User login
Crisis in Medicine: Part 3. The Physician as the Captain—A Personal Touch
"Report to the Administrator’s Office for a discussion 7:00 am sharp,” reads the email on your phone. The phone log sheet from your administrator is handed to you as you are running to the operating room and reads, “Call back Mr. Smith’s health insurance company because your patient stayed overnight unexpectedly in the hospital, and if the return phone call is not received by 8:40 am the complete hospital stay will be disallowed.” The text message reads, “The head nurse from the emergency department wants to have a discussion with you tomorrow about what transpired in room 23 last night at 1:33 am.” Your physician assistant calls you because a recent history and physical examination from the out-of-state internist has not been cosigned by you, and, therefore, the patient is still in the admitting office; the admitting officer is waiting to go home and won’t accept the physician assistant’s signature.
This simple illustration of a surgeon’s typical morning is hardly hyperbole. Demands and finger-pointing are routine aspects of care, with a concurrent need to attribute blame and create a hostile work environment whether in the office, operating room, or floor of the hospital by anyone who can proudly say to the physician, “Gotcha!” The environment that produces this ethos is toxic and needs to be changed. While all members of a patient care team must be accountable, no member should be antagonistic toward another, and each member must feel a part of a working whole that is led by a competent, caring, and identifiable physician. Yes, the doctor must be the team captain; he or she must take back the reins of care immediately in order to provide the patient with the best possible outcome.
The loss of leadership can be traced back to the rise of regulatory controls put in place by government entities or local hospital administration to contain costs and limit liability. While the target goals of such measures are laudable, the negative impact on the doctor–patient relationship has been palpable and problematic and requires reassessment. The profession itself will be preserved by refocusing on the doctor–patient relationship and returning the physician to the role of team leader. Our patients deserve to feel as though their health care resides in the hands of the physician as the leader of a team that is pursuing a common goal: patient care with minimal distractions.
What, though, makes a great captain or leader? Sociologists have said that in a stable environment a “participatory model” of leadership is appropriate, while in a high-growth or changing environment, like the one in which we presently live, an “authoritative model” can be used to right the ship.1,2 Many types of leaders exist within both models. Leaders who are “innovators” will design and bring new ideas and original thought but may generate too many ideas that can’t be implemented practically in the hospital setting. Leaders who are “developers” will build and move forward to achieve challenging goals but may be impatient when ideas do not work and may be perceived in many interdisciplinary meetings as unruly. “Bureaucratic” leaders, presently seen in many leadership positions, can be classified as stabilizers and, while they may maintain equilibrium and keep things running smoothly, they often insist on a policy for every situation, resulting in stasis and sometimes even paralysis of the surgical center or hospital system.
I believe that health management and patient care require the simultaneous use of the authoritative and participatory models to encourage innovation, set attainable short- and long-term goals, and maintain the physician as the team leader. To lead effectively under this hybrid model, the physician must be accessible and fair, a teacher and a student, and a risk-taker, but, ultimately, at the end of every day, the physician must be accountable.
The time has come for physician leaders to assemble the troops: administrators, clinical providers, and nonclinical support staff. To paraphrase John Quincy Adams, in your actions inspire others to dream more and become more; then, and only then, are you an excellent leader. A secret to effective leadership is in finding one’s voice and acknowledging strengths and weaknesses. The leader must recruit other leaders who are very different from himself or herself and must listen to them deeply and trust them completely. One of our former first ladies said wisely, “A leader takes people where they want to go. A great leader takes people where they don’t necessarily want to go, but ought to be.” To truly find this leadership model, we as busy surgeons must spend some concentrated time away from our patients and exciting research to sit in the room with our nurses, administrators, and all other members of the health care community and listen to their thoughts and understand their concerns. We must understand policy to assess if it is reasonable and, if it is not, to reject it and propose more effective and appropriate rules for good care. We must remove from leadership positions those that do not have the interest of the patient as their primary concern. We must challenge any policy that does not have the patient’s interest and health as its raison d’etre. We must be proactive and not reactive. We must be ready to stand tall and politely question when dictated to unless evidence-based medical reasons can be presented.
You may ask, therefore, where should we lead? The answer is obvious! We need to be involved in every aspect of this great profession. We need to be the leaders of hospital systems, we need to be in charge of research institutions, and, as always, we need to be the chief of the operating room and the chief within each room as the team leader for the nurse, anesthesiologist, and nonclinical staff in order to safely guide our patients through the stress of a medical crisis or routine intervention. We need to find those of us with other degrees, whether MPH, MBA, MHA, or JD, and place those physicians in positions of business and political leadership as well as in leadership positions in hospitals and private practitioner offices. We need to encourage our medical students, residents, and fellows to continue their rigorous training to include an understanding of health care policy and economics so as to help manage and resolve the crisis at hand.
We must now navigate the sea of change to allow for continuity of care and not throw up our arms in despair. The role of physician as private practitioner or as full-time faculty member has its origins deeply imbedded in the roots of our profession, and this traditional role as caretaker and scientist must continue. But in this century, we need to be leaders in the political and business communities as well. This vision requires a new and fresh momentum. We cannot sit idly by as patient care becomes increasingly managed by nonphysicians. The time has come to use our unique position as doctors to frame the debate, participate in the discussion, and lead our profession and the management of health care toward calmer waters with compassion, science, and responsibility. To do this, we must demand transparency, proceed with respect, and require excellence from everyone around us and make sure it is demanded from all of us.◾
1. Morgan G. Developing the art of organizational analysis. In: Morgan G. Images of Organization. Beverly Hills, CA: Sage Publications; 1986:321-337.
2. Cherry KA. Leadership styles. About.com website. http://psychology.about.com/od/leadership/a/leadstyles.htm. Published 2006. Accessed October 20, 2015.
"Report to the Administrator’s Office for a discussion 7:00 am sharp,” reads the email on your phone. The phone log sheet from your administrator is handed to you as you are running to the operating room and reads, “Call back Mr. Smith’s health insurance company because your patient stayed overnight unexpectedly in the hospital, and if the return phone call is not received by 8:40 am the complete hospital stay will be disallowed.” The text message reads, “The head nurse from the emergency department wants to have a discussion with you tomorrow about what transpired in room 23 last night at 1:33 am.” Your physician assistant calls you because a recent history and physical examination from the out-of-state internist has not been cosigned by you, and, therefore, the patient is still in the admitting office; the admitting officer is waiting to go home and won’t accept the physician assistant’s signature.
This simple illustration of a surgeon’s typical morning is hardly hyperbole. Demands and finger-pointing are routine aspects of care, with a concurrent need to attribute blame and create a hostile work environment whether in the office, operating room, or floor of the hospital by anyone who can proudly say to the physician, “Gotcha!” The environment that produces this ethos is toxic and needs to be changed. While all members of a patient care team must be accountable, no member should be antagonistic toward another, and each member must feel a part of a working whole that is led by a competent, caring, and identifiable physician. Yes, the doctor must be the team captain; he or she must take back the reins of care immediately in order to provide the patient with the best possible outcome.
The loss of leadership can be traced back to the rise of regulatory controls put in place by government entities or local hospital administration to contain costs and limit liability. While the target goals of such measures are laudable, the negative impact on the doctor–patient relationship has been palpable and problematic and requires reassessment. The profession itself will be preserved by refocusing on the doctor–patient relationship and returning the physician to the role of team leader. Our patients deserve to feel as though their health care resides in the hands of the physician as the leader of a team that is pursuing a common goal: patient care with minimal distractions.
What, though, makes a great captain or leader? Sociologists have said that in a stable environment a “participatory model” of leadership is appropriate, while in a high-growth or changing environment, like the one in which we presently live, an “authoritative model” can be used to right the ship.1,2 Many types of leaders exist within both models. Leaders who are “innovators” will design and bring new ideas and original thought but may generate too many ideas that can’t be implemented practically in the hospital setting. Leaders who are “developers” will build and move forward to achieve challenging goals but may be impatient when ideas do not work and may be perceived in many interdisciplinary meetings as unruly. “Bureaucratic” leaders, presently seen in many leadership positions, can be classified as stabilizers and, while they may maintain equilibrium and keep things running smoothly, they often insist on a policy for every situation, resulting in stasis and sometimes even paralysis of the surgical center or hospital system.
I believe that health management and patient care require the simultaneous use of the authoritative and participatory models to encourage innovation, set attainable short- and long-term goals, and maintain the physician as the team leader. To lead effectively under this hybrid model, the physician must be accessible and fair, a teacher and a student, and a risk-taker, but, ultimately, at the end of every day, the physician must be accountable.
The time has come for physician leaders to assemble the troops: administrators, clinical providers, and nonclinical support staff. To paraphrase John Quincy Adams, in your actions inspire others to dream more and become more; then, and only then, are you an excellent leader. A secret to effective leadership is in finding one’s voice and acknowledging strengths and weaknesses. The leader must recruit other leaders who are very different from himself or herself and must listen to them deeply and trust them completely. One of our former first ladies said wisely, “A leader takes people where they want to go. A great leader takes people where they don’t necessarily want to go, but ought to be.” To truly find this leadership model, we as busy surgeons must spend some concentrated time away from our patients and exciting research to sit in the room with our nurses, administrators, and all other members of the health care community and listen to their thoughts and understand their concerns. We must understand policy to assess if it is reasonable and, if it is not, to reject it and propose more effective and appropriate rules for good care. We must remove from leadership positions those that do not have the interest of the patient as their primary concern. We must challenge any policy that does not have the patient’s interest and health as its raison d’etre. We must be proactive and not reactive. We must be ready to stand tall and politely question when dictated to unless evidence-based medical reasons can be presented.
You may ask, therefore, where should we lead? The answer is obvious! We need to be involved in every aspect of this great profession. We need to be the leaders of hospital systems, we need to be in charge of research institutions, and, as always, we need to be the chief of the operating room and the chief within each room as the team leader for the nurse, anesthesiologist, and nonclinical staff in order to safely guide our patients through the stress of a medical crisis or routine intervention. We need to find those of us with other degrees, whether MPH, MBA, MHA, or JD, and place those physicians in positions of business and political leadership as well as in leadership positions in hospitals and private practitioner offices. We need to encourage our medical students, residents, and fellows to continue their rigorous training to include an understanding of health care policy and economics so as to help manage and resolve the crisis at hand.
We must now navigate the sea of change to allow for continuity of care and not throw up our arms in despair. The role of physician as private practitioner or as full-time faculty member has its origins deeply imbedded in the roots of our profession, and this traditional role as caretaker and scientist must continue. But in this century, we need to be leaders in the political and business communities as well. This vision requires a new and fresh momentum. We cannot sit idly by as patient care becomes increasingly managed by nonphysicians. The time has come to use our unique position as doctors to frame the debate, participate in the discussion, and lead our profession and the management of health care toward calmer waters with compassion, science, and responsibility. To do this, we must demand transparency, proceed with respect, and require excellence from everyone around us and make sure it is demanded from all of us.◾
"Report to the Administrator’s Office for a discussion 7:00 am sharp,” reads the email on your phone. The phone log sheet from your administrator is handed to you as you are running to the operating room and reads, “Call back Mr. Smith’s health insurance company because your patient stayed overnight unexpectedly in the hospital, and if the return phone call is not received by 8:40 am the complete hospital stay will be disallowed.” The text message reads, “The head nurse from the emergency department wants to have a discussion with you tomorrow about what transpired in room 23 last night at 1:33 am.” Your physician assistant calls you because a recent history and physical examination from the out-of-state internist has not been cosigned by you, and, therefore, the patient is still in the admitting office; the admitting officer is waiting to go home and won’t accept the physician assistant’s signature.
This simple illustration of a surgeon’s typical morning is hardly hyperbole. Demands and finger-pointing are routine aspects of care, with a concurrent need to attribute blame and create a hostile work environment whether in the office, operating room, or floor of the hospital by anyone who can proudly say to the physician, “Gotcha!” The environment that produces this ethos is toxic and needs to be changed. While all members of a patient care team must be accountable, no member should be antagonistic toward another, and each member must feel a part of a working whole that is led by a competent, caring, and identifiable physician. Yes, the doctor must be the team captain; he or she must take back the reins of care immediately in order to provide the patient with the best possible outcome.
The loss of leadership can be traced back to the rise of regulatory controls put in place by government entities or local hospital administration to contain costs and limit liability. While the target goals of such measures are laudable, the negative impact on the doctor–patient relationship has been palpable and problematic and requires reassessment. The profession itself will be preserved by refocusing on the doctor–patient relationship and returning the physician to the role of team leader. Our patients deserve to feel as though their health care resides in the hands of the physician as the leader of a team that is pursuing a common goal: patient care with minimal distractions.
What, though, makes a great captain or leader? Sociologists have said that in a stable environment a “participatory model” of leadership is appropriate, while in a high-growth or changing environment, like the one in which we presently live, an “authoritative model” can be used to right the ship.1,2 Many types of leaders exist within both models. Leaders who are “innovators” will design and bring new ideas and original thought but may generate too many ideas that can’t be implemented practically in the hospital setting. Leaders who are “developers” will build and move forward to achieve challenging goals but may be impatient when ideas do not work and may be perceived in many interdisciplinary meetings as unruly. “Bureaucratic” leaders, presently seen in many leadership positions, can be classified as stabilizers and, while they may maintain equilibrium and keep things running smoothly, they often insist on a policy for every situation, resulting in stasis and sometimes even paralysis of the surgical center or hospital system.
I believe that health management and patient care require the simultaneous use of the authoritative and participatory models to encourage innovation, set attainable short- and long-term goals, and maintain the physician as the team leader. To lead effectively under this hybrid model, the physician must be accessible and fair, a teacher and a student, and a risk-taker, but, ultimately, at the end of every day, the physician must be accountable.
The time has come for physician leaders to assemble the troops: administrators, clinical providers, and nonclinical support staff. To paraphrase John Quincy Adams, in your actions inspire others to dream more and become more; then, and only then, are you an excellent leader. A secret to effective leadership is in finding one’s voice and acknowledging strengths and weaknesses. The leader must recruit other leaders who are very different from himself or herself and must listen to them deeply and trust them completely. One of our former first ladies said wisely, “A leader takes people where they want to go. A great leader takes people where they don’t necessarily want to go, but ought to be.” To truly find this leadership model, we as busy surgeons must spend some concentrated time away from our patients and exciting research to sit in the room with our nurses, administrators, and all other members of the health care community and listen to their thoughts and understand their concerns. We must understand policy to assess if it is reasonable and, if it is not, to reject it and propose more effective and appropriate rules for good care. We must remove from leadership positions those that do not have the interest of the patient as their primary concern. We must challenge any policy that does not have the patient’s interest and health as its raison d’etre. We must be proactive and not reactive. We must be ready to stand tall and politely question when dictated to unless evidence-based medical reasons can be presented.
You may ask, therefore, where should we lead? The answer is obvious! We need to be involved in every aspect of this great profession. We need to be the leaders of hospital systems, we need to be in charge of research institutions, and, as always, we need to be the chief of the operating room and the chief within each room as the team leader for the nurse, anesthesiologist, and nonclinical staff in order to safely guide our patients through the stress of a medical crisis or routine intervention. We need to find those of us with other degrees, whether MPH, MBA, MHA, or JD, and place those physicians in positions of business and political leadership as well as in leadership positions in hospitals and private practitioner offices. We need to encourage our medical students, residents, and fellows to continue their rigorous training to include an understanding of health care policy and economics so as to help manage and resolve the crisis at hand.
We must now navigate the sea of change to allow for continuity of care and not throw up our arms in despair. The role of physician as private practitioner or as full-time faculty member has its origins deeply imbedded in the roots of our profession, and this traditional role as caretaker and scientist must continue. But in this century, we need to be leaders in the political and business communities as well. This vision requires a new and fresh momentum. We cannot sit idly by as patient care becomes increasingly managed by nonphysicians. The time has come to use our unique position as doctors to frame the debate, participate in the discussion, and lead our profession and the management of health care toward calmer waters with compassion, science, and responsibility. To do this, we must demand transparency, proceed with respect, and require excellence from everyone around us and make sure it is demanded from all of us.◾
1. Morgan G. Developing the art of organizational analysis. In: Morgan G. Images of Organization. Beverly Hills, CA: Sage Publications; 1986:321-337.
2. Cherry KA. Leadership styles. About.com website. http://psychology.about.com/od/leadership/a/leadstyles.htm. Published 2006. Accessed October 20, 2015.
1. Morgan G. Developing the art of organizational analysis. In: Morgan G. Images of Organization. Beverly Hills, CA: Sage Publications; 1986:321-337.
2. Cherry KA. Leadership styles. About.com website. http://psychology.about.com/od/leadership/a/leadstyles.htm. Published 2006. Accessed October 20, 2015.
Excision of Symptomatic Spinous Process Nonunion in Adolescent Athletes
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
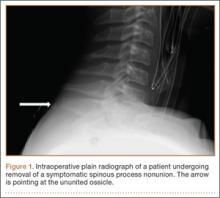
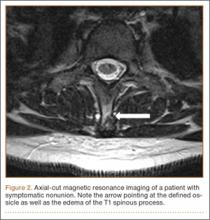
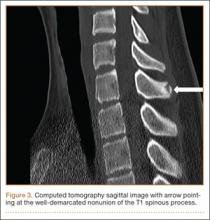
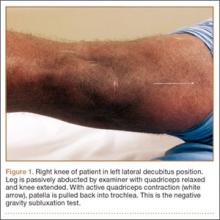
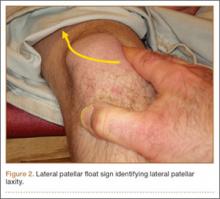
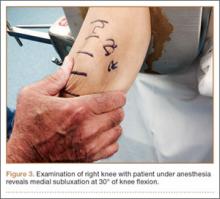
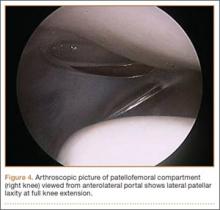
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
Fractures of the spinous process of the lower cervical spine or upper thoracic spine are frequently referred to as clay-shoveler’s fractures. Originally reported by Hall1 in 1940, these fractures were described in workers in Australia who dug drains in clay soil and threw the clay overhead with long shovels. Occasionally, the mud would not release from the shovel, causing excess force to be transmitted to the supraspinous ligaments and resulting in a forceful avulsion fracture of one or multiple spinous processes. The few reports following the earliest description in the literature frequently describe the mechanism of injury as being athletic in nature.2-4 The forceful contraction of the paraspinal and trapezius muscles on the supraspinous ligaments and the resultant attachment to the spinous processes make this a not uncommon injury during athletics, especially with a flexed position of the neck and shoulders. The resultant fracture or apophyseal avulsion is painful and often necessitates a visit to the physician, with plain films, computed tomography (CT) scans, or magnetic resonance imaging (MRI) confirming the diagnosis.5
Treatment of these fractures has not been well described, but frequently a period of rest followed by physical therapy will allow a return to activity. We present a series of adolescent athletes who developed nonunion of the fracture of the T1 spinous process with continued symptoms, despite rest and conservative therapy, and who underwent surgical excision of the ununited fragment.
Materials and Methods
We obtained institutional review board permission for this study and searched the surgical database between 2006 and 2013 for patients who had undergone resection of a spinous process nonunion. We collected demographic data on the patients, evaluated the radiographic studies, and reviewed operative reports and follow-up patient data.
Results
Dr. Hedequist operated on 3 patients with a spinous process nonunion over the study time period. The average age of the patients was 14 years; the location of the spinous process fracture was the T1 vertebra in all patients. Two patients sustained the injury while playing hockey and 1 during wrestling. The average duration of symptoms prior to operation was 10 months; all patients had seen physicians without a diagnosis prior to evaluation at out institution. All patients had a trial of physical therapy before surgery, and all had been unable to return to sport after injury secondary to pain.
Examination of all patients revealed pain directly over the fracture site and accentuated by forward flexion of the neck and shoulders. Evaluation of injury plain films revealed a fracture fragment in 2 patients (Figure 1). All 3 patients underwent MRI and CT scans confirming the diagnosis. MRI confirmed areas of increased signal at the tip of the T1 spinous process, with inflammation in the supraspinous ligament directly at that region (Figure 2). The CT scans confirmed the presence of a bony fragment correlating with the tip of the T1 spinous process (Figure 3).
Surgery was performed under general endotracheal anesthesia via a midline incision over the affected area down to the spinous process. The supraspinous ligament was opened revealing an easily identified and definable ununited ossicle, which was removed without taking down the interspinous ligament. All 3 nonunions were noted to be atrophic with no evidence of surrounding inflammatory tissue or bursa. The residual end of the spinous process was smoothed down with a rongeur. Standard closure was performed. There were no surgical complications.
All patients had complete relief of pain at follow-up; 1 patient returned to full sports activity at 6 weeks and the other 2 returned to full sports activity at 3 months. There was no loss of cervical motion or trapezial strength at follow-up. All patients voiced satisfaction with the decision for surgical intervention.
Discussion
Clay-shoveler’s fracture is an injury well known to orthopedists. This fracture is thought to be caused by a forceful contraction of the thoracic paraspinal and trapezial muscles, causing an avulsion fracture with pain and frequently a “pop” experienced by the patient.1 Usually considered self-limiting injuries, treatment involves a period of rest and activity modification with occasional physical therapy. Return to sports has been reported with occasional pain but with patient satisfaction.3,5,6
Our series of patients represent a group of adolescent athletes who sustained spinous process fractures of the T1 vertebra and, despite a significant period of rest and activity modification, were unable to return to sports given their pain. The examination of these patients revealed focal tenderness at the tip of the spinous process. The diagnosis is made clinically, with radiographic studies confirming the diagnosis. In our series of patients, MRI was the original modality used to confirm injury to the area, with hyperintensity seen in the area of the supraspinous ligament and tip of the spinous process. CT confirmed the nonunion and presence of an ossicle in all patients. Surgical exposure of that area easily exposed the ununited ossicle, which was removed in all patients.
To our knowledge, this is the first report in the literature describing surgical excision of an ununited spinous process fracture in adolescent athletes. The original descriptive case series by Hall1 states “in the minds of surgeons who have seen many of these cases that early operative removal of the fragments is the proper routine treatment.” Since that original series, we have not found articles in the literature that support surgical removal; however, persistent symptoms after fracture are described.5 It is not surprising that these patients developed pain at the site of the fracture given the forces acting in that area. The trapezial and paraspinal muscles acting on that area are forceful and repetitive during activities, especially sports. All our patients had pain with attempts at activity and all had had a significant period of rest. In a recent article, this injury was described in adolescents without the patients having clear relief of symptoms despite a period of inactivity.5 While physical therapy is therapeutic in some patients experiencing pain, it can be a source of aggravation due to neck and shoulder motion and muscle contraction. It is not surprising that therapy would not help in most cases, as neck and shoulder motion and muscle contraction are the sources of continuing discomfort.
Clinical practice suggests that most patients with spinous process fractures will become pain-free; however, that is not universal. This series demonstrates that a small subset of patients with this injury will continue to have significant symptoms despite a period of rest. In those patients who desire a pain-free return to sports, we recommend consideration of surgical excision after confirmation of nonunion with radiographic studies. The inherent risks of surgical treatment are minimal with this procedure, and the benefits include return to pain-free sports activity, with the resultant physical and psychosocial benefits for adolescent athletes.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
1. Hall RDM. Clay-shoveler’s fracture. J Bone Joint Surg Am. 1940;22(1):63-75.
2. Herrick RT. Clay-shoveler’s fracture in power-lifting. A case report. Am J Sports Med. 1981;9(1):29-30.
3. Hetsroni I, Mann G, Dolev E, Morgenstern D, Nyska M. Clay shoveler’s fracture in a volleyball player. Phys Sportsmed. 2005;33(7):38-42.
4. Kaloostian PE, Kim JE, Calabresi PA, Bydon A, Witham T. Clay-shoveler’s fracture during indoor rock climbing. Orthopedics. 2013;36(3):e381-e383.
5. Yamaguchi KT Jr, Myung KS, Alonso MA, Skaggs DL. Clay-shoveler’s fracture equivalent in children. Spine. 2012;37(26):e1672-e1675.
6. Kang DH, Lee SH. Multiple spinous process fractures of the thoracic vertebrae (clay-shoveler’s fracture) in a beginning golfer: a case report. Spine. 2009;34(15):e534-e537.
Academic Characteristics of Orthopedic Team Physicians Affiliated With High School, Collegiate, and Professional Teams
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
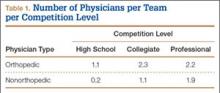
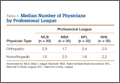
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
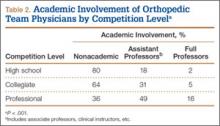
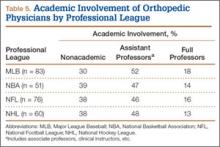
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
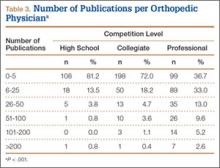
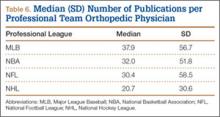
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
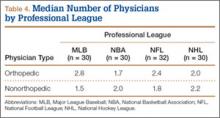
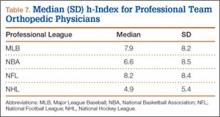
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
The responsibilities of team physicians have increased dramatically since the early 19th century, when these physicians first appeared on the sidelines during football games.1 Although the primary role of the team physician is to care for the athlete, other responsibilities include administrative and legal duties, equipment- and environment-related duties, teaching, and communication with parents, coaches, and other physicians.2-4 These responsibilities differ greatly by the level of the athlete and the team being covered. For example, compared with high school and collegiate sport physicians, physicians caring for professional athletes may have increased interaction with the media.5
Despite the increasing demands and responsibilities of team physicians, it is important that they continue to advance the field of sports medicine through teaching and research.3,6 Team physicians have direct access to athletes at multiple levels of competition, from novice to professional, and therefore have a unique understanding of the injuries that commonly affect these athletes. Efforts to both teach and study the prevention, diagnosis, and treatment of these injuries have dramatically advanced the field of sports medicine. In fact, several advancements in sports medicine have come from team physicians, including advancements in anterior cruciate ligament reconstruction,7,8 shoulder arthroscopy,9 and “Tommy John” surgery,10 to name a few.
Given the important role of team physicians (particularly orthopedic team physicians) in advancing sports medicine, it is important to understand the degree to which team physicians at all levels of sport contribute to teaching and research.
We conducted a study to determine the overall academic involvement of orthopedic team physicians at all levels of sport, including the degree to which these physicians are affiliated with academic medical centers (by level of sport and by professional sport) and the quantity and impact of these physicians’ scientific publications. We hypothesized that orthopedic physician academic involvement would be higher at the professional level of sport than at the collegiate or high school level and that the degree of physician academic involvement would differ between professional sporting leagues.
Materials and Methods
In August 2012, we performed a comprehensive telephone- and Internet-based search to identify a sample of team physicians caring for athletes at the high school, collegiate, and professional levels of sport. Data were collected on all team physicians, regardless of medical specialty. We defined a physician as any person listed as having either a doctor of medicine (MD) or a doctor of osteopathic medicine (DO) degree. A physician listed as a team physician at 2 different levels of competition (high school, college, professional) was included in both cohorts. A physician listed as a team physician in 2 different professional sports leagues was included independently for both leagues. All other medical personnel, including athletic trainers, therapists, and nursing staff, were excluded. Data on our sample population were collected as follows:
1. High school. Performing a comprehensive database search through the US Department of Education, we generated a list of all 20,989 US schools that include grades 9 to 12.11 We then used a random number generator (random.org) to randomly select a sample of 120 high schools. These schools were contacted by telephone and asked to identify the team physician(s) for their sports teams. Twenty of these schools reported not having an athletic team, so we randomly generated a list of 20 additional high schools. High schools that had an athletic team but denied having a team physician were included in the analysis.
2. College. We used the National Collegiate Athletic Association (NCAA) website (ncaa.org) to generate a list of all colleges affiliated with the NCAA. Of these colleges, 347 were Division I, 316 were Division II, and 443 were Division III. The random.org random number generator was used to generate a list of 40 schools for each division, for a total of 120 schools. An Internet-based search was then performed to identify any and all team physicians caring for athletes at that particular school. In select cases, telephone calls were made to determine all the team physicians involved in the care of athletes at that institution.
3. Professional. Team physician data were collected for 4 of the most popular professional sporting leagues12: Major League Baseball (MLB), National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL). Each team’s official website was identified through its league website (mlb.com, nba.com, nfl.com, nhl.com), and the roster or directory listing of all team physicians was recorded. In 2 cases, the team’s medical personnel listing could not be retrieved through the Internet, and a telephone call had to be made to identify all team physicians. Team physicians were identified for 122 professional teams: 30 MLB, 30 NBA, 32 NFL, and 30 NHL.
For this study, all physicians were classified as either orthopedic or nonorthopedic. Orthopedic surgeons—the focus of this study—were defined as those who completed residency training in orthopedic surgery. Median number of orthopedic and nonorthopedic surgeons per team was calculated at the high school, collegiate, and professional levels.
After identifying all orthopedic team physicians, we performed additional Internet searches to determine any affiliation between each physician and an applicable academic medical center. Physicians were placed in 1 of 3 different categories based on “level” of academic affiliation. Orthopedists with no identifiable connection to an academic medical center were listed under none. The first 100 search results were studied before this determination was made. Orthopedists with any academic affiliation below the level of full professorship were placed in the category associate/assistant/adjunct professor, which included any physician who was an associate professor, adjunct professor, clinical instructor, or volunteer instructor at an academic medical center. Last, orthopedists listed as full professors were placed in the professor category.
Number of publications written by each orthopedic team physician was then calculated using SciVerse Scopus (scopus.com), a comprehensive abstract and citation database of research literature that offers complete coverage of the Medline and Embase databases.13 Scopus offers a Scopus Author Identifier, which assigns each author in Scopus a unique identification number.14 This number is based on an “algorithm that matches author names based on their affiliation, address, subject area, source title, dates of publication citations, and co-authors.”14 Authors whose names did not appear in Scopus were assumed to have no publications, and this was reported after cross-referencing with Medline to ensure no documents were missed. This study included all publications: original research articles, reviews, letters, and commentaries. Any level of authorship (first, second, etc) was included. All publications were scanned, and duplicate listings were not included. Median number of publications per orthopedic team physician was calculated at the high school, college, and professional levels.
We also determined the h-index for each orthopedic team physician. The h-index is used to measure the impact of the published work of a scholar: “A scientist has index h if h of his/her papers have at least h citations each, and the other papers have no more than h citations each.”15 For example, an h-index of 12 means that, out of an author’s total number of publications, 12 have been cited at least 12 times, and all of his or her other publications have been cited fewer than 12 times. All authors in Scopus are automatically assigned h-indexes, and we collected these numbers.16 Of note, citations for articles published before 1996 are not included in the h-index calculation. Median h-index score per orthopedic team physician was calculated at the high school, college, and professional levels.
Analysis of variance was used to compare continuous data (eg, number of publications per surgeon) across different groups (eg, physicians from respective sports). Chi-square tests were used to detect whole-number differences between groups (eg, difference in number of physicians per team across the various professional sports leagues). Statistical significance was set at P < .05.
Results
We identified 1054 team physicians among the 362 total high schools, colleges, and professional sports teams included in this study. Of the 1054 physicians, 678 (64%) were orthopedic surgeons (Table 1). Seventy-two (60%) of the 120 high schools did not have a team physician, whereas all the colleges and professional teams did. Number of orthopedic surgeons per team was higher at the collegiate level (2.29; range, 0-11) and professional level (2.21; range, 1-9) than at the high school level (1.11; range, 0-24) (Table 1). Median number of nonorthopedic surgeons was highest in professional sports (1.88; range, 0-9) followed by college sports (1.06; range, 0-9) and high school sports (0.16; range, 0-2) (Table 1).
Of the 678 orthopedic team physicians, 298 (44%) were officially affiliated with an academic medical center, either as clinical instructor, associate/adjunct professor, or full professor. Percentage of orthopedists affiliated with an academic medical center was highest in professional sports (173/270, 64%) followed by collegiate sports (98/275, 36%) and high school sports (27/133, 20%) (P < .001, Table 2). Percentage of orthopedists identified as full professors was highest at the professional level (42/270, 16%) followed by the collegiate level (14/275, 5.1%) and the high school level (3/133, 2.3%) (P < .001, Table 2).
We found 12,036 publications written by the 678 orthopedic team physicians included in this study. Median number of publications per orthopedist was significantly higher in professional sports (30.6; range, 0-460) than in collegiate sports (10.7; range, 0-581) and high school sports (6.0; range, 0-220) (P < .001). Number of authors with more than 25 publications was highest at the professional level (82) followed by the collegiate level (27) and the high school level (7) (Table 3). Median number of publications per orthopedist was also higher at the professional level (12) than at the collegiate level (2) and high school level (1). Median h-index was higher among orthopedists in professional sports (7.1; range, 0-50) than at colleges (2.7; range, 0-63) and high schools (1.8; range, 0-32) (P < .001). Median h-index was also significantly higher at the professional level (5) than at the collegiate level (1) and high school level (0).
At the professional level of sports, we identified 499 team physicians (270 orthopedic, 54%; 229 nonorthopedic, 46%). Median number of orthopedic team physicians varied by sport, with MLB (2.8; range, 1-8) and the NFL (2.4; range, 1-4) having relatively more of these physicians than the NHL (2.0; range, 1-6) and the NBA (1.7; range, 1-9) (Table 4). Percentage of orthopedic team physicians affiliated with academic medical centers was highest in MLB (58/83, 69.9%) followed by the NFL (47/76, 61.8%), the NHL (37/60, 61.7%), and the NBA (31/51, 60.8%) (Table 5). Median number of publications by orthopedists also varied by sport, with the highest number in MLB (37.9; range, 0-225) followed by the NBA (32.0; range, 0-227) and the NFL (30.4; range, 0-460), with the lowest number in the NHL (20.7; range, 0-144) (Table 6). Median number of publications was the same (17.5) in MLB and the NFL and lower in the NBA (11) and the NHL (7.5). Median h-index was highest in the NFL (8.2; range, 0-50) and MLB (7.9; range, 0-32) followed by the NBA (6.6; range, 0-35) and the NHL (4.9; range, 0-20) (Table 7) Median h-index was the same (6) in MLB and the NFL and lower (3) in the NBA and the NHL.
Discussion
To our knowledge, this is the first study of academic involvement and the research activities of orthopedic team physicians at the high school, college, and professional levels of sport. We found that, on average, there were almost twice as many orthopedists at the collegiate and professional levels than at the high school level—likely because 72 of the 120 high schools randomly selected did not have a team physician, despite having sports teams. We can attribute this to the organizational structure of teams in a high school setting, where it is fairly common that no medically educated health care provider is readily available for the student athletes.5 Although the median number of orthopedists was similar at the collegiate and professional levels, the number of nonorthopedic team physicians was higher at the professional level than at the collegiate level. Although most collegiate and professional teams have an internist and an orthopedist on staff, medical staff at the professional level may also include several subspecialists from a variety of medical fields (eg, dental medicine, ophthalmology, neurology).17
We found that a significantly larger proportion of orthopedists at the professional level (64%) were affiliated with academic medical centers as associate/adjunct professors and full professors compared with orthopedists at the collegiate level (36%) and high school level (20%). The academic relationship with collegiate teams was much lower than expected. Regarding professional sports, however, this finding confirmed our hypothesis, and the explanation is likely multifactorial and historical. Moreover, the median number of publications was higher for orthopedists at the professional level (30.8) than at the collegiate level (10.7) and high school level (6). In the late 1940s and early 1950s, many orthopedic team physicians entered into contracts with major universities.4 For many physicians, this contractual relationship increased their prestige, and some orthopedic groups were alleged to have endorsed scholarships at those schools.4 Given the high level of publicity and scrutiny surrounding medical decisions at the professional level of sports, it is possible that professional sports teams specifically seek orthopedists who are well respected within academia. Moreover, contracts between universities/academic medical centers and professional teams may mandate that a faculty member from that organization provide the orthopedic/medical care for the team. This may also increase the likelihood of professional teams being paired with academic orthopedic physicians. However, such contractual agreements are made between professional teams and large private medical groups as well.
In addition to measuring quantity of publications, we used the h-index to measure their quality. Following the same pattern as the publication rate, median h-index per orthopedic team physician was significantly higher at the professional level (7.1) than at the collegiate level (2.7) and high school level (1.8). As with publication volume, this is not entirely surprising, as h-index has been shown to correlate with academic rank in other surgical specialties,18 and there was a higher percentage of academic physicians at the professional level than at the collegiate and high school levels.
At the professional level of sports, 56% of all team physicians were orthopedic surgeons. Orthopedists caring for MLB teams had the highest median number of publications (37.9), followed by the NBA (32.0), the NFL (30.4), and the NHL (20.7). One likely explanation is the higher percentage of MLB physicians affiliated with academic medical centers. Regarding the h-index, MLB and NFL physicians had the highest values (7.9 and 8.2, respectively).
Our study had several limitations. First, we may not have captured data on all the team physicians at the high school, college, and professional levels. By following a detailed protocol in identifying surgeons, however, we tried to minimize the impact of any such omissions. In addition, teams may have had many unofficial consultants acting as team physicians, whether orthopedic or nonorthopedic, and, if these physicians were not listed in an official capacity, they may have been omitted from this study. We further realize that a true measure of academic productivity should also include book chapters and books published, research grants awarded, and patents registered. By including only peer-reviewed articles, we omitted these other criteria.
To our knowledge, the data presented here represent the first attempt to quantify the academic involvement and research productivity of orthopedic team physicians at the high school, college, and professional levels of sport. These data help us understand how research productivity varies by orthopedic team physicians at different levels of sport and may be useful to those considering a career as a team physician, as they can better evaluate their own productivity in the context of team physicians across different levels of competition.
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
1. Thorndike A. Athletic Injuries: Prevention, Diagnosis, and Treatment. Philadelphia, PA: Lea & Febiger; 1956.
2. The team physician. A statement of the Committee on the Medical Aspects of Sports of the American Medical Association, September 1967. J School Health. 1967;37(10):510-514.
3. Team physician consensus statement. Am J Sports Med. 2000;28(3):440-441.
4. Whiteside J, Andrews JR. Trends for the future as a team physician: Herodicus to hereafter. Clin Sports Med. 2007;26(2):285-304.
5. Goforth M, Almquist J, Matney M, et al. Understanding organization structures of the college, university, high school, clinical, and professional settings. Clin Sports Med. 2007;26(2):201-226.
6. Hughston JC. Want to be in sports medicine? Get involved. Am J Sports Med. 1979;7(2):79-80.
7. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
8. Clancy WG Jr, Nelson DA, Reider B, Narechania RG. Anterior cruciate ligament reconstruction using one-third of the patellar ligament, augmented by extra-articular tendon transfers. J Bone Joint Surg Am. 1982;64(3):352-359.
9. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
10. Indelicato PA, Jobe FW, Kerlan RK, Carter VS, Shields CL, Lombardo SJ. Correctable elbow lesions in professional baseball players: a review of 25 cases. Am J Sports Med. 1979;7(1):72-75.
11. Elementary/Secondary Information System (EISi). National Center for Education Statistics, Institute of Education Sciences, US Department of Education website. http://nces.ed.gov/ccd/elsi/. Accessed September 21, 2015.
12. Corso RA; Harris Interactive. Football is America’s favorite sport as lead over baseball continues to grow; college football and auto racing come next. Harris Interactive website. http://www.harrisinteractive.com/vault/Harris Poll 9 - Favorite sport_1.25.12.pdf. Harris Poll 9, January 25, 2012. Accessed September 21, 2015.
13. [Scopus content]. Elsevier website. http://www.elsevier.com/solutions/scopus/content. Accessed September 21, 2015.
14. Scopus Author Identifier. Scopus website. http://help.scopus.com/Content/h_autsrch_intro.htm. Accessed October 5, 2015.
15. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569-16572.
16. Author Evaluator h Index Tab. Scopus website. http://help.scopus.com/Content/h_auteval_hindex.htm. Accessed October 5, 2015.
17. Boyd JL. Understanding the politics of being a team physician. Clin Sports Med. 2007;26(2):161-172.
18. Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387-
Conflict of Interest in Sports Medicine: Does It Affect Our Judgment?
As defined by the American Academy of Orthopaedic Surgeons (AAOS) in 1996, conflict of interest (COI) is the “circumstance that exists when, because of personal financial gain, an individual has the potential to be less than objective when called on to reach a judgment or interpret a result.”1 In medical research, COIs often occur in relationships between physician-researchers and pharmaceutical, medical device, and biotechnology companies. These relationships usually take the form of research grants but can also arise when the researcher has a financial interest in the product being tested or in the company that manufactures the product.
Although constructive collaboration between academic medicine and industry has worked to improve health care and ultimately benefit patients, potential drawbacks of such relationships include sequestration and suppression of results that may be disadvantageous to the industry sponsor,2 increased likelihood of reporting positive results (pro-industry),3-7 and biased study designs.8 The nature of such relationships may threaten the integrity of scientific studies, the objectivity of medical education, the quality of patient care, and the public’s trust in medicine.9
Financial relationships and affiliations are increasing as we seek to answer a growing number of clinical questions—with funding often being a limiting factor. At national scientific meetings, the number of presentations reporting COIs reflects this trend. Paper and poster presentations accepted for annual meetings of the Orthopaedic Trauma Association (OTA) and reporting a COI increased from 7.6% in 1993 to 12.6% in 2002 (P = .0129).2
Medical subspecialties outside of orthopedics are experiencing similar trends. Most notable is the American Psychiatric Association (APA). After the APA published a mandatory financial COI disclosure policy in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the percentage of task force members reporting industry relationships increased by 12%.10 Analysis of the DSM-5 panels demonstrated that the panels with the largest percentage of reported COIs are those for which pharmacological treatment is the first-line intervention, including the panels for mood disorders (67%), psychotic disorders (83%) and sleep/wake disorders (100%).10 Moreover, the industry ties reported are to the pharmaceutical companies that manufacture the medications used to treat these disorders or to companies that service the pharmaceutical industry.10
The degree to which financial COIs affect the interpretation of the orthopedic literature has never been quantified. Although it is clear that COIs can confound the results and reporting of data, how the medical community uses disclosures when interpreting the literature and when formulating opinions that may or may not affect their practice patterns is largely unknown.
We conducted a study to evaluate how a hypothetical financial COI disclosure would influence the interpretation of data by orthopedic clinicians. We also wanted to determine the reliability of the data as perceived in association with different study designs, levels of evidence, research institutional settings, and reporting of positive or negative results.
Methods
We asked members of the Arthroscopy Association of North America (AANA) and the American Orthopaedic Society for Sports Medicine (AOSSM) to complete a multiple-choice situational questionnaire (Table). The questionnaire assesses the degree to which respondents use COI disclosures when interpreting the literature. It further explores the perceived clinical value of a study with a given reported COI, assuming variations in study design, research institutional setting, and significance of results. The fictional research team disclosed the project was funded by a pharmaceutical company and all team members received consulting compensation. The survey and study were reviewed and approved by our institutional review board. The survey consisted of 14 multiple-choice questions that allowed for only 1 answer selection per person and allowed survey takers to skip questions they did not wish to answer. The survey questions and associated response options appear in edited form in the Table. A link to the questionnaire (https://www.surveymonkey.com/s/MPCCLCX) was sent with a message explaining the study. The responses to the questionnaire constituted the data.
Results
We sent a request to participate in the survey to 750 physicians and received 522 responses (overall response rate, 70%). The response rate for each question equaled or exceeded 98%.
The majority of respondents (95.6%) were male. Ninety-nine percent of respondents were orthopedic surgeons. The Northeast (US) was the most common geographical practice location of respondents (32%), followed by the Midwest (19.1%) and the Southeast (16.6%). Most respondents (40%) had been in practice for more than 20 years; 67% had been in practice a minimum of 10 years. The majority (68.8%) were employed by private practice groups, either single specialty (57.8%) or multispecialty (11%).
Eighty percent of respondents strongly agreed that COI disclosure is important when interpreting study results, 62% reported always reading the disclosure slide during academy or other meeting presentations, and 41% reported always using this information when deciding how to interpret scientific data.
Seventy-five percent of respondents thought the study—an academic-center case series with significant results in favor of the pharmaceutical company funding the study—was biased (42% indicated biased with merit, 33% biased without merit). Twenty-three percent thought the study was possibly biased, but likely trustworthy given the academic institutional affiliation. When the study setting was changed to community hospital, 95% thought the study was biased (51% biased with merit, 44% biased without merit). With the same study performed at an academic center, and no statistically significant results (not in favor of the pharmaceutical company funding the study), 88% thought the study had merit (46% biased with merit, 42% unbiased with merit).
When the study design was changed to a randomized controlled trial (level I evidence) conducted at an academic center with negative results, an overwhelming 95% of respondents thought the study had merit (33% biased with merit, 62% unbiased with merit). Given the same study design at an academic center, with positive results, 78% still thought the study had merit (39% biased with merit, 39% unbiased with merit). An additional 18% thought the study was biased, but still likely trustworthy given the academic institutional affiliation. Finally, given a randomized controlled trial and positive results, but with the research setting a small community practice, 90% thought the study had merit (51% biased with merit, 39% unbiased with merit). The percentage of respondents who found the study biased and likely without merit increased from 3.7% to 9.5% when the institutional affiliation changed from academic to community.
Discussion
As governmental funding sources become increasingly limited, the role of industry sponsorship of orthopedic research has grown. Potential drawbacks and biases of such research support have been well described—most notably, increased positive result reporting, suppression of results that may be disadvantageous to the industry sponsor, and biased study designs.2-8 However, the extent to which financial COIs affect the orthopedic medical community’s interpretation of the literature has never been quantified. To our knowledge, the present study is the first to quantify the impact of reported COI on study interpretation.
Our goal was to examine how reported financial COIs influence the interpretation of the literature by the orthopedic medical community. Moreover, we wanted to determine the perceived reliability of the data when variables (study design, institutional affiliation, positive vs negative results) were changed. The results of our survey indicate that, when a financial COI is reported, study reliability is perceived as highest when negative results were found.
Our survey noted a discrepancy between the documented importance of the hypothetical research team’s COI disclosure and the use of such disclosures when interpreting study results. Eighty percent of respondents agreed that COI disclosure is important when interpreting study results, but only 62% reported always reading disclosures, and even fewer (41%) reported always using the information when interpreting results. It is unclear exactly why this trend exists, as one would expect the percentages to be more similar. These particular survey questions were formed around using COI disclosures when interpreting study results during academic presentations at national meetings and not during the review of published literature. It is possible that positioning the COI disclosure at the beginning of a presentation has an effect, but only 3.7% of respondents indicated they seldom remembered the disclosure by the end of the presentation. The results of our survey may have varied if the questions had targeted reading and interpreting the literature.
Interestingly, the results of these survey questions tended to be more consistent with rates of reported financial COI by presenters at national orthopedic meetings. A study published in the New England Journal of Medicine found that less than 80% of orthopedic surgeons reported their disclosures at a large annual meeting (AAOS), even when the disclosure involved payments pertinent to the research they were presenting.5 When the payments were indirectly related to the research, the percentage of surgeons reporting disclosures was 50%, almost the same as the disclosure rate for unrelated payments.5
When the study was changed to a level I randomized controlled trial, more survey respondents found it to be less biased and have more merit. Although it would seem intuitive for a study with a higher level of evidence to carry more clinical value during interpretation, this may not hold true in the setting of industry-sponsored clinical trials. Several studies have documented a significant association between the reporting of positive results and industry-sponsored randomized clinical trials. In 2008, Khan and colleagues3 examined 100 orthopedic randomized clinical trials reported in 5 major orthopedic subspecialty journals over a 2-year period. The association between industry funding and favorable outcome in all original randomized clinical trials was strong and significant (P < .001). This is not surprising, given the amount of time and money required for a well-designed clinical study. Commercial products with preclinical promise are pushed to testing in a clinical trial, whereas resources would not be wasted on products lacking preclinical merit.
The most important variable affecting interpretation of study merit by survey respondents was the reporting of negative results. As more researchers are developing COIs, many studies are discovering a relationship between COIs and outcomes of research studies. Reviewing the adult total joint literature, Ezzet8 found an industry funding rate of 50%. Positive results were reported in 93% of cases in commercially funded studies versus 37% of cases in independently funded studies. Furthermore, no negative results were reported by investigators who were receiving royalties from the respective companies.
Studies across the medical literature have also found this association between industry sponsorship and reporting of positive results. One such study, reported by Valachis and colleagues7 in the Journal of Clinical Oncology, examined more than 80 economic analyses of targeted oncologic therapies and found the studies funded by pharmaceutical companies were more likely to report favorable qualitative cost estimates. In addition, when studies with a COI disclosure were examined, those reporting any financial relationship with a manufacturer (eg, author affiliation, funding) were more likely than those without such a relationship to report favorable results.
Our study had several limitations. First, as most of the survey respondents were orthopedic surgeons, extrapolating their data to the medical community at large may not be appropriate, as each specialty may view industry affiliations differently. In addition, respondents were asked to base their interpretations of a study on conclusions we predetermined—no direct visualization of the data set or statistical testing methods. It is possible that these responses may have been different had the respondents had the opportunity to further evaluate the study in question. In a recent study, Altwairgi and colleagues11 found that 10% of randomized clinical trials involving lung cancer treatment were reported with different conclusions in their full manuscripts relative to their abstracts. We think our survey design perhaps best mimics an annual meeting environment in which participants have very limited ability to interpret studies and may rely more heavily on the factors we investigated—study design, significance of findings, and setting, all similar to information presented in an abstract—when making informed decisions. Although our response rate was only 70%, this is comparable to or better than the rates in similar survey studies that used email-based questionnaires.12,13
Another limitation was that our survey may have forced respondents into answers they did not entirely agree with, given the limited options of the multiple-choice response format and the specific wording of the questions. Our conclusions may have been more dramatic when we were evaluating whether the study was deemed meritorious or not. However, there is no adopted standard for evaluating the extent of bias perceived by a clinician. We thought it was important to include answer options indicating a study had merit despite obvious bias in design and execution. That a study had merit can mean different things. It may change clinical practice, may require further study and reproducibility, or may not be significant enough to matter. Asking follow-up questions to evaluate this perception among the respondents could have provided validity to the term merit. Further studies in this field are needed to determine how studies are interpreted and translated into clinical practice by various clinicians.
Conclusion
Although the present study is not a quantitative analysis of the determination of bias in the orthopedic community, it is the first to evaluate orthopedic surgeons’ perceptions on the basis of key fundamentals of orthopedic research relative to COI. It is clear from our study results that introducing levels of evidence to the orthopedic milieu has had a significant impact both on the quality of research and on the foundational use of deductive reasoning when interpreting the literature. Reporting negative outcomes is perhaps the most important factor in eliminating the perception of bias among orthopedic surgeons. To what extent a perceived COI plays into medical decision-making and the ultimate treatment of patients is still relatively unknown.
1. Lubahn JD, Mankin CJ, Mankin HJ, Kuhn PJ. Orthopaedics, ethics, and industry. Appropriateness of gifts, grants, and awards. Clin Orthop Relat Res. 2000;(371):256-263.
2. Kubiak EN, Park SS, Egol K, Zuckerman JD, Koval KJ. Increasingly conflicted: an analysis of conflicts of interest reported at the annual meetings of the Orthopaedic Trauma Association. Bull Hosp Jt Dis. 2006;63(3-4):83-87.
3. Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop. 2008;37(12):E205-E212.
4. Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research. An association between findings and funding in scientific presentations. J Bone Joint Surg Am. 2007;89(3):608-613.
5. Okike K, Kocher MS, Wei EX, Mehlman CT, Bhandari M. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361(15):1466-1474.
6. Shah RV, Albert TJ, Bruegel-Sanchez V, Vaccaro AR, Hilibrand AS, Grauer JN. Industry support and correlation to study outcome for papers published in Spine. Spine. 2005;30(9):1099-1104.
7. Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30(12):1316-1320.
8. Ezzet KA. The prevalence of corporate funding in adult lower extremity research and its correlation with reported results. J Arthroplasty. 2003;18(7 suppl 1):138-145.
9. Lo B, Field MJ, eds; Institute of Medicine, Committee on Conflict of Interest in Medical Research, Education, and Practice, Board on Health Sciences Policy. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press; 2009. http://www.ncbi.nlm.nih.gov/books/NBK22942. Accessed September 29, 2015.
10. Cosgrove L, Krimsky S. A comparison of DSM-IV and DSM-5 panel members’ financial associations with industry: a pernicious problem persists. PLoS Med. 2012;9(3):e1001190.
11. Altwairgi AK, Booth CM, Hopman WM, Baetz TD. Discordance between conclusions stated in the abstract and conclusions in the article: analysis of published randomized controlled trials of systemic therapy in lung cancer. J Clin Oncol. 2012;30(28):3552-3557.
12. Decoster LC, Vailas JC, Swartz WG. Functional ACL bracing. A survey of current opinion and practice. Am J Orthop. 1995;24(11):838-843.
13. Mann BJ, Grana WA, Indelicato PA, O’Neill DF, George SZ. A survey of sports medicine physicians regarding psychological issues in patient-athletes. Am J Sports Med. 2007;35(12):2140-2147.
As defined by the American Academy of Orthopaedic Surgeons (AAOS) in 1996, conflict of interest (COI) is the “circumstance that exists when, because of personal financial gain, an individual has the potential to be less than objective when called on to reach a judgment or interpret a result.”1 In medical research, COIs often occur in relationships between physician-researchers and pharmaceutical, medical device, and biotechnology companies. These relationships usually take the form of research grants but can also arise when the researcher has a financial interest in the product being tested or in the company that manufactures the product.
Although constructive collaboration between academic medicine and industry has worked to improve health care and ultimately benefit patients, potential drawbacks of such relationships include sequestration and suppression of results that may be disadvantageous to the industry sponsor,2 increased likelihood of reporting positive results (pro-industry),3-7 and biased study designs.8 The nature of such relationships may threaten the integrity of scientific studies, the objectivity of medical education, the quality of patient care, and the public’s trust in medicine.9
Financial relationships and affiliations are increasing as we seek to answer a growing number of clinical questions—with funding often being a limiting factor. At national scientific meetings, the number of presentations reporting COIs reflects this trend. Paper and poster presentations accepted for annual meetings of the Orthopaedic Trauma Association (OTA) and reporting a COI increased from 7.6% in 1993 to 12.6% in 2002 (P = .0129).2
Medical subspecialties outside of orthopedics are experiencing similar trends. Most notable is the American Psychiatric Association (APA). After the APA published a mandatory financial COI disclosure policy in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the percentage of task force members reporting industry relationships increased by 12%.10 Analysis of the DSM-5 panels demonstrated that the panels with the largest percentage of reported COIs are those for which pharmacological treatment is the first-line intervention, including the panels for mood disorders (67%), psychotic disorders (83%) and sleep/wake disorders (100%).10 Moreover, the industry ties reported are to the pharmaceutical companies that manufacture the medications used to treat these disorders or to companies that service the pharmaceutical industry.10
The degree to which financial COIs affect the interpretation of the orthopedic literature has never been quantified. Although it is clear that COIs can confound the results and reporting of data, how the medical community uses disclosures when interpreting the literature and when formulating opinions that may or may not affect their practice patterns is largely unknown.
We conducted a study to evaluate how a hypothetical financial COI disclosure would influence the interpretation of data by orthopedic clinicians. We also wanted to determine the reliability of the data as perceived in association with different study designs, levels of evidence, research institutional settings, and reporting of positive or negative results.
Methods
We asked members of the Arthroscopy Association of North America (AANA) and the American Orthopaedic Society for Sports Medicine (AOSSM) to complete a multiple-choice situational questionnaire (Table). The questionnaire assesses the degree to which respondents use COI disclosures when interpreting the literature. It further explores the perceived clinical value of a study with a given reported COI, assuming variations in study design, research institutional setting, and significance of results. The fictional research team disclosed the project was funded by a pharmaceutical company and all team members received consulting compensation. The survey and study were reviewed and approved by our institutional review board. The survey consisted of 14 multiple-choice questions that allowed for only 1 answer selection per person and allowed survey takers to skip questions they did not wish to answer. The survey questions and associated response options appear in edited form in the Table. A link to the questionnaire (https://www.surveymonkey.com/s/MPCCLCX) was sent with a message explaining the study. The responses to the questionnaire constituted the data.
Results
We sent a request to participate in the survey to 750 physicians and received 522 responses (overall response rate, 70%). The response rate for each question equaled or exceeded 98%.
The majority of respondents (95.6%) were male. Ninety-nine percent of respondents were orthopedic surgeons. The Northeast (US) was the most common geographical practice location of respondents (32%), followed by the Midwest (19.1%) and the Southeast (16.6%). Most respondents (40%) had been in practice for more than 20 years; 67% had been in practice a minimum of 10 years. The majority (68.8%) were employed by private practice groups, either single specialty (57.8%) or multispecialty (11%).
Eighty percent of respondents strongly agreed that COI disclosure is important when interpreting study results, 62% reported always reading the disclosure slide during academy or other meeting presentations, and 41% reported always using this information when deciding how to interpret scientific data.
Seventy-five percent of respondents thought the study—an academic-center case series with significant results in favor of the pharmaceutical company funding the study—was biased (42% indicated biased with merit, 33% biased without merit). Twenty-three percent thought the study was possibly biased, but likely trustworthy given the academic institutional affiliation. When the study setting was changed to community hospital, 95% thought the study was biased (51% biased with merit, 44% biased without merit). With the same study performed at an academic center, and no statistically significant results (not in favor of the pharmaceutical company funding the study), 88% thought the study had merit (46% biased with merit, 42% unbiased with merit).
When the study design was changed to a randomized controlled trial (level I evidence) conducted at an academic center with negative results, an overwhelming 95% of respondents thought the study had merit (33% biased with merit, 62% unbiased with merit). Given the same study design at an academic center, with positive results, 78% still thought the study had merit (39% biased with merit, 39% unbiased with merit). An additional 18% thought the study was biased, but still likely trustworthy given the academic institutional affiliation. Finally, given a randomized controlled trial and positive results, but with the research setting a small community practice, 90% thought the study had merit (51% biased with merit, 39% unbiased with merit). The percentage of respondents who found the study biased and likely without merit increased from 3.7% to 9.5% when the institutional affiliation changed from academic to community.
Discussion
As governmental funding sources become increasingly limited, the role of industry sponsorship of orthopedic research has grown. Potential drawbacks and biases of such research support have been well described—most notably, increased positive result reporting, suppression of results that may be disadvantageous to the industry sponsor, and biased study designs.2-8 However, the extent to which financial COIs affect the orthopedic medical community’s interpretation of the literature has never been quantified. To our knowledge, the present study is the first to quantify the impact of reported COI on study interpretation.
Our goal was to examine how reported financial COIs influence the interpretation of the literature by the orthopedic medical community. Moreover, we wanted to determine the perceived reliability of the data when variables (study design, institutional affiliation, positive vs negative results) were changed. The results of our survey indicate that, when a financial COI is reported, study reliability is perceived as highest when negative results were found.
Our survey noted a discrepancy between the documented importance of the hypothetical research team’s COI disclosure and the use of such disclosures when interpreting study results. Eighty percent of respondents agreed that COI disclosure is important when interpreting study results, but only 62% reported always reading disclosures, and even fewer (41%) reported always using the information when interpreting results. It is unclear exactly why this trend exists, as one would expect the percentages to be more similar. These particular survey questions were formed around using COI disclosures when interpreting study results during academic presentations at national meetings and not during the review of published literature. It is possible that positioning the COI disclosure at the beginning of a presentation has an effect, but only 3.7% of respondents indicated they seldom remembered the disclosure by the end of the presentation. The results of our survey may have varied if the questions had targeted reading and interpreting the literature.
Interestingly, the results of these survey questions tended to be more consistent with rates of reported financial COI by presenters at national orthopedic meetings. A study published in the New England Journal of Medicine found that less than 80% of orthopedic surgeons reported their disclosures at a large annual meeting (AAOS), even when the disclosure involved payments pertinent to the research they were presenting.5 When the payments were indirectly related to the research, the percentage of surgeons reporting disclosures was 50%, almost the same as the disclosure rate for unrelated payments.5
When the study was changed to a level I randomized controlled trial, more survey respondents found it to be less biased and have more merit. Although it would seem intuitive for a study with a higher level of evidence to carry more clinical value during interpretation, this may not hold true in the setting of industry-sponsored clinical trials. Several studies have documented a significant association between the reporting of positive results and industry-sponsored randomized clinical trials. In 2008, Khan and colleagues3 examined 100 orthopedic randomized clinical trials reported in 5 major orthopedic subspecialty journals over a 2-year period. The association between industry funding and favorable outcome in all original randomized clinical trials was strong and significant (P < .001). This is not surprising, given the amount of time and money required for a well-designed clinical study. Commercial products with preclinical promise are pushed to testing in a clinical trial, whereas resources would not be wasted on products lacking preclinical merit.
The most important variable affecting interpretation of study merit by survey respondents was the reporting of negative results. As more researchers are developing COIs, many studies are discovering a relationship between COIs and outcomes of research studies. Reviewing the adult total joint literature, Ezzet8 found an industry funding rate of 50%. Positive results were reported in 93% of cases in commercially funded studies versus 37% of cases in independently funded studies. Furthermore, no negative results were reported by investigators who were receiving royalties from the respective companies.
Studies across the medical literature have also found this association between industry sponsorship and reporting of positive results. One such study, reported by Valachis and colleagues7 in the Journal of Clinical Oncology, examined more than 80 economic analyses of targeted oncologic therapies and found the studies funded by pharmaceutical companies were more likely to report favorable qualitative cost estimates. In addition, when studies with a COI disclosure were examined, those reporting any financial relationship with a manufacturer (eg, author affiliation, funding) were more likely than those without such a relationship to report favorable results.
Our study had several limitations. First, as most of the survey respondents were orthopedic surgeons, extrapolating their data to the medical community at large may not be appropriate, as each specialty may view industry affiliations differently. In addition, respondents were asked to base their interpretations of a study on conclusions we predetermined—no direct visualization of the data set or statistical testing methods. It is possible that these responses may have been different had the respondents had the opportunity to further evaluate the study in question. In a recent study, Altwairgi and colleagues11 found that 10% of randomized clinical trials involving lung cancer treatment were reported with different conclusions in their full manuscripts relative to their abstracts. We think our survey design perhaps best mimics an annual meeting environment in which participants have very limited ability to interpret studies and may rely more heavily on the factors we investigated—study design, significance of findings, and setting, all similar to information presented in an abstract—when making informed decisions. Although our response rate was only 70%, this is comparable to or better than the rates in similar survey studies that used email-based questionnaires.12,13
Another limitation was that our survey may have forced respondents into answers they did not entirely agree with, given the limited options of the multiple-choice response format and the specific wording of the questions. Our conclusions may have been more dramatic when we were evaluating whether the study was deemed meritorious or not. However, there is no adopted standard for evaluating the extent of bias perceived by a clinician. We thought it was important to include answer options indicating a study had merit despite obvious bias in design and execution. That a study had merit can mean different things. It may change clinical practice, may require further study and reproducibility, or may not be significant enough to matter. Asking follow-up questions to evaluate this perception among the respondents could have provided validity to the term merit. Further studies in this field are needed to determine how studies are interpreted and translated into clinical practice by various clinicians.
Conclusion
Although the present study is not a quantitative analysis of the determination of bias in the orthopedic community, it is the first to evaluate orthopedic surgeons’ perceptions on the basis of key fundamentals of orthopedic research relative to COI. It is clear from our study results that introducing levels of evidence to the orthopedic milieu has had a significant impact both on the quality of research and on the foundational use of deductive reasoning when interpreting the literature. Reporting negative outcomes is perhaps the most important factor in eliminating the perception of bias among orthopedic surgeons. To what extent a perceived COI plays into medical decision-making and the ultimate treatment of patients is still relatively unknown.
As defined by the American Academy of Orthopaedic Surgeons (AAOS) in 1996, conflict of interest (COI) is the “circumstance that exists when, because of personal financial gain, an individual has the potential to be less than objective when called on to reach a judgment or interpret a result.”1 In medical research, COIs often occur in relationships between physician-researchers and pharmaceutical, medical device, and biotechnology companies. These relationships usually take the form of research grants but can also arise when the researcher has a financial interest in the product being tested or in the company that manufactures the product.
Although constructive collaboration between academic medicine and industry has worked to improve health care and ultimately benefit patients, potential drawbacks of such relationships include sequestration and suppression of results that may be disadvantageous to the industry sponsor,2 increased likelihood of reporting positive results (pro-industry),3-7 and biased study designs.8 The nature of such relationships may threaten the integrity of scientific studies, the objectivity of medical education, the quality of patient care, and the public’s trust in medicine.9
Financial relationships and affiliations are increasing as we seek to answer a growing number of clinical questions—with funding often being a limiting factor. At national scientific meetings, the number of presentations reporting COIs reflects this trend. Paper and poster presentations accepted for annual meetings of the Orthopaedic Trauma Association (OTA) and reporting a COI increased from 7.6% in 1993 to 12.6% in 2002 (P = .0129).2
Medical subspecialties outside of orthopedics are experiencing similar trends. Most notable is the American Psychiatric Association (APA). After the APA published a mandatory financial COI disclosure policy in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), the percentage of task force members reporting industry relationships increased by 12%.10 Analysis of the DSM-5 panels demonstrated that the panels with the largest percentage of reported COIs are those for which pharmacological treatment is the first-line intervention, including the panels for mood disorders (67%), psychotic disorders (83%) and sleep/wake disorders (100%).10 Moreover, the industry ties reported are to the pharmaceutical companies that manufacture the medications used to treat these disorders or to companies that service the pharmaceutical industry.10
The degree to which financial COIs affect the interpretation of the orthopedic literature has never been quantified. Although it is clear that COIs can confound the results and reporting of data, how the medical community uses disclosures when interpreting the literature and when formulating opinions that may or may not affect their practice patterns is largely unknown.
We conducted a study to evaluate how a hypothetical financial COI disclosure would influence the interpretation of data by orthopedic clinicians. We also wanted to determine the reliability of the data as perceived in association with different study designs, levels of evidence, research institutional settings, and reporting of positive or negative results.
Methods
We asked members of the Arthroscopy Association of North America (AANA) and the American Orthopaedic Society for Sports Medicine (AOSSM) to complete a multiple-choice situational questionnaire (Table). The questionnaire assesses the degree to which respondents use COI disclosures when interpreting the literature. It further explores the perceived clinical value of a study with a given reported COI, assuming variations in study design, research institutional setting, and significance of results. The fictional research team disclosed the project was funded by a pharmaceutical company and all team members received consulting compensation. The survey and study were reviewed and approved by our institutional review board. The survey consisted of 14 multiple-choice questions that allowed for only 1 answer selection per person and allowed survey takers to skip questions they did not wish to answer. The survey questions and associated response options appear in edited form in the Table. A link to the questionnaire (https://www.surveymonkey.com/s/MPCCLCX) was sent with a message explaining the study. The responses to the questionnaire constituted the data.
Results
We sent a request to participate in the survey to 750 physicians and received 522 responses (overall response rate, 70%). The response rate for each question equaled or exceeded 98%.
The majority of respondents (95.6%) were male. Ninety-nine percent of respondents were orthopedic surgeons. The Northeast (US) was the most common geographical practice location of respondents (32%), followed by the Midwest (19.1%) and the Southeast (16.6%). Most respondents (40%) had been in practice for more than 20 years; 67% had been in practice a minimum of 10 years. The majority (68.8%) were employed by private practice groups, either single specialty (57.8%) or multispecialty (11%).
Eighty percent of respondents strongly agreed that COI disclosure is important when interpreting study results, 62% reported always reading the disclosure slide during academy or other meeting presentations, and 41% reported always using this information when deciding how to interpret scientific data.
Seventy-five percent of respondents thought the study—an academic-center case series with significant results in favor of the pharmaceutical company funding the study—was biased (42% indicated biased with merit, 33% biased without merit). Twenty-three percent thought the study was possibly biased, but likely trustworthy given the academic institutional affiliation. When the study setting was changed to community hospital, 95% thought the study was biased (51% biased with merit, 44% biased without merit). With the same study performed at an academic center, and no statistically significant results (not in favor of the pharmaceutical company funding the study), 88% thought the study had merit (46% biased with merit, 42% unbiased with merit).
When the study design was changed to a randomized controlled trial (level I evidence) conducted at an academic center with negative results, an overwhelming 95% of respondents thought the study had merit (33% biased with merit, 62% unbiased with merit). Given the same study design at an academic center, with positive results, 78% still thought the study had merit (39% biased with merit, 39% unbiased with merit). An additional 18% thought the study was biased, but still likely trustworthy given the academic institutional affiliation. Finally, given a randomized controlled trial and positive results, but with the research setting a small community practice, 90% thought the study had merit (51% biased with merit, 39% unbiased with merit). The percentage of respondents who found the study biased and likely without merit increased from 3.7% to 9.5% when the institutional affiliation changed from academic to community.
Discussion
As governmental funding sources become increasingly limited, the role of industry sponsorship of orthopedic research has grown. Potential drawbacks and biases of such research support have been well described—most notably, increased positive result reporting, suppression of results that may be disadvantageous to the industry sponsor, and biased study designs.2-8 However, the extent to which financial COIs affect the orthopedic medical community’s interpretation of the literature has never been quantified. To our knowledge, the present study is the first to quantify the impact of reported COI on study interpretation.
Our goal was to examine how reported financial COIs influence the interpretation of the literature by the orthopedic medical community. Moreover, we wanted to determine the perceived reliability of the data when variables (study design, institutional affiliation, positive vs negative results) were changed. The results of our survey indicate that, when a financial COI is reported, study reliability is perceived as highest when negative results were found.
Our survey noted a discrepancy between the documented importance of the hypothetical research team’s COI disclosure and the use of such disclosures when interpreting study results. Eighty percent of respondents agreed that COI disclosure is important when interpreting study results, but only 62% reported always reading disclosures, and even fewer (41%) reported always using the information when interpreting results. It is unclear exactly why this trend exists, as one would expect the percentages to be more similar. These particular survey questions were formed around using COI disclosures when interpreting study results during academic presentations at national meetings and not during the review of published literature. It is possible that positioning the COI disclosure at the beginning of a presentation has an effect, but only 3.7% of respondents indicated they seldom remembered the disclosure by the end of the presentation. The results of our survey may have varied if the questions had targeted reading and interpreting the literature.
Interestingly, the results of these survey questions tended to be more consistent with rates of reported financial COI by presenters at national orthopedic meetings. A study published in the New England Journal of Medicine found that less than 80% of orthopedic surgeons reported their disclosures at a large annual meeting (AAOS), even when the disclosure involved payments pertinent to the research they were presenting.5 When the payments were indirectly related to the research, the percentage of surgeons reporting disclosures was 50%, almost the same as the disclosure rate for unrelated payments.5
When the study was changed to a level I randomized controlled trial, more survey respondents found it to be less biased and have more merit. Although it would seem intuitive for a study with a higher level of evidence to carry more clinical value during interpretation, this may not hold true in the setting of industry-sponsored clinical trials. Several studies have documented a significant association between the reporting of positive results and industry-sponsored randomized clinical trials. In 2008, Khan and colleagues3 examined 100 orthopedic randomized clinical trials reported in 5 major orthopedic subspecialty journals over a 2-year period. The association between industry funding and favorable outcome in all original randomized clinical trials was strong and significant (P < .001). This is not surprising, given the amount of time and money required for a well-designed clinical study. Commercial products with preclinical promise are pushed to testing in a clinical trial, whereas resources would not be wasted on products lacking preclinical merit.
The most important variable affecting interpretation of study merit by survey respondents was the reporting of negative results. As more researchers are developing COIs, many studies are discovering a relationship between COIs and outcomes of research studies. Reviewing the adult total joint literature, Ezzet8 found an industry funding rate of 50%. Positive results were reported in 93% of cases in commercially funded studies versus 37% of cases in independently funded studies. Furthermore, no negative results were reported by investigators who were receiving royalties from the respective companies.
Studies across the medical literature have also found this association between industry sponsorship and reporting of positive results. One such study, reported by Valachis and colleagues7 in the Journal of Clinical Oncology, examined more than 80 economic analyses of targeted oncologic therapies and found the studies funded by pharmaceutical companies were more likely to report favorable qualitative cost estimates. In addition, when studies with a COI disclosure were examined, those reporting any financial relationship with a manufacturer (eg, author affiliation, funding) were more likely than those without such a relationship to report favorable results.
Our study had several limitations. First, as most of the survey respondents were orthopedic surgeons, extrapolating their data to the medical community at large may not be appropriate, as each specialty may view industry affiliations differently. In addition, respondents were asked to base their interpretations of a study on conclusions we predetermined—no direct visualization of the data set or statistical testing methods. It is possible that these responses may have been different had the respondents had the opportunity to further evaluate the study in question. In a recent study, Altwairgi and colleagues11 found that 10% of randomized clinical trials involving lung cancer treatment were reported with different conclusions in their full manuscripts relative to their abstracts. We think our survey design perhaps best mimics an annual meeting environment in which participants have very limited ability to interpret studies and may rely more heavily on the factors we investigated—study design, significance of findings, and setting, all similar to information presented in an abstract—when making informed decisions. Although our response rate was only 70%, this is comparable to or better than the rates in similar survey studies that used email-based questionnaires.12,13
Another limitation was that our survey may have forced respondents into answers they did not entirely agree with, given the limited options of the multiple-choice response format and the specific wording of the questions. Our conclusions may have been more dramatic when we were evaluating whether the study was deemed meritorious or not. However, there is no adopted standard for evaluating the extent of bias perceived by a clinician. We thought it was important to include answer options indicating a study had merit despite obvious bias in design and execution. That a study had merit can mean different things. It may change clinical practice, may require further study and reproducibility, or may not be significant enough to matter. Asking follow-up questions to evaluate this perception among the respondents could have provided validity to the term merit. Further studies in this field are needed to determine how studies are interpreted and translated into clinical practice by various clinicians.
Conclusion
Although the present study is not a quantitative analysis of the determination of bias in the orthopedic community, it is the first to evaluate orthopedic surgeons’ perceptions on the basis of key fundamentals of orthopedic research relative to COI. It is clear from our study results that introducing levels of evidence to the orthopedic milieu has had a significant impact both on the quality of research and on the foundational use of deductive reasoning when interpreting the literature. Reporting negative outcomes is perhaps the most important factor in eliminating the perception of bias among orthopedic surgeons. To what extent a perceived COI plays into medical decision-making and the ultimate treatment of patients is still relatively unknown.
1. Lubahn JD, Mankin CJ, Mankin HJ, Kuhn PJ. Orthopaedics, ethics, and industry. Appropriateness of gifts, grants, and awards. Clin Orthop Relat Res. 2000;(371):256-263.
2. Kubiak EN, Park SS, Egol K, Zuckerman JD, Koval KJ. Increasingly conflicted: an analysis of conflicts of interest reported at the annual meetings of the Orthopaedic Trauma Association. Bull Hosp Jt Dis. 2006;63(3-4):83-87.
3. Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop. 2008;37(12):E205-E212.
4. Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research. An association between findings and funding in scientific presentations. J Bone Joint Surg Am. 2007;89(3):608-613.
5. Okike K, Kocher MS, Wei EX, Mehlman CT, Bhandari M. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361(15):1466-1474.
6. Shah RV, Albert TJ, Bruegel-Sanchez V, Vaccaro AR, Hilibrand AS, Grauer JN. Industry support and correlation to study outcome for papers published in Spine. Spine. 2005;30(9):1099-1104.
7. Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30(12):1316-1320.
8. Ezzet KA. The prevalence of corporate funding in adult lower extremity research and its correlation with reported results. J Arthroplasty. 2003;18(7 suppl 1):138-145.
9. Lo B, Field MJ, eds; Institute of Medicine, Committee on Conflict of Interest in Medical Research, Education, and Practice, Board on Health Sciences Policy. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press; 2009. http://www.ncbi.nlm.nih.gov/books/NBK22942. Accessed September 29, 2015.
10. Cosgrove L, Krimsky S. A comparison of DSM-IV and DSM-5 panel members’ financial associations with industry: a pernicious problem persists. PLoS Med. 2012;9(3):e1001190.
11. Altwairgi AK, Booth CM, Hopman WM, Baetz TD. Discordance between conclusions stated in the abstract and conclusions in the article: analysis of published randomized controlled trials of systemic therapy in lung cancer. J Clin Oncol. 2012;30(28):3552-3557.
12. Decoster LC, Vailas JC, Swartz WG. Functional ACL bracing. A survey of current opinion and practice. Am J Orthop. 1995;24(11):838-843.
13. Mann BJ, Grana WA, Indelicato PA, O’Neill DF, George SZ. A survey of sports medicine physicians regarding psychological issues in patient-athletes. Am J Sports Med. 2007;35(12):2140-2147.
1. Lubahn JD, Mankin CJ, Mankin HJ, Kuhn PJ. Orthopaedics, ethics, and industry. Appropriateness of gifts, grants, and awards. Clin Orthop Relat Res. 2000;(371):256-263.
2. Kubiak EN, Park SS, Egol K, Zuckerman JD, Koval KJ. Increasingly conflicted: an analysis of conflicts of interest reported at the annual meetings of the Orthopaedic Trauma Association. Bull Hosp Jt Dis. 2006;63(3-4):83-87.
3. Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop. 2008;37(12):E205-E212.
4. Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research. An association between findings and funding in scientific presentations. J Bone Joint Surg Am. 2007;89(3):608-613.
5. Okike K, Kocher MS, Wei EX, Mehlman CT, Bhandari M. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361(15):1466-1474.
6. Shah RV, Albert TJ, Bruegel-Sanchez V, Vaccaro AR, Hilibrand AS, Grauer JN. Industry support and correlation to study outcome for papers published in Spine. Spine. 2005;30(9):1099-1104.
7. Valachis A, Polyzos NP, Nearchou A, Lind P, Mauri D. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol. 2012;30(12):1316-1320.
8. Ezzet KA. The prevalence of corporate funding in adult lower extremity research and its correlation with reported results. J Arthroplasty. 2003;18(7 suppl 1):138-145.
9. Lo B, Field MJ, eds; Institute of Medicine, Committee on Conflict of Interest in Medical Research, Education, and Practice, Board on Health Sciences Policy. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press; 2009. http://www.ncbi.nlm.nih.gov/books/NBK22942. Accessed September 29, 2015.
10. Cosgrove L, Krimsky S. A comparison of DSM-IV and DSM-5 panel members’ financial associations with industry: a pernicious problem persists. PLoS Med. 2012;9(3):e1001190.
11. Altwairgi AK, Booth CM, Hopman WM, Baetz TD. Discordance between conclusions stated in the abstract and conclusions in the article: analysis of published randomized controlled trials of systemic therapy in lung cancer. J Clin Oncol. 2012;30(28):3552-3557.
12. Decoster LC, Vailas JC, Swartz WG. Functional ACL bracing. A survey of current opinion and practice. Am J Orthop. 1995;24(11):838-843.
13. Mann BJ, Grana WA, Indelicato PA, O’Neill DF, George SZ. A survey of sports medicine physicians regarding psychological issues in patient-athletes. Am J Sports Med. 2007;35(12):2140-2147.
Medial Patellar Subluxation: Diagnosis and Treatment
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
Diagnostic Arthroscopy
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
Diagnostic Arthroscopy
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
Medial patellar subluxation (MPS) is a disabling condition caused by an imbalance in the medial and lateral forces in the normal knee, allowing the patella to displace medially. Normally, the patella glides appropriately in the femoral trochlea, but alteration in this medial–lateral equilibrium can lead to pain and instability.1 MPS was first described in 1987 by Betz and colleagues2 as a complication of lateral retinacular release. Since then, multiple cases of iatrogenic, traumatic, and isolated medial subluxation have been reported.3–15 However, MPS after lateral release is the most common cause, accounting for the majority of published cases, whereas only 8 cases of isolated MPS have been reported to date.
Optimal treatment for MPS is not well understood. To better comprehend and manage MPS, we must fully appreciate the pathoanatomy, biomechanics, and current research. In this review, we focus on the anatomy of the lateral retinaculum, diagnosis and treatment of MPS, and outcomes of current treatment techniques.
Anatomy
In 1980, Fulkerson and Gossling16 delineated the anatomy of the knee joint lateral retinaculum. They described a 2-layered system with separate distinct anatomical structures. The lateral retinaculum is oriented longitudinally with the knee extended but exerts a posterolateral force on the lateral aspect of the patella as the knee is flexed. The superficial layer is composed of oblique fibers of the lateral retinaculum originating from the iliotibial band and the vastus lateralis fascia and inserting into the lateral margin of the patella and the patella tendon. The deep layer of the retinaculum consists of several structures, including the deep transverse retinaculum, lateral patellofemoral ligament (LPFL), and the patellotibial band.
Over the years, several studies have described the importance of the lateral retinaculum and, in particular, the LPFL. Examining the functional anatomy of the knee in 1962, Kaplan17 first described the lateral epicondylopatellar ligament as a palpable thickening of the joint capsule. Reider and colleagues18 later named this structure the lateral patellofemoral ligament in their anatomical study of 21 fresh cadaver knees. They described its width as ranging from 3 to 10 mm. In a comprehensive cadaveric study of the LPFL, Navarro and colleagues19,20 found it to be a distinct structure present in all 20 of their dissected specimens. They found its femoral insertion at the lateral epicondyle with a fanlike expansion of the fibers predominantly in the posterior region proximal to the lateral epicondyle. The patellar insertion was found in the posterior half and upper lateral aspect, also with expanded fibers. Mean length of the LPFL is 42.1 mm, and mean width is 16.1 mm.
Medial and lateral forces are balanced in a normal knee, and the patella glides appropriately in the femoral trochlea. Alteration in this medial–lateral equilibrium can lead to pain and instability.1 Normally, the patella lies laterally with the knee extended, but in early flexion the patella moves medially as it engages in the trochlea. As the knee continues to flex, the patella flexes and translates distally.21 By 45°, the patella is fully engaged in the trochlear groove throughout the remainder of the knee’s range of motion (ROM).
Lateral release procedures, as described in the literature, result in sectioning of both layers of the lateral retinaculum. In a biomechanical study, Merican and colleagues22 found that staged release of the lateral retinaculum reduced the medial stability of the patellofemoral joint progressively, making it easier to push the patella medially. At 30° of flexion, the transverse fibers of the midsection of the lateral retinaculum were found to be the main contributor to the lateral restraint of the patella. When the release extends too far proximally, the transverse fibers that anchor the lateral patella and the vastus lateralis oblique tendon to the iliotibial band are disrupted. Subsequent loss of a dynamic muscular pull in the orientation of the lateral stabilizing structures results in medial subluxation in a range from full knee extension to about 30° of flexion.
Furthermore, the attachments of the LPFL and the orientation of its fibers suggest that the LPFL may have a significant role in limiting medial excursion of the patella. Vieira and colleagues23 resected the LPFL in 10 fresh cadaver knees. They noticed that, after resection, the patella spontaneously traveled medially, demonstrating the importance of this ligament in patellar stability. In cases of isolated MPS, there have been no reports of associated pathology, such as muscular imbalance or coronal/rotational malalignment of the lower extremity. With an intact lateral retinaculum, medial subluxation is likely caused by pathology in the normal histologic structure of the LPFL and lateral retinaculum. However, the histologic structure of the LPFL and its contribution to the understanding of the pathoetiology of MPS have not been documented.
Diagnosis
MPS diagnosis can be challenging. Often, clinical examination findings are subtle, and radiographs may not show significant pathology. The most accurate diagnosis is obtained by combining patient history, physical examination findings, imaging studies, and diagnostic arthroscopy.
Patient History
Patients with MPS report chronic pain localized to the inferior medial patella and anterior-medial joint line. Occasionally, they complain of crepitus and intermittent swelling. Other symptoms include pain with knee flexion activity, such as squatting and climbing or descending stairs. Some patients describe episodes of giving way and feelings of instability. Often, they are aware the direction of instability is medial. The pain typically is not relieved by medication, physical therapy, or bracing.
Physical Examination
MPS must be identified by clinical examination. Peripatellar tenderness is typically noted. There is often no effusion or crepitus, but the patella is unstable in early flexion. Active and passive ROM is painful through the first 30° of knee flexion. The patient may have a positive medial apprehension test7 in which he or she experiences apprehension of the patella being subluxated with a medially directed force on the lateral border of the patella.
The gravity subluxation test described by Nonweiler and DeLee6 is useful in detecting MPS after lateral release and indicates that the vastus lateralis muscle has been detached from the patella and that the lateral retinaculum is lax. In this test, the patient is positioned in the lateral decubitus position with the involved knee farthest from the table. In this position, gravity causes the patella to subluxate out of the trochlea. The test is positive for MPS when a voluntary contraction of the quadriceps does not center the patella into the trochlear groove. Patients with MPS without previous lateral release can have the patella subluxate medially in the lateral decubitus position, but it is pulled back into the trochlea with active quadriceps contraction (Figure 1).
Patients with MPS often have lateral patellar laxity (LPL), which allows the patella to rotate upward on the lateral side and skid across the medial facet of the femoral trochlea. A physical examination sign combining lateral patellar glide and tilt was described by Shneider24 to identify LPL. This “lateral patellar float” sign is present when the patella translates laterally and rotates or tilts upward with medial pressure on the patella (Figure 2). Another maneuver to test for subtle MPS involves manually centering the patella in the trochlea during active knee flexion and extension. The involved knee is examined in the seated position. The examiner attempts to center the patella in the trochlea with a laterally directed force from the examiner’s thumb on the medial border of the patella. This will usually provide immediate relief as the patient actively ranges the knee.
Imaging Studies
Diagnostic imaging is a crucial component of the evaluation and treatment decision process. Plain radiographs often are not helpful in diagnosing MPS but may provide additional information.5 A variety of radiographic measurements have been described as indicators of structural disease, but there is a lack of comprehensive information recommending radiographic evaluation and interpretation of patients with patellofemoral dysfunction. It is crucial that orthopedic surgeons have common and consistent radiographic views for plain radiographic assessment that can serve as a basis for accurate diagnosis and surgical decision-making.
Standard knee radiographs should include a standing anteroposterior view of bilateral knees, a standing lateral view of the symptomatic knee in 30° of flexion, a patellar axial view, and a tunnel view. These views, occasionally combined with magnetic resonance imaging (MRI), can yield information vital to surgical decision-making. Image quality is highly technique-dependent, and variability in patient positioning can substantially affect the ability to properly diagnose structural abnormalities. For improved diagnostic accuracy and disease classification, radiographs must be obtained with use of the same standardized imaging protocol.
Kinetic MRI was shown by Shellock and colleagues25 to provide diagnostic information related to patellar malalignment. As kinetic MRI can image the patellofemoral joint within the initial 20° to 30° of flexion, it is useful in detecting some of the more subtle patellar tracking problems. In their study of 43 knees (40 patients) with symptoms after lateral release, Shellock and colleagues25 found that 27 knees (63%) had medial subluxation of the patella as the knee moved from extension to flexion. Furthermore, MPS was noted on the contralateral, unoperated knee in 17 (43%) of the 40 patients.
Diagnostic Arthroscopy
Once MPS is suspected after a thorough history and physical examination, examination under anesthesia accompanied by diagnostic arthroscopy confirms the diagnosis. Lateral forces are applied to the patella in full knee extension and 30° of flexion (Figure 3). During arthroscopy, the patellofemoral compartment is viewed from the anterolateral portal. With the knee at full extension, the lateral laxity and medial tilt of the patella can be identified (Figure 4). As the knee is flexed to 30°, the patella moves medially and can subluxate over the edge of the medial facet of the trochlea (Figure 5).
Treatment
Nonsurgical Management
Treatment of MPS depends entirely on making an accurate diagnosis and determining the degree of impairment. Patients with symptomatic MPS should initially undergo supervised rehabilitation focusing on balancing the medial and lateral forces that influence patellar tracking. Patients should be evaluated for specific muscle tightness, weakness, and biomechanical abnormalities. Each problem should be addressed with an individualized rehabilitation prescription. Emphasis is placed on balance, proprioception, and strengthening of the quadriceps, hip abductors/external rotators, and abdominal core muscle groups.
In some patients, symptomatic MPS may be reduced with a patella-stabilizing brace with a medial buttress.3,5,26 Although bracing should be regarded as an adjuvant to a structured physical therapy program, it can also be helpful in confirming the diagnosis of MPS. Shannon and Keene3 reported that all patients in their study experienced significant pain relief and decreased medial patellar subluxations when they wore a medial patella–stabilizing brace. Shellock and colleagues25 used kinematic MRI to investigate the effect of a patella-realignment brace and found that bracing counteracted patellar subluxation in the majority of knees studied.
Surgical Management
When conservative management fails and patients continue to experience pain and instability, surgical intervention is often required. Although various surgical techniques have been used (Table),3–6,8–10,14,15,27,28 the optimal surgical treatment for MPS has not been identified.
Lateral Retinaculum Imbrication. Lateral retinaculum imbrication has been used to centralize patella tracking and stabilize the patella. Richman and Scheller5 reported on a 17-year-old patient who had isolated medial subluxation of the patella without having undergone a previous lateral release. At 3-month follow-up, there was no recurrent instability; there was only intermittent medial knee soreness with weight-bearing activity.
Lateral Retinaculum Repair/Reconstruction. Hughston and colleagues8 treated 65 knees for MPS. Most had undergone lateral release. Of the 65 knees, 39 were treated with direct repair of the lateral retinaculum, and 26 with reconstruction of the lateral patellotibial ligament using locally available tissue, such as strips of iliotibial band or patellar tendon. Results were good to excellent in 80% of patients at a mean follow-up of 53.7 months. Nonweiler and DeLee6 reconstructed the lateral retinaculum in 5 patients with MPS that developed after isolated lateral retinacular release. Four (80%) of the 5 patients had no symptoms or physical signs of instability at a mean follow-up of 3.3 years. Results were excellent (3 knees) and good (2 knees) according to the Merchant and Mercer rating scale. Akşahin and colleagues28 reported on a single case of spontaneous medial patellar instability. At surgery, imbrication of the lateral structures failed to prevent the medial subluxation. Lateral patellotibial ligament augmentation was performed using an iliotibial band flap that effectively corrected the instability. At 1 year, the patient was characterized as engaging in vigorous recreational activity, according to the clinical score defined by Hughston and colleagues.8 He had mild pain with competitive sports but no pain with daily activity. Abhaykumar and Craig9 reported on 4 surgically treated knees with medial instability. They reconstructed the lateral retinaculum using a strip of fascia lata. By follow-up (5-7 years), each knee had its instability resolved and full ROM restored. Johnson and Wakeley26 reported on a case of iatrogenic MPS after lateral release. Treatment consisted of mobilization and direct repair of the lateral retinaculum. At 12-month follow-up, there was no instability. Although symptom-free with light activity, the patient had patellofemoral pain with strenuous activity. Sanchis-Alfonso and colleagues14 reported the results of isolated lateral retinacular reconstruction for iatrogenic MPS in 17 patients. At mean follow-up of 56 months, results were good or excellent in 65% of patients, and the Lysholm score improved from 36.4 preoperatively to 86.1 postoperatively.
Medial Retinaculum Release. Medial retinaculum release has been used as an alternative to open reconstruction. Shannon and Keene3 reported the results of medial retinacular release procedures on 9 knees. Four (44%) of the 9 patients had either spontaneous or traumatic onset of instability. All cases were treated with arthroscopic medial retinacular release, extending 2 cm medial to the superior pole of the patella down to the anteromedial portal. This avoided releasing the attachment of the vastus medialis oblique muscle to the patella and removing its dynamic medial stabilizing force. At a mean follow-up of 2.7 years, both medial subluxation and knee pain were relieved in all 9 knees without complications or further realignment surgery. Results were excellent in 6 knees (66.7%) and good in 3 knees (33.3%). Shannon and Keene3 emphasized that the procedure should not be used in patients with hypermobile patellae or in cases of failed lateral retinacular releases in which MPS is not clearly and carefully documented.
LPFL Reconstruction. Before coming to our practice, most patients have tried several months of formal physical rehabilitation, medications, and bracing. Many have already had surgical procedures, including arthroscopy, lateral release, and tibial tubercle transfer. When the diagnosis of MPS is suspected after a thorough history and physical examination, LPFL reconstruction is offered. Management of MPS with LPFL reconstruction has yielded excellent and reliable clinical results. Teitge and Torga Spak10 described an LPFL reconstruction technique that is used as a salvage procedure in managing medial iatrogenic patellar instability (the patient’s own quadriceps tendon is used). In their experience, direct repair or imbrication of the lateral retinaculum failed to provide long-term stability because medial excursion usually appeared after 1 year. The 60 patients’ outcomes were excellent with respect to patellar stability, and there were no cases of recurrent subluxation. Borbas and colleagues15 reported a case of LPFL reconstruction in a symptomatic medial subluxated patella resulting from TKA and extended lateral release. Using a free gracilis autograft through patellar bone tunnels to reconstruct the LPFL, the patient was free of pain and very satisfied with the result at 1 year postoperatively. Our current strategy is anatomical reconstruction of the LPFL using a quadriceps tendon graft and no bone tunnels, screws, or anchors in the patella.27 We previously reported a single case of isolated medial instability.4 At 2-year follow-up, there was no recurrent instability, and the functional outcome was excellent. This LPFL reconstruction method has been used in 10 patients with isolated MPS. There has been no residual medial subluxation on follow-up ranging from 3 months to 2 years. Outcome studies are in progress.
Rehabilitation. The initial goal of rehabilitation after surgical reconstruction of the lateral retinaculum or LPFL is to protect the healing soft tissues, restore normal knee ROM, and normalize gait. The knee is immobilized in a brace for weight-bearing activity for 4 to 6 weeks, until limb control is sufficient to prevent rotational stress on the knee. Gradual increase to full weight-bearing without bracing is permitted as quadriceps strength is restored. As motion is regained, strength, balance, and proprioception are emphasized for the entire lower extremity and core.
Functional limb training, including rotational activity, begins at 12 weeks. As strength and neuromuscular control progress, single-leg activity may be started with particular attention to proper alignment of the pelvis and the entire lower extremity. For competitive or recreational athletes, the final stages of rehabilitation focus on dynamic lower extremity control during sport-specific movements. Patients return to unrestricted activity by 6 months to 1 year after surgery.
Summary
MPS is a disabling condition that can limit daily functional activity because of apprehension and pain. Initially described as a complication of lateral retinacular release, isolated MPS can occur in the absence of a previous lateral release. Thorough physical examination and identification during arthroscopy are crucial for proper MPS diagnosis and management. When nonsurgical measures fail, LPFL reconstruction can provide patellofemoral stability and excellent functional outcomes.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
1. Marumoto JM, Jordan C, Akins R. A biomechanical comparison of lateral retinacular releases. Am J Sports Med. 1995;23(2):151-155.
2. Betz RR, Magill JT, Lonergan RP. The percutaneous lateral retinacular release. Am J Sports Med. 1987;15(5):477-482.
3. Shannon BD, Keene JS. Results of arthroscopic medial retinacular release for treatment of medial subluxation of the patella. Am J Sports Med. 2007;35(7):1180-1187.
4. Saper MG, Shneider DA. Medial patellar subluxation without previous lateral release: a case report. J Pediatr Orthop B. 2014;23(4):350-353.
5. Richman NM, Scheller AD Jr. Medial subluxation of the patella without previous lateral retinacular release. Orthopedics. 1998;21(7):810-813.
6. Nonweiler DE, DeLee JC. The diagnosis and treatment of medial subluxation of the patella after lateral retinacular release. Am J Sports Med. 1994;22(5):680-686.
7. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
8. Hughston JC, Flandry F, Brinker MR, Terry GC, Mills JC 3rd. Surgical correction of medial subluxation of the patella. Am J Sports Med. 1996;24(4):486-491.
9. Abhaykumar S, Craig DM. Fascia lata sling reconstruction for recurrent medial dislocation of the patella. The Knee. 1999;6(1):55-57.
10. Teitge RA, Torga Spak R. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20(9):998-1002.
11. Kusano M, Horibe S, Tanaka Y, et al. Simultaneous MPFL and LPFL reconstruction for recurrent lateral patellar dislocation with medial patellofemoral instability. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2014;1:42-46.
12. Saper MG, Shneider DA. Simultaneous medial and lateral patellofemoral ligament reconstruction for combined medial and lateral patellar subluxation. Arthrosc Tech. 2014,3(2):e227-e231.
13. Udagawa K, Niki Y, Matsumoto H, et al. Lateral patellar retinaculum reconstruction for medial patellar instability following lateral retinacular release: a case report. Knee. 2014;21(1):336-339.
14. Sanchis-Alfonso V, Montesinos-Berry E, Monllau JC, Merchant AC. Results of isolated lateral retinacular reconstruction for iatrogenic medial patellar instability. Arthroscopy. 2015;31(3):422-427.
15. Borbas P, Koch PP, Fucentese SF. Lateral patellofemoral ligament reconstruction using a free gracilis autograft. Orthopedics. 2014;37(7):e665-e668.
16. Fulkerson JP, Gossling H. Anatomy of the knee joint lateral retinaculum. Clin Orthop Relat Res. 1980;153:183-188.
17. Kaplan E. Some aspects of functional anatomy of the human knee joint. Clin Orthop Relat Res. 1962;23:18-29.
18. Reider B, Marshall J, Koslin B, Ring B, Girgis F. The anterior aspect of the knee joint. J Bone Joint Surg Am. 1981;63(3):351-356.
19. Navarro MS, Navarro RD, Akita Junior J, Cohen M. Anatomical study of the lateral patellofemoral ligament in cadaver knees. Rev Bras Ortop. 2008;43(7):300-307.
20. Navarro MS, Beltrani Filho CA, Akita Junior J, Navarro RD, Cohen M. Relationship between the lateral patellofemoral ligament and the width of the lateral patellar facet. Acta Ortop Bras. 2010;18(1):19-22.
21. Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277-284.
22. Merican AM, Kondo E, Amis AA. The effect on patellofemoral joint stability of selective cutting of lateral retinacular and capsular structures. J Biomech. 2009;42(3):291-296.
23. Vieira EL, Vieira EÁ, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269-274.
24. Shneider DA. Lateral patellar laxity—identification, significance, treatment. Poster session presented at: Annual Meeting of the American Academy of Orthopaedic Surgeons; February 25-28, 2009; Las Vegas, NV.
25. Shellock FG, Mink JH, Deutsch A, Fox JM, Ferkel RD. Evaluation of patients with persistent symptoms after lateral retinacular release by kinematic magnetic resonance imaging of the patellofemoral joint. Arthroscopy. 1990;6(3):226-234.
26. Johnson DP, Wakeley C. Reconstruction of the lateral patellar retinaculum following lateral release: a case report. Knee Surg Sports Traumatol Arthrosc. 2002;10(6):361-363.
27. Saper MG, Shneider DA. Lateral patellofemoral ligament reconstruction using a quadriceps tendon graft. Arthrosc Tech. 2014;3(4):e445-e448.
28. Akşahin E, Yumrukçal F, Yüksel HY, Doğruyol D, Celebi L. Role of pathophysiology of patellofemoral instability in the treatment of spontaneous medial patellofemoral subluxation: a case report. J Med Case Rep. 2010;4:148.
Biceps Tenodesis and Superior Labrum Anterior to Posterior (SLAP) Tears
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
Injuries of the superior labrum–biceps complex (SLBC) have been recognized as a cause of shoulder pain since they were first described by Andrews and colleagues1 in 1985. Superior labrum anterior to posterior (SLAP) tears are relatively uncommon injuries of the shoulder, and their true incidence is difficult to establish. However, recently there has been a significant increase in the reported incidence and operative treatment of SLAP tears.2 SLAP tears can occur in isolation, but they are commonly seen in association with other shoulder lesions, including rotator cuff tear, Bankart lesion, glenohumeral arthritis, acromioclavicular joint pathology, and subacromial impingement.
Although SLAP tears are well described and classified,3-6 our understanding of symptomatic SLAP tears and of their contribution to glenohumeral instability is limited. Diagnosing a SLAP tear on the basis of history and physical examination is a clinical challenge. Pain is the most common presentation of SLAP tears, though localization and characterization of pain are variable and nonspecific.7 The mechanism of injury is helpful in acute presentation (traction injury; fall on outstretched, abducted arm), but an overhead athlete may present with no distinct mechanism other than chronic, repetitive use of the shoulder.8-11 Numerous provocative physical examination tests have been used to assist in the diagnosis of SLAP tear, yet there is no consensus regarding the ideal physical examination test, with high sensitivity, specificity, and accuracy.12-14 Magnetic resonance arthrography, the gold standard imaging modality, is highly sensitive and specific (>95%) for diagnosing SLAP tears.
SLAP tear management is based on lesion type and severity, age, functional demands, and presence of coexisting intra-articular lesions. Management options include nonoperative treatment, débridement or repair of SLBC, biceps tenotomy, and biceps tenodesis.15-19
In this 5-point review, we present an evidence-based analysis of the role of the SLBC in glenohumeral stability and the role of biceps tenodesis in the management of SLAP tears.
1. Role of SLBC in stability of glenohumeral joint
The anatomy of the SLBC has been well described,20,21 and there is consensus that SLBC pathology can be a source of shoulder pain. The superior labrum is relatively more mobile than the rest of the glenoid labrum, and it provides attachment to the long head of the biceps tendon (LHBT) and the superior glenohumeral and middle glenohumeral ligaments.
The functional role of the SLBC in glenohumeral stability and its contribution to the pathogenesis of shoulder instability are not clearly defined. Our understanding of SLBC function is largely derived from simulated cadaveric experiments of SLAP tears. Controlled laboratory studies with simulated type II SLAP tears in cadavers have shown significantly increased glenohumeral translation in the anterior-posterior and superior-inferior directions, suggesting a role of the superior labrum in maintaining glenohumeral stability.22-26 Interestingly, there is conflicting evidence regarding restoration of normal glenohumeral translation in cadaveric shoulders after repair of simulated SLAP lesions in the presence or absence of simulated anterior capsular laxity.22,25-27 However, it is important to understand the limitations of cadaveric experiments in order to appreciate and truly comprehend the results of these experiments. There are inconsistencies in the size of simulated type II SLAP lesions in different studies, which can affect the degree of glenohumeral translation and the results of repair.23-25,28 The amount of glenohumeral translation noticed after simulated SLAP tears in cadavers, though statistically significant, is small in amplitude, and its relevance may not translate to a clinically significant level. The impact of dynamic components of stability (eg, rotator cuff muscles), capsular stretch, and other in vivo variables that affect glenohumeral stability are unaccounted for during cadaveric experiments.
LHBT is a recognized cause of shoulder pain, but its contribution to shoulder stability is a point of continued debate. According to one school of thought, LHBT is a vestigial structure that can be sacrificed without any loss of stability. Another school of thought holds that LHBT is an important active stabilizer of the glenohumeral joint. Cadaveric studies have demonstrated that loading the LHBT decreases glenohumeral translation and rotational range of motion, especially in lower and mid ranges of abduction.23,29,30 Furthermore, LHBT contributes to anterior glenohumeral stability by resisting torsional forces in the abducted and externally rotated shoulder and reducing stress on the inferior glenohumeral ligaments.31-33 Strauss and colleagues22 recently found that simulated anterior and posterior type II SLAP lesions in cadaveric shoulders increased glenohumeral translation in all planes, and biceps tenodesis did not further worsen this abnormal glenohumeral translation. Furthermore, repair of posterior SLAP lesions along with biceps tenodesis restored abnormal glenohumeral translation with no significant difference from the baseline in any plane of motion. Again, the limitations of cadaveric studies should be considered when interpreting these results and applying them clinically.
2. Biceps tenodesis as primary treatment for SLAP tears
A growing body of evidence suggests that primary tenodesis of LHBT may be an effective alternative treatment to SLAP repairs in select patients.34-36 However, the evidence is weak, and high-quality studies comparing SLAP repair and primary biceps tenodesis are required in order to make a strong recommendation for one technique over another. Gupta and colleagues35 retrospectively analyzed 28 cases of concomitant SLAP tear and biceps tendonitis treated with primary open subpectoral biceps tenodesis. There was significant improvement in patients’ functional outcome scores postoperatively [SANE (Single Assessment Numeric Evaluation), ASES (American Shoulder and Elbow Surgeons shoulder index), SST (Simple Shoulder Test), VAS (visual analog scale), and SF-12 (Short Form-12)]. In addition, 80% of patients were satisfied with their outcome. Mean age was 43.7 years. Forty-two percent of patients had a worker’s compensation claim. Interestingly, 15 patients in this cohort had a type I SLAP tear. Boileau and colleagues34 prospectively followed 25 cases of type II SLAP tear treated with either SLAP repair (10 patients; mean age, 37 years) or primary arthroscopic biceps tenodesis (15 patients; mean age, 52 years). Compared with the SLAP repair group, the biceps tenodesis group had significantly higher rates of satisfaction and return to previous level of sports participation. However, group assignments were nonrandomized, and the decision to treat a patient with SLAP repair versus biceps tenodesis was made by the senior surgeon purely on the basis of age (SLAP repair for patients under 30 years). Ek and colleagues36 retrospectively compared the cases of 10 patients who underwent SLAP repair (mean age, 32 years) and 15 who underwent biceps tenodesis (mean age, 47 years) for type II SLAP tear. There was no significant difference between the groups with respect to outcome scores, return to play or preinjury activity level, or complications.
There continues to be significant debate as to which patient will benefit from primary SLAP repair versus biceps tenodesis. Multiple factors are involved: age, presence of associated shoulder pathology, occupation, preinjury activity level, and worker’s compensation status. Age has convincingly been shown to affect the outcomes of treatment of type II SLAP tears.34,35,37-40 There is consensus that patients over age 40 years will benefit from primary biceps tenodesis for SLAP tears. However, the evidence for this recommendation is weak.
3. Biceps tenodesis and failed SLAP repair
The definition of a failed SLAP repair is not well documented in the literature, but dissatisfaction after SLAP repair can result from continued shoulder pain, poor shoulder function, or inability to return to preinjury functional level.15,41 The etiologic determination and treatment of a failed SLAP repair are challenging, and outcomes of revision SLAP repair are not very promising.42,43 Biceps tenodesis has been proposed as an alternative treatment to revision SLAP repair for failed SLAP repair. McCormick and colleagues41 prospectively evaluated 42 patients (mean age, 39.2 years; minimum follow-up, 2 years) with failed type II SLAP repairs that were treated with open subpectoral biceps tenodesis. There was significant improvement in ASES, SANE, and Western Ontario Shoulder Instability Index (WOSI) outcome scores and in postoperative shoulder range of motion at a mean follow-up of 3.6 years. One patient had transient musculocutaneous neurapraxia after surgery. In a retrospective cohort study, Gupta and colleagues44 found significant improvement in ASES, SANE, SST, SF-12, and VAS outcome scores in 11 patients who underwent open subpectoral biceps tenodesis for failed arthroscopic SLAP repair (mean age at surgery, 40 years; mean follow-up, 26 months). Three of the 11 patients had worker’s compensation claims, and there were no complications and no revision surgeries required after biceps tenodesis. Werner and colleagues16 retrospectively evaluated 17 patients who underwent biceps tenodesis for failed SLAP repair (mean age, 39 years; minimum follow-up, 2 years). Twenty-nine percent of patients had worker’s compensation claims. Compared with the contralateral shoulder, the treated shoulder had better postoperative ASES, SANE, SST, and Veteran RAND 36-item health survey outcome scores; range of motion was near normal.
There are no high-quality studies comparing revision SLAP repair and biceps tenodesis in the management of failed SLAP repair.16,41-44 Case series studies have found improved outcomes and pain relief after biceps tenodesis for failed SLAP repair, but the quality of evidence has been poor (level IV evidence).16,41-44 The senior author recommends treating failed SLAP repairs with biceps tenodesis.
4. Biceps tenodesis as treatment option for SLAP tear in overhead throwing athletes
Biceps tenodesis is a potential alternative treatment to SLAP repair in overhead throwing athletes. Although outcome scores and satisfaction rates after SLAP repair are high in overhead athletes, the rates of return to sport are relatively low, especially in baseball players.38,45-47 In a level III cohort study, Boileau and colleagues34 found that 13 (87%) of 15 patients with type II SLAP tears, including 8 overhead athletes, had returned to their previous level of activity by a mean of 30 months after biceps tenodesis. In contrast, only 2 of 10 patients returned to their previous level of activity after SLAP repair. Interestingly, 3 patients who underwent biceps tenodesis for failed SLAP repair returned to overhead sports. Schöffl and colleagues48 reported on the outcomes of biceps tenodesis for SLAP lesions in 6 high-level rock climbers. By a mean follow-up of 6 months, all 6 patients had returned to their previous level of climbing. Their satisfaction rate was 96.8%. Gupta and colleagues35 reported on a cohort of 28 patients who underwent biceps tenodesis for SLAP tears and concomitant biceps tendonitis. Of the 8 athletes in the group, 5 were able to return to their previous level of play, and 1 was able to return to a lower level of sporting activity. There was significant improvement from preoperative to postoperative scores on ASES, SST, SANE, VAS, SF-12 overall, and SF-12 components.
Chalmers and colleagues49 recently described motion analyses with simultaneous surface electromyographic measurements in 18 baseball pitchers. Of these 18 players, 7 were uninjured (controls), 6 were pitching after SLAP repair, and 5 were pitching after subpectoral biceps tenodesis. There were no significant differences between controls and postoperative patients with respect to pitching kinematics. Interestingly, compared with the controls and the patients who underwent open biceps tenodesis, the patients who underwent SLAP repair had altered patterns of thoracic rotation during pitching. However, the clinical significance of this finding and the impact of this finding on pitching efficacy are not currently known.
Biceps tenodesis as a primary procedure for type II SLAP lesion in an overhead athlete is a concept in evolution. Increasing evidence suggests a role for primary biceps tenodesis in an overhead athlete with type II SLAP lesion and concomitant biceps pathology. However, this evidence is of poor quality, and the strength of the recommendation is weak. Still to be determined is whether return to preinjury performance level is better with primary biceps tenodesis or with SLAP repair in overhead athletes with type II SLAP lesion. As per the senior author’s treatment algorithm, we prefer SLAP repair for overhead athletes with type II SLAP tears and reserve biceps tenodesis for cases involving significant biceps pathology and/or clinical symptoms involving the bicipital groove consistent with extra-articular biceps pain.
5. Biceps tenodesis for type II SLAP tear in contact athletes and occupations demanding heavy labor (blue-collar jobs)
SLAP tears are less common in contact athletes, and there is general agreement that SLAP repair outcomes are better in contact athletes than in overhead athletes. In a retrospective review of 18 rugby players with SLAP tears, Funk and Snow50 reported excellent results and quicker return to sport after SLAP repair. Patients with isolated SLAP tears had the earliest return to play. Enad and colleagues51 reported SLAP repair outcomes in an active military population. SLAP tears are more common in the military versus the general population because of the unique physical demands placed on military personnel. The authors retrospectively reviewed 27 cases of type II SLAP tears treated with SLAP repair and suture anchors. Outcomes were measured at a mean of 30.5 months after surgery. Twenty-four (89%) of the 27 patients had good to excellent results, and 94% had returned to active duty by a mean of 4.4 months after SLAP repair.
Given the poor-quality evidence in the literature, we believe that biceps tenodesis should be reserved for revision surgery in contact athletes. There is insufficient evidence to recommend biceps tenodesis as primary treatment for type II SLAP tears in contact athletes. SLAP repair should be performed for primary SLAP lesions in contact athletes and for patients in physically demanding professions (eg, military, laborer, weightlifter).
Conclusion
SLAP tears can result in persistent shoulder pain and dysfunction. SLAP tear management depends on lesion type and severity, age, and functional demands. SLAP repair is the treatment of choice for type II SLAP lesions in young, active patients. Biceps tenodesis is a preferred alternative to SLAP repair in failed SLAP repair and in type II SLAP patients who are older than 40 years and who are less active and have a worker’s compensation claim. These recommendations are based on poor-quality evidence. There is an unmet need for randomized clinical studies comparing SLAP repair with biceps tenodesis for type II SLAP tears in different patient populations so as to optimize the current decision-making algorithm for SLAP tears.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
1. Andrews JR, Carson WG Jr, McLeod WD. Glenoid labrum tears related to the long head of the biceps. Am J Sports Med. 1985;13(5):337-341.
2. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopaedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
3. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
4. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14(6):553-565.
5. Powell SE, Nord KD, Ryu RKN. The diagnosis, classification, and treatment of SLAP lesions. Oper Tech Sports Med. 2012;20(1):46-56.
6. Maffet MW, Gartsman GM, Moseley B. Superior labrum-biceps tendon complex lesions of the shoulder. Am J Sports Med. 1995;23(1):93-98.
7. Kim TK, Queale WS, Cosgarea AJ, McFarland EG. Clinical features of the different types of SLAP lesions: an analysis of one hundred and thirty-nine cases. J Bone Joint Surg Am. 2003;85(1):66-71.
8. Abrams GD, Safran MR. Diagnosis and management of superior labrum anterior posterior lesions in overhead athletes. Br J Sports Med. 2010;44(5):311-318.
9. Keener JD, Brophy RH. Superior labral tears of the shoulder: pathogenesis, evaluation, and treatment. J Am Acad Orthop Surg. 2009;17(10):627-637.
10. Abrams GD, Hussey KE, Harris JD, Cole BJ. Clinical results of combined meniscus and femoral osteochondral allograft transplantation: minimum 2-year follow-up. Arthroscopy. 2014;30(8):964-970.e1.
11. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
12. Virk MS, Arciero RA. Superior labrum anterior to posterior tears and glenohumeral instability. Instr Course Lect. 2013;62:501-514.
13. Calvert E, Chambers GK, Regan W, Hawkins RH, Leith JM. Special physical examination tests for superior labrum anterior posterior shoulder tears are clinically limited and invalid: a diagnostic systematic review. J Clin Epidemiol. 2009;62(5):558-563.
14. Jones GL, Galluch DB. Clinical assessment of superior glenoid labral lesions: a systematic review. Clin Orthop Relat Res. 2007;455:45-51.
15. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
16. Werner BC, Pehlivan HC, Hart JM, et al. Biceps tenodesis is a viable option for salvage of failed SLAP repair. J Shoulder Elbow Surg. 2014;23(8):e179-e184.
17. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
18. Huri G, Hyun YS, Garbis NG, McFarland EG. Treatment of superior labrum anterior posterior lesions: a literature review. Acta Orthop Traumatol Turc. 2014;48(3):290-297.
19. Li X, Lin TJ, Jager M, et al. Management of type II superior labrum anterior posterior lesions: a review of the literature. Orthop Rev. 2010;2(1):e6.
20. Cooper DE, Arnoczky SP, O’Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992;74(1):46-52.
21. Vangsness CT, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
22. Strauss EJ, Salata MJ, Sershon RA, et al. Role of the superior labrum after biceps tenodesis in glenohumeral stability. J Shoulder Elbow Surg. 2014;23(4):485-491.
23. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, Altchek DW. Effect of lesions of the superior portion of the glenoid labrum on glenohumeral translation. J Bone Joint Surg Am. 1995;77(7):1003-1010.
24. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13(1):39-44.
25. Burkart A, Debski R, Musahl V, McMahon P, Woo SL. Biomechanical tests for type II SLAP lesions of the shoulder joint before and after arthroscopic repair [in German]. Orthopade. 2003;32(7):600-607.
26. Panossian VR, Mihata T, Tibone JE, Fitzpatrick MJ, McGarry MH, Lee TQ. Biomechanical analysis of isolated type II SLAP lesions and repair. J Shoulder Elbow Surg. 2005;14(5):529-534.
27. Mihata T, McGarry MH, Tibone JE, Fitzpatrick MJ, Kinoshita M, Lee TQ. Biomechanical assessment of type II superior labral anterior-posterior (SLAP) lesions associated with anterior shoulder capsular laxity as seen in throwers: a cadaveric study. Am J Sports Med. 2008;36(8):1604-1610.
28. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36(4):767-774.
29. Youm T, ElAttrache NS, Tibone JE, McGarry MH, Lee TQ. The effect of the long head of the biceps on glenohumeral kinematics. J Shoulder Elbow Surg. 2009;18(1):122-129.
30. McGarry MH, Nguyen ML, Quigley RJ, Hanypsiak B, Gupta R, Lee TQ. The effect of long and short head biceps loading on glenohumeral joint rotational range of motion and humeral head position [published online ahead of print September 26, 2014]. Knee Surg Sports Traumatol Arthrosc.
31. Glousman R, Jobe F, Tibone J, Moynes D, Antonelli D, Perry J. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am. 1988;70(2):220-226.
32. Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching. Professional versus amateur pitchers. Am J Sports Med. 1987;15(6):586-590.
33. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
34. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37(5):929-936.
35. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
36. Ek ET, Shi LL, Tompson JD, Freehill MT, Warner JJ. Surgical treatment of isolated type II superior labrum anterior-posterior (SLAP) lesions: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2014;23(7):1059-1065.
37. Alpert JM, Wuerz TH, O’Donnell TF, Carroll KM, Brucker NN, Gill TJ. The effect of age on the outcomes of arthroscopic repair of type II superior labral anterior and posterior lesions. Am J Sports Med. 2010;38(11):2299-2303.
38. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
39. Denard PJ, Lädermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy. 2012;28(4):451-457.
40. Burns JP, Bahk M, Snyder SJ. Superior labral tears: repair versus biceps tenodesis. J Shoulder Elbow Surg. 2011;20(2 suppl):S2-S8.
41. McCormick F, Nwachukwu BU, Solomon D, et al. The efficacy of biceps tenodesis in the treatment of failed superior labral anterior posterior repairs. Am J Sports Med. 2014;42(4):820-825.
42. Katz LM, Hsu S, Miller SL, et al. Poor outcomes after SLAP repair: descriptive analysis and prognosis. Arthroscopy. 2009;25(8):849-855.
43. Park S, Glousman RE. Outcomes of revision arthroscopic type II superior labral anterior posterior repairs. Am J Sports Med. 2011;39(6):1290-1294.
44. Gupta AK, Bruce B, Klosterman EL, McCormick F, Harris J, Romeo AA. Subpectoral biceps tenodesis for failed type II SLAP repair. Orthopedics. 2013;36(6):e723-e728.
45. Neuman BJ, Boisvert CB, Reiter B, Lawson K, Ciccotti MG, Cohen SB. Results of arthroscopic repair of type II superior labral anterior posterior lesions in overhead athletes: assessment of return to preinjury playing level and satisfaction. Am J Sports Med. 2011;39(9):1883-1888.
46. Fedoriw WW, Ramkumar P, McCulloch PC, Lintner DM. Return to play after treatment of superior labral tears in professional baseball players. Am J Sports Med. 2014;42(5):1155-1160.
47. Park JY, Chung SW, Jeon SH, Lee JG, Oh KS. Clinical and radiological outcomes of type 2 superior labral anterior posterior repairs in elite overhead athletes. Am J Sports Med. 2013;41(6):1372-1379.
48. Schöffl V, Popp D, Dickschass J, Küpper T. Superior labral anterior-posterior lesions in rock climbers—primary double tenodesis? Clin J Sport Med. 2011;21(3):261-263.
49. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
50. Funk L, Snow M. SLAP tears of the glenoid labrum in contact athletes. Clin J Sport Med. 2007;17(1):1-4.
51. Enad JG, Gaines RJ, White SM, Kurtz CA. Arthroscopic superior labrum anterior-posterior repair in military patients. J Shoulder Elbow Surg. 2007;16(3):300-305.
Do Women With Knee Osteoarthritis Experience Greater Pain Sensitivity Than Men?
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Does Arthritis Contribute to Higher Rates of Poverty In Women?
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
AAOS Guidelines Sum-Up Prevention and Treatment Strategies for ACL Injuries
The American Academy of Orthopaedic Surgeons (AAOS) Board of Directors has approved Appropriate Use Criteria (AUCs) for anterior cruciate ligament (ACL) injury prevention programs and treatment, as well as rehabilitation and function checklists to help guide and ensure a safe return to sports for the treated athlete. The AUCs and checklists are available online at http://www.orthoguidelines.org/go/auc/.
“Both prevention and treatment of ACL injuries can be confusing given the diversity of injured patients—from skeletally immature youth to older adults, low- and high-risk athletes playing a variety of sports, and patients with and without arthritis,” said Robert Quinn, MD, AUC Section Leader on the Committee on Evidence-Based Quality and Value.
Last year, the AAOS released the Clinical Practice Guideline (CPG) titled “Management of Anterior Cruciate Ligament Injuries.” The guideline recommends, with “moderate” supporting evidence, that reconstructive surgery occur within 5 months of an ACL injury to protect the knee joint. In addition, the CPG states that in young adults, ages 18 to 35, use of a patient's own tissue is preferable over donor tissue to repair an ACL tear.
The new “Appropriate Use Guideline for the Treatment of Anterior Cruciate Ligament Injuries" provides more specific guidance to orthopedic surgeons based on a patient’s various indications, including age, activity level, presence of advanced arthritis, and the status of the ACL tear. The guideline recommends specific next steps and procedures to ensure optimal recovery. Each treatment recommendation is ranked by level of appropriateness.
“The good news for patients and practitioners is that ACL reconstruction with autograft or allograft tissue is very successful,” said Dr. Quinn. “What these guidelines do is delineate, in a very easy-to-maneuver way, what the most appropriate treatments are in each category. It actually gives you the specific circumstances to plug in, and highlights where the evidence matches the recommendations.”
Most patients, especially high-level athletes, are eager to return to play following ACL surgery. However, there is a significant amount of post-surgical rehabilitation and functional recovery required before an athlete can resume sports play.
The new ACL Reconstruction Surgery “Return to Play” and “Postoperative Rehabilitation” checklists “are evidence-based lists on what should be going on before an athlete returns to play, and are constructed in a way that realistically sets expectations for what needs to be accomplished,” said Dr. Quinn.
The “Postoperative Rehabilitation” checklist outlines the post-surgical protocol, from early range of motion, weight bearing and closed and open chain quad and hamstring therapy, to optional rehabilitative bracing and neuromuscular stimulation.
According to the “Return to Play” checklist, a patient should feel confident that he or she can return to their sport of interest, and have been advised to participate in an ongoing ACL-prevention/movement-retraining program before resuming activities. In addition, the graft and surgical site have fully healed; and range of motion, balance, knee stability, strength and functional skills, have been restored.
For athletes involved in competitive or recreational athletics with no prior history of ACL reconstruction and no current history of ACL deficiency, the “Appropriate Use Guideline for ACL Injury Prevention Programs” provides advice regarding a supervised ACL injury prevention program, utilizing the best available scientific evidence and expert opinion.
“Injury prevention programs are very successful,” said Dr. Quinn. “This AUC helps alleviate some of the controversy about when these good options are most applicable.”
Like the treatment AUC, the injury prevention guidelines use patient indications and classifications. For example, sex, growth status, activity level, sports participation, and athlete risk can help determine whether or not a particular, supervised ACL injury prevention program is optimal.
The American Academy of Orthopaedic Surgeons (AAOS) Board of Directors has approved Appropriate Use Criteria (AUCs) for anterior cruciate ligament (ACL) injury prevention programs and treatment, as well as rehabilitation and function checklists to help guide and ensure a safe return to sports for the treated athlete. The AUCs and checklists are available online at http://www.orthoguidelines.org/go/auc/.
“Both prevention and treatment of ACL injuries can be confusing given the diversity of injured patients—from skeletally immature youth to older adults, low- and high-risk athletes playing a variety of sports, and patients with and without arthritis,” said Robert Quinn, MD, AUC Section Leader on the Committee on Evidence-Based Quality and Value.
Last year, the AAOS released the Clinical Practice Guideline (CPG) titled “Management of Anterior Cruciate Ligament Injuries.” The guideline recommends, with “moderate” supporting evidence, that reconstructive surgery occur within 5 months of an ACL injury to protect the knee joint. In addition, the CPG states that in young adults, ages 18 to 35, use of a patient's own tissue is preferable over donor tissue to repair an ACL tear.
The new “Appropriate Use Guideline for the Treatment of Anterior Cruciate Ligament Injuries" provides more specific guidance to orthopedic surgeons based on a patient’s various indications, including age, activity level, presence of advanced arthritis, and the status of the ACL tear. The guideline recommends specific next steps and procedures to ensure optimal recovery. Each treatment recommendation is ranked by level of appropriateness.
“The good news for patients and practitioners is that ACL reconstruction with autograft or allograft tissue is very successful,” said Dr. Quinn. “What these guidelines do is delineate, in a very easy-to-maneuver way, what the most appropriate treatments are in each category. It actually gives you the specific circumstances to plug in, and highlights where the evidence matches the recommendations.”
Most patients, especially high-level athletes, are eager to return to play following ACL surgery. However, there is a significant amount of post-surgical rehabilitation and functional recovery required before an athlete can resume sports play.
The new ACL Reconstruction Surgery “Return to Play” and “Postoperative Rehabilitation” checklists “are evidence-based lists on what should be going on before an athlete returns to play, and are constructed in a way that realistically sets expectations for what needs to be accomplished,” said Dr. Quinn.
The “Postoperative Rehabilitation” checklist outlines the post-surgical protocol, from early range of motion, weight bearing and closed and open chain quad and hamstring therapy, to optional rehabilitative bracing and neuromuscular stimulation.
According to the “Return to Play” checklist, a patient should feel confident that he or she can return to their sport of interest, and have been advised to participate in an ongoing ACL-prevention/movement-retraining program before resuming activities. In addition, the graft and surgical site have fully healed; and range of motion, balance, knee stability, strength and functional skills, have been restored.
For athletes involved in competitive or recreational athletics with no prior history of ACL reconstruction and no current history of ACL deficiency, the “Appropriate Use Guideline for ACL Injury Prevention Programs” provides advice regarding a supervised ACL injury prevention program, utilizing the best available scientific evidence and expert opinion.
“Injury prevention programs are very successful,” said Dr. Quinn. “This AUC helps alleviate some of the controversy about when these good options are most applicable.”
Like the treatment AUC, the injury prevention guidelines use patient indications and classifications. For example, sex, growth status, activity level, sports participation, and athlete risk can help determine whether or not a particular, supervised ACL injury prevention program is optimal.
The American Academy of Orthopaedic Surgeons (AAOS) Board of Directors has approved Appropriate Use Criteria (AUCs) for anterior cruciate ligament (ACL) injury prevention programs and treatment, as well as rehabilitation and function checklists to help guide and ensure a safe return to sports for the treated athlete. The AUCs and checklists are available online at http://www.orthoguidelines.org/go/auc/.
“Both prevention and treatment of ACL injuries can be confusing given the diversity of injured patients—from skeletally immature youth to older adults, low- and high-risk athletes playing a variety of sports, and patients with and without arthritis,” said Robert Quinn, MD, AUC Section Leader on the Committee on Evidence-Based Quality and Value.
Last year, the AAOS released the Clinical Practice Guideline (CPG) titled “Management of Anterior Cruciate Ligament Injuries.” The guideline recommends, with “moderate” supporting evidence, that reconstructive surgery occur within 5 months of an ACL injury to protect the knee joint. In addition, the CPG states that in young adults, ages 18 to 35, use of a patient's own tissue is preferable over donor tissue to repair an ACL tear.
The new “Appropriate Use Guideline for the Treatment of Anterior Cruciate Ligament Injuries" provides more specific guidance to orthopedic surgeons based on a patient’s various indications, including age, activity level, presence of advanced arthritis, and the status of the ACL tear. The guideline recommends specific next steps and procedures to ensure optimal recovery. Each treatment recommendation is ranked by level of appropriateness.
“The good news for patients and practitioners is that ACL reconstruction with autograft or allograft tissue is very successful,” said Dr. Quinn. “What these guidelines do is delineate, in a very easy-to-maneuver way, what the most appropriate treatments are in each category. It actually gives you the specific circumstances to plug in, and highlights where the evidence matches the recommendations.”
Most patients, especially high-level athletes, are eager to return to play following ACL surgery. However, there is a significant amount of post-surgical rehabilitation and functional recovery required before an athlete can resume sports play.
The new ACL Reconstruction Surgery “Return to Play” and “Postoperative Rehabilitation” checklists “are evidence-based lists on what should be going on before an athlete returns to play, and are constructed in a way that realistically sets expectations for what needs to be accomplished,” said Dr. Quinn.
The “Postoperative Rehabilitation” checklist outlines the post-surgical protocol, from early range of motion, weight bearing and closed and open chain quad and hamstring therapy, to optional rehabilitative bracing and neuromuscular stimulation.
According to the “Return to Play” checklist, a patient should feel confident that he or she can return to their sport of interest, and have been advised to participate in an ongoing ACL-prevention/movement-retraining program before resuming activities. In addition, the graft and surgical site have fully healed; and range of motion, balance, knee stability, strength and functional skills, have been restored.
For athletes involved in competitive or recreational athletics with no prior history of ACL reconstruction and no current history of ACL deficiency, the “Appropriate Use Guideline for ACL Injury Prevention Programs” provides advice regarding a supervised ACL injury prevention program, utilizing the best available scientific evidence and expert opinion.
“Injury prevention programs are very successful,” said Dr. Quinn. “This AUC helps alleviate some of the controversy about when these good options are most applicable.”
Like the treatment AUC, the injury prevention guidelines use patient indications and classifications. For example, sex, growth status, activity level, sports participation, and athlete risk can help determine whether or not a particular, supervised ACL injury prevention program is optimal.
Is There a Greater Risk of Mortality Following Hip Fracture Surgery Compared With Hip Replacement Surgery?
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.