User login
Traumatic Fractures Should Trigger Osteoporosis Assessment in Postmenopausal Women
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
Study Overview
Objective. To compare the risk of subsequent fractures after an initial traumatic or nontraumatic fracture in postmenopausal women.
Design. A prospective observational study utilizing data from the Women’s Health Initiative (WHI) Study, WHI Clinical Trials (WHI-CT), and WHI Bone Density Substudy to evaluate rates at which patients who suffered a traumatic fracture vs nontraumatic fracture develop a subsequent fracture.
Setting and participants. The WHI study, implemented at 40 United States clinical sites, enrolled 161 808 postmenopausal women aged 50 to 79 years at baseline between 1993 and 1998. The study cohort consisted of 75 335 patients who had self-reported fractures from September 1994 to December 1998 that were confirmed by the WHI Bone Density Substudy and WHI-CT. Of these participants, 253 (0.3%) were excluded because of a lack of follow-up information regarding incident fractures, and 8208 (10.9%) were excluded due to incomplete information on covariates, thus resulting in an analytic sample of 66 874 (88.8%) participants. Prospective fracture ascertainment with participants was conducted at least annually and the mechanism of fracture was assessed to differentiate traumatic vs nontraumatic incident fractures. Traumatic fractures were defined as fractures caused by motor vehicle collisions, falls from a height, falls downstairs, or sports injury. Nontraumatic fractures were defined as fractures caused by a trip and fall.
Main outcome measures. The primary outcome was an incident fracture at an anatomically distinct body part. Fractures were classified as upper extremity (carpal, elbow, lower or upper end of humerus, shaft of humerus, upper radius/ulna, or radius/ulna), lower extremity (ankle, hip, patella, pelvis, shaft of femur, tibia/fibula, or tibial plateau), or spine (lumbar and/or thoracic spine). Self-reported fractures were verified via medical chart review by WHI study physicians; hip fractures were confirmed by review of written reports of radiographic studies; and nonhip fractures were confirmed by review of radiography reports or clinical documentations.
Main results. In total, 66 874 women in the study (mean [SD] age) 63.1 (7.0) years without clinical fracture and 65.3 (7.2) years with clinical fracture at baseline were followed for 8.1 (1.6) years. Of these participants, 7142 (10.7%) experienced incident fracture during the study follow-up period (13.9 per 1000 person-years), and 721 (10.1%) of whom had a subsequent fracture. The adjusted hazard ratio (aHR) of subsequent fracture after an initial fracture was 1.49 (95% CI, 1.38-1.61, P < .001). Covariates adjusted were age, race, ethnicity, body mass index, treated diabetes, frequency of falls in the previous year, and physical function and activity. In women with initial traumatic fracture, the association between initial and subsequent fracture was increased (aHR, 1.25; 95% CI, 1.06-1.48, P = .01). Among women with initial nontraumatic fracture, the association between initial and subsequent fracture was also increased (aHR, 1.52; 95% CI, 1.37-1.68, P < .001). The confidence intervals for the 2 preceding associations for traumatic and nontraumatic initial fracture strata were overlapping.
Conclusion. Fractures, regardless of mechanism of injury, are similarly associated with an increased risk of subsequent fractures in postmenopausal women aged 50 years and older. Findings from this study provide evidence to support reevaluation of current clinical guidelines to include traumatic fracture as a trigger for osteoporosis screening.
Commentary
Osteoporosis is one of the most common age-associated disease that affects 1 in 4 women and 1 in 20 men over the age of 65.1 It increases the risk of fracture, and its clinical sequelae include reduced mobility, health decline, and increased all-cause mortality. The high prevalence of osteoporosis poses a clinical challenge as the global population continues to age. Pharmacological treatments such as bisphosphonates are highly effective in preventing or slowing bone mineral density (BMD) loss and reducing risk of fragility fractures (eg, nontraumatic fractures of the vertebra, hip, and femur) and are commonly used to mitigate adverse effects of degenerative bone changes secondary to osteoporosis.1
The high prevalence of osteoporosis and effectiveness of bisphosphonates raises the question of how to optimally identify adults at risk for osteoporosis so that pharmacologic therapy can be promptly initiated to prevent disease progression. Multiple osteoporosis screening guidelines, including those from the United States Preventive Services Task Force (USPSTF), American Association of Family Physicians, and National Osteoporosis Foundation, are widely used in the clinical setting to address this important clinical question. In general, the prevailing wisdom is to screen osteoporosis in postmenopausal women over the age of 65, women under the age of 65 who have a significant 10-year fracture risk, or women over the age of 50 who have experienced a fragility fracture.1 In the study reported by Crandall et al, it was shown that the risks of having subsequent fractures were similar after an initial traumatic or nontraumatic (fragility) fracture in postmenopausal women aged 50 years and older.2 This finding brings into question whether traumatic fractures should be viewed any differently than nontraumatic fractures in women over the age of 50 in light of evaluation for osteoporosis. Furthermore, these results suggest that most fractures in postmenopausal women may indicate decreased bone integrity, thus adding to the rationale that osteoporosis screening needs to be considered and expanded to include postmenopausal women under the age of 65 who endured a traumatic fracture.
Per current guidelines, a woman under the age of 65 is recommended for osteoporosis screening only if she has an increased 10-year fracture risk compared to women aged 65 years and older. This risk is calculated based on the World Health Organization fracture-risk algorithm (WHO FRAX) tool which uses multiple factors such as age, weight, and history of fragility fractures to predict whether an individual is at risk of developing a fracture in the next 10 years. The WHO FRAX tool does not include traumatic fractures in its risk calculation and current clinical guidelines do not account for traumatic fractures as a red flag to initiate osteoporosis screening. Therefore, postmenopausal women under the age of 65 are less likely to be screened for osteoporosis when they experience a traumatic fracture compared to a fragility fracture, despite being at a demonstrably higher risk for subsequent fracture. As an unintended consequence, this may lead to the under diagnosis of osteoporosis in postmenopausal women under the age of 65. Thus, Crandall et al conclude that a fracture due to any cause warrants follow up evaluation for osteoporosis including BMD testing in women older than 50 years of age.
Older men constitute another population who are commonly under screened for osteoporosis. The current USPSTF guidelines indicate that there is an insufficient body of evidence to screen men for osteoporosis given its lower prevalence.1 However, it is important to note that men have significantly increased mortality after a hip fracture, are less likely to be on pharmacological treatment for osteoporosis, and are under diagnosed for osteoporosis.3 Consistent with findings from the current study, Leslie et al showed that high-trauma and low-trauma fractures have similarly elevated subsequent fracture risk in both men and women over the age of 40 in a Canadian study.4 Moreover, in the same study, BMD was decreased in both men and women who suffered a fracture regardless of the injury mechanism. This finding further underscores a need to consider traumatic fractures as a risk factor for osteoporosis. Taken together, given that men are under screened and treated for osteoporosis but have increased mortality post-fracture, considerations to initiate osteoporosis evaluation should be similarly given to men who endured a traumatic fracture.
The study conducted by Crandall et al has several strengths. It is noteworthy for the large size of the WHI cohort with participants from across the United States which enables the capture of a wider range of age groups as women under the age of 65 are not common participants of osteoporosis studies. Additionally, data ascertainment and outcome adjudication utilizing medical records and physician review assure data quality. A limitation of the study is that the study cohort consists exclusively of women and therefore the findings are not generalizable to men. However, findings from this study echo those from other studies that investigate the relationship between fracture mechanisms and subsequent fracture risk in men and women.3,4 Collectively, these comparable findings highlight the need for additional research to validate traumatic fracture as a risk factor for osteoporosis and to incorporate it into clinical guidelines for osteoporosis screening.
Applications for Clinical Practice
The findings from the current study indicate that traumatic and fragility fractures may be more alike than previously recognized in regards to bone health and subsequent fracture prevention in postmenopausal women. If validated, these results may lead to changes in clinical practice whereby all fractures in postmenopausal women could trigger osteoporosis screening, assessment, and treatment if indicated for the secondary prevention of fractures.
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
1. US Preventive Services Task Force, Curry SJ, Krist Ah, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–2531. doi:10.1001/jama.2018.7498
2. Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern Med. Published online June 7, 2021. doi:10.1001/jamainternmed.2021.2617
3. Mackey DC, Lui L, Cawthon PM, et al. High-Trauma Fractures and Low Bone Mineral Density in Older Women and Men. JAMA. 2007;298(20):2381–2388. doi:10.1001/jama.298.20.2381
4. Leslie WD, Schousboe JT, Morin SN, et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31(6):1059–1067. doi:10.1007/s00198-019-05274-2
Occipital nerve stimulation offers relief for patients with intractable chronic cluster headache
Occipital nerve stimulation may help safely prevent attacks of medically intractable chronic cluster headache, according to a new study.
Medically intractable chronic cluster headaches are unilateral headaches that cause excruciating pain during attacks, which may happen as frequently as eight times per day. They are refractory to, or intolerant of, preventive medications typically used in chronic cluster headaches.
“ONS was associated with a major, rapid, and sustained improvement of severe and long-lasting medically intractable chronic cluster headache, both at high and low intensity,” Leopoldine A. Wilbrink, MD, of Leiden (the Netherlands) University Medical Centre, and coauthors wrote in their paper.
The findings were published online.
The multicenter, randomized, double-blind, phase 3 clinical trial was carried out at seven hospitals in the Netherlands, Belgium, Germany, and Hungary. A total of 150 patients with suspected medically intractable chronic cluster headache were enrolled between October 2010 and December 2017, and observed for 12 weeks at baseline. Of those initially enrolled, 131 patients with at least four medically intractable chronic cluster headache attacks per week and a history of nonresponsiveness to at least three standard preventive medications were randomly allocated to one of two groups: Sixty-five patients received 24 weeks of ONS at high intensity (100% intensity, or the intensity 10% below the threshold of discomfort as reported by the patient) while 66 received low-intensity (30%) ONS. At 25-48 weeks, the patients received open-label ONS.
Safe and well tolerated
“Because ONS causes paraesthesia, preventing masked comparison versus placebo, we compared high-intensity versus low-intensity ONS, which are hypothesised to cause similar paraesthesia, but with different efficacy,” wrote Dr. Wilbrink and colleagues.
From baseline to weeks 21-24, the median weekly mean attack frequencies decreased to 7.38 (95% confidence interval [CI]: 2.5-18.5, P < .0001). A median decrease in 5.21 attacks per week (–11.18 to –0.19, P < .0001) was observed.
The 100% ONS group saw a decrease in mean attack frequency from 17.58 at baseline (range, 9.83-29.33) to 9.5 (3-21.25) at 21-24 weeks with a median change of –4.08 (–11.92 to –0.25). In the 30% ONS group, the mean attack frequency decreased from 15 (9.25 to 22.33) to 6.75 (1.5-16.5) with a median change of –6.5 (–10.83 to –0.08).
At weeks 21-24, the difference in median weekly mean attack frequency between the groups was –2.42 (–5.17 to 3.33).
The authors stated that, in both groups, ONS was “safe and well tolerated.” A total of 129 adverse events were reported in the 100% ONS group and 95 in the 30% ONS group, of which 17 and 9 were considered serious, respectively. The serious adverse events required a short hospital stay to resolve minor hardware issues. The adverse events most frequently observed were local pain, impaired wound healing, neck stiffness, and hardware damage.
Low intensity stimulation may be best
“The main limitation of the study comes from the difficulty in defining the electrical dose, which was not constant across patients within each group, but individually adjusted depending on the perception of the ONS-induced paraesthesia,” Denys Fontaine, MD, and Michel Lanteri-Minet, MD, both from Université Cote D’Azur in France, wrote in an accompanying editorial.
Given that the primary outcome did not differ significantly between the treatment groups, the editorialists stated that “the lowest stimulation intensity that induces paraesthesia is sufficient to obtain an effect in the patients who respond. Increasing the electrical dose or intensity does not seem to bring better efficacy and might even induce discomfort (painful paraesthesia or shock-like sensations) that might substantially reduce the tolerance of this approach.”
While the trial did not provide convincing evidence of high intensity ONS in medically intractable chronic cluster headache, the editorialists are otherwise optimistic about the findings: “… considering the significant difference between baseline and the end of the randomised stimulation phase in both groups (about half of the patients showed a 50% decrease in attack frequency), the findings of this study support the favourable results of previous real-world studies, and indicate that a substantial proportion of patients with intractable chronic cluster headache, although not all, could have their condition substantially improved by ONS.” Dr. Fontaine and Dr. Lanteri-Minet added that they hope that “these data will help health authorities to recognise the efficacy of ONS and consider its approval for use in patients with intractable chronic cluster headache.”
Priorities for future research in this area should “focus on optimising stimulation protocols and disentangling the underlying mechanism of action,” Dr. Wilbrink and colleagues wrote.
The study was funded by the Spinoza 2009 Lifetime Scientific Research Achievement Premium, the Netherlands Organisation for Scientific Research, the Dutch Ministry of Health (as part of a national provisional reimbursement program for promising new treatments), the NutsOhra Foundation from the Dutch Health Insurance Companies, and an unrestricted grant from Medtronic, all to Dr. Ferrari.
Occipital nerve stimulation may help safely prevent attacks of medically intractable chronic cluster headache, according to a new study.
Medically intractable chronic cluster headaches are unilateral headaches that cause excruciating pain during attacks, which may happen as frequently as eight times per day. They are refractory to, or intolerant of, preventive medications typically used in chronic cluster headaches.
“ONS was associated with a major, rapid, and sustained improvement of severe and long-lasting medically intractable chronic cluster headache, both at high and low intensity,” Leopoldine A. Wilbrink, MD, of Leiden (the Netherlands) University Medical Centre, and coauthors wrote in their paper.
The findings were published online.
The multicenter, randomized, double-blind, phase 3 clinical trial was carried out at seven hospitals in the Netherlands, Belgium, Germany, and Hungary. A total of 150 patients with suspected medically intractable chronic cluster headache were enrolled between October 2010 and December 2017, and observed for 12 weeks at baseline. Of those initially enrolled, 131 patients with at least four medically intractable chronic cluster headache attacks per week and a history of nonresponsiveness to at least three standard preventive medications were randomly allocated to one of two groups: Sixty-five patients received 24 weeks of ONS at high intensity (100% intensity, or the intensity 10% below the threshold of discomfort as reported by the patient) while 66 received low-intensity (30%) ONS. At 25-48 weeks, the patients received open-label ONS.
Safe and well tolerated
“Because ONS causes paraesthesia, preventing masked comparison versus placebo, we compared high-intensity versus low-intensity ONS, which are hypothesised to cause similar paraesthesia, but with different efficacy,” wrote Dr. Wilbrink and colleagues.
From baseline to weeks 21-24, the median weekly mean attack frequencies decreased to 7.38 (95% confidence interval [CI]: 2.5-18.5, P < .0001). A median decrease in 5.21 attacks per week (–11.18 to –0.19, P < .0001) was observed.
The 100% ONS group saw a decrease in mean attack frequency from 17.58 at baseline (range, 9.83-29.33) to 9.5 (3-21.25) at 21-24 weeks with a median change of –4.08 (–11.92 to –0.25). In the 30% ONS group, the mean attack frequency decreased from 15 (9.25 to 22.33) to 6.75 (1.5-16.5) with a median change of –6.5 (–10.83 to –0.08).
At weeks 21-24, the difference in median weekly mean attack frequency between the groups was –2.42 (–5.17 to 3.33).
The authors stated that, in both groups, ONS was “safe and well tolerated.” A total of 129 adverse events were reported in the 100% ONS group and 95 in the 30% ONS group, of which 17 and 9 were considered serious, respectively. The serious adverse events required a short hospital stay to resolve minor hardware issues. The adverse events most frequently observed were local pain, impaired wound healing, neck stiffness, and hardware damage.
Low intensity stimulation may be best
“The main limitation of the study comes from the difficulty in defining the electrical dose, which was not constant across patients within each group, but individually adjusted depending on the perception of the ONS-induced paraesthesia,” Denys Fontaine, MD, and Michel Lanteri-Minet, MD, both from Université Cote D’Azur in France, wrote in an accompanying editorial.
Given that the primary outcome did not differ significantly between the treatment groups, the editorialists stated that “the lowest stimulation intensity that induces paraesthesia is sufficient to obtain an effect in the patients who respond. Increasing the electrical dose or intensity does not seem to bring better efficacy and might even induce discomfort (painful paraesthesia or shock-like sensations) that might substantially reduce the tolerance of this approach.”
While the trial did not provide convincing evidence of high intensity ONS in medically intractable chronic cluster headache, the editorialists are otherwise optimistic about the findings: “… considering the significant difference between baseline and the end of the randomised stimulation phase in both groups (about half of the patients showed a 50% decrease in attack frequency), the findings of this study support the favourable results of previous real-world studies, and indicate that a substantial proportion of patients with intractable chronic cluster headache, although not all, could have their condition substantially improved by ONS.” Dr. Fontaine and Dr. Lanteri-Minet added that they hope that “these data will help health authorities to recognise the efficacy of ONS and consider its approval for use in patients with intractable chronic cluster headache.”
Priorities for future research in this area should “focus on optimising stimulation protocols and disentangling the underlying mechanism of action,” Dr. Wilbrink and colleagues wrote.
The study was funded by the Spinoza 2009 Lifetime Scientific Research Achievement Premium, the Netherlands Organisation for Scientific Research, the Dutch Ministry of Health (as part of a national provisional reimbursement program for promising new treatments), the NutsOhra Foundation from the Dutch Health Insurance Companies, and an unrestricted grant from Medtronic, all to Dr. Ferrari.
Occipital nerve stimulation may help safely prevent attacks of medically intractable chronic cluster headache, according to a new study.
Medically intractable chronic cluster headaches are unilateral headaches that cause excruciating pain during attacks, which may happen as frequently as eight times per day. They are refractory to, or intolerant of, preventive medications typically used in chronic cluster headaches.
“ONS was associated with a major, rapid, and sustained improvement of severe and long-lasting medically intractable chronic cluster headache, both at high and low intensity,” Leopoldine A. Wilbrink, MD, of Leiden (the Netherlands) University Medical Centre, and coauthors wrote in their paper.
The findings were published online.
The multicenter, randomized, double-blind, phase 3 clinical trial was carried out at seven hospitals in the Netherlands, Belgium, Germany, and Hungary. A total of 150 patients with suspected medically intractable chronic cluster headache were enrolled between October 2010 and December 2017, and observed for 12 weeks at baseline. Of those initially enrolled, 131 patients with at least four medically intractable chronic cluster headache attacks per week and a history of nonresponsiveness to at least three standard preventive medications were randomly allocated to one of two groups: Sixty-five patients received 24 weeks of ONS at high intensity (100% intensity, or the intensity 10% below the threshold of discomfort as reported by the patient) while 66 received low-intensity (30%) ONS. At 25-48 weeks, the patients received open-label ONS.
Safe and well tolerated
“Because ONS causes paraesthesia, preventing masked comparison versus placebo, we compared high-intensity versus low-intensity ONS, which are hypothesised to cause similar paraesthesia, but with different efficacy,” wrote Dr. Wilbrink and colleagues.
From baseline to weeks 21-24, the median weekly mean attack frequencies decreased to 7.38 (95% confidence interval [CI]: 2.5-18.5, P < .0001). A median decrease in 5.21 attacks per week (–11.18 to –0.19, P < .0001) was observed.
The 100% ONS group saw a decrease in mean attack frequency from 17.58 at baseline (range, 9.83-29.33) to 9.5 (3-21.25) at 21-24 weeks with a median change of –4.08 (–11.92 to –0.25). In the 30% ONS group, the mean attack frequency decreased from 15 (9.25 to 22.33) to 6.75 (1.5-16.5) with a median change of –6.5 (–10.83 to –0.08).
At weeks 21-24, the difference in median weekly mean attack frequency between the groups was –2.42 (–5.17 to 3.33).
The authors stated that, in both groups, ONS was “safe and well tolerated.” A total of 129 adverse events were reported in the 100% ONS group and 95 in the 30% ONS group, of which 17 and 9 were considered serious, respectively. The serious adverse events required a short hospital stay to resolve minor hardware issues. The adverse events most frequently observed were local pain, impaired wound healing, neck stiffness, and hardware damage.
Low intensity stimulation may be best
“The main limitation of the study comes from the difficulty in defining the electrical dose, which was not constant across patients within each group, but individually adjusted depending on the perception of the ONS-induced paraesthesia,” Denys Fontaine, MD, and Michel Lanteri-Minet, MD, both from Université Cote D’Azur in France, wrote in an accompanying editorial.
Given that the primary outcome did not differ significantly between the treatment groups, the editorialists stated that “the lowest stimulation intensity that induces paraesthesia is sufficient to obtain an effect in the patients who respond. Increasing the electrical dose or intensity does not seem to bring better efficacy and might even induce discomfort (painful paraesthesia or shock-like sensations) that might substantially reduce the tolerance of this approach.”
While the trial did not provide convincing evidence of high intensity ONS in medically intractable chronic cluster headache, the editorialists are otherwise optimistic about the findings: “… considering the significant difference between baseline and the end of the randomised stimulation phase in both groups (about half of the patients showed a 50% decrease in attack frequency), the findings of this study support the favourable results of previous real-world studies, and indicate that a substantial proportion of patients with intractable chronic cluster headache, although not all, could have their condition substantially improved by ONS.” Dr. Fontaine and Dr. Lanteri-Minet added that they hope that “these data will help health authorities to recognise the efficacy of ONS and consider its approval for use in patients with intractable chronic cluster headache.”
Priorities for future research in this area should “focus on optimising stimulation protocols and disentangling the underlying mechanism of action,” Dr. Wilbrink and colleagues wrote.
The study was funded by the Spinoza 2009 Lifetime Scientific Research Achievement Premium, the Netherlands Organisation for Scientific Research, the Dutch Ministry of Health (as part of a national provisional reimbursement program for promising new treatments), the NutsOhra Foundation from the Dutch Health Insurance Companies, and an unrestricted grant from Medtronic, all to Dr. Ferrari.
FROM LANCET NEUROLOGY
Hand ulceration
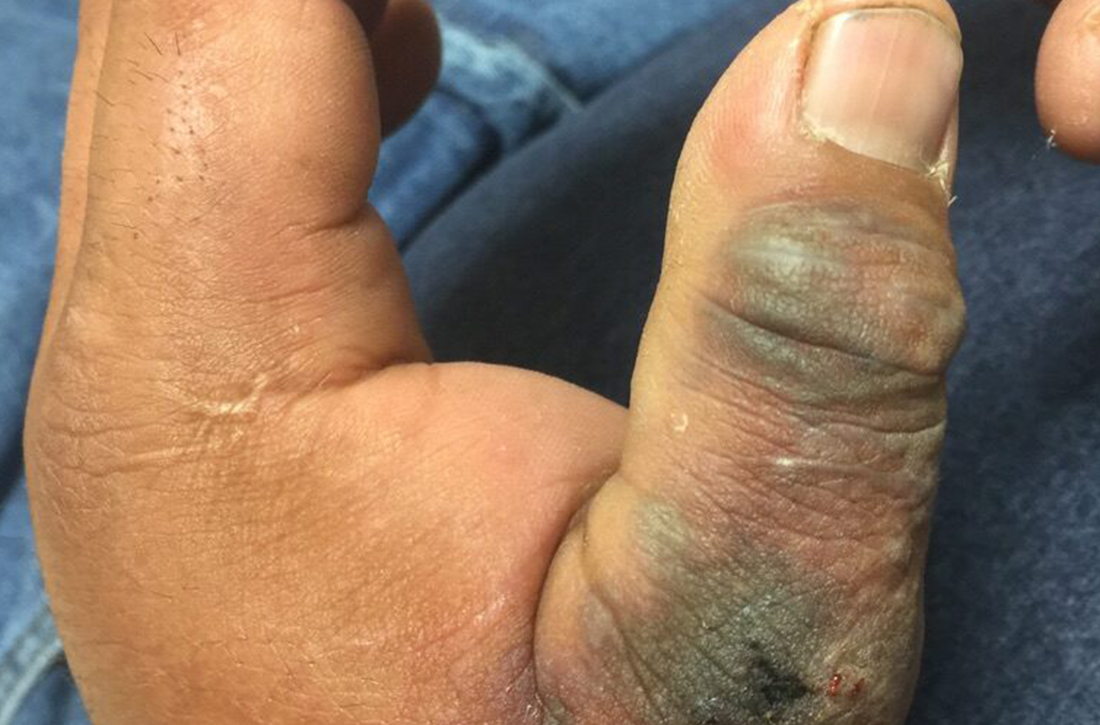
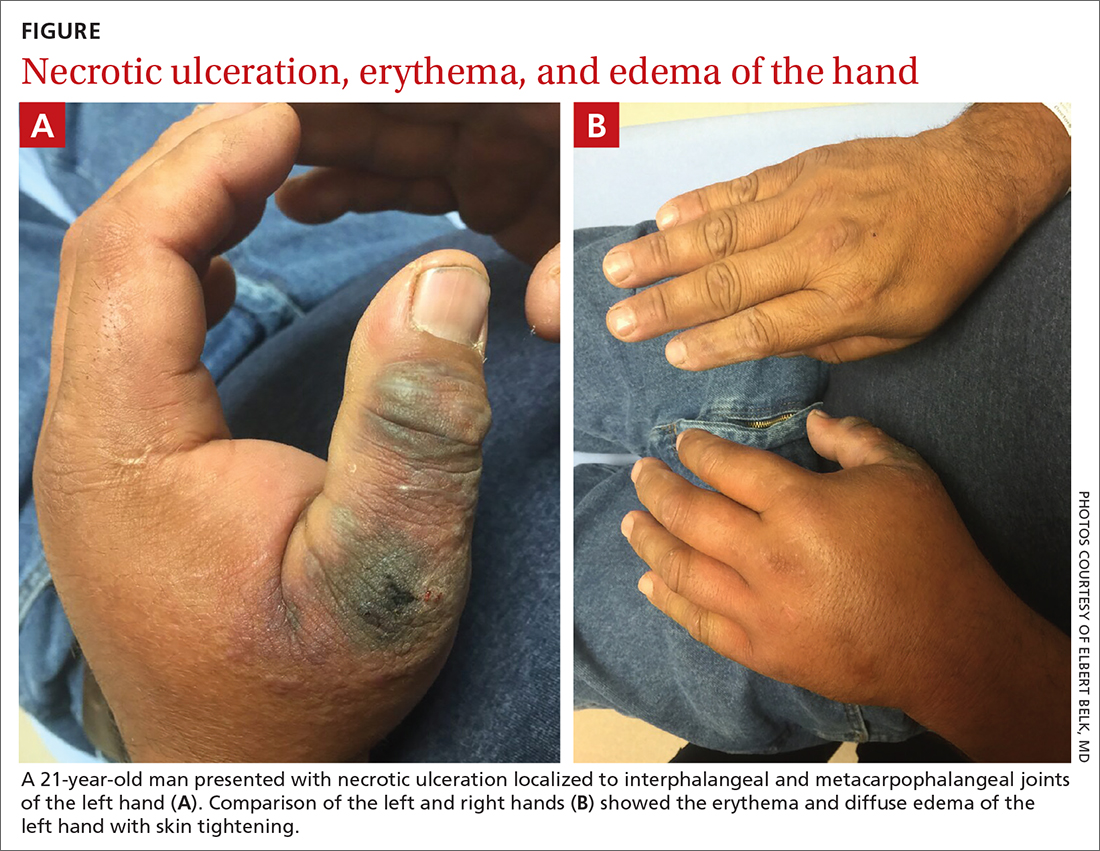
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
A 45-year-old man presented to a south Texas emergency department with a red, tender, edematous left hand. Earlier that day, he had been working in an oil field when his hand suddenly began to hurt.
On physical exam, puncture wounds were visible at the metacarpophalangeal joint of the thumb and the interphalangeal joint, dorsal aspect; the area was surrounded by necrotic black tissue (FIGURE). Additionally, erythema with extensive edema extended distally to the proximal interphalangeal joints of each digit. Upon palpation, the area was warm, firm, and tender, with the edema tracking proximally to his mid-forearm.
The patient had a temperature of 99.5 °F; his other vital signs were normal. His past medical history included hypertension.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Cellulitis, compartment syndrome by scorpion sting
Based on the necrotic puncture wounds, unilateral distribution of the swelling, and the patient’s acknowledgement that he’d seen a scorpion in his work environment prior to symptom onset, he was given a diagnosis of cellulitis with secondary compartment syndrome following a scorpion sting.
A geographic problem
In the United States, there are approximately 17,000 reported cases of scorpion stings every year, with fewer than 11 related deaths reported between 1999 and 2014.1 These cases tend to follow a geographic distribution along the American Southwest, with the highest incidence occurring in Arizona, followed by Texas; the majority of cases occur during the summer months.1
The most clinically relevant scorpion in the United States is the Centruroides sculpturatus, also known as the Arizona bark scorpion.2Centruroides spp can be recognized by a slender, yellow to light brown or tan body measuring 1.3 cm to 7.6 cm in length. There is a tubercule at the base of the stringer, a defining characteristic of the species.3
Urgent care is necessary for more severe symptoms
The most common complaint following a scorpion sting tends to be pain (88.7%), followed by numbness, edema, and erythema.1 Other signs and symptoms include muscle spasms, hypertension, and salivation. Symptoms can persist for 10 to 48 hours. Cardiovascular collapse and disseminated intravascular coagulation are 2 potentially fatal complications of a scorpion sting.
The diagnosis is made clinically based on history and physical exam findings; a complete blood count, coagulation panel, and creatine kinase and amylase/lipase bloodwork may be ordered to assess for end-organ complications. Local serious complications, such as compartment syndrome, should be urgently referred for surgical management.
Continue to: Signs of compartment syndrome...
Signs of compartment syndrome include tense, swollen compartments and pain with passive stretching of muscles within the compartment. Rapid progression of symptoms, as seen in this case, is also a red flag.
Differential diagnosis includes necrotizing fasciitis
The differential diagnosis includes uncomplicated cellulitis, as well as necrotizing fasciitis and methicillin-resistant Staphylococcus aureus (MRSA) cellulitis.
Necrotizing fasciitis. The lack of subcutaneous crackles and pain that is out of proportion to touch, as well as relatively normal vital signs, ruled out a diagnosis of necrotizing fasciitis in this case.
Community-acquired MRSA is seen with purulent cellulitis. However, this patient had no purulent discharge.
Antivenom is only needed for severe cases
Treatment is primarily supportive; all patients should have the wound thoroughly cleaned, and pain can be controlled using nonsteroidal anti-inflammatory drugs or opioid therapy.2 Tetanus prophylaxis should be given. The Centruroides antivenom, Anascorp, should be considered for patients with severe symptoms, including loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, and vomiting.4 In most cases, local poison control centers should be consulted for advice on management and to answer questions about antivenom availability.
Our patient was admitted to the hospital and an urgent surgery consult was obtained. The surgeon performed a fasciotomy to treat the compartment syndrome, and the patient survived without loss of his hand or arm.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
1. Kang AM, Brooks DE. Nationwide scorpion exposures reported to US poison control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165. doi: 10.1007/s13181-016-0594-0
2. Barish RA, Arnold T. Scorpion Stings. Merck Manual. Updated April 2020. Accessed June 26, 2021. https://www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/scorpion-stings
3. González-Santillán E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Trop. 2018;187:264-274. doi: 10.1016/j.actatropica.2018.08.002
4. Anascorp. Package insert. Accredo Health Group, Inc; 2011.
Moving patients beyond injury and back to work
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
This month, JFP tackles a topic—work disability—that might, at first, seem a bit outside our usual wheelhouse of clinical review articles. Work disability is, however, a very important topic. The authors point out that “... primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.” This statement suggests that we need to learn more about managing work-related disability and how to influence patients’ outcomes in a positive manner.
I suspect that we tend to be pessimistic about our ability to influence patient outcomes because we are uncertain about the best course of action. The authors of this article provide excellent information about how we can—and should—help ill and injured patients return to work.
As I read the article, I reflected on my own experience providing patients with advice about returning to work. Two points, in particular, struck a chord with me.
1. Many factors in the process are beyond our control. The physician’s role in helping patients return to work after an injury or illness is limited. The authors remind us that there are many patient and employer factors that are beyond our control and that influence patients’ successful return to work. Patient factors include motivation, mental health, and job satisfaction. Employer factors include job flexibility and disability benefits and policies. And of course, there are system factors that include laws governing work-related disability.
2. Our role, while limited, is important. By putting forth a positive attitude toward recovery and providing encouragement to patients, we can facilitate an earlier return to work.
I am cognizant of the pivotal role we can play with back injuries, a frequent cause of work disability. A great deal of excellent research over the past 20 years guides us regarding treatment and prognosis. Most back injuries are due to musculoskeletal injury and improve quickly during the first week, no matter what the therapy. By steering these patients clear of narcotics, telling them to remain as physically active as their pain will allow, and letting them know they will recover, we can pave the way for an early return to work.
Let us all take full advantage, then, of these important conversations with our patients. Armed with the strategies in this month’s article, we can increase the likelihood of our patients’ success.
Managing work disability to help patients return to the job
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
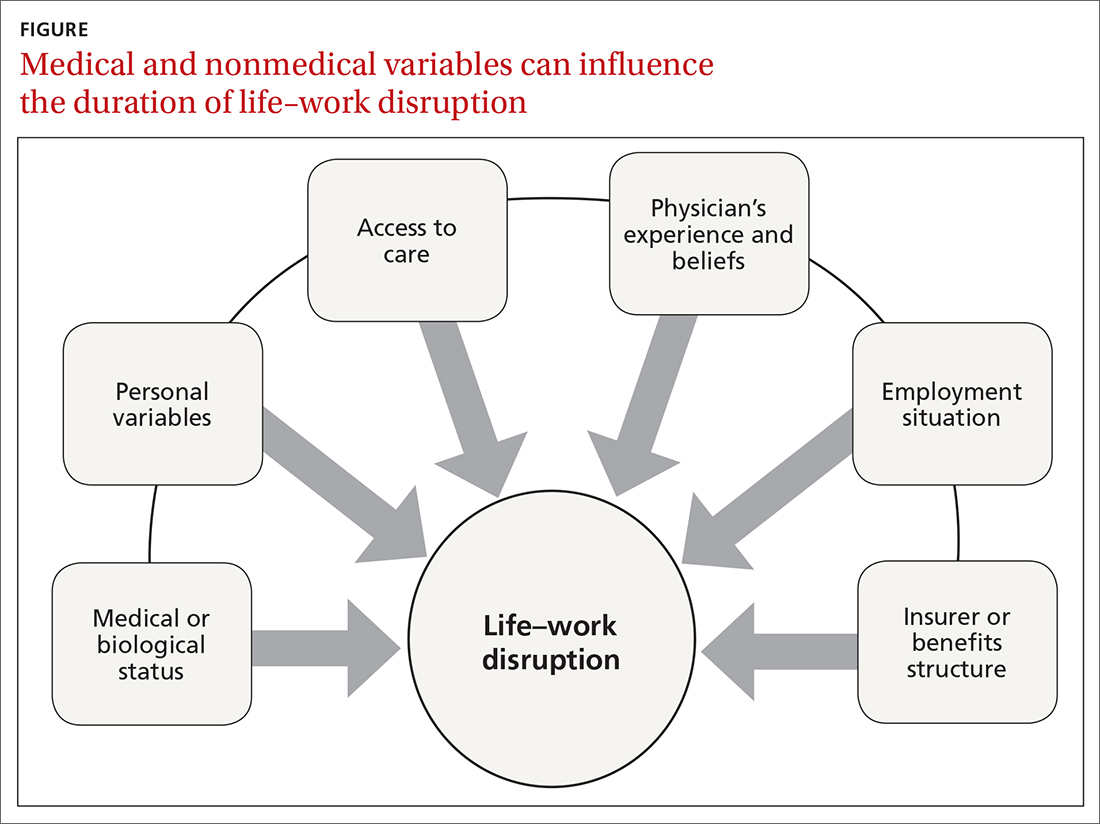
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).
Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.
Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).

Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.

Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
All clinicians who have patients who are employed play an essential role in work disability programs—whether or not those clinicians have received formal training in occupational health. A study found that primary care clinicians are asked to provide guidance about work activities in nearly 10% of their patient encounters; however, 25% of those clinicians thought they had little influence over work disability outcomes.1
In this article, we explain why it is important for family physicians to better manage work disability at the point of care, to help patients return to their pre-injury or pre-illness level of activity.
Why managing the duration of work disability matters
Each year, millions of American workers leave their jobs—temporarily or permanently—because of illness, injury, or the effects of a chronic condition.2 It is estimated that 893 million workdays are lost annually due to a new medical problem; an additional 527 million workdays are lost due to the impact of chronic health conditions on the ability to perform at work.3 The great majority of these lost workdays are the result of personal health conditions, not work-related problems; patients must therefore cope with the accompanying disruption of life and work.
Significant injury and illness can create a life crisis, especially when there is uncertainty about future livelihood, such as an income shortfall during a lengthy recovery. Only 40% of the US workforce is covered by a short-term disability insurance program; only 10% of low-wage and low-skill workers have this type of coverage.4 Benefits rarely replace loss of income entirely, and worker compensation insurance programs provide only partial wage replacement.
In short, work disability is destabilizing and can threaten overall well-being.5
Furthermore, the longer a person remains on temporary disability, the more likely that person is to move to a publicly funded disability program or leave the workforce entirely—thus, potentially losing future earnings and self-identity related to being a working member of society.6-8
Most of the annual cost of poor health for US employers derives from medical and wage benefits ($226 billion) and impaired or reduced employee performance ($223 billion).3 In addition, temporarily disabled workers likely account for a disproportionate share of health care costs: A study found that one-half of medical and pharmacy payments were paid out to the one-quarter of employees requiring disability benefits.9
Continue to: Benefits of staying on the job
Benefits of staying on the job. Research shows that there are physical and mental health benefits to remaining at, or returning to, work after an injury or illness.10,11 For example, in a longitudinal cohort of people with low back pain, immediate or early return to work (in 1-7 days) was associated with reduced pain and improved functioning at 3 months.12 Physicians who can guide patients safely back to normal activities, including work, minimize the physical and mental health impact of the injury or illness and avoid chronicity.13
Emphasizing the importance of health, not disease or injury
Health researchers have found that diagnosis, cause, and extent of morbidity do not adequately explain observed variability in the impact of health conditions, utilization of resources, or need for services. A wider view of the functional implications of an injury or illness is therefore required for physicians to effectively recommend disability duration.
The World Health Organization recommends a shift toward a more holistic view of health, impairment, and disability, including an emphasis on functional ability, intrinsic capacity, and environmental context.14 The American Medical Association, American College of Occupational and Environmental Medicine, and Canadian Medical Association emphasize that prolonged absence from one’s normal role can be detrimental to mental, physical, and social well-being.8 These advisory groups recommend that physicians encourage patients who are unable to work to (1) focus on restoring the rhythm of their everyday life in a stepwise fashion and (2) resume their usual responsibilities as soon as possible.
Advising a patient to focus on “what you can do,” not “what you can’t do,” might make all the difference in their return to productivity. Keeping the patient’s—as well as your own—attention focused on the positive process of recovery and documenting evidence of functional progress is an important addition to (or substitute for) detailed inquiries about pain and dysfunction.
Why does duration of disability vary so much from case to case?
Disability duration is influenced by the individual patient, employer, physician, jurisdiction, insurer or benefits structure, and access to care.15 For you to effectively manage a patient who is out of work for a medical reason, it is important to understand how nonmedical variables often influence the pace of recovery and the timing of return to work (FIGURE).

Continue to: Deficient communication
Deficient communication. Often, employers, insurers, third-party administrators, and clinicians—each a key stakeholder in disability care—are disconnected from one another, resulting in poor communication with the injured worker. Such fragmented communication can delay treatment and recovery.16 Data systems are not designed to measure the duration of disability or provide proactive notification for key stakeholders who might intervene to facilitate a patient’s recovery.
Alternatively, a collaborative approach to disability management has been shown to improve outcomes.17,18 Communication among the various professionals involved can be coordinated and expedited by a case manager or disability manager hired by the medical practice, the employer, or the insurance company.
Psychosocial and economic influences can radically affect the time it takes to return to pre-injury or pre-illness functional status. Demographic variables (age, sex, income, education, and support system) influence how a person responds to a debilitating injury or illness.19 Fear of re-injury, anxiety over the intensity of pain upon movement, worry over dependency on others, and resiliency play an important role when a patient is attempting to return to full activity.20,21
Job satisfaction has been identified as the most significant variable associated with prompt return to work.15 Work has many health-enhancing aspects, including socioeconomic status, psychosocial support, and self-identity22; however, not everyone wants, or feels ready, to go back to work even once they are physically able. Workplace variables, such as the patient–employee’s dislike of the position, coworkers, or manager, have been cited by physicians as leading barriers to returning to work at an appropriate time.23,24
Other external variables. Physicians should formulate activity prescriptions and medical restrictions based on the impact the medical condition has on the usual ability to function, as well as the anticipated impact of specific activities on the body’s natural healing process. However, Rainville and colleagues found that external variables—patient requests, employer characteristics, and jurisdiction issues—considerably influence physicians’ recommendations.20 For example, benefit structure might influence how long a patient wants to remain out of work—thus altering the requests they make to their physician. Jurisdictional characteristics, such as health care systems, state workers’ compensation departments, and payer systems, all influence a patient’s recovery timeline and time away from work.25
Continue to: What does your patient need so that they can recover?
What does your patient need so that they can recover? Individual and systemic factors must be appropriately addressed to minimize the impact that recovery from a disability has on a person’s life. Successful functional recovery enables the person to self-manage symptoms, reduce disruption-associated stress, preserve mental health, and maintain healthy relationships at home and work. An example is the patient who has successfully coped with the entire predicament that their medical condition posed and resumed their usual daily routine and responsibilities at home and at work—albeit sometimes with temporary or permanent modification necessitated by their specific condition.
Strategies that help patients stay at, or return to, their job
Physicians who anticipate, monitor, and actively manage the duration of a work disability can improve patient outcomes by minimizing life disruption, avoiding unnecessary medical care, and shortening the period of absence from work.
Key strategy: Set expectations for functional recovery early in the episode, including a forecast of how long it will take to get life and work back to normal.26,27 This is similar to discussing expectations about pain before surgery, which has been shown to decrease subsequent requests for opioids.28 It is crucial to educate the patient about timelines, define functional outcomes, and encourage them to set goals for recovery.29
Devise an evidence-based treatment plan. A fundamental way to reduce disability duration is to (1) devise a treatment plan that is evidence based and (2) take the most effective route to recovery. Given the pace with which medical research changes the understanding of diseases and treatments, it is essential to rely on up-to-date, comprehensive, independent, and authoritative resources to support your care decisions.
Aligning clinical practice with evidence-based medicine (EBM) is a good way to accomplish that goal. By definition, EBM practice guidelines recommend the safest and most effective treatments after unbiased assessment of the best available research. Increasingly, EBM is adopted to improve clinical and functional outcomes, establish national standards of care, and set criteria to evaluate clinical performance.30
Continue to: Utilize established guidelines
Utilize established guidelines. A tactic that can make it easier to discuss return to work with patients is to rely on an independent and authoritative reference set of codified disability duration guidelines, which, typically, can be searched by diagnosis, procedure, or presenting symptoms. Such guidelines provide a condition-specific expected duration of work disability in the form of number of days, with shortest, typical, and maximum durations for different levels of job demands. If necessary, you can then adjust the guideline’s estimated duration to account for the patient’s age, underlying state of health, comorbidities, and so forth.
The use of disability duration guidelines at the point of care can facilitate the process of setting early and appropriate expectations for a patient’s recovery. If a patient is confrontational in response to your recommendation on the duration of work disability, guidelines can be used to address specific objections and facilitate understanding of functional recovery.
Consider the employer’s needs. To support return-to-work efforts, your guidance about work should consider the employer’s business needs. Employers require that the patient’s abilities, restrictions, and limitations be described in concrete terms because they must decide which specific tasks are unsafe and which ones they can reasonably expect the recovering worker to perform. However, employers often fail to send information to the physician about the patient’s job tasks—such that the clinician must rely on patient self-reporting, which might be inaccurate, incomplete, or biased.15 When a patient needs protection against foreseeable harm, highlight specific activities that are currently unsafe on the recovery timeline.
Employers rely on the physician to (1) estimate what the patient can do and (2) describe work ability in clear, objective terms that both patient and employer can interpret (TABLE). For example, “no heavy lifting” might be hard for an employer to interpret; “may lift 10 pounds from the floor to the waist as many as 12 times an hour” might be applied in a more practical manner to help a patient return to work safely.31 Including specific numbers, rates, and metrics in activity restrictions can also help demonstrate improvement over the course of treatment.

Be clear and specific on work restrictions. During recovery, it is important to tell the patient which temporary work restrictions are intended to prevent further injury or recurrence (prophylactic work restrictions) and which are an estimate of what they are able to do safely at work (capacity-based restrictions). Your written work restrictions form should be kept separate from private medical information because those restrictions will be the basis of subsequent conversations between patient and employer, who should be invited to give feedback if the guidance needs revision or clarification.
Continue to: Employer programs
Employer programs, such as modified duty, transitional duty, or early return to work programs, have been found to resolve claims faster and improve recovery outcomes.10,12 Such programs might also reduce occupational stress and improve productivity when an employee realizes that their functional abilities are matched to realistic job expectations during recovery.16 You can play an important role in empowering your patients to seek out these support programs.
What’s ahead for managing disability durations?
Work disability duration is influenced by the complex mix of biological, psychosocial, and economic variables that we have touched on here. All stakeholders involved in the recovery process should support the patient’s ability to live life with as few restrictions as possible; you play a key role in their recovery by focusing on ability, highlighting remaining capabilities, emphasizing activities that are safe to perform, and encouraging acceptance of, and adaptation to, any irrevocable losses.
This is a holistic approach that might help patients overcome the stress and anxiety associated with major life events arising from illness or injury that trigger disability benefits. Open communication and establishing a shared goal, among all involved, of the best possible outcome increases the likelihood that working patients will return to their familiar life or find another positive path forward.
Using EBM and disability duration guidelines can help decrease the length of life–work disruption by ensuring that patients are given a diagnosis, treated, and managed appropriately.32,33 Although these practices have been adopted by some physicians, health care systems, and insurers, they are not being implemented systematically and are unlikely to become ubiquitous unless they are mandated by payers or by law.
Family physicians are front-line providers for America’s workforce. They are distinctly situated to help patients achieve their best life at home and work. Improving the timeliness and quality of work guidance provided by the physician is an important way to minimize the impact of health problems on working people’s lives and livelihoods—and to help them stay employed.
CORRESPONDENCE
Kerri Wizner, MPH, 10355 Westmoor Drive, Westminster, CO 80021; [email protected].
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
1. Pransky G, Katz JN, Benjamin K, et al. Improving the physician role in evaluating work ability and managing disability: A survey of primary care practitioners. Disabil Rehabil. 2002;24:867-874. doi: 10.1080/09638280210142176
2. Hollenbeck K. Promoting Retention or Reemployment of Workers After a Significant Injury or Illness. Mathematica Policy Research; October 22, 2015. Accessed June 1, 2021. https://mathematica.org/publications/promoting-retention-or-reemployment-of-workers-after-a-significant-injury-or-illness
3. Poor health costs us employers $530 billion and 1.4 billion work days of absence and impaired performance according to Integrated Benefits Institute. Press release. November 15, 2018. Accessed June 1, 2021. www.ibiweb.org/poor-health-costs-us-employers-530-billion-and-1-4-billion-work-days-of-absence-and-impaired-performance
4. US Bureau of Labor Statistics. Life and disability insurance benefits: How extensive is the employer-provided safety net? BLS looks at life and disability benefits. Program Perspectives. 2010;2:7:1-4. Accessed June 8, 2021. www.bls.gov/opub/btn/archive/program-perspectives-on-life-and-disability-insurance-benefits.pdf
5. Kettlewell N, Morris RW, Ho N, et al. The differential impact of major life events on cognitive and affective wellbeing. SSM Popul Health. 2019;10:100533. doi: 10.1016/j.ssmph.2019.100533
6. Contreary K, Ben-Shalom Y, Gifford B. Using predictive analytics for early identification of short-term disability claimants who exhaust their benefits. J Occup Rehabil. 2018;28:584-596. doi: 10.1007/s10926-018-9815-5
7. Hultin H, Lindholm C, Möller J. Is there an association between long-term sick leave and disability pension and unemployment beyond the effect of health status? – A cohort study. PLoS One. 2012;7:e35614. doi: 10.1371/journal.pone.0035614
8. Canadian Medical Association. CMA policy: The treating physician’s role in helping patients return to work after an illness or injury (update 2013); 2013:1-6. Accessed June 1, 2021. https://policybase.cma.ca/documents/policypdf/PD13-05.pdf
9. Gifford B. Temporarily disabled workers account for a disproportionate share of health care payments. Health Aff (Millwood). 2017;36:245-249. doi:10.1377/hlthaff.2016.1013
10. Rueda S, Chambers L, Wilson M, et al. Association of returning to work with better health in working-aged adults: a systematic review. Am J Public Health. 2012;102:541-556. doi: 10.2105/AJPH.2011.300401
11. Modini M, Joyce S, Mykletun A, et al. The mental health benefits of employment: results of a systematic meta-review. Australas Psychiatry. 2016;24:331-336. doi: 10.1177/1039856215618523
12. Shaw WS, Nelson CC, Woiszwillo MJ, et al. Early return to work has benefits for relief of back pain and functional recovery after controlling for multiple confounds. J Occup Environ Med. 2018;60:901-910. doi: 10.1097/JOM.0000000000001380
13. Jurisic M, Bean M, Harbaugh J, et al. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59:e125-e131. doi: 10.1097/JOM.0000000000001055
14. World Health Organization. Towards a common language for functioning, disability and health. ICF: The International Classification of Functioning, Disability and Health. 2002. Accessed June 2, 2021. www.who.int/classifications/icf/icfbeginnersguide.pdf
15. Talmage JB, Melhorn JM, Hyman MH. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
16. Harrell M. Psychological factors and workforce health. In: Lee LP, Martin DW, Kancelbaum B. Occupational Medicine: A Basic Guide. American College of Occupational and Environmental Medicine; 2019. Accessed June 1, 2021. https://ohguides.acoem.org/07-psychological-factors-and-workforce-health-stress-management
17. Wickizer TM, Franklin GM, Fulton-Kehoe D. Innovations in occupational health care delivery can prevent entry into permanent disability: 8-year follow-up of the Washington State Centers for Occupational Health and Education. Med Care. 2018;56:1018-1023. doi: 10.1097/MLR.0000000000000991
18. Christian J, Wickizer T, Burton K. Implementing a community-focused health & work service. SSDI Solution Initiative, Fiscal Institute of the Committee for a Responsible Federal Budget. May 2019. Accessed June 2, 2021. www.crfb.org/sites/default/files/Implementing_a_Community-Focused_HWS.pdf
19. Macpherson RA, Koehoorn M, Fan J, et al. Do differences in work disability duration between men and women vary by province in Canada? J Occup Rehabil. 2018;29:560-568. doi: 10.1007/s10926-018-9819-1
20. Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine (Phila Pa 1976). 2005;30:2579-2584. doi: 10.1097/01.brs.0000186589.69382.1d
21. Jay K, Thorsen SV, Sundstrup E, et al. Fear avoidance beliefs and risk of long-term sickness absence: prospective cohort study among workers with musculoskeletal pain. Pain Res Treat. 2018;2018:8347120. doi: 10.1155/2018/8347120
22. Burgard S, Lin KY. Bad jobs, bad health? How work and working conditions contribute to health disparities. Am Behav Sci. 2013;57:10.1177/0002764213487347. doi: 10.1177/0002764213487347
23. Soklaridis S, Tang G, Cartmill C, et al. “Can you go back to work?” Family physicians’ experiences with assessing patients’ functional ability to return to work. Can Fam Physician. 2011;57:202-209.
24. Peters SE, Truong AP, Johnston V. Stakeholders identify similar barriers but different strategies to facilitate return-to-work: a vignette of a worker with an upper extremity condition. Work. 2018;59:401-412. doi: 10.3233/WOR-182692
25. Shraim M, Cifuentes M, Willetts JL, et al. Regional socioeconomic disparities in outcomes for workers with low back pain in the United States. Am J Ind Med. 2017;60:472-483. doi: 10.1002/ajim.22712
26. Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys Ther. 2011;91:712-721. doi: 10.2522/ptj.20100280
27. Aasdahl L, Pape K, Jensen C, et al. Associations between the readiness for return to work scale and return to work: a prospective study. J Occup Rehabil. 2018;28:97-106. doi: 10.1007/s10926-017-9705-2
28. Pino C, Covington M. Prescription of opioids for acute pain in opioid naïve patients. UpToDate Web site. February 9, 2021. Accessed June 2, 2021. www.uptodate.com/contents/prescription-of-opioids-for-acute-pain-in-opioid-naive-patients
29. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24:32. doi: 10.1186/s12998-016-0113-z
30. Lewis SJ, Orland BI. The importance and impact of evidence-based medicine. J Manag Care Pharm. 2004;10(5 suppl A):S3-S5. doi: 10.18553/jmcp.2004.10.S5-A.S3
31. Rupe KL. Work restrictions: documenting a patient’s return to work. Nurse Pract. 2010;35:49-53. doi: 10.1097/01.NPR.0000388901.49604.a8
32. Owens JD, Hegmann KT, Thiese MS, et al. Impacts of adherence to evidence-based medicine guidelines for the management of acute low back pain on costs of worker's compensation claims. J Occup Environ Med. 2019;61:445-452. doi: 10.1097/JOM.0000000000001593
33. Gaspar FW, Kownacki R, Zaidel CS, et al. Reducing disability durations and medical costs for patients with a carpal tunnel release surgery through the use of opioid prescribing guidelines. J Occup Environ Med. 2017;59:1180-1187. doi: 10.1097/JOM.0000000000001168
PRACTICE RECOMMENDATIONS
› Set appropriate expectations for the patient at the start of any episode of work disability: Estimate the course of functional recovery over time and the total duration of life–work disruption. A
› Include detailed activity prescriptions in the treatment plan, with stepwise progression over time toward full recovery. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sharp decrease in opioid access for dying U.S. cancer patients
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
FDA OKs spinal cord stimulation for diabetic neuropathy pain
The Food and Drug Administration has approved the first high-frequency spinal cord stimulation (SCS) therapy for treating painful diabetic neuropathy (PDN).
The approval is specific for the treatment of chronic pain associated with PDN using the Nevro’s Senza System with 10 kHz stimulation. It is intended for patients whose pain is refractory to, or who can’t tolerate, conventional medical treatment. According to the company, there are currently about 2.3 million individuals with refractory PDN in the United States.
The 10 kHz device, called HFX, involves minimally invasive epidural implantation of the stimulator device, which delivers mild electrical impulses to the nerves to interrupt pain signal to the brain. Such spinal cord stimulation “is a straightforward, well-established treatment for chronic pain that’s been used for over 30 years,” according to the company, although this is the first approval of the modality specifically for PDN.
Asked to comment, Rodica Pop-Busui MD, PhD, the Larry D. Soderquist Professor in Diabetes at the University of Michigan, Ann Arbor, said that “the approval of the Nevro 10kHz high-frequency spinal cord stimulation to treat pain associated with diabetic neuropathy has the potential for benefit for many patients with diabetes and painful diabetic peripheral neuropathy.”
She noted that, “although there are several other pharmacological agents that currently carry the FDA approval for PDN, this is a condition that is notoriously difficult to treat, particularly when taking into account the actual number needed to treat with a specific agent to achieve a clinically meaningful pain reduction, as well as the spectrum of side effects and drug-drug interactions in a patient population that require many other additional agents to manage diabetes and comorbidities on a daily basis. Thus, this new therapeutic approach besides effective pain reduction has the additional benefit of bypassing drug interactions.” Dr. Pop-Busui was the lead author on the American Diabetes Association’s 2017 position statement on diabetic neuropathy.
She also cautioned, on the other hand, that “it is not very clear yet how easy it will be for all eligible patients to have access to this technology, what will be the actual costs, the insurance coverage, or the acceptance by patients across various sociodemographic backgrounds from the at-large clinical care. However, given the challenges we encounter to treat diabetic neuropathy and particularly the pain associated with it, it is quite encouraging to see that the tools available to help our patients are now broader.”
Both 6-and 12-month results show benefit
The FDA approval was based on 6-month data from a prospective, multicenter, open-label randomized clinical trial published in JAMA Neurology.
Use of the 10-kHz SCS device was compared with conventional treatment alone in 216 patients with PDN refractory to gabapentinoids and at least one other analgesic class and lower limb pain intensity of 5 cm or more on a 10-cm visual analog scale.
The primary endpoint, percentage of participants reporting 50% pain relief or more without worsening of baseline neurologic deficits at 3 months, was met by 5 of 94 (5%) patients in the conventional group, compared with 75 of 95 (79%) with the 10-kHz SCS plus conventional treatment (P < .001).
Infections requiring device explant occurred in two patients in the 10-kHz SCS group (2%).
At 12 months, those in the original SCS group plus 86% of subjects given the option to cross over from the conventional treatment group showed “clear and sustained” benefits of the 10-kHz SCS with regard to lower-limb pain, pain interference with daily living, sleep quality, and activity, Erika Petersen, MD, director of the section of functional and restorative neurosurgery at the University of Arkansas for Medical Sciences, Little Rock , reported at the 2021 annual scientific sessions of the ADA.
Infection was the most common study-related adverse event, affecting 8 of 154 patients with the SCS implants (5.2%). Three resolved with conservative treatment and five (3.2%) required removal of the device.
The patients will be followed for a total of 24 months.
Commercial launch of HFX in the United States will begin immediately, the company said.
Dr. Pop-Busui has received consultant fees in the last 12 months from Averitas Pharma, Boehringer Ingelheim, Nevro, and Novo Nordisk. Dr. Petersen has financial relationships with Nevro, Medtronic, and several other neuromodulator makers.
The Food and Drug Administration has approved the first high-frequency spinal cord stimulation (SCS) therapy for treating painful diabetic neuropathy (PDN).
The approval is specific for the treatment of chronic pain associated with PDN using the Nevro’s Senza System with 10 kHz stimulation. It is intended for patients whose pain is refractory to, or who can’t tolerate, conventional medical treatment. According to the company, there are currently about 2.3 million individuals with refractory PDN in the United States.
The 10 kHz device, called HFX, involves minimally invasive epidural implantation of the stimulator device, which delivers mild electrical impulses to the nerves to interrupt pain signal to the brain. Such spinal cord stimulation “is a straightforward, well-established treatment for chronic pain that’s been used for over 30 years,” according to the company, although this is the first approval of the modality specifically for PDN.
Asked to comment, Rodica Pop-Busui MD, PhD, the Larry D. Soderquist Professor in Diabetes at the University of Michigan, Ann Arbor, said that “the approval of the Nevro 10kHz high-frequency spinal cord stimulation to treat pain associated with diabetic neuropathy has the potential for benefit for many patients with diabetes and painful diabetic peripheral neuropathy.”
She noted that, “although there are several other pharmacological agents that currently carry the FDA approval for PDN, this is a condition that is notoriously difficult to treat, particularly when taking into account the actual number needed to treat with a specific agent to achieve a clinically meaningful pain reduction, as well as the spectrum of side effects and drug-drug interactions in a patient population that require many other additional agents to manage diabetes and comorbidities on a daily basis. Thus, this new therapeutic approach besides effective pain reduction has the additional benefit of bypassing drug interactions.” Dr. Pop-Busui was the lead author on the American Diabetes Association’s 2017 position statement on diabetic neuropathy.
She also cautioned, on the other hand, that “it is not very clear yet how easy it will be for all eligible patients to have access to this technology, what will be the actual costs, the insurance coverage, or the acceptance by patients across various sociodemographic backgrounds from the at-large clinical care. However, given the challenges we encounter to treat diabetic neuropathy and particularly the pain associated with it, it is quite encouraging to see that the tools available to help our patients are now broader.”
Both 6-and 12-month results show benefit
The FDA approval was based on 6-month data from a prospective, multicenter, open-label randomized clinical trial published in JAMA Neurology.
Use of the 10-kHz SCS device was compared with conventional treatment alone in 216 patients with PDN refractory to gabapentinoids and at least one other analgesic class and lower limb pain intensity of 5 cm or more on a 10-cm visual analog scale.
The primary endpoint, percentage of participants reporting 50% pain relief or more without worsening of baseline neurologic deficits at 3 months, was met by 5 of 94 (5%) patients in the conventional group, compared with 75 of 95 (79%) with the 10-kHz SCS plus conventional treatment (P < .001).
Infections requiring device explant occurred in two patients in the 10-kHz SCS group (2%).
At 12 months, those in the original SCS group plus 86% of subjects given the option to cross over from the conventional treatment group showed “clear and sustained” benefits of the 10-kHz SCS with regard to lower-limb pain, pain interference with daily living, sleep quality, and activity, Erika Petersen, MD, director of the section of functional and restorative neurosurgery at the University of Arkansas for Medical Sciences, Little Rock , reported at the 2021 annual scientific sessions of the ADA.
Infection was the most common study-related adverse event, affecting 8 of 154 patients with the SCS implants (5.2%). Three resolved with conservative treatment and five (3.2%) required removal of the device.
The patients will be followed for a total of 24 months.
Commercial launch of HFX in the United States will begin immediately, the company said.
Dr. Pop-Busui has received consultant fees in the last 12 months from Averitas Pharma, Boehringer Ingelheim, Nevro, and Novo Nordisk. Dr. Petersen has financial relationships with Nevro, Medtronic, and several other neuromodulator makers.
The Food and Drug Administration has approved the first high-frequency spinal cord stimulation (SCS) therapy for treating painful diabetic neuropathy (PDN).
The approval is specific for the treatment of chronic pain associated with PDN using the Nevro’s Senza System with 10 kHz stimulation. It is intended for patients whose pain is refractory to, or who can’t tolerate, conventional medical treatment. According to the company, there are currently about 2.3 million individuals with refractory PDN in the United States.
The 10 kHz device, called HFX, involves minimally invasive epidural implantation of the stimulator device, which delivers mild electrical impulses to the nerves to interrupt pain signal to the brain. Such spinal cord stimulation “is a straightforward, well-established treatment for chronic pain that’s been used for over 30 years,” according to the company, although this is the first approval of the modality specifically for PDN.
Asked to comment, Rodica Pop-Busui MD, PhD, the Larry D. Soderquist Professor in Diabetes at the University of Michigan, Ann Arbor, said that “the approval of the Nevro 10kHz high-frequency spinal cord stimulation to treat pain associated with diabetic neuropathy has the potential for benefit for many patients with diabetes and painful diabetic peripheral neuropathy.”
She noted that, “although there are several other pharmacological agents that currently carry the FDA approval for PDN, this is a condition that is notoriously difficult to treat, particularly when taking into account the actual number needed to treat with a specific agent to achieve a clinically meaningful pain reduction, as well as the spectrum of side effects and drug-drug interactions in a patient population that require many other additional agents to manage diabetes and comorbidities on a daily basis. Thus, this new therapeutic approach besides effective pain reduction has the additional benefit of bypassing drug interactions.” Dr. Pop-Busui was the lead author on the American Diabetes Association’s 2017 position statement on diabetic neuropathy.
She also cautioned, on the other hand, that “it is not very clear yet how easy it will be for all eligible patients to have access to this technology, what will be the actual costs, the insurance coverage, or the acceptance by patients across various sociodemographic backgrounds from the at-large clinical care. However, given the challenges we encounter to treat diabetic neuropathy and particularly the pain associated with it, it is quite encouraging to see that the tools available to help our patients are now broader.”
Both 6-and 12-month results show benefit
The FDA approval was based on 6-month data from a prospective, multicenter, open-label randomized clinical trial published in JAMA Neurology.
Use of the 10-kHz SCS device was compared with conventional treatment alone in 216 patients with PDN refractory to gabapentinoids and at least one other analgesic class and lower limb pain intensity of 5 cm or more on a 10-cm visual analog scale.
The primary endpoint, percentage of participants reporting 50% pain relief or more without worsening of baseline neurologic deficits at 3 months, was met by 5 of 94 (5%) patients in the conventional group, compared with 75 of 95 (79%) with the 10-kHz SCS plus conventional treatment (P < .001).
Infections requiring device explant occurred in two patients in the 10-kHz SCS group (2%).
At 12 months, those in the original SCS group plus 86% of subjects given the option to cross over from the conventional treatment group showed “clear and sustained” benefits of the 10-kHz SCS with regard to lower-limb pain, pain interference with daily living, sleep quality, and activity, Erika Petersen, MD, director of the section of functional and restorative neurosurgery at the University of Arkansas for Medical Sciences, Little Rock , reported at the 2021 annual scientific sessions of the ADA.
Infection was the most common study-related adverse event, affecting 8 of 154 patients with the SCS implants (5.2%). Three resolved with conservative treatment and five (3.2%) required removal of the device.
The patients will be followed for a total of 24 months.
Commercial launch of HFX in the United States will begin immediately, the company said.
Dr. Pop-Busui has received consultant fees in the last 12 months from Averitas Pharma, Boehringer Ingelheim, Nevro, and Novo Nordisk. Dr. Petersen has financial relationships with Nevro, Medtronic, and several other neuromodulator makers.
Widely prescribed meds ineffective for low back pain?
Results of a large systematic review and meta-analysis of randomized controlled trials show very “low certainty evidence” that non-benzodiazepine antispasmodics provide meaningful improvement in pain intensity in patients with low back pain – and may actually increase adverse event risk.
“We found that muscle relaxants might reduce pain in the short term, but on average, the effect is probably too small to be important, and most patients wouldn’t be able to feel any difference in their pain compared to taking a placebo,” study investigator Aidan Cashin, PhD, with the Center for Pain IMPACT, Neuroscience Research Australia, and University of New South Wales, Sydney, told this news organization. “There is also an increased risk of side effects,” he added.
The study was published online July 7 in The BMJ.
Global problem
Low back pain is a major global public health problem that burdens individuals, health care systems, and societies.
“Most people, around 80%, will have at least one episode of low back pain during their life,” Dr. Cashin noted.
Muscle relaxants, a broad class of drugs that include non-benzodiazepine antispasmodics and antispastics, are often prescribed for low back pain. In 2020 alone, prescriptions exceeded 1.3 million in England and topped 30 million in the United States.
“However, clinical practice guidelines have provided conflicting recommendations for the use of muscle relaxants to treat low back pain,” Dr. Cashin said.
To assess the efficacy and safety of muscle relaxants, the researchers conducted a detailed analysis of 31 randomized controlled trials that compared muscle relaxants with placebo, usual care, or no treatment in a total of 6,505 adults with nonspecific low back pain.
For acute low back pain, they found “very low certainty evidence” that non-benzodiazepine antispasmodics might reduce pain intensity at 2 weeks or less, but the effect is small – less than 8 points on a 0 to 100 point scale – and not clinically meaningful.
They found little to no effect of non-benzodiazepine antispasmodics on pain intensity at 3 to 13 weeks or on disability at any follow-up time points. None of the trials assessed the effect of muscle relaxants on long-term outcomes.
There was also low-certainty and very-low-certainty evidence that non-benzodiazepine antispasmodics might increase the risk of an adverse event, commonly dizziness, drowsiness, headache, and nausea (relative risk 1.6; 95% confidence interval, 1.2-2.0).
Better research needed
“We were surprised by the findings, as earlier research suggested that muscle relaxants did reduce pain intensity. But when we included all of the most up-to-date research, the results became much less certain,” said Dr. Cashin.
“We were also surprised to see that so much of the research wasn’t done very well, which means that we can’t be very certain in the results. There is a clear need to improve how research is done for low back pain so that we better understand whether medicines can help people or not,” Dr. Cashin said.
“We would encourage clinicians to discuss this uncertainty in the efficacy and safety of muscle relaxants with patients, sharing information about the possibility for a worthwhile benefit in pain reduction but increased risk of experiencing a nonserious adverse event, to allow them to make informed treatment decisions,” corresponding author James McAuley, PhD, University of New South Wales, said in an interview.
“We know that no matter what medicines people with low back pain are taking, they should avoid staying in bed, and they should try to be active and continue with their usual activities, including work, as much as they can. High-quality research shows that people who do this are more likely to recover faster and more completely,” said Dr. McAuley.
A symptom, not a diagnosis
Reached for comment, Andrew Hecht, MD, chief of spine surgery at Mount Sinai Health System, New York, noted that acute low back pain is “a symptom, not a diagnosis, and most episodes of acute low back pain without leg pain will resolve within a few weeks no matter what you do.”
“For people who have an episode of acute low back pain, we typically use anti-inflammatory medications, combined with a short, low dose course of a muscle relaxant if necessary, depending on the severity of symptoms, to help get you over the worst part of it,” Dr. Hecht said.
“We are trying to help the patient feel better in the short term and get more physically strong with therapy to try to reduce the frequency of these attacks in the future,” he added.
“But each patient is different. It’s not one-size-fits-all, and we don’t give prolonged courses of muscle relaxants because they have some side effects, like sedation,” Dr. Hecht cautioned.
The study had no specific funding. Dr. Cashin, Dr. McAuley, and Dr. Hecht have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large systematic review and meta-analysis of randomized controlled trials show very “low certainty evidence” that non-benzodiazepine antispasmodics provide meaningful improvement in pain intensity in patients with low back pain – and may actually increase adverse event risk.
“We found that muscle relaxants might reduce pain in the short term, but on average, the effect is probably too small to be important, and most patients wouldn’t be able to feel any difference in their pain compared to taking a placebo,” study investigator Aidan Cashin, PhD, with the Center for Pain IMPACT, Neuroscience Research Australia, and University of New South Wales, Sydney, told this news organization. “There is also an increased risk of side effects,” he added.
The study was published online July 7 in The BMJ.
Global problem
Low back pain is a major global public health problem that burdens individuals, health care systems, and societies.
“Most people, around 80%, will have at least one episode of low back pain during their life,” Dr. Cashin noted.
Muscle relaxants, a broad class of drugs that include non-benzodiazepine antispasmodics and antispastics, are often prescribed for low back pain. In 2020 alone, prescriptions exceeded 1.3 million in England and topped 30 million in the United States.
“However, clinical practice guidelines have provided conflicting recommendations for the use of muscle relaxants to treat low back pain,” Dr. Cashin said.
To assess the efficacy and safety of muscle relaxants, the researchers conducted a detailed analysis of 31 randomized controlled trials that compared muscle relaxants with placebo, usual care, or no treatment in a total of 6,505 adults with nonspecific low back pain.
For acute low back pain, they found “very low certainty evidence” that non-benzodiazepine antispasmodics might reduce pain intensity at 2 weeks or less, but the effect is small – less than 8 points on a 0 to 100 point scale – and not clinically meaningful.
They found little to no effect of non-benzodiazepine antispasmodics on pain intensity at 3 to 13 weeks or on disability at any follow-up time points. None of the trials assessed the effect of muscle relaxants on long-term outcomes.
There was also low-certainty and very-low-certainty evidence that non-benzodiazepine antispasmodics might increase the risk of an adverse event, commonly dizziness, drowsiness, headache, and nausea (relative risk 1.6; 95% confidence interval, 1.2-2.0).
Better research needed
“We were surprised by the findings, as earlier research suggested that muscle relaxants did reduce pain intensity. But when we included all of the most up-to-date research, the results became much less certain,” said Dr. Cashin.
“We were also surprised to see that so much of the research wasn’t done very well, which means that we can’t be very certain in the results. There is a clear need to improve how research is done for low back pain so that we better understand whether medicines can help people or not,” Dr. Cashin said.
“We would encourage clinicians to discuss this uncertainty in the efficacy and safety of muscle relaxants with patients, sharing information about the possibility for a worthwhile benefit in pain reduction but increased risk of experiencing a nonserious adverse event, to allow them to make informed treatment decisions,” corresponding author James McAuley, PhD, University of New South Wales, said in an interview.
“We know that no matter what medicines people with low back pain are taking, they should avoid staying in bed, and they should try to be active and continue with their usual activities, including work, as much as they can. High-quality research shows that people who do this are more likely to recover faster and more completely,” said Dr. McAuley.
A symptom, not a diagnosis
Reached for comment, Andrew Hecht, MD, chief of spine surgery at Mount Sinai Health System, New York, noted that acute low back pain is “a symptom, not a diagnosis, and most episodes of acute low back pain without leg pain will resolve within a few weeks no matter what you do.”
“For people who have an episode of acute low back pain, we typically use anti-inflammatory medications, combined with a short, low dose course of a muscle relaxant if necessary, depending on the severity of symptoms, to help get you over the worst part of it,” Dr. Hecht said.
“We are trying to help the patient feel better in the short term and get more physically strong with therapy to try to reduce the frequency of these attacks in the future,” he added.
“But each patient is different. It’s not one-size-fits-all, and we don’t give prolonged courses of muscle relaxants because they have some side effects, like sedation,” Dr. Hecht cautioned.
The study had no specific funding. Dr. Cashin, Dr. McAuley, and Dr. Hecht have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large systematic review and meta-analysis of randomized controlled trials show very “low certainty evidence” that non-benzodiazepine antispasmodics provide meaningful improvement in pain intensity in patients with low back pain – and may actually increase adverse event risk.
“We found that muscle relaxants might reduce pain in the short term, but on average, the effect is probably too small to be important, and most patients wouldn’t be able to feel any difference in their pain compared to taking a placebo,” study investigator Aidan Cashin, PhD, with the Center for Pain IMPACT, Neuroscience Research Australia, and University of New South Wales, Sydney, told this news organization. “There is also an increased risk of side effects,” he added.
The study was published online July 7 in The BMJ.
Global problem
Low back pain is a major global public health problem that burdens individuals, health care systems, and societies.
“Most people, around 80%, will have at least one episode of low back pain during their life,” Dr. Cashin noted.
Muscle relaxants, a broad class of drugs that include non-benzodiazepine antispasmodics and antispastics, are often prescribed for low back pain. In 2020 alone, prescriptions exceeded 1.3 million in England and topped 30 million in the United States.
“However, clinical practice guidelines have provided conflicting recommendations for the use of muscle relaxants to treat low back pain,” Dr. Cashin said.
To assess the efficacy and safety of muscle relaxants, the researchers conducted a detailed analysis of 31 randomized controlled trials that compared muscle relaxants with placebo, usual care, or no treatment in a total of 6,505 adults with nonspecific low back pain.
For acute low back pain, they found “very low certainty evidence” that non-benzodiazepine antispasmodics might reduce pain intensity at 2 weeks or less, but the effect is small – less than 8 points on a 0 to 100 point scale – and not clinically meaningful.
They found little to no effect of non-benzodiazepine antispasmodics on pain intensity at 3 to 13 weeks or on disability at any follow-up time points. None of the trials assessed the effect of muscle relaxants on long-term outcomes.
There was also low-certainty and very-low-certainty evidence that non-benzodiazepine antispasmodics might increase the risk of an adverse event, commonly dizziness, drowsiness, headache, and nausea (relative risk 1.6; 95% confidence interval, 1.2-2.0).
Better research needed
“We were surprised by the findings, as earlier research suggested that muscle relaxants did reduce pain intensity. But when we included all of the most up-to-date research, the results became much less certain,” said Dr. Cashin.
“We were also surprised to see that so much of the research wasn’t done very well, which means that we can’t be very certain in the results. There is a clear need to improve how research is done for low back pain so that we better understand whether medicines can help people or not,” Dr. Cashin said.
“We would encourage clinicians to discuss this uncertainty in the efficacy and safety of muscle relaxants with patients, sharing information about the possibility for a worthwhile benefit in pain reduction but increased risk of experiencing a nonserious adverse event, to allow them to make informed treatment decisions,” corresponding author James McAuley, PhD, University of New South Wales, said in an interview.
“We know that no matter what medicines people with low back pain are taking, they should avoid staying in bed, and they should try to be active and continue with their usual activities, including work, as much as they can. High-quality research shows that people who do this are more likely to recover faster and more completely,” said Dr. McAuley.
A symptom, not a diagnosis
Reached for comment, Andrew Hecht, MD, chief of spine surgery at Mount Sinai Health System, New York, noted that acute low back pain is “a symptom, not a diagnosis, and most episodes of acute low back pain without leg pain will resolve within a few weeks no matter what you do.”
“For people who have an episode of acute low back pain, we typically use anti-inflammatory medications, combined with a short, low dose course of a muscle relaxant if necessary, depending on the severity of symptoms, to help get you over the worst part of it,” Dr. Hecht said.
“We are trying to help the patient feel better in the short term and get more physically strong with therapy to try to reduce the frequency of these attacks in the future,” he added.
“But each patient is different. It’s not one-size-fits-all, and we don’t give prolonged courses of muscle relaxants because they have some side effects, like sedation,” Dr. Hecht cautioned.
The study had no specific funding. Dr. Cashin, Dr. McAuley, and Dr. Hecht have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sen. Schumer backs federal decriminalization of marijuana
U.S. Sen. Chuck Schumer, the Senate majority leader, is cosponsoring legislation that would decriminalize marijuana at the federal level.
The Cannabis Administration & Opportunity Act would allow the federal government to regulate and tax marijuana sales for the first time and would stop the federal prosecution of people for possessing and selling the drug, The New York Times reported. States could still make their own marijuana laws, however.
The bill calls for using money raised by taxing marijuana to help poor people and communities of color that have been unduly affected by marijuana laws.
Arrests and convictions for nonviolent marijuana offenses would be automatically expunged, The New York Times reported.
“The War on Drugs has been a war on people – particularly people of color,” a draft of the bill said, adding that the bill “aims to end the decades of harm inflicted on communities of color by removing cannabis from the federal list of controlled substances and empowering states to implement their own cannabis laws.”
But passage of the bill is highly uncertain because of strong Republican opposition in the Senate, where Democrats hold a narrow majority, according to The New York Times.
Sen. Schumer signaled his intentions when he spoke on April 20, the unofficial holiday for marijuana smokers.
“Hopefully, the next time this unofficial holiday of 4/20 rolls around, our country will have made progress in addressing the massive overcriminalization of marijuana in a meaningful and comprehensive way,” he said at the time, the newspaper reported.
Cosponsors were U.S. Sen. Cory Booker of New Jersey and U.S. Sen. Ron Wyden of Oregon, chairman of the Senate Finance Committee.
A version of this article first appeared on WebMD.com.
U.S. Sen. Chuck Schumer, the Senate majority leader, is cosponsoring legislation that would decriminalize marijuana at the federal level.
The Cannabis Administration & Opportunity Act would allow the federal government to regulate and tax marijuana sales for the first time and would stop the federal prosecution of people for possessing and selling the drug, The New York Times reported. States could still make their own marijuana laws, however.
The bill calls for using money raised by taxing marijuana to help poor people and communities of color that have been unduly affected by marijuana laws.
Arrests and convictions for nonviolent marijuana offenses would be automatically expunged, The New York Times reported.
“The War on Drugs has been a war on people – particularly people of color,” a draft of the bill said, adding that the bill “aims to end the decades of harm inflicted on communities of color by removing cannabis from the federal list of controlled substances and empowering states to implement their own cannabis laws.”
But passage of the bill is highly uncertain because of strong Republican opposition in the Senate, where Democrats hold a narrow majority, according to The New York Times.
Sen. Schumer signaled his intentions when he spoke on April 20, the unofficial holiday for marijuana smokers.
“Hopefully, the next time this unofficial holiday of 4/20 rolls around, our country will have made progress in addressing the massive overcriminalization of marijuana in a meaningful and comprehensive way,” he said at the time, the newspaper reported.
Cosponsors were U.S. Sen. Cory Booker of New Jersey and U.S. Sen. Ron Wyden of Oregon, chairman of the Senate Finance Committee.
A version of this article first appeared on WebMD.com.
U.S. Sen. Chuck Schumer, the Senate majority leader, is cosponsoring legislation that would decriminalize marijuana at the federal level.
The Cannabis Administration & Opportunity Act would allow the federal government to regulate and tax marijuana sales for the first time and would stop the federal prosecution of people for possessing and selling the drug, The New York Times reported. States could still make their own marijuana laws, however.
The bill calls for using money raised by taxing marijuana to help poor people and communities of color that have been unduly affected by marijuana laws.
Arrests and convictions for nonviolent marijuana offenses would be automatically expunged, The New York Times reported.
“The War on Drugs has been a war on people – particularly people of color,” a draft of the bill said, adding that the bill “aims to end the decades of harm inflicted on communities of color by removing cannabis from the federal list of controlled substances and empowering states to implement their own cannabis laws.”
But passage of the bill is highly uncertain because of strong Republican opposition in the Senate, where Democrats hold a narrow majority, according to The New York Times.
Sen. Schumer signaled his intentions when he spoke on April 20, the unofficial holiday for marijuana smokers.
“Hopefully, the next time this unofficial holiday of 4/20 rolls around, our country will have made progress in addressing the massive overcriminalization of marijuana in a meaningful and comprehensive way,” he said at the time, the newspaper reported.
Cosponsors were U.S. Sen. Cory Booker of New Jersey and U.S. Sen. Ron Wyden of Oregon, chairman of the Senate Finance Committee.
A version of this article first appeared on WebMD.com.
Updated consensus statement assesses new migraine treatments
“Because the benefit–risk profiles of newer treatments will continue to evolve as clinical trial and real-world data accrue, the American Headache Society intends to review this statement regularly and update, if appropriate, based on the emergence of evidence with implications for clinical practice,” wrote lead author Jessica Ailani, MD, of the department of neurology at Medstar Georgetown University Hospital, Washington, and colleagues. The statement was published in Headache.
To assess recent data on the efficacy, safety, and clinical use of newly introduced acute and preventive migraine treatments, the AHS convened a small task force to review relevant literature published from December 2018 through February 2021. The society’s board of directors, along with patients and patient advocates associated with the American Migraine Foundation, also provided pertinent commentary.
New migraine treatment
Five recently approved acute migraine treatments were specifically noted: two small-molecule calcitonin gene-related peptide (CGRP) receptor antagonists – rimegepant and ubrogepant – along with the nonsteroidal anti-inflammatory drug celecoxib, the serotonin 5-HT1F agonist lasmiditan, and remote electrical neuromodulation (REN). Highlighted risks include serious cardiovascular thrombotic events in patients on celecoxib, along with driving impairment, sleepiness, and the possibility of overuse in patients on lasmiditan. The authors added, however, that REN “has shown good tolerability and safety in clinical trials” and that frequent use of rimegepant or ubrogepant does not appear to lead to medication-overuse headache.
Regarding acute treatment overall, the statement recommended nonsteroidal anti-inflammatory drugs (NSAIDs), nonopioid analgesics, acetaminophen, or caffeinated analgesic combinations – such as aspirin plus acetaminophen plus caffeine – for mild to moderate attacks. For moderate or severe attacks, they recommended migraine-specific agents such as triptans, small-molecule CGRP receptor antagonists (gepants), or selective serotonin 5-HT1F receptor agonists (ditans). No matter the prescribed treatment, the statement pushed for patients to “treat at the first sign of pain to improve the probability of achieving freedom from pain and reduce attack-related disability.”
The authors added that 30% of patients on triptans have an “insufficient response” and as such may benefit from a second triptan or – if certain criteria are met – switching to a gepant, a ditan, or a neuromodulatory device. They also recommended a nonoral formulation for patients whose attacks are often accompanied by severe nausea or vomiting.
More broadly, they addressed the tolerability and safety issues associated with certain treatments, including the gastrointestinal and cardiovascular side effects of NSAIDs and the dangers of using triptans in patients with coronary artery disease or other vascular disorders. And while gepants and ditans appeared in clinical trials to be safe choices for patients with stable cardiovascular disease, “benefit-risk should be assessed in each patient as the real-world database for these therapies grows,” they wrote.
Only one recently approved preventive treatment – eptinezumab, an intravenous anti-CGRP ligand monoclonal antibody (MAB) – was highlighted. The authors noted that its benefits can begin within 24 hours, and it can reduce acute medication use and therefore the risk of medication-overuse headache.
Regarding preventive treatments overall, the authors stated that prevention should be offered if patients suffer from 6 or more days of headache per month, or 3-4 days of headache plus some-to-severe disability. Preventive treatments should be considered in patients who range from at least 2 days of headache per month plus severe disability to 4 or 5 days of headache. Prevention should also be considered in patients with uncommon migraine subtypes, including hemiplegic migraine, migraine with brainstem aura, and migraine with prolonged aura.
Initiating treatment
When considering initiation of treatment with one of the four Food and Drug Administration–approved CGRP MABs – eptinezumab, erenumab, fremanezumab, or galcanezumab – the authors recommend their use if migraine patients show an inability to tolerate or respond to a trial of two or more older oral medications or other established effective therapies. Though they emphasized that oral preventive medications should be started at a low dose and titrated slowly until the target response is reached or tolerability issues emerge, no such need was specified for the parenteral treatments. They also endorsed the approach of patients staying on oral preventive drugs for a minimum of 8 weeks to determine effectiveness or a lack thereof; at that point, switching to another treatment is recommended.
The dual use of therapies such as neuromodulation, biobehavioral therapies, and gepants were also examined, including gepants’ potential as a “continuum between the acute and preventive treatment of migraine” and the limited use of neuromodulatory devices in clinical practice despite clear benefits in patients who prefer to avoid medication or those suffering from frequent attacks and subsequent medication overuse. In addition, it was stated that biobehavioral therapies have “grade A evidence” supporting their use in patients who either prefer nonpharmacologic treatments or have an adverse or poor reaction to the drugs.
From the patient perspective, one of the six reviewers shared concerns about migraine patients being required to try two established preventive medications before starting a recently introduced option, noting that the older drugs have lower efficacy and tolerability. Two reviewers would have liked to see the statement focus more on nonpharmacologic and device-related therapies, and one reviewer noted the possible value in guidance regarding “exploratory approaches” such as cannabis.
The authors acknowledged numerous potential conflicts of interest, including receiving speaking and consulting fees, grants, personal fees, and honoraria from various pharmaceutical and publishing companies.
Not everyone agrees
Commenting on the AHS consensus statement, James A Charles, MD, and Ira Turner, MD, had this to say: “This Consensus Statement incorporates the best available evidence including the newer CGRP therapies as well as the older treatments. The AHS posture is that the CGRP abortive and preventive treatments have a lesser amount of data and experience than the older treatments which have a wealth of literature and data because they have been around longer. As a result, there are 2 statements in these guidelines that the insurance companies quote in their manual of policies:
1. Inadequate response to two or more oral triptans before using a gepant as abortive treatment
2. Inadequate response to an 8-week trial at a dose established to be potentially effective of two or more of the following before using CGRP MAB for preventive treatment: topiramate, divalproex sodium/valproate sodium; beta-blocker: metoprolol, propranolol, timolol, atenolol, nadolol; tricyclic antidepressant: amitriptyline, nortriptyline; serotonin-norepinephrine reuptake inhibitor: venlafaxine, duloxetine; other Level A or B treatments.”
Dr. Charles, who is affiliated with Holy Name Medical Center in Teaneck N.J., and Dr. Turner, who is affiliated with the Center for Headache Care and Research at Island Neurological Associates in Plainview, N.Y., further said that “giving the CGRP MABs and gepants second-class status because they have not been around as long as the old boys is an insult to the research, development, and successful execution of gepant and CGRP MAB therapies in the last several years. The authors omitted the Hepp study and the long list of adverse effects of triptans leading to high discontinuance rates, and how trying a second triptan will probably not work.” Importantly, they said, “the authors have given the insurance carriers a weapon to deny direct access to gepants and CGRP MABs making direct access to these agents difficult for patients and physicians and their staffs.”
Dr. Charles and Dr. Turner point out that the AHS guidelines use the term “cost effective” – that it is better to use the cheaper, older drugs first. “Ineffective treatment of a patient for 8 weeks before using CGRP blocking therapies and using 2 triptans before a gepant is cost ineffective,” they said. “Inadequate delayed treatment results in loss of work productivity and loss of school and family participation and excessive use of ER visits. These guidelines forget that we ameliorate current disability and prevent chronification by treating with the most effective abortive and preventive therapies which may not commence with the cheaper old drugs.”
They explain: “Of course, we would use a beta-blocker for comorbid hypertension and/or anxiety, and venlafaxine for comorbid depression. And if a patient is pain free in 2 hrs with no adverse effects from a triptan used less than 10 times a month, it would not be appropriate to switch to a gepant. However, a treatment naive migraineur with accelerating migraine should have the option of going directly to a gepant and CGRP blocking MAB.” Dr. Charles and Dr. Turner concur that the phrase in the AHS consensus statement regarding the staging of therapy – two triptans before a gepant and two oral preventatives for 8 weeks before a CGRP MAB – “should be removed so that the CGRP drugs get the equal credit they deserve, as can be attested to by the migraine voices of lives saved by the sound research that led to their development and approval by the FDA.”
Ultimately, Dr. Charles and Dr. Turner said, “the final decision on treatment should be made by the physician and patient, not the insurance company or consensus statements.”
Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, former president of the International Headache Society, and editor-in-chief of Neurology Reviews, said, “Although I think the consensus statement is well done, and the authors have the right to make the statements they have made, Drs. Charles and Turner are excellent experienced clinicians and they should be heard. They properly state that the restrictive statements highlighted by the authors have already been used by insurance companies to prevent access to the more expensive but more effective therapies with fewer adverse effects.”
Dr. Rapoport goes on to say, “I believe that the patient’s individual headache history and past responses to therapies must be analyzed by the treating physician and an appropriate treatment be agreed upon between the patient and doctor. It is time to let experienced headache-interested doctors make their own correct decision about treatment without the heavy hand of the insurance company, which is often more intent on saving money than helping the patient.
Suggested reading
Hepp Z et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478-88.
Alam A et al. Triptan use and discontinuation in a representative sample of persons with migraine: Results from Migraine in America Symptoms and Treatment (MAST) study. Headache. 2018;58:68‐69.
Buse DC et al. Adding additional acute medications to a triptan regimen for migraine and observed changes in headache-related disability: Results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2015 Jun;55(6):825-39.
“Because the benefit–risk profiles of newer treatments will continue to evolve as clinical trial and real-world data accrue, the American Headache Society intends to review this statement regularly and update, if appropriate, based on the emergence of evidence with implications for clinical practice,” wrote lead author Jessica Ailani, MD, of the department of neurology at Medstar Georgetown University Hospital, Washington, and colleagues. The statement was published in Headache.
To assess recent data on the efficacy, safety, and clinical use of newly introduced acute and preventive migraine treatments, the AHS convened a small task force to review relevant literature published from December 2018 through February 2021. The society’s board of directors, along with patients and patient advocates associated with the American Migraine Foundation, also provided pertinent commentary.
New migraine treatment
Five recently approved acute migraine treatments were specifically noted: two small-molecule calcitonin gene-related peptide (CGRP) receptor antagonists – rimegepant and ubrogepant – along with the nonsteroidal anti-inflammatory drug celecoxib, the serotonin 5-HT1F agonist lasmiditan, and remote electrical neuromodulation (REN). Highlighted risks include serious cardiovascular thrombotic events in patients on celecoxib, along with driving impairment, sleepiness, and the possibility of overuse in patients on lasmiditan. The authors added, however, that REN “has shown good tolerability and safety in clinical trials” and that frequent use of rimegepant or ubrogepant does not appear to lead to medication-overuse headache.
Regarding acute treatment overall, the statement recommended nonsteroidal anti-inflammatory drugs (NSAIDs), nonopioid analgesics, acetaminophen, or caffeinated analgesic combinations – such as aspirin plus acetaminophen plus caffeine – for mild to moderate attacks. For moderate or severe attacks, they recommended migraine-specific agents such as triptans, small-molecule CGRP receptor antagonists (gepants), or selective serotonin 5-HT1F receptor agonists (ditans). No matter the prescribed treatment, the statement pushed for patients to “treat at the first sign of pain to improve the probability of achieving freedom from pain and reduce attack-related disability.”
The authors added that 30% of patients on triptans have an “insufficient response” and as such may benefit from a second triptan or – if certain criteria are met – switching to a gepant, a ditan, or a neuromodulatory device. They also recommended a nonoral formulation for patients whose attacks are often accompanied by severe nausea or vomiting.
More broadly, they addressed the tolerability and safety issues associated with certain treatments, including the gastrointestinal and cardiovascular side effects of NSAIDs and the dangers of using triptans in patients with coronary artery disease or other vascular disorders. And while gepants and ditans appeared in clinical trials to be safe choices for patients with stable cardiovascular disease, “benefit-risk should be assessed in each patient as the real-world database for these therapies grows,” they wrote.
Only one recently approved preventive treatment – eptinezumab, an intravenous anti-CGRP ligand monoclonal antibody (MAB) – was highlighted. The authors noted that its benefits can begin within 24 hours, and it can reduce acute medication use and therefore the risk of medication-overuse headache.
Regarding preventive treatments overall, the authors stated that prevention should be offered if patients suffer from 6 or more days of headache per month, or 3-4 days of headache plus some-to-severe disability. Preventive treatments should be considered in patients who range from at least 2 days of headache per month plus severe disability to 4 or 5 days of headache. Prevention should also be considered in patients with uncommon migraine subtypes, including hemiplegic migraine, migraine with brainstem aura, and migraine with prolonged aura.
Initiating treatment
When considering initiation of treatment with one of the four Food and Drug Administration–approved CGRP MABs – eptinezumab, erenumab, fremanezumab, or galcanezumab – the authors recommend their use if migraine patients show an inability to tolerate or respond to a trial of two or more older oral medications or other established effective therapies. Though they emphasized that oral preventive medications should be started at a low dose and titrated slowly until the target response is reached or tolerability issues emerge, no such need was specified for the parenteral treatments. They also endorsed the approach of patients staying on oral preventive drugs for a minimum of 8 weeks to determine effectiveness or a lack thereof; at that point, switching to another treatment is recommended.
The dual use of therapies such as neuromodulation, biobehavioral therapies, and gepants were also examined, including gepants’ potential as a “continuum between the acute and preventive treatment of migraine” and the limited use of neuromodulatory devices in clinical practice despite clear benefits in patients who prefer to avoid medication or those suffering from frequent attacks and subsequent medication overuse. In addition, it was stated that biobehavioral therapies have “grade A evidence” supporting their use in patients who either prefer nonpharmacologic treatments or have an adverse or poor reaction to the drugs.
From the patient perspective, one of the six reviewers shared concerns about migraine patients being required to try two established preventive medications before starting a recently introduced option, noting that the older drugs have lower efficacy and tolerability. Two reviewers would have liked to see the statement focus more on nonpharmacologic and device-related therapies, and one reviewer noted the possible value in guidance regarding “exploratory approaches” such as cannabis.
The authors acknowledged numerous potential conflicts of interest, including receiving speaking and consulting fees, grants, personal fees, and honoraria from various pharmaceutical and publishing companies.
Not everyone agrees
Commenting on the AHS consensus statement, James A Charles, MD, and Ira Turner, MD, had this to say: “This Consensus Statement incorporates the best available evidence including the newer CGRP therapies as well as the older treatments. The AHS posture is that the CGRP abortive and preventive treatments have a lesser amount of data and experience than the older treatments which have a wealth of literature and data because they have been around longer. As a result, there are 2 statements in these guidelines that the insurance companies quote in their manual of policies:
1. Inadequate response to two or more oral triptans before using a gepant as abortive treatment
2. Inadequate response to an 8-week trial at a dose established to be potentially effective of two or more of the following before using CGRP MAB for preventive treatment: topiramate, divalproex sodium/valproate sodium; beta-blocker: metoprolol, propranolol, timolol, atenolol, nadolol; tricyclic antidepressant: amitriptyline, nortriptyline; serotonin-norepinephrine reuptake inhibitor: venlafaxine, duloxetine; other Level A or B treatments.”
Dr. Charles, who is affiliated with Holy Name Medical Center in Teaneck N.J., and Dr. Turner, who is affiliated with the Center for Headache Care and Research at Island Neurological Associates in Plainview, N.Y., further said that “giving the CGRP MABs and gepants second-class status because they have not been around as long as the old boys is an insult to the research, development, and successful execution of gepant and CGRP MAB therapies in the last several years. The authors omitted the Hepp study and the long list of adverse effects of triptans leading to high discontinuance rates, and how trying a second triptan will probably not work.” Importantly, they said, “the authors have given the insurance carriers a weapon to deny direct access to gepants and CGRP MABs making direct access to these agents difficult for patients and physicians and their staffs.”
Dr. Charles and Dr. Turner point out that the AHS guidelines use the term “cost effective” – that it is better to use the cheaper, older drugs first. “Ineffective treatment of a patient for 8 weeks before using CGRP blocking therapies and using 2 triptans before a gepant is cost ineffective,” they said. “Inadequate delayed treatment results in loss of work productivity and loss of school and family participation and excessive use of ER visits. These guidelines forget that we ameliorate current disability and prevent chronification by treating with the most effective abortive and preventive therapies which may not commence with the cheaper old drugs.”
They explain: “Of course, we would use a beta-blocker for comorbid hypertension and/or anxiety, and venlafaxine for comorbid depression. And if a patient is pain free in 2 hrs with no adverse effects from a triptan used less than 10 times a month, it would not be appropriate to switch to a gepant. However, a treatment naive migraineur with accelerating migraine should have the option of going directly to a gepant and CGRP blocking MAB.” Dr. Charles and Dr. Turner concur that the phrase in the AHS consensus statement regarding the staging of therapy – two triptans before a gepant and two oral preventatives for 8 weeks before a CGRP MAB – “should be removed so that the CGRP drugs get the equal credit they deserve, as can be attested to by the migraine voices of lives saved by the sound research that led to their development and approval by the FDA.”
Ultimately, Dr. Charles and Dr. Turner said, “the final decision on treatment should be made by the physician and patient, not the insurance company or consensus statements.”
Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, former president of the International Headache Society, and editor-in-chief of Neurology Reviews, said, “Although I think the consensus statement is well done, and the authors have the right to make the statements they have made, Drs. Charles and Turner are excellent experienced clinicians and they should be heard. They properly state that the restrictive statements highlighted by the authors have already been used by insurance companies to prevent access to the more expensive but more effective therapies with fewer adverse effects.”
Dr. Rapoport goes on to say, “I believe that the patient’s individual headache history and past responses to therapies must be analyzed by the treating physician and an appropriate treatment be agreed upon between the patient and doctor. It is time to let experienced headache-interested doctors make their own correct decision about treatment without the heavy hand of the insurance company, which is often more intent on saving money than helping the patient.
Suggested reading
Hepp Z et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478-88.
Alam A et al. Triptan use and discontinuation in a representative sample of persons with migraine: Results from Migraine in America Symptoms and Treatment (MAST) study. Headache. 2018;58:68‐69.
Buse DC et al. Adding additional acute medications to a triptan regimen for migraine and observed changes in headache-related disability: Results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2015 Jun;55(6):825-39.
“Because the benefit–risk profiles of newer treatments will continue to evolve as clinical trial and real-world data accrue, the American Headache Society intends to review this statement regularly and update, if appropriate, based on the emergence of evidence with implications for clinical practice,” wrote lead author Jessica Ailani, MD, of the department of neurology at Medstar Georgetown University Hospital, Washington, and colleagues. The statement was published in Headache.
To assess recent data on the efficacy, safety, and clinical use of newly introduced acute and preventive migraine treatments, the AHS convened a small task force to review relevant literature published from December 2018 through February 2021. The society’s board of directors, along with patients and patient advocates associated with the American Migraine Foundation, also provided pertinent commentary.
New migraine treatment
Five recently approved acute migraine treatments were specifically noted: two small-molecule calcitonin gene-related peptide (CGRP) receptor antagonists – rimegepant and ubrogepant – along with the nonsteroidal anti-inflammatory drug celecoxib, the serotonin 5-HT1F agonist lasmiditan, and remote electrical neuromodulation (REN). Highlighted risks include serious cardiovascular thrombotic events in patients on celecoxib, along with driving impairment, sleepiness, and the possibility of overuse in patients on lasmiditan. The authors added, however, that REN “has shown good tolerability and safety in clinical trials” and that frequent use of rimegepant or ubrogepant does not appear to lead to medication-overuse headache.
Regarding acute treatment overall, the statement recommended nonsteroidal anti-inflammatory drugs (NSAIDs), nonopioid analgesics, acetaminophen, or caffeinated analgesic combinations – such as aspirin plus acetaminophen plus caffeine – for mild to moderate attacks. For moderate or severe attacks, they recommended migraine-specific agents such as triptans, small-molecule CGRP receptor antagonists (gepants), or selective serotonin 5-HT1F receptor agonists (ditans). No matter the prescribed treatment, the statement pushed for patients to “treat at the first sign of pain to improve the probability of achieving freedom from pain and reduce attack-related disability.”
The authors added that 30% of patients on triptans have an “insufficient response” and as such may benefit from a second triptan or – if certain criteria are met – switching to a gepant, a ditan, or a neuromodulatory device. They also recommended a nonoral formulation for patients whose attacks are often accompanied by severe nausea or vomiting.
More broadly, they addressed the tolerability and safety issues associated with certain treatments, including the gastrointestinal and cardiovascular side effects of NSAIDs and the dangers of using triptans in patients with coronary artery disease or other vascular disorders. And while gepants and ditans appeared in clinical trials to be safe choices for patients with stable cardiovascular disease, “benefit-risk should be assessed in each patient as the real-world database for these therapies grows,” they wrote.
Only one recently approved preventive treatment – eptinezumab, an intravenous anti-CGRP ligand monoclonal antibody (MAB) – was highlighted. The authors noted that its benefits can begin within 24 hours, and it can reduce acute medication use and therefore the risk of medication-overuse headache.
Regarding preventive treatments overall, the authors stated that prevention should be offered if patients suffer from 6 or more days of headache per month, or 3-4 days of headache plus some-to-severe disability. Preventive treatments should be considered in patients who range from at least 2 days of headache per month plus severe disability to 4 or 5 days of headache. Prevention should also be considered in patients with uncommon migraine subtypes, including hemiplegic migraine, migraine with brainstem aura, and migraine with prolonged aura.
Initiating treatment
When considering initiation of treatment with one of the four Food and Drug Administration–approved CGRP MABs – eptinezumab, erenumab, fremanezumab, or galcanezumab – the authors recommend their use if migraine patients show an inability to tolerate or respond to a trial of two or more older oral medications or other established effective therapies. Though they emphasized that oral preventive medications should be started at a low dose and titrated slowly until the target response is reached or tolerability issues emerge, no such need was specified for the parenteral treatments. They also endorsed the approach of patients staying on oral preventive drugs for a minimum of 8 weeks to determine effectiveness or a lack thereof; at that point, switching to another treatment is recommended.
The dual use of therapies such as neuromodulation, biobehavioral therapies, and gepants were also examined, including gepants’ potential as a “continuum between the acute and preventive treatment of migraine” and the limited use of neuromodulatory devices in clinical practice despite clear benefits in patients who prefer to avoid medication or those suffering from frequent attacks and subsequent medication overuse. In addition, it was stated that biobehavioral therapies have “grade A evidence” supporting their use in patients who either prefer nonpharmacologic treatments or have an adverse or poor reaction to the drugs.
From the patient perspective, one of the six reviewers shared concerns about migraine patients being required to try two established preventive medications before starting a recently introduced option, noting that the older drugs have lower efficacy and tolerability. Two reviewers would have liked to see the statement focus more on nonpharmacologic and device-related therapies, and one reviewer noted the possible value in guidance regarding “exploratory approaches” such as cannabis.
The authors acknowledged numerous potential conflicts of interest, including receiving speaking and consulting fees, grants, personal fees, and honoraria from various pharmaceutical and publishing companies.
Not everyone agrees
Commenting on the AHS consensus statement, James A Charles, MD, and Ira Turner, MD, had this to say: “This Consensus Statement incorporates the best available evidence including the newer CGRP therapies as well as the older treatments. The AHS posture is that the CGRP abortive and preventive treatments have a lesser amount of data and experience than the older treatments which have a wealth of literature and data because they have been around longer. As a result, there are 2 statements in these guidelines that the insurance companies quote in their manual of policies:
1. Inadequate response to two or more oral triptans before using a gepant as abortive treatment
2. Inadequate response to an 8-week trial at a dose established to be potentially effective of two or more of the following before using CGRP MAB for preventive treatment: topiramate, divalproex sodium/valproate sodium; beta-blocker: metoprolol, propranolol, timolol, atenolol, nadolol; tricyclic antidepressant: amitriptyline, nortriptyline; serotonin-norepinephrine reuptake inhibitor: venlafaxine, duloxetine; other Level A or B treatments.”
Dr. Charles, who is affiliated with Holy Name Medical Center in Teaneck N.J., and Dr. Turner, who is affiliated with the Center for Headache Care and Research at Island Neurological Associates in Plainview, N.Y., further said that “giving the CGRP MABs and gepants second-class status because they have not been around as long as the old boys is an insult to the research, development, and successful execution of gepant and CGRP MAB therapies in the last several years. The authors omitted the Hepp study and the long list of adverse effects of triptans leading to high discontinuance rates, and how trying a second triptan will probably not work.” Importantly, they said, “the authors have given the insurance carriers a weapon to deny direct access to gepants and CGRP MABs making direct access to these agents difficult for patients and physicians and their staffs.”
Dr. Charles and Dr. Turner point out that the AHS guidelines use the term “cost effective” – that it is better to use the cheaper, older drugs first. “Ineffective treatment of a patient for 8 weeks before using CGRP blocking therapies and using 2 triptans before a gepant is cost ineffective,” they said. “Inadequate delayed treatment results in loss of work productivity and loss of school and family participation and excessive use of ER visits. These guidelines forget that we ameliorate current disability and prevent chronification by treating with the most effective abortive and preventive therapies which may not commence with the cheaper old drugs.”
They explain: “Of course, we would use a beta-blocker for comorbid hypertension and/or anxiety, and venlafaxine for comorbid depression. And if a patient is pain free in 2 hrs with no adverse effects from a triptan used less than 10 times a month, it would not be appropriate to switch to a gepant. However, a treatment naive migraineur with accelerating migraine should have the option of going directly to a gepant and CGRP blocking MAB.” Dr. Charles and Dr. Turner concur that the phrase in the AHS consensus statement regarding the staging of therapy – two triptans before a gepant and two oral preventatives for 8 weeks before a CGRP MAB – “should be removed so that the CGRP drugs get the equal credit they deserve, as can be attested to by the migraine voices of lives saved by the sound research that led to their development and approval by the FDA.”
Ultimately, Dr. Charles and Dr. Turner said, “the final decision on treatment should be made by the physician and patient, not the insurance company or consensus statements.”
Alan Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, former president of the International Headache Society, and editor-in-chief of Neurology Reviews, said, “Although I think the consensus statement is well done, and the authors have the right to make the statements they have made, Drs. Charles and Turner are excellent experienced clinicians and they should be heard. They properly state that the restrictive statements highlighted by the authors have already been used by insurance companies to prevent access to the more expensive but more effective therapies with fewer adverse effects.”
Dr. Rapoport goes on to say, “I believe that the patient’s individual headache history and past responses to therapies must be analyzed by the treating physician and an appropriate treatment be agreed upon between the patient and doctor. It is time to let experienced headache-interested doctors make their own correct decision about treatment without the heavy hand of the insurance company, which is often more intent on saving money than helping the patient.
Suggested reading
Hepp Z et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478-88.
Alam A et al. Triptan use and discontinuation in a representative sample of persons with migraine: Results from Migraine in America Symptoms and Treatment (MAST) study. Headache. 2018;58:68‐69.
Buse DC et al. Adding additional acute medications to a triptan regimen for migraine and observed changes in headache-related disability: Results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2015 Jun;55(6):825-39.
FROM HEADACHE