User login
CDC clarifies opioid prescribing guidelines in cancer, sickle cell disease
Officials at the Centers for Disease Control and Prevention have clarified the agency’s guidelines on opioid prescribing after a trio of organizations raised concerns that insurers were inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The CDC guidelines, released in March 2016, address when to initiate or continue opioids for chronic pain, opioid selection, dosage, duration, follow-up, and discontinuation, and assess risk and harms of opioid use. Although the guidelines clearly state they are intended for clinicians prescribing opioids outside of active cancer treatment, insurance companies are still applying the guidelines to opioid coverage decisions for patients with active cancer, according to a Feb. 13, 2019, letter sent to the CDC from leaders at the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology.
Additionally, the associations wrote that the CDC’s recommendations pose coverage problems for sickle cell patients and select groups of cancer survivors who may benefit from opioids for pain management. The groups asked the CDC to issue a clarification to ensure appropriate implementation of the opioid recommendations.
In a Feb. 28, 2019, letter to ASCO, NCCN, and ASH, Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control took note of the concerns, clarifying that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
The CDC guidance may apply to cancer survivors in certain conditions, Dr. Dowell wrote, namely when survivors experience chronic pain after cancer treatment completion, are in clinical remission, and are under cancer surveillance only. However, she agreed that, for select groups of cancer survivors with persistent pain caused by past cancer, the ratio of opioid benefits to risks for chronic pain is unique. She referred health providers to guidelines by ASCO on chronic pain management for adult cancer survivors and NCCN guidance on managing adult cancer pain when considering opioids for pain control in such populations.
Special considerations in sickle cell disease may also change the balance of opioid risks to benefits for pain management, Dr. Dowell wrote, referring providers and insurers to additional guidance on sickle cell disease from the National Institute of Health when making treatment and reimbursement decisions.
“Clinical decision making should be based on the relationship between the clinician and patient, with an understanding of the patient’s clinical situation, functioning, and life context, as well as careful consideration of the benefits and risk of all treatment options, including opioid therapy,” Dr. Dowell wrote. “CDC encourages physicians to continue using their clinical judgment and base treatment on what they know about their patients, including the use of opioids if determined to be the best course of treatment.”
Clifford A. Hudis, MD, CEO of ASCO, praised the clarification, calling the letter necessary to clear up confusion and prevent inappropriate coverage decisions.
“This clarification from CDC is critically important because, while the agency’s guideline clearly states that it is not intended to apply to patients during active cancer and sickle cell disease treatment, many payers have been inappropriately using it to make opioid coverage determinations for those exact populations,” Dr. Hudis said in a statement.
Sickle cell patients suffer from severe, chronic pain, which is debilitating on its own without the added burden of having to constantly appeal coverage denials, added ASH President Roy Silverstein, MD.
“We appreciate CDC’s acknowledgment that the challenges of managing severe and chronic pain in conditions, such as sickle cell disease, require special consideration, and we hope payers will take the CDC’s clarification into account to ensure that patients’ pain management needs are covered,” he said in the same statement.
Officials at the Centers for Disease Control and Prevention have clarified the agency’s guidelines on opioid prescribing after a trio of organizations raised concerns that insurers were inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The CDC guidelines, released in March 2016, address when to initiate or continue opioids for chronic pain, opioid selection, dosage, duration, follow-up, and discontinuation, and assess risk and harms of opioid use. Although the guidelines clearly state they are intended for clinicians prescribing opioids outside of active cancer treatment, insurance companies are still applying the guidelines to opioid coverage decisions for patients with active cancer, according to a Feb. 13, 2019, letter sent to the CDC from leaders at the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology.
Additionally, the associations wrote that the CDC’s recommendations pose coverage problems for sickle cell patients and select groups of cancer survivors who may benefit from opioids for pain management. The groups asked the CDC to issue a clarification to ensure appropriate implementation of the opioid recommendations.
In a Feb. 28, 2019, letter to ASCO, NCCN, and ASH, Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control took note of the concerns, clarifying that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
The CDC guidance may apply to cancer survivors in certain conditions, Dr. Dowell wrote, namely when survivors experience chronic pain after cancer treatment completion, are in clinical remission, and are under cancer surveillance only. However, she agreed that, for select groups of cancer survivors with persistent pain caused by past cancer, the ratio of opioid benefits to risks for chronic pain is unique. She referred health providers to guidelines by ASCO on chronic pain management for adult cancer survivors and NCCN guidance on managing adult cancer pain when considering opioids for pain control in such populations.
Special considerations in sickle cell disease may also change the balance of opioid risks to benefits for pain management, Dr. Dowell wrote, referring providers and insurers to additional guidance on sickle cell disease from the National Institute of Health when making treatment and reimbursement decisions.
“Clinical decision making should be based on the relationship between the clinician and patient, with an understanding of the patient’s clinical situation, functioning, and life context, as well as careful consideration of the benefits and risk of all treatment options, including opioid therapy,” Dr. Dowell wrote. “CDC encourages physicians to continue using their clinical judgment and base treatment on what they know about their patients, including the use of opioids if determined to be the best course of treatment.”
Clifford A. Hudis, MD, CEO of ASCO, praised the clarification, calling the letter necessary to clear up confusion and prevent inappropriate coverage decisions.
“This clarification from CDC is critically important because, while the agency’s guideline clearly states that it is not intended to apply to patients during active cancer and sickle cell disease treatment, many payers have been inappropriately using it to make opioid coverage determinations for those exact populations,” Dr. Hudis said in a statement.
Sickle cell patients suffer from severe, chronic pain, which is debilitating on its own without the added burden of having to constantly appeal coverage denials, added ASH President Roy Silverstein, MD.
“We appreciate CDC’s acknowledgment that the challenges of managing severe and chronic pain in conditions, such as sickle cell disease, require special consideration, and we hope payers will take the CDC’s clarification into account to ensure that patients’ pain management needs are covered,” he said in the same statement.
Officials at the Centers for Disease Control and Prevention have clarified the agency’s guidelines on opioid prescribing after a trio of organizations raised concerns that insurers were inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The CDC guidelines, released in March 2016, address when to initiate or continue opioids for chronic pain, opioid selection, dosage, duration, follow-up, and discontinuation, and assess risk and harms of opioid use. Although the guidelines clearly state they are intended for clinicians prescribing opioids outside of active cancer treatment, insurance companies are still applying the guidelines to opioid coverage decisions for patients with active cancer, according to a Feb. 13, 2019, letter sent to the CDC from leaders at the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology.
Additionally, the associations wrote that the CDC’s recommendations pose coverage problems for sickle cell patients and select groups of cancer survivors who may benefit from opioids for pain management. The groups asked the CDC to issue a clarification to ensure appropriate implementation of the opioid recommendations.
In a Feb. 28, 2019, letter to ASCO, NCCN, and ASH, Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control took note of the concerns, clarifying that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
The CDC guidance may apply to cancer survivors in certain conditions, Dr. Dowell wrote, namely when survivors experience chronic pain after cancer treatment completion, are in clinical remission, and are under cancer surveillance only. However, she agreed that, for select groups of cancer survivors with persistent pain caused by past cancer, the ratio of opioid benefits to risks for chronic pain is unique. She referred health providers to guidelines by ASCO on chronic pain management for adult cancer survivors and NCCN guidance on managing adult cancer pain when considering opioids for pain control in such populations.
Special considerations in sickle cell disease may also change the balance of opioid risks to benefits for pain management, Dr. Dowell wrote, referring providers and insurers to additional guidance on sickle cell disease from the National Institute of Health when making treatment and reimbursement decisions.
“Clinical decision making should be based on the relationship between the clinician and patient, with an understanding of the patient’s clinical situation, functioning, and life context, as well as careful consideration of the benefits and risk of all treatment options, including opioid therapy,” Dr. Dowell wrote. “CDC encourages physicians to continue using their clinical judgment and base treatment on what they know about their patients, including the use of opioids if determined to be the best course of treatment.”
Clifford A. Hudis, MD, CEO of ASCO, praised the clarification, calling the letter necessary to clear up confusion and prevent inappropriate coverage decisions.
“This clarification from CDC is critically important because, while the agency’s guideline clearly states that it is not intended to apply to patients during active cancer and sickle cell disease treatment, many payers have been inappropriately using it to make opioid coverage determinations for those exact populations,” Dr. Hudis said in a statement.
Sickle cell patients suffer from severe, chronic pain, which is debilitating on its own without the added burden of having to constantly appeal coverage denials, added ASH President Roy Silverstein, MD.
“We appreciate CDC’s acknowledgment that the challenges of managing severe and chronic pain in conditions, such as sickle cell disease, require special consideration, and we hope payers will take the CDC’s clarification into account to ensure that patients’ pain management needs are covered,” he said in the same statement.
Treating the pregnant patient with opioid addiction
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
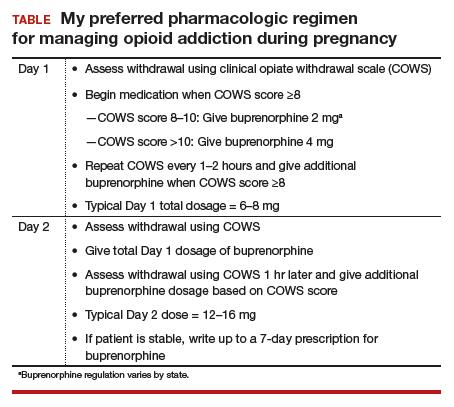
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
OBG Management : How has the opioid crisis affected women in general?
Mishka Terplan, MD: Everyone is aware that we are experiencing a massive opioid crisis in the United States, and from a historical perspective, this is at least the third or fourth significant opioid epidemic in our nation’s history.1 It is similar in some ways to the very first one, which also featured a large proportion of women and also was driven initially by physician prescribing practices. However, the magnitude of this crisis is unparalleled compared with prior opioid epidemics.
There are lots of reasons why women are overrepresented in this crisis. There are gender-based differences in pain—chronic pain syndromes are more common in women. In addition, we have a gender bias in prescribing opioids and prescribe more opioids to women (especially older women) than to men. Cultural differences also contribute. As providers, we tend not to think of women as people who use drugs or people who develop addictions the same way as we think of these risks and behaviors for men. Therefore, compared with men, we are less likely to screen, assess, or refer women for substance use, misuse, and addiction. All of this adds up to creating a crisis in which women are increasingly the face of the epidemic.
OBG Management : What are the concerns about opioid addiction and pregnant women specifically?
Dr. Terplan: Addiction is a chronic condition, just like diabetes or depression, and the same principles that we think of in terms of optimizing maternal and newborn health apply to addiction. Ideally, we want, for women with chronic diseases to have stable disease at the time of conception and through pregnancy. We know this maximizes birth outcomes.
Unfortunately, there is a massive treatment gap in the United States. Most people with addiction receive no treatment. Only 11% of people with a substance use disorder report receipt of treatment. By contrast, more than 70% of people with depression, hypertension, or diabetes receive care. This treatment gap is also present in pregnancy. Among use disorders, treatment receipt is highest for opioid use disorder; however, nationally, at best, 25% of pregnant women with opioid addiction receive any care.
In other words, when we encounter addiction clinically, it is often untreated addiction. Therefore, many times providers will have women presenting to care who are both pregnant and have untreated addiction. From both a public health and a clinical practice perspective, the salient distinction is not between people with addiction and those without but between people with treated disease and people with untreated disease.
Untreated addiction is a serious medical condition. It is associated with preterm delivery and low birth weight infants. It is associated with acquisition and transmission of HIV and hepatitis C. It is associated with overdose and overdose death. By contrast, treated addiction is associated with term delivery and normal weight infants. Pharmacotherapies for opioid use disorder stabilize the intrauterine environment and allow for normal fetal growth. Pharmacotherapies for opioid use disorder help to structure and stabilize the mom’s social circumstance, providing a platform to deliver prenatal care and essential social services. And pharmacotherapies for opioid use disorder protect women and their fetuses from overdose and from overdose deaths. The goal of management of addiction in pregnancy is treatment of the underlying condition, treating the addiction.
Continue to: OBG Management...
OBG Management : What should the ObGyn do when faced with a patient who might have an addiction?
Dr. Terplan: The good news is that there are lots of recently published guidance documents from the World Health Organization,2 the American College of Obstetricians and Gynecologists (ACOG),3 and the Substance Abuse and Mental Health Services Administration (SAMHSA),4 and there have been a whole series of trainings throughout the United States organized by both ACOG and SAMHSA.
There is also a collaboration between ACOG and the American Society of Addiction Medicine (ASAM) to provide buprenorphine waiver trainings specifically designed for ObGyns. Check both the ACOG and ASAM pages for details. I encourage every provider to get a waiver to prescribe buprenorphine. There are about 30 ObGyns who are also board certified in addiction medicine in the United States, and all of us are more than happy to help our colleagues in the clinical care of this population, a population that all of us really enjoy taking care of.
Although care in pregnancy is important, we must not forget about the postpartum period. Generally speaking, women do quite well during pregnancy in terms of treatment. Postpartum, however, is a vulnerable period, where relapse happens, where gaps in care happen, where child welfare involvement and sometimes child removal happens, which can be very stressful for anyone much less somebody with a substance use disorder. Recent data demonstrate that one of the leading causes of maternal mortality in the US in from overdose, and most of these deaths occur in the postpartum period.5 Regardless of what happens during pregnancy, it is essential that we be able to link and continue care for women with opioid use disorder throughout the postpartum period.
OBG Management : How do you treat opioid use disorder in pregnancy?
Dr. Terplan: The standard of care for treatment of opioid use disorder in pregnancy is pharmacotherapy with either methadone or buprenorphine (TABLE) plus behavioral counseling—ideally, co-located with prenatal care. The evidence base for pharmacotherapy for opioid use disorder in pregnancy is supported by every single professional society that has ever issued guidance on this, from the World Health Organization to ACOG, to ASAM, to the Royal College in the UK as well as Canadian and Australian obstetrics and gynecology societies; literally every single professional society supports medication.
The core principle of maternal fetal medicine rests upon the fact that chronic conditions need to be treated and that treated illness improves birth outcomes. For both maternal and fetal health, treated addiction is way better than untreated addiction. One concern people have regarding methadone and buprenorphine is the development of dependence. Dependence is a physiologic effect of medication and occurs with opioids, as well as with many other medications, such as antidepressants and most hypertensive agents. For the fetus, dependence means that at the time of delivery, the infant may go into withdrawal, which is also called neonatal abstinence syndrome. Neonatal abstinence syndrome is an expected outcome of in-utero opioid exposure. It is a time-limited and treatable condition. Prospective data do not demonstrate any long-term harms among infants whose mothers received pharmacotherapy for opioid use disorder during pregnancy.6
The treatment for neonatal abstinence syndrome is costly, especially when in a neonatal intensive care unit. It can be quite concerning to a new mother to have an infant that has to spend extra time in the hospital and sometimes be medicated for management of withdrawal.
There has been a renewed interest amongst ObGyns in investigating medically-supervised withdrawal during pregnancy. Although there are remaining questions, overall, the literature does not support withdrawal during pregnancy—mostly because withdrawal is associated with relapse, and relapse is associated with cessation of care (both prenatal care and addiction treatment), acquisition and transmission of HIV and Hepatitis C, and overdose and overdose death. The pertinent clinical and public health goal is the treatment of the chronic condition of addiction during pregnancy. The standard of care remains pharmacotherapy plus behavioral counseling for the treatment of opioid use disorder in pregnancy.
Clinical care, however, is both evidence-based and person-centered. All of us who have worked in this field, long before there was attention to the opioid crisis, all of us have provided medically-supervised withdrawal of a pregnant person, and that is because we understand the principles of care. When evidence-based care conflicts with person-centered care, the ethical course is the provision of person-centered care. Patients have the right of refusal. If someone wants to discontinue medication, I have tapered the medication during pregnancy, but continued to provide (and often increase) behavioral counseling and prenatal care.
Treated addiction is better for the fetus than untreated addiction. Untreated opioid addiction is associated with preterm birth and low birth weight. These obstetric risks are not because of the opioid per se, but because of the repeated cycles of withdrawal that an individual with untreated addiction experiences. People with untreated addiction are not getting “high” when they use, they are just becoming a little bit less sick. It is this repeated cycle of withdrawal that stresses the fetus, which leads to preterm delivery and low birth weight.
Medications for opioid use disorder are long-acting and dosed daily. In contrast to the repeated cycles of fetal withdrawal in untreated addiction, pharmacotherapy stabilizes the intrauterine environment. There is no cyclic, repeated, stressful withdrawal, and consequentially, the fetus grows normally and delivers at term. Obstetric risk is from repeated cyclic withdrawal more than from opioid exposure itself.
Continue to: OBG Management...
OBG Management : Research reports that women are not using all of the opioids that are prescribed to them after a cesarean delivery. What are the risks for addiction in this setting?
Dr. Terplan: I mark a distinction between use (ie, using something as prescribed) and misuse, which means using a prescribed medication not in the manner in which it was prescribed, or using somebody else’s medications, or using an illicit substance. And I differentiate use and misuse from addiction, which is a behavioral condition, a disease. There has been a lot of attention paid to opioid prescribing in general and in particular postdelivery and post–cesarean delivery, which is one of the most common operative procedures in the United States.
It seems clear from the literature that we have overprescribed opioids postdelivery, and a small number of women, about 1 in 300 will continue an opioid script.7 This means that 1 in 300 women who received an opioid prescription following delivery present for care and get another opioid prescription filled. Now, that is a small number at the level of the individual, but because we do so many cesarean deliveries, this is a large number of women at the level of the population. This does not mean, however, that 1 in 300 women who received opioids after cesarean delivery are going to become addicted to them. It just means that 1 in 300 will continue the prescription. Prescription continuation is a risk factor for opioid misuse, and opioid misuse is on the pathway toward addiction.
Most people who use substances do not develop an addiction to that substance. We know from the opioid literature that at most only 10% of people who receive chronic opioid therapy will meet criteria for opioid use disorder.8 Now 10% is not 100%, nor is it 0%, but because we prescribed so many opioids to so many people for so long, the absolute number of people with opioid use disorder from physician opioid prescribing is large, even though the risk at the level of the individual is not as large as people think.
OBG Management : From your experience in treating addiction during pregnancy, are there clinical pearls you would like to share with ObGyns?
Dr. Terplan: There are a couple of takeaways. One is that all women are motivated to maximize their health and that of their baby to be, and every pregnant woman engages in behavioral change; in fact most women quit or cutback substance use during pregnancy. But some can’t. Those that can’t likely have a substance use disorder. We think of addiction as a chronic condition, centered in the brain, but the primary symptoms of addiction are behaviors. The salient feature of addiction is continued use despite adverse consequences; using something that you know is harming yourself and others but you can’t stop using it. In other words, continuing substance use during pregnancy. When we see clinically a pregnant woman who is using a substance, 99% of the time we are seeing a pregnant woman who has the condition of addiction, and what she needs is treatment. She does not need to be told that injecting heroin is unsafe for her and her fetus, she knows that. What she needs is treatment.
The second point is that pregnant women who use drugs and pregnant women with addiction experience a real specific and strong form of discrimination by providers, by other people with addiction, by the legal system, and by their friends and families. Caring for people who have substance use disorder is grounded in human rights, which means treating people with dignity and respect. It is important for providers to have empathy, especially for pregnant people who use drugs, to counter the discrimination they experience from society and from other health care providers.
Continue to: OBG Management...
OBG Management : Are there specific ways in which ObGyns can show empathy when speaking with a pregnant woman who likely has addiction?
Dr. Terplan: In general when we talk to people about drug use, it is important to ask their permission to talk about it. For example, “Is it okay if I ask you some questions about smoking, drinking, and other drugs?” If someone says, “No, I don’t want you to ask those questions,” we have to respect that. Assessment of substance use should be a universal part of all medical care, as substance use, misuse, and addiction are essential domains of wellness, but I think we should ask permission before screening.
One of the really good things about prenatal care is that people come back; we have multiple visits across the gestational period. The behavioral work of addiction treatment rests upon a strong therapeutic alliance. If you do not respect your patient, then there is no way you can achieve a therapeutic alliance. Asking permission, and then respecting somebody’s answers, I think goes a really long way to establishing a strong therapeutic alliance, which is the basis of any medical care.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.
- Terplan M. Women and the opioid crisis: historical context and public health solutions. Fertil Steril. 2017;108:195-199.
- Management of substance abuse. World Health Organization website. https://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/. Accessed March 20, 2019.
- Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
- Substance Abuse and Mental Health Services Administration. Clinical guidance for treating pregnant and parenting women with opioid use disorder and their infants. HHS Publication No. (SMA) 18-5054. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- Metz TD, Royner P, Hoffman MC, et al. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128:1233-1240.
- Kaltenbach K, O’Grady E, Heil SH, et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40-49.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215:353.e1-353.e18.
FDA to expand opioid labeling with instructions on proper tapering
The Food and Drug Administration is making changes to opioid analgesic labeling to give better information to clinicians on how to properly taper patients dependent on opioid use, according to Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research.
, such as withdrawal symptoms, uncontrolled pain, and suicide. Both the FDA and the Centers for Disease Control and Prevention offer guidelines on how to properly taper opioids, Dr. Throckmorton said, but more needs to be done to ensure that patients are being provided with the correct advice and care.
The changes to the labels will include expanded information to health care clinicians and are intended to be used when both the clinician and patient have agreed to reduce the opioid dosage. When this is discussed, factors that should be considered include the dose of the drug, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient.
Other actions the FDA is pursuing to combat opioid use disorder include working with the National Academies of Sciences, Engineering, and Medicine on guidelines for the proper opioid analgesic prescribing for acute pain resulting from specific conditions or procedures, and advancing policies that make immediate-release opioid formulations available in fixed-quantity packaging for 1 or 2 days.
“The FDA remains committed to addressing the opioid crisis on all fronts, with a significant focus on decreasing unnecessary exposure to opioids and preventing new addiction; supporting the treatment of those with opioid use disorder; fostering the development of novel pain treatment therapies and opioids more resistant to abuse and misuse; and taking action against those involved in the illegal importation and sale of opioids,” Dr. Throckmorton said.
Find the full statement by Dr. Throckmorton on the FDA website.
The Food and Drug Administration is making changes to opioid analgesic labeling to give better information to clinicians on how to properly taper patients dependent on opioid use, according to Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research.
, such as withdrawal symptoms, uncontrolled pain, and suicide. Both the FDA and the Centers for Disease Control and Prevention offer guidelines on how to properly taper opioids, Dr. Throckmorton said, but more needs to be done to ensure that patients are being provided with the correct advice and care.
The changes to the labels will include expanded information to health care clinicians and are intended to be used when both the clinician and patient have agreed to reduce the opioid dosage. When this is discussed, factors that should be considered include the dose of the drug, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient.
Other actions the FDA is pursuing to combat opioid use disorder include working with the National Academies of Sciences, Engineering, and Medicine on guidelines for the proper opioid analgesic prescribing for acute pain resulting from specific conditions or procedures, and advancing policies that make immediate-release opioid formulations available in fixed-quantity packaging for 1 or 2 days.
“The FDA remains committed to addressing the opioid crisis on all fronts, with a significant focus on decreasing unnecessary exposure to opioids and preventing new addiction; supporting the treatment of those with opioid use disorder; fostering the development of novel pain treatment therapies and opioids more resistant to abuse and misuse; and taking action against those involved in the illegal importation and sale of opioids,” Dr. Throckmorton said.
Find the full statement by Dr. Throckmorton on the FDA website.
The Food and Drug Administration is making changes to opioid analgesic labeling to give better information to clinicians on how to properly taper patients dependent on opioid use, according to Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research.
, such as withdrawal symptoms, uncontrolled pain, and suicide. Both the FDA and the Centers for Disease Control and Prevention offer guidelines on how to properly taper opioids, Dr. Throckmorton said, but more needs to be done to ensure that patients are being provided with the correct advice and care.
The changes to the labels will include expanded information to health care clinicians and are intended to be used when both the clinician and patient have agreed to reduce the opioid dosage. When this is discussed, factors that should be considered include the dose of the drug, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient.
Other actions the FDA is pursuing to combat opioid use disorder include working with the National Academies of Sciences, Engineering, and Medicine on guidelines for the proper opioid analgesic prescribing for acute pain resulting from specific conditions or procedures, and advancing policies that make immediate-release opioid formulations available in fixed-quantity packaging for 1 or 2 days.
“The FDA remains committed to addressing the opioid crisis on all fronts, with a significant focus on decreasing unnecessary exposure to opioids and preventing new addiction; supporting the treatment of those with opioid use disorder; fostering the development of novel pain treatment therapies and opioids more resistant to abuse and misuse; and taking action against those involved in the illegal importation and sale of opioids,” Dr. Throckmorton said.
Find the full statement by Dr. Throckmorton on the FDA website.
NIH’s HEAL initiative seeks coordinated effort to tackle pain, addiction
MILWAUKEE – Congress has allocated a half billion dollars annually to the National Institutes of Health for a program that seeks to end America’s opioid crisis. The agency is putting in place over two-dozen projects spanning basic and translational research, clinical trials, and implementation of new strategies to address pain and fight addiction.
The , said Walter Koroshetz, MD, speaking at the scientific meeting of the American Pain Society. This represents carryover from 2018, a planning year for the initiative, along with the 2019 $500 million annual supplement to the NIH’s base appropriation.
In 2018, NIH and other federal agencies successfully convinced Congress that funding a coordinated use of resources was necessary to overcome the country’s dual opioid and chronic pain crises. “Luck happens to the prepared,” said Dr. Koroshetz, director of the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Md., adding that many hours went into putting together a national pain strategy that is multidisciplinary and multi-layered, and involves multiple players.
The two aims of research under the initiative are to improve treatments for misuse and addiction, and to enhance pain management. Focusing on this latter aim, Dr. Koroshetz said that the initiative has several research priorities to enhance pain management.
First, the biological basis for chronic pain needs to be understood in order to formulate effective therapies and interventions. “We need to understand the transition from acute to chronic pain,” he commented. “We need to see if we can learn about the risk factors for developing chronic pain; if we get really lucky, we might identify some biological markers” that identify who is at risk for this transition “in a high-risk acute pain situation.”
Next, a key request of industry and academia will be development of more drugs that avoid the dual-target program of opioids, which affect reward circuitry along with pain circuitry. “Drugs affecting the pain circuit and the reward circuit will always result in addiction” potential, said Dr. Koroshetz. “We’re still using drugs for pain from the poppy plant that were discovered 8,000 years ago.”
The hope with the HEAL initiative is to bring together academic centers with patient populations and research capabilities with industry, to accelerate moving nonaddictive treatments through to phase 3 trials.
The initiative also aims to promote discovery of new biologic targets for safe and effective pain treatment. New understanding of the physiology of pain has led to a multitude of candidate targets, said Dr. Koroshetz: “The good news is that there are so many potential targets. When I started in neurology in the ‘90s, I wouldn’t have said there were many, but now I’d say the list is long.”
Support for this work will require the development of human cell and tissue models, such as induced pluripotent stem cells, 3D printed organoids, and tissue chips. Several HEAL-funded grant mechanisms also seek research-industry collaboration to move investigational drugs for new targets through the pipeline quickly. The agency is hoping to see grantees apply new technologies, such as artificial intelligence, which can help identify new chemical structures and pinpoint new therapeutic targets for drug repurposing.
In addition to rapid drug discovery and accelerated clinical trials, Dr. Koroshetz said that HEAL leaders are hoping to see cross-pollination from two other NIH initiatives to boost pain-targeted medical device development. Both the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) and the Stimulating Peripheral Activity to Relieve Conditions (SPARC) initiatives have already shown promise in identifying targets for effective, noninvasive pain relief devices, he said. Technologies being developed from these programs are “truly amazing,” he added.
A new focus on data and asset sharing among industry, academia, and NIH will “improve the quality, consistency, and efficiency of early-phase pain clinical trials,” Dr. Koroshetz continued. The Early Phase Pain Investigation Clinical Network (EPPIC-Net) will coordinate data and biosample hosting.
Through a competitive submission process, EPPIC-net will review dossiers from institutions or consortia that can serve as assets around which clinical trials can be designed and executed. These early-phase trials will focus on well-defined pain conditions with unmet need, such as chronic regional pain syndrome and tic douloureux, he said.
“We want to find patients who have well-defined conditions. We know the phenotypes, we know the natural history. We’re looking for clinical sites to work on these projects as part of one large team to bring new therapies to patients,” noted Dr. Koroshetz.
Further along the spectrum of research, comparative effectiveness research networks will provide a reality check to compare both pharmacologic and nonpharmacologic interventions all along the spectrum from acute to chronic pain. Here, data elements and storage will also be coordinated through EPPIC-Net.
Implementation science research will fine-tune the practicalities of bringing research to practice as the final piece of the puzzle, said Dr. Koroshetz.
Under NIH director Francis Collins, MD, PhD, Dr. Koroshetz is co-leading the HEAL initiative, along with Nora Volkow, MD, director of the National Institute on Drug Abuse. They wrote about the initiative in JAMA last year (JAMA. 2018 Jul 10;320[2]:129-30).
Dr. Koroshetz reported no conflicts of interest.
MILWAUKEE – Congress has allocated a half billion dollars annually to the National Institutes of Health for a program that seeks to end America’s opioid crisis. The agency is putting in place over two-dozen projects spanning basic and translational research, clinical trials, and implementation of new strategies to address pain and fight addiction.
The , said Walter Koroshetz, MD, speaking at the scientific meeting of the American Pain Society. This represents carryover from 2018, a planning year for the initiative, along with the 2019 $500 million annual supplement to the NIH’s base appropriation.
In 2018, NIH and other federal agencies successfully convinced Congress that funding a coordinated use of resources was necessary to overcome the country’s dual opioid and chronic pain crises. “Luck happens to the prepared,” said Dr. Koroshetz, director of the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Md., adding that many hours went into putting together a national pain strategy that is multidisciplinary and multi-layered, and involves multiple players.
The two aims of research under the initiative are to improve treatments for misuse and addiction, and to enhance pain management. Focusing on this latter aim, Dr. Koroshetz said that the initiative has several research priorities to enhance pain management.
First, the biological basis for chronic pain needs to be understood in order to formulate effective therapies and interventions. “We need to understand the transition from acute to chronic pain,” he commented. “We need to see if we can learn about the risk factors for developing chronic pain; if we get really lucky, we might identify some biological markers” that identify who is at risk for this transition “in a high-risk acute pain situation.”
Next, a key request of industry and academia will be development of more drugs that avoid the dual-target program of opioids, which affect reward circuitry along with pain circuitry. “Drugs affecting the pain circuit and the reward circuit will always result in addiction” potential, said Dr. Koroshetz. “We’re still using drugs for pain from the poppy plant that were discovered 8,000 years ago.”
The hope with the HEAL initiative is to bring together academic centers with patient populations and research capabilities with industry, to accelerate moving nonaddictive treatments through to phase 3 trials.
The initiative also aims to promote discovery of new biologic targets for safe and effective pain treatment. New understanding of the physiology of pain has led to a multitude of candidate targets, said Dr. Koroshetz: “The good news is that there are so many potential targets. When I started in neurology in the ‘90s, I wouldn’t have said there were many, but now I’d say the list is long.”
Support for this work will require the development of human cell and tissue models, such as induced pluripotent stem cells, 3D printed organoids, and tissue chips. Several HEAL-funded grant mechanisms also seek research-industry collaboration to move investigational drugs for new targets through the pipeline quickly. The agency is hoping to see grantees apply new technologies, such as artificial intelligence, which can help identify new chemical structures and pinpoint new therapeutic targets for drug repurposing.
In addition to rapid drug discovery and accelerated clinical trials, Dr. Koroshetz said that HEAL leaders are hoping to see cross-pollination from two other NIH initiatives to boost pain-targeted medical device development. Both the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) and the Stimulating Peripheral Activity to Relieve Conditions (SPARC) initiatives have already shown promise in identifying targets for effective, noninvasive pain relief devices, he said. Technologies being developed from these programs are “truly amazing,” he added.
A new focus on data and asset sharing among industry, academia, and NIH will “improve the quality, consistency, and efficiency of early-phase pain clinical trials,” Dr. Koroshetz continued. The Early Phase Pain Investigation Clinical Network (EPPIC-Net) will coordinate data and biosample hosting.
Through a competitive submission process, EPPIC-net will review dossiers from institutions or consortia that can serve as assets around which clinical trials can be designed and executed. These early-phase trials will focus on well-defined pain conditions with unmet need, such as chronic regional pain syndrome and tic douloureux, he said.
“We want to find patients who have well-defined conditions. We know the phenotypes, we know the natural history. We’re looking for clinical sites to work on these projects as part of one large team to bring new therapies to patients,” noted Dr. Koroshetz.
Further along the spectrum of research, comparative effectiveness research networks will provide a reality check to compare both pharmacologic and nonpharmacologic interventions all along the spectrum from acute to chronic pain. Here, data elements and storage will also be coordinated through EPPIC-Net.
Implementation science research will fine-tune the practicalities of bringing research to practice as the final piece of the puzzle, said Dr. Koroshetz.
Under NIH director Francis Collins, MD, PhD, Dr. Koroshetz is co-leading the HEAL initiative, along with Nora Volkow, MD, director of the National Institute on Drug Abuse. They wrote about the initiative in JAMA last year (JAMA. 2018 Jul 10;320[2]:129-30).
Dr. Koroshetz reported no conflicts of interest.
MILWAUKEE – Congress has allocated a half billion dollars annually to the National Institutes of Health for a program that seeks to end America’s opioid crisis. The agency is putting in place over two-dozen projects spanning basic and translational research, clinical trials, and implementation of new strategies to address pain and fight addiction.
The , said Walter Koroshetz, MD, speaking at the scientific meeting of the American Pain Society. This represents carryover from 2018, a planning year for the initiative, along with the 2019 $500 million annual supplement to the NIH’s base appropriation.
In 2018, NIH and other federal agencies successfully convinced Congress that funding a coordinated use of resources was necessary to overcome the country’s dual opioid and chronic pain crises. “Luck happens to the prepared,” said Dr. Koroshetz, director of the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Md., adding that many hours went into putting together a national pain strategy that is multidisciplinary and multi-layered, and involves multiple players.
The two aims of research under the initiative are to improve treatments for misuse and addiction, and to enhance pain management. Focusing on this latter aim, Dr. Koroshetz said that the initiative has several research priorities to enhance pain management.
First, the biological basis for chronic pain needs to be understood in order to formulate effective therapies and interventions. “We need to understand the transition from acute to chronic pain,” he commented. “We need to see if we can learn about the risk factors for developing chronic pain; if we get really lucky, we might identify some biological markers” that identify who is at risk for this transition “in a high-risk acute pain situation.”
Next, a key request of industry and academia will be development of more drugs that avoid the dual-target program of opioids, which affect reward circuitry along with pain circuitry. “Drugs affecting the pain circuit and the reward circuit will always result in addiction” potential, said Dr. Koroshetz. “We’re still using drugs for pain from the poppy plant that were discovered 8,000 years ago.”
The hope with the HEAL initiative is to bring together academic centers with patient populations and research capabilities with industry, to accelerate moving nonaddictive treatments through to phase 3 trials.
The initiative also aims to promote discovery of new biologic targets for safe and effective pain treatment. New understanding of the physiology of pain has led to a multitude of candidate targets, said Dr. Koroshetz: “The good news is that there are so many potential targets. When I started in neurology in the ‘90s, I wouldn’t have said there were many, but now I’d say the list is long.”
Support for this work will require the development of human cell and tissue models, such as induced pluripotent stem cells, 3D printed organoids, and tissue chips. Several HEAL-funded grant mechanisms also seek research-industry collaboration to move investigational drugs for new targets through the pipeline quickly. The agency is hoping to see grantees apply new technologies, such as artificial intelligence, which can help identify new chemical structures and pinpoint new therapeutic targets for drug repurposing.
In addition to rapid drug discovery and accelerated clinical trials, Dr. Koroshetz said that HEAL leaders are hoping to see cross-pollination from two other NIH initiatives to boost pain-targeted medical device development. Both the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) and the Stimulating Peripheral Activity to Relieve Conditions (SPARC) initiatives have already shown promise in identifying targets for effective, noninvasive pain relief devices, he said. Technologies being developed from these programs are “truly amazing,” he added.
A new focus on data and asset sharing among industry, academia, and NIH will “improve the quality, consistency, and efficiency of early-phase pain clinical trials,” Dr. Koroshetz continued. The Early Phase Pain Investigation Clinical Network (EPPIC-Net) will coordinate data and biosample hosting.
Through a competitive submission process, EPPIC-net will review dossiers from institutions or consortia that can serve as assets around which clinical trials can be designed and executed. These early-phase trials will focus on well-defined pain conditions with unmet need, such as chronic regional pain syndrome and tic douloureux, he said.
“We want to find patients who have well-defined conditions. We know the phenotypes, we know the natural history. We’re looking for clinical sites to work on these projects as part of one large team to bring new therapies to patients,” noted Dr. Koroshetz.
Further along the spectrum of research, comparative effectiveness research networks will provide a reality check to compare both pharmacologic and nonpharmacologic interventions all along the spectrum from acute to chronic pain. Here, data elements and storage will also be coordinated through EPPIC-Net.
Implementation science research will fine-tune the practicalities of bringing research to practice as the final piece of the puzzle, said Dr. Koroshetz.
Under NIH director Francis Collins, MD, PhD, Dr. Koroshetz is co-leading the HEAL initiative, along with Nora Volkow, MD, director of the National Institute on Drug Abuse. They wrote about the initiative in JAMA last year (JAMA. 2018 Jul 10;320[2]:129-30).
Dr. Koroshetz reported no conflicts of interest.
REPORTING FROM APS 2019
Alternative regimen reduces narcotic use after pelvic reconstructive surgery
TUCSON, ARIZ. – compared with a standard regimen, with no difference in patient satisfaction scores.
The new study extends findings from other surgical procedures to pelvic reconstructive surgery.
“This can limit both inpatient and outpatient narcotic use. It uses oral Toradol on an outpatient basis. It’s totally underutilized. People are afraid of it, people think it causes more bleeding, and maybe there’s a cost issue,” Andrey Petrikovets, MD, a urogynecologist in Los Angeles, said in an interview.
The regimen, which he calls ICE-T, relies in part on 16 tablets of Toradol sent home with the patient – 4 days’ worth. “It’s just 16 tablets, so it’s cheap, and patients do great with it. If you really use Toradol appropriately, especially on an outpatient basis, you can pretty much eliminate outpatient narcotic use,” said Dr. Petrikovets, who presented the work at the annual scientific meeting of the Society of Gynecologic Surgeons.
He believes that ICE-T is a good option for vaginal surgery. It’s a possibility for benign laparoscopic and perhaps robotic surgery, although those applications need to be studied. ICE-T should be avoided in patients with chronic pain, as well as patients with contraindications to any of the regimen’s medications, Dr. Petrikovets said.
According to the protocol, until hospital discharge, patients receive 20 minutes of ice to the perineum every 2 hours, 30 mg IV Toradol every 6 hours, 1,000 mg oral Tylenol every 6 hours, and 0.2 mg IV Dilaudid every 3 hours as needed for breakthrough pain. The constant pain management is important, said Dr. Petrikovets. “Patients don’t have an opportunity for the pain to get really high,” he said. At-home management includes 1,000 mg oral Tylenol every 6 hours, as needed (pain level 1-5, 60 tablets), and 10 mg Toradol every 6 hours as needed (pain level 6-10, 16 tablets).
The trial was conducted at two centers, where 63 patients were randomized to ICE-T or a standard regimen, which at the hospital included 600 mg ibuprofen every 6 hours as needed for pain levels 1-3, one tablet of Percocet (5/325 mg) every 4-6 hours as needed for pain levels 4-6, two tablets of Percocet for pain levels 7-10, and 0.2 mg IV Dilaudid every 3 hours as needed for breakthrough pain. At-home management consisted of 600 mg ibuprofen every 6 hours for pain levels 1-5 (60 tablets), and Percocet 5/325 mg every 6 hours for pain levels 6-10 (16 tablets).
Using the visual analog scale, researchers found that the 30 patients in the ICE-T arm of the study had less morning pain (VAS score, 20 mm vs. 40 mm; P = .03), and lower numerical pain score at 96 hours (2 vs. 3; P = .04). During the mornings and at 96 hours, the two groups had similar quality of recovery and satisfaction scores.
Narcotic use, measured as oral morphine equivalents, was significantly lower in the ICE-T arm between exit from the postanesthesia care unit (PACU) and hospital discharge (3 vs. 20; P less than .001) and through PACU all the way to discharge (17 vs. 38; P less than .001); 70% of patients in the ICE-T arm required no narcotics after PACU discharge, compared with 12% in the standard care arm (P less than .001).
At 96 hours, there was no significant difference between the two groups in the number of emergency department visits, percentage who had a bowel movement since surgery, or the number of Percocet/Toradol tablets taken. The ICE-T group took more Tylenol tablets than did the standard group took ibuprofen (11 vs. 6; P = .012).
SOURCE: Petrikovets A et al. SGS 2019, Abstract 07.
TUCSON, ARIZ. – compared with a standard regimen, with no difference in patient satisfaction scores.
The new study extends findings from other surgical procedures to pelvic reconstructive surgery.
“This can limit both inpatient and outpatient narcotic use. It uses oral Toradol on an outpatient basis. It’s totally underutilized. People are afraid of it, people think it causes more bleeding, and maybe there’s a cost issue,” Andrey Petrikovets, MD, a urogynecologist in Los Angeles, said in an interview.
The regimen, which he calls ICE-T, relies in part on 16 tablets of Toradol sent home with the patient – 4 days’ worth. “It’s just 16 tablets, so it’s cheap, and patients do great with it. If you really use Toradol appropriately, especially on an outpatient basis, you can pretty much eliminate outpatient narcotic use,” said Dr. Petrikovets, who presented the work at the annual scientific meeting of the Society of Gynecologic Surgeons.
He believes that ICE-T is a good option for vaginal surgery. It’s a possibility for benign laparoscopic and perhaps robotic surgery, although those applications need to be studied. ICE-T should be avoided in patients with chronic pain, as well as patients with contraindications to any of the regimen’s medications, Dr. Petrikovets said.
According to the protocol, until hospital discharge, patients receive 20 minutes of ice to the perineum every 2 hours, 30 mg IV Toradol every 6 hours, 1,000 mg oral Tylenol every 6 hours, and 0.2 mg IV Dilaudid every 3 hours as needed for breakthrough pain. The constant pain management is important, said Dr. Petrikovets. “Patients don’t have an opportunity for the pain to get really high,” he said. At-home management includes 1,000 mg oral Tylenol every 6 hours, as needed (pain level 1-5, 60 tablets), and 10 mg Toradol every 6 hours as needed (pain level 6-10, 16 tablets).
The trial was conducted at two centers, where 63 patients were randomized to ICE-T or a standard regimen, which at the hospital included 600 mg ibuprofen every 6 hours as needed for pain levels 1-3, one tablet of Percocet (5/325 mg) every 4-6 hours as needed for pain levels 4-6, two tablets of Percocet for pain levels 7-10, and 0.2 mg IV Dilaudid every 3 hours as needed for breakthrough pain. At-home management consisted of 600 mg ibuprofen every 6 hours for pain levels 1-5 (60 tablets), and Percocet 5/325 mg every 6 hours for pain levels 6-10 (16 tablets).
Using the visual analog scale, researchers found that the 30 patients in the ICE-T arm of the study had less morning pain (VAS score, 20 mm vs. 40 mm; P = .03), and lower numerical pain score at 96 hours (2 vs. 3; P = .04). During the mornings and at 96 hours, the two groups had similar quality of recovery and satisfaction scores.
Narcotic use, measured as oral morphine equivalents, was significantly lower in the ICE-T arm between exit from the postanesthesia care unit (PACU) and hospital discharge (3 vs. 20; P less than .001) and through PACU all the way to discharge (17 vs. 38; P less than .001); 70% of patients in the ICE-T arm required no narcotics after PACU discharge, compared with 12% in the standard care arm (P less than .001).
At 96 hours, there was no significant difference between the two groups in the number of emergency department visits, percentage who had a bowel movement since surgery, or the number of Percocet/Toradol tablets taken. The ICE-T group took more Tylenol tablets than did the standard group took ibuprofen (11 vs. 6; P = .012).
SOURCE: Petrikovets A et al. SGS 2019, Abstract 07.
TUCSON, ARIZ. – compared with a standard regimen, with no difference in patient satisfaction scores.
The new study extends findings from other surgical procedures to pelvic reconstructive surgery.
“This can limit both inpatient and outpatient narcotic use. It uses oral Toradol on an outpatient basis. It’s totally underutilized. People are afraid of it, people think it causes more bleeding, and maybe there’s a cost issue,” Andrey Petrikovets, MD, a urogynecologist in Los Angeles, said in an interview.
The regimen, which he calls ICE-T, relies in part on 16 tablets of Toradol sent home with the patient – 4 days’ worth. “It’s just 16 tablets, so it’s cheap, and patients do great with it. If you really use Toradol appropriately, especially on an outpatient basis, you can pretty much eliminate outpatient narcotic use,” said Dr. Petrikovets, who presented the work at the annual scientific meeting of the Society of Gynecologic Surgeons.
He believes that ICE-T is a good option for vaginal surgery. It’s a possibility for benign laparoscopic and perhaps robotic surgery, although those applications need to be studied. ICE-T should be avoided in patients with chronic pain, as well as patients with contraindications to any of the regimen’s medications, Dr. Petrikovets said.
According to the protocol, until hospital discharge, patients receive 20 minutes of ice to the perineum every 2 hours, 30 mg IV Toradol every 6 hours, 1,000 mg oral Tylenol every 6 hours, and 0.2 mg IV Dilaudid every 3 hours as needed for breakthrough pain. The constant pain management is important, said Dr. Petrikovets. “Patients don’t have an opportunity for the pain to get really high,” he said. At-home management includes 1,000 mg oral Tylenol every 6 hours, as needed (pain level 1-5, 60 tablets), and 10 mg Toradol every 6 hours as needed (pain level 6-10, 16 tablets).
The trial was conducted at two centers, where 63 patients were randomized to ICE-T or a standard regimen, which at the hospital included 600 mg ibuprofen every 6 hours as needed for pain levels 1-3, one tablet of Percocet (5/325 mg) every 4-6 hours as needed for pain levels 4-6, two tablets of Percocet for pain levels 7-10, and 0.2 mg IV Dilaudid every 3 hours as needed for breakthrough pain. At-home management consisted of 600 mg ibuprofen every 6 hours for pain levels 1-5 (60 tablets), and Percocet 5/325 mg every 6 hours for pain levels 6-10 (16 tablets).
Using the visual analog scale, researchers found that the 30 patients in the ICE-T arm of the study had less morning pain (VAS score, 20 mm vs. 40 mm; P = .03), and lower numerical pain score at 96 hours (2 vs. 3; P = .04). During the mornings and at 96 hours, the two groups had similar quality of recovery and satisfaction scores.
Narcotic use, measured as oral morphine equivalents, was significantly lower in the ICE-T arm between exit from the postanesthesia care unit (PACU) and hospital discharge (3 vs. 20; P less than .001) and through PACU all the way to discharge (17 vs. 38; P less than .001); 70% of patients in the ICE-T arm required no narcotics after PACU discharge, compared with 12% in the standard care arm (P less than .001).
At 96 hours, there was no significant difference between the two groups in the number of emergency department visits, percentage who had a bowel movement since surgery, or the number of Percocet/Toradol tablets taken. The ICE-T group took more Tylenol tablets than did the standard group took ibuprofen (11 vs. 6; P = .012).
SOURCE: Petrikovets A et al. SGS 2019, Abstract 07.
REPORTING FROM SGS 2019
Report calls for focus on ‘subpopulations’ to fight opioid epidemic
Most people who could benefit from FDA-approved medications for opioid use disorder do not receive them, and access to those treatments is not equitable, according to a new consensus study report from the National Academies of Sciences, Engineering, and Medicine.
“Methadone, buprenorphine, and extended-release naltrexone are safe and highly effective medications that are already approved by the U.S. Food and Drug Administration to treat OUD,” the report said. “These medications save lives, but the majority of people with OUD in the United States receive no treatment at all.”
It also said additional research will be needed to address opioid use disorder among subpopulations in the United States, such as adolescents, older adults, people with comorbidities, racial and ethnic groups, and people with low socioeconomic status. The National Academies’ report was sponsored by NIDA and SAMHSA.
A few weeks before the release of National Academies report, the National Academy of Medicine (NAM) held a webinar providing details on its Action Collaborative on Countering the U.S. Opioid Epidemic. The collaborative, a partnership of public and private stakeholders, aims to address the opioid crisis through a multidisciplinary, cross-sector effort.
The collaborative is represented by federal agencies, state and local governments, health care systems, provider groups, nonprofits, payers, industry, academia, patient organizations, and communities across about 55 organizations, according to Victor J. Dzau, MD, chair of the Action Collaborative and current NAM president. Over a 2-year period, the collaborative’s goal is to accelerate progress in overcoming the opioid crisis by recognizing the challenges, research gaps, and needs of organizations involved in the crisis and “elevate and accelerate evidence-based, multisectoral, and interprofessional solutions,” he said.
“This is not a problem that can be solved by a single sector. It is truly a whole of society problem,” said Adm. Brett P. Giroir, MD, assistant secretary for health at the U.S. Department of Health and Human Services, said during the webinar. “And the only way that we are going to be able to begin making inroads to reverse the trends of this crisis is if we work together.” Dr. Giroir also serves as cochair of the steering committee for the collaborative.
In its overview of the collaborative, the NAM outlined four working groups developed through a series of surveys and planning meetings that would identify the resources that currently exist to combat the opioid epidemic and determine which resources still need to be developed. In the Health Professional Education and Training Working Group, for example, the objective is to examine what is being taught to health professionals about acute and chronic pain management at an accreditation, certification, and regulatory level to develop educational tools based around knowledge gaps in those areas and analyze how the new resources are affecting health professions after they have been adopted, said Steve Singer, PhD, vice president of education and outreach at the Accreditation Council for Graduate Medical Education and colead of the working group.“Our goal is really to provide guidance and resources across the continuum of health professions and education with an interprofessional – and patient-informed view,” he said.
The Opioid Prescribing Guidelines and Evidence Standards Working Group plans to address the disparities in prescribing and tapering guidelines for acute and chronic pain as well as identify where pain management guidelines in different specialties “cannot be justified,” based on available evidence.
“Further, we think it’s really important to not just have guidelines that will sit on a shelf, but we also want to think about how we can support implementation of these guidelines into practice ... ” said Helen Burstin, MD, MPH, executive vice president and CEO for the Council of Medical Specialty Societies and colead of the working group.
Alonzo L. Plough, PhD, MPH, vice president of research-evaluation-learning at the Robert Wood Johnson Foundation and colead of the Prevention, Treatment, and Recovery Services Working Group, explained that the goal of his group is to identify the “essential elements and components” and best practices of prevention, treatment, and recovery for OUD. He noted that, although the working group will not be able to reach all patient populations affected by OUD, it has discussed targeting vulnerable high-risk populations, such as those involved in the criminal justice system, homeless veterans, mothers, and children.
“This is an ecosystem that requires great concentration and effort to make sure that there are integrated approaches throughout the continuum that work for patients and clients from different walks of life, and I think that our overall guidance is how we can recognize and use evidence to find those approaches and build on them for guidance,” he said.
The Research, Data, and Metrics Needs Working Group is tasked with collaborating with the other groups to obtain currently available information and identify what barriers exist to greater transparency, sharing and interoperability of data as well as what gaps in research currently exist that would further the collaborative’s mission, said Kelly J. Clark, MD, MBA, of the ASAM. “It is simply critical for us to utilize the data that’s out there, to pool it into more actionable information – and then to act on it,” Dr. Clark said.
The NAM is seeking new organizations interested in joining the collaborative as a network organization, which would receive updates and provide input on the collaborative but would not be a part of the working groups.
The first public meeting of the Action Collaborative on Countering the U.S. Opioid Epidemic will take place on April 30, 2019, in Washington.
Most people who could benefit from FDA-approved medications for opioid use disorder do not receive them, and access to those treatments is not equitable, according to a new consensus study report from the National Academies of Sciences, Engineering, and Medicine.
“Methadone, buprenorphine, and extended-release naltrexone are safe and highly effective medications that are already approved by the U.S. Food and Drug Administration to treat OUD,” the report said. “These medications save lives, but the majority of people with OUD in the United States receive no treatment at all.”
It also said additional research will be needed to address opioid use disorder among subpopulations in the United States, such as adolescents, older adults, people with comorbidities, racial and ethnic groups, and people with low socioeconomic status. The National Academies’ report was sponsored by NIDA and SAMHSA.
A few weeks before the release of National Academies report, the National Academy of Medicine (NAM) held a webinar providing details on its Action Collaborative on Countering the U.S. Opioid Epidemic. The collaborative, a partnership of public and private stakeholders, aims to address the opioid crisis through a multidisciplinary, cross-sector effort.
The collaborative is represented by federal agencies, state and local governments, health care systems, provider groups, nonprofits, payers, industry, academia, patient organizations, and communities across about 55 organizations, according to Victor J. Dzau, MD, chair of the Action Collaborative and current NAM president. Over a 2-year period, the collaborative’s goal is to accelerate progress in overcoming the opioid crisis by recognizing the challenges, research gaps, and needs of organizations involved in the crisis and “elevate and accelerate evidence-based, multisectoral, and interprofessional solutions,” he said.
“This is not a problem that can be solved by a single sector. It is truly a whole of society problem,” said Adm. Brett P. Giroir, MD, assistant secretary for health at the U.S. Department of Health and Human Services, said during the webinar. “And the only way that we are going to be able to begin making inroads to reverse the trends of this crisis is if we work together.” Dr. Giroir also serves as cochair of the steering committee for the collaborative.
In its overview of the collaborative, the NAM outlined four working groups developed through a series of surveys and planning meetings that would identify the resources that currently exist to combat the opioid epidemic and determine which resources still need to be developed. In the Health Professional Education and Training Working Group, for example, the objective is to examine what is being taught to health professionals about acute and chronic pain management at an accreditation, certification, and regulatory level to develop educational tools based around knowledge gaps in those areas and analyze how the new resources are affecting health professions after they have been adopted, said Steve Singer, PhD, vice president of education and outreach at the Accreditation Council for Graduate Medical Education and colead of the working group.“Our goal is really to provide guidance and resources across the continuum of health professions and education with an interprofessional – and patient-informed view,” he said.
The Opioid Prescribing Guidelines and Evidence Standards Working Group plans to address the disparities in prescribing and tapering guidelines for acute and chronic pain as well as identify where pain management guidelines in different specialties “cannot be justified,” based on available evidence.
“Further, we think it’s really important to not just have guidelines that will sit on a shelf, but we also want to think about how we can support implementation of these guidelines into practice ... ” said Helen Burstin, MD, MPH, executive vice president and CEO for the Council of Medical Specialty Societies and colead of the working group.
Alonzo L. Plough, PhD, MPH, vice president of research-evaluation-learning at the Robert Wood Johnson Foundation and colead of the Prevention, Treatment, and Recovery Services Working Group, explained that the goal of his group is to identify the “essential elements and components” and best practices of prevention, treatment, and recovery for OUD. He noted that, although the working group will not be able to reach all patient populations affected by OUD, it has discussed targeting vulnerable high-risk populations, such as those involved in the criminal justice system, homeless veterans, mothers, and children.
“This is an ecosystem that requires great concentration and effort to make sure that there are integrated approaches throughout the continuum that work for patients and clients from different walks of life, and I think that our overall guidance is how we can recognize and use evidence to find those approaches and build on them for guidance,” he said.
The Research, Data, and Metrics Needs Working Group is tasked with collaborating with the other groups to obtain currently available information and identify what barriers exist to greater transparency, sharing and interoperability of data as well as what gaps in research currently exist that would further the collaborative’s mission, said Kelly J. Clark, MD, MBA, of the ASAM. “It is simply critical for us to utilize the data that’s out there, to pool it into more actionable information – and then to act on it,” Dr. Clark said.
The NAM is seeking new organizations interested in joining the collaborative as a network organization, which would receive updates and provide input on the collaborative but would not be a part of the working groups.
The first public meeting of the Action Collaborative on Countering the U.S. Opioid Epidemic will take place on April 30, 2019, in Washington.
Most people who could benefit from FDA-approved medications for opioid use disorder do not receive them, and access to those treatments is not equitable, according to a new consensus study report from the National Academies of Sciences, Engineering, and Medicine.
“Methadone, buprenorphine, and extended-release naltrexone are safe and highly effective medications that are already approved by the U.S. Food and Drug Administration to treat OUD,” the report said. “These medications save lives, but the majority of people with OUD in the United States receive no treatment at all.”
It also said additional research will be needed to address opioid use disorder among subpopulations in the United States, such as adolescents, older adults, people with comorbidities, racial and ethnic groups, and people with low socioeconomic status. The National Academies’ report was sponsored by NIDA and SAMHSA.
A few weeks before the release of National Academies report, the National Academy of Medicine (NAM) held a webinar providing details on its Action Collaborative on Countering the U.S. Opioid Epidemic. The collaborative, a partnership of public and private stakeholders, aims to address the opioid crisis through a multidisciplinary, cross-sector effort.
The collaborative is represented by federal agencies, state and local governments, health care systems, provider groups, nonprofits, payers, industry, academia, patient organizations, and communities across about 55 organizations, according to Victor J. Dzau, MD, chair of the Action Collaborative and current NAM president. Over a 2-year period, the collaborative’s goal is to accelerate progress in overcoming the opioid crisis by recognizing the challenges, research gaps, and needs of organizations involved in the crisis and “elevate and accelerate evidence-based, multisectoral, and interprofessional solutions,” he said.
“This is not a problem that can be solved by a single sector. It is truly a whole of society problem,” said Adm. Brett P. Giroir, MD, assistant secretary for health at the U.S. Department of Health and Human Services, said during the webinar. “And the only way that we are going to be able to begin making inroads to reverse the trends of this crisis is if we work together.” Dr. Giroir also serves as cochair of the steering committee for the collaborative.
In its overview of the collaborative, the NAM outlined four working groups developed through a series of surveys and planning meetings that would identify the resources that currently exist to combat the opioid epidemic and determine which resources still need to be developed. In the Health Professional Education and Training Working Group, for example, the objective is to examine what is being taught to health professionals about acute and chronic pain management at an accreditation, certification, and regulatory level to develop educational tools based around knowledge gaps in those areas and analyze how the new resources are affecting health professions after they have been adopted, said Steve Singer, PhD, vice president of education and outreach at the Accreditation Council for Graduate Medical Education and colead of the working group.“Our goal is really to provide guidance and resources across the continuum of health professions and education with an interprofessional – and patient-informed view,” he said.
The Opioid Prescribing Guidelines and Evidence Standards Working Group plans to address the disparities in prescribing and tapering guidelines for acute and chronic pain as well as identify where pain management guidelines in different specialties “cannot be justified,” based on available evidence.
“Further, we think it’s really important to not just have guidelines that will sit on a shelf, but we also want to think about how we can support implementation of these guidelines into practice ... ” said Helen Burstin, MD, MPH, executive vice president and CEO for the Council of Medical Specialty Societies and colead of the working group.
Alonzo L. Plough, PhD, MPH, vice president of research-evaluation-learning at the Robert Wood Johnson Foundation and colead of the Prevention, Treatment, and Recovery Services Working Group, explained that the goal of his group is to identify the “essential elements and components” and best practices of prevention, treatment, and recovery for OUD. He noted that, although the working group will not be able to reach all patient populations affected by OUD, it has discussed targeting vulnerable high-risk populations, such as those involved in the criminal justice system, homeless veterans, mothers, and children.
“This is an ecosystem that requires great concentration and effort to make sure that there are integrated approaches throughout the continuum that work for patients and clients from different walks of life, and I think that our overall guidance is how we can recognize and use evidence to find those approaches and build on them for guidance,” he said.
The Research, Data, and Metrics Needs Working Group is tasked with collaborating with the other groups to obtain currently available information and identify what barriers exist to greater transparency, sharing and interoperability of data as well as what gaps in research currently exist that would further the collaborative’s mission, said Kelly J. Clark, MD, MBA, of the ASAM. “It is simply critical for us to utilize the data that’s out there, to pool it into more actionable information – and then to act on it,” Dr. Clark said.
The NAM is seeking new organizations interested in joining the collaborative as a network organization, which would receive updates and provide input on the collaborative but would not be a part of the working groups.
The first public meeting of the Action Collaborative on Countering the U.S. Opioid Epidemic will take place on April 30, 2019, in Washington.
Postcesarean pain relief better on nonopioid regimen
LAS VEGAS – Women who had cesarean delivery and received a nonopioid pain control regimen at hospital discharge had lower pain scores by 4 weeks post partum than those who also received opioids, according to study results shared during a fellows session at the meeting presented by the Society for Maternal-Fetal Medicine.
At 2-4 weeks post partum, the mean pain score on a visual analog scale (VAS) was 12/100 mm for women on the nonopioid regimen, compared with 16/100 mm for women who received opioids, using an intention-to-treat analysis. The median pain score for those in the nonopioid arm was 0, compared with 6 for those in the opioid arm.
The findings surprised Jenifer Dinis, MD, a maternal-fetal medicine fellow at the University of Texas, Houston, and her collaborators, because they had hypothesized merely that the two groups would have similar pain scores 2-4 weeks after delivery.
Although women in the nonopioid arm were able to obtain a rescue hydrocodone prescription through the study, and some women obtained opioids from their private physician, they still used less than half as much opioid medication as women in the opioid arm (21 versus 43 morphine milligram equivalents, P less than .01).
However, women in the nonopioid arm did not use significantly more ibuprofen or acetaminophen, and there was no difference in patient satisfaction with the outpatient postpartum analgesic regimen between study arms. Somnolence was more common in the opioid arm (P = .03); no other medication side effects were significantly more common in one group than the other.
Overall, 22 of 76 (29%) women in the nonopioid arm took any opioids after discharge, compared with 59/81 (73%) in the opioid arm (P less than .01).
After cesarean delivery, the 170 participating women had an inpatient pain control regimen determined by their primary ob.gyn., Dr. Dinis said in her presentation. Patients were randomized 1:1 to their outpatient analgesia regimens on postoperative day 2 or 3, with appropriate prescriptions placed in patient charts. Participants received either a nonopioid regimen with prescriptions for 60 ibuprofen tablets (600 mg) and 60 acetaminophen tablets (325 mg), or to an opioid regimen that included ibuprofen plus hydrocodone/acetaminophen 5 (325 mg) 1-2 tablets every 4 hours.
Pain scores were assessed between 2 and 4 weeks after delivery, either at an in-person appointment or by means of a phone call and a provided email link.
The single-site study was designed as a parallel-group equivalence trial, to show noninferiority of one pain control regimen over the other. Women between the ages of 18 and 50 years were included if they had a cesarean delivery; both English- and Spanish-speaking women were enrolled.
Allowing for attrition and crossover, Dr. Dinis and her colleagues enrolled 85 patients per study arm to achieve sufficient statistical power to detect the difference needed. The investigators planned both an intention-to-treat and a per-protocol analysis in their registered clinical trial.
Postpartum pain assessments were not obtained for 12 patients in the nonopioid group, and 9 in the opioid group, leaving 73 and 76 patients in each group for the per-protocol analysis, respectively.
At baseline, patients were a mean 28 years old, and a little over a quarter (28%) were nulliparous. Participants were overall about half African American and 34%-40% Hispanic. Over half (62%-72%) received Medicaid; most women (62%-75%) had body mass indices of 30 kg/m2 or more.
The mean gestational age at delivery was a little more than 36 weeks, with about half of deliveries being the participant’s first cesarean delivery. About 90% of women had a Pfannenstiel skin incision, with a low transverse uterine incision.
Patients were aware of their allocation, and the study results aren’t applicable to women with opioid or benzodiazepine use disorder, she noted. However, the study was pragmatic, included all types of cesarean deliveries, and was adequately powered to detect “the smallest clinically significant difference.”
Dr. Dinis reported no outside sources of funding and no conflicts of interest.
SOURCE: Dinis J et al. Am J Obstet Gynecol. 2019 Jan;220(1):S34, Abstract 42.
LAS VEGAS – Women who had cesarean delivery and received a nonopioid pain control regimen at hospital discharge had lower pain scores by 4 weeks post partum than those who also received opioids, according to study results shared during a fellows session at the meeting presented by the Society for Maternal-Fetal Medicine.
At 2-4 weeks post partum, the mean pain score on a visual analog scale (VAS) was 12/100 mm for women on the nonopioid regimen, compared with 16/100 mm for women who received opioids, using an intention-to-treat analysis. The median pain score for those in the nonopioid arm was 0, compared with 6 for those in the opioid arm.
The findings surprised Jenifer Dinis, MD, a maternal-fetal medicine fellow at the University of Texas, Houston, and her collaborators, because they had hypothesized merely that the two groups would have similar pain scores 2-4 weeks after delivery.
Although women in the nonopioid arm were able to obtain a rescue hydrocodone prescription through the study, and some women obtained opioids from their private physician, they still used less than half as much opioid medication as women in the opioid arm (21 versus 43 morphine milligram equivalents, P less than .01).
However, women in the nonopioid arm did not use significantly more ibuprofen or acetaminophen, and there was no difference in patient satisfaction with the outpatient postpartum analgesic regimen between study arms. Somnolence was more common in the opioid arm (P = .03); no other medication side effects were significantly more common in one group than the other.
Overall, 22 of 76 (29%) women in the nonopioid arm took any opioids after discharge, compared with 59/81 (73%) in the opioid arm (P less than .01).
After cesarean delivery, the 170 participating women had an inpatient pain control regimen determined by their primary ob.gyn., Dr. Dinis said in her presentation. Patients were randomized 1:1 to their outpatient analgesia regimens on postoperative day 2 or 3, with appropriate prescriptions placed in patient charts. Participants received either a nonopioid regimen with prescriptions for 60 ibuprofen tablets (600 mg) and 60 acetaminophen tablets (325 mg), or to an opioid regimen that included ibuprofen plus hydrocodone/acetaminophen 5 (325 mg) 1-2 tablets every 4 hours.
Pain scores were assessed between 2 and 4 weeks after delivery, either at an in-person appointment or by means of a phone call and a provided email link.
The single-site study was designed as a parallel-group equivalence trial, to show noninferiority of one pain control regimen over the other. Women between the ages of 18 and 50 years were included if they had a cesarean delivery; both English- and Spanish-speaking women were enrolled.
Allowing for attrition and crossover, Dr. Dinis and her colleagues enrolled 85 patients per study arm to achieve sufficient statistical power to detect the difference needed. The investigators planned both an intention-to-treat and a per-protocol analysis in their registered clinical trial.
Postpartum pain assessments were not obtained for 12 patients in the nonopioid group, and 9 in the opioid group, leaving 73 and 76 patients in each group for the per-protocol analysis, respectively.
At baseline, patients were a mean 28 years old, and a little over a quarter (28%) were nulliparous. Participants were overall about half African American and 34%-40% Hispanic. Over half (62%-72%) received Medicaid; most women (62%-75%) had body mass indices of 30 kg/m2 or more.
The mean gestational age at delivery was a little more than 36 weeks, with about half of deliveries being the participant’s first cesarean delivery. About 90% of women had a Pfannenstiel skin incision, with a low transverse uterine incision.
Patients were aware of their allocation, and the study results aren’t applicable to women with opioid or benzodiazepine use disorder, she noted. However, the study was pragmatic, included all types of cesarean deliveries, and was adequately powered to detect “the smallest clinically significant difference.”
Dr. Dinis reported no outside sources of funding and no conflicts of interest.
SOURCE: Dinis J et al. Am J Obstet Gynecol. 2019 Jan;220(1):S34, Abstract 42.
LAS VEGAS – Women who had cesarean delivery and received a nonopioid pain control regimen at hospital discharge had lower pain scores by 4 weeks post partum than those who also received opioids, according to study results shared during a fellows session at the meeting presented by the Society for Maternal-Fetal Medicine.
At 2-4 weeks post partum, the mean pain score on a visual analog scale (VAS) was 12/100 mm for women on the nonopioid regimen, compared with 16/100 mm for women who received opioids, using an intention-to-treat analysis. The median pain score for those in the nonopioid arm was 0, compared with 6 for those in the opioid arm.
The findings surprised Jenifer Dinis, MD, a maternal-fetal medicine fellow at the University of Texas, Houston, and her collaborators, because they had hypothesized merely that the two groups would have similar pain scores 2-4 weeks after delivery.
Although women in the nonopioid arm were able to obtain a rescue hydrocodone prescription through the study, and some women obtained opioids from their private physician, they still used less than half as much opioid medication as women in the opioid arm (21 versus 43 morphine milligram equivalents, P less than .01).
However, women in the nonopioid arm did not use significantly more ibuprofen or acetaminophen, and there was no difference in patient satisfaction with the outpatient postpartum analgesic regimen between study arms. Somnolence was more common in the opioid arm (P = .03); no other medication side effects were significantly more common in one group than the other.
Overall, 22 of 76 (29%) women in the nonopioid arm took any opioids after discharge, compared with 59/81 (73%) in the opioid arm (P less than .01).
After cesarean delivery, the 170 participating women had an inpatient pain control regimen determined by their primary ob.gyn., Dr. Dinis said in her presentation. Patients were randomized 1:1 to their outpatient analgesia regimens on postoperative day 2 or 3, with appropriate prescriptions placed in patient charts. Participants received either a nonopioid regimen with prescriptions for 60 ibuprofen tablets (600 mg) and 60 acetaminophen tablets (325 mg), or to an opioid regimen that included ibuprofen plus hydrocodone/acetaminophen 5 (325 mg) 1-2 tablets every 4 hours.
Pain scores were assessed between 2 and 4 weeks after delivery, either at an in-person appointment or by means of a phone call and a provided email link.
The single-site study was designed as a parallel-group equivalence trial, to show noninferiority of one pain control regimen over the other. Women between the ages of 18 and 50 years were included if they had a cesarean delivery; both English- and Spanish-speaking women were enrolled.
Allowing for attrition and crossover, Dr. Dinis and her colleagues enrolled 85 patients per study arm to achieve sufficient statistical power to detect the difference needed. The investigators planned both an intention-to-treat and a per-protocol analysis in their registered clinical trial.
Postpartum pain assessments were not obtained for 12 patients in the nonopioid group, and 9 in the opioid group, leaving 73 and 76 patients in each group for the per-protocol analysis, respectively.
At baseline, patients were a mean 28 years old, and a little over a quarter (28%) were nulliparous. Participants were overall about half African American and 34%-40% Hispanic. Over half (62%-72%) received Medicaid; most women (62%-75%) had body mass indices of 30 kg/m2 or more.
The mean gestational age at delivery was a little more than 36 weeks, with about half of deliveries being the participant’s first cesarean delivery. About 90% of women had a Pfannenstiel skin incision, with a low transverse uterine incision.
Patients were aware of their allocation, and the study results aren’t applicable to women with opioid or benzodiazepine use disorder, she noted. However, the study was pragmatic, included all types of cesarean deliveries, and was adequately powered to detect “the smallest clinically significant difference.”
Dr. Dinis reported no outside sources of funding and no conflicts of interest.
SOURCE: Dinis J et al. Am J Obstet Gynecol. 2019 Jan;220(1):S34, Abstract 42.
REPORTING FROM THE PREGNANCY MEETING
Opioid overdose risk greater among HIV patients
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
REPORTING FROM CROI 2019
Teens likely to mimic parents’ opioid use
reported Pamela C. Griesler, PhD, of Columbia University, New York, and her associates.
Given the significant link between parental and adolescent smoking and adolescent nonmedical prescription opioid (NMPO) use, smoking also should be included in targeted interventions, they wrote in Pediatrics.
Dr. Griesler and her colleagues noted that there actually are three classes of factors influencing the association between parent and adolescent NMPO use: phenotypic heritability, parental role modeling, and parental socialization and other environmental influences.
In the first known study to explore the relationship of parent-adolescent NMPO use within a nationally representative sampling of parent-child dyads taken from the National Surveys on Drug Use and Health, Dr. Griesler and her colleagues examined the intergenerational association of lifetime NMPO use among 35,000 parent-adolescent dyads (21,200 mothers, 13,800 fathers). Of the 35,000 children aged 12-17 years included in the sample, 90% were biological, 8% were stepchildren, and 2% were adopted.
Given the absence of previous studies exploring the relationship between parent-adolescent NMPO use, Dr. Griesler and her associates used established findings for smoking and substance use to hypothesize that there would be stronger associations for mothers than fathers, daughters than sons, and for whites than African Americans.
The investigators posed three questions that formed the basis of their research: 1) What is the association between lifetime parental and child NMPO use? 2) What is the unique association between parental and child NMPO use, controlling for other factors? 3) Do parental/adolescent NMPO use associations differ by parent/child gender and race and/or ethnicity?
About 14% of parents reported ever using an NMPO; fathers (14%) had slightly higher rates of usage than mothers (13%), and white parents had higher rates of use (16%) than African American (10%) or Hispanic (9%) parents. Among adolescents, 9% reported ever having used an NMPO; this included similar rates for boys (9%) and girls (9%), as well as whites (9%), Hispanics (9%), and African Americans (8%). Use increased with age over time, from 4% among 12-year-olds to 15% among 17-year-olds.
Dr. Griesler and her colleagues did find “a significant positive association between NMPO use by parents and adolescents.” Adolescents were more likely to use an NMPO in their lifetime (14%) if a parent had a history of any use than adolescents whose parents did not have a history (8%). This association persisted even when controlling for other factors (adjusted odds ratio, 1.3).
Adolescent reporting identified low levels of parental support and monitoring, as well as parent approval of drug use, as the primary factors contributing to perceptions of subpar parent-child relationship quality and subsequent NMPO use. Additional adolescent behaviors contributing to increased risk of drug use included delinquency, depression, anxiety, reduced academic and religious involvement, and perceptions around peer drug use and approval of drug use, as well as being older.
Consistent with their original hypothesis, “only maternal NMPO use was significantly associated with adolescent NMPO use,” the investigators wrote (aOR, 1.62), which was not correlated either way concerning the gender of the child. The authors did note, however, “a marginally significant negative association among sons, [aOR, 0.71],” even though no overall paternal-child NMPO correlation was found (aOR, 0.98). They speculated that this negative association might be explained “by the father’s use of other drugs, particularly marijuana.”
Parental factors independently associated with adolescent NMPO use included smoking, alcohol and/or marijuana use, as well as other illicit drug use. When controlling for their use of different drugs and other covariates, only smoking remained associated with adolescent NMPO use (aOR, 1.24). Importantly, higher NMPO usage was observed in cases of poor parenting quality, especially for low levels of monitoring and high incidence of conflict between parents and adolescents. Adolescent NMPO usage were conversely lower in cases where parents self-reported their belief that drug use was risky.
Adolescent behaviors that predicted lifetime NMPO use included starting to smoke cigarettes or marijuana before using NMPO, being depressed or delinquent, having the perception that most peers use drugs, and being older in age. Dr. Griesler and her associates also observed that adolescents who began using alcohol before NMPO were likely to experiment first with smoking cigarettes and marijuana before NMPO.
The lack of differences observed with regard to child gender, race, or ethnicity warrants further investigation, but the authors speculated that “such differences might be detected with measures of current or heavy use.”
One limitation of the study was the focus on lifetime use, Dr. Griesler and her colleagues wrote. Observing patterns of current or heavy use, as well as disorder and “genetically informative samples,” might shed light on the role that familial environmental and genetic influences could play. Additionally, limiting households to one parent and one adolescent discounts the possible combined influence of mother and father NMPO usage on adolescent usage. The research also did not explore the role that adolescent NMPO use could play in influencing “parent-child interactions.”
The authors reported no financial relationships or potential conflicts of interest. The study was supported by grants from the National Institute on Drug Abuse and the New York State Psychiatric Institute; it was funded by the National Institutes of Health.
SOURCE: Griesler PC et al. Pediatrics. 2019;143(3):e20182354.
reported Pamela C. Griesler, PhD, of Columbia University, New York, and her associates.
Given the significant link between parental and adolescent smoking and adolescent nonmedical prescription opioid (NMPO) use, smoking also should be included in targeted interventions, they wrote in Pediatrics.
Dr. Griesler and her colleagues noted that there actually are three classes of factors influencing the association between parent and adolescent NMPO use: phenotypic heritability, parental role modeling, and parental socialization and other environmental influences.
In the first known study to explore the relationship of parent-adolescent NMPO use within a nationally representative sampling of parent-child dyads taken from the National Surveys on Drug Use and Health, Dr. Griesler and her colleagues examined the intergenerational association of lifetime NMPO use among 35,000 parent-adolescent dyads (21,200 mothers, 13,800 fathers). Of the 35,000 children aged 12-17 years included in the sample, 90% were biological, 8% were stepchildren, and 2% were adopted.
Given the absence of previous studies exploring the relationship between parent-adolescent NMPO use, Dr. Griesler and her associates used established findings for smoking and substance use to hypothesize that there would be stronger associations for mothers than fathers, daughters than sons, and for whites than African Americans.
The investigators posed three questions that formed the basis of their research: 1) What is the association between lifetime parental and child NMPO use? 2) What is the unique association between parental and child NMPO use, controlling for other factors? 3) Do parental/adolescent NMPO use associations differ by parent/child gender and race and/or ethnicity?
About 14% of parents reported ever using an NMPO; fathers (14%) had slightly higher rates of usage than mothers (13%), and white parents had higher rates of use (16%) than African American (10%) or Hispanic (9%) parents. Among adolescents, 9% reported ever having used an NMPO; this included similar rates for boys (9%) and girls (9%), as well as whites (9%), Hispanics (9%), and African Americans (8%). Use increased with age over time, from 4% among 12-year-olds to 15% among 17-year-olds.
Dr. Griesler and her colleagues did find “a significant positive association between NMPO use by parents and adolescents.” Adolescents were more likely to use an NMPO in their lifetime (14%) if a parent had a history of any use than adolescents whose parents did not have a history (8%). This association persisted even when controlling for other factors (adjusted odds ratio, 1.3).
Adolescent reporting identified low levels of parental support and monitoring, as well as parent approval of drug use, as the primary factors contributing to perceptions of subpar parent-child relationship quality and subsequent NMPO use. Additional adolescent behaviors contributing to increased risk of drug use included delinquency, depression, anxiety, reduced academic and religious involvement, and perceptions around peer drug use and approval of drug use, as well as being older.
Consistent with their original hypothesis, “only maternal NMPO use was significantly associated with adolescent NMPO use,” the investigators wrote (aOR, 1.62), which was not correlated either way concerning the gender of the child. The authors did note, however, “a marginally significant negative association among sons, [aOR, 0.71],” even though no overall paternal-child NMPO correlation was found (aOR, 0.98). They speculated that this negative association might be explained “by the father’s use of other drugs, particularly marijuana.”
Parental factors independently associated with adolescent NMPO use included smoking, alcohol and/or marijuana use, as well as other illicit drug use. When controlling for their use of different drugs and other covariates, only smoking remained associated with adolescent NMPO use (aOR, 1.24). Importantly, higher NMPO usage was observed in cases of poor parenting quality, especially for low levels of monitoring and high incidence of conflict between parents and adolescents. Adolescent NMPO usage were conversely lower in cases where parents self-reported their belief that drug use was risky.
Adolescent behaviors that predicted lifetime NMPO use included starting to smoke cigarettes or marijuana before using NMPO, being depressed or delinquent, having the perception that most peers use drugs, and being older in age. Dr. Griesler and her associates also observed that adolescents who began using alcohol before NMPO were likely to experiment first with smoking cigarettes and marijuana before NMPO.
The lack of differences observed with regard to child gender, race, or ethnicity warrants further investigation, but the authors speculated that “such differences might be detected with measures of current or heavy use.”
One limitation of the study was the focus on lifetime use, Dr. Griesler and her colleagues wrote. Observing patterns of current or heavy use, as well as disorder and “genetically informative samples,” might shed light on the role that familial environmental and genetic influences could play. Additionally, limiting households to one parent and one adolescent discounts the possible combined influence of mother and father NMPO usage on adolescent usage. The research also did not explore the role that adolescent NMPO use could play in influencing “parent-child interactions.”
The authors reported no financial relationships or potential conflicts of interest. The study was supported by grants from the National Institute on Drug Abuse and the New York State Psychiatric Institute; it was funded by the National Institutes of Health.
SOURCE: Griesler PC et al. Pediatrics. 2019;143(3):e20182354.
reported Pamela C. Griesler, PhD, of Columbia University, New York, and her associates.
Given the significant link between parental and adolescent smoking and adolescent nonmedical prescription opioid (NMPO) use, smoking also should be included in targeted interventions, they wrote in Pediatrics.
Dr. Griesler and her colleagues noted that there actually are three classes of factors influencing the association between parent and adolescent NMPO use: phenotypic heritability, parental role modeling, and parental socialization and other environmental influences.
In the first known study to explore the relationship of parent-adolescent NMPO use within a nationally representative sampling of parent-child dyads taken from the National Surveys on Drug Use and Health, Dr. Griesler and her colleagues examined the intergenerational association of lifetime NMPO use among 35,000 parent-adolescent dyads (21,200 mothers, 13,800 fathers). Of the 35,000 children aged 12-17 years included in the sample, 90% were biological, 8% were stepchildren, and 2% were adopted.
Given the absence of previous studies exploring the relationship between parent-adolescent NMPO use, Dr. Griesler and her associates used established findings for smoking and substance use to hypothesize that there would be stronger associations for mothers than fathers, daughters than sons, and for whites than African Americans.
The investigators posed three questions that formed the basis of their research: 1) What is the association between lifetime parental and child NMPO use? 2) What is the unique association between parental and child NMPO use, controlling for other factors? 3) Do parental/adolescent NMPO use associations differ by parent/child gender and race and/or ethnicity?
About 14% of parents reported ever using an NMPO; fathers (14%) had slightly higher rates of usage than mothers (13%), and white parents had higher rates of use (16%) than African American (10%) or Hispanic (9%) parents. Among adolescents, 9% reported ever having used an NMPO; this included similar rates for boys (9%) and girls (9%), as well as whites (9%), Hispanics (9%), and African Americans (8%). Use increased with age over time, from 4% among 12-year-olds to 15% among 17-year-olds.
Dr. Griesler and her colleagues did find “a significant positive association between NMPO use by parents and adolescents.” Adolescents were more likely to use an NMPO in their lifetime (14%) if a parent had a history of any use than adolescents whose parents did not have a history (8%). This association persisted even when controlling for other factors (adjusted odds ratio, 1.3).
Adolescent reporting identified low levels of parental support and monitoring, as well as parent approval of drug use, as the primary factors contributing to perceptions of subpar parent-child relationship quality and subsequent NMPO use. Additional adolescent behaviors contributing to increased risk of drug use included delinquency, depression, anxiety, reduced academic and religious involvement, and perceptions around peer drug use and approval of drug use, as well as being older.
Consistent with their original hypothesis, “only maternal NMPO use was significantly associated with adolescent NMPO use,” the investigators wrote (aOR, 1.62), which was not correlated either way concerning the gender of the child. The authors did note, however, “a marginally significant negative association among sons, [aOR, 0.71],” even though no overall paternal-child NMPO correlation was found (aOR, 0.98). They speculated that this negative association might be explained “by the father’s use of other drugs, particularly marijuana.”
Parental factors independently associated with adolescent NMPO use included smoking, alcohol and/or marijuana use, as well as other illicit drug use. When controlling for their use of different drugs and other covariates, only smoking remained associated with adolescent NMPO use (aOR, 1.24). Importantly, higher NMPO usage was observed in cases of poor parenting quality, especially for low levels of monitoring and high incidence of conflict between parents and adolescents. Adolescent NMPO usage were conversely lower in cases where parents self-reported their belief that drug use was risky.
Adolescent behaviors that predicted lifetime NMPO use included starting to smoke cigarettes or marijuana before using NMPO, being depressed or delinquent, having the perception that most peers use drugs, and being older in age. Dr. Griesler and her associates also observed that adolescents who began using alcohol before NMPO were likely to experiment first with smoking cigarettes and marijuana before NMPO.
The lack of differences observed with regard to child gender, race, or ethnicity warrants further investigation, but the authors speculated that “such differences might be detected with measures of current or heavy use.”
One limitation of the study was the focus on lifetime use, Dr. Griesler and her colleagues wrote. Observing patterns of current or heavy use, as well as disorder and “genetically informative samples,” might shed light on the role that familial environmental and genetic influences could play. Additionally, limiting households to one parent and one adolescent discounts the possible combined influence of mother and father NMPO usage on adolescent usage. The research also did not explore the role that adolescent NMPO use could play in influencing “parent-child interactions.”
The authors reported no financial relationships or potential conflicts of interest. The study was supported by grants from the National Institute on Drug Abuse and the New York State Psychiatric Institute; it was funded by the National Institutes of Health.
SOURCE: Griesler PC et al. Pediatrics. 2019;143(3):e20182354.
FROM PEDIATRICS
Obstetric patients with opioid use disorder fare well with medication-assisted treatment
LAS VEGAS – A prospective study of pregnant women with opioid use disorder showed good success with tapering or discontinuation of medication-assisted treatment (MAT) for women who wished to reduce or eliminate opioids while pregnant.
In related work, naltrexone showed promise for MAT in pregnancy, with rapid fetal clearance and no neonatal abstinence syndrome (NAS) seen in women who were adherent to naltrexone.
Presenting early experiences from an obstetric clinic dedicated to care of women with opioid use disorder (OUD), Craig Towers, MD, said that the clinic began seeing patients in November, 2016. “Eastern Tennessee has a high rate of opioid use disorder,” so the clinic at the University of Tennessee, Knoxville, fills an unmet need, he said, speaking during a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who enroll at the clinic are offered MAT; those who are stable on MAT and adherent to prenatal care also are offered the choice to taper from MAT and detoxify, said Dr. Towers, professor in the division of maternal-fetal medicine in the department of obstetrics and gynecology at the University of Tennessee – Knoxville.
The prospective observational cohort study found that, of a total of 367 compliant patients, 286 (78%) chose opioid tapering/detoxification, and of these, 152 (53%) did detoxify fully. Of these patients, 126 (83%) were taking no opioids at delivery, and their infants experienced no NAS.
At the time of delivery, 26 patients (17%) were taking opioids at delivery; 18 were back on MAT, and 8 were using illicit opioids. A total of 116 patients chose to continue MAT, whether they stayed on the regimen or converted back to stable MAT doses after beginning to taper.
Another option offered women at the OUD-dedicated obstetric clinic is the use of the Bridge device, a percutaneous nerve field stimulator, for OUD. Dr. Tower said that, in early use in 14 patients, the Bridge was successful in helping women transition off opioids completely in 10 (71%) patients.
About the larger study, Dr. Towers said, “This is the first study to prospectively report outcome data on a designated OUD clinic that offers detoxification during pregnancy.
“With a structured program that includes behavioral health and offers the option of detoxification, fetal risks are negligible, relapse rates by delivery are low, and NAS rates are greatly decreased,” wrote Dr. Towers and colleagues in the abstract accompanying the study.
In a related poster presentation, Dr. Towers reported outcomes for a subset of pregnant women with OUD who received naltrexone as MAT. Previously, said Dr. Towers, retrospective work showed no significant harm using naltrexone, which is one of the three approved options for MAT to manage OUD, along with buprenorphine and methadone. Naltrexone has the advantage of helping reduce cravings.
Of the 108 patients, 82 (76%) remained on naltrexone until delivery; in these pregnancies, there were no cases of NAS. However, among the 26 pregnancies in which women stopped taking naltrexone before delivery, there were 6 (23%) NAS cases.
Fifty-one patients were started on naltrexone before 24 weeks’ gestation, and of those patients, no changes were seen with fetal monitoring. There were no instances of spontaneous abortion or intrauterine demise in any participants.
The investigators tracked gestational age at the point of full detoxification and gestational age at the point naltrexone was started. Additionally, fetal response to naltrexone and maternal and neonatal outcomes were recorded.
“This is the first prospective study and largest to date on the use of naltrexone in pregnancy,” Dr. Towers and his colleagues wrote in the poster accompanying the presentation. “These data demonstrate that naltrexone MAT is a viable option for managing OUD in pregnancy.”
Dr. Towers reported no conflicts of interest and no outside sources of funding.
LAS VEGAS – A prospective study of pregnant women with opioid use disorder showed good success with tapering or discontinuation of medication-assisted treatment (MAT) for women who wished to reduce or eliminate opioids while pregnant.
In related work, naltrexone showed promise for MAT in pregnancy, with rapid fetal clearance and no neonatal abstinence syndrome (NAS) seen in women who were adherent to naltrexone.
Presenting early experiences from an obstetric clinic dedicated to care of women with opioid use disorder (OUD), Craig Towers, MD, said that the clinic began seeing patients in November, 2016. “Eastern Tennessee has a high rate of opioid use disorder,” so the clinic at the University of Tennessee, Knoxville, fills an unmet need, he said, speaking during a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who enroll at the clinic are offered MAT; those who are stable on MAT and adherent to prenatal care also are offered the choice to taper from MAT and detoxify, said Dr. Towers, professor in the division of maternal-fetal medicine in the department of obstetrics and gynecology at the University of Tennessee – Knoxville.
The prospective observational cohort study found that, of a total of 367 compliant patients, 286 (78%) chose opioid tapering/detoxification, and of these, 152 (53%) did detoxify fully. Of these patients, 126 (83%) were taking no opioids at delivery, and their infants experienced no NAS.
At the time of delivery, 26 patients (17%) were taking opioids at delivery; 18 were back on MAT, and 8 were using illicit opioids. A total of 116 patients chose to continue MAT, whether they stayed on the regimen or converted back to stable MAT doses after beginning to taper.
Another option offered women at the OUD-dedicated obstetric clinic is the use of the Bridge device, a percutaneous nerve field stimulator, for OUD. Dr. Tower said that, in early use in 14 patients, the Bridge was successful in helping women transition off opioids completely in 10 (71%) patients.
About the larger study, Dr. Towers said, “This is the first study to prospectively report outcome data on a designated OUD clinic that offers detoxification during pregnancy.
“With a structured program that includes behavioral health and offers the option of detoxification, fetal risks are negligible, relapse rates by delivery are low, and NAS rates are greatly decreased,” wrote Dr. Towers and colleagues in the abstract accompanying the study.
In a related poster presentation, Dr. Towers reported outcomes for a subset of pregnant women with OUD who received naltrexone as MAT. Previously, said Dr. Towers, retrospective work showed no significant harm using naltrexone, which is one of the three approved options for MAT to manage OUD, along with buprenorphine and methadone. Naltrexone has the advantage of helping reduce cravings.
Of the 108 patients, 82 (76%) remained on naltrexone until delivery; in these pregnancies, there were no cases of NAS. However, among the 26 pregnancies in which women stopped taking naltrexone before delivery, there were 6 (23%) NAS cases.
Fifty-one patients were started on naltrexone before 24 weeks’ gestation, and of those patients, no changes were seen with fetal monitoring. There were no instances of spontaneous abortion or intrauterine demise in any participants.
The investigators tracked gestational age at the point of full detoxification and gestational age at the point naltrexone was started. Additionally, fetal response to naltrexone and maternal and neonatal outcomes were recorded.
“This is the first prospective study and largest to date on the use of naltrexone in pregnancy,” Dr. Towers and his colleagues wrote in the poster accompanying the presentation. “These data demonstrate that naltrexone MAT is a viable option for managing OUD in pregnancy.”
Dr. Towers reported no conflicts of interest and no outside sources of funding.
LAS VEGAS – A prospective study of pregnant women with opioid use disorder showed good success with tapering or discontinuation of medication-assisted treatment (MAT) for women who wished to reduce or eliminate opioids while pregnant.
In related work, naltrexone showed promise for MAT in pregnancy, with rapid fetal clearance and no neonatal abstinence syndrome (NAS) seen in women who were adherent to naltrexone.
Presenting early experiences from an obstetric clinic dedicated to care of women with opioid use disorder (OUD), Craig Towers, MD, said that the clinic began seeing patients in November, 2016. “Eastern Tennessee has a high rate of opioid use disorder,” so the clinic at the University of Tennessee, Knoxville, fills an unmet need, he said, speaking during a poster session at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Women who enroll at the clinic are offered MAT; those who are stable on MAT and adherent to prenatal care also are offered the choice to taper from MAT and detoxify, said Dr. Towers, professor in the division of maternal-fetal medicine in the department of obstetrics and gynecology at the University of Tennessee – Knoxville.
The prospective observational cohort study found that, of a total of 367 compliant patients, 286 (78%) chose opioid tapering/detoxification, and of these, 152 (53%) did detoxify fully. Of these patients, 126 (83%) were taking no opioids at delivery, and their infants experienced no NAS.
At the time of delivery, 26 patients (17%) were taking opioids at delivery; 18 were back on MAT, and 8 were using illicit opioids. A total of 116 patients chose to continue MAT, whether they stayed on the regimen or converted back to stable MAT doses after beginning to taper.
Another option offered women at the OUD-dedicated obstetric clinic is the use of the Bridge device, a percutaneous nerve field stimulator, for OUD. Dr. Tower said that, in early use in 14 patients, the Bridge was successful in helping women transition off opioids completely in 10 (71%) patients.
About the larger study, Dr. Towers said, “This is the first study to prospectively report outcome data on a designated OUD clinic that offers detoxification during pregnancy.
“With a structured program that includes behavioral health and offers the option of detoxification, fetal risks are negligible, relapse rates by delivery are low, and NAS rates are greatly decreased,” wrote Dr. Towers and colleagues in the abstract accompanying the study.
In a related poster presentation, Dr. Towers reported outcomes for a subset of pregnant women with OUD who received naltrexone as MAT. Previously, said Dr. Towers, retrospective work showed no significant harm using naltrexone, which is one of the three approved options for MAT to manage OUD, along with buprenorphine and methadone. Naltrexone has the advantage of helping reduce cravings.
Of the 108 patients, 82 (76%) remained on naltrexone until delivery; in these pregnancies, there were no cases of NAS. However, among the 26 pregnancies in which women stopped taking naltrexone before delivery, there were 6 (23%) NAS cases.
Fifty-one patients were started on naltrexone before 24 weeks’ gestation, and of those patients, no changes were seen with fetal monitoring. There were no instances of spontaneous abortion or intrauterine demise in any participants.
The investigators tracked gestational age at the point of full detoxification and gestational age at the point naltrexone was started. Additionally, fetal response to naltrexone and maternal and neonatal outcomes were recorded.
“This is the first prospective study and largest to date on the use of naltrexone in pregnancy,” Dr. Towers and his colleagues wrote in the poster accompanying the presentation. “These data demonstrate that naltrexone MAT is a viable option for managing OUD in pregnancy.”
Dr. Towers reported no conflicts of interest and no outside sources of funding.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Over two-thirds of MAT-adherent patients chose detoxification.
Major finding:
Study details: Prospective single-center cohort study of 367 pregnant women with opioid use disorder.
Disclosures: Dr. Towers reported no outside sources of funding and no relevant conflicts of interest.
Source: Towers C. et al. SMFM 2019, Posters 141 & 142.