User login
FDA okays prophylactic Pradaxa for VTE in hip replacement
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
Postsurgical Analgesic Found to Decrease Opioid Use, Hospital Stay, and Readmission Rates After Knee Replacement Surgery
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
DALLAS—Positive data about the use of Exparel (bupivacaine liposome injectable suspension) as a postsurgical analgesic following total knee replacement surgery was presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
The study, which compared the use of bupivacaine liposome injectable suspension infiltration to the standard of care in 1,110 patients, found that bupivacaine liposome injectable suspension was associated with significant improvements in a variety of patient and health economic outcomes, including opioid use, hospital stay, and readmission rate.
Patients who underwent total knee arthroplasty (TKA) received identical pre-, intra-, and postoperative pain management protocols, with the exception of 527 patients who received bupivacaine liposome injectable suspension infiltration in place of a femoral nerve block.
The study authors compared several patient and cost-related outcomes. Opioid use during hospitalization was statistically significantly reduced in the bupivacaine liposome injectable suspension group. Other key findings included:
• Shorter hospital length of stay (2.93 days for the bupivacaine liposome injectable suspension group vs 3.19 days for the femoral nerve block group, P<0.001)
• Increased rate of discharge to home (77.8% for the bupivacaine liposome injectable suspension group vs 72.21% for the femoral nerve block group, P=0.032)
• Reduced inpatient fall rate (0.56% for the bupivacaine liposome injectable suspension group vs 2.11% for the femoral nerve block group, P=0.03)
• Lower 30-day all-cause readmission rate (0.95% for the bupivacaine liposome injectable suspension group vs 2.57% for the femoral nerve block group, P=0.041)
“Based on our analysis, incorporating liposomal bupivacaine into the postsurgical analgesic protocol following total knee arthroplasty has significant and quantifiable benefits to both the patient and the institution,” said Richard Iorio, MD, Professor of Orthopaedic Surgery at NYU School of Medicine in New York. “The measurable opioid-sparing effect of this new regimen has enabled us to virtually eliminate intravenous patient-controlled analgesia, or PCA, devices from the standard of care in total joint arthroplasty patients, without compromising patient comfort. In addition, we found that the incremental cost of adding this new modality was offset by meaningful savings from shorter anesthesia induction time in the operating room, shorter hospital stays and lower rates of 30-day readmission.”
Patients With Sacroiliac Joint Pain Helped With Implant Procedure
A minimally invasive implant procedure is highly effective in reducing pain and disability for patients with sacroiliac joint dysfunction (SIJ), according to a study published in the November issue of Neurosurgery. The randomized controlled trial showed superior outcomes in patients undergoing minimally invasive sacroiliac joint fusion using triangular titanium implants, compared with nonsurgical management, according to lead author David W. Polly, MD, a professor in the Departments of Orthopedic Surgery and Neurosurgery at the University of Minnesota in Minneapolis.
This study included 148 patients with low back pain caused by confirmed SIJ dysfunction, treated at 19 spine surgery clinics in the United States. SIJ disruption, also known as osteoarthritis, is estimated to cause 15% to 23% of cases of chronic low back pain.
Study participants had severe SIJ pain, with an average pain score of 82 on a 0-to-100-point scale. Average pain duration was longer than 6 years, and about two-thirds of subjects were taking opioid medications. Many study participants had previously received nonsurgical SIJ treatments and many had a history of prior spinal surgery.
Two-thirds of subjects were randomly assigned to undergo minimally invasive SIJ fusion. In this procedure, triangular titanium implants were placed through a small incision to stabilize and fuse the SIJ. Procedures were unilateral in most cases, but some subjects underwent bilateral treatment. The remaining subjects received nonsurgical treatments, such as physical therapy, steroid injections, and/or radiofrequency ablation of sacral nerve root lateral branches.
Pain and other outcomes were compared at baseline and at 1, 3, 6, and 12 months. At 6 months, subjects in the nonsurgical group had the option to “cross over” to the implant procedure.
Based on reduction in pain and absence of complications at 6 months, treatment was rated successful in 81% of subjects assigned to the SIJ implant procedure, compared with 26% of people with nonsurgical treatment. The average pain score decreased to 30 in the surgical group compared with 72 in the nonsurgical group. A total of 73% of subjects undergoing the implant procedure had “clinically significant” reduction in disability scores, compared with 14% in the nonsurgical group.
After 1 year, subjects assigned to SIJ fusion still had significant reductions in pain and disability, as well as improved quality of life. Thirty-five subjects from the nonsurgical group opted to undergo the implant procedure, with similarly good results.
The minimally invasive SIJ implant approach that was evaluated in this trial has been cleared by the FDA. The study is the first randomized controlled trial to directly compare the results of surgical and nonsurgical treatment for SIJ dysfunction.
The results show “clinically and statistically important” improvements in clinical outcomes for patients undergoing the SIJ implant procedure, according to Dr. Polly and colleagues, with “profound differences” between the surgical and nonsurgical groups. The implant procedure is minimally invasive, has few complications, and produces significant and lasting improvements in pain, disability, and quality of life.
The study authors noted some important limitations of their trial, including the lack of long-term outcomes in the nonsurgical group due to the high crossover rate.
Investigators plan further analyses, including 2-year follow-up CT scans and a cost-effectiveness comparison of SIJ fusion versus nonsurgical treatment.
Suggested Reading
Polly DW, Cher DJ, Wine KD, et al. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-691.
A minimally invasive implant procedure is highly effective in reducing pain and disability for patients with sacroiliac joint dysfunction (SIJ), according to a study published in the November issue of Neurosurgery. The randomized controlled trial showed superior outcomes in patients undergoing minimally invasive sacroiliac joint fusion using triangular titanium implants, compared with nonsurgical management, according to lead author David W. Polly, MD, a professor in the Departments of Orthopedic Surgery and Neurosurgery at the University of Minnesota in Minneapolis.
This study included 148 patients with low back pain caused by confirmed SIJ dysfunction, treated at 19 spine surgery clinics in the United States. SIJ disruption, also known as osteoarthritis, is estimated to cause 15% to 23% of cases of chronic low back pain.
Study participants had severe SIJ pain, with an average pain score of 82 on a 0-to-100-point scale. Average pain duration was longer than 6 years, and about two-thirds of subjects were taking opioid medications. Many study participants had previously received nonsurgical SIJ treatments and many had a history of prior spinal surgery.
Two-thirds of subjects were randomly assigned to undergo minimally invasive SIJ fusion. In this procedure, triangular titanium implants were placed through a small incision to stabilize and fuse the SIJ. Procedures were unilateral in most cases, but some subjects underwent bilateral treatment. The remaining subjects received nonsurgical treatments, such as physical therapy, steroid injections, and/or radiofrequency ablation of sacral nerve root lateral branches.
Pain and other outcomes were compared at baseline and at 1, 3, 6, and 12 months. At 6 months, subjects in the nonsurgical group had the option to “cross over” to the implant procedure.
Based on reduction in pain and absence of complications at 6 months, treatment was rated successful in 81% of subjects assigned to the SIJ implant procedure, compared with 26% of people with nonsurgical treatment. The average pain score decreased to 30 in the surgical group compared with 72 in the nonsurgical group. A total of 73% of subjects undergoing the implant procedure had “clinically significant” reduction in disability scores, compared with 14% in the nonsurgical group.
After 1 year, subjects assigned to SIJ fusion still had significant reductions in pain and disability, as well as improved quality of life. Thirty-five subjects from the nonsurgical group opted to undergo the implant procedure, with similarly good results.
The minimally invasive SIJ implant approach that was evaluated in this trial has been cleared by the FDA. The study is the first randomized controlled trial to directly compare the results of surgical and nonsurgical treatment for SIJ dysfunction.
The results show “clinically and statistically important” improvements in clinical outcomes for patients undergoing the SIJ implant procedure, according to Dr. Polly and colleagues, with “profound differences” between the surgical and nonsurgical groups. The implant procedure is minimally invasive, has few complications, and produces significant and lasting improvements in pain, disability, and quality of life.
The study authors noted some important limitations of their trial, including the lack of long-term outcomes in the nonsurgical group due to the high crossover rate.
Investigators plan further analyses, including 2-year follow-up CT scans and a cost-effectiveness comparison of SIJ fusion versus nonsurgical treatment.
A minimally invasive implant procedure is highly effective in reducing pain and disability for patients with sacroiliac joint dysfunction (SIJ), according to a study published in the November issue of Neurosurgery. The randomized controlled trial showed superior outcomes in patients undergoing minimally invasive sacroiliac joint fusion using triangular titanium implants, compared with nonsurgical management, according to lead author David W. Polly, MD, a professor in the Departments of Orthopedic Surgery and Neurosurgery at the University of Minnesota in Minneapolis.
This study included 148 patients with low back pain caused by confirmed SIJ dysfunction, treated at 19 spine surgery clinics in the United States. SIJ disruption, also known as osteoarthritis, is estimated to cause 15% to 23% of cases of chronic low back pain.
Study participants had severe SIJ pain, with an average pain score of 82 on a 0-to-100-point scale. Average pain duration was longer than 6 years, and about two-thirds of subjects were taking opioid medications. Many study participants had previously received nonsurgical SIJ treatments and many had a history of prior spinal surgery.
Two-thirds of subjects were randomly assigned to undergo minimally invasive SIJ fusion. In this procedure, triangular titanium implants were placed through a small incision to stabilize and fuse the SIJ. Procedures were unilateral in most cases, but some subjects underwent bilateral treatment. The remaining subjects received nonsurgical treatments, such as physical therapy, steroid injections, and/or radiofrequency ablation of sacral nerve root lateral branches.
Pain and other outcomes were compared at baseline and at 1, 3, 6, and 12 months. At 6 months, subjects in the nonsurgical group had the option to “cross over” to the implant procedure.
Based on reduction in pain and absence of complications at 6 months, treatment was rated successful in 81% of subjects assigned to the SIJ implant procedure, compared with 26% of people with nonsurgical treatment. The average pain score decreased to 30 in the surgical group compared with 72 in the nonsurgical group. A total of 73% of subjects undergoing the implant procedure had “clinically significant” reduction in disability scores, compared with 14% in the nonsurgical group.
After 1 year, subjects assigned to SIJ fusion still had significant reductions in pain and disability, as well as improved quality of life. Thirty-five subjects from the nonsurgical group opted to undergo the implant procedure, with similarly good results.
The minimally invasive SIJ implant approach that was evaluated in this trial has been cleared by the FDA. The study is the first randomized controlled trial to directly compare the results of surgical and nonsurgical treatment for SIJ dysfunction.
The results show “clinically and statistically important” improvements in clinical outcomes for patients undergoing the SIJ implant procedure, according to Dr. Polly and colleagues, with “profound differences” between the surgical and nonsurgical groups. The implant procedure is minimally invasive, has few complications, and produces significant and lasting improvements in pain, disability, and quality of life.
The study authors noted some important limitations of their trial, including the lack of long-term outcomes in the nonsurgical group due to the high crossover rate.
Investigators plan further analyses, including 2-year follow-up CT scans and a cost-effectiveness comparison of SIJ fusion versus nonsurgical treatment.
Suggested Reading
Polly DW, Cher DJ, Wine KD, et al. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-691.
Suggested Reading
Polly DW, Cher DJ, Wine KD, et al. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-691.
Irisin Increases Cortical Bone Mass
A recently identified molecule produced by skeletal muscle in response to exercise has been shown to increase bone mass, according to a study published online ahead of print September 29 in the Proceedings of the National Academy of Sciences. “This is a novel finding, and offers promise in the lab, and in the clinic,” said co-lead study author Mone Zaidi, MD, PhD, Professor of Medicine and of Structural and Chemical Biology at the Icahn School of Medicine at Mount Sinai, and Director of the Mount Sinai Bone Program in New York. “It establishes for the first time [that] a molecule released from muscle during exercise can act directly on long bones to increase their strength. These are the bones utilized during exercise, and also the ones most likely to break.”
In this study, young male mice, chosen because researchers could best see bone accrual at this age, were injected with irisin. In the injected mice, researchers saw significant increases in bone mass and strength, specifically cortical bone. The action of the recently identified signaling molecule, irisin, was mediated primarily through bone growth.
The study suggests irisin is fundamental to muscle–bone communication, and likely translates the well-known skeletal anabolic action of exercise by directly stimulating new bone synthesis by osteoblasts.
According to the study authors, identifying irisin as a molecule responsible for muscle-bone connectivity during exercise could lead to the development of future therapies for sarcopenia and osteoporosis.
“These diseases often occur together, and both muscle and bone loss are common medical problems in the elderly that cause significant disability. Understanding this molecular connection between muscle and bone gives us hope for treating age-related bone and muscle loss at the same time, with the same agent,” said Dr. Zaidi.
Suggested Reading
Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112(39):12157-12162. [Epub ahead of print].
A recently identified molecule produced by skeletal muscle in response to exercise has been shown to increase bone mass, according to a study published online ahead of print September 29 in the Proceedings of the National Academy of Sciences. “This is a novel finding, and offers promise in the lab, and in the clinic,” said co-lead study author Mone Zaidi, MD, PhD, Professor of Medicine and of Structural and Chemical Biology at the Icahn School of Medicine at Mount Sinai, and Director of the Mount Sinai Bone Program in New York. “It establishes for the first time [that] a molecule released from muscle during exercise can act directly on long bones to increase their strength. These are the bones utilized during exercise, and also the ones most likely to break.”
In this study, young male mice, chosen because researchers could best see bone accrual at this age, were injected with irisin. In the injected mice, researchers saw significant increases in bone mass and strength, specifically cortical bone. The action of the recently identified signaling molecule, irisin, was mediated primarily through bone growth.
The study suggests irisin is fundamental to muscle–bone communication, and likely translates the well-known skeletal anabolic action of exercise by directly stimulating new bone synthesis by osteoblasts.
According to the study authors, identifying irisin as a molecule responsible for muscle-bone connectivity during exercise could lead to the development of future therapies for sarcopenia and osteoporosis.
“These diseases often occur together, and both muscle and bone loss are common medical problems in the elderly that cause significant disability. Understanding this molecular connection between muscle and bone gives us hope for treating age-related bone and muscle loss at the same time, with the same agent,” said Dr. Zaidi.
A recently identified molecule produced by skeletal muscle in response to exercise has been shown to increase bone mass, according to a study published online ahead of print September 29 in the Proceedings of the National Academy of Sciences. “This is a novel finding, and offers promise in the lab, and in the clinic,” said co-lead study author Mone Zaidi, MD, PhD, Professor of Medicine and of Structural and Chemical Biology at the Icahn School of Medicine at Mount Sinai, and Director of the Mount Sinai Bone Program in New York. “It establishes for the first time [that] a molecule released from muscle during exercise can act directly on long bones to increase their strength. These are the bones utilized during exercise, and also the ones most likely to break.”
In this study, young male mice, chosen because researchers could best see bone accrual at this age, were injected with irisin. In the injected mice, researchers saw significant increases in bone mass and strength, specifically cortical bone. The action of the recently identified signaling molecule, irisin, was mediated primarily through bone growth.
The study suggests irisin is fundamental to muscle–bone communication, and likely translates the well-known skeletal anabolic action of exercise by directly stimulating new bone synthesis by osteoblasts.
According to the study authors, identifying irisin as a molecule responsible for muscle-bone connectivity during exercise could lead to the development of future therapies for sarcopenia and osteoporosis.
“These diseases often occur together, and both muscle and bone loss are common medical problems in the elderly that cause significant disability. Understanding this molecular connection between muscle and bone gives us hope for treating age-related bone and muscle loss at the same time, with the same agent,” said Dr. Zaidi.
Suggested Reading
Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112(39):12157-12162. [Epub ahead of print].
Suggested Reading
Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA. 2015;112(39):12157-12162. [Epub ahead of print].
Do Heavier Patients Require Fewer Blood Transfusions In Hip, Knee Replacement Surgery?
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
VIENNA—Blood transfusion rates in hip and knee replacement surgery are lower in overweight or obese patients than in patients with a normal weight, according to a study presented at the 2015 International Society for Technology in Arthroplasty conference.
In this retrospective study, which included 2,399 participants, researchers sought to evaluate the impact of BMI on blood transfusions and postsurgical complications in hip and knee replacement surgery. In all, 1,503 patients underwent knee replacement and 896 patients underwent hip surgery between January 1, 2011, and November 1, 2013.
Patients were classified into groups according to BMI—normal (< 25 BMI), overweight (25 to 29.9 BMI), and obese (> 30 BMI).
Among the study’s findings were:
• A 34.8% blood transfusion rate for normal BMI patients compared with 21.9% for obese BMI patients for hip replacement.
• A 17.3% blood transfusion rate for normal BMI patients compared with 8.3% for obese BMI patients for knee replacement.
• A trend towards increased rates of deep surgical site infections in obese BMI patients.
“The results were surprising to us. It goes against the normal thought process,” said Craig Silverton, DO, a joint replacement surgeon at Henry Ford Hospital in Detroit and the study’s lead author. “It’s hard to explain but one theory could be that heavier patients have larger blood volume than patients of normal weight.”
Researchers also found no correlation between the heavier patients and post-surgical complications such as blood clots and heart attacks.
An estimated 78.6 million adult Americans are obese, and their weight problems are closely linked with an increased demand for hip and knee replacement surgery, according to government and research figures.
Patients who undergo a hip replacement typically lose about 2 pints of blood during surgery. For a knee replacement, patients usually lose about 1 pint of blood.
Surgical Management of Gorham-Stout Disease of the Pelvis Refractory to Medical and Radiation Therapy
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
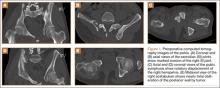
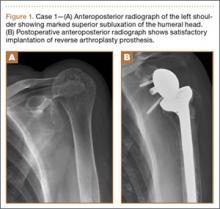
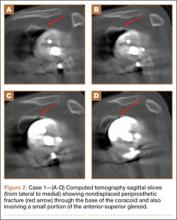
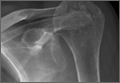
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
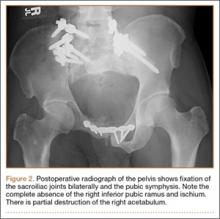
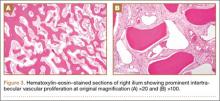
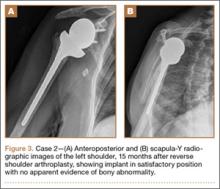
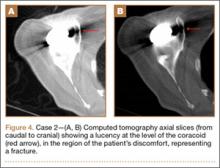
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
Gorham-Stout disease (GSD) is a rare condition characterized by spontaneous idiopathic resorption of bone with lymphovascular proliferation and an absence of malignant features. It was originally described by Jackson1 in an 1838 report of a 36-year-old man whose “arm bone, between the shoulder and elbow” had completely vanished after 2 fractures. The disease was defined and its pathology characterized by Gorham and Stout2 in 1955 in a series of 24 patients. Despite about 200 reported cases in the literature,3 its etiology remains unclear. Any bone in the skeleton may be affected by GSD, although there is a predilection for the skull, humerus, clavicle, ribs, pelvis, and femur.4-6 It commonly manifests within the first 3 decades of life, but case reports range from as early as 2 months of age to the eighth decade.5,7
Gorham-Stout disease is a diagnosis of exclusion that requires careful consideration of the clinical context, radiographic findings, and histopathology. Typical histopathologic findings include benign lymphatic or vascular proliferation, involution of adipose tissue within the bone marrow, and thinning of bony trabeculae.6 Fibrous tissue may replace vascular tissue after the initial vasoproliferative, osteolytic phase.6 Some authors describe the disease as having 2 phases, the first with massive osteolysis followed by relative dormancy and the second without progression or re-ossification.8,9 Treatment remains controversial and is guided by management of the disease’s complications. Options range from careful observation and supportive management to aggressive surgical resection and reconstruction, with positive outcomes reported using many different modalities.10 Most treatment successes, however, hinge on halting bony resorption using medical and radiation therapy. Surgery is usually reserved as a salvage option for patients who have failed medical modalities and have residual symptoms or functional limitations.6
This case report describes the successful surgical management of a patient with pelvic GSD who had progressive pain and functional limitation despite exhaustive medical and radiation therapy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 27-year-old man sought medical attention after a fall while mowing his lawn that resulted in difficulty ambulating. Radiographic studies showed discontinuous lytic lesions in the right periacetabular region and the right sacroiliac (SI) joint. Biopsy at an outside institution revealed an infiltration of thin-walled branching vascular channels involving intertrabecular marrow spaces and periosteal connective tissue. The vessels were devoid of a muscular coat and lined by flattened epithelium; these features were seen as consistent with GSD.
The patient was managed medically at the outside institution for approximately 2 years, with regimens consisting of zoledronate, denosumab, sorafenib, vincristine, sirolimus, and bevacizumab. Because there is no standard chemotherapy protocol for GSD, this broad regimen was likely an attempt by treating physicians to control disease progression before considering radiation or surgery. Zoledronate, a bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor κβ ligand (RANKL), both inhibit bone resorption, making them logical choices in treating an osteolytic disease. Sorafenib, vincristine, sirolimus, and bevacizumab may be of clinical benefit in GSD via inhibition of vascular proliferation, which is a key histologic feature in GSD. Sorafenib inhibits the vascular endothelial growth factor (VEGF) receptor, vincristine and sirolimus inhibit VEGF production, and bevacizumab is a monoclonal antibody targeting VEGF.
The patient’s disease continued to involve more of his right hemipelvis despite this extensive regimen of chemotherapy, and he experienced significant functional decline about 2 years after initial presentation, when he was no longer able to ambulate unassisted. Radiation therapy to the pelvis was attempted at the outside institution (6/15 MV photons, 5040 cGy, 28 fractions) without improvement. Three years after his initial injury, he presented to our clinic.
Now age 30 years, the patient ambulated only with crutches and endorsed minimal improvement in his pain over 3 years of treatment. Physical examination of the patient revealed that he was a tall, thin man in visible discomfort. Sensation was intact to light touch in the bilateral L1 to S1 nerve distributions. There was marked weakness of the right lower extremity, and his examination was limited by pain. He could not perform a straight leg raise on the right side. Right quadriceps strength was 4/5, and right hamstrings strength was 3/5. There was no weakness in the left leg. Reflexes were normal and symmetric bilaterally at the patellar and gastrocnemius soleus tendons. Distal circulatory status in both extremities was normal, and there were no deformities of the skin.
Figure 1 shows the patient’s computed tomography (CT) scan. Figures 1A and 1B reveal fragmentation of the posterior ilia and sacrum along both SI joints. Dislocation of the pubic symphysis is shown in Figures 1C and 1D, and discontinuous involvement of the ischium and posterior wall of the acetabulum is visible in Figure 1E.
Serum studies, including C-reactive protein, erythrocyte sedimentation rate, and a complete blood count, were within normal limits. A CT-guided core needle biopsy and aspiration of the right SI joint revealed no infection; pathology was nondiagnostic. Anesthetic injection of the hip joint resulted in no relief. As this man was severely functionally limited and had exhausted all medical and radiation treatment options, a collaborative decision was made to proceed with surgical management. Surgical options included spinopelvic fusion unilaterally or bilaterally, hip arthroplasty, or sacropelvic resection with or without reconstruction. The patient opted for intralesional surgery and spinopelvic fusion in place of more radical options.
Thirty-seven months after his initial presentation, he underwent posterior spinal fusion L5 to S1, SI fusion, and anterior locking plate fixation of the pubic symphysis, as seen in Figure 2. Pathology from surgical specimens, seen at original magnification ×20 and ×100 in Figures 3A and 3B, respectively, showed prominent vascular proliferation in the right ilium, with reactive bone changes in the left ilium and right sacrum. A lytic lesion showed fibrous tissue with an embedded fragment of necrotic bone.
Six weeks after surgery, the patient had substantial improvement in his pain and was partially weight-bearing. He was able to ambulate with crutches and returned to work. The patient’s overall clinical status continued to improve throughout the postoperative course. He developed low back pain 7 months after surgery and was found to have a sacrococcygeal abscess and coccygeal fracture anterior to the sacrum. He underwent irrigation and débridement of the abscess and distal coccygectomy and was treated with 6 weeks of intravenous cefazolin and long-term suppression with levofloxacin and rifampin for methicillin-sensitive Staphylococcus aureus hardware infection and osteomyelitis. The patient’s clinical course subsequently improved. At latest follow-up 16 months after the index operation, pain was reported as manageable and mostly an annoyance. He was prescribed up to 40 mg of oxycodone daily for pain. The patient returned to work, ambulates with a cane (no other assistive devices), and reports being able to get around without any difficulty.
Discussion
Gorham-Stout disease is an exceedingly rare condition resulting in spontaneous osteolysis. Approximately 200 cases have been reported with no apparent gender, race, or familial predilection or systemic symptoms differentiating it from other etiologies of idiopathic osteolysis.6 These patients often seek medical attention after sustaining a pathologic fracture,6 when a broad differential diagnosis narrows to GSD only after biopsy excludes other possibilities and demonstrates characteristic angiomatosis without malignant features.2,4,6,8,10 Gorham-Stout disease appears more frequently at particular sites within the skeleton, and pelvic involvement is common—more than 20% of cases in 1 review.5,10 Limitations in the patient’s ability to ambulate invariably result from osteolysis of the pelvis, which is concerning considering the young age at which GSD typically presents. A variety of treatment modalities have been described for pelvic GSD, but surgery has been undertaken in relatively few cases.5
The diagnosis is one of exclusion after considering the clinical context and radiologic and pathologic findings. In this case, a pathologic fracture was discovered with osteolytic lesions throughout the hemipelvis. Biopsy excluded malignancy and demonstrated the key hemangiomatous vascular proliferation with thin-walled vessels that is classic for GSD. While our patient initially appeared to have 2 sites of disease, the surgical specimen revealed a primary site of vascular proliferation in the right ilium from which 2 apparent foci had spread, consistent with the typical monocentric presentation of GSD.11 A broad differential diagnosis must be considered at initial presentation, including osteomyelitis, metastatic disease, multiple myeloma, and primary bone sarcoma. Upon identifying a primary osteolytic process, several considerations besides GSD remain, such as Hajdu-Cheney syndrome, Winchester syndrome, multicentric osteolysis with nephropathy, familial osteolysis, Farber disease, and neurogenic osteolysis; most of these etiologies involve familial predispositions and/or systemic symptoms.
Treatment options for GSD include supportive care, medical therapy, radiation, and surgery. For pelvic GSD, numerous reports have demonstrated good outcomes with supportive management, since osteolysis often spontaneously arrests.8,9,12 Others have had success with medical treatments in attempts to halt bone resorption.6,13-15 Bisphosphonates are the cornerstone of medical therapy in GSD, as they appear to halt further osteoclastic bone breakdown. The levels of VEGF have been shown to be elevated in GSD,13 likely consistent with the vascular proliferation evident on pathology, and therapies such as bevacizumab and interferon α-2b have been used to target osteolysis via this pathway with good outcome.13,14,16 External beam-radiation therapy has been shown to prevent local progression of osteolysis in up to 80% of cases.4 However, even with arrest of bone resorption, damage to affected bone may have progressed to the point of significant functional limitation. This may be especially true in the pelvis.
We present a case of a patient who continued to deteriorate after maximal medical and radiation therapy. Many reported cases of pelvic GSD have had good outcomes with some combination of conservative management, medical therapy, and radiation. However, in our patient, the pelvis and lumbosacral spine were unstable as a result of significant bone loss and fracture, and his clinical deterioration was dramatic. We considered reasonable surgical approaches, including local intralesional débridement and massive en bloc resection with structural allograft. We chose the less radical procedure given the patient’s age, minimal surgical history, and personal preference. Although structural pelvic allograft has been successful in a few cases, there remains a high risk of complications, such as fracture, resorption, or infection.17 We considered the addition of hip arthroplasty with either scenario, but we elected not to perform this component given his young age and lack of symptomatic improvement with diagnostic anesthetic hip injection. The key to this patient’s surgical reconstruction, aside from eliminating gross disease, was the stabilization of the spinopelvic junction and pelvic ring. His functional improvement as early as 6 weeks after surgery demonstrates that surgery can have an important role for patients with pelvic GSD who fail medical and radiation therapy.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
1. Jackson JBS. A boneless arm. Boston Med Surg J. 1838;18:368-369.
2. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
3. Lehmann G, Pfeil A, Böttcher J, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129(7):967-972.
4. Heyd R, Micke O, Surholt C, et al; German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Radiation therapy for Gorham-Stout syndrome: results of a national patterns-of-care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81(3):e179-e185.
5. Kulenkampff HA, Richter GM, Hasse WE, Adler CP. Massive pelvic osteolysis in the Gorham-Stout syndrome. Int Orthop. 1990;14(4):361-366.
6. Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: the experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40(11):1391-1397.
7. Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18(6):985-989.
8. Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24(5):551-555.
9. Malde R, Agrawal HM, Ghosh SL, Dinshaw KA. Vanishing bone disease involving the pelvis. J Cancer Res Ther. 2005;1(4):227-228.
10. Kuriyama DK, McElligott SC, Glaser DW, Thompson KS. Treatment of Gorham-Stout disease with zoledronic acid and interferon-α: a case report and literature review. J Pediatr Hematol Oncol. 2010;32(8):579-584.
11. Tie ML, Poland GA, Rosenow EC III. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105(1):208-213.
12. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
13. Dupond JL, Bermont L, Runge M, de Billy M. Plasma VEGF determination in disseminated lymphangiomatosis—Gorham-Stout syndrome: a marker of activity? A case report with a 5-year follow-up. Bone. 2010;46(3):873-876.
14. Wang JD, Chang TK, Cheng YY, et al. A child with dyspnea and unstable gait. Pediatr Hemat Oncol. 2007;24(4):321-324.
15. Zheng MW, Yang M, Qiu JX, et al. Gorham-Stout syndrome presenting in a 5-year-old girl with a successful bisphosphonate therapeutic effect. Exp Ther Med. 2012;4(3):449-451.
16. Timke C, Krause MF, Oppermann HC, Leuschner I, Claviez A. Interferon alpha 2b treatment in an eleven-year-old boy with disseminated lymphangiomatosis. Pediatr Blood Cancer. 2007;48(1):108-111.
17. Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114(4):207-210.
Coracoid Fracture After Reverse Total Shoulder Arthroplasty: A Report of 2 Cases
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
Posterior Reversible Encephalopathy Syndrome: Temporary Visual Loss After Spinal Deformity Surgery
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
Posttraumatic Saphenous Neuroma After Open Tibial Fracture
Neuralgia and neuroma secondary to iatrogenic saphenous nerve injury have been described in the setting of orthopedic surgical interventions using a medial parapatellar approach, and in vascular surgery procedures for harvest of the saphenous vein.1-3 However, postoperative neuropathic pain caused by saphenous neuroma in the setting of orthopedic trauma has not been described.
We present a case of symptomatic posttraumatic saphenous neuroma after a displaced and laterally angulated open distal one-third tibial fracture. This unreported cause of postinjury neuralgia is an important complication to address as other similar and more common conditions, such as peripheral neuropathy and complex regional pain syndrome (CRPS), can present in a similar manner. Reaching the correct diagnosis can be challenging for clinicians unfamiliar with this condition or its clinical presentation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 43-year-old woman presented to our practice 2 years after an open distal one-third metadiaphyseal fracture of the tibia with associated segmental fibular fracture (Gustilo-Anderson type II)4 after an automobile/bicycle accident. At the time of injury, she was noted to have a complex medial wound in the region of an open fracture at the junction of the middle and distal thirds of her tibial shaft. She underwent definitive treatment at an outside facility with initial irrigation and débridement and primary wound closure, followed by staged intramedullary nail fixation. Both soft-tissue and bony injuries healed within the expected time frame, and the patient was discharged from orthopedic care.
Approximately 1 year after her initial injury, the patient began to complain of progressive and persistent anteromedial knee pain as well as gradual-onset, medial-sided leg pain. The leg pain began at the level of her previous fracture site, at the distal one-third metadiaphyseal tibial junction, and radiated from the site of her previous medial open wound distally to the medial aspect of her foot. The pain was burning and tingling in nature, with associated hyperesthesia of the affected area. A diagnosis of CRPS was made, and the patient was prescribed a course of desensitization therapy, oral neuromodulating agents, and physical therapy. After 3 months’ therapy, she remained symptomatic and underwent removal of her proximal tibial interlocking screw fixation (Figure 1). When these measures failed to provide symptomatic relief, and having seen several therapists and physicians, including physiatrists, pain management specialists, and orthopedic surgeons, she presented to our clinic for consultation.
Diagnostic Assessment
On presentation, the patient’s surgical incisions were well healed. At the junction of the middle and distal thirds of the tibia, a 4-cm oblique scar was noted over the anteromedial border of the tibia, the site of her previous open fracture. She demonstrated decreased sensation along the length of this oblique scar, as well as in the distribution of the saphenous nerve distally. Further examination of the previously injured region revealed a positive Tinel sign over the course of the saphenous nerve, with radiating pain down the medial aspect of her leg, recreating her symptoms. She otherwise had full range of motion at the knee with mild tenderness to palpation at the medial joint line and patellar tendon. Her lower extremity motor examination, reflexes, and the remainder of her sensory examination were benign.
These findings were consistent with isolated saphenous neuralgia, and selective injection of the saphenous distribution over the injury site was performed. This injection provided immediate symptomatic relief, with the patient reporting preinjection and postinjection pain scores of 7/10 and 2/10, respectively. Because of the clinical improvement demonstrated with selective injection, surgical intervention with exploration and neurolysis of the saphenous nerve was recommended.
Therapeutic Intervention
The patient underwent surgical exploration of her saphenous nerve at the level of her original open fracture. This was done concurrently with a left-knee diagnostic arthroscopy and removal of her intramedullary tibial implant both to exclude intra-articular pathology (given her medial joint-line tenderness and the limitation of magnetic resonance imaging to diagnose meniscus tear in the presence of her tibial hardware) and to remove any potential hardware irritation in the setting of her anterior knee pain.5,6
Preoperatively, the path of the saphenous nerve was marked using the saphenous vein as a guide. An incision overlying the presumed saphenous nerve course was made at the site of her previous open wound and clinical Tinel sign. The saphenous nerve was carefully dissected with loupe magnification, and the distal divisions of the anterior and posterior branches were identified. The anterior branch was found to be in continuity but encased in fibrotic neuroma. Selective neurolysis of this anterior branch was performed. The posterior branch was found to have been traumatically severed, with both the proximal and distal ends encased in neuromatous scar (Figure 2). Neurectomy of the posterior branch was performed and the severed proximal end of the nerve was buried into the adjacent medial gastrocnemius muscle beneath the fascia of the superficial posterior compartment.7-9
Postoperative Course and Outcome
Upon transport to the postsurgical care unit and emergence from sedation, the patient experienced immediate resolution of her neuralgic symptoms. Pathology of the operative specimen showed a benign, disorganized arrangement of axons, Schwann cells, and perineural fibroblasts amidst a fibrous stroma, consistent with traumatic neuroma. At 1-month and 6-month follow-up visits, the patient remained symptom-free, aside from some continued anterior left knee pain near the site of intramedullary nail entry. Her positive Tinel sign had completely resolved, as did her neuralgic symptoms down the medial aspect of her leg. This proved consistent with a diagnosis of neuroma as the cause of the majority of her symptoms. Subjectively, she reported excellent overall pain relief and satisfaction with her treatment and postoperative course.
Discussion
Postoperative pain after intramedullary fixation of tibial shaft fractures is common and can be caused by several clinical entities.5,10 Anterior knee pain is a well-known complication present in up to 73% of patients treated with tibial nailing.10 Osteoarthritis of the knee or ankle as well as nonarthritic ipsilateral ankle pain are also common complaints, often resulting from tibial malunion or malrotation, leading to altered joint kinematics.11 Additionally, superficial peroneal nerve and tibial neurovascular bundle injuries have been reported as potential complications of distal interlocking screw placement, and should be considered in such patients.12
Another consideration for the development of postoperative pain is CRPS, which is thought to be caused by postinjury sympathetic activation that produces pain out of proportion to clinical examination findings.13 Although no postoperative incidence of CRPS in the setting of tibial nailing has been reported, it is a known contributor to poor functional outcomes after fractures or crush injuries to the lower extremity.9 When attempting to diagnose and treat chronic postoperative pain after tibial nailing, the clinician must keep these common etiologies in mind as well as an understanding of the adjacent anatomy.
The saphenous nerve originates from the third and fourth lumbar nerve roots, coursing beneath the inguinal ligament as part of the femoral nerve. As the terminal branch of the femoral nerve, the saphenous nerve runs in the Hunter canal beneath the fascia of the sartorius muscle. It is bordered laterally by the vastus medialis muscle, and posteriorly and laterally by the adductor longus and magnus muscles. The saphenous nerve then crosses the femoral artery superficially from medial to lateral as it courses distally in the canal. As it emerges from the adductor hiatus, the saphenous nerve runs superficial to the gracilis muscle around the posterior border of the sartorius muscle with the descending genicular artery, and becomes a subcutaneous structure at the level of the knee joint. The infrapatellar branch of the saphenous nerve provides sensation to the medial knee, and continues in a subcutaneous course just medial to the posterior aspect of the tibial shaft with the great saphenous vein. The nerve distally supplies sensory input from the medial foot and ankle.1,3,14
There are several causes of saphenous neuralgia related to surgical and nonsurgical trauma.2,3,15,16 The most common cause of nerve injury is iatrogenic traction or transection causing neuralgic sequelae from subsequent neuroma formation. The anatomy of the saphenous nerve puts it at particular risk when performing saphenectomy for vascular procedures, and its infrapatellar branch is at particular risk when performing a medial parapatellar approach for total knee arthroplasty.2,3 In the case of the surgically naïve patient, saphenous nerve entrapment syndromes have also been described, and occur most frequently at the level of the adductor hiatus or as the saphenous nerve courses between the sartorius and gracilis muscles proximal to the knee joint.16
As is illustrated in the present case, orthopedic trauma may be an additional cause of saphenous neuroma formation, leading to symptomatic neuralgia. This case suggests that symptomatic neuroma should be included in the differential diagnosis of posttraumatic pain in the orthopedic trauma patient. It is important to note that, although this case occurred after a severe injury, the intimate association of the saphenous nerve with the tibia places it in a vulnerable position, and traumatic transection is possible after closed injuries to the tibial metadiaphyseal junction or tibial shaft.
Neuroma formation occurs in response to damage to the endoneurium and axon. For an axon to repair properly, the damaged proximal segment must join with, and reenter, the distal stump. As axons attempt to regenerate, occasionally the proximal stump can escape into the surrounding tissue and form a painful neuroma consisting of a disorganized mass of Schwann cells, fibroblasts, blood vessels, and axons with various degrees of myelination. The subsequent neuralgia associated with neuroma formation is caused by chemical or mechanical stimulation of the damaged axons or by spontaneously evoked potentials in the damaged axons. These signals can manifest as a variety of symptoms, including paresthesia and allodynia.17,18
Making the diagnosis of neuroma-related neuralgia can be challenging and nebulous. A characteristic history and positive Tinel sign over the affected area are helpful clinical indicators. However, the clinical finding most predictive of favorable surgical outcome is symptomatic relief after local injection of 1% lidocaine to the affected area. This is an important diagnostic test, especially when attempting to differentiate painful neuroma from other causes of posttraumatic lower extremity pain (eg, CRPS). Such an injection should be performed in the diagnosis and treatment of symptomatic neuroma, and some authors would suggest that insufficient relief of symptoms with diagnostic nerve block is a contraindication to surgical treatment.19
Several treatments for painful neuromas have been described, with variable results.19,20 The most widely accepted treatment of a complete nerve transection with associated neuroma is neurectomy with reimplantation of the proximal end into adjacent bone, muscle, or vein.14,15 Balcin and colleagues21 suggest that vein transposition produces the most favorable outcomes. Simple neurolysis of in-continuity neuromas has also been described with favorable results.
Conclusion
Neuralgia-producing neuromas of the saphenous nerve are relatively uncommon but can lead to persistent pain and frustrating symptoms for the patient. As noted, the diagnosis may elude clinicians, especially in patients with less obvious clinical presentations. We suggest the following algorithm to help distinguish between painful neuroma and other causes of posttraumatic leg pain: (1) physical examination (including testing for instability, joint line tenderness, patellofemoral pain, Tinel sign, and Semmes-Weinstein testing) should be performed, and plain radiographs taken of the involved bones and joints; (2) if all of the above reveal no abnormality, and there is a positive Tinel sign directly over the course of a nerve, an injection of lidocaine over the region of the potential neuroma can be diagnostic; (3) should several abnormalities be present, further investigation using magnetic resonance imaging, bone scan, and/or electromyography may provide additional information that leads to a diagnosis.
1. Senegor M. Iatrogenic saphenous neuralgia: successful treatment with neuroma resection. J Neurosurg. 1991;28(2):295-298.
2. Mountney J, Wilkinson GA. Saphenous neuralgia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 1999;16(4):440-443.
3. Kachar SM, Williams KM, Finn HA. Neuroma of the infrapatellar branch of the saphenous nerve: a cause of reversible knee stiffness after total knee arthroplasty. J Arthroplasty. 2008;23(6):927-930.
4. Gustilo RB, Anderson AB. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
5. Keating JF, Orfaly R, O’Brien PJ. Knee pain after tibial nailing. J Orthop Trauma. 1997;11(1):10-13.
6. Chen CY, Lin KC, Yang SW, Tarng YW, Hsu CJ, Renn JH. Influence of nail prominence and insertion point on anterior knee pain after tibial intramedullary nailing. Orthopedics. 2014;37(3):e221-e225.
7. Lewin-Kowalik J, Marcol W, Kotulska K, Mandera M, Klimczak A. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46(2):62-67.
8. Otfinowski J, Pawelec A, Kaluza J. Implantation of peripheral neural stump into muscle and its effect on the development of posttraumatic neuroma. Pol J Pathol. 1994;45:195-202.
9. Van Beek AL. Management of nerve compression syndromes and painful neuromas. In: McCarthy JG, May JW Jr, Littler JW, eds. Plastic Surgery. Philadelphia, PA: WB Saunders; 1990:4817-4858.
10. Lefaivre KA, Guy P, Chan H, Blachut PA. Long-term follow-up of tibial shaft fractures treated with intramedullary nailing. J Orthop Trauma. 2008;22(8):525-529.
11. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
12. Roberts CS, King D, Wang M, Seligson D, Voor MJ. Should distal interlocking of tibial nails be performed from medial or lateral direction? Anatomical and biomechanical considerations. J Orthop Trauma. 1999;13(1):27-32.
13. Hogan CJ, Hurwitz SR. Treatment of complex regional pain syndrome of the lower extremity. J Am Acad Orthop Surg. 2002;10(4):281-289.
14. Gray H, Lewis WH. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger, 1918. Bartleby.com website. http://www.bartleby.com/br/107.html. Accessed September 29, 2015.
15. Myerson MS, McGarvey WC, Henderson MR, Hakim J. Morbidity after crush injuries to the foot. J Orthop Trauma. 1994;8(4):343-349.
16. Kalenak A. Saphenous nerve entrapment. Op Tech Sports Med. 1996;4(1):40-45.
17. Wolf SW, Hotchkiss RN, Pederson WC, Kozin SH. The peripheral neuroma. In: Green DP, Wolfe SW, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone, 2011;1063-1071.
18. Thordarson DB, Shean CJ. Nerve and tendon lacerations about the foot and ankle. J Am Acad Orthop Surg. 2005;13(3):186-196.
19. Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862-869.
20. Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78(5):714-719.
21. Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 2009;91(6):803-808.
Neuralgia and neuroma secondary to iatrogenic saphenous nerve injury have been described in the setting of orthopedic surgical interventions using a medial parapatellar approach, and in vascular surgery procedures for harvest of the saphenous vein.1-3 However, postoperative neuropathic pain caused by saphenous neuroma in the setting of orthopedic trauma has not been described.
We present a case of symptomatic posttraumatic saphenous neuroma after a displaced and laterally angulated open distal one-third tibial fracture. This unreported cause of postinjury neuralgia is an important complication to address as other similar and more common conditions, such as peripheral neuropathy and complex regional pain syndrome (CRPS), can present in a similar manner. Reaching the correct diagnosis can be challenging for clinicians unfamiliar with this condition or its clinical presentation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 43-year-old woman presented to our practice 2 years after an open distal one-third metadiaphyseal fracture of the tibia with associated segmental fibular fracture (Gustilo-Anderson type II)4 after an automobile/bicycle accident. At the time of injury, she was noted to have a complex medial wound in the region of an open fracture at the junction of the middle and distal thirds of her tibial shaft. She underwent definitive treatment at an outside facility with initial irrigation and débridement and primary wound closure, followed by staged intramedullary nail fixation. Both soft-tissue and bony injuries healed within the expected time frame, and the patient was discharged from orthopedic care.
Approximately 1 year after her initial injury, the patient began to complain of progressive and persistent anteromedial knee pain as well as gradual-onset, medial-sided leg pain. The leg pain began at the level of her previous fracture site, at the distal one-third metadiaphyseal tibial junction, and radiated from the site of her previous medial open wound distally to the medial aspect of her foot. The pain was burning and tingling in nature, with associated hyperesthesia of the affected area. A diagnosis of CRPS was made, and the patient was prescribed a course of desensitization therapy, oral neuromodulating agents, and physical therapy. After 3 months’ therapy, she remained symptomatic and underwent removal of her proximal tibial interlocking screw fixation (Figure 1). When these measures failed to provide symptomatic relief, and having seen several therapists and physicians, including physiatrists, pain management specialists, and orthopedic surgeons, she presented to our clinic for consultation.
Diagnostic Assessment
On presentation, the patient’s surgical incisions were well healed. At the junction of the middle and distal thirds of the tibia, a 4-cm oblique scar was noted over the anteromedial border of the tibia, the site of her previous open fracture. She demonstrated decreased sensation along the length of this oblique scar, as well as in the distribution of the saphenous nerve distally. Further examination of the previously injured region revealed a positive Tinel sign over the course of the saphenous nerve, with radiating pain down the medial aspect of her leg, recreating her symptoms. She otherwise had full range of motion at the knee with mild tenderness to palpation at the medial joint line and patellar tendon. Her lower extremity motor examination, reflexes, and the remainder of her sensory examination were benign.
These findings were consistent with isolated saphenous neuralgia, and selective injection of the saphenous distribution over the injury site was performed. This injection provided immediate symptomatic relief, with the patient reporting preinjection and postinjection pain scores of 7/10 and 2/10, respectively. Because of the clinical improvement demonstrated with selective injection, surgical intervention with exploration and neurolysis of the saphenous nerve was recommended.
Therapeutic Intervention
The patient underwent surgical exploration of her saphenous nerve at the level of her original open fracture. This was done concurrently with a left-knee diagnostic arthroscopy and removal of her intramedullary tibial implant both to exclude intra-articular pathology (given her medial joint-line tenderness and the limitation of magnetic resonance imaging to diagnose meniscus tear in the presence of her tibial hardware) and to remove any potential hardware irritation in the setting of her anterior knee pain.5,6
Preoperatively, the path of the saphenous nerve was marked using the saphenous vein as a guide. An incision overlying the presumed saphenous nerve course was made at the site of her previous open wound and clinical Tinel sign. The saphenous nerve was carefully dissected with loupe magnification, and the distal divisions of the anterior and posterior branches were identified. The anterior branch was found to be in continuity but encased in fibrotic neuroma. Selective neurolysis of this anterior branch was performed. The posterior branch was found to have been traumatically severed, with both the proximal and distal ends encased in neuromatous scar (Figure 2). Neurectomy of the posterior branch was performed and the severed proximal end of the nerve was buried into the adjacent medial gastrocnemius muscle beneath the fascia of the superficial posterior compartment.7-9
Postoperative Course and Outcome
Upon transport to the postsurgical care unit and emergence from sedation, the patient experienced immediate resolution of her neuralgic symptoms. Pathology of the operative specimen showed a benign, disorganized arrangement of axons, Schwann cells, and perineural fibroblasts amidst a fibrous stroma, consistent with traumatic neuroma. At 1-month and 6-month follow-up visits, the patient remained symptom-free, aside from some continued anterior left knee pain near the site of intramedullary nail entry. Her positive Tinel sign had completely resolved, as did her neuralgic symptoms down the medial aspect of her leg. This proved consistent with a diagnosis of neuroma as the cause of the majority of her symptoms. Subjectively, she reported excellent overall pain relief and satisfaction with her treatment and postoperative course.
Discussion
Postoperative pain after intramedullary fixation of tibial shaft fractures is common and can be caused by several clinical entities.5,10 Anterior knee pain is a well-known complication present in up to 73% of patients treated with tibial nailing.10 Osteoarthritis of the knee or ankle as well as nonarthritic ipsilateral ankle pain are also common complaints, often resulting from tibial malunion or malrotation, leading to altered joint kinematics.11 Additionally, superficial peroneal nerve and tibial neurovascular bundle injuries have been reported as potential complications of distal interlocking screw placement, and should be considered in such patients.12
Another consideration for the development of postoperative pain is CRPS, which is thought to be caused by postinjury sympathetic activation that produces pain out of proportion to clinical examination findings.13 Although no postoperative incidence of CRPS in the setting of tibial nailing has been reported, it is a known contributor to poor functional outcomes after fractures or crush injuries to the lower extremity.9 When attempting to diagnose and treat chronic postoperative pain after tibial nailing, the clinician must keep these common etiologies in mind as well as an understanding of the adjacent anatomy.
The saphenous nerve originates from the third and fourth lumbar nerve roots, coursing beneath the inguinal ligament as part of the femoral nerve. As the terminal branch of the femoral nerve, the saphenous nerve runs in the Hunter canal beneath the fascia of the sartorius muscle. It is bordered laterally by the vastus medialis muscle, and posteriorly and laterally by the adductor longus and magnus muscles. The saphenous nerve then crosses the femoral artery superficially from medial to lateral as it courses distally in the canal. As it emerges from the adductor hiatus, the saphenous nerve runs superficial to the gracilis muscle around the posterior border of the sartorius muscle with the descending genicular artery, and becomes a subcutaneous structure at the level of the knee joint. The infrapatellar branch of the saphenous nerve provides sensation to the medial knee, and continues in a subcutaneous course just medial to the posterior aspect of the tibial shaft with the great saphenous vein. The nerve distally supplies sensory input from the medial foot and ankle.1,3,14
There are several causes of saphenous neuralgia related to surgical and nonsurgical trauma.2,3,15,16 The most common cause of nerve injury is iatrogenic traction or transection causing neuralgic sequelae from subsequent neuroma formation. The anatomy of the saphenous nerve puts it at particular risk when performing saphenectomy for vascular procedures, and its infrapatellar branch is at particular risk when performing a medial parapatellar approach for total knee arthroplasty.2,3 In the case of the surgically naïve patient, saphenous nerve entrapment syndromes have also been described, and occur most frequently at the level of the adductor hiatus or as the saphenous nerve courses between the sartorius and gracilis muscles proximal to the knee joint.16
As is illustrated in the present case, orthopedic trauma may be an additional cause of saphenous neuroma formation, leading to symptomatic neuralgia. This case suggests that symptomatic neuroma should be included in the differential diagnosis of posttraumatic pain in the orthopedic trauma patient. It is important to note that, although this case occurred after a severe injury, the intimate association of the saphenous nerve with the tibia places it in a vulnerable position, and traumatic transection is possible after closed injuries to the tibial metadiaphyseal junction or tibial shaft.
Neuroma formation occurs in response to damage to the endoneurium and axon. For an axon to repair properly, the damaged proximal segment must join with, and reenter, the distal stump. As axons attempt to regenerate, occasionally the proximal stump can escape into the surrounding tissue and form a painful neuroma consisting of a disorganized mass of Schwann cells, fibroblasts, blood vessels, and axons with various degrees of myelination. The subsequent neuralgia associated with neuroma formation is caused by chemical or mechanical stimulation of the damaged axons or by spontaneously evoked potentials in the damaged axons. These signals can manifest as a variety of symptoms, including paresthesia and allodynia.17,18
Making the diagnosis of neuroma-related neuralgia can be challenging and nebulous. A characteristic history and positive Tinel sign over the affected area are helpful clinical indicators. However, the clinical finding most predictive of favorable surgical outcome is symptomatic relief after local injection of 1% lidocaine to the affected area. This is an important diagnostic test, especially when attempting to differentiate painful neuroma from other causes of posttraumatic lower extremity pain (eg, CRPS). Such an injection should be performed in the diagnosis and treatment of symptomatic neuroma, and some authors would suggest that insufficient relief of symptoms with diagnostic nerve block is a contraindication to surgical treatment.19
Several treatments for painful neuromas have been described, with variable results.19,20 The most widely accepted treatment of a complete nerve transection with associated neuroma is neurectomy with reimplantation of the proximal end into adjacent bone, muscle, or vein.14,15 Balcin and colleagues21 suggest that vein transposition produces the most favorable outcomes. Simple neurolysis of in-continuity neuromas has also been described with favorable results.
Conclusion
Neuralgia-producing neuromas of the saphenous nerve are relatively uncommon but can lead to persistent pain and frustrating symptoms for the patient. As noted, the diagnosis may elude clinicians, especially in patients with less obvious clinical presentations. We suggest the following algorithm to help distinguish between painful neuroma and other causes of posttraumatic leg pain: (1) physical examination (including testing for instability, joint line tenderness, patellofemoral pain, Tinel sign, and Semmes-Weinstein testing) should be performed, and plain radiographs taken of the involved bones and joints; (2) if all of the above reveal no abnormality, and there is a positive Tinel sign directly over the course of a nerve, an injection of lidocaine over the region of the potential neuroma can be diagnostic; (3) should several abnormalities be present, further investigation using magnetic resonance imaging, bone scan, and/or electromyography may provide additional information that leads to a diagnosis.
Neuralgia and neuroma secondary to iatrogenic saphenous nerve injury have been described in the setting of orthopedic surgical interventions using a medial parapatellar approach, and in vascular surgery procedures for harvest of the saphenous vein.1-3 However, postoperative neuropathic pain caused by saphenous neuroma in the setting of orthopedic trauma has not been described.
We present a case of symptomatic posttraumatic saphenous neuroma after a displaced and laterally angulated open distal one-third tibial fracture. This unreported cause of postinjury neuralgia is an important complication to address as other similar and more common conditions, such as peripheral neuropathy and complex regional pain syndrome (CRPS), can present in a similar manner. Reaching the correct diagnosis can be challenging for clinicians unfamiliar with this condition or its clinical presentation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 43-year-old woman presented to our practice 2 years after an open distal one-third metadiaphyseal fracture of the tibia with associated segmental fibular fracture (Gustilo-Anderson type II)4 after an automobile/bicycle accident. At the time of injury, she was noted to have a complex medial wound in the region of an open fracture at the junction of the middle and distal thirds of her tibial shaft. She underwent definitive treatment at an outside facility with initial irrigation and débridement and primary wound closure, followed by staged intramedullary nail fixation. Both soft-tissue and bony injuries healed within the expected time frame, and the patient was discharged from orthopedic care.
Approximately 1 year after her initial injury, the patient began to complain of progressive and persistent anteromedial knee pain as well as gradual-onset, medial-sided leg pain. The leg pain began at the level of her previous fracture site, at the distal one-third metadiaphyseal tibial junction, and radiated from the site of her previous medial open wound distally to the medial aspect of her foot. The pain was burning and tingling in nature, with associated hyperesthesia of the affected area. A diagnosis of CRPS was made, and the patient was prescribed a course of desensitization therapy, oral neuromodulating agents, and physical therapy. After 3 months’ therapy, she remained symptomatic and underwent removal of her proximal tibial interlocking screw fixation (Figure 1). When these measures failed to provide symptomatic relief, and having seen several therapists and physicians, including physiatrists, pain management specialists, and orthopedic surgeons, she presented to our clinic for consultation.
Diagnostic Assessment
On presentation, the patient’s surgical incisions were well healed. At the junction of the middle and distal thirds of the tibia, a 4-cm oblique scar was noted over the anteromedial border of the tibia, the site of her previous open fracture. She demonstrated decreased sensation along the length of this oblique scar, as well as in the distribution of the saphenous nerve distally. Further examination of the previously injured region revealed a positive Tinel sign over the course of the saphenous nerve, with radiating pain down the medial aspect of her leg, recreating her symptoms. She otherwise had full range of motion at the knee with mild tenderness to palpation at the medial joint line and patellar tendon. Her lower extremity motor examination, reflexes, and the remainder of her sensory examination were benign.
These findings were consistent with isolated saphenous neuralgia, and selective injection of the saphenous distribution over the injury site was performed. This injection provided immediate symptomatic relief, with the patient reporting preinjection and postinjection pain scores of 7/10 and 2/10, respectively. Because of the clinical improvement demonstrated with selective injection, surgical intervention with exploration and neurolysis of the saphenous nerve was recommended.
Therapeutic Intervention
The patient underwent surgical exploration of her saphenous nerve at the level of her original open fracture. This was done concurrently with a left-knee diagnostic arthroscopy and removal of her intramedullary tibial implant both to exclude intra-articular pathology (given her medial joint-line tenderness and the limitation of magnetic resonance imaging to diagnose meniscus tear in the presence of her tibial hardware) and to remove any potential hardware irritation in the setting of her anterior knee pain.5,6
Preoperatively, the path of the saphenous nerve was marked using the saphenous vein as a guide. An incision overlying the presumed saphenous nerve course was made at the site of her previous open wound and clinical Tinel sign. The saphenous nerve was carefully dissected with loupe magnification, and the distal divisions of the anterior and posterior branches were identified. The anterior branch was found to be in continuity but encased in fibrotic neuroma. Selective neurolysis of this anterior branch was performed. The posterior branch was found to have been traumatically severed, with both the proximal and distal ends encased in neuromatous scar (Figure 2). Neurectomy of the posterior branch was performed and the severed proximal end of the nerve was buried into the adjacent medial gastrocnemius muscle beneath the fascia of the superficial posterior compartment.7-9
Postoperative Course and Outcome
Upon transport to the postsurgical care unit and emergence from sedation, the patient experienced immediate resolution of her neuralgic symptoms. Pathology of the operative specimen showed a benign, disorganized arrangement of axons, Schwann cells, and perineural fibroblasts amidst a fibrous stroma, consistent with traumatic neuroma. At 1-month and 6-month follow-up visits, the patient remained symptom-free, aside from some continued anterior left knee pain near the site of intramedullary nail entry. Her positive Tinel sign had completely resolved, as did her neuralgic symptoms down the medial aspect of her leg. This proved consistent with a diagnosis of neuroma as the cause of the majority of her symptoms. Subjectively, she reported excellent overall pain relief and satisfaction with her treatment and postoperative course.
Discussion
Postoperative pain after intramedullary fixation of tibial shaft fractures is common and can be caused by several clinical entities.5,10 Anterior knee pain is a well-known complication present in up to 73% of patients treated with tibial nailing.10 Osteoarthritis of the knee or ankle as well as nonarthritic ipsilateral ankle pain are also common complaints, often resulting from tibial malunion or malrotation, leading to altered joint kinematics.11 Additionally, superficial peroneal nerve and tibial neurovascular bundle injuries have been reported as potential complications of distal interlocking screw placement, and should be considered in such patients.12
Another consideration for the development of postoperative pain is CRPS, which is thought to be caused by postinjury sympathetic activation that produces pain out of proportion to clinical examination findings.13 Although no postoperative incidence of CRPS in the setting of tibial nailing has been reported, it is a known contributor to poor functional outcomes after fractures or crush injuries to the lower extremity.9 When attempting to diagnose and treat chronic postoperative pain after tibial nailing, the clinician must keep these common etiologies in mind as well as an understanding of the adjacent anatomy.
The saphenous nerve originates from the third and fourth lumbar nerve roots, coursing beneath the inguinal ligament as part of the femoral nerve. As the terminal branch of the femoral nerve, the saphenous nerve runs in the Hunter canal beneath the fascia of the sartorius muscle. It is bordered laterally by the vastus medialis muscle, and posteriorly and laterally by the adductor longus and magnus muscles. The saphenous nerve then crosses the femoral artery superficially from medial to lateral as it courses distally in the canal. As it emerges from the adductor hiatus, the saphenous nerve runs superficial to the gracilis muscle around the posterior border of the sartorius muscle with the descending genicular artery, and becomes a subcutaneous structure at the level of the knee joint. The infrapatellar branch of the saphenous nerve provides sensation to the medial knee, and continues in a subcutaneous course just medial to the posterior aspect of the tibial shaft with the great saphenous vein. The nerve distally supplies sensory input from the medial foot and ankle.1,3,14
There are several causes of saphenous neuralgia related to surgical and nonsurgical trauma.2,3,15,16 The most common cause of nerve injury is iatrogenic traction or transection causing neuralgic sequelae from subsequent neuroma formation. The anatomy of the saphenous nerve puts it at particular risk when performing saphenectomy for vascular procedures, and its infrapatellar branch is at particular risk when performing a medial parapatellar approach for total knee arthroplasty.2,3 In the case of the surgically naïve patient, saphenous nerve entrapment syndromes have also been described, and occur most frequently at the level of the adductor hiatus or as the saphenous nerve courses between the sartorius and gracilis muscles proximal to the knee joint.16
As is illustrated in the present case, orthopedic trauma may be an additional cause of saphenous neuroma formation, leading to symptomatic neuralgia. This case suggests that symptomatic neuroma should be included in the differential diagnosis of posttraumatic pain in the orthopedic trauma patient. It is important to note that, although this case occurred after a severe injury, the intimate association of the saphenous nerve with the tibia places it in a vulnerable position, and traumatic transection is possible after closed injuries to the tibial metadiaphyseal junction or tibial shaft.
Neuroma formation occurs in response to damage to the endoneurium and axon. For an axon to repair properly, the damaged proximal segment must join with, and reenter, the distal stump. As axons attempt to regenerate, occasionally the proximal stump can escape into the surrounding tissue and form a painful neuroma consisting of a disorganized mass of Schwann cells, fibroblasts, blood vessels, and axons with various degrees of myelination. The subsequent neuralgia associated with neuroma formation is caused by chemical or mechanical stimulation of the damaged axons or by spontaneously evoked potentials in the damaged axons. These signals can manifest as a variety of symptoms, including paresthesia and allodynia.17,18
Making the diagnosis of neuroma-related neuralgia can be challenging and nebulous. A characteristic history and positive Tinel sign over the affected area are helpful clinical indicators. However, the clinical finding most predictive of favorable surgical outcome is symptomatic relief after local injection of 1% lidocaine to the affected area. This is an important diagnostic test, especially when attempting to differentiate painful neuroma from other causes of posttraumatic lower extremity pain (eg, CRPS). Such an injection should be performed in the diagnosis and treatment of symptomatic neuroma, and some authors would suggest that insufficient relief of symptoms with diagnostic nerve block is a contraindication to surgical treatment.19
Several treatments for painful neuromas have been described, with variable results.19,20 The most widely accepted treatment of a complete nerve transection with associated neuroma is neurectomy with reimplantation of the proximal end into adjacent bone, muscle, or vein.14,15 Balcin and colleagues21 suggest that vein transposition produces the most favorable outcomes. Simple neurolysis of in-continuity neuromas has also been described with favorable results.
Conclusion
Neuralgia-producing neuromas of the saphenous nerve are relatively uncommon but can lead to persistent pain and frustrating symptoms for the patient. As noted, the diagnosis may elude clinicians, especially in patients with less obvious clinical presentations. We suggest the following algorithm to help distinguish between painful neuroma and other causes of posttraumatic leg pain: (1) physical examination (including testing for instability, joint line tenderness, patellofemoral pain, Tinel sign, and Semmes-Weinstein testing) should be performed, and plain radiographs taken of the involved bones and joints; (2) if all of the above reveal no abnormality, and there is a positive Tinel sign directly over the course of a nerve, an injection of lidocaine over the region of the potential neuroma can be diagnostic; (3) should several abnormalities be present, further investigation using magnetic resonance imaging, bone scan, and/or electromyography may provide additional information that leads to a diagnosis.
1. Senegor M. Iatrogenic saphenous neuralgia: successful treatment with neuroma resection. J Neurosurg. 1991;28(2):295-298.
2. Mountney J, Wilkinson GA. Saphenous neuralgia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 1999;16(4):440-443.
3. Kachar SM, Williams KM, Finn HA. Neuroma of the infrapatellar branch of the saphenous nerve: a cause of reversible knee stiffness after total knee arthroplasty. J Arthroplasty. 2008;23(6):927-930.
4. Gustilo RB, Anderson AB. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
5. Keating JF, Orfaly R, O’Brien PJ. Knee pain after tibial nailing. J Orthop Trauma. 1997;11(1):10-13.
6. Chen CY, Lin KC, Yang SW, Tarng YW, Hsu CJ, Renn JH. Influence of nail prominence and insertion point on anterior knee pain after tibial intramedullary nailing. Orthopedics. 2014;37(3):e221-e225.
7. Lewin-Kowalik J, Marcol W, Kotulska K, Mandera M, Klimczak A. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46(2):62-67.
8. Otfinowski J, Pawelec A, Kaluza J. Implantation of peripheral neural stump into muscle and its effect on the development of posttraumatic neuroma. Pol J Pathol. 1994;45:195-202.
9. Van Beek AL. Management of nerve compression syndromes and painful neuromas. In: McCarthy JG, May JW Jr, Littler JW, eds. Plastic Surgery. Philadelphia, PA: WB Saunders; 1990:4817-4858.
10. Lefaivre KA, Guy P, Chan H, Blachut PA. Long-term follow-up of tibial shaft fractures treated with intramedullary nailing. J Orthop Trauma. 2008;22(8):525-529.
11. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
12. Roberts CS, King D, Wang M, Seligson D, Voor MJ. Should distal interlocking of tibial nails be performed from medial or lateral direction? Anatomical and biomechanical considerations. J Orthop Trauma. 1999;13(1):27-32.
13. Hogan CJ, Hurwitz SR. Treatment of complex regional pain syndrome of the lower extremity. J Am Acad Orthop Surg. 2002;10(4):281-289.
14. Gray H, Lewis WH. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger, 1918. Bartleby.com website. http://www.bartleby.com/br/107.html. Accessed September 29, 2015.
15. Myerson MS, McGarvey WC, Henderson MR, Hakim J. Morbidity after crush injuries to the foot. J Orthop Trauma. 1994;8(4):343-349.
16. Kalenak A. Saphenous nerve entrapment. Op Tech Sports Med. 1996;4(1):40-45.
17. Wolf SW, Hotchkiss RN, Pederson WC, Kozin SH. The peripheral neuroma. In: Green DP, Wolfe SW, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone, 2011;1063-1071.
18. Thordarson DB, Shean CJ. Nerve and tendon lacerations about the foot and ankle. J Am Acad Orthop Surg. 2005;13(3):186-196.
19. Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862-869.
20. Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78(5):714-719.
21. Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 2009;91(6):803-808.
1. Senegor M. Iatrogenic saphenous neuralgia: successful treatment with neuroma resection. J Neurosurg. 1991;28(2):295-298.
2. Mountney J, Wilkinson GA. Saphenous neuralgia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 1999;16(4):440-443.
3. Kachar SM, Williams KM, Finn HA. Neuroma of the infrapatellar branch of the saphenous nerve: a cause of reversible knee stiffness after total knee arthroplasty. J Arthroplasty. 2008;23(6):927-930.
4. Gustilo RB, Anderson AB. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
5. Keating JF, Orfaly R, O’Brien PJ. Knee pain after tibial nailing. J Orthop Trauma. 1997;11(1):10-13.
6. Chen CY, Lin KC, Yang SW, Tarng YW, Hsu CJ, Renn JH. Influence of nail prominence and insertion point on anterior knee pain after tibial intramedullary nailing. Orthopedics. 2014;37(3):e221-e225.
7. Lewin-Kowalik J, Marcol W, Kotulska K, Mandera M, Klimczak A. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46(2):62-67.
8. Otfinowski J, Pawelec A, Kaluza J. Implantation of peripheral neural stump into muscle and its effect on the development of posttraumatic neuroma. Pol J Pathol. 1994;45:195-202.
9. Van Beek AL. Management of nerve compression syndromes and painful neuromas. In: McCarthy JG, May JW Jr, Littler JW, eds. Plastic Surgery. Philadelphia, PA: WB Saunders; 1990:4817-4858.
10. Lefaivre KA, Guy P, Chan H, Blachut PA. Long-term follow-up of tibial shaft fractures treated with intramedullary nailing. J Orthop Trauma. 2008;22(8):525-529.
11. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
12. Roberts CS, King D, Wang M, Seligson D, Voor MJ. Should distal interlocking of tibial nails be performed from medial or lateral direction? Anatomical and biomechanical considerations. J Orthop Trauma. 1999;13(1):27-32.
13. Hogan CJ, Hurwitz SR. Treatment of complex regional pain syndrome of the lower extremity. J Am Acad Orthop Surg. 2002;10(4):281-289.
14. Gray H, Lewis WH. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger, 1918. Bartleby.com website. http://www.bartleby.com/br/107.html. Accessed September 29, 2015.
15. Myerson MS, McGarvey WC, Henderson MR, Hakim J. Morbidity after crush injuries to the foot. J Orthop Trauma. 1994;8(4):343-349.
16. Kalenak A. Saphenous nerve entrapment. Op Tech Sports Med. 1996;4(1):40-45.
17. Wolf SW, Hotchkiss RN, Pederson WC, Kozin SH. The peripheral neuroma. In: Green DP, Wolfe SW, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone, 2011;1063-1071.
18. Thordarson DB, Shean CJ. Nerve and tendon lacerations about the foot and ankle. J Am Acad Orthop Surg. 2005;13(3):186-196.
19. Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862-869.
20. Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78(5):714-719.
21. Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 2009;91(6):803-808.
Acute Multiple Flexor Tendon Injury and Carpal Tunnel Syndrome After Open Distal Radius Fracture
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.