User login
IBD: Inpatient opioids linked with outpatient use
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Patients with inflammatory bowel disease (IBD) who receive opioids while hospitalized are three times as likely to be prescribed opioids after discharge.
Major finding: Patients who were given intravenous opioids while hospitalized were three times as likely to receive a postdischarge opioid prescription, compared with patients who did not receive inpatient intravenous opioids (odds ratio, 3.3).
Study details: A retrospective cohort study involving 862 patients with inflammatory bowel disease.
Disclosures: The investigators disclosed relationships Abbott, Gilead, Romark, and others.
Source: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
Adding cannabinoids to opioids doesn’t improve cancer pain control
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
Adding cannabinoids to opioids does not appear to improve control of cancer-related pain in adults with late-stage disease, authors of a systematic review and meta-analysis contend.
Among 1,442 participants in five randomized controlled trials of cannabinoids compared with placebo, there was no significant difference in the primary outcome of pain intensity scores, reported Elaine G. Boland, MD, PhD, of Hull University Teaching Hospitals NHS Trust in Cottingham, England, and colleagues.
“For a medication to be useful, there needs to be a net overall benefit, with the positive effects (analgesia) outweighing adverse effects. None of the included phase III studies show benefit of cannabinoids,” they wrote. Their report is in BMJ Supportive & Palliative Care.
According to NORML, the National Organization for the Reform of Marijuana Laws, 33 U.S. states currently have legalized medical use of marijuana or cannabinoids, and Dr. Boland and coauthors report that medical marijuana is legal in some 40 nations worldwide.
Survey data and a randomized sample of urine tests from a cancer center in Washington State, were marijuana is legal, show that cannabis or cannabinoid use is common among cancer patients. Despite its widespread use, good quality evidence of the efficacy of cannabis for control of cancer pain is sparse, the investigators said.-
They designed a systematic review and meta-analysis to identify randomized controlled trials with a low risk for bias, eventually settling on five with a total of 1,442 patients. Four of the studies evaluated nabiximols (Sativex), an oromucosal formulation of delta-9-tetrahydrocannabinol:cannabidiol (THC:CBD), and one tested THC:CBD or THC abstract vs. placebo.
To bolster confidence in their results, the investigators contacted the authors of the included studies to obtain additional findings and information about each study’s design.
They found that in the pooled data there was no significant difference between cannabinoids and placebo for the difference in average pain on a Numeric Rating Scale (NRS). The mean difference was –0.21 (P = .14) and did not reach significance when the analysis was restricted to phase 3 trials (mean difference –.02, P = .80).
For the secondary outcomes of adverse events and dropouts, they found that cannabinoids were associated with significantly higher risk for somnolence (odds ratio [OR] 2.69, P less than .001) and dizziness (OR 1.58, P = .05), and that dropouts due to adverse events were more frequent in the cannabinoid arms.
The investigators acknowledged that the study was limited by its reliance on the NRS pain score “as this simple instrument does not capture the complexity of pain especially when it has been [a] long-standing problem,” and by the possibility that vagaries in the use of the oromucosal spray might affect the absorption and efficacy of the cannabinoids.
The authors did not report a funding source. No conflicts of interest were reported.
SOURCE: Boland EG et al. BMJ Supportive & Palliative Care 2020 Jan 20. doi: 10.1136/bmjspcare-2019-002032.
FROM BMJ SUPPORTIVE & PALLIATIVE CARE
We can achieve opioid-free analgesia after childbirth: Stop prescribing opioids after vaginal delivery and reduce their use after cesarean
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)
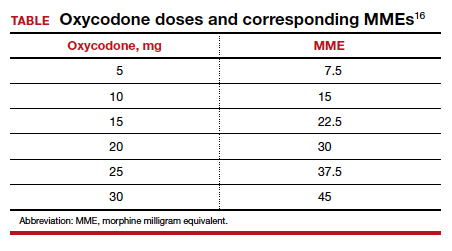
A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)

A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
CASE New mother receives unneeded opioids after CD
A house officer wrote orders for a healthy patient who had just had an uncomplicated cesarean delivery (CD). The hospital’s tradition dictates orders for oxycodone plus acetaminophen tablets in addition to ibuprofen for all new mothers. At the time of the patient’s discharge, the same house officer prescribed 30 tablets of oxycodone plus acetaminophen “just in case,” although the patient had required only a few tablets while in the hospital on postoperative day 2 and none on the day of discharge.
Stuck in the habit
Prescribing postpartum opioids in the United States is almost habitual. Both optimizing patient satisfaction and minimizing patient phone calls may be driving this well-established pattern. Interestingly, a survey study of obstetric providers in 14 countries found that clinicians in 13 countries prescribe opioids “almost never” after vaginal delivery.1 The United States was the 1 outlier, with providers reporting prescribing opioids “on a regular basis” after vaginal birth. Similarly, providers in 10 countries reported prescribing opioids “almost never” after CD, while those in the United States reported prescribing opioids “almost always” in this context.
Moreover, mounting data suggest that many patients do not require the quantity of opioids prescribed and that our overprescribing may be causing more harm than good.
The problem of overprescribing opioids after childbirth
Opioid analgesia has long been the mainstay of treatment for postpartum pain, which when poorly controlled is associated with the development of postpartum depression and chronic pain.2 However, common adverse effects of opioids, including nausea, drowsiness, and dizziness, similarly can interfere with self-care and infant care. Of additional concern, a 2016 claims data study found that 1 of 300 opioid-naïve women who were prescribed opioids at discharge after CD used these medications persistently in the first year postpartum.3
Many women do not use the opioids that are prescribed to them at discharge, thus making tablets available for potential diversion into the community—a commonly recognized source of opioid misuse and abuse.4,5 In a 2018 Committee Opinion on postpartum pain management, the American College of Obstetricians and Gynecologists (ACOG) stated that “a stepwise, multimodal approach emphasizing nonopioid analgesia as first-line therapy is safe and effective for vaginal deliveries and cesarean deliveries.”6 The Committee Opinion also asserted that “opioid medication is an adjunct for patients with uncontrolled pain despite adequate first-line therapy.”6
Despite efforts by the Centers for Disease Control and Prevention (CDC) and ACOG to improve opioid prescribing patterns after childbirth, the vast majority of women receive opioids in the hospital and at discharge not only after CD, but after vaginal delivery as well.4,7 Why has tradition prevailed over data, and why have we not changed?
Continue to: Common misconceptions about reducing opioid use...
Common misconceptions about reducing opioid use
Two misconceptions persist regarding reducing opioid prescriptions for postpartum pain.
Misconception #1: Patients will be in pain
Randomized controlled trials that compared nonopioid with opioid regimens in the emergency room setting and opioid use after outpatient general surgery procedures have demonstrated that pain control for patients receiving opioids was equivalent to that for patients with pain managed with nonopioid regimens.8-10 In the obstetric setting, a survey study of 720 women who underwent CD found that higher quantities of opioid tablets prescribed at discharge were not associated with improved pain, higher satisfaction, or lower refill rates at 2 weeks postpartum.4 However, greater quantities of opioids prescribed at the time of discharge were associated with greater opioid consumption.
Recently, several quality improvement studies implemented various interventions and successfully decreased postpartum opioid consumption without compromising pain management. One quality improvement project eliminated the routine use of opioids after CD and decreased the proportion of patients using any opioids in the hospital from 68% to 45%, with no changes in pain scores.11 A similar study implemented an enhanced recovery after surgery (ERAS) program for women after CD; mean in-patient opioid use decreased from 10.7 to 5.4 average daily morphine equivalents, with improvement in the proportion of time that patients reported their pain as acceptable.12
Misconception #2: Clinicians will be overwhelmed with pages and phone calls
Providers commonly fear that decreasing opioid use will lead to an increased volume of pages and phone calls from patients requesting additional medication. However, data suggest otherwise. For example, a quality improvement study that eliminated the routine use of opioids after CD tracked the number of phone calls that were received requesting rescue opioid prescriptions after discharge.11 Although the percentage of women discharged with opioids decreased from 90.6% to 40.3%, the requests for rescue opioid prescriptions did not change. Of 191 women, 4 requested a rescue prescription prior to the intervention compared with no women after the intervention. At the same time, according to unpublished data (Dr. Holland), satisfaction among nurses, house staff, and faculty did not change.
Similarly, a quality improvement project that implemented shared decision-making to inform the quantity of opioids prescribed at discharge demonstrated that the number of tablets prescribed decreased from 33.2 to 26.5, and there was no change in the rate of patients requesting opioid refills.13
Success stories: Strategies for reducing opioid use after childbirth
While overall rates of opioid prescribing after vaginal delivery and CD remain high throughout the United States, various institutions have developed successful and reproducible strategies to reduce opioid use after childbirth both in the hospital and at discharge. We highlight 3 strategies below.
Strategy 1: ERAS initiatives
An integrated health care system in northern California studied the effects of an ERAS protocol for CD across 15 medical centers.12 The intervention centered on 4 pillars: multimodal pain management, early mobility, optimal nutrition, and patient engagement through education. Specifically, multimodal pain management consisted of the following:
- intrathecal opioids during CD
- scheduled intravenous acetaminophen for 24 hours followed by oral acetaminophen every 6 hours
- nonsteroidal anti-inflammatory drugs (NSAIDs) every 6 hours
- oral oxycodone for breakthrough pain
- decoupling of opioid medication from nonopioids in the post-CD order set
- decoupling of opioid and nonopioid medications in the discharge order set along with a reduction from 30 to 20 tablets as the default discharge quantity.
Continue to: Among 4,689 and 4,624 patients who underwent CD...
Among 4,689 and 4,624 patients who underwent CD before and after the intervention, the daily morphine milligram equivalents (MME) consumed in the hospital decreased from 10.7 to 5.4. The percentage of women who required no opioids while in the hospital increased from 8.3% to 21.4% after ERAS implementation, while the percentage of time that patients reported acceptable pain scores increased from 82.1% to 86.4%. The average number of opioid tablets prescribed at discharge also decreased, from 37 to 26 MME.12 (The TABLE shows oxycodone doses converted to MMEs.)

A similar initiative at a network of 5 hospitals in Texas showed that implementation of a “multimodal pain power plan” (which incorporated postpartum activity goals with standardized order sets) decreased opioid use after both vaginal delivery and CD.14
Strategy 2: Order set change to eliminate routine use of opioids
A tertiary care center in Boston, Massachusetts, implemented a quality improvement project aimed at eliminating the routine use of opioid medication after CD through an order set change.11 The intervention consisted of the following:
- intrathecal morphine
- multimodal postoperative pain management including scheduled oral acetaminophen for 72 hours followed by as-needed oral acetaminophen, scheduled NSAIDs for 72 hours followed by as-needed NSAIDs
- no postoperative order for opioids unless the patient had a contraindication to acetaminophen or NSAIDs, had a history of opioid dependence, or underwent complex surgery
- counseling patients that opioids were available for breakthrough pain if needed. In this case, nursing staff would page the responding clinician, who would order oxycodone 5 mg every 6 hours for 6 doses.
- specific criteria for discharge quantities of opioids: if the patient required no opioids in the hospital, she received no opioids at discharge; if the patient required opioids in the hospital but none at the time of discharge, she received no more than 10 tablets of oxycodone 5 mg; if the patient required opioids at the time of discharge, she received a maximum of 20 tablets of oxycodone 5 mg.
Among 191 and 181 women undergoing CD before and after the intervention, the percentage of patients who received any opioids in the hospital decreased from 68.1% to 45.3%.11 Similarly, the percentage of patients receiving a discharge prescription for opioids decreased from 90.6% to 40.3%, while patient pain scores and satisfaction with pain control remained unchanged.
Strategy 3: Shared decision-making tool
Another tertiary care center in Boston evaluated the effects of a shared decision-making tool on opioid discharge prescribing after CD.15 The intervention consisted of a 10-minute clinician-facilitated session incorporating:
- education around anticipated patterns of postoperative pain
- expected outpatient opioid use after CD
- risks and benefits of opioids and nonopioids
- education around opioid disposal and access to refills.
Among the 50 women enrolled in the study, the number of oxycodone 5-mg tablets prescribed at discharge decreased from the institutional standard of 40 to 20. Ninety percent of women reported being satisfied or very satisfied with their pain control, while only 4 of 50 women required an opioid refill. A follow-up quality improvement project, which implemented the shared decision-making model along with a standardized multimodal pain management protocol, demonstrated a similar decrease in the quantity of opioids prescribed at discharge.13
Continue to: Change is here to stay: A new culture of postpartum analgesia...
Change is here to stay: A new culture of postpartum analgesia
The CDC continues to champion responsible opioid prescribing, while ACOG advocates for a reassessment of the way that opioids are utilized postpartum. The majority of women in the United States, however, continue to receive opioids after both vaginal delivery and CD. Consciously or not, we clinicians may be contributing to an outdated tradition that is potentially harmful both to patients and society. Reproducible strategies exist to reduce opioid use without compromising pain control or overwhelming clinicians with phone calls. It is time to embrace the change.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
- Wong CA, Girard T. Undertreated or overtreated? Opioids for postdelivery analgesia. Br J Anaesth. 2018;121:339-342.
- Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87-94.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1- 353.e18.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36-41.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 742: postpartum pain management. Obstet Gynecol. 2018;132:e35-e43.
- Mills JR, Huizinga MM, Robinson SB, et al. Draft opioid prescribing guidelines for uncomplicated normal spontaneous vaginal birth. Obstet Gynecol. 2019;133:81-90.
- Chang AK, Bijur PE, Esses D, et al. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318:1661-1667.
- Mitchell A, van Zanten SV, Inglis K, et al. A randomized controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine after outpatient general surgery. J Am Coll Surg. 2008;206:472-479.
- Mitchell A, McCrea P, Inglis K, et al. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19:3792-3800.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97.
- Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
- Prabhu M, Dubois H, James K, et al. Implementation of a quality improvement initiative to decrease opioid prescribing after cesarean delivery. Obstet Gynecol. 2018;132:631-636.
- Rogers RG, Nix M, Chipman Z, et al. Decreasing opioid use postpartum: a quality improvement initiative. Obstet Gynecol. 2019;134:932-940.
- Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42-46.
- Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. www.cdc.gov/ drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed December 31, 2019.
Lofexidine: An option for treating opioid withdrawal
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
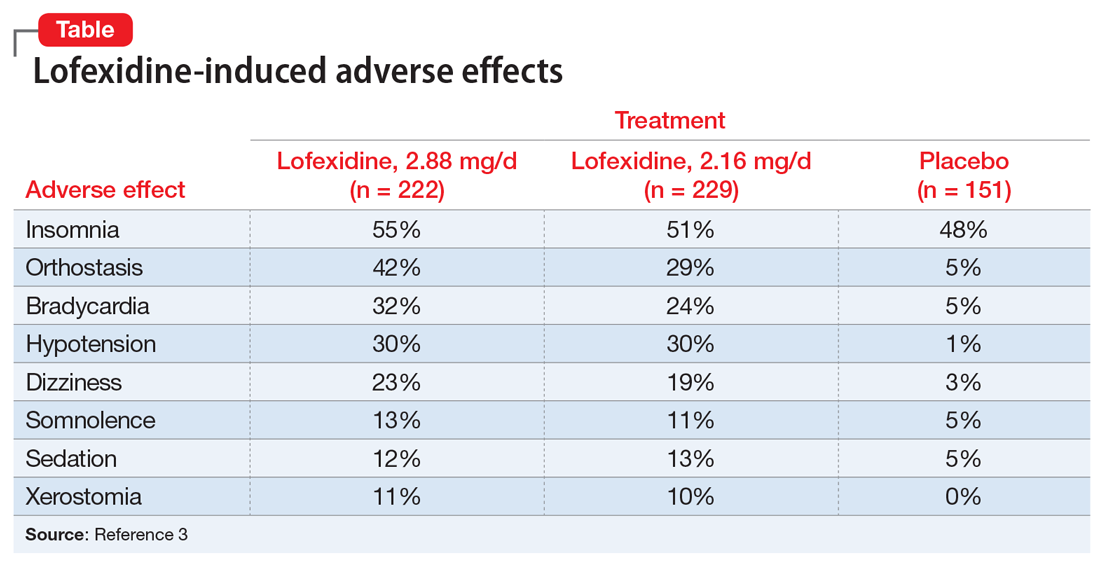
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).

Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).

Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
In addiction, abusive partners can wreak havoc
Gender-based violence could be driver of opioid epidemic, expert suggests
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
Gender-based violence could be driver of opioid epidemic, expert suggests
Gender-based violence could be driver of opioid epidemic, expert suggests
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
REPORTING FROM AAAP 2019
Survey: Cancer-related pain, opioid use up since 2018
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Depression linked to persistent opioid use after hysterectomy
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
VANCOUVER –
Women with depression had an 8% increased risk of perioperative opioid use but a 43% increased risk of persistent use, defined as at least one perioperative prescription followed by at least one prescription 90 days or longer after surgery.
Opioid prescriptions after surgery have been on the rise in recent years, and this has led to a focus on how chronic pain disorders are managed. But studies have shown that patients undergoing general surgery, both minor and major, are at increased risk of persistent opioid use, even after a single surgery, according to Erin Carey, MD, director of the division of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, who presented the research at the meeting sponsored by AAGL.
“We also know that preoperative depression has been linked to adverse outcomes after hysterectomy, both acute postoperative pain in the first 2 days after surgery, and increasing the risk of chronic postoperative pain,” Dr. Carey said.
That prompted her and her team to look at whether preoperative depression might influence the risk of new persistent opioid use after hysterectomy. They analyzed data from the IBM Watson/Truven Health Analytics MarketScan database of claims-based data, which collects information from a variety of sources, including electronic medical records and workplace records such as absences, disability, and long-term disability.
“So it does allow for long-term tracking, which makes it optimal for this type of study,” said Dr. Carey.
The study included 382,078 hysterectomies performed between 2001 and 2015 on women who had continuous prescription plans 180 days before to 180 days after the procedure, excluding anyone who had an opioid prescription in the previous 180 days; 60% of the procedures were minimally invasive. About 20% of women were considered to have depression before the procedure, based on a diagnosis (55%), an antidepressant prescription (22%), or both (23%).
There were some differences at baseline between the two populations: Women with preoperative depression were more likely to have a comorbid pain disorder, compared with patients without depression (20% vs. 14%), another psychiatric disorder (2% vs. less than 1%), and a Charlson comorbidity (12% vs. 9%). They also were less likely to undergo a minimally invasive procedure than women without depression (66% vs. 79%). There was an increase in the prevalence of depression over time, from 16% to 23%.
Overall, 74% of women were prescribed an opioid during the perioperative period; 17% were filled before the hysterectomy was performed. Preoperative fills also increased over time, from 4% in 2001 to 21% in 2015.
Women with preoperative depression were at a slightly greater risk for perioperative opioid use (risk ratio, 1.08), but a greater risk for persistent postoperative opioid use (11% vs. 8%; RR, 1.43). The heightened risk for opioid use was similar whether the surgery was performed on an outpatient or inpatient basis.
The presence of other comorbidities in women with diagnosed depression or prescribed antidepressants complicates the findings, according to Dr. Carey. “There may be additional chronic pain factors that are confounding this data, but it is consistent with other data that de novo postoperative opioid dependence may be a higher risk for these patients, so it’s important for us to look at that critically.”
Dr. Carey has been a consultant for Teleflex Medical and a speaker for Med-IQ.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Pediatricians uniquely qualified to treat adolescents with opioid use disorder
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
EXPERT ANALYSIS FROM AAP 19
Opioid reduction works after minimally invasive gynecologic surgery
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Fentanyl-related deaths show strong regional pattern
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.
Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.

Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.

Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.









