User login
Consider neurodevelopmental impacts of hyperemesis gravidarum
Hyperemesis gravidarum (HG) affects just 1%-2% of pregnant women, but it’s clinical consequences are significant, with excess vomiting and dehydration, hospitalization, and the need for intravenous fluids being common in that group. In extreme cases, repeated vomiting has led to tears in the esophagus and severe dehydration has caused acute renal failure. All of that leaves aside the obvious suffering and distress it causes for women with the condition.
While studies continue to support the long-held theory that mild-to-moderate nausea and vomiting has a protective effect in pregnancy, that does not appear to be true for HG. Rather, the medical literature shows that HG is associated with small-for-gestational-age neonates, low birth weight, higher rates of preterm birth, and lower Apgar scores at 5 minutes.
I was one of the investigators on a study that prospectively followed more than 200 women with nausea and vomiting in pregnancy from 2006 to 2012. We found that children whose mothers were hospitalized for their symptoms – 22 in all – had significantly lower IQ scores at 3.5 years to 7 years, compared with children whose mothers were not hospitalized. Verbal IQ scores were 107.2 points vs. 112.7 (P = .04), performance IQ scores were 105.6 vs. 112.3 (P = .03), and full scale IQ was 108.7 vs. 114.2 (P = .05).
The study cohort included three groups: women treated with more than four tablets per day of doxylamine/pyridoxine (Diclegis); women treated with up to four tablets per day of the drug; and women who did not receive pharmacotherapy (Obstet Gynecol. 2015. doi: 10.1097/01.AOG.0000463229.81803.1a).
Hospitalized women in the study received antiemetics about a week later, experienced more severe symptoms, and were more likely to report depression. Overall, we found that duration of hospitalization, maternal depression, and maternal IQ all were significant predictors for these outcomes. However, daily antiemetic therapy was not associated with adverse outcomes.
Another study, published the same year, found that children exposed to HG had a more than three times increased risk for a neurodevelopmental diagnosis, including attention disorders, speech and language delays, and sensory disorders. The changes were more prevalent when women experienced symptoms early in pregnancy – prior to 5 weeks of gestation (Eur J Obstet Gynecol Reprod Biol. 2015 Jun;189:79-84).
The study compared neurodevelopmental outcomes for 312 children from 203 women with HG, with 169 children from 89 unaffected mothers. The findings are similar to those of our study, despite the differences in methodologies. Both studies found that the antiemetics were not associated with adverse outcomes, but the symptoms of HG appear to be the culprit.
While more research is needed to confirm these findings, it makes sense that the nutritional deficiencies created by excess vomiting and inability to eat are having an impact on the fetus.
It also raises an important question for the ob.gyn. about when to intervene in these women. Often, clinicians take a wait-and-see approach to nausea and vomiting in pregnancy, but the developing research suggests that earlier intervention would lead to better outcomes for mother and baby. One guide to determining that preventive antiemetics are necessary is to consider whether your patient has had HG in a previous pregnancy or if her mother or sister has experienced HG.
Another consideration is treating the nutritional deficiency that develops in women whose HG symptoms persist. These women are not simply in need of fluids and electrolytes but are missing essential vitamins and proteins. This is an area where much more research is needed, but clinicians can take a proactive approach by providing team care that includes consultation with a dietitians or nutritionist.
Finally, we cannot forget that maternal depression also appears to be significant predictor of poor fetal outcomes, so providing appropriate psychiatric treatment is essential.
Dr. Koren is professor of physiology/pharmacology and pediatrics at Western University in Ontario. He is the founder of the Motherisk Program. Dr. Koren was a principal investigator in the U.S. study that resulted in the approval of Diclegis, marketed by Duchesnay USA, and has served as a consultant to Duchesnay.
Hyperemesis gravidarum (HG) affects just 1%-2% of pregnant women, but it’s clinical consequences are significant, with excess vomiting and dehydration, hospitalization, and the need for intravenous fluids being common in that group. In extreme cases, repeated vomiting has led to tears in the esophagus and severe dehydration has caused acute renal failure. All of that leaves aside the obvious suffering and distress it causes for women with the condition.
While studies continue to support the long-held theory that mild-to-moderate nausea and vomiting has a protective effect in pregnancy, that does not appear to be true for HG. Rather, the medical literature shows that HG is associated with small-for-gestational-age neonates, low birth weight, higher rates of preterm birth, and lower Apgar scores at 5 minutes.
I was one of the investigators on a study that prospectively followed more than 200 women with nausea and vomiting in pregnancy from 2006 to 2012. We found that children whose mothers were hospitalized for their symptoms – 22 in all – had significantly lower IQ scores at 3.5 years to 7 years, compared with children whose mothers were not hospitalized. Verbal IQ scores were 107.2 points vs. 112.7 (P = .04), performance IQ scores were 105.6 vs. 112.3 (P = .03), and full scale IQ was 108.7 vs. 114.2 (P = .05).
The study cohort included three groups: women treated with more than four tablets per day of doxylamine/pyridoxine (Diclegis); women treated with up to four tablets per day of the drug; and women who did not receive pharmacotherapy (Obstet Gynecol. 2015. doi: 10.1097/01.AOG.0000463229.81803.1a).
Hospitalized women in the study received antiemetics about a week later, experienced more severe symptoms, and were more likely to report depression. Overall, we found that duration of hospitalization, maternal depression, and maternal IQ all were significant predictors for these outcomes. However, daily antiemetic therapy was not associated with adverse outcomes.
Another study, published the same year, found that children exposed to HG had a more than three times increased risk for a neurodevelopmental diagnosis, including attention disorders, speech and language delays, and sensory disorders. The changes were more prevalent when women experienced symptoms early in pregnancy – prior to 5 weeks of gestation (Eur J Obstet Gynecol Reprod Biol. 2015 Jun;189:79-84).
The study compared neurodevelopmental outcomes for 312 children from 203 women with HG, with 169 children from 89 unaffected mothers. The findings are similar to those of our study, despite the differences in methodologies. Both studies found that the antiemetics were not associated with adverse outcomes, but the symptoms of HG appear to be the culprit.
While more research is needed to confirm these findings, it makes sense that the nutritional deficiencies created by excess vomiting and inability to eat are having an impact on the fetus.
It also raises an important question for the ob.gyn. about when to intervene in these women. Often, clinicians take a wait-and-see approach to nausea and vomiting in pregnancy, but the developing research suggests that earlier intervention would lead to better outcomes for mother and baby. One guide to determining that preventive antiemetics are necessary is to consider whether your patient has had HG in a previous pregnancy or if her mother or sister has experienced HG.
Another consideration is treating the nutritional deficiency that develops in women whose HG symptoms persist. These women are not simply in need of fluids and electrolytes but are missing essential vitamins and proteins. This is an area where much more research is needed, but clinicians can take a proactive approach by providing team care that includes consultation with a dietitians or nutritionist.
Finally, we cannot forget that maternal depression also appears to be significant predictor of poor fetal outcomes, so providing appropriate psychiatric treatment is essential.
Dr. Koren is professor of physiology/pharmacology and pediatrics at Western University in Ontario. He is the founder of the Motherisk Program. Dr. Koren was a principal investigator in the U.S. study that resulted in the approval of Diclegis, marketed by Duchesnay USA, and has served as a consultant to Duchesnay.
Hyperemesis gravidarum (HG) affects just 1%-2% of pregnant women, but it’s clinical consequences are significant, with excess vomiting and dehydration, hospitalization, and the need for intravenous fluids being common in that group. In extreme cases, repeated vomiting has led to tears in the esophagus and severe dehydration has caused acute renal failure. All of that leaves aside the obvious suffering and distress it causes for women with the condition.
While studies continue to support the long-held theory that mild-to-moderate nausea and vomiting has a protective effect in pregnancy, that does not appear to be true for HG. Rather, the medical literature shows that HG is associated with small-for-gestational-age neonates, low birth weight, higher rates of preterm birth, and lower Apgar scores at 5 minutes.
I was one of the investigators on a study that prospectively followed more than 200 women with nausea and vomiting in pregnancy from 2006 to 2012. We found that children whose mothers were hospitalized for their symptoms – 22 in all – had significantly lower IQ scores at 3.5 years to 7 years, compared with children whose mothers were not hospitalized. Verbal IQ scores were 107.2 points vs. 112.7 (P = .04), performance IQ scores were 105.6 vs. 112.3 (P = .03), and full scale IQ was 108.7 vs. 114.2 (P = .05).
The study cohort included three groups: women treated with more than four tablets per day of doxylamine/pyridoxine (Diclegis); women treated with up to four tablets per day of the drug; and women who did not receive pharmacotherapy (Obstet Gynecol. 2015. doi: 10.1097/01.AOG.0000463229.81803.1a).
Hospitalized women in the study received antiemetics about a week later, experienced more severe symptoms, and were more likely to report depression. Overall, we found that duration of hospitalization, maternal depression, and maternal IQ all were significant predictors for these outcomes. However, daily antiemetic therapy was not associated with adverse outcomes.
Another study, published the same year, found that children exposed to HG had a more than three times increased risk for a neurodevelopmental diagnosis, including attention disorders, speech and language delays, and sensory disorders. The changes were more prevalent when women experienced symptoms early in pregnancy – prior to 5 weeks of gestation (Eur J Obstet Gynecol Reprod Biol. 2015 Jun;189:79-84).
The study compared neurodevelopmental outcomes for 312 children from 203 women with HG, with 169 children from 89 unaffected mothers. The findings are similar to those of our study, despite the differences in methodologies. Both studies found that the antiemetics were not associated with adverse outcomes, but the symptoms of HG appear to be the culprit.
While more research is needed to confirm these findings, it makes sense that the nutritional deficiencies created by excess vomiting and inability to eat are having an impact on the fetus.
It also raises an important question for the ob.gyn. about when to intervene in these women. Often, clinicians take a wait-and-see approach to nausea and vomiting in pregnancy, but the developing research suggests that earlier intervention would lead to better outcomes for mother and baby. One guide to determining that preventive antiemetics are necessary is to consider whether your patient has had HG in a previous pregnancy or if her mother or sister has experienced HG.
Another consideration is treating the nutritional deficiency that develops in women whose HG symptoms persist. These women are not simply in need of fluids and electrolytes but are missing essential vitamins and proteins. This is an area where much more research is needed, but clinicians can take a proactive approach by providing team care that includes consultation with a dietitians or nutritionist.
Finally, we cannot forget that maternal depression also appears to be significant predictor of poor fetal outcomes, so providing appropriate psychiatric treatment is essential.
Dr. Koren is professor of physiology/pharmacology and pediatrics at Western University in Ontario. He is the founder of the Motherisk Program. Dr. Koren was a principal investigator in the U.S. study that resulted in the approval of Diclegis, marketed by Duchesnay USA, and has served as a consultant to Duchesnay.
Zika-related birth defects up in recent weeks
Zika virus infection has been occurring in pregnant women at a slow but steady clip over the last couple of months, but cases of liveborn infants with Zika-related birth defects have jumped in recent weeks, according to the Centers for Disease Control and Prevention.
Eight liveborn infants with Zika-related birth defects were reported to the U.S. Zika Pregnancy Registry during the 2 weeks ending May 23, more than any other 2-week period this year, and that was after six such infants were reported for the 2 weeks ending May 9. The total for the 50 states and the District of Columbia is now 72 for 2016-2017. No new pregnancy losses with birth defects were reported over the same 4-week span, so the 50 state/D.C. total remained at eight for 2016-2017, CDC data show.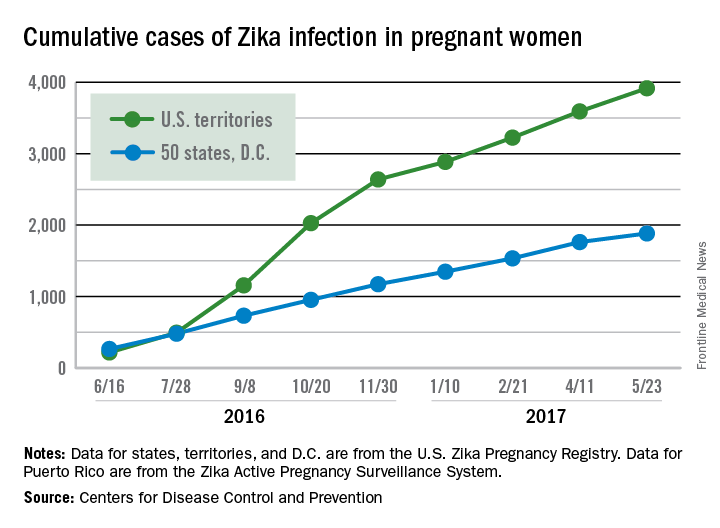
The CDC notes that these are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
Zika virus infection has been occurring in pregnant women at a slow but steady clip over the last couple of months, but cases of liveborn infants with Zika-related birth defects have jumped in recent weeks, according to the Centers for Disease Control and Prevention.
Eight liveborn infants with Zika-related birth defects were reported to the U.S. Zika Pregnancy Registry during the 2 weeks ending May 23, more than any other 2-week period this year, and that was after six such infants were reported for the 2 weeks ending May 9. The total for the 50 states and the District of Columbia is now 72 for 2016-2017. No new pregnancy losses with birth defects were reported over the same 4-week span, so the 50 state/D.C. total remained at eight for 2016-2017, CDC data show.
The CDC notes that these are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
Zika virus infection has been occurring in pregnant women at a slow but steady clip over the last couple of months, but cases of liveborn infants with Zika-related birth defects have jumped in recent weeks, according to the Centers for Disease Control and Prevention.
Eight liveborn infants with Zika-related birth defects were reported to the U.S. Zika Pregnancy Registry during the 2 weeks ending May 23, more than any other 2-week period this year, and that was after six such infants were reported for the 2 weeks ending May 9. The total for the 50 states and the District of Columbia is now 72 for 2016-2017. No new pregnancy losses with birth defects were reported over the same 4-week span, so the 50 state/D.C. total remained at eight for 2016-2017, CDC data show.
The CDC notes that these are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
Cord gas analysis can be beneficial but has drawbacks
“HOW AND WHEN UMBILICAL CORD GAS ANALYSIS CAN JUSTIFY YOUR OBSTETRIC MANAGEMENT”
MICHAEL G. ROSS, MD, MPH (MARCH 2017)
Cord gas analysis can be beneficial but has drawbacks
In his article, Dr. Ross makes a few statements I would like to challenge. He gives a list of indications for cord gas analysis, even with a vigorous newborn. I would suggest that doing so is not only unnecessary, but could get the delivering provider in trouble. Normal gases with a vigorous infant are not actionable, and neither are abnormal gases with a vigorous infant. The latter situation could, however, lower the bar for a lawsuit if any neurologic pathology is diagnosed in the child.
At our hospital, blood gas assessments generate charges of $90 for each arterial and venous sample. The author states that gases are helpful for staff education. If that is the purposeof measuring the gases when Apgar scores are normal, then the bill for the gases should be sent to the staff, not the patient or insurance company.
The precise reason for doing cord gases is to prove you are a good doctor. If the Apgar scores are low, a healthy set of gases shows that your interventions were timely and appropriate. Normal gases prevent lawsuits in this situation.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Ross responds
I appreciate the comments of Dr. Walsh, who suggests that we should not obtain cord gases in vigorous infants due, in part, to the hospital charges. There are several reasons for the indications detailed in the article. Although normal Apgar scores would appear to negate the potential for severe metabolic acidosis, Apgar scoring accuracy has been challenged in medical legal cases. Furthermore, there may be newborn complications (eg, pre-existing hypoxic injury, intraventricular bleed) that may not be recognized immediately, yet hypoxemia and acidosis may be alleged to have contributed to the outcome. The actual cost of running a blood gas sample is far less than the $90 hospital charges. Nevertheless, if hospital charge is a concern, I recommend that the physician obtain a cord gas sample immediately following the delivery and determine whether to run the sample after the 5-minute Apgar score is obtained.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“HOW AND WHEN UMBILICAL CORD GAS ANALYSIS CAN JUSTIFY YOUR OBSTETRIC MANAGEMENT”
MICHAEL G. ROSS, MD, MPH (MARCH 2017)
Cord gas analysis can be beneficial but has drawbacks
In his article, Dr. Ross makes a few statements I would like to challenge. He gives a list of indications for cord gas analysis, even with a vigorous newborn. I would suggest that doing so is not only unnecessary, but could get the delivering provider in trouble. Normal gases with a vigorous infant are not actionable, and neither are abnormal gases with a vigorous infant. The latter situation could, however, lower the bar for a lawsuit if any neurologic pathology is diagnosed in the child.
At our hospital, blood gas assessments generate charges of $90 for each arterial and venous sample. The author states that gases are helpful for staff education. If that is the purposeof measuring the gases when Apgar scores are normal, then the bill for the gases should be sent to the staff, not the patient or insurance company.
The precise reason for doing cord gases is to prove you are a good doctor. If the Apgar scores are low, a healthy set of gases shows that your interventions were timely and appropriate. Normal gases prevent lawsuits in this situation.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Ross responds
I appreciate the comments of Dr. Walsh, who suggests that we should not obtain cord gases in vigorous infants due, in part, to the hospital charges. There are several reasons for the indications detailed in the article. Although normal Apgar scores would appear to negate the potential for severe metabolic acidosis, Apgar scoring accuracy has been challenged in medical legal cases. Furthermore, there may be newborn complications (eg, pre-existing hypoxic injury, intraventricular bleed) that may not be recognized immediately, yet hypoxemia and acidosis may be alleged to have contributed to the outcome. The actual cost of running a blood gas sample is far less than the $90 hospital charges. Nevertheless, if hospital charge is a concern, I recommend that the physician obtain a cord gas sample immediately following the delivery and determine whether to run the sample after the 5-minute Apgar score is obtained.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“HOW AND WHEN UMBILICAL CORD GAS ANALYSIS CAN JUSTIFY YOUR OBSTETRIC MANAGEMENT”
MICHAEL G. ROSS, MD, MPH (MARCH 2017)
Cord gas analysis can be beneficial but has drawbacks
In his article, Dr. Ross makes a few statements I would like to challenge. He gives a list of indications for cord gas analysis, even with a vigorous newborn. I would suggest that doing so is not only unnecessary, but could get the delivering provider in trouble. Normal gases with a vigorous infant are not actionable, and neither are abnormal gases with a vigorous infant. The latter situation could, however, lower the bar for a lawsuit if any neurologic pathology is diagnosed in the child.
At our hospital, blood gas assessments generate charges of $90 for each arterial and venous sample. The author states that gases are helpful for staff education. If that is the purposeof measuring the gases when Apgar scores are normal, then the bill for the gases should be sent to the staff, not the patient or insurance company.
The precise reason for doing cord gases is to prove you are a good doctor. If the Apgar scores are low, a healthy set of gases shows that your interventions were timely and appropriate. Normal gases prevent lawsuits in this situation.
Joe Walsh, MD
Philadelphia, Pennsylvania
Dr. Ross responds
I appreciate the comments of Dr. Walsh, who suggests that we should not obtain cord gases in vigorous infants due, in part, to the hospital charges. There are several reasons for the indications detailed in the article. Although normal Apgar scores would appear to negate the potential for severe metabolic acidosis, Apgar scoring accuracy has been challenged in medical legal cases. Furthermore, there may be newborn complications (eg, pre-existing hypoxic injury, intraventricular bleed) that may not be recognized immediately, yet hypoxemia and acidosis may be alleged to have contributed to the outcome. The actual cost of running a blood gas sample is far less than the $90 hospital charges. Nevertheless, if hospital charge is a concern, I recommend that the physician obtain a cord gas sample immediately following the delivery and determine whether to run the sample after the 5-minute Apgar score is obtained.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Universal cervical length screening–saving babies lives
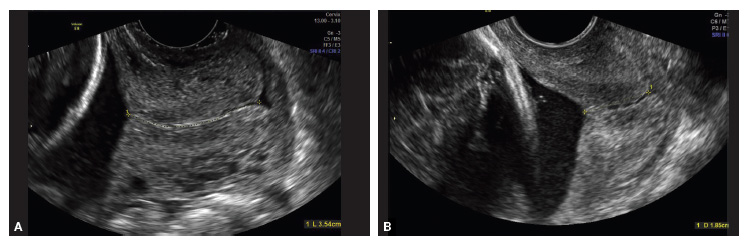
Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
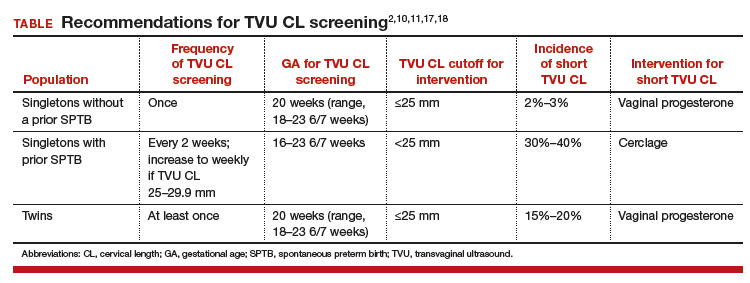
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).
Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.

Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).

Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Transvaginal ultrasound (TVU) cervical length (CL) screening for prediction and prevention of spontaneous preterm birth (SPTB) is among the most transformative clinical changes in obstetrics in the last decades. TVU CL screening should now be offered to all pregnant women: hence the appellative ‘universal CL screening.’
TVU CL screening is an excellent screening test for several reasons. It screens for SPTB, which is a clinically important, well-defined disease whose prevalence and natural history is known, and has an early recognizable asymptomatic phase in CL shortening detected by TVU. TVU CL screening is a well-described technique, safe and acceptable, with a reasonable cutoff (25 mm) now identified for all populations, and results are reproducible and accurate. There are hundreds of studies proving these facts. In the last 10 years, TVU measurement of CL as a screening test has been accepted1,2: it identifies women at risk for SPTB, and an early intervention (progesterone or cerclage depending on the clinical situation) is effective in preventing SPTB. Screening and treatment of short cervix is cost-effective and readily available as an early intervention (progesterone or cerclage depending on the clinical situation), is effective in preventing the outcome (SPTB), treating abnormal results is cost-effective, and facilities for screening are available and treatments are readily available.3–5 It is also important to emphasize that CL screening for prevention of SPTB should be done by TVU, and not by transabdominal ultrasound.6It is best to review TVU CL screening by populations: singletons without prior SPTB, singletons with prior SPTB, and twins (Table).

Related Article:
Can transabdominal ultrasound exclude short cervix?
Singletons without prior SPTB
Women with no previous SPTB who are carrying a singleton pregnancy is the population in which TVU CL could have the greatest impact on decreasing SPTB, for several reasons:
- Up to 60% to 90% of SPTB occur in this population.
- More than 90% of these women have risk factors for SPTB.7,8
- Vaginal progesterone has been associated with a significant 39% decrease in PTB at <33 weeks of gestation and a significant 38% decrease in perinatal morbidity and mortality in a meta-analysis of randomized controlled trials (RCTs) including 606 women without prior PTB.9,10
- Cost-effectiveness studies have shown that TVU CL screening in this specific population prevents thousands of preterm births, saves or improves from death or major morbidity 350 babies’ lives annually, and saves approximately $320,000 per year in the US alone.3 These numbers may be even higher now as the TVU CL cutoff for offering vaginal progesterone has moved in many centers from ≤20 mm to ≤25 mm, including more women (from about 0.8% to about 2% to 3%, respectively11) who benefit from screening.
- Real-world implementation studies have indeed shown significant decreases in SPTB when a policy of universal TVU CL screening in this specific population is implemented.12,13
Universal TVU CL screening recently called into question
In a recent article published in the Journal of the American Medical Association,14 TVU CL screening in this population, in particular for nulliparous women, has come under interrogation. The authors found only an 8% sensitivity of TVU CL screening for SPTB using a cutoff of ≤25 mm at 16 0/7 to 22 6/7 weeks of gestation in 9,410 nulliparous women. This result is different compared with other previous cohort studies in this area, however, and is likely related to a number of issues in the methodology.
First, TVU CL screening was done in many women at too early a gestational age. The earlier the CL screening, the lower the sensitivity of the procedure. Data at 16 and 17 weeks of gestation should have been excluded, as almost all RCTs and other studies on universal TVU CL screening in this population recommended doing screening at about 18 0/7 to 23 6/7 weeks.
Second, women with TVU CL <15 mm received vaginal progesterone. This would decrease the incidence of PTB and, therefore, sensitivity.
Third, outcomes data were not available for 469 women and, compared with women analyzed, these women were at higher risk for SPTB as they were more likely to be aged 21 years or younger, black, with less than a high school education, and single, all significant risk factors for SPTB. (Not all risk factors for SPTB were reported in this study.)
Fourth, pregnancy losses before 20 weeks were excluded, and these could have been early SPTB; therefore, the sensitivity could have been decreased if women with this outcome were excluded.
Fifth, prior studies have shown that TVU CL screening in singletons without prior SPTB has a sensitivity of about 30% to 40%.15,16 In nulliparas, the sensitivity of TVU CL ≤20 mm had been reported previously to be 20%.16 Additional data from 2012–2014 at our institution demonstrate that the incidence of CL ≤25 mm is about 2.8% in nulliparous women, with a sensitivity of 19.5% for SPTB <37 weeks. These numbers show again that 8% sensitivity was low in the JAMA study14 due the shortcomings we just highlighted. Furthermore, the reported sensitivity of TVU CL ≤25 mm for PTB <32 weeks was 24% in Esplin and colleagues’ study,14 while 60% in our data. Given that early preterm births are the most significant source of neonatal morbidity and mortality, women with a singleton gestation and no prior SPTB but with a short TVU CL are perhaps the most important subgroup to identify.
Sixth, a low sensitivity in and of itself is not reflective of a poor screening test. We have known for a long time that SPTB has many etiologies. No one screening test, and no one intervention, would independently prevent all SPTBs. In a population that accounts for more than half of PTBs and for whom no other screening test has been found to be effective, much less cost effective, it is important not to cast aside the dramatic potential clinical benefit to TVU CL screening.
Related Article:
A stepwise approach to cervical cerclage
Singletons with a prior SPTB
This is the first population in which TVU CL screening was first proven beneficial for prevention of SPTB. These women all should receive progesterone starting at 16 weeks because of the prior SPTB. In these women, TVU CL screening should be initiated at 16 weeks, and repeated every 2 weeks (weekly if TVU CL is found to be 25 mm to 29 mm) until 23 6/7 weeks. If the TVU CL is identified to be <25 mm before 24 weeks, cerclage should be recommended.1,2,17
Twins
Twins are the most recent population in which an intervention based on TVU CL screening has been shown to be beneficial. Vaginal progesterone has been associated with a significant decrease in SPTB as well as in some neonatal outcomes in twin gestations found to have a TVU CL <25 mm in the midtrimester in a meta-analysis of RCTs.18 Based on these results, we at our institution recently have started offering TVU CL screening at the time of the anatomy scan (about 20 weeks) to twin gestations.
Related Article:
Which perioperative strategies for transvaginal cervical cerclage are backed by data?
Bottom line
In summary, universal second trimester TVU CL screening of both singletons and twin gestations should be considered seriously by obstetric practitioners to successfully decrease the grave burden of SPTB.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.
- Berghella V. Progesterone and preterm birth prevention: Translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206(5):376-386.
- Committee on Practice Bulletins--Obstetrics, The American College of Obstetricians and Gynecologists. Practice Bulletin No. 130: Prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
- Werner EF, Hamel MS, Orzechowski K, Berghella V, Thung SF. Cost-effectiveness of transvaginal ultrasound cervical length screening in singletons without a prior preterm birth: an update. Am J Obstet Gynecol. 2015;213(4):554.e1-e6.
- Einerson BD, Grobman WA, Miller ES. Cost-effectiveness of risk-based screening for cervical length to prevent preterm birth. Am J Obstet Gynecol. 2016;215(1):100.e1-e7.
- McIntosh J, Feltovich H, Berghella V, Manuck T; Society for Maternal-Fetal medicine. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2-B7.
- Khalifeh A, Quist-Nelson J. Current implementation of universal cervical length screening for preterm birth prevention in the United States. Obstet Gynecol. 2016;127(suppl 1):7S.
- Mella MT, Mackeen AD, Gache D, Baxter JK, Berghella V. The utility of screening for historical risk factors for preterm birth in women with known second trimester cervical length. J Matern Fetal Neonatal Med. 2013;26(7):710-715.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(5):572-591.
- Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1-e19.
- Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth ≤34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016;48(3):308-317.
- Orzechoski KM, Boelig RC, Baxter JK, Berghella V. A universal transvaginal cervical length screening program for preterm birth prevention. Obstet Gynecol. 2014;124(3):520-525.
- Son M, Grobman WA, Ayala NK, Miller ES. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(3):365.e1-e5.
- Temming LA, Durst JK, Tuuli MG, et al. Universal cervical length screening: implementation and outcomes. Am J Obstet Gynecol. 2016;214(4):523.e1-e8.
- Esplin MS, Elovitz MA, Iams JD, et al; njMoM2b Network. Predictive accuracy of serial ttransvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047-1056.
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567-572.
- Orzechowski KM, Boelig R, Nicholas SS, Baxter J, Berghella V. Is universal cervical length screening indicated in women with prior term birth? Am J Obstet Gynecol. 2015;212(2):234.e1-e5.
- Preterm labour and birth. National Institute for Health and Care Excellence website. https://www.nice.org.uk/guidance/ng25?unlid=9291036072016213201257. Published November 2015. Accessed May 18, 2017.
- Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49(3):303-314.
First trimester use of inactivated flu vaccine didn’t cause birth defects
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
, said Elyse Olshen Kharbanda, MD, of HealthPartners Institute, Minneapolis, and her associates.
Data from seven participating Vaccine Safety Datalink sites in six states were used to identify 52,856 women who received IIV in the first trimester of pregnancy (12% of the study total) and 373,088 not exposed to the flu vaccine in the first trimester (88%). A total of 865 women in the IIV-exposed group had an infant with 1 of the 50 selected major structural defects (1.6 per 100 live births), versus 5,730 in the unexposed group (1.5 per 100 live births).
Among the strengths of the study were the large population, which allowed the researchers to examine subgroups of major structural birth defects; their findings were consistent across all those subgroups. In addition, the investigators were able to exclude women at increased risk for major birth defects because of comorbidities such as diabetes, drug exposures, diagnosed chromosomal abnormalities, or congenital infections. Finally, the study authors were able to exclude women with potential exposure to teratogenic medications.
“Because IIV is currently recommended for all women who will be pregnant during periods of influenza circulation, these data should provide reassurance for women considering first trimester vaccination,” said Dr. Kharbanda and her associates.
Read more in the Journal of Pediatrics (2017 May 24. doi: 10.1016/j.jpeds.2017.04.039).
FROM THE JOURNAL OF PEDIATRICS
Infections up the risk for pregnancy-associated stroke in preeclampsia
A host of factors, some of them preventable or treatable, increase the risk of pregnancy-related stroke among women hospitalized for preeclampsia, according to findings from a case-control study of nearly 800 preeclamptic women in New York.
Women who experienced a stroke were roughly seven times more likely to have severe preeclampsia or eclampsia, and about three to four times more likely to have an infection, a prothrombotic state, a coagulopathy, or chronic hypertension, according to the findings (Stroke. 2017 May 25. doi: 10.1161/STROKEAHA.117.017374).
“Prospective studies are needed to confirm these findings and develop interventions aimed at preventing strokes in this uniquely vulnerable group,” they added.
For the study, the investigators used billing data from the 2003-2012 New York State Department of Health inpatient database to identify women aged 12-55 years admitted with preeclampsia.
They matched each woman who experienced pregnancy-associated stroke with three randomly selected controls of the same age, race/ethnicity, and insurance status. They then compared the groups on a set of predefined risk factors.
Results showed that of 88,857 women admitted for preeclampsia during the study period, 0.2% experienced pregnancy-associated stroke, translating to a cumulative incidence of 222 per 100,000 preeclamptic women, a value more than six times that seen in the general pregnant population, the investigators noted.
The majority of strokes occurred post partum (66.5%), but more than a quarter occurred before delivery (27.9%). The single most common type of stroke was hemorrhagic (46.7%).
The 197 women with preeclampsia who experienced pregnancy-associated stroke had a sharply higher rate of in-hospital mortality (13.2%), compared with the 591 controls (0.2%).
In multivariate analysis, women with preeclampsia experiencing stroke were more likely to have severe preeclampsia or eclampsia (odds ratio, 7.2; 95% confidence interval, 4.6-11.3), or infections at the time of admission (OR, 3.0; 95% CI, 1.6-5.8), predominantly genitourinary infections.
Other risk factors for pregnancy-associated stroke included prothrombotic states (OR, 3.5; 95% CI, 1.3-9.2), coagulopathies (OR, 3.1; 95% CI, 1.3-7.1), or chronic hypertension (OR, 3.2; 95% CI, 1.8-5.5).
The findings were similar when women were matched by the severity of preeclampsia, when women with eclampsia were excluded, or when women with only postpartum stroke were included.
Heart disease, multiple gestation, and previous pregnancies were not significantly independently associated with the risk of pregnancy-associated stroke.
“The ethnic and regional diversity of New York State increases the generalizability of our findings,” the investigators wrote. “Matching of cases and controls allowed for nuanced analysis of other risk factors.”
But the study may have missed some cases of preeclampsia not formally diagnosed, and the timing of infections relative to stroke was unknown, they acknowledged. Additionally, they noted that causality cannot be inferred from the observational study, and therefore the results should be interpreted cautiously.
The investigators reported research support from the National Institutes of Health. They had no other financial disclosures.
A host of factors, some of them preventable or treatable, increase the risk of pregnancy-related stroke among women hospitalized for preeclampsia, according to findings from a case-control study of nearly 800 preeclamptic women in New York.
Women who experienced a stroke were roughly seven times more likely to have severe preeclampsia or eclampsia, and about three to four times more likely to have an infection, a prothrombotic state, a coagulopathy, or chronic hypertension, according to the findings (Stroke. 2017 May 25. doi: 10.1161/STROKEAHA.117.017374).
“Prospective studies are needed to confirm these findings and develop interventions aimed at preventing strokes in this uniquely vulnerable group,” they added.
For the study, the investigators used billing data from the 2003-2012 New York State Department of Health inpatient database to identify women aged 12-55 years admitted with preeclampsia.
They matched each woman who experienced pregnancy-associated stroke with three randomly selected controls of the same age, race/ethnicity, and insurance status. They then compared the groups on a set of predefined risk factors.
Results showed that of 88,857 women admitted for preeclampsia during the study period, 0.2% experienced pregnancy-associated stroke, translating to a cumulative incidence of 222 per 100,000 preeclamptic women, a value more than six times that seen in the general pregnant population, the investigators noted.
The majority of strokes occurred post partum (66.5%), but more than a quarter occurred before delivery (27.9%). The single most common type of stroke was hemorrhagic (46.7%).
The 197 women with preeclampsia who experienced pregnancy-associated stroke had a sharply higher rate of in-hospital mortality (13.2%), compared with the 591 controls (0.2%).
In multivariate analysis, women with preeclampsia experiencing stroke were more likely to have severe preeclampsia or eclampsia (odds ratio, 7.2; 95% confidence interval, 4.6-11.3), or infections at the time of admission (OR, 3.0; 95% CI, 1.6-5.8), predominantly genitourinary infections.
Other risk factors for pregnancy-associated stroke included prothrombotic states (OR, 3.5; 95% CI, 1.3-9.2), coagulopathies (OR, 3.1; 95% CI, 1.3-7.1), or chronic hypertension (OR, 3.2; 95% CI, 1.8-5.5).
The findings were similar when women were matched by the severity of preeclampsia, when women with eclampsia were excluded, or when women with only postpartum stroke were included.
Heart disease, multiple gestation, and previous pregnancies were not significantly independently associated with the risk of pregnancy-associated stroke.
“The ethnic and regional diversity of New York State increases the generalizability of our findings,” the investigators wrote. “Matching of cases and controls allowed for nuanced analysis of other risk factors.”
But the study may have missed some cases of preeclampsia not formally diagnosed, and the timing of infections relative to stroke was unknown, they acknowledged. Additionally, they noted that causality cannot be inferred from the observational study, and therefore the results should be interpreted cautiously.
The investigators reported research support from the National Institutes of Health. They had no other financial disclosures.
A host of factors, some of them preventable or treatable, increase the risk of pregnancy-related stroke among women hospitalized for preeclampsia, according to findings from a case-control study of nearly 800 preeclamptic women in New York.
Women who experienced a stroke were roughly seven times more likely to have severe preeclampsia or eclampsia, and about three to four times more likely to have an infection, a prothrombotic state, a coagulopathy, or chronic hypertension, according to the findings (Stroke. 2017 May 25. doi: 10.1161/STROKEAHA.117.017374).
“Prospective studies are needed to confirm these findings and develop interventions aimed at preventing strokes in this uniquely vulnerable group,” they added.
For the study, the investigators used billing data from the 2003-2012 New York State Department of Health inpatient database to identify women aged 12-55 years admitted with preeclampsia.
They matched each woman who experienced pregnancy-associated stroke with three randomly selected controls of the same age, race/ethnicity, and insurance status. They then compared the groups on a set of predefined risk factors.
Results showed that of 88,857 women admitted for preeclampsia during the study period, 0.2% experienced pregnancy-associated stroke, translating to a cumulative incidence of 222 per 100,000 preeclamptic women, a value more than six times that seen in the general pregnant population, the investigators noted.
The majority of strokes occurred post partum (66.5%), but more than a quarter occurred before delivery (27.9%). The single most common type of stroke was hemorrhagic (46.7%).
The 197 women with preeclampsia who experienced pregnancy-associated stroke had a sharply higher rate of in-hospital mortality (13.2%), compared with the 591 controls (0.2%).
In multivariate analysis, women with preeclampsia experiencing stroke were more likely to have severe preeclampsia or eclampsia (odds ratio, 7.2; 95% confidence interval, 4.6-11.3), or infections at the time of admission (OR, 3.0; 95% CI, 1.6-5.8), predominantly genitourinary infections.
Other risk factors for pregnancy-associated stroke included prothrombotic states (OR, 3.5; 95% CI, 1.3-9.2), coagulopathies (OR, 3.1; 95% CI, 1.3-7.1), or chronic hypertension (OR, 3.2; 95% CI, 1.8-5.5).
The findings were similar when women were matched by the severity of preeclampsia, when women with eclampsia were excluded, or when women with only postpartum stroke were included.
Heart disease, multiple gestation, and previous pregnancies were not significantly independently associated with the risk of pregnancy-associated stroke.
“The ethnic and regional diversity of New York State increases the generalizability of our findings,” the investigators wrote. “Matching of cases and controls allowed for nuanced analysis of other risk factors.”
But the study may have missed some cases of preeclampsia not formally diagnosed, and the timing of infections relative to stroke was unknown, they acknowledged. Additionally, they noted that causality cannot be inferred from the observational study, and therefore the results should be interpreted cautiously.
The investigators reported research support from the National Institutes of Health. They had no other financial disclosures.
FROM STROKE
Key clinical point:
Major finding: Independent risk factors for pregnancy-associated stroke were severe preeclampsia or eclampsia (OR, 7.2), infections (OR, 3.0), prothrombotic states (OR, 3.5), coagulopathies (OR, 3.1), or chronic hypertension (OR, 3.2).
Data source: A matched, case-control study of 788 women from a New York inpatient database who were hospitalized for preeclampsia.
Disclosures: The investigators reported research support from the National Institutes of Health. They had no other financial disclosures.
Inpatient prenatal yoga found feasible for high-risk women
AT ACOG 2017
SAN DIEGO – Inpatient prenatal yoga is a feasible and acceptable intervention for high-risk women admitted to the hospital, results from a single-center study suggested.
“We know that outside of obstetrics, yoga is beneficial to stress relief, musculoskeletal pain, and sleep quality,” Veronica Demtchouk, MD, said in an interview at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Inpatient high-risk obstetrics patients have very limited physical activity that they feel is safe to do.”
In an effort to investigate the feasibility of establishing an inpatient prenatal yoga program, the researchers recruited 40 women with anticipated admission to the antepartum service for at least 72 hours and who received medical clearance from their primary obstetrician. One of the medical center’s nurse practitioners, who is also a certified yoga instructor, taught a 30-minute prenatal yoga session once a week in a waiting room.
“It was a large enough space; we moved away the furniture and did the yoga sessions there,” Dr. Demtchouk said.
Study participants completed a questionnaire after each yoga session and at hospital discharge, while 14 nurses completed questionnaires regarding patient care and patient satisfaction. Of the 40 patients, 16 completed one or more yoga sessions; 24 did not participate because of scheduling conflicts with ultrasound or fetal testing, change in clinical status, lack of interest on the day of the session, and delivery or discharge prior to the yoga session.
Of the 16 study participants, 8 reported a decreased level of stress, 4 reported better sleep, 4 reported applying the yoga techniques outside of class, and 3 reported decreased pain/discomfort.
“Not a single woman complained or was displeased with the yoga sessions,” Dr. Demtchouk said. “The biggest challenge was the timing of the yoga session. It was just once a week, which limited the number of women who could attend.”
Of the 14 nurses who completed questionnaires, all viewed yoga as beneficial to their patients, none found it disruptive to providing patient care, and all indicated they would recommend an inpatient prenatal yoga program to other hospitals with an antepartum service.
“I think having several sessions throughout the week is essential for having adequate patient participation,” Dr. Demtchouk added. “It’s essential to have the nurses on board with it.”
She reported having no relevant financial disclosures.
AT ACOG 2017
SAN DIEGO – Inpatient prenatal yoga is a feasible and acceptable intervention for high-risk women admitted to the hospital, results from a single-center study suggested.
“We know that outside of obstetrics, yoga is beneficial to stress relief, musculoskeletal pain, and sleep quality,” Veronica Demtchouk, MD, said in an interview at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Inpatient high-risk obstetrics patients have very limited physical activity that they feel is safe to do.”
In an effort to investigate the feasibility of establishing an inpatient prenatal yoga program, the researchers recruited 40 women with anticipated admission to the antepartum service for at least 72 hours and who received medical clearance from their primary obstetrician. One of the medical center’s nurse practitioners, who is also a certified yoga instructor, taught a 30-minute prenatal yoga session once a week in a waiting room.
“It was a large enough space; we moved away the furniture and did the yoga sessions there,” Dr. Demtchouk said.
Study participants completed a questionnaire after each yoga session and at hospital discharge, while 14 nurses completed questionnaires regarding patient care and patient satisfaction. Of the 40 patients, 16 completed one or more yoga sessions; 24 did not participate because of scheduling conflicts with ultrasound or fetal testing, change in clinical status, lack of interest on the day of the session, and delivery or discharge prior to the yoga session.
Of the 16 study participants, 8 reported a decreased level of stress, 4 reported better sleep, 4 reported applying the yoga techniques outside of class, and 3 reported decreased pain/discomfort.
“Not a single woman complained or was displeased with the yoga sessions,” Dr. Demtchouk said. “The biggest challenge was the timing of the yoga session. It was just once a week, which limited the number of women who could attend.”
Of the 14 nurses who completed questionnaires, all viewed yoga as beneficial to their patients, none found it disruptive to providing patient care, and all indicated they would recommend an inpatient prenatal yoga program to other hospitals with an antepartum service.
“I think having several sessions throughout the week is essential for having adequate patient participation,” Dr. Demtchouk added. “It’s essential to have the nurses on board with it.”
She reported having no relevant financial disclosures.
AT ACOG 2017
SAN DIEGO – Inpatient prenatal yoga is a feasible and acceptable intervention for high-risk women admitted to the hospital, results from a single-center study suggested.
“We know that outside of obstetrics, yoga is beneficial to stress relief, musculoskeletal pain, and sleep quality,” Veronica Demtchouk, MD, said in an interview at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “Inpatient high-risk obstetrics patients have very limited physical activity that they feel is safe to do.”
In an effort to investigate the feasibility of establishing an inpatient prenatal yoga program, the researchers recruited 40 women with anticipated admission to the antepartum service for at least 72 hours and who received medical clearance from their primary obstetrician. One of the medical center’s nurse practitioners, who is also a certified yoga instructor, taught a 30-minute prenatal yoga session once a week in a waiting room.
“It was a large enough space; we moved away the furniture and did the yoga sessions there,” Dr. Demtchouk said.
Study participants completed a questionnaire after each yoga session and at hospital discharge, while 14 nurses completed questionnaires regarding patient care and patient satisfaction. Of the 40 patients, 16 completed one or more yoga sessions; 24 did not participate because of scheduling conflicts with ultrasound or fetal testing, change in clinical status, lack of interest on the day of the session, and delivery or discharge prior to the yoga session.
Of the 16 study participants, 8 reported a decreased level of stress, 4 reported better sleep, 4 reported applying the yoga techniques outside of class, and 3 reported decreased pain/discomfort.
“Not a single woman complained or was displeased with the yoga sessions,” Dr. Demtchouk said. “The biggest challenge was the timing of the yoga session. It was just once a week, which limited the number of women who could attend.”
Of the 14 nurses who completed questionnaires, all viewed yoga as beneficial to their patients, none found it disruptive to providing patient care, and all indicated they would recommend an inpatient prenatal yoga program to other hospitals with an antepartum service.
“I think having several sessions throughout the week is essential for having adequate patient participation,” Dr. Demtchouk added. “It’s essential to have the nurses on board with it.”
She reported having no relevant financial disclosures.
Key clinical point:
Major finding: Of the 16 study participants, 8 reported a decreased level of stress, 4 reported better sleep, 4 reported applying the yoga techniques outside of class, and 3 reported decreased pain/discomfort.
Data source: A feasibility study of 16 hospitalized high-risk pregnant women.
Disclosures: Dr. Demtchouk reported having no relevant financial disclosures.
Prepping the vagina before cesarean delivery
"SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?"
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Prepping the vagina before cesarean delivery
I enjoyed your review of the topic. I am interested in using vaginal preparation prior to cesarean in the settings of active-phase and second-stage arrest. This should be most valuable since we anticipate possible prolonged attempt at head delivery. There may be a need for head elevation as well. Of course, we have become enthusiastic about using reverse breech extraction in difficult cases since your article a few years ago. I have yet to do a Patwardhan maneuver. That seems to rely on rotating the spine anteriorly to get the second arm out. With the head impaction, there is limited range for neck rotation. With vaginal preparation, is there any concern about fetal exposure to iodine?
Kimberly Harney, MD
Stanford, California
Dr. Barbieri responds
Dr. Harney raises the important issue of the potential adverse effects of povidone-iodine surgical preparation when used on a pregnant woman with ruptured membranes. There is very little direct evidence of a toxic effect of povidone-iodine on the fetus, but studies on women report that there is a transient increase in circulating iodine and iodine excretion following a vaginal povidone-iodine preparation.1 The American College of Obstetricians and Gynecologists has suggested that chlorhexidine might be a superior vaginal disinfectant than povidone-iodine,2 but chlorhexidine is not approved by the US Food and Drug Administration for use in the vagina, and many surgical nursing directors favor the use of povidone-iodine in the vagina.3
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Another way to prevent post-cesarean delivery infections
After 40 years in ObGyn practice (I am now retired), I find it interesting that experts have ignored a major potential source of infection--the operation team. Back in the day of Phisohex (hexachlorophene) use, we scrubbed our hands, arms, and fingers for a finite time--10 minutes--systematically and religiously. Our infection rates increased only when house staff rather than surgical assistants "helped" us. When scrubbing, I was always amazed that the house staff appeared at the sink long after I did and left before I had completed my presurgical ritual. (This was not true of non-MD assistants.) And my private practice postoperative infection rate reflected the difference. So perhaps the evidence is skewed away from this source of infection, which I submit may well be the major one!
Steve Melkin, MD
Phoenix, Arizona
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Velasco I, Naranjo S, Lopez-Pedrera C, Garriga MJ, Garcia-Fuentes E, Soriquer F. Use of povidine-iodine during the first trimester of pregnancy: a correct practice? BJOG. 2009;116(3):452-455.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 571: solutions for surgical preparation of the vagina. Obstet Gynecol. 2013;122(3):718-720.
- Guideline for preoperative patient skin antisepsis. In: Guidelines for perioperative practice. Denver, CO: Association of Perioperative Registered Nurses, Inc; 2014.
"SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?"
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Prepping the vagina before cesarean delivery
I enjoyed your review of the topic. I am interested in using vaginal preparation prior to cesarean in the settings of active-phase and second-stage arrest. This should be most valuable since we anticipate possible prolonged attempt at head delivery. There may be a need for head elevation as well. Of course, we have become enthusiastic about using reverse breech extraction in difficult cases since your article a few years ago. I have yet to do a Patwardhan maneuver. That seems to rely on rotating the spine anteriorly to get the second arm out. With the head impaction, there is limited range for neck rotation. With vaginal preparation, is there any concern about fetal exposure to iodine?
Kimberly Harney, MD
Stanford, California
Dr. Barbieri responds
Dr. Harney raises the important issue of the potential adverse effects of povidone-iodine surgical preparation when used on a pregnant woman with ruptured membranes. There is very little direct evidence of a toxic effect of povidone-iodine on the fetus, but studies on women report that there is a transient increase in circulating iodine and iodine excretion following a vaginal povidone-iodine preparation.1 The American College of Obstetricians and Gynecologists has suggested that chlorhexidine might be a superior vaginal disinfectant than povidone-iodine,2 but chlorhexidine is not approved by the US Food and Drug Administration for use in the vagina, and many surgical nursing directors favor the use of povidone-iodine in the vagina.3
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Another way to prevent post-cesarean delivery infections
After 40 years in ObGyn practice (I am now retired), I find it interesting that experts have ignored a major potential source of infection--the operation team. Back in the day of Phisohex (hexachlorophene) use, we scrubbed our hands, arms, and fingers for a finite time--10 minutes--systematically and religiously. Our infection rates increased only when house staff rather than surgical assistants "helped" us. When scrubbing, I was always amazed that the house staff appeared at the sink long after I did and left before I had completed my presurgical ritual. (This was not true of non-MD assistants.) And my private practice postoperative infection rate reflected the difference. So perhaps the evidence is skewed away from this source of infection, which I submit may well be the major one!
Steve Melkin, MD
Phoenix, Arizona
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
"SHOULD YOU ADOPT THE PRACTICE OF VAGINAL CLEANSING WITH POVIDONE-IODINE PRIOR TO CESAREAN DELIVERY?"
ROBERT L. BARBIERI, MD (EDITORIAL; JANUARY 2016)
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Prepping the vagina before cesarean delivery
I enjoyed your review of the topic. I am interested in using vaginal preparation prior to cesarean in the settings of active-phase and second-stage arrest. This should be most valuable since we anticipate possible prolonged attempt at head delivery. There may be a need for head elevation as well. Of course, we have become enthusiastic about using reverse breech extraction in difficult cases since your article a few years ago. I have yet to do a Patwardhan maneuver. That seems to rely on rotating the spine anteriorly to get the second arm out. With the head impaction, there is limited range for neck rotation. With vaginal preparation, is there any concern about fetal exposure to iodine?
Kimberly Harney, MD
Stanford, California
Dr. Barbieri responds
Dr. Harney raises the important issue of the potential adverse effects of povidone-iodine surgical preparation when used on a pregnant woman with ruptured membranes. There is very little direct evidence of a toxic effect of povidone-iodine on the fetus, but studies on women report that there is a transient increase in circulating iodine and iodine excretion following a vaginal povidone-iodine preparation.1 The American College of Obstetricians and Gynecologists has suggested that chlorhexidine might be a superior vaginal disinfectant than povidone-iodine,2 but chlorhexidine is not approved by the US Food and Drug Administration for use in the vagina, and many surgical nursing directors favor the use of povidone-iodine in the vagina.3
"PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER"
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMBER 2016)
Another way to prevent post-cesarean delivery infections
After 40 years in ObGyn practice (I am now retired), I find it interesting that experts have ignored a major potential source of infection--the operation team. Back in the day of Phisohex (hexachlorophene) use, we scrubbed our hands, arms, and fingers for a finite time--10 minutes--systematically and religiously. Our infection rates increased only when house staff rather than surgical assistants "helped" us. When scrubbing, I was always amazed that the house staff appeared at the sink long after I did and left before I had completed my presurgical ritual. (This was not true of non-MD assistants.) And my private practice postoperative infection rate reflected the difference. So perhaps the evidence is skewed away from this source of infection, which I submit may well be the major one!
Steve Melkin, MD
Phoenix, Arizona
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Velasco I, Naranjo S, Lopez-Pedrera C, Garriga MJ, Garcia-Fuentes E, Soriquer F. Use of povidine-iodine during the first trimester of pregnancy: a correct practice? BJOG. 2009;116(3):452-455.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 571: solutions for surgical preparation of the vagina. Obstet Gynecol. 2013;122(3):718-720.
- Guideline for preoperative patient skin antisepsis. In: Guidelines for perioperative practice. Denver, CO: Association of Perioperative Registered Nurses, Inc; 2014.
- Velasco I, Naranjo S, Lopez-Pedrera C, Garriga MJ, Garcia-Fuentes E, Soriquer F. Use of povidine-iodine during the first trimester of pregnancy: a correct practice? BJOG. 2009;116(3):452-455.
- Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. Committee Opinion No. 571: solutions for surgical preparation of the vagina. Obstet Gynecol. 2013;122(3):718-720.
- Guideline for preoperative patient skin antisepsis. In: Guidelines for perioperative practice. Denver, CO: Association of Perioperative Registered Nurses, Inc; 2014.
Experts bust common sports medicine myths
SAN DIEGO – Is it okay for pregnant women to attend CrossFit classes? Are patients who run for fun at increased risk for osteoarthritis? Does stretching before exercise provide any benefits to athletes?
Experts discussed these topics during a session titled “Mythbusters in sports medicine” at the annual meeting of the American Medical Society for Sports Medicine.
Does exercise negatively impact pregnancy?
The idea that strenuous exercise during pregnancy can harm a baby’s health is a myth, according to Elizabeth A. Joy, MD.
“Women having healthy, uncomplicated pregnancies should be encouraged to be physically active throughout pregnancy, with a goal of achieving 150 minutes per week of moderate-intensity activity,” she said. “Fit pregnant women who were habitually performing high-intensity exercise before pregnancy can continue to do so during pregnancy, assuming an otherwise healthy, uncomplicated pregnancy.”
Results from more than 600 studies in the medical literature indicate that exercise during pregnancy is safe for moms and babies, noted Dr. Joy, medical director for community health and food and nutrition at Intermountain Healthcare in Salt Lake City, Utah.
In fact, the 2008 Physical Activity Guidelines for Americans state that pregnant women who habitually engage in vigorous-intensity aerobic activity or who are highly active “can continue physical activity during pregnancy and the postpartum period, provided that they remain healthy and discuss with their health care provider how and when activity should be adjusted over time.”
Such advice wasn’t always supported by the medical profession. In fact, 1985 guidelines from the American College of Obstetricians and Gynecologists recommended that women limit exercise to no more than 15 minutes at a time during pregnancy and keep their maternal heart rate less than 140 beats per minute. ACOG also discouraged previously sedentary women from beginning an exercise program during pregnancy.
“Sadly, women are still getting this advice,” said Dr. Joy, who is also president of the American College of Sports Medicine. According to the 2005-2010 National Ambulatory Medical Care Survey, only 18% of pregnant women reported receiving counseling to be physically active during their pregnancies (Matern Child Health J. 2014 Sep;18[7]:1610-18). “That is just unacceptable,” she said.
In a prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birth weight, researchers evaluated 1,647 births among primiparous women (Matern Child Health J. 2012 Jul;16[5]:1031-44).
They conducted telephone interviews with the women between 7-20 weeks gestation and assigned metabolic equivalent of task values to self-reported levels of physical activity. Of the 1,647 births, 7% were preterm.
Slightly more than one-third of the women (35%) performed first-trimester vigorous physical activity. The average total vigorous activity reported was 76 minutes per week, 38% of which was vigorous recreational activity.
Women who performed first-trimester vigorous recreational physical activity tended to have lower odds of preterm birth. They also tended to have lighter-weight babies, but this did not reach statistical significance (P = .08). The authors concluded that first-trimester vigorous physical activity “does not appear to be detrimental to the timing of birth or birth weight.”
In a separate analysis, researchers evaluated acute fetal responses to individually prescribed exercise according to existing physical activity guidelines in active and inactive pregnant women (Obstet Gynecol. 2012 Mar;119[3]:603-10).
Of the 45 study participants, 15 were classified as nonexercisers, 15 were regularly active, and 15 were highly active. The women underwent treadmill assessment between 28 weeks and 33 weeks, while fetal assessment included umbilical artery Doppler, fetal heart tracing and rate, and biophysical profile at rest and immediately post exercise.
The researchers observed no differences between the groups in mode of delivery, birth weight, and Apgar scores. During vigorous-intensity exercise, all umbilical artery indices showed decreases post exercise.
“Although statistically significant, this decrease is likely not clinically significant,” the researchers wrote. “We did not identify any adverse acute fetal responses to current exercise recommendations.” They went on to conclude that the potential health benefits of exercise “are too great for [physicians] to miss the opportunity to effectively counsel pregnant women about this important heath-enhancing behavior.”
In a more recent randomized study, Swedish researchers evaluated the efficacy of moderate-to-vigorous resistance exercise in 92 pregnant women (Acta Obstet Gynecol Scand. 2015 Jan; 94[1]:35-42).
The intervention group received supervised resistance exercise twice per week at moderate-to-vigorous intensity between 14-25 weeks of their pregnancy, while the control group received a generalized home exercise program. Outcome measures included health-related quality of life, physical strength, pain, weight, blood pressure, functional status, activity level, and perinatal data.
The researchers found no significant differences between the two groups and concluded that “supervised regular, moderate-to-vigorous resistance exercise performed twice per week does not adversely impact childbirth outcome, pain, or blood pressure.”
Despite all that’s known about exercise during pregnancy, a few practice and research gaps remain.
For one, Dr. Joy said, the relationship between performing physically demanding work during pregnancy in combination with moderate-to-vigorous exercise remains largely unknown.
“Even within health care, you have residents, nurses, and others working in hospitals,” she said. “That’s demanding work, but we don’t know whether or not moderate-to-vigorous exercise in combination with that kind of work is safe. Also, although women tend to thermoregulate better during pregnancy, we still don’t fully understand the impact of elevated core body temperature, which may occur with regularly performed vigorous-intensity exercise over the course of pregnancy.”
Does running cause knee OA?
During another talk at the meeting, William O. Roberts, MD, characterized the notion that running causes knee osteoarthritis as largely a myth for recreational runners. However, elite runners and athletes who participate in other sports may face an increased risk of developing the condition.
Well-established risk factors for knee OA include post–joint injury proteases and cytokines and injury load stress on articular cartilage. “Other risk factors include overweight and obesity, a family history of OA, exercise, heavy work that involved squatting and kneeling, and being female,” said Dr. Roberts, professor of family medicine and community health at the University of Minnesota, Minneapolis.
He discussed three articles on the topic drawn from medical literature. One was a retrospective cross-sectional analysis of 2,637 Osteoarthritis Initiative participants, 45-79 years of age, who had knee-specific pain or knee x-ray data 4 years into the 10-year–long study (Arthritis Care Res. 2017 Feb;69[2]:183-91).
More than half of the participants (56%) were female, their mean body mass index was 28.5 kg/m2, only 20% reported more than 2,000 bouts of running during their lifetime, and about 5% had run competitively.
Adjusted odds ratios of pain, radiographic OA, and symptomatic OA for those prior runners and current runners, compared with those who never ran, were 0.82 and 0.76 (P for trend = .02), 0.98 and 0.91 (P for trend = .05), and 0.88 and 0.71 (P for trend = .03), respectively.
The authors concluded that running does not appear to be detrimental to the knees, and the strength of recommendation taxonomy was rated as 2B.
In a separate analysis, researchers performed a systematic review and meta-analysis of 11 cohort (6 retrospective) and 4 case-control studies related to running and knee arthritis (Am J Sports Med. 2016 May;45[6]:1447-57). The mean ages of subjects at outcome assessment ranged from 27 years to 69 years, and the sample size ranged from 15 to 1,279 participants. The four case control studies assessed exposure by mailed questionnaire or by personal interview.
The meta-analysis suggests that runners have a 50% reduced odds of requiring a total knee replacement because of OA.
“It contradicts some previous studies, and there were confounders,” Dr. Roberts said of the analysis. “The one that I noticed is that people would delay surgery to keep running. That’s what I find in my practice.”
The researchers were unable to link running to knee OA development. Moderate- to low-quality evidence suggests a positive association with OA diagnosis but a negative association with requirement for a total knee replacement.
Based on published evidence, they concluded there is no clear advice to give regarding the potential effect of running on musculoskeletal health and rated the strength of evidence as 1A.
A third study Dr. Roberts discussed investigated the association between specific sports participation and knee OA (J Athl Train. 2015 Jan. 9. doi: 10.4085/1062-6050-50.2.08). After locating nearly 18,000 articles on the topic, the researchers limited their meta-analysis to 17 published studies.
They found that the overall risk of knee OA prevalence in sports participants was 7.7%, compared with 7.3% among nonexposed controls (odds ratio, 0.9). However, risks for knee OA were elevated among those who participated in the following sports: soccer (OR, 3.5), elite long-distance running (OR, 3.3), competitive weightlifting (OR, 6.9), and wrestling (OR, 3.8). The researchers concluded that athletes who participate in those sports “should be targeted for risk-reduction strategies.”
“So, does running cause knee OA? It depends,” said Dr. Roberts, who is also medical director of Twin Cities in Motion. “There is a potential risk to high-volume, high-intensity, and long-distance runners, but there does not appear to be a risk in fitness or recreational runners. Of course, you can’t erase your genetics.”
He called for more research on the topic, including prospective longitudinal outcomes studies, those that study the role of genetics/epigenetics in runners and nonrunners who develop knee OA and those focused on the knee joint “chemical environment,” referring to recent work that suggests that running appears to decrease knee intra-articular proinflammatory cytokine concentration (Eur J Appl Physiol. 2016 Dec;116[11-12]:2305-14).
“It’s okay to run for fitness, because the health benefits far outweigh the risk of knee OA,” Dr. Roberts said. “If you run hard and long, it could be a problem. We probably should be screening for neuromuscular control to reduce anterior cruciate ligament disruption.”
Does it help to stretch before exercise?
Stretching before engaging in exercise is a common practice often recommended by coaches and clinicians – but it appears to have no role in preventing injuries during exercise itself.
Several decades ago, investigators subscribed to muscle spasm theory, which held that unaccustomed exercise caused muscle spasms.
“The thought was that muscle spasms impeded blood flow to the muscle, causing ischemic pain and further spasm,” Valerie E. Cothran, MD, said during a presentation at the meeting. “Stretching the muscle was thought to restore blood flow to the muscle and interrupt the pain-spasm-pain cycle. This theory has been discredited for 40 years, but the practice of stretching before exercise persists.”
According to Dr. Cothran, of the department of family and community medicine at the University of Maryland, Baltimore, a limited number of randomized, controlled trials exist on the topic – and many are fraught with limitations, such as the evaluation of multiple stretching methods and variable types of sports activities and the inclusion of multiple cointerventions.
One systematic review evaluated 361 randomized, controlled trials and cohort studies of interventions that included stretching and that appeared in the medical literature from 1966 to 2002 (Med Sci Sports Exerc. 2004 Mar;36[3]:371-8). Studies with no controls were excluded from the analysis, as were those in which stretching could not be assessed independently or those that did not include people engaged in sports or fitness activities.
The researchers determined that stretching was not significantly associated with a reduction in total injuries (OR, 0.93). “There is not sufficient evidence to endorse or discontinue routine stretching before or after exercise to prevent injury among competitive or recreational athletes,” they concluded.
The following year, Lawrence Hart, MBBch, of McMaster University, Hamilton, Ont., assessed the same set of data but eliminated some of the confounding factors of the previous analysis, including studies that had limited statistical power (Clin J Sport Med. 2005 Mar;15[2]:113). The final meta-analysis included six studies.
Dr. Hart found that neither stretching of specific leg-muscle groups or multiple muscle groups led to a reduction in total injuries, such as shin splints, tibial stress reaction, or sprains/strains (OR, 0.93). In addition, reduction in injuries was not significantly greater for stretching of specific muscles or multiple muscle groups (OR, 0.80, and OR, 0.96, respectively). “Limited evidence showed stretching had no effects on injuries,” he concluded.
A more recent systematic review analyzed the efficacy of static stretching as part of a warm-up for the prevention of exercise-related injury (Res Sports Med. 2008;16[3]:213-31). The researchers reviewed 364 studies published after 1990 but before 2008, and they included seven in the final analysis: four randomized, controlled trials and three controlled trials.
All four randomized, controlled trials concluded that static stretching was ineffective in reducing the incidence of exercise-related injury, and only one of the three controlled trials concluded that static stretching reduced the incidence of exercise-related injury. In addition, three of the seven studies reported significant reductions in musculotendinous and ligament injuries following a static stretching protocol.
“There is moderate to strong evidence that routine application of static stretching does not reduce overall injury rates,” the researchers concluded. “There is preliminary evidence, however, that static stretching may reduce musculotendinous injuries.”
The final study Dr. Cothran discussed was a systematic review of two randomized, controlled trials and two prospective cohort studies on the effect of stretching in sports injury prevention that appeared in the literature between 1998 and 2008 (J Comm Health Sci. 2008;3[1]:51-8).
One cohort study found that stretching reduced the incidence of exercise-related injuries, while two randomized, controlled trials and one cohort study found that stretching did not produce a practical reduction on the occurrence of injuries. The researchers concluded that stretching exercises “do not give a practical, useful reduction in the risk of injuries.”
Some studies have demonstrated that explosive athletic performance such as sprinting may be compromised by acute stretching, noted Dr. Cothran, who is also program director of the primary care sports medicine fellowship at the University of Maryland, Baltimore. Current practice and research gaps include few recent randomized, controlled trials; few studies isolating stretching alone; and few that compare the different forms of stretching, such as dynamic and static stretching, she added.
“There is moderate to strong evidence that routine stretching before exercise will not reduce injury rates,” she concluded. “There is evidence that stretching before exercise may negatively affect performance. Flexibility training can be beneficial but should take place at alternative times and not before exercise.”
Dr. Joy disclosed that she receives funding from Savvysherpa and Dexcom for a project on the prevention of gestational diabetes. Dr. Roberts and Dr. Cothran reported having no financial disclosures.
SAN DIEGO – Is it okay for pregnant women to attend CrossFit classes? Are patients who run for fun at increased risk for osteoarthritis? Does stretching before exercise provide any benefits to athletes?
Experts discussed these topics during a session titled “Mythbusters in sports medicine” at the annual meeting of the American Medical Society for Sports Medicine.
Does exercise negatively impact pregnancy?
The idea that strenuous exercise during pregnancy can harm a baby’s health is a myth, according to Elizabeth A. Joy, MD.
“Women having healthy, uncomplicated pregnancies should be encouraged to be physically active throughout pregnancy, with a goal of achieving 150 minutes per week of moderate-intensity activity,” she said. “Fit pregnant women who were habitually performing high-intensity exercise before pregnancy can continue to do so during pregnancy, assuming an otherwise healthy, uncomplicated pregnancy.”
Results from more than 600 studies in the medical literature indicate that exercise during pregnancy is safe for moms and babies, noted Dr. Joy, medical director for community health and food and nutrition at Intermountain Healthcare in Salt Lake City, Utah.
In fact, the 2008 Physical Activity Guidelines for Americans state that pregnant women who habitually engage in vigorous-intensity aerobic activity or who are highly active “can continue physical activity during pregnancy and the postpartum period, provided that they remain healthy and discuss with their health care provider how and when activity should be adjusted over time.”
Such advice wasn’t always supported by the medical profession. In fact, 1985 guidelines from the American College of Obstetricians and Gynecologists recommended that women limit exercise to no more than 15 minutes at a time during pregnancy and keep their maternal heart rate less than 140 beats per minute. ACOG also discouraged previously sedentary women from beginning an exercise program during pregnancy.
“Sadly, women are still getting this advice,” said Dr. Joy, who is also president of the American College of Sports Medicine. According to the 2005-2010 National Ambulatory Medical Care Survey, only 18% of pregnant women reported receiving counseling to be physically active during their pregnancies (Matern Child Health J. 2014 Sep;18[7]:1610-18). “That is just unacceptable,” she said.
In a prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birth weight, researchers evaluated 1,647 births among primiparous women (Matern Child Health J. 2012 Jul;16[5]:1031-44).
They conducted telephone interviews with the women between 7-20 weeks gestation and assigned metabolic equivalent of task values to self-reported levels of physical activity. Of the 1,647 births, 7% were preterm.
Slightly more than one-third of the women (35%) performed first-trimester vigorous physical activity. The average total vigorous activity reported was 76 minutes per week, 38% of which was vigorous recreational activity.
Women who performed first-trimester vigorous recreational physical activity tended to have lower odds of preterm birth. They also tended to have lighter-weight babies, but this did not reach statistical significance (P = .08). The authors concluded that first-trimester vigorous physical activity “does not appear to be detrimental to the timing of birth or birth weight.”
In a separate analysis, researchers evaluated acute fetal responses to individually prescribed exercise according to existing physical activity guidelines in active and inactive pregnant women (Obstet Gynecol. 2012 Mar;119[3]:603-10).
Of the 45 study participants, 15 were classified as nonexercisers, 15 were regularly active, and 15 were highly active. The women underwent treadmill assessment between 28 weeks and 33 weeks, while fetal assessment included umbilical artery Doppler, fetal heart tracing and rate, and biophysical profile at rest and immediately post exercise.
The researchers observed no differences between the groups in mode of delivery, birth weight, and Apgar scores. During vigorous-intensity exercise, all umbilical artery indices showed decreases post exercise.
“Although statistically significant, this decrease is likely not clinically significant,” the researchers wrote. “We did not identify any adverse acute fetal responses to current exercise recommendations.” They went on to conclude that the potential health benefits of exercise “are too great for [physicians] to miss the opportunity to effectively counsel pregnant women about this important heath-enhancing behavior.”
In a more recent randomized study, Swedish researchers evaluated the efficacy of moderate-to-vigorous resistance exercise in 92 pregnant women (Acta Obstet Gynecol Scand. 2015 Jan; 94[1]:35-42).
The intervention group received supervised resistance exercise twice per week at moderate-to-vigorous intensity between 14-25 weeks of their pregnancy, while the control group received a generalized home exercise program. Outcome measures included health-related quality of life, physical strength, pain, weight, blood pressure, functional status, activity level, and perinatal data.
The researchers found no significant differences between the two groups and concluded that “supervised regular, moderate-to-vigorous resistance exercise performed twice per week does not adversely impact childbirth outcome, pain, or blood pressure.”
Despite all that’s known about exercise during pregnancy, a few practice and research gaps remain.
For one, Dr. Joy said, the relationship between performing physically demanding work during pregnancy in combination with moderate-to-vigorous exercise remains largely unknown.
“Even within health care, you have residents, nurses, and others working in hospitals,” she said. “That’s demanding work, but we don’t know whether or not moderate-to-vigorous exercise in combination with that kind of work is safe. Also, although women tend to thermoregulate better during pregnancy, we still don’t fully understand the impact of elevated core body temperature, which may occur with regularly performed vigorous-intensity exercise over the course of pregnancy.”
Does running cause knee OA?
During another talk at the meeting, William O. Roberts, MD, characterized the notion that running causes knee osteoarthritis as largely a myth for recreational runners. However, elite runners and athletes who participate in other sports may face an increased risk of developing the condition.
Well-established risk factors for knee OA include post–joint injury proteases and cytokines and injury load stress on articular cartilage. “Other risk factors include overweight and obesity, a family history of OA, exercise, heavy work that involved squatting and kneeling, and being female,” said Dr. Roberts, professor of family medicine and community health at the University of Minnesota, Minneapolis.
He discussed three articles on the topic drawn from medical literature. One was a retrospective cross-sectional analysis of 2,637 Osteoarthritis Initiative participants, 45-79 years of age, who had knee-specific pain or knee x-ray data 4 years into the 10-year–long study (Arthritis Care Res. 2017 Feb;69[2]:183-91).
More than half of the participants (56%) were female, their mean body mass index was 28.5 kg/m2, only 20% reported more than 2,000 bouts of running during their lifetime, and about 5% had run competitively.
Adjusted odds ratios of pain, radiographic OA, and symptomatic OA for those prior runners and current runners, compared with those who never ran, were 0.82 and 0.76 (P for trend = .02), 0.98 and 0.91 (P for trend = .05), and 0.88 and 0.71 (P for trend = .03), respectively.
The authors concluded that running does not appear to be detrimental to the knees, and the strength of recommendation taxonomy was rated as 2B.
In a separate analysis, researchers performed a systematic review and meta-analysis of 11 cohort (6 retrospective) and 4 case-control studies related to running and knee arthritis (Am J Sports Med. 2016 May;45[6]:1447-57). The mean ages of subjects at outcome assessment ranged from 27 years to 69 years, and the sample size ranged from 15 to 1,279 participants. The four case control studies assessed exposure by mailed questionnaire or by personal interview.
The meta-analysis suggests that runners have a 50% reduced odds of requiring a total knee replacement because of OA.
“It contradicts some previous studies, and there were confounders,” Dr. Roberts said of the analysis. “The one that I noticed is that people would delay surgery to keep running. That’s what I find in my practice.”
The researchers were unable to link running to knee OA development. Moderate- to low-quality evidence suggests a positive association with OA diagnosis but a negative association with requirement for a total knee replacement.
Based on published evidence, they concluded there is no clear advice to give regarding the potential effect of running on musculoskeletal health and rated the strength of evidence as 1A.
A third study Dr. Roberts discussed investigated the association between specific sports participation and knee OA (J Athl Train. 2015 Jan. 9. doi: 10.4085/1062-6050-50.2.08). After locating nearly 18,000 articles on the topic, the researchers limited their meta-analysis to 17 published studies.
They found that the overall risk of knee OA prevalence in sports participants was 7.7%, compared with 7.3% among nonexposed controls (odds ratio, 0.9). However, risks for knee OA were elevated among those who participated in the following sports: soccer (OR, 3.5), elite long-distance running (OR, 3.3), competitive weightlifting (OR, 6.9), and wrestling (OR, 3.8). The researchers concluded that athletes who participate in those sports “should be targeted for risk-reduction strategies.”
“So, does running cause knee OA? It depends,” said Dr. Roberts, who is also medical director of Twin Cities in Motion. “There is a potential risk to high-volume, high-intensity, and long-distance runners, but there does not appear to be a risk in fitness or recreational runners. Of course, you can’t erase your genetics.”
He called for more research on the topic, including prospective longitudinal outcomes studies, those that study the role of genetics/epigenetics in runners and nonrunners who develop knee OA and those focused on the knee joint “chemical environment,” referring to recent work that suggests that running appears to decrease knee intra-articular proinflammatory cytokine concentration (Eur J Appl Physiol. 2016 Dec;116[11-12]:2305-14).
“It’s okay to run for fitness, because the health benefits far outweigh the risk of knee OA,” Dr. Roberts said. “If you run hard and long, it could be a problem. We probably should be screening for neuromuscular control to reduce anterior cruciate ligament disruption.”
Does it help to stretch before exercise?
Stretching before engaging in exercise is a common practice often recommended by coaches and clinicians – but it appears to have no role in preventing injuries during exercise itself.
Several decades ago, investigators subscribed to muscle spasm theory, which held that unaccustomed exercise caused muscle spasms.
“The thought was that muscle spasms impeded blood flow to the muscle, causing ischemic pain and further spasm,” Valerie E. Cothran, MD, said during a presentation at the meeting. “Stretching the muscle was thought to restore blood flow to the muscle and interrupt the pain-spasm-pain cycle. This theory has been discredited for 40 years, but the practice of stretching before exercise persists.”
According to Dr. Cothran, of the department of family and community medicine at the University of Maryland, Baltimore, a limited number of randomized, controlled trials exist on the topic – and many are fraught with limitations, such as the evaluation of multiple stretching methods and variable types of sports activities and the inclusion of multiple cointerventions.
One systematic review evaluated 361 randomized, controlled trials and cohort studies of interventions that included stretching and that appeared in the medical literature from 1966 to 2002 (Med Sci Sports Exerc. 2004 Mar;36[3]:371-8). Studies with no controls were excluded from the analysis, as were those in which stretching could not be assessed independently or those that did not include people engaged in sports or fitness activities.
The researchers determined that stretching was not significantly associated with a reduction in total injuries (OR, 0.93). “There is not sufficient evidence to endorse or discontinue routine stretching before or after exercise to prevent injury among competitive or recreational athletes,” they concluded.
The following year, Lawrence Hart, MBBch, of McMaster University, Hamilton, Ont., assessed the same set of data but eliminated some of the confounding factors of the previous analysis, including studies that had limited statistical power (Clin J Sport Med. 2005 Mar;15[2]:113). The final meta-analysis included six studies.
Dr. Hart found that neither stretching of specific leg-muscle groups or multiple muscle groups led to a reduction in total injuries, such as shin splints, tibial stress reaction, or sprains/strains (OR, 0.93). In addition, reduction in injuries was not significantly greater for stretching of specific muscles or multiple muscle groups (OR, 0.80, and OR, 0.96, respectively). “Limited evidence showed stretching had no effects on injuries,” he concluded.
A more recent systematic review analyzed the efficacy of static stretching as part of a warm-up for the prevention of exercise-related injury (Res Sports Med. 2008;16[3]:213-31). The researchers reviewed 364 studies published after 1990 but before 2008, and they included seven in the final analysis: four randomized, controlled trials and three controlled trials.
All four randomized, controlled trials concluded that static stretching was ineffective in reducing the incidence of exercise-related injury, and only one of the three controlled trials concluded that static stretching reduced the incidence of exercise-related injury. In addition, three of the seven studies reported significant reductions in musculotendinous and ligament injuries following a static stretching protocol.
“There is moderate to strong evidence that routine application of static stretching does not reduce overall injury rates,” the researchers concluded. “There is preliminary evidence, however, that static stretching may reduce musculotendinous injuries.”
The final study Dr. Cothran discussed was a systematic review of two randomized, controlled trials and two prospective cohort studies on the effect of stretching in sports injury prevention that appeared in the literature between 1998 and 2008 (J Comm Health Sci. 2008;3[1]:51-8).
One cohort study found that stretching reduced the incidence of exercise-related injuries, while two randomized, controlled trials and one cohort study found that stretching did not produce a practical reduction on the occurrence of injuries. The researchers concluded that stretching exercises “do not give a practical, useful reduction in the risk of injuries.”
Some studies have demonstrated that explosive athletic performance such as sprinting may be compromised by acute stretching, noted Dr. Cothran, who is also program director of the primary care sports medicine fellowship at the University of Maryland, Baltimore. Current practice and research gaps include few recent randomized, controlled trials; few studies isolating stretching alone; and few that compare the different forms of stretching, such as dynamic and static stretching, she added.
“There is moderate to strong evidence that routine stretching before exercise will not reduce injury rates,” she concluded. “There is evidence that stretching before exercise may negatively affect performance. Flexibility training can be beneficial but should take place at alternative times and not before exercise.”
Dr. Joy disclosed that she receives funding from Savvysherpa and Dexcom for a project on the prevention of gestational diabetes. Dr. Roberts and Dr. Cothran reported having no financial disclosures.
SAN DIEGO – Is it okay for pregnant women to attend CrossFit classes? Are patients who run for fun at increased risk for osteoarthritis? Does stretching before exercise provide any benefits to athletes?
Experts discussed these topics during a session titled “Mythbusters in sports medicine” at the annual meeting of the American Medical Society for Sports Medicine.
Does exercise negatively impact pregnancy?
The idea that strenuous exercise during pregnancy can harm a baby’s health is a myth, according to Elizabeth A. Joy, MD.
“Women having healthy, uncomplicated pregnancies should be encouraged to be physically active throughout pregnancy, with a goal of achieving 150 minutes per week of moderate-intensity activity,” she said. “Fit pregnant women who were habitually performing high-intensity exercise before pregnancy can continue to do so during pregnancy, assuming an otherwise healthy, uncomplicated pregnancy.”
Results from more than 600 studies in the medical literature indicate that exercise during pregnancy is safe for moms and babies, noted Dr. Joy, medical director for community health and food and nutrition at Intermountain Healthcare in Salt Lake City, Utah.
In fact, the 2008 Physical Activity Guidelines for Americans state that pregnant women who habitually engage in vigorous-intensity aerobic activity or who are highly active “can continue physical activity during pregnancy and the postpartum period, provided that they remain healthy and discuss with their health care provider how and when activity should be adjusted over time.”
Such advice wasn’t always supported by the medical profession. In fact, 1985 guidelines from the American College of Obstetricians and Gynecologists recommended that women limit exercise to no more than 15 minutes at a time during pregnancy and keep their maternal heart rate less than 140 beats per minute. ACOG also discouraged previously sedentary women from beginning an exercise program during pregnancy.
“Sadly, women are still getting this advice,” said Dr. Joy, who is also president of the American College of Sports Medicine. According to the 2005-2010 National Ambulatory Medical Care Survey, only 18% of pregnant women reported receiving counseling to be physically active during their pregnancies (Matern Child Health J. 2014 Sep;18[7]:1610-18). “That is just unacceptable,” she said.
In a prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birth weight, researchers evaluated 1,647 births among primiparous women (Matern Child Health J. 2012 Jul;16[5]:1031-44).
They conducted telephone interviews with the women between 7-20 weeks gestation and assigned metabolic equivalent of task values to self-reported levels of physical activity. Of the 1,647 births, 7% were preterm.
Slightly more than one-third of the women (35%) performed first-trimester vigorous physical activity. The average total vigorous activity reported was 76 minutes per week, 38% of which was vigorous recreational activity.
Women who performed first-trimester vigorous recreational physical activity tended to have lower odds of preterm birth. They also tended to have lighter-weight babies, but this did not reach statistical significance (P = .08). The authors concluded that first-trimester vigorous physical activity “does not appear to be detrimental to the timing of birth or birth weight.”
In a separate analysis, researchers evaluated acute fetal responses to individually prescribed exercise according to existing physical activity guidelines in active and inactive pregnant women (Obstet Gynecol. 2012 Mar;119[3]:603-10).
Of the 45 study participants, 15 were classified as nonexercisers, 15 were regularly active, and 15 were highly active. The women underwent treadmill assessment between 28 weeks and 33 weeks, while fetal assessment included umbilical artery Doppler, fetal heart tracing and rate, and biophysical profile at rest and immediately post exercise.
The researchers observed no differences between the groups in mode of delivery, birth weight, and Apgar scores. During vigorous-intensity exercise, all umbilical artery indices showed decreases post exercise.
“Although statistically significant, this decrease is likely not clinically significant,” the researchers wrote. “We did not identify any adverse acute fetal responses to current exercise recommendations.” They went on to conclude that the potential health benefits of exercise “are too great for [physicians] to miss the opportunity to effectively counsel pregnant women about this important heath-enhancing behavior.”
In a more recent randomized study, Swedish researchers evaluated the efficacy of moderate-to-vigorous resistance exercise in 92 pregnant women (Acta Obstet Gynecol Scand. 2015 Jan; 94[1]:35-42).
The intervention group received supervised resistance exercise twice per week at moderate-to-vigorous intensity between 14-25 weeks of their pregnancy, while the control group received a generalized home exercise program. Outcome measures included health-related quality of life, physical strength, pain, weight, blood pressure, functional status, activity level, and perinatal data.
The researchers found no significant differences between the two groups and concluded that “supervised regular, moderate-to-vigorous resistance exercise performed twice per week does not adversely impact childbirth outcome, pain, or blood pressure.”
Despite all that’s known about exercise during pregnancy, a few practice and research gaps remain.
For one, Dr. Joy said, the relationship between performing physically demanding work during pregnancy in combination with moderate-to-vigorous exercise remains largely unknown.
“Even within health care, you have residents, nurses, and others working in hospitals,” she said. “That’s demanding work, but we don’t know whether or not moderate-to-vigorous exercise in combination with that kind of work is safe. Also, although women tend to thermoregulate better during pregnancy, we still don’t fully understand the impact of elevated core body temperature, which may occur with regularly performed vigorous-intensity exercise over the course of pregnancy.”
Does running cause knee OA?
During another talk at the meeting, William O. Roberts, MD, characterized the notion that running causes knee osteoarthritis as largely a myth for recreational runners. However, elite runners and athletes who participate in other sports may face an increased risk of developing the condition.
Well-established risk factors for knee OA include post–joint injury proteases and cytokines and injury load stress on articular cartilage. “Other risk factors include overweight and obesity, a family history of OA, exercise, heavy work that involved squatting and kneeling, and being female,” said Dr. Roberts, professor of family medicine and community health at the University of Minnesota, Minneapolis.
He discussed three articles on the topic drawn from medical literature. One was a retrospective cross-sectional analysis of 2,637 Osteoarthritis Initiative participants, 45-79 years of age, who had knee-specific pain or knee x-ray data 4 years into the 10-year–long study (Arthritis Care Res. 2017 Feb;69[2]:183-91).
More than half of the participants (56%) were female, their mean body mass index was 28.5 kg/m2, only 20% reported more than 2,000 bouts of running during their lifetime, and about 5% had run competitively.
Adjusted odds ratios of pain, radiographic OA, and symptomatic OA for those prior runners and current runners, compared with those who never ran, were 0.82 and 0.76 (P for trend = .02), 0.98 and 0.91 (P for trend = .05), and 0.88 and 0.71 (P for trend = .03), respectively.
The authors concluded that running does not appear to be detrimental to the knees, and the strength of recommendation taxonomy was rated as 2B.
In a separate analysis, researchers performed a systematic review and meta-analysis of 11 cohort (6 retrospective) and 4 case-control studies related to running and knee arthritis (Am J Sports Med. 2016 May;45[6]:1447-57). The mean ages of subjects at outcome assessment ranged from 27 years to 69 years, and the sample size ranged from 15 to 1,279 participants. The four case control studies assessed exposure by mailed questionnaire or by personal interview.
The meta-analysis suggests that runners have a 50% reduced odds of requiring a total knee replacement because of OA.
“It contradicts some previous studies, and there were confounders,” Dr. Roberts said of the analysis. “The one that I noticed is that people would delay surgery to keep running. That’s what I find in my practice.”
The researchers were unable to link running to knee OA development. Moderate- to low-quality evidence suggests a positive association with OA diagnosis but a negative association with requirement for a total knee replacement.
Based on published evidence, they concluded there is no clear advice to give regarding the potential effect of running on musculoskeletal health and rated the strength of evidence as 1A.
A third study Dr. Roberts discussed investigated the association between specific sports participation and knee OA (J Athl Train. 2015 Jan. 9. doi: 10.4085/1062-6050-50.2.08). After locating nearly 18,000 articles on the topic, the researchers limited their meta-analysis to 17 published studies.
They found that the overall risk of knee OA prevalence in sports participants was 7.7%, compared with 7.3% among nonexposed controls (odds ratio, 0.9). However, risks for knee OA were elevated among those who participated in the following sports: soccer (OR, 3.5), elite long-distance running (OR, 3.3), competitive weightlifting (OR, 6.9), and wrestling (OR, 3.8). The researchers concluded that athletes who participate in those sports “should be targeted for risk-reduction strategies.”
“So, does running cause knee OA? It depends,” said Dr. Roberts, who is also medical director of Twin Cities in Motion. “There is a potential risk to high-volume, high-intensity, and long-distance runners, but there does not appear to be a risk in fitness or recreational runners. Of course, you can’t erase your genetics.”
He called for more research on the topic, including prospective longitudinal outcomes studies, those that study the role of genetics/epigenetics in runners and nonrunners who develop knee OA and those focused on the knee joint “chemical environment,” referring to recent work that suggests that running appears to decrease knee intra-articular proinflammatory cytokine concentration (Eur J Appl Physiol. 2016 Dec;116[11-12]:2305-14).
“It’s okay to run for fitness, because the health benefits far outweigh the risk of knee OA,” Dr. Roberts said. “If you run hard and long, it could be a problem. We probably should be screening for neuromuscular control to reduce anterior cruciate ligament disruption.”
Does it help to stretch before exercise?
Stretching before engaging in exercise is a common practice often recommended by coaches and clinicians – but it appears to have no role in preventing injuries during exercise itself.
Several decades ago, investigators subscribed to muscle spasm theory, which held that unaccustomed exercise caused muscle spasms.
“The thought was that muscle spasms impeded blood flow to the muscle, causing ischemic pain and further spasm,” Valerie E. Cothran, MD, said during a presentation at the meeting. “Stretching the muscle was thought to restore blood flow to the muscle and interrupt the pain-spasm-pain cycle. This theory has been discredited for 40 years, but the practice of stretching before exercise persists.”
According to Dr. Cothran, of the department of family and community medicine at the University of Maryland, Baltimore, a limited number of randomized, controlled trials exist on the topic – and many are fraught with limitations, such as the evaluation of multiple stretching methods and variable types of sports activities and the inclusion of multiple cointerventions.
One systematic review evaluated 361 randomized, controlled trials and cohort studies of interventions that included stretching and that appeared in the medical literature from 1966 to 2002 (Med Sci Sports Exerc. 2004 Mar;36[3]:371-8). Studies with no controls were excluded from the analysis, as were those in which stretching could not be assessed independently or those that did not include people engaged in sports or fitness activities.
The researchers determined that stretching was not significantly associated with a reduction in total injuries (OR, 0.93). “There is not sufficient evidence to endorse or discontinue routine stretching before or after exercise to prevent injury among competitive or recreational athletes,” they concluded.
The following year, Lawrence Hart, MBBch, of McMaster University, Hamilton, Ont., assessed the same set of data but eliminated some of the confounding factors of the previous analysis, including studies that had limited statistical power (Clin J Sport Med. 2005 Mar;15[2]:113). The final meta-analysis included six studies.
Dr. Hart found that neither stretching of specific leg-muscle groups or multiple muscle groups led to a reduction in total injuries, such as shin splints, tibial stress reaction, or sprains/strains (OR, 0.93). In addition, reduction in injuries was not significantly greater for stretching of specific muscles or multiple muscle groups (OR, 0.80, and OR, 0.96, respectively). “Limited evidence showed stretching had no effects on injuries,” he concluded.
A more recent systematic review analyzed the efficacy of static stretching as part of a warm-up for the prevention of exercise-related injury (Res Sports Med. 2008;16[3]:213-31). The researchers reviewed 364 studies published after 1990 but before 2008, and they included seven in the final analysis: four randomized, controlled trials and three controlled trials.
All four randomized, controlled trials concluded that static stretching was ineffective in reducing the incidence of exercise-related injury, and only one of the three controlled trials concluded that static stretching reduced the incidence of exercise-related injury. In addition, three of the seven studies reported significant reductions in musculotendinous and ligament injuries following a static stretching protocol.
“There is moderate to strong evidence that routine application of static stretching does not reduce overall injury rates,” the researchers concluded. “There is preliminary evidence, however, that static stretching may reduce musculotendinous injuries.”
The final study Dr. Cothran discussed was a systematic review of two randomized, controlled trials and two prospective cohort studies on the effect of stretching in sports injury prevention that appeared in the literature between 1998 and 2008 (J Comm Health Sci. 2008;3[1]:51-8).
One cohort study found that stretching reduced the incidence of exercise-related injuries, while two randomized, controlled trials and one cohort study found that stretching did not produce a practical reduction on the occurrence of injuries. The researchers concluded that stretching exercises “do not give a practical, useful reduction in the risk of injuries.”
Some studies have demonstrated that explosive athletic performance such as sprinting may be compromised by acute stretching, noted Dr. Cothran, who is also program director of the primary care sports medicine fellowship at the University of Maryland, Baltimore. Current practice and research gaps include few recent randomized, controlled trials; few studies isolating stretching alone; and few that compare the different forms of stretching, such as dynamic and static stretching, she added.
“There is moderate to strong evidence that routine stretching before exercise will not reduce injury rates,” she concluded. “There is evidence that stretching before exercise may negatively affect performance. Flexibility training can be beneficial but should take place at alternative times and not before exercise.”
Dr. Joy disclosed that she receives funding from Savvysherpa and Dexcom for a project on the prevention of gestational diabetes. Dr. Roberts and Dr. Cothran reported having no financial disclosures.
Pregnancy boosts risk of ventral hernia recurrence
Pregnancy is associated with a significant increase in the risk of ventral hernia recurrence after repair, according to a population-based cohort study published online in the American Journal of Surgery.
Analysis of registry data from 3,578 Danish women of reproductive age who had previously undergone ventral hernia repair showed that subsequent pregnancy was associated with a 56% higher risk of recurrence (95% confidence interval, 1.09-2.25; P = .016), compared with women who did not get pregnant (Am J Surg. 2017 April 5. doi: 10.1016/j.amjsurg.2017.03.044).
They noted that few studies have directly reported on the rate of ventral hernia recurrence after pregnancy, and the results that do exist are conflicting.
The overall rate of ventral hernia recurrence in the cohort was 12.5%, 67.9% of whom subsequently underwent reoperation to repair. The median time from hernia repair to pregnancy was 1.1 years, and median follow-up was 3.1 years.
Umbilical and incisional hernia repair were independently associated with a higher risk of recurrence (hazard ratio, 1.55 and 3.30, respectively) than epigastric repair, while larger hernia defects also increased the risk of recurrence.
“According to Laplace’s law, both the abdominal wall stretch and the raised intra-abdominal pressure theoretically strain the repaired ventral hernia site and are likely involved in the associated increased risk of recurrence,” the authors wrote. “Furthermore, prolonged duration of the second stage of labor and the use of manual fundal pressure might increase the risk of ventral hernia recurrence.”
The authors pointed out that inadequate fixation and lateral detachment of the mesh material were the most commonly reported mechanisms involved in ventral hernia recurrence after mesh repair. The fact that most mesh materials are far less elastic than the abdominal wall could account for the association between pregnancy and recurrence.
Based on the findings, they suggested that elective surgery for incisional or umbilical hernia repair be postponed until after the last planned pregnancy and that female patients of reproductive age be counseled on the increased risk of recurrence with pregnancy should they choose to undergo ventral hernia repair. They also noted that the natural course of an untreated ventral hernia, how it responds to pregnancy, and the risk of emergency repair during pregnancy need further investigation.
The study was supported by grants from Edgar Schnohr, MD, Dr MSc & Wife Gilberte Schnohr’s Foundation, and Bispebjerg Hospital. No conflicts of interest were declared.
Pregnancy is associated with a significant increase in the risk of ventral hernia recurrence after repair, according to a population-based cohort study published online in the American Journal of Surgery.
Analysis of registry data from 3,578 Danish women of reproductive age who had previously undergone ventral hernia repair showed that subsequent pregnancy was associated with a 56% higher risk of recurrence (95% confidence interval, 1.09-2.25; P = .016), compared with women who did not get pregnant (Am J Surg. 2017 April 5. doi: 10.1016/j.amjsurg.2017.03.044).
They noted that few studies have directly reported on the rate of ventral hernia recurrence after pregnancy, and the results that do exist are conflicting.
The overall rate of ventral hernia recurrence in the cohort was 12.5%, 67.9% of whom subsequently underwent reoperation to repair. The median time from hernia repair to pregnancy was 1.1 years, and median follow-up was 3.1 years.
Umbilical and incisional hernia repair were independently associated with a higher risk of recurrence (hazard ratio, 1.55 and 3.30, respectively) than epigastric repair, while larger hernia defects also increased the risk of recurrence.
“According to Laplace’s law, both the abdominal wall stretch and the raised intra-abdominal pressure theoretically strain the repaired ventral hernia site and are likely involved in the associated increased risk of recurrence,” the authors wrote. “Furthermore, prolonged duration of the second stage of labor and the use of manual fundal pressure might increase the risk of ventral hernia recurrence.”
The authors pointed out that inadequate fixation and lateral detachment of the mesh material were the most commonly reported mechanisms involved in ventral hernia recurrence after mesh repair. The fact that most mesh materials are far less elastic than the abdominal wall could account for the association between pregnancy and recurrence.
Based on the findings, they suggested that elective surgery for incisional or umbilical hernia repair be postponed until after the last planned pregnancy and that female patients of reproductive age be counseled on the increased risk of recurrence with pregnancy should they choose to undergo ventral hernia repair. They also noted that the natural course of an untreated ventral hernia, how it responds to pregnancy, and the risk of emergency repair during pregnancy need further investigation.
The study was supported by grants from Edgar Schnohr, MD, Dr MSc & Wife Gilberte Schnohr’s Foundation, and Bispebjerg Hospital. No conflicts of interest were declared.
Pregnancy is associated with a significant increase in the risk of ventral hernia recurrence after repair, according to a population-based cohort study published online in the American Journal of Surgery.
Analysis of registry data from 3,578 Danish women of reproductive age who had previously undergone ventral hernia repair showed that subsequent pregnancy was associated with a 56% higher risk of recurrence (95% confidence interval, 1.09-2.25; P = .016), compared with women who did not get pregnant (Am J Surg. 2017 April 5. doi: 10.1016/j.amjsurg.2017.03.044).
They noted that few studies have directly reported on the rate of ventral hernia recurrence after pregnancy, and the results that do exist are conflicting.
The overall rate of ventral hernia recurrence in the cohort was 12.5%, 67.9% of whom subsequently underwent reoperation to repair. The median time from hernia repair to pregnancy was 1.1 years, and median follow-up was 3.1 years.
Umbilical and incisional hernia repair were independently associated with a higher risk of recurrence (hazard ratio, 1.55 and 3.30, respectively) than epigastric repair, while larger hernia defects also increased the risk of recurrence.
“According to Laplace’s law, both the abdominal wall stretch and the raised intra-abdominal pressure theoretically strain the repaired ventral hernia site and are likely involved in the associated increased risk of recurrence,” the authors wrote. “Furthermore, prolonged duration of the second stage of labor and the use of manual fundal pressure might increase the risk of ventral hernia recurrence.”
The authors pointed out that inadequate fixation and lateral detachment of the mesh material were the most commonly reported mechanisms involved in ventral hernia recurrence after mesh repair. The fact that most mesh materials are far less elastic than the abdominal wall could account for the association between pregnancy and recurrence.
Based on the findings, they suggested that elective surgery for incisional or umbilical hernia repair be postponed until after the last planned pregnancy and that female patients of reproductive age be counseled on the increased risk of recurrence with pregnancy should they choose to undergo ventral hernia repair. They also noted that the natural course of an untreated ventral hernia, how it responds to pregnancy, and the risk of emergency repair during pregnancy need further investigation.
The study was supported by grants from Edgar Schnohr, MD, Dr MSc & Wife Gilberte Schnohr’s Foundation, and Bispebjerg Hospital. No conflicts of interest were declared.
FROM THE AMERICAN JOURNAL OF SURGERY
Key clinical point: Pregnancy after ventral hernia repair can significantly increase the risk of recurrence.
Major finding: Pregnancy is associated with a 56% increase in the risk of recurrence of ventral hernia after repair.
Data source: A population-based cohort study of 3,578 women of reproductive age who underwent ventral hernia repair.
Disclosures: The study was supported by grants from Edgar Schnohr MD, Dr MSc & Wife Gilberte Schnohr’s Foundation, and Bispebjerg Hospital. No conflicts of interest were declared.