User login
‘Deeper dive’ into opioid overdose deaths during COVID pandemic
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
EDs saw more benzodiazepine overdoses, but fewer patients overall, in 2020
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
FROM MMWR
PA gets prison time for knowingly prescribing unneeded addictive drugs
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
Atogepant reduces migraine days: ADVANCE trial results published
full results from a phase 3 trial suggest.
AbbVie, the company developing the oral therapy, announced topline results of the ADVANCE trial of atogepant last year. Safety results were presented in April at the 2021 annual meeting of the American Academy of Neurology.
The full results were published online Aug. 19 in the New England Journal of Medicine ahead of the upcoming target action date of the U.S. Food and Drug Administration.
The multicenter study included nearly 900 patients who were randomly assigned to receive either placebo or one of three doses of atogepant for 12 weeks. The mean number of monthly migraine days decreased by about 4 for all three doses of the active treatment, compared with a reduction of 2.5 days with placebo.
“Overall, this study showed us that atogepant was safe and surprisingly seems to be pretty effective regardless of the dose,” said lead author Jessica Ailani, MD, director of MedStar Georgetown Headache Center and associate professor of neurology at Georgetown University, Washington.
All doses effective
The study included 873 patients with episodic migraine with or without aura. Patients who were not assigned to the placebo control group received either 10 mg, 30 mg, or 60 mg of atogepant once daily.
After a 4-week screening period, all patients received treatment for 12 weeks and then entered a 4-week safety follow-up period. In total, the participants completed eight scheduled clinical visits.
The mean reduction from baseline in the mean number of migraine days per month was 3.7 with the 10-mg dose of atogepant, 3.9 with the 30-mg dose, 4.2 with the 60-mg dose, and 2.5 with placebo. The differences between each active dose and placebo was statistically significant (P < .001).
Treatment with the CGRP inhibitor was also associated with a reduction in the mean number of headache days per month. The mean reduction from baseline was 3.9 days for the 10-mg dose, 4.0 days for the 30-mg dose, 4.2 days for the 60-mg dose, and 2.5 days for placebo (P < .001 for all comparisons with placebo).
In addition, for 55.6% of the 10-mg group, 58.7% of the 30-mg group, 60.8% of the 60-mg group, and 29.0% of the control group, there was a reduction of at least 50% in the 3-month average number of migraine days per month (P < .001 for each vs. placebo).
The most commonly reported adverse events (AEs) among patients who received atogepant were constipation (6.9%-7.7% across doses), nausea (4.4%-6.1%), and upper respiratory tract infection (1.4%-3.9%). Frequency of AEs did not differ between the active-treatment groups and the control group, and no relationships between AEs and atogepant dose were observed.
Multidose flexibility
“Side effects were pretty even across the board,” said Dr. Ailani. She noted that the reported AEs were expected because of atogepant’s mechanism of action. In addition, the rate of discontinuation in the study was low.
The proportion of participants who experienced a reduction in monthly migraine days of at least 50% grew as time passed. “By the end of this study, your chance of having a greater than 50% response is about 75%,” Dr. Ailani said.
“Imagine telling your patient, ‘You stick on this drug for 3 months, and I can almost guarantee you that you’re going to get better,’” she added.
Although the treatment has no drug-drug contraindications, drug-drug interactions may occur. “The availability of various doses would allow clinicians to adjust treatment to avoid potential drug-drug interactions,” said Dr. Ailani. “That multidose flexibility is very important.”
An FDA decision on atogepant could be made in the coming months. “I’m hopeful, as a clinician, that it is positive news, because we really have waited a long time for something like this,” Dr. Ailani said.
“You can easily identify patients who would do well on this medication,” she added.
In a different study of atogepant among patients with chronic migraine, there were recruitment delays because of the pandemic. That study is now almost complete, Dr. Ailani reported.
“Well-conducted study”
Commenting on the findings, Kathleen B. Digre, MD, chief of the division of headache and neuro-ophthalmology at the University of Utah Health, Salt Lake City, expressed enthusiasm for the experimental drug. “I’m excited to see another treatment modality for migraine,” said Dr. Digre, who was not involved with the research. “It was a very well-conducted study,” she added.
The treatment arms were almost identical in regard to disease severity, and all the doses showed an effect. Although the difference in reduction of monthly migraine days in comparison with placebo was numerically small, “for people who have frequent migraine, it’s important,” Dr. Digre said.
The results for atogepant should be viewed in a larger context, however. “Even though it’s a treatment that works better than placebo for well-matched controls, it may not be a medication that everybody’s going to respond to,” she noted. “And we can’t generalize it for some of the most disabled people, which is for chronic migraine,” she said.
It is significant that the study was published in the New England Journal of Medicine, Dr. Digre noted. “Sometimes migraine is dismissed as not important and not affecting people’s lives,” she said. “That makes me very happy to see migraine being taken seriously by our major journals.”
In addition, she noted that the prospects for FDA approval of atogepant seem favorable. “I’m hopeful that they will approve it, because it’s got a low side-effect profile, plus it’s effective.”
Migraine-specific preventive therapy has emerged only in the past few years. “I’m so excited to see this surge of preventive medicine for migraine,” Dr. Digre said. “It’s so important, because we see so many people who are disabled by migraine,” she added.
The study was funded by Allergan before atogepant was acquired by AbbVie. Dr. Ailani has received honoraria from AbbVie for consulting, has received compensation from Allergan and AbbVie for participating in a speakers’ bureau, and has received clinical trial grants from Allergan. Dr. Digre has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
full results from a phase 3 trial suggest.
AbbVie, the company developing the oral therapy, announced topline results of the ADVANCE trial of atogepant last year. Safety results were presented in April at the 2021 annual meeting of the American Academy of Neurology.
The full results were published online Aug. 19 in the New England Journal of Medicine ahead of the upcoming target action date of the U.S. Food and Drug Administration.
The multicenter study included nearly 900 patients who were randomly assigned to receive either placebo or one of three doses of atogepant for 12 weeks. The mean number of monthly migraine days decreased by about 4 for all three doses of the active treatment, compared with a reduction of 2.5 days with placebo.
“Overall, this study showed us that atogepant was safe and surprisingly seems to be pretty effective regardless of the dose,” said lead author Jessica Ailani, MD, director of MedStar Georgetown Headache Center and associate professor of neurology at Georgetown University, Washington.
All doses effective
The study included 873 patients with episodic migraine with or without aura. Patients who were not assigned to the placebo control group received either 10 mg, 30 mg, or 60 mg of atogepant once daily.
After a 4-week screening period, all patients received treatment for 12 weeks and then entered a 4-week safety follow-up period. In total, the participants completed eight scheduled clinical visits.
The mean reduction from baseline in the mean number of migraine days per month was 3.7 with the 10-mg dose of atogepant, 3.9 with the 30-mg dose, 4.2 with the 60-mg dose, and 2.5 with placebo. The differences between each active dose and placebo was statistically significant (P < .001).
Treatment with the CGRP inhibitor was also associated with a reduction in the mean number of headache days per month. The mean reduction from baseline was 3.9 days for the 10-mg dose, 4.0 days for the 30-mg dose, 4.2 days for the 60-mg dose, and 2.5 days for placebo (P < .001 for all comparisons with placebo).
In addition, for 55.6% of the 10-mg group, 58.7% of the 30-mg group, 60.8% of the 60-mg group, and 29.0% of the control group, there was a reduction of at least 50% in the 3-month average number of migraine days per month (P < .001 for each vs. placebo).
The most commonly reported adverse events (AEs) among patients who received atogepant were constipation (6.9%-7.7% across doses), nausea (4.4%-6.1%), and upper respiratory tract infection (1.4%-3.9%). Frequency of AEs did not differ between the active-treatment groups and the control group, and no relationships between AEs and atogepant dose were observed.
Multidose flexibility
“Side effects were pretty even across the board,” said Dr. Ailani. She noted that the reported AEs were expected because of atogepant’s mechanism of action. In addition, the rate of discontinuation in the study was low.
The proportion of participants who experienced a reduction in monthly migraine days of at least 50% grew as time passed. “By the end of this study, your chance of having a greater than 50% response is about 75%,” Dr. Ailani said.
“Imagine telling your patient, ‘You stick on this drug for 3 months, and I can almost guarantee you that you’re going to get better,’” she added.
Although the treatment has no drug-drug contraindications, drug-drug interactions may occur. “The availability of various doses would allow clinicians to adjust treatment to avoid potential drug-drug interactions,” said Dr. Ailani. “That multidose flexibility is very important.”
An FDA decision on atogepant could be made in the coming months. “I’m hopeful, as a clinician, that it is positive news, because we really have waited a long time for something like this,” Dr. Ailani said.
“You can easily identify patients who would do well on this medication,” she added.
In a different study of atogepant among patients with chronic migraine, there were recruitment delays because of the pandemic. That study is now almost complete, Dr. Ailani reported.
“Well-conducted study”
Commenting on the findings, Kathleen B. Digre, MD, chief of the division of headache and neuro-ophthalmology at the University of Utah Health, Salt Lake City, expressed enthusiasm for the experimental drug. “I’m excited to see another treatment modality for migraine,” said Dr. Digre, who was not involved with the research. “It was a very well-conducted study,” she added.
The treatment arms were almost identical in regard to disease severity, and all the doses showed an effect. Although the difference in reduction of monthly migraine days in comparison with placebo was numerically small, “for people who have frequent migraine, it’s important,” Dr. Digre said.
The results for atogepant should be viewed in a larger context, however. “Even though it’s a treatment that works better than placebo for well-matched controls, it may not be a medication that everybody’s going to respond to,” she noted. “And we can’t generalize it for some of the most disabled people, which is for chronic migraine,” she said.
It is significant that the study was published in the New England Journal of Medicine, Dr. Digre noted. “Sometimes migraine is dismissed as not important and not affecting people’s lives,” she said. “That makes me very happy to see migraine being taken seriously by our major journals.”
In addition, she noted that the prospects for FDA approval of atogepant seem favorable. “I’m hopeful that they will approve it, because it’s got a low side-effect profile, plus it’s effective.”
Migraine-specific preventive therapy has emerged only in the past few years. “I’m so excited to see this surge of preventive medicine for migraine,” Dr. Digre said. “It’s so important, because we see so many people who are disabled by migraine,” she added.
The study was funded by Allergan before atogepant was acquired by AbbVie. Dr. Ailani has received honoraria from AbbVie for consulting, has received compensation from Allergan and AbbVie for participating in a speakers’ bureau, and has received clinical trial grants from Allergan. Dr. Digre has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
full results from a phase 3 trial suggest.
AbbVie, the company developing the oral therapy, announced topline results of the ADVANCE trial of atogepant last year. Safety results were presented in April at the 2021 annual meeting of the American Academy of Neurology.
The full results were published online Aug. 19 in the New England Journal of Medicine ahead of the upcoming target action date of the U.S. Food and Drug Administration.
The multicenter study included nearly 900 patients who were randomly assigned to receive either placebo or one of three doses of atogepant for 12 weeks. The mean number of monthly migraine days decreased by about 4 for all three doses of the active treatment, compared with a reduction of 2.5 days with placebo.
“Overall, this study showed us that atogepant was safe and surprisingly seems to be pretty effective regardless of the dose,” said lead author Jessica Ailani, MD, director of MedStar Georgetown Headache Center and associate professor of neurology at Georgetown University, Washington.
All doses effective
The study included 873 patients with episodic migraine with or without aura. Patients who were not assigned to the placebo control group received either 10 mg, 30 mg, or 60 mg of atogepant once daily.
After a 4-week screening period, all patients received treatment for 12 weeks and then entered a 4-week safety follow-up period. In total, the participants completed eight scheduled clinical visits.
The mean reduction from baseline in the mean number of migraine days per month was 3.7 with the 10-mg dose of atogepant, 3.9 with the 30-mg dose, 4.2 with the 60-mg dose, and 2.5 with placebo. The differences between each active dose and placebo was statistically significant (P < .001).
Treatment with the CGRP inhibitor was also associated with a reduction in the mean number of headache days per month. The mean reduction from baseline was 3.9 days for the 10-mg dose, 4.0 days for the 30-mg dose, 4.2 days for the 60-mg dose, and 2.5 days for placebo (P < .001 for all comparisons with placebo).
In addition, for 55.6% of the 10-mg group, 58.7% of the 30-mg group, 60.8% of the 60-mg group, and 29.0% of the control group, there was a reduction of at least 50% in the 3-month average number of migraine days per month (P < .001 for each vs. placebo).
The most commonly reported adverse events (AEs) among patients who received atogepant were constipation (6.9%-7.7% across doses), nausea (4.4%-6.1%), and upper respiratory tract infection (1.4%-3.9%). Frequency of AEs did not differ between the active-treatment groups and the control group, and no relationships between AEs and atogepant dose were observed.
Multidose flexibility
“Side effects were pretty even across the board,” said Dr. Ailani. She noted that the reported AEs were expected because of atogepant’s mechanism of action. In addition, the rate of discontinuation in the study was low.
The proportion of participants who experienced a reduction in monthly migraine days of at least 50% grew as time passed. “By the end of this study, your chance of having a greater than 50% response is about 75%,” Dr. Ailani said.
“Imagine telling your patient, ‘You stick on this drug for 3 months, and I can almost guarantee you that you’re going to get better,’” she added.
Although the treatment has no drug-drug contraindications, drug-drug interactions may occur. “The availability of various doses would allow clinicians to adjust treatment to avoid potential drug-drug interactions,” said Dr. Ailani. “That multidose flexibility is very important.”
An FDA decision on atogepant could be made in the coming months. “I’m hopeful, as a clinician, that it is positive news, because we really have waited a long time for something like this,” Dr. Ailani said.
“You can easily identify patients who would do well on this medication,” she added.
In a different study of atogepant among patients with chronic migraine, there were recruitment delays because of the pandemic. That study is now almost complete, Dr. Ailani reported.
“Well-conducted study”
Commenting on the findings, Kathleen B. Digre, MD, chief of the division of headache and neuro-ophthalmology at the University of Utah Health, Salt Lake City, expressed enthusiasm for the experimental drug. “I’m excited to see another treatment modality for migraine,” said Dr. Digre, who was not involved with the research. “It was a very well-conducted study,” she added.
The treatment arms were almost identical in regard to disease severity, and all the doses showed an effect. Although the difference in reduction of monthly migraine days in comparison with placebo was numerically small, “for people who have frequent migraine, it’s important,” Dr. Digre said.
The results for atogepant should be viewed in a larger context, however. “Even though it’s a treatment that works better than placebo for well-matched controls, it may not be a medication that everybody’s going to respond to,” she noted. “And we can’t generalize it for some of the most disabled people, which is for chronic migraine,” she said.
It is significant that the study was published in the New England Journal of Medicine, Dr. Digre noted. “Sometimes migraine is dismissed as not important and not affecting people’s lives,” she said. “That makes me very happy to see migraine being taken seriously by our major journals.”
In addition, she noted that the prospects for FDA approval of atogepant seem favorable. “I’m hopeful that they will approve it, because it’s got a low side-effect profile, plus it’s effective.”
Migraine-specific preventive therapy has emerged only in the past few years. “I’m so excited to see this surge of preventive medicine for migraine,” Dr. Digre said. “It’s so important, because we see so many people who are disabled by migraine,” she added.
The study was funded by Allergan before atogepant was acquired by AbbVie. Dr. Ailani has received honoraria from AbbVie for consulting, has received compensation from Allergan and AbbVie for participating in a speakers’ bureau, and has received clinical trial grants from Allergan. Dr. Digre has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Doctor wins restraining order against CVS after prescription ban
In an Aug. 11 decision, District Court Judge William Bertelsman ordered CVS to stop refusing prescriptions written by Kendall E. Hansen, MD. Judge Bertelsman ruled that Dr. Hansen is likely to succeed in his claim that CVS barred his prescriptions without evidence that he violated any law or professional protocol. The restraining order will remain in place while Dr. Hansen’s lawsuit against CVS Pharmacy proceeds.
Ronald W. Chapman II, an attorney representing Dr. Hansen, said the order is groundbreaking and that, to his knowledge, it’s the first time a federal court has overturned a pharmacy’s decision to block a prescriber.
“We believe that CVS’ decision was based solely on algorithms they use to analyze prescriber practices and not an any individual review of patient records,” Mr. Chapman said. “In fact, we invited CVS to come out to Dr. Hansen’s practice and look at how he was treating patients and ensure things were compliant, but they refused. Instead, they had a phone call with him then cut his patients off.”
Michael DeAngelis, a spokesman for CVS, said the court’s order illustrates the proverbial rock and hard place that pharmacies are placed between in the country’s fight against the misuse of prescription opioids.
“It is alleged in many lawsuits that pharmacies fill too many opioid prescriptions and should operate programs that use data to block prescriptions written by some doctors,” Mr. DeAngelis told this news organization. “And yet other lawsuits, including this one, argue that we should not operate programs that may block prescriptions. Such contradictions are grossly unfair to the pharmacy profession.”
Mr. DeAngelis declined to comment about Dr. Hansen’s claims or specify what led CVS to refuse his prescriptions.
Dr. Hansen declined to comment for this story through his attorney.
Dr. Hansen is no stranger to the spotlight. The Northern Kentucky pain doctor made headlines in 2012 when two of his horses, Fast and Accurate, and Hansen, ran in the Kentucky Derby. In February 2019, he drew media attention when his practice, Interventional Pain Specialists in Crestview Hills, Ky., was raided by federal agents. Dr. Hansen owns and operates the facility, which serves patients in Kentucky, Ohio, and Indiana.
The search yielded no findings, and no charges were filed, according to Mr. Chapman. Scott Hardcorn, director of the Northern Kentucky Drug Strike Force, confirmed that his agency assisted in the operation but said he was unaware of the outcome and that his officers generated no reports from the investigation. A spokesperson for the Drug Enforcement Administration would not comment about the investigation and directed a reporter for this news organization to the DEA website where enforcement actions are listed. No records or actions against Dr. Hansen can be found.
The CVS complaint stems from actions taken by the pharmacy against Dr. Hansen earlier this year. In June, a pharmacy representative allegedly contacted Dr. Hansen by phone and asked him questions about his practice and his prescribing practices, according to his lawsuit filed in U.S. District Court for the Eastern District of Kentucky. During the call, the representative did not inform Dr. Hansen that any of his prescriptions were in question or were suspected of being medically unnecessary, the complaint alleges.
On July 28, 2021, CVS sent Dr. Hansen a letter announcing that its pharmacies would no longer be honoring his prescriptions. The letter, entered as an exhibit in the lawsuit, states that CVS contacted Dr. Hansen twice in 2021 about his prescribing practices, once in May and again in June.
“Despite our attempts to resolve the concerns with your controlled substance prescribing patterns, these concerns persist,” Kahra Lutkiewicz, director of CVS’ retail pharmacy professional practice, wrote in the letter. “Thus, we are writing to inform you that effective Aug. 5, 2021, CVS/pharmacy stores will no longer be able to fill prescriptions that you write for controlled substances. We take our compliance obligations very seriously, and after careful consideration, find it necessary to take this action.”
The letter does not explain the details behind CVS’ concerns.
Dr. Hansen sued CVS on Aug. 4 for tortious interference with a business relationship and defamation, among other claims. His complaint alleges that Dr. Hansen and his patients will suffer irreparable injury if the prescription decision stands. More than 250 of Dr. Hansen’s patients use CVS pharmacies for their prescriptions, and some are locked into using the pharmacy because of insurance contracts, Mr. Chapman said.
“There really is nowhere else for these patients to go,” Mr. Chapman said. “They would have to go to a new doctor and establish a new relationship, and obviously that has devastating consequences when we’re talking about people who need their medication.”
CVS has not yet issued a written response to the lawsuit. In his order, Judge Bertelsman stated that a preliminary conference was held in which all parties were represented and stated their positions to the judge.
“Plaintiffs are likely to succeed on the merits of their claims that defendant has interfered with plaintiffs’ relationships with their patients by refusing to fill prescriptions written by plaintiffs, and defendant has done so without evidence that plaintiffs have violated any law or professional protocol related to such prescriptions,” Judge Bertelsman wrote. “The balance of the hardships between the parties weighs in favor of issuing a temporary restraining order inasmuch as defendant’s actions pose a threat to plaintiffs’ professional reputation and livelihood and ... because plaintiffs’ patients’ medical care is implicated by defendant’s actions, the public interest weighs in favor of issuance of the temporary restraining order.”
Dr. Hansen is currently embroiled in several other legal battles as both a plaintiff and a defendant.
In 2019, a patient sued him for negligence and fraud for allegedly performing medically unnecessary and excessive injection therapy. The suit claims the patient was required to undergo injection therapy on a continuing basis in order to receive her narcotic pain medication, according to the lawsuit filed in Kenton Circuit Court. The complaint alleges that Dr. Hansen made false representations to the patient and to her insurers that the injections were necessary for the treatment of the patient’s chronic pain.
The federal government is not involved in the case.
The negligence lawsuit is in the discovery stage, and attorneys plan to collect Dr. Hansen’s deposition soon, said Eric Deters, a spokesman for Deters Law, a law firm based in Independence, Ky., that is representing the patient.
“The crux is that he performs unnecessary pain procedures and forces you to get an unnecessary procedure before giving you your medication,” Mr. Deters said.
However, Dr. Hansen’s and Mr. Deters’ history together includes a recent riff, according to an August 2021 lawsuit filed by Dr. Hansen against the law firm. Dr. Hansen was a former medical expert in cases for Deters and Associates, but the relationship turned sour when attorneys believed Dr. Hansen was retained as an expert in a case against their clients, according to Dr. Hansen’s suit. Dr. Hansen claims that as retribution, Deters and Associates issued a medical malpractice lawsuit against him in 2020, even though attorneys allegedly knew the statute of limitations had run out. A trial court dismissed the 2020 lawsuit against Dr. Hansen as being untimely filed. Dr. Hansen’s lawsuit alleges wrongful use of civil proceedings and requests compensatory, punitive damages and court costs from the law firm.
The law firm has faced trouble in the past. In August 2021, the Ohio Supreme Court ordered that Mr. Deters pay a $6,500 fine for engaging in the unauthorized practice of law. Mr. Deters’ Kentucky law license has been suspended since 2013 for ethics infractions, according to court records. He retired from law in 2014 and now acts as a spokesperson and office manager for the law firm. The fine resulted from legal advice given by Mr. Deters to two clients at the law firm, according to the Ohio Supreme Court decision.
As for the CVS lawsuit, an upcoming hearing will determine whether the federal court issues a permanent injunction against CVS’s actions. CVS officials have not said whether they will fight the temporary restraining order or the withdrawal of their prescription ban against Dr. Hansen.
A version of this article first appeared on Medscape.com.
In an Aug. 11 decision, District Court Judge William Bertelsman ordered CVS to stop refusing prescriptions written by Kendall E. Hansen, MD. Judge Bertelsman ruled that Dr. Hansen is likely to succeed in his claim that CVS barred his prescriptions without evidence that he violated any law or professional protocol. The restraining order will remain in place while Dr. Hansen’s lawsuit against CVS Pharmacy proceeds.
Ronald W. Chapman II, an attorney representing Dr. Hansen, said the order is groundbreaking and that, to his knowledge, it’s the first time a federal court has overturned a pharmacy’s decision to block a prescriber.
“We believe that CVS’ decision was based solely on algorithms they use to analyze prescriber practices and not an any individual review of patient records,” Mr. Chapman said. “In fact, we invited CVS to come out to Dr. Hansen’s practice and look at how he was treating patients and ensure things were compliant, but they refused. Instead, they had a phone call with him then cut his patients off.”
Michael DeAngelis, a spokesman for CVS, said the court’s order illustrates the proverbial rock and hard place that pharmacies are placed between in the country’s fight against the misuse of prescription opioids.
“It is alleged in many lawsuits that pharmacies fill too many opioid prescriptions and should operate programs that use data to block prescriptions written by some doctors,” Mr. DeAngelis told this news organization. “And yet other lawsuits, including this one, argue that we should not operate programs that may block prescriptions. Such contradictions are grossly unfair to the pharmacy profession.”
Mr. DeAngelis declined to comment about Dr. Hansen’s claims or specify what led CVS to refuse his prescriptions.
Dr. Hansen declined to comment for this story through his attorney.
Dr. Hansen is no stranger to the spotlight. The Northern Kentucky pain doctor made headlines in 2012 when two of his horses, Fast and Accurate, and Hansen, ran in the Kentucky Derby. In February 2019, he drew media attention when his practice, Interventional Pain Specialists in Crestview Hills, Ky., was raided by federal agents. Dr. Hansen owns and operates the facility, which serves patients in Kentucky, Ohio, and Indiana.
The search yielded no findings, and no charges were filed, according to Mr. Chapman. Scott Hardcorn, director of the Northern Kentucky Drug Strike Force, confirmed that his agency assisted in the operation but said he was unaware of the outcome and that his officers generated no reports from the investigation. A spokesperson for the Drug Enforcement Administration would not comment about the investigation and directed a reporter for this news organization to the DEA website where enforcement actions are listed. No records or actions against Dr. Hansen can be found.
The CVS complaint stems from actions taken by the pharmacy against Dr. Hansen earlier this year. In June, a pharmacy representative allegedly contacted Dr. Hansen by phone and asked him questions about his practice and his prescribing practices, according to his lawsuit filed in U.S. District Court for the Eastern District of Kentucky. During the call, the representative did not inform Dr. Hansen that any of his prescriptions were in question or were suspected of being medically unnecessary, the complaint alleges.
On July 28, 2021, CVS sent Dr. Hansen a letter announcing that its pharmacies would no longer be honoring his prescriptions. The letter, entered as an exhibit in the lawsuit, states that CVS contacted Dr. Hansen twice in 2021 about his prescribing practices, once in May and again in June.
“Despite our attempts to resolve the concerns with your controlled substance prescribing patterns, these concerns persist,” Kahra Lutkiewicz, director of CVS’ retail pharmacy professional practice, wrote in the letter. “Thus, we are writing to inform you that effective Aug. 5, 2021, CVS/pharmacy stores will no longer be able to fill prescriptions that you write for controlled substances. We take our compliance obligations very seriously, and after careful consideration, find it necessary to take this action.”
The letter does not explain the details behind CVS’ concerns.
Dr. Hansen sued CVS on Aug. 4 for tortious interference with a business relationship and defamation, among other claims. His complaint alleges that Dr. Hansen and his patients will suffer irreparable injury if the prescription decision stands. More than 250 of Dr. Hansen’s patients use CVS pharmacies for their prescriptions, and some are locked into using the pharmacy because of insurance contracts, Mr. Chapman said.
“There really is nowhere else for these patients to go,” Mr. Chapman said. “They would have to go to a new doctor and establish a new relationship, and obviously that has devastating consequences when we’re talking about people who need their medication.”
CVS has not yet issued a written response to the lawsuit. In his order, Judge Bertelsman stated that a preliminary conference was held in which all parties were represented and stated their positions to the judge.
“Plaintiffs are likely to succeed on the merits of their claims that defendant has interfered with plaintiffs’ relationships with their patients by refusing to fill prescriptions written by plaintiffs, and defendant has done so without evidence that plaintiffs have violated any law or professional protocol related to such prescriptions,” Judge Bertelsman wrote. “The balance of the hardships between the parties weighs in favor of issuing a temporary restraining order inasmuch as defendant’s actions pose a threat to plaintiffs’ professional reputation and livelihood and ... because plaintiffs’ patients’ medical care is implicated by defendant’s actions, the public interest weighs in favor of issuance of the temporary restraining order.”
Dr. Hansen is currently embroiled in several other legal battles as both a plaintiff and a defendant.
In 2019, a patient sued him for negligence and fraud for allegedly performing medically unnecessary and excessive injection therapy. The suit claims the patient was required to undergo injection therapy on a continuing basis in order to receive her narcotic pain medication, according to the lawsuit filed in Kenton Circuit Court. The complaint alleges that Dr. Hansen made false representations to the patient and to her insurers that the injections were necessary for the treatment of the patient’s chronic pain.
The federal government is not involved in the case.
The negligence lawsuit is in the discovery stage, and attorneys plan to collect Dr. Hansen’s deposition soon, said Eric Deters, a spokesman for Deters Law, a law firm based in Independence, Ky., that is representing the patient.
“The crux is that he performs unnecessary pain procedures and forces you to get an unnecessary procedure before giving you your medication,” Mr. Deters said.
However, Dr. Hansen’s and Mr. Deters’ history together includes a recent riff, according to an August 2021 lawsuit filed by Dr. Hansen against the law firm. Dr. Hansen was a former medical expert in cases for Deters and Associates, but the relationship turned sour when attorneys believed Dr. Hansen was retained as an expert in a case against their clients, according to Dr. Hansen’s suit. Dr. Hansen claims that as retribution, Deters and Associates issued a medical malpractice lawsuit against him in 2020, even though attorneys allegedly knew the statute of limitations had run out. A trial court dismissed the 2020 lawsuit against Dr. Hansen as being untimely filed. Dr. Hansen’s lawsuit alleges wrongful use of civil proceedings and requests compensatory, punitive damages and court costs from the law firm.
The law firm has faced trouble in the past. In August 2021, the Ohio Supreme Court ordered that Mr. Deters pay a $6,500 fine for engaging in the unauthorized practice of law. Mr. Deters’ Kentucky law license has been suspended since 2013 for ethics infractions, according to court records. He retired from law in 2014 and now acts as a spokesperson and office manager for the law firm. The fine resulted from legal advice given by Mr. Deters to two clients at the law firm, according to the Ohio Supreme Court decision.
As for the CVS lawsuit, an upcoming hearing will determine whether the federal court issues a permanent injunction against CVS’s actions. CVS officials have not said whether they will fight the temporary restraining order or the withdrawal of their prescription ban against Dr. Hansen.
A version of this article first appeared on Medscape.com.
In an Aug. 11 decision, District Court Judge William Bertelsman ordered CVS to stop refusing prescriptions written by Kendall E. Hansen, MD. Judge Bertelsman ruled that Dr. Hansen is likely to succeed in his claim that CVS barred his prescriptions without evidence that he violated any law or professional protocol. The restraining order will remain in place while Dr. Hansen’s lawsuit against CVS Pharmacy proceeds.
Ronald W. Chapman II, an attorney representing Dr. Hansen, said the order is groundbreaking and that, to his knowledge, it’s the first time a federal court has overturned a pharmacy’s decision to block a prescriber.
“We believe that CVS’ decision was based solely on algorithms they use to analyze prescriber practices and not an any individual review of patient records,” Mr. Chapman said. “In fact, we invited CVS to come out to Dr. Hansen’s practice and look at how he was treating patients and ensure things were compliant, but they refused. Instead, they had a phone call with him then cut his patients off.”
Michael DeAngelis, a spokesman for CVS, said the court’s order illustrates the proverbial rock and hard place that pharmacies are placed between in the country’s fight against the misuse of prescription opioids.
“It is alleged in many lawsuits that pharmacies fill too many opioid prescriptions and should operate programs that use data to block prescriptions written by some doctors,” Mr. DeAngelis told this news organization. “And yet other lawsuits, including this one, argue that we should not operate programs that may block prescriptions. Such contradictions are grossly unfair to the pharmacy profession.”
Mr. DeAngelis declined to comment about Dr. Hansen’s claims or specify what led CVS to refuse his prescriptions.
Dr. Hansen declined to comment for this story through his attorney.
Dr. Hansen is no stranger to the spotlight. The Northern Kentucky pain doctor made headlines in 2012 when two of his horses, Fast and Accurate, and Hansen, ran in the Kentucky Derby. In February 2019, he drew media attention when his practice, Interventional Pain Specialists in Crestview Hills, Ky., was raided by federal agents. Dr. Hansen owns and operates the facility, which serves patients in Kentucky, Ohio, and Indiana.
The search yielded no findings, and no charges were filed, according to Mr. Chapman. Scott Hardcorn, director of the Northern Kentucky Drug Strike Force, confirmed that his agency assisted in the operation but said he was unaware of the outcome and that his officers generated no reports from the investigation. A spokesperson for the Drug Enforcement Administration would not comment about the investigation and directed a reporter for this news organization to the DEA website where enforcement actions are listed. No records or actions against Dr. Hansen can be found.
The CVS complaint stems from actions taken by the pharmacy against Dr. Hansen earlier this year. In June, a pharmacy representative allegedly contacted Dr. Hansen by phone and asked him questions about his practice and his prescribing practices, according to his lawsuit filed in U.S. District Court for the Eastern District of Kentucky. During the call, the representative did not inform Dr. Hansen that any of his prescriptions were in question or were suspected of being medically unnecessary, the complaint alleges.
On July 28, 2021, CVS sent Dr. Hansen a letter announcing that its pharmacies would no longer be honoring his prescriptions. The letter, entered as an exhibit in the lawsuit, states that CVS contacted Dr. Hansen twice in 2021 about his prescribing practices, once in May and again in June.
“Despite our attempts to resolve the concerns with your controlled substance prescribing patterns, these concerns persist,” Kahra Lutkiewicz, director of CVS’ retail pharmacy professional practice, wrote in the letter. “Thus, we are writing to inform you that effective Aug. 5, 2021, CVS/pharmacy stores will no longer be able to fill prescriptions that you write for controlled substances. We take our compliance obligations very seriously, and after careful consideration, find it necessary to take this action.”
The letter does not explain the details behind CVS’ concerns.
Dr. Hansen sued CVS on Aug. 4 for tortious interference with a business relationship and defamation, among other claims. His complaint alleges that Dr. Hansen and his patients will suffer irreparable injury if the prescription decision stands. More than 250 of Dr. Hansen’s patients use CVS pharmacies for their prescriptions, and some are locked into using the pharmacy because of insurance contracts, Mr. Chapman said.
“There really is nowhere else for these patients to go,” Mr. Chapman said. “They would have to go to a new doctor and establish a new relationship, and obviously that has devastating consequences when we’re talking about people who need their medication.”
CVS has not yet issued a written response to the lawsuit. In his order, Judge Bertelsman stated that a preliminary conference was held in which all parties were represented and stated their positions to the judge.
“Plaintiffs are likely to succeed on the merits of their claims that defendant has interfered with plaintiffs’ relationships with their patients by refusing to fill prescriptions written by plaintiffs, and defendant has done so without evidence that plaintiffs have violated any law or professional protocol related to such prescriptions,” Judge Bertelsman wrote. “The balance of the hardships between the parties weighs in favor of issuing a temporary restraining order inasmuch as defendant’s actions pose a threat to plaintiffs’ professional reputation and livelihood and ... because plaintiffs’ patients’ medical care is implicated by defendant’s actions, the public interest weighs in favor of issuance of the temporary restraining order.”
Dr. Hansen is currently embroiled in several other legal battles as both a plaintiff and a defendant.
In 2019, a patient sued him for negligence and fraud for allegedly performing medically unnecessary and excessive injection therapy. The suit claims the patient was required to undergo injection therapy on a continuing basis in order to receive her narcotic pain medication, according to the lawsuit filed in Kenton Circuit Court. The complaint alleges that Dr. Hansen made false representations to the patient and to her insurers that the injections were necessary for the treatment of the patient’s chronic pain.
The federal government is not involved in the case.
The negligence lawsuit is in the discovery stage, and attorneys plan to collect Dr. Hansen’s deposition soon, said Eric Deters, a spokesman for Deters Law, a law firm based in Independence, Ky., that is representing the patient.
“The crux is that he performs unnecessary pain procedures and forces you to get an unnecessary procedure before giving you your medication,” Mr. Deters said.
However, Dr. Hansen’s and Mr. Deters’ history together includes a recent riff, according to an August 2021 lawsuit filed by Dr. Hansen against the law firm. Dr. Hansen was a former medical expert in cases for Deters and Associates, but the relationship turned sour when attorneys believed Dr. Hansen was retained as an expert in a case against their clients, according to Dr. Hansen’s suit. Dr. Hansen claims that as retribution, Deters and Associates issued a medical malpractice lawsuit against him in 2020, even though attorneys allegedly knew the statute of limitations had run out. A trial court dismissed the 2020 lawsuit against Dr. Hansen as being untimely filed. Dr. Hansen’s lawsuit alleges wrongful use of civil proceedings and requests compensatory, punitive damages and court costs from the law firm.
The law firm has faced trouble in the past. In August 2021, the Ohio Supreme Court ordered that Mr. Deters pay a $6,500 fine for engaging in the unauthorized practice of law. Mr. Deters’ Kentucky law license has been suspended since 2013 for ethics infractions, according to court records. He retired from law in 2014 and now acts as a spokesperson and office manager for the law firm. The fine resulted from legal advice given by Mr. Deters to two clients at the law firm, according to the Ohio Supreme Court decision.
As for the CVS lawsuit, an upcoming hearing will determine whether the federal court issues a permanent injunction against CVS’s actions. CVS officials have not said whether they will fight the temporary restraining order or the withdrawal of their prescription ban against Dr. Hansen.
A version of this article first appeared on Medscape.com.
Pandemic derails small success in lowering diabetes-related amputations
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.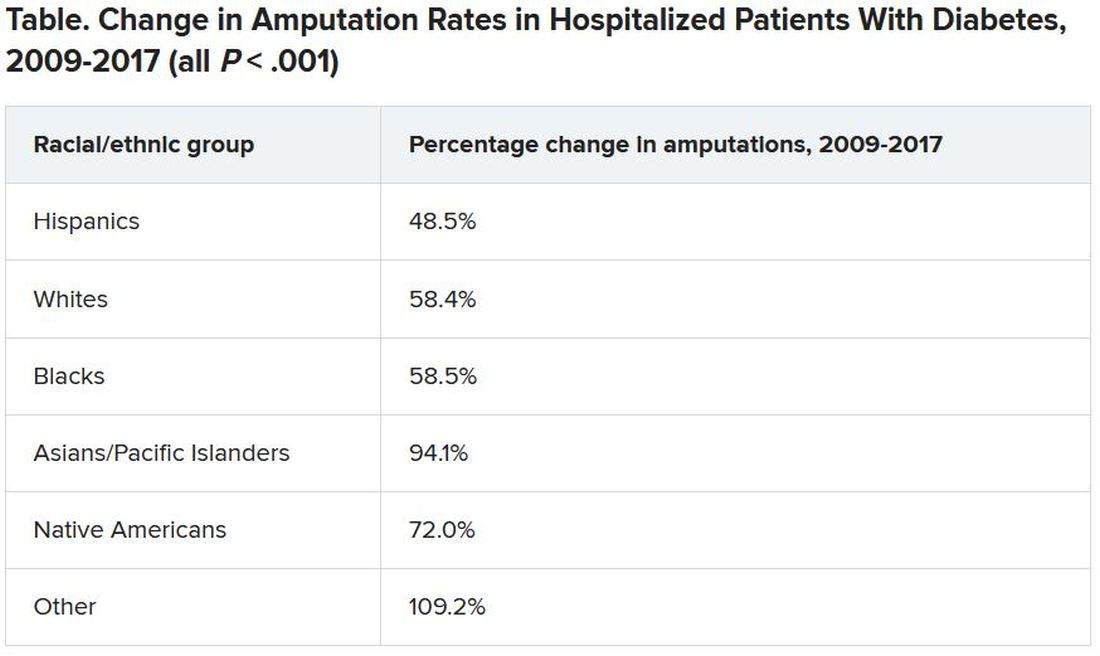
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An Interdisciplinary Approach to Metastatic Pancreatic Cancer and Comorbid Opioid Use Disorder Treatment Within a VA Health Care System
A multidisciplinary approach provided safe and feasible cancer treatment in a patient with advanced pancreatic cancer and coexisting active substance use disorder.
Substance use disorders (SUDs) are an important but understudied aspect of treating patients diagnosed with cancer. Substance use can affect cancer treatment outcomes, including morbidity and mortality.1,2 Additionally, patients with cancer and SUD may have unique psychosocial needs that require close attention and management. There is a paucity of data regarding the best approach to treating such patients. For example, cocaine use may increase the cardiovascular and hematologic risk of some traditional chemotherapy agents.3,4 Newer targeted agents and immunotherapies remain understudied with respect to SUD risk.
Although the US Department of Veterans Affairs (VA) has established helpful clinical practice guidelines for the treatment of SUD, there are no guidelines for treating patients with SUD and cancer.5 Clinicians have limited confidence in treatment approach, and treatment is inconsistent among oncologists nationwide even within the same practice. Furthermore, it can be challenging to safely prescribe opioids for cancer-related pain in individuals with SUD. There is a high risk of SUD and mental health disorders in veterans, making this population particularly vulnerable. We report a case of a male with metastatic pancreatic cancer, severe opioid use disorder (OUD) and moderate cocaine use disorder (CUD) who received pain management and cancer treatment under the direction of a multidisciplinary team approach.
Case Report
A 63-year-old male with a medical history of HIV treated with highly active antiretroviral therapy (HAART), compensated cirrhosis, severe OUD, moderate CUD, and sedative use disorder in sustained remission was admitted to the West Haven campus of the VA Connecticut Healthcare System (VACHS) with abdominal pain, weight loss and fatigue. He used heroin 1 month prior to his admission and reported regular cocaine and marijuana use (Table 1). He was diagnosed with HIV in 1989, and his medical history included herpes zoster and oral candidiasis but no other opportunistic infections. Several months prior to this admission, he had an undetectable viral load and CD4 count of 688.
At the time of this admission, the patient was adherent to methadone treatment. He reported increased abdominal pain. Computed tomography (CT) showed a 2.4-cm mass in the pancreatic uncinate process, multiple liver metastases, retroperitoneal lymphadenopathy, and small lung nodules. A CT-guided liver biopsy showed adenocarcinoma consistent with a primary cancer of the pancreas. Given the complexity of the case, a multidisciplinary team approach was used to treat his cancer and the sequelae safely, including the oncology team, community living center team, palliative care team, and interprofessional opioid reassessment clinic team (ORC).
Cancer Treatment
Chemotherapy with FOLFIRINOX (leucovorin calcium, fluorouracil, irinotecan hydrochloride, and oxaliplatin) was recommended. The first cycle of treatment originally was planned for the outpatient setting, and a peripherally inserted central catheter (PICC) line was placed. However, after a urine toxicology test was positive for cocaine, the PICC line was removed due to concern for possible use of PICC line for nonprescribed substance use. The patient expressed suicidal ideation at the time and was admitted for psychiatric consult and pain control. Cycle 1 FOLFIRINOX was started during this admission. A PICC line was again put in place and then removed before discharge. A celiac plexus block was performed several days after this admission for pain control.
Given concern about cocaine use increasing the risk of cardiac toxicity with FOLFIRINOX treatment, treating providers sconsulted with the community living center (CLC) about possible admission for future chemotherapy administration and pain management. The CLC at VACHS has 38 beds for rehabilitation, long-term care, and hospice with the mission to restore each veteran to his or her highest level of well-being. After discussion with this patient and CLC staff, he agreed to a CLC admission. The patient agreed to remain in the facility, wear a secure care device, and not leave without staff accompaniment. He was able to obtain a 2-hour pass to pay bills and rent. During the 2 months he was admitted to the CLC he would present to the VACHS Cancer Center for chemotherapy every 2 weeks. He completed 6 cycles of chemotherapy while admitted. During the admission, he was transferred to active medical service for 2 days for fever and malaise, and then returned to the CLC. The patient elected to leave the CLC after 2 months as the inability to see close friends was interfering with his quality of life.
Upon being discharged from the CLC, shared decision making took place with the patient to establish a new treatment plan. In collaboration with the patient, a plan was made to admit him every 2 weeks for continued chemotherapy. A PICC line was placed on each day of admission and removed prior to discharge. It was also agreed that treatment would be delayed if a urine drug test was positive for cocaine on the morning of admission. The patient was also seen by ORC every 2 weeks after being discharged from the CLC.
Imaging after cycle 6 showed decreased size of liver metastases, retroperitoneal lymph nodes, and pancreas mass. Cancer antigen 19-9 (CA19-9) tumor marker was reduced from 3513 U/mL pretreatment to 50 U/mL after cycle 7. Chemotherapy cycle 7 was delayed 6 days due to active cocaine and heroin use. A repeat urine was obtained several days later, which was negative for cocaine, and he was admitted for cycle 7 chemotherapy. Using this treatment approach of admissions for every cycle, the patient was able to receive 11 cycles of FOLFIRINOX with clinical benefit.
Palliative Care/Pain Management
Safely treating the patient’s malignant pain in the context of his OUD was critically important. In order to do this the palliative care team worked closely alongside ORC, is a multidisciplinary team consisting of health care providers (HCPs) from addiction psychiatry, internal medicine, health psychology and pharmacy who are consulted to evaluate veterans’ current opioid regimens and make recommendations to optimize both safety and efficacy. ORC followed this particular veteran as an outpatient and consulted on pain issues during his admission. They recommended the continuation of methadone at 120 mg daily and increased oral oxycodone to 30 mg every 6 hours, and then further increased to 45 mg every 6 hours. He continued to have increased pain despite higher doses of oxycodone, and pain medication was changed to oral hydromorphone 28 mg every 6 hours with the continuation of methadone. ORC and the palliative care team obtained consent from the veteran and a release of Information form signed by the patient to contact his community methadone clinic for further collaboration around pain management throughout the time caring for the veteran.
Even with improvement in disease based on imaging and tumor markers, opioid medications could not be decreased in this case. This is likely in part due to the multidimensional nature of pain. Careful assessment of the biologic, emotional, social, and spiritual contributors to pain is needed in the management of pain, especially at end of life.6 Nonpharmacologic pain management strategies used in this case included a transcutaneous electrical nerve stimulation unit, moist heat, celiac plexus block, and emotional support.
Psychosocial Issues/Substance Use
Psychosocial support for the patient was provided by the interdisciplinary palliative care team and the ORC team in both the inpatient and outpatient settings. Despite efforts from case management to get the veteran home services once discharged from the CLC, he declined repeatedly. Thus, the CLC social worker obtained a guardian alert for the veteran on discharge.
Close outpatient follow-up for medical and psychosocial support was very critical. When an outpatient, the veteran was scheduled for biweekly appointments with palliative care or ORC. When admitted to the hospital, the palliative care team medical director and psychologist conducted joint visits with him. Although he denied depressed mood and anxiety throughout his treatment, he often reflected on regrets that he had as he faced the end of his life. Specifically, he shared thoughts about being estranged from his surviving brother given his long struggle with substance use. Although he did not think a relationship was possible with his brother at the end of life, he still cared deeply for him and wanted to make him aware of his pancreatic cancer diagnosis. This was particularly important to him because their late brother had also died of pancreatic cancer. It was the patient’s wish at the end of his life to alert his surviving brother of his diagnosis so he and his children could get adequate screening throughout their lives. Although he had spoken of this desire often, it wasn’t until his disease progressed and he elected to transition to hospice that he felt ready to write the letter. The palliative care team assisted the veteran in writing and mailing a letter to his brother informing him of his diagnosis and transition to hospice as well as communicating that his brother and his family had been in his thoughts at the end of his life. The patient’s brother received this letter and with assistance from the CLC social worker made arrangements to visit the veteran at bedside at the inpatient CLC hospice unit the final days of his life.
Discussion
There are very little data on the safety of cancer-directed therapy in patients with active SUD. The limited studies that have been done showed conflicting results.
A retrospective study among women with co-occurring SUD and locally advanced cervical cancer who were undergoing primary radiation therapy found that SUD was not associated with a difference in toxicity or survival outcomes.7 However, other research suggests that SUD may be associated with an increase in all-cause mortality as well as other adverse outcomes for patients and health care systems (eg, emergency department visits, hospitalizations).8 A retrospective study of patients with a history of SUD and nonsmall cell lung cancer showed that these patients had higher rates of depression, less family support, increased rates of missed appointments, more emergency department visits and more hospitalizations.9 Patients with chronic myeloid leukemia or myelodysplastic syndromes who had long-term cocaine use had a 6-fold increased risk of death, which was not found in patients who had long-term alcohol or marijuana use.2
The limited data highlight the need for careful consideration of ways to mitigate potentially adverse outcomes in this population while still providing clinically indicated cancer treatment. Integrated VA health care systems provide unique resources that can maximize veteran safety during cancer treatment. Utilization of VA resources and close interdisciplinary collaboration across VA HCPs can help to ensure equitable access to state-of-the-art cancer therapies for veterans with comorbid SUD.
VA Services for Patients With Comorbidities
This case highlights several distinct aspects of VA health care that make it possible to safely treat individuals with complex comorbidities. One important aspect of this was collaboration with the CLC to admit the veteran for his initial treatment after a positive cocaine test. CLC admission was nonpunitive and allowed ongoing involvement in the VA community. This provided an essential, safe, and structured environment in which 6 cycles of chemotherapy could be delivered.
Although the patient left the CLC after 2 months due to floor restrictions negatively impacting his quality of life and ability to spend time with close friends, several important events occurred during this stay. First, the patient established close relationships with the CLC staff and the palliative care team; both groups followed him throughout his inpatient and outpatient care. These relationships proved essential throughout his care as they were the foundation of difficult conversations about substance use, treatment adherence, and eventually, transition to hospice.
In addition, the opportunity to administer 6 cycles of chemotherapy at the CLC was enough to lead to clinical benefit and radiographic response to treatment. Clinical benefits while in the CLC included maintenance of a good appetite, 15-lb weight gain and preserved performance status (ECOG [Eastern Cooperative Group]-1), which allowed him to actively participate in multiple social and recreational activities while in the CLC. From early conversations, this patient was clear that he wanted treatment as long as his life could be prolonged with good quality of life. Having evidence of the benefit of treatment, at least initially, increased the patient’s confidence in treatment. There were a few conversations when the challenges of treatment mounted (eg, pain, needs for abstinence from cocaine prior to admission for chemotherapy, frequent doctor appointments), and the patient would remind himself of these data to recommit himself to treatment. The opportunity to admit him to the inpatient VA facility, including bed availability for 3 days during his treatment once he left the CLC was important. This plan to admit the patient following a negative urine toxicology test for cocaine was made collaboratively with the veteran and the oncology and palliative care teams. The plan allowed the patient to achieve his treatment goals while maintaining his safety and reducing theoretical cardiac toxicities with his cancer treatment.
Finally, the availability of a multidisciplinary team approach including palliative care, oncology, psychology, addiction medicine and addiction psychiatry, was critical for addressing the veteran’s malignant pain. Palliative care worked in close collaboration with the ORC to prescribe and renew pain medications. ORC offered ongoing consultation on pain management in the context of OUD. As the veteran’s cancer progressed and functional decline prohibited his daily attendance at the community methadone clinic, palliative care and ORC met with the methadone clinic to arrange a less frequent methadone pickup schedule (the patient previously needed daily pickup). Non-VA settings may not have access to these resources to safely treat the biopsychosocial issues that arise in complex cases.
Substance Use and Cancer Treatments
This case raises several critical questions for oncologic care. Cocaine and fluorouracil are both associated with cardiotoxicity, and many oncologists would not feel it is safe to administer a regimen containing fluorouracil to a patient with active cocaine use. The National Comprehensive Cancer Network (NCCN) panel recommends FOLFIRINOX as a preferred category 1 recommendation for first-line treatment of patients with advanced pancreas cancer with good performance status.10 This recommendation is based on the PRODIGE trial, which has shown improved overall survival (OS): 11.1 vs 6.8 months for patients who received single-agent gemcitabine.11 If patients are not candidates for FOLFIRINOX and have good performance status, the NCCN recommends gemcitabine plus albumin-bound paclitaxel with category 1 level of evidence based on the IMPACT trial, which showed improvement in OS (8.7 vs 6.6 months compared with single-agent gemcitabine).12
Some oncologists may have additional concerns administering fluorouracil treatment alternatives (such as gemcitabine and albumin-bound paclitaxel) to individuals with active SUD because of concerns about altered mental status impacting the ability to report important adverse effects. In the absence of sufficient data, HCPs must determine whether they feel it is safe to administer these agents in individuals with active cocaine use. However, denying these patients the possible benefits of standard-of-care life-prolonging therapies without established data raises concerns regarding the ethics of such practices. There is concern that the stigma surrounding cocaine use might contribute to withholding treatment, while treatment is continued for individuals taking prescribed stimulant medications that also have cardiotoxicity risks. VA health care facilities are uniquely situated to use all available resources to address these issues using interprofessional patient-centered care and determine the most optimal treatment based on a risk/benefit discussion between the patient and the HCP.
Similarly, this case also raised questions among HCPs about the safety of using an indwelling port for treatment in a patient with SUD. In the current case there was concern about keeping in a port for a patient with a history of IV drug use; therefore, a PICC line was initiated and removed at each admission. Without guidelines in these situations, HCPs are left to weigh the risks and benefits of using a port or a PICC for individuals with recent or current substance use without formal data, which can lead to inconsistent access to care. More guidance is needed for these situations.
SUD Screening
This case begs the question of whether oncologists are adequately screening for a range of SUDs, and when they encounter an issue, how they are addressing it. Many oncologists do not receive adequate training on assessment of current or recent substance use. There are health care and systems-level practices that may increase patient safety for individuals with ongoing substance use who are undergoing cancer treatment. Training on obtaining appropriate substance use histories, motivational interviewing to resolve ambivalence about substance use in the direction of change, and shared decision making about treatment options could increase confidence in understanding and addressing substance use issues. It is also important to educate oncologists on how to address patients who return to or continued substance use during treatment. In this case the collaboration from palliative care, psychology, addiction medicine, and addiction psychiatry through the ORC was essential in assisting with ongoing assessment of substance use, guiding difficult conversations about the impact of substance use on the treatment plan, and identifying risk-mitigation strategies. Close collaboration and full utilization of all VA resources allowed this patient to receive first-line treatment for pancreatic cancer in order to reach his goal of prolonging his life while maintaining acceptable quality of life. Table 2 provides best practices for management of patients with comorbid SUD and cancer.
More research is needed into cancer treatment for patients with SUD, especially in the current era of cancer care using novel cancer treatments leading to significantly improved survival in many cancer types. Ideally, oncologists should be routinely or consistently screening patients for substance use, including alcohol. The patient should participate in this decision-making process after being educated about the risks and benefits. These patients can be followed using a multimodal approach to increase their rates of success and improve their quality of life. Although the literature is limited and no formal guidelines are available, VA oncologists are fortunate to have a range of resources available to them to navigate these difficult cases. Veterans have elevated rates of SUD, making this a critical issue to consider in the VA.13 It is the hope that this case can highlight how to take advantage of the many VA resources in order to ensure equitable cancer care for all veterans.
Conclusions
This case demonstrates that cancer-directed treatment is safe and feasible in a patient with advanced pancreatic cancer and coexisting active SUD by using a multidisciplinary approach. The multidisciplinary team included palliative care, oncology, psychology, addiction medicine, and addiction psychiatry. Critical steps for a successful outcome include gathering history about SUD; motivational interviewing to resolve ambivalence about treatment for SUD; shared decision making about cancer treatment; and risk-reduction strategies in pain and SUD management.
Treatment advancements in many cancer types have led to significantly longer survival, and it is critical to develop safe protocols to treat patients with active SUD so they also can derive benefit from these very significant medical advancements.
Acknowledgments
Michal Rose, MD, Director of VACHS Cancer Center, and Chandrika Kumar, MD, Director of VACHS Community Living Center, for their collaboration in care for this veteran.
1. Chang G, Meadows ME, Jones JA, Antin JH, Orav EJ. Substance use and survival after treatment for chronic myelogenous leukemia (CML) or myelodysplastic syndrome (MDS). Am J Drug Alcohol Ab. 2010;36(1):1-6. doi:10.3109/00952990903490758
2. Stagno S, Busby K, Shapiro A, Kotz M. Patients at risk: addressing addiction in patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2008;42(4):221-226. doi:10.1038/bmt.2008.211
3. Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345(1):45-51. doi:10.1097/MAJ.0b013e31825b2b50
4. Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122(24):2558-2569. doi:10.1161/CIRCULATIONAHA.110.940569
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. Published 2015. Accessed July 8, 2021. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPGRevised22216.pdf
6. Mehta A, Chan LS. Understanding of the concept of “total pain”: a prerequisite for pain control. J Hosp Palliat Nurs. 2008;10(1):26-32. doi:10.1097/01.NJH.0000306714.50539.1a
7. Rubinsak LA, Terplan M, Martin CE, Fields EC, McGuire WP, Temkin SM. Co-occurring substance use disorder: The impact on treatment adherence in women with locally advanced cervical cancer. Gynecol Oncol Rep. 2019;28:116-119. Published 2019 Mar 27. doi:10.1016/j.gore.2019.03.016
8. Chhatre S, Metzger DS, Malkowicz SB, Woody G, Jayadevappa R. Substance use disorder and its effects on outcomes in men with advanced-stage prostate cancer. Cancer. 2014;120(21):3338-3345. doi:10.1002/cncr.28861
9. Concannon K, Thayer JH, Hicks R, et al. Outcomes among patients with a history of substance abuse in non-small cell lung cancer: a county hospital experience. J Clin Onc. 2019;37(15)(suppl):e20031-e20031. doi:10.1200/JCO.2019.37.15
10. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma. Version 2.2021. Updated February 25, 2021. Accessed July 8, 2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
11. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi:10.1056/NEJMoa1011923
12. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703. doi:10.1056/NEJMoa1304369
13. Seal KH, Cohen G, Waldrop A, Cohen BE, Maguen S, Ren L. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: Implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116(1-3):93-101. doi:10.1016/j.drugalcdep.2010.11.027
14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association; 2013.
A multidisciplinary approach provided safe and feasible cancer treatment in a patient with advanced pancreatic cancer and coexisting active substance use disorder.
A multidisciplinary approach provided safe and feasible cancer treatment in a patient with advanced pancreatic cancer and coexisting active substance use disorder.
Substance use disorders (SUDs) are an important but understudied aspect of treating patients diagnosed with cancer. Substance use can affect cancer treatment outcomes, including morbidity and mortality.1,2 Additionally, patients with cancer and SUD may have unique psychosocial needs that require close attention and management. There is a paucity of data regarding the best approach to treating such patients. For example, cocaine use may increase the cardiovascular and hematologic risk of some traditional chemotherapy agents.3,4 Newer targeted agents and immunotherapies remain understudied with respect to SUD risk.
Although the US Department of Veterans Affairs (VA) has established helpful clinical practice guidelines for the treatment of SUD, there are no guidelines for treating patients with SUD and cancer.5 Clinicians have limited confidence in treatment approach, and treatment is inconsistent among oncologists nationwide even within the same practice. Furthermore, it can be challenging to safely prescribe opioids for cancer-related pain in individuals with SUD. There is a high risk of SUD and mental health disorders in veterans, making this population particularly vulnerable. We report a case of a male with metastatic pancreatic cancer, severe opioid use disorder (OUD) and moderate cocaine use disorder (CUD) who received pain management and cancer treatment under the direction of a multidisciplinary team approach.
Case Report
A 63-year-old male with a medical history of HIV treated with highly active antiretroviral therapy (HAART), compensated cirrhosis, severe OUD, moderate CUD, and sedative use disorder in sustained remission was admitted to the West Haven campus of the VA Connecticut Healthcare System (VACHS) with abdominal pain, weight loss and fatigue. He used heroin 1 month prior to his admission and reported regular cocaine and marijuana use (Table 1). He was diagnosed with HIV in 1989, and his medical history included herpes zoster and oral candidiasis but no other opportunistic infections. Several months prior to this admission, he had an undetectable viral load and CD4 count of 688.
At the time of this admission, the patient was adherent to methadone treatment. He reported increased abdominal pain. Computed tomography (CT) showed a 2.4-cm mass in the pancreatic uncinate process, multiple liver metastases, retroperitoneal lymphadenopathy, and small lung nodules. A CT-guided liver biopsy showed adenocarcinoma consistent with a primary cancer of the pancreas. Given the complexity of the case, a multidisciplinary team approach was used to treat his cancer and the sequelae safely, including the oncology team, community living center team, palliative care team, and interprofessional opioid reassessment clinic team (ORC).
Cancer Treatment
Chemotherapy with FOLFIRINOX (leucovorin calcium, fluorouracil, irinotecan hydrochloride, and oxaliplatin) was recommended. The first cycle of treatment originally was planned for the outpatient setting, and a peripherally inserted central catheter (PICC) line was placed. However, after a urine toxicology test was positive for cocaine, the PICC line was removed due to concern for possible use of PICC line for nonprescribed substance use. The patient expressed suicidal ideation at the time and was admitted for psychiatric consult and pain control. Cycle 1 FOLFIRINOX was started during this admission. A PICC line was again put in place and then removed before discharge. A celiac plexus block was performed several days after this admission for pain control.
Given concern about cocaine use increasing the risk of cardiac toxicity with FOLFIRINOX treatment, treating providers sconsulted with the community living center (CLC) about possible admission for future chemotherapy administration and pain management. The CLC at VACHS has 38 beds for rehabilitation, long-term care, and hospice with the mission to restore each veteran to his or her highest level of well-being. After discussion with this patient and CLC staff, he agreed to a CLC admission. The patient agreed to remain in the facility, wear a secure care device, and not leave without staff accompaniment. He was able to obtain a 2-hour pass to pay bills and rent. During the 2 months he was admitted to the CLC he would present to the VACHS Cancer Center for chemotherapy every 2 weeks. He completed 6 cycles of chemotherapy while admitted. During the admission, he was transferred to active medical service for 2 days for fever and malaise, and then returned to the CLC. The patient elected to leave the CLC after 2 months as the inability to see close friends was interfering with his quality of life.
Upon being discharged from the CLC, shared decision making took place with the patient to establish a new treatment plan. In collaboration with the patient, a plan was made to admit him every 2 weeks for continued chemotherapy. A PICC line was placed on each day of admission and removed prior to discharge. It was also agreed that treatment would be delayed if a urine drug test was positive for cocaine on the morning of admission. The patient was also seen by ORC every 2 weeks after being discharged from the CLC.
Imaging after cycle 6 showed decreased size of liver metastases, retroperitoneal lymph nodes, and pancreas mass. Cancer antigen 19-9 (CA19-9) tumor marker was reduced from 3513 U/mL pretreatment to 50 U/mL after cycle 7. Chemotherapy cycle 7 was delayed 6 days due to active cocaine and heroin use. A repeat urine was obtained several days later, which was negative for cocaine, and he was admitted for cycle 7 chemotherapy. Using this treatment approach of admissions for every cycle, the patient was able to receive 11 cycles of FOLFIRINOX with clinical benefit.
Palliative Care/Pain Management
Safely treating the patient’s malignant pain in the context of his OUD was critically important. In order to do this the palliative care team worked closely alongside ORC, is a multidisciplinary team consisting of health care providers (HCPs) from addiction psychiatry, internal medicine, health psychology and pharmacy who are consulted to evaluate veterans’ current opioid regimens and make recommendations to optimize both safety and efficacy. ORC followed this particular veteran as an outpatient and consulted on pain issues during his admission. They recommended the continuation of methadone at 120 mg daily and increased oral oxycodone to 30 mg every 6 hours, and then further increased to 45 mg every 6 hours. He continued to have increased pain despite higher doses of oxycodone, and pain medication was changed to oral hydromorphone 28 mg every 6 hours with the continuation of methadone. ORC and the palliative care team obtained consent from the veteran and a release of Information form signed by the patient to contact his community methadone clinic for further collaboration around pain management throughout the time caring for the veteran.
Even with improvement in disease based on imaging and tumor markers, opioid medications could not be decreased in this case. This is likely in part due to the multidimensional nature of pain. Careful assessment of the biologic, emotional, social, and spiritual contributors to pain is needed in the management of pain, especially at end of life.6 Nonpharmacologic pain management strategies used in this case included a transcutaneous electrical nerve stimulation unit, moist heat, celiac plexus block, and emotional support.
Psychosocial Issues/Substance Use
Psychosocial support for the patient was provided by the interdisciplinary palliative care team and the ORC team in both the inpatient and outpatient settings. Despite efforts from case management to get the veteran home services once discharged from the CLC, he declined repeatedly. Thus, the CLC social worker obtained a guardian alert for the veteran on discharge.
Close outpatient follow-up for medical and psychosocial support was very critical. When an outpatient, the veteran was scheduled for biweekly appointments with palliative care or ORC. When admitted to the hospital, the palliative care team medical director and psychologist conducted joint visits with him. Although he denied depressed mood and anxiety throughout his treatment, he often reflected on regrets that he had as he faced the end of his life. Specifically, he shared thoughts about being estranged from his surviving brother given his long struggle with substance use. Although he did not think a relationship was possible with his brother at the end of life, he still cared deeply for him and wanted to make him aware of his pancreatic cancer diagnosis. This was particularly important to him because their late brother had also died of pancreatic cancer. It was the patient’s wish at the end of his life to alert his surviving brother of his diagnosis so he and his children could get adequate screening throughout their lives. Although he had spoken of this desire often, it wasn’t until his disease progressed and he elected to transition to hospice that he felt ready to write the letter. The palliative care team assisted the veteran in writing and mailing a letter to his brother informing him of his diagnosis and transition to hospice as well as communicating that his brother and his family had been in his thoughts at the end of his life. The patient’s brother received this letter and with assistance from the CLC social worker made arrangements to visit the veteran at bedside at the inpatient CLC hospice unit the final days of his life.
Discussion
There are very little data on the safety of cancer-directed therapy in patients with active SUD. The limited studies that have been done showed conflicting results.
A retrospective study among women with co-occurring SUD and locally advanced cervical cancer who were undergoing primary radiation therapy found that SUD was not associated with a difference in toxicity or survival outcomes.7 However, other research suggests that SUD may be associated with an increase in all-cause mortality as well as other adverse outcomes for patients and health care systems (eg, emergency department visits, hospitalizations).8 A retrospective study of patients with a history of SUD and nonsmall cell lung cancer showed that these patients had higher rates of depression, less family support, increased rates of missed appointments, more emergency department visits and more hospitalizations.9 Patients with chronic myeloid leukemia or myelodysplastic syndromes who had long-term cocaine use had a 6-fold increased risk of death, which was not found in patients who had long-term alcohol or marijuana use.2
The limited data highlight the need for careful consideration of ways to mitigate potentially adverse outcomes in this population while still providing clinically indicated cancer treatment. Integrated VA health care systems provide unique resources that can maximize veteran safety during cancer treatment. Utilization of VA resources and close interdisciplinary collaboration across VA HCPs can help to ensure equitable access to state-of-the-art cancer therapies for veterans with comorbid SUD.
VA Services for Patients With Comorbidities
This case highlights several distinct aspects of VA health care that make it possible to safely treat individuals with complex comorbidities. One important aspect of this was collaboration with the CLC to admit the veteran for his initial treatment after a positive cocaine test. CLC admission was nonpunitive and allowed ongoing involvement in the VA community. This provided an essential, safe, and structured environment in which 6 cycles of chemotherapy could be delivered.
Although the patient left the CLC after 2 months due to floor restrictions negatively impacting his quality of life and ability to spend time with close friends, several important events occurred during this stay. First, the patient established close relationships with the CLC staff and the palliative care team; both groups followed him throughout his inpatient and outpatient care. These relationships proved essential throughout his care as they were the foundation of difficult conversations about substance use, treatment adherence, and eventually, transition to hospice.
In addition, the opportunity to administer 6 cycles of chemotherapy at the CLC was enough to lead to clinical benefit and radiographic response to treatment. Clinical benefits while in the CLC included maintenance of a good appetite, 15-lb weight gain and preserved performance status (ECOG [Eastern Cooperative Group]-1), which allowed him to actively participate in multiple social and recreational activities while in the CLC. From early conversations, this patient was clear that he wanted treatment as long as his life could be prolonged with good quality of life. Having evidence of the benefit of treatment, at least initially, increased the patient’s confidence in treatment. There were a few conversations when the challenges of treatment mounted (eg, pain, needs for abstinence from cocaine prior to admission for chemotherapy, frequent doctor appointments), and the patient would remind himself of these data to recommit himself to treatment. The opportunity to admit him to the inpatient VA facility, including bed availability for 3 days during his treatment once he left the CLC was important. This plan to admit the patient following a negative urine toxicology test for cocaine was made collaboratively with the veteran and the oncology and palliative care teams. The plan allowed the patient to achieve his treatment goals while maintaining his safety and reducing theoretical cardiac toxicities with his cancer treatment.
Finally, the availability of a multidisciplinary team approach including palliative care, oncology, psychology, addiction medicine and addiction psychiatry, was critical for addressing the veteran’s malignant pain. Palliative care worked in close collaboration with the ORC to prescribe and renew pain medications. ORC offered ongoing consultation on pain management in the context of OUD. As the veteran’s cancer progressed and functional decline prohibited his daily attendance at the community methadone clinic, palliative care and ORC met with the methadone clinic to arrange a less frequent methadone pickup schedule (the patient previously needed daily pickup). Non-VA settings may not have access to these resources to safely treat the biopsychosocial issues that arise in complex cases.
Substance Use and Cancer Treatments
This case raises several critical questions for oncologic care. Cocaine and fluorouracil are both associated with cardiotoxicity, and many oncologists would not feel it is safe to administer a regimen containing fluorouracil to a patient with active cocaine use. The National Comprehensive Cancer Network (NCCN) panel recommends FOLFIRINOX as a preferred category 1 recommendation for first-line treatment of patients with advanced pancreas cancer with good performance status.10 This recommendation is based on the PRODIGE trial, which has shown improved overall survival (OS): 11.1 vs 6.8 months for patients who received single-agent gemcitabine.11 If patients are not candidates for FOLFIRINOX and have good performance status, the NCCN recommends gemcitabine plus albumin-bound paclitaxel with category 1 level of evidence based on the IMPACT trial, which showed improvement in OS (8.7 vs 6.6 months compared with single-agent gemcitabine).12
Some oncologists may have additional concerns administering fluorouracil treatment alternatives (such as gemcitabine and albumin-bound paclitaxel) to individuals with active SUD because of concerns about altered mental status impacting the ability to report important adverse effects. In the absence of sufficient data, HCPs must determine whether they feel it is safe to administer these agents in individuals with active cocaine use. However, denying these patients the possible benefits of standard-of-care life-prolonging therapies without established data raises concerns regarding the ethics of such practices. There is concern that the stigma surrounding cocaine use might contribute to withholding treatment, while treatment is continued for individuals taking prescribed stimulant medications that also have cardiotoxicity risks. VA health care facilities are uniquely situated to use all available resources to address these issues using interprofessional patient-centered care and determine the most optimal treatment based on a risk/benefit discussion between the patient and the HCP.
Similarly, this case also raised questions among HCPs about the safety of using an indwelling port for treatment in a patient with SUD. In the current case there was concern about keeping in a port for a patient with a history of IV drug use; therefore, a PICC line was initiated and removed at each admission. Without guidelines in these situations, HCPs are left to weigh the risks and benefits of using a port or a PICC for individuals with recent or current substance use without formal data, which can lead to inconsistent access to care. More guidance is needed for these situations.
SUD Screening
This case begs the question of whether oncologists are adequately screening for a range of SUDs, and when they encounter an issue, how they are addressing it. Many oncologists do not receive adequate training on assessment of current or recent substance use. There are health care and systems-level practices that may increase patient safety for individuals with ongoing substance use who are undergoing cancer treatment. Training on obtaining appropriate substance use histories, motivational interviewing to resolve ambivalence about substance use in the direction of change, and shared decision making about treatment options could increase confidence in understanding and addressing substance use issues. It is also important to educate oncologists on how to address patients who return to or continued substance use during treatment. In this case the collaboration from palliative care, psychology, addiction medicine, and addiction psychiatry through the ORC was essential in assisting with ongoing assessment of substance use, guiding difficult conversations about the impact of substance use on the treatment plan, and identifying risk-mitigation strategies. Close collaboration and full utilization of all VA resources allowed this patient to receive first-line treatment for pancreatic cancer in order to reach his goal of prolonging his life while maintaining acceptable quality of life. Table 2 provides best practices for management of patients with comorbid SUD and cancer.
More research is needed into cancer treatment for patients with SUD, especially in the current era of cancer care using novel cancer treatments leading to significantly improved survival in many cancer types. Ideally, oncologists should be routinely or consistently screening patients for substance use, including alcohol. The patient should participate in this decision-making process after being educated about the risks and benefits. These patients can be followed using a multimodal approach to increase their rates of success and improve their quality of life. Although the literature is limited and no formal guidelines are available, VA oncologists are fortunate to have a range of resources available to them to navigate these difficult cases. Veterans have elevated rates of SUD, making this a critical issue to consider in the VA.13 It is the hope that this case can highlight how to take advantage of the many VA resources in order to ensure equitable cancer care for all veterans.
Conclusions
This case demonstrates that cancer-directed treatment is safe and feasible in a patient with advanced pancreatic cancer and coexisting active SUD by using a multidisciplinary approach. The multidisciplinary team included palliative care, oncology, psychology, addiction medicine, and addiction psychiatry. Critical steps for a successful outcome include gathering history about SUD; motivational interviewing to resolve ambivalence about treatment for SUD; shared decision making about cancer treatment; and risk-reduction strategies in pain and SUD management.
Treatment advancements in many cancer types have led to significantly longer survival, and it is critical to develop safe protocols to treat patients with active SUD so they also can derive benefit from these very significant medical advancements.
Acknowledgments
Michal Rose, MD, Director of VACHS Cancer Center, and Chandrika Kumar, MD, Director of VACHS Community Living Center, for their collaboration in care for this veteran.
Substance use disorders (SUDs) are an important but understudied aspect of treating patients diagnosed with cancer. Substance use can affect cancer treatment outcomes, including morbidity and mortality.1,2 Additionally, patients with cancer and SUD may have unique psychosocial needs that require close attention and management. There is a paucity of data regarding the best approach to treating such patients. For example, cocaine use may increase the cardiovascular and hematologic risk of some traditional chemotherapy agents.3,4 Newer targeted agents and immunotherapies remain understudied with respect to SUD risk.
Although the US Department of Veterans Affairs (VA) has established helpful clinical practice guidelines for the treatment of SUD, there are no guidelines for treating patients with SUD and cancer.5 Clinicians have limited confidence in treatment approach, and treatment is inconsistent among oncologists nationwide even within the same practice. Furthermore, it can be challenging to safely prescribe opioids for cancer-related pain in individuals with SUD. There is a high risk of SUD and mental health disorders in veterans, making this population particularly vulnerable. We report a case of a male with metastatic pancreatic cancer, severe opioid use disorder (OUD) and moderate cocaine use disorder (CUD) who received pain management and cancer treatment under the direction of a multidisciplinary team approach.
Case Report
A 63-year-old male with a medical history of HIV treated with highly active antiretroviral therapy (HAART), compensated cirrhosis, severe OUD, moderate CUD, and sedative use disorder in sustained remission was admitted to the West Haven campus of the VA Connecticut Healthcare System (VACHS) with abdominal pain, weight loss and fatigue. He used heroin 1 month prior to his admission and reported regular cocaine and marijuana use (Table 1). He was diagnosed with HIV in 1989, and his medical history included herpes zoster and oral candidiasis but no other opportunistic infections. Several months prior to this admission, he had an undetectable viral load and CD4 count of 688.
At the time of this admission, the patient was adherent to methadone treatment. He reported increased abdominal pain. Computed tomography (CT) showed a 2.4-cm mass in the pancreatic uncinate process, multiple liver metastases, retroperitoneal lymphadenopathy, and small lung nodules. A CT-guided liver biopsy showed adenocarcinoma consistent with a primary cancer of the pancreas. Given the complexity of the case, a multidisciplinary team approach was used to treat his cancer and the sequelae safely, including the oncology team, community living center team, palliative care team, and interprofessional opioid reassessment clinic team (ORC).
Cancer Treatment
Chemotherapy with FOLFIRINOX (leucovorin calcium, fluorouracil, irinotecan hydrochloride, and oxaliplatin) was recommended. The first cycle of treatment originally was planned for the outpatient setting, and a peripherally inserted central catheter (PICC) line was placed. However, after a urine toxicology test was positive for cocaine, the PICC line was removed due to concern for possible use of PICC line for nonprescribed substance use. The patient expressed suicidal ideation at the time and was admitted for psychiatric consult and pain control. Cycle 1 FOLFIRINOX was started during this admission. A PICC line was again put in place and then removed before discharge. A celiac plexus block was performed several days after this admission for pain control.
Given concern about cocaine use increasing the risk of cardiac toxicity with FOLFIRINOX treatment, treating providers sconsulted with the community living center (CLC) about possible admission for future chemotherapy administration and pain management. The CLC at VACHS has 38 beds for rehabilitation, long-term care, and hospice with the mission to restore each veteran to his or her highest level of well-being. After discussion with this patient and CLC staff, he agreed to a CLC admission. The patient agreed to remain in the facility, wear a secure care device, and not leave without staff accompaniment. He was able to obtain a 2-hour pass to pay bills and rent. During the 2 months he was admitted to the CLC he would present to the VACHS Cancer Center for chemotherapy every 2 weeks. He completed 6 cycles of chemotherapy while admitted. During the admission, he was transferred to active medical service for 2 days for fever and malaise, and then returned to the CLC. The patient elected to leave the CLC after 2 months as the inability to see close friends was interfering with his quality of life.
Upon being discharged from the CLC, shared decision making took place with the patient to establish a new treatment plan. In collaboration with the patient, a plan was made to admit him every 2 weeks for continued chemotherapy. A PICC line was placed on each day of admission and removed prior to discharge. It was also agreed that treatment would be delayed if a urine drug test was positive for cocaine on the morning of admission. The patient was also seen by ORC every 2 weeks after being discharged from the CLC.
Imaging after cycle 6 showed decreased size of liver metastases, retroperitoneal lymph nodes, and pancreas mass. Cancer antigen 19-9 (CA19-9) tumor marker was reduced from 3513 U/mL pretreatment to 50 U/mL after cycle 7. Chemotherapy cycle 7 was delayed 6 days due to active cocaine and heroin use. A repeat urine was obtained several days later, which was negative for cocaine, and he was admitted for cycle 7 chemotherapy. Using this treatment approach of admissions for every cycle, the patient was able to receive 11 cycles of FOLFIRINOX with clinical benefit.
Palliative Care/Pain Management
Safely treating the patient’s malignant pain in the context of his OUD was critically important. In order to do this the palliative care team worked closely alongside ORC, is a multidisciplinary team consisting of health care providers (HCPs) from addiction psychiatry, internal medicine, health psychology and pharmacy who are consulted to evaluate veterans’ current opioid regimens and make recommendations to optimize both safety and efficacy. ORC followed this particular veteran as an outpatient and consulted on pain issues during his admission. They recommended the continuation of methadone at 120 mg daily and increased oral oxycodone to 30 mg every 6 hours, and then further increased to 45 mg every 6 hours. He continued to have increased pain despite higher doses of oxycodone, and pain medication was changed to oral hydromorphone 28 mg every 6 hours with the continuation of methadone. ORC and the palliative care team obtained consent from the veteran and a release of Information form signed by the patient to contact his community methadone clinic for further collaboration around pain management throughout the time caring for the veteran.
Even with improvement in disease based on imaging and tumor markers, opioid medications could not be decreased in this case. This is likely in part due to the multidimensional nature of pain. Careful assessment of the biologic, emotional, social, and spiritual contributors to pain is needed in the management of pain, especially at end of life.6 Nonpharmacologic pain management strategies used in this case included a transcutaneous electrical nerve stimulation unit, moist heat, celiac plexus block, and emotional support.
Psychosocial Issues/Substance Use
Psychosocial support for the patient was provided by the interdisciplinary palliative care team and the ORC team in both the inpatient and outpatient settings. Despite efforts from case management to get the veteran home services once discharged from the CLC, he declined repeatedly. Thus, the CLC social worker obtained a guardian alert for the veteran on discharge.
Close outpatient follow-up for medical and psychosocial support was very critical. When an outpatient, the veteran was scheduled for biweekly appointments with palliative care or ORC. When admitted to the hospital, the palliative care team medical director and psychologist conducted joint visits with him. Although he denied depressed mood and anxiety throughout his treatment, he often reflected on regrets that he had as he faced the end of his life. Specifically, he shared thoughts about being estranged from his surviving brother given his long struggle with substance use. Although he did not think a relationship was possible with his brother at the end of life, he still cared deeply for him and wanted to make him aware of his pancreatic cancer diagnosis. This was particularly important to him because their late brother had also died of pancreatic cancer. It was the patient’s wish at the end of his life to alert his surviving brother of his diagnosis so he and his children could get adequate screening throughout their lives. Although he had spoken of this desire often, it wasn’t until his disease progressed and he elected to transition to hospice that he felt ready to write the letter. The palliative care team assisted the veteran in writing and mailing a letter to his brother informing him of his diagnosis and transition to hospice as well as communicating that his brother and his family had been in his thoughts at the end of his life. The patient’s brother received this letter and with assistance from the CLC social worker made arrangements to visit the veteran at bedside at the inpatient CLC hospice unit the final days of his life.
Discussion
There are very little data on the safety of cancer-directed therapy in patients with active SUD. The limited studies that have been done showed conflicting results.
A retrospective study among women with co-occurring SUD and locally advanced cervical cancer who were undergoing primary radiation therapy found that SUD was not associated with a difference in toxicity or survival outcomes.7 However, other research suggests that SUD may be associated with an increase in all-cause mortality as well as other adverse outcomes for patients and health care systems (eg, emergency department visits, hospitalizations).8 A retrospective study of patients with a history of SUD and nonsmall cell lung cancer showed that these patients had higher rates of depression, less family support, increased rates of missed appointments, more emergency department visits and more hospitalizations.9 Patients with chronic myeloid leukemia or myelodysplastic syndromes who had long-term cocaine use had a 6-fold increased risk of death, which was not found in patients who had long-term alcohol or marijuana use.2
The limited data highlight the need for careful consideration of ways to mitigate potentially adverse outcomes in this population while still providing clinically indicated cancer treatment. Integrated VA health care systems provide unique resources that can maximize veteran safety during cancer treatment. Utilization of VA resources and close interdisciplinary collaboration across VA HCPs can help to ensure equitable access to state-of-the-art cancer therapies for veterans with comorbid SUD.
VA Services for Patients With Comorbidities
This case highlights several distinct aspects of VA health care that make it possible to safely treat individuals with complex comorbidities. One important aspect of this was collaboration with the CLC to admit the veteran for his initial treatment after a positive cocaine test. CLC admission was nonpunitive and allowed ongoing involvement in the VA community. This provided an essential, safe, and structured environment in which 6 cycles of chemotherapy could be delivered.
Although the patient left the CLC after 2 months due to floor restrictions negatively impacting his quality of life and ability to spend time with close friends, several important events occurred during this stay. First, the patient established close relationships with the CLC staff and the palliative care team; both groups followed him throughout his inpatient and outpatient care. These relationships proved essential throughout his care as they were the foundation of difficult conversations about substance use, treatment adherence, and eventually, transition to hospice.
In addition, the opportunity to administer 6 cycles of chemotherapy at the CLC was enough to lead to clinical benefit and radiographic response to treatment. Clinical benefits while in the CLC included maintenance of a good appetite, 15-lb weight gain and preserved performance status (ECOG [Eastern Cooperative Group]-1), which allowed him to actively participate in multiple social and recreational activities while in the CLC. From early conversations, this patient was clear that he wanted treatment as long as his life could be prolonged with good quality of life. Having evidence of the benefit of treatment, at least initially, increased the patient’s confidence in treatment. There were a few conversations when the challenges of treatment mounted (eg, pain, needs for abstinence from cocaine prior to admission for chemotherapy, frequent doctor appointments), and the patient would remind himself of these data to recommit himself to treatment. The opportunity to admit him to the inpatient VA facility, including bed availability for 3 days during his treatment once he left the CLC was important. This plan to admit the patient following a negative urine toxicology test for cocaine was made collaboratively with the veteran and the oncology and palliative care teams. The plan allowed the patient to achieve his treatment goals while maintaining his safety and reducing theoretical cardiac toxicities with his cancer treatment.
Finally, the availability of a multidisciplinary team approach including palliative care, oncology, psychology, addiction medicine and addiction psychiatry, was critical for addressing the veteran’s malignant pain. Palliative care worked in close collaboration with the ORC to prescribe and renew pain medications. ORC offered ongoing consultation on pain management in the context of OUD. As the veteran’s cancer progressed and functional decline prohibited his daily attendance at the community methadone clinic, palliative care and ORC met with the methadone clinic to arrange a less frequent methadone pickup schedule (the patient previously needed daily pickup). Non-VA settings may not have access to these resources to safely treat the biopsychosocial issues that arise in complex cases.
Substance Use and Cancer Treatments
This case raises several critical questions for oncologic care. Cocaine and fluorouracil are both associated with cardiotoxicity, and many oncologists would not feel it is safe to administer a regimen containing fluorouracil to a patient with active cocaine use. The National Comprehensive Cancer Network (NCCN) panel recommends FOLFIRINOX as a preferred category 1 recommendation for first-line treatment of patients with advanced pancreas cancer with good performance status.10 This recommendation is based on the PRODIGE trial, which has shown improved overall survival (OS): 11.1 vs 6.8 months for patients who received single-agent gemcitabine.11 If patients are not candidates for FOLFIRINOX and have good performance status, the NCCN recommends gemcitabine plus albumin-bound paclitaxel with category 1 level of evidence based on the IMPACT trial, which showed improvement in OS (8.7 vs 6.6 months compared with single-agent gemcitabine).12
Some oncologists may have additional concerns administering fluorouracil treatment alternatives (such as gemcitabine and albumin-bound paclitaxel) to individuals with active SUD because of concerns about altered mental status impacting the ability to report important adverse effects. In the absence of sufficient data, HCPs must determine whether they feel it is safe to administer these agents in individuals with active cocaine use. However, denying these patients the possible benefits of standard-of-care life-prolonging therapies without established data raises concerns regarding the ethics of such practices. There is concern that the stigma surrounding cocaine use might contribute to withholding treatment, while treatment is continued for individuals taking prescribed stimulant medications that also have cardiotoxicity risks. VA health care facilities are uniquely situated to use all available resources to address these issues using interprofessional patient-centered care and determine the most optimal treatment based on a risk/benefit discussion between the patient and the HCP.
Similarly, this case also raised questions among HCPs about the safety of using an indwelling port for treatment in a patient with SUD. In the current case there was concern about keeping in a port for a patient with a history of IV drug use; therefore, a PICC line was initiated and removed at each admission. Without guidelines in these situations, HCPs are left to weigh the risks and benefits of using a port or a PICC for individuals with recent or current substance use without formal data, which can lead to inconsistent access to care. More guidance is needed for these situations.
SUD Screening
This case begs the question of whether oncologists are adequately screening for a range of SUDs, and when they encounter an issue, how they are addressing it. Many oncologists do not receive adequate training on assessment of current or recent substance use. There are health care and systems-level practices that may increase patient safety for individuals with ongoing substance use who are undergoing cancer treatment. Training on obtaining appropriate substance use histories, motivational interviewing to resolve ambivalence about substance use in the direction of change, and shared decision making about treatment options could increase confidence in understanding and addressing substance use issues. It is also important to educate oncologists on how to address patients who return to or continued substance use during treatment. In this case the collaboration from palliative care, psychology, addiction medicine, and addiction psychiatry through the ORC was essential in assisting with ongoing assessment of substance use, guiding difficult conversations about the impact of substance use on the treatment plan, and identifying risk-mitigation strategies. Close collaboration and full utilization of all VA resources allowed this patient to receive first-line treatment for pancreatic cancer in order to reach his goal of prolonging his life while maintaining acceptable quality of life. Table 2 provides best practices for management of patients with comorbid SUD and cancer.
More research is needed into cancer treatment for patients with SUD, especially in the current era of cancer care using novel cancer treatments leading to significantly improved survival in many cancer types. Ideally, oncologists should be routinely or consistently screening patients for substance use, including alcohol. The patient should participate in this decision-making process after being educated about the risks and benefits. These patients can be followed using a multimodal approach to increase their rates of success and improve their quality of life. Although the literature is limited and no formal guidelines are available, VA oncologists are fortunate to have a range of resources available to them to navigate these difficult cases. Veterans have elevated rates of SUD, making this a critical issue to consider in the VA.13 It is the hope that this case can highlight how to take advantage of the many VA resources in order to ensure equitable cancer care for all veterans.
Conclusions
This case demonstrates that cancer-directed treatment is safe and feasible in a patient with advanced pancreatic cancer and coexisting active SUD by using a multidisciplinary approach. The multidisciplinary team included palliative care, oncology, psychology, addiction medicine, and addiction psychiatry. Critical steps for a successful outcome include gathering history about SUD; motivational interviewing to resolve ambivalence about treatment for SUD; shared decision making about cancer treatment; and risk-reduction strategies in pain and SUD management.
Treatment advancements in many cancer types have led to significantly longer survival, and it is critical to develop safe protocols to treat patients with active SUD so they also can derive benefit from these very significant medical advancements.
Acknowledgments
Michal Rose, MD, Director of VACHS Cancer Center, and Chandrika Kumar, MD, Director of VACHS Community Living Center, for their collaboration in care for this veteran.
1. Chang G, Meadows ME, Jones JA, Antin JH, Orav EJ. Substance use and survival after treatment for chronic myelogenous leukemia (CML) or myelodysplastic syndrome (MDS). Am J Drug Alcohol Ab. 2010;36(1):1-6. doi:10.3109/00952990903490758
2. Stagno S, Busby K, Shapiro A, Kotz M. Patients at risk: addressing addiction in patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2008;42(4):221-226. doi:10.1038/bmt.2008.211
3. Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345(1):45-51. doi:10.1097/MAJ.0b013e31825b2b50
4. Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122(24):2558-2569. doi:10.1161/CIRCULATIONAHA.110.940569
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. Published 2015. Accessed July 8, 2021. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPGRevised22216.pdf
6. Mehta A, Chan LS. Understanding of the concept of “total pain”: a prerequisite for pain control. J Hosp Palliat Nurs. 2008;10(1):26-32. doi:10.1097/01.NJH.0000306714.50539.1a
7. Rubinsak LA, Terplan M, Martin CE, Fields EC, McGuire WP, Temkin SM. Co-occurring substance use disorder: The impact on treatment adherence in women with locally advanced cervical cancer. Gynecol Oncol Rep. 2019;28:116-119. Published 2019 Mar 27. doi:10.1016/j.gore.2019.03.016
8. Chhatre S, Metzger DS, Malkowicz SB, Woody G, Jayadevappa R. Substance use disorder and its effects on outcomes in men with advanced-stage prostate cancer. Cancer. 2014;120(21):3338-3345. doi:10.1002/cncr.28861
9. Concannon K, Thayer JH, Hicks R, et al. Outcomes among patients with a history of substance abuse in non-small cell lung cancer: a county hospital experience. J Clin Onc. 2019;37(15)(suppl):e20031-e20031. doi:10.1200/JCO.2019.37.15
10. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma. Version 2.2021. Updated February 25, 2021. Accessed July 8, 2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
11. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi:10.1056/NEJMoa1011923
12. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703. doi:10.1056/NEJMoa1304369
13. Seal KH, Cohen G, Waldrop A, Cohen BE, Maguen S, Ren L. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: Implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116(1-3):93-101. doi:10.1016/j.drugalcdep.2010.11.027
14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association; 2013.
1. Chang G, Meadows ME, Jones JA, Antin JH, Orav EJ. Substance use and survival after treatment for chronic myelogenous leukemia (CML) or myelodysplastic syndrome (MDS). Am J Drug Alcohol Ab. 2010;36(1):1-6. doi:10.3109/00952990903490758
2. Stagno S, Busby K, Shapiro A, Kotz M. Patients at risk: addressing addiction in patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2008;42(4):221-226. doi:10.1038/bmt.2008.211
3. Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345(1):45-51. doi:10.1097/MAJ.0b013e31825b2b50
4. Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122(24):2558-2569. doi:10.1161/CIRCULATIONAHA.110.940569
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. Published 2015. Accessed July 8, 2021. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPGRevised22216.pdf
6. Mehta A, Chan LS. Understanding of the concept of “total pain”: a prerequisite for pain control. J Hosp Palliat Nurs. 2008;10(1):26-32. doi:10.1097/01.NJH.0000306714.50539.1a
7. Rubinsak LA, Terplan M, Martin CE, Fields EC, McGuire WP, Temkin SM. Co-occurring substance use disorder: The impact on treatment adherence in women with locally advanced cervical cancer. Gynecol Oncol Rep. 2019;28:116-119. Published 2019 Mar 27. doi:10.1016/j.gore.2019.03.016
8. Chhatre S, Metzger DS, Malkowicz SB, Woody G, Jayadevappa R. Substance use disorder and its effects on outcomes in men with advanced-stage prostate cancer. Cancer. 2014;120(21):3338-3345. doi:10.1002/cncr.28861
9. Concannon K, Thayer JH, Hicks R, et al. Outcomes among patients with a history of substance abuse in non-small cell lung cancer: a county hospital experience. J Clin Onc. 2019;37(15)(suppl):e20031-e20031. doi:10.1200/JCO.2019.37.15
10. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma. Version 2.2021. Updated February 25, 2021. Accessed July 8, 2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
11. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi:10.1056/NEJMoa1011923
12. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703. doi:10.1056/NEJMoa1304369
13. Seal KH, Cohen G, Waldrop A, Cohen BE, Maguen S, Ren L. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: Implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116(1-3):93-101. doi:10.1016/j.drugalcdep.2010.11.027
14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association; 2013.
Opioid prescribing laws having an impact
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
State laws capping initial opioid prescriptions to 7 days or less have led to a reduction in opioid prescribing, a new analysis of Medicare data shows.
While overall opioid prescribing has decreased, the reduction in states with legislation restricting opioid prescribing was “significantly greater than in states without such legislation,” study investigator Michael Brenner, MD, University of Michigan, Ann Arbor, said in an interview.
The study was published online August 9 in JAMA Internal Medicine.
Significant but limited effect
Because of rising concern around the opioid crisis, 23 states representing 43% of the U.S. population passed laws from 2016 through 2018 limiting initial opioid prescription to 7 days or less.
Using Medicare data from 2013 through 2018, Dr. Brenner and colleagues conducted a before-and-after study to assess the effect of these laws.
They found that on average, the number of days an opioid was prescribed for each Medicare beneficiary decreased by 11.6 days (from 44.2 days in 2013 to 32.7 days in 2018) in states that imposed duration limits, compared with 10.1 days in states without these laws (from 43.4 days in 2013 to 33.3 days in 2018).
Prior to the start of duration limits in 2016, days an opioid was prescribed were comparable among states.
After adjusting for state-level differences in race, urbanization, median income, tobacco and alcohol use, serious mental illness, and other factors, state laws limiting opioid prescriptions to 7 days or less were associated with a reduction in prescribing of 1.7 days per enrollee, “suggesting a significant but limited outcome” for these laws, the researchers note.
, but this was not significantly different in states with limit laws versus those without. However, state laws limiting duration led to a significant reduction in days of opioid prescribed among surgeons, dentists, pain specialists, and other specialists.
Inadequate pain control?
The researchers note the study was limited to Medicare beneficiaries; however, excess opioid prescribing is prevalent across all patient populations.
In addition, it’s not possible to tell from the data whether acute pain was adequately controlled with fewer pills.
“The question of adequacy of pain control is a crucial one that has been investigated extensively in prior work but was not possible to evaluate in this particular study,” said Dr. Brenner.
However, “ample evidence supports a role for reducing opioid prescribing and that such reduction can be achieved while ensuring that pain is adequately controlled with fewer pills,” he noted.
“A persistent misconception is that opioids are uniquely powerful and effective for controlling pain. Patients may perceive that effective analgesia is being withheld when opioids are not included in a regimen,” Dr. Brenner added.
“Yet, the evidence from meta-analyses derived from large numbers of randomized clinical trials finds that [nonsteroidal anti-inflammatory drugs] NSAIDS combined with acetaminophen provide similar or improved acute pain when compared to commonly prescribed opioid regimens, based on number-needed-to-treat analyses,” he added.
In a related editorial, Deborah Grady, MD, MPH, with University of California, San Francisco, and Mitchell H. Katz, MD, president and CEO of NYC Health + Hospitals, say the decrease in opioid prescribing with duration limits was “small but probably meaningful.”
Restricting initial prescriptions to seven or fewer days is “reasonable because patients with new onset of pain should be re-evaluated in a week if the pain continues,” they write.
However, Dr. Grady and Dr. Katz “worry” that restricting initial prescriptions to shorter periods, such as 3 or 5 days, as has occurred in six states, “may result in patients with acute pain going untreated or having to go to extraordinary effort to obtain adequate pain relief.”
In their view, the data from this study suggest that limiting initial prescriptions to seven or fewer days is “helpful, but we would not restrict any further given that we do not know how it affected patients with acute pain.”
The study had no specific funding. Dr. Brenner, Dr. Grady, and Dr. Katz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physician ‘predator’ sentenced for opioid-related patient death; more
Doctor’s illegal opioid distribution results in patient death
Thomas K. Ballard III, MD, of Jackson, Tenn., pleaded guilty to causing the death of one of his patients in 2015 by illegally prescribing hydrocodone. He faces a minimum of 20 years in prison for one count of illegal drug distribution resulting in death. He will be sentenced in September.
Dr. Ballard, 63, owned and operated the Ballard Clinic, from which he prescribed dangerous and addictive drugs without legitimate medical purpose. according to the U.S. Department of Justice.
Dr. Ballard’s treatment records show that he believed a patient had psychiatric problems and was abusing her medication, evidenced by positive drug screens and prescriptions obtained elsewhere for suboxone, a drug used to treat opioid dependency. However, Dr. Ballard continued to prescribe hydrocodone to the patient, including on May 28, 2015, when the patient fatally overdosed on the drug.
“Ballard has proven himself to be nothing more than a predator in a white lab coat, and he should expect to be punished accordingly,” said Special Agent in Charge J. Todd Scott of the Drug Enforcement Administration’s Louisville Division. “Doctors take an oath to first do no harm, and instead, Ballard chose to put his own licentious interests above his patients’ well-being.”
Clinical researchers falsify drug trial data
Eduardo Navarro, of Miami, and Nayade Varona, of Port St. Lucie, Fla., pleaded guilty to conspiring to falsify clinical trial data. Mr. Navarro, 52, was sentenced to 46 months in prison and Ms. Varona, 50, received 30 months in prison.
The court also ordered the defendants to pay $2,134,503 in restitution.
Mr. Navarro and Ms. Varona both worked for Tellus Clinical Research, where Mr. Navarro was a subinvestigator and nurse practitioner, and Ms. Varona was an assistant study coordinator. They admitted to agreeing with one another and others to falsify data in medical records for two clinical trials that were evaluating a treatment for irritable bowel syndrome. Mr. Navarro and Ms. Varona falsified data to make it appear as though patients were participating in the trials, which never occurred.
Doctor faces decade in prison for $6 million health care fraud
Keyvan Amirikhorheh, MD, a family physician in Seal Beach, Calif., pleaded guilty to conspiring to commit health care fraud. He faces a maximum of 10 years in prison.
While working at Los Angeles Community Clinic, Dr. Amirikhorheh submitted fraudulent claims for family planning services, diagnostic testing, and prescriptions for nonexistent patients, defrauding the Family Planning, Access, Care and Treatment (Family PACT) program administered by Medi-Cal, the California Medicaid program.
Between March 2016 and April 2019, Los Angeles Community Clinic and associated laboratories and pharmacies submitted approximately $8,406,204 in claims to Medi-Cal and were paid approximately $6,660,028. Dr. Amirikhorheh, 61, is the final defendant of five to plead guilty in the case, according to the DOJ.
Dentist office sued for HIV discrimination
Night and Day Dental, of North Carolina, settled with the DOJ to resolve a claim that it discriminated against a woman with HIV in violation of the Americans With Disabilities Act.
Title III of the ADA prohibits health care professionals from discriminating against people with disabilities, including those with HIV. The DOJ found that Night and Day Dental refused to accept a woman as a new patient because of her HIV-positive status. The patient was seeking routine dental care, including a cleaning and check-up. Night and Day Dental additionally requires certain blood work results from patients with HIV before deciding whether to provide dental care, when requiring such results is not medically necessary.
They will pay $30,000 to the victim of the discrimination, train their staff on the ADA, develop an antidiscrimination policy, and report to the DOJ every time they refuse to treat a person with HIV or stop providing treatment after learning of a patient’s HIV-positive status. “Turning away patients with HIV or requiring them to provide information that is not medically recommended creates unfair barriers to health care for people with HIV,” said Kristen Clarke, assistant attorney general in the DOJ’s Civil Rights Division.
A version of this article first appeared on Medscape.com.
This article was updated 8/12/21.
Doctor’s illegal opioid distribution results in patient death
Thomas K. Ballard III, MD, of Jackson, Tenn., pleaded guilty to causing the death of one of his patients in 2015 by illegally prescribing hydrocodone. He faces a minimum of 20 years in prison for one count of illegal drug distribution resulting in death. He will be sentenced in September.
Dr. Ballard, 63, owned and operated the Ballard Clinic, from which he prescribed dangerous and addictive drugs without legitimate medical purpose. according to the U.S. Department of Justice.
Dr. Ballard’s treatment records show that he believed a patient had psychiatric problems and was abusing her medication, evidenced by positive drug screens and prescriptions obtained elsewhere for suboxone, a drug used to treat opioid dependency. However, Dr. Ballard continued to prescribe hydrocodone to the patient, including on May 28, 2015, when the patient fatally overdosed on the drug.
“Ballard has proven himself to be nothing more than a predator in a white lab coat, and he should expect to be punished accordingly,” said Special Agent in Charge J. Todd Scott of the Drug Enforcement Administration’s Louisville Division. “Doctors take an oath to first do no harm, and instead, Ballard chose to put his own licentious interests above his patients’ well-being.”
Clinical researchers falsify drug trial data
Eduardo Navarro, of Miami, and Nayade Varona, of Port St. Lucie, Fla., pleaded guilty to conspiring to falsify clinical trial data. Mr. Navarro, 52, was sentenced to 46 months in prison and Ms. Varona, 50, received 30 months in prison.
The court also ordered the defendants to pay $2,134,503 in restitution.
Mr. Navarro and Ms. Varona both worked for Tellus Clinical Research, where Mr. Navarro was a subinvestigator and nurse practitioner, and Ms. Varona was an assistant study coordinator. They admitted to agreeing with one another and others to falsify data in medical records for two clinical trials that were evaluating a treatment for irritable bowel syndrome. Mr. Navarro and Ms. Varona falsified data to make it appear as though patients were participating in the trials, which never occurred.
Doctor faces decade in prison for $6 million health care fraud
Keyvan Amirikhorheh, MD, a family physician in Seal Beach, Calif., pleaded guilty to conspiring to commit health care fraud. He faces a maximum of 10 years in prison.
While working at Los Angeles Community Clinic, Dr. Amirikhorheh submitted fraudulent claims for family planning services, diagnostic testing, and prescriptions for nonexistent patients, defrauding the Family Planning, Access, Care and Treatment (Family PACT) program administered by Medi-Cal, the California Medicaid program.
Between March 2016 and April 2019, Los Angeles Community Clinic and associated laboratories and pharmacies submitted approximately $8,406,204 in claims to Medi-Cal and were paid approximately $6,660,028. Dr. Amirikhorheh, 61, is the final defendant of five to plead guilty in the case, according to the DOJ.
Dentist office sued for HIV discrimination
Night and Day Dental, of North Carolina, settled with the DOJ to resolve a claim that it discriminated against a woman with HIV in violation of the Americans With Disabilities Act.
Title III of the ADA prohibits health care professionals from discriminating against people with disabilities, including those with HIV. The DOJ found that Night and Day Dental refused to accept a woman as a new patient because of her HIV-positive status. The patient was seeking routine dental care, including a cleaning and check-up. Night and Day Dental additionally requires certain blood work results from patients with HIV before deciding whether to provide dental care, when requiring such results is not medically necessary.
They will pay $30,000 to the victim of the discrimination, train their staff on the ADA, develop an antidiscrimination policy, and report to the DOJ every time they refuse to treat a person with HIV or stop providing treatment after learning of a patient’s HIV-positive status. “Turning away patients with HIV or requiring them to provide information that is not medically recommended creates unfair barriers to health care for people with HIV,” said Kristen Clarke, assistant attorney general in the DOJ’s Civil Rights Division.
A version of this article first appeared on Medscape.com.
This article was updated 8/12/21.
Doctor’s illegal opioid distribution results in patient death
Thomas K. Ballard III, MD, of Jackson, Tenn., pleaded guilty to causing the death of one of his patients in 2015 by illegally prescribing hydrocodone. He faces a minimum of 20 years in prison for one count of illegal drug distribution resulting in death. He will be sentenced in September.
Dr. Ballard, 63, owned and operated the Ballard Clinic, from which he prescribed dangerous and addictive drugs without legitimate medical purpose. according to the U.S. Department of Justice.
Dr. Ballard’s treatment records show that he believed a patient had psychiatric problems and was abusing her medication, evidenced by positive drug screens and prescriptions obtained elsewhere for suboxone, a drug used to treat opioid dependency. However, Dr. Ballard continued to prescribe hydrocodone to the patient, including on May 28, 2015, when the patient fatally overdosed on the drug.
“Ballard has proven himself to be nothing more than a predator in a white lab coat, and he should expect to be punished accordingly,” said Special Agent in Charge J. Todd Scott of the Drug Enforcement Administration’s Louisville Division. “Doctors take an oath to first do no harm, and instead, Ballard chose to put his own licentious interests above his patients’ well-being.”
Clinical researchers falsify drug trial data
Eduardo Navarro, of Miami, and Nayade Varona, of Port St. Lucie, Fla., pleaded guilty to conspiring to falsify clinical trial data. Mr. Navarro, 52, was sentenced to 46 months in prison and Ms. Varona, 50, received 30 months in prison.
The court also ordered the defendants to pay $2,134,503 in restitution.
Mr. Navarro and Ms. Varona both worked for Tellus Clinical Research, where Mr. Navarro was a subinvestigator and nurse practitioner, and Ms. Varona was an assistant study coordinator. They admitted to agreeing with one another and others to falsify data in medical records for two clinical trials that were evaluating a treatment for irritable bowel syndrome. Mr. Navarro and Ms. Varona falsified data to make it appear as though patients were participating in the trials, which never occurred.
Doctor faces decade in prison for $6 million health care fraud
Keyvan Amirikhorheh, MD, a family physician in Seal Beach, Calif., pleaded guilty to conspiring to commit health care fraud. He faces a maximum of 10 years in prison.
While working at Los Angeles Community Clinic, Dr. Amirikhorheh submitted fraudulent claims for family planning services, diagnostic testing, and prescriptions for nonexistent patients, defrauding the Family Planning, Access, Care and Treatment (Family PACT) program administered by Medi-Cal, the California Medicaid program.
Between March 2016 and April 2019, Los Angeles Community Clinic and associated laboratories and pharmacies submitted approximately $8,406,204 in claims to Medi-Cal and were paid approximately $6,660,028. Dr. Amirikhorheh, 61, is the final defendant of five to plead guilty in the case, according to the DOJ.
Dentist office sued for HIV discrimination
Night and Day Dental, of North Carolina, settled with the DOJ to resolve a claim that it discriminated against a woman with HIV in violation of the Americans With Disabilities Act.
Title III of the ADA prohibits health care professionals from discriminating against people with disabilities, including those with HIV. The DOJ found that Night and Day Dental refused to accept a woman as a new patient because of her HIV-positive status. The patient was seeking routine dental care, including a cleaning and check-up. Night and Day Dental additionally requires certain blood work results from patients with HIV before deciding whether to provide dental care, when requiring such results is not medically necessary.
They will pay $30,000 to the victim of the discrimination, train their staff on the ADA, develop an antidiscrimination policy, and report to the DOJ every time they refuse to treat a person with HIV or stop providing treatment after learning of a patient’s HIV-positive status. “Turning away patients with HIV or requiring them to provide information that is not medically recommended creates unfair barriers to health care for people with HIV,” said Kristen Clarke, assistant attorney general in the DOJ’s Civil Rights Division.
A version of this article first appeared on Medscape.com.
This article was updated 8/12/21.
Feasibility of Risk Stratification of Patients Presenting to the Emergency Department With Chest Pain Using HEART Score
From the Department of Internal Medicine, Mount Sinai Health System, Icahn School of Medicine at Mount Sinai, New York, NY (Dr. Gandhi), and the School of Medicine, Seth Gordhandas Sunderdas Medical College, and King Edward Memorial Hospital, Mumbai, India (Drs. Gandhi and Tiwari).
Objective: Calculation of HEART score to (1) stratify patients as low-risk, intermediate-risk, high-risk, and to predict the short-term incidence of major adverse cardiovascular events (MACE), and (2) demonstrate feasibility of HEART score in our local settings.
Design: A prospective cohort study of patients with a chief complaint of chest pain concerning for acute coronary syndrome.
Setting: Participants were recruited from the emergency department (ED) of King Edward Memorial Hospital, a tertiary care academic medical center and a resource-limited setting in Mumbai, India.
Participants: We evaluated 141 patients aged 18 years and older presenting to the ED and stratified them using the HEART score. To assess patients’ progress, a follow-up phone call was made within 6 weeks after presentation to the ED.
Measurements: The primary outcomes were a risk stratification, 6-week occurrence of MACE, and performance of unscheduled revascularization or stress testing. The secondary outcomes were discharge or death.
Results: The 141 participants were stratified into low-risk, intermediate-risk, and high-risk groups: 67 (47.52%), 44 (31.21%), and 30 (21.28%), respectively. The 6-week incidence of MACE in each category was 1.49%, 18.18%, and 90%, respectively. An acute myocardial infarction was diagnosed in 24 patients (17.02%), 15 patients (10.64%) underwent percutaneous coronary intervention (PCI), and 4 patients (2.84%) underwent coronary artery bypass graft (CABG). Overall, 98.5% of low-risk patients and 93.33% of high-risk patients had an uneventful recovery following discharge; therefore, extrapolation based on results demonstrated reduced health care utilization. All the survey respondents found the HEART score to be feasible. The patient characteristics and risk profile of the patients with and without MACE demonstrated that: patients with MACE were older and had a higher proportion of males, hypertension, type 2 diabetes mellitus, smoking, hypercholesterolemia, prior history of PCI/CABG, and history of stroke.
Conclusion: The HEART score seems to be a useful tool for risk stratification and a reliable predictor of outcomes in chest pain patients and can therefore be used for triage.
Keywords: chest pain; emergency department; HEART score; acute coronary syndrome; major adverse cardiac events; myocardial infarction; revascularization.
Cardiovascular diseases (CVDs), especially coronary heart disease (CHD), have epidemic proportions worldwide. Globally, in 2012, CVD led to 17.5 million deaths,1,2 with more than 75% of them occurring in developing countries. In contrast to developed countries, where mortality from CHD is rapidly declining, it is increasing in developing countries.1,3 Current estimates from epidemiologic studies from various parts of India indicate the prevalence of CHD in India to be between 7% and 13% in urban populations and 2% and 7% in rural populations.4
Premature mortality in terms of years of life lost because of CVD in India increased by 59% over a 20-year span, from 23.2 million in 1990 to 37 million in 2010.5 Studies conducted in Mumbai (Mumbai Cohort Study) reported very high CVD mortality rates, approaching 500 per 100 000 for men and 250 per 100 000 for women.6,7 However, to the best of our knowledge, in the Indian population, there are minimal data on utilization of a triage score, such as the HEART score, in chest pain patients in the emergency department (ED) in a resource-limited setting.
The most common reason for admitting patients to the ED is chest pain.8 There are various cardiac and noncardiac etiologies of chest pain presentation. Acute coronary syndrome (ACS) needs to be ruled out first in every patient presenting with chest pain. However, 80% of patients with ACS have no clear diagnostic features on presentation.9 The timely diagnosis and treatment of patients with ACS improves their prognosis. Therefore, clinicians tend to start each patient on ACS treatment to reduce the risk, which often leads to increased costs due to unnecessary, time-consuming diagnostic procedures that may place burdens on both the health care system and the patient.10
Several risk-stratifying tools have been developed in the last few years. Both the GRACE and TIMI risk scores have been designed for risk stratification of patients with proven ACS and not for the chest pain population at the ED.11 Some of these tools are applicable to patients with all types of chest pain presenting to the ED, such as the Manchester Triage System. Other, more selective systems are devoted to the risk stratification of suspected ACS in the ED. One is the HEART score.12
The first study on the HEART score—an acronym that stands for History, Electrocardiogram, Age, Risk factors, and Troponin—was done by Backus et al, who proved that the HEART score is an easy, quick, and reliable predictor of outcomes in chest pain patients.10 The HEART score predicts the short-term incidence of major adverse cardiac events (MACE), which allows clinicians to stratify patients as low-risk, intermediate-risk, and high-risk and to guide their clinical decision-making accordingly. It was developed to provide clinicians with a simple, reliable predictor of cardiac risk on the basis of the lowest score of 0 (very low-risk) up to a score of 10 (very high-risk).
We studied the clinical performance of the HEART score in patients with chest pain, focusing on the efficacy and safety of rapidly identifying patients at risk of MACE. We aimed to determine (1) whether the HEART score is a reliable predictor of outcomes of chest pain patients presenting to the ED; (2) whether the score is feasible in our local settings; and (3) whether it describes the risk profile of patients with and without MACE.
Methods
Setting
Participants were recruited from the ED of King Edward Memorial Hospital, a municipal teaching hospital in Mumbai. The study institute is a tertiary care academic medical center located in Parel, Mumbai, Maharashtra, and is a resource-limited setting serving urban, suburban, and rural populations. Participants requiring urgent attention are first seen by a casualty officer and then referred to the emergency ward. Here, the physician on duty evaluates them and decides on admission to the various wards, like the general ward, medical intensive care unit (ICU), coronary care unit (CCU), etc. The specialist’s opinion may also be obtained before admission. Critically ill patients are initially admitted to the emergency ward and stabilized before being shifted to other areas of the hospital.
Participants
Patients aged 18 years and older presenting with symptoms of acute chest pain or suspected ACS were stratified by priority using the chest pain scoring system—the HEART score. Only patients presenting to the ED were eligible for the study. Informed consent from the patient or next of kin was mandatory for participation in the study.
Patients were determined ineligible for the following reasons: a clear cause for chest pain other than ACS (eg, trauma, diagnosed aortic dissection), persisting or recurrent chest pain caused by rheumatic diseases or cancer (a terminal illness), pregnancy, unable or unwilling to provide informed consent, or incomplete data.
Study design
We conducted a
We conducted our study to determine the importance of calculating the HEART score in each patient, which will help to correctly place them into low-, intermediate-, and high-risk groups for clinically important, irreversible adverse cardiac events and guide the clinical decision-making. Patients with low risk will avoid costly tests and hospital admissions, thus decreasing the cost of treatment and ensuring timely discharge from the ED. Patients with high risk will be treated immediately, to possibly prevent a life-threatening, ACS-related incident. Thus, the HEART score will serve as a quick and reliable predictor of outcomes in chest pain patients and help clinicians to make accurate diagnostic and therapeutic choices in uncertain situations.
HEART score
The total number of points for History, Electrocardiogram (ECG), Age, Risk factors, and Troponin was noted as the HEART score (Table 1).
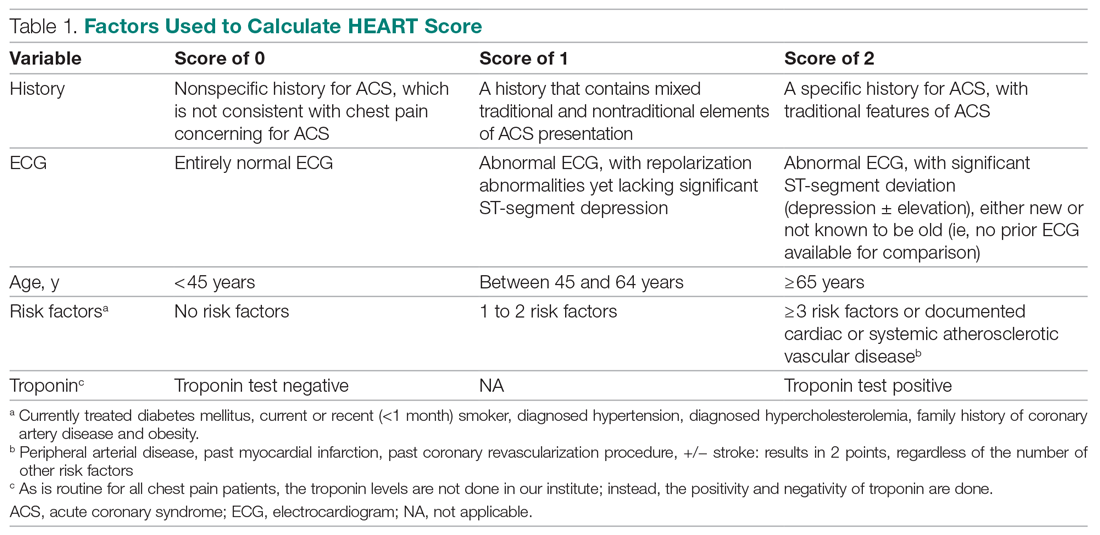
For this study, the patient’s history and ECGs were interpreted by internal medicine attending physicians in the ED. The ECG taken in the emergency room was reviewed and classified, and a copy of the admission ECG was added to the file. The recommendation for patients with a HEART score in a particular range was evaluated. Notably, those with a score of 3 or lower led to a recommendation of reassurance and early discharge. Those with a HEART score in the intermediate range (4-6) were admitted to the hospital for further clinical observation and testing, whereas a high HEART score (7-10) led to admission for intensive monitoring and early intervention. In the analysis of HEART score data, we only used those patients having records for all 5 parameters, excluding patients without an ECG or troponin test.
Results
Myocardial infarction (MI) was defined based on Universal Definition of Myocardial Infarction.13 Coronary revascularization was defined as angioplasty with or without stent placement or coronary artery bypass surgery.14 Percutaneous coronary intervention (PCI) was defined as any therapeutic catheter intervention in the coronary arteries. Coronary artery bypass graft (CABG) surgery was defined as any cardiac surgery in which coronary arteries were operated on.
The primary outcomes in this study were the (1) risk stratification of chest pain patients into low-risk, intermediate-risk, and high-risk categories; (2) incidence of a MACE within 6 weeks of initial presentation. MACE consists of acute myocardial infarction (AMI), PCI, CABG, coronary angiography revealing procedurally correctable stenosis managed conservatively, and death due to any cause.
Our secondary outcomes were discharge or death due to any cause within 6 weeks after presentation.
Follow-up
Within 6 weeks after presentation to the ED, a follow-up phone call was placed to assess the patient’s progress. The follow-up focused on the endpoint of MACE, comprising all-cause death, MI, and revascularization. No patient was lost to follow-up.
Statistical analysis
We aimed to find a difference in the 6-week MACE between low-, intermediate-, and high-risk categories of the HEART score. The prevalence of CHD in India is 10%,4 and assuming an α of 0.05, we needed a sample of 141 patients from the ED patient population. Continuous variables were presented by mean (SD), and categorical variables as percentages. We used t test and the Mann-Whitney U test for comparison of means for continuous variables, χ2 for categorical variables, and Fisher’s exact test for comparison of the categorical variables. Results with P < .05 were considered statistically significant.
We evaluated 141 patients presenting to the ED with chest pain concerning for ACS during the study period, from July 2019 to October 2019.
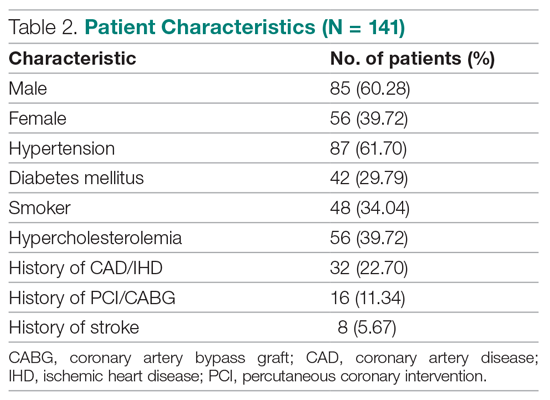
Primary outcomes
The risk stratification of the HEART score in chest pain patients and the incidence of 6-week MACE are outlined in Table 3
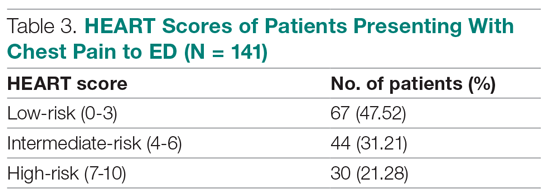
The distribution of the HEART score’s 5 elements in the groups with or without MACE endpoints is shown in Table 5. Notice the significant differences between the groups. A follow-up phone call was made within 6 weeks after the presentation to the ED to assess the patient’s progress. The 6-week follow-up call data are included in Table 6.
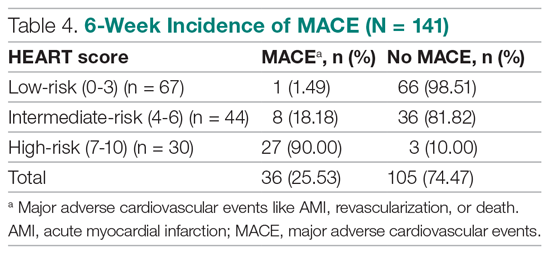
Of 141 patients, 36 patients (25.53%) were diagnosed with MACE within 6 weeks of presentation.
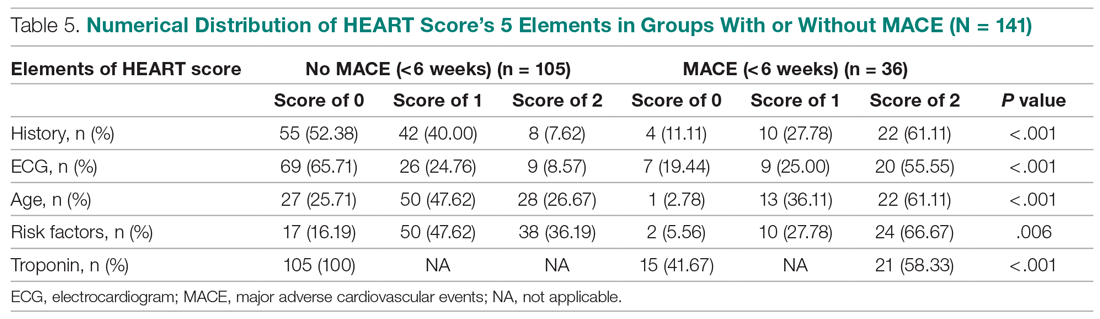
Myocardial infarction—An AMI was diagnosed in 24 of the 141 patients (17.02%). Twenty-one of those already had positive markers on admission (apparently, these AMI had started before their arrival to the emergency room). One AMI occurred 2 days after admission in a 66-year-old male, and another occurred 10 days after discharge. A further AMI occurred 2 weeks after discharge. All 3 patients belonged to the intermediate-risk group.
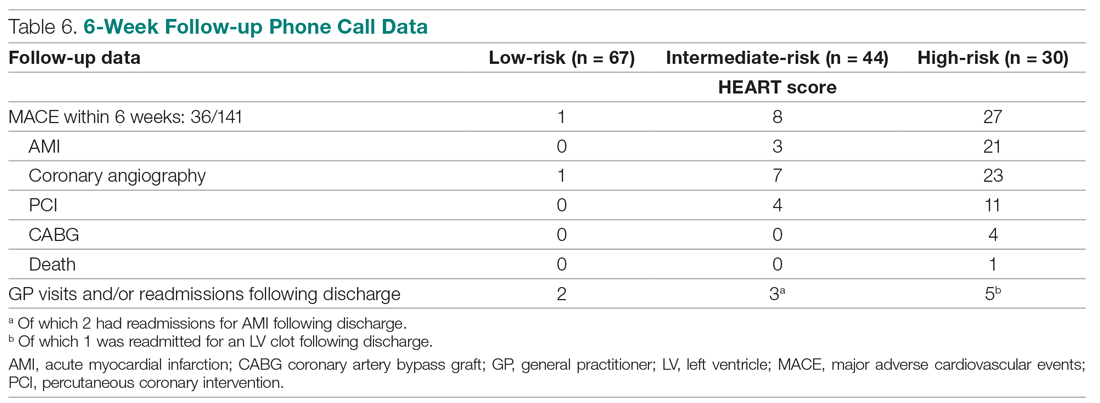
Revascularization—Coronary angiography was performed in 31 of 141 patients (21.99%). Revascularization was performed in 19 patients (13.48%), of which 15 were PCIs (10.64%) and 4 were CABGs (2.84%).
Mortality—One patient died from the study population. He was a 72-year-old male who died 14 days after admission. He had a HEART score of 8.
Among the 67 low-risk patients:
- MACE: Coronary angiography was performed in 1 patient (1.49%). Among the 67 patients in the low-risk category, there was no cases of AMI or deaths. The remaining 66 patients (98.51%) had an uneventful recovery following discharge.
- General practitioner (GP) visits/readmissions following discharge: Two of 67 patients (2.99%) had GP visits following discharge, of which 1 was uneventful. The other patient, a 64-year-old male, was readmitted due to a recurrent history of chest pain and underwent coronary angiography.
Among the 44 intermediate-risk patients:
- MACE: Of the 7 of 44 patients (15.91%) who had coronary angiography, 3 patients (6.82%) had AMI, of which 1 occurred 2 days after admission in a 66-year-old male. Two patients had AMI following discharge. There were no deaths. Overall, 42 of 44 patients (95.55%) had an uneventful recovery following discharge.
- GP visits/readmissions following discharge: Three of 44 patients (6.82%) had repeated visits following discharge. One was a GP visit that was uneventful. The remaining 2 patients were diagnosed with AMI and readmitted following discharge. One AMI occurred 10 days after discharge in a patient with a HEART score of 6; another occurred 2 weeks after discharge in a patient with a HEART score of 5.
Among the 30 high-risk patients:
- MACE: Twenty-three of 30 patients (76.67%) underwent coronary angiography. One patient died 5 days after discharge. The patient had a HEART score of 8. Most patients however, had an uneventful recovery following discharge (28, 93.33%).
- GP visits/readmissions following discharge: Five of 30 patients (16.67%) had repeated visits following discharge. Two were uneventful. Two patients had a history of recurrent chest pain that resolved on Sorbitrate. One patient was readmitted 2 weeks following discharge due to a complication: a left ventricular clot was found. The patient had a HEART score of 10.
Secondary outcome—Overall, 140 of 141 patients were discharged. One patient died: a 72-year-old male with a HEART score of 8.
Feasibility—To determine the ease and feasibility of performing a HEART score in chest pain patients presenting to the ED, a survey was distributed to the internal medicine physicians in the ED. In the survey, the Likert scale was used to rate the ease of utilizing the HEART score and whether the physicians found it feasible to use it for risk stratification of their chest pain patients. A total of 12 of 15 respondents (80%) found it “easy” to use. Of the remaining 3 respondents, 2 (13.33%) rated the HEART score “very easy” to use, while 1 (6.66%) considered it “difficult” to work with. None of the respondents said that it was not feasible to perform a HEART score in the ED.
Risk factors for reaching an endpoint:
We compared risk profiles between the patient groups with and without an endpoint. The group of patients with MACE were older and had a higher proportion of males than the group of patients without MACE. Moreover, they also had a higher prevalence of hypertension, type 2 diabetes mellitus, smoking, hypercholesterolemia, prior history of PCI/CABG, and history of stroke. These also showed a significant association with MACE. Obesity was not included in our risk factors as we did not have data collected to measure body mass index. Results are represented in Table 7.
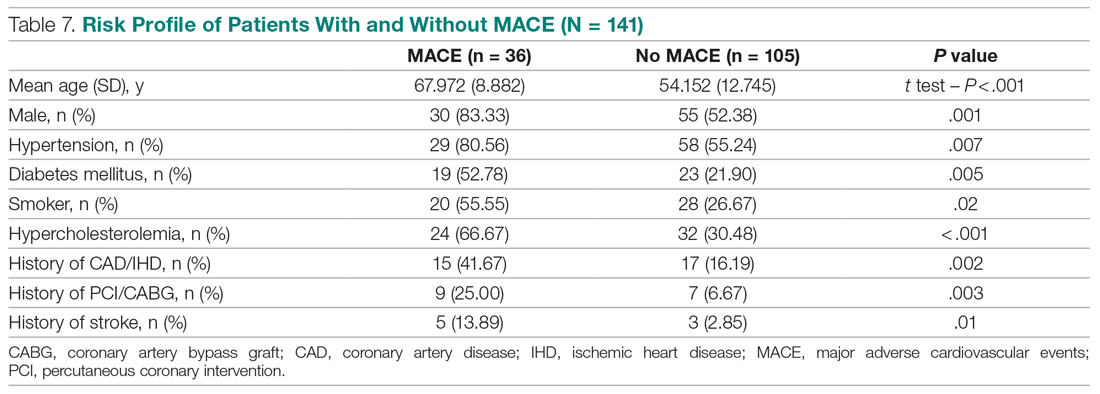
Discussion
Our study described a patient population presenting to an ED with chest pain as their primary complaint. The results of this prospective study confirm that the HEART score is an excellent system to triage chest pain patients. It provides the clinician with a reliable predictor of the outcome (MACE) after the patient’s arrival, based on available clinical data and in a resource-limited setting like ours.
Cardiovascular epidemiology studies indicate that this has become a significant public health problem in India.1 Several risk scores for ACS have been published in European and American guidelines. However, in the Indian population, minimal data are available on utilization of such a triage score (HEART score) in chest pain patients in the ED in a resource-limited setting, to the best of our knowledge. In India, only 1 such study is reported,15 at the Sundaram Medical Foundation, a 170-bed community hospital in Chennai. In this study, 13 of 14 patients (92.86%) with a high HEART score had MACE, indicating a sensitivity of 92.86%; in the 44 patients with a low HEART score, 1 patient (2.22%) had MACE, indicating a specificity of 97.78%; and in the 28 patients with a moderate HEART score, 12 patients (42.86%) had MACE.
In looking for the optimal risk-stratifying system for chest pain patients, we analyzed the HEART score. The first study on the HEART score was done Backus et al, proving that the HEART score is an easy, quick, and reliable predictor of outcomes in chest pain patients.10 The HEART score had good discriminatory power, too. The C statistic for the HEART score for ACS occurrence shows a value of 0.83. This signifies a good-to-excellent ability to stratify all-cause chest pain patients in the ED for their risk of MACE. The application of the HEART score to our patient population demonstrated that the majority of the patients belonged to the low-risk category, as reported in the first cohort study that applied the HEART score.8 The relationship between the HEART score category and occurrence of MACE within 6 weeks showed a curve with 3 different patterns, corresponding to the 3 risk categories defined in the literature.11,12 The risk stratification of chest pain patients using the 3 categories (0-3, 4-6, 7-10) identified MACE with an incidence similar to the multicenter study of Backus et al,10,11 but with a greater risk of MACE in the high-risk category (Figure).
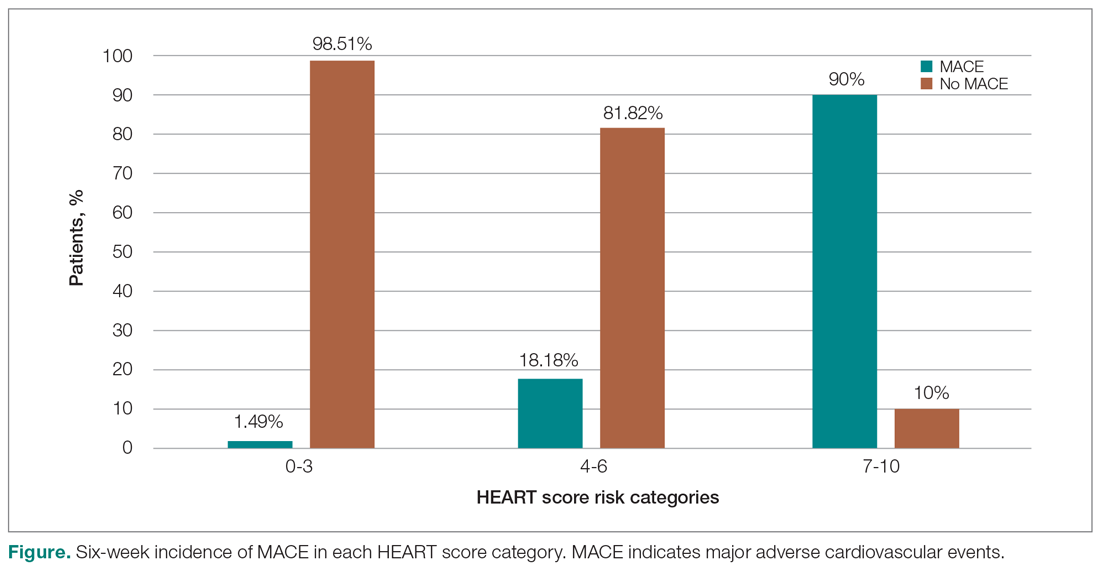
Thus, our study confirmed the utility of the HEART score categories to predict the 6-week incidence of MACE. The sensitivity, specificity, and positive and negative predictive values for the established cut-off scores of 4 and 7 are shown in Table 8. The patients in the low-risk category, corresponding to a score < 4, had a very high negative predictive value, thus identifying a small-risk population. The patients in the high-risk category (score ≥ 7) showed a high positive predictive value, allowing the identification of a high-risk population, even in patients with more atypical presentations. Therefore, the HEART score may help clinicians to make accurate management choices by being a strong predictor of both event-free survival and potentially life-threatening cardiac events.11,12
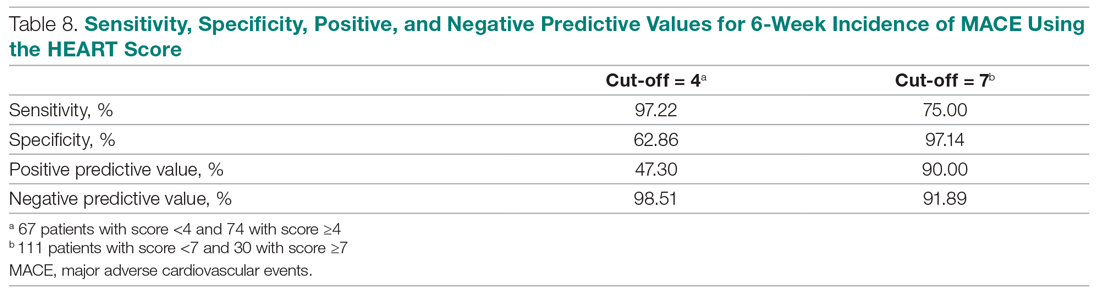
Our study tested the efficacy of the HEART score pathway in helping clinicians make smart diagnostic and therapeutic choices. It confirmed that the HEART score was accurate in predicting the short-term incidence of MACE, thus stratifying patients according to their risk severity. In our study, 67 of 141 patients (47.52%) had low-risk HEART scores, and we found the 6-week incidence of MACE to be 1.49%. We omitted the diagnostic and treatment evaluation for patients in the low-risk category and moved onto discharge. Overall, 66 of 67 patients (98.51%) in the low-risk category had an uneventful recovery following discharge. Only 2 of 67 these patients (2.99%) of patients had health care utilization following discharge. Therefore, extrapolation based on results demonstrates reduced health care utilization. Previous studies have shown similar results.9,12,14,16 For instance, in a prospective study conducted in the Netherlands, low-risk patients representing 36.4% of the total were found to have a low MACE rate (1.7%).9 These low-risk patients were categorized as appropriate and safe for ED discharge without additional cardiac evaluation or inpatient admission.9 Another retrospective study in Portugal,12 and one in Chennai, India,15 found the 6-week incidence of MACE to be 2.00% and 2.22%, respectively. The results of the first HEART Pathway Randomized Control Trial14 showed that the HEART score pathway reduces health care utilization (cardiac testing, hospitalization, and hospital length of stay). The study also showed that these gains occurred without any of the patients that were identified for early discharge, suffering from MACE at 30 days, or secondary increase in cardiac-related hospitalizations. Similar results were obtained by a randomized trial conducted in North Carolina17 that also demonstrated a reduction in objective cardiac testing, a doubling of the rate of early discharge from the ED, and a reduced length of stay by half a day. Another study using a modified HEART score also demonstrated that when low-risk patients are evaluated with cardiac testing, the likelihood for false positives is high.16 Hoffman et al also reported that patients randomized to coronary computed tomographic angiography (CCTA) received > 2.5 times more radiation exposure.16 Thus, low-risk patients may be safely discharged without the need for stress testing or CCTA.
In our study, 30 out of 141 patients (21.28%) had high-risk HEART scores (7-10), and we found the 6-week incidence of MACE to be 90%. Based on the pathway leading to inpatient admission and intensive treatment, 23 of 30 patients (76.67%) patients in our study underwent coronary angiography and further therapeutic treatment. In the high-risk category, 28 of 30 patients (93.33%) patients had an uneventful recovery following discharge. Previous studies have shown similar results. A retrospective study in Portugal showed that 76.9% of the high-risk patients had a 6-week incidence of MACE.12 In a study in the Netherlands,9 72.7% of high-risk patients had a 6-week incidence of MACE. Therefore, a HEART score of ≥ 7 in patients implies early aggressive treatment, including invasive strategies, when necessary, without noninvasive treatment preceding it.8
In terms of intermediate risk, in our study 44 of 141 patients (31.21%) patients had an intermediate-risk HEART score (4-6), and we found the 6-week incidence of MACE to be 18.18%. Based on the pathway, they were kept in the observation ward on admission. In our study, 7 of 44 patients (15.91%) underwent coronary angiography and further treatment; 42 of 44 patients (95.55%) had an uneventful recovery following discharge. In a prospective study in the Netherlands, 46.1% of patients with an intermediate score had a 6-week MACE incidence of 16.6%.10 Similarly, in another retrospective study in Portugal, the incidence of 6-week MACE in intermediate-risk patients (36.7%) was found to be 15.6%.12 Therefore, in patients with a HEART score of 4-6 points, immediate discharge is not an option, as this figure indicates a risk of 18.18% for an adverse outcome. These patients should be admitted for clinical observation, treated as an ACS awaiting final diagnosis, and subjected to noninvasive investigations, such as repeated troponin. Using the HEART score as guidance in the treatment of chest pain patients will benefit patients on both sides of the spectrum.11,12
Our sample presented a male predominance, a wide range of age, and a mean age similar to that of previous studies.12.16 Some risk factors, we found, can increase significantly the odds of chest pain being of cardiovascular origin, such as male gender, smoking, hypertension, type 2 diabetes mellitus, and hypercholesterolemia. Other studies also reported similar findings.8,12,16 Risk factors for premature CHD have been quantified in the case-control INTERHEART study.1 In the INTERHEART study, 8 common risk factors explained > 90% of AMIs in South Asian and Indian patients. The risk factors include dyslipidemia, smoking or tobacco use, known hypertension, known diabetes, abdominal obesity, physical inactivity, low fruit and vegetable intake, and psychosocial stress.1 Regarding the feasibility of treating physicians using the HEART score in the ED, we observed that, based on the Likert scale, 80% of survey respondents found it easy to use, and 100% found it feasible in the ED.
However, there were certain limitations to our study. It involved a single academic medical center and a small sample size, which limit generalizability of the findings. In addition, troponin levels are not calculated at our institution, as it is a resource-limited setting; therefore, we used a positive and negative as +2 and 0, respectively.
Conclusion
The HEART score provides the clinician with a quick and reliable predictor of outcome of patients with chest pain after arrival to the ED and can be used for triage. For patients with low HEART scores (0-3), short-term MACE can be excluded with greater than 98% certainty. In these patients, one may consider reserved treatment and discharge policies that may also reduce health care utilization. In patients with high HEART scores (7-10), the high risk of MACE (90%) may indicate early aggressive treatment, including invasive strategies, when necessary. Therefore, the HEART score may help clinicians make accurate management choices by being a strong predictor of both event-free survival and potentially life-threatening cardiac events. Age, gender, and cardiovascular risk factors may also be considered in the assessment of patients. This study confirmed the utility of the HEART score categories to predict the 6-week incidence of MACE.
Corresponding author: Smrati Bajpai Tiwari, MD, DNB, FAIMER, Department of Medicine, Seth Gordhandas Sunderdas Medical College and King Edward Memorial Hospital, Acharya Donde Marg, Parel, Mumbai 400 012, Maharashtra, India; [email protected].
Financial disclosures: None.
1. Gupta R, Mohan I, Narula J. Trends in coronary heart disease epidemiology in India. Ann Glob Health. 2016;82:307-315.
2. World Health Organization. Global status report on non-communicable diseases 2014. Accessed June 22, 2021. https://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf
3. Fuster V, Kelly BB, eds. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Institutes of Medicine; 2010.
4. Krishnan MN. Coronary heart disease and risk factors in India—on the brink of an epidemic. Indian Heart J. 2012;64:364-367.
5. Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India: current epidemiology and future directions. Circulation. 2016;133:1605-1620.
6. Aeri B, Chauhan S. The rising incidence of cardiovascular diseases in India: assessing its economic impact. J Prev Cardiol. 2015;4:735-740.
7. Pednekar M, Gupta R, Gupta PC. Illiteracy, low educational status and cardiovascular mortality in India. BMC Public Health. 2011;11:567.
8. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196.
9. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168;2153-2158.
10. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART score. Crit Pathw Cardiol. 2010;9:164-169.
11. Backus BE, Six AJ, Kelder JH, et al. Risk scores for patients with chest pain: evaluation in the emergency department. Curr Cardiol Rev. 2011;7:2-8.
12. Leite L, Baptista R, Leitão J, et al. Chest pain in the emergency department: risk stratification with Manchester triage system and HEART score. BMC Cardiovasc Disord. 2015;15:48.
13. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction. Circulation. 2018;138:e618-e651.
14. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8:195-203.
15. Natarajan B, Mallick P, Thangalvadi TA, Rajavelu P. Validation of the HEART score in Indian population. Int J Emerg Med. 2015,8(suppl 1):P5.
16. McCord J, Cabrera R, Lindahl B, et al. Prognostic utility of a modified HEART score in chest pain patients in the emergency department. Circ Cardiovasc Qual Outcomes. 2017;10:e003101.
17. Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2012;168:795-802.
From the Department of Internal Medicine, Mount Sinai Health System, Icahn School of Medicine at Mount Sinai, New York, NY (Dr. Gandhi), and the School of Medicine, Seth Gordhandas Sunderdas Medical College, and King Edward Memorial Hospital, Mumbai, India (Drs. Gandhi and Tiwari).
Objective: Calculation of HEART score to (1) stratify patients as low-risk, intermediate-risk, high-risk, and to predict the short-term incidence of major adverse cardiovascular events (MACE), and (2) demonstrate feasibility of HEART score in our local settings.
Design: A prospective cohort study of patients with a chief complaint of chest pain concerning for acute coronary syndrome.
Setting: Participants were recruited from the emergency department (ED) of King Edward Memorial Hospital, a tertiary care academic medical center and a resource-limited setting in Mumbai, India.
Participants: We evaluated 141 patients aged 18 years and older presenting to the ED and stratified them using the HEART score. To assess patients’ progress, a follow-up phone call was made within 6 weeks after presentation to the ED.
Measurements: The primary outcomes were a risk stratification, 6-week occurrence of MACE, and performance of unscheduled revascularization or stress testing. The secondary outcomes were discharge or death.
Results: The 141 participants were stratified into low-risk, intermediate-risk, and high-risk groups: 67 (47.52%), 44 (31.21%), and 30 (21.28%), respectively. The 6-week incidence of MACE in each category was 1.49%, 18.18%, and 90%, respectively. An acute myocardial infarction was diagnosed in 24 patients (17.02%), 15 patients (10.64%) underwent percutaneous coronary intervention (PCI), and 4 patients (2.84%) underwent coronary artery bypass graft (CABG). Overall, 98.5% of low-risk patients and 93.33% of high-risk patients had an uneventful recovery following discharge; therefore, extrapolation based on results demonstrated reduced health care utilization. All the survey respondents found the HEART score to be feasible. The patient characteristics and risk profile of the patients with and without MACE demonstrated that: patients with MACE were older and had a higher proportion of males, hypertension, type 2 diabetes mellitus, smoking, hypercholesterolemia, prior history of PCI/CABG, and history of stroke.
Conclusion: The HEART score seems to be a useful tool for risk stratification and a reliable predictor of outcomes in chest pain patients and can therefore be used for triage.
Keywords: chest pain; emergency department; HEART score; acute coronary syndrome; major adverse cardiac events; myocardial infarction; revascularization.
Cardiovascular diseases (CVDs), especially coronary heart disease (CHD), have epidemic proportions worldwide. Globally, in 2012, CVD led to 17.5 million deaths,1,2 with more than 75% of them occurring in developing countries. In contrast to developed countries, where mortality from CHD is rapidly declining, it is increasing in developing countries.1,3 Current estimates from epidemiologic studies from various parts of India indicate the prevalence of CHD in India to be between 7% and 13% in urban populations and 2% and 7% in rural populations.4
Premature mortality in terms of years of life lost because of CVD in India increased by 59% over a 20-year span, from 23.2 million in 1990 to 37 million in 2010.5 Studies conducted in Mumbai (Mumbai Cohort Study) reported very high CVD mortality rates, approaching 500 per 100 000 for men and 250 per 100 000 for women.6,7 However, to the best of our knowledge, in the Indian population, there are minimal data on utilization of a triage score, such as the HEART score, in chest pain patients in the emergency department (ED) in a resource-limited setting.
The most common reason for admitting patients to the ED is chest pain.8 There are various cardiac and noncardiac etiologies of chest pain presentation. Acute coronary syndrome (ACS) needs to be ruled out first in every patient presenting with chest pain. However, 80% of patients with ACS have no clear diagnostic features on presentation.9 The timely diagnosis and treatment of patients with ACS improves their prognosis. Therefore, clinicians tend to start each patient on ACS treatment to reduce the risk, which often leads to increased costs due to unnecessary, time-consuming diagnostic procedures that may place burdens on both the health care system and the patient.10
Several risk-stratifying tools have been developed in the last few years. Both the GRACE and TIMI risk scores have been designed for risk stratification of patients with proven ACS and not for the chest pain population at the ED.11 Some of these tools are applicable to patients with all types of chest pain presenting to the ED, such as the Manchester Triage System. Other, more selective systems are devoted to the risk stratification of suspected ACS in the ED. One is the HEART score.12
The first study on the HEART score—an acronym that stands for History, Electrocardiogram, Age, Risk factors, and Troponin—was done by Backus et al, who proved that the HEART score is an easy, quick, and reliable predictor of outcomes in chest pain patients.10 The HEART score predicts the short-term incidence of major adverse cardiac events (MACE), which allows clinicians to stratify patients as low-risk, intermediate-risk, and high-risk and to guide their clinical decision-making accordingly. It was developed to provide clinicians with a simple, reliable predictor of cardiac risk on the basis of the lowest score of 0 (very low-risk) up to a score of 10 (very high-risk).
We studied the clinical performance of the HEART score in patients with chest pain, focusing on the efficacy and safety of rapidly identifying patients at risk of MACE. We aimed to determine (1) whether the HEART score is a reliable predictor of outcomes of chest pain patients presenting to the ED; (2) whether the score is feasible in our local settings; and (3) whether it describes the risk profile of patients with and without MACE.
Methods
Setting
Participants were recruited from the ED of King Edward Memorial Hospital, a municipal teaching hospital in Mumbai. The study institute is a tertiary care academic medical center located in Parel, Mumbai, Maharashtra, and is a resource-limited setting serving urban, suburban, and rural populations. Participants requiring urgent attention are first seen by a casualty officer and then referred to the emergency ward. Here, the physician on duty evaluates them and decides on admission to the various wards, like the general ward, medical intensive care unit (ICU), coronary care unit (CCU), etc. The specialist’s opinion may also be obtained before admission. Critically ill patients are initially admitted to the emergency ward and stabilized before being shifted to other areas of the hospital.
Participants
Patients aged 18 years and older presenting with symptoms of acute chest pain or suspected ACS were stratified by priority using the chest pain scoring system—the HEART score. Only patients presenting to the ED were eligible for the study. Informed consent from the patient or next of kin was mandatory for participation in the study.
Patients were determined ineligible for the following reasons: a clear cause for chest pain other than ACS (eg, trauma, diagnosed aortic dissection), persisting or recurrent chest pain caused by rheumatic diseases or cancer (a terminal illness), pregnancy, unable or unwilling to provide informed consent, or incomplete data.
Study design
We conducted a
We conducted our study to determine the importance of calculating the HEART score in each patient, which will help to correctly place them into low-, intermediate-, and high-risk groups for clinically important, irreversible adverse cardiac events and guide the clinical decision-making. Patients with low risk will avoid costly tests and hospital admissions, thus decreasing the cost of treatment and ensuring timely discharge from the ED. Patients with high risk will be treated immediately, to possibly prevent a life-threatening, ACS-related incident. Thus, the HEART score will serve as a quick and reliable predictor of outcomes in chest pain patients and help clinicians to make accurate diagnostic and therapeutic choices in uncertain situations.
HEART score
The total number of points for History, Electrocardiogram (ECG), Age, Risk factors, and Troponin was noted as the HEART score (Table 1).

For this study, the patient’s history and ECGs were interpreted by internal medicine attending physicians in the ED. The ECG taken in the emergency room was reviewed and classified, and a copy of the admission ECG was added to the file. The recommendation for patients with a HEART score in a particular range was evaluated. Notably, those with a score of 3 or lower led to a recommendation of reassurance and early discharge. Those with a HEART score in the intermediate range (4-6) were admitted to the hospital for further clinical observation and testing, whereas a high HEART score (7-10) led to admission for intensive monitoring and early intervention. In the analysis of HEART score data, we only used those patients having records for all 5 parameters, excluding patients without an ECG or troponin test.
Results
Myocardial infarction (MI) was defined based on Universal Definition of Myocardial Infarction.13 Coronary revascularization was defined as angioplasty with or without stent placement or coronary artery bypass surgery.14 Percutaneous coronary intervention (PCI) was defined as any therapeutic catheter intervention in the coronary arteries. Coronary artery bypass graft (CABG) surgery was defined as any cardiac surgery in which coronary arteries were operated on.
The primary outcomes in this study were the (1) risk stratification of chest pain patients into low-risk, intermediate-risk, and high-risk categories; (2) incidence of a MACE within 6 weeks of initial presentation. MACE consists of acute myocardial infarction (AMI), PCI, CABG, coronary angiography revealing procedurally correctable stenosis managed conservatively, and death due to any cause.
Our secondary outcomes were discharge or death due to any cause within 6 weeks after presentation.
Follow-up
Within 6 weeks after presentation to the ED, a follow-up phone call was placed to assess the patient’s progress. The follow-up focused on the endpoint of MACE, comprising all-cause death, MI, and revascularization. No patient was lost to follow-up.
Statistical analysis
We aimed to find a difference in the 6-week MACE between low-, intermediate-, and high-risk categories of the HEART score. The prevalence of CHD in India is 10%,4 and assuming an α of 0.05, we needed a sample of 141 patients from the ED patient population. Continuous variables were presented by mean (SD), and categorical variables as percentages. We used t test and the Mann-Whitney U test for comparison of means for continuous variables, χ2 for categorical variables, and Fisher’s exact test for comparison of the categorical variables. Results with P < .05 were considered statistically significant.
We evaluated 141 patients presenting to the ED with chest pain concerning for ACS during the study period, from July 2019 to October 2019.

Primary outcomes
The risk stratification of the HEART score in chest pain patients and the incidence of 6-week MACE are outlined in Table 3

The distribution of the HEART score’s 5 elements in the groups with or without MACE endpoints is shown in Table 5. Notice the significant differences between the groups. A follow-up phone call was made within 6 weeks after the presentation to the ED to assess the patient’s progress. The 6-week follow-up call data are included in Table 6.

Of 141 patients, 36 patients (25.53%) were diagnosed with MACE within 6 weeks of presentation.

Myocardial infarction—An AMI was diagnosed in 24 of the 141 patients (17.02%). Twenty-one of those already had positive markers on admission (apparently, these AMI had started before their arrival to the emergency room). One AMI occurred 2 days after admission in a 66-year-old male, and another occurred 10 days after discharge. A further AMI occurred 2 weeks after discharge. All 3 patients belonged to the intermediate-risk group.

Revascularization—Coronary angiography was performed in 31 of 141 patients (21.99%). Revascularization was performed in 19 patients (13.48%), of which 15 were PCIs (10.64%) and 4 were CABGs (2.84%).
Mortality—One patient died from the study population. He was a 72-year-old male who died 14 days after admission. He had a HEART score of 8.
Among the 67 low-risk patients:
- MACE: Coronary angiography was performed in 1 patient (1.49%). Among the 67 patients in the low-risk category, there was no cases of AMI or deaths. The remaining 66 patients (98.51%) had an uneventful recovery following discharge.
- General practitioner (GP) visits/readmissions following discharge: Two of 67 patients (2.99%) had GP visits following discharge, of which 1 was uneventful. The other patient, a 64-year-old male, was readmitted due to a recurrent history of chest pain and underwent coronary angiography.
Among the 44 intermediate-risk patients:
- MACE: Of the 7 of 44 patients (15.91%) who had coronary angiography, 3 patients (6.82%) had AMI, of which 1 occurred 2 days after admission in a 66-year-old male. Two patients had AMI following discharge. There were no deaths. Overall, 42 of 44 patients (95.55%) had an uneventful recovery following discharge.
- GP visits/readmissions following discharge: Three of 44 patients (6.82%) had repeated visits following discharge. One was a GP visit that was uneventful. The remaining 2 patients were diagnosed with AMI and readmitted following discharge. One AMI occurred 10 days after discharge in a patient with a HEART score of 6; another occurred 2 weeks after discharge in a patient with a HEART score of 5.
Among the 30 high-risk patients:
- MACE: Twenty-three of 30 patients (76.67%) underwent coronary angiography. One patient died 5 days after discharge. The patient had a HEART score of 8. Most patients however, had an uneventful recovery following discharge (28, 93.33%).
- GP visits/readmissions following discharge: Five of 30 patients (16.67%) had repeated visits following discharge. Two were uneventful. Two patients had a history of recurrent chest pain that resolved on Sorbitrate. One patient was readmitted 2 weeks following discharge due to a complication: a left ventricular clot was found. The patient had a HEART score of 10.
Secondary outcome—Overall, 140 of 141 patients were discharged. One patient died: a 72-year-old male with a HEART score of 8.
Feasibility—To determine the ease and feasibility of performing a HEART score in chest pain patients presenting to the ED, a survey was distributed to the internal medicine physicians in the ED. In the survey, the Likert scale was used to rate the ease of utilizing the HEART score and whether the physicians found it feasible to use it for risk stratification of their chest pain patients. A total of 12 of 15 respondents (80%) found it “easy” to use. Of the remaining 3 respondents, 2 (13.33%) rated the HEART score “very easy” to use, while 1 (6.66%) considered it “difficult” to work with. None of the respondents said that it was not feasible to perform a HEART score in the ED.
Risk factors for reaching an endpoint:
We compared risk profiles between the patient groups with and without an endpoint. The group of patients with MACE were older and had a higher proportion of males than the group of patients without MACE. Moreover, they also had a higher prevalence of hypertension, type 2 diabetes mellitus, smoking, hypercholesterolemia, prior history of PCI/CABG, and history of stroke. These also showed a significant association with MACE. Obesity was not included in our risk factors as we did not have data collected to measure body mass index. Results are represented in Table 7.

Discussion
Our study described a patient population presenting to an ED with chest pain as their primary complaint. The results of this prospective study confirm that the HEART score is an excellent system to triage chest pain patients. It provides the clinician with a reliable predictor of the outcome (MACE) after the patient’s arrival, based on available clinical data and in a resource-limited setting like ours.
Cardiovascular epidemiology studies indicate that this has become a significant public health problem in India.1 Several risk scores for ACS have been published in European and American guidelines. However, in the Indian population, minimal data are available on utilization of such a triage score (HEART score) in chest pain patients in the ED in a resource-limited setting, to the best of our knowledge. In India, only 1 such study is reported,15 at the Sundaram Medical Foundation, a 170-bed community hospital in Chennai. In this study, 13 of 14 patients (92.86%) with a high HEART score had MACE, indicating a sensitivity of 92.86%; in the 44 patients with a low HEART score, 1 patient (2.22%) had MACE, indicating a specificity of 97.78%; and in the 28 patients with a moderate HEART score, 12 patients (42.86%) had MACE.
In looking for the optimal risk-stratifying system for chest pain patients, we analyzed the HEART score. The first study on the HEART score was done Backus et al, proving that the HEART score is an easy, quick, and reliable predictor of outcomes in chest pain patients.10 The HEART score had good discriminatory power, too. The C statistic for the HEART score for ACS occurrence shows a value of 0.83. This signifies a good-to-excellent ability to stratify all-cause chest pain patients in the ED for their risk of MACE. The application of the HEART score to our patient population demonstrated that the majority of the patients belonged to the low-risk category, as reported in the first cohort study that applied the HEART score.8 The relationship between the HEART score category and occurrence of MACE within 6 weeks showed a curve with 3 different patterns, corresponding to the 3 risk categories defined in the literature.11,12 The risk stratification of chest pain patients using the 3 categories (0-3, 4-6, 7-10) identified MACE with an incidence similar to the multicenter study of Backus et al,10,11 but with a greater risk of MACE in the high-risk category (Figure).

Thus, our study confirmed the utility of the HEART score categories to predict the 6-week incidence of MACE. The sensitivity, specificity, and positive and negative predictive values for the established cut-off scores of 4 and 7 are shown in Table 8. The patients in the low-risk category, corresponding to a score < 4, had a very high negative predictive value, thus identifying a small-risk population. The patients in the high-risk category (score ≥ 7) showed a high positive predictive value, allowing the identification of a high-risk population, even in patients with more atypical presentations. Therefore, the HEART score may help clinicians to make accurate management choices by being a strong predictor of both event-free survival and potentially life-threatening cardiac events.11,12

Our study tested the efficacy of the HEART score pathway in helping clinicians make smart diagnostic and therapeutic choices. It confirmed that the HEART score was accurate in predicting the short-term incidence of MACE, thus stratifying patients according to their risk severity. In our study, 67 of 141 patients (47.52%) had low-risk HEART scores, and we found the 6-week incidence of MACE to be 1.49%. We omitted the diagnostic and treatment evaluation for patients in the low-risk category and moved onto discharge. Overall, 66 of 67 patients (98.51%) in the low-risk category had an uneventful recovery following discharge. Only 2 of 67 these patients (2.99%) of patients had health care utilization following discharge. Therefore, extrapolation based on results demonstrates reduced health care utilization. Previous studies have shown similar results.9,12,14,16 For instance, in a prospective study conducted in the Netherlands, low-risk patients representing 36.4% of the total were found to have a low MACE rate (1.7%).9 These low-risk patients were categorized as appropriate and safe for ED discharge without additional cardiac evaluation or inpatient admission.9 Another retrospective study in Portugal,12 and one in Chennai, India,15 found the 6-week incidence of MACE to be 2.00% and 2.22%, respectively. The results of the first HEART Pathway Randomized Control Trial14 showed that the HEART score pathway reduces health care utilization (cardiac testing, hospitalization, and hospital length of stay). The study also showed that these gains occurred without any of the patients that were identified for early discharge, suffering from MACE at 30 days, or secondary increase in cardiac-related hospitalizations. Similar results were obtained by a randomized trial conducted in North Carolina17 that also demonstrated a reduction in objective cardiac testing, a doubling of the rate of early discharge from the ED, and a reduced length of stay by half a day. Another study using a modified HEART score also demonstrated that when low-risk patients are evaluated with cardiac testing, the likelihood for false positives is high.16 Hoffman et al also reported that patients randomized to coronary computed tomographic angiography (CCTA) received > 2.5 times more radiation exposure.16 Thus, low-risk patients may be safely discharged without the need for stress testing or CCTA.
In our study, 30 out of 141 patients (21.28%) had high-risk HEART scores (7-10), and we found the 6-week incidence of MACE to be 90%. Based on the pathway leading to inpatient admission and intensive treatment, 23 of 30 patients (76.67%) patients in our study underwent coronary angiography and further therapeutic treatment. In the high-risk category, 28 of 30 patients (93.33%) patients had an uneventful recovery following discharge. Previous studies have shown similar results. A retrospective study in Portugal showed that 76.9% of the high-risk patients had a 6-week incidence of MACE.12 In a study in the Netherlands,9 72.7% of high-risk patients had a 6-week incidence of MACE. Therefore, a HEART score of ≥ 7 in patients implies early aggressive treatment, including invasive strategies, when necessary, without noninvasive treatment preceding it.8
In terms of intermediate risk, in our study 44 of 141 patients (31.21%) patients had an intermediate-risk HEART score (4-6), and we found the 6-week incidence of MACE to be 18.18%. Based on the pathway, they were kept in the observation ward on admission. In our study, 7 of 44 patients (15.91%) underwent coronary angiography and further treatment; 42 of 44 patients (95.55%) had an uneventful recovery following discharge. In a prospective study in the Netherlands, 46.1% of patients with an intermediate score had a 6-week MACE incidence of 16.6%.10 Similarly, in another retrospective study in Portugal, the incidence of 6-week MACE in intermediate-risk patients (36.7%) was found to be 15.6%.12 Therefore, in patients with a HEART score of 4-6 points, immediate discharge is not an option, as this figure indicates a risk of 18.18% for an adverse outcome. These patients should be admitted for clinical observation, treated as an ACS awaiting final diagnosis, and subjected to noninvasive investigations, such as repeated troponin. Using the HEART score as guidance in the treatment of chest pain patients will benefit patients on both sides of the spectrum.11,12
Our sample presented a male predominance, a wide range of age, and a mean age similar to that of previous studies.12.16 Some risk factors, we found, can increase significantly the odds of chest pain being of cardiovascular origin, such as male gender, smoking, hypertension, type 2 diabetes mellitus, and hypercholesterolemia. Other studies also reported similar findings.8,12,16 Risk factors for premature CHD have been quantified in the case-control INTERHEART study.1 In the INTERHEART study, 8 common risk factors explained > 90% of AMIs in South Asian and Indian patients. The risk factors include dyslipidemia, smoking or tobacco use, known hypertension, known diabetes, abdominal obesity, physical inactivity, low fruit and vegetable intake, and psychosocial stress.1 Regarding the feasibility of treating physicians using the HEART score in the ED, we observed that, based on the Likert scale, 80% of survey respondents found it easy to use, and 100% found it feasible in the ED.
However, there were certain limitations to our study. It involved a single academic medical center and a small sample size, which limit generalizability of the findings. In addition, troponin levels are not calculated at our institution, as it is a resource-limited setting; therefore, we used a positive and negative as +2 and 0, respectively.
Conclusion
The HEART score provides the clinician with a quick and reliable predictor of outcome of patients with chest pain after arrival to the ED and can be used for triage. For patients with low HEART scores (0-3), short-term MACE can be excluded with greater than 98% certainty. In these patients, one may consider reserved treatment and discharge policies that may also reduce health care utilization. In patients with high HEART scores (7-10), the high risk of MACE (90%) may indicate early aggressive treatment, including invasive strategies, when necessary. Therefore, the HEART score may help clinicians make accurate management choices by being a strong predictor of both event-free survival and potentially life-threatening cardiac events. Age, gender, and cardiovascular risk factors may also be considered in the assessment of patients. This study confirmed the utility of the HEART score categories to predict the 6-week incidence of MACE.
Corresponding author: Smrati Bajpai Tiwari, MD, DNB, FAIMER, Department of Medicine, Seth Gordhandas Sunderdas Medical College and King Edward Memorial Hospital, Acharya Donde Marg, Parel, Mumbai 400 012, Maharashtra, India; [email protected].
Financial disclosures: None.
From the Department of Internal Medicine, Mount Sinai Health System, Icahn School of Medicine at Mount Sinai, New York, NY (Dr. Gandhi), and the School of Medicine, Seth Gordhandas Sunderdas Medical College, and King Edward Memorial Hospital, Mumbai, India (Drs. Gandhi and Tiwari).
Objective: Calculation of HEART score to (1) stratify patients as low-risk, intermediate-risk, high-risk, and to predict the short-term incidence of major adverse cardiovascular events (MACE), and (2) demonstrate feasibility of HEART score in our local settings.
Design: A prospective cohort study of patients with a chief complaint of chest pain concerning for acute coronary syndrome.
Setting: Participants were recruited from the emergency department (ED) of King Edward Memorial Hospital, a tertiary care academic medical center and a resource-limited setting in Mumbai, India.
Participants: We evaluated 141 patients aged 18 years and older presenting to the ED and stratified them using the HEART score. To assess patients’ progress, a follow-up phone call was made within 6 weeks after presentation to the ED.
Measurements: The primary outcomes were a risk stratification, 6-week occurrence of MACE, and performance of unscheduled revascularization or stress testing. The secondary outcomes were discharge or death.
Results: The 141 participants were stratified into low-risk, intermediate-risk, and high-risk groups: 67 (47.52%), 44 (31.21%), and 30 (21.28%), respectively. The 6-week incidence of MACE in each category was 1.49%, 18.18%, and 90%, respectively. An acute myocardial infarction was diagnosed in 24 patients (17.02%), 15 patients (10.64%) underwent percutaneous coronary intervention (PCI), and 4 patients (2.84%) underwent coronary artery bypass graft (CABG). Overall, 98.5% of low-risk patients and 93.33% of high-risk patients had an uneventful recovery following discharge; therefore, extrapolation based on results demonstrated reduced health care utilization. All the survey respondents found the HEART score to be feasible. The patient characteristics and risk profile of the patients with and without MACE demonstrated that: patients with MACE were older and had a higher proportion of males, hypertension, type 2 diabetes mellitus, smoking, hypercholesterolemia, prior history of PCI/CABG, and history of stroke.
Conclusion: The HEART score seems to be a useful tool for risk stratification and a reliable predictor of outcomes in chest pain patients and can therefore be used for triage.
Keywords: chest pain; emergency department; HEART score; acute coronary syndrome; major adverse cardiac events; myocardial infarction; revascularization.
Cardiovascular diseases (CVDs), especially coronary heart disease (CHD), have epidemic proportions worldwide. Globally, in 2012, CVD led to 17.5 million deaths,1,2 with more than 75% of them occurring in developing countries. In contrast to developed countries, where mortality from CHD is rapidly declining, it is increasing in developing countries.1,3 Current estimates from epidemiologic studies from various parts of India indicate the prevalence of CHD in India to be between 7% and 13% in urban populations and 2% and 7% in rural populations.4
Premature mortality in terms of years of life lost because of CVD in India increased by 59% over a 20-year span, from 23.2 million in 1990 to 37 million in 2010.5 Studies conducted in Mumbai (Mumbai Cohort Study) reported very high CVD mortality rates, approaching 500 per 100 000 for men and 250 per 100 000 for women.6,7 However, to the best of our knowledge, in the Indian population, there are minimal data on utilization of a triage score, such as the HEART score, in chest pain patients in the emergency department (ED) in a resource-limited setting.
The most common reason for admitting patients to the ED is chest pain.8 There are various cardiac and noncardiac etiologies of chest pain presentation. Acute coronary syndrome (ACS) needs to be ruled out first in every patient presenting with chest pain. However, 80% of patients with ACS have no clear diagnostic features on presentation.9 The timely diagnosis and treatment of patients with ACS improves their prognosis. Therefore, clinicians tend to start each patient on ACS treatment to reduce the risk, which often leads to increased costs due to unnecessary, time-consuming diagnostic procedures that may place burdens on both the health care system and the patient.10
Several risk-stratifying tools have been developed in the last few years. Both the GRACE and TIMI risk scores have been designed for risk stratification of patients with proven ACS and not for the chest pain population at the ED.11 Some of these tools are applicable to patients with all types of chest pain presenting to the ED, such as the Manchester Triage System. Other, more selective systems are devoted to the risk stratification of suspected ACS in the ED. One is the HEART score.12
The first study on the HEART score—an acronym that stands for History, Electrocardiogram, Age, Risk factors, and Troponin—was done by Backus et al, who proved that the HEART score is an easy, quick, and reliable predictor of outcomes in chest pain patients.10 The HEART score predicts the short-term incidence of major adverse cardiac events (MACE), which allows clinicians to stratify patients as low-risk, intermediate-risk, and high-risk and to guide their clinical decision-making accordingly. It was developed to provide clinicians with a simple, reliable predictor of cardiac risk on the basis of the lowest score of 0 (very low-risk) up to a score of 10 (very high-risk).
We studied the clinical performance of the HEART score in patients with chest pain, focusing on the efficacy and safety of rapidly identifying patients at risk of MACE. We aimed to determine (1) whether the HEART score is a reliable predictor of outcomes of chest pain patients presenting to the ED; (2) whether the score is feasible in our local settings; and (3) whether it describes the risk profile of patients with and without MACE.
Methods
Setting
Participants were recruited from the ED of King Edward Memorial Hospital, a municipal teaching hospital in Mumbai. The study institute is a tertiary care academic medical center located in Parel, Mumbai, Maharashtra, and is a resource-limited setting serving urban, suburban, and rural populations. Participants requiring urgent attention are first seen by a casualty officer and then referred to the emergency ward. Here, the physician on duty evaluates them and decides on admission to the various wards, like the general ward, medical intensive care unit (ICU), coronary care unit (CCU), etc. The specialist’s opinion may also be obtained before admission. Critically ill patients are initially admitted to the emergency ward and stabilized before being shifted to other areas of the hospital.
Participants
Patients aged 18 years and older presenting with symptoms of acute chest pain or suspected ACS were stratified by priority using the chest pain scoring system—the HEART score. Only patients presenting to the ED were eligible for the study. Informed consent from the patient or next of kin was mandatory for participation in the study.
Patients were determined ineligible for the following reasons: a clear cause for chest pain other than ACS (eg, trauma, diagnosed aortic dissection), persisting or recurrent chest pain caused by rheumatic diseases or cancer (a terminal illness), pregnancy, unable or unwilling to provide informed consent, or incomplete data.
Study design
We conducted a
We conducted our study to determine the importance of calculating the HEART score in each patient, which will help to correctly place them into low-, intermediate-, and high-risk groups for clinically important, irreversible adverse cardiac events and guide the clinical decision-making. Patients with low risk will avoid costly tests and hospital admissions, thus decreasing the cost of treatment and ensuring timely discharge from the ED. Patients with high risk will be treated immediately, to possibly prevent a life-threatening, ACS-related incident. Thus, the HEART score will serve as a quick and reliable predictor of outcomes in chest pain patients and help clinicians to make accurate diagnostic and therapeutic choices in uncertain situations.
HEART score
The total number of points for History, Electrocardiogram (ECG), Age, Risk factors, and Troponin was noted as the HEART score (Table 1).

For this study, the patient’s history and ECGs were interpreted by internal medicine attending physicians in the ED. The ECG taken in the emergency room was reviewed and classified, and a copy of the admission ECG was added to the file. The recommendation for patients with a HEART score in a particular range was evaluated. Notably, those with a score of 3 or lower led to a recommendation of reassurance and early discharge. Those with a HEART score in the intermediate range (4-6) were admitted to the hospital for further clinical observation and testing, whereas a high HEART score (7-10) led to admission for intensive monitoring and early intervention. In the analysis of HEART score data, we only used those patients having records for all 5 parameters, excluding patients without an ECG or troponin test.
Results
Myocardial infarction (MI) was defined based on Universal Definition of Myocardial Infarction.13 Coronary revascularization was defined as angioplasty with or without stent placement or coronary artery bypass surgery.14 Percutaneous coronary intervention (PCI) was defined as any therapeutic catheter intervention in the coronary arteries. Coronary artery bypass graft (CABG) surgery was defined as any cardiac surgery in which coronary arteries were operated on.
The primary outcomes in this study were the (1) risk stratification of chest pain patients into low-risk, intermediate-risk, and high-risk categories; (2) incidence of a MACE within 6 weeks of initial presentation. MACE consists of acute myocardial infarction (AMI), PCI, CABG, coronary angiography revealing procedurally correctable stenosis managed conservatively, and death due to any cause.
Our secondary outcomes were discharge or death due to any cause within 6 weeks after presentation.
Follow-up
Within 6 weeks after presentation to the ED, a follow-up phone call was placed to assess the patient’s progress. The follow-up focused on the endpoint of MACE, comprising all-cause death, MI, and revascularization. No patient was lost to follow-up.
Statistical analysis
We aimed to find a difference in the 6-week MACE between low-, intermediate-, and high-risk categories of the HEART score. The prevalence of CHD in India is 10%,4 and assuming an α of 0.05, we needed a sample of 141 patients from the ED patient population. Continuous variables were presented by mean (SD), and categorical variables as percentages. We used t test and the Mann-Whitney U test for comparison of means for continuous variables, χ2 for categorical variables, and Fisher’s exact test for comparison of the categorical variables. Results with P < .05 were considered statistically significant.
We evaluated 141 patients presenting to the ED with chest pain concerning for ACS during the study period, from July 2019 to October 2019.

Primary outcomes
The risk stratification of the HEART score in chest pain patients and the incidence of 6-week MACE are outlined in Table 3

The distribution of the HEART score’s 5 elements in the groups with or without MACE endpoints is shown in Table 5. Notice the significant differences between the groups. A follow-up phone call was made within 6 weeks after the presentation to the ED to assess the patient’s progress. The 6-week follow-up call data are included in Table 6.

Of 141 patients, 36 patients (25.53%) were diagnosed with MACE within 6 weeks of presentation.

Myocardial infarction—An AMI was diagnosed in 24 of the 141 patients (17.02%). Twenty-one of those already had positive markers on admission (apparently, these AMI had started before their arrival to the emergency room). One AMI occurred 2 days after admission in a 66-year-old male, and another occurred 10 days after discharge. A further AMI occurred 2 weeks after discharge. All 3 patients belonged to the intermediate-risk group.

Revascularization—Coronary angiography was performed in 31 of 141 patients (21.99%). Revascularization was performed in 19 patients (13.48%), of which 15 were PCIs (10.64%) and 4 were CABGs (2.84%).
Mortality—One patient died from the study population. He was a 72-year-old male who died 14 days after admission. He had a HEART score of 8.
Among the 67 low-risk patients:
- MACE: Coronary angiography was performed in 1 patient (1.49%). Among the 67 patients in the low-risk category, there was no cases of AMI or deaths. The remaining 66 patients (98.51%) had an uneventful recovery following discharge.
- General practitioner (GP) visits/readmissions following discharge: Two of 67 patients (2.99%) had GP visits following discharge, of which 1 was uneventful. The other patient, a 64-year-old male, was readmitted due to a recurrent history of chest pain and underwent coronary angiography.
Among the 44 intermediate-risk patients:
- MACE: Of the 7 of 44 patients (15.91%) who had coronary angiography, 3 patients (6.82%) had AMI, of which 1 occurred 2 days after admission in a 66-year-old male. Two patients had AMI following discharge. There were no deaths. Overall, 42 of 44 patients (95.55%) had an uneventful recovery following discharge.
- GP visits/readmissions following discharge: Three of 44 patients (6.82%) had repeated visits following discharge. One was a GP visit that was uneventful. The remaining 2 patients were diagnosed with AMI and readmitted following discharge. One AMI occurred 10 days after discharge in a patient with a HEART score of 6; another occurred 2 weeks after discharge in a patient with a HEART score of 5.
Among the 30 high-risk patients:
- MACE: Twenty-three of 30 patients (76.67%) underwent coronary angiography. One patient died 5 days after discharge. The patient had a HEART score of 8. Most patients however, had an uneventful recovery following discharge (28, 93.33%).
- GP visits/readmissions following discharge: Five of 30 patients (16.67%) had repeated visits following discharge. Two were uneventful. Two patients had a history of recurrent chest pain that resolved on Sorbitrate. One patient was readmitted 2 weeks following discharge due to a complication: a left ventricular clot was found. The patient had a HEART score of 10.
Secondary outcome—Overall, 140 of 141 patients were discharged. One patient died: a 72-year-old male with a HEART score of 8.
Feasibility—To determine the ease and feasibility of performing a HEART score in chest pain patients presenting to the ED, a survey was distributed to the internal medicine physicians in the ED. In the survey, the Likert scale was used to rate the ease of utilizing the HEART score and whether the physicians found it feasible to use it for risk stratification of their chest pain patients. A total of 12 of 15 respondents (80%) found it “easy” to use. Of the remaining 3 respondents, 2 (13.33%) rated the HEART score “very easy” to use, while 1 (6.66%) considered it “difficult” to work with. None of the respondents said that it was not feasible to perform a HEART score in the ED.
Risk factors for reaching an endpoint:
We compared risk profiles between the patient groups with and without an endpoint. The group of patients with MACE were older and had a higher proportion of males than the group of patients without MACE. Moreover, they also had a higher prevalence of hypertension, type 2 diabetes mellitus, smoking, hypercholesterolemia, prior history of PCI/CABG, and history of stroke. These also showed a significant association with MACE. Obesity was not included in our risk factors as we did not have data collected to measure body mass index. Results are represented in Table 7.

Discussion
Our study described a patient population presenting to an ED with chest pain as their primary complaint. The results of this prospective study confirm that the HEART score is an excellent system to triage chest pain patients. It provides the clinician with a reliable predictor of the outcome (MACE) after the patient’s arrival, based on available clinical data and in a resource-limited setting like ours.
Cardiovascular epidemiology studies indicate that this has become a significant public health problem in India.1 Several risk scores for ACS have been published in European and American guidelines. However, in the Indian population, minimal data are available on utilization of such a triage score (HEART score) in chest pain patients in the ED in a resource-limited setting, to the best of our knowledge. In India, only 1 such study is reported,15 at the Sundaram Medical Foundation, a 170-bed community hospital in Chennai. In this study, 13 of 14 patients (92.86%) with a high HEART score had MACE, indicating a sensitivity of 92.86%; in the 44 patients with a low HEART score, 1 patient (2.22%) had MACE, indicating a specificity of 97.78%; and in the 28 patients with a moderate HEART score, 12 patients (42.86%) had MACE.
In looking for the optimal risk-stratifying system for chest pain patients, we analyzed the HEART score. The first study on the HEART score was done Backus et al, proving that the HEART score is an easy, quick, and reliable predictor of outcomes in chest pain patients.10 The HEART score had good discriminatory power, too. The C statistic for the HEART score for ACS occurrence shows a value of 0.83. This signifies a good-to-excellent ability to stratify all-cause chest pain patients in the ED for their risk of MACE. The application of the HEART score to our patient population demonstrated that the majority of the patients belonged to the low-risk category, as reported in the first cohort study that applied the HEART score.8 The relationship between the HEART score category and occurrence of MACE within 6 weeks showed a curve with 3 different patterns, corresponding to the 3 risk categories defined in the literature.11,12 The risk stratification of chest pain patients using the 3 categories (0-3, 4-6, 7-10) identified MACE with an incidence similar to the multicenter study of Backus et al,10,11 but with a greater risk of MACE in the high-risk category (Figure).

Thus, our study confirmed the utility of the HEART score categories to predict the 6-week incidence of MACE. The sensitivity, specificity, and positive and negative predictive values for the established cut-off scores of 4 and 7 are shown in Table 8. The patients in the low-risk category, corresponding to a score < 4, had a very high negative predictive value, thus identifying a small-risk population. The patients in the high-risk category (score ≥ 7) showed a high positive predictive value, allowing the identification of a high-risk population, even in patients with more atypical presentations. Therefore, the HEART score may help clinicians to make accurate management choices by being a strong predictor of both event-free survival and potentially life-threatening cardiac events.11,12

Our study tested the efficacy of the HEART score pathway in helping clinicians make smart diagnostic and therapeutic choices. It confirmed that the HEART score was accurate in predicting the short-term incidence of MACE, thus stratifying patients according to their risk severity. In our study, 67 of 141 patients (47.52%) had low-risk HEART scores, and we found the 6-week incidence of MACE to be 1.49%. We omitted the diagnostic and treatment evaluation for patients in the low-risk category and moved onto discharge. Overall, 66 of 67 patients (98.51%) in the low-risk category had an uneventful recovery following discharge. Only 2 of 67 these patients (2.99%) of patients had health care utilization following discharge. Therefore, extrapolation based on results demonstrates reduced health care utilization. Previous studies have shown similar results.9,12,14,16 For instance, in a prospective study conducted in the Netherlands, low-risk patients representing 36.4% of the total were found to have a low MACE rate (1.7%).9 These low-risk patients were categorized as appropriate and safe for ED discharge without additional cardiac evaluation or inpatient admission.9 Another retrospective study in Portugal,12 and one in Chennai, India,15 found the 6-week incidence of MACE to be 2.00% and 2.22%, respectively. The results of the first HEART Pathway Randomized Control Trial14 showed that the HEART score pathway reduces health care utilization (cardiac testing, hospitalization, and hospital length of stay). The study also showed that these gains occurred without any of the patients that were identified for early discharge, suffering from MACE at 30 days, or secondary increase in cardiac-related hospitalizations. Similar results were obtained by a randomized trial conducted in North Carolina17 that also demonstrated a reduction in objective cardiac testing, a doubling of the rate of early discharge from the ED, and a reduced length of stay by half a day. Another study using a modified HEART score also demonstrated that when low-risk patients are evaluated with cardiac testing, the likelihood for false positives is high.16 Hoffman et al also reported that patients randomized to coronary computed tomographic angiography (CCTA) received > 2.5 times more radiation exposure.16 Thus, low-risk patients may be safely discharged without the need for stress testing or CCTA.
In our study, 30 out of 141 patients (21.28%) had high-risk HEART scores (7-10), and we found the 6-week incidence of MACE to be 90%. Based on the pathway leading to inpatient admission and intensive treatment, 23 of 30 patients (76.67%) patients in our study underwent coronary angiography and further therapeutic treatment. In the high-risk category, 28 of 30 patients (93.33%) patients had an uneventful recovery following discharge. Previous studies have shown similar results. A retrospective study in Portugal showed that 76.9% of the high-risk patients had a 6-week incidence of MACE.12 In a study in the Netherlands,9 72.7% of high-risk patients had a 6-week incidence of MACE. Therefore, a HEART score of ≥ 7 in patients implies early aggressive treatment, including invasive strategies, when necessary, without noninvasive treatment preceding it.8
In terms of intermediate risk, in our study 44 of 141 patients (31.21%) patients had an intermediate-risk HEART score (4-6), and we found the 6-week incidence of MACE to be 18.18%. Based on the pathway, they were kept in the observation ward on admission. In our study, 7 of 44 patients (15.91%) underwent coronary angiography and further treatment; 42 of 44 patients (95.55%) had an uneventful recovery following discharge. In a prospective study in the Netherlands, 46.1% of patients with an intermediate score had a 6-week MACE incidence of 16.6%.10 Similarly, in another retrospective study in Portugal, the incidence of 6-week MACE in intermediate-risk patients (36.7%) was found to be 15.6%.12 Therefore, in patients with a HEART score of 4-6 points, immediate discharge is not an option, as this figure indicates a risk of 18.18% for an adverse outcome. These patients should be admitted for clinical observation, treated as an ACS awaiting final diagnosis, and subjected to noninvasive investigations, such as repeated troponin. Using the HEART score as guidance in the treatment of chest pain patients will benefit patients on both sides of the spectrum.11,12
Our sample presented a male predominance, a wide range of age, and a mean age similar to that of previous studies.12.16 Some risk factors, we found, can increase significantly the odds of chest pain being of cardiovascular origin, such as male gender, smoking, hypertension, type 2 diabetes mellitus, and hypercholesterolemia. Other studies also reported similar findings.8,12,16 Risk factors for premature CHD have been quantified in the case-control INTERHEART study.1 In the INTERHEART study, 8 common risk factors explained > 90% of AMIs in South Asian and Indian patients. The risk factors include dyslipidemia, smoking or tobacco use, known hypertension, known diabetes, abdominal obesity, physical inactivity, low fruit and vegetable intake, and psychosocial stress.1 Regarding the feasibility of treating physicians using the HEART score in the ED, we observed that, based on the Likert scale, 80% of survey respondents found it easy to use, and 100% found it feasible in the ED.
However, there were certain limitations to our study. It involved a single academic medical center and a small sample size, which limit generalizability of the findings. In addition, troponin levels are not calculated at our institution, as it is a resource-limited setting; therefore, we used a positive and negative as +2 and 0, respectively.
Conclusion
The HEART score provides the clinician with a quick and reliable predictor of outcome of patients with chest pain after arrival to the ED and can be used for triage. For patients with low HEART scores (0-3), short-term MACE can be excluded with greater than 98% certainty. In these patients, one may consider reserved treatment and discharge policies that may also reduce health care utilization. In patients with high HEART scores (7-10), the high risk of MACE (90%) may indicate early aggressive treatment, including invasive strategies, when necessary. Therefore, the HEART score may help clinicians make accurate management choices by being a strong predictor of both event-free survival and potentially life-threatening cardiac events. Age, gender, and cardiovascular risk factors may also be considered in the assessment of patients. This study confirmed the utility of the HEART score categories to predict the 6-week incidence of MACE.
Corresponding author: Smrati Bajpai Tiwari, MD, DNB, FAIMER, Department of Medicine, Seth Gordhandas Sunderdas Medical College and King Edward Memorial Hospital, Acharya Donde Marg, Parel, Mumbai 400 012, Maharashtra, India; [email protected].
Financial disclosures: None.
1. Gupta R, Mohan I, Narula J. Trends in coronary heart disease epidemiology in India. Ann Glob Health. 2016;82:307-315.
2. World Health Organization. Global status report on non-communicable diseases 2014. Accessed June 22, 2021. https://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf
3. Fuster V, Kelly BB, eds. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Institutes of Medicine; 2010.
4. Krishnan MN. Coronary heart disease and risk factors in India—on the brink of an epidemic. Indian Heart J. 2012;64:364-367.
5. Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India: current epidemiology and future directions. Circulation. 2016;133:1605-1620.
6. Aeri B, Chauhan S. The rising incidence of cardiovascular diseases in India: assessing its economic impact. J Prev Cardiol. 2015;4:735-740.
7. Pednekar M, Gupta R, Gupta PC. Illiteracy, low educational status and cardiovascular mortality in India. BMC Public Health. 2011;11:567.
8. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196.
9. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168;2153-2158.
10. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART score. Crit Pathw Cardiol. 2010;9:164-169.
11. Backus BE, Six AJ, Kelder JH, et al. Risk scores for patients with chest pain: evaluation in the emergency department. Curr Cardiol Rev. 2011;7:2-8.
12. Leite L, Baptista R, Leitão J, et al. Chest pain in the emergency department: risk stratification with Manchester triage system and HEART score. BMC Cardiovasc Disord. 2015;15:48.
13. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction. Circulation. 2018;138:e618-e651.
14. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8:195-203.
15. Natarajan B, Mallick P, Thangalvadi TA, Rajavelu P. Validation of the HEART score in Indian population. Int J Emerg Med. 2015,8(suppl 1):P5.
16. McCord J, Cabrera R, Lindahl B, et al. Prognostic utility of a modified HEART score in chest pain patients in the emergency department. Circ Cardiovasc Qual Outcomes. 2017;10:e003101.
17. Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2012;168:795-802.
1. Gupta R, Mohan I, Narula J. Trends in coronary heart disease epidemiology in India. Ann Glob Health. 2016;82:307-315.
2. World Health Organization. Global status report on non-communicable diseases 2014. Accessed June 22, 2021. https://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf
3. Fuster V, Kelly BB, eds. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Institutes of Medicine; 2010.
4. Krishnan MN. Coronary heart disease and risk factors in India—on the brink of an epidemic. Indian Heart J. 2012;64:364-367.
5. Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India: current epidemiology and future directions. Circulation. 2016;133:1605-1620.
6. Aeri B, Chauhan S. The rising incidence of cardiovascular diseases in India: assessing its economic impact. J Prev Cardiol. 2015;4:735-740.
7. Pednekar M, Gupta R, Gupta PC. Illiteracy, low educational status and cardiovascular mortality in India. BMC Public Health. 2011;11:567.
8. Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16:191-196.
9. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168;2153-2158.
10. Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART score. Crit Pathw Cardiol. 2010;9:164-169.
11. Backus BE, Six AJ, Kelder JH, et al. Risk scores for patients with chest pain: evaluation in the emergency department. Curr Cardiol Rev. 2011;7:2-8.
12. Leite L, Baptista R, Leitão J, et al. Chest pain in the emergency department: risk stratification with Manchester triage system and HEART score. BMC Cardiovasc Disord. 2015;15:48.
13. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction. Circulation. 2018;138:e618-e651.
14. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8:195-203.
15. Natarajan B, Mallick P, Thangalvadi TA, Rajavelu P. Validation of the HEART score in Indian population. Int J Emerg Med. 2015,8(suppl 1):P5.
16. McCord J, Cabrera R, Lindahl B, et al. Prognostic utility of a modified HEART score in chest pain patients in the emergency department. Circ Cardiovasc Qual Outcomes. 2017;10:e003101.
17. Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2012;168:795-802.









