User login
Telemedicine: A primer for today’s ObGyn
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
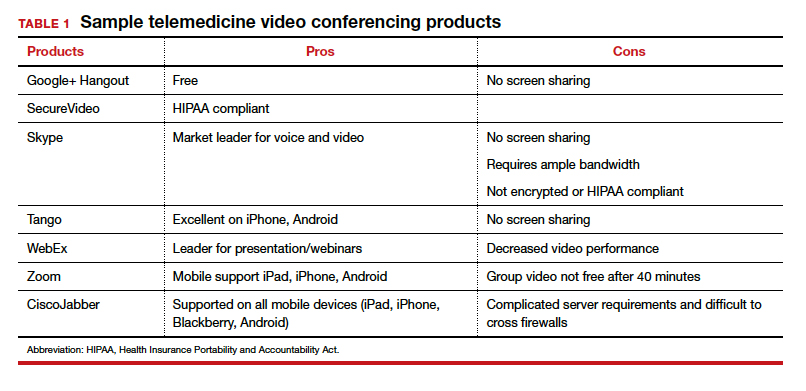
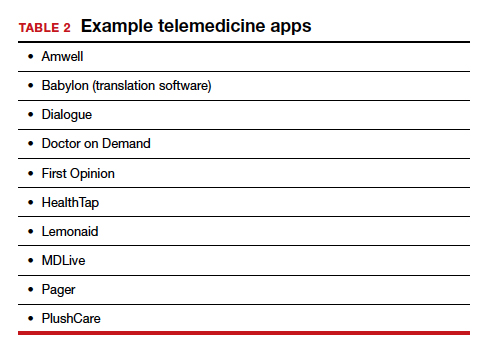
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.
Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
COVID-19: We are in a war, without the most effective weapons to fight a novel viral pathogen
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
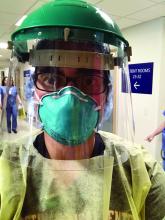
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
On June 17, 1775, American colonists, defending a forward redoubt on Breed’s Hill, ran out of gunpowder, and their position was overrun by British troops. The Battle of Bunker Hill resulted in the death of 140 colonists and 226 British soldiers, setting the stage for major combat throughout the colonies. American colonists lacked many necessary weapons. They had almost no gunpowder, few field cannons, and no warships. Yet, they fought on with the weapons at hand for 6 long years.
In the spring of 2020, American society has been shaken by the COVID-19 pandemic. Hospitals have been overrun with thousands of people infected with the disease. Some hospitals are breaking under the crush of intensely ill people filling up and spilling out of intensive care units. We are in a war, fighting a viral disease with a limited supply of weapons. We do not have access to the most powerful medical munitions: easily available rapid testing, proven antiviral medications, and an effective vaccine. Nevertheless, clinicians and patients are courageous, and we will continue the fight with the limited weapons we have until the pandemic is brought to an end.

The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The virus is aptly named because it is usually transmitted through close contact with respiratory droplets. The disease can progress acutely, and some people experience a remarkably severe respiratory syndrome, including tachypnea, hypoxia, and interstitial and alveolar opacities on chest x-ray, necessitating ventilatory support. The virus is an encapsulated single-stranded RNA virus. When viewed by electron microscopy, the virus appears to have a halo or crown, hence it is named “coronavirus.” Among infected individuals, the virus is present in the upper respiratory system and in feces but not in urine.1 The World Health Organization (WHO) believes that respiratory droplets and contaminated surfaces are the major routes of transmission.2 The highest risk of developing severe COVID-19 disease occurs in people with one or more of the following characteristics: age greater than 70 years, hypertension, diabetes, respiratory disease, heart disease, and immunosuppression.3,4 Pregnant women do not appear to be at increased risk for severe COVID-19 disease.4 The case fatality rate is highest in people 80 years of age or older.5
Who is infected with SARS-CoV-2?
Rapid high-fidelity testing for SARS-CoV-2 nucleic acid sequences would be the best approach to identifying people with COVID-19 disease. At the beginning of the pandemic, testing was strictly rationed because of lack of reagents and test swabs. Clinicians were permitted to test only a minority of people who had symptoms. Asymptomatic individuals were not eligible to be tested. This terribly flawed approach to screening permitted a vast army of SARS-CoV-2–positive asymptomatic and mildly symptomatic people to circulate unchecked in the general population, infecting dozens of other people, some of whom developed moderate or severe disease. The Centers for Disease Control and Prevention (CDC) has reported on 7 independent clusters of COVID-19 disease, each of which appear to have been caused by one asymptomatic infected individual.6 Another cluster of COVID-19 disease from China appears to have been caused by one asymptomatic infected individual.7 Based on limited data, it appears that there may be a 1- to 3-day window where an individual with COVID-19 may be asymptomatic and able to infect others. I suspect that we will soon discover, based on testing for the presence of high-titre anti SARS-CoV-2 antibodies, that many people with no history of illness and people with mild respiratory symptoms had an undiagnosed COVID-19 infection.
As testing capacity expands we likely will be testing all women, including asymptomatic women, before they arrive at the hospital for childbirth or gynecologic surgery, as well as all inpatients and women with respiratory symptoms having an ambulatory encounter.
With expanded testing capability, some pregnant women who were symptomatic and tested positive for SARS-CoV-2 have had sequential long-term follow-up testing. A frequent observation is that over one to two weeks the viral symptoms resolve and the nasopharyngeal test becomes negative for SARS-CoV-2 on multiple sequential tests, only to become positive at a later date. The cause of the positive-negative-negative-positive test results is unknown, but it raises the possibility that once a person tests positive for SARS-CoV-2, they may be able to transmit the infection over many weeks, even after viral symptoms resolve.
Continue to: COVID-19: Respiratory droplet or aerosol transmission?
COVID-19: Respiratory droplet or aerosol transmission?
Respiratory droplets are large particles (> 5 µm in diameter) that tend to be pulled to the ground or furniture surfaces by gravity. Respiratory droplets do not circulate in the air for an extended period of time. Droplet nuclei are small particles less than 5 µm in diameter. These small particles may become aerosolized and float through the air for an extended period of time. The CDC and WHO believe that under ordinary conditions, SARS-CoV-2 is transmitted through respiratory droplets and contact routes.2 In an analysis of more than 75,000 COVID-19 cases in China there were no reports of transmission by aerosolized airborne virus. Therefore, under ordinary conditions, surgical masks, face shields, gowns, and gloves provide a high level of protection from infection.8
In contrast to the WHO’s perspective, Dr. Harvey Fineberg, Chair of the National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats, wrote a letter to the federal Office of Science and Technology Policy warning that normal breathing might generate aerosolization of the SARS-CoV-2 virus and result in airborne transmission.9 A report from the University of Nebraska Medical Center supports the concept of airborne transmission of SARS-CoV-2. In a study of 13 patients with COVID-19, room surfaces, toilet facilities, and air had evidence of viral contamination.10 The investigators concluded that disease spreads through respiratory droplets, person-to-person touch, contaminated surfaces, and airborne routes. Other investigators also have reported that aersolization of SARS-CoV-2 may occur.11 Professional societies recommend that all medical staff caring for potential or confirmed COVID-19 patients should use personal protective equipment (PPE), including respirators (N95 respirators) when available. Importantly, all medical staff should be trained in and adhere to proper donning and doffing of PPE. The controversy about the modes of transmission of SARS-CoV-2 will continue, but as clinicians we need to work within the constraints of the equipment we have.
Certain medical procedures and devices are known to generate aerosolization of respiratory secretions. These procedures and devices include: bronchoscopy, intubation, extubation, cardiopulmonary resuscitation, nebulization, high-flow oxygen masks, and continuous- and bilevel-positive airway pressure devices. When aerosols are generated during the care of a patient with COVID-19, surgical masks are not sufficient protection against infection. When an aerosol is generated maximal protection of health care workers from viral transmission requires use of a negative-pressure room and an N95 respirator or powered air-purifying respirator (PAPR) device. However, negative-pressure rooms, N95 masks, and PAPRs are in very short supply or are unavailable in some health systems. We are lucky at our hospital that all of the labor rooms can be configured to operate in a negative-pressure mode, limiting potential airborne spread of the virus on the unit. Many hospitals restrict the use of N95 masks to anesthesiologists, leaving nurses, ObGyns, and surgical technicians without the best protective equipment, risking their health. As one action to reduce aerosolization of virus, obstetricians can markedly reduce the use of oxygen masks and nasal cannulas by laboring women.
Universal use of surgical masks and mouth-nose coverings
During the entire COVID-19 pandemic, PPE has been in short supply, including severe shortages of N95 masks, PAPRs, and in some health systems, surgical masks, gowns, eye protection, and face shields. Given the severe shortages, some clinicians have needed to conserve PPE, using the same PPE across multiple patient encounters and across multiple work shifts.
Given that the virus is transmitted by respiratory droplets and contaminated surfaces, use of face coverings, including surgical masks, face shields, and gloves is critically important. Scrupulous hand hygiene is a simple approach to reducing infection risk. In my health system, all employees are required to wear a surgical mask, all day every day, requiring distribution of 35,000 masks daily.12 We also require every patient and visitor to our health care facilities to use a face mask. The purpose of the procedure or surgical mask is to prevent presymptomatic spread of COVID-19 from an asymptomatic health care worker to an uninfected patient or a colleague by reducing the transmission of respiratory droplets. Another benefit is to protect the uninfected health care worker from patients and colleagues who are infected and not yet diagnosed with COVID-19. The CDC now recommends that all people wear a mouth and nose covering when they are outside of their residence. America may become a nation where wearing masks in public becomes a routine practice. Since SARS-CoV-2 is transmitted by respiratory droplets, social distancing is an important preventive measure.
Continue to: Obstetric care...
Obstetric care
Can it be repeated too often? No. Containing COVID-19 disease requires social distancing, fastidious hand hygiene, and using a mask that covers the mouth and nose.
Pregnant women should be advised to assiduously practice social distancing and to wear a face covering or mask in public. Hand hygiene should be emphasized. Pregnant women with children should be advised to not allow their children to play with non‒cohabiting children because children may be asymptomatic vectors for COVID-19.
Pregnant health care workers should stop face-to-face contact with patients after 36 weeks’ gestation to avoid a late pregnancy infection that might cause the mother to be separated from her newborn. Based on data currently available, pregnancy in the absence of another risk factor is not a major risk factor for developing severe COVID-19 disease.13
Hyperthermia is a common feature of COVID-19. Acetaminophen is recommended treatment to suppress pyrexia during pregnancy.
The COVID-19 pandemic has transformed prenatal care from a series of face-to-face encounters at a health care facility to telemedicine either by telephone or a videoconferencing portal. Many factors contributed to the rapid switch to telemedicine, including orders by governors to restrict unnecessary travel, patients’ fear of contracting COVID-19 at their clinicians’ offices, clinicians’ fear of contracting COVID-19 from patients, and insurers’ rapid implementation of policies to pay for telemedicine visits. Most prenatal visits can be provided through telemedicine as long as the patient has a home blood pressure cuff and can reliably use the instrument. In-person visits may be required for blood testing, ultrasound assessment, anti-Rh immunoglobulin administration, and group B streptococcal infection screening. One regimen is to limit in-person prenatal visits to encounters at 12, 20, 28, and 36 weeks’ gestation when blood testing and ultrasound examinations are needed. The postpartum visit also may be conducted using telemedicine.
Pregnant women with COVID-19 and pneumonia are reported to have high rates of preterm birth less than 37 weeks (41%) and preterm prelabor rupture of membranes (19%).14
The rate of vertical transmission from mother to fetus is probably very low (<1%).15 However, based on serological studies, an occasional newborn has been reported to have IgM and IgG antibodies to the SARS-CoV-2 nucleoprotein at birth.16,17
Pregnant women should be consistently and regularly screened for symptoms of an upper respiratory infection, including: fever, new cough, new runny nose or nasal congestion, new sore throat, shortness of breath, muscle aches, and anosmia. A report of any of these symptoms should result in nucleic acid testing of a nasal swab for SARS-CoV-2 of all pregnant women. Given limited testing resources, however, symptomatic pregnant women with the following characteristics should be prioritized for testing: if the woman is more than 36 weeks pregnant, intrapartum, or in the hospital after delivery. Ambulatory pregnant women with symptoms who do not need medical care should quarantine themselves at home, if possible, or at another secure location away from their families. In some regions, testing of ambulatory patients with upper respiratory symptoms is limited.
All women scheduled for induction or cesarean delivery (CD) and their support person should have a symptom screen 24 to 48 hours before arrival to the hospital and should be rescreened prior to entry to labor and delivery. In this situation if the pregnant woman screens positive, she should be tested for SARS-CoV-2, and if the test result is positive, the scheduled induction and CD should be rescheduled, if possible. All hospitalized women and their support persons should be screened for symptoms daily. If the pregnant woman screens positive she should have a nucleic acid test for SARS-CoV-2. If the support person screens positive, he or she should be sent home.
Systemic glucocorticoids may worsen the course of COVID-19. For pregnant women with COVID-19 disease, betamethasone administration should be limited to women at high risk for preterm delivery within 7 days and only given to women between 23 weeks to 33 weeks 6 days of gestation. Women at risk for preterm delivery at 34 weeks to 36 weeks and 6 days of gestation should not be given betamethasone.
If cervical ripening is required, outpatient regimens should be prioritized.
One support person plays an important role in optimal labor outcome and should be permitted at the hospital. All support persons should wear a surgical or procedure mask.
Nitrous oxide for labor anesthesia should not be used during the pandemic because it might cause aerosolization of respiratory secretions, endangering health care workers. Neuraxial anesthesia is an optimal approach to labor anesthesia.
Labor management and timing of delivery does not need to be altered during the COVID-19 pandemic. However, pregnant women with moderate or severe COVID-19 disease who are not improving may have a modest improvement in respiratory function if they are delivered preterm.
At the beginning of the COVID pandemic, the CDC recommended separation of a COVID-positive mother and her newborn until the mother’s respiratory symptoms resolved. However, the CDC now recommends that, for a COVID-positive mother, joint decision-making should be used to decide whether to support the baby rooming-in with the mother or to practice separation of mother and baby at birth to reduce the risk for postnatal infection from mother to newborn. There is no evidence that breast milk contains virus that can cause an infection. One option is for the mother who recently tested positive for SARS-CoV-2 to provide newborn nutrition with expressed breast milk.
Pregnant women with COVID-19 may be at increased risk for venous thromboembolism. Some experts recommend that hospitalized pregnant women and postpartum women with COVID-19 receive thromboembolism prophylaxis.
The Chinese Centers for Disease Control and Prevention described a classification system for COVID-19 disease, including 3 categories18:
- mild: no dyspnea, no pneumonia, or mild pneumonia
- severe: dyspnea, respiratory frequency ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50% within 48 hours of onset of symptoms
- critical: respiratory failure, septic shock, or multiple organ dysfunction or failure.
Among 72,314 cases in China, 81% had mild disease, 14% had severe disease, and 5% had critical disease. In a report of 118 pregnant women in China, 92% of the women had mild disease; 8% had severe disease (hypoxemia), one of whom developed critical disease requiring mechanical ventilation.19 In this cohort, the most common presenting symptoms were fever (75%), cough (73%), chest tightness (18%), fatigue (17%), shortness of breath (7%), diarrhea (7%), and headache (6%). Lymphopenia was present in 44% of the women.
Severe and critical COVID-19 disease are associated with elevations in D-dimer, C-reactive protein, troponin, ferritin, and creatine phosphokinase levels. These markers return to the normal range with resolution of disease.
Continue to: Gynecologic care...
Gynecologic care
Gynecologists are highly impacted by the COVID-19 pandemic. Most state governments have requested that all elective surgery be suspended for the duration of the pandemic in order to redeploy health resources to the care of COVID-19 patients. Except for high-priority gynecologic surgery, including cancer surgery, treatment of heavy vaginal bleeding, and surgical care of ectopic pregnancy and miscarriage, most gynecologic surgery has ceased.
All office visits for routine gynecologic care have been suspended. Video and telephone visits can be used for contraceptive counseling and prescribing and for managing problems associated with the menopause, endometriosis, and vaginitis. Cervical cancer screening can be deferred for 3 to 6 months, depending on patient risk factors.
Medicines to treat COVID-19 infections
There are many highly effective medicines to manage HIV infection and medicines that cure hepatitis C. There is an urgent need to develop precision medicines to treat this disease. Early in the pandemic some experts thought that hydroxychloroquine might be helpful in the treatment of COVID-19 disease. But recent evidence suggests that hydroxychloroquine is probably not an effective treatment. As the pandemic has evolved, there is evidence that remdesivir may have modest efficacy in treating COVID-19 disease.20 Remdesivir has received emergency-use authorization by the FDA to treat COVID-19 infection.
Remdesivir
Based on expert opinion, in the absence of high-quality clinical trial evidence, our current practice is to offer pregnant women with severe or critical COVID-19 disease treatment with remdesivir.
Remdesivir (Gilead Sciences, Inc) is a nucleoside analog that inhibits RNA synthesis. A dose regimen for remdesivir is a 200-mg loading dose given intravenously, followed by 100 mg daily given intravenously for 5 to 10 days. Remdesivir may cause elevation of hepatic enzymes. Remdesivir has been administered to a few pregnant women to treat Ebola and Marburg virus disease.21
Experts in infectious disease are important resources for determining optimal medication regimens for the treatment of COVID-19 disease in pregnant women.
Continue to: Convalescent serum...
Convalescent serum
There are no high-quality studies demonstrating the efficacy of convalescent serum for treatment of COVID-19. A small case series suggests that there may be modest benefit to treatment of people with severe COVID-19 disease with convalescent serum.22
Testing for anti-SARS-CoV-2 IgM and IgG antibodies
We may have a serious problem in our current approach to detecting COVID-19 disease. Based on measurement of IgM and IgG antibodies to SARS-CoV-2 nucleocapsid protein, our current nucleic acid tests for SARS-CoV-2 may detect less than 80% of infections early in the course of disease. In two studies of IgM and IgG antibodies to the SARS-CoV-2 nucleocapsid protein, a single polymerase chain reaction test for SARS-CoV-2 had less than a 60% sensitivity for detecting the virus.23,24 During the second week of COVID-19 illness, IgM or IgG antibodies were detected in greater than 89% of infected patients.23 Severe disease resulted in high concentrations of antibody.
When testing for IgM and IgG antibodies is widely available, it may become an option to test all health care workers. This will permit the assignment of those health care workers with the highest levels of antibody to frontline duties with COVID-19 patients during the next disease outbreak, likely to occur at some point during the next 12 months.
A COVID-19 vaccine
Dozens of research teams, including pharmaceutical and biotechnology companies and many academic laboratories, are working on developing and testing vaccines to prevent COVID-19 disease. An effective vaccine would reduce the number of people who develop severe disease during the next outbreak, reducing deaths, avoiding a shutdown of the country, and allowing the health systems to function normally. A vaccine is unlikely to be widely available until sometime early in 2021.
Facing COVID-19 well-being and mental health
SARS-CoV-2, like all viral particles, is incredibly small. Remarkably, it has changed permanently life on earth. COVID-19 is affecting our physical health, psychological well-being, economics, and patterns of social interaction. As clinicians it is difficult to face a viral enemy that cannot be stopped from causing the death of more than 100,000 people, including some of our clinical colleagues, within a short period of time.
- F—focus on what is in your control
- A—acknowledge your thoughts and feelings
- C—come back to a focus on your body
- E—engage in what you are doing
- C—commit to acting effectively based on your core values
- O—opening up to difficult feelings and being kind to yourself and others
- V—values should guide your actions
- I—identify resources for help, assistance, support, and advice
- D—disinfect and practice social distancing.
This war will come to an end
During the American Revolution, colonists faced housing and food insecurity, epidemics of typhus and smallpox, traumatic injury including amputation of limbs, and a complete disruption of normal life activities. They persevered and, against the odds, successfully concluded the war. Unlike the colonists, who did not know if their conflict would end with success or failure, we clinicians know that the COVID-19 pandemic will end. We also know that eventually the global community of clinicians will develop and deploy the effective weapons we need to prevent a recurrence of this traumatic pandemic: population-wide testing for both the SARS-CoV-2 virus and serologic testing for IgG and IgM antibodies to the virus, effective antiviral medications, and a potent vaccine. ●
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA . doi: 10.1001/ jama . 2020 .3786.
- World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. March 29, 2020. https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed April 16, 2020.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State [published online March 19, 2020]. JAMA . doi: 10.1001/ jama . 2020 .4326.
- Guan WJ, Liang WH, Zhao Y, et al; China Medical Treatment Expert Group for Covid-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis [published online March 26, 2020]. Eur Respir J . doi: 10.1183/13993003.00547- 2020 .
- Onder G, Rezza G, Brusaferro S. Case fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4683.
- Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23 to March 16, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:411-415.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19 [published online February 21, 2020]. JAMA. doi: 10.1001/ jama . 2020 .2565.
- Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient [published online March 4, 2020]. JAMA . doi: 10.1001/ jama .2020.3227.
- Fineberg HV. Rapid expert consultation on the possibility of bioaerosol spread of SARS-CoV-2 for the COVID-19 pandemic. April 1, 2020. https://www.nap.edu/read/25769/chapter/1. Accessed April 16, 2020.
- Santarpia JL, River DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. March 26, 2020. doi.org10.1101/2020.03.23.20039466.
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan Hospitals during COVID-19 outbreak. BioRxiv. March 10, 2020. doi.org/10.1101/2020.03.08.982637.
- Klompas M, Morris CA, Sinclair J, et al. Universal masking in hospitals in the COVID-19 era [published online April 1, 2020]. N Engl J Med. doi: 10.1056/NEJMp2006372.
- Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1-6. doi: 10.2214/AJR.20.23072.
- Di Mascio D, Khalik A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol. doi:10.1016/j.ajogmf.2020.
100107. - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786.
- Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4621.
- Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia [published online March 26, 2020]. JAMA. doi: 10.1001/ jama .2020.4861.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Diease 2019 (COVID-19) outbreak in China. Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA . doi: 10.1001/jama.2020.2648.
- Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China [published online April 17, 2020]. N Engl J Med. doi 10.1056/NEJMc2009226.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. April 10, 2020. https://doi.org/10.1101/2020.03.22.20040758.
- Maulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293-2303.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma [published online March 27, 2020]. JAMA. doi: 10.1001/ jama . 2020 .4783.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019 [published online March 29, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa344.
- Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) [published online March 21, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa310.
NSDUH data might underestimate substance use by pregnant women
New study suggests rate of alcohol use might be almost 19%
The use of alcohol, tobacco products, and drugs by pregnant women is a substantial problem that may be more prevalent than previously thought, according to researcher Kimberly Yonkers, MD.
Higher levels of substance use during pregnancy means more negative impacts on maternal and fetal, neonatal, and child health. However, one bit of good news is that pregnant women still are less likely than nonpregnant women to engage in such behavior, said Dr. Yonkers, director of psychological medicine and the Center for Wellbeing of Women and Mothers at Yale University, New Haven, Conn.
Dr. Yonkers said in a featured presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Tobacco predominates among substances of concern used by pregnant women, with 11.6% reporting past-month use in 2018, according to the National Survey on Drug Use and Health, Dr. Yonkers said. Alcohol was next, with 9.9% of pregnant women reporting use in the past month, followed by drugs at 5.4%, of which marijuana was the most common.
Those numbers may jump much higher when focusing on substance use that’s biologically verified, she added, referring to a recent three-center cross-sectional study she and her colleagues published in Addiction (2019 Jun 19. doi: 10.1111/add.14651). In that study, alcohol use was as high as 18.9% among pregnant women who either had positive urine or self-reported use. Similarly, rates of nicotine or nicotine byproduct detected were 27% at one center in the study, and tetrahydrocannabinol reached 29.4% at that same center.
“These numbers are impressive,” Dr. Yonkers told attendees at the meeting. “So what we may be seeing in terms of the National Survey on Drug Use and Health, as valuable as it is, is in all likelihood it underestimates the use of substances in pregnancy.”
Substance use goes down in pregnancy as some women become more mindful of perinatal health, though unfortunately, that abstinence is offset by a dramatic rise in substance use in the 6-12 months’ post partum, research suggests.
Interestingly, big differences are found in both abstinence and relapse rates, with some data sets showing that, while alcohol is stopped fairly early, cigarettes are stopped much later, if at all.
On the postpartum side of the equation, relapse rates look similar for cigarettes, alcohol, and marijuana, but for some reason, cocaine relapse rates are much lower “That’s kind of nice, and we’d like to be able to understand what it is about this whole process that enabled that relative period of wellness,” Dr. Yonkers said.
Opioid use disorder is rising among pregnant women, just like it is in the general population, and 50% – or possibly even as high as 80% – of babies born to these women will experience neonatal opioid withdrawal, Dr. Yonkers said.
Maternal mortality in the United States increased by 34% from 2008 to 2016; while that’s a sobering statistic, Dr. Yonkers said, opioid-related maternal mortality doubled over that same time period.
“We really have to be mindful that we’re not just talking about taking care of kids and offspring, but we have to take care of moms – it’s really critical,” she said.
With the increasing legalization of cannabis, it’s expected that a lot more cannabis-exposed pregnancies will be seen in clinical practice, and some studies are starting to show an increase in prevalence in the preconception, prenatal, and postpartum period.
While some people feel that cannabis is benign, more data are needed, according to Dr. Yonkers, who said that cannabis and its metabolites cross the blood/placenta and blood/milk barriers, and that cannabinoid receptors are “very important” to fertility, implantation, and fetal development. One study recently published linked cannabis use in pregnancy to significant increases in preterm birth rates (JAMA. 2019 Jun 18;322[2]:145-52).
“We don’t really have a context for this, so we don’t really know what’s going to have an impact, and what’s not,” Dr. Yonkers said.
While both standard and novel treatments could help in the quest to achieve and maintain well-being in this unique patient population, Dr. Yonkers said that health equity and universal approaches to care might be needed to more comprehensively address the problem, which means taking a close look at how much money women have, the resources available to them, and where they live.
In many communities, eliminating inequalities in care will be critical to successfully addressing substance use issues in pregnant women, agreed Maureen Sayres Van Niel, MD, a specialist in women’s psychiatry in Boston and president of the Women’s Caucus of the APA.
“What we see is such a disparity in the delivery of care to women who are poor and living in communities where the socioeconomic and financial problems are very severe,” Dr. Van Niel said in an interview. “Unless we address these disparities, women will not be getting the kind of health care that they really need to have in the perinatal period.”
Dr. Yonkers reported a disclosure related to UpToDate.
New study suggests rate of alcohol use might be almost 19%
New study suggests rate of alcohol use might be almost 19%
The use of alcohol, tobacco products, and drugs by pregnant women is a substantial problem that may be more prevalent than previously thought, according to researcher Kimberly Yonkers, MD.
Higher levels of substance use during pregnancy means more negative impacts on maternal and fetal, neonatal, and child health. However, one bit of good news is that pregnant women still are less likely than nonpregnant women to engage in such behavior, said Dr. Yonkers, director of psychological medicine and the Center for Wellbeing of Women and Mothers at Yale University, New Haven, Conn.
Dr. Yonkers said in a featured presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Tobacco predominates among substances of concern used by pregnant women, with 11.6% reporting past-month use in 2018, according to the National Survey on Drug Use and Health, Dr. Yonkers said. Alcohol was next, with 9.9% of pregnant women reporting use in the past month, followed by drugs at 5.4%, of which marijuana was the most common.
Those numbers may jump much higher when focusing on substance use that’s biologically verified, she added, referring to a recent three-center cross-sectional study she and her colleagues published in Addiction (2019 Jun 19. doi: 10.1111/add.14651). In that study, alcohol use was as high as 18.9% among pregnant women who either had positive urine or self-reported use. Similarly, rates of nicotine or nicotine byproduct detected were 27% at one center in the study, and tetrahydrocannabinol reached 29.4% at that same center.
“These numbers are impressive,” Dr. Yonkers told attendees at the meeting. “So what we may be seeing in terms of the National Survey on Drug Use and Health, as valuable as it is, is in all likelihood it underestimates the use of substances in pregnancy.”
Substance use goes down in pregnancy as some women become more mindful of perinatal health, though unfortunately, that abstinence is offset by a dramatic rise in substance use in the 6-12 months’ post partum, research suggests.
Interestingly, big differences are found in both abstinence and relapse rates, with some data sets showing that, while alcohol is stopped fairly early, cigarettes are stopped much later, if at all.
On the postpartum side of the equation, relapse rates look similar for cigarettes, alcohol, and marijuana, but for some reason, cocaine relapse rates are much lower “That’s kind of nice, and we’d like to be able to understand what it is about this whole process that enabled that relative period of wellness,” Dr. Yonkers said.
Opioid use disorder is rising among pregnant women, just like it is in the general population, and 50% – or possibly even as high as 80% – of babies born to these women will experience neonatal opioid withdrawal, Dr. Yonkers said.
Maternal mortality in the United States increased by 34% from 2008 to 2016; while that’s a sobering statistic, Dr. Yonkers said, opioid-related maternal mortality doubled over that same time period.
“We really have to be mindful that we’re not just talking about taking care of kids and offspring, but we have to take care of moms – it’s really critical,” she said.
With the increasing legalization of cannabis, it’s expected that a lot more cannabis-exposed pregnancies will be seen in clinical practice, and some studies are starting to show an increase in prevalence in the preconception, prenatal, and postpartum period.
While some people feel that cannabis is benign, more data are needed, according to Dr. Yonkers, who said that cannabis and its metabolites cross the blood/placenta and blood/milk barriers, and that cannabinoid receptors are “very important” to fertility, implantation, and fetal development. One study recently published linked cannabis use in pregnancy to significant increases in preterm birth rates (JAMA. 2019 Jun 18;322[2]:145-52).
“We don’t really have a context for this, so we don’t really know what’s going to have an impact, and what’s not,” Dr. Yonkers said.
While both standard and novel treatments could help in the quest to achieve and maintain well-being in this unique patient population, Dr. Yonkers said that health equity and universal approaches to care might be needed to more comprehensively address the problem, which means taking a close look at how much money women have, the resources available to them, and where they live.
In many communities, eliminating inequalities in care will be critical to successfully addressing substance use issues in pregnant women, agreed Maureen Sayres Van Niel, MD, a specialist in women’s psychiatry in Boston and president of the Women’s Caucus of the APA.
“What we see is such a disparity in the delivery of care to women who are poor and living in communities where the socioeconomic and financial problems are very severe,” Dr. Van Niel said in an interview. “Unless we address these disparities, women will not be getting the kind of health care that they really need to have in the perinatal period.”
Dr. Yonkers reported a disclosure related to UpToDate.
The use of alcohol, tobacco products, and drugs by pregnant women is a substantial problem that may be more prevalent than previously thought, according to researcher Kimberly Yonkers, MD.
Higher levels of substance use during pregnancy means more negative impacts on maternal and fetal, neonatal, and child health. However, one bit of good news is that pregnant women still are less likely than nonpregnant women to engage in such behavior, said Dr. Yonkers, director of psychological medicine and the Center for Wellbeing of Women and Mothers at Yale University, New Haven, Conn.
Dr. Yonkers said in a featured presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Tobacco predominates among substances of concern used by pregnant women, with 11.6% reporting past-month use in 2018, according to the National Survey on Drug Use and Health, Dr. Yonkers said. Alcohol was next, with 9.9% of pregnant women reporting use in the past month, followed by drugs at 5.4%, of which marijuana was the most common.
Those numbers may jump much higher when focusing on substance use that’s biologically verified, she added, referring to a recent three-center cross-sectional study she and her colleagues published in Addiction (2019 Jun 19. doi: 10.1111/add.14651). In that study, alcohol use was as high as 18.9% among pregnant women who either had positive urine or self-reported use. Similarly, rates of nicotine or nicotine byproduct detected were 27% at one center in the study, and tetrahydrocannabinol reached 29.4% at that same center.
“These numbers are impressive,” Dr. Yonkers told attendees at the meeting. “So what we may be seeing in terms of the National Survey on Drug Use and Health, as valuable as it is, is in all likelihood it underestimates the use of substances in pregnancy.”
Substance use goes down in pregnancy as some women become more mindful of perinatal health, though unfortunately, that abstinence is offset by a dramatic rise in substance use in the 6-12 months’ post partum, research suggests.
Interestingly, big differences are found in both abstinence and relapse rates, with some data sets showing that, while alcohol is stopped fairly early, cigarettes are stopped much later, if at all.
On the postpartum side of the equation, relapse rates look similar for cigarettes, alcohol, and marijuana, but for some reason, cocaine relapse rates are much lower “That’s kind of nice, and we’d like to be able to understand what it is about this whole process that enabled that relative period of wellness,” Dr. Yonkers said.
Opioid use disorder is rising among pregnant women, just like it is in the general population, and 50% – or possibly even as high as 80% – of babies born to these women will experience neonatal opioid withdrawal, Dr. Yonkers said.
Maternal mortality in the United States increased by 34% from 2008 to 2016; while that’s a sobering statistic, Dr. Yonkers said, opioid-related maternal mortality doubled over that same time period.
“We really have to be mindful that we’re not just talking about taking care of kids and offspring, but we have to take care of moms – it’s really critical,” she said.
With the increasing legalization of cannabis, it’s expected that a lot more cannabis-exposed pregnancies will be seen in clinical practice, and some studies are starting to show an increase in prevalence in the preconception, prenatal, and postpartum period.
While some people feel that cannabis is benign, more data are needed, according to Dr. Yonkers, who said that cannabis and its metabolites cross the blood/placenta and blood/milk barriers, and that cannabinoid receptors are “very important” to fertility, implantation, and fetal development. One study recently published linked cannabis use in pregnancy to significant increases in preterm birth rates (JAMA. 2019 Jun 18;322[2]:145-52).
“We don’t really have a context for this, so we don’t really know what’s going to have an impact, and what’s not,” Dr. Yonkers said.
While both standard and novel treatments could help in the quest to achieve and maintain well-being in this unique patient population, Dr. Yonkers said that health equity and universal approaches to care might be needed to more comprehensively address the problem, which means taking a close look at how much money women have, the resources available to them, and where they live.
In many communities, eliminating inequalities in care will be critical to successfully addressing substance use issues in pregnant women, agreed Maureen Sayres Van Niel, MD, a specialist in women’s psychiatry in Boston and president of the Women’s Caucus of the APA.
“What we see is such a disparity in the delivery of care to women who are poor and living in communities where the socioeconomic and financial problems are very severe,” Dr. Van Niel said in an interview. “Unless we address these disparities, women will not be getting the kind of health care that they really need to have in the perinatal period.”
Dr. Yonkers reported a disclosure related to UpToDate.
EXPERT ANALYSIS FROM APA 2020
The American maternal mortality crisis: The role of racism and bias
April 11-17 marked the third annual national Black Maternal Health Week, an event launched in 2017 by the Atlanta-based Black Mamas Matter Alliance (BMMA), in part to “deepen the national conversation about black maternal health.”
Around the same time, emerging data showing higher mortality rates among black patients versus patients of other races with COVID-19 opened similar dialogue fraught with questions about what might explain the disturbing health disparities.
“It’s kind of surprising to me that people are shocked by these [COVID-19] disparities,” Rebekah Gee, MD, an ob.gyn. who is director of the Louisiana State University Health System in New Orleans and a driving force behind initiatives addressing racial disparities in maternal health, said in an interview. If this is it, great – and certainly every moment is a moment for learning – but these COVID-19 disparities should not be surprising to people who have been looking at data.”
Veronica Gillispie, MD, an ob.gyn. and medical director of the Louisiana Perinatal Quality Collaborative and Pregnancy-Associated Mortality Review, was similarly baffled that the news was treated as a revelation.
That news includes outcomes data from New York showing that in March there were 92.3 and 74.3 deaths per 100,000 black and Hispanic COVID-19 patients, respectively, compared with 45.2 per 100,000 white patients.
“Now there’s a task force and all these initiatives to look at why this is happening, and I think those of us who work in maternal mortality are all saying, ‘We know why it’s happening,’ ” she said. “It’s the same thing we’ve been telling people why it’s been happening in maternal mortality.
“It’s implicit bias and structural racism.”
Facing hard numbers, harder conversations
The U.S. maternal mortality rate in 2018 was 17 per 100,000 live births – the highest of any similarly wealthy industrialized nation, the Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS) reported in January. That’s a striking statistic in its own right. Perhaps more striking is the breakdown by race.
Hispanic women had the lowest maternal mortality rate at 12 per 100,000 live births, followed by non-Hispanic white women at 15.
The rate for non-Hispanic black women was 37 per 100,000 live births.
Numerous factors contribute to these disparities. Among those listed by the American College of Obstetricians and Gynecologists’ chief executive officer Maureen G. Phipps, MD, in a press statement on the NCHS data, are care access issues, lack of standardization of care, bias, and racism. All of these must be addressed if the disparities in maternal and other areas of care are to be eliminated, according to Dr. Phipps.
“The NCHS data confirmed what we have known from other data sources: The rate of maternal deaths for non-Hispanic black women is substantially higher than the rates for non-Hispanic white women,” she wrote. “Continued efforts to improve the standardization of data and review processes related to U.S. maternal mortality are a necessary step to achieving the goal of eliminating disparities and preventable maternal mortality.”
However, such efforts frequently encounter roadblocks constructed by the reluctance among “many academics, policy makers, scientists, elected officials, journalists, and others responsible for defining and responding to the public discourse” to identify racism as a root cause of health disparities, according to Zinzi D. Bailey, ScD, former director of research and evaluation for the New York City Department of Health and Mental Hygiene, and colleagues.
In the third of a three-part conceptual report in The Lancet, entitled America: Equity and Equality in Health, Dr. Bailey and colleagues argued that advancing health equity requires a focus on structural racism – which they defined as “the totality of ways in which societies foster racial discrimination via mutually reinforcing inequitable systems (e.g., in housing, education, employment, earning, benefits, credit, media, healthcare, and criminal justice, etc.) that in turn reinforce discriminatory beliefs, values, and distribution of resources.”
In their series, the authors peeled back layer upon layer of sociological and political contributors to structural racism throughout history, revealing how each laid a foundation for health inequity over time. They particularly home in on health care quality and access.
“Interpersonal racism, bias, and discrimination in healthcare settings can directly affect health through poor health care,” they wrote, noting that “almost 15 years ago, the Institute of Medicine report entitled Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, documented systematic and pervasive bias in the treatment of people of color resulting in substandard care.”
That report concluded that “bias, stereotyping, prejudice, and clinical uncertainty on the part of healthcare providers” likely play a role in the continuation of health disparities. More recent data – including the NCHS maternal mortality data – show an ongoing crisis.
A study of 210 experienced primary care providers and 190 community members in the Denver area, for example, found substantial evidence of implicit bias against both Latino and African American patients. The authors defined implicit bias as “unintentional and even unconscious” negative evaluation of one group and its members relative to another that is expressed implicitly, such as through negative nonverbal behavior.
“Activated by situational cues (e.g., a person’s skin color), implicit bias can quickly and unknowingly exert its influence on perception, memory, and behavior,” they wrote.
In their study, Implicit Association Test and self-report measures of bias showed similar rates of implicit bias among the providers and community members, with only a slight weakening of ethnic/racial bias among providers after adjustment for background characteristics, which suggests “a wider societal problem,” they said.
A specific example of how implicit bias can manifest was described in a 2016 report addressing the well-documented under-treatment of pain among black versus white patients. Kelly M. Hoffman, PhD, and colleagues demonstrated that a substantial number of individuals with at least some medical training endorse false beliefs regarding biological differences between black and white patients. For example, 25% of 28 white residents surveyed agreed black individuals have thicker skin, and 4% believed black individuals have faster blood coagulation and less sensitivity in their nerve endings.
Those who more strongly endorsed such erroneous beliefs were more likely to underestimate and undertreat pain among black patients, the authors found.
Another study, which underscored the insidiousness of structural racism, was reported in Science. The authors identified significant racial bias in an algorithm widely used by health systems, insurers, and practitioners to allocate health care resources for patients with complex health needs. The algorithm, which affects millions of patients, uses predictions of future health care costs rather than future illness to determine who should receive extra medical care.
The problem is that unequal care access for black patients skews lower the foundational cost data used for making those predictions. Correcting the algorithm would increase the percentage of black patients receiving additional medical help from 17.7% to 46.5%, the authors concluded.
This evidence of persistent racism and bias in medicine, however, doesn’t mean progress is lacking.
ACOG has partnered with numerous other organizations to promote awareness and change, including through legislation. A recent win was the enactment of the Preventing Maternal Deaths Act of 2018, a bipartisan bill designed to promote and support maternal mortality review committees in every state. A major focus of BMMA’s Black Maternal Health Week was the Black Maternal Health Momnibus Act of 2020, a nine-bill package introduced in March to comprehensively address the crisis.
But efforts like these, whether they aim to elucidate the contributors to health disparities or to directly target structural and overt racism and root out implicit bias in medical care, are nothing new. As Dr. Bailey and colleagues noted, a challenge is getting the message across because efforts to avoid tough conversations around these topics are nothing new, either.
Dr. Gee attested to that during a maternal mortality panel discussion at the 2019 ACOG meeting where she spoke about the resistance she encountered in 2016 when she was appointed secretary of the Louisiana Health Department and worked to make racism and bias a foundational part of the discussion on improving maternal and fetal outcomes.
She established the first Office of Health Equity in the state – and the first in the nation to not only require measurement outcomes by race but also explicitly address racial bias at the outset. The goal wasn’t just to talk about it but to “plan for addressing equity in every single aspect of what we do with ... our case equity action teams.”
“At our first maternal mortality quality meetings we insisted on focusing on equity at the very outset, and we had people that left when we started talking about racism,” she said, noting that others said it was “too political” to discuss during an election year or that equity was something to address later.
“We said no. We insisted on it, and I think that was very important because all ships don’t rise with the tide, not with health disparities,” she said, recounting an earlier experience when she led the Louisiana Birth Outcomes Initiative: “I asked to have a brown-bag focused on racism at the department so we could talk about the impact of implicit bias on decision making, and I was told that I was a Yankee who didn’t understand the South and that racism didn’t really exist here, and what did I know about it – and I couldn’t have the brown-bag.”
That was in 2011.
Fast-forward to April 24, 2020. As Dr. Gee shared her perspective on addressing racism and bias in medicine, she was preparing for a call regarding the racial disparities in COVID-19 outcomes – the first health equity action sanctioned by Louisiana Governor John Bel Edwards (D).
“I think we really set the stage for these discussions,” she said.
Addressing equity to enact change
The efforts in Louisiana also set the stage for better maternal outcomes. At the 2019 ACOG meeting where she spoke as part of the President’s Panel, Dr. Gee said Louisiana had the highest maternal mortality rates in the nation. The NCHC data released in January, however, suggest that may no longer be the case.
Inconsistencies in how the latest and prior data were reported, including in how maternal mortality was defined, make direct comparison impossible. But in the latest report, Louisiana ranked seventh among states with available data.
“Ninety percent of the deliveries in the state happen at hospitals that we worked with,” Dr. Gee said, highlighting the reach of the efforts to improve outcomes there.
She also described a recent case involving an anemic patient whose bleeding risk was identified early thanks to the programs put in place. That enabled early preparation in the event of complications.
The patient experienced a massive hemorrhage, but the preparation, including having units of blood on hand in case of such an emergency, saved her life.
“So we clearly have not just data, but individual stories of people whose lives have been saved by this work,” she said.
More tangible data on maternal morbidity further show that the efforts in the state are making a difference, Dr. Gillispie said, citing preliminary outcomes data from the Pregnancy-Associated Mortality Review launched in 2018.
“We started with an initial goal of reducing severe maternal morbidity related to hypertension and hemorrhage by 20%, as well as reducing the black/white disparity gap by Mother’s Day 2020,” she said.
Final analyses have been delayed because of COVID-19, but early assessments showed a reduction in the disparity gap, she said, again highlighting the importance of focusing on equity.
“Definitely from the standpoint of the Quality Collaborative side ... we’ve been working with our facilities to make them aware of what implicit bias is, helping them to also do the Harvard Implicit Bias Test so they can figure out what their own biases are, start working to acknowledge them and address them, and start working to fight against letting that bias change how they treat individuals,” Dr. Gillispie said.
The work started through these initiatives will continue because there is much left to be done, she said.
Indeed, the surprised reactions in recent weeks to the reports of disparities in COVID-19 outcomes further underscore that reality, and the maternal mortality statistics – with use of the voices of those directly affected by structural and overt racism and bias in maternal care as a megaphone – speak for themselves.
Hearing implicit bias from patients’ perspective
Just ask Timoria McQueen Saba, a black woman who nearly died from a postpartum hemorrhage in 2010. At ACOG 2019, she spoke about how she had to switch ob.gyns. three times during her first pregnancy because she felt she had not received quality care – one doctor neglected to tell her she had placenta previa. She also experienced excessive wait times at prenatal appointments and had been on the receiving end of microaggressions and degrading questions such as “Are you still married?” and “Is your husband your baby’s father?” – and these are all things her white friends who recommended those physicians never experienced, she said.
“The health care system has just sometimes beaten people down so much, just like the world has – people of color, especially – to where you’re dismissed, your concerns are invalidated,” she said. “Some doctors don’t even think black people feel pain [or that] our pain is less.”
Mrs. Saba also spoke about how her health care “improved a billion percent” when her white husband accompanied her to appointments.
Just ask Charles S. Johnson IV, whose wife Kira Dixon Johnson died in 2016 during surgery for postpartum bleeding complications – after he and other family members spent 10 hours pleading for help for her.
Speaking at the ACOG panel discussion with Mrs. Saba, Mr. Johnson described “a clear disconnect” between the medical staff at the hospital and the way they viewed and valued Kira. He shared his frustration in wanting to advocate for his wife, but knowing that, as an African American male, he risked being seen as a threat and removed from the hospital if he didn’t stay calm, if he “tapped into those natural instincts as a man and a husband who wants to just protect his family.”
He fought back emotions, struggling to get the words out, saying that’s what haunts him and keeps him up at night – wondering if he should have “fought harder, grabbed the doctor by the collar, raised his voice, slammed on the counter.
“Maybe they would have done something,” he said.
Such experiences cross all socioeconomic boundaries. Ask U.S. Track and Field Olympic gold medalist Allyson Felix, who testified at a U.S. House Ways and Means Committee hearing on May 16, 2019 after developing severe preeclampsia that threatened her life and that of her baby. Ask tennis champion Serena Williams, who demanded assessment for pulmonary embolism following the birth of her child; she knew the signs, but her health care providers initially dismissed her concerns.
Their experiences aren’t just anecdotal. Data consistently show how racism and bias affect patient treatment and outcomes. Dr. Gee, for example, shared findings from a retrospective assessment of 47 confirmed pregnancy-related deaths in Louisiana between 2011 and 2016 that looked specifically at whether the deaths could potentially have been prevented if blood was given sooner, cardiomyopathy was recognized sooner, hypertension was treated on time, or other changes were made to care.
The answer was “Yes” in 9% of cases involving white patients – and in 59% of cases involving black patients (odds ratio, 14.6).
The study, reported in February in Obstetrics & Gynecology, showed that 27 of the deaths (58%) occurred at level III or IV birth facilities and that those deaths were not less likely than those at level I or II facilities to be categorized as preventable (OR, 2.0).
Findings from the Giving Voice to Mothers study, published in Reproductive Health in 2019, showed how mistreatment during childbirth might contribute to such outcomes.
In an online cross-sectional survey of more than 2,100 U.S. women, one in six reported at least one type of mistreatment, such as loss of autonomy, being yelled at or threatened, being ignored or having requests for help ignored, Saraswathi Vedam, SciD, of the Birth Place Lab at the University of British Columbia, Vancouver, and colleagues reported.
Race was among the factors associated with likelihood of mistreatment, and the rates of mistreatment for women of color were consistently higher – even when looking at interactions between race and other characteristics, such as socioeconomic status (SES). For example, 27.2% of women of color with low SES, compared with 18.7% of white women with low SES, reported mistreatment. Having a partner who was black, regardless of maternal race, was also associated with an increased rate of mistreatment, the authors found.
“I often get the question, ‘Do you think Kira would be alive if she was white,’ ” Mr. Johnson said. “The first way I respond to that question is [by saying that] the simple fact that you have to ask me is a problem.
“When this first happened, I was in so much pain that I couldn’t process the fact that something so egregious and outrageous happened to my wife because of the color of her skin, but as I began to process and really think about it and unpack this scenario, I have to be really frank ... do I think that she would have sat there for 10 hours while we begged and pleaded? Absolutely not.”
He stressed that his words aren’t “an indictment of the profession.”
“This is not an indictment saying that all people are racist or prejudiced,” he said. “But here’s the reality: If you are in this profession, if you are responsible for the well-being of patients and their families, and you are not able to see them in the same way that you see your mother, your wife, your sister, you have two options – you need to find something else to do or you need to take steps to get better.”
Fixing systems, finding solutions
Dr. Gee acknowledged the work that physicians need to do to help improve outcomes.
“The average time we give a patient to talk is 11 seconds before we interrupt them,” she said, as one example. “We have to recognize that.”
But efforts to improve outcomes shouldn’t just focus on changing physician behavior, she said.
“We really need to focus, as has the U.K. – very effectively – on using midwives, doulas, other health care professionals as complements to physicians to make sure that we have women-centered birth experiences.
“So, instead of just blaming the doctors, I think we need to change the system,” Dr. Gee emphasized.
The disruptions in health systems caused by COVID-19 present a unique opportunity to do that, she said. There is now an opportunity to build them back.
“We have a chance to build the systems back, and when we do so, we ought to build them back correcting for implicit bias and some of the systemic issues that lead to poor outcomes for people of color in our country,” she said.
Solutions proposed by Dr. Gee and others include more diversity in the workforce, more inclusion of patient advocates in maternal care, development of culturally appropriate literacy and numeracy communications, measurement by race (and action on the outcomes), standardization of care, and development of new ways to improve care access.
We will focus more specifically on these solutions in Part 2 of this article in our maternal mortality series. Previous articles in the series are available at mdedge.com/obgyn/maternal-mortality.
April 11-17 marked the third annual national Black Maternal Health Week, an event launched in 2017 by the Atlanta-based Black Mamas Matter Alliance (BMMA), in part to “deepen the national conversation about black maternal health.”
Around the same time, emerging data showing higher mortality rates among black patients versus patients of other races with COVID-19 opened similar dialogue fraught with questions about what might explain the disturbing health disparities.
“It’s kind of surprising to me that people are shocked by these [COVID-19] disparities,” Rebekah Gee, MD, an ob.gyn. who is director of the Louisiana State University Health System in New Orleans and a driving force behind initiatives addressing racial disparities in maternal health, said in an interview. If this is it, great – and certainly every moment is a moment for learning – but these COVID-19 disparities should not be surprising to people who have been looking at data.”
Veronica Gillispie, MD, an ob.gyn. and medical director of the Louisiana Perinatal Quality Collaborative and Pregnancy-Associated Mortality Review, was similarly baffled that the news was treated as a revelation.
That news includes outcomes data from New York showing that in March there were 92.3 and 74.3 deaths per 100,000 black and Hispanic COVID-19 patients, respectively, compared with 45.2 per 100,000 white patients.
“Now there’s a task force and all these initiatives to look at why this is happening, and I think those of us who work in maternal mortality are all saying, ‘We know why it’s happening,’ ” she said. “It’s the same thing we’ve been telling people why it’s been happening in maternal mortality.
“It’s implicit bias and structural racism.”
Facing hard numbers, harder conversations
The U.S. maternal mortality rate in 2018 was 17 per 100,000 live births – the highest of any similarly wealthy industrialized nation, the Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS) reported in January. That’s a striking statistic in its own right. Perhaps more striking is the breakdown by race.
Hispanic women had the lowest maternal mortality rate at 12 per 100,000 live births, followed by non-Hispanic white women at 15.
The rate for non-Hispanic black women was 37 per 100,000 live births.
Numerous factors contribute to these disparities. Among those listed by the American College of Obstetricians and Gynecologists’ chief executive officer Maureen G. Phipps, MD, in a press statement on the NCHS data, are care access issues, lack of standardization of care, bias, and racism. All of these must be addressed if the disparities in maternal and other areas of care are to be eliminated, according to Dr. Phipps.
“The NCHS data confirmed what we have known from other data sources: The rate of maternal deaths for non-Hispanic black women is substantially higher than the rates for non-Hispanic white women,” she wrote. “Continued efforts to improve the standardization of data and review processes related to U.S. maternal mortality are a necessary step to achieving the goal of eliminating disparities and preventable maternal mortality.”
However, such efforts frequently encounter roadblocks constructed by the reluctance among “many academics, policy makers, scientists, elected officials, journalists, and others responsible for defining and responding to the public discourse” to identify racism as a root cause of health disparities, according to Zinzi D. Bailey, ScD, former director of research and evaluation for the New York City Department of Health and Mental Hygiene, and colleagues.
In the third of a three-part conceptual report in The Lancet, entitled America: Equity and Equality in Health, Dr. Bailey and colleagues argued that advancing health equity requires a focus on structural racism – which they defined as “the totality of ways in which societies foster racial discrimination via mutually reinforcing inequitable systems (e.g., in housing, education, employment, earning, benefits, credit, media, healthcare, and criminal justice, etc.) that in turn reinforce discriminatory beliefs, values, and distribution of resources.”
In their series, the authors peeled back layer upon layer of sociological and political contributors to structural racism throughout history, revealing how each laid a foundation for health inequity over time. They particularly home in on health care quality and access.
“Interpersonal racism, bias, and discrimination in healthcare settings can directly affect health through poor health care,” they wrote, noting that “almost 15 years ago, the Institute of Medicine report entitled Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, documented systematic and pervasive bias in the treatment of people of color resulting in substandard care.”
That report concluded that “bias, stereotyping, prejudice, and clinical uncertainty on the part of healthcare providers” likely play a role in the continuation of health disparities. More recent data – including the NCHS maternal mortality data – show an ongoing crisis.
A study of 210 experienced primary care providers and 190 community members in the Denver area, for example, found substantial evidence of implicit bias against both Latino and African American patients. The authors defined implicit bias as “unintentional and even unconscious” negative evaluation of one group and its members relative to another that is expressed implicitly, such as through negative nonverbal behavior.
“Activated by situational cues (e.g., a person’s skin color), implicit bias can quickly and unknowingly exert its influence on perception, memory, and behavior,” they wrote.
In their study, Implicit Association Test and self-report measures of bias showed similar rates of implicit bias among the providers and community members, with only a slight weakening of ethnic/racial bias among providers after adjustment for background characteristics, which suggests “a wider societal problem,” they said.
A specific example of how implicit bias can manifest was described in a 2016 report addressing the well-documented under-treatment of pain among black versus white patients. Kelly M. Hoffman, PhD, and colleagues demonstrated that a substantial number of individuals with at least some medical training endorse false beliefs regarding biological differences between black and white patients. For example, 25% of 28 white residents surveyed agreed black individuals have thicker skin, and 4% believed black individuals have faster blood coagulation and less sensitivity in their nerve endings.
Those who more strongly endorsed such erroneous beliefs were more likely to underestimate and undertreat pain among black patients, the authors found.
Another study, which underscored the insidiousness of structural racism, was reported in Science. The authors identified significant racial bias in an algorithm widely used by health systems, insurers, and practitioners to allocate health care resources for patients with complex health needs. The algorithm, which affects millions of patients, uses predictions of future health care costs rather than future illness to determine who should receive extra medical care.
The problem is that unequal care access for black patients skews lower the foundational cost data used for making those predictions. Correcting the algorithm would increase the percentage of black patients receiving additional medical help from 17.7% to 46.5%, the authors concluded.
This evidence of persistent racism and bias in medicine, however, doesn’t mean progress is lacking.
ACOG has partnered with numerous other organizations to promote awareness and change, including through legislation. A recent win was the enactment of the Preventing Maternal Deaths Act of 2018, a bipartisan bill designed to promote and support maternal mortality review committees in every state. A major focus of BMMA’s Black Maternal Health Week was the Black Maternal Health Momnibus Act of 2020, a nine-bill package introduced in March to comprehensively address the crisis.
But efforts like these, whether they aim to elucidate the contributors to health disparities or to directly target structural and overt racism and root out implicit bias in medical care, are nothing new. As Dr. Bailey and colleagues noted, a challenge is getting the message across because efforts to avoid tough conversations around these topics are nothing new, either.
Dr. Gee attested to that during a maternal mortality panel discussion at the 2019 ACOG meeting where she spoke about the resistance she encountered in 2016 when she was appointed secretary of the Louisiana Health Department and worked to make racism and bias a foundational part of the discussion on improving maternal and fetal outcomes.
She established the first Office of Health Equity in the state – and the first in the nation to not only require measurement outcomes by race but also explicitly address racial bias at the outset. The goal wasn’t just to talk about it but to “plan for addressing equity in every single aspect of what we do with ... our case equity action teams.”
“At our first maternal mortality quality meetings we insisted on focusing on equity at the very outset, and we had people that left when we started talking about racism,” she said, noting that others said it was “too political” to discuss during an election year or that equity was something to address later.
“We said no. We insisted on it, and I think that was very important because all ships don’t rise with the tide, not with health disparities,” she said, recounting an earlier experience when she led the Louisiana Birth Outcomes Initiative: “I asked to have a brown-bag focused on racism at the department so we could talk about the impact of implicit bias on decision making, and I was told that I was a Yankee who didn’t understand the South and that racism didn’t really exist here, and what did I know about it – and I couldn’t have the brown-bag.”
That was in 2011.
Fast-forward to April 24, 2020. As Dr. Gee shared her perspective on addressing racism and bias in medicine, she was preparing for a call regarding the racial disparities in COVID-19 outcomes – the first health equity action sanctioned by Louisiana Governor John Bel Edwards (D).
“I think we really set the stage for these discussions,” she said.
Addressing equity to enact change
The efforts in Louisiana also set the stage for better maternal outcomes. At the 2019 ACOG meeting where she spoke as part of the President’s Panel, Dr. Gee said Louisiana had the highest maternal mortality rates in the nation. The NCHC data released in January, however, suggest that may no longer be the case.
Inconsistencies in how the latest and prior data were reported, including in how maternal mortality was defined, make direct comparison impossible. But in the latest report, Louisiana ranked seventh among states with available data.
“Ninety percent of the deliveries in the state happen at hospitals that we worked with,” Dr. Gee said, highlighting the reach of the efforts to improve outcomes there.
She also described a recent case involving an anemic patient whose bleeding risk was identified early thanks to the programs put in place. That enabled early preparation in the event of complications.
The patient experienced a massive hemorrhage, but the preparation, including having units of blood on hand in case of such an emergency, saved her life.
“So we clearly have not just data, but individual stories of people whose lives have been saved by this work,” she said.
More tangible data on maternal morbidity further show that the efforts in the state are making a difference, Dr. Gillispie said, citing preliminary outcomes data from the Pregnancy-Associated Mortality Review launched in 2018.
“We started with an initial goal of reducing severe maternal morbidity related to hypertension and hemorrhage by 20%, as well as reducing the black/white disparity gap by Mother’s Day 2020,” she said.
Final analyses have been delayed because of COVID-19, but early assessments showed a reduction in the disparity gap, she said, again highlighting the importance of focusing on equity.
“Definitely from the standpoint of the Quality Collaborative side ... we’ve been working with our facilities to make them aware of what implicit bias is, helping them to also do the Harvard Implicit Bias Test so they can figure out what their own biases are, start working to acknowledge them and address them, and start working to fight against letting that bias change how they treat individuals,” Dr. Gillispie said.
The work started through these initiatives will continue because there is much left to be done, she said.
Indeed, the surprised reactions in recent weeks to the reports of disparities in COVID-19 outcomes further underscore that reality, and the maternal mortality statistics – with use of the voices of those directly affected by structural and overt racism and bias in maternal care as a megaphone – speak for themselves.
Hearing implicit bias from patients’ perspective
Just ask Timoria McQueen Saba, a black woman who nearly died from a postpartum hemorrhage in 2010. At ACOG 2019, she spoke about how she had to switch ob.gyns. three times during her first pregnancy because she felt she had not received quality care – one doctor neglected to tell her she had placenta previa. She also experienced excessive wait times at prenatal appointments and had been on the receiving end of microaggressions and degrading questions such as “Are you still married?” and “Is your husband your baby’s father?” – and these are all things her white friends who recommended those physicians never experienced, she said.
“The health care system has just sometimes beaten people down so much, just like the world has – people of color, especially – to where you’re dismissed, your concerns are invalidated,” she said. “Some doctors don’t even think black people feel pain [or that] our pain is less.”
Mrs. Saba also spoke about how her health care “improved a billion percent” when her white husband accompanied her to appointments.
Just ask Charles S. Johnson IV, whose wife Kira Dixon Johnson died in 2016 during surgery for postpartum bleeding complications – after he and other family members spent 10 hours pleading for help for her.
Speaking at the ACOG panel discussion with Mrs. Saba, Mr. Johnson described “a clear disconnect” between the medical staff at the hospital and the way they viewed and valued Kira. He shared his frustration in wanting to advocate for his wife, but knowing that, as an African American male, he risked being seen as a threat and removed from the hospital if he didn’t stay calm, if he “tapped into those natural instincts as a man and a husband who wants to just protect his family.”
He fought back emotions, struggling to get the words out, saying that’s what haunts him and keeps him up at night – wondering if he should have “fought harder, grabbed the doctor by the collar, raised his voice, slammed on the counter.
“Maybe they would have done something,” he said.
Such experiences cross all socioeconomic boundaries. Ask U.S. Track and Field Olympic gold medalist Allyson Felix, who testified at a U.S. House Ways and Means Committee hearing on May 16, 2019 after developing severe preeclampsia that threatened her life and that of her baby. Ask tennis champion Serena Williams, who demanded assessment for pulmonary embolism following the birth of her child; she knew the signs, but her health care providers initially dismissed her concerns.
Their experiences aren’t just anecdotal. Data consistently show how racism and bias affect patient treatment and outcomes. Dr. Gee, for example, shared findings from a retrospective assessment of 47 confirmed pregnancy-related deaths in Louisiana between 2011 and 2016 that looked specifically at whether the deaths could potentially have been prevented if blood was given sooner, cardiomyopathy was recognized sooner, hypertension was treated on time, or other changes were made to care.
The answer was “Yes” in 9% of cases involving white patients – and in 59% of cases involving black patients (odds ratio, 14.6).
The study, reported in February in Obstetrics & Gynecology, showed that 27 of the deaths (58%) occurred at level III or IV birth facilities and that those deaths were not less likely than those at level I or II facilities to be categorized as preventable (OR, 2.0).
Findings from the Giving Voice to Mothers study, published in Reproductive Health in 2019, showed how mistreatment during childbirth might contribute to such outcomes.
In an online cross-sectional survey of more than 2,100 U.S. women, one in six reported at least one type of mistreatment, such as loss of autonomy, being yelled at or threatened, being ignored or having requests for help ignored, Saraswathi Vedam, SciD, of the Birth Place Lab at the University of British Columbia, Vancouver, and colleagues reported.
Race was among the factors associated with likelihood of mistreatment, and the rates of mistreatment for women of color were consistently higher – even when looking at interactions between race and other characteristics, such as socioeconomic status (SES). For example, 27.2% of women of color with low SES, compared with 18.7% of white women with low SES, reported mistreatment. Having a partner who was black, regardless of maternal race, was also associated with an increased rate of mistreatment, the authors found.
“I often get the question, ‘Do you think Kira would be alive if she was white,’ ” Mr. Johnson said. “The first way I respond to that question is [by saying that] the simple fact that you have to ask me is a problem.
“When this first happened, I was in so much pain that I couldn’t process the fact that something so egregious and outrageous happened to my wife because of the color of her skin, but as I began to process and really think about it and unpack this scenario, I have to be really frank ... do I think that she would have sat there for 10 hours while we begged and pleaded? Absolutely not.”
He stressed that his words aren’t “an indictment of the profession.”
“This is not an indictment saying that all people are racist or prejudiced,” he said. “But here’s the reality: If you are in this profession, if you are responsible for the well-being of patients and their families, and you are not able to see them in the same way that you see your mother, your wife, your sister, you have two options – you need to find something else to do or you need to take steps to get better.”
Fixing systems, finding solutions
Dr. Gee acknowledged the work that physicians need to do to help improve outcomes.
“The average time we give a patient to talk is 11 seconds before we interrupt them,” she said, as one example. “We have to recognize that.”
But efforts to improve outcomes shouldn’t just focus on changing physician behavior, she said.
“We really need to focus, as has the U.K. – very effectively – on using midwives, doulas, other health care professionals as complements to physicians to make sure that we have women-centered birth experiences.
“So, instead of just blaming the doctors, I think we need to change the system,” Dr. Gee emphasized.
The disruptions in health systems caused by COVID-19 present a unique opportunity to do that, she said. There is now an opportunity to build them back.
“We have a chance to build the systems back, and when we do so, we ought to build them back correcting for implicit bias and some of the systemic issues that lead to poor outcomes for people of color in our country,” she said.
Solutions proposed by Dr. Gee and others include more diversity in the workforce, more inclusion of patient advocates in maternal care, development of culturally appropriate literacy and numeracy communications, measurement by race (and action on the outcomes), standardization of care, and development of new ways to improve care access.
We will focus more specifically on these solutions in Part 2 of this article in our maternal mortality series. Previous articles in the series are available at mdedge.com/obgyn/maternal-mortality.
April 11-17 marked the third annual national Black Maternal Health Week, an event launched in 2017 by the Atlanta-based Black Mamas Matter Alliance (BMMA), in part to “deepen the national conversation about black maternal health.”
Around the same time, emerging data showing higher mortality rates among black patients versus patients of other races with COVID-19 opened similar dialogue fraught with questions about what might explain the disturbing health disparities.
“It’s kind of surprising to me that people are shocked by these [COVID-19] disparities,” Rebekah Gee, MD, an ob.gyn. who is director of the Louisiana State University Health System in New Orleans and a driving force behind initiatives addressing racial disparities in maternal health, said in an interview. If this is it, great – and certainly every moment is a moment for learning – but these COVID-19 disparities should not be surprising to people who have been looking at data.”
Veronica Gillispie, MD, an ob.gyn. and medical director of the Louisiana Perinatal Quality Collaborative and Pregnancy-Associated Mortality Review, was similarly baffled that the news was treated as a revelation.
That news includes outcomes data from New York showing that in March there were 92.3 and 74.3 deaths per 100,000 black and Hispanic COVID-19 patients, respectively, compared with 45.2 per 100,000 white patients.
“Now there’s a task force and all these initiatives to look at why this is happening, and I think those of us who work in maternal mortality are all saying, ‘We know why it’s happening,’ ” she said. “It’s the same thing we’ve been telling people why it’s been happening in maternal mortality.
“It’s implicit bias and structural racism.”
Facing hard numbers, harder conversations
The U.S. maternal mortality rate in 2018 was 17 per 100,000 live births – the highest of any similarly wealthy industrialized nation, the Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS) reported in January. That’s a striking statistic in its own right. Perhaps more striking is the breakdown by race.
Hispanic women had the lowest maternal mortality rate at 12 per 100,000 live births, followed by non-Hispanic white women at 15.
The rate for non-Hispanic black women was 37 per 100,000 live births.
Numerous factors contribute to these disparities. Among those listed by the American College of Obstetricians and Gynecologists’ chief executive officer Maureen G. Phipps, MD, in a press statement on the NCHS data, are care access issues, lack of standardization of care, bias, and racism. All of these must be addressed if the disparities in maternal and other areas of care are to be eliminated, according to Dr. Phipps.
“The NCHS data confirmed what we have known from other data sources: The rate of maternal deaths for non-Hispanic black women is substantially higher than the rates for non-Hispanic white women,” she wrote. “Continued efforts to improve the standardization of data and review processes related to U.S. maternal mortality are a necessary step to achieving the goal of eliminating disparities and preventable maternal mortality.”
However, such efforts frequently encounter roadblocks constructed by the reluctance among “many academics, policy makers, scientists, elected officials, journalists, and others responsible for defining and responding to the public discourse” to identify racism as a root cause of health disparities, according to Zinzi D. Bailey, ScD, former director of research and evaluation for the New York City Department of Health and Mental Hygiene, and colleagues.
In the third of a three-part conceptual report in The Lancet, entitled America: Equity and Equality in Health, Dr. Bailey and colleagues argued that advancing health equity requires a focus on structural racism – which they defined as “the totality of ways in which societies foster racial discrimination via mutually reinforcing inequitable systems (e.g., in housing, education, employment, earning, benefits, credit, media, healthcare, and criminal justice, etc.) that in turn reinforce discriminatory beliefs, values, and distribution of resources.”
In their series, the authors peeled back layer upon layer of sociological and political contributors to structural racism throughout history, revealing how each laid a foundation for health inequity over time. They particularly home in on health care quality and access.
“Interpersonal racism, bias, and discrimination in healthcare settings can directly affect health through poor health care,” they wrote, noting that “almost 15 years ago, the Institute of Medicine report entitled Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care, documented systematic and pervasive bias in the treatment of people of color resulting in substandard care.”
That report concluded that “bias, stereotyping, prejudice, and clinical uncertainty on the part of healthcare providers” likely play a role in the continuation of health disparities. More recent data – including the NCHS maternal mortality data – show an ongoing crisis.
A study of 210 experienced primary care providers and 190 community members in the Denver area, for example, found substantial evidence of implicit bias against both Latino and African American patients. The authors defined implicit bias as “unintentional and even unconscious” negative evaluation of one group and its members relative to another that is expressed implicitly, such as through negative nonverbal behavior.
“Activated by situational cues (e.g., a person’s skin color), implicit bias can quickly and unknowingly exert its influence on perception, memory, and behavior,” they wrote.
In their study, Implicit Association Test and self-report measures of bias showed similar rates of implicit bias among the providers and community members, with only a slight weakening of ethnic/racial bias among providers after adjustment for background characteristics, which suggests “a wider societal problem,” they said.
A specific example of how implicit bias can manifest was described in a 2016 report addressing the well-documented under-treatment of pain among black versus white patients. Kelly M. Hoffman, PhD, and colleagues demonstrated that a substantial number of individuals with at least some medical training endorse false beliefs regarding biological differences between black and white patients. For example, 25% of 28 white residents surveyed agreed black individuals have thicker skin, and 4% believed black individuals have faster blood coagulation and less sensitivity in their nerve endings.
Those who more strongly endorsed such erroneous beliefs were more likely to underestimate and undertreat pain among black patients, the authors found.
Another study, which underscored the insidiousness of structural racism, was reported in Science. The authors identified significant racial bias in an algorithm widely used by health systems, insurers, and practitioners to allocate health care resources for patients with complex health needs. The algorithm, which affects millions of patients, uses predictions of future health care costs rather than future illness to determine who should receive extra medical care.
The problem is that unequal care access for black patients skews lower the foundational cost data used for making those predictions. Correcting the algorithm would increase the percentage of black patients receiving additional medical help from 17.7% to 46.5%, the authors concluded.
This evidence of persistent racism and bias in medicine, however, doesn’t mean progress is lacking.
ACOG has partnered with numerous other organizations to promote awareness and change, including through legislation. A recent win was the enactment of the Preventing Maternal Deaths Act of 2018, a bipartisan bill designed to promote and support maternal mortality review committees in every state. A major focus of BMMA’s Black Maternal Health Week was the Black Maternal Health Momnibus Act of 2020, a nine-bill package introduced in March to comprehensively address the crisis.
But efforts like these, whether they aim to elucidate the contributors to health disparities or to directly target structural and overt racism and root out implicit bias in medical care, are nothing new. As Dr. Bailey and colleagues noted, a challenge is getting the message across because efforts to avoid tough conversations around these topics are nothing new, either.
Dr. Gee attested to that during a maternal mortality panel discussion at the 2019 ACOG meeting where she spoke about the resistance she encountered in 2016 when she was appointed secretary of the Louisiana Health Department and worked to make racism and bias a foundational part of the discussion on improving maternal and fetal outcomes.
She established the first Office of Health Equity in the state – and the first in the nation to not only require measurement outcomes by race but also explicitly address racial bias at the outset. The goal wasn’t just to talk about it but to “plan for addressing equity in every single aspect of what we do with ... our case equity action teams.”
“At our first maternal mortality quality meetings we insisted on focusing on equity at the very outset, and we had people that left when we started talking about racism,” she said, noting that others said it was “too political” to discuss during an election year or that equity was something to address later.
“We said no. We insisted on it, and I think that was very important because all ships don’t rise with the tide, not with health disparities,” she said, recounting an earlier experience when she led the Louisiana Birth Outcomes Initiative: “I asked to have a brown-bag focused on racism at the department so we could talk about the impact of implicit bias on decision making, and I was told that I was a Yankee who didn’t understand the South and that racism didn’t really exist here, and what did I know about it – and I couldn’t have the brown-bag.”
That was in 2011.
Fast-forward to April 24, 2020. As Dr. Gee shared her perspective on addressing racism and bias in medicine, she was preparing for a call regarding the racial disparities in COVID-19 outcomes – the first health equity action sanctioned by Louisiana Governor John Bel Edwards (D).
“I think we really set the stage for these discussions,” she said.
Addressing equity to enact change
The efforts in Louisiana also set the stage for better maternal outcomes. At the 2019 ACOG meeting where she spoke as part of the President’s Panel, Dr. Gee said Louisiana had the highest maternal mortality rates in the nation. The NCHC data released in January, however, suggest that may no longer be the case.
Inconsistencies in how the latest and prior data were reported, including in how maternal mortality was defined, make direct comparison impossible. But in the latest report, Louisiana ranked seventh among states with available data.
“Ninety percent of the deliveries in the state happen at hospitals that we worked with,” Dr. Gee said, highlighting the reach of the efforts to improve outcomes there.
She also described a recent case involving an anemic patient whose bleeding risk was identified early thanks to the programs put in place. That enabled early preparation in the event of complications.
The patient experienced a massive hemorrhage, but the preparation, including having units of blood on hand in case of such an emergency, saved her life.
“So we clearly have not just data, but individual stories of people whose lives have been saved by this work,” she said.
More tangible data on maternal morbidity further show that the efforts in the state are making a difference, Dr. Gillispie said, citing preliminary outcomes data from the Pregnancy-Associated Mortality Review launched in 2018.
“We started with an initial goal of reducing severe maternal morbidity related to hypertension and hemorrhage by 20%, as well as reducing the black/white disparity gap by Mother’s Day 2020,” she said.
Final analyses have been delayed because of COVID-19, but early assessments showed a reduction in the disparity gap, she said, again highlighting the importance of focusing on equity.
“Definitely from the standpoint of the Quality Collaborative side ... we’ve been working with our facilities to make them aware of what implicit bias is, helping them to also do the Harvard Implicit Bias Test so they can figure out what their own biases are, start working to acknowledge them and address them, and start working to fight against letting that bias change how they treat individuals,” Dr. Gillispie said.
The work started through these initiatives will continue because there is much left to be done, she said.
Indeed, the surprised reactions in recent weeks to the reports of disparities in COVID-19 outcomes further underscore that reality, and the maternal mortality statistics – with use of the voices of those directly affected by structural and overt racism and bias in maternal care as a megaphone – speak for themselves.
Hearing implicit bias from patients’ perspective
Just ask Timoria McQueen Saba, a black woman who nearly died from a postpartum hemorrhage in 2010. At ACOG 2019, she spoke about how she had to switch ob.gyns. three times during her first pregnancy because she felt she had not received quality care – one doctor neglected to tell her she had placenta previa. She also experienced excessive wait times at prenatal appointments and had been on the receiving end of microaggressions and degrading questions such as “Are you still married?” and “Is your husband your baby’s father?” – and these are all things her white friends who recommended those physicians never experienced, she said.
“The health care system has just sometimes beaten people down so much, just like the world has – people of color, especially – to where you’re dismissed, your concerns are invalidated,” she said. “Some doctors don’t even think black people feel pain [or that] our pain is less.”
Mrs. Saba also spoke about how her health care “improved a billion percent” when her white husband accompanied her to appointments.
Just ask Charles S. Johnson IV, whose wife Kira Dixon Johnson died in 2016 during surgery for postpartum bleeding complications – after he and other family members spent 10 hours pleading for help for her.
Speaking at the ACOG panel discussion with Mrs. Saba, Mr. Johnson described “a clear disconnect” between the medical staff at the hospital and the way they viewed and valued Kira. He shared his frustration in wanting to advocate for his wife, but knowing that, as an African American male, he risked being seen as a threat and removed from the hospital if he didn’t stay calm, if he “tapped into those natural instincts as a man and a husband who wants to just protect his family.”
He fought back emotions, struggling to get the words out, saying that’s what haunts him and keeps him up at night – wondering if he should have “fought harder, grabbed the doctor by the collar, raised his voice, slammed on the counter.
“Maybe they would have done something,” he said.
Such experiences cross all socioeconomic boundaries. Ask U.S. Track and Field Olympic gold medalist Allyson Felix, who testified at a U.S. House Ways and Means Committee hearing on May 16, 2019 after developing severe preeclampsia that threatened her life and that of her baby. Ask tennis champion Serena Williams, who demanded assessment for pulmonary embolism following the birth of her child; she knew the signs, but her health care providers initially dismissed her concerns.
Their experiences aren’t just anecdotal. Data consistently show how racism and bias affect patient treatment and outcomes. Dr. Gee, for example, shared findings from a retrospective assessment of 47 confirmed pregnancy-related deaths in Louisiana between 2011 and 2016 that looked specifically at whether the deaths could potentially have been prevented if blood was given sooner, cardiomyopathy was recognized sooner, hypertension was treated on time, or other changes were made to care.
The answer was “Yes” in 9% of cases involving white patients – and in 59% of cases involving black patients (odds ratio, 14.6).
The study, reported in February in Obstetrics & Gynecology, showed that 27 of the deaths (58%) occurred at level III or IV birth facilities and that those deaths were not less likely than those at level I or II facilities to be categorized as preventable (OR, 2.0).
Findings from the Giving Voice to Mothers study, published in Reproductive Health in 2019, showed how mistreatment during childbirth might contribute to such outcomes.
In an online cross-sectional survey of more than 2,100 U.S. women, one in six reported at least one type of mistreatment, such as loss of autonomy, being yelled at or threatened, being ignored or having requests for help ignored, Saraswathi Vedam, SciD, of the Birth Place Lab at the University of British Columbia, Vancouver, and colleagues reported.
Race was among the factors associated with likelihood of mistreatment, and the rates of mistreatment for women of color were consistently higher – even when looking at interactions between race and other characteristics, such as socioeconomic status (SES). For example, 27.2% of women of color with low SES, compared with 18.7% of white women with low SES, reported mistreatment. Having a partner who was black, regardless of maternal race, was also associated with an increased rate of mistreatment, the authors found.
“I often get the question, ‘Do you think Kira would be alive if she was white,’ ” Mr. Johnson said. “The first way I respond to that question is [by saying that] the simple fact that you have to ask me is a problem.
“When this first happened, I was in so much pain that I couldn’t process the fact that something so egregious and outrageous happened to my wife because of the color of her skin, but as I began to process and really think about it and unpack this scenario, I have to be really frank ... do I think that she would have sat there for 10 hours while we begged and pleaded? Absolutely not.”
He stressed that his words aren’t “an indictment of the profession.”
“This is not an indictment saying that all people are racist or prejudiced,” he said. “But here’s the reality: If you are in this profession, if you are responsible for the well-being of patients and their families, and you are not able to see them in the same way that you see your mother, your wife, your sister, you have two options – you need to find something else to do or you need to take steps to get better.”
Fixing systems, finding solutions
Dr. Gee acknowledged the work that physicians need to do to help improve outcomes.
“The average time we give a patient to talk is 11 seconds before we interrupt them,” she said, as one example. “We have to recognize that.”
But efforts to improve outcomes shouldn’t just focus on changing physician behavior, she said.
“We really need to focus, as has the U.K. – very effectively – on using midwives, doulas, other health care professionals as complements to physicians to make sure that we have women-centered birth experiences.
“So, instead of just blaming the doctors, I think we need to change the system,” Dr. Gee emphasized.
The disruptions in health systems caused by COVID-19 present a unique opportunity to do that, she said. There is now an opportunity to build them back.
“We have a chance to build the systems back, and when we do so, we ought to build them back correcting for implicit bias and some of the systemic issues that lead to poor outcomes for people of color in our country,” she said.
Solutions proposed by Dr. Gee and others include more diversity in the workforce, more inclusion of patient advocates in maternal care, development of culturally appropriate literacy and numeracy communications, measurement by race (and action on the outcomes), standardization of care, and development of new ways to improve care access.
We will focus more specifically on these solutions in Part 2 of this article in our maternal mortality series. Previous articles in the series are available at mdedge.com/obgyn/maternal-mortality.
Primary care physicians reshuffle their work, lives in a pandemic
During his shift at a COVID-19 drive-through triage screening area set up outside the University of Arkansas for Medical Sciences in Little Rock, Robert Hopkins Jr., MD, noticed a woman bowled over in the front seat of her car.
A nurse practitioner had just informed her that she had met the criteria for undergoing testing for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
“She was very upset and was crying nearly inconsolably,” said Dr. Hopkins, who directs the division of general internal medicine at the University of Arkansas Medical Sciences College of Medicine. “I went over and visited with her for a few minutes. She was scared to death that we [had] told her she was going to die. In her mind, if she had COVID-19 that meant a death sentence, and if we were testing her that meant she was likely to not survive.”
Dr. Hopkins tried his best to put testing in perspective for the woman. “At least she came to a level of comfort and realized that we were doing this for her, that this was not a death sentence, that this was not her fault,” he said. “She was worried about infecting her kids and her grandkids and ending up in the hospital and being a burden. Being able to spend that few minutes with her and help to bring down her level of anxiety – I think that’s where we need to put our efforts as physicians right now, helping people understand, ‘Yes, this is serious. Yes, we need to continue to social distance. Yes, we need to be cautious. But, we will get through this if we all work together to do so.’ ”
Prior to the COVID-19 pandemic, Dr. Hopkins spent part of his time seeing patients in the university’s main hospital, but most of it in an outpatient clinic where he and about 20 other primary care physicians care for patients and precept medical residents. Now, medical residents have been deployed to other services, primarily in the hospital, and he and his physician colleagues are conducting 80%-90% of patient visits by video conferencing or by telephone. It’s a whole new world.
“We’ve gone from a relatively traditional inpatient/outpatient practice where we’re seeing patients face to face to doing some face-to-face visits, but an awful lot of what we do now is in the technology domain,” said Dr. Hopkins, who also assisted with health care relief efforts during hurricanes Rita and Katrina.
“A group of six of us has been redeployed to assist with the surge unit for the inpatient facility, so our outpatient duties are being taken on by some of our partners.”
He also pitches in at the drive-through COVID-19 screening clinic, which was set up on March 27 and operates between 8 a.m. and 8 p.m., 7 days a week. “We’re able to measure people’s temperature, take a quick screening history, decide whether their risk is such that we need to do a COVID-19 PCR [polymerase chain reaction] test,” he said. “Then we make a determination of whether they need to go home on quarantine awaiting those results, or if they don’t have anything that needs to be evaluated, or whether they need to be triaged to an urgent care setting or to the emergency department.”
To minimize his risk of acquiring COVID-19, he follows personal hygiene practices recommended by the Centers for Disease Control and Prevention. He also places his work shoes in a shoebox, which he keeps in his car. “I put them on when I get to the parking deck at work, do my work, and then I put them in the shoebox, slip on another pair of shoes and drive home so I’m not tracking in things I potentially had on me,” said Dr. Hopkins, who is married and a father to two college-aged sons and a daughter in fourth grade. “When I get home I immediately shower, and then I exercise or have dinner with my family.”
Despite the longer-than-usual work hours and upheaval to the traditional medical practice model brought on by the pandemic, Dr. Hopkins, a self-described “glass half full person,” said that he does his best to keep watch over his patients and colleagues. “I’m trying to keep an eye out on my team members – physicians, nurses, medical assistants, and folks at the front desk – trying to make sure that people are getting rest, trying to make sure that people are not overcommitting,” he said. “Because if we’re not all working together and working for the long term, we’re going to be in trouble. This is not going to be a sprint; this is going to be a marathon for us to get through.”
To keep mentally centered, he engages in at least 40 minutes of exercise each day on his bicycle or on the elliptical machine at home. Dr. Hopkins hopes that the current efforts to redeploy resources, expand clinician skill sets, and forge relationships with colleagues in other disciplines will carry over into the delivery of health care when COVID-19 is a distant memory. “I hope that some of those relationships are going to continue and result in better care for all of our patients,” he said.
"We are in dire need of hugs"
Typically, Dr. Dakkak, a family physician at Boston University, practices a mix of clinic-based family medicine and obstetrics, and works in inpatient medicine 6 weeks a year. Currently, she is leading a COVID-19 team full time at Boston Medical Center, a 300-bed safety-net hospital located on the campus of Boston University Medical Center.
COVID-19 has also shaken up her life at home.
When Dr. Dakkak volunteered to take on her new role, the first thing that came to her mind was how making the switch would affect the well-being of her 8-year-old son and 10-year-old daughter.
“I thought, ‘How do I get my children somewhere where I don’t have to worry about them?’ ” Dr. Dakkak said.
She floated the idea with her husband of flying their children out to stay with her recently retired parents, who live outside of Sacramento, Calif., until the pandemic eases up. “I was thinking to myself, ‘Am I overreacting? Is the pandemic not going to be that bad?’ because the rest of the country seemed to be in some amount of denial,” she said. “So, I called my dad, who’s a retired pediatric anesthesiologist. He’s from Egypt so he’s done crisis medicine in his time. He encouraged me to send the kids.”
On the same day that Dr. Dakkak began her first 12-hour COVID-19 shift at the hospital, her husband and children boarded a plane to California, where the kids remain in the care of her parents. Her husband returned after staying there for 2 weeks. “Every day when I’m working, I validate my decision,” she said. “When I first started, I worked 5 nights in a row, had 2 days off, and then worked 6 nights in a row. I was busy so I didn’t think about [being away from my kids], but at the same time I was grateful that I didn’t have to come home and worry about homeschooling the kids or infecting them.”
She checks in with them as she can via cell phone or FaceTime. “My son has been very honest,” Dr. Dakkak said. “He says, ‘FaceTime makes me miss you more, and I don’t like it,’ which I understand. I’ll call my mom, and if they want to talk to me, they’ll talk to me. If they don’t want to talk to me, I’m okay. This is about them being healthy and safe. I sent them a care package a few days ago with cards and some workbooks. I’m optimistic that in June I can at least see them if not bring them home.”
Dr. Dakkak describes leading a COVID-19 team as a grueling experience that challenges her medical know-how nearly every day, with seemingly ever-changing algorithms. “Our knowledge of this disease is five steps behind, and changing at lightning speed,” said Dr. Dakkak, who completed a fellowship in surgical and high-risk obstetrics. “It’s hard to balance continuing to teach evidence-based medicine for everything else in medicine [with continuing] to practice minimal and ever-changing evidence-based COVID medicine. We just don’t know enough [about the virus] yet. This is nothing like we were taught in medical school. Everyone has elevated d-dimers with COVID-19, and we don’t get CT pulmonary angiograms [CTPAs] on all of them; we wouldn’t physically be able to. Some patients have d-dimers in the thousands, and only some are stable to get CTPAs. We are also finding pulmonary embolisms. Now we’re basing our algorithm on anticoagulation due to d-dimers because sometimes you can’t always do a CTPA even if you want to. On the other hand, we have people who are coming into the hospital too late. We’ve had a few who have come in after having days of stroke symptoms. I worry about our patients at home who hesitate to come in when they really should.”
Sometimes she feels sad for the medical residents on her team because their instinct is to go in and check on each patient, “but I don’t want them to get exposed,” she said. “So, we check in by phone, or if they need a physical check-in, we minimize the check-ins; only one of us goes in. I’m more willing to put myself in the room than to put them in the room. I also feel for them because they came into medicine for the humanity of medicine – not the charting or the ordering of medicine. I also worry about the acuity and sadness they’re seeing. This is a rough introduction to medicine for them.”
When interviewed for this story in late April, Dr. Dakkak had kept track of her intubated COVID-19 patients. “Most of my patients get to go home without having been intubated, but those aren’t the ones I worry about,” she said. “I have two patients I have been watching. One of them has just been extubated and I’m still worried about him, but I’m hoping he’s going to be fine. The other one is the first pregnant woman we intubated. She is now extubated, doing really well, and went home. Her fetus is doing well, never had any issues while she was intubated. Those cases make me happy. They were both under the age of 35. It is nice to follow those intubations and find that the majority are doing okay.”
The first patient she had cared for who died was a young man “who was always in good spirits,” she recalled. “We called his brother right before intubating him. After intubation, his oxygen saturation didn’t jump up, which made me worry a bit.” About a week later, the young man died. “I kept thinking, ‘We intubated him when he was still comfortable talking. Should I have put it off and had him call more people to say goodbye? Should I have known that he wasn’t going to wake up?’ ” said Dr. Dakkak, who is also women’s health director at Manet Community Health Centers. “A lot of us have worked on our end-of-life discussions in the past month, just being able to tell somebody, ‘This might be your last time to call family. Call family and talk to whoever you want.’ Guilt isn’t the right word, but it’s unsettling if I’m the last person a patient talks to. I feel that, if that’s the case, then I didn’t do a good enough job trying to get them to their family or friends. If I am worried about a patient’s clinical status declining, I tell families now, when I call them, ‘I hope I’m wrong; I hope they don’t need to be intubated, but I think this is the time to talk.’ ”
To keep herself grounded during off hours, Dr. Dakkak spends time resting, checking in with her family, journaling “to get a lot of feelings out,” gardening, hiking, and joining Zoom chats with friends. Once recentered, she draws from a sense of obligation to others as she prepares for her next shift caring for COVID-19 patients.
“I have a lot of love for the world that I get to expend by doing this hard work,” she said. “I love humanity and I love humanity in times of crisis. The interactions I have with patients and their families are still central to why I do this work. I love my medical teams, and I would never want to let them down. It is nice to feel the sense of teamwork across the hospital. The nurses that I sit with and experience this with are amazing. I keep saying that the only thing I want to do when this pandemic is over is hug everyone. I think we are in dire need of hugs.”
Finding light in the darkness
Internist Katie Jobbins, DO, also has worked in a professional role that was created because of COVID-19.
Shortly before Dr. Jobbins was deployed to Baystate Medical Center in Springfield, Ma., for 2 weeks in April of 2020 to help clinicians with an anticipated surge of COVID-19 cases, she encountered a patient who walked into Baystate’s High Street Health Center.
“I think I have COVID-19,” the patient proclaimed to her, at the outpatient clinic that serves mostly inner-city, Medicaid patients.
Prior to becoming an ambulatory internist, Dr. Jobbins was a surgical resident. “So I went into that mode of ‘I need to do this, this, and this,’ ” she said. “I went through a checklist in my head to make sure I was prepared to take care of the patient.”
She applied that same systems approach during her redeployment assignment in the tertiary care hospital, which typically involved 10-hour shifts overseeing internal medicine residents in a medical telemetry unit. “We would take care of people under investigation for COVID-19, but we were not assigned to the actual COVID unit,” said Dr. Jobbins, who is also associate program director for the internal medicine residency program at the University of Massachusetts Medical School–Baystate Springfield. “They tried to redeploy other people to those units who had special training, and we were trying to back fill into where those people that got moved to the COVID units or the ICU units were actually working. We were taking more of the medical side of the floors.”
Even so, one patient on the unit was suspected of having COVID-19, so Dr. Jobbins suited up with personal protective equipment and conducted a thorough exam with residents waiting outside the patient’s room, a safe distance away. “I explained everything I found on the exam to the residents, trying to give them some educational benefit, even though they couldn’t physically examine the patient because we’re trying to protect them since they’re in training,” she said. “It was anxiety provoking, on some level, knowing that there’s a potential risk of exposure [to the virus], but knowing that Baystate Health has gone to extraordinary measures to make sure we have the correct PPE and support us is reassuring. I knew I had the right equipment and the right tools to take care of the patient, which calmed my nerves and made me feel like I could do the job. That’s the most important thing as a physician during this time: knowing that you have people supporting you who have your back at all times.”
Like Dr. Dakkak, Dr. Jobbins had to make some adjustments to her interaction with her family.
Before she began the deployment, Dr. Jobbins engaged in a frank discussion with her husband and her two young boys about the risks she faced working in a hospital caring for patients with COVID-19. “My husband and I made sure our wills were up to date, and we talked about what we would do if either of us got the virus,” she said. To minimize the potential risk of transmitting the virus to her loved ones during the two-week deployment, she considered living away from her family in a nearby home owned by her father, but decided against that and to “take it day by day.” Following her hospital shifts, Dr. Jobbins changed into a fresh set of clothes before leaving the hospital. Once she arrived home, she showered to reduce the risk of possibly becoming a vector to her family.
She had to tell her kids: “You can’t kiss me right now.”
“As much as it’s hard for them to understand, we had a conversation [in which I explained] ‘This is a virus. It will go away eventually, but it’s a virus we’re fighting.’ It’s interesting to watch a 3-year-old try to process that and take his play samurai sword or Marvel toys and decide he’s going to run around the neighborhood and try to kill the virus.”
At the High Street Health Center, Dr. Jobbins and her colleagues have transitioned to conducting most patient encounters via telephone or video appointments. “We have tried to maintain as much continuity for our patients to address their chronic medical needs through these visits, such as hypertension management and diabetes care,” she said. “We have begun a rigorous screening process to triage and treat patients suspicious for COVID-19 through telehealth in hopes of keeping them safe and in their own homes. We also continue to see patients for nonrespiratory urgent care needs in person once they have screened negative for COVID-19.”
“In terms of the inpatient setting, we’ve noticed that a lot of people are choosing not to go to the hospital now, unless they’re extremely ill,” Dr. Jobbins noted. “We’re going to need to find a balance with when do people truly need to go to the hospital and when do they not? What can we manage as an outpatient versus having someone go to the emergency department? That’s really the role of the primary care physician. We need to help people understand, ‘You don’t need to go to the ED for everything, but here are the things you really need to go for.’ ”
“It will be interesting to see what health care looks like in 6 months or a year. I’m excited to see where we land,” Dr. Jobbins added.
Hopes for the Future of Telemedicine
When the practice of medicine enters a post–COVID-19 era, Dr. Jobbins hopes that telemedicine will be incorporated more into the delivery of patient care. “I’ve found that many of my patients who often are no-shows to the inpatient version of their visits have had a higher success rate of follow-through when we do the telephone visits,” she said. “It’s been very successful. I hope that the insurance companies and [Centers for Medicare & Medicaid] will continue to reimburse this as they see this is a benefit to our patients.
Dr. Hopkins is also hopeful that physicians will be able to successfully see patients via telemedicine in the postpandemic world.
“For the ups and downs we’ve had with telemedicine, I’d love for us to be able to enhance the positives and incorporate that into our practice going forward. If we can reach our patients and help treat them where they are, rather than them having to come to us, that may be a plus,” he said.
In the meantime, Dr. Jobbins presses on as the curve of COVID-19 cases flattens in Western Massachusetts and remains grateful that she chose to practice medicine.
“The commitment I have to being an educator in addition to being a physician is part of why I keep doing this,” Dr. Jobbins said. “I find this to be one of the most fulfilling jobs and careers you could ever have: being there for people when they need you the most. That’s really what a physician’s job is: being there for people when a family member has passed away or when they just need to talk because they’re having anxiety. At the end of the day, if we can impart that to those we work with and bring in a positive attitude, it’s infectious and it makes people see this is a reason we keep doing what we’re doing.”
She’s also been heartened by the kindness of strangers during this pandemic, from those who made and donated face shields when they were in short supply, to those who delivered food to the hospital as a gesture of thanks.
“I had a patient who made homemade masks and sent them to my office,” she said. “There’s obviously good and bad during this time, but I get hope from seeing all of the good things that are coming out of this, the whole idea of finding the light in the darkness.”
During his shift at a COVID-19 drive-through triage screening area set up outside the University of Arkansas for Medical Sciences in Little Rock, Robert Hopkins Jr., MD, noticed a woman bowled over in the front seat of her car.
A nurse practitioner had just informed her that she had met the criteria for undergoing testing for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
“She was very upset and was crying nearly inconsolably,” said Dr. Hopkins, who directs the division of general internal medicine at the University of Arkansas Medical Sciences College of Medicine. “I went over and visited with her for a few minutes. She was scared to death that we [had] told her she was going to die. In her mind, if she had COVID-19 that meant a death sentence, and if we were testing her that meant she was likely to not survive.”
Dr. Hopkins tried his best to put testing in perspective for the woman. “At least she came to a level of comfort and realized that we were doing this for her, that this was not a death sentence, that this was not her fault,” he said. “She was worried about infecting her kids and her grandkids and ending up in the hospital and being a burden. Being able to spend that few minutes with her and help to bring down her level of anxiety – I think that’s where we need to put our efforts as physicians right now, helping people understand, ‘Yes, this is serious. Yes, we need to continue to social distance. Yes, we need to be cautious. But, we will get through this if we all work together to do so.’ ”
Prior to the COVID-19 pandemic, Dr. Hopkins spent part of his time seeing patients in the university’s main hospital, but most of it in an outpatient clinic where he and about 20 other primary care physicians care for patients and precept medical residents. Now, medical residents have been deployed to other services, primarily in the hospital, and he and his physician colleagues are conducting 80%-90% of patient visits by video conferencing or by telephone. It’s a whole new world.
“We’ve gone from a relatively traditional inpatient/outpatient practice where we’re seeing patients face to face to doing some face-to-face visits, but an awful lot of what we do now is in the technology domain,” said Dr. Hopkins, who also assisted with health care relief efforts during hurricanes Rita and Katrina.
“A group of six of us has been redeployed to assist with the surge unit for the inpatient facility, so our outpatient duties are being taken on by some of our partners.”
He also pitches in at the drive-through COVID-19 screening clinic, which was set up on March 27 and operates between 8 a.m. and 8 p.m., 7 days a week. “We’re able to measure people’s temperature, take a quick screening history, decide whether their risk is such that we need to do a COVID-19 PCR [polymerase chain reaction] test,” he said. “Then we make a determination of whether they need to go home on quarantine awaiting those results, or if they don’t have anything that needs to be evaluated, or whether they need to be triaged to an urgent care setting or to the emergency department.”
To minimize his risk of acquiring COVID-19, he follows personal hygiene practices recommended by the Centers for Disease Control and Prevention. He also places his work shoes in a shoebox, which he keeps in his car. “I put them on when I get to the parking deck at work, do my work, and then I put them in the shoebox, slip on another pair of shoes and drive home so I’m not tracking in things I potentially had on me,” said Dr. Hopkins, who is married and a father to two college-aged sons and a daughter in fourth grade. “When I get home I immediately shower, and then I exercise or have dinner with my family.”
Despite the longer-than-usual work hours and upheaval to the traditional medical practice model brought on by the pandemic, Dr. Hopkins, a self-described “glass half full person,” said that he does his best to keep watch over his patients and colleagues. “I’m trying to keep an eye out on my team members – physicians, nurses, medical assistants, and folks at the front desk – trying to make sure that people are getting rest, trying to make sure that people are not overcommitting,” he said. “Because if we’re not all working together and working for the long term, we’re going to be in trouble. This is not going to be a sprint; this is going to be a marathon for us to get through.”
To keep mentally centered, he engages in at least 40 minutes of exercise each day on his bicycle or on the elliptical machine at home. Dr. Hopkins hopes that the current efforts to redeploy resources, expand clinician skill sets, and forge relationships with colleagues in other disciplines will carry over into the delivery of health care when COVID-19 is a distant memory. “I hope that some of those relationships are going to continue and result in better care for all of our patients,” he said.
"We are in dire need of hugs"
Typically, Dr. Dakkak, a family physician at Boston University, practices a mix of clinic-based family medicine and obstetrics, and works in inpatient medicine 6 weeks a year. Currently, she is leading a COVID-19 team full time at Boston Medical Center, a 300-bed safety-net hospital located on the campus of Boston University Medical Center.
COVID-19 has also shaken up her life at home.
When Dr. Dakkak volunteered to take on her new role, the first thing that came to her mind was how making the switch would affect the well-being of her 8-year-old son and 10-year-old daughter.
“I thought, ‘How do I get my children somewhere where I don’t have to worry about them?’ ” Dr. Dakkak said.
She floated the idea with her husband of flying their children out to stay with her recently retired parents, who live outside of Sacramento, Calif., until the pandemic eases up. “I was thinking to myself, ‘Am I overreacting? Is the pandemic not going to be that bad?’ because the rest of the country seemed to be in some amount of denial,” she said. “So, I called my dad, who’s a retired pediatric anesthesiologist. He’s from Egypt so he’s done crisis medicine in his time. He encouraged me to send the kids.”
On the same day that Dr. Dakkak began her first 12-hour COVID-19 shift at the hospital, her husband and children boarded a plane to California, where the kids remain in the care of her parents. Her husband returned after staying there for 2 weeks. “Every day when I’m working, I validate my decision,” she said. “When I first started, I worked 5 nights in a row, had 2 days off, and then worked 6 nights in a row. I was busy so I didn’t think about [being away from my kids], but at the same time I was grateful that I didn’t have to come home and worry about homeschooling the kids or infecting them.”
She checks in with them as she can via cell phone or FaceTime. “My son has been very honest,” Dr. Dakkak said. “He says, ‘FaceTime makes me miss you more, and I don’t like it,’ which I understand. I’ll call my mom, and if they want to talk to me, they’ll talk to me. If they don’t want to talk to me, I’m okay. This is about them being healthy and safe. I sent them a care package a few days ago with cards and some workbooks. I’m optimistic that in June I can at least see them if not bring them home.”
Dr. Dakkak describes leading a COVID-19 team as a grueling experience that challenges her medical know-how nearly every day, with seemingly ever-changing algorithms. “Our knowledge of this disease is five steps behind, and changing at lightning speed,” said Dr. Dakkak, who completed a fellowship in surgical and high-risk obstetrics. “It’s hard to balance continuing to teach evidence-based medicine for everything else in medicine [with continuing] to practice minimal and ever-changing evidence-based COVID medicine. We just don’t know enough [about the virus] yet. This is nothing like we were taught in medical school. Everyone has elevated d-dimers with COVID-19, and we don’t get CT pulmonary angiograms [CTPAs] on all of them; we wouldn’t physically be able to. Some patients have d-dimers in the thousands, and only some are stable to get CTPAs. We are also finding pulmonary embolisms. Now we’re basing our algorithm on anticoagulation due to d-dimers because sometimes you can’t always do a CTPA even if you want to. On the other hand, we have people who are coming into the hospital too late. We’ve had a few who have come in after having days of stroke symptoms. I worry about our patients at home who hesitate to come in when they really should.”
Sometimes she feels sad for the medical residents on her team because their instinct is to go in and check on each patient, “but I don’t want them to get exposed,” she said. “So, we check in by phone, or if they need a physical check-in, we minimize the check-ins; only one of us goes in. I’m more willing to put myself in the room than to put them in the room. I also feel for them because they came into medicine for the humanity of medicine – not the charting or the ordering of medicine. I also worry about the acuity and sadness they’re seeing. This is a rough introduction to medicine for them.”
When interviewed for this story in late April, Dr. Dakkak had kept track of her intubated COVID-19 patients. “Most of my patients get to go home without having been intubated, but those aren’t the ones I worry about,” she said. “I have two patients I have been watching. One of them has just been extubated and I’m still worried about him, but I’m hoping he’s going to be fine. The other one is the first pregnant woman we intubated. She is now extubated, doing really well, and went home. Her fetus is doing well, never had any issues while she was intubated. Those cases make me happy. They were both under the age of 35. It is nice to follow those intubations and find that the majority are doing okay.”
The first patient she had cared for who died was a young man “who was always in good spirits,” she recalled. “We called his brother right before intubating him. After intubation, his oxygen saturation didn’t jump up, which made me worry a bit.” About a week later, the young man died. “I kept thinking, ‘We intubated him when he was still comfortable talking. Should I have put it off and had him call more people to say goodbye? Should I have known that he wasn’t going to wake up?’ ” said Dr. Dakkak, who is also women’s health director at Manet Community Health Centers. “A lot of us have worked on our end-of-life discussions in the past month, just being able to tell somebody, ‘This might be your last time to call family. Call family and talk to whoever you want.’ Guilt isn’t the right word, but it’s unsettling if I’m the last person a patient talks to. I feel that, if that’s the case, then I didn’t do a good enough job trying to get them to their family or friends. If I am worried about a patient’s clinical status declining, I tell families now, when I call them, ‘I hope I’m wrong; I hope they don’t need to be intubated, but I think this is the time to talk.’ ”
To keep herself grounded during off hours, Dr. Dakkak spends time resting, checking in with her family, journaling “to get a lot of feelings out,” gardening, hiking, and joining Zoom chats with friends. Once recentered, she draws from a sense of obligation to others as she prepares for her next shift caring for COVID-19 patients.
“I have a lot of love for the world that I get to expend by doing this hard work,” she said. “I love humanity and I love humanity in times of crisis. The interactions I have with patients and their families are still central to why I do this work. I love my medical teams, and I would never want to let them down. It is nice to feel the sense of teamwork across the hospital. The nurses that I sit with and experience this with are amazing. I keep saying that the only thing I want to do when this pandemic is over is hug everyone. I think we are in dire need of hugs.”
Finding light in the darkness
Internist Katie Jobbins, DO, also has worked in a professional role that was created because of COVID-19.
Shortly before Dr. Jobbins was deployed to Baystate Medical Center in Springfield, Ma., for 2 weeks in April of 2020 to help clinicians with an anticipated surge of COVID-19 cases, she encountered a patient who walked into Baystate’s High Street Health Center.
“I think I have COVID-19,” the patient proclaimed to her, at the outpatient clinic that serves mostly inner-city, Medicaid patients.
Prior to becoming an ambulatory internist, Dr. Jobbins was a surgical resident. “So I went into that mode of ‘I need to do this, this, and this,’ ” she said. “I went through a checklist in my head to make sure I was prepared to take care of the patient.”
She applied that same systems approach during her redeployment assignment in the tertiary care hospital, which typically involved 10-hour shifts overseeing internal medicine residents in a medical telemetry unit. “We would take care of people under investigation for COVID-19, but we were not assigned to the actual COVID unit,” said Dr. Jobbins, who is also associate program director for the internal medicine residency program at the University of Massachusetts Medical School–Baystate Springfield. “They tried to redeploy other people to those units who had special training, and we were trying to back fill into where those people that got moved to the COVID units or the ICU units were actually working. We were taking more of the medical side of the floors.”
Even so, one patient on the unit was suspected of having COVID-19, so Dr. Jobbins suited up with personal protective equipment and conducted a thorough exam with residents waiting outside the patient’s room, a safe distance away. “I explained everything I found on the exam to the residents, trying to give them some educational benefit, even though they couldn’t physically examine the patient because we’re trying to protect them since they’re in training,” she said. “It was anxiety provoking, on some level, knowing that there’s a potential risk of exposure [to the virus], but knowing that Baystate Health has gone to extraordinary measures to make sure we have the correct PPE and support us is reassuring. I knew I had the right equipment and the right tools to take care of the patient, which calmed my nerves and made me feel like I could do the job. That’s the most important thing as a physician during this time: knowing that you have people supporting you who have your back at all times.”
Like Dr. Dakkak, Dr. Jobbins had to make some adjustments to her interaction with her family.
Before she began the deployment, Dr. Jobbins engaged in a frank discussion with her husband and her two young boys about the risks she faced working in a hospital caring for patients with COVID-19. “My husband and I made sure our wills were up to date, and we talked about what we would do if either of us got the virus,” she said. To minimize the potential risk of transmitting the virus to her loved ones during the two-week deployment, she considered living away from her family in a nearby home owned by her father, but decided against that and to “take it day by day.” Following her hospital shifts, Dr. Jobbins changed into a fresh set of clothes before leaving the hospital. Once she arrived home, she showered to reduce the risk of possibly becoming a vector to her family.
She had to tell her kids: “You can’t kiss me right now.”
“As much as it’s hard for them to understand, we had a conversation [in which I explained] ‘This is a virus. It will go away eventually, but it’s a virus we’re fighting.’ It’s interesting to watch a 3-year-old try to process that and take his play samurai sword or Marvel toys and decide he’s going to run around the neighborhood and try to kill the virus.”
At the High Street Health Center, Dr. Jobbins and her colleagues have transitioned to conducting most patient encounters via telephone or video appointments. “We have tried to maintain as much continuity for our patients to address their chronic medical needs through these visits, such as hypertension management and diabetes care,” she said. “We have begun a rigorous screening process to triage and treat patients suspicious for COVID-19 through telehealth in hopes of keeping them safe and in their own homes. We also continue to see patients for nonrespiratory urgent care needs in person once they have screened negative for COVID-19.”
“In terms of the inpatient setting, we’ve noticed that a lot of people are choosing not to go to the hospital now, unless they’re extremely ill,” Dr. Jobbins noted. “We’re going to need to find a balance with when do people truly need to go to the hospital and when do they not? What can we manage as an outpatient versus having someone go to the emergency department? That’s really the role of the primary care physician. We need to help people understand, ‘You don’t need to go to the ED for everything, but here are the things you really need to go for.’ ”
“It will be interesting to see what health care looks like in 6 months or a year. I’m excited to see where we land,” Dr. Jobbins added.
Hopes for the Future of Telemedicine
When the practice of medicine enters a post–COVID-19 era, Dr. Jobbins hopes that telemedicine will be incorporated more into the delivery of patient care. “I’ve found that many of my patients who often are no-shows to the inpatient version of their visits have had a higher success rate of follow-through when we do the telephone visits,” she said. “It’s been very successful. I hope that the insurance companies and [Centers for Medicare & Medicaid] will continue to reimburse this as they see this is a benefit to our patients.
Dr. Hopkins is also hopeful that physicians will be able to successfully see patients via telemedicine in the postpandemic world.
“For the ups and downs we’ve had with telemedicine, I’d love for us to be able to enhance the positives and incorporate that into our practice going forward. If we can reach our patients and help treat them where they are, rather than them having to come to us, that may be a plus,” he said.
In the meantime, Dr. Jobbins presses on as the curve of COVID-19 cases flattens in Western Massachusetts and remains grateful that she chose to practice medicine.
“The commitment I have to being an educator in addition to being a physician is part of why I keep doing this,” Dr. Jobbins said. “I find this to be one of the most fulfilling jobs and careers you could ever have: being there for people when they need you the most. That’s really what a physician’s job is: being there for people when a family member has passed away or when they just need to talk because they’re having anxiety. At the end of the day, if we can impart that to those we work with and bring in a positive attitude, it’s infectious and it makes people see this is a reason we keep doing what we’re doing.”
She’s also been heartened by the kindness of strangers during this pandemic, from those who made and donated face shields when they were in short supply, to those who delivered food to the hospital as a gesture of thanks.
“I had a patient who made homemade masks and sent them to my office,” she said. “There’s obviously good and bad during this time, but I get hope from seeing all of the good things that are coming out of this, the whole idea of finding the light in the darkness.”
During his shift at a COVID-19 drive-through triage screening area set up outside the University of Arkansas for Medical Sciences in Little Rock, Robert Hopkins Jr., MD, noticed a woman bowled over in the front seat of her car.
A nurse practitioner had just informed her that she had met the criteria for undergoing testing for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
“She was very upset and was crying nearly inconsolably,” said Dr. Hopkins, who directs the division of general internal medicine at the University of Arkansas Medical Sciences College of Medicine. “I went over and visited with her for a few minutes. She was scared to death that we [had] told her she was going to die. In her mind, if she had COVID-19 that meant a death sentence, and if we were testing her that meant she was likely to not survive.”
Dr. Hopkins tried his best to put testing in perspective for the woman. “At least she came to a level of comfort and realized that we were doing this for her, that this was not a death sentence, that this was not her fault,” he said. “She was worried about infecting her kids and her grandkids and ending up in the hospital and being a burden. Being able to spend that few minutes with her and help to bring down her level of anxiety – I think that’s where we need to put our efforts as physicians right now, helping people understand, ‘Yes, this is serious. Yes, we need to continue to social distance. Yes, we need to be cautious. But, we will get through this if we all work together to do so.’ ”
Prior to the COVID-19 pandemic, Dr. Hopkins spent part of his time seeing patients in the university’s main hospital, but most of it in an outpatient clinic where he and about 20 other primary care physicians care for patients and precept medical residents. Now, medical residents have been deployed to other services, primarily in the hospital, and he and his physician colleagues are conducting 80%-90% of patient visits by video conferencing or by telephone. It’s a whole new world.
“We’ve gone from a relatively traditional inpatient/outpatient practice where we’re seeing patients face to face to doing some face-to-face visits, but an awful lot of what we do now is in the technology domain,” said Dr. Hopkins, who also assisted with health care relief efforts during hurricanes Rita and Katrina.
“A group of six of us has been redeployed to assist with the surge unit for the inpatient facility, so our outpatient duties are being taken on by some of our partners.”
He also pitches in at the drive-through COVID-19 screening clinic, which was set up on March 27 and operates between 8 a.m. and 8 p.m., 7 days a week. “We’re able to measure people’s temperature, take a quick screening history, decide whether their risk is such that we need to do a COVID-19 PCR [polymerase chain reaction] test,” he said. “Then we make a determination of whether they need to go home on quarantine awaiting those results, or if they don’t have anything that needs to be evaluated, or whether they need to be triaged to an urgent care setting or to the emergency department.”
To minimize his risk of acquiring COVID-19, he follows personal hygiene practices recommended by the Centers for Disease Control and Prevention. He also places his work shoes in a shoebox, which he keeps in his car. “I put them on when I get to the parking deck at work, do my work, and then I put them in the shoebox, slip on another pair of shoes and drive home so I’m not tracking in things I potentially had on me,” said Dr. Hopkins, who is married and a father to two college-aged sons and a daughter in fourth grade. “When I get home I immediately shower, and then I exercise or have dinner with my family.”
Despite the longer-than-usual work hours and upheaval to the traditional medical practice model brought on by the pandemic, Dr. Hopkins, a self-described “glass half full person,” said that he does his best to keep watch over his patients and colleagues. “I’m trying to keep an eye out on my team members – physicians, nurses, medical assistants, and folks at the front desk – trying to make sure that people are getting rest, trying to make sure that people are not overcommitting,” he said. “Because if we’re not all working together and working for the long term, we’re going to be in trouble. This is not going to be a sprint; this is going to be a marathon for us to get through.”
To keep mentally centered, he engages in at least 40 minutes of exercise each day on his bicycle or on the elliptical machine at home. Dr. Hopkins hopes that the current efforts to redeploy resources, expand clinician skill sets, and forge relationships with colleagues in other disciplines will carry over into the delivery of health care when COVID-19 is a distant memory. “I hope that some of those relationships are going to continue and result in better care for all of our patients,” he said.
"We are in dire need of hugs"
Typically, Dr. Dakkak, a family physician at Boston University, practices a mix of clinic-based family medicine and obstetrics, and works in inpatient medicine 6 weeks a year. Currently, she is leading a COVID-19 team full time at Boston Medical Center, a 300-bed safety-net hospital located on the campus of Boston University Medical Center.
COVID-19 has also shaken up her life at home.
When Dr. Dakkak volunteered to take on her new role, the first thing that came to her mind was how making the switch would affect the well-being of her 8-year-old son and 10-year-old daughter.
“I thought, ‘How do I get my children somewhere where I don’t have to worry about them?’ ” Dr. Dakkak said.
She floated the idea with her husband of flying their children out to stay with her recently retired parents, who live outside of Sacramento, Calif., until the pandemic eases up. “I was thinking to myself, ‘Am I overreacting? Is the pandemic not going to be that bad?’ because the rest of the country seemed to be in some amount of denial,” she said. “So, I called my dad, who’s a retired pediatric anesthesiologist. He’s from Egypt so he’s done crisis medicine in his time. He encouraged me to send the kids.”
On the same day that Dr. Dakkak began her first 12-hour COVID-19 shift at the hospital, her husband and children boarded a plane to California, where the kids remain in the care of her parents. Her husband returned after staying there for 2 weeks. “Every day when I’m working, I validate my decision,” she said. “When I first started, I worked 5 nights in a row, had 2 days off, and then worked 6 nights in a row. I was busy so I didn’t think about [being away from my kids], but at the same time I was grateful that I didn’t have to come home and worry about homeschooling the kids or infecting them.”
She checks in with them as she can via cell phone or FaceTime. “My son has been very honest,” Dr. Dakkak said. “He says, ‘FaceTime makes me miss you more, and I don’t like it,’ which I understand. I’ll call my mom, and if they want to talk to me, they’ll talk to me. If they don’t want to talk to me, I’m okay. This is about them being healthy and safe. I sent them a care package a few days ago with cards and some workbooks. I’m optimistic that in June I can at least see them if not bring them home.”
Dr. Dakkak describes leading a COVID-19 team as a grueling experience that challenges her medical know-how nearly every day, with seemingly ever-changing algorithms. “Our knowledge of this disease is five steps behind, and changing at lightning speed,” said Dr. Dakkak, who completed a fellowship in surgical and high-risk obstetrics. “It’s hard to balance continuing to teach evidence-based medicine for everything else in medicine [with continuing] to practice minimal and ever-changing evidence-based COVID medicine. We just don’t know enough [about the virus] yet. This is nothing like we were taught in medical school. Everyone has elevated d-dimers with COVID-19, and we don’t get CT pulmonary angiograms [CTPAs] on all of them; we wouldn’t physically be able to. Some patients have d-dimers in the thousands, and only some are stable to get CTPAs. We are also finding pulmonary embolisms. Now we’re basing our algorithm on anticoagulation due to d-dimers because sometimes you can’t always do a CTPA even if you want to. On the other hand, we have people who are coming into the hospital too late. We’ve had a few who have come in after having days of stroke symptoms. I worry about our patients at home who hesitate to come in when they really should.”
Sometimes she feels sad for the medical residents on her team because their instinct is to go in and check on each patient, “but I don’t want them to get exposed,” she said. “So, we check in by phone, or if they need a physical check-in, we minimize the check-ins; only one of us goes in. I’m more willing to put myself in the room than to put them in the room. I also feel for them because they came into medicine for the humanity of medicine – not the charting or the ordering of medicine. I also worry about the acuity and sadness they’re seeing. This is a rough introduction to medicine for them.”
When interviewed for this story in late April, Dr. Dakkak had kept track of her intubated COVID-19 patients. “Most of my patients get to go home without having been intubated, but those aren’t the ones I worry about,” she said. “I have two patients I have been watching. One of them has just been extubated and I’m still worried about him, but I’m hoping he’s going to be fine. The other one is the first pregnant woman we intubated. She is now extubated, doing really well, and went home. Her fetus is doing well, never had any issues while she was intubated. Those cases make me happy. They were both under the age of 35. It is nice to follow those intubations and find that the majority are doing okay.”
The first patient she had cared for who died was a young man “who was always in good spirits,” she recalled. “We called his brother right before intubating him. After intubation, his oxygen saturation didn’t jump up, which made me worry a bit.” About a week later, the young man died. “I kept thinking, ‘We intubated him when he was still comfortable talking. Should I have put it off and had him call more people to say goodbye? Should I have known that he wasn’t going to wake up?’ ” said Dr. Dakkak, who is also women’s health director at Manet Community Health Centers. “A lot of us have worked on our end-of-life discussions in the past month, just being able to tell somebody, ‘This might be your last time to call family. Call family and talk to whoever you want.’ Guilt isn’t the right word, but it’s unsettling if I’m the last person a patient talks to. I feel that, if that’s the case, then I didn’t do a good enough job trying to get them to their family or friends. If I am worried about a patient’s clinical status declining, I tell families now, when I call them, ‘I hope I’m wrong; I hope they don’t need to be intubated, but I think this is the time to talk.’ ”
To keep herself grounded during off hours, Dr. Dakkak spends time resting, checking in with her family, journaling “to get a lot of feelings out,” gardening, hiking, and joining Zoom chats with friends. Once recentered, she draws from a sense of obligation to others as she prepares for her next shift caring for COVID-19 patients.
“I have a lot of love for the world that I get to expend by doing this hard work,” she said. “I love humanity and I love humanity in times of crisis. The interactions I have with patients and their families are still central to why I do this work. I love my medical teams, and I would never want to let them down. It is nice to feel the sense of teamwork across the hospital. The nurses that I sit with and experience this with are amazing. I keep saying that the only thing I want to do when this pandemic is over is hug everyone. I think we are in dire need of hugs.”
Finding light in the darkness
Internist Katie Jobbins, DO, also has worked in a professional role that was created because of COVID-19.
Shortly before Dr. Jobbins was deployed to Baystate Medical Center in Springfield, Ma., for 2 weeks in April of 2020 to help clinicians with an anticipated surge of COVID-19 cases, she encountered a patient who walked into Baystate’s High Street Health Center.
“I think I have COVID-19,” the patient proclaimed to her, at the outpatient clinic that serves mostly inner-city, Medicaid patients.
Prior to becoming an ambulatory internist, Dr. Jobbins was a surgical resident. “So I went into that mode of ‘I need to do this, this, and this,’ ” she said. “I went through a checklist in my head to make sure I was prepared to take care of the patient.”
She applied that same systems approach during her redeployment assignment in the tertiary care hospital, which typically involved 10-hour shifts overseeing internal medicine residents in a medical telemetry unit. “We would take care of people under investigation for COVID-19, but we were not assigned to the actual COVID unit,” said Dr. Jobbins, who is also associate program director for the internal medicine residency program at the University of Massachusetts Medical School–Baystate Springfield. “They tried to redeploy other people to those units who had special training, and we were trying to back fill into where those people that got moved to the COVID units or the ICU units were actually working. We were taking more of the medical side of the floors.”
Even so, one patient on the unit was suspected of having COVID-19, so Dr. Jobbins suited up with personal protective equipment and conducted a thorough exam with residents waiting outside the patient’s room, a safe distance away. “I explained everything I found on the exam to the residents, trying to give them some educational benefit, even though they couldn’t physically examine the patient because we’re trying to protect them since they’re in training,” she said. “It was anxiety provoking, on some level, knowing that there’s a potential risk of exposure [to the virus], but knowing that Baystate Health has gone to extraordinary measures to make sure we have the correct PPE and support us is reassuring. I knew I had the right equipment and the right tools to take care of the patient, which calmed my nerves and made me feel like I could do the job. That’s the most important thing as a physician during this time: knowing that you have people supporting you who have your back at all times.”
Like Dr. Dakkak, Dr. Jobbins had to make some adjustments to her interaction with her family.
Before she began the deployment, Dr. Jobbins engaged in a frank discussion with her husband and her two young boys about the risks she faced working in a hospital caring for patients with COVID-19. “My husband and I made sure our wills were up to date, and we talked about what we would do if either of us got the virus,” she said. To minimize the potential risk of transmitting the virus to her loved ones during the two-week deployment, she considered living away from her family in a nearby home owned by her father, but decided against that and to “take it day by day.” Following her hospital shifts, Dr. Jobbins changed into a fresh set of clothes before leaving the hospital. Once she arrived home, she showered to reduce the risk of possibly becoming a vector to her family.
She had to tell her kids: “You can’t kiss me right now.”
“As much as it’s hard for them to understand, we had a conversation [in which I explained] ‘This is a virus. It will go away eventually, but it’s a virus we’re fighting.’ It’s interesting to watch a 3-year-old try to process that and take his play samurai sword or Marvel toys and decide he’s going to run around the neighborhood and try to kill the virus.”
At the High Street Health Center, Dr. Jobbins and her colleagues have transitioned to conducting most patient encounters via telephone or video appointments. “We have tried to maintain as much continuity for our patients to address their chronic medical needs through these visits, such as hypertension management and diabetes care,” she said. “We have begun a rigorous screening process to triage and treat patients suspicious for COVID-19 through telehealth in hopes of keeping them safe and in their own homes. We also continue to see patients for nonrespiratory urgent care needs in person once they have screened negative for COVID-19.”
“In terms of the inpatient setting, we’ve noticed that a lot of people are choosing not to go to the hospital now, unless they’re extremely ill,” Dr. Jobbins noted. “We’re going to need to find a balance with when do people truly need to go to the hospital and when do they not? What can we manage as an outpatient versus having someone go to the emergency department? That’s really the role of the primary care physician. We need to help people understand, ‘You don’t need to go to the ED for everything, but here are the things you really need to go for.’ ”
“It will be interesting to see what health care looks like in 6 months or a year. I’m excited to see where we land,” Dr. Jobbins added.
Hopes for the Future of Telemedicine
When the practice of medicine enters a post–COVID-19 era, Dr. Jobbins hopes that telemedicine will be incorporated more into the delivery of patient care. “I’ve found that many of my patients who often are no-shows to the inpatient version of their visits have had a higher success rate of follow-through when we do the telephone visits,” she said. “It’s been very successful. I hope that the insurance companies and [Centers for Medicare & Medicaid] will continue to reimburse this as they see this is a benefit to our patients.
Dr. Hopkins is also hopeful that physicians will be able to successfully see patients via telemedicine in the postpandemic world.
“For the ups and downs we’ve had with telemedicine, I’d love for us to be able to enhance the positives and incorporate that into our practice going forward. If we can reach our patients and help treat them where they are, rather than them having to come to us, that may be a plus,” he said.
In the meantime, Dr. Jobbins presses on as the curve of COVID-19 cases flattens in Western Massachusetts and remains grateful that she chose to practice medicine.
“The commitment I have to being an educator in addition to being a physician is part of why I keep doing this,” Dr. Jobbins said. “I find this to be one of the most fulfilling jobs and careers you could ever have: being there for people when they need you the most. That’s really what a physician’s job is: being there for people when a family member has passed away or when they just need to talk because they’re having anxiety. At the end of the day, if we can impart that to those we work with and bring in a positive attitude, it’s infectious and it makes people see this is a reason we keep doing what we’re doing.”
She’s also been heartened by the kindness of strangers during this pandemic, from those who made and donated face shields when they were in short supply, to those who delivered food to the hospital as a gesture of thanks.
“I had a patient who made homemade masks and sent them to my office,” she said. “There’s obviously good and bad during this time, but I get hope from seeing all of the good things that are coming out of this, the whole idea of finding the light in the darkness.”
Progress report: Elimination of neonatal tetanus
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
Worldwide cases of neonatal tetanus fell by 90% from 2000 to 2018, deaths dropped by 85%, and 45 countries achieved elimination of maternal and neonatal tetanus (MNT), according to the Centers for Disease Control and Prevention.
“Despite this progress, some countries that achieved elimination are still struggling to sustain performance indicators; war and insecurity pose challenges in countries that have not achieved MNT elimination,” Henry N. Njuguna, MD, of the CDC’s global immunization division, and associates wrote in the Morbidity and Mortality Weekly Report.
Other worldwide measures also improved from 2000 to 2018: and the percentage of deliveries attended by a skilled birth attendant increased from 62% during 2000-2005 to 81% in 2013-2018, they reported.
The MNT elimination initiative, which began in 1999 and targeted 59 priority countries, immunized approximately 154 million women of reproductive age with at least two doses of tetanus toxoid–containing vaccine from 2000 to 2018, the investigators wrote, based on data from the World Health Organization and the United Nations Children’s Fund.
With 14 of the priority countries – including Nigeria, Pakistan, and Yemen – still dealing with MNT, however, numerous challenges remain, they noted. About 47 million women and their babies are still unprotected, and 49 million women have not received tetanus toxoid–containing vaccine.
This lack of coverage “can be attributed to weak health systems, including conflict and security issues that limit access to vaccination services, competing priorities that limit the implementation of planned MNT elimination activities, and withdrawal of donor funding,” Dr. Njuguna and associates wrote.
SOURCE: Njuguna HN et al. MMWR. 2020 May 1;69(17):515-20.
FROM MMWR
COVID-19 and pregnancy: Is miscarriage a risk?

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020
Ob.gyns., peds, other PCPs seeking COVID-19 financial relief from feds
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
Decreased fetal movement: Time to educate patients and ourselves
We have all as providers experienced the tragic stillbirth of a term fetus for one of our patients. Too often no fetal movement was felt for days, but the patient never called. Or the patient did call, but the nonstress test (NST) was reactive or the ultrasound showed normal growth and fluid or the biophysical profile (BPP) was 8/8. Yet the patient still presented with a stillborn fetus a day later. Was the first patient simply so fearful of the likely deceased child within her that she did not call? Or did she simply not know to report it because she was not educated about what decreased fetal movement could mean? Could the second example have been prevented even though the testing was normal? I believe both scenarios could have been prevented with better education for both providers and patients.
The national stillbirth rate has remained relatively stagnant since 2000, despite many improvements in guidelines for the management of higher risk pregnancies.1 We follow the growth of these pregnancies, do NSTs, and often induce these patients prior to the due date. We do this in the hope of having a healthy mom and baby. However, an analysis of 614 stillbirth cases and 1,816 control deliveries found that 81% of patients presenting with a stillborn baby had no risks factors that required additional monitoring.2 Nearly 66% of 1,714 patients with a late stillbirth reported decreased fetal movement, no fetal movement, or a concerning increase in fetal movement in the days leading up to their baby’s death.3 Studies have suggested that persistent decreased fetal movement has an odds ratio for stillbirth of 4.51,4 which is higher than hypertensive disease and diabetes for this same outcome by nearly a factor of two. Yet there are no formal guidelines on education for patients or management of this chief complaint.
We assess fetal movement at every prenatal visit but patients who experienced stillbirth will say they didn’t know why. This is because as a culture and a profession we are afraid to talk about such a taboo subject as stillbirth. We are afraid we will scare our patients if we tell them that a decrease in fetal movement or no fetal movement may be because their baby is at risk for this dreaded complication. On one level this argument makes sense, but as soon as the baby is born we give parents plenty of education and advice to keep their children safe. Telling a parent to remove all bedding, put their baby on their back, and keep their baby from being too warm to prevent sudden infant death syndrome (SIDS) is very scary. However, this education is necessary. If moms simply know the reason why we ask about fetal movements, they may not wait 2 days before they call. We must have faith that pregnant women can handle this education about decreased fetal movement.
Next most important is our response to the complaint of decreased fetal movement. Often when the NST is reactive or the ultrasound is normal, we assume the baby is at no risk and we reassure the mother that everything is fine. We often tell moms the false myth that babies slow down at the end or advise kick counts after this complaint despite studies failing to show their utility. Because the education about kick count is frequency is what matters, a mother may not call if there is a change in pattern or strength – even if she is very worried about this. A baby may “pass” a kick count, but a mom still may be very worried, yet she will not call because the baby “passed.”
Protocols from the United Kingdom and Australia focus on the assumption that the complaint of decreased fetal movement may be the only warning sign of impending stillbirth. Harvey Kliman, MD, PhD, director of reproductive and placental research unit at Yale University, New Haven, Conn. said an analogy to this is a car driving 55 miles per hour despite only 10 miles of gas being left in the tank.* The car is running fine even when it is almost out of gas. That may be why we all have seen a fetus with recent reassuring tests in the last few days who presents stillborn. Perhaps the only warning sign is decreased fetal movement – not a nonreactive NST or low score BPP. Placental insufficiency is often the cause of initially unexplained stillbirth, far more common than “cord accidents.” If we liken the placenta to the “gas tank” for the pregnancy, then decreased fetal movement may be the “low gas” signal on the dashboard. After this patient has a reactive NST and/or reassuring ultrasound, we need to ask her if she is reassured. Data from a study of 380 women found that women who had a gut instinct that something was wrong were 23 times more likely to experience a stillbirth, according to the unadjusted odds ratio from the logistic regression model.5 We should follow up closely with moms who are not reassured and consider induction if they are over 39 weeks. We should tell every mom who presents with a concern about fetal movement that she did the right thing, and we want to hear from her again immediately if the movement is decreased again or persists. We cannot make women feel silly for calling. We should do an ultrasound for worried moms even if the NST is reactive to make sure we are not missing oligohydramnios or fetal growth restriction; the latter is the biggest known risk factor for stillbirth. We also should perform an ultrasound for moms with risk factors for stillbirth such as advanced maternal age or black race.
The education about and plan for management of decreased fetal movement are two components of the UK Saving Babies Lives Protocol; one study in the United Kingdom has shown a 20% decline in stillbirths from 2010 to 2017. The other two components are making sure to catch all fetal growth restricted babies and smoking cessation. We incorporated this protocol in my practice several months ago, and we have had very positive feedback from patients. We are not getting an increase in concerns/visits and have not had any patients call and say that they were upset about receiving this education. The Word Health Organization calls stillbirth a “neglected tragedy.” The United States has the lowest annual reduction of its stillbirth rate for all high-income nations in the Lancet 2015 series on stillbirth.6
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. The Lancet. 2016, Jan 18;387(10018):587-603.
2. JAMA. 2011 Dec 14;306(22):2469-79.
3. BMC Pregnancy Childbirth. 2015 Aug 15;15:172.
4. BMJ Open. 2018 Jul 6;8(7):e020031.
5. Midwifery. 2018 Jul;62:171-6.
6. The Lancet. 2016, Jan 18;387(10019):691-702.
*This article was updated on 5/4/2020.
We have all as providers experienced the tragic stillbirth of a term fetus for one of our patients. Too often no fetal movement was felt for days, but the patient never called. Or the patient did call, but the nonstress test (NST) was reactive or the ultrasound showed normal growth and fluid or the biophysical profile (BPP) was 8/8. Yet the patient still presented with a stillborn fetus a day later. Was the first patient simply so fearful of the likely deceased child within her that she did not call? Or did she simply not know to report it because she was not educated about what decreased fetal movement could mean? Could the second example have been prevented even though the testing was normal? I believe both scenarios could have been prevented with better education for both providers and patients.
The national stillbirth rate has remained relatively stagnant since 2000, despite many improvements in guidelines for the management of higher risk pregnancies.1 We follow the growth of these pregnancies, do NSTs, and often induce these patients prior to the due date. We do this in the hope of having a healthy mom and baby. However, an analysis of 614 stillbirth cases and 1,816 control deliveries found that 81% of patients presenting with a stillborn baby had no risks factors that required additional monitoring.2 Nearly 66% of 1,714 patients with a late stillbirth reported decreased fetal movement, no fetal movement, or a concerning increase in fetal movement in the days leading up to their baby’s death.3 Studies have suggested that persistent decreased fetal movement has an odds ratio for stillbirth of 4.51,4 which is higher than hypertensive disease and diabetes for this same outcome by nearly a factor of two. Yet there are no formal guidelines on education for patients or management of this chief complaint.
We assess fetal movement at every prenatal visit but patients who experienced stillbirth will say they didn’t know why. This is because as a culture and a profession we are afraid to talk about such a taboo subject as stillbirth. We are afraid we will scare our patients if we tell them that a decrease in fetal movement or no fetal movement may be because their baby is at risk for this dreaded complication. On one level this argument makes sense, but as soon as the baby is born we give parents plenty of education and advice to keep their children safe. Telling a parent to remove all bedding, put their baby on their back, and keep their baby from being too warm to prevent sudden infant death syndrome (SIDS) is very scary. However, this education is necessary. If moms simply know the reason why we ask about fetal movements, they may not wait 2 days before they call. We must have faith that pregnant women can handle this education about decreased fetal movement.
Next most important is our response to the complaint of decreased fetal movement. Often when the NST is reactive or the ultrasound is normal, we assume the baby is at no risk and we reassure the mother that everything is fine. We often tell moms the false myth that babies slow down at the end or advise kick counts after this complaint despite studies failing to show their utility. Because the education about kick count is frequency is what matters, a mother may not call if there is a change in pattern or strength – even if she is very worried about this. A baby may “pass” a kick count, but a mom still may be very worried, yet she will not call because the baby “passed.”
Protocols from the United Kingdom and Australia focus on the assumption that the complaint of decreased fetal movement may be the only warning sign of impending stillbirth. Harvey Kliman, MD, PhD, director of reproductive and placental research unit at Yale University, New Haven, Conn. said an analogy to this is a car driving 55 miles per hour despite only 10 miles of gas being left in the tank.* The car is running fine even when it is almost out of gas. That may be why we all have seen a fetus with recent reassuring tests in the last few days who presents stillborn. Perhaps the only warning sign is decreased fetal movement – not a nonreactive NST or low score BPP. Placental insufficiency is often the cause of initially unexplained stillbirth, far more common than “cord accidents.” If we liken the placenta to the “gas tank” for the pregnancy, then decreased fetal movement may be the “low gas” signal on the dashboard. After this patient has a reactive NST and/or reassuring ultrasound, we need to ask her if she is reassured. Data from a study of 380 women found that women who had a gut instinct that something was wrong were 23 times more likely to experience a stillbirth, according to the unadjusted odds ratio from the logistic regression model.5 We should follow up closely with moms who are not reassured and consider induction if they are over 39 weeks. We should tell every mom who presents with a concern about fetal movement that she did the right thing, and we want to hear from her again immediately if the movement is decreased again or persists. We cannot make women feel silly for calling. We should do an ultrasound for worried moms even if the NST is reactive to make sure we are not missing oligohydramnios or fetal growth restriction; the latter is the biggest known risk factor for stillbirth. We also should perform an ultrasound for moms with risk factors for stillbirth such as advanced maternal age or black race.
The education about and plan for management of decreased fetal movement are two components of the UK Saving Babies Lives Protocol; one study in the United Kingdom has shown a 20% decline in stillbirths from 2010 to 2017. The other two components are making sure to catch all fetal growth restricted babies and smoking cessation. We incorporated this protocol in my practice several months ago, and we have had very positive feedback from patients. We are not getting an increase in concerns/visits and have not had any patients call and say that they were upset about receiving this education. The Word Health Organization calls stillbirth a “neglected tragedy.” The United States has the lowest annual reduction of its stillbirth rate for all high-income nations in the Lancet 2015 series on stillbirth.6
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. The Lancet. 2016, Jan 18;387(10018):587-603.
2. JAMA. 2011 Dec 14;306(22):2469-79.
3. BMC Pregnancy Childbirth. 2015 Aug 15;15:172.
4. BMJ Open. 2018 Jul 6;8(7):e020031.
5. Midwifery. 2018 Jul;62:171-6.
6. The Lancet. 2016, Jan 18;387(10019):691-702.
*This article was updated on 5/4/2020.
We have all as providers experienced the tragic stillbirth of a term fetus for one of our patients. Too often no fetal movement was felt for days, but the patient never called. Or the patient did call, but the nonstress test (NST) was reactive or the ultrasound showed normal growth and fluid or the biophysical profile (BPP) was 8/8. Yet the patient still presented with a stillborn fetus a day later. Was the first patient simply so fearful of the likely deceased child within her that she did not call? Or did she simply not know to report it because she was not educated about what decreased fetal movement could mean? Could the second example have been prevented even though the testing was normal? I believe both scenarios could have been prevented with better education for both providers and patients.
The national stillbirth rate has remained relatively stagnant since 2000, despite many improvements in guidelines for the management of higher risk pregnancies.1 We follow the growth of these pregnancies, do NSTs, and often induce these patients prior to the due date. We do this in the hope of having a healthy mom and baby. However, an analysis of 614 stillbirth cases and 1,816 control deliveries found that 81% of patients presenting with a stillborn baby had no risks factors that required additional monitoring.2 Nearly 66% of 1,714 patients with a late stillbirth reported decreased fetal movement, no fetal movement, or a concerning increase in fetal movement in the days leading up to their baby’s death.3 Studies have suggested that persistent decreased fetal movement has an odds ratio for stillbirth of 4.51,4 which is higher than hypertensive disease and diabetes for this same outcome by nearly a factor of two. Yet there are no formal guidelines on education for patients or management of this chief complaint.
We assess fetal movement at every prenatal visit but patients who experienced stillbirth will say they didn’t know why. This is because as a culture and a profession we are afraid to talk about such a taboo subject as stillbirth. We are afraid we will scare our patients if we tell them that a decrease in fetal movement or no fetal movement may be because their baby is at risk for this dreaded complication. On one level this argument makes sense, but as soon as the baby is born we give parents plenty of education and advice to keep their children safe. Telling a parent to remove all bedding, put their baby on their back, and keep their baby from being too warm to prevent sudden infant death syndrome (SIDS) is very scary. However, this education is necessary. If moms simply know the reason why we ask about fetal movements, they may not wait 2 days before they call. We must have faith that pregnant women can handle this education about decreased fetal movement.
Next most important is our response to the complaint of decreased fetal movement. Often when the NST is reactive or the ultrasound is normal, we assume the baby is at no risk and we reassure the mother that everything is fine. We often tell moms the false myth that babies slow down at the end or advise kick counts after this complaint despite studies failing to show their utility. Because the education about kick count is frequency is what matters, a mother may not call if there is a change in pattern or strength – even if she is very worried about this. A baby may “pass” a kick count, but a mom still may be very worried, yet she will not call because the baby “passed.”
Protocols from the United Kingdom and Australia focus on the assumption that the complaint of decreased fetal movement may be the only warning sign of impending stillbirth. Harvey Kliman, MD, PhD, director of reproductive and placental research unit at Yale University, New Haven, Conn. said an analogy to this is a car driving 55 miles per hour despite only 10 miles of gas being left in the tank.* The car is running fine even when it is almost out of gas. That may be why we all have seen a fetus with recent reassuring tests in the last few days who presents stillborn. Perhaps the only warning sign is decreased fetal movement – not a nonreactive NST or low score BPP. Placental insufficiency is often the cause of initially unexplained stillbirth, far more common than “cord accidents.” If we liken the placenta to the “gas tank” for the pregnancy, then decreased fetal movement may be the “low gas” signal on the dashboard. After this patient has a reactive NST and/or reassuring ultrasound, we need to ask her if she is reassured. Data from a study of 380 women found that women who had a gut instinct that something was wrong were 23 times more likely to experience a stillbirth, according to the unadjusted odds ratio from the logistic regression model.5 We should follow up closely with moms who are not reassured and consider induction if they are over 39 weeks. We should tell every mom who presents with a concern about fetal movement that she did the right thing, and we want to hear from her again immediately if the movement is decreased again or persists. We cannot make women feel silly for calling. We should do an ultrasound for worried moms even if the NST is reactive to make sure we are not missing oligohydramnios or fetal growth restriction; the latter is the biggest known risk factor for stillbirth. We also should perform an ultrasound for moms with risk factors for stillbirth such as advanced maternal age or black race.
The education about and plan for management of decreased fetal movement are two components of the UK Saving Babies Lives Protocol; one study in the United Kingdom has shown a 20% decline in stillbirths from 2010 to 2017. The other two components are making sure to catch all fetal growth restricted babies and smoking cessation. We incorporated this protocol in my practice several months ago, and we have had very positive feedback from patients. We are not getting an increase in concerns/visits and have not had any patients call and say that they were upset about receiving this education. The Word Health Organization calls stillbirth a “neglected tragedy.” The United States has the lowest annual reduction of its stillbirth rate for all high-income nations in the Lancet 2015 series on stillbirth.6
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures.
References
1. The Lancet. 2016, Jan 18;387(10018):587-603.
2. JAMA. 2011 Dec 14;306(22):2469-79.
3. BMC Pregnancy Childbirth. 2015 Aug 15;15:172.
4. BMJ Open. 2018 Jul 6;8(7):e020031.
5. Midwifery. 2018 Jul;62:171-6.
6. The Lancet. 2016, Jan 18;387(10019):691-702.
*This article was updated on 5/4/2020.
Postcesarean recovery protocols reduce opioid use
based on data from cohorts of women before and after the introduction of the protocols.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
ERAS protocols have been introduced in surgical specialties including colorectal, urologic, gynecologic, and hepatobiliary – with noted benefits to patients and the health care system, wrote Nnamdi I. Gwacham, DO, of Saint Barnabas Medical Center, Livingston, N.J., and colleagues.
The researchers explored the impact of ERAS on reduction in opioid use after cesarean sections at a community teaching hospital in a retrospective study also published in Obstetrics & Gynecology.
The study population included a historical cohort of 2,109 patients from 2018 before the establishment of the ERAS pathway and 1,463 patients since the ERAS pathway was established in 2019.*
Significantly fewer patients in the ERAS group required opioids, compared with the historical group (1,766 vs. 341). A total of 8,082 opioid units were used before the introduction of the ERAS pathway, compared with 803 units used since its introduction, Dr. Gwacham and associates reported. The study was a Donald F. Richardson Prize Paper.
The ERAS pathway consisted of received transversus abdominis plane blocks in the immediate postoperative period (given to 98% of the patients), and all patients were started on “a scheduled multimodal analgesia with a combination of ibuprofen, acetaminophen, and dextromethorphan until discharge,” the researchers wrote. Patients received opioids only if their pain was not well controlled with the ERAS protocol.
In addition, patients who received ERAS had significantly shorter hospital stays than the historical group (3.19 days vs. 2.63 days) and incurred a significantly lower average direct cost ($4,290 vs. $3,957).
The groups were not significantly different in age, race, or body mass index.
“Given the current opioid epidemic in America, researching ways to reduce their use is an urgent matter,” Angela Martin, MD, of the University of Kansas, Kansas City, said in an interview.
She thought the study findings were to be expected based on research in other areas. “Given the trends and ability to reduced opioid use with ERAS in other specialties, it does not surprise me that women recovering from cesarean sections are similar.”
The take-home message for clinicians: “Begin thinking outside of the box when it comes to pain control,” emphasized Dr. Martin, who was not a part of this study. “Opioids don’t have to be the first line medications for postoperative pain management.”
She added that additional directions for research could include the patient perspective on postoperative pain management after a cesarean delivery. “Alternative options to opioids would be even more enticing if the inpatient experience was also improved,” said Dr. Martin, who is a member of the Ob.Gyn News editorial advisory board.
The researchers had no financial conflicts to disclose. Dr. Martin had no relevant financial disclosures.
SOURCE: Gwacham NI et al. Obstet Gynecol. 2020 May;135:2S. doi: 10.1097/01.AOG.0000662880.08512.6b.
*This article was updated on 4/29/2020.
based on data from cohorts of women before and after the introduction of the protocols.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
ERAS protocols have been introduced in surgical specialties including colorectal, urologic, gynecologic, and hepatobiliary – with noted benefits to patients and the health care system, wrote Nnamdi I. Gwacham, DO, of Saint Barnabas Medical Center, Livingston, N.J., and colleagues.
The researchers explored the impact of ERAS on reduction in opioid use after cesarean sections at a community teaching hospital in a retrospective study also published in Obstetrics & Gynecology.
The study population included a historical cohort of 2,109 patients from 2018 before the establishment of the ERAS pathway and 1,463 patients since the ERAS pathway was established in 2019.*
Significantly fewer patients in the ERAS group required opioids, compared with the historical group (1,766 vs. 341). A total of 8,082 opioid units were used before the introduction of the ERAS pathway, compared with 803 units used since its introduction, Dr. Gwacham and associates reported. The study was a Donald F. Richardson Prize Paper.
The ERAS pathway consisted of received transversus abdominis plane blocks in the immediate postoperative period (given to 98% of the patients), and all patients were started on “a scheduled multimodal analgesia with a combination of ibuprofen, acetaminophen, and dextromethorphan until discharge,” the researchers wrote. Patients received opioids only if their pain was not well controlled with the ERAS protocol.
In addition, patients who received ERAS had significantly shorter hospital stays than the historical group (3.19 days vs. 2.63 days) and incurred a significantly lower average direct cost ($4,290 vs. $3,957).
The groups were not significantly different in age, race, or body mass index.
“Given the current opioid epidemic in America, researching ways to reduce their use is an urgent matter,” Angela Martin, MD, of the University of Kansas, Kansas City, said in an interview.
She thought the study findings were to be expected based on research in other areas. “Given the trends and ability to reduced opioid use with ERAS in other specialties, it does not surprise me that women recovering from cesarean sections are similar.”
The take-home message for clinicians: “Begin thinking outside of the box when it comes to pain control,” emphasized Dr. Martin, who was not a part of this study. “Opioids don’t have to be the first line medications for postoperative pain management.”
She added that additional directions for research could include the patient perspective on postoperative pain management after a cesarean delivery. “Alternative options to opioids would be even more enticing if the inpatient experience was also improved,” said Dr. Martin, who is a member of the Ob.Gyn News editorial advisory board.
The researchers had no financial conflicts to disclose. Dr. Martin had no relevant financial disclosures.
SOURCE: Gwacham NI et al. Obstet Gynecol. 2020 May;135:2S. doi: 10.1097/01.AOG.0000662880.08512.6b.
*This article was updated on 4/29/2020.
based on data from cohorts of women before and after the introduction of the protocols.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
ERAS protocols have been introduced in surgical specialties including colorectal, urologic, gynecologic, and hepatobiliary – with noted benefits to patients and the health care system, wrote Nnamdi I. Gwacham, DO, of Saint Barnabas Medical Center, Livingston, N.J., and colleagues.
The researchers explored the impact of ERAS on reduction in opioid use after cesarean sections at a community teaching hospital in a retrospective study also published in Obstetrics & Gynecology.
The study population included a historical cohort of 2,109 patients from 2018 before the establishment of the ERAS pathway and 1,463 patients since the ERAS pathway was established in 2019.*
Significantly fewer patients in the ERAS group required opioids, compared with the historical group (1,766 vs. 341). A total of 8,082 opioid units were used before the introduction of the ERAS pathway, compared with 803 units used since its introduction, Dr. Gwacham and associates reported. The study was a Donald F. Richardson Prize Paper.
The ERAS pathway consisted of received transversus abdominis plane blocks in the immediate postoperative period (given to 98% of the patients), and all patients were started on “a scheduled multimodal analgesia with a combination of ibuprofen, acetaminophen, and dextromethorphan until discharge,” the researchers wrote. Patients received opioids only if their pain was not well controlled with the ERAS protocol.
In addition, patients who received ERAS had significantly shorter hospital stays than the historical group (3.19 days vs. 2.63 days) and incurred a significantly lower average direct cost ($4,290 vs. $3,957).
The groups were not significantly different in age, race, or body mass index.
“Given the current opioid epidemic in America, researching ways to reduce their use is an urgent matter,” Angela Martin, MD, of the University of Kansas, Kansas City, said in an interview.
She thought the study findings were to be expected based on research in other areas. “Given the trends and ability to reduced opioid use with ERAS in other specialties, it does not surprise me that women recovering from cesarean sections are similar.”
The take-home message for clinicians: “Begin thinking outside of the box when it comes to pain control,” emphasized Dr. Martin, who was not a part of this study. “Opioids don’t have to be the first line medications for postoperative pain management.”
She added that additional directions for research could include the patient perspective on postoperative pain management after a cesarean delivery. “Alternative options to opioids would be even more enticing if the inpatient experience was also improved,” said Dr. Martin, who is a member of the Ob.Gyn News editorial advisory board.
The researchers had no financial conflicts to disclose. Dr. Martin had no relevant financial disclosures.
SOURCE: Gwacham NI et al. Obstet Gynecol. 2020 May;135:2S. doi: 10.1097/01.AOG.0000662880.08512.6b.
*This article was updated on 4/29/2020.
REPORTING FROM ACOG 2020