 6 months and 12 months after researchers used multiple imputation to fill in missing data. While there was no significant difference in caregiver burden between groups at 6 months, there was a statistically significant difference at 12 months favoring the palliative care group (between-group difference, −2.60; 95% CI, −4.58 to −0.61; P = .01).
Patients receiving palliative care also had better nonmotor symptom burden, motor symptom severity, and were more likely to complete advance directives, compared with patients receiving standard care alone. “We hypothesize that motor improvements may have reflected an unanticipated benefit of our palliative care team’s general goal of encouraging activities that promoted joy, meaning, and connection,” Dr. Kluger and colleagues said. Researchers also noted that the intervention patients with greater need for palliative care tended to benefit more than patients with patients with lower palliative care needs.
“Because the palliative care intervention is time-intensive and resource-intensive, future studies should optimize triage tools and consider alternative models of care delivery, such as telemedicine or care navigators, to provide key aspects of the intervention at lower cost,” they said.
In a related editorial, Bastiaan R. Bloem, MD, PhD, from the Center of Expertise for Parkinson & Movement Disorders, at Radboud University Medical Center, in the Netherlands, and colleagues acknowledged that the study by Kluger et al. is “timely and practical” because it introduces a system for outpatient palliative care for patients with PDRD at a time when there is “growing awareness that palliative care may also benefit persons with neurodegenerative diseases like Parkinson’s disease.”
The study is also important because it highlights that patients at varying stages of disease, including mild disease, may benefit from an integrated outpatient palliative model, which is not usually considered when determining candidates for palliative care in other scenarios, such as in patients with cancer. Future studies are warranted to assess how palliative care models can be implemented in different disease states and health care settings, they said.
“These new studies should continue to highlight the fact that palliative care is not about terminal diseases and dying,” Dr. Bloem and colleagues concluded. “As Kluger and colleagues indicate in their important clinical trial, palliative care is about how to live well.”
Six authors reported receiving a grant from the Patient-Centered Outcomes Research Institute, which was the funding source for the study. Two authors reported receiving grants from the University Hospital Foundation during the study. One author reported receiving grants from Allergan and Merz Pharma and is a consultant for GE Pharmaceuticals and Sunovion Pharmaceuticals; another reported receiving grants from the Archstone Foundation, the California Health Care Foundation, the Cambia Health Foundation, the Gordon and Betty Moore Foundation, the National Institute of Nursing Research, the Stupski Foundation, and the UniHealth Foundation. Dr. Bloem and a colleague reported their institution received a center of excellence grant from the Parkinson’s Foundation.
SOURCE: Kluger B et al. JAMA Neurol. doi: 10.1001/jamaneurol.2019.4992.
, according to results from a randomized clinical trial in JAMA Neurology.
The benefits of palliative care even extended to patients’ caregivers, who also appeared to benefit from outpatient palliative care at the 12-month mark, according to lead author Benzi M. Kluger, MD, of the department of neurology, University of Colorado at Denver, Aurora, and colleagues.
Between November 2015 and September 2017, Dr. Kluger and colleagues included 210 patients into the trial from three participating academic tertiary care centers who had “moderate to high palliative care needs” as assessed by the Palliative Care Needs Assessment Tool, which the researchers said are “common reasons for referral” and “reflect a desire to meet patient-centered needs rather than disease-centered markers.” Patients were primarily non-Hispanic white men with a mean age of about 70 years. The researchers also included 175 caregivers in the trial, most of whom were women, spouses to the patients, and in their caregiver role for over 5.5 years.
Patients with PDRD were randomized to receive standard care – usual care through their primary care physician and a neurologist – or “integrated outpatient palliative care,” from a team consisting of a palliative neurologist, nurse, social worker, chaplain, and board-certified palliative medicine physician. The goal of palliative care was addressing “nonmotor symptoms, goals of care, anticipatory guidance, difficult emotions, and caregiver support,” which patients received every 3 months through an in-person outpatient visit or telemedicine.
Quality of life for patients was measured through the Quality of Life in Alzheimer’s Disease (QoL-AD) scale, and caregiver burden was assessed with the Zarit Burden Interview (ZBI-12). The researchers also measured symptom burden and other QoL measures using the Edmonton Symptom Assessment Scale–Revised for Parkinson’s Disease, Parkinson’s Disease Questionnaire, Hospital Anxiety and Depression Scale, Prolonged Grief Disorder questionnaire, and Functional Assessment of Chronic Illness Therapy–Spiritual Well-Being.
Overall, 87 of 105 (82.1%) of patients in the palliative care group went to all their outpatient palliative care visits, and 19 of 106 (17.9%) patients received palliative care through telemedicine. Patients in the palliative care group also attended more neurology visits (4.66 visits) than those in the standard care (3.16 visits) group.
Quality of life significantly improved for patients in the palliative care group, compared with patients receiving standard care only at 6 months (0.66 vs. –0.84; between-group difference, 1.87; 95% confidence interval, 0.47-3.27; P = .009) and at 12 months (0.68 vs. –0.42; between-group difference, 1.36; 95% CI, −0.01 to 2.73; P = .05). These results remained significant at 6 months and 12 months after researchers used multiple imputation to fill in missing data. While there was no significant difference in caregiver burden between groups at 6 months, there was a statistically significant difference at 12 months favoring the palliative care group (between-group difference, −2.60; 95% CI, −4.58 to −0.61; P = .01).
Patients receiving palliative care also had better nonmotor symptom burden, motor symptom severity, and were more likely to complete advance directives, compared with patients receiving standard care alone. “We hypothesize that motor improvements may have reflected an unanticipated benefit of our palliative care team’s general goal of encouraging activities that promoted joy, meaning, and connection,” Dr. Kluger and colleagues said. Researchers also noted that the intervention patients with greater need for palliative care tended to benefit more than patients with patients with lower palliative care needs.
“Because the palliative care intervention is time-intensive and resource-intensive, future studies should optimize triage tools and consider alternative models of care delivery, such as telemedicine or care navigators, to provide key aspects of the intervention at lower cost,” they said.
In a related editorial, Bastiaan R. Bloem, MD, PhD, from the Center of Expertise for Parkinson & Movement Disorders, at Radboud University Medical Center, in the Netherlands, and colleagues acknowledged that the study by Kluger et al. is “timely and practical” because it introduces a system for outpatient palliative care for patients with PDRD at a time when there is “growing awareness that palliative care may also benefit persons with neurodegenerative diseases like Parkinson’s disease.”
The study is also important because it highlights that patients at varying stages of disease, including mild disease, may benefit from an integrated outpatient palliative model, which is not usually considered when determining candidates for palliative care in other scenarios, such as in patients with cancer. Future studies are warranted to assess how palliative care models can be implemented in different disease states and health care settings, they said.
“These new studies should continue to highlight the fact that palliative care is not about terminal diseases and dying,” Dr. Bloem and colleagues concluded. “As Kluger and colleagues indicate in their important clinical trial, palliative care is about how to live well.”
Six authors reported receiving a grant from the Patient-Centered Outcomes Research Institute, which was the funding source for the study. Two authors reported receiving grants from the University Hospital Foundation during the study. One author reported receiving grants from Allergan and Merz Pharma and is a consultant for GE Pharmaceuticals and Sunovion Pharmaceuticals; another reported receiving grants from the Archstone Foundation, the California Health Care Foundation, the Cambia Health Foundation, the Gordon and Betty Moore Foundation, the National Institute of Nursing Research, the Stupski Foundation, and the UniHealth Foundation. Dr. Bloem and a colleague reported their institution received a center of excellence grant from the Parkinson’s Foundation.
SOURCE: Kluger B et al. JAMA Neurol. doi: 10.1001/jamaneurol.2019.4992.
FROM JAMA NEUROLOGY
APOE genotype directly regulates alpha-synuclein accumulation
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
Apolipoprotein E epsilon 4 (APOE4) directly and independently exacerbates accumulation of alpha-synuclein in patients with Lewy body dementia, whereas APOE2 may have a protective effect, based on two recent studies involving mouse models and human patients.
These insights confirm the importance of APOE in synucleinopathies, and may lead to new treatments, according to Eliezer Masliah, MD, director of the division of neuroscience at the National Institute on Aging.
“These [studies] definitely implicate a role of APOE4,” Dr. Masliah said in an interview.
According to Dr. Masliah, previous studies linked the APOE4 genotype with cognitive decline in synucleinopathies, but underlying molecular mechanisms remained unknown.
“We [now] have more direct confirmation [based on] different experimental animal models,” Dr. Masliah said. “It also means that APOE4 could be a therapeutic target for dementia with Lewy bodies.”
The two studies were published simultaneously in Science Translational Medicine. The first study was conducted by Albert A. Davis, MD, PhD, of Washington University, St. Louis, and colleagues; the second was led by Na Zhao, MD, PhD, of the Mayo Clinic in Jacksonville, Fla.
“The studies are very synergistic, but used different techniques,” said Dr. Masliah, who was not involved in the studies.
Both studies involved mice that expressed a human variant of APOE: APOE2, APOE3, or APOE4. Three independent techniques were used to concurrently overexpress alpha-synuclein; Dr. Davis and colleagues used a transgenic approach, as well as striatal injection of alpha-synuclein preformed fibrils, whereas Dr. Zhao and colleagues turned to a viral vector. Regardless of technique, each APOE variant had a distinct impact on the level of alpha-synuclein accumulation.
“In a nutshell, [Dr. Davis and colleagues] found that those mice that have synuclein and APOE4 have a much more rapid progression of the disease,” Dr. Masliah said. “They become Parkinsonian much faster, but also, they become cognitively impaired much faster, and they have more synuclein in the brain. Remarkably, on the opposite side, those that were expressing APOE2, which we know is a protective allele, actually were far less impaired. So that’s really a remarkable finding.”
The study at the Mayo Clinic echoed these findings.
“Essentially, [Dr. Zhao and colleagues] had very similar results,” Dr. Masliah said. “[In mice expressing] APOE4, synuclein accumulation was worse and pathology was worse, and with APOE2, there was relative protection.”
Both studies found that the exacerbating effect of APOE4 translated to human patients.
Dr. Davis and colleagues evaluated data from 251 patients in the Parkinson’s Progression Markers Initiative. A multivariate model showed that patients with the APOE4 genotype had faster cognitive decline, an impact that was independent of other variables, including cerebrospinal fluid concentrations of amyloid beta and tau protein (P = .0119). This finding was further supported by additional analyses involving 177 patients with Parkinson’s disease from the Washington University Movement Disorders Center, and another 1,030 patients enrolled in the NeuroGenetics Research Consortium study.
Dr. Zhao and colleagues evaluated postmortem samples from patients with Lewy body dementia who had minimal amyloid pathology. Comparing 22 APOE4 carriers versus 22 age- and sex-matched noncarriers, they found that carriers had significantly greater accumulations of alpha-synuclein (P less than .05).
According to the investigators, these findings could have both prognostic and therapeutic implications.
“[I]t is intriguing to speculate whether APOE and other potential genetic risk or resilience genes could be useful as screening tools to stratify risk for individual patients,” Dr. Davis and colleagues wrote in their paper. They went on to suggest that APOE genotyping may one day be used to personalize treatments for patients with neurodegenerative disease.
According to Dr. Masliah, several treatment strategies are under investigation.
“There are some pharmaceutical companies and also some academic groups that have been developing antibodies against APOE4 for Alzheimer’s disease, but certainly that could also be used for dementia with Lewy bodies,” he said. “There are other ways. One could [be] to suppress the expression of APOE4 with antisense or other technologies.
“There is also a very innovative technology that has been developed by the group at the Gladstone Institutes in San Francisco, which is to switch APOE4 to APOE3.” This technique, Dr. Masliah explained, is accomplished by breaking a disulfide bond in APOE4, which opens the structure into an isoform that mimics APOE3. “They have developed small molecules that actually can break that bond and essentially chemically switch APOE4 to APOE3,” he said.
Although multiple techniques are feasible, Dr. Masliah stressed that these therapeutic efforts are still in their infancy.
“We need to better understand the mechanisms as to how APOE4 and alpha-synuclein interact,” he said. “I think we need a lot more work in this area.”
The Davis study was funded by the American Academy of Neurology/American Brain Foundation, the BrightFocus Foundation, the Mary E. Groff Charitable Trust, and others; the investigators reported additional relationships with Biogen, Alector, Parabon, and others. The Zhao study was funded by the National Institutes of Health and the Lewy Body Dementia Center Without Walls; the investigators reported no competing interests. Dr. Masliah reported no conflicts of interest.
SOURCES: Davis AA et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay3069; Zhao N et al. Sci Transl Med. 2020 Feb 5. doi: 10.1126/scitranslmed.aay1809.
FROM SCIENCE TRANSLATIONAL MEDICINE
Synaptic pruning deficits may cause tremor in essential tremor
according to an investigation published January 15 in Science Translational Medicine. These synaptic pruning deficits result from insufficiency of glutamate receptor delta 2 (GluR[delta]2) protein. The findings indicate molecular, structural, physiological, and behavioral factors that contribute to tremor and might influence future treatment of essential tremor, the authors wrote.

Essential tremor has a complex etiology that includes genetic and environmental factors. Its pathophysiology is poorly understood. First author Ming-Kai Pan, MD, assistant professor of medical research and neurology at National Taiwan University Hospital in Taipei, and colleagues previously observed pruning deficits of CF-PC synapses in the cerebellum of deceased patients with essential tremor. An excess of CF-PC synapses are a prominent feature of essential tremor, but not of other cerebellar degenerative disorders. Researchers have observed this pathology consistently in patients with essential tremor who have diverse clinical features. Dr. Pan and colleagues therefore chose to examine these synaptic changes to clarify the pathophysiology of essential tremor.
Patients had more CF synapses than did controls
The investigators performed a pathological examination of postmortem cerebellar tissue from patients with essential tremor and controls to identify microstructural changes in essential tremor. Next, they applied these changes to mouse models of essential tremor and examined the corresponding structural, electrophysiologic, and behavioral changes. Finally, Dr. Pan and colleagues used cerebellar EEG to validate their findings in patients with essential tremor.
Compared with age-matched controls, patients with essential tremor had more CF synapses in the parallel-fiber synaptic territory on PC dendrites. Patients also had an approximately 75% reduction in mean GluR(delta)2 expression, compared with controls. The amount of GluR(delta)2 was inversely correlated with the percentage of CFs extending to parallel-fiber synaptic territory. The findings suggest that PC synaptic pathology in essential tremor might be related to reduced GluR(delta)2 expression, Dr. Pan and colleagues wrote.
The investigators examined a mouse model that produces 10% of full-length GluR(delta)2 protein. These mice had significant reduction of GluR(delta)2 in the cerebellar cortex and the PC dendrites. In addition, the mice consistently developed CF synapses innervating distal, thin PC dendrites. The investigators observed a 20-Hz tremor in the mice that occurred mainly during action and rarely during rest.
Dr. Pan and colleagues injected a virus containing GluR(delta)2 protein into the mice’s brains to test the protein’s relationship to tremor. Five days after the injection, the mice’s brains were expressing GluR(delta)2 protein reliably. By 4-6 days after injection, the mice’s tremor had been reduced. It returned to baseline levels at 12-14 days after injection. Injecting a control virus did not affect tremor.
Cerebellar oscillatory indexes were correlated with tremor scores
When the researchers examined local field potentials in mouse cerebellum, they found cerebellar oscillations at 20 Hz that were consistent with the observed tremor. “Putting the evidence together, GluR(delta)2 insufficiency causes CF synaptic pruning deficits, and the surplus CF-PC synaptic activity generates excessive cerebellar oscillations, which drive tremor,” Dr. Pan and colleagues reported.
Next, the researchers performed cerebellar EEG in 10 patients with essential tremor and 10 age-matched controls. Patients had cerebellar oscillations at 4-12 Hz, which are the human tremor frequencies. In an expanded cohort of 20 patients with essential tremor and 20 controls, the cerebellar oscillatory indexes were correlated with tremor scores in patients, which showed that the former could be an index of tremor severity. “Currently, diagnosis of essential tremor is based on pure clinical tremor phenomenology and direct tremor measurement, without a physiological marker indicating the underlying brain circuitry abnormalities,” they wrote. “Cerebellar oscillations can be a physiological signature and a therapeutic target for essential tremor.”
The research was funded by grants from the National Institutes of Health, the Parkinson’s Foundation, the International Essential Tremor Foundation, the Ministry of Science and Technology in Taiwan, and the National Taiwan University Hospital. The authors declared that they had no competing interests.
SOURCE: Pan M-K et al. Sci Transl Med. 2020;12:eaay1769. doi: 10.1126/scitranslmed.aay1769.
according to an investigation published January 15 in Science Translational Medicine. These synaptic pruning deficits result from insufficiency of glutamate receptor delta 2 (GluR[delta]2) protein. The findings indicate molecular, structural, physiological, and behavioral factors that contribute to tremor and might influence future treatment of essential tremor, the authors wrote.

Essential tremor has a complex etiology that includes genetic and environmental factors. Its pathophysiology is poorly understood. First author Ming-Kai Pan, MD, assistant professor of medical research and neurology at National Taiwan University Hospital in Taipei, and colleagues previously observed pruning deficits of CF-PC synapses in the cerebellum of deceased patients with essential tremor. An excess of CF-PC synapses are a prominent feature of essential tremor, but not of other cerebellar degenerative disorders. Researchers have observed this pathology consistently in patients with essential tremor who have diverse clinical features. Dr. Pan and colleagues therefore chose to examine these synaptic changes to clarify the pathophysiology of essential tremor.
Patients had more CF synapses than did controls
The investigators performed a pathological examination of postmortem cerebellar tissue from patients with essential tremor and controls to identify microstructural changes in essential tremor. Next, they applied these changes to mouse models of essential tremor and examined the corresponding structural, electrophysiologic, and behavioral changes. Finally, Dr. Pan and colleagues used cerebellar EEG to validate their findings in patients with essential tremor.
Compared with age-matched controls, patients with essential tremor had more CF synapses in the parallel-fiber synaptic territory on PC dendrites. Patients also had an approximately 75% reduction in mean GluR(delta)2 expression, compared with controls. The amount of GluR(delta)2 was inversely correlated with the percentage of CFs extending to parallel-fiber synaptic territory. The findings suggest that PC synaptic pathology in essential tremor might be related to reduced GluR(delta)2 expression, Dr. Pan and colleagues wrote.
The investigators examined a mouse model that produces 10% of full-length GluR(delta)2 protein. These mice had significant reduction of GluR(delta)2 in the cerebellar cortex and the PC dendrites. In addition, the mice consistently developed CF synapses innervating distal, thin PC dendrites. The investigators observed a 20-Hz tremor in the mice that occurred mainly during action and rarely during rest.
Dr. Pan and colleagues injected a virus containing GluR(delta)2 protein into the mice’s brains to test the protein’s relationship to tremor. Five days after the injection, the mice’s brains were expressing GluR(delta)2 protein reliably. By 4-6 days after injection, the mice’s tremor had been reduced. It returned to baseline levels at 12-14 days after injection. Injecting a control virus did not affect tremor.
Cerebellar oscillatory indexes were correlated with tremor scores
When the researchers examined local field potentials in mouse cerebellum, they found cerebellar oscillations at 20 Hz that were consistent with the observed tremor. “Putting the evidence together, GluR(delta)2 insufficiency causes CF synaptic pruning deficits, and the surplus CF-PC synaptic activity generates excessive cerebellar oscillations, which drive tremor,” Dr. Pan and colleagues reported.
Next, the researchers performed cerebellar EEG in 10 patients with essential tremor and 10 age-matched controls. Patients had cerebellar oscillations at 4-12 Hz, which are the human tremor frequencies. In an expanded cohort of 20 patients with essential tremor and 20 controls, the cerebellar oscillatory indexes were correlated with tremor scores in patients, which showed that the former could be an index of tremor severity. “Currently, diagnosis of essential tremor is based on pure clinical tremor phenomenology and direct tremor measurement, without a physiological marker indicating the underlying brain circuitry abnormalities,” they wrote. “Cerebellar oscillations can be a physiological signature and a therapeutic target for essential tremor.”
The research was funded by grants from the National Institutes of Health, the Parkinson’s Foundation, the International Essential Tremor Foundation, the Ministry of Science and Technology in Taiwan, and the National Taiwan University Hospital. The authors declared that they had no competing interests.
SOURCE: Pan M-K et al. Sci Transl Med. 2020;12:eaay1769. doi: 10.1126/scitranslmed.aay1769.
according to an investigation published January 15 in Science Translational Medicine. These synaptic pruning deficits result from insufficiency of glutamate receptor delta 2 (GluR[delta]2) protein. The findings indicate molecular, structural, physiological, and behavioral factors that contribute to tremor and might influence future treatment of essential tremor, the authors wrote.

Essential tremor has a complex etiology that includes genetic and environmental factors. Its pathophysiology is poorly understood. First author Ming-Kai Pan, MD, assistant professor of medical research and neurology at National Taiwan University Hospital in Taipei, and colleagues previously observed pruning deficits of CF-PC synapses in the cerebellum of deceased patients with essential tremor. An excess of CF-PC synapses are a prominent feature of essential tremor, but not of other cerebellar degenerative disorders. Researchers have observed this pathology consistently in patients with essential tremor who have diverse clinical features. Dr. Pan and colleagues therefore chose to examine these synaptic changes to clarify the pathophysiology of essential tremor.
Patients had more CF synapses than did controls
The investigators performed a pathological examination of postmortem cerebellar tissue from patients with essential tremor and controls to identify microstructural changes in essential tremor. Next, they applied these changes to mouse models of essential tremor and examined the corresponding structural, electrophysiologic, and behavioral changes. Finally, Dr. Pan and colleagues used cerebellar EEG to validate their findings in patients with essential tremor.
Compared with age-matched controls, patients with essential tremor had more CF synapses in the parallel-fiber synaptic territory on PC dendrites. Patients also had an approximately 75% reduction in mean GluR(delta)2 expression, compared with controls. The amount of GluR(delta)2 was inversely correlated with the percentage of CFs extending to parallel-fiber synaptic territory. The findings suggest that PC synaptic pathology in essential tremor might be related to reduced GluR(delta)2 expression, Dr. Pan and colleagues wrote.
The investigators examined a mouse model that produces 10% of full-length GluR(delta)2 protein. These mice had significant reduction of GluR(delta)2 in the cerebellar cortex and the PC dendrites. In addition, the mice consistently developed CF synapses innervating distal, thin PC dendrites. The investigators observed a 20-Hz tremor in the mice that occurred mainly during action and rarely during rest.
Dr. Pan and colleagues injected a virus containing GluR(delta)2 protein into the mice’s brains to test the protein’s relationship to tremor. Five days after the injection, the mice’s brains were expressing GluR(delta)2 protein reliably. By 4-6 days after injection, the mice’s tremor had been reduced. It returned to baseline levels at 12-14 days after injection. Injecting a control virus did not affect tremor.
Cerebellar oscillatory indexes were correlated with tremor scores
When the researchers examined local field potentials in mouse cerebellum, they found cerebellar oscillations at 20 Hz that were consistent with the observed tremor. “Putting the evidence together, GluR(delta)2 insufficiency causes CF synaptic pruning deficits, and the surplus CF-PC synaptic activity generates excessive cerebellar oscillations, which drive tremor,” Dr. Pan and colleagues reported.
Next, the researchers performed cerebellar EEG in 10 patients with essential tremor and 10 age-matched controls. Patients had cerebellar oscillations at 4-12 Hz, which are the human tremor frequencies. In an expanded cohort of 20 patients with essential tremor and 20 controls, the cerebellar oscillatory indexes were correlated with tremor scores in patients, which showed that the former could be an index of tremor severity. “Currently, diagnosis of essential tremor is based on pure clinical tremor phenomenology and direct tremor measurement, without a physiological marker indicating the underlying brain circuitry abnormalities,” they wrote. “Cerebellar oscillations can be a physiological signature and a therapeutic target for essential tremor.”
The research was funded by grants from the National Institutes of Health, the Parkinson’s Foundation, the International Essential Tremor Foundation, the Ministry of Science and Technology in Taiwan, and the National Taiwan University Hospital. The authors declared that they had no competing interests.
SOURCE: Pan M-K et al. Sci Transl Med. 2020;12:eaay1769. doi: 10.1126/scitranslmed.aay1769.
FROM SCIENCE TRANSLATIONAL MEDICINE
Schizophrenia, bipolar disorder associated with increased risk of secondary TD
Psychiatric inpatients, particularly those with schizophrenia or bipolar disorder, have both a greater risk of having a secondary diagnosis of tardive dyskinesia and having worse illness when tardive dyskinesia is also present, according to results of a case-control study of more than 77,000 inpatients.
For the study, the investigators conducted an analysis of 77,022 adults from the Nationwide Inpatient Sample who had been admitted between January 2010 and December 2014 for mood disorders and schizophrenia; 38,382 patients in this group also had a secondary diagnosis of tardive dyskinesia (TD), reported Rikinkumar S. Patel, MD, of the department of psychiatry at Griffin Memorial Hospital in Norman, Okla., and associates. The study was published in Heliyon.
They investigators found that patients with schizophrenia and bipolar disorder were four to five times more likely to also have TD, and patients with TD were six times more likely to have severe morbidity because of a major loss of function. Compared with non-TD controls, patients with TD had a longer hospital length of stay by 6.36 days and higher cost by $20,415.
More than 60% of TD patients came from below the 50th percentile in median household income, compared with less than 40% of the non-TD group. Dr. Patel and associates also found that almost half of the patients with TD were aged 40-60 years and that the prevalence of TD in the study population increased with age.
“Our findings support the previous evidence that advanced age is a risk factor for the development of TD,” they wrote, citing research by Criscely L. Go, MD, and associates (Parkinsonism Relat Disord. 2019. 15[9]:655-9).
Dr. Patel and associates concluded that more systematic research is needed to prevent TD and “optimize inpatient outcomes in psychiatric patients with TD.”
The study authors reported having no conflicts of interest.
SOURCE: Patel RS et al. Heliyon. 2019. doi: 10.1016/j.heliyon.2019.e01745.
Psychiatric inpatients, particularly those with schizophrenia or bipolar disorder, have both a greater risk of having a secondary diagnosis of tardive dyskinesia and having worse illness when tardive dyskinesia is also present, according to results of a case-control study of more than 77,000 inpatients.
For the study, the investigators conducted an analysis of 77,022 adults from the Nationwide Inpatient Sample who had been admitted between January 2010 and December 2014 for mood disorders and schizophrenia; 38,382 patients in this group also had a secondary diagnosis of tardive dyskinesia (TD), reported Rikinkumar S. Patel, MD, of the department of psychiatry at Griffin Memorial Hospital in Norman, Okla., and associates. The study was published in Heliyon.
They investigators found that patients with schizophrenia and bipolar disorder were four to five times more likely to also have TD, and patients with TD were six times more likely to have severe morbidity because of a major loss of function. Compared with non-TD controls, patients with TD had a longer hospital length of stay by 6.36 days and higher cost by $20,415.
More than 60% of TD patients came from below the 50th percentile in median household income, compared with less than 40% of the non-TD group. Dr. Patel and associates also found that almost half of the patients with TD were aged 40-60 years and that the prevalence of TD in the study population increased with age.
“Our findings support the previous evidence that advanced age is a risk factor for the development of TD,” they wrote, citing research by Criscely L. Go, MD, and associates (Parkinsonism Relat Disord. 2019. 15[9]:655-9).
Dr. Patel and associates concluded that more systematic research is needed to prevent TD and “optimize inpatient outcomes in psychiatric patients with TD.”
The study authors reported having no conflicts of interest.
SOURCE: Patel RS et al. Heliyon. 2019. doi: 10.1016/j.heliyon.2019.e01745.
Psychiatric inpatients, particularly those with schizophrenia or bipolar disorder, have both a greater risk of having a secondary diagnosis of tardive dyskinesia and having worse illness when tardive dyskinesia is also present, according to results of a case-control study of more than 77,000 inpatients.
For the study, the investigators conducted an analysis of 77,022 adults from the Nationwide Inpatient Sample who had been admitted between January 2010 and December 2014 for mood disorders and schizophrenia; 38,382 patients in this group also had a secondary diagnosis of tardive dyskinesia (TD), reported Rikinkumar S. Patel, MD, of the department of psychiatry at Griffin Memorial Hospital in Norman, Okla., and associates. The study was published in Heliyon.
They investigators found that patients with schizophrenia and bipolar disorder were four to five times more likely to also have TD, and patients with TD were six times more likely to have severe morbidity because of a major loss of function. Compared with non-TD controls, patients with TD had a longer hospital length of stay by 6.36 days and higher cost by $20,415.
More than 60% of TD patients came from below the 50th percentile in median household income, compared with less than 40% of the non-TD group. Dr. Patel and associates also found that almost half of the patients with TD were aged 40-60 years and that the prevalence of TD in the study population increased with age.
“Our findings support the previous evidence that advanced age is a risk factor for the development of TD,” they wrote, citing research by Criscely L. Go, MD, and associates (Parkinsonism Relat Disord. 2019. 15[9]:655-9).
Dr. Patel and associates concluded that more systematic research is needed to prevent TD and “optimize inpatient outcomes in psychiatric patients with TD.”
The study authors reported having no conflicts of interest.
SOURCE: Patel RS et al. Heliyon. 2019. doi: 10.1016/j.heliyon.2019.e01745.
FROM HELIYON
Pimavanserin reduced dementia-related psychotic symptoms without affecting cognition
SAN DIEGO – Pimavanserin, a second-generation antipsychotic approved for hallucinations and delusions in patients with Parkinson’s disease, may also be helpful for psychotic symptoms in other dementia patients, Erin P. Foff, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
In fact, the phase 3 HARMONY trial was stopped early, after an interim efficacy analysis determined that treatment with pimavanserin (Nuplazid) had achieved its primary endpoint – a statistically significant threefold reduction in the risk of relapse (P less than .0033).
Importantly, pimavanserin didn’t significantly affect cognition nor, at least in this controlled setting, did it appear to increase falls or other adverse events often seen with antipsychotic use in elderly patients, said Dr. Foff, clinical lead for the dementia-related psychosis program at Acadia Pharmaceuticals, which makes the drug and sponsored the study.
Based on the positive results, Acadia intends to submit a supplemental new drug application for this indication, according to an investor presentation posted on the company website.
“There is a critical need for an intervention [for psychosis symptoms] in this population,” Dr. Foff said. “We saw a robust response that was well tolerated and well maintained with no negative impact on cognitive scores.”
The second-generation antipsychotic was approved in 2016 for treating hallucinations and delusions in patients with Parkinson’s disease.
The drug is a selective antagonist of 5-HT2 receptors, with low affinity for dopamine receptors. This slightly differentiates it from other second-generation antipsychotics that affect dopamine receptors as well as 5-HT2 receptors.
HARMONY was not a typical placebo-controlled, randomized efficacy trial. Rather, it employed a two-phase design: an open-label treatment response period followed by a placebo-controlled randomization limited to open-label responders. Overall, HARMONY involved 392 patients with mild to severe dementia of numerous etiologies, including Alzheimer’s disease (66.8%), Parkinson’s disease dementia (14.3%), frontotemporal dementia (1.8%), vascular dementia (9.7%), and dementia with Lewy bodies (7.4%). All patients entered a 12-week, open-label period during which they received pimavanserin 34 mg daily. The primary endpoint was a combination of least a 30% reduction on the total Scale for the Assessment of Positive Symptom–Hallucinations and Delusions (SAPS-HD) scale plus a score of 1-2 on the Clinical Global Impressions–Improvement (CGI-I) scale, meaning better or very much better.
At 12 weeks, all responders were then randomized to placebo or continued therapy for 26 weeks. The primary endpoint was relapse, defined as at least a 30% worsening of the SAPS-HD relative to open-label baseline, plus a CGI-I score of 6-7 (worse or very much worse).
Patients were aged a mean of 74 years. Most (about 90%) were living at home. Visual hallucinations occurred in 80% and delusions in 83%. At baseline, the mean SAPS-HD score was 24.4, and the mean CGI-Severity score was 4.7. The mean Mini-Mental State Exam (MMSE) score was 16.7.
In the open-label period, pimavanserin reduced the SAPS-HD score at 12 weeks by a mean of 75%. Symptoms began to decline in the first week of treatment, with continuing improvement throughout the treatment period. By week 4, 30% had hit the response target. This number increased steadily, with 51% responding by week 4, 75% by week 8, and 88% by week 12.
By probable diagnosis, response rates were 59.8% in Alzheimer’s patients, 45.5% for those with Lewy body dementia, 71.2% among patients with Parkinson’s disease, 71% in patients with vascular dementia, and 50% in patients with frontotemporal dementia. In the final analysis, 80% of patients overall were considered responders.
The randomized potion began immediately thereafter with no washout period. About 62% (194) of the entire cohort – all responders – entered into the placebo-controlled phase. The remaining patients were either not responders (20%), dropped out because of an adverse event (7.7%), or left the study for unspecified reasons (10%). There was one death, which was not related to the study medication. A total of 41 patients were still being treated when the study was discontinued, and they were excluded from the final analysis.
When the randomized study ended, relapses had occurred in 28.3% of those taking placebo and in 12.6% of those taking pimavanserin – a statistically significant difference (hazard ratio, 0.353). This translated to a 180% reduction in relapse.
The rate of adverse events was similar in both active and placebo groups (41% vs. 36.6%). Serious adverse events occurred in 4.8% and 3.6%, respectively. The most commonly reported adverse events were headache (9.5% vs. 4.5%) and urinary tract infection (6.7% vs. 3.6%). Asthenia occurred in 2.9% of treated patients and 0.9% of placebo patients, but no falls were reported. Anxiety and dizziness were also reported in three patients taking the study medication.
Three patients (2.9%) experienced a prolonged QT phase on ECG, with a mean delay of 5.4 milliseconds from baseline. “Pimavanserin is known to have this effect of QT prolongation,” Dr. Foff said. “This 5.4-ms change is exactly in line with what we already know about pimavanserin and is not clinically significant. We saw no effect on motor function, consistent with the mechanism of action, and very low levels of agitation or aggression.”
Pimavanserin didn’t significantly change cognition from baseline in the open-label period, and in the randomized period, MMSE never differed significantly between groups.
The company also conducted an exploratory subgroup analysis that looked at placebo versus pimavanserin relapse by probable clinical diagnosis. Among the types of dementia, relapse rates for placebo versus pimavanserin were 23% versus 13% among Alzheimer’s patients, 67% versus 0% in Lewy body dementia patients, 50% versus 7% in patients with Parkinson’s, and 17% each among vascular dementia patients. Only one patient in the randomized period had frontotemporal dementia, and that patient relapsed on treatment.
Whether pimavanserin is effective specifically for psychosis in Alzheimer’s disease patients, however, remains in question. In 2018, Acadia published a negative phase 2 trial in a targeted group of 181 Alzheimer’s patients. The primary outcome in each study was mean change on the Neuropsychiatric Inventory–Nursing Home Version psychosis score (NPI-NH-PS). Clive Ballard, MD, of the University of Exeter (England), was the primary investigator.
After 6 weeks, those taking pimavanserin had a 3.76-point change in the NPI-NH-PS, compared with a 1.93-point change in the placebo group. The mean 1.84-point difference was not statistically significant.
This Alzheimer’s-only cohort group also experienced more adverse events than the HARMONY mixed-diagnosis cohort did, although the differences between pimavanserin and placebo groups were not significant. Adverse events included falls (23% of each group) and agitation (21% with pimavanserin vs. 14% with placebo). Cognition was unaffected.
Later that year, Acadia published a subgroup analysis of the same cohort parsing response by symptom severity, again with Dr. Ballard as the lead investigator.
The analysis focused on 57 patients with a baseline NPI-NH-PS of at least 12, indicating severe symptoms of psychosis.
Treatment effects were more pronounced in this group, significantly favoring pimavanserin. On the NPI-NH-PS, 88.9% of the pimavanserin group and 43.3% of the placebo group had at least a 30% improvement; 77.8% and 43.3% experienced at least a 50% improvement. The rate of serious adverse events was similar (18% with pimavanserin and 17% with placebo) and cognition was unaffected. Falls occurred in 14% of the treated group and 20% of the placebo group.
“These findings coupled with the results from other studies of pimavanserin suggest a potential role for pimavanserin in treating psychosis in patients across a range of neuropsychiatric conditions,” Dr. Ballard wrote.
SOURCE: Foff EP et al. CTAD 2019, Late-breaker 1
SAN DIEGO – Pimavanserin, a second-generation antipsychotic approved for hallucinations and delusions in patients with Parkinson’s disease, may also be helpful for psychotic symptoms in other dementia patients, Erin P. Foff, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
In fact, the phase 3 HARMONY trial was stopped early, after an interim efficacy analysis determined that treatment with pimavanserin (Nuplazid) had achieved its primary endpoint – a statistically significant threefold reduction in the risk of relapse (P less than .0033).
Importantly, pimavanserin didn’t significantly affect cognition nor, at least in this controlled setting, did it appear to increase falls or other adverse events often seen with antipsychotic use in elderly patients, said Dr. Foff, clinical lead for the dementia-related psychosis program at Acadia Pharmaceuticals, which makes the drug and sponsored the study.
Based on the positive results, Acadia intends to submit a supplemental new drug application for this indication, according to an investor presentation posted on the company website.
“There is a critical need for an intervention [for psychosis symptoms] in this population,” Dr. Foff said. “We saw a robust response that was well tolerated and well maintained with no negative impact on cognitive scores.”
The second-generation antipsychotic was approved in 2016 for treating hallucinations and delusions in patients with Parkinson’s disease.
The drug is a selective antagonist of 5-HT2 receptors, with low affinity for dopamine receptors. This slightly differentiates it from other second-generation antipsychotics that affect dopamine receptors as well as 5-HT2 receptors.
HARMONY was not a typical placebo-controlled, randomized efficacy trial. Rather, it employed a two-phase design: an open-label treatment response period followed by a placebo-controlled randomization limited to open-label responders. Overall, HARMONY involved 392 patients with mild to severe dementia of numerous etiologies, including Alzheimer’s disease (66.8%), Parkinson’s disease dementia (14.3%), frontotemporal dementia (1.8%), vascular dementia (9.7%), and dementia with Lewy bodies (7.4%). All patients entered a 12-week, open-label period during which they received pimavanserin 34 mg daily. The primary endpoint was a combination of least a 30% reduction on the total Scale for the Assessment of Positive Symptom–Hallucinations and Delusions (SAPS-HD) scale plus a score of 1-2 on the Clinical Global Impressions–Improvement (CGI-I) scale, meaning better or very much better.
At 12 weeks, all responders were then randomized to placebo or continued therapy for 26 weeks. The primary endpoint was relapse, defined as at least a 30% worsening of the SAPS-HD relative to open-label baseline, plus a CGI-I score of 6-7 (worse or very much worse).
Patients were aged a mean of 74 years. Most (about 90%) were living at home. Visual hallucinations occurred in 80% and delusions in 83%. At baseline, the mean SAPS-HD score was 24.4, and the mean CGI-Severity score was 4.7. The mean Mini-Mental State Exam (MMSE) score was 16.7.
In the open-label period, pimavanserin reduced the SAPS-HD score at 12 weeks by a mean of 75%. Symptoms began to decline in the first week of treatment, with continuing improvement throughout the treatment period. By week 4, 30% had hit the response target. This number increased steadily, with 51% responding by week 4, 75% by week 8, and 88% by week 12.
By probable diagnosis, response rates were 59.8% in Alzheimer’s patients, 45.5% for those with Lewy body dementia, 71.2% among patients with Parkinson’s disease, 71% in patients with vascular dementia, and 50% in patients with frontotemporal dementia. In the final analysis, 80% of patients overall were considered responders.
The randomized potion began immediately thereafter with no washout period. About 62% (194) of the entire cohort – all responders – entered into the placebo-controlled phase. The remaining patients were either not responders (20%), dropped out because of an adverse event (7.7%), or left the study for unspecified reasons (10%). There was one death, which was not related to the study medication. A total of 41 patients were still being treated when the study was discontinued, and they were excluded from the final analysis.
When the randomized study ended, relapses had occurred in 28.3% of those taking placebo and in 12.6% of those taking pimavanserin – a statistically significant difference (hazard ratio, 0.353). This translated to a 180% reduction in relapse.
The rate of adverse events was similar in both active and placebo groups (41% vs. 36.6%). Serious adverse events occurred in 4.8% and 3.6%, respectively. The most commonly reported adverse events were headache (9.5% vs. 4.5%) and urinary tract infection (6.7% vs. 3.6%). Asthenia occurred in 2.9% of treated patients and 0.9% of placebo patients, but no falls were reported. Anxiety and dizziness were also reported in three patients taking the study medication.
Three patients (2.9%) experienced a prolonged QT phase on ECG, with a mean delay of 5.4 milliseconds from baseline. “Pimavanserin is known to have this effect of QT prolongation,” Dr. Foff said. “This 5.4-ms change is exactly in line with what we already know about pimavanserin and is not clinically significant. We saw no effect on motor function, consistent with the mechanism of action, and very low levels of agitation or aggression.”
Pimavanserin didn’t significantly change cognition from baseline in the open-label period, and in the randomized period, MMSE never differed significantly between groups.
The company also conducted an exploratory subgroup analysis that looked at placebo versus pimavanserin relapse by probable clinical diagnosis. Among the types of dementia, relapse rates for placebo versus pimavanserin were 23% versus 13% among Alzheimer’s patients, 67% versus 0% in Lewy body dementia patients, 50% versus 7% in patients with Parkinson’s, and 17% each among vascular dementia patients. Only one patient in the randomized period had frontotemporal dementia, and that patient relapsed on treatment.
Whether pimavanserin is effective specifically for psychosis in Alzheimer’s disease patients, however, remains in question. In 2018, Acadia published a negative phase 2 trial in a targeted group of 181 Alzheimer’s patients. The primary outcome in each study was mean change on the Neuropsychiatric Inventory–Nursing Home Version psychosis score (NPI-NH-PS). Clive Ballard, MD, of the University of Exeter (England), was the primary investigator.
After 6 weeks, those taking pimavanserin had a 3.76-point change in the NPI-NH-PS, compared with a 1.93-point change in the placebo group. The mean 1.84-point difference was not statistically significant.
This Alzheimer’s-only cohort group also experienced more adverse events than the HARMONY mixed-diagnosis cohort did, although the differences between pimavanserin and placebo groups were not significant. Adverse events included falls (23% of each group) and agitation (21% with pimavanserin vs. 14% with placebo). Cognition was unaffected.
Later that year, Acadia published a subgroup analysis of the same cohort parsing response by symptom severity, again with Dr. Ballard as the lead investigator.
The analysis focused on 57 patients with a baseline NPI-NH-PS of at least 12, indicating severe symptoms of psychosis.
Treatment effects were more pronounced in this group, significantly favoring pimavanserin. On the NPI-NH-PS, 88.9% of the pimavanserin group and 43.3% of the placebo group had at least a 30% improvement; 77.8% and 43.3% experienced at least a 50% improvement. The rate of serious adverse events was similar (18% with pimavanserin and 17% with placebo) and cognition was unaffected. Falls occurred in 14% of the treated group and 20% of the placebo group.
“These findings coupled with the results from other studies of pimavanserin suggest a potential role for pimavanserin in treating psychosis in patients across a range of neuropsychiatric conditions,” Dr. Ballard wrote.
SOURCE: Foff EP et al. CTAD 2019, Late-breaker 1
SAN DIEGO – Pimavanserin, a second-generation antipsychotic approved for hallucinations and delusions in patients with Parkinson’s disease, may also be helpful for psychotic symptoms in other dementia patients, Erin P. Foff, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
In fact, the phase 3 HARMONY trial was stopped early, after an interim efficacy analysis determined that treatment with pimavanserin (Nuplazid) had achieved its primary endpoint – a statistically significant threefold reduction in the risk of relapse (P less than .0033).
Importantly, pimavanserin didn’t significantly affect cognition nor, at least in this controlled setting, did it appear to increase falls or other adverse events often seen with antipsychotic use in elderly patients, said Dr. Foff, clinical lead for the dementia-related psychosis program at Acadia Pharmaceuticals, which makes the drug and sponsored the study.
Based on the positive results, Acadia intends to submit a supplemental new drug application for this indication, according to an investor presentation posted on the company website.
“There is a critical need for an intervention [for psychosis symptoms] in this population,” Dr. Foff said. “We saw a robust response that was well tolerated and well maintained with no negative impact on cognitive scores.”
The second-generation antipsychotic was approved in 2016 for treating hallucinations and delusions in patients with Parkinson’s disease.
The drug is a selective antagonist of 5-HT2 receptors, with low affinity for dopamine receptors. This slightly differentiates it from other second-generation antipsychotics that affect dopamine receptors as well as 5-HT2 receptors.
HARMONY was not a typical placebo-controlled, randomized efficacy trial. Rather, it employed a two-phase design: an open-label treatment response period followed by a placebo-controlled randomization limited to open-label responders. Overall, HARMONY involved 392 patients with mild to severe dementia of numerous etiologies, including Alzheimer’s disease (66.8%), Parkinson’s disease dementia (14.3%), frontotemporal dementia (1.8%), vascular dementia (9.7%), and dementia with Lewy bodies (7.4%). All patients entered a 12-week, open-label period during which they received pimavanserin 34 mg daily. The primary endpoint was a combination of least a 30% reduction on the total Scale for the Assessment of Positive Symptom–Hallucinations and Delusions (SAPS-HD) scale plus a score of 1-2 on the Clinical Global Impressions–Improvement (CGI-I) scale, meaning better or very much better.
At 12 weeks, all responders were then randomized to placebo or continued therapy for 26 weeks. The primary endpoint was relapse, defined as at least a 30% worsening of the SAPS-HD relative to open-label baseline, plus a CGI-I score of 6-7 (worse or very much worse).
Patients were aged a mean of 74 years. Most (about 90%) were living at home. Visual hallucinations occurred in 80% and delusions in 83%. At baseline, the mean SAPS-HD score was 24.4, and the mean CGI-Severity score was 4.7. The mean Mini-Mental State Exam (MMSE) score was 16.7.
In the open-label period, pimavanserin reduced the SAPS-HD score at 12 weeks by a mean of 75%. Symptoms began to decline in the first week of treatment, with continuing improvement throughout the treatment period. By week 4, 30% had hit the response target. This number increased steadily, with 51% responding by week 4, 75% by week 8, and 88% by week 12.
By probable diagnosis, response rates were 59.8% in Alzheimer’s patients, 45.5% for those with Lewy body dementia, 71.2% among patients with Parkinson’s disease, 71% in patients with vascular dementia, and 50% in patients with frontotemporal dementia. In the final analysis, 80% of patients overall were considered responders.
The randomized potion began immediately thereafter with no washout period. About 62% (194) of the entire cohort – all responders – entered into the placebo-controlled phase. The remaining patients were either not responders (20%), dropped out because of an adverse event (7.7%), or left the study for unspecified reasons (10%). There was one death, which was not related to the study medication. A total of 41 patients were still being treated when the study was discontinued, and they were excluded from the final analysis.
When the randomized study ended, relapses had occurred in 28.3% of those taking placebo and in 12.6% of those taking pimavanserin – a statistically significant difference (hazard ratio, 0.353). This translated to a 180% reduction in relapse.
The rate of adverse events was similar in both active and placebo groups (41% vs. 36.6%). Serious adverse events occurred in 4.8% and 3.6%, respectively. The most commonly reported adverse events were headache (9.5% vs. 4.5%) and urinary tract infection (6.7% vs. 3.6%). Asthenia occurred in 2.9% of treated patients and 0.9% of placebo patients, but no falls were reported. Anxiety and dizziness were also reported in three patients taking the study medication.
Three patients (2.9%) experienced a prolonged QT phase on ECG, with a mean delay of 5.4 milliseconds from baseline. “Pimavanserin is known to have this effect of QT prolongation,” Dr. Foff said. “This 5.4-ms change is exactly in line with what we already know about pimavanserin and is not clinically significant. We saw no effect on motor function, consistent with the mechanism of action, and very low levels of agitation or aggression.”
Pimavanserin didn’t significantly change cognition from baseline in the open-label period, and in the randomized period, MMSE never differed significantly between groups.
The company also conducted an exploratory subgroup analysis that looked at placebo versus pimavanserin relapse by probable clinical diagnosis. Among the types of dementia, relapse rates for placebo versus pimavanserin were 23% versus 13% among Alzheimer’s patients, 67% versus 0% in Lewy body dementia patients, 50% versus 7% in patients with Parkinson’s, and 17% each among vascular dementia patients. Only one patient in the randomized period had frontotemporal dementia, and that patient relapsed on treatment.
Whether pimavanserin is effective specifically for psychosis in Alzheimer’s disease patients, however, remains in question. In 2018, Acadia published a negative phase 2 trial in a targeted group of 181 Alzheimer’s patients. The primary outcome in each study was mean change on the Neuropsychiatric Inventory–Nursing Home Version psychosis score (NPI-NH-PS). Clive Ballard, MD, of the University of Exeter (England), was the primary investigator.
After 6 weeks, those taking pimavanserin had a 3.76-point change in the NPI-NH-PS, compared with a 1.93-point change in the placebo group. The mean 1.84-point difference was not statistically significant.
This Alzheimer’s-only cohort group also experienced more adverse events than the HARMONY mixed-diagnosis cohort did, although the differences between pimavanserin and placebo groups were not significant. Adverse events included falls (23% of each group) and agitation (21% with pimavanserin vs. 14% with placebo). Cognition was unaffected.
Later that year, Acadia published a subgroup analysis of the same cohort parsing response by symptom severity, again with Dr. Ballard as the lead investigator.
The analysis focused on 57 patients with a baseline NPI-NH-PS of at least 12, indicating severe symptoms of psychosis.
Treatment effects were more pronounced in this group, significantly favoring pimavanserin. On the NPI-NH-PS, 88.9% of the pimavanserin group and 43.3% of the placebo group had at least a 30% improvement; 77.8% and 43.3% experienced at least a 50% improvement. The rate of serious adverse events was similar (18% with pimavanserin and 17% with placebo) and cognition was unaffected. Falls occurred in 14% of the treated group and 20% of the placebo group.
“These findings coupled with the results from other studies of pimavanserin suggest a potential role for pimavanserin in treating psychosis in patients across a range of neuropsychiatric conditions,” Dr. Ballard wrote.
SOURCE: Foff EP et al. CTAD 2019, Late-breaker 1
REPORTING FROM CTAD 2019




