User login
Hypertensive disorders screening recommended for all pregnant women
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
FROM JAMA
Hepatitis B infection in pregnancy: Essentials of antiviral therapy and immunoprophylaxis
Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.

Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●

Hepatitis B is one of the more common infections encountered in the daily practice of obstetrics. It is responsible for 40% to 45% of all cases of viral hepatitis.1,2 Hepatitis B may cause serious complications in both the infected mother and neonate.
In this article, I review the virology, epidemiology, and clinical presentation of hepatitis B and then discuss the key diagnostic tests and, subsequently, the clinical management for both the mother and neonate. I focus particular attention on relatively new information about the value of specific antiviral medication to enhance the protective effect of conventional neonatal immunoprophylaxis.
To set the framework for the discussion, consider the following 2 case studies.
CASE 1 Undetectable level of hepatitis B surface antibody in a pregnant woman
A 25-year-old healthy primigravid woman at 10 weeks’ gestation had a series of laboratory studies that included a test for hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (HBsAb). The test for the surface antigen was negative. The test for the surface antibody was below the level of detection. Upon questioning, the patient indicates that she received the 3-dose hepatitis B vaccine when she was age 13 years.
- What treatment, if any, is indicated for this patient?
- What treatment is indicated for her neonate?
CASE 2 Pregnant woman tests positive for hepatitis B surface antigen
A 31-year-old woman (G3P2002) at 12 weeks’ gestation tested positive for HBsAg. She indicates that she never has had symptomatic hepatitis and that she considers herself to be in excellent health.
- What additional laboratory tests are indicated at this time?
- What additional laboratory test should be performed at the end of the second trimester?
- What treatment is indicated for the mother and neonate?
Virology and epidemiology of hepatitis B
Hepatitis B is caused by a double-stranded, enveloped DNA virus. The virus has 10 genotypes and 24 subtypes.3 The organism contains 3 major antigens. Detection of these antigens and their corresponding antibodies is an essential step in the diagnostic workup of patients who may be infected.
The surface antigen (HBsAg) confers infectivity and is the most valuable serologic marker of infection. The e antigen (HBeAg) is not present in every infected patient. It is secreted from infected cells, but it is not incorporated into the viral particle. When present, it denotes a high level of viral replication and exceptionally high infectivity. The core antigen (HBcAg) is a valuable serologic marker for distinguishing between acute and chronic infection.1-3
Hepatitis B is highly infectious, much more so than HIV or hepatitis C. The virus has an incubation period of 4 weeks to 6 months, and the duration of incubation is inversely related to the size of the viral inoculum. The virus is transmitted in 3 principal ways: sexual contact with contaminated genital tract secretions, contact with infected blood from sharing contaminated drug-injecting paraphernalia or from receiving a blood transfusion (extremely rare today), and transmission from an infected mother to her neonate. Perinatal transmission occurs primarily during the delivery process as opposed to transplacental infection. Transmission also can occur by more casual household contact, such as sharing eating utensils, kissing, and handling an infant.1,2,4,5
Worldwide, more than 400 million people have chronic hepatitis B infection. In the United States, approximately 1.25 to 1.5 million individuals are infected. Several groups are at particularly high risk for being infected, including1-3:
- Asians
- Alaska Natives
- sub-Saharan Africans
- sex workers
- intravenous drug users
- individuals with hemophilia
- international travelers
- staff and residents of long-term care facilities
- tattoo recipients.
Continue to: Clinical presentation...
Clinical presentation
Approximately 90% of adult patients who contract hepatitis B, either symptomatically or asymptomatically, will develop protective levels of antibody and clear the virus from their system. They will then have lifelong immunity to reinfection. Approximately 10% of patients will fail to develop protective levels of antibody and will become chronically infected, posing a risk to their household members, sexual contacts, and their fetus if they become pregnant. Persistence of the surface antigen in the patient’s serum for more than 6 months denotes chronic infection. A very small number of individuals—less than 1%—will develop acute liver failure and experience a fatal outcome.1-3,5
In the United States, the prevalence of acute hepatitis B in pregnancy is 1 to 2 per 1,000. Clinical manifestations typically include anorexia, nausea, low-grade fever, right upper quadrant pain and tenderness, passage of clay-colored stools, and jaundice.
The prevalence of chronic infection in pregnancy is significantly higher, approximately 5 to 15 per 1,000. Over the long term, patients with chronic infection are at risk for progressive liver injury, including cirrhosis and even hepatocellular carcinoma. These serious sequelae are particularly likely to occur when the patient is co-infected with hepatitis C, D, or both. The overall risk of progression to chronic cirrhosis is approximately 15% to 30%. In patients who progress to cirrhosis, the annual incidence of hepatocellular carcinoma is 10%.1-3
Diagnosis of hepatitis B infection
Patients with acute hepatitis B will test positive for HBsAg and immunoglobulin M (IgM) antibody to the core antigen. Some patients will also test positive for HBeAg. Assessment of the patient’s serum by polymerase chain reaction (PCR) allows quantitation of the viral load, which often is expressed as viral copies per milliliter. Alternatively, the quantitative hepatitis B DNA concentration may be expressed as international units per milliliter (IU/mL). The World Health Organization recommends this latter quantitative method. Multiplying the DNA in IU/mL by 5.6 provides the conversion to viral copies per milliliter.
Patients with chronic hepatitis B infection will test positive for the HBsAg and for immunoglobulin G (IgG) antibody to the core antigen. They may also have a positive test for the HBeAg, and PCR may be used to quantify the viral load.1-3
Managing hepatitis B infection in pregnancy
General supportive measures. All pregnant patients should be tested for the HBsAg and HBsAb at the time of the first prenatal appointment. The tests should be repeated at the beginning of the third trimester in high-risk patients. Seropositive patients should have a hepatitis B genotype, a test for the e antigen, and tests for other sexually transmissible infections (gonorrhea, chlamydia, syphilis, HIV) and for hepatitis C and D. Liver function tests should be performed to assess for elevations in the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Patients with elevated transaminase enzymes should have a coagulation profile to be certain they are not at risk for a coagulopathy.
At the end of the second trimester, patients should have a PCR assessment to determine the viral load. This assessment will be important for deciding if specific antiviral therapy is indicated during the third trimester to enhance the effects of neonatal immunoprophylaxis (see below). Of note, patients who are positive for the e antigen may have a very high viral load and yet have normal or near-normal transaminase levels. This seemingly paradoxical finding reflects the non-cytopathic nature of hepatitis B.
The patient should optimize her nutrition and sleep. She should avoid, or at least minimize, medications such as acetaminophen that could cause further liver injury. Without question, she should refrain from consuming even small amounts of alcohol. She should be tested for immunity to hepatitis A; if found to be susceptible, she should be vaccinated with the hepatitis A vaccine. This agent is an inactivated vaccine and is safe for administration at any time in pregnancy.1,2,5
Household contacts. In addition to the measures outlined above, the patient’s household contacts, particularly her sexual partner(s), should be tested for immunity to hepatitis B. If they do not have immunity by virtue of natural infection or previous vaccination, they should receive the hepatitis B vaccine series. It is also prudent to provide the sexual partner(s) with an initial dose of hepatitis B immune globulin (HBIG) to provide a temporary level of passive immunity.
Postdelivery care. After delivery, the patient should be referred to an infectious disease specialist or hepatologist for consideration of long-term treatment with antiviral agents, such as interferon alfa, pegylated interferon alfa, lamivudine, adefovir, entecavir, telbivudine, or tenofovir.6 The principal candidates for treatment are those who have cirrhosis and detectable levels of hepatitis B DNA. The ultimate goal of treatment is to reduce the serum hepatitis B DNA concentration to an undetectable level. Once the surface antigenemia is cleared, treatment can be stopped. A cure is defined when the absence of hepa-titis B DNA in the serum is sustained.
- Hepatitis B is a DNA virus that is transmitted via sexual contact, exposure to infected blood, and from an infected mother to her fetus.
- Most patients in our practice will most likely have chronic, asymptomatic infection, and the diagnosis will be established by detection of HBsAg in the patient’s serum.
- All obstetric patients should be tested for both HBsAg and HBsAb.
- Patients who are positive for the surface antigen should be tested for HIV infection and hepatitis C and D. They also should have a determination of the hepatitis B genotype and viral load and assessment of liver function (ALT, AST).
- Patients who are chronically infected with hepatitis B should be vaccinated against hepatitis A to prevent further liver injury. They also should avoid medications that might cause hepatic injury.
- Patients who have a viral DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL should be treated with tenofovir, 300 mg daily, from week 28 until 4 to 8 weeks after delivery.
- Infants delivered to infected mothers should receive HBIG within 12 hours of birth and then begin the 3-dose hepatitis B vaccine series. The first dose should be administered prior to hospital discharge.
- Infants delivered to mothers who are negative for the surface antigen should begin the hepatitis B vaccine series prior to discharge from the hospital.
- Mothers who test negative for HBsAb should be questioned about prior vaccination. If they have never been vaccinated, they should receive the 3-dose vaccine series. If they have been vaccinated, they should receive a single hepatitis B vaccine booster. The vaccine is safe for administration at any time during pregnancy.
- Infected mothers may breastfeed as long as they do not have cracked or bleeding nipples or exudative skin lesions near the nipple(s).
Neonatal immunoprophylaxis
The Centers for Disease Control and Prevention recommends universal hepatitis B vaccination for all newborns. The first dose of the vaccine should be administered prior to hospital discharge. The second and third doses should be administered 1 and 6 months later.1,2,5 There are few, if any, medical contraindications to neonatal vaccination. For the vast majority of infants, the immunity induced by vaccination is lifelong. For a small number, immunity may wane over time. Thus, reassessment of the HBsAb concentration is indicated in selected situations, for example, acute high-risk exposure to an infected person, development of an immunosuppressive disorder, or pregnancy.
Infants delivered to mothers who are infected with hepatitis B also should receive HBIG in addition to the vaccine. HBIG provides passive immunization to counteract the high viral inoculum encountered by the neonate during delivery. This preparation should be administered within 12 hours of birth.1,2,5
In the absence of immunoprophylaxis, a neonate delivered to a mother who is seropositive for HBsAg has a 20% to 30% probability of becoming chronically infected. If the mother is positive for both the surface antigen and the e antigen, the risk of chronic infection increases to almost 90%. Approximately 90% of infants who are infected in the perinatal period subsequently develop chronic infection. However, with appropriate immunoprophylaxis in the neonatal period, the risk of perinatal transmission is reduced by 85% to 95%.1,2,5
Cesarean delivery offers no additional protection beyond that provided by immunoprophylaxis. Moreover, because immunoprophylaxis is so effective, infected mothers may breastfeed without fear of transmitting infection to their infant. Shi and colleagues published a systematic review and meta-analysis of the risk associated with breastfeeding in hepatitis B–infected mothers.7 Infants who breastfed did not have a higher rate of mother-to-child transmission, regardless of whether they received combined immunoprophylaxis or only hepatitis B vaccine and regardless of whether the HBsAg was detected in the mother’s breast milk. The only precaution is the need to avoid breastfeeding if the nipples are cracked or bleeding or if exudative lesions are present on the skin near the nipple.
Continue to: Maternal antiviral therapy...
Maternal antiviral therapy
As noted above, neonatal immunoprophylaxis is 85% to 95% effective in preventing perinatal transmission of hepatitis B infection. Failures of prophylaxis are primarily due to antenatal transmission in patients who have exceptionally high viral loads. Several cutoffs have been used to define “high viral load,” including greater than 1 to 2 million copies/mL and a hepatitis B DNA concentration greater than 200,000 IU/mL. There is not a perfect consensus on the appropriate cutoff.
In essence, 2 different approaches have been tried to further reduce the risk of perinatal transmission in these high-risk patients.8 The first major initiative was administration of HBIG (100–200 IU) intramuscularly to the patient at 28, 32, and 36 weeks. The outcomes with this approach have been inconsistent, due, at least in part, to varying doses of the agent and various cutoffs for defining “high risk,” and this intervention is no longer recommended.1,2
The second major approach is administration of specific antiviral drugs to the mother during the third trimester. The first agent widely used in clinical practice was lamivudine. In a systematic review and meta-analysis, Shi and colleagues reported that, in infants whose mothers received lamivudine plus conventional neonatal immunuprophylaxis, the risk of perinatal infection was significantly reduced compared with infants who received only immunoprophylaxis.9
Although lamivudine is effective, there is considerable concern about the rapid development of viral resistance to the medication. Accordingly, most attention today is focused on the use of tenofovir to prevent perinatal transmission.
In an important early investigation, Pan and colleagues reported the results of a randomized controlled trial conducted in China in women with a hepatitis B DNA concentration greater than 200,000 IU/mL (viral load > 1,120,000 copies/mL).10 Patients also were positive for the e antigen. Ninety-two patients were assigned to tenofovir disoproxil fumarate (TDF), 300 mg daily, from 30 to 32 weeks until postpartum week 4 plus conventional neonatal immunoprophylaxis, and 100 patients were assigned to immunoprophylaxis alone. In the intention-to-treat analysis, 18 neonates in the control group were infected compared with 5 in the treatment group (P = .007). In the per-protocol analysis, 7 neonates in the control group were infected compared with 0 in the treatment group (P = .01). No clinically significant adverse maternal or neonatal effects occurred in the treatment group.
Subsequently, Jourdain and colleagues reported a multicenter, double-blind trial conducted in 17 public health hospitals in Thailand.11 TDF (300 mg daily) or placebo was administered from 28 weeks’ gestation until 8 weeks postpartum. Patients in both arms of the study were positive for the e antigen; 87% to 90% of the patients had a serum hepatitis B DNA concentration greater than 200,000 IU/mL.Following birth, infants in both groups received an injection of HBIG and then 4 doses of hepatitis B vaccine (0, 1, 2, 4, and 6 months). Both the HBIG and hepatitis B vaccine were administered very promptly after birth (median time, 1.2–1.3 hours).
At 6 months after delivery, 2% of infants in the placebo group (3 of 147) were HBsAg-positive compared with none of the infants in the treatment arm.11 No serious adverse effects occurred in infants in the TDF group. This difference in outcome was not statistically significant, but the overall rate of infection was so low in both groups that the sample size was definitely too small to exclude a type 2 statistical error. Moreover, the fourth dose of neonatal hepatitis B vaccine may have contributed to the surprisingly low rate of perinatal transmission. Of note, the serum hepatitis B DNA concentration in the TDF group declined from a mean of 7.6 log10 IU/mL to a mean of 4.0 log10 IU/mL at delivery.
In the most recent report, Wang and colleagues reported the results of a prospective cohort study in patients with a hepatitis B virus DNA concentration greater than 200,000 IU/mL.12 Beginning at either 24 or 32 weeks, patients were assigned to treatment with either oral TDF (300 mg daily) or oral telbivudine (LdT, 600 mg daily). The medications were continued for 4 weeks postpartum. In the intention-to-treat analysis, the rates of perinatal transmission were comparable, 1.5% versus 1.8%. In the per-protocol analysis, no infants in either group were infected. However, the predelivery decline in hepatitis Bvirus DNA concentration was greater in the TDF group. The ALT elevation rate was also lower in the TDF group. Patients in the LdT group had fewer problems with anorexia but more instances of arthralgia compared with those in the TDF group.
Based primarily on these 3 investigations, I recommend that all infected patients with a hepatitis B DNA concentration greater than 200,000 IU/mL or a viral load greater than 1,120,000 million copies/mL receive oral TDF, 300 mg daily, from 28 weeks until at least 4 to 8 weeks postpartum. The decision about duration of postpartum treatment should be made in consultation with an infectious disease specialist or hepatologist.
Case studies resolved
CASE 1 No protective level of surface antibody
This patient should promptly receive a single booster dose of the hepatitis B vaccine. The vaccine is an inactivated agent and is safe for administration at any time in pregnancy. Following delivery and prior to discharge from the hospital, the neonate should receive the first dose of the hepatitis B vaccine. A second dose should be administered 1 month later, and a third dose should be administered 6 months after the first dose.
CASE 2 Mother is seropositive for HBsAg
This patient should be tested immediately for HIV infection and hepatitis C and D. The hepatitis B viral genotype should be determined. She also should have a panel of liver function tests. If any of these tests are abnormal, a coagulation profile should be obtained to be certain that the patient is not at risk for a coagulopathy. Near the end of the second trimester, a hepatitis B viral load should be obtained. If the viral DNA concentration is greater than 200,000 IU/mLor a viral load greater than 1,120,000 million copies/mL, the patient should be treated with tenofovir, 300 mg daily, from week 28 until at least 4 weeks after delivery. The neonate should receive an injection of HBIG within 12 hours of birth and the first dose of the hepatitis B vaccine prior to discharge from the hospital. Two additional doses of the vaccine should be administered 1 and 6 months later. ●
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862.
- Bernstein HB, Lee MJ. Maternal and perinatal infection in pregnancy: viral. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics. Normal and Problem Pregnancies. 8th ed. Elsevier; 2021;1092.
- Dusheiko G, Agarwal K, Maini MK. New approaches to chronic hepatitis B. N Engl J Med. 2023;388:55-69.
- Ma L, Alla NR, Li X, et al. Mother to child transmission of HBV: review of current clinical management and prevention strategies. Rev Med Virol. 2014; 24: 396-406.
- Society for Maternal-Fetal Medicine; Dionne-Odom J, Tita ATN, Silverman NS. SMFM consult: preventing vertical transmission of hepatitis B. Contemporary OB/GYN. September 22, 2015. Accessed August 21, 2023. https://www .contemporaryobgyn.net/view/smfm-consult-preventing -vertical-transmission-hepatitis-b
- Lok ASF. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743-2746.
- Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846.
- Dusheiko G. A shift in thinking to reduce mother-to-infant transmission of hepatitis B. N Engl J Med. 2018;378:952-953.
- Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159.
- Pan C, Duan Z, Dai E, et al; China Study Group for the Motherto-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374:2324-2334.
- Jourdain G, Ngo-Giang-Huong N, Harrison L, et al. Tenofovir versus placebo to prevent perinatal transmission of hepatitis B. N Engl J Med. 2018;378:911-923.
- Wang M, Ran R, Zhu Y, et al. Comparison of tenofovir disoproxil fumarate and telbivudine in preventing hepatitis B transmission in mothers with high viral load. Int J Gynaecol Obstet. 2023:160:646-652.
In utero SSRI exposure tied to lower brain volume in kids
However, the investigators, led by Henning Tiemeier, MD, PhD, professor of social and behavioral sciences at Harvard School of Public Health in Boston, note that the findings should be interpreted cautiously because the size of the study population who received brain MRI was relatively small.
Dr. Tiemeier said in an interview that the associations detected were small and could not show causality between prenatal SSRI use and a decrease in gray and white matter across certain areas of the brain.
“Women who are pregnant and on maintenance therapy should consult their therapist if preventive therapy is still needed and if there are alternatives. This choice must be carefully considered, and women should be carefully advised,” he said.
The study was published online in JAMA Psychiatry.
An important decision
The investigators note that the decision to prescribe antidepressants, particularly SSRIs, during pregnancy is challenging. Though SSRI use during pregnancy is generally considered safe, some previous research suggests an association with negative outcomes in offspring, including adverse effects on neurodevelopment.
However, the researchers also note that it’s possible that pregnant women who use SSRIs may have other factors, including more severe depressive symptoms, which may be independently associated with adverse outcomes in offspring.
To investigate the link between intrauterine SSRI exposure and brain development, the researchers conducted a prospective, population-based study that included 3,198 pregnant individuals with an expected delivery date between April 2002 and January 2006. Study participants were divided into five groups: 41 who used SSRIs during pregnancy, 257 who did not use the medications but had depressive symptoms during pregnancy, 77 who used SSRIs prenatally, 74 who developed depressive symptoms after giving birth, and 2,749 controls with no SSRI use or depressive symptoms. Participants had a mean age of 31 years, and all identified as women.
Of those who took SSRIs during pregnancy, 20 used them during the first trimester only, and 21 used them the first or in one or two additional trimesters. The SSRIs used included paroxetine, fluoxetine, sertraline, fluvoxamine, and citalopram.
Offspring of the women enrolled in the study received MRIs at three different times between the ages 7 and 15 years.
The 41 children born to the women who took SSRIs prenatally had 80 scans in total, the 257 with mothers who did not use SSRIs yet had depressive symptoms while pregnant had 477 MRIs, the 77 children born to the mothers who took SSRIs before pregnancy had 126 MRIs, the 74 born to mothers with postnatal depression only had 128 MRIs, and the 2,749 children born to the mothers with no SSRI use or depression had 4,813 MRIs.
The study’s primary outcome was brain morphometry in offspring including global and cortical brain volumes, measured by three MRI assessments from ages 7 to 15 years.
Reduced brain volume
Compared with children with no in utero SSRI exposure, those who were exposed had reduced gray and white matter volume that persisted up to 15 years of age (P = .006), particularly in the corticolimbic circuit.
Investigators observed a “persistent association between prenatal SSRI exposure and less cortical volumes across the 10-year follow-up period, including in the superior frontal cortex, medial orbitofrontal cortex, parahippocampal gyrus, rostral anterior cingulate cortex, and posterior cingulate.”
Investigators noted that prenatal SSRI exposure was consistently associated with 5%-10% lower brain volume in the frontal, cingulate, and temporal cortex throughout the age range studied.
In a couple of areas of the brain, however, the brain volume gradually increased back to levels seen in non-SSRI exposed children. For instance, smaller amygdala volumes had increased by age 15 years, so children who were exposed to SSRIs were not any different from control children.
Among the group of women with postnatal depression using an SSRI before or during pregnancy who had depressive symptoms post natally, neonates had a reduced fusiform gyrus (P = .002)
Dr. Tiemeier could not speculate on the effects of the volume differences on children’s development, although the parts of the brain found to be reduced are primarily responsible for emotion regulation.
Investigators noted there was limited ability to investigate trimester-specific outcomes of SSRI use and assess associations with specific SSRIs due to low prevalence of SSRI use.
In addition, research on the long-term behavioral and psychological outcomes associated with demonstrated brain changes is needed, investigators noted.
Clinical significance ‘unclear’
In an accompanying editorial, Ardesheer Talati, PhD, Columbia University, New York, noted that though the research enhances understanding of how brain development through adolescence may be associated with SSRI exposure, “the clinical significance was unclear, especially as key limbic regions, including the amygdala, normalized over time.”
If future evidence links brain anomalies to adverse youth outcomes, Dr. Talati writes, this will need to be “calibrated into the risk-benefit profile.” Until then, he said, the findings must not be overinterpreted “to either promote or discourage antidepressant medication use during the critical period of pregnancy.”
The study was funded by the Netherlands Organization for Scientific Research, European Union’s Horizon Research and Innovation Program, the Netherlands Organization for Health Research and Development, the Sophia Foundation for Neuroimaging, and the European Union’s Horizon Research and Innovation 5 Program. Dr. Talati reported receiving grants from the National Institutes of Health outside of the submitted work.
A version of this article first appeared on Medscape.com.
However, the investigators, led by Henning Tiemeier, MD, PhD, professor of social and behavioral sciences at Harvard School of Public Health in Boston, note that the findings should be interpreted cautiously because the size of the study population who received brain MRI was relatively small.
Dr. Tiemeier said in an interview that the associations detected were small and could not show causality between prenatal SSRI use and a decrease in gray and white matter across certain areas of the brain.
“Women who are pregnant and on maintenance therapy should consult their therapist if preventive therapy is still needed and if there are alternatives. This choice must be carefully considered, and women should be carefully advised,” he said.
The study was published online in JAMA Psychiatry.
An important decision
The investigators note that the decision to prescribe antidepressants, particularly SSRIs, during pregnancy is challenging. Though SSRI use during pregnancy is generally considered safe, some previous research suggests an association with negative outcomes in offspring, including adverse effects on neurodevelopment.
However, the researchers also note that it’s possible that pregnant women who use SSRIs may have other factors, including more severe depressive symptoms, which may be independently associated with adverse outcomes in offspring.
To investigate the link between intrauterine SSRI exposure and brain development, the researchers conducted a prospective, population-based study that included 3,198 pregnant individuals with an expected delivery date between April 2002 and January 2006. Study participants were divided into five groups: 41 who used SSRIs during pregnancy, 257 who did not use the medications but had depressive symptoms during pregnancy, 77 who used SSRIs prenatally, 74 who developed depressive symptoms after giving birth, and 2,749 controls with no SSRI use or depressive symptoms. Participants had a mean age of 31 years, and all identified as women.
Of those who took SSRIs during pregnancy, 20 used them during the first trimester only, and 21 used them the first or in one or two additional trimesters. The SSRIs used included paroxetine, fluoxetine, sertraline, fluvoxamine, and citalopram.
Offspring of the women enrolled in the study received MRIs at three different times between the ages 7 and 15 years.
The 41 children born to the women who took SSRIs prenatally had 80 scans in total, the 257 with mothers who did not use SSRIs yet had depressive symptoms while pregnant had 477 MRIs, the 77 children born to the mothers who took SSRIs before pregnancy had 126 MRIs, the 74 born to mothers with postnatal depression only had 128 MRIs, and the 2,749 children born to the mothers with no SSRI use or depression had 4,813 MRIs.
The study’s primary outcome was brain morphometry in offspring including global and cortical brain volumes, measured by three MRI assessments from ages 7 to 15 years.
Reduced brain volume
Compared with children with no in utero SSRI exposure, those who were exposed had reduced gray and white matter volume that persisted up to 15 years of age (P = .006), particularly in the corticolimbic circuit.
Investigators observed a “persistent association between prenatal SSRI exposure and less cortical volumes across the 10-year follow-up period, including in the superior frontal cortex, medial orbitofrontal cortex, parahippocampal gyrus, rostral anterior cingulate cortex, and posterior cingulate.”
Investigators noted that prenatal SSRI exposure was consistently associated with 5%-10% lower brain volume in the frontal, cingulate, and temporal cortex throughout the age range studied.
In a couple of areas of the brain, however, the brain volume gradually increased back to levels seen in non-SSRI exposed children. For instance, smaller amygdala volumes had increased by age 15 years, so children who were exposed to SSRIs were not any different from control children.
Among the group of women with postnatal depression using an SSRI before or during pregnancy who had depressive symptoms post natally, neonates had a reduced fusiform gyrus (P = .002)
Dr. Tiemeier could not speculate on the effects of the volume differences on children’s development, although the parts of the brain found to be reduced are primarily responsible for emotion regulation.
Investigators noted there was limited ability to investigate trimester-specific outcomes of SSRI use and assess associations with specific SSRIs due to low prevalence of SSRI use.
In addition, research on the long-term behavioral and psychological outcomes associated with demonstrated brain changes is needed, investigators noted.
Clinical significance ‘unclear’
In an accompanying editorial, Ardesheer Talati, PhD, Columbia University, New York, noted that though the research enhances understanding of how brain development through adolescence may be associated with SSRI exposure, “the clinical significance was unclear, especially as key limbic regions, including the amygdala, normalized over time.”
If future evidence links brain anomalies to adverse youth outcomes, Dr. Talati writes, this will need to be “calibrated into the risk-benefit profile.” Until then, he said, the findings must not be overinterpreted “to either promote or discourage antidepressant medication use during the critical period of pregnancy.”
The study was funded by the Netherlands Organization for Scientific Research, European Union’s Horizon Research and Innovation Program, the Netherlands Organization for Health Research and Development, the Sophia Foundation for Neuroimaging, and the European Union’s Horizon Research and Innovation 5 Program. Dr. Talati reported receiving grants from the National Institutes of Health outside of the submitted work.
A version of this article first appeared on Medscape.com.
However, the investigators, led by Henning Tiemeier, MD, PhD, professor of social and behavioral sciences at Harvard School of Public Health in Boston, note that the findings should be interpreted cautiously because the size of the study population who received brain MRI was relatively small.
Dr. Tiemeier said in an interview that the associations detected were small and could not show causality between prenatal SSRI use and a decrease in gray and white matter across certain areas of the brain.
“Women who are pregnant and on maintenance therapy should consult their therapist if preventive therapy is still needed and if there are alternatives. This choice must be carefully considered, and women should be carefully advised,” he said.
The study was published online in JAMA Psychiatry.
An important decision
The investigators note that the decision to prescribe antidepressants, particularly SSRIs, during pregnancy is challenging. Though SSRI use during pregnancy is generally considered safe, some previous research suggests an association with negative outcomes in offspring, including adverse effects on neurodevelopment.
However, the researchers also note that it’s possible that pregnant women who use SSRIs may have other factors, including more severe depressive symptoms, which may be independently associated with adverse outcomes in offspring.
To investigate the link between intrauterine SSRI exposure and brain development, the researchers conducted a prospective, population-based study that included 3,198 pregnant individuals with an expected delivery date between April 2002 and January 2006. Study participants were divided into five groups: 41 who used SSRIs during pregnancy, 257 who did not use the medications but had depressive symptoms during pregnancy, 77 who used SSRIs prenatally, 74 who developed depressive symptoms after giving birth, and 2,749 controls with no SSRI use or depressive symptoms. Participants had a mean age of 31 years, and all identified as women.
Of those who took SSRIs during pregnancy, 20 used them during the first trimester only, and 21 used them the first or in one or two additional trimesters. The SSRIs used included paroxetine, fluoxetine, sertraline, fluvoxamine, and citalopram.
Offspring of the women enrolled in the study received MRIs at three different times between the ages 7 and 15 years.
The 41 children born to the women who took SSRIs prenatally had 80 scans in total, the 257 with mothers who did not use SSRIs yet had depressive symptoms while pregnant had 477 MRIs, the 77 children born to the mothers who took SSRIs before pregnancy had 126 MRIs, the 74 born to mothers with postnatal depression only had 128 MRIs, and the 2,749 children born to the mothers with no SSRI use or depression had 4,813 MRIs.
The study’s primary outcome was brain morphometry in offspring including global and cortical brain volumes, measured by three MRI assessments from ages 7 to 15 years.
Reduced brain volume
Compared with children with no in utero SSRI exposure, those who were exposed had reduced gray and white matter volume that persisted up to 15 years of age (P = .006), particularly in the corticolimbic circuit.
Investigators observed a “persistent association between prenatal SSRI exposure and less cortical volumes across the 10-year follow-up period, including in the superior frontal cortex, medial orbitofrontal cortex, parahippocampal gyrus, rostral anterior cingulate cortex, and posterior cingulate.”
Investigators noted that prenatal SSRI exposure was consistently associated with 5%-10% lower brain volume in the frontal, cingulate, and temporal cortex throughout the age range studied.
In a couple of areas of the brain, however, the brain volume gradually increased back to levels seen in non-SSRI exposed children. For instance, smaller amygdala volumes had increased by age 15 years, so children who were exposed to SSRIs were not any different from control children.
Among the group of women with postnatal depression using an SSRI before or during pregnancy who had depressive symptoms post natally, neonates had a reduced fusiform gyrus (P = .002)
Dr. Tiemeier could not speculate on the effects of the volume differences on children’s development, although the parts of the brain found to be reduced are primarily responsible for emotion regulation.
Investigators noted there was limited ability to investigate trimester-specific outcomes of SSRI use and assess associations with specific SSRIs due to low prevalence of SSRI use.
In addition, research on the long-term behavioral and psychological outcomes associated with demonstrated brain changes is needed, investigators noted.
Clinical significance ‘unclear’
In an accompanying editorial, Ardesheer Talati, PhD, Columbia University, New York, noted that though the research enhances understanding of how brain development through adolescence may be associated with SSRI exposure, “the clinical significance was unclear, especially as key limbic regions, including the amygdala, normalized over time.”
If future evidence links brain anomalies to adverse youth outcomes, Dr. Talati writes, this will need to be “calibrated into the risk-benefit profile.” Until then, he said, the findings must not be overinterpreted “to either promote or discourage antidepressant medication use during the critical period of pregnancy.”
The study was funded by the Netherlands Organization for Scientific Research, European Union’s Horizon Research and Innovation Program, the Netherlands Organization for Health Research and Development, the Sophia Foundation for Neuroimaging, and the European Union’s Horizon Research and Innovation 5 Program. Dr. Talati reported receiving grants from the National Institutes of Health outside of the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Social media use may promote depression in pregnancy
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Depressive symptoms among pregnant women have risen in recent years, but the potential impact of social media use on depression in pregnancy has not been well studied, wrote Lotte Muskens, a PhD candidate at Tilburg (the Netherlands) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers surveyed 697 pregnant women aged 19-42 years who were part of a larger longitudinal prospective study (the Brabant Study) in the Netherlands. The mean age of the participants was 31 years; 96% were employed, 99% had a partner, and 71% had a bachelor’s degree or higher. Depressive symptoms were assessed at 12, 20, and 28 weeks of pregnancy using the Dutch version of the 10-item Edinburgh Depression Scale (EDS).
The researchers categorized the participants into trajectories of depressive symptoms during pregnancy, with 489 identified as low stable (mean EDS scores 2.8-3.0), 183 as intermediate stable (mean EDS scores 8.4-8.8), and 25 as high stable (mean EDS scores 15.1-16.9).
Problematic SMU was identified using the six-item Bergen Social Media Addiction Scale (BSMAS) at 12 weeks of pregnancy; scores ranged from 6 to 30, with higher scores representing more problematic SMU.
The mean BSMAS scores were 9.0, 10.7, and 12.6 for the low-stable, intermediate-stable, and high-stable depression groups, respectively.
Data on social media use (SMU) were collected at 12 weeks of pregnancy. Social media was defined as common platforms including Facebook, Instagram, LinkedIn, Pinterest, Twitter, and YouTube.
SMU was defined in terms of intensity, measured by time and frequency. Time was measured by asking participants to list how many hours per day they used social media on a scale of 1 (no use of social media) to 9 (7 or more hours per day). Frequency was measured by asking how often participants visited the various social media platforms, on a scale of 1 (no use of social media) to 7 (five or more visits per day). Overall, the participants averaged 1.6 hours per day and 19.5 visits per week on SMU.
Increased time and frequency of SMU was significantly associated with increased odds of being in the high-stable group, compared with the low-stable group in an adjusted analysis (odds ratios, 1.51 and 1.05, respectively; P = .017 and P = .019, respectively).
In addition, problematic SMU (as defined by higher BSMAS scores) remained significantly associated with increased odds of belonging to the intermediate-stable or high-stable classes in an adjusted analysis (odds ratios, 1.17 and 1.31; P < .001 for both).
“While our results suggest that SMU can have negative consequences for pregnant women’s mental wellbeing, it is important to note that SMU during pregnancy may also be helpful for some pregnant women,” as many women, especially first-time mothers, find information and support through social media, the researchers wrote in their discussion.
The findings were limited by several factors, including the variation in group sizes for depressive symptoms, reliance on self-reports, and the collection of data during the COVID-19 pandemic, which may have affected the results, the researchers noted.
However, the results were strengthened by the large sample size and longitudinal design that allowed measurement of trajectories. More research is needed to determine causal relationships, but the data indicate an association between higher levels of depression during pregnancy and more intense and problematic SMU use, and health care providers should discuss SMU in addition to other risk factors for depression in pregnant women, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Freezing the biological clock: A 2023 update on preserving fertility
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.
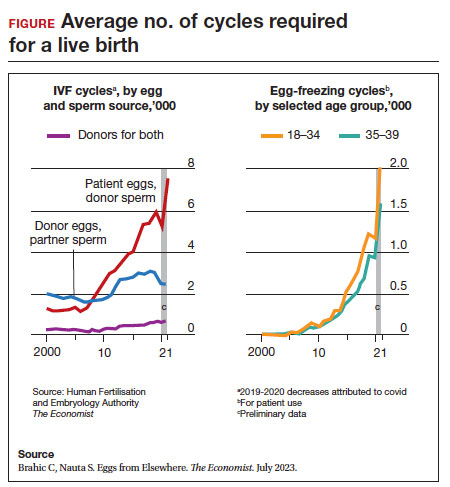
Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.

Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.

Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
Does remote blood pressure monitoring improve patient outcomes postpartum?
Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
EXPERT COMMENTARY
Courtney Bisson, MD, is a Maternal-Fetal Medicine Fellow, University of Chicago/NorthShore University HealthSystem, Chicago, Illinois.
Sarosh Rana, MD, MPH, is Professor of Obstetrics and Gynecology and Section Chief, Maternal-Fetal Medicine, University of Chicago.
Hypertensive disorders of pregnancy account for a significant amount of morbidity during pregnancy and postpartum. In the pregnant population, data have shown that the implementation of a standardized blood pressure education program, provision of a blood pressure cuff, and assistance with postpartum follow-up result in improved blood pressures and postpartum follow-up for up to 6 weeks. In the nonpregnant population, literature suggests that RBPM in patients with hypertension results in improved outcomes, although the long-term impact of RBPM in the postpartum population remains unclear.
Recently, Hirshberg and colleagues published the results of a retrospective cohort study that assessed the impact of RBPM with text message reminders for 10 days postpartum on a composite of adverse maternal outcomes, readmissions, and follow-up within 1 year postpartum.1

Details of the study
The retrospective cohort study was conducted during 2017–2021 based on insurance claims of patients with hypertensive disorders of pregnancy who were enrolled in a twice-daily text message–based RBPM program for 10 days postpartum.
Data from 1,700 patients enrolled in RBPM were compared with that of propensity score matched controls that included 2,297 women not enrolled in RBPM. Of these controls, 1,276 patients (cohort C) simultaneously received care at other institutions without RBPM, and 1,021 patients (cohort A) received care at the same institution prior to implementation of RBPM.
Results. Patients in the RBPM group were found to have a significantly lower rate of composite adverse maternal outcomes compared with their matched cohorts in the year after delivery. (Individual adverse outcomes included stroke, disseminated intravascular coagulation, eclampsia, pulmonary edema, renal injury or liver failure, HELLP [hemolysis, elevated liver enzymes, low platelet count] syndrome, myocardial infarction, and cardiomyopathy.) Rates were 2.9% versus 4.7% (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.40–0.98) in the RBPM group compared with cohort A; rates in the RBPM group compared with cohort C were 3.2% versus 4.5% (OR, 0.71; 95% CI, 0.47–1.07).
Although not statistically significant, rates of emergency department visits and readmissions also were lower in the RBPM patients. Those enrolled in the RBPM program were more likely to have follow-up with cardiologists or specialist visits within 6 months postpartum. Fewer emergency department visits and readmissions resulted in lower health care utilization costs.
Study strengths and limitations
This study’s strength lies in its design and implementation of standardized protocols that allowed assessment of clinically meaningful outcomes postpartum. Although the program for RBPM was for only 10 days postpartum, it showed effects beyond the timeframe of the direct care. No such prior data exist evaluating a program’s effectiveness in improving postpartum clinical outcomes and costs through 1 year postdelivery.
Study limitations include residual bias from unobserved confounders, analysis of only 1 payer type, lack of patient level data, and evaluation of disparity. ●
Previous work by Suresh and colleagues illustrated that a standardized postpartum blood pressure monitoring quality improvement initiative resulted in better blood pressures, improved postpartum visit adherence, and reduced disparity.2 The study by Hirshberg and colleagues furthers these findings, illustrating how uniform protocols surrounding preeclampsia management in the postpartum setting could further improve morbidity and mortality in the year following childbirth. Such protocols should be incorporated hospital-wide in standard obstetrical management.
COURTNEY BISSON, MD; SAROSH RANA, MD, MPH
- Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
- Suresh SC, Duncan C, Kaur H, et al. Postpartum outcomes with systematic treatment and management of postpartum hypertension. Obstet Gynecol. 2021;138:777-787. doi:10.1097 /AOG.0000000000004574.
Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
EXPERT COMMENTARY
Courtney Bisson, MD, is a Maternal-Fetal Medicine Fellow, University of Chicago/NorthShore University HealthSystem, Chicago, Illinois.
Sarosh Rana, MD, MPH, is Professor of Obstetrics and Gynecology and Section Chief, Maternal-Fetal Medicine, University of Chicago.
Hypertensive disorders of pregnancy account for a significant amount of morbidity during pregnancy and postpartum. In the pregnant population, data have shown that the implementation of a standardized blood pressure education program, provision of a blood pressure cuff, and assistance with postpartum follow-up result in improved blood pressures and postpartum follow-up for up to 6 weeks. In the nonpregnant population, literature suggests that RBPM in patients with hypertension results in improved outcomes, although the long-term impact of RBPM in the postpartum population remains unclear.
Recently, Hirshberg and colleagues published the results of a retrospective cohort study that assessed the impact of RBPM with text message reminders for 10 days postpartum on a composite of adverse maternal outcomes, readmissions, and follow-up within 1 year postpartum.1

Details of the study
The retrospective cohort study was conducted during 2017–2021 based on insurance claims of patients with hypertensive disorders of pregnancy who were enrolled in a twice-daily text message–based RBPM program for 10 days postpartum.
Data from 1,700 patients enrolled in RBPM were compared with that of propensity score matched controls that included 2,297 women not enrolled in RBPM. Of these controls, 1,276 patients (cohort C) simultaneously received care at other institutions without RBPM, and 1,021 patients (cohort A) received care at the same institution prior to implementation of RBPM.
Results. Patients in the RBPM group were found to have a significantly lower rate of composite adverse maternal outcomes compared with their matched cohorts in the year after delivery. (Individual adverse outcomes included stroke, disseminated intravascular coagulation, eclampsia, pulmonary edema, renal injury or liver failure, HELLP [hemolysis, elevated liver enzymes, low platelet count] syndrome, myocardial infarction, and cardiomyopathy.) Rates were 2.9% versus 4.7% (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.40–0.98) in the RBPM group compared with cohort A; rates in the RBPM group compared with cohort C were 3.2% versus 4.5% (OR, 0.71; 95% CI, 0.47–1.07).
Although not statistically significant, rates of emergency department visits and readmissions also were lower in the RBPM patients. Those enrolled in the RBPM program were more likely to have follow-up with cardiologists or specialist visits within 6 months postpartum. Fewer emergency department visits and readmissions resulted in lower health care utilization costs.
Study strengths and limitations
This study’s strength lies in its design and implementation of standardized protocols that allowed assessment of clinically meaningful outcomes postpartum. Although the program for RBPM was for only 10 days postpartum, it showed effects beyond the timeframe of the direct care. No such prior data exist evaluating a program’s effectiveness in improving postpartum clinical outcomes and costs through 1 year postdelivery.
Study limitations include residual bias from unobserved confounders, analysis of only 1 payer type, lack of patient level data, and evaluation of disparity. ●
Previous work by Suresh and colleagues illustrated that a standardized postpartum blood pressure monitoring quality improvement initiative resulted in better blood pressures, improved postpartum visit adherence, and reduced disparity.2 The study by Hirshberg and colleagues furthers these findings, illustrating how uniform protocols surrounding preeclampsia management in the postpartum setting could further improve morbidity and mortality in the year following childbirth. Such protocols should be incorporated hospital-wide in standard obstetrical management.
COURTNEY BISSON, MD; SAROSH RANA, MD, MPH
Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
EXPERT COMMENTARY
Courtney Bisson, MD, is a Maternal-Fetal Medicine Fellow, University of Chicago/NorthShore University HealthSystem, Chicago, Illinois.
Sarosh Rana, MD, MPH, is Professor of Obstetrics and Gynecology and Section Chief, Maternal-Fetal Medicine, University of Chicago.
Hypertensive disorders of pregnancy account for a significant amount of morbidity during pregnancy and postpartum. In the pregnant population, data have shown that the implementation of a standardized blood pressure education program, provision of a blood pressure cuff, and assistance with postpartum follow-up result in improved blood pressures and postpartum follow-up for up to 6 weeks. In the nonpregnant population, literature suggests that RBPM in patients with hypertension results in improved outcomes, although the long-term impact of RBPM in the postpartum population remains unclear.
Recently, Hirshberg and colleagues published the results of a retrospective cohort study that assessed the impact of RBPM with text message reminders for 10 days postpartum on a composite of adverse maternal outcomes, readmissions, and follow-up within 1 year postpartum.1

Details of the study
The retrospective cohort study was conducted during 2017–2021 based on insurance claims of patients with hypertensive disorders of pregnancy who were enrolled in a twice-daily text message–based RBPM program for 10 days postpartum.
Data from 1,700 patients enrolled in RBPM were compared with that of propensity score matched controls that included 2,297 women not enrolled in RBPM. Of these controls, 1,276 patients (cohort C) simultaneously received care at other institutions without RBPM, and 1,021 patients (cohort A) received care at the same institution prior to implementation of RBPM.
Results. Patients in the RBPM group were found to have a significantly lower rate of composite adverse maternal outcomes compared with their matched cohorts in the year after delivery. (Individual adverse outcomes included stroke, disseminated intravascular coagulation, eclampsia, pulmonary edema, renal injury or liver failure, HELLP [hemolysis, elevated liver enzymes, low platelet count] syndrome, myocardial infarction, and cardiomyopathy.) Rates were 2.9% versus 4.7% (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.40–0.98) in the RBPM group compared with cohort A; rates in the RBPM group compared with cohort C were 3.2% versus 4.5% (OR, 0.71; 95% CI, 0.47–1.07).
Although not statistically significant, rates of emergency department visits and readmissions also were lower in the RBPM patients. Those enrolled in the RBPM program were more likely to have follow-up with cardiologists or specialist visits within 6 months postpartum. Fewer emergency department visits and readmissions resulted in lower health care utilization costs.
Study strengths and limitations
This study’s strength lies in its design and implementation of standardized protocols that allowed assessment of clinically meaningful outcomes postpartum. Although the program for RBPM was for only 10 days postpartum, it showed effects beyond the timeframe of the direct care. No such prior data exist evaluating a program’s effectiveness in improving postpartum clinical outcomes and costs through 1 year postdelivery.
Study limitations include residual bias from unobserved confounders, analysis of only 1 payer type, lack of patient level data, and evaluation of disparity. ●
Previous work by Suresh and colleagues illustrated that a standardized postpartum blood pressure monitoring quality improvement initiative resulted in better blood pressures, improved postpartum visit adherence, and reduced disparity.2 The study by Hirshberg and colleagues furthers these findings, illustrating how uniform protocols surrounding preeclampsia management in the postpartum setting could further improve morbidity and mortality in the year following childbirth. Such protocols should be incorporated hospital-wide in standard obstetrical management.
COURTNEY BISSON, MD; SAROSH RANA, MD, MPH
- Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
- Suresh SC, Duncan C, Kaur H, et al. Postpartum outcomes with systematic treatment and management of postpartum hypertension. Obstet Gynecol. 2021;138:777-787. doi:10.1097 /AOG.0000000000004574.
- Hirshberg A, Zhu Y, Smith-McLallen A, et al. Association of a remote blood pressure monitoring program with postpartum adverse outcomes. Obstet Gynecol. 2023;141:1163-1170. doi:10.1097/AOG.0000000000005197.
- Suresh SC, Duncan C, Kaur H, et al. Postpartum outcomes with systematic treatment and management of postpartum hypertension. Obstet Gynecol. 2021;138:777-787. doi:10.1097 /AOG.0000000000004574.
How ob.gyn. programs provide abortion training post Dobbs
to fulfill required clinical rotations in the procedure.
The Accreditation Council for Graduate Medical Education requires ob.gyn. residents – unless they have a religious or moral exemption – to undergo abortion training to complete their programs. In states with bans or restrictions on family planning services or abortions, resident training must be received at institutions that are out of state.
Some residency programs are just beginning to coordinate out-of-state training, while others are further along in their offerings. There’s no formal matching process, and it remains unclear who will cover the costs of residents training elsewhere for a month.
These uncertainties, along with lack of coordination about malpractice, clinical rotations, and limited faculty, leave some program directors skeptical they’ll be able to keep up with demand for out-of-state slots. They are also wary of harming their own residents’ educational and clinical opportunities.
A 3rd-year ob.gyn. resident, who didn’t want to give her name or residency program for fear of backlash against her home institution, told this news organization that the Catholic-affiliated site is trying to avoid drawing attention to its minimal abortion training in a restrictive Midwest state. She knew after the Supreme Court’s decision in Dobbs v. Jackson she’d have to look outside the program for more complex abortion training.
While she could learn dilation and curettage or other first-trimester or early–second-trimester procedures at the Midwest program, she said she couldn’t learn dilation and evacuation.
A mentor at her program connected her with a residency program at the University of New Mexico, where she recently started a 5-week family planning rotation. She is the first out-of-state resident hosted by UNM. Currently, UNM has six ob.gyn. residents per class year, for a total of 36, and six family planning fellows.
The ob.gyn. resident is staying with a friend at no cost, and her home institution still pays her salary. But she still must pay the mortgage on a home she can’t live in while away and misses being part of a community where she’s built a life over the past 2 years.
“There’s a part of you that’s just angry that you can’t do this for the women ... in your state,” she said. “Unfortunately, there isn’t a formalized program for ob.gyn. residents interested in more advanced training to be matched with a program that has the ability to offer that training. It’s very much a word-of-mouth and who-you-know situation. For people without those connections, it can be difficult to obtain this training unless they are interested in a formal fellowship.”
This year, about 1,500 ob.gyn. residents matched into 280 residency programs, according to the National Residency Matching Program.
Alyssa Colwill, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University and director of the ob.gyn. Ryan Residency Program at OHSU, estimated that 1,000 ob.gyn. residents per year will seek out-of-state abortion training. The estimate is based on the number of residents in programs in states with restrictions.
The Ryan Program, which began in 1999, helps ob.gyn. residency programs provide training in abortion and contraception care (family planning) as a required rotation.
Connecting programs
Ryan-affiliated residencies have been helping connect programs in states with abortion bans and restrictions to programs in states with more liberal laws.
Twelve of the 100 Ryan programs sent residents out of state in the past academic year, and 15 will follow this year. More are expected soon, said Kristin Simonson, MA, director of programs and operations at the Ryan Residency Program, headquartered at the Bixby Center for Global Reproductive Health at the University of California, San Francisco.
Before the Dobbs decision, very few programs considered next steps to train ob.gyn. residents if abortions became illegal, Ms. Simonson said. “I think a lot of people just kind of were waiting and seeing ... and hoping that they wouldn’t have to make any drastic plans. It was hard to motivate people to have a plan B ready to go,” she said.
“Almost all of us working in this field had a really bad feeling,” said Courtney G. Forbis, MD, UNM assistant professor of ob.gyn. and Ryan Residency director. She and colleagues began planning for the future months ahead of the court decision. But the program wasn’t able to begin accepting out-of-state residents until now, she said. “We are trying to use this experience to see what we can accommodate in the future.”
OHSU also began planning for alternative training when it learned of the leaked Supreme Court decision, Dr. Colwill said. “We decided that we had the bandwidth and opportunity to train more individuals that were going to lose access to services and educational opportunities,” she said.
The university ran a 4-week test rotation last fall. So far, six residents and one fellow have come from out of the state, said Dr. Colwill. OHSU hopes to have 10 more in the coming year. The out-of-state learners will join 32 ob.gyn. residents and 12 fellows who were already in the program, she said.
To ease residents’ integration into an away program, the Ryan Program – along with the American College of Obstetricians and Gynecologists, the Council on Resident Education in Obstetrics and Gynecology, and Innovating Education in Reproductive Health – recently began offering a free, web-based patient-centered abortion education curriculum.
The course supplements in-person clinical training in abortion care and prepares residents traveling and transitioning into another program to begin learning new skills on their first day, AnnaMarie Connolly, MD, ACOG’s chief of education and academic affairs, said in a prepared statement.
Training costs
Residents and their institutions also face additional costs. The home institution that loses a resident for a few weeks to a month has to determine how to cover the care not provided while they are away, Ms. Simonson said. Residents may incur expenses for transportation, housing, food, and other things while out of state.
OHSU covers transportation and housing through its abortion care and training fund, but there are other factors to consider, Dr. Colwill said. For example, the home and host programs have to coordinate licensing, malpractice, and line up rotation dates, she said.
Among other complications, UNM wasn’t able to set up an agreement so that its new resident could participate in a rotation at Planned Parenthood. “We have the clinical volume to accommodate another learner,” Dr. Forbis said. But the program has to balance resources, such as “trying to make sure we don’t have one faculty [member] assigned to too many learners at one time,” she said.
Given the logistic and financial challenges, programs may not be able to ensure that all residents who need abortion training receive it, said Ms. Simonson.
The Ryan Program, for instance, can’t help the more than 100 residency programs in states where abortions are currently illegal, she said.
UNM is trying to partner with specific programs, such as those in the state of Texas where abortion is banned, to train its residents each year, Dr. Forbis said.
OHSU also will look for opportunities to train as many residents as possible, Dr. Colwill said, “but I don’t think we’ll ever be able to fill that gap of 1,000 residents that need this training.”
A version of this article first appeared on Medscape.com.
to fulfill required clinical rotations in the procedure.
The Accreditation Council for Graduate Medical Education requires ob.gyn. residents – unless they have a religious or moral exemption – to undergo abortion training to complete their programs. In states with bans or restrictions on family planning services or abortions, resident training must be received at institutions that are out of state.
Some residency programs are just beginning to coordinate out-of-state training, while others are further along in their offerings. There’s no formal matching process, and it remains unclear who will cover the costs of residents training elsewhere for a month.
These uncertainties, along with lack of coordination about malpractice, clinical rotations, and limited faculty, leave some program directors skeptical they’ll be able to keep up with demand for out-of-state slots. They are also wary of harming their own residents’ educational and clinical opportunities.
A 3rd-year ob.gyn. resident, who didn’t want to give her name or residency program for fear of backlash against her home institution, told this news organization that the Catholic-affiliated site is trying to avoid drawing attention to its minimal abortion training in a restrictive Midwest state. She knew after the Supreme Court’s decision in Dobbs v. Jackson she’d have to look outside the program for more complex abortion training.
While she could learn dilation and curettage or other first-trimester or early–second-trimester procedures at the Midwest program, she said she couldn’t learn dilation and evacuation.
A mentor at her program connected her with a residency program at the University of New Mexico, where she recently started a 5-week family planning rotation. She is the first out-of-state resident hosted by UNM. Currently, UNM has six ob.gyn. residents per class year, for a total of 36, and six family planning fellows.
The ob.gyn. resident is staying with a friend at no cost, and her home institution still pays her salary. But she still must pay the mortgage on a home she can’t live in while away and misses being part of a community where she’s built a life over the past 2 years.
“There’s a part of you that’s just angry that you can’t do this for the women ... in your state,” she said. “Unfortunately, there isn’t a formalized program for ob.gyn. residents interested in more advanced training to be matched with a program that has the ability to offer that training. It’s very much a word-of-mouth and who-you-know situation. For people without those connections, it can be difficult to obtain this training unless they are interested in a formal fellowship.”
This year, about 1,500 ob.gyn. residents matched into 280 residency programs, according to the National Residency Matching Program.
Alyssa Colwill, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University and director of the ob.gyn. Ryan Residency Program at OHSU, estimated that 1,000 ob.gyn. residents per year will seek out-of-state abortion training. The estimate is based on the number of residents in programs in states with restrictions.
The Ryan Program, which began in 1999, helps ob.gyn. residency programs provide training in abortion and contraception care (family planning) as a required rotation.
Connecting programs
Ryan-affiliated residencies have been helping connect programs in states with abortion bans and restrictions to programs in states with more liberal laws.
Twelve of the 100 Ryan programs sent residents out of state in the past academic year, and 15 will follow this year. More are expected soon, said Kristin Simonson, MA, director of programs and operations at the Ryan Residency Program, headquartered at the Bixby Center for Global Reproductive Health at the University of California, San Francisco.
Before the Dobbs decision, very few programs considered next steps to train ob.gyn. residents if abortions became illegal, Ms. Simonson said. “I think a lot of people just kind of were waiting and seeing ... and hoping that they wouldn’t have to make any drastic plans. It was hard to motivate people to have a plan B ready to go,” she said.
“Almost all of us working in this field had a really bad feeling,” said Courtney G. Forbis, MD, UNM assistant professor of ob.gyn. and Ryan Residency director. She and colleagues began planning for the future months ahead of the court decision. But the program wasn’t able to begin accepting out-of-state residents until now, she said. “We are trying to use this experience to see what we can accommodate in the future.”
OHSU also began planning for alternative training when it learned of the leaked Supreme Court decision, Dr. Colwill said. “We decided that we had the bandwidth and opportunity to train more individuals that were going to lose access to services and educational opportunities,” she said.
The university ran a 4-week test rotation last fall. So far, six residents and one fellow have come from out of the state, said Dr. Colwill. OHSU hopes to have 10 more in the coming year. The out-of-state learners will join 32 ob.gyn. residents and 12 fellows who were already in the program, she said.
To ease residents’ integration into an away program, the Ryan Program – along with the American College of Obstetricians and Gynecologists, the Council on Resident Education in Obstetrics and Gynecology, and Innovating Education in Reproductive Health – recently began offering a free, web-based patient-centered abortion education curriculum.
The course supplements in-person clinical training in abortion care and prepares residents traveling and transitioning into another program to begin learning new skills on their first day, AnnaMarie Connolly, MD, ACOG’s chief of education and academic affairs, said in a prepared statement.
Training costs
Residents and their institutions also face additional costs. The home institution that loses a resident for a few weeks to a month has to determine how to cover the care not provided while they are away, Ms. Simonson said. Residents may incur expenses for transportation, housing, food, and other things while out of state.
OHSU covers transportation and housing through its abortion care and training fund, but there are other factors to consider, Dr. Colwill said. For example, the home and host programs have to coordinate licensing, malpractice, and line up rotation dates, she said.
Among other complications, UNM wasn’t able to set up an agreement so that its new resident could participate in a rotation at Planned Parenthood. “We have the clinical volume to accommodate another learner,” Dr. Forbis said. But the program has to balance resources, such as “trying to make sure we don’t have one faculty [member] assigned to too many learners at one time,” she said.
Given the logistic and financial challenges, programs may not be able to ensure that all residents who need abortion training receive it, said Ms. Simonson.
The Ryan Program, for instance, can’t help the more than 100 residency programs in states where abortions are currently illegal, she said.
UNM is trying to partner with specific programs, such as those in the state of Texas where abortion is banned, to train its residents each year, Dr. Forbis said.
OHSU also will look for opportunities to train as many residents as possible, Dr. Colwill said, “but I don’t think we’ll ever be able to fill that gap of 1,000 residents that need this training.”
A version of this article first appeared on Medscape.com.
to fulfill required clinical rotations in the procedure.
The Accreditation Council for Graduate Medical Education requires ob.gyn. residents – unless they have a religious or moral exemption – to undergo abortion training to complete their programs. In states with bans or restrictions on family planning services or abortions, resident training must be received at institutions that are out of state.
Some residency programs are just beginning to coordinate out-of-state training, while others are further along in their offerings. There’s no formal matching process, and it remains unclear who will cover the costs of residents training elsewhere for a month.
These uncertainties, along with lack of coordination about malpractice, clinical rotations, and limited faculty, leave some program directors skeptical they’ll be able to keep up with demand for out-of-state slots. They are also wary of harming their own residents’ educational and clinical opportunities.
A 3rd-year ob.gyn. resident, who didn’t want to give her name or residency program for fear of backlash against her home institution, told this news organization that the Catholic-affiliated site is trying to avoid drawing attention to its minimal abortion training in a restrictive Midwest state. She knew after the Supreme Court’s decision in Dobbs v. Jackson she’d have to look outside the program for more complex abortion training.
While she could learn dilation and curettage or other first-trimester or early–second-trimester procedures at the Midwest program, she said she couldn’t learn dilation and evacuation.
A mentor at her program connected her with a residency program at the University of New Mexico, where she recently started a 5-week family planning rotation. She is the first out-of-state resident hosted by UNM. Currently, UNM has six ob.gyn. residents per class year, for a total of 36, and six family planning fellows.
The ob.gyn. resident is staying with a friend at no cost, and her home institution still pays her salary. But she still must pay the mortgage on a home she can’t live in while away and misses being part of a community where she’s built a life over the past 2 years.
“There’s a part of you that’s just angry that you can’t do this for the women ... in your state,” she said. “Unfortunately, there isn’t a formalized program for ob.gyn. residents interested in more advanced training to be matched with a program that has the ability to offer that training. It’s very much a word-of-mouth and who-you-know situation. For people without those connections, it can be difficult to obtain this training unless they are interested in a formal fellowship.”
This year, about 1,500 ob.gyn. residents matched into 280 residency programs, according to the National Residency Matching Program.
Alyssa Colwill, MD, assistant professor of obstetrics and gynecology at Oregon Health and Science University and director of the ob.gyn. Ryan Residency Program at OHSU, estimated that 1,000 ob.gyn. residents per year will seek out-of-state abortion training. The estimate is based on the number of residents in programs in states with restrictions.
The Ryan Program, which began in 1999, helps ob.gyn. residency programs provide training in abortion and contraception care (family planning) as a required rotation.
Connecting programs
Ryan-affiliated residencies have been helping connect programs in states with abortion bans and restrictions to programs in states with more liberal laws.
Twelve of the 100 Ryan programs sent residents out of state in the past academic year, and 15 will follow this year. More are expected soon, said Kristin Simonson, MA, director of programs and operations at the Ryan Residency Program, headquartered at the Bixby Center for Global Reproductive Health at the University of California, San Francisco.
Before the Dobbs decision, very few programs considered next steps to train ob.gyn. residents if abortions became illegal, Ms. Simonson said. “I think a lot of people just kind of were waiting and seeing ... and hoping that they wouldn’t have to make any drastic plans. It was hard to motivate people to have a plan B ready to go,” she said.
“Almost all of us working in this field had a really bad feeling,” said Courtney G. Forbis, MD, UNM assistant professor of ob.gyn. and Ryan Residency director. She and colleagues began planning for the future months ahead of the court decision. But the program wasn’t able to begin accepting out-of-state residents until now, she said. “We are trying to use this experience to see what we can accommodate in the future.”
OHSU also began planning for alternative training when it learned of the leaked Supreme Court decision, Dr. Colwill said. “We decided that we had the bandwidth and opportunity to train more individuals that were going to lose access to services and educational opportunities,” she said.
The university ran a 4-week test rotation last fall. So far, six residents and one fellow have come from out of the state, said Dr. Colwill. OHSU hopes to have 10 more in the coming year. The out-of-state learners will join 32 ob.gyn. residents and 12 fellows who were already in the program, she said.
To ease residents’ integration into an away program, the Ryan Program – along with the American College of Obstetricians and Gynecologists, the Council on Resident Education in Obstetrics and Gynecology, and Innovating Education in Reproductive Health – recently began offering a free, web-based patient-centered abortion education curriculum.
The course supplements in-person clinical training in abortion care and prepares residents traveling and transitioning into another program to begin learning new skills on their first day, AnnaMarie Connolly, MD, ACOG’s chief of education and academic affairs, said in a prepared statement.
Training costs
Residents and their institutions also face additional costs. The home institution that loses a resident for a few weeks to a month has to determine how to cover the care not provided while they are away, Ms. Simonson said. Residents may incur expenses for transportation, housing, food, and other things while out of state.
OHSU covers transportation and housing through its abortion care and training fund, but there are other factors to consider, Dr. Colwill said. For example, the home and host programs have to coordinate licensing, malpractice, and line up rotation dates, she said.
Among other complications, UNM wasn’t able to set up an agreement so that its new resident could participate in a rotation at Planned Parenthood. “We have the clinical volume to accommodate another learner,” Dr. Forbis said. But the program has to balance resources, such as “trying to make sure we don’t have one faculty [member] assigned to too many learners at one time,” she said.
Given the logistic and financial challenges, programs may not be able to ensure that all residents who need abortion training receive it, said Ms. Simonson.
The Ryan Program, for instance, can’t help the more than 100 residency programs in states where abortions are currently illegal, she said.
UNM is trying to partner with specific programs, such as those in the state of Texas where abortion is banned, to train its residents each year, Dr. Forbis said.
OHSU also will look for opportunities to train as many residents as possible, Dr. Colwill said, “but I don’t think we’ll ever be able to fill that gap of 1,000 residents that need this training.”
A version of this article first appeared on Medscape.com.
Nurses maintain more stigma toward pregnant women with OUD
Opioid use disorder among pregnant women continues to rise, and untreated opioid use is associated with complications including preterm delivery, placental abruption, and stillbirth, wrote Alexis Braverman, MD, of the University of Illinois, Chicago, and colleagues. However, many perinatal women who seek care and medications for opioid use disorder (OUD) report stigma that limits their ability to reduce these risks.
In a study published in the American Journal on Addictions , the researchers conducted an anonymous survey of 132 health care workers at six outpatient locations and a main hospital of an urban medical center. The survey was designed to assess attitudes toward pregnant women who were using opioids. The 119 complete responses in the final analysis included 40 nurses and 79 clinicians across ob.gyn., family medicine, and pediatrics. A total of 19 respondents were waivered to prescribe outpatient buprenorphine for OUD.
Nurses were significantly less likely than clinicians to agree that OUD is a chronic illness, to feel sympathy for women who use opioids during pregnancy, and to see pregnancy as an opportunity for behavior change (P = .000, P = .003, and P = .001, respectively).
Overall, family medicine providers and clinicians with 11-20 years of practice experience were significantly more sympathetic to pregnant women who used opioids, compared with providers from other departments and with fewer years of practice (P = .025 and P = .039, respectively).
Providers in pediatrics departments were significantly more likely than those from other departments to agree strongly with feeling anger at pregnant women who use opioids (P = .009), and that these women should not be allowed to parent (P = .013). However, providers in pediatrics were significantly more comfortable than those in other departments with discussing the involvement of social services in patient care (P = .020) and with counseling patients on neonatal opioid withdrawal syndrome, known as NOWS (P = .027).
“We hypothesize that nurses who perform more acute, inpatient work rather than outpatient work may not be exposed as frequently to a patient’s personal progress on their journey with OUD,” and therefore might not be exposed to the rewarding experiences and progress made by patients, the researchers wrote in their discussion.
However, the overall low level of comfort in discussing NOWS and social service involvement across provider groups (one-quarter for pediatrics, one-fifth for ob.gyn, and one-sixth for family medicine) highlights the need for further training in this area, they said.
The findings were limited by several factors, including the potential for responder bias; however, the results identify a need for greater training in stigma reduction and in counseling families on issues related to OUD, the researchers said. More studies are needed to examine attitude changes after the implementation of stigma reduction strategies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Opioid use disorder among pregnant women continues to rise, and untreated opioid use is associated with complications including preterm delivery, placental abruption, and stillbirth, wrote Alexis Braverman, MD, of the University of Illinois, Chicago, and colleagues. However, many perinatal women who seek care and medications for opioid use disorder (OUD) report stigma that limits their ability to reduce these risks.
In a study published in the American Journal on Addictions , the researchers conducted an anonymous survey of 132 health care workers at six outpatient locations and a main hospital of an urban medical center. The survey was designed to assess attitudes toward pregnant women who were using opioids. The 119 complete responses in the final analysis included 40 nurses and 79 clinicians across ob.gyn., family medicine, and pediatrics. A total of 19 respondents were waivered to prescribe outpatient buprenorphine for OUD.
Nurses were significantly less likely than clinicians to agree that OUD is a chronic illness, to feel sympathy for women who use opioids during pregnancy, and to see pregnancy as an opportunity for behavior change (P = .000, P = .003, and P = .001, respectively).
Overall, family medicine providers and clinicians with 11-20 years of practice experience were significantly more sympathetic to pregnant women who used opioids, compared with providers from other departments and with fewer years of practice (P = .025 and P = .039, respectively).
Providers in pediatrics departments were significantly more likely than those from other departments to agree strongly with feeling anger at pregnant women who use opioids (P = .009), and that these women should not be allowed to parent (P = .013). However, providers in pediatrics were significantly more comfortable than those in other departments with discussing the involvement of social services in patient care (P = .020) and with counseling patients on neonatal opioid withdrawal syndrome, known as NOWS (P = .027).
“We hypothesize that nurses who perform more acute, inpatient work rather than outpatient work may not be exposed as frequently to a patient’s personal progress on their journey with OUD,” and therefore might not be exposed to the rewarding experiences and progress made by patients, the researchers wrote in their discussion.
However, the overall low level of comfort in discussing NOWS and social service involvement across provider groups (one-quarter for pediatrics, one-fifth for ob.gyn, and one-sixth for family medicine) highlights the need for further training in this area, they said.
The findings were limited by several factors, including the potential for responder bias; however, the results identify a need for greater training in stigma reduction and in counseling families on issues related to OUD, the researchers said. More studies are needed to examine attitude changes after the implementation of stigma reduction strategies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Opioid use disorder among pregnant women continues to rise, and untreated opioid use is associated with complications including preterm delivery, placental abruption, and stillbirth, wrote Alexis Braverman, MD, of the University of Illinois, Chicago, and colleagues. However, many perinatal women who seek care and medications for opioid use disorder (OUD) report stigma that limits their ability to reduce these risks.
In a study published in the American Journal on Addictions , the researchers conducted an anonymous survey of 132 health care workers at six outpatient locations and a main hospital of an urban medical center. The survey was designed to assess attitudes toward pregnant women who were using opioids. The 119 complete responses in the final analysis included 40 nurses and 79 clinicians across ob.gyn., family medicine, and pediatrics. A total of 19 respondents were waivered to prescribe outpatient buprenorphine for OUD.
Nurses were significantly less likely than clinicians to agree that OUD is a chronic illness, to feel sympathy for women who use opioids during pregnancy, and to see pregnancy as an opportunity for behavior change (P = .000, P = .003, and P = .001, respectively).
Overall, family medicine providers and clinicians with 11-20 years of practice experience were significantly more sympathetic to pregnant women who used opioids, compared with providers from other departments and with fewer years of practice (P = .025 and P = .039, respectively).
Providers in pediatrics departments were significantly more likely than those from other departments to agree strongly with feeling anger at pregnant women who use opioids (P = .009), and that these women should not be allowed to parent (P = .013). However, providers in pediatrics were significantly more comfortable than those in other departments with discussing the involvement of social services in patient care (P = .020) and with counseling patients on neonatal opioid withdrawal syndrome, known as NOWS (P = .027).
“We hypothesize that nurses who perform more acute, inpatient work rather than outpatient work may not be exposed as frequently to a patient’s personal progress on their journey with OUD,” and therefore might not be exposed to the rewarding experiences and progress made by patients, the researchers wrote in their discussion.
However, the overall low level of comfort in discussing NOWS and social service involvement across provider groups (one-quarter for pediatrics, one-fifth for ob.gyn, and one-sixth for family medicine) highlights the need for further training in this area, they said.
The findings were limited by several factors, including the potential for responder bias; however, the results identify a need for greater training in stigma reduction and in counseling families on issues related to OUD, the researchers said. More studies are needed to examine attitude changes after the implementation of stigma reduction strategies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE AMERICAN JOURNAL ON ADDICTIONS
New European restrictions on topiramate in pregnancy
While it’s well known that topiramate can cause major congenital malformations and fetal growth restriction when used during pregnancy, recent data also suggest a possibly increased risk for neurodevelopmental disorders when topiramate is used during pregnancy, the EMA said in a statement.
The data include two observational studies that showed children born to mothers with epilepsy and who were exposed to topiramate in the womb may have a two- to threefold higher risk for neurodevelopmental disorders, in particular autism spectrum disorders (ASD), intellectual disability, or attention deficit hyperactivity disorder (ADHD), compared with children born to mothers with epilepsy not taking antiepileptic medication.
For patients using topiramate for the treatment of epilepsy, the PRAC now recommends that the medicine not be used during pregnancy unless no other suitable treatment is available.
The PRAC had also recommended a pregnancy prevention program to avoid exposure of the developing fetus to topiramate. “These measures will inform any woman or girl who is able to have children of the risks of taking topiramate during pregnancy and the need to avoid becoming pregnant while taking topiramate,” the EMA said.
Regardless of indication, the agency said topiramate should be used in women of childbearing age only when the following conditions of the pregnancy prevention program are met:
- A pregnancy test before starting treatment.
- Counseling about the risks of topiramate treatment and the need for highly effective contraception throughout treatment.
- A review of ongoing treatment at least annually by completion of a risk awareness form.
The PRAC recommends that health care professionals ensure women of childbearing age are fully aware of the risks of taking topiramate during pregnancy. The committee noted that alternative treatment options should be considered and the need for topiramate treatment should be reassessed at least annually.
The product information for topiramate-containing medicines will be updated to further highlight the risks for neurodevelopmental disorders and the additional safety measures to be taken.
Patients and health care professionals will be provided with educational materials regarding the risks of using topiramate during pregnancy, and a patient card will be provided to the patient with each medicine package. A visible warning will also be added to the outer packaging of the medicine.
The new PRAC recommendations will be sent to the Coordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh), which will adopt a position.
A version of this article first appeared on Medscape.com.
While it’s well known that topiramate can cause major congenital malformations and fetal growth restriction when used during pregnancy, recent data also suggest a possibly increased risk for neurodevelopmental disorders when topiramate is used during pregnancy, the EMA said in a statement.
The data include two observational studies that showed children born to mothers with epilepsy and who were exposed to topiramate in the womb may have a two- to threefold higher risk for neurodevelopmental disorders, in particular autism spectrum disorders (ASD), intellectual disability, or attention deficit hyperactivity disorder (ADHD), compared with children born to mothers with epilepsy not taking antiepileptic medication.
For patients using topiramate for the treatment of epilepsy, the PRAC now recommends that the medicine not be used during pregnancy unless no other suitable treatment is available.
The PRAC had also recommended a pregnancy prevention program to avoid exposure of the developing fetus to topiramate. “These measures will inform any woman or girl who is able to have children of the risks of taking topiramate during pregnancy and the need to avoid becoming pregnant while taking topiramate,” the EMA said.
Regardless of indication, the agency said topiramate should be used in women of childbearing age only when the following conditions of the pregnancy prevention program are met:
- A pregnancy test before starting treatment.
- Counseling about the risks of topiramate treatment and the need for highly effective contraception throughout treatment.
- A review of ongoing treatment at least annually by completion of a risk awareness form.
The PRAC recommends that health care professionals ensure women of childbearing age are fully aware of the risks of taking topiramate during pregnancy. The committee noted that alternative treatment options should be considered and the need for topiramate treatment should be reassessed at least annually.
The product information for topiramate-containing medicines will be updated to further highlight the risks for neurodevelopmental disorders and the additional safety measures to be taken.
Patients and health care professionals will be provided with educational materials regarding the risks of using topiramate during pregnancy, and a patient card will be provided to the patient with each medicine package. A visible warning will also be added to the outer packaging of the medicine.
The new PRAC recommendations will be sent to the Coordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh), which will adopt a position.
A version of this article first appeared on Medscape.com.
While it’s well known that topiramate can cause major congenital malformations and fetal growth restriction when used during pregnancy, recent data also suggest a possibly increased risk for neurodevelopmental disorders when topiramate is used during pregnancy, the EMA said in a statement.
The data include two observational studies that showed children born to mothers with epilepsy and who were exposed to topiramate in the womb may have a two- to threefold higher risk for neurodevelopmental disorders, in particular autism spectrum disorders (ASD), intellectual disability, or attention deficit hyperactivity disorder (ADHD), compared with children born to mothers with epilepsy not taking antiepileptic medication.
For patients using topiramate for the treatment of epilepsy, the PRAC now recommends that the medicine not be used during pregnancy unless no other suitable treatment is available.
The PRAC had also recommended a pregnancy prevention program to avoid exposure of the developing fetus to topiramate. “These measures will inform any woman or girl who is able to have children of the risks of taking topiramate during pregnancy and the need to avoid becoming pregnant while taking topiramate,” the EMA said.
Regardless of indication, the agency said topiramate should be used in women of childbearing age only when the following conditions of the pregnancy prevention program are met:
- A pregnancy test before starting treatment.
- Counseling about the risks of topiramate treatment and the need for highly effective contraception throughout treatment.
- A review of ongoing treatment at least annually by completion of a risk awareness form.
The PRAC recommends that health care professionals ensure women of childbearing age are fully aware of the risks of taking topiramate during pregnancy. The committee noted that alternative treatment options should be considered and the need for topiramate treatment should be reassessed at least annually.
The product information for topiramate-containing medicines will be updated to further highlight the risks for neurodevelopmental disorders and the additional safety measures to be taken.
Patients and health care professionals will be provided with educational materials regarding the risks of using topiramate during pregnancy, and a patient card will be provided to the patient with each medicine package. A visible warning will also be added to the outer packaging of the medicine.
The new PRAC recommendations will be sent to the Coordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh), which will adopt a position.
A version of this article first appeared on Medscape.com.
MS drugs during pregnancy show no safety signals
AURORA, COLO. – Several drugs for multiple sclerosis (MS) that are contraindicated during pregnancy nevertheless have not shown concerning safety signals in a series of small studies presented as posters at the annual meeting of the Consortium of Multiple Sclerosis Centers. The industry-sponsored research included an assessment of pregnancy and infant outcomes for cladribine, ocrelizumab, ofatumumab, and ozanimod, all of which are not recommended during pregnancy based primarily on minimal data that suggests, but does not confirm, possible teratogenicity.
“When these new medications hit the market, maternal-fetal medicine physicians and obstetricians are left with very scant data on how to counsel patients, and it’s often based on theory, case reports, or animal studies,” said Teodora Kolarova, MD, a maternal-fetal medicine physician at the University of Washington, Seattle, who was not involved in any of the research. “Although these sample sizes seem small, the population they are sampling from – patients with MS who take immunomodulators who then experience a pregnancy – is much smaller than all pregnant patients.”
Taken together, the findings suggest no increased risk of miscarriage or congenital malformation, compared with baseline risk, Dr. Kolarova said.
“As a whole, these studies are overall reassuring with, of course, some caveats, including timing of medication exposure, limited sample size, and limited outcome data,” Dr. Kolarova said. She noted that embryonic organ formation is complete by 10 weeks gestation, by which time an unplanned pregnancy may not have been recognized yet. “In the subset of patients in the studies that were exposed during the first trimester, there was no increase in congenital malformations from a baseline risk of about 2%-3% in the general population, which is helpful for patient counseling.”
Counseling during the childbearing years
That kind of counseling is important yet absent for many people capable of pregnancy, suggests separate research also presented at the conference by Suma Shah, MD, an associate professor of neurology at Duke University, Durham, N.C. Dr. Shah gave 13-question surveys to female MS patients of all ages at her institution and presented an analysis of data from 38 completed surveys. Among those taking disease-modifying therapies, their medications included ocrelizumab, rituximab, teriflunomide, fingolimod, fumarates, interferons, natalizumab, and cladribine.
“MS disproportionately impacts women among 20 to 40 years, and that’s a really big part of their childbearing years when there are big decisions being made about whether they’re going to choose to grow family or not,” said Dr. Shah. The average age of those who completed the survey was 44. Dr. Shah noted that a lot of research has looked at the safety of older disease-modifying agents in pregnancy, but that information doesn’t appear to be filtering down to patients. “What I really wanted to look at is what do our parent patients understand about whether or not they can even think about pregnancy – and there’s a lot of work to be done.”
Just under a third of survey respondents said they did not have as many children as they would like, and a quarter said they were told they couldn’t have children if they had a diagnosis of MS.
“That was a little heartbreaking to hear because that’s not the truth,” Dr. Shah said. She said it’s necessary to have a more detailed conversation looking at tailored decisions for patients. “Both of those things – patients not being able to grow their family to the number that they desire, and not feeling like they can grow a family – I would think in 2023 we would have come farther than that, and there’s still a lot of room there to improve.”
She advised clinicians not to assume that MS patients know what their options are regarding family planning. “There’s still a lot of room for conversations,” she said. She also explicitly recommends discussing family planning and pregnancy planning with every patient, no matter their gender, early and often.
Cladribine shows no miscarriage, malformations
Dr. Kolarova noted that one of the studies, on cladribine, had a fairly robust sample size with its 180 pregnancy exposures. In that study, led by Kerstin Hellwig, MD, of Ruhr University in Bochum, Germany, data came from the global surveillance program MAPLE-MS, established to assess cladribine effects on pregnancy and infant outcomes. The researchers analyzed data from 76 mothers and 9 fathers who, at any time from 2017 to 2022, were taking cladribine during pregnancy or up to 6 months before pregnancy. Outcomes included live birth, miscarriage, stillbirth, elective abortion, ectopic pregnancy, and major congenital anomalies.
Just over half the mothers (53.9%) were exposed before pregnancy, and about a quarter (26.3%) were exposed during the first trimester. The timing was unknown for most of the other mothers (18.4%). Among the fathers, two-thirds (66.7%) were exposed before pregnancy, and one-third had unknown timing.
Among the 180 pregnancies in the maternal cohort, 42.2% had known outcomes. Nearly half the women (48.7%) taking cladribine had live births, 28.9% had elective abortions, and 21.1% had miscarriages. Only 9 of the 22 pregnancies in the paternal cohort had known outcomes, which included 88.9% live births and 11.1% miscarriages. None of the pregnancies resulted in stillbirth or in a live birth with major congenital anomalies.
”Robust conclusions cannot be made about the risks of adverse pregnancy outcomes with cladribine tablets, but no increase has been signaled thus far,” the researchers reported. ”It is necessary to counsel patients to prevent pregnancy and to use effective contraception during cladribine tablets intake and for at least 6 months after the last cladribine tablet intake in each treatment year.”
Emily Evans, MD, MBE, medical director at U.S. Neurology and Immunology in Rockland, Mass., speaking on behalf of the findings, said they were fairly encouraging.
“Of course, we don’t encourage patients to get pregnant within 6 months of their last dose of cladribine tablets,” Dr. Evans said, but “within those individuals who have gotten pregnant within 6 months of their last dose of cladribine, or who have fathered a child within 6 months of their last dose of cladribine tablets, we’re seeing overall encouraging outcomes. We’re specifically not seeing any differences in the rates of spontaneous abortions, and we’re not seeing any differences in the rates of congenital malformations.”
Ocrelizumab and ofatumumab: No infections so far
Current recommendations for ocrelizumab are to avoid pregnancy for 6 months after the last infusion and stop any breastfeeding during therapy. Yet these recommendations are only because of insufficient data rather than evidence of risk, according to Lana Zhovtis Ryerson, MD, of the NYU Multiple Sclerosis Comprehensive Care Center in New York. She and her colleagues identified all women of childbearing age who had received ocrelizumab within 1 year of pregnancy at their NYU institution. A retrospective chart review found 18 women, with an average age of 35, an average 11 years of an MS diagnosis, and an average 11 months taking ocrelizumab.
Among the 18 pregnancies, four women had a first trimester miscarriage, one had a second trimester miscarriage, and one had an abortion. The miscarriage rate could have been partly influenced by the older maternal population, the authors noted. Of the remaining 12 live births, one infant was premature at 34 weeks, and three infants stayed in NICU but were discharged within 2 weeks.
One patient experienced an MS relapse postpartum, despite receiving ocrelizumab within 45 days of delivery. Of the 16 women who agreed to participate in a Pregnancy Assessment Monitoring System (PRAMS) developed by the CDC, two women chose to breastfeed, and seven said their neurologist recommended against breastfeeding. None of the children’s pediatricians advised delaying vaccinations.
“This small sample observational study has not identified a potential additional risk with ocrelizumab for an adverse pregnancy outcome,” the authors concluded, but they added that ongoing studies, MINORE and SOPRANINO, can help guide future recommendations.
Though still limited, slightly more data exists on ofatumumab during pregnancy, including transient B-cell depletion and lymphopenia in infants whose mothers received anti-CD20 antibodies during pregnancy. However, research has found minimal IgG transfer in the first trimester, though it begins rising in the second trimester, and in utero ofatumumab exposure did not lead to any maternal toxicity or adverse prenatal or postnatal developmental effects in cynomolgus monkeys.
Riley Love, MD, of the University of California, San Francisco, Weill Institute for Neuroscience, and her colleagues both prospectively and retrospectively examined pregnancy and infant outcomes for up to 1 year post partum in women with MS who took ofatumumab during pregnancy or in the 6 months leading up to pregnancy. Their population included 104 prospective cases, most of which (84%) included first trimester exposure, and 14 retrospective cases. One in five of the prospective cases occurred during a clinical trial, while the remaining 80% occurred in postmarketing surveillance.
The prospectively followed women were an average 32 years old and were an average 7 weeks pregnant at the time of reporting. Among the 106 fetuses (including two twin pregnancies), only 30 outcomes had data at the cutoff time, including 16 live births, 9 abortions, and 5 miscarriages. None of the live births had congenital anomalies or serious infections. Another 30 pregnancies were lost to follow-up, and 46 were ongoing.
In the 14 retrospective cases, 57% of women were exposed in the first trimester, and 43% were exposed leading up to pregnancy. Half the cases occurred during clinical trials, and half in postmarketing surveillance. The women were an average 32 years old and were an average 10 weeks pregnant at reporting. Among the 14 pregnancies, nine were miscarriages, one was aborted, and four were born live with no congenital anomalies.
The authors did not draw any conclusions from the findings; they cited too little data and an ongoing study by Novartis to investigate ofatumumab in pregnancy.
“Therapies such as ofatumumab and ocrelizumab can lead to increased risk of infection due to transient B-cell depletion in neonates, but the two studies looking at this did not demonstrate increased infectious morbidity for these infants,” Dr. Kolarova said. “As with all poster presentations, I look forward to reading the full papers once they are published as they will often include a lot more detail about when during pregnancy medication exposure occurred and more detailed outcome data that was assessed.”
Ozanimod outcomes within general population’s ‘expected ranges’
The final study looked at outcomes of pregnancies in people taking ozanimod and in the partners of people taking ozanimod in a clinical trial setting. The findings show low rates of miscarriage, preterm birth, and congenital anomalies that the authors concluded were within the typical range expected for the general population.
“While pregnancy should be avoided when taking and for 3 months after stopping ozanimod to allow for drug elimination, there is no evidence to date of increased occurrence of adverse pregnancy outcomes with ozanimod exposure during early pregnancy,” wrote Anthony Krakovich, of Bristol Myers Squibb in Princeton, N.J., and his associates.
Ozanimod is an oral sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator whose therapeutic mechanism is not fully understood “but may involve the reduction of lymphocyte migration into the central nervous system and intestine,” the authors wrote. S1P receptors are involved in vascular formation during embryogenesis, and animal studies in rats and rabbits have shown toxicity to the embryo and fetus from S1P receptor modulators, including death and malformations. S1P receptor modulator labels therefore note potential fetal risk and the need for effective contraception while taking the drug.
The study prospectively tracked clinical trial participants taking ozanimod as healthy volunteers or for relapsing MS, ulcerative colitis, or Crohn’s disease. Most of the participants who became pregnant (73%) had relapsing MS, while 18% had ulcerative colitis and 8% had Crohn’s disease.
In female patients receiving ozanimod, 78 pregnancies resulted in 12 miscarriages (including one twin), 15 abortions, and 42 live births, with 6 pregnancies ongoing at the time of reporting and no data available for the remaining 4 pregnancies. Among the 42 live births, 4 were premature but otherwise healthy, 1 had a duplex kidney, and the other 37 infants were typical with no apparent health concerns. These rates of miscarriage, preterm birth, and congenital anomalies were within the expected ranges for the general population, the researchers wrote.
The researchers also assessed pregnancy outcomes for partners of male participants taking ozanimod. The 29 partner pregnancies resulted in 21 live births and one miscarriage, with one pregnancy ongoing and no information available for the other seven. The live births included 5 premature infants (including twins), 13 typical and healthy infants, 1 with Hirschsprung’s disease, 1 with a congenital hydrocele, and 1 with a partial atrioventricular septal defect. Again, the researchers concluded that these rates were within the typical range for the general population and that “no teratogenicity was observed.”
“We often encourage patients with MS, regardless of disease activity and therapies, to seek preconception evaluations with Maternal-Fetal Medicine and their neurologists in order to make plans for pregnancy and postpartum care,” Dr. Kolarova said. “That being said, access to subspecialized health care is not available to all, and pregnancy prior to such consultation does occur. These studies provide novel information that we have not had access to in the past and can improve patient counseling regarding their risks and options.”
The study on cladribine was funded by Merck KGaA, at which two authors are employed. Dr. Hellwig reported consulting, speaker, and/or research support from Bayer, Biogen, Teva, Novartis, Roche, Sanofi, Schering Healthcare, Serono, and Merck, and one author is a former employee of EMD Serono. The study on ocrelizumab was funded by Genentech. Dr. Zhovtis Ryerson reported personal fees from Biogen, Genentech, and Novartis, and research grants from Biogen, Genentech, and CMSC. The other authors had no disclosures. The study on ofatumumab was funded by Novartis. Dr. Bove has received research funds from Biogen, Novartis, and Roche Genentech, and consulting fees from EMD Serono, Horizon, Janssen, and TG Therapeutics; she has an ownership interest in Global Consult MD. Five authors are Novartis employees. Her coauthors, including Dr. Hellwig, reported advisory, consulting, research, speaking, or traveling fees from Alexion, Bayer, Biogen, Celgene BMS, EMD Serono, Horizon, Janssen, Lundbeck, Merck, Pfizer, Roche Genentech, Sanofi Genzyme, Schering Healthcare, Teva, TG Therapeutics, and Novartis. The study on ozanimod was funded by Bristol Myers Squibb. Dr. Krakovich and another author are employees and/or shareholders of Bristol Myers Squibb. The other authors reported consulting, speaking, advisory board, and/or research fees from AbbVie, Almirall, Arena, Biogen, Boehringer Ingelhei, Celgene, Celltrion, EXCEMED, Falk Benelux, Ferring, Forward Pharma, Genentech, Genzyme, Gilead, Janssen, Lilly, Merck, Novartis, Ono Pharma, Pfizer, Prometheus Labs, Protagonist, Roche, Sanofi, Synthon, Takeda, and Teva. Dr. Kolarova had no disclosures. Dr. Shah has received research support from Biogen and VeraSci.
AURORA, COLO. – Several drugs for multiple sclerosis (MS) that are contraindicated during pregnancy nevertheless have not shown concerning safety signals in a series of small studies presented as posters at the annual meeting of the Consortium of Multiple Sclerosis Centers. The industry-sponsored research included an assessment of pregnancy and infant outcomes for cladribine, ocrelizumab, ofatumumab, and ozanimod, all of which are not recommended during pregnancy based primarily on minimal data that suggests, but does not confirm, possible teratogenicity.
“When these new medications hit the market, maternal-fetal medicine physicians and obstetricians are left with very scant data on how to counsel patients, and it’s often based on theory, case reports, or animal studies,” said Teodora Kolarova, MD, a maternal-fetal medicine physician at the University of Washington, Seattle, who was not involved in any of the research. “Although these sample sizes seem small, the population they are sampling from – patients with MS who take immunomodulators who then experience a pregnancy – is much smaller than all pregnant patients.”
Taken together, the findings suggest no increased risk of miscarriage or congenital malformation, compared with baseline risk, Dr. Kolarova said.
“As a whole, these studies are overall reassuring with, of course, some caveats, including timing of medication exposure, limited sample size, and limited outcome data,” Dr. Kolarova said. She noted that embryonic organ formation is complete by 10 weeks gestation, by which time an unplanned pregnancy may not have been recognized yet. “In the subset of patients in the studies that were exposed during the first trimester, there was no increase in congenital malformations from a baseline risk of about 2%-3% in the general population, which is helpful for patient counseling.”
Counseling during the childbearing years
That kind of counseling is important yet absent for many people capable of pregnancy, suggests separate research also presented at the conference by Suma Shah, MD, an associate professor of neurology at Duke University, Durham, N.C. Dr. Shah gave 13-question surveys to female MS patients of all ages at her institution and presented an analysis of data from 38 completed surveys. Among those taking disease-modifying therapies, their medications included ocrelizumab, rituximab, teriflunomide, fingolimod, fumarates, interferons, natalizumab, and cladribine.
“MS disproportionately impacts women among 20 to 40 years, and that’s a really big part of their childbearing years when there are big decisions being made about whether they’re going to choose to grow family or not,” said Dr. Shah. The average age of those who completed the survey was 44. Dr. Shah noted that a lot of research has looked at the safety of older disease-modifying agents in pregnancy, but that information doesn’t appear to be filtering down to patients. “What I really wanted to look at is what do our parent patients understand about whether or not they can even think about pregnancy – and there’s a lot of work to be done.”
Just under a third of survey respondents said they did not have as many children as they would like, and a quarter said they were told they couldn’t have children if they had a diagnosis of MS.
“That was a little heartbreaking to hear because that’s not the truth,” Dr. Shah said. She said it’s necessary to have a more detailed conversation looking at tailored decisions for patients. “Both of those things – patients not being able to grow their family to the number that they desire, and not feeling like they can grow a family – I would think in 2023 we would have come farther than that, and there’s still a lot of room there to improve.”
She advised clinicians not to assume that MS patients know what their options are regarding family planning. “There’s still a lot of room for conversations,” she said. She also explicitly recommends discussing family planning and pregnancy planning with every patient, no matter their gender, early and often.
Cladribine shows no miscarriage, malformations
Dr. Kolarova noted that one of the studies, on cladribine, had a fairly robust sample size with its 180 pregnancy exposures. In that study, led by Kerstin Hellwig, MD, of Ruhr University in Bochum, Germany, data came from the global surveillance program MAPLE-MS, established to assess cladribine effects on pregnancy and infant outcomes. The researchers analyzed data from 76 mothers and 9 fathers who, at any time from 2017 to 2022, were taking cladribine during pregnancy or up to 6 months before pregnancy. Outcomes included live birth, miscarriage, stillbirth, elective abortion, ectopic pregnancy, and major congenital anomalies.
Just over half the mothers (53.9%) were exposed before pregnancy, and about a quarter (26.3%) were exposed during the first trimester. The timing was unknown for most of the other mothers (18.4%). Among the fathers, two-thirds (66.7%) were exposed before pregnancy, and one-third had unknown timing.
Among the 180 pregnancies in the maternal cohort, 42.2% had known outcomes. Nearly half the women (48.7%) taking cladribine had live births, 28.9% had elective abortions, and 21.1% had miscarriages. Only 9 of the 22 pregnancies in the paternal cohort had known outcomes, which included 88.9% live births and 11.1% miscarriages. None of the pregnancies resulted in stillbirth or in a live birth with major congenital anomalies.
”Robust conclusions cannot be made about the risks of adverse pregnancy outcomes with cladribine tablets, but no increase has been signaled thus far,” the researchers reported. ”It is necessary to counsel patients to prevent pregnancy and to use effective contraception during cladribine tablets intake and for at least 6 months after the last cladribine tablet intake in each treatment year.”
Emily Evans, MD, MBE, medical director at U.S. Neurology and Immunology in Rockland, Mass., speaking on behalf of the findings, said they were fairly encouraging.
“Of course, we don’t encourage patients to get pregnant within 6 months of their last dose of cladribine tablets,” Dr. Evans said, but “within those individuals who have gotten pregnant within 6 months of their last dose of cladribine, or who have fathered a child within 6 months of their last dose of cladribine tablets, we’re seeing overall encouraging outcomes. We’re specifically not seeing any differences in the rates of spontaneous abortions, and we’re not seeing any differences in the rates of congenital malformations.”
Ocrelizumab and ofatumumab: No infections so far
Current recommendations for ocrelizumab are to avoid pregnancy for 6 months after the last infusion and stop any breastfeeding during therapy. Yet these recommendations are only because of insufficient data rather than evidence of risk, according to Lana Zhovtis Ryerson, MD, of the NYU Multiple Sclerosis Comprehensive Care Center in New York. She and her colleagues identified all women of childbearing age who had received ocrelizumab within 1 year of pregnancy at their NYU institution. A retrospective chart review found 18 women, with an average age of 35, an average 11 years of an MS diagnosis, and an average 11 months taking ocrelizumab.
Among the 18 pregnancies, four women had a first trimester miscarriage, one had a second trimester miscarriage, and one had an abortion. The miscarriage rate could have been partly influenced by the older maternal population, the authors noted. Of the remaining 12 live births, one infant was premature at 34 weeks, and three infants stayed in NICU but were discharged within 2 weeks.
One patient experienced an MS relapse postpartum, despite receiving ocrelizumab within 45 days of delivery. Of the 16 women who agreed to participate in a Pregnancy Assessment Monitoring System (PRAMS) developed by the CDC, two women chose to breastfeed, and seven said their neurologist recommended against breastfeeding. None of the children’s pediatricians advised delaying vaccinations.
“This small sample observational study has not identified a potential additional risk with ocrelizumab for an adverse pregnancy outcome,” the authors concluded, but they added that ongoing studies, MINORE and SOPRANINO, can help guide future recommendations.
Though still limited, slightly more data exists on ofatumumab during pregnancy, including transient B-cell depletion and lymphopenia in infants whose mothers received anti-CD20 antibodies during pregnancy. However, research has found minimal IgG transfer in the first trimester, though it begins rising in the second trimester, and in utero ofatumumab exposure did not lead to any maternal toxicity or adverse prenatal or postnatal developmental effects in cynomolgus monkeys.
Riley Love, MD, of the University of California, San Francisco, Weill Institute for Neuroscience, and her colleagues both prospectively and retrospectively examined pregnancy and infant outcomes for up to 1 year post partum in women with MS who took ofatumumab during pregnancy or in the 6 months leading up to pregnancy. Their population included 104 prospective cases, most of which (84%) included first trimester exposure, and 14 retrospective cases. One in five of the prospective cases occurred during a clinical trial, while the remaining 80% occurred in postmarketing surveillance.
The prospectively followed women were an average 32 years old and were an average 7 weeks pregnant at the time of reporting. Among the 106 fetuses (including two twin pregnancies), only 30 outcomes had data at the cutoff time, including 16 live births, 9 abortions, and 5 miscarriages. None of the live births had congenital anomalies or serious infections. Another 30 pregnancies were lost to follow-up, and 46 were ongoing.
In the 14 retrospective cases, 57% of women were exposed in the first trimester, and 43% were exposed leading up to pregnancy. Half the cases occurred during clinical trials, and half in postmarketing surveillance. The women were an average 32 years old and were an average 10 weeks pregnant at reporting. Among the 14 pregnancies, nine were miscarriages, one was aborted, and four were born live with no congenital anomalies.
The authors did not draw any conclusions from the findings; they cited too little data and an ongoing study by Novartis to investigate ofatumumab in pregnancy.
“Therapies such as ofatumumab and ocrelizumab can lead to increased risk of infection due to transient B-cell depletion in neonates, but the two studies looking at this did not demonstrate increased infectious morbidity for these infants,” Dr. Kolarova said. “As with all poster presentations, I look forward to reading the full papers once they are published as they will often include a lot more detail about when during pregnancy medication exposure occurred and more detailed outcome data that was assessed.”
Ozanimod outcomes within general population’s ‘expected ranges’
The final study looked at outcomes of pregnancies in people taking ozanimod and in the partners of people taking ozanimod in a clinical trial setting. The findings show low rates of miscarriage, preterm birth, and congenital anomalies that the authors concluded were within the typical range expected for the general population.
“While pregnancy should be avoided when taking and for 3 months after stopping ozanimod to allow for drug elimination, there is no evidence to date of increased occurrence of adverse pregnancy outcomes with ozanimod exposure during early pregnancy,” wrote Anthony Krakovich, of Bristol Myers Squibb in Princeton, N.J., and his associates.
Ozanimod is an oral sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator whose therapeutic mechanism is not fully understood “but may involve the reduction of lymphocyte migration into the central nervous system and intestine,” the authors wrote. S1P receptors are involved in vascular formation during embryogenesis, and animal studies in rats and rabbits have shown toxicity to the embryo and fetus from S1P receptor modulators, including death and malformations. S1P receptor modulator labels therefore note potential fetal risk and the need for effective contraception while taking the drug.
The study prospectively tracked clinical trial participants taking ozanimod as healthy volunteers or for relapsing MS, ulcerative colitis, or Crohn’s disease. Most of the participants who became pregnant (73%) had relapsing MS, while 18% had ulcerative colitis and 8% had Crohn’s disease.
In female patients receiving ozanimod, 78 pregnancies resulted in 12 miscarriages (including one twin), 15 abortions, and 42 live births, with 6 pregnancies ongoing at the time of reporting and no data available for the remaining 4 pregnancies. Among the 42 live births, 4 were premature but otherwise healthy, 1 had a duplex kidney, and the other 37 infants were typical with no apparent health concerns. These rates of miscarriage, preterm birth, and congenital anomalies were within the expected ranges for the general population, the researchers wrote.
The researchers also assessed pregnancy outcomes for partners of male participants taking ozanimod. The 29 partner pregnancies resulted in 21 live births and one miscarriage, with one pregnancy ongoing and no information available for the other seven. The live births included 5 premature infants (including twins), 13 typical and healthy infants, 1 with Hirschsprung’s disease, 1 with a congenital hydrocele, and 1 with a partial atrioventricular septal defect. Again, the researchers concluded that these rates were within the typical range for the general population and that “no teratogenicity was observed.”
“We often encourage patients with MS, regardless of disease activity and therapies, to seek preconception evaluations with Maternal-Fetal Medicine and their neurologists in order to make plans for pregnancy and postpartum care,” Dr. Kolarova said. “That being said, access to subspecialized health care is not available to all, and pregnancy prior to such consultation does occur. These studies provide novel information that we have not had access to in the past and can improve patient counseling regarding their risks and options.”
The study on cladribine was funded by Merck KGaA, at which two authors are employed. Dr. Hellwig reported consulting, speaker, and/or research support from Bayer, Biogen, Teva, Novartis, Roche, Sanofi, Schering Healthcare, Serono, and Merck, and one author is a former employee of EMD Serono. The study on ocrelizumab was funded by Genentech. Dr. Zhovtis Ryerson reported personal fees from Biogen, Genentech, and Novartis, and research grants from Biogen, Genentech, and CMSC. The other authors had no disclosures. The study on ofatumumab was funded by Novartis. Dr. Bove has received research funds from Biogen, Novartis, and Roche Genentech, and consulting fees from EMD Serono, Horizon, Janssen, and TG Therapeutics; she has an ownership interest in Global Consult MD. Five authors are Novartis employees. Her coauthors, including Dr. Hellwig, reported advisory, consulting, research, speaking, or traveling fees from Alexion, Bayer, Biogen, Celgene BMS, EMD Serono, Horizon, Janssen, Lundbeck, Merck, Pfizer, Roche Genentech, Sanofi Genzyme, Schering Healthcare, Teva, TG Therapeutics, and Novartis. The study on ozanimod was funded by Bristol Myers Squibb. Dr. Krakovich and another author are employees and/or shareholders of Bristol Myers Squibb. The other authors reported consulting, speaking, advisory board, and/or research fees from AbbVie, Almirall, Arena, Biogen, Boehringer Ingelhei, Celgene, Celltrion, EXCEMED, Falk Benelux, Ferring, Forward Pharma, Genentech, Genzyme, Gilead, Janssen, Lilly, Merck, Novartis, Ono Pharma, Pfizer, Prometheus Labs, Protagonist, Roche, Sanofi, Synthon, Takeda, and Teva. Dr. Kolarova had no disclosures. Dr. Shah has received research support from Biogen and VeraSci.
AURORA, COLO. – Several drugs for multiple sclerosis (MS) that are contraindicated during pregnancy nevertheless have not shown concerning safety signals in a series of small studies presented as posters at the annual meeting of the Consortium of Multiple Sclerosis Centers. The industry-sponsored research included an assessment of pregnancy and infant outcomes for cladribine, ocrelizumab, ofatumumab, and ozanimod, all of which are not recommended during pregnancy based primarily on minimal data that suggests, but does not confirm, possible teratogenicity.
“When these new medications hit the market, maternal-fetal medicine physicians and obstetricians are left with very scant data on how to counsel patients, and it’s often based on theory, case reports, or animal studies,” said Teodora Kolarova, MD, a maternal-fetal medicine physician at the University of Washington, Seattle, who was not involved in any of the research. “Although these sample sizes seem small, the population they are sampling from – patients with MS who take immunomodulators who then experience a pregnancy – is much smaller than all pregnant patients.”
Taken together, the findings suggest no increased risk of miscarriage or congenital malformation, compared with baseline risk, Dr. Kolarova said.
“As a whole, these studies are overall reassuring with, of course, some caveats, including timing of medication exposure, limited sample size, and limited outcome data,” Dr. Kolarova said. She noted that embryonic organ formation is complete by 10 weeks gestation, by which time an unplanned pregnancy may not have been recognized yet. “In the subset of patients in the studies that were exposed during the first trimester, there was no increase in congenital malformations from a baseline risk of about 2%-3% in the general population, which is helpful for patient counseling.”
Counseling during the childbearing years
That kind of counseling is important yet absent for many people capable of pregnancy, suggests separate research also presented at the conference by Suma Shah, MD, an associate professor of neurology at Duke University, Durham, N.C. Dr. Shah gave 13-question surveys to female MS patients of all ages at her institution and presented an analysis of data from 38 completed surveys. Among those taking disease-modifying therapies, their medications included ocrelizumab, rituximab, teriflunomide, fingolimod, fumarates, interferons, natalizumab, and cladribine.
“MS disproportionately impacts women among 20 to 40 years, and that’s a really big part of their childbearing years when there are big decisions being made about whether they’re going to choose to grow family or not,” said Dr. Shah. The average age of those who completed the survey was 44. Dr. Shah noted that a lot of research has looked at the safety of older disease-modifying agents in pregnancy, but that information doesn’t appear to be filtering down to patients. “What I really wanted to look at is what do our parent patients understand about whether or not they can even think about pregnancy – and there’s a lot of work to be done.”
Just under a third of survey respondents said they did not have as many children as they would like, and a quarter said they were told they couldn’t have children if they had a diagnosis of MS.
“That was a little heartbreaking to hear because that’s not the truth,” Dr. Shah said. She said it’s necessary to have a more detailed conversation looking at tailored decisions for patients. “Both of those things – patients not being able to grow their family to the number that they desire, and not feeling like they can grow a family – I would think in 2023 we would have come farther than that, and there’s still a lot of room there to improve.”
She advised clinicians not to assume that MS patients know what their options are regarding family planning. “There’s still a lot of room for conversations,” she said. She also explicitly recommends discussing family planning and pregnancy planning with every patient, no matter their gender, early and often.
Cladribine shows no miscarriage, malformations
Dr. Kolarova noted that one of the studies, on cladribine, had a fairly robust sample size with its 180 pregnancy exposures. In that study, led by Kerstin Hellwig, MD, of Ruhr University in Bochum, Germany, data came from the global surveillance program MAPLE-MS, established to assess cladribine effects on pregnancy and infant outcomes. The researchers analyzed data from 76 mothers and 9 fathers who, at any time from 2017 to 2022, were taking cladribine during pregnancy or up to 6 months before pregnancy. Outcomes included live birth, miscarriage, stillbirth, elective abortion, ectopic pregnancy, and major congenital anomalies.
Just over half the mothers (53.9%) were exposed before pregnancy, and about a quarter (26.3%) were exposed during the first trimester. The timing was unknown for most of the other mothers (18.4%). Among the fathers, two-thirds (66.7%) were exposed before pregnancy, and one-third had unknown timing.
Among the 180 pregnancies in the maternal cohort, 42.2% had known outcomes. Nearly half the women (48.7%) taking cladribine had live births, 28.9% had elective abortions, and 21.1% had miscarriages. Only 9 of the 22 pregnancies in the paternal cohort had known outcomes, which included 88.9% live births and 11.1% miscarriages. None of the pregnancies resulted in stillbirth or in a live birth with major congenital anomalies.
”Robust conclusions cannot be made about the risks of adverse pregnancy outcomes with cladribine tablets, but no increase has been signaled thus far,” the researchers reported. ”It is necessary to counsel patients to prevent pregnancy and to use effective contraception during cladribine tablets intake and for at least 6 months after the last cladribine tablet intake in each treatment year.”
Emily Evans, MD, MBE, medical director at U.S. Neurology and Immunology in Rockland, Mass., speaking on behalf of the findings, said they were fairly encouraging.
“Of course, we don’t encourage patients to get pregnant within 6 months of their last dose of cladribine tablets,” Dr. Evans said, but “within those individuals who have gotten pregnant within 6 months of their last dose of cladribine, or who have fathered a child within 6 months of their last dose of cladribine tablets, we’re seeing overall encouraging outcomes. We’re specifically not seeing any differences in the rates of spontaneous abortions, and we’re not seeing any differences in the rates of congenital malformations.”
Ocrelizumab and ofatumumab: No infections so far
Current recommendations for ocrelizumab are to avoid pregnancy for 6 months after the last infusion and stop any breastfeeding during therapy. Yet these recommendations are only because of insufficient data rather than evidence of risk, according to Lana Zhovtis Ryerson, MD, of the NYU Multiple Sclerosis Comprehensive Care Center in New York. She and her colleagues identified all women of childbearing age who had received ocrelizumab within 1 year of pregnancy at their NYU institution. A retrospective chart review found 18 women, with an average age of 35, an average 11 years of an MS diagnosis, and an average 11 months taking ocrelizumab.
Among the 18 pregnancies, four women had a first trimester miscarriage, one had a second trimester miscarriage, and one had an abortion. The miscarriage rate could have been partly influenced by the older maternal population, the authors noted. Of the remaining 12 live births, one infant was premature at 34 weeks, and three infants stayed in NICU but were discharged within 2 weeks.
One patient experienced an MS relapse postpartum, despite receiving ocrelizumab within 45 days of delivery. Of the 16 women who agreed to participate in a Pregnancy Assessment Monitoring System (PRAMS) developed by the CDC, two women chose to breastfeed, and seven said their neurologist recommended against breastfeeding. None of the children’s pediatricians advised delaying vaccinations.
“This small sample observational study has not identified a potential additional risk with ocrelizumab for an adverse pregnancy outcome,” the authors concluded, but they added that ongoing studies, MINORE and SOPRANINO, can help guide future recommendations.
Though still limited, slightly more data exists on ofatumumab during pregnancy, including transient B-cell depletion and lymphopenia in infants whose mothers received anti-CD20 antibodies during pregnancy. However, research has found minimal IgG transfer in the first trimester, though it begins rising in the second trimester, and in utero ofatumumab exposure did not lead to any maternal toxicity or adverse prenatal or postnatal developmental effects in cynomolgus monkeys.
Riley Love, MD, of the University of California, San Francisco, Weill Institute for Neuroscience, and her colleagues both prospectively and retrospectively examined pregnancy and infant outcomes for up to 1 year post partum in women with MS who took ofatumumab during pregnancy or in the 6 months leading up to pregnancy. Their population included 104 prospective cases, most of which (84%) included first trimester exposure, and 14 retrospective cases. One in five of the prospective cases occurred during a clinical trial, while the remaining 80% occurred in postmarketing surveillance.
The prospectively followed women were an average 32 years old and were an average 7 weeks pregnant at the time of reporting. Among the 106 fetuses (including two twin pregnancies), only 30 outcomes had data at the cutoff time, including 16 live births, 9 abortions, and 5 miscarriages. None of the live births had congenital anomalies or serious infections. Another 30 pregnancies were lost to follow-up, and 46 were ongoing.
In the 14 retrospective cases, 57% of women were exposed in the first trimester, and 43% were exposed leading up to pregnancy. Half the cases occurred during clinical trials, and half in postmarketing surveillance. The women were an average 32 years old and were an average 10 weeks pregnant at reporting. Among the 14 pregnancies, nine were miscarriages, one was aborted, and four were born live with no congenital anomalies.
The authors did not draw any conclusions from the findings; they cited too little data and an ongoing study by Novartis to investigate ofatumumab in pregnancy.
“Therapies such as ofatumumab and ocrelizumab can lead to increased risk of infection due to transient B-cell depletion in neonates, but the two studies looking at this did not demonstrate increased infectious morbidity for these infants,” Dr. Kolarova said. “As with all poster presentations, I look forward to reading the full papers once they are published as they will often include a lot more detail about when during pregnancy medication exposure occurred and more detailed outcome data that was assessed.”
Ozanimod outcomes within general population’s ‘expected ranges’
The final study looked at outcomes of pregnancies in people taking ozanimod and in the partners of people taking ozanimod in a clinical trial setting. The findings show low rates of miscarriage, preterm birth, and congenital anomalies that the authors concluded were within the typical range expected for the general population.
“While pregnancy should be avoided when taking and for 3 months after stopping ozanimod to allow for drug elimination, there is no evidence to date of increased occurrence of adverse pregnancy outcomes with ozanimod exposure during early pregnancy,” wrote Anthony Krakovich, of Bristol Myers Squibb in Princeton, N.J., and his associates.
Ozanimod is an oral sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator whose therapeutic mechanism is not fully understood “but may involve the reduction of lymphocyte migration into the central nervous system and intestine,” the authors wrote. S1P receptors are involved in vascular formation during embryogenesis, and animal studies in rats and rabbits have shown toxicity to the embryo and fetus from S1P receptor modulators, including death and malformations. S1P receptor modulator labels therefore note potential fetal risk and the need for effective contraception while taking the drug.
The study prospectively tracked clinical trial participants taking ozanimod as healthy volunteers or for relapsing MS, ulcerative colitis, or Crohn’s disease. Most of the participants who became pregnant (73%) had relapsing MS, while 18% had ulcerative colitis and 8% had Crohn’s disease.
In female patients receiving ozanimod, 78 pregnancies resulted in 12 miscarriages (including one twin), 15 abortions, and 42 live births, with 6 pregnancies ongoing at the time of reporting and no data available for the remaining 4 pregnancies. Among the 42 live births, 4 were premature but otherwise healthy, 1 had a duplex kidney, and the other 37 infants were typical with no apparent health concerns. These rates of miscarriage, preterm birth, and congenital anomalies were within the expected ranges for the general population, the researchers wrote.
The researchers also assessed pregnancy outcomes for partners of male participants taking ozanimod. The 29 partner pregnancies resulted in 21 live births and one miscarriage, with one pregnancy ongoing and no information available for the other seven. The live births included 5 premature infants (including twins), 13 typical and healthy infants, 1 with Hirschsprung’s disease, 1 with a congenital hydrocele, and 1 with a partial atrioventricular septal defect. Again, the researchers concluded that these rates were within the typical range for the general population and that “no teratogenicity was observed.”
“We often encourage patients with MS, regardless of disease activity and therapies, to seek preconception evaluations with Maternal-Fetal Medicine and their neurologists in order to make plans for pregnancy and postpartum care,” Dr. Kolarova said. “That being said, access to subspecialized health care is not available to all, and pregnancy prior to such consultation does occur. These studies provide novel information that we have not had access to in the past and can improve patient counseling regarding their risks and options.”
The study on cladribine was funded by Merck KGaA, at which two authors are employed. Dr. Hellwig reported consulting, speaker, and/or research support from Bayer, Biogen, Teva, Novartis, Roche, Sanofi, Schering Healthcare, Serono, and Merck, and one author is a former employee of EMD Serono. The study on ocrelizumab was funded by Genentech. Dr. Zhovtis Ryerson reported personal fees from Biogen, Genentech, and Novartis, and research grants from Biogen, Genentech, and CMSC. The other authors had no disclosures. The study on ofatumumab was funded by Novartis. Dr. Bove has received research funds from Biogen, Novartis, and Roche Genentech, and consulting fees from EMD Serono, Horizon, Janssen, and TG Therapeutics; she has an ownership interest in Global Consult MD. Five authors are Novartis employees. Her coauthors, including Dr. Hellwig, reported advisory, consulting, research, speaking, or traveling fees from Alexion, Bayer, Biogen, Celgene BMS, EMD Serono, Horizon, Janssen, Lundbeck, Merck, Pfizer, Roche Genentech, Sanofi Genzyme, Schering Healthcare, Teva, TG Therapeutics, and Novartis. The study on ozanimod was funded by Bristol Myers Squibb. Dr. Krakovich and another author are employees and/or shareholders of Bristol Myers Squibb. The other authors reported consulting, speaking, advisory board, and/or research fees from AbbVie, Almirall, Arena, Biogen, Boehringer Ingelhei, Celgene, Celltrion, EXCEMED, Falk Benelux, Ferring, Forward Pharma, Genentech, Genzyme, Gilead, Janssen, Lilly, Merck, Novartis, Ono Pharma, Pfizer, Prometheus Labs, Protagonist, Roche, Sanofi, Synthon, Takeda, and Teva. Dr. Kolarova had no disclosures. Dr. Shah has received research support from Biogen and VeraSci.
FROM CMSC 2023