User login
Chronic opioid use linked to low testosterone levels
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
REPORTING FROM ENDO 2019
Direct pharmacy dispensing of naloxone linked to drop in fatal overdoses
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
investigators reported.
By contrast, state laws that stopped short of allowing pharmacists to directly dispense the opioid antagonist did not appear to impact mortality, according to the report, which appears in JAMA Internal Medicine (2019 May 6. doi: 10.1001/jamainternmed.2019.0272).
The report, based on state-level trends tracked from 2005 to 2016, indicates that fatal opioid overdoses fell by nearly one-third in states that adopted direct dispensing laws as compared with states that adopted other naloxone laws.
That finding suggests that the policy type determines whether a naloxone law is useful in combating fatal opioid overdoses, said Rahi Abouk, PhD, of William Paterson University, Wayne, N.J. and co-authors of the paper.
“Enabling distribution through various sources, or requiring gatekeepers, will not be as beneficial,” Dr. Abouk and co-authors said in their report.
The current rate of deaths from fentanyl, heroin, and prescription analgesic overdose has outpaced all previous drug epidemics on record, and even surpasses the number of deaths in the peak year of the HIV epidemic of the 1980s, Dr. Abouk and colleagues wrote in their paper.
The number of states with naloxone access laws grew from just 2 in 2005 to 47 by 2016, including 9 states that granted direct authority to pharmacists and 38 that granted indirect authority, according to the researchers.
The analysis of overdose trends from 2005 to 2016 was based on naloxone distribution data from state Medicaid agencies and opioid-related mortality data from a national statistics system. Forty percent of nonelderly adults with an opioid addiction are covered by Medicaid, the researchers said.
They found that naloxone laws granting pharmacists direct dispensing authority were linked to a drop in opioid deaths that increased in magnitude over time, according to researchers. The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws, relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Emergency department visits related to opioids increased by 15% in direct authority states 3 or more years after adoption, as compared to states that did not adopt direct authority laws. According to investigators, that translated into 15 additional opioid-related emergency department visits each month.
That increase suggests that, alongside direct dispensing laws, “useful interventions” and connections to treatment are needed for the emergency department, according to Dr. Abouk and colleagues.
“This is the location where such programs may be the most effective,” they said in their report.
Future research should be done to determine whether removing gatekeepers increases the value of naloxone distribution policies, they concluded in the report.
Dr. Abouk had no disclosures. Co-authors on the study reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
SOURCE: Abouk R, et al. JAMA Intern Med. 2019 May 6. doi:10.1001/jamainternmed.2019.0272.
FROM JAMA Internal Medicine
Key clinical point: State laws granting pharmacists direct authority to dispense naloxone were linked to significant drops in opioid-related fatal overdoses.
Major finding: The mean number of opioid deaths dropped by 27% in the second year after adoption of direct authority laws relative to opioid deaths in states with indirect access laws, while in subsequent years, deaths dropped by 34%.
Study details: Analysis of naloxone distribution data and opioid-related mortality data from 2005 to 2016 for all 50 states and the District of Columbia.
Disclosures: Study authors reported funding and conflict of interest disclosures related to the National Institute on Drug Abuse and the Centers for Disease Control and Prevention.
Source: Abouk R, et al. JAMA Intern Med. 2019 May 6.
Only 1.5% of individuals at high risk of opioid overdose receive naloxone
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
The vast majority of individuals at high risk for opioid overdose do not receive naloxone, despite numerous opportunities, according to Sarah Follman and associates from the University of Chicago.
In a retrospective study published in JAMA Network Open, the study authors analyzed data from individuals in the Truven Health MarketScan Research Database who had ICD-10 codes related to opioid use, misuse, dependence, and overdose. Data from Oct. 1, 2015, through Dec. 31, 2016, were included; a total of 138,108 high-risk individuals were identified as interacting with the health care system nearly 1.2 million times (88,618 hospitalizations, 229,680 ED visits, 298,058 internal medicine visits, and 568,448 family practice visits).
Of the 138,108 individuals in the study, only 2,135 (1.5%) were prescribed naloxone during the study period. Patients who had prior diagnoses of both opioid misuse/dependence and overdose were significantly more likely to receive naloxone than were those who only had a history of opioid dependence (odds ratio, 2.32; 95% confidence interval, 1.98-2.72; P less than .001). In addition, having a history of overdose alone was associated with a decreased chance of receiving naloxone, compared with those with a history of opioid misuse alone (OR, 0.73; 95% CI, 0.57-0.94; P = .01).
Other factors that significantly reduced the odds of receiving naloxone included being aged 30-44 years and being from the Midwest or West. Factors that reduced the odds include having received treatment for opioid use disorder, visiting a detoxification facility, receiving other substance use disorder treatment; and having received outpatient care from a pain specialist, psychologist, or surgeon.
“Most individuals at high risk of opioid overdose do not receive naloxone through direct prescribing,” Ms. Follman and associates wrote. “Clinicians can address this gap by regularly prescribing naloxone to eligible patients. To address barriers to prescribing, hospital systems and medical schools can support clinicians by improving education on screening and treating substance use disorders, clarifying legal concerns, and developing policies and protocols to guide implementation of increased prescribing.
No conflicts of interest were reported; one coauthor reported receiving a grant from the National Institutes of Health.
SOURCE: Follman S et al. JAMA Netw Open. 2019 May 3. doi: 10.1001/jamanetworkopen.2019.3209.
FROM JAMA NETWORK OPEN
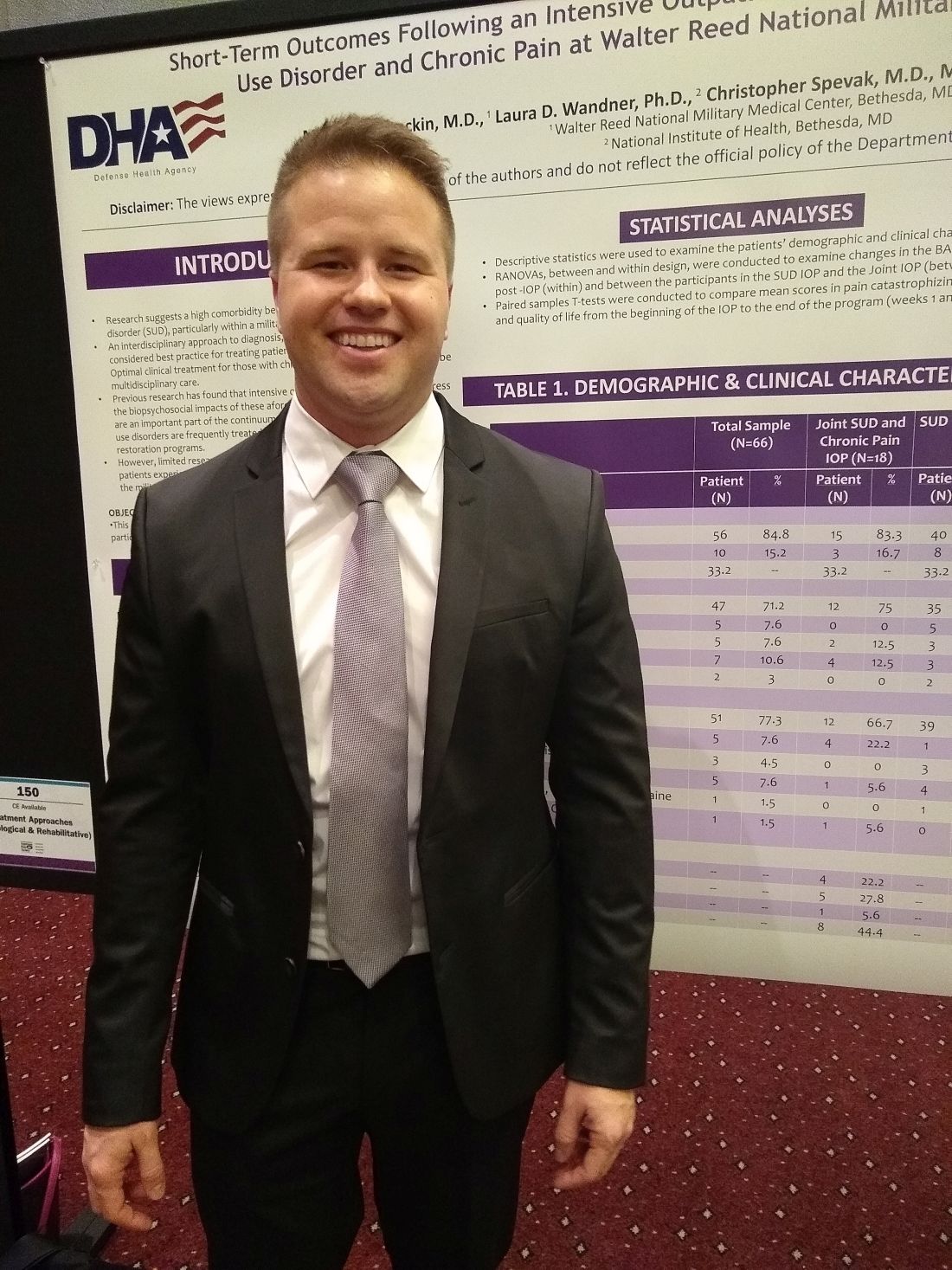
Outpatient program successfully tackles substance use and chronic pain
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
MILWAUKEE – An interdisciplinary intensive outpatient treatment program addressing chronic pain and substance use disorder effectively addressed both diagnoses in a military population.
Intensive outpatient programs (IOPs) frequently address these conditions within a biopsychosocial format, but it’s not common for IOPs to have this dual focus on chronic pain and substance use disorder (SUD), said Michael Stockin, MD, speaking in an interview at the scientific meeting of the American Pain Society.
Dr. Stockin said he and his collaborators recognized that, especially among a military population, the two conditions have considerable overlap, so it made sense to integrate behavioral treatment for both conditions in an intensive outpatient program. “Our hypothesis was that if you can use an intensive outpatient program to address substance use disorder, maybe you can actually add a chronic pain curriculum – like a functional restoration program to it.
“As a result of our study, we did find that there were significant differences in worst pain scores as a result of the program. In the people who took both the substance use disorder and chronic pain curriculum, we found significant reductions in total impairment, worst pain, and they also had less … substance use as well,” said Dr. Stockin.
In a quality improvement project, Dr. Stockin and collaborators compared short-term outcomes for patients who received IOP treatment addressing both chronic pain and SUD with those receiving SUD-only IOP.
For those participating in the joint IOP, scores indicating worst pain on the 0-10 numeric rating scale were reduced significantly, from 7.55 to 6.23 (P = .013). Scores on a functional measure of impairment, the Pain Outcomes Questionnaire Short Form (POQ-SF) also dropped significantly, from 84.92 to 63.50 (P = .034). The vitality domain of the POQ-SF also showed that patients had less impairment after participation in the joint IOP, with scores in that domain dropping from 20.17 to 17.25 (P = .024).
Looking at the total cohort, patient scores on the Brief Addiction Monitor (BAM) dropped significantly from baseline to the end of the intervention, indicating reduced substance use (P = .041). Mean scores for participants in the joint IOP were higher at baseline than for those in the SUD-only IOP (1.000 vs. 0.565). However, those participating in the joint IOP had lower mean postintervention BAM scores than the SUD-only cohort (0.071 vs. 0.174).
American veterans experience more severe pain and have a higher prevalence of chronic pain than nonveterans. Similarly, wrote Dr. Stockin, a chronic pain fellow in pain management at Walter Reed National Military Medical Center, Bethesda, Md., and colleagues in the poster presentation.
The project enrolled a total of 66 patients (10 female and 56 male). Of these, 18 participated in the joint SUD–chronic pain program, and 48 received usual treatment of the SUD-only IOP treatment. The mean overall age was 33.2 years, and 71.2% of participants were white.
Overall, 51 patients (77.3%) of participants had alcohol use disorder. Participants included active duty service members, veterans, and their dependents. Opioid and cannabis use disorders were experienced by a total of eight patients, and seven more patients had diagnoses of alcohol use disorder along with other substance use disorders.
All patients completed the BAM and received urine toxicology and alcohol breath testing at enrollment; drug and alcohol screening was completed at other points during the IOP treatment for both groups as well.
The joint IOP ran 3 full days a week, with a substance use curriculum in the morning and a pain management program in the afternoon; the SUD-only participants had three morning sessions weekly. Both interventions lasted 6 weeks, and Dr. Stockin said he and his colleagues would like to acquire longitudinal data to assess the durability of gains seen from the joint IOP.
The multidisciplinary team running the joint IOP was made up of an addiction/pain medicine physician, a clinical health psychologist, a physical therapist, social workers, and a nurse.
“This project is the first of its kind to find a significant reduction in pain burden while concurrently treating addiction and pain in an outpatient military health care setting,” Dr. Stockin and colleagues wrote in the poster accompanying the presentation.
“We had outcomes in both substance use and chronic pain that were positive, so it suggests that in the military health system, people may actually benefit from treating both chronic pain and substance use disorder concurrently. If you could harmonize those programs, you might be able to get good outcomes for soldiers and their families,” Dr. Stockin said.
Dr. Stockin reported no conflicts of interest. The project was funded by the Defense Health Agency.
REPORTING FROM APS 2019
Key clinical point: An intensive, 6-week joint substance use disorder and chronic pain intensive outpatient program significantly reduced both substance use and pain.
Major finding: Patients had less pain and reduced substance use after completing the program, compared with baseline (P = .013 and .041, respectively).
Study details: A quality improvement project including 66 patients at a military health facility.
Disclosures: The study was sponsored by the Defense Health Agency. Dr. Stockin reported no conflicts of interest.
Marijuana during prenatal OUD treatment increases premature birth
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
BALTIMORE – Marijuana is a not a good idea during pregnancy, and it’s an even worse idea when women are being treated for opioid addiction, according to an investigation from East Tennessee State University, Mountain Home.
Marijuana use may become more common as legalization rolls out across the country, and legalization, in turn, may add to the perception that pot is harmless, and maybe a good way to take the edge off during pregnancy and prevent morning sickness, said neonatologist Darshan Shaw, MD, of the department of pediatrics at the university.
Dr. Shaw wondered how that trend might impact treatment of opioid use disorder (OUD) during pregnancy, which has also become more common. The take-home is that “if you have a pregnant patient on medically assistant therapy” for opioid addition, “you should warn them against use of marijuana. It increases the risk of prematurity and low birth weight,” he said at the Pediatric Academic Societies annual meeting.
He and his team reviewed 2,375 opioid-exposed pregnancies at six hospitals in south-central Appalachia from July 2011 to June 2016. All of the women had used opioids during pregnancy, some illegally and others for opioid use disorder (OUD) treatment or other medical issues; 108 had urine screens that were positive for tetrahydrocannabinol (THC) at the time of delivery.
Infants were born a mean of 3 days earlier in the marijuana group, and a mean of 265 g lighter. They were also more likely to be born before 37 weeks’ gestation (14% versus 6.5%); born weighing less than 2,500 g (17.6% versus 7.3%); and more likely to be admitted to the neonatal ICU (17.5% versus 7.1%).
On logistic regression to control for parity, maternal status, and tobacco and benzodiazepine use, prenatal marijuana exposure more than doubled the risk of prematurity (odds ratio, 2.35; 95% confidence interval, 1.3-4.23); tobacco and benzodiazepines did not increase the risk. Marijuana also doubled the risk of low birth weight (OR, 2.02; 95% CI, 1.18-3.47), about the same as tobacco and benzodiazepines.
The study had limitations. There was no controlling for a major confounder: the amount of opioids woman took while pregnant. These data were not available, Dr. Shaw said.
Neonatal abstinence syndrome was more common in the marijuana group (33.3% versus 18.1%), so it’s possible that women who used marijuana also used more opioids. “We suspect that opioid exposure was not uniform among all infants,” he said. There were also no data on the amount or way marijuana was used.
Marijuana-positive women were more likely to be unmarried, nulliparous, and use tobacco and benzodiazepines.
There was no industry funding for the work, and Dr. Shaw had no disclosures.
REPORTING FROM PAS 2019
Key clinical point: Warn pregnant women being treated for opioid use disorder to stay away from marijuana.
Major finding: Marijuana use more than doubled the risk of prematurity and low birth weight.
Study details: Review of 2,375 opioid-exposed pregnancies at six hospitals
Disclosures: There was no industry funding for the work, and the lead investigator had no disclosures.
EU authorization recommended for buprenorphine implant
The European Medicines Agency announced April 26 that its human medicines committee has recommended granting a marketing authorization for Sixmo, a long-lasting implant delivering buprenorphine as treatment for opioid use disorder (OUD).
This recommendation is a step toward making the product available to patients with OUD in the European Union, according to a press release from the EMA. Safety and efficacy of the implant were studied in three trials with a total of 628 patients.
Standard treatment of OUD includes psychological and social counseling, as well as substitution opioid therapy – such as methadone or buprenorphine. The Sixmo implant involves four small rods implanted in the patient’s upper arm under local anesthetic.
The most common adverse events associated with the medicine were in keeping with the known events associated with buprenorphine – headache, constipation, and insomnia. Insertion and removal were associated with pain, severe itching, and hematoma at the implant site.
The full release can be found on the EMA website.
The European Medicines Agency announced April 26 that its human medicines committee has recommended granting a marketing authorization for Sixmo, a long-lasting implant delivering buprenorphine as treatment for opioid use disorder (OUD).
This recommendation is a step toward making the product available to patients with OUD in the European Union, according to a press release from the EMA. Safety and efficacy of the implant were studied in three trials with a total of 628 patients.
Standard treatment of OUD includes psychological and social counseling, as well as substitution opioid therapy – such as methadone or buprenorphine. The Sixmo implant involves four small rods implanted in the patient’s upper arm under local anesthetic.
The most common adverse events associated with the medicine were in keeping with the known events associated with buprenorphine – headache, constipation, and insomnia. Insertion and removal were associated with pain, severe itching, and hematoma at the implant site.
The full release can be found on the EMA website.
The European Medicines Agency announced April 26 that its human medicines committee has recommended granting a marketing authorization for Sixmo, a long-lasting implant delivering buprenorphine as treatment for opioid use disorder (OUD).
This recommendation is a step toward making the product available to patients with OUD in the European Union, according to a press release from the EMA. Safety and efficacy of the implant were studied in three trials with a total of 628 patients.
Standard treatment of OUD includes psychological and social counseling, as well as substitution opioid therapy – such as methadone or buprenorphine. The Sixmo implant involves four small rods implanted in the patient’s upper arm under local anesthetic.
The most common adverse events associated with the medicine were in keeping with the known events associated with buprenorphine – headache, constipation, and insomnia. Insertion and removal were associated with pain, severe itching, and hematoma at the implant site.
The full release can be found on the EMA website.
Medical cannabis relieved pain, decreased opioid use in elderly
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
FROM AAN 2019
Deadly overlap of fentanyl and stimulants on the rise
Rates of a potentially deadly overlap between use of nonprescribed fentanyl and use of either cocaine or methamphetamine have been increasing, a cross-sectional study of 1 million urine drug tests shows.
Leah LaRue, PharmD, of Millennium Health in San Diego, and colleagues performed the study, which sampled 1 million urine drug tests submitted by health care professionals “as part of routine care” during Jan. 1, 2013–Sept. 30, 2018. They isolated tests that were positive for either cocaine or methamphetamine – but not positive for both – and then determined how many in each group were also positive for nonprescribed fentanyl. Their analyses showed that the rate of cocaine-positive tests that also were positive for nonprescribed fentanyl increased from 0.9% in 2013 (n = 84; 95% confidence interval, 0.7%-1.1%) to 17.6% in 2018 (n = 427; 95% CI, 16.1%-19.1%), an increase of 1,850% (P less than .001). The rate of methamphetamine-positive tests that also were positive for nonprescribed fentanyl also started at 0.9% in 2013 (n = 29; 95% CI, 0.6%-1.2%) but rose to 7.9% in 2018 (n = 344; 95% CI, 7.1%-8.7%, a 798% increase (P less than .001). The study was published in JAMA Network Open.
The investigators suggested two explanations for these increases: intentional combination of drugs for “speedball effects” of combining stimulants and depressants and/or unintentional exposure on the part of users through contamination of substances. There have been increases in both cocaine-related and methamphetamine-related deaths, and the investigators of this study suspect these increases could be explained in part by overlap with opioids such as fentanyl. Part of the overdose risk inherent in these combinations is that, as the stimulant wears off, the fentanyl increasingly depresses the respiratory system, according to investigators; alternatively, opioid-naive stimulant users might be exposed to high levels of fentanyl with no opioid tolerance, which also can lead to overdose.
The study’s limitations include how samples were submitted – by health care professionals as part of routine care – and the possibility that individuals’ list of prescribed medications could have been incomplete or inaccurate such that the presence of prescribed fentanyl was counted as nonprescribed.
“The combination of nonprescribed fentanyl with cocaine or methamphetamine places an individual at increased risk of overdose,” they concluded.
[email protected]
SOURCE: LaRue L et al. JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2851.
Rates of a potentially deadly overlap between use of nonprescribed fentanyl and use of either cocaine or methamphetamine have been increasing, a cross-sectional study of 1 million urine drug tests shows.
Leah LaRue, PharmD, of Millennium Health in San Diego, and colleagues performed the study, which sampled 1 million urine drug tests submitted by health care professionals “as part of routine care” during Jan. 1, 2013–Sept. 30, 2018. They isolated tests that were positive for either cocaine or methamphetamine – but not positive for both – and then determined how many in each group were also positive for nonprescribed fentanyl. Their analyses showed that the rate of cocaine-positive tests that also were positive for nonprescribed fentanyl increased from 0.9% in 2013 (n = 84; 95% confidence interval, 0.7%-1.1%) to 17.6% in 2018 (n = 427; 95% CI, 16.1%-19.1%), an increase of 1,850% (P less than .001). The rate of methamphetamine-positive tests that also were positive for nonprescribed fentanyl also started at 0.9% in 2013 (n = 29; 95% CI, 0.6%-1.2%) but rose to 7.9% in 2018 (n = 344; 95% CI, 7.1%-8.7%, a 798% increase (P less than .001). The study was published in JAMA Network Open.
The investigators suggested two explanations for these increases: intentional combination of drugs for “speedball effects” of combining stimulants and depressants and/or unintentional exposure on the part of users through contamination of substances. There have been increases in both cocaine-related and methamphetamine-related deaths, and the investigators of this study suspect these increases could be explained in part by overlap with opioids such as fentanyl. Part of the overdose risk inherent in these combinations is that, as the stimulant wears off, the fentanyl increasingly depresses the respiratory system, according to investigators; alternatively, opioid-naive stimulant users might be exposed to high levels of fentanyl with no opioid tolerance, which also can lead to overdose.
The study’s limitations include how samples were submitted – by health care professionals as part of routine care – and the possibility that individuals’ list of prescribed medications could have been incomplete or inaccurate such that the presence of prescribed fentanyl was counted as nonprescribed.
“The combination of nonprescribed fentanyl with cocaine or methamphetamine places an individual at increased risk of overdose,” they concluded.
[email protected]
SOURCE: LaRue L et al. JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2851.
Rates of a potentially deadly overlap between use of nonprescribed fentanyl and use of either cocaine or methamphetamine have been increasing, a cross-sectional study of 1 million urine drug tests shows.
Leah LaRue, PharmD, of Millennium Health in San Diego, and colleagues performed the study, which sampled 1 million urine drug tests submitted by health care professionals “as part of routine care” during Jan. 1, 2013–Sept. 30, 2018. They isolated tests that were positive for either cocaine or methamphetamine – but not positive for both – and then determined how many in each group were also positive for nonprescribed fentanyl. Their analyses showed that the rate of cocaine-positive tests that also were positive for nonprescribed fentanyl increased from 0.9% in 2013 (n = 84; 95% confidence interval, 0.7%-1.1%) to 17.6% in 2018 (n = 427; 95% CI, 16.1%-19.1%), an increase of 1,850% (P less than .001). The rate of methamphetamine-positive tests that also were positive for nonprescribed fentanyl also started at 0.9% in 2013 (n = 29; 95% CI, 0.6%-1.2%) but rose to 7.9% in 2018 (n = 344; 95% CI, 7.1%-8.7%, a 798% increase (P less than .001). The study was published in JAMA Network Open.
The investigators suggested two explanations for these increases: intentional combination of drugs for “speedball effects” of combining stimulants and depressants and/or unintentional exposure on the part of users through contamination of substances. There have been increases in both cocaine-related and methamphetamine-related deaths, and the investigators of this study suspect these increases could be explained in part by overlap with opioids such as fentanyl. Part of the overdose risk inherent in these combinations is that, as the stimulant wears off, the fentanyl increasingly depresses the respiratory system, according to investigators; alternatively, opioid-naive stimulant users might be exposed to high levels of fentanyl with no opioid tolerance, which also can lead to overdose.
The study’s limitations include how samples were submitted – by health care professionals as part of routine care – and the possibility that individuals’ list of prescribed medications could have been incomplete or inaccurate such that the presence of prescribed fentanyl was counted as nonprescribed.
“The combination of nonprescribed fentanyl with cocaine or methamphetamine places an individual at increased risk of overdose,” they concluded.
[email protected]
SOURCE: LaRue L et al. JAMA Netw Open. 2019 Apr 26. doi: 10.1001/jamanetworkopen.2019.2851.
FROM jama network open
CDC warns against misuse of opioid-prescribing guideline
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
FDA approves generic naloxone spray for opioid overdose treatment
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.