User login
Your role in early diagnosis & Tx of metastatic bone disease
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
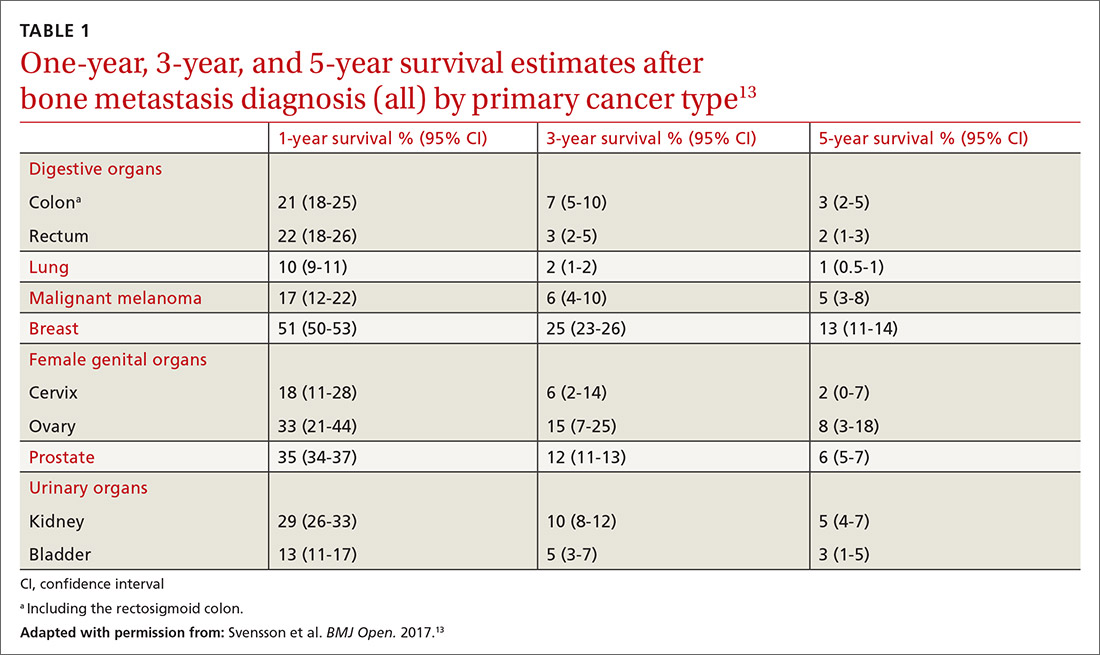
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.
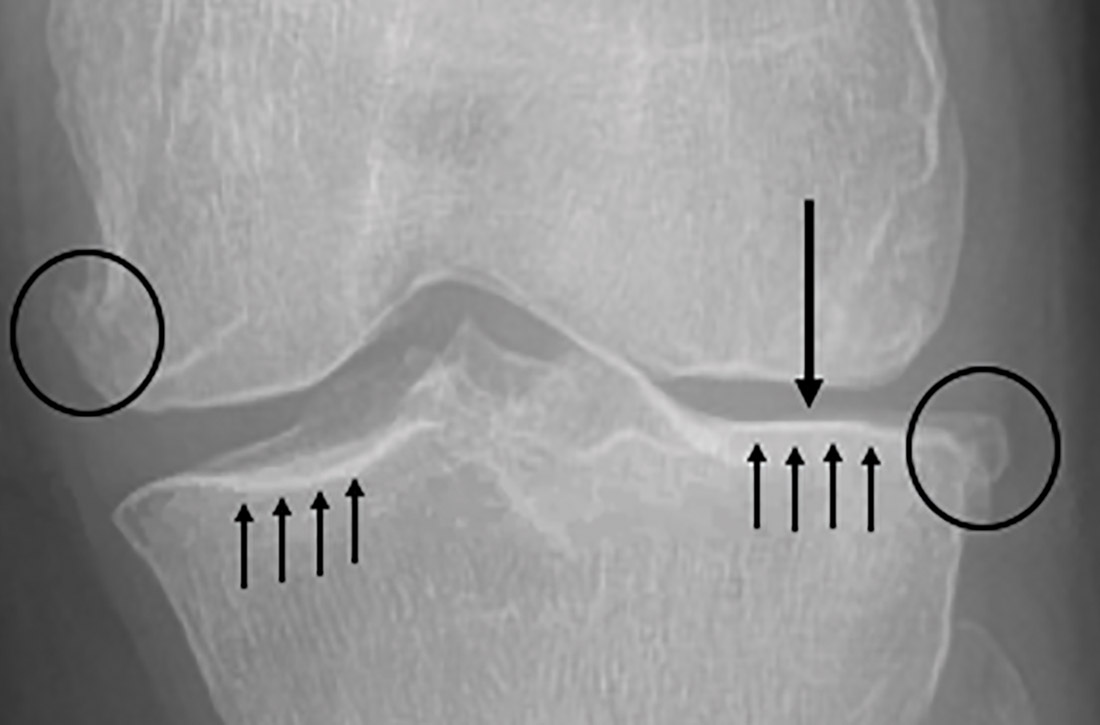
When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
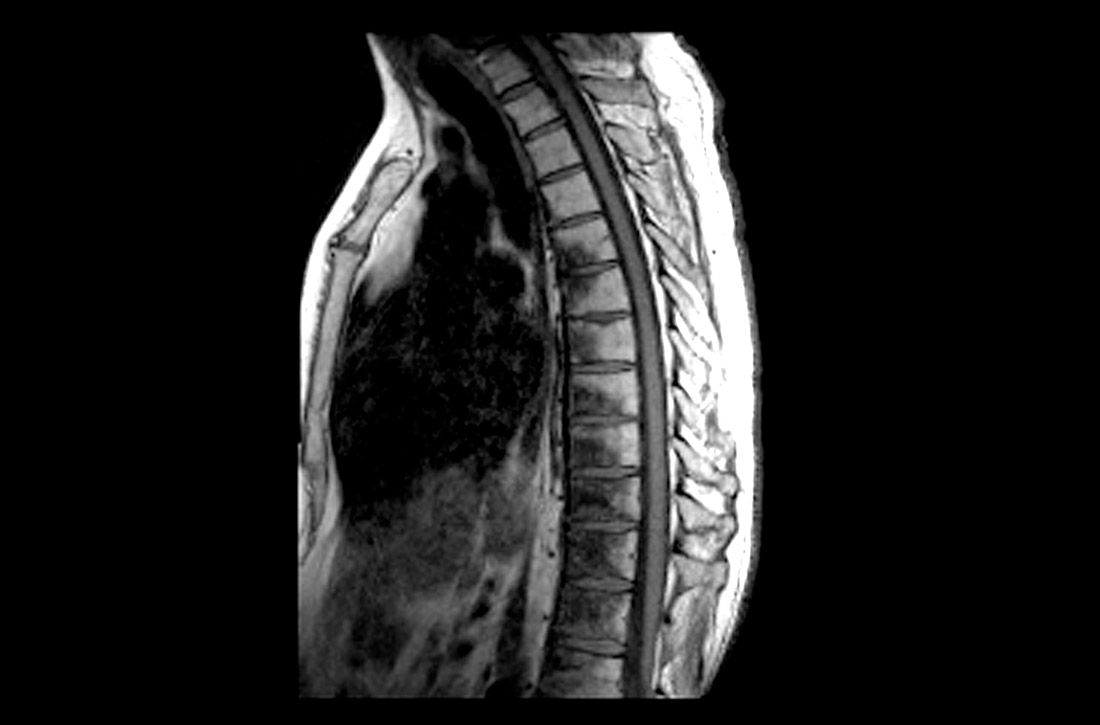
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
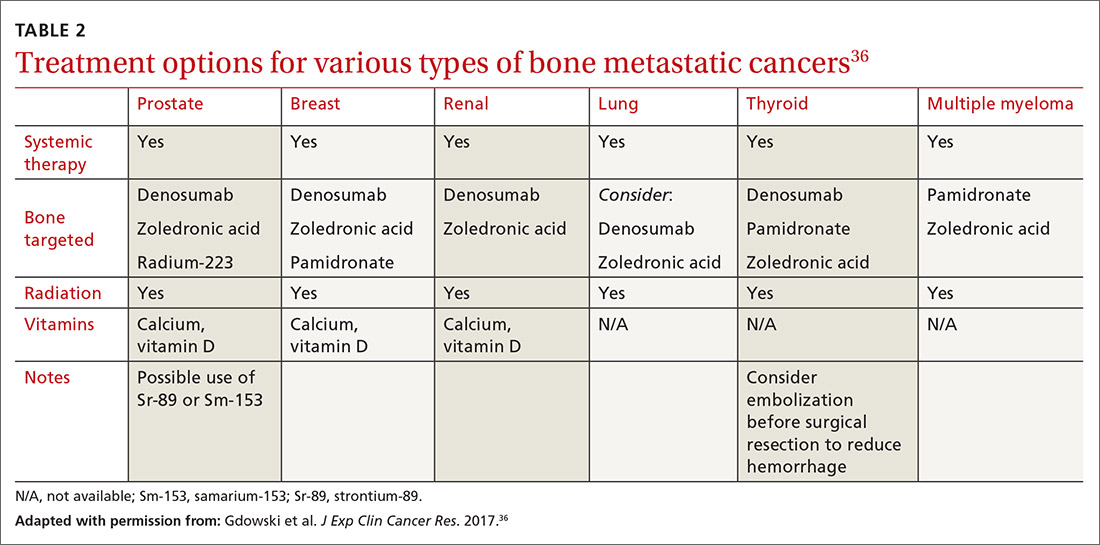
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36
Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; [email protected].
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.

When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36

Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; [email protected].
Since the early 1990s, modern treatments have steadily reduced overall cancer mortality from primary tumors.1 Consequently, more people are at risk of metastatic bone disease, with subsequent pain and pathologic fractures1,2 and death from metastasis.3 Patients who have bone metastases present with a variety of signs and symptoms including pain, fractures, and metabolic derangements. The primary care approach to work-up and diagnosis described in this article enables prompt treatment, either surgical or nonsurgical, to maintain a high quality of life for patients.
Primary tumors determine types of metastases and prognosis
Metastasis, a complex pathologic process in which cancerous cells migrate to distant organs, implant, and grow,3 is a poor prognostic indicator in cancer patients. Bone is the third most common site of metastasis, behind the liver and lungs.4 While the true prevalence of metastatic bone cancer is unknown, studies have estimated it to be > 280,000 cases in the United States.5
Bone metastases interfere with normal bone metabolism and turnover in several different characteristic patterns. These changes—radiographically defined as osteoblastic, osteolytic, or mixed lesions—are determined by the primary tumor type.
- Osteoblastic lesions, comprised of new, disorganized bone formation, often occur secondary to prostate cancer, small cell lung cancer, and carcinoid malignancies, among others.
- Osteolytic lesions, in which bone is destroyed, are more common with breast cancer, renal cell carcinoma, melanoma, and multiple myeloma.
- Mixed lesions, in which areas of bone destruction and growth are simultaneously found, occur with some GI cancers and a few breast cancers.6,7
Most bone metastases result from carcinomas, of which up to 50% eventually spread to bone, although this process can take 10 to 15 years.8,9 The likelihood of bone metastasis depends on the primary tumor and its stage. Breast and prostate cancer account for most skeletal metastases, although these lesions are often asymptomatic.6,9 Other malignancies, such as ovarian and gastrointestinal, metastasize to bone much less frequently.7,10 Virtually any cancer at an advanced stage can spread to bone. These metastases are usually multifocal and incurable, with the patient’s prognosis varying from a few months to years.6,11,12
Factors that influence prognosis. Metastatic bone disease arising from melanoma and lung cancers has the shortest life expectancy of roughly 6 months from initial diagnosis; metastasis following prostate, breast, and thyroid cancers has the longest, usually 2 to 4 years.11TABLE 113 shows survival estimates from a large Danish population at various time points following bone metastasis diagnosis for several primary cancer types.

When surgical intervention for bony metastasis is required, prognosis is generally poorer, likely due to more advanced disease. The overall 1-year survival following surgery varies, but several large studies have found a rate of around 40% when considering all primary tumors.14,15 The most common metastases, from breast and prostate cancers, have 1-year survivals of around 50% and 30%, respectively, following surgical intervention.16-18
What you’re likely to see on presentation
Bone metastases are one of the leading causes of morbidity in cancer patients from resultant pain, pathologic fractures, metabolic derangements, and reduced activities of daily living.8,19 The most common cause of cancer pain is bone involvement.6 Patients report pain that is usually worse at night, poorly localized, and not alleviated with rest. They often mistakenly relate the pain to an injury.20 The pathophysiology of bone pain is not completely understood but is likely multifactorial and includes inflammatory and mechanical processes.7,21 Spine involvement can lead to stenosis or nerve root compression, with symptoms dependent on level and severity of nerve or cord compromise.20 Overall, the most common site of bone metastasis is the thoracic spine, followed by the ribs, pelvis, and proximal long bones.20
Continue to: Pathologic fractures
Pathologic fractures occur frequently in cancer patients. Bone destruction leads to a loss of mechanical support which, in turn, causes microfractures and pain. These microfractures can proliferate and coalesce, causing a pathologic fracture, often in weight-bearing bones.6 Breast cancer with lytic lesions is the single leading cause of all pathologic fractures.22 Lung cancer with its short survival time and prostate cancer with blastic lesions are less common causes.23 In the appendicular skeleton, the vast majority of these fractures occur in the femur and humerus.11
Symptomatic metabolic derangements. The most common metabolic disorder is hypercalcemia, found predominantly in patients with hematologic malignancies, squamous cell lung cancer, renal cell cancer, and breast cancer.6,7,12,24 The clinical presentation is nonspecific and can include polyuria, polydipsia, fatigue, constipation, and confusion. The prevalence is estimated to be 13% in breast cancer, 4% in lung cancers, and 1% in prostate cancer, although results in individual studies vary.12 The pathophysiology is multifactorial and often includes osteolytic lesions and an increased circulating level of parathyroid hormone–related peptide, although other mechanisms contribute.25,26 Ultimately, severe hypercalcemia may be fatal secondary to renal failure and cardiac arrhythmias.6,7,12 Paraneoplastic hypercalcemia independently decreases survival; 1 study found the median survival to be 10 to 12 weeks.11
Primary care work-up and diagnosis
When a patient presents with signs and symptoms suggestive of metastatic bone disease, inquire about a history of cancer. Even if such a history is remote, it is important—particularly so if the patient received chemotherapy or radiation, which can lead to secondary cancers such as leukemia or sarcoma.20 If a primary site of malignancy is unknown, pursue a general review of systems. Clues to the primary site of disease could be a history of chest pain, shortness of breath, hemoptysis, heat/cold intolerance, or changes in bowel/bladder habits. Also ask about risk factors such as smoking, chemical exposure, and sun exposure.
Pointers on radiographic imaging. If you suspect a destructive bone lesion, order appropriate radiographic imaging. Arrange for plain radiographs with at least 2 views of the specific area of interest that include the entire bone along with the joints above and below. Importantly, the entire bone must be imaged before any surgical procedure to avoid periprosthetic fractures from undetected bone metastases around hardware.20 Keep in mind that plain films can miss early lesions, and computed tomography (CT) or magnetic resonance imaging (MRI) may be needed if suspicion of a pathologic process is still strong and especially if a primary malignancy is known.27
Working back to a primary diagnosis
If imaging confirms a suspicious lesion and the patient has no known primary tumor, order labs, a CT scan with contrast of the chest, abdomen, and pelvis, and a bone scan, and refer the patient to an oncologist. If the bone lesion is painful, initiate protected weight-bearing and additionally refer the patient to an orthopedic surgeon.
Continue to: Appropriate laboratory evaluation
Appropriate laboratory evaluation entails a complete blood count; metabolic panel that includes serum calcium and phosphorus, vitamin D, alkaline phosphatase, thyroid-stimulating hormone, and parathyroid hormone; and serum protein electrophoresis to rule out multiple myeloma.7,11 Tumor markers are useful to monitor a patient’s response to cancer treatment or to determine recurrence, but they play only a limited role in the initial work-up of an unknown bone tumor.28
Further imaging. A CT scan with intravenous contrast of the chest, abdomen, and pelvis is done to screen for visceral malignancy; however, 15% of bone lesions in patients with an unknown primary lesion never have a source identified.29 Bone scans can be useful in identifying the extent of a single lesion seen on plain films and to assess for additional asymptomatic lesions. Additional imaging—eg, CT or MRI of the lesion, or positron emission tomography (PET)—can be left to the discretion of the oncologist or surgeon.
CT scans have significantly higher sensitivity than radiographs and offer better visualization of bone quality, bone destruction, and soft-tissue extension.30 MRI can be used to assess changes in bone marrow and soft-tissue involvement. PET scans, which detect tumors by quantifying metabolic activity, remain controversial. PET is superior to bone scans in detecting bone metastases from lung and breast cancers, but worse in renal and prostate cancers due to slow growth of metastases.31-33
Caveat.
Treatment options
Metastatic bone disease is typically managed nonsurgically with radiation, chemo- or immunotherapies, hormone suppression, bone-modifying agents, or ablation.36 An overview of the cancer treatment guidelines for bone metastasis from the 2017 National Comprehensive Cancer Network is shown in TABLE 2.36

Continue to: Radiotherapy
Radiotherapy can take the form of external-beam or radioisotope radiation. With localized irradiation, most patients who have painful lesions experience at least partial relief, often within a few weeks.12,37 It may be used postoperatively, as well, to decrease the chances of disease progession.20
Systemic therapies include chemo- and hormone therapies. Chemotherapy effectiveness is highly dependent on the primary tumor type. For example, renal cell carcinoma and melanoma are often resistant, while lymphoma and germ-cell tumors may be eliminated and sometimes even cured.7 Hormone therapy can be highly effective in selective cancers, primarily breast and prostate cancers. Immunotherapy options may also be used to specifically target bone metastasis sites.
Bone-modifying agents include bisphosphonates and denosumab (Prolia, Xgeva). These are generally initiated at the discretion of the oncologist, but primary care physicians should be familiar with their use. Bisphosphonates, which includes zoledronic acid, pamidronate, and other agents, are analogues of pyrophosphate that inhibit bone demineralization.38 These agents target bone resorption through incorporation into osteoclasts and have been effective in the treatment of hypercalcemia and bone lesions.6,12,39 Not only do they reduce the incidence of all skeleton-related events, including pathologic fractures and pain, they also appear to have antitumor activity with prolonged survival in certain cancers.7,12
Denosumab, which has a much shorter half-life than bisphosphonates, is a monoclonal antibody that targets the gene RANKL, a key activator of osteoclasts, and thereby prevents the development of osteoclasts and related bone resorption.40
Radiofrequency ablation or cryoablation, using image-guided needle placement, specifically targets individual bone lesions, destroying tumor cells with extreme heat or cold, respectively. This has been shown to reduce pain and opioid consumption.41
Continue to: Managing pain
Managing pain
Pain management can be difficult, especially as patients live longer and undergo additional treatments such as surgery, radiation, and chemotherapy, each with the potential to produce chronic pain.42 A multidisciplinary team with a stepwise and multimodal approach can improve the patient’s function and comfort while decreasing drug adverse effects.43
For mild-to-moderate pain, nonsteroidal anti-inflammatory drugs, acetaminophen, and tramadol may provide effective relief. For more severe pain, narcotics are often required on a fixed-dose schedule along with breakthrough options such as short-acting hydromorphone, oxycodone, or transmucosal fentanyl.42-44 Opioid adverse effects such as constipation and nausea/vomiting must be managed with laxatives and metoclopramide/antidopaminergics, respectively.
Other important non-narcotic therapies are corticosteroids, tricyclic antidepressants, gabapentin, neuroleptics, and nerve blocks.45 Physical therapy and acupuncture may also be useful, depending on the patient’s needs and desires. Despite the wide range of options, most patients continue to have a significant amount of pain that can impact daily activities and even cause them to feel that their quality of life was not an important factor in physician decision making.46
Surgery options
Surgical intervention for metastatic bone disease differs from its use in primary bone tumors in that clinical indications are not clearly defined. In general, surgery for metastatic disease is used in patients who have pathologic fractures, a risk of pathologic fracture, or uncontrolled cancer-induced bone pain. Keep in mind that the overarching goal of surgery is to reduce morbidity, not mortality, although exceptions exist. Metastatic renal cell carcinoma is one such exception: improved survival may be achieved via aggressive surgical resection for solitary or oligometastatic lesions.47
Before deciding on surgery, engage the patient in goals-of-care discussions and take into account factors specific to the individual, as operative complications can be devasting. Risk of postoperative infection is high, given that these patients are often immunocompromised and that irradiated tissue is prone to wound healing issues.8 Complications may require a pause in chemotherapy and a subsequent decrease in life expectancy.
Continue to: Another factor in surgical decision making...
Another factor in surgical decision making is that newer systemic therapies are leading to longer survival for those with various types of metastatic cancer.48 Older methods of fixation designed to last a few years may now fail during the patient’s prolonged lifespan. As novel therapies continue to improve survival and complicate surgical indications, it may be prudent for the surgical management of metastatic bone disease to be handled by fellowship-trained orthopedic oncologists.
Factors that affect timing. Surgical intervention ideally occurs before the development of a pathologic fracture. Outcomes research has shown that intervention before fracture leads to reduced blood loss and length of hospital stay with improved functional recovery and survival.12,49 Despite these improved outcomes, an adequate scoring system to guide surgical intervention has yet to be developed. Mirels’ criteria are cited most often, yet this scoring system fails to account for many important considerations such as primary tumor type, life expectancy, and other factors.50,51
Given the deleterious effects of fractures in cancer patients and the inadequacy of closed reduction and immobilization, surgical intervention is often warranted.52 Surgical technology has continued to progress; however, intramedullary nailing, plating, and endoprostheses are still the most commonly used methods.53
Intramedullary nailing is commonly used in the prophylactic treatment of pathologic lesions and fractures of long bones in patients whose expected survival is as little as 6 to 12 weeks.54 Plate and screw fixation is a viable alternative to intramedullary nailing when tumor resection is desired. Endoprostheses replacement is used when a tumor involves joint surfaces or if biological reconstruction cannot be achieved by nailing or plating.
Explicit communication with patients is critical
Of vital importance is your participation with patients and families in shared decision making throughout the diagnostic and treatment process, ensuring clear communication. Misunderstandings about cancer stages and prognoses are not uncommon and are sometimes due to insufficient explanation.55,56 Additionally, expectations of survival and adverse effects of treatment often differ greatly between physicians and patients, which can lead to patient dissatisfaction.57
Continue to: Finally, the long-term care...
Finally, the long-term care of patients with metastatic cancers necessarily involves multidisciplinary teams, which further complicates communication. To ensure that patients are receiving an appropriate course of treatment, evaluate their health literacy, confirm their understanding of the disease, and acknowledge their desires.
CORRESPONDENCE
Kyle Sweeney, MD, University of Kansas Medical Center, Department of Orthopedic Surgery, 3901 Rainbow Boulevard, MS 3017, Kansas City, KS 66160; [email protected].
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289.
3. Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243-255.
4. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165-176.
5. Li S, Peng Y, Weinhandl ED, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87-93.
6. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s.
7. Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
8. Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081-4089.
9. Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(suppl 1):S5.
10. Suva LJ, Washam C, Nicholas RW, et al. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208-218.
11. Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365-378.
12. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037.
13. Svensson E, Christiansen CF, Ulrichsen SP, et al. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7 e016022.
14. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132-138.
15. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11-15.
16. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res. 2002:191-196.
17. Weiss RJ, Tullberg E, Forsberg JA, et al. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast. 2014;23:286-290.
18. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012;83:74-79.
19. Nathan SS, Chan L, Tan WL, et al. The need for a system of prognostication in skeletal metastasis to decide best end-of-life care - a call to arms. Ann Acad Med Singapore. 2010;39:476-481.
20. Weber KL. Evaluation of the adult patient (aged > 40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18:169-179.
21. Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 suppl):866-873.
22. McDuffee LA, Colterjohn N, Singh G. Bone metastasis and pathological fractures. In: Singh G, Rabbani SA, eds. Bone Metastasis. Experimental and Clinical Therapeutics. Totowa, NJ: Humana Press; 2005:229-241.
23. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509-524.
24. Maisano R, Pergolizzi S, Cascinu S. Novel therapeutic approaches to cancer patients with bone metastasis. Crit Rev Oncol Hematol. 2001;40:239-250.
25. Marino MT, Asp AA, Budayer AA, et al. Hypercalcaemia and elevated levels of parathyroid hormone-related protein in cutaneous squamous/basal cell carcinoma. J Intern Med. 1993;233:205-207.
26. Grill V, Ho P, Body JJ, et al. Parathyroid hormone-related protein: elevated levels in both humoral hypercalcemia of malignancy and hypercalcemia complicating metastatic breast cancer. J Clin Endocrinol Metab. 1991;73:1309-1315.
27. Jehn CF, Diel IJ, Overkamp F, et al. Management of metastatic bone disease algorithms for diagnostics and treatment. Anticancer Res. 2016;36:2631-2637.
28. Molina R, Bosch X, Auge JM, et al. Utility of serum tumor markers as an aid in the differential diagnosis of patients with clinical suspicion of cancer and in patients with cancer of unknown primary site. Tumour Biol. 2012;33:463-474.
29. Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276-1281.
30. Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53-64.
31. Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803-809.
32. Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
33. Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623-1629.
34. Adams SC, Potter BK, Mahmood Z, et al. Consequences and prevention of inadvertent internal fixation of primary osseous sarcomas. Clin Orthop Relat Res. 2009;467:519-525.
35. Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J Am Acad Orthop Surg. 2014;22:90-100.
36. Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108.
37. Yoon F, Morton GC. Single fraction radiotherapy versus multiple fraction radiotherapy for bone metastases in prostate cancer patients: comparative effectiveness. Cancer Manag Res. 2014;6:451-457.
38. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-340.
39. Van Acker HH, Anguille S, Willemen Y, et al. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40.
40. Castellano D, Sepulveda JM, Garcia-Escobar I, et al. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136-145.
41. Guenette JP, Lopez MJ, Kim E, et al. Solitary painful osseous metastases: correlation of imaging features with pain palliation after radiofrequency ablation—a multicenter American College of Radiology imaging network study. Radiology. 2013;268:907-915.
42. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
43. ASATFCPM, ASRAPM. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810-833.
44. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166-iv191.
45. Kvale PA, Simoff M, Prakash UBS, ACCP. Lung cancer. Palliative care. Chest. 2003;123(1 suppl):284S-311S.
46. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420-1433.
47. Kato S, Murakami H, Takeuchi A, et al. Fifteen-year survivor of renal cell carcinoma after metastasectomies for multiple bone metastases. Orthopedics. 2013;36:e1454-e1457.
48. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 suppl):1614-1627.
49. Ristevski B, Jenkinson RJ, Stephen DJG, et al. Mortality and complications following stabilization of femoral metastatic lesions: a population-based study of regional variation and outcome. Can J Surg. 2009;52:302-308.
50. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. 1989. Clin Orthop Relat Res. 2003(415 suppl):S4-S13.
51. Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res. 2010;468:2825-2827.
52. Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297-302.
53. Bird JE. “Advances in the surgical management of bone tumors.” Curr Oncol Rep. 2014;16:392.
54. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503-1516.
55. Kim SH, Shin DW, Kim SY, et al. Terminal versus advanced cancer: do the general population and health care professionals share a common language? Cancer Res Treat. 2016;48:759-767.
56. Lee JK, Yun YH, An AR, et al. The understanding of terminal cancer and its relationship with attitudes toward end-of-life care issues. Med Decis Making. 2014;34:720-730.
57. Lux MP, Bayer CM, Loehberg CR, et al. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429-440.
PRACTICE RECOMMENDATIONS
› Initiate appropriate lab and imaging work-ups for any patient without known malignancy who has a suspicious bone lesion. C
› Prescribe protected weight-bearing for the patient who has a painful bone lesion, and refer promptly to an orthopedic surgeon to prevent pathologic fracture. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
One in seven high schoolers is misusing opioids
according to an analysis from the Centers for Disease Control and Prevention.
That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
according to an analysis from the Centers for Disease Control and Prevention.

That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
according to an analysis from the Centers for Disease Control and Prevention.

That type of opioid use/misuse, reported by 14.3% of respondents to the 2019 Youth Risk Behavior Survey, was more common among females (16.1%) than males (12.4%) and even more prevalent among nonheterosexuals and those who are unsure about their sexual identity, Christopher M. Jones, PharmD, DrPH, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
The YRBS data show that 18.5% of gay or lesbian students had, at some point in their lives, used a prescription opioid differently than a physician had told them to or taken one without a prescription. That figure was slightly higher (19.1%) for those unsure of their sexual identity, considerably higher (25.4%) for bisexuals, and lower for heterosexuals (12.7%), they reported.
The pattern for current use/misuse of opioids, defined as use one or more times in the 30 days before the survey, was similar to ever use but somewhat less pronounced in 2019. Prevalence was 7.2% for all students in grades 9-12, 8.3% for females, and 6.1% for males. By sexual identity, prevalence was 6.4% for heterosexuals, 7.6% for gays or lesbians, 11.5% for those unsure about their sexual identity, and 13.1% for bisexuals, based on the YRBS data.
This increased misuse of opioids among sexual minority youths, “even after controlling for other demographic and substance use characteristics ... emphasizes the importance of identifying tailored prevention strategies to address disparities among this vulnerable population,” the CDC researchers wrote.
SOURCE: Jones CM et al. MMWR Suppl. 2020 Aug 21;69(1):38-46.
FROM MMWR
A practical approach to knee OA
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8

Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)
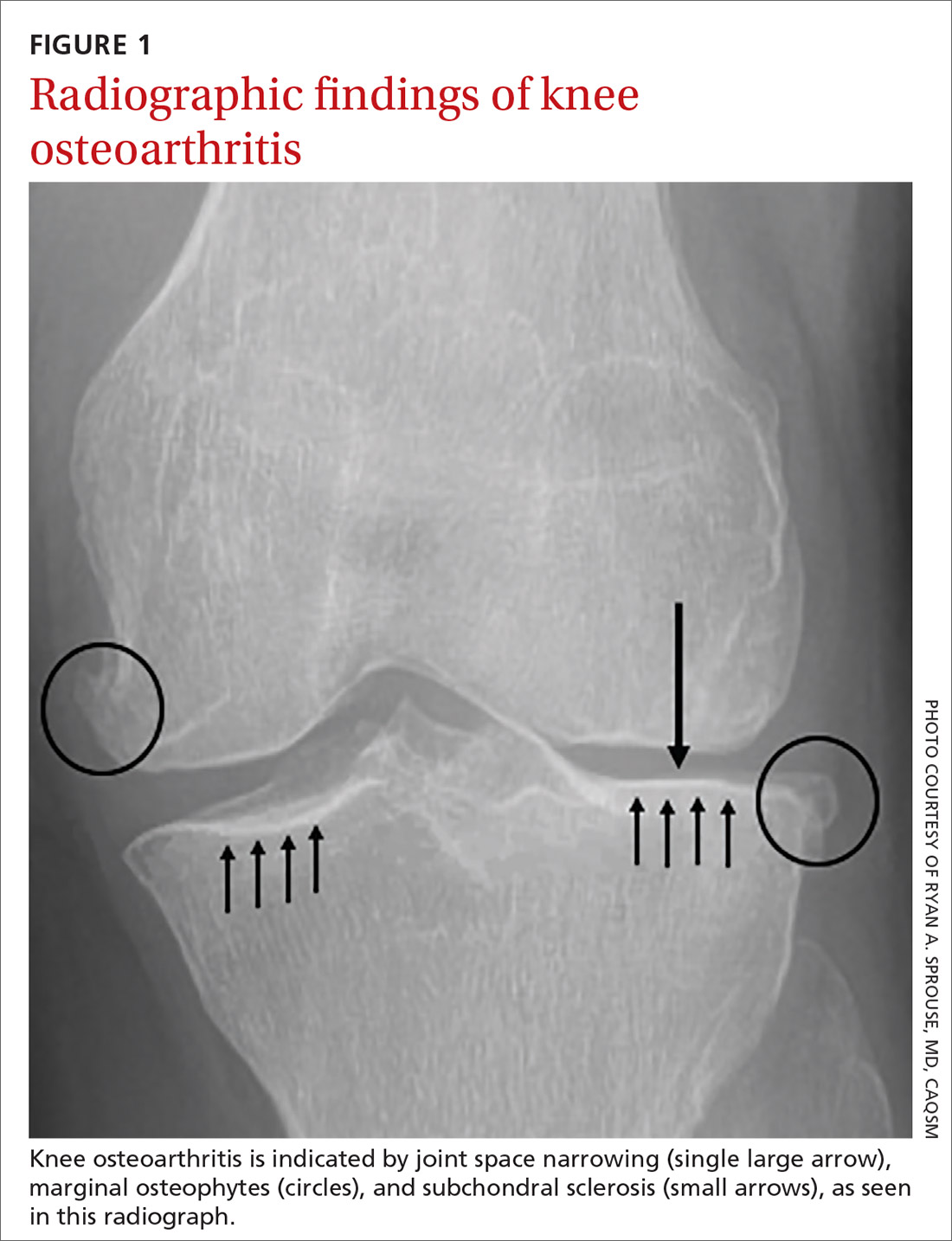
Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.
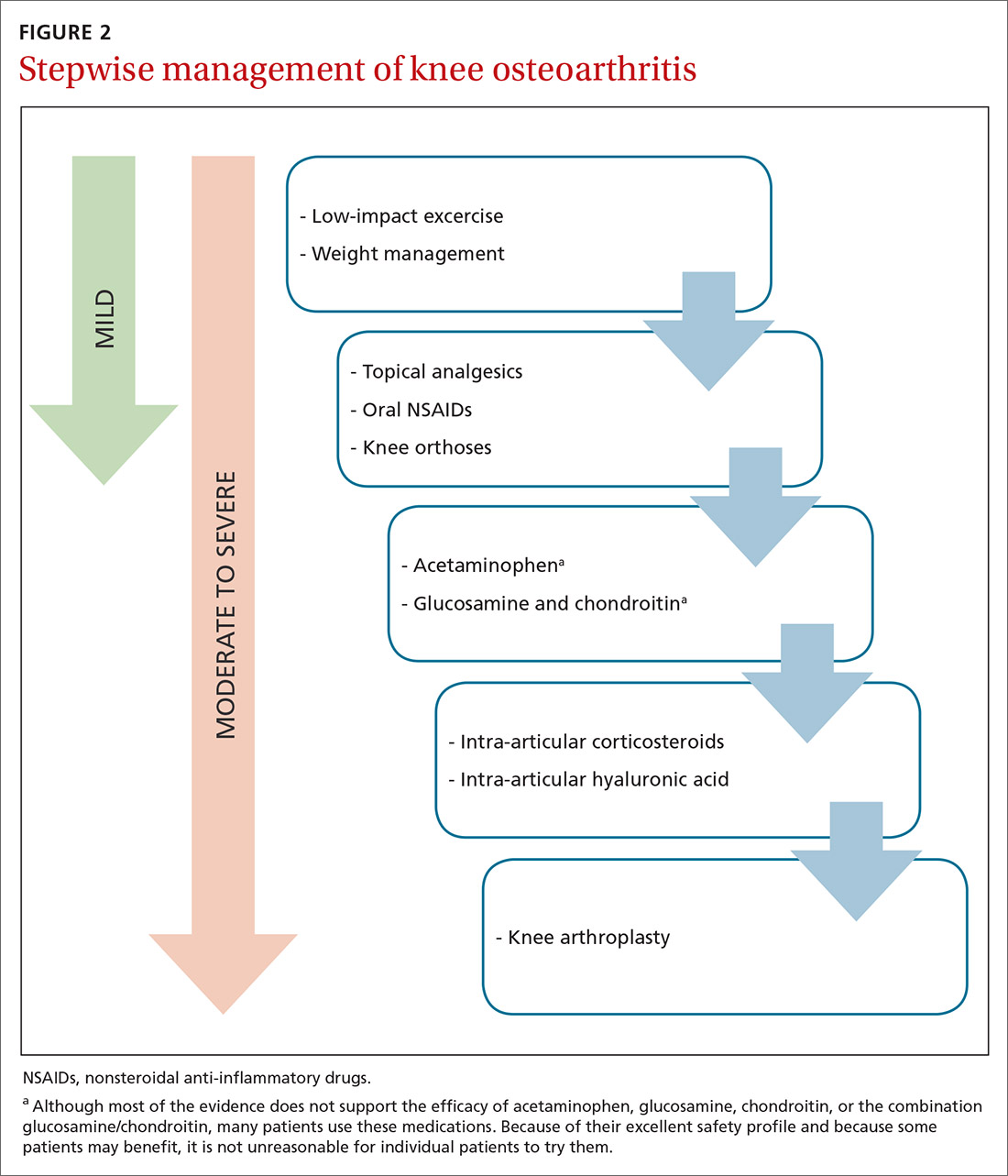
Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8

Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)

Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.

Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
CASE A 73-year-old woman presents to your clinic with 1 year of gradual-onset left knee pain. The pain is worse at the medial knee and at the beginning and end of the day, with some mild improvement after activity in the morning. The patient has already tried oral acetaminophen, an over-the-counter menthol cream, and a soft elastic knee brace, but these interventions have helped only minimally.
On physical exam, there is no obvious deformity of the knee. There is a bit of small joint effusion without redness or warmth. There is mild tenderness to palpation of the medial joint line. Radiographic findings include osteophytes of the medial and lateral tibial plateaus and medial and lateral femoral condyles with mild joint-space narrowing of the medial compartment, consistent with mild osteoarthritis.
How would you manage this patient’s care?
The knee is the most common joint to be affected by osteoarthritis (OA) and accounts for the majority of the disease’s total burden.1 More than 19% of American adults ages ≥ 45 years have knee OA,1,2 and more than half of the people with symptomatic knee OA in the United States are younger than 65 years of age.3 Longer lifespan and increasing rates of obesity are thought to be driving the increasing prevalence of knee OA, although this remains debated.1 Risk factors for knee OA are outlined in TABLE.1,4-8

Diagnosis: Radiographs are helpful, not essential
The diagnosis of knee OA is relatively straightforward. Gradual onset of knee joint pain is present most days, with pain worse after activity and better with rest. Patients are usually middle-aged or older and/or have a distant history of knee joint injury. Other signs, symptoms, and physical exam findings associated with knee OA include: morning stiffness < 30 minutes, crepitus, instability, range-of-motion deficit, varus or valgus deformity, bony exostosis, joint-line tenderness, joint swelling/effusion, and the absence of erythema/warmth.1,9,10
Although radiographs are not necessary to diagnose knee OA, they can be helpful in confirming the diagnosis by assessing the degree and location of OA and ruling out other pathology. Standing, weight-bearing radiographs are particularly helpful for assessing the degree of joint-space narrowing. In addition to joint-space narrowing, radiographic findings indicative of knee OA include marginal osteophytes, subchondral sclerosis, and subchondral cysts. (See FIGURE 1.)

Keep in mind that radiographs are less sensitive for early OA, that the degree of OA seen on radiographs does not correlate well with symptoms, and that radiographic evidence of OA is a common incidental finding—especially in elderly individuals.11 Although not routinely utilized for knee OA diagnosis, magnetic resonance imaging (MRI) can be used to assess for earlier stages of the disease and to rule out pathology associated with the soft tissue and cartilage that is not directly associated with OA.
Continue to: Management
Management: Decrease pain, improve function, slow progression
Because there is no cure for OA, the primary goals of treatment are to decrease pain, improve function of the joint, and slow progression of the disease. As a result, a multifaceted treatment approach is usually undertaken that includes weight reduction and exercise therapy and may include pharmacotherapy, depending on the degree of symptoms. FIGURE 2 contains a summary of the stepwise management of knee OA.

Weight management can slow progression of the disease
Obesity is a causative factor in knee OA.12,13 Patients with knee OA who achieve and maintain an appropriate body weight can potentially slow progression of the disease.13,14 One pound of weight loss can lead to a 4-fold reduction in the load exerted on the knee per step.15
Specific methods of weight reduction are beyond the scope of this article; however, one randomized controlled trial (RCT) involving 399 overweight and obese adults with knee OA found that individuals who participated in a dietary intervention or a combined diet and exercise intervention achieved more weight loss than those who undertook exercise alone.16 Additionally, the diet group had greater reductions in knee compression forces compared to the exercise group, and the combined diet and exercise group had less pain and better function than both the diet group and the exercise group.16 This would suggest that both diet and exercise interventions should be employed in the treatment of knee OA, not only for weight management, but also for knee joint health.
What kind of exercise? Evidence exists to support the utilization of various forms of exercise. In general, land-based therapeutic exercise improves knee pain, physical function, and quality of life, but these benefits often last less than 1 year because people often fail to maintain exercise programs for the long term.17
Specific therapies such as yoga, Tai Chi, balance training, and aquatic exercise have shown some minor improvement in symptoms related to knee OA.18-22 Weight-bearing strength training, non–weight-bearing strength training, and aerobic exercise have all been shown to be effective for short-term pain relief in knee OA, with non–weight-bearing strength training being the most effective.23
Continue to: Strengthening of the upper leg muscles...
Strengthening of the upper leg muscles is thought to be one of the factors involved in reducing pain associated with knee OA.24 Strength training, Tai Chi, and aerobic exercise have also been shown to decrease fall risk in the elderly with knee OA.25 In general, lower impact activities (eg, walking, swimming, biking, yoga) are preferred over higher impact activities (eg, running, jumping) in order to lessen pain with exercise.26-28
Knee orthoses: Many forms and mixed findings
Knee braces come in many forms, including soft braces (eg, elastic sleeves, simple hinged braces) and unloading braces. Many of these braces have been purported to help with knee OA although the evidence remains mixed, with a lack of high-quality trials. A systematic review of RCTs comparing various knee braces, foot orthotics, and conservative treatment for the management of medial compartment OA concluded that the optimal choice for orthosis remains unclear, and long-term evidence is lacking.29
The medial unloading (valgus) knee brace is often used to treat medial compartment OA and varus malalignment of the knee by applying a valgus force, thereby reducing the load on the medial compartment. One recent systematic review concluded that medial unloading braces improve pain from medial compartment OA, but whether they improve function and stiffness is unclear.30 Another study showed that compared to conservative treatment alone, valgus knee bracing has some benefit in decreasing pain and improving knee function.31 Additionally, an 8-year prospective study found that the valgus unloading brace can delay the time before patients need to undergo knee arthroplasty.32 However, another prospective study examining the efficacy of valgus bracing at 2.7 years and 11.2 years showed short-term but not long-term benefit.33
Soft knee braces include a variety of elastic sleeves and simple hinged knee braces. These braces are available commercially at most pharmacies and athletic retail stores. Soft braces are thought to improve pain by a thermal and compressive effect, and to provide stability to the knee joint. One systematic review concluded that soft knee braces have a moderate effect on pain and a small-to-moderate effect on self-reported physical function.34 A small trial showed that soft knee braces reduced pain and dynamic instability in individuals with knee OA.35
In summary, many types of soft knee braces exist, but the evidence for recommending them individually or collectively is limited, as high-quality trials are lacking. However, the available evidence does suggest some mild benefit with regard to pain and function with no concern for adverse effects.
Continue to: Pharmacotherapy
Pharmacotherapy: Oral agents
Acetaminophen. Although people commonly use this over-the-counter analgesic for knee OA pain, recent meta-analyses have shown that acetaminophen provides little to no benefit.36,37 Furthermore, although many believe acetaminophen causes fewer adverse effects than oral nonsteroidal anti-inflammatory drugs (NSAIDs), liver, gastrointestinal, and renal complications are not uncommon with long-term acetaminophen use. Nevertheless, a trial of acetaminophen may be beneficial in patients with cardiovascular disease or who are taking oral anticoagulants.
Oral NSAIDs. Many studies have concluded that NSAIDs are more effective at controlling pain from knee OA than acetaminophen.37,38 They are among the most commonly prescribed treatments for knee OA, but patients and their physicians should be cautious about long-term use because of potential cardiac, renal, gastrointestinal, and other adverse effects. Although evidence regarding optimal frequency of use is scarce, oral NSAIDs should be used intermittently and at the minimal effective dose in order to decrease the risk of adverse events.
One recent meta-analysis of RCTs concluded that diclofenac at a dose of 150 mg/d is the most effective NSAID for improving pain and function associated with knee OA.37 Another recent systematic review and meta-analysis analyzing multiple pharmacologic treatments found an association between celecoxib and decreased pain from knee OA.39 However, this study also concluded that uncertainty surrounded all of the estimates of effect size for change in pain compared to placebo for all of the pharmacologic treatments included in the study.39
A meta-analysis of RCTs comparing celecoxib to no treatment, placebo, naproxen, and diclofenac concluded that celecoxib is slightly better than placebo and the aforementioned NSAIDs in reducing pain and improving function in general OA. However, the authors had reservations regarding pharmaceutical industry involvement in the studies and overall limited data.40
With all of that said, the American Academy of Orthopaedic Surgeons (AAOS) recommends strongly for the use of oral NSAIDs in the management of knee OA.41
Continue to: Glucosamine and chondroitin
Glucosamine and chondroitin. Glucosamine and chondroitin are supplements that have gained popularity in the treatment of knee OA. These constituents are found naturally in articular cartilage, which explains the rationale for their use. Glucosamine and chondroitin (or a combination of the 2) are associated with few adverse effects, but the evidence to support their use in knee OA management is mixed.
One large double-blind RCT (the Glucosamine/Chondroitin Arthritis Intervention Trial [GAIT]) concluded that glucosamine, chondroitin, or the combination of the 2 did not have a significant effect on reducing pain from knee OA compared to placebo and did not slow structural joint disease.42 However, this same study found that in a subset of patients with moderate-to-severe knee OA, the combination of glucosamine and chondroitin was mildly effective in reducing pain.42
Multiple studies have shown either no benefit, inconsistent results, or limited benefit of glucosamine and chondroitin in the treatment of knee OA, with the patented crystalline form of glucosamine showing the most efficacy.43-47 The AAOS and the American College of Rheumatology (ACR) do not recommend glucosamine and chondroitin for knee OA management.10,41
In summary, the evidence for glucosamine, chondroitin, or a combination of the 2 for knee OA is mixed with likely limited benefit, but because they are associated with few adverse effects, patients may be offered a 3- to 6-month trial of these supplements if other effective options are exhausted.
Injections
Limited-quality evidence suggests that oral NSAIDs and intra-articular (IA) hyaluronic acid (HA) injections are equally efficacious for knee OA pain.38,48 There is insufficient evidence directly comparing oral NSAIDs with IA corticosteroid (CS) injections.
Continue to: HA is found naturally...
HA is found naturally in articular cartilage, which explains the rationale behind its use. A network meta-analysis performed by the American Medical Society for Sports Medicine concluded that knee OA is more likely to respond to IAHA than to IACS or IA placebo, leading the society to recommend the use of IAHA in knee OA management, especially for patients > 60 years with mild-to-moderate knee OA.9 Conversely, the AAOS does not recommend the use of IAHA, and the ACR does not recommend for or against the use of IAHA.10,41
IACSs are commonly used to provide pain relief in those with moderate-to-severe knee OA. There is evidence that a single IACS injection provides mild pain relief for up to 6 weeks.49 However, there is some concern that repetitive IACS injections may speed cartilage loss. A 2-year randomized double-blind placebo-controlled trial comparing the effectiveness of repetitive IA triamcinolone vs saline in knee OA found no difference in pain severity and concluded that there was greater cartilage volume loss in the triamcinolone group.50
AAOS does not recommend for or against the use of IACSs, whereas the ACR does recommend for the use of IACSs.10,41 Given the available evidence, conservative use of IACS injections remains an option for patients with refractory moderate-to-severe knee OA.
Topicals
Topical analgesics are often utilized for knee OA because of their efficacy, tolerability, low risk of adverse effects, and ease of use. They are generally recommended over oral NSAIDs in the elderly and in individuals at risk for cardiac, renal, and gastrointestinal complications from oral NSAIDs.
One review found that topical diclofenac and topical ketoprofen were comparable to the oral forms of these medications.51 One RCT concluded that topical and oral diclofenac were equally efficacious in treating knee OA symptoms, although topical diclofenac was associated with significantly fewer gastrointestinal adverse effects.52 In multiple randomized trials, topical diclofenac has shown efficacy compared to placebo.53-55 A recent systematic review and meta-analysis of RCTs concluded that topical NSAIDs were safe and effective for treating general OA compared to placebo, with diclofenac patches most effective for pain relief and piroxicam most effective for functional improvement.56
Continue to: Topical capsaicin has shown...
Topical capsaicin has shown some efficacy in treating pain associated with knee OA.57 One meta-analysis of RCTs concluded that topical NSAIDs and capsaicin may be equally efficacious for OA-associated pain relief, although none of the RCTs directly compared the two.58 The major limitation of capsaicin is a patient-reported mild-to-moderate burning sensation with application that may decrease compliance.
Emerging treatments: IA PRP & extended-release IA triamcinolone acetonide
IA platelet-rich plasma (PRP) has been investigated for efficacy in treating knee OA. PRP is thought to decrease inflammation in the joint, although its exact mechanism remains unknown.59 Multiple studies have shown some benefit of PRP in reducing pain and improving function in individuals with knee OA, but nearly all of these studies have failed to show a clear benefit of PRP over HA injections.59-63 Additionally, the authors of most of these studies mention a high risk of bias. PRP therapy is expensive and generally is not covered by insurance companies, which precludes its use for many people.
Extended-release (ER) IA triamcinolone acetonide (Zilretta) has shown some superiority to standard IA triamcinolone acetonide in both degree and duration of pain relief for knee OA.64-66 The ER version tolerability did not differ from placebo and also showed prolonged synovial presence, lower systemic absorption, and lower blood glucose elevations compared with standard triamcinolone.64-66
Surgical intervention: A last resort
Select patients with severe pain and disability from knee OA that is refractory to conservative management options should be referred for consideration of knee arthroplasty. Age, weight, OA location, and degree of OA are all considered with respect to knee arthroplasty timing and technique.
There is good evidence that arthroscopy with debridement, on the other hand, is no more effective than conservative management.67
Continue to: Unicompartmental or "partial"...
Unicompartmental or “partial” knee replacements are reserved for select cases when 1 knee compartment has a significantly higher degree of degenerative change.
CASE After reviewing the therapeutic options with your patient, you agree that she will undergo a course of physical therapy and try using topical diclofenac along with a hinged knee brace. Because of the patient’s age and co-morbidities of cardiovascular disease and mild chronic kidney disease, oral NSAIDs are avoided at this time.
The patient returns to the office in 2 months reporting mild improvement in her pain. To provide additional pain relief, an ultrasound-guided IA steroid injection is attempted. The patient also continues home physical therapy, activity modification, topical diclofenac, and use of a hinged knee brace.
She returns to the office 2 months later, reporting continued improvement in her pain. No further intervention is undertaken at this time.
CORRESPONDENCE
Ryan A. Sprouse, MD, CAQSM, West Virginia University School of Medicine–Eastern Campus, WVU Medicine Orthopaedics and Sports Medicine, 912 Somerset Boulevard, Charles Town, WV 25414; [email protected].
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
1. Wallace IJ, Worthington S,Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci. 2017;114:9332-9336.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167.
4. Warner SC, Valdes AM. Genetic association studies in osteoarthritis: is it fairytale? Curr Opin Rheumatol. 2017;29:103-109.
5. Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-781.
6. Palazzo C, Nguyen C, Lefevre-Colau MM, et al. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138.
7. Tanamas S, Hanna FS, Cicuttini FM, et al. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61:459-467.
8. Yucesoy B, Charles LE, Baker B, et al. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50:261-273.
9. Trojian TH, Concoff AL, Joy SM, et al. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92.
10. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012;64:465-474.
11. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116.
12. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
13. Yusuf E, Bijsterbosch J, Slagboom PE, et al. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1117-1122.
14. Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329-335.
15. Messier SP, Gutekunst DJ, Davis C, et al. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-2032.
16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263-1273.
17. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med.
18. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
19. Chang WD, Chen S, Lee CL, et al. The effects of tai chi chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1813979.
20. Takacs J, Krowchuk NM, Garland SJ, et al. Dynamic balance training improves physical function in individuals with knee osteoarthritis: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2017;98:1586-1593.
21. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
22. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
23. Tanaka R, Ozawa J, Kito N, et al. Efficacy of strengthening or aerobic exercise on pain relief in people with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27:1059-1071.
24. Knoop J, Steultjens MP, Roorda LD, et al. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy. 2015;101:171-177.
25. Mat S, Tan MP, Kamaruzzaman SB, et al. Physical therapies for improving balance and reducing falls risk in osteoarthritis of the knee: a systematic review. Age Ageing. 2015;44:16-24.
26. Peeler J, Christian M, Cooper J, et al. Managing knee osteoarthritis: the effects of body weight supported physical activity on joint pain, function, and thigh muscle strength. Clin J Sport Med. 2015;25:518-523.
27. Peeler J, Ripat J. The effect of low-load exercise on joint pain, function, and activities of daily living in patients with knee osteoarthritis. Knee. 2018;25:135-145.
28. Takacs J, Anderson JE, Leiter JR, et al. Lower body positive pressure: an emerging technology in the battle against knee osteoarthritis? Clin Interv Aging. 2013;8:983-991.
29. Duivenvoorden T, Brouwer RW, van Raaij TM, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(3):CD004020.
30. Gohal C, Shanmugaraj A, Tate P, et al. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10:500-514.
31. Brouwer RW, van Raaij TM, Verhaar JA, et al. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthritis Cartilage. 2006;14:777-783.
32. Lee PY, Winfield TG, Harris SR, et al. Unloading knee brace is a cost-effective method to bridge and delay surgery in unicompartmental knee arthritis. BMJ Open Sport Exerc Med. 2017;2:e000195.
33. Wilson B, Rankin H, Barnes CL. Long-term results of an unloader brace in patients with unicompartmental knee osteoarthritis. Orthopedics. 2011;34:334-347.
34. Cudejko T, van der Esch M, van der Leeden M, et al. Effect of soft braces on pain and physical function in patients with knee osteoarthritis: systematic review with meta-analyses. Arch Phys Med Rehabil. 2018;99:153-163.
35. Cudejko T, van der Esch M, van den Noort JC. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in persons with knee osteoarthritis. Arthritis Care Res (Hoboken). 2019;71:1036-1043.
36. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
37. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21-e33.
38. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
39. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579.
40. Puljak L, Marin A, Vrdoljak D, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;(5):CD009865.
41. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;9:571-576.
42. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
43. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
44. Yang S, Eaton CB, McAlindon TE, et al. Effects of glucosamine and chondroitin on treating knee osteoarthritis: an analysis with marginal structural models. Arthritis Rheumatol. 2015;67:714-723.
45. Ogata T, Yuki Ideno Y, Masami Akai M,et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol. 2018;37:2479-2487.
46. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946.
47. Bruyèreetal O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 suppl):S3-S11.
48. Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial. Arthritis Res Ther. 2014;16:R18.
49. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328.
50. McAlindon TE, LaValley MP, Harvey FW, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975.
51. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;(4):CD007400.
52. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31:2002-2012.
53. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32:241-250.
54. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.
55. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44.
56. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. 2018;52:642-650.
57. Guedes V, Castro JP, Brito I. Topical capsaicin for pain in osteoarthritis: a literature review. Reumatol Clin. 2018;14:40-45.
58. Persson MSM, Stocks J, Walsh DA, et al. The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthritis Cartilage. 2018;26:1575-1582.
59. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339-346.
60. Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
61. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418-1429.
62. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
63. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47:347-354.
64. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877-888.
65. Conaghan PG, Cohen SB, Berenbaum F, et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70:204-211.
66. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Jt Surg Am. 2018;100:666–677.
67. Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
PRACTICE RECOMMENDATIONS
› Treat pain from knee osteoarthritis (OA) with weight management and low-impact exercise to decrease the risk of disease progression. A
› Prescribe oral or topical nonsteroidal anti-inflammatory drugs to relieve pain from knee OA, as both forms are equally effective. B
› Recommend a medial unloading (valgus) knee brace for short-term relief of medial knee OA. B
› Consider a trial of intra-articular corticosteroids or intra-articular hyaluronic acid derivatives for short-term relief of knee OA pain. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic abdominal pain and diarrhea
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.
Small-fiber polyneuropathy may underlie dysautonomia in ME/CFS
A significant proportion of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia may have potentially treatable underlying autoimmune-associated small-fiber polyneuropathy (aaSFPN), pilot data suggest.
The findings, from a single-site study of 61 patients with ME/CFS, were presented August 21 at the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis by Ryan Whelan, BS, a research assistant at Simmaron Research Institute, Incline Village, Nevada.
Recent evidence suggests an autoimmune etiology for some patients with ME/CFS, which is defined as experiencing for a period of at least 6 months profound, unexplained fatigue, postexertional malaise, and unrefreshing sleep, as well as cognitive dysfunction and/or orthostatic intolerance (OI).
OI is part of a spectrum of autonomic dysfunction commonly seen in ME/CFS patients, which may also include postural orthostatic tachycardia (POTS), peripheral temperature dysregulation and light sensitivity, neuropathic pain, and gastrointestinal complaints. Many of these symptoms overlap those reported by patients with aaSFPN, a common but underdiagnosed neurodegenerative disorder characterized by the loss of peripheral autonomic nerve fibers, Whelan explained.
Findings from the current study show that in more than half of ME/CFS patients, levels of at least one autoantibody were elevated. A majority had comorbid POTS or OI, and over a third had biopsy-confirmed aaSFPN.
“Given the overlap of symptoms and common etiological basis, it may be important to identify ME/CFS patients who present with comorbid aaSFPN, as it has been shown that immune modulatory agents, including intravenous gamma globulin [IVIG], reduce the autonomic symptom burden in aaSFPN patients,” Whelan said.
He noted that Anne Louise Oaklander, MD, a neurologist at Massachusetts General Hospital, Harvard Medical School, Boston, and colleagues previously linked aaSFPN with fibromyalgia. In addition, they’ve found a connection between small-fiber dysfunction and postexertional malaise, which is a hallmark ME/CFS symptom.
Asked to comment on Whelan’s presentation, IACFSME co-president Lily Chu, MD, told Medscape Medical News that the new findings are “valuable, because ME/CFS has always been looked upon as just subjective symptoms. When people have laboratory abnormalities, it can be due to a bunch of other causes, but...here’s pathology, here’s a biopsy of actual damage. It’s not just a transient finding. You can actually see it. ... It’s a solid concrete piece of evidence vs something that can fluctuate.”
Autoantibodies, Autonomic Dysfunction, and Small-Fiber Polyneuropathy
Whelan and colleagues conducted an extensive analysis of medical records of 364 patients with ME/CFS (72% female) to identify potential aaSFPN comorbidity. Such identifications were made on the basis of progress notes documenting autonomic dysfunction, laboratory results for serum autoantibodies, and questionnaire symptom self-reports.
They identified 61 patients as possibly having comorbid aaSFPN. Of those, 52% tested positive for at least 1 of 4 autoantibodies, including antimuscarinic cholinergic receptor 4 (47%), anti-beta-2 adrenergic (27%), antimuscarinic cholinergic 3 (25%), and anti-beta-1 adrenergic (13%). These autoantibodies were linked to ME/CFS in a recent Swedish cohort study.
“Evidence supports that these autoantibodies may bind to receptor sites, blocking ligands from reaching these receptors. Disturbances of adrenergic and cholinergic receptors by these autoantibodies may contribute to symptoms of autonomic dysfunction in ME/CFS,” Whelan said.
Although 22% of patients in the study group had POTS and 59% had OI, the authors found no correlation between autoantibody levels and either OI or POTS. However, 38% were confirmed to have small-fiber polyneuropathy on skin biopsy, and the vast majority of those patients (93%) had either POTS or OI.
IVIG May Be a Potential Treatment
Whelan notes that some data suggest that IVIG might help patients with small-fiber neuropathy, including those with autoimmunity.
In addition, he described anecdotal data from a single patient with ME/CFS who had neuropathic symptoms. The patient was treated at Simmaron. The 56-year-old received two IVIG infusions given 6 months apart. The patient experienced a dramatic reduction in levels of all four of the relevant autoantibodies and favorable symptom reduction, as shown in clinician follow-up records. “With the success of this case study, we intend to further evaluate IVIG as a potential treatment in ME/CFS patients. With this research, we hope to identify a subset of ME/CFS patients who will respond favorably to IVIG,” Whelan concluded.
Regarding use of IVIG, Chu commented, “We don’t know exactly how it works, but it seems to help certain conditions.” She pointed to another recent small study that reported clinical improvement in patients with ME/CFS through a different approach, immunoadsorption, for reducing the autoantibody levels.
Overall, Chu said, this line of research “is important because it shows there’s some type of abnormal biomarker for ME/CFS. And, it may lay a path toward understanding the pathophysiology of the disease and why people have certain symptoms, and could be used to target therapies. ... It’s intriguing.”
Whelan and Chu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A significant proportion of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia may have potentially treatable underlying autoimmune-associated small-fiber polyneuropathy (aaSFPN), pilot data suggest.
The findings, from a single-site study of 61 patients with ME/CFS, were presented August 21 at the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis by Ryan Whelan, BS, a research assistant at Simmaron Research Institute, Incline Village, Nevada.
Recent evidence suggests an autoimmune etiology for some patients with ME/CFS, which is defined as experiencing for a period of at least 6 months profound, unexplained fatigue, postexertional malaise, and unrefreshing sleep, as well as cognitive dysfunction and/or orthostatic intolerance (OI).
OI is part of a spectrum of autonomic dysfunction commonly seen in ME/CFS patients, which may also include postural orthostatic tachycardia (POTS), peripheral temperature dysregulation and light sensitivity, neuropathic pain, and gastrointestinal complaints. Many of these symptoms overlap those reported by patients with aaSFPN, a common but underdiagnosed neurodegenerative disorder characterized by the loss of peripheral autonomic nerve fibers, Whelan explained.
Findings from the current study show that in more than half of ME/CFS patients, levels of at least one autoantibody were elevated. A majority had comorbid POTS or OI, and over a third had biopsy-confirmed aaSFPN.
“Given the overlap of symptoms and common etiological basis, it may be important to identify ME/CFS patients who present with comorbid aaSFPN, as it has been shown that immune modulatory agents, including intravenous gamma globulin [IVIG], reduce the autonomic symptom burden in aaSFPN patients,” Whelan said.
He noted that Anne Louise Oaklander, MD, a neurologist at Massachusetts General Hospital, Harvard Medical School, Boston, and colleagues previously linked aaSFPN with fibromyalgia. In addition, they’ve found a connection between small-fiber dysfunction and postexertional malaise, which is a hallmark ME/CFS symptom.
Asked to comment on Whelan’s presentation, IACFSME co-president Lily Chu, MD, told Medscape Medical News that the new findings are “valuable, because ME/CFS has always been looked upon as just subjective symptoms. When people have laboratory abnormalities, it can be due to a bunch of other causes, but...here’s pathology, here’s a biopsy of actual damage. It’s not just a transient finding. You can actually see it. ... It’s a solid concrete piece of evidence vs something that can fluctuate.”
Autoantibodies, Autonomic Dysfunction, and Small-Fiber Polyneuropathy
Whelan and colleagues conducted an extensive analysis of medical records of 364 patients with ME/CFS (72% female) to identify potential aaSFPN comorbidity. Such identifications were made on the basis of progress notes documenting autonomic dysfunction, laboratory results for serum autoantibodies, and questionnaire symptom self-reports.
They identified 61 patients as possibly having comorbid aaSFPN. Of those, 52% tested positive for at least 1 of 4 autoantibodies, including antimuscarinic cholinergic receptor 4 (47%), anti-beta-2 adrenergic (27%), antimuscarinic cholinergic 3 (25%), and anti-beta-1 adrenergic (13%). These autoantibodies were linked to ME/CFS in a recent Swedish cohort study.
“Evidence supports that these autoantibodies may bind to receptor sites, blocking ligands from reaching these receptors. Disturbances of adrenergic and cholinergic receptors by these autoantibodies may contribute to symptoms of autonomic dysfunction in ME/CFS,” Whelan said.
Although 22% of patients in the study group had POTS and 59% had OI, the authors found no correlation between autoantibody levels and either OI or POTS. However, 38% were confirmed to have small-fiber polyneuropathy on skin biopsy, and the vast majority of those patients (93%) had either POTS or OI.
IVIG May Be a Potential Treatment
Whelan notes that some data suggest that IVIG might help patients with small-fiber neuropathy, including those with autoimmunity.
In addition, he described anecdotal data from a single patient with ME/CFS who had neuropathic symptoms. The patient was treated at Simmaron. The 56-year-old received two IVIG infusions given 6 months apart. The patient experienced a dramatic reduction in levels of all four of the relevant autoantibodies and favorable symptom reduction, as shown in clinician follow-up records. “With the success of this case study, we intend to further evaluate IVIG as a potential treatment in ME/CFS patients. With this research, we hope to identify a subset of ME/CFS patients who will respond favorably to IVIG,” Whelan concluded.
Regarding use of IVIG, Chu commented, “We don’t know exactly how it works, but it seems to help certain conditions.” She pointed to another recent small study that reported clinical improvement in patients with ME/CFS through a different approach, immunoadsorption, for reducing the autoantibody levels.
Overall, Chu said, this line of research “is important because it shows there’s some type of abnormal biomarker for ME/CFS. And, it may lay a path toward understanding the pathophysiology of the disease and why people have certain symptoms, and could be used to target therapies. ... It’s intriguing.”
Whelan and Chu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A significant proportion of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia may have potentially treatable underlying autoimmune-associated small-fiber polyneuropathy (aaSFPN), pilot data suggest.
The findings, from a single-site study of 61 patients with ME/CFS, were presented August 21 at the virtual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis by Ryan Whelan, BS, a research assistant at Simmaron Research Institute, Incline Village, Nevada.
Recent evidence suggests an autoimmune etiology for some patients with ME/CFS, which is defined as experiencing for a period of at least 6 months profound, unexplained fatigue, postexertional malaise, and unrefreshing sleep, as well as cognitive dysfunction and/or orthostatic intolerance (OI).
OI is part of a spectrum of autonomic dysfunction commonly seen in ME/CFS patients, which may also include postural orthostatic tachycardia (POTS), peripheral temperature dysregulation and light sensitivity, neuropathic pain, and gastrointestinal complaints. Many of these symptoms overlap those reported by patients with aaSFPN, a common but underdiagnosed neurodegenerative disorder characterized by the loss of peripheral autonomic nerve fibers, Whelan explained.
Findings from the current study show that in more than half of ME/CFS patients, levels of at least one autoantibody were elevated. A majority had comorbid POTS or OI, and over a third had biopsy-confirmed aaSFPN.
“Given the overlap of symptoms and common etiological basis, it may be important to identify ME/CFS patients who present with comorbid aaSFPN, as it has been shown that immune modulatory agents, including intravenous gamma globulin [IVIG], reduce the autonomic symptom burden in aaSFPN patients,” Whelan said.
He noted that Anne Louise Oaklander, MD, a neurologist at Massachusetts General Hospital, Harvard Medical School, Boston, and colleagues previously linked aaSFPN with fibromyalgia. In addition, they’ve found a connection between small-fiber dysfunction and postexertional malaise, which is a hallmark ME/CFS symptom.
Asked to comment on Whelan’s presentation, IACFSME co-president Lily Chu, MD, told Medscape Medical News that the new findings are “valuable, because ME/CFS has always been looked upon as just subjective symptoms. When people have laboratory abnormalities, it can be due to a bunch of other causes, but...here’s pathology, here’s a biopsy of actual damage. It’s not just a transient finding. You can actually see it. ... It’s a solid concrete piece of evidence vs something that can fluctuate.”
Autoantibodies, Autonomic Dysfunction, and Small-Fiber Polyneuropathy
Whelan and colleagues conducted an extensive analysis of medical records of 364 patients with ME/CFS (72% female) to identify potential aaSFPN comorbidity. Such identifications were made on the basis of progress notes documenting autonomic dysfunction, laboratory results for serum autoantibodies, and questionnaire symptom self-reports.
They identified 61 patients as possibly having comorbid aaSFPN. Of those, 52% tested positive for at least 1 of 4 autoantibodies, including antimuscarinic cholinergic receptor 4 (47%), anti-beta-2 adrenergic (27%), antimuscarinic cholinergic 3 (25%), and anti-beta-1 adrenergic (13%). These autoantibodies were linked to ME/CFS in a recent Swedish cohort study.
“Evidence supports that these autoantibodies may bind to receptor sites, blocking ligands from reaching these receptors. Disturbances of adrenergic and cholinergic receptors by these autoantibodies may contribute to symptoms of autonomic dysfunction in ME/CFS,” Whelan said.
Although 22% of patients in the study group had POTS and 59% had OI, the authors found no correlation between autoantibody levels and either OI or POTS. However, 38% were confirmed to have small-fiber polyneuropathy on skin biopsy, and the vast majority of those patients (93%) had either POTS or OI.
IVIG May Be a Potential Treatment
Whelan notes that some data suggest that IVIG might help patients with small-fiber neuropathy, including those with autoimmunity.
In addition, he described anecdotal data from a single patient with ME/CFS who had neuropathic symptoms. The patient was treated at Simmaron. The 56-year-old received two IVIG infusions given 6 months apart. The patient experienced a dramatic reduction in levels of all four of the relevant autoantibodies and favorable symptom reduction, as shown in clinician follow-up records. “With the success of this case study, we intend to further evaluate IVIG as a potential treatment in ME/CFS patients. With this research, we hope to identify a subset of ME/CFS patients who will respond favorably to IVIG,” Whelan concluded.
Regarding use of IVIG, Chu commented, “We don’t know exactly how it works, but it seems to help certain conditions.” She pointed to another recent small study that reported clinical improvement in patients with ME/CFS through a different approach, immunoadsorption, for reducing the autoantibody levels.
Overall, Chu said, this line of research “is important because it shows there’s some type of abnormal biomarker for ME/CFS. And, it may lay a path toward understanding the pathophysiology of the disease and why people have certain symptoms, and could be used to target therapies. ... It’s intriguing.”
Whelan and Chu have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Appendix may be common site of endometriosis
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
FROM SGS 2020
AHA on cannabis: No evidence of heart benefits, but potential harms
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
Evidence for a link between cannabis use and cardiovascular health remains unsupported, and the potential risks outweigh any potential benefits, according to a scientific statement from the American Heart Association.
The increased legalization of cannabis and cannabis products in the United States has driven medical professionals to evaluate the safety and efficacy of cannabis in relation to health conditions, wrote Robert L. Page II, PharmD, of the University of Colorado, Aurora, and colleagues.
In a statement published in Circulation, the researchers noted that although cannabis has been shown to relieve pain and other symptoms in certain conditions, clinicians in the United States have been limited from studying its health effects because of federal law restrictions. “Cannabis remains a schedule I controlled substance, deeming no accepted medical use, a high potential for abuse, and an unacceptable safety profile,” the researchers wrote.
The statement addresses issues with the use of cannabis by individuals with cardiovascular disease or those at increased risk. Observational studies have shown no cardiovascular benefits associated with cannabis, the writers noted. The most common chemicals in cannabis include THC (tetrahydrocannabinolic acid) and CBD (cannabidiol).
Some research has shown associations between CBD cardiovascular features including lower blood pressure and reduced inflammation, the writers noted. However, THC, the component of cannabis associated with a “high” or intoxication, has been associated with heart rhythm abnormalities. The writers cited data suggesting an increased risk of heart attacks, atrial fibrillation and heart failure, although more research is needed.
The statement outlines common cannabis formulations including plant-based, extracts, crystalline forms, edible products, and tinctures. In addition, the statement notes that synthetic cannabis products are marketed and used in the United States without subject to regulation.
“Over the past 5 years, we have seen a surge in cannabis use, particularly during the COVID-19 pandemic here in Colorado, especially among adolescents and young adults,” Dr. Page said in an interview. Because of the surge, health care practitioners need to familiarize themselves with not only the benefits, but risks associated with cannabis use regardless of the formulation,” he said. As heart disease remains a leading cause of death in the United States, understanding the cardiovascular risks associated with cannabis is crucial at this time.
Dr. Page noted that popular attitudes about cannabis could pose risks to users’ cardiovascular health. “One leading misconception about cannabis is because it is ‘natural’ it must be safe,” Dr. Page said. “As with all medications, cannabis has side effects, some of which can be cardiovascular in nature,” he said. “Significant drug-drug interactions can occur as CBD and THC, both found in cannabis, inhibit CYP3A4, which metabolizes a large number of medications used to treat many cardiovascular conditions,” he noted.
“Unfortunately, much of the published data is observational in nature due to the federal restrictions on cannabis as a schedule I drug,” said Dr. Page. “Nonetheless, safety signals have emerged regarding cannabis use and adverse cardiovascular outcomes, including myocardial infarction, heart failure, and atrial fibrillation. Carefully designed prospective short- and long-term studies regarding cannabis use and cardiovascular safety are needed,” he emphasized.
Areas in particular need of additional research include the cardiovascular effects of cannabis in several vulnerable populations such as adolescents, older adults, pregnant women, transplant recipients, and those with underlying cardiovascular disease, said Dr. Page.
“Nonetheless, based on the safety signals described within this Clinical Science Statement, an open discussion regarding the risks of using cannabis needs to occur between patient and health care providers,” he said. “Furthermore, patients must be transparent regarding their cannabis use with their cardiologist and primary care provider. The cannabis story will continue to evolve and is a rapidly moving/changing target,” he said.
“Whether cannabis use is a definitive risk factor for cardiovascular disease as with tobacco use is still unknown, and both acute and long-term studies are desperately needed to address this issue,” he said.
Dr. Page had no relevant financial conflicts to disclose.
SOURCE: Page et al. Circulation. 2020 Aug 5. doi: 10.1161/CIR.0000000000000883.
FROM CIRCULATION
All NSAIDs raise post-MI risk but some are safer than others: Next chapter
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Exploring cannabis use by older adults
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
Older Americans – people aged 65 or older – make up 15% of the U.S. population, according to the Census Bureau. By the end of this decade, or the year 2030, this proportion will increase to 21% – and all “baby boomers,” those born between 1946 and 1964, will be older than 65.1 Those demographic developments are occurring alongside a change in societal, legal, and public attitudes on cannabis.
Liberalization of cannabis laws across the United States allows for ever easier access to medicinal and recreational cannabis. Traditionally, cannabis use, its effects, and related considerations in the adolescent and young adult populations have commanded significant research attention. Cannabis use in older adults, however, is not as well studied.2 An exploration of trends in cannabis use by older adults and potential impact in terms of health is timely and important.
According to data from the National Survey on Drug Use and Health, cannabis use in adults aged 65 years and older appears to have been increasing steadily over the past 2 decades. Use among this group rose from 0.4% in 2006 and 2007, to 2.9% in 2015 and 2016.2 And, most recently, use climbed from 3.7% in 2017 to 4.2% in 2018.2
Cannabis use also has risen among other adults. For those aged 50-64, cannabis use increased from 2.8% in 2006-2007 to 4.8% in 2012-2013.2,3 Meanwhile, from 2015 to 2016, that number increased to 9.0%.3,4
Past-year cannabis use in the groups of those aged 50-64 and those aged 65 and older appears to be higher in individuals with mental health problems, alcohol use disorder, and nicotine dependence.5,6 Being male and being unmarried appear to be correlated with past-year cannabis use. Multimorbidity does not appear to be associated with past-year cannabis use. Those using cannabis tend to be long-term users and have first use at a much younger age, typically before age 21.
Older adults use cannabis for both recreational and perceived medical benefits. Arthritis, chronic back pain, anxiety, depression, relaxation, stress reduction, and enhancement in terms of creativity are all purported reasons for use. However, there is limited to no evidence for the efficacy of cannabis in helping with those conditions and purposes. Clinical trials have shown that cannabis can be beneficial in managing pain and nausea, but those trials have not been conducted in older adults.7,8
There is a real risk of cannabis use having a negative impact on the health of older adults. To begin with, the cannabis consumed today is significantly higher in potency than the cannabis that baby boomers were introduced to in their youth. The higher potency, combined with an age-related decline in function experienced by some older adults, makes them vulnerable to its known side effects, such as anxiety, dry mouth, tachycardia, high blood pressure, palpitations, wheezing, confusion, and dizziness.
Cannabis use is reported to bring a fourfold increase in cardiac events within the first hour of ingestion.9 Cognitive decline and memory impairment are well known adverse effects of cannabis use. Research has shown significant self-reported cognitive decline in older adults in relation to cannabis use.Cannabis metabolites are known to have an effect on cytochrome P450 enzymes, affecting the metabolism of medication, and increasing the susceptibility of older adults who use cannabis to adverse effects of polypharmacy. Finally, as research on emergency department visits by older adults shows, cannabis use can increase the risk of injury among this cohort.
As in the United States, cannabis use among older adults in Canada has increased significantly. The percentage of older adults who use cannabis in the Canadian province of Ontario, for example, reportedly doubled from 2005 to 2015. In response to this increase, and in anticipation of a rise in problematic use of cannabis and cannabis use disorder in older adults, the Canadian Coalition for Seniors’ Mental Health (through financial support from Substance Use and Addictions Program of Health Canada) has created guidelines on the prevention, assessment, and management of cannabis use disorder in older adults.
In the absence of a set of guidelines specific to the United States, the recommendations made by the coalition should be helpful in the care of older Americans. Among other recommendations, the guidelines highlight the needs for primary care physicians to build a better knowledge base around the use of cannabis in older adults, to screen older adults for cannabis use, and to educate older adults and their families about the risk of cannabis use.9
Cannabis use is increasingly popular among older adults10 for both medicinal and recreational purposes. Research and data supporting its medical benefits are limited, and the potential of harm from its use among older adults is present and significant. Importantly, many older adults who use marijuana have co-occurring mental health issues and substance use disorder(s).
Often, our older patients learn about benefits and harms of cannabis from friends and the Internet rather than from physicians and other clinicians.9 We must do our part to make sure that older patients understand the potential negative health impact that cannabis can have on their health. Physicians should screen older adults for marijuana use. Building a better knowledge base around changing trends and views in/on the use and accessibility of cannabis will help physicians better address cannabis use in older adults.
Mr. Kaleka is a medical student in the class of 2021 at Central Michigan University College of Medicine, Mount Pleasant. He has no disclosures. Mr. Kaleka would like to thank his mentor, Furhut Janssen, DO, for her continued guidance and support in research on mental health in vulnerable populations.
References
1. Vespa J et al. Demographic turning points for the United States: Population projections for 2020 to 2060. Current Population Reports. Washington: U.S. Census Bureau. 2020 Feb.
2. Han BH et al. Addiction. 2016 Oct 21. doi: 10.1111/add.13670.
3. Han BH and Palamar JJ. Drug Alcohol Depend. 2018 Oct;191:374-81.
4. Han BH and Palamar JJ. JAMA Intern Med. 2020 Feb 4;180(4):609-11.
5. Choi NG et al. Drug Alcohol Abuse. 2018;44(2):215-23.
6. Reynolds IR et al. J Am Griatr Soc. 2018 Nov;66(11):2167-71.
7. Ahmed AIA et al. J Am Geriatr Soc. 2014 Feb;62(2):410-1.
8. Lum HD et al. Gerontol Geriatr Med. 2019 Jan-Dec;5:2333721419843707.
9. Bertram JR et al. Can Geriatr J. 2020 Mar;23(1):135-42.
10. Baumbusch J and Yip IS. Clin Gerontol. 2020 Mar 29;1-7.
NSAID continuation linked to less knee OA pain
in a randomized trial.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score was 6.7 out of a possible total of 20 for patients who continued meloxicam for 4 weeks versus 7.8 in those who stopped and switched to a placebo. The estimated mean difference in pain score was 1.4 (P = .92 for noninferiority), which is below the threshold of 2.1 that is considered to be the minimum clinically important difference.
Furthermore, patients who had switched to placebo and then subsequently participated in a telephone-based cognitive behavior therapy (CBT) program for another 10 weeks had higher pain levels compared with those who continued meloxicam. WOMAC scores were 12.1 and 11.8, respectively with a mean difference of 0.8 (P = .28 for noninferiority).
“Among patients with knee osteoarthritis, placebo and CBT (after placebo) are inferior to meloxicam,” Liana Fraenkel, MD, MPH, of Yale University, New Haven, Conn., and coinvestigators concluded in their article, published in JAMA Internal Medicine.
They observed that the WOMAC pain score differences between the two groups were small, however, and that there were no statistically significant differences in participants’ global impression of change or function after 14 weeks.
“Although the overall results of the trial are negative, they provide clinicians with data to support shared decision-making and reassure patients willing to taper NSAIDs and consider self-management approaches such as CBT,” Dr. Fraenkel and coauthors suggested.
The Stopping NSAIDs for Arthritis Pain trial had ultimately included 364 participants, 86% of whom were men, recruited from four veterans affairs health care systems. All had been taking NSAIDs for knee OA pain for at least 3 months and had participated in a 2-week run-in period where the NSAID they had been taking was switched to meloxicam, 15 mg once daily.
The aim of the trial had been to see if discontinuing NSAIDs and starting a CBT program would be noninferior to continuing NSAIDs in patients with knee OA.
The trial does not provide robust information on the use of CBT, David Walsh, a rheumatologist and director of the Pain Centre Versus Arthritis at the University of Nottingham, England, said in an interview.
“It can’t tell you about efficacy of CBT,” Dr. Walsh said as the CBT part of the study was not randomized, was not controlled, and was unblinded. ”It would be a different task to design a CBT trial aiming to help people to stop taking tablets,” he added.
Dr. Fraenkel and coinvestigators had reported that, at week 14, the adjusted mean difference in WOMAC pain score between the placebo (followed by CBT) and meloxicam groups was 0.8 (P = .28 for noninferiority).
“What the trial’s really doing is seeing whether people who’ve been on long-term nonsteroidals, can they just stop them without getting any worse? The conclusion for that is actually they are more likely to get worse than not if you just stop the nonsteroidals,” Dr. Walsh said.
“The withdrawal trial protocol is an important one. You can’t run a prospective trial for years to see whether something works for years. It is just not feasible. So actually, the protocol they’ve got of switching to placebo, or continuing with a nonsteroidal, is probably the best way of working out if an anti-inflammatory still has a pharmacological effect after actually being on it for X years,” Dr. Walsh said.
Dr. Walsh, who was not involved in the trial, observed that while the difference in pain scores between the groups was small, the deterioration in scores might be important for individual patients. Some may do worse, although granted that there may be some that might do better, he said.
“It is suggesting to me that nonsteroidals are still working in people who are on long-term treatment. It is not a very big pharmacological effect, but we already know from the RCTs of anti-inflammatory tablets, that they can be beneficial,” Dr. Walsh noted.
He also pointed out that patients’ pain had been improved after being switched from their current NSAID to meloxicam – the overall WOMAC pain score at recruitment was 9.6 and was 5.6 after the 2-week meloxicam run-in phase.
“Now, whether that’s because they’ve been switched to meloxicam, or whether it’s because they’re in a trial,” is an important question, Dr. Walsh suggested, adding that “it looks as though it’s more likely to be because they’re in a trial, because improvement was maintained during the following 4 weeks on placebo.”
Another point he made was that there was a higher percentage of patients in the placebo group that started taking other types of painkillers, just under half (46%) used acetaminophen versus a quarter (26%) of those who continued using meloxicam.
It is an interesting trial, “trying to tackle some really difficult questions and I think that there are really important implications from it that we can build on, but is it actually going to change the lives of patients at the moment? Not massively,” Dr. Walsh said, ”but it’s another step in the right direction.”
Dr. Fraenkel disclosed receiving research funding from the VA Office of Research and Development, the sponsor of the trial.
SOURCE: Fraenkel L et al. JAMA Intern Med. 2020 Jul 20. doi:10.1001/jamainternmed.2020.2821.
in a randomized trial.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score was 6.7 out of a possible total of 20 for patients who continued meloxicam for 4 weeks versus 7.8 in those who stopped and switched to a placebo. The estimated mean difference in pain score was 1.4 (P = .92 for noninferiority), which is below the threshold of 2.1 that is considered to be the minimum clinically important difference.
Furthermore, patients who had switched to placebo and then subsequently participated in a telephone-based cognitive behavior therapy (CBT) program for another 10 weeks had higher pain levels compared with those who continued meloxicam. WOMAC scores were 12.1 and 11.8, respectively with a mean difference of 0.8 (P = .28 for noninferiority).
“Among patients with knee osteoarthritis, placebo and CBT (after placebo) are inferior to meloxicam,” Liana Fraenkel, MD, MPH, of Yale University, New Haven, Conn., and coinvestigators concluded in their article, published in JAMA Internal Medicine.
They observed that the WOMAC pain score differences between the two groups were small, however, and that there were no statistically significant differences in participants’ global impression of change or function after 14 weeks.
“Although the overall results of the trial are negative, they provide clinicians with data to support shared decision-making and reassure patients willing to taper NSAIDs and consider self-management approaches such as CBT,” Dr. Fraenkel and coauthors suggested.
The Stopping NSAIDs for Arthritis Pain trial had ultimately included 364 participants, 86% of whom were men, recruited from four veterans affairs health care systems. All had been taking NSAIDs for knee OA pain for at least 3 months and had participated in a 2-week run-in period where the NSAID they had been taking was switched to meloxicam, 15 mg once daily.
The aim of the trial had been to see if discontinuing NSAIDs and starting a CBT program would be noninferior to continuing NSAIDs in patients with knee OA.
The trial does not provide robust information on the use of CBT, David Walsh, a rheumatologist and director of the Pain Centre Versus Arthritis at the University of Nottingham, England, said in an interview.
“It can’t tell you about efficacy of CBT,” Dr. Walsh said as the CBT part of the study was not randomized, was not controlled, and was unblinded. ”It would be a different task to design a CBT trial aiming to help people to stop taking tablets,” he added.
Dr. Fraenkel and coinvestigators had reported that, at week 14, the adjusted mean difference in WOMAC pain score between the placebo (followed by CBT) and meloxicam groups was 0.8 (P = .28 for noninferiority).
“What the trial’s really doing is seeing whether people who’ve been on long-term nonsteroidals, can they just stop them without getting any worse? The conclusion for that is actually they are more likely to get worse than not if you just stop the nonsteroidals,” Dr. Walsh said.
“The withdrawal trial protocol is an important one. You can’t run a prospective trial for years to see whether something works for years. It is just not feasible. So actually, the protocol they’ve got of switching to placebo, or continuing with a nonsteroidal, is probably the best way of working out if an anti-inflammatory still has a pharmacological effect after actually being on it for X years,” Dr. Walsh said.
Dr. Walsh, who was not involved in the trial, observed that while the difference in pain scores between the groups was small, the deterioration in scores might be important for individual patients. Some may do worse, although granted that there may be some that might do better, he said.
“It is suggesting to me that nonsteroidals are still working in people who are on long-term treatment. It is not a very big pharmacological effect, but we already know from the RCTs of anti-inflammatory tablets, that they can be beneficial,” Dr. Walsh noted.
He also pointed out that patients’ pain had been improved after being switched from their current NSAID to meloxicam – the overall WOMAC pain score at recruitment was 9.6 and was 5.6 after the 2-week meloxicam run-in phase.
“Now, whether that’s because they’ve been switched to meloxicam, or whether it’s because they’re in a trial,” is an important question, Dr. Walsh suggested, adding that “it looks as though it’s more likely to be because they’re in a trial, because improvement was maintained during the following 4 weeks on placebo.”
Another point he made was that there was a higher percentage of patients in the placebo group that started taking other types of painkillers, just under half (46%) used acetaminophen versus a quarter (26%) of those who continued using meloxicam.
It is an interesting trial, “trying to tackle some really difficult questions and I think that there are really important implications from it that we can build on, but is it actually going to change the lives of patients at the moment? Not massively,” Dr. Walsh said, ”but it’s another step in the right direction.”
Dr. Fraenkel disclosed receiving research funding from the VA Office of Research and Development, the sponsor of the trial.
SOURCE: Fraenkel L et al. JAMA Intern Med. 2020 Jul 20. doi:10.1001/jamainternmed.2020.2821.
in a randomized trial.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score was 6.7 out of a possible total of 20 for patients who continued meloxicam for 4 weeks versus 7.8 in those who stopped and switched to a placebo. The estimated mean difference in pain score was 1.4 (P = .92 for noninferiority), which is below the threshold of 2.1 that is considered to be the minimum clinically important difference.
Furthermore, patients who had switched to placebo and then subsequently participated in a telephone-based cognitive behavior therapy (CBT) program for another 10 weeks had higher pain levels compared with those who continued meloxicam. WOMAC scores were 12.1 and 11.8, respectively with a mean difference of 0.8 (P = .28 for noninferiority).
“Among patients with knee osteoarthritis, placebo and CBT (after placebo) are inferior to meloxicam,” Liana Fraenkel, MD, MPH, of Yale University, New Haven, Conn., and coinvestigators concluded in their article, published in JAMA Internal Medicine.
They observed that the WOMAC pain score differences between the two groups were small, however, and that there were no statistically significant differences in participants’ global impression of change or function after 14 weeks.
“Although the overall results of the trial are negative, they provide clinicians with data to support shared decision-making and reassure patients willing to taper NSAIDs and consider self-management approaches such as CBT,” Dr. Fraenkel and coauthors suggested.
The Stopping NSAIDs for Arthritis Pain trial had ultimately included 364 participants, 86% of whom were men, recruited from four veterans affairs health care systems. All had been taking NSAIDs for knee OA pain for at least 3 months and had participated in a 2-week run-in period where the NSAID they had been taking was switched to meloxicam, 15 mg once daily.
The aim of the trial had been to see if discontinuing NSAIDs and starting a CBT program would be noninferior to continuing NSAIDs in patients with knee OA.
The trial does not provide robust information on the use of CBT, David Walsh, a rheumatologist and director of the Pain Centre Versus Arthritis at the University of Nottingham, England, said in an interview.
“It can’t tell you about efficacy of CBT,” Dr. Walsh said as the CBT part of the study was not randomized, was not controlled, and was unblinded. ”It would be a different task to design a CBT trial aiming to help people to stop taking tablets,” he added.
Dr. Fraenkel and coinvestigators had reported that, at week 14, the adjusted mean difference in WOMAC pain score between the placebo (followed by CBT) and meloxicam groups was 0.8 (P = .28 for noninferiority).
“What the trial’s really doing is seeing whether people who’ve been on long-term nonsteroidals, can they just stop them without getting any worse? The conclusion for that is actually they are more likely to get worse than not if you just stop the nonsteroidals,” Dr. Walsh said.
“The withdrawal trial protocol is an important one. You can’t run a prospective trial for years to see whether something works for years. It is just not feasible. So actually, the protocol they’ve got of switching to placebo, or continuing with a nonsteroidal, is probably the best way of working out if an anti-inflammatory still has a pharmacological effect after actually being on it for X years,” Dr. Walsh said.
Dr. Walsh, who was not involved in the trial, observed that while the difference in pain scores between the groups was small, the deterioration in scores might be important for individual patients. Some may do worse, although granted that there may be some that might do better, he said.
“It is suggesting to me that nonsteroidals are still working in people who are on long-term treatment. It is not a very big pharmacological effect, but we already know from the RCTs of anti-inflammatory tablets, that they can be beneficial,” Dr. Walsh noted.
He also pointed out that patients’ pain had been improved after being switched from their current NSAID to meloxicam – the overall WOMAC pain score at recruitment was 9.6 and was 5.6 after the 2-week meloxicam run-in phase.
“Now, whether that’s because they’ve been switched to meloxicam, or whether it’s because they’re in a trial,” is an important question, Dr. Walsh suggested, adding that “it looks as though it’s more likely to be because they’re in a trial, because improvement was maintained during the following 4 weeks on placebo.”
Another point he made was that there was a higher percentage of patients in the placebo group that started taking other types of painkillers, just under half (46%) used acetaminophen versus a quarter (26%) of those who continued using meloxicam.
It is an interesting trial, “trying to tackle some really difficult questions and I think that there are really important implications from it that we can build on, but is it actually going to change the lives of patients at the moment? Not massively,” Dr. Walsh said, ”but it’s another step in the right direction.”
Dr. Fraenkel disclosed receiving research funding from the VA Office of Research and Development, the sponsor of the trial.
SOURCE: Fraenkel L et al. JAMA Intern Med. 2020 Jul 20. doi:10.1001/jamainternmed.2020.2821.
FROM JAMA INTERNAL MEDICINE