User login
New biomarker linked to chronic kidney disease
Elevated plasma soluble urokinase-type plasminogen activator receptor (suPAR) levels are associated with declines in estimated glomerular filtration rate (eGFR) and may predict progression to clinical chronic kidney disease, new research suggests.
Data from the Emory Cardiovascular Biobank – a prospective registry of 3,683 patients undergoing cardiac catheterization – showed individuals in the highest quartile of baseline suPAR had a significantly greater annual decline in eGFR than those in the lowest quartile (-4.2 mL/min per 1.73 m2 vs. -0.9mL/min per 1.73 m2).
Similarly, those in the highest suPAR quartile had a threefold greater risk of incident chronic kidney disease than those in the lowest quartile, independent of their race or diabetes status and excluding those with acute kidney injury, according to a paper presented at the meeting sponsored by the American Society of Nephrology and published simultaneously online in the Nov. 5 edition of the New England Journal of Medicine.
The relationship between elevated suPAR and eGFR decline was strongest in patients who had a normal eGFR at baseline (N Engl J Med. 2015 Nov 5 [doi:10.1056/NEJMoa1506362]).
The risk classification for chronic kidney disease using suPAR was greater than existing and well-established risk factors such as C-reactive protein and B-type natriuretic peptide, wrote Dr. Salim S. Hayek, from Emory University, Atlanta, Sanja Sever, Ph.D., of Harvard Medical School, Boston, and their coauthors.
“Our results suggest that suPAR meets critical requirements for a biomarker of chronic kidney disease,” the authors said.
The study was supported by the Abraham J. and Phyllis Katz Foundation, the Robert W. Woodruff Health Sciences Center Fund, Emory Heart and Vascular Center, and the National Institutes of Health. Several authors declared grants and personal fees and committee involvement with private industry, and patents relevant to the work.
Research on suPAR is controversial because several studies have not confirmed an association of an elevated suPAR level with nephropathy. However, these findings should engender validation studies in additional cohorts.
Improving outcome is the ultimate goal for biomarker studies in major progressive diseases, and studies that show that intervention based on suPAR levels is beneficial could cement a potential relationship between suPAR and chronic kidney disease.
Dr. Karl L. Skorecki is with the Technion–Israel Institute of Technology, Haifa, Israel, and Dr. Barry I. Freedman is with Wake Forest School of Medicine, Winston-Salem, N.C. These comments are taken from an accompanying editorial (N Engl J Med. 2015 Nov 5 [doi:10.1056/NEJMe1512997]). Dr. Freedman declared a grant from Novartis.
Research on suPAR is controversial because several studies have not confirmed an association of an elevated suPAR level with nephropathy. However, these findings should engender validation studies in additional cohorts.
Improving outcome is the ultimate goal for biomarker studies in major progressive diseases, and studies that show that intervention based on suPAR levels is beneficial could cement a potential relationship between suPAR and chronic kidney disease.
Dr. Karl L. Skorecki is with the Technion–Israel Institute of Technology, Haifa, Israel, and Dr. Barry I. Freedman is with Wake Forest School of Medicine, Winston-Salem, N.C. These comments are taken from an accompanying editorial (N Engl J Med. 2015 Nov 5 [doi:10.1056/NEJMe1512997]). Dr. Freedman declared a grant from Novartis.
Research on suPAR is controversial because several studies have not confirmed an association of an elevated suPAR level with nephropathy. However, these findings should engender validation studies in additional cohorts.
Improving outcome is the ultimate goal for biomarker studies in major progressive diseases, and studies that show that intervention based on suPAR levels is beneficial could cement a potential relationship between suPAR and chronic kidney disease.
Dr. Karl L. Skorecki is with the Technion–Israel Institute of Technology, Haifa, Israel, and Dr. Barry I. Freedman is with Wake Forest School of Medicine, Winston-Salem, N.C. These comments are taken from an accompanying editorial (N Engl J Med. 2015 Nov 5 [doi:10.1056/NEJMe1512997]). Dr. Freedman declared a grant from Novartis.
Elevated plasma soluble urokinase-type plasminogen activator receptor (suPAR) levels are associated with declines in estimated glomerular filtration rate (eGFR) and may predict progression to clinical chronic kidney disease, new research suggests.
Data from the Emory Cardiovascular Biobank – a prospective registry of 3,683 patients undergoing cardiac catheterization – showed individuals in the highest quartile of baseline suPAR had a significantly greater annual decline in eGFR than those in the lowest quartile (-4.2 mL/min per 1.73 m2 vs. -0.9mL/min per 1.73 m2).
Similarly, those in the highest suPAR quartile had a threefold greater risk of incident chronic kidney disease than those in the lowest quartile, independent of their race or diabetes status and excluding those with acute kidney injury, according to a paper presented at the meeting sponsored by the American Society of Nephrology and published simultaneously online in the Nov. 5 edition of the New England Journal of Medicine.
The relationship between elevated suPAR and eGFR decline was strongest in patients who had a normal eGFR at baseline (N Engl J Med. 2015 Nov 5 [doi:10.1056/NEJMoa1506362]).
The risk classification for chronic kidney disease using suPAR was greater than existing and well-established risk factors such as C-reactive protein and B-type natriuretic peptide, wrote Dr. Salim S. Hayek, from Emory University, Atlanta, Sanja Sever, Ph.D., of Harvard Medical School, Boston, and their coauthors.
“Our results suggest that suPAR meets critical requirements for a biomarker of chronic kidney disease,” the authors said.
The study was supported by the Abraham J. and Phyllis Katz Foundation, the Robert W. Woodruff Health Sciences Center Fund, Emory Heart and Vascular Center, and the National Institutes of Health. Several authors declared grants and personal fees and committee involvement with private industry, and patents relevant to the work.
Elevated plasma soluble urokinase-type plasminogen activator receptor (suPAR) levels are associated with declines in estimated glomerular filtration rate (eGFR) and may predict progression to clinical chronic kidney disease, new research suggests.
Data from the Emory Cardiovascular Biobank – a prospective registry of 3,683 patients undergoing cardiac catheterization – showed individuals in the highest quartile of baseline suPAR had a significantly greater annual decline in eGFR than those in the lowest quartile (-4.2 mL/min per 1.73 m2 vs. -0.9mL/min per 1.73 m2).
Similarly, those in the highest suPAR quartile had a threefold greater risk of incident chronic kidney disease than those in the lowest quartile, independent of their race or diabetes status and excluding those with acute kidney injury, according to a paper presented at the meeting sponsored by the American Society of Nephrology and published simultaneously online in the Nov. 5 edition of the New England Journal of Medicine.
The relationship between elevated suPAR and eGFR decline was strongest in patients who had a normal eGFR at baseline (N Engl J Med. 2015 Nov 5 [doi:10.1056/NEJMoa1506362]).
The risk classification for chronic kidney disease using suPAR was greater than existing and well-established risk factors such as C-reactive protein and B-type natriuretic peptide, wrote Dr. Salim S. Hayek, from Emory University, Atlanta, Sanja Sever, Ph.D., of Harvard Medical School, Boston, and their coauthors.
“Our results suggest that suPAR meets critical requirements for a biomarker of chronic kidney disease,” the authors said.
The study was supported by the Abraham J. and Phyllis Katz Foundation, the Robert W. Woodruff Health Sciences Center Fund, Emory Heart and Vascular Center, and the National Institutes of Health. Several authors declared grants and personal fees and committee involvement with private industry, and patents relevant to the work.
FROM KIDNEY WEEK 2015
Key clinical point:Elevated plasma soluble urokinase-type plasminogen activator receptor (suPAR) levels may predict clinical chronic kidney disease.
Major finding: Patients in the highest suPAR quartile had a threefold greater risk of incident chronic kidney disease than those in the lowest quartile.
Data source: The Emory Cardiovascular Biobank, a prospective registry of 3,683 patients undergoing cardiac catheterization.
Disclosures: The study was supported by the Abraham J. and Phyllis Katz Foundation, the Robert W. Woodruff Health Sciences Center Fund, Emory Heart and Vascular Center, and the National Institutes of Health. Several authors declared grants and personal fees and committee involvement with private industry, and patents relevant to the work.
Shorter sleep linked to faster kidney function decline
SAN DIEGO – Shorter sleep duration is prospectively and independently associated with a faster decline in renal function, according to results from a large observational study.
“We know that people with chronic kidney disease have a disrupted sleep pattern, particularly those on dialysis,” lead study author Dr. Ciaran Joseph McMullan said in an interview in advance of Kidney Week 2015.
“Disrupted sleep is actually one of the symptoms of end-stage renal disease,” Dr. McMullan noted, “but what hasn’t been studied as much is when the sleep disturbances occur in the progression from normal kidney function to chronic kidney disease and further on to end-stage renal disease. It’s unclear at what point those abnormal sleep patterns develop.”
Medical research in the past decade has demonstrated that disrupting people’s sleep can affect their metabolism in profound ways, explained Dr. McMullan of the renal division at Brigham and Women’s Hospital, Boston.
“You can take healthy people, reduce their sleep each night to around 5 hours, and they can develop characteristics of diabetes,” he said. “In the long term, we know that people who sleep less are at increased risk of developing hypertension, an increased risk of developing diabetes, and an increased mortality overall. Two of the most important risk factors for kidney disease are diabetes and hypertension.”
For the current study, Dr. McMullan and his associates prospectively evaluated 4,238 participants from the Nurses’ Health Study who had their renal function measured on at least two occasions and had their sleep function reported in a 24-hour period between 1989 and 2000.
Sleep duration was based on self-report and included four categories: 5 hours or fewer per night, 6 hours per night, 7-8 hours per night, and 9 or more hours per night. Rapid decline in renal function was defined as a decline of estimated glomerular filtration rate (eGFR) of 25% or more over the 11-year period.
The researchers found that, compared with sleeping 7-8 hours per night, the adjusted odds ratios for a rapid decline in renal function were 1.65 for sleeping 5 or fewer hours per night, 1.31 for sleeping 6 hours per night, and 0.78 for sleeping 9 or more hours per night.
At the same time, the adjusted annualized decline in eGFR was 1.2 mL/min per 1.73 m2 per year among those sleeping 5 or fewer hours per night, 0.9 mL/ per 1.73 m2 per year among those sleeping 6 hours per night, and 0.8 mL/ per 1.73 m2 per year among those sleeping 7-8 hours per night, as well as those sleeping 9 or more hours per night (P = .02 for trend).
“This is the first time that we’ve studied people longitudinally to see how their kidney function changes over time based on how much they sleep per night,” Dr. McMullan said.
While he acknowledged that the study is limited by its observational design, the findings “lead us to consider if normal kidney function is disrupted by short sleep that may cause an irreversible decline in kidney function over time.
“I think we need to repeat this study using more accurate measurements of sleep, [such as] polysomnography to measure sleep duration and quality of sleep more accurately,” he said. “This would help us answer the question, ‘Why do these individual with short sleep duration have a more rapid decline of their kidney function?’ ”
Dr. McMullan added that “we also don’t know if lengthening sleep duration in individuals who have habitual sleep restriction is beneficial for their kidney function. This would be important to know before we can make any kind of clinical recommendation.”
The study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McMullan reported having no financial disclosures.
SAN DIEGO – Shorter sleep duration is prospectively and independently associated with a faster decline in renal function, according to results from a large observational study.
“We know that people with chronic kidney disease have a disrupted sleep pattern, particularly those on dialysis,” lead study author Dr. Ciaran Joseph McMullan said in an interview in advance of Kidney Week 2015.
“Disrupted sleep is actually one of the symptoms of end-stage renal disease,” Dr. McMullan noted, “but what hasn’t been studied as much is when the sleep disturbances occur in the progression from normal kidney function to chronic kidney disease and further on to end-stage renal disease. It’s unclear at what point those abnormal sleep patterns develop.”
Medical research in the past decade has demonstrated that disrupting people’s sleep can affect their metabolism in profound ways, explained Dr. McMullan of the renal division at Brigham and Women’s Hospital, Boston.
“You can take healthy people, reduce their sleep each night to around 5 hours, and they can develop characteristics of diabetes,” he said. “In the long term, we know that people who sleep less are at increased risk of developing hypertension, an increased risk of developing diabetes, and an increased mortality overall. Two of the most important risk factors for kidney disease are diabetes and hypertension.”
For the current study, Dr. McMullan and his associates prospectively evaluated 4,238 participants from the Nurses’ Health Study who had their renal function measured on at least two occasions and had their sleep function reported in a 24-hour period between 1989 and 2000.
Sleep duration was based on self-report and included four categories: 5 hours or fewer per night, 6 hours per night, 7-8 hours per night, and 9 or more hours per night. Rapid decline in renal function was defined as a decline of estimated glomerular filtration rate (eGFR) of 25% or more over the 11-year period.
The researchers found that, compared with sleeping 7-8 hours per night, the adjusted odds ratios for a rapid decline in renal function were 1.65 for sleeping 5 or fewer hours per night, 1.31 for sleeping 6 hours per night, and 0.78 for sleeping 9 or more hours per night.
At the same time, the adjusted annualized decline in eGFR was 1.2 mL/min per 1.73 m2 per year among those sleeping 5 or fewer hours per night, 0.9 mL/ per 1.73 m2 per year among those sleeping 6 hours per night, and 0.8 mL/ per 1.73 m2 per year among those sleeping 7-8 hours per night, as well as those sleeping 9 or more hours per night (P = .02 for trend).
“This is the first time that we’ve studied people longitudinally to see how their kidney function changes over time based on how much they sleep per night,” Dr. McMullan said.
While he acknowledged that the study is limited by its observational design, the findings “lead us to consider if normal kidney function is disrupted by short sleep that may cause an irreversible decline in kidney function over time.
“I think we need to repeat this study using more accurate measurements of sleep, [such as] polysomnography to measure sleep duration and quality of sleep more accurately,” he said. “This would help us answer the question, ‘Why do these individual with short sleep duration have a more rapid decline of their kidney function?’ ”
Dr. McMullan added that “we also don’t know if lengthening sleep duration in individuals who have habitual sleep restriction is beneficial for their kidney function. This would be important to know before we can make any kind of clinical recommendation.”
The study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McMullan reported having no financial disclosures.
SAN DIEGO – Shorter sleep duration is prospectively and independently associated with a faster decline in renal function, according to results from a large observational study.
“We know that people with chronic kidney disease have a disrupted sleep pattern, particularly those on dialysis,” lead study author Dr. Ciaran Joseph McMullan said in an interview in advance of Kidney Week 2015.
“Disrupted sleep is actually one of the symptoms of end-stage renal disease,” Dr. McMullan noted, “but what hasn’t been studied as much is when the sleep disturbances occur in the progression from normal kidney function to chronic kidney disease and further on to end-stage renal disease. It’s unclear at what point those abnormal sleep patterns develop.”
Medical research in the past decade has demonstrated that disrupting people’s sleep can affect their metabolism in profound ways, explained Dr. McMullan of the renal division at Brigham and Women’s Hospital, Boston.
“You can take healthy people, reduce their sleep each night to around 5 hours, and they can develop characteristics of diabetes,” he said. “In the long term, we know that people who sleep less are at increased risk of developing hypertension, an increased risk of developing diabetes, and an increased mortality overall. Two of the most important risk factors for kidney disease are diabetes and hypertension.”
For the current study, Dr. McMullan and his associates prospectively evaluated 4,238 participants from the Nurses’ Health Study who had their renal function measured on at least two occasions and had their sleep function reported in a 24-hour period between 1989 and 2000.
Sleep duration was based on self-report and included four categories: 5 hours or fewer per night, 6 hours per night, 7-8 hours per night, and 9 or more hours per night. Rapid decline in renal function was defined as a decline of estimated glomerular filtration rate (eGFR) of 25% or more over the 11-year period.
The researchers found that, compared with sleeping 7-8 hours per night, the adjusted odds ratios for a rapid decline in renal function were 1.65 for sleeping 5 or fewer hours per night, 1.31 for sleeping 6 hours per night, and 0.78 for sleeping 9 or more hours per night.
At the same time, the adjusted annualized decline in eGFR was 1.2 mL/min per 1.73 m2 per year among those sleeping 5 or fewer hours per night, 0.9 mL/ per 1.73 m2 per year among those sleeping 6 hours per night, and 0.8 mL/ per 1.73 m2 per year among those sleeping 7-8 hours per night, as well as those sleeping 9 or more hours per night (P = .02 for trend).
“This is the first time that we’ve studied people longitudinally to see how their kidney function changes over time based on how much they sleep per night,” Dr. McMullan said.
While he acknowledged that the study is limited by its observational design, the findings “lead us to consider if normal kidney function is disrupted by short sleep that may cause an irreversible decline in kidney function over time.
“I think we need to repeat this study using more accurate measurements of sleep, [such as] polysomnography to measure sleep duration and quality of sleep more accurately,” he said. “This would help us answer the question, ‘Why do these individual with short sleep duration have a more rapid decline of their kidney function?’ ”
Dr. McMullan added that “we also don’t know if lengthening sleep duration in individuals who have habitual sleep restriction is beneficial for their kidney function. This would be important to know before we can make any kind of clinical recommendation.”
The study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McMullan reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: Kidney function may be compromised when sleep is disrupted.
Major finding: Compared with sleeping 7-8 hours per night, the adjusted odds ratio for a rapid decline in renal function was 1.65 for sleeping 5 or fewer hours per night.
Data source: A prospective evaluation of 4,238 participants from the Nurses’ Health Study who had their renal function measured on at least two occasions and had their sleep function reported in a 24-hour period between 1989 and 2000.
Disclosures: The study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McMullan reported having no financial disclosures.
Renal Denervation
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Predictive Factors for CKD
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
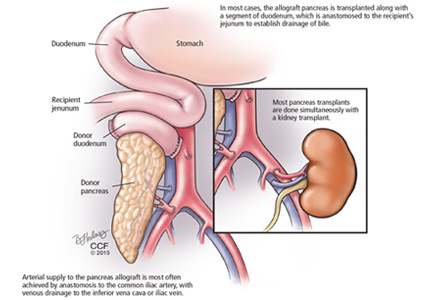
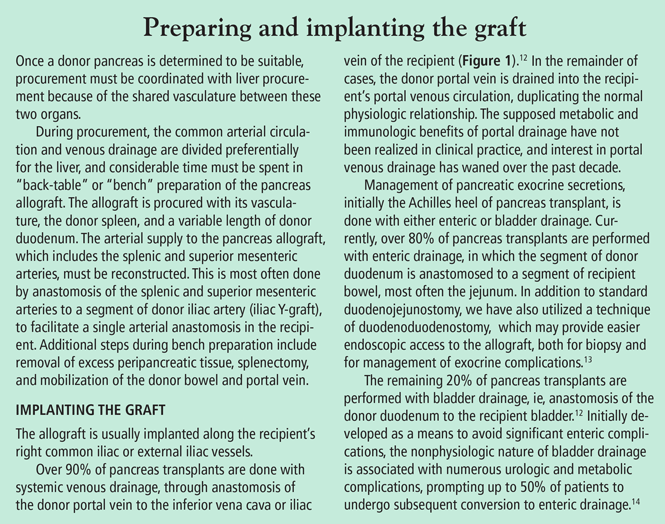
Pancreas transplant for diabetes mellitus
Pancreas transplant is the only long-term diabetes treatment that consistently results in normal hemoglobin A1c levels without the risk of severe hypoglycemia. Additionally, pancreas transplant may prevent, halt, or even reverse the complications of diabetes.
Here, we explore the indications, options, and outcomes of pancreas transplant as a treatment for diabetes mellitus.
DIABETES IS COMMON, AND OFTEN NOT WELL CONTROLLED
Diabetes mellitus affects more than 25 million people in the United States (8.3% of the population) and is the leading cause of kidney failure, nontraumatic lower-limb amputation, and adult-onset blindness. In 2007, nearly $116 billion was spent on diabetes treatment, not counting another $58 billion in indirect costs such as disability, work loss, and premature death.1
Despite the tremendous expenditure in human, material, and financial resources, only about 50% of patients achieve their diabetes treatment goals. In 2013, a large US population-based study2 reported that 52.2% of patients were achieving the American Diabetes Association treatment goal of hemoglobin A1c lower than 7%. A similar study in South Korea3 found that 45.6% were at this goal.
Most of the patients in these studies had type 2 diabetes, and the data suggested that attaining glycemic goals is more difficult in insulin-treated patients. Studies of patients with type 1 diabetes found hemoglobin A1c levels lower than 7% in only 8.1% of hospitalized patients with type 1 diabetes, and in only 13% in an outpatient diabetes clinic.4,5
YET RATES OF PANCREAS TRANSPLANT ARE DECLINING
Pancreas transplant was first performed more than 40 years ago at the University of Minnesota.6 Since then, dramatic changes in immunosuppression, organ preservation, surgical technique, and donor and recipient selection have brought about significant progress.
Currently, more than 13,000 patients are alive with a functioning pancreas allograft. After reaching a peak in 2004, the annual number of pancreas transplants performed in the United States has declined steadily, whereas the procedure continues to increase in popularity outside North America.7 The primary reason for the decline is recognition of donor factors that lead to success—surgeons are refusing to transplant organs they might have accepted previously, because experience suggests they would yield poor results. In the United States, 1,043 pancreas transplants were performed in 2012, and more than 3,100 patients were on the waiting list.8
Islet cell transplant—a different procedure involving harvesting, encapsulating, and implanting insulin-producing beta cells—has not gained widespread application due to very low long-term success rates.
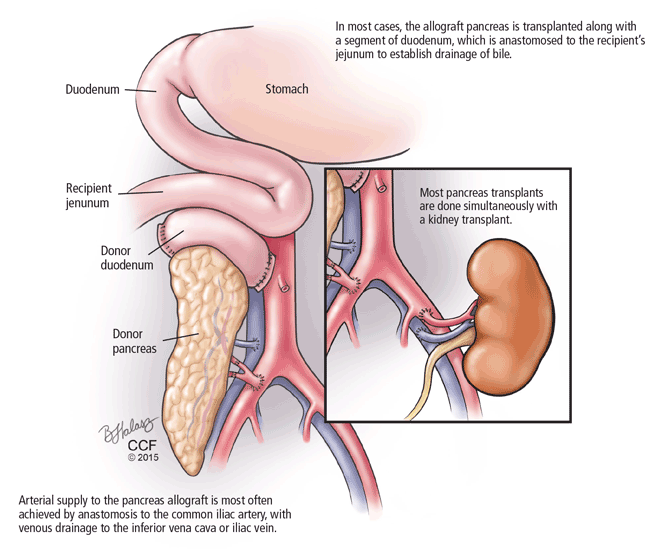
THREE CATEGORIES OF PANCREAS TRANSPLANT
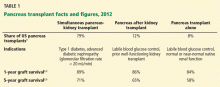
Pancreas transplant can be categorized according to whether the patient is also receiving or has already received a kidney graft (Table 1).
Simultaneous kidney and pancreas transplant is performed in patients who have type 1 diabetes with advanced chronic kidney disease due to diabetic nephropathy. This remains the most commonly performed type, accounting for 79% of all pancreas transplants in 2012.8
Pancreas-after-kidney transplant is most often done after a living-donor kidney transplant. This procedure accounted for most of the increase in pancreas transplants during the first decade of the 2000s. However, the number of these procedures has steadily decreased since 2004, and in 2012 accounted for only 12% of pancreas transplants.8
Pancreas transplant alone is performed in nonuremic diabetic patients who have labile blood sugar control. Performed in patients with preserved renal function but severe complications of “brittle” diabetes, such as hypoglycemic unawareness, this type accounts for 8% of pancreas transplants.9
Indications for pancreas transplant
A small number of these procedures are done for indications unrelated to diabetes mellitus. In most of these cases, the pancreas is transplanted as part of a multivisceral transplant to facilitate the technical (surgical) aspect of the procedure—the pancreas, liver, stomach, gallbladder, and part of the intestines are transplanted en bloc to maintain the native vasculature. Very infrequently, pancreas transplant is done to replace exocrine pancreatic function.
A small, select group of patients with type 2 diabetes and low body mass index (BMI) may be eligible for pancreas transplant, and they accounted for 8.2% of active candidates in 2012.8 However, most pancreas transplants are performed in patients with type 1 diabetes.
WHAT MAKES A GOOD ALLOGRAFT?
Pancreas allografts are procured as whole organs from brain-dead organ donors. Relatively few pancreas allografts (3.1% in 2012) are from cardiac-death donors, because of concern about warm ischemic injury during the period of circulatory arrest.8
Proper donor selection is critical to the success of pancreas transplant, as donor factors including medical history, age, BMI, and cause of death can significantly affect the outcome. In general, transplant of a pancreas allograft from a young donor (age < 30) with excellent organ function, low BMI, and traumatic cause of death provides the best chance of success.
The Pancreas Donor Risk Index (PDRI)10 was developed after analysis of objective donor criteria, transplant type, and ischemic time in grafts transplanted between 2000 and 2006. One-year graft survival was directly related to the PDRI and ranged between 77% and 87% in recipients of “standard” pancreas allografts (PDRI score of 1.0). Use of grafts from the highest (worst) three quintiles of PDRI (PDRI score > 1.16) was associated with 1-year graft survival rates of 67% to 82%, significantly inferior to that seen with “higher- quality” grafts, again emphasizing the need for rigorous donor selection.10
In addition to these objective measures, visual assessment of pancreas quality at the time of procurement remains an equally important predictor of success. Determination of subjective features, such as fatty infiltration and glandular fibrosis, requires surgical experience developed over several years. In a 2010 analysis, dissatisfaction with the quality of the donor graft on inspection accounted for more than 80% of refusals of potential pancreas donors.11 These studies illustrate an ill-defined aspect of pancreas transplant, ie, even when the pancreas donor is perceived to be suitable, the outcome may be markedly different.
SURGICAL COMPLICATIONS
Surgical complications have long been considered a limiting factor in the growth of pancreas transplant. Technical failure or loss of the graft within 90 days is most commonly due to graft thrombosis, leakage of the enteric anastomosis, or severe peripancreatic infection. The rate of technical failure has declined across all recipient categories and is currently about 9%.8
DO RECIPIENT FACTORS AFFECT OUTCOMES?
As mentioned above, the PDRI identifies donor factors that influence the 1-year graft survival rate. Recipient factors are also thought to play a role, although the influence of these factors has not been consistently demonstrated.
Humar et al15 found that recipient obesity (defined in this study as BMI > 25 kg/m2) and donor age over 40 were risk factors for early laparotomy after pancreas transplant.15 Moreover, patients undergoing early laparotomy had poorer graft survival outcomes.
This finding was reinforced by an analysis of 5,725 primary simultaneous pancreas-kidney recipients between 2000 and 2007. Obesity (BMI 30 ≥ kg/m2) was associated with increased rates of patient death, pancreas graft loss, and kidney graft loss at 3 years.16
More recently, Finger et al17 did not find a statistically significant association between recipient BMI and technical failure, but they did notice a trend toward increased graft loss with a BMI greater than 25 kg/m2. Similarly, others have not found a clear adverse association between recipient BMI and pancreas graft survival.
Intuitively, obesity and other recipient factors such as age, vascular disease, duration of diabetes, and dialysis should influence pancreas graft survival but have not been shown in analyses to carry an adverse effect.18 The inability to consistently find adverse effects of recipient characteristics is most likely due to the relative similarity between the vast majority of pancreas transplant recipients and the relatively small numbers of adverse events. In 98 consecutive pancreas transplants at our center between 2009 and 2014, the technical loss rate was 1.8% (unpublished data).
Acute rejection most commonly occurs during the first year and is usually reversible. More than 1 year after transplant, graft loss is due to chronic rejection, and death is usually from underlying cardiovascular disease.
The immunosuppressive regimens used in pancreas transplant are similar to those in kidney transplant. Since the pancreas is considered to be more immunogenic than other organs, most centers employ a strategy of induction immunosuppression with T-cell–depleting or interleukin 2-receptor antibodies. Maintenance immunosuppression consists of a calcineurin inhibitor (tacrolimus or cyclosporine), an antimetabolite (mycophenolate), and a corticosteroid.8
Immunosuppressive complications occur at a rate similar to that seen in other solid-organ transplants and include an increased risk of opportunistic infection and malignancy. The risk of these complications must be balanced against the patient’s risk of health decline with dialysis and insulin-based therapies.
OVERALL OUTCOMES ARE GOOD
The success rate of pancreas transplant is currently at its highest since the inception of the procedure. The unadjusted patient survival rate for all groups is over 96% at 1 year, and over 80% at 5 years.8 One-year patient survival after pancreas transplant alone, at better than 96%, is the highest of all organ transplant procedures.9
Several recently published single-center reviews of pancreas transplant since 2000 report patient survival rates of 96% to 100% at 1 year and 88% to 100% at 5 years.19–22 This variability is likely closely linked to donor and recipient selection, as centers performing smaller numbers of transplants tend to be more selective and, in turn, report higher patient survival rates.19,21
Long-term patient survival outcomes can be gathered from larger, registry-based reviews, accepting limitations in assessing causes of patient death. Siskind et al23 analyzed the outcomes of 20,854 US pancreas transplants done between 1996 and 2012 and found the 10-year patient survival rate ranged from 43% to 77% and was highly dependent on patient age at the time of the procedure.23 Patient survival after transplant must be balanced against the generally poor long-term survival prospects of diabetic patients on dialysis.
By type of transplant, pancreas graft survival rates at 1 year are 89% for simultaneous pancreas-kidney transplant, 86% for pancreas-after-kidney transplant, and 84% for pancreas-alone transplant. Graft survival rates at 5 years are 71% for simultaneous pancreas-kidney transplant, 65% for pancreas-after-kidney transplant, and 58% for pancreas-alone transplant.8,9
Simultaneous pancreas-kidney transplant has been shown to improve the survival rate compared with cadaveric kidney transplant alone in patients with type 1 diabetes and chronic kidney disease.24,25 The survival benefit of isolated pancreas transplant (after kidney transplant and alone) is not evident at 4-year follow-up compared with patients on the waiting list. However, the benefit for the individual patient must be considered by weighing the incapacities experienced with insulin-based treatments against the risks of surgery and immunosuppression.26,27 For patients who have experienced frequent and significant hypoglycemic episodes, particularly those requiring third-party assistance, pancreas transplant can be a lifesaving procedure.
Effects on secondary diabetic complications
Notwithstanding the effect on the patient’s life span, data from several studies of long-term pancreas transplant recipients suggest that secondary diabetic complications can be halted or even improved. Most of these studies examined the effect of restoring euglycemia in nephropathy and the subsequent influence on renal function.
Effect on renal function. Kleinclauss et al28 examined renal allograft function in type 1 diabetic recipients of living-donor kidney transplants. Comparing kidney allograft survival and function in patients who received a subsequent pancreas-after-kidney transplant vs those who did not, graft survival was superior after 5 years, and the estimated glomerular filtration rate was 10 mL/min higher in pancreas-after-kidney recipients.28 This improvement in renal function was not seen immediately after the pancreas transplant but became evident more than 4 years after establishment of normoglycemia. Somewhat similarly, reversal of diabetic changes in native kidney biopsies has been seen 10 years after pancreas transplant.29
Effect on neuropathy. In other studies, reversal of autonomic neuropathy and hypoglycemic unawareness and improvements in peripheral sensory-motor neuropathy have also been observed.30–32
Effect on retinopathy. Improvements in early-stage nonproliferative diabetic retinopathy and laser-treated proliferative lesions have been seen, even within short periods of follow-up.33 Other groups have shown a significantly higher proportion of improvement or stability of advanced diabetic retinopathy at 3 years after simultaneous pancreas-kidney transplant, compared with kidney transplant alone in patients with type 1 diabetes.34
Effect on heart disease. Salutary effects on cardiovascular risk factors and amelioration of cardiac morphology and functional cardiac indices have been seen within the first posttransplant year.35 Moreover, with longer follow-up (nearly 4 years), simultaneous pancreas-kidney recipients with functioning pancreas grafts were found to have less progression of coronary atherosclerosis than simultaneous pancreas-kidney recipients with early pancreas graft loss.36 These data provide a potential pathophysiologic mechanism for the long-term survival advantage seen in uremic type 1 diabetic patients undergoing simultaneous pancreas-kidney transplant.
In the aggregate, these findings suggest that, in the absence of surgical and immunosuppression-related complications, a functioning pancreas allograft can alter the progress of diabetic complications. As an extension of these results, pancreas transplant done earlier in the course of diabetes may have an even greater impact.
- Centers for Disease Control and Prevention (CDC). National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 12, 2015.
- Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med 2013; 368:1613–1624.
- Jeon JY, Kim DJ, Ko SH, et al; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J 2014; 38:197–203.
- Govan L, Wu O, Briggs A, et al; Scottish Diabetes Research Network Epidemiology Group. Achieved levels of HbA1c and likelihood of hospital admission in people with type 1 diabetes in the Scottish population: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetes Care 2011; 34:1992–1997.
- Bryant W, Greenfield JR, Chisholm DJ, Campbell LV. Diabetes guidelines: easier to preach than to practise? Med J Aust 2006; 185:305–309.
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837.
- Gruessner AC, Gruessner RW. Pancreas transplant outcomes for United States and non United States cases as reported to the United Network for Organ Sharing and the International Pancreas Transplant Registry as of December 2011. Clin Transpl 2012: 23–40.
- Israni AK, Skeans MA, Gustafson SK, et al. OPTN/SRTR 2012 Annual Data Report: pancreas. Am J Transplant 2014; 14(suppl 1):45–68
- Gruessner RW, Gruessner AC. Pancreas transplant alone: a procedure coming of age. Diabetes Care 2013; 36:2440–2447.
- Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant 2010; 10:837–845.
- Wiseman AC, Wainright JL, Sleeman E, et al. An analysis of the lack of donor pancreas utilization from younger adult organ donors. Transplantation 2010; 90:475–480.
- Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol 2013; 9:555–562.
- Gunasekaran G, Wee A, Rabets J, Winans C, Krishnamurthi V. Duodenoduodenostomy in pancreas transplantation. Clin Transplant 2012; 26:550–557.
- Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg 2009; 250:618–630.
- Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 2000; 231:269–275.
- Sampaio MS, Reddy PN, Kuo HT, et al. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation 2010; 89:1117–1125.
- Finger EB, Radosevich DM, Dunn TB, et al. A composite risk model for predicting technical failure in pancreas transplantation. Am J Transplant 2013; 13:1840–1849.
- Fridell JA, Mangus RS, Taber TE, et al. Growth of a nation part II: impact of recipient obesity on whole-organ pancreas transplantation. Clin Transplant 2011; 25:E366–E374.
- Tai DS, Hong J, Busuttil RW, Lipshutz GS. Low rates of short- and long-term graft loss after kidney-pancreas transplant from a single center. JAMA Surg 2013; 148:368–373.
- Bazerbachi F, Selzner M, Marquez MA, et al. Pancreas-after-kidney versus synchronous pancreas-kidney transplantation: comparison of intermediate-term results. Transplantation 2013; 95:489–494.
- Laftavi MR, Pankewycz O, Gruessner A, et al. Long-term outcomes of pancreas after kidney transplantation in small centers: is it justified? Transplant Proc 2014; 46:1920–1923.
- Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, Rogers J. Similar results with solitary pancreas transplantation compared with simultaneous pancreas-kidney transplantation in the new millennium. Transplant Proc 2014; 46:1924–1927.
- Siskind E, Maloney C, Akerman M, et al. An analysis of pancreas transplantation outcomes based on age groupings—an update of the UNOS database. Clin Transplant 2014; 28:990–994.
- Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 2001; 71:82–90.
- Reddy KS, Stablein D, Taranto S, et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis 2003; 41:464–470.
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290:2817–2823.
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4:2018–2026.
- Kleinclauss F, Fauda M, Sutherland DE, et al. Pancreas after living donor kidney transplants in diabetic patients: impact on long-term kidney graft function. Clin Transplant 2009; 23:437–446.
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339:69–75.
- Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia 1996; 39:1415–1424.
- Robertson RP. Update on transplanting beta cells for reversing type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39:655–667.
- Robertson RP, Holohan TV, Genuth S. Therapeutic controversy: pancreas transplantation for type I diabetes. J Clin Endocrinol Metab 1998; 83:1868–1674.
- Giannarelli R, Coppelli A, Sartini MS, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia 2006; 49:2977–2982.
- Koznarová R, Saudek F, Sosna T, et al. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant 2000; 9:903–908.
- Coppelli A, Giannarelli R, Mariotti R, et al. Pancreas transplant alone determines early improvement of cardiovascular risk factors and cardiac function in type 1 diabetic patients. Transplantation 2003; 76:974–976.
- Jukema JW, Smets YF, van der Pijl JW, et al. Impact of simultaneous pancreas and kidney transplantation on progression of coronary atherosclerosis in patients with end-stage renal failure due to type 1 diabetes. Diabetes Care 2002; 25:906–911.
Pancreas transplant is the only long-term diabetes treatment that consistently results in normal hemoglobin A1c levels without the risk of severe hypoglycemia. Additionally, pancreas transplant may prevent, halt, or even reverse the complications of diabetes.
Here, we explore the indications, options, and outcomes of pancreas transplant as a treatment for diabetes mellitus.
DIABETES IS COMMON, AND OFTEN NOT WELL CONTROLLED
Diabetes mellitus affects more than 25 million people in the United States (8.3% of the population) and is the leading cause of kidney failure, nontraumatic lower-limb amputation, and adult-onset blindness. In 2007, nearly $116 billion was spent on diabetes treatment, not counting another $58 billion in indirect costs such as disability, work loss, and premature death.1
Despite the tremendous expenditure in human, material, and financial resources, only about 50% of patients achieve their diabetes treatment goals. In 2013, a large US population-based study2 reported that 52.2% of patients were achieving the American Diabetes Association treatment goal of hemoglobin A1c lower than 7%. A similar study in South Korea3 found that 45.6% were at this goal.
Most of the patients in these studies had type 2 diabetes, and the data suggested that attaining glycemic goals is more difficult in insulin-treated patients. Studies of patients with type 1 diabetes found hemoglobin A1c levels lower than 7% in only 8.1% of hospitalized patients with type 1 diabetes, and in only 13% in an outpatient diabetes clinic.4,5
YET RATES OF PANCREAS TRANSPLANT ARE DECLINING
Pancreas transplant was first performed more than 40 years ago at the University of Minnesota.6 Since then, dramatic changes in immunosuppression, organ preservation, surgical technique, and donor and recipient selection have brought about significant progress.
Currently, more than 13,000 patients are alive with a functioning pancreas allograft. After reaching a peak in 2004, the annual number of pancreas transplants performed in the United States has declined steadily, whereas the procedure continues to increase in popularity outside North America.7 The primary reason for the decline is recognition of donor factors that lead to success—surgeons are refusing to transplant organs they might have accepted previously, because experience suggests they would yield poor results. In the United States, 1,043 pancreas transplants were performed in 2012, and more than 3,100 patients were on the waiting list.8
Islet cell transplant—a different procedure involving harvesting, encapsulating, and implanting insulin-producing beta cells—has not gained widespread application due to very low long-term success rates.
THREE CATEGORIES OF PANCREAS TRANSPLANT
Pancreas transplant can be categorized according to whether the patient is also receiving or has already received a kidney graft (Table 1).
Simultaneous kidney and pancreas transplant is performed in patients who have type 1 diabetes with advanced chronic kidney disease due to diabetic nephropathy. This remains the most commonly performed type, accounting for 79% of all pancreas transplants in 2012.8
Pancreas-after-kidney transplant is most often done after a living-donor kidney transplant. This procedure accounted for most of the increase in pancreas transplants during the first decade of the 2000s. However, the number of these procedures has steadily decreased since 2004, and in 2012 accounted for only 12% of pancreas transplants.8
Pancreas transplant alone is performed in nonuremic diabetic patients who have labile blood sugar control. Performed in patients with preserved renal function but severe complications of “brittle” diabetes, such as hypoglycemic unawareness, this type accounts for 8% of pancreas transplants.9
Indications for pancreas transplant
A small number of these procedures are done for indications unrelated to diabetes mellitus. In most of these cases, the pancreas is transplanted as part of a multivisceral transplant to facilitate the technical (surgical) aspect of the procedure—the pancreas, liver, stomach, gallbladder, and part of the intestines are transplanted en bloc to maintain the native vasculature. Very infrequently, pancreas transplant is done to replace exocrine pancreatic function.
A small, select group of patients with type 2 diabetes and low body mass index (BMI) may be eligible for pancreas transplant, and they accounted for 8.2% of active candidates in 2012.8 However, most pancreas transplants are performed in patients with type 1 diabetes.
WHAT MAKES A GOOD ALLOGRAFT?
Pancreas allografts are procured as whole organs from brain-dead organ donors. Relatively few pancreas allografts (3.1% in 2012) are from cardiac-death donors, because of concern about warm ischemic injury during the period of circulatory arrest.8


Proper donor selection is critical to the success of pancreas transplant, as donor factors including medical history, age, BMI, and cause of death can significantly affect the outcome. In general, transplant of a pancreas allograft from a young donor (age < 30) with excellent organ function, low BMI, and traumatic cause of death provides the best chance of success.
The Pancreas Donor Risk Index (PDRI)10 was developed after analysis of objective donor criteria, transplant type, and ischemic time in grafts transplanted between 2000 and 2006. One-year graft survival was directly related to the PDRI and ranged between 77% and 87% in recipients of “standard” pancreas allografts (PDRI score of 1.0). Use of grafts from the highest (worst) three quintiles of PDRI (PDRI score > 1.16) was associated with 1-year graft survival rates of 67% to 82%, significantly inferior to that seen with “higher- quality” grafts, again emphasizing the need for rigorous donor selection.10
In addition to these objective measures, visual assessment of pancreas quality at the time of procurement remains an equally important predictor of success. Determination of subjective features, such as fatty infiltration and glandular fibrosis, requires surgical experience developed over several years. In a 2010 analysis, dissatisfaction with the quality of the donor graft on inspection accounted for more than 80% of refusals of potential pancreas donors.11 These studies illustrate an ill-defined aspect of pancreas transplant, ie, even when the pancreas donor is perceived to be suitable, the outcome may be markedly different.
SURGICAL COMPLICATIONS
Surgical complications have long been considered a limiting factor in the growth of pancreas transplant. Technical failure or loss of the graft within 90 days is most commonly due to graft thrombosis, leakage of the enteric anastomosis, or severe peripancreatic infection. The rate of technical failure has declined across all recipient categories and is currently about 9%.8
DO RECIPIENT FACTORS AFFECT OUTCOMES?
As mentioned above, the PDRI identifies donor factors that influence the 1-year graft survival rate. Recipient factors are also thought to play a role, although the influence of these factors has not been consistently demonstrated.
Humar et al15 found that recipient obesity (defined in this study as BMI > 25 kg/m2) and donor age over 40 were risk factors for early laparotomy after pancreas transplant.15 Moreover, patients undergoing early laparotomy had poorer graft survival outcomes.
This finding was reinforced by an analysis of 5,725 primary simultaneous pancreas-kidney recipients between 2000 and 2007. Obesity (BMI 30 ≥ kg/m2) was associated with increased rates of patient death, pancreas graft loss, and kidney graft loss at 3 years.16
More recently, Finger et al17 did not find a statistically significant association between recipient BMI and technical failure, but they did notice a trend toward increased graft loss with a BMI greater than 25 kg/m2. Similarly, others have not found a clear adverse association between recipient BMI and pancreas graft survival.
Intuitively, obesity and other recipient factors such as age, vascular disease, duration of diabetes, and dialysis should influence pancreas graft survival but have not been shown in analyses to carry an adverse effect.18 The inability to consistently find adverse effects of recipient characteristics is most likely due to the relative similarity between the vast majority of pancreas transplant recipients and the relatively small numbers of adverse events. In 98 consecutive pancreas transplants at our center between 2009 and 2014, the technical loss rate was 1.8% (unpublished data).
Acute rejection most commonly occurs during the first year and is usually reversible. More than 1 year after transplant, graft loss is due to chronic rejection, and death is usually from underlying cardiovascular disease.
The immunosuppressive regimens used in pancreas transplant are similar to those in kidney transplant. Since the pancreas is considered to be more immunogenic than other organs, most centers employ a strategy of induction immunosuppression with T-cell–depleting or interleukin 2-receptor antibodies. Maintenance immunosuppression consists of a calcineurin inhibitor (tacrolimus or cyclosporine), an antimetabolite (mycophenolate), and a corticosteroid.8
Immunosuppressive complications occur at a rate similar to that seen in other solid-organ transplants and include an increased risk of opportunistic infection and malignancy. The risk of these complications must be balanced against the patient’s risk of health decline with dialysis and insulin-based therapies.
OVERALL OUTCOMES ARE GOOD
The success rate of pancreas transplant is currently at its highest since the inception of the procedure. The unadjusted patient survival rate for all groups is over 96% at 1 year, and over 80% at 5 years.8 One-year patient survival after pancreas transplant alone, at better than 96%, is the highest of all organ transplant procedures.9
Several recently published single-center reviews of pancreas transplant since 2000 report patient survival rates of 96% to 100% at 1 year and 88% to 100% at 5 years.19–22 This variability is likely closely linked to donor and recipient selection, as centers performing smaller numbers of transplants tend to be more selective and, in turn, report higher patient survival rates.19,21
Long-term patient survival outcomes can be gathered from larger, registry-based reviews, accepting limitations in assessing causes of patient death. Siskind et al23 analyzed the outcomes of 20,854 US pancreas transplants done between 1996 and 2012 and found the 10-year patient survival rate ranged from 43% to 77% and was highly dependent on patient age at the time of the procedure.23 Patient survival after transplant must be balanced against the generally poor long-term survival prospects of diabetic patients on dialysis.
By type of transplant, pancreas graft survival rates at 1 year are 89% for simultaneous pancreas-kidney transplant, 86% for pancreas-after-kidney transplant, and 84% for pancreas-alone transplant. Graft survival rates at 5 years are 71% for simultaneous pancreas-kidney transplant, 65% for pancreas-after-kidney transplant, and 58% for pancreas-alone transplant.8,9
Simultaneous pancreas-kidney transplant has been shown to improve the survival rate compared with cadaveric kidney transplant alone in patients with type 1 diabetes and chronic kidney disease.24,25 The survival benefit of isolated pancreas transplant (after kidney transplant and alone) is not evident at 4-year follow-up compared with patients on the waiting list. However, the benefit for the individual patient must be considered by weighing the incapacities experienced with insulin-based treatments against the risks of surgery and immunosuppression.26,27 For patients who have experienced frequent and significant hypoglycemic episodes, particularly those requiring third-party assistance, pancreas transplant can be a lifesaving procedure.
Effects on secondary diabetic complications
Notwithstanding the effect on the patient’s life span, data from several studies of long-term pancreas transplant recipients suggest that secondary diabetic complications can be halted or even improved. Most of these studies examined the effect of restoring euglycemia in nephropathy and the subsequent influence on renal function.
Effect on renal function. Kleinclauss et al28 examined renal allograft function in type 1 diabetic recipients of living-donor kidney transplants. Comparing kidney allograft survival and function in patients who received a subsequent pancreas-after-kidney transplant vs those who did not, graft survival was superior after 5 years, and the estimated glomerular filtration rate was 10 mL/min higher in pancreas-after-kidney recipients.28 This improvement in renal function was not seen immediately after the pancreas transplant but became evident more than 4 years after establishment of normoglycemia. Somewhat similarly, reversal of diabetic changes in native kidney biopsies has been seen 10 years after pancreas transplant.29
Effect on neuropathy. In other studies, reversal of autonomic neuropathy and hypoglycemic unawareness and improvements in peripheral sensory-motor neuropathy have also been observed.30–32
Effect on retinopathy. Improvements in early-stage nonproliferative diabetic retinopathy and laser-treated proliferative lesions have been seen, even within short periods of follow-up.33 Other groups have shown a significantly higher proportion of improvement or stability of advanced diabetic retinopathy at 3 years after simultaneous pancreas-kidney transplant, compared with kidney transplant alone in patients with type 1 diabetes.34
Effect on heart disease. Salutary effects on cardiovascular risk factors and amelioration of cardiac morphology and functional cardiac indices have been seen within the first posttransplant year.35 Moreover, with longer follow-up (nearly 4 years), simultaneous pancreas-kidney recipients with functioning pancreas grafts were found to have less progression of coronary atherosclerosis than simultaneous pancreas-kidney recipients with early pancreas graft loss.36 These data provide a potential pathophysiologic mechanism for the long-term survival advantage seen in uremic type 1 diabetic patients undergoing simultaneous pancreas-kidney transplant.
In the aggregate, these findings suggest that, in the absence of surgical and immunosuppression-related complications, a functioning pancreas allograft can alter the progress of diabetic complications. As an extension of these results, pancreas transplant done earlier in the course of diabetes may have an even greater impact.
Pancreas transplant is the only long-term diabetes treatment that consistently results in normal hemoglobin A1c levels without the risk of severe hypoglycemia. Additionally, pancreas transplant may prevent, halt, or even reverse the complications of diabetes.
Here, we explore the indications, options, and outcomes of pancreas transplant as a treatment for diabetes mellitus.
DIABETES IS COMMON, AND OFTEN NOT WELL CONTROLLED
Diabetes mellitus affects more than 25 million people in the United States (8.3% of the population) and is the leading cause of kidney failure, nontraumatic lower-limb amputation, and adult-onset blindness. In 2007, nearly $116 billion was spent on diabetes treatment, not counting another $58 billion in indirect costs such as disability, work loss, and premature death.1
Despite the tremendous expenditure in human, material, and financial resources, only about 50% of patients achieve their diabetes treatment goals. In 2013, a large US population-based study2 reported that 52.2% of patients were achieving the American Diabetes Association treatment goal of hemoglobin A1c lower than 7%. A similar study in South Korea3 found that 45.6% were at this goal.
Most of the patients in these studies had type 2 diabetes, and the data suggested that attaining glycemic goals is more difficult in insulin-treated patients. Studies of patients with type 1 diabetes found hemoglobin A1c levels lower than 7% in only 8.1% of hospitalized patients with type 1 diabetes, and in only 13% in an outpatient diabetes clinic.4,5
YET RATES OF PANCREAS TRANSPLANT ARE DECLINING
Pancreas transplant was first performed more than 40 years ago at the University of Minnesota.6 Since then, dramatic changes in immunosuppression, organ preservation, surgical technique, and donor and recipient selection have brought about significant progress.
Currently, more than 13,000 patients are alive with a functioning pancreas allograft. After reaching a peak in 2004, the annual number of pancreas transplants performed in the United States has declined steadily, whereas the procedure continues to increase in popularity outside North America.7 The primary reason for the decline is recognition of donor factors that lead to success—surgeons are refusing to transplant organs they might have accepted previously, because experience suggests they would yield poor results. In the United States, 1,043 pancreas transplants were performed in 2012, and more than 3,100 patients were on the waiting list.8
Islet cell transplant—a different procedure involving harvesting, encapsulating, and implanting insulin-producing beta cells—has not gained widespread application due to very low long-term success rates.
THREE CATEGORIES OF PANCREAS TRANSPLANT
Pancreas transplant can be categorized according to whether the patient is also receiving or has already received a kidney graft (Table 1).
Simultaneous kidney and pancreas transplant is performed in patients who have type 1 diabetes with advanced chronic kidney disease due to diabetic nephropathy. This remains the most commonly performed type, accounting for 79% of all pancreas transplants in 2012.8
Pancreas-after-kidney transplant is most often done after a living-donor kidney transplant. This procedure accounted for most of the increase in pancreas transplants during the first decade of the 2000s. However, the number of these procedures has steadily decreased since 2004, and in 2012 accounted for only 12% of pancreas transplants.8
Pancreas transplant alone is performed in nonuremic diabetic patients who have labile blood sugar control. Performed in patients with preserved renal function but severe complications of “brittle” diabetes, such as hypoglycemic unawareness, this type accounts for 8% of pancreas transplants.9
Indications for pancreas transplant
A small number of these procedures are done for indications unrelated to diabetes mellitus. In most of these cases, the pancreas is transplanted as part of a multivisceral transplant to facilitate the technical (surgical) aspect of the procedure—the pancreas, liver, stomach, gallbladder, and part of the intestines are transplanted en bloc to maintain the native vasculature. Very infrequently, pancreas transplant is done to replace exocrine pancreatic function.
A small, select group of patients with type 2 diabetes and low body mass index (BMI) may be eligible for pancreas transplant, and they accounted for 8.2% of active candidates in 2012.8 However, most pancreas transplants are performed in patients with type 1 diabetes.
WHAT MAKES A GOOD ALLOGRAFT?
Pancreas allografts are procured as whole organs from brain-dead organ donors. Relatively few pancreas allografts (3.1% in 2012) are from cardiac-death donors, because of concern about warm ischemic injury during the period of circulatory arrest.8


Proper donor selection is critical to the success of pancreas transplant, as donor factors including medical history, age, BMI, and cause of death can significantly affect the outcome. In general, transplant of a pancreas allograft from a young donor (age < 30) with excellent organ function, low BMI, and traumatic cause of death provides the best chance of success.
The Pancreas Donor Risk Index (PDRI)10 was developed after analysis of objective donor criteria, transplant type, and ischemic time in grafts transplanted between 2000 and 2006. One-year graft survival was directly related to the PDRI and ranged between 77% and 87% in recipients of “standard” pancreas allografts (PDRI score of 1.0). Use of grafts from the highest (worst) three quintiles of PDRI (PDRI score > 1.16) was associated with 1-year graft survival rates of 67% to 82%, significantly inferior to that seen with “higher- quality” grafts, again emphasizing the need for rigorous donor selection.10
In addition to these objective measures, visual assessment of pancreas quality at the time of procurement remains an equally important predictor of success. Determination of subjective features, such as fatty infiltration and glandular fibrosis, requires surgical experience developed over several years. In a 2010 analysis, dissatisfaction with the quality of the donor graft on inspection accounted for more than 80% of refusals of potential pancreas donors.11 These studies illustrate an ill-defined aspect of pancreas transplant, ie, even when the pancreas donor is perceived to be suitable, the outcome may be markedly different.
SURGICAL COMPLICATIONS
Surgical complications have long been considered a limiting factor in the growth of pancreas transplant. Technical failure or loss of the graft within 90 days is most commonly due to graft thrombosis, leakage of the enteric anastomosis, or severe peripancreatic infection. The rate of technical failure has declined across all recipient categories and is currently about 9%.8
DO RECIPIENT FACTORS AFFECT OUTCOMES?
As mentioned above, the PDRI identifies donor factors that influence the 1-year graft survival rate. Recipient factors are also thought to play a role, although the influence of these factors has not been consistently demonstrated.
Humar et al15 found that recipient obesity (defined in this study as BMI > 25 kg/m2) and donor age over 40 were risk factors for early laparotomy after pancreas transplant.15 Moreover, patients undergoing early laparotomy had poorer graft survival outcomes.
This finding was reinforced by an analysis of 5,725 primary simultaneous pancreas-kidney recipients between 2000 and 2007. Obesity (BMI 30 ≥ kg/m2) was associated with increased rates of patient death, pancreas graft loss, and kidney graft loss at 3 years.16
More recently, Finger et al17 did not find a statistically significant association between recipient BMI and technical failure, but they did notice a trend toward increased graft loss with a BMI greater than 25 kg/m2. Similarly, others have not found a clear adverse association between recipient BMI and pancreas graft survival.
Intuitively, obesity and other recipient factors such as age, vascular disease, duration of diabetes, and dialysis should influence pancreas graft survival but have not been shown in analyses to carry an adverse effect.18 The inability to consistently find adverse effects of recipient characteristics is most likely due to the relative similarity between the vast majority of pancreas transplant recipients and the relatively small numbers of adverse events. In 98 consecutive pancreas transplants at our center between 2009 and 2014, the technical loss rate was 1.8% (unpublished data).
Acute rejection most commonly occurs during the first year and is usually reversible. More than 1 year after transplant, graft loss is due to chronic rejection, and death is usually from underlying cardiovascular disease.
The immunosuppressive regimens used in pancreas transplant are similar to those in kidney transplant. Since the pancreas is considered to be more immunogenic than other organs, most centers employ a strategy of induction immunosuppression with T-cell–depleting or interleukin 2-receptor antibodies. Maintenance immunosuppression consists of a calcineurin inhibitor (tacrolimus or cyclosporine), an antimetabolite (mycophenolate), and a corticosteroid.8
Immunosuppressive complications occur at a rate similar to that seen in other solid-organ transplants and include an increased risk of opportunistic infection and malignancy. The risk of these complications must be balanced against the patient’s risk of health decline with dialysis and insulin-based therapies.
OVERALL OUTCOMES ARE GOOD
The success rate of pancreas transplant is currently at its highest since the inception of the procedure. The unadjusted patient survival rate for all groups is over 96% at 1 year, and over 80% at 5 years.8 One-year patient survival after pancreas transplant alone, at better than 96%, is the highest of all organ transplant procedures.9
Several recently published single-center reviews of pancreas transplant since 2000 report patient survival rates of 96% to 100% at 1 year and 88% to 100% at 5 years.19–22 This variability is likely closely linked to donor and recipient selection, as centers performing smaller numbers of transplants tend to be more selective and, in turn, report higher patient survival rates.19,21
Long-term patient survival outcomes can be gathered from larger, registry-based reviews, accepting limitations in assessing causes of patient death. Siskind et al23 analyzed the outcomes of 20,854 US pancreas transplants done between 1996 and 2012 and found the 10-year patient survival rate ranged from 43% to 77% and was highly dependent on patient age at the time of the procedure.23 Patient survival after transplant must be balanced against the generally poor long-term survival prospects of diabetic patients on dialysis.
By type of transplant, pancreas graft survival rates at 1 year are 89% for simultaneous pancreas-kidney transplant, 86% for pancreas-after-kidney transplant, and 84% for pancreas-alone transplant. Graft survival rates at 5 years are 71% for simultaneous pancreas-kidney transplant, 65% for pancreas-after-kidney transplant, and 58% for pancreas-alone transplant.8,9
Simultaneous pancreas-kidney transplant has been shown to improve the survival rate compared with cadaveric kidney transplant alone in patients with type 1 diabetes and chronic kidney disease.24,25 The survival benefit of isolated pancreas transplant (after kidney transplant and alone) is not evident at 4-year follow-up compared with patients on the waiting list. However, the benefit for the individual patient must be considered by weighing the incapacities experienced with insulin-based treatments against the risks of surgery and immunosuppression.26,27 For patients who have experienced frequent and significant hypoglycemic episodes, particularly those requiring third-party assistance, pancreas transplant can be a lifesaving procedure.
Effects on secondary diabetic complications
Notwithstanding the effect on the patient’s life span, data from several studies of long-term pancreas transplant recipients suggest that secondary diabetic complications can be halted or even improved. Most of these studies examined the effect of restoring euglycemia in nephropathy and the subsequent influence on renal function.
Effect on renal function. Kleinclauss et al28 examined renal allograft function in type 1 diabetic recipients of living-donor kidney transplants. Comparing kidney allograft survival and function in patients who received a subsequent pancreas-after-kidney transplant vs those who did not, graft survival was superior after 5 years, and the estimated glomerular filtration rate was 10 mL/min higher in pancreas-after-kidney recipients.28 This improvement in renal function was not seen immediately after the pancreas transplant but became evident more than 4 years after establishment of normoglycemia. Somewhat similarly, reversal of diabetic changes in native kidney biopsies has been seen 10 years after pancreas transplant.29
Effect on neuropathy. In other studies, reversal of autonomic neuropathy and hypoglycemic unawareness and improvements in peripheral sensory-motor neuropathy have also been observed.30–32
Effect on retinopathy. Improvements in early-stage nonproliferative diabetic retinopathy and laser-treated proliferative lesions have been seen, even within short periods of follow-up.33 Other groups have shown a significantly higher proportion of improvement or stability of advanced diabetic retinopathy at 3 years after simultaneous pancreas-kidney transplant, compared with kidney transplant alone in patients with type 1 diabetes.34
Effect on heart disease. Salutary effects on cardiovascular risk factors and amelioration of cardiac morphology and functional cardiac indices have been seen within the first posttransplant year.35 Moreover, with longer follow-up (nearly 4 years), simultaneous pancreas-kidney recipients with functioning pancreas grafts were found to have less progression of coronary atherosclerosis than simultaneous pancreas-kidney recipients with early pancreas graft loss.36 These data provide a potential pathophysiologic mechanism for the long-term survival advantage seen in uremic type 1 diabetic patients undergoing simultaneous pancreas-kidney transplant.
In the aggregate, these findings suggest that, in the absence of surgical and immunosuppression-related complications, a functioning pancreas allograft can alter the progress of diabetic complications. As an extension of these results, pancreas transplant done earlier in the course of diabetes may have an even greater impact.
- Centers for Disease Control and Prevention (CDC). National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 12, 2015.
- Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med 2013; 368:1613–1624.
- Jeon JY, Kim DJ, Ko SH, et al; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J 2014; 38:197–203.
- Govan L, Wu O, Briggs A, et al; Scottish Diabetes Research Network Epidemiology Group. Achieved levels of HbA1c and likelihood of hospital admission in people with type 1 diabetes in the Scottish population: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetes Care 2011; 34:1992–1997.
- Bryant W, Greenfield JR, Chisholm DJ, Campbell LV. Diabetes guidelines: easier to preach than to practise? Med J Aust 2006; 185:305–309.
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837.
- Gruessner AC, Gruessner RW. Pancreas transplant outcomes for United States and non United States cases as reported to the United Network for Organ Sharing and the International Pancreas Transplant Registry as of December 2011. Clin Transpl 2012: 23–40.
- Israni AK, Skeans MA, Gustafson SK, et al. OPTN/SRTR 2012 Annual Data Report: pancreas. Am J Transplant 2014; 14(suppl 1):45–68
- Gruessner RW, Gruessner AC. Pancreas transplant alone: a procedure coming of age. Diabetes Care 2013; 36:2440–2447.
- Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant 2010; 10:837–845.
- Wiseman AC, Wainright JL, Sleeman E, et al. An analysis of the lack of donor pancreas utilization from younger adult organ donors. Transplantation 2010; 90:475–480.
- Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol 2013; 9:555–562.
- Gunasekaran G, Wee A, Rabets J, Winans C, Krishnamurthi V. Duodenoduodenostomy in pancreas transplantation. Clin Transplant 2012; 26:550–557.
- Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg 2009; 250:618–630.
- Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 2000; 231:269–275.
- Sampaio MS, Reddy PN, Kuo HT, et al. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation 2010; 89:1117–1125.
- Finger EB, Radosevich DM, Dunn TB, et al. A composite risk model for predicting technical failure in pancreas transplantation. Am J Transplant 2013; 13:1840–1849.
- Fridell JA, Mangus RS, Taber TE, et al. Growth of a nation part II: impact of recipient obesity on whole-organ pancreas transplantation. Clin Transplant 2011; 25:E366–E374.
- Tai DS, Hong J, Busuttil RW, Lipshutz GS. Low rates of short- and long-term graft loss after kidney-pancreas transplant from a single center. JAMA Surg 2013; 148:368–373.
- Bazerbachi F, Selzner M, Marquez MA, et al. Pancreas-after-kidney versus synchronous pancreas-kidney transplantation: comparison of intermediate-term results. Transplantation 2013; 95:489–494.
- Laftavi MR, Pankewycz O, Gruessner A, et al. Long-term outcomes of pancreas after kidney transplantation in small centers: is it justified? Transplant Proc 2014; 46:1920–1923.
- Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, Rogers J. Similar results with solitary pancreas transplantation compared with simultaneous pancreas-kidney transplantation in the new millennium. Transplant Proc 2014; 46:1924–1927.
- Siskind E, Maloney C, Akerman M, et al. An analysis of pancreas transplantation outcomes based on age groupings—an update of the UNOS database. Clin Transplant 2014; 28:990–994.
- Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 2001; 71:82–90.
- Reddy KS, Stablein D, Taranto S, et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis 2003; 41:464–470.
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290:2817–2823.
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4:2018–2026.
- Kleinclauss F, Fauda M, Sutherland DE, et al. Pancreas after living donor kidney transplants in diabetic patients: impact on long-term kidney graft function. Clin Transplant 2009; 23:437–446.
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339:69–75.
- Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia 1996; 39:1415–1424.
- Robertson RP. Update on transplanting beta cells for reversing type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39:655–667.
- Robertson RP, Holohan TV, Genuth S. Therapeutic controversy: pancreas transplantation for type I diabetes. J Clin Endocrinol Metab 1998; 83:1868–1674.
- Giannarelli R, Coppelli A, Sartini MS, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia 2006; 49:2977–2982.
- Koznarová R, Saudek F, Sosna T, et al. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant 2000; 9:903–908.
- Coppelli A, Giannarelli R, Mariotti R, et al. Pancreas transplant alone determines early improvement of cardiovascular risk factors and cardiac function in type 1 diabetic patients. Transplantation 2003; 76:974–976.
- Jukema JW, Smets YF, van der Pijl JW, et al. Impact of simultaneous pancreas and kidney transplantation on progression of coronary atherosclerosis in patients with end-stage renal failure due to type 1 diabetes. Diabetes Care 2002; 25:906–911.
- Centers for Disease Control and Prevention (CDC). National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 12, 2015.
- Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med 2013; 368:1613–1624.
- Jeon JY, Kim DJ, Ko SH, et al; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J 2014; 38:197–203.
- Govan L, Wu O, Briggs A, et al; Scottish Diabetes Research Network Epidemiology Group. Achieved levels of HbA1c and likelihood of hospital admission in people with type 1 diabetes in the Scottish population: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetes Care 2011; 34:1992–1997.
- Bryant W, Greenfield JR, Chisholm DJ, Campbell LV. Diabetes guidelines: easier to preach than to practise? Med J Aust 2006; 185:305–309.
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837.
- Gruessner AC, Gruessner RW. Pancreas transplant outcomes for United States and non United States cases as reported to the United Network for Organ Sharing and the International Pancreas Transplant Registry as of December 2011. Clin Transpl 2012: 23–40.
- Israni AK, Skeans MA, Gustafson SK, et al. OPTN/SRTR 2012 Annual Data Report: pancreas. Am J Transplant 2014; 14(suppl 1):45–68
- Gruessner RW, Gruessner AC. Pancreas transplant alone: a procedure coming of age. Diabetes Care 2013; 36:2440–2447.
- Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant 2010; 10:837–845.
- Wiseman AC, Wainright JL, Sleeman E, et al. An analysis of the lack of donor pancreas utilization from younger adult organ donors. Transplantation 2010; 90:475–480.
- Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol 2013; 9:555–562.
- Gunasekaran G, Wee A, Rabets J, Winans C, Krishnamurthi V. Duodenoduodenostomy in pancreas transplantation. Clin Transplant 2012; 26:550–557.
- Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg 2009; 250:618–630.
- Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 2000; 231:269–275.
- Sampaio MS, Reddy PN, Kuo HT, et al. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation 2010; 89:1117–1125.
- Finger EB, Radosevich DM, Dunn TB, et al. A composite risk model for predicting technical failure in pancreas transplantation. Am J Transplant 2013; 13:1840–1849.
- Fridell JA, Mangus RS, Taber TE, et al. Growth of a nation part II: impact of recipient obesity on whole-organ pancreas transplantation. Clin Transplant 2011; 25:E366–E374.
- Tai DS, Hong J, Busuttil RW, Lipshutz GS. Low rates of short- and long-term graft loss after kidney-pancreas transplant from a single center. JAMA Surg 2013; 148:368–373.
- Bazerbachi F, Selzner M, Marquez MA, et al. Pancreas-after-kidney versus synchronous pancreas-kidney transplantation: comparison of intermediate-term results. Transplantation 2013; 95:489–494.
- Laftavi MR, Pankewycz O, Gruessner A, et al. Long-term outcomes of pancreas after kidney transplantation in small centers: is it justified? Transplant Proc 2014; 46:1920–1923.
- Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, Rogers J. Similar results with solitary pancreas transplantation compared with simultaneous pancreas-kidney transplantation in the new millennium. Transplant Proc 2014; 46:1924–1927.
- Siskind E, Maloney C, Akerman M, et al. An analysis of pancreas transplantation outcomes based on age groupings—an update of the UNOS database. Clin Transplant 2014; 28:990–994.
- Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 2001; 71:82–90.
- Reddy KS, Stablein D, Taranto S, et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis 2003; 41:464–470.
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290:2817–2823.
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4:2018–2026.
- Kleinclauss F, Fauda M, Sutherland DE, et al. Pancreas after living donor kidney transplants in diabetic patients: impact on long-term kidney graft function. Clin Transplant 2009; 23:437–446.
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339:69–75.
- Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia 1996; 39:1415–1424.
- Robertson RP. Update on transplanting beta cells for reversing type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39:655–667.
- Robertson RP, Holohan TV, Genuth S. Therapeutic controversy: pancreas transplantation for type I diabetes. J Clin Endocrinol Metab 1998; 83:1868–1674.
- Giannarelli R, Coppelli A, Sartini MS, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia 2006; 49:2977–2982.
- Koznarová R, Saudek F, Sosna T, et al. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant 2000; 9:903–908.
- Coppelli A, Giannarelli R, Mariotti R, et al. Pancreas transplant alone determines early improvement of cardiovascular risk factors and cardiac function in type 1 diabetic patients. Transplantation 2003; 76:974–976.
- Jukema JW, Smets YF, van der Pijl JW, et al. Impact of simultaneous pancreas and kidney transplantation on progression of coronary atherosclerosis in patients with end-stage renal failure due to type 1 diabetes. Diabetes Care 2002; 25:906–911.
KEY POINTS
- Current options are simultaneous pancreas-kidney transplant, pancreas-after-kidney transplant, and pancreas-alone transplant.
- Simultaneous pancreas-kidney transplant provides a significant survival benefit over insulin- and dialysis-based therapies.
- Isolated pancreas transplant for diabetic patients without uremia can prevent hypoglycemic unawareness.
The color purple
A 58-year-old man with a history of cystoprostatectomy for prostate cancer, end-stage renal disease on hemodialysis, and distal ureteral obstruction requiring bilateral nephrostomy tubes noticed that one of the nephrostomy bags looked “purple” (Figure 1). A specimen collected from one bag was reddish purple (Figure 2). The urine in the other bag was normal. The condition was diagnosed as purple urine bag syndrome.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome, a relatively rare condition that appears after 2 to 3 months of indwelling urinary catheterization, is usually asymptomatic, the only signs being the purplish urine and staining of the urinary bags and catheters. However, it should be considered a sign of underlying urinary tract infection, which can disseminate causing local complications (Fournier gangrene), systemic complications (septicemia), and death.1–3
The syndrome, first described in 1978 in children with spina bifida and urinary diversion,4 is more prevalent in women than in men, possibly because of the shorter urethra and closer proximity to the anus, which predispose women to bacterial colonization of the urinary tract. Predisposing conditions include dementia,5 female sex, increased dietary tryptophan, bacteriuria, urinary tract infection, constipation, older age, immobility, and alkaline urine.6–8
The cause of the discoloration
The purple color is from indigo and indirubin compounds in the urine, the result of the breakdown of dietary tryptophan. The color varies depending on the proportions of the two pigments.
Dietary tryptophan is broken down into indole by colonic bacteria. After reaching the portal circulation, it is excreted into the urine as indoxyl sulfate, which is broken down to indoxyl by sulfatase-producing bacteria (eg, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, Providencia species, Morganella morganii). Indoxyl is then oxidized to indigo and indirubin.
These compounds do not discolor the urine directly, but rather precipitate after interacting with the lining of the urinary catheter and bags, thereby imparting a purple color.1,9–13
Management
Effective initial measures are improved urinary hygiene (eg, frequent, careful changing of the urinary catheter) and management of constipation, as constipation leads to increased colonization of the intestine by bacteria that metabolize dietary tryptophan into indoxyl. Antibiotics should be given for symptomatic urinary tract infection (fever, increased urinary frequency, dysuria, abdominal pain) but not for color change alone. Coverage should be for gram-negative bacilli, although methicillin-resistant Staphylococcus aureus, which is gram-positive, has also been reported to cause purple urine bag syndrome.
In most cases, purple urine bag syndrome is benign and requires no therapy other than that mentioned above.3,13–15 However, in rare cases, immunocompromised patients (eg, people with diabetes) can develop local complications and sepsis from dissemination of bacterial infection, requiring aggressive therapy.14 Therefore, purple urine bag syndrome should be recognized as an indicator of an underlying urinary tract infection and should be treated if symptomatic. Nevertheless, the long-term prognosis is generally good.
OUR PATIENT’S MANAGEMENT
Our patient was confirmed to have urinary colonization with P aeruginosa and E coli, and alkaline urine. He underwent replacement of the nephrostomy tubes and urinary bag during his hospital stay (he was already in the hospital for another indication), but he continued to produce purple-colored urine from his right side and normal-colored urine from his left side. The unilateral involvement was likely from selective colonization of the right-sided nephrostomy tube with gram-negative bacteria.
- Kang KH, Jeong KH, Baik SK, et al. Purple urine bag syndrome: case report and literature review. Clin Nephrol 2011; 75:557–559.
- Ribeiro JP, Marcelino P, Marum S, Fernandes AP, Grilo A. Case report: purple urine bag syndrome. Crit Care 2004; 8:R137.
- Robinson J. Purple urinary bag syndrome: a harmless but alarming problem. Br J Community Nurs 2003; 8:263–266.
- Barlow GB, Dickson JAS. Purple urine bags. Lancet 1978; 1:220–221.
- Ga H, Kojima T. Purple urine bag syndrome. JAMA 2012; 307:1912–1913.
- Ishida T, Ogura S, Kawakami Y. Five cases of purple urine bag syndrome in a geriatric ward. Nihon Ronen Igakkai Zasshi 1999; 36:826–829. Japanese.
- Gautam G, Kothari A, Kumar R, Dogra PN. Purple urine bag syndrome: a rare clinical entity in patients with long term indwelling catheters. Int Urol Nephrol 2007; 39:155–156.
- Shiao CC, Weng CY, Chuang JC, Huang MS, Chen ZY. Purple urine bag syndrome: a community-based study and literature review. Nephrology (Carlton) 2008; 13:554–559.
- Chong VH. Purple urine bag syndrome: it is the urine bag and not the urine that is discolored purple. South Med J 2012; 105:446.
- Chung SD, Liao CH, Sun HD. Purple urine bag syndrome with acidic urine. Int J Infect Dis 2008; 12:526–527.
- Wu HH, Yang WC, Lin CC. Purple urine bag syndrome. Am J Med Sci 2009; 337:368.
- Achtergael W, Michielsen D, Gorus FK, Gerlo E. Indoxyl sulphate and the purple urine bag syndrome: a case report. Acta Clin Belg 2006; 61:38–41.
- Hadano Y, Shimizu T, Takada S, Inoue T, Sorano S. An update on purple urine bag syndrome. Int J Gen Med 2012; 5:707–710.
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Ferrara F, D’Angelo G, Costantino G. Monolateral purple urine bag syndrome in bilateral nephrostomy. Postgrad Med J 2010; 86:627.
A 58-year-old man with a history of cystoprostatectomy for prostate cancer, end-stage renal disease on hemodialysis, and distal ureteral obstruction requiring bilateral nephrostomy tubes noticed that one of the nephrostomy bags looked “purple” (Figure 1). A specimen collected from one bag was reddish purple (Figure 2). The urine in the other bag was normal. The condition was diagnosed as purple urine bag syndrome.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome, a relatively rare condition that appears after 2 to 3 months of indwelling urinary catheterization, is usually asymptomatic, the only signs being the purplish urine and staining of the urinary bags and catheters. However, it should be considered a sign of underlying urinary tract infection, which can disseminate causing local complications (Fournier gangrene), systemic complications (septicemia), and death.1–3
The syndrome, first described in 1978 in children with spina bifida and urinary diversion,4 is more prevalent in women than in men, possibly because of the shorter urethra and closer proximity to the anus, which predispose women to bacterial colonization of the urinary tract. Predisposing conditions include dementia,5 female sex, increased dietary tryptophan, bacteriuria, urinary tract infection, constipation, older age, immobility, and alkaline urine.6–8
The cause of the discoloration
The purple color is from indigo and indirubin compounds in the urine, the result of the breakdown of dietary tryptophan. The color varies depending on the proportions of the two pigments.
Dietary tryptophan is broken down into indole by colonic bacteria. After reaching the portal circulation, it is excreted into the urine as indoxyl sulfate, which is broken down to indoxyl by sulfatase-producing bacteria (eg, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, Providencia species, Morganella morganii). Indoxyl is then oxidized to indigo and indirubin.
These compounds do not discolor the urine directly, but rather precipitate after interacting with the lining of the urinary catheter and bags, thereby imparting a purple color.1,9–13
Management
Effective initial measures are improved urinary hygiene (eg, frequent, careful changing of the urinary catheter) and management of constipation, as constipation leads to increased colonization of the intestine by bacteria that metabolize dietary tryptophan into indoxyl. Antibiotics should be given for symptomatic urinary tract infection (fever, increased urinary frequency, dysuria, abdominal pain) but not for color change alone. Coverage should be for gram-negative bacilli, although methicillin-resistant Staphylococcus aureus, which is gram-positive, has also been reported to cause purple urine bag syndrome.
In most cases, purple urine bag syndrome is benign and requires no therapy other than that mentioned above.3,13–15 However, in rare cases, immunocompromised patients (eg, people with diabetes) can develop local complications and sepsis from dissemination of bacterial infection, requiring aggressive therapy.14 Therefore, purple urine bag syndrome should be recognized as an indicator of an underlying urinary tract infection and should be treated if symptomatic. Nevertheless, the long-term prognosis is generally good.
OUR PATIENT’S MANAGEMENT
Our patient was confirmed to have urinary colonization with P aeruginosa and E coli, and alkaline urine. He underwent replacement of the nephrostomy tubes and urinary bag during his hospital stay (he was already in the hospital for another indication), but he continued to produce purple-colored urine from his right side and normal-colored urine from his left side. The unilateral involvement was likely from selective colonization of the right-sided nephrostomy tube with gram-negative bacteria.
A 58-year-old man with a history of cystoprostatectomy for prostate cancer, end-stage renal disease on hemodialysis, and distal ureteral obstruction requiring bilateral nephrostomy tubes noticed that one of the nephrostomy bags looked “purple” (Figure 1). A specimen collected from one bag was reddish purple (Figure 2). The urine in the other bag was normal. The condition was diagnosed as purple urine bag syndrome.
PURPLE URINE BAG SYNDROME
Purple urine bag syndrome, a relatively rare condition that appears after 2 to 3 months of indwelling urinary catheterization, is usually asymptomatic, the only signs being the purplish urine and staining of the urinary bags and catheters. However, it should be considered a sign of underlying urinary tract infection, which can disseminate causing local complications (Fournier gangrene), systemic complications (septicemia), and death.1–3
The syndrome, first described in 1978 in children with spina bifida and urinary diversion,4 is more prevalent in women than in men, possibly because of the shorter urethra and closer proximity to the anus, which predispose women to bacterial colonization of the urinary tract. Predisposing conditions include dementia,5 female sex, increased dietary tryptophan, bacteriuria, urinary tract infection, constipation, older age, immobility, and alkaline urine.6–8
The cause of the discoloration
The purple color is from indigo and indirubin compounds in the urine, the result of the breakdown of dietary tryptophan. The color varies depending on the proportions of the two pigments.
Dietary tryptophan is broken down into indole by colonic bacteria. After reaching the portal circulation, it is excreted into the urine as indoxyl sulfate, which is broken down to indoxyl by sulfatase-producing bacteria (eg, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, Providencia species, Morganella morganii). Indoxyl is then oxidized to indigo and indirubin.
These compounds do not discolor the urine directly, but rather precipitate after interacting with the lining of the urinary catheter and bags, thereby imparting a purple color.1,9–13
Management
Effective initial measures are improved urinary hygiene (eg, frequent, careful changing of the urinary catheter) and management of constipation, as constipation leads to increased colonization of the intestine by bacteria that metabolize dietary tryptophan into indoxyl. Antibiotics should be given for symptomatic urinary tract infection (fever, increased urinary frequency, dysuria, abdominal pain) but not for color change alone. Coverage should be for gram-negative bacilli, although methicillin-resistant Staphylococcus aureus, which is gram-positive, has also been reported to cause purple urine bag syndrome.
In most cases, purple urine bag syndrome is benign and requires no therapy other than that mentioned above.3,13–15 However, in rare cases, immunocompromised patients (eg, people with diabetes) can develop local complications and sepsis from dissemination of bacterial infection, requiring aggressive therapy.14 Therefore, purple urine bag syndrome should be recognized as an indicator of an underlying urinary tract infection and should be treated if symptomatic. Nevertheless, the long-term prognosis is generally good.
OUR PATIENT’S MANAGEMENT
Our patient was confirmed to have urinary colonization with P aeruginosa and E coli, and alkaline urine. He underwent replacement of the nephrostomy tubes and urinary bag during his hospital stay (he was already in the hospital for another indication), but he continued to produce purple-colored urine from his right side and normal-colored urine from his left side. The unilateral involvement was likely from selective colonization of the right-sided nephrostomy tube with gram-negative bacteria.
- Kang KH, Jeong KH, Baik SK, et al. Purple urine bag syndrome: case report and literature review. Clin Nephrol 2011; 75:557–559.
- Ribeiro JP, Marcelino P, Marum S, Fernandes AP, Grilo A. Case report: purple urine bag syndrome. Crit Care 2004; 8:R137.
- Robinson J. Purple urinary bag syndrome: a harmless but alarming problem. Br J Community Nurs 2003; 8:263–266.
- Barlow GB, Dickson JAS. Purple urine bags. Lancet 1978; 1:220–221.
- Ga H, Kojima T. Purple urine bag syndrome. JAMA 2012; 307:1912–1913.
- Ishida T, Ogura S, Kawakami Y. Five cases of purple urine bag syndrome in a geriatric ward. Nihon Ronen Igakkai Zasshi 1999; 36:826–829. Japanese.
- Gautam G, Kothari A, Kumar R, Dogra PN. Purple urine bag syndrome: a rare clinical entity in patients with long term indwelling catheters. Int Urol Nephrol 2007; 39:155–156.
- Shiao CC, Weng CY, Chuang JC, Huang MS, Chen ZY. Purple urine bag syndrome: a community-based study and literature review. Nephrology (Carlton) 2008; 13:554–559.
- Chong VH. Purple urine bag syndrome: it is the urine bag and not the urine that is discolored purple. South Med J 2012; 105:446.
- Chung SD, Liao CH, Sun HD. Purple urine bag syndrome with acidic urine. Int J Infect Dis 2008; 12:526–527.
- Wu HH, Yang WC, Lin CC. Purple urine bag syndrome. Am J Med Sci 2009; 337:368.
- Achtergael W, Michielsen D, Gorus FK, Gerlo E. Indoxyl sulphate and the purple urine bag syndrome: a case report. Acta Clin Belg 2006; 61:38–41.
- Hadano Y, Shimizu T, Takada S, Inoue T, Sorano S. An update on purple urine bag syndrome. Int J Gen Med 2012; 5:707–710.
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Ferrara F, D’Angelo G, Costantino G. Monolateral purple urine bag syndrome in bilateral nephrostomy. Postgrad Med J 2010; 86:627.
- Kang KH, Jeong KH, Baik SK, et al. Purple urine bag syndrome: case report and literature review. Clin Nephrol 2011; 75:557–559.
- Ribeiro JP, Marcelino P, Marum S, Fernandes AP, Grilo A. Case report: purple urine bag syndrome. Crit Care 2004; 8:R137.
- Robinson J. Purple urinary bag syndrome: a harmless but alarming problem. Br J Community Nurs 2003; 8:263–266.
- Barlow GB, Dickson JAS. Purple urine bags. Lancet 1978; 1:220–221.
- Ga H, Kojima T. Purple urine bag syndrome. JAMA 2012; 307:1912–1913.
- Ishida T, Ogura S, Kawakami Y. Five cases of purple urine bag syndrome in a geriatric ward. Nihon Ronen Igakkai Zasshi 1999; 36:826–829. Japanese.
- Gautam G, Kothari A, Kumar R, Dogra PN. Purple urine bag syndrome: a rare clinical entity in patients with long term indwelling catheters. Int Urol Nephrol 2007; 39:155–156.
- Shiao CC, Weng CY, Chuang JC, Huang MS, Chen ZY. Purple urine bag syndrome: a community-based study and literature review. Nephrology (Carlton) 2008; 13:554–559.
- Chong VH. Purple urine bag syndrome: it is the urine bag and not the urine that is discolored purple. South Med J 2012; 105:446.
- Chung SD, Liao CH, Sun HD. Purple urine bag syndrome with acidic urine. Int J Infect Dis 2008; 12:526–527.
- Wu HH, Yang WC, Lin CC. Purple urine bag syndrome. Am J Med Sci 2009; 337:368.
- Achtergael W, Michielsen D, Gorus FK, Gerlo E. Indoxyl sulphate and the purple urine bag syndrome: a case report. Acta Clin Belg 2006; 61:38–41.
- Hadano Y, Shimizu T, Takada S, Inoue T, Sorano S. An update on purple urine bag syndrome. Int J Gen Med 2012; 5:707–710.
- Tasi YM, Huang MS, Yang CJ, Yeh SM, Liu CC. Purple urine bag syndrome, not always a benign process. Am J Emerg Med 2009; 27:895–897.
- Ferrara F, D’Angelo G, Costantino G. Monolateral purple urine bag syndrome in bilateral nephrostomy. Postgrad Med J 2010; 86:627.
Stellate ulceration in a nonuremic patient
A 64-year-old man was admitted for extensive painful ulceration of the left lower leg (Figure 1) that occurred after a fall and that had worsened over the last 4 months.
His medical history included hyperuricemia, hypertension, and type 2 diabetes mellitus. He had no known cardiac or renal disease.
Results of initial laboratory testing showed the following:
- Hemoglobin 10.9 g/dL (reference range 13.5–17.5); red blood cells were normocytic and normochromic
- White blood cell count 10.2 × 109/L (4.5–11.0)
- Neutrophil count 9.11 × 109/L (2.0–8.5)
- C-reactive protein 259 mg/L (< 5)
- Creatinine, urea, sodium, potassium, calcium, and phosphate were within normal limits.
Doppler ultrasonography of the legs showed mild diffuse atheromatous arterial disease without significant blockage of blood flow, in addition to mild bilateral venous insufficiency.
Cutaneous biopsy showed intravascular calcium deposition in the hypodermis (Figure 2) and reticular dermis, erythrocyte extravasation in the superficial dermis, and epidermal necrosis, thus establishing the diagnosis of nonuremic calciphylaxis. The vascular occlusion with spreading necrosis gave the characteristic stellate appearance.
Aside from diabetes, our patient had none of the conditions usually associated with nonuremic calciphylaxis—namely, hyperparathyroidism, previous corticosteroid therapy, warfarin use, connective tissue disease, or malignancy.
A POORLY UNDERSTOOD SMALL-VESSEL VASCULOPATHY
Calciphylaxis is a poorly understood small-vessel vasculopathy, most often associated with end-stage renal disease, with a prevalence of 1% to 4% in patients on dialysis.1 It carries a high risk of death, most often from sepsis.
The cause is still unclear, but several conditions have been implicated, including primary hyperparathyroidism, malignancies, alcoholic liver disease, connective tissue disease, and diabetes.2
Making the diagnosis may be challenging, especially in nonuremic patients. It is a rare condition, the presentation is not always typical, and it can occur with fully normal kidney function and normal indicators of calcium and phosphate metabolism.
The differential diagnosis includes:
- Vasculitis, either primary or secondary to an autoimmune disorder such as rheumatoid arthritis, systemic lupus erythematosus, or cryoglobulinemia
- Peripheral vascular disease
- Other inflammatory conditions such as pyoderma gangrenosum and panniculitis
- Infections such as cellulitis and necrotizing fasciitis
- Iatrogenic disorders such as warfarin necrosis and early-stage nephrogenic systemic fibrosis.3,4
The current approach to treatment is multidisciplinary and is based only on case reports and small case series, since no randomized prospective trial has been done. The goal is optimal control of calcium and phosphate homeostasis and correction of hypercoagulability.5 Available data4,5 support appropriate wound care and surgical debridement.4,5 Intravenous sodium thiosulfate is the most widely used medical treatment and can be given regardless of the level of renal function. Resolution rates have been greater than 90% in patients with normal renal function, whereas improvement in cutaneous ulcers and pain has been observed in 70% of hemodialysis patients.4 However, it does not reduce the associated mortality rate.4
Awareness of nonuremic calciphylaxis and a high index of suspicion are needed when any patient presents with a leg ulcer and no clear cause. It should be considered in the differential diagnosis of leg ulcer in patients with chronic renal failure even if they have risk factors for more common causes of ulcers, and even occasionally in patients such as ours without chronic kidney disease or other risk factors for this condition.
OUR PATIENT’S MANAGEMENT
The patient developed profuse diarrhea, and infection with Clostridium difficile was confirmed. Despite treatment with metronidazole and vancomycin, he died several days later. No treatment directed to calciphylaxis was ever started because of the patient’s unstable condition during the entire hospitalization.
- Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med 2008; 75:772–777.
- Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol 2008; 3:1139–1143.
- Lee JL, Naguwa SM, Cheema G, Gershwin ME. Recognizing calcific uremic arteriolopathy in autoimmune disease: An emerging mimicker of vasculitis. Autoimmun Rev 2008; 7:638–643.
- Wollina U. Update on cutaneous calciphylaxis. Indian J Dermatol 2013; 58:87–92.
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
A 64-year-old man was admitted for extensive painful ulceration of the left lower leg (Figure 1) that occurred after a fall and that had worsened over the last 4 months.
His medical history included hyperuricemia, hypertension, and type 2 diabetes mellitus. He had no known cardiac or renal disease.
Results of initial laboratory testing showed the following:
- Hemoglobin 10.9 g/dL (reference range 13.5–17.5); red blood cells were normocytic and normochromic
- White blood cell count 10.2 × 109/L (4.5–11.0)
- Neutrophil count 9.11 × 109/L (2.0–8.5)
- C-reactive protein 259 mg/L (< 5)
- Creatinine, urea, sodium, potassium, calcium, and phosphate were within normal limits.
Doppler ultrasonography of the legs showed mild diffuse atheromatous arterial disease without significant blockage of blood flow, in addition to mild bilateral venous insufficiency.
Cutaneous biopsy showed intravascular calcium deposition in the hypodermis (Figure 2) and reticular dermis, erythrocyte extravasation in the superficial dermis, and epidermal necrosis, thus establishing the diagnosis of nonuremic calciphylaxis. The vascular occlusion with spreading necrosis gave the characteristic stellate appearance.
Aside from diabetes, our patient had none of the conditions usually associated with nonuremic calciphylaxis—namely, hyperparathyroidism, previous corticosteroid therapy, warfarin use, connective tissue disease, or malignancy.
A POORLY UNDERSTOOD SMALL-VESSEL VASCULOPATHY
Calciphylaxis is a poorly understood small-vessel vasculopathy, most often associated with end-stage renal disease, with a prevalence of 1% to 4% in patients on dialysis.1 It carries a high risk of death, most often from sepsis.
The cause is still unclear, but several conditions have been implicated, including primary hyperparathyroidism, malignancies, alcoholic liver disease, connective tissue disease, and diabetes.2
Making the diagnosis may be challenging, especially in nonuremic patients. It is a rare condition, the presentation is not always typical, and it can occur with fully normal kidney function and normal indicators of calcium and phosphate metabolism.
The differential diagnosis includes:
- Vasculitis, either primary or secondary to an autoimmune disorder such as rheumatoid arthritis, systemic lupus erythematosus, or cryoglobulinemia
- Peripheral vascular disease
- Other inflammatory conditions such as pyoderma gangrenosum and panniculitis
- Infections such as cellulitis and necrotizing fasciitis
- Iatrogenic disorders such as warfarin necrosis and early-stage nephrogenic systemic fibrosis.3,4
The current approach to treatment is multidisciplinary and is based only on case reports and small case series, since no randomized prospective trial has been done. The goal is optimal control of calcium and phosphate homeostasis and correction of hypercoagulability.5 Available data4,5 support appropriate wound care and surgical debridement.4,5 Intravenous sodium thiosulfate is the most widely used medical treatment and can be given regardless of the level of renal function. Resolution rates have been greater than 90% in patients with normal renal function, whereas improvement in cutaneous ulcers and pain has been observed in 70% of hemodialysis patients.4 However, it does not reduce the associated mortality rate.4
Awareness of nonuremic calciphylaxis and a high index of suspicion are needed when any patient presents with a leg ulcer and no clear cause. It should be considered in the differential diagnosis of leg ulcer in patients with chronic renal failure even if they have risk factors for more common causes of ulcers, and even occasionally in patients such as ours without chronic kidney disease or other risk factors for this condition.
OUR PATIENT’S MANAGEMENT
The patient developed profuse diarrhea, and infection with Clostridium difficile was confirmed. Despite treatment with metronidazole and vancomycin, he died several days later. No treatment directed to calciphylaxis was ever started because of the patient’s unstable condition during the entire hospitalization.
A 64-year-old man was admitted for extensive painful ulceration of the left lower leg (Figure 1) that occurred after a fall and that had worsened over the last 4 months.
His medical history included hyperuricemia, hypertension, and type 2 diabetes mellitus. He had no known cardiac or renal disease.
Results of initial laboratory testing showed the following:
- Hemoglobin 10.9 g/dL (reference range 13.5–17.5); red blood cells were normocytic and normochromic
- White blood cell count 10.2 × 109/L (4.5–11.0)
- Neutrophil count 9.11 × 109/L (2.0–8.5)
- C-reactive protein 259 mg/L (< 5)
- Creatinine, urea, sodium, potassium, calcium, and phosphate were within normal limits.
Doppler ultrasonography of the legs showed mild diffuse atheromatous arterial disease without significant blockage of blood flow, in addition to mild bilateral venous insufficiency.
Cutaneous biopsy showed intravascular calcium deposition in the hypodermis (Figure 2) and reticular dermis, erythrocyte extravasation in the superficial dermis, and epidermal necrosis, thus establishing the diagnosis of nonuremic calciphylaxis. The vascular occlusion with spreading necrosis gave the characteristic stellate appearance.
Aside from diabetes, our patient had none of the conditions usually associated with nonuremic calciphylaxis—namely, hyperparathyroidism, previous corticosteroid therapy, warfarin use, connective tissue disease, or malignancy.
A POORLY UNDERSTOOD SMALL-VESSEL VASCULOPATHY
Calciphylaxis is a poorly understood small-vessel vasculopathy, most often associated with end-stage renal disease, with a prevalence of 1% to 4% in patients on dialysis.1 It carries a high risk of death, most often from sepsis.
The cause is still unclear, but several conditions have been implicated, including primary hyperparathyroidism, malignancies, alcoholic liver disease, connective tissue disease, and diabetes.2
Making the diagnosis may be challenging, especially in nonuremic patients. It is a rare condition, the presentation is not always typical, and it can occur with fully normal kidney function and normal indicators of calcium and phosphate metabolism.
The differential diagnosis includes:
- Vasculitis, either primary or secondary to an autoimmune disorder such as rheumatoid arthritis, systemic lupus erythematosus, or cryoglobulinemia
- Peripheral vascular disease
- Other inflammatory conditions such as pyoderma gangrenosum and panniculitis
- Infections such as cellulitis and necrotizing fasciitis
- Iatrogenic disorders such as warfarin necrosis and early-stage nephrogenic systemic fibrosis.3,4
The current approach to treatment is multidisciplinary and is based only on case reports and small case series, since no randomized prospective trial has been done. The goal is optimal control of calcium and phosphate homeostasis and correction of hypercoagulability.5 Available data4,5 support appropriate wound care and surgical debridement.4,5 Intravenous sodium thiosulfate is the most widely used medical treatment and can be given regardless of the level of renal function. Resolution rates have been greater than 90% in patients with normal renal function, whereas improvement in cutaneous ulcers and pain has been observed in 70% of hemodialysis patients.4 However, it does not reduce the associated mortality rate.4
Awareness of nonuremic calciphylaxis and a high index of suspicion are needed when any patient presents with a leg ulcer and no clear cause. It should be considered in the differential diagnosis of leg ulcer in patients with chronic renal failure even if they have risk factors for more common causes of ulcers, and even occasionally in patients such as ours without chronic kidney disease or other risk factors for this condition.
OUR PATIENT’S MANAGEMENT
The patient developed profuse diarrhea, and infection with Clostridium difficile was confirmed. Despite treatment with metronidazole and vancomycin, he died several days later. No treatment directed to calciphylaxis was ever started because of the patient’s unstable condition during the entire hospitalization.
- Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med 2008; 75:772–777.
- Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol 2008; 3:1139–1143.
- Lee JL, Naguwa SM, Cheema G, Gershwin ME. Recognizing calcific uremic arteriolopathy in autoimmune disease: An emerging mimicker of vasculitis. Autoimmun Rev 2008; 7:638–643.
- Wollina U. Update on cutaneous calciphylaxis. Indian J Dermatol 2013; 58:87–92.
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
- Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med 2008; 75:772–777.
- Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol 2008; 3:1139–1143.
- Lee JL, Naguwa SM, Cheema G, Gershwin ME. Recognizing calcific uremic arteriolopathy in autoimmune disease: An emerging mimicker of vasculitis. Autoimmun Rev 2008; 7:638–643.
- Wollina U. Update on cutaneous calciphylaxis. Indian J Dermatol 2013; 58:87–92.
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
Should all patients with significant proteinuria take a renin-angiotensin inhibitor?
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
EADV: Latest gruesome twosome: Psoriasis spawns renal disease
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
COPENHAGEN – Severe psoriasis is an independent risk factor for serious renal disease – and psoriatic arthritis multiplies that risk even further, according to a Taiwanese national study.
“We think this might be related to the higher inflammatory status associated with psoriatic arthritis. For those with mild psoriasis without psoriatic arthritis, the risk was not increased,” Dr. Ching-Chi Chi said at the annual congress of the European Academy of Dermatology and Venereology.
The past decade has brought a mountain of data documenting that psoriasis, a chronic inflammatory dermatosis, is associated with increased risks of cardiovascular disease, diabetes, and other metabolic abnormalities, noted Dr. Chi, professor of dermatology at Chang Gung University in Taoyuan.
He presented a nationwide retrospective cohort study utilizing Taiwan’s national health insurance system, which covers more than 99% of the population. The study included 4,633 patients with psoriasis diagnosed by a dermatologist or rheumatologist since 2005 and 922,534 controls. A total of 453 patients were classified as having severe psoriasis based upon ever having received systemic treatment or phototherapy; the other 4,180 were classified as having mild psoriasis.
Among the controls there were 36,615 incident cases of chronic kidney disease (CKD) and 9,493 new cases of end-stage renal disease (ESRD) during the study period, translating to rates of 676 per 1,000,000 person-years and 172 per 1,000,000 person-years, respectively. In contrast, patients with severe psoriasis had an incident CKD rate of 2,160 per 1,000,000 person-years and an ESRD rate of 876 per 1,000,000 person-years.
In a Cox regression analysis adjusted for potential confounders including age, gender, comorbid cardiovascular disease, gout, hypertension, dyslipidemia, and use of NSAIDs, severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD.
Although mild psoriasis alone wasn’t associated with increased risk of renal disease, the 254 patients with mild psoriasis and psoriatic arthritis were at 1.3-fold increased risk of CKD and 2.5-fold increased risk of incident ESRD, compared with controls. Moreover, among the 93 patients with severe psoriasis plus psoriatic arthritis, the risks of incident CKD and ESRD were increased by 2.6- and 6.7-fold over the control subjects.
The new Taiwanese national study confirms earlier work by Dr. Joel M. Gelfand and his coinvestigators at the University of Pennsylvania, Philadelphia. Their population-based cohort study and nested cross-sectional study utilizing a huge U.K. electronic medical records database analyzed 136,529 patients with mild psoriasis, 7,354 with severe psoriasis, and nearly 690,000 matched controls and concluded that moderate to severe psoriasis is associated with an increased risk of stage 3-5 chronic kidney disease (BMJ. 2013 Oct 15;347:f5961).
He reported having no financial conflicts regarding this study.
This article was updated October 29, 2015.
AT THE EADV CONGRESS
Key clinical point: Patients with severe but not mild psoriasis are at increased risk of new-onset chronic kidney disease and end-stage renal disease.
Major finding: Severe psoriasis was independently associated with a 1.9-fold increased risk of new-onset CKD and a 3-fold increased risk of ESRD. Comorbid psoriatic arthritis further boosted those risks.
Data source: This retrospective cohort study included 4,633 consecutive patients diagnosed with psoriasis in Taiwan.
Disclosures: Dr. Chi reported having no financial conflicts regarding this government-funded study.
The Role of the Kidney in Glycemic Control
Ji Hyun (CJ) Chun highlights the reasons for targeting the kidney for lowering blood glucose levels.
This video provides three takeaways in less than three minutes from the presentation at the 2015 MEDS conference, entitled “Looking Beyond Insulin-Based Therapies in Type 2 Diabetes: The Role of the Kidney in Glycemic Control,” co-presented by Mary D. Knudtson, DNSc, FNP/PNP-BC, FAAN and Creative Educational Concepts, Inc., and supported by an independent educational grant from AstraZeneca.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Ji Hyun (CJ) Chun highlights the reasons for targeting the kidney for lowering blood glucose levels.
This video provides three takeaways in less than three minutes from the presentation at the 2015 MEDS conference, entitled “Looking Beyond Insulin-Based Therapies in Type 2 Diabetes: The Role of the Kidney in Glycemic Control,” co-presented by Mary D. Knudtson, DNSc, FNP/PNP-BC, FAAN and Creative Educational Concepts, Inc., and supported by an independent educational grant from AstraZeneca.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Ji Hyun (CJ) Chun highlights the reasons for targeting the kidney for lowering blood glucose levels.
This video provides three takeaways in less than three minutes from the presentation at the 2015 MEDS conference, entitled “Looking Beyond Insulin-Based Therapies in Type 2 Diabetes: The Role of the Kidney in Glycemic Control,” co-presented by Mary D. Knudtson, DNSc, FNP/PNP-BC, FAAN and Creative Educational Concepts, Inc., and supported by an independent educational grant from AstraZeneca.