User login
AF tied to 45% increase in mild cognitive impairment
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- From over 4.3 million people in the UK primary electronic health record (EHR) database, researchers identified 233,833 (5.4%) with AF (mean age, 74.2 years) and randomly selected one age- and sex-matched control person without AF for each AF case patient.
- The primary outcome was incidence of mild cognitive impairment (MCI).
- The authors adjusted for age, sex, year at study entry, socioeconomic status, smoking, and a number of comorbid conditions.
- During a median of 5.3 years of follow-up, there were 4,269 incident MCI cases among both AF and non-AF patients.
TAKEAWAY:
- Individuals with AF had a higher risk of MCI than that of those without AF (adjusted hazard ratio [aHR], 1.45; 95% confidence interval [CI], 1.35-1.56).
- Besides AF, older age (risk ratio [RR], 1.08) and history of depression (RR, 1.44) were associated with greater risk of MCI, as were female sex, greater socioeconomic deprivation, stroke, and multimorbidity, including, for example, diabetes, hypercholesterolemia, and peripheral artery disease (all P < .001).
- Individuals with AF who received oral anticoagulants or amiodarone were not at increased risk of MCI, as was the case for those treated with digoxin.
- Individuals with AF and MCI were at greater risk of dementia (aHR, 1.25; 95% CI, 1.09-1.42). Sex, smoking, chronic kidney disease, and multi-comorbidity were among factors linked to elevated dementia risk.
IN PRACTICE:
The findings emphasize the association of multi-comorbidity and cardiovascular risk factors with development of MCI and progression to dementia in AF patients, the authors wrote. They noted that the data suggest combining anticoagulation and symptom and comorbidity management may prevent cognitive deterioration.
SOURCE:
The study was conducted by Sheng-Chia Chung, PhD, Institute of Health informatics Research, University College London, and colleagues. It was published online Oct. 25, 2023, as a research letter in the Journal of the American College of Cardiology (JACC): Advances.
LIMITATIONS:
The EHR dataset may have lacked granularity and detail, and some risk factors or comorbidities may not have been measured. While those with AF receiving digoxin or amiodarone treatment had no higher risk of MCI than their non-AF peers, the study’s observational design and very wide confidence intervals for these subgroups prevent making solid inferences about causality or a potential protective role of these drugs.
DISCLOSURES:
Dr. Chung is supported by the National Institute of Health and Care Research (NIHR) Author Rui Providencia, MD, PhD, of the Institute of Health informatics Research, University College London, is supported by the University College London British Heart Foundation and NIHR. All other authors report no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- From over 4.3 million people in the UK primary electronic health record (EHR) database, researchers identified 233,833 (5.4%) with AF (mean age, 74.2 years) and randomly selected one age- and sex-matched control person without AF for each AF case patient.
- The primary outcome was incidence of mild cognitive impairment (MCI).
- The authors adjusted for age, sex, year at study entry, socioeconomic status, smoking, and a number of comorbid conditions.
- During a median of 5.3 years of follow-up, there were 4,269 incident MCI cases among both AF and non-AF patients.
TAKEAWAY:
- Individuals with AF had a higher risk of MCI than that of those without AF (adjusted hazard ratio [aHR], 1.45; 95% confidence interval [CI], 1.35-1.56).
- Besides AF, older age (risk ratio [RR], 1.08) and history of depression (RR, 1.44) were associated with greater risk of MCI, as were female sex, greater socioeconomic deprivation, stroke, and multimorbidity, including, for example, diabetes, hypercholesterolemia, and peripheral artery disease (all P < .001).
- Individuals with AF who received oral anticoagulants or amiodarone were not at increased risk of MCI, as was the case for those treated with digoxin.
- Individuals with AF and MCI were at greater risk of dementia (aHR, 1.25; 95% CI, 1.09-1.42). Sex, smoking, chronic kidney disease, and multi-comorbidity were among factors linked to elevated dementia risk.
IN PRACTICE:
The findings emphasize the association of multi-comorbidity and cardiovascular risk factors with development of MCI and progression to dementia in AF patients, the authors wrote. They noted that the data suggest combining anticoagulation and symptom and comorbidity management may prevent cognitive deterioration.
SOURCE:
The study was conducted by Sheng-Chia Chung, PhD, Institute of Health informatics Research, University College London, and colleagues. It was published online Oct. 25, 2023, as a research letter in the Journal of the American College of Cardiology (JACC): Advances.
LIMITATIONS:
The EHR dataset may have lacked granularity and detail, and some risk factors or comorbidities may not have been measured. While those with AF receiving digoxin or amiodarone treatment had no higher risk of MCI than their non-AF peers, the study’s observational design and very wide confidence intervals for these subgroups prevent making solid inferences about causality or a potential protective role of these drugs.
DISCLOSURES:
Dr. Chung is supported by the National Institute of Health and Care Research (NIHR) Author Rui Providencia, MD, PhD, of the Institute of Health informatics Research, University College London, is supported by the University College London British Heart Foundation and NIHR. All other authors report no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- From over 4.3 million people in the UK primary electronic health record (EHR) database, researchers identified 233,833 (5.4%) with AF (mean age, 74.2 years) and randomly selected one age- and sex-matched control person without AF for each AF case patient.
- The primary outcome was incidence of mild cognitive impairment (MCI).
- The authors adjusted for age, sex, year at study entry, socioeconomic status, smoking, and a number of comorbid conditions.
- During a median of 5.3 years of follow-up, there were 4,269 incident MCI cases among both AF and non-AF patients.
TAKEAWAY:
- Individuals with AF had a higher risk of MCI than that of those without AF (adjusted hazard ratio [aHR], 1.45; 95% confidence interval [CI], 1.35-1.56).
- Besides AF, older age (risk ratio [RR], 1.08) and history of depression (RR, 1.44) were associated with greater risk of MCI, as were female sex, greater socioeconomic deprivation, stroke, and multimorbidity, including, for example, diabetes, hypercholesterolemia, and peripheral artery disease (all P < .001).
- Individuals with AF who received oral anticoagulants or amiodarone were not at increased risk of MCI, as was the case for those treated with digoxin.
- Individuals with AF and MCI were at greater risk of dementia (aHR, 1.25; 95% CI, 1.09-1.42). Sex, smoking, chronic kidney disease, and multi-comorbidity were among factors linked to elevated dementia risk.
IN PRACTICE:
The findings emphasize the association of multi-comorbidity and cardiovascular risk factors with development of MCI and progression to dementia in AF patients, the authors wrote. They noted that the data suggest combining anticoagulation and symptom and comorbidity management may prevent cognitive deterioration.
SOURCE:
The study was conducted by Sheng-Chia Chung, PhD, Institute of Health informatics Research, University College London, and colleagues. It was published online Oct. 25, 2023, as a research letter in the Journal of the American College of Cardiology (JACC): Advances.
LIMITATIONS:
The EHR dataset may have lacked granularity and detail, and some risk factors or comorbidities may not have been measured. While those with AF receiving digoxin or amiodarone treatment had no higher risk of MCI than their non-AF peers, the study’s observational design and very wide confidence intervals for these subgroups prevent making solid inferences about causality or a potential protective role of these drugs.
DISCLOSURES:
Dr. Chung is supported by the National Institute of Health and Care Research (NIHR) Author Rui Providencia, MD, PhD, of the Institute of Health informatics Research, University College London, is supported by the University College London British Heart Foundation and NIHR. All other authors report no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Nightmare on CIL Street: A Simulation Series to Increase Confidence and Skill in Responding to Clinical Emergencies
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
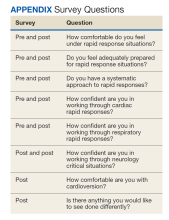
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
More evidence metformin may be neuroprotective
TOPLINE:
New research suggests terminating metformin may raise the risk for dementia in older adults with type 2 diabetes, providing more evidence of metformin’s potential neuroprotective effects.
METHODOLOGY:
- Researchers evaluated the association between discontinuing metformin for reasons unrelated to kidney dysfunction and dementia incidence.
- The cohort included 12,220 Kaiser Permanente Northern California members who stopped metformin early (with normal kidney function) and 29,126 routine metformin users.
- The cohort of early terminators was 46% women with an average age of 59 years at the start of metformin prescription. The cohort continuing metformin was 47% women, with a start age of 61 years.
TAKEAWAY:
- Adults who stopped metformin early were 21% more likely to be diagnosed with dementia during follow up (hazard ratio, 1.21; 95% confidence interval, 1.12-1.30), compared with routine metformin users.
- This association was largely independent of changes in A1c level and insulin usage.
IN PRACTICE:
The findings “corroborate the largely consistent evidence from other observational studies showing an association between metformin use and lower dementia incidence [and] may have important implications for clinical treatment of adults with diabetes,” the authors write.
SOURCE:
The study, with first author Scott Zimmerman, MPH, University of California, San Francisco, was published online in JAMA Network Open.
LIMITATIONS:
Dementia diagnosis was obtained based on medical records. Factors such as race, ethnicity, or time on metformin were not evaluated. Information on the exact reason for stopping metformin was not available.
DISCLOSURES:
The study was funded by grants from the National Institutes of Health, National Institute on Aging. Mr. Zimmerman owns stock in AbbVie, Gilead Sciences, CRISPR Therapeutics, and Abbott Laboratories. Disclosure for the other study authors can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
New research suggests terminating metformin may raise the risk for dementia in older adults with type 2 diabetes, providing more evidence of metformin’s potential neuroprotective effects.
METHODOLOGY:
- Researchers evaluated the association between discontinuing metformin for reasons unrelated to kidney dysfunction and dementia incidence.
- The cohort included 12,220 Kaiser Permanente Northern California members who stopped metformin early (with normal kidney function) and 29,126 routine metformin users.
- The cohort of early terminators was 46% women with an average age of 59 years at the start of metformin prescription. The cohort continuing metformin was 47% women, with a start age of 61 years.
TAKEAWAY:
- Adults who stopped metformin early were 21% more likely to be diagnosed with dementia during follow up (hazard ratio, 1.21; 95% confidence interval, 1.12-1.30), compared with routine metformin users.
- This association was largely independent of changes in A1c level and insulin usage.
IN PRACTICE:
The findings “corroborate the largely consistent evidence from other observational studies showing an association between metformin use and lower dementia incidence [and] may have important implications for clinical treatment of adults with diabetes,” the authors write.
SOURCE:
The study, with first author Scott Zimmerman, MPH, University of California, San Francisco, was published online in JAMA Network Open.
LIMITATIONS:
Dementia diagnosis was obtained based on medical records. Factors such as race, ethnicity, or time on metformin were not evaluated. Information on the exact reason for stopping metformin was not available.
DISCLOSURES:
The study was funded by grants from the National Institutes of Health, National Institute on Aging. Mr. Zimmerman owns stock in AbbVie, Gilead Sciences, CRISPR Therapeutics, and Abbott Laboratories. Disclosure for the other study authors can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
New research suggests terminating metformin may raise the risk for dementia in older adults with type 2 diabetes, providing more evidence of metformin’s potential neuroprotective effects.
METHODOLOGY:
- Researchers evaluated the association between discontinuing metformin for reasons unrelated to kidney dysfunction and dementia incidence.
- The cohort included 12,220 Kaiser Permanente Northern California members who stopped metformin early (with normal kidney function) and 29,126 routine metformin users.
- The cohort of early terminators was 46% women with an average age of 59 years at the start of metformin prescription. The cohort continuing metformin was 47% women, with a start age of 61 years.
TAKEAWAY:
- Adults who stopped metformin early were 21% more likely to be diagnosed with dementia during follow up (hazard ratio, 1.21; 95% confidence interval, 1.12-1.30), compared with routine metformin users.
- This association was largely independent of changes in A1c level and insulin usage.
IN PRACTICE:
The findings “corroborate the largely consistent evidence from other observational studies showing an association between metformin use and lower dementia incidence [and] may have important implications for clinical treatment of adults with diabetes,” the authors write.
SOURCE:
The study, with first author Scott Zimmerman, MPH, University of California, San Francisco, was published online in JAMA Network Open.
LIMITATIONS:
Dementia diagnosis was obtained based on medical records. Factors such as race, ethnicity, or time on metformin were not evaluated. Information on the exact reason for stopping metformin was not available.
DISCLOSURES:
The study was funded by grants from the National Institutes of Health, National Institute on Aging. Mr. Zimmerman owns stock in AbbVie, Gilead Sciences, CRISPR Therapeutics, and Abbott Laboratories. Disclosure for the other study authors can be found with the original article.
A version of this article first appeared on Medscape.com.
Brain structural and cognitive changes during pregnancy
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
Pregnancy is unquestionably a major milestone in a woman’s life. During gestation, her body shape noticeably changes, but the invisible structural and cognitive changes in her brain are more striking. Some of those neurobiological changes are short-term, while others are long-lasting, well beyond delivery, and even into old age.
Physiological changes during pregnancy are extraordinary. The dramatic increases in estrogen, progesterone, and glucocorticoids help maintain pregnancy, ensure safe delivery of the baby, and trigger maternal behavior. However, other important changes also occur in the mother’s cardiac output, blood volume, renal function, respiratory output, and immune adaptations to accommodate the growth of the fetus. Gene expression also occurs to accomplish those changes, and there are lifelong repercussions from those drastic physiological changes.
During pregnancy, the brain is exposed to escalating levels of hormones released from the placenta, which the woman had never experienced. Those hormones regulate neuroplasticity, neuroinflammation, behavior, and cognition.
Structural brain changes1-6
Brain volume declines during pregnancy, reaching a nadir at the time of parturition. However, recovery occurs within 5 months after delivery. During the postpartum period, gray matter volume increases in the first 3 to 4 weeks, especially in areas involved in maternal behavior, including the amygdala, prefrontal cortex, and hypothalamus. Hippocampal gray matter decreases at 2 months postpartum compared to preconception levels, and reductions can still be observed up to 2 years following delivery. Gray matter reductions occur in multiple brain regions involved in social cognition, including the superior temporal gyrus, medial and inferior frontal cortex, fusiform areas, and hippocampus. Those changes correlate with positive maternal attachment. It is noteworthy that neural activity is highest in areas with reduced gray volume, so a decline in brain volume is associated with enhanced maternal attachment. Interestingly, those changes occur in fathers, too.
Childbearing improves stroke outcomes in middle age, but body weight will increase. The risk of Alzheimer’s disease increases with a higher number of gestations, but longevity is higher if the pregnancy occurs at an older age. Reproduction is also associated with shorter telomeres, which can elevate the risk of cancer, inflammation, diabetes, and dementia.
Cognitive changes7-10
The term “pregnancy brain” refers to cognitive changes during pregnancy and postpartum; these include decreased memory and concentration, absent-mindedness, heightened reactivity to threatening stimuli, and a decrease in motivation and executive functions. After delivery a mother has increased empathy (sometimes referred to as Theory of Mind) and greater activation in brain structures involved in empathy, including the paracingulate cortex, the posterior cingulate, and the insula. Also, the mirror neuron system becomes more activated in response to a woman’s own children compared to unfamiliar children. This incudes the ventral premotor cortex, the inferior frontal gyrus, and the posterior parietal cortex.
Certain forms of memory are impaired during pregnancy and early postpartum, including verbal free recall and working memory, as well as executive functions. Those are believed to correlate with glucocorticoids and estrogen levels.
Continue to: The following cognitive functions...
The following cognitive functions increase between the first and second trimester: verbal memory, attention, executive functions processing speed, verbal, and visuospatial abilities. Interestingly, mothers of a male fetus outperformed mothers of a female fetus on working memory and spatial ability.
Other changes11-16
- Cells from the fetus can traffic to the mother’s body and create microchimeric cells, which have short-term benefits (healing some of the other’s organs as stem cells do) but long-term downsides include future brain disorders such as Parkinson’s disease or Alzheimer’s disease, as well as autoimmune diseases and various types of cancer. The reverse also occurs with cells transferring from the mother to the fetus, persisting into infancy and childhood.
- Postpartum psychosis is associated with reductions in the volumes of the anterior cingulate, left parahippocampal gyrus, and superior temporal gyrus.
- A woman’s white matter increases during pregnancy compared to preconception. This is attributed to the high levels of prolactin, which proliferates oligodendrocytes, the glial cells that continuously manufacture myelin.
- The pituitary gland increases by 200% to 300% during pregnancy and returns to pre-pregnancy levels approximately 8 months following delivery. Prolactin also mediates the production of brain cells in the hippocampus (ie, neurogenesis).
- Sexual activity, even without pregnancy, increases neurogenesis. Plasma levels of prolactin increase significantly following an orgasm in both men and women, which indicates that sexual activity has beneficial brain effects.
- With pregnancy, the immune system shifts from proinflammatory to anti-inflammatory signaling. This protects the fetus from being attacked and rejected as foreign tissue. However, at the end of pregnancy, there is a “burst” of proinflammatory signaling, which serves as a major trigger to induce uterine contractions and initiate labor (to expel the foreign tissue).
- Brain levels of the anti-inflammatory cytokine interleukin-6 increase in the postpartum period, which represents a significant modification in the neuroimmune environment, and the maternal brain assumes an inflammatory-resistant state, which has cognitive and neuroplasticity implications. However, this neuroimmune dysregulation is implicated in postpartum depression and anxiety.
- Older females who were never pregnant or only had 1 pregnancy had better overall cognitive functioning than females who became pregnant at an young age.
- In animal studies, reproduction alleviates the negative effects of aging on several hippocampal functions, especially neurogenesis. Dendritic spine density in the CA1 region of the hippocampus is higher in pregnancy and early postpartum period compared to nulliparous females (based on animal studies).
Pregnancy is indispensable for the perpetuation of the species. Its hormonal, physiologic, neurobiological, and cognitive correlates are extensive. The cognitive changes in the postpartum period are designed by evolution to prepare a woman to care for her newborn and to ensure its survival. But the biological sequelae of pregnancy extend to the rest of a woman’s life and may predispose her to immune and brain disorders as she ages.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
1. Barba-Müller E, Craddock S, Carmona S, et al. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch Womens Ment Health. 2019;22(2):289-299.
2. Pawluski JL, Hoekzema E, Leuner B, et al. Less can be more: fine tuning the maternal brain. Neurosci Biobehav Rev. 2022;133:104475. doi:10.1016/j.neubiorev.2021.11.045
3. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
4. Cárdenas EF, Kujawa A, Humphreys KL. Neurobiological changes during the peripartum period: implications for health and behavior. Soc Cogn Affect Neurosci. 2020;15(10):1097-1110.
5. Eid RS, Chaiton JA, Lieblich SE, et al. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol Aging. 2019;78:1-17.
6. Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26(10):641-648.
7. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126(1):73-85.
8. Barda G, Mizrachi Y, Borokchovich I, et al. The effect of pregnancy on maternal cognition. Sci Rep. 2011;11(1)12187. doi:10.1038/s41598-021-91504-9
9. Davies SJ, Lum JA, Skouteris H, et al. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 2018;208(1):35-40.
10. Pownall M, Hutter RRC, Rockliffe L, et al. Memory and mood changes in pregnancy: a qualitative content analysis of women’s first-hand accounts. J Reprod Infant Psychol. 2023;41(5):516-527.
11. Hoekzema E, Barba-Müller E, Pozzobon C, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20(2):287-296.
12. Duarte-Guterman P, Leuner B, Galea LAM. The long and short term effects of motherhood on the brain. Front Neuroendocrinol. 2019;53:100740. doi:10.1016/j.yfrne.2019.02.004
13. Haim A, Julian D, Albin-Brooks C, et al. A survey of neuroimmune changes in pregnant and postpartum female rats. Brain Behav Immun. 2017;59:67-78.
14. Benson JC, Malyuk DF, Madhavan A, et al. Pituitary volume changes in pregnancy and the post-partum period. Neuroradiol J. 2023. doi:10.1177/19714009231196470
15. Schepanski S, Chini M, Sternemann V, et al. Pregnancy-induced maternal microchimerism shapes neurodevelopment and behavior in mice. Nat Commun. 2022;13(1):4571. doi:10.1038/s41467-022-32230-2
16. Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26(2):201-209.
Higher triglycerides linked to lower dementia risk
TOPLINE:
a large study of community-dwelling older adults suggests.
METHODOLOGY:
- The analysis included 18,294 participants, median age 75 years and median triglyceride level 106 mg/dL, from the Aspirin in Reducing Events in the Elderly (ASPREE) study, a placebo-controlled, randomized trial of daily low-dose aspirin in older people without dementia or history of cardiovascular disease (CVD) at recruitment.
- Researchers repeated their main analyses in a sub-cohort of 13,976 subjects with APOE epsilon-4 genetic data, and an external cohort of 68,200 participants, mean age 66.9 years and a median nonfasting triglyceride of 139 mg/dL, from the UK biobank, followed for a median of 12.5 years.
- The main outcome was incident dementia over 6.4 years and secondary outcomes included changes in composite cognitive function and domain-specific cognition.
- Researchers controlled for a number of potential confounders, including age, sex, race, smoking, alcohol consumption, education, family history of dementia, diabetes, hypertension, and statin use.
TAKEAWAY:
- Every doubling of baseline triglycerides was associated with an 18% lower risk of incident dementia across the entire study cohort (adjusted hazard ratio, 0.82) and in participants with genotypic data (aHR, 0.82) and a 17% lower risk in the external UK Biobank cohort (aHR, 0.83) (P ≤ .01 for all).
- In the entire cohort, the risk for dementia was 15% lower in those with triglyceride levels at 63-106 mg/dL (aHR, 0.85); 24% lower in those at 107-186 mg/dL (aHR, 0.76); and 36% lower for those with levels higher than 187 mg/dL (aHR, 0.64), compared with individuals with levels below 62 mg/dL (P for trend <.001).
- The direction and magnitude of the inverse association between triglycerides and dementia risk were not modified by age, sex, or risk factors related to triglycerides or dementia.
- In the entire study cohort, higher triglyceride levels were significantly associated with slower decline in global cognition (P = .02), composite cognition (P = .03), and a borderline significantly slower decline in episodic memory (P = .05).
IN PRACTICE:
“Triglyceride levels may serve as a useful predictor for dementia risk and cognitive decline in older populations,” the investigators write. Higher triglyceride levels may reflect better overall health and/or lifestyle behaviors that protect against dementia.
SOURCE:
The study was led by Zhen Zhou, of Monash University, Melbourne. It was published online in Neurology.
LIMITATIONS:
The study can’t establish a causal relationship between triglyceride levels and dementia or fully exclude reverse causality. As most ASPREE participants had normal to high-normal triglyceride levels, the results can’t be generalized to those with severe hypertriglyceridemia. The findings are unique to older people without CVD and may not be generalizable to other populations.
DISCLOSURES:
The study received support from the Royal Australian College of General Practitioners (RACGP)/HCF Research Foundation. Dr. Zhou reported receiving salary from the RACGP/HCF Research Foundation.
A version of this article first appeared on Medscape.com.
TOPLINE:
a large study of community-dwelling older adults suggests.
METHODOLOGY:
- The analysis included 18,294 participants, median age 75 years and median triglyceride level 106 mg/dL, from the Aspirin in Reducing Events in the Elderly (ASPREE) study, a placebo-controlled, randomized trial of daily low-dose aspirin in older people without dementia or history of cardiovascular disease (CVD) at recruitment.
- Researchers repeated their main analyses in a sub-cohort of 13,976 subjects with APOE epsilon-4 genetic data, and an external cohort of 68,200 participants, mean age 66.9 years and a median nonfasting triglyceride of 139 mg/dL, from the UK biobank, followed for a median of 12.5 years.
- The main outcome was incident dementia over 6.4 years and secondary outcomes included changes in composite cognitive function and domain-specific cognition.
- Researchers controlled for a number of potential confounders, including age, sex, race, smoking, alcohol consumption, education, family history of dementia, diabetes, hypertension, and statin use.
TAKEAWAY:
- Every doubling of baseline triglycerides was associated with an 18% lower risk of incident dementia across the entire study cohort (adjusted hazard ratio, 0.82) and in participants with genotypic data (aHR, 0.82) and a 17% lower risk in the external UK Biobank cohort (aHR, 0.83) (P ≤ .01 for all).
- In the entire cohort, the risk for dementia was 15% lower in those with triglyceride levels at 63-106 mg/dL (aHR, 0.85); 24% lower in those at 107-186 mg/dL (aHR, 0.76); and 36% lower for those with levels higher than 187 mg/dL (aHR, 0.64), compared with individuals with levels below 62 mg/dL (P for trend <.001).
- The direction and magnitude of the inverse association between triglycerides and dementia risk were not modified by age, sex, or risk factors related to triglycerides or dementia.
- In the entire study cohort, higher triglyceride levels were significantly associated with slower decline in global cognition (P = .02), composite cognition (P = .03), and a borderline significantly slower decline in episodic memory (P = .05).
IN PRACTICE:
“Triglyceride levels may serve as a useful predictor for dementia risk and cognitive decline in older populations,” the investigators write. Higher triglyceride levels may reflect better overall health and/or lifestyle behaviors that protect against dementia.
SOURCE:
The study was led by Zhen Zhou, of Monash University, Melbourne. It was published online in Neurology.
LIMITATIONS:
The study can’t establish a causal relationship between triglyceride levels and dementia or fully exclude reverse causality. As most ASPREE participants had normal to high-normal triglyceride levels, the results can’t be generalized to those with severe hypertriglyceridemia. The findings are unique to older people without CVD and may not be generalizable to other populations.
DISCLOSURES:
The study received support from the Royal Australian College of General Practitioners (RACGP)/HCF Research Foundation. Dr. Zhou reported receiving salary from the RACGP/HCF Research Foundation.
A version of this article first appeared on Medscape.com.
TOPLINE:
a large study of community-dwelling older adults suggests.
METHODOLOGY:
- The analysis included 18,294 participants, median age 75 years and median triglyceride level 106 mg/dL, from the Aspirin in Reducing Events in the Elderly (ASPREE) study, a placebo-controlled, randomized trial of daily low-dose aspirin in older people without dementia or history of cardiovascular disease (CVD) at recruitment.
- Researchers repeated their main analyses in a sub-cohort of 13,976 subjects with APOE epsilon-4 genetic data, and an external cohort of 68,200 participants, mean age 66.9 years and a median nonfasting triglyceride of 139 mg/dL, from the UK biobank, followed for a median of 12.5 years.
- The main outcome was incident dementia over 6.4 years and secondary outcomes included changes in composite cognitive function and domain-specific cognition.
- Researchers controlled for a number of potential confounders, including age, sex, race, smoking, alcohol consumption, education, family history of dementia, diabetes, hypertension, and statin use.
TAKEAWAY:
- Every doubling of baseline triglycerides was associated with an 18% lower risk of incident dementia across the entire study cohort (adjusted hazard ratio, 0.82) and in participants with genotypic data (aHR, 0.82) and a 17% lower risk in the external UK Biobank cohort (aHR, 0.83) (P ≤ .01 for all).
- In the entire cohort, the risk for dementia was 15% lower in those with triglyceride levels at 63-106 mg/dL (aHR, 0.85); 24% lower in those at 107-186 mg/dL (aHR, 0.76); and 36% lower for those with levels higher than 187 mg/dL (aHR, 0.64), compared with individuals with levels below 62 mg/dL (P for trend <.001).
- The direction and magnitude of the inverse association between triglycerides and dementia risk were not modified by age, sex, or risk factors related to triglycerides or dementia.
- In the entire study cohort, higher triglyceride levels were significantly associated with slower decline in global cognition (P = .02), composite cognition (P = .03), and a borderline significantly slower decline in episodic memory (P = .05).
IN PRACTICE:
“Triglyceride levels may serve as a useful predictor for dementia risk and cognitive decline in older populations,” the investigators write. Higher triglyceride levels may reflect better overall health and/or lifestyle behaviors that protect against dementia.
SOURCE:
The study was led by Zhen Zhou, of Monash University, Melbourne. It was published online in Neurology.
LIMITATIONS:
The study can’t establish a causal relationship between triglyceride levels and dementia or fully exclude reverse causality. As most ASPREE participants had normal to high-normal triglyceride levels, the results can’t be generalized to those with severe hypertriglyceridemia. The findings are unique to older people without CVD and may not be generalizable to other populations.
DISCLOSURES:
The study received support from the Royal Australian College of General Practitioners (RACGP)/HCF Research Foundation. Dr. Zhou reported receiving salary from the RACGP/HCF Research Foundation.
A version of this article first appeared on Medscape.com.
Urgent need to improve early detection of mild cognitive impairment in primary care
TOPLINE:
Detection rates of mild cognitive impairment (MCI) in primary care are extremely low, with only about 8% of expected cases diagnosed on average, a finding that points to an urgent need to improve early detection in primary care.
METHODOLOGY:
- Researchers estimated MCI detection rates among 226,756 primary care clinicians and 54,597 practices that had at least 25 patients enrolled in Medicare between 2017 and 2019.
- They compared the expected number of MCI cases, based on a predictive model, to actual diagnosed cases as documented in claims and encounter data.
- They accounted for uncertainty in these estimates to determine whether detection rates are within the expected range or significantly higher or lower.
TAKEAWAY:
- More than 25% of clinicians and practices did not have a single patient with diagnosed MCI; the average detection rate was 0.01 for both clinicians and practices.
- The modeled expected MCI detection rate, however, was much higher (average 0.19 for clinicians and 0.20 for practices).
- Average detection rates for clinicians and practices was 0.08, with more than 99% of clinicians and practices underdiagnosing MCI; clinicians practicing geriatric medicine had higher detection rates than others.
IN PRACTICE:
The findings are “concerning not only because patients might not get identified for a disease-modifying AD treatment in time, but also because numerous causes of MCI – such as hypothyroidism and medication side effects – are reversible, and the condition itself can be stabilized by lifestyle modification interventions,” the authors write.
SOURCE:
The study was published online in the Journal of Prevention of Alzheimer’s Disease. The first author was Ying Liu, PhD, of the University of Southern California, Los Angeles.
LIMITATIONS:
The predictive model based on demographic information has only moderate accuracy. Expected prevalence of MCI was based on cognitive test scores, which is not the same as a true clinical diagnosis.
DISCLOSURES:
The study was partially funded by a contract from Genentech to the University of Southern California. Coauthors Soeren Mattke and Christopher Wallick have disclosed relationships with Genentech.
A version of this article appeared on Medscape.com.
TOPLINE:
Detection rates of mild cognitive impairment (MCI) in primary care are extremely low, with only about 8% of expected cases diagnosed on average, a finding that points to an urgent need to improve early detection in primary care.
METHODOLOGY:
- Researchers estimated MCI detection rates among 226,756 primary care clinicians and 54,597 practices that had at least 25 patients enrolled in Medicare between 2017 and 2019.
- They compared the expected number of MCI cases, based on a predictive model, to actual diagnosed cases as documented in claims and encounter data.
- They accounted for uncertainty in these estimates to determine whether detection rates are within the expected range or significantly higher or lower.
TAKEAWAY:
- More than 25% of clinicians and practices did not have a single patient with diagnosed MCI; the average detection rate was 0.01 for both clinicians and practices.
- The modeled expected MCI detection rate, however, was much higher (average 0.19 for clinicians and 0.20 for practices).
- Average detection rates for clinicians and practices was 0.08, with more than 99% of clinicians and practices underdiagnosing MCI; clinicians practicing geriatric medicine had higher detection rates than others.
IN PRACTICE:
The findings are “concerning not only because patients might not get identified for a disease-modifying AD treatment in time, but also because numerous causes of MCI – such as hypothyroidism and medication side effects – are reversible, and the condition itself can be stabilized by lifestyle modification interventions,” the authors write.
SOURCE:
The study was published online in the Journal of Prevention of Alzheimer’s Disease. The first author was Ying Liu, PhD, of the University of Southern California, Los Angeles.
LIMITATIONS:
The predictive model based on demographic information has only moderate accuracy. Expected prevalence of MCI was based on cognitive test scores, which is not the same as a true clinical diagnosis.
DISCLOSURES:
The study was partially funded by a contract from Genentech to the University of Southern California. Coauthors Soeren Mattke and Christopher Wallick have disclosed relationships with Genentech.
A version of this article appeared on Medscape.com.
TOPLINE:
Detection rates of mild cognitive impairment (MCI) in primary care are extremely low, with only about 8% of expected cases diagnosed on average, a finding that points to an urgent need to improve early detection in primary care.
METHODOLOGY:
- Researchers estimated MCI detection rates among 226,756 primary care clinicians and 54,597 practices that had at least 25 patients enrolled in Medicare between 2017 and 2019.
- They compared the expected number of MCI cases, based on a predictive model, to actual diagnosed cases as documented in claims and encounter data.
- They accounted for uncertainty in these estimates to determine whether detection rates are within the expected range or significantly higher or lower.
TAKEAWAY:
- More than 25% of clinicians and practices did not have a single patient with diagnosed MCI; the average detection rate was 0.01 for both clinicians and practices.
- The modeled expected MCI detection rate, however, was much higher (average 0.19 for clinicians and 0.20 for practices).
- Average detection rates for clinicians and practices was 0.08, with more than 99% of clinicians and practices underdiagnosing MCI; clinicians practicing geriatric medicine had higher detection rates than others.
IN PRACTICE:
The findings are “concerning not only because patients might not get identified for a disease-modifying AD treatment in time, but also because numerous causes of MCI – such as hypothyroidism and medication side effects – are reversible, and the condition itself can be stabilized by lifestyle modification interventions,” the authors write.
SOURCE:
The study was published online in the Journal of Prevention of Alzheimer’s Disease. The first author was Ying Liu, PhD, of the University of Southern California, Los Angeles.
LIMITATIONS:
The predictive model based on demographic information has only moderate accuracy. Expected prevalence of MCI was based on cognitive test scores, which is not the same as a true clinical diagnosis.
DISCLOSURES:
The study was partially funded by a contract from Genentech to the University of Southern California. Coauthors Soeren Mattke and Christopher Wallick have disclosed relationships with Genentech.
A version of this article appeared on Medscape.com.
No benefit of colchicine after stroke, TIA: CHANCE-3
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
FROM WSC 2023
Is it time to scrap ultraprocessed foods?
Ultraprocessed foods (UPFs) make up nearly three-quarters of the entire U.S. food supply and about 60% of Americans’ daily caloric intake. A significant body of research has tied consumption of these foods – awash in added sugar, salt, fat, artificial colors, or preservatives – to cancer, diabetes, and heart disease.
Now, a growing number of studies also link them to poor brain health, including an increased risk of dementia, depression, and anxiety, and some experts are calling for public health policies aimed at reducing UPF consumption.
Under srutiny
A mainstay of diets in countries around the world, UPFs have come under increasing scrutiny because of their link to major diseases. The ingredients in UPFs add little or no nutritional value. Their primary function is to increase a product’s shelf life and palatability. Some recent evidence suggests these foods may be as addictive as tobacco. In addition, two pooled analysis studies using the Yale Food Addiction Scale showed that 14% of adults and 12% of children in the United States may have a UPF addiction.
The most widely used measure of what is, and what is not, a UPF was developed in 2009 by researchers in Brazil. The NOVA food classification system assigns food and beverages to one of four groups:
- Unprocessed and minimally processed foods, such as fruits, vegetables, milk, and meat.
- Processed culinary ingredients, including white sugar, butter, and oils derived from seeds, nuts, and fruits.
- Processed foods, such as tomato paste, bacon, canned tuna, and wine.
- Ultraprocessed foods, such as soda, ice cream, breakfast cereal, and prepackaged meals.
Those sounding the alarm about the potential harmful effects of UPFs are particularly concerned about their consumption by young people. The National Health and Nutrition Examination Survey showed that from 1999 to 2018, highly processed foods accounted for the majority of energy intake in those aged 2-19 years.
One of the most commonly used additives in UPFs, the artificial sweetener aspartame, garnered headlines this summer when the World Health Organization classified it as a likely carcinogen in humans. Aspartame is used in thousands of products, from soda to chewing gum to chewable vitamins.
The U.S. Food and Drug Administration strongly disagreed with the WHO’s position and is sticking by its recommended daily limit of 50 mg/kg of body weight – equivalent to 75 packets of the sweetener Equal – as safe for human consumption.
“Aspartame is one of the most studied food additives in the human food supply,” FDA officials said in a statement, adding that the agency found “significant shortcomings” in the studies the WHO used to justify the new classification. “FDA scientists do not have safety concerns when aspartame is used under the approved conditions.”
Increased attention to consumption of UPFs in general and aspartame particularly in recent years has yielded several studies pointing to the foods’ association with compromised brain health.
Link to depression, dementia
A recent report on UPF consumption and mental well-being among nearly 300,000 people across 70 countries showed that 53% of those who consumed UPFs several times a day were distressed or were struggling with their mental well-being, compared with 18% of those who rarely or never consumed UPFs.
Part of the Global Mind Project run by the nonprofit Sapien Labs in Arlington, Va., the report also showed that individuals with the highest rates of UPF consumption reported higher levels of confusion, slowed thinking, unwanted or obsessive thoughts, irritability, and feelings of sadness.
“There seems to be a much broader effect than just depression symptoms,” Tara Thiagarajan, PhD, founder and chief scientist of Sapien Labs and coauthor of the report, said in an interview.
The report, which has not been peer reviewed, comes on the heels of several other studies, including one from the Nurses Health Study II that showed that participants who consumed more than eight servings of UPFs daily had about a 50% higher depression risk, compared with those who consumed half that much.
“We found that UPFs in general, and artificial sweeteners and beverages in particular, were associated with increased risk,” said lead investigator Andrew T. Chan, MD, MPH, professor of medicine at Harvard Medical School and chief of the clinical and translational epidemiology unit, Massachusetts General Hospital, both in Boston.
“This was an interesting finding that correlates with data from animal studies that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” he told this news organization.
Cognition may also be affected. An analysis of more than 72,000 people in the UK Biobank showed that those who consumed a high levels of UPFs were 50% more likely to develop dementia than those who consumed fewer processed foods. For every 10% increase in UPF consumption, the odds of developing any kind of dementia increased by 25%.
Another study of nearly 11,000 people showed that higher UPF consumption was associated with a significantly faster decline in executive and global cognitive function.
Epigenetic changes
While these and other studies suggest a link between UPF consumption and brain health, they are designed to demonstrate correlation. To date, no human study has proven that eating highly processed foods directly causes a decline in mental health or cognition.
Animal studies could provide that causal link. Earlier this year, researchers at Florida State University in Tallahassee reported learning and memory deficits in two groups of male mice that completed a maze test after being fed water mixed with aspartame for about 20% of their adult lives, compared with a group of mice that drank water only. Animals that ingested aspartame could finish the test, but it took them longer, and they needed help.
The amount of aspartame used in the study was just 7% and 15% of the FDA’s recommended maximum intake of aspartame (equivalent to two to four 8-ounce diet sodas daily).
Most intriguing was that offspring of the mice in the aspartame groups demonstrated the same levels of cognitive decline and anxiety as their fathers, even though they had never ingested the artificial sweetener. Researchers theorize that in addition to changes in brain gene expression, aspartame also caused epigenetic changes in germ cells.
“Epigenetic changes in germ cells due to environmental exposures are both good and bad,” lead investigator Pradeep G. Bhide, PhD, professor of developmental neuroscience and director of the Center for Brain Repair at FSU, told this news organization. “They are bad because the next generation is affected. But they’re good because as long as the exposure no longer occurs, 2 or 3 generations later, that’s gone.”
The mice, which lacked taste receptors for aspartame, were the same age and weight in all three groups. Because the only difference was exposure to the artificial sweetener, Dr. Bhide says it suggests a causal link.
“Extrapolation of data from well-controlled laboratory experiments in mice to humans is always risky,” Dr. Bhide said. “The extrapolations give us insights into what could happen rather than what will happen.”
Potential mechanisms
Although scientists can’t say for certain how UPFs affect brain health, there are several theories. UPFs may influence an inflammatory immune response, which has been linked to depression and dementia. Consumption of highly processed foods may also disrupt the gut microbiome, Dr. Chan said, which, in turn, may increase depression risk.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” he said.
In addition, with UPFs that contain aspartame, there could be a more direct link to brain function. In the gastrointestinal track, the sweetener is quickly broken down into methanol, aspartic acid, and phenylalanine. All three enter the bloodstream, cross the blood-brain barrier, and are neuroactive.
“Phenylalanine is a precursor for neurotransmitters in the brain, and aspartic acid activates the glutamate excitatory neurotransmitter receptor,” Dr. Bhide said. “The effects we’ve seen could be due to these metabolites that have a direct effect on the brain function.”
Time to act?
Some researchers are building a case for classifying UPFs as addictive substances. Others are calling for additional research on UPF safety that is conducted outside the food industry.
There has also been some discussion of placing warning labels on UPFs. However, there is disagreement about what information should be included and how consumers might interpret it. The question of which food products are UPFs and which are not also isn’t settled. The NOVA system may be widely used, but it still has its detractors who believe it misclassifies some healthy foods as ultraprocessed.
Dr. Chan and other experts say the research conducted thus far requires additional corroboration to inform appropriate public health interventions. That would likely take the form of a large, randomized trial with one group of participants eating a healthy diet and the other consuming large amounts of UPFs.
“This type of study is extremely challenging given the number of people that would have to be willing to participate and be willing to eat a very specific diet over a long period of time,” Dr. Chan said. “I am also not sure it would be ethical to assign people to such a diet, given what we already know about the potential health effects of UPFs.”
Dr. Thiagarajan and others have called on funding agencies to direct more grant monies toward studies of UPFs to better understand their effect on brain health.
“Given the magnitude of the problem and given that there is a fair bit of evidence that points to a potential causal link, then we damn well better put money into this and get to the bottom of it,” she said.
Others are looking to the FDA to increase the agency’s scrutiny of food additives. While some additives such as artificial sweeteners have a place in diets of people with diabetes or obesity, Dr. Bhide suggests it may be wise for healthy individuals to reduce their daily intake of UPFs.
“Our data raise this to a different level because of the transgenerational transmission, which has never been shown before,” he said. “We are saying that the FDA should look in preclinical models at germ cells and maybe transgenerational transmission before approving any food additive.”
A version of this article first appeared on Medscape.com.
Ultraprocessed foods (UPFs) make up nearly three-quarters of the entire U.S. food supply and about 60% of Americans’ daily caloric intake. A significant body of research has tied consumption of these foods – awash in added sugar, salt, fat, artificial colors, or preservatives – to cancer, diabetes, and heart disease.
Now, a growing number of studies also link them to poor brain health, including an increased risk of dementia, depression, and anxiety, and some experts are calling for public health policies aimed at reducing UPF consumption.
Under srutiny
A mainstay of diets in countries around the world, UPFs have come under increasing scrutiny because of their link to major diseases. The ingredients in UPFs add little or no nutritional value. Their primary function is to increase a product’s shelf life and palatability. Some recent evidence suggests these foods may be as addictive as tobacco. In addition, two pooled analysis studies using the Yale Food Addiction Scale showed that 14% of adults and 12% of children in the United States may have a UPF addiction.
The most widely used measure of what is, and what is not, a UPF was developed in 2009 by researchers in Brazil. The NOVA food classification system assigns food and beverages to one of four groups:
- Unprocessed and minimally processed foods, such as fruits, vegetables, milk, and meat.
- Processed culinary ingredients, including white sugar, butter, and oils derived from seeds, nuts, and fruits.
- Processed foods, such as tomato paste, bacon, canned tuna, and wine.
- Ultraprocessed foods, such as soda, ice cream, breakfast cereal, and prepackaged meals.
Those sounding the alarm about the potential harmful effects of UPFs are particularly concerned about their consumption by young people. The National Health and Nutrition Examination Survey showed that from 1999 to 2018, highly processed foods accounted for the majority of energy intake in those aged 2-19 years.
One of the most commonly used additives in UPFs, the artificial sweetener aspartame, garnered headlines this summer when the World Health Organization classified it as a likely carcinogen in humans. Aspartame is used in thousands of products, from soda to chewing gum to chewable vitamins.
The U.S. Food and Drug Administration strongly disagreed with the WHO’s position and is sticking by its recommended daily limit of 50 mg/kg of body weight – equivalent to 75 packets of the sweetener Equal – as safe for human consumption.
“Aspartame is one of the most studied food additives in the human food supply,” FDA officials said in a statement, adding that the agency found “significant shortcomings” in the studies the WHO used to justify the new classification. “FDA scientists do not have safety concerns when aspartame is used under the approved conditions.”
Increased attention to consumption of UPFs in general and aspartame particularly in recent years has yielded several studies pointing to the foods’ association with compromised brain health.
Link to depression, dementia
A recent report on UPF consumption and mental well-being among nearly 300,000 people across 70 countries showed that 53% of those who consumed UPFs several times a day were distressed or were struggling with their mental well-being, compared with 18% of those who rarely or never consumed UPFs.
Part of the Global Mind Project run by the nonprofit Sapien Labs in Arlington, Va., the report also showed that individuals with the highest rates of UPF consumption reported higher levels of confusion, slowed thinking, unwanted or obsessive thoughts, irritability, and feelings of sadness.
“There seems to be a much broader effect than just depression symptoms,” Tara Thiagarajan, PhD, founder and chief scientist of Sapien Labs and coauthor of the report, said in an interview.
The report, which has not been peer reviewed, comes on the heels of several other studies, including one from the Nurses Health Study II that showed that participants who consumed more than eight servings of UPFs daily had about a 50% higher depression risk, compared with those who consumed half that much.
“We found that UPFs in general, and artificial sweeteners and beverages in particular, were associated with increased risk,” said lead investigator Andrew T. Chan, MD, MPH, professor of medicine at Harvard Medical School and chief of the clinical and translational epidemiology unit, Massachusetts General Hospital, both in Boston.
“This was an interesting finding that correlates with data from animal studies that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” he told this news organization.
Cognition may also be affected. An analysis of more than 72,000 people in the UK Biobank showed that those who consumed a high levels of UPFs were 50% more likely to develop dementia than those who consumed fewer processed foods. For every 10% increase in UPF consumption, the odds of developing any kind of dementia increased by 25%.
Another study of nearly 11,000 people showed that higher UPF consumption was associated with a significantly faster decline in executive and global cognitive function.
Epigenetic changes
While these and other studies suggest a link between UPF consumption and brain health, they are designed to demonstrate correlation. To date, no human study has proven that eating highly processed foods directly causes a decline in mental health or cognition.
Animal studies could provide that causal link. Earlier this year, researchers at Florida State University in Tallahassee reported learning and memory deficits in two groups of male mice that completed a maze test after being fed water mixed with aspartame for about 20% of their adult lives, compared with a group of mice that drank water only. Animals that ingested aspartame could finish the test, but it took them longer, and they needed help.
The amount of aspartame used in the study was just 7% and 15% of the FDA’s recommended maximum intake of aspartame (equivalent to two to four 8-ounce diet sodas daily).
Most intriguing was that offspring of the mice in the aspartame groups demonstrated the same levels of cognitive decline and anxiety as their fathers, even though they had never ingested the artificial sweetener. Researchers theorize that in addition to changes in brain gene expression, aspartame also caused epigenetic changes in germ cells.
“Epigenetic changes in germ cells due to environmental exposures are both good and bad,” lead investigator Pradeep G. Bhide, PhD, professor of developmental neuroscience and director of the Center for Brain Repair at FSU, told this news organization. “They are bad because the next generation is affected. But they’re good because as long as the exposure no longer occurs, 2 or 3 generations later, that’s gone.”
The mice, which lacked taste receptors for aspartame, were the same age and weight in all three groups. Because the only difference was exposure to the artificial sweetener, Dr. Bhide says it suggests a causal link.
“Extrapolation of data from well-controlled laboratory experiments in mice to humans is always risky,” Dr. Bhide said. “The extrapolations give us insights into what could happen rather than what will happen.”
Potential mechanisms
Although scientists can’t say for certain how UPFs affect brain health, there are several theories. UPFs may influence an inflammatory immune response, which has been linked to depression and dementia. Consumption of highly processed foods may also disrupt the gut microbiome, Dr. Chan said, which, in turn, may increase depression risk.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” he said.
In addition, with UPFs that contain aspartame, there could be a more direct link to brain function. In the gastrointestinal track, the sweetener is quickly broken down into methanol, aspartic acid, and phenylalanine. All three enter the bloodstream, cross the blood-brain barrier, and are neuroactive.
“Phenylalanine is a precursor for neurotransmitters in the brain, and aspartic acid activates the glutamate excitatory neurotransmitter receptor,” Dr. Bhide said. “The effects we’ve seen could be due to these metabolites that have a direct effect on the brain function.”
Time to act?
Some researchers are building a case for classifying UPFs as addictive substances. Others are calling for additional research on UPF safety that is conducted outside the food industry.
There has also been some discussion of placing warning labels on UPFs. However, there is disagreement about what information should be included and how consumers might interpret it. The question of which food products are UPFs and which are not also isn’t settled. The NOVA system may be widely used, but it still has its detractors who believe it misclassifies some healthy foods as ultraprocessed.
Dr. Chan and other experts say the research conducted thus far requires additional corroboration to inform appropriate public health interventions. That would likely take the form of a large, randomized trial with one group of participants eating a healthy diet and the other consuming large amounts of UPFs.
“This type of study is extremely challenging given the number of people that would have to be willing to participate and be willing to eat a very specific diet over a long period of time,” Dr. Chan said. “I am also not sure it would be ethical to assign people to such a diet, given what we already know about the potential health effects of UPFs.”
Dr. Thiagarajan and others have called on funding agencies to direct more grant monies toward studies of UPFs to better understand their effect on brain health.
“Given the magnitude of the problem and given that there is a fair bit of evidence that points to a potential causal link, then we damn well better put money into this and get to the bottom of it,” she said.
Others are looking to the FDA to increase the agency’s scrutiny of food additives. While some additives such as artificial sweeteners have a place in diets of people with diabetes or obesity, Dr. Bhide suggests it may be wise for healthy individuals to reduce their daily intake of UPFs.
“Our data raise this to a different level because of the transgenerational transmission, which has never been shown before,” he said. “We are saying that the FDA should look in preclinical models at germ cells and maybe transgenerational transmission before approving any food additive.”
A version of this article first appeared on Medscape.com.
Ultraprocessed foods (UPFs) make up nearly three-quarters of the entire U.S. food supply and about 60% of Americans’ daily caloric intake. A significant body of research has tied consumption of these foods – awash in added sugar, salt, fat, artificial colors, or preservatives – to cancer, diabetes, and heart disease.
Now, a growing number of studies also link them to poor brain health, including an increased risk of dementia, depression, and anxiety, and some experts are calling for public health policies aimed at reducing UPF consumption.
Under srutiny
A mainstay of diets in countries around the world, UPFs have come under increasing scrutiny because of their link to major diseases. The ingredients in UPFs add little or no nutritional value. Their primary function is to increase a product’s shelf life and palatability. Some recent evidence suggests these foods may be as addictive as tobacco. In addition, two pooled analysis studies using the Yale Food Addiction Scale showed that 14% of adults and 12% of children in the United States may have a UPF addiction.
The most widely used measure of what is, and what is not, a UPF was developed in 2009 by researchers in Brazil. The NOVA food classification system assigns food and beverages to one of four groups:
- Unprocessed and minimally processed foods, such as fruits, vegetables, milk, and meat.
- Processed culinary ingredients, including white sugar, butter, and oils derived from seeds, nuts, and fruits.
- Processed foods, such as tomato paste, bacon, canned tuna, and wine.
- Ultraprocessed foods, such as soda, ice cream, breakfast cereal, and prepackaged meals.
Those sounding the alarm about the potential harmful effects of UPFs are particularly concerned about their consumption by young people. The National Health and Nutrition Examination Survey showed that from 1999 to 2018, highly processed foods accounted for the majority of energy intake in those aged 2-19 years.
One of the most commonly used additives in UPFs, the artificial sweetener aspartame, garnered headlines this summer when the World Health Organization classified it as a likely carcinogen in humans. Aspartame is used in thousands of products, from soda to chewing gum to chewable vitamins.
The U.S. Food and Drug Administration strongly disagreed with the WHO’s position and is sticking by its recommended daily limit of 50 mg/kg of body weight – equivalent to 75 packets of the sweetener Equal – as safe for human consumption.
“Aspartame is one of the most studied food additives in the human food supply,” FDA officials said in a statement, adding that the agency found “significant shortcomings” in the studies the WHO used to justify the new classification. “FDA scientists do not have safety concerns when aspartame is used under the approved conditions.”
Increased attention to consumption of UPFs in general and aspartame particularly in recent years has yielded several studies pointing to the foods’ association with compromised brain health.
Link to depression, dementia
A recent report on UPF consumption and mental well-being among nearly 300,000 people across 70 countries showed that 53% of those who consumed UPFs several times a day were distressed or were struggling with their mental well-being, compared with 18% of those who rarely or never consumed UPFs.
Part of the Global Mind Project run by the nonprofit Sapien Labs in Arlington, Va., the report also showed that individuals with the highest rates of UPF consumption reported higher levels of confusion, slowed thinking, unwanted or obsessive thoughts, irritability, and feelings of sadness.
“There seems to be a much broader effect than just depression symptoms,” Tara Thiagarajan, PhD, founder and chief scientist of Sapien Labs and coauthor of the report, said in an interview.
The report, which has not been peer reviewed, comes on the heels of several other studies, including one from the Nurses Health Study II that showed that participants who consumed more than eight servings of UPFs daily had about a 50% higher depression risk, compared with those who consumed half that much.
“We found that UPFs in general, and artificial sweeteners and beverages in particular, were associated with increased risk,” said lead investigator Andrew T. Chan, MD, MPH, professor of medicine at Harvard Medical School and chief of the clinical and translational epidemiology unit, Massachusetts General Hospital, both in Boston.
“This was an interesting finding that correlates with data from animal studies that artificial sweeteners may trigger the transmission of particular signaling molecules in the brain that are important for mood,” he told this news organization.
Cognition may also be affected. An analysis of more than 72,000 people in the UK Biobank showed that those who consumed a high levels of UPFs were 50% more likely to develop dementia than those who consumed fewer processed foods. For every 10% increase in UPF consumption, the odds of developing any kind of dementia increased by 25%.
Another study of nearly 11,000 people showed that higher UPF consumption was associated with a significantly faster decline in executive and global cognitive function.
Epigenetic changes
While these and other studies suggest a link between UPF consumption and brain health, they are designed to demonstrate correlation. To date, no human study has proven that eating highly processed foods directly causes a decline in mental health or cognition.
Animal studies could provide that causal link. Earlier this year, researchers at Florida State University in Tallahassee reported learning and memory deficits in two groups of male mice that completed a maze test after being fed water mixed with aspartame for about 20% of their adult lives, compared with a group of mice that drank water only. Animals that ingested aspartame could finish the test, but it took them longer, and they needed help.
The amount of aspartame used in the study was just 7% and 15% of the FDA’s recommended maximum intake of aspartame (equivalent to two to four 8-ounce diet sodas daily).
Most intriguing was that offspring of the mice in the aspartame groups demonstrated the same levels of cognitive decline and anxiety as their fathers, even though they had never ingested the artificial sweetener. Researchers theorize that in addition to changes in brain gene expression, aspartame also caused epigenetic changes in germ cells.
“Epigenetic changes in germ cells due to environmental exposures are both good and bad,” lead investigator Pradeep G. Bhide, PhD, professor of developmental neuroscience and director of the Center for Brain Repair at FSU, told this news organization. “They are bad because the next generation is affected. But they’re good because as long as the exposure no longer occurs, 2 or 3 generations later, that’s gone.”
The mice, which lacked taste receptors for aspartame, were the same age and weight in all three groups. Because the only difference was exposure to the artificial sweetener, Dr. Bhide says it suggests a causal link.
“Extrapolation of data from well-controlled laboratory experiments in mice to humans is always risky,” Dr. Bhide said. “The extrapolations give us insights into what could happen rather than what will happen.”
Potential mechanisms
Although scientists can’t say for certain how UPFs affect brain health, there are several theories. UPFs may influence an inflammatory immune response, which has been linked to depression and dementia. Consumption of highly processed foods may also disrupt the gut microbiome, Dr. Chan said, which, in turn, may increase depression risk.
“This is an important potential mechanism linking ultraprocessed food to depression since there is emerging evidence that microbes in the gut have been linked with mood through their role in metabolizing and producing proteins that have activity in the brain,” he said.
In addition, with UPFs that contain aspartame, there could be a more direct link to brain function. In the gastrointestinal track, the sweetener is quickly broken down into methanol, aspartic acid, and phenylalanine. All three enter the bloodstream, cross the blood-brain barrier, and are neuroactive.
“Phenylalanine is a precursor for neurotransmitters in the brain, and aspartic acid activates the glutamate excitatory neurotransmitter receptor,” Dr. Bhide said. “The effects we’ve seen could be due to these metabolites that have a direct effect on the brain function.”
Time to act?
Some researchers are building a case for classifying UPFs as addictive substances. Others are calling for additional research on UPF safety that is conducted outside the food industry.
There has also been some discussion of placing warning labels on UPFs. However, there is disagreement about what information should be included and how consumers might interpret it. The question of which food products are UPFs and which are not also isn’t settled. The NOVA system may be widely used, but it still has its detractors who believe it misclassifies some healthy foods as ultraprocessed.
Dr. Chan and other experts say the research conducted thus far requires additional corroboration to inform appropriate public health interventions. That would likely take the form of a large, randomized trial with one group of participants eating a healthy diet and the other consuming large amounts of UPFs.
“This type of study is extremely challenging given the number of people that would have to be willing to participate and be willing to eat a very specific diet over a long period of time,” Dr. Chan said. “I am also not sure it would be ethical to assign people to such a diet, given what we already know about the potential health effects of UPFs.”
Dr. Thiagarajan and others have called on funding agencies to direct more grant monies toward studies of UPFs to better understand their effect on brain health.
“Given the magnitude of the problem and given that there is a fair bit of evidence that points to a potential causal link, then we damn well better put money into this and get to the bottom of it,” she said.
Others are looking to the FDA to increase the agency’s scrutiny of food additives. While some additives such as artificial sweeteners have a place in diets of people with diabetes or obesity, Dr. Bhide suggests it may be wise for healthy individuals to reduce their daily intake of UPFs.
“Our data raise this to a different level because of the transgenerational transmission, which has never been shown before,” he said. “We are saying that the FDA should look in preclinical models at germ cells and maybe transgenerational transmission before approving any food additive.”
A version of this article first appeared on Medscape.com.
FDA okays drug for Duchenne muscular dystrophy
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .
Duchenne muscular dystrophy (DMD) in patients as young as age 2 years, the company has announced Vamorolone is a structurally unique steroidal anti-inflammatory drug that potently inhibits proinflammatory NFkB pathways via high-affinity binding to the glucocorticoid receptor.
“Corticosteroids have been a first line treatment for DMD for many years, but their utility has always been limited by the side effect profile, which includes weight gain, short stature, and decreased bone density, among others,” Sharon Hesterlee, PhD, chief research officer for the Muscular Dystrophy Association, said in a statement.
The approval of vamorolone “provides people living with Duchenne, and their families, a powerful tool to treat the disease, while limiting some negative side effects associated with corticosteroids,” Dr. Hesterlee added.
The approval was based on data from the phase 2b VISION-DMD study, supplemented with safety information collected from three open-label studies.
Vamorolone was administered at doses ranging from 2-6 mg/kg/d for a period of up to 48 months.
Vamorolone demonstrated efficacy similar to that of traditional corticosteroids, with data suggesting a reduction in adverse events – notably related to bone health, growth trajectory, and behavior.
Vamorolone had received orphan drug status for DMD, as well as fast track and rare pediatric disease designations. It will be made available in the United States by Catalyst Pharmaceuticals.
A version of this article first appeared on Medscape.com .
Neurologic nuggets of wisdom for pediatric practice
WASHINGTON – Get the back story before rushing to diagnose a seizure disorder in a child, Michael Strunc, MD, said in a presentation at the annual meeting of the American Academy of Pediatrics.
Clinicians should ask parents or caregivers about the child’s behavior before the suspected seizure, whether there were any triggers, and if so, what might they have been, according to Dr. Strunc, a child neurologist and sleep medicine specialist at Children’s Hospital of the King’s Daughters, Norfolk, Va.
“Most seizures don’t have triggers,” he said. Rather, patients often become stiff, experience a motor event that builds in intensity then slows and stops, and finally, the patient is sleepy and tired. Clinicians should also find out whether the event had a beginning, middle, and end.
Approximately 0.6% of children younger than 17 years in the United States have active epilepsy, according to the Centers for Disease Control and Prevention.
Dr. Strunc offered a few more tips for diagnosing a child:
- Ask whether the patient’s eyes were open during the event. If the eyes were closed or squished closed, “it is almost never a seizure,” he said.
- Find out whether the patient was awake or asleep, and how, if at all, caregivers attempted to stop the event.
- Ask if the child’s experiences were repeating and predictable, and inquire about a family history of seizures or other events.
- Inquire about any developmental changes and other changes in the child, such as irritability, regression, or ataxia.
The differential diagnosis for a seizure includes nonepileptic events that occur with and without changes in consciousness or sleep. These events range from breath-holding and hyperventilation to night terrors, narcolepsy, migraine, and attention-deficit/hyperactivity disorder, he said.
Is it epilepsy?
Dr. Strunc shared several cases of neurologic “events” ranging from simple to severe.
In one case, a 10-month-old infant girl with a potential tonic/staring seizure presented with a history of events that involved getting stuck in a stiff position, usually while sitting in a car seat or highchair, with adducting of legs, redness of face, and “zoned-out” expression. The infant was healthy, smart, and precocious, with no illness, fever, or trauma, but the mother was very concerned, Dr. Strunc said.
The diagnosis: Self-gratification, which is benign and usually outgrown, although it can become extreme, he said.
By contrast, “absence,” also known as idiopathic generalized epilepsy, presents as brief events of 4-10 seconds that may occur up to hundreds of times a day. This type of epilepsy is associated with the sudden onset of impaired consciousness and unresponsiveness. These events end abruptly, and the child may be unaware. Absence is more common in girls. It usually occurs after age 4 and usually remits by about age 12, Dr. Strunc said.
However, the onset of absence in patients younger than age 3 is associated with increased odds of neurodevelopmental abnormalities “and probably represents another epilepsy syndrome,” he said.
Absence symptoms may mirror those of children who are simply daydreamers, Dr. Strunc noted. One way to confirm absence is by provoking hyperventilation, which will bring on an episode of absence if present, he said. EEGs provide evidence as well.
Acute ataxia in children has a wide differential that sends kids and families to the pediatrician or emergency department, Dr. Strunc said. Acute cerebellar ataxia is characterized by abrupt and symmetric symptoms, with no mental status changes, no fever, no meningitis, and no headache. A wide, unstable gait is a distinguishing feature, Dr. Strunc said.
However, other causes of acute ataxia should be ruled out, including toxic ingestion, tick paralysis, central nervous system infections, vascular conditions, and genetic conditions.
Don’t miss those ticks
Especially during periods when kids are outdoors, clinicians should consider a tick bite as a source of ataxia and neurologic symptoms in children, Dr. Strunc emphasized. Tick paralysis notably resembles many symptoms of Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy).
Dr. Strunc described a case involving a 5-year-old girl who developed sudden problems with gait. The problems worsened quickly and prompted an emergency department visit.
The girl had an unremarkable history, she had not experienced mental status changes, her strength was normal, and she had just returned from a Girl Scouts trip. The patient was presumed to have Guillain-Barré. IVIG was initiated when an emergency nurse found a tick on her scalp. The tick was removed, and the patient left the hospital within 24 hours.
Children with tick paralysis are usually symptomatic after 5-7 days with the tick attached, Dr. Strunc said. They recover within a day after tick removal.
Overall, actual seizures are less common than other neurologic events in children, according to Dr. Strunc. Details on history, lack or presence of neurologic feature, and normal child development can help guide evaluation.
Take advantage of videos, he emphasized, as many parents and caregivers record a child’s neurologic events.
“Ataxia is scary, but exam and associated findings will help you with etiology,” he said.
Dr. Strunc has received research support from Jazz and Harmony and has served on the speakers’ bureau for Jazz Pharmaceuticals, Harmony Biosciences, and Avadel, unrelated to his presentation.
A version of this article first appeared on Medscape.com.
WASHINGTON – Get the back story before rushing to diagnose a seizure disorder in a child, Michael Strunc, MD, said in a presentation at the annual meeting of the American Academy of Pediatrics.
Clinicians should ask parents or caregivers about the child’s behavior before the suspected seizure, whether there were any triggers, and if so, what might they have been, according to Dr. Strunc, a child neurologist and sleep medicine specialist at Children’s Hospital of the King’s Daughters, Norfolk, Va.
“Most seizures don’t have triggers,” he said. Rather, patients often become stiff, experience a motor event that builds in intensity then slows and stops, and finally, the patient is sleepy and tired. Clinicians should also find out whether the event had a beginning, middle, and end.
Approximately 0.6% of children younger than 17 years in the United States have active epilepsy, according to the Centers for Disease Control and Prevention.
Dr. Strunc offered a few more tips for diagnosing a child:
- Ask whether the patient’s eyes were open during the event. If the eyes were closed or squished closed, “it is almost never a seizure,” he said.
- Find out whether the patient was awake or asleep, and how, if at all, caregivers attempted to stop the event.
- Ask if the child’s experiences were repeating and predictable, and inquire about a family history of seizures or other events.
- Inquire about any developmental changes and other changes in the child, such as irritability, regression, or ataxia.
The differential diagnosis for a seizure includes nonepileptic events that occur with and without changes in consciousness or sleep. These events range from breath-holding and hyperventilation to night terrors, narcolepsy, migraine, and attention-deficit/hyperactivity disorder, he said.
Is it epilepsy?
Dr. Strunc shared several cases of neurologic “events” ranging from simple to severe.
In one case, a 10-month-old infant girl with a potential tonic/staring seizure presented with a history of events that involved getting stuck in a stiff position, usually while sitting in a car seat or highchair, with adducting of legs, redness of face, and “zoned-out” expression. The infant was healthy, smart, and precocious, with no illness, fever, or trauma, but the mother was very concerned, Dr. Strunc said.
The diagnosis: Self-gratification, which is benign and usually outgrown, although it can become extreme, he said.
By contrast, “absence,” also known as idiopathic generalized epilepsy, presents as brief events of 4-10 seconds that may occur up to hundreds of times a day. This type of epilepsy is associated with the sudden onset of impaired consciousness and unresponsiveness. These events end abruptly, and the child may be unaware. Absence is more common in girls. It usually occurs after age 4 and usually remits by about age 12, Dr. Strunc said.
However, the onset of absence in patients younger than age 3 is associated with increased odds of neurodevelopmental abnormalities “and probably represents another epilepsy syndrome,” he said.
Absence symptoms may mirror those of children who are simply daydreamers, Dr. Strunc noted. One way to confirm absence is by provoking hyperventilation, which will bring on an episode of absence if present, he said. EEGs provide evidence as well.
Acute ataxia in children has a wide differential that sends kids and families to the pediatrician or emergency department, Dr. Strunc said. Acute cerebellar ataxia is characterized by abrupt and symmetric symptoms, with no mental status changes, no fever, no meningitis, and no headache. A wide, unstable gait is a distinguishing feature, Dr. Strunc said.
However, other causes of acute ataxia should be ruled out, including toxic ingestion, tick paralysis, central nervous system infections, vascular conditions, and genetic conditions.
Don’t miss those ticks
Especially during periods when kids are outdoors, clinicians should consider a tick bite as a source of ataxia and neurologic symptoms in children, Dr. Strunc emphasized. Tick paralysis notably resembles many symptoms of Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy).
Dr. Strunc described a case involving a 5-year-old girl who developed sudden problems with gait. The problems worsened quickly and prompted an emergency department visit.
The girl had an unremarkable history, she had not experienced mental status changes, her strength was normal, and she had just returned from a Girl Scouts trip. The patient was presumed to have Guillain-Barré. IVIG was initiated when an emergency nurse found a tick on her scalp. The tick was removed, and the patient left the hospital within 24 hours.
Children with tick paralysis are usually symptomatic after 5-7 days with the tick attached, Dr. Strunc said. They recover within a day after tick removal.
Overall, actual seizures are less common than other neurologic events in children, according to Dr. Strunc. Details on history, lack or presence of neurologic feature, and normal child development can help guide evaluation.
Take advantage of videos, he emphasized, as many parents and caregivers record a child’s neurologic events.
“Ataxia is scary, but exam and associated findings will help you with etiology,” he said.
Dr. Strunc has received research support from Jazz and Harmony and has served on the speakers’ bureau for Jazz Pharmaceuticals, Harmony Biosciences, and Avadel, unrelated to his presentation.
A version of this article first appeared on Medscape.com.
WASHINGTON – Get the back story before rushing to diagnose a seizure disorder in a child, Michael Strunc, MD, said in a presentation at the annual meeting of the American Academy of Pediatrics.
Clinicians should ask parents or caregivers about the child’s behavior before the suspected seizure, whether there were any triggers, and if so, what might they have been, according to Dr. Strunc, a child neurologist and sleep medicine specialist at Children’s Hospital of the King’s Daughters, Norfolk, Va.
“Most seizures don’t have triggers,” he said. Rather, patients often become stiff, experience a motor event that builds in intensity then slows and stops, and finally, the patient is sleepy and tired. Clinicians should also find out whether the event had a beginning, middle, and end.
Approximately 0.6% of children younger than 17 years in the United States have active epilepsy, according to the Centers for Disease Control and Prevention.
Dr. Strunc offered a few more tips for diagnosing a child:
- Ask whether the patient’s eyes were open during the event. If the eyes were closed or squished closed, “it is almost never a seizure,” he said.
- Find out whether the patient was awake or asleep, and how, if at all, caregivers attempted to stop the event.
- Ask if the child’s experiences were repeating and predictable, and inquire about a family history of seizures or other events.
- Inquire about any developmental changes and other changes in the child, such as irritability, regression, or ataxia.
The differential diagnosis for a seizure includes nonepileptic events that occur with and without changes in consciousness or sleep. These events range from breath-holding and hyperventilation to night terrors, narcolepsy, migraine, and attention-deficit/hyperactivity disorder, he said.
Is it epilepsy?
Dr. Strunc shared several cases of neurologic “events” ranging from simple to severe.
In one case, a 10-month-old infant girl with a potential tonic/staring seizure presented with a history of events that involved getting stuck in a stiff position, usually while sitting in a car seat or highchair, with adducting of legs, redness of face, and “zoned-out” expression. The infant was healthy, smart, and precocious, with no illness, fever, or trauma, but the mother was very concerned, Dr. Strunc said.
The diagnosis: Self-gratification, which is benign and usually outgrown, although it can become extreme, he said.
By contrast, “absence,” also known as idiopathic generalized epilepsy, presents as brief events of 4-10 seconds that may occur up to hundreds of times a day. This type of epilepsy is associated with the sudden onset of impaired consciousness and unresponsiveness. These events end abruptly, and the child may be unaware. Absence is more common in girls. It usually occurs after age 4 and usually remits by about age 12, Dr. Strunc said.
However, the onset of absence in patients younger than age 3 is associated with increased odds of neurodevelopmental abnormalities “and probably represents another epilepsy syndrome,” he said.
Absence symptoms may mirror those of children who are simply daydreamers, Dr. Strunc noted. One way to confirm absence is by provoking hyperventilation, which will bring on an episode of absence if present, he said. EEGs provide evidence as well.
Acute ataxia in children has a wide differential that sends kids and families to the pediatrician or emergency department, Dr. Strunc said. Acute cerebellar ataxia is characterized by abrupt and symmetric symptoms, with no mental status changes, no fever, no meningitis, and no headache. A wide, unstable gait is a distinguishing feature, Dr. Strunc said.
However, other causes of acute ataxia should be ruled out, including toxic ingestion, tick paralysis, central nervous system infections, vascular conditions, and genetic conditions.
Don’t miss those ticks
Especially during periods when kids are outdoors, clinicians should consider a tick bite as a source of ataxia and neurologic symptoms in children, Dr. Strunc emphasized. Tick paralysis notably resembles many symptoms of Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy).
Dr. Strunc described a case involving a 5-year-old girl who developed sudden problems with gait. The problems worsened quickly and prompted an emergency department visit.
The girl had an unremarkable history, she had not experienced mental status changes, her strength was normal, and she had just returned from a Girl Scouts trip. The patient was presumed to have Guillain-Barré. IVIG was initiated when an emergency nurse found a tick on her scalp. The tick was removed, and the patient left the hospital within 24 hours.
Children with tick paralysis are usually symptomatic after 5-7 days with the tick attached, Dr. Strunc said. They recover within a day after tick removal.
Overall, actual seizures are less common than other neurologic events in children, according to Dr. Strunc. Details on history, lack or presence of neurologic feature, and normal child development can help guide evaluation.
Take advantage of videos, he emphasized, as many parents and caregivers record a child’s neurologic events.
“Ataxia is scary, but exam and associated findings will help you with etiology,” he said.
Dr. Strunc has received research support from Jazz and Harmony and has served on the speakers’ bureau for Jazz Pharmaceuticals, Harmony Biosciences, and Avadel, unrelated to his presentation.
A version of this article first appeared on Medscape.com.
FROM AAP 2023