User login
Is the Orthopedic Fellowship Interview Process Broken? A Survey of Program Directors and Residents
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
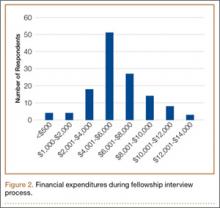
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
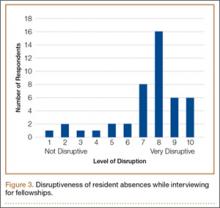
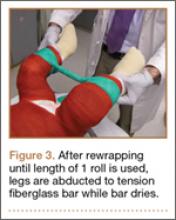
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
Risk Factors for Discharge to Rehabilitation Among Hip Fracture Patients
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
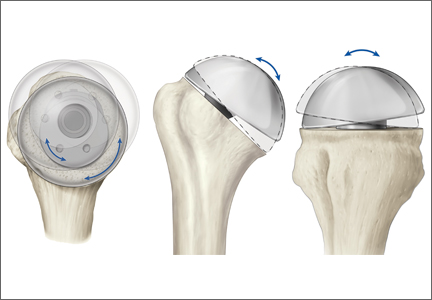
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
Incidence and Functional Outcomes of Malunion of Nonoperatively Treated Humeral Shaft Fractures
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Long-Term Elastic Durability of Polymer Matrix Composite Materials After Repeated Steam Sterilization
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
Reinforcing a Spica Cast With a Fiberglass Bar
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Professional Dissatisfaction: Are Orthopedic Surgeons Spoiled?
Several years ago, I was on the American Academy of Orthopaedic Surgeons leadership fellow committee, reviewing fellowship applications. The committee had been poised to very favorably rule on an application until a new member spoke up, stating that he had been in the applicant’s department and that points made in the recommending letter bore little resemblance to the person’s performance. Further study confirmed the dishonesty in the letter, and a more fit candidate was selected instead.
I was puzzled. Why would a leader in the field do such a thing? The question led me to a personal investigation into the monumental topic of professionalism and, more specifically, professionalism among orthopedic surgeons.
Physicians, Especially Orthopedists, Are Not Happy
Physicians, in general, are not a happy lot. According to a 2012 survey by the Physicians Foundation,1 77.4% of practicing physicians were pessimistic about the future of medicine, and 82% thought they had little ability to change the health care system. Sources of pessimism included “too much regulation/paperwork, loss of clinical autonomy, physicians not compensated for quality, erosion of physician/patient relationship, and money trumps patient care.” We are now in the age of “organizational physicians,” who, subject to institutional management, are experiencing a distressing loss of autonomy.
What sustains, or does not sustain, surgeons’ career satisfaction? Commonly stated positive factors include the ability to provide quality care, time with patients, income, and financial incentives2; reported negative factors include threat of malpractice, lack of autonomy, excessive administrative tasks, and high patient volume. Early-career physicians have the lowest career satisfaction, but physicians in mid-career have the highest rate of burnout and likelihood of leaving medical practice.3 Work–home conflict is most difficult in the early career, when families have young children, and the conflict generally goes unresolved. Burnout and low satisfaction with specialty choice are most common in mid-career.
Despite all the negative factors acting on medical practices, orthopedic surgeons have fared well financially, but not as well in career satisfaction. The Medscape Physician Compensation Report 20144 places orthopedics compensation first among 25 specialties listed, without a close second, but orthopedists rank 15th in thinking they are fairly compensated, and next to last in indicating they would choose medicine again as a career. A separate study of physician career satisfaction ranked orthopedics 32nd of 42 specialties studied.5
What is our problem, and what can we do about it? It’s hard to digest this information and not feel that orthopedists are, for lack of a better word, spoiled.
DeBotton6 wrote about status anxiety, which arises over and over again in daily life. Essentially, it is the envy or dissatisfaction one feels when a peer gets a better deal that does not seem just. A remarkable aspect of Medscape’s compensation report4 is that family medicine physicians, whose annual income was well under half that of orthopedic surgeons, were more likely to view themselves as fairly compensated. On this basis, we have to conclude that orthopedic surgeons have status anxiety. But why?
Humanism
Osler, the quintessential physician, counseled medical students: “Nothing will sustain you more potently in your humdrum routine … than the power to recognize the true poetry of life—the poetry of the commonplace, of the ordinary man, of the plain, toilworn woman, with their loves and their joys, their sorrows and their griefs.”7 In short, take the time to know your patients. In a study of physicians who were regarded as clinically excellent, several traits were noted: honest, nonjudgmental, genuinely caring, treating all patients equally, and constantly striving for excellence.8 A century after Osler, Stellato9 echoed the sentiment: “Listen to your patients, not just about their illness, but about their life.”
Humanism, then, is the trait underlying professionalism.10,11 Communication skills are essential to humanism.12 However, a study of specialty physicians in Spain “showed scarce empathic behaviours or behaviours that foster a shared decision making process.”13 In addition, a recent survey placed the communication skills of orthopedists last among 28 specialties.14 Assessment was based on how often a physician explains things, listens carefully, gives easy-to-understand instructions, shows respect, and spends enough time.
Could it be that orthopedists are not satisfied with their income because as a group they lack the communication skills and humanistic characteristics of lower-paid physicians?
Residency and the Academic Medical Center
The education of the orthopedic surgeon starts with the selection process. Simon15 noted that “the brightest, but not always the best” are selected largely because objective criteria are an excellent measure of cognitive achievement but not of character. Also noting that 10% of examinees pass part I of the board but fail part II, Simon opined that they “lack clinical judgment, communication skills, and, in some instances, ethics.” A 1999 team of authors found that 18% of research citations listed by orthopedic residency applicants were misrepresented, and a follow-up study by the same authors in 2007 noted a rate increase, to 20.6%.16 Both sets of authors wrote of a need for a better selection process and a better evaluative process during residency.
The residency process has been substantially altered by work-hour restrictions. The 20th-century residency, which emphasizes taking responsibility for the patient throughout a hospital stay, has now been dismissed as “nostalgic professionalism.” Residents are now advised to avoid such activities as checking laboratory results from home and coming to work when they are not feeling well.17 However, there has been considerable pushback against diminishing nostalgic professionalism, primarily from surgeons.18 “Teaching residents that they should go home to rest at the end of their shift without regard for the circumstances of their cases in progress is not an acceptable example for training.”19 Current promulgated restrictions on duty hours move concern for the “circumstances of their cases” to the back burner—the shift ends, the physician leaves. Residents are pulled one way by forces telling them to leave (Accreditation Council for Graduate Medical Education) and the other way by forces telling them to stay (their conscience).
How do residents develop their surgical identities and concepts of humanism and professionalism? There is a substantial body of evidence that the so-called hidden curriculum is the dominant factor: trainees emulate what their faculty say and do.20 As Gofton and Regehr21 noted, “It is vital for members of the surgical academic community to recognize [that] the attitudes, beliefs, and values implicit in every action, every word, every inaction, and every silence are not only shaping the attitudes, beliefs, and values of one’s protégés, but also are shaping the decisions of students who are considering the possibility of becoming one’s protégés.” It is not easy being a surgical role model given the conflicts affecting academic surgeons. For example, should a surgeon allot extra time so a trainee can do a case properly, or should the case be finished expeditiously in order to avoid canceling the next case, or to get to a committee meeting or a kid’s ballgame on time? Monetary pressures, along with the possibility of losing operative time because the schedule was not full, can influence the decision to operate or not.22 Trainees absorb what they hear and see.
In 2003, Inui23 published A Flag in the Wind: Educating for Professionalism in Medicine, in which he stated, “There can be little doubt that physicians in general as well as the leadership of the organization of medicine have been preoccupied with finances and the economics of medical care. … The topics and the language of academic leadership [have] shifted in the last twenty years. … Core functions of the academic medical center became ‘enterprises.’” He also noted, “The most difficult challenge of all may be the need to understand—and to be explicitly mindful of, and articulate about—medical education as a special form of personal and professional formation that is rooted in the daily activities of individuals and groups in academic medical communities.”23 In addition, the “institutional environment we create … [is] a reflection of the values we hold as a professional community.”23 In effect, the academic medical center is part of the hidden curriculum.
Curiously, academic institutions tend not to reward clinical excellence—a self-defeating measure for any institution that recognizes the importance of the hidden curriculum.24 A peer evaluation of hospitalists revealed that the most highly regarded were highly associated with humanism and a passion for clinical medicine.25 At a prominent institution, however, it was found that clinical educators were less likely than research faculty to hold a higher rank.26
Of the factors affecting physician dissatisfaction, workplace stress is predominant.27 In this age of organizational physicians, job satisfaction correlates with how a physician feels about his or her ability to function as a physician. In a study by Wai and colleagues,28 “surgical faculty reported low satisfaction with a number of questions about communication in their medical schools and their clinical practice locations.” The authors indicated that “medical school and department governance are critical determinants of faculty satisfaction within academic surgical centers.” Pololi and colleagues29 extensively studied the culture of academic medicine and summarized the sources of discontent: “competitive individualism, undervaluing of humanistic qualities, deprecation, and the erosion of trust.” In another study,30 they studied the incidence (~25%) of, and reasons for, considering to leave academic medicine. Reasons included feeling isolated in the department, lack of institutional support, poor communication with administrators, and a perceived difference between the stated culture of the institution and what was observed on a daily basis.30
What Can We Do?
The obvious starting point is the selection process—focusing more on finding the “best,” not necessarily the “brightest.”15 This is not easy. Recommendation letters are often based on limited contact and may or may not reflect applicants’ true character. Numerous websites advise resident applicants on what questions to expect and how to prepare and practice for them. I have found questions of current events very illuminating, as they can probe how applicants view the world. Given the high income of orthopedic surgeons, some applicants likely are attracted to that aspect of the specialty. These applicants are not the “best.”
Residents who exhibit questionable ethical reasoning or behavior must be identified and not be allowed to finish their program. It is the responsibility of the program, not the board, to ensure that those entering practice exhibit a high degree of professionalism. Faculty must seriously recognize, every day, that everything they do is part of the hidden curriculum.
As noted, the academic medical environment can be inimical. Faculty who experience dissonance must be able to effectively confront administrative leadership to express their concerns, and they need to feel their concerns are recognized. Leaders of academic medical centers must guide their institutions in such a way that the day-to-day functions are compatible with the stated mission and values.31
Chervenak and colleagues32 forcefully stated that “appropriate ethical values” are the core component that academic leadership needs in order to respond to the opposing forces of increasing pressures of patient satisfaction, compliance, liability, and other administrative demands on one hand and diminishing resources on the other hand. They listed 4 “professional virtues” that characterize responsible professional leadership: self-effacement, which obligates physician leaders to be unbiased; self-sacrifice, the willingness to risk individual and organizational self-interest, especially in the economic domain; compassion, or “What can I do to help?”; and integrity. The principles of effective leadership are not that complicated, but implementing them requires conviction and courage.33
Physicians increasingly are practicing in the organization setting. They need to increase their involvement in the organization in order to promulgate the needs of physicians. Organizational executive leadership is primarily driven by budgetary and capital planning processes; physician input is essential to ensure resources are directed toward better patient care. A feeling of loss of control over one’s practice is a primary cause of physician dissatisfaction. The schism between physicians and administrators traditionally has been characterized by a lack of trust; a more trusting relationship, reinforced by frequent constructive dialogue, will result in more physician control of the practice.34 This will be difficult, but it is necessary for improving professional satisfaction.
For practicing physicians, Wynia35 made the compelling case that professionalism demands self-regulation, which involves identifying and reporting impaired or incompetent physicians—another task that requires conviction and courage.
But the core issue is how an orthopedist regards the day-to-day aspects of his or her practice. Shanafelt and colleagues36 concluded that surgeons are not very good at assessing their own well-being and stress levels. Certainly high stress can affect well-being, which in turn can affect professionalism. West and Shanafelt37 uniquely described this relationship: “The effect of distress on professionalism in medicine has become clear in recent years. The well-documented decline of crucial elements of professionalism, including empathy and humanism, during medical training appears to be related in part to personal distress experienced during medical school and residency. Unfortunately, this decline continues as physicians move into practice, where distress also is associated with decreased compassion and empathy.” This description sounds completely synchronized with the current career dissatisfaction of orthopedic surgeons.
Improving orthopedists’ status requires ethical and involved leadership, both in academia and in our professional organizations, which too often seem mired in the (not so effective) status quo. Recognizing that the resident selection process is fallible is the first step in taking action—engaging in scrupulous role modeling and insisting that residents demonstrate professionalism and communication skills in their daily work. Becoming involved in organizational management is preferable to becoming angry and dissatisfied. Getting to know one’s patients is its own reward in terms of career satisfaction. Orthopedic surgeons have a well-earned macho image—that image can be enhanced with a dose of humanism. The result would be a true professional who enjoys his or her practice and has a satisfying career.
1. The Physicians Foundation. A Survey of America’s Physicians: Practice Patterns and Perspectives. An Examination of the Professional Morale, Practice Patterns, Career Plans, and Healthcare Perspectives of Today’s Physicians, Aggregated by Age, Gender, Primary Care/Specialists, and Practice Owners/Employees. http://www.physiciansfoundation.org/uploads/default/Physicians_Foundation_2012_Biennial_Survey.pdf. Published September 2012. Accessed September 26, 2015.
2. Deshpande SP, Deshpande SS. Career satisfaction of surgical specialties. Ann Surg. 2011;253(5):1011-1016.
3. Dyrbye LN, Varkey P, Boone SL, Satele DV, Sloan JA, Shanafelt TD. Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358-1367.
4. Medscape Physician Compensation Report 2014. New York, NY: Medscape; 2014.
5. Leigh JP, Tancredi DJ, Kravitz RL. Physician career satisfaction within specialties. BMC Health Serv Res. 2009;9:166.
6. deBotton A. Status Anxiety. New York, NY: Vintage Books; 2004.
7. Golden RL. William Osler at 150: an overview of a life. JAMA. 1999;282(23):2252-2258.
8. Christmas C, Kravet SJ, Durso SC, Wright SM. Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989-994.
9. Stellato TA. Humanism and the art of surgery. Surgery. 2007;142(4):433-438.
10. Gold A, Gold S. Humanism in medicine from the perspective of the Arnold Gold Foundation: challenges to maintaining the care in health care. J Child Neurol. 2006;21(6):546-549.
11. Cohen JJ. Viewpoint: linking professionalism to humanism: what it means, why it matters. Acad Med. 2007;82(11):1029-1032.
12. Holt GR. Bioethics and humanism in head and neck cancer. Arch Facial Plast Surg. 2010;12(2):85-86.
13. Ruiz-Moral R, Pérez Rodríguez E, Pérula de Torres LA, de la Torre J. Physician–patient communication: a study on the observed behaviours of specialty physicians and the ways their patients perceive them. Patient Educ Couns. 2006;64(1-3):242-248.
14. Quigley DD, Elliott MN, Farley DO, Burkhart Q, Skootsky SA, Hays RD. Specialties differ in which aspects of doctor communication predict overall physician ratings. J Gen Intern Med. 2014;29(3):447-454.
15. Simon MA. The education of future orthopaedists—dèjá vu. J Bone Joint Surg Am. 2001;83(9):1416-1423.
16. Konstantakos EK, Laughlin RT, Markert RJ, Crosby LA. Follow-up on misrepresentation of research activity by orthopaedic residency applicants: has anything changed? J Bone Joint Surg Am. 2007;89(9):2084-2088.
17. Arora VM, Farnan JM, Humphrey HJ. Professionalism in the era of duty hours: time for a shift change? JAMA. 2012;308(21):2195-2196.
18. Corlew S, Lineaweaver W. New professionalism, nostalgic professionalism, pejoratives, and evidence-based persuasion. Ann Plast Surg. 2014;72(3):263-264.
19. Rohrich RJ, Persing JA, Phillips L. Mandating shorter work hours and enhancing patient safety: a new challenge for resident education. Plast Reconstr Surg. 2003;111(1):395-397.
20. Jin CJ, Martimianakis MA, Kitto S, Moulton CA. Pressures to “measure up” in surgery: managing your image and managing your patient. Ann Surg. 2012;256(6):989-993.
21. Gofton W, Regehr G. Factors in optimizing the learning environment for surgical training. Clin Orthop Relat Res. 2006;(449):100-107.
22. Leung A, Luu S, Regehr G, Murnaghan ML, Gallinger S, Moulton CA. “First, do no harm”: balancing competing priorities in surgical practice. Acad Med. 2012;87(10):1368-1374.
23. Inui TS. A Flag in the Wind: Educating for Professionalism in Medicine. Washington, DC: Association of American Medical Colleges; 2003. http://www.bumc.bu.edu/mec/files/2010/06/AAMC_Inui_2003.pdf. Accessed September 26, 2015.
24. Durso SC, Christmas C, Kravet SJ, Parsons G, Wright SM. Implications of academic medicine’s failure to recognize clinical excellence. Clin Med Res. 2009;7(4):127-133.
25. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258.
26. Thomas PA, Diener-West M, Canto MI, Martin DR, Post WS, Streiff MB. Results of an academic promotion and career path survey of faculty at the Johns Hopkins University School of Medicine. Acad Med. 2004;79(3):258-264.
27. Williams ES, Konrad TR, Scheckler WE, et al. Understanding physicians’ intentions to withdraw from practice: the role of job satisfaction, job stress, mental and physical health. 2001. Health Care Manage Rev. 2010;35(2):105-115.
28. Wai PY, Dandar V, Radosevich DM, Brubaker L, Kuo PC. Engagement, workplace satisfaction, and retention of surgical specialists in academic medicine in the United States. J Am Coll Surg. 2014;219(1):31-42.
29. Pololi LH, Kern DE, Carr P, Conrad P, Knight S. The culture of academic medicine: faculty perceptions of the lack of alignment between individual and institutional values. J Gen Intern Med. 2009;24(12):1289-1295.
30. Pololi LH, Krupat E, Civian JT, Ash AS, Brennan RT. Why are a quarter of faculty considering leaving academic medicine? A study of their perceptions of institutional culture and intentions to leave at 26 representiative U.S. medical schools. Acad Med. 2012;87(7):859-869.
31. Beckerle MC, Reed KL, Scott RP, et al. Medical faculty development: a modern-day Odyssey. Sci Transl Med. 2011;3(104):104cm31.
32. Chervenak FA, McCullough LB, Brent RL. The professional responsibility model of physician leadership. Am J Obstet Gynecol. 2013;208(2):97-101.
33. Gross RH. The coaching model for educational leadership principles. J Bone Joint Surg Am. 2004;86(9):2082-2084.
34. Mullins LA. Hospital–physician relationships: a synergy that must work. Front Health Serv Manage. 2003;20(2):37-41.
35. Wynia MK. The role of professionalism and self-regulation in detecting impaired or incompetent physicians. JAMA. 2010;304(2):210-212.
36. Shanafelt TD, Kaups KL, Nelson H, et al. An interactive individualized intervention to promote behavioral change to increase personal well-being in US surgeons. Ann Surg. 2014;259(1):82-88.
37. West CP, Shanafelt TD. Physician well-being and professionalism. Minn Med. 2007;90(8):44-46.
Several years ago, I was on the American Academy of Orthopaedic Surgeons leadership fellow committee, reviewing fellowship applications. The committee had been poised to very favorably rule on an application until a new member spoke up, stating that he had been in the applicant’s department and that points made in the recommending letter bore little resemblance to the person’s performance. Further study confirmed the dishonesty in the letter, and a more fit candidate was selected instead.
I was puzzled. Why would a leader in the field do such a thing? The question led me to a personal investigation into the monumental topic of professionalism and, more specifically, professionalism among orthopedic surgeons.
Physicians, Especially Orthopedists, Are Not Happy
Physicians, in general, are not a happy lot. According to a 2012 survey by the Physicians Foundation,1 77.4% of practicing physicians were pessimistic about the future of medicine, and 82% thought they had little ability to change the health care system. Sources of pessimism included “too much regulation/paperwork, loss of clinical autonomy, physicians not compensated for quality, erosion of physician/patient relationship, and money trumps patient care.” We are now in the age of “organizational physicians,” who, subject to institutional management, are experiencing a distressing loss of autonomy.
What sustains, or does not sustain, surgeons’ career satisfaction? Commonly stated positive factors include the ability to provide quality care, time with patients, income, and financial incentives2; reported negative factors include threat of malpractice, lack of autonomy, excessive administrative tasks, and high patient volume. Early-career physicians have the lowest career satisfaction, but physicians in mid-career have the highest rate of burnout and likelihood of leaving medical practice.3 Work–home conflict is most difficult in the early career, when families have young children, and the conflict generally goes unresolved. Burnout and low satisfaction with specialty choice are most common in mid-career.
Despite all the negative factors acting on medical practices, orthopedic surgeons have fared well financially, but not as well in career satisfaction. The Medscape Physician Compensation Report 20144 places orthopedics compensation first among 25 specialties listed, without a close second, but orthopedists rank 15th in thinking they are fairly compensated, and next to last in indicating they would choose medicine again as a career. A separate study of physician career satisfaction ranked orthopedics 32nd of 42 specialties studied.5
What is our problem, and what can we do about it? It’s hard to digest this information and not feel that orthopedists are, for lack of a better word, spoiled.
DeBotton6 wrote about status anxiety, which arises over and over again in daily life. Essentially, it is the envy or dissatisfaction one feels when a peer gets a better deal that does not seem just. A remarkable aspect of Medscape’s compensation report4 is that family medicine physicians, whose annual income was well under half that of orthopedic surgeons, were more likely to view themselves as fairly compensated. On this basis, we have to conclude that orthopedic surgeons have status anxiety. But why?
Humanism
Osler, the quintessential physician, counseled medical students: “Nothing will sustain you more potently in your humdrum routine … than the power to recognize the true poetry of life—the poetry of the commonplace, of the ordinary man, of the plain, toilworn woman, with their loves and their joys, their sorrows and their griefs.”7 In short, take the time to know your patients. In a study of physicians who were regarded as clinically excellent, several traits were noted: honest, nonjudgmental, genuinely caring, treating all patients equally, and constantly striving for excellence.8 A century after Osler, Stellato9 echoed the sentiment: “Listen to your patients, not just about their illness, but about their life.”
Humanism, then, is the trait underlying professionalism.10,11 Communication skills are essential to humanism.12 However, a study of specialty physicians in Spain “showed scarce empathic behaviours or behaviours that foster a shared decision making process.”13 In addition, a recent survey placed the communication skills of orthopedists last among 28 specialties.14 Assessment was based on how often a physician explains things, listens carefully, gives easy-to-understand instructions, shows respect, and spends enough time.
Could it be that orthopedists are not satisfied with their income because as a group they lack the communication skills and humanistic characteristics of lower-paid physicians?
Residency and the Academic Medical Center
The education of the orthopedic surgeon starts with the selection process. Simon15 noted that “the brightest, but not always the best” are selected largely because objective criteria are an excellent measure of cognitive achievement but not of character. Also noting that 10% of examinees pass part I of the board but fail part II, Simon opined that they “lack clinical judgment, communication skills, and, in some instances, ethics.” A 1999 team of authors found that 18% of research citations listed by orthopedic residency applicants were misrepresented, and a follow-up study by the same authors in 2007 noted a rate increase, to 20.6%.16 Both sets of authors wrote of a need for a better selection process and a better evaluative process during residency.
The residency process has been substantially altered by work-hour restrictions. The 20th-century residency, which emphasizes taking responsibility for the patient throughout a hospital stay, has now been dismissed as “nostalgic professionalism.” Residents are now advised to avoid such activities as checking laboratory results from home and coming to work when they are not feeling well.17 However, there has been considerable pushback against diminishing nostalgic professionalism, primarily from surgeons.18 “Teaching residents that they should go home to rest at the end of their shift without regard for the circumstances of their cases in progress is not an acceptable example for training.”19 Current promulgated restrictions on duty hours move concern for the “circumstances of their cases” to the back burner—the shift ends, the physician leaves. Residents are pulled one way by forces telling them to leave (Accreditation Council for Graduate Medical Education) and the other way by forces telling them to stay (their conscience).
How do residents develop their surgical identities and concepts of humanism and professionalism? There is a substantial body of evidence that the so-called hidden curriculum is the dominant factor: trainees emulate what their faculty say and do.20 As Gofton and Regehr21 noted, “It is vital for members of the surgical academic community to recognize [that] the attitudes, beliefs, and values implicit in every action, every word, every inaction, and every silence are not only shaping the attitudes, beliefs, and values of one’s protégés, but also are shaping the decisions of students who are considering the possibility of becoming one’s protégés.” It is not easy being a surgical role model given the conflicts affecting academic surgeons. For example, should a surgeon allot extra time so a trainee can do a case properly, or should the case be finished expeditiously in order to avoid canceling the next case, or to get to a committee meeting or a kid’s ballgame on time? Monetary pressures, along with the possibility of losing operative time because the schedule was not full, can influence the decision to operate or not.22 Trainees absorb what they hear and see.
In 2003, Inui23 published A Flag in the Wind: Educating for Professionalism in Medicine, in which he stated, “There can be little doubt that physicians in general as well as the leadership of the organization of medicine have been preoccupied with finances and the economics of medical care. … The topics and the language of academic leadership [have] shifted in the last twenty years. … Core functions of the academic medical center became ‘enterprises.’” He also noted, “The most difficult challenge of all may be the need to understand—and to be explicitly mindful of, and articulate about—medical education as a special form of personal and professional formation that is rooted in the daily activities of individuals and groups in academic medical communities.”23 In addition, the “institutional environment we create … [is] a reflection of the values we hold as a professional community.”23 In effect, the academic medical center is part of the hidden curriculum.
Curiously, academic institutions tend not to reward clinical excellence—a self-defeating measure for any institution that recognizes the importance of the hidden curriculum.24 A peer evaluation of hospitalists revealed that the most highly regarded were highly associated with humanism and a passion for clinical medicine.25 At a prominent institution, however, it was found that clinical educators were less likely than research faculty to hold a higher rank.26
Of the factors affecting physician dissatisfaction, workplace stress is predominant.27 In this age of organizational physicians, job satisfaction correlates with how a physician feels about his or her ability to function as a physician. In a study by Wai and colleagues,28 “surgical faculty reported low satisfaction with a number of questions about communication in their medical schools and their clinical practice locations.” The authors indicated that “medical school and department governance are critical determinants of faculty satisfaction within academic surgical centers.” Pololi and colleagues29 extensively studied the culture of academic medicine and summarized the sources of discontent: “competitive individualism, undervaluing of humanistic qualities, deprecation, and the erosion of trust.” In another study,30 they studied the incidence (~25%) of, and reasons for, considering to leave academic medicine. Reasons included feeling isolated in the department, lack of institutional support, poor communication with administrators, and a perceived difference between the stated culture of the institution and what was observed on a daily basis.30
What Can We Do?
The obvious starting point is the selection process—focusing more on finding the “best,” not necessarily the “brightest.”15 This is not easy. Recommendation letters are often based on limited contact and may or may not reflect applicants’ true character. Numerous websites advise resident applicants on what questions to expect and how to prepare and practice for them. I have found questions of current events very illuminating, as they can probe how applicants view the world. Given the high income of orthopedic surgeons, some applicants likely are attracted to that aspect of the specialty. These applicants are not the “best.”
Residents who exhibit questionable ethical reasoning or behavior must be identified and not be allowed to finish their program. It is the responsibility of the program, not the board, to ensure that those entering practice exhibit a high degree of professionalism. Faculty must seriously recognize, every day, that everything they do is part of the hidden curriculum.
As noted, the academic medical environment can be inimical. Faculty who experience dissonance must be able to effectively confront administrative leadership to express their concerns, and they need to feel their concerns are recognized. Leaders of academic medical centers must guide their institutions in such a way that the day-to-day functions are compatible with the stated mission and values.31
Chervenak and colleagues32 forcefully stated that “appropriate ethical values” are the core component that academic leadership needs in order to respond to the opposing forces of increasing pressures of patient satisfaction, compliance, liability, and other administrative demands on one hand and diminishing resources on the other hand. They listed 4 “professional virtues” that characterize responsible professional leadership: self-effacement, which obligates physician leaders to be unbiased; self-sacrifice, the willingness to risk individual and organizational self-interest, especially in the economic domain; compassion, or “What can I do to help?”; and integrity. The principles of effective leadership are not that complicated, but implementing them requires conviction and courage.33
Physicians increasingly are practicing in the organization setting. They need to increase their involvement in the organization in order to promulgate the needs of physicians. Organizational executive leadership is primarily driven by budgetary and capital planning processes; physician input is essential to ensure resources are directed toward better patient care. A feeling of loss of control over one’s practice is a primary cause of physician dissatisfaction. The schism between physicians and administrators traditionally has been characterized by a lack of trust; a more trusting relationship, reinforced by frequent constructive dialogue, will result in more physician control of the practice.34 This will be difficult, but it is necessary for improving professional satisfaction.
For practicing physicians, Wynia35 made the compelling case that professionalism demands self-regulation, which involves identifying and reporting impaired or incompetent physicians—another task that requires conviction and courage.
But the core issue is how an orthopedist regards the day-to-day aspects of his or her practice. Shanafelt and colleagues36 concluded that surgeons are not very good at assessing their own well-being and stress levels. Certainly high stress can affect well-being, which in turn can affect professionalism. West and Shanafelt37 uniquely described this relationship: “The effect of distress on professionalism in medicine has become clear in recent years. The well-documented decline of crucial elements of professionalism, including empathy and humanism, during medical training appears to be related in part to personal distress experienced during medical school and residency. Unfortunately, this decline continues as physicians move into practice, where distress also is associated with decreased compassion and empathy.” This description sounds completely synchronized with the current career dissatisfaction of orthopedic surgeons.
Improving orthopedists’ status requires ethical and involved leadership, both in academia and in our professional organizations, which too often seem mired in the (not so effective) status quo. Recognizing that the resident selection process is fallible is the first step in taking action—engaging in scrupulous role modeling and insisting that residents demonstrate professionalism and communication skills in their daily work. Becoming involved in organizational management is preferable to becoming angry and dissatisfied. Getting to know one’s patients is its own reward in terms of career satisfaction. Orthopedic surgeons have a well-earned macho image—that image can be enhanced with a dose of humanism. The result would be a true professional who enjoys his or her practice and has a satisfying career.
Several years ago, I was on the American Academy of Orthopaedic Surgeons leadership fellow committee, reviewing fellowship applications. The committee had been poised to very favorably rule on an application until a new member spoke up, stating that he had been in the applicant’s department and that points made in the recommending letter bore little resemblance to the person’s performance. Further study confirmed the dishonesty in the letter, and a more fit candidate was selected instead.
I was puzzled. Why would a leader in the field do such a thing? The question led me to a personal investigation into the monumental topic of professionalism and, more specifically, professionalism among orthopedic surgeons.
Physicians, Especially Orthopedists, Are Not Happy
Physicians, in general, are not a happy lot. According to a 2012 survey by the Physicians Foundation,1 77.4% of practicing physicians were pessimistic about the future of medicine, and 82% thought they had little ability to change the health care system. Sources of pessimism included “too much regulation/paperwork, loss of clinical autonomy, physicians not compensated for quality, erosion of physician/patient relationship, and money trumps patient care.” We are now in the age of “organizational physicians,” who, subject to institutional management, are experiencing a distressing loss of autonomy.
What sustains, or does not sustain, surgeons’ career satisfaction? Commonly stated positive factors include the ability to provide quality care, time with patients, income, and financial incentives2; reported negative factors include threat of malpractice, lack of autonomy, excessive administrative tasks, and high patient volume. Early-career physicians have the lowest career satisfaction, but physicians in mid-career have the highest rate of burnout and likelihood of leaving medical practice.3 Work–home conflict is most difficult in the early career, when families have young children, and the conflict generally goes unresolved. Burnout and low satisfaction with specialty choice are most common in mid-career.
Despite all the negative factors acting on medical practices, orthopedic surgeons have fared well financially, but not as well in career satisfaction. The Medscape Physician Compensation Report 20144 places orthopedics compensation first among 25 specialties listed, without a close second, but orthopedists rank 15th in thinking they are fairly compensated, and next to last in indicating they would choose medicine again as a career. A separate study of physician career satisfaction ranked orthopedics 32nd of 42 specialties studied.5
What is our problem, and what can we do about it? It’s hard to digest this information and not feel that orthopedists are, for lack of a better word, spoiled.
DeBotton6 wrote about status anxiety, which arises over and over again in daily life. Essentially, it is the envy or dissatisfaction one feels when a peer gets a better deal that does not seem just. A remarkable aspect of Medscape’s compensation report4 is that family medicine physicians, whose annual income was well under half that of orthopedic surgeons, were more likely to view themselves as fairly compensated. On this basis, we have to conclude that orthopedic surgeons have status anxiety. But why?
Humanism
Osler, the quintessential physician, counseled medical students: “Nothing will sustain you more potently in your humdrum routine … than the power to recognize the true poetry of life—the poetry of the commonplace, of the ordinary man, of the plain, toilworn woman, with their loves and their joys, their sorrows and their griefs.”7 In short, take the time to know your patients. In a study of physicians who were regarded as clinically excellent, several traits were noted: honest, nonjudgmental, genuinely caring, treating all patients equally, and constantly striving for excellence.8 A century after Osler, Stellato9 echoed the sentiment: “Listen to your patients, not just about their illness, but about their life.”
Humanism, then, is the trait underlying professionalism.10,11 Communication skills are essential to humanism.12 However, a study of specialty physicians in Spain “showed scarce empathic behaviours or behaviours that foster a shared decision making process.”13 In addition, a recent survey placed the communication skills of orthopedists last among 28 specialties.14 Assessment was based on how often a physician explains things, listens carefully, gives easy-to-understand instructions, shows respect, and spends enough time.
Could it be that orthopedists are not satisfied with their income because as a group they lack the communication skills and humanistic characteristics of lower-paid physicians?
Residency and the Academic Medical Center
The education of the orthopedic surgeon starts with the selection process. Simon15 noted that “the brightest, but not always the best” are selected largely because objective criteria are an excellent measure of cognitive achievement but not of character. Also noting that 10% of examinees pass part I of the board but fail part II, Simon opined that they “lack clinical judgment, communication skills, and, in some instances, ethics.” A 1999 team of authors found that 18% of research citations listed by orthopedic residency applicants were misrepresented, and a follow-up study by the same authors in 2007 noted a rate increase, to 20.6%.16 Both sets of authors wrote of a need for a better selection process and a better evaluative process during residency.
The residency process has been substantially altered by work-hour restrictions. The 20th-century residency, which emphasizes taking responsibility for the patient throughout a hospital stay, has now been dismissed as “nostalgic professionalism.” Residents are now advised to avoid such activities as checking laboratory results from home and coming to work when they are not feeling well.17 However, there has been considerable pushback against diminishing nostalgic professionalism, primarily from surgeons.18 “Teaching residents that they should go home to rest at the end of their shift without regard for the circumstances of their cases in progress is not an acceptable example for training.”19 Current promulgated restrictions on duty hours move concern for the “circumstances of their cases” to the back burner—the shift ends, the physician leaves. Residents are pulled one way by forces telling them to leave (Accreditation Council for Graduate Medical Education) and the other way by forces telling them to stay (their conscience).
How do residents develop their surgical identities and concepts of humanism and professionalism? There is a substantial body of evidence that the so-called hidden curriculum is the dominant factor: trainees emulate what their faculty say and do.20 As Gofton and Regehr21 noted, “It is vital for members of the surgical academic community to recognize [that] the attitudes, beliefs, and values implicit in every action, every word, every inaction, and every silence are not only shaping the attitudes, beliefs, and values of one’s protégés, but also are shaping the decisions of students who are considering the possibility of becoming one’s protégés.” It is not easy being a surgical role model given the conflicts affecting academic surgeons. For example, should a surgeon allot extra time so a trainee can do a case properly, or should the case be finished expeditiously in order to avoid canceling the next case, or to get to a committee meeting or a kid’s ballgame on time? Monetary pressures, along with the possibility of losing operative time because the schedule was not full, can influence the decision to operate or not.22 Trainees absorb what they hear and see.
In 2003, Inui23 published A Flag in the Wind: Educating for Professionalism in Medicine, in which he stated, “There can be little doubt that physicians in general as well as the leadership of the organization of medicine have been preoccupied with finances and the economics of medical care. … The topics and the language of academic leadership [have] shifted in the last twenty years. … Core functions of the academic medical center became ‘enterprises.’” He also noted, “The most difficult challenge of all may be the need to understand—and to be explicitly mindful of, and articulate about—medical education as a special form of personal and professional formation that is rooted in the daily activities of individuals and groups in academic medical communities.”23 In addition, the “institutional environment we create … [is] a reflection of the values we hold as a professional community.”23 In effect, the academic medical center is part of the hidden curriculum.
Curiously, academic institutions tend not to reward clinical excellence—a self-defeating measure for any institution that recognizes the importance of the hidden curriculum.24 A peer evaluation of hospitalists revealed that the most highly regarded were highly associated with humanism and a passion for clinical medicine.25 At a prominent institution, however, it was found that clinical educators were less likely than research faculty to hold a higher rank.26
Of the factors affecting physician dissatisfaction, workplace stress is predominant.27 In this age of organizational physicians, job satisfaction correlates with how a physician feels about his or her ability to function as a physician. In a study by Wai and colleagues,28 “surgical faculty reported low satisfaction with a number of questions about communication in their medical schools and their clinical practice locations.” The authors indicated that “medical school and department governance are critical determinants of faculty satisfaction within academic surgical centers.” Pololi and colleagues29 extensively studied the culture of academic medicine and summarized the sources of discontent: “competitive individualism, undervaluing of humanistic qualities, deprecation, and the erosion of trust.” In another study,30 they studied the incidence (~25%) of, and reasons for, considering to leave academic medicine. Reasons included feeling isolated in the department, lack of institutional support, poor communication with administrators, and a perceived difference between the stated culture of the institution and what was observed on a daily basis.30
What Can We Do?
The obvious starting point is the selection process—focusing more on finding the “best,” not necessarily the “brightest.”15 This is not easy. Recommendation letters are often based on limited contact and may or may not reflect applicants’ true character. Numerous websites advise resident applicants on what questions to expect and how to prepare and practice for them. I have found questions of current events very illuminating, as they can probe how applicants view the world. Given the high income of orthopedic surgeons, some applicants likely are attracted to that aspect of the specialty. These applicants are not the “best.”
Residents who exhibit questionable ethical reasoning or behavior must be identified and not be allowed to finish their program. It is the responsibility of the program, not the board, to ensure that those entering practice exhibit a high degree of professionalism. Faculty must seriously recognize, every day, that everything they do is part of the hidden curriculum.
As noted, the academic medical environment can be inimical. Faculty who experience dissonance must be able to effectively confront administrative leadership to express their concerns, and they need to feel their concerns are recognized. Leaders of academic medical centers must guide their institutions in such a way that the day-to-day functions are compatible with the stated mission and values.31
Chervenak and colleagues32 forcefully stated that “appropriate ethical values” are the core component that academic leadership needs in order to respond to the opposing forces of increasing pressures of patient satisfaction, compliance, liability, and other administrative demands on one hand and diminishing resources on the other hand. They listed 4 “professional virtues” that characterize responsible professional leadership: self-effacement, which obligates physician leaders to be unbiased; self-sacrifice, the willingness to risk individual and organizational self-interest, especially in the economic domain; compassion, or “What can I do to help?”; and integrity. The principles of effective leadership are not that complicated, but implementing them requires conviction and courage.33
Physicians increasingly are practicing in the organization setting. They need to increase their involvement in the organization in order to promulgate the needs of physicians. Organizational executive leadership is primarily driven by budgetary and capital planning processes; physician input is essential to ensure resources are directed toward better patient care. A feeling of loss of control over one’s practice is a primary cause of physician dissatisfaction. The schism between physicians and administrators traditionally has been characterized by a lack of trust; a more trusting relationship, reinforced by frequent constructive dialogue, will result in more physician control of the practice.34 This will be difficult, but it is necessary for improving professional satisfaction.
For practicing physicians, Wynia35 made the compelling case that professionalism demands self-regulation, which involves identifying and reporting impaired or incompetent physicians—another task that requires conviction and courage.
But the core issue is how an orthopedist regards the day-to-day aspects of his or her practice. Shanafelt and colleagues36 concluded that surgeons are not very good at assessing their own well-being and stress levels. Certainly high stress can affect well-being, which in turn can affect professionalism. West and Shanafelt37 uniquely described this relationship: “The effect of distress on professionalism in medicine has become clear in recent years. The well-documented decline of crucial elements of professionalism, including empathy and humanism, during medical training appears to be related in part to personal distress experienced during medical school and residency. Unfortunately, this decline continues as physicians move into practice, where distress also is associated with decreased compassion and empathy.” This description sounds completely synchronized with the current career dissatisfaction of orthopedic surgeons.
Improving orthopedists’ status requires ethical and involved leadership, both in academia and in our professional organizations, which too often seem mired in the (not so effective) status quo. Recognizing that the resident selection process is fallible is the first step in taking action—engaging in scrupulous role modeling and insisting that residents demonstrate professionalism and communication skills in their daily work. Becoming involved in organizational management is preferable to becoming angry and dissatisfied. Getting to know one’s patients is its own reward in terms of career satisfaction. Orthopedic surgeons have a well-earned macho image—that image can be enhanced with a dose of humanism. The result would be a true professional who enjoys his or her practice and has a satisfying career.
1. The Physicians Foundation. A Survey of America’s Physicians: Practice Patterns and Perspectives. An Examination of the Professional Morale, Practice Patterns, Career Plans, and Healthcare Perspectives of Today’s Physicians, Aggregated by Age, Gender, Primary Care/Specialists, and Practice Owners/Employees. http://www.physiciansfoundation.org/uploads/default/Physicians_Foundation_2012_Biennial_Survey.pdf. Published September 2012. Accessed September 26, 2015.
2. Deshpande SP, Deshpande SS. Career satisfaction of surgical specialties. Ann Surg. 2011;253(5):1011-1016.
3. Dyrbye LN, Varkey P, Boone SL, Satele DV, Sloan JA, Shanafelt TD. Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358-1367.
4. Medscape Physician Compensation Report 2014. New York, NY: Medscape; 2014.
5. Leigh JP, Tancredi DJ, Kravitz RL. Physician career satisfaction within specialties. BMC Health Serv Res. 2009;9:166.
6. deBotton A. Status Anxiety. New York, NY: Vintage Books; 2004.
7. Golden RL. William Osler at 150: an overview of a life. JAMA. 1999;282(23):2252-2258.
8. Christmas C, Kravet SJ, Durso SC, Wright SM. Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989-994.
9. Stellato TA. Humanism and the art of surgery. Surgery. 2007;142(4):433-438.
10. Gold A, Gold S. Humanism in medicine from the perspective of the Arnold Gold Foundation: challenges to maintaining the care in health care. J Child Neurol. 2006;21(6):546-549.
11. Cohen JJ. Viewpoint: linking professionalism to humanism: what it means, why it matters. Acad Med. 2007;82(11):1029-1032.
12. Holt GR. Bioethics and humanism in head and neck cancer. Arch Facial Plast Surg. 2010;12(2):85-86.
13. Ruiz-Moral R, Pérez Rodríguez E, Pérula de Torres LA, de la Torre J. Physician–patient communication: a study on the observed behaviours of specialty physicians and the ways their patients perceive them. Patient Educ Couns. 2006;64(1-3):242-248.
14. Quigley DD, Elliott MN, Farley DO, Burkhart Q, Skootsky SA, Hays RD. Specialties differ in which aspects of doctor communication predict overall physician ratings. J Gen Intern Med. 2014;29(3):447-454.
15. Simon MA. The education of future orthopaedists—dèjá vu. J Bone Joint Surg Am. 2001;83(9):1416-1423.
16. Konstantakos EK, Laughlin RT, Markert RJ, Crosby LA. Follow-up on misrepresentation of research activity by orthopaedic residency applicants: has anything changed? J Bone Joint Surg Am. 2007;89(9):2084-2088.
17. Arora VM, Farnan JM, Humphrey HJ. Professionalism in the era of duty hours: time for a shift change? JAMA. 2012;308(21):2195-2196.
18. Corlew S, Lineaweaver W. New professionalism, nostalgic professionalism, pejoratives, and evidence-based persuasion. Ann Plast Surg. 2014;72(3):263-264.
19. Rohrich RJ, Persing JA, Phillips L. Mandating shorter work hours and enhancing patient safety: a new challenge for resident education. Plast Reconstr Surg. 2003;111(1):395-397.
20. Jin CJ, Martimianakis MA, Kitto S, Moulton CA. Pressures to “measure up” in surgery: managing your image and managing your patient. Ann Surg. 2012;256(6):989-993.
21. Gofton W, Regehr G. Factors in optimizing the learning environment for surgical training. Clin Orthop Relat Res. 2006;(449):100-107.
22. Leung A, Luu S, Regehr G, Murnaghan ML, Gallinger S, Moulton CA. “First, do no harm”: balancing competing priorities in surgical practice. Acad Med. 2012;87(10):1368-1374.
23. Inui TS. A Flag in the Wind: Educating for Professionalism in Medicine. Washington, DC: Association of American Medical Colleges; 2003. http://www.bumc.bu.edu/mec/files/2010/06/AAMC_Inui_2003.pdf. Accessed September 26, 2015.
24. Durso SC, Christmas C, Kravet SJ, Parsons G, Wright SM. Implications of academic medicine’s failure to recognize clinical excellence. Clin Med Res. 2009;7(4):127-133.
25. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258.
26. Thomas PA, Diener-West M, Canto MI, Martin DR, Post WS, Streiff MB. Results of an academic promotion and career path survey of faculty at the Johns Hopkins University School of Medicine. Acad Med. 2004;79(3):258-264.
27. Williams ES, Konrad TR, Scheckler WE, et al. Understanding physicians’ intentions to withdraw from practice: the role of job satisfaction, job stress, mental and physical health. 2001. Health Care Manage Rev. 2010;35(2):105-115.
28. Wai PY, Dandar V, Radosevich DM, Brubaker L, Kuo PC. Engagement, workplace satisfaction, and retention of surgical specialists in academic medicine in the United States. J Am Coll Surg. 2014;219(1):31-42.
29. Pololi LH, Kern DE, Carr P, Conrad P, Knight S. The culture of academic medicine: faculty perceptions of the lack of alignment between individual and institutional values. J Gen Intern Med. 2009;24(12):1289-1295.
30. Pololi LH, Krupat E, Civian JT, Ash AS, Brennan RT. Why are a quarter of faculty considering leaving academic medicine? A study of their perceptions of institutional culture and intentions to leave at 26 representiative U.S. medical schools. Acad Med. 2012;87(7):859-869.
31. Beckerle MC, Reed KL, Scott RP, et al. Medical faculty development: a modern-day Odyssey. Sci Transl Med. 2011;3(104):104cm31.
32. Chervenak FA, McCullough LB, Brent RL. The professional responsibility model of physician leadership. Am J Obstet Gynecol. 2013;208(2):97-101.
33. Gross RH. The coaching model for educational leadership principles. J Bone Joint Surg Am. 2004;86(9):2082-2084.
34. Mullins LA. Hospital–physician relationships: a synergy that must work. Front Health Serv Manage. 2003;20(2):37-41.
35. Wynia MK. The role of professionalism and self-regulation in detecting impaired or incompetent physicians. JAMA. 2010;304(2):210-212.
36. Shanafelt TD, Kaups KL, Nelson H, et al. An interactive individualized intervention to promote behavioral change to increase personal well-being in US surgeons. Ann Surg. 2014;259(1):82-88.
37. West CP, Shanafelt TD. Physician well-being and professionalism. Minn Med. 2007;90(8):44-46.
1. The Physicians Foundation. A Survey of America’s Physicians: Practice Patterns and Perspectives. An Examination of the Professional Morale, Practice Patterns, Career Plans, and Healthcare Perspectives of Today’s Physicians, Aggregated by Age, Gender, Primary Care/Specialists, and Practice Owners/Employees. http://www.physiciansfoundation.org/uploads/default/Physicians_Foundation_2012_Biennial_Survey.pdf. Published September 2012. Accessed September 26, 2015.
2. Deshpande SP, Deshpande SS. Career satisfaction of surgical specialties. Ann Surg. 2011;253(5):1011-1016.
3. Dyrbye LN, Varkey P, Boone SL, Satele DV, Sloan JA, Shanafelt TD. Physician satisfaction and burnout at different career stages. Mayo Clin Proc. 2013;88(12):1358-1367.
4. Medscape Physician Compensation Report 2014. New York, NY: Medscape; 2014.
5. Leigh JP, Tancredi DJ, Kravitz RL. Physician career satisfaction within specialties. BMC Health Serv Res. 2009;9:166.
6. deBotton A. Status Anxiety. New York, NY: Vintage Books; 2004.
7. Golden RL. William Osler at 150: an overview of a life. JAMA. 1999;282(23):2252-2258.
8. Christmas C, Kravet SJ, Durso SC, Wright SM. Clinical excellence in academia: perspectives from masterful academic clinicians. Mayo Clin Proc. 2008;83(9):989-994.
9. Stellato TA. Humanism and the art of surgery. Surgery. 2007;142(4):433-438.
10. Gold A, Gold S. Humanism in medicine from the perspective of the Arnold Gold Foundation: challenges to maintaining the care in health care. J Child Neurol. 2006;21(6):546-549.
11. Cohen JJ. Viewpoint: linking professionalism to humanism: what it means, why it matters. Acad Med. 2007;82(11):1029-1032.
12. Holt GR. Bioethics and humanism in head and neck cancer. Arch Facial Plast Surg. 2010;12(2):85-86.
13. Ruiz-Moral R, Pérez Rodríguez E, Pérula de Torres LA, de la Torre J. Physician–patient communication: a study on the observed behaviours of specialty physicians and the ways their patients perceive them. Patient Educ Couns. 2006;64(1-3):242-248.
14. Quigley DD, Elliott MN, Farley DO, Burkhart Q, Skootsky SA, Hays RD. Specialties differ in which aspects of doctor communication predict overall physician ratings. J Gen Intern Med. 2014;29(3):447-454.
15. Simon MA. The education of future orthopaedists—dèjá vu. J Bone Joint Surg Am. 2001;83(9):1416-1423.
16. Konstantakos EK, Laughlin RT, Markert RJ, Crosby LA. Follow-up on misrepresentation of research activity by orthopaedic residency applicants: has anything changed? J Bone Joint Surg Am. 2007;89(9):2084-2088.
17. Arora VM, Farnan JM, Humphrey HJ. Professionalism in the era of duty hours: time for a shift change? JAMA. 2012;308(21):2195-2196.
18. Corlew S, Lineaweaver W. New professionalism, nostalgic professionalism, pejoratives, and evidence-based persuasion. Ann Plast Surg. 2014;72(3):263-264.
19. Rohrich RJ, Persing JA, Phillips L. Mandating shorter work hours and enhancing patient safety: a new challenge for resident education. Plast Reconstr Surg. 2003;111(1):395-397.
20. Jin CJ, Martimianakis MA, Kitto S, Moulton CA. Pressures to “measure up” in surgery: managing your image and managing your patient. Ann Surg. 2012;256(6):989-993.
21. Gofton W, Regehr G. Factors in optimizing the learning environment for surgical training. Clin Orthop Relat Res. 2006;(449):100-107.
22. Leung A, Luu S, Regehr G, Murnaghan ML, Gallinger S, Moulton CA. “First, do no harm”: balancing competing priorities in surgical practice. Acad Med. 2012;87(10):1368-1374.
23. Inui TS. A Flag in the Wind: Educating for Professionalism in Medicine. Washington, DC: Association of American Medical Colleges; 2003. http://www.bumc.bu.edu/mec/files/2010/06/AAMC_Inui_2003.pdf. Accessed September 26, 2015.
24. Durso SC, Christmas C, Kravet SJ, Parsons G, Wright SM. Implications of academic medicine’s failure to recognize clinical excellence. Clin Med Res. 2009;7(4):127-133.
25. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258.
26. Thomas PA, Diener-West M, Canto MI, Martin DR, Post WS, Streiff MB. Results of an academic promotion and career path survey of faculty at the Johns Hopkins University School of Medicine. Acad Med. 2004;79(3):258-264.
27. Williams ES, Konrad TR, Scheckler WE, et al. Understanding physicians’ intentions to withdraw from practice: the role of job satisfaction, job stress, mental and physical health. 2001. Health Care Manage Rev. 2010;35(2):105-115.
28. Wai PY, Dandar V, Radosevich DM, Brubaker L, Kuo PC. Engagement, workplace satisfaction, and retention of surgical specialists in academic medicine in the United States. J Am Coll Surg. 2014;219(1):31-42.
29. Pololi LH, Kern DE, Carr P, Conrad P, Knight S. The culture of academic medicine: faculty perceptions of the lack of alignment between individual and institutional values. J Gen Intern Med. 2009;24(12):1289-1295.
30. Pololi LH, Krupat E, Civian JT, Ash AS, Brennan RT. Why are a quarter of faculty considering leaving academic medicine? A study of their perceptions of institutional culture and intentions to leave at 26 representiative U.S. medical schools. Acad Med. 2012;87(7):859-869.
31. Beckerle MC, Reed KL, Scott RP, et al. Medical faculty development: a modern-day Odyssey. Sci Transl Med. 2011;3(104):104cm31.
32. Chervenak FA, McCullough LB, Brent RL. The professional responsibility model of physician leadership. Am J Obstet Gynecol. 2013;208(2):97-101.
33. Gross RH. The coaching model for educational leadership principles. J Bone Joint Surg Am. 2004;86(9):2082-2084.
34. Mullins LA. Hospital–physician relationships: a synergy that must work. Front Health Serv Manage. 2003;20(2):37-41.
35. Wynia MK. The role of professionalism and self-regulation in detecting impaired or incompetent physicians. JAMA. 2010;304(2):210-212.
36. Shanafelt TD, Kaups KL, Nelson H, et al. An interactive individualized intervention to promote behavioral change to increase personal well-being in US surgeons. Ann Surg. 2014;259(1):82-88.
37. West CP, Shanafelt TD. Physician well-being and professionalism. Minn Med. 2007;90(8):44-46.
Total Shoulder Arthroplasty Outcome for Treatment of Osteoarthritis: A Multicenter Study Using a Contemporary Implant
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Collagenase Enzymatic Fasciotomy for Dupuytren Contracture in Patients on Chronic Immunosuppression
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
The incidence of Dupuytren disease increases with advancing age,1 as do the medical comorbidities of patients seeking treatment for disabling hand contractures. For patients with significant comorbidities, open surgical fasciectomy, the current standard of treatment for Dupuytren disease,2,3 may be associated with increased perioperative risks.
Collagenase enzymatic fasciotomy has become an accepted nonsurgical treatment alternative to traditional fasciectomy or surgical fasciotomy for significant digital contractures caused by Dupuytren disease.4-6 Clostridium histolyticum collagenase (CHC) is a foreign protein, made up of 2 collagenases isolated from the bacteria C histolyticum.7 The collagenases are zinc-dependent matrix metalloproteinases that cleave the triple helical structure of collagen molecules.8 Also known as Xiaflex (Auxilium Pharmaceuticals), CHC was approved by the US Food and Drug Administration (FDA) in February 2010 for use in patients with Dupuytren contractures.
Enzymatic rupture is safe and efficacious at midterm follow-up and offers the theoretical advantage of avoiding palmar and digital fasciectomy and the associated risks of surgical-site infection and wound-healing complications.6 The risks of surgical wound complications are magnified in immunosuppressed patients, particularly those on chronic steroid therapy; wound-healing complication rates may be increased 2 to 5 times compared with controls.9 In a pooled literature review, wound-healing complications were reported after 22.9% of open primary fasciectomies, with infection occurring in 2.4%.10 A nonsurgical alternative is therefore particularly appealing for a patient cohort that may be at higher risk for a frequently described complication of surgery for Dupuytren contracture.
The exclusion criteria in the trials for FDA approval were extensive and included breast-feeding, pregnancy, bleeding disorder, recent stroke, use of tetracycline derivative within 14 days before start of study, use of anticoagulant within 7 days before start of study, allergy to collagenase, and chronic muscular, neurologic, or neuromuscular disorder affecting the hands.6 Safety and efficacy of collagenase in patients requiring chronic immunosuppressive therapy for medical comorbidities have not been previously documented. Furthermore, although skin tears were reported in 11% of patients after manual cord rupture in the CORD (Collagenase Option for the Reduction of Dupuytren’s) I trial,6 the likelihood of deep and superficial infection and delayed wound healing has not been quantitated.
In this article, we report on outcomes of 13 collagenase enzymatic fasciotomies performed in 8 patients who were on chronic immunosuppressive therapy.
Methods
Institutional review board approval was obtained at both academic hand surgery institutions. We retrospectively reviewed prospectively collected clinical data within our 2 centers’ databases of patients with Dupuytren disease. Eight patients on chronic immunosuppressive therapies treated with collagenase for metacarpophalangeal (MP) or proximal interphalangeal (PIP) joint contractures between February 2010 and December 2011 were identified. Three of these patients received collagenase injections into 2 or more separate Dupuytren cords at different encounters, resulting in a total of 13 individual collagenase enzymatic fasciotomies.
Collagenase injections were administered following CORD I trial protocol,6 except we injected Dupuytren cords crossing the PIP joint using a lateral approach to minimize risk of flexor tendon rupture. Manipulation of the treated joint was performed between 24 and 48 hours after collagenase injection under local anesthesia with 3 mL of 1% mepivacaine or lidocaine without epinephrine. After manipulation and cord rupture, patients were placed in a hand-based extension splint to wear at night for up to 3 months. Patients were followed at 1 and 12 months.
Results
Patients’ baseline characteristics are summarized in Table 1. Four patients were maintained on chronic prednisone therapy, 3 on methotrexate, and 1 on azathioprine. Therapy duration, medication dose, and diagnoses requiring immunosuppressant therapy varied among patients.
Outcomes and adverse events are summarized in Table 2. Mean number of joint contractures per hand treated was 2.8 (MP, 1.4; PIP, 1.4). However, not all joints met the intervention criteria. Of the 13 joints treated, 7 were MP joints, and 6 were PIP joints. Mean preinjection contracture of the treated joints was 53.0° (range, 20°-90°). Twelve of the 13 joint contractures improved. At mean follow-up of 6.7 months (range, 1-22 months), mean magnitude of contracture improved to 12.9° (range, 0°-45°). Mean MP joint contracture improved from 42.0° to 4.2° (range, 0°-10°), and mean PIP joint contracture improved from 65.8° to 21.7° (range, 0°-45°).
All 13 collagenase injections were well tolerated, and there were no systemic reactions. Injection-site pain was common. Mild injection-site bruising and edema were reported in all cases. Enzymatic fasciotomy was performed in all patients, and immediate improvement in contracture after manipulation 24 to 48 hours after injection was recorded.
Three of the 13 injections were complicated by skin tears during manipulation and cord rupture. All 3 skin tears were treated with local wound care, which included use of povidone-iodine and wet-to-dry dressings. There was no evidence of subsequent superficial or deep, local or regional infection. In 2 cases, the wound healed within 1 week; in the third case, wound healing was present by 2 weeks. Once the wounds showed early re-epithelialization, hand-based extension splinting in a position of comfort was used at night for up to 3 months after injection. Two of the 13 injections were complicated by small blood blisters. These were treated with observation and resolved spontaneously.
Discussion
Collagenase enzymatic fasciotomy appeared to be a safe and efficacious alternative to surgical treatment of Dupuytren contractures in this cohort of patients maintained on chronic immunosuppressive agents. MP contractures responded more substantially than PIP contractures did, as expected.6 No previously undescribed adverse outcomes were noted in these 8 patients on chronic immunosuppressive therapy beyond those reported in the CORD I trial. Three (23%) of the 13 collagenase injections in our series were complicated by skin tears after manipulation. Skins tears were reported in 22 (11%) of 204 patients after manual cord rupture in the CORD I trial.6 Given the limited numbers in this series, it remains unclear if chronic immunosuppression truly increases the risk of skin tears in this subset of patients. Other common treatment-related adverse events seen in the CORD I trial—injection-site hemorrhage (37%), pruritis (11%) and lymphadenopathy (10%)—were not seen after the 13 injections in our case series. We are prospectively following all patients with Dupuytren disease, and this is an area of ongoing research at our centers.
The immunosuppressive actions of prednisone, azathioprine, and methotrexate are well documented. Prednisone is a glucocorticoid, converted in the liver to prednisolone, which suppresses inflammation and immune responses by regulation of gene expression. Its immunosuppressive actions are multifactorial, relating to inhibition of lymphocytes, neutrophils, and monocytes. These effects are dose- and time-dependent11 and may become evident in patients receiving low doses over prolonged periods. Skin atrophy12 and delayed wound healing9 are side effects of long-term prednisone use. Skin atrophy may make the prednisone-treated patient more susceptible to skin tears after collagenase injection and manipulation. Azathioprine inhibits purine synthesis, which is especially important in the proliferation of immune cells.13 It has been shown to inhibit both cellular immunity at low doses and humoral immunity at higher doses.14 Methotrexate inhibits lymphocyte folic acid metabolism. The immunosuppressive properties of low-dose methotrexate have been linked to the induction of apoptosis in activated T cells.15
A more complex process in immunosuppressed patients is the immunogenicity of injected collagenase. As CHC in current use is a mixture of 2 foreign proteins, an immunologic response is expected in the host after injection. It has been shown that, after 3 injections of CHC into Dupuytren cords, 100% of patients developed antibodies to both enzymes in their serum.6 More than 85% demonstrated anti-CHC antibodies after a single injection. However, no patients showed signs of anaphylaxis or allergic reaction, and there was no correlation between serum levels of anti-CHC and adverse events. It has been hypothesized that there is a potential for cross-reactivity of the anti-CHC antibodies with human matrix metalloproteinases, causing enzymatic dysfunction within the host.16 This has yet to be reported clinically, and Xiaflex is currently under postmarketing surveillance. Immunocompromised people, with suppressed humoral and cellular immune responses, may produce less of an antibody response to the foreign CHC proteins. Whether this conclusively leads to a change in the side effect profile of the medication in these individuals is beyond the scope of this article. However, we identified no new side effects in this small but higher risk cohort. The issue should be continually monitored as collagenase is used in wider clinical settings.
Collagenase enzymatic fasciotomy is a new nonsurgical therapeutic option for Dupuytren disease. Indications and guidelines for use continue to evolve. This case series highlights the use of collagenase in 8 patients who were on long-term immunosuppressive therapy. This study has the limitations inherent to retrospective analyses. It is difficult to generalize results across broader immunosuppressed populations. A larger cohort, with long-term follow-up assessing recurrence of contracture, is needed to make definitive conclusions about use of collagenase in this challenging subset of patients. Based on our observations in this limited cohort, it appears appropriate to pursue further studies on use of collagenase enzymatic fasciotomy. A randomized, prospective or case–control series comparing surgical fasciectomy with enzymatic fasciotomy would yield further meaningful data. As more patients seek nonsurgical treatment for Dupuytren disease, its safety and efficacy in select cohorts of patients should continue to be evaluated.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
1. Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60.
2. Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57(1):13-17.
3. Sennwald GR. Fasciectomy for treatment of Dupuytren’s disease and early complications. J Hand Surg Am. 1990;15(5):755-761.
4. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25(4):629-636.
5. Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788-798.
6. Hurst LC, Badalamente MA, Hentz VR, et al; CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968-979.
7. Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116-126.
8. Watanabe K. Collagenolytic proteases from bacteria. Appl Microbiol Biotechnol. 2004;63(5):520-526.
9. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410-417.
10. Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. Eplasty. 2010;10:e15.
11. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963.
12. Oikarinen A, Autio P. New aspects of the mechanism of corticosteroid-induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416-419.
13. Makinodan T, Santos GW, Quinn RP. Immunosuppressive drugs. Pharmacol Rev. 1970;22(2):189-247.
14. Röllinghoff M, Schrader J, Wagner H. Effect of azathioprine and cytosine arabinoside on humoral and cellular immunity in vitro. Clin Exp Immunol. 1973;15(2):261-269.
15. Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102(2):322-328.
16. Desai SS, Hentz VR. Collagenase Clostridium histolyticum for Dupuytren’s contracture. Expert Opin Biol Ther. 2010;10(9):1395-1404.
Open Carpal Tunnel Release With Use of a Nasal Turbinate Speculum
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
Carpal tunnel syndrome (CTS) is a disorder characterized by entrapment of the median nerve at the wrist, which may lead to symptoms of pain, paresthesia, and, ultimately, thenar muscle atrophy. Surgical intervention is indicated with persistent or progressive symptoms despite nonoperative management. Timely surgical decompression aims to halt progression of this disorder and prevent permanent peripheral nerve injury.
Carpal tunnel release (CTR) is the most common hand and wrist surgery in the United States, with about 400,000 operations performed annually.1,2 Several methods of decompressing the carpal tunnel have been described.3 These include standard open CTR (OCTR), mini-open approaches, and various endoscopic techniques. OCTR was initially described by Sir James Learmonth in 1933,4 and it remains the gold-standard surgical treatment for patients with symptomatic CTS. Uniform excellent results with high patient satisfaction and low complication rates have been reported in several series.5-9 Common to all techniques is complete proximal-to-distal division of the transverse carpal ligament (TCL). Magnetic resonance imaging studies have shown that TCL transection and the resulting diastasis between the radial and ulnar leaflets cause a significant increase in the volume of the carpal tunnel, leading to decreased pressure.10,11
Endoscopic CTR (ECTR) techniques were developed in an effort to reduce complications, scar sensitivity, and pillar pain and facilitate more rapid return to work.12-17 Outcome studies have demonstrated that both open and endoscopic releases yield patient-reported subjective improvements over preoperative symptoms.18-22 A randomized, controlled trial by Trumble and colleagues23 in 2002 found that ECTR led to improved patient outcomes in the early postoperative period (first 3 months), though differences in outcomes were reduced at final follow-up. More recently (2007), a Cochrane review of 33 trials concluded there was no strong evidence favoring use of alternative techniques over OCTR.3 Further, OCTR has been found to be technically less demanding and associated with decreased complications and costs.24
Indications
The benefit of median nerve decompression at the wrist for CTS is clear.6,7 Indications for surgery in patients with CTS include persistent symptoms despite nonoperative treatment, objective sensory disturbance or motor weakness, and thenar atrophy. Symptomatic response to corticosteroid injection is predictive of success after carpal tunnel surgery.25 More than 87% of patients who gain symptomatic relief from corticosteroid injection have an excellent surgical outcome.
Technique
OCTR allows direct visualization of the TCL and the distal volar forearm fascia (DVFF) and evaluation for the presence of anomalous branching patterns of the median nerve. OCTR traditionally was performed through a 4- to 5-cm longitudinal incision extending from the wrist crease proximally to the Kaplan cardinal line distally. The mini-open technique is identical with the exception of incision length. We routinely use a 2.5- to 3-cm incision. Regardless of incision length, each OCTR should proceed through the same reproducible steps.
We perform OCTR under tourniquet control. Choice of anesthesia is surgeon and patient preference. We prefer local anesthesia with conscious sedation. After conscious sedation is administered, we infiltrate the carpal tunnel and surrounding subcutaneous tissue with 10 mL of a 50:50 mixture of 0.5% bupivacaine and 1% lidocaine without epinephrine.
A 2.5- to 3-cm longitudinal incision is made along the axis of the radial border of the ring finger from the Kaplan cardinal line26 and extending about 3 cm proximally toward the wrist flexion crease ulnar to the palmaris longus if present (Figure 1).
After the skin is incised longitudinally, the subcutaneous fat is mobilized and cutaneous sensory branches identified and protected. The underlying superficial palmar fascia is incised in line with the skin incision. The underlying midportion of the TCL is now visualized.
Transverse Carpal Ligament Release
Occasionally, the investing fascia along the ulnar edge of the thenar musculature is mobilized radialward (if the thenar musculature is well developed) to visualize the proximal limb of the TCL. Injury to any anomalous motor branch of the median nerve is avoided by directly visualizing and then incising the TCL (Figure 2). The TCL is incised along its ulnar border just radial to the hook of hamate from distal to proximal in line with the radial border of the ring finger. Staying near the ulnar attachment of the TCL keeps the plane of ligament division farther away from the median nerve and its recurrent motor branches. Although the ulnar neurovascular bundle typically resides ulnar to the hook of hamate in the canal of Guyon, the surgeon must be aware that it can be located radial to the hook in some instances.27,28 In the elderly, the ulnar artery may be tortuous and enter the field and require retraction. The TCL is incised distally until the sentinel fat pad, which marks the superficial palmar arterial arch, is visualized. This bed of adipose tissue marks the distal edge of the TCL.29
Proximally, subcutaneous tissues above the proximal limb of the TCL and DVFF are mobilized to about 2 cm proximal to the wrist flexion crease to create a plane for the fine long nasal turbinate speculum. The nasal turbinate speculum is then inserted into this plane above the proximal limb of the TCL and DVFF (Figure 3). Once inserted to the level of the confluence of the TCL and the DVFF, the speculum is opened.
Topside visualization is now encountered with the ulnar neurovascular bundle protected by the ulnar blade of the speculum. A long-handle scalpel is used to incise the TCL and the DVFF under direct visualization from proximal to distal in line with the previously completed distal release (Figure 4). As the nasal turbinate speculum is stretching the TCL and putting it under tension, the TCL can be heard splitting as it is being incised. Once the TCL and the DVFF are divided, the speculum is slowly closed and removed. Wide diastasis of the radial and ulnar leaflets of the TCL and the DVFF is directly visualized. Complete decompression of the median nerve from the distal forearm fascia to the superficial palmar arch is confirmed.
Adhesions between the undersurface of the radial leaflet and the flexor tendons and median nerve are mobilized. The median nerve is assessed for “hourglass” morphology or atrophy. The flexor tendons can be swept radialward with a free elevator to inspect the floor of the carpal tunnel. Flexor tenosynovectomy is not routinely performed. The incision is closed with interrupted simple sutures using 4-0 nylon.
Study Results
This study was conducted at Hand Surgery PC, Newton-Wellesley Hospital, Tufts University School of Medicine. Over a 10-month interval, 101 consecutive mini-OCTRs (63 right hands, 38 left hands) were performed with this proximal release modification in 88 patients (51 females, 37 males) by Dr. Ruchelsman and Dr. Belsky (Table). CTRs performed in the setting of wrist and/or carpal trauma were excluded. Mean age was 62.8 years. Mean follow-up was 11.3 weeks (~3 months). For isolated cases of CTR, mean tourniquet time was 16 minutes. CTS symptoms were relieved in all patients with a high degree of satisfaction as measured with history and examination findings at follow-up visits. There were no major complications (eg, infection, neural or vascular damage, severe residual pain). Four patients reported minor residual numbness in the fingers at latest follow-up but nevertheless had major improvement over preoperative baseline. These 4 patients had preoperative electromyograms or nerve conduction studies documenting the extent of their disease. There was 1 case of minor wound complication. Three weeks after surgery, the patient had a 1-cm wound opening, which closed with local wound care. The patient did not develop any drainage, infection, bleeding, or neurologic symptoms.
Discussion
Open release of the TCL—the gold standard of surgical treatment for CTS—produces reliable symptom relief in the vast majority of patients.25,30 Given that the most common complication of carpal tunnel surgery is incomplete release of the TCL,31,32 this technique, which uses a nasal turbinate speculum to better visualize the median nerve, could potentially reduce the reoperation rate. The nasal turbinate speculum allows the surgeon to see the confluence of the TCL and the DVFF. In addition, as the complete release can be visualized, there is minimal chance of injury.
The 2007 Cochrane review3 found no strong evidence supporting replacing OCTR with endoscopic techniques. Previous investigators have questioned the utility of ECTR given that it is higher in cost and more resource-intensive than OCTR1,33,34 and is associated with higher rates of certain complications.5,22,35-37 A 2004 meta-analysis of 13 randomized, controlled trials found a higher rate of reversible nerve damage with an odds ratio of 3.1 for ECTR versus OCTR.35 A more recent (2006) review of more than 80 studies found transient neurapraxias in 1.45% of ECTR cases and 0.25% of OCTR cases.5 The same study reported overall complication rates (reversible and major neurovascular structural injuries) of 0.74% for OCTR and 1.63% for ECTR (P < .005). Another limitation of ECTR is that endoscopic techniques require a higher degree of surgical skill, which makes teaching residents and fellows more challenging.
The novel nasal turbinate speculum technique presented here is easily reproducible and allows first-time surgeons to visualize all important structures. Given that this technique does not require an endoscope or an endoscope-viewing tower, it is likely more cost-effective and requires less time for turnover between cases. Patients obtain good relief of their CTS symptoms with this technique, and most return to their daily activities within weeks after operation.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
1. Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. Int J Gen Med. 2010;3(4):255-261.
2. Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plast Reconstr Surg. 2000;105(5):1662-1665.
3. Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, de Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;(4):CD003905.
4. In memoriam Sir James Learmonth, K.C.V.O., C.B.E., hon. F.R.C.S. (1895-1967). Ann R Coll Surg Engl. 1967;41(5):438-439.
5. Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919-924, 924.e1-e2.
6. Jarvik JG, Comstock BA, Kliot M, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
7. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;(4):CD001552.
8. Garland H, Langworth EP, Taverner D, et al. Surgical treatment for the carpal tunnel syndrome. Lancet. 1964;1(7343):1129-1130.
9. Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288(10):1245-1251.
10. Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380-383.
11. Sucher BM. Myofascial manipulative release of carpal tunnel syndrome: documentation with magnetic resonance imaging. J Am Osteopath Assoc. 1993;93(12):1273-1278.
12. Pereira EE, Miranda DA, Sere I, et al. Endoscopic release of the carpal tunnel: a 2-portal-modified technique. Tech Hand Up Extrem Surg. 2010;14(4):263-265.
13. Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985;62(3):352-356.
14. Mirza MA, King ET Jr, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1):82-90.
15. Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17(6):1009-1011.
16. Agee JM, McCarroll HR Jr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17(6):987-995.
17. Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11-18.
18. Ghaly RF, Saban KL, Haley DA, et al. Endoscopic carpal tunnel release surgery: report of patient satisfaction. Neurol Res. 2000;22(6):551-555.
19. Lee WP, Plancher KD, Strickland JW. Carpal tunnel release with a small palmar incision. Hand Clin. 1996;12(2):271-284.
20. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334.
21. Brown RA, Gelberman RH, Seiler JG 3rd, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275.
22. Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6(4):288-296.
23. Trumble TE, Diao E, Abrams RA, et al. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107-1115.
24. Gerritsen AA, Uitdehaag BM, van Geldere D, et al. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 2001;88(10):1285-1295.
25. Edgell SE, McCabe SJ, Breidenbach WC, et al. Predicting the outcome of carpal tunnel release. J Hand Surg Am. 2003;28(2):255-261.
26. Vella JC, Hartigan BJ, Stern PJ. Kaplan’s cardinal line. J Hand Surg Am. 2006;31(6):912-918.
27. Kwon JY, Kim JY, Hong JT, et al. Position change of the neurovascular structures around the carpal tunnel with dynamic wrist motion. J Korean Neurosurg Soc. 2011;50(4):377-380.
28. Netscher D, Polsen C, Thornby J, et al. Anatomic delineation of the ulnar nerve and ulnar artery in relation to the carpal tunnel by axial magnetic resonance imaging scanning. J Hand Surg Am. 1996;21(2):273-276.
29. Madhav TJ, To P, Stern PJ. The palmar fat pad is a reliable intraoperative landmark during carpal tunnel release. J Hand Surg Am. 2009;34(7):1204-1209.
30. Kulick MI, Gordillo G, Javidi T, et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11(1):59-66.
31. Bland JD. Treatment of carpal tunnel syndrome. Muscle Nerve. 2007;36(2):167-171.
32. MacDonald RI, Lichtman DM, Hanlon JJ, et al. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am. 1978;3(1):70-76.
33. Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473.
34. Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002;84(3):375-379.
35. Thoma A, Veltri K, Haines T, et al. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg. 2004;114(5):1137-1146.
36. Murphy RX Jr, Jennings JF, Wukich DK. Major neurovascular complications of endoscopic carpal tunnel release. J Hand Surg Am. 1994;19(1):114-118.
37. Palmer DH, Paulson JC, Lane-Larsen CL, et al. Endoscopic carpal tunnel release: a comparison of two techniques with open release. Arthroscopy. 1993;9(5):498-508.
Crisis in Medicine: Part 3. The Physician as the Captain—A Personal Touch
"Report to the Administrator’s Office for a discussion 7:00 am sharp,” reads the email on your phone. The phone log sheet from your administrator is handed to you as you are running to the operating room and reads, “Call back Mr. Smith’s health insurance company because your patient stayed overnight unexpectedly in the hospital, and if the return phone call is not received by 8:40 am the complete hospital stay will be disallowed.” The text message reads, “The head nurse from the emergency department wants to have a discussion with you tomorrow about what transpired in room 23 last night at 1:33 am.” Your physician assistant calls you because a recent history and physical examination from the out-of-state internist has not been cosigned by you, and, therefore, the patient is still in the admitting office; the admitting officer is waiting to go home and won’t accept the physician assistant’s signature.
This simple illustration of a surgeon’s typical morning is hardly hyperbole. Demands and finger-pointing are routine aspects of care, with a concurrent need to attribute blame and create a hostile work environment whether in the office, operating room, or floor of the hospital by anyone who can proudly say to the physician, “Gotcha!” The environment that produces this ethos is toxic and needs to be changed. While all members of a patient care team must be accountable, no member should be antagonistic toward another, and each member must feel a part of a working whole that is led by a competent, caring, and identifiable physician. Yes, the doctor must be the team captain; he or she must take back the reins of care immediately in order to provide the patient with the best possible outcome.
The loss of leadership can be traced back to the rise of regulatory controls put in place by government entities or local hospital administration to contain costs and limit liability. While the target goals of such measures are laudable, the negative impact on the doctor–patient relationship has been palpable and problematic and requires reassessment. The profession itself will be preserved by refocusing on the doctor–patient relationship and returning the physician to the role of team leader. Our patients deserve to feel as though their health care resides in the hands of the physician as the leader of a team that is pursuing a common goal: patient care with minimal distractions.
What, though, makes a great captain or leader? Sociologists have said that in a stable environment a “participatory model” of leadership is appropriate, while in a high-growth or changing environment, like the one in which we presently live, an “authoritative model” can be used to right the ship.1,2 Many types of leaders exist within both models. Leaders who are “innovators” will design and bring new ideas and original thought but may generate too many ideas that can’t be implemented practically in the hospital setting. Leaders who are “developers” will build and move forward to achieve challenging goals but may be impatient when ideas do not work and may be perceived in many interdisciplinary meetings as unruly. “Bureaucratic” leaders, presently seen in many leadership positions, can be classified as stabilizers and, while they may maintain equilibrium and keep things running smoothly, they often insist on a policy for every situation, resulting in stasis and sometimes even paralysis of the surgical center or hospital system.
I believe that health management and patient care require the simultaneous use of the authoritative and participatory models to encourage innovation, set attainable short- and long-term goals, and maintain the physician as the team leader. To lead effectively under this hybrid model, the physician must be accessible and fair, a teacher and a student, and a risk-taker, but, ultimately, at the end of every day, the physician must be accountable.
The time has come for physician leaders to assemble the troops: administrators, clinical providers, and nonclinical support staff. To paraphrase John Quincy Adams, in your actions inspire others to dream more and become more; then, and only then, are you an excellent leader. A secret to effective leadership is in finding one’s voice and acknowledging strengths and weaknesses. The leader must recruit other leaders who are very different from himself or herself and must listen to them deeply and trust them completely. One of our former first ladies said wisely, “A leader takes people where they want to go. A great leader takes people where they don’t necessarily want to go, but ought to be.” To truly find this leadership model, we as busy surgeons must spend some concentrated time away from our patients and exciting research to sit in the room with our nurses, administrators, and all other members of the health care community and listen to their thoughts and understand their concerns. We must understand policy to assess if it is reasonable and, if it is not, to reject it and propose more effective and appropriate rules for good care. We must remove from leadership positions those that do not have the interest of the patient as their primary concern. We must challenge any policy that does not have the patient’s interest and health as its raison d’etre. We must be proactive and not reactive. We must be ready to stand tall and politely question when dictated to unless evidence-based medical reasons can be presented.
You may ask, therefore, where should we lead? The answer is obvious! We need to be involved in every aspect of this great profession. We need to be the leaders of hospital systems, we need to be in charge of research institutions, and, as always, we need to be the chief of the operating room and the chief within each room as the team leader for the nurse, anesthesiologist, and nonclinical staff in order to safely guide our patients through the stress of a medical crisis or routine intervention. We need to find those of us with other degrees, whether MPH, MBA, MHA, or JD, and place those physicians in positions of business and political leadership as well as in leadership positions in hospitals and private practitioner offices. We need to encourage our medical students, residents, and fellows to continue their rigorous training to include an understanding of health care policy and economics so as to help manage and resolve the crisis at hand.
We must now navigate the sea of change to allow for continuity of care and not throw up our arms in despair. The role of physician as private practitioner or as full-time faculty member has its origins deeply imbedded in the roots of our profession, and this traditional role as caretaker and scientist must continue. But in this century, we need to be leaders in the political and business communities as well. This vision requires a new and fresh momentum. We cannot sit idly by as patient care becomes increasingly managed by nonphysicians. The time has come to use our unique position as doctors to frame the debate, participate in the discussion, and lead our profession and the management of health care toward calmer waters with compassion, science, and responsibility. To do this, we must demand transparency, proceed with respect, and require excellence from everyone around us and make sure it is demanded from all of us.◾
1. Morgan G. Developing the art of organizational analysis. In: Morgan G. Images of Organization. Beverly Hills, CA: Sage Publications; 1986:321-337.
2. Cherry KA. Leadership styles. About.com website. http://psychology.about.com/od/leadership/a/leadstyles.htm. Published 2006. Accessed October 20, 2015.
"Report to the Administrator’s Office for a discussion 7:00 am sharp,” reads the email on your phone. The phone log sheet from your administrator is handed to you as you are running to the operating room and reads, “Call back Mr. Smith’s health insurance company because your patient stayed overnight unexpectedly in the hospital, and if the return phone call is not received by 8:40 am the complete hospital stay will be disallowed.” The text message reads, “The head nurse from the emergency department wants to have a discussion with you tomorrow about what transpired in room 23 last night at 1:33 am.” Your physician assistant calls you because a recent history and physical examination from the out-of-state internist has not been cosigned by you, and, therefore, the patient is still in the admitting office; the admitting officer is waiting to go home and won’t accept the physician assistant’s signature.
This simple illustration of a surgeon’s typical morning is hardly hyperbole. Demands and finger-pointing are routine aspects of care, with a concurrent need to attribute blame and create a hostile work environment whether in the office, operating room, or floor of the hospital by anyone who can proudly say to the physician, “Gotcha!” The environment that produces this ethos is toxic and needs to be changed. While all members of a patient care team must be accountable, no member should be antagonistic toward another, and each member must feel a part of a working whole that is led by a competent, caring, and identifiable physician. Yes, the doctor must be the team captain; he or she must take back the reins of care immediately in order to provide the patient with the best possible outcome.
The loss of leadership can be traced back to the rise of regulatory controls put in place by government entities or local hospital administration to contain costs and limit liability. While the target goals of such measures are laudable, the negative impact on the doctor–patient relationship has been palpable and problematic and requires reassessment. The profession itself will be preserved by refocusing on the doctor–patient relationship and returning the physician to the role of team leader. Our patients deserve to feel as though their health care resides in the hands of the physician as the leader of a team that is pursuing a common goal: patient care with minimal distractions.
What, though, makes a great captain or leader? Sociologists have said that in a stable environment a “participatory model” of leadership is appropriate, while in a high-growth or changing environment, like the one in which we presently live, an “authoritative model” can be used to right the ship.1,2 Many types of leaders exist within both models. Leaders who are “innovators” will design and bring new ideas and original thought but may generate too many ideas that can’t be implemented practically in the hospital setting. Leaders who are “developers” will build and move forward to achieve challenging goals but may be impatient when ideas do not work and may be perceived in many interdisciplinary meetings as unruly. “Bureaucratic” leaders, presently seen in many leadership positions, can be classified as stabilizers and, while they may maintain equilibrium and keep things running smoothly, they often insist on a policy for every situation, resulting in stasis and sometimes even paralysis of the surgical center or hospital system.
I believe that health management and patient care require the simultaneous use of the authoritative and participatory models to encourage innovation, set attainable short- and long-term goals, and maintain the physician as the team leader. To lead effectively under this hybrid model, the physician must be accessible and fair, a teacher and a student, and a risk-taker, but, ultimately, at the end of every day, the physician must be accountable.
The time has come for physician leaders to assemble the troops: administrators, clinical providers, and nonclinical support staff. To paraphrase John Quincy Adams, in your actions inspire others to dream more and become more; then, and only then, are you an excellent leader. A secret to effective leadership is in finding one’s voice and acknowledging strengths and weaknesses. The leader must recruit other leaders who are very different from himself or herself and must listen to them deeply and trust them completely. One of our former first ladies said wisely, “A leader takes people where they want to go. A great leader takes people where they don’t necessarily want to go, but ought to be.” To truly find this leadership model, we as busy surgeons must spend some concentrated time away from our patients and exciting research to sit in the room with our nurses, administrators, and all other members of the health care community and listen to their thoughts and understand their concerns. We must understand policy to assess if it is reasonable and, if it is not, to reject it and propose more effective and appropriate rules for good care. We must remove from leadership positions those that do not have the interest of the patient as their primary concern. We must challenge any policy that does not have the patient’s interest and health as its raison d’etre. We must be proactive and not reactive. We must be ready to stand tall and politely question when dictated to unless evidence-based medical reasons can be presented.
You may ask, therefore, where should we lead? The answer is obvious! We need to be involved in every aspect of this great profession. We need to be the leaders of hospital systems, we need to be in charge of research institutions, and, as always, we need to be the chief of the operating room and the chief within each room as the team leader for the nurse, anesthesiologist, and nonclinical staff in order to safely guide our patients through the stress of a medical crisis or routine intervention. We need to find those of us with other degrees, whether MPH, MBA, MHA, or JD, and place those physicians in positions of business and political leadership as well as in leadership positions in hospitals and private practitioner offices. We need to encourage our medical students, residents, and fellows to continue their rigorous training to include an understanding of health care policy and economics so as to help manage and resolve the crisis at hand.
We must now navigate the sea of change to allow for continuity of care and not throw up our arms in despair. The role of physician as private practitioner or as full-time faculty member has its origins deeply imbedded in the roots of our profession, and this traditional role as caretaker and scientist must continue. But in this century, we need to be leaders in the political and business communities as well. This vision requires a new and fresh momentum. We cannot sit idly by as patient care becomes increasingly managed by nonphysicians. The time has come to use our unique position as doctors to frame the debate, participate in the discussion, and lead our profession and the management of health care toward calmer waters with compassion, science, and responsibility. To do this, we must demand transparency, proceed with respect, and require excellence from everyone around us and make sure it is demanded from all of us.◾
"Report to the Administrator’s Office for a discussion 7:00 am sharp,” reads the email on your phone. The phone log sheet from your administrator is handed to you as you are running to the operating room and reads, “Call back Mr. Smith’s health insurance company because your patient stayed overnight unexpectedly in the hospital, and if the return phone call is not received by 8:40 am the complete hospital stay will be disallowed.” The text message reads, “The head nurse from the emergency department wants to have a discussion with you tomorrow about what transpired in room 23 last night at 1:33 am.” Your physician assistant calls you because a recent history and physical examination from the out-of-state internist has not been cosigned by you, and, therefore, the patient is still in the admitting office; the admitting officer is waiting to go home and won’t accept the physician assistant’s signature.
This simple illustration of a surgeon’s typical morning is hardly hyperbole. Demands and finger-pointing are routine aspects of care, with a concurrent need to attribute blame and create a hostile work environment whether in the office, operating room, or floor of the hospital by anyone who can proudly say to the physician, “Gotcha!” The environment that produces this ethos is toxic and needs to be changed. While all members of a patient care team must be accountable, no member should be antagonistic toward another, and each member must feel a part of a working whole that is led by a competent, caring, and identifiable physician. Yes, the doctor must be the team captain; he or she must take back the reins of care immediately in order to provide the patient with the best possible outcome.
The loss of leadership can be traced back to the rise of regulatory controls put in place by government entities or local hospital administration to contain costs and limit liability. While the target goals of such measures are laudable, the negative impact on the doctor–patient relationship has been palpable and problematic and requires reassessment. The profession itself will be preserved by refocusing on the doctor–patient relationship and returning the physician to the role of team leader. Our patients deserve to feel as though their health care resides in the hands of the physician as the leader of a team that is pursuing a common goal: patient care with minimal distractions.
What, though, makes a great captain or leader? Sociologists have said that in a stable environment a “participatory model” of leadership is appropriate, while in a high-growth or changing environment, like the one in which we presently live, an “authoritative model” can be used to right the ship.1,2 Many types of leaders exist within both models. Leaders who are “innovators” will design and bring new ideas and original thought but may generate too many ideas that can’t be implemented practically in the hospital setting. Leaders who are “developers” will build and move forward to achieve challenging goals but may be impatient when ideas do not work and may be perceived in many interdisciplinary meetings as unruly. “Bureaucratic” leaders, presently seen in many leadership positions, can be classified as stabilizers and, while they may maintain equilibrium and keep things running smoothly, they often insist on a policy for every situation, resulting in stasis and sometimes even paralysis of the surgical center or hospital system.
I believe that health management and patient care require the simultaneous use of the authoritative and participatory models to encourage innovation, set attainable short- and long-term goals, and maintain the physician as the team leader. To lead effectively under this hybrid model, the physician must be accessible and fair, a teacher and a student, and a risk-taker, but, ultimately, at the end of every day, the physician must be accountable.
The time has come for physician leaders to assemble the troops: administrators, clinical providers, and nonclinical support staff. To paraphrase John Quincy Adams, in your actions inspire others to dream more and become more; then, and only then, are you an excellent leader. A secret to effective leadership is in finding one’s voice and acknowledging strengths and weaknesses. The leader must recruit other leaders who are very different from himself or herself and must listen to them deeply and trust them completely. One of our former first ladies said wisely, “A leader takes people where they want to go. A great leader takes people where they don’t necessarily want to go, but ought to be.” To truly find this leadership model, we as busy surgeons must spend some concentrated time away from our patients and exciting research to sit in the room with our nurses, administrators, and all other members of the health care community and listen to their thoughts and understand their concerns. We must understand policy to assess if it is reasonable and, if it is not, to reject it and propose more effective and appropriate rules for good care. We must remove from leadership positions those that do not have the interest of the patient as their primary concern. We must challenge any policy that does not have the patient’s interest and health as its raison d’etre. We must be proactive and not reactive. We must be ready to stand tall and politely question when dictated to unless evidence-based medical reasons can be presented.
You may ask, therefore, where should we lead? The answer is obvious! We need to be involved in every aspect of this great profession. We need to be the leaders of hospital systems, we need to be in charge of research institutions, and, as always, we need to be the chief of the operating room and the chief within each room as the team leader for the nurse, anesthesiologist, and nonclinical staff in order to safely guide our patients through the stress of a medical crisis or routine intervention. We need to find those of us with other degrees, whether MPH, MBA, MHA, or JD, and place those physicians in positions of business and political leadership as well as in leadership positions in hospitals and private practitioner offices. We need to encourage our medical students, residents, and fellows to continue their rigorous training to include an understanding of health care policy and economics so as to help manage and resolve the crisis at hand.
We must now navigate the sea of change to allow for continuity of care and not throw up our arms in despair. The role of physician as private practitioner or as full-time faculty member has its origins deeply imbedded in the roots of our profession, and this traditional role as caretaker and scientist must continue. But in this century, we need to be leaders in the political and business communities as well. This vision requires a new and fresh momentum. We cannot sit idly by as patient care becomes increasingly managed by nonphysicians. The time has come to use our unique position as doctors to frame the debate, participate in the discussion, and lead our profession and the management of health care toward calmer waters with compassion, science, and responsibility. To do this, we must demand transparency, proceed with respect, and require excellence from everyone around us and make sure it is demanded from all of us.◾
1. Morgan G. Developing the art of organizational analysis. In: Morgan G. Images of Organization. Beverly Hills, CA: Sage Publications; 1986:321-337.
2. Cherry KA. Leadership styles. About.com website. http://psychology.about.com/od/leadership/a/leadstyles.htm. Published 2006. Accessed October 20, 2015.
1. Morgan G. Developing the art of organizational analysis. In: Morgan G. Images of Organization. Beverly Hills, CA: Sage Publications; 1986:321-337.
2. Cherry KA. Leadership styles. About.com website. http://psychology.about.com/od/leadership/a/leadstyles.htm. Published 2006. Accessed October 20, 2015.