User login
Reworked OxyContin fails to cut overall opioid abuse, FDA panel says
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
One in seven high schoolers is misusing opioids
according to an analysis from the Centers for Disease Control and Prevention.
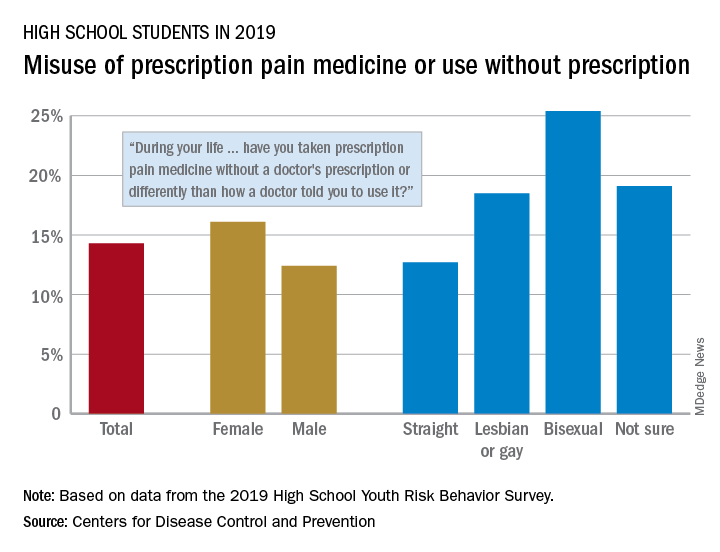
That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
according to an analysis from the Centers for Disease Control and Prevention.

That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
according to an analysis from the Centers for Disease Control and Prevention.

That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
FROM MMWR
Deaths, despair tied to drug dependence are accelerating amid COVID-19
Patients with OUDs need assistance now more than ever.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
Patients with OUDs need assistance now more than ever.
Patients with OUDs need assistance now more than ever.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
The Centers for Disease Control and Prevention reported recently that opioid overdose deaths will increase to a new U.S. record, and more are expected as pandemic-related overdose deaths are yet to be counted.1
Specifically, according to the CDC, 70,980 people died from fatal overdoses in 2019,2 which is record high. Experts such as Bruce A. Goldberger, PhD, fear that the 2020 numbers could rise even higher, exacerbated by the coronavirus pandemic.
Deaths from drug overdoses remain higher than the peak yearly death totals ever recorded for car accidents, guns, or AIDS. Overdose deaths have accelerated further – pushing down overall life expectancy in the United States.3 Headlines purporting to identify good news in drug death figures don’t always get below top-level data. Deaths and despair tied to drug dependence are indeed accelerating. I am concerned about these alarmingly dangerous trends.
Synthetic opioids such as fentanyl accounted for about 3,000 deaths in 2013. By 2019, they accounted for more than 37,137.4 In addition, 16,539 deaths involved stimulants such as methamphetamine, and 16,196 deaths involved cocaine, the most recent CDC reporting shows. Opioids continue to play a role in U.S. “deaths of despair,” or rising fatalities from drugs, suicides, and alcohol among Americans without employment, hope of job opportunities, or college degrees.5 As the American Medical Association has warned,6 more people are dying from overdoses amid the COVID-19 pandemic. Clinicians need to be aware of trends so that we can help our patients navigate these challenges.
Fentanyl presents dangers
Experts had predicted that the pandemic, by limiting access to treatment, rescue, or overdose services, and increasing time at home and in the neighborhood, would result in more tragedy. In addition, the shift from prescription opioids to heroin and now to fentanyl has made deaths more common.
Fentanyls – synthetic opioids – are involved in more than half of overdose deaths, and in many of the cocaine and methamphetamine-related deaths, which also are on the rise. Fentanyl is about 100 times more potent than morphine and 50 times more potent than heroin. Breathing can stop after use of just 2 mg of fentanyl, which is about as much as trace amounts of table salt. Fentanyl has replaced heroin in many cities as the pandemic changed the relative ease of importing raw drugs such as heroin.
Another important trend is that fentanyl production and distribution throughout the United States have expanded. The ease of manufacture in unregulated sectors of the Chinese and Mexican economies is difficult for U.S. authorities to curb or eliminate. The Internet promotes novel strategies for synthesizing the substance, spreading its production across many labs; suppliers use the U.S. Postal Service for distribution, and e-commerce helps to get the drug from manufacturers to U.S. consumers for fentanyl transactions.
A recent RAND report observes that, for only $10 through the postal service, suppliers can ship a 1-kg parcel from China to the United States, and private shipments cost about $100.7 And with large volumes of legal trade between the two countries making rigorous scrutiny of products difficult, especially given the light weight of fentanyl, suppliers find it relatively easy to hide illicit substances in licit shipments. Opioid users have made the switch to fentanyl, and have seen fentanyl added to cocaine and methamphetamine they buy on the streets.
OUD and buprenorphine
Fentanyl is one part of the overdose crisis. Opioid use disorder (OUD) is the other. Both need to be addressed if we are to make any progress in this epidemic of death and dependency.
The OUD crisis continues amid the pandemic – and isn’t going away.8 Slips, relapses, and overdoses are all too common. Medication-assisted treatment (MAT) and OUD treatment programs are essential parts of our response to overdose initiatives. After naloxone rescue, the best anti-overdose response is to get the OUD patient into treatment with MATs. Patients with OUD have continuously high risks of overdose. The best outcomes appear to be related to treatment duration of greater than 2 years. But it is common to see patients with OUDs who have been in treatment multiple times, taking MATs, dropping out, overdosing, and dying. Some have been described as treatment resistant.9 It is clear that treatment can work, but also that even evidence-based treatments often fail.10
A recent study compared OUD patients who continued treatment for 6-9 months to those patients who had continued MAT treatment for 15-18 months. The longer the treatment, the fewer emergencies, prescriptions, or hospitalizations.11
But this study reminds us that all OUD patients, whether they are currently buprenorphine treated or not, experience overdoses and emergency department interventions. Short and longer treatment groups have a similar nonfatal overdose rate, about 6%, and went to the emergency department at a high rate, above 40%. Discontinuation of buprenorphine treatment is a major risk factor in opioid relapse, emergency department visits, and overdose. Cures are not common. Whether an OUD patient is being treated or has been treated in the past, carrying naloxone (brand name Narcan), makes sense and can save lives.
Methadone still considered most effective
Methadone is a synthetic opioid first studied as a treatment for OUD at Rockefeller University in New York City in the 1960s. Methadone may be the most effective treatment for OUD in promoting treatment retention for years, decreasing intravenous drug use, and decreasing deaths.12 It has been studied and safely used in treatment programs for decades. Methadone is typically administered in a clinic, daily, and with observation. In addition, methadone patients periodically take urine drug tests, which can distinguish methadone from substances of abuse. They also receive counseling. But methadone can be prescribed and administered only in methadone clinics in the United States. It is available for prescription in primary care clinics in Great Britain, Canada, and Australia.13 Numerous experts have suggested passing new legislation aimed at changing how methadone can be prescribed. Allowing primary care to administer methadone, just like buprenorphine, can improve access and benefit OUD patients.12
Availability of Narcan is critical
A comprehensive treatment model for OUDs includes prescribing naloxone, encouraging those patients with an OUD and their loved ones to have naloxone with them, and providing MATs and appropriate therapies, such as counseling.
As described by Allison L. Pitt and colleagues at Stanford (Calif.) University,14 the United States might be on track to have up to 500,000 deaths tied to opioid overdoses that might occur over the next 5 years. They modeled the effect on overdose of a long list of interventions, but only a few had an impact. At the top of the list was naloxone availability. We need to focus on saving lives by increasing naloxone availability, improving initiation, and expanding access to MAT, and increasing psychosocial treatment to improve outcomes, increase life-years and quality-adjusted life-years, and reduce opioid-related deaths. When Ms. Pitt and colleagues looked at what would make the most impact in reducing OUD deaths, it was naloxone. Pain patients on higher doses of opioids, nonprescription opioid users, OUD patients should be given naloxone prescriptions. While many can give a Heimlich to a choking person or CPR, few have naloxone to rescue a person who has overdosed on opioids. If an overdose is suspected, it should be administered by anyone who has it, as soon as possible. Then, the person who is intervening should call 911.
What we can do today
At this moment, clinicians can follow the Surgeon General’s advice,15 and prescribe naloxone.
We should give naloxone to OUD patients and their families, to pain patients at dosages of greater than or equal to 50 MME. Our top priorities should be patients with comorbid pain syndromes, those being treated with benzodiazepines and sleeping medications, and patients with alcohol use disorders. This is also an important intervention for those who binge drink, and have sleep apnea, and heart and respiratory diseases.
Naloxone is available without a prescription in at least 43 states. Naloxone is available in harm reduction programs and in hospitals, and is carried by emergency medical staff, law enforcement, and EMTs. It also is available on the streets, though it does not appear to have a dollar value like opioids or even buprenorphine. Also, the availability of naloxone in pharmacies has made it easier for family members and caregivers of pain patients or those with OUD to have it to administer in an emergency.
An excellent place for MDs to start is to do more to encourage all patients with OUD to carry naloxone, for their loved ones to carry naloxone, and for their homes to have naloxone nearby in the bedroom or bathroom. It is not logical to expect a person with an OUD to rescue themselves. Current and past OUD patients, as well as their loved ones, are at high risk – and should have naloxone nearby at all times.
Naloxone reverses an opioid overdose, but it should be thought about like cardioversion or CPR rather than a treatment for an underlying disease. Increasing access to buprenorphine, buprenorphine + naloxone, and naltrexone treatment for OUDs is an important organizing principle. Initiation of MAT treatment in the emergency setting or most anywhere and any place a patient with an OUD can begin treatment is necessary. Treatment with buprenorphine or methadone reduces opioid overdose and opioid-related acute care use.16
Reducing racial disparities in OUD treatment is necessary, because buprenorphine treatment is concentrated among White patients who either use private insurance or are self-pay.17 Reducing barriers to methadone program licenses, expanding sites for distribution,18 prescribing methadone in an office setting might help. Clinicians can do a better job of explaining the risks associated with opioid prescriptions, including diversion and overdose, and the benefits of OUD treatment. So, To reduce opioid overdoses, we must increase physician competencies in addiction medicine.
Dr. Gold is professor of psychiatry (adjunct) at Washington University, St. Louis. He is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville. For more than 40 years, Dr. Gold has worked on developing models for understanding the effects of opioid, tobacco, cocaine, and other drugs, as well as food, on the brain and behavior. He disclosed financial ties with ADAPT Pharma and Magstim Ltd.
References
1. Kamp J. Overdose deaths rise, may reach record level, federal data show. Wall Street Journal. 2020 Jul 15.
2. 12 month–ending provisional number of drug overdose drugs. Centers for Disease Control and Prevention. 2020 Jul 5.
3. Katz J et al. In shadow of pandemic, U.S. drug overdose deaths resurge to record. New York Times. 2020 Jul 15.
4. Gold MS. The fentanyl crisis is only getting worse. Addiction Policy Forum. Updated 2020 Mar 12.
5. Gold MS. Mo Med. 2020-Mar-Apr;117(2):99-101.
6. Reports of increases in opioid-related overdoses and other concerns during the COVID-19 pandemic. American Medical Association. Issue brief. Updated 2020 Jul 20.
7. Pardo B et al. The future of fentanyl and other synthetic opioids. RAND report.
8. Gold MS. New challenges in the opioid epidemic. Addiction Policy Forum. 2020 Jun 4.
9. Patterson Silver Wolf DA and Gold MS. J Neurol Sci. 2020;411:116718.
10. Oesterle TS et al. Mayo Clin Proc. 2019;94(10):2072-86.
11. Connery HS and Weiss RD. Am J Psychiatry. 2020;177(2):104-6.
12. Kleber HD. JAMA. 2008;300(19):2303-5.
13. Samet JH et al. N Engl J Med. 2018;379(1):7-8.
14. Pitt AL et al. Am J Public Health. 2018;108(10):1394-1400.
15. U.S. Surgeon General’s Advisory on Naloxone and Opioid Overdose. hhs.gov.
16. Wakeman SE et al. JAMA Netw Open. 2020;3(2):e1920622.
17. Lagisetty PA et al. JAMA Psychiatry. 2019;76(9):979-81.
18. Kleinman RA. JAMA Psychiatry. 2020 Jul 15. doi: 10.1001/jamapsychiatry.2020.1624.
AMA urges change after dramatic increase in illicit opioid fatalities
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
In the past 5 years, there has been a significant drop in the use of prescription opioids and in deaths associated with such use; but at the same time there’s been a dramatic increase in fatalities involving illicit opioids and stimulants, a new report from the American Medical Association (AMA) Opioid Task Force shows.
Although the medical community has made some important progress against the opioid epidemic, with a 37% reduction in opioid prescribing since 2013, illicit drugs are now the dominant reason why drug overdoses kill more than 70,000 people each year, the report says.
In an effort to improve the situation, the AMA Opioid Task Force is urging the removal of barriers to evidence-based care for patients who have pain and for those who have substance use disorders (SUDs). The report notes that “red tape and misguided policies are grave dangers” to these patients.
“It is critically important as we see drug overdoses increasing that we work towards reducing barriers of care for substance use abusers,” Task Force Chair Patrice A. Harris, MD, said in an interview.
“At present, the status quo is killing far too many of our loved ones and wreaking havoc in our communities,” she said.
Dr. Harris noted that “a more coordinated/integrated approach” is needed to help individuals with SUDs.
“It is vitally important that these individuals can get access to treatment. Everyone deserves the opportunity for care,” she added.
Dramatic increases
The report cites figures from the Centers for Disease Control and Prevention that indicate the following regarding the period from the beginning of 2015 to the end of 2019:
- Deaths involving illicitly manufactured and fentanyl analogues increased from 5,766 to 36,509.
- Deaths involving stimulants such as increased from 4,402 to 16,279.
- Deaths involving cocaine increased from 5,496 to 15,974.
- Deaths involving heroin increased from 10,788 to 14,079.
- Deaths involving prescription opioids decreased from 12,269 to 11,904.
The report notes that deaths involving prescription opioids peaked in July 2017 at 15,003.
Some good news
In addition to the 37% reduction in opioid prescribing in recent years, the AMA lists other points of progress, such as a large increase in prescription drug monitoring program registrations. More than 1.8 million physicians and other healthcare professionals now participate in these programs.
Also, more physicians are now certified to treat opioid use disorder. More than 85,000 physicians, as well as a growing number of nurse practitioners and physician assistants, are now certified to treat patients in the office with buprenorphine. This represents an increase of more than 50,000 from 2017.
Access to naloxone is also increasing. More than 1 million naloxone prescriptions were dispensed in 2019 – nearly double the amount in 2018. This represents a 649% increase from 2017.
“We have made some good progress, but we can’t declare victory, and there are far too many barriers to getting treatment for substance use disorder,” Dr. Harris said.
“Policymakers, public health officials, and insurance companies need to come together to create a system where there are no barriers to care for people with substance use disorder and for those needing pain medications,” she added.
At present, prior authorization is often needed before these patients can receive medication. “This involves quite a bit of administration, filling in forms, making phone calls, and this is stopping people getting the care they need,” said Dr. Harris.
“This is a highly regulated environment. There are also regulations on the amount of methadone that can be prescribed and for the prescription of buprenorphine, which has to be initiated in person,” she said.
Will COVID-19 bring change?
Dr. Harris noted that some of these regulations have been relaxed during the COVID-19 crisis so that physicians could ensure that patients have continued access to medication, and she suggested that this may pave the way for the future.
“We need now to look at this carefully and have a conversation about whether these relaxations can be continued. But this would have to be evidence based. Perhaps we can use experience from the COVID-19 period to guide future policy on this,” she said.
The report highlights that despite medical society and patient advocacy, only 21 states and the District of Columbia have enacted laws that limit public and private insurers from imposing prior authorization requirements on SUD services or medications.
The Task Force urges removal of remaining prior authorizations, step therapy, and other inappropriate administrative burdens that delay or deny care for Food and Drug Administration–approved medications used as part of medication-assisted treatment for opioid use disorder.
The organization is also calling for better implementation of mental health and substance use disorder parity laws that require health insurers to provide the same level of benefits for mental health and SUD treatment and services that they do for medical/surgical care.
At present, only a few states have taken meaningful action to enact or enforce those laws, the report notes.
The Task Force also recommends the implementation of systems to track overdose and mortality trends to provide equitable public health interventions. These measures would include comprehensive, disaggregated racial and ethnic data collection related to testing, hospitalization, and mortality associated with opioids and other substances.
“We know that ending the drug overdose epidemic will not be easy, but if policymakers allow the status quo to continue, it will be impossible,” Dr. Harris said.
“ Physicians will continue to do our part. We urge policymakers to do theirs,” she added.
A version of this article originally appeared on Medscape.com.
Some women use prescription opioids during pregnancy
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.
Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
and almost a third of those women did not receive counseling from a provider on the effects of opioids on their unborn children, according to analysis from the Centers for Disease Control and Prevention.

Data from the Pregnancy Risk Assessment Monitoring System 2019 survey show that 7% of the nearly 21,000 respondents reported using an opioid pain reliever during pregnancy, considerably lower than the fill rates of 14%-22% seen in studies of pharmacy dispensing, Jean Y. Ko, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
In the current analysis, opioid use during pregnancy varied by age – the rate was highest, 10%, in those aged 19 years and under and dropped as age increased to 6% among those aged 35 and older – and by race/ethnicity – 9% of black women reported use, compared with 7% of Hispanics, 6% of whites, and 7% of all others, the investigators reported.
Use of prescription opioids was significantly higher for two specific groups. Women who smoked cigarettes during the last 3 months of their pregnancy had a 16% rate of opioid use, and those with depression during pregnancy had a rate of 13%, they said.
Physicians caring for pregnant women should seek to identify and address substance use and misuse, and mental health conditions such as depression, history of trauma, posttraumatic stress disorder, and anxiety, the CDC researchers pointed out.
The CDC and the American College of Obstetricians and Gynecologists both recommend that caregivers and patients also need to “discuss and carefully weigh risks and benefits when considering initiation of opioid therapy for chronic pain during pregnancy,” Dr. Ko and associates wrote.
That sort of counseling, however, was not always offered: 32% of the women with self-reported prescription opioid use during their pregnancy said that they had not been counseled about the drugs’ effect on an infant. Some variation was seen by age or race/ethnicity, but the differences were not significant, the researchers reported.
“Opioid prescribing consistent with clinical practice guidelines can ensure that patients, particularly those who are pregnant, have access to safer, more effective chronic pain treatment and reduce the number of persons at risk for opioid misuse, opioid use disorder, and overdose,” the investigators concluded.
Survey data from 32 jurisdictions (30 states, along with the District of Columbia and Puerto Rico) that participate in the monitoring system were included in the analysis, as were data from California and Ohio, which do not participate. All of the respondents had a live birth in the preceding 2-6 months, the researchers explained.
SOURCE: Ko JY et al. MMWR. 2020 Jul 17;69(28):897-903.
FROM MMWR
Travel times to opioid addiction programs drive a lack of access to treatment
If US pharmacies were permitted to dispense methadone for opioid use disorder (OUD) it would improve national access to treatment and save costs, new research suggests.
Under current federal regulations, only opioid treatment programs (OTPs) are permitted to dispense methadone maintenance treatment. This stands in sharp contrast to how methadone is dispensed in Canada, Australia, and the United Kingdom, where patients can obtain daily doses of methadone maintenance from community pharmacies.
“It’s challenging for patients in many parts of the US to access methadone treatment,” Robert Kleinman, MD, of Stanford University School of Medicine, Stanford, California, said in a JAMA Psychiatry podcast.
“It’s important for policymakers to consider strategies that enhance access to methadone maintenance treatment, in that it’s associated with large reductions in mortality from opioid use disorder. One possibility is to use pharmacies as dispensing sites,” said Kleinman.
The study was published online July 15 in JAMA Psychiatry.
An Hour vs 10 Minutes
Kleinman examined how pharmacy-based dispensing would affect drive times to the nearest OTP for the general US population. The analysis included all 1682 OTP locations, 69,475 unique pharmacy locations, and 72,443 census tracts.
The average drive time to OTPs in the US is 20.4 minutes vs a drive time of 4.5 minutes to pharmacies.
Driving times to OTPs are particularly long in nonmetropolitan counties while pharmacies remain “relatively easily accessible” in nonmetropolitan counties, he said.
In “micropolitan” counties, for example, the drive time to OTPs was 48.4 minutes vs 7 minutes to pharmacies. In the most rural counties, the drive time to OTPs is 60.9 minutes vs 9.1 minutes to pharmacies.
“This suggests that pharmacy-based dispensing has the potential to reduce urban or rural inequities, and access to methadone treatment,” Kleinman said.
In a mileage cost analysis, Kleinman determined that the average cost of one-way trip to an OTP in the US is $3.12 compared with 45 cents to a pharmacy. In the most rural counties, the average cost one-way is $11.10 vs $1.27 to a pharmacy.
Kleinman says decreasing drive times, distance, and costs for patients seeking methadone treatment by allowing pharmacies to dispense the medication may help achieve several public health goals.
“Patients dissuaded from obtaining treatment because of extended travel, particularly patients with disabilities, unreliable access to transportation, or from rural regions, would have reduced barriers to care. Quality of life may be increased for the more than 380,000 individuals currently receiving methadone treatment if less time is spent commuting,” he writes.
Time for Change
The authors of an accompanying editorial, say the “regulatory burden” on methadone provision in the US “effectively prohibits the integration of methadone prescribing into primary care, even in rural communities where there may exist no specialty substance use treatment options.”
However, federal and state agencies are starting to take action to expand geographic access to methadone treatment, note Paul Joudrey, MD, MPH, and coauthors from Yale School of Medicine, New Haven, Connecticut.
, and Ohio and Kentucky have passed laws to allow greater use of federally qualified health centers and other facilities for methadone dispensing.
“While these policies are welcomed, the results here by Kleinman and others suggest they fall short of needed expansion if patients’ rights to evidence-based care for OUD are to be ensured. Importantly, even with broad adoption of mobile or pharmacy-based dispensing, patients would still face a long drive time to a central OTP before starting methadone,” Joudrey and colleagues write.
In their view, the only way to address this barrier is to modify federal law, and this “should be urgently pursued in the context of the ongoing overdose epidemic. It is time for policies that truly support methadone treatment for OUD as opposed to focusing on diversion.”
This article first appeared on Medscape.com.
If US pharmacies were permitted to dispense methadone for opioid use disorder (OUD) it would improve national access to treatment and save costs, new research suggests.
Under current federal regulations, only opioid treatment programs (OTPs) are permitted to dispense methadone maintenance treatment. This stands in sharp contrast to how methadone is dispensed in Canada, Australia, and the United Kingdom, where patients can obtain daily doses of methadone maintenance from community pharmacies.
“It’s challenging for patients in many parts of the US to access methadone treatment,” Robert Kleinman, MD, of Stanford University School of Medicine, Stanford, California, said in a JAMA Psychiatry podcast.
“It’s important for policymakers to consider strategies that enhance access to methadone maintenance treatment, in that it’s associated with large reductions in mortality from opioid use disorder. One possibility is to use pharmacies as dispensing sites,” said Kleinman.
The study was published online July 15 in JAMA Psychiatry.
An Hour vs 10 Minutes
Kleinman examined how pharmacy-based dispensing would affect drive times to the nearest OTP for the general US population. The analysis included all 1682 OTP locations, 69,475 unique pharmacy locations, and 72,443 census tracts.
The average drive time to OTPs in the US is 20.4 minutes vs a drive time of 4.5 minutes to pharmacies.
Driving times to OTPs are particularly long in nonmetropolitan counties while pharmacies remain “relatively easily accessible” in nonmetropolitan counties, he said.
In “micropolitan” counties, for example, the drive time to OTPs was 48.4 minutes vs 7 minutes to pharmacies. In the most rural counties, the drive time to OTPs is 60.9 minutes vs 9.1 minutes to pharmacies.
“This suggests that pharmacy-based dispensing has the potential to reduce urban or rural inequities, and access to methadone treatment,” Kleinman said.
In a mileage cost analysis, Kleinman determined that the average cost of one-way trip to an OTP in the US is $3.12 compared with 45 cents to a pharmacy. In the most rural counties, the average cost one-way is $11.10 vs $1.27 to a pharmacy.
Kleinman says decreasing drive times, distance, and costs for patients seeking methadone treatment by allowing pharmacies to dispense the medication may help achieve several public health goals.
“Patients dissuaded from obtaining treatment because of extended travel, particularly patients with disabilities, unreliable access to transportation, or from rural regions, would have reduced barriers to care. Quality of life may be increased for the more than 380,000 individuals currently receiving methadone treatment if less time is spent commuting,” he writes.
Time for Change
The authors of an accompanying editorial, say the “regulatory burden” on methadone provision in the US “effectively prohibits the integration of methadone prescribing into primary care, even in rural communities where there may exist no specialty substance use treatment options.”
However, federal and state agencies are starting to take action to expand geographic access to methadone treatment, note Paul Joudrey, MD, MPH, and coauthors from Yale School of Medicine, New Haven, Connecticut.
, and Ohio and Kentucky have passed laws to allow greater use of federally qualified health centers and other facilities for methadone dispensing.
“While these policies are welcomed, the results here by Kleinman and others suggest they fall short of needed expansion if patients’ rights to evidence-based care for OUD are to be ensured. Importantly, even with broad adoption of mobile or pharmacy-based dispensing, patients would still face a long drive time to a central OTP before starting methadone,” Joudrey and colleagues write.
In their view, the only way to address this barrier is to modify federal law, and this “should be urgently pursued in the context of the ongoing overdose epidemic. It is time for policies that truly support methadone treatment for OUD as opposed to focusing on diversion.”
This article first appeared on Medscape.com.
If US pharmacies were permitted to dispense methadone for opioid use disorder (OUD) it would improve national access to treatment and save costs, new research suggests.
Under current federal regulations, only opioid treatment programs (OTPs) are permitted to dispense methadone maintenance treatment. This stands in sharp contrast to how methadone is dispensed in Canada, Australia, and the United Kingdom, where patients can obtain daily doses of methadone maintenance from community pharmacies.
“It’s challenging for patients in many parts of the US to access methadone treatment,” Robert Kleinman, MD, of Stanford University School of Medicine, Stanford, California, said in a JAMA Psychiatry podcast.
“It’s important for policymakers to consider strategies that enhance access to methadone maintenance treatment, in that it’s associated with large reductions in mortality from opioid use disorder. One possibility is to use pharmacies as dispensing sites,” said Kleinman.
The study was published online July 15 in JAMA Psychiatry.
An Hour vs 10 Minutes
Kleinman examined how pharmacy-based dispensing would affect drive times to the nearest OTP for the general US population. The analysis included all 1682 OTP locations, 69,475 unique pharmacy locations, and 72,443 census tracts.
The average drive time to OTPs in the US is 20.4 minutes vs a drive time of 4.5 minutes to pharmacies.
Driving times to OTPs are particularly long in nonmetropolitan counties while pharmacies remain “relatively easily accessible” in nonmetropolitan counties, he said.
In “micropolitan” counties, for example, the drive time to OTPs was 48.4 minutes vs 7 minutes to pharmacies. In the most rural counties, the drive time to OTPs is 60.9 minutes vs 9.1 minutes to pharmacies.
“This suggests that pharmacy-based dispensing has the potential to reduce urban or rural inequities, and access to methadone treatment,” Kleinman said.
In a mileage cost analysis, Kleinman determined that the average cost of one-way trip to an OTP in the US is $3.12 compared with 45 cents to a pharmacy. In the most rural counties, the average cost one-way is $11.10 vs $1.27 to a pharmacy.
Kleinman says decreasing drive times, distance, and costs for patients seeking methadone treatment by allowing pharmacies to dispense the medication may help achieve several public health goals.
“Patients dissuaded from obtaining treatment because of extended travel, particularly patients with disabilities, unreliable access to transportation, or from rural regions, would have reduced barriers to care. Quality of life may be increased for the more than 380,000 individuals currently receiving methadone treatment if less time is spent commuting,” he writes.
Time for Change
The authors of an accompanying editorial, say the “regulatory burden” on methadone provision in the US “effectively prohibits the integration of methadone prescribing into primary care, even in rural communities where there may exist no specialty substance use treatment options.”
However, federal and state agencies are starting to take action to expand geographic access to methadone treatment, note Paul Joudrey, MD, MPH, and coauthors from Yale School of Medicine, New Haven, Connecticut.
, and Ohio and Kentucky have passed laws to allow greater use of federally qualified health centers and other facilities for methadone dispensing.
“While these policies are welcomed, the results here by Kleinman and others suggest they fall short of needed expansion if patients’ rights to evidence-based care for OUD are to be ensured. Importantly, even with broad adoption of mobile or pharmacy-based dispensing, patients would still face a long drive time to a central OTP before starting methadone,” Joudrey and colleagues write.
In their view, the only way to address this barrier is to modify federal law, and this “should be urgently pursued in the context of the ongoing overdose epidemic. It is time for policies that truly support methadone treatment for OUD as opposed to focusing on diversion.”
This article first appeared on Medscape.com.
Medication-assisted treatment in corrections: A life-saving intervention
Opioid overdose deaths in the United States have more than tripled in recent years, from 6.1 deaths per 100,000 individuals in 1999 to 20.7 per 100,000 individuals in 2018.1 Although the availability of medication-assisted treatment (MAT) has expanded over the past decade, this lifesaving treatment remains largely inaccessible to some of the most vulnerable members of our communities: opioid users facing reentry after incarceration.
Just as abstinence in the community brings a loss of tolerance to opioids, individuals who are incarcerated lose tolerance as well. Clinicians who treat patients with opioid use disorders (OUD) are accustomed to warning patients about the risk of returning to prior levels of use too quickly. Harm reduction strategies include using slowly, using with friends, and having naloxone on hand to prevent unintended overdose.
The risks of opioid use are magnified for those facing reentry; incarceration contributes to a loss of employment, social supports, and connection to care. Those changes can create an exceptionally stressful reentry period – one that places individuals at an acutely high risk of relapse and overdose. Within the first 2 years of release, an individual with a history of incarceration has a risk of death 3.5 times higher than that of someone in the general population. Within the first 2 weeks, those recently incarcerated are 129 times more likely to overdose on opioids and 12.7 times more likely to die than members of the general population.2
Treatment with MAT dramatically reduces deaths during this crucial period. In England, large national studies have shown a similar 75% decrease in all-cause mortality within the first 4 weeks of release among individuals with OUD.4 In California, the counties with the highest overdose death rates are consistently those with fewer opioid treatment programs, which suggests that access to treatment is necessary to prolong the lives of those suffering from OUD.5 In-custody overdose deaths are quite rare, and access to MAT during incarceration has decreased in-custody deaths by 74%.6
Decreased opioid overdose deaths is not the only outcome of MAT. Pharmacotherapy for OUD also has been shown to increase treatment retention,7 reduce reincarceration,8 prevent communicable infections,9 and decrease use of other illicit substances.10 The provision of MAT also has been shown to be cost effective.11
Despite those benefits, as of 2017, only 30 out of 5,100 jails and prisons in the United States provided treatment with methadone or buprenorphine.12 When individuals on maintenance therapy are incarcerated, most correctional facilities force them to taper and discontinue those medications. This practice can cause distressing withdrawal symptoms and actively increase the risk of death for these individuals.
Concerns related to the provision of MAT, and specifically buprenorphine, in the correctional health setting often are related to diversion. Although safe administration of opioid full and partial agonists is a priority, recent literature has suggested that buprenorphine is not a medication frequently used for euphoric properties. In fact, the literature suggests that individuals using illicit buprenorphine primarily do so to treat withdrawal symptoms and that illicit use diminishes with access to formal treatment.13,14
Another concern is that pharmacotherapy for OUD should not be used without adjunctive psychotherapies and social supports. While dual pharmacotherapy and psychotherapy is ideal, the American Society for Addiction Medicine 2020 National Practice Guidelines for the treatment of OUD state: “a patient’s decision to decline psychosocial treatment or the absence of available psychosocial treatment should not preclude or delay pharmacotherapy, with appropriate medication management.”15 Just as some patients wish to engage in mutual help or psychotherapeutic modalities only, some patients wish to engage only in psychopharmacologic interventions. Declaring one modality of treatment better, or worse, or more worthwhile is not borne out by the literature and often places clinicians’ preferences over the preferences of patients.
Individuals who suffer from substance use disorders are at high risk of incarceration, relapse, and overdose death. These patients also suffer from stigmatization from peers and health care workers alike, making the process of engaging in care incredibly burdensome. Because of the disease of addiction, many of our patients cannot envision a healthy future: a future with the potential for intimate relationships, meaningful community engagement, and a rich inner life. The provision of MAT is lifesaving and improves the chances of a successful reentry – an intuitive first step in a long, but worthwhile, journey.
References
1. Hedegaard H et al; National Center for Health Statistics. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, 2020 Jan, No. 356.
2. Binswanger IA et al. N Engl J Med. 2007;356:157-65.
3. Green TC et al. JAMA Psychiatry. 2018;75(4):405-7.
4. Marsden J et al. Addiction. 2017;112(8):1408-18.
5. Joshi V and Urada D. State Targeted Response to the Opioid Crisis: California Strategic Plan. 2017 Aug 30.
6. Larney S et al. BMJ Open. 2014. doi: 10.1136/bmjopen-2013-004666.
7. Rich JD et al. Lancet. 2015;386(9991):350-9.
8. Deck D et al. J Addict Dis. 2009. 28(2):89-102.
9. MacArthur GJ et al. BMJ. 2012. doi: 10.1136/bmj.e5945.
10. Tsui J et al. J Subst Abuse Treat. 2019. 109:80-5.
11. Gisev N et al. Addiction. 2015 Dec;110(12):1975-84.
12. National Mental Health and Substance Use Policy Laboratory. “Use of Medication-Assisted Treatment for Opioid Use Disorder in Criminal Justice Settings.” HHS Publication No. PEP19-MATUSECJS. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2019.
13. Bazazi AR et al. J Addict Med. 2011;5(3):175-80.
14. Schuman-Olivier Z. et al. J Subst Abuse Treat. 2010 Jul;39(1):41-50.
15. Crotty K et al. J Addict Med. 2020;14(2)99-112.
Dr. Barnes is chief resident at San Mateo County Behavioral Health and Recovery Services in California. He disclosed no relevant financial relationships. Dr. Lenane is resident* at San Mateo County Behavioral Health and Recovery Services. He disclosed no relevant financial relationships. The opinions shared in this article represent the viewpoints of the authors and are not necessarily representative of the viewpoints or policies of their academic program or employer.
*This article was updated 7/9/2020.
Opioid overdose deaths in the United States have more than tripled in recent years, from 6.1 deaths per 100,000 individuals in 1999 to 20.7 per 100,000 individuals in 2018.1 Although the availability of medication-assisted treatment (MAT) has expanded over the past decade, this lifesaving treatment remains largely inaccessible to some of the most vulnerable members of our communities: opioid users facing reentry after incarceration.
Just as abstinence in the community brings a loss of tolerance to opioids, individuals who are incarcerated lose tolerance as well. Clinicians who treat patients with opioid use disorders (OUD) are accustomed to warning patients about the risk of returning to prior levels of use too quickly. Harm reduction strategies include using slowly, using with friends, and having naloxone on hand to prevent unintended overdose.
The risks of opioid use are magnified for those facing reentry; incarceration contributes to a loss of employment, social supports, and connection to care. Those changes can create an exceptionally stressful reentry period – one that places individuals at an acutely high risk of relapse and overdose. Within the first 2 years of release, an individual with a history of incarceration has a risk of death 3.5 times higher than that of someone in the general population. Within the first 2 weeks, those recently incarcerated are 129 times more likely to overdose on opioids and 12.7 times more likely to die than members of the general population.2
Treatment with MAT dramatically reduces deaths during this crucial period. In England, large national studies have shown a similar 75% decrease in all-cause mortality within the first 4 weeks of release among individuals with OUD.4 In California, the counties with the highest overdose death rates are consistently those with fewer opioid treatment programs, which suggests that access to treatment is necessary to prolong the lives of those suffering from OUD.5 In-custody overdose deaths are quite rare, and access to MAT during incarceration has decreased in-custody deaths by 74%.6
Decreased opioid overdose deaths is not the only outcome of MAT. Pharmacotherapy for OUD also has been shown to increase treatment retention,7 reduce reincarceration,8 prevent communicable infections,9 and decrease use of other illicit substances.10 The provision of MAT also has been shown to be cost effective.11
Despite those benefits, as of 2017, only 30 out of 5,100 jails and prisons in the United States provided treatment with methadone or buprenorphine.12 When individuals on maintenance therapy are incarcerated, most correctional facilities force them to taper and discontinue those medications. This practice can cause distressing withdrawal symptoms and actively increase the risk of death for these individuals.
Concerns related to the provision of MAT, and specifically buprenorphine, in the correctional health setting often are related to diversion. Although safe administration of opioid full and partial agonists is a priority, recent literature has suggested that buprenorphine is not a medication frequently used for euphoric properties. In fact, the literature suggests that individuals using illicit buprenorphine primarily do so to treat withdrawal symptoms and that illicit use diminishes with access to formal treatment.13,14
Another concern is that pharmacotherapy for OUD should not be used without adjunctive psychotherapies and social supports. While dual pharmacotherapy and psychotherapy is ideal, the American Society for Addiction Medicine 2020 National Practice Guidelines for the treatment of OUD state: “a patient’s decision to decline psychosocial treatment or the absence of available psychosocial treatment should not preclude or delay pharmacotherapy, with appropriate medication management.”15 Just as some patients wish to engage in mutual help or psychotherapeutic modalities only, some patients wish to engage only in psychopharmacologic interventions. Declaring one modality of treatment better, or worse, or more worthwhile is not borne out by the literature and often places clinicians’ preferences over the preferences of patients.
Individuals who suffer from substance use disorders are at high risk of incarceration, relapse, and overdose death. These patients also suffer from stigmatization from peers and health care workers alike, making the process of engaging in care incredibly burdensome. Because of the disease of addiction, many of our patients cannot envision a healthy future: a future with the potential for intimate relationships, meaningful community engagement, and a rich inner life. The provision of MAT is lifesaving and improves the chances of a successful reentry – an intuitive first step in a long, but worthwhile, journey.
References
1. Hedegaard H et al; National Center for Health Statistics. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, 2020 Jan, No. 356.
2. Binswanger IA et al. N Engl J Med. 2007;356:157-65.
3. Green TC et al. JAMA Psychiatry. 2018;75(4):405-7.
4. Marsden J et al. Addiction. 2017;112(8):1408-18.
5. Joshi V and Urada D. State Targeted Response to the Opioid Crisis: California Strategic Plan. 2017 Aug 30.
6. Larney S et al. BMJ Open. 2014. doi: 10.1136/bmjopen-2013-004666.
7. Rich JD et al. Lancet. 2015;386(9991):350-9.
8. Deck D et al. J Addict Dis. 2009. 28(2):89-102.
9. MacArthur GJ et al. BMJ. 2012. doi: 10.1136/bmj.e5945.
10. Tsui J et al. J Subst Abuse Treat. 2019. 109:80-5.
11. Gisev N et al. Addiction. 2015 Dec;110(12):1975-84.
12. National Mental Health and Substance Use Policy Laboratory. “Use of Medication-Assisted Treatment for Opioid Use Disorder in Criminal Justice Settings.” HHS Publication No. PEP19-MATUSECJS. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2019.
13. Bazazi AR et al. J Addict Med. 2011;5(3):175-80.
14. Schuman-Olivier Z. et al. J Subst Abuse Treat. 2010 Jul;39(1):41-50.
15. Crotty K et al. J Addict Med. 2020;14(2)99-112.
Dr. Barnes is chief resident at San Mateo County Behavioral Health and Recovery Services in California. He disclosed no relevant financial relationships. Dr. Lenane is resident* at San Mateo County Behavioral Health and Recovery Services. He disclosed no relevant financial relationships. The opinions shared in this article represent the viewpoints of the authors and are not necessarily representative of the viewpoints or policies of their academic program or employer.
*This article was updated 7/9/2020.
Opioid overdose deaths in the United States have more than tripled in recent years, from 6.1 deaths per 100,000 individuals in 1999 to 20.7 per 100,000 individuals in 2018.1 Although the availability of medication-assisted treatment (MAT) has expanded over the past decade, this lifesaving treatment remains largely inaccessible to some of the most vulnerable members of our communities: opioid users facing reentry after incarceration.
Just as abstinence in the community brings a loss of tolerance to opioids, individuals who are incarcerated lose tolerance as well. Clinicians who treat patients with opioid use disorders (OUD) are accustomed to warning patients about the risk of returning to prior levels of use too quickly. Harm reduction strategies include using slowly, using with friends, and having naloxone on hand to prevent unintended overdose.
The risks of opioid use are magnified for those facing reentry; incarceration contributes to a loss of employment, social supports, and connection to care. Those changes can create an exceptionally stressful reentry period – one that places individuals at an acutely high risk of relapse and overdose. Within the first 2 years of release, an individual with a history of incarceration has a risk of death 3.5 times higher than that of someone in the general population. Within the first 2 weeks, those recently incarcerated are 129 times more likely to overdose on opioids and 12.7 times more likely to die than members of the general population.2
Treatment with MAT dramatically reduces deaths during this crucial period. In England, large national studies have shown a similar 75% decrease in all-cause mortality within the first 4 weeks of release among individuals with OUD.4 In California, the counties with the highest overdose death rates are consistently those with fewer opioid treatment programs, which suggests that access to treatment is necessary to prolong the lives of those suffering from OUD.5 In-custody overdose deaths are quite rare, and access to MAT during incarceration has decreased in-custody deaths by 74%.6
Decreased opioid overdose deaths is not the only outcome of MAT. Pharmacotherapy for OUD also has been shown to increase treatment retention,7 reduce reincarceration,8 prevent communicable infections,9 and decrease use of other illicit substances.10 The provision of MAT also has been shown to be cost effective.11
Despite those benefits, as of 2017, only 30 out of 5,100 jails and prisons in the United States provided treatment with methadone or buprenorphine.12 When individuals on maintenance therapy are incarcerated, most correctional facilities force them to taper and discontinue those medications. This practice can cause distressing withdrawal symptoms and actively increase the risk of death for these individuals.
Concerns related to the provision of MAT, and specifically buprenorphine, in the correctional health setting often are related to diversion. Although safe administration of opioid full and partial agonists is a priority, recent literature has suggested that buprenorphine is not a medication frequently used for euphoric properties. In fact, the literature suggests that individuals using illicit buprenorphine primarily do so to treat withdrawal symptoms and that illicit use diminishes with access to formal treatment.13,14
Another concern is that pharmacotherapy for OUD should not be used without adjunctive psychotherapies and social supports. While dual pharmacotherapy and psychotherapy is ideal, the American Society for Addiction Medicine 2020 National Practice Guidelines for the treatment of OUD state: “a patient’s decision to decline psychosocial treatment or the absence of available psychosocial treatment should not preclude or delay pharmacotherapy, with appropriate medication management.”15 Just as some patients wish to engage in mutual help or psychotherapeutic modalities only, some patients wish to engage only in psychopharmacologic interventions. Declaring one modality of treatment better, or worse, or more worthwhile is not borne out by the literature and often places clinicians’ preferences over the preferences of patients.
Individuals who suffer from substance use disorders are at high risk of incarceration, relapse, and overdose death. These patients also suffer from stigmatization from peers and health care workers alike, making the process of engaging in care incredibly burdensome. Because of the disease of addiction, many of our patients cannot envision a healthy future: a future with the potential for intimate relationships, meaningful community engagement, and a rich inner life. The provision of MAT is lifesaving and improves the chances of a successful reentry – an intuitive first step in a long, but worthwhile, journey.
References
1. Hedegaard H et al; National Center for Health Statistics. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, 2020 Jan, No. 356.
2. Binswanger IA et al. N Engl J Med. 2007;356:157-65.
3. Green TC et al. JAMA Psychiatry. 2018;75(4):405-7.
4. Marsden J et al. Addiction. 2017;112(8):1408-18.
5. Joshi V and Urada D. State Targeted Response to the Opioid Crisis: California Strategic Plan. 2017 Aug 30.
6. Larney S et al. BMJ Open. 2014. doi: 10.1136/bmjopen-2013-004666.
7. Rich JD et al. Lancet. 2015;386(9991):350-9.
8. Deck D et al. J Addict Dis. 2009. 28(2):89-102.
9. MacArthur GJ et al. BMJ. 2012. doi: 10.1136/bmj.e5945.
10. Tsui J et al. J Subst Abuse Treat. 2019. 109:80-5.
11. Gisev N et al. Addiction. 2015 Dec;110(12):1975-84.
12. National Mental Health and Substance Use Policy Laboratory. “Use of Medication-Assisted Treatment for Opioid Use Disorder in Criminal Justice Settings.” HHS Publication No. PEP19-MATUSECJS. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2019.
13. Bazazi AR et al. J Addict Med. 2011;5(3):175-80.
14. Schuman-Olivier Z. et al. J Subst Abuse Treat. 2010 Jul;39(1):41-50.
15. Crotty K et al. J Addict Med. 2020;14(2)99-112.
Dr. Barnes is chief resident at San Mateo County Behavioral Health and Recovery Services in California. He disclosed no relevant financial relationships. Dr. Lenane is resident* at San Mateo County Behavioral Health and Recovery Services. He disclosed no relevant financial relationships. The opinions shared in this article represent the viewpoints of the authors and are not necessarily representative of the viewpoints or policies of their academic program or employer.
*This article was updated 7/9/2020.
Amid pandemic, Virginia hospital’s opioid overdoses up nearly 10-fold
Opioid overdoses have shot up by almost 10-fold at a Virginia ED since March, a new report finds. The report provides more evidence that the coronavirus pandemic is sparking a severe medical crisis among illicit drug users.
“Health care providers should closely monitor the number of overdoses coming into their hospitals and in the surrounding community during this time,” study lead author and postdoctoral research fellow Taylor Ochalek, PhD, said in an interview. “If they do notice an increasing trend of overdoses, they should spread awareness in the community to the general public, and offer resources and information for those that may be seeking help and/or may be at a high risk of overdosing.”
Dr. Ochalek presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
According to the report, opioid overdoses at the VCU Medical Center in Richmond, Va., grew from an average of six a month from February to December 2019 to 50, 57, and 63 in March, April, and May 2020. Of the 171 cases in the later time frame, the average age was 44 years, 72% were male, and 82% were African American.
“The steep increase in overdoses began primarily in March,” said Dr. Ochalek, of Virginia Commonwealth University in Richmond. “This timing coincides with the Virginia governor’s state of emergency declaration, stay-at-home order, and closure of nonessential businesses order.”
The researchers did not provide details about the types of opioids used, the patient outcomes, or whether the patients tested positive for COVID-19. It’s unclear whether the pandemic directly spawned a higher number of overdoses, but there are growing signs of a stark nationwide trend.
“Nationwide, federal and local officials are reporting alarming spikes in drug overdoses – a hidden epidemic within the coronavirus pandemic,” the Washington Post reported on July 1, pointing to increases in Kentucky, Virginia, and the Chicago area.
Meanwhile, the federal Overdose Detection Mapping Application Program, which tracks overdoses nationwide, issued 191% more “spike alerts” in January to April 2020 than in the same time period in 2019. However, the spike alerts began to increase in January, weeks before the pandemic began to take hold.
The findings are consistent with trends in Houston, where overdose calls were up 31% in the first 3 months of 2020, compared with 2019, said psychologist James Bray, PhD, of the University of Texas, San Antonio, in an interview. More recent data suggest that the numbers are rising even higher, said Dr. Bray, who works with Houston first responders and has analyzed data.
Dr. Bray said.
Another potential factor is the disruption in the illicit drug supply chain because of limits on crossings at the southern border, said ED physician Scott Weiner, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston. “As a result, opioids of extremely variable potency have infiltrated markets, and people using drugs may not be used to the new doses, especially if they are high-potency fentanyl analogues.”
Moving forward, Dr. Bray said, “people need continued access to treatment. Telehealth and other virtual services need to be provided so that people can continue to have access to treatment even during the pandemic.”
Dr. Weiner also emphasized the importance of treatment for patients who overdose on opioids. “In my previous work, we discovered that about 1 in 20 patients who are treated in an emergency department and survive would die within 1 year. That number will likely increase drastically during COVID,” he said. “When a patient presents after overdose, we must intervene aggressively with buprenorphine and other harm-reduction techniques to save these lives.”
The study was funded by the National Institutes of Health. Dr. Ochalek, Dr. Weiner, and Dr. Bray reported no relevant disclosures.
Opioid overdoses have shot up by almost 10-fold at a Virginia ED since March, a new report finds. The report provides more evidence that the coronavirus pandemic is sparking a severe medical crisis among illicit drug users.
“Health care providers should closely monitor the number of overdoses coming into their hospitals and in the surrounding community during this time,” study lead author and postdoctoral research fellow Taylor Ochalek, PhD, said in an interview. “If they do notice an increasing trend of overdoses, they should spread awareness in the community to the general public, and offer resources and information for those that may be seeking help and/or may be at a high risk of overdosing.”
Dr. Ochalek presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
According to the report, opioid overdoses at the VCU Medical Center in Richmond, Va., grew from an average of six a month from February to December 2019 to 50, 57, and 63 in March, April, and May 2020. Of the 171 cases in the later time frame, the average age was 44 years, 72% were male, and 82% were African American.
“The steep increase in overdoses began primarily in March,” said Dr. Ochalek, of Virginia Commonwealth University in Richmond. “This timing coincides with the Virginia governor’s state of emergency declaration, stay-at-home order, and closure of nonessential businesses order.”
The researchers did not provide details about the types of opioids used, the patient outcomes, or whether the patients tested positive for COVID-19. It’s unclear whether the pandemic directly spawned a higher number of overdoses, but there are growing signs of a stark nationwide trend.
“Nationwide, federal and local officials are reporting alarming spikes in drug overdoses – a hidden epidemic within the coronavirus pandemic,” the Washington Post reported on July 1, pointing to increases in Kentucky, Virginia, and the Chicago area.
Meanwhile, the federal Overdose Detection Mapping Application Program, which tracks overdoses nationwide, issued 191% more “spike alerts” in January to April 2020 than in the same time period in 2019. However, the spike alerts began to increase in January, weeks before the pandemic began to take hold.
The findings are consistent with trends in Houston, where overdose calls were up 31% in the first 3 months of 2020, compared with 2019, said psychologist James Bray, PhD, of the University of Texas, San Antonio, in an interview. More recent data suggest that the numbers are rising even higher, said Dr. Bray, who works with Houston first responders and has analyzed data.
Dr. Bray said.
Another potential factor is the disruption in the illicit drug supply chain because of limits on crossings at the southern border, said ED physician Scott Weiner, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston. “As a result, opioids of extremely variable potency have infiltrated markets, and people using drugs may not be used to the new doses, especially if they are high-potency fentanyl analogues.”
Moving forward, Dr. Bray said, “people need continued access to treatment. Telehealth and other virtual services need to be provided so that people can continue to have access to treatment even during the pandemic.”
Dr. Weiner also emphasized the importance of treatment for patients who overdose on opioids. “In my previous work, we discovered that about 1 in 20 patients who are treated in an emergency department and survive would die within 1 year. That number will likely increase drastically during COVID,” he said. “When a patient presents after overdose, we must intervene aggressively with buprenorphine and other harm-reduction techniques to save these lives.”
The study was funded by the National Institutes of Health. Dr. Ochalek, Dr. Weiner, and Dr. Bray reported no relevant disclosures.
Opioid overdoses have shot up by almost 10-fold at a Virginia ED since March, a new report finds. The report provides more evidence that the coronavirus pandemic is sparking a severe medical crisis among illicit drug users.
“Health care providers should closely monitor the number of overdoses coming into their hospitals and in the surrounding community during this time,” study lead author and postdoctoral research fellow Taylor Ochalek, PhD, said in an interview. “If they do notice an increasing trend of overdoses, they should spread awareness in the community to the general public, and offer resources and information for those that may be seeking help and/or may be at a high risk of overdosing.”
Dr. Ochalek presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
According to the report, opioid overdoses at the VCU Medical Center in Richmond, Va., grew from an average of six a month from February to December 2019 to 50, 57, and 63 in March, April, and May 2020. Of the 171 cases in the later time frame, the average age was 44 years, 72% were male, and 82% were African American.
“The steep increase in overdoses began primarily in March,” said Dr. Ochalek, of Virginia Commonwealth University in Richmond. “This timing coincides with the Virginia governor’s state of emergency declaration, stay-at-home order, and closure of nonessential businesses order.”
The researchers did not provide details about the types of opioids used, the patient outcomes, or whether the patients tested positive for COVID-19. It’s unclear whether the pandemic directly spawned a higher number of overdoses, but there are growing signs of a stark nationwide trend.
“Nationwide, federal and local officials are reporting alarming spikes in drug overdoses – a hidden epidemic within the coronavirus pandemic,” the Washington Post reported on July 1, pointing to increases in Kentucky, Virginia, and the Chicago area.
Meanwhile, the federal Overdose Detection Mapping Application Program, which tracks overdoses nationwide, issued 191% more “spike alerts” in January to April 2020 than in the same time period in 2019. However, the spike alerts began to increase in January, weeks before the pandemic began to take hold.
The findings are consistent with trends in Houston, where overdose calls were up 31% in the first 3 months of 2020, compared with 2019, said psychologist James Bray, PhD, of the University of Texas, San Antonio, in an interview. More recent data suggest that the numbers are rising even higher, said Dr. Bray, who works with Houston first responders and has analyzed data.
Dr. Bray said.
Another potential factor is the disruption in the illicit drug supply chain because of limits on crossings at the southern border, said ED physician Scott Weiner, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston. “As a result, opioids of extremely variable potency have infiltrated markets, and people using drugs may not be used to the new doses, especially if they are high-potency fentanyl analogues.”
Moving forward, Dr. Bray said, “people need continued access to treatment. Telehealth and other virtual services need to be provided so that people can continue to have access to treatment even during the pandemic.”
Dr. Weiner also emphasized the importance of treatment for patients who overdose on opioids. “In my previous work, we discovered that about 1 in 20 patients who are treated in an emergency department and survive would die within 1 year. That number will likely increase drastically during COVID,” he said. “When a patient presents after overdose, we must intervene aggressively with buprenorphine and other harm-reduction techniques to save these lives.”
The study was funded by the National Institutes of Health. Dr. Ochalek, Dr. Weiner, and Dr. Bray reported no relevant disclosures.
FROM CPDD 2020
Use of nonopioid pain meds is on the rise
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
High percentage of stimulant use found in opioid ED cases
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
FROM CPDD 2020









