User login
Opportunities and limits in universal screening for perinatal depression
The American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice recently published a revised opinion on screening for perinatal depression, recommending that “clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms using a standard, validated tool.” The statement adds that “women with current depression or anxiety, a history of perinatal mood disorders, or risk factors for perinatal mood disorders warrant particularly close monitoring, evaluation, and assessment.” A list of validated depression screening tools is included (Obstet. Gynecol. 2015;125:1268-71). In previous iterations, the committee had not recommended formal screening for perinatal depression (referred to as major or minor depressive episodes occurring during pregnancy or during the first 12 months after delivery) and left the utility of screening as an open question to the field.
Noting that screening alone cannot improve clinical outcomes, the ACOG opinion says that it “must be coupled with appropriate follow-up and treatment when indicated,” and – most critically – adds that clinical staff in the practice “should be prepared to initiate medical therapy, refer patients to appropriate health resources when indicated, or both.” The latter recommendation is followed by the statement that “systems should be in place to ensure follow-up for diagnosis and treatment.”
Many states have initiated programs for screening for perinatal depression, which is intuitive given the prevalence of mood and anxiety disorders in women of reproductive age. Unfortunately, to date, there are no data indicating whether screening results in improved outcomes, or what type of treatment women receive as a result of screening; the ACOG opinion notes that definitive evidence on the benefit of screening is “limited.”
In prevalence studies, maternal morbidity associated with untreated perinatal mood and anxiety disorders clearly exceeds the morbidity associated with hemorrhage and pregnancy-induced hypertension, with significant effects on families and children as well. Therefore, even in the absence of an evidence base, there is support for routine screening and for ob.gyns. to initiate treatment and to facilitate referrals to appropriate settings.
In Massachusetts, where I practice, screening is not mandatory but is becoming increasingly popular, and resources to manage those with positive screening results are being developed.
The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms was established to enhance screening for perinatal depression and to provide screening and educational tools, as well as free telephone backup, consultation, and referral service for ob.gyn. practices. MCPAP for Moms is coupled with an extensive community-based perinatal mood and anxiety service network: mental health providers, including social workers; specialized nurses with expertise in perinatal mental health; and support groups for women suffering from perinatal mood and anxiety disorders. The program is new and has promise, although evidence supporting its effectiveness is not yet available.
Some argue that screening and treatment of perinatal depression by nonpsychiatric providers opens up a “Pandora’s box.” But should the box be opened nonetheless?
Obvious problems might include many women with positive screening results not being referred for appropriate treatment or, if referred, receiving incomplete treatment – all very valid concerns. But one could also argue that with a highly prevalent illness that presents during a discrete period of time, the opportunity to screen in the obstetric setting (or in the pediatric setting, a separate topic) is an opportunity to at least help mitigate some of the suffering associated with perinatal depression.
The clinician in the community who will screen these women will need to manage the substantial responsibility of initiating treatment for patients with perinatal depression or referring them for management. The main question following diagnosis of perinatal depression is really not necessarily how “best” to treat a patient with perinatal depression. An evidence base exists supporting efficacy for treatments, including medication and certain psychotherapies. Perhaps the greatest pitfall inherent in an opinion like the one from ACOG relates to the incomplete infrastructure and associated resources in many parts of the country – and in our health care system – needed to accommodate and effectively manage the increasing number of women who will be diagnosed with perinatal mood and anxiety disorders as a consequence of more widespread screening.
Whether community-based ob.gyns. will be comfortable with direct treatment of perinatal psychiatric illness or the extent to which they view this as part of their clinical responsibility remains to be seen. It is possible that they will follow suit, just as primary care physicians became increasingly comfortable prescribing antidepressants in the early 1990s as easy and safe antidepressant treatments became available, particularly for patients with relatively straightforward major depression.
This committee opinion is an incremental advance, compared with previous opinions, and most critically, puts the conversation back on the national scene at an important time, as population health management is becoming an increasingly proximate reality.
The opinion leaves many unanswered questions regarding implementation on a national level, which may be beyond the scope of the committee’s task. But the recommendations, if carried out, will increase the likelihood of mitigating at least some of the substantial suffering associated with a highly prevalent illness.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. To comment, e-mail him at [email protected].
The American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice recently published a revised opinion on screening for perinatal depression, recommending that “clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms using a standard, validated tool.” The statement adds that “women with current depression or anxiety, a history of perinatal mood disorders, or risk factors for perinatal mood disorders warrant particularly close monitoring, evaluation, and assessment.” A list of validated depression screening tools is included (Obstet. Gynecol. 2015;125:1268-71). In previous iterations, the committee had not recommended formal screening for perinatal depression (referred to as major or minor depressive episodes occurring during pregnancy or during the first 12 months after delivery) and left the utility of screening as an open question to the field.
Noting that screening alone cannot improve clinical outcomes, the ACOG opinion says that it “must be coupled with appropriate follow-up and treatment when indicated,” and – most critically – adds that clinical staff in the practice “should be prepared to initiate medical therapy, refer patients to appropriate health resources when indicated, or both.” The latter recommendation is followed by the statement that “systems should be in place to ensure follow-up for diagnosis and treatment.”
Many states have initiated programs for screening for perinatal depression, which is intuitive given the prevalence of mood and anxiety disorders in women of reproductive age. Unfortunately, to date, there are no data indicating whether screening results in improved outcomes, or what type of treatment women receive as a result of screening; the ACOG opinion notes that definitive evidence on the benefit of screening is “limited.”
In prevalence studies, maternal morbidity associated with untreated perinatal mood and anxiety disorders clearly exceeds the morbidity associated with hemorrhage and pregnancy-induced hypertension, with significant effects on families and children as well. Therefore, even in the absence of an evidence base, there is support for routine screening and for ob.gyns. to initiate treatment and to facilitate referrals to appropriate settings.
In Massachusetts, where I practice, screening is not mandatory but is becoming increasingly popular, and resources to manage those with positive screening results are being developed.
The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms was established to enhance screening for perinatal depression and to provide screening and educational tools, as well as free telephone backup, consultation, and referral service for ob.gyn. practices. MCPAP for Moms is coupled with an extensive community-based perinatal mood and anxiety service network: mental health providers, including social workers; specialized nurses with expertise in perinatal mental health; and support groups for women suffering from perinatal mood and anxiety disorders. The program is new and has promise, although evidence supporting its effectiveness is not yet available.
Some argue that screening and treatment of perinatal depression by nonpsychiatric providers opens up a “Pandora’s box.” But should the box be opened nonetheless?
Obvious problems might include many women with positive screening results not being referred for appropriate treatment or, if referred, receiving incomplete treatment – all very valid concerns. But one could also argue that with a highly prevalent illness that presents during a discrete period of time, the opportunity to screen in the obstetric setting (or in the pediatric setting, a separate topic) is an opportunity to at least help mitigate some of the suffering associated with perinatal depression.
The clinician in the community who will screen these women will need to manage the substantial responsibility of initiating treatment for patients with perinatal depression or referring them for management. The main question following diagnosis of perinatal depression is really not necessarily how “best” to treat a patient with perinatal depression. An evidence base exists supporting efficacy for treatments, including medication and certain psychotherapies. Perhaps the greatest pitfall inherent in an opinion like the one from ACOG relates to the incomplete infrastructure and associated resources in many parts of the country – and in our health care system – needed to accommodate and effectively manage the increasing number of women who will be diagnosed with perinatal mood and anxiety disorders as a consequence of more widespread screening.
Whether community-based ob.gyns. will be comfortable with direct treatment of perinatal psychiatric illness or the extent to which they view this as part of their clinical responsibility remains to be seen. It is possible that they will follow suit, just as primary care physicians became increasingly comfortable prescribing antidepressants in the early 1990s as easy and safe antidepressant treatments became available, particularly for patients with relatively straightforward major depression.
This committee opinion is an incremental advance, compared with previous opinions, and most critically, puts the conversation back on the national scene at an important time, as population health management is becoming an increasingly proximate reality.
The opinion leaves many unanswered questions regarding implementation on a national level, which may be beyond the scope of the committee’s task. But the recommendations, if carried out, will increase the likelihood of mitigating at least some of the substantial suffering associated with a highly prevalent illness.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. To comment, e-mail him at [email protected].
The American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice recently published a revised opinion on screening for perinatal depression, recommending that “clinicians screen patients at least once during the perinatal period for depression and anxiety symptoms using a standard, validated tool.” The statement adds that “women with current depression or anxiety, a history of perinatal mood disorders, or risk factors for perinatal mood disorders warrant particularly close monitoring, evaluation, and assessment.” A list of validated depression screening tools is included (Obstet. Gynecol. 2015;125:1268-71). In previous iterations, the committee had not recommended formal screening for perinatal depression (referred to as major or minor depressive episodes occurring during pregnancy or during the first 12 months after delivery) and left the utility of screening as an open question to the field.
Noting that screening alone cannot improve clinical outcomes, the ACOG opinion says that it “must be coupled with appropriate follow-up and treatment when indicated,” and – most critically – adds that clinical staff in the practice “should be prepared to initiate medical therapy, refer patients to appropriate health resources when indicated, or both.” The latter recommendation is followed by the statement that “systems should be in place to ensure follow-up for diagnosis and treatment.”
Many states have initiated programs for screening for perinatal depression, which is intuitive given the prevalence of mood and anxiety disorders in women of reproductive age. Unfortunately, to date, there are no data indicating whether screening results in improved outcomes, or what type of treatment women receive as a result of screening; the ACOG opinion notes that definitive evidence on the benefit of screening is “limited.”
In prevalence studies, maternal morbidity associated with untreated perinatal mood and anxiety disorders clearly exceeds the morbidity associated with hemorrhage and pregnancy-induced hypertension, with significant effects on families and children as well. Therefore, even in the absence of an evidence base, there is support for routine screening and for ob.gyns. to initiate treatment and to facilitate referrals to appropriate settings.
In Massachusetts, where I practice, screening is not mandatory but is becoming increasingly popular, and resources to manage those with positive screening results are being developed.
The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms was established to enhance screening for perinatal depression and to provide screening and educational tools, as well as free telephone backup, consultation, and referral service for ob.gyn. practices. MCPAP for Moms is coupled with an extensive community-based perinatal mood and anxiety service network: mental health providers, including social workers; specialized nurses with expertise in perinatal mental health; and support groups for women suffering from perinatal mood and anxiety disorders. The program is new and has promise, although evidence supporting its effectiveness is not yet available.
Some argue that screening and treatment of perinatal depression by nonpsychiatric providers opens up a “Pandora’s box.” But should the box be opened nonetheless?
Obvious problems might include many women with positive screening results not being referred for appropriate treatment or, if referred, receiving incomplete treatment – all very valid concerns. But one could also argue that with a highly prevalent illness that presents during a discrete period of time, the opportunity to screen in the obstetric setting (or in the pediatric setting, a separate topic) is an opportunity to at least help mitigate some of the suffering associated with perinatal depression.
The clinician in the community who will screen these women will need to manage the substantial responsibility of initiating treatment for patients with perinatal depression or referring them for management. The main question following diagnosis of perinatal depression is really not necessarily how “best” to treat a patient with perinatal depression. An evidence base exists supporting efficacy for treatments, including medication and certain psychotherapies. Perhaps the greatest pitfall inherent in an opinion like the one from ACOG relates to the incomplete infrastructure and associated resources in many parts of the country – and in our health care system – needed to accommodate and effectively manage the increasing number of women who will be diagnosed with perinatal mood and anxiety disorders as a consequence of more widespread screening.
Whether community-based ob.gyns. will be comfortable with direct treatment of perinatal psychiatric illness or the extent to which they view this as part of their clinical responsibility remains to be seen. It is possible that they will follow suit, just as primary care physicians became increasingly comfortable prescribing antidepressants in the early 1990s as easy and safe antidepressant treatments became available, particularly for patients with relatively straightforward major depression.
This committee opinion is an incremental advance, compared with previous opinions, and most critically, puts the conversation back on the national scene at an important time, as population health management is becoming an increasingly proximate reality.
The opinion leaves many unanswered questions regarding implementation on a national level, which may be beyond the scope of the committee’s task. But the recommendations, if carried out, will increase the likelihood of mitigating at least some of the substantial suffering associated with a highly prevalent illness.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. To comment, e-mail him at [email protected].
Q&A with the FDA: Implementing the Pregnancy and Lactation Labeling Rule
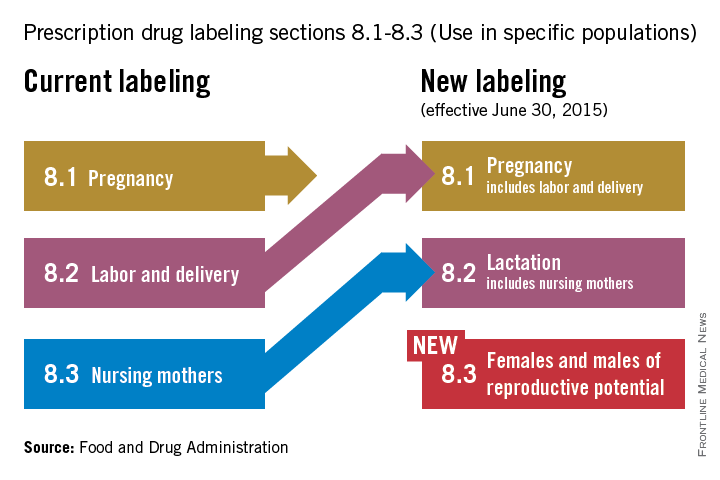
On June 30, pharmaceutical and biological manufacturers will begin implementing the Food and Drug Administration’s revised rules for pregnancy and lactation drug labeling. The Pregnancy and Lactation Labeling Rule (PLLR) amends the Physician Labeling Rule (PLR, issued in 2006) and new labels aimed at providing clearer and more accurate information on drug risks will gradually roll out over the next 5 years.
The new rule – several years in the making – replaces pregnancy categories A, B, C, D, and X with short, integrated summaries that provide physicians with more information for discussing with patients the risks and benefits of a medication. The summaries will be brief and easy to read, and will contain evidence-based information that specifically addresses drug risks for women during pregnancy and lactation, as well as for men and women of reproductive potential. Under the PLLR, the pregnancy subsection of the risk summary will include for the first time information for any drug that has a pregnancy exposure registry.
Dr. John Whyte, director of Professional Affairs and Stakeholder Engagment (PASE) at the FDA Center for Drug Evaluation and Research, answers questions about the new rule from Ob.Gyn. News journalists and our Drugs, Pregnancy & Lactation columnists.
Question: Instead of the traditional letter categories, prescribers will now have to read the pregnancy and lactation subsections of the labeling. What can the agency do to ensure prescribers are reading all of the new contextual information in the drug label?
Answer: The agency has initiated outreach efforts to help prescribers become familiar with the new information in labeling. The most important information is now under a new heading called Risk Summary. This narrative summary replaces the pregnancy letter categories with a summary of information that is known about a product. The new labeling rule explains, based on available information, the potential benefits and risks for the mother, the fetus, and the breastfeeding child. The decision to replace the pregnancy letter categories with the summary paragraph was reached after extensive consultation with experts and stakeholders who were concerned that the traditional letter categories were overly simplistic.
Q: PLLR praises the value of pregnancy exposure registries and mandates that if a registry exists for an approved product, then the label must include the registry’s website address. There is no requirement, however, that industry actually fund these registries. Why did the agency stop short of requiring companies to provide ongoing support for pregnancy exposure registries?
A: FDA has the authority to require the establishment of pregnancy registries when there is a safety concern that would benefit from the collection of data in a postapproval study, or when a particular product will be used by a large number of females of reproductive age. However, FDA does not have the authority to require companies to fund existing pregnancy exposure registries.
Q: How does the FDA plan to review all of the summary statements of available data regarding reproductive safety considering the breadth of drugs in various therapeutic areas that will need to be reviewed?
A: All labeling changes, including the changes required by the PLLR, must be submitted to the FDA for review and approval. Changes to the Pregnancy and Lactation subsections of labeling have been integrated into standard labeling review processes. In addition, the Division of Pediatric and Maternal Health, in the Office of New Drugs, works with the primary review divisions to help coordinate PLLR review processes.
Q: Are the manufacturers of generic drugs expected to “piggyback” the label for a branded molecule with respect to the pregnancy label?
A: Generic drug products (Abbreviated New Drug Applications) are required by regulation to have the “same” labeling as the reference drug listed. When the labeling is revised for the referenced drug, generic drug manufacturers also are required to update their labeling.
Q: In the new system, how will labeling stay up to date when important new data are published on a drug sometimes two to three times a year?
A: When new information becomes available that causes the labeling to be inaccurate, false, or misleading, drug manufacturers are required by regulation to update labeling.
Q: The rule is phased in over time. Drugs already on the market are given more time to switch to the new system. How long will physicians need to deal simultaneously with the old and the new formats?
A: There have been two labeling formats in use for some time as products approved prior to 2001 are not required to conform to either PLLR or PLR. The agency encourages manufacturers to voluntarily convert labeling of older products into PLR format. However, products approved prior to 2001 will be required to remove the pregnancy letter category by June 30, 2018, (3 years after the implementation of the PLLR).
Q: Does the FDA have any plans to address labeling for over-the-counter products in terms of their impact on pregnancy, lactation, and reproductive potential?
A: The PLLR does not apply to over-the-counter products. However, the agency is continually reviewing the safety of products used over the counter, including impacts on pregnancy, lactation, and reproductive potential.
Q: How does the FDA plan to assess over time the usefulness of the new labeling for prescribers and patients and make revisions?
A: The draft guidance was issued concurrently with PLLR. Based on the comments received from the public on the draft, as well as learning from the initial revisions of labeling, the guidance will be revised as needed. Guidance statements issued by FDA are regularly reviewed and revised as needed.
Dr. Whyte, a board-certified internist, is the director of Professional Affairs and Stakeholder Engagement at the FDA. Do you have other questions about the PLLR? Send them to [email protected].
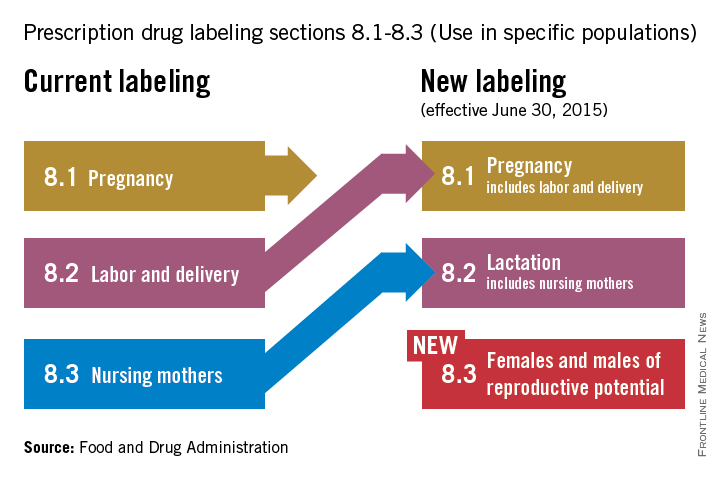
On June 30, pharmaceutical and biological manufacturers will begin implementing the Food and Drug Administration’s revised rules for pregnancy and lactation drug labeling. The Pregnancy and Lactation Labeling Rule (PLLR) amends the Physician Labeling Rule (PLR, issued in 2006) and new labels aimed at providing clearer and more accurate information on drug risks will gradually roll out over the next 5 years.
The new rule – several years in the making – replaces pregnancy categories A, B, C, D, and X with short, integrated summaries that provide physicians with more information for discussing with patients the risks and benefits of a medication. The summaries will be brief and easy to read, and will contain evidence-based information that specifically addresses drug risks for women during pregnancy and lactation, as well as for men and women of reproductive potential. Under the PLLR, the pregnancy subsection of the risk summary will include for the first time information for any drug that has a pregnancy exposure registry.

Dr. John Whyte, director of Professional Affairs and Stakeholder Engagment (PASE) at the FDA Center for Drug Evaluation and Research, answers questions about the new rule from Ob.Gyn. News journalists and our Drugs, Pregnancy & Lactation columnists.
Question: Instead of the traditional letter categories, prescribers will now have to read the pregnancy and lactation subsections of the labeling. What can the agency do to ensure prescribers are reading all of the new contextual information in the drug label?
Answer: The agency has initiated outreach efforts to help prescribers become familiar with the new information in labeling. The most important information is now under a new heading called Risk Summary. This narrative summary replaces the pregnancy letter categories with a summary of information that is known about a product. The new labeling rule explains, based on available information, the potential benefits and risks for the mother, the fetus, and the breastfeeding child. The decision to replace the pregnancy letter categories with the summary paragraph was reached after extensive consultation with experts and stakeholders who were concerned that the traditional letter categories were overly simplistic.
Q: PLLR praises the value of pregnancy exposure registries and mandates that if a registry exists for an approved product, then the label must include the registry’s website address. There is no requirement, however, that industry actually fund these registries. Why did the agency stop short of requiring companies to provide ongoing support for pregnancy exposure registries?
A: FDA has the authority to require the establishment of pregnancy registries when there is a safety concern that would benefit from the collection of data in a postapproval study, or when a particular product will be used by a large number of females of reproductive age. However, FDA does not have the authority to require companies to fund existing pregnancy exposure registries.
Q: How does the FDA plan to review all of the summary statements of available data regarding reproductive safety considering the breadth of drugs in various therapeutic areas that will need to be reviewed?
A: All labeling changes, including the changes required by the PLLR, must be submitted to the FDA for review and approval. Changes to the Pregnancy and Lactation subsections of labeling have been integrated into standard labeling review processes. In addition, the Division of Pediatric and Maternal Health, in the Office of New Drugs, works with the primary review divisions to help coordinate PLLR review processes.
Q: Are the manufacturers of generic drugs expected to “piggyback” the label for a branded molecule with respect to the pregnancy label?
A: Generic drug products (Abbreviated New Drug Applications) are required by regulation to have the “same” labeling as the reference drug listed. When the labeling is revised for the referenced drug, generic drug manufacturers also are required to update their labeling.
Q: In the new system, how will labeling stay up to date when important new data are published on a drug sometimes two to three times a year?
A: When new information becomes available that causes the labeling to be inaccurate, false, or misleading, drug manufacturers are required by regulation to update labeling.
Q: The rule is phased in over time. Drugs already on the market are given more time to switch to the new system. How long will physicians need to deal simultaneously with the old and the new formats?
A: There have been two labeling formats in use for some time as products approved prior to 2001 are not required to conform to either PLLR or PLR. The agency encourages manufacturers to voluntarily convert labeling of older products into PLR format. However, products approved prior to 2001 will be required to remove the pregnancy letter category by June 30, 2018, (3 years after the implementation of the PLLR).
Q: Does the FDA have any plans to address labeling for over-the-counter products in terms of their impact on pregnancy, lactation, and reproductive potential?
A: The PLLR does not apply to over-the-counter products. However, the agency is continually reviewing the safety of products used over the counter, including impacts on pregnancy, lactation, and reproductive potential.
Q: How does the FDA plan to assess over time the usefulness of the new labeling for prescribers and patients and make revisions?
A: The draft guidance was issued concurrently with PLLR. Based on the comments received from the public on the draft, as well as learning from the initial revisions of labeling, the guidance will be revised as needed. Guidance statements issued by FDA are regularly reviewed and revised as needed.
Dr. Whyte, a board-certified internist, is the director of Professional Affairs and Stakeholder Engagement at the FDA. Do you have other questions about the PLLR? Send them to [email protected].
On June 30, pharmaceutical and biological manufacturers will begin implementing the Food and Drug Administration’s revised rules for pregnancy and lactation drug labeling. The Pregnancy and Lactation Labeling Rule (PLLR) amends the Physician Labeling Rule (PLR, issued in 2006) and new labels aimed at providing clearer and more accurate information on drug risks will gradually roll out over the next 5 years.
The new rule – several years in the making – replaces pregnancy categories A, B, C, D, and X with short, integrated summaries that provide physicians with more information for discussing with patients the risks and benefits of a medication. The summaries will be brief and easy to read, and will contain evidence-based information that specifically addresses drug risks for women during pregnancy and lactation, as well as for men and women of reproductive potential. Under the PLLR, the pregnancy subsection of the risk summary will include for the first time information for any drug that has a pregnancy exposure registry.

Dr. John Whyte, director of Professional Affairs and Stakeholder Engagment (PASE) at the FDA Center for Drug Evaluation and Research, answers questions about the new rule from Ob.Gyn. News journalists and our Drugs, Pregnancy & Lactation columnists.
Question: Instead of the traditional letter categories, prescribers will now have to read the pregnancy and lactation subsections of the labeling. What can the agency do to ensure prescribers are reading all of the new contextual information in the drug label?
Answer: The agency has initiated outreach efforts to help prescribers become familiar with the new information in labeling. The most important information is now under a new heading called Risk Summary. This narrative summary replaces the pregnancy letter categories with a summary of information that is known about a product. The new labeling rule explains, based on available information, the potential benefits and risks for the mother, the fetus, and the breastfeeding child. The decision to replace the pregnancy letter categories with the summary paragraph was reached after extensive consultation with experts and stakeholders who were concerned that the traditional letter categories were overly simplistic.
Q: PLLR praises the value of pregnancy exposure registries and mandates that if a registry exists for an approved product, then the label must include the registry’s website address. There is no requirement, however, that industry actually fund these registries. Why did the agency stop short of requiring companies to provide ongoing support for pregnancy exposure registries?
A: FDA has the authority to require the establishment of pregnancy registries when there is a safety concern that would benefit from the collection of data in a postapproval study, or when a particular product will be used by a large number of females of reproductive age. However, FDA does not have the authority to require companies to fund existing pregnancy exposure registries.
Q: How does the FDA plan to review all of the summary statements of available data regarding reproductive safety considering the breadth of drugs in various therapeutic areas that will need to be reviewed?
A: All labeling changes, including the changes required by the PLLR, must be submitted to the FDA for review and approval. Changes to the Pregnancy and Lactation subsections of labeling have been integrated into standard labeling review processes. In addition, the Division of Pediatric and Maternal Health, in the Office of New Drugs, works with the primary review divisions to help coordinate PLLR review processes.
Q: Are the manufacturers of generic drugs expected to “piggyback” the label for a branded molecule with respect to the pregnancy label?
A: Generic drug products (Abbreviated New Drug Applications) are required by regulation to have the “same” labeling as the reference drug listed. When the labeling is revised for the referenced drug, generic drug manufacturers also are required to update their labeling.
Q: In the new system, how will labeling stay up to date when important new data are published on a drug sometimes two to three times a year?
A: When new information becomes available that causes the labeling to be inaccurate, false, or misleading, drug manufacturers are required by regulation to update labeling.
Q: The rule is phased in over time. Drugs already on the market are given more time to switch to the new system. How long will physicians need to deal simultaneously with the old and the new formats?
A: There have been two labeling formats in use for some time as products approved prior to 2001 are not required to conform to either PLLR or PLR. The agency encourages manufacturers to voluntarily convert labeling of older products into PLR format. However, products approved prior to 2001 will be required to remove the pregnancy letter category by June 30, 2018, (3 years after the implementation of the PLLR).
Q: Does the FDA have any plans to address labeling for over-the-counter products in terms of their impact on pregnancy, lactation, and reproductive potential?
A: The PLLR does not apply to over-the-counter products. However, the agency is continually reviewing the safety of products used over the counter, including impacts on pregnancy, lactation, and reproductive potential.
Q: How does the FDA plan to assess over time the usefulness of the new labeling for prescribers and patients and make revisions?
A: The draft guidance was issued concurrently with PLLR. Based on the comments received from the public on the draft, as well as learning from the initial revisions of labeling, the guidance will be revised as needed. Guidance statements issued by FDA are regularly reviewed and revised as needed.
Dr. Whyte, a board-certified internist, is the director of Professional Affairs and Stakeholder Engagement at the FDA. Do you have other questions about the PLLR? Send them to [email protected].
ADA: Stress may up risk for excess gestational weight gain
BOSTON – Psychosocial stress is an independent risk factor for excess weight gain among women with gestational diabetes mellitus, findings from the Gestational Diabetes Effects on Moms (GEM) study suggest.
Among nearly 1,300 women in the cluster randomized trial conducted within Kaiser Permanent Northern California, perceived stress near the time of gestational diabetes diagnosis at around 32 weeks’ gestation was significantly associated with a risk of excess gestational weight gain.
After adjusting for gestational and maternal age at the time of gestational diabetes diagnosis, education level, pregravid body mass index, and race/ethnicity, an upper-quartile score on the Perceived Stress Scale (PSS), compared with a lower score, was associated with a 54% increased odds of weight gain that exceeded Institute of Medicine recommendations (odds ratio, 1.54), Ai Kubo, Ph.D., of Kaiser Permanente in Oakland, Calif., reported at the annual scientific sessions of the American Diabetes Association.
A significant trend between PSS score and gestational weight gain was seen, and the association was not attenuated by inclusion of other lifestyle variables, including diet and physical activity, Dr. Kubo said, noting that no association was seen between PSS and the odds of gaining weight at a level below the IOM recommendations.
Dr. Kubo and her colleagues examined the relationship between stress and weight gain using baseline data from the GEM study. Subjects were women who delivered a term singleton at greater than 37 weeks’ gestation. Total gestational weight gain (about 20 pounds on average) and prepregnancy body mass index were obtained from electronic health records. Weight gain was categorized according to 2009 IOM recommendations.
Women with lower socioeconomic status tended to be in the highest stress group, and although there was no significant differences in prepregnancy weight between the highest and lowest stress groups, more of those with BMIs over 35 were in the highest stress group, she noted.
“Excess gestational weight gain has become an important public health concern,” Dr. Kubo said, noting that nearly 60% of women exceed IOM recommendations for weight gain, which increases the risk of adverse health outcomes, including obesity, in both women and their offspring.
Gestational diabetes mellitus occurs in about 8% of all pregnant women and also increases the risk of adverse outcomes in both mothers and offspring, she said.
The current findings suggest that stress reduction interventions may be warranted in women with gestational diabetes mellitus to optimize weight gain and possibly reduce the risks to both mother and offspring, she said, noting that the study is limited by a lack of assessment regarding the timing of stress relative to weight gain and gestational diabetes diagnosis.
“For future studies, use of a [non–gestational diabetes] population, and assessment of stress at the beginning of the pregnancy or even prior to the pregnancy would help us better understand the role of psychosocial stress on weight gain during pregnancy,” she said.
Dr. Kubo reported having no disclosures.
BOSTON – Psychosocial stress is an independent risk factor for excess weight gain among women with gestational diabetes mellitus, findings from the Gestational Diabetes Effects on Moms (GEM) study suggest.
Among nearly 1,300 women in the cluster randomized trial conducted within Kaiser Permanent Northern California, perceived stress near the time of gestational diabetes diagnosis at around 32 weeks’ gestation was significantly associated with a risk of excess gestational weight gain.
After adjusting for gestational and maternal age at the time of gestational diabetes diagnosis, education level, pregravid body mass index, and race/ethnicity, an upper-quartile score on the Perceived Stress Scale (PSS), compared with a lower score, was associated with a 54% increased odds of weight gain that exceeded Institute of Medicine recommendations (odds ratio, 1.54), Ai Kubo, Ph.D., of Kaiser Permanente in Oakland, Calif., reported at the annual scientific sessions of the American Diabetes Association.
A significant trend between PSS score and gestational weight gain was seen, and the association was not attenuated by inclusion of other lifestyle variables, including diet and physical activity, Dr. Kubo said, noting that no association was seen between PSS and the odds of gaining weight at a level below the IOM recommendations.
Dr. Kubo and her colleagues examined the relationship between stress and weight gain using baseline data from the GEM study. Subjects were women who delivered a term singleton at greater than 37 weeks’ gestation. Total gestational weight gain (about 20 pounds on average) and prepregnancy body mass index were obtained from electronic health records. Weight gain was categorized according to 2009 IOM recommendations.
Women with lower socioeconomic status tended to be in the highest stress group, and although there was no significant differences in prepregnancy weight between the highest and lowest stress groups, more of those with BMIs over 35 were in the highest stress group, she noted.
“Excess gestational weight gain has become an important public health concern,” Dr. Kubo said, noting that nearly 60% of women exceed IOM recommendations for weight gain, which increases the risk of adverse health outcomes, including obesity, in both women and their offspring.
Gestational diabetes mellitus occurs in about 8% of all pregnant women and also increases the risk of adverse outcomes in both mothers and offspring, she said.
The current findings suggest that stress reduction interventions may be warranted in women with gestational diabetes mellitus to optimize weight gain and possibly reduce the risks to both mother and offspring, she said, noting that the study is limited by a lack of assessment regarding the timing of stress relative to weight gain and gestational diabetes diagnosis.
“For future studies, use of a [non–gestational diabetes] population, and assessment of stress at the beginning of the pregnancy or even prior to the pregnancy would help us better understand the role of psychosocial stress on weight gain during pregnancy,” she said.
Dr. Kubo reported having no disclosures.
BOSTON – Psychosocial stress is an independent risk factor for excess weight gain among women with gestational diabetes mellitus, findings from the Gestational Diabetes Effects on Moms (GEM) study suggest.
Among nearly 1,300 women in the cluster randomized trial conducted within Kaiser Permanent Northern California, perceived stress near the time of gestational diabetes diagnosis at around 32 weeks’ gestation was significantly associated with a risk of excess gestational weight gain.
After adjusting for gestational and maternal age at the time of gestational diabetes diagnosis, education level, pregravid body mass index, and race/ethnicity, an upper-quartile score on the Perceived Stress Scale (PSS), compared with a lower score, was associated with a 54% increased odds of weight gain that exceeded Institute of Medicine recommendations (odds ratio, 1.54), Ai Kubo, Ph.D., of Kaiser Permanente in Oakland, Calif., reported at the annual scientific sessions of the American Diabetes Association.
A significant trend between PSS score and gestational weight gain was seen, and the association was not attenuated by inclusion of other lifestyle variables, including diet and physical activity, Dr. Kubo said, noting that no association was seen between PSS and the odds of gaining weight at a level below the IOM recommendations.
Dr. Kubo and her colleagues examined the relationship between stress and weight gain using baseline data from the GEM study. Subjects were women who delivered a term singleton at greater than 37 weeks’ gestation. Total gestational weight gain (about 20 pounds on average) and prepregnancy body mass index were obtained from electronic health records. Weight gain was categorized according to 2009 IOM recommendations.
Women with lower socioeconomic status tended to be in the highest stress group, and although there was no significant differences in prepregnancy weight between the highest and lowest stress groups, more of those with BMIs over 35 were in the highest stress group, she noted.
“Excess gestational weight gain has become an important public health concern,” Dr. Kubo said, noting that nearly 60% of women exceed IOM recommendations for weight gain, which increases the risk of adverse health outcomes, including obesity, in both women and their offspring.
Gestational diabetes mellitus occurs in about 8% of all pregnant women and also increases the risk of adverse outcomes in both mothers and offspring, she said.
The current findings suggest that stress reduction interventions may be warranted in women with gestational diabetes mellitus to optimize weight gain and possibly reduce the risks to both mother and offspring, she said, noting that the study is limited by a lack of assessment regarding the timing of stress relative to weight gain and gestational diabetes diagnosis.
“For future studies, use of a [non–gestational diabetes] population, and assessment of stress at the beginning of the pregnancy or even prior to the pregnancy would help us better understand the role of psychosocial stress on weight gain during pregnancy,” she said.
Dr. Kubo reported having no disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Psychosocial stress is an independent risk factor for excess weight gain among women with gestational diabetes mellitus, findings from the Gestational Diabetes Effects on Moms (GEM) study suggest.
Major finding: An upper-quartile score on the Perceived Stress Scale, compared with a lower score, was associated with a 54% increased odds of weight gain that exceeded Institute of Medicine recommendations (odds ratio, 1.54).
Data source: The cluster randomized GEM study of nearly 1,300 women.
Disclosures: Dr. Kubo reported having no disclosures.
Uncomplicated pregnancies in women with lupus may not boost risk for CV events
Pregnancy may not increase the risk of a cardiovascular event (CVE) in women with systemic lupus erythematosus as much as disease-related morbidities, according to findings from a large, retrospective study presented at the European Congress of Rheumatology.
In fact, uncomplicated pregnancy may be a positive marker for later cardiovascular health in lupus patients, Dr. May Ching Soh reported at the meeting.
“Physicians and patients may derive some reassurance that perhaps a pregnancy uncomplicated by maternal-placental pathology may be associated with lower risk for future cardiovascular events,” Dr. Soh said in an interview. “However, we cannot at this time recommend relaxing on our laurels and not screening and actively managing cardiovascular risk factors in all patients with systemic lupus erythematosus.”
Dr. Soh, an obstetrician in the Women’s Centre at Oxford Radcliffe Hospital, part of the Oxford (England) University Hospitals NHS Trust, extracted data from linked Swedish population registries on systemic lupus erythematosus (SLE) patients’ parity status, the occurrence of features of maternal-placental syndrome (MPS, defined as hypertensive disorders of pregnancy, small-for-gestational-age, stillbirth, and placental abruption), SLE-related morbidities (in-patient admissions, renal disease, malignancies, and infections), and CVE outcomes (coronary artery disease, stroke, peripheral vascular disease, and death from these causes).
The final cohort comprised 3,232 women with SLE who had been born in 1951-1971. A total of 72% had children.
The mean age at follow-up was 49 years. Nulliparous women had more SLE-related morbidities, more cardiovascular risk factors, and more cardiovascular events than did parous women.
CVEs were most common among those women who had never given birth (3.4 per 1,000 person-years), followed by women with pregnancies complicated by MPS (2.8 per 1,000 person-years). Compared with women who had an uncomplicated pregnancy, the risk of a CVE was doubled in the nulliparous group (hazard ratio, 2.2) and close to double in the MPS-pregnancy group (HR, 1.8).
The time to first CVE also was significantly delayed in women who had uncomplicated pregnancies. By age 30, almost none had occurred in these women, but 5% of those with MPS-complicated pregnancies and 10% of the nulliparous women had experienced an event by that age. The separation continued as women aged. By age 40, an event had occurred in about 4% of the MPS-free women, 8% of the MPS group, and 10% of the nulliparous group. The rates at age 45 were 5%, 8%, and 15%, respectively.
“Our nonparous cohort did develop cardiovascular events earlier, but the MPS cohort also had accelerated development compared to the women who had uncomplicated pregnancies,” Dr. Soh said. “In fact, an adverse pregnancy outcome should serve as a red flag for physicians to start screening early for cardiovascular disease and actively managing risk factors.”
She had no financial disclosures.
On Twitter @Alz_Gal
Pregnancy may not increase the risk of a cardiovascular event (CVE) in women with systemic lupus erythematosus as much as disease-related morbidities, according to findings from a large, retrospective study presented at the European Congress of Rheumatology.
In fact, uncomplicated pregnancy may be a positive marker for later cardiovascular health in lupus patients, Dr. May Ching Soh reported at the meeting.
“Physicians and patients may derive some reassurance that perhaps a pregnancy uncomplicated by maternal-placental pathology may be associated with lower risk for future cardiovascular events,” Dr. Soh said in an interview. “However, we cannot at this time recommend relaxing on our laurels and not screening and actively managing cardiovascular risk factors in all patients with systemic lupus erythematosus.”
Dr. Soh, an obstetrician in the Women’s Centre at Oxford Radcliffe Hospital, part of the Oxford (England) University Hospitals NHS Trust, extracted data from linked Swedish population registries on systemic lupus erythematosus (SLE) patients’ parity status, the occurrence of features of maternal-placental syndrome (MPS, defined as hypertensive disorders of pregnancy, small-for-gestational-age, stillbirth, and placental abruption), SLE-related morbidities (in-patient admissions, renal disease, malignancies, and infections), and CVE outcomes (coronary artery disease, stroke, peripheral vascular disease, and death from these causes).
The final cohort comprised 3,232 women with SLE who had been born in 1951-1971. A total of 72% had children.
The mean age at follow-up was 49 years. Nulliparous women had more SLE-related morbidities, more cardiovascular risk factors, and more cardiovascular events than did parous women.
CVEs were most common among those women who had never given birth (3.4 per 1,000 person-years), followed by women with pregnancies complicated by MPS (2.8 per 1,000 person-years). Compared with women who had an uncomplicated pregnancy, the risk of a CVE was doubled in the nulliparous group (hazard ratio, 2.2) and close to double in the MPS-pregnancy group (HR, 1.8).
The time to first CVE also was significantly delayed in women who had uncomplicated pregnancies. By age 30, almost none had occurred in these women, but 5% of those with MPS-complicated pregnancies and 10% of the nulliparous women had experienced an event by that age. The separation continued as women aged. By age 40, an event had occurred in about 4% of the MPS-free women, 8% of the MPS group, and 10% of the nulliparous group. The rates at age 45 were 5%, 8%, and 15%, respectively.
“Our nonparous cohort did develop cardiovascular events earlier, but the MPS cohort also had accelerated development compared to the women who had uncomplicated pregnancies,” Dr. Soh said. “In fact, an adverse pregnancy outcome should serve as a red flag for physicians to start screening early for cardiovascular disease and actively managing risk factors.”
She had no financial disclosures.
On Twitter @Alz_Gal
Pregnancy may not increase the risk of a cardiovascular event (CVE) in women with systemic lupus erythematosus as much as disease-related morbidities, according to findings from a large, retrospective study presented at the European Congress of Rheumatology.
In fact, uncomplicated pregnancy may be a positive marker for later cardiovascular health in lupus patients, Dr. May Ching Soh reported at the meeting.
“Physicians and patients may derive some reassurance that perhaps a pregnancy uncomplicated by maternal-placental pathology may be associated with lower risk for future cardiovascular events,” Dr. Soh said in an interview. “However, we cannot at this time recommend relaxing on our laurels and not screening and actively managing cardiovascular risk factors in all patients with systemic lupus erythematosus.”
Dr. Soh, an obstetrician in the Women’s Centre at Oxford Radcliffe Hospital, part of the Oxford (England) University Hospitals NHS Trust, extracted data from linked Swedish population registries on systemic lupus erythematosus (SLE) patients’ parity status, the occurrence of features of maternal-placental syndrome (MPS, defined as hypertensive disorders of pregnancy, small-for-gestational-age, stillbirth, and placental abruption), SLE-related morbidities (in-patient admissions, renal disease, malignancies, and infections), and CVE outcomes (coronary artery disease, stroke, peripheral vascular disease, and death from these causes).
The final cohort comprised 3,232 women with SLE who had been born in 1951-1971. A total of 72% had children.
The mean age at follow-up was 49 years. Nulliparous women had more SLE-related morbidities, more cardiovascular risk factors, and more cardiovascular events than did parous women.
CVEs were most common among those women who had never given birth (3.4 per 1,000 person-years), followed by women with pregnancies complicated by MPS (2.8 per 1,000 person-years). Compared with women who had an uncomplicated pregnancy, the risk of a CVE was doubled in the nulliparous group (hazard ratio, 2.2) and close to double in the MPS-pregnancy group (HR, 1.8).
The time to first CVE also was significantly delayed in women who had uncomplicated pregnancies. By age 30, almost none had occurred in these women, but 5% of those with MPS-complicated pregnancies and 10% of the nulliparous women had experienced an event by that age. The separation continued as women aged. By age 40, an event had occurred in about 4% of the MPS-free women, 8% of the MPS group, and 10% of the nulliparous group. The rates at age 45 were 5%, 8%, and 15%, respectively.
“Our nonparous cohort did develop cardiovascular events earlier, but the MPS cohort also had accelerated development compared to the women who had uncomplicated pregnancies,” Dr. Soh said. “In fact, an adverse pregnancy outcome should serve as a red flag for physicians to start screening early for cardiovascular disease and actively managing risk factors.”
She had no financial disclosures.
On Twitter @Alz_Gal
FROM THE EULAR 2015 CONGRESS
Key clinical point: An uncomplicated pregnancy doesn’t appear to accelerate the risk of cardiovascular events in women with systemic lupus erythematosus.
Major finding: Nulliparous women or women who had a pregnancy complicated by lupus had twice the risk for a cardiovascular event, compared with women who had an uncomplicated pregnancy.
Data source: The retrospective cohort study involved 3,232 women.
Disclosures: Dr. Soh had no financial disclosures.
Diet, exercise in pregnancy keep excessive weight gain in check
Expectant mothers can reduce the likelihood that they will gain too much weight during pregnancy by improving their diet, increasing their exercise, or using a combined diet and exercise program, according to an updated Cochrane review.
The original review published in 2012 found too little evidence to conclude that diet and exercise interventions could benefit pregnant women and their newborns, but the update includes an additional 41 randomized controlled studies published between October 2011 and November 2014, along with 24 studies from the original review.
“Moderate-intensity exercise appears to be an important part of controlling weight gain in pregnancy; however, the evidence on the risk of preterm birth is limited, and more research is needed to establish safe guidelines,” wrote Benja Muktabhant of Khon Kaen (Thailand) University and associates (Cochrane Database Syst. Rev. 2015 June 10 [doi:10.1002/14651858.CD007145.pub3]).
Excessive weight gain in pregnancy is well known to increase the risk for poor maternal and neonatal outcomes, including gestational diabetes, hypertension, cesarean delivery, macrosomia, and stillbirth. But research shows that pregnant women continue to struggle to stay within recommended weight limits. In 2009, the Institute of Medicine (IOM) recommended that normal weight women with singleton pregnancies gain 25-35 pounds, while overweight women should gain 15-25 pounds. For obese women with a body mass index of 30 kg/m2 or greater, the IOM recommendation is 11-20 pounds of weight gain.
In the current review, the researchers identified a total of 65 randomized controlled trials that focused on using exercise, diet, or both to prevent excessive gestational weight gain. The 49 studies that were part of the quantitative meta-analysis included 11,444 women, but the methodologies varied greatly among the studies, and 20 studies had a moderate to high risk of bias. Further, the majority of studies included participants from high-income countries, primarily the United States, Canada, Australia, and European countries, making generalization to lower-income populations difficult to assess.
Using diet, exercise, or both reduced the risk of excessive weight gain in pregnancy by 20% based on 24 studies involving 7,096 participants. This evidence, rated high quality, found that low-glycemic-load diets, exercise with and without supervision, and combined diet and exercise programs all reduced women’s weights by similar amounts. Diet and/or exercise programs also increased the likelihood by 14% that women would have a low gestational weight gain, based on 4,422 participants in 11 studies with moderate-quality evidence.
Exercise programs included moderate exercise such as walking, dance, or aerobics.
The risk of maternal hypertension was lower in the diet and/or exercise intervention groups based on 11 studies involving 5,162 participants, but the researchers graded the evidence as low quality because of inconsistency and risk of bias concerns. The researchers found no reduced risk for preeclampsia in the 15 studies that included it as an outcome.
Similarly, the 16 studies assessing preterm birth and the 28 studies assessing risk of cesarean delivery found no difference between the intervention and control groups. However, when the researchers looked only at a combined diet and exercise intervention, they found a 13% reduction in cesarean delivery, which had borderline statistical significance.
The potential risk reduction of infant macrosomia depended on a woman’s prepregnancy weight, with only the findings for overweight or obese women, or those with or at risk for gestational diabetes, barely reaching statistical significance. Among the 27 studies with 8,598 participants that included macrosomia as an outcome, the 7% lower risk for oversized newborns just missed statistical significance. Among high-risk women, combined diet and exercise counseling reduced macrosomia risk by 15% (P = .05) with moderate-quality evidence from 3,252 participants in nine studies.
There was no difference between the two groups in risk for shoulder dystocia, neonatal hypoglycemia, hyperbilirubinemia, and birth trauma, according to the review.
The project was supported by the National Institute for Health Research in the United Kingdom, and the World Health Organization. The researchers reported having no relevant financial conflicts.
Expectant mothers can reduce the likelihood that they will gain too much weight during pregnancy by improving their diet, increasing their exercise, or using a combined diet and exercise program, according to an updated Cochrane review.
The original review published in 2012 found too little evidence to conclude that diet and exercise interventions could benefit pregnant women and their newborns, but the update includes an additional 41 randomized controlled studies published between October 2011 and November 2014, along with 24 studies from the original review.
“Moderate-intensity exercise appears to be an important part of controlling weight gain in pregnancy; however, the evidence on the risk of preterm birth is limited, and more research is needed to establish safe guidelines,” wrote Benja Muktabhant of Khon Kaen (Thailand) University and associates (Cochrane Database Syst. Rev. 2015 June 10 [doi:10.1002/14651858.CD007145.pub3]).
Excessive weight gain in pregnancy is well known to increase the risk for poor maternal and neonatal outcomes, including gestational diabetes, hypertension, cesarean delivery, macrosomia, and stillbirth. But research shows that pregnant women continue to struggle to stay within recommended weight limits. In 2009, the Institute of Medicine (IOM) recommended that normal weight women with singleton pregnancies gain 25-35 pounds, while overweight women should gain 15-25 pounds. For obese women with a body mass index of 30 kg/m2 or greater, the IOM recommendation is 11-20 pounds of weight gain.
In the current review, the researchers identified a total of 65 randomized controlled trials that focused on using exercise, diet, or both to prevent excessive gestational weight gain. The 49 studies that were part of the quantitative meta-analysis included 11,444 women, but the methodologies varied greatly among the studies, and 20 studies had a moderate to high risk of bias. Further, the majority of studies included participants from high-income countries, primarily the United States, Canada, Australia, and European countries, making generalization to lower-income populations difficult to assess.
Using diet, exercise, or both reduced the risk of excessive weight gain in pregnancy by 20% based on 24 studies involving 7,096 participants. This evidence, rated high quality, found that low-glycemic-load diets, exercise with and without supervision, and combined diet and exercise programs all reduced women’s weights by similar amounts. Diet and/or exercise programs also increased the likelihood by 14% that women would have a low gestational weight gain, based on 4,422 participants in 11 studies with moderate-quality evidence.
Exercise programs included moderate exercise such as walking, dance, or aerobics.
The risk of maternal hypertension was lower in the diet and/or exercise intervention groups based on 11 studies involving 5,162 participants, but the researchers graded the evidence as low quality because of inconsistency and risk of bias concerns. The researchers found no reduced risk for preeclampsia in the 15 studies that included it as an outcome.
Similarly, the 16 studies assessing preterm birth and the 28 studies assessing risk of cesarean delivery found no difference between the intervention and control groups. However, when the researchers looked only at a combined diet and exercise intervention, they found a 13% reduction in cesarean delivery, which had borderline statistical significance.
The potential risk reduction of infant macrosomia depended on a woman’s prepregnancy weight, with only the findings for overweight or obese women, or those with or at risk for gestational diabetes, barely reaching statistical significance. Among the 27 studies with 8,598 participants that included macrosomia as an outcome, the 7% lower risk for oversized newborns just missed statistical significance. Among high-risk women, combined diet and exercise counseling reduced macrosomia risk by 15% (P = .05) with moderate-quality evidence from 3,252 participants in nine studies.
There was no difference between the two groups in risk for shoulder dystocia, neonatal hypoglycemia, hyperbilirubinemia, and birth trauma, according to the review.
The project was supported by the National Institute for Health Research in the United Kingdom, and the World Health Organization. The researchers reported having no relevant financial conflicts.
Expectant mothers can reduce the likelihood that they will gain too much weight during pregnancy by improving their diet, increasing their exercise, or using a combined diet and exercise program, according to an updated Cochrane review.
The original review published in 2012 found too little evidence to conclude that diet and exercise interventions could benefit pregnant women and their newborns, but the update includes an additional 41 randomized controlled studies published between October 2011 and November 2014, along with 24 studies from the original review.
“Moderate-intensity exercise appears to be an important part of controlling weight gain in pregnancy; however, the evidence on the risk of preterm birth is limited, and more research is needed to establish safe guidelines,” wrote Benja Muktabhant of Khon Kaen (Thailand) University and associates (Cochrane Database Syst. Rev. 2015 June 10 [doi:10.1002/14651858.CD007145.pub3]).
Excessive weight gain in pregnancy is well known to increase the risk for poor maternal and neonatal outcomes, including gestational diabetes, hypertension, cesarean delivery, macrosomia, and stillbirth. But research shows that pregnant women continue to struggle to stay within recommended weight limits. In 2009, the Institute of Medicine (IOM) recommended that normal weight women with singleton pregnancies gain 25-35 pounds, while overweight women should gain 15-25 pounds. For obese women with a body mass index of 30 kg/m2 or greater, the IOM recommendation is 11-20 pounds of weight gain.
In the current review, the researchers identified a total of 65 randomized controlled trials that focused on using exercise, diet, or both to prevent excessive gestational weight gain. The 49 studies that were part of the quantitative meta-analysis included 11,444 women, but the methodologies varied greatly among the studies, and 20 studies had a moderate to high risk of bias. Further, the majority of studies included participants from high-income countries, primarily the United States, Canada, Australia, and European countries, making generalization to lower-income populations difficult to assess.
Using diet, exercise, or both reduced the risk of excessive weight gain in pregnancy by 20% based on 24 studies involving 7,096 participants. This evidence, rated high quality, found that low-glycemic-load diets, exercise with and without supervision, and combined diet and exercise programs all reduced women’s weights by similar amounts. Diet and/or exercise programs also increased the likelihood by 14% that women would have a low gestational weight gain, based on 4,422 participants in 11 studies with moderate-quality evidence.
Exercise programs included moderate exercise such as walking, dance, or aerobics.
The risk of maternal hypertension was lower in the diet and/or exercise intervention groups based on 11 studies involving 5,162 participants, but the researchers graded the evidence as low quality because of inconsistency and risk of bias concerns. The researchers found no reduced risk for preeclampsia in the 15 studies that included it as an outcome.
Similarly, the 16 studies assessing preterm birth and the 28 studies assessing risk of cesarean delivery found no difference between the intervention and control groups. However, when the researchers looked only at a combined diet and exercise intervention, they found a 13% reduction in cesarean delivery, which had borderline statistical significance.
The potential risk reduction of infant macrosomia depended on a woman’s prepregnancy weight, with only the findings for overweight or obese women, or those with or at risk for gestational diabetes, barely reaching statistical significance. Among the 27 studies with 8,598 participants that included macrosomia as an outcome, the 7% lower risk for oversized newborns just missed statistical significance. Among high-risk women, combined diet and exercise counseling reduced macrosomia risk by 15% (P = .05) with moderate-quality evidence from 3,252 participants in nine studies.
There was no difference between the two groups in risk for shoulder dystocia, neonatal hypoglycemia, hyperbilirubinemia, and birth trauma, according to the review.
The project was supported by the National Institute for Health Research in the United Kingdom, and the World Health Organization. The researchers reported having no relevant financial conflicts.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point: Diet and/or exercise programs reduce the risk of excessive gestational weight gain.
Major finding: Women using diet and/or exercise interventions had a 20% lower risk of excessive weight gain during pregnancy.
Data source: An updated Cochrane review including 65 studies through November 2014, 49 of which were part of the overall meta-analysis with a combined 11,444 participants.
Disclosures: The project was supported by the National Institute for Health Research in the United Kingdom, and the World Health Organization. The researchers reported having no relevant financial conflicts.
Pregnancy weight changes infant metabolic profiles
BOSTON – Maternal obesity and increased weight gain during pregnancy are associated with changes to newborns’ cardiometabolic markers, and these associations are largely independent of infant adiposity, according to new research findings.
Maternal obesity and weight gain during pregnancy are established risk factors for childhood obesity, but the reasons for this remain little understood.
In utero programming of infant metabolism may be occurring, providing reasons beyond fetal growth and fat accretion for the differences in lipid and hormonal profiles among infants, judging from the findings reported at the annual scientific sessions of the American Diabetes Association.
Presenting data from a cohort of 753 mother-infant pairs, Dominick J. Lemas, Ph.D., of the University of Colorado, Denver, said that samples of cord blood revealed “striking” associations.
Dr. Lemas and his colleagues found higher levels of cord blood glucose (P = .03), and leptin (P < .001) in newborns among mothers who gained more weight during pregnancy. Infants of women with higher body mass index when they became pregnant saw higher levels of leptin (P < .001) in cord blood and lower levels of HDL cholesterol (P = .05), even after adjusting for infant adiposity.
The leptin finding was particularly surprising, Dr. Lemas said. Leptin, a hormone that regulates food intake, energy output, and other functions, is synthesized by fatty tissues and therefore expected to be found in higher levels among infants with fatter body composition.
In this study, however, “the association actually became stronger when we adjusted for the adiposity of the infant,” he said.
The investigators looked carefully at cord blood lipids, including total and high-density lipoprotein (HDL) cholesterol, triglycerides, and free fatty acids. The finding that prepregnancy BMI was negatively associated with HDL cholesterol “was something very interesting for our group,” Dr. Lemas said at the conference. “In adults, abnormal lipid patterns are a very strong predictor of cardiovascular disease.”
The findings raise two main concerns, Dr. Lemas said. First, “what factors beyond maternal BMI and gestational weight gain contribute to the changes to these cardiometabolic biomarkers at delivery?” And second, he said, it remains to be seen if these markers persist or result in clinically meaningful differences: “It’s possible cord-blood biomarkers don’t predict the metabolic risk of these individuals a few years down the road.”
The investigators used data from Healthy Start, a cohort study funded by the National Institute of Diabetes and Digestive and Kidney Diseases. They disclosed no conflicts of interest, and the full paper on their findings will be published in the International Journal of Obesity.
BOSTON – Maternal obesity and increased weight gain during pregnancy are associated with changes to newborns’ cardiometabolic markers, and these associations are largely independent of infant adiposity, according to new research findings.
Maternal obesity and weight gain during pregnancy are established risk factors for childhood obesity, but the reasons for this remain little understood.
In utero programming of infant metabolism may be occurring, providing reasons beyond fetal growth and fat accretion for the differences in lipid and hormonal profiles among infants, judging from the findings reported at the annual scientific sessions of the American Diabetes Association.
Presenting data from a cohort of 753 mother-infant pairs, Dominick J. Lemas, Ph.D., of the University of Colorado, Denver, said that samples of cord blood revealed “striking” associations.
Dr. Lemas and his colleagues found higher levels of cord blood glucose (P = .03), and leptin (P < .001) in newborns among mothers who gained more weight during pregnancy. Infants of women with higher body mass index when they became pregnant saw higher levels of leptin (P < .001) in cord blood and lower levels of HDL cholesterol (P = .05), even after adjusting for infant adiposity.
The leptin finding was particularly surprising, Dr. Lemas said. Leptin, a hormone that regulates food intake, energy output, and other functions, is synthesized by fatty tissues and therefore expected to be found in higher levels among infants with fatter body composition.
In this study, however, “the association actually became stronger when we adjusted for the adiposity of the infant,” he said.
The investigators looked carefully at cord blood lipids, including total and high-density lipoprotein (HDL) cholesterol, triglycerides, and free fatty acids. The finding that prepregnancy BMI was negatively associated with HDL cholesterol “was something very interesting for our group,” Dr. Lemas said at the conference. “In adults, abnormal lipid patterns are a very strong predictor of cardiovascular disease.”
The findings raise two main concerns, Dr. Lemas said. First, “what factors beyond maternal BMI and gestational weight gain contribute to the changes to these cardiometabolic biomarkers at delivery?” And second, he said, it remains to be seen if these markers persist or result in clinically meaningful differences: “It’s possible cord-blood biomarkers don’t predict the metabolic risk of these individuals a few years down the road.”
The investigators used data from Healthy Start, a cohort study funded by the National Institute of Diabetes and Digestive and Kidney Diseases. They disclosed no conflicts of interest, and the full paper on their findings will be published in the International Journal of Obesity.
BOSTON – Maternal obesity and increased weight gain during pregnancy are associated with changes to newborns’ cardiometabolic markers, and these associations are largely independent of infant adiposity, according to new research findings.
Maternal obesity and weight gain during pregnancy are established risk factors for childhood obesity, but the reasons for this remain little understood.
In utero programming of infant metabolism may be occurring, providing reasons beyond fetal growth and fat accretion for the differences in lipid and hormonal profiles among infants, judging from the findings reported at the annual scientific sessions of the American Diabetes Association.
Presenting data from a cohort of 753 mother-infant pairs, Dominick J. Lemas, Ph.D., of the University of Colorado, Denver, said that samples of cord blood revealed “striking” associations.
Dr. Lemas and his colleagues found higher levels of cord blood glucose (P = .03), and leptin (P < .001) in newborns among mothers who gained more weight during pregnancy. Infants of women with higher body mass index when they became pregnant saw higher levels of leptin (P < .001) in cord blood and lower levels of HDL cholesterol (P = .05), even after adjusting for infant adiposity.
The leptin finding was particularly surprising, Dr. Lemas said. Leptin, a hormone that regulates food intake, energy output, and other functions, is synthesized by fatty tissues and therefore expected to be found in higher levels among infants with fatter body composition.
In this study, however, “the association actually became stronger when we adjusted for the adiposity of the infant,” he said.
The investigators looked carefully at cord blood lipids, including total and high-density lipoprotein (HDL) cholesterol, triglycerides, and free fatty acids. The finding that prepregnancy BMI was negatively associated with HDL cholesterol “was something very interesting for our group,” Dr. Lemas said at the conference. “In adults, abnormal lipid patterns are a very strong predictor of cardiovascular disease.”
The findings raise two main concerns, Dr. Lemas said. First, “what factors beyond maternal BMI and gestational weight gain contribute to the changes to these cardiometabolic biomarkers at delivery?” And second, he said, it remains to be seen if these markers persist or result in clinically meaningful differences: “It’s possible cord-blood biomarkers don’t predict the metabolic risk of these individuals a few years down the road.”
The investigators used data from Healthy Start, a cohort study funded by the National Institute of Diabetes and Digestive and Kidney Diseases. They disclosed no conflicts of interest, and the full paper on their findings will be published in the International Journal of Obesity.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Mothers’ prepregnancy BMI and gestational weight gain are both associated with changes in neonatal cardiometabolic biomarkers largely independent of neonatal adiposity.
Major finding: Gestational weight gain was associated with significant increases in cord blood glucose and leptin in newborns even after adjusting for newborn adiposity. Prepregnancy BMI was associated with higher cord blood leptin and inversely associated with cord blood HDL cholesterol after adjusting for adiposity.
Data source: A longitudinal, prebirth cohort of 753 ethnically diverse pregnant women 16 years of age and older and their newborns recruited from university hospital obstetric clinics in Colorado from 2009 to 2014, with prepregnancy weight extracted from medical records or self reported, and subsequent gains recorded. Cord blood samples and adiposity measurements were taken from newborns.
Disclosures: Study funded with grants from the National Institute of Diabetes and Digestive and Kidney Diseases. The investigators disclosed no conflicts of interest.
Slow spindle assembly in human oocyte meiosis leads to frequent aneuploidy
The high rate of aneuploidy in human eggs could be explained by an unusually long meiotic spindle assembly period which favors chromosome segregation errors, according to a new study published in Science.
Dr. Zuzana Holubcova and her colleagues established an experimental system for ex vivo high-resolution fluorescence microscopy of human oocytes that allowed them to observe the major stages of meiosis in the laboratory.
Human oocyte spindle assembly was controlled primarily by chromosomes and the small guanosine triphosphatase Ran and was independent of centrosomes or other microtubule organization. This process took about 16 hours to complete, considerably longer than 30 minutes needed for spindle assembly in cells undergoing mitosis or the 3-5 hours required for mouse oocyte meiosis.
While nearly all oocytes progressed into anaphase with bipolar spindles, over the course of spindle assembly, 44% of spindles showed moderate instability with an apolar phase, and 38% of spindles showed severe instability with a multipolar phase. Oocytes with abnormal spindles were more likely to have persistent lagging chromosomes during anaphase, which were associated with abnormal kinetochore-microtubule attachments.
“Spindle instability may reflect attempts of the chromosomes to establish stable bipolar microtubule attachments, which could be more challenging in human oocytes, possibly due to structural features of their chromosomes abnormal attachments,” the researchers wrote. “Progression into anaphase with these abnormal attachments would put the oocyte at risk of chromosome segregation errors, providing at least one mechanism for the relatively frequent aneuploidy of eggs, even in young women.”
Find the full study in Science (doi: 10.1126/science.aaa9529).
Watch a video of spindle assembly here.
This video shows 3D volume reconstructions of kinetochores, chromosomes, and microtubules in cold-treated human oocytes fixed during early (left) or late (right) spindle assembly.
Credit: Zuzana Holubcova and Melina Schuh, MRC Laboratory of Molecular Biology
The high rate of aneuploidy in human eggs could be explained by an unusually long meiotic spindle assembly period which favors chromosome segregation errors, according to a new study published in Science.
Dr. Zuzana Holubcova and her colleagues established an experimental system for ex vivo high-resolution fluorescence microscopy of human oocytes that allowed them to observe the major stages of meiosis in the laboratory.
Human oocyte spindle assembly was controlled primarily by chromosomes and the small guanosine triphosphatase Ran and was independent of centrosomes or other microtubule organization. This process took about 16 hours to complete, considerably longer than 30 minutes needed for spindle assembly in cells undergoing mitosis or the 3-5 hours required for mouse oocyte meiosis.
While nearly all oocytes progressed into anaphase with bipolar spindles, over the course of spindle assembly, 44% of spindles showed moderate instability with an apolar phase, and 38% of spindles showed severe instability with a multipolar phase. Oocytes with abnormal spindles were more likely to have persistent lagging chromosomes during anaphase, which were associated with abnormal kinetochore-microtubule attachments.
“Spindle instability may reflect attempts of the chromosomes to establish stable bipolar microtubule attachments, which could be more challenging in human oocytes, possibly due to structural features of their chromosomes abnormal attachments,” the researchers wrote. “Progression into anaphase with these abnormal attachments would put the oocyte at risk of chromosome segregation errors, providing at least one mechanism for the relatively frequent aneuploidy of eggs, even in young women.”
Find the full study in Science (doi: 10.1126/science.aaa9529).
Watch a video of spindle assembly here.
This video shows 3D volume reconstructions of kinetochores, chromosomes, and microtubules in cold-treated human oocytes fixed during early (left) or late (right) spindle assembly.
Credit: Zuzana Holubcova and Melina Schuh, MRC Laboratory of Molecular Biology
The high rate of aneuploidy in human eggs could be explained by an unusually long meiotic spindle assembly period which favors chromosome segregation errors, according to a new study published in Science.
Dr. Zuzana Holubcova and her colleagues established an experimental system for ex vivo high-resolution fluorescence microscopy of human oocytes that allowed them to observe the major stages of meiosis in the laboratory.
Human oocyte spindle assembly was controlled primarily by chromosomes and the small guanosine triphosphatase Ran and was independent of centrosomes or other microtubule organization. This process took about 16 hours to complete, considerably longer than 30 minutes needed for spindle assembly in cells undergoing mitosis or the 3-5 hours required for mouse oocyte meiosis.
While nearly all oocytes progressed into anaphase with bipolar spindles, over the course of spindle assembly, 44% of spindles showed moderate instability with an apolar phase, and 38% of spindles showed severe instability with a multipolar phase. Oocytes with abnormal spindles were more likely to have persistent lagging chromosomes during anaphase, which were associated with abnormal kinetochore-microtubule attachments.
“Spindle instability may reflect attempts of the chromosomes to establish stable bipolar microtubule attachments, which could be more challenging in human oocytes, possibly due to structural features of their chromosomes abnormal attachments,” the researchers wrote. “Progression into anaphase with these abnormal attachments would put the oocyte at risk of chromosome segregation errors, providing at least one mechanism for the relatively frequent aneuploidy of eggs, even in young women.”
Find the full study in Science (doi: 10.1126/science.aaa9529).
Watch a video of spindle assembly here.
This video shows 3D volume reconstructions of kinetochores, chromosomes, and microtubules in cold-treated human oocytes fixed during early (left) or late (right) spindle assembly.
Credit: Zuzana Holubcova and Melina Schuh, MRC Laboratory of Molecular Biology
Asthma exacerbations in pregnancy linked to obesity, weight gain
DENVER – Obesity and weight gain of more than 5 kg during the first trimester in women with asthma are significant predictors of asthma exacerbation during pregnancy, according to findings from the Management of Asthma during Pregnancy (MAP) study.
Of 1,018 women in the prospective study, 370 experienced a total of 407 asthma exacerbations. The risk of exacerbation was greatest in those with body mass index over 30 kg/m2 (odds ratio, 2.2) and in those with gestational weight gain of more than 5 kg in the first trimester (OR, 8.2), Zarqa Ali of Hvidovre Hospital, Copenhagen, and colleagues reported in a poster at an international conference of the American Thoracic Society.
Other risk factors for exacerbation included previous asthma exacerbations (OR, 1.3), uncontrolled asthma (OR, 2), inhaled corticosteroid treatment (OR, 5.8), and current and former smoking (OR, 1.9), the investigators said.
Risk factors for severe exacerbation of asthma, which occurred in 157 women, included gestational weight gain of greater than 5 kg (OR, 4.1), and age (OR, 1.1).
After excluding women with a prepregnancy body mass index above the mean of 24.1, risk factors for exacerbation included first-trimester weight gain greater than 5 kg (OR, 13.1) and lower prepregnancy body weight (OR, 0.9), they noted.
Women included in the study had a diagnosis of asthma, had been prescribed rescue bronchodilator therapy, had their first outpatient clinic visit within the first 18 weeks of pregnancy, and were seen for scheduled visits about every 4 weeks during pregnancy and for 3 months post partum. The women had a mean age of 31 years, 73% had never smoked, and their mean forced expiratory volume in 1 second (FEV1) % predicted was 92, and mean exhaled nitric oxide was 20 ppb.
FEV1% predicted was significantly greater at baseline in those without exacerbations vs. with exacerbations (93 vs. 90), and exhaled nitric oxide was significantly lower in the groups, respectively (18 vs. 23 ppb).
The findings are important, as acute exacerbations of asthma during pregnancy may be the most significant risk factor for unfavorable pregnancy outcomes in women with asthma, the investigators said, noting that exacerbations occur in about 30% of pregnant women with asthma and are associated with complications such as preterm delivery, low birth weight, excessive antepartum hemorrhage, and cesarean delivery.
Risk factors for exacerbation identified in prior studies include severe asthma, viral infections, nonadherence to controller medication, tobacco exposure, and possibly fetal gender and maternal body mass index, the investigators said.
The investigators reported having no disclosures.
DENVER – Obesity and weight gain of more than 5 kg during the first trimester in women with asthma are significant predictors of asthma exacerbation during pregnancy, according to findings from the Management of Asthma during Pregnancy (MAP) study.
Of 1,018 women in the prospective study, 370 experienced a total of 407 asthma exacerbations. The risk of exacerbation was greatest in those with body mass index over 30 kg/m2 (odds ratio, 2.2) and in those with gestational weight gain of more than 5 kg in the first trimester (OR, 8.2), Zarqa Ali of Hvidovre Hospital, Copenhagen, and colleagues reported in a poster at an international conference of the American Thoracic Society.
Other risk factors for exacerbation included previous asthma exacerbations (OR, 1.3), uncontrolled asthma (OR, 2), inhaled corticosteroid treatment (OR, 5.8), and current and former smoking (OR, 1.9), the investigators said.
Risk factors for severe exacerbation of asthma, which occurred in 157 women, included gestational weight gain of greater than 5 kg (OR, 4.1), and age (OR, 1.1).
After excluding women with a prepregnancy body mass index above the mean of 24.1, risk factors for exacerbation included first-trimester weight gain greater than 5 kg (OR, 13.1) and lower prepregnancy body weight (OR, 0.9), they noted.
Women included in the study had a diagnosis of asthma, had been prescribed rescue bronchodilator therapy, had their first outpatient clinic visit within the first 18 weeks of pregnancy, and were seen for scheduled visits about every 4 weeks during pregnancy and for 3 months post partum. The women had a mean age of 31 years, 73% had never smoked, and their mean forced expiratory volume in 1 second (FEV1) % predicted was 92, and mean exhaled nitric oxide was 20 ppb.
FEV1% predicted was significantly greater at baseline in those without exacerbations vs. with exacerbations (93 vs. 90), and exhaled nitric oxide was significantly lower in the groups, respectively (18 vs. 23 ppb).
The findings are important, as acute exacerbations of asthma during pregnancy may be the most significant risk factor for unfavorable pregnancy outcomes in women with asthma, the investigators said, noting that exacerbations occur in about 30% of pregnant women with asthma and are associated with complications such as preterm delivery, low birth weight, excessive antepartum hemorrhage, and cesarean delivery.
Risk factors for exacerbation identified in prior studies include severe asthma, viral infections, nonadherence to controller medication, tobacco exposure, and possibly fetal gender and maternal body mass index, the investigators said.
The investigators reported having no disclosures.
DENVER – Obesity and weight gain of more than 5 kg during the first trimester in women with asthma are significant predictors of asthma exacerbation during pregnancy, according to findings from the Management of Asthma during Pregnancy (MAP) study.
Of 1,018 women in the prospective study, 370 experienced a total of 407 asthma exacerbations. The risk of exacerbation was greatest in those with body mass index over 30 kg/m2 (odds ratio, 2.2) and in those with gestational weight gain of more than 5 kg in the first trimester (OR, 8.2), Zarqa Ali of Hvidovre Hospital, Copenhagen, and colleagues reported in a poster at an international conference of the American Thoracic Society.
Other risk factors for exacerbation included previous asthma exacerbations (OR, 1.3), uncontrolled asthma (OR, 2), inhaled corticosteroid treatment (OR, 5.8), and current and former smoking (OR, 1.9), the investigators said.
Risk factors for severe exacerbation of asthma, which occurred in 157 women, included gestational weight gain of greater than 5 kg (OR, 4.1), and age (OR, 1.1).
After excluding women with a prepregnancy body mass index above the mean of 24.1, risk factors for exacerbation included first-trimester weight gain greater than 5 kg (OR, 13.1) and lower prepregnancy body weight (OR, 0.9), they noted.
Women included in the study had a diagnosis of asthma, had been prescribed rescue bronchodilator therapy, had their first outpatient clinic visit within the first 18 weeks of pregnancy, and were seen for scheduled visits about every 4 weeks during pregnancy and for 3 months post partum. The women had a mean age of 31 years, 73% had never smoked, and their mean forced expiratory volume in 1 second (FEV1) % predicted was 92, and mean exhaled nitric oxide was 20 ppb.
FEV1% predicted was significantly greater at baseline in those without exacerbations vs. with exacerbations (93 vs. 90), and exhaled nitric oxide was significantly lower in the groups, respectively (18 vs. 23 ppb).
The findings are important, as acute exacerbations of asthma during pregnancy may be the most significant risk factor for unfavorable pregnancy outcomes in women with asthma, the investigators said, noting that exacerbations occur in about 30% of pregnant women with asthma and are associated with complications such as preterm delivery, low birth weight, excessive antepartum hemorrhage, and cesarean delivery.
Risk factors for exacerbation identified in prior studies include severe asthma, viral infections, nonadherence to controller medication, tobacco exposure, and possibly fetal gender and maternal body mass index, the investigators said.
The investigators reported having no disclosures.
AT ATS 2015
Key clinical point: Baseline weight and gestational weight gain predict asthma exacerbations in pregnancy.
Major finding: Asthma exacerbation risk was greatest in those with body mass index over 30 kg/m2 and with gestational weight gain of more than 5 kg in the first trimester.
Data source: The prospective MAP study involving 1,018 women.
Disclosures: The investigators reported having no disclosures.
Worsening migraine in pregnancy linked to adverse outcomes
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
VALENCIA, SPAIN – Women who present with acute severe migraine during pregnancy are at increased risk for adverse pregnancy outcomes and should be seen in a high-risk pregnancy clinic, Dr. Tracy B. Grossman asserted at the International Headache Congress.
“We should not be seeing these patients in a regular ob.gyn./generalist’s office because oftentimes we need input from neurology, and we need extra surveillance for both the fetus and the mother,” she said at the meeting sponsored by the International Headache Society and the American Headache Society.
Dr. Grossman presented a retrospective study of 90 consecutive pregnant patients who presented with acute severe migraine and obtained a neurology consult at Montefiore Medical Center, New York, where she is an ob.gyn. resident.
“These patients are different from most migraine patients because most migraine patients actually see improvement of symptoms during pregnancy. So this is a special group of patients with worsening and refractory migraine,” she noted in an interview.
Most were in their third trimester and had migraine without aura. Twenty-four presented with status migrainosus, a migraine for 15 or more days a month for more than 3 months.
Forty-nine of the 90 patients (54%) experienced one or more adverse pregnancy outcomes. Of note, the 28% preterm delivery rate was nearly three times the national average of 11% as reported by the March of Dimes. The preeclampsia rate was 20.5%, compared with a national rate of just 3%-4%. The 19.2% low birth weight rate was more than double the 8% national average. The cesarean section rate was 30.8%.
The study hypothesis was that the migraine-with-aura group would have higher preeclampsia, preterm delivery, and low birth weight rates, as has been reported by some other investigators in what is a sparse literature. Not so, Dr. Grossman said, because most of these patients didn’t have aura.
“So it can’t be purely an aura/vascular phenomenon that’s resulting in these adverse outcomes. These high rates of adverse pregnancy outcomes aren’t easily explainable. There’s something going on here that we haven’t teased out yet as to why these migraine patients are special,” she continued.
Their risk of adverse pregnancy outcomes wasn’t related to the headache medications they took. Sixty-two patients received a combination of oral and intravenous therapy with acetaminophen, metoclopramide, and dihydroergotamine. But 30% of patients were briefly on barbiturates, and 30% were on oxycodone or codeine; these are drugs of concern during pregnancy, yet there was no associated increase in adverse pregnancy outcomes, compared with the women who weren’t on those drugs or who indeed weren’t on any headache medications at all.
Dr. Grossman’s own therapeutic preference in patients with severe migraine in pregnancy is a peripheral nerve block with bupivacaine and lidocaine.
“It works for the majority of people – we don’t quite know why – and it’s a local therapy that avoids fetal exposure to systemic medications,” she observed.
Dr. Grossman reported no financial conflicts with regard to her study, which was carried out without industry support.
AT IHC 2015
Key clinical point: Patients who present with acute severe migraine during pregnancy should be seen in a high-risk pregnancy clinic.
Major finding: Forty-nine of 90 (54%) consecutive pregnant women who presented with acute severe migraine subsequently had one or more adverse pregnancy outcomes.
Data source: This was a retrospective single-center study.
Disclosures: Dr. Grossman reported having no financial conflicts regarding this study, carried out free of commercial support.
Expert: Don’t discourage pregnancy in MS patients; manage it
INDIANAPOLIS – If you feel overwhelmed by the notion of managing multiple sclerosis patients who seek your guidance in navigating their pregnancy, you’re not alone.
Comprehensive management programs for pregnant MS patients currently do not exist, even within most dedicated MS centers and clinics, Dr. Maria K. Houtchens said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Individual providers have an interest in this area, but there are no guidelines for most physicians or nurse practitioners to follow yet,” she said. “The level of care these patients receive varies dramatically, from one part of the country to another and from one provider to the next.”
An estimated 50% of all pregnancies in the United States and about 40% of all pregnancies worldwide are unplanned.
“I believe that women with MS should receive support and counseling from their MS specialist on issues about possible pregnancy,” said Dr. Houtchens, a neurologist who directs the Women’s Health Program at the Partners MS Center at Brigham and Women’s Hospital, Boston. “I believe that we should all feel comfortable discussing with our patients the effects of pregnancy on their MS course and the reciprocal effect on pregnancy outcomes of their disease, the genetic risk of disease in their offspring, and optimal conception timing. We need to be able to talk to them freely about disease control before, during, and after pregnancy.”
Dr. Houtchens, one of the authors of a recent multinational systematic review on the topic noted that increasing numbers of MS patients with stable disease are choosing not to become pregnant (Obstet. Gynecol. 2014;124:1157-68). Others “want to get pregnant and feel cheated out of their life goal,” she said.
A large survey of female patients with MS from Canada found that more than three-quarters had not become pregnant since being diagnosed with the disease. The most common contributing factor across both MS-related and non–MS-related categories was completion of families prior to an MS diagnosis (53%). The top MS-related reasons that contributed to not having children were symptoms interfering with parenting, burdening the partner, and finances (Mult. Scler. 2013;19:351-8).
“It’s only in the last 2-3 decades that this issue has come to the forefront in caring for MS patients,” she said. “Previous to that, a lot of colleagues would discourage patients from becoming pregnant. Some of those attitudes persist to this day. There’s a perception of disapproval from health care providers on the part of the patient, and from their family and peers, and historic misconceptions. Our goal as physicians is to help women live lives to their fullest potential with a disease that can’t be cured. We should not discourage our patients from becoming pregnant.”
To optimize chances of conception, she recommended that oral contraceptives be stopped 2-3 months prior to conception attempts, and patients should be advised to transition to mechanical birth control. The optimal “fertility window” is a 6-day period, ending with the ovulation day. This window can be estimated based on duration of menstrual cycle, cervical mucus, and basal body temperature, as well as commercially available ovulation kits. Intercourse is most likely going to result in a pregnancy if attempted within a 3-day period, ending with the ovulation day. Moderate alcohol consumption, smoking, drug use, and vaginal lubricant use decrease the chance of contraception, she said.
Assisted reproductive technologies, if associated with failed pregnancy attempts, can lead to an increased relapse rate.
“If somebody really wants to have a child and are not able to conceive on their own, they may certainly consider ART; it’s all about education,” Dr. Houtchens said. “You just need to tell them what to expect and that they might have a higher risk of relapses if their attempt is unsuccessful.”
According to the medical literature, an MS patient’s prepregnancy annualized relapse rate predicts her risk for relapse during the postpartum period. “From that perspective, if you have the luxury of time, you ideally try to make sure that her disease is stabilized for the year before she becomes pregnant,” she said. “That means if she has an active MRI or has had a couple of attacks on whatever drug she’s taking, you talk to her about that, and you change the medication and repeat the MRI after the medication is changed to try to make sure that her disease is stable before she becomes pregnant. Hopefully that will help prevent postpartum attacks.”
Dr. Houtchens recommends standard preconception care including prenatal vitamins with 0.4 mg-1 mg of daily folate, smoking and alcohol cessation, improved sleep hygiene, and vitamin D3 supplementation. Low levels of vitamin D3 are associated with adverse pregnancy outcomes, and a poorer clinical and radiologic MS course. Low levels may also be associated with increased MS risk in offspring.
“A pregnancy test should be administered prior to every treatment with a chemotherapeutic or cytotoxic agent in a woman of child-bearing age, even if she thinks her periods have ceased as a result of chemotherapy treatment,” she said. “They may have ceased due to pregnancy, and we don’t want to treat our pregnant patients with chemotherapy.”
The approximate risk of MS is 1 in 500 in the general population, 1 in 100 in people with an affected second-degree relative, 1 in 50 with an affected first-degree relative, and 1 in 4 in monozygotic twins born to an affected mother, according to Dr. Houtchens.
Babies born to MS moms face a risk of being slightly smaller for gestational age by weight (odds ratio, 1.45) but their Apgar scores are the same as their healthy mom counterpart babies. MS moms face a slight risk for operative deliveries but no increased risk for birth defects or other adverse fetal outcomes specifically related to MS. The method of labor and delivery does not impact the postpartum course of the disease, she said.
“Epidural anesthesia is perfectly safe and does not impact the postpartum course of MS,” Dr. Houtchens said. “There’s still an ongoing misconception [about this], especially among obstetric providers and anesthesiologists. Multiple studies have been published stating that there is no increased risk of relapse in these patients after they get an epidural. If they don’t want an epidural, that’s okay, but they shouldn’t be denied it because they have MS.”
Secondary MS symptoms may be affected by pregnancy, including fatigue, bladder symptoms, and mobility difficulty due to increased weight. IV corticosteroids are used widely to treat MS relapses during pregnancy, as well as in obstetrics to speed fetal lung maturity.
Steroids “cross the placental barrier and may increase the risk of cleft palate when used in the first trimester or may cause lower birth weight in the baby and earlier than expected delivery. They could also in theory delay healing for the mother after giving birth,” she said.
However, prednisone, prednisolone, and methylprednisolone can be administered with low levels of fetal exposure. “These agents are metabolized to inactive forms by 11 beta-hydroxysteroid dehydrogenase in the placenta, allowing less than 10% of the maternal dose to reach the fetus,” she said. Betamethasone and dexamethasone cross the placenta with minimal metabolism, leading to direct full-dose effects on the fetus.
Dr. Houtchens cautioned that none of the available MS drugs should be used in pregnant patients.
“It appears that pregnancy itself is protective enough that we don’t need to use a drug to keep them healthier,” she said.
For a nonlactating patient, it’s safe to resume MS therapy within 1 week after birth. For a lactating patient with previously active disease, it may be safe to administer monthly steroids or monthly IVIG, instructing them to discontinue breastfeeding for 24 hours after treatment. In breast milk, beta-interferon agents are found at 0.006% of the maternal dose. Oral small molecules such as fingolimod and dimethyl fumarate “are freely passed in breast milk at a lower level than in sera but are more likely to directly affect the infant’s immune/neurological systems,” Dr. Houtchens said. “Hepatic clearance is slower in infants. We don’t have anyone at our MS center taking any MS medications and breastfeeding. Is this the right thing to do? I don’t know. But if you have someone who really wants to breastfeed, you could theoretically put them on an injectable medication.”
MRI should be repeated within 6 months postpartum to assess radiographic disease activity, she recommended.
Dr. Houtchers pointed out that postpartum depression is common in mothers with MS, “and it’s probably under-studied in general.” In fact, the lifetime prevalence of major depressive disorder in people with MS is estimated to be approximately 50%, while the rate of suicides among people with MS is 7.5 times greater than that of the general population.
Helping MS patients navigate conception, pregnancy, and the postpartum period is just the beginning.
“You’re still going to be her doctor,” Dr. Houtchens said. “She’s still going to have that child for the rest of her life. How is she going to deal with raising the child with all of the symptoms of her disease over time? How is she going to relate to her child? You’re going to walk this road with your patients, as one of their most important health care providers.”
Dr. Houtchens disclosed that she has received research grants from Genzyme Sanofi, Biogen Idec, and Novartis. She has also served as a consultant for Teva Pharmaceuticals, Genzyme Sanofi, Questcor, Biogen Idec, and Novartis.
On Twitter @dougbrunk
INDIANAPOLIS – If you feel overwhelmed by the notion of managing multiple sclerosis patients who seek your guidance in navigating their pregnancy, you’re not alone.
Comprehensive management programs for pregnant MS patients currently do not exist, even within most dedicated MS centers and clinics, Dr. Maria K. Houtchens said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Individual providers have an interest in this area, but there are no guidelines for most physicians or nurse practitioners to follow yet,” she said. “The level of care these patients receive varies dramatically, from one part of the country to another and from one provider to the next.”
An estimated 50% of all pregnancies in the United States and about 40% of all pregnancies worldwide are unplanned.
“I believe that women with MS should receive support and counseling from their MS specialist on issues about possible pregnancy,” said Dr. Houtchens, a neurologist who directs the Women’s Health Program at the Partners MS Center at Brigham and Women’s Hospital, Boston. “I believe that we should all feel comfortable discussing with our patients the effects of pregnancy on their MS course and the reciprocal effect on pregnancy outcomes of their disease, the genetic risk of disease in their offspring, and optimal conception timing. We need to be able to talk to them freely about disease control before, during, and after pregnancy.”
Dr. Houtchens, one of the authors of a recent multinational systematic review on the topic noted that increasing numbers of MS patients with stable disease are choosing not to become pregnant (Obstet. Gynecol. 2014;124:1157-68). Others “want to get pregnant and feel cheated out of their life goal,” she said.
A large survey of female patients with MS from Canada found that more than three-quarters had not become pregnant since being diagnosed with the disease. The most common contributing factor across both MS-related and non–MS-related categories was completion of families prior to an MS diagnosis (53%). The top MS-related reasons that contributed to not having children were symptoms interfering with parenting, burdening the partner, and finances (Mult. Scler. 2013;19:351-8).
“It’s only in the last 2-3 decades that this issue has come to the forefront in caring for MS patients,” she said. “Previous to that, a lot of colleagues would discourage patients from becoming pregnant. Some of those attitudes persist to this day. There’s a perception of disapproval from health care providers on the part of the patient, and from their family and peers, and historic misconceptions. Our goal as physicians is to help women live lives to their fullest potential with a disease that can’t be cured. We should not discourage our patients from becoming pregnant.”
To optimize chances of conception, she recommended that oral contraceptives be stopped 2-3 months prior to conception attempts, and patients should be advised to transition to mechanical birth control. The optimal “fertility window” is a 6-day period, ending with the ovulation day. This window can be estimated based on duration of menstrual cycle, cervical mucus, and basal body temperature, as well as commercially available ovulation kits. Intercourse is most likely going to result in a pregnancy if attempted within a 3-day period, ending with the ovulation day. Moderate alcohol consumption, smoking, drug use, and vaginal lubricant use decrease the chance of contraception, she said.
Assisted reproductive technologies, if associated with failed pregnancy attempts, can lead to an increased relapse rate.
“If somebody really wants to have a child and are not able to conceive on their own, they may certainly consider ART; it’s all about education,” Dr. Houtchens said. “You just need to tell them what to expect and that they might have a higher risk of relapses if their attempt is unsuccessful.”
According to the medical literature, an MS patient’s prepregnancy annualized relapse rate predicts her risk for relapse during the postpartum period. “From that perspective, if you have the luxury of time, you ideally try to make sure that her disease is stabilized for the year before she becomes pregnant,” she said. “That means if she has an active MRI or has had a couple of attacks on whatever drug she’s taking, you talk to her about that, and you change the medication and repeat the MRI after the medication is changed to try to make sure that her disease is stable before she becomes pregnant. Hopefully that will help prevent postpartum attacks.”
Dr. Houtchens recommends standard preconception care including prenatal vitamins with 0.4 mg-1 mg of daily folate, smoking and alcohol cessation, improved sleep hygiene, and vitamin D3 supplementation. Low levels of vitamin D3 are associated with adverse pregnancy outcomes, and a poorer clinical and radiologic MS course. Low levels may also be associated with increased MS risk in offspring.
“A pregnancy test should be administered prior to every treatment with a chemotherapeutic or cytotoxic agent in a woman of child-bearing age, even if she thinks her periods have ceased as a result of chemotherapy treatment,” she said. “They may have ceased due to pregnancy, and we don’t want to treat our pregnant patients with chemotherapy.”
The approximate risk of MS is 1 in 500 in the general population, 1 in 100 in people with an affected second-degree relative, 1 in 50 with an affected first-degree relative, and 1 in 4 in monozygotic twins born to an affected mother, according to Dr. Houtchens.
Babies born to MS moms face a risk of being slightly smaller for gestational age by weight (odds ratio, 1.45) but their Apgar scores are the same as their healthy mom counterpart babies. MS moms face a slight risk for operative deliveries but no increased risk for birth defects or other adverse fetal outcomes specifically related to MS. The method of labor and delivery does not impact the postpartum course of the disease, she said.
“Epidural anesthesia is perfectly safe and does not impact the postpartum course of MS,” Dr. Houtchens said. “There’s still an ongoing misconception [about this], especially among obstetric providers and anesthesiologists. Multiple studies have been published stating that there is no increased risk of relapse in these patients after they get an epidural. If they don’t want an epidural, that’s okay, but they shouldn’t be denied it because they have MS.”
Secondary MS symptoms may be affected by pregnancy, including fatigue, bladder symptoms, and mobility difficulty due to increased weight. IV corticosteroids are used widely to treat MS relapses during pregnancy, as well as in obstetrics to speed fetal lung maturity.
Steroids “cross the placental barrier and may increase the risk of cleft palate when used in the first trimester or may cause lower birth weight in the baby and earlier than expected delivery. They could also in theory delay healing for the mother after giving birth,” she said.
However, prednisone, prednisolone, and methylprednisolone can be administered with low levels of fetal exposure. “These agents are metabolized to inactive forms by 11 beta-hydroxysteroid dehydrogenase in the placenta, allowing less than 10% of the maternal dose to reach the fetus,” she said. Betamethasone and dexamethasone cross the placenta with minimal metabolism, leading to direct full-dose effects on the fetus.
Dr. Houtchens cautioned that none of the available MS drugs should be used in pregnant patients.
“It appears that pregnancy itself is protective enough that we don’t need to use a drug to keep them healthier,” she said.
For a nonlactating patient, it’s safe to resume MS therapy within 1 week after birth. For a lactating patient with previously active disease, it may be safe to administer monthly steroids or monthly IVIG, instructing them to discontinue breastfeeding for 24 hours after treatment. In breast milk, beta-interferon agents are found at 0.006% of the maternal dose. Oral small molecules such as fingolimod and dimethyl fumarate “are freely passed in breast milk at a lower level than in sera but are more likely to directly affect the infant’s immune/neurological systems,” Dr. Houtchens said. “Hepatic clearance is slower in infants. We don’t have anyone at our MS center taking any MS medications and breastfeeding. Is this the right thing to do? I don’t know. But if you have someone who really wants to breastfeed, you could theoretically put them on an injectable medication.”
MRI should be repeated within 6 months postpartum to assess radiographic disease activity, she recommended.
Dr. Houtchers pointed out that postpartum depression is common in mothers with MS, “and it’s probably under-studied in general.” In fact, the lifetime prevalence of major depressive disorder in people with MS is estimated to be approximately 50%, while the rate of suicides among people with MS is 7.5 times greater than that of the general population.
Helping MS patients navigate conception, pregnancy, and the postpartum period is just the beginning.
“You’re still going to be her doctor,” Dr. Houtchens said. “She’s still going to have that child for the rest of her life. How is she going to deal with raising the child with all of the symptoms of her disease over time? How is she going to relate to her child? You’re going to walk this road with your patients, as one of their most important health care providers.”
Dr. Houtchens disclosed that she has received research grants from Genzyme Sanofi, Biogen Idec, and Novartis. She has also served as a consultant for Teva Pharmaceuticals, Genzyme Sanofi, Questcor, Biogen Idec, and Novartis.
On Twitter @dougbrunk
INDIANAPOLIS – If you feel overwhelmed by the notion of managing multiple sclerosis patients who seek your guidance in navigating their pregnancy, you’re not alone.
Comprehensive management programs for pregnant MS patients currently do not exist, even within most dedicated MS centers and clinics, Dr. Maria K. Houtchens said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Individual providers have an interest in this area, but there are no guidelines for most physicians or nurse practitioners to follow yet,” she said. “The level of care these patients receive varies dramatically, from one part of the country to another and from one provider to the next.”
An estimated 50% of all pregnancies in the United States and about 40% of all pregnancies worldwide are unplanned.
“I believe that women with MS should receive support and counseling from their MS specialist on issues about possible pregnancy,” said Dr. Houtchens, a neurologist who directs the Women’s Health Program at the Partners MS Center at Brigham and Women’s Hospital, Boston. “I believe that we should all feel comfortable discussing with our patients the effects of pregnancy on their MS course and the reciprocal effect on pregnancy outcomes of their disease, the genetic risk of disease in their offspring, and optimal conception timing. We need to be able to talk to them freely about disease control before, during, and after pregnancy.”
Dr. Houtchens, one of the authors of a recent multinational systematic review on the topic noted that increasing numbers of MS patients with stable disease are choosing not to become pregnant (Obstet. Gynecol. 2014;124:1157-68). Others “want to get pregnant and feel cheated out of their life goal,” she said.
A large survey of female patients with MS from Canada found that more than three-quarters had not become pregnant since being diagnosed with the disease. The most common contributing factor across both MS-related and non–MS-related categories was completion of families prior to an MS diagnosis (53%). The top MS-related reasons that contributed to not having children were symptoms interfering with parenting, burdening the partner, and finances (Mult. Scler. 2013;19:351-8).
“It’s only in the last 2-3 decades that this issue has come to the forefront in caring for MS patients,” she said. “Previous to that, a lot of colleagues would discourage patients from becoming pregnant. Some of those attitudes persist to this day. There’s a perception of disapproval from health care providers on the part of the patient, and from their family and peers, and historic misconceptions. Our goal as physicians is to help women live lives to their fullest potential with a disease that can’t be cured. We should not discourage our patients from becoming pregnant.”
To optimize chances of conception, she recommended that oral contraceptives be stopped 2-3 months prior to conception attempts, and patients should be advised to transition to mechanical birth control. The optimal “fertility window” is a 6-day period, ending with the ovulation day. This window can be estimated based on duration of menstrual cycle, cervical mucus, and basal body temperature, as well as commercially available ovulation kits. Intercourse is most likely going to result in a pregnancy if attempted within a 3-day period, ending with the ovulation day. Moderate alcohol consumption, smoking, drug use, and vaginal lubricant use decrease the chance of contraception, she said.
Assisted reproductive technologies, if associated with failed pregnancy attempts, can lead to an increased relapse rate.
“If somebody really wants to have a child and are not able to conceive on their own, they may certainly consider ART; it’s all about education,” Dr. Houtchens said. “You just need to tell them what to expect and that they might have a higher risk of relapses if their attempt is unsuccessful.”
According to the medical literature, an MS patient’s prepregnancy annualized relapse rate predicts her risk for relapse during the postpartum period. “From that perspective, if you have the luxury of time, you ideally try to make sure that her disease is stabilized for the year before she becomes pregnant,” she said. “That means if she has an active MRI or has had a couple of attacks on whatever drug she’s taking, you talk to her about that, and you change the medication and repeat the MRI after the medication is changed to try to make sure that her disease is stable before she becomes pregnant. Hopefully that will help prevent postpartum attacks.”
Dr. Houtchens recommends standard preconception care including prenatal vitamins with 0.4 mg-1 mg of daily folate, smoking and alcohol cessation, improved sleep hygiene, and vitamin D3 supplementation. Low levels of vitamin D3 are associated with adverse pregnancy outcomes, and a poorer clinical and radiologic MS course. Low levels may also be associated with increased MS risk in offspring.
“A pregnancy test should be administered prior to every treatment with a chemotherapeutic or cytotoxic agent in a woman of child-bearing age, even if she thinks her periods have ceased as a result of chemotherapy treatment,” she said. “They may have ceased due to pregnancy, and we don’t want to treat our pregnant patients with chemotherapy.”
The approximate risk of MS is 1 in 500 in the general population, 1 in 100 in people with an affected second-degree relative, 1 in 50 with an affected first-degree relative, and 1 in 4 in monozygotic twins born to an affected mother, according to Dr. Houtchens.
Babies born to MS moms face a risk of being slightly smaller for gestational age by weight (odds ratio, 1.45) but their Apgar scores are the same as their healthy mom counterpart babies. MS moms face a slight risk for operative deliveries but no increased risk for birth defects or other adverse fetal outcomes specifically related to MS. The method of labor and delivery does not impact the postpartum course of the disease, she said.
“Epidural anesthesia is perfectly safe and does not impact the postpartum course of MS,” Dr. Houtchens said. “There’s still an ongoing misconception [about this], especially among obstetric providers and anesthesiologists. Multiple studies have been published stating that there is no increased risk of relapse in these patients after they get an epidural. If they don’t want an epidural, that’s okay, but they shouldn’t be denied it because they have MS.”
Secondary MS symptoms may be affected by pregnancy, including fatigue, bladder symptoms, and mobility difficulty due to increased weight. IV corticosteroids are used widely to treat MS relapses during pregnancy, as well as in obstetrics to speed fetal lung maturity.
Steroids “cross the placental barrier and may increase the risk of cleft palate when used in the first trimester or may cause lower birth weight in the baby and earlier than expected delivery. They could also in theory delay healing for the mother after giving birth,” she said.
However, prednisone, prednisolone, and methylprednisolone can be administered with low levels of fetal exposure. “These agents are metabolized to inactive forms by 11 beta-hydroxysteroid dehydrogenase in the placenta, allowing less than 10% of the maternal dose to reach the fetus,” she said. Betamethasone and dexamethasone cross the placenta with minimal metabolism, leading to direct full-dose effects on the fetus.
Dr. Houtchens cautioned that none of the available MS drugs should be used in pregnant patients.
“It appears that pregnancy itself is protective enough that we don’t need to use a drug to keep them healthier,” she said.
For a nonlactating patient, it’s safe to resume MS therapy within 1 week after birth. For a lactating patient with previously active disease, it may be safe to administer monthly steroids or monthly IVIG, instructing them to discontinue breastfeeding for 24 hours after treatment. In breast milk, beta-interferon agents are found at 0.006% of the maternal dose. Oral small molecules such as fingolimod and dimethyl fumarate “are freely passed in breast milk at a lower level than in sera but are more likely to directly affect the infant’s immune/neurological systems,” Dr. Houtchens said. “Hepatic clearance is slower in infants. We don’t have anyone at our MS center taking any MS medications and breastfeeding. Is this the right thing to do? I don’t know. But if you have someone who really wants to breastfeed, you could theoretically put them on an injectable medication.”
MRI should be repeated within 6 months postpartum to assess radiographic disease activity, she recommended.
Dr. Houtchers pointed out that postpartum depression is common in mothers with MS, “and it’s probably under-studied in general.” In fact, the lifetime prevalence of major depressive disorder in people with MS is estimated to be approximately 50%, while the rate of suicides among people with MS is 7.5 times greater than that of the general population.
Helping MS patients navigate conception, pregnancy, and the postpartum period is just the beginning.
“You’re still going to be her doctor,” Dr. Houtchens said. “She’s still going to have that child for the rest of her life. How is she going to deal with raising the child with all of the symptoms of her disease over time? How is she going to relate to her child? You’re going to walk this road with your patients, as one of their most important health care providers.”
Dr. Houtchens disclosed that she has received research grants from Genzyme Sanofi, Biogen Idec, and Novartis. She has also served as a consultant for Teva Pharmaceuticals, Genzyme Sanofi, Questcor, Biogen Idec, and Novartis.
On Twitter @dougbrunk
EXPERT ANALYSIS AT THE CMSC ANNUAL MEETING