User login
Nonopioid med promising for neuropathic pain
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Step-wise medical therapy is cost effective for endometriosis
For patients with endometriosis-related dysmenorrhea, it is cost effective to use medical therapy before surgery, according to investigators.
A stepwise strategy involving two medications, then surgery, was associated with the lowest cost per quality-adjusted life-years (QALYs), reported lead author, Jacqueline A. Bohn, MD, of Oregon Health & Science University, Portland, and colleagues.
“In 2009, the medical costs associated with endometriosis in the United States were estimated at $69.4 billion annually,” the investigators wrote in Obstetrics and Gynecology. “Despite the recognized cost burden of this disease, cost-effectiveness data on the various treatment strategies is limited. Previous studies have investigated the direct and indirect costs regarding endometriosis; however, there are no prior studies that evaluate the cost-effectiveness of a stepwise regimen to guide management.”
To fill this knowledge gap, Dr. Bohn and colleagues created a cost-effectiveness model comparing four treatment strategies:
NSAIDs, then surgery
NSAIDs, then short-acting reversible contraceptives or long-acting reversible contraceptives (LARCs), then surgery
NSAIDs, then a short-acting reversible contraceptive or a LARC, then a LARC or gonadotropin-releasing hormone (GnRH) modulator, then surgery
Surgery alone
The analysis, which compared costs, QALYs, and incremental cost-effectiveness ratios, involved a theoretical cohort of 4,817,894 women aged 18-45 years, representing the estimated number of reproductive-age women in the United States with endometriosis-related dysmenorrhea. Costs were determined from published literature and inflated to 2019 dollars. Medical treatments were theoretically given for 6 months each, and the cost of laparoscopic surgery incorporated 12 months of postoperative care.
Of the four strategies, the two-medication approach was most cost effective, with a cost per QALY of $1,158. This was followed closely by the three-medication regimen, at $1,158, the single-medication regimen, at $2,108, and finally, surgery alone, at $4,338.
“We found that, although cost effective, requiring trial of a third medication offered little comparative advantage before proceeding directly to surgery after the second therapy fails,” the investigators wrote. “Yet, for the woman who is anxious about surgical intervention, or when a prolonged wait for a surgical specialist occurs, trial of a GnRH modulator may be worthwhile.”
Compared with surgery alone, each regimen starting with medical therapy remained below the standard willingness-to-pay threshold of $100,000 per QALY; however, the investigators recommend against trying more than three medications.
“Delaying surgical management in a woman with pain refractory to more than three medications may decrease quality of life and further increase cost,” they wrote.
To make surgery alone the most cost-effective option, surgery success would need to exceed 83%, Dr. Bohn and colleagues concluded.
According to Hugh Taylor, MD, of Yale University, New Haven, Conn., it’s unlikely that this surgery success threshold will be met, since surgery alone typically leads to recurrence.
“We know there’s a very high relapse rate after surgery,” Dr. Taylor said in an interview. “Even if the surgery may be initially successful, there’s roughly a 50% recurrence rate after about 2 years. So, finding the right medical therapy will give you more chance for long-term success.”
Dr. Taylor said it’s “really nice” that Dr. Bohn and colleagues conducted a sequential analysis because the findings support the most common approach in real-world practice.
“It confirms that starting with a medical therapy prior to surgery is an appropriate, successful treatment for endometriosis, which is something that many, many people in the community do, but we haven’t had a real trial to show that,” he said.
Dr. Taylor offered two areas of improvement for similar studies in the future: First, he suggested separating LARCs from oral contraceptives because LARCs may be less effective for some patients with endometriosis; and second, he suggested that limiting the third medication to a GnRH antagonist would be more applicable to real-world practice than using the broader category of GnRH modulators.
Although the three-medication approach involving a GnRH modulator was slightly more expensive than the two-medication approach, Dr. Taylor said the costs were so similar that a three-medication approach is “still reasonable,” particularly because it could spare patients from surgery.
Dr. Taylor also speculated that trying a GnRH antagonist could become more cost effective soon. Although only one GnRH antagonist is currently on the market, he noted that a second agent is poised for Food and Drug Administration approval, while a third is in the pipeline, and this competition may decrease drug prices.
The investigators disclosed support from the National Institutes of Health, Arnold Ventures, the World Health Organization, Merck, and others. Dr. Taylor reported that Yale University receives funding for endometriosis biomarker research from AbbVie.
For patients with endometriosis-related dysmenorrhea, it is cost effective to use medical therapy before surgery, according to investigators.
A stepwise strategy involving two medications, then surgery, was associated with the lowest cost per quality-adjusted life-years (QALYs), reported lead author, Jacqueline A. Bohn, MD, of Oregon Health & Science University, Portland, and colleagues.
“In 2009, the medical costs associated with endometriosis in the United States were estimated at $69.4 billion annually,” the investigators wrote in Obstetrics and Gynecology. “Despite the recognized cost burden of this disease, cost-effectiveness data on the various treatment strategies is limited. Previous studies have investigated the direct and indirect costs regarding endometriosis; however, there are no prior studies that evaluate the cost-effectiveness of a stepwise regimen to guide management.”
To fill this knowledge gap, Dr. Bohn and colleagues created a cost-effectiveness model comparing four treatment strategies:
NSAIDs, then surgery
NSAIDs, then short-acting reversible contraceptives or long-acting reversible contraceptives (LARCs), then surgery
NSAIDs, then a short-acting reversible contraceptive or a LARC, then a LARC or gonadotropin-releasing hormone (GnRH) modulator, then surgery
Surgery alone
The analysis, which compared costs, QALYs, and incremental cost-effectiveness ratios, involved a theoretical cohort of 4,817,894 women aged 18-45 years, representing the estimated number of reproductive-age women in the United States with endometriosis-related dysmenorrhea. Costs were determined from published literature and inflated to 2019 dollars. Medical treatments were theoretically given for 6 months each, and the cost of laparoscopic surgery incorporated 12 months of postoperative care.
Of the four strategies, the two-medication approach was most cost effective, with a cost per QALY of $1,158. This was followed closely by the three-medication regimen, at $1,158, the single-medication regimen, at $2,108, and finally, surgery alone, at $4,338.
“We found that, although cost effective, requiring trial of a third medication offered little comparative advantage before proceeding directly to surgery after the second therapy fails,” the investigators wrote. “Yet, for the woman who is anxious about surgical intervention, or when a prolonged wait for a surgical specialist occurs, trial of a GnRH modulator may be worthwhile.”
Compared with surgery alone, each regimen starting with medical therapy remained below the standard willingness-to-pay threshold of $100,000 per QALY; however, the investigators recommend against trying more than three medications.
“Delaying surgical management in a woman with pain refractory to more than three medications may decrease quality of life and further increase cost,” they wrote.
To make surgery alone the most cost-effective option, surgery success would need to exceed 83%, Dr. Bohn and colleagues concluded.
According to Hugh Taylor, MD, of Yale University, New Haven, Conn., it’s unlikely that this surgery success threshold will be met, since surgery alone typically leads to recurrence.
“We know there’s a very high relapse rate after surgery,” Dr. Taylor said in an interview. “Even if the surgery may be initially successful, there’s roughly a 50% recurrence rate after about 2 years. So, finding the right medical therapy will give you more chance for long-term success.”
Dr. Taylor said it’s “really nice” that Dr. Bohn and colleagues conducted a sequential analysis because the findings support the most common approach in real-world practice.
“It confirms that starting with a medical therapy prior to surgery is an appropriate, successful treatment for endometriosis, which is something that many, many people in the community do, but we haven’t had a real trial to show that,” he said.
Dr. Taylor offered two areas of improvement for similar studies in the future: First, he suggested separating LARCs from oral contraceptives because LARCs may be less effective for some patients with endometriosis; and second, he suggested that limiting the third medication to a GnRH antagonist would be more applicable to real-world practice than using the broader category of GnRH modulators.
Although the three-medication approach involving a GnRH modulator was slightly more expensive than the two-medication approach, Dr. Taylor said the costs were so similar that a three-medication approach is “still reasonable,” particularly because it could spare patients from surgery.
Dr. Taylor also speculated that trying a GnRH antagonist could become more cost effective soon. Although only one GnRH antagonist is currently on the market, he noted that a second agent is poised for Food and Drug Administration approval, while a third is in the pipeline, and this competition may decrease drug prices.
The investigators disclosed support from the National Institutes of Health, Arnold Ventures, the World Health Organization, Merck, and others. Dr. Taylor reported that Yale University receives funding for endometriosis biomarker research from AbbVie.
For patients with endometriosis-related dysmenorrhea, it is cost effective to use medical therapy before surgery, according to investigators.
A stepwise strategy involving two medications, then surgery, was associated with the lowest cost per quality-adjusted life-years (QALYs), reported lead author, Jacqueline A. Bohn, MD, of Oregon Health & Science University, Portland, and colleagues.
“In 2009, the medical costs associated with endometriosis in the United States were estimated at $69.4 billion annually,” the investigators wrote in Obstetrics and Gynecology. “Despite the recognized cost burden of this disease, cost-effectiveness data on the various treatment strategies is limited. Previous studies have investigated the direct and indirect costs regarding endometriosis; however, there are no prior studies that evaluate the cost-effectiveness of a stepwise regimen to guide management.”
To fill this knowledge gap, Dr. Bohn and colleagues created a cost-effectiveness model comparing four treatment strategies:
NSAIDs, then surgery
NSAIDs, then short-acting reversible contraceptives or long-acting reversible contraceptives (LARCs), then surgery
NSAIDs, then a short-acting reversible contraceptive or a LARC, then a LARC or gonadotropin-releasing hormone (GnRH) modulator, then surgery
Surgery alone
The analysis, which compared costs, QALYs, and incremental cost-effectiveness ratios, involved a theoretical cohort of 4,817,894 women aged 18-45 years, representing the estimated number of reproductive-age women in the United States with endometriosis-related dysmenorrhea. Costs were determined from published literature and inflated to 2019 dollars. Medical treatments were theoretically given for 6 months each, and the cost of laparoscopic surgery incorporated 12 months of postoperative care.
Of the four strategies, the two-medication approach was most cost effective, with a cost per QALY of $1,158. This was followed closely by the three-medication regimen, at $1,158, the single-medication regimen, at $2,108, and finally, surgery alone, at $4,338.
“We found that, although cost effective, requiring trial of a third medication offered little comparative advantage before proceeding directly to surgery after the second therapy fails,” the investigators wrote. “Yet, for the woman who is anxious about surgical intervention, or when a prolonged wait for a surgical specialist occurs, trial of a GnRH modulator may be worthwhile.”
Compared with surgery alone, each regimen starting with medical therapy remained below the standard willingness-to-pay threshold of $100,000 per QALY; however, the investigators recommend against trying more than three medications.
“Delaying surgical management in a woman with pain refractory to more than three medications may decrease quality of life and further increase cost,” they wrote.
To make surgery alone the most cost-effective option, surgery success would need to exceed 83%, Dr. Bohn and colleagues concluded.
According to Hugh Taylor, MD, of Yale University, New Haven, Conn., it’s unlikely that this surgery success threshold will be met, since surgery alone typically leads to recurrence.
“We know there’s a very high relapse rate after surgery,” Dr. Taylor said in an interview. “Even if the surgery may be initially successful, there’s roughly a 50% recurrence rate after about 2 years. So, finding the right medical therapy will give you more chance for long-term success.”
Dr. Taylor said it’s “really nice” that Dr. Bohn and colleagues conducted a sequential analysis because the findings support the most common approach in real-world practice.
“It confirms that starting with a medical therapy prior to surgery is an appropriate, successful treatment for endometriosis, which is something that many, many people in the community do, but we haven’t had a real trial to show that,” he said.
Dr. Taylor offered two areas of improvement for similar studies in the future: First, he suggested separating LARCs from oral contraceptives because LARCs may be less effective for some patients with endometriosis; and second, he suggested that limiting the third medication to a GnRH antagonist would be more applicable to real-world practice than using the broader category of GnRH modulators.
Although the three-medication approach involving a GnRH modulator was slightly more expensive than the two-medication approach, Dr. Taylor said the costs were so similar that a three-medication approach is “still reasonable,” particularly because it could spare patients from surgery.
Dr. Taylor also speculated that trying a GnRH antagonist could become more cost effective soon. Although only one GnRH antagonist is currently on the market, he noted that a second agent is poised for Food and Drug Administration approval, while a third is in the pipeline, and this competition may decrease drug prices.
The investigators disclosed support from the National Institutes of Health, Arnold Ventures, the World Health Organization, Merck, and others. Dr. Taylor reported that Yale University receives funding for endometriosis biomarker research from AbbVie.
FROM OBSTETRICS & GYNECOLOGY
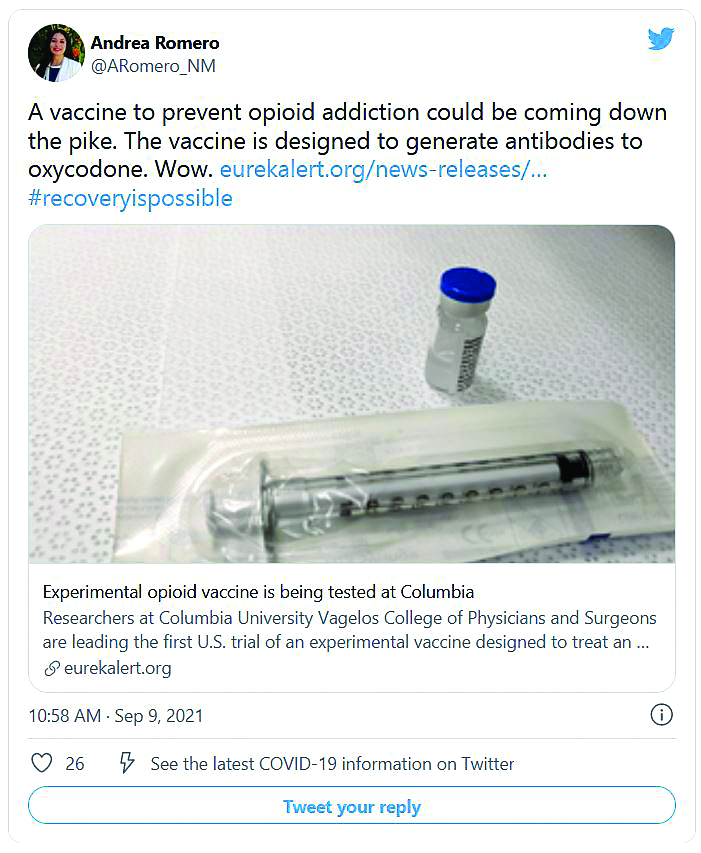
As opioid deaths climb, human trials begin for vaccine
Opioid-related drug overdose deaths in the United States exploded to an estimated record high of 69,031 people in 2020, topping the 49,860 deaths logged in 2019, according to a new report from the Centers for Disease Control and Prevention. Most of the deaths involved synthetic opioids such as fentanyl.
President Joe Biden has pledged more than $10 billion to expand access to prevention, treatment, and recovery services. The money is important as people receiving treatment for opioid use disorder have a high risk for relapse, and that means a high risk for opioid overdose.
Now, researchers are studying a possible bridge to successful recovery: A vaccine that could blunt the drugs’ ability to cause harm.
The first such vaccines are now entering clinical trials, raising hopes of adding another tool to the antiaddiction armamentarium. But even if the vaccines prove safe and effective, their success could generate some new problems to solve.
An advantage of vaccines is that their effects can last for several months, said trial investigator Sandra Comer, PhD, professor of neurobiology and psychiatry at Columbia University Irving Medical Center, New York. Dropout rates for existing medical therapies for opioid use disorder are as high as 50% at 6 months, and a vaccine could protect people from overdose and give them time to re-enter treatment.
“It serves as a bit of a safety net,” she said.
The first vaccine to enter a trial targets oxycodone. Volunteers are being recruited who have a diagnosis of opioid use disorder but are not being medically treated and are still using opioids. A third of them will receive a placebo vaccine, a third will receive a low-dose injection of vaccine, and the other third will receive a high-dose vaccine.
A shot against oxycodone
Researchers are primarily tracking the safety of the shot, but they’re also looking at whether vaccination prevents the euphoria that opioids usually produce. They expect to enroll 24 people initially but expand to 45 if results look promising.
In response to the shot, the body produces antibodies, proteins that tag oxycodone and keep it from reaching the brain. If the drug can’t reach brain cells, it can’t produce euphoria. And more important for lifesaving effects, it can’t block the brain’s signals to the body to breathe. The vaccine has already performed well in animal studies.
Previous trials of vaccines for cocaine and nicotine failed. Those vaccines made it to the last clinical trial stage, but didn’t prove effective overall. So this time, investigators plan to track antibody levels in participants, examining blood samples for signs of a good immune response to the vaccine.
But even though earlier cocaine and nicotine vaccines didn’t work for everybody, there were some people they seemed to help. This is why investigators involved in opioid vaccine trials want to track immune responses, said Marco Pravetoni, PhD, associate professor of pharmacology and medicine at the University of Minnesota, Minneapolis, whose team will be assessing the blood samples. Ultimately, a doctor might even be able to use this information to tailor vaccine selection to a specific person.
Dr. Pravetoni also said that oxycodone is one of three vaccine targets – the other two are heroin and fentanyl – that researchers hope to combine into a single shot. Recipients might need to have one shot a month for the first 3 to 4 months and then receive annual boosters.
Stopping the pain
The vaccines also raise some issues that need attention, said Cody Wenthur, PharmD, PhD, assistant professor of pharmacy at the University of Wisconsin–Madison, who is not involved in the vaccine trials.
“If you’re vaccinated against oxycodone, you might not have access to adequate pain control if you get into a car accident, for example,” he said.
Clinicians could use other opioids for pain management, but limiting the opioids that the vaccine targets is a “double-edged sword,” said Dr. Wenthur, because vaccinated people could just switch their opioid of choice to one that a vaccine does not inhibit.
Although these issues need to be addressed, vaccines, if successful, will have an important role. Dr. Wenthur noted a survey of pharmacists and pharmacy students that he and his group conducted showing that respondents “overwhelmingly” viewed a potential vaccine as helpful.
said Dr. Pravetoni. He mentioned the 2002 incident when terrorists took over a theater in Moscow and Russian special forces are thought to have used an aerosolized form of fentanyl to incapacitate everyone in the room. More than 100 of the hostages died, and the episode raised the specter of opioids being used in chemical attacks.
Dr. Pravetoni said vaccination could offer protection for first responders, law enforcement or other people whose professions place them at risk for inhalation, either accidentally or through such attacks.
These or other real-world applications for people at risk for exposure are several years away. Dr. Pravetoni said it took 10 years to get to this phase and estimates that, in about 5 years, a vaccine that targets multiple opioid drugs might enter the first clinical trial.
A version of this article first appeared on WebMD.com.
Opioid-related drug overdose deaths in the United States exploded to an estimated record high of 69,031 people in 2020, topping the 49,860 deaths logged in 2019, according to a new report from the Centers for Disease Control and Prevention. Most of the deaths involved synthetic opioids such as fentanyl.
President Joe Biden has pledged more than $10 billion to expand access to prevention, treatment, and recovery services. The money is important as people receiving treatment for opioid use disorder have a high risk for relapse, and that means a high risk for opioid overdose.
Now, researchers are studying a possible bridge to successful recovery: A vaccine that could blunt the drugs’ ability to cause harm.
The first such vaccines are now entering clinical trials, raising hopes of adding another tool to the antiaddiction armamentarium. But even if the vaccines prove safe and effective, their success could generate some new problems to solve.
An advantage of vaccines is that their effects can last for several months, said trial investigator Sandra Comer, PhD, professor of neurobiology and psychiatry at Columbia University Irving Medical Center, New York. Dropout rates for existing medical therapies for opioid use disorder are as high as 50% at 6 months, and a vaccine could protect people from overdose and give them time to re-enter treatment.
“It serves as a bit of a safety net,” she said.
The first vaccine to enter a trial targets oxycodone. Volunteers are being recruited who have a diagnosis of opioid use disorder but are not being medically treated and are still using opioids. A third of them will receive a placebo vaccine, a third will receive a low-dose injection of vaccine, and the other third will receive a high-dose vaccine.
A shot against oxycodone
Researchers are primarily tracking the safety of the shot, but they’re also looking at whether vaccination prevents the euphoria that opioids usually produce. They expect to enroll 24 people initially but expand to 45 if results look promising.
In response to the shot, the body produces antibodies, proteins that tag oxycodone and keep it from reaching the brain. If the drug can’t reach brain cells, it can’t produce euphoria. And more important for lifesaving effects, it can’t block the brain’s signals to the body to breathe. The vaccine has already performed well in animal studies.
Previous trials of vaccines for cocaine and nicotine failed. Those vaccines made it to the last clinical trial stage, but didn’t prove effective overall. So this time, investigators plan to track antibody levels in participants, examining blood samples for signs of a good immune response to the vaccine.
But even though earlier cocaine and nicotine vaccines didn’t work for everybody, there were some people they seemed to help. This is why investigators involved in opioid vaccine trials want to track immune responses, said Marco Pravetoni, PhD, associate professor of pharmacology and medicine at the University of Minnesota, Minneapolis, whose team will be assessing the blood samples. Ultimately, a doctor might even be able to use this information to tailor vaccine selection to a specific person.
Dr. Pravetoni also said that oxycodone is one of three vaccine targets – the other two are heroin and fentanyl – that researchers hope to combine into a single shot. Recipients might need to have one shot a month for the first 3 to 4 months and then receive annual boosters.
Stopping the pain
The vaccines also raise some issues that need attention, said Cody Wenthur, PharmD, PhD, assistant professor of pharmacy at the University of Wisconsin–Madison, who is not involved in the vaccine trials.
“If you’re vaccinated against oxycodone, you might not have access to adequate pain control if you get into a car accident, for example,” he said.
Clinicians could use other opioids for pain management, but limiting the opioids that the vaccine targets is a “double-edged sword,” said Dr. Wenthur, because vaccinated people could just switch their opioid of choice to one that a vaccine does not inhibit.
Although these issues need to be addressed, vaccines, if successful, will have an important role. Dr. Wenthur noted a survey of pharmacists and pharmacy students that he and his group conducted showing that respondents “overwhelmingly” viewed a potential vaccine as helpful.
said Dr. Pravetoni. He mentioned the 2002 incident when terrorists took over a theater in Moscow and Russian special forces are thought to have used an aerosolized form of fentanyl to incapacitate everyone in the room. More than 100 of the hostages died, and the episode raised the specter of opioids being used in chemical attacks.
Dr. Pravetoni said vaccination could offer protection for first responders, law enforcement or other people whose professions place them at risk for inhalation, either accidentally or through such attacks.
These or other real-world applications for people at risk for exposure are several years away. Dr. Pravetoni said it took 10 years to get to this phase and estimates that, in about 5 years, a vaccine that targets multiple opioid drugs might enter the first clinical trial.
A version of this article first appeared on WebMD.com.
Opioid-related drug overdose deaths in the United States exploded to an estimated record high of 69,031 people in 2020, topping the 49,860 deaths logged in 2019, according to a new report from the Centers for Disease Control and Prevention. Most of the deaths involved synthetic opioids such as fentanyl.
President Joe Biden has pledged more than $10 billion to expand access to prevention, treatment, and recovery services. The money is important as people receiving treatment for opioid use disorder have a high risk for relapse, and that means a high risk for opioid overdose.
Now, researchers are studying a possible bridge to successful recovery: A vaccine that could blunt the drugs’ ability to cause harm.
The first such vaccines are now entering clinical trials, raising hopes of adding another tool to the antiaddiction armamentarium. But even if the vaccines prove safe and effective, their success could generate some new problems to solve.
An advantage of vaccines is that their effects can last for several months, said trial investigator Sandra Comer, PhD, professor of neurobiology and psychiatry at Columbia University Irving Medical Center, New York. Dropout rates for existing medical therapies for opioid use disorder are as high as 50% at 6 months, and a vaccine could protect people from overdose and give them time to re-enter treatment.
“It serves as a bit of a safety net,” she said.
The first vaccine to enter a trial targets oxycodone. Volunteers are being recruited who have a diagnosis of opioid use disorder but are not being medically treated and are still using opioids. A third of them will receive a placebo vaccine, a third will receive a low-dose injection of vaccine, and the other third will receive a high-dose vaccine.
A shot against oxycodone
Researchers are primarily tracking the safety of the shot, but they’re also looking at whether vaccination prevents the euphoria that opioids usually produce. They expect to enroll 24 people initially but expand to 45 if results look promising.
In response to the shot, the body produces antibodies, proteins that tag oxycodone and keep it from reaching the brain. If the drug can’t reach brain cells, it can’t produce euphoria. And more important for lifesaving effects, it can’t block the brain’s signals to the body to breathe. The vaccine has already performed well in animal studies.
Previous trials of vaccines for cocaine and nicotine failed. Those vaccines made it to the last clinical trial stage, but didn’t prove effective overall. So this time, investigators plan to track antibody levels in participants, examining blood samples for signs of a good immune response to the vaccine.
But even though earlier cocaine and nicotine vaccines didn’t work for everybody, there were some people they seemed to help. This is why investigators involved in opioid vaccine trials want to track immune responses, said Marco Pravetoni, PhD, associate professor of pharmacology and medicine at the University of Minnesota, Minneapolis, whose team will be assessing the blood samples. Ultimately, a doctor might even be able to use this information to tailor vaccine selection to a specific person.
Dr. Pravetoni also said that oxycodone is one of three vaccine targets – the other two are heroin and fentanyl – that researchers hope to combine into a single shot. Recipients might need to have one shot a month for the first 3 to 4 months and then receive annual boosters.
Stopping the pain
The vaccines also raise some issues that need attention, said Cody Wenthur, PharmD, PhD, assistant professor of pharmacy at the University of Wisconsin–Madison, who is not involved in the vaccine trials.
“If you’re vaccinated against oxycodone, you might not have access to adequate pain control if you get into a car accident, for example,” he said.
Clinicians could use other opioids for pain management, but limiting the opioids that the vaccine targets is a “double-edged sword,” said Dr. Wenthur, because vaccinated people could just switch their opioid of choice to one that a vaccine does not inhibit.
Although these issues need to be addressed, vaccines, if successful, will have an important role. Dr. Wenthur noted a survey of pharmacists and pharmacy students that he and his group conducted showing that respondents “overwhelmingly” viewed a potential vaccine as helpful.
said Dr. Pravetoni. He mentioned the 2002 incident when terrorists took over a theater in Moscow and Russian special forces are thought to have used an aerosolized form of fentanyl to incapacitate everyone in the room. More than 100 of the hostages died, and the episode raised the specter of opioids being used in chemical attacks.
Dr. Pravetoni said vaccination could offer protection for first responders, law enforcement or other people whose professions place them at risk for inhalation, either accidentally or through such attacks.
These or other real-world applications for people at risk for exposure are several years away. Dr. Pravetoni said it took 10 years to get to this phase and estimates that, in about 5 years, a vaccine that targets multiple opioid drugs might enter the first clinical trial.
A version of this article first appeared on WebMD.com.
Opioid overdoses tied to lasting cognitive impairment
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
Opioid overdoses usually aren’t fatal, but a new review of numerous studies, mostly case reports and case series, suggests that they can have long-lasting effects on cognition, possibly because of hypoxia resulting from respiratory depression.
Erin L. Winstanley, PhD, MA, and associates noted in the review that opioids cause about 80% of worldwide deaths from illicit drug use, and the Centers for Disease Control and Prevention’s provisional August 2021 number of more than 88,000 opioid-caused deaths in the United States is the highest ever recorded – a 27% increase over what was reported last December. That number suggests that the opioid epidemic continues to rage, but the study results also show that the neurological consequences of nonfatal overdoses are an important public health problem.
And that’s something that may be overlooked, according to Mark S. Gold, MD, who was not involved with the study and was asked to comment on the review, which was published in the Journal of Addiction Science.
“Assuming that an overdose has no effect on the brain, mood, and behavior is not supported by experience or the literature. He is a University of Florida, Gainesville, Emeritus Eminent Scholar, adjunct professor of psychiatry at Washington University in St. Louis, and a member of the clinical council of Washington University’s Public Health Institute.
A common pattern among patients with opioid use disorder (OUD) is that they undergo treatment with medication-assisted therapy (MAT), only to drop out of treatment and then repeat the treatment at a later date. That suggests that physicians should take a harder look at the limitations of MAT and other treatments, Dr. Gold said.
Although the review found some associations between neurocognitive deficits and opioid overdose, the authors point out that it is difficult to make direct comparisons because of biases and differences in methodology among the included studies. They were not able to reach conclusions about the prevalence of brain injuries following nonfatal opioid overdoses. Few included studies controlled for confounding factors that might contribute to or explain neurocognitive impairments, reported Dr. Winstanley, associate professor in the department of behavioral medicine and psychiatry at the University of West Virginia, Morgantown, and associates.
Still, distinct patterns emerged from the analysis of almost 3,500 subjects in 79 studies in 21 countries. Twenty-nine studies reported diagnoses of leukoencephalopathy, which affects white matter. Spongiform leukoencephalopathy is known to occur secondarily after exposure to a variety of toxic agents, including carbon monoxide poisoning and drugs of abuse. The damage can lead to erosion of higher cerebral function. The condition can occur from 2 to 180 days after a hypoxic brain injury, potentially complicating efforts to attribute it specifically to an opioid overdose. Amnestic syndrome was also reported in some studies. One study found that about 39% of people seeking buprenorphine treatment suffered from neurocognitive impairment.
Dr. Gold called the study’s findings novel and of public health importance. “Each overdose takes a toll on the body, and especially the brain,” he said.
Better documentation needed
The variability in symptoms, as well as their timing, present challenges to initial treatment, which often occur before a patient reaches the hospital. This is a vital window because the length of time of inadequate respiration because of opioid overdose is likely to predict the extent of brain injury. The duration of inadequate respiration may not be captured in electronic medical records, and emergency departments don’t typically collect toxicology information, which may lead health care providers to attribute neurocognitive impairments to ongoing drug use rather than an acute anoxic or hypoxic episode. Further neurocognitive damage may have a delayed onset, and better documentation of these events could help physicians determine whether those symptoms stem from the acute event.
Dr. Winstanley and associates called for more research, including prospective case-control studies to identify brain changes following opioid-related overdose.
The authors also suggested that physicians might want to consider screening patients who experience prolonged anoxia or hypoxia for neurocognitive impairments and brain injuries. Dr. Gold agreed.
“Clinicians working with OUD patients should take these data to heart and take a comprehensive history of previous overdoses, loss of consciousness, head trauma, and following up on the history with neuropsychological and other tests of brain function,” Dr. Gold said. “After an assessment, rehabilitation and treatment might then be more personalized and effective.”
Dr. Gold had no relevant financial disclosures.
FROM THE JOURNAL OF ADDICTION SCIENCE
Assessing headache severity via migraine symptoms can help predict outcomes
according to an analysis of data from thousands of headache sufferers who recorded variables like pain and duration in a daily digital diary.
“Our hope is that this work serves as foundational basis for better understanding the complexity of headache as a symptom-based condition,” James S. McGinley, PhD, of Vector Psychometric Group in Chapel Hill, N.C., and coauthors wrote. The study was published in Cephalalgia.
To evaluate whether keeping track of daily headache features can produce a useful, predictive score, the researchers reviewed data from migraine patients that were collected via N1‑Headache, a commercial digital health platform. Ultimately, information from 4,380 adults with a self-reported migraine diagnosis was analyzed; the sample was 90% female and their mean age was 37 years. Study participants reported an average of 33 headaches per month over the last 3 months. Nine patient-reported variables were initially considered in calculating the Headache Day Severity (HDS) score: pain intensity, headache duration, aura, pulsating/throbbing pain, unilateral pain, pain aggravation by activity, nausea/vomiting, photophobia, and phonophobia.
After determining that unilateral pain was not a meaningful variable, the researchers’ model found that, for every 1 standard deviation increase in HDS, the patient’s odds of physician visit increased by 71% (odds ratio, 1.71; 95% confidence interval, 1.32-2.21) and the odds of an ED visit increased by 342% (OR, 4.42; 95% CI, 2.23-7.60). They also found that the likelihood of missed work or school increased by 190% (OR, 2.90; 95% CI, 2.56-3.29), the chances of missing household work increased by 237% (OR, 3.37; 95% CI, 3.06-3.72) and the odds of missing other leisure or social activity increased by 228% (OR, 3.28; 95% CI, 2.97-3.64).
Tracking multiple variables
“We encourage all of our patients to monitor their headaches; there are just too many variables to try to keep it in your head,” Robert Cowan, MD, professor of neurology and chief of the division of headache medicine at Stanford (Calif.) University, said in an interview. He referenced a previous study from the University of Washington where patients were asked to track their headaches; that data was then compared against their self-reported headaches at a quarterly physician visit.
“What they found was there was absolutely no correlation with reported frequency of headache at the visit and what was seen in the tracker,” he said. “If patients had a headache in the previous 3 days before their visit, they felt that their headaches were poorly controlled. If they hadn’t, they thought their headaches were under good control. So the value of tracking is pretty clear.”
He added that, while not every headache sufferer needs to track their daily routines and symptoms, once those symptoms interfere with your life on a day-to-day basis, it’s probably time to consider keeping tabs on yourself with a tool of some sort. And while this study’s calculated HDS score supports the idea of migraine’s complexity, it also leaves unanswered the question of how to treat patients with severe symptoms.
“Frequently,” he said, “we’ll see patients who say: ‘I can deal with the pain, but the nausea makes it impossible to work, or the light sensitivity makes it impossible to go outside.’ The big question within the headache community is, can you treat migraine and have it address the whole spectrum, from dizziness to light sensitivity to sound sensitivity to vertigo, or should you be going after individual symptoms? That’s a controversy that rages on; I think most of us go for a combination. We’re in a polypharmacy phase: ‘If nausea is a big problem, take this, but we also try to prevent the whole migraine complex, so take this as well.’ ”
The authors acknowledged their study’s limitations, including the inability to determine how many participants’ migraines were formally diagnosed by a trained medical professional and the lack of generalizability of data from a convenience sample, though they added that patients who independently track their own headaches “may be representative of those who would participate in a clinical trial.” In addition, as seven of the nine features were collected in N1‑Headache on a yes/no scale, they recognized that “increasing the number of response options for each item may improve our ability to measure HDS.”
The study was funded by Amgen through the Competitive Grant Program in Migraine Research. The authors declared several potential conflicts of interest, including receiving funding, research support, salary, and honoraria from various pharmaceutical companies.
according to an analysis of data from thousands of headache sufferers who recorded variables like pain and duration in a daily digital diary.
“Our hope is that this work serves as foundational basis for better understanding the complexity of headache as a symptom-based condition,” James S. McGinley, PhD, of Vector Psychometric Group in Chapel Hill, N.C., and coauthors wrote. The study was published in Cephalalgia.
To evaluate whether keeping track of daily headache features can produce a useful, predictive score, the researchers reviewed data from migraine patients that were collected via N1‑Headache, a commercial digital health platform. Ultimately, information from 4,380 adults with a self-reported migraine diagnosis was analyzed; the sample was 90% female and their mean age was 37 years. Study participants reported an average of 33 headaches per month over the last 3 months. Nine patient-reported variables were initially considered in calculating the Headache Day Severity (HDS) score: pain intensity, headache duration, aura, pulsating/throbbing pain, unilateral pain, pain aggravation by activity, nausea/vomiting, photophobia, and phonophobia.
After determining that unilateral pain was not a meaningful variable, the researchers’ model found that, for every 1 standard deviation increase in HDS, the patient’s odds of physician visit increased by 71% (odds ratio, 1.71; 95% confidence interval, 1.32-2.21) and the odds of an ED visit increased by 342% (OR, 4.42; 95% CI, 2.23-7.60). They also found that the likelihood of missed work or school increased by 190% (OR, 2.90; 95% CI, 2.56-3.29), the chances of missing household work increased by 237% (OR, 3.37; 95% CI, 3.06-3.72) and the odds of missing other leisure or social activity increased by 228% (OR, 3.28; 95% CI, 2.97-3.64).
Tracking multiple variables
“We encourage all of our patients to monitor their headaches; there are just too many variables to try to keep it in your head,” Robert Cowan, MD, professor of neurology and chief of the division of headache medicine at Stanford (Calif.) University, said in an interview. He referenced a previous study from the University of Washington where patients were asked to track their headaches; that data was then compared against their self-reported headaches at a quarterly physician visit.
“What they found was there was absolutely no correlation with reported frequency of headache at the visit and what was seen in the tracker,” he said. “If patients had a headache in the previous 3 days before their visit, they felt that their headaches were poorly controlled. If they hadn’t, they thought their headaches were under good control. So the value of tracking is pretty clear.”
He added that, while not every headache sufferer needs to track their daily routines and symptoms, once those symptoms interfere with your life on a day-to-day basis, it’s probably time to consider keeping tabs on yourself with a tool of some sort. And while this study’s calculated HDS score supports the idea of migraine’s complexity, it also leaves unanswered the question of how to treat patients with severe symptoms.
“Frequently,” he said, “we’ll see patients who say: ‘I can deal with the pain, but the nausea makes it impossible to work, or the light sensitivity makes it impossible to go outside.’ The big question within the headache community is, can you treat migraine and have it address the whole spectrum, from dizziness to light sensitivity to sound sensitivity to vertigo, or should you be going after individual symptoms? That’s a controversy that rages on; I think most of us go for a combination. We’re in a polypharmacy phase: ‘If nausea is a big problem, take this, but we also try to prevent the whole migraine complex, so take this as well.’ ”
The authors acknowledged their study’s limitations, including the inability to determine how many participants’ migraines were formally diagnosed by a trained medical professional and the lack of generalizability of data from a convenience sample, though they added that patients who independently track their own headaches “may be representative of those who would participate in a clinical trial.” In addition, as seven of the nine features were collected in N1‑Headache on a yes/no scale, they recognized that “increasing the number of response options for each item may improve our ability to measure HDS.”
The study was funded by Amgen through the Competitive Grant Program in Migraine Research. The authors declared several potential conflicts of interest, including receiving funding, research support, salary, and honoraria from various pharmaceutical companies.
according to an analysis of data from thousands of headache sufferers who recorded variables like pain and duration in a daily digital diary.
“Our hope is that this work serves as foundational basis for better understanding the complexity of headache as a symptom-based condition,” James S. McGinley, PhD, of Vector Psychometric Group in Chapel Hill, N.C., and coauthors wrote. The study was published in Cephalalgia.
To evaluate whether keeping track of daily headache features can produce a useful, predictive score, the researchers reviewed data from migraine patients that were collected via N1‑Headache, a commercial digital health platform. Ultimately, information from 4,380 adults with a self-reported migraine diagnosis was analyzed; the sample was 90% female and their mean age was 37 years. Study participants reported an average of 33 headaches per month over the last 3 months. Nine patient-reported variables were initially considered in calculating the Headache Day Severity (HDS) score: pain intensity, headache duration, aura, pulsating/throbbing pain, unilateral pain, pain aggravation by activity, nausea/vomiting, photophobia, and phonophobia.
After determining that unilateral pain was not a meaningful variable, the researchers’ model found that, for every 1 standard deviation increase in HDS, the patient’s odds of physician visit increased by 71% (odds ratio, 1.71; 95% confidence interval, 1.32-2.21) and the odds of an ED visit increased by 342% (OR, 4.42; 95% CI, 2.23-7.60). They also found that the likelihood of missed work or school increased by 190% (OR, 2.90; 95% CI, 2.56-3.29), the chances of missing household work increased by 237% (OR, 3.37; 95% CI, 3.06-3.72) and the odds of missing other leisure or social activity increased by 228% (OR, 3.28; 95% CI, 2.97-3.64).
Tracking multiple variables
“We encourage all of our patients to monitor their headaches; there are just too many variables to try to keep it in your head,” Robert Cowan, MD, professor of neurology and chief of the division of headache medicine at Stanford (Calif.) University, said in an interview. He referenced a previous study from the University of Washington where patients were asked to track their headaches; that data was then compared against their self-reported headaches at a quarterly physician visit.
“What they found was there was absolutely no correlation with reported frequency of headache at the visit and what was seen in the tracker,” he said. “If patients had a headache in the previous 3 days before their visit, they felt that their headaches were poorly controlled. If they hadn’t, they thought their headaches were under good control. So the value of tracking is pretty clear.”
He added that, while not every headache sufferer needs to track their daily routines and symptoms, once those symptoms interfere with your life on a day-to-day basis, it’s probably time to consider keeping tabs on yourself with a tool of some sort. And while this study’s calculated HDS score supports the idea of migraine’s complexity, it also leaves unanswered the question of how to treat patients with severe symptoms.
“Frequently,” he said, “we’ll see patients who say: ‘I can deal with the pain, but the nausea makes it impossible to work, or the light sensitivity makes it impossible to go outside.’ The big question within the headache community is, can you treat migraine and have it address the whole spectrum, from dizziness to light sensitivity to sound sensitivity to vertigo, or should you be going after individual symptoms? That’s a controversy that rages on; I think most of us go for a combination. We’re in a polypharmacy phase: ‘If nausea is a big problem, take this, but we also try to prevent the whole migraine complex, so take this as well.’ ”
The authors acknowledged their study’s limitations, including the inability to determine how many participants’ migraines were formally diagnosed by a trained medical professional and the lack of generalizability of data from a convenience sample, though they added that patients who independently track their own headaches “may be representative of those who would participate in a clinical trial.” In addition, as seven of the nine features were collected in N1‑Headache on a yes/no scale, they recognized that “increasing the number of response options for each item may improve our ability to measure HDS.”
The study was funded by Amgen through the Competitive Grant Program in Migraine Research. The authors declared several potential conflicts of interest, including receiving funding, research support, salary, and honoraria from various pharmaceutical companies.
FROM CEPHALALGIA
Most muscle pain on statins not a drug effect: SAMSON in print
but by the expectation of such adverse effects, conclude researchers behind the randomized SAMSON trial, now fully published .
It’s common for patients to stop taking their statin because of muscle pain and their belief that the drug itself is to blame. That can sometimes be true, but the SAMSON trial, owing to its unusual design, makes a strong case that such symptoms are usually a nocebo effect.
That is, most statin-related muscle symptoms are likely “driven by the act of taking tablets rather than whether the tablets contain a statin,” concludes the report, which appears in the September 21 issue of the Journal of the American College of Cardiology, with lead authors James P. Howard, PhD, and Frances A. Wood, MPhil, Imperial College London.
SAMSON had been presented at the American Heart Association Scientific Sessions 2020 virtual meeting, covered at the time by this news organization, and simultaneously published in abbreviated form as correspondence in the New England Journal of Medicine.
“SAMSON suggests that the bulk of statin-related intolerable side effects arise from the taking of a tablet, not from statin therapy per se,” agrees an editorial accompanying the new publication.
“The study also demonstrates that the informal experimentation of stopping and restarting a statin to evaluate symptom resolution and reinduction without use of a placebo leads to nocebo symptoms misattributed to the statin,” writes Peter P. Toth, MD, PhD, Johns Hopkins University, Baltimore.
Statin intolerance, he continues, “warrants considerable further investigation, because it undermines standard of care for a very large number of patients worldwide,” leaving them vulnerable to atherosclerotic cardiovascular disease events. “Aches and pains are a fact of life; just because a patient has them does not mean they should be attributed to their statin.”
SAMSON assigned 35 men and 25 women to take atorvastatin 20 mg/day, its matching placebo, or neither pill each for 1 month in randomly alternating order for 12 months, with double-blinding, such that each of the three regimens was maintained for a total of 4 months.
The patients, 77% of whom were prescribed statins for primary prevention and all of whom had a history of stopping the drugs because of adverse effects, documented the severity of any perceived adverse effects on a smartphone app, with a “symptom score” ranging from 0 to 100.
The symptom score averaged 8.0 in months when no tablet was taken, but it was much higher in other months: 15.4 in placebo-pill months and 16.3 in months when atorvastatin was taken. The no-tablet score was significantly lower (P < .001) than either of the two other scores, which themselves were not significantly different from each other.
Eleven patients were unable to complete all 12 one-month segments of the trial, including five because of severe symptoms, but discontinuation was no more likely to occur in the atorvastatin group than in the placebo group.
The authors calculated an overall 0.90 “nocebo ratio” for the study, defined as the difference between symptom intensity on placebo and on no pill, divided by the difference between symptom intensity on atorvastatin and on no pill.
That means, the authors propose, that 90% of the symptom burden felt by patients receiving atorvastatin was also felt on the placebo pill and could be attributed to the nocebo effect.
“Prompt onset and offset of symptoms after starting and stopping tablets is often interpreted by patients and clinicians as evidence of causation. Our data indicate that this is true,” the authors write, but “the causation is from taking a tablet, rather than from the tablet being a statin.”
SAMSON was funded by the British Heart Foundation and supported by the National Institute for Health Research Imperial Biomedical Research Centre and the Imperial Clinical Trials Unit. Dr. Howard is supported by the Wellcome Trust. Dr. Wood declared no conflicts. Disclosures for the other authors are in the report. Dr. Toth discloses serving as a consultant to Amarin, Amgen, AstraZeneca, nio89, Kowa, Merck, Resverlogix, and Theravance; and serving on a speaker’s bureau for Amarin, Amgen, Esperion, and NovoNordisk.
A version of this article first appeared on Medscape.com.
but by the expectation of such adverse effects, conclude researchers behind the randomized SAMSON trial, now fully published .
It’s common for patients to stop taking their statin because of muscle pain and their belief that the drug itself is to blame. That can sometimes be true, but the SAMSON trial, owing to its unusual design, makes a strong case that such symptoms are usually a nocebo effect.
That is, most statin-related muscle symptoms are likely “driven by the act of taking tablets rather than whether the tablets contain a statin,” concludes the report, which appears in the September 21 issue of the Journal of the American College of Cardiology, with lead authors James P. Howard, PhD, and Frances A. Wood, MPhil, Imperial College London.
SAMSON had been presented at the American Heart Association Scientific Sessions 2020 virtual meeting, covered at the time by this news organization, and simultaneously published in abbreviated form as correspondence in the New England Journal of Medicine.
“SAMSON suggests that the bulk of statin-related intolerable side effects arise from the taking of a tablet, not from statin therapy per se,” agrees an editorial accompanying the new publication.
“The study also demonstrates that the informal experimentation of stopping and restarting a statin to evaluate symptom resolution and reinduction without use of a placebo leads to nocebo symptoms misattributed to the statin,” writes Peter P. Toth, MD, PhD, Johns Hopkins University, Baltimore.
Statin intolerance, he continues, “warrants considerable further investigation, because it undermines standard of care for a very large number of patients worldwide,” leaving them vulnerable to atherosclerotic cardiovascular disease events. “Aches and pains are a fact of life; just because a patient has them does not mean they should be attributed to their statin.”
SAMSON assigned 35 men and 25 women to take atorvastatin 20 mg/day, its matching placebo, or neither pill each for 1 month in randomly alternating order for 12 months, with double-blinding, such that each of the three regimens was maintained for a total of 4 months.
The patients, 77% of whom were prescribed statins for primary prevention and all of whom had a history of stopping the drugs because of adverse effects, documented the severity of any perceived adverse effects on a smartphone app, with a “symptom score” ranging from 0 to 100.
The symptom score averaged 8.0 in months when no tablet was taken, but it was much higher in other months: 15.4 in placebo-pill months and 16.3 in months when atorvastatin was taken. The no-tablet score was significantly lower (P < .001) than either of the two other scores, which themselves were not significantly different from each other.
Eleven patients were unable to complete all 12 one-month segments of the trial, including five because of severe symptoms, but discontinuation was no more likely to occur in the atorvastatin group than in the placebo group.
The authors calculated an overall 0.90 “nocebo ratio” for the study, defined as the difference between symptom intensity on placebo and on no pill, divided by the difference between symptom intensity on atorvastatin and on no pill.
That means, the authors propose, that 90% of the symptom burden felt by patients receiving atorvastatin was also felt on the placebo pill and could be attributed to the nocebo effect.
“Prompt onset and offset of symptoms after starting and stopping tablets is often interpreted by patients and clinicians as evidence of causation. Our data indicate that this is true,” the authors write, but “the causation is from taking a tablet, rather than from the tablet being a statin.”
SAMSON was funded by the British Heart Foundation and supported by the National Institute for Health Research Imperial Biomedical Research Centre and the Imperial Clinical Trials Unit. Dr. Howard is supported by the Wellcome Trust. Dr. Wood declared no conflicts. Disclosures for the other authors are in the report. Dr. Toth discloses serving as a consultant to Amarin, Amgen, AstraZeneca, nio89, Kowa, Merck, Resverlogix, and Theravance; and serving on a speaker’s bureau for Amarin, Amgen, Esperion, and NovoNordisk.
A version of this article first appeared on Medscape.com.
but by the expectation of such adverse effects, conclude researchers behind the randomized SAMSON trial, now fully published .
It’s common for patients to stop taking their statin because of muscle pain and their belief that the drug itself is to blame. That can sometimes be true, but the SAMSON trial, owing to its unusual design, makes a strong case that such symptoms are usually a nocebo effect.
That is, most statin-related muscle symptoms are likely “driven by the act of taking tablets rather than whether the tablets contain a statin,” concludes the report, which appears in the September 21 issue of the Journal of the American College of Cardiology, with lead authors James P. Howard, PhD, and Frances A. Wood, MPhil, Imperial College London.
SAMSON had been presented at the American Heart Association Scientific Sessions 2020 virtual meeting, covered at the time by this news organization, and simultaneously published in abbreviated form as correspondence in the New England Journal of Medicine.
“SAMSON suggests that the bulk of statin-related intolerable side effects arise from the taking of a tablet, not from statin therapy per se,” agrees an editorial accompanying the new publication.
“The study also demonstrates that the informal experimentation of stopping and restarting a statin to evaluate symptom resolution and reinduction without use of a placebo leads to nocebo symptoms misattributed to the statin,” writes Peter P. Toth, MD, PhD, Johns Hopkins University, Baltimore.
Statin intolerance, he continues, “warrants considerable further investigation, because it undermines standard of care for a very large number of patients worldwide,” leaving them vulnerable to atherosclerotic cardiovascular disease events. “Aches and pains are a fact of life; just because a patient has them does not mean they should be attributed to their statin.”
SAMSON assigned 35 men and 25 women to take atorvastatin 20 mg/day, its matching placebo, or neither pill each for 1 month in randomly alternating order for 12 months, with double-blinding, such that each of the three regimens was maintained for a total of 4 months.
The patients, 77% of whom were prescribed statins for primary prevention and all of whom had a history of stopping the drugs because of adverse effects, documented the severity of any perceived adverse effects on a smartphone app, with a “symptom score” ranging from 0 to 100.
The symptom score averaged 8.0 in months when no tablet was taken, but it was much higher in other months: 15.4 in placebo-pill months and 16.3 in months when atorvastatin was taken. The no-tablet score was significantly lower (P < .001) than either of the two other scores, which themselves were not significantly different from each other.
Eleven patients were unable to complete all 12 one-month segments of the trial, including five because of severe symptoms, but discontinuation was no more likely to occur in the atorvastatin group than in the placebo group.
The authors calculated an overall 0.90 “nocebo ratio” for the study, defined as the difference between symptom intensity on placebo and on no pill, divided by the difference between symptom intensity on atorvastatin and on no pill.
That means, the authors propose, that 90% of the symptom burden felt by patients receiving atorvastatin was also felt on the placebo pill and could be attributed to the nocebo effect.
“Prompt onset and offset of symptoms after starting and stopping tablets is often interpreted by patients and clinicians as evidence of causation. Our data indicate that this is true,” the authors write, but “the causation is from taking a tablet, rather than from the tablet being a statin.”
SAMSON was funded by the British Heart Foundation and supported by the National Institute for Health Research Imperial Biomedical Research Centre and the Imperial Clinical Trials Unit. Dr. Howard is supported by the Wellcome Trust. Dr. Wood declared no conflicts. Disclosures for the other authors are in the report. Dr. Toth discloses serving as a consultant to Amarin, Amgen, AstraZeneca, nio89, Kowa, Merck, Resverlogix, and Theravance; and serving on a speaker’s bureau for Amarin, Amgen, Esperion, and NovoNordisk.
A version of this article first appeared on Medscape.com.
Provider Perceptions of Opioid Safety Measures in VHA Emergency Departments and Urgent Care Centers
The United States is facing an opioid crisis in which approximately 10 million people have misused opioids in the past year, and an estimated 2 million people have an opioid use disorder (OUD).1 Compared with the general population, veterans treated in the Veterans Health Administration (VHA) facilities are at nearly twice the risk for accidental opioid overdose.2 The implementation of opioid safety measures in VHA facilities across all care settings is a priority in addressing this public health crisis. Hence, VHA leadership is working to minimize veteran risk of fatal opioid overdoses and to increase veteran access to medication-assisted treatments (MAT) for OUD.3
Since the administration of our survey, the VHA has shifted to using the term medication for opioid use disorder (MOUD) instead of MAT for OUD. However, for consistency with the survey we distributed, we use MAT in this analysis.
Acute care settings represent an opportunity to offer appropriate opioid care and treatment options to patients at risk for OUD or opioid-related overdose. VHA facilities offer 2 outpatient acute care settings for emergent ambulatory care: emergency departments (EDs) and urgent care centers (UCCs). Annually, these settings see an estimated 2.5 million patients each year, making EDs and UCCs critical access points of OUD care for veterans. Partnering with key national VHA stakeholders from Pharmacy Benefits Management (PBM), the Office of Emergency Medicine, and Academic Detailing Services (ADS), we developed the Emergency Department Opioid Safety Initiative (ED OSI) aimed at implementing and evaluating opioid safety measures in VHA outpatient acute care settings.
The US Department of Veterans Affairs (VA)/Department of Defense (DoD) Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (CPG) makes recommendations for the initiation and continuation of opioids, risk mitigation, taper of opioids, and opioid therapy for acute pain in VHA facilities.4 Using these recommendations, we developed the broad aims of the ED OSI quality improvement (QI) program. The CPG is clear about the prioritization of safe opioid prescribing practices. New opioid prescriptions written in the ED have been associated with continued and chronic opioid use.5 At the time of prescription, patients not currently and chronically on opioids who receive more than a 3-day supply are at increased risk of becoming long-term opioid users.6 Given the annual volume of patients seen, VHA ED/UCCs are a crucial area for implementing better opioid prescribing practices.
The CPG also includes recommendations for the prescribing or coprescribing of naloxone rescue kits. The administration of naloxone following opioid overdose has been found to be an effective measure against fatal overdose. Increasing provider awareness of common risk factors for opioid-related overdose (eg, frequent ED visits or hospitalizations) helps facilitate a discussion on naloxone prescribing at discharge. Prior studies provide evidence that naloxone distribution and accompanying education also are effective in reducing opioid overdose mortalityand ED visits related to adverse opioid-related events.7,8
Similarly, the guidelines provide recommendations for the use of MAT for veterans with OUD. MAT for OUD is considered a first-line treatment option for patients with moderate-to-severe OUD. When used to treat patients with unsafe opioid use, this treatment helps alleviate symptoms of withdrawal, which can increase opioid taper adherence and has a protective effect against opioid overdose mortality.9 MAT initiated in the ED can increase patient engagement to addiction services.10
These 3 CPG recommendations serve as the basis for the broad goals of the ED OSI program. We aim to develop, implement, and evaluate programs and initiatives to (aim 1) reduce inappropriate opioid prescribing from VHA EDs; (aim 2) increase naloxone distribution from VHA EDs; and (aim 3) increase access to MAT initiation from VHA EDs through the implementation of ED-based MAT-initiation programs with EDs across the VHA. Aim 1 was a focused and strategic QI effort to implement an ED-based program to reduce inappropriate opioid prescribing. The ED OSI prescribing program offered a 4-step bundled approach: (1) sharing of opioid prescribing dashboard data with ED medical director and academic detailer; (2) education of ED providers and implementation of toolkit resources; (3) academic detailers conduct audit and feedback session(s) with highest prescribers; and (4) quarterly reports of opioid prescribing data to ED providers.
Results from the pilot suggested that our program was associated with accelerating the rate at which ED prescribing rates decreased.11 In addition, the pilot found that ED-based QI initiatives in VHA facilities are a feasible practice. As we work to develop and implement the next 2 phases of the QI program, a major consideration is to identify facilitators and address any existing barriers to the implementation of naloxone distribution (aim 2) and MAT-initiation (aim 3) programs for treatment-naïve patients from VHA EDs. To date, there have been no recent published studies examining the barriers and facilitators to use or implementation of MAT initiation or naloxone distribution in VHA facilities or, more specifically, from VHA EDs.12 As part of our QI program, we set out to better understand VHA ED provider perceptions of barriers and facilitators to implementation of programs aimed at increasing naloxone distribution and initiation of MAT for treatment-naïve patients in the ED.
Methods
This project received a QI designation from the Office of PBM Academic Detailing Service Institutional Review Board at the Edward Hines, Jr. Veterans Affairs Hospital VA Medical Center (VAMC). This designation was reviewed and approved by the Rocky Mountain Regional VAMC Research and Development service. In addition, we received national union approval to disseminate this survey nationally across all VA Integrated Service Networks (VISNs).
Survey
We worked with VHA subject matter experts, key stakeholders, and the VA Collaborative Evaluation Center (VACE) to develop the survey. Subject matter experts and stakeholders included VHA emergency medicine leadership, ADS leadership, and mental health and substance treatment providers. VACE is an interdisciplinary group of mixed-method researchers. The survey questions aimed to capture perceptions and experiences regarding naloxone distribution and new MAT initiation of VHA ED/UCC providers.
We used a variety of survey question formats. Close-ended questions with a predefined list of answer options were used to capture discrete domains, such as demographic information, comfort level, and experience level. To capture health care provider (HCP) perceptions on barriers and facilitators, we used multiple-answer multiple-choice questions. Built into this question format was a free-response option, which allowed respondents to offer additional barriers or facilitators. Respondents also had the option of not answering individual questions.
We identified physicians, nurse practitioners (NPs), and physician assistants (PAs) who saw at least 100 patients in the ED or UCC in at least one 3-month period in the prior year and obtained an email address for each. In total, 2228 ED or UCC providers across 132 facilities were emailed a survey; 1883 (84.5%) were ED providers and 345 (15.5%) were UCC providers.
We used Research Electronic Data Capture (REDCap) software to build and disseminate the survey via email. Surveys were initially disseminated in late January 2019. During the 3-month survey period, recipients received 3 automated email reminders from REDCap to complete the survey. Survey data were exported from REDCap. Results were analyzed using descriptive statistics analyses with Microsoft Excel.
Results
One respondent received the survey in error and was excluded from the analysis. The survey response rate was 16.7%: 372 responses from 103 unique facilities. Each VISN had a mean 20 respondents. The majority of respondents (n = 286, 76.9%) worked in highly complex level 1 facilities characterized by high patient volume and more high-risk patients and were teaching and research facilities. Respondents were asked to describe their most recent ED or UCC role. While 281 respondents (75.5%) were medical doctors, 61 respondents (16.4%) were NPs, 30 (8.1%) were PAs, and 26 (7.0%) were ED/UCC chiefs or medical directors (Table 1). Most respondents (80.4%) reported at least 10 years of health care experience.
The majority of respondents (72.9%) believed that HCPs at their VHA facility should be prescribing naloxone. When asked to specify which HCPs should be prescribing naloxone, most HCP respondents selected pharmacists (76.4%) and substance abuse providers (71.6%). Less than half of respondents (45.0%) felt that VA ED/UCC providers also should be prescribing naloxone. However, 58.1% of most HCP respondents reported being comfortable or very comfortable with prescribing naloxone to a patient in the ED or UCC who already had an existing prescription of opioids. Similarly, 52.7% of respondents reported being comfortable or very comfortable with coprescribing naloxone when discharging a patient with an opioid prescription from the ED/UCC. Notably, while 36.7% of PAs reported being comfortable/very comfortable coprescribing naloxone, 46.7% reported being comfortable/very comfortable prescribing naloxone to a patient with an existing opioid prescription. Physicians and NPs expressed similar levels of comfort with coprescribing and prescribing naloxone.
Respondents across provider types indicated a number of barriers to prescribing naloxone to medically appropriate patients (Table 2). Many respondents indicated prescribing naloxone was beyond the ED/UCC provider scope of practice (35.2%), followed by the perceived stigma associated with naloxone (33.3%), time required to prescribe naloxone (23.9%), and concern with patient’s ability to use naloxone (22.8%).
Facilitators for prescribing naloxone to medically appropriate patients identified by HCP respondents included pharmacist help and education (44.6%), patient knowledge of medication options (31.7%), societal shift away from opioids for pain management (28.0%), facility leadership (26.9%), and patient interest in safe opioid usage (26.6%) (Table 3). In addition, NPs specifically endorsed
Less than 6.8% of HCP respondents indicated that they were comfortable using MAT. Meanwhile, 42.1% of respondents reported being aware of MAT but not familiar with it, and 23.5% reported that they were unaware of MAT. Correspondingly, 301 of the 372 (88.5%) HCP respondents indicated that they had not prescribed MAT in the past year. Across HCP types, only 24.1% indicated that it is the role of VA ED or UCC providers to prescribe MAT when medically appropriate and subsequently refer patients to substance abuse treatment for follow-up (just 7.1% of PAs endorsed this). Furthermore, 6.5% and 18.8% of HCP respondents indicated that their facility leadership was very supportive and supportive, respectively, of MAT for OUD prescribing.
Barriers to MAT initiation indicated by HCP respondents included limited scope of ED and UCC practice (53.2%), unclear follow-up/referral process (50.3%), time (29.8%), and discomfort (28.2%). Nearly one-third of NPs (27.9%) identified patient willingness/ability as a barrier to MAT initiation (Table 4).
Facilitators of MAT initiation in the ED or UCC included VHA same-day treatment options (34.9%), patient desire (32.5%), pharmacist help/education (27.4%), and psychiatric social workers in the ED or UCC (25.3%). Some NPs (23.0%) and PAs (26.7%) also indicated that having time to educate veterans about the medication would be a facilitator (Table 5). Facility leadership support was considered a facilitator by 30% of PAs.
Discussion
To the best of our knowledge, there have not been any studies examining HCP perceptions of the barriers and facilitators to naloxone distribution or the initiation of MAT in VHA ED and UCCs. Veterans are at an increased risk of overdose when compared with the general population, and increasing access to opioid safety measures (eg, safer prescribing practices, naloxone distribution) and treatment with MAT for OUD across all clinical settings has been a VHA priority.3
National guidance from VHA leadership, the Centers for Disease Control and Prevention (CDC), the US Surgeon General, and the US Department of Health and Human Services (HHS) call for an all-hands-on-deck approach to combatting opioid overdose with naloxone distribution or MAT (such as buprenorphine) initiation.13 VHA ED and UCC settings provide acute outpatient care to patients with medical or psychiatric illnesses or injuries that the patient believes requires emergent or immediate medical attention or for which there is a critical need for treatment to prevent deterioration of the condition or the possible impairment of recovery.14 However, ED and UCC environments are often regarded as settings meant to stabilize a patient until they can be seen by a primary care or long-term care provider.
A major barrier identified by HCPs was that MAT for OUD was outside their ED/UCC scope of practice, which suggests a need for a top-down or peer-to-peer reexamination of the role of HCPs in ED/UCC settings. Any naloxone distribution and/or MAT-initiation program in VHA ED/UCCs should consider education about the role of ED/UCC HCPs in opioid safety and treatment.
Only 25.3% of HCPs reported that their facility leadership was supportive or very supportive of MAT prescribing. This suggests that facility leadership should be engaged in any efforts to implement a MAT-initiation program in the facility’s ED. Engaging leadership in efforts to implement ED-based MAT programs will allow for a better understanding of leadership goals as related to opioid safety and an opportunity to address concerns regarding prescribing MAT in the ED. We recommend engaging facility leadership early in MAT implementation efforts. Respectively, 12.4% and 28.2% of HCP respondents reported discomfort prescribing naloxone or using MAT, suggesting a need for more education. Similarly, only 6.8% of HCPs reported comfort with using MAT.
A consideration for implementing ED/UCC-based MAT should be the inclusion of a training component. An evidence-based clinical treatment pathway that is appropriate to the ED/UCC setting and facility on the administration of MAT also could be beneficial. A clinical treatment pathway that includes ED/UCC-initiated discharge recommendations would address HCP concerns of unclear follow-up plans and system for referral of care. To this end, a key implementation task is coordinating with other outpatient services (eg, pain management clinic, substance use disorder treatment clinic) equipped for long-term patient follow-up to develop a system for referral of care. For example, as part of the clinical treatment pathway, an ED can develop a system of referral for patients initiated on MAT in the ED in which patients are referred for follow-up at the facility’s substance use disorder treatment clinic to be seen within 72 hours to continue the administration of MAT (such as buprenorphine).
In addition to HCP education, results suggest that patient/veteran education regarding naloxone and/or MAT should be considered. HCPs indicated that having help from a pharmacist to educate the patient about the medications would be a facilitator to naloxone distribution and MAT initiation. Similarly, patient knowledge of the medications also was endorsed as a facilitator. As such, a consideration for any future ED/UCC-based naloxone distribution or MAT-initiation programs in the VHA should be patient education whether by a clinically trained professional or an educational campaign for veterans.
Expanded naloxone distribution and initiation of MAT for OUD for EDs/UCCs across the VHA could impact the lives of veterans on long-term opioid therapy, with OUD, or who are otherwise at risk for opioid overdose. Steps taken to address the barriers and leverage the facilitators identified by HCP respondents can greatly reduce current obstacles to widespread implementation of ED/UCC-based naloxone distribution and MAT initiation nationally within the VHA.
Limitations
This survey had a low response rate (16.7%). One potential explanation for the low response rate is that when the survey was deployed, many of the VHA ED/UCC physicians were per-diem employees. Per-diem physicians may be less engaged and aware of site facilitators or barriers to naloxone and MAT prescribing. This, too, may have potentially skewed the collected data. However, the survey did not ask HCPs to disclose their employment status; thus, exact rates of per diem respondents are unknown.
We aimed to capture only self-perceived barriers to prescribing naloxone and MAT in the ED, but we did not capture or measure HCP respondent’s actual prescribing rates of MAT or naloxone. Understanding HCP perceptions of naloxone distribution and MAT initiation in the ED may have been further informed by comparing HCP responses to their actual clinical practice as related to their prescribing of these medications. In future research, we will link HCPs with the actual numbers of naloxone and MAT medications prescribed. Additionally, we do not know how many of these barriers or proposed facilitators will impact clinical practice.
Conclusions
A key aim for VHA leadership is to increase veteran access to naloxone distribution and MAT for OUD across clinical areas. The present study aimed to identify HCP perceptions of barriers and facilitators to the naloxone distribution and MAT-initiation programs in VHA ED/UCCs to inform the development of a targeted QI program to implement these opioid safety measures. Although the survey yielded a low response rate, results allowed us to identify important action items for our QI program, such as the development of clear protocols, follow-up plans, and systems for referral of care and HCP educational materials related to MAT and naloxone. We hope this work will serve as the basis for ED/UCC-tailored programs that can provide customized educational programs for HCPs designed to overcome known barriers to naloxone and MAT initiation.
Acknowledgments
This work was supported by the VA Office of Specialty Care Services 10P11 and through funding provided by the Comprehensive Addiction and Recovery Act (CARA).
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the united states: results from the 2018 National Survey on Drug Use and Health. Published August 2019. Accessed August 20, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
2. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. US Department of Veterans Affairs, Pharmacy Benefits Management Service. Recommendations for issuing naloxone rescue for the VA opioid overdose education and naloxone distribution (OEND) program. Published August 2016. Accessed August 20, 2021. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/Naloxone_HCl_Rescue_Kits_Recommendations_for_Use.pdf
4. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. doi:10.1056/NEJMsa1610524
6. Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265-269. Published 2017 Mar 17. doi:10.15585/mmwr.mm6610a1
7. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163. doi:10.1097/ADM.0000000000000034
8. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for Pain. Ann Intern Med. 2016;165(4):245-252. doi:10.7326/M15-2771
9. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
10. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
11. Dieujuste N, Johnson-Koenke R, Christopher M, et al. Feasibility study of a quasi-experimental regional opioid safety prescribing program in Veterans Health Administration emergency departments. Acad Emerg Med. 2020;27(8):734-741. doi:10.1111/acem.13980
12. Mackey K, Veazie S, Anderson J, Bourne D, Peterson K. Evidence brief: barriers and facilitators to use of medications for opioid use disorder. Published July 2017. Accessed August 20, 2021. http://www.ncbi.nlm.nih.gov/books/NBK549203/
13. US Department of Health and Human Services, Office of the Surgeon General. Naloxone: the opioid reversal drug that saves lives. Published December 2018. Accessed August 20, 2021. https://www.hhs.gov/opioids/sites/default/files/2018-12/naloxone-coprescribing-guidance.pdf
14. US Department of Veterans Affairs, Veterans Health Administration. Chapter 256: Emergency department (ED) and urgent care clinic (UCC). Updated October 3, 2016. Accessed August 20, 2021. https://www.cfm.va.gov/til/space/spChapter256.pdf.
The United States is facing an opioid crisis in which approximately 10 million people have misused opioids in the past year, and an estimated 2 million people have an opioid use disorder (OUD).1 Compared with the general population, veterans treated in the Veterans Health Administration (VHA) facilities are at nearly twice the risk for accidental opioid overdose.2 The implementation of opioid safety measures in VHA facilities across all care settings is a priority in addressing this public health crisis. Hence, VHA leadership is working to minimize veteran risk of fatal opioid overdoses and to increase veteran access to medication-assisted treatments (MAT) for OUD.3
Since the administration of our survey, the VHA has shifted to using the term medication for opioid use disorder (MOUD) instead of MAT for OUD. However, for consistency with the survey we distributed, we use MAT in this analysis.
Acute care settings represent an opportunity to offer appropriate opioid care and treatment options to patients at risk for OUD or opioid-related overdose. VHA facilities offer 2 outpatient acute care settings for emergent ambulatory care: emergency departments (EDs) and urgent care centers (UCCs). Annually, these settings see an estimated 2.5 million patients each year, making EDs and UCCs critical access points of OUD care for veterans. Partnering with key national VHA stakeholders from Pharmacy Benefits Management (PBM), the Office of Emergency Medicine, and Academic Detailing Services (ADS), we developed the Emergency Department Opioid Safety Initiative (ED OSI) aimed at implementing and evaluating opioid safety measures in VHA outpatient acute care settings.
The US Department of Veterans Affairs (VA)/Department of Defense (DoD) Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (CPG) makes recommendations for the initiation and continuation of opioids, risk mitigation, taper of opioids, and opioid therapy for acute pain in VHA facilities.4 Using these recommendations, we developed the broad aims of the ED OSI quality improvement (QI) program. The CPG is clear about the prioritization of safe opioid prescribing practices. New opioid prescriptions written in the ED have been associated with continued and chronic opioid use.5 At the time of prescription, patients not currently and chronically on opioids who receive more than a 3-day supply are at increased risk of becoming long-term opioid users.6 Given the annual volume of patients seen, VHA ED/UCCs are a crucial area for implementing better opioid prescribing practices.
The CPG also includes recommendations for the prescribing or coprescribing of naloxone rescue kits. The administration of naloxone following opioid overdose has been found to be an effective measure against fatal overdose. Increasing provider awareness of common risk factors for opioid-related overdose (eg, frequent ED visits or hospitalizations) helps facilitate a discussion on naloxone prescribing at discharge. Prior studies provide evidence that naloxone distribution and accompanying education also are effective in reducing opioid overdose mortalityand ED visits related to adverse opioid-related events.7,8
Similarly, the guidelines provide recommendations for the use of MAT for veterans with OUD. MAT for OUD is considered a first-line treatment option for patients with moderate-to-severe OUD. When used to treat patients with unsafe opioid use, this treatment helps alleviate symptoms of withdrawal, which can increase opioid taper adherence and has a protective effect against opioid overdose mortality.9 MAT initiated in the ED can increase patient engagement to addiction services.10
These 3 CPG recommendations serve as the basis for the broad goals of the ED OSI program. We aim to develop, implement, and evaluate programs and initiatives to (aim 1) reduce inappropriate opioid prescribing from VHA EDs; (aim 2) increase naloxone distribution from VHA EDs; and (aim 3) increase access to MAT initiation from VHA EDs through the implementation of ED-based MAT-initiation programs with EDs across the VHA. Aim 1 was a focused and strategic QI effort to implement an ED-based program to reduce inappropriate opioid prescribing. The ED OSI prescribing program offered a 4-step bundled approach: (1) sharing of opioid prescribing dashboard data with ED medical director and academic detailer; (2) education of ED providers and implementation of toolkit resources; (3) academic detailers conduct audit and feedback session(s) with highest prescribers; and (4) quarterly reports of opioid prescribing data to ED providers.
Results from the pilot suggested that our program was associated with accelerating the rate at which ED prescribing rates decreased.11 In addition, the pilot found that ED-based QI initiatives in VHA facilities are a feasible practice. As we work to develop and implement the next 2 phases of the QI program, a major consideration is to identify facilitators and address any existing barriers to the implementation of naloxone distribution (aim 2) and MAT-initiation (aim 3) programs for treatment-naïve patients from VHA EDs. To date, there have been no recent published studies examining the barriers and facilitators to use or implementation of MAT initiation or naloxone distribution in VHA facilities or, more specifically, from VHA EDs.12 As part of our QI program, we set out to better understand VHA ED provider perceptions of barriers and facilitators to implementation of programs aimed at increasing naloxone distribution and initiation of MAT for treatment-naïve patients in the ED.
Methods
This project received a QI designation from the Office of PBM Academic Detailing Service Institutional Review Board at the Edward Hines, Jr. Veterans Affairs Hospital VA Medical Center (VAMC). This designation was reviewed and approved by the Rocky Mountain Regional VAMC Research and Development service. In addition, we received national union approval to disseminate this survey nationally across all VA Integrated Service Networks (VISNs).
Survey
We worked with VHA subject matter experts, key stakeholders, and the VA Collaborative Evaluation Center (VACE) to develop the survey. Subject matter experts and stakeholders included VHA emergency medicine leadership, ADS leadership, and mental health and substance treatment providers. VACE is an interdisciplinary group of mixed-method researchers. The survey questions aimed to capture perceptions and experiences regarding naloxone distribution and new MAT initiation of VHA ED/UCC providers.
We used a variety of survey question formats. Close-ended questions with a predefined list of answer options were used to capture discrete domains, such as demographic information, comfort level, and experience level. To capture health care provider (HCP) perceptions on barriers and facilitators, we used multiple-answer multiple-choice questions. Built into this question format was a free-response option, which allowed respondents to offer additional barriers or facilitators. Respondents also had the option of not answering individual questions.
We identified physicians, nurse practitioners (NPs), and physician assistants (PAs) who saw at least 100 patients in the ED or UCC in at least one 3-month period in the prior year and obtained an email address for each. In total, 2228 ED or UCC providers across 132 facilities were emailed a survey; 1883 (84.5%) were ED providers and 345 (15.5%) were UCC providers.
We used Research Electronic Data Capture (REDCap) software to build and disseminate the survey via email. Surveys were initially disseminated in late January 2019. During the 3-month survey period, recipients received 3 automated email reminders from REDCap to complete the survey. Survey data were exported from REDCap. Results were analyzed using descriptive statistics analyses with Microsoft Excel.
Results
One respondent received the survey in error and was excluded from the analysis. The survey response rate was 16.7%: 372 responses from 103 unique facilities. Each VISN had a mean 20 respondents. The majority of respondents (n = 286, 76.9%) worked in highly complex level 1 facilities characterized by high patient volume and more high-risk patients and were teaching and research facilities. Respondents were asked to describe their most recent ED or UCC role. While 281 respondents (75.5%) were medical doctors, 61 respondents (16.4%) were NPs, 30 (8.1%) were PAs, and 26 (7.0%) were ED/UCC chiefs or medical directors (Table 1). Most respondents (80.4%) reported at least 10 years of health care experience.
The majority of respondents (72.9%) believed that HCPs at their VHA facility should be prescribing naloxone. When asked to specify which HCPs should be prescribing naloxone, most HCP respondents selected pharmacists (76.4%) and substance abuse providers (71.6%). Less than half of respondents (45.0%) felt that VA ED/UCC providers also should be prescribing naloxone. However, 58.1% of most HCP respondents reported being comfortable or very comfortable with prescribing naloxone to a patient in the ED or UCC who already had an existing prescription of opioids. Similarly, 52.7% of respondents reported being comfortable or very comfortable with coprescribing naloxone when discharging a patient with an opioid prescription from the ED/UCC. Notably, while 36.7% of PAs reported being comfortable/very comfortable coprescribing naloxone, 46.7% reported being comfortable/very comfortable prescribing naloxone to a patient with an existing opioid prescription. Physicians and NPs expressed similar levels of comfort with coprescribing and prescribing naloxone.
Respondents across provider types indicated a number of barriers to prescribing naloxone to medically appropriate patients (Table 2). Many respondents indicated prescribing naloxone was beyond the ED/UCC provider scope of practice (35.2%), followed by the perceived stigma associated with naloxone (33.3%), time required to prescribe naloxone (23.9%), and concern with patient’s ability to use naloxone (22.8%).
Facilitators for prescribing naloxone to medically appropriate patients identified by HCP respondents included pharmacist help and education (44.6%), patient knowledge of medication options (31.7%), societal shift away from opioids for pain management (28.0%), facility leadership (26.9%), and patient interest in safe opioid usage (26.6%) (Table 3). In addition, NPs specifically endorsed
Less than 6.8% of HCP respondents indicated that they were comfortable using MAT. Meanwhile, 42.1% of respondents reported being aware of MAT but not familiar with it, and 23.5% reported that they were unaware of MAT. Correspondingly, 301 of the 372 (88.5%) HCP respondents indicated that they had not prescribed MAT in the past year. Across HCP types, only 24.1% indicated that it is the role of VA ED or UCC providers to prescribe MAT when medically appropriate and subsequently refer patients to substance abuse treatment for follow-up (just 7.1% of PAs endorsed this). Furthermore, 6.5% and 18.8% of HCP respondents indicated that their facility leadership was very supportive and supportive, respectively, of MAT for OUD prescribing.
Barriers to MAT initiation indicated by HCP respondents included limited scope of ED and UCC practice (53.2%), unclear follow-up/referral process (50.3%), time (29.8%), and discomfort (28.2%). Nearly one-third of NPs (27.9%) identified patient willingness/ability as a barrier to MAT initiation (Table 4).
Facilitators of MAT initiation in the ED or UCC included VHA same-day treatment options (34.9%), patient desire (32.5%), pharmacist help/education (27.4%), and psychiatric social workers in the ED or UCC (25.3%). Some NPs (23.0%) and PAs (26.7%) also indicated that having time to educate veterans about the medication would be a facilitator (Table 5). Facility leadership support was considered a facilitator by 30% of PAs.
Discussion
To the best of our knowledge, there have not been any studies examining HCP perceptions of the barriers and facilitators to naloxone distribution or the initiation of MAT in VHA ED and UCCs. Veterans are at an increased risk of overdose when compared with the general population, and increasing access to opioid safety measures (eg, safer prescribing practices, naloxone distribution) and treatment with MAT for OUD across all clinical settings has been a VHA priority.3
National guidance from VHA leadership, the Centers for Disease Control and Prevention (CDC), the US Surgeon General, and the US Department of Health and Human Services (HHS) call for an all-hands-on-deck approach to combatting opioid overdose with naloxone distribution or MAT (such as buprenorphine) initiation.13 VHA ED and UCC settings provide acute outpatient care to patients with medical or psychiatric illnesses or injuries that the patient believes requires emergent or immediate medical attention or for which there is a critical need for treatment to prevent deterioration of the condition or the possible impairment of recovery.14 However, ED and UCC environments are often regarded as settings meant to stabilize a patient until they can be seen by a primary care or long-term care provider.
A major barrier identified by HCPs was that MAT for OUD was outside their ED/UCC scope of practice, which suggests a need for a top-down or peer-to-peer reexamination of the role of HCPs in ED/UCC settings. Any naloxone distribution and/or MAT-initiation program in VHA ED/UCCs should consider education about the role of ED/UCC HCPs in opioid safety and treatment.
Only 25.3% of HCPs reported that their facility leadership was supportive or very supportive of MAT prescribing. This suggests that facility leadership should be engaged in any efforts to implement a MAT-initiation program in the facility’s ED. Engaging leadership in efforts to implement ED-based MAT programs will allow for a better understanding of leadership goals as related to opioid safety and an opportunity to address concerns regarding prescribing MAT in the ED. We recommend engaging facility leadership early in MAT implementation efforts. Respectively, 12.4% and 28.2% of HCP respondents reported discomfort prescribing naloxone or using MAT, suggesting a need for more education. Similarly, only 6.8% of HCPs reported comfort with using MAT.
A consideration for implementing ED/UCC-based MAT should be the inclusion of a training component. An evidence-based clinical treatment pathway that is appropriate to the ED/UCC setting and facility on the administration of MAT also could be beneficial. A clinical treatment pathway that includes ED/UCC-initiated discharge recommendations would address HCP concerns of unclear follow-up plans and system for referral of care. To this end, a key implementation task is coordinating with other outpatient services (eg, pain management clinic, substance use disorder treatment clinic) equipped for long-term patient follow-up to develop a system for referral of care. For example, as part of the clinical treatment pathway, an ED can develop a system of referral for patients initiated on MAT in the ED in which patients are referred for follow-up at the facility’s substance use disorder treatment clinic to be seen within 72 hours to continue the administration of MAT (such as buprenorphine).
In addition to HCP education, results suggest that patient/veteran education regarding naloxone and/or MAT should be considered. HCPs indicated that having help from a pharmacist to educate the patient about the medications would be a facilitator to naloxone distribution and MAT initiation. Similarly, patient knowledge of the medications also was endorsed as a facilitator. As such, a consideration for any future ED/UCC-based naloxone distribution or MAT-initiation programs in the VHA should be patient education whether by a clinically trained professional or an educational campaign for veterans.
Expanded naloxone distribution and initiation of MAT for OUD for EDs/UCCs across the VHA could impact the lives of veterans on long-term opioid therapy, with OUD, or who are otherwise at risk for opioid overdose. Steps taken to address the barriers and leverage the facilitators identified by HCP respondents can greatly reduce current obstacles to widespread implementation of ED/UCC-based naloxone distribution and MAT initiation nationally within the VHA.
Limitations
This survey had a low response rate (16.7%). One potential explanation for the low response rate is that when the survey was deployed, many of the VHA ED/UCC physicians were per-diem employees. Per-diem physicians may be less engaged and aware of site facilitators or barriers to naloxone and MAT prescribing. This, too, may have potentially skewed the collected data. However, the survey did not ask HCPs to disclose their employment status; thus, exact rates of per diem respondents are unknown.
We aimed to capture only self-perceived barriers to prescribing naloxone and MAT in the ED, but we did not capture or measure HCP respondent’s actual prescribing rates of MAT or naloxone. Understanding HCP perceptions of naloxone distribution and MAT initiation in the ED may have been further informed by comparing HCP responses to their actual clinical practice as related to their prescribing of these medications. In future research, we will link HCPs with the actual numbers of naloxone and MAT medications prescribed. Additionally, we do not know how many of these barriers or proposed facilitators will impact clinical practice.
Conclusions
A key aim for VHA leadership is to increase veteran access to naloxone distribution and MAT for OUD across clinical areas. The present study aimed to identify HCP perceptions of barriers and facilitators to the naloxone distribution and MAT-initiation programs in VHA ED/UCCs to inform the development of a targeted QI program to implement these opioid safety measures. Although the survey yielded a low response rate, results allowed us to identify important action items for our QI program, such as the development of clear protocols, follow-up plans, and systems for referral of care and HCP educational materials related to MAT and naloxone. We hope this work will serve as the basis for ED/UCC-tailored programs that can provide customized educational programs for HCPs designed to overcome known barriers to naloxone and MAT initiation.
Acknowledgments
This work was supported by the VA Office of Specialty Care Services 10P11 and through funding provided by the Comprehensive Addiction and Recovery Act (CARA).
The United States is facing an opioid crisis in which approximately 10 million people have misused opioids in the past year, and an estimated 2 million people have an opioid use disorder (OUD).1 Compared with the general population, veterans treated in the Veterans Health Administration (VHA) facilities are at nearly twice the risk for accidental opioid overdose.2 The implementation of opioid safety measures in VHA facilities across all care settings is a priority in addressing this public health crisis. Hence, VHA leadership is working to minimize veteran risk of fatal opioid overdoses and to increase veteran access to medication-assisted treatments (MAT) for OUD.3
Since the administration of our survey, the VHA has shifted to using the term medication for opioid use disorder (MOUD) instead of MAT for OUD. However, for consistency with the survey we distributed, we use MAT in this analysis.
Acute care settings represent an opportunity to offer appropriate opioid care and treatment options to patients at risk for OUD or opioid-related overdose. VHA facilities offer 2 outpatient acute care settings for emergent ambulatory care: emergency departments (EDs) and urgent care centers (UCCs). Annually, these settings see an estimated 2.5 million patients each year, making EDs and UCCs critical access points of OUD care for veterans. Partnering with key national VHA stakeholders from Pharmacy Benefits Management (PBM), the Office of Emergency Medicine, and Academic Detailing Services (ADS), we developed the Emergency Department Opioid Safety Initiative (ED OSI) aimed at implementing and evaluating opioid safety measures in VHA outpatient acute care settings.
The US Department of Veterans Affairs (VA)/Department of Defense (DoD) Clinical Practice Guidelines for Opioid Therapy for Chronic Pain (CPG) makes recommendations for the initiation and continuation of opioids, risk mitigation, taper of opioids, and opioid therapy for acute pain in VHA facilities.4 Using these recommendations, we developed the broad aims of the ED OSI quality improvement (QI) program. The CPG is clear about the prioritization of safe opioid prescribing practices. New opioid prescriptions written in the ED have been associated with continued and chronic opioid use.5 At the time of prescription, patients not currently and chronically on opioids who receive more than a 3-day supply are at increased risk of becoming long-term opioid users.6 Given the annual volume of patients seen, VHA ED/UCCs are a crucial area for implementing better opioid prescribing practices.
The CPG also includes recommendations for the prescribing or coprescribing of naloxone rescue kits. The administration of naloxone following opioid overdose has been found to be an effective measure against fatal overdose. Increasing provider awareness of common risk factors for opioid-related overdose (eg, frequent ED visits or hospitalizations) helps facilitate a discussion on naloxone prescribing at discharge. Prior studies provide evidence that naloxone distribution and accompanying education also are effective in reducing opioid overdose mortalityand ED visits related to adverse opioid-related events.7,8
Similarly, the guidelines provide recommendations for the use of MAT for veterans with OUD. MAT for OUD is considered a first-line treatment option for patients with moderate-to-severe OUD. When used to treat patients with unsafe opioid use, this treatment helps alleviate symptoms of withdrawal, which can increase opioid taper adherence and has a protective effect against opioid overdose mortality.9 MAT initiated in the ED can increase patient engagement to addiction services.10
These 3 CPG recommendations serve as the basis for the broad goals of the ED OSI program. We aim to develop, implement, and evaluate programs and initiatives to (aim 1) reduce inappropriate opioid prescribing from VHA EDs; (aim 2) increase naloxone distribution from VHA EDs; and (aim 3) increase access to MAT initiation from VHA EDs through the implementation of ED-based MAT-initiation programs with EDs across the VHA. Aim 1 was a focused and strategic QI effort to implement an ED-based program to reduce inappropriate opioid prescribing. The ED OSI prescribing program offered a 4-step bundled approach: (1) sharing of opioid prescribing dashboard data with ED medical director and academic detailer; (2) education of ED providers and implementation of toolkit resources; (3) academic detailers conduct audit and feedback session(s) with highest prescribers; and (4) quarterly reports of opioid prescribing data to ED providers.
Results from the pilot suggested that our program was associated with accelerating the rate at which ED prescribing rates decreased.11 In addition, the pilot found that ED-based QI initiatives in VHA facilities are a feasible practice. As we work to develop and implement the next 2 phases of the QI program, a major consideration is to identify facilitators and address any existing barriers to the implementation of naloxone distribution (aim 2) and MAT-initiation (aim 3) programs for treatment-naïve patients from VHA EDs. To date, there have been no recent published studies examining the barriers and facilitators to use or implementation of MAT initiation or naloxone distribution in VHA facilities or, more specifically, from VHA EDs.12 As part of our QI program, we set out to better understand VHA ED provider perceptions of barriers and facilitators to implementation of programs aimed at increasing naloxone distribution and initiation of MAT for treatment-naïve patients in the ED.
Methods
This project received a QI designation from the Office of PBM Academic Detailing Service Institutional Review Board at the Edward Hines, Jr. Veterans Affairs Hospital VA Medical Center (VAMC). This designation was reviewed and approved by the Rocky Mountain Regional VAMC Research and Development service. In addition, we received national union approval to disseminate this survey nationally across all VA Integrated Service Networks (VISNs).
Survey
We worked with VHA subject matter experts, key stakeholders, and the VA Collaborative Evaluation Center (VACE) to develop the survey. Subject matter experts and stakeholders included VHA emergency medicine leadership, ADS leadership, and mental health and substance treatment providers. VACE is an interdisciplinary group of mixed-method researchers. The survey questions aimed to capture perceptions and experiences regarding naloxone distribution and new MAT initiation of VHA ED/UCC providers.
We used a variety of survey question formats. Close-ended questions with a predefined list of answer options were used to capture discrete domains, such as demographic information, comfort level, and experience level. To capture health care provider (HCP) perceptions on barriers and facilitators, we used multiple-answer multiple-choice questions. Built into this question format was a free-response option, which allowed respondents to offer additional barriers or facilitators. Respondents also had the option of not answering individual questions.
We identified physicians, nurse practitioners (NPs), and physician assistants (PAs) who saw at least 100 patients in the ED or UCC in at least one 3-month period in the prior year and obtained an email address for each. In total, 2228 ED or UCC providers across 132 facilities were emailed a survey; 1883 (84.5%) were ED providers and 345 (15.5%) were UCC providers.
We used Research Electronic Data Capture (REDCap) software to build and disseminate the survey via email. Surveys were initially disseminated in late January 2019. During the 3-month survey period, recipients received 3 automated email reminders from REDCap to complete the survey. Survey data were exported from REDCap. Results were analyzed using descriptive statistics analyses with Microsoft Excel.
Results
One respondent received the survey in error and was excluded from the analysis. The survey response rate was 16.7%: 372 responses from 103 unique facilities. Each VISN had a mean 20 respondents. The majority of respondents (n = 286, 76.9%) worked in highly complex level 1 facilities characterized by high patient volume and more high-risk patients and were teaching and research facilities. Respondents were asked to describe their most recent ED or UCC role. While 281 respondents (75.5%) were medical doctors, 61 respondents (16.4%) were NPs, 30 (8.1%) were PAs, and 26 (7.0%) were ED/UCC chiefs or medical directors (Table 1). Most respondents (80.4%) reported at least 10 years of health care experience.
The majority of respondents (72.9%) believed that HCPs at their VHA facility should be prescribing naloxone. When asked to specify which HCPs should be prescribing naloxone, most HCP respondents selected pharmacists (76.4%) and substance abuse providers (71.6%). Less than half of respondents (45.0%) felt that VA ED/UCC providers also should be prescribing naloxone. However, 58.1% of most HCP respondents reported being comfortable or very comfortable with prescribing naloxone to a patient in the ED or UCC who already had an existing prescription of opioids. Similarly, 52.7% of respondents reported being comfortable or very comfortable with coprescribing naloxone when discharging a patient with an opioid prescription from the ED/UCC. Notably, while 36.7% of PAs reported being comfortable/very comfortable coprescribing naloxone, 46.7% reported being comfortable/very comfortable prescribing naloxone to a patient with an existing opioid prescription. Physicians and NPs expressed similar levels of comfort with coprescribing and prescribing naloxone.
Respondents across provider types indicated a number of barriers to prescribing naloxone to medically appropriate patients (Table 2). Many respondents indicated prescribing naloxone was beyond the ED/UCC provider scope of practice (35.2%), followed by the perceived stigma associated with naloxone (33.3%), time required to prescribe naloxone (23.9%), and concern with patient’s ability to use naloxone (22.8%).
Facilitators for prescribing naloxone to medically appropriate patients identified by HCP respondents included pharmacist help and education (44.6%), patient knowledge of medication options (31.7%), societal shift away from opioids for pain management (28.0%), facility leadership (26.9%), and patient interest in safe opioid usage (26.6%) (Table 3). In addition, NPs specifically endorsed
Less than 6.8% of HCP respondents indicated that they were comfortable using MAT. Meanwhile, 42.1% of respondents reported being aware of MAT but not familiar with it, and 23.5% reported that they were unaware of MAT. Correspondingly, 301 of the 372 (88.5%) HCP respondents indicated that they had not prescribed MAT in the past year. Across HCP types, only 24.1% indicated that it is the role of VA ED or UCC providers to prescribe MAT when medically appropriate and subsequently refer patients to substance abuse treatment for follow-up (just 7.1% of PAs endorsed this). Furthermore, 6.5% and 18.8% of HCP respondents indicated that their facility leadership was very supportive and supportive, respectively, of MAT for OUD prescribing.
Barriers to MAT initiation indicated by HCP respondents included limited scope of ED and UCC practice (53.2%), unclear follow-up/referral process (50.3%), time (29.8%), and discomfort (28.2%). Nearly one-third of NPs (27.9%) identified patient willingness/ability as a barrier to MAT initiation (Table 4).
Facilitators of MAT initiation in the ED or UCC included VHA same-day treatment options (34.9%), patient desire (32.5%), pharmacist help/education (27.4%), and psychiatric social workers in the ED or UCC (25.3%). Some NPs (23.0%) and PAs (26.7%) also indicated that having time to educate veterans about the medication would be a facilitator (Table 5). Facility leadership support was considered a facilitator by 30% of PAs.
Discussion
To the best of our knowledge, there have not been any studies examining HCP perceptions of the barriers and facilitators to naloxone distribution or the initiation of MAT in VHA ED and UCCs. Veterans are at an increased risk of overdose when compared with the general population, and increasing access to opioid safety measures (eg, safer prescribing practices, naloxone distribution) and treatment with MAT for OUD across all clinical settings has been a VHA priority.3
National guidance from VHA leadership, the Centers for Disease Control and Prevention (CDC), the US Surgeon General, and the US Department of Health and Human Services (HHS) call for an all-hands-on-deck approach to combatting opioid overdose with naloxone distribution or MAT (such as buprenorphine) initiation.13 VHA ED and UCC settings provide acute outpatient care to patients with medical or psychiatric illnesses or injuries that the patient believes requires emergent or immediate medical attention or for which there is a critical need for treatment to prevent deterioration of the condition or the possible impairment of recovery.14 However, ED and UCC environments are often regarded as settings meant to stabilize a patient until they can be seen by a primary care or long-term care provider.
A major barrier identified by HCPs was that MAT for OUD was outside their ED/UCC scope of practice, which suggests a need for a top-down or peer-to-peer reexamination of the role of HCPs in ED/UCC settings. Any naloxone distribution and/or MAT-initiation program in VHA ED/UCCs should consider education about the role of ED/UCC HCPs in opioid safety and treatment.
Only 25.3% of HCPs reported that their facility leadership was supportive or very supportive of MAT prescribing. This suggests that facility leadership should be engaged in any efforts to implement a MAT-initiation program in the facility’s ED. Engaging leadership in efforts to implement ED-based MAT programs will allow for a better understanding of leadership goals as related to opioid safety and an opportunity to address concerns regarding prescribing MAT in the ED. We recommend engaging facility leadership early in MAT implementation efforts. Respectively, 12.4% and 28.2% of HCP respondents reported discomfort prescribing naloxone or using MAT, suggesting a need for more education. Similarly, only 6.8% of HCPs reported comfort with using MAT.
A consideration for implementing ED/UCC-based MAT should be the inclusion of a training component. An evidence-based clinical treatment pathway that is appropriate to the ED/UCC setting and facility on the administration of MAT also could be beneficial. A clinical treatment pathway that includes ED/UCC-initiated discharge recommendations would address HCP concerns of unclear follow-up plans and system for referral of care. To this end, a key implementation task is coordinating with other outpatient services (eg, pain management clinic, substance use disorder treatment clinic) equipped for long-term patient follow-up to develop a system for referral of care. For example, as part of the clinical treatment pathway, an ED can develop a system of referral for patients initiated on MAT in the ED in which patients are referred for follow-up at the facility’s substance use disorder treatment clinic to be seen within 72 hours to continue the administration of MAT (such as buprenorphine).
In addition to HCP education, results suggest that patient/veteran education regarding naloxone and/or MAT should be considered. HCPs indicated that having help from a pharmacist to educate the patient about the medications would be a facilitator to naloxone distribution and MAT initiation. Similarly, patient knowledge of the medications also was endorsed as a facilitator. As such, a consideration for any future ED/UCC-based naloxone distribution or MAT-initiation programs in the VHA should be patient education whether by a clinically trained professional or an educational campaign for veterans.
Expanded naloxone distribution and initiation of MAT for OUD for EDs/UCCs across the VHA could impact the lives of veterans on long-term opioid therapy, with OUD, or who are otherwise at risk for opioid overdose. Steps taken to address the barriers and leverage the facilitators identified by HCP respondents can greatly reduce current obstacles to widespread implementation of ED/UCC-based naloxone distribution and MAT initiation nationally within the VHA.
Limitations
This survey had a low response rate (16.7%). One potential explanation for the low response rate is that when the survey was deployed, many of the VHA ED/UCC physicians were per-diem employees. Per-diem physicians may be less engaged and aware of site facilitators or barriers to naloxone and MAT prescribing. This, too, may have potentially skewed the collected data. However, the survey did not ask HCPs to disclose their employment status; thus, exact rates of per diem respondents are unknown.
We aimed to capture only self-perceived barriers to prescribing naloxone and MAT in the ED, but we did not capture or measure HCP respondent’s actual prescribing rates of MAT or naloxone. Understanding HCP perceptions of naloxone distribution and MAT initiation in the ED may have been further informed by comparing HCP responses to their actual clinical practice as related to their prescribing of these medications. In future research, we will link HCPs with the actual numbers of naloxone and MAT medications prescribed. Additionally, we do not know how many of these barriers or proposed facilitators will impact clinical practice.
Conclusions
A key aim for VHA leadership is to increase veteran access to naloxone distribution and MAT for OUD across clinical areas. The present study aimed to identify HCP perceptions of barriers and facilitators to the naloxone distribution and MAT-initiation programs in VHA ED/UCCs to inform the development of a targeted QI program to implement these opioid safety measures. Although the survey yielded a low response rate, results allowed us to identify important action items for our QI program, such as the development of clear protocols, follow-up plans, and systems for referral of care and HCP educational materials related to MAT and naloxone. We hope this work will serve as the basis for ED/UCC-tailored programs that can provide customized educational programs for HCPs designed to overcome known barriers to naloxone and MAT initiation.
Acknowledgments
This work was supported by the VA Office of Specialty Care Services 10P11 and through funding provided by the Comprehensive Addiction and Recovery Act (CARA).
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the united states: results from the 2018 National Survey on Drug Use and Health. Published August 2019. Accessed August 20, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
2. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. US Department of Veterans Affairs, Pharmacy Benefits Management Service. Recommendations for issuing naloxone rescue for the VA opioid overdose education and naloxone distribution (OEND) program. Published August 2016. Accessed August 20, 2021. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/Naloxone_HCl_Rescue_Kits_Recommendations_for_Use.pdf
4. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. doi:10.1056/NEJMsa1610524
6. Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265-269. Published 2017 Mar 17. doi:10.15585/mmwr.mm6610a1
7. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163. doi:10.1097/ADM.0000000000000034
8. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for Pain. Ann Intern Med. 2016;165(4):245-252. doi:10.7326/M15-2771
9. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
10. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
11. Dieujuste N, Johnson-Koenke R, Christopher M, et al. Feasibility study of a quasi-experimental regional opioid safety prescribing program in Veterans Health Administration emergency departments. Acad Emerg Med. 2020;27(8):734-741. doi:10.1111/acem.13980
12. Mackey K, Veazie S, Anderson J, Bourne D, Peterson K. Evidence brief: barriers and facilitators to use of medications for opioid use disorder. Published July 2017. Accessed August 20, 2021. http://www.ncbi.nlm.nih.gov/books/NBK549203/
13. US Department of Health and Human Services, Office of the Surgeon General. Naloxone: the opioid reversal drug that saves lives. Published December 2018. Accessed August 20, 2021. https://www.hhs.gov/opioids/sites/default/files/2018-12/naloxone-coprescribing-guidance.pdf
14. US Department of Veterans Affairs, Veterans Health Administration. Chapter 256: Emergency department (ED) and urgent care clinic (UCC). Updated October 3, 2016. Accessed August 20, 2021. https://www.cfm.va.gov/til/space/spChapter256.pdf.
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the united states: results from the 2018 National Survey on Drug Use and Health. Published August 2019. Accessed August 20, 2021. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
2. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. US Department of Veterans Affairs, Pharmacy Benefits Management Service. Recommendations for issuing naloxone rescue for the VA opioid overdose education and naloxone distribution (OEND) program. Published August 2016. Accessed August 20, 2021. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/Naloxone_HCl_Rescue_Kits_Recommendations_for_Use.pdf
4. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
5. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. doi:10.1056/NEJMsa1610524
6. Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265-269. Published 2017 Mar 17. doi:10.15585/mmwr.mm6610a1
7. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163. doi:10.1097/ADM.0000000000000034
8. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for Pain. Ann Intern Med. 2016;165(4):245-252. doi:10.7326/M15-2771
9. Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868-1883. doi:10.1038/s41380-018-0094-5
10. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
11. Dieujuste N, Johnson-Koenke R, Christopher M, et al. Feasibility study of a quasi-experimental regional opioid safety prescribing program in Veterans Health Administration emergency departments. Acad Emerg Med. 2020;27(8):734-741. doi:10.1111/acem.13980
12. Mackey K, Veazie S, Anderson J, Bourne D, Peterson K. Evidence brief: barriers and facilitators to use of medications for opioid use disorder. Published July 2017. Accessed August 20, 2021. http://www.ncbi.nlm.nih.gov/books/NBK549203/
13. US Department of Health and Human Services, Office of the Surgeon General. Naloxone: the opioid reversal drug that saves lives. Published December 2018. Accessed August 20, 2021. https://www.hhs.gov/opioids/sites/default/files/2018-12/naloxone-coprescribing-guidance.pdf
14. US Department of Veterans Affairs, Veterans Health Administration. Chapter 256: Emergency department (ED) and urgent care clinic (UCC). Updated October 3, 2016. Accessed August 20, 2021. https://www.cfm.va.gov/til/space/spChapter256.pdf.
Case: Patient with statin-associated muscle symptoms
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021
‘Lopioid protocol’ – low-dose opioids – proposed for fracture surgery
In a paper presented at the annual meeting of the American Academy of Orthopaedic Surgeons, researchers from NYU reported on the implementation of their multimodal strategy, dubbed the “lopioid protocol.”
According to the 2019 National Survey on Drug Use and Health, orthopedic surgeons are the third-highest opioid prescribers in the United States.
Kennneth A. Egol, MD, vice chair of the department of orthopedic surgery at NYU, who is the first author of the study, was motivated to help create the protocol following misconceptions that orthopedic surgeons were helping to fuel the opioid epidemic.
Dr. Egol pointed to the year 1995, when pain became the fifth vital sign after body temperature, pulse rate, respiratory rate, and blood pressure.
Since then, in light of the opioid epidemic, the focus of physicians has shifted away from prescribing strong pain medication and reducing pain scores to zero to instead reducing pain to a manageable level.
Reducing opioid prescriptions can be challenging when patients are prescribed an anti-inflammatory and they subsequently ask their physician for a “pain pill.” Patients sometimes don’t understand that inflammation is what causes pain.
It can also be difficult to convince patients that medications that they can buy over the counter can adequately control their pain, as confirmed in numerous studies.
Multimodal pain therapy aims to reduce the need for opioids by supplementing their use with other oral medications and, at times, long-lasting regional nerve blocks.
Anti-inflammatories act at the site of injury or surgery where inflammation is occurring. Nerves then carry the pain signal to the brain. These signals can be dampened by medications such as gabapentin that act on the nerves themselves. The pain signal is received in the brain, where opioids act by binding to receptors in the brain.
The so-called lopioid protocol does not eliminate opioids completely but rather uses “safer” opioids, such as tramadol, in lieu of stronger narcotics.
The protocol began at NYU on Jan. 1, 2019. It consists in the prescribing of tramadol, meloxicam, gabapentin, and acetaminophen.
The study presented at the AAOS meeting demonstrated statistically significant reductions in visual analogue pain scores at discharge and subsequent medication refills for the 931 patients in the lopioid group, compared with a group of 848 patients who received narcotic prescriptions containing oxycodone from the year prior to the protocol initiation.
Educating patients on the rationale for the prescription combination can help to allay their fears. Dr. Egol thinks it’s important for physicians to explain the dangers of opioids to patients. He said in an interview that he also believes surgeons need to “give [patients] an understanding of why we are pursuing these protocols. They also need to know we will not ignore their pain and concerns.”
Brannon Orton, MD, is an orthopedic surgeon at Confluence Health, in Moses Lake, Wash. He sees a large number of trauma patients and thinks NYU is doing a good job of addressing a difficult problem in orthopedics – especially in the field of trauma.
He said in an interview: “Managing narcotics postoperatively can be challenging due to the fact that many people come into these fractures with a history of narcotic use.” Not only are they used to turning to opioids for pain relief, but they also may have built up a tolerance to them.
Although he hasn’t been using the lopioid protocol specifically, he has been following a multimodal approach regarding the postoperative use of narcotics. Of the study by Dr. Egol and colleagues, he said, “I think their paper presents an effective way of decreasing use of oral narcotics and still adequately managing patients’ pain postoperatively.” Dr. Orton’s own practice utilizes tramadol, acetaminophen, and ibuprofen after fracture surgery.
From Dr. Orton’s perspective, a significant challenge in implementing the lopioid protocol in practice is simply sticking to the plan. “It can become difficult when patients are pressuring staff or physicians for more narcotics. However, I feel that if everybody is on the same page with the plan, then it can be very doable.”
Dr. Egol and NYU try to limit narcotic prescriptions beginning with the patient’s initial visit to the ED. The ED physicians at his institution only “prescribe small amounts of narcotics. Our ED people really limit the amount of opioids prescribed.”
Dr. Egol recommends that all practitioners begin with nonnarcotic medication, even if treating a fracture nonoperatively. “Start low and go higher. I always try to start with NSAIDs and Tylenol,” he said.
Dr. Egol and Dr. Orton reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a paper presented at the annual meeting of the American Academy of Orthopaedic Surgeons, researchers from NYU reported on the implementation of their multimodal strategy, dubbed the “lopioid protocol.”
According to the 2019 National Survey on Drug Use and Health, orthopedic surgeons are the third-highest opioid prescribers in the United States.
Kennneth A. Egol, MD, vice chair of the department of orthopedic surgery at NYU, who is the first author of the study, was motivated to help create the protocol following misconceptions that orthopedic surgeons were helping to fuel the opioid epidemic.
Dr. Egol pointed to the year 1995, when pain became the fifth vital sign after body temperature, pulse rate, respiratory rate, and blood pressure.
Since then, in light of the opioid epidemic, the focus of physicians has shifted away from prescribing strong pain medication and reducing pain scores to zero to instead reducing pain to a manageable level.
Reducing opioid prescriptions can be challenging when patients are prescribed an anti-inflammatory and they subsequently ask their physician for a “pain pill.” Patients sometimes don’t understand that inflammation is what causes pain.
It can also be difficult to convince patients that medications that they can buy over the counter can adequately control their pain, as confirmed in numerous studies.
Multimodal pain therapy aims to reduce the need for opioids by supplementing their use with other oral medications and, at times, long-lasting regional nerve blocks.
Anti-inflammatories act at the site of injury or surgery where inflammation is occurring. Nerves then carry the pain signal to the brain. These signals can be dampened by medications such as gabapentin that act on the nerves themselves. The pain signal is received in the brain, where opioids act by binding to receptors in the brain.
The so-called lopioid protocol does not eliminate opioids completely but rather uses “safer” opioids, such as tramadol, in lieu of stronger narcotics.
The protocol began at NYU on Jan. 1, 2019. It consists in the prescribing of tramadol, meloxicam, gabapentin, and acetaminophen.
The study presented at the AAOS meeting demonstrated statistically significant reductions in visual analogue pain scores at discharge and subsequent medication refills for the 931 patients in the lopioid group, compared with a group of 848 patients who received narcotic prescriptions containing oxycodone from the year prior to the protocol initiation.
Educating patients on the rationale for the prescription combination can help to allay their fears. Dr. Egol thinks it’s important for physicians to explain the dangers of opioids to patients. He said in an interview that he also believes surgeons need to “give [patients] an understanding of why we are pursuing these protocols. They also need to know we will not ignore their pain and concerns.”
Brannon Orton, MD, is an orthopedic surgeon at Confluence Health, in Moses Lake, Wash. He sees a large number of trauma patients and thinks NYU is doing a good job of addressing a difficult problem in orthopedics – especially in the field of trauma.
He said in an interview: “Managing narcotics postoperatively can be challenging due to the fact that many people come into these fractures with a history of narcotic use.” Not only are they used to turning to opioids for pain relief, but they also may have built up a tolerance to them.
Although he hasn’t been using the lopioid protocol specifically, he has been following a multimodal approach regarding the postoperative use of narcotics. Of the study by Dr. Egol and colleagues, he said, “I think their paper presents an effective way of decreasing use of oral narcotics and still adequately managing patients’ pain postoperatively.” Dr. Orton’s own practice utilizes tramadol, acetaminophen, and ibuprofen after fracture surgery.
From Dr. Orton’s perspective, a significant challenge in implementing the lopioid protocol in practice is simply sticking to the plan. “It can become difficult when patients are pressuring staff or physicians for more narcotics. However, I feel that if everybody is on the same page with the plan, then it can be very doable.”
Dr. Egol and NYU try to limit narcotic prescriptions beginning with the patient’s initial visit to the ED. The ED physicians at his institution only “prescribe small amounts of narcotics. Our ED people really limit the amount of opioids prescribed.”
Dr. Egol recommends that all practitioners begin with nonnarcotic medication, even if treating a fracture nonoperatively. “Start low and go higher. I always try to start with NSAIDs and Tylenol,” he said.
Dr. Egol and Dr. Orton reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a paper presented at the annual meeting of the American Academy of Orthopaedic Surgeons, researchers from NYU reported on the implementation of their multimodal strategy, dubbed the “lopioid protocol.”
According to the 2019 National Survey on Drug Use and Health, orthopedic surgeons are the third-highest opioid prescribers in the United States.
Kennneth A. Egol, MD, vice chair of the department of orthopedic surgery at NYU, who is the first author of the study, was motivated to help create the protocol following misconceptions that orthopedic surgeons were helping to fuel the opioid epidemic.
Dr. Egol pointed to the year 1995, when pain became the fifth vital sign after body temperature, pulse rate, respiratory rate, and blood pressure.
Since then, in light of the opioid epidemic, the focus of physicians has shifted away from prescribing strong pain medication and reducing pain scores to zero to instead reducing pain to a manageable level.
Reducing opioid prescriptions can be challenging when patients are prescribed an anti-inflammatory and they subsequently ask their physician for a “pain pill.” Patients sometimes don’t understand that inflammation is what causes pain.
It can also be difficult to convince patients that medications that they can buy over the counter can adequately control their pain, as confirmed in numerous studies.
Multimodal pain therapy aims to reduce the need for opioids by supplementing their use with other oral medications and, at times, long-lasting regional nerve blocks.
Anti-inflammatories act at the site of injury or surgery where inflammation is occurring. Nerves then carry the pain signal to the brain. These signals can be dampened by medications such as gabapentin that act on the nerves themselves. The pain signal is received in the brain, where opioids act by binding to receptors in the brain.
The so-called lopioid protocol does not eliminate opioids completely but rather uses “safer” opioids, such as tramadol, in lieu of stronger narcotics.
The protocol began at NYU on Jan. 1, 2019. It consists in the prescribing of tramadol, meloxicam, gabapentin, and acetaminophen.
The study presented at the AAOS meeting demonstrated statistically significant reductions in visual analogue pain scores at discharge and subsequent medication refills for the 931 patients in the lopioid group, compared with a group of 848 patients who received narcotic prescriptions containing oxycodone from the year prior to the protocol initiation.
Educating patients on the rationale for the prescription combination can help to allay their fears. Dr. Egol thinks it’s important for physicians to explain the dangers of opioids to patients. He said in an interview that he also believes surgeons need to “give [patients] an understanding of why we are pursuing these protocols. They also need to know we will not ignore their pain and concerns.”
Brannon Orton, MD, is an orthopedic surgeon at Confluence Health, in Moses Lake, Wash. He sees a large number of trauma patients and thinks NYU is doing a good job of addressing a difficult problem in orthopedics – especially in the field of trauma.
He said in an interview: “Managing narcotics postoperatively can be challenging due to the fact that many people come into these fractures with a history of narcotic use.” Not only are they used to turning to opioids for pain relief, but they also may have built up a tolerance to them.
Although he hasn’t been using the lopioid protocol specifically, he has been following a multimodal approach regarding the postoperative use of narcotics. Of the study by Dr. Egol and colleagues, he said, “I think their paper presents an effective way of decreasing use of oral narcotics and still adequately managing patients’ pain postoperatively.” Dr. Orton’s own practice utilizes tramadol, acetaminophen, and ibuprofen after fracture surgery.
From Dr. Orton’s perspective, a significant challenge in implementing the lopioid protocol in practice is simply sticking to the plan. “It can become difficult when patients are pressuring staff or physicians for more narcotics. However, I feel that if everybody is on the same page with the plan, then it can be very doable.”
Dr. Egol and NYU try to limit narcotic prescriptions beginning with the patient’s initial visit to the ED. The ED physicians at his institution only “prescribe small amounts of narcotics. Our ED people really limit the amount of opioids prescribed.”
Dr. Egol recommends that all practitioners begin with nonnarcotic medication, even if treating a fracture nonoperatively. “Start low and go higher. I always try to start with NSAIDs and Tylenol,” he said.
Dr. Egol and Dr. Orton reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.