User login
Hourly air pollution exposure: A risk factor for stroke
TOPLINE:
METHODOLOGY:
- Limited studies have investigated the association between hourly exposure to air pollutants and specific stroke subtypes, especially in regions with moderate to high levels of air pollution.
- The multicenter case-crossover study evaluated the association between hourly exposure to air pollution and stroke among 86,635 emergency admissions for stroke across 10 hospitals in 3 cities.
- Of 86,635 admissions, 79,478 were admitted for ischemic stroke, 3,122 for hemorrhagic stroke, and 4,035 for undetermined type of stroke.
- Hourly levels of fine particulate matter (PM2.5), respirable PM (PM10), nitrogen dioxide (NO2), and sulfur dioxide (SO2) were collected from the China National Environmental Monitoring Center.
TAKEAWAY:
- Exposure to NO2 and SO2 increased the risk for emergency admission for stroke shortly after exposure by 3.34% (95% confidence interval, 1.41%-5.31%) and 2.81% (95% CI, 1.15%-4.51%), respectively.
- Among men, exposure to PM2.5 and PM10 increased the risk for emergency admission for stroke by 3.40% (95% CI, 1.21%-5.64%) and 4.33% (95% CI, 2.18%-6.53%), respectively.
- Among patients aged less than 65 years, exposure to PM10 and NO2 increased the risk for emergency admissions for stroke shortly after exposure by 4.88% (95% CI, 2.29%-7.54%) and 5.59% (95% CI, 2.34%-8.93%), respectively.
IN PRACTICE:
“These variations in susceptibility highlight the importance of implementing effective health protection measures to reduce exposure to air pollution and mitigate the risk of stroke in younger and male populations,” wrote the authors.
SOURCE:
The study was led by Xin Lv, MD, department of epidemiology and biostatistics, School of Public Health, Capital Medical University, Beijing. It was published online in the journal Stroke.
LIMITATIONS:
- Using data from the nearest monitoring site to the hospital address may lead to localized variations in pollution concentrations when assessing exposure.
- There may be a possibility of residual confounding resulting from time-varying lifestyle-related factors.
DISCLOSURES:
This study was supported by the Zhejiang Provincial Project for Medical Research and Health Sciences. No disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Limited studies have investigated the association between hourly exposure to air pollutants and specific stroke subtypes, especially in regions with moderate to high levels of air pollution.
- The multicenter case-crossover study evaluated the association between hourly exposure to air pollution and stroke among 86,635 emergency admissions for stroke across 10 hospitals in 3 cities.
- Of 86,635 admissions, 79,478 were admitted for ischemic stroke, 3,122 for hemorrhagic stroke, and 4,035 for undetermined type of stroke.
- Hourly levels of fine particulate matter (PM2.5), respirable PM (PM10), nitrogen dioxide (NO2), and sulfur dioxide (SO2) were collected from the China National Environmental Monitoring Center.
TAKEAWAY:
- Exposure to NO2 and SO2 increased the risk for emergency admission for stroke shortly after exposure by 3.34% (95% confidence interval, 1.41%-5.31%) and 2.81% (95% CI, 1.15%-4.51%), respectively.
- Among men, exposure to PM2.5 and PM10 increased the risk for emergency admission for stroke by 3.40% (95% CI, 1.21%-5.64%) and 4.33% (95% CI, 2.18%-6.53%), respectively.
- Among patients aged less than 65 years, exposure to PM10 and NO2 increased the risk for emergency admissions for stroke shortly after exposure by 4.88% (95% CI, 2.29%-7.54%) and 5.59% (95% CI, 2.34%-8.93%), respectively.
IN PRACTICE:
“These variations in susceptibility highlight the importance of implementing effective health protection measures to reduce exposure to air pollution and mitigate the risk of stroke in younger and male populations,” wrote the authors.
SOURCE:
The study was led by Xin Lv, MD, department of epidemiology and biostatistics, School of Public Health, Capital Medical University, Beijing. It was published online in the journal Stroke.
LIMITATIONS:
- Using data from the nearest monitoring site to the hospital address may lead to localized variations in pollution concentrations when assessing exposure.
- There may be a possibility of residual confounding resulting from time-varying lifestyle-related factors.
DISCLOSURES:
This study was supported by the Zhejiang Provincial Project for Medical Research and Health Sciences. No disclosures were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Limited studies have investigated the association between hourly exposure to air pollutants and specific stroke subtypes, especially in regions with moderate to high levels of air pollution.
- The multicenter case-crossover study evaluated the association between hourly exposure to air pollution and stroke among 86,635 emergency admissions for stroke across 10 hospitals in 3 cities.
- Of 86,635 admissions, 79,478 were admitted for ischemic stroke, 3,122 for hemorrhagic stroke, and 4,035 for undetermined type of stroke.
- Hourly levels of fine particulate matter (PM2.5), respirable PM (PM10), nitrogen dioxide (NO2), and sulfur dioxide (SO2) were collected from the China National Environmental Monitoring Center.
TAKEAWAY:
- Exposure to NO2 and SO2 increased the risk for emergency admission for stroke shortly after exposure by 3.34% (95% confidence interval, 1.41%-5.31%) and 2.81% (95% CI, 1.15%-4.51%), respectively.
- Among men, exposure to PM2.5 and PM10 increased the risk for emergency admission for stroke by 3.40% (95% CI, 1.21%-5.64%) and 4.33% (95% CI, 2.18%-6.53%), respectively.
- Among patients aged less than 65 years, exposure to PM10 and NO2 increased the risk for emergency admissions for stroke shortly after exposure by 4.88% (95% CI, 2.29%-7.54%) and 5.59% (95% CI, 2.34%-8.93%), respectively.
IN PRACTICE:
“These variations in susceptibility highlight the importance of implementing effective health protection measures to reduce exposure to air pollution and mitigate the risk of stroke in younger and male populations,” wrote the authors.
SOURCE:
The study was led by Xin Lv, MD, department of epidemiology and biostatistics, School of Public Health, Capital Medical University, Beijing. It was published online in the journal Stroke.
LIMITATIONS:
- Using data from the nearest monitoring site to the hospital address may lead to localized variations in pollution concentrations when assessing exposure.
- There may be a possibility of residual confounding resulting from time-varying lifestyle-related factors.
DISCLOSURES:
This study was supported by the Zhejiang Provincial Project for Medical Research and Health Sciences. No disclosures were reported.
A version of this article first appeared on Medscape.com.
Fibromyalgia, CFS more prevalent in patients with IBS
TOPLINE:
METHODOLOGY:
- The authors conducted a retrospective cohort study to investigate the prevalence and predictors of fibromyalgia and CFS in patients hospitalized with IBS vs people without IBS.
- The researchers used ICD-10 codes to analyze U.S. National Inpatient Sample (NIS) data from 2016-2019.
- A subgroup analysis investigated associations with IBS-diarrhea (IBS-D), IBS-constipation (IBS-C), and IBS-mixed types.
- Variables included patient age, sex, ethnicity, race, household income, insurance status, and hospital-level characteristics (including location, bed size, and teaching status).
TAKEAWAY:
- Among 1.2 million patients with IBS included in the study, 10.7% also had fibromyalgia and 0.4% had CFS. The majority of fibromyalgia (96.5%) and CFS (89.9%) patients were female and White (86.5%). CFS prevalence also was highest among White persons (90.7%).
- The prevalence of fibromyalgia and CFS was significantly higher in patients with IBS compared to those without IBS (adjusted odds ratio [AOR], 5.33 for fibromyalgia and AOR, 5.4 for CFS).
- IBS-D, IBS-C, and IBS-mixed types were independently associated with increased odds of fibromyalgia and CFS.
- Independent predictors of increased odds of fibromyalgia and CFS, respectively, were increasing age (AOR, 1.02 for both), female sex (AOR, 11.2; AOR, 1.86) and White race (AOR, 2.04; AOR, 1.69).
- Overall, White race, lower socioeconomic status, smoking, alcohol use, obesity, and hyperlipidemia were associated with increased odds of fibromyalgia. For CFS, increased odds were associated with White race, higher socioeconomic status, smoking, obesity, and hyperlipidemia.
IN PRACTICE:
“In current clinical practice, there is a high risk of neglecting multi-syndromic patients. We as clinicians should integrate in our practice with regular screening for other somatic disorders in the IBS population and determine the need to consult other specialties like rheumatology and psychiatry to improve the overall health outcome in IBS patients,” the authors wrote.
SOURCE:
Zahid Ijaz Tarar, MD, University of Missouri, Columbia, led the study, which was published online in Biomedicines.
LIMITATIONS:
The retrospective design of the study can only show associations, not a causal relationship. Lack of blinding and randomization in the data creates bias. The NIS database does not provide medication and laboratory data, so the effect of pharmaceutical therapies cannot be measured.
DISCLOSURES:
The research received no external funding. The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
TOPLINE:
METHODOLOGY:
- The authors conducted a retrospective cohort study to investigate the prevalence and predictors of fibromyalgia and CFS in patients hospitalized with IBS vs people without IBS.
- The researchers used ICD-10 codes to analyze U.S. National Inpatient Sample (NIS) data from 2016-2019.
- A subgroup analysis investigated associations with IBS-diarrhea (IBS-D), IBS-constipation (IBS-C), and IBS-mixed types.
- Variables included patient age, sex, ethnicity, race, household income, insurance status, and hospital-level characteristics (including location, bed size, and teaching status).
TAKEAWAY:
- Among 1.2 million patients with IBS included in the study, 10.7% also had fibromyalgia and 0.4% had CFS. The majority of fibromyalgia (96.5%) and CFS (89.9%) patients were female and White (86.5%). CFS prevalence also was highest among White persons (90.7%).
- The prevalence of fibromyalgia and CFS was significantly higher in patients with IBS compared to those without IBS (adjusted odds ratio [AOR], 5.33 for fibromyalgia and AOR, 5.4 for CFS).
- IBS-D, IBS-C, and IBS-mixed types were independently associated with increased odds of fibromyalgia and CFS.
- Independent predictors of increased odds of fibromyalgia and CFS, respectively, were increasing age (AOR, 1.02 for both), female sex (AOR, 11.2; AOR, 1.86) and White race (AOR, 2.04; AOR, 1.69).
- Overall, White race, lower socioeconomic status, smoking, alcohol use, obesity, and hyperlipidemia were associated with increased odds of fibromyalgia. For CFS, increased odds were associated with White race, higher socioeconomic status, smoking, obesity, and hyperlipidemia.
IN PRACTICE:
“In current clinical practice, there is a high risk of neglecting multi-syndromic patients. We as clinicians should integrate in our practice with regular screening for other somatic disorders in the IBS population and determine the need to consult other specialties like rheumatology and psychiatry to improve the overall health outcome in IBS patients,” the authors wrote.
SOURCE:
Zahid Ijaz Tarar, MD, University of Missouri, Columbia, led the study, which was published online in Biomedicines.
LIMITATIONS:
The retrospective design of the study can only show associations, not a causal relationship. Lack of blinding and randomization in the data creates bias. The NIS database does not provide medication and laboratory data, so the effect of pharmaceutical therapies cannot be measured.
DISCLOSURES:
The research received no external funding. The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
TOPLINE:
METHODOLOGY:
- The authors conducted a retrospective cohort study to investigate the prevalence and predictors of fibromyalgia and CFS in patients hospitalized with IBS vs people without IBS.
- The researchers used ICD-10 codes to analyze U.S. National Inpatient Sample (NIS) data from 2016-2019.
- A subgroup analysis investigated associations with IBS-diarrhea (IBS-D), IBS-constipation (IBS-C), and IBS-mixed types.
- Variables included patient age, sex, ethnicity, race, household income, insurance status, and hospital-level characteristics (including location, bed size, and teaching status).
TAKEAWAY:
- Among 1.2 million patients with IBS included in the study, 10.7% also had fibromyalgia and 0.4% had CFS. The majority of fibromyalgia (96.5%) and CFS (89.9%) patients were female and White (86.5%). CFS prevalence also was highest among White persons (90.7%).
- The prevalence of fibromyalgia and CFS was significantly higher in patients with IBS compared to those without IBS (adjusted odds ratio [AOR], 5.33 for fibromyalgia and AOR, 5.4 for CFS).
- IBS-D, IBS-C, and IBS-mixed types were independently associated with increased odds of fibromyalgia and CFS.
- Independent predictors of increased odds of fibromyalgia and CFS, respectively, were increasing age (AOR, 1.02 for both), female sex (AOR, 11.2; AOR, 1.86) and White race (AOR, 2.04; AOR, 1.69).
- Overall, White race, lower socioeconomic status, smoking, alcohol use, obesity, and hyperlipidemia were associated with increased odds of fibromyalgia. For CFS, increased odds were associated with White race, higher socioeconomic status, smoking, obesity, and hyperlipidemia.
IN PRACTICE:
“In current clinical practice, there is a high risk of neglecting multi-syndromic patients. We as clinicians should integrate in our practice with regular screening for other somatic disorders in the IBS population and determine the need to consult other specialties like rheumatology and psychiatry to improve the overall health outcome in IBS patients,” the authors wrote.
SOURCE:
Zahid Ijaz Tarar, MD, University of Missouri, Columbia, led the study, which was published online in Biomedicines.
LIMITATIONS:
The retrospective design of the study can only show associations, not a causal relationship. Lack of blinding and randomization in the data creates bias. The NIS database does not provide medication and laboratory data, so the effect of pharmaceutical therapies cannot be measured.
DISCLOSURES:
The research received no external funding. The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Spinal cord stimulator restores Parkinson patient’s gait
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Atrial fibrillation linked to dementia, especially when diagnosed before age 65 years
TOPLINE:
Adults with atrial fibrillation (AFib) are at increased risk for dementia, especially when AFib occurs before age 65 years, new research shows. Investigators note the findings highlight the importance of monitoring cognitive function in adults with AF.
METHODOLOGY:
- This prospective, population-based cohort study leveraged data from 433,746 UK Biobank participants (55% women), including 30,601 with AFib, who were followed for a median of 12.6 years
- Incident cases of dementia were determined through linkage from multiple databases.
- Cox proportional hazards models and propensity score matching were used to estimate the association between age at onset of AFib and incident dementia.
TAKEAWAY:
- During follow-up, new-onset dementia occurred in 5,898 participants (2,546 with Alzheimer’s disease [AD] and 1,211 with vascular dementia [VD]), of which, 1,031 had AFib (350 with AD; 320 with VD).
- Compared with participants without AFib, those with AFib had a 42% higher risk for all-cause dementia (adjusted hazard ratio, 1.42; P < .001) and more than double the risk for VD (aHR, 2.06; P < .001), but no significantly higher risk for AD.
- Younger age at AFib onset was associated with higher risks for all-cause dementia, AD and VD, with aHRs per 10-year decrease of 1.23, 1.27, and 1.35, respectively (P < .001 for all).
- After propensity score matching, AFib onset before age 65 years had the highest risk for all-cause dementia (aHR, 1.82; P < .001), followed by AF onset at age 65-74 years (aHR, 1.47; P < .001). Similar results were seen in AD and VD.
IN PRACTICE:
“The findings indicate that careful monitoring of cognitive function for patients with a younger [AFib] onset age, particularly those diagnosed with [AFib] before age 65 years, is important to attenuate the risk of subsequent dementia,” the authors write.
SOURCE:
The study, with first author Wenya Zhang, with the Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Because the study was observational, a cause-effect relationship cannot be established. Despite the adjustment for many underlying confounders, residual unidentified confounders may still exist. The vast majority of participants were White. The analyses did not consider the potential impact of effective treatment of AFib on dementia risk.
DISCLOSURES:
The study had no commercial funding. The authors have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Adults with atrial fibrillation (AFib) are at increased risk for dementia, especially when AFib occurs before age 65 years, new research shows. Investigators note the findings highlight the importance of monitoring cognitive function in adults with AF.
METHODOLOGY:
- This prospective, population-based cohort study leveraged data from 433,746 UK Biobank participants (55% women), including 30,601 with AFib, who were followed for a median of 12.6 years
- Incident cases of dementia were determined through linkage from multiple databases.
- Cox proportional hazards models and propensity score matching were used to estimate the association between age at onset of AFib and incident dementia.
TAKEAWAY:
- During follow-up, new-onset dementia occurred in 5,898 participants (2,546 with Alzheimer’s disease [AD] and 1,211 with vascular dementia [VD]), of which, 1,031 had AFib (350 with AD; 320 with VD).
- Compared with participants without AFib, those with AFib had a 42% higher risk for all-cause dementia (adjusted hazard ratio, 1.42; P < .001) and more than double the risk for VD (aHR, 2.06; P < .001), but no significantly higher risk for AD.
- Younger age at AFib onset was associated with higher risks for all-cause dementia, AD and VD, with aHRs per 10-year decrease of 1.23, 1.27, and 1.35, respectively (P < .001 for all).
- After propensity score matching, AFib onset before age 65 years had the highest risk for all-cause dementia (aHR, 1.82; P < .001), followed by AF onset at age 65-74 years (aHR, 1.47; P < .001). Similar results were seen in AD and VD.
IN PRACTICE:
“The findings indicate that careful monitoring of cognitive function for patients with a younger [AFib] onset age, particularly those diagnosed with [AFib] before age 65 years, is important to attenuate the risk of subsequent dementia,” the authors write.
SOURCE:
The study, with first author Wenya Zhang, with the Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Because the study was observational, a cause-effect relationship cannot be established. Despite the adjustment for many underlying confounders, residual unidentified confounders may still exist. The vast majority of participants were White. The analyses did not consider the potential impact of effective treatment of AFib on dementia risk.
DISCLOSURES:
The study had no commercial funding. The authors have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Adults with atrial fibrillation (AFib) are at increased risk for dementia, especially when AFib occurs before age 65 years, new research shows. Investigators note the findings highlight the importance of monitoring cognitive function in adults with AF.
METHODOLOGY:
- This prospective, population-based cohort study leveraged data from 433,746 UK Biobank participants (55% women), including 30,601 with AFib, who were followed for a median of 12.6 years
- Incident cases of dementia were determined through linkage from multiple databases.
- Cox proportional hazards models and propensity score matching were used to estimate the association between age at onset of AFib and incident dementia.
TAKEAWAY:
- During follow-up, new-onset dementia occurred in 5,898 participants (2,546 with Alzheimer’s disease [AD] and 1,211 with vascular dementia [VD]), of which, 1,031 had AFib (350 with AD; 320 with VD).
- Compared with participants without AFib, those with AFib had a 42% higher risk for all-cause dementia (adjusted hazard ratio, 1.42; P < .001) and more than double the risk for VD (aHR, 2.06; P < .001), but no significantly higher risk for AD.
- Younger age at AFib onset was associated with higher risks for all-cause dementia, AD and VD, with aHRs per 10-year decrease of 1.23, 1.27, and 1.35, respectively (P < .001 for all).
- After propensity score matching, AFib onset before age 65 years had the highest risk for all-cause dementia (aHR, 1.82; P < .001), followed by AF onset at age 65-74 years (aHR, 1.47; P < .001). Similar results were seen in AD and VD.
IN PRACTICE:
“The findings indicate that careful monitoring of cognitive function for patients with a younger [AFib] onset age, particularly those diagnosed with [AFib] before age 65 years, is important to attenuate the risk of subsequent dementia,” the authors write.
SOURCE:
The study, with first author Wenya Zhang, with the Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, was published online in JAMA Network Open.
LIMITATIONS:
Because the study was observational, a cause-effect relationship cannot be established. Despite the adjustment for many underlying confounders, residual unidentified confounders may still exist. The vast majority of participants were White. The analyses did not consider the potential impact of effective treatment of AFib on dementia risk.
DISCLOSURES:
The study had no commercial funding. The authors have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Blood test could predict future disability in MS
a new study suggests.
Rising NfL levels are a known indicator of neuroaxonal injury and correlate with MS disease activity. Levels rise in the presence of an MS relapse or MRI activity and fall following treatment with disease-modifying therapies. But the link between NfL levels and worsening disability was less understood.
This new analysis of NfL in two large MS cohorts found that elevated levels of the neuronal protein at baseline were associated with large increases in future disability risk, even in patients with no clinical relapse.
“This rising of NfL up to 2 years before signs of disability worsening represents the window when interventions may prevent worsening,” lead investigator Ahmed Abdelhak, MD, department of neurology, University of California, San Francisco, said in a press release.
The findings were published online in JAMA Neurology.
Early warning system?
The study included data on 1,899 patients with nearly 13,000 patient visits from two observational, long-term, real-world cohorts: the U.S.-based Expression, Proteomics, Imaging, Clinical (EPIC) study (n = 609 patients), and the Swiss Multiple Sclerosis Cohort trial (SMSC; n = 1,290 patients).
Investigators analyzed longitudinal serum NfL measurements in conjunction with clinical disability worsening, defined as 6 months or more of increased impairment as measured by the Expanded Disability Status Scale.
Researchers also assessed the temporal association between NfL measurements and the risk of increased disability and distinguished between disability with and without relapse.
Worsening disability was reported in 227 patients in the EPIC group and 435 in the SMSC trial.
Elevated NfL at baseline was associated with a 70% higher risk for worsening disability with relapse about 11 months later in the SMSC study (hazard ratio, 1.70; P = .02). In the EPIC trial, there was trend toward a 91% higher risk for worsening disability with relapse at 12.6 months, although the findings did not meet statistical significance (HR, 1.91; P = .07).
The risk of future disability progression independent of clinical relapse was 40% higher in those with high NfL at baseline in the EPIC study 12 months after baseline (HR, 1.40; P = .02) and 49% higher in the SMSC trial 21 months later (HR, 1.49; P < .001).
The early elevation of NfL levels suggests a slower degradation of nerve cells and could be a possible early warning system of future progression of disability, allowing time for interventions that could slow or even halt further disability.
“Monitoring NfL levels might be able to detect disease activity with higher sensitivity than clinical exam or conventional imaging,” senior author Jens Kuhle, MD, PhD, leader of the Swiss cohort and head of the Multiple Sclerosis Center at University Hospital and University of Basel, said in a statement.
Challenges for clinicians
Commenting on the findings, Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research, Neurological Institute, Cleveland Clinic, said that, while there is a clinical test to measure NfL levels, incorporating that test into standard of care isn’t straightforward.
“The challenge for the practicing clinician is to translate these population-level studies to individual patient management decisions,” said Dr. Fox, who was not a part of the study.
“The published prediction curves corrected for age, sex, disease course, disease-modifying treatment, relapse within the past 90 days, and current disability status, the combination of which makes it rather challenging to calculate and interpret adjusted z score NfL levels in routine practice and then use it in clinical decision-making.”
The investigators said the study underscores the importance of NfL as an MS biomarker and “points to the existence of different windows of dynamic central nervous system pathology” that precedes worsening disability with or without relapse. But there may be a simpler explanation, Dr. Fox suggested.
“We know MRI activity occurs 5-10 times more frequently than relapses, and we know that MRI activity is associated with both NfL increases and future disability progression,” Dr. Fox said. “It is quite likely that the elevations in NfL seen here are reflective of new MRI disease activity, which frequently is seen without symptoms of an MS relapse,” he said
The study was funded by the Westridge Foundation, F. Hoffmann–La Roche, the Fishman Family, the Swiss National Research Foundation, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and the Valhalla Foundation. Dr. Abdelhak reported receiving grants from the German Multiple Sclerosis Society and the Weill Institute for Neurosciences outside the submitted work. Dr. Kuhle has received grants from Swiss MS Society, the Swiss National Research Foundation, the Progressive MS Alliance, Biogen, Merck, Celgene, Bristol-Myers Squibb, Novartis, Octave Bioscience, Roche, Sanofi, Alnylam, Bayer, Immunic, Quanterix, Neurogenesis, Stata DX, and the University of Basel outside the submitted work. Dr. Fox reported receiving consulting fees from Siemens and Roche.
A version of this article appeared on Medscape.com.
a new study suggests.
Rising NfL levels are a known indicator of neuroaxonal injury and correlate with MS disease activity. Levels rise in the presence of an MS relapse or MRI activity and fall following treatment with disease-modifying therapies. But the link between NfL levels and worsening disability was less understood.
This new analysis of NfL in two large MS cohorts found that elevated levels of the neuronal protein at baseline were associated with large increases in future disability risk, even in patients with no clinical relapse.
“This rising of NfL up to 2 years before signs of disability worsening represents the window when interventions may prevent worsening,” lead investigator Ahmed Abdelhak, MD, department of neurology, University of California, San Francisco, said in a press release.
The findings were published online in JAMA Neurology.
Early warning system?
The study included data on 1,899 patients with nearly 13,000 patient visits from two observational, long-term, real-world cohorts: the U.S.-based Expression, Proteomics, Imaging, Clinical (EPIC) study (n = 609 patients), and the Swiss Multiple Sclerosis Cohort trial (SMSC; n = 1,290 patients).
Investigators analyzed longitudinal serum NfL measurements in conjunction with clinical disability worsening, defined as 6 months or more of increased impairment as measured by the Expanded Disability Status Scale.
Researchers also assessed the temporal association between NfL measurements and the risk of increased disability and distinguished between disability with and without relapse.
Worsening disability was reported in 227 patients in the EPIC group and 435 in the SMSC trial.
Elevated NfL at baseline was associated with a 70% higher risk for worsening disability with relapse about 11 months later in the SMSC study (hazard ratio, 1.70; P = .02). In the EPIC trial, there was trend toward a 91% higher risk for worsening disability with relapse at 12.6 months, although the findings did not meet statistical significance (HR, 1.91; P = .07).
The risk of future disability progression independent of clinical relapse was 40% higher in those with high NfL at baseline in the EPIC study 12 months after baseline (HR, 1.40; P = .02) and 49% higher in the SMSC trial 21 months later (HR, 1.49; P < .001).
The early elevation of NfL levels suggests a slower degradation of nerve cells and could be a possible early warning system of future progression of disability, allowing time for interventions that could slow or even halt further disability.
“Monitoring NfL levels might be able to detect disease activity with higher sensitivity than clinical exam or conventional imaging,” senior author Jens Kuhle, MD, PhD, leader of the Swiss cohort and head of the Multiple Sclerosis Center at University Hospital and University of Basel, said in a statement.
Challenges for clinicians
Commenting on the findings, Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research, Neurological Institute, Cleveland Clinic, said that, while there is a clinical test to measure NfL levels, incorporating that test into standard of care isn’t straightforward.
“The challenge for the practicing clinician is to translate these population-level studies to individual patient management decisions,” said Dr. Fox, who was not a part of the study.
“The published prediction curves corrected for age, sex, disease course, disease-modifying treatment, relapse within the past 90 days, and current disability status, the combination of which makes it rather challenging to calculate and interpret adjusted z score NfL levels in routine practice and then use it in clinical decision-making.”
The investigators said the study underscores the importance of NfL as an MS biomarker and “points to the existence of different windows of dynamic central nervous system pathology” that precedes worsening disability with or without relapse. But there may be a simpler explanation, Dr. Fox suggested.
“We know MRI activity occurs 5-10 times more frequently than relapses, and we know that MRI activity is associated with both NfL increases and future disability progression,” Dr. Fox said. “It is quite likely that the elevations in NfL seen here are reflective of new MRI disease activity, which frequently is seen without symptoms of an MS relapse,” he said
The study was funded by the Westridge Foundation, F. Hoffmann–La Roche, the Fishman Family, the Swiss National Research Foundation, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and the Valhalla Foundation. Dr. Abdelhak reported receiving grants from the German Multiple Sclerosis Society and the Weill Institute for Neurosciences outside the submitted work. Dr. Kuhle has received grants from Swiss MS Society, the Swiss National Research Foundation, the Progressive MS Alliance, Biogen, Merck, Celgene, Bristol-Myers Squibb, Novartis, Octave Bioscience, Roche, Sanofi, Alnylam, Bayer, Immunic, Quanterix, Neurogenesis, Stata DX, and the University of Basel outside the submitted work. Dr. Fox reported receiving consulting fees from Siemens and Roche.
A version of this article appeared on Medscape.com.
a new study suggests.
Rising NfL levels are a known indicator of neuroaxonal injury and correlate with MS disease activity. Levels rise in the presence of an MS relapse or MRI activity and fall following treatment with disease-modifying therapies. But the link between NfL levels and worsening disability was less understood.
This new analysis of NfL in two large MS cohorts found that elevated levels of the neuronal protein at baseline were associated with large increases in future disability risk, even in patients with no clinical relapse.
“This rising of NfL up to 2 years before signs of disability worsening represents the window when interventions may prevent worsening,” lead investigator Ahmed Abdelhak, MD, department of neurology, University of California, San Francisco, said in a press release.
The findings were published online in JAMA Neurology.
Early warning system?
The study included data on 1,899 patients with nearly 13,000 patient visits from two observational, long-term, real-world cohorts: the U.S.-based Expression, Proteomics, Imaging, Clinical (EPIC) study (n = 609 patients), and the Swiss Multiple Sclerosis Cohort trial (SMSC; n = 1,290 patients).
Investigators analyzed longitudinal serum NfL measurements in conjunction with clinical disability worsening, defined as 6 months or more of increased impairment as measured by the Expanded Disability Status Scale.
Researchers also assessed the temporal association between NfL measurements and the risk of increased disability and distinguished between disability with and without relapse.
Worsening disability was reported in 227 patients in the EPIC group and 435 in the SMSC trial.
Elevated NfL at baseline was associated with a 70% higher risk for worsening disability with relapse about 11 months later in the SMSC study (hazard ratio, 1.70; P = .02). In the EPIC trial, there was trend toward a 91% higher risk for worsening disability with relapse at 12.6 months, although the findings did not meet statistical significance (HR, 1.91; P = .07).
The risk of future disability progression independent of clinical relapse was 40% higher in those with high NfL at baseline in the EPIC study 12 months after baseline (HR, 1.40; P = .02) and 49% higher in the SMSC trial 21 months later (HR, 1.49; P < .001).
The early elevation of NfL levels suggests a slower degradation of nerve cells and could be a possible early warning system of future progression of disability, allowing time for interventions that could slow or even halt further disability.
“Monitoring NfL levels might be able to detect disease activity with higher sensitivity than clinical exam or conventional imaging,” senior author Jens Kuhle, MD, PhD, leader of the Swiss cohort and head of the Multiple Sclerosis Center at University Hospital and University of Basel, said in a statement.
Challenges for clinicians
Commenting on the findings, Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research, Neurological Institute, Cleveland Clinic, said that, while there is a clinical test to measure NfL levels, incorporating that test into standard of care isn’t straightforward.
“The challenge for the practicing clinician is to translate these population-level studies to individual patient management decisions,” said Dr. Fox, who was not a part of the study.
“The published prediction curves corrected for age, sex, disease course, disease-modifying treatment, relapse within the past 90 days, and current disability status, the combination of which makes it rather challenging to calculate and interpret adjusted z score NfL levels in routine practice and then use it in clinical decision-making.”
The investigators said the study underscores the importance of NfL as an MS biomarker and “points to the existence of different windows of dynamic central nervous system pathology” that precedes worsening disability with or without relapse. But there may be a simpler explanation, Dr. Fox suggested.
“We know MRI activity occurs 5-10 times more frequently than relapses, and we know that MRI activity is associated with both NfL increases and future disability progression,” Dr. Fox said. “It is quite likely that the elevations in NfL seen here are reflective of new MRI disease activity, which frequently is seen without symptoms of an MS relapse,” he said
The study was funded by the Westridge Foundation, F. Hoffmann–La Roche, the Fishman Family, the Swiss National Research Foundation, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and the Valhalla Foundation. Dr. Abdelhak reported receiving grants from the German Multiple Sclerosis Society and the Weill Institute for Neurosciences outside the submitted work. Dr. Kuhle has received grants from Swiss MS Society, the Swiss National Research Foundation, the Progressive MS Alliance, Biogen, Merck, Celgene, Bristol-Myers Squibb, Novartis, Octave Bioscience, Roche, Sanofi, Alnylam, Bayer, Immunic, Quanterix, Neurogenesis, Stata DX, and the University of Basel outside the submitted work. Dr. Fox reported receiving consulting fees from Siemens and Roche.
A version of this article appeared on Medscape.com.
FROM JAMA NEUROLOGY
Short steroid taper tested with tocilizumab for giant cell arteritis
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
TOPLINE:
A combination of tocilizumab (Actemra) and 8 weeks of tapering prednisone was effective for inducing and maintaining disease remission in adults with giant cell arteritis (GCA).
METHODOLOGY:
- In a single-center, single-arm, open-label pilot study, 30 adults (mean age, 73.7 years) with GCA received 162 mg of tocilizumab as a subcutaneous injection once a week for 52 weeks, plus prednisone starting between 20 mg and 60 mg with a prespecified 8-week taper off the glucocorticoid.
- Patients had to be at least 50 years of age and could have either new-onset (diagnosis within 6 weeks of baseline) or relapsing disease (diagnosis > 6 weeks from baseline).
- The primary endpoint was sustained, prednisone-free remission at 52 weeks, defined by an erythrocyte sedimentation rate of less than 40 mm/h, C-reactive protein level less than 10 mg/L, and adherence to the prednisone taper; secondary endpoints included the proportions of patients in remission and relapse, cumulative prednisone dose, and glucocorticoid toxicity.
TAKEAWAY:
- At 52 weeks, 23 patients (77%) met the criteria for sustained remission after weaning off prednisone within 8 weeks of starting tocilizumab; 7 relapsed after a mean of 15.8 weeks.
- Of the patients who relapsed, six underwent a second prednisone taper for 8 weeks with a mean initial daily dose of 32.1 mg, four regained and maintained remission, and two experienced a second relapse and withdrew from the study.
- The mean cumulative prednisone dose at week 52 was 1,051.5 mg for responders and 1,673.1 mg for nonresponders.
- All 30 patients had at least one adverse event; four patients had a serious adverse event likely related to tocilizumab, prednisone, or both.
IN PRACTICE:
Studies such as this “are highly valuable as proof of concept, but of course cannot be definitive guides to treatment decisions without a comparator group,” according to authors of an editorial accompanying the study.
SOURCE:
The study, by Sebastian Unizony, MD, Harvard Medical School, Boston, and colleagues, was published in The Lancet Rheumatology .
LIMITATIONS:
The small size and open-label design with no control group were limiting factors; more research is needed to confirm the findings before this treatment strategy can be recommended for clinical practice.
DISCLOSURES:
The study was funded by Genentech. Two authors reported financial relationships with pharmaceutical companies outside of this report.
A version of this article first appeared on Medscape.com.
An FP’s guide to caring for patients with seizure and epilepsy
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).
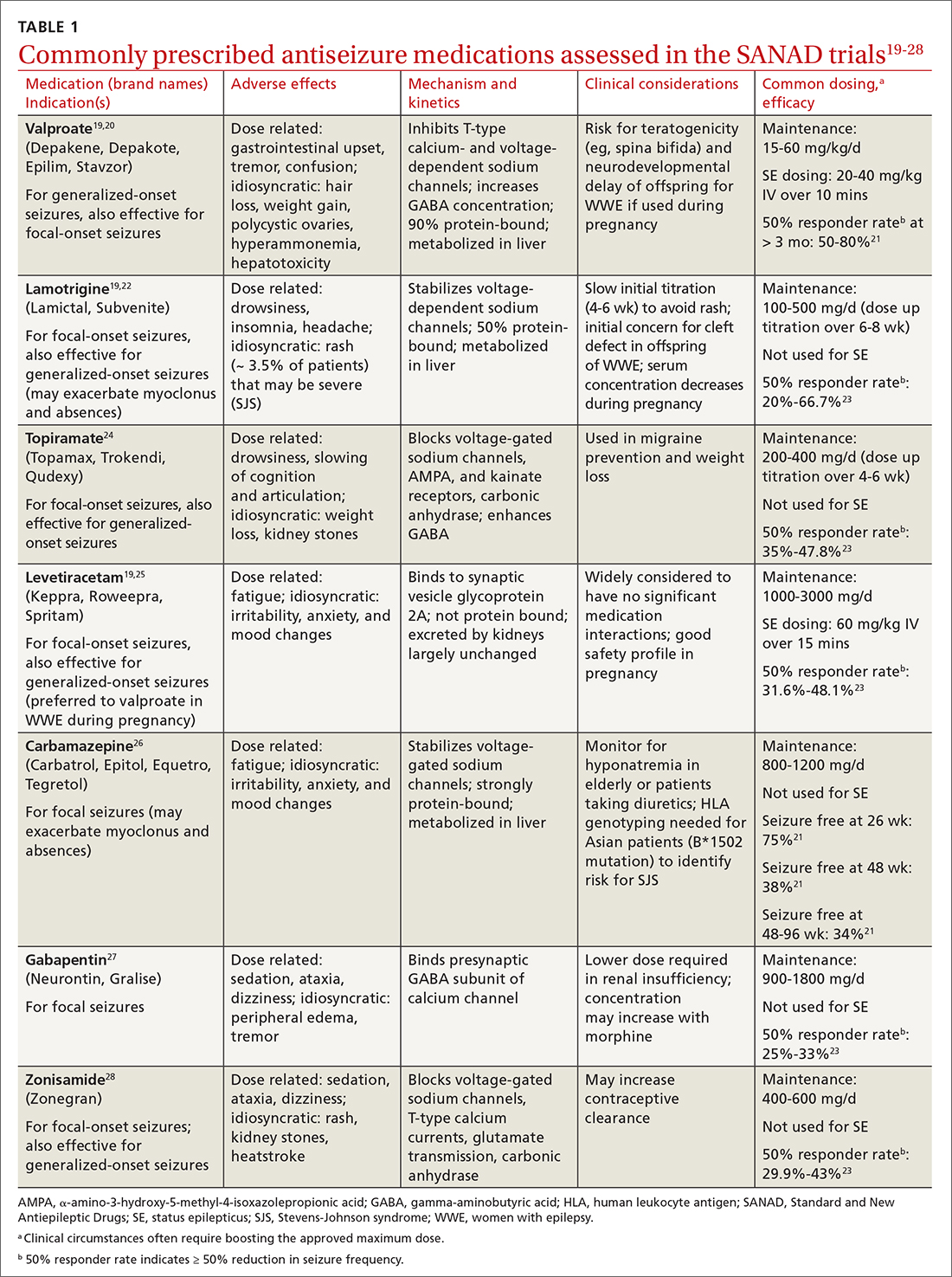
Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals
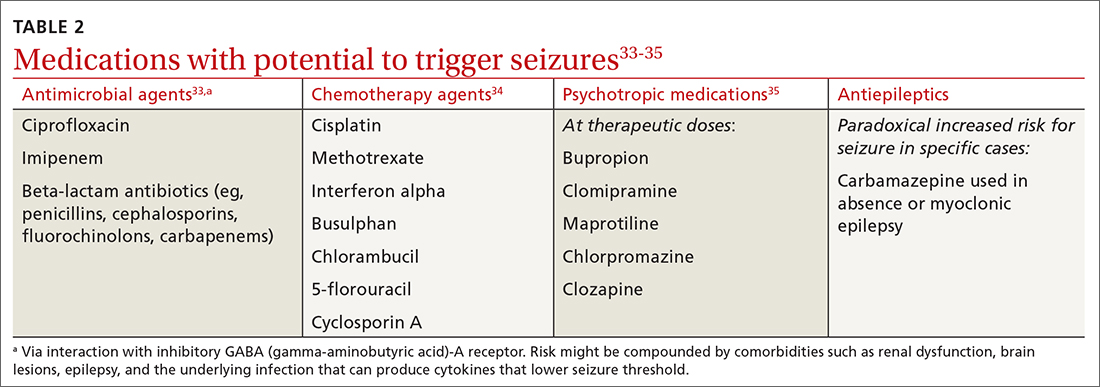
Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).

Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals

Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).

Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals

Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
PRACTICE RECOMMENDATIONS
› Consider treating a first-time seizure if electroencephalography shows particular epileptiform activity, if the neurologic exam or computerized tomography or magnetic resonance imaging results are abnormal, if the seizure is focal or nocturnal, or if there is a family history of seizures. A
› Consider valproate (except for women of childbearing age) and levetiracetam as first-line agents for generalized or unclassified epilepsy, and lamotrigine for focal epilepsies. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke patients benefit from neurologic music therapy
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM WCN 2023
‘Hidden’ cognitive impairments in DMD may worsen outcomes
A new tool from the National Institutes of Health, called NIH Toolbox, could improve that outcome, according to Mathula Thangarajh, MD, PhD, who has conducted research in the field.
“When we talk to families and parents, they are able to identify that even during infancy that [children with DMD] have delayed cognitive function. This includes speech delay, but also language and adaptive skills. We also know that those children with speech delay, which is really a very commonly reported phenotype in up to 50%, go on to have school-based needs. They may repeat [grades] in elementary years, but they also use more resources at school,” said Dr. Thangarajh, who is an assistant professor of neurology at the Children’s Hospital of Richmond at Virginia Commonwealth University, Richmond, during a talk at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
A previous natural history study that utilized the Pediatric Quality of Life assessment also showed that DMD patients reported the lowest scores in brain health, including emotional health and school performance.
Other research has shown a correlation between cognitive function and survival in DMD. “This suggests that health maintenance may play an important role [in outcomes],” said Dr. Thangarajh. Another study found a correlation between psychomotor delay that required school-based interventions and earlier loss of ambulation, lower cardiac ejection fraction, and worse pulmonary function. The researchers also found that boys with cognitive delay were diagnosed at an earlier age, and yet had delays in diagnosis and worse motor function, pulmonary health, and cardiac health outcomes. On average, they lost ambulatory ability 2 years earlier.
A study by Dr. Thangarajh’s group showed that patients with speech delay and lower IQ had lower performance in timed tests, including 6-minute walk test distance and scored an average of 2 points lower on the North Star Ambulatory Assessment.
A tool for continuous cognitive assessment
The Centers for Disease Control and Prevention–supported DMD CARE guidelines only say that neuropsychological evaluations should be considered at diagnosis, but is essential if concerns arise about developmental progress. However, the Muscular Dystrophy Association has found barriers both in access to specialists, with an average wait time of 1-2 years, and burdensome out-of-pocket costs.
Those issues prompted Dr. Thangarajh to look for an alternative solution. At the time that she embarked on this work, the NIH was interested in technologies to assess neurobehavioral issues across different diseases. The resulting NIH Toolbox iPad app was driven largely by failed clinical trials in dementia, and the aim was to be able to provide continuous assessment over time. “It will allow for assessments across the lifespan, so you can use the same construct from age 3 to 80-plus,” said Dr. Thangarajh. It can also normalize population factors, such as annual household income and mother’s IQ.
She set out to validate the NIH Toolbox in children with DMD. The toolbox includes measures of crystalized cognition and fluid cognition. The former encompasses vocabulary and reading ability, which are strongly predicted by socioeconomic status and maternal IQ. On the other hand, fluid cognition includes cognitive features that develop across the lifespan and is directly related to academic underperformance in DMD patients.
Dr. Thangarajh’s group assessed 30 boys with DMD and found that crystallized cognition was normal, but they had a deficit in fluid cognition. They found deficits within several subdomains of fluid cognition. “This tells us that the NIH Toolbox was able to replicate what we had known in the literature, that these boys really have lower intellectual capacity, but they also have significant weakness in fluid cognition,” she said.
She also wanted to examine changes over time by testing the boys at a 1-year interview. “What we found was that they are not making as much gain in fluid cognition as we would like. They are just making marginal improvements over time. This has implications on how often we should screen them, but also not be over reliant on using school-based resources for them to get tested,” said Dr. Thangarajh.
Her group’s analysis of a dataset of 55 boys provided by PTC Therapeutics revealed a difference by age in a test of working memory. “What we found was that boys who are actually greater than 9 years, compared with those who are less than 9 years, they actually had a reversal of development-based improvement. The older they get, they were not making as much gains as you would expect,” said Dr. Thangarajh.
She went on to discuss psychosocial determinants of cognitive health in DMD. It is known that women who are carriers of the dystrophin mutation can underperform in cognitively stressful tasks, leading her to wonder if this could lead to transgenerational risk to offspring with DMD. Her group tested women who were carriers of the mutation with the NIH Toolbox and found that they had lower fluid cognition than noncarriers. They then tested 65 dyads of mothers and children, and found a correlation, but only when it came to inhibitory control, which required the individual to note the direction of an arrow while ignoring surrounding arrows pointing in various directions.
Next, the researchers examined neighborhoods and their impact on cognitive health, which can be affected by the presence of green spaces, access to public transportation and good nutrition, and other factors. There were significant deficits associated with residence zip codes. “We were pretty shocked. Someone who is not in a socially vulnerable region is scoring slightly below average, but someone who is in a very socially vulnerable neighborhood is only scoring 75 [age-adjusted score] on the NIH toolbox. So with this, we can conclude that carrier women are vulnerable in certain cognitive domains, but also children who come from socially vulnerable [situations] have poor cognitive control. This, again, has implications on how often we should screen and how much we should overly rely on school-based resources for these individuals,” said Dr. Thangarajh.
Overcoming a significant barrier
The NIH Toolbox has a lot of potential to improve DMD care, according to Dianna Quan, MD, who is the incoming president of AANEM, and professor of neurology at the University of Colorado at Denver, Aurora. “There’s this huge problem in terms of getting people in to see neuropsychologists and having formal evaluations. I think that’s a huge barrier. If we have people able to access this toolkit, which is simple and easily and universally accessible, how wonderful is that? I think that will be a really great improvement on what’s going on right now. It allows people to easily screen for these cognitive disabilities and make sure that we address them,” Dr. Quan said in an interview.
Asked how the tool could specifically improve care, Dr. Quan suggested that the first step is to understand the contributing factors to cognitive issues, whether they are biological, social, or a combination. “Some of them we can modify, potentially, through addressing the social environment. Some of those biologic factors may also be modifiable with many of the new drug studies that are coming.”
Dr. Thangarajh has received speaker honoraria from NS Pharma and PTC Therapeutics. Dr. Quan has received funding from Alnylam, Pfizer, Cytokinetics, Momenta, and Argenx.
A new tool from the National Institutes of Health, called NIH Toolbox, could improve that outcome, according to Mathula Thangarajh, MD, PhD, who has conducted research in the field.
“When we talk to families and parents, they are able to identify that even during infancy that [children with DMD] have delayed cognitive function. This includes speech delay, but also language and adaptive skills. We also know that those children with speech delay, which is really a very commonly reported phenotype in up to 50%, go on to have school-based needs. They may repeat [grades] in elementary years, but they also use more resources at school,” said Dr. Thangarajh, who is an assistant professor of neurology at the Children’s Hospital of Richmond at Virginia Commonwealth University, Richmond, during a talk at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
A previous natural history study that utilized the Pediatric Quality of Life assessment also showed that DMD patients reported the lowest scores in brain health, including emotional health and school performance.
Other research has shown a correlation between cognitive function and survival in DMD. “This suggests that health maintenance may play an important role [in outcomes],” said Dr. Thangarajh. Another study found a correlation between psychomotor delay that required school-based interventions and earlier loss of ambulation, lower cardiac ejection fraction, and worse pulmonary function. The researchers also found that boys with cognitive delay were diagnosed at an earlier age, and yet had delays in diagnosis and worse motor function, pulmonary health, and cardiac health outcomes. On average, they lost ambulatory ability 2 years earlier.
A study by Dr. Thangarajh’s group showed that patients with speech delay and lower IQ had lower performance in timed tests, including 6-minute walk test distance and scored an average of 2 points lower on the North Star Ambulatory Assessment.
A tool for continuous cognitive assessment
The Centers for Disease Control and Prevention–supported DMD CARE guidelines only say that neuropsychological evaluations should be considered at diagnosis, but is essential if concerns arise about developmental progress. However, the Muscular Dystrophy Association has found barriers both in access to specialists, with an average wait time of 1-2 years, and burdensome out-of-pocket costs.
Those issues prompted Dr. Thangarajh to look for an alternative solution. At the time that she embarked on this work, the NIH was interested in technologies to assess neurobehavioral issues across different diseases. The resulting NIH Toolbox iPad app was driven largely by failed clinical trials in dementia, and the aim was to be able to provide continuous assessment over time. “It will allow for assessments across the lifespan, so you can use the same construct from age 3 to 80-plus,” said Dr. Thangarajh. It can also normalize population factors, such as annual household income and mother’s IQ.
She set out to validate the NIH Toolbox in children with DMD. The toolbox includes measures of crystalized cognition and fluid cognition. The former encompasses vocabulary and reading ability, which are strongly predicted by socioeconomic status and maternal IQ. On the other hand, fluid cognition includes cognitive features that develop across the lifespan and is directly related to academic underperformance in DMD patients.
Dr. Thangarajh’s group assessed 30 boys with DMD and found that crystallized cognition was normal, but they had a deficit in fluid cognition. They found deficits within several subdomains of fluid cognition. “This tells us that the NIH Toolbox was able to replicate what we had known in the literature, that these boys really have lower intellectual capacity, but they also have significant weakness in fluid cognition,” she said.
She also wanted to examine changes over time by testing the boys at a 1-year interview. “What we found was that they are not making as much gain in fluid cognition as we would like. They are just making marginal improvements over time. This has implications on how often we should screen them, but also not be over reliant on using school-based resources for them to get tested,” said Dr. Thangarajh.
Her group’s analysis of a dataset of 55 boys provided by PTC Therapeutics revealed a difference by age in a test of working memory. “What we found was that boys who are actually greater than 9 years, compared with those who are less than 9 years, they actually had a reversal of development-based improvement. The older they get, they were not making as much gains as you would expect,” said Dr. Thangarajh.
She went on to discuss psychosocial determinants of cognitive health in DMD. It is known that women who are carriers of the dystrophin mutation can underperform in cognitively stressful tasks, leading her to wonder if this could lead to transgenerational risk to offspring with DMD. Her group tested women who were carriers of the mutation with the NIH Toolbox and found that they had lower fluid cognition than noncarriers. They then tested 65 dyads of mothers and children, and found a correlation, but only when it came to inhibitory control, which required the individual to note the direction of an arrow while ignoring surrounding arrows pointing in various directions.
Next, the researchers examined neighborhoods and their impact on cognitive health, which can be affected by the presence of green spaces, access to public transportation and good nutrition, and other factors. There were significant deficits associated with residence zip codes. “We were pretty shocked. Someone who is not in a socially vulnerable region is scoring slightly below average, but someone who is in a very socially vulnerable neighborhood is only scoring 75 [age-adjusted score] on the NIH toolbox. So with this, we can conclude that carrier women are vulnerable in certain cognitive domains, but also children who come from socially vulnerable [situations] have poor cognitive control. This, again, has implications on how often we should screen and how much we should overly rely on school-based resources for these individuals,” said Dr. Thangarajh.
Overcoming a significant barrier
The NIH Toolbox has a lot of potential to improve DMD care, according to Dianna Quan, MD, who is the incoming president of AANEM, and professor of neurology at the University of Colorado at Denver, Aurora. “There’s this huge problem in terms of getting people in to see neuropsychologists and having formal evaluations. I think that’s a huge barrier. If we have people able to access this toolkit, which is simple and easily and universally accessible, how wonderful is that? I think that will be a really great improvement on what’s going on right now. It allows people to easily screen for these cognitive disabilities and make sure that we address them,” Dr. Quan said in an interview.
Asked how the tool could specifically improve care, Dr. Quan suggested that the first step is to understand the contributing factors to cognitive issues, whether they are biological, social, or a combination. “Some of them we can modify, potentially, through addressing the social environment. Some of those biologic factors may also be modifiable with many of the new drug studies that are coming.”
Dr. Thangarajh has received speaker honoraria from NS Pharma and PTC Therapeutics. Dr. Quan has received funding from Alnylam, Pfizer, Cytokinetics, Momenta, and Argenx.
A new tool from the National Institutes of Health, called NIH Toolbox, could improve that outcome, according to Mathula Thangarajh, MD, PhD, who has conducted research in the field.
“When we talk to families and parents, they are able to identify that even during infancy that [children with DMD] have delayed cognitive function. This includes speech delay, but also language and adaptive skills. We also know that those children with speech delay, which is really a very commonly reported phenotype in up to 50%, go on to have school-based needs. They may repeat [grades] in elementary years, but they also use more resources at school,” said Dr. Thangarajh, who is an assistant professor of neurology at the Children’s Hospital of Richmond at Virginia Commonwealth University, Richmond, during a talk at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
A previous natural history study that utilized the Pediatric Quality of Life assessment also showed that DMD patients reported the lowest scores in brain health, including emotional health and school performance.
Other research has shown a correlation between cognitive function and survival in DMD. “This suggests that health maintenance may play an important role [in outcomes],” said Dr. Thangarajh. Another study found a correlation between psychomotor delay that required school-based interventions and earlier loss of ambulation, lower cardiac ejection fraction, and worse pulmonary function. The researchers also found that boys with cognitive delay were diagnosed at an earlier age, and yet had delays in diagnosis and worse motor function, pulmonary health, and cardiac health outcomes. On average, they lost ambulatory ability 2 years earlier.
A study by Dr. Thangarajh’s group showed that patients with speech delay and lower IQ had lower performance in timed tests, including 6-minute walk test distance and scored an average of 2 points lower on the North Star Ambulatory Assessment.
A tool for continuous cognitive assessment
The Centers for Disease Control and Prevention–supported DMD CARE guidelines only say that neuropsychological evaluations should be considered at diagnosis, but is essential if concerns arise about developmental progress. However, the Muscular Dystrophy Association has found barriers both in access to specialists, with an average wait time of 1-2 years, and burdensome out-of-pocket costs.
Those issues prompted Dr. Thangarajh to look for an alternative solution. At the time that she embarked on this work, the NIH was interested in technologies to assess neurobehavioral issues across different diseases. The resulting NIH Toolbox iPad app was driven largely by failed clinical trials in dementia, and the aim was to be able to provide continuous assessment over time. “It will allow for assessments across the lifespan, so you can use the same construct from age 3 to 80-plus,” said Dr. Thangarajh. It can also normalize population factors, such as annual household income and mother’s IQ.
She set out to validate the NIH Toolbox in children with DMD. The toolbox includes measures of crystalized cognition and fluid cognition. The former encompasses vocabulary and reading ability, which are strongly predicted by socioeconomic status and maternal IQ. On the other hand, fluid cognition includes cognitive features that develop across the lifespan and is directly related to academic underperformance in DMD patients.
Dr. Thangarajh’s group assessed 30 boys with DMD and found that crystallized cognition was normal, but they had a deficit in fluid cognition. They found deficits within several subdomains of fluid cognition. “This tells us that the NIH Toolbox was able to replicate what we had known in the literature, that these boys really have lower intellectual capacity, but they also have significant weakness in fluid cognition,” she said.
She also wanted to examine changes over time by testing the boys at a 1-year interview. “What we found was that they are not making as much gain in fluid cognition as we would like. They are just making marginal improvements over time. This has implications on how often we should screen them, but also not be over reliant on using school-based resources for them to get tested,” said Dr. Thangarajh.
Her group’s analysis of a dataset of 55 boys provided by PTC Therapeutics revealed a difference by age in a test of working memory. “What we found was that boys who are actually greater than 9 years, compared with those who are less than 9 years, they actually had a reversal of development-based improvement. The older they get, they were not making as much gains as you would expect,” said Dr. Thangarajh.
She went on to discuss psychosocial determinants of cognitive health in DMD. It is known that women who are carriers of the dystrophin mutation can underperform in cognitively stressful tasks, leading her to wonder if this could lead to transgenerational risk to offspring with DMD. Her group tested women who were carriers of the mutation with the NIH Toolbox and found that they had lower fluid cognition than noncarriers. They then tested 65 dyads of mothers and children, and found a correlation, but only when it came to inhibitory control, which required the individual to note the direction of an arrow while ignoring surrounding arrows pointing in various directions.
Next, the researchers examined neighborhoods and their impact on cognitive health, which can be affected by the presence of green spaces, access to public transportation and good nutrition, and other factors. There were significant deficits associated with residence zip codes. “We were pretty shocked. Someone who is not in a socially vulnerable region is scoring slightly below average, but someone who is in a very socially vulnerable neighborhood is only scoring 75 [age-adjusted score] on the NIH toolbox. So with this, we can conclude that carrier women are vulnerable in certain cognitive domains, but also children who come from socially vulnerable [situations] have poor cognitive control. This, again, has implications on how often we should screen and how much we should overly rely on school-based resources for these individuals,” said Dr. Thangarajh.
Overcoming a significant barrier
The NIH Toolbox has a lot of potential to improve DMD care, according to Dianna Quan, MD, who is the incoming president of AANEM, and professor of neurology at the University of Colorado at Denver, Aurora. “There’s this huge problem in terms of getting people in to see neuropsychologists and having formal evaluations. I think that’s a huge barrier. If we have people able to access this toolkit, which is simple and easily and universally accessible, how wonderful is that? I think that will be a really great improvement on what’s going on right now. It allows people to easily screen for these cognitive disabilities and make sure that we address them,” Dr. Quan said in an interview.
Asked how the tool could specifically improve care, Dr. Quan suggested that the first step is to understand the contributing factors to cognitive issues, whether they are biological, social, or a combination. “Some of them we can modify, potentially, through addressing the social environment. Some of those biologic factors may also be modifiable with many of the new drug studies that are coming.”
Dr. Thangarajh has received speaker honoraria from NS Pharma and PTC Therapeutics. Dr. Quan has received funding from Alnylam, Pfizer, Cytokinetics, Momenta, and Argenx.
FROM AANEM 2023
Duchenne muscular dystrophy gene therapy safe, effective at 4 years
PHOENIX – compared with untreated patients who showed significant decline over the same time period, new research shows.
“Functional assessments demonstrated long-term sustained stabilization of motor function that was clinically meaningful, at ages where functional decline would be expected based on natural history,” the investigators noted in their abstract. Furthermore, the treatment, known as delandistrogene moxeparvovec-rokl (SRP-9001), was well tolerated 4 years post treatment.
The study was presented at the annual meeting of the American Association of Neuromuscular Electrodiagnostic Medicine.
Severe type of DMD
Considered one of the most severe forms of muscular dystrophy, DMD causes progressive muscle wasting stemming from the root genetic cause of missing dystrophin in muscle cells. Often referred to as a molecular “shock absorber,” dystrophin stabilizes the sarcolemma during muscle contractions to prevent degeneration.
SRP-9001, a single-dose recombinant gene therapy administered as an intravenous infusion, was designed to deliver a trimmed down form of dystrophin to compensate for the deficit.
In July, the adeno-associated virus vector (AAV)–based SRP-9001 gene therapy was granted accelerated approval by the Food and Drug Administration for the treatment of ambulatory pediatric patients aged 4-5 years with DMD with a confirmed mutation in the DMD gene.
The therapy is administered over 1-2 hours at a dose of 133 trillion vector genomes per kilogram of body weight.
For Study 101, one of several evaluating the novel therapy, a research team led by senior investigator Jerry Mendell, MD, an attending neurologist at Nationwide Children’s Hospital and professor of pediatrics and neurology at Ohio State University, both in Columbus,evaluated data on four ambulatory male patients aged 4-8 years who received a single IV infusion of the therapy.
All patients also received prednisone 1 mg/kg, 1 day preinfusion and 30 days post infusion.
At 4 years post treatment, there were no new safety events. All treatment-related adverse events occurred mainly within the first 70 days, and all resolved.
The most commonly reported adverse reactions of the gene therapy include vomiting, nausea, increases in liver enzymes, pyrexia (fever), and thrombocytopenia, all of which occurred within 90 days of infusion and been manageable.
Risk mitigation strategies for hepatotoxicity or acute liver injury include pre- and postinfusion monitoring of liver enzymes, the authors noted.
No serious abnormalities were observed in hematologic or chemistry panels, and while three patients had elevated gamma-glutamyl transpeptidase in the first 3 months post treatment, those cases resolved with oral steroid treatment.
Significant improvements in function were observed, with a mean improvement in North Star Ambulatory Assessment (NSAA) scores from baseline of 7.0 points (range, 4-11).
Exploratory analyses further showed that, compared with a propensity score–weighted external control cohort of 21 patients with DMD who did not receive the therapy, those receiving SRP-9001 had a statistically significant difference of 9.4 points in least-squares mean change from baseline to 4 years on the NSAA score (P = .0125).
Similar trends were observed in improvement from baseline in key measures of time to rise, 4-stair climb, and 10- and 100-meter walk/run function tests.
Other reported adverse events include acute serious liver injury, immune-mediated myositis, and myocarditis. Because of the latter risk, the therapy is contraindicated in patients with any deletion in exon 8 and/or exon 9 in the DMD gene.
The current 4-year update on SRP-9001 adds to clinical trial results that have been reported on more than 80 patients treated to date, with favorable results and consistent safety profiles reported at other time points.
Continued FDA approval for the therapy will be contingent upon verification of a clinical benefit in the confirmatory trials, including the EMBARK trial.
Increased function, long-term stability
Discussing the research at the meeting, Craig McDonald, MD, professor and chair of physical medicine & rehabilitation, a professor of pediatrics and study chair of the CINRG Duchenne Natural History Study at University of California Davis Health, noted that top-line results from the ongoing, confirmatory phase 3 EMBARK trial show functional benefits of SRP-9001 not only in 4- to 5-year-olds but also in other older age groups.
“What’s really striking, and in my mind the most impressive, is that when you follow these patients out 3 or 4 years ... you see there is this bump in function followed by long-term stability, whereas the external control cohort predictably shows really quite significant declines in their [NSAA] functional values,” he said in his presentation.
“When you look at each individually treated patient versus their own predicted trajectory using their baseline values on the time function test, each of the patients actually has a really quite impressive stabilization of function over their predicted disease trajectory,” he added.
A caveat that SRP-9001 shares with other gene therapies is the issue of cost – reported in the range of $2 million–$3 million.
In the context of racial and socioeconomic disparities in access to diagnosis and care reported in DMD, Emma Ciafaloni, MD, a professor of neurology and pediatrics at the University of Rochester (N.Y.) Medical Center, underscored the need to consider approval versus access to gene therapies and how to optimize access to the novel treatments.
“We need to consider what the cost is, how it’s going to be accessed, and whether there is a sustainable model,” said Ciafaloni, who was not associated with the study. “There will need to be institutional readiness and support for specialized multidisciplinary clinics for gene therapy.”
She also noted “we need to consider how we can do better on a broader level, because this is not a provider problem or a manufacturer problem — it’s a society problem.”
The study was funded by Sarepta Therapeutics. McDonald reported consulting work for Sarepta Therapeutics and has been an investigator in SRP-9001 research. Ciafaloni reported serving on advisory boards or other relationships with Alexion, Argenx, Biogen, Amicus, Momenta, Medscape, Pfizer, Sanofi/Genzyme, Sarepta, Jansen, NS Pharma, CureSMA, Orphazyme, the Patient-Centered Outcomes Research Institute, PPMD, PTC Therapeutics, and Santhera.
A version of this article first appeared on Medscape.com.
PHOENIX – compared with untreated patients who showed significant decline over the same time period, new research shows.
“Functional assessments demonstrated long-term sustained stabilization of motor function that was clinically meaningful, at ages where functional decline would be expected based on natural history,” the investigators noted in their abstract. Furthermore, the treatment, known as delandistrogene moxeparvovec-rokl (SRP-9001), was well tolerated 4 years post treatment.
The study was presented at the annual meeting of the American Association of Neuromuscular Electrodiagnostic Medicine.
Severe type of DMD
Considered one of the most severe forms of muscular dystrophy, DMD causes progressive muscle wasting stemming from the root genetic cause of missing dystrophin in muscle cells. Often referred to as a molecular “shock absorber,” dystrophin stabilizes the sarcolemma during muscle contractions to prevent degeneration.
SRP-9001, a single-dose recombinant gene therapy administered as an intravenous infusion, was designed to deliver a trimmed down form of dystrophin to compensate for the deficit.
In July, the adeno-associated virus vector (AAV)–based SRP-9001 gene therapy was granted accelerated approval by the Food and Drug Administration for the treatment of ambulatory pediatric patients aged 4-5 years with DMD with a confirmed mutation in the DMD gene.
The therapy is administered over 1-2 hours at a dose of 133 trillion vector genomes per kilogram of body weight.
For Study 101, one of several evaluating the novel therapy, a research team led by senior investigator Jerry Mendell, MD, an attending neurologist at Nationwide Children’s Hospital and professor of pediatrics and neurology at Ohio State University, both in Columbus,evaluated data on four ambulatory male patients aged 4-8 years who received a single IV infusion of the therapy.
All patients also received prednisone 1 mg/kg, 1 day preinfusion and 30 days post infusion.
At 4 years post treatment, there were no new safety events. All treatment-related adverse events occurred mainly within the first 70 days, and all resolved.
The most commonly reported adverse reactions of the gene therapy include vomiting, nausea, increases in liver enzymes, pyrexia (fever), and thrombocytopenia, all of which occurred within 90 days of infusion and been manageable.
Risk mitigation strategies for hepatotoxicity or acute liver injury include pre- and postinfusion monitoring of liver enzymes, the authors noted.
No serious abnormalities were observed in hematologic or chemistry panels, and while three patients had elevated gamma-glutamyl transpeptidase in the first 3 months post treatment, those cases resolved with oral steroid treatment.
Significant improvements in function were observed, with a mean improvement in North Star Ambulatory Assessment (NSAA) scores from baseline of 7.0 points (range, 4-11).
Exploratory analyses further showed that, compared with a propensity score–weighted external control cohort of 21 patients with DMD who did not receive the therapy, those receiving SRP-9001 had a statistically significant difference of 9.4 points in least-squares mean change from baseline to 4 years on the NSAA score (P = .0125).
Similar trends were observed in improvement from baseline in key measures of time to rise, 4-stair climb, and 10- and 100-meter walk/run function tests.
Other reported adverse events include acute serious liver injury, immune-mediated myositis, and myocarditis. Because of the latter risk, the therapy is contraindicated in patients with any deletion in exon 8 and/or exon 9 in the DMD gene.
The current 4-year update on SRP-9001 adds to clinical trial results that have been reported on more than 80 patients treated to date, with favorable results and consistent safety profiles reported at other time points.
Continued FDA approval for the therapy will be contingent upon verification of a clinical benefit in the confirmatory trials, including the EMBARK trial.
Increased function, long-term stability
Discussing the research at the meeting, Craig McDonald, MD, professor and chair of physical medicine & rehabilitation, a professor of pediatrics and study chair of the CINRG Duchenne Natural History Study at University of California Davis Health, noted that top-line results from the ongoing, confirmatory phase 3 EMBARK trial show functional benefits of SRP-9001 not only in 4- to 5-year-olds but also in other older age groups.
“What’s really striking, and in my mind the most impressive, is that when you follow these patients out 3 or 4 years ... you see there is this bump in function followed by long-term stability, whereas the external control cohort predictably shows really quite significant declines in their [NSAA] functional values,” he said in his presentation.
“When you look at each individually treated patient versus their own predicted trajectory using their baseline values on the time function test, each of the patients actually has a really quite impressive stabilization of function over their predicted disease trajectory,” he added.
A caveat that SRP-9001 shares with other gene therapies is the issue of cost – reported in the range of $2 million–$3 million.
In the context of racial and socioeconomic disparities in access to diagnosis and care reported in DMD, Emma Ciafaloni, MD, a professor of neurology and pediatrics at the University of Rochester (N.Y.) Medical Center, underscored the need to consider approval versus access to gene therapies and how to optimize access to the novel treatments.
“We need to consider what the cost is, how it’s going to be accessed, and whether there is a sustainable model,” said Ciafaloni, who was not associated with the study. “There will need to be institutional readiness and support for specialized multidisciplinary clinics for gene therapy.”
She also noted “we need to consider how we can do better on a broader level, because this is not a provider problem or a manufacturer problem — it’s a society problem.”
The study was funded by Sarepta Therapeutics. McDonald reported consulting work for Sarepta Therapeutics and has been an investigator in SRP-9001 research. Ciafaloni reported serving on advisory boards or other relationships with Alexion, Argenx, Biogen, Amicus, Momenta, Medscape, Pfizer, Sanofi/Genzyme, Sarepta, Jansen, NS Pharma, CureSMA, Orphazyme, the Patient-Centered Outcomes Research Institute, PPMD, PTC Therapeutics, and Santhera.
A version of this article first appeared on Medscape.com.
PHOENIX – compared with untreated patients who showed significant decline over the same time period, new research shows.
“Functional assessments demonstrated long-term sustained stabilization of motor function that was clinically meaningful, at ages where functional decline would be expected based on natural history,” the investigators noted in their abstract. Furthermore, the treatment, known as delandistrogene moxeparvovec-rokl (SRP-9001), was well tolerated 4 years post treatment.
The study was presented at the annual meeting of the American Association of Neuromuscular Electrodiagnostic Medicine.
Severe type of DMD
Considered one of the most severe forms of muscular dystrophy, DMD causes progressive muscle wasting stemming from the root genetic cause of missing dystrophin in muscle cells. Often referred to as a molecular “shock absorber,” dystrophin stabilizes the sarcolemma during muscle contractions to prevent degeneration.
SRP-9001, a single-dose recombinant gene therapy administered as an intravenous infusion, was designed to deliver a trimmed down form of dystrophin to compensate for the deficit.
In July, the adeno-associated virus vector (AAV)–based SRP-9001 gene therapy was granted accelerated approval by the Food and Drug Administration for the treatment of ambulatory pediatric patients aged 4-5 years with DMD with a confirmed mutation in the DMD gene.
The therapy is administered over 1-2 hours at a dose of 133 trillion vector genomes per kilogram of body weight.
For Study 101, one of several evaluating the novel therapy, a research team led by senior investigator Jerry Mendell, MD, an attending neurologist at Nationwide Children’s Hospital and professor of pediatrics and neurology at Ohio State University, both in Columbus,evaluated data on four ambulatory male patients aged 4-8 years who received a single IV infusion of the therapy.
All patients also received prednisone 1 mg/kg, 1 day preinfusion and 30 days post infusion.
At 4 years post treatment, there were no new safety events. All treatment-related adverse events occurred mainly within the first 70 days, and all resolved.
The most commonly reported adverse reactions of the gene therapy include vomiting, nausea, increases in liver enzymes, pyrexia (fever), and thrombocytopenia, all of which occurred within 90 days of infusion and been manageable.
Risk mitigation strategies for hepatotoxicity or acute liver injury include pre- and postinfusion monitoring of liver enzymes, the authors noted.
No serious abnormalities were observed in hematologic or chemistry panels, and while three patients had elevated gamma-glutamyl transpeptidase in the first 3 months post treatment, those cases resolved with oral steroid treatment.
Significant improvements in function were observed, with a mean improvement in North Star Ambulatory Assessment (NSAA) scores from baseline of 7.0 points (range, 4-11).
Exploratory analyses further showed that, compared with a propensity score–weighted external control cohort of 21 patients with DMD who did not receive the therapy, those receiving SRP-9001 had a statistically significant difference of 9.4 points in least-squares mean change from baseline to 4 years on the NSAA score (P = .0125).
Similar trends were observed in improvement from baseline in key measures of time to rise, 4-stair climb, and 10- and 100-meter walk/run function tests.
Other reported adverse events include acute serious liver injury, immune-mediated myositis, and myocarditis. Because of the latter risk, the therapy is contraindicated in patients with any deletion in exon 8 and/or exon 9 in the DMD gene.
The current 4-year update on SRP-9001 adds to clinical trial results that have been reported on more than 80 patients treated to date, with favorable results and consistent safety profiles reported at other time points.
Continued FDA approval for the therapy will be contingent upon verification of a clinical benefit in the confirmatory trials, including the EMBARK trial.
Increased function, long-term stability
Discussing the research at the meeting, Craig McDonald, MD, professor and chair of physical medicine & rehabilitation, a professor of pediatrics and study chair of the CINRG Duchenne Natural History Study at University of California Davis Health, noted that top-line results from the ongoing, confirmatory phase 3 EMBARK trial show functional benefits of SRP-9001 not only in 4- to 5-year-olds but also in other older age groups.
“What’s really striking, and in my mind the most impressive, is that when you follow these patients out 3 or 4 years ... you see there is this bump in function followed by long-term stability, whereas the external control cohort predictably shows really quite significant declines in their [NSAA] functional values,” he said in his presentation.
“When you look at each individually treated patient versus their own predicted trajectory using their baseline values on the time function test, each of the patients actually has a really quite impressive stabilization of function over their predicted disease trajectory,” he added.
A caveat that SRP-9001 shares with other gene therapies is the issue of cost – reported in the range of $2 million–$3 million.
In the context of racial and socioeconomic disparities in access to diagnosis and care reported in DMD, Emma Ciafaloni, MD, a professor of neurology and pediatrics at the University of Rochester (N.Y.) Medical Center, underscored the need to consider approval versus access to gene therapies and how to optimize access to the novel treatments.
“We need to consider what the cost is, how it’s going to be accessed, and whether there is a sustainable model,” said Ciafaloni, who was not associated with the study. “There will need to be institutional readiness and support for specialized multidisciplinary clinics for gene therapy.”
She also noted “we need to consider how we can do better on a broader level, because this is not a provider problem or a manufacturer problem — it’s a society problem.”
The study was funded by Sarepta Therapeutics. McDonald reported consulting work for Sarepta Therapeutics and has been an investigator in SRP-9001 research. Ciafaloni reported serving on advisory boards or other relationships with Alexion, Argenx, Biogen, Amicus, Momenta, Medscape, Pfizer, Sanofi/Genzyme, Sarepta, Jansen, NS Pharma, CureSMA, Orphazyme, the Patient-Centered Outcomes Research Institute, PPMD, PTC Therapeutics, and Santhera.
A version of this article first appeared on Medscape.com.
AT AANEM 2023