User login
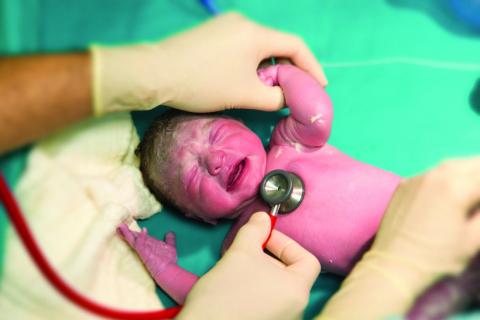
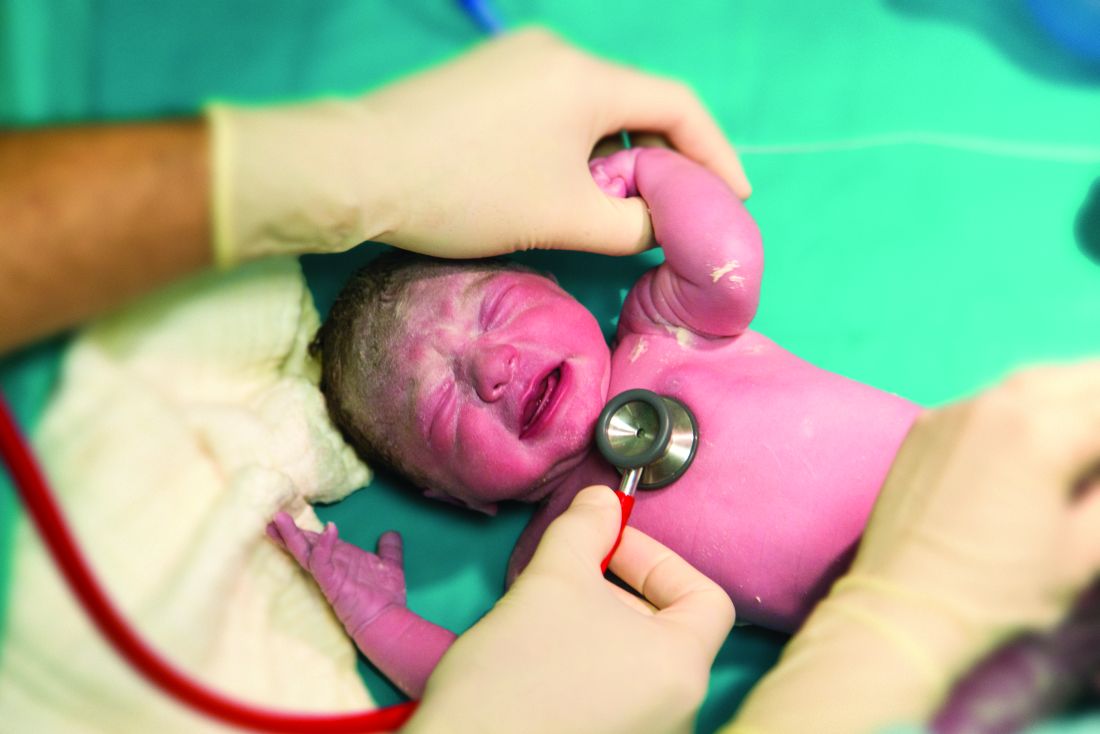
Rural areas with local obstetrical care have better perinatal outcomes
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
FROM ANNALS OF FAMILY MEDICINE
Key clinical point: The absence of labor and delivery (L&D) services in rural counties predicts adverse outcomes, including higher child mortality.
Major finding: In the absence of L&D units, the risk of perinatal mortality per 1,000 live births is 19% higher (5.67 vs. 4.74; P = .0034).
Data Source: Retrospective cohort study.
Disclosures: Potential conflicts of interest involving this topic were not reported.
Source: Waits JB et al. Ann Fam Med. 2020;18:446-51.
How to build your identity as a physician online
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
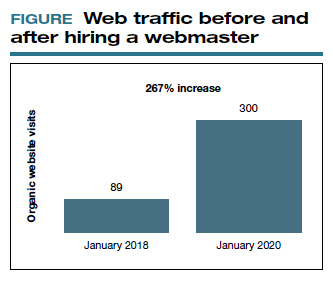
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.
Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.

Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.

Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
Major changes in Medicare billing are planned for January 2021: Some specialties fare better than others
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.
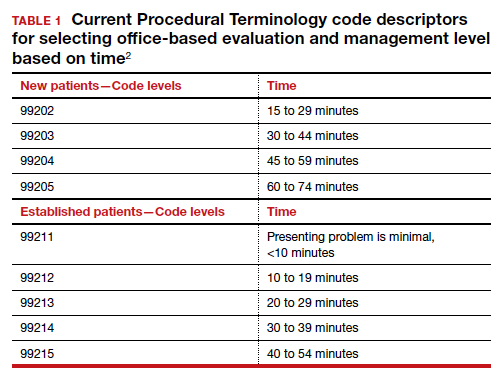
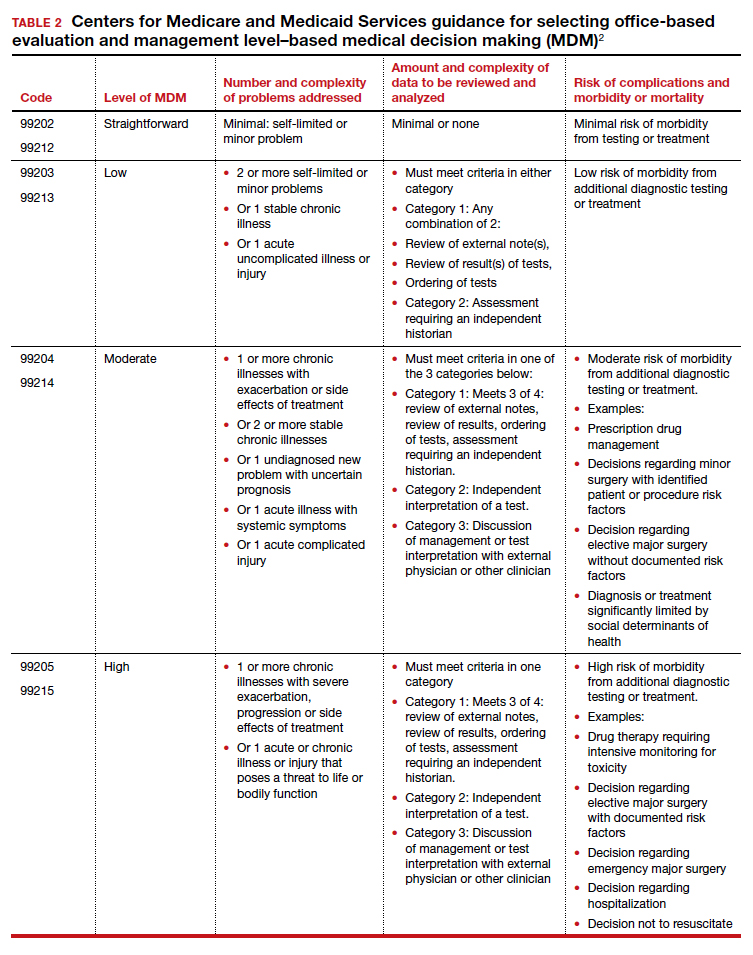
Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.
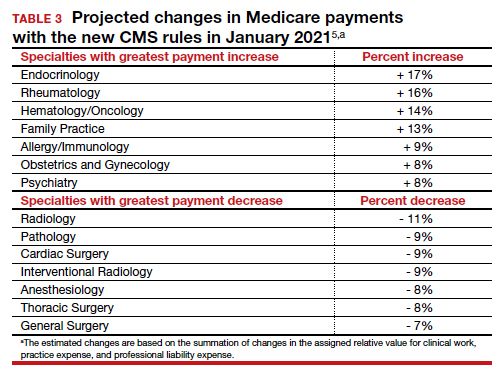
Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.
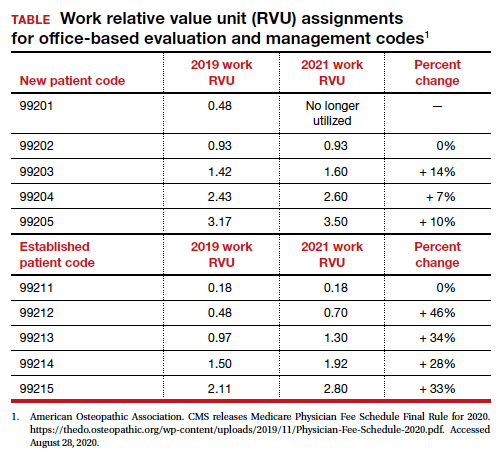
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

The Centers for Medicare and Medicaid Services (CMS) finalized an increase in the relative value of evaluation and management (E/M) service codes effective January 1, 2021, which results in an overall decrease in the payment for procedural services in the Medicare program. (Due to the mandate for budget neutrality, an increase in relative value units [RVUs] for E/M resulted in a large decrease in the conversion factor—the number of dollars per RVU). This has increased payments for endocrinologists, rheumatologists, and family medicine clinicians and decreased payments for radiologists, pathologists, and surgeons.
In a major win for physicians, CMS proposes to simplify documentation requirements for billing and focus on the complexity of the medical decision making (MDM) or the total time needed to care for the patient on the date of the service as the foundation for determining the relative value of the service. Therefore, there is no more counting bullets—ie, we don’t have to perform a comprehensive physical exam or review of systems to achieve a high level code! Prior to this change, time was only available for coding purposes when counseling and coordination of care was the predominant service (>50%), and only face-to-face time with the patient was considered. Effective January 1, for office and other outpatient services, total time on the calendar date of the encounter will be used. This acknowledges the intensity and value of non–face-to-face work.
Acting through CMS, the federal government influences greatly the US health care system. CMS is an agency in the Department of Health and Human Services that administers the Medicare program and partners with state governments to administer the Health Insurance Exchanges, Medicaid, and the Children’s Health Insurance programs (CHIP).1 In addition, CMS is responsible for enforcing quality care standards in long-term care facilities and clinical laboratories and the implementation of the Health Insurance Portability and Accountability Act.1
In January, CMS plans the following major changes to coding and documentation2,3:
- Selection of the level of E/M service will no longer require documentation of bullet points in the history, physical exam, and MDM. The simplified system allows physicians and qualified health care professionals to code either by total time (both face-to-face and non–face-to-face) on the date of the encounter or by level of MDM.
- For established office patients, 5 levels of office-based evaluation and management services will be retained. CMS had initially proposed to reduce the number of office-based E/M codes from 5 to 3, combining code levels 2, 3, and 4 into 1 code.4 However, after receiving feedback from professional societies and the public, CMS abandoned the plan for radical simplification of coding levels.2,3 Implementation of their proposal would have resulted in the same payment for treatment of a hang nail as for a complex gyn patient with multiple medical problems. Both patient advocacy groups and professional societies argued that incentives originally were misaligned.
- For new office patients, since both 99201 and 99202 require straightforward MDM, the level 1 code (99201) has been eliminated, reducing the number of code levels from 5 to 4.
- History and physical exam will no longer be used to determine code level for office E/M codes. These elements will be required only as medically appropriate. This means that documentation review will no longer focus on “bean counting” the elements in the history and physical exam.
- Following a reassessment of the actual time required to provide E/M services in real-life practice, CMS plans to markedly increase the relative value of office visits for established patients and modestly increase the relative value of office visits for new patients. CMS operates under the principle of “neutral budgeting,” meaning that an increase of the relative value of E/M codes will result in a decrease in the payment for procedural codes. The actual RVUs for procedural services do not change; however, budget neutrality requires a decrease in the dollar conversion factor. The proposed changes will increase the payment for E/M services and decrease payments for procedural services.
Continue to: Refocusing practice on MDM complexity...
Refocusing practice on MDM complexity
The practice of medicine is a calling with great rewards. Prominent among those rewards are improving the health of women, children, and the community, developing deep and trusting relationships with patients, families, and clinical colleagues. The practice of medicine is also replete with a host of punishing administrative burdens, including prior authorizations, clunky electronic medical records, poorly designed quality metrics that are applied to clinicians, and billing compliance rules that emphasize the repetitive documentation of clinical information with minimal value.
Some of the most irritating aspects of medical practice are the CMS rules governing medical record documentation required for billing ambulatory office visits. Current coding compliance focuses on counting the number of systems reviewed in the review of systems; the documentation of past history, social history, and family history; the number of organs and organ elements examined during the physical examination; and the complexity of MDM.
In January 2021, CMS plans to adopt new Current Procedural Terminology (CPT) code descriptors for the office and other outpatient E/M services that sunset most of the “bean-counting” metrics and emphasize the importance of the complexity of MDM in guiding selection of a correct code.2 Beginning in January 2021, clinicians will have the option of selecting an E/M code level based on the total amount of time required to provide the office visit service or the complexity of MDM. When selecting a code level based on MDM the new guidance emphasizes the importance of reviewing notes from other clinicians, reviewing test results, ordering of tests, and discussing and coordinating the care of the patient with other treating physicians. These changes reflect a better understanding of what is most important in good medical practice, promoting better patient care. TABLES 1 and 2 provide the initial guidance from CMS concerning selection of E/M code level based on time and MDM, respectively.2 The guidance for using MDM to select an E/M code level is likely to evolve following implementation, so stay tuned. When using MDM to select a code, 2 of the 3 general categories are required to select that level of service.


Increase in the valuation of office-based E/M services
The Medicare Physician Fee Schedule uses a resource-based relative value system to determine time and intensity of the work of clinical practice. This system recognizes 3 major factors that influence the resources required to provide a service:
- work of the clinician
- practice expense for technical components
- cost of professional liability insurance.
Many primary care professional associations have long contended that CMS has undervalued office-based E/M services relative to procedures, resulting in the devaluing of primary care practice. After the CPT code descriptors were updated by the CPT editorial panel, 52 specialty societies surveyed their members to provide inputs to CMS on the time and intensity of the office and other outpatient E/M codes as currently practiced. The American Medical Association’s Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) reviewed the surveys and provided new inputs via open comment to CMS. CMS has responded to this feedback with a review of the intensity of clinical work required to provide an ambulatory visit service. In response to the review, CMS proposes to accept the recommendations of the RUC representing the house of medicine and increase the work and practice expense relative value assigned to new and established office visit codes. Overall, the combination of changes in relative values assigned for the work of the clinician and the expense of practice, increases the total value of office-based E/M codes for new patients by 7% to 14% and for established patients from 28% to 46% (see supplemental table in the sidebar at the end of this article).
Continue to: Decreased payments for procedural services...
Decreased payments for procedural services
Medicare is required to offset increased payment in one arena of health care delivery with decreased payment in other arenas of care, thereby achieving “budget-neutrality.” As detailed above, CMS plans to increase Medicare payments for office-based E/M services. Payment for services is calculated by multiplying the total RVUs for a particular service by a “conversion factor” (ie, number of dollars per RVU). To achieve budget-neutrality, CMS has proposed substantially reducing the conversion factor for 2021 (from $36.09 to $32.26), which will effectively decrease Medicare payments for procedural services since their RVUs have not changed. While the AMA RUC and many specialty societies continue to strongly advocate for the E/M work RVU increases to be included in the E/M components of 10- and 90-day global services, CMS has proposed to implement them only for “stand alone” E/M services.
Organizations are lobbying to delay or prevent the planned decrease in conversion factor, which results in substantial declines in payment for procedural services. (See "What do the Medicare billing changes mean for the Obstetrical Bundled services?" at the end of this article.) Due to the economic and clinical practice challenges caused by the coronavirus disease 2019 (COVID-19) pandemic it would be best if CMS did not reduce payments to physicians who are experts in procedural health care, thereby avoiding the risk of reduced access to these vital services.
If the current CMS changes in payment are implemented, endocrinologists, rheumatologists, and family physicians will have an increase in payment, and radiologists, pathologists, and surgeons will have a decrease in payment (TABLE 3).6 Obstetrics and gynecology is projected to have an 8% increase in Medicare payment. However, if an obstetrician-gynecologist derives most of their Medicare payments from surgical procedures, they are likely to have a decrease in payment from Medicare. Other payers will be incorporating the new coding structure for 2021; however, their payment structures and conversion factors are likely to vary. It is important to note that the RVUs for procedures have not changed. The budget neutrality adjustment resulted in a much lower conversion factor and therefore a decrease in payment for those specialties whose RVUs did not increase.

Bottom line
Working through the Medicare, Medicaid, and CHIP programs, CMS can influence greatly the practice of medicine including medical record documentation practices and payment rates for every clinical service. CMS proposes to end the onerous “bean counting” approach to billing compliance and refocus on the complexity of MDM as the foundation for selecting a billing code level. This change is long overdue, valuing the effective management of complex patients in office practice. Hopefully, CMS will reverse the planned reduction in the payment for procedural services, preserving patient access to important health care services. ●
The CY 2020 Medicare Physician Fee Schedule Final Rule was published electronically in the Federal Register on November 1, 2019. This final rule aligns the evaluation and management (E/M) coding and payment with changes recommended by the Current Procedural Terminology (CPT) Editorial Panel and American Medical Association’s (AMA) Specialty Society Resource-Based Relative Value Scale Update Committee (RUC) for office/outpatient E/M visits. Unfortunately, the Centers for Medicare and Medicaid Services (CMS) did not agree with the RUC, AMA, and specialty societies that the E/M payment changes should be applicable across all global services that incorporate E/M visits—despite the fact that the values proposed by the RUC incorporated survey data from 52 specialties, representing most of medicine (including those specialties that predominantly perform procedures). Specifically, CMS expressed the view that the number of E/M visits within the 10- and 90-day global codes, as well as the maternity care bundle, were difficult to validate; therefore, the increased values would not be distributed to those procedural services.
Many professional societies expressed significant concerns about the resulting budget neutrality adjustments that would occur effective January 2021. The great news for ObGyns is that the American College of Obstetricians and Gynecologists (ACOG) was able to respond directly to CMS’s concerns with data to support the number of prenatal visits within the Obstetrical Bundle. Tapping into a de-identified, cloud-based data set of prenatal records—representing more than 1,100 obstetric providers with close to 30,000 recently completed pregnancies—ACOG was able to document both a mean and median number of prenatal visits across a broad geographic, payer, and patient demographic that supported the 13 prenatal visits in the Obstetrical Bundle.
With ACOG’s advocacy and ability to provide data to CMS, the proposed physician fee schedule rule for 2021 has proposed to incorporate the E/M increased reimbursement into the prenatal care codes. Now we urge the CMS to finalize this proposal. Although Medicare pays for a tiny number of pregnancies annually, we hope that all payers, including Medicaid and managed care plans, will agree with this acknowledgement of the increased work of evaluation and management that obstetricians provide during prenatal care. Join ACOG in telling CMS to finalize their proposal to increase the values of the global obstetric codes: https://acog.quorum.us/campaign/28579/.

- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
- Centers for Medicare and Medicaid Services. https://www.cms.gov/. Accessed August 28, 2020.
- American Medical Association. CPT Evaluation and Management (E/M) Office or Other Outpatient (99202-99215) and Prolonged Services (99354, 99355, 99356, 99XXX) Code and Guideline Changes. 2019. https://www.ama-assn.org /system/files/2019-06/cpt-office-prolonged-svs -code-changes.pdf. Accessed August 28, 2020.
- The American Academy of Family Physicians. Family medicine updates. Ann Fam Med. 2020;18:84-85. doi: 10.1370/afm.2508.
- Centers for Medicare and Medicaid Services. Final policy, payment and quality provisions changes to the Medicare Physician Fee Schedule for calendar year 2019. November 1, 2018. https://www.cms.gov/newsroom/fact-sheets /final-policy-payment-and-quality-provisionschanges-medicare-physician-fee-schedulecalendar-year. Accessed August 28, 2020.
- Department of Health and Human Services; Centers for Medicare and Medicaid Services. 42 CFR Parts 410, 414, 415, 423, 424, and 425. Federal Register. 2020;85(159). https://www.govinfo.gov /content/pkg/FR-2020-08-17/pdf/2020-17127 .pdf. Accessed August 28, 2020.
Lifting the restrictions on mifepristone during COVID-19: A step in the right direction
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Psoriasis, PsA, and pregnancy: Tailoring treatment with increasing data
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.
With an average age of diagnosis of 28 years, and one of two incidence peaks occurring at 15-30 years, psoriasis affects many women in the midst of their reproductive years. The prospect of pregnancy – or the reality of a surprise pregnancy – drives questions about heritability of the disease in offspring, the impact of the disease on pregnancy outcomes and breastfeeding, and how to best balance risks of treatments with risks of uncontrolled psoriasis and/or psoriatic arthritis (PsA).
While answers to these questions are not always clear, discussions about pregnancy and psoriasis management “shouldn’t be scary,” said Jenny E. Murase, MD, a dermatologist who speaks and writes widely about her research and experience with psoriasis and pregnancy. “We have access to information and data and educational resources to [work with] and reassure our patients – we just need to use it. Right now, there’s unnecessary suffering [with some patients unnecessarily stopping all treatment].”
Much has been learned in the past 2 decades about the course of psoriasis in pregnancy, and pregnancy outcomes data on the safety of biologics during pregnancy are increasingly emerging – particularly for tumor necrosis factor (TNF)–alpha inhibitors.
Ideally, since half of all pregnancies are unplanned, the implications of therapeutic options should be discussed with all women with psoriasis who are of reproductive age, whether they are sexually active or not. “The onus is on us to make sure that we’re considering the possibility [that our patient] could become pregnant without consulting us first,” said Dr. Murase, associate professor of dermatology at the University of California, San Francisco, and director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif.
Lisa R. Sammaritano, MD, associate professor of clinical medicine at Weill Cornell Medicine and a rheumatologist at the Hospital for Special Surgery, both in New York, urges similar attention for PsA. “Pregnancy is best planned while patients have quiescent disease on pregnancy-compatible medications,” she said. “We encourage [more] rheumatologists to be actively involved in pregnancy planning [in order] to guide therapy.”
The impact of estrogen
Dr. Murase was inspired to study psoriasis and pregnancy in part by a patient she met as a medical student. “She had severe psoriasis covering her body, and she said that the only times her psoriasis cleared was during her three pregnancies,” Dr. Murase recalled. “I wondered: What about the pregnancies resulted in such a substantial reduction of her psoriasis?”
She subsequently led a study, published in 2005, of 47 pregnant and 27 nonpregnant patients with psoriasis. More than half of the patients – 55% – reported improvements in their psoriasis during pregnancy, 21% reported no change, and 23% reported worsening. Among the 16 patients who had 10% or greater psoriatic body surface area (BSA) involvement and reported improvements, lesions decreased by 84%.
In the postpartum period, only 9% reported improvement, 26% reported no change, and 65% reported worsening. The increased BSA values observed 6 weeks postpartum did not exceed those of the first trimester, suggesting a return to the patients’ baseline status.
Earlier and smaller retrospective studies had also shown that approximately half of patients improve during pregnancy, and it was believed that progesterone was most likely responsible for this improvement. Dr. Murase’s study moved the needle in that it examined BSA in pregnancy and the postpartum period. It also turned the spotlight on estrogen: Patients who had higher levels of improvement also had higher levels of estradiol, estrone, and the ratio of estrogen to progesterone. However, there was no correlation between psoriatic change and levels of progesterone.
To promote fetal survival, pregnancy triggers a shift from Th1 cell–mediated immunity – and Th17 immunity – to Th2 immunity. While there’s no proof of a causative effect, increased estrogen appears to play a role in this shift and in the reduced production of Th1 and Th17 cytokines. Psoriasis is believed to be primarily a Th17-mediated disease, with some Th1 involvement, so this down-regulation can result in improved disease status, Dr. Murase said. (A host of other autoimmune diseases categorized as Th1 mediated similarly tend to improve during pregnancy, she added.)
Information on the effect of pregnancy on PsA is “conflicting,” Dr. Sammaritano said. “Some [of a limited number of studies] suggest a beneficial effect as is generally seen for rheumatoid arthritis. Others, however, have found an increased risk of disease activity during pregnancy ... It may be that psoriatic arthritis can be quite variable from patient to patient in its clinical presentation.”
At least one study, Dr. Sammaritano added, “has shown that the arthritis in pregnancy patients with PsA did not improve, compared to control nonpregnant patients, while the psoriasis rash did improve.”
The mixed findings don’t surprise Dr. Murase. “It harder to quantify joint disease in general,” she said. “And during pregnancy, physiologic changes relating to the pregnancy itself can cause discomfort – your joints ache. The numbers [of improved] cases aren’t as high with PsA, but it’s a more complex question.”
In the postpartum period, however, research findings “all suggest an increased risk of flare” of PsA, Dr. Sammaritano said, just as with psoriasis.
Assessing risk of treatment
Understanding the immunologic effects of pregnancy on psoriasis and PsA – and appreciating the concept of a hormonal component – is an important part of treatment decision making. So is understanding pregnancy outcomes data.
Researchers have looked at a host of pregnancy outcomes – including congenital malformations, preterm birth, spontaneous abortion, low birth weight, macrosomia, and gestational diabetes and hypertension – in women with psoriasis or psoriasis/PsA, compared with control groups. Some studies have suggested a link between disease activity and pregnancy complications or adverse pregnancy outcomes, “just as a result of having moderate to severe disease,” while others have found no evidence of increased risk, Dr. Murase said.
“It’s a bit unclear and a difficult question to answer; it depends on what study you look at and what data you believe. It would be nice to have some clarity, but basically the jury is still out,” said Dr. Murase, who, with coauthors Alice B. Gottlieb, MD, PhD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and Caitriona Ryan, MD, of the Blackrock Clinic and Charles Institute of Dermatology, University College Dublin, discussed the pregnancy outcomes data in a recently published review of psoriasis in women.
“In my opinion, because we have therapies that are so low risk and well tolerated, it’s better to make sure that the inflammatory cascade and inflammation created by psoriasis is under control,” she said. “So whether or not the pregnancy itself causes the patient to go into remission, or whether you have to use therapy to help the patient stay in remission, it’s important to control the inflammation.”
Contraindicated in pregnancy are oral psoralen, methotrexate, and acitretin, the latter of which should be avoided for several years before pregnancy and “therefore shouldn’t be used in a woman of childbearing age,” said Dr. Murase. Methotrexate, said Dr. Sammaritano, should generally be stopped 1-3 months prior to conception.
For psoriasis, the therapy that’s “classically considered the safest in pregnancy is UVB light therapy, specifically the 300-nm wavelength of light, which works really well as an anti-inflammatory,” Dr. Murase said. Because of the potential for maternal folate degradation with phototherapy and the long-known association of folate deficiency with neural tube defects, women of childbearing age who are receiving light therapy should take daily folic acid supplementation. (She prescribes a daily prenatal vitamin containing at least 1 mg of folic acid for women who are utilizing light therapy.)
Many topical agents can be used during pregnancy, Dr. Murase said. Topical corticosteroids, she noted, have the most safety-affirming data of any topical medication.
Regarding oral therapies, Dr. Murase recommends against the use of apremilast (Otezla) for her patients. “It’s not contraindicated, but the animal studies don’t look promising, so I don’t use that one in women of childbearing age just in case. There’s just very little data to support the safety of this medication [in pregnancy].”
There are no therapeutic guidelines in the United States for guiding the management of psoriasis in women who are considering pregnancy. In 2012, the medical board of the National Psoriasis Foundation published a review of treatment options for psoriasis in pregnant or lactating women, the “closest thing to guidelines that we’ve had,” said Dr. Murase. (Now almost a decade old, the review addresses TNF inhibitors but does not cover the anti-interleukin agents more recently approved for moderate to severe psoriasis and PsA.)
For treating PsA, rheumatologists now have the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases to reference. The 2020 guideline does not address PsA specifically, but its section on pregnancy and lactation includes recommendations on biologic and other therapies used to treat the disease.
Guidelines aside, physician-patient discussions over drug safety have the potential to be much more meaningful now that drug labels offer clinical summaries, data, and risk summaries regarding potential use in pregnancy. The labels have “more of a narrative, which is a more useful way to counsel patients and make risk-benefit decisions” than the former system of five-letter categories, said Dr. Murase. (The changes were made per the Pregnancy and Lactation Labeling Rule of 2015.)
MothertoBaby, a service of the nonprofit Organization of Teratology Information Specialists, also provides good evidence-based information to physicians and mothers, Dr. Sammaritano noted.
The use of biologic therapies
In a 2017 review of biologic safety for patients with psoriasis during pregnancy, Alexa B. Kimball, MD, MPH, professor of dermatology at Harvard Medical School, Boston; Martina L. Porter, MD, currently with the department of dermatology at Beth Israel Deaconess Medical Center, Boston; and Stephen J. Lockwood, MD, MPH, of the department of dermatology at Harvard Medical School, concluded that an increasing body of literature suggests that biologic agents can be used during pregnancy and breastfeeding. Anti-TNF agents “should be considered over IL-12/23 and IL-17 inhibitors due to the increased availability of long-term data,” they wrote.
“In general,” said Dr. Murase, “there’s more and more data coming out from gastroenterology and rheumatology to reassure patients and prescribing physicians that the TNF-blocker class is likely safe to use in pregnancy,” particularly during the first trimester and early second trimester, when the transport of maternal antibodies across the placenta is “essentially nonexistent.” In the third trimester, the active transport of IgG antibodies increases rapidly.
If possible, said Dr. Sammaritano, who served as lead author of the ACR’s reproductive health guideline, TNF inhibitors “will be stopped prior to the third trimester to avoid [the possibility of] high drug levels in the infant at birth, which raises concern for immunosuppression in the newborn. If disease is very active, however, they can be continued throughout the pregnancy.”
The TNF inhibitor certolizumab pegol (Cimzia) has the advantage of being transported only minimally across the placenta, if at all, she and Dr. Murase both explained. “To be actively carried across, antibodies need what’s called an Fc region for the placenta to grab onto,” Dr. Murase said. Certolizumab – a pegylated anti–binding fragment antibody – lacks this Fc region.
Two recent studies – CRIB and a UCB Pharma safety database analysis – showed “essentially no medication crossing – there were barely detectable levels,” Dr. Murase said. Certolizumab’s label contains this information and other clinical trial data as well as findings from safety database analyses/surveillance registries.
“Before we had much data for the biologics, I’d advise transitioning patients to light therapy from their biologics and a lot of times their psoriasis would improve, but it was more of a dance,” she said. “Now we tend to look at [certolizumab] when they’re of childbearing age and keep them on the treatment. I know that the baby is not being immunosuppressed.”
Consideration of the use of certolizumab when treatment with biologic agents is required throughout the pregnancy is a recommendation included in Dr. Kimball’s 2017 review.
As newer anti-interleukin agents – the IL-12/23 and IL-17 inhibitors – play a growing role in the treatment of psoriasis and PsA, questions loom about their safety profile. Dr. Murase and Dr. Sammaritano are waiting for more data. “In general,” Dr. Sammaritano said, “we recommend stopping them at the time pregnancy is detected, based on a lack of data at this time.”
Small-molecule drugs are also less well studied, she noted. “Because of their low molecular weight, we anticipate they will easily cross the placenta, so we recommend avoiding use during pregnancy until more information is available.”
Postpartum care
The good news, both experts say, is that the vast majority of medications, including biologics, are safe to use during breastfeeding. Methotrexate should be avoided, Dr. Sammaritano pointed out, and the impact of novel small-molecule therapies on breast milk has not been studied.
In her 2019 review of psoriasis in women, Dr. Murase and coauthors wrote that too many dermatologists believe that breastfeeding women should either not be on biologics or are uncertain about biologic use during breastfeeding. However, “biologics are considered compatible for use while breastfeeding due to their large molecular size and the proteolytic environment in the neonatal gastrointestinal tract,” they added.
Counseling and support for breastfeeding is especially important for women with psoriasis, Dr. Murase emphasized. “Breastfeeding is very traumatizing to the skin, and psoriasis can form in skin that’s injured. I have my patients set up an office visit very soon after the pregnancy to make sure they’re doing alright with their breastfeeding and that they’re coating their nipple area with some type of moisturizer and keeping the health of their nipples in good shape.”
Timely reviews of therapy and adjustments are also a priority, she said. “We need to prepare for 6 weeks post partum” when psoriasis will often flare without treatment.
Dr. Murase disclosed that she is a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron. She is also coeditor in chief of the International Journal of Women’s Dermatology. Dr. Sammaritano reported that she has no disclosures relating to the treatment of PsA.
More research needed on how fetal exposure affects later development
The number of genes in humans seems inadequate to account for the diversity seen in people. While maternal and paternal factors do play a role in the development of offspring, increased attention is being paid to the forces that express these genes and the impact they have on the health of a person, including development of psychiatric conditions, according to Dolores Malaspina, MD.
Epigenetics, or changes that occur in a fetal phenotype that do not involve changes to the genotype, involve factors such as DNA methylation to control gene expression, histone modification or the wrapping of genes, or the silencing and activation of certain genes with noncoding RNA-associated factors, said Dr. Malaspina of the Icahn School of Medicine at Mount Sinai, New York.
When this occurs during pregnancy, “the fetus does not simply develop from a genetic blueprint of the genes from its father and mother. Instead, signals are received throughout the pregnancy as to the health of the mother and signals about the environment,” she said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
There is an evolutionary advantage to this so-called survival phenotype. “If, during the pregnancy, there’s a deficit of available nutrition, that may be a signal to the fetus that food will be scarce. In the setting of food scarcity, certain physiological adaptations during development can make the fetus more likely to survive to adulthood,” Dr. Malaspina said at the meeting, presented by Global Academy for Medical Education. But a fetus programmed to adapt to scarcity of food may also develop cardiovascular disease, metabolic disease, or mortality later in life if the prediction of scarce nutrition proved incorrect.
This approach to thinking about the developmental origins of health and disease, which examines how prenatal and perinatal exposure to environmental factors affect disease in adulthood, has also found a link between some exposures and psychiatric disorders. The most famous example, the Dutch Hunger Winter Families Study, found an increased risk of schizophrenia among children born during the height of the famine (Int J Epidemiol. 2007 Dec;36[6]:1196-204). During the Arab-Israeli war of 1967 (the Six-Day War), which took place in June, the fetuses of mothers who were pregnant during that month had a higher risk of schizophrenia if the fetus was in the second month (relative risk, 2.3; 95% confidence interval, 1.1-4.7) or third month (RR, 2.5; 95% CI, 1.2-5.2) of fetal life during June 1967, Dr. Malaspina and associates wrote (BMC Psychiatry. 2008 Aug 21;8:71).
“The key aspect is the ascertainment of individuals during a circumscribed period, the assessment and then the longitudinal follow-up,” she said. “Obviously, these are not easy studies to do, but enough of them have been done such that for the last decade at least, the general population should be aware of the developmental origins of health and disease.”
Maternal depression is another psychiatric condition that can serve as a prenatal exposure to adversity. A recent review found that children of women with untreated depression were 56% more likely to be born preterm and 96% more likely to have a low birth weight (Pediatr Res. 2019 Jan;85[2]:134-45). “Preterm birth and early birth along with low birth weight, these have ramifying effects throughout life, not only on neonatal and infant mortality, but on developmental disorders and lifetime morbidity,” she said. “These effects of maternal depression withstand all sorts of accounting for other correlated exposures, including maternal age and her medical complications or substance use.”
“The modulation of mood and affect can affect temperament and affect mental health. Studies exist linking maternal depression to autism, attention-deficit disorder, developmental delay, behavioral problems, sleep problems, externalizing behavior and depression, showing a very large effect of maternal depression on offspring well-being.”
To complicate matters, at least 15% of women will experience major depression during pregnancy, but of these, major depression is not being addressed in about half. Nonpharmacologic interventions can include cognitive-behavioral therapy and relaxation practices, but medication should be considered as well. “There’s an ongoing debate about whether antidepressant medications are harmful for the offspring,” she said. However, reviews conducted by Dr. Malaspina’s group have found low evidence of serious harm.
“My summary would be the depression itself holds much more evidence for disrupting offspring health and development than medications,” Dr. Malaspina said. “Most studies find no adverse birth effects when they properly controlled accounting for maternal age and the other conditions and other medications.”
Global Academy and this news organization are owned by the same parent company. Dr. Malaspina reported no relevant conflicts of interest.
The number of genes in humans seems inadequate to account for the diversity seen in people. While maternal and paternal factors do play a role in the development of offspring, increased attention is being paid to the forces that express these genes and the impact they have on the health of a person, including development of psychiatric conditions, according to Dolores Malaspina, MD.
Epigenetics, or changes that occur in a fetal phenotype that do not involve changes to the genotype, involve factors such as DNA methylation to control gene expression, histone modification or the wrapping of genes, or the silencing and activation of certain genes with noncoding RNA-associated factors, said Dr. Malaspina of the Icahn School of Medicine at Mount Sinai, New York.
When this occurs during pregnancy, “the fetus does not simply develop from a genetic blueprint of the genes from its father and mother. Instead, signals are received throughout the pregnancy as to the health of the mother and signals about the environment,” she said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
There is an evolutionary advantage to this so-called survival phenotype. “If, during the pregnancy, there’s a deficit of available nutrition, that may be a signal to the fetus that food will be scarce. In the setting of food scarcity, certain physiological adaptations during development can make the fetus more likely to survive to adulthood,” Dr. Malaspina said at the meeting, presented by Global Academy for Medical Education. But a fetus programmed to adapt to scarcity of food may also develop cardiovascular disease, metabolic disease, or mortality later in life if the prediction of scarce nutrition proved incorrect.
This approach to thinking about the developmental origins of health and disease, which examines how prenatal and perinatal exposure to environmental factors affect disease in adulthood, has also found a link between some exposures and psychiatric disorders. The most famous example, the Dutch Hunger Winter Families Study, found an increased risk of schizophrenia among children born during the height of the famine (Int J Epidemiol. 2007 Dec;36[6]:1196-204). During the Arab-Israeli war of 1967 (the Six-Day War), which took place in June, the fetuses of mothers who were pregnant during that month had a higher risk of schizophrenia if the fetus was in the second month (relative risk, 2.3; 95% confidence interval, 1.1-4.7) or third month (RR, 2.5; 95% CI, 1.2-5.2) of fetal life during June 1967, Dr. Malaspina and associates wrote (BMC Psychiatry. 2008 Aug 21;8:71).
“The key aspect is the ascertainment of individuals during a circumscribed period, the assessment and then the longitudinal follow-up,” she said. “Obviously, these are not easy studies to do, but enough of them have been done such that for the last decade at least, the general population should be aware of the developmental origins of health and disease.”
Maternal depression is another psychiatric condition that can serve as a prenatal exposure to adversity. A recent review found that children of women with untreated depression were 56% more likely to be born preterm and 96% more likely to have a low birth weight (Pediatr Res. 2019 Jan;85[2]:134-45). “Preterm birth and early birth along with low birth weight, these have ramifying effects throughout life, not only on neonatal and infant mortality, but on developmental disorders and lifetime morbidity,” she said. “These effects of maternal depression withstand all sorts of accounting for other correlated exposures, including maternal age and her medical complications or substance use.”
“The modulation of mood and affect can affect temperament and affect mental health. Studies exist linking maternal depression to autism, attention-deficit disorder, developmental delay, behavioral problems, sleep problems, externalizing behavior and depression, showing a very large effect of maternal depression on offspring well-being.”
To complicate matters, at least 15% of women will experience major depression during pregnancy, but of these, major depression is not being addressed in about half. Nonpharmacologic interventions can include cognitive-behavioral therapy and relaxation practices, but medication should be considered as well. “There’s an ongoing debate about whether antidepressant medications are harmful for the offspring,” she said. However, reviews conducted by Dr. Malaspina’s group have found low evidence of serious harm.
“My summary would be the depression itself holds much more evidence for disrupting offspring health and development than medications,” Dr. Malaspina said. “Most studies find no adverse birth effects when they properly controlled accounting for maternal age and the other conditions and other medications.”
Global Academy and this news organization are owned by the same parent company. Dr. Malaspina reported no relevant conflicts of interest.
The number of genes in humans seems inadequate to account for the diversity seen in people. While maternal and paternal factors do play a role in the development of offspring, increased attention is being paid to the forces that express these genes and the impact they have on the health of a person, including development of psychiatric conditions, according to Dolores Malaspina, MD.
Epigenetics, or changes that occur in a fetal phenotype that do not involve changes to the genotype, involve factors such as DNA methylation to control gene expression, histone modification or the wrapping of genes, or the silencing and activation of certain genes with noncoding RNA-associated factors, said Dr. Malaspina of the Icahn School of Medicine at Mount Sinai, New York.
When this occurs during pregnancy, “the fetus does not simply develop from a genetic blueprint of the genes from its father and mother. Instead, signals are received throughout the pregnancy as to the health of the mother and signals about the environment,” she said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
There is an evolutionary advantage to this so-called survival phenotype. “If, during the pregnancy, there’s a deficit of available nutrition, that may be a signal to the fetus that food will be scarce. In the setting of food scarcity, certain physiological adaptations during development can make the fetus more likely to survive to adulthood,” Dr. Malaspina said at the meeting, presented by Global Academy for Medical Education. But a fetus programmed to adapt to scarcity of food may also develop cardiovascular disease, metabolic disease, or mortality later in life if the prediction of scarce nutrition proved incorrect.
This approach to thinking about the developmental origins of health and disease, which examines how prenatal and perinatal exposure to environmental factors affect disease in adulthood, has also found a link between some exposures and psychiatric disorders. The most famous example, the Dutch Hunger Winter Families Study, found an increased risk of schizophrenia among children born during the height of the famine (Int J Epidemiol. 2007 Dec;36[6]:1196-204). During the Arab-Israeli war of 1967 (the Six-Day War), which took place in June, the fetuses of mothers who were pregnant during that month had a higher risk of schizophrenia if the fetus was in the second month (relative risk, 2.3; 95% confidence interval, 1.1-4.7) or third month (RR, 2.5; 95% CI, 1.2-5.2) of fetal life during June 1967, Dr. Malaspina and associates wrote (BMC Psychiatry. 2008 Aug 21;8:71).
“The key aspect is the ascertainment of individuals during a circumscribed period, the assessment and then the longitudinal follow-up,” she said. “Obviously, these are not easy studies to do, but enough of them have been done such that for the last decade at least, the general population should be aware of the developmental origins of health and disease.”
Maternal depression is another psychiatric condition that can serve as a prenatal exposure to adversity. A recent review found that children of women with untreated depression were 56% more likely to be born preterm and 96% more likely to have a low birth weight (Pediatr Res. 2019 Jan;85[2]:134-45). “Preterm birth and early birth along with low birth weight, these have ramifying effects throughout life, not only on neonatal and infant mortality, but on developmental disorders and lifetime morbidity,” she said. “These effects of maternal depression withstand all sorts of accounting for other correlated exposures, including maternal age and her medical complications or substance use.”
“The modulation of mood and affect can affect temperament and affect mental health. Studies exist linking maternal depression to autism, attention-deficit disorder, developmental delay, behavioral problems, sleep problems, externalizing behavior and depression, showing a very large effect of maternal depression on offspring well-being.”
To complicate matters, at least 15% of women will experience major depression during pregnancy, but of these, major depression is not being addressed in about half. Nonpharmacologic interventions can include cognitive-behavioral therapy and relaxation practices, but medication should be considered as well. “There’s an ongoing debate about whether antidepressant medications are harmful for the offspring,” she said. However, reviews conducted by Dr. Malaspina’s group have found low evidence of serious harm.
“My summary would be the depression itself holds much more evidence for disrupting offspring health and development than medications,” Dr. Malaspina said. “Most studies find no adverse birth effects when they properly controlled accounting for maternal age and the other conditions and other medications.”
Global Academy and this news organization are owned by the same parent company. Dr. Malaspina reported no relevant conflicts of interest.
FROM FOCUS ON NEUROPSYCHIATRY 2020
SARS-CoV-2 appears unlikely to pass through breast milk
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
FROM JAMA
Reducing maternal mortality with prenatal care
As in its typical fashion, the question sprang out from a young Black patient after some meandering conversation during preconception counseling: “How do y’all prevent Black maternal mortality?” At the beginning of my career, I used to think preparing a patient for pregnancy involved recommending prenatal vitamins and rubella immunity screening. Now, having worked in a society with substantial racial health disparities for 14 years, there is greater awareness that pregnancy can be a matter of life or death that disproportionately affects people of color.
For Black patients in the United States, the maternal mortality ratio is almost four times higher than the ratio for White patients, 42 deaths versus 13 deaths per 100,000 live births, respectively.1 Georgia has the highest maternal mortality ratio in the United States at 67 maternal deaths per 100,000 live births. However, if you are a Black woman in Georgia, your chance of dying of pregnancy-related causes is 2.7 times that of a non-Hispanic White woman living in Georgia.2
Black patients often are not taken seriously, even when they are wealthy, have attained high levels of education, or are famous. Serena Williams, a Black woman and one of the most talented tennis players of all time, was ignored when complaining that she felt a blood clot had returned in her lungs post partum. As a recognition of this crisis, the Centers for Disease Control and Prevention has a new campaign to improve recognition of the warning signs of problems in pregnancy called the HEAR HER campaign. This issue is a pervasive problem in our lives that runs across the spectrum of Black experience. I have had Black friends, patients, and colleagues who have been ignored when complaining about labor pain, workplace discrimination, and even when trying to advocate for their patients. We need to uplift Black voices so they can be heard and support the initiatives and interventions they are asking for.
We practice standardized responses to emergencies and to health conditions. We use drills to practice our responses to life-threatening emergencies such as STAT cesarean delivery, shoulder dystocia, obstetrical hemorrhage, or treatment of preeclampsia and eclampsia. The Alliance for Innovation on Maternal Health has organized evidence-driven protocols called AIM bundles to reduce preventable maternal morbidity and mortality when implemented. Standardization is an important component of equitable treatment and reduction of disparities. The concept has been used across industries to reduce error and bias. The Alliance for Innovation on Maternal Health bundles even include a section on Reduction of Peripartum and Ethnic Disparities.
We admit that bias exists and that we need training to recognize and eliminate it. According to a study in the Proceedings of the National Academy of Sciences of the United States of America about racial bias in pain assessment more than 20% of White residents and medical students surveyed believed that Black people had less sensitive nerve endings than Whites.3 Studies show that this stereotype leads to inappropriate pain management in Black patients, a chief concern when considering how patients are treated on labor and delivery or after surgery.4 Additionally, unconscious bias can be addressed by hiring a diverse workforce at all levels. Familiarity with a diverse group can help us learn from one another in our day-to-day lives.
We need to offer the same high-quality preconception counseling to all of our patients. A patient’s perceived race or ethnicity is a poor indicator of their actual health needs. The amount of melanin in our skin is highly variable but our genetics are remarkably similar, therefore our health concerns are similar. All patients deserve a focus on prevention. Folic acid supplements in the form of prenatal vitamins should be recommended. Routine vaccinations and rubella immunity checks should be offered. Basic carrier screening for diseases of hemoglobin (which includes sickle cell trait), fragile X, spinomuscular atrophy, and cystic fibrosis should be offered. Finally, an emphasis on safety, mental health, and daily low-level exercise (i.e., walking) should be promoted to help prevent illness and injury in this age group. The leading causes of death for people of reproductive age are accidents, suicide, homicide, and heart disease – all preventable.
We treat the social determinants of health, not just the patient in front of us. When “race” is a risk factor for disease, it’s usually racism that’s the problem. As stated earlier, how much melanin is in our skin has little to do with our genetics – if we removed our skin, we’d have similar life expectancies and die of similar things. However, it has everything to do with how we navigate our society and access health care. The stress associated with being Black in America is the likely cause of preterm birth rates – leading to infant illness and death – and maternal mortality being higher in Black patients. This is referred to as “weathering” – the cumulative effects of stress as we age. It explains why Black women are more likely to die in pregnancy despite higher levels of education and increasing age – factors that are protective for other groups. Improving access to quality education, reforming the criminal justice system, affordable housing and child care, living wages, family planning, and universal basic health care exemplify the intersectionality of some of our greatest societal challenges. Addressing these root causes will reduce weathering and ultimately, save Black lives.
We strive to train more “underrepresented minorities” in medicine. According to the American Association of Medical Colleges, only 7.3% of medical students in 2019-2020 identified as Black or African American. This is way below their representation of 13% of the U.S. population. I’m proud that my division and department as a whole have hired and promoted diverse faculty with 30% of my generalist ob.gyn. colleagues being people of color. This shows that we have the input of diverse experiences as well as recognize the special concerns of patients of color. Underrepresented students interested in the health professions need us to do more to get their “foot in the door.” They are less likely to have connections to the field of medicine (family members, mentors), have access to prep courses or advisors, or have the finances to support the expensive application process. Reach out to your alma maters and ask how you can help mentor students at a young age and continue through adulthood, support scholarships, support unpaid internship recipients, and promote interconnectedness throughout this community.
I hope I answered my patient’s question in that moment, but I know what needs to be done is bigger that taking care of one patient. It will require small progress, by us, every single day. Until these interventions and others reshape our society, I’ll still have Black patients who say: “Don’t let me die, okay?” with a look right into my soul and a tight grip on my hand. And I’ll feel the immense weight of that trust, and squeeze the hand back.
Dr. Collins (she/her/hers) is assistant professor of obstetrics and gynecology, generalist division, at Emory University, Atlanta. She has no relevant financial disclosures. Email Dr. Collins at [email protected].
References
1. CDC Pregnancy Mortality Surveillance System, 2016. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
2. Maternal Mortality Fact Sheet, 2012-2015. https://dph.georgia.gov/maternal-mortality.
3. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4296-301.
4. Pain Med. 2012 Feb;13(2):150-74.
As in its typical fashion, the question sprang out from a young Black patient after some meandering conversation during preconception counseling: “How do y’all prevent Black maternal mortality?” At the beginning of my career, I used to think preparing a patient for pregnancy involved recommending prenatal vitamins and rubella immunity screening. Now, having worked in a society with substantial racial health disparities for 14 years, there is greater awareness that pregnancy can be a matter of life or death that disproportionately affects people of color.
For Black patients in the United States, the maternal mortality ratio is almost four times higher than the ratio for White patients, 42 deaths versus 13 deaths per 100,000 live births, respectively.1 Georgia has the highest maternal mortality ratio in the United States at 67 maternal deaths per 100,000 live births. However, if you are a Black woman in Georgia, your chance of dying of pregnancy-related causes is 2.7 times that of a non-Hispanic White woman living in Georgia.2
Black patients often are not taken seriously, even when they are wealthy, have attained high levels of education, or are famous. Serena Williams, a Black woman and one of the most talented tennis players of all time, was ignored when complaining that she felt a blood clot had returned in her lungs post partum. As a recognition of this crisis, the Centers for Disease Control and Prevention has a new campaign to improve recognition of the warning signs of problems in pregnancy called the HEAR HER campaign. This issue is a pervasive problem in our lives that runs across the spectrum of Black experience. I have had Black friends, patients, and colleagues who have been ignored when complaining about labor pain, workplace discrimination, and even when trying to advocate for their patients. We need to uplift Black voices so they can be heard and support the initiatives and interventions they are asking for.
We practice standardized responses to emergencies and to health conditions. We use drills to practice our responses to life-threatening emergencies such as STAT cesarean delivery, shoulder dystocia, obstetrical hemorrhage, or treatment of preeclampsia and eclampsia. The Alliance for Innovation on Maternal Health has organized evidence-driven protocols called AIM bundles to reduce preventable maternal morbidity and mortality when implemented. Standardization is an important component of equitable treatment and reduction of disparities. The concept has been used across industries to reduce error and bias. The Alliance for Innovation on Maternal Health bundles even include a section on Reduction of Peripartum and Ethnic Disparities.
We admit that bias exists and that we need training to recognize and eliminate it. According to a study in the Proceedings of the National Academy of Sciences of the United States of America about racial bias in pain assessment more than 20% of White residents and medical students surveyed believed that Black people had less sensitive nerve endings than Whites.3 Studies show that this stereotype leads to inappropriate pain management in Black patients, a chief concern when considering how patients are treated on labor and delivery or after surgery.4 Additionally, unconscious bias can be addressed by hiring a diverse workforce at all levels. Familiarity with a diverse group can help us learn from one another in our day-to-day lives.
We need to offer the same high-quality preconception counseling to all of our patients. A patient’s perceived race or ethnicity is a poor indicator of their actual health needs. The amount of melanin in our skin is highly variable but our genetics are remarkably similar, therefore our health concerns are similar. All patients deserve a focus on prevention. Folic acid supplements in the form of prenatal vitamins should be recommended. Routine vaccinations and rubella immunity checks should be offered. Basic carrier screening for diseases of hemoglobin (which includes sickle cell trait), fragile X, spinomuscular atrophy, and cystic fibrosis should be offered. Finally, an emphasis on safety, mental health, and daily low-level exercise (i.e., walking) should be promoted to help prevent illness and injury in this age group. The leading causes of death for people of reproductive age are accidents, suicide, homicide, and heart disease – all preventable.
We treat the social determinants of health, not just the patient in front of us. When “race” is a risk factor for disease, it’s usually racism that’s the problem. As stated earlier, how much melanin is in our skin has little to do with our genetics – if we removed our skin, we’d have similar life expectancies and die of similar things. However, it has everything to do with how we navigate our society and access health care. The stress associated with being Black in America is the likely cause of preterm birth rates – leading to infant illness and death – and maternal mortality being higher in Black patients. This is referred to as “weathering” – the cumulative effects of stress as we age. It explains why Black women are more likely to die in pregnancy despite higher levels of education and increasing age – factors that are protective for other groups. Improving access to quality education, reforming the criminal justice system, affordable housing and child care, living wages, family planning, and universal basic health care exemplify the intersectionality of some of our greatest societal challenges. Addressing these root causes will reduce weathering and ultimately, save Black lives.
We strive to train more “underrepresented minorities” in medicine. According to the American Association of Medical Colleges, only 7.3% of medical students in 2019-2020 identified as Black or African American. This is way below their representation of 13% of the U.S. population. I’m proud that my division and department as a whole have hired and promoted diverse faculty with 30% of my generalist ob.gyn. colleagues being people of color. This shows that we have the input of diverse experiences as well as recognize the special concerns of patients of color. Underrepresented students interested in the health professions need us to do more to get their “foot in the door.” They are less likely to have connections to the field of medicine (family members, mentors), have access to prep courses or advisors, or have the finances to support the expensive application process. Reach out to your alma maters and ask how you can help mentor students at a young age and continue through adulthood, support scholarships, support unpaid internship recipients, and promote interconnectedness throughout this community.
I hope I answered my patient’s question in that moment, but I know what needs to be done is bigger that taking care of one patient. It will require small progress, by us, every single day. Until these interventions and others reshape our society, I’ll still have Black patients who say: “Don’t let me die, okay?” with a look right into my soul and a tight grip on my hand. And I’ll feel the immense weight of that trust, and squeeze the hand back.
Dr. Collins (she/her/hers) is assistant professor of obstetrics and gynecology, generalist division, at Emory University, Atlanta. She has no relevant financial disclosures. Email Dr. Collins at [email protected].
References
1. CDC Pregnancy Mortality Surveillance System, 2016. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
2. Maternal Mortality Fact Sheet, 2012-2015. https://dph.georgia.gov/maternal-mortality.
3. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4296-301.
4. Pain Med. 2012 Feb;13(2):150-74.
As in its typical fashion, the question sprang out from a young Black patient after some meandering conversation during preconception counseling: “How do y’all prevent Black maternal mortality?” At the beginning of my career, I used to think preparing a patient for pregnancy involved recommending prenatal vitamins and rubella immunity screening. Now, having worked in a society with substantial racial health disparities for 14 years, there is greater awareness that pregnancy can be a matter of life or death that disproportionately affects people of color.
For Black patients in the United States, the maternal mortality ratio is almost four times higher than the ratio for White patients, 42 deaths versus 13 deaths per 100,000 live births, respectively.1 Georgia has the highest maternal mortality ratio in the United States at 67 maternal deaths per 100,000 live births. However, if you are a Black woman in Georgia, your chance of dying of pregnancy-related causes is 2.7 times that of a non-Hispanic White woman living in Georgia.2
Black patients often are not taken seriously, even when they are wealthy, have attained high levels of education, or are famous. Serena Williams, a Black woman and one of the most talented tennis players of all time, was ignored when complaining that she felt a blood clot had returned in her lungs post partum. As a recognition of this crisis, the Centers for Disease Control and Prevention has a new campaign to improve recognition of the warning signs of problems in pregnancy called the HEAR HER campaign. This issue is a pervasive problem in our lives that runs across the spectrum of Black experience. I have had Black friends, patients, and colleagues who have been ignored when complaining about labor pain, workplace discrimination, and even when trying to advocate for their patients. We need to uplift Black voices so they can be heard and support the initiatives and interventions they are asking for.
We practice standardized responses to emergencies and to health conditions. We use drills to practice our responses to life-threatening emergencies such as STAT cesarean delivery, shoulder dystocia, obstetrical hemorrhage, or treatment of preeclampsia and eclampsia. The Alliance for Innovation on Maternal Health has organized evidence-driven protocols called AIM bundles to reduce preventable maternal morbidity and mortality when implemented. Standardization is an important component of equitable treatment and reduction of disparities. The concept has been used across industries to reduce error and bias. The Alliance for Innovation on Maternal Health bundles even include a section on Reduction of Peripartum and Ethnic Disparities.
We admit that bias exists and that we need training to recognize and eliminate it. According to a study in the Proceedings of the National Academy of Sciences of the United States of America about racial bias in pain assessment more than 20% of White residents and medical students surveyed believed that Black people had less sensitive nerve endings than Whites.3 Studies show that this stereotype leads to inappropriate pain management in Black patients, a chief concern when considering how patients are treated on labor and delivery or after surgery.4 Additionally, unconscious bias can be addressed by hiring a diverse workforce at all levels. Familiarity with a diverse group can help us learn from one another in our day-to-day lives.
We need to offer the same high-quality preconception counseling to all of our patients. A patient’s perceived race or ethnicity is a poor indicator of their actual health needs. The amount of melanin in our skin is highly variable but our genetics are remarkably similar, therefore our health concerns are similar. All patients deserve a focus on prevention. Folic acid supplements in the form of prenatal vitamins should be recommended. Routine vaccinations and rubella immunity checks should be offered. Basic carrier screening for diseases of hemoglobin (which includes sickle cell trait), fragile X, spinomuscular atrophy, and cystic fibrosis should be offered. Finally, an emphasis on safety, mental health, and daily low-level exercise (i.e., walking) should be promoted to help prevent illness and injury in this age group. The leading causes of death for people of reproductive age are accidents, suicide, homicide, and heart disease – all preventable.
We treat the social determinants of health, not just the patient in front of us. When “race” is a risk factor for disease, it’s usually racism that’s the problem. As stated earlier, how much melanin is in our skin has little to do with our genetics – if we removed our skin, we’d have similar life expectancies and die of similar things. However, it has everything to do with how we navigate our society and access health care. The stress associated with being Black in America is the likely cause of preterm birth rates – leading to infant illness and death – and maternal mortality being higher in Black patients. This is referred to as “weathering” – the cumulative effects of stress as we age. It explains why Black women are more likely to die in pregnancy despite higher levels of education and increasing age – factors that are protective for other groups. Improving access to quality education, reforming the criminal justice system, affordable housing and child care, living wages, family planning, and universal basic health care exemplify the intersectionality of some of our greatest societal challenges. Addressing these root causes will reduce weathering and ultimately, save Black lives.
We strive to train more “underrepresented minorities” in medicine. According to the American Association of Medical Colleges, only 7.3% of medical students in 2019-2020 identified as Black or African American. This is way below their representation of 13% of the U.S. population. I’m proud that my division and department as a whole have hired and promoted diverse faculty with 30% of my generalist ob.gyn. colleagues being people of color. This shows that we have the input of diverse experiences as well as recognize the special concerns of patients of color. Underrepresented students interested in the health professions need us to do more to get their “foot in the door.” They are less likely to have connections to the field of medicine (family members, mentors), have access to prep courses or advisors, or have the finances to support the expensive application process. Reach out to your alma maters and ask how you can help mentor students at a young age and continue through adulthood, support scholarships, support unpaid internship recipients, and promote interconnectedness throughout this community.
I hope I answered my patient’s question in that moment, but I know what needs to be done is bigger that taking care of one patient. It will require small progress, by us, every single day. Until these interventions and others reshape our society, I’ll still have Black patients who say: “Don’t let me die, okay?” with a look right into my soul and a tight grip on my hand. And I’ll feel the immense weight of that trust, and squeeze the hand back.
Dr. Collins (she/her/hers) is assistant professor of obstetrics and gynecology, generalist division, at Emory University, Atlanta. She has no relevant financial disclosures. Email Dr. Collins at [email protected].
References
1. CDC Pregnancy Mortality Surveillance System, 2016. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
2. Maternal Mortality Fact Sheet, 2012-2015. https://dph.georgia.gov/maternal-mortality.
3. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4296-301.
4. Pain Med. 2012 Feb;13(2):150-74.
Rooting out systemic racism: Equal access to maternal, prenatal care
Against the backdrop of the COVID-19 pandemic and ongoing national conversations around racial injustice, it is more important than ever that we identify and root out systemic discrimination – including in our health care system. As an ob.gyn., I’ve spent my entire career making sure that women receive the best care, and have witnessed firsthand the results of a system that provides differing levels of care based on one’s socioeconomic level, race, or ethnicity.
This disparity is borne out in this country’s maternal health outcomes. For example, the latest figures from the Centers for Disease Control and Prevention indicate that the maternal mortality rate for black women, 37.1 deaths per 100,000 live births, is more than double the rate for white women at 14.7. In addition, the black infant mortality rate, at 11.4 per 1,000 live births, is also more than double the white infant mortality rate, 4.9. While many of these differences stem from discriminatory levels of coverage and care after delivery, just as important is the coverage and care before delivery: prenatal care.
Prenatal care includes a variety of screening tests, including those that can help expecting mothers identify whether the baby is more or less likely to have certain genetic disorders. These tests include traditional and less accurate options like serum or combined screening, and newer noninvasive prenatal testing (NIPT) options that use blood samples from the mother to analyze the baby’s DNA.
Research already has demonstrated that NIPT is the most accurate and effective screening option for common chromosomal abnormalities (Ont Health Technol Assess Ser. 2019;19[4]:1-166). A 2015 New England Journal of Medicine study showed that, without access to NIPT, 22% of pregnancies with Down syndrome were missed. With older screening methods, 5.4% of positive results for Down syndrome were false, compared with 0.06% of the NIPT tests (N Engl J Med 2015;372:1589-97). Older, less accurate screening tests cause unnecessary referrals to specialists for possible invasive testing, resulting in additional costs and undue stress on women and their families.
And yet, troubling new data have shown that black and Hispanic women have less access to NIPT than white women. Currently, NIPT is available to all California women through the state-funded prenatal screening program as a second-tier test. Many women, however, decide to opt out or go outside of the state program to have NIPT as a first-tier test, choosing private payer or other plans instead. New data shared by the California Department of Public Health with ob.gyns. and maternal-fetal medicine physicians in California showed that white women who opted out of California’s state-funded prenatal screening program were more than twice as likely to gain access to NIPT as black and Hispanic women (39%-17%). We can assume this to be true of women outside California as well – women who have no access to a state-funded program like California’s and depend solely on private payer or other health care plans. In fact, although some commercial insurance companies do cover NIPT, noninvasive prenatal screening is not covered by large insurance companies like Aetna and UnitedHealthcare.
As ob.gyns., physicians, and health professionals, we need to ask ourselves: Why is there such a great disparity in the access of superior and more effective NIPT options for black and Hispanic women?
Many reasons are apparent. There are significant differences by ethnic and racial groups in their knowledge of the availability of prenatal testing. Furthermore, there are higher levels of mistrust along certain racial and ethnic lines of the medical system in this country; specific religious or ethnic beliefs also may obviate the use of prenatal testing or diagnosis. Significant differences also exist in the types of health coverage by race and ethnicity, ultimately impacting the ability to have prenatal testing. Finally, there are different physician group recommendations. While medical societies such as the American College of Medical Genetics, International Society for Prenatal Diagnosis, and the American Society of Human Genetics all have long endorsed newer NIPT option for all pregnant women, two of the national physician groups that make recommendations about what care pregnant women should be able to access – the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – only recently have recommended broad access to NIPT. As a result, some state Medicaid programs have not made NIPT available to patients.
We know that racial disparities are a public health crisis in America. The recent data from the California Department of Public Health, paired with COVID-19’s disproportionate impact on blacks and Hispanics, only further illustrate the existing health disparities experienced by this country’s communities of color.
We need to root out systemic discrimination in health care and we can begin with our maternal health care. Providing equitable access to the most accurate and consistent prenatal screenings, including NIPT options, regardless of insurance plan, socioeconomic level, race, or ethnicity is paramount in starting this work.
Dr. Gaither is a double board–certified physician in ob.gyn. and maternal-fetal medicine. She is director of perinatal services for NYC Health+Hospitals/Lincoln. She reports no conflicts of interest. Email her at [email protected].
Against the backdrop of the COVID-19 pandemic and ongoing national conversations around racial injustice, it is more important than ever that we identify and root out systemic discrimination – including in our health care system. As an ob.gyn., I’ve spent my entire career making sure that women receive the best care, and have witnessed firsthand the results of a system that provides differing levels of care based on one’s socioeconomic level, race, or ethnicity.
This disparity is borne out in this country’s maternal health outcomes. For example, the latest figures from the Centers for Disease Control and Prevention indicate that the maternal mortality rate for black women, 37.1 deaths per 100,000 live births, is more than double the rate for white women at 14.7. In addition, the black infant mortality rate, at 11.4 per 1,000 live births, is also more than double the white infant mortality rate, 4.9. While many of these differences stem from discriminatory levels of coverage and care after delivery, just as important is the coverage and care before delivery: prenatal care.
Prenatal care includes a variety of screening tests, including those that can help expecting mothers identify whether the baby is more or less likely to have certain genetic disorders. These tests include traditional and less accurate options like serum or combined screening, and newer noninvasive prenatal testing (NIPT) options that use blood samples from the mother to analyze the baby’s DNA.
Research already has demonstrated that NIPT is the most accurate and effective screening option for common chromosomal abnormalities (Ont Health Technol Assess Ser. 2019;19[4]:1-166). A 2015 New England Journal of Medicine study showed that, without access to NIPT, 22% of pregnancies with Down syndrome were missed. With older screening methods, 5.4% of positive results for Down syndrome were false, compared with 0.06% of the NIPT tests (N Engl J Med 2015;372:1589-97). Older, less accurate screening tests cause unnecessary referrals to specialists for possible invasive testing, resulting in additional costs and undue stress on women and their families.
And yet, troubling new data have shown that black and Hispanic women have less access to NIPT than white women. Currently, NIPT is available to all California women through the state-funded prenatal screening program as a second-tier test. Many women, however, decide to opt out or go outside of the state program to have NIPT as a first-tier test, choosing private payer or other plans instead. New data shared by the California Department of Public Health with ob.gyns. and maternal-fetal medicine physicians in California showed that white women who opted out of California’s state-funded prenatal screening program were more than twice as likely to gain access to NIPT as black and Hispanic women (39%-17%). We can assume this to be true of women outside California as well – women who have no access to a state-funded program like California’s and depend solely on private payer or other health care plans. In fact, although some commercial insurance companies do cover NIPT, noninvasive prenatal screening is not covered by large insurance companies like Aetna and UnitedHealthcare.
As ob.gyns., physicians, and health professionals, we need to ask ourselves: Why is there such a great disparity in the access of superior and more effective NIPT options for black and Hispanic women?
Many reasons are apparent. There are significant differences by ethnic and racial groups in their knowledge of the availability of prenatal testing. Furthermore, there are higher levels of mistrust along certain racial and ethnic lines of the medical system in this country; specific religious or ethnic beliefs also may obviate the use of prenatal testing or diagnosis. Significant differences also exist in the types of health coverage by race and ethnicity, ultimately impacting the ability to have prenatal testing. Finally, there are different physician group recommendations. While medical societies such as the American College of Medical Genetics, International Society for Prenatal Diagnosis, and the American Society of Human Genetics all have long endorsed newer NIPT option for all pregnant women, two of the national physician groups that make recommendations about what care pregnant women should be able to access – the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – only recently have recommended broad access to NIPT. As a result, some state Medicaid programs have not made NIPT available to patients.
We know that racial disparities are a public health crisis in America. The recent data from the California Department of Public Health, paired with COVID-19’s disproportionate impact on blacks and Hispanics, only further illustrate the existing health disparities experienced by this country’s communities of color.
We need to root out systemic discrimination in health care and we can begin with our maternal health care. Providing equitable access to the most accurate and consistent prenatal screenings, including NIPT options, regardless of insurance plan, socioeconomic level, race, or ethnicity is paramount in starting this work.
Dr. Gaither is a double board–certified physician in ob.gyn. and maternal-fetal medicine. She is director of perinatal services for NYC Health+Hospitals/Lincoln. She reports no conflicts of interest. Email her at [email protected].
Against the backdrop of the COVID-19 pandemic and ongoing national conversations around racial injustice, it is more important than ever that we identify and root out systemic discrimination – including in our health care system. As an ob.gyn., I’ve spent my entire career making sure that women receive the best care, and have witnessed firsthand the results of a system that provides differing levels of care based on one’s socioeconomic level, race, or ethnicity.
This disparity is borne out in this country’s maternal health outcomes. For example, the latest figures from the Centers for Disease Control and Prevention indicate that the maternal mortality rate for black women, 37.1 deaths per 100,000 live births, is more than double the rate for white women at 14.7. In addition, the black infant mortality rate, at 11.4 per 1,000 live births, is also more than double the white infant mortality rate, 4.9. While many of these differences stem from discriminatory levels of coverage and care after delivery, just as important is the coverage and care before delivery: prenatal care.
Prenatal care includes a variety of screening tests, including those that can help expecting mothers identify whether the baby is more or less likely to have certain genetic disorders. These tests include traditional and less accurate options like serum or combined screening, and newer noninvasive prenatal testing (NIPT) options that use blood samples from the mother to analyze the baby’s DNA.
Research already has demonstrated that NIPT is the most accurate and effective screening option for common chromosomal abnormalities (Ont Health Technol Assess Ser. 2019;19[4]:1-166). A 2015 New England Journal of Medicine study showed that, without access to NIPT, 22% of pregnancies with Down syndrome were missed. With older screening methods, 5.4% of positive results for Down syndrome were false, compared with 0.06% of the NIPT tests (N Engl J Med 2015;372:1589-97). Older, less accurate screening tests cause unnecessary referrals to specialists for possible invasive testing, resulting in additional costs and undue stress on women and their families.
And yet, troubling new data have shown that black and Hispanic women have less access to NIPT than white women. Currently, NIPT is available to all California women through the state-funded prenatal screening program as a second-tier test. Many women, however, decide to opt out or go outside of the state program to have NIPT as a first-tier test, choosing private payer or other plans instead. New data shared by the California Department of Public Health with ob.gyns. and maternal-fetal medicine physicians in California showed that white women who opted out of California’s state-funded prenatal screening program were more than twice as likely to gain access to NIPT as black and Hispanic women (39%-17%). We can assume this to be true of women outside California as well – women who have no access to a state-funded program like California’s and depend solely on private payer or other health care plans. In fact, although some commercial insurance companies do cover NIPT, noninvasive prenatal screening is not covered by large insurance companies like Aetna and UnitedHealthcare.
As ob.gyns., physicians, and health professionals, we need to ask ourselves: Why is there such a great disparity in the access of superior and more effective NIPT options for black and Hispanic women?
Many reasons are apparent. There are significant differences by ethnic and racial groups in their knowledge of the availability of prenatal testing. Furthermore, there are higher levels of mistrust along certain racial and ethnic lines of the medical system in this country; specific religious or ethnic beliefs also may obviate the use of prenatal testing or diagnosis. Significant differences also exist in the types of health coverage by race and ethnicity, ultimately impacting the ability to have prenatal testing. Finally, there are different physician group recommendations. While medical societies such as the American College of Medical Genetics, International Society for Prenatal Diagnosis, and the American Society of Human Genetics all have long endorsed newer NIPT option for all pregnant women, two of the national physician groups that make recommendations about what care pregnant women should be able to access – the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – only recently have recommended broad access to NIPT. As a result, some state Medicaid programs have not made NIPT available to patients.
We know that racial disparities are a public health crisis in America. The recent data from the California Department of Public Health, paired with COVID-19’s disproportionate impact on blacks and Hispanics, only further illustrate the existing health disparities experienced by this country’s communities of color.
We need to root out systemic discrimination in health care and we can begin with our maternal health care. Providing equitable access to the most accurate and consistent prenatal screenings, including NIPT options, regardless of insurance plan, socioeconomic level, race, or ethnicity is paramount in starting this work.
Dr. Gaither is a double board–certified physician in ob.gyn. and maternal-fetal medicine. She is director of perinatal services for NYC Health+Hospitals/Lincoln. She reports no conflicts of interest. Email her at [email protected].
Mitigating psychiatric disorder relapse in pregnancy during pandemic
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.