User login
Intense intervention may boost addiction program retention
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
Shared medical appointments may bridge the opioid treatment gap
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New coalition demands urgent action on COVID-19 mental health crisis
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
COVID-19 fuels surge in overdose-related cardiac arrests
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
How to identify, evaluate, and treat patients with ‘Percocet use disorder’
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
Rapid relief of opioid-induced constipation with MNTX
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.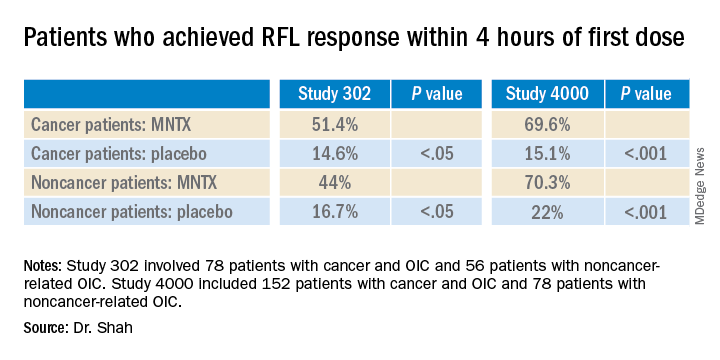
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.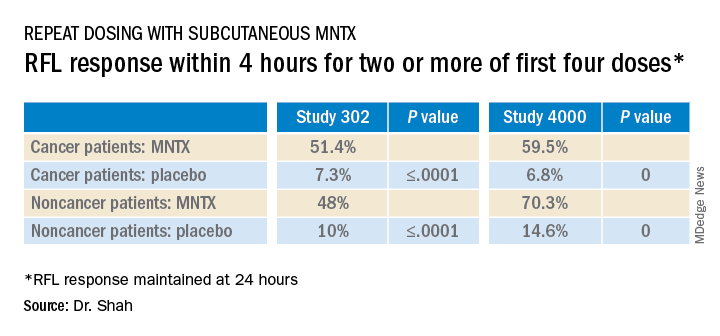
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
How mental health care would look under a Trump vs. Biden administration
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
Children’s opioid harms vary by race, location
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.
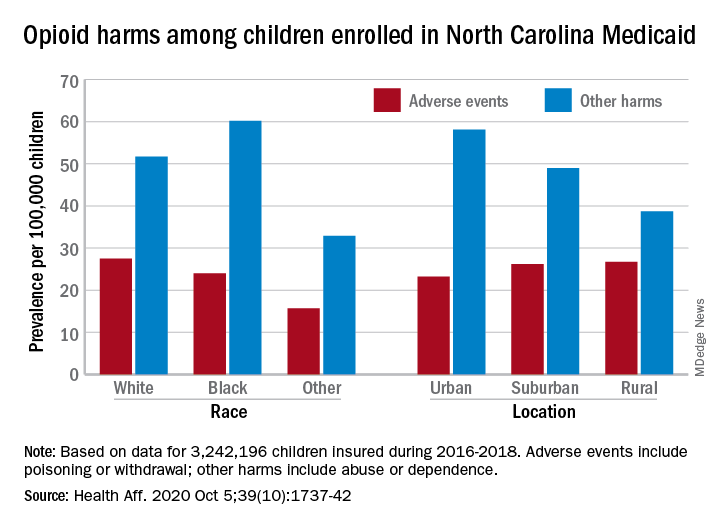
Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
FROM HEALTH AFFAIRS
FDA orders stronger warnings on benzodiazepines
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
New acute pain guidelines from the ACP and AAFP have limitations
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.









