User login
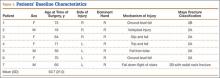
Practice Transition Planning: When Is the Right Time?
If you are a solo orthopedic surgeon or practice in a small group and are 55 years or older, this article is for you. The answer to the question “When is the right time to begin planning for the transition out of practice?” is now. And planning is the most important word in that sentence.
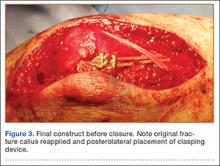
Joining your peers who’ve quit, often rather quickly, because of Obamacare, electronic health records (EHRs), or the implementation of ICD-10 (International Classification of Diseases, Tenth Revision) may prove unsatisfying. As the saying goes, “act in haste, repent at leisure.” And as a gerontologist friend of mine liked to say, “Retiring from medicine without retiring to something is risky.” He often quipped that golf didn’t count.
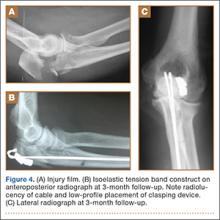
Recent survey results help support his point. In the 2014 Medscape Orthopedist Compensation Report,1 respondents were asked:
What is the most rewarding part of your job?
- Gratitude/relationships with patients 43%
- Being very good at what I do/Finding answers, diagnoses 31%
- Making good money at a job that I like 10%
- Knowing that I’m making the world a better place 7%
- Being proud of being a doctor 4%
- Nothing 1%
- Let’s hope you are not part of the 1%!
In the often-quoted Deloitte 2013 Survey of U.S. Physicians,2 6 in 10 respondents predicted that many physicians will retire earlier than planned in the next 1 to 3 years. Yet even in that survey, 41% of surgical specialists said that patient relationships were the most satisfying factor about practicing medicine. Protecting and promoting individual health was second (33%), and intellectual stimulation was third (16%).2
As Steve Marsh, managing partner at The Medicus Firm, Dallas, was quoted as saying about this data, “For older doctors, being a physician is much more of a lifestyle than a job.”3 In my 40 years of working with physicians, I agree. And that’s why you, dear readers, must begin the transition planning process now, if you are 55 years or older, or soon, if you are approaching this age. Unraveling yourself from the patient relationships and the profession you have enjoyed for so long will feel like a big loss for the majority of you. There will be a grieving process. You’re not just leaving a “job,” you’re leaving your “life’s work,” and the sooner you begin planning for this shift, the less uncomfortable it will be.
Transition Planning Timeline
As the Chinese saying goes, “the longest journey begins with a single step.” The first, most challenging step in transition planning is deciding to address the issue head on—whether you see yourself practicing well into your late 60s or stepping aside 3 years from now.
Here are 7 questions to get you started. Discuss them with your spouse and a trusted advisor or mentor.
1. Have you done everything that you wanted to accomplish professionally? What’s left on your “to-do” list?
2. Are you satisfied with the legacy you are leaving to your community, partners, or employees?
3. What does your spouse think? His or her age and stage may dictate some choices. One wife said she believed in Henny Youngman’s advice: “Promise to take your wife for better or worse, but not for lunch.” Younger spouses in satisfying careers may not be ready to quit or slow down.
4. What could fill the void of, as the Medscape survey indicated, the “gratitude/patient relationships” and “being good at what you do” that you would be leaving behind? Could going on medical missions satisfy your need to keep your hands in? Or volunteering for the community clinic?
5. If you were to retire within the next 6 months, what would your routine look like? Because the first year is often filled with travel and long-postponed fun, think beyond that and describe year 3. (Assume good health and adequate finances.)
6. Are there options for part-time practice? Could you ease out instead of going basically full throttle until your retirement date?
7. Are challenges such as stress, fatigue, cognitive decline, or a feeling of burnout a reality for you? Be honest with yourself. These are real issues that not only impact your decision about when to transition, but also patient safety and care.
If you’ve reached 60 years of age and haven’t thought about questions like these, you aren’t alone. Many orthopedic surgeons delay this planning exercise for the same reasons other business owners do:
You are too busy spending all of your time putting out fires. Who has time to plan? Learning the new ICD-10 codes for local coverage determinations (LCDs), hiring a new physician assistant, firing the receptionist, and, oh by the way, taking care of a full schedule of patients, takes time and reduces the time to plan.
You think “it’s not time yet.” We often hear surgeons say, “Gosh, I don’t feel __ years old!” or “I plan to work until I’m 70.” Sound familiar?
You’re afraid to think about what life would be like without your profession. So you do nothing. Imagining a life without being needed on a daily basis can be daunting. Reread the survey results above. If you don’t have interesting and emotionally rewarding activities that will fill the void, that can cause anxiety. And the fact is, the demands on physicians, especially those in solo practice, haven’t left much time for outside interests.
Discussing personal goals and financial matters with others is messy or taboo. Transitioning out of practice is an awkward and uncomfortable topic. Plus, whom do you call for help with planning the next stage of your life?
These and others on a long list of excuses and anxieties result in fewer than 70% of all surgical specialists we talk with having a viable transition plan. Many, of course, have done a superb job of funding their retirement plans and have the assets set aside to fund a comfortable lifestyle. A lot has been written on the financial aspects of retirement. Your financial advisor, broker, or banker has formulas, tools, and advice that you’ve probably been following for decades. The 2014 Medscape Orthopedist Compensation Report shows the average salary is $413,000, with private practice doctors earning even more, $439,000 on average.1 Although such salaries should ensure the funding of retirement savings plans, undeniably, the financial crisis and stock market collapse of 2008 delayed many surgeons’ retirement. Even today, some surgeons who are considering their practice finish line are looking over their shoulder at market returns with a sense of insecurity.
Recruitment Is More Likely Than Cash Out
Thinking you can sell your practice for big bucks is a false hope. In the 1970s and early 1980s, before the onslaught of managed care, it was possible to sell your practice. A young surgeon would welcome having space, staff, and patients at the ready. This is no longer the case, since patient loyalty is now impacted by health insurance plan membership.
Pocketing a hefty sum from selling the office building may not be much of a windfall either. It depends on that all-important real estate formula: location, location, location. In addition, dividends from and investment in a surgery center rarely continue once you are no longer operating.
To maximize the profit potential that remains in this last phase of practice—which in turn can attract surgical talent as you transition—you’ve got to sharpen the sword and pay attention. One surgeon attributed a revenue decline of about 30% over the last 5 years to a combination of lesser insurance reimbursements, his taking more time off, and failing to pay attention to his staff’s write-off habits. Revenue cycle, management, coding, and practice operations must be finely tuned to optimize profitability, and failing to manage your practice effectively will make it less attractive when recruiting a younger surgeon to take your place or assume the patient base. Consider a practice evaluation regardless of where you are in your planning, which will help the practice prioritize improvements that deliver the best benefit and value within the context of your transition plan.
And if recruitment is part of that plan, be prepared to spend significant time on the search. Solo and small groups are finding it challenging to recruit just-out-of-training associates. This generation of new physicians values work-life balance and is more likely to prefer employment to entrepreneurship. Additionally, established physicians who have not invested in or adopted new technologies, such as EHR, will have a tough time attracting top talent. Having been trained using EHRs, few, if any, young doctors will find a reversion to paper records acceptable—and, in fact, most find it a turnoff. Thus, depending on your transition plan and your age and stage, updating technology may be a necessary investment.
Stepping Down But Not Out
If you’re thinking about slowing down but not ceasing practice completely just yet, 2 options are worth considering: practicing part-time and/or becoming a nonoperative orthopedist.
The 2014 Orthopaedic Practice in the United States (OPUS) report issued by the American Academy of Orthopaedic Surgeons shows that the average age of part-time surgeons is 69.14 years and that 48.6% are generalists.5 Part-time surgeons surveyed reported working an average of 23 hours per week and performing 5 procedures per month, compared with full-time surgeons who clock in at 56 hours per week and perform 31 procedures per month.5
Senior surgeons who want to pull back their hours or become nonoperative orthopedists may be quite marketable to group practices. There are several reasons for this. First, population growth will not be supported by the number of physicians graduating from Medicare-sponsored residency slots—which have not increased since 1997. Second, the physician workforce is growing older, and younger surgeons are harder to recruit. They tend to emphasize work-life balance over working the countless hours their senior counterparts did, and, thus, don’t treat as many patients as older colleagues did. And, third, a nonoperative or part-time physician may be more appealing to patients than nonphysician providers, yet accomplish the same purpose of keeping operating surgeons out of the office and in the operating room. So, that former competitor down the street may become a potential employer. You won’t be a voting partner, but that may be a low priority as you step into part-time practice.
We imagine an opportunity for nonoperative orthopedists similar to concierge internists, who go out of network and charge reasonable fees for longer appointments and less paperwork hassle. And this opportunity isn’t only for those practicing in groups. Solo orthopedists may see this change in practice attractive, as it offers reduced professional liability premiums, holds some clear attraction for patients not eager to go under the knife, and makes it easier to arrange time off for the doctor.
As I often tell clients about their business: “Plan your work, and work your plan.” This same maxim holds true of planning for retirement. The intangible aspects of leaving your livelihood require thought and contemplation. My hope is that you’ll put pen to paper and document the answers to the questions posed in this article, so they begin to become as important as the financial aspects of your retirement planning. Of course, the plan may be waylaid midstream owing to reimbursement challenges, an offer you can’t refuse from the hospital, or a change in your health or that of your spouse. However, taking that single step and starting your plan will give you the foundation necessary to move forward or pivot in the journey ahead.
1. Peckham C. Medscape Orthopedist Compensation Report 2014. Medscape website. http://www.medscape.com/features/slideshow/compensation/2014/orthopedics#1. Published April 15, 2014. Accessed October 29, 2015.
2. Deloitte 2013 Survey of U.S. Physicians: Physician Perspectives About Health Care Reform and the Future of the Medical Profession. Deloitte Center for Health Solutions website. http://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-deloitte-2013-physician-survey-10012014.pdf. Accessed October 29, 2015.
3. Hyden M. Are physicians retiring early? MGMA Connection Plus. Medical Group Management Association website. http://www.mgma.com/practice-resources/mgma-connection-plus/online-only/2015/august-(1)/are-physicians-retiring-early. Published July 28, 2015. Accessed November 10, 2015.
4. The Doctor is Out: A Physician’s Guide to Closing a Practice. North Carolina Medical Board website. http://www.ncmedboard.org/images/uploads/article_images/Physicians_Guide_to_Closing_a_Practice_05_12_2014.pdf. Published May 12, 2014. Accessed October 29, 2015.
5. Oreluk H, Cherf J. Practice settings and productivity. AAOS Now. 2015;9(10). http://www.aaos.org/news/aaosnow/oct15/managing1.asp. Accessed October 29, 2015.
If you are a solo orthopedic surgeon or practice in a small group and are 55 years or older, this article is for you. The answer to the question “When is the right time to begin planning for the transition out of practice?” is now. And planning is the most important word in that sentence.
Joining your peers who’ve quit, often rather quickly, because of Obamacare, electronic health records (EHRs), or the implementation of ICD-10 (International Classification of Diseases, Tenth Revision) may prove unsatisfying. As the saying goes, “act in haste, repent at leisure.” And as a gerontologist friend of mine liked to say, “Retiring from medicine without retiring to something is risky.” He often quipped that golf didn’t count.
Recent survey results help support his point. In the 2014 Medscape Orthopedist Compensation Report,1 respondents were asked:
What is the most rewarding part of your job?
- Gratitude/relationships with patients 43%
- Being very good at what I do/Finding answers, diagnoses 31%
- Making good money at a job that I like 10%
- Knowing that I’m making the world a better place 7%
- Being proud of being a doctor 4%
- Nothing 1%
- Let’s hope you are not part of the 1%!
In the often-quoted Deloitte 2013 Survey of U.S. Physicians,2 6 in 10 respondents predicted that many physicians will retire earlier than planned in the next 1 to 3 years. Yet even in that survey, 41% of surgical specialists said that patient relationships were the most satisfying factor about practicing medicine. Protecting and promoting individual health was second (33%), and intellectual stimulation was third (16%).2
As Steve Marsh, managing partner at The Medicus Firm, Dallas, was quoted as saying about this data, “For older doctors, being a physician is much more of a lifestyle than a job.”3 In my 40 years of working with physicians, I agree. And that’s why you, dear readers, must begin the transition planning process now, if you are 55 years or older, or soon, if you are approaching this age. Unraveling yourself from the patient relationships and the profession you have enjoyed for so long will feel like a big loss for the majority of you. There will be a grieving process. You’re not just leaving a “job,” you’re leaving your “life’s work,” and the sooner you begin planning for this shift, the less uncomfortable it will be.
Transition Planning Timeline
As the Chinese saying goes, “the longest journey begins with a single step.” The first, most challenging step in transition planning is deciding to address the issue head on—whether you see yourself practicing well into your late 60s or stepping aside 3 years from now.
Here are 7 questions to get you started. Discuss them with your spouse and a trusted advisor or mentor.
1. Have you done everything that you wanted to accomplish professionally? What’s left on your “to-do” list?
2. Are you satisfied with the legacy you are leaving to your community, partners, or employees?
3. What does your spouse think? His or her age and stage may dictate some choices. One wife said she believed in Henny Youngman’s advice: “Promise to take your wife for better or worse, but not for lunch.” Younger spouses in satisfying careers may not be ready to quit or slow down.
4. What could fill the void of, as the Medscape survey indicated, the “gratitude/patient relationships” and “being good at what you do” that you would be leaving behind? Could going on medical missions satisfy your need to keep your hands in? Or volunteering for the community clinic?
5. If you were to retire within the next 6 months, what would your routine look like? Because the first year is often filled with travel and long-postponed fun, think beyond that and describe year 3. (Assume good health and adequate finances.)
6. Are there options for part-time practice? Could you ease out instead of going basically full throttle until your retirement date?
7. Are challenges such as stress, fatigue, cognitive decline, or a feeling of burnout a reality for you? Be honest with yourself. These are real issues that not only impact your decision about when to transition, but also patient safety and care.
If you’ve reached 60 years of age and haven’t thought about questions like these, you aren’t alone. Many orthopedic surgeons delay this planning exercise for the same reasons other business owners do:
You are too busy spending all of your time putting out fires. Who has time to plan? Learning the new ICD-10 codes for local coverage determinations (LCDs), hiring a new physician assistant, firing the receptionist, and, oh by the way, taking care of a full schedule of patients, takes time and reduces the time to plan.
You think “it’s not time yet.” We often hear surgeons say, “Gosh, I don’t feel __ years old!” or “I plan to work until I’m 70.” Sound familiar?
You’re afraid to think about what life would be like without your profession. So you do nothing. Imagining a life without being needed on a daily basis can be daunting. Reread the survey results above. If you don’t have interesting and emotionally rewarding activities that will fill the void, that can cause anxiety. And the fact is, the demands on physicians, especially those in solo practice, haven’t left much time for outside interests.
Discussing personal goals and financial matters with others is messy or taboo. Transitioning out of practice is an awkward and uncomfortable topic. Plus, whom do you call for help with planning the next stage of your life?
These and others on a long list of excuses and anxieties result in fewer than 70% of all surgical specialists we talk with having a viable transition plan. Many, of course, have done a superb job of funding their retirement plans and have the assets set aside to fund a comfortable lifestyle. A lot has been written on the financial aspects of retirement. Your financial advisor, broker, or banker has formulas, tools, and advice that you’ve probably been following for decades. The 2014 Medscape Orthopedist Compensation Report shows the average salary is $413,000, with private practice doctors earning even more, $439,000 on average.1 Although such salaries should ensure the funding of retirement savings plans, undeniably, the financial crisis and stock market collapse of 2008 delayed many surgeons’ retirement. Even today, some surgeons who are considering their practice finish line are looking over their shoulder at market returns with a sense of insecurity.
Recruitment Is More Likely Than Cash Out
Thinking you can sell your practice for big bucks is a false hope. In the 1970s and early 1980s, before the onslaught of managed care, it was possible to sell your practice. A young surgeon would welcome having space, staff, and patients at the ready. This is no longer the case, since patient loyalty is now impacted by health insurance plan membership.
Pocketing a hefty sum from selling the office building may not be much of a windfall either. It depends on that all-important real estate formula: location, location, location. In addition, dividends from and investment in a surgery center rarely continue once you are no longer operating.
To maximize the profit potential that remains in this last phase of practice—which in turn can attract surgical talent as you transition—you’ve got to sharpen the sword and pay attention. One surgeon attributed a revenue decline of about 30% over the last 5 years to a combination of lesser insurance reimbursements, his taking more time off, and failing to pay attention to his staff’s write-off habits. Revenue cycle, management, coding, and practice operations must be finely tuned to optimize profitability, and failing to manage your practice effectively will make it less attractive when recruiting a younger surgeon to take your place or assume the patient base. Consider a practice evaluation regardless of where you are in your planning, which will help the practice prioritize improvements that deliver the best benefit and value within the context of your transition plan.
And if recruitment is part of that plan, be prepared to spend significant time on the search. Solo and small groups are finding it challenging to recruit just-out-of-training associates. This generation of new physicians values work-life balance and is more likely to prefer employment to entrepreneurship. Additionally, established physicians who have not invested in or adopted new technologies, such as EHR, will have a tough time attracting top talent. Having been trained using EHRs, few, if any, young doctors will find a reversion to paper records acceptable—and, in fact, most find it a turnoff. Thus, depending on your transition plan and your age and stage, updating technology may be a necessary investment.
Stepping Down But Not Out
If you’re thinking about slowing down but not ceasing practice completely just yet, 2 options are worth considering: practicing part-time and/or becoming a nonoperative orthopedist.
The 2014 Orthopaedic Practice in the United States (OPUS) report issued by the American Academy of Orthopaedic Surgeons shows that the average age of part-time surgeons is 69.14 years and that 48.6% are generalists.5 Part-time surgeons surveyed reported working an average of 23 hours per week and performing 5 procedures per month, compared with full-time surgeons who clock in at 56 hours per week and perform 31 procedures per month.5
Senior surgeons who want to pull back their hours or become nonoperative orthopedists may be quite marketable to group practices. There are several reasons for this. First, population growth will not be supported by the number of physicians graduating from Medicare-sponsored residency slots—which have not increased since 1997. Second, the physician workforce is growing older, and younger surgeons are harder to recruit. They tend to emphasize work-life balance over working the countless hours their senior counterparts did, and, thus, don’t treat as many patients as older colleagues did. And, third, a nonoperative or part-time physician may be more appealing to patients than nonphysician providers, yet accomplish the same purpose of keeping operating surgeons out of the office and in the operating room. So, that former competitor down the street may become a potential employer. You won’t be a voting partner, but that may be a low priority as you step into part-time practice.
We imagine an opportunity for nonoperative orthopedists similar to concierge internists, who go out of network and charge reasonable fees for longer appointments and less paperwork hassle. And this opportunity isn’t only for those practicing in groups. Solo orthopedists may see this change in practice attractive, as it offers reduced professional liability premiums, holds some clear attraction for patients not eager to go under the knife, and makes it easier to arrange time off for the doctor.
As I often tell clients about their business: “Plan your work, and work your plan.” This same maxim holds true of planning for retirement. The intangible aspects of leaving your livelihood require thought and contemplation. My hope is that you’ll put pen to paper and document the answers to the questions posed in this article, so they begin to become as important as the financial aspects of your retirement planning. Of course, the plan may be waylaid midstream owing to reimbursement challenges, an offer you can’t refuse from the hospital, or a change in your health or that of your spouse. However, taking that single step and starting your plan will give you the foundation necessary to move forward or pivot in the journey ahead.
If you are a solo orthopedic surgeon or practice in a small group and are 55 years or older, this article is for you. The answer to the question “When is the right time to begin planning for the transition out of practice?” is now. And planning is the most important word in that sentence.
Joining your peers who’ve quit, often rather quickly, because of Obamacare, electronic health records (EHRs), or the implementation of ICD-10 (International Classification of Diseases, Tenth Revision) may prove unsatisfying. As the saying goes, “act in haste, repent at leisure.” And as a gerontologist friend of mine liked to say, “Retiring from medicine without retiring to something is risky.” He often quipped that golf didn’t count.
Recent survey results help support his point. In the 2014 Medscape Orthopedist Compensation Report,1 respondents were asked:
What is the most rewarding part of your job?
- Gratitude/relationships with patients 43%
- Being very good at what I do/Finding answers, diagnoses 31%
- Making good money at a job that I like 10%
- Knowing that I’m making the world a better place 7%
- Being proud of being a doctor 4%
- Nothing 1%
- Let’s hope you are not part of the 1%!
In the often-quoted Deloitte 2013 Survey of U.S. Physicians,2 6 in 10 respondents predicted that many physicians will retire earlier than planned in the next 1 to 3 years. Yet even in that survey, 41% of surgical specialists said that patient relationships were the most satisfying factor about practicing medicine. Protecting and promoting individual health was second (33%), and intellectual stimulation was third (16%).2
As Steve Marsh, managing partner at The Medicus Firm, Dallas, was quoted as saying about this data, “For older doctors, being a physician is much more of a lifestyle than a job.”3 In my 40 years of working with physicians, I agree. And that’s why you, dear readers, must begin the transition planning process now, if you are 55 years or older, or soon, if you are approaching this age. Unraveling yourself from the patient relationships and the profession you have enjoyed for so long will feel like a big loss for the majority of you. There will be a grieving process. You’re not just leaving a “job,” you’re leaving your “life’s work,” and the sooner you begin planning for this shift, the less uncomfortable it will be.
Transition Planning Timeline
As the Chinese saying goes, “the longest journey begins with a single step.” The first, most challenging step in transition planning is deciding to address the issue head on—whether you see yourself practicing well into your late 60s or stepping aside 3 years from now.
Here are 7 questions to get you started. Discuss them with your spouse and a trusted advisor or mentor.
1. Have you done everything that you wanted to accomplish professionally? What’s left on your “to-do” list?
2. Are you satisfied with the legacy you are leaving to your community, partners, or employees?
3. What does your spouse think? His or her age and stage may dictate some choices. One wife said she believed in Henny Youngman’s advice: “Promise to take your wife for better or worse, but not for lunch.” Younger spouses in satisfying careers may not be ready to quit or slow down.
4. What could fill the void of, as the Medscape survey indicated, the “gratitude/patient relationships” and “being good at what you do” that you would be leaving behind? Could going on medical missions satisfy your need to keep your hands in? Or volunteering for the community clinic?
5. If you were to retire within the next 6 months, what would your routine look like? Because the first year is often filled with travel and long-postponed fun, think beyond that and describe year 3. (Assume good health and adequate finances.)
6. Are there options for part-time practice? Could you ease out instead of going basically full throttle until your retirement date?
7. Are challenges such as stress, fatigue, cognitive decline, or a feeling of burnout a reality for you? Be honest with yourself. These are real issues that not only impact your decision about when to transition, but also patient safety and care.
If you’ve reached 60 years of age and haven’t thought about questions like these, you aren’t alone. Many orthopedic surgeons delay this planning exercise for the same reasons other business owners do:
You are too busy spending all of your time putting out fires. Who has time to plan? Learning the new ICD-10 codes for local coverage determinations (LCDs), hiring a new physician assistant, firing the receptionist, and, oh by the way, taking care of a full schedule of patients, takes time and reduces the time to plan.
You think “it’s not time yet.” We often hear surgeons say, “Gosh, I don’t feel __ years old!” or “I plan to work until I’m 70.” Sound familiar?
You’re afraid to think about what life would be like without your profession. So you do nothing. Imagining a life without being needed on a daily basis can be daunting. Reread the survey results above. If you don’t have interesting and emotionally rewarding activities that will fill the void, that can cause anxiety. And the fact is, the demands on physicians, especially those in solo practice, haven’t left much time for outside interests.
Discussing personal goals and financial matters with others is messy or taboo. Transitioning out of practice is an awkward and uncomfortable topic. Plus, whom do you call for help with planning the next stage of your life?
These and others on a long list of excuses and anxieties result in fewer than 70% of all surgical specialists we talk with having a viable transition plan. Many, of course, have done a superb job of funding their retirement plans and have the assets set aside to fund a comfortable lifestyle. A lot has been written on the financial aspects of retirement. Your financial advisor, broker, or banker has formulas, tools, and advice that you’ve probably been following for decades. The 2014 Medscape Orthopedist Compensation Report shows the average salary is $413,000, with private practice doctors earning even more, $439,000 on average.1 Although such salaries should ensure the funding of retirement savings plans, undeniably, the financial crisis and stock market collapse of 2008 delayed many surgeons’ retirement. Even today, some surgeons who are considering their practice finish line are looking over their shoulder at market returns with a sense of insecurity.
Recruitment Is More Likely Than Cash Out
Thinking you can sell your practice for big bucks is a false hope. In the 1970s and early 1980s, before the onslaught of managed care, it was possible to sell your practice. A young surgeon would welcome having space, staff, and patients at the ready. This is no longer the case, since patient loyalty is now impacted by health insurance plan membership.
Pocketing a hefty sum from selling the office building may not be much of a windfall either. It depends on that all-important real estate formula: location, location, location. In addition, dividends from and investment in a surgery center rarely continue once you are no longer operating.
To maximize the profit potential that remains in this last phase of practice—which in turn can attract surgical talent as you transition—you’ve got to sharpen the sword and pay attention. One surgeon attributed a revenue decline of about 30% over the last 5 years to a combination of lesser insurance reimbursements, his taking more time off, and failing to pay attention to his staff’s write-off habits. Revenue cycle, management, coding, and practice operations must be finely tuned to optimize profitability, and failing to manage your practice effectively will make it less attractive when recruiting a younger surgeon to take your place or assume the patient base. Consider a practice evaluation regardless of where you are in your planning, which will help the practice prioritize improvements that deliver the best benefit and value within the context of your transition plan.
And if recruitment is part of that plan, be prepared to spend significant time on the search. Solo and small groups are finding it challenging to recruit just-out-of-training associates. This generation of new physicians values work-life balance and is more likely to prefer employment to entrepreneurship. Additionally, established physicians who have not invested in or adopted new technologies, such as EHR, will have a tough time attracting top talent. Having been trained using EHRs, few, if any, young doctors will find a reversion to paper records acceptable—and, in fact, most find it a turnoff. Thus, depending on your transition plan and your age and stage, updating technology may be a necessary investment.
Stepping Down But Not Out
If you’re thinking about slowing down but not ceasing practice completely just yet, 2 options are worth considering: practicing part-time and/or becoming a nonoperative orthopedist.
The 2014 Orthopaedic Practice in the United States (OPUS) report issued by the American Academy of Orthopaedic Surgeons shows that the average age of part-time surgeons is 69.14 years and that 48.6% are generalists.5 Part-time surgeons surveyed reported working an average of 23 hours per week and performing 5 procedures per month, compared with full-time surgeons who clock in at 56 hours per week and perform 31 procedures per month.5
Senior surgeons who want to pull back their hours or become nonoperative orthopedists may be quite marketable to group practices. There are several reasons for this. First, population growth will not be supported by the number of physicians graduating from Medicare-sponsored residency slots—which have not increased since 1997. Second, the physician workforce is growing older, and younger surgeons are harder to recruit. They tend to emphasize work-life balance over working the countless hours their senior counterparts did, and, thus, don’t treat as many patients as older colleagues did. And, third, a nonoperative or part-time physician may be more appealing to patients than nonphysician providers, yet accomplish the same purpose of keeping operating surgeons out of the office and in the operating room. So, that former competitor down the street may become a potential employer. You won’t be a voting partner, but that may be a low priority as you step into part-time practice.
We imagine an opportunity for nonoperative orthopedists similar to concierge internists, who go out of network and charge reasonable fees for longer appointments and less paperwork hassle. And this opportunity isn’t only for those practicing in groups. Solo orthopedists may see this change in practice attractive, as it offers reduced professional liability premiums, holds some clear attraction for patients not eager to go under the knife, and makes it easier to arrange time off for the doctor.
As I often tell clients about their business: “Plan your work, and work your plan.” This same maxim holds true of planning for retirement. The intangible aspects of leaving your livelihood require thought and contemplation. My hope is that you’ll put pen to paper and document the answers to the questions posed in this article, so they begin to become as important as the financial aspects of your retirement planning. Of course, the plan may be waylaid midstream owing to reimbursement challenges, an offer you can’t refuse from the hospital, or a change in your health or that of your spouse. However, taking that single step and starting your plan will give you the foundation necessary to move forward or pivot in the journey ahead.
1. Peckham C. Medscape Orthopedist Compensation Report 2014. Medscape website. http://www.medscape.com/features/slideshow/compensation/2014/orthopedics#1. Published April 15, 2014. Accessed October 29, 2015.
2. Deloitte 2013 Survey of U.S. Physicians: Physician Perspectives About Health Care Reform and the Future of the Medical Profession. Deloitte Center for Health Solutions website. http://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-deloitte-2013-physician-survey-10012014.pdf. Accessed October 29, 2015.
3. Hyden M. Are physicians retiring early? MGMA Connection Plus. Medical Group Management Association website. http://www.mgma.com/practice-resources/mgma-connection-plus/online-only/2015/august-(1)/are-physicians-retiring-early. Published July 28, 2015. Accessed November 10, 2015.
4. The Doctor is Out: A Physician’s Guide to Closing a Practice. North Carolina Medical Board website. http://www.ncmedboard.org/images/uploads/article_images/Physicians_Guide_to_Closing_a_Practice_05_12_2014.pdf. Published May 12, 2014. Accessed October 29, 2015.
5. Oreluk H, Cherf J. Practice settings and productivity. AAOS Now. 2015;9(10). http://www.aaos.org/news/aaosnow/oct15/managing1.asp. Accessed October 29, 2015.
1. Peckham C. Medscape Orthopedist Compensation Report 2014. Medscape website. http://www.medscape.com/features/slideshow/compensation/2014/orthopedics#1. Published April 15, 2014. Accessed October 29, 2015.
2. Deloitte 2013 Survey of U.S. Physicians: Physician Perspectives About Health Care Reform and the Future of the Medical Profession. Deloitte Center for Health Solutions website. http://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-deloitte-2013-physician-survey-10012014.pdf. Accessed October 29, 2015.
3. Hyden M. Are physicians retiring early? MGMA Connection Plus. Medical Group Management Association website. http://www.mgma.com/practice-resources/mgma-connection-plus/online-only/2015/august-(1)/are-physicians-retiring-early. Published July 28, 2015. Accessed November 10, 2015.
4. The Doctor is Out: A Physician’s Guide to Closing a Practice. North Carolina Medical Board website. http://www.ncmedboard.org/images/uploads/article_images/Physicians_Guide_to_Closing_a_Practice_05_12_2014.pdf. Published May 12, 2014. Accessed October 29, 2015.
5. Oreluk H, Cherf J. Practice settings and productivity. AAOS Now. 2015;9(10). http://www.aaos.org/news/aaosnow/oct15/managing1.asp. Accessed October 29, 2015.
Magnetic Resonance Imaging of Complications of Anterior Cruciate Ligament Reconstruction
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
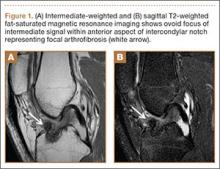
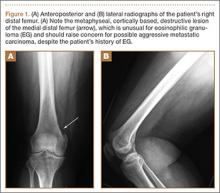
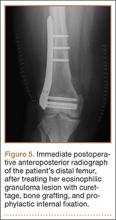
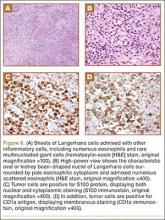
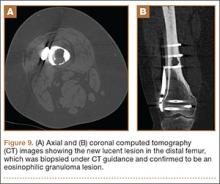
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
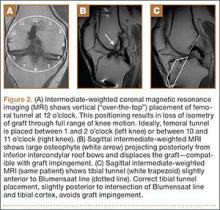
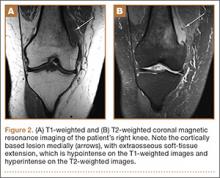
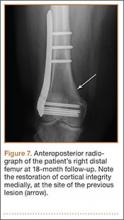
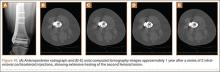
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
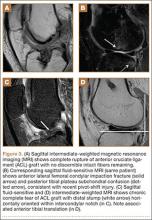
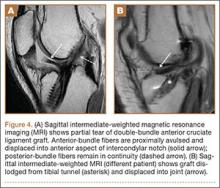
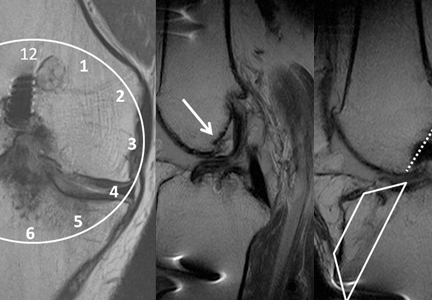
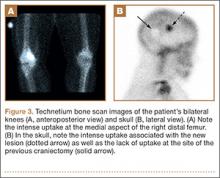
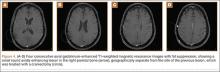
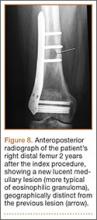
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
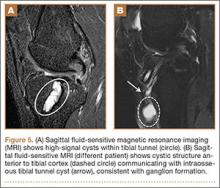
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
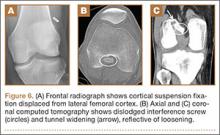
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
Magnetic resonance imaging (MRI) is the preferred modality in the evaluation of complications of anterior cruciate ligament reconstruction (ACL-R).1-3 ACL-R complications may be broadly characterized as those resulting in decreased range of motion (ROM), eg, arthrofibrosis and impingement, and those resulting in increased laxity, ie, graft disruption.4 Short tau inversion recovery (STIR) sequences best minimize artifact related to field inhomogeneity in the presence of metal-containing fixation devices. Patients with contraindications to MRI may undergo high-resolution computed tomographic arthrography of the knee for evaluation of postoperative graft abnormalities.1
Arthrofibrosis refers to focal or diffuse synovial scar tissue, which may limit ROM. Preoperative irritation, preoperative limited ROM, and reconstruction within 4 weeks of trauma may all play a role in the development of arthrofibrosis.5,6 The focal form, cyclops lesion, named for its arthroscopic appearance, has been reported in 1% to 10% of patients with ACL-R.1 On MRI, focal arthrofibrosis may be seen as a focal or diffuse intermediate signal lesion in the anterior intercondylar notch extending linearly along the intercondylar roof1 (Figure 1).
MRI can be used to accurately determine the position of the femoral and tibial tunnels. Correct femoral tunnel position results in isometry of the graft during full ROM of the knee. Graft impingement can occur when the tibial tunnel is placed too far anteriorly such that the graft contacts the roof of the intercondylar notch before the knee is able to fully extend.7 A tibial tunnel placed anterior to the intersection of the Blumensaat line and the tibia is at higher risk for impingement.1,4 Impingement may be accompanied by signal change in the graft on intermediate-weighted and fluid-sensitive sequences. The signal abnormality is usually focal and persists longer than the expected signal changes related to revascularization of immature grafts within the first year (Figure 2). If left untreated, impingement may progress to graft rupture.4
Complete graft rupture is diagnosed on the basis of discontinuity of the graft fibers. MRI findings include fluid-filled defect or absence of intact graft fibers. Other reliable signs include large joint effusion, anterior tibial translation, pivot-shift–type marrow edema pattern, and horizontal orientation, laxity, or resorption of the graft fibers.1,8,9 The diagnosis of partial graft rupture may be challenging, as there are several other causes of increased graft signal, including revascularization (within 12 months after procedure), signal heterogeneity between individual bundles of hamstring grafts, and focal signal changes related to impingment (Figures 3, 4).
Fluid within the tunnels is a normal finding after surgery and typically resolves within the first 18 months.1 Cyst formation within the tibial tunnel is an uncommon complication of ACL-R and may be incidental to or present with clinical symptoms caused by extension into the pretibial soft tissues or expansion of the tunnel (Figure 5). Communication of cyst with joint space is important, as a noncommunicating cyst requires simple excision without need for bone grafting.7
Hardware-related complications (eg, loosening of fixation devices) are uncommon but may require revision surgery (Figure 6). Septic arthritis after ACL-R has a cumulative incidence of 0.1% to 0.9% and may be difficult to diagnose clinically because of the lack of classic symptoms of a septic joint.1 Diagnosis requires joint aspiration.
MRI is reliably and accurately used to assess ACL-R complications. The clinical history helps in stratifying complications that result in decreased ROM or increased laxity.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
1. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115-2126.
2. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22(4):765-774.
3. Papakonstantinou O, Chung CB, Chanchairujira K, Resnick DL. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13(5):1106-1117.
4. Meyers AB, Haims AH, Menn K, Moukaddam H. Imaging of anterior cruciate ligament repair and its complications. AJR Am J Roentgenol. 2010;194(2):476-484.
5. Kwok CS, Harrison T, Servant C. The optimal timing for anterior cruciate ligament reconstruction with respect to the risk of postoperative stiffness. Arthroscopy. 2013;29(3):556-565.
6. Mayr HO, Weig TG, Plitz W. Arthrofibrosis following ACL reconstruction—reasons and outcome. Arch Orthop Trauma Surg. 2004;124(8):518-522.
7. Ghazikhanian V, Beltran J, Nikac V, Feldman M, Bencardino JT. Tibial tunnel and pretibial cysts following ACL graft reconstruction: MR imaging diagnosis. Skeletal Radiol. 2012;41(11):1375-1379.
8. Collins MS, Unruh KP, Bond JR, Mandrekar JN. Magnetic resonance imaging of surgically confirmed anterior cruciate ligament graft disruption. Skeletal Radiol. 2008;37(3):233-243.
9. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249(2):581-590.
Orthopedic Implant Waste: Analysis and Quantification
The cost of health care in the United States is increasing at an unsustainable rate.1-3 To decrease or even reverse this trend, we must decrease the cost of care without adversely affecting quality. Porter4 defined value as the quality of care divided by its cost. The economics of total joint arthroplasty (TJA) has received a great deal of attention because of both increasing demand and increasing cost.5-9 About 33% of all orthopedic surgeries and the majority of TJAs are paid for by Medicare.9 In recent years, the rate of reimbursement for orthopedic cases has steadily declined while the cost of implants has increased.3,10,11 Given the significant cost of implants, health care providers in some subspecialties have focused on implant costs as a potential area for cost reduction.12 For example, in TJA this has proved effective in reducing the overall cost, as has decreasing length of stay after surgery.8,10,13-16
With little evidence suggesting any specific orthopedic implant has outcomes superior to those of others, with the exception of select poorly performing outliers, we must increase value of care by lowering the cost when considering these devices.17,18 In addition, some experts have suggested that intraoperative waste is a significant factor in TJA cost, and it does contribute to the average implant cost for a TJA case.6,19 Using data collected from 72 institutions, Zywiel and colleagues19 estimated the annual cost of wasted hip and knee arthroplasty implants to be more than $36 million in the United States.
However, considering the aging US population, TJA is not the only orthopedic surgery with increased demand. An estimated 600,000 spine surgeries are performed each year in the United States.20 Between 1992 and 2003, Medicare spending for lumbar spinal fusion increased 500%.21 In addition, in a 15-month observational study of incidence of intraoperative waste in spine surgery, Soroceanu and colleagues22 reported waste occurring in 20% of spine procedures.
Although these studies have described implant waste in TJA and spine surgeries, little has been published on the cost of wasted implants in a center performing the full range of orthopedic procedures. In this article, we detail the implant waste costs incurred by surgeons for all orthopedic subspecialties at a single orthopedic specialty hospital over a 1-year period. Our study goals were to identify types of implants wasted, and incidence and cost of implant waste, for all total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and lumbar spinal fusions performed at the hospital and to determine whether case volume or years in surgical practice affect the rate or cost of implants wasted.
Methods
We performed a retrospective economic analysis of 1 year of administrative implant data from our institution. Collected data were quantified and analyzed for factors that might explain any variance in implant waste among surgeons. We were granted exempt institutional review board status, as no patient information was involved in this study.
We reviewed the administrative implant data for the 12-month period beginning June 2012 and ending May 2013. For that period, number of cases in which an implant was used and number of cases in which an implant was wasted were recorded. For each instance of waste, type and cost of the wasted implant were entered into the administrative database. In addition, overall cost of implants for the year and cost of wasted implants were determined. Data were available for 81 surgeons across 8 orthopedic divisions (subspecialties). From this information, we determined percentage of cases in which waste occurred, percentage of total implant cost wasted, average cost of waste per case, and most commonly wasted implants. All 3 variables were also calculated for THAs, TKAs, and lumbar spinal fusion procedures.
Statistical Analysis
The data were analyzed to determine if surgeon case volume or years in surgical practice affected implant waste. All analyses were performed at department, division (subspecialty), and surgeon levels. Case volume was analyzed in 3 groups: top 25%, middle 50%, and lower 25%. Number of years in surgical practice was analyzed in 3 groups: fewer than 10 years, 10 to 19 years, and 20 years or more. Normality assumption of variables was tested using the Shapiro-Wilk test (P < .05). For between-group differences, 1-way analysis of variance and the Tukey honestly significant difference post hoc test were performed for variables with a normal distribution, and the Kruskal-Wallis and Mann-Whitney tests were performed for variables without a normal distribution.
For the subspecialty-level analyses, only the Adult Reconstruction, Sports Medicine, and Spine divisions were analyzed for the effects of volume, and only the Sports Medicine and Spine divisions were analyzed for the effect of surgical experience, as surgeon numbers were insufficient for adequate grouping(s).
Data are presented as means with corresponding 95% confidence intervals (CIs). Categorical variables are presented as counts with percentages. All statistical analyses were performed with SPSS Version 21.0 (IBM SPSS) statistical software. Statistical significance was set at .05.
Results
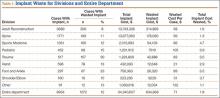
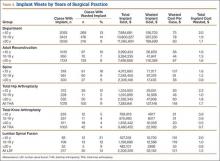
During the 1-year period, 8954 department cases involved an implant of any type. Waste occurred in 12% (1072) of these cases. The rate ranged from 8% in the Adult Reconstruction division to 30% in the Trauma division (Table 1), and the rate for individual surgeons ranged from 3% to 100%, though the surgeon with 100% performed only 1 case, and the next highest rate was 50%.
Total implant cost for our hospital during the period was $34,340,607. Of that total cost, 1.8% ($634,668) was lost because of implant waste. Percentage of total implant cost wasted ranged from 1.6% in the Adult Reconstruction division to 4.7% in the Sports Medicine division (Table 1). Percentage of total implant cost wasted for individual surgeons ranged from 0.2% to 16.1%. Tables 2 and 3 list the most commonly wasted implants by count and cost, respectively.
When total cost of wasted implants was averaged over all implant cases performed during the period, the loss resulting from waste amounted to $71 per case for the department and ranged from $21 per case for the Hand division to $105 per case for the Pediatric division (Table 1). For individual surgeons, the loss ranged from $4 to $250 per case.
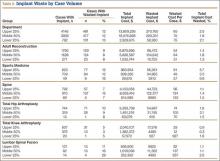
During the period studied, an implant was wasted in 9% (100) of the 1076 primary THAs performed, 4% (42) of the 1003 primary TKAs, and 14% (30) of the 217 lumbar spinal fusions (Tables 4, 5).
There was no significant difference between groups for department (P = .46) or for the Adult Reconstruction (P = .83), Spine (P = .10), or Sports Medicine (P = .69) division. Analyzing for variance by years in surgical practice, we found a significant difference for department (P = .01) but not for the Adult Reconstruction (P = .12) or Spine (P = .14) division. The department difference resulted from a significant difference (P = .001; 95% CI, 1.112-17.408) between surgeons (<10 years of surgical practice) who wasted implants in 12.8% of their cases and surgeons (>20 years of surgical practice) who wasted implants in 9% of their cases (Table 4).
There was no significant difference between groups for department (P = .83) or for the Adult Reconstruction (P = .29) or Spine (P = .41) division when analyzed by years in surgical practice. Analyzing by case volume, we found a significant difference for the Sports Medicine division (P = .004): Percentage of total implant waste was significantly higher (P = .003; 95% CI, –12.61 to –2.97) for surgeons with the lower 25% of case volume (9.8%) than for surgeons with the middle 50% of case volume (3.5%) (Table 5). No other significant difference was found for department (P = .52) or for the Adult Reconstruction (P = .69) or Spine (P = .45) division.
Analyzing by case volume and years in surgical practice, we found no significant difference for department (case volume, P = .76; years in surgical practice, P = .07), Adult Reconstruction division (case volume, P = .47; years in surgical practice, P = .78), Spine division (case volume, P = .11; years in surgical practice, P = .15), or Sports Medicine division (case volume, P = .08).
Selected Procedures
Total Hip Arthroplasty. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .072; years in practice, P = .076), percentage of total implant cost wasted (volume, P = .074; years in practice, P = .12), cost of waste per case (volume, P = .075; years in practice, P = .32).
Total Knee Arthroplasty. Regarding variance by years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (P = .38), percentage of total implant cost wasted (P = .50), cost of waste per case (P = .50). Regarding variance by volume, there was no significant difference for percentage of cases with waste (P = .70) or cost of waste per case (P = .05), but we found a significant difference for percentage of total implant cost wasted (P = .038). That difference was caused by an outlier: One surgeon with the lower 25% of case volume wasted an implant in the only TKA he performed that year. Correction for the outlier removed the significance.
Posterior Lumbar Spinal Fusion. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .36; years in surgical practice, P = .22), percentage of total implant cost wasted (volume, P = .33; years in surgical practice, P = .41), cost of waste per case (volume, P = .34; years in practice, P = .15).
Discussion
The steadily increasing demand for orthopedic surgeries and declining rates of reimbursement by Medicare and other insurance providers have led many hospitals to look for ways to control the cost of these surgeries. Reducing operating room costs, lowering implant prices, and shortening hospital stays have all proved successful.6,15,20,23 One area that has not been thoroughly explored is the cost burden of wasted implants. Our findings suggest implant waste contributes significantly to the cost of orthopedic surgeries.
One weakness of this study is that its data, though encompassing all orthopedic subspecialties and procedures, come from a single teaching institution and therefore are less representative of all orthopedic departments across the United States. However, the findings are useful in that the analysis was performed across multiple specialties at a high-volume institution and may be applied to similar institutions. Another weakness of this study is that the data cover only 1 year. Collecting data over a longer period could improve the magnitude and power of the analysis. Nonetheless, 1 year of data is a good starting point in identifying the issues and guiding the initiation of measures to address them. Last, we did not explore the reason for each instance of waste during the period reviewed. Knowing the reason for implant waste would be helpful in developing strategies to reduce implant waste.
Our study results showed that, in 1 year, implant waste occurred in 1.8% of procedures that required an implant—representing a loss of $634,000. Other studies have quantified implant waste for selected procedures or single departments, but to our knowledge none has quantified implant waste for an entire orthopedic department or hospital. It is therefore difficult to compare our institutional results with other results. For instance, definitions of waste differ. A study that found waste in 20% of spine surgery cases22 included all intraoperative waste, whereas our 11% of spine cases were implant waste only. Similarly, though rates of implant waste in trauma cases differed significantly between a multi-institution study by Zywiel and colleagues24 (0.6%) and our institution (30%), their study excluded arthroplasty cases from the trauma subset and reported implant waste for a single vendor, whereas we included arthroplasty cases and a wide array of implant vendors. In addition, costs cannot be directly compared because, in our study, implants wasted may have differed. Although the Trauma division had the highest incidence of waste (30%) in our analysis, it did not have the highest waste-related costs. Instead, the Adult Reconstruction division, with waste in 8% of cases, had the highest waste cost, $214,869. The cost difference is certainly the result of the difference in type of implants wasted. The implants most commonly wasted in the Trauma division were screws, which cost between $17 and $150; a single femoral stem, though wasted less often, cost significantly more, $2000 to $6000.
Our results showed a combined implant waste incidence of 6.8% for primary THA and primary TKA cases over the year. In their multi-institution study, Zywiel and colleagues19 reported a combined incidence of implant waste in 2% of THA and TKA cases. The difference is that Zywiel and colleagues19 reported data from a single implant vendor and included revision surgeries, hip hemiarthroplasties, and unicondylar knee arthroplasties. Another study reported implant waste in 5.7% of all TKA cases but did not specify whether revision or unicondylar arthroplasties were included.25 For lumbar spinal fusion, we found an implant waste incidence of 14%. Given the lack of studies in this area, we cannot make a comparison of results.
To our knowledge, there has been no other study of the effects of case volume and years in surgical practice on implant waste. Our analysis showed that waste incidence was not related to surgeon case volume but was related to years in surgical practice. Incidence of waste was significantly lower among surgeons practicing 20 years or more than among surgeons practicing fewer than 10 years. The difference may be a reflection that case volume during a single year is not totally indicative of a surgeon’s lifetime case volume. For example, several surgeons with many years of experience and a significant lifetime case volume had an annual case volume in the lower 25% of the department because they were approaching retirement or had only recently joined the institution. More rigorous prospective studies are needed to further understand this relationship.
Conclusions
Our study demonstrated significant costs related to implant waste. These costs are important to consider not only for traditional cases, such as total joint and spine procedures, in which implant costs are routinely scrutinized, but for all subspecialties, such as sports medicine, in which the majority of cases are performed on an outpatient basis. Considering the estimated $36 million wasted during THAs and TKAs and $126 million wasted on spine surgeries in the United States annually, and the significant waste we observed in other orthopedic subspecialties, decreasing the rate of intraoperative waste during orthopedic surgeries represents another area that could provide significant cost reduction through implant cost savings.19,22 A few successful programs have been reported. Soroceanu and colleagues22 found an almost 50% decrease in intraoperative waste during spine surgery after an educational program was used to address such waste. Elsewhere, use of a computer-based system (e.Label and Compatibility) led to an estimated cost reduction of $75,000 in implant waste.25 Efforts to develop and implement other programs to reduce implant waste are needed and should be part of any orthopedic operating room cost reduction strategy.
1. Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole.” N Engl J Med. 2012;366(4):289-291.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Keehan SP, Sisko AM, Truffer CJ, et al. National health spending projections through 2020: economic recovery and reform drive faster spending growth. Health Aff. 2011;30(8):1594-1605.
4. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
5. Belatti DA, Phisitkul P. Trends in orthopedics: an analysis of Medicare claims, 2000–2010. Orthopedics. 2013;36(3):e366-e372.
6. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
7. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(5):23-27.
8. Mendenhall S. 2003 hip and knee implant review. Orthop Network News. 2003;14(3):2.
9. Mendenhall S. 2008 hip and knee implant review. Orthop Network News. 2008;19(3):20.
10. Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011;469(1):87-94.
11. Mendenhall S. 2007 hip and knee implant review. Orthop Network News. 2007;18(3):16.
12. Iorio R, Davis CM 3rd, Healy WL, Fehring TK, O’Connor MI, York S. Impact of the economic downturn on adult reconstruction surgery: a survey of the American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25(7):1005-1014.
13. Healy WL, Iorio R, Ko J, Appleby D, Lemos DW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84(3):348-353.
14. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
15. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop Relat Res. 2011;469(2):355-361.
16. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
17. de Steiger RN, Miller LN, Davidson DC, Ryan P, Graves SE. Joint registry approach for identification of outlier prostheses. Acta Orthop. 2013;84(4):348-352.
18. Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80(4):393-401.
19. Zywiel MG, Ulrich SD, Suda AJ, Duncan JL, McGrath MS, Mont MA. Incidence and cost of intraoperative waste of hip and knee arthroplasty implants. J Arthroplasty. 2010;25(4):558-562.
20. Kim P, Kurokawa R, Itoki K. Technical advancements and utilization of spine surgery—international disparities in trend-dynamics between Japan, Korea, and the USA. Neurol Med Chir. 2010;50(9):853-858.
21. Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31(23):2707-2714.
22. Soroceanu A, Canacari E, Brown E, Robinson A, McGuire KJ. Intraoperative waste in spine surgery: incidence, cost, and effectiveness of an educational program. Spine. 2011;36(19):E1270-E1273.
23. Bosco JA, Alvarado CM, Slover JD, Iorio R, Hutzler LH. Decreasing total joint implant costs and physician specific cost variation through negotiation. J Arthroplasty. 2014;29(4):678-680.
24. Zywiel MG, Delanois RE, McGrath MS, Ulrich SD, Duncan JL, Mont MA. Intraoperative waste of trauma implants: a cost burden to hospitals worth addressing? J Orthop Trauma. 2009;23(10):710-715.
25. Ast MP, Mayman DJ, Su EP, Gonzalez Della Valle AM, Parks ML, Haas SB. The reduction of implant-related errors and waste in total knee arthroplasty using a novel, computer based, e.Label and Compatibility system. J Arthroplasty. 2014;29(1):132-136.
The cost of health care in the United States is increasing at an unsustainable rate.1-3 To decrease or even reverse this trend, we must decrease the cost of care without adversely affecting quality. Porter4 defined value as the quality of care divided by its cost. The economics of total joint arthroplasty (TJA) has received a great deal of attention because of both increasing demand and increasing cost.5-9 About 33% of all orthopedic surgeries and the majority of TJAs are paid for by Medicare.9 In recent years, the rate of reimbursement for orthopedic cases has steadily declined while the cost of implants has increased.3,10,11 Given the significant cost of implants, health care providers in some subspecialties have focused on implant costs as a potential area for cost reduction.12 For example, in TJA this has proved effective in reducing the overall cost, as has decreasing length of stay after surgery.8,10,13-16
With little evidence suggesting any specific orthopedic implant has outcomes superior to those of others, with the exception of select poorly performing outliers, we must increase value of care by lowering the cost when considering these devices.17,18 In addition, some experts have suggested that intraoperative waste is a significant factor in TJA cost, and it does contribute to the average implant cost for a TJA case.6,19 Using data collected from 72 institutions, Zywiel and colleagues19 estimated the annual cost of wasted hip and knee arthroplasty implants to be more than $36 million in the United States.
However, considering the aging US population, TJA is not the only orthopedic surgery with increased demand. An estimated 600,000 spine surgeries are performed each year in the United States.20 Between 1992 and 2003, Medicare spending for lumbar spinal fusion increased 500%.21 In addition, in a 15-month observational study of incidence of intraoperative waste in spine surgery, Soroceanu and colleagues22 reported waste occurring in 20% of spine procedures.
Although these studies have described implant waste in TJA and spine surgeries, little has been published on the cost of wasted implants in a center performing the full range of orthopedic procedures. In this article, we detail the implant waste costs incurred by surgeons for all orthopedic subspecialties at a single orthopedic specialty hospital over a 1-year period. Our study goals were to identify types of implants wasted, and incidence and cost of implant waste, for all total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and lumbar spinal fusions performed at the hospital and to determine whether case volume or years in surgical practice affect the rate or cost of implants wasted.
Methods
We performed a retrospective economic analysis of 1 year of administrative implant data from our institution. Collected data were quantified and analyzed for factors that might explain any variance in implant waste among surgeons. We were granted exempt institutional review board status, as no patient information was involved in this study.
We reviewed the administrative implant data for the 12-month period beginning June 2012 and ending May 2013. For that period, number of cases in which an implant was used and number of cases in which an implant was wasted were recorded. For each instance of waste, type and cost of the wasted implant were entered into the administrative database. In addition, overall cost of implants for the year and cost of wasted implants were determined. Data were available for 81 surgeons across 8 orthopedic divisions (subspecialties). From this information, we determined percentage of cases in which waste occurred, percentage of total implant cost wasted, average cost of waste per case, and most commonly wasted implants. All 3 variables were also calculated for THAs, TKAs, and lumbar spinal fusion procedures.
Statistical Analysis
The data were analyzed to determine if surgeon case volume or years in surgical practice affected implant waste. All analyses were performed at department, division (subspecialty), and surgeon levels. Case volume was analyzed in 3 groups: top 25%, middle 50%, and lower 25%. Number of years in surgical practice was analyzed in 3 groups: fewer than 10 years, 10 to 19 years, and 20 years or more. Normality assumption of variables was tested using the Shapiro-Wilk test (P < .05). For between-group differences, 1-way analysis of variance and the Tukey honestly significant difference post hoc test were performed for variables with a normal distribution, and the Kruskal-Wallis and Mann-Whitney tests were performed for variables without a normal distribution.
For the subspecialty-level analyses, only the Adult Reconstruction, Sports Medicine, and Spine divisions were analyzed for the effects of volume, and only the Sports Medicine and Spine divisions were analyzed for the effect of surgical experience, as surgeon numbers were insufficient for adequate grouping(s).
Data are presented as means with corresponding 95% confidence intervals (CIs). Categorical variables are presented as counts with percentages. All statistical analyses were performed with SPSS Version 21.0 (IBM SPSS) statistical software. Statistical significance was set at .05.
Results
During the 1-year period, 8954 department cases involved an implant of any type. Waste occurred in 12% (1072) of these cases. The rate ranged from 8% in the Adult Reconstruction division to 30% in the Trauma division (Table 1), and the rate for individual surgeons ranged from 3% to 100%, though the surgeon with 100% performed only 1 case, and the next highest rate was 50%.
Total implant cost for our hospital during the period was $34,340,607. Of that total cost, 1.8% ($634,668) was lost because of implant waste. Percentage of total implant cost wasted ranged from 1.6% in the Adult Reconstruction division to 4.7% in the Sports Medicine division (Table 1). Percentage of total implant cost wasted for individual surgeons ranged from 0.2% to 16.1%. Tables 2 and 3 list the most commonly wasted implants by count and cost, respectively.
When total cost of wasted implants was averaged over all implant cases performed during the period, the loss resulting from waste amounted to $71 per case for the department and ranged from $21 per case for the Hand division to $105 per case for the Pediatric division (Table 1). For individual surgeons, the loss ranged from $4 to $250 per case.
During the period studied, an implant was wasted in 9% (100) of the 1076 primary THAs performed, 4% (42) of the 1003 primary TKAs, and 14% (30) of the 217 lumbar spinal fusions (Tables 4, 5).
There was no significant difference between groups for department (P = .46) or for the Adult Reconstruction (P = .83), Spine (P = .10), or Sports Medicine (P = .69) division. Analyzing for variance by years in surgical practice, we found a significant difference for department (P = .01) but not for the Adult Reconstruction (P = .12) or Spine (P = .14) division. The department difference resulted from a significant difference (P = .001; 95% CI, 1.112-17.408) between surgeons (<10 years of surgical practice) who wasted implants in 12.8% of their cases and surgeons (>20 years of surgical practice) who wasted implants in 9% of their cases (Table 4).
There was no significant difference between groups for department (P = .83) or for the Adult Reconstruction (P = .29) or Spine (P = .41) division when analyzed by years in surgical practice. Analyzing by case volume, we found a significant difference for the Sports Medicine division (P = .004): Percentage of total implant waste was significantly higher (P = .003; 95% CI, –12.61 to –2.97) for surgeons with the lower 25% of case volume (9.8%) than for surgeons with the middle 50% of case volume (3.5%) (Table 5). No other significant difference was found for department (P = .52) or for the Adult Reconstruction (P = .69) or Spine (P = .45) division.
Analyzing by case volume and years in surgical practice, we found no significant difference for department (case volume, P = .76; years in surgical practice, P = .07), Adult Reconstruction division (case volume, P = .47; years in surgical practice, P = .78), Spine division (case volume, P = .11; years in surgical practice, P = .15), or Sports Medicine division (case volume, P = .08).
Selected Procedures
Total Hip Arthroplasty. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .072; years in practice, P = .076), percentage of total implant cost wasted (volume, P = .074; years in practice, P = .12), cost of waste per case (volume, P = .075; years in practice, P = .32).
Total Knee Arthroplasty. Regarding variance by years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (P = .38), percentage of total implant cost wasted (P = .50), cost of waste per case (P = .50). Regarding variance by volume, there was no significant difference for percentage of cases with waste (P = .70) or cost of waste per case (P = .05), but we found a significant difference for percentage of total implant cost wasted (P = .038). That difference was caused by an outlier: One surgeon with the lower 25% of case volume wasted an implant in the only TKA he performed that year. Correction for the outlier removed the significance.
Posterior Lumbar Spinal Fusion. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .36; years in surgical practice, P = .22), percentage of total implant cost wasted (volume, P = .33; years in surgical practice, P = .41), cost of waste per case (volume, P = .34; years in practice, P = .15).
Discussion
The steadily increasing demand for orthopedic surgeries and declining rates of reimbursement by Medicare and other insurance providers have led many hospitals to look for ways to control the cost of these surgeries. Reducing operating room costs, lowering implant prices, and shortening hospital stays have all proved successful.6,15,20,23 One area that has not been thoroughly explored is the cost burden of wasted implants. Our findings suggest implant waste contributes significantly to the cost of orthopedic surgeries.
One weakness of this study is that its data, though encompassing all orthopedic subspecialties and procedures, come from a single teaching institution and therefore are less representative of all orthopedic departments across the United States. However, the findings are useful in that the analysis was performed across multiple specialties at a high-volume institution and may be applied to similar institutions. Another weakness of this study is that the data cover only 1 year. Collecting data over a longer period could improve the magnitude and power of the analysis. Nonetheless, 1 year of data is a good starting point in identifying the issues and guiding the initiation of measures to address them. Last, we did not explore the reason for each instance of waste during the period reviewed. Knowing the reason for implant waste would be helpful in developing strategies to reduce implant waste.
Our study results showed that, in 1 year, implant waste occurred in 1.8% of procedures that required an implant—representing a loss of $634,000. Other studies have quantified implant waste for selected procedures or single departments, but to our knowledge none has quantified implant waste for an entire orthopedic department or hospital. It is therefore difficult to compare our institutional results with other results. For instance, definitions of waste differ. A study that found waste in 20% of spine surgery cases22 included all intraoperative waste, whereas our 11% of spine cases were implant waste only. Similarly, though rates of implant waste in trauma cases differed significantly between a multi-institution study by Zywiel and colleagues24 (0.6%) and our institution (30%), their study excluded arthroplasty cases from the trauma subset and reported implant waste for a single vendor, whereas we included arthroplasty cases and a wide array of implant vendors. In addition, costs cannot be directly compared because, in our study, implants wasted may have differed. Although the Trauma division had the highest incidence of waste (30%) in our analysis, it did not have the highest waste-related costs. Instead, the Adult Reconstruction division, with waste in 8% of cases, had the highest waste cost, $214,869. The cost difference is certainly the result of the difference in type of implants wasted. The implants most commonly wasted in the Trauma division were screws, which cost between $17 and $150; a single femoral stem, though wasted less often, cost significantly more, $2000 to $6000.
Our results showed a combined implant waste incidence of 6.8% for primary THA and primary TKA cases over the year. In their multi-institution study, Zywiel and colleagues19 reported a combined incidence of implant waste in 2% of THA and TKA cases. The difference is that Zywiel and colleagues19 reported data from a single implant vendor and included revision surgeries, hip hemiarthroplasties, and unicondylar knee arthroplasties. Another study reported implant waste in 5.7% of all TKA cases but did not specify whether revision or unicondylar arthroplasties were included.25 For lumbar spinal fusion, we found an implant waste incidence of 14%. Given the lack of studies in this area, we cannot make a comparison of results.
To our knowledge, there has been no other study of the effects of case volume and years in surgical practice on implant waste. Our analysis showed that waste incidence was not related to surgeon case volume but was related to years in surgical practice. Incidence of waste was significantly lower among surgeons practicing 20 years or more than among surgeons practicing fewer than 10 years. The difference may be a reflection that case volume during a single year is not totally indicative of a surgeon’s lifetime case volume. For example, several surgeons with many years of experience and a significant lifetime case volume had an annual case volume in the lower 25% of the department because they were approaching retirement or had only recently joined the institution. More rigorous prospective studies are needed to further understand this relationship.
Conclusions
Our study demonstrated significant costs related to implant waste. These costs are important to consider not only for traditional cases, such as total joint and spine procedures, in which implant costs are routinely scrutinized, but for all subspecialties, such as sports medicine, in which the majority of cases are performed on an outpatient basis. Considering the estimated $36 million wasted during THAs and TKAs and $126 million wasted on spine surgeries in the United States annually, and the significant waste we observed in other orthopedic subspecialties, decreasing the rate of intraoperative waste during orthopedic surgeries represents another area that could provide significant cost reduction through implant cost savings.19,22 A few successful programs have been reported. Soroceanu and colleagues22 found an almost 50% decrease in intraoperative waste during spine surgery after an educational program was used to address such waste. Elsewhere, use of a computer-based system (e.Label and Compatibility) led to an estimated cost reduction of $75,000 in implant waste.25 Efforts to develop and implement other programs to reduce implant waste are needed and should be part of any orthopedic operating room cost reduction strategy.
The cost of health care in the United States is increasing at an unsustainable rate.1-3 To decrease or even reverse this trend, we must decrease the cost of care without adversely affecting quality. Porter4 defined value as the quality of care divided by its cost. The economics of total joint arthroplasty (TJA) has received a great deal of attention because of both increasing demand and increasing cost.5-9 About 33% of all orthopedic surgeries and the majority of TJAs are paid for by Medicare.9 In recent years, the rate of reimbursement for orthopedic cases has steadily declined while the cost of implants has increased.3,10,11 Given the significant cost of implants, health care providers in some subspecialties have focused on implant costs as a potential area for cost reduction.12 For example, in TJA this has proved effective in reducing the overall cost, as has decreasing length of stay after surgery.8,10,13-16
With little evidence suggesting any specific orthopedic implant has outcomes superior to those of others, with the exception of select poorly performing outliers, we must increase value of care by lowering the cost when considering these devices.17,18 In addition, some experts have suggested that intraoperative waste is a significant factor in TJA cost, and it does contribute to the average implant cost for a TJA case.6,19 Using data collected from 72 institutions, Zywiel and colleagues19 estimated the annual cost of wasted hip and knee arthroplasty implants to be more than $36 million in the United States.
However, considering the aging US population, TJA is not the only orthopedic surgery with increased demand. An estimated 600,000 spine surgeries are performed each year in the United States.20 Between 1992 and 2003, Medicare spending for lumbar spinal fusion increased 500%.21 In addition, in a 15-month observational study of incidence of intraoperative waste in spine surgery, Soroceanu and colleagues22 reported waste occurring in 20% of spine procedures.
Although these studies have described implant waste in TJA and spine surgeries, little has been published on the cost of wasted implants in a center performing the full range of orthopedic procedures. In this article, we detail the implant waste costs incurred by surgeons for all orthopedic subspecialties at a single orthopedic specialty hospital over a 1-year period. Our study goals were to identify types of implants wasted, and incidence and cost of implant waste, for all total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and lumbar spinal fusions performed at the hospital and to determine whether case volume or years in surgical practice affect the rate or cost of implants wasted.
Methods
We performed a retrospective economic analysis of 1 year of administrative implant data from our institution. Collected data were quantified and analyzed for factors that might explain any variance in implant waste among surgeons. We were granted exempt institutional review board status, as no patient information was involved in this study.
We reviewed the administrative implant data for the 12-month period beginning June 2012 and ending May 2013. For that period, number of cases in which an implant was used and number of cases in which an implant was wasted were recorded. For each instance of waste, type and cost of the wasted implant were entered into the administrative database. In addition, overall cost of implants for the year and cost of wasted implants were determined. Data were available for 81 surgeons across 8 orthopedic divisions (subspecialties). From this information, we determined percentage of cases in which waste occurred, percentage of total implant cost wasted, average cost of waste per case, and most commonly wasted implants. All 3 variables were also calculated for THAs, TKAs, and lumbar spinal fusion procedures.
Statistical Analysis
The data were analyzed to determine if surgeon case volume or years in surgical practice affected implant waste. All analyses were performed at department, division (subspecialty), and surgeon levels. Case volume was analyzed in 3 groups: top 25%, middle 50%, and lower 25%. Number of years in surgical practice was analyzed in 3 groups: fewer than 10 years, 10 to 19 years, and 20 years or more. Normality assumption of variables was tested using the Shapiro-Wilk test (P < .05). For between-group differences, 1-way analysis of variance and the Tukey honestly significant difference post hoc test were performed for variables with a normal distribution, and the Kruskal-Wallis and Mann-Whitney tests were performed for variables without a normal distribution.
For the subspecialty-level analyses, only the Adult Reconstruction, Sports Medicine, and Spine divisions were analyzed for the effects of volume, and only the Sports Medicine and Spine divisions were analyzed for the effect of surgical experience, as surgeon numbers were insufficient for adequate grouping(s).
Data are presented as means with corresponding 95% confidence intervals (CIs). Categorical variables are presented as counts with percentages. All statistical analyses were performed with SPSS Version 21.0 (IBM SPSS) statistical software. Statistical significance was set at .05.
Results
During the 1-year period, 8954 department cases involved an implant of any type. Waste occurred in 12% (1072) of these cases. The rate ranged from 8% in the Adult Reconstruction division to 30% in the Trauma division (Table 1), and the rate for individual surgeons ranged from 3% to 100%, though the surgeon with 100% performed only 1 case, and the next highest rate was 50%.
Total implant cost for our hospital during the period was $34,340,607. Of that total cost, 1.8% ($634,668) was lost because of implant waste. Percentage of total implant cost wasted ranged from 1.6% in the Adult Reconstruction division to 4.7% in the Sports Medicine division (Table 1). Percentage of total implant cost wasted for individual surgeons ranged from 0.2% to 16.1%. Tables 2 and 3 list the most commonly wasted implants by count and cost, respectively.
When total cost of wasted implants was averaged over all implant cases performed during the period, the loss resulting from waste amounted to $71 per case for the department and ranged from $21 per case for the Hand division to $105 per case for the Pediatric division (Table 1). For individual surgeons, the loss ranged from $4 to $250 per case.
During the period studied, an implant was wasted in 9% (100) of the 1076 primary THAs performed, 4% (42) of the 1003 primary TKAs, and 14% (30) of the 217 lumbar spinal fusions (Tables 4, 5).
There was no significant difference between groups for department (P = .46) or for the Adult Reconstruction (P = .83), Spine (P = .10), or Sports Medicine (P = .69) division. Analyzing for variance by years in surgical practice, we found a significant difference for department (P = .01) but not for the Adult Reconstruction (P = .12) or Spine (P = .14) division. The department difference resulted from a significant difference (P = .001; 95% CI, 1.112-17.408) between surgeons (<10 years of surgical practice) who wasted implants in 12.8% of their cases and surgeons (>20 years of surgical practice) who wasted implants in 9% of their cases (Table 4).
There was no significant difference between groups for department (P = .83) or for the Adult Reconstruction (P = .29) or Spine (P = .41) division when analyzed by years in surgical practice. Analyzing by case volume, we found a significant difference for the Sports Medicine division (P = .004): Percentage of total implant waste was significantly higher (P = .003; 95% CI, –12.61 to –2.97) for surgeons with the lower 25% of case volume (9.8%) than for surgeons with the middle 50% of case volume (3.5%) (Table 5). No other significant difference was found for department (P = .52) or for the Adult Reconstruction (P = .69) or Spine (P = .45) division.
Analyzing by case volume and years in surgical practice, we found no significant difference for department (case volume, P = .76; years in surgical practice, P = .07), Adult Reconstruction division (case volume, P = .47; years in surgical practice, P = .78), Spine division (case volume, P = .11; years in surgical practice, P = .15), or Sports Medicine division (case volume, P = .08).
Selected Procedures
Total Hip Arthroplasty. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .072; years in practice, P = .076), percentage of total implant cost wasted (volume, P = .074; years in practice, P = .12), cost of waste per case (volume, P = .075; years in practice, P = .32).
Total Knee Arthroplasty. Regarding variance by years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (P = .38), percentage of total implant cost wasted (P = .50), cost of waste per case (P = .50). Regarding variance by volume, there was no significant difference for percentage of cases with waste (P = .70) or cost of waste per case (P = .05), but we found a significant difference for percentage of total implant cost wasted (P = .038). That difference was caused by an outlier: One surgeon with the lower 25% of case volume wasted an implant in the only TKA he performed that year. Correction for the outlier removed the significance.
Posterior Lumbar Spinal Fusion. Regarding variance by case volume and years in surgical practice, we found no significant difference for any variable analyzed: percentage of cases with waste (volume, P = .36; years in surgical practice, P = .22), percentage of total implant cost wasted (volume, P = .33; years in surgical practice, P = .41), cost of waste per case (volume, P = .34; years in practice, P = .15).
Discussion
The steadily increasing demand for orthopedic surgeries and declining rates of reimbursement by Medicare and other insurance providers have led many hospitals to look for ways to control the cost of these surgeries. Reducing operating room costs, lowering implant prices, and shortening hospital stays have all proved successful.6,15,20,23 One area that has not been thoroughly explored is the cost burden of wasted implants. Our findings suggest implant waste contributes significantly to the cost of orthopedic surgeries.
One weakness of this study is that its data, though encompassing all orthopedic subspecialties and procedures, come from a single teaching institution and therefore are less representative of all orthopedic departments across the United States. However, the findings are useful in that the analysis was performed across multiple specialties at a high-volume institution and may be applied to similar institutions. Another weakness of this study is that the data cover only 1 year. Collecting data over a longer period could improve the magnitude and power of the analysis. Nonetheless, 1 year of data is a good starting point in identifying the issues and guiding the initiation of measures to address them. Last, we did not explore the reason for each instance of waste during the period reviewed. Knowing the reason for implant waste would be helpful in developing strategies to reduce implant waste.
Our study results showed that, in 1 year, implant waste occurred in 1.8% of procedures that required an implant—representing a loss of $634,000. Other studies have quantified implant waste for selected procedures or single departments, but to our knowledge none has quantified implant waste for an entire orthopedic department or hospital. It is therefore difficult to compare our institutional results with other results. For instance, definitions of waste differ. A study that found waste in 20% of spine surgery cases22 included all intraoperative waste, whereas our 11% of spine cases were implant waste only. Similarly, though rates of implant waste in trauma cases differed significantly between a multi-institution study by Zywiel and colleagues24 (0.6%) and our institution (30%), their study excluded arthroplasty cases from the trauma subset and reported implant waste for a single vendor, whereas we included arthroplasty cases and a wide array of implant vendors. In addition, costs cannot be directly compared because, in our study, implants wasted may have differed. Although the Trauma division had the highest incidence of waste (30%) in our analysis, it did not have the highest waste-related costs. Instead, the Adult Reconstruction division, with waste in 8% of cases, had the highest waste cost, $214,869. The cost difference is certainly the result of the difference in type of implants wasted. The implants most commonly wasted in the Trauma division were screws, which cost between $17 and $150; a single femoral stem, though wasted less often, cost significantly more, $2000 to $6000.
Our results showed a combined implant waste incidence of 6.8% for primary THA and primary TKA cases over the year. In their multi-institution study, Zywiel and colleagues19 reported a combined incidence of implant waste in 2% of THA and TKA cases. The difference is that Zywiel and colleagues19 reported data from a single implant vendor and included revision surgeries, hip hemiarthroplasties, and unicondylar knee arthroplasties. Another study reported implant waste in 5.7% of all TKA cases but did not specify whether revision or unicondylar arthroplasties were included.25 For lumbar spinal fusion, we found an implant waste incidence of 14%. Given the lack of studies in this area, we cannot make a comparison of results.
To our knowledge, there has been no other study of the effects of case volume and years in surgical practice on implant waste. Our analysis showed that waste incidence was not related to surgeon case volume but was related to years in surgical practice. Incidence of waste was significantly lower among surgeons practicing 20 years or more than among surgeons practicing fewer than 10 years. The difference may be a reflection that case volume during a single year is not totally indicative of a surgeon’s lifetime case volume. For example, several surgeons with many years of experience and a significant lifetime case volume had an annual case volume in the lower 25% of the department because they were approaching retirement or had only recently joined the institution. More rigorous prospective studies are needed to further understand this relationship.
Conclusions
Our study demonstrated significant costs related to implant waste. These costs are important to consider not only for traditional cases, such as total joint and spine procedures, in which implant costs are routinely scrutinized, but for all subspecialties, such as sports medicine, in which the majority of cases are performed on an outpatient basis. Considering the estimated $36 million wasted during THAs and TKAs and $126 million wasted on spine surgeries in the United States annually, and the significant waste we observed in other orthopedic subspecialties, decreasing the rate of intraoperative waste during orthopedic surgeries represents another area that could provide significant cost reduction through implant cost savings.19,22 A few successful programs have been reported. Soroceanu and colleagues22 found an almost 50% decrease in intraoperative waste during spine surgery after an educational program was used to address such waste. Elsewhere, use of a computer-based system (e.Label and Compatibility) led to an estimated cost reduction of $75,000 in implant waste.25 Efforts to develop and implement other programs to reduce implant waste are needed and should be part of any orthopedic operating room cost reduction strategy.
1. Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole.” N Engl J Med. 2012;366(4):289-291.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Keehan SP, Sisko AM, Truffer CJ, et al. National health spending projections through 2020: economic recovery and reform drive faster spending growth. Health Aff. 2011;30(8):1594-1605.
4. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
5. Belatti DA, Phisitkul P. Trends in orthopedics: an analysis of Medicare claims, 2000–2010. Orthopedics. 2013;36(3):e366-e372.
6. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
7. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(5):23-27.
8. Mendenhall S. 2003 hip and knee implant review. Orthop Network News. 2003;14(3):2.
9. Mendenhall S. 2008 hip and knee implant review. Orthop Network News. 2008;19(3):20.
10. Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011;469(1):87-94.
11. Mendenhall S. 2007 hip and knee implant review. Orthop Network News. 2007;18(3):16.
12. Iorio R, Davis CM 3rd, Healy WL, Fehring TK, O’Connor MI, York S. Impact of the economic downturn on adult reconstruction surgery: a survey of the American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25(7):1005-1014.
13. Healy WL, Iorio R, Ko J, Appleby D, Lemos DW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84(3):348-353.
14. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
15. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop Relat Res. 2011;469(2):355-361.
16. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
17. de Steiger RN, Miller LN, Davidson DC, Ryan P, Graves SE. Joint registry approach for identification of outlier prostheses. Acta Orthop. 2013;84(4):348-352.
18. Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80(4):393-401.
19. Zywiel MG, Ulrich SD, Suda AJ, Duncan JL, McGrath MS, Mont MA. Incidence and cost of intraoperative waste of hip and knee arthroplasty implants. J Arthroplasty. 2010;25(4):558-562.
20. Kim P, Kurokawa R, Itoki K. Technical advancements and utilization of spine surgery—international disparities in trend-dynamics between Japan, Korea, and the USA. Neurol Med Chir. 2010;50(9):853-858.
21. Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31(23):2707-2714.
22. Soroceanu A, Canacari E, Brown E, Robinson A, McGuire KJ. Intraoperative waste in spine surgery: incidence, cost, and effectiveness of an educational program. Spine. 2011;36(19):E1270-E1273.
23. Bosco JA, Alvarado CM, Slover JD, Iorio R, Hutzler LH. Decreasing total joint implant costs and physician specific cost variation through negotiation. J Arthroplasty. 2014;29(4):678-680.
24. Zywiel MG, Delanois RE, McGrath MS, Ulrich SD, Duncan JL, Mont MA. Intraoperative waste of trauma implants: a cost burden to hospitals worth addressing? J Orthop Trauma. 2009;23(10):710-715.
25. Ast MP, Mayman DJ, Su EP, Gonzalez Della Valle AM, Parks ML, Haas SB. The reduction of implant-related errors and waste in total knee arthroplasty using a novel, computer based, e.Label and Compatibility system. J Arthroplasty. 2014;29(1):132-136.
1. Alhassani A, Chandra A, Chernew ME. The sources of the SGR “hole.” N Engl J Med. 2012;366(4):289-291.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Keehan SP, Sisko AM, Truffer CJ, et al. National health spending projections through 2020: economic recovery and reform drive faster spending growth. Health Aff. 2011;30(8):1594-1605.
4. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481.
5. Belatti DA, Phisitkul P. Trends in orthopedics: an analysis of Medicare claims, 2000–2010. Orthopedics. 2013;36(3):e366-e372.
6. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
7. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(5):23-27.
8. Mendenhall S. 2003 hip and knee implant review. Orthop Network News. 2003;14(3):2.
9. Mendenhall S. 2008 hip and knee implant review. Orthop Network News. 2008;19(3):20.
10. Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011;469(1):87-94.
11. Mendenhall S. 2007 hip and knee implant review. Orthop Network News. 2007;18(3):16.
12. Iorio R, Davis CM 3rd, Healy WL, Fehring TK, O’Connor MI, York S. Impact of the economic downturn on adult reconstruction surgery: a survey of the American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25(7):1005-1014.
13. Healy WL, Iorio R, Ko J, Appleby D, Lemos DW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84(3):348-353.
14. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
15. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop Relat Res. 2011;469(2):355-361.
16. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
17. de Steiger RN, Miller LN, Davidson DC, Ryan P, Graves SE. Joint registry approach for identification of outlier prostheses. Acta Orthop. 2013;84(4):348-352.
18. Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80(4):393-401.
19. Zywiel MG, Ulrich SD, Suda AJ, Duncan JL, McGrath MS, Mont MA. Incidence and cost of intraoperative waste of hip and knee arthroplasty implants. J Arthroplasty. 2010;25(4):558-562.
20. Kim P, Kurokawa R, Itoki K. Technical advancements and utilization of spine surgery—international disparities in trend-dynamics between Japan, Korea, and the USA. Neurol Med Chir. 2010;50(9):853-858.
21. Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31(23):2707-2714.
22. Soroceanu A, Canacari E, Brown E, Robinson A, McGuire KJ. Intraoperative waste in spine surgery: incidence, cost, and effectiveness of an educational program. Spine. 2011;36(19):E1270-E1273.
23. Bosco JA, Alvarado CM, Slover JD, Iorio R, Hutzler LH. Decreasing total joint implant costs and physician specific cost variation through negotiation. J Arthroplasty. 2014;29(4):678-680.
24. Zywiel MG, Delanois RE, McGrath MS, Ulrich SD, Duncan JL, Mont MA. Intraoperative waste of trauma implants: a cost burden to hospitals worth addressing? J Orthop Trauma. 2009;23(10):710-715.
25. Ast MP, Mayman DJ, Su EP, Gonzalez Della Valle AM, Parks ML, Haas SB. The reduction of implant-related errors and waste in total knee arthroplasty using a novel, computer based, e.Label and Compatibility system. J Arthroplasty. 2014;29(1):132-136.
Technique Using Isoelastic Tension Band for Treatment of Olecranon Fractures
Olecranon fractures are relatively common in adults and constitute 10% of all upper extremity injuries.1,2 An olecranon fracture may be sustained either directly (from blunt trauma or a fall onto the tip of the elbow) or indirectly (as a result of forceful hyperextension of the triceps during a fall onto an outstretched arm). Displaced olecranon fractures with extensor discontinuity require reduction and stabilization. One treatment option is tension band wiring (TBW), which is used to manage noncomminuted fractures.3 TBW, first described by Weber and Vasey4 in 1963, involves transforming the distractive forces of the triceps into dynamic compression forces across the olecranon articular surface using 2 intramedullary Kirschner wires (K-wires) and stainless steel wires looped in figure-of-8 fashion.
Various modifications of the TBW technique of Weber and Vasey4 have been proposed to reduce the frequency of complications. These modifications include substituting screws for K-wires, aiming the angle of the K-wires into the anterior coronoid cortex or loop configuration of the stainless steel wire, using double knots and twisting procedures to finalize fixation, and using alternative materials for the loop construct.5-8 In the literature and in our experience, patients often complain after surgery about prominent K-wires and the twisted knots used to tension the construct.9-12 Surgeons also must address the technical difficulties of positioning the brittle wire without kinking, and avoiding slack while tensioning.
In this article, we report on the clinical outcomes of a series of 7 patients with olecranon fracture treated with a US Food and Drug Administration–approved novel isoelastic ultrahigh-molecular-weight polyethylene (UHMWPE) cerclage cable (Iso-Elastic Cerclage System, Kinamed).
Materials and Methods
Surgical Technique
The patient is arranged in a sloppy lateral position to allow access to the posterior elbow. A nonsterile tourniquet is placed on the upper arm, and the limb is sterilely prepared and draped in standard fashion. A posterolateral incision is made around the olecranon and extended proximally 6 cm and distally 6 cm along the subcutaneous border of the ulna. The fracture is visualized and comminution identified.
To provide anchorage for a pointed reduction clamp, the surgeon drills a 2.5-mm hole in the subcutaneous border of the ulnar shaft. The fracture is reduced in extension and the clamp affixed. The elbow is then flexed and the reduction confirmed visually and by imaging. After realignment of the articular surfaces, 2 longitudinal, parallel K-wires (diameter, 1.6-2.0 mm) are passed in antegrade direction through the proximal olecranon within the medullary canal of the shaft. The proximal ends must not cross the cortex so they may fully capture the figure-of-8 wire during subsequent, final advancement, and the distal ends must not pierce the anterior cortex. A 2.5-mm transverse hole is created distal to the fracture in the dorsal aspect of the ulnar shaft from medial to lateral at 2 times the distance from the tip of the olecranon to the fracture site. This hole is expanded with a 3.5-mm drill bit, allowing both strands of the cable to be passed simultaneously medial to lateral, making the figure-of-8. The 3.5-mm hole represents about 20% of the overall width of the bone, which we have not found to create a significant stress riser in either laboratory or clinical tests of this construct. Proximally, the cables are placed on the periosteum of the olecranon but deep to the triceps tendon and adjacent to the K-wires. The locking clip is placed on the posterolateral aspect of the elbow joint in a location where it can be covered with local tissue for adequate padding. The cable is then threaded through the clamping bracket and tightened slowly and gradually with a tensioning device to low torque level (Figure 1). At this stage, tension may be released to make any necessary adjustments. Last, the locking clip is deployed, securing the tension band in the clip, and the excess cable is trimmed with a scalpel. Softening and pliability of the cable during its insertion and tensioning should be noted.
The ends of the K-wires are now curved in a hook configuration. The tines of the hooks should be parallel to accommodate the cable, and then the triceps is sharply incised to bone. If the bone is hard, an awl is used to create a pilot hole so the hook may be impaled into bone while capturing the cable. Next, the triceps is closed over the pins, minimizing the potential for pin migration and backout. The 2 K-wires are left in place to keep the fragments in proper anatomical alignment during healing and to prevent displacement with elbow motion. Figure 2 is a schematic of the final construct, and Figure 3 shows the construct in a patient.
Reduction of the olecranon fracture is assessed by imaging in full extension to check for possible implant impingement. Last, we apply the previously harvested fracture callus to the fracture site. Layered closure is performed, and bulky soft dressings are applied. Postoperative immobilization with a splint is used. Gentle range-of-motion exercises begin in about 2 weeks and progress as pain allows.
A case example with preoperative and postoperative images taken at 3-month follow-up is provided in Figure 4. The entire surgical technique can be viewed in the Video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clinical Cases
Between July 2007 and February 2011, 7 patients with displaced olecranon fractures underwent osteosynthesis using the isoelastic tension band (Table 1). According to the Mayo classification system, 5 of these patients had type 2A fractures, 1 had a type 2B fracture with an ipsilateral nondisplaced radial neck fracture, and 1 had a type 3B fracture. There were 4 female and 3 male patients. The injury was on the dominant side in 3 patients. All patients gave informed consent to evaluation at subsequent office visits and completed outcomes questionnaires by mail several years after surgery. Mean follow-up at which outcome measures questionnaires were obtained was 3.3 years (range, 2.1-6.8 years). Exclusion criteria were age under 18 years and inability to provide informed consent, fracture patterns with extensive articular comminution, and open fractures. Permission to conduct this research was granted by institutional review board.
At each visit, patients completed the Disabilities of the Arm, Shoulder, and Hand (DASH) functional outcome survey and were evaluated according to Broberg and Morrey’s elbow scoring system.13,14 Chart review consisted of evaluation of medical records, including radiographs and orthopedic physician notes in which preoperative examination was documented, mechanism of injury was noted, radiologic fracture pattern was evaluated, and time to bony union was recorded. Elbow motion was documented. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan) set at level 2, as delineated in Broberg and Morrey’s functional elbow scoring system.
Results
The 7 patients were assessed at a mean final follow-up of 19 months after surgery and received a mean Broberg and Morrey score of good (92.2/100) (Table 2). Restoration of motion and strength was excellent; compared with contralateral extremity, mean flexion arc was 96%, and mean forearm rotation was 96%. Grip was 99% of the noninjured side, perhaps the result of increased conditioning from physical therapy. Patients completed outcomes questionnaires at a mean of 3.3 years after surgery. Mean (SD) DASH score at this longest follow-up was 12.6 (17.2) (Table 2). Patients were satisfied (mean, 9.8/10; range, 9.5-10) and had little pain (mean, 0.8/10; range, 0-3). All fractures united, and there were no infections. One patient had a satisfactory union with complete restoration of motion and continued to play sports vocationally but developed pain over the locking clip 5 years after the index procedure and decided to have the implant removed. He had no radiographic evidence of K-wire or implant migration. Another patient had a minor degree of implant irritation at longest follow-up but did not request hardware removal.
Discussion
Stainless steel wire is often used in TBW because of its widespread availability, low cost, lack of immunogenicity, and relative strength.7 However, stainless steel wire has several disadvantages. It is susceptible to low-cycle fatigue failure, and fatigue strength may be seriously reduced secondary to incidental trauma to the wire on implantation.15,16 Other complications are kinking, skin irritation, implant prominence, fixation loss caused by wire loosening, and inadequate initial reduction potentially requiring revision.10,12,17-21
Isoelastic cable is a new type of cerclage cable that consists of UHMWPE strands braided over a nylon core. The particular property profile of the isoelastic tension band gives the cable intrinsic elastic and pliable qualities. In addition, unlike stainless steel, the band maintains a uniform, continuous compression force across a fracture site.22 Multifilament braided cables fatigue and fray, but the isoelastic cerclage cable showed no evidence of fraying or breakage after 1 million loading cycles.22,23 Compared with metal wire or braided metal cable, the band also has higher fatigue strength and higher ultimate tensile strength.7 Furthermore, the cable is less abrasive than stainless steel, so theoretically it is less irritating to surrounding subcutaneous tissue. Last, the pliability of the band allows the surgeon to create multiple loops of cable without the wire-failure side effects related to kinking, which is common with the metal construct.
In 2010, Ting and colleagues24 retrospectively studied implant failure complications associated with use of isoelastic cerclage cables in the treatment of periprosthetic fractures in total hip arthroplasty. They reported a breakage rate of 0% and noted that previously published breakage data for metallic cerclage devices ranged from 0% to 44%. They concluded that isoelastic cables were not associated with material failure, and there were no direct complications related to the cables. Similarly, Edwards and colleagues25 evaluated the same type of cable used in revision shoulder arthroplasty and reported excellent success and no failures. Although these data stem from use in the femur and humerus, we think the noted benefits apply to fractures of the elbow as well, as we observed a similar breakage rate (0%).
Various studies have addressed the clinical complaints and reoperation rates associated with retained metal implants after olecranon fixation. Traditional AO (Arbeitsgemeinschaft für Osteosynthesefragen) technique involves subcutaneous placement of stainless steel wires, which often results in tissue irritation. Reoperation rates as high as 80% have been reported, and a proportion of implant removals may in fact be caused by factors related to the subcutaneous placement of the metallic implants rather than K-wire migration alone.5,12,18 A nonmetallic isoelastic tension band can provide a more comfortable and less irritating implant, which could reduce the need for secondary intervention related to painful subcutaneous implant. One of our 7 patients had a symptomatic implant removed 5 years after surgery. This patient complained of pain over the area of the tension band device clip, so after fracture healing the entire fixation device was removed in the operating room. If reoperation is necessary, removal of intramedullary K-wires is relatively simple using a minimal incision; removal of stainless steel TBW may require a larger approach if the twisted knots cannot be easily retrieved.
A study of compression forces created by stainless steel wire demonstrated that a “finely tuned mechanical sense” was needed to produce optimal fixation compression when using stainless steel wire.26 It was observed that a submaximal twist created insufficient compressive force, while an ostensibly minimal increase in twisting force above optimum abruptly caused wire failure through breakage. Cerclage cables using clasping devices, such as the current isoelastic cerclage cable, were superior in ease of application. Furthermore, a clasping device allows for cable tension readjustment that is not possible with stainless steel wire. The clasping mechanism precludes the surgeon from having to bury the stainless steel knot and allows for the objective cable-tensioning not possible with stainless steel wire. Last, the tensioning device is titratable, which allows the surgeon to set the construct at a predetermined quantitative tension, which is of benefit in patients with osteopenia.
One limitation of this study is that it did not resolve the potential for K-wire migration, and we agree with previous recommendations that careful attention to surgical technique may avoid such a complication.10 In addition, the sample was small, and the study lacked a control group; a larger sample and a control group would have boosted study power. Nevertheless, the physical and functional outcomes associated with use of this technique were excellent. These results demonstrate an efficacious attempt to decrease secondary surgery rates and are therefore proof of concept that the isoelastic tension band may be used as an alternative to stainless steel in the TBW of displaced olecranon fractures with minimal or no comminution.
Conclusion
This easily reproducible technique for use of an isoelastic tension band in olecranon fracture fixation was associated with excellent physical and functional outcomes in a series of 7 patients. The rate of secondary intervention was slightly better for these patients than for patients treated with wire tension band fixation. Although more rigorous study of this device is needed, we think it is a promising alternative to wire tension band techniques.
1. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
2. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
3. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
4. Weber BG, Vasey H. Osteosynthesis in olecranon fractures [in German]. Z Unfallmed Berufskr. 1963;56:90-96.
5. Netz P, Strömberg L. Non-sliding pins in traction absorbing wiring of fractures: a modified technique. Acta Orthop Scand. 1982;53(3):355-360.
6. Prayson MJ, Williams JL, Marshall MP, Scilaris TA, Lingenfelter EJ. Biomechanical comparison of fixation methods in transverse olecranon fractures: a cadaveric study. J Orthop Trauma. 1997;11(8):565-572.
7. Rothaug PG, Boston RC, Richardson DW, Nunamaker DM. A comparison of ultra-high-molecular weight polyethylene cable and stainless steel wire using two fixation techniques for repair of equine midbody sesamoid fractures: an in vitro biomechanical study. Vet Surg. 2002;31(5):445-454.
8. Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
9. Nimura A, Nakagawa T, Wakabayashi Y, Sekiya I, Okawa A, Muneta T. Repair of olecranon fractures using FiberWire without metallic implants: report of two cases. J Orthop Surg Res. 2010;5:73.
10. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
11. Helm RH, Hornby R, Miller SW. The complications of surgical treatment of displaced fractures of the olecranon. Injury. 1987;18(1):48-50.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128-146.
14. Broberg MA, Morrey BF. Results of delayed excision of the radial head after fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
15. Bostrom MP, Asnis SE, Ernberg JJ, et al. Fatigue testing of cerclage stainless steel wire fixation. J Orthop Trauma. 1994;8(5):422-428.
16. Oh I, Sander TW, Treharne RW. The fatigue resistance of orthopaedic wire. Clin Orthop Relat Res. 1985;(192):228-236.
17. Amstutz HC, Maki S. Complications of trochanteric osteotomy in total hip replacement. J Bone Joint Surg Am. 1978;60(2):214-216.
18. Jensen CM, Olsen BB. Drawbacks of traction-absorbing wiring (TAW) in displaced fractures of the olecranon. Injury. 1986;17(3):174-175.
19. Kumar G, Mereddy PK, Hakkalamani S, Donnachie NJ. Implant removal following surgical stabilization of patella fracture. Orthopedics. 2010;33(5).
20. Hume MC, Wiss DA. Olecranon fractures. A clinical and radiographic comparison of tension band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
21. Wolfgang G, Burke F, Bush D, et al. Surgical treatment of displaced olecranon fractures by tension band wiring technique. Clin Orthop Relat Res. 1987;(224):192-204.
22. Sarin VK, Mattchen TM, Hack B. A novel iso-elastic cerclage cable for treatment of fractures. Paper presented at: Annual Meeting of the Orthopaedic Research Society; February 20-23, 2005; Washington, DC. Paper 739.
23. Silverton CD, Jacobs JJ, Rosenberg AG, Kull L, Conley A, Galante JO. Complications of a cable grip system. J Arthroplasty. 1996;11(4):400-404.
24. Ting NT, Wera GD, Levine BR, Della Valle CJ. Early experience with a novel nonmetallic cable in reconstructive hip surgery. Clin Orthop Relat Res. 2010;468(9):2382-2386.
25. Edwards TB, Stuart KD, Trappey GJ, O’Connor DP, Sarin VK. Utility of polymer cerclage cables in revision shoulder arthroplasty. Orthopedics. 2011;34(4).
26. Shaw JA, Daubert HB. Compression capability of cerclage fixation systems. A biomechanical study. Orthopedics. 1988;11(8):1169-1174.
Olecranon fractures are relatively common in adults and constitute 10% of all upper extremity injuries.1,2 An olecranon fracture may be sustained either directly (from blunt trauma or a fall onto the tip of the elbow) or indirectly (as a result of forceful hyperextension of the triceps during a fall onto an outstretched arm). Displaced olecranon fractures with extensor discontinuity require reduction and stabilization. One treatment option is tension band wiring (TBW), which is used to manage noncomminuted fractures.3 TBW, first described by Weber and Vasey4 in 1963, involves transforming the distractive forces of the triceps into dynamic compression forces across the olecranon articular surface using 2 intramedullary Kirschner wires (K-wires) and stainless steel wires looped in figure-of-8 fashion.
Various modifications of the TBW technique of Weber and Vasey4 have been proposed to reduce the frequency of complications. These modifications include substituting screws for K-wires, aiming the angle of the K-wires into the anterior coronoid cortex or loop configuration of the stainless steel wire, using double knots and twisting procedures to finalize fixation, and using alternative materials for the loop construct.5-8 In the literature and in our experience, patients often complain after surgery about prominent K-wires and the twisted knots used to tension the construct.9-12 Surgeons also must address the technical difficulties of positioning the brittle wire without kinking, and avoiding slack while tensioning.
In this article, we report on the clinical outcomes of a series of 7 patients with olecranon fracture treated with a US Food and Drug Administration–approved novel isoelastic ultrahigh-molecular-weight polyethylene (UHMWPE) cerclage cable (Iso-Elastic Cerclage System, Kinamed).
Materials and Methods
Surgical Technique
The patient is arranged in a sloppy lateral position to allow access to the posterior elbow. A nonsterile tourniquet is placed on the upper arm, and the limb is sterilely prepared and draped in standard fashion. A posterolateral incision is made around the olecranon and extended proximally 6 cm and distally 6 cm along the subcutaneous border of the ulna. The fracture is visualized and comminution identified.
To provide anchorage for a pointed reduction clamp, the surgeon drills a 2.5-mm hole in the subcutaneous border of the ulnar shaft. The fracture is reduced in extension and the clamp affixed. The elbow is then flexed and the reduction confirmed visually and by imaging. After realignment of the articular surfaces, 2 longitudinal, parallel K-wires (diameter, 1.6-2.0 mm) are passed in antegrade direction through the proximal olecranon within the medullary canal of the shaft. The proximal ends must not cross the cortex so they may fully capture the figure-of-8 wire during subsequent, final advancement, and the distal ends must not pierce the anterior cortex. A 2.5-mm transverse hole is created distal to the fracture in the dorsal aspect of the ulnar shaft from medial to lateral at 2 times the distance from the tip of the olecranon to the fracture site. This hole is expanded with a 3.5-mm drill bit, allowing both strands of the cable to be passed simultaneously medial to lateral, making the figure-of-8. The 3.5-mm hole represents about 20% of the overall width of the bone, which we have not found to create a significant stress riser in either laboratory or clinical tests of this construct. Proximally, the cables are placed on the periosteum of the olecranon but deep to the triceps tendon and adjacent to the K-wires. The locking clip is placed on the posterolateral aspect of the elbow joint in a location where it can be covered with local tissue for adequate padding. The cable is then threaded through the clamping bracket and tightened slowly and gradually with a tensioning device to low torque level (Figure 1). At this stage, tension may be released to make any necessary adjustments. Last, the locking clip is deployed, securing the tension band in the clip, and the excess cable is trimmed with a scalpel. Softening and pliability of the cable during its insertion and tensioning should be noted.
The ends of the K-wires are now curved in a hook configuration. The tines of the hooks should be parallel to accommodate the cable, and then the triceps is sharply incised to bone. If the bone is hard, an awl is used to create a pilot hole so the hook may be impaled into bone while capturing the cable. Next, the triceps is closed over the pins, minimizing the potential for pin migration and backout. The 2 K-wires are left in place to keep the fragments in proper anatomical alignment during healing and to prevent displacement with elbow motion. Figure 2 is a schematic of the final construct, and Figure 3 shows the construct in a patient.
Reduction of the olecranon fracture is assessed by imaging in full extension to check for possible implant impingement. Last, we apply the previously harvested fracture callus to the fracture site. Layered closure is performed, and bulky soft dressings are applied. Postoperative immobilization with a splint is used. Gentle range-of-motion exercises begin in about 2 weeks and progress as pain allows.
A case example with preoperative and postoperative images taken at 3-month follow-up is provided in Figure 4. The entire surgical technique can be viewed in the Video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clinical Cases
Between July 2007 and February 2011, 7 patients with displaced olecranon fractures underwent osteosynthesis using the isoelastic tension band (Table 1). According to the Mayo classification system, 5 of these patients had type 2A fractures, 1 had a type 2B fracture with an ipsilateral nondisplaced radial neck fracture, and 1 had a type 3B fracture. There were 4 female and 3 male patients. The injury was on the dominant side in 3 patients. All patients gave informed consent to evaluation at subsequent office visits and completed outcomes questionnaires by mail several years after surgery. Mean follow-up at which outcome measures questionnaires were obtained was 3.3 years (range, 2.1-6.8 years). Exclusion criteria were age under 18 years and inability to provide informed consent, fracture patterns with extensive articular comminution, and open fractures. Permission to conduct this research was granted by institutional review board.
At each visit, patients completed the Disabilities of the Arm, Shoulder, and Hand (DASH) functional outcome survey and were evaluated according to Broberg and Morrey’s elbow scoring system.13,14 Chart review consisted of evaluation of medical records, including radiographs and orthopedic physician notes in which preoperative examination was documented, mechanism of injury was noted, radiologic fracture pattern was evaluated, and time to bony union was recorded. Elbow motion was documented. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan) set at level 2, as delineated in Broberg and Morrey’s functional elbow scoring system.
Results
The 7 patients were assessed at a mean final follow-up of 19 months after surgery and received a mean Broberg and Morrey score of good (92.2/100) (Table 2). Restoration of motion and strength was excellent; compared with contralateral extremity, mean flexion arc was 96%, and mean forearm rotation was 96%. Grip was 99% of the noninjured side, perhaps the result of increased conditioning from physical therapy. Patients completed outcomes questionnaires at a mean of 3.3 years after surgery. Mean (SD) DASH score at this longest follow-up was 12.6 (17.2) (Table 2). Patients were satisfied (mean, 9.8/10; range, 9.5-10) and had little pain (mean, 0.8/10; range, 0-3). All fractures united, and there were no infections. One patient had a satisfactory union with complete restoration of motion and continued to play sports vocationally but developed pain over the locking clip 5 years after the index procedure and decided to have the implant removed. He had no radiographic evidence of K-wire or implant migration. Another patient had a minor degree of implant irritation at longest follow-up but did not request hardware removal.
Discussion
Stainless steel wire is often used in TBW because of its widespread availability, low cost, lack of immunogenicity, and relative strength.7 However, stainless steel wire has several disadvantages. It is susceptible to low-cycle fatigue failure, and fatigue strength may be seriously reduced secondary to incidental trauma to the wire on implantation.15,16 Other complications are kinking, skin irritation, implant prominence, fixation loss caused by wire loosening, and inadequate initial reduction potentially requiring revision.10,12,17-21
Isoelastic cable is a new type of cerclage cable that consists of UHMWPE strands braided over a nylon core. The particular property profile of the isoelastic tension band gives the cable intrinsic elastic and pliable qualities. In addition, unlike stainless steel, the band maintains a uniform, continuous compression force across a fracture site.22 Multifilament braided cables fatigue and fray, but the isoelastic cerclage cable showed no evidence of fraying or breakage after 1 million loading cycles.22,23 Compared with metal wire or braided metal cable, the band also has higher fatigue strength and higher ultimate tensile strength.7 Furthermore, the cable is less abrasive than stainless steel, so theoretically it is less irritating to surrounding subcutaneous tissue. Last, the pliability of the band allows the surgeon to create multiple loops of cable without the wire-failure side effects related to kinking, which is common with the metal construct.
In 2010, Ting and colleagues24 retrospectively studied implant failure complications associated with use of isoelastic cerclage cables in the treatment of periprosthetic fractures in total hip arthroplasty. They reported a breakage rate of 0% and noted that previously published breakage data for metallic cerclage devices ranged from 0% to 44%. They concluded that isoelastic cables were not associated with material failure, and there were no direct complications related to the cables. Similarly, Edwards and colleagues25 evaluated the same type of cable used in revision shoulder arthroplasty and reported excellent success and no failures. Although these data stem from use in the femur and humerus, we think the noted benefits apply to fractures of the elbow as well, as we observed a similar breakage rate (0%).
Various studies have addressed the clinical complaints and reoperation rates associated with retained metal implants after olecranon fixation. Traditional AO (Arbeitsgemeinschaft für Osteosynthesefragen) technique involves subcutaneous placement of stainless steel wires, which often results in tissue irritation. Reoperation rates as high as 80% have been reported, and a proportion of implant removals may in fact be caused by factors related to the subcutaneous placement of the metallic implants rather than K-wire migration alone.5,12,18 A nonmetallic isoelastic tension band can provide a more comfortable and less irritating implant, which could reduce the need for secondary intervention related to painful subcutaneous implant. One of our 7 patients had a symptomatic implant removed 5 years after surgery. This patient complained of pain over the area of the tension band device clip, so after fracture healing the entire fixation device was removed in the operating room. If reoperation is necessary, removal of intramedullary K-wires is relatively simple using a minimal incision; removal of stainless steel TBW may require a larger approach if the twisted knots cannot be easily retrieved.
A study of compression forces created by stainless steel wire demonstrated that a “finely tuned mechanical sense” was needed to produce optimal fixation compression when using stainless steel wire.26 It was observed that a submaximal twist created insufficient compressive force, while an ostensibly minimal increase in twisting force above optimum abruptly caused wire failure through breakage. Cerclage cables using clasping devices, such as the current isoelastic cerclage cable, were superior in ease of application. Furthermore, a clasping device allows for cable tension readjustment that is not possible with stainless steel wire. The clasping mechanism precludes the surgeon from having to bury the stainless steel knot and allows for the objective cable-tensioning not possible with stainless steel wire. Last, the tensioning device is titratable, which allows the surgeon to set the construct at a predetermined quantitative tension, which is of benefit in patients with osteopenia.
One limitation of this study is that it did not resolve the potential for K-wire migration, and we agree with previous recommendations that careful attention to surgical technique may avoid such a complication.10 In addition, the sample was small, and the study lacked a control group; a larger sample and a control group would have boosted study power. Nevertheless, the physical and functional outcomes associated with use of this technique were excellent. These results demonstrate an efficacious attempt to decrease secondary surgery rates and are therefore proof of concept that the isoelastic tension band may be used as an alternative to stainless steel in the TBW of displaced olecranon fractures with minimal or no comminution.
Conclusion
This easily reproducible technique for use of an isoelastic tension band in olecranon fracture fixation was associated with excellent physical and functional outcomes in a series of 7 patients. The rate of secondary intervention was slightly better for these patients than for patients treated with wire tension band fixation. Although more rigorous study of this device is needed, we think it is a promising alternative to wire tension band techniques.
Olecranon fractures are relatively common in adults and constitute 10% of all upper extremity injuries.1,2 An olecranon fracture may be sustained either directly (from blunt trauma or a fall onto the tip of the elbow) or indirectly (as a result of forceful hyperextension of the triceps during a fall onto an outstretched arm). Displaced olecranon fractures with extensor discontinuity require reduction and stabilization. One treatment option is tension band wiring (TBW), which is used to manage noncomminuted fractures.3 TBW, first described by Weber and Vasey4 in 1963, involves transforming the distractive forces of the triceps into dynamic compression forces across the olecranon articular surface using 2 intramedullary Kirschner wires (K-wires) and stainless steel wires looped in figure-of-8 fashion.
Various modifications of the TBW technique of Weber and Vasey4 have been proposed to reduce the frequency of complications. These modifications include substituting screws for K-wires, aiming the angle of the K-wires into the anterior coronoid cortex or loop configuration of the stainless steel wire, using double knots and twisting procedures to finalize fixation, and using alternative materials for the loop construct.5-8 In the literature and in our experience, patients often complain after surgery about prominent K-wires and the twisted knots used to tension the construct.9-12 Surgeons also must address the technical difficulties of positioning the brittle wire without kinking, and avoiding slack while tensioning.
In this article, we report on the clinical outcomes of a series of 7 patients with olecranon fracture treated with a US Food and Drug Administration–approved novel isoelastic ultrahigh-molecular-weight polyethylene (UHMWPE) cerclage cable (Iso-Elastic Cerclage System, Kinamed).
Materials and Methods
Surgical Technique
The patient is arranged in a sloppy lateral position to allow access to the posterior elbow. A nonsterile tourniquet is placed on the upper arm, and the limb is sterilely prepared and draped in standard fashion. A posterolateral incision is made around the olecranon and extended proximally 6 cm and distally 6 cm along the subcutaneous border of the ulna. The fracture is visualized and comminution identified.
To provide anchorage for a pointed reduction clamp, the surgeon drills a 2.5-mm hole in the subcutaneous border of the ulnar shaft. The fracture is reduced in extension and the clamp affixed. The elbow is then flexed and the reduction confirmed visually and by imaging. After realignment of the articular surfaces, 2 longitudinal, parallel K-wires (diameter, 1.6-2.0 mm) are passed in antegrade direction through the proximal olecranon within the medullary canal of the shaft. The proximal ends must not cross the cortex so they may fully capture the figure-of-8 wire during subsequent, final advancement, and the distal ends must not pierce the anterior cortex. A 2.5-mm transverse hole is created distal to the fracture in the dorsal aspect of the ulnar shaft from medial to lateral at 2 times the distance from the tip of the olecranon to the fracture site. This hole is expanded with a 3.5-mm drill bit, allowing both strands of the cable to be passed simultaneously medial to lateral, making the figure-of-8. The 3.5-mm hole represents about 20% of the overall width of the bone, which we have not found to create a significant stress riser in either laboratory or clinical tests of this construct. Proximally, the cables are placed on the periosteum of the olecranon but deep to the triceps tendon and adjacent to the K-wires. The locking clip is placed on the posterolateral aspect of the elbow joint in a location where it can be covered with local tissue for adequate padding. The cable is then threaded through the clamping bracket and tightened slowly and gradually with a tensioning device to low torque level (Figure 1). At this stage, tension may be released to make any necessary adjustments. Last, the locking clip is deployed, securing the tension band in the clip, and the excess cable is trimmed with a scalpel. Softening and pliability of the cable during its insertion and tensioning should be noted.
The ends of the K-wires are now curved in a hook configuration. The tines of the hooks should be parallel to accommodate the cable, and then the triceps is sharply incised to bone. If the bone is hard, an awl is used to create a pilot hole so the hook may be impaled into bone while capturing the cable. Next, the triceps is closed over the pins, minimizing the potential for pin migration and backout. The 2 K-wires are left in place to keep the fragments in proper anatomical alignment during healing and to prevent displacement with elbow motion. Figure 2 is a schematic of the final construct, and Figure 3 shows the construct in a patient.
Reduction of the olecranon fracture is assessed by imaging in full extension to check for possible implant impingement. Last, we apply the previously harvested fracture callus to the fracture site. Layered closure is performed, and bulky soft dressings are applied. Postoperative immobilization with a splint is used. Gentle range-of-motion exercises begin in about 2 weeks and progress as pain allows.
A case example with preoperative and postoperative images taken at 3-month follow-up is provided in Figure 4. The entire surgical technique can be viewed in the Video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clinical Cases
Between July 2007 and February 2011, 7 patients with displaced olecranon fractures underwent osteosynthesis using the isoelastic tension band (Table 1). According to the Mayo classification system, 5 of these patients had type 2A fractures, 1 had a type 2B fracture with an ipsilateral nondisplaced radial neck fracture, and 1 had a type 3B fracture. There were 4 female and 3 male patients. The injury was on the dominant side in 3 patients. All patients gave informed consent to evaluation at subsequent office visits and completed outcomes questionnaires by mail several years after surgery. Mean follow-up at which outcome measures questionnaires were obtained was 3.3 years (range, 2.1-6.8 years). Exclusion criteria were age under 18 years and inability to provide informed consent, fracture patterns with extensive articular comminution, and open fractures. Permission to conduct this research was granted by institutional review board.
At each visit, patients completed the Disabilities of the Arm, Shoulder, and Hand (DASH) functional outcome survey and were evaluated according to Broberg and Morrey’s elbow scoring system.13,14 Chart review consisted of evaluation of medical records, including radiographs and orthopedic physician notes in which preoperative examination was documented, mechanism of injury was noted, radiologic fracture pattern was evaluated, and time to bony union was recorded. Elbow motion was documented. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan) set at level 2, as delineated in Broberg and Morrey’s functional elbow scoring system.
Results
The 7 patients were assessed at a mean final follow-up of 19 months after surgery and received a mean Broberg and Morrey score of good (92.2/100) (Table 2). Restoration of motion and strength was excellent; compared with contralateral extremity, mean flexion arc was 96%, and mean forearm rotation was 96%. Grip was 99% of the noninjured side, perhaps the result of increased conditioning from physical therapy. Patients completed outcomes questionnaires at a mean of 3.3 years after surgery. Mean (SD) DASH score at this longest follow-up was 12.6 (17.2) (Table 2). Patients were satisfied (mean, 9.8/10; range, 9.5-10) and had little pain (mean, 0.8/10; range, 0-3). All fractures united, and there were no infections. One patient had a satisfactory union with complete restoration of motion and continued to play sports vocationally but developed pain over the locking clip 5 years after the index procedure and decided to have the implant removed. He had no radiographic evidence of K-wire or implant migration. Another patient had a minor degree of implant irritation at longest follow-up but did not request hardware removal.
Discussion
Stainless steel wire is often used in TBW because of its widespread availability, low cost, lack of immunogenicity, and relative strength.7 However, stainless steel wire has several disadvantages. It is susceptible to low-cycle fatigue failure, and fatigue strength may be seriously reduced secondary to incidental trauma to the wire on implantation.15,16 Other complications are kinking, skin irritation, implant prominence, fixation loss caused by wire loosening, and inadequate initial reduction potentially requiring revision.10,12,17-21
Isoelastic cable is a new type of cerclage cable that consists of UHMWPE strands braided over a nylon core. The particular property profile of the isoelastic tension band gives the cable intrinsic elastic and pliable qualities. In addition, unlike stainless steel, the band maintains a uniform, continuous compression force across a fracture site.22 Multifilament braided cables fatigue and fray, but the isoelastic cerclage cable showed no evidence of fraying or breakage after 1 million loading cycles.22,23 Compared with metal wire or braided metal cable, the band also has higher fatigue strength and higher ultimate tensile strength.7 Furthermore, the cable is less abrasive than stainless steel, so theoretically it is less irritating to surrounding subcutaneous tissue. Last, the pliability of the band allows the surgeon to create multiple loops of cable without the wire-failure side effects related to kinking, which is common with the metal construct.
In 2010, Ting and colleagues24 retrospectively studied implant failure complications associated with use of isoelastic cerclage cables in the treatment of periprosthetic fractures in total hip arthroplasty. They reported a breakage rate of 0% and noted that previously published breakage data for metallic cerclage devices ranged from 0% to 44%. They concluded that isoelastic cables were not associated with material failure, and there were no direct complications related to the cables. Similarly, Edwards and colleagues25 evaluated the same type of cable used in revision shoulder arthroplasty and reported excellent success and no failures. Although these data stem from use in the femur and humerus, we think the noted benefits apply to fractures of the elbow as well, as we observed a similar breakage rate (0%).
Various studies have addressed the clinical complaints and reoperation rates associated with retained metal implants after olecranon fixation. Traditional AO (Arbeitsgemeinschaft für Osteosynthesefragen) technique involves subcutaneous placement of stainless steel wires, which often results in tissue irritation. Reoperation rates as high as 80% have been reported, and a proportion of implant removals may in fact be caused by factors related to the subcutaneous placement of the metallic implants rather than K-wire migration alone.5,12,18 A nonmetallic isoelastic tension band can provide a more comfortable and less irritating implant, which could reduce the need for secondary intervention related to painful subcutaneous implant. One of our 7 patients had a symptomatic implant removed 5 years after surgery. This patient complained of pain over the area of the tension band device clip, so after fracture healing the entire fixation device was removed in the operating room. If reoperation is necessary, removal of intramedullary K-wires is relatively simple using a minimal incision; removal of stainless steel TBW may require a larger approach if the twisted knots cannot be easily retrieved.
A study of compression forces created by stainless steel wire demonstrated that a “finely tuned mechanical sense” was needed to produce optimal fixation compression when using stainless steel wire.26 It was observed that a submaximal twist created insufficient compressive force, while an ostensibly minimal increase in twisting force above optimum abruptly caused wire failure through breakage. Cerclage cables using clasping devices, such as the current isoelastic cerclage cable, were superior in ease of application. Furthermore, a clasping device allows for cable tension readjustment that is not possible with stainless steel wire. The clasping mechanism precludes the surgeon from having to bury the stainless steel knot and allows for the objective cable-tensioning not possible with stainless steel wire. Last, the tensioning device is titratable, which allows the surgeon to set the construct at a predetermined quantitative tension, which is of benefit in patients with osteopenia.
One limitation of this study is that it did not resolve the potential for K-wire migration, and we agree with previous recommendations that careful attention to surgical technique may avoid such a complication.10 In addition, the sample was small, and the study lacked a control group; a larger sample and a control group would have boosted study power. Nevertheless, the physical and functional outcomes associated with use of this technique were excellent. These results demonstrate an efficacious attempt to decrease secondary surgery rates and are therefore proof of concept that the isoelastic tension band may be used as an alternative to stainless steel in the TBW of displaced olecranon fractures with minimal or no comminution.
Conclusion
This easily reproducible technique for use of an isoelastic tension band in olecranon fracture fixation was associated with excellent physical and functional outcomes in a series of 7 patients. The rate of secondary intervention was slightly better for these patients than for patients treated with wire tension band fixation. Although more rigorous study of this device is needed, we think it is a promising alternative to wire tension band techniques.
1. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
2. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
3. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
4. Weber BG, Vasey H. Osteosynthesis in olecranon fractures [in German]. Z Unfallmed Berufskr. 1963;56:90-96.
5. Netz P, Strömberg L. Non-sliding pins in traction absorbing wiring of fractures: a modified technique. Acta Orthop Scand. 1982;53(3):355-360.
6. Prayson MJ, Williams JL, Marshall MP, Scilaris TA, Lingenfelter EJ. Biomechanical comparison of fixation methods in transverse olecranon fractures: a cadaveric study. J Orthop Trauma. 1997;11(8):565-572.
7. Rothaug PG, Boston RC, Richardson DW, Nunamaker DM. A comparison of ultra-high-molecular weight polyethylene cable and stainless steel wire using two fixation techniques for repair of equine midbody sesamoid fractures: an in vitro biomechanical study. Vet Surg. 2002;31(5):445-454.
8. Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
9. Nimura A, Nakagawa T, Wakabayashi Y, Sekiya I, Okawa A, Muneta T. Repair of olecranon fractures using FiberWire without metallic implants: report of two cases. J Orthop Surg Res. 2010;5:73.
10. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
11. Helm RH, Hornby R, Miller SW. The complications of surgical treatment of displaced fractures of the olecranon. Injury. 1987;18(1):48-50.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128-146.
14. Broberg MA, Morrey BF. Results of delayed excision of the radial head after fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
15. Bostrom MP, Asnis SE, Ernberg JJ, et al. Fatigue testing of cerclage stainless steel wire fixation. J Orthop Trauma. 1994;8(5):422-428.
16. Oh I, Sander TW, Treharne RW. The fatigue resistance of orthopaedic wire. Clin Orthop Relat Res. 1985;(192):228-236.
17. Amstutz HC, Maki S. Complications of trochanteric osteotomy in total hip replacement. J Bone Joint Surg Am. 1978;60(2):214-216.
18. Jensen CM, Olsen BB. Drawbacks of traction-absorbing wiring (TAW) in displaced fractures of the olecranon. Injury. 1986;17(3):174-175.
19. Kumar G, Mereddy PK, Hakkalamani S, Donnachie NJ. Implant removal following surgical stabilization of patella fracture. Orthopedics. 2010;33(5).
20. Hume MC, Wiss DA. Olecranon fractures. A clinical and radiographic comparison of tension band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
21. Wolfgang G, Burke F, Bush D, et al. Surgical treatment of displaced olecranon fractures by tension band wiring technique. Clin Orthop Relat Res. 1987;(224):192-204.
22. Sarin VK, Mattchen TM, Hack B. A novel iso-elastic cerclage cable for treatment of fractures. Paper presented at: Annual Meeting of the Orthopaedic Research Society; February 20-23, 2005; Washington, DC. Paper 739.
23. Silverton CD, Jacobs JJ, Rosenberg AG, Kull L, Conley A, Galante JO. Complications of a cable grip system. J Arthroplasty. 1996;11(4):400-404.
24. Ting NT, Wera GD, Levine BR, Della Valle CJ. Early experience with a novel nonmetallic cable in reconstructive hip surgery. Clin Orthop Relat Res. 2010;468(9):2382-2386.
25. Edwards TB, Stuart KD, Trappey GJ, O’Connor DP, Sarin VK. Utility of polymer cerclage cables in revision shoulder arthroplasty. Orthopedics. 2011;34(4).
26. Shaw JA, Daubert HB. Compression capability of cerclage fixation systems. A biomechanical study. Orthopedics. 1988;11(8):1169-1174.
1. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
2. Veillette CJ, Steinmann SP. Olecranon fractures. Orthop Clin North Am. 2008;39(2):229-236.
3. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
4. Weber BG, Vasey H. Osteosynthesis in olecranon fractures [in German]. Z Unfallmed Berufskr. 1963;56:90-96.
5. Netz P, Strömberg L. Non-sliding pins in traction absorbing wiring of fractures: a modified technique. Acta Orthop Scand. 1982;53(3):355-360.
6. Prayson MJ, Williams JL, Marshall MP, Scilaris TA, Lingenfelter EJ. Biomechanical comparison of fixation methods in transverse olecranon fractures: a cadaveric study. J Orthop Trauma. 1997;11(8):565-572.
7. Rothaug PG, Boston RC, Richardson DW, Nunamaker DM. A comparison of ultra-high-molecular weight polyethylene cable and stainless steel wire using two fixation techniques for repair of equine midbody sesamoid fractures: an in vitro biomechanical study. Vet Surg. 2002;31(5):445-454.
8. Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
9. Nimura A, Nakagawa T, Wakabayashi Y, Sekiya I, Okawa A, Muneta T. Repair of olecranon fractures using FiberWire without metallic implants: report of two cases. J Orthop Surg Res. 2010;5:73.
10. Macko D, Szabo RM. Complications of tension-band wiring of olecranon fractures. J Bone Joint Surg Am. 1985;67(9):1396-1401.
11. Helm RH, Hornby R, Miller SW. The complications of surgical treatment of displaced fractures of the olecranon. Injury. 1987;18(1):48-50.
12. Romero JM, Miran A, Jensen CH. Complications and re-operation rate after tension-band wiring of olecranon fractures. J Orthop Sci. 2000;5(4):318-320.
13. Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14(2):128-146.
14. Broberg MA, Morrey BF. Results of delayed excision of the radial head after fracture. J Bone Joint Surg Am. 1986;68(5):669-674.
15. Bostrom MP, Asnis SE, Ernberg JJ, et al. Fatigue testing of cerclage stainless steel wire fixation. J Orthop Trauma. 1994;8(5):422-428.
16. Oh I, Sander TW, Treharne RW. The fatigue resistance of orthopaedic wire. Clin Orthop Relat Res. 1985;(192):228-236.
17. Amstutz HC, Maki S. Complications of trochanteric osteotomy in total hip replacement. J Bone Joint Surg Am. 1978;60(2):214-216.
18. Jensen CM, Olsen BB. Drawbacks of traction-absorbing wiring (TAW) in displaced fractures of the olecranon. Injury. 1986;17(3):174-175.
19. Kumar G, Mereddy PK, Hakkalamani S, Donnachie NJ. Implant removal following surgical stabilization of patella fracture. Orthopedics. 2010;33(5).
20. Hume MC, Wiss DA. Olecranon fractures. A clinical and radiographic comparison of tension band wiring and plate fixation. Clin Orthop Relat Res. 1992;(285):229-235.
21. Wolfgang G, Burke F, Bush D, et al. Surgical treatment of displaced olecranon fractures by tension band wiring technique. Clin Orthop Relat Res. 1987;(224):192-204.
22. Sarin VK, Mattchen TM, Hack B. A novel iso-elastic cerclage cable for treatment of fractures. Paper presented at: Annual Meeting of the Orthopaedic Research Society; February 20-23, 2005; Washington, DC. Paper 739.
23. Silverton CD, Jacobs JJ, Rosenberg AG, Kull L, Conley A, Galante JO. Complications of a cable grip system. J Arthroplasty. 1996;11(4):400-404.
24. Ting NT, Wera GD, Levine BR, Della Valle CJ. Early experience with a novel nonmetallic cable in reconstructive hip surgery. Clin Orthop Relat Res. 2010;468(9):2382-2386.
25. Edwards TB, Stuart KD, Trappey GJ, O’Connor DP, Sarin VK. Utility of polymer cerclage cables in revision shoulder arthroplasty. Orthopedics. 2011;34(4).
26. Shaw JA, Daubert HB. Compression capability of cerclage fixation systems. A biomechanical study. Orthopedics. 1988;11(8):1169-1174.
Orthopedics in US Health Care
In the United States, the landscape of health care is changing. Health care reform and fluctuating political and economic climates have affected and will continue to affect the practice of orthopedic surgery. Demand for musculoskeletal care and the costs of providing this care are exceeding available resources—which has led to an evolution in how we practice as individuals and in the institutions where we provide care. Patient safety, quality, and value have become the outcomes of importance. Orthopedic surgeons, as experts in musculoskeletal care, must be a part of these changes. In this review, we offer perspective on the changing face of orthopedic surgery in the modern US health care system.
1. Meeting the demand
Musculoskeletal conditions represent one of the most common and costly health issues in the United States, affecting individuals medically and economically and compromising their quality of life.1,2 In 2008, more than 110 million US adults (1 in 2) reported having a musculoskeletal condition for more than 3 months, and almost 7% reported that a chronic musculoskeletal condition made routine activities of daily living significantly difficult.1 Overall, in the United States, some of the most common chronic conditions are musculoskeletal in origin. These conditions include osteoarthritis and back pain.
Osteoarthritis is the leading cause of chronic pain and disability. Physician-diagnosed arthritis is expected to affect 25% of US adults by 2030,3 and in more than one-third of these patients arthritis limits work or other activity.4 Back pain is another of the most common debilitating conditions in the United States.3,5 St Sauver and colleagues6 found that back pain is the third most common condition (23.9%) that prompts patients to seek health care—following skin-related problems (42.7%) and osteoarthritis/joint pain (33.6%).
As life expectancy increases, so do expectations of enjoying higher levels of activity into the later years. Patients expect to be as active in their geriatric years as they were in middle age, and many are able to do so. Amid the growing obesity epidemic and increased incidence of chronic comorbidities, however, the aging population not only is at substantial risk for developing a chronic musculoskeletal disorder but may face new challenges in accessing care.
Although orthopedic surgeons specialize in treating musculoskeletal conditions, up to 90% of common nonsurgical musculoskeletal complaints are thought to be manageable in the primary care setting.7 With a disproportionate increase in musculoskeletal demand against a relatively constant number of orthopedic providers,8 it is becoming increasingly important for nonorthopedists to adequately manage musculoskeletal conditions. Physiatrists, rheumatologists, internists, family practitioners, and the expanding field of sports medicine specialists provide primary care of musculoskeletal conditions. To meet the growing demand and to ensure that patients receive quality, sustainable, effective, and efficient care, orthopedic surgeons should be actively involved in training these providers. As high as the cost of managing musculoskeletal conditions can be, it is far less than the cost resulting from inadequate or improper management. There is already justification for formal development of a specialization in nonoperative management of musculoskeletal care. Establishing this specialization requires a multidisciplinary approach, with orthopedic surgery taking a lead role.
2. The cost equation
As the prevalence of orthopedic conditions increases, so does the cost of delivering musculoskeletal care. The economic implications of meeting this growing demand are an important area of concern for our health care system. Steadily increasing hospital expenses for personnel and services, rising costs of pharmaceuticals and laboratory tests, constant evolution of costly technology, and insurance/reimbursement rates that do not keep pace with rising costs all contribute to the rapid escalation of the “cost of care.”
Health care expenditures accounted for 17.2% of the US gross domestic product (GDP) in 2012 and are expected to represent 19.3% by 2023.9 For musculoskeletal disease, direct costs alone are expected to approach $510 billion, equaling 5% of GDP and representing almost 30% of all health care expenditures. In Medicare patients, osteoarthritis is the most expensive condition to treat overall, and 3 other musculoskeletal problems rank highly as well: femoral neck fractures (3rd), back pain (10th), and fractures of all types (16th).10 Clearly, musculoskeletal care is one of the most prevalent and expensive health conditions in the United States.
Part of the direct costs of care that consistently increase each year are the steadily increasing costs of technology, which is often considered synonymous with orthopedic care. Promotion of new and more costly implants is common in the absence of evidence supporting their use. However, use of new implants and technology is being scrutinized in an effort to strike the proper cost–benefit balance.
To change the slope of the cost curve, orthopedic surgeons should utilize technological advances that are proven to be clinically significant and economically feasible and should avoid modest improvements with limited clinical benefit and higher price tags. Unfortunately, this approach is not being taken. Minor modifications of implant designs are often marketed as “new and improved” to justify increased costs, and these implants often gain widespread use. A few may prove to be clinically better, but most will be only comparable to older, less expensive designs, and some may end up being clinical failures, discovered at great cost to patients and the health care system.11,12
Orthopedic surgeons have an important role in this decision-making. We should strive for the best, most cost-effective outcomes for our patients. We should reject new technology that does not clearly improve outcomes. At the least, we should use the technology in a manufacturer-supported clinical trial to determine its superiority. Whether the improvement is in technique, implant design, or workflow efficiency, orthopedic surgeons must be actively involved in researching and developing the latest innovations and must help determine their prospective value by considering not only their potential clinical benefits but also their economic implications.
As the political and economic environment becomes more directed at the cost-containment and sustainability of care, there has been a clear shift in focus to quality and value rather than volume, giving rise to the “value-based care” approach. The “value equation,” in which value equals quality divided by cost, requires a clear measure of outcomes and an equally clear understanding of costs. Delivering high-quality care in a cost-conscious environment is an approach that every orthopedic surgeon should adopt. Widespread adoption of the value-based strategy by hospital systems and insurance companies is resulting in a paradigm shift away from more traditional volume-based metrics and in favor of value-based metrics, including quality measures, patient-reported outcomes, Hospital Consumer Assessment of Healthcare Providers and Systems, and physician-specific outcome measures.
The new paradigm has brought the bundled payment initiative (BPI), a strategy included in the Patient Protection and Affordable Care Act. The philosophy behind the BPI model is for hospital systems and physicians to control costs while maintaining and improving the quality of care. Measured by patient metrics (eg, clinical outcomes, patient satisfaction) and hospital metrics (eg, readmission rates, cost of care), bundled payments reimburse hospitals on the basis of cost of an entire episode of care rather than on the basis of individual procedures and services. This approach provides incentives for both physicians and hospitals to promote value-based care while emphasizing coordination of care among all members of the health care team.
Providing the best possible care for our patients while holding our practice to the highest standards is a central tenet of the practice of orthopedic surgery and should be independent of reimbursement strategies. Thus, to increase the value of care, we must establish practice models and strategies to optimize cost-efficiency while improving outcomes. As explained by Porter and Teisberg,13 it is important to be conscientious about cost, but above all we must not allow quality of health care delivery to be compromised when trying to improve the “value” of care. Through evidence-based management and a clear understanding of costs, we must develop cost-efficient practice models that sustainably deliver the highest value of care.
3. Evolving practice models
As the health care landscape continues to change, physician practice models evolve accordingly. Although the private practice model once dominated the physician workforce, this is no longer true, as there has been a significant shift to employer-based practice models. The multiple factors at work relate to changing patterns of reimbursement, increasing government regulations, and a general change in recent residency graduates’ expectations regarding work–life balance. Other catalysts are the shift from volume- to value-based care and the recognition that cost-effective health care is more easily achieved when physicians and their institutions are in alignment. Ultimately, physician–institution alignment is crucial in improving care and outcomes.
Physician–institution alignment requires further discussion. Ideally, it should strike the proper balance between physician autonomy and institutional priorities to ensure the highest quality care. Physicians and their institutions should align their interests in terms of patient safety, quality, and economics to create a work environment conducive to both patient/physician satisfaction and institutional success.14 As identified by Page and colleagues,15 the primary drivers of physician–institution alignment, specific to orthopedic surgery, are economic, regulatory, and cultural. In economics, implant selection and ancillary services are the important issues; in the regulatory area, cooperative efforts to address expanding state and federal requirements are needed; last, the primary cultural driver is delivery of care to an expanding, diverse patient population.
Physician–institution alignment brings opportunities for “gainsharing,” which can directly benefit individual physicians, physician groups, and departments. Gainsharing is classically defined as “arrangements in which a hospital gives physicians a percentage share of any reduction in the hospital’s costs for patient care attributable in part to the physicians’ efforts.”16 Modern gainsharing programs can be used by institutions to align the economic interests of physicians and hospitals, with the ultimate goal being to achieve a sustainable increase in the value and quality of care delivered to patients.13 Examples include efforts to reduce the cost of orthopedic implants, which is a major cost driver in orthopedic surgery. Our institution realized significant savings when surgeons were directly involved in the implant contracting process with strategic sourcing personnel. These savings were shared with the department to enhance research and education programs. BPI, a risk-sharing program in which Medicare and hospitals participate, incorporates gainsharing opportunities in which each participating physician can receive up to 50% of his or her previous Medicare billings when specific targets are achieved. BPI included 27 musculoskeletal diagnosis–related groups that could be developed into a bundled payment proposal. Our institution participated in a 90-day episode, for primary hip and knee arthroplasty and non–cervical spine fusion, that had very promising results.
Gainsharing offers physicians incentives to meet institution goals of improved outcomes and increased patient satisfaction while increasing oversight and accountability. When physician-specific outcomes do not meet the established goals in key areas (readmissions, thromboembolic complications, infections), it is only logical that steps will be taken to improve outcomes. Although physicians may not be used to this increased scrutiny, the goal of improving outcomes, even if it necessitates a change in an established approach to care, should be welcomed.
Physicians should be rewarded for good outcomes but not suboptimal outcomes. When outcomes are suboptimal, physicians should take a constructive approach to improve them. On the other hand, not being rewarded for unachieved goals can be perceived as being penalized. Additional monitoring may paradoxically lead physicians to avoid more “complex” cases, such as those of patients at higher risk for complications and poorer outcomes. An example is found in patient selection for surgery, in which issues like obesity, diabetes, and heart disease are known to negatively affect outcomes. In these models, “cherry-picking” is a well-recognized risk17,18 that can compromise our ethical obligation to provide equal access for all patients. To offset this tendency, we should use a risk-stratification model in which all patients are not considered equal in the risks they present. A risk-adjustment approach benefits both patients and providers by identifying modifiable risk factors that can be addressed to positively affect outcomes. This risk-stratification approach further incentivizes the orthopedist to closely work with other health care providers to address the medical comorbidities that may negatively affect surgical outcomes.
4. Patient and physician expectations
Living in a technology-driven society in the age of information has had a major impact on patients’ attitudes and expectations about their care—and therefore on physicians’ practice methods. It is uncommon to evaluate a patient who has not already consulted the Internet about a problem. Patients now have much more information they can use to make decisions about their treatment, and, though many question the accuracy of Internet information, there is no argument that being more informed is beneficial. In this time of shared decision-making, it is absolutely essential that patients keep themselves informed.
It is crucial to align the expectations of both physicians and patients in order to achieve the best outcomes. Gaining a clear understanding of treatment goals, management, and potential complications consistently leads to improved patient satisfaction, more favorable clinical outcomes, and reduced risk of litigation.19-22 Addressing patient concerns and expectations is significantly enhanced by a strong patient–physician relationship through clinical models focused on patient-centered care.
Now considered a standard of care, the patient-centered model has changed the way we practice. The foundation of the patient-centered approach is to strengthen the patient–physician relationship by empowering patients to become active decision-makers in the management of their own health. The role of orthopedists in this model is to provide patients with information and insight into their conditions in order to facilitate shared decision-making. Our role should be to guide patients to make educated and informed decisions. Doing so enhances communication, thereby strengthening the patient–physician relationship, and places both patient and physician expectations in perspective. Patient-reported outcomes, satisfaction rates, symptomatic burdens, and costs of care are all positively correlated with strong communication and realistic expectations achieved through a patient-centered approach.21,23
The evolution of clinical practice has been influenced by factors ranging from external forces (eg, changing political and economic climates) to social trends (use of social media and the Internet). Technology has been a driving force in our rapidly changing clinical environment, significantly altering the way we practice. Although we must be careful in how we use it, new technology can certainly work to our advantage. We have a plethora of medical information at our fingertips, and, with physician-directed guidance, our patients can become more informed than ever before. This is the principle of patient-centered medicine and shared decision-making, and its utility will only increase in importance.
5. The role of advocacy
The central tenet of orthopedic practice has always been a focus on patients. We continually strive to improve patient outcomes, reduce costs, and work efficiently in our practices and facilities. Although we can focus on our individual practices, we cannot ignore the influence and impact of the political system on our performance. Federal and state regulations give physicians and insurance companies an uneven playing field. This imbalance requires that physicians be more active in health care policymaking and advocacy. Although we are more involved than ever before, our influence is far less than what we would like it to be, perhaps partly because of the nature of the political process but perhaps also because of physicians’ resistance to becoming involved.
As experts in the treatment of musculoskeletal conditions, we should be at the forefront of health care policy development—a position we have not been able to attain. Although many factors contribute to our lack of a “seat at the table,” we must recognize our reluctance as a group to support advocacy, either financially or through personal time commitment. The American Association of Orthopaedic Surgeons (AAOS) Orthopaedic Political Action Committee has never been able to obtain donations from more than 30% of AAOS members. Although this committee historically has been successful, we could be much more so if we had financial support from 90% of members. There are many ways to be actively involved in advocacy. One way is to join local and state orthopedic societies and support their advocacy efforts. State orthopedic societies work closely with the AAOS Office of Government Relations to coordinate advocacy and direct efforts and resources to areas of greatest need. Knowing local congressional representatives and communicating with them about issues we face in our practices make our issues “real.” Some of our colleagues have even successfully run for office in Congress, and they certainly deserve our support. Advocacy will absolutely play an increasingly important role as federal and state governments expand their involvement in health care. Our role should be to get involved, at least to some degree. We need to recognize that our strength is in our numbers, as the few cannot accomplish nearly as much as the many.
Summary
Orthopedic surgeons are practicing in the midst of almost constant change—evolving patient care, shifts in employment models, advances in technology, modern patient expectations, and an increasingly complex regulatory environment. Even in this context, however, our goal remains unchanged: to give our patients the highest-quality care possible. Our core values as orthopedic surgeons and physicians are dedication, commitment, and service to patients and to our profession. As US health care continues to evolve, we must evolve as well, with an emphasis on expanding our role in the health care policy debate.
1. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. Rosemont, IL: US Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed October 26, 2015.
2. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. 2nd ed. Rosemont, IL: US Bone and Joint Initiative; 2011. http://www.boneandjointburden.org. Accessed October 26, 2015.
3. Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986-995.e1.
4. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
5. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
6. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56-67.
7. Anderson BC. Office Orthopedics for Primary Care: Diagnosis and Treatment. 2nd ed. Philadelphia, PA: Saunders; 1999.
8. American Academy of Orthopaedic Surgeons, Department of Research and Scientific Affairs. Orthopaedic Practice in the U.S. 2012 [2012 Orthopaedic Surgeon Census Report]. Rosemont, IL: American Academy of Orthopaedic Surgeons; January 2013.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. NHE [National Health Expenditure] Fact Sheet, 2014. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Updated July 28, 2015. Accessed October 26, 2015.
10. Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075-1077.
11. Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46.
12. Dahlstrand H, Stark A, Anissian L, Hailer NP. Elevated serum concentrations of cobalt, chromium, nickel, and manganese after metal-on-metal alloarthroplasty of the hip: a prospective randomized study. J Arthroplasty. 2009;24(6):837-845.
13. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
14. American Association of Orthopaedic Surgeons. Alignment of physician and facility payment and incentives. Position statement 1171. American Association of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/position/1171.asp. Published September 2006. Revised February 2009. Accessed October 26, 2015.
15. Page AE, Butler CA, Bozic KJ. Factors driving physician–hospital alignment in orthopaedic surgery. Clin Orthop Relat Res. 2013;471(6):1809-1817.
16. US Department of Health and Human Services, Office of Inspector General. Gainsharing arrangements and CMPs for hospital payments to physicians to reduce or limit services to beneficiaries [special advisory bulletin]. Office of Inspector General website. http://oig.hhs.gov/fraud/docs/alertsandbulletins/gainsh.htm. Published July 1999. Accessed October 26, 2015.
17. Bronson WH, Fewer M, Godlewski K, et al. The ethics of patient risk modification prior to elective joint replacement surgery. J Bone Joint Surg Am. 2014;96(13):e113.
18. Bosco J. To cherry pick or not: the unintended ethical consequences of pay for performance. Presented at: New York University Colloquium on Medical Ethics; New York, NY; November 2014.
19. Hageman MG, Briët JP, Bossen JK, Blok RD, Ring DC, Vranceanu AM. Do previsit expectations correlate with satisfaction of new patients presenting for evaluation with an orthopaedic surgical practice? Clin Orthop Relat Res. 2015;473(2):716-721.
20. Jourdan C, Poiraudeau S, Descamps S, et al. Comparison of patient and surgeon expectations of total hip arthroplasty. PLoS One. 2012;7(1):e30195.
21. McMillan S, Kendall E, Sav A, et al. Patient-centered approaches to health care: a systematic review of randomized controlled trials. Med Care Res Rev. 2013;70(6):567-596.
22. Forster HP, Schwartz J, DeRenzo E. Reducing legal risk by practicing patient-centered medicine. Arch Intern Med. 2002;162(11):1217-1219.
23. Van Citters AD, Fahlman C, Goldmann DA, et al. Developing a pathway for high-value, patient-centered total joint arthroplasty. Clin Orthop Relat Res. 2014;472(5):1619-1635.
In the United States, the landscape of health care is changing. Health care reform and fluctuating political and economic climates have affected and will continue to affect the practice of orthopedic surgery. Demand for musculoskeletal care and the costs of providing this care are exceeding available resources—which has led to an evolution in how we practice as individuals and in the institutions where we provide care. Patient safety, quality, and value have become the outcomes of importance. Orthopedic surgeons, as experts in musculoskeletal care, must be a part of these changes. In this review, we offer perspective on the changing face of orthopedic surgery in the modern US health care system.
1. Meeting the demand
Musculoskeletal conditions represent one of the most common and costly health issues in the United States, affecting individuals medically and economically and compromising their quality of life.1,2 In 2008, more than 110 million US adults (1 in 2) reported having a musculoskeletal condition for more than 3 months, and almost 7% reported that a chronic musculoskeletal condition made routine activities of daily living significantly difficult.1 Overall, in the United States, some of the most common chronic conditions are musculoskeletal in origin. These conditions include osteoarthritis and back pain.
Osteoarthritis is the leading cause of chronic pain and disability. Physician-diagnosed arthritis is expected to affect 25% of US adults by 2030,3 and in more than one-third of these patients arthritis limits work or other activity.4 Back pain is another of the most common debilitating conditions in the United States.3,5 St Sauver and colleagues6 found that back pain is the third most common condition (23.9%) that prompts patients to seek health care—following skin-related problems (42.7%) and osteoarthritis/joint pain (33.6%).
As life expectancy increases, so do expectations of enjoying higher levels of activity into the later years. Patients expect to be as active in their geriatric years as they were in middle age, and many are able to do so. Amid the growing obesity epidemic and increased incidence of chronic comorbidities, however, the aging population not only is at substantial risk for developing a chronic musculoskeletal disorder but may face new challenges in accessing care.
Although orthopedic surgeons specialize in treating musculoskeletal conditions, up to 90% of common nonsurgical musculoskeletal complaints are thought to be manageable in the primary care setting.7 With a disproportionate increase in musculoskeletal demand against a relatively constant number of orthopedic providers,8 it is becoming increasingly important for nonorthopedists to adequately manage musculoskeletal conditions. Physiatrists, rheumatologists, internists, family practitioners, and the expanding field of sports medicine specialists provide primary care of musculoskeletal conditions. To meet the growing demand and to ensure that patients receive quality, sustainable, effective, and efficient care, orthopedic surgeons should be actively involved in training these providers. As high as the cost of managing musculoskeletal conditions can be, it is far less than the cost resulting from inadequate or improper management. There is already justification for formal development of a specialization in nonoperative management of musculoskeletal care. Establishing this specialization requires a multidisciplinary approach, with orthopedic surgery taking a lead role.
2. The cost equation
As the prevalence of orthopedic conditions increases, so does the cost of delivering musculoskeletal care. The economic implications of meeting this growing demand are an important area of concern for our health care system. Steadily increasing hospital expenses for personnel and services, rising costs of pharmaceuticals and laboratory tests, constant evolution of costly technology, and insurance/reimbursement rates that do not keep pace with rising costs all contribute to the rapid escalation of the “cost of care.”
Health care expenditures accounted for 17.2% of the US gross domestic product (GDP) in 2012 and are expected to represent 19.3% by 2023.9 For musculoskeletal disease, direct costs alone are expected to approach $510 billion, equaling 5% of GDP and representing almost 30% of all health care expenditures. In Medicare patients, osteoarthritis is the most expensive condition to treat overall, and 3 other musculoskeletal problems rank highly as well: femoral neck fractures (3rd), back pain (10th), and fractures of all types (16th).10 Clearly, musculoskeletal care is one of the most prevalent and expensive health conditions in the United States.
Part of the direct costs of care that consistently increase each year are the steadily increasing costs of technology, which is often considered synonymous with orthopedic care. Promotion of new and more costly implants is common in the absence of evidence supporting their use. However, use of new implants and technology is being scrutinized in an effort to strike the proper cost–benefit balance.
To change the slope of the cost curve, orthopedic surgeons should utilize technological advances that are proven to be clinically significant and economically feasible and should avoid modest improvements with limited clinical benefit and higher price tags. Unfortunately, this approach is not being taken. Minor modifications of implant designs are often marketed as “new and improved” to justify increased costs, and these implants often gain widespread use. A few may prove to be clinically better, but most will be only comparable to older, less expensive designs, and some may end up being clinical failures, discovered at great cost to patients and the health care system.11,12
Orthopedic surgeons have an important role in this decision-making. We should strive for the best, most cost-effective outcomes for our patients. We should reject new technology that does not clearly improve outcomes. At the least, we should use the technology in a manufacturer-supported clinical trial to determine its superiority. Whether the improvement is in technique, implant design, or workflow efficiency, orthopedic surgeons must be actively involved in researching and developing the latest innovations and must help determine their prospective value by considering not only their potential clinical benefits but also their economic implications.
As the political and economic environment becomes more directed at the cost-containment and sustainability of care, there has been a clear shift in focus to quality and value rather than volume, giving rise to the “value-based care” approach. The “value equation,” in which value equals quality divided by cost, requires a clear measure of outcomes and an equally clear understanding of costs. Delivering high-quality care in a cost-conscious environment is an approach that every orthopedic surgeon should adopt. Widespread adoption of the value-based strategy by hospital systems and insurance companies is resulting in a paradigm shift away from more traditional volume-based metrics and in favor of value-based metrics, including quality measures, patient-reported outcomes, Hospital Consumer Assessment of Healthcare Providers and Systems, and physician-specific outcome measures.
The new paradigm has brought the bundled payment initiative (BPI), a strategy included in the Patient Protection and Affordable Care Act. The philosophy behind the BPI model is for hospital systems and physicians to control costs while maintaining and improving the quality of care. Measured by patient metrics (eg, clinical outcomes, patient satisfaction) and hospital metrics (eg, readmission rates, cost of care), bundled payments reimburse hospitals on the basis of cost of an entire episode of care rather than on the basis of individual procedures and services. This approach provides incentives for both physicians and hospitals to promote value-based care while emphasizing coordination of care among all members of the health care team.
Providing the best possible care for our patients while holding our practice to the highest standards is a central tenet of the practice of orthopedic surgery and should be independent of reimbursement strategies. Thus, to increase the value of care, we must establish practice models and strategies to optimize cost-efficiency while improving outcomes. As explained by Porter and Teisberg,13 it is important to be conscientious about cost, but above all we must not allow quality of health care delivery to be compromised when trying to improve the “value” of care. Through evidence-based management and a clear understanding of costs, we must develop cost-efficient practice models that sustainably deliver the highest value of care.
3. Evolving practice models
As the health care landscape continues to change, physician practice models evolve accordingly. Although the private practice model once dominated the physician workforce, this is no longer true, as there has been a significant shift to employer-based practice models. The multiple factors at work relate to changing patterns of reimbursement, increasing government regulations, and a general change in recent residency graduates’ expectations regarding work–life balance. Other catalysts are the shift from volume- to value-based care and the recognition that cost-effective health care is more easily achieved when physicians and their institutions are in alignment. Ultimately, physician–institution alignment is crucial in improving care and outcomes.
Physician–institution alignment requires further discussion. Ideally, it should strike the proper balance between physician autonomy and institutional priorities to ensure the highest quality care. Physicians and their institutions should align their interests in terms of patient safety, quality, and economics to create a work environment conducive to both patient/physician satisfaction and institutional success.14 As identified by Page and colleagues,15 the primary drivers of physician–institution alignment, specific to orthopedic surgery, are economic, regulatory, and cultural. In economics, implant selection and ancillary services are the important issues; in the regulatory area, cooperative efforts to address expanding state and federal requirements are needed; last, the primary cultural driver is delivery of care to an expanding, diverse patient population.
Physician–institution alignment brings opportunities for “gainsharing,” which can directly benefit individual physicians, physician groups, and departments. Gainsharing is classically defined as “arrangements in which a hospital gives physicians a percentage share of any reduction in the hospital’s costs for patient care attributable in part to the physicians’ efforts.”16 Modern gainsharing programs can be used by institutions to align the economic interests of physicians and hospitals, with the ultimate goal being to achieve a sustainable increase in the value and quality of care delivered to patients.13 Examples include efforts to reduce the cost of orthopedic implants, which is a major cost driver in orthopedic surgery. Our institution realized significant savings when surgeons were directly involved in the implant contracting process with strategic sourcing personnel. These savings were shared with the department to enhance research and education programs. BPI, a risk-sharing program in which Medicare and hospitals participate, incorporates gainsharing opportunities in which each participating physician can receive up to 50% of his or her previous Medicare billings when specific targets are achieved. BPI included 27 musculoskeletal diagnosis–related groups that could be developed into a bundled payment proposal. Our institution participated in a 90-day episode, for primary hip and knee arthroplasty and non–cervical spine fusion, that had very promising results.
Gainsharing offers physicians incentives to meet institution goals of improved outcomes and increased patient satisfaction while increasing oversight and accountability. When physician-specific outcomes do not meet the established goals in key areas (readmissions, thromboembolic complications, infections), it is only logical that steps will be taken to improve outcomes. Although physicians may not be used to this increased scrutiny, the goal of improving outcomes, even if it necessitates a change in an established approach to care, should be welcomed.
Physicians should be rewarded for good outcomes but not suboptimal outcomes. When outcomes are suboptimal, physicians should take a constructive approach to improve them. On the other hand, not being rewarded for unachieved goals can be perceived as being penalized. Additional monitoring may paradoxically lead physicians to avoid more “complex” cases, such as those of patients at higher risk for complications and poorer outcomes. An example is found in patient selection for surgery, in which issues like obesity, diabetes, and heart disease are known to negatively affect outcomes. In these models, “cherry-picking” is a well-recognized risk17,18 that can compromise our ethical obligation to provide equal access for all patients. To offset this tendency, we should use a risk-stratification model in which all patients are not considered equal in the risks they present. A risk-adjustment approach benefits both patients and providers by identifying modifiable risk factors that can be addressed to positively affect outcomes. This risk-stratification approach further incentivizes the orthopedist to closely work with other health care providers to address the medical comorbidities that may negatively affect surgical outcomes.
4. Patient and physician expectations
Living in a technology-driven society in the age of information has had a major impact on patients’ attitudes and expectations about their care—and therefore on physicians’ practice methods. It is uncommon to evaluate a patient who has not already consulted the Internet about a problem. Patients now have much more information they can use to make decisions about their treatment, and, though many question the accuracy of Internet information, there is no argument that being more informed is beneficial. In this time of shared decision-making, it is absolutely essential that patients keep themselves informed.
It is crucial to align the expectations of both physicians and patients in order to achieve the best outcomes. Gaining a clear understanding of treatment goals, management, and potential complications consistently leads to improved patient satisfaction, more favorable clinical outcomes, and reduced risk of litigation.19-22 Addressing patient concerns and expectations is significantly enhanced by a strong patient–physician relationship through clinical models focused on patient-centered care.
Now considered a standard of care, the patient-centered model has changed the way we practice. The foundation of the patient-centered approach is to strengthen the patient–physician relationship by empowering patients to become active decision-makers in the management of their own health. The role of orthopedists in this model is to provide patients with information and insight into their conditions in order to facilitate shared decision-making. Our role should be to guide patients to make educated and informed decisions. Doing so enhances communication, thereby strengthening the patient–physician relationship, and places both patient and physician expectations in perspective. Patient-reported outcomes, satisfaction rates, symptomatic burdens, and costs of care are all positively correlated with strong communication and realistic expectations achieved through a patient-centered approach.21,23
The evolution of clinical practice has been influenced by factors ranging from external forces (eg, changing political and economic climates) to social trends (use of social media and the Internet). Technology has been a driving force in our rapidly changing clinical environment, significantly altering the way we practice. Although we must be careful in how we use it, new technology can certainly work to our advantage. We have a plethora of medical information at our fingertips, and, with physician-directed guidance, our patients can become more informed than ever before. This is the principle of patient-centered medicine and shared decision-making, and its utility will only increase in importance.
5. The role of advocacy
The central tenet of orthopedic practice has always been a focus on patients. We continually strive to improve patient outcomes, reduce costs, and work efficiently in our practices and facilities. Although we can focus on our individual practices, we cannot ignore the influence and impact of the political system on our performance. Federal and state regulations give physicians and insurance companies an uneven playing field. This imbalance requires that physicians be more active in health care policymaking and advocacy. Although we are more involved than ever before, our influence is far less than what we would like it to be, perhaps partly because of the nature of the political process but perhaps also because of physicians’ resistance to becoming involved.
As experts in the treatment of musculoskeletal conditions, we should be at the forefront of health care policy development—a position we have not been able to attain. Although many factors contribute to our lack of a “seat at the table,” we must recognize our reluctance as a group to support advocacy, either financially or through personal time commitment. The American Association of Orthopaedic Surgeons (AAOS) Orthopaedic Political Action Committee has never been able to obtain donations from more than 30% of AAOS members. Although this committee historically has been successful, we could be much more so if we had financial support from 90% of members. There are many ways to be actively involved in advocacy. One way is to join local and state orthopedic societies and support their advocacy efforts. State orthopedic societies work closely with the AAOS Office of Government Relations to coordinate advocacy and direct efforts and resources to areas of greatest need. Knowing local congressional representatives and communicating with them about issues we face in our practices make our issues “real.” Some of our colleagues have even successfully run for office in Congress, and they certainly deserve our support. Advocacy will absolutely play an increasingly important role as federal and state governments expand their involvement in health care. Our role should be to get involved, at least to some degree. We need to recognize that our strength is in our numbers, as the few cannot accomplish nearly as much as the many.
Summary
Orthopedic surgeons are practicing in the midst of almost constant change—evolving patient care, shifts in employment models, advances in technology, modern patient expectations, and an increasingly complex regulatory environment. Even in this context, however, our goal remains unchanged: to give our patients the highest-quality care possible. Our core values as orthopedic surgeons and physicians are dedication, commitment, and service to patients and to our profession. As US health care continues to evolve, we must evolve as well, with an emphasis on expanding our role in the health care policy debate.
In the United States, the landscape of health care is changing. Health care reform and fluctuating political and economic climates have affected and will continue to affect the practice of orthopedic surgery. Demand for musculoskeletal care and the costs of providing this care are exceeding available resources—which has led to an evolution in how we practice as individuals and in the institutions where we provide care. Patient safety, quality, and value have become the outcomes of importance. Orthopedic surgeons, as experts in musculoskeletal care, must be a part of these changes. In this review, we offer perspective on the changing face of orthopedic surgery in the modern US health care system.
1. Meeting the demand
Musculoskeletal conditions represent one of the most common and costly health issues in the United States, affecting individuals medically and economically and compromising their quality of life.1,2 In 2008, more than 110 million US adults (1 in 2) reported having a musculoskeletal condition for more than 3 months, and almost 7% reported that a chronic musculoskeletal condition made routine activities of daily living significantly difficult.1 Overall, in the United States, some of the most common chronic conditions are musculoskeletal in origin. These conditions include osteoarthritis and back pain.
Osteoarthritis is the leading cause of chronic pain and disability. Physician-diagnosed arthritis is expected to affect 25% of US adults by 2030,3 and in more than one-third of these patients arthritis limits work or other activity.4 Back pain is another of the most common debilitating conditions in the United States.3,5 St Sauver and colleagues6 found that back pain is the third most common condition (23.9%) that prompts patients to seek health care—following skin-related problems (42.7%) and osteoarthritis/joint pain (33.6%).
As life expectancy increases, so do expectations of enjoying higher levels of activity into the later years. Patients expect to be as active in their geriatric years as they were in middle age, and many are able to do so. Amid the growing obesity epidemic and increased incidence of chronic comorbidities, however, the aging population not only is at substantial risk for developing a chronic musculoskeletal disorder but may face new challenges in accessing care.
Although orthopedic surgeons specialize in treating musculoskeletal conditions, up to 90% of common nonsurgical musculoskeletal complaints are thought to be manageable in the primary care setting.7 With a disproportionate increase in musculoskeletal demand against a relatively constant number of orthopedic providers,8 it is becoming increasingly important for nonorthopedists to adequately manage musculoskeletal conditions. Physiatrists, rheumatologists, internists, family practitioners, and the expanding field of sports medicine specialists provide primary care of musculoskeletal conditions. To meet the growing demand and to ensure that patients receive quality, sustainable, effective, and efficient care, orthopedic surgeons should be actively involved in training these providers. As high as the cost of managing musculoskeletal conditions can be, it is far less than the cost resulting from inadequate or improper management. There is already justification for formal development of a specialization in nonoperative management of musculoskeletal care. Establishing this specialization requires a multidisciplinary approach, with orthopedic surgery taking a lead role.
2. The cost equation
As the prevalence of orthopedic conditions increases, so does the cost of delivering musculoskeletal care. The economic implications of meeting this growing demand are an important area of concern for our health care system. Steadily increasing hospital expenses for personnel and services, rising costs of pharmaceuticals and laboratory tests, constant evolution of costly technology, and insurance/reimbursement rates that do not keep pace with rising costs all contribute to the rapid escalation of the “cost of care.”
Health care expenditures accounted for 17.2% of the US gross domestic product (GDP) in 2012 and are expected to represent 19.3% by 2023.9 For musculoskeletal disease, direct costs alone are expected to approach $510 billion, equaling 5% of GDP and representing almost 30% of all health care expenditures. In Medicare patients, osteoarthritis is the most expensive condition to treat overall, and 3 other musculoskeletal problems rank highly as well: femoral neck fractures (3rd), back pain (10th), and fractures of all types (16th).10 Clearly, musculoskeletal care is one of the most prevalent and expensive health conditions in the United States.
Part of the direct costs of care that consistently increase each year are the steadily increasing costs of technology, which is often considered synonymous with orthopedic care. Promotion of new and more costly implants is common in the absence of evidence supporting their use. However, use of new implants and technology is being scrutinized in an effort to strike the proper cost–benefit balance.
To change the slope of the cost curve, orthopedic surgeons should utilize technological advances that are proven to be clinically significant and economically feasible and should avoid modest improvements with limited clinical benefit and higher price tags. Unfortunately, this approach is not being taken. Minor modifications of implant designs are often marketed as “new and improved” to justify increased costs, and these implants often gain widespread use. A few may prove to be clinically better, but most will be only comparable to older, less expensive designs, and some may end up being clinical failures, discovered at great cost to patients and the health care system.11,12
Orthopedic surgeons have an important role in this decision-making. We should strive for the best, most cost-effective outcomes for our patients. We should reject new technology that does not clearly improve outcomes. At the least, we should use the technology in a manufacturer-supported clinical trial to determine its superiority. Whether the improvement is in technique, implant design, or workflow efficiency, orthopedic surgeons must be actively involved in researching and developing the latest innovations and must help determine their prospective value by considering not only their potential clinical benefits but also their economic implications.
As the political and economic environment becomes more directed at the cost-containment and sustainability of care, there has been a clear shift in focus to quality and value rather than volume, giving rise to the “value-based care” approach. The “value equation,” in which value equals quality divided by cost, requires a clear measure of outcomes and an equally clear understanding of costs. Delivering high-quality care in a cost-conscious environment is an approach that every orthopedic surgeon should adopt. Widespread adoption of the value-based strategy by hospital systems and insurance companies is resulting in a paradigm shift away from more traditional volume-based metrics and in favor of value-based metrics, including quality measures, patient-reported outcomes, Hospital Consumer Assessment of Healthcare Providers and Systems, and physician-specific outcome measures.
The new paradigm has brought the bundled payment initiative (BPI), a strategy included in the Patient Protection and Affordable Care Act. The philosophy behind the BPI model is for hospital systems and physicians to control costs while maintaining and improving the quality of care. Measured by patient metrics (eg, clinical outcomes, patient satisfaction) and hospital metrics (eg, readmission rates, cost of care), bundled payments reimburse hospitals on the basis of cost of an entire episode of care rather than on the basis of individual procedures and services. This approach provides incentives for both physicians and hospitals to promote value-based care while emphasizing coordination of care among all members of the health care team.
Providing the best possible care for our patients while holding our practice to the highest standards is a central tenet of the practice of orthopedic surgery and should be independent of reimbursement strategies. Thus, to increase the value of care, we must establish practice models and strategies to optimize cost-efficiency while improving outcomes. As explained by Porter and Teisberg,13 it is important to be conscientious about cost, but above all we must not allow quality of health care delivery to be compromised when trying to improve the “value” of care. Through evidence-based management and a clear understanding of costs, we must develop cost-efficient practice models that sustainably deliver the highest value of care.
3. Evolving practice models
As the health care landscape continues to change, physician practice models evolve accordingly. Although the private practice model once dominated the physician workforce, this is no longer true, as there has been a significant shift to employer-based practice models. The multiple factors at work relate to changing patterns of reimbursement, increasing government regulations, and a general change in recent residency graduates’ expectations regarding work–life balance. Other catalysts are the shift from volume- to value-based care and the recognition that cost-effective health care is more easily achieved when physicians and their institutions are in alignment. Ultimately, physician–institution alignment is crucial in improving care and outcomes.
Physician–institution alignment requires further discussion. Ideally, it should strike the proper balance between physician autonomy and institutional priorities to ensure the highest quality care. Physicians and their institutions should align their interests in terms of patient safety, quality, and economics to create a work environment conducive to both patient/physician satisfaction and institutional success.14 As identified by Page and colleagues,15 the primary drivers of physician–institution alignment, specific to orthopedic surgery, are economic, regulatory, and cultural. In economics, implant selection and ancillary services are the important issues; in the regulatory area, cooperative efforts to address expanding state and federal requirements are needed; last, the primary cultural driver is delivery of care to an expanding, diverse patient population.
Physician–institution alignment brings opportunities for “gainsharing,” which can directly benefit individual physicians, physician groups, and departments. Gainsharing is classically defined as “arrangements in which a hospital gives physicians a percentage share of any reduction in the hospital’s costs for patient care attributable in part to the physicians’ efforts.”16 Modern gainsharing programs can be used by institutions to align the economic interests of physicians and hospitals, with the ultimate goal being to achieve a sustainable increase in the value and quality of care delivered to patients.13 Examples include efforts to reduce the cost of orthopedic implants, which is a major cost driver in orthopedic surgery. Our institution realized significant savings when surgeons were directly involved in the implant contracting process with strategic sourcing personnel. These savings were shared with the department to enhance research and education programs. BPI, a risk-sharing program in which Medicare and hospitals participate, incorporates gainsharing opportunities in which each participating physician can receive up to 50% of his or her previous Medicare billings when specific targets are achieved. BPI included 27 musculoskeletal diagnosis–related groups that could be developed into a bundled payment proposal. Our institution participated in a 90-day episode, for primary hip and knee arthroplasty and non–cervical spine fusion, that had very promising results.
Gainsharing offers physicians incentives to meet institution goals of improved outcomes and increased patient satisfaction while increasing oversight and accountability. When physician-specific outcomes do not meet the established goals in key areas (readmissions, thromboembolic complications, infections), it is only logical that steps will be taken to improve outcomes. Although physicians may not be used to this increased scrutiny, the goal of improving outcomes, even if it necessitates a change in an established approach to care, should be welcomed.
Physicians should be rewarded for good outcomes but not suboptimal outcomes. When outcomes are suboptimal, physicians should take a constructive approach to improve them. On the other hand, not being rewarded for unachieved goals can be perceived as being penalized. Additional monitoring may paradoxically lead physicians to avoid more “complex” cases, such as those of patients at higher risk for complications and poorer outcomes. An example is found in patient selection for surgery, in which issues like obesity, diabetes, and heart disease are known to negatively affect outcomes. In these models, “cherry-picking” is a well-recognized risk17,18 that can compromise our ethical obligation to provide equal access for all patients. To offset this tendency, we should use a risk-stratification model in which all patients are not considered equal in the risks they present. A risk-adjustment approach benefits both patients and providers by identifying modifiable risk factors that can be addressed to positively affect outcomes. This risk-stratification approach further incentivizes the orthopedist to closely work with other health care providers to address the medical comorbidities that may negatively affect surgical outcomes.
4. Patient and physician expectations
Living in a technology-driven society in the age of information has had a major impact on patients’ attitudes and expectations about their care—and therefore on physicians’ practice methods. It is uncommon to evaluate a patient who has not already consulted the Internet about a problem. Patients now have much more information they can use to make decisions about their treatment, and, though many question the accuracy of Internet information, there is no argument that being more informed is beneficial. In this time of shared decision-making, it is absolutely essential that patients keep themselves informed.
It is crucial to align the expectations of both physicians and patients in order to achieve the best outcomes. Gaining a clear understanding of treatment goals, management, and potential complications consistently leads to improved patient satisfaction, more favorable clinical outcomes, and reduced risk of litigation.19-22 Addressing patient concerns and expectations is significantly enhanced by a strong patient–physician relationship through clinical models focused on patient-centered care.
Now considered a standard of care, the patient-centered model has changed the way we practice. The foundation of the patient-centered approach is to strengthen the patient–physician relationship by empowering patients to become active decision-makers in the management of their own health. The role of orthopedists in this model is to provide patients with information and insight into their conditions in order to facilitate shared decision-making. Our role should be to guide patients to make educated and informed decisions. Doing so enhances communication, thereby strengthening the patient–physician relationship, and places both patient and physician expectations in perspective. Patient-reported outcomes, satisfaction rates, symptomatic burdens, and costs of care are all positively correlated with strong communication and realistic expectations achieved through a patient-centered approach.21,23
The evolution of clinical practice has been influenced by factors ranging from external forces (eg, changing political and economic climates) to social trends (use of social media and the Internet). Technology has been a driving force in our rapidly changing clinical environment, significantly altering the way we practice. Although we must be careful in how we use it, new technology can certainly work to our advantage. We have a plethora of medical information at our fingertips, and, with physician-directed guidance, our patients can become more informed than ever before. This is the principle of patient-centered medicine and shared decision-making, and its utility will only increase in importance.
5. The role of advocacy
The central tenet of orthopedic practice has always been a focus on patients. We continually strive to improve patient outcomes, reduce costs, and work efficiently in our practices and facilities. Although we can focus on our individual practices, we cannot ignore the influence and impact of the political system on our performance. Federal and state regulations give physicians and insurance companies an uneven playing field. This imbalance requires that physicians be more active in health care policymaking and advocacy. Although we are more involved than ever before, our influence is far less than what we would like it to be, perhaps partly because of the nature of the political process but perhaps also because of physicians’ resistance to becoming involved.
As experts in the treatment of musculoskeletal conditions, we should be at the forefront of health care policy development—a position we have not been able to attain. Although many factors contribute to our lack of a “seat at the table,” we must recognize our reluctance as a group to support advocacy, either financially or through personal time commitment. The American Association of Orthopaedic Surgeons (AAOS) Orthopaedic Political Action Committee has never been able to obtain donations from more than 30% of AAOS members. Although this committee historically has been successful, we could be much more so if we had financial support from 90% of members. There are many ways to be actively involved in advocacy. One way is to join local and state orthopedic societies and support their advocacy efforts. State orthopedic societies work closely with the AAOS Office of Government Relations to coordinate advocacy and direct efforts and resources to areas of greatest need. Knowing local congressional representatives and communicating with them about issues we face in our practices make our issues “real.” Some of our colleagues have even successfully run for office in Congress, and they certainly deserve our support. Advocacy will absolutely play an increasingly important role as federal and state governments expand their involvement in health care. Our role should be to get involved, at least to some degree. We need to recognize that our strength is in our numbers, as the few cannot accomplish nearly as much as the many.
Summary
Orthopedic surgeons are practicing in the midst of almost constant change—evolving patient care, shifts in employment models, advances in technology, modern patient expectations, and an increasingly complex regulatory environment. Even in this context, however, our goal remains unchanged: to give our patients the highest-quality care possible. Our core values as orthopedic surgeons and physicians are dedication, commitment, and service to patients and to our profession. As US health care continues to evolve, we must evolve as well, with an emphasis on expanding our role in the health care policy debate.
1. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. Rosemont, IL: US Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed October 26, 2015.
2. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. 2nd ed. Rosemont, IL: US Bone and Joint Initiative; 2011. http://www.boneandjointburden.org. Accessed October 26, 2015.
3. Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986-995.e1.
4. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
5. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
6. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56-67.
7. Anderson BC. Office Orthopedics for Primary Care: Diagnosis and Treatment. 2nd ed. Philadelphia, PA: Saunders; 1999.
8. American Academy of Orthopaedic Surgeons, Department of Research and Scientific Affairs. Orthopaedic Practice in the U.S. 2012 [2012 Orthopaedic Surgeon Census Report]. Rosemont, IL: American Academy of Orthopaedic Surgeons; January 2013.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. NHE [National Health Expenditure] Fact Sheet, 2014. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Updated July 28, 2015. Accessed October 26, 2015.
10. Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075-1077.
11. Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46.
12. Dahlstrand H, Stark A, Anissian L, Hailer NP. Elevated serum concentrations of cobalt, chromium, nickel, and manganese after metal-on-metal alloarthroplasty of the hip: a prospective randomized study. J Arthroplasty. 2009;24(6):837-845.
13. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
14. American Association of Orthopaedic Surgeons. Alignment of physician and facility payment and incentives. Position statement 1171. American Association of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/position/1171.asp. Published September 2006. Revised February 2009. Accessed October 26, 2015.
15. Page AE, Butler CA, Bozic KJ. Factors driving physician–hospital alignment in orthopaedic surgery. Clin Orthop Relat Res. 2013;471(6):1809-1817.
16. US Department of Health and Human Services, Office of Inspector General. Gainsharing arrangements and CMPs for hospital payments to physicians to reduce or limit services to beneficiaries [special advisory bulletin]. Office of Inspector General website. http://oig.hhs.gov/fraud/docs/alertsandbulletins/gainsh.htm. Published July 1999. Accessed October 26, 2015.
17. Bronson WH, Fewer M, Godlewski K, et al. The ethics of patient risk modification prior to elective joint replacement surgery. J Bone Joint Surg Am. 2014;96(13):e113.
18. Bosco J. To cherry pick or not: the unintended ethical consequences of pay for performance. Presented at: New York University Colloquium on Medical Ethics; New York, NY; November 2014.
19. Hageman MG, Briët JP, Bossen JK, Blok RD, Ring DC, Vranceanu AM. Do previsit expectations correlate with satisfaction of new patients presenting for evaluation with an orthopaedic surgical practice? Clin Orthop Relat Res. 2015;473(2):716-721.
20. Jourdan C, Poiraudeau S, Descamps S, et al. Comparison of patient and surgeon expectations of total hip arthroplasty. PLoS One. 2012;7(1):e30195.
21. McMillan S, Kendall E, Sav A, et al. Patient-centered approaches to health care: a systematic review of randomized controlled trials. Med Care Res Rev. 2013;70(6):567-596.
22. Forster HP, Schwartz J, DeRenzo E. Reducing legal risk by practicing patient-centered medicine. Arch Intern Med. 2002;162(11):1217-1219.
23. Van Citters AD, Fahlman C, Goldmann DA, et al. Developing a pathway for high-value, patient-centered total joint arthroplasty. Clin Orthop Relat Res. 2014;472(5):1619-1635.
1. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. Rosemont, IL: US Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed October 26, 2015.
2. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. 2nd ed. Rosemont, IL: US Bone and Joint Initiative; 2011. http://www.boneandjointburden.org. Accessed October 26, 2015.
3. Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986-995.e1.
4. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
5. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
6. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56-67.
7. Anderson BC. Office Orthopedics for Primary Care: Diagnosis and Treatment. 2nd ed. Philadelphia, PA: Saunders; 1999.
8. American Academy of Orthopaedic Surgeons, Department of Research and Scientific Affairs. Orthopaedic Practice in the U.S. 2012 [2012 Orthopaedic Surgeon Census Report]. Rosemont, IL: American Academy of Orthopaedic Surgeons; January 2013.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. NHE [National Health Expenditure] Fact Sheet, 2014. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Updated July 28, 2015. Accessed October 26, 2015.
10. Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075-1077.
11. Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46.
12. Dahlstrand H, Stark A, Anissian L, Hailer NP. Elevated serum concentrations of cobalt, chromium, nickel, and manganese after metal-on-metal alloarthroplasty of the hip: a prospective randomized study. J Arthroplasty. 2009;24(6):837-845.
13. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
14. American Association of Orthopaedic Surgeons. Alignment of physician and facility payment and incentives. Position statement 1171. American Association of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/position/1171.asp. Published September 2006. Revised February 2009. Accessed October 26, 2015.
15. Page AE, Butler CA, Bozic KJ. Factors driving physician–hospital alignment in orthopaedic surgery. Clin Orthop Relat Res. 2013;471(6):1809-1817.
16. US Department of Health and Human Services, Office of Inspector General. Gainsharing arrangements and CMPs for hospital payments to physicians to reduce or limit services to beneficiaries [special advisory bulletin]. Office of Inspector General website. http://oig.hhs.gov/fraud/docs/alertsandbulletins/gainsh.htm. Published July 1999. Accessed October 26, 2015.
17. Bronson WH, Fewer M, Godlewski K, et al. The ethics of patient risk modification prior to elective joint replacement surgery. J Bone Joint Surg Am. 2014;96(13):e113.
18. Bosco J. To cherry pick or not: the unintended ethical consequences of pay for performance. Presented at: New York University Colloquium on Medical Ethics; New York, NY; November 2014.
19. Hageman MG, Briët JP, Bossen JK, Blok RD, Ring DC, Vranceanu AM. Do previsit expectations correlate with satisfaction of new patients presenting for evaluation with an orthopaedic surgical practice? Clin Orthop Relat Res. 2015;473(2):716-721.
20. Jourdan C, Poiraudeau S, Descamps S, et al. Comparison of patient and surgeon expectations of total hip arthroplasty. PLoS One. 2012;7(1):e30195.
21. McMillan S, Kendall E, Sav A, et al. Patient-centered approaches to health care: a systematic review of randomized controlled trials. Med Care Res Rev. 2013;70(6):567-596.
22. Forster HP, Schwartz J, DeRenzo E. Reducing legal risk by practicing patient-centered medicine. Arch Intern Med. 2002;162(11):1217-1219.
23. Van Citters AD, Fahlman C, Goldmann DA, et al. Developing a pathway for high-value, patient-centered total joint arthroplasty. Clin Orthop Relat Res. 2014;472(5):1619-1635.
Value and the Orthopedic Surgeon
Health care financing and the nature of orthopedic practice have changed dramatically in recent years and will continue to do so. Driving these changes is the emphasis on “value,” defined by Porter1 as the quality of care divided by the cost of care, as opposed to the traditional volume-based care, in which reimbursement is based on a fee for services rendered. Exploring this concept of value in orthopedic care is a favorite topic of mine, succinctly summarized by Black and Warner2 in their 2013 article in The American Journal of Orthopedics. Two papers in this current issue of The American Journal of Orthopedics make important points regarding value and the orthopedic surgeon.
In “Orthopedic Implant Waste: Analysis and Quantification” (pages 554-560), Payne and colleagues examine the costs of wasted implants across 8 orthopedic subspecialties at 1 academic institution over the course of 12 months. The take-home points were these: wasted implants accounted for nearly 2% of the implant cost of the institution; the incidence of waste was related to surgeons with less experience (in practice less than 10 years) but not case volumes (ie, busier surgeons); and nearly two-thirds of the cost of wasted implants occurred in total joint and spine fusion cases.
At my institution, orthopedic implants represent one of the 3 major costs of inpatient hospital care (the other 2 being operating room time and length of stay). Hence, a 2% savings of total implant costs by minimizing waste can make a significant difference in an institution’s profit margin. Since the attending surgeon makes the intraoperative decision on implant type, the burden of minimizing implant waste falls primarily on the orthopedic surgeon. This is just one example of how the individual orthopedic surgeon can improve “value” by decreasing the “cost” of care.
In “Orthopedics in US Health Care” (pages 538-541), Yu and Zuckerman review 5 points on the evolving role orthopedic surgery plays in the changing landscape of US health care. Among many important topics reviewed, the authors raise 2 important issues specifically related to value and the orthopedic surgeon that I believe warrant special attention.
In point 2, “The Cost Equation,” Yu and Zuckerman state that new technology (always more expensive than existing technology!) must “clearly improve outcomes” prior to its introduction to the market. The adage “newer is better” is sometimes true, but new and more expensive technology (which increases the denominator of the “value” quotient) must afford even greater improvement in quality outcomes to justify its widespread use. Hence, as practicing orthopedic surgeons, we should resist the temptation to embrace new technology without clear evidence that said new technology actually improves the quality of care.
The second topic of interest to me is how we measure “outcomes” in this new value-driven health care world. While many important outcome metrics can be measured by hospital data systems, such as length of stay, unscheduled returns to the operating room, transfusion and infection rates, and 30-day readmissions, equally important clinical outcomes (eg, pain and function scores, joint range of motion and strength, and radiographic findings) are obtained primarily from office-based outpatient medical records. These clinically based quality metrics are far more difficult to obtain for individual practicing orthopedic surgeons and require an investment of time and staff to gather meaningful data. How to record and incorporate these clinical outcomes remains a challenge for the practicing orthopedic surgeon, especially in the nonacademic setting, but these clinical metrics must be a component in the “value equation.”
The concept of value in orthopedic surgery will be the primary driver of future health care financing and policies. To succeed in this changing world, orthopedic surgeons will need to not only understand this new paradigm “value = quality/cost,” but be fundamentally involved in the process, institutionally and politically, that both defines and rewards value.
1. Porter ME. What is value in health care? N Engl J Med. 2010;363(26): 2477-2481.
2. Black EM, Warner JJP. 5 points on value in orthopedic surgery. Am J Orthop. 2013:42(1):22-25.
Health care financing and the nature of orthopedic practice have changed dramatically in recent years and will continue to do so. Driving these changes is the emphasis on “value,” defined by Porter1 as the quality of care divided by the cost of care, as opposed to the traditional volume-based care, in which reimbursement is based on a fee for services rendered. Exploring this concept of value in orthopedic care is a favorite topic of mine, succinctly summarized by Black and Warner2 in their 2013 article in The American Journal of Orthopedics. Two papers in this current issue of The American Journal of Orthopedics make important points regarding value and the orthopedic surgeon.
In “Orthopedic Implant Waste: Analysis and Quantification” (pages 554-560), Payne and colleagues examine the costs of wasted implants across 8 orthopedic subspecialties at 1 academic institution over the course of 12 months. The take-home points were these: wasted implants accounted for nearly 2% of the implant cost of the institution; the incidence of waste was related to surgeons with less experience (in practice less than 10 years) but not case volumes (ie, busier surgeons); and nearly two-thirds of the cost of wasted implants occurred in total joint and spine fusion cases.
At my institution, orthopedic implants represent one of the 3 major costs of inpatient hospital care (the other 2 being operating room time and length of stay). Hence, a 2% savings of total implant costs by minimizing waste can make a significant difference in an institution’s profit margin. Since the attending surgeon makes the intraoperative decision on implant type, the burden of minimizing implant waste falls primarily on the orthopedic surgeon. This is just one example of how the individual orthopedic surgeon can improve “value” by decreasing the “cost” of care.
In “Orthopedics in US Health Care” (pages 538-541), Yu and Zuckerman review 5 points on the evolving role orthopedic surgery plays in the changing landscape of US health care. Among many important topics reviewed, the authors raise 2 important issues specifically related to value and the orthopedic surgeon that I believe warrant special attention.
In point 2, “The Cost Equation,” Yu and Zuckerman state that new technology (always more expensive than existing technology!) must “clearly improve outcomes” prior to its introduction to the market. The adage “newer is better” is sometimes true, but new and more expensive technology (which increases the denominator of the “value” quotient) must afford even greater improvement in quality outcomes to justify its widespread use. Hence, as practicing orthopedic surgeons, we should resist the temptation to embrace new technology without clear evidence that said new technology actually improves the quality of care.
The second topic of interest to me is how we measure “outcomes” in this new value-driven health care world. While many important outcome metrics can be measured by hospital data systems, such as length of stay, unscheduled returns to the operating room, transfusion and infection rates, and 30-day readmissions, equally important clinical outcomes (eg, pain and function scores, joint range of motion and strength, and radiographic findings) are obtained primarily from office-based outpatient medical records. These clinically based quality metrics are far more difficult to obtain for individual practicing orthopedic surgeons and require an investment of time and staff to gather meaningful data. How to record and incorporate these clinical outcomes remains a challenge for the practicing orthopedic surgeon, especially in the nonacademic setting, but these clinical metrics must be a component in the “value equation.”
The concept of value in orthopedic surgery will be the primary driver of future health care financing and policies. To succeed in this changing world, orthopedic surgeons will need to not only understand this new paradigm “value = quality/cost,” but be fundamentally involved in the process, institutionally and politically, that both defines and rewards value.
Health care financing and the nature of orthopedic practice have changed dramatically in recent years and will continue to do so. Driving these changes is the emphasis on “value,” defined by Porter1 as the quality of care divided by the cost of care, as opposed to the traditional volume-based care, in which reimbursement is based on a fee for services rendered. Exploring this concept of value in orthopedic care is a favorite topic of mine, succinctly summarized by Black and Warner2 in their 2013 article in The American Journal of Orthopedics. Two papers in this current issue of The American Journal of Orthopedics make important points regarding value and the orthopedic surgeon.
In “Orthopedic Implant Waste: Analysis and Quantification” (pages 554-560), Payne and colleagues examine the costs of wasted implants across 8 orthopedic subspecialties at 1 academic institution over the course of 12 months. The take-home points were these: wasted implants accounted for nearly 2% of the implant cost of the institution; the incidence of waste was related to surgeons with less experience (in practice less than 10 years) but not case volumes (ie, busier surgeons); and nearly two-thirds of the cost of wasted implants occurred in total joint and spine fusion cases.
At my institution, orthopedic implants represent one of the 3 major costs of inpatient hospital care (the other 2 being operating room time and length of stay). Hence, a 2% savings of total implant costs by minimizing waste can make a significant difference in an institution’s profit margin. Since the attending surgeon makes the intraoperative decision on implant type, the burden of minimizing implant waste falls primarily on the orthopedic surgeon. This is just one example of how the individual orthopedic surgeon can improve “value” by decreasing the “cost” of care.
In “Orthopedics in US Health Care” (pages 538-541), Yu and Zuckerman review 5 points on the evolving role orthopedic surgery plays in the changing landscape of US health care. Among many important topics reviewed, the authors raise 2 important issues specifically related to value and the orthopedic surgeon that I believe warrant special attention.
In point 2, “The Cost Equation,” Yu and Zuckerman state that new technology (always more expensive than existing technology!) must “clearly improve outcomes” prior to its introduction to the market. The adage “newer is better” is sometimes true, but new and more expensive technology (which increases the denominator of the “value” quotient) must afford even greater improvement in quality outcomes to justify its widespread use. Hence, as practicing orthopedic surgeons, we should resist the temptation to embrace new technology without clear evidence that said new technology actually improves the quality of care.
The second topic of interest to me is how we measure “outcomes” in this new value-driven health care world. While many important outcome metrics can be measured by hospital data systems, such as length of stay, unscheduled returns to the operating room, transfusion and infection rates, and 30-day readmissions, equally important clinical outcomes (eg, pain and function scores, joint range of motion and strength, and radiographic findings) are obtained primarily from office-based outpatient medical records. These clinically based quality metrics are far more difficult to obtain for individual practicing orthopedic surgeons and require an investment of time and staff to gather meaningful data. How to record and incorporate these clinical outcomes remains a challenge for the practicing orthopedic surgeon, especially in the nonacademic setting, but these clinical metrics must be a component in the “value equation.”
The concept of value in orthopedic surgery will be the primary driver of future health care financing and policies. To succeed in this changing world, orthopedic surgeons will need to not only understand this new paradigm “value = quality/cost,” but be fundamentally involved in the process, institutionally and politically, that both defines and rewards value.
1. Porter ME. What is value in health care? N Engl J Med. 2010;363(26): 2477-2481.
2. Black EM, Warner JJP. 5 points on value in orthopedic surgery. Am J Orthop. 2013:42(1):22-25.
1. Porter ME. What is value in health care? N Engl J Med. 2010;363(26): 2477-2481.
2. Black EM, Warner JJP. 5 points on value in orthopedic surgery. Am J Orthop. 2013:42(1):22-25.
Multifocal Langerhans Cell Histiocytosis in an Adult
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
Lipoma of the Tendon Sheath in the Fourth Extensor Compartment of the Hand
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Osteosarcoma: A Meta-Analysis and Review of the Literature
Osteosarcoma, a primary malignant tumor of the skeleton, is characterized by direct formation of immature bone or osteoid tissue by tumor cells. The World Health Organization histologic classification of bone tumors divides osteosarcoma into central and surface tumors and recognizes a number of subtypes within each group.1 The present review refers only to the classic central high-grade primary osteosarcoma of bone, which represents about 90% of all osteosarcoma cases. Classic osteosarcoma represents about 15% of all biopsy-analyzed primary bone tumors.1 It is the third most common type of neoplasia, preceded by leukemia and lymphoma among older children and adolescents aged 12 to 18 years.2 High-grade primary osteosarcoma is the most common primary skeletal tumor of childhood and adolescence, with an overall annual incidence of 5.6 cases per million children under age 15 years.3-5 Peak incidence is in the second decade of life, and males are affected slightly more often than females.2,6 The period of highest incidence coincides with the growth spurt of the long bones. Osteosarcoma preferentially affects the metaphysis of long bones, the 3 main sites being distal femur, tibia, and proximal humerus.2
Historical Perspective
For most of the 20th century, the 5-year survival rate for classic primary osteosarcoma was under 20%.7 In the 1970s, the first revolution in osteosarcoma treatment arrived with the introduction of adjuvant chemotherapy, which increased survival rates to 50%.8-10 During this expansion of research, several chemotherapeutics (eg, vincristine, bleomycin, dactinomycin) were discarded for poor effectiveness, and others (eg, cisplatin, ifosfamide) were added to doxorubicin and methotrexate, improving 5-year disease-free survival to about 70% in patients with nonmetastatic osteosarcoma. In another significant advance, adjuvant chemotherapy was supplemented with intensive preoperative chemotherapy, resulting in 5-year tumor-free survival that has ranged from 50% to 75% for high-grade osteosarcoma.5,11,12 Adding neoadjuvant chemotherapy and histologic response has allowed for evaluation of surgical margins and early treatment of microscopic disease. Thus, effective limb-sparing procedures can be performed, and the incidence of amputation has decreased from 90% to between 10% and 20%.13,14 However, statistical improvements in survival associated with neoadjuvant treatment may simply delay time of recurrence and metastasis.15 In addition, though chemotherapy has improved survival in osteogenic sarcoma, many have written that this improvement appears to reflect mainly the increase in the intensity of the chemotherapy used, which also leads to a higher propensity for side effects.16
Despite research and advances in chemotherapy regimens, the prognosis of patients with osteosarcoma remains highly variable and often dismal. Mirabello and colleagues17 examined osteosarcoma incidence and survival rates between 1973 and 2004 and found that, with the introduction of neoadjuvant chemotherapy, survival rates improved significantly between 1973 and 1983 and between 1984 and 1993, but there was little improvement between 1993 and 2004.
The long-term outcome for patients with metastatic disease is poor. Investigators have found that 11% to 20% of patients have pulmonary metastasis at initial diagnosis. About half of patients without pulmonary metastases develop them later in the disease course.18 Survival rates for patients with metastasis at initial presentation have ranged from 10% to 40%.19 Recurrent disease still occurs in 30% to 40% of patients, and more than 70% of them die of the tumor.15 The survivors of osteosarcoma are then at increased risk for chronic medical conditions and adverse health status because of the osteosarcoma-related treatments.20
Prognostic Factors
It is important to understand and exploit the influences of different prognostic factors in treating patients with osteosarcoma.7 These factors are important in establishing the best treatment for the individual. Thus, more aggressive treatments can be started in patients with prognostic factors that pose a higher risk of relapse.21 A number of clinical and pathologic features (eg, tumor site, size, subtype; patient sex and age; high alkaline phosphatase or high lactate dehydrogenase [LDH] values; multidrug resistance; genetic variations) have prognostic significance but often with contradictory results because of lack of uniformity in patient analyses and methods.15
Survival for patients with primary osteosarcoma has been analyzed with respect to tumor size and location.7 Studies have found higher survival rates for patients with smaller tumors (<10 cm) and more distal tumor locations.7 These superior survival rates may be the result of earlier detection of tumors and more options for surgical resection of smaller, distal tumors.
Serum LDH levels have helped in risk stratification of patients. High LDH often occurred at time of relapse, and relapse with high LDH correlated with poor prognosis. Meyers and colleagues22 found that 5-year disease-free survival was 72% for patients with normal LDH at presentation and 54% for patients with elevated LDH at presentation.
Several studies have shown that percentage of tumor necrosis on histology is strongly correlated with good prognosis.21 Most groups now define a good histologic response as less than 10% viable tumor cells at time of surgery, and a poor response as more than 10%.23 Results of the Pediatric Oncology Group (POG) protocol for localized osteosarcoma (POG 9351), or Children’s Cancer Group (CCG) 7921, found 45% of patients had favorable responses (>90% necrosis) after preoperative chemotherapy.24 However, several clinicians have recently questioned this finding.
Overall, the prognosis for classic osteosarcoma of the extremity remains highly variable, and there has been little improvement over the past 20 years. The prognosis for younger patients, patients with spinal disease, and patients with metastatic disease remains poor. Although some prognostic factors have been identified and shown to predict a good outcome, it seems few patients have these positive factors. In this article, we describe the literature review and meta-analysis we performed to better define recent survival trends for patients with primary osteosarcoma.
Methods
The MEDLINE, PubMed, and Cochrane databases were searched for eligible studies published in English between 2000 and 2011—a decade of recently reported research. We applied the search strategy [“osteosarcoma” OR “osteogenic sarcoma”] AND [“prognosis” OR “treatment” OR “survival”] and selected reports that specifically addressed factors predicting survival in patients with osteosarcoma—reports that were limited to primary osteosarcoma of the pelvis or extremity and provided 5-year overall survival (OS) data. Abstracts of the selected articles were independently reviewed, and the inclusion and exclusion criteria were applied. We excluded basic science studies and those without pediatric patients, those without primary osteosarcoma, those with periosteal or parosteal osteosarcoma, and those that did not report 5-year OS data.
Statistical Analysis
Number or proportion of patients (whichever was reported) with 5-year OS and number or proportion of patients with 90% necrosis were extracted from each study. For each trial, proportion of patients with 5-year OS and 95% confidence intervals (CIs) and proportion of patients achieving 90% necrosis and 95% CIs were determined. We also calculated proportion of patients with 5-year OS and proportion of patients with 90% necrosis with corresponding 95% CIs of studies that included patients with nonmetastatic disease.
We assessed statistical heterogeneity among trials included in the meta-analysis using the Cochran Q test. Inconsistency was quantified with the I2 statistic, which estimates percentage of total across-studies variation caused by heterogeneity rather than chance.25 We considered I2 higher than 50% as indicating substantial heterogeneity. When substantial heterogeneity was not found, the pooled estimate calculated on the basis of the fixed-effects model was reported using the inverse variance method. When substantial heterogeneity was found, the pooled estimate calculated on the basis of a random-effects model was reported using the DerSimonian and Laird26 method, which takes both within- and between-study variations into account.
Publication bias was assessed through funnel plots and with Begg and Egger tests.27,28 Two-tailed P < .05 was considered statistically significant. All statistical analyses were performed with Stata/SE Version 11.0 (StataCorp).
Results
Our literature search yielded 597 articles. We cross-referenced these articles with the MEDLINE, PubMed, and Cochrane search results using the same keywords and discarded the duplicates. The abstracts of these articles were then reviewed in detail. The 40 articles4,6,11,12,14,15,17-19,21,29-58 that met our study inclusion criteria reported on studies that included patients with metastatic and nonmetastatic osteosarcoma. Because of the significant difference in OS of patients with metastatic disease, we also analyzed articles that included only patients with nonmetastatic disease. Sixteen articles6,14,15,29,32-35,39,47,48,51,53,54,55,57 were included in the analysis of patients with nonmetastatic disease.
Figure 1 shows 5-year OS for each of the 40 studies. For studies that compared survival of different groups of patients, the survival of each group is shown separately. For example, Bacci and colleagues39 divided patients into adolescent and preadolescent groups and reported 5-year OS for each. In our analysis, we treated each group independently and reported their 5-year OS separately. For each study, 5-year OS, weight of study, and CI are included. Five-year OS ranged from 19% to 94%. Analysis was performed to determine 5-year OS for all studies based on weight given to each study. The random-effects model used for this analysis (heterogeneity test, Q = 656.23; P < .001; I2 = 93.4%) showed 5-year OS of 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma.
Figure 2 shows 5-year OS (range, 53%-94%) for each of the 16 studies that included only patients with nonmetastatic disease. The random-effects model used for this analysis (heterogeneity test, Q = 142.08; P < .001; I2 = 89.4%) showed 5-year OS of 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic disease.
We then examined percentage of patients achieving 90% necrosis on histology in each study. Several studies included in the OS analysis did not report percentage necrosis, leaving 29 studies for the necrosis analysis. Of these 29 studies, all 29 included patients with metastatic and nonmetastatic disease,4,6,11,14,15,18,19,21,29,31-36,37,39,40,43-47,49,50,54-57,59 and 13 included only patients with nonmetastatic disease.6,14,15,29,32-35,40,47,54,55,57 Again, because of the known difference in prognosis between patients with metastatic disease and patients with nonmetastatic disease, we performed separate analyses, one for the combined dataset of all 29 studies (Figure 3) and the other for the 13 nonmetastatic studies (Figure 4). Random-effects models showed 90% necrosis for 50% of patients in both analyses: studies that included patients with metastatic and nonmetastatic disease (95% CI, 45%-54%; heterogeneity test, Q = 692.88; P < .001; I2 = 95.5%) and nonmetastatic studies (95% CI, 41%-59%; heterogeneity test, Q = 385.42; P < .001; I2 = 96.9%).
We also performed a meta-regression analysis that included necrosis as a continuous variable for both the overall dataset and the nonmetastatic dataset. Five-year OS was plotted against percentage of patients achieving 90% tumor necrosis for each study. The results are plotted in Figure 5 (combined dataset).
No evidence of publication bias was detected for 5-year OS or percentage necrosis for the analyses of the combined datasets by either Egger test or Begg test. For 5-year OS, Ps were .21 (Egger) and .19 (Begg); for percentage necrosis, Ps were .10 (Egger) and .62 (Begg). In addition, no evidence of publication bias was detected for the analyses of the nonmetastatic studies by either test. For 5-year OS, Ps were .55 (Egger) and .41 (Begg); for percentage necrosis, Ps were .42 (Egger) and .95 (Begg).
Discussion
Five-year OS was 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma and 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic osteosarcoma. These percentages fall within the range found in the literature. Mankin and colleagues37 reviewed 648 cases of patients with osteosarcoma treated at Massachusetts General Hospital in 2004; OS was 68%. In 2011, Sampo and colleagues60 reported 10-year OS of 63% for patients with metastatic and nonmetastatic disease and 73% for patients with local disease at presentation. Five-year OS rates in the literature are consistently about 70%. Ferrari and colleagues61 reported 5-year OS of 73% and 74% for 230 patients treated with 2 different neoadjuvant chemotherapy regimens between 2001 and 2006. The consistency in 5-year OS suggests OS of pediatric patients with osteosarcoma has plateaued, and there has been no significant improvement in survival of patients with osteosarcoma over the past 30 years.
Histologic response to preoperative chemotherapy is strongly associated with survival in pediatric osteosarcoma. Bielack and colleagues31 reported 5-year OS of 75% to 80% for patients who responded well to preoperative chemotherapy (>90% tumor necrosis) and 45% to 55% for patients who responded poorly (<10% necrosis). In our meta-analysis of studies that included patients with nonmetastatic osteosarcoma, 50% achieved necrosis of more than 90%. Percentage of patients achieving necrosis of more than 90% has been about 45%, according to past reports. In 2012, Ferrari and colleagues61 reported that 45% of 230 patients treated with neoadjuvant chemotherapy achieved more than 90% tumor necrosis. Therefore, 5-year OS and percentage of patients achieving 90% necrosis are consistent with previous reports, though this also suggests these numbers have remained constant over the past several decades.
Despite its expansive scale, our study has several important limitations. Data were extracted from published studies, and individual patient data were not available, so we were not able to assess the effects of risk factors (eg, tumor size, location) on 5-year OS. We could not correlate the proportion of patients with 90% necrosis to 5-year OS, as studies did not report OS by necrosis strata. Also, because our numbers were derived from published studies, they may not accurately represent outcomes in the community as a whole. In addition, several successive studies may contain duplicate patient cases. We limited our search to studies published since 2000 to include patients recently diagnosed and treated for osteosarcoma; however, several studies published after 2000 also included patients diagnosed and treated before 2000. Several of these studies are from countries outside the United States and may have a significantly different incidence of osteosarcoma as well as treatment methods and survival rates.
Although this meta-analysis suggests 5-year OS remains about 70% for patients with primary nonmetastatic osteosarcoma, we cannot settle on this conclusion because of the many differences between the studies we included. Therefore, more studies of patients diagnosed and treated within the past 10 years are needed to confirm our beliefs about patient survival.
1. Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
2. Cho WH, Song WS, Jeon DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010;17(3):702-708.
3. Abate ME, Longhi A, Galletti S, Ferrari S, Bacci G. Non-metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatr Blood Cancer. 2010;55(4):652-654.
4. Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011-2018.
5. Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45(13):2367-2375.
6. Kaste SC, Liu T, Billups CA, Daw NC, Pratt CB, Meyer WH. Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer. 2004;43(7):723-728.
7. Brostrom LA, Strander H, Nilsonne U. Survival in osteosarcoma in relation to tumor size and location. Clin Orthop Relat Res. 1982;167:250-254.
8. Harvei S, Solheim O. The prognosis in osteosarcoma: Norwegian national data. Cancer. 1981;48(8):1719-1723.
9. Sutow WW, Sullivan MP, Fernbach DJ, Cangir A, George SL. Adjuvant chemotherapy in primary treatment of osteogenic sarcoma. A Southwest Oncology Group study. Cancer. 1975;36(5):1598-1602.
10. Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5(1):21-26.
11. Hsieh MY, Hung GY, Yen HJ, Chen WM, Chen TH. Osteosarcoma in preadolescent patients: experience in a single institute in Taiwan. J Chin Med Assoc. 2009;72(9):455-461.
12. Longhi A, Pasini E, Bertoni F, Pignotti E, Ferrari C, Bacci G. Twenty-year follow-up of osteosarcoma of the extremity treated with adjuvant chemotherapy. J Chemother. 2004;16(6):582-588.
13. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
14. Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/Osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18(24):4016-4027.
15. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154-1161.
16. Cohen IJ, Kaplinsky C, Katz K, et al. Improved results in osteogenic sarcoma 1973–79 vs. 1980–86: analysis of results from a single center. Isr J Med Sci. 1993;29(1):27-29.
17. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results program. Cancer. 2009;115(7):1531-1543.
18. Kager L, Zoubek A, Dominkus M, et al. Osteosarcoma in very young children: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2010;116(22):5316-5324.
19. Szendroi M, Papai Z, Koos R, Illes T. Limb-saving surgery, survival, and prognostic factors for osteosarcoma: the Hungarian experience. J Surg Oncol. 2000;73(2):87-94.
20. Nagarajan R, Kamruzzaman A, Ness KK, et al. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(3):625-634.
21. Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori. 2004;90(5):478-484.
22. Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5-15.
23. Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422-441.
24. Hendershot E, Pappo A, Malkin D, Sung L. Tumor necrosis in pediatric osteosarcoma: impact of modern therapies. J Pediatr Oncol Nurs. 2006;23(4):176-181.
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101.
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634.
29. Bacci G, Ferrari S, Longhi A, Mellano D, Giacomini S, Forni C. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86(3):204-206.
30. Bacci G, Ferrari S, Longhi A, et al. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother. 2001;13(1):93-99.
31. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790.
32. Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38(9):1218-1225.
33. Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg Am. 2002;84(1):49-57.
34. Wilkins RM, Cullen JW, Odom L, et al. Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity. Ann Surg Oncol. 2003;10(5):498-507.
35. Smeland S, Muller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488-494.
36. Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21(2):334-341.
37. Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286-291.
38. Donati D, Giacomini S, Gozzi E, et al. Osteosarcoma of the pelvis. Eur J Surg Oncol. 2004;30(3):332-340.
39. Bacci G, Longhi A, Bertoni F, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients. J Pediatr Hematol Oncol. 2005;27(3):129-134.
40. Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41(18):2836-2845.
41. Matsuo T, Sugita T, Sato K, et al. Clinical outcomes of 54 pelvic osteosarcomas registered by Japanese musculoskeletal oncology group. Oncology. 2005;68(4-6):375-381.
42. Kuhelj D, Jereb B. Pediatric osteosarcoma: a 35-year experience in Slovenia. Pediatr Hematol Oncol. 2005;22(4):335-343.
43. Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104(5):1100-1109.
44. Daecke W, Bielack S, Martini AK, et al. Osteosarcoma of the hand and forearm: experience of the Cooperative Osteosarcoma Study Group. Ann Surg Oncol. 2005;12(4):322-331.
45. Cho WH, Lee SY, Song WS, Park JH. Osteosarcoma in pre-adolescent patients. J Int Med Res. 2006;34(6):676-681.
46. Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161-1168.
47. Kim MS, Lee SY, Cho WH, et al. Growth patterns of osteosarcoma predict patient survival. Arch Orthop Trauma Surg. 2009;129(9):1189-1196.
48. Lee JA, Kim MS, Kim DH, et al. Osteosarcoma developed in the period of maximal growth rate have inferior prognosis. J Pediatr Hematol Oncol. 2008;30(6):419-424.
49. Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39(8):514-522.
50. Ayan I, Kebudi R, Ozger H. Childhood osteosarcoma: multimodal therapy in a single-institution Turkish series. Cancer Treat Res. 2009;152:319-338.
51. Bruland OS, Bauer H, Alvegaard T, Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat Res. 2009;152:309-318.
52. Bielack S, Jurgens H, Jundt G, et al. Osteosarcoma: the COSS experience. Cancer Treat Res. 2009;152:289-308.
53. Bispo Júnior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo). 2009;64(12):1177-1186.
54. Gonzalez-Billalabeitia E, Hitt R, Fernandez J, et al. Pre-treatment serum lactate dehydrogenase level is an important prognostic factor in high-grade extremity osteosarcoma. Clin Transl Oncol. 2009;11(7):479-483.
55. Kong CB, Kim MS, Lee SY, et al. Prognostic effect of diaphyseal location in osteosarcoma: a cohort case–control study at a single institute. Ann Surg Oncol. 2009;16(11):3094-3100.
56. Kim MS, Lee SY, Cho WH, et al. Prognostic effects of doctor-associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg. 2009;129(10):1421-1425.
57. Lee JA, Kim MS, Kim DH, et al. Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer. 2009;52(3):340-345.
58. Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Osteosarcoma in children 5 years of age or younger at initial diagnosis. Pediatr Blood Cancer. 2010;55(2):285-289.
59. Munajat I, Zulmi W, Norazman MZ, Wan Faisham WI. Tumour volume and lung metastasis in patients with osteosarcoma. J Orthop Surg (Hong Kong). 2008;16(2):182-185.
60. Sampo M, Koivikko M, Taskinen M, et al. Incidence, epidemiology and treatment results of osteosarcoma in Finland - a nationwide population-based study. Acta Oncol. 2011;50(8):1206-1214.
61. Ferrari S, Ruggieri P, Cefalo G, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian Sarcoma Group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112-2118.
Osteosarcoma, a primary malignant tumor of the skeleton, is characterized by direct formation of immature bone or osteoid tissue by tumor cells. The World Health Organization histologic classification of bone tumors divides osteosarcoma into central and surface tumors and recognizes a number of subtypes within each group.1 The present review refers only to the classic central high-grade primary osteosarcoma of bone, which represents about 90% of all osteosarcoma cases. Classic osteosarcoma represents about 15% of all biopsy-analyzed primary bone tumors.1 It is the third most common type of neoplasia, preceded by leukemia and lymphoma among older children and adolescents aged 12 to 18 years.2 High-grade primary osteosarcoma is the most common primary skeletal tumor of childhood and adolescence, with an overall annual incidence of 5.6 cases per million children under age 15 years.3-5 Peak incidence is in the second decade of life, and males are affected slightly more often than females.2,6 The period of highest incidence coincides with the growth spurt of the long bones. Osteosarcoma preferentially affects the metaphysis of long bones, the 3 main sites being distal femur, tibia, and proximal humerus.2
Historical Perspective
For most of the 20th century, the 5-year survival rate for classic primary osteosarcoma was under 20%.7 In the 1970s, the first revolution in osteosarcoma treatment arrived with the introduction of adjuvant chemotherapy, which increased survival rates to 50%.8-10 During this expansion of research, several chemotherapeutics (eg, vincristine, bleomycin, dactinomycin) were discarded for poor effectiveness, and others (eg, cisplatin, ifosfamide) were added to doxorubicin and methotrexate, improving 5-year disease-free survival to about 70% in patients with nonmetastatic osteosarcoma. In another significant advance, adjuvant chemotherapy was supplemented with intensive preoperative chemotherapy, resulting in 5-year tumor-free survival that has ranged from 50% to 75% for high-grade osteosarcoma.5,11,12 Adding neoadjuvant chemotherapy and histologic response has allowed for evaluation of surgical margins and early treatment of microscopic disease. Thus, effective limb-sparing procedures can be performed, and the incidence of amputation has decreased from 90% to between 10% and 20%.13,14 However, statistical improvements in survival associated with neoadjuvant treatment may simply delay time of recurrence and metastasis.15 In addition, though chemotherapy has improved survival in osteogenic sarcoma, many have written that this improvement appears to reflect mainly the increase in the intensity of the chemotherapy used, which also leads to a higher propensity for side effects.16
Despite research and advances in chemotherapy regimens, the prognosis of patients with osteosarcoma remains highly variable and often dismal. Mirabello and colleagues17 examined osteosarcoma incidence and survival rates between 1973 and 2004 and found that, with the introduction of neoadjuvant chemotherapy, survival rates improved significantly between 1973 and 1983 and between 1984 and 1993, but there was little improvement between 1993 and 2004.
The long-term outcome for patients with metastatic disease is poor. Investigators have found that 11% to 20% of patients have pulmonary metastasis at initial diagnosis. About half of patients without pulmonary metastases develop them later in the disease course.18 Survival rates for patients with metastasis at initial presentation have ranged from 10% to 40%.19 Recurrent disease still occurs in 30% to 40% of patients, and more than 70% of them die of the tumor.15 The survivors of osteosarcoma are then at increased risk for chronic medical conditions and adverse health status because of the osteosarcoma-related treatments.20
Prognostic Factors
It is important to understand and exploit the influences of different prognostic factors in treating patients with osteosarcoma.7 These factors are important in establishing the best treatment for the individual. Thus, more aggressive treatments can be started in patients with prognostic factors that pose a higher risk of relapse.21 A number of clinical and pathologic features (eg, tumor site, size, subtype; patient sex and age; high alkaline phosphatase or high lactate dehydrogenase [LDH] values; multidrug resistance; genetic variations) have prognostic significance but often with contradictory results because of lack of uniformity in patient analyses and methods.15
Survival for patients with primary osteosarcoma has been analyzed with respect to tumor size and location.7 Studies have found higher survival rates for patients with smaller tumors (<10 cm) and more distal tumor locations.7 These superior survival rates may be the result of earlier detection of tumors and more options for surgical resection of smaller, distal tumors.
Serum LDH levels have helped in risk stratification of patients. High LDH often occurred at time of relapse, and relapse with high LDH correlated with poor prognosis. Meyers and colleagues22 found that 5-year disease-free survival was 72% for patients with normal LDH at presentation and 54% for patients with elevated LDH at presentation.
Several studies have shown that percentage of tumor necrosis on histology is strongly correlated with good prognosis.21 Most groups now define a good histologic response as less than 10% viable tumor cells at time of surgery, and a poor response as more than 10%.23 Results of the Pediatric Oncology Group (POG) protocol for localized osteosarcoma (POG 9351), or Children’s Cancer Group (CCG) 7921, found 45% of patients had favorable responses (>90% necrosis) after preoperative chemotherapy.24 However, several clinicians have recently questioned this finding.
Overall, the prognosis for classic osteosarcoma of the extremity remains highly variable, and there has been little improvement over the past 20 years. The prognosis for younger patients, patients with spinal disease, and patients with metastatic disease remains poor. Although some prognostic factors have been identified and shown to predict a good outcome, it seems few patients have these positive factors. In this article, we describe the literature review and meta-analysis we performed to better define recent survival trends for patients with primary osteosarcoma.
Methods
The MEDLINE, PubMed, and Cochrane databases were searched for eligible studies published in English between 2000 and 2011—a decade of recently reported research. We applied the search strategy [“osteosarcoma” OR “osteogenic sarcoma”] AND [“prognosis” OR “treatment” OR “survival”] and selected reports that specifically addressed factors predicting survival in patients with osteosarcoma—reports that were limited to primary osteosarcoma of the pelvis or extremity and provided 5-year overall survival (OS) data. Abstracts of the selected articles were independently reviewed, and the inclusion and exclusion criteria were applied. We excluded basic science studies and those without pediatric patients, those without primary osteosarcoma, those with periosteal or parosteal osteosarcoma, and those that did not report 5-year OS data.
Statistical Analysis
Number or proportion of patients (whichever was reported) with 5-year OS and number or proportion of patients with 90% necrosis were extracted from each study. For each trial, proportion of patients with 5-year OS and 95% confidence intervals (CIs) and proportion of patients achieving 90% necrosis and 95% CIs were determined. We also calculated proportion of patients with 5-year OS and proportion of patients with 90% necrosis with corresponding 95% CIs of studies that included patients with nonmetastatic disease.
We assessed statistical heterogeneity among trials included in the meta-analysis using the Cochran Q test. Inconsistency was quantified with the I2 statistic, which estimates percentage of total across-studies variation caused by heterogeneity rather than chance.25 We considered I2 higher than 50% as indicating substantial heterogeneity. When substantial heterogeneity was not found, the pooled estimate calculated on the basis of the fixed-effects model was reported using the inverse variance method. When substantial heterogeneity was found, the pooled estimate calculated on the basis of a random-effects model was reported using the DerSimonian and Laird26 method, which takes both within- and between-study variations into account.
Publication bias was assessed through funnel plots and with Begg and Egger tests.27,28 Two-tailed P < .05 was considered statistically significant. All statistical analyses were performed with Stata/SE Version 11.0 (StataCorp).
Results
Our literature search yielded 597 articles. We cross-referenced these articles with the MEDLINE, PubMed, and Cochrane search results using the same keywords and discarded the duplicates. The abstracts of these articles were then reviewed in detail. The 40 articles4,6,11,12,14,15,17-19,21,29-58 that met our study inclusion criteria reported on studies that included patients with metastatic and nonmetastatic osteosarcoma. Because of the significant difference in OS of patients with metastatic disease, we also analyzed articles that included only patients with nonmetastatic disease. Sixteen articles6,14,15,29,32-35,39,47,48,51,53,54,55,57 were included in the analysis of patients with nonmetastatic disease.
Figure 1 shows 5-year OS for each of the 40 studies. For studies that compared survival of different groups of patients, the survival of each group is shown separately. For example, Bacci and colleagues39 divided patients into adolescent and preadolescent groups and reported 5-year OS for each. In our analysis, we treated each group independently and reported their 5-year OS separately. For each study, 5-year OS, weight of study, and CI are included. Five-year OS ranged from 19% to 94%. Analysis was performed to determine 5-year OS for all studies based on weight given to each study. The random-effects model used for this analysis (heterogeneity test, Q = 656.23; P < .001; I2 = 93.4%) showed 5-year OS of 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma.
Figure 2 shows 5-year OS (range, 53%-94%) for each of the 16 studies that included only patients with nonmetastatic disease. The random-effects model used for this analysis (heterogeneity test, Q = 142.08; P < .001; I2 = 89.4%) showed 5-year OS of 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic disease.
We then examined percentage of patients achieving 90% necrosis on histology in each study. Several studies included in the OS analysis did not report percentage necrosis, leaving 29 studies for the necrosis analysis. Of these 29 studies, all 29 included patients with metastatic and nonmetastatic disease,4,6,11,14,15,18,19,21,29,31-36,37,39,40,43-47,49,50,54-57,59 and 13 included only patients with nonmetastatic disease.6,14,15,29,32-35,40,47,54,55,57 Again, because of the known difference in prognosis between patients with metastatic disease and patients with nonmetastatic disease, we performed separate analyses, one for the combined dataset of all 29 studies (Figure 3) and the other for the 13 nonmetastatic studies (Figure 4). Random-effects models showed 90% necrosis for 50% of patients in both analyses: studies that included patients with metastatic and nonmetastatic disease (95% CI, 45%-54%; heterogeneity test, Q = 692.88; P < .001; I2 = 95.5%) and nonmetastatic studies (95% CI, 41%-59%; heterogeneity test, Q = 385.42; P < .001; I2 = 96.9%).
We also performed a meta-regression analysis that included necrosis as a continuous variable for both the overall dataset and the nonmetastatic dataset. Five-year OS was plotted against percentage of patients achieving 90% tumor necrosis for each study. The results are plotted in Figure 5 (combined dataset).
No evidence of publication bias was detected for 5-year OS or percentage necrosis for the analyses of the combined datasets by either Egger test or Begg test. For 5-year OS, Ps were .21 (Egger) and .19 (Begg); for percentage necrosis, Ps were .10 (Egger) and .62 (Begg). In addition, no evidence of publication bias was detected for the analyses of the nonmetastatic studies by either test. For 5-year OS, Ps were .55 (Egger) and .41 (Begg); for percentage necrosis, Ps were .42 (Egger) and .95 (Begg).
Discussion
Five-year OS was 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma and 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic osteosarcoma. These percentages fall within the range found in the literature. Mankin and colleagues37 reviewed 648 cases of patients with osteosarcoma treated at Massachusetts General Hospital in 2004; OS was 68%. In 2011, Sampo and colleagues60 reported 10-year OS of 63% for patients with metastatic and nonmetastatic disease and 73% for patients with local disease at presentation. Five-year OS rates in the literature are consistently about 70%. Ferrari and colleagues61 reported 5-year OS of 73% and 74% for 230 patients treated with 2 different neoadjuvant chemotherapy regimens between 2001 and 2006. The consistency in 5-year OS suggests OS of pediatric patients with osteosarcoma has plateaued, and there has been no significant improvement in survival of patients with osteosarcoma over the past 30 years.
Histologic response to preoperative chemotherapy is strongly associated with survival in pediatric osteosarcoma. Bielack and colleagues31 reported 5-year OS of 75% to 80% for patients who responded well to preoperative chemotherapy (>90% tumor necrosis) and 45% to 55% for patients who responded poorly (<10% necrosis). In our meta-analysis of studies that included patients with nonmetastatic osteosarcoma, 50% achieved necrosis of more than 90%. Percentage of patients achieving necrosis of more than 90% has been about 45%, according to past reports. In 2012, Ferrari and colleagues61 reported that 45% of 230 patients treated with neoadjuvant chemotherapy achieved more than 90% tumor necrosis. Therefore, 5-year OS and percentage of patients achieving 90% necrosis are consistent with previous reports, though this also suggests these numbers have remained constant over the past several decades.
Despite its expansive scale, our study has several important limitations. Data were extracted from published studies, and individual patient data were not available, so we were not able to assess the effects of risk factors (eg, tumor size, location) on 5-year OS. We could not correlate the proportion of patients with 90% necrosis to 5-year OS, as studies did not report OS by necrosis strata. Also, because our numbers were derived from published studies, they may not accurately represent outcomes in the community as a whole. In addition, several successive studies may contain duplicate patient cases. We limited our search to studies published since 2000 to include patients recently diagnosed and treated for osteosarcoma; however, several studies published after 2000 also included patients diagnosed and treated before 2000. Several of these studies are from countries outside the United States and may have a significantly different incidence of osteosarcoma as well as treatment methods and survival rates.
Although this meta-analysis suggests 5-year OS remains about 70% for patients with primary nonmetastatic osteosarcoma, we cannot settle on this conclusion because of the many differences between the studies we included. Therefore, more studies of patients diagnosed and treated within the past 10 years are needed to confirm our beliefs about patient survival.
Osteosarcoma, a primary malignant tumor of the skeleton, is characterized by direct formation of immature bone or osteoid tissue by tumor cells. The World Health Organization histologic classification of bone tumors divides osteosarcoma into central and surface tumors and recognizes a number of subtypes within each group.1 The present review refers only to the classic central high-grade primary osteosarcoma of bone, which represents about 90% of all osteosarcoma cases. Classic osteosarcoma represents about 15% of all biopsy-analyzed primary bone tumors.1 It is the third most common type of neoplasia, preceded by leukemia and lymphoma among older children and adolescents aged 12 to 18 years.2 High-grade primary osteosarcoma is the most common primary skeletal tumor of childhood and adolescence, with an overall annual incidence of 5.6 cases per million children under age 15 years.3-5 Peak incidence is in the second decade of life, and males are affected slightly more often than females.2,6 The period of highest incidence coincides with the growth spurt of the long bones. Osteosarcoma preferentially affects the metaphysis of long bones, the 3 main sites being distal femur, tibia, and proximal humerus.2
Historical Perspective
For most of the 20th century, the 5-year survival rate for classic primary osteosarcoma was under 20%.7 In the 1970s, the first revolution in osteosarcoma treatment arrived with the introduction of adjuvant chemotherapy, which increased survival rates to 50%.8-10 During this expansion of research, several chemotherapeutics (eg, vincristine, bleomycin, dactinomycin) were discarded for poor effectiveness, and others (eg, cisplatin, ifosfamide) were added to doxorubicin and methotrexate, improving 5-year disease-free survival to about 70% in patients with nonmetastatic osteosarcoma. In another significant advance, adjuvant chemotherapy was supplemented with intensive preoperative chemotherapy, resulting in 5-year tumor-free survival that has ranged from 50% to 75% for high-grade osteosarcoma.5,11,12 Adding neoadjuvant chemotherapy and histologic response has allowed for evaluation of surgical margins and early treatment of microscopic disease. Thus, effective limb-sparing procedures can be performed, and the incidence of amputation has decreased from 90% to between 10% and 20%.13,14 However, statistical improvements in survival associated with neoadjuvant treatment may simply delay time of recurrence and metastasis.15 In addition, though chemotherapy has improved survival in osteogenic sarcoma, many have written that this improvement appears to reflect mainly the increase in the intensity of the chemotherapy used, which also leads to a higher propensity for side effects.16
Despite research and advances in chemotherapy regimens, the prognosis of patients with osteosarcoma remains highly variable and often dismal. Mirabello and colleagues17 examined osteosarcoma incidence and survival rates between 1973 and 2004 and found that, with the introduction of neoadjuvant chemotherapy, survival rates improved significantly between 1973 and 1983 and between 1984 and 1993, but there was little improvement between 1993 and 2004.
The long-term outcome for patients with metastatic disease is poor. Investigators have found that 11% to 20% of patients have pulmonary metastasis at initial diagnosis. About half of patients without pulmonary metastases develop them later in the disease course.18 Survival rates for patients with metastasis at initial presentation have ranged from 10% to 40%.19 Recurrent disease still occurs in 30% to 40% of patients, and more than 70% of them die of the tumor.15 The survivors of osteosarcoma are then at increased risk for chronic medical conditions and adverse health status because of the osteosarcoma-related treatments.20
Prognostic Factors
It is important to understand and exploit the influences of different prognostic factors in treating patients with osteosarcoma.7 These factors are important in establishing the best treatment for the individual. Thus, more aggressive treatments can be started in patients with prognostic factors that pose a higher risk of relapse.21 A number of clinical and pathologic features (eg, tumor site, size, subtype; patient sex and age; high alkaline phosphatase or high lactate dehydrogenase [LDH] values; multidrug resistance; genetic variations) have prognostic significance but often with contradictory results because of lack of uniformity in patient analyses and methods.15
Survival for patients with primary osteosarcoma has been analyzed with respect to tumor size and location.7 Studies have found higher survival rates for patients with smaller tumors (<10 cm) and more distal tumor locations.7 These superior survival rates may be the result of earlier detection of tumors and more options for surgical resection of smaller, distal tumors.
Serum LDH levels have helped in risk stratification of patients. High LDH often occurred at time of relapse, and relapse with high LDH correlated with poor prognosis. Meyers and colleagues22 found that 5-year disease-free survival was 72% for patients with normal LDH at presentation and 54% for patients with elevated LDH at presentation.
Several studies have shown that percentage of tumor necrosis on histology is strongly correlated with good prognosis.21 Most groups now define a good histologic response as less than 10% viable tumor cells at time of surgery, and a poor response as more than 10%.23 Results of the Pediatric Oncology Group (POG) protocol for localized osteosarcoma (POG 9351), or Children’s Cancer Group (CCG) 7921, found 45% of patients had favorable responses (>90% necrosis) after preoperative chemotherapy.24 However, several clinicians have recently questioned this finding.
Overall, the prognosis for classic osteosarcoma of the extremity remains highly variable, and there has been little improvement over the past 20 years. The prognosis for younger patients, patients with spinal disease, and patients with metastatic disease remains poor. Although some prognostic factors have been identified and shown to predict a good outcome, it seems few patients have these positive factors. In this article, we describe the literature review and meta-analysis we performed to better define recent survival trends for patients with primary osteosarcoma.
Methods
The MEDLINE, PubMed, and Cochrane databases were searched for eligible studies published in English between 2000 and 2011—a decade of recently reported research. We applied the search strategy [“osteosarcoma” OR “osteogenic sarcoma”] AND [“prognosis” OR “treatment” OR “survival”] and selected reports that specifically addressed factors predicting survival in patients with osteosarcoma—reports that were limited to primary osteosarcoma of the pelvis or extremity and provided 5-year overall survival (OS) data. Abstracts of the selected articles were independently reviewed, and the inclusion and exclusion criteria were applied. We excluded basic science studies and those without pediatric patients, those without primary osteosarcoma, those with periosteal or parosteal osteosarcoma, and those that did not report 5-year OS data.
Statistical Analysis
Number or proportion of patients (whichever was reported) with 5-year OS and number or proportion of patients with 90% necrosis were extracted from each study. For each trial, proportion of patients with 5-year OS and 95% confidence intervals (CIs) and proportion of patients achieving 90% necrosis and 95% CIs were determined. We also calculated proportion of patients with 5-year OS and proportion of patients with 90% necrosis with corresponding 95% CIs of studies that included patients with nonmetastatic disease.
We assessed statistical heterogeneity among trials included in the meta-analysis using the Cochran Q test. Inconsistency was quantified with the I2 statistic, which estimates percentage of total across-studies variation caused by heterogeneity rather than chance.25 We considered I2 higher than 50% as indicating substantial heterogeneity. When substantial heterogeneity was not found, the pooled estimate calculated on the basis of the fixed-effects model was reported using the inverse variance method. When substantial heterogeneity was found, the pooled estimate calculated on the basis of a random-effects model was reported using the DerSimonian and Laird26 method, which takes both within- and between-study variations into account.
Publication bias was assessed through funnel plots and with Begg and Egger tests.27,28 Two-tailed P < .05 was considered statistically significant. All statistical analyses were performed with Stata/SE Version 11.0 (StataCorp).
Results
Our literature search yielded 597 articles. We cross-referenced these articles with the MEDLINE, PubMed, and Cochrane search results using the same keywords and discarded the duplicates. The abstracts of these articles were then reviewed in detail. The 40 articles4,6,11,12,14,15,17-19,21,29-58 that met our study inclusion criteria reported on studies that included patients with metastatic and nonmetastatic osteosarcoma. Because of the significant difference in OS of patients with metastatic disease, we also analyzed articles that included only patients with nonmetastatic disease. Sixteen articles6,14,15,29,32-35,39,47,48,51,53,54,55,57 were included in the analysis of patients with nonmetastatic disease.
Figure 1 shows 5-year OS for each of the 40 studies. For studies that compared survival of different groups of patients, the survival of each group is shown separately. For example, Bacci and colleagues39 divided patients into adolescent and preadolescent groups and reported 5-year OS for each. In our analysis, we treated each group independently and reported their 5-year OS separately. For each study, 5-year OS, weight of study, and CI are included. Five-year OS ranged from 19% to 94%. Analysis was performed to determine 5-year OS for all studies based on weight given to each study. The random-effects model used for this analysis (heterogeneity test, Q = 656.23; P < .001; I2 = 93.4%) showed 5-year OS of 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma.
Figure 2 shows 5-year OS (range, 53%-94%) for each of the 16 studies that included only patients with nonmetastatic disease. The random-effects model used for this analysis (heterogeneity test, Q = 142.08; P < .001; I2 = 89.4%) showed 5-year OS of 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic disease.
We then examined percentage of patients achieving 90% necrosis on histology in each study. Several studies included in the OS analysis did not report percentage necrosis, leaving 29 studies for the necrosis analysis. Of these 29 studies, all 29 included patients with metastatic and nonmetastatic disease,4,6,11,14,15,18,19,21,29,31-36,37,39,40,43-47,49,50,54-57,59 and 13 included only patients with nonmetastatic disease.6,14,15,29,32-35,40,47,54,55,57 Again, because of the known difference in prognosis between patients with metastatic disease and patients with nonmetastatic disease, we performed separate analyses, one for the combined dataset of all 29 studies (Figure 3) and the other for the 13 nonmetastatic studies (Figure 4). Random-effects models showed 90% necrosis for 50% of patients in both analyses: studies that included patients with metastatic and nonmetastatic disease (95% CI, 45%-54%; heterogeneity test, Q = 692.88; P < .001; I2 = 95.5%) and nonmetastatic studies (95% CI, 41%-59%; heterogeneity test, Q = 385.42; P < .001; I2 = 96.9%).
We also performed a meta-regression analysis that included necrosis as a continuous variable for both the overall dataset and the nonmetastatic dataset. Five-year OS was plotted against percentage of patients achieving 90% tumor necrosis for each study. The results are plotted in Figure 5 (combined dataset).
No evidence of publication bias was detected for 5-year OS or percentage necrosis for the analyses of the combined datasets by either Egger test or Begg test. For 5-year OS, Ps were .21 (Egger) and .19 (Begg); for percentage necrosis, Ps were .10 (Egger) and .62 (Begg). In addition, no evidence of publication bias was detected for the analyses of the nonmetastatic studies by either test. For 5-year OS, Ps were .55 (Egger) and .41 (Begg); for percentage necrosis, Ps were .42 (Egger) and .95 (Begg).
Discussion
Five-year OS was 63% (95% CI, 60%-66%) for studies that included patients with metastatic and nonmetastatic osteosarcoma and 71% (95% CI, 67%-76%) for studies that included only patients with nonmetastatic osteosarcoma. These percentages fall within the range found in the literature. Mankin and colleagues37 reviewed 648 cases of patients with osteosarcoma treated at Massachusetts General Hospital in 2004; OS was 68%. In 2011, Sampo and colleagues60 reported 10-year OS of 63% for patients with metastatic and nonmetastatic disease and 73% for patients with local disease at presentation. Five-year OS rates in the literature are consistently about 70%. Ferrari and colleagues61 reported 5-year OS of 73% and 74% for 230 patients treated with 2 different neoadjuvant chemotherapy regimens between 2001 and 2006. The consistency in 5-year OS suggests OS of pediatric patients with osteosarcoma has plateaued, and there has been no significant improvement in survival of patients with osteosarcoma over the past 30 years.
Histologic response to preoperative chemotherapy is strongly associated with survival in pediatric osteosarcoma. Bielack and colleagues31 reported 5-year OS of 75% to 80% for patients who responded well to preoperative chemotherapy (>90% tumor necrosis) and 45% to 55% for patients who responded poorly (<10% necrosis). In our meta-analysis of studies that included patients with nonmetastatic osteosarcoma, 50% achieved necrosis of more than 90%. Percentage of patients achieving necrosis of more than 90% has been about 45%, according to past reports. In 2012, Ferrari and colleagues61 reported that 45% of 230 patients treated with neoadjuvant chemotherapy achieved more than 90% tumor necrosis. Therefore, 5-year OS and percentage of patients achieving 90% necrosis are consistent with previous reports, though this also suggests these numbers have remained constant over the past several decades.
Despite its expansive scale, our study has several important limitations. Data were extracted from published studies, and individual patient data were not available, so we were not able to assess the effects of risk factors (eg, tumor size, location) on 5-year OS. We could not correlate the proportion of patients with 90% necrosis to 5-year OS, as studies did not report OS by necrosis strata. Also, because our numbers were derived from published studies, they may not accurately represent outcomes in the community as a whole. In addition, several successive studies may contain duplicate patient cases. We limited our search to studies published since 2000 to include patients recently diagnosed and treated for osteosarcoma; however, several studies published after 2000 also included patients diagnosed and treated before 2000. Several of these studies are from countries outside the United States and may have a significantly different incidence of osteosarcoma as well as treatment methods and survival rates.
Although this meta-analysis suggests 5-year OS remains about 70% for patients with primary nonmetastatic osteosarcoma, we cannot settle on this conclusion because of the many differences between the studies we included. Therefore, more studies of patients diagnosed and treated within the past 10 years are needed to confirm our beliefs about patient survival.
1. Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
2. Cho WH, Song WS, Jeon DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010;17(3):702-708.
3. Abate ME, Longhi A, Galletti S, Ferrari S, Bacci G. Non-metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatr Blood Cancer. 2010;55(4):652-654.
4. Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011-2018.
5. Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45(13):2367-2375.
6. Kaste SC, Liu T, Billups CA, Daw NC, Pratt CB, Meyer WH. Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer. 2004;43(7):723-728.
7. Brostrom LA, Strander H, Nilsonne U. Survival in osteosarcoma in relation to tumor size and location. Clin Orthop Relat Res. 1982;167:250-254.
8. Harvei S, Solheim O. The prognosis in osteosarcoma: Norwegian national data. Cancer. 1981;48(8):1719-1723.
9. Sutow WW, Sullivan MP, Fernbach DJ, Cangir A, George SL. Adjuvant chemotherapy in primary treatment of osteogenic sarcoma. A Southwest Oncology Group study. Cancer. 1975;36(5):1598-1602.
10. Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5(1):21-26.
11. Hsieh MY, Hung GY, Yen HJ, Chen WM, Chen TH. Osteosarcoma in preadolescent patients: experience in a single institute in Taiwan. J Chin Med Assoc. 2009;72(9):455-461.
12. Longhi A, Pasini E, Bertoni F, Pignotti E, Ferrari C, Bacci G. Twenty-year follow-up of osteosarcoma of the extremity treated with adjuvant chemotherapy. J Chemother. 2004;16(6):582-588.
13. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
14. Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/Osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18(24):4016-4027.
15. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154-1161.
16. Cohen IJ, Kaplinsky C, Katz K, et al. Improved results in osteogenic sarcoma 1973–79 vs. 1980–86: analysis of results from a single center. Isr J Med Sci. 1993;29(1):27-29.
17. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results program. Cancer. 2009;115(7):1531-1543.
18. Kager L, Zoubek A, Dominkus M, et al. Osteosarcoma in very young children: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2010;116(22):5316-5324.
19. Szendroi M, Papai Z, Koos R, Illes T. Limb-saving surgery, survival, and prognostic factors for osteosarcoma: the Hungarian experience. J Surg Oncol. 2000;73(2):87-94.
20. Nagarajan R, Kamruzzaman A, Ness KK, et al. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(3):625-634.
21. Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori. 2004;90(5):478-484.
22. Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5-15.
23. Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422-441.
24. Hendershot E, Pappo A, Malkin D, Sung L. Tumor necrosis in pediatric osteosarcoma: impact of modern therapies. J Pediatr Oncol Nurs. 2006;23(4):176-181.
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101.
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634.
29. Bacci G, Ferrari S, Longhi A, Mellano D, Giacomini S, Forni C. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86(3):204-206.
30. Bacci G, Ferrari S, Longhi A, et al. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother. 2001;13(1):93-99.
31. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790.
32. Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38(9):1218-1225.
33. Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg Am. 2002;84(1):49-57.
34. Wilkins RM, Cullen JW, Odom L, et al. Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity. Ann Surg Oncol. 2003;10(5):498-507.
35. Smeland S, Muller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488-494.
36. Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21(2):334-341.
37. Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286-291.
38. Donati D, Giacomini S, Gozzi E, et al. Osteosarcoma of the pelvis. Eur J Surg Oncol. 2004;30(3):332-340.
39. Bacci G, Longhi A, Bertoni F, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients. J Pediatr Hematol Oncol. 2005;27(3):129-134.
40. Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41(18):2836-2845.
41. Matsuo T, Sugita T, Sato K, et al. Clinical outcomes of 54 pelvic osteosarcomas registered by Japanese musculoskeletal oncology group. Oncology. 2005;68(4-6):375-381.
42. Kuhelj D, Jereb B. Pediatric osteosarcoma: a 35-year experience in Slovenia. Pediatr Hematol Oncol. 2005;22(4):335-343.
43. Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104(5):1100-1109.
44. Daecke W, Bielack S, Martini AK, et al. Osteosarcoma of the hand and forearm: experience of the Cooperative Osteosarcoma Study Group. Ann Surg Oncol. 2005;12(4):322-331.
45. Cho WH, Lee SY, Song WS, Park JH. Osteosarcoma in pre-adolescent patients. J Int Med Res. 2006;34(6):676-681.
46. Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161-1168.
47. Kim MS, Lee SY, Cho WH, et al. Growth patterns of osteosarcoma predict patient survival. Arch Orthop Trauma Surg. 2009;129(9):1189-1196.
48. Lee JA, Kim MS, Kim DH, et al. Osteosarcoma developed in the period of maximal growth rate have inferior prognosis. J Pediatr Hematol Oncol. 2008;30(6):419-424.
49. Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39(8):514-522.
50. Ayan I, Kebudi R, Ozger H. Childhood osteosarcoma: multimodal therapy in a single-institution Turkish series. Cancer Treat Res. 2009;152:319-338.
51. Bruland OS, Bauer H, Alvegaard T, Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat Res. 2009;152:309-318.
52. Bielack S, Jurgens H, Jundt G, et al. Osteosarcoma: the COSS experience. Cancer Treat Res. 2009;152:289-308.
53. Bispo Júnior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo). 2009;64(12):1177-1186.
54. Gonzalez-Billalabeitia E, Hitt R, Fernandez J, et al. Pre-treatment serum lactate dehydrogenase level is an important prognostic factor in high-grade extremity osteosarcoma. Clin Transl Oncol. 2009;11(7):479-483.
55. Kong CB, Kim MS, Lee SY, et al. Prognostic effect of diaphyseal location in osteosarcoma: a cohort case–control study at a single institute. Ann Surg Oncol. 2009;16(11):3094-3100.
56. Kim MS, Lee SY, Cho WH, et al. Prognostic effects of doctor-associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg. 2009;129(10):1421-1425.
57. Lee JA, Kim MS, Kim DH, et al. Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer. 2009;52(3):340-345.
58. Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Osteosarcoma in children 5 years of age or younger at initial diagnosis. Pediatr Blood Cancer. 2010;55(2):285-289.
59. Munajat I, Zulmi W, Norazman MZ, Wan Faisham WI. Tumour volume and lung metastasis in patients with osteosarcoma. J Orthop Surg (Hong Kong). 2008;16(2):182-185.
60. Sampo M, Koivikko M, Taskinen M, et al. Incidence, epidemiology and treatment results of osteosarcoma in Finland - a nationwide population-based study. Acta Oncol. 2011;50(8):1206-1214.
61. Ferrari S, Ruggieri P, Cefalo G, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian Sarcoma Group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112-2118.
1. Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
2. Cho WH, Song WS, Jeon DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010;17(3):702-708.
3. Abate ME, Longhi A, Galletti S, Ferrari S, Bacci G. Non-metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatr Blood Cancer. 2010;55(4):652-654.
4. Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011-2018.
5. Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45(13):2367-2375.
6. Kaste SC, Liu T, Billups CA, Daw NC, Pratt CB, Meyer WH. Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer. 2004;43(7):723-728.
7. Brostrom LA, Strander H, Nilsonne U. Survival in osteosarcoma in relation to tumor size and location. Clin Orthop Relat Res. 1982;167:250-254.
8. Harvei S, Solheim O. The prognosis in osteosarcoma: Norwegian national data. Cancer. 1981;48(8):1719-1723.
9. Sutow WW, Sullivan MP, Fernbach DJ, Cangir A, George SL. Adjuvant chemotherapy in primary treatment of osteogenic sarcoma. A Southwest Oncology Group study. Cancer. 1975;36(5):1598-1602.
10. Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5(1):21-26.
11. Hsieh MY, Hung GY, Yen HJ, Chen WM, Chen TH. Osteosarcoma in preadolescent patients: experience in a single institute in Taiwan. J Chin Med Assoc. 2009;72(9):455-461.
12. Longhi A, Pasini E, Bertoni F, Pignotti E, Ferrari C, Bacci G. Twenty-year follow-up of osteosarcoma of the extremity treated with adjuvant chemotherapy. J Chemother. 2004;16(6):582-588.
13. Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84(1):88-92.
14. Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/Osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18(24):4016-4027.
15. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154-1161.
16. Cohen IJ, Kaplinsky C, Katz K, et al. Improved results in osteogenic sarcoma 1973–79 vs. 1980–86: analysis of results from a single center. Isr J Med Sci. 1993;29(1):27-29.
17. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results program. Cancer. 2009;115(7):1531-1543.
18. Kager L, Zoubek A, Dominkus M, et al. Osteosarcoma in very young children: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2010;116(22):5316-5324.
19. Szendroi M, Papai Z, Koos R, Illes T. Limb-saving surgery, survival, and prognostic factors for osteosarcoma: the Hungarian experience. J Surg Oncol. 2000;73(2):87-94.
20. Nagarajan R, Kamruzzaman A, Ness KK, et al. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117(3):625-634.
21. Bacci G, Longhi A, Ferrari S, et al. Prognostic significance of serum lactate dehydrogenase in osteosarcoma of the extremity: experience at Rizzoli on 1421 patients treated over the last 30 years. Tumori. 2004;90(5):478-484.
22. Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5-15.
23. Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422-441.
24. Hendershot E, Pappo A, Malkin D, Sung L. Tumor necrosis in pediatric osteosarcoma: impact of modern therapies. J Pediatr Oncol Nurs. 2006;23(4):176-181.
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101.
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634.
29. Bacci G, Ferrari S, Longhi A, Mellano D, Giacomini S, Forni C. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86(3):204-206.
30. Bacci G, Ferrari S, Longhi A, et al. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother. 2001;13(1):93-99.
31. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790.
32. Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38(9):1218-1225.
33. Scully SP, Ghert MA, Zurakowski D, Thompson RC, Gebhardt MC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg Am. 2002;84(1):49-57.
34. Wilkins RM, Cullen JW, Odom L, et al. Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity. Ann Surg Oncol. 2003;10(5):498-507.
35. Smeland S, Muller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488-494.
36. Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21(2):334-341.
37. Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286-291.
38. Donati D, Giacomini S, Gozzi E, et al. Osteosarcoma of the pelvis. Eur J Surg Oncol. 2004;30(3):332-340.
39. Bacci G, Longhi A, Bertoni F, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients. J Pediatr Hematol Oncol. 2005;27(3):129-134.
40. Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41(18):2836-2845.
41. Matsuo T, Sugita T, Sato K, et al. Clinical outcomes of 54 pelvic osteosarcomas registered by Japanese musculoskeletal oncology group. Oncology. 2005;68(4-6):375-381.
42. Kuhelj D, Jereb B. Pediatric osteosarcoma: a 35-year experience in Slovenia. Pediatr Hematol Oncol. 2005;22(4):335-343.
43. Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104(5):1100-1109.
44. Daecke W, Bielack S, Martini AK, et al. Osteosarcoma of the hand and forearm: experience of the Cooperative Osteosarcoma Study Group. Ann Surg Oncol. 2005;12(4):322-331.
45. Cho WH, Lee SY, Song WS, Park JH. Osteosarcoma in pre-adolescent patients. J Int Med Res. 2006;34(6):676-681.
46. Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161-1168.
47. Kim MS, Lee SY, Cho WH, et al. Growth patterns of osteosarcoma predict patient survival. Arch Orthop Trauma Surg. 2009;129(9):1189-1196.
48. Lee JA, Kim MS, Kim DH, et al. Osteosarcoma developed in the period of maximal growth rate have inferior prognosis. J Pediatr Hematol Oncol. 2008;30(6):419-424.
49. Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39(8):514-522.
50. Ayan I, Kebudi R, Ozger H. Childhood osteosarcoma: multimodal therapy in a single-institution Turkish series. Cancer Treat Res. 2009;152:319-338.
51. Bruland OS, Bauer H, Alvegaard T, Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat Res. 2009;152:309-318.
52. Bielack S, Jurgens H, Jundt G, et al. Osteosarcoma: the COSS experience. Cancer Treat Res. 2009;152:289-308.
53. Bispo Júnior RZ, Camargo OP. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clinics (Sao Paulo). 2009;64(12):1177-1186.
54. Gonzalez-Billalabeitia E, Hitt R, Fernandez J, et al. Pre-treatment serum lactate dehydrogenase level is an important prognostic factor in high-grade extremity osteosarcoma. Clin Transl Oncol. 2009;11(7):479-483.
55. Kong CB, Kim MS, Lee SY, et al. Prognostic effect of diaphyseal location in osteosarcoma: a cohort case–control study at a single institute. Ann Surg Oncol. 2009;16(11):3094-3100.
56. Kim MS, Lee SY, Cho WH, et al. Prognostic effects of doctor-associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg. 2009;129(10):1421-1425.
57. Lee JA, Kim MS, Kim DH, et al. Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer. 2009;52(3):340-345.
58. Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Osteosarcoma in children 5 years of age or younger at initial diagnosis. Pediatr Blood Cancer. 2010;55(2):285-289.
59. Munajat I, Zulmi W, Norazman MZ, Wan Faisham WI. Tumour volume and lung metastasis in patients with osteosarcoma. J Orthop Surg (Hong Kong). 2008;16(2):182-185.
60. Sampo M, Koivikko M, Taskinen M, et al. Incidence, epidemiology and treatment results of osteosarcoma in Finland - a nationwide population-based study. Acta Oncol. 2011;50(8):1206-1214.
61. Ferrari S, Ruggieri P, Cefalo G, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian Sarcoma Group trial ISG/OS-1. J Clin Oncol. 2012;30(17):2112-2118.
Psoriasis Cohort Reveals High Arthritis Risk
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
FROM ARTHRITIS & RHEUMATOLOGY