User login
Vaginal cleansing at cesarean delivery works in practice
Vaginal cleansing before cesarean delivery was successfully implemented – and significantly decreased the rate of surgical site infections (SSI) – in a quality improvement study done at Thomas Jefferson University Hospital in Philadelphia.
“Our goal was not to prove that vaginal preparation [before cesarean section] works, because that’s already been shown in large randomized, controlled trials, but to show that we can implement it and that we can see the same results in real life,” lead investigator Johanna Quist-Nelson, MD, said in an interview.
Dr. Quist-Nelson, a third-year fellow at the hospital and the department of obstetrics and gynecology at Sidney Kimmel Medical College, Philadelphia, was scheduled to present the findings at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
Resident and staff physicians as well as nursing and operating room staff were educated/reminded through a multipronged intervention about the benefits of vaginal cleansing with a sponge stick preparation of 10% povidone-iodine solution (Betadine) – and later about the potential benefits of intravenous azithromycin – immediately before cesarean delivery for women in labor and women with ruptured membranes.
Dr. Quist-Nelson and coinvestigators compared three periods of time: 12 months preintervention, 14 months with vaginal cleansing promoted for infection prophylaxis, and 16 months of instructions for both vaginal cleansing and intravenous azithromycin. The three periods captured 1,033 patients. The researchers used control charts – a tool “often used in implementation science,” she said – to analyze monthly data and assess trends for SSI rates and for compliance.
The rate of SSI – as defined by the Centers for Disease Control and Prevention – decreased by 33%, they found, from 23% to 15%. The drop occurred mainly 4 months into the vaginal cleansing portion of the study and was sustained during the following 26 months. The addition of intravenous azithromycin education did not result in any further change in the SSI rate, Dr. Quist-Nelson and associates reported in the study – the abstract for which was published in Obstetrics & Gynecology. It won a third-place prize among the papers on current clinical and basic investigation.
Compliance with the vaginal cleansing protocol increased from 60% at the start of the vaginal cleansing phase to 85% 1 year later. Azithromycin compliance rose to 75% over the third phase of the intervention.
Vaginal cleansing has received attention at Thomas Jefferson for several years. In 2017, researchers there collaborated with investigators in Italy on a systemic review and meta-analysis which concluded that women who received vaginal cleansing before cesarean delivery – most commonly with 10% povidine-iodine – had a significantly lower incidence of endometritis (Obstet Gynecol. 2017 Sep;130[3]:527-38).
A subgroup analysis showed that Dr. Quist-Nelson said.
Azithromycin similarly was found to reduce the risk of postoperative infection in women undergoing nonelective cesarean deliveries in a randomized trial published in 2016 (N Engl J Med. 2016 Sep 29;375[13]:1231-41). While the new quality improvement study did not suggest any additional benefit to intravenous azithromycin, “we continue to offer it [at our hospital] because it has been shown [in prior research] to be beneficial and because our study wasn’t [designed] to show benefit,” Dr. Quist-Nelson said.
The quality improvement intervention included hands-on training on vaginal cleansing for resident physicians and e-mail reminders for physician staff, and daily reviews for 1 week on intravenous azithromycin for resident physicians and EMR “best practice advisory” reminders for physician staff. “We also wrote a protocol available online, and put reminders in our OR notes, as well as trained the nursing staff and OR staff,” she said.
Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview that SSI rates are “problematic [in obstetrics], not only because of morbidity but also potential cost because of rehospitalization.” The study shows that vaginal cleansing “is absolutely a good target for quality improvement,” she said. “It’s promising, and very exciting to see something like this have such a dramatic positive result.” Dr. Cansino, who is a member of the Ob.Gyn News editorial advisory board, was not involved in this study.
Thomas Jefferson Hospital has had relatively high SSI rates, Dr. Quist-Nelson noted.
Dr. Quist-Nelson and coinvestigators did not report any potential conflicts of interest. Dr. Cansino also did not report any potential conflicts of interest.
SOURCE: Quist-Nelson J et al. Obstet. Gynecol. 2020 May;135:1S. doi: 10.1097/01.AOG.0000662876.23603.13.
Vaginal cleansing before cesarean delivery was successfully implemented – and significantly decreased the rate of surgical site infections (SSI) – in a quality improvement study done at Thomas Jefferson University Hospital in Philadelphia.
“Our goal was not to prove that vaginal preparation [before cesarean section] works, because that’s already been shown in large randomized, controlled trials, but to show that we can implement it and that we can see the same results in real life,” lead investigator Johanna Quist-Nelson, MD, said in an interview.
Dr. Quist-Nelson, a third-year fellow at the hospital and the department of obstetrics and gynecology at Sidney Kimmel Medical College, Philadelphia, was scheduled to present the findings at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
Resident and staff physicians as well as nursing and operating room staff were educated/reminded through a multipronged intervention about the benefits of vaginal cleansing with a sponge stick preparation of 10% povidone-iodine solution (Betadine) – and later about the potential benefits of intravenous azithromycin – immediately before cesarean delivery for women in labor and women with ruptured membranes.
Dr. Quist-Nelson and coinvestigators compared three periods of time: 12 months preintervention, 14 months with vaginal cleansing promoted for infection prophylaxis, and 16 months of instructions for both vaginal cleansing and intravenous azithromycin. The three periods captured 1,033 patients. The researchers used control charts – a tool “often used in implementation science,” she said – to analyze monthly data and assess trends for SSI rates and for compliance.
The rate of SSI – as defined by the Centers for Disease Control and Prevention – decreased by 33%, they found, from 23% to 15%. The drop occurred mainly 4 months into the vaginal cleansing portion of the study and was sustained during the following 26 months. The addition of intravenous azithromycin education did not result in any further change in the SSI rate, Dr. Quist-Nelson and associates reported in the study – the abstract for which was published in Obstetrics & Gynecology. It won a third-place prize among the papers on current clinical and basic investigation.
Compliance with the vaginal cleansing protocol increased from 60% at the start of the vaginal cleansing phase to 85% 1 year later. Azithromycin compliance rose to 75% over the third phase of the intervention.
Vaginal cleansing has received attention at Thomas Jefferson for several years. In 2017, researchers there collaborated with investigators in Italy on a systemic review and meta-analysis which concluded that women who received vaginal cleansing before cesarean delivery – most commonly with 10% povidine-iodine – had a significantly lower incidence of endometritis (Obstet Gynecol. 2017 Sep;130[3]:527-38).
A subgroup analysis showed that Dr. Quist-Nelson said.
Azithromycin similarly was found to reduce the risk of postoperative infection in women undergoing nonelective cesarean deliveries in a randomized trial published in 2016 (N Engl J Med. 2016 Sep 29;375[13]:1231-41). While the new quality improvement study did not suggest any additional benefit to intravenous azithromycin, “we continue to offer it [at our hospital] because it has been shown [in prior research] to be beneficial and because our study wasn’t [designed] to show benefit,” Dr. Quist-Nelson said.
The quality improvement intervention included hands-on training on vaginal cleansing for resident physicians and e-mail reminders for physician staff, and daily reviews for 1 week on intravenous azithromycin for resident physicians and EMR “best practice advisory” reminders for physician staff. “We also wrote a protocol available online, and put reminders in our OR notes, as well as trained the nursing staff and OR staff,” she said.
Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview that SSI rates are “problematic [in obstetrics], not only because of morbidity but also potential cost because of rehospitalization.” The study shows that vaginal cleansing “is absolutely a good target for quality improvement,” she said. “It’s promising, and very exciting to see something like this have such a dramatic positive result.” Dr. Cansino, who is a member of the Ob.Gyn News editorial advisory board, was not involved in this study.
Thomas Jefferson Hospital has had relatively high SSI rates, Dr. Quist-Nelson noted.
Dr. Quist-Nelson and coinvestigators did not report any potential conflicts of interest. Dr. Cansino also did not report any potential conflicts of interest.
SOURCE: Quist-Nelson J et al. Obstet. Gynecol. 2020 May;135:1S. doi: 10.1097/01.AOG.0000662876.23603.13.
Vaginal cleansing before cesarean delivery was successfully implemented – and significantly decreased the rate of surgical site infections (SSI) – in a quality improvement study done at Thomas Jefferson University Hospital in Philadelphia.
“Our goal was not to prove that vaginal preparation [before cesarean section] works, because that’s already been shown in large randomized, controlled trials, but to show that we can implement it and that we can see the same results in real life,” lead investigator Johanna Quist-Nelson, MD, said in an interview.
Dr. Quist-Nelson, a third-year fellow at the hospital and the department of obstetrics and gynecology at Sidney Kimmel Medical College, Philadelphia, was scheduled to present the findings at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
Resident and staff physicians as well as nursing and operating room staff were educated/reminded through a multipronged intervention about the benefits of vaginal cleansing with a sponge stick preparation of 10% povidone-iodine solution (Betadine) – and later about the potential benefits of intravenous azithromycin – immediately before cesarean delivery for women in labor and women with ruptured membranes.
Dr. Quist-Nelson and coinvestigators compared three periods of time: 12 months preintervention, 14 months with vaginal cleansing promoted for infection prophylaxis, and 16 months of instructions for both vaginal cleansing and intravenous azithromycin. The three periods captured 1,033 patients. The researchers used control charts – a tool “often used in implementation science,” she said – to analyze monthly data and assess trends for SSI rates and for compliance.
The rate of SSI – as defined by the Centers for Disease Control and Prevention – decreased by 33%, they found, from 23% to 15%. The drop occurred mainly 4 months into the vaginal cleansing portion of the study and was sustained during the following 26 months. The addition of intravenous azithromycin education did not result in any further change in the SSI rate, Dr. Quist-Nelson and associates reported in the study – the abstract for which was published in Obstetrics & Gynecology. It won a third-place prize among the papers on current clinical and basic investigation.
Compliance with the vaginal cleansing protocol increased from 60% at the start of the vaginal cleansing phase to 85% 1 year later. Azithromycin compliance rose to 75% over the third phase of the intervention.
Vaginal cleansing has received attention at Thomas Jefferson for several years. In 2017, researchers there collaborated with investigators in Italy on a systemic review and meta-analysis which concluded that women who received vaginal cleansing before cesarean delivery – most commonly with 10% povidine-iodine – had a significantly lower incidence of endometritis (Obstet Gynecol. 2017 Sep;130[3]:527-38).
A subgroup analysis showed that Dr. Quist-Nelson said.
Azithromycin similarly was found to reduce the risk of postoperative infection in women undergoing nonelective cesarean deliveries in a randomized trial published in 2016 (N Engl J Med. 2016 Sep 29;375[13]:1231-41). While the new quality improvement study did not suggest any additional benefit to intravenous azithromycin, “we continue to offer it [at our hospital] because it has been shown [in prior research] to be beneficial and because our study wasn’t [designed] to show benefit,” Dr. Quist-Nelson said.
The quality improvement intervention included hands-on training on vaginal cleansing for resident physicians and e-mail reminders for physician staff, and daily reviews for 1 week on intravenous azithromycin for resident physicians and EMR “best practice advisory” reminders for physician staff. “We also wrote a protocol available online, and put reminders in our OR notes, as well as trained the nursing staff and OR staff,” she said.
Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview that SSI rates are “problematic [in obstetrics], not only because of morbidity but also potential cost because of rehospitalization.” The study shows that vaginal cleansing “is absolutely a good target for quality improvement,” she said. “It’s promising, and very exciting to see something like this have such a dramatic positive result.” Dr. Cansino, who is a member of the Ob.Gyn News editorial advisory board, was not involved in this study.
Thomas Jefferson Hospital has had relatively high SSI rates, Dr. Quist-Nelson noted.
Dr. Quist-Nelson and coinvestigators did not report any potential conflicts of interest. Dr. Cansino also did not report any potential conflicts of interest.
SOURCE: Quist-Nelson J et al. Obstet. Gynecol. 2020 May;135:1S. doi: 10.1097/01.AOG.0000662876.23603.13.
REPORTING FROM ACOG 2020
COVID-19 registry tracks pregnant women, newborns
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
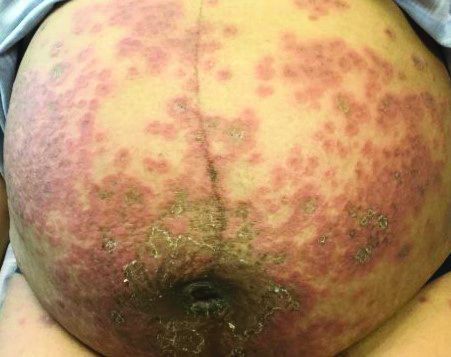
Itchy, vesicular rash
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Addressing CVD’s role in U.S. maternal mortality: Multispecialty collaboration is needed
Nearly 700 women died from pregnancy-related complications in the United States in 2018, and almost a third of those deaths were associated with cardiovascular disease, according to the latest data from the Centers for Disease Control and Prevention.
Strikingly, studies suggest that up to half of cardiovascular disease–related maternal deaths are preventable, yet CVD remains the leading cause of maternal morbidity and mortality – and the incidence has been rising steadily for 2 decades.
The American College of Obstetricians and Gynecologists says that acquired heart disease is the likely culprit in the rise in incidence of maternal mortality as women enter pregnancy with an increasingly heavy burden of CVD risk factors, including older age, obesity, diabetes, and hypertension.
“They are entering pregnancy while already at risk, and that has led to an increase in morbidity and mortality during pregnancy,” Renee Patrice Bullock-Palmer, MD, a cardiologist and director of the Women’s Heart Center at Deborah Heart and Lung Center in Browns Mills, N.J., explained in an interview. “Unfortunately, among developed countries, the U.S. has the highest rates of maternal morbidity and mortality, and that’s shocking.”
It’s a problem that requires collaboration between obstetricians, cardiologists, and others involved in the care of pregnant women, she said.
The data and the depth of the crisis
The maternal mortality rate in 1987 – the year the CDC’s Pregnancy Mortality Surveillance System was implemented – was 7.2 per 100,000 live births. The rate in 2016 was more than double that at 16.9, and the rate in 2018, the most recent year for which data are available, was 17.4 – and significant racial and ethnic disparities in those rates have persisted over time.
In an August 2019 article published on the American Heart Association website, Dr. Bullock-Palmer addressed the cardiovascular state of health for pregnant women and the role of the cardiologists in their care, noting that there is a “role for increased collaboration between the cardiologist and the obstetrician with regards to a pregnancy heart team.”
“It is vital that mothers who are at increased risk for CVD or have established CVD be referred to a cardiologist for cardiovascular assessment and management,” she wrote, adding it is important to raise awareness among ob.gyns. and to improve cardiologists’ recognition of women at risk when they present for care for the first time.
These referrals should be made in the antepartum and early postpartum period, she said in an interview. More attention also must be paid to racial and ethnic disparities, and the role of cardiologists in addressing these disparities.
The CDC has emphasized racial and ethnic disparities in maternal mortality, noting in a 2019 Morbidity and Mortality Weekly report that, compared with white women, black and American Indian/Alaskan Native women aged over 30 years have a 300%-400% higher rate of pregnancy-related deaths (Morb Mortal Wkly Rep. 2019 Sep 6;68[35]:762-5).
With regard to disparities, Dr. Bullock-Palmer said the causes are multifold and may be related to a higher prevalence of CVD risk factors like obesity and hypertension in non-Hispanic black women.
“There may also be limited access to adequate postpartum care in this patient population,” she wrote, adding that some attention has been paid to addressing disparities, but that “there is a lot of work left to be done in resolving these inequities in maternal health care.”
Partnerships across specialties will help in addressing most of the factors associated with CVD and maternal death, she said.
The urgent need for these partnerships is underscored by the latest findings on CVD-related complications in pregnancy. A study published in March 2020 in the Journal of the American College of Cardiology, for example, looked specifically at the incidence of serious cardiac events (SCEs) in pregnant women with heart disease, and whether the events were preventable.
In a prospective cohort of 1,315 pregnancies among women with heart disease, Birgit Pfaller, MD, of the University of Toronto Pregnancy and Heart Disease Research Program, and colleagues found that SCEs occurred in 3.6% of cases (47 women) – most often during the antepartum period – that 49% were preventable, and that 74% were related to provider management factors.
The most common SCEs were cardiac death or arrest, heart failure, arrhythmias, and urgent intervention, and they were more likely to occur in women with acquired heart disease, severe aortic or mitral stenosis, mechanical valves, and systemic ventricular dysfunction. Adverse fetal and neonatal outcomes more than doubled in cases involving SCEs, compared with those without (62% vs. 29%), and adverse obstetric events occurred most often in women with severe preeclampsia.
“The majority of the preventable events occurred due to provider management factors, including: failure to identify the patient condition prior to pregnancy, failure to identify the patient as high risk, late recognition in cardiac deterioration, delay in treatment/intervention, inappropriate treatment, and lack of preconception counseling,” Melinda Davis, MD, of the University of Michigan, Ann Arbor, wrote in a summary and editorial published in the Journal of the American College of Cardiology.
Some preventable events were attributable to patient failure to seek care, noncompliance with care recommendations, and lack of access to care, Dr. Davis noted.
“These findings suggest that provider training, patient education, and health care advocacy are all important interventions to improve outcomes among pregnant women,” she wrote, adding that “the development of multidisciplinary cardio-obstetric clinics at tertiary care centers may also be helpful.”
Dr. Bullock-Palmer added the need for greater risk-prediction tools to the list, explaining that these are needed to assess CVD risk in the prenatal, antenatal, and postnatal period.
“The recently concluded Cardiac Disease in Pregnancy [CARPREG II] study indicated that there were 10 predictors that could be utilized to asses maternal CVD risk,” she noted.
The CARPREG II authors identified five general predictors (prior cardiac events or arrhythmias, poor functional class or cyanosis, high-risk valve disease/left ventricular outflow tract obstruction, systemic ventricular dysfunction, no prior cardiac interventions), four lesion-specific predictors (mechanical valves, high-risk aortopathies, pulmonary hypertension, coronary artery disease), and one delivery-of-care predictor (late pregnancy assessment), and incorporated them into a risk index.
“It is hopeful that these new initiatives will assist providers in improving their ability to appropriately risk stratify women,” Dr. Bullock-Palmer said.
Ongoing efforts
Efforts also are ongoing to develop the types of cardio-obstetric clinics mentioned by Dr. Davis and to establish collaborations and “pregnancy heart teams” as attention is increasingly focused on the U.S. maternal mortality crisis.
In fact, such teams are a cornerstone of ACOG’s guidance on pregnancy and heart disease. In May 2019 the college released a Practice Bulletin with 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period.
Pregnant women and postpartum women with known or suspected CVD should undergo evaluation by a “pregnancy heart team that includes a cardiologist and maternal-fetal medicine subspecialist, or both, and other subspecialists as necessary,” according to the bulletin.
In a recent interview, Lisa Hollier, MD, immediate past president of ACOG and an instrumental figure in the push to better address maternal mortality – and in particular the cardiovascular contributors to the crisis – said she is “seeing a strengthening of that” with numerous organizations establishing pregnancy health teams.
Dr. Bullock-Palmer said she also is seeing progress, and added that collaboration should be prioritized even in the absence of dedicated pregnancy heart teams and clinics.
“Heart disease in pregnancy requires a multidisciplinary approach. You can’t just see the patient from the cardiac perspective – you also have to interact and team up with the obstetrician who is handling the pregnancy,” she said, adding that, without a dedicated team, coordination takes more effort, but is imperative for improving outcomes. “You have to collaborate at times when it is beyond the expertise of the institution or the physician; you have to know when to refer these higher-risk patients, particularly women with adult congenital heart disease.”
This referral should occur early – preferably in the antenatal period, she added.
The most important thing, however, is “recognizing these women ... even before the pregnancy,” Dr. Bullock-Palmer said, explaining that this can facilitate the necessary management – and in some cases, postponement – of pregnancy for women whose cardiac issues need to be addressed first.
Among other efforts to address maternal mortality are several programs developed by ACOG, and the Heart Outcomes in Pregnancy: Expectations for Mom and Baby Registry (HOPE) project of the Saint Luke’s Health System in Kansas.
“Hopefully the [HOPE] research collaborative ... which aims to address key clinical questions surrounding the preconception period, antenatal care, delivery planning and outcomes, and long-term postpartum care and outcomes of women will help to address the knowledge gaps and disparities in the care of women with heart disease in pregnancy,” Dr. Bullock-Palmer wrote in her article.
CVD-related risks in the post partum
Dr. Bullock-Palmer has particular concern for postpartum follow-up, given the increased risk for future heart disease among women with CVD-related pregnancy complications and the heightened risk of certain CVD-related events in the postpartum period.
That’s a component of the crisis that also was addressed during a press briefing at the 2019 ACOG annual meeting when the Pregnancy and Heart Disease Practice Bulletin was released.
James Martin, MD, chair of ACOG’s Pregnancy and Heart Disease Task Force and a past ACOG president, explained during the briefing that CVD-related risks may accelerate and persist in the days and weeks after delivery, underscoring the need for follow-up and postpartum care.
Cardiomyopathy is a particular concern during this time – it’s the major cause of maternal mortality after 42 days, he noted. An emphasis on postpartum care also is especially important given that some data suggest up to 40% of women don’t return for that care.
“That is a very sad statistic and perhaps it reflects on our need to change payment models so that physicians and patients realize the importance of coming back for continuing care, because this really may be the end of pregnancy, but it is the beginning of the rest of their life,” he said. “And if they have cardiovascular disease or the risk factors ... they are going to possibly become worse over the course of their lifetime.”
Nearly 700 women died from pregnancy-related complications in the United States in 2018, and almost a third of those deaths were associated with cardiovascular disease, according to the latest data from the Centers for Disease Control and Prevention.
Strikingly, studies suggest that up to half of cardiovascular disease–related maternal deaths are preventable, yet CVD remains the leading cause of maternal morbidity and mortality – and the incidence has been rising steadily for 2 decades.
The American College of Obstetricians and Gynecologists says that acquired heart disease is the likely culprit in the rise in incidence of maternal mortality as women enter pregnancy with an increasingly heavy burden of CVD risk factors, including older age, obesity, diabetes, and hypertension.
“They are entering pregnancy while already at risk, and that has led to an increase in morbidity and mortality during pregnancy,” Renee Patrice Bullock-Palmer, MD, a cardiologist and director of the Women’s Heart Center at Deborah Heart and Lung Center in Browns Mills, N.J., explained in an interview. “Unfortunately, among developed countries, the U.S. has the highest rates of maternal morbidity and mortality, and that’s shocking.”
It’s a problem that requires collaboration between obstetricians, cardiologists, and others involved in the care of pregnant women, she said.
The data and the depth of the crisis
The maternal mortality rate in 1987 – the year the CDC’s Pregnancy Mortality Surveillance System was implemented – was 7.2 per 100,000 live births. The rate in 2016 was more than double that at 16.9, and the rate in 2018, the most recent year for which data are available, was 17.4 – and significant racial and ethnic disparities in those rates have persisted over time.
In an August 2019 article published on the American Heart Association website, Dr. Bullock-Palmer addressed the cardiovascular state of health for pregnant women and the role of the cardiologists in their care, noting that there is a “role for increased collaboration between the cardiologist and the obstetrician with regards to a pregnancy heart team.”
“It is vital that mothers who are at increased risk for CVD or have established CVD be referred to a cardiologist for cardiovascular assessment and management,” she wrote, adding it is important to raise awareness among ob.gyns. and to improve cardiologists’ recognition of women at risk when they present for care for the first time.
These referrals should be made in the antepartum and early postpartum period, she said in an interview. More attention also must be paid to racial and ethnic disparities, and the role of cardiologists in addressing these disparities.
The CDC has emphasized racial and ethnic disparities in maternal mortality, noting in a 2019 Morbidity and Mortality Weekly report that, compared with white women, black and American Indian/Alaskan Native women aged over 30 years have a 300%-400% higher rate of pregnancy-related deaths (Morb Mortal Wkly Rep. 2019 Sep 6;68[35]:762-5).
With regard to disparities, Dr. Bullock-Palmer said the causes are multifold and may be related to a higher prevalence of CVD risk factors like obesity and hypertension in non-Hispanic black women.
“There may also be limited access to adequate postpartum care in this patient population,” she wrote, adding that some attention has been paid to addressing disparities, but that “there is a lot of work left to be done in resolving these inequities in maternal health care.”
Partnerships across specialties will help in addressing most of the factors associated with CVD and maternal death, she said.
The urgent need for these partnerships is underscored by the latest findings on CVD-related complications in pregnancy. A study published in March 2020 in the Journal of the American College of Cardiology, for example, looked specifically at the incidence of serious cardiac events (SCEs) in pregnant women with heart disease, and whether the events were preventable.
In a prospective cohort of 1,315 pregnancies among women with heart disease, Birgit Pfaller, MD, of the University of Toronto Pregnancy and Heart Disease Research Program, and colleagues found that SCEs occurred in 3.6% of cases (47 women) – most often during the antepartum period – that 49% were preventable, and that 74% were related to provider management factors.
The most common SCEs were cardiac death or arrest, heart failure, arrhythmias, and urgent intervention, and they were more likely to occur in women with acquired heart disease, severe aortic or mitral stenosis, mechanical valves, and systemic ventricular dysfunction. Adverse fetal and neonatal outcomes more than doubled in cases involving SCEs, compared with those without (62% vs. 29%), and adverse obstetric events occurred most often in women with severe preeclampsia.
“The majority of the preventable events occurred due to provider management factors, including: failure to identify the patient condition prior to pregnancy, failure to identify the patient as high risk, late recognition in cardiac deterioration, delay in treatment/intervention, inappropriate treatment, and lack of preconception counseling,” Melinda Davis, MD, of the University of Michigan, Ann Arbor, wrote in a summary and editorial published in the Journal of the American College of Cardiology.
Some preventable events were attributable to patient failure to seek care, noncompliance with care recommendations, and lack of access to care, Dr. Davis noted.
“These findings suggest that provider training, patient education, and health care advocacy are all important interventions to improve outcomes among pregnant women,” she wrote, adding that “the development of multidisciplinary cardio-obstetric clinics at tertiary care centers may also be helpful.”
Dr. Bullock-Palmer added the need for greater risk-prediction tools to the list, explaining that these are needed to assess CVD risk in the prenatal, antenatal, and postnatal period.
“The recently concluded Cardiac Disease in Pregnancy [CARPREG II] study indicated that there were 10 predictors that could be utilized to asses maternal CVD risk,” she noted.
The CARPREG II authors identified five general predictors (prior cardiac events or arrhythmias, poor functional class or cyanosis, high-risk valve disease/left ventricular outflow tract obstruction, systemic ventricular dysfunction, no prior cardiac interventions), four lesion-specific predictors (mechanical valves, high-risk aortopathies, pulmonary hypertension, coronary artery disease), and one delivery-of-care predictor (late pregnancy assessment), and incorporated them into a risk index.
“It is hopeful that these new initiatives will assist providers in improving their ability to appropriately risk stratify women,” Dr. Bullock-Palmer said.
Ongoing efforts
Efforts also are ongoing to develop the types of cardio-obstetric clinics mentioned by Dr. Davis and to establish collaborations and “pregnancy heart teams” as attention is increasingly focused on the U.S. maternal mortality crisis.
In fact, such teams are a cornerstone of ACOG’s guidance on pregnancy and heart disease. In May 2019 the college released a Practice Bulletin with 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period.
Pregnant women and postpartum women with known or suspected CVD should undergo evaluation by a “pregnancy heart team that includes a cardiologist and maternal-fetal medicine subspecialist, or both, and other subspecialists as necessary,” according to the bulletin.
In a recent interview, Lisa Hollier, MD, immediate past president of ACOG and an instrumental figure in the push to better address maternal mortality – and in particular the cardiovascular contributors to the crisis – said she is “seeing a strengthening of that” with numerous organizations establishing pregnancy health teams.
Dr. Bullock-Palmer said she also is seeing progress, and added that collaboration should be prioritized even in the absence of dedicated pregnancy heart teams and clinics.
“Heart disease in pregnancy requires a multidisciplinary approach. You can’t just see the patient from the cardiac perspective – you also have to interact and team up with the obstetrician who is handling the pregnancy,” she said, adding that, without a dedicated team, coordination takes more effort, but is imperative for improving outcomes. “You have to collaborate at times when it is beyond the expertise of the institution or the physician; you have to know when to refer these higher-risk patients, particularly women with adult congenital heart disease.”
This referral should occur early – preferably in the antenatal period, she added.
The most important thing, however, is “recognizing these women ... even before the pregnancy,” Dr. Bullock-Palmer said, explaining that this can facilitate the necessary management – and in some cases, postponement – of pregnancy for women whose cardiac issues need to be addressed first.
Among other efforts to address maternal mortality are several programs developed by ACOG, and the Heart Outcomes in Pregnancy: Expectations for Mom and Baby Registry (HOPE) project of the Saint Luke’s Health System in Kansas.
“Hopefully the [HOPE] research collaborative ... which aims to address key clinical questions surrounding the preconception period, antenatal care, delivery planning and outcomes, and long-term postpartum care and outcomes of women will help to address the knowledge gaps and disparities in the care of women with heart disease in pregnancy,” Dr. Bullock-Palmer wrote in her article.
CVD-related risks in the post partum
Dr. Bullock-Palmer has particular concern for postpartum follow-up, given the increased risk for future heart disease among women with CVD-related pregnancy complications and the heightened risk of certain CVD-related events in the postpartum period.
That’s a component of the crisis that also was addressed during a press briefing at the 2019 ACOG annual meeting when the Pregnancy and Heart Disease Practice Bulletin was released.
James Martin, MD, chair of ACOG’s Pregnancy and Heart Disease Task Force and a past ACOG president, explained during the briefing that CVD-related risks may accelerate and persist in the days and weeks after delivery, underscoring the need for follow-up and postpartum care.
Cardiomyopathy is a particular concern during this time – it’s the major cause of maternal mortality after 42 days, he noted. An emphasis on postpartum care also is especially important given that some data suggest up to 40% of women don’t return for that care.
“That is a very sad statistic and perhaps it reflects on our need to change payment models so that physicians and patients realize the importance of coming back for continuing care, because this really may be the end of pregnancy, but it is the beginning of the rest of their life,” he said. “And if they have cardiovascular disease or the risk factors ... they are going to possibly become worse over the course of their lifetime.”
Nearly 700 women died from pregnancy-related complications in the United States in 2018, and almost a third of those deaths were associated with cardiovascular disease, according to the latest data from the Centers for Disease Control and Prevention.
Strikingly, studies suggest that up to half of cardiovascular disease–related maternal deaths are preventable, yet CVD remains the leading cause of maternal morbidity and mortality – and the incidence has been rising steadily for 2 decades.
The American College of Obstetricians and Gynecologists says that acquired heart disease is the likely culprit in the rise in incidence of maternal mortality as women enter pregnancy with an increasingly heavy burden of CVD risk factors, including older age, obesity, diabetes, and hypertension.
“They are entering pregnancy while already at risk, and that has led to an increase in morbidity and mortality during pregnancy,” Renee Patrice Bullock-Palmer, MD, a cardiologist and director of the Women’s Heart Center at Deborah Heart and Lung Center in Browns Mills, N.J., explained in an interview. “Unfortunately, among developed countries, the U.S. has the highest rates of maternal morbidity and mortality, and that’s shocking.”
It’s a problem that requires collaboration between obstetricians, cardiologists, and others involved in the care of pregnant women, she said.
The data and the depth of the crisis
The maternal mortality rate in 1987 – the year the CDC’s Pregnancy Mortality Surveillance System was implemented – was 7.2 per 100,000 live births. The rate in 2016 was more than double that at 16.9, and the rate in 2018, the most recent year for which data are available, was 17.4 – and significant racial and ethnic disparities in those rates have persisted over time.
In an August 2019 article published on the American Heart Association website, Dr. Bullock-Palmer addressed the cardiovascular state of health for pregnant women and the role of the cardiologists in their care, noting that there is a “role for increased collaboration between the cardiologist and the obstetrician with regards to a pregnancy heart team.”
“It is vital that mothers who are at increased risk for CVD or have established CVD be referred to a cardiologist for cardiovascular assessment and management,” she wrote, adding it is important to raise awareness among ob.gyns. and to improve cardiologists’ recognition of women at risk when they present for care for the first time.
These referrals should be made in the antepartum and early postpartum period, she said in an interview. More attention also must be paid to racial and ethnic disparities, and the role of cardiologists in addressing these disparities.
The CDC has emphasized racial and ethnic disparities in maternal mortality, noting in a 2019 Morbidity and Mortality Weekly report that, compared with white women, black and American Indian/Alaskan Native women aged over 30 years have a 300%-400% higher rate of pregnancy-related deaths (Morb Mortal Wkly Rep. 2019 Sep 6;68[35]:762-5).
With regard to disparities, Dr. Bullock-Palmer said the causes are multifold and may be related to a higher prevalence of CVD risk factors like obesity and hypertension in non-Hispanic black women.
“There may also be limited access to adequate postpartum care in this patient population,” she wrote, adding that some attention has been paid to addressing disparities, but that “there is a lot of work left to be done in resolving these inequities in maternal health care.”
Partnerships across specialties will help in addressing most of the factors associated with CVD and maternal death, she said.
The urgent need for these partnerships is underscored by the latest findings on CVD-related complications in pregnancy. A study published in March 2020 in the Journal of the American College of Cardiology, for example, looked specifically at the incidence of serious cardiac events (SCEs) in pregnant women with heart disease, and whether the events were preventable.
In a prospective cohort of 1,315 pregnancies among women with heart disease, Birgit Pfaller, MD, of the University of Toronto Pregnancy and Heart Disease Research Program, and colleagues found that SCEs occurred in 3.6% of cases (47 women) – most often during the antepartum period – that 49% were preventable, and that 74% were related to provider management factors.
The most common SCEs were cardiac death or arrest, heart failure, arrhythmias, and urgent intervention, and they were more likely to occur in women with acquired heart disease, severe aortic or mitral stenosis, mechanical valves, and systemic ventricular dysfunction. Adverse fetal and neonatal outcomes more than doubled in cases involving SCEs, compared with those without (62% vs. 29%), and adverse obstetric events occurred most often in women with severe preeclampsia.
“The majority of the preventable events occurred due to provider management factors, including: failure to identify the patient condition prior to pregnancy, failure to identify the patient as high risk, late recognition in cardiac deterioration, delay in treatment/intervention, inappropriate treatment, and lack of preconception counseling,” Melinda Davis, MD, of the University of Michigan, Ann Arbor, wrote in a summary and editorial published in the Journal of the American College of Cardiology.
Some preventable events were attributable to patient failure to seek care, noncompliance with care recommendations, and lack of access to care, Dr. Davis noted.
“These findings suggest that provider training, patient education, and health care advocacy are all important interventions to improve outcomes among pregnant women,” she wrote, adding that “the development of multidisciplinary cardio-obstetric clinics at tertiary care centers may also be helpful.”
Dr. Bullock-Palmer added the need for greater risk-prediction tools to the list, explaining that these are needed to assess CVD risk in the prenatal, antenatal, and postnatal period.
“The recently concluded Cardiac Disease in Pregnancy [CARPREG II] study indicated that there were 10 predictors that could be utilized to asses maternal CVD risk,” she noted.
The CARPREG II authors identified five general predictors (prior cardiac events or arrhythmias, poor functional class or cyanosis, high-risk valve disease/left ventricular outflow tract obstruction, systemic ventricular dysfunction, no prior cardiac interventions), four lesion-specific predictors (mechanical valves, high-risk aortopathies, pulmonary hypertension, coronary artery disease), and one delivery-of-care predictor (late pregnancy assessment), and incorporated them into a risk index.
“It is hopeful that these new initiatives will assist providers in improving their ability to appropriately risk stratify women,” Dr. Bullock-Palmer said.
Ongoing efforts
Efforts also are ongoing to develop the types of cardio-obstetric clinics mentioned by Dr. Davis and to establish collaborations and “pregnancy heart teams” as attention is increasingly focused on the U.S. maternal mortality crisis.
In fact, such teams are a cornerstone of ACOG’s guidance on pregnancy and heart disease. In May 2019 the college released a Practice Bulletin with 27 specific recommendations and conclusions relating to screening, diagnosis, and management of CVD for women during the prepregnancy period through the postpartum period.
Pregnant women and postpartum women with known or suspected CVD should undergo evaluation by a “pregnancy heart team that includes a cardiologist and maternal-fetal medicine subspecialist, or both, and other subspecialists as necessary,” according to the bulletin.
In a recent interview, Lisa Hollier, MD, immediate past president of ACOG and an instrumental figure in the push to better address maternal mortality – and in particular the cardiovascular contributors to the crisis – said she is “seeing a strengthening of that” with numerous organizations establishing pregnancy health teams.
Dr. Bullock-Palmer said she also is seeing progress, and added that collaboration should be prioritized even in the absence of dedicated pregnancy heart teams and clinics.
“Heart disease in pregnancy requires a multidisciplinary approach. You can’t just see the patient from the cardiac perspective – you also have to interact and team up with the obstetrician who is handling the pregnancy,” she said, adding that, without a dedicated team, coordination takes more effort, but is imperative for improving outcomes. “You have to collaborate at times when it is beyond the expertise of the institution or the physician; you have to know when to refer these higher-risk patients, particularly women with adult congenital heart disease.”
This referral should occur early – preferably in the antenatal period, she added.
The most important thing, however, is “recognizing these women ... even before the pregnancy,” Dr. Bullock-Palmer said, explaining that this can facilitate the necessary management – and in some cases, postponement – of pregnancy for women whose cardiac issues need to be addressed first.
Among other efforts to address maternal mortality are several programs developed by ACOG, and the Heart Outcomes in Pregnancy: Expectations for Mom and Baby Registry (HOPE) project of the Saint Luke’s Health System in Kansas.
“Hopefully the [HOPE] research collaborative ... which aims to address key clinical questions surrounding the preconception period, antenatal care, delivery planning and outcomes, and long-term postpartum care and outcomes of women will help to address the knowledge gaps and disparities in the care of women with heart disease in pregnancy,” Dr. Bullock-Palmer wrote in her article.
CVD-related risks in the post partum
Dr. Bullock-Palmer has particular concern for postpartum follow-up, given the increased risk for future heart disease among women with CVD-related pregnancy complications and the heightened risk of certain CVD-related events in the postpartum period.
That’s a component of the crisis that also was addressed during a press briefing at the 2019 ACOG annual meeting when the Pregnancy and Heart Disease Practice Bulletin was released.
James Martin, MD, chair of ACOG’s Pregnancy and Heart Disease Task Force and a past ACOG president, explained during the briefing that CVD-related risks may accelerate and persist in the days and weeks after delivery, underscoring the need for follow-up and postpartum care.
Cardiomyopathy is a particular concern during this time – it’s the major cause of maternal mortality after 42 days, he noted. An emphasis on postpartum care also is especially important given that some data suggest up to 40% of women don’t return for that care.
“That is a very sad statistic and perhaps it reflects on our need to change payment models so that physicians and patients realize the importance of coming back for continuing care, because this really may be the end of pregnancy, but it is the beginning of the rest of their life,” he said. “And if they have cardiovascular disease or the risk factors ... they are going to possibly become worse over the course of their lifetime.”
N.Y. universal testing: Many COVID-19+ pregnant women are asymptomatic
based on data from 215 pregnant women in New York City.
“The obstetrical population presents a unique challenge during this pandemic, since these patients have multiple interactions with the health care system and eventually most are admitted to the hospital for delivery,” wrote Desmond Sutton, MD, and colleagues at Columbia University Irving Medical Center, New York
In a letter published in the New England Journal of Medicine, the researchers reviewed their experiences with 215 pregnant women who delivered infants during March 22–April 4, 2020, at the New York–Presbyterian Allen Hospital and Columbia University Irving Medical Center. All the women were screened for symptoms of the COVID-19 infection on admission.
Overall, four women (1.9%) had fevers or other symptoms on admission, and all of these women tested positive for the virus that causes COVID-19. The other 211 women were afebrile and asymptomatic at admission, and 210 of them were tested via nasopharyngeal swabs. A total of 29 asymptomatic women (13.7%) tested positive for COVID-19 infection.
“Thus, 29 of the 33 patients who were positive for SARS-CoV-2 at admission (87.9%) had no symptoms of COVID-19 at presentation,” Dr. Sutton and colleagues wrote.
Three of the 29 COVID-19-positive women who were asymptomatic on admission developed fevers before they were discharged from the hospital after a median stay of 2 days. Of these, two received antibiotics for presumed endomyometritis and one patient with presumed COVID-19 infection received supportive care. In addition, one patient who was initially negative developed COVID-19 symptoms after delivery and tested positive 3 days after her initial negative test.
“Our use of universal SARS-CoV-2 testing in all pregnant patients presenting for delivery revealed that at this point in the pandemic in New York City, most of the patients who were positive for SARS-CoV-2 at delivery were asymptomatic,” Dr. Sutton and colleagues said.
Although their numbers may not be generalizable to areas with lower infection rates, they highlight the risk of COVID-19 infection in asymptomatic pregnant women, they noted.
“The potential benefits of a universal testing approach include the ability to use COVID-19 status to determine hospital isolation practices and bed assignments, inform neonatal care, and guide the use of personal protective equipment,” they concluded.
Continuing challenges
“What I have seen in our institute is the debate about rapid testing and the inherent problems with false negatives and false positives,” Catherine Cansino, MD, of the University of California, Davis, said in an interview. “I think there is definitely a role for universal testing, especially in areas with high prevalence,” and the New York clinicians have made a strong case.
However, the challenge remains of obtaining quick test results that would still be reliable, as many rapid tests have a false-negative rate of as much as 20%, noted Dr. Cansino, who was not involved in the New York study.
Her institution is using a test with a higher level of accuracy, “but it can take several hours or a day to get the results,” at which point the women may have gone through labor and delivery and been in contact with multiple health care workers who have used personal protective equipment accordingly if they don’t know a patient’s status.
To help guide policies, Dr. Cansino said that outcome data would be useful. “It’s hard to know how outcomes are different, and it would be good to know how transmission rates differ between symptomatic carriers and those who are asymptomatic.”
“As SARS-CoV-2, the virus responsible for COVID-19, continues to spread, pregnant women remain a unique population with required frequent health system contacts and ultimate need for delivery,” Iris Krishna, MD, of the Emory Healthcare Network in Atlanta, said in an interview. “This report in a high prevalence area demonstrated 1 out of 8 asymptomatic pregnant patients presenting for delivery were SARS-CoV-2 positive, illustrating a need for universal screening.
“As this pandemic evolves, we are learning more and more, and it is important to expand our understanding of asymptomatic transmission and the risk this may pose,” said Dr. Krishna, who was not part of the New York study.
“Key benefits to universal screening are the capability for labor and delivery units to implement best hospital practices in their care of mothers and babies, such as admitting positive patients to cohort units,” she noted. Such units would “allow for closer monitoring of mothers and babies, as well as ensuring proper use of personal protective equipment by health care teams” and also would help preserve supplies of personal protective equipment.
Dr. Krishna cited hospital testing capacity as an obvious barrier to universal screening of pregnant women, as well as factors including the need for additional protective equipment to be used during swab collection. Also, “If you get a negative result and there is a strong suspicion for COVID-19 infection, when do you retest?” she asked. “These are key questions or areas of assessment that should be considered before embarking on universal screening for pregnant women.” In addition, some patients may refuse testing out of fear of stigma or separation from their newborn.
“Implementing an ‘opt out’ approach to screening is encouraged, whereby a patient is informed that a test will be included in standard preventive screening, and they may decline the test,” Dr. Krishna said. “Routine, opt-out screening approaches have proven to be highly effective as it removes the stigma associated with testing, fosters earlier diagnosis and treatment, reduces risk of transmission, and has proven to be cost effective. Pregnant women should be reassured that universal screening is beneficial for their care and the care of their newborn baby,” she emphasized.
“Institutions should consider implementing universal screening on labor and delivery as several geographic areas are predicted to reach their peak time of COVID-19 transmission, and it is clear that asymptomatic individuals continue to play a role in its transmission,” Dr. Krishna concluded.
Dr. Sutton and associates had no financial conflicts to disclose. Neither Dr. Cansino nor Dr. Krishna had any financial conflicts to disclose. Dr. Cansino and Dr. Krishna are members of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Sutton D et al. N Engl J Med. 2020 Apr 13. doi: 10.1056/NEJMc2009316.
based on data from 215 pregnant women in New York City.
“The obstetrical population presents a unique challenge during this pandemic, since these patients have multiple interactions with the health care system and eventually most are admitted to the hospital for delivery,” wrote Desmond Sutton, MD, and colleagues at Columbia University Irving Medical Center, New York
In a letter published in the New England Journal of Medicine, the researchers reviewed their experiences with 215 pregnant women who delivered infants during March 22–April 4, 2020, at the New York–Presbyterian Allen Hospital and Columbia University Irving Medical Center. All the women were screened for symptoms of the COVID-19 infection on admission.
Overall, four women (1.9%) had fevers or other symptoms on admission, and all of these women tested positive for the virus that causes COVID-19. The other 211 women were afebrile and asymptomatic at admission, and 210 of them were tested via nasopharyngeal swabs. A total of 29 asymptomatic women (13.7%) tested positive for COVID-19 infection.
“Thus, 29 of the 33 patients who were positive for SARS-CoV-2 at admission (87.9%) had no symptoms of COVID-19 at presentation,” Dr. Sutton and colleagues wrote.
Three of the 29 COVID-19-positive women who were asymptomatic on admission developed fevers before they were discharged from the hospital after a median stay of 2 days. Of these, two received antibiotics for presumed endomyometritis and one patient with presumed COVID-19 infection received supportive care. In addition, one patient who was initially negative developed COVID-19 symptoms after delivery and tested positive 3 days after her initial negative test.
“Our use of universal SARS-CoV-2 testing in all pregnant patients presenting for delivery revealed that at this point in the pandemic in New York City, most of the patients who were positive for SARS-CoV-2 at delivery were asymptomatic,” Dr. Sutton and colleagues said.
Although their numbers may not be generalizable to areas with lower infection rates, they highlight the risk of COVID-19 infection in asymptomatic pregnant women, they noted.
“The potential benefits of a universal testing approach include the ability to use COVID-19 status to determine hospital isolation practices and bed assignments, inform neonatal care, and guide the use of personal protective equipment,” they concluded.
Continuing challenges
“What I have seen in our institute is the debate about rapid testing and the inherent problems with false negatives and false positives,” Catherine Cansino, MD, of the University of California, Davis, said in an interview. “I think there is definitely a role for universal testing, especially in areas with high prevalence,” and the New York clinicians have made a strong case.
However, the challenge remains of obtaining quick test results that would still be reliable, as many rapid tests have a false-negative rate of as much as 20%, noted Dr. Cansino, who was not involved in the New York study.
Her institution is using a test with a higher level of accuracy, “but it can take several hours or a day to get the results,” at which point the women may have gone through labor and delivery and been in contact with multiple health care workers who have used personal protective equipment accordingly if they don’t know a patient’s status.
To help guide policies, Dr. Cansino said that outcome data would be useful. “It’s hard to know how outcomes are different, and it would be good to know how transmission rates differ between symptomatic carriers and those who are asymptomatic.”
“As SARS-CoV-2, the virus responsible for COVID-19, continues to spread, pregnant women remain a unique population with required frequent health system contacts and ultimate need for delivery,” Iris Krishna, MD, of the Emory Healthcare Network in Atlanta, said in an interview. “This report in a high prevalence area demonstrated 1 out of 8 asymptomatic pregnant patients presenting for delivery were SARS-CoV-2 positive, illustrating a need for universal screening.
“As this pandemic evolves, we are learning more and more, and it is important to expand our understanding of asymptomatic transmission and the risk this may pose,” said Dr. Krishna, who was not part of the New York study.
“Key benefits to universal screening are the capability for labor and delivery units to implement best hospital practices in their care of mothers and babies, such as admitting positive patients to cohort units,” she noted. Such units would “allow for closer monitoring of mothers and babies, as well as ensuring proper use of personal protective equipment by health care teams” and also would help preserve supplies of personal protective equipment.
Dr. Krishna cited hospital testing capacity as an obvious barrier to universal screening of pregnant women, as well as factors including the need for additional protective equipment to be used during swab collection. Also, “If you get a negative result and there is a strong suspicion for COVID-19 infection, when do you retest?” she asked. “These are key questions or areas of assessment that should be considered before embarking on universal screening for pregnant women.” In addition, some patients may refuse testing out of fear of stigma or separation from their newborn.
“Implementing an ‘opt out’ approach to screening is encouraged, whereby a patient is informed that a test will be included in standard preventive screening, and they may decline the test,” Dr. Krishna said. “Routine, opt-out screening approaches have proven to be highly effective as it removes the stigma associated with testing, fosters earlier diagnosis and treatment, reduces risk of transmission, and has proven to be cost effective. Pregnant women should be reassured that universal screening is beneficial for their care and the care of their newborn baby,” she emphasized.
“Institutions should consider implementing universal screening on labor and delivery as several geographic areas are predicted to reach their peak time of COVID-19 transmission, and it is clear that asymptomatic individuals continue to play a role in its transmission,” Dr. Krishna concluded.
Dr. Sutton and associates had no financial conflicts to disclose. Neither Dr. Cansino nor Dr. Krishna had any financial conflicts to disclose. Dr. Cansino and Dr. Krishna are members of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Sutton D et al. N Engl J Med. 2020 Apr 13. doi: 10.1056/NEJMc2009316.
based on data from 215 pregnant women in New York City.
“The obstetrical population presents a unique challenge during this pandemic, since these patients have multiple interactions with the health care system and eventually most are admitted to the hospital for delivery,” wrote Desmond Sutton, MD, and colleagues at Columbia University Irving Medical Center, New York
In a letter published in the New England Journal of Medicine, the researchers reviewed their experiences with 215 pregnant women who delivered infants during March 22–April 4, 2020, at the New York–Presbyterian Allen Hospital and Columbia University Irving Medical Center. All the women were screened for symptoms of the COVID-19 infection on admission.
Overall, four women (1.9%) had fevers or other symptoms on admission, and all of these women tested positive for the virus that causes COVID-19. The other 211 women were afebrile and asymptomatic at admission, and 210 of them were tested via nasopharyngeal swabs. A total of 29 asymptomatic women (13.7%) tested positive for COVID-19 infection.
“Thus, 29 of the 33 patients who were positive for SARS-CoV-2 at admission (87.9%) had no symptoms of COVID-19 at presentation,” Dr. Sutton and colleagues wrote.
Three of the 29 COVID-19-positive women who were asymptomatic on admission developed fevers before they were discharged from the hospital after a median stay of 2 days. Of these, two received antibiotics for presumed endomyometritis and one patient with presumed COVID-19 infection received supportive care. In addition, one patient who was initially negative developed COVID-19 symptoms after delivery and tested positive 3 days after her initial negative test.
“Our use of universal SARS-CoV-2 testing in all pregnant patients presenting for delivery revealed that at this point in the pandemic in New York City, most of the patients who were positive for SARS-CoV-2 at delivery were asymptomatic,” Dr. Sutton and colleagues said.
Although their numbers may not be generalizable to areas with lower infection rates, they highlight the risk of COVID-19 infection in asymptomatic pregnant women, they noted.
“The potential benefits of a universal testing approach include the ability to use COVID-19 status to determine hospital isolation practices and bed assignments, inform neonatal care, and guide the use of personal protective equipment,” they concluded.
Continuing challenges
“What I have seen in our institute is the debate about rapid testing and the inherent problems with false negatives and false positives,” Catherine Cansino, MD, of the University of California, Davis, said in an interview. “I think there is definitely a role for universal testing, especially in areas with high prevalence,” and the New York clinicians have made a strong case.
However, the challenge remains of obtaining quick test results that would still be reliable, as many rapid tests have a false-negative rate of as much as 20%, noted Dr. Cansino, who was not involved in the New York study.
Her institution is using a test with a higher level of accuracy, “but it can take several hours or a day to get the results,” at which point the women may have gone through labor and delivery and been in contact with multiple health care workers who have used personal protective equipment accordingly if they don’t know a patient’s status.
To help guide policies, Dr. Cansino said that outcome data would be useful. “It’s hard to know how outcomes are different, and it would be good to know how transmission rates differ between symptomatic carriers and those who are asymptomatic.”
“As SARS-CoV-2, the virus responsible for COVID-19, continues to spread, pregnant women remain a unique population with required frequent health system contacts and ultimate need for delivery,” Iris Krishna, MD, of the Emory Healthcare Network in Atlanta, said in an interview. “This report in a high prevalence area demonstrated 1 out of 8 asymptomatic pregnant patients presenting for delivery were SARS-CoV-2 positive, illustrating a need for universal screening.
“As this pandemic evolves, we are learning more and more, and it is important to expand our understanding of asymptomatic transmission and the risk this may pose,” said Dr. Krishna, who was not part of the New York study.
“Key benefits to universal screening are the capability for labor and delivery units to implement best hospital practices in their care of mothers and babies, such as admitting positive patients to cohort units,” she noted. Such units would “allow for closer monitoring of mothers and babies, as well as ensuring proper use of personal protective equipment by health care teams” and also would help preserve supplies of personal protective equipment.
Dr. Krishna cited hospital testing capacity as an obvious barrier to universal screening of pregnant women, as well as factors including the need for additional protective equipment to be used during swab collection. Also, “If you get a negative result and there is a strong suspicion for COVID-19 infection, when do you retest?” she asked. “These are key questions or areas of assessment that should be considered before embarking on universal screening for pregnant women.” In addition, some patients may refuse testing out of fear of stigma or separation from their newborn.
“Implementing an ‘opt out’ approach to screening is encouraged, whereby a patient is informed that a test will be included in standard preventive screening, and they may decline the test,” Dr. Krishna said. “Routine, opt-out screening approaches have proven to be highly effective as it removes the stigma associated with testing, fosters earlier diagnosis and treatment, reduces risk of transmission, and has proven to be cost effective. Pregnant women should be reassured that universal screening is beneficial for their care and the care of their newborn baby,” she emphasized.
“Institutions should consider implementing universal screening on labor and delivery as several geographic areas are predicted to reach their peak time of COVID-19 transmission, and it is clear that asymptomatic individuals continue to play a role in its transmission,” Dr. Krishna concluded.
Dr. Sutton and associates had no financial conflicts to disclose. Neither Dr. Cansino nor Dr. Krishna had any financial conflicts to disclose. Dr. Cansino and Dr. Krishna are members of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Sutton D et al. N Engl J Med. 2020 Apr 13. doi: 10.1056/NEJMc2009316.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Universal COVID-19 testing for pregnant women entering hospitals for delivery could better protect patients and staff.
Major finding: Approximately 88% of 33 pregnant women who tested positive for COVID-19 infection at hospital admission were asymptomatic; about 14% of the 215 women overall tested positive for the novel coronavirus.
Study details: The data come from a review of 215 pregnant women who delivered infants between March 22 and April 4, 2020, in New York City.
Disclosures: The authors had no financial conflicts to disclose.
Source: Sutton D et al. N Engl J Med. 2020 Apr 13. doi: 10.1056/NEJMc2009316.
COVID-19: Press pause on assisted reproduction?
The SARS-CoV-2 novel coronavirus has dramatically altered specialty practice across the board, including the practice of infertility treatment. Reproductive medicine societies recommend suspending new infertility treatment cycles during this time. Women and couples who have already invested time and money in their treatment may be understandably frustrated and worried about the impact of this enforced – and indefinite – delay on their chances of conceiving. This puts the physician, who can’t even guarantee when treatment can resume, in the difficult position of trying to balance the patient’s needs with expert recommendations and government mandates.
Infertility Care During COVID-19
European and American reproductive medicine societies have both offered guidelines regarding infertility care during the pandemic. Both recommend shifting to the use of telehealth rather than in-person visits when possible for initial consultations and follow-up discussions.
With respect to infertility treatments during the COVID-19 pandemic, the American Society for Reproductive Medicine (ASRM) advises the following:
- Suspend initiation of new treatment cycles, including ovulation induction; intrauterine insemination; and in vitro fertilization, including retrievals and frozen embryo transfers, and suspend nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
In most countries, including the United States, all healthcare providers have been asked to put elective and nonurgent medical interventions on hold to ensure that personal protective equipment and other resources are available for the management of patients with COVID-19.
Infertility is a disease and, as such, not all infertility care should be considered elective. Still, for most patients, the overall chances of conceiving will not be compromised by a short delay (1-3 months) in treatment. A longer wait could have more impact on older patients or those who already have reduced ovarian reserve, but these are not indications for urgent fertility treatment.
There are clearly some cases in which infertility treatment cannot be delayed: for example, fertility preservation (oocyte or embryo vitrification) for patients who need to undergo immediate gonadotoxic oncology treatment. These patients need to be able to freeze oocytes/embryos so that later on, they have the option of having a family.
Another situation that could require new infertility treatment is a woman who needs urgent surgery for a condition such as severe symptomatic endometriosis causing ureteral or bowel stenosis/obstruction. Because the surgery itself can compromise fertility, the patient may elect to undergo oocyte embryo cryopreservation or ovarian tissue cryopreservation before the surgical procedure.
Pregnancy and COVID-19
As a precautionary measure during the COVID-19 pandemic, it is recommended that planned pregnancy be avoided. The available data on the risks presented by planning a pregnancy during the COVID pandemic are reassuring but limited.
Pregnancy itself has not been shown to alter the course of COVID-19, and most affected pregnant women will experience only mild or moderate flulike symptoms. Patients with cardiovascular or metabolic comorbidities or those requiring immunosuppressants are expected to be at increased risk for more severe forms of the infection. Currently, no strong evidence suggests a higher risk for miscarriage, stillbirth, or adverse neonatal outcomes with maternal COVID-19 infection.
A report based on 38 cases found no evidence for vertical transmission from mother to fetus, and all neonatal specimens (placental tissue) tested negative for the virus. Moreover, no maternal deaths were reported among these 38 infected women. Another study of 11 infected pregnant women likewise found no increased risk for perinatal morbidity or mortality.
On the other hand, a recent article on the perinatal outcomes of 33 neonates born to mothers with confirmed COVID-19 reported three cases of neonatal COVID-19 as a result of possible vertical transmission. In two cases, symptoms were mild and initial positive coronavirus test results turned negative within a few days. The third case – a pregnancy delivered by emergency cesarean section at 31 weeks for fetal distress – was complicated by bacterial sepsis, thrombocytopenia, and coagulopathy, but once again, the initially positive coronavirus test was negative by day 7.
No neonatal deaths were reported in these 33 cases. The authors could not rule out the possibility of vertical transmission in the three COVID-positive newborns because strict infection control measures were implemented during the care of the patients.
Counseling Patients About Suspending Infertility Treatments
Counseling women is the key to acceptance of the need to suspend or postpone infertility treatments during the pandemic. In addition to the economic hardships that some patients may face as a consequence of the pandemic, an obvious source of frustration stems from not knowing how long delays in treatment might be necessary. A discussion with patients or couples may reassure them that delaying conception is the safest route. For some women, other treatment options might be offered, such as the use of a donor gamete.
Some patients, even when counseled appropriately, may elect to accept the unknown risks. These patients should be counseled about the benefits of cryopreservation with delayed transfer. This could be a compromise, because their overall chances of pregnancy will not be affected but they will have to wait to become pregnant.
Counseling patients about the true impact of delaying treatment in their individual circumstances, providing them with emotional and (if needed) psychological support is important while they wait for their treatment to start. For now, the vast majority of the patients understand the need for delay, appreciate the opportunity to consult the physician over the phone, and are demonstrating patience as they wait for their treatment to start or resume.
Resuming Infertility Care
Recommendations could change as the pandemic continues and more information becomes available about the impact of coronavirus infection during pregnancy and the overall capacity of the healthcare system improves. ASRM acknowledges that “reproductive care professionals, in consultation with their patients, will have to consider reassessing the criteria of what represents urgent and non-urgent care.” If the data remain reassuring and social distancing measures are able to slow down the spread of the disease, the infertility care of those couples who would be most affected by a delay in their treatment could gradually be resumed. On April 14, ASRM updated its recommendations about resuming infertility treatment: “ While it is not yet prudent to resume nonemergency infertility procedures, the Task Force recognizes it is also time to begin to consider strategies and best practices for resuming time-sensitive fertility treatments in the face of COVID-19.”
It is likely that the return to “normal” daily practice will be done in a stepwise fashion. I expect the practices first to open for diagnostic infertility testing, then for the less invasive procedures (frozen embryo transfer, intrauterine insemination) and finally for the more invasive lengthy procedures (stimulation with retrieval and embryo transfer). During the reopening of practice, strict infection control measures will need to be observed.
Dr. Kovacs is the medical director of Kaali Institute IVF Center in Budapest, Hungary. He has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The SARS-CoV-2 novel coronavirus has dramatically altered specialty practice across the board, including the practice of infertility treatment. Reproductive medicine societies recommend suspending new infertility treatment cycles during this time. Women and couples who have already invested time and money in their treatment may be understandably frustrated and worried about the impact of this enforced – and indefinite – delay on their chances of conceiving. This puts the physician, who can’t even guarantee when treatment can resume, in the difficult position of trying to balance the patient’s needs with expert recommendations and government mandates.
Infertility Care During COVID-19
European and American reproductive medicine societies have both offered guidelines regarding infertility care during the pandemic. Both recommend shifting to the use of telehealth rather than in-person visits when possible for initial consultations and follow-up discussions.
With respect to infertility treatments during the COVID-19 pandemic, the American Society for Reproductive Medicine (ASRM) advises the following:
- Suspend initiation of new treatment cycles, including ovulation induction; intrauterine insemination; and in vitro fertilization, including retrievals and frozen embryo transfers, and suspend nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
In most countries, including the United States, all healthcare providers have been asked to put elective and nonurgent medical interventions on hold to ensure that personal protective equipment and other resources are available for the management of patients with COVID-19.
Infertility is a disease and, as such, not all infertility care should be considered elective. Still, for most patients, the overall chances of conceiving will not be compromised by a short delay (1-3 months) in treatment. A longer wait could have more impact on older patients or those who already have reduced ovarian reserve, but these are not indications for urgent fertility treatment.
There are clearly some cases in which infertility treatment cannot be delayed: for example, fertility preservation (oocyte or embryo vitrification) for patients who need to undergo immediate gonadotoxic oncology treatment. These patients need to be able to freeze oocytes/embryos so that later on, they have the option of having a family.
Another situation that could require new infertility treatment is a woman who needs urgent surgery for a condition such as severe symptomatic endometriosis causing ureteral or bowel stenosis/obstruction. Because the surgery itself can compromise fertility, the patient may elect to undergo oocyte embryo cryopreservation or ovarian tissue cryopreservation before the surgical procedure.
Pregnancy and COVID-19
As a precautionary measure during the COVID-19 pandemic, it is recommended that planned pregnancy be avoided. The available data on the risks presented by planning a pregnancy during the COVID pandemic are reassuring but limited.
Pregnancy itself has not been shown to alter the course of COVID-19, and most affected pregnant women will experience only mild or moderate flulike symptoms. Patients with cardiovascular or metabolic comorbidities or those requiring immunosuppressants are expected to be at increased risk for more severe forms of the infection. Currently, no strong evidence suggests a higher risk for miscarriage, stillbirth, or adverse neonatal outcomes with maternal COVID-19 infection.
A report based on 38 cases found no evidence for vertical transmission from mother to fetus, and all neonatal specimens (placental tissue) tested negative for the virus. Moreover, no maternal deaths were reported among these 38 infected women. Another study of 11 infected pregnant women likewise found no increased risk for perinatal morbidity or mortality.
On the other hand, a recent article on the perinatal outcomes of 33 neonates born to mothers with confirmed COVID-19 reported three cases of neonatal COVID-19 as a result of possible vertical transmission. In two cases, symptoms were mild and initial positive coronavirus test results turned negative within a few days. The third case – a pregnancy delivered by emergency cesarean section at 31 weeks for fetal distress – was complicated by bacterial sepsis, thrombocytopenia, and coagulopathy, but once again, the initially positive coronavirus test was negative by day 7.
No neonatal deaths were reported in these 33 cases. The authors could not rule out the possibility of vertical transmission in the three COVID-positive newborns because strict infection control measures were implemented during the care of the patients.
Counseling Patients About Suspending Infertility Treatments
Counseling women is the key to acceptance of the need to suspend or postpone infertility treatments during the pandemic. In addition to the economic hardships that some patients may face as a consequence of the pandemic, an obvious source of frustration stems from not knowing how long delays in treatment might be necessary. A discussion with patients or couples may reassure them that delaying conception is the safest route. For some women, other treatment options might be offered, such as the use of a donor gamete.
Some patients, even when counseled appropriately, may elect to accept the unknown risks. These patients should be counseled about the benefits of cryopreservation with delayed transfer. This could be a compromise, because their overall chances of pregnancy will not be affected but they will have to wait to become pregnant.
Counseling patients about the true impact of delaying treatment in their individual circumstances, providing them with emotional and (if needed) psychological support is important while they wait for their treatment to start. For now, the vast majority of the patients understand the need for delay, appreciate the opportunity to consult the physician over the phone, and are demonstrating patience as they wait for their treatment to start or resume.
Resuming Infertility Care
Recommendations could change as the pandemic continues and more information becomes available about the impact of coronavirus infection during pregnancy and the overall capacity of the healthcare system improves. ASRM acknowledges that “reproductive care professionals, in consultation with their patients, will have to consider reassessing the criteria of what represents urgent and non-urgent care.” If the data remain reassuring and social distancing measures are able to slow down the spread of the disease, the infertility care of those couples who would be most affected by a delay in their treatment could gradually be resumed. On April 14, ASRM updated its recommendations about resuming infertility treatment: “ While it is not yet prudent to resume nonemergency infertility procedures, the Task Force recognizes it is also time to begin to consider strategies and best practices for resuming time-sensitive fertility treatments in the face of COVID-19.”
It is likely that the return to “normal” daily practice will be done in a stepwise fashion. I expect the practices first to open for diagnostic infertility testing, then for the less invasive procedures (frozen embryo transfer, intrauterine insemination) and finally for the more invasive lengthy procedures (stimulation with retrieval and embryo transfer). During the reopening of practice, strict infection control measures will need to be observed.
Dr. Kovacs is the medical director of Kaali Institute IVF Center in Budapest, Hungary. He has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The SARS-CoV-2 novel coronavirus has dramatically altered specialty practice across the board, including the practice of infertility treatment. Reproductive medicine societies recommend suspending new infertility treatment cycles during this time. Women and couples who have already invested time and money in their treatment may be understandably frustrated and worried about the impact of this enforced – and indefinite – delay on their chances of conceiving. This puts the physician, who can’t even guarantee when treatment can resume, in the difficult position of trying to balance the patient’s needs with expert recommendations and government mandates.
Infertility Care During COVID-19
European and American reproductive medicine societies have both offered guidelines regarding infertility care during the pandemic. Both recommend shifting to the use of telehealth rather than in-person visits when possible for initial consultations and follow-up discussions.
With respect to infertility treatments during the COVID-19 pandemic, the American Society for Reproductive Medicine (ASRM) advises the following:
- Suspend initiation of new treatment cycles, including ovulation induction; intrauterine insemination; and in vitro fertilization, including retrievals and frozen embryo transfers, and suspend nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
In most countries, including the United States, all healthcare providers have been asked to put elective and nonurgent medical interventions on hold to ensure that personal protective equipment and other resources are available for the management of patients with COVID-19.
Infertility is a disease and, as such, not all infertility care should be considered elective. Still, for most patients, the overall chances of conceiving will not be compromised by a short delay (1-3 months) in treatment. A longer wait could have more impact on older patients or those who already have reduced ovarian reserve, but these are not indications for urgent fertility treatment.
There are clearly some cases in which infertility treatment cannot be delayed: for example, fertility preservation (oocyte or embryo vitrification) for patients who need to undergo immediate gonadotoxic oncology treatment. These patients need to be able to freeze oocytes/embryos so that later on, they have the option of having a family.
Another situation that could require new infertility treatment is a woman who needs urgent surgery for a condition such as severe symptomatic endometriosis causing ureteral or bowel stenosis/obstruction. Because the surgery itself can compromise fertility, the patient may elect to undergo oocyte embryo cryopreservation or ovarian tissue cryopreservation before the surgical procedure.
Pregnancy and COVID-19
As a precautionary measure during the COVID-19 pandemic, it is recommended that planned pregnancy be avoided. The available data on the risks presented by planning a pregnancy during the COVID pandemic are reassuring but limited.
Pregnancy itself has not been shown to alter the course of COVID-19, and most affected pregnant women will experience only mild or moderate flulike symptoms. Patients with cardiovascular or metabolic comorbidities or those requiring immunosuppressants are expected to be at increased risk for more severe forms of the infection. Currently, no strong evidence suggests a higher risk for miscarriage, stillbirth, or adverse neonatal outcomes with maternal COVID-19 infection.
A report based on 38 cases found no evidence for vertical transmission from mother to fetus, and all neonatal specimens (placental tissue) tested negative for the virus. Moreover, no maternal deaths were reported among these 38 infected women. Another study of 11 infected pregnant women likewise found no increased risk for perinatal morbidity or mortality.
On the other hand, a recent article on the perinatal outcomes of 33 neonates born to mothers with confirmed COVID-19 reported three cases of neonatal COVID-19 as a result of possible vertical transmission. In two cases, symptoms were mild and initial positive coronavirus test results turned negative within a few days. The third case – a pregnancy delivered by emergency cesarean section at 31 weeks for fetal distress – was complicated by bacterial sepsis, thrombocytopenia, and coagulopathy, but once again, the initially positive coronavirus test was negative by day 7.
No neonatal deaths were reported in these 33 cases. The authors could not rule out the possibility of vertical transmission in the three COVID-positive newborns because strict infection control measures were implemented during the care of the patients.
Counseling Patients About Suspending Infertility Treatments
Counseling women is the key to acceptance of the need to suspend or postpone infertility treatments during the pandemic. In addition to the economic hardships that some patients may face as a consequence of the pandemic, an obvious source of frustration stems from not knowing how long delays in treatment might be necessary. A discussion with patients or couples may reassure them that delaying conception is the safest route. For some women, other treatment options might be offered, such as the use of a donor gamete.
Some patients, even when counseled appropriately, may elect to accept the unknown risks. These patients should be counseled about the benefits of cryopreservation with delayed transfer. This could be a compromise, because their overall chances of pregnancy will not be affected but they will have to wait to become pregnant.
Counseling patients about the true impact of delaying treatment in their individual circumstances, providing them with emotional and (if needed) psychological support is important while they wait for their treatment to start. For now, the vast majority of the patients understand the need for delay, appreciate the opportunity to consult the physician over the phone, and are demonstrating patience as they wait for their treatment to start or resume.
Resuming Infertility Care
Recommendations could change as the pandemic continues and more information becomes available about the impact of coronavirus infection during pregnancy and the overall capacity of the healthcare system improves. ASRM acknowledges that “reproductive care professionals, in consultation with their patients, will have to consider reassessing the criteria of what represents urgent and non-urgent care.” If the data remain reassuring and social distancing measures are able to slow down the spread of the disease, the infertility care of those couples who would be most affected by a delay in their treatment could gradually be resumed. On April 14, ASRM updated its recommendations about resuming infertility treatment: “ While it is not yet prudent to resume nonemergency infertility procedures, the Task Force recognizes it is also time to begin to consider strategies and best practices for resuming time-sensitive fertility treatments in the face of COVID-19.”
It is likely that the return to “normal” daily practice will be done in a stepwise fashion. I expect the practices first to open for diagnostic infertility testing, then for the less invasive procedures (frozen embryo transfer, intrauterine insemination) and finally for the more invasive lengthy procedures (stimulation with retrieval and embryo transfer). During the reopening of practice, strict infection control measures will need to be observed.
Dr. Kovacs is the medical director of Kaali Institute IVF Center in Budapest, Hungary. He has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Bone density slow to rebound after lactation in women with HIV
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
Women with HIV had more bone mobilization during lactation, and attenuated skeletal recovery after lactation, compared with HIV-negative women, according to research presented during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The study “demonstrated that there were reductions as expected in BMD during breastfeeding, and there was recovery at the end of breastfeeding, which was higher among women who were not HIV-infected compared to HIV-infected women,” said Mary Glenn Fowler, MD, speaking in a video presentation during the virtual conference. The differences between women who had HIV and the HIV-negative reference group were statistically significant (P = .003 for lumbar spine and P less than .001 for whole-body aBMD).
“We also saw that for whole-body BMD, there was recovery at the end of breastfeeding for women who were not HIV infected, but a dampened response of recovery for BMD for HIV-infected women,” she went on, adding: “These findings held after adjustment for parity, age, body mass, breastfeeding practices, duration of breastfeeding, use of [injectable medroxyprogesterone acetate], and resumption of menses.”
Dr. Fowler presented the study’s results on behalf of lead author Florence Nabwire, PhD, an investigator scientist in the nutrition and bone health group of the United Kingdom’s Medical Research Council (Cambridge).
Although it’s known that antiretroviral therapy (ART) is associated with bone loss, Dr. Fowler explained that there are only limited data in HIV-positive women who are lactating. It’s important to see what happens during lactation for this group of women because of the potential sequelae later in life of insufficient recovery from the physiological bone mobilization that occurs during lactation. The study looked at changes in areal bone mineral density (aBMD) both during and after lactation for women with HIV living in Uganda who were taking Option B+ ART, a regimen that includes tenofovir, 3TC, and efavirenz. These women were compared with a reference group of HIV-negative women.
In all, 95 women with HIV and 96 HIV negative women were recruited into the study during pregnancy. Participants were followed postpartum at weeks 2, 14, and 26, and at a final visit that occurred 14 weeks after lactation stopped.
In addition to lumbar spine, total hip, and femoral neck aBMD measurements, the investigators also obtained whole body-less-head reading.
For total hip and femoral neck aBMD, the nadir of density was seen at 26 postpartum, when a drop of about 6% was seen from baseline readings. By the final post-lactation visit, women without HIV had recovered to their baseline; for women with HIV, some recovery also occurred, but the effect was dampened, with a persistent bone density deficit of about 3% from baseline. The differences between HIV-positive and HIV-negative women in these measurements were also statistically significant, at P less than .001 for total hip aBMD differences and P = .0008 for femoral neck differences. Again, correction for multiple confounders didn’t attenuate the results, said Dr. Fowler.
“In conclusion, these data showed accentuated mobilization of hip and whole body aBMD during lactation,” said Dr. Fowler, who also noted “slower skeletal recovery post lactation for HIV-infected women.” Clinical implications of these findings aren’t currently known, she said. Further ongoing studies are aiming to tease out both mechanisms and longer-term consequences for the bone health of HIV-infected women and their children, who may also see differences in bone mineral accretion and growth.
Session moderator Risa Hoffman, MD, in introductory remarks, set the findings in some context. “As we know, HIV-positive adults have low bone mineral density, and this appears to be a result of interactions of HIV, traditional risk factors for loss of bone density, and antiretroviral therapy,” said Dr. Hoffman, director of the global health program at the University of California, Los Angeles. She added that previous work had shown that “middle-aged HIV-positive women have higher 10-year fracture incidence compared to their HIV-negative counterparts.” The current study, she said, “has both short- and long-term implications for women as they go through multiple pregnancies and multiple periods of breastfeeding.”
The study was funded by the United Kingdom’s Medical Research Council and Department for International Development as well as the Alborada Trust and the Gates Cambridge Scholarship. The authors reported no conflicts of interest.
SOURCE: Nabwire F et al. CROI 2020, Abstract 768.
FROM CROI 2020
With mild or stable lupus, few patients flare during, after pregnancy
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
FROM ARTHRITIS RESEARCH & THERAPY
Learning to live with COVID-19: Postpandemic life will be reflected in how effectively we leverage this crisis
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
CDC: Screen nearly all adults, including pregnant women, for HCV
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).