User login
Biomechanical Evaluation of Two Arthroscopic Biceps Tenodesis Techniques: Proximal Interference Screw and Modified Percutaneous Intra-Articular Transtendon
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
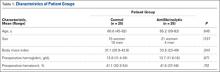
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
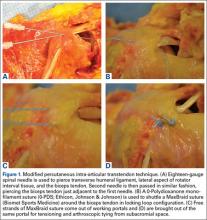
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
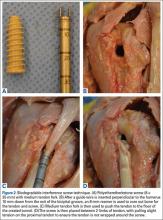
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
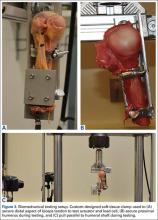
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
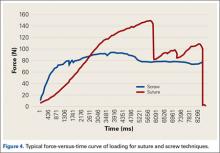
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
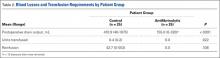
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
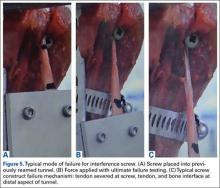
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
Is a Persistent Vacuum Phenomenon a Sign of Pseudarthrosis After Posterolateral Spinal Fusion?
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
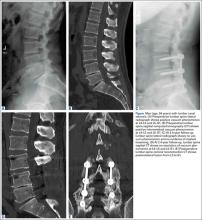
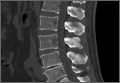
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
Using Aminocaproic Acid to Reduce Blood Loss After Primary Unilateral Total Knee Arthroplasty
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
Postop delirium linked to greater long-term cognitive decline
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
Patients with postoperative delirium have significantly worse preoperative short-term cognitive performance and significantly greater long-term cognitive decline, compared with patients without delirium, according to Sharon K. Inouye, MD, and her associates.
In a prospective cohort study of 560 patients aged 70 years and older, 134 patients were selected for the delirium group and 426 for the nondelirium group. The delirium group had a significantly greater decline (–1.03 points) at 1 month, compared with those without delirium (P = .003). After cognitive function had recovered at 2 months, there were no significant differences between groups (P = 0.99). After 2 months, both groups decline on average; however, the delirium group declined significantly more (–1.07) in adjusted mean scores at 36 months (P =.02).
From baseline to 36 months, there was a significant change for the delirium group (–1.30, P less than .01) and no significant change for the group without delirium (–0.23, P = .30). Researchers noted that the effect of delirium remains undiminished after consecutive rehospitalizations, intercurrent illnesses, and major postoperative complications were controlled for.
The patients underwent major noncardiac surgery, such as total hip or knee replacement, open abdominal aortic aneurysm repair, colectomy, and lower-extremity arterial bypass.
“This study provides a novel presentation of the biphasic relationship of delirium and cognitive trajectory, both its well-recognized acute effects but also long-term effects,” the researchers wrote. “Our results suggest that after a period of initial recovery, patients with delirium experience a substantially accelerated trajectory of cognitive aging.”
Read the full study in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association (doi:10.1016/j.jalz.2016.03.005).
FROM ALZHEIMER’S & DEMENTIA
Location of UCL Tears May Help Determine If Surgery Is Needed
The location of ligament tears within a pitcher’s elbow can be key to predicting the success of non-operative treatment for injuries, according to the results of a study presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine.
Researchers examined 38 pitchers from one professional baseball organization (both major and minor league teams) who sustained ulnar collateral ligament (UCL) injuries between 2006 and 2015,. Thirty-two players (84%) received non-operative treatment for partial ligament tears. A proximal tear of the UCL was identified in 81% of the patients who were successfully treated non-operatively. By contrast, a distal tear of the UCL was detected in 90% of patients who failed non-operative treatment and required surgery.
Suggested Reading
Frangiamore S, Lynch TS, Vaugh MD, Soloff L, Schickendantz MS. MRI predictors of failure in non-operative management of ulnar collateral ligament injuries in professional baseball pitchers. Paper presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine. Available at: http://apps.sportsmed.org/meetings/am2016/files/Paper_116.pdf. Accessed July 29, 2016.
The location of ligament tears within a pitcher’s elbow can be key to predicting the success of non-operative treatment for injuries, according to the results of a study presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine.
Researchers examined 38 pitchers from one professional baseball organization (both major and minor league teams) who sustained ulnar collateral ligament (UCL) injuries between 2006 and 2015,. Thirty-two players (84%) received non-operative treatment for partial ligament tears. A proximal tear of the UCL was identified in 81% of the patients who were successfully treated non-operatively. By contrast, a distal tear of the UCL was detected in 90% of patients who failed non-operative treatment and required surgery.
The location of ligament tears within a pitcher’s elbow can be key to predicting the success of non-operative treatment for injuries, according to the results of a study presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine.
Researchers examined 38 pitchers from one professional baseball organization (both major and minor league teams) who sustained ulnar collateral ligament (UCL) injuries between 2006 and 2015,. Thirty-two players (84%) received non-operative treatment for partial ligament tears. A proximal tear of the UCL was identified in 81% of the patients who were successfully treated non-operatively. By contrast, a distal tear of the UCL was detected in 90% of patients who failed non-operative treatment and required surgery.
Suggested Reading
Frangiamore S, Lynch TS, Vaugh MD, Soloff L, Schickendantz MS. MRI predictors of failure in non-operative management of ulnar collateral ligament injuries in professional baseball pitchers. Paper presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine. Available at: http://apps.sportsmed.org/meetings/am2016/files/Paper_116.pdf. Accessed July 29, 2016.
Suggested Reading
Frangiamore S, Lynch TS, Vaugh MD, Soloff L, Schickendantz MS. MRI predictors of failure in non-operative management of ulnar collateral ligament injuries in professional baseball pitchers. Paper presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine. Available at: http://apps.sportsmed.org/meetings/am2016/files/Paper_116.pdf. Accessed July 29, 2016.
Women Under Age 25 at Greater Risk for ACL Re-Tear
After anterior cruciate ligament (ACL) reconstruction, women younger than age 25 with a graft size of <8 mm have an increased change of re-tearing their ACL, according to the results of a study presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine.
Researchers studied 503 athletes (235 women and 268 men; average age 27) undergoing primary, autograft hamstring ACL reconstruction. The surgeries were all performed at a single center by a single surgeon between September through December 2012. Patients were followed for 2 years. Overall, the rate of re-tears was 6% and the mean graft size was 7.9 mm.
Graft size <8 mm and age < 25 years were significantly predictive of re‐tear. Female sex was correlated with re‐tear but was not significant.
Suggested Reading
Nguyen D. Sex, age, and graft size as predictors of ACL: re‐tear: a multivariate logistic regression of a cohort of 503 athletes. Paper presented at 2016 annual meeting of the American Orthopedic Society of Sports Medicine. Available at: http://apps.sportsmed.org/meetings/am2016/files/Paper_111.pdf. Accessed July 29, 2016.
After anterior cruciate ligament (ACL) reconstruction, women younger than age 25 with a graft size of <8 mm have an increased change of re-tearing their ACL, according to the results of a study presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine.
Researchers studied 503 athletes (235 women and 268 men; average age 27) undergoing primary, autograft hamstring ACL reconstruction. The surgeries were all performed at a single center by a single surgeon between September through December 2012. Patients were followed for 2 years. Overall, the rate of re-tears was 6% and the mean graft size was 7.9 mm.
Graft size <8 mm and age < 25 years were significantly predictive of re‐tear. Female sex was correlated with re‐tear but was not significant.
After anterior cruciate ligament (ACL) reconstruction, women younger than age 25 with a graft size of <8 mm have an increased change of re-tearing their ACL, according to the results of a study presented at the 2016 annual meeting of the American Orthopedic Society of Sports Medicine.
Researchers studied 503 athletes (235 women and 268 men; average age 27) undergoing primary, autograft hamstring ACL reconstruction. The surgeries were all performed at a single center by a single surgeon between September through December 2012. Patients were followed for 2 years. Overall, the rate of re-tears was 6% and the mean graft size was 7.9 mm.
Graft size <8 mm and age < 25 years were significantly predictive of re‐tear. Female sex was correlated with re‐tear but was not significant.
Suggested Reading
Nguyen D. Sex, age, and graft size as predictors of ACL: re‐tear: a multivariate logistic regression of a cohort of 503 athletes. Paper presented at 2016 annual meeting of the American Orthopedic Society of Sports Medicine. Available at: http://apps.sportsmed.org/meetings/am2016/files/Paper_111.pdf. Accessed July 29, 2016.
Suggested Reading
Nguyen D. Sex, age, and graft size as predictors of ACL: re‐tear: a multivariate logistic regression of a cohort of 503 athletes. Paper presented at 2016 annual meeting of the American Orthopedic Society of Sports Medicine. Available at: http://apps.sportsmed.org/meetings/am2016/files/Paper_111.pdf. Accessed July 29, 2016.
How Do Age, Sex Affect Outcomes After Arthroscopy for Hip Impingement?
Although both men and women generally do well after having arthroscopic surgery for hip impingement, patients over age 45, particularly women over 45, don’t fare quite as well, according to a study published May 18 in The Journal of Bone and Joint Surgery.
Researchers examined 150 men and women of various ages, who underwent hip arthroscopy to treat femoroacetabular impingement (FAI). Patients were divided into groups based on age and sex. Outcomes were evaluated based on results from several instruments, include the Hip Outcome Score Activities of Daily Living Subscale, Hip Outcome Score Sport-Specific Subscale, and modified Harris hip score, as well as by clinical improvement at follow-up.
Researchers found that while all patients had significant improvements after hip arthroscopy for FAI, patients under age 45 had better overall results and fewer complications compared with people over age 45. Women older than age 45 had lower outcome scores than their male counterparts.
Suggested Reading
Frank MR, Lee S, Bush-Joseph C, et al. Outcomes for hip arthroscopy according to sex and age. J Bone Joint Surg Am. 2016;98(10):797-804.
Although both men and women generally do well after having arthroscopic surgery for hip impingement, patients over age 45, particularly women over 45, don’t fare quite as well, according to a study published May 18 in The Journal of Bone and Joint Surgery.
Researchers examined 150 men and women of various ages, who underwent hip arthroscopy to treat femoroacetabular impingement (FAI). Patients were divided into groups based on age and sex. Outcomes were evaluated based on results from several instruments, include the Hip Outcome Score Activities of Daily Living Subscale, Hip Outcome Score Sport-Specific Subscale, and modified Harris hip score, as well as by clinical improvement at follow-up.
Researchers found that while all patients had significant improvements after hip arthroscopy for FAI, patients under age 45 had better overall results and fewer complications compared with people over age 45. Women older than age 45 had lower outcome scores than their male counterparts.
Although both men and women generally do well after having arthroscopic surgery for hip impingement, patients over age 45, particularly women over 45, don’t fare quite as well, according to a study published May 18 in The Journal of Bone and Joint Surgery.
Researchers examined 150 men and women of various ages, who underwent hip arthroscopy to treat femoroacetabular impingement (FAI). Patients were divided into groups based on age and sex. Outcomes were evaluated based on results from several instruments, include the Hip Outcome Score Activities of Daily Living Subscale, Hip Outcome Score Sport-Specific Subscale, and modified Harris hip score, as well as by clinical improvement at follow-up.
Researchers found that while all patients had significant improvements after hip arthroscopy for FAI, patients under age 45 had better overall results and fewer complications compared with people over age 45. Women older than age 45 had lower outcome scores than their male counterparts.
Suggested Reading
Frank MR, Lee S, Bush-Joseph C, et al. Outcomes for hip arthroscopy according to sex and age. J Bone Joint Surg Am. 2016;98(10):797-804.
Suggested Reading
Frank MR, Lee S, Bush-Joseph C, et al. Outcomes for hip arthroscopy according to sex and age. J Bone Joint Surg Am. 2016;98(10):797-804.
The Relationship Between Sustained Gripping and the Development of Carpal Tunnel Syndrome
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
Prevention of Periprosthetic Joint Infections of the Hip and Knee
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
Nearly 2% of patients who undergo total knee arthroplasty (TKA) or total hip arthroplasty (THA) develop a periprosthetic joint infection (PJI) within 20 years of surgery, and 41% of these infections occur within the first 2 years.1 PJI is the most common cause of TKA failure and the third leading complication of THA.2 The estimated total hospital cost of treating PJI increased from $320 million in 2001 to $566 million in 2009, which can be extrapolated to $1.62 billion in 2020.3 By 2030, the projected increase in demand for TKA and THA will be 673% and 174% of what it was in 2005, respectively.4 Treatment of PJI of the knee is estimated to cost 3 to 4 times more than a primary TKA, and the cost of revision THA for PJI is almost $6000 more than that of revision TKA for PJI.3
In this article, we review the numerous preoperative, intraoperative, and postoperative methods of decreasing PJI incidence after total joint arthroplasty (TJA).
Preoperative Risk Prevention
Medical Comorbidities
Preoperative medical optimization is a key element in PJI prevention (Table 1). An American Society of Anesthesiologists classification score of 3 or more has been associated with doubled risk for surgical site infections (SSIs) after THA.5 Autoimmune conditions confer a particularly higher risk. In a retrospective double-cohort study of 924 subjects, Bongartz and colleagues6 found that, compared with osteoarthritis, rheumatoid arthritis tripled the risk of PJI. Small case series originally suggested a higher risk of PJI in patients with psoriasis,7,8 but more recent studies have contradicted that finding.9,10 Nevertheless, psoriatic plaques have elevated bacterial counts,11 and planned incisions should circumvent these areas.
Diabetes mellitus is a clear risk factor for PJI.12-16 Regarding whether preoperative glucose control affects risk, findings have been mixed. Mraovic and colleagues17 showed preoperative hyperglycemia to be an independent risk factor; Jämsen and colleagues,15 in a single-center analysis of more than 7000 TJAs, suggested preoperative blood glucose levels were not independently associated with PJI; and Iorio and colleagues16 found no association between surgical infections and hemoglobin A1c levels.
TJA incidence is higher in patients with chronic kidney disease (CKD) than in the general population.18 Dialysis users have a post-THA PJI rate as high as 13% to 19%.19,20 Early clinical data suggested that outcomes are improved in dialysis users who undergo renal transplant, but this finding recently has been questioned.19,21 Deegan and colleagues22 found an increased PJA rate of 3.5% even in low-level CKD (stage 1, 2, or 3), but this may be confounded by the increased association of CKD with other PJI-predisposing comorbidities.
Given a higher incidence of urinary tract infections (UTIs) among patients with PJI, some surgeons think UTIs predispose to PJIs by hematogenous seeding.12,23,24 Symptomatic UTIs should be cleared before surgery and confirmed on urinalysis. Obstructive symptoms should prompt urologic evaluation. As asymptomatic pyuria and bacteriuria (colony counts, >1 × 105/mL) do not predispose to PJI, patients without symptoms do not require intervention.25,26 Past history of malignancy may also have a role in PJI. In a case-control study of the Mayo Clinic arthroplasty experience from 1969 to 1991, Berbari and colleagues1 found an association between malignancy and PJI (odds ratio, 2.4). They theorized the immunosuppressive effects of cancer treatment might be responsible for this increased risk.
Immunocompromising Medications
Immunocompromising medications are modifiable and should be adjusted before surgery. Stopping any disease-modifying antirheumatic drug (DMARD) more than 4 weeks before surgery is not recommended.27
Corticosteroid use can lead to immunosuppression and increased protein catabolism, which impairs soft-tissue healing. To avoid flares or adrenal insufficiency, however, chronic corticosteroid users should continue their regular doses perioperatively.28 On the day of surgery, they should also receive a stress dose of hydrocortisone 50 to 75 mg (for primary arthroplasty) or 100 to 150 mg (for revision arthroplasty), followed by expeditious tapering over 1 to 2 days.29 DMARDs are increasingly used by rheumatologists. One of the most effective DMARDs is methotrexate. Despite its immunocompromising activity, methotrexate should be continued perioperatively, as stopping for even 2 days may increase flare-related complications.30 Hydroxychloroquine can be continued perioperatively and has even been shown, by Johnson and Charnley,31 to prevent deep vein thromboses. Sulfasalazine can also be continued perioperatively—but with caution, as it may elevate international normalized ratio (INR) levels in patients receiving warfarin.29 Most other DMARDs should be temporarily discontinued. Leflunomide and interleukin 1 antagonists, such as anakinra, should be stopped 1 to 2 days before surgery and restarted 10 to 14 days after surgery.29 Rituximab should be stopped 1 week before surgery and restarted 10 to 14 days after surgery. Tumor necrosis factor α inhibitors should be discontinued for 2 half-lives before and after surgery.32 Etanercept has a half-life of 3 to 5 days; infliximab, 8 to 10 days; and adalimumab, 10 to 13 days. Most surgeons schedule surgery for the end of a dosing cycle and discontinue these biologic agents for another 10 to 14 days after surgery.
Metabolic Factors
Obese patients are susceptible to longer surgeries, more extensive dissection, poorly vascularized subcutaneous tissue, and higher requirements of weight-adjusted antibiotic dosing.13 Body mass index (BMI) of 40 kg/m2 or more (morbid obesity) and BMI over 50 kg/m2 have been associated with 9 times and 21.3 times increased risk of PJI, respectively.13,14 Delaying surgery with dietary consultation has been suggested,33,34 and bariatric surgery before TKA may decrease infection rates by 3.5 times.35
Nutritional markers are considered before arthroplasty. According to most laboratories, a serum transferrin level under 200 mg/dL, albumin level under 3.5 g/dL, and total lymphocyte count under 1500 cells/mm3 indicate malnourishment, which can increase the incidence of wound complications by 5 to 7 times.36 Patients should also have sufficient protein, vitamin, and mineral supplementation, particularly vitamins A and C, zinc, and copper.37Smokers who cease smoking at least 4 to 6 weeks before surgery lower their wound complication rate by up to 26%.38,39 When nicotine leaves the bloodstream, vasodilation occurs, oxygenation improves, and the immune system recovers.39 Studies have found more SSIs in patients who abuse alcohol,40 and numerous authors have confirmed this finding in the arthroplasty population.24,41,42 Alcohol inhibits platelet function and may predispose to a postoperative hematoma. In contrast to smoking cessation evidence, evidence regarding alcohol interventions in preventing postoperative infections is less conclusive.43,44
MRSA Colonization
Methicillin-resistant Staphylococcus aureus (MRSA) is a particularly difficult bacterium to eradicate in PJI. As the mean cost of treating a single case of MRSA-related prosthetic infection is $107,264 vs $68,053 for susceptible strains,45,46 many infection-containment strategies focus on addressing benign MRSA colonization before surgery.
MRSA is present in the nares of 25 million people in the United States. Nasal colonization increases the risk of bacteremia 4-fold47 and SSI 2- to 9-fold.48,49 Nasal swabs are analyzed with either a rapid polymerase chain reaction (PCR) test, which provides results in 2 hours, or a bacterial culture, which provides results in 1 to 4 days. The PCR test is more expensive.
Eradication of MRSA colonization is increasingly prevalent. Several Scandinavian countries have instituted strict practices by which patients are denied elective surgery until negative nasal swabs are obtained.49 Nasal decontamination is one method of colonization reduction. Topical mupirocin, which yields eradication in 91% of nasal carriers immediately after treatment and in 87% after 4 weeks,50 is effective in reducing SSI rates only when used in conjunction with a body wash, which is used to clean the axilla and groin.51 There is no consensus on optimal timing, but Bode and colleagues52 found a significant decrease in deep SSIs when decontamination occurred just 24 hours before surgery.
Povidone-iodine showers went out of favor with the realization that chlorhexidine gluconate acts longer on the skin surface.53,54 Preoperative showers involve rinsing with liquid chlorhexidine soap 24 to 48 hours before surgery. However, chlorhexidine binds preferentially to the cotton in washcloths instead of the skin. Edmiston and colleagues54,55 found that 4% chlorhexidine liquid soaps achieve much lower skin chlorhexidine concentrations than 2% polyester cloths do. Use of these “chlorhexidine wipes” the night before and the day of surgery has decreased PJI after TKA from 2.2% to 0.6%.56,57
Intraoperative Risk Prevention
Preparation
Which preoperative antibiotic to use is one of the first operative considerations in PJI prophylaxis (Table 2). Cefazolin is recommended as a first-line agent for its excellent soft-tissue penetration, long half-life, and activity against gram-positive bacteria such as skin flora.58 Clindamycin may be considered for patients allergic to β-lactam antibiotics. Vancomycin may be considered for adjunctive use with cephalosporins in cases of known MRSA colonization. Vancomycin infusion should be started earlier than infusion with other antibiotics, as vancomycin must be infused slowly and takes longer to become therapeutic.
Antibiotic dosing should be based on local antibiograms, adjusted dosing weight, or BMI.59 For revision arthroplasty, preoperative prophylaxis should not be stopped out of fear of affecting operative cultures.60 Some surgeons pause antibiotic use if a preoperative joint aspirate has not been obtained. Infusion within 1 hour of incision is part of the pay-for-performance guidelines established by the US Centers for Medicare & Medicaid Services.61 An antibiotic should be redosed if the operation will take longer than 2 half-lives of the drug.59 Surgeons should consider administering a dose every 4 hours or whenever blood loss exceeds 1000 mL.62 Engesæter and colleagues63 found that antibiotic prophylaxis was most effective given 4 times perioperatively (1 time before surgery, 3 times after surgery). Postoperative antibiotics should not be administered longer than 24 hours, as prolonged dosing confers no benefit.58 Operating room conditions must be optimized for prophylaxis. More people and operating room traffic in nonsterile corridors increase contamination of instruments open to air.64 Laminar airflow systems are commonly used. Although there is little dispute that laminar flow decreases the bacterial load of air, there are mixed results regarding its benefit in preventing PJI.65-68 Skin preparation may address patient risk factors. Hair clipping is preferred to shaving, which may cause microabrasions and increased susceptibility to skin flora.69 Patients should be prepared with antiseptic solution. One randomized controlled trial found that 2% chlorhexidine gluconate mixed with 70% isopropyl alcohol was superior to 10% povidone-iodine in preventing SSIs.70 However, a recent cohort study showed a lower rate of superficial wound infections when 1% povidone-iodine (vs 0.5% chlorhexidine) was used with alcohol.71 This finding may indicate the need for alcohol preparation, higher concentrations of chlorhexidine, or both.
Proper scrubbing and protective gear are needed to reduce surgeon risk factors. Hand washing is a routine part of any surgery. Alcohol-based hand scrubs are as effective as hand scrubbing.65 They reduce local skin flora by 95% immediately and by 99% with repeated applications.72 Lidwell and colleagues73 found a 75% reduction in infection when body exhaust suits were used in combination with laminar flow in a multicenter randomized controlled trial of 8052 patients. Sterile draping with impermeable drapes should be done over properly prepared skin. Ioban drapes (3M) are often used as a protective barrier. Interestingly, a Cochrane review found no benefit in using plastic adhesives impregnated with iodine over sterilely prepared skin.74
Operative Considerations
Surgical gloves become contaminated in almost one third of cases, half the time during draping.75 For this reason, many surgeons change gloves after draping. In addition, double gloving prevents a breech of aseptic technique should the outer glove become perforated.76 Demircay and colleagues77 assessed double latex gloving in arthroplasty and found the outer and inner gloves perforated in 18.4% and 8.4% of cases, respectively. Punctures are most common along the nondominant index finger, and then the dominant thumb.77,78 Perforation is more common when 2 latex gloves are worn—vs 1 latex glove plus an outer cloth glove—and the chance of perforation increases with surgery duration. The inner glove may become punctured in up to 100% of operations that last over 3 hours.79 Although Dodds and colleagues80 found no change in bacterial counts on surgeons’ hands or gloves after perforation, precautions are still recommended. Al-Maiyah and colleagues81 went as far as to recommend glove changes at 20-minute intervals and before cementation.
Surgical instruments can be sources of contamination. Some authors change the suction tip every hour to minimize the risk of deep wound infection.82-85 Others change it before femoral canal preparation and prosthesis insertion during THA.86 The splash basin is frequently contaminated, and instruments placed in it should not be returned to the operative field.87 Hargrove and colleagues88 suggested pulsatile lavage decreases PJI more than bulb syringe irrigation does, whereas others argued that high-pressure lavage allows bacteria to penetrate more deeply, which could lead to retention of more bacteria.89 Minimizing operating room time was found by Kurtz and colleagues90 and Peersman and colleagues91 to decrease PJI incidence. Carroll and colleagues71 correlated longer tourniquet use with a higher rate of infection after TKA; proposed mechanisms include local tissue hypoxia and lowered concentrations of prophylactic antibiotics.
Similarly, minimizing blood loss and transfusion needs is another strategy for preventing infection. Allogenic transfusion may increase the risk of PJI 2 times.23,71,92 The mechanism seems to be immune system modulation by allogenic blood, which impairs microcirculation and oxygen delivery at the surgical site.23,75 Transfusions should be approached with caution, and consideration given to preoperative optimization and autologous blood donation. Cherian and colleagues93 reviewed different blood management strategies and found preoperative iron therapy, intravenous erythropoietin, and autologous blood donation to be equally effective in reducing the need for allogenic transfusions. Numerous studies of tranexamic acid, thrombin-based hemostatic matrix (Floseal; Baxter Inc), and bipolar sealer with radiofrequency ablation (Aquamantys; Medtronic Inc) have found no alterations in infection rates, but most have used calculated blood loss, not PJI, as the primary endpoint.94-105 Antibiotic cement also can be used to block infection.63,106-110 Although liquid gentamicin may weaken bone cement,111 most antibiotics, including powdered tobramycin and vancomycin, do not weaken its fatigue strength.111-114 A recent meta-analysis by Parvizi and colleagues115 revealed that deep infection rates dropped from 2.3% to 1.2% with use of antibiotic cement for primary THAs. Cummins and colleagues,116 however, reported the limited cost-effectiveness of antibiotic cement in primary arthroplasty. Performing povidone-iodine lavage at the end of the case may be a more inexpensive alternative. Brown and colleagues117 found that rinsing with dilute povidone-iodine (.35%) for 3 minutes significantly decreased the incidence of PJI.
Closure techniques and sutures have been a focus of much of the recent literature. Winiarsky and colleagues34 advocated using a longer incision for obese patients and augmenting closure in fattier areas with vertical mattress retention sutures, which are removed after 5 days. A barbed monofilament suture (Quill; Angiotech Inc) is gaining in popularity. Laboratory research has shown that bacteria adhere less to barbed monofilament sutures than to braided sutures.118 Smith and colleagues119 found a statistically nonsignificant higher rate of wound complications with barbed monofilament sutures, whereas Ting and colleagues120 found no difference in complications. These studies were powered to detect differences in time and cost, not postoperative complications. Skin adhesive (Dermabond; Ethicon Inc), also used in closure, may be superior to staples in avoiding superficial skin abscesses.121 Although expensive, silver-impregnated dressing has antimicrobial activity that reduces PJI incidence by up to 74%.122 One brand of this dressing (Aquacel; ConvaTec Inc) has a polyurethane waterproof barrier that allows it to be worn for 7 days.
Three factors commonly mentioned in PJI prevention show little supporting evidence. Drains, which are often used, may create a passage for postoperative infection and are associated with increased transfusion needs.123,124 Adding antibiotics to irrigation solution125 and routinely changing scalpel blades126-129 also have little supporting evidence. In 2014, the utility of changing scalpel blades after incision was studied by Lee and colleagues,130 who reported persistence of Propionibacterium acnes in the dermal layer after skin preparation. Their study, however, was isolated to the upper back region, not the hip or knee.
Postoperative Risk Prevention
Most arthroplasty patients receive anticoagulation after surgery, but it must be used with caution. Large hematomas can predispose to wound complications. Parvizi and colleagues131 associated wound drainage, hematoma, and subsequent PJI with an INR above 1.5 in the early postoperative period. Therefore, balanced anticoagulation is crucial. Postoperative glucose control is also essential, particularly for patients with diabetes. Although preoperative blood glucose levels may or may not affect PJI risk,15,17,132 postoperative blood glucose levels of 126 mg/dL or higher are strongly associated with joint infections.133 Even nondiabetic patients with postoperative morning levels over 140 mg/dL are 3 times more likely to develop an infection.17
Efforts should be made to discharge patients as soon as it is safe to do so. With longer hospital stays, patients are more exposed to nosocomial organisms and increased antibiotic resistance.5,23,134 Outpatient antibiotics should be considered for dental, gastrointestinal, and genitourinary procedures. Oral antibiotic prophylaxis is controversial, as there is some evidence that dental procedures increase the risk of PJI only minimally.10,135-138
Conclusion
PJI is a potentially devastating complication of TJA. For this reason, much research has been devoted to proper diagnosis and treatment. Although the literature on PJI prophylaxis is abundant, there is relatively little consensus on appropriate PJI precautions. Preoperative considerations should include medical comorbidities, use of immunocompromising medications, obesity, nutritional factors, smoking, alcohol use, and MRSA colonization. Surgeons must have a consistent intraoperative method of antibiotic administration, skin preparation, scrubbing, draping, gloving, instrument exchange, blood loss management, cementing, and closure. In addition, monitoring of postoperative anticoagulation and blood glucose management is important. Having a thorough understanding of PJI risk factors may help reduce the incidence of this devastating complication.
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
1. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247-1254.
2. Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection. J Bone Joint Surg Br. 2012;94(11 suppl A):42-46.
3. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Ridgeway S. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
6. Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59(12):1713-1720.
7. Menon TJ, Wroblewski BM. Charnley low-friction arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1983;(176):127-128.
8. Stern SH, Insall JN, Windsor RE, Inglis AE, Dines DM. Total knee arthroplasty in patients with psoriasis. Clin Orthop Relat Res. 1989;(248):108-100.
9. Beyer CA, Hanssen AD, Lewallen DG, Pittelkow MR. Primary total knee arthroplasty in patients with psoriasis. J Bone Joint Surg Br. 1991;73(2):258-259.
10. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case–control study. Clin Infect Dis. 2010;50(1):8-16.
11. Singh G, Rao DJ. Bacteriology of psoriatic plaques. Dermatologica. 1978;157(1):21-27.
12. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574-583.
13. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 suppl):84-88.
14. Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577-1581.
15. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
16. Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726-729.e1.
17. Mraovic B, Suh D, Jacovides C. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412-418.
18. Abbott KC, Bucci JR, Agodoa LY. Total hip arthroplasty in chronic dialysis patients in the United States. J Nephrol. 2003;16(1):34-39.
19. Lieberman JR, Fuchs MD, Haas SB, et al. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10(2):191-195.
20. Sakalkale DP, Hozack WJ, Rothman RH. Total hip arthroplasty in patients on long-term renal dialysis. J Arthroplasty. 1999;14(5):571-575.
21. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329.
22. Deegan BF, Richard RD, Bowen TR, Perkins RM, Graham JH, Foltzer MA. Impact of chronic kidney disease stage on lower-extremity arthroplasty. Orthopedics. 2014;37(7):e613-e618.
23. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
24. Tomás T. Patient-related risk factors for infected total arthroplasty. Acta Chir Orthop. 2008;75(6):451-456.
25. Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10(3):467-469.
26. Gou W, Chen J, Jia Y, Wang Y. Preoperative asymptomatic leucocyturia and early prosthetic joint infections in patients undergoing joint arthroplasty. J Arthroplasty. 2014;29(3):473-476.
27. Goodman SM, Paget S. Perioperative drug safety in patients with rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(4):747-759.
28. Salem M, Tainsh RE Jr, Bromberg J, Loriaux DL, Chernow B. Perioperative glucocorticoid coverage. A reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425.
29. Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551.
30. Grennan DM. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217.
31. Johnson R, Charnley J. Hydroxychloroquine in prophylaxis of pulmonary embolism following hip arthroplasty. Clin Orthop Relat Res. 1979;(144):174-177.
32. Mushtaq S, Goodman SM, Scanzello CR. Perioperative management of biologic agents used in treatment of rheumatoid arthritis. Am J Ther. 2011;18(5):426-434.
33. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46-50.
34. Winiarsky R, Barth P, Lotke PA. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80(12):1770-1774.
35. Kulkarni A, Jameson SS, James P, Woodcock S, Muller S, Reed MR. Does bariatric surgery prior to lower limb joint replacement reduce complications? Surgeon. 2011;9(1):18-21.
36. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. J Arthroplasty. 1991;6(4):321-325.
37. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults. JAMA. 2002;287(23):3116.
38. Kwiatkowski TC, Hanley EN Jr, Ramp WK. Cigarette smoking and its orthopedic consequences. Am J Orthop. 1996;25(9):590-597.
39. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114-117.
40. Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386.
41. Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9(4):e95300.
42. Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res. 2010;468(12):3268-3277.
43. Tønnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ. 1999;318(7194):1311-1316.
44. Shourie S, Conigrave KM, Proude EM, Ward JE, Wutzke SE, Haber PS. The effectiveness of a tailored intervention for excessive alcohol consumption prior to elective surgery. Alcohol Alcohol. 2006;41(6):643-649.
45. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133.
46. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
47. Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121(4):310-315.
48. American Academy of Orthopaedic Surgeons Patient Safety Committee, Evans RP. Surgical site infection prevention and control: an emerging paradigm. J Bone Joint Surg Am. 2009;91(suppl 6):2-9.
49. Goyal N, Aggarwal V, Parvizi J. Methicillin-resistant Staphylococcus aureus screening in total joint arthroplasty: a worthwhile endeavor. J Knee Surg. 2012;25(1):37-43.
50. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505-520.
51. Wilcox MH, Hall J, Pike H, et al. Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect. 2003;54(3):196-201.
52. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9-17.
53. Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN J. 2002;75(1):184-187.
54. Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate–impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89-96.
55. Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233-239.
56. Johnson AJ, Kapadia BH, Daley JA, Molina CB, Mont MA. Chlorhexidine reduces infections in knee arthroplasty. J Knee Surg. 2013;26(3):213-218.
57. Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty. 2010;25(6 suppl):98-102.
58. Mauerhan DR, Nelson CL, Smith DL, et al. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J Bone Joint Surg Am. 1994;76(1):39-45.
59. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395-404.
60. Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472(1):52-56.
61. Illingworth KD, Mihalko WM, Parvizi J, et al. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(8):e50.
62. Bannister GC, Auchincloss JM, Johnson DP, Newman JH. The timing of tourniquet application in relation to prophylactic antibiotic administration. J Bone Joint Surg Br. 1988;70(2):322-324.
63. Engesæter LB, Lie SA, Espehaug B, Furnes O, Vollset SE, Havelin LI. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74(6):644-651.
64. Ritter MA. Operating room environment. Clin Orthop Relat Res. 1999;(369):103-109.
65. Brandt C, Hott U, Sohr D, Daschner F, Gastmeier P, Rüden H. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg. 2008;248(5):695-700.
66. Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51(2):79-84.
67. Hamilton HW, Booth AD, Lone FJ, Clark N. Penetration of gown material by organisms from the surgical team. Clin Orthop Relat Res. 1979;(141):237-246.
68. Da Costa AR, Kothari A, Bannister GC, Blom AW. Investigating bacterial growth in surgical theatres: establishing the effect of laminar airflow on bacterial growth on plastic, metal and wood surfaces. Ann R Coll Surg Engl. 2008;90(5):417-419.
69. Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;(2):CD004122.
70. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18-26.
71. Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect. 2013;20(2):130-135.
72. Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-27.
73. Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Lowe D. Extended follow-up of patients suspected of having joint sepsis after total joint replacement. J Hyg (Lond). 1985;95(3):655-664.
74. Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;(4):CD006353.
75. Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014;27(4):251-258.
76. Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2002;(3):CD003087.
77. Demircay E, Unay K, Bilgili MG, Alataca G. Glove perforation in hip and knee arthroplasty. J Orthop Sci. 2010;15(6):790-794.
78. Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007;73(6):760-764.
79. Sanders R, Fortin P, Ross E, Helfet D. Outer gloves in orthopaedic procedures. Cloth compared with latex. J Bone Joint Surg Am. 1990;72(6):914-917.
80. Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966-968.
81. Al-Maiyah M, Bajwa A, Mackenney P, et al. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87(4):556-559.
82. Insull PJ, Hudson J. Suction tip: a potential source of infection in clean orthopaedic procedures. ANZ J Surg. 2012;82(3):185-186.
83. Givissis P, Karataglis D, Antonarakos P, Symeonidis PD, Christodoulou A. Suction during orthopaedic surgery. How safe is the suction tip? Acta Orthop Belg. 2008;74(4):531-533.
84. Meals RA, Knoke L. The surgical suction top—a contaminated instrument. J Bone Joint Surg Am. 1978;60(3):409-410.
85. Strange-Vognsen MH, Klareskov B. Bacteriologic contamination of suction tips during hip arthroplasty. Acta Orthop Scand. 1988;59(4):410-411.
86. Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68(1):151-153.
87. Baird RA, Nickel FR, Thrupp LD, Rucker S, Hawkins B. Splash basin contamination in orthopaedic surgery. Clin Orthop Relat Res. 1984;(187):129-133.
88. Hargrove R, Ridgeway S, Russell R, Norris M, Packham I, Levy B. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62(4):446-449.
89. Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;(439):27-31.
90. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56.
91. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement. Clin Orthop Relat Res. 2001;(392):15-23.
92. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
93. Cherian JJ, Kapadia BH, Issa K, et al. Preoperative blood management strategies for total hip arthroplasty. Surg Technol Int. 2013;23:261-266.
94. Issa K, Banerjee S, Rifai A, et al. Blood management strategies in primary and revision total knee arthroplasty for Jehovah’s Witness patients. J Knee Surg. 2013;26(6):401-404.
95. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2010;93(1):39-46.
96. Berger V, Alperson S. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79-88.
97. Chimento GF, Huff T, Ochsner JL, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
98. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty. 2014;29(3):501-503.
99. Kim HJ, Fraser MR, Kahn B, Lyman S, Figgie MP. The efficacy of a thrombin-based hemostatic agent in unilateral total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(13):1160-1165.
100. Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950-1955.
101. Romanò CL, Monti L, Logoluso N, Romanò D, Drago L. Does a thrombin-based topical haemostatic agent reduce blood loss and transfusion requirements after total knee revision surgery? A randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3337-3342.
102. Falez F, Meo A, Panegrossi G, Favetti F, Cava F, Casella F. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213-1217.
103. Morris MJ, Barrett M, Lombardi AV, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
104. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
105. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
106. Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10(4):470-475.
107. Srivastav A, Nadkarni B, Srivastav S, Mittal V, Agarwal S. Prophylactic use of antibiotic-loaded bone cement in primary total knee arthroplasty: justified or not? Indian J Orthop. 2009;43(3):259-263.
108. Dunbar MJ. Antibiotic bone cements: their use in routine primary total joint arthroplasty is justified. Orthopedics. 2009;32(9).
109. Merollini KM, Zheng H, Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41(3):221-226.
110. Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38-47.
111. Seldes RM, Winiarsky R, Jordan LC, et al. Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg Am. 2005;87(2):268-272.
112. Wright TM, Sullivan DJ, Arnoczky SP. The effect of antibiotic additions on the fracture properties of bone cements. Acta Orthop Scand. 1984;55(4):414-418.
113. Baleani M, Persson C, Zolezzi C, Andollina A, Borrelli AM, Tigani D. Biological and biomechanical effects of vancomycin and meropenem in acrylic bone cement. J Arthroplasty. 2008;23(8):1232-1238.
114. Baleani M, Cristofolini L, Minari C, Toni A. Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulphate vs pure PMMA. Proc Inst Mech Eng H. 2005;217(1):9-12.
115. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop Scand. 2008;79(3):335-341.
116. Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesæter LB, Finlayson SRG. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91(3):634-641.
117. Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute Betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27-30.
118. Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013;471(2):665-671.
119. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287.
120. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788.
121. Miller AG, Swank ML. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop. 2010;39(10):476-478.
122. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
123. Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185-189.
124. Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86(6):1146-1152.
125. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36-46.
126. Ritter MA, French ML, Eitzen HE. Bacterial contamination of the surgical knife. Clin Orthop Relat Res. 1975;(108):158-160.
127. Fairclough JA, Mackie IG, Mintowt-Czyz W, Phillips GE. The contaminated skin-knife. A surgical myth. J Bone Joint Surg Br. 1983;65(2):210.
128. Ramón R, García S, Combalía A, Puig de la Bellacasa J, Segur JM. Bacteriological study of surgical knives: is the use of two blades necessary? Arch Orthop Trauma Surg. 1994;113(3):157-158.
129. Hasselgren PO, Hagberg E, Malmer H, Säljö A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917-920.
130. Lee MJ, Pottinger PS, Butler-Wu S, Bumgarner RE, Russ SM, Matsen FA 3rd. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96(17):1447-1450.
131. Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(6 suppl 2):24-28.
132. Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am. 2009;91(7):1621-1629.
133. Reátegui D, Sanchez-Etayo G, Núñez E, et al. Perioperative hyperglycaemia and incidence of post-operative complications in patients undergoing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2026-2031.
134. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216-1222.e1-e3.
135. Friedlander AH. Oral cavity staphylococci are a potential source of prosthetic joint infection. Clin Infect Dis. 2010;50(12):1682-1683.
136. Zimmerli W, Sendi P. Antibiotics for prevention of periprosthetic joint infection following dentistry: time to focus on data. Clin Infect Dis. 2010;50(1):17-19.
137. Young H, Hirsh J, Hammerberg EM, Price CS. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(2):162-168.
138. Simmons NA, Ball AP, Cawson RA, et al. Case against antibiotic prophylaxis for dental treatment of
Quality and Quantity of the Elbow Arthroscopy Literature: A Systematic Review and Meta-Analysis
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.