User login
Not all exercise is beneficial: The physical activity paradox explained
In the pursuit of optimal health, regular physical activity (PA) is recommended to protect against dementia, cardiovascular disease (CVD), cancer, and other noncommunicable diseases. A significant body of research suggests the benefits of PA are positively correlated with higher frequency and intensity – with more often deemed better. This research has spawned a focus on increasing step counts and investing in standing desks and other interventions aimed at keeping people active.
But , but they also actually experience an increased risk for the very conditions that PA is intended to prevent.
A study published recently in The Lancet Regional Health – Europe used registry data from more than 7,000 adults in Norway, following them from age 33 to 65 years, to assess PA trajectories and risks for later-life mild cognitive impairment (MCI) and dementia at age 70 or older.
“Incorporating a life-course perspective gives a broader picture of how participants’ occupational histories relate to cognitive impairment later in life,” principal investigator Vegard Skirbekk, PhD, Columbia University Mailman School of Public Health, New York, said in an interview. Other studies typically have assessed occupational PA at a single time point, often close to the end of an individual’s career, and largely relied on self-report, he said.
Study participants worked in more than 300 different occupations. General physical activities performed on the included jobs required “considerable” use of arms and legs and moving the whole body, such as climbing, lifting, balancing, walking, stooping, and handling of materials.
Dr. Skirbekk and colleagues grouped participants into four PA trajectories over the 44-year study period: stable low, increasing then decreasing, stable intermediate, and stable high.
A total of 902 individuals were diagnosed with dementia and 2,407 with MCI at age 70 years or older. After adjustment, risks for MCI and dementia were 15.5% for those with higher occupational PA scores in the latter part of their working life and 9% for those with lower physical demands. The researchers concluded that “consistently working in an occupation with intermediate or high occupational PA was linked to an increased risk of cognitive impairment.”
The findings support those of the Copenhagen Male Study. Published in 2020, this longitudinal study compared leisure-time and occupational PA among more than 4,000 men in Denmark aged 40-59 at baseline in 1970-1971 and followed them until they turned 60. After adjustment, participants with high occupational PA had a 55% greater risk of developing dementia compared with those doing sedentary work.
Good vs. bad PA
“[T]he WHO [World Health Organization] guide to preventing dementia and disease on the whole mentions physical activity as an important factor. But our study suggests that it must be a ‘good’ form of physical activity, which hard physical work is not,” said Kirsten Nabe-Nielsen, PhD, lead author of this study
Beyond dementia, another recent study adds to a wealth of data on associations between occupational PA and cardiovascular risks. The cross-sectional analysis of U.S. data from the National Institute for Occupational Safety and Health showed that odds of CVD were higher when participants were “always” performing total occupational activity (odds ratio [OR], 1.99), occupational exertion (OR, 2.15), or occupational standing and walking around (OR, 1.84) compared with “never” engaging in these activities.
The contrasting effects of leisure-time vs. occupational PA constitute the “physical activity paradox” hypothesis. Starting in 2011, multiple studies by Andreas Holtermann, PhD, of the National Research Centre for the Working Environment lend support to the PA paradox theory, as do subsequent studies by others.
Although only “marginally considered” until a few years ago, recent large cohort studies seem to confirm the paradox, Pier Luigi Temporelli, MD wrote in a recent editorial.
In separate interviews, Dr. Skirbekk and lead author Tyler Quinn, PhD, MS, West Virginia University, Morgantown, pointed to the PA paradox as an explanation for their own recent findings, suggesting that the mechanisms that underlie it probably are responsible for the associated deleterious effects of occupational PA on the brain and heart, and even mortality.
“It’s well established that PA in your leisure time can be positive, but in the workplace, the results are quite the opposite,” Dr. Skirbekk said. “The specific mechanisms for why occupational PA is associated with elevated dementia risk are still not well understood and we need more knowledge. But we know that higher occupational physical demands have been linked to smaller hippocampal volume and poorer memory performance.”
Furthermore, he said, individuals working in jobs with high demands, both psychological and physical, combined with low job control perform more poorly on cognitive testing later in life.
“We looked mainly at professions where people have heavy workloads and you have much less autonomy, such as nursing assistants, office cleaners, childcare workers, and other personal care workers,” he said. “You cannot sit. You have somebody relying on you. It’s not all pleasure, and it can be very hard. That’s where we find the associations.”
Lack of autonomy
Specific characteristics indirectly associated with high occupational PA jobs – low cognitive stimuli, lifestyle factors, and socioeconomic influences – as well as factors directly related to high occupational PA, such as long hours, repetitive tasks, low levels of control, and stress, could also adversely affect cognitive trajectories, Dr. Skirbekk explained.
“By contrast, leisure-time physical activities tend to be of much shorter duration; are associated with socialization, play, [and] positive emotions; and [include] the opportunity to take breaks or shift to other types of activities if one prefers,” he said. “It may also be that too little or too much PA could be adversely related to cognitive outcomes – hence moderate activity levels, for example 10,000 steps a day, are still likely beneficial for cognitive functioning.”
Dr. Quinn said most of the CVD risk linked to occupational PA has to do with long periods of exertion such as lifting and carrying objects. While occupational standing and walking all day are also linked to CVD risk, they’re not as risky as lifting and carrying, he said.
Like Dr. Skirbekk, Dr. Quinn noted that individuals can take a break from leisure-time PA when they are tired, but occupational PA doesn’t have that same autonomy to allow for recovery.
“So, in many cases, individuals are not getting the recovery their body needs to actually experience PA benefits, because those benefits come during rest,” Dr. Quinn said.
“We’ve shown that PA at work raises acute cardiovascular responses, which are related to cardiovascular risk. For example, 24-hour and waking heart rate and diastolic blood pressure, as well as nonwork diastolic blood pressure, all were significantly higher on workdays versus non-workdays,” he said.
Dr. Quinn also said that psychological stress at work amplifies risk. “A person who does PA at work and is stressed is likely to be at greater risk than someone who has a physically active job but doesn’t have psychological stress combined with it.”
Research gaps
However, Dr. Skirbekk noted that there are strategies that can reduce the risk for MCI and dementia despite high levels of occupational PA. “It is often difficult to change professions, and even if you do, it won’t immediately affect cognition. But altering one’s lifestyle is likely to have effects on cognitive development across the life cycle.
“Many clinicians say they always advise lifestyle changes, but nothing happens. But it makes sense to emphasize that these changes – stopping smoking, eating well, getting proper sleep, etc. – affect not only cardiovascular risk but also cognition. And I think clinicians should also take a patient’s occupation into account during any evaluation,” Dr. Skirbekk noted.
Dr. Quinn said it isn’t realistic to expect workers to come up with solutions to the PA paradox because many don’t have the autonomy to be able to mitigate their occupational risk.
“I think administrative controls and policy changes eventually will be the levers of change. We’re not quite there yet, but those are the types of things we should do when we’re trying to reduce loads in some way, or reduce the time that people spend doing certain tasks we know are potentially bad,” he said.
However, not everyone agrees that occupational PA doesn’t confer the same benefits of leisure-time PA, at least with respect to cardiovascular risk. For example, the Prospective Urban Rural Epidemiology (PURE) study, which includes a cohort of 130,000 people from 17 high-income, middle-income, and low-income countries, concluded that both higher recreational and nonrecreational PA were associated with a lower risk for mortality and CVD events.
What additional research is needed to clarify the effects of occupational and leisure-time activity and to address conflicting findings?
“Even studies coming out now regarding the effects of occupational PA have mainly used older data,” Dr. Skirbekk noted. “Labor markets and job demands have changed over time. There are different types of tasks and skills required now than there were 20 or 40 years ago. And of course, working from home is a recent phenomenon that’s happened on a large scale and might affect daily routines, sleep patterns, and also cognition. We need a better understanding of what the consequences might be.”
Health inequity issue
More research is also necessary to understand the social determinants of cognitive decline, impairment, and dementia, he said. “Many of the studies we see today are based on self-report of what someone has done in the past, which is particularly problematic for individuals who are impaired or who give interviews with others, which can induce biases.”
Dr. Quinn suggests that PA guidelines may need to differentiate between occupational and leisure-time PA to better reflect current research findings.
Meanwhile, Dr. Skirbekk and Dr. Quinn both point to the toll that occupational PA takes on the brain and body in lower-income workers as an important health equity issue.
“Our national guidelines for PA include occupational activity,” said Dr. Quinn. “But it’s clear that a lot of people who are getting a lot of occupational PA, particularly socioeconomic and racial/ethnic minorities, are not benefiting from it.”
Dr. Holtermann, who has arguably done the most research to date on the PA paradox, noted in a recent editorial that the majority of workers with high occupational PA have a low socioeconomic position and therefore “improving our understanding of the underlying mechanisms behind the PA health paradox and identifying new intervention targets along those pathways will be an important step to reduce socioeconomic health inequalities across the globe.”
A version of this article first appeared on Medscape.com.
In the pursuit of optimal health, regular physical activity (PA) is recommended to protect against dementia, cardiovascular disease (CVD), cancer, and other noncommunicable diseases. A significant body of research suggests the benefits of PA are positively correlated with higher frequency and intensity – with more often deemed better. This research has spawned a focus on increasing step counts and investing in standing desks and other interventions aimed at keeping people active.
But , but they also actually experience an increased risk for the very conditions that PA is intended to prevent.
A study published recently in The Lancet Regional Health – Europe used registry data from more than 7,000 adults in Norway, following them from age 33 to 65 years, to assess PA trajectories and risks for later-life mild cognitive impairment (MCI) and dementia at age 70 or older.
“Incorporating a life-course perspective gives a broader picture of how participants’ occupational histories relate to cognitive impairment later in life,” principal investigator Vegard Skirbekk, PhD, Columbia University Mailman School of Public Health, New York, said in an interview. Other studies typically have assessed occupational PA at a single time point, often close to the end of an individual’s career, and largely relied on self-report, he said.
Study participants worked in more than 300 different occupations. General physical activities performed on the included jobs required “considerable” use of arms and legs and moving the whole body, such as climbing, lifting, balancing, walking, stooping, and handling of materials.
Dr. Skirbekk and colleagues grouped participants into four PA trajectories over the 44-year study period: stable low, increasing then decreasing, stable intermediate, and stable high.
A total of 902 individuals were diagnosed with dementia and 2,407 with MCI at age 70 years or older. After adjustment, risks for MCI and dementia were 15.5% for those with higher occupational PA scores in the latter part of their working life and 9% for those with lower physical demands. The researchers concluded that “consistently working in an occupation with intermediate or high occupational PA was linked to an increased risk of cognitive impairment.”
The findings support those of the Copenhagen Male Study. Published in 2020, this longitudinal study compared leisure-time and occupational PA among more than 4,000 men in Denmark aged 40-59 at baseline in 1970-1971 and followed them until they turned 60. After adjustment, participants with high occupational PA had a 55% greater risk of developing dementia compared with those doing sedentary work.
Good vs. bad PA
“[T]he WHO [World Health Organization] guide to preventing dementia and disease on the whole mentions physical activity as an important factor. But our study suggests that it must be a ‘good’ form of physical activity, which hard physical work is not,” said Kirsten Nabe-Nielsen, PhD, lead author of this study
Beyond dementia, another recent study adds to a wealth of data on associations between occupational PA and cardiovascular risks. The cross-sectional analysis of U.S. data from the National Institute for Occupational Safety and Health showed that odds of CVD were higher when participants were “always” performing total occupational activity (odds ratio [OR], 1.99), occupational exertion (OR, 2.15), or occupational standing and walking around (OR, 1.84) compared with “never” engaging in these activities.
The contrasting effects of leisure-time vs. occupational PA constitute the “physical activity paradox” hypothesis. Starting in 2011, multiple studies by Andreas Holtermann, PhD, of the National Research Centre for the Working Environment lend support to the PA paradox theory, as do subsequent studies by others.
Although only “marginally considered” until a few years ago, recent large cohort studies seem to confirm the paradox, Pier Luigi Temporelli, MD wrote in a recent editorial.
In separate interviews, Dr. Skirbekk and lead author Tyler Quinn, PhD, MS, West Virginia University, Morgantown, pointed to the PA paradox as an explanation for their own recent findings, suggesting that the mechanisms that underlie it probably are responsible for the associated deleterious effects of occupational PA on the brain and heart, and even mortality.
“It’s well established that PA in your leisure time can be positive, but in the workplace, the results are quite the opposite,” Dr. Skirbekk said. “The specific mechanisms for why occupational PA is associated with elevated dementia risk are still not well understood and we need more knowledge. But we know that higher occupational physical demands have been linked to smaller hippocampal volume and poorer memory performance.”
Furthermore, he said, individuals working in jobs with high demands, both psychological and physical, combined with low job control perform more poorly on cognitive testing later in life.
“We looked mainly at professions where people have heavy workloads and you have much less autonomy, such as nursing assistants, office cleaners, childcare workers, and other personal care workers,” he said. “You cannot sit. You have somebody relying on you. It’s not all pleasure, and it can be very hard. That’s where we find the associations.”
Lack of autonomy
Specific characteristics indirectly associated with high occupational PA jobs – low cognitive stimuli, lifestyle factors, and socioeconomic influences – as well as factors directly related to high occupational PA, such as long hours, repetitive tasks, low levels of control, and stress, could also adversely affect cognitive trajectories, Dr. Skirbekk explained.
“By contrast, leisure-time physical activities tend to be of much shorter duration; are associated with socialization, play, [and] positive emotions; and [include] the opportunity to take breaks or shift to other types of activities if one prefers,” he said. “It may also be that too little or too much PA could be adversely related to cognitive outcomes – hence moderate activity levels, for example 10,000 steps a day, are still likely beneficial for cognitive functioning.”
Dr. Quinn said most of the CVD risk linked to occupational PA has to do with long periods of exertion such as lifting and carrying objects. While occupational standing and walking all day are also linked to CVD risk, they’re not as risky as lifting and carrying, he said.
Like Dr. Skirbekk, Dr. Quinn noted that individuals can take a break from leisure-time PA when they are tired, but occupational PA doesn’t have that same autonomy to allow for recovery.
“So, in many cases, individuals are not getting the recovery their body needs to actually experience PA benefits, because those benefits come during rest,” Dr. Quinn said.
“We’ve shown that PA at work raises acute cardiovascular responses, which are related to cardiovascular risk. For example, 24-hour and waking heart rate and diastolic blood pressure, as well as nonwork diastolic blood pressure, all were significantly higher on workdays versus non-workdays,” he said.
Dr. Quinn also said that psychological stress at work amplifies risk. “A person who does PA at work and is stressed is likely to be at greater risk than someone who has a physically active job but doesn’t have psychological stress combined with it.”
Research gaps
However, Dr. Skirbekk noted that there are strategies that can reduce the risk for MCI and dementia despite high levels of occupational PA. “It is often difficult to change professions, and even if you do, it won’t immediately affect cognition. But altering one’s lifestyle is likely to have effects on cognitive development across the life cycle.
“Many clinicians say they always advise lifestyle changes, but nothing happens. But it makes sense to emphasize that these changes – stopping smoking, eating well, getting proper sleep, etc. – affect not only cardiovascular risk but also cognition. And I think clinicians should also take a patient’s occupation into account during any evaluation,” Dr. Skirbekk noted.
Dr. Quinn said it isn’t realistic to expect workers to come up with solutions to the PA paradox because many don’t have the autonomy to be able to mitigate their occupational risk.
“I think administrative controls and policy changes eventually will be the levers of change. We’re not quite there yet, but those are the types of things we should do when we’re trying to reduce loads in some way, or reduce the time that people spend doing certain tasks we know are potentially bad,” he said.
However, not everyone agrees that occupational PA doesn’t confer the same benefits of leisure-time PA, at least with respect to cardiovascular risk. For example, the Prospective Urban Rural Epidemiology (PURE) study, which includes a cohort of 130,000 people from 17 high-income, middle-income, and low-income countries, concluded that both higher recreational and nonrecreational PA were associated with a lower risk for mortality and CVD events.
What additional research is needed to clarify the effects of occupational and leisure-time activity and to address conflicting findings?
“Even studies coming out now regarding the effects of occupational PA have mainly used older data,” Dr. Skirbekk noted. “Labor markets and job demands have changed over time. There are different types of tasks and skills required now than there were 20 or 40 years ago. And of course, working from home is a recent phenomenon that’s happened on a large scale and might affect daily routines, sleep patterns, and also cognition. We need a better understanding of what the consequences might be.”
Health inequity issue
More research is also necessary to understand the social determinants of cognitive decline, impairment, and dementia, he said. “Many of the studies we see today are based on self-report of what someone has done in the past, which is particularly problematic for individuals who are impaired or who give interviews with others, which can induce biases.”
Dr. Quinn suggests that PA guidelines may need to differentiate between occupational and leisure-time PA to better reflect current research findings.
Meanwhile, Dr. Skirbekk and Dr. Quinn both point to the toll that occupational PA takes on the brain and body in lower-income workers as an important health equity issue.
“Our national guidelines for PA include occupational activity,” said Dr. Quinn. “But it’s clear that a lot of people who are getting a lot of occupational PA, particularly socioeconomic and racial/ethnic minorities, are not benefiting from it.”
Dr. Holtermann, who has arguably done the most research to date on the PA paradox, noted in a recent editorial that the majority of workers with high occupational PA have a low socioeconomic position and therefore “improving our understanding of the underlying mechanisms behind the PA health paradox and identifying new intervention targets along those pathways will be an important step to reduce socioeconomic health inequalities across the globe.”
A version of this article first appeared on Medscape.com.
In the pursuit of optimal health, regular physical activity (PA) is recommended to protect against dementia, cardiovascular disease (CVD), cancer, and other noncommunicable diseases. A significant body of research suggests the benefits of PA are positively correlated with higher frequency and intensity – with more often deemed better. This research has spawned a focus on increasing step counts and investing in standing desks and other interventions aimed at keeping people active.
But , but they also actually experience an increased risk for the very conditions that PA is intended to prevent.
A study published recently in The Lancet Regional Health – Europe used registry data from more than 7,000 adults in Norway, following them from age 33 to 65 years, to assess PA trajectories and risks for later-life mild cognitive impairment (MCI) and dementia at age 70 or older.
“Incorporating a life-course perspective gives a broader picture of how participants’ occupational histories relate to cognitive impairment later in life,” principal investigator Vegard Skirbekk, PhD, Columbia University Mailman School of Public Health, New York, said in an interview. Other studies typically have assessed occupational PA at a single time point, often close to the end of an individual’s career, and largely relied on self-report, he said.
Study participants worked in more than 300 different occupations. General physical activities performed on the included jobs required “considerable” use of arms and legs and moving the whole body, such as climbing, lifting, balancing, walking, stooping, and handling of materials.
Dr. Skirbekk and colleagues grouped participants into four PA trajectories over the 44-year study period: stable low, increasing then decreasing, stable intermediate, and stable high.
A total of 902 individuals were diagnosed with dementia and 2,407 with MCI at age 70 years or older. After adjustment, risks for MCI and dementia were 15.5% for those with higher occupational PA scores in the latter part of their working life and 9% for those with lower physical demands. The researchers concluded that “consistently working in an occupation with intermediate or high occupational PA was linked to an increased risk of cognitive impairment.”
The findings support those of the Copenhagen Male Study. Published in 2020, this longitudinal study compared leisure-time and occupational PA among more than 4,000 men in Denmark aged 40-59 at baseline in 1970-1971 and followed them until they turned 60. After adjustment, participants with high occupational PA had a 55% greater risk of developing dementia compared with those doing sedentary work.
Good vs. bad PA
“[T]he WHO [World Health Organization] guide to preventing dementia and disease on the whole mentions physical activity as an important factor. But our study suggests that it must be a ‘good’ form of physical activity, which hard physical work is not,” said Kirsten Nabe-Nielsen, PhD, lead author of this study
Beyond dementia, another recent study adds to a wealth of data on associations between occupational PA and cardiovascular risks. The cross-sectional analysis of U.S. data from the National Institute for Occupational Safety and Health showed that odds of CVD were higher when participants were “always” performing total occupational activity (odds ratio [OR], 1.99), occupational exertion (OR, 2.15), or occupational standing and walking around (OR, 1.84) compared with “never” engaging in these activities.
The contrasting effects of leisure-time vs. occupational PA constitute the “physical activity paradox” hypothesis. Starting in 2011, multiple studies by Andreas Holtermann, PhD, of the National Research Centre for the Working Environment lend support to the PA paradox theory, as do subsequent studies by others.
Although only “marginally considered” until a few years ago, recent large cohort studies seem to confirm the paradox, Pier Luigi Temporelli, MD wrote in a recent editorial.
In separate interviews, Dr. Skirbekk and lead author Tyler Quinn, PhD, MS, West Virginia University, Morgantown, pointed to the PA paradox as an explanation for their own recent findings, suggesting that the mechanisms that underlie it probably are responsible for the associated deleterious effects of occupational PA on the brain and heart, and even mortality.
“It’s well established that PA in your leisure time can be positive, but in the workplace, the results are quite the opposite,” Dr. Skirbekk said. “The specific mechanisms for why occupational PA is associated with elevated dementia risk are still not well understood and we need more knowledge. But we know that higher occupational physical demands have been linked to smaller hippocampal volume and poorer memory performance.”
Furthermore, he said, individuals working in jobs with high demands, both psychological and physical, combined with low job control perform more poorly on cognitive testing later in life.
“We looked mainly at professions where people have heavy workloads and you have much less autonomy, such as nursing assistants, office cleaners, childcare workers, and other personal care workers,” he said. “You cannot sit. You have somebody relying on you. It’s not all pleasure, and it can be very hard. That’s where we find the associations.”
Lack of autonomy
Specific characteristics indirectly associated with high occupational PA jobs – low cognitive stimuli, lifestyle factors, and socioeconomic influences – as well as factors directly related to high occupational PA, such as long hours, repetitive tasks, low levels of control, and stress, could also adversely affect cognitive trajectories, Dr. Skirbekk explained.
“By contrast, leisure-time physical activities tend to be of much shorter duration; are associated with socialization, play, [and] positive emotions; and [include] the opportunity to take breaks or shift to other types of activities if one prefers,” he said. “It may also be that too little or too much PA could be adversely related to cognitive outcomes – hence moderate activity levels, for example 10,000 steps a day, are still likely beneficial for cognitive functioning.”
Dr. Quinn said most of the CVD risk linked to occupational PA has to do with long periods of exertion such as lifting and carrying objects. While occupational standing and walking all day are also linked to CVD risk, they’re not as risky as lifting and carrying, he said.
Like Dr. Skirbekk, Dr. Quinn noted that individuals can take a break from leisure-time PA when they are tired, but occupational PA doesn’t have that same autonomy to allow for recovery.
“So, in many cases, individuals are not getting the recovery their body needs to actually experience PA benefits, because those benefits come during rest,” Dr. Quinn said.
“We’ve shown that PA at work raises acute cardiovascular responses, which are related to cardiovascular risk. For example, 24-hour and waking heart rate and diastolic blood pressure, as well as nonwork diastolic blood pressure, all were significantly higher on workdays versus non-workdays,” he said.
Dr. Quinn also said that psychological stress at work amplifies risk. “A person who does PA at work and is stressed is likely to be at greater risk than someone who has a physically active job but doesn’t have psychological stress combined with it.”
Research gaps
However, Dr. Skirbekk noted that there are strategies that can reduce the risk for MCI and dementia despite high levels of occupational PA. “It is often difficult to change professions, and even if you do, it won’t immediately affect cognition. But altering one’s lifestyle is likely to have effects on cognitive development across the life cycle.
“Many clinicians say they always advise lifestyle changes, but nothing happens. But it makes sense to emphasize that these changes – stopping smoking, eating well, getting proper sleep, etc. – affect not only cardiovascular risk but also cognition. And I think clinicians should also take a patient’s occupation into account during any evaluation,” Dr. Skirbekk noted.
Dr. Quinn said it isn’t realistic to expect workers to come up with solutions to the PA paradox because many don’t have the autonomy to be able to mitigate their occupational risk.
“I think administrative controls and policy changes eventually will be the levers of change. We’re not quite there yet, but those are the types of things we should do when we’re trying to reduce loads in some way, or reduce the time that people spend doing certain tasks we know are potentially bad,” he said.
However, not everyone agrees that occupational PA doesn’t confer the same benefits of leisure-time PA, at least with respect to cardiovascular risk. For example, the Prospective Urban Rural Epidemiology (PURE) study, which includes a cohort of 130,000 people from 17 high-income, middle-income, and low-income countries, concluded that both higher recreational and nonrecreational PA were associated with a lower risk for mortality and CVD events.
What additional research is needed to clarify the effects of occupational and leisure-time activity and to address conflicting findings?
“Even studies coming out now regarding the effects of occupational PA have mainly used older data,” Dr. Skirbekk noted. “Labor markets and job demands have changed over time. There are different types of tasks and skills required now than there were 20 or 40 years ago. And of course, working from home is a recent phenomenon that’s happened on a large scale and might affect daily routines, sleep patterns, and also cognition. We need a better understanding of what the consequences might be.”
Health inequity issue
More research is also necessary to understand the social determinants of cognitive decline, impairment, and dementia, he said. “Many of the studies we see today are based on self-report of what someone has done in the past, which is particularly problematic for individuals who are impaired or who give interviews with others, which can induce biases.”
Dr. Quinn suggests that PA guidelines may need to differentiate between occupational and leisure-time PA to better reflect current research findings.
Meanwhile, Dr. Skirbekk and Dr. Quinn both point to the toll that occupational PA takes on the brain and body in lower-income workers as an important health equity issue.
“Our national guidelines for PA include occupational activity,” said Dr. Quinn. “But it’s clear that a lot of people who are getting a lot of occupational PA, particularly socioeconomic and racial/ethnic minorities, are not benefiting from it.”
Dr. Holtermann, who has arguably done the most research to date on the PA paradox, noted in a recent editorial that the majority of workers with high occupational PA have a low socioeconomic position and therefore “improving our understanding of the underlying mechanisms behind the PA health paradox and identifying new intervention targets along those pathways will be an important step to reduce socioeconomic health inequalities across the globe.”
A version of this article first appeared on Medscape.com.
The Lancet Regional Health – Europe
Alzheimer’s blood test coming within 5 years, UK group pledges
Alzheimer’s Research UK, the Alzheimer’s Society, and the National Institute for Health and Care Research (NIHR) are collaborating and leading AD researchers to bring a diagnostic blood test to the UK’s National Health Service (NHS).
“Dementia affects around 900,000 people in the UK today, and that number is expected to rise to 1.4 million by 2040. It is the UK’s biggest killer,” Fiona Carragher, with the Alzheimer’s Society, said during a media briefing announcing the project.
Yet, many people face a very long wait of up to 2-4 years to get a dementia diagnosis, and many cases remain undiagnosed, she noted.
A chief reason is lack of access to specialized diagnostic testing. Currently, only 2% of people in the United Kingdom have access to advanced diagnostic tests such as PET scans and lumbar punctures owing to limited availability.
“Getting an early and accurate diagnosis is the pivotal first step to getting help today and unlocking hope for the future” and blood biomarkers provide a “real opportunity to disrupt the diagnostic paradigm,” Ms. Carragher said. It also offers greater opportunities to participate in research and clinical trials, she added.
Attitude shift
Susan Kohlhaas, PhD, with Alzheimer’s Research UK, noted that attitudes toward dementia diagnosis have changed in the past few years. The days when people may have not wanted to know if they have dementia are gone.
Data from the latest wave of the Alzheimer’s Research UK Dementia Attitudes Monitor survey show that 9 in 10 people would seek a diagnosis from their provider. “That’s been driven by awareness of treatments and things that people can proactively do to try and slow disease progression,” Dr. Kohlhaas said.
“As new treatments for dementia become available there will to be a surge in people seeking a diagnosis. At the moment, we don’t have adequate infrastructure to cope with that demand,” Dr. Kohlhaas added.
She noted that blood tests are starting to show their potential as an effective part of the diagnosis and are widely used in research.
“In some cases, they are similar in sensitivity to gold-standard PET scans and lumbar punctures, and they’re less expensive and potentially more scalable on the NHS. What we need to do over the next several years is to understand how they fit into the clinical pathway,” Dr. Kohlhaas said.
The project will involve working with leading dementia researchers to pilot the implementation of potential blood tests in the NHS that can give an early and accurate diagnose of dementia.
The project, which kicks off in January 2024, will receive £5 million ($6.13 million) awarded by the UK Postcode Dream Fund. Specific details regarding the leadership team, participating centers, and specific blood biomarker tests to be trialed will be announced then.
Ms. Carragher and Dr. Kohlhaas reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
Alzheimer’s Research UK, the Alzheimer’s Society, and the National Institute for Health and Care Research (NIHR) are collaborating and leading AD researchers to bring a diagnostic blood test to the UK’s National Health Service (NHS).
“Dementia affects around 900,000 people in the UK today, and that number is expected to rise to 1.4 million by 2040. It is the UK’s biggest killer,” Fiona Carragher, with the Alzheimer’s Society, said during a media briefing announcing the project.
Yet, many people face a very long wait of up to 2-4 years to get a dementia diagnosis, and many cases remain undiagnosed, she noted.
A chief reason is lack of access to specialized diagnostic testing. Currently, only 2% of people in the United Kingdom have access to advanced diagnostic tests such as PET scans and lumbar punctures owing to limited availability.
“Getting an early and accurate diagnosis is the pivotal first step to getting help today and unlocking hope for the future” and blood biomarkers provide a “real opportunity to disrupt the diagnostic paradigm,” Ms. Carragher said. It also offers greater opportunities to participate in research and clinical trials, she added.
Attitude shift
Susan Kohlhaas, PhD, with Alzheimer’s Research UK, noted that attitudes toward dementia diagnosis have changed in the past few years. The days when people may have not wanted to know if they have dementia are gone.
Data from the latest wave of the Alzheimer’s Research UK Dementia Attitudes Monitor survey show that 9 in 10 people would seek a diagnosis from their provider. “That’s been driven by awareness of treatments and things that people can proactively do to try and slow disease progression,” Dr. Kohlhaas said.
“As new treatments for dementia become available there will to be a surge in people seeking a diagnosis. At the moment, we don’t have adequate infrastructure to cope with that demand,” Dr. Kohlhaas added.
She noted that blood tests are starting to show their potential as an effective part of the diagnosis and are widely used in research.
“In some cases, they are similar in sensitivity to gold-standard PET scans and lumbar punctures, and they’re less expensive and potentially more scalable on the NHS. What we need to do over the next several years is to understand how they fit into the clinical pathway,” Dr. Kohlhaas said.
The project will involve working with leading dementia researchers to pilot the implementation of potential blood tests in the NHS that can give an early and accurate diagnose of dementia.
The project, which kicks off in January 2024, will receive £5 million ($6.13 million) awarded by the UK Postcode Dream Fund. Specific details regarding the leadership team, participating centers, and specific blood biomarker tests to be trialed will be announced then.
Ms. Carragher and Dr. Kohlhaas reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
Alzheimer’s Research UK, the Alzheimer’s Society, and the National Institute for Health and Care Research (NIHR) are collaborating and leading AD researchers to bring a diagnostic blood test to the UK’s National Health Service (NHS).
“Dementia affects around 900,000 people in the UK today, and that number is expected to rise to 1.4 million by 2040. It is the UK’s biggest killer,” Fiona Carragher, with the Alzheimer’s Society, said during a media briefing announcing the project.
Yet, many people face a very long wait of up to 2-4 years to get a dementia diagnosis, and many cases remain undiagnosed, she noted.
A chief reason is lack of access to specialized diagnostic testing. Currently, only 2% of people in the United Kingdom have access to advanced diagnostic tests such as PET scans and lumbar punctures owing to limited availability.
“Getting an early and accurate diagnosis is the pivotal first step to getting help today and unlocking hope for the future” and blood biomarkers provide a “real opportunity to disrupt the diagnostic paradigm,” Ms. Carragher said. It also offers greater opportunities to participate in research and clinical trials, she added.
Attitude shift
Susan Kohlhaas, PhD, with Alzheimer’s Research UK, noted that attitudes toward dementia diagnosis have changed in the past few years. The days when people may have not wanted to know if they have dementia are gone.
Data from the latest wave of the Alzheimer’s Research UK Dementia Attitudes Monitor survey show that 9 in 10 people would seek a diagnosis from their provider. “That’s been driven by awareness of treatments and things that people can proactively do to try and slow disease progression,” Dr. Kohlhaas said.
“As new treatments for dementia become available there will to be a surge in people seeking a diagnosis. At the moment, we don’t have adequate infrastructure to cope with that demand,” Dr. Kohlhaas added.
She noted that blood tests are starting to show their potential as an effective part of the diagnosis and are widely used in research.
“In some cases, they are similar in sensitivity to gold-standard PET scans and lumbar punctures, and they’re less expensive and potentially more scalable on the NHS. What we need to do over the next several years is to understand how they fit into the clinical pathway,” Dr. Kohlhaas said.
The project will involve working with leading dementia researchers to pilot the implementation of potential blood tests in the NHS that can give an early and accurate diagnose of dementia.
The project, which kicks off in January 2024, will receive £5 million ($6.13 million) awarded by the UK Postcode Dream Fund. Specific details regarding the leadership team, participating centers, and specific blood biomarker tests to be trialed will be announced then.
Ms. Carragher and Dr. Kohlhaas reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
Military service linked to Alzheimer’s neuropathology
TOPLINE:
METHODOLOGY:
- The study included 597 male decedents who donated their brains to one of two Alzheimer’s Disease Research Center (ADRC) brain bank programs between 1986 and 2018.
- Researchers conducted public data tracing for historical information on military history, which included searching online commercial genealogical databases and paper archives.
- They evaluated tau tangles (using a B score of neurofibrillary tangle deposition in four stages: B0 [not present], B1 [transentorhinal stages], B2 [limbic stages], and B3 [isocortical stages]) and amyloid plaque pathology (using a C score that classifies neuritic amyloid plaque into four categories: no plaques, sparse, moderate, or frequent).
- The study involved three B score comparisons (1, 2, 3 vs. 0; 2, 3 vs. 0, 1; and 3 vs. 0, 1, 2) and two C score comparisons (sparse, moderate, or frequent vs. no plaques, and moderate or frequent vs. no plaque or sparse).
TAKEAWAY:
- Public record tracing determined that 60% of the sample of male decedents had a history of military service; the median year of birth was 1923 and the median year of death was 2007.
- After adjustment for age and year of death, those with a military service history had a 26% increased risk for a higher neuritic amyloid plaque C score compared with those without such history (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.06-1.49), an increase that applied for both relevant comparisons.
- A history of military service was also associated with a 10% greater adjusted odds of a higher neurofibrillary tangle B score (OR, 1.10; 95% CI, 1.08-1.12), with the increase applying to all three comparisons.
- A sensitivity analysis that included both the male decedents and 556 female decedents (increasing the overall sample to 1,153) and was adjusted for sex in addition to age and year of death showed similar results to the male-only sample estimations for both B and C score comparisons.
IN PRACTICE:
Understanding how military service affects AD biological processes is “essential” from a research perspective, the investigators noted. These new findings “emphasize that targeted AD therapies in the veteran population are urgently needed.”
SOURCE:
The study was conducted by W. Ryan Powell, Center for Health Disparities Research and Department of Medicine, Geriatrics Division, University of Wisconsin School of Medicine and Public Health, and colleagues. It was published online in Alzheimer’s & Dementia.
LIMITATIONS:
Selection bias in brain donation is likely because ADRC cohorts are recruitment based. The study was unable to rigorously identify factors that may explain why individuals with military service are at greater risk of having amyloid and tau neuropathology (including the interplay between environmental and genetic risk factors such as apolipoprotein E status).
DISCLOSURES:
The study was supported by the National Institute on Aging. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The study included 597 male decedents who donated their brains to one of two Alzheimer’s Disease Research Center (ADRC) brain bank programs between 1986 and 2018.
- Researchers conducted public data tracing for historical information on military history, which included searching online commercial genealogical databases and paper archives.
- They evaluated tau tangles (using a B score of neurofibrillary tangle deposition in four stages: B0 [not present], B1 [transentorhinal stages], B2 [limbic stages], and B3 [isocortical stages]) and amyloid plaque pathology (using a C score that classifies neuritic amyloid plaque into four categories: no plaques, sparse, moderate, or frequent).
- The study involved three B score comparisons (1, 2, 3 vs. 0; 2, 3 vs. 0, 1; and 3 vs. 0, 1, 2) and two C score comparisons (sparse, moderate, or frequent vs. no plaques, and moderate or frequent vs. no plaque or sparse).
TAKEAWAY:
- Public record tracing determined that 60% of the sample of male decedents had a history of military service; the median year of birth was 1923 and the median year of death was 2007.
- After adjustment for age and year of death, those with a military service history had a 26% increased risk for a higher neuritic amyloid plaque C score compared with those without such history (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.06-1.49), an increase that applied for both relevant comparisons.
- A history of military service was also associated with a 10% greater adjusted odds of a higher neurofibrillary tangle B score (OR, 1.10; 95% CI, 1.08-1.12), with the increase applying to all three comparisons.
- A sensitivity analysis that included both the male decedents and 556 female decedents (increasing the overall sample to 1,153) and was adjusted for sex in addition to age and year of death showed similar results to the male-only sample estimations for both B and C score comparisons.
IN PRACTICE:
Understanding how military service affects AD biological processes is “essential” from a research perspective, the investigators noted. These new findings “emphasize that targeted AD therapies in the veteran population are urgently needed.”
SOURCE:
The study was conducted by W. Ryan Powell, Center for Health Disparities Research and Department of Medicine, Geriatrics Division, University of Wisconsin School of Medicine and Public Health, and colleagues. It was published online in Alzheimer’s & Dementia.
LIMITATIONS:
Selection bias in brain donation is likely because ADRC cohorts are recruitment based. The study was unable to rigorously identify factors that may explain why individuals with military service are at greater risk of having amyloid and tau neuropathology (including the interplay between environmental and genetic risk factors such as apolipoprotein E status).
DISCLOSURES:
The study was supported by the National Institute on Aging. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The study included 597 male decedents who donated their brains to one of two Alzheimer’s Disease Research Center (ADRC) brain bank programs between 1986 and 2018.
- Researchers conducted public data tracing for historical information on military history, which included searching online commercial genealogical databases and paper archives.
- They evaluated tau tangles (using a B score of neurofibrillary tangle deposition in four stages: B0 [not present], B1 [transentorhinal stages], B2 [limbic stages], and B3 [isocortical stages]) and amyloid plaque pathology (using a C score that classifies neuritic amyloid plaque into four categories: no plaques, sparse, moderate, or frequent).
- The study involved three B score comparisons (1, 2, 3 vs. 0; 2, 3 vs. 0, 1; and 3 vs. 0, 1, 2) and two C score comparisons (sparse, moderate, or frequent vs. no plaques, and moderate or frequent vs. no plaque or sparse).
TAKEAWAY:
- Public record tracing determined that 60% of the sample of male decedents had a history of military service; the median year of birth was 1923 and the median year of death was 2007.
- After adjustment for age and year of death, those with a military service history had a 26% increased risk for a higher neuritic amyloid plaque C score compared with those without such history (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.06-1.49), an increase that applied for both relevant comparisons.
- A history of military service was also associated with a 10% greater adjusted odds of a higher neurofibrillary tangle B score (OR, 1.10; 95% CI, 1.08-1.12), with the increase applying to all three comparisons.
- A sensitivity analysis that included both the male decedents and 556 female decedents (increasing the overall sample to 1,153) and was adjusted for sex in addition to age and year of death showed similar results to the male-only sample estimations for both B and C score comparisons.
IN PRACTICE:
Understanding how military service affects AD biological processes is “essential” from a research perspective, the investigators noted. These new findings “emphasize that targeted AD therapies in the veteran population are urgently needed.”
SOURCE:
The study was conducted by W. Ryan Powell, Center for Health Disparities Research and Department of Medicine, Geriatrics Division, University of Wisconsin School of Medicine and Public Health, and colleagues. It was published online in Alzheimer’s & Dementia.
LIMITATIONS:
Selection bias in brain donation is likely because ADRC cohorts are recruitment based. The study was unable to rigorously identify factors that may explain why individuals with military service are at greater risk of having amyloid and tau neuropathology (including the interplay between environmental and genetic risk factors such as apolipoprotein E status).
DISCLOSURES:
The study was supported by the National Institute on Aging. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
Pandemic tied to a 50% drop in memory, executive function in older adults
TOPLINE:
This was attributed to an increase in known dementia risk factors, including increased alcohol use and a more sedentary lifestyle. This trend persisted into the second year of the pandemic, after social restrictions had eased.
METHODOLOGY:
- In total, 3,140 participants (54% women; mean age, 68 years) in the PROTECT study, a longitudinal aging study in the United Kingdom, completed annual cognitive assessments and self-reported questionnaires related to mental health and lifestyle.
- Investigators analyzed cognition across three time periods: during the year before the pandemic (March 2019 to February 2020), during pandemic year 1 (March 2020 to February 2021), and pandemic year 2 (March 2021 to February 2022).
- Investigators conducted a subanalysis on those with mild cognitive impairment and those with a history of COVID-19 (n = 752).
TAKEAWAY:
- During the first year of the pandemic, when there were societal lockdowns totaling 6 months, significant worsening of executive function and working memory was seen across the entire cohort (effect sizes, 0.15 and 0.51, respectively), in people with mild cognitive impairment (effect sizes, 0.13 and 0.40, respectively), and in those with a previous history of COVID-19 (effect sizes, 0.24 and 0.46, respectively).
- Worsening of working memory was sustained across the whole cohort in the second year of the pandemic after lockdowns were lifted (effect size, 0.47).
- Even after investigators removed data on people with mild cognitive impairment and COVID-19, the decline in executive function (effect size, 0.15; P < .0001) and working memory (effect size, 0.53; P < .0001) persisted.
- Cognitive decline was significantly associated with known risk factors for dementia, such as reduced exercise (P = .0049) and increased alcohol use (P = .049), across the whole cohort, as well as depression (P = .011) in those with a history of COVID-19 and loneliness (P = .0038) in those with mild cognitive impairment.
IN PRACTICE:
Investigators noted that these data add to existing knowledge of long-standing health consequences of COVID-19, especially for older people with memory problems. “On the positive note, there is evidence that lifestyle changes and improved health management can positively influence mental functioning,” study coauthor Dag Aarsland, MD, PhD, professor of old age psychiatry at the Institute of Psychiatry, Psychology & Neuroscience of King’s College London, said in a press release. “The current study underlines the importance of careful monitoring of people at risk during major events such as the pandemic.”
SOURCE:
The study was led by Anne Corbett, PhD, of University of Exeter, and was published online in The Lancet Healthy Longevity. The research was funded by the National Institute for Health and Care Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR Exeter Biomedical Research Centre.
LIMITATIONS:
The study relied on self-reported data. In addition, the PROTECT cohort is self-selected and may skew toward participants with higher education levels.
DISCLOSURES:
Dr. Corbett reported receiving funding from the NIHR and grants from Synexus, reMYND, and Novo Nordisk. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
This was attributed to an increase in known dementia risk factors, including increased alcohol use and a more sedentary lifestyle. This trend persisted into the second year of the pandemic, after social restrictions had eased.
METHODOLOGY:
- In total, 3,140 participants (54% women; mean age, 68 years) in the PROTECT study, a longitudinal aging study in the United Kingdom, completed annual cognitive assessments and self-reported questionnaires related to mental health and lifestyle.
- Investigators analyzed cognition across three time periods: during the year before the pandemic (March 2019 to February 2020), during pandemic year 1 (March 2020 to February 2021), and pandemic year 2 (March 2021 to February 2022).
- Investigators conducted a subanalysis on those with mild cognitive impairment and those with a history of COVID-19 (n = 752).
TAKEAWAY:
- During the first year of the pandemic, when there were societal lockdowns totaling 6 months, significant worsening of executive function and working memory was seen across the entire cohort (effect sizes, 0.15 and 0.51, respectively), in people with mild cognitive impairment (effect sizes, 0.13 and 0.40, respectively), and in those with a previous history of COVID-19 (effect sizes, 0.24 and 0.46, respectively).
- Worsening of working memory was sustained across the whole cohort in the second year of the pandemic after lockdowns were lifted (effect size, 0.47).
- Even after investigators removed data on people with mild cognitive impairment and COVID-19, the decline in executive function (effect size, 0.15; P < .0001) and working memory (effect size, 0.53; P < .0001) persisted.
- Cognitive decline was significantly associated with known risk factors for dementia, such as reduced exercise (P = .0049) and increased alcohol use (P = .049), across the whole cohort, as well as depression (P = .011) in those with a history of COVID-19 and loneliness (P = .0038) in those with mild cognitive impairment.
IN PRACTICE:
Investigators noted that these data add to existing knowledge of long-standing health consequences of COVID-19, especially for older people with memory problems. “On the positive note, there is evidence that lifestyle changes and improved health management can positively influence mental functioning,” study coauthor Dag Aarsland, MD, PhD, professor of old age psychiatry at the Institute of Psychiatry, Psychology & Neuroscience of King’s College London, said in a press release. “The current study underlines the importance of careful monitoring of people at risk during major events such as the pandemic.”
SOURCE:
The study was led by Anne Corbett, PhD, of University of Exeter, and was published online in The Lancet Healthy Longevity. The research was funded by the National Institute for Health and Care Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR Exeter Biomedical Research Centre.
LIMITATIONS:
The study relied on self-reported data. In addition, the PROTECT cohort is self-selected and may skew toward participants with higher education levels.
DISCLOSURES:
Dr. Corbett reported receiving funding from the NIHR and grants from Synexus, reMYND, and Novo Nordisk. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
This was attributed to an increase in known dementia risk factors, including increased alcohol use and a more sedentary lifestyle. This trend persisted into the second year of the pandemic, after social restrictions had eased.
METHODOLOGY:
- In total, 3,140 participants (54% women; mean age, 68 years) in the PROTECT study, a longitudinal aging study in the United Kingdom, completed annual cognitive assessments and self-reported questionnaires related to mental health and lifestyle.
- Investigators analyzed cognition across three time periods: during the year before the pandemic (March 2019 to February 2020), during pandemic year 1 (March 2020 to February 2021), and pandemic year 2 (March 2021 to February 2022).
- Investigators conducted a subanalysis on those with mild cognitive impairment and those with a history of COVID-19 (n = 752).
TAKEAWAY:
- During the first year of the pandemic, when there were societal lockdowns totaling 6 months, significant worsening of executive function and working memory was seen across the entire cohort (effect sizes, 0.15 and 0.51, respectively), in people with mild cognitive impairment (effect sizes, 0.13 and 0.40, respectively), and in those with a previous history of COVID-19 (effect sizes, 0.24 and 0.46, respectively).
- Worsening of working memory was sustained across the whole cohort in the second year of the pandemic after lockdowns were lifted (effect size, 0.47).
- Even after investigators removed data on people with mild cognitive impairment and COVID-19, the decline in executive function (effect size, 0.15; P < .0001) and working memory (effect size, 0.53; P < .0001) persisted.
- Cognitive decline was significantly associated with known risk factors for dementia, such as reduced exercise (P = .0049) and increased alcohol use (P = .049), across the whole cohort, as well as depression (P = .011) in those with a history of COVID-19 and loneliness (P = .0038) in those with mild cognitive impairment.
IN PRACTICE:
Investigators noted that these data add to existing knowledge of long-standing health consequences of COVID-19, especially for older people with memory problems. “On the positive note, there is evidence that lifestyle changes and improved health management can positively influence mental functioning,” study coauthor Dag Aarsland, MD, PhD, professor of old age psychiatry at the Institute of Psychiatry, Psychology & Neuroscience of King’s College London, said in a press release. “The current study underlines the importance of careful monitoring of people at risk during major events such as the pandemic.”
SOURCE:
The study was led by Anne Corbett, PhD, of University of Exeter, and was published online in The Lancet Healthy Longevity. The research was funded by the National Institute for Health and Care Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR Exeter Biomedical Research Centre.
LIMITATIONS:
The study relied on self-reported data. In addition, the PROTECT cohort is self-selected and may skew toward participants with higher education levels.
DISCLOSURES:
Dr. Corbett reported receiving funding from the NIHR and grants from Synexus, reMYND, and Novo Nordisk. Other disclosures are noted in the original article.
A version of this article appeared on Medscape.com.
Is most Parkinson’s disease man-made and therefore preventable?
This transcript has been edited for clarity.
Indu Subramanian, MD: It’s my pleasure to have Ray Dorsey on our program today. Ray is a professor of neurology at the University of Rochester and has been doing some amazing advocacy work in largely the space of trying to end Parkinson’s disease.
E. Ray Dorsey, MD: Thanks very much for having me, Indu. I’m delighted to be with you.
Trichloroethylene and PD
Dr. Subramanian: I wanted to first that we talk about as something that is in pretty much everywhere. This paper came out, and you wrote a commentary in JAMA Neurology as well. Perhaps we can summarize the paper and its findings.
Dr. Dorsey: Like most people, I didn’t know what TCE was until about 5 or 6 years ago. TCE is a very simple molecule. It’s got six atoms – two carbon atoms, one hydrogen atom, and three chlorine atoms — hence, its name “trichloroethylene.” There’s a very similar chemical called perchloroethylene, which is widely used in dry cleaning. It’s got one additional chlorine atom, and the prefix “per-” means “four.” I’ll talk about TCE predominantly, but both of these chemicals probably have similar toxicity with respect to Parkinson’s disease.
Research done by Dr. Carlie Tanner and Dr. Sam Goldman about a decade ago showed that in twins who were exposed to this through their work (it’s widely used as a degreasing agent) or hobbies (it’s used in printing and painting, by varnish workers, or by anyone that needs it as a solvent) had a 500% increased risk of developing Parkinson’s disease. Importantly, in that study, they showed that there was a lag time of 10-40 years between exposure to that chemical and the diagnosis of the disease. Because TCE was so widely used, they said that public health implications could be substantial.
What’s Camp Lejeune? Camp Lejeune is a Marine base in North Carolina where many Marines are trained. Between 1953 and 1987 at that Marine base, the drinking water was contaminated with TCE, perchloroethylene, and other toxic chemicals. The reason Camp Lejeune is so infamous is because the Marines knew about the contamination for many years and covered it up.
Indeed, this story only came to the forefront because Jennie Ensminger, the daughter of a Marine drill instructor, developed leukemia at age 6 and died at age 9. Her father, Jerry Ensminger, a retired master sergeant, found out after the fact that these cancer-causing chemicals, including TCE, a known carcinogen, were found at the Marine base and could be an explanation for why his daughter developed and died of leukemia.
Dr. Sam Goldman and Dr. Carlie Tanner and colleagues from UCSF looked at the rates of Parkinson’s among Marines who served at Camp Lejeune during the 1970s and compared that with rates in Marines who served Camp Pendleton on the West Coast. It turned out that the Marines who served at Camp Lejeune had a 70% higher risk of developing Parkinson’s disease than the Marines who served at Camp Pendleton.
Importantly, these Marines, by definition, were healthy. They were young. They were only 20 years old, on average, when they were at Camp Lejeune. They stayed at a Marine base for a short period of time, so on average, they were only there for 2 years. Yet 30 years later, they had a 70% increased risk of developing Parkinson’s disease.
Ending Parkinson’s disease
Dr. Subramanian: Wow, that’s pretty profound. You’ve done a large amount of work, and in fact you, along with some of our colleagues wrote a book about ending Parkinson’s disease. I read that book when it came out a couple of years ago, and I was really struck by a few things. Parkinson’s has doubled in the past 40 years and is going to double again in the next 20 years. Can you tell me a little bit about that statistic and why that is? It’s not just because people are aging. What is the sense of that? How do we interpret that?
Dr. Dorsey: According to the Global Burden of Disease study, which I was fortunate to be part of, the number of people with Parkinson’s disease has more than doubled in the past 25 years. A conservative projection based on aging alone suggests that it’s going to double again unless we change something about it. It’s now the world’s fastest-growing brain disease, and it is growing faster than can be explained by aging alone.
If you look at the map of Parkinson’s disease, if you thought it was purely genetic, you would have a relatively uniform map of rates of Parkinson’s disease. In fact, we don’t see that. Rates of Parkinson’s are five times higher in industrialized parts of the world, like the United States and Canada, than they are in sub-Saharan Africa. Rates of Parkinson’s disease are increasing most rapidly in areas of world that are undergoing the most rapid industrialization, such as India and China, where adjusted for age, the rates of Parkinson’s have more than doubled in the past 25 years.
The thesis of our book is that much of Parkinson’s disease is man-made. Work done by your colleagues at UCLA, including Jeff Bronstein and Beate Ritz, have demonstrated that air pollution and certain pesticides are likely fueling the rise of Parkinson’s disease.
Given that in the United States, rates of Parkinson’s disease are actually higher in urban and suburban areas than they are in rural areas, I think that this dry-cleaning chemical – which was widely used in the 1970s in everything from typewriter correction fluid to decaffeinated coffee and [over] 2 pounds per American [was produced] – could be one of the most important causes or contributing factors to Parkinson’s disease.
What to tell patients
Dr. Subramanian: For the general neurologists or practitioners out there watching this, what can they do? If you have a patient whom you suspect may have been exposed to toxins, what should we tell people who aren’t patients yet who are at risk? What are some things that you think would be helpful?
Dr. Dorsey: I think one of the shortcomings of American medicine is that we often just go from diagnosis to treatment. You’re depressed, you get an antidepressant; you have Parkinson’s disease, you get levodopa; you have seizures, you get put on an antiepileptic medication.
I think we need to spend a couple of minutes at least, maybe at the beginning, to go to the diagnosis of the condition and why you have this disease. If you just do a brief occupational history, after you start the exam – things like finding out what people do for a living or did for a living or how they spend their time – I think you’ll find many of these risk factors are actually present.
It’s pretty easy to identify whether people grew up in a rural area and drank well water, which is prone to be contaminated with pesticides. We know that people who drink [contaminated] well water have about a 75% increased risk of developing Parkinson’s disease. I think you can find for people, especially when they grew up, when they were young, that the most relevant exposure might be that when people were young children.
It’s a little bit harder to identify all exposure to TCE. The Marines at Camp Lejeune didn’t know they were drinking the water that was contaminated with this and only found out about it after the fact because Jerry Ensminger launched a 26-year campaign to bring justice for the Marines and their dependents.
Some people who know that they work with chemicals or with solvents might know about this. In New York City, these chemicals are widely used in dry cleaning. They’re readily volatile. These chemicals can evaporate from dry-cleaning buildings and go into the indoor air of apartments above dry cleaners, for example, in New York City. That can be in toxic levels. These readily dissolve in fat, hence their use in degreasing.
There have been studies, for example, in Germany, that found that supermarkets that are simply near a dry cleaner will have TCE or perchloroethylene in the butter and the cheese that they’re selling.
It gets even worse. For example, you bring your daughter into the dry-cleaning building and she’s eating an ice cream cone. When she leaves, she’s eating perchloroethylene and TCE.
It’s a little bit harder to find it, but I think it’s relevant because some people might be still being exposed and some people might still be drinking well water and they rarely have their well tested. For those people, I recommend they get their well tested and I recommend all my patients to get a carbon filter to decrease exposure to pesticides and chemicals. A carbon filter is just like what Brita and Pure and other brands are.
Because they’re chemicals known to cause cancer, I get a little bit concerned about cancer screening. This is most strongly tied to non-Hodgkin lymphoma, liver cancer, and renal cancer. It’s also linked to multiple myeloma, prostate cancer, probably brain cancer, and probably breast cancer, especially in men.
I tell people to be concerned about those, and then I tell people to avoid pesticides if they have Parkinson’s disease in all its forms, not only in the drinking water but in the produce you buy, the food you eat, what you put on your lawn, what’s on the golf course where you play, and the like.
Dr. Subramanian: I would say, just from the wellness perspective, if people are at risk for degenerative disease in terms of their brain health, things like sleep, mind-body practices, exercise, diet (Mediterranean or organic, if you can), and avoiding pesticides are all important. Social connection is important as well – the things that we think are helpful in general as people age and to prevent Alzheimer’s and other things like that.
Dr. Dorsey: These are fantastic ways to modify disease course. The evidence for them is only increasing. There’s an analogy I like to use. If someone is diagnosed with lung cancer, the first thing we tell them to do is to stop smoking. If someone’s diagnosed with Parkinson’s, we don’t tell them to stop getting exposure to pesticides. We don’t tell them to stop dry cleaning their clothes. We don’t tell them to avoid air pollution. These are all risk factors that are increasingly well established for Parkinson’s disease.
I think Parkinson’s disease, fundamentally for the vast majority of people, is an entirely preventable disease. We’re not taking actions to prevent people from getting this very disabling and very deadly disease.
Advocacy work
Dr. Subramanian: You and I are quite interested in the sense of being advocates as neurologists, and I think it fuels our passion and helps us to wake up every morning feeling like we have something that is meaningful and purposeful in our lives. Could you describe this as your passion and how it may prevent burnout and what it’s given you as a neurologist?
Dr. Dorsey: The credit for much of this is Dr. Carlie Tanner at UC San Francisco. I had the gift of sabbatical and I started reading the literature, I started reading her literature, and I came away with that, over the past 25 years, she detailed these environmental risk factors that are linked to Parkinson’s disease. Pesticides, these dry-cleaning chemicals, and air pollution. When I read it, I just realized that this was the case.
The same time I was reading her work, I read this book called “How to Survive a Plague,” by David France, who was a member of a group called Act Up, which was a group of men in New York City who reacted to the emergence of HIV in the 1980s. If you remember the 1980s, there was no federal response to HIV. People were blamed for the diseases that they were developing. It was only because brave men and women in New York City and in San Francisco banded together and organized that they changed the course of HIV.
They didn’t just do it for themselves. They did it for all of us. You and I and many people may not have HIV because of their courage. They made HIV a treatable condition. It’s actually more treatable than Parkinson’s disease. It’s associated with a near-normal life expectancy. They also made it a preventable disease. Thousands, if not millions, of us don’t have HIV because of their work. It’s an increasingly less common disease. Rates of HIV are actually decreasing, which is something that you or I would never have expected when we were in medical training.
I can’t think of a better outcome for a neurologist or any physician than to make the diseases that they’re caring for nonexistent ... than if we lived in a world that didn’t have HIV, we lived in a world where lung cancer largely didn’t exist. We’ve had worlds in the past where Parkinson’s probably didn’t exist or existed in extremely small numbers. That might be true for diffuse Lewy body disease and others, and if these diseases are preventable, we can take actions as individuals and as a society to lower our risk.
What a wonderful gift for future generations and many generations to come, hopefully, to live in a world that’s largely devoid of Parkinson’s disease. Just like we live in a world free of typhus. We live in a world free of smallpox. We live in a world where polio is extraordinarily uncommon. We don’t even have treatments for polio because we just don’t have polio. I think we can do the same thing for Parkinson’s disease for the vast majority.
Dr. Subramanian: Thank you so much, Ray, for your advocacy. We’re getting to the point in neurology, which is exciting to me, of possibly primary prevention of some of these disorders. I think we have a role in that, which is exciting for the future.
Dr. Dorsey: Absolutely.
Dr. Subramanian is clinical professor, department of neurology, University of California Los Angeles, and director of PADRECC (Parkinson’s Disease Research, Education, and Clinical Centers), West Los Angeles Veterans Association, Los Angeles. She disclosed ties with Acorda Pharma. Dr. Dorsey is the David M. Levy Professor of Neurology, University of Rochester (N.Y.). He disclosed ties to Abbott, AbbVie, Acadia, Acorda Therapeutics, Averitas Pharma, Biogen, BioSensics, Boehringer Ingelheim, Burroughs Wellcome Fund, Caraway Therapeutics, CuraSen, DConsult2, Denali Therapeutics, Eli Lilly, Genentech, Health & Wellness Partners, HMP Education, Included Health, Karger, KOL Groups, Life Sciences, Mediflix, Medrhythms, Merck; MJH Holdings, North American Center for Continuing Medical Education, Novartis, Otsuka, Pfizer, Photopharmics, Praxis Medicine, Roche, Safra Foundation, Sanofi, Seelos Therapeutics, SemCap, Spark Therapeutics, Springer Healthcare, Synapticure, Theravance Biopharmaceuticals, and WebMD.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Indu Subramanian, MD: It’s my pleasure to have Ray Dorsey on our program today. Ray is a professor of neurology at the University of Rochester and has been doing some amazing advocacy work in largely the space of trying to end Parkinson’s disease.
E. Ray Dorsey, MD: Thanks very much for having me, Indu. I’m delighted to be with you.
Trichloroethylene and PD
Dr. Subramanian: I wanted to first that we talk about as something that is in pretty much everywhere. This paper came out, and you wrote a commentary in JAMA Neurology as well. Perhaps we can summarize the paper and its findings.
Dr. Dorsey: Like most people, I didn’t know what TCE was until about 5 or 6 years ago. TCE is a very simple molecule. It’s got six atoms – two carbon atoms, one hydrogen atom, and three chlorine atoms — hence, its name “trichloroethylene.” There’s a very similar chemical called perchloroethylene, which is widely used in dry cleaning. It’s got one additional chlorine atom, and the prefix “per-” means “four.” I’ll talk about TCE predominantly, but both of these chemicals probably have similar toxicity with respect to Parkinson’s disease.
Research done by Dr. Carlie Tanner and Dr. Sam Goldman about a decade ago showed that in twins who were exposed to this through their work (it’s widely used as a degreasing agent) or hobbies (it’s used in printing and painting, by varnish workers, or by anyone that needs it as a solvent) had a 500% increased risk of developing Parkinson’s disease. Importantly, in that study, they showed that there was a lag time of 10-40 years between exposure to that chemical and the diagnosis of the disease. Because TCE was so widely used, they said that public health implications could be substantial.
What’s Camp Lejeune? Camp Lejeune is a Marine base in North Carolina where many Marines are trained. Between 1953 and 1987 at that Marine base, the drinking water was contaminated with TCE, perchloroethylene, and other toxic chemicals. The reason Camp Lejeune is so infamous is because the Marines knew about the contamination for many years and covered it up.
Indeed, this story only came to the forefront because Jennie Ensminger, the daughter of a Marine drill instructor, developed leukemia at age 6 and died at age 9. Her father, Jerry Ensminger, a retired master sergeant, found out after the fact that these cancer-causing chemicals, including TCE, a known carcinogen, were found at the Marine base and could be an explanation for why his daughter developed and died of leukemia.
Dr. Sam Goldman and Dr. Carlie Tanner and colleagues from UCSF looked at the rates of Parkinson’s among Marines who served at Camp Lejeune during the 1970s and compared that with rates in Marines who served Camp Pendleton on the West Coast. It turned out that the Marines who served at Camp Lejeune had a 70% higher risk of developing Parkinson’s disease than the Marines who served at Camp Pendleton.
Importantly, these Marines, by definition, were healthy. They were young. They were only 20 years old, on average, when they were at Camp Lejeune. They stayed at a Marine base for a short period of time, so on average, they were only there for 2 years. Yet 30 years later, they had a 70% increased risk of developing Parkinson’s disease.
Ending Parkinson’s disease
Dr. Subramanian: Wow, that’s pretty profound. You’ve done a large amount of work, and in fact you, along with some of our colleagues wrote a book about ending Parkinson’s disease. I read that book when it came out a couple of years ago, and I was really struck by a few things. Parkinson’s has doubled in the past 40 years and is going to double again in the next 20 years. Can you tell me a little bit about that statistic and why that is? It’s not just because people are aging. What is the sense of that? How do we interpret that?
Dr. Dorsey: According to the Global Burden of Disease study, which I was fortunate to be part of, the number of people with Parkinson’s disease has more than doubled in the past 25 years. A conservative projection based on aging alone suggests that it’s going to double again unless we change something about it. It’s now the world’s fastest-growing brain disease, and it is growing faster than can be explained by aging alone.
If you look at the map of Parkinson’s disease, if you thought it was purely genetic, you would have a relatively uniform map of rates of Parkinson’s disease. In fact, we don’t see that. Rates of Parkinson’s are five times higher in industrialized parts of the world, like the United States and Canada, than they are in sub-Saharan Africa. Rates of Parkinson’s disease are increasing most rapidly in areas of world that are undergoing the most rapid industrialization, such as India and China, where adjusted for age, the rates of Parkinson’s have more than doubled in the past 25 years.
The thesis of our book is that much of Parkinson’s disease is man-made. Work done by your colleagues at UCLA, including Jeff Bronstein and Beate Ritz, have demonstrated that air pollution and certain pesticides are likely fueling the rise of Parkinson’s disease.
Given that in the United States, rates of Parkinson’s disease are actually higher in urban and suburban areas than they are in rural areas, I think that this dry-cleaning chemical – which was widely used in the 1970s in everything from typewriter correction fluid to decaffeinated coffee and [over] 2 pounds per American [was produced] – could be one of the most important causes or contributing factors to Parkinson’s disease.
What to tell patients
Dr. Subramanian: For the general neurologists or practitioners out there watching this, what can they do? If you have a patient whom you suspect may have been exposed to toxins, what should we tell people who aren’t patients yet who are at risk? What are some things that you think would be helpful?
Dr. Dorsey: I think one of the shortcomings of American medicine is that we often just go from diagnosis to treatment. You’re depressed, you get an antidepressant; you have Parkinson’s disease, you get levodopa; you have seizures, you get put on an antiepileptic medication.
I think we need to spend a couple of minutes at least, maybe at the beginning, to go to the diagnosis of the condition and why you have this disease. If you just do a brief occupational history, after you start the exam – things like finding out what people do for a living or did for a living or how they spend their time – I think you’ll find many of these risk factors are actually present.
It’s pretty easy to identify whether people grew up in a rural area and drank well water, which is prone to be contaminated with pesticides. We know that people who drink [contaminated] well water have about a 75% increased risk of developing Parkinson’s disease. I think you can find for people, especially when they grew up, when they were young, that the most relevant exposure might be that when people were young children.
It’s a little bit harder to identify all exposure to TCE. The Marines at Camp Lejeune didn’t know they were drinking the water that was contaminated with this and only found out about it after the fact because Jerry Ensminger launched a 26-year campaign to bring justice for the Marines and their dependents.
Some people who know that they work with chemicals or with solvents might know about this. In New York City, these chemicals are widely used in dry cleaning. They’re readily volatile. These chemicals can evaporate from dry-cleaning buildings and go into the indoor air of apartments above dry cleaners, for example, in New York City. That can be in toxic levels. These readily dissolve in fat, hence their use in degreasing.
There have been studies, for example, in Germany, that found that supermarkets that are simply near a dry cleaner will have TCE or perchloroethylene in the butter and the cheese that they’re selling.
It gets even worse. For example, you bring your daughter into the dry-cleaning building and she’s eating an ice cream cone. When she leaves, she’s eating perchloroethylene and TCE.
It’s a little bit harder to find it, but I think it’s relevant because some people might be still being exposed and some people might still be drinking well water and they rarely have their well tested. For those people, I recommend they get their well tested and I recommend all my patients to get a carbon filter to decrease exposure to pesticides and chemicals. A carbon filter is just like what Brita and Pure and other brands are.
Because they’re chemicals known to cause cancer, I get a little bit concerned about cancer screening. This is most strongly tied to non-Hodgkin lymphoma, liver cancer, and renal cancer. It’s also linked to multiple myeloma, prostate cancer, probably brain cancer, and probably breast cancer, especially in men.
I tell people to be concerned about those, and then I tell people to avoid pesticides if they have Parkinson’s disease in all its forms, not only in the drinking water but in the produce you buy, the food you eat, what you put on your lawn, what’s on the golf course where you play, and the like.
Dr. Subramanian: I would say, just from the wellness perspective, if people are at risk for degenerative disease in terms of their brain health, things like sleep, mind-body practices, exercise, diet (Mediterranean or organic, if you can), and avoiding pesticides are all important. Social connection is important as well – the things that we think are helpful in general as people age and to prevent Alzheimer’s and other things like that.
Dr. Dorsey: These are fantastic ways to modify disease course. The evidence for them is only increasing. There’s an analogy I like to use. If someone is diagnosed with lung cancer, the first thing we tell them to do is to stop smoking. If someone’s diagnosed with Parkinson’s, we don’t tell them to stop getting exposure to pesticides. We don’t tell them to stop dry cleaning their clothes. We don’t tell them to avoid air pollution. These are all risk factors that are increasingly well established for Parkinson’s disease.
I think Parkinson’s disease, fundamentally for the vast majority of people, is an entirely preventable disease. We’re not taking actions to prevent people from getting this very disabling and very deadly disease.
Advocacy work
Dr. Subramanian: You and I are quite interested in the sense of being advocates as neurologists, and I think it fuels our passion and helps us to wake up every morning feeling like we have something that is meaningful and purposeful in our lives. Could you describe this as your passion and how it may prevent burnout and what it’s given you as a neurologist?
Dr. Dorsey: The credit for much of this is Dr. Carlie Tanner at UC San Francisco. I had the gift of sabbatical and I started reading the literature, I started reading her literature, and I came away with that, over the past 25 years, she detailed these environmental risk factors that are linked to Parkinson’s disease. Pesticides, these dry-cleaning chemicals, and air pollution. When I read it, I just realized that this was the case.
The same time I was reading her work, I read this book called “How to Survive a Plague,” by David France, who was a member of a group called Act Up, which was a group of men in New York City who reacted to the emergence of HIV in the 1980s. If you remember the 1980s, there was no federal response to HIV. People were blamed for the diseases that they were developing. It was only because brave men and women in New York City and in San Francisco banded together and organized that they changed the course of HIV.
They didn’t just do it for themselves. They did it for all of us. You and I and many people may not have HIV because of their courage. They made HIV a treatable condition. It’s actually more treatable than Parkinson’s disease. It’s associated with a near-normal life expectancy. They also made it a preventable disease. Thousands, if not millions, of us don’t have HIV because of their work. It’s an increasingly less common disease. Rates of HIV are actually decreasing, which is something that you or I would never have expected when we were in medical training.
I can’t think of a better outcome for a neurologist or any physician than to make the diseases that they’re caring for nonexistent ... than if we lived in a world that didn’t have HIV, we lived in a world where lung cancer largely didn’t exist. We’ve had worlds in the past where Parkinson’s probably didn’t exist or existed in extremely small numbers. That might be true for diffuse Lewy body disease and others, and if these diseases are preventable, we can take actions as individuals and as a society to lower our risk.
What a wonderful gift for future generations and many generations to come, hopefully, to live in a world that’s largely devoid of Parkinson’s disease. Just like we live in a world free of typhus. We live in a world free of smallpox. We live in a world where polio is extraordinarily uncommon. We don’t even have treatments for polio because we just don’t have polio. I think we can do the same thing for Parkinson’s disease for the vast majority.
Dr. Subramanian: Thank you so much, Ray, for your advocacy. We’re getting to the point in neurology, which is exciting to me, of possibly primary prevention of some of these disorders. I think we have a role in that, which is exciting for the future.
Dr. Dorsey: Absolutely.
Dr. Subramanian is clinical professor, department of neurology, University of California Los Angeles, and director of PADRECC (Parkinson’s Disease Research, Education, and Clinical Centers), West Los Angeles Veterans Association, Los Angeles. She disclosed ties with Acorda Pharma. Dr. Dorsey is the David M. Levy Professor of Neurology, University of Rochester (N.Y.). He disclosed ties to Abbott, AbbVie, Acadia, Acorda Therapeutics, Averitas Pharma, Biogen, BioSensics, Boehringer Ingelheim, Burroughs Wellcome Fund, Caraway Therapeutics, CuraSen, DConsult2, Denali Therapeutics, Eli Lilly, Genentech, Health & Wellness Partners, HMP Education, Included Health, Karger, KOL Groups, Life Sciences, Mediflix, Medrhythms, Merck; MJH Holdings, North American Center for Continuing Medical Education, Novartis, Otsuka, Pfizer, Photopharmics, Praxis Medicine, Roche, Safra Foundation, Sanofi, Seelos Therapeutics, SemCap, Spark Therapeutics, Springer Healthcare, Synapticure, Theravance Biopharmaceuticals, and WebMD.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Indu Subramanian, MD: It’s my pleasure to have Ray Dorsey on our program today. Ray is a professor of neurology at the University of Rochester and has been doing some amazing advocacy work in largely the space of trying to end Parkinson’s disease.
E. Ray Dorsey, MD: Thanks very much for having me, Indu. I’m delighted to be with you.
Trichloroethylene and PD
Dr. Subramanian: I wanted to first that we talk about as something that is in pretty much everywhere. This paper came out, and you wrote a commentary in JAMA Neurology as well. Perhaps we can summarize the paper and its findings.
Dr. Dorsey: Like most people, I didn’t know what TCE was until about 5 or 6 years ago. TCE is a very simple molecule. It’s got six atoms – two carbon atoms, one hydrogen atom, and three chlorine atoms — hence, its name “trichloroethylene.” There’s a very similar chemical called perchloroethylene, which is widely used in dry cleaning. It’s got one additional chlorine atom, and the prefix “per-” means “four.” I’ll talk about TCE predominantly, but both of these chemicals probably have similar toxicity with respect to Parkinson’s disease.
Research done by Dr. Carlie Tanner and Dr. Sam Goldman about a decade ago showed that in twins who were exposed to this through their work (it’s widely used as a degreasing agent) or hobbies (it’s used in printing and painting, by varnish workers, or by anyone that needs it as a solvent) had a 500% increased risk of developing Parkinson’s disease. Importantly, in that study, they showed that there was a lag time of 10-40 years between exposure to that chemical and the diagnosis of the disease. Because TCE was so widely used, they said that public health implications could be substantial.
What’s Camp Lejeune? Camp Lejeune is a Marine base in North Carolina where many Marines are trained. Between 1953 and 1987 at that Marine base, the drinking water was contaminated with TCE, perchloroethylene, and other toxic chemicals. The reason Camp Lejeune is so infamous is because the Marines knew about the contamination for many years and covered it up.
Indeed, this story only came to the forefront because Jennie Ensminger, the daughter of a Marine drill instructor, developed leukemia at age 6 and died at age 9. Her father, Jerry Ensminger, a retired master sergeant, found out after the fact that these cancer-causing chemicals, including TCE, a known carcinogen, were found at the Marine base and could be an explanation for why his daughter developed and died of leukemia.
Dr. Sam Goldman and Dr. Carlie Tanner and colleagues from UCSF looked at the rates of Parkinson’s among Marines who served at Camp Lejeune during the 1970s and compared that with rates in Marines who served Camp Pendleton on the West Coast. It turned out that the Marines who served at Camp Lejeune had a 70% higher risk of developing Parkinson’s disease than the Marines who served at Camp Pendleton.
Importantly, these Marines, by definition, were healthy. They were young. They were only 20 years old, on average, when they were at Camp Lejeune. They stayed at a Marine base for a short period of time, so on average, they were only there for 2 years. Yet 30 years later, they had a 70% increased risk of developing Parkinson’s disease.
Ending Parkinson’s disease
Dr. Subramanian: Wow, that’s pretty profound. You’ve done a large amount of work, and in fact you, along with some of our colleagues wrote a book about ending Parkinson’s disease. I read that book when it came out a couple of years ago, and I was really struck by a few things. Parkinson’s has doubled in the past 40 years and is going to double again in the next 20 years. Can you tell me a little bit about that statistic and why that is? It’s not just because people are aging. What is the sense of that? How do we interpret that?
Dr. Dorsey: According to the Global Burden of Disease study, which I was fortunate to be part of, the number of people with Parkinson’s disease has more than doubled in the past 25 years. A conservative projection based on aging alone suggests that it’s going to double again unless we change something about it. It’s now the world’s fastest-growing brain disease, and it is growing faster than can be explained by aging alone.
If you look at the map of Parkinson’s disease, if you thought it was purely genetic, you would have a relatively uniform map of rates of Parkinson’s disease. In fact, we don’t see that. Rates of Parkinson’s are five times higher in industrialized parts of the world, like the United States and Canada, than they are in sub-Saharan Africa. Rates of Parkinson’s disease are increasing most rapidly in areas of world that are undergoing the most rapid industrialization, such as India and China, where adjusted for age, the rates of Parkinson’s have more than doubled in the past 25 years.
The thesis of our book is that much of Parkinson’s disease is man-made. Work done by your colleagues at UCLA, including Jeff Bronstein and Beate Ritz, have demonstrated that air pollution and certain pesticides are likely fueling the rise of Parkinson’s disease.
Given that in the United States, rates of Parkinson’s disease are actually higher in urban and suburban areas than they are in rural areas, I think that this dry-cleaning chemical – which was widely used in the 1970s in everything from typewriter correction fluid to decaffeinated coffee and [over] 2 pounds per American [was produced] – could be one of the most important causes or contributing factors to Parkinson’s disease.
What to tell patients
Dr. Subramanian: For the general neurologists or practitioners out there watching this, what can they do? If you have a patient whom you suspect may have been exposed to toxins, what should we tell people who aren’t patients yet who are at risk? What are some things that you think would be helpful?
Dr. Dorsey: I think one of the shortcomings of American medicine is that we often just go from diagnosis to treatment. You’re depressed, you get an antidepressant; you have Parkinson’s disease, you get levodopa; you have seizures, you get put on an antiepileptic medication.
I think we need to spend a couple of minutes at least, maybe at the beginning, to go to the diagnosis of the condition and why you have this disease. If you just do a brief occupational history, after you start the exam – things like finding out what people do for a living or did for a living or how they spend their time – I think you’ll find many of these risk factors are actually present.
It’s pretty easy to identify whether people grew up in a rural area and drank well water, which is prone to be contaminated with pesticides. We know that people who drink [contaminated] well water have about a 75% increased risk of developing Parkinson’s disease. I think you can find for people, especially when they grew up, when they were young, that the most relevant exposure might be that when people were young children.
It’s a little bit harder to identify all exposure to TCE. The Marines at Camp Lejeune didn’t know they were drinking the water that was contaminated with this and only found out about it after the fact because Jerry Ensminger launched a 26-year campaign to bring justice for the Marines and their dependents.
Some people who know that they work with chemicals or with solvents might know about this. In New York City, these chemicals are widely used in dry cleaning. They’re readily volatile. These chemicals can evaporate from dry-cleaning buildings and go into the indoor air of apartments above dry cleaners, for example, in New York City. That can be in toxic levels. These readily dissolve in fat, hence their use in degreasing.
There have been studies, for example, in Germany, that found that supermarkets that are simply near a dry cleaner will have TCE or perchloroethylene in the butter and the cheese that they’re selling.
It gets even worse. For example, you bring your daughter into the dry-cleaning building and she’s eating an ice cream cone. When she leaves, she’s eating perchloroethylene and TCE.
It’s a little bit harder to find it, but I think it’s relevant because some people might be still being exposed and some people might still be drinking well water and they rarely have their well tested. For those people, I recommend they get their well tested and I recommend all my patients to get a carbon filter to decrease exposure to pesticides and chemicals. A carbon filter is just like what Brita and Pure and other brands are.
Because they’re chemicals known to cause cancer, I get a little bit concerned about cancer screening. This is most strongly tied to non-Hodgkin lymphoma, liver cancer, and renal cancer. It’s also linked to multiple myeloma, prostate cancer, probably brain cancer, and probably breast cancer, especially in men.
I tell people to be concerned about those, and then I tell people to avoid pesticides if they have Parkinson’s disease in all its forms, not only in the drinking water but in the produce you buy, the food you eat, what you put on your lawn, what’s on the golf course where you play, and the like.
Dr. Subramanian: I would say, just from the wellness perspective, if people are at risk for degenerative disease in terms of their brain health, things like sleep, mind-body practices, exercise, diet (Mediterranean or organic, if you can), and avoiding pesticides are all important. Social connection is important as well – the things that we think are helpful in general as people age and to prevent Alzheimer’s and other things like that.
Dr. Dorsey: These are fantastic ways to modify disease course. The evidence for them is only increasing. There’s an analogy I like to use. If someone is diagnosed with lung cancer, the first thing we tell them to do is to stop smoking. If someone’s diagnosed with Parkinson’s, we don’t tell them to stop getting exposure to pesticides. We don’t tell them to stop dry cleaning their clothes. We don’t tell them to avoid air pollution. These are all risk factors that are increasingly well established for Parkinson’s disease.
I think Parkinson’s disease, fundamentally for the vast majority of people, is an entirely preventable disease. We’re not taking actions to prevent people from getting this very disabling and very deadly disease.
Advocacy work
Dr. Subramanian: You and I are quite interested in the sense of being advocates as neurologists, and I think it fuels our passion and helps us to wake up every morning feeling like we have something that is meaningful and purposeful in our lives. Could you describe this as your passion and how it may prevent burnout and what it’s given you as a neurologist?
Dr. Dorsey: The credit for much of this is Dr. Carlie Tanner at UC San Francisco. I had the gift of sabbatical and I started reading the literature, I started reading her literature, and I came away with that, over the past 25 years, she detailed these environmental risk factors that are linked to Parkinson’s disease. Pesticides, these dry-cleaning chemicals, and air pollution. When I read it, I just realized that this was the case.
The same time I was reading her work, I read this book called “How to Survive a Plague,” by David France, who was a member of a group called Act Up, which was a group of men in New York City who reacted to the emergence of HIV in the 1980s. If you remember the 1980s, there was no federal response to HIV. People were blamed for the diseases that they were developing. It was only because brave men and women in New York City and in San Francisco banded together and organized that they changed the course of HIV.
They didn’t just do it for themselves. They did it for all of us. You and I and many people may not have HIV because of their courage. They made HIV a treatable condition. It’s actually more treatable than Parkinson’s disease. It’s associated with a near-normal life expectancy. They also made it a preventable disease. Thousands, if not millions, of us don’t have HIV because of their work. It’s an increasingly less common disease. Rates of HIV are actually decreasing, which is something that you or I would never have expected when we were in medical training.
I can’t think of a better outcome for a neurologist or any physician than to make the diseases that they’re caring for nonexistent ... than if we lived in a world that didn’t have HIV, we lived in a world where lung cancer largely didn’t exist. We’ve had worlds in the past where Parkinson’s probably didn’t exist or existed in extremely small numbers. That might be true for diffuse Lewy body disease and others, and if these diseases are preventable, we can take actions as individuals and as a society to lower our risk.
What a wonderful gift for future generations and many generations to come, hopefully, to live in a world that’s largely devoid of Parkinson’s disease. Just like we live in a world free of typhus. We live in a world free of smallpox. We live in a world where polio is extraordinarily uncommon. We don’t even have treatments for polio because we just don’t have polio. I think we can do the same thing for Parkinson’s disease for the vast majority.
Dr. Subramanian: Thank you so much, Ray, for your advocacy. We’re getting to the point in neurology, which is exciting to me, of possibly primary prevention of some of these disorders. I think we have a role in that, which is exciting for the future.
Dr. Dorsey: Absolutely.
Dr. Subramanian is clinical professor, department of neurology, University of California Los Angeles, and director of PADRECC (Parkinson’s Disease Research, Education, and Clinical Centers), West Los Angeles Veterans Association, Los Angeles. She disclosed ties with Acorda Pharma. Dr. Dorsey is the David M. Levy Professor of Neurology, University of Rochester (N.Y.). He disclosed ties to Abbott, AbbVie, Acadia, Acorda Therapeutics, Averitas Pharma, Biogen, BioSensics, Boehringer Ingelheim, Burroughs Wellcome Fund, Caraway Therapeutics, CuraSen, DConsult2, Denali Therapeutics, Eli Lilly, Genentech, Health & Wellness Partners, HMP Education, Included Health, Karger, KOL Groups, Life Sciences, Mediflix, Medrhythms, Merck; MJH Holdings, North American Center for Continuing Medical Education, Novartis, Otsuka, Pfizer, Photopharmics, Praxis Medicine, Roche, Safra Foundation, Sanofi, Seelos Therapeutics, SemCap, Spark Therapeutics, Springer Healthcare, Synapticure, Theravance Biopharmaceuticals, and WebMD.
A version of this article appeared on Medscape.com.
What to tell your patients about anti-amyloids for Alzheimer’s disease
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Headache after drinking red wine? This could be why
This transcript has been edited for clarity.
Robert Louis Stevenson famously said, “Wine is bottled poetry.” And I think it works quite well. I’ve had wines that are simple, elegant, and unpretentious like Emily Dickinson, and passionate and mysterious like Pablo Neruda. And I’ve had wines that are more analogous to the limerick you might read scrawled on a rest-stop bathroom wall. Those ones give me headaches.
– and apparently it’s not just the alcohol.
Headaches are common, and headaches after drinking alcohol are particularly common. An interesting epidemiologic phenomenon, not yet adequately explained, is why red wine is associated with more headache than other forms of alcohol. There have been many studies fingering many suspects, from sulfites to tannins to various phenolic compounds, but none have really provided a concrete explanation for what might be going on.
A new hypothesis came to the fore on Nov. 20 in the journal Scientific Reports:
To understand the idea, first a reminder of what happens when you drink alcohol, physiologically.
Alcohol is metabolized by the enzyme alcohol dehydrogenase in the gut and then in the liver. That turns it into acetaldehyde, a toxic metabolite. In most of us, aldehyde dehydrogenase (ALDH) quickly metabolizes acetaldehyde to the inert acetate, which can be safely excreted.
I say “most of us” because some populations, particularly those with East Asian ancestry, have a mutation in the ALDH gene which can lead to accumulation of toxic acetaldehyde with alcohol consumption – leading to facial flushing, nausea, and headache.
We can also inhibit the enzyme medically. That’s what the drug disulfiram, also known as Antabuse, does. It doesn’t prevent you from wanting to drink; it makes the consequences of drinking incredibly aversive.
The researchers focused in on the aldehyde dehydrogenase enzyme and conducted a screening study. Are there any compounds in red wine that naturally inhibit ALDH?
The results pointed squarely at quercetin, and particularly its metabolite quercetin glucuronide, which, at 20 micromolar concentrations, inhibited about 80% of ALDH activity.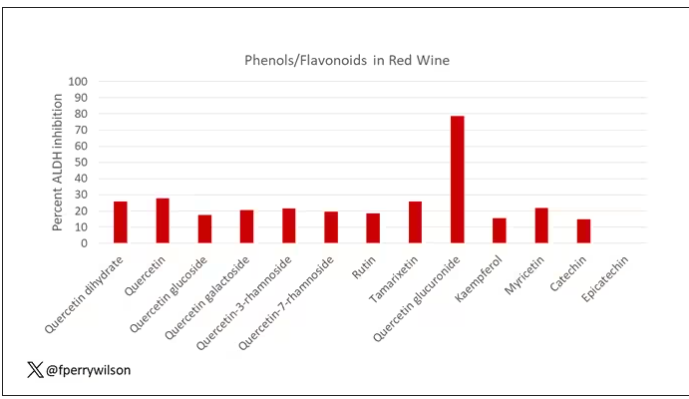
Quercetin is a flavonoid – a compound that gives color to a variety of vegetables and fruits, including grapes. In a test tube, it is an antioxidant, which is enough evidence to spawn a small quercetin-as-supplement industry, but there is no convincing evidence that it is medically useful. The authors then examined the concentration of quercetin glucuronide to achieve various inhibitions of ALDH, as you can see in this graph here.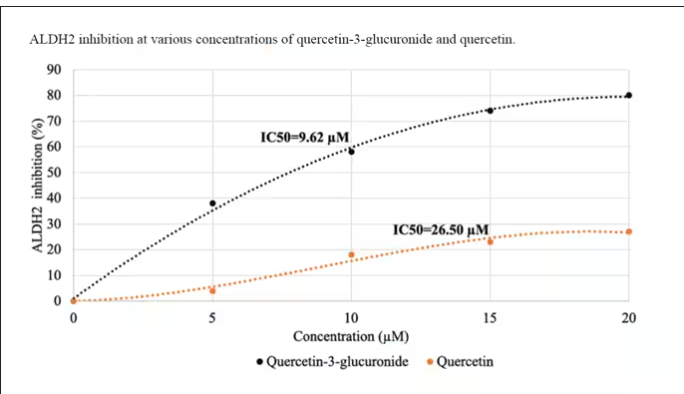
By about 10 micromolar, we see a decent amount of inhibition. Disulfiram is about 10 times more potent than that, but then again, you don’t drink three glasses of disulfiram with Thanksgiving dinner.
This is where this study stops. But it obviously tells us very little about what might be happening in the human body. For that, we need to ask the question: Can we get our quercetin levels to 10 micromolar? Is that remotely achievable?
Let’s start with how much quercetin there is in red wine. Like all things wine, it varies, but this study examining Australian wines found mean concentrations of 11 mg/L. The highest value I saw was close to 50 mg/L.
So let’s do some math. To make the numbers easy, let’s say you drank a liter of Australian wine, taking in 50 mg of quercetin glucuronide.
How much of that gets into your bloodstream? Some studies suggest a bioavailability of less than 1%, which basically means none and should probably put the quercetin hypothesis to bed. But there is some variation here too; it seems to depend on the form of quercetin you ingest.
Let’s say all 50 mg gets into your bloodstream. What blood concentration would that lead to? Well, I’ll keep the stoichiometry in the graphics and just say that if we assume that the volume of distribution of the compound is restricted to plasma alone, then you could achieve similar concentrations to what was done in petri dishes during this study.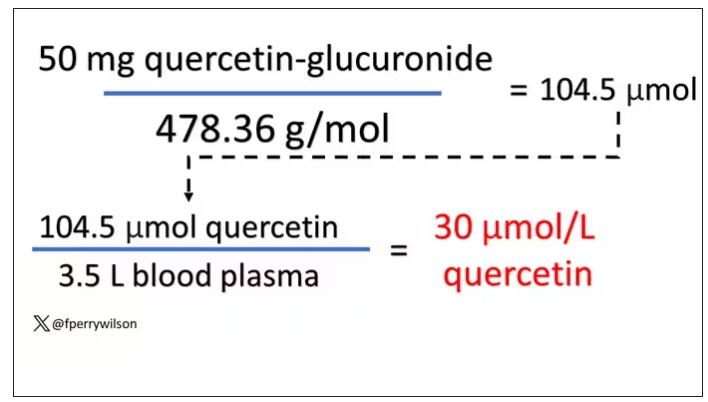
Of course, if quercetin is really the culprit behind red wine headache, I have some questions: Why aren’t the Amazon reviews of quercetin supplements chock full of warnings not to take them with alcohol? And other foods have way higher quercetin concentration than wine, but you don’t hear people warning not to take your red onions with alcohol, or your capers, or lingonberries.
There’s some more work to be done here – most importantly, some human studies. Let’s give people wine with different amounts of quercetin and see what happens. Sign me up. Seriously.
As for Thanksgiving, it’s worth noting that cranberries have a lot of quercetin in them. So between the cranberry sauce, the Beaujolais, and your uncle ranting about the contrails again, the probability of headache is pretty darn high. Stay safe out there, and Happy Thanksgiving.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Robert Louis Stevenson famously said, “Wine is bottled poetry.” And I think it works quite well. I’ve had wines that are simple, elegant, and unpretentious like Emily Dickinson, and passionate and mysterious like Pablo Neruda. And I’ve had wines that are more analogous to the limerick you might read scrawled on a rest-stop bathroom wall. Those ones give me headaches.
– and apparently it’s not just the alcohol.
Headaches are common, and headaches after drinking alcohol are particularly common. An interesting epidemiologic phenomenon, not yet adequately explained, is why red wine is associated with more headache than other forms of alcohol. There have been many studies fingering many suspects, from sulfites to tannins to various phenolic compounds, but none have really provided a concrete explanation for what might be going on.
A new hypothesis came to the fore on Nov. 20 in the journal Scientific Reports:
To understand the idea, first a reminder of what happens when you drink alcohol, physiologically.
Alcohol is metabolized by the enzyme alcohol dehydrogenase in the gut and then in the liver. That turns it into acetaldehyde, a toxic metabolite. In most of us, aldehyde dehydrogenase (ALDH) quickly metabolizes acetaldehyde to the inert acetate, which can be safely excreted.
I say “most of us” because some populations, particularly those with East Asian ancestry, have a mutation in the ALDH gene which can lead to accumulation of toxic acetaldehyde with alcohol consumption – leading to facial flushing, nausea, and headache.
We can also inhibit the enzyme medically. That’s what the drug disulfiram, also known as Antabuse, does. It doesn’t prevent you from wanting to drink; it makes the consequences of drinking incredibly aversive.
The researchers focused in on the aldehyde dehydrogenase enzyme and conducted a screening study. Are there any compounds in red wine that naturally inhibit ALDH?
The results pointed squarely at quercetin, and particularly its metabolite quercetin glucuronide, which, at 20 micromolar concentrations, inhibited about 80% of ALDH activity.
Quercetin is a flavonoid – a compound that gives color to a variety of vegetables and fruits, including grapes. In a test tube, it is an antioxidant, which is enough evidence to spawn a small quercetin-as-supplement industry, but there is no convincing evidence that it is medically useful. The authors then examined the concentration of quercetin glucuronide to achieve various inhibitions of ALDH, as you can see in this graph here.
By about 10 micromolar, we see a decent amount of inhibition. Disulfiram is about 10 times more potent than that, but then again, you don’t drink three glasses of disulfiram with Thanksgiving dinner.
This is where this study stops. But it obviously tells us very little about what might be happening in the human body. For that, we need to ask the question: Can we get our quercetin levels to 10 micromolar? Is that remotely achievable?
Let’s start with how much quercetin there is in red wine. Like all things wine, it varies, but this study examining Australian wines found mean concentrations of 11 mg/L. The highest value I saw was close to 50 mg/L.
So let’s do some math. To make the numbers easy, let’s say you drank a liter of Australian wine, taking in 50 mg of quercetin glucuronide.
How much of that gets into your bloodstream? Some studies suggest a bioavailability of less than 1%, which basically means none and should probably put the quercetin hypothesis to bed. But there is some variation here too; it seems to depend on the form of quercetin you ingest.
Let’s say all 50 mg gets into your bloodstream. What blood concentration would that lead to? Well, I’ll keep the stoichiometry in the graphics and just say that if we assume that the volume of distribution of the compound is restricted to plasma alone, then you could achieve similar concentrations to what was done in petri dishes during this study.
Of course, if quercetin is really the culprit behind red wine headache, I have some questions: Why aren’t the Amazon reviews of quercetin supplements chock full of warnings not to take them with alcohol? And other foods have way higher quercetin concentration than wine, but you don’t hear people warning not to take your red onions with alcohol, or your capers, or lingonberries.
There’s some more work to be done here – most importantly, some human studies. Let’s give people wine with different amounts of quercetin and see what happens. Sign me up. Seriously.
As for Thanksgiving, it’s worth noting that cranberries have a lot of quercetin in them. So between the cranberry sauce, the Beaujolais, and your uncle ranting about the contrails again, the probability of headache is pretty darn high. Stay safe out there, and Happy Thanksgiving.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Robert Louis Stevenson famously said, “Wine is bottled poetry.” And I think it works quite well. I’ve had wines that are simple, elegant, and unpretentious like Emily Dickinson, and passionate and mysterious like Pablo Neruda. And I’ve had wines that are more analogous to the limerick you might read scrawled on a rest-stop bathroom wall. Those ones give me headaches.
– and apparently it’s not just the alcohol.
Headaches are common, and headaches after drinking alcohol are particularly common. An interesting epidemiologic phenomenon, not yet adequately explained, is why red wine is associated with more headache than other forms of alcohol. There have been many studies fingering many suspects, from sulfites to tannins to various phenolic compounds, but none have really provided a concrete explanation for what might be going on.
A new hypothesis came to the fore on Nov. 20 in the journal Scientific Reports:
To understand the idea, first a reminder of what happens when you drink alcohol, physiologically.
Alcohol is metabolized by the enzyme alcohol dehydrogenase in the gut and then in the liver. That turns it into acetaldehyde, a toxic metabolite. In most of us, aldehyde dehydrogenase (ALDH) quickly metabolizes acetaldehyde to the inert acetate, which can be safely excreted.
I say “most of us” because some populations, particularly those with East Asian ancestry, have a mutation in the ALDH gene which can lead to accumulation of toxic acetaldehyde with alcohol consumption – leading to facial flushing, nausea, and headache.
We can also inhibit the enzyme medically. That’s what the drug disulfiram, also known as Antabuse, does. It doesn’t prevent you from wanting to drink; it makes the consequences of drinking incredibly aversive.
The researchers focused in on the aldehyde dehydrogenase enzyme and conducted a screening study. Are there any compounds in red wine that naturally inhibit ALDH?
The results pointed squarely at quercetin, and particularly its metabolite quercetin glucuronide, which, at 20 micromolar concentrations, inhibited about 80% of ALDH activity.
Quercetin is a flavonoid – a compound that gives color to a variety of vegetables and fruits, including grapes. In a test tube, it is an antioxidant, which is enough evidence to spawn a small quercetin-as-supplement industry, but there is no convincing evidence that it is medically useful. The authors then examined the concentration of quercetin glucuronide to achieve various inhibitions of ALDH, as you can see in this graph here.
By about 10 micromolar, we see a decent amount of inhibition. Disulfiram is about 10 times more potent than that, but then again, you don’t drink three glasses of disulfiram with Thanksgiving dinner.
This is where this study stops. But it obviously tells us very little about what might be happening in the human body. For that, we need to ask the question: Can we get our quercetin levels to 10 micromolar? Is that remotely achievable?
Let’s start with how much quercetin there is in red wine. Like all things wine, it varies, but this study examining Australian wines found mean concentrations of 11 mg/L. The highest value I saw was close to 50 mg/L.
So let’s do some math. To make the numbers easy, let’s say you drank a liter of Australian wine, taking in 50 mg of quercetin glucuronide.
How much of that gets into your bloodstream? Some studies suggest a bioavailability of less than 1%, which basically means none and should probably put the quercetin hypothesis to bed. But there is some variation here too; it seems to depend on the form of quercetin you ingest.
Let’s say all 50 mg gets into your bloodstream. What blood concentration would that lead to? Well, I’ll keep the stoichiometry in the graphics and just say that if we assume that the volume of distribution of the compound is restricted to plasma alone, then you could achieve similar concentrations to what was done in petri dishes during this study.
Of course, if quercetin is really the culprit behind red wine headache, I have some questions: Why aren’t the Amazon reviews of quercetin supplements chock full of warnings not to take them with alcohol? And other foods have way higher quercetin concentration than wine, but you don’t hear people warning not to take your red onions with alcohol, or your capers, or lingonberries.
There’s some more work to be done here – most importantly, some human studies. Let’s give people wine with different amounts of quercetin and see what happens. Sign me up. Seriously.
As for Thanksgiving, it’s worth noting that cranberries have a lot of quercetin in them. So between the cranberry sauce, the Beaujolais, and your uncle ranting about the contrails again, the probability of headache is pretty darn high. Stay safe out there, and Happy Thanksgiving.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Adolescents with migraine need smooth handoff to adult care
, according to a headache specialist who treats adults and children and spoke at the 2023 Scottsdale Headache Symposium.
“I would start at about the age of 15 or 16,” said Hope L. O’Brien, MD, Headache Center of Hope, University of Cincinnati.
Describing the steps that she thinks should be included in an effective transition, Dr. O’Brien maintained, “you will have a greater chance of successful transition and lessen the likelihood of the chronicity and the poor outcomes that we see in adults.”
Dr. O’Brien, who developed a headache clinic that serves individuals between the ages of 15 and 27, has substantial experience with headache patients in this age range. She acknowledged that there are no guideline recommendations for how best to guide the transition from pediatric to adult care, but she has developed some strategies at her own institution, including a tool for determining when the transition should be considered.
“Transition readiness is something that you need to think about,” she said. “You don’t just do it [automatically] at the age of 18.”
TRAQ questionnaire is helpful
The Transition Readiness Assessment Questionnaire (TRAQ) is one tool that can be helpful, according to Dr. O’Brien, This tool, which can be used to evaluate whether young patients feel prepared to describe their own health status and needs and advocate on their own behalf, is not specific to headache, but the principle is particularly important in headache because of the importance of the patient’s history. Dr. O’Brien said that a fellow in her program, Allyson Bazarsky, MD, who is now affiliated with the University of Vermont Medical Center, Burlington, validated TRAQ for headache about 6 years ago.
“TRAQ is available online. It’s free. You can download it as a PDF,” Dr. O’Brien said. In fact, several age-specific versions can now be found readily on a web search for TRAQ questionnaire.
Ultimately, TRAQ helps the clinician to gauge what patients know about their disease, the medications they are taking, and the relevance of any comorbidities, such as mood disorders. It also provides insight about the ability to understand their health issues and to communicate well with caregivers.
Dr. O’Brien sees this as a process over time, rather than something to be implemented a few months before the transition.
“It is important to start making the shift during childhood and talking directly to the child,” Dr. O’Brien said. If education about the disease and its triggers are started relatively early in adolescence, the transition will not only be easier, but patients might have a chance to understand and control their disease at an earlier age.
With this kind of approach, most children are at least in the preparation stage by age 18 years. However, the age at which patients are suitable for transition varies substantially. Many patients 18 years of age or older are in the “action phase,” meaning it is time to take steps to transition.
Again, based on the interrelationship between headache and comorbidities, particularly mood disorders, such as depression and anxiety, the goal should not be limited to headache. Young adults should be educated about taking responsibility for their overall health.
In addition to educating the patient, Dr. O’Brien recommended preparing a transfer packet, such as the one described in an article published in Headache. Geared for communicating with the clinician who will take over care, the contents should include a detailed medical history along with the current treatment plan and list of medications that have been effective and those that have failed, according to Dr. O’Brien.
“An emergency plan in the form of an emergency department letter in case the patient needs to seek emergent care at an outside facility” is also appropriate, Dr. O’Brien said.
The patient should be aware of what is in the transfer pack in order to participate in an informed discussion of health care with the adult neurologist.
Poor transition linked to poor outcomes
A substantial proportion of adolescents with migraine continue to experience episodes as an adult, particularly those with a delayed diagnosis of migraine, those with a first degree relative who has migraine, and those with poor health habits, but this is not inevitable. Dr. O’Brien noted that “unsuccessful transition of care” into adulthood is a factor associated with poorer outcomes, making it an appropriate target for optimizing outcomes.
“Have that discussion on transfer of care with an action plan and do that early, especially in those with chronic or persistent disability headaches,” Dr. O’Brien emphasized.
This is pertinent advice, according to Amy A. Gelfand, MD, director of the child and adolescent headache program at Benioff Children’s Hospitals, University of California, San Francisco. Senior author of a comprehensive review article on pediatric migraine in Neurologic Clinics, Dr. Gelfand said the practical value of young adults learning what medications they are taking, and why, can place them in a better position to monitor their disease and to understand when a clinical visit is appropriate.
“I agree that it is important to help young adults (i.e., 18- or 19-year-olds) to prepare for the transition from the pediatric health care environment to the adult one,” said Dr. Gelfand, who has written frequently on this and related topics, such as the impact of comorbidities on outcome.
Dr. O’Brien reports financial relationships with AbbVie, Eli Lilly, Guidepoint, Pfizer, and Vector Psychometric Group. Dr. Gelfand reports financial relationships with Allergan, Eli Lilly, EMKinetics, eNeura, Teva and Zosano.
, according to a headache specialist who treats adults and children and spoke at the 2023 Scottsdale Headache Symposium.
“I would start at about the age of 15 or 16,” said Hope L. O’Brien, MD, Headache Center of Hope, University of Cincinnati.
Describing the steps that she thinks should be included in an effective transition, Dr. O’Brien maintained, “you will have a greater chance of successful transition and lessen the likelihood of the chronicity and the poor outcomes that we see in adults.”
Dr. O’Brien, who developed a headache clinic that serves individuals between the ages of 15 and 27, has substantial experience with headache patients in this age range. She acknowledged that there are no guideline recommendations for how best to guide the transition from pediatric to adult care, but she has developed some strategies at her own institution, including a tool for determining when the transition should be considered.
“Transition readiness is something that you need to think about,” she said. “You don’t just do it [automatically] at the age of 18.”
TRAQ questionnaire is helpful
The Transition Readiness Assessment Questionnaire (TRAQ) is one tool that can be helpful, according to Dr. O’Brien, This tool, which can be used to evaluate whether young patients feel prepared to describe their own health status and needs and advocate on their own behalf, is not specific to headache, but the principle is particularly important in headache because of the importance of the patient’s history. Dr. O’Brien said that a fellow in her program, Allyson Bazarsky, MD, who is now affiliated with the University of Vermont Medical Center, Burlington, validated TRAQ for headache about 6 years ago.
“TRAQ is available online. It’s free. You can download it as a PDF,” Dr. O’Brien said. In fact, several age-specific versions can now be found readily on a web search for TRAQ questionnaire.
Ultimately, TRAQ helps the clinician to gauge what patients know about their disease, the medications they are taking, and the relevance of any comorbidities, such as mood disorders. It also provides insight about the ability to understand their health issues and to communicate well with caregivers.
Dr. O’Brien sees this as a process over time, rather than something to be implemented a few months before the transition.
“It is important to start making the shift during childhood and talking directly to the child,” Dr. O’Brien said. If education about the disease and its triggers are started relatively early in adolescence, the transition will not only be easier, but patients might have a chance to understand and control their disease at an earlier age.
With this kind of approach, most children are at least in the preparation stage by age 18 years. However, the age at which patients are suitable for transition varies substantially. Many patients 18 years of age or older are in the “action phase,” meaning it is time to take steps to transition.
Again, based on the interrelationship between headache and comorbidities, particularly mood disorders, such as depression and anxiety, the goal should not be limited to headache. Young adults should be educated about taking responsibility for their overall health.
In addition to educating the patient, Dr. O’Brien recommended preparing a transfer packet, such as the one described in an article published in Headache. Geared for communicating with the clinician who will take over care, the contents should include a detailed medical history along with the current treatment plan and list of medications that have been effective and those that have failed, according to Dr. O’Brien.
“An emergency plan in the form of an emergency department letter in case the patient needs to seek emergent care at an outside facility” is also appropriate, Dr. O’Brien said.
The patient should be aware of what is in the transfer pack in order to participate in an informed discussion of health care with the adult neurologist.
Poor transition linked to poor outcomes
A substantial proportion of adolescents with migraine continue to experience episodes as an adult, particularly those with a delayed diagnosis of migraine, those with a first degree relative who has migraine, and those with poor health habits, but this is not inevitable. Dr. O’Brien noted that “unsuccessful transition of care” into adulthood is a factor associated with poorer outcomes, making it an appropriate target for optimizing outcomes.
“Have that discussion on transfer of care with an action plan and do that early, especially in those with chronic or persistent disability headaches,” Dr. O’Brien emphasized.
This is pertinent advice, according to Amy A. Gelfand, MD, director of the child and adolescent headache program at Benioff Children’s Hospitals, University of California, San Francisco. Senior author of a comprehensive review article on pediatric migraine in Neurologic Clinics, Dr. Gelfand said the practical value of young adults learning what medications they are taking, and why, can place them in a better position to monitor their disease and to understand when a clinical visit is appropriate.
“I agree that it is important to help young adults (i.e., 18- or 19-year-olds) to prepare for the transition from the pediatric health care environment to the adult one,” said Dr. Gelfand, who has written frequently on this and related topics, such as the impact of comorbidities on outcome.
Dr. O’Brien reports financial relationships with AbbVie, Eli Lilly, Guidepoint, Pfizer, and Vector Psychometric Group. Dr. Gelfand reports financial relationships with Allergan, Eli Lilly, EMKinetics, eNeura, Teva and Zosano.
, according to a headache specialist who treats adults and children and spoke at the 2023 Scottsdale Headache Symposium.
“I would start at about the age of 15 or 16,” said Hope L. O’Brien, MD, Headache Center of Hope, University of Cincinnati.
Describing the steps that she thinks should be included in an effective transition, Dr. O’Brien maintained, “you will have a greater chance of successful transition and lessen the likelihood of the chronicity and the poor outcomes that we see in adults.”
Dr. O’Brien, who developed a headache clinic that serves individuals between the ages of 15 and 27, has substantial experience with headache patients in this age range. She acknowledged that there are no guideline recommendations for how best to guide the transition from pediatric to adult care, but she has developed some strategies at her own institution, including a tool for determining when the transition should be considered.
“Transition readiness is something that you need to think about,” she said. “You don’t just do it [automatically] at the age of 18.”
TRAQ questionnaire is helpful
The Transition Readiness Assessment Questionnaire (TRAQ) is one tool that can be helpful, according to Dr. O’Brien, This tool, which can be used to evaluate whether young patients feel prepared to describe their own health status and needs and advocate on their own behalf, is not specific to headache, but the principle is particularly important in headache because of the importance of the patient’s history. Dr. O’Brien said that a fellow in her program, Allyson Bazarsky, MD, who is now affiliated with the University of Vermont Medical Center, Burlington, validated TRAQ for headache about 6 years ago.
“TRAQ is available online. It’s free. You can download it as a PDF,” Dr. O’Brien said. In fact, several age-specific versions can now be found readily on a web search for TRAQ questionnaire.
Ultimately, TRAQ helps the clinician to gauge what patients know about their disease, the medications they are taking, and the relevance of any comorbidities, such as mood disorders. It also provides insight about the ability to understand their health issues and to communicate well with caregivers.
Dr. O’Brien sees this as a process over time, rather than something to be implemented a few months before the transition.
“It is important to start making the shift during childhood and talking directly to the child,” Dr. O’Brien said. If education about the disease and its triggers are started relatively early in adolescence, the transition will not only be easier, but patients might have a chance to understand and control their disease at an earlier age.
With this kind of approach, most children are at least in the preparation stage by age 18 years. However, the age at which patients are suitable for transition varies substantially. Many patients 18 years of age or older are in the “action phase,” meaning it is time to take steps to transition.
Again, based on the interrelationship between headache and comorbidities, particularly mood disorders, such as depression and anxiety, the goal should not be limited to headache. Young adults should be educated about taking responsibility for their overall health.
In addition to educating the patient, Dr. O’Brien recommended preparing a transfer packet, such as the one described in an article published in Headache. Geared for communicating with the clinician who will take over care, the contents should include a detailed medical history along with the current treatment plan and list of medications that have been effective and those that have failed, according to Dr. O’Brien.
“An emergency plan in the form of an emergency department letter in case the patient needs to seek emergent care at an outside facility” is also appropriate, Dr. O’Brien said.
The patient should be aware of what is in the transfer pack in order to participate in an informed discussion of health care with the adult neurologist.
Poor transition linked to poor outcomes
A substantial proportion of adolescents with migraine continue to experience episodes as an adult, particularly those with a delayed diagnosis of migraine, those with a first degree relative who has migraine, and those with poor health habits, but this is not inevitable. Dr. O’Brien noted that “unsuccessful transition of care” into adulthood is a factor associated with poorer outcomes, making it an appropriate target for optimizing outcomes.
“Have that discussion on transfer of care with an action plan and do that early, especially in those with chronic or persistent disability headaches,” Dr. O’Brien emphasized.
This is pertinent advice, according to Amy A. Gelfand, MD, director of the child and adolescent headache program at Benioff Children’s Hospitals, University of California, San Francisco. Senior author of a comprehensive review article on pediatric migraine in Neurologic Clinics, Dr. Gelfand said the practical value of young adults learning what medications they are taking, and why, can place them in a better position to monitor their disease and to understand when a clinical visit is appropriate.
“I agree that it is important to help young adults (i.e., 18- or 19-year-olds) to prepare for the transition from the pediatric health care environment to the adult one,” said Dr. Gelfand, who has written frequently on this and related topics, such as the impact of comorbidities on outcome.
Dr. O’Brien reports financial relationships with AbbVie, Eli Lilly, Guidepoint, Pfizer, and Vector Psychometric Group. Dr. Gelfand reports financial relationships with Allergan, Eli Lilly, EMKinetics, eNeura, Teva and Zosano.
FROM THE 2023 SCOTTSDALE HEADACHE SYMPOSIUM
Memory-enhancing intervention may help boost confidence, not necessarily memory, in older adults, study suggests
A novel approach aimed at enhancing everyday memory may lead older adults to feel more confident that they can accurately recollect phone numbers, names, and other information, according to findings from a small randomized controlled trial that were presented at the annual meeting of the Gerontological Society of America.
The tool, called Everyday Memory and Metacognitive Intervention (EMMI), trains people to be more mindful of memories, like where they parked their car, by repeating information at increasing intervals and self-testing.
EMMI “is a very important approach, focused on everyday memory,” said George W. Rebok, PhD, professor emeritus in the department of mental health at Johns Hopkins University, Baltimore, who was not involved with the study. “Many times, when we do memory interventions, we only focus on improving objective memories,” such as recalling major life events or one-time occurrences.
Everyday memory was defined as recalling basic facts including names, phone numbers, and daily appointments. The research, led by Ann Pearman, MD, associate director of adult psychology at Case Western Reserve University School of Medicine at MetroHealth Medical Center, Cleveland, Ohio, expanded on previous work she conducted with colleagues. That study found that EMMI may help improve confidence in the ability to recollect information and functional independence among older adults.
The current study was of 62 of the same participants in the earlier research, with one group that received EMMI (n = 30) and another that underwent traditional memory strategy training ([MSC]; n = 32). Both groups underwent four 3-hour virtual training sessions in their designated intervention over 2 weeks.
“One of the most important parts of the study is the [training] period,” when participants build new habits to help recall their everyday memories, Dr. Pearman said.
For 7 weeks, participants reported errors in everyday memories on a smartphone and submitted diary entries for each. Dr. Rebok that said tracking can help identify patterns or circumstances under which a person is likely to experience a memory lapse.
The study found mixed results when comparing EMMI with MSC, with the latter group demonstrating greater improvements in associative memory, such as pairing of a name to a face, highlighting the effectiveness of traditional MCS.
However, participants who underwent EMMI reported an increase in self-confidence that they were able to remember things, compared with those in the MSC group (4.92, confidence interval 95%, P = .30).
The EMMI intervention also was not uniformly effective in reducing memory errors across all participants in the group, which is to be expected, experts note. “In memory training, as with any kind of cognitive training, one size doesn’t fit all,” Dr. Rebok said.
“The mixed findings may highlight the need for a holistic approach to memory improvement and brain health, especially in older adults,” said Krystal L. Culler, DBH, founder of the Virtual Brain Health Center in Cleveland, who was not involved with the study.
EMMI could potentially be part of a broader strategy that includes lifestyle factors like sleep hygiene, physical exercise, diet, and social engagement to support optimal memory care, Dr. Culler said.
Patients who noticed some change in their memory and who are interested in making some positive changes in their daily cognitive functioning may benefit most from EMMI, according to Dr. Pearman.
“Making proactive decisions about memory challenges [patients] in their thinking and doing in everyday life,” she said.
Dr. Pearman shared that she and her colleagues are now looking into a combined EMMI and traditional memory strategy training to maximize the benefits of both interventions.
The study was supported by the Retirement Research Foundation (2018-2019); and the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK111024) from the Georgia Center for Diabetes Translation Research. The study authors report no relevant conflicts. Dr. Culler and Dr. Rebok report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel approach aimed at enhancing everyday memory may lead older adults to feel more confident that they can accurately recollect phone numbers, names, and other information, according to findings from a small randomized controlled trial that were presented at the annual meeting of the Gerontological Society of America.
The tool, called Everyday Memory and Metacognitive Intervention (EMMI), trains people to be more mindful of memories, like where they parked their car, by repeating information at increasing intervals and self-testing.
EMMI “is a very important approach, focused on everyday memory,” said George W. Rebok, PhD, professor emeritus in the department of mental health at Johns Hopkins University, Baltimore, who was not involved with the study. “Many times, when we do memory interventions, we only focus on improving objective memories,” such as recalling major life events or one-time occurrences.
Everyday memory was defined as recalling basic facts including names, phone numbers, and daily appointments. The research, led by Ann Pearman, MD, associate director of adult psychology at Case Western Reserve University School of Medicine at MetroHealth Medical Center, Cleveland, Ohio, expanded on previous work she conducted with colleagues. That study found that EMMI may help improve confidence in the ability to recollect information and functional independence among older adults.
The current study was of 62 of the same participants in the earlier research, with one group that received EMMI (n = 30) and another that underwent traditional memory strategy training ([MSC]; n = 32). Both groups underwent four 3-hour virtual training sessions in their designated intervention over 2 weeks.
“One of the most important parts of the study is the [training] period,” when participants build new habits to help recall their everyday memories, Dr. Pearman said.
For 7 weeks, participants reported errors in everyday memories on a smartphone and submitted diary entries for each. Dr. Rebok that said tracking can help identify patterns or circumstances under which a person is likely to experience a memory lapse.
The study found mixed results when comparing EMMI with MSC, with the latter group demonstrating greater improvements in associative memory, such as pairing of a name to a face, highlighting the effectiveness of traditional MCS.
However, participants who underwent EMMI reported an increase in self-confidence that they were able to remember things, compared with those in the MSC group (4.92, confidence interval 95%, P = .30).
The EMMI intervention also was not uniformly effective in reducing memory errors across all participants in the group, which is to be expected, experts note. “In memory training, as with any kind of cognitive training, one size doesn’t fit all,” Dr. Rebok said.
“The mixed findings may highlight the need for a holistic approach to memory improvement and brain health, especially in older adults,” said Krystal L. Culler, DBH, founder of the Virtual Brain Health Center in Cleveland, who was not involved with the study.
EMMI could potentially be part of a broader strategy that includes lifestyle factors like sleep hygiene, physical exercise, diet, and social engagement to support optimal memory care, Dr. Culler said.
Patients who noticed some change in their memory and who are interested in making some positive changes in their daily cognitive functioning may benefit most from EMMI, according to Dr. Pearman.
“Making proactive decisions about memory challenges [patients] in their thinking and doing in everyday life,” she said.
Dr. Pearman shared that she and her colleagues are now looking into a combined EMMI and traditional memory strategy training to maximize the benefits of both interventions.
The study was supported by the Retirement Research Foundation (2018-2019); and the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK111024) from the Georgia Center for Diabetes Translation Research. The study authors report no relevant conflicts. Dr. Culler and Dr. Rebok report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel approach aimed at enhancing everyday memory may lead older adults to feel more confident that they can accurately recollect phone numbers, names, and other information, according to findings from a small randomized controlled trial that were presented at the annual meeting of the Gerontological Society of America.
The tool, called Everyday Memory and Metacognitive Intervention (EMMI), trains people to be more mindful of memories, like where they parked their car, by repeating information at increasing intervals and self-testing.
EMMI “is a very important approach, focused on everyday memory,” said George W. Rebok, PhD, professor emeritus in the department of mental health at Johns Hopkins University, Baltimore, who was not involved with the study. “Many times, when we do memory interventions, we only focus on improving objective memories,” such as recalling major life events or one-time occurrences.
Everyday memory was defined as recalling basic facts including names, phone numbers, and daily appointments. The research, led by Ann Pearman, MD, associate director of adult psychology at Case Western Reserve University School of Medicine at MetroHealth Medical Center, Cleveland, Ohio, expanded on previous work she conducted with colleagues. That study found that EMMI may help improve confidence in the ability to recollect information and functional independence among older adults.
The current study was of 62 of the same participants in the earlier research, with one group that received EMMI (n = 30) and another that underwent traditional memory strategy training ([MSC]; n = 32). Both groups underwent four 3-hour virtual training sessions in their designated intervention over 2 weeks.
“One of the most important parts of the study is the [training] period,” when participants build new habits to help recall their everyday memories, Dr. Pearman said.
For 7 weeks, participants reported errors in everyday memories on a smartphone and submitted diary entries for each. Dr. Rebok that said tracking can help identify patterns or circumstances under which a person is likely to experience a memory lapse.
The study found mixed results when comparing EMMI with MSC, with the latter group demonstrating greater improvements in associative memory, such as pairing of a name to a face, highlighting the effectiveness of traditional MCS.
However, participants who underwent EMMI reported an increase in self-confidence that they were able to remember things, compared with those in the MSC group (4.92, confidence interval 95%, P = .30).
The EMMI intervention also was not uniformly effective in reducing memory errors across all participants in the group, which is to be expected, experts note. “In memory training, as with any kind of cognitive training, one size doesn’t fit all,” Dr. Rebok said.
“The mixed findings may highlight the need for a holistic approach to memory improvement and brain health, especially in older adults,” said Krystal L. Culler, DBH, founder of the Virtual Brain Health Center in Cleveland, who was not involved with the study.
EMMI could potentially be part of a broader strategy that includes lifestyle factors like sleep hygiene, physical exercise, diet, and social engagement to support optimal memory care, Dr. Culler said.
Patients who noticed some change in their memory and who are interested in making some positive changes in their daily cognitive functioning may benefit most from EMMI, according to Dr. Pearman.
“Making proactive decisions about memory challenges [patients] in their thinking and doing in everyday life,” she said.
Dr. Pearman shared that she and her colleagues are now looking into a combined EMMI and traditional memory strategy training to maximize the benefits of both interventions.
The study was supported by the Retirement Research Foundation (2018-2019); and the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK111024) from the Georgia Center for Diabetes Translation Research. The study authors report no relevant conflicts. Dr. Culler and Dr. Rebok report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GSA 2023
Avoid adding to minority stress when treating headache in LGBTQIA+ patients
It is “important not to assume that just because someone is a member of the LGBTQ+ community they will need psychiatric or behavioral health support,” said Maya A. Marzouk, PhD, division of behavioral medicine and clinical psychology, Cincinnati Children’s Hospital Medical Center.
Instead, it is useful not to make any assumptions. There is a potential association between minority status and headache susceptibility, but it is more reasonable initially to address the diagnosis and treatment of headache in LGBTQIA+ patients the same way it is addressed in any other patient, Dr. Marzouk said at the 2023 Scottsdale Headache Symposium.
The acronym to describe individuals with gender identities different from male and female and sexual orientations not limited to heterosexuality has been in almost constant evolution over several decades. An addition sign that accompanies LGBTQIA refers to those who do not identify with any letters in the acronym (lesbian, gay, bisexual, transsexual, queer/questioning, intersex, and asexual).
Take steps to normalize the interaction
Although many clinicians have been acclimated to these diverse identifies, not all have risen above preconceptions that become obstacles to effective care, according to Dr. Marzouk. In the context of headache management, Dr. Marzouk emphasized the need to be respectful of the range of gender identities and sexual orientations and to take steps to normalize the interaction.
For example, Dr. Marzouk advised using gender-neutral language at the start of each patient encounter and ask open-ended questions about gender, sexual identify, and pronouns to avoid patient discomfort from misidentification. In turn, the clinicians can establish their own gender identification and preferred pronouns to reinforce the idea that doing so is normal behavior.
This change in approach should be made “for all patients. Do not try to guess who needs them,” she said.
Intake forms and office atmosphere, such as signs and images, should also be welcoming to all patients, she added. Rather than trying to make adjustments for a LGBTQIA+ visit, Dr. Marzouk said a uniform approach helps normalize the experience of LGBTQIA+ patients without singling them out.
Despite the effort to provide an open and welcoming environment, Dr. Marzouk acknowledged that mistakes are difficult to avoid for those with limited experience serving the LGBTQIA+ community. When mistakes are made, she advised clinicians to immediately acknowledge the mistake and ask for guidance from the patient.
The potential offense is making the patient feel “other” or abnormal.
A higher rate of migraine
The interactions that LBGTQIA+ patients have with others outside their community is a possible explanation for the substantial rate of headache as well as headache with comorbid psychiatric disorders in this population.
In a survey published in 2020, the rate of migraine was 19.7% in heterosexual women, 26.7% in lesbians, and 36.8% in bisexual women. Among men, it rose from 9.8% in heterosexuals to 14.8% in gays and then to 22.8% in bisexuals.
Migraine relative to headache is also associated with more mood disorders among LGBTQIA+ individuals. In a study published in 2022, LGBTQIA+ patients with migraine relative to those with headache were more likely to have depression (46.4% vs. 22.3%; P < .001), anxiety (72.1% vs. 51.6%; P < .001), and posttraumatic stress disorder (37.5% vs. 21.4%; P < .001).
A vicious cycle of underdiagnosis and undertreatment
These associations are consistent with minority stress theory, according to Dr. Marzouk. This theory postulates that the associated stress of discrimination, rejection, and microaggressions, such as explicit efforts to make LGBTQIA+ individuals to feel “other,” produces epigenetic changes and dysregulation of the hypothalamic-pituitary-adrenal axis. In turn, this plays a role in the pathogenesis of migraine.
The inconsistency with which minority stress affects LGBTQIA+ patients might be due to relative differences in social support, coping skills, an innate resilience to these effects, Dr. Marzouk explained.
Dr. Marzouk characterized the LGBTQIA+ community as “underserved” for treatment of headache. She suggested that medical mistrust and self-blame among LGBTQIA+ individuals might be factors contributing to a vicious cycle of underdiagnosis and undertreatment. Efforts by the medical community to reach out to the LGBTQIA+ community are appropriate to address an unmet need.
“Individuals with psychiatric comorbidities may experience even more benefit from migraine care,” she said.
Clinical studies should be more inclusive
While agreeing in principle with these remarks, Eric A. Kaiser, MD, PhD, department of neurology, University of Pennsylvania, Philadelphia, said that this area would be better advanced if studies routinely included patients with diverse-gender identities and sexual orientations. Speaking about how to organize these studies, Dr. Kaiser suggested that enrollment criteria should explicitly seek these individuals and that these differences should be captured in the baseline characteristics.
“For example, gender options could include man, woman, non-binary, gender diverse, gender nonconforming, or gender nonspecified,” he said.
To close “the significant knowledge gap that exists in managing headache disorders in sexually- and gender- diverse people,” Dr. Kaiser said that clinical research studies, like patient treatment of diverse populations, “should be conducted with welcoming and affirming practices.”
Dr. Marzouk reported no potential conflicts of interest. Dr. Kaiser reported financial relationships with Amgen and Lundbeck.
It is “important not to assume that just because someone is a member of the LGBTQ+ community they will need psychiatric or behavioral health support,” said Maya A. Marzouk, PhD, division of behavioral medicine and clinical psychology, Cincinnati Children’s Hospital Medical Center.
Instead, it is useful not to make any assumptions. There is a potential association between minority status and headache susceptibility, but it is more reasonable initially to address the diagnosis and treatment of headache in LGBTQIA+ patients the same way it is addressed in any other patient, Dr. Marzouk said at the 2023 Scottsdale Headache Symposium.
The acronym to describe individuals with gender identities different from male and female and sexual orientations not limited to heterosexuality has been in almost constant evolution over several decades. An addition sign that accompanies LGBTQIA refers to those who do not identify with any letters in the acronym (lesbian, gay, bisexual, transsexual, queer/questioning, intersex, and asexual).
Take steps to normalize the interaction
Although many clinicians have been acclimated to these diverse identifies, not all have risen above preconceptions that become obstacles to effective care, according to Dr. Marzouk. In the context of headache management, Dr. Marzouk emphasized the need to be respectful of the range of gender identities and sexual orientations and to take steps to normalize the interaction.
For example, Dr. Marzouk advised using gender-neutral language at the start of each patient encounter and ask open-ended questions about gender, sexual identify, and pronouns to avoid patient discomfort from misidentification. In turn, the clinicians can establish their own gender identification and preferred pronouns to reinforce the idea that doing so is normal behavior.
This change in approach should be made “for all patients. Do not try to guess who needs them,” she said.
Intake forms and office atmosphere, such as signs and images, should also be welcoming to all patients, she added. Rather than trying to make adjustments for a LGBTQIA+ visit, Dr. Marzouk said a uniform approach helps normalize the experience of LGBTQIA+ patients without singling them out.
Despite the effort to provide an open and welcoming environment, Dr. Marzouk acknowledged that mistakes are difficult to avoid for those with limited experience serving the LGBTQIA+ community. When mistakes are made, she advised clinicians to immediately acknowledge the mistake and ask for guidance from the patient.
The potential offense is making the patient feel “other” or abnormal.
A higher rate of migraine
The interactions that LBGTQIA+ patients have with others outside their community is a possible explanation for the substantial rate of headache as well as headache with comorbid psychiatric disorders in this population.
In a survey published in 2020, the rate of migraine was 19.7% in heterosexual women, 26.7% in lesbians, and 36.8% in bisexual women. Among men, it rose from 9.8% in heterosexuals to 14.8% in gays and then to 22.8% in bisexuals.
Migraine relative to headache is also associated with more mood disorders among LGBTQIA+ individuals. In a study published in 2022, LGBTQIA+ patients with migraine relative to those with headache were more likely to have depression (46.4% vs. 22.3%; P < .001), anxiety (72.1% vs. 51.6%; P < .001), and posttraumatic stress disorder (37.5% vs. 21.4%; P < .001).
A vicious cycle of underdiagnosis and undertreatment
These associations are consistent with minority stress theory, according to Dr. Marzouk. This theory postulates that the associated stress of discrimination, rejection, and microaggressions, such as explicit efforts to make LGBTQIA+ individuals to feel “other,” produces epigenetic changes and dysregulation of the hypothalamic-pituitary-adrenal axis. In turn, this plays a role in the pathogenesis of migraine.
The inconsistency with which minority stress affects LGBTQIA+ patients might be due to relative differences in social support, coping skills, an innate resilience to these effects, Dr. Marzouk explained.
Dr. Marzouk characterized the LGBTQIA+ community as “underserved” for treatment of headache. She suggested that medical mistrust and self-blame among LGBTQIA+ individuals might be factors contributing to a vicious cycle of underdiagnosis and undertreatment. Efforts by the medical community to reach out to the LGBTQIA+ community are appropriate to address an unmet need.
“Individuals with psychiatric comorbidities may experience even more benefit from migraine care,” she said.
Clinical studies should be more inclusive
While agreeing in principle with these remarks, Eric A. Kaiser, MD, PhD, department of neurology, University of Pennsylvania, Philadelphia, said that this area would be better advanced if studies routinely included patients with diverse-gender identities and sexual orientations. Speaking about how to organize these studies, Dr. Kaiser suggested that enrollment criteria should explicitly seek these individuals and that these differences should be captured in the baseline characteristics.
“For example, gender options could include man, woman, non-binary, gender diverse, gender nonconforming, or gender nonspecified,” he said.
To close “the significant knowledge gap that exists in managing headache disorders in sexually- and gender- diverse people,” Dr. Kaiser said that clinical research studies, like patient treatment of diverse populations, “should be conducted with welcoming and affirming practices.”
Dr. Marzouk reported no potential conflicts of interest. Dr. Kaiser reported financial relationships with Amgen and Lundbeck.
It is “important not to assume that just because someone is a member of the LGBTQ+ community they will need psychiatric or behavioral health support,” said Maya A. Marzouk, PhD, division of behavioral medicine and clinical psychology, Cincinnati Children’s Hospital Medical Center.
Instead, it is useful not to make any assumptions. There is a potential association between minority status and headache susceptibility, but it is more reasonable initially to address the diagnosis and treatment of headache in LGBTQIA+ patients the same way it is addressed in any other patient, Dr. Marzouk said at the 2023 Scottsdale Headache Symposium.
The acronym to describe individuals with gender identities different from male and female and sexual orientations not limited to heterosexuality has been in almost constant evolution over several decades. An addition sign that accompanies LGBTQIA refers to those who do not identify with any letters in the acronym (lesbian, gay, bisexual, transsexual, queer/questioning, intersex, and asexual).
Take steps to normalize the interaction
Although many clinicians have been acclimated to these diverse identifies, not all have risen above preconceptions that become obstacles to effective care, according to Dr. Marzouk. In the context of headache management, Dr. Marzouk emphasized the need to be respectful of the range of gender identities and sexual orientations and to take steps to normalize the interaction.
For example, Dr. Marzouk advised using gender-neutral language at the start of each patient encounter and ask open-ended questions about gender, sexual identify, and pronouns to avoid patient discomfort from misidentification. In turn, the clinicians can establish their own gender identification and preferred pronouns to reinforce the idea that doing so is normal behavior.
This change in approach should be made “for all patients. Do not try to guess who needs them,” she said.
Intake forms and office atmosphere, such as signs and images, should also be welcoming to all patients, she added. Rather than trying to make adjustments for a LGBTQIA+ visit, Dr. Marzouk said a uniform approach helps normalize the experience of LGBTQIA+ patients without singling them out.
Despite the effort to provide an open and welcoming environment, Dr. Marzouk acknowledged that mistakes are difficult to avoid for those with limited experience serving the LGBTQIA+ community. When mistakes are made, she advised clinicians to immediately acknowledge the mistake and ask for guidance from the patient.
The potential offense is making the patient feel “other” or abnormal.
A higher rate of migraine
The interactions that LBGTQIA+ patients have with others outside their community is a possible explanation for the substantial rate of headache as well as headache with comorbid psychiatric disorders in this population.
In a survey published in 2020, the rate of migraine was 19.7% in heterosexual women, 26.7% in lesbians, and 36.8% in bisexual women. Among men, it rose from 9.8% in heterosexuals to 14.8% in gays and then to 22.8% in bisexuals.
Migraine relative to headache is also associated with more mood disorders among LGBTQIA+ individuals. In a study published in 2022, LGBTQIA+ patients with migraine relative to those with headache were more likely to have depression (46.4% vs. 22.3%; P < .001), anxiety (72.1% vs. 51.6%; P < .001), and posttraumatic stress disorder (37.5% vs. 21.4%; P < .001).
A vicious cycle of underdiagnosis and undertreatment
These associations are consistent with minority stress theory, according to Dr. Marzouk. This theory postulates that the associated stress of discrimination, rejection, and microaggressions, such as explicit efforts to make LGBTQIA+ individuals to feel “other,” produces epigenetic changes and dysregulation of the hypothalamic-pituitary-adrenal axis. In turn, this plays a role in the pathogenesis of migraine.
The inconsistency with which minority stress affects LGBTQIA+ patients might be due to relative differences in social support, coping skills, an innate resilience to these effects, Dr. Marzouk explained.
Dr. Marzouk characterized the LGBTQIA+ community as “underserved” for treatment of headache. She suggested that medical mistrust and self-blame among LGBTQIA+ individuals might be factors contributing to a vicious cycle of underdiagnosis and undertreatment. Efforts by the medical community to reach out to the LGBTQIA+ community are appropriate to address an unmet need.
“Individuals with psychiatric comorbidities may experience even more benefit from migraine care,” she said.
Clinical studies should be more inclusive
While agreeing in principle with these remarks, Eric A. Kaiser, MD, PhD, department of neurology, University of Pennsylvania, Philadelphia, said that this area would be better advanced if studies routinely included patients with diverse-gender identities and sexual orientations. Speaking about how to organize these studies, Dr. Kaiser suggested that enrollment criteria should explicitly seek these individuals and that these differences should be captured in the baseline characteristics.
“For example, gender options could include man, woman, non-binary, gender diverse, gender nonconforming, or gender nonspecified,” he said.
To close “the significant knowledge gap that exists in managing headache disorders in sexually- and gender- diverse people,” Dr. Kaiser said that clinical research studies, like patient treatment of diverse populations, “should be conducted with welcoming and affirming practices.”
Dr. Marzouk reported no potential conflicts of interest. Dr. Kaiser reported financial relationships with Amgen and Lundbeck.
FROM THE 2023 SCOTTSDALE HEADACHE SYMPOSIUM