User login
Pregnant women commonly refuse the influenza vaccine
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Although almost all ob.gyns. recommend the influenza and Tdap vaccines for pregnant women, both commonly are refused.
Major finding: A total of 62% of ob.gyns. reported that 10% or greater of their pregnant patients refused the influenza vaccine; 32% reported this for Tdap vaccine.
Study details: An email and mail survey sent to a national network of ob.gyns. between March and June 2016.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
Source: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Uptick in adult syphilis means congenital syphilis may be lurking
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.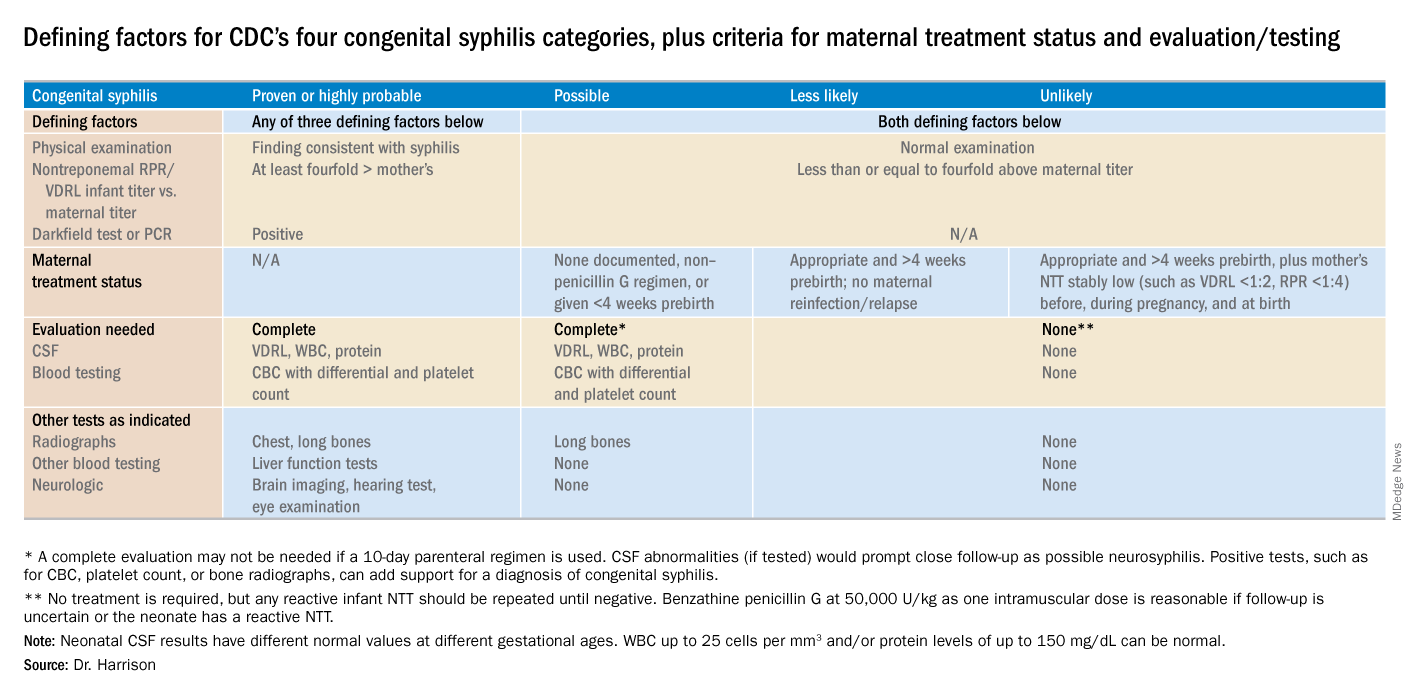
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.
While many pediatric clinicians have not frequently managed newborns of mothers with reactive syphilis serology, increased adult syphilis may change that.1
Diagnosing/managing congenital syphilis is not always clear cut. A positive rapid plasma reagin (RPR) titer in a newborn may not indicate congenital infection but merely may reflect transplacental, passively acquired maternal IgG from the mother’s current or previous infection rather than antibodies produced by the newborn. Because currently no IgM assay for syphilis is recommended by the Centers for Disease Control and Prevention for newborn testing, we must deal with IgG test results.
Often initial management decisions are needed while the infant’s status is evolving. The questions to answer to make final decisions include the following2:
- Was the mother actively infected with Treponema pallidum during pregnancy?
- If so, was the mother appropriately treated and when?
- Does the infant have any clinical, laboratory, or radiographic evidence of syphilis?
- How do the mother’s and infant’s nontreponemal serologic titers (NTT) compare at delivery using the same test?
Note: All infants assessed for congenital syphilis need a full evaluation for HIV.
Managing the infant of a mother with positive tests3,4
All such neonates need an examination for evidence of congenital syphilis. The clinical signs of congenital syphilis in neonates include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of extremity. Also, consider dark-field examination or polymerase chain reaction (PCR) of lesions (such as bullae) or secretions (nasal). If available, have the placenta examined histologically (silver stain) or by PCR (Clinical Laboratory Improvement Amendments–validated test). Skeletal radiographic surveys are more useful for stillborn than live born infants. (The complete algorithm can be found in Figure 3.10 of reference 4.)
Order a quantitative NTT, using the Venereal Disease Research Laboratory (VDRL) test or RPR test on neonatal serum. Umbilical cord blood is not appropriate because of potential maternal blood contamination, which could give a false-positive result, or Wharton’s jelly, which could give a false-negative result. Use of treponemal-specific tests that are used for maternal diagnosis – such as T. pallidum particle agglutination (TP-PA), T. pallidum enzyme-linked immunosorbent assay (TP-EIA), fluorescent treponemal antibody absorption (FTA-ABS) test, or T. pallidum chemiluminescence immunoassay (TP-CIA) – on neonatal serum is not recommended because of difficulties in interpretation.
Diagnostic results allow designation of an infant into one of four CDC categories: proven/highly probable syphilis; possible syphilis; syphilis less likely; and syphilis unlikely. Treatment recommendations are based on these categories.
Proven or highly probable syphilis
There are two alternative recommended 10-day treatment regimens.
A. Aqueous crystalline penicillin G 100,000-150,000 U/kg per day by IV at 50,000 U/kg per dose, given every 12 hours through 7 days of age or every 8 hours if greater than 7 days old.
B. Procaine penicillin G at 50,000 U/kg per dose intramuscularly in one dose each day.
More than 1 day of missed therapy requires restarting a new 10-day course. Use of other antimicrobial agents (such as ampicillin) is not validated, so any empiric ampicillin initially given for possible sepsis does not count toward the 10-day penicillin regimen. If nonpenicillin drugs must be used, close serologic follow-up must occur to ensure adequacy of response to therapy.
Possible syphilis
There are three alternative regimens, the same two as in proven/highly probable syphilis (above) plus a single-dose option
A. Aqueous crystalline penicillin G, as described above.
B. Procaine penicillin G, as described above.
C. Benzathine penicillin G at 50,000 U/kg per dose intramuscularly in a single dose.
Note: To be eligible for regimen C, an infant must have a complete evaluation that is normal (cerebrospinal fluid [CSF] examination, long-bone radiographs, and complete blood count with platelet count) and follow-up must be assured. Exception: Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Less likely syphilis
One antibiotic regimen is available, but no treatment also may be an option.
A. Benzathine penicillin G as described above.
B. If mother’s NTT has decreased at least fourfold after appropriate early syphilis therapy or remained stably low, which indicates latent syphilis (VDRL less than 1:2; RPR less than 1:4), no treatment is an option but requires repeat serology every 2-3 months until infant is 6 months old.
Unlikely syphilis
No treatment is recommended unless follow-up is uncertain, in which case it is appropriate to give the infant benzathine penicillin G as described above.
Infant with positive NTT at birth
All neonates with reactive NTT need careful follow-up examinations and repeat NTT every 2-3 months until nonreactive. NTT in infants who are not treated because of less likely or unlikely syphilis status should drop by 3 months and be nonreactive by 6 months; this indicates NTT was passively transferred maternal IgG. If NTT remains reactive at 6 months, the infant is likely infected and needs treatment. Persistent NTT at 6-12 months in treated neonates should trigger repeat CSF examination and infectious diseases consultation about a possible repeat of the 10-day penicillin G regimen. If the mother was seroreactive, but the newborn’s NTT was negative at birth, testing of the infant’s NTT needs repeating at 3 months to exclude the possibility that the congenital syphilis was incubating when prior testing occurred at birth. Note: Treponemal-specific tests are not useful in assessing treatment because detectable maternal IgG treponemal antibody can persist at least 15 months.
Neonates with abnormal CSF at birth
Repeat cerebrospinal fluid evaluation every 6 months until results normalize. Persistently reactive CSF VDRL or abnormal CSF indexes not caused by another known cause requires retreatment for possible neurosyphilis, as well as consultation with an expert.
Summary
NTT are the essential test for newborns and some degree of laboratory or imaging work up often are needed. Consider consulting an expert in infectious diseases and/or perinatology if the gray areas do not readily become clear. Treatment of the correct patients with the right drug for the right duration remains the goal, as usual.
Dr. Harrison is a professor of pediatrics at University of Missouri-Kansas City and Director of Research Affairs in the pediatric infectious diseases division at Children’s Mercy Hospital – Kansas City. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2015 Nov 13;64(44);1241-5.
2. “Congenital Syphilis,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
3. “Syphilis During Pregnancy,” 2015 Sexually Transmitted Diseases Treatment Guidelines.
4. Syphilis – Section 3: Summaries of Infectious Diseases. Red Book Online. 2018.
New syphilis cases for pregnant women rose 61% over 5 years
Syphilis cases increased by 61% between 2012 and 2016 among pregnant women, and the proportion of syphilis cases was higher for women who were non-Hispanic black race and Hispanic ethnicity, according to research in Obstetrics & Gynecology.
“These findings support current recommendations for universal syphilis screening at the first prenatal visit and indicate that it may be necessary to include population context when determining whether to implement repeat screening during pregnancy,” Shivika Trivedi, MD, MSc, of the CDC Foundation and the Division of STD Prevention at the Centers for Disease Control and Prevention and colleagues wrote.
Dr. Trivedi and colleagues identified 9,883 pregnant women with reported syphilis in the CDC National Notifiable Diseases Surveillance System during 2012-2016. During that time, there was an increase in the number of female syphilis cases from 9,551 cases in 2012 to 14,838 cases in 2016 (55%), while there was an increase in the number of syphilis cases for pregnant women from 1,561 cases in 2012 to 2,508 cases in 2016 (61%). Of the risk factors reported for syphilis, 49% reported no risk factors within 12 priors before diagnosis, 43% said they had had at least one sexually transmitted disease, and 30% reported more than one sexual partner within the last year.
The greatest prevalence for syphilis was among women who were in their 20s (59%), located in the South (56%), and were non-Hispanic black (49%) or Hispanic (28%). However, researchers noted the rates of syphilis increased among all women between 18 years and 45 years and in every race and ethnicity group between 2012 and 2016. Early syphilis cases increased from 35% in 2012 to 58% in 2016, while late latent cases decreased from 65% in 2012 to 42% in 2016.
Researchers noted several limitations in the study, including case-based surveillance data, which potentially underreported the rates of syphilis, and a lack of pregnancy outcomes for pregnant women with syphilitic infections. However, they noted the data do show a trend of syphilis infections in pregnant women because the live birth rate “was relatively stable and did not fluctuate more than” 1.5% between 2012 and 2016.
“Through an increased awareness of the rising syphilis cases among pregnant women as well as these trend data, health care providers can be better informed to ensure they are following national guidelines and state policies for syphilis screening in pregnancy,” Dr. Trivedi and colleagues concluded.
The authors reported no relevant conflicts of interest.
SOURCE: Trivedi S et al. Obstet Gynecol. 2018. doi: 10.1097/AOG.0000000000003000.
I think this is an important topic of which pregnant women and their providers should be aware. It is possible the rising incidence is a result of increased screening and awareness; however, regardless of whether this is the case, it is important to identify the cases of congenital syphilis as preventable.
It is important for providers to be aware of their local syphilis prevalence and regulations on prenatal syphilis screening because given the effects of congenital syphilis and the ease of treatment.
Martina L. Badell, MD, is an assistant professor in the department of gynecology and obstetrics and maternal-fetal medicine at Emory University in Atlanta. She reported no relevant conflicts of interest.
I think this is an important topic of which pregnant women and their providers should be aware. It is possible the rising incidence is a result of increased screening and awareness; however, regardless of whether this is the case, it is important to identify the cases of congenital syphilis as preventable.
It is important for providers to be aware of their local syphilis prevalence and regulations on prenatal syphilis screening because given the effects of congenital syphilis and the ease of treatment.
Martina L. Badell, MD, is an assistant professor in the department of gynecology and obstetrics and maternal-fetal medicine at Emory University in Atlanta. She reported no relevant conflicts of interest.
I think this is an important topic of which pregnant women and their providers should be aware. It is possible the rising incidence is a result of increased screening and awareness; however, regardless of whether this is the case, it is important to identify the cases of congenital syphilis as preventable.
It is important for providers to be aware of their local syphilis prevalence and regulations on prenatal syphilis screening because given the effects of congenital syphilis and the ease of treatment.
Martina L. Badell, MD, is an assistant professor in the department of gynecology and obstetrics and maternal-fetal medicine at Emory University in Atlanta. She reported no relevant conflicts of interest.
Syphilis cases increased by 61% between 2012 and 2016 among pregnant women, and the proportion of syphilis cases was higher for women who were non-Hispanic black race and Hispanic ethnicity, according to research in Obstetrics & Gynecology.
“These findings support current recommendations for universal syphilis screening at the first prenatal visit and indicate that it may be necessary to include population context when determining whether to implement repeat screening during pregnancy,” Shivika Trivedi, MD, MSc, of the CDC Foundation and the Division of STD Prevention at the Centers for Disease Control and Prevention and colleagues wrote.
Dr. Trivedi and colleagues identified 9,883 pregnant women with reported syphilis in the CDC National Notifiable Diseases Surveillance System during 2012-2016. During that time, there was an increase in the number of female syphilis cases from 9,551 cases in 2012 to 14,838 cases in 2016 (55%), while there was an increase in the number of syphilis cases for pregnant women from 1,561 cases in 2012 to 2,508 cases in 2016 (61%). Of the risk factors reported for syphilis, 49% reported no risk factors within 12 priors before diagnosis, 43% said they had had at least one sexually transmitted disease, and 30% reported more than one sexual partner within the last year.
The greatest prevalence for syphilis was among women who were in their 20s (59%), located in the South (56%), and were non-Hispanic black (49%) or Hispanic (28%). However, researchers noted the rates of syphilis increased among all women between 18 years and 45 years and in every race and ethnicity group between 2012 and 2016. Early syphilis cases increased from 35% in 2012 to 58% in 2016, while late latent cases decreased from 65% in 2012 to 42% in 2016.
Researchers noted several limitations in the study, including case-based surveillance data, which potentially underreported the rates of syphilis, and a lack of pregnancy outcomes for pregnant women with syphilitic infections. However, they noted the data do show a trend of syphilis infections in pregnant women because the live birth rate “was relatively stable and did not fluctuate more than” 1.5% between 2012 and 2016.
“Through an increased awareness of the rising syphilis cases among pregnant women as well as these trend data, health care providers can be better informed to ensure they are following national guidelines and state policies for syphilis screening in pregnancy,” Dr. Trivedi and colleagues concluded.
The authors reported no relevant conflicts of interest.
SOURCE: Trivedi S et al. Obstet Gynecol. 2018. doi: 10.1097/AOG.0000000000003000.
Syphilis cases increased by 61% between 2012 and 2016 among pregnant women, and the proportion of syphilis cases was higher for women who were non-Hispanic black race and Hispanic ethnicity, according to research in Obstetrics & Gynecology.
“These findings support current recommendations for universal syphilis screening at the first prenatal visit and indicate that it may be necessary to include population context when determining whether to implement repeat screening during pregnancy,” Shivika Trivedi, MD, MSc, of the CDC Foundation and the Division of STD Prevention at the Centers for Disease Control and Prevention and colleagues wrote.
Dr. Trivedi and colleagues identified 9,883 pregnant women with reported syphilis in the CDC National Notifiable Diseases Surveillance System during 2012-2016. During that time, there was an increase in the number of female syphilis cases from 9,551 cases in 2012 to 14,838 cases in 2016 (55%), while there was an increase in the number of syphilis cases for pregnant women from 1,561 cases in 2012 to 2,508 cases in 2016 (61%). Of the risk factors reported for syphilis, 49% reported no risk factors within 12 priors before diagnosis, 43% said they had had at least one sexually transmitted disease, and 30% reported more than one sexual partner within the last year.
The greatest prevalence for syphilis was among women who were in their 20s (59%), located in the South (56%), and were non-Hispanic black (49%) or Hispanic (28%). However, researchers noted the rates of syphilis increased among all women between 18 years and 45 years and in every race and ethnicity group between 2012 and 2016. Early syphilis cases increased from 35% in 2012 to 58% in 2016, while late latent cases decreased from 65% in 2012 to 42% in 2016.
Researchers noted several limitations in the study, including case-based surveillance data, which potentially underreported the rates of syphilis, and a lack of pregnancy outcomes for pregnant women with syphilitic infections. However, they noted the data do show a trend of syphilis infections in pregnant women because the live birth rate “was relatively stable and did not fluctuate more than” 1.5% between 2012 and 2016.
“Through an increased awareness of the rising syphilis cases among pregnant women as well as these trend data, health care providers can be better informed to ensure they are following national guidelines and state policies for syphilis screening in pregnancy,” Dr. Trivedi and colleagues concluded.
The authors reported no relevant conflicts of interest.
SOURCE: Trivedi S et al. Obstet Gynecol. 2018. doi: 10.1097/AOG.0000000000003000.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Syphilis rates rose more in pregnant women between 2012 and 2016, compared with women in the general population.
Major finding: There was an increase of syphilis cases by 61% among pregnant women, compared with a 55% increase among women overall.
Study details: A study of national case report data from 9,883 pregnant women with reported syphilis during 2012-2016.
Disclosures: The authors reported no relevant conflicts of interest.
Source: Trivedi S et al. Obstet Gynecol. 2018. doi: 10.1097/AOG.0000000000003000.
Brazil sees first live birth from deceased-donor uterus transplant
The healthy 2,550-g infant girl was born in December 2017 via a planned cesarean delivery at about 36 weeks’ gestation. Her mother, the transplant recipient, has congenital absence of the uterus from Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Removal of the transplanted uterus at the time of delivery allowed the woman to stop taking the immunosuppressive medications that she’d been on since the transplantation, which had been performed less than a year and a half previously.
The uterus had been retrieved from a 45-year-old donor who experienced a subarachnoid hemorrhage and subsequent brain death. The donor had three vaginal deliveries, and no history of reproductive issues or sexually transmitted infection, wrote Dani Ejzenberg, MD, and his colleagues at the University of São Paolo, Brazil.
The retrieval and transplantation procedures were done at the university’s hospital, in accordance with a research protocol approved by the university, a Brazilian national ethics committee, and the country’s national transplantation system. Thorough psychological evaluation was part of the research protocol, and the patient and her partner had monthly psychological counseling from therapists with expertise in transplant and fertility, wrote Dr. Ejzenberg and his colleagues.
In preparation for the transplantation, which occurred when the recipient was 32 years old, she had in vitro fertilization several months before the procedure. Eight “good-quality” blastocysts were retrieved and cryopreserved, said Dr. Ejzenberg and his coauthors. The recipient’s menstrual cycle resumed 37 days after transplantation, and one of the cryopreserved embryos was transferred about 7 months after the uterine transplantation procedure, resulting in the pregnancy.
The donor and recipient were matched only by ABO blood type, with no further tissue typing being done, wrote Dr. Ejzenberg and his colleagues. The immunosuppressive regimen paralleled that used in previous successful uterine transplantations from live donors in Sweden, with induction via 1 g intraoperative methylprednisolone and 1.5 mg/kg of thymoglobulin. Thereafter, the recipient received tacrolimus titrated to a trough of 8-10 ng/mL, along with mycophenolate mofetil 720 mg twice daily. Five months after her transplantation, the mycophenolate mofetil was replaced with 100 mg azathioprine and 10 mg prednisone daily, a regimen that she stayed on until cesarean delivery.
Broad-spectrum antibiotics, antifungals, and anthelmintics were administered during the patient’s hospital stay. Prophylactic antibiotics were continued for 6 months, and antiviral medication was given prophylactically for 3 months. The recipient had one episode of vaginal discharge, treated with antifungal medication, and one episode of pyelonephritis during pregnancy, treated during a brief inpatient stay.
Enoxaparin and aspirin were used for inpatient venous thromboembolism prophylaxis, and heparin and aspirin thereafter. Aspirin was discontinued at 34 weeks, and heparin the day before delivery.
Swedish and American teams involved in uterine transplantation are working to develop standardization of surgical techniques, immunosuppression protocol, and methods to monitor rejection.
However, pointed out Dr. Ejzenberg and his coauthors, some technical aspects were unique to the deceased donor transplantation. These included managing total ischemic time for the donor tissue because heart, liver, and kidney retrieval all were given priority.
One downstream effect of this was longer-than-expected procedure and anesthesia time for the recipient, because coordinating donor uterus retrieval and preparation of the surgical bed in the live recipient was tricky; surgery time was about 10.5 hours. Also, there was prolonged warm-ischemia time because six small-vessel anastomoses needed to be performed, wrote the investigators.
After reperfusion of the implanted uterus, there was brisk bleeding from a number of small vessels that had not been ligated on retrieval because of concerns about ischemic time. These were identified and sutured or cauterized, but the total estimated blood loss during the procedures was 1,200 mL, with most of that coming from the uterus, said Dr. Ejzenberg and his coauthors.
The donor uterus had a total of almost 8 hours of ischemic time, exceeding the previously published live donor maximum uterine ischemic time of 3 hours, 25 minutes. This experience can inform surgical teams considering future uterine transplantations.
Dr. Ejzenberg and his colleagues also said that they cast a broad net with immunosuppression, erring on the side of caution. With more experience may come the ability to scale back immunosuppressive regimens, they noted.
The explantation of the uterus and associated blood vessels after delivery afforded the opportunity for pathological examination of the uterus and other tissues, which showed no signs of rejection. The uterine arteries did have mild intimal fibrous hyperplasia that was likely related to the age of the donor, said Dr. Ejzenberg and his coauthors.
This successful completion of a deceased-donor uterine transplantation demonstrates the feasibility of accessing “a much wider potential donor population, as the numbers of people willing and committed to donate organs upon their own deaths are far larger than those of potential live donors,” wrote Dr. Ejzenberg and his colleagues. “Further incidental but substantial benefits of the use of deceased donors include lower costs and avoidance of live donors’ surgical risks.”
In 2011, a uterine transplantation from a deceased donor resulted in pregnancy, but ended in miscarriage.
Funding was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo and the Hospital das Clínicas of University of São Paulo School of Medicine. Dr. Ejzenberg and his colleagues reported that they had no conflicts of interest.
SOURCE: Ejzenberg D. et al. Lancet. 2018 Dec. doi: 10.1015/S0140-6736(18)31766-5.
Among the advances seen in this deceased-donor uterus transplant is a demonstration that ischemic time of nearly 8 hours – four times the average seen in live donation – does not preclude a successful transplantation.
Also, the timetable for transplantation seen here did not involve the year-long waiting period between transplantation and pregnancy that has been the norm in live uterine transplantation.
However, uterine transplantation, whether from a living or deceased donor, is still in its early stages. Among the many unsettled questions are whether live or deceased donor transplantations yield superior results. Additional technical aspects to be further studied include best surgical approach for the donor uterus, best anastomosis technique, and optimal immunosuppression and antimicrobial/antifungal/antiviral regimens.
Continued work needs to be done to standardize these and other aspects of the peri- and postoperative care of women undergoing uterine transplantation.
In addition, long-term tracking of children born from transplanted uteri is needed, so outcomes can be assessed over the lifespan.
Going forward, it could be that uterine transplantation may be offered to an expanded cohort of women, including those with bulky, nonoperable uterine fibroids, those who have received pelvic radiotherapy, and even those who have had multiple unexplained problems with implantation during fertility treatments. In all cases, researchers should work toward achieving the highest live birth rate at the lowest risk to donors and patients, while also working to make more organs available; establishing registries, and encouraging prospective registration and transparent reporting of uterus transplantation procedures.
Cesar Diaz-Garcia, MD, is medical director of IVI-London, and Antonio Pellicer, MD, is professor of obstetrics and gynecology at the University of Valencia, Spain. These remarks were drawn from their editorial accompanying the report by Ejzenberg et al. (Lancet. 2018 Dec. doi: 10.1016/50140-6736(18)32106-8).
Among the advances seen in this deceased-donor uterus transplant is a demonstration that ischemic time of nearly 8 hours – four times the average seen in live donation – does not preclude a successful transplantation.
Also, the timetable for transplantation seen here did not involve the year-long waiting period between transplantation and pregnancy that has been the norm in live uterine transplantation.
However, uterine transplantation, whether from a living or deceased donor, is still in its early stages. Among the many unsettled questions are whether live or deceased donor transplantations yield superior results. Additional technical aspects to be further studied include best surgical approach for the donor uterus, best anastomosis technique, and optimal immunosuppression and antimicrobial/antifungal/antiviral regimens.
Continued work needs to be done to standardize these and other aspects of the peri- and postoperative care of women undergoing uterine transplantation.
In addition, long-term tracking of children born from transplanted uteri is needed, so outcomes can be assessed over the lifespan.
Going forward, it could be that uterine transplantation may be offered to an expanded cohort of women, including those with bulky, nonoperable uterine fibroids, those who have received pelvic radiotherapy, and even those who have had multiple unexplained problems with implantation during fertility treatments. In all cases, researchers should work toward achieving the highest live birth rate at the lowest risk to donors and patients, while also working to make more organs available; establishing registries, and encouraging prospective registration and transparent reporting of uterus transplantation procedures.
Cesar Diaz-Garcia, MD, is medical director of IVI-London, and Antonio Pellicer, MD, is professor of obstetrics and gynecology at the University of Valencia, Spain. These remarks were drawn from their editorial accompanying the report by Ejzenberg et al. (Lancet. 2018 Dec. doi: 10.1016/50140-6736(18)32106-8).
Among the advances seen in this deceased-donor uterus transplant is a demonstration that ischemic time of nearly 8 hours – four times the average seen in live donation – does not preclude a successful transplantation.
Also, the timetable for transplantation seen here did not involve the year-long waiting period between transplantation and pregnancy that has been the norm in live uterine transplantation.
However, uterine transplantation, whether from a living or deceased donor, is still in its early stages. Among the many unsettled questions are whether live or deceased donor transplantations yield superior results. Additional technical aspects to be further studied include best surgical approach for the donor uterus, best anastomosis technique, and optimal immunosuppression and antimicrobial/antifungal/antiviral regimens.
Continued work needs to be done to standardize these and other aspects of the peri- and postoperative care of women undergoing uterine transplantation.
In addition, long-term tracking of children born from transplanted uteri is needed, so outcomes can be assessed over the lifespan.
Going forward, it could be that uterine transplantation may be offered to an expanded cohort of women, including those with bulky, nonoperable uterine fibroids, those who have received pelvic radiotherapy, and even those who have had multiple unexplained problems with implantation during fertility treatments. In all cases, researchers should work toward achieving the highest live birth rate at the lowest risk to donors and patients, while also working to make more organs available; establishing registries, and encouraging prospective registration and transparent reporting of uterus transplantation procedures.
Cesar Diaz-Garcia, MD, is medical director of IVI-London, and Antonio Pellicer, MD, is professor of obstetrics and gynecology at the University of Valencia, Spain. These remarks were drawn from their editorial accompanying the report by Ejzenberg et al. (Lancet. 2018 Dec. doi: 10.1016/50140-6736(18)32106-8).
The healthy 2,550-g infant girl was born in December 2017 via a planned cesarean delivery at about 36 weeks’ gestation. Her mother, the transplant recipient, has congenital absence of the uterus from Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Removal of the transplanted uterus at the time of delivery allowed the woman to stop taking the immunosuppressive medications that she’d been on since the transplantation, which had been performed less than a year and a half previously.
The uterus had been retrieved from a 45-year-old donor who experienced a subarachnoid hemorrhage and subsequent brain death. The donor had three vaginal deliveries, and no history of reproductive issues or sexually transmitted infection, wrote Dani Ejzenberg, MD, and his colleagues at the University of São Paolo, Brazil.
The retrieval and transplantation procedures were done at the university’s hospital, in accordance with a research protocol approved by the university, a Brazilian national ethics committee, and the country’s national transplantation system. Thorough psychological evaluation was part of the research protocol, and the patient and her partner had monthly psychological counseling from therapists with expertise in transplant and fertility, wrote Dr. Ejzenberg and his colleagues.
In preparation for the transplantation, which occurred when the recipient was 32 years old, she had in vitro fertilization several months before the procedure. Eight “good-quality” blastocysts were retrieved and cryopreserved, said Dr. Ejzenberg and his coauthors. The recipient’s menstrual cycle resumed 37 days after transplantation, and one of the cryopreserved embryos was transferred about 7 months after the uterine transplantation procedure, resulting in the pregnancy.
The donor and recipient were matched only by ABO blood type, with no further tissue typing being done, wrote Dr. Ejzenberg and his colleagues. The immunosuppressive regimen paralleled that used in previous successful uterine transplantations from live donors in Sweden, with induction via 1 g intraoperative methylprednisolone and 1.5 mg/kg of thymoglobulin. Thereafter, the recipient received tacrolimus titrated to a trough of 8-10 ng/mL, along with mycophenolate mofetil 720 mg twice daily. Five months after her transplantation, the mycophenolate mofetil was replaced with 100 mg azathioprine and 10 mg prednisone daily, a regimen that she stayed on until cesarean delivery.
Broad-spectrum antibiotics, antifungals, and anthelmintics were administered during the patient’s hospital stay. Prophylactic antibiotics were continued for 6 months, and antiviral medication was given prophylactically for 3 months. The recipient had one episode of vaginal discharge, treated with antifungal medication, and one episode of pyelonephritis during pregnancy, treated during a brief inpatient stay.
Enoxaparin and aspirin were used for inpatient venous thromboembolism prophylaxis, and heparin and aspirin thereafter. Aspirin was discontinued at 34 weeks, and heparin the day before delivery.
Swedish and American teams involved in uterine transplantation are working to develop standardization of surgical techniques, immunosuppression protocol, and methods to monitor rejection.
However, pointed out Dr. Ejzenberg and his coauthors, some technical aspects were unique to the deceased donor transplantation. These included managing total ischemic time for the donor tissue because heart, liver, and kidney retrieval all were given priority.
One downstream effect of this was longer-than-expected procedure and anesthesia time for the recipient, because coordinating donor uterus retrieval and preparation of the surgical bed in the live recipient was tricky; surgery time was about 10.5 hours. Also, there was prolonged warm-ischemia time because six small-vessel anastomoses needed to be performed, wrote the investigators.
After reperfusion of the implanted uterus, there was brisk bleeding from a number of small vessels that had not been ligated on retrieval because of concerns about ischemic time. These were identified and sutured or cauterized, but the total estimated blood loss during the procedures was 1,200 mL, with most of that coming from the uterus, said Dr. Ejzenberg and his coauthors.
The donor uterus had a total of almost 8 hours of ischemic time, exceeding the previously published live donor maximum uterine ischemic time of 3 hours, 25 minutes. This experience can inform surgical teams considering future uterine transplantations.
Dr. Ejzenberg and his colleagues also said that they cast a broad net with immunosuppression, erring on the side of caution. With more experience may come the ability to scale back immunosuppressive regimens, they noted.
The explantation of the uterus and associated blood vessels after delivery afforded the opportunity for pathological examination of the uterus and other tissues, which showed no signs of rejection. The uterine arteries did have mild intimal fibrous hyperplasia that was likely related to the age of the donor, said Dr. Ejzenberg and his coauthors.
This successful completion of a deceased-donor uterine transplantation demonstrates the feasibility of accessing “a much wider potential donor population, as the numbers of people willing and committed to donate organs upon their own deaths are far larger than those of potential live donors,” wrote Dr. Ejzenberg and his colleagues. “Further incidental but substantial benefits of the use of deceased donors include lower costs and avoidance of live donors’ surgical risks.”
In 2011, a uterine transplantation from a deceased donor resulted in pregnancy, but ended in miscarriage.
Funding was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo and the Hospital das Clínicas of University of São Paulo School of Medicine. Dr. Ejzenberg and his colleagues reported that they had no conflicts of interest.
SOURCE: Ejzenberg D. et al. Lancet. 2018 Dec. doi: 10.1015/S0140-6736(18)31766-5.
The healthy 2,550-g infant girl was born in December 2017 via a planned cesarean delivery at about 36 weeks’ gestation. Her mother, the transplant recipient, has congenital absence of the uterus from Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Removal of the transplanted uterus at the time of delivery allowed the woman to stop taking the immunosuppressive medications that she’d been on since the transplantation, which had been performed less than a year and a half previously.
The uterus had been retrieved from a 45-year-old donor who experienced a subarachnoid hemorrhage and subsequent brain death. The donor had three vaginal deliveries, and no history of reproductive issues or sexually transmitted infection, wrote Dani Ejzenberg, MD, and his colleagues at the University of São Paolo, Brazil.
The retrieval and transplantation procedures were done at the university’s hospital, in accordance with a research protocol approved by the university, a Brazilian national ethics committee, and the country’s national transplantation system. Thorough psychological evaluation was part of the research protocol, and the patient and her partner had monthly psychological counseling from therapists with expertise in transplant and fertility, wrote Dr. Ejzenberg and his colleagues.
In preparation for the transplantation, which occurred when the recipient was 32 years old, she had in vitro fertilization several months before the procedure. Eight “good-quality” blastocysts were retrieved and cryopreserved, said Dr. Ejzenberg and his coauthors. The recipient’s menstrual cycle resumed 37 days after transplantation, and one of the cryopreserved embryos was transferred about 7 months after the uterine transplantation procedure, resulting in the pregnancy.
The donor and recipient were matched only by ABO blood type, with no further tissue typing being done, wrote Dr. Ejzenberg and his colleagues. The immunosuppressive regimen paralleled that used in previous successful uterine transplantations from live donors in Sweden, with induction via 1 g intraoperative methylprednisolone and 1.5 mg/kg of thymoglobulin. Thereafter, the recipient received tacrolimus titrated to a trough of 8-10 ng/mL, along with mycophenolate mofetil 720 mg twice daily. Five months after her transplantation, the mycophenolate mofetil was replaced with 100 mg azathioprine and 10 mg prednisone daily, a regimen that she stayed on until cesarean delivery.
Broad-spectrum antibiotics, antifungals, and anthelmintics were administered during the patient’s hospital stay. Prophylactic antibiotics were continued for 6 months, and antiviral medication was given prophylactically for 3 months. The recipient had one episode of vaginal discharge, treated with antifungal medication, and one episode of pyelonephritis during pregnancy, treated during a brief inpatient stay.
Enoxaparin and aspirin were used for inpatient venous thromboembolism prophylaxis, and heparin and aspirin thereafter. Aspirin was discontinued at 34 weeks, and heparin the day before delivery.
Swedish and American teams involved in uterine transplantation are working to develop standardization of surgical techniques, immunosuppression protocol, and methods to monitor rejection.
However, pointed out Dr. Ejzenberg and his coauthors, some technical aspects were unique to the deceased donor transplantation. These included managing total ischemic time for the donor tissue because heart, liver, and kidney retrieval all were given priority.
One downstream effect of this was longer-than-expected procedure and anesthesia time for the recipient, because coordinating donor uterus retrieval and preparation of the surgical bed in the live recipient was tricky; surgery time was about 10.5 hours. Also, there was prolonged warm-ischemia time because six small-vessel anastomoses needed to be performed, wrote the investigators.
After reperfusion of the implanted uterus, there was brisk bleeding from a number of small vessels that had not been ligated on retrieval because of concerns about ischemic time. These were identified and sutured or cauterized, but the total estimated blood loss during the procedures was 1,200 mL, with most of that coming from the uterus, said Dr. Ejzenberg and his coauthors.
The donor uterus had a total of almost 8 hours of ischemic time, exceeding the previously published live donor maximum uterine ischemic time of 3 hours, 25 minutes. This experience can inform surgical teams considering future uterine transplantations.
Dr. Ejzenberg and his colleagues also said that they cast a broad net with immunosuppression, erring on the side of caution. With more experience may come the ability to scale back immunosuppressive regimens, they noted.
The explantation of the uterus and associated blood vessels after delivery afforded the opportunity for pathological examination of the uterus and other tissues, which showed no signs of rejection. The uterine arteries did have mild intimal fibrous hyperplasia that was likely related to the age of the donor, said Dr. Ejzenberg and his coauthors.
This successful completion of a deceased-donor uterine transplantation demonstrates the feasibility of accessing “a much wider potential donor population, as the numbers of people willing and committed to donate organs upon their own deaths are far larger than those of potential live donors,” wrote Dr. Ejzenberg and his colleagues. “Further incidental but substantial benefits of the use of deceased donors include lower costs and avoidance of live donors’ surgical risks.”
In 2011, a uterine transplantation from a deceased donor resulted in pregnancy, but ended in miscarriage.
Funding was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo and the Hospital das Clínicas of University of São Paulo School of Medicine. Dr. Ejzenberg and his colleagues reported that they had no conflicts of interest.
SOURCE: Ejzenberg D. et al. Lancet. 2018 Dec. doi: 10.1015/S0140-6736(18)31766-5.
FROM THE LANCET
Don’t push women into preterm delivery after myomectomy
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: Vaginal trial of labor is safe after myomectomy, at least if the uterine cavity wasn’t entered.
Major finding: Sixty-five percent of women who didn’t have their uterine cavities entered had vaginal deliveries, a success rate similar to labor after C-section.
Study details: Review of 102 pregnancies with live births after myomectomy at Northwestern University, Chicago
Disclosures: There was no external funding, and the lead investigator didn’t have any disclosures.
Source: King N et al. 2018 AAGL Global Congress, Abstract 162.
Self-report of prenatal marijuana use not very reliable
Even in the setting of legalized marijuana use, estimated prevalence of marijuana use during pregnancy was lower by self-report than it was by umbilical cord testing.
Torri D. Metz, MD, of the University of Utah Health, Salt Lake City, and her colleagues surveyed women at two urban hospitals in Colorado, which has legalized both medical and recreational use of marijuana. They found that, while 6% of the 116 women in the study reported using marijuana in the past 30 days, umbilical cord testing showed as many as 22% had detectable levels of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic, and 10% had levels above quantification.
The majority of studies of maternal marijuana use during pregnancy rely on self-report, so this could affect attempts to assess the effects of such prenatal use, they said.
Adverse outcomes associated with marijuana use during pregnancy include fetal growth restriction, small for gestational age, preterm birth, and adverse neurodevelopmental outcomes, studies have shown.
Read more in Obstetrics & Gynecology.
Even in the setting of legalized marijuana use, estimated prevalence of marijuana use during pregnancy was lower by self-report than it was by umbilical cord testing.
Torri D. Metz, MD, of the University of Utah Health, Salt Lake City, and her colleagues surveyed women at two urban hospitals in Colorado, which has legalized both medical and recreational use of marijuana. They found that, while 6% of the 116 women in the study reported using marijuana in the past 30 days, umbilical cord testing showed as many as 22% had detectable levels of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic, and 10% had levels above quantification.
The majority of studies of maternal marijuana use during pregnancy rely on self-report, so this could affect attempts to assess the effects of such prenatal use, they said.
Adverse outcomes associated with marijuana use during pregnancy include fetal growth restriction, small for gestational age, preterm birth, and adverse neurodevelopmental outcomes, studies have shown.
Read more in Obstetrics & Gynecology.
Even in the setting of legalized marijuana use, estimated prevalence of marijuana use during pregnancy was lower by self-report than it was by umbilical cord testing.
Torri D. Metz, MD, of the University of Utah Health, Salt Lake City, and her colleagues surveyed women at two urban hospitals in Colorado, which has legalized both medical and recreational use of marijuana. They found that, while 6% of the 116 women in the study reported using marijuana in the past 30 days, umbilical cord testing showed as many as 22% had detectable levels of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic, and 10% had levels above quantification.
The majority of studies of maternal marijuana use during pregnancy rely on self-report, so this could affect attempts to assess the effects of such prenatal use, they said.
Adverse outcomes associated with marijuana use during pregnancy include fetal growth restriction, small for gestational age, preterm birth, and adverse neurodevelopmental outcomes, studies have shown.
Read more in Obstetrics & Gynecology.
FROM OBSTETRICS & GYNECOLOGY
Common AEDs confer modestly increased risk of major congenital malformations
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
REPORTING FROM AES 2018
Key clinical point:
Major finding: The malformation rate was 5% in exposed pregnancies.
Study details: The MONEAD study comprised 351 pregnant women with epilepsy, 109 nonpregnant women with epilepsy, and 105 healthy pregnant women.
Disclosures: The National Institutes of Health funded the study; Dr. Meador reported no financial disclosures.
Source: Meador KJ et al. AES 2018, Abstract 3.231.
Breastfeeding with MS: Good for mom, too
BERLIN – In the changing multiple sclerosis landscape, more women are having babies, and more are asking questions. With these women, what’s the best way to address the complicated interplay among pregnancy, relapse risk, breastfeeding, and medication resumption? A starting point is to recognize that “women with MS are very different today than they were 25 years ago,” said Annette Langer-Gould, MD, PhD. Not only have diagnostic criteria changed but also highly effective treatments now exist that were not available when the first pregnancy cohorts were studied, she pointed out, speaking at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
The existing literature, said Dr. Langer-Gould, has addressed one controversy: “Most women with MS can have normal pregnancies – and breastfeed – without incurring harm,” though it’s true that severe rebound relapses are possible if natalizumab (Tysabri) or fingolimod (Gilenya) are stopped before pregnancy. In any case, new small-molecule MS medications need to be stopped during pregnancy and breastfeeding, she pointed out. “We didn’t have to worry about that too much when we only had injectables and monoclonal antibodies because they were larger and didn’t cross the placenta.”
Since the 1980s, the conversation about pregnancy and MS has moved from asking “Is pregnancy bad for women with MS?” to the current MS landscape, in which sicker women are able to become pregnant, Dr. Langer-Gould said, adding that how women with MS fare through pregnancy and in the postpartum period is changing over time as well. She and her colleagues’ experience with pregnancy in a cohort of women with MS in the Kaiser Permanente care system, where she is a clinical neurologist and regional research lead, revealed a relapse rate of 8.4%. “So it was pretty rare for a woman to have a relapse during pregnancy,” Dr. Langer-Gould said.
Most women with MS who become pregnant, whether their care is received in a referral center or is community based, are now doing so while on a disease-modifying therapy (DMT), Dr. Langer-Gould said. On these highly effective treatments, “women who were too sick to get pregnant are now well controlled and having babies.”
As more women with MS become pregnant, more conversations about breastfeeding will inevitably crop up, she said. And the discussion about breastfeeding has now begun to acknowledge the “strong benefits to mom and the baby of not just breastfeeding, but longer breastfeeding,” as well.
“Because of this baby-friendly push in a lot of hospitals in the United States, where they’re trying to encourage all women to breastfeed,” a full 87% of women breastfed their infants at least some of the time, and over a third of women (35%) breastfed exclusively for at least 2 months, Dr. Langer-Gould said.
“There’s no one clear explanation of why the women seem to be healthier and doing better through pregnancy as a group, but it’s probably a combination of having milder disease, breastfeeding more, and they’ve got better controlled disease before pregnancy,” she said.
At least eight studies to date have examined the relationship between postpartum MS relapses and breastfeeding, Dr. Langer-Gould said.
“The thing to take away ... is that, even though we’ve studied this many, many times, no one can show that it’s harmful,” she said. For mothers who want to breastfeed, “you can support them in the breastfeeding choice, because they are not going to have more severe disease because of that.”
Whether breastfeeding is exclusive or not has not always been tracked in studies of childbearing women with MS, but when it was captured in the data, exclusive breastfeeding has exerted a protective effect, with about a 50% reduction in risk for postpartum relapse seen in one study (JAMA Neurol. 2015 Oct;72[10]:1132-8).
There is a hormonal rationale for exclusive breastfeeding exerting a protective effect on MS: With exclusive breastfeeding comes more frequent, intense suckling, with more profound elevations in prolactin, and larger drops in follicle-stimulating hormone, luteinizing hormone, progesterone, and estradiol. All these hormonal changes work together to produce more prolonged amenorrhea and anovulation, Dr. Langer-Gould said, with potentially beneficial immunologic effects.
When other, more general maternal and infant health benefits of breastfeeding also are taken into account, there’s strong evidence for the benefits of breastfeeding for women with MS whose medication profile allows them to breastfeed, she said.
However, the “treatment” effect of exclusive breastfeeding is only effective until the infant starts taking regular supplemental feedings, including the introduction of table food at around 6 months of age. “Once regular supplemental feedings are introduced, relapses return,” Dr. Langer-Gould said.
There is some suggestion that, in women without MS, prolonged breastfeeding may be associated with reduced risk of MS. In the MS Sunshine study, breastfeeding for 15 months or longer decreased the risk of later MS by 23%-53% (Nutrients. 2018 Feb 27;10[3]:268). The investigators, led by Dr. Langer-Gould, summed the total months of breastfeeding across all children, so that the 15-month threshold could be reached by breastfeeding one child for 15 months, or three children for 5 months each. “It’s a single study; I wouldn’t make too much out of it,” Dr. Langer-Gould said.
Open questions still remain, she said: “So far, no one has been able to demonstrate a clear beneficial effect in reducing the risk of postpartum relapse if they resume their DMT early in the postpartum period.” Dr. Langer-Gould noted that the literature in this area is hampered by heterogeneity and by the fact that newer, more highly active DMTs have not been well studied.
Also, the link between postpartum relapses and long-term prognosis is not completely delineated. Indirect evidence, she said, points to a postpartum relapse as being “overall, a low-impact event.”
Dr. Langer-Gould reported that she has been the site principal investigator for clinical trials sponsored by Roche and Biogen.
SOURCE: Langer-Gould A. ECTRIMS 2018, Abstract 5.
BERLIN – In the changing multiple sclerosis landscape, more women are having babies, and more are asking questions. With these women, what’s the best way to address the complicated interplay among pregnancy, relapse risk, breastfeeding, and medication resumption? A starting point is to recognize that “women with MS are very different today than they were 25 years ago,” said Annette Langer-Gould, MD, PhD. Not only have diagnostic criteria changed but also highly effective treatments now exist that were not available when the first pregnancy cohorts were studied, she pointed out, speaking at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
The existing literature, said Dr. Langer-Gould, has addressed one controversy: “Most women with MS can have normal pregnancies – and breastfeed – without incurring harm,” though it’s true that severe rebound relapses are possible if natalizumab (Tysabri) or fingolimod (Gilenya) are stopped before pregnancy. In any case, new small-molecule MS medications need to be stopped during pregnancy and breastfeeding, she pointed out. “We didn’t have to worry about that too much when we only had injectables and monoclonal antibodies because they were larger and didn’t cross the placenta.”
Since the 1980s, the conversation about pregnancy and MS has moved from asking “Is pregnancy bad for women with MS?” to the current MS landscape, in which sicker women are able to become pregnant, Dr. Langer-Gould said, adding that how women with MS fare through pregnancy and in the postpartum period is changing over time as well. She and her colleagues’ experience with pregnancy in a cohort of women with MS in the Kaiser Permanente care system, where she is a clinical neurologist and regional research lead, revealed a relapse rate of 8.4%. “So it was pretty rare for a woman to have a relapse during pregnancy,” Dr. Langer-Gould said.
Most women with MS who become pregnant, whether their care is received in a referral center or is community based, are now doing so while on a disease-modifying therapy (DMT), Dr. Langer-Gould said. On these highly effective treatments, “women who were too sick to get pregnant are now well controlled and having babies.”
As more women with MS become pregnant, more conversations about breastfeeding will inevitably crop up, she said. And the discussion about breastfeeding has now begun to acknowledge the “strong benefits to mom and the baby of not just breastfeeding, but longer breastfeeding,” as well.
“Because of this baby-friendly push in a lot of hospitals in the United States, where they’re trying to encourage all women to breastfeed,” a full 87% of women breastfed their infants at least some of the time, and over a third of women (35%) breastfed exclusively for at least 2 months, Dr. Langer-Gould said.
“There’s no one clear explanation of why the women seem to be healthier and doing better through pregnancy as a group, but it’s probably a combination of having milder disease, breastfeeding more, and they’ve got better controlled disease before pregnancy,” she said.
At least eight studies to date have examined the relationship between postpartum MS relapses and breastfeeding, Dr. Langer-Gould said.
“The thing to take away ... is that, even though we’ve studied this many, many times, no one can show that it’s harmful,” she said. For mothers who want to breastfeed, “you can support them in the breastfeeding choice, because they are not going to have more severe disease because of that.”
Whether breastfeeding is exclusive or not has not always been tracked in studies of childbearing women with MS, but when it was captured in the data, exclusive breastfeeding has exerted a protective effect, with about a 50% reduction in risk for postpartum relapse seen in one study (JAMA Neurol. 2015 Oct;72[10]:1132-8).
There is a hormonal rationale for exclusive breastfeeding exerting a protective effect on MS: With exclusive breastfeeding comes more frequent, intense suckling, with more profound elevations in prolactin, and larger drops in follicle-stimulating hormone, luteinizing hormone, progesterone, and estradiol. All these hormonal changes work together to produce more prolonged amenorrhea and anovulation, Dr. Langer-Gould said, with potentially beneficial immunologic effects.
When other, more general maternal and infant health benefits of breastfeeding also are taken into account, there’s strong evidence for the benefits of breastfeeding for women with MS whose medication profile allows them to breastfeed, she said.
However, the “treatment” effect of exclusive breastfeeding is only effective until the infant starts taking regular supplemental feedings, including the introduction of table food at around 6 months of age. “Once regular supplemental feedings are introduced, relapses return,” Dr. Langer-Gould said.
There is some suggestion that, in women without MS, prolonged breastfeeding may be associated with reduced risk of MS. In the MS Sunshine study, breastfeeding for 15 months or longer decreased the risk of later MS by 23%-53% (Nutrients. 2018 Feb 27;10[3]:268). The investigators, led by Dr. Langer-Gould, summed the total months of breastfeeding across all children, so that the 15-month threshold could be reached by breastfeeding one child for 15 months, or three children for 5 months each. “It’s a single study; I wouldn’t make too much out of it,” Dr. Langer-Gould said.
Open questions still remain, she said: “So far, no one has been able to demonstrate a clear beneficial effect in reducing the risk of postpartum relapse if they resume their DMT early in the postpartum period.” Dr. Langer-Gould noted that the literature in this area is hampered by heterogeneity and by the fact that newer, more highly active DMTs have not been well studied.
Also, the link between postpartum relapses and long-term prognosis is not completely delineated. Indirect evidence, she said, points to a postpartum relapse as being “overall, a low-impact event.”
Dr. Langer-Gould reported that she has been the site principal investigator for clinical trials sponsored by Roche and Biogen.
SOURCE: Langer-Gould A. ECTRIMS 2018, Abstract 5.
BERLIN – In the changing multiple sclerosis landscape, more women are having babies, and more are asking questions. With these women, what’s the best way to address the complicated interplay among pregnancy, relapse risk, breastfeeding, and medication resumption? A starting point is to recognize that “women with MS are very different today than they were 25 years ago,” said Annette Langer-Gould, MD, PhD. Not only have diagnostic criteria changed but also highly effective treatments now exist that were not available when the first pregnancy cohorts were studied, she pointed out, speaking at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
The existing literature, said Dr. Langer-Gould, has addressed one controversy: “Most women with MS can have normal pregnancies – and breastfeed – without incurring harm,” though it’s true that severe rebound relapses are possible if natalizumab (Tysabri) or fingolimod (Gilenya) are stopped before pregnancy. In any case, new small-molecule MS medications need to be stopped during pregnancy and breastfeeding, she pointed out. “We didn’t have to worry about that too much when we only had injectables and monoclonal antibodies because they were larger and didn’t cross the placenta.”
Since the 1980s, the conversation about pregnancy and MS has moved from asking “Is pregnancy bad for women with MS?” to the current MS landscape, in which sicker women are able to become pregnant, Dr. Langer-Gould said, adding that how women with MS fare through pregnancy and in the postpartum period is changing over time as well. She and her colleagues’ experience with pregnancy in a cohort of women with MS in the Kaiser Permanente care system, where she is a clinical neurologist and regional research lead, revealed a relapse rate of 8.4%. “So it was pretty rare for a woman to have a relapse during pregnancy,” Dr. Langer-Gould said.
Most women with MS who become pregnant, whether their care is received in a referral center or is community based, are now doing so while on a disease-modifying therapy (DMT), Dr. Langer-Gould said. On these highly effective treatments, “women who were too sick to get pregnant are now well controlled and having babies.”
As more women with MS become pregnant, more conversations about breastfeeding will inevitably crop up, she said. And the discussion about breastfeeding has now begun to acknowledge the “strong benefits to mom and the baby of not just breastfeeding, but longer breastfeeding,” as well.
“Because of this baby-friendly push in a lot of hospitals in the United States, where they’re trying to encourage all women to breastfeed,” a full 87% of women breastfed their infants at least some of the time, and over a third of women (35%) breastfed exclusively for at least 2 months, Dr. Langer-Gould said.
“There’s no one clear explanation of why the women seem to be healthier and doing better through pregnancy as a group, but it’s probably a combination of having milder disease, breastfeeding more, and they’ve got better controlled disease before pregnancy,” she said.
At least eight studies to date have examined the relationship between postpartum MS relapses and breastfeeding, Dr. Langer-Gould said.
“The thing to take away ... is that, even though we’ve studied this many, many times, no one can show that it’s harmful,” she said. For mothers who want to breastfeed, “you can support them in the breastfeeding choice, because they are not going to have more severe disease because of that.”
Whether breastfeeding is exclusive or not has not always been tracked in studies of childbearing women with MS, but when it was captured in the data, exclusive breastfeeding has exerted a protective effect, with about a 50% reduction in risk for postpartum relapse seen in one study (JAMA Neurol. 2015 Oct;72[10]:1132-8).
There is a hormonal rationale for exclusive breastfeeding exerting a protective effect on MS: With exclusive breastfeeding comes more frequent, intense suckling, with more profound elevations in prolactin, and larger drops in follicle-stimulating hormone, luteinizing hormone, progesterone, and estradiol. All these hormonal changes work together to produce more prolonged amenorrhea and anovulation, Dr. Langer-Gould said, with potentially beneficial immunologic effects.
When other, more general maternal and infant health benefits of breastfeeding also are taken into account, there’s strong evidence for the benefits of breastfeeding for women with MS whose medication profile allows them to breastfeed, she said.
However, the “treatment” effect of exclusive breastfeeding is only effective until the infant starts taking regular supplemental feedings, including the introduction of table food at around 6 months of age. “Once regular supplemental feedings are introduced, relapses return,” Dr. Langer-Gould said.
There is some suggestion that, in women without MS, prolonged breastfeeding may be associated with reduced risk of MS. In the MS Sunshine study, breastfeeding for 15 months or longer decreased the risk of later MS by 23%-53% (Nutrients. 2018 Feb 27;10[3]:268). The investigators, led by Dr. Langer-Gould, summed the total months of breastfeeding across all children, so that the 15-month threshold could be reached by breastfeeding one child for 15 months, or three children for 5 months each. “It’s a single study; I wouldn’t make too much out of it,” Dr. Langer-Gould said.
Open questions still remain, she said: “So far, no one has been able to demonstrate a clear beneficial effect in reducing the risk of postpartum relapse if they resume their DMT early in the postpartum period.” Dr. Langer-Gould noted that the literature in this area is hampered by heterogeneity and by the fact that newer, more highly active DMTs have not been well studied.
Also, the link between postpartum relapses and long-term prognosis is not completely delineated. Indirect evidence, she said, points to a postpartum relapse as being “overall, a low-impact event.”
Dr. Langer-Gould reported that she has been the site principal investigator for clinical trials sponsored by Roche and Biogen.
SOURCE: Langer-Gould A. ECTRIMS 2018, Abstract 5.
REPORTING FROM ECTRIMS 2018
Maternal health benefits of breastfeeding
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In the past decade, breastfeeding rates have increased substantially. Between 2000 and 2015, the proportion of infants who continued to breastfeed at 12 months increased from 16% to 36%. The proportion of infants who had any breastfeeding increased from 71% to 83%.1 While the infant health benefits of breastfeeding are widely recognized, the maternal health benefits of breastfeeding are many and likely underappreciated.
Infant health benefits of breastfeeding
There are no large-scale, randomized studies of the long-term health benefits of breastfeeding versus formula feeding. The evidence supporting the health benefits of breastfeeding is derived from case-control and cohort studies. Breastfeeding directly benefits newborn and infant nutrition, gastrointestinal function, host defense, and psychological well-being. Compared with formula-fed newborns, breastfed infants have a reduced risk of infectious diseases including otitis media, gastroenteritis, respiratory infections, sudden infant death syndrome, and metabolic disease. These benefits alone strongly support the public health benefit of breastfeeding.2 In addition, breastfeeding greatly benefits maternal health.
Maternal health benefits of breastfeeding
Breastfeeding reduces a woman’s risk for type 2 diabetes, hypertension, and coronary artery disease, myocardial infarction, as well as breast, ovarian, and endometrial cancer. There are few exposures that have such a multitude of positive health benefits.
filler
Type 2 diabetes
In a prospective cohort study of 1,238 women without diabetes in 1985–1986, 182 women developed type 2 diabetes after 30 years of follow-up. Compared with never breastfeeding, breastfeeding for 0 to 6 months, >6 months to <12 months, or ≥12 months reduced the risk of type 2 diabetes by 25%, 48%, and 69% respectively.3 In the prospective Nurses’ Health Study, among parous women, each additional year of breastfeeding decreased the risk of type 2 diabetes by 15% compared with women who did not breastfeed.4

Hypertension

In the Women’s Health Initiative (WHI) study of postmenopausal women, a lifetime history of breastfeeding for 12 months or more was associated with a 12% decrease in the risk of hypertension.5 For parous women, the prevalence of hypertension among breastfeeding (≥12 months) and never breastfeeding women was estimated to be 38.6% versus 42.1%.5 Similar results were observed in the Nurses’ Health Study II.6
Myocardial infarction and coronary heart disease
In the prospective Nurses’ Health Study, during 1,350,965 person-years of follow-up, 2,540 women had a myocardial infarction (MI). Women who had breastfed for ≥ 2 years had a 37% decreased risk of MI compared with women who never breastfed. After adjustment for family history, lifestyle factors, and adiposity, the observed reduction in risk was 23%.7 In the WHI (observational study plus controlled trial), women with a single live birth who breastfed for 7 to 12 months had a lower risk of cardiovascular disease than women with a single live birth who did not breastfeed (hazard ratio, 0.72; 95% confidence interval, 0.53–97).5

Breast cancer

In a systematic review and meta-analysis of 100 publications, breastfeeding >12 months reduced the risk of breast cancer by 26%.8 In a systematic review of 47 studies, the relative risk of breast cancer decreased by 4.7% for every 12 months of breastfeeding.9 In a systematic review and meta-analysis of 3 studies, ever breastfeeding was associated with a 28% reduced risk for triple-negative (ER-, PR-, HER2-) breast cancer among parous women.10 Triple-negative breast cancer generally has a poorer prognosis than receptor-positive breast cancers.
Continue to: Ovarian Cancer
Ovarian cancer
In a systematic review and meta-analysis of 40 publications, ever breastfeeding was associated with a 37% reduction in the risk of ovarian cancer.8 In a prospective study of 1.1 million women in the United Kingdom, 8,719 developed ovarian cancer. Among parous women, ovarian cancer risk was reduced by 10% for every 12 months of breastfeeding.11

Endometrial cancer

In a meta-analysis of 17 publications, including 8,981 cases and 17,241 controls, ever breastfeeding was associated with an 11% reduction in breast cancer risk.12 In a meta-analysis of 15 publications with 6,704 cases, breastfeeding was associated with a 26% reduction in endometrial cancer. After controlling for hormone use and body mass index, the reduced risk was in the range of 35%. A linear relationship between breastfeeding and reduced risk of endometrial cancer was observed, with 1 month of breastfeeding being associated with a 1.2% reduction in the risk of endometrial cancer.13
Let’s support our patients’ health by encouraging successful breastfeeding
Obstetrician-gynecologists play an important role in helping women make informed decisions about breastfeeding. Most professional organizations, including the American College of Obstetricians and Gynecologists, recommend exclusive breastfeeding for the first 6 months of life, with continued breastfeeding and introduction of complementary food from 6 to 12 months.14,15 Birth practices that help to increase successful breastfeeding include:
- inform all pregnant women about the newborn and maternal health benefits and management of breastfeeding
- initiate skin-to-skin contact at birth
- encourage the initiation of breastfeeding within 1 hour of birth
- ensure that breastfeeding newborns do not receive any food or drink other than breast milk, unless medically indicated
- encourage breastfeeding women to not use pacifiers or artificial nipples.15
When women are discharged from the maternity center, providing information about community-based lactation support is helpful in ensuring continuation of successful breastfeeding.16
Most patients know that exercise and maintaining a healthy weight can reduce the risk of developing many prevalent diseases. However, far fewer patients know that breastfeeding can reduce the risk of developing type 2 diabetes, hypertension, and coronary artery disease, as well as breast, ovarian, and endometrial cancers. Educating our patients about these health benefits may help them to more fully commit to breastfeeding.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2009–2015, CDC National Immunization Survey. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Updated August 2018. Accessed November 19, 2018.
- Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 (suppl 1):S17.
- Gunderson Ep, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Int Med. 2018;178:328-337.
- Stuebe AM, Rich-Edwards JW, Willett WC, et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601-2610.
- Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
- Stuebe Am, Schwarz EB, Grewen K, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174:1147-1158.
- Stuebe AM, Michels KB, Willett WC, et al. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200:138.e1-e8.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96-113.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries including 50,302 women with breast cancer and 96,973 women without the disease. Lancet. 2002;360:187-195.
- Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. 2015;26:2398-2407.
- Gaitskell K, Green J, Pirie K, et al. Million Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the Million Women Study. Int J Cancer. 2018;142:281-289.
- Jordan SJ, Na R, Johnatty SE, et al. Breastfeeding and endometrial cancer risk: an analysis from the epidemiology of endometrial cancer consortium. Obstet Gynecol. 2017;129:1059-1067.
- Zhan B, Liu X, Li F, Zhang D, et al. Breastfeeding and the incidence of endometrial cancer: a meta-analysis. Oncotarget. 2015;6:38398-38409.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;CD003517.
- ACOG Committee Opinion No. 756. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e187-e196.
- McFadden A, Gavine A, Renfrew M, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2017;CD001141.
Empowering women through self-managed abortion
Consider Ashley, a 22-year-old G3P2, 8 weeks pregnant, on Medicaid and living in rural Arkansas. The victim of intimate partner violence, she just broke up with her boyfriend and feels she does not have the financial or emotional resources to raise another child; she has no family in town to turn to and wants to be the best parent she can be to her 10-month-old and 3-year old.
In Arkansas, as in many other states and the District of Columbia, Medicaid covers abortion only for rape, incest, or danger to the woman’s life. Arkansas, as well as many other states, requires women to wait 48 hours following counseling before they can proceed with abortion. Waiting periods exacerbate Ashley’s tenuous situation. Will her boss give her time off from work? How will she get to the clinic? Who will watch her children? And lost wages and greater expenses are not the only problems she faces. Arkansas requires a legal contract between the abortion provider and a physician with hospital admitting privileges to provide medical abortion. The result: Only one clinic in Arkansas can legally provide medical abortion for its entire female population. For our impoverished young mother of two, the best choice is the most difficult. And she is far from alone.
Since 2010, many states have passed numerous laws restricting access to safe abortion. As geography plays a growing role in determining access, women and health care providers actively seek ways to circumvent barriers. Telemedicine, initially designed to expedite primary care for patients whose access was hampered by Boston traffic, now brings quality health care to areas lacking providers.1 Telemedicine works for a variety of medical services, from prescribing antibiotics to performing neurosurgery; reproductive health care is part of this digital revolution.2 In 2008, Iowa’s Planned Parenthood of the Heartland began using telemedicine to offer medical abortion.3
As approved by the Food and Drug Administration, medical abortion is the termination of a pregnancy of up to 10 weeks’ gestation using a combination of mifepristone and misoprostol, the former taken to block progesterone receptors, the latter to cause expulsion of the pregnancy. Today, about a third of all abortions in the United States are medical abortions. Because current FDA regulations require that mifepristone be dispensed by a physician, patients usually receive the medications after an in-person evaluation by a health care provider in a clinic.
Two models of telemedicine could improve access for Ashley.
In the first, like the Iowa Planned Parenthood model, remote clinic staff evaluate patients with history and physical examination, ultrasonography, and hemoglobin measurement; the information is forwarded to an off-site physician who has a video discussion with the patient and remotely dispenses the medication for eligible candidates. Between 2008 and 2015, Iowa Planned Parenthood provided 8,765 medical abortions using this model.3 Clinically adverse events, such as hospital admission, surgery, blood transfusion, and death occurred in 16 (0.18%) with no ectopic pregnancies or death.3 For comparison, the rate of severe maternal morbidity in the United States is 1.4%, approximately 10 times the rate with this model of medical abortion.4
In the second model of fully self-managed telemedicine abortion, patients complete a checklist that is reviewed by a provider who sends the medications through the mail. For safety, women must be able to determine their eligibility through the checklist, manage the medications, and self-assess for abortion completion. The World Health Organization endorses self-managed abortion as an option when there is “a source of accurate information and access to a health care provider should they need or want it at any stage of the process.”5 Women on Web, an organization that has provided telemedicine abortion to women globally, has recently begun providing services to the United States after sweeping restrictions vastly increased the number of requests from U.S. women. The U.S. service, Aid Access, operates similarly and for $95 provides online consultation, shipping of the medications, and Skype or phone calls for questions.6
Self-managed abortion has a bad reputation, in part from anti-abortion activists who seek to punish women who attempt to end their pregnancies themselves, but also because of its association with pre–Roe v. Wade “back alley” unsafe abortions. Neither perspective recognizes the benefits of safe self-managed abortion. Some states have criminalized self-induced abortion; both the American College of Obstetricians and Gynecologists and the American Medical Association have voiced opposition to such laws to ensure that women do not fear prosecution for seeking medical care for complications.
Given the landscape of abortion access in the United States, where legal constraints, lack of insurance, and a dearth of providers may create insurmountable barriers, we support self-managed abortion for the following reasons:
- Access barriers: The complexity and number of legal restrictions to abortion care have made it unavailable/unaffordable through traditional clinic visits in many parts of the United States. With the addition of Justice Brett M. Kavanaugh to the Supreme Court, restrictions are likely to increase.
- Safety: The evidence-based assessment of the World Health Organization is that in-person clinical evaluation is unnecessary if the appropriate checklists, educational information, and access to a provider are available.
- Autonomy and equity: Even without the barriers mentioned above, self-managed telemedicine abortion remains a patient-centered option. Often more accessible and less expensive, inherently more private, it is bound to appeal to many women.
This decade has seen unprecedented challenges to comprehensive safe reproductive health care, with no relief in sight. In the decades prior to Roe v. Wade, illegal abortions were responsible for 20% of all maternal mortality in the United States. As government, national medical organizations, and the public become more aware of our intolerably high maternal mortality rate, these actors are increasingly driven to bring our maternal health to parity with our industrialized peers. Restricting access to safe abortion runs counter to that goal. Two hundred forty years of American history teach us that legal restrictions do not prevent abortions, because they do not eliminate the reasons for which women seek abortion. Legal restrictions do, however, prevent women from ending pregnancies in the safest manner possible. The inability to obtain safe abortions invariably leads to dead women – our mothers, daughters, sisters, and wives. In this country’s harsh political climate, we must protect a woman’s right to choose. By advocating for innovative approaches to protect women’s reproductive choices, we empower women and save lives.
Dr. Anwar is an obstetrician/gynecologist at Michigan State University in Flint and Dr. Espey is professor and chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. Neither of them have conflicts of interest. Email them at [email protected].
References
1. “How a ‘Stupid Idea’ Gave Birth to Telemedicine,” MedPageToday. Dec 15, .
2. J Neurosurg Pediatr. 2016 Dec;25(6):753-7.
3. Obstet Gynecol. 2017 Oct;130(4):778-82.
4. Centers for Disease Control and Prevention. Severe Maternal Morbidity in the United States.
5. Guttmacher Rep Public Policy. 2018;21:41-7.
6. “International ‘safe abortions by mail’ service can now ship to women in US,” The Hill, Nov 7, 2018.
Consider Ashley, a 22-year-old G3P2, 8 weeks pregnant, on Medicaid and living in rural Arkansas. The victim of intimate partner violence, she just broke up with her boyfriend and feels she does not have the financial or emotional resources to raise another child; she has no family in town to turn to and wants to be the best parent she can be to her 10-month-old and 3-year old.
In Arkansas, as in many other states and the District of Columbia, Medicaid covers abortion only for rape, incest, or danger to the woman’s life. Arkansas, as well as many other states, requires women to wait 48 hours following counseling before they can proceed with abortion. Waiting periods exacerbate Ashley’s tenuous situation. Will her boss give her time off from work? How will she get to the clinic? Who will watch her children? And lost wages and greater expenses are not the only problems she faces. Arkansas requires a legal contract between the abortion provider and a physician with hospital admitting privileges to provide medical abortion. The result: Only one clinic in Arkansas can legally provide medical abortion for its entire female population. For our impoverished young mother of two, the best choice is the most difficult. And she is far from alone.
Since 2010, many states have passed numerous laws restricting access to safe abortion. As geography plays a growing role in determining access, women and health care providers actively seek ways to circumvent barriers. Telemedicine, initially designed to expedite primary care for patients whose access was hampered by Boston traffic, now brings quality health care to areas lacking providers.1 Telemedicine works for a variety of medical services, from prescribing antibiotics to performing neurosurgery; reproductive health care is part of this digital revolution.2 In 2008, Iowa’s Planned Parenthood of the Heartland began using telemedicine to offer medical abortion.3
As approved by the Food and Drug Administration, medical abortion is the termination of a pregnancy of up to 10 weeks’ gestation using a combination of mifepristone and misoprostol, the former taken to block progesterone receptors, the latter to cause expulsion of the pregnancy. Today, about a third of all abortions in the United States are medical abortions. Because current FDA regulations require that mifepristone be dispensed by a physician, patients usually receive the medications after an in-person evaluation by a health care provider in a clinic.
Two models of telemedicine could improve access for Ashley.
In the first, like the Iowa Planned Parenthood model, remote clinic staff evaluate patients with history and physical examination, ultrasonography, and hemoglobin measurement; the information is forwarded to an off-site physician who has a video discussion with the patient and remotely dispenses the medication for eligible candidates. Between 2008 and 2015, Iowa Planned Parenthood provided 8,765 medical abortions using this model.3 Clinically adverse events, such as hospital admission, surgery, blood transfusion, and death occurred in 16 (0.18%) with no ectopic pregnancies or death.3 For comparison, the rate of severe maternal morbidity in the United States is 1.4%, approximately 10 times the rate with this model of medical abortion.4
In the second model of fully self-managed telemedicine abortion, patients complete a checklist that is reviewed by a provider who sends the medications through the mail. For safety, women must be able to determine their eligibility through the checklist, manage the medications, and self-assess for abortion completion. The World Health Organization endorses self-managed abortion as an option when there is “a source of accurate information and access to a health care provider should they need or want it at any stage of the process.”5 Women on Web, an organization that has provided telemedicine abortion to women globally, has recently begun providing services to the United States after sweeping restrictions vastly increased the number of requests from U.S. women. The U.S. service, Aid Access, operates similarly and for $95 provides online consultation, shipping of the medications, and Skype or phone calls for questions.6
Self-managed abortion has a bad reputation, in part from anti-abortion activists who seek to punish women who attempt to end their pregnancies themselves, but also because of its association with pre–Roe v. Wade “back alley” unsafe abortions. Neither perspective recognizes the benefits of safe self-managed abortion. Some states have criminalized self-induced abortion; both the American College of Obstetricians and Gynecologists and the American Medical Association have voiced opposition to such laws to ensure that women do not fear prosecution for seeking medical care for complications.
Given the landscape of abortion access in the United States, where legal constraints, lack of insurance, and a dearth of providers may create insurmountable barriers, we support self-managed abortion for the following reasons:
- Access barriers: The complexity and number of legal restrictions to abortion care have made it unavailable/unaffordable through traditional clinic visits in many parts of the United States. With the addition of Justice Brett M. Kavanaugh to the Supreme Court, restrictions are likely to increase.
- Safety: The evidence-based assessment of the World Health Organization is that in-person clinical evaluation is unnecessary if the appropriate checklists, educational information, and access to a provider are available.
- Autonomy and equity: Even without the barriers mentioned above, self-managed telemedicine abortion remains a patient-centered option. Often more accessible and less expensive, inherently more private, it is bound to appeal to many women.
This decade has seen unprecedented challenges to comprehensive safe reproductive health care, with no relief in sight. In the decades prior to Roe v. Wade, illegal abortions were responsible for 20% of all maternal mortality in the United States. As government, national medical organizations, and the public become more aware of our intolerably high maternal mortality rate, these actors are increasingly driven to bring our maternal health to parity with our industrialized peers. Restricting access to safe abortion runs counter to that goal. Two hundred forty years of American history teach us that legal restrictions do not prevent abortions, because they do not eliminate the reasons for which women seek abortion. Legal restrictions do, however, prevent women from ending pregnancies in the safest manner possible. The inability to obtain safe abortions invariably leads to dead women – our mothers, daughters, sisters, and wives. In this country’s harsh political climate, we must protect a woman’s right to choose. By advocating for innovative approaches to protect women’s reproductive choices, we empower women and save lives.
Dr. Anwar is an obstetrician/gynecologist at Michigan State University in Flint and Dr. Espey is professor and chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. Neither of them have conflicts of interest. Email them at [email protected].
References
1. “How a ‘Stupid Idea’ Gave Birth to Telemedicine,” MedPageToday. Dec 15, .
2. J Neurosurg Pediatr. 2016 Dec;25(6):753-7.
3. Obstet Gynecol. 2017 Oct;130(4):778-82.
4. Centers for Disease Control and Prevention. Severe Maternal Morbidity in the United States.
5. Guttmacher Rep Public Policy. 2018;21:41-7.
6. “International ‘safe abortions by mail’ service can now ship to women in US,” The Hill, Nov 7, 2018.
Consider Ashley, a 22-year-old G3P2, 8 weeks pregnant, on Medicaid and living in rural Arkansas. The victim of intimate partner violence, she just broke up with her boyfriend and feels she does not have the financial or emotional resources to raise another child; she has no family in town to turn to and wants to be the best parent she can be to her 10-month-old and 3-year old.
In Arkansas, as in many other states and the District of Columbia, Medicaid covers abortion only for rape, incest, or danger to the woman’s life. Arkansas, as well as many other states, requires women to wait 48 hours following counseling before they can proceed with abortion. Waiting periods exacerbate Ashley’s tenuous situation. Will her boss give her time off from work? How will she get to the clinic? Who will watch her children? And lost wages and greater expenses are not the only problems she faces. Arkansas requires a legal contract between the abortion provider and a physician with hospital admitting privileges to provide medical abortion. The result: Only one clinic in Arkansas can legally provide medical abortion for its entire female population. For our impoverished young mother of two, the best choice is the most difficult. And she is far from alone.
Since 2010, many states have passed numerous laws restricting access to safe abortion. As geography plays a growing role in determining access, women and health care providers actively seek ways to circumvent barriers. Telemedicine, initially designed to expedite primary care for patients whose access was hampered by Boston traffic, now brings quality health care to areas lacking providers.1 Telemedicine works for a variety of medical services, from prescribing antibiotics to performing neurosurgery; reproductive health care is part of this digital revolution.2 In 2008, Iowa’s Planned Parenthood of the Heartland began using telemedicine to offer medical abortion.3
As approved by the Food and Drug Administration, medical abortion is the termination of a pregnancy of up to 10 weeks’ gestation using a combination of mifepristone and misoprostol, the former taken to block progesterone receptors, the latter to cause expulsion of the pregnancy. Today, about a third of all abortions in the United States are medical abortions. Because current FDA regulations require that mifepristone be dispensed by a physician, patients usually receive the medications after an in-person evaluation by a health care provider in a clinic.
Two models of telemedicine could improve access for Ashley.
In the first, like the Iowa Planned Parenthood model, remote clinic staff evaluate patients with history and physical examination, ultrasonography, and hemoglobin measurement; the information is forwarded to an off-site physician who has a video discussion with the patient and remotely dispenses the medication for eligible candidates. Between 2008 and 2015, Iowa Planned Parenthood provided 8,765 medical abortions using this model.3 Clinically adverse events, such as hospital admission, surgery, blood transfusion, and death occurred in 16 (0.18%) with no ectopic pregnancies or death.3 For comparison, the rate of severe maternal morbidity in the United States is 1.4%, approximately 10 times the rate with this model of medical abortion.4
In the second model of fully self-managed telemedicine abortion, patients complete a checklist that is reviewed by a provider who sends the medications through the mail. For safety, women must be able to determine their eligibility through the checklist, manage the medications, and self-assess for abortion completion. The World Health Organization endorses self-managed abortion as an option when there is “a source of accurate information and access to a health care provider should they need or want it at any stage of the process.”5 Women on Web, an organization that has provided telemedicine abortion to women globally, has recently begun providing services to the United States after sweeping restrictions vastly increased the number of requests from U.S. women. The U.S. service, Aid Access, operates similarly and for $95 provides online consultation, shipping of the medications, and Skype or phone calls for questions.6
Self-managed abortion has a bad reputation, in part from anti-abortion activists who seek to punish women who attempt to end their pregnancies themselves, but also because of its association with pre–Roe v. Wade “back alley” unsafe abortions. Neither perspective recognizes the benefits of safe self-managed abortion. Some states have criminalized self-induced abortion; both the American College of Obstetricians and Gynecologists and the American Medical Association have voiced opposition to such laws to ensure that women do not fear prosecution for seeking medical care for complications.
Given the landscape of abortion access in the United States, where legal constraints, lack of insurance, and a dearth of providers may create insurmountable barriers, we support self-managed abortion for the following reasons:
- Access barriers: The complexity and number of legal restrictions to abortion care have made it unavailable/unaffordable through traditional clinic visits in many parts of the United States. With the addition of Justice Brett M. Kavanaugh to the Supreme Court, restrictions are likely to increase.
- Safety: The evidence-based assessment of the World Health Organization is that in-person clinical evaluation is unnecessary if the appropriate checklists, educational information, and access to a provider are available.
- Autonomy and equity: Even without the barriers mentioned above, self-managed telemedicine abortion remains a patient-centered option. Often more accessible and less expensive, inherently more private, it is bound to appeal to many women.
This decade has seen unprecedented challenges to comprehensive safe reproductive health care, with no relief in sight. In the decades prior to Roe v. Wade, illegal abortions were responsible for 20% of all maternal mortality in the United States. As government, national medical organizations, and the public become more aware of our intolerably high maternal mortality rate, these actors are increasingly driven to bring our maternal health to parity with our industrialized peers. Restricting access to safe abortion runs counter to that goal. Two hundred forty years of American history teach us that legal restrictions do not prevent abortions, because they do not eliminate the reasons for which women seek abortion. Legal restrictions do, however, prevent women from ending pregnancies in the safest manner possible. The inability to obtain safe abortions invariably leads to dead women – our mothers, daughters, sisters, and wives. In this country’s harsh political climate, we must protect a woman’s right to choose. By advocating for innovative approaches to protect women’s reproductive choices, we empower women and save lives.
Dr. Anwar is an obstetrician/gynecologist at Michigan State University in Flint and Dr. Espey is professor and chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. Neither of them have conflicts of interest. Email them at [email protected].
References
1. “How a ‘Stupid Idea’ Gave Birth to Telemedicine,” MedPageToday. Dec 15, .
2. J Neurosurg Pediatr. 2016 Dec;25(6):753-7.
3. Obstet Gynecol. 2017 Oct;130(4):778-82.
4. Centers for Disease Control and Prevention. Severe Maternal Morbidity in the United States.
5. Guttmacher Rep Public Policy. 2018;21:41-7.
6. “International ‘safe abortions by mail’ service can now ship to women in US,” The Hill, Nov 7, 2018.