User login
A Pilot With Electrical Pain in the Face
An intracranial epidermoid cyst is an unusual but treatable cause of trigeminal neuralgia.
A 25-year-old male student pilot presented to his flight surgeon in Corpus Christi, Texas, with a 1-year history of episodic left-sided facial pain. He described the pain as electric-like with subsequent tingling sensation. These symptoms were always located on the left side of his tongue and lower lip. They were provoked by chewing, touching, or brushing his teeth. The most recent episode had lasted for 3 days before resolving. He noted 2 similar episodes a few months earlier that he related to periods of high stress.
On physical examination, the student pilot was well appearing with unremarkable vital signs. There were no skin lesions of the head or neck region. His tongue was midline without cutaneous lesions or atrophy. There was no facial numbness or weakness of the mastication muscles. There were no oropharyngeal mucosal or anatomic abnormalities. He had no lymphadenopathy. The remainder of the physical examination was unremarkable. He was seen by both dental medicine and oral surgery providers who did not identify an underlying cause for his symptoms.
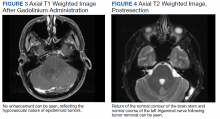
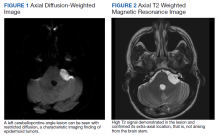
When symptoms recurred a fourth time, the student was referred for a magnetic resonance imaging (MRI) of the brain with and without contrast. The MRI demonstrated a left-sided extra-axial mass, involving the cerebellopontine angle (CPA), with imaging features most consistent with an epidermoid cyst (Figures 1, 2, and 3). An audiogram performed at the time of diagnosis revealed no sensorineural hearing loss.
Discussion
Epidermoid cysts are extra-axial tumors that are benign and slow growing. They constitute about 1% of all intracranial tumors.1 They most commonly occur at the CPA but can also arise in the fourth ventricle and suprasellar regions.2 Epidermoid cysts constitute about 5 to 7% of all CPA tumors.3,4 The 2 most common presenting symptoms of these tumors are headache and cranial nerve dysfunction.1 Other presenting symptoms may include ataxia, hemiparesis, and tinnitus.
On computed tomography (CT), epidermoid cysts can be identical in density to cerebrospinal fluid, making early detection difficult. On MRI, the lesion is easily seen on diffusion-weighted imaging, due to hyperintensity and restricted diffusion. The cysts rarely enhance, unlike the more common tumors in this region, vestibular schwannomas and meningiomas.5
Total neurosurgical resection of the epidermoid cyst is the optimal treatment and is possible in most cases.6 Management of these cysts may prove difficult because of their close proximity to the cranial nerves and brain stem. A near-total excision may be necessary for those tumors that have strong adhesions to neurovascular structures.7 Literature reports that recurrence after surgery is rare in cases of subtotal removal.8,9 Reported postoperative complications may include aseptic meningitis and cranial nerve dysfunction.10
Management
The patient was informed of the presumed diagnosis of brain epidermoid cyst and sent for neurosurgery evaluation. Surgery was indicated and via a retrosigmoid craniotomy, the tumor was removed in total with no complications. On 3-month postoperative follow-up, MRI showed no evidence of residual epidermoid (Figure 4). On physical examination at the follow-up, he was alert and oriented. His surgical incision was well healed. He was neurologically intact with a normal gait. He was released without restrictions from neurosurgical care.
The patient wished to continue flying after successful resection of his cyst. The neurosurgical procedure for removal of the epidermoid cyst medically disqualified him for military aviation.11 As the patient had no neurologic deficits, a waiver was submitted on his behalf to the Naval Aerospace Medical Institute. The waiver was granted for flying duties, and the patient returned to training. He has had no return of symptoms to date.
Conclusions
An intracranial epidermoid cyst is an unusual but treatable cause of trigeminal neuralgia. Gross total removal, without cranial nerve or cerebellar deficits, resulted in the patient’s complete return to health and training as a pilot.
1. Farhoud A, Khedr W, Aboul-Enein H. Surgical resection of cerebellopontine epidermoid cysts: limitations and outcome. J Neurol Surg B Skull Base. 2018;79(2):167‐172. doi:10.1055/s-0037-1606220
2. Hung LC, Wu CS, Lin CC, Fang WK, Hsu YC. Epidermoid cyst presenting as isolated trigeminal neuralgia - two case reports. Acta Neurol Taiwan. 2013;22(3):133‐137.
3. Feng R, Gu X, Hu J, et al. Surgical treatment and radiotherapy of epidermoid cyst with malignant transformation in cerebellopontine angle. Int J Clin Exp Med. 2014;7(1):312‐315.
4. Friedmann DR, Grobelny B, Golfinos JG, Roland JT. Nonschwannoma tumors of the cerebellopontine angle. Otolaryngol Clin North Am. 2015;48(3):461-475. doi:10.1016/j.ote.2015.02.006
5. CPA-IAC In: Harnsberger HR, Glastonbury CM, Michel MA, Koch BL Branstetter BF IV. Diagnostic Imaging: Head and Neck, 2nd ed. Amirsys, Inc; 2011:VI(8):6-9 6. Hasegawa M, Nouri M, Nagahisa S, et al. Cerebellopontine angle epidermoid cysts: clinical presentations and surgical outcome. Neurosurg Rev. 2016;39(2):259‐267. doi:10.1007/s10143-015-0684-5
7. Safavi-Abbasi S, Di Rocco F, Bambakidis N, et al. Has management of epidermoid tumors of the cerebellopontine angle improved? A surgical synopsis of the past and present. Skull Base. 2008;18(2):85‐98. doi:10.1055/s-2007-991108
8. Son DW, Choi CH, Cha SH. Epidermoid tumors in the cerebellopontine angle presenting with trigeminal neuralgia. J Korean Neurosurg Soc. 2010;47(4):271‐277. doi:10.3340/jkns.2010.47.4.271
9. Schiefer TK, Link MJ. Epidermoids of the cerebellopontine angle: a 20-year experience. Surg Neurol. 2008;70(6):584-590; discussion 590. doi:10.1016/j.surneu.2007.12.021
10. Meng L, Yuguang L, Feng L, Wandong S, Shugan Z, Chengyuan W. Cerebellopontine angle epidermoids presenting with trigeminal neuralgia. J Clin Neurosci. 2005;12(7):784‐786. doi:10.1016/j.jocn.2004.09.023
11. Naval Aerospace Medical Institute. US Navy Aeromedical reference and waiver guide. Updated March 31, 2021. Accessed June 17, 2021. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf
An intracranial epidermoid cyst is an unusual but treatable cause of trigeminal neuralgia.
An intracranial epidermoid cyst is an unusual but treatable cause of trigeminal neuralgia.
A 25-year-old male student pilot presented to his flight surgeon in Corpus Christi, Texas, with a 1-year history of episodic left-sided facial pain. He described the pain as electric-like with subsequent tingling sensation. These symptoms were always located on the left side of his tongue and lower lip. They were provoked by chewing, touching, or brushing his teeth. The most recent episode had lasted for 3 days before resolving. He noted 2 similar episodes a few months earlier that he related to periods of high stress.
On physical examination, the student pilot was well appearing with unremarkable vital signs. There were no skin lesions of the head or neck region. His tongue was midline without cutaneous lesions or atrophy. There was no facial numbness or weakness of the mastication muscles. There were no oropharyngeal mucosal or anatomic abnormalities. He had no lymphadenopathy. The remainder of the physical examination was unremarkable. He was seen by both dental medicine and oral surgery providers who did not identify an underlying cause for his symptoms.
When symptoms recurred a fourth time, the student was referred for a magnetic resonance imaging (MRI) of the brain with and without contrast. The MRI demonstrated a left-sided extra-axial mass, involving the cerebellopontine angle (CPA), with imaging features most consistent with an epidermoid cyst (Figures 1, 2, and 3). An audiogram performed at the time of diagnosis revealed no sensorineural hearing loss.
Discussion
Epidermoid cysts are extra-axial tumors that are benign and slow growing. They constitute about 1% of all intracranial tumors.1 They most commonly occur at the CPA but can also arise in the fourth ventricle and suprasellar regions.2 Epidermoid cysts constitute about 5 to 7% of all CPA tumors.3,4 The 2 most common presenting symptoms of these tumors are headache and cranial nerve dysfunction.1 Other presenting symptoms may include ataxia, hemiparesis, and tinnitus.
On computed tomography (CT), epidermoid cysts can be identical in density to cerebrospinal fluid, making early detection difficult. On MRI, the lesion is easily seen on diffusion-weighted imaging, due to hyperintensity and restricted diffusion. The cysts rarely enhance, unlike the more common tumors in this region, vestibular schwannomas and meningiomas.5
Total neurosurgical resection of the epidermoid cyst is the optimal treatment and is possible in most cases.6 Management of these cysts may prove difficult because of their close proximity to the cranial nerves and brain stem. A near-total excision may be necessary for those tumors that have strong adhesions to neurovascular structures.7 Literature reports that recurrence after surgery is rare in cases of subtotal removal.8,9 Reported postoperative complications may include aseptic meningitis and cranial nerve dysfunction.10
Management
The patient was informed of the presumed diagnosis of brain epidermoid cyst and sent for neurosurgery evaluation. Surgery was indicated and via a retrosigmoid craniotomy, the tumor was removed in total with no complications. On 3-month postoperative follow-up, MRI showed no evidence of residual epidermoid (Figure 4). On physical examination at the follow-up, he was alert and oriented. His surgical incision was well healed. He was neurologically intact with a normal gait. He was released without restrictions from neurosurgical care.
The patient wished to continue flying after successful resection of his cyst. The neurosurgical procedure for removal of the epidermoid cyst medically disqualified him for military aviation.11 As the patient had no neurologic deficits, a waiver was submitted on his behalf to the Naval Aerospace Medical Institute. The waiver was granted for flying duties, and the patient returned to training. He has had no return of symptoms to date.
Conclusions
An intracranial epidermoid cyst is an unusual but treatable cause of trigeminal neuralgia. Gross total removal, without cranial nerve or cerebellar deficits, resulted in the patient’s complete return to health and training as a pilot.
A 25-year-old male student pilot presented to his flight surgeon in Corpus Christi, Texas, with a 1-year history of episodic left-sided facial pain. He described the pain as electric-like with subsequent tingling sensation. These symptoms were always located on the left side of his tongue and lower lip. They were provoked by chewing, touching, or brushing his teeth. The most recent episode had lasted for 3 days before resolving. He noted 2 similar episodes a few months earlier that he related to periods of high stress.
On physical examination, the student pilot was well appearing with unremarkable vital signs. There were no skin lesions of the head or neck region. His tongue was midline without cutaneous lesions or atrophy. There was no facial numbness or weakness of the mastication muscles. There were no oropharyngeal mucosal or anatomic abnormalities. He had no lymphadenopathy. The remainder of the physical examination was unremarkable. He was seen by both dental medicine and oral surgery providers who did not identify an underlying cause for his symptoms.
When symptoms recurred a fourth time, the student was referred for a magnetic resonance imaging (MRI) of the brain with and without contrast. The MRI demonstrated a left-sided extra-axial mass, involving the cerebellopontine angle (CPA), with imaging features most consistent with an epidermoid cyst (Figures 1, 2, and 3). An audiogram performed at the time of diagnosis revealed no sensorineural hearing loss.
Discussion
Epidermoid cysts are extra-axial tumors that are benign and slow growing. They constitute about 1% of all intracranial tumors.1 They most commonly occur at the CPA but can also arise in the fourth ventricle and suprasellar regions.2 Epidermoid cysts constitute about 5 to 7% of all CPA tumors.3,4 The 2 most common presenting symptoms of these tumors are headache and cranial nerve dysfunction.1 Other presenting symptoms may include ataxia, hemiparesis, and tinnitus.
On computed tomography (CT), epidermoid cysts can be identical in density to cerebrospinal fluid, making early detection difficult. On MRI, the lesion is easily seen on diffusion-weighted imaging, due to hyperintensity and restricted diffusion. The cysts rarely enhance, unlike the more common tumors in this region, vestibular schwannomas and meningiomas.5
Total neurosurgical resection of the epidermoid cyst is the optimal treatment and is possible in most cases.6 Management of these cysts may prove difficult because of their close proximity to the cranial nerves and brain stem. A near-total excision may be necessary for those tumors that have strong adhesions to neurovascular structures.7 Literature reports that recurrence after surgery is rare in cases of subtotal removal.8,9 Reported postoperative complications may include aseptic meningitis and cranial nerve dysfunction.10
Management
The patient was informed of the presumed diagnosis of brain epidermoid cyst and sent for neurosurgery evaluation. Surgery was indicated and via a retrosigmoid craniotomy, the tumor was removed in total with no complications. On 3-month postoperative follow-up, MRI showed no evidence of residual epidermoid (Figure 4). On physical examination at the follow-up, he was alert and oriented. His surgical incision was well healed. He was neurologically intact with a normal gait. He was released without restrictions from neurosurgical care.
The patient wished to continue flying after successful resection of his cyst. The neurosurgical procedure for removal of the epidermoid cyst medically disqualified him for military aviation.11 As the patient had no neurologic deficits, a waiver was submitted on his behalf to the Naval Aerospace Medical Institute. The waiver was granted for flying duties, and the patient returned to training. He has had no return of symptoms to date.
Conclusions
An intracranial epidermoid cyst is an unusual but treatable cause of trigeminal neuralgia. Gross total removal, without cranial nerve or cerebellar deficits, resulted in the patient’s complete return to health and training as a pilot.
1. Farhoud A, Khedr W, Aboul-Enein H. Surgical resection of cerebellopontine epidermoid cysts: limitations and outcome. J Neurol Surg B Skull Base. 2018;79(2):167‐172. doi:10.1055/s-0037-1606220
2. Hung LC, Wu CS, Lin CC, Fang WK, Hsu YC. Epidermoid cyst presenting as isolated trigeminal neuralgia - two case reports. Acta Neurol Taiwan. 2013;22(3):133‐137.
3. Feng R, Gu X, Hu J, et al. Surgical treatment and radiotherapy of epidermoid cyst with malignant transformation in cerebellopontine angle. Int J Clin Exp Med. 2014;7(1):312‐315.
4. Friedmann DR, Grobelny B, Golfinos JG, Roland JT. Nonschwannoma tumors of the cerebellopontine angle. Otolaryngol Clin North Am. 2015;48(3):461-475. doi:10.1016/j.ote.2015.02.006
5. CPA-IAC In: Harnsberger HR, Glastonbury CM, Michel MA, Koch BL Branstetter BF IV. Diagnostic Imaging: Head and Neck, 2nd ed. Amirsys, Inc; 2011:VI(8):6-9 6. Hasegawa M, Nouri M, Nagahisa S, et al. Cerebellopontine angle epidermoid cysts: clinical presentations and surgical outcome. Neurosurg Rev. 2016;39(2):259‐267. doi:10.1007/s10143-015-0684-5
7. Safavi-Abbasi S, Di Rocco F, Bambakidis N, et al. Has management of epidermoid tumors of the cerebellopontine angle improved? A surgical synopsis of the past and present. Skull Base. 2008;18(2):85‐98. doi:10.1055/s-2007-991108
8. Son DW, Choi CH, Cha SH. Epidermoid tumors in the cerebellopontine angle presenting with trigeminal neuralgia. J Korean Neurosurg Soc. 2010;47(4):271‐277. doi:10.3340/jkns.2010.47.4.271
9. Schiefer TK, Link MJ. Epidermoids of the cerebellopontine angle: a 20-year experience. Surg Neurol. 2008;70(6):584-590; discussion 590. doi:10.1016/j.surneu.2007.12.021
10. Meng L, Yuguang L, Feng L, Wandong S, Shugan Z, Chengyuan W. Cerebellopontine angle epidermoids presenting with trigeminal neuralgia. J Clin Neurosci. 2005;12(7):784‐786. doi:10.1016/j.jocn.2004.09.023
11. Naval Aerospace Medical Institute. US Navy Aeromedical reference and waiver guide. Updated March 31, 2021. Accessed June 17, 2021. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf
1. Farhoud A, Khedr W, Aboul-Enein H. Surgical resection of cerebellopontine epidermoid cysts: limitations and outcome. J Neurol Surg B Skull Base. 2018;79(2):167‐172. doi:10.1055/s-0037-1606220
2. Hung LC, Wu CS, Lin CC, Fang WK, Hsu YC. Epidermoid cyst presenting as isolated trigeminal neuralgia - two case reports. Acta Neurol Taiwan. 2013;22(3):133‐137.
3. Feng R, Gu X, Hu J, et al. Surgical treatment and radiotherapy of epidermoid cyst with malignant transformation in cerebellopontine angle. Int J Clin Exp Med. 2014;7(1):312‐315.
4. Friedmann DR, Grobelny B, Golfinos JG, Roland JT. Nonschwannoma tumors of the cerebellopontine angle. Otolaryngol Clin North Am. 2015;48(3):461-475. doi:10.1016/j.ote.2015.02.006
5. CPA-IAC In: Harnsberger HR, Glastonbury CM, Michel MA, Koch BL Branstetter BF IV. Diagnostic Imaging: Head and Neck, 2nd ed. Amirsys, Inc; 2011:VI(8):6-9 6. Hasegawa M, Nouri M, Nagahisa S, et al. Cerebellopontine angle epidermoid cysts: clinical presentations and surgical outcome. Neurosurg Rev. 2016;39(2):259‐267. doi:10.1007/s10143-015-0684-5
7. Safavi-Abbasi S, Di Rocco F, Bambakidis N, et al. Has management of epidermoid tumors of the cerebellopontine angle improved? A surgical synopsis of the past and present. Skull Base. 2008;18(2):85‐98. doi:10.1055/s-2007-991108
8. Son DW, Choi CH, Cha SH. Epidermoid tumors in the cerebellopontine angle presenting with trigeminal neuralgia. J Korean Neurosurg Soc. 2010;47(4):271‐277. doi:10.3340/jkns.2010.47.4.271
9. Schiefer TK, Link MJ. Epidermoids of the cerebellopontine angle: a 20-year experience. Surg Neurol. 2008;70(6):584-590; discussion 590. doi:10.1016/j.surneu.2007.12.021
10. Meng L, Yuguang L, Feng L, Wandong S, Shugan Z, Chengyuan W. Cerebellopontine angle epidermoids presenting with trigeminal neuralgia. J Clin Neurosci. 2005;12(7):784‐786. doi:10.1016/j.jocn.2004.09.023
11. Naval Aerospace Medical Institute. US Navy Aeromedical reference and waiver guide. Updated March 31, 2021. Accessed June 17, 2021. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf
‘Long haul’ COVID recovery worse than cancer rehab for some: CDC
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
Infusion centers may best EDs for treating sickle cell crises
At infusion centers, patients received pain medication an average of 70 minutes faster compared with patients treated in EDs (62 vs. 132 minutes), according to a study published online in the Annals of Internal Medicine. In addition, patients at infusion centers were 3.8 times more likely to have their pain reassessed within 30 minutes of the first dose. And they were 4 times more likely to be discharged home, the researchers found.
“It’s not that the emergency room doctors don’t want to do the right thing,” study author Sophie Lanzkron, MD, said in an interview. “They do, but they aren’t experts in sickle cell disease. They work in an emergency room, which is an incredibly busy, stressful place where they see trauma and heart attacks and strokes and all of these things that need emergency care. And so it just is not the right setting to treat people with sickle cell disease.”
To assess whether care at specialty infusion centers or EDs leads to better outcomes for patients with sickle cell disease with uncomplicated vaso-occlusive crises, Dr. Lanzkron, director of the Sickle Cell Center for Adults at the Johns Hopkins Hospital, Baltimore, and colleagues conducted the ESCAPED (Examining Sickle Cell Acute Pain in the Emergency vs. Day Hospital) study.
The trial included 483 adults with sickle cell disease who lived within 60 miles of an infusion center in four U.S. cities: Baltimore, Maryland; Cleveland, Ohio; Milwaukee, Wisconsin; and Baton Rouge, Louisiana. Investigators recruited patients between April 2015 and December 2016 and followed them for 18 months after enrollment.
The present analysis focused on data from 269 participants who had infusion center visits or ED visits that occurred during weekdays when infusion centers were open. Two sites had infusion centers solely for adults with sickle cell disease (Baltimore and Milwaukee), and two infusion centers shared infusion space with other hematology-oncology patients. All four sites were in hospitals that also had EDs.
Although participants may have received comprehensive care at one of the sites with an infusion center, those who lived farther from an infusion center were likely to receive care for acute pain at an ED closer to home, the authors explain in the article.
The investigators used propensity score methodology to balance patient characteristics in the study groups.
Quick, effective pain reduction is beneficial
The results suggest that infusion centers “are more likely to provide guideline-based care than EDs,” and this care “can improve overall outcomes,” the authors write.
Although the specialty infusion centers the researchers studied used various models, similar outcomes were seen at all of them.
The study did not include patients who had complications of sickle cell disease in addition to vaso-occlusive crisis, the researchers note.
“[Because] the magnitude of the treatment effects estimated in our study is large and we have captured most of the important potential confounders, an unmeasured confounder that can nullify the treatment effect is unlikely to exist,” the authors write.
“Sickle cell disease is a complicated condition that affects multiple organs. Patients who present with acute pain will have better outcomes being treated under providers who know and understand the disease,” commented Julie Kanter, MD, director of the adult sickle cell disease program and codirector of the Comprehensive Sickle Cell Center at the University of Alabama at Birmingham. “Specialized infusion centers offer the opportunity to both improve outcomes and decrease the cost of care. Most importantly, it is better for the individual with sickle cell disease,” she said.
Dr. Kanter wrote an accompanying editorial about the ESCAPED findings. The editorialist notes that “opioid medications are the only option to reduce the pain caused by microvascular injury” in patients with sickle cell crisis, although these treatments do not reduce the underlying damage and have substantial side effects and risks. Nevertheless, “quick and effective reduction of pain can allow patients to more easily move, stretch, and breathe ... important to increase oxygenation and restore blood flow, which will eventually abate the crisis,” Dr. Kanter wrote.
The study shows that the infusion center treatment approach can benefit patients across different settings, commented John J. Strouse, MD, PhD, medical director of the adult sickle cell program at Duke University Sickle Cell Center, Durham, N.C., who was not involved in the study.
“They show that they can definitely get closer to the recommendations of guidelines for acute pain management and sickle cell disease” in a setting that is focused on one problem, he said. “The other piece that is really important is that people are much more likely to go home if you follow the guideline.”
Infusion centers are scarce
“These systems need to be built,” Dr. Lanzkron said. “In most places, patients don’t have access to the infusion center model for their care. And in some places, it is not going to be practical.” Still, there may be ways to establish infusion locations, such as at oncology centers. And while there are challenges to delivering sickle cell disease care in EDs, “emergency rooms need to try to meet the needs of this patient population as best as they can,” Dr. Lanzkron said.
“Structural racism has played a role in the quality of care delivered” to patients with sickle cell disease, Dr. Lanzkron said. “The big message is [that] there is a better way to do this.”
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Lanzkron’s disclosures included grants or contracts with government agencies and companies that are paid to her institution, as well as consulting fees from Bluebird Bio, Novo Nordisk, and Pfizer. Coauthors have disclosed working with sickle cell organizations and various medical companies. Dr. Kanter and Dr. Strouse have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At infusion centers, patients received pain medication an average of 70 minutes faster compared with patients treated in EDs (62 vs. 132 minutes), according to a study published online in the Annals of Internal Medicine. In addition, patients at infusion centers were 3.8 times more likely to have their pain reassessed within 30 minutes of the first dose. And they were 4 times more likely to be discharged home, the researchers found.
“It’s not that the emergency room doctors don’t want to do the right thing,” study author Sophie Lanzkron, MD, said in an interview. “They do, but they aren’t experts in sickle cell disease. They work in an emergency room, which is an incredibly busy, stressful place where they see trauma and heart attacks and strokes and all of these things that need emergency care. And so it just is not the right setting to treat people with sickle cell disease.”
To assess whether care at specialty infusion centers or EDs leads to better outcomes for patients with sickle cell disease with uncomplicated vaso-occlusive crises, Dr. Lanzkron, director of the Sickle Cell Center for Adults at the Johns Hopkins Hospital, Baltimore, and colleagues conducted the ESCAPED (Examining Sickle Cell Acute Pain in the Emergency vs. Day Hospital) study.
The trial included 483 adults with sickle cell disease who lived within 60 miles of an infusion center in four U.S. cities: Baltimore, Maryland; Cleveland, Ohio; Milwaukee, Wisconsin; and Baton Rouge, Louisiana. Investigators recruited patients between April 2015 and December 2016 and followed them for 18 months after enrollment.
The present analysis focused on data from 269 participants who had infusion center visits or ED visits that occurred during weekdays when infusion centers were open. Two sites had infusion centers solely for adults with sickle cell disease (Baltimore and Milwaukee), and two infusion centers shared infusion space with other hematology-oncology patients. All four sites were in hospitals that also had EDs.
Although participants may have received comprehensive care at one of the sites with an infusion center, those who lived farther from an infusion center were likely to receive care for acute pain at an ED closer to home, the authors explain in the article.
The investigators used propensity score methodology to balance patient characteristics in the study groups.
Quick, effective pain reduction is beneficial
The results suggest that infusion centers “are more likely to provide guideline-based care than EDs,” and this care “can improve overall outcomes,” the authors write.
Although the specialty infusion centers the researchers studied used various models, similar outcomes were seen at all of them.
The study did not include patients who had complications of sickle cell disease in addition to vaso-occlusive crisis, the researchers note.
“[Because] the magnitude of the treatment effects estimated in our study is large and we have captured most of the important potential confounders, an unmeasured confounder that can nullify the treatment effect is unlikely to exist,” the authors write.
“Sickle cell disease is a complicated condition that affects multiple organs. Patients who present with acute pain will have better outcomes being treated under providers who know and understand the disease,” commented Julie Kanter, MD, director of the adult sickle cell disease program and codirector of the Comprehensive Sickle Cell Center at the University of Alabama at Birmingham. “Specialized infusion centers offer the opportunity to both improve outcomes and decrease the cost of care. Most importantly, it is better for the individual with sickle cell disease,” she said.
Dr. Kanter wrote an accompanying editorial about the ESCAPED findings. The editorialist notes that “opioid medications are the only option to reduce the pain caused by microvascular injury” in patients with sickle cell crisis, although these treatments do not reduce the underlying damage and have substantial side effects and risks. Nevertheless, “quick and effective reduction of pain can allow patients to more easily move, stretch, and breathe ... important to increase oxygenation and restore blood flow, which will eventually abate the crisis,” Dr. Kanter wrote.
The study shows that the infusion center treatment approach can benefit patients across different settings, commented John J. Strouse, MD, PhD, medical director of the adult sickle cell program at Duke University Sickle Cell Center, Durham, N.C., who was not involved in the study.
“They show that they can definitely get closer to the recommendations of guidelines for acute pain management and sickle cell disease” in a setting that is focused on one problem, he said. “The other piece that is really important is that people are much more likely to go home if you follow the guideline.”
Infusion centers are scarce
“These systems need to be built,” Dr. Lanzkron said. “In most places, patients don’t have access to the infusion center model for their care. And in some places, it is not going to be practical.” Still, there may be ways to establish infusion locations, such as at oncology centers. And while there are challenges to delivering sickle cell disease care in EDs, “emergency rooms need to try to meet the needs of this patient population as best as they can,” Dr. Lanzkron said.
“Structural racism has played a role in the quality of care delivered” to patients with sickle cell disease, Dr. Lanzkron said. “The big message is [that] there is a better way to do this.”
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Lanzkron’s disclosures included grants or contracts with government agencies and companies that are paid to her institution, as well as consulting fees from Bluebird Bio, Novo Nordisk, and Pfizer. Coauthors have disclosed working with sickle cell organizations and various medical companies. Dr. Kanter and Dr. Strouse have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At infusion centers, patients received pain medication an average of 70 minutes faster compared with patients treated in EDs (62 vs. 132 minutes), according to a study published online in the Annals of Internal Medicine. In addition, patients at infusion centers were 3.8 times more likely to have their pain reassessed within 30 minutes of the first dose. And they were 4 times more likely to be discharged home, the researchers found.
“It’s not that the emergency room doctors don’t want to do the right thing,” study author Sophie Lanzkron, MD, said in an interview. “They do, but they aren’t experts in sickle cell disease. They work in an emergency room, which is an incredibly busy, stressful place where they see trauma and heart attacks and strokes and all of these things that need emergency care. And so it just is not the right setting to treat people with sickle cell disease.”
To assess whether care at specialty infusion centers or EDs leads to better outcomes for patients with sickle cell disease with uncomplicated vaso-occlusive crises, Dr. Lanzkron, director of the Sickle Cell Center for Adults at the Johns Hopkins Hospital, Baltimore, and colleagues conducted the ESCAPED (Examining Sickle Cell Acute Pain in the Emergency vs. Day Hospital) study.
The trial included 483 adults with sickle cell disease who lived within 60 miles of an infusion center in four U.S. cities: Baltimore, Maryland; Cleveland, Ohio; Milwaukee, Wisconsin; and Baton Rouge, Louisiana. Investigators recruited patients between April 2015 and December 2016 and followed them for 18 months after enrollment.
The present analysis focused on data from 269 participants who had infusion center visits or ED visits that occurred during weekdays when infusion centers were open. Two sites had infusion centers solely for adults with sickle cell disease (Baltimore and Milwaukee), and two infusion centers shared infusion space with other hematology-oncology patients. All four sites were in hospitals that also had EDs.
Although participants may have received comprehensive care at one of the sites with an infusion center, those who lived farther from an infusion center were likely to receive care for acute pain at an ED closer to home, the authors explain in the article.
The investigators used propensity score methodology to balance patient characteristics in the study groups.
Quick, effective pain reduction is beneficial
The results suggest that infusion centers “are more likely to provide guideline-based care than EDs,” and this care “can improve overall outcomes,” the authors write.
Although the specialty infusion centers the researchers studied used various models, similar outcomes were seen at all of them.
The study did not include patients who had complications of sickle cell disease in addition to vaso-occlusive crisis, the researchers note.
“[Because] the magnitude of the treatment effects estimated in our study is large and we have captured most of the important potential confounders, an unmeasured confounder that can nullify the treatment effect is unlikely to exist,” the authors write.
“Sickle cell disease is a complicated condition that affects multiple organs. Patients who present with acute pain will have better outcomes being treated under providers who know and understand the disease,” commented Julie Kanter, MD, director of the adult sickle cell disease program and codirector of the Comprehensive Sickle Cell Center at the University of Alabama at Birmingham. “Specialized infusion centers offer the opportunity to both improve outcomes and decrease the cost of care. Most importantly, it is better for the individual with sickle cell disease,” she said.
Dr. Kanter wrote an accompanying editorial about the ESCAPED findings. The editorialist notes that “opioid medications are the only option to reduce the pain caused by microvascular injury” in patients with sickle cell crisis, although these treatments do not reduce the underlying damage and have substantial side effects and risks. Nevertheless, “quick and effective reduction of pain can allow patients to more easily move, stretch, and breathe ... important to increase oxygenation and restore blood flow, which will eventually abate the crisis,” Dr. Kanter wrote.
The study shows that the infusion center treatment approach can benefit patients across different settings, commented John J. Strouse, MD, PhD, medical director of the adult sickle cell program at Duke University Sickle Cell Center, Durham, N.C., who was not involved in the study.
“They show that they can definitely get closer to the recommendations of guidelines for acute pain management and sickle cell disease” in a setting that is focused on one problem, he said. “The other piece that is really important is that people are much more likely to go home if you follow the guideline.”
Infusion centers are scarce
“These systems need to be built,” Dr. Lanzkron said. “In most places, patients don’t have access to the infusion center model for their care. And in some places, it is not going to be practical.” Still, there may be ways to establish infusion locations, such as at oncology centers. And while there are challenges to delivering sickle cell disease care in EDs, “emergency rooms need to try to meet the needs of this patient population as best as they can,” Dr. Lanzkron said.
“Structural racism has played a role in the quality of care delivered” to patients with sickle cell disease, Dr. Lanzkron said. “The big message is [that] there is a better way to do this.”
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Lanzkron’s disclosures included grants or contracts with government agencies and companies that are paid to her institution, as well as consulting fees from Bluebird Bio, Novo Nordisk, and Pfizer. Coauthors have disclosed working with sickle cell organizations and various medical companies. Dr. Kanter and Dr. Strouse have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Opioid prescriptions decrease in young kids, long dosages increase
The opioid prescription rates have significantly decreased for children, teens, and younger adults between 2006 and 2018, according to new research.
“What’s important about this new study is that it documented that these improvements were also occurring for children and young adults specifically,” said Kao-Ping Chua, MD, PhD, primary care physician and assistant professor of pediatrics at the University of Michigan, Ann Arbor, who was not involved in the study. “The reason that’s important is that changes in medical practice for adults aren’t always reflected in pediatrics.”
The study, published in JAMA Pediatrics, found that dispensed opioid prescriptions for this population have decreased by 15% annually since 2013. However, the study also examined specific prescribing variables, such as duration of opioid prescription and high-dosage prescriptions. Researchers found reduced rates of high-dosage and long-duration prescriptions for adolescents and younger adults. However, these types of prescription practices increased in children aged 0-5 years.
“I think [the findings are] promising, suggesting that opiate prescribing practices may be improving,” study author Madeline Renny, MD, pediatric emergency medicine doctor at New York University Langone Health, said in an interview. “But we did find that there were increases in the young children for the practice variables, which we didn’t expect. I think that was kind of one of the findings that we were a bit surprised about and want to explore further.”
Previous studies have linked prescription opioid use in children and teens to an increased risk of future opioid misuse. A 2015 study published in Pediatrics found that using prescribed opioids before the 12th grade is associated with a 33% increase in the risk of future opioid misuse by the age of 23. The study also found that for those with a low predicted risk of future opioid misuse, an opioid prescription increases the risk for misuse after high school threefold.
Furthermore, a 2018 study published in JAMA Network Open found that, between 1999 and 2016, the annual estimated mortality rate for all children and adolescents from prescription and illicit opioid use rose 268.2%.
In the new study, Dr. Renny and colleagues examined data from 2006 to 2018 from IQVIA Longitudinal Prescription Data, which captured 74%-92% of U.S. retail outpatient opioid prescriptions dispensed to people up to the age of 24. Researchers also examined prescribing practice variables, which included opioid dispensing rates, average amount of opioid dispensed per prescription, duration of opioid prescription, high-dosage opioid prescription for individuals, and the rate in which extended-release or long-acting opioids are prescribed.
Researchers found that between 2006 and 2018, the total U.S. annual opioid prescriptions dispensed to patients younger than 25 years was highest in 2007 at 15,689,779 prescriptions, and since 2012 has steadily decreased to 6,705,478 in 2018.
“Our study did show that there were declines, but opioids remain readily dispensed,” Dr. Renny said. “And I think it’s good that rates have gone down, but I think opioids are still commonly dispensed to children and adolescents and young adults and all of our age groups.”
Dr. Chua said that the study was important, but when it came to younger children, it didn’t account for the fact that “the underlying population of patients who were getting opioids changed because it’s not the same group of children.”
“Maybe at the beginning there were more surgical patients who are getting shorter duration, lower dosage opioids,” he added. “Now some of those surgical exceptions kind of went away and who’s left in the population of people who get opioids is a sicker population.”
“Who are the 0 to 5-year-olds who are getting opioids now?” Dr. Chua asked. “Well, some of them are going to be cancer or surgical patients. If you think about it, over time their surgeons may be more judicious and they stop prescribing opioids for some things like circumcision or something like that. So that means that who’s left in the population of children who get opiate prescriptions are the cancer patients. Cancer patients’ opioid dosages are going to be higher because they have chronic pain.”
Dr. Chua said it is important to remember that the number of children who are affected by those high-risk prescriptions are lower because the overall number of opioid prescriptions has gone down. He added that the key piece of missing information is the absolute number of prescriptions that were high risk.
Researchers of the current study suggested that, because of the differences between pediatric and adult pain and indications for opioid prescribing, there should be national guidelines on general opioid prescribing for children and adolescents.
Experts did not disclose relevant financial relationships.
The opioid prescription rates have significantly decreased for children, teens, and younger adults between 2006 and 2018, according to new research.
“What’s important about this new study is that it documented that these improvements were also occurring for children and young adults specifically,” said Kao-Ping Chua, MD, PhD, primary care physician and assistant professor of pediatrics at the University of Michigan, Ann Arbor, who was not involved in the study. “The reason that’s important is that changes in medical practice for adults aren’t always reflected in pediatrics.”
The study, published in JAMA Pediatrics, found that dispensed opioid prescriptions for this population have decreased by 15% annually since 2013. However, the study also examined specific prescribing variables, such as duration of opioid prescription and high-dosage prescriptions. Researchers found reduced rates of high-dosage and long-duration prescriptions for adolescents and younger adults. However, these types of prescription practices increased in children aged 0-5 years.
“I think [the findings are] promising, suggesting that opiate prescribing practices may be improving,” study author Madeline Renny, MD, pediatric emergency medicine doctor at New York University Langone Health, said in an interview. “But we did find that there were increases in the young children for the practice variables, which we didn’t expect. I think that was kind of one of the findings that we were a bit surprised about and want to explore further.”
Previous studies have linked prescription opioid use in children and teens to an increased risk of future opioid misuse. A 2015 study published in Pediatrics found that using prescribed opioids before the 12th grade is associated with a 33% increase in the risk of future opioid misuse by the age of 23. The study also found that for those with a low predicted risk of future opioid misuse, an opioid prescription increases the risk for misuse after high school threefold.
Furthermore, a 2018 study published in JAMA Network Open found that, between 1999 and 2016, the annual estimated mortality rate for all children and adolescents from prescription and illicit opioid use rose 268.2%.
In the new study, Dr. Renny and colleagues examined data from 2006 to 2018 from IQVIA Longitudinal Prescription Data, which captured 74%-92% of U.S. retail outpatient opioid prescriptions dispensed to people up to the age of 24. Researchers also examined prescribing practice variables, which included opioid dispensing rates, average amount of opioid dispensed per prescription, duration of opioid prescription, high-dosage opioid prescription for individuals, and the rate in which extended-release or long-acting opioids are prescribed.
Researchers found that between 2006 and 2018, the total U.S. annual opioid prescriptions dispensed to patients younger than 25 years was highest in 2007 at 15,689,779 prescriptions, and since 2012 has steadily decreased to 6,705,478 in 2018.
“Our study did show that there were declines, but opioids remain readily dispensed,” Dr. Renny said. “And I think it’s good that rates have gone down, but I think opioids are still commonly dispensed to children and adolescents and young adults and all of our age groups.”
Dr. Chua said that the study was important, but when it came to younger children, it didn’t account for the fact that “the underlying population of patients who were getting opioids changed because it’s not the same group of children.”
“Maybe at the beginning there were more surgical patients who are getting shorter duration, lower dosage opioids,” he added. “Now some of those surgical exceptions kind of went away and who’s left in the population of people who get opioids is a sicker population.”
“Who are the 0 to 5-year-olds who are getting opioids now?” Dr. Chua asked. “Well, some of them are going to be cancer or surgical patients. If you think about it, over time their surgeons may be more judicious and they stop prescribing opioids for some things like circumcision or something like that. So that means that who’s left in the population of children who get opiate prescriptions are the cancer patients. Cancer patients’ opioid dosages are going to be higher because they have chronic pain.”
Dr. Chua said it is important to remember that the number of children who are affected by those high-risk prescriptions are lower because the overall number of opioid prescriptions has gone down. He added that the key piece of missing information is the absolute number of prescriptions that were high risk.
Researchers of the current study suggested that, because of the differences between pediatric and adult pain and indications for opioid prescribing, there should be national guidelines on general opioid prescribing for children and adolescents.
Experts did not disclose relevant financial relationships.
The opioid prescription rates have significantly decreased for children, teens, and younger adults between 2006 and 2018, according to new research.
“What’s important about this new study is that it documented that these improvements were also occurring for children and young adults specifically,” said Kao-Ping Chua, MD, PhD, primary care physician and assistant professor of pediatrics at the University of Michigan, Ann Arbor, who was not involved in the study. “The reason that’s important is that changes in medical practice for adults aren’t always reflected in pediatrics.”
The study, published in JAMA Pediatrics, found that dispensed opioid prescriptions for this population have decreased by 15% annually since 2013. However, the study also examined specific prescribing variables, such as duration of opioid prescription and high-dosage prescriptions. Researchers found reduced rates of high-dosage and long-duration prescriptions for adolescents and younger adults. However, these types of prescription practices increased in children aged 0-5 years.
“I think [the findings are] promising, suggesting that opiate prescribing practices may be improving,” study author Madeline Renny, MD, pediatric emergency medicine doctor at New York University Langone Health, said in an interview. “But we did find that there were increases in the young children for the practice variables, which we didn’t expect. I think that was kind of one of the findings that we were a bit surprised about and want to explore further.”
Previous studies have linked prescription opioid use in children and teens to an increased risk of future opioid misuse. A 2015 study published in Pediatrics found that using prescribed opioids before the 12th grade is associated with a 33% increase in the risk of future opioid misuse by the age of 23. The study also found that for those with a low predicted risk of future opioid misuse, an opioid prescription increases the risk for misuse after high school threefold.
Furthermore, a 2018 study published in JAMA Network Open found that, between 1999 and 2016, the annual estimated mortality rate for all children and adolescents from prescription and illicit opioid use rose 268.2%.
In the new study, Dr. Renny and colleagues examined data from 2006 to 2018 from IQVIA Longitudinal Prescription Data, which captured 74%-92% of U.S. retail outpatient opioid prescriptions dispensed to people up to the age of 24. Researchers also examined prescribing practice variables, which included opioid dispensing rates, average amount of opioid dispensed per prescription, duration of opioid prescription, high-dosage opioid prescription for individuals, and the rate in which extended-release or long-acting opioids are prescribed.
Researchers found that between 2006 and 2018, the total U.S. annual opioid prescriptions dispensed to patients younger than 25 years was highest in 2007 at 15,689,779 prescriptions, and since 2012 has steadily decreased to 6,705,478 in 2018.
“Our study did show that there were declines, but opioids remain readily dispensed,” Dr. Renny said. “And I think it’s good that rates have gone down, but I think opioids are still commonly dispensed to children and adolescents and young adults and all of our age groups.”
Dr. Chua said that the study was important, but when it came to younger children, it didn’t account for the fact that “the underlying population of patients who were getting opioids changed because it’s not the same group of children.”
“Maybe at the beginning there were more surgical patients who are getting shorter duration, lower dosage opioids,” he added. “Now some of those surgical exceptions kind of went away and who’s left in the population of people who get opioids is a sicker population.”
“Who are the 0 to 5-year-olds who are getting opioids now?” Dr. Chua asked. “Well, some of them are going to be cancer or surgical patients. If you think about it, over time their surgeons may be more judicious and they stop prescribing opioids for some things like circumcision or something like that. So that means that who’s left in the population of children who get opiate prescriptions are the cancer patients. Cancer patients’ opioid dosages are going to be higher because they have chronic pain.”
Dr. Chua said it is important to remember that the number of children who are affected by those high-risk prescriptions are lower because the overall number of opioid prescriptions has gone down. He added that the key piece of missing information is the absolute number of prescriptions that were high risk.
Researchers of the current study suggested that, because of the differences between pediatric and adult pain and indications for opioid prescribing, there should be national guidelines on general opioid prescribing for children and adolescents.
Experts did not disclose relevant financial relationships.
FROM JAMA PEDIATRICS
Novel NSAID–triptan drug effectively relieves migraine pain
, new research suggests. Results from the phase 3 INTERCEPT trial show that the treatment, known as AXS-07 (Axsome Therapeutics), also provided greater relief from the patients’ most bothersome symptom (MBS) compared with placebo.
In addition, about 74% of patients who received AXS-07 experienced no progression of pain from 2 to 24 hours after dosing and were less than half as likely to use rescue medication through 24 hours than those who received placebo.
Similar to a previous formulation combining naproxen sodium and sumatriptan, AXS-07 combines a nonsteroidal anti-inflammatory drug with a triptan. The combination is synergistic, investigators note, because one drug addresses pain mechanisms that the other does not.
“Rizatriptan’s primary mechanism is peripheral, and NSAIDs have both peripheral and central benefit,” said study investigator Stewart J. Tepper, MD, professor of neurology, Geisel School of Medicine at Dartmouth, Hanover, N.H. “That is why the whole is greater than the sum of the parts,” Dr. Tepper added.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Acute treatments needed
For many patients, current migraine treatments are inadequate. In addition, suboptimal acute treatment can increase risk for progression from episodic migraine to chronic migraine. It also increases the risk for medication-overuse headache.
The search for optimal acute treatments is therefore “really important for patients,” Dr. Tepper noted.
Because it contains rizatriptan, AXS-07 is believed to inhibit the release of calcitonin gene-related peptide, reverse the vasodilation that it causes, and decrease the transmission of pain signals. Meloxicam, on the other hand, is thought to reduce neuroinflammation and reverse central sensitization, which maintains chronic pain.
In the phase 3, double-blind INTERCEPT trial, the investigators examined AXS-07 for early treatment of migraine. Eligible patients were aged 18 to 65 years, had been diagnosed with migraine in accordance with ICHD-3 criteria, and averaged two to eight migraines per month.
The researchers randomly assigned a single dose of AXS-07 (n = 152) or placebo (n = 150). Participants were asked to administer treatment to themselves at the earliest sign of migraine pain.
The trial’s two primary endpoints were pain freedom and freedom from the MBS 2 hours after dosing. Secondary endpoints included sustained pain freedom and freedom from pain progression, functional disability, and use of rescue medication.
Demographic characteristics of the study population reflected those of the general population of people with migraine, according to the researchers. More than 85% of participants were women, and the study group’s mean age was 41 years. There were no demographic differences between the two treatment groups.
Reduced pain progression
Results showed that 2 hours after treatment, rate of pain freedom was 32.6% in the AXS-07 group and 16.3% in the placebo group (P = .002). At the same time point, rate of freedom from MBS was 43.9% and 26.7%, respectively (P = .003).
Approximately 64% of patients who received AXS-07 were pain free at 12 hours, and 69% were pain free at 24 hours. In contrast, 42% of the placebo group were pain free at 12 hours, and 47% were pain free at 24 hours (P < .001 for both comparisons).
The benefits AXS-07 provided were sustained; 22.7% of the active-treatment group achieved sustained pain freedom from 2 to 24 hours after treatment, compared with 12.6% of the placebo group (P = .03). Results were similar for sustained pain freedom from 2 to 48 hours after treatment (20.5% vs. 9.6%; P = .013).
In addition, 73.5% of patients who received AXS-07 had freedom from pain progression from 2 to 24 hours after treatment, versus 47.4% of those who received placebo (P < .001). The rate of rescue medication use through 24 hours was 15.3% and 42.2%, respectively (P < .001).
AXS-07 was also linked to significant reductions in functional disability. About 74% of patients who received it reported no disability at 24 hours, compared with 47% of patients who received placebo (P < .001). Scores on the Patient Global Impression of Change scale were very much improved or much improved 2 hours after dosing for 52.4% of the AXS-07 group, versus 27.7% of the placebo group (P < .001).
The overall rate of treatment-emergent adverse events (AEs) was 17.9% in the active group and 7.7% among the control group. The rate of somnolence was 4.3%, versus 2.1%; the rate of dizziness was 2.9%, versus 1.4%; and the rate of paresthesia was 2.1%, versus 0%. There were no serious AEs.
“Unexpectedly, and it’s hard to interpret this, but the nausea associated with the use of AXS-07 is less than with either of the active components or the placebo,” said Dr. Tepper. “It’s not dramatically different for dizziness.”
Improved adherence?
Meloxicam is generally used not as an acute medication but for prevention, Dr. Tepper noted. The drug is often administered to reduce inflammation in conditions such as chronic arthritis.
AXS-07 incorporates an altered pharmacokinetic delivery system to provide a quicker onset of effect for meloxicam.
“Most headache specialists would say that of all the oral triptans, rizatriptan is the fastest,” said Dr. Tepper.
The idea for the new agent was to hasten the onset of meloxicam’s effect so that both active components would work rapidly. “We know that there is a synergy between NSAIDs and triptans, in terms of complete headache response,” Dr. Tepper said.
Data indicate that when neurologists recommend that patients take an NSAID and triptan together at the beginning of an attack, patients rarely comply. “It’s a big adherence issue,” said Dr. Tepper. “They’re more likely to get a complete response if they take them together, especially if the tablet is designed to deliver the two products together in an optimal way.”
Uncertain therapeutic advantage
Commenting on the findings, Robert Shapiro, MD, PhD, professor of neurologic science at the University of Vermont, Burlington, noted that because of favorable data from past studies for the combination of 85 mg of sumatriptan with 500 mg of naproxen sodium, the coadministration of a triptan with an NSAID has been a standard of care for the past decade.
“It’s therefore unsurprising that a combination of rizatriptan 10 mg plus meloxicam 20 mg in a proprietary MoSEIC formulation might also prove to be more effective than either individual medication taken alone for acute migraine attacks,” said Dr. Shapiro, who was not involved with the research.
It is not possible to compare the efficacy and tolerability of AXS-07 with those of sumatriptan–naproxen sodium without head-to-head trials. However, the available data suggest that the latter formulation is superior, he added.
In 2008, researchers conducted two parallel-group, placebo-controlled trials of sumatriptan–naproxen sodium taken early in a migraine attack. These trials had protocols comparable to that of the current INTERCEPT trial for AXS-07, said Dr. Shapiro.
For the key primary endpoint of 2-hour pain freedom, the two sumatriptan–naproxen sodium trials found therapeutic gains of 35% and 36%, respectively, versus 16.3% for the AXS-07 trial. The placebo response rates (17% and 15% for sumatriptan–naproxen sodium, vs. 16.3% for the AXS-07 trial) were comparable.
Similarly, for the endpoint of 2- to 24-hour pain freedom, the sumatriptan–naproxen sodium trials found therapeutic gains of 33% and 26%, respectively, versus 15.1% for the AXS-07 trial. Again, response rates for placebo were comparable (12% and 14% for sumatriptan–naproxen sodium, vs. 12.6% for AXS-07).
The placebo-adjusted differences for reporting any treatment-emergent AE, otherwise known as “therapeutic penalty,” was 10.2% for AXS-07 in the INTERCEPT trial, versus 7% and 5%, respectively for participants in the two sumatriptan–naproxen sodium trials.
“In light of these data, it’s not immediately apparent what advantage AXS-07 might offer over sumatriptan–naproxen sodium,” said Dr. Shapiro.
“Furthermore, sumatriptan–naproxen sodium is currently available in generic form,” he added.
The study was funded by Axsome Therapeutics. Dr. Tepper is a consultant to Axsome Therapeutics. Dr. Shapiro has previously performed research consulting for Lilly and Lundbeck.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from the phase 3 INTERCEPT trial show that the treatment, known as AXS-07 (Axsome Therapeutics), also provided greater relief from the patients’ most bothersome symptom (MBS) compared with placebo.
In addition, about 74% of patients who received AXS-07 experienced no progression of pain from 2 to 24 hours after dosing and were less than half as likely to use rescue medication through 24 hours than those who received placebo.
Similar to a previous formulation combining naproxen sodium and sumatriptan, AXS-07 combines a nonsteroidal anti-inflammatory drug with a triptan. The combination is synergistic, investigators note, because one drug addresses pain mechanisms that the other does not.
“Rizatriptan’s primary mechanism is peripheral, and NSAIDs have both peripheral and central benefit,” said study investigator Stewart J. Tepper, MD, professor of neurology, Geisel School of Medicine at Dartmouth, Hanover, N.H. “That is why the whole is greater than the sum of the parts,” Dr. Tepper added.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Acute treatments needed
For many patients, current migraine treatments are inadequate. In addition, suboptimal acute treatment can increase risk for progression from episodic migraine to chronic migraine. It also increases the risk for medication-overuse headache.
The search for optimal acute treatments is therefore “really important for patients,” Dr. Tepper noted.
Because it contains rizatriptan, AXS-07 is believed to inhibit the release of calcitonin gene-related peptide, reverse the vasodilation that it causes, and decrease the transmission of pain signals. Meloxicam, on the other hand, is thought to reduce neuroinflammation and reverse central sensitization, which maintains chronic pain.
In the phase 3, double-blind INTERCEPT trial, the investigators examined AXS-07 for early treatment of migraine. Eligible patients were aged 18 to 65 years, had been diagnosed with migraine in accordance with ICHD-3 criteria, and averaged two to eight migraines per month.
The researchers randomly assigned a single dose of AXS-07 (n = 152) or placebo (n = 150). Participants were asked to administer treatment to themselves at the earliest sign of migraine pain.
The trial’s two primary endpoints were pain freedom and freedom from the MBS 2 hours after dosing. Secondary endpoints included sustained pain freedom and freedom from pain progression, functional disability, and use of rescue medication.
Demographic characteristics of the study population reflected those of the general population of people with migraine, according to the researchers. More than 85% of participants were women, and the study group’s mean age was 41 years. There were no demographic differences between the two treatment groups.
Reduced pain progression
Results showed that 2 hours after treatment, rate of pain freedom was 32.6% in the AXS-07 group and 16.3% in the placebo group (P = .002). At the same time point, rate of freedom from MBS was 43.9% and 26.7%, respectively (P = .003).
Approximately 64% of patients who received AXS-07 were pain free at 12 hours, and 69% were pain free at 24 hours. In contrast, 42% of the placebo group were pain free at 12 hours, and 47% were pain free at 24 hours (P < .001 for both comparisons).
The benefits AXS-07 provided were sustained; 22.7% of the active-treatment group achieved sustained pain freedom from 2 to 24 hours after treatment, compared with 12.6% of the placebo group (P = .03). Results were similar for sustained pain freedom from 2 to 48 hours after treatment (20.5% vs. 9.6%; P = .013).
In addition, 73.5% of patients who received AXS-07 had freedom from pain progression from 2 to 24 hours after treatment, versus 47.4% of those who received placebo (P < .001). The rate of rescue medication use through 24 hours was 15.3% and 42.2%, respectively (P < .001).
AXS-07 was also linked to significant reductions in functional disability. About 74% of patients who received it reported no disability at 24 hours, compared with 47% of patients who received placebo (P < .001). Scores on the Patient Global Impression of Change scale were very much improved or much improved 2 hours after dosing for 52.4% of the AXS-07 group, versus 27.7% of the placebo group (P < .001).
The overall rate of treatment-emergent adverse events (AEs) was 17.9% in the active group and 7.7% among the control group. The rate of somnolence was 4.3%, versus 2.1%; the rate of dizziness was 2.9%, versus 1.4%; and the rate of paresthesia was 2.1%, versus 0%. There were no serious AEs.
“Unexpectedly, and it’s hard to interpret this, but the nausea associated with the use of AXS-07 is less than with either of the active components or the placebo,” said Dr. Tepper. “It’s not dramatically different for dizziness.”
Improved adherence?
Meloxicam is generally used not as an acute medication but for prevention, Dr. Tepper noted. The drug is often administered to reduce inflammation in conditions such as chronic arthritis.
AXS-07 incorporates an altered pharmacokinetic delivery system to provide a quicker onset of effect for meloxicam.
“Most headache specialists would say that of all the oral triptans, rizatriptan is the fastest,” said Dr. Tepper.
The idea for the new agent was to hasten the onset of meloxicam’s effect so that both active components would work rapidly. “We know that there is a synergy between NSAIDs and triptans, in terms of complete headache response,” Dr. Tepper said.
Data indicate that when neurologists recommend that patients take an NSAID and triptan together at the beginning of an attack, patients rarely comply. “It’s a big adherence issue,” said Dr. Tepper. “They’re more likely to get a complete response if they take them together, especially if the tablet is designed to deliver the two products together in an optimal way.”
Uncertain therapeutic advantage
Commenting on the findings, Robert Shapiro, MD, PhD, professor of neurologic science at the University of Vermont, Burlington, noted that because of favorable data from past studies for the combination of 85 mg of sumatriptan with 500 mg of naproxen sodium, the coadministration of a triptan with an NSAID has been a standard of care for the past decade.
“It’s therefore unsurprising that a combination of rizatriptan 10 mg plus meloxicam 20 mg in a proprietary MoSEIC formulation might also prove to be more effective than either individual medication taken alone for acute migraine attacks,” said Dr. Shapiro, who was not involved with the research.
It is not possible to compare the efficacy and tolerability of AXS-07 with those of sumatriptan–naproxen sodium without head-to-head trials. However, the available data suggest that the latter formulation is superior, he added.
In 2008, researchers conducted two parallel-group, placebo-controlled trials of sumatriptan–naproxen sodium taken early in a migraine attack. These trials had protocols comparable to that of the current INTERCEPT trial for AXS-07, said Dr. Shapiro.
For the key primary endpoint of 2-hour pain freedom, the two sumatriptan–naproxen sodium trials found therapeutic gains of 35% and 36%, respectively, versus 16.3% for the AXS-07 trial. The placebo response rates (17% and 15% for sumatriptan–naproxen sodium, vs. 16.3% for the AXS-07 trial) were comparable.
Similarly, for the endpoint of 2- to 24-hour pain freedom, the sumatriptan–naproxen sodium trials found therapeutic gains of 33% and 26%, respectively, versus 15.1% for the AXS-07 trial. Again, response rates for placebo were comparable (12% and 14% for sumatriptan–naproxen sodium, vs. 12.6% for AXS-07).
The placebo-adjusted differences for reporting any treatment-emergent AE, otherwise known as “therapeutic penalty,” was 10.2% for AXS-07 in the INTERCEPT trial, versus 7% and 5%, respectively for participants in the two sumatriptan–naproxen sodium trials.
“In light of these data, it’s not immediately apparent what advantage AXS-07 might offer over sumatriptan–naproxen sodium,” said Dr. Shapiro.
“Furthermore, sumatriptan–naproxen sodium is currently available in generic form,” he added.
The study was funded by Axsome Therapeutics. Dr. Tepper is a consultant to Axsome Therapeutics. Dr. Shapiro has previously performed research consulting for Lilly and Lundbeck.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from the phase 3 INTERCEPT trial show that the treatment, known as AXS-07 (Axsome Therapeutics), also provided greater relief from the patients’ most bothersome symptom (MBS) compared with placebo.
In addition, about 74% of patients who received AXS-07 experienced no progression of pain from 2 to 24 hours after dosing and were less than half as likely to use rescue medication through 24 hours than those who received placebo.
Similar to a previous formulation combining naproxen sodium and sumatriptan, AXS-07 combines a nonsteroidal anti-inflammatory drug with a triptan. The combination is synergistic, investigators note, because one drug addresses pain mechanisms that the other does not.
“Rizatriptan’s primary mechanism is peripheral, and NSAIDs have both peripheral and central benefit,” said study investigator Stewart J. Tepper, MD, professor of neurology, Geisel School of Medicine at Dartmouth, Hanover, N.H. “That is why the whole is greater than the sum of the parts,” Dr. Tepper added.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Acute treatments needed
For many patients, current migraine treatments are inadequate. In addition, suboptimal acute treatment can increase risk for progression from episodic migraine to chronic migraine. It also increases the risk for medication-overuse headache.
The search for optimal acute treatments is therefore “really important for patients,” Dr. Tepper noted.
Because it contains rizatriptan, AXS-07 is believed to inhibit the release of calcitonin gene-related peptide, reverse the vasodilation that it causes, and decrease the transmission of pain signals. Meloxicam, on the other hand, is thought to reduce neuroinflammation and reverse central sensitization, which maintains chronic pain.
In the phase 3, double-blind INTERCEPT trial, the investigators examined AXS-07 for early treatment of migraine. Eligible patients were aged 18 to 65 years, had been diagnosed with migraine in accordance with ICHD-3 criteria, and averaged two to eight migraines per month.
The researchers randomly assigned a single dose of AXS-07 (n = 152) or placebo (n = 150). Participants were asked to administer treatment to themselves at the earliest sign of migraine pain.
The trial’s two primary endpoints were pain freedom and freedom from the MBS 2 hours after dosing. Secondary endpoints included sustained pain freedom and freedom from pain progression, functional disability, and use of rescue medication.
Demographic characteristics of the study population reflected those of the general population of people with migraine, according to the researchers. More than 85% of participants were women, and the study group’s mean age was 41 years. There were no demographic differences between the two treatment groups.
Reduced pain progression
Results showed that 2 hours after treatment, rate of pain freedom was 32.6% in the AXS-07 group and 16.3% in the placebo group (P = .002). At the same time point, rate of freedom from MBS was 43.9% and 26.7%, respectively (P = .003).
Approximately 64% of patients who received AXS-07 were pain free at 12 hours, and 69% were pain free at 24 hours. In contrast, 42% of the placebo group were pain free at 12 hours, and 47% were pain free at 24 hours (P < .001 for both comparisons).
The benefits AXS-07 provided were sustained; 22.7% of the active-treatment group achieved sustained pain freedom from 2 to 24 hours after treatment, compared with 12.6% of the placebo group (P = .03). Results were similar for sustained pain freedom from 2 to 48 hours after treatment (20.5% vs. 9.6%; P = .013).
In addition, 73.5% of patients who received AXS-07 had freedom from pain progression from 2 to 24 hours after treatment, versus 47.4% of those who received placebo (P < .001). The rate of rescue medication use through 24 hours was 15.3% and 42.2%, respectively (P < .001).
AXS-07 was also linked to significant reductions in functional disability. About 74% of patients who received it reported no disability at 24 hours, compared with 47% of patients who received placebo (P < .001). Scores on the Patient Global Impression of Change scale were very much improved or much improved 2 hours after dosing for 52.4% of the AXS-07 group, versus 27.7% of the placebo group (P < .001).
The overall rate of treatment-emergent adverse events (AEs) was 17.9% in the active group and 7.7% among the control group. The rate of somnolence was 4.3%, versus 2.1%; the rate of dizziness was 2.9%, versus 1.4%; and the rate of paresthesia was 2.1%, versus 0%. There were no serious AEs.
“Unexpectedly, and it’s hard to interpret this, but the nausea associated with the use of AXS-07 is less than with either of the active components or the placebo,” said Dr. Tepper. “It’s not dramatically different for dizziness.”
Improved adherence?
Meloxicam is generally used not as an acute medication but for prevention, Dr. Tepper noted. The drug is often administered to reduce inflammation in conditions such as chronic arthritis.
AXS-07 incorporates an altered pharmacokinetic delivery system to provide a quicker onset of effect for meloxicam.
“Most headache specialists would say that of all the oral triptans, rizatriptan is the fastest,” said Dr. Tepper.
The idea for the new agent was to hasten the onset of meloxicam’s effect so that both active components would work rapidly. “We know that there is a synergy between NSAIDs and triptans, in terms of complete headache response,” Dr. Tepper said.
Data indicate that when neurologists recommend that patients take an NSAID and triptan together at the beginning of an attack, patients rarely comply. “It’s a big adherence issue,” said Dr. Tepper. “They’re more likely to get a complete response if they take them together, especially if the tablet is designed to deliver the two products together in an optimal way.”
Uncertain therapeutic advantage
Commenting on the findings, Robert Shapiro, MD, PhD, professor of neurologic science at the University of Vermont, Burlington, noted that because of favorable data from past studies for the combination of 85 mg of sumatriptan with 500 mg of naproxen sodium, the coadministration of a triptan with an NSAID has been a standard of care for the past decade.
“It’s therefore unsurprising that a combination of rizatriptan 10 mg plus meloxicam 20 mg in a proprietary MoSEIC formulation might also prove to be more effective than either individual medication taken alone for acute migraine attacks,” said Dr. Shapiro, who was not involved with the research.
It is not possible to compare the efficacy and tolerability of AXS-07 with those of sumatriptan–naproxen sodium without head-to-head trials. However, the available data suggest that the latter formulation is superior, he added.
In 2008, researchers conducted two parallel-group, placebo-controlled trials of sumatriptan–naproxen sodium taken early in a migraine attack. These trials had protocols comparable to that of the current INTERCEPT trial for AXS-07, said Dr. Shapiro.
For the key primary endpoint of 2-hour pain freedom, the two sumatriptan–naproxen sodium trials found therapeutic gains of 35% and 36%, respectively, versus 16.3% for the AXS-07 trial. The placebo response rates (17% and 15% for sumatriptan–naproxen sodium, vs. 16.3% for the AXS-07 trial) were comparable.
Similarly, for the endpoint of 2- to 24-hour pain freedom, the sumatriptan–naproxen sodium trials found therapeutic gains of 33% and 26%, respectively, versus 15.1% for the AXS-07 trial. Again, response rates for placebo were comparable (12% and 14% for sumatriptan–naproxen sodium, vs. 12.6% for AXS-07).
The placebo-adjusted differences for reporting any treatment-emergent AE, otherwise known as “therapeutic penalty,” was 10.2% for AXS-07 in the INTERCEPT trial, versus 7% and 5%, respectively for participants in the two sumatriptan–naproxen sodium trials.
“In light of these data, it’s not immediately apparent what advantage AXS-07 might offer over sumatriptan–naproxen sodium,” said Dr. Shapiro.
“Furthermore, sumatriptan–naproxen sodium is currently available in generic form,” he added.
The study was funded by Axsome Therapeutics. Dr. Tepper is a consultant to Axsome Therapeutics. Dr. Shapiro has previously performed research consulting for Lilly and Lundbeck.
A version of this article first appeared on Medscape.com.
From AHS 2021
Diaphragmatic endometriosis diagnosed many years after symptom onset
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Foot rash and joint pain
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
Photobiomodulation: Evaluation in a wide range of medical specialties underway
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
FROM ASLMS 2021
Chronic headache pain in veterans linked to suicide attempts
, according to findings presented at the American Headache Society’s 2021 annual meeting. Risk rose even more in those with chronic headache pain and a comorbid traumatic brain injury (TBI).
“In addition, as expected, veterans with psychiatric conditions have increased risk of suicide attempt with the exception of anxiety in men and dependent personality in women,” said X. Michelle Androulakis, MD, associate professor of neurology at the University of South Carolina, Columbia.
‘Surprising’ findings
“These findings are eye-opening but not surprising since we know that veterans in general and people with chronic pain are at higher risk for suicidal behaviors compared with their civilian counterparts,” said Amy. S Grinberg, PhD, a clinical health psychologist who practices in New Rochelle, N.Y. Dr. Grinberg, who also works at VA Connecticut Healthcare System, was not involved in the study.
“It is, however, very interesting that suicidal attempts are higher in veterans with chronic headache compared with other chronic pain disorders, such as chronic neck and back pain,” Dr Grinberg said. “This really highlights the impact of living with a chronic headache disorder, and emphasizes the continued efforts that should be put into place to support veterans with chronic headache, including improved access to a range of treatment options and continued funding for future research.”
Veterans with chronic pain
The researchers retrospectively analyzed Veterans Health Administration electronic health records of 3,252,704 veterans, predominantly male and White, who had been diagnosed with any type of chronic pain from 2000 to 2010.
The researchers looked at overall headache diagnoses instead of specific diagnoses, such as migraine, cluster headache, or posttraumatic headache, since specific headache disorders are frequently underdiagnosed.
The population included 14.7% of patients with chronic headache, 14.9% with chronic neck pain, 59.2% with chronic back pain, and 60.2% with other types of chronic pain, including arthritis, fibromyalgia, joint pain, and reflex sympathetic dystrophy.
Traumatic brain injury occurred in 11.2% of those with chronic headaches, compared with 6.8% of those with chronic back pain, 8.5% of those with chronic neck pain, and 5.9% of those with other chronic pain.
More than half (56.4%) of those with chronic headache had depression, the most common comorbidity in the group, followed by 31.5% who had posttraumatic stress disorder (PTSD), and 21.8% who had adjustment disorder. Other rates of psychiatric disorders were all below 10%. Prevalence of depression occurred in 44.5% of those with back pain, 52.4% of those with neck pain, and 39% of those with other chronic pain. PTSD rates were also lower in those with back (22%), neck (27.2%), or other chronic pain (18.6%).
“Interestingly, this study found that those veterans with a history of traumatic brain injury and psychiatric comorbidities, such as depression, are at greater risk for suicide attempts,” said Dr. Grinberg. “The good news is that these are modifiable risk factors, and evidence-based treatments for depression, PTSD, and headache, for example, are widely disseminated within the VA.”
The majority of headache diagnoses were not otherwise specified (80.1%). Half (50.2%) were migraine headaches while rates were much lower for tension-type headache (8.8%), trigeminal neuralgia (5%), cluster headache (0.8%), and posttraumatic headache (0.7%).
The highest incidence of suicide attempts occurred among those with chronic headaches, ranging from 329 to 396 per 100,000, aside from a peak of 482 per 100,000 in 2005. Suicide attempts peaked among all patients with chronic pain in 2005, “likely related to the deployment and policy changes in the Veterans Health Administration,” Dr. Androulakis said.
Those with neck pain had the next highest rate of suicide attempts, ranging from 263 to 314 per 100,000, excluding the peak of 398 per 100,000 in 2005.
Male veterans with chronic headaches had a 1.5 times greater likelihood of a suicide attempt than did those with back or neck pain (relative risk [RR] = 1.5), which increased to a relative risk of 2.8 greater for those with concurrent TBI. Among female veterans, chronic headache was associated with a 1.6 times greater risk of a suicide attempt, which rose to 2.15 times greater with concurrent TBI.
“Knowing that veterans with chronic headache disorders have an elevated rate of suicide, it is imperative that doctors and other clinical providers continue to conduct in-depth risk assessments and implement strategies to support those veterans who are at risk,” said Dr. Grinberg. “Clinical providers should continue in their efforts to reduce stigma associated with headache disorders and mental health treatment in order to effectively engage veterans in evidence-based treatments that are likely a step towards reducing symptoms and suicidal attempts.”
No external funding was noted. Dr. Androulakis and Dr. Grinberg had no disclosures.
, according to findings presented at the American Headache Society’s 2021 annual meeting. Risk rose even more in those with chronic headache pain and a comorbid traumatic brain injury (TBI).
“In addition, as expected, veterans with psychiatric conditions have increased risk of suicide attempt with the exception of anxiety in men and dependent personality in women,” said X. Michelle Androulakis, MD, associate professor of neurology at the University of South Carolina, Columbia.
‘Surprising’ findings
“These findings are eye-opening but not surprising since we know that veterans in general and people with chronic pain are at higher risk for suicidal behaviors compared with their civilian counterparts,” said Amy. S Grinberg, PhD, a clinical health psychologist who practices in New Rochelle, N.Y. Dr. Grinberg, who also works at VA Connecticut Healthcare System, was not involved in the study.
“It is, however, very interesting that suicidal attempts are higher in veterans with chronic headache compared with other chronic pain disorders, such as chronic neck and back pain,” Dr Grinberg said. “This really highlights the impact of living with a chronic headache disorder, and emphasizes the continued efforts that should be put into place to support veterans with chronic headache, including improved access to a range of treatment options and continued funding for future research.”
Veterans with chronic pain
The researchers retrospectively analyzed Veterans Health Administration electronic health records of 3,252,704 veterans, predominantly male and White, who had been diagnosed with any type of chronic pain from 2000 to 2010.
The researchers looked at overall headache diagnoses instead of specific diagnoses, such as migraine, cluster headache, or posttraumatic headache, since specific headache disorders are frequently underdiagnosed.
The population included 14.7% of patients with chronic headache, 14.9% with chronic neck pain, 59.2% with chronic back pain, and 60.2% with other types of chronic pain, including arthritis, fibromyalgia, joint pain, and reflex sympathetic dystrophy.
Traumatic brain injury occurred in 11.2% of those with chronic headaches, compared with 6.8% of those with chronic back pain, 8.5% of those with chronic neck pain, and 5.9% of those with other chronic pain.
More than half (56.4%) of those with chronic headache had depression, the most common comorbidity in the group, followed by 31.5% who had posttraumatic stress disorder (PTSD), and 21.8% who had adjustment disorder. Other rates of psychiatric disorders were all below 10%. Prevalence of depression occurred in 44.5% of those with back pain, 52.4% of those with neck pain, and 39% of those with other chronic pain. PTSD rates were also lower in those with back (22%), neck (27.2%), or other chronic pain (18.6%).
“Interestingly, this study found that those veterans with a history of traumatic brain injury and psychiatric comorbidities, such as depression, are at greater risk for suicide attempts,” said Dr. Grinberg. “The good news is that these are modifiable risk factors, and evidence-based treatments for depression, PTSD, and headache, for example, are widely disseminated within the VA.”
The majority of headache diagnoses were not otherwise specified (80.1%). Half (50.2%) were migraine headaches while rates were much lower for tension-type headache (8.8%), trigeminal neuralgia (5%), cluster headache (0.8%), and posttraumatic headache (0.7%).
The highest incidence of suicide attempts occurred among those with chronic headaches, ranging from 329 to 396 per 100,000, aside from a peak of 482 per 100,000 in 2005. Suicide attempts peaked among all patients with chronic pain in 2005, “likely related to the deployment and policy changes in the Veterans Health Administration,” Dr. Androulakis said.
Those with neck pain had the next highest rate of suicide attempts, ranging from 263 to 314 per 100,000, excluding the peak of 398 per 100,000 in 2005.
Male veterans with chronic headaches had a 1.5 times greater likelihood of a suicide attempt than did those with back or neck pain (relative risk [RR] = 1.5), which increased to a relative risk of 2.8 greater for those with concurrent TBI. Among female veterans, chronic headache was associated with a 1.6 times greater risk of a suicide attempt, which rose to 2.15 times greater with concurrent TBI.
“Knowing that veterans with chronic headache disorders have an elevated rate of suicide, it is imperative that doctors and other clinical providers continue to conduct in-depth risk assessments and implement strategies to support those veterans who are at risk,” said Dr. Grinberg. “Clinical providers should continue in their efforts to reduce stigma associated with headache disorders and mental health treatment in order to effectively engage veterans in evidence-based treatments that are likely a step towards reducing symptoms and suicidal attempts.”
No external funding was noted. Dr. Androulakis and Dr. Grinberg had no disclosures.
, according to findings presented at the American Headache Society’s 2021 annual meeting. Risk rose even more in those with chronic headache pain and a comorbid traumatic brain injury (TBI).
“In addition, as expected, veterans with psychiatric conditions have increased risk of suicide attempt with the exception of anxiety in men and dependent personality in women,” said X. Michelle Androulakis, MD, associate professor of neurology at the University of South Carolina, Columbia.
‘Surprising’ findings
“These findings are eye-opening but not surprising since we know that veterans in general and people with chronic pain are at higher risk for suicidal behaviors compared with their civilian counterparts,” said Amy. S Grinberg, PhD, a clinical health psychologist who practices in New Rochelle, N.Y. Dr. Grinberg, who also works at VA Connecticut Healthcare System, was not involved in the study.
“It is, however, very interesting that suicidal attempts are higher in veterans with chronic headache compared with other chronic pain disorders, such as chronic neck and back pain,” Dr Grinberg said. “This really highlights the impact of living with a chronic headache disorder, and emphasizes the continued efforts that should be put into place to support veterans with chronic headache, including improved access to a range of treatment options and continued funding for future research.”
Veterans with chronic pain
The researchers retrospectively analyzed Veterans Health Administration electronic health records of 3,252,704 veterans, predominantly male and White, who had been diagnosed with any type of chronic pain from 2000 to 2010.
The researchers looked at overall headache diagnoses instead of specific diagnoses, such as migraine, cluster headache, or posttraumatic headache, since specific headache disorders are frequently underdiagnosed.
The population included 14.7% of patients with chronic headache, 14.9% with chronic neck pain, 59.2% with chronic back pain, and 60.2% with other types of chronic pain, including arthritis, fibromyalgia, joint pain, and reflex sympathetic dystrophy.
Traumatic brain injury occurred in 11.2% of those with chronic headaches, compared with 6.8% of those with chronic back pain, 8.5% of those with chronic neck pain, and 5.9% of those with other chronic pain.
More than half (56.4%) of those with chronic headache had depression, the most common comorbidity in the group, followed by 31.5% who had posttraumatic stress disorder (PTSD), and 21.8% who had adjustment disorder. Other rates of psychiatric disorders were all below 10%. Prevalence of depression occurred in 44.5% of those with back pain, 52.4% of those with neck pain, and 39% of those with other chronic pain. PTSD rates were also lower in those with back (22%), neck (27.2%), or other chronic pain (18.6%).
“Interestingly, this study found that those veterans with a history of traumatic brain injury and psychiatric comorbidities, such as depression, are at greater risk for suicide attempts,” said Dr. Grinberg. “The good news is that these are modifiable risk factors, and evidence-based treatments for depression, PTSD, and headache, for example, are widely disseminated within the VA.”
The majority of headache diagnoses were not otherwise specified (80.1%). Half (50.2%) were migraine headaches while rates were much lower for tension-type headache (8.8%), trigeminal neuralgia (5%), cluster headache (0.8%), and posttraumatic headache (0.7%).
The highest incidence of suicide attempts occurred among those with chronic headaches, ranging from 329 to 396 per 100,000, aside from a peak of 482 per 100,000 in 2005. Suicide attempts peaked among all patients with chronic pain in 2005, “likely related to the deployment and policy changes in the Veterans Health Administration,” Dr. Androulakis said.
Those with neck pain had the next highest rate of suicide attempts, ranging from 263 to 314 per 100,000, excluding the peak of 398 per 100,000 in 2005.
Male veterans with chronic headaches had a 1.5 times greater likelihood of a suicide attempt than did those with back or neck pain (relative risk [RR] = 1.5), which increased to a relative risk of 2.8 greater for those with concurrent TBI. Among female veterans, chronic headache was associated with a 1.6 times greater risk of a suicide attempt, which rose to 2.15 times greater with concurrent TBI.
“Knowing that veterans with chronic headache disorders have an elevated rate of suicide, it is imperative that doctors and other clinical providers continue to conduct in-depth risk assessments and implement strategies to support those veterans who are at risk,” said Dr. Grinberg. “Clinical providers should continue in their efforts to reduce stigma associated with headache disorders and mental health treatment in order to effectively engage veterans in evidence-based treatments that are likely a step towards reducing symptoms and suicidal attempts.”
No external funding was noted. Dr. Androulakis and Dr. Grinberg had no disclosures.
FROM AHS 2021
e-TNS device passes at-home test
The study also demonstrated that the device, manufactured by Cefaly and cleared in 2020 by the Food and Drug Administration for over-the-counter use, can be safely and effectively used at home.
The study also explored the benefits of 2 hours of use, rather than the 1 hour of use tested in a previous study. “The programming on the device is currently [set to] turn off at 1 hour. As a result of this study, I tell patients if they don’t have adequate relief, and they’re tolerating it, that they can activate it again for a second hour,” Stewart Tepper, MD, said in an interview. Dr. Tepper is a professor of neurology at Geisel School of Medicine at Dartmouth, Hanover, N.H., and a coauthor of the study that was presented by Deena Kuruvilla, MD, at the American Headache Society’s 2021 annual meeting. Dr. Kuruvilla is a neurologist and director of the Westport (Conn.) Headache Institute.
The improvements seen over the sham were significant but not overwhelming, according to Deborah Friedman, MD, MPH, professor of neurology and ophthalmology at the University of Texas, Dallas.
“The numbers are not super impressive when you compare them with other devices. I thought it was interesting that the most bothersome symptom went away in a much higher percentage of people than the headache. That was actually pretty impressive,” said Dr. Friedman, who was asked to comment on the study. She also wondered if the sham device may have inadvertently provided a small amount of stimulation, which could explain the smaller than expected efficacy difference. “It just kind of makes me wonder because I would expect to see a larger separation, even though it was statistically significant.”
The study was an overall success according to Dr. Tepper, who noted that the efficacy of pain freedom was comparable with what has been seen with calcitonin gene-related peptide receptor antagonists (gepants), as well as relieving the most bothersome symptom at 2 hours. The device failed to reduce the usage of rescue medication, suggesting that it might be a candidate to combine with rescue medications. “I think the main thing is it works. It works in a sham-controlled trial, it works at home, and it works comparably to acute medication. And it is further evidence that the lack of access is something that needs to be addressed,” said Dr. Tepper.
Access will depend on insurance companies, who have so far been reluctant to pay for the device. Dr. Tepper is not optimistic they will come around on their own. “My feeling about it is that the only way that payers will finally start to cover this is with a concerted, organized advocacy campaign by patients. The analogy is that when the disease-modifying therapies became available for multiple sclerosis, the National MS Society organized the MS patients and they demanded that the payers cover the disease modifying therapies. That’s the kind of intense focus of advocacy that needs to be done for these noninvasive neuromodulation devices,” said Dr. Tepper.
The TEAM study was a double blind, randomized, sham-controlled trial of 538 patients who were asked to use neurostimulation for a 2-hour, continuous session within 4 hours of a moderate to severe migraine accompanied by at least one migraine-associated symptom. At 2 hours, 25.5% of those using the device achieved pain freedom, compared with 18.3% of those using the sham (P < .05). Among those using the device, 56.4% had freedom from most bothersome symptom, compared with 42.3% of those using the sham (P < .01).
Pain relief at 2 hours was more common in the device group (69.5% vs. 55.2%; P < .01), as was absence of all migraine-associated symptoms at 2 hours (42.5% vs. 34.1%; P < .05), sustained pain freedom at 24 hours (22.8% vs. 15.8%; P < .05), and sustained pain relief at 24 hours (45.9% vs. 34.4%; P < .01). There was no statistically significant between-group difference in use of rescue medications.
In the device group, 8.5% of patients experienced an adverse event, versus 2.9% in the sham group (P = .004). The only adverse reaction that occurred more frequently in the device group was forehead paresthesia, discomfort, and burning (3.5% vs. 0.4%; P = .009).
The study was funded by Cefaly. Dr. Tepper and Dr. Friedman have no relevant financial disclosures.
The study also demonstrated that the device, manufactured by Cefaly and cleared in 2020 by the Food and Drug Administration for over-the-counter use, can be safely and effectively used at home.
The study also explored the benefits of 2 hours of use, rather than the 1 hour of use tested in a previous study. “The programming on the device is currently [set to] turn off at 1 hour. As a result of this study, I tell patients if they don’t have adequate relief, and they’re tolerating it, that they can activate it again for a second hour,” Stewart Tepper, MD, said in an interview. Dr. Tepper is a professor of neurology at Geisel School of Medicine at Dartmouth, Hanover, N.H., and a coauthor of the study that was presented by Deena Kuruvilla, MD, at the American Headache Society’s 2021 annual meeting. Dr. Kuruvilla is a neurologist and director of the Westport (Conn.) Headache Institute.
The improvements seen over the sham were significant but not overwhelming, according to Deborah Friedman, MD, MPH, professor of neurology and ophthalmology at the University of Texas, Dallas.
“The numbers are not super impressive when you compare them with other devices. I thought it was interesting that the most bothersome symptom went away in a much higher percentage of people than the headache. That was actually pretty impressive,” said Dr. Friedman, who was asked to comment on the study. She also wondered if the sham device may have inadvertently provided a small amount of stimulation, which could explain the smaller than expected efficacy difference. “It just kind of makes me wonder because I would expect to see a larger separation, even though it was statistically significant.”
The study was an overall success according to Dr. Tepper, who noted that the efficacy of pain freedom was comparable with what has been seen with calcitonin gene-related peptide receptor antagonists (gepants), as well as relieving the most bothersome symptom at 2 hours. The device failed to reduce the usage of rescue medication, suggesting that it might be a candidate to combine with rescue medications. “I think the main thing is it works. It works in a sham-controlled trial, it works at home, and it works comparably to acute medication. And it is further evidence that the lack of access is something that needs to be addressed,” said Dr. Tepper.
Access will depend on insurance companies, who have so far been reluctant to pay for the device. Dr. Tepper is not optimistic they will come around on their own. “My feeling about it is that the only way that payers will finally start to cover this is with a concerted, organized advocacy campaign by patients. The analogy is that when the disease-modifying therapies became available for multiple sclerosis, the National MS Society organized the MS patients and they demanded that the payers cover the disease modifying therapies. That’s the kind of intense focus of advocacy that needs to be done for these noninvasive neuromodulation devices,” said Dr. Tepper.
The TEAM study was a double blind, randomized, sham-controlled trial of 538 patients who were asked to use neurostimulation for a 2-hour, continuous session within 4 hours of a moderate to severe migraine accompanied by at least one migraine-associated symptom. At 2 hours, 25.5% of those using the device achieved pain freedom, compared with 18.3% of those using the sham (P < .05). Among those using the device, 56.4% had freedom from most bothersome symptom, compared with 42.3% of those using the sham (P < .01).
Pain relief at 2 hours was more common in the device group (69.5% vs. 55.2%; P < .01), as was absence of all migraine-associated symptoms at 2 hours (42.5% vs. 34.1%; P < .05), sustained pain freedom at 24 hours (22.8% vs. 15.8%; P < .05), and sustained pain relief at 24 hours (45.9% vs. 34.4%; P < .01). There was no statistically significant between-group difference in use of rescue medications.
In the device group, 8.5% of patients experienced an adverse event, versus 2.9% in the sham group (P = .004). The only adverse reaction that occurred more frequently in the device group was forehead paresthesia, discomfort, and burning (3.5% vs. 0.4%; P = .009).
The study was funded by Cefaly. Dr. Tepper and Dr. Friedman have no relevant financial disclosures.
The study also demonstrated that the device, manufactured by Cefaly and cleared in 2020 by the Food and Drug Administration for over-the-counter use, can be safely and effectively used at home.
The study also explored the benefits of 2 hours of use, rather than the 1 hour of use tested in a previous study. “The programming on the device is currently [set to] turn off at 1 hour. As a result of this study, I tell patients if they don’t have adequate relief, and they’re tolerating it, that they can activate it again for a second hour,” Stewart Tepper, MD, said in an interview. Dr. Tepper is a professor of neurology at Geisel School of Medicine at Dartmouth, Hanover, N.H., and a coauthor of the study that was presented by Deena Kuruvilla, MD, at the American Headache Society’s 2021 annual meeting. Dr. Kuruvilla is a neurologist and director of the Westport (Conn.) Headache Institute.
The improvements seen over the sham were significant but not overwhelming, according to Deborah Friedman, MD, MPH, professor of neurology and ophthalmology at the University of Texas, Dallas.
“The numbers are not super impressive when you compare them with other devices. I thought it was interesting that the most bothersome symptom went away in a much higher percentage of people than the headache. That was actually pretty impressive,” said Dr. Friedman, who was asked to comment on the study. She also wondered if the sham device may have inadvertently provided a small amount of stimulation, which could explain the smaller than expected efficacy difference. “It just kind of makes me wonder because I would expect to see a larger separation, even though it was statistically significant.”
The study was an overall success according to Dr. Tepper, who noted that the efficacy of pain freedom was comparable with what has been seen with calcitonin gene-related peptide receptor antagonists (gepants), as well as relieving the most bothersome symptom at 2 hours. The device failed to reduce the usage of rescue medication, suggesting that it might be a candidate to combine with rescue medications. “I think the main thing is it works. It works in a sham-controlled trial, it works at home, and it works comparably to acute medication. And it is further evidence that the lack of access is something that needs to be addressed,” said Dr. Tepper.
Access will depend on insurance companies, who have so far been reluctant to pay for the device. Dr. Tepper is not optimistic they will come around on their own. “My feeling about it is that the only way that payers will finally start to cover this is with a concerted, organized advocacy campaign by patients. The analogy is that when the disease-modifying therapies became available for multiple sclerosis, the National MS Society organized the MS patients and they demanded that the payers cover the disease modifying therapies. That’s the kind of intense focus of advocacy that needs to be done for these noninvasive neuromodulation devices,” said Dr. Tepper.
The TEAM study was a double blind, randomized, sham-controlled trial of 538 patients who were asked to use neurostimulation for a 2-hour, continuous session within 4 hours of a moderate to severe migraine accompanied by at least one migraine-associated symptom. At 2 hours, 25.5% of those using the device achieved pain freedom, compared with 18.3% of those using the sham (P < .05). Among those using the device, 56.4% had freedom from most bothersome symptom, compared with 42.3% of those using the sham (P < .01).
Pain relief at 2 hours was more common in the device group (69.5% vs. 55.2%; P < .01), as was absence of all migraine-associated symptoms at 2 hours (42.5% vs. 34.1%; P < .05), sustained pain freedom at 24 hours (22.8% vs. 15.8%; P < .05), and sustained pain relief at 24 hours (45.9% vs. 34.4%; P < .01). There was no statistically significant between-group difference in use of rescue medications.
In the device group, 8.5% of patients experienced an adverse event, versus 2.9% in the sham group (P = .004). The only adverse reaction that occurred more frequently in the device group was forehead paresthesia, discomfort, and burning (3.5% vs. 0.4%; P = .009).
The study was funded by Cefaly. Dr. Tepper and Dr. Friedman have no relevant financial disclosures.
FROM AHS 2021