User login
Multidisciplinary Transitional Pain Service for the Veteran Population
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
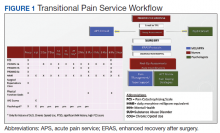
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
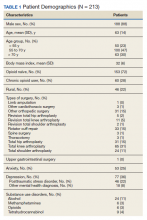
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
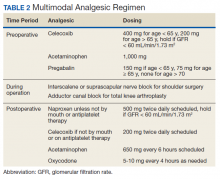
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
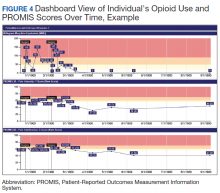
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
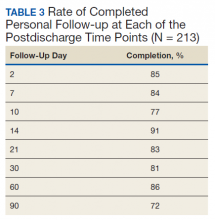
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
Despite advancements in techniques, postsurgical pain continues to be a prominent part of the patient experience. Often this experience can lead to developing chronic postsurgical pain that interferes with quality of life after the expected time to recovery.1-3 As many as 14% of patients who undergo surgery without any history of opioid use develop chronic opioid use that persists after recovery from their operation.4-8 For patients with existing chronic opioid use or a history of substance use disorder (SUD), surgeons, primary care providers, or addiction providers often do not provide sufficient presurgical planning or postsurgical coordination of care. This lack of pain care coordination can increase the risk of inadequate pain control, opioid use escalation, or SUD relapse after surgery.
Convincing arguments have been made that a perioperative surgical home can improve significantly the quality of perioperative care.9-14 This report describes our experience implementing a perioperative surgical home at the US Department of Veterans Affairs (VA) Salt Lake City VA Medical Center (SLCVAMC), focusing on pain management extending from the preoperative period until 6 months or more after surgery. This type of Transitional Pain Service (TPS) has been described previously.15-17 Our service differs from those described previously by enrolling all patients before surgery rather than select postsurgical enrollment of only patients with a history of opioid use or SUD or patients who struggle with persistent postsurgical pain.
Methods
In January 2018, we developed and implemented a new TPS at the SLCVAMC. The transitional pain team consisted of an anesthesiologist with specialization in acute pain management, a nurse practitioner (NP) with experience in both acute and chronic pain management, 2 nurse care coordinators, and a psychologist (Figure 1). Before implementation, a needs assessment took place with these key stakeholders and others at SLCVAMC to identify the following specific goals of the TPS: (1) reduce pain through pharmacologic and nonpharmacologic interventions; (2) eliminate new chronic opioid use in previously nonopioid user (NOU) patients; (3) address chronic opioid use in previous chronic opioid users (COUs) by providing support for opioid taper and alternative analgesic therapies for their chronic pain conditions; and (4) improve continuity of care by close coordination with the surgical team, primary care providers (PCPs), and mental health or chronic pain providers as needed.
Once these TPS goals were defined, the Consolidated Framework for Implementation Research (CFIR) guided the implementation. CFIR is a theory-based implementation framework consisting of 5 domains: intervention characteristics, inner setting, outer setting, characteristics of individuals, and process. These domains were used to identify barriers and facilitators during the early implementation process and helped refine TPS as it was put into clinical practice.
Patient Selection
During the initial implementation of TPS, enrollment was limited to patients scheduled for elective primary or revision knee, hip, or shoulder replacement as well as rotator cuff repair surgery. But as the TPS workflow became established after iterative refinement, we expanded the program to enroll patients with established risk factors for OUD having other types of surgery (Table 1). The diagnosis of risk factors, such as history of SUD, chronic opioid use, or significant mental health disorders (ie, history of suicidal ideation or attempt, posttraumatic stress disorder, and inpatient psychiatric care) were confirmed through both in-person interviews and electronic health record (EHR) documentation. The overall goal was to identify all at-risk patients as soon as they were indicated for surgery, to allow time for evaluation, education, developing an individualized pain plan, and opioid taper prior to surgery if indicated.
Preoperative Procedures
Once identified, patients were contacted by a TPS team member and invited to attend a onetime 90-minute presurgical expectations class held at SLCVAMC. The education curriculum was developed by the whole team, and classes were taught primarily by the TPS psychologist. The class included education about expectations for postoperative pain, available analgesic therapies, opioid education, appropriate use of opioids, and the effect of psychological factors on pain. Pain coping strategies were introduced using a mindfulness-based intervention (MBI) and the Acceptance and Commitment Therapy (ACT) matrix. Classes were offered multiple times a week to help maximize convenience for patients and were separate from the anesthesia preoperative evaluation. Patients attended class only once. High-risk patients (patients with chronic opioid therapy, recent history of or current SUDs, significant comorbid mental health issues) were encouraged to attend this class one-on-one with the TPS psychologist rather than in the group setting, so individual attention to mental health and SUD issues could be addressed directly.
Baseline history, morphine equivalent daily dose (MEDD), and patient-reported outcomes using measures from the Patient-Reported Outcome Measurement System (PROMIS) for pain intensity (PROMIS 3a), pain interference (PROMIS 6b), and physical function (PROMIS 8b), and a pain-catastrophizing scale (PCS) score were obtained on all patients.18 PROMIS measures are validated questionnaires developed with the National Institutes of Health to standardize and quantify patient-reported outcomes in many domains.19 Patients with a history of SUD or COU met with the anesthesiologist and/or NP, and a personalized pain plan was developed that included preoperative opioid taper, buprenorphine use strategy, or opioid-free strategies.
Hospital Procedures
On the day of surgery, the TPS team met with the patient preoperatively and implemented an individualized pain plan that included multimodal analgesic techniques with nonsteroidal anti-inflammatory drugs, acetaminophen, gabapentinoids, and regional anesthesia, where appropriate (Table 2). Enhanced recovery after surgery protocols were developed in conjunction with the surgeons to include local infiltration analgesia by the surgeon, postoperative multimodal analgesic strategies, and intensive physical therapy starting the day of surgery for inpatient procedures.
After surgery, the TPS team followed up with patients daily and provided recommendations for analgesic therapies. Patients were offered daily sessions with the psychologist to reinforce and practice nonpharmacologic pain-coping strategies, such as meditation and relaxation. Prior to patient discharge, the TPS team provided recommendations for discharge medications and an opioid taper plan. For some patients taking buprenorphine before surgery who had stopped this therapy prior to or during their hospital stay, TPS providers transitioned them back to buprenorphine before discharge.
Postoperative Procedures
Patients were called by the nurse care coordinators at postdischarge days 2, 7, 10, 14, 21, 28, and then monthly for ≥ 6 months. For patients who had not stopped opioid use or returned to their preoperative baseline opioid dose, weekly calls were made until opioid taper goals were achieved. At each call, nurses collected PROMIS scores for the previous 24 hours, the most recent 24-hour MEDD, the date of last opioid use, and the number of remaining opioid tablets after opioid cessation. In addition, nurses provided active listening and supportive care and encouragement as well as care coordination for issues related to rehabilitation facilities, physical therapy, transportation, medication questions, and wound questions. Nurses notified the anesthesiologist or NP when patients were unable to taper opioid use or had poor pain control as indicated by their PROMIS scores, opioid use, or directly expressed by the patient.
The TPS team prescribed alternative analgesic therapies, opioid taper plans, and communicated with surgeons and primary care providers if limited continued opioid therapy was recommended. Individual sessions with the psychologist were available to patients after discharge with a focus on ACT-matrix therapy and consultation with long-term mental health and/or substance abuse providers as indicated. Frequent communication and care coordination were maintained with the surgical team, the PCP, and other providers on the mental health or chronic pain services. This care coordination often included postsurgical joint clinic appointments in which TPS providers and nurses would be present with the surgeon or the PCP.
For patients with inadequately treated chronic pain conditions or who required long-term opioid tapers, we developed a combined clinic with the TPS and Anesthesia Chronic Pain group. This clinic allows patients to be seen by both services in the same setting, allowing a warm handoff by TPS to the chronic pain team.
Heath and Decision Support Tools
An electronic dashboard registry of surgical episodes managed by TPS was developed to achieve clinical, administrative, and quality improvement goals. The dashboard registry consists of surgical episode data, opioid doses, patient-reported outcomes, and clinical decision-making processes. Custom-built note templates capture pertinent data through embedded data labels, called health factors. Data are captured as part of routine clinical care, recorded in Computerized Patient Record System as health factors. They are available in the VA Corporate Data Warehouse as structured data. Workflows are executed daily to keep the dashboard registry current, clean, and able to process new data. Information displays direct daily clinical workflow and support point-of-care clinical decision making (Figures 2, 3, and 4). Data are aggregated across patient-care encounters and allow nurse care coordinators to concisely review pertinent patient data prior to delivering care. These data include surgical history, comorbidities, timeline of opioid use, and PROMIS scores during their course of recovery. This system allows TPS to optimize care delivery by providing longitudinal data across the surgical episode, thereby reducing the time needed to review records. Secondary purposes of captured data include measuring clinic performance and quality improvement to improve care delivery.
Results
The TPS intervention was implemented January 1, 2018. Two-hundred thirteen patients were enrolled between January and December 2018, which included 60 (28%) patients with a history of chronic opioid use and 153 (72%) patients who were considered opioid naïve. A total of 99% of patients had ≥ 1 successful follow-up within 14 days after discharge, 96% had ≥ 1 follow-up between 14 and 30 days after surgery, and 72% had completed personal follow-up 90 days after discharge (Table 3). For patients who TPS was unable to contact in person or by phone, 90-day MEDD was obtained using prescription and Controlled Substance Database reviews. The protocol for this retrospective analysis was approved by the University of Utah Institutional Review Board and the VA Research Review Committee.
By 90 days after surgery, 26 (43.3%) COUs were off opioids completely, 17 (28.3%) had decreased their opioid dose from their preoperative baseline MEDD (120 [SD, 108] vs 55 [SD, 45]), 14 (23.3%) returned to their baseline dose, and 3 (5%) increased from their baseline dose. Of the 153 patients who were NOUs before surgery, only 1 (0.7%) was taking opioids after 90 days. TPS continued to work closely with the patient and their PCP and that patient was finally able to stop opioid use 262 days after discharge. Ten patients had an additional surgery within 90 days of the initial surgery. Of these, 6 were COU, of whom 3 stopped all opioids by 90 days from their original surgery, 2 had no change in MEDD at 90 days, and 1 had a lower MEDD at 90 days. Of the 4 NOU who had additional surgery, all were off opioids by 90 days from the original surgery.
Although difficult to quantify, a meaningful outcome of TPS has been to improve satisfaction substantially among health care providers caring for complex patients at risk for chronic opioid abuse. This group includes the many members of the surgical team, PCPs, and addiction specialists who appreciate the close care coordination and assistance in caring for patients with difficult issues, especially with opioid tapers or SUDs. We also have noticed changes in prescribing practices among surgeons and PCPs for their patients who are not part of TPS.
Discussion
With any new clinical service, there are obstacles and challenges. TPS requires a considerable investment in personnel, and currently no mechanism is in place for obtaining payment for many of the provided services. We were fortunate the VA Whole Health Initiative, the VA Office of Rural Health, and the VA Centers of Innovation provided support for the development, implementation, and pilot evaluation of TPS. After we presented our initial results to hospital leadership, we also received hospital support to expand TPS service to include a total of 4 nurse care coordinators and 2 psychologists. We are currently performing a cost analysis of the service but recognize that this model may be difficult to reproduce at other institutions without a change in reimbursement standards.
Developing a working relationship with the surgical and primary care services required a concerted effort from the TPS team and a number of months to become effective. As most veterans receive primary care, mental health care, and surgical care within the VA system, this model lends itself to close care coordination. Initially there was skepticism about TPS recommendations to reduce opioid use, especially from PCPs who had cared for complex patients over many years. But this uncertainty went away as we showed evidence of close patient follow-up and detailed communication. TPS soon became the designated service for both primary care and surgical providers who were otherwise uncomfortable with how to approach opioid tapers and nonopioid pain strategies. In fact, a substantial portion of our referrals now come directly from the PCP who is referring a high-risk patient for evaluation for surgery rather than from the surgeons, and joint visits with TPS and primary care have become commonplace.
Challenges abound when working with patients with substance abuse history, opioid use history, high anxiety, significant pain catastrophizing, and those who have had previous negative experiences with surgery. We have found that the most important facet of our service comes from the amount of time and effort team members, especially the nurses, spend helping patients. Much of the nurses' work focuses on nonpain-related issues, such as assisting patients with finding transportation, housing issues, questions about medications, help scheduling appointments, etc. Through this concerted effort, patients gain trust in TPS providers and are willing to listen to and experiment with our recommendations. Many patients who were initially extremely unreceptive to the presurgery education asked for our support weeks after surgery to help with postsurgery pain.
Another challenge we continue to experience comes from the success of the program.
Conclusions
The multidisciplinary TPS supports greater preoperative to postoperative longitudinal care for surgical patients. This endeavor has resulted in better patient preparation before surgery and improved care coordination after surgery, with specific improvements in appropriate use of opioid medications and smooth transitions of care for patients with ongoing and complex needs. Development of sophisticated note templates and customized health information technology allows for accurate follow-through and data gathering for quality improvement, facilitating data-driven improvements and proving value to the facility.
Given that TPS is a multidisciplinary program with multiple interventions, it is difficult to pinpoint which specific aspects of TPS are most effective in achieving success. For example, although we have little doubt that the work our psychologists do with our patients is beneficial and even essential for the success we have had with some of our most difficult patients, it is less clear whether it matters if they use mindfulness, ACT matrix, or cognitive behavioral therapy. We think that an important part of TPS is the frequent human interaction with a caring individual. Therefore, as TPS continues to grow, maintaining the ability to provide frequent personal interaction is a priority.
The role of opioids in acute pain deserves further scrutiny. In 2018, with TPS use of opioids after orthopedic surgery decreased by > 40% from the previous year. Despite this more restricted use of opioids, pain interference and physical function scores indicated that surgical patients do not seem to experience increased pain or reduced physical function. In addition, stopping opioid use for COUs did not seem to affect the quality of recovery, pain, or physical function. Future prospective controlled studies of TPS are needed to confirm these findings and identify which aspects of TPS are most effective in improving functional recovery of patients. Also, more evidence is needed to determine the appropriateness or need for opioids in acute postsurgical pain.
TPS has expanded to include all surgical specialties. Given the high burden and limited resources, we have chosen to focus on patients at higher risk for chronic postsurgical pain by type of surgery (eg, thoracotomy, open abdominal, limb amputation, major joint surgery) and/or history of substance abuse or chronic opioid use. To better direct scarce resources where it would be of most benefit, we are now enrolling only NOUs without other risk factors postoperatively if they request a refill of opioids or are otherwise struggling with pain control after surgery. Whether this approach affects the success we had in the first year in preventing new COUs after surgery remains to be seen.
It is unlikely that any single model of a perioperative surgical home will fit the needs of the many different types of medical systems that exist. The TPS model fits well in large hospital systems, like the VA, where patients receive most of their care within the same system. However, it seems to us that the optimal TPS program in any health system will provide education, support, and care coordination beginning preoperatively to prepare the patient for surgery and then to facilitate care coordination to transition patients back to their PCPs or on to specialized chronic care.
Acknowledgments
We would like to acknowledge the contributions of Candice Harmon, RN; David Merrill, RN; Amy Beckstead, RN, who have provided invaluable assistance with establishing the TPS program at the VA Salt Lake City and helping with the evaluation process.
Funding for the implementation and evaluation of the TPS was received from the VA Whole Health Initiative, the VA Center of Innovation, the VA Office of Rural Health, and National Institutes of Health Grant UL1TR002538.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
1. Ilfeld BM, Madison SJ, Suresh PJ. Persistent postmastectomy pain and pain-related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1-year follow-up assessment of a randomized, triple-masked, placebo-controlled study. Ann Surg Oncol. 2015;22(6):2017-2025. doi:10.1245/s10434-014-4248-7
2. Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain. Anesthesiology. 2018;129(3):590-607. doi:10.1097/aln.0000000000002238
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537-1546. doi:10.1016/s0140-6736(19)30352-6
4. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surgery. 2017;152(6):e170504-e170504. doi:10.1001/jamasurg.2017.0504
5. Swenson CW, Kamdar NS, Seiler K, Morgan DM, Lin P, As-Sanie S. Definition development and prevalence of new persistent opioid use following hysterectomy. Am J Obstet Gynecol. 2018;219(5):486.e1-486.e7. doi:10.1016/j.ajog.2018.06.010
6. Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - a retrospective cohort study. Drug Alcohol Depend. 2018;187:61-65. doi:10.1016/j.drugalcdep.2018.02.013
7. Bedard N, DeMik D, Dowdle S, Callaghan J. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi:10.1302/0301-620x.100b1.bjj-2017-0547.r1
8. Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, Seyler T. Trends in opioid utilization before and after total knee arthroplasty. J Arthroplasty. 2018;33(7S):S147-S153.e1. doi:10.1016/j.arth.2017.10.060
9. Mariano ER, Walters TL, Kim ET, Kain ZN. Why the perioperative surgical home makes sense for Veterans Affairs health care. Anesth Analg. 2015;120(5):1163-1166. doi:10.1213/ane.0000000000000712
10. Walters TL, Howard SK, Kou A, et al. Design and implementation of a perioperative surgical home at a Veterans Affairs hospital. Semin Cardiothorac Vasc Anesth. 2016;20(2):133-140. doi:10.1177/1089253215607066
11. Walters TL, Mariano ER, Clark DJ. Perioperative surgical home and the integral role of pain medicine. Pain Med. 2015;16(9):1666-1672. doi:10.1111/pme.12796
12. Vetter TR, Kain ZN. Role of the perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. doi:10.1213/ane.0000000000002280
13. Shafer SL. Anesthesia & Analgesia’s 2015 collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966-967. doi:10.1213/ane.0000000000000696
14. Wenzel JT, Schwenk ES, Baratta JL, Viscusi ER. Managing opioid-tolerant patients in the perioperative surgical home. Anesthesiol Clin. 2016;34(2):287-301. doi:10.1016/j.anclin.2016.01.005
15. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/jpr.s91924
16. Tiippana E, Hamunen K, Heiskanen T, Nieminen T, Kalso E, Kontinen VK. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain. 2016;12(1):19-24. doi:10.1016/j.sjpain.2016.02.008
17. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49-58. doi:10.1080/24740527.2019.1574537
18. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
19. HealthMeasures. Intro to PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed September 28, 2020.
Children’s opioid harms vary by race, location
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.
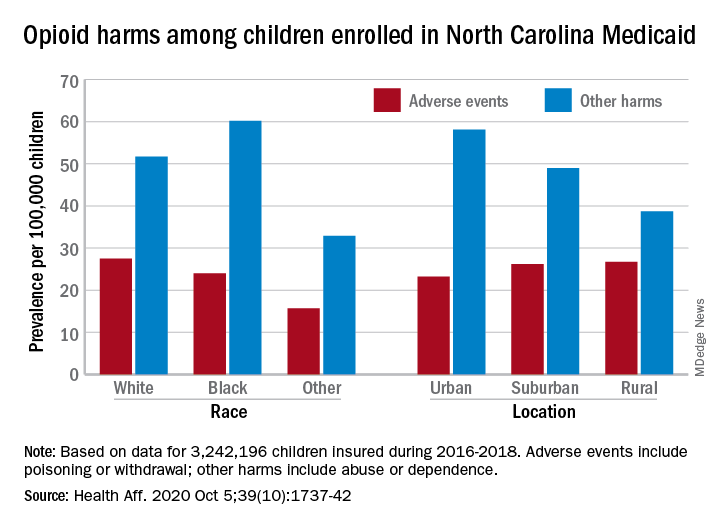
Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
or dependence, compared with their White or rural/suburban counterparts, according to a study of 3.2 million Medicaid-enrolled children in North Carolina.

Analysis of the almost 138,000 prescription fills also showed that Black and urban children in North Carolina were less likely to fill a opioid prescription, suggesting a need “for future studies to explore racial and geographic opioid-related inequities in children,” Kelby W. Brown, MA, and associates at Duke University, Durham, N.C., said Oct. 5 in Health Affairs.
In 2016-2018, the prevalence of opioid-related adverse events, such as poisoning or withdrawal, was 24.0 per 100,000 children among Blacks aged 1-17 years, compared with 27.5 per 100,000 for whites. For other opioid-related harms such as abuse or dependence, the order was reversed: 60.2 for Blacks and 51.7 for Whites, the investigators reported. Children of all other races were lowest in both measures.
Geography also appears to play a part. The children in urban areas had the lowest rate of adverse events – 23.2 per 100,000 vs. 26.2 (suburban) and 26.7 (rural) – and the highest rate of other opioid-related harms – 58.1 vs. 49.0 (suburban) and 38.7 (rural), the Medicaid claims data showed.
Analysis of prescription fills revealed that black children aged 1-17 years had a significantly lower rate (2.7%) than Whites (3.1%) or those of other races (3.0%) and that urban children were significantly less likely to fill a prescription (2.7%) for opioids than the other two groups (suburban, 3.1%; rural, 3.4%), Mr. Brown and associates said.
The prescription data also showed that 48.4% of children aged 6-17 years who had an adverse event had filled a prescription for an opioid in the previous 6 months, compared with just 9.4% of those with other opioid-related harms. The median length of time since the last fill? Three days for children with an adverse event and 67 days for those with other harms, they said.
And those prescriptions, it turns out, were not coming just from the physicians of North Carolina. Physicians, with 35.5% of the prescription load, were the main source, but 33.3% of opioid fills in 2016-2018 came from dentists, and another 17.7% were written by advanced practice providers. Among physicians, the leading opioid-prescribing specialists were surgeons, with 17.3% of the total, the investigators reported.
“The distinct and separate groups of clinicians who prescribe opioids to children suggest the need for pediatric opioid prescribing guidelines, particularly for postprocedural pain,” Mr. Brown and associates wrote.
SOURCE: Brown KW et al. Health Aff. 2020;39(10):1737-42.
FROM HEALTH AFFAIRS
Music’s charms may soothe heart failure’s effects
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.
Music listening and singing each showed early, promising evidence for producing cardiovascular benefits, part of a burgeoning area of research that is exploring and documenting ways to effectively use music to improve health.
A study run at four centers in Italy randomized 159 patients with heart failure, primarily New York Heart Association class I or II disease, to either a daily regimen of at least 30 minutes spent listening to music daily or to a control group that received usual care with no music prescription. After 3 months, the 82 patients in the daily music-listening group had a statistically significant improvement in their Minnesota Living with Heart Failure Questionnaire scores, compared with 77 controls for the study’s primary outcome measure. The results also showed significant benefits, compared with placebo, for other, secondary efficacy measures including improvements in anxiety, depression, sleep quality, and cognition.
Although the results are considered preliminary, they drew significant attention when published in July 2020 (J Card Fail. 2020 Jul 1;26[7]:541-9), where it was accompanied by two editorials in the same issue as well as an editor’s statement. All these commentators as well as other experts interested in music as medicine gathered to further discuss the topic during a panel session at the virtual annual meeting of the Heart Failure Society of America.
Music as a calming influence
The source of the primary benefits seen in this Italian study likely involved “emotional, psychological, and relaxation,” suggested Jerome L. Fleg, MD, program officer for clinical cardiovascular disease at the National Heart, Lung, and Blood Institute in Bethesda, Md. Researchers had used calming potential as a major criterion when selecting the 80 classical pieces that the heart failure patients in the intervention arm of the study could shuffle on their play lists.
“The tempo/rhythm was set up in a range between 60 and 80 beats per minute, because this range mirrors the human heart rate and facilitates relaxation,” the investigators said in their published report. Unfortunately, noted Dr. Fleg, the study lacked physiologic and biomarker measurements that could have provided objective evidence of effects from music. And the study failed to include a control arm of patients instructed to spend 30 minutes a day resting and relaxing without instruction to listen to music, he noted.
Dr. Fleg had authored one of the July editorials, where he said “It is hoped that findings from these studies and others can expand the scientific evidence for music-based interventions and bring these therapies into clinical practice. The current study from Burrai et al. is a positive step in this direction for patients with heart failure.” (J Card Fail. 2020 Jul 1;26[7]: 550-1). What’s needed now, he added during the virtual session, are “more objective data” to better and more comprehensively document the benefits from a music-based intervention in patients with heart failure.
An add-on to standard care
The findings in heart failure patients follows a growing literature that’s shown music can generate a restful state by doing things like activating autonomic parasympathetic outflow while dampening sympathetic outflow. This produces moderation in mood and emotion as well as depressed heart rate, lowered blood pressure, and slowed respiration, commented Emmeline Edwards, PhD director of the division of extramural research of the National Center for Complementary and Integrative Health in Bethesda, Md. Music also seems able to stimulate higher-order brain regions that can result in reduced psychological stress, anxiety, and depression.
“It’s a promising protective intervention to add to standard care for cardiac patients,” Dr. Edwards said during the virtual session. “Music is part of the toolbox for managing symptoms and improving health and well-being.”
“Music is not a substitute for standard therapy, but could add to it,” declared Dr. Fleg.
The already-established intervention known as music therapy has identified music’s ability to modulate breathing as an important mediator of music’s effect.
“Breathing is one of the few physiological processes that can be voluntarily controlled making it a viable target for intervention,” noted opera soprano Renée Fleming and Sheri L. Robb, PhD, in the second editorial that accompanied the Italian heart failure report (J Card Fail. 2020 Jul 1;26[7]:552-4). The music-listening intervention “may have had more effect if they had used compositional features [of the music] to teach patients how to structure their breathing,” said Dr. Robb, a music therapist at Indiana University–Purdue University Indianapolis, during the virtual session.
Another variable to consider is the type of music. “What is the emotional response to the music, and how does that affect heart rate,” wondered Dr. Robb, a professor at the Indiana University School of Nursing in Indianapolis.
Music as exercise
The division that Dr. Edwards directs recently funded a pilot study that assessed the feasibility of using music to stimulate activity and improve breathing another way, by repurposing singing as a novel form of rehabilitative exercise.
The pilot study enrolled patients with coronary disease into a randomized study that tested whether a 14-minute session of supervised singing could produce acute improvement in vascular function, “a biomarker for the risk of future cardiovascular disease events,” explained Jacqueline P. Kulinski, MD, a preventive cardiologist at the Medical College of Wisconsin in Milwaukee. Dr. Kulinski did not report details of her yet-unpublished study, but said that her initial findings held promise for developing musical activities such as singing as a novel way to stimulate therapeutic physical activity in patients with heart disease.
“It’s exciting to see this signal” of benefit. “I envision music therapy as a part of cardiac rehabilitation, or an alternative for patients who can’t participate in traditional rehab,” Dr. Kulinski said during the virtual session. “I think of singing as a physical activity, as exercise, and using this exercise as medicine.”
Harmonizing with the NIH
“Singing is like swimming: You need to hold your breath,” agreed Ms. Fleming, who participated on the virtual panel and has spearheaded a collaboration between the National Institutes of Health and the Kennedy Center for the Performing Arts, the Sound Health Initiative, that’s coordinating research into the connections between music and health. Ms. Fleming helped launch the Sound Health Initiative in 2017 by coauthoring a JAMA article with the NIH director that spelled out the rationale and goals of the project (JAMA. 2017 Jun 27;317[24]:2470-1), and by launching a lecture tour on the topic in a presentation she calls Music and the Mind.
Ms. Fleming has given her talk in more than 30 locations worldwide, and she’s found that “audiences love” the combination of neuroscience and music that her talks cover, she said. Her lectures highlight that, in addition to cardiovascular disease, the potential for music therapy and related interventions has been shown in patients with disorders that include autism, psychosis, pain, Parkinson’s disease, Alzheimer’s disease, and epilepsy.
The research highlighted in the session “opens new doors to prevention and treatment strategies using music for patients with heart failure and cardiovascular disease,” summed up Biykem Bozkurt, MD, professor of medicine at the Baylor College of Medicine in Houston and president of the Heart Failure Society of America, who helped organize the virtual session.
Dr. Fleg, Dr. Edwards, Dr. Robb, Dr Kulinski, Ms. Fleming, and Dr. Bozkurt had no relevant financial disclosures.
‘Overwhelming evidence’ FDA’s opioid approval process is shoddy
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
Despite the ongoing epidemic of misuse, overuse, and diversion of opioids, the Food and Drug Administration has set a low bar for approval of these medications over the past 20 years, new research suggests.
The study results also show that the FDA did not require manufacturers to collect safety data on tolerance, withdrawal, overdose, misuse, and diversion in any rigorous fashion.
In addition, during the study period, 17 of the 39 new drug applications (NDAs) (only one was an innovator product, known as a new molecular entity) for chronic pain were approved with an “enriched enrollment randomized withdrawal” (EERW) trial design. Such a design, in this case, allowed manufacturers to exclude 32%-43% of the initially enrolled patients from the double-blind treatment phase.
“The question for regulators, policy makers, and others is: How did we get to a point where these approvals took place based on trials that were by design unlikely to yield some of the most important information about safety and efficacy that patients and clinicians would care about?” study investigator G. Caleb Alexander, MD, Johns Hopkins University, Baltimore, said in an interview.
The study was published online Sept. 29 in the Annals of Internal Medicine.
‘Cooking the books’
Little is known about the evidence required by the FDA for new approvals of opioid analgesics.
To characterize the quality of safety and efficacy data in NDAs for opioid analgesics approved by the FDA between 1997 and 2018, the investigators conducted the cross-sectional analysis using data from ClinicalTrials.gov, FDA reviews, and peer-reviewed publications regarding phase 3 pivotal trials.
The investigators examined the key characteristics of each NDA, including the number, size, and duration of pivotal trials, trial control groups, use of EERW, and systematically measured safety outcomes.
Results showed that most of the 48 NDAs evaluated were for new dosage forms (52.1%) or new formulations (18.8%). Only one (2.1%) was for a new molecular entity.
Of 39 NDAs approved for the treatment of chronic pain, only 21 products were supported by at least one pivotal trial. The mean duration of these 28 trials was 84 days, and they enrolled a median of 299 patients.
Results showed that, for 17 of the 39 opioids approved for chronic pain, pivotal trials had an EERW design. For the latest period – 2012-2018 – trials of all eight of the approved opioids used the EERW method.
This EERW design allows the manufacturer to assess efficacy “among a subset of patients most likely to respond and least likely to have adverse effects, reducing generalizability to real-world settings,” the investigators noted.
They called on the FDA to stop relying on this type of trial to assess opioid efficacy.
In an August 2020 article, Andrew Kolodny, MD, pointed out the pitfalls of the EERW approach. In such a study, all participants are made physiologically dependent on the opioid in a 4- to 6-week open-label phase. Only those who tolerate the drug and find it helpful are included in the randomized study. Dr. Kolodny is codirector of opioid policy research at Brandeis University, Waltham, Mass.
“Critics of EERW have correctly described this methodology as ‘cooking the books,’ ” Dr. Kolodny writes.
He noted that the agency’s decision to rely on EERW trials for opioids was “based on discussions at private meetings between FDA officials and pharmaceutical company executives hosted by an organization called Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.” The 2013 meetings were reported in an article published in the Washington Post.
Little sign of change
Among NDAs for chronic pain, the investigators found that eight (20.5%) included pooled safety reviews that reported systematic assessment of diversion. Seven (17.9%) reported systematic measurement of nonmedical use, and 15 (38.5%) assessed incident tolerance.
The study revealed that eight of nine products that were approved for acute pain were supported by at least one pivotal trial. The median duration of these 19 trials was 1 day, and they enrolled a median of 329 patients.
The investigators noted that the findings “underscore the evidence gaps that have limited clinicians’ and patients’ understanding and appreciation of the inherent risks of prescription opioid analgesics.”
Dr. Alexander, who has been an FDA advisory committee chairman and currently serves as a consultant to plaintiffs who are suing opioid manufacturers in federal multidistrict litigation, said the study “is a story about missed opportunities to improve the safety and to improve the regulatory review of these products.”
Coinvestigator Peter Lurie, MD, who was an official at the FDA from 2009 to 2017, said that “there’s not a lot of signs that things are changing” at the agency.
The study shows that the FDA has “accepted what the companies have been presenting,” said Dr. Lurie, who is president of the Center for Science in the Public Interest.
The FDA “absolutely has the authority” to require manufacturers to undertake more rigorous trials, but agency culture keeps it from making such demands, especially if doing so means a new applicant might have to conduct trials that weren’t previously required, Dr. Lurie said in an interview.
“FDA is pretty rigorous about trying to establish a level playing field. That’s a virtuous thing, but it becomes problematic when that prevents change,” said Dr. Lurie.
The most recent FDA guidance to manufacturers, issued in 2019, does not provide advice on criteria for endpoints, study duration, or which populations are most likely to benefit from opioid treatment. The agency also does not require drug manufacturers to formally collect data on safety, tolerance, overdose symptoms, or constipation.
The guidance does suggest that the agency would likely take into account public health considerations when evaluating opioids, such as the risk to the overall population for overdose and diversion.
‘Overwhelming evidence’
Dr. Kolodny said that, as far as he is aware, “this is the first scientific publication in a peer-reviewed journal demonstrating clearly the problems with FDA’s opioid approval process.”
The article offers “overwhelming evidence that they are improperly approving the most dangerous medications – medications that killed more people than any other medication on the market,” added Dr. Kolodny, who is also president of Physicians for Responsible Opioid Prescribing.
Asked to respond to the study findings, FDA spokesperson Charles Kohler said the agency “does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
A version of this article originally appeared on Medscape.com.
Recurrent leg lesions
Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.

Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
FDA orders stronger warnings on benzodiazepines
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration wants updated boxed warnings on benzodiazepines to reflect the “serious” risks of abuse, misuse, addiction, physical dependence, and withdrawal reactions associated with these medications.
“The current prescribing information for benzodiazepines does not provide adequate warnings about these serious risks and harms associated with these medicines so they may be prescribed and used inappropriately,” the FDA said in a safety communication.
The FDA also wants revisions to the patient medication guides for benzodiazepines to help educate patients and caregivers about these risks.
“While benzodiazepines are important therapies for many Americans, they are also commonly abused and misused, often together with opioid pain relievers and other medicines, alcohol, and illicit drugs,” FDA Commissioner Stephen M. Hahn, MD, said in a statement.
“We are taking measures and requiring new labeling information to help health care professionals and patients better understand that, while benzodiazepines have many treatment benefits, they also carry with them an increased risk of abuse, misuse, addiction, and dependence,” said Dr. Hahn.
Ninety-two million prescriptions in 2019
Benzodiazepines are widely used to treat anxiety, insomnia, seizures, and other conditions, often for extended periods of time.
According to the FDA, in 2019, an estimated 92 million benzodiazepine prescriptions were dispensed from U.S. outpatient pharmacies, most commonly alprazolam, clonazepam, and lorazepam.
Data from 2018 show that roughly 5.4 million people in the United States 12 years and older abused or misused benzodiazepines in the previous year.
Although the precise risk of benzodiazepine addiction remains unclear, population data “clearly indicate that both primary benzodiazepine use disorders and polysubstance addiction involving benzodiazepines do occur,” the FDA said.
Data from the National Survey on Drug Use and Health from 2015-2016 suggest that half million community-dwelling U.S. adults were estimated to have a benzodiazepine use disorder.
Jump in overdose deaths
Overdose deaths involving benzodiazepines jumped from 1,298 in 2010 to 11,537 in 2017 – an increase of more 780%. Most of these deaths involved benzodiazepines taken with prescription opioids.
the FDA said.
The agency urged particular caution when prescribing benzodiazepines with opioids and other central nervous system depressants, which has resulted in serious adverse events including severe respiratory depression and death.
The FDA also says patients and caregivers should be warned about the risks of abuse, misuse, addiction, dependence, and withdrawal with benzodiazepines and the associated signs and symptoms.
Physicians are encouraged to report adverse events involving benzodiazepines or other medicines to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
Health Care Disparities Among Adolescents and Adults With Sickle Cell Disease: A Community-Based Needs Assessment to Inform Intervention Strategies
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23
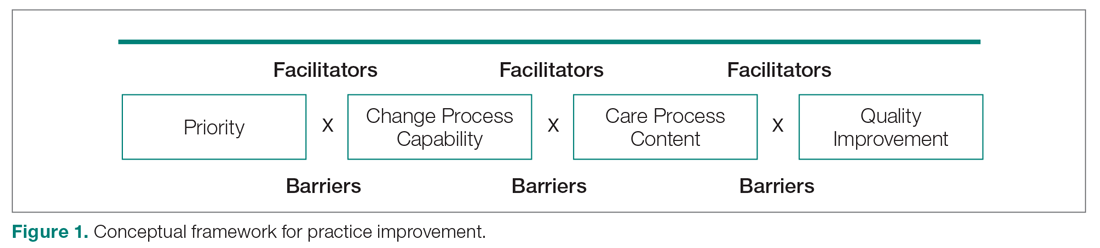
The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.
Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.
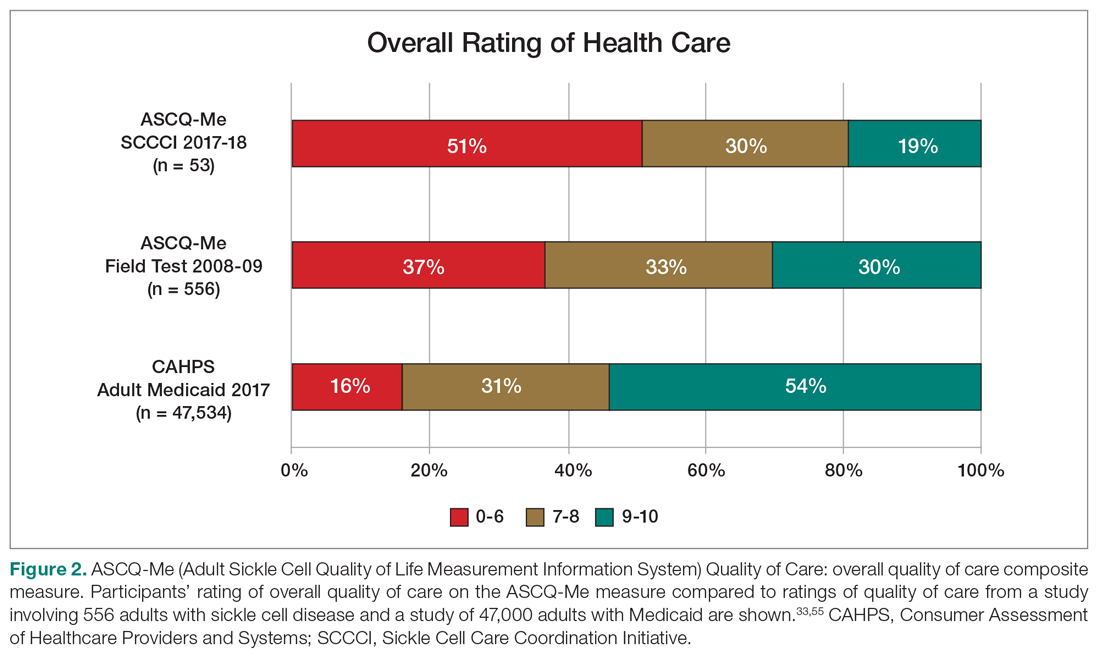
Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”
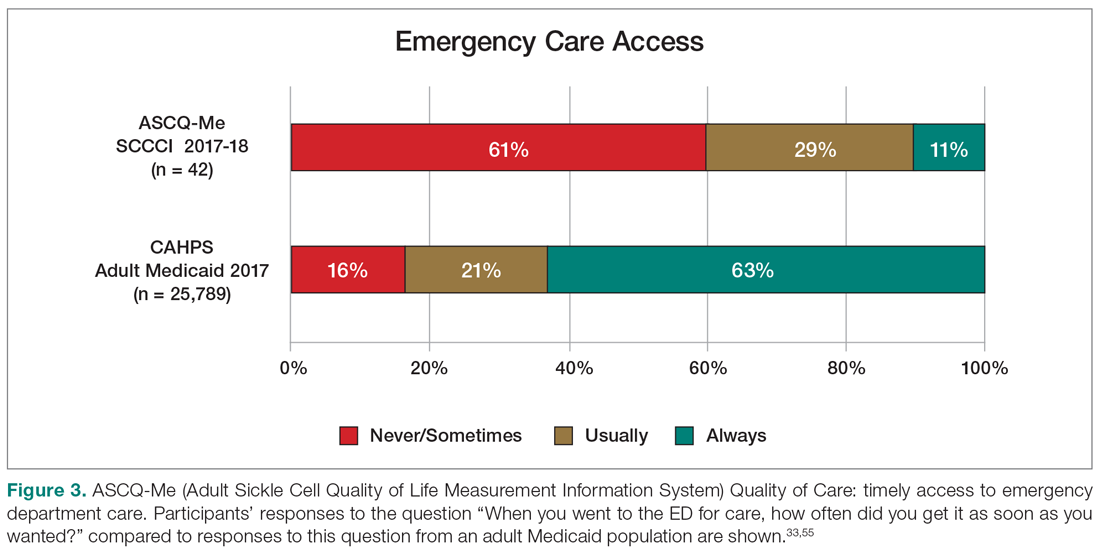
On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.
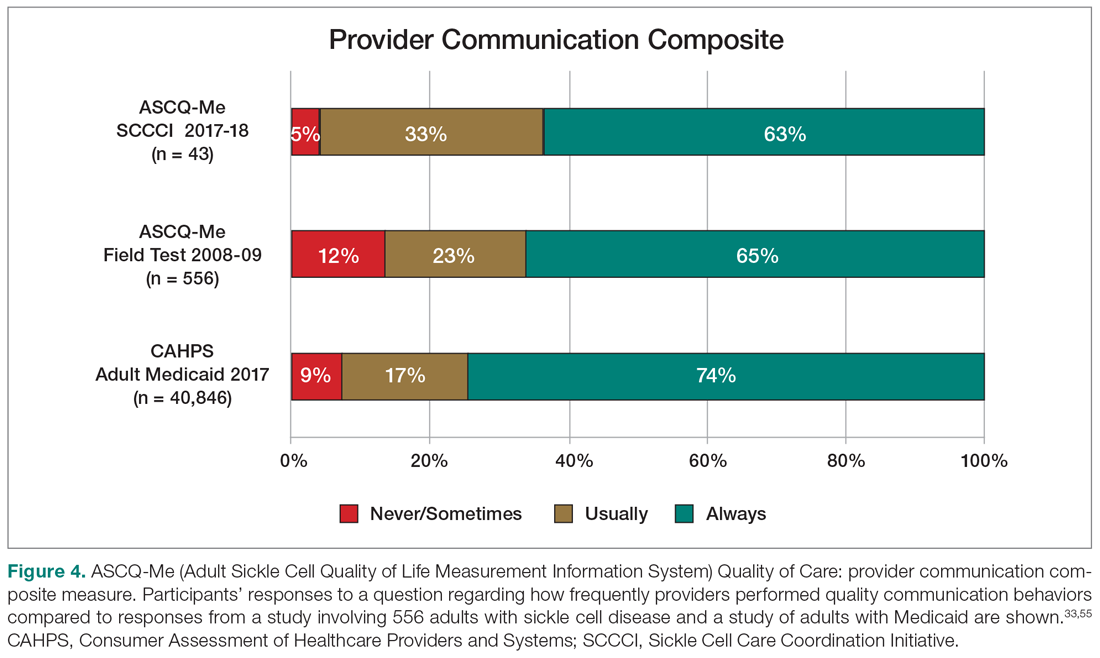
Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).
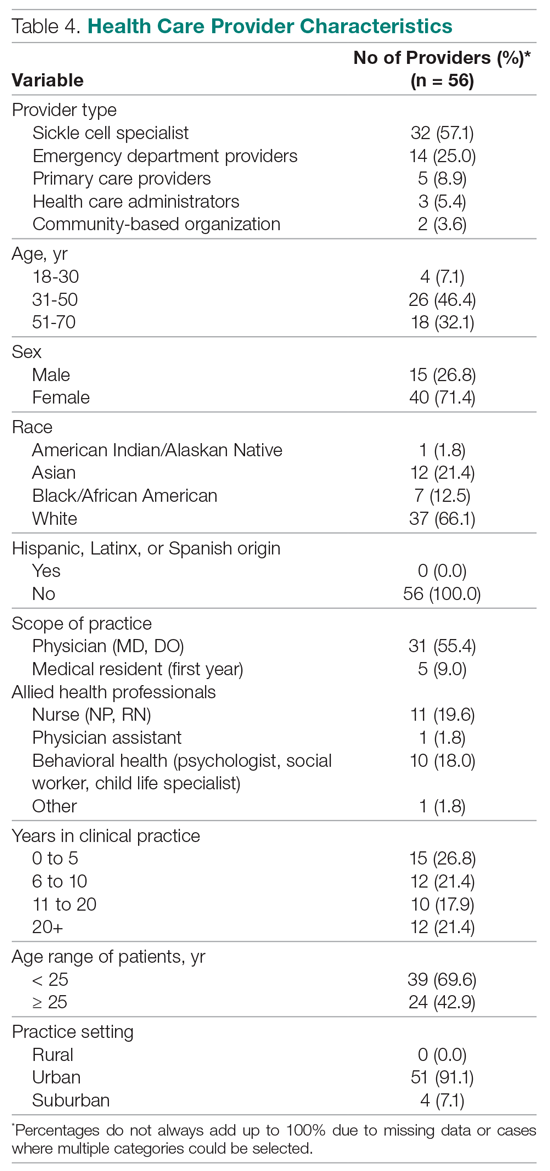
Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23

The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.

Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.

Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”

On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.

Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).

Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23

The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.

Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.

Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”

On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.

Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).

Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
New acute pain guidelines from the ACP and AAFP have limitations
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.
The American College of Physicians and the American Academy of Family Physicians recently authored a guideline regarding the treatment of acute, non–low back, musculoskeletal injuries in adults in the outpatient setting.
According to the authors, musculoskeletal injuries result in more than 65 million medical visits a year with an annual estimated cost of $176.1 billion in 2010.
In summary, the guideline, which was published in the Annals of Internal Medicine, is based on a review of the best available evidence. The research reviewed by the guideline authors showed favorable results with topical NSAIDs, oral NSAIDs, oral acetaminophen, acupressure, and transcutaneous electrical nerve stimulation in reducing pain and/or improving function. The guideline authors “recommend that clinicians treat patients with acute pain from non–low back, musculoskeletal injuries with topical [NSAIDs] with or without gel as first-line therapy to reduce or relieve symptoms, including pain; improve physical function; and improve the patient’s treatment satisfaction (Grade: strong recommendation; moderate-certainty evidence).” Additionally, the guideline recommends against treating acute pain from non–low back, musculoskeletal injuries with opioids, including tramadol (Grade: conditional recommendation; low-certainty evidence).
The guideline also mentions improving function in relation to decreasing pain, which can be multifactorial.
Treating pain requires a multipronged approach. Many patients require more than one therapy to treat their pain, such as NSAIDs plus physical therapy. The ACP and AAFP did not make any recommendations for combination therapies in this guideline.
When physical therapy is needed
Nonopioid pain medications can do a great job of reducing a patient’s physical discomfort, which the evidence for these guideline demonstrates. However, much of the dysfunction caused by musculoskeletal injuries will not improve by reducing the pain alone. Physical therapy, exercise, and mobilization did not show a significant benefit in reducing symptoms in the systematic review and meta-analysis of randomized trials that appeared alongside the guideline. The type of pain, however, was not evaluated in relation to the effectiveness of these treatments. A fractured bone, for example, may heal just fine with casting and pain management, without the need for additional therapies. However, the muscles surrounding that bone can atrophy and become weak from not being used. Physical therapy may be needed to restrengthen those muscles. Therefore, a multifaceted approach is often needed, even for uncomplicated conditions.
Mental pain often comes with physical pain, and this is an aspect of care that is often neglected. It can be quite devastating for patients to not be able to do the things they were previously able to do. While this is easily recognized in professional athletes when they can no longer play, it is not so readily apparent with a mother who is just trying to take care of her kids. As doctors, especially those of us in family medicine, we should be addressing more than just physical pain.
Patients can also do activities that exacerbate their pain. As doctors, we need to be asking questions that help us determine whether a patient’s pain is caused by a particular action. Maybe that increase in shoulder pain is due to nothing more than lifting something heavy rather than a failure in a prescribed medication. Pain diaries are helpful, and clinicians don’t use them often enough.
How pain affects mental health
Acute injuries can also lead to disability. Many patients become quite distressed about being unable to work. They often need Famiy & Medical Leave Act forms filled out, and this task usually falls to the primary care doctor. In addition to assessing the pain, we need to be evaluating, at each visit, a patient’s level of functioning and their ability to do their job.
Every patient responds to pain differently, and it is important to evaluate patients’ mindsets regarding theirs. A patient may be in severe pain and may try to ignore it for a variety of reasons. A patient may “catastrophize” their pain, believing only the worst outcome will happen to them. Helping patients set appropriate expectations and having a positive mindset can help.
Overall, the new recommendations are a great tool as a guideline, but they are not complete enough to be the only ones used in managing acute, non–low back, musculoskeletal pain in adults.
They are very important for clinicians who may be prescribing opioid medications for patients with this type of pain. Amid an opioid crisis, it is the responsibility of every doctor to prescribe these medications appropriately. The evidence clearly shows they provide little benefit and place patients at risk of addiction.
We should all be following these recommendations as the baseline of care for acute pain. However, we need to delve deeper and manage all the components involved. We would be ignoring very real suffering in our patients if we limited our focus to only the physical discomfort.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Rutgers RWJ Medical School.
SOURCE: Ann Intern Med. 2020 Aug 18. doi: 10.7326/M19-3602.
Reworked OxyContin fails to cut overall opioid abuse, FDA panel says
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
The long-awaited postmarketing studies of the abuse-deterrent formulation (ADF) of OxyContin (Perdue Pharma) received mixed reviews from a Food and Drug Administration joint advisory committee.
After a 2-day discussion of new research submitted by Perdue, as well as other relevant published data, most members of the Drug Safety and Risk Management and Anesthetic and Analgesic Drug Products advisory committees came to the conclusion that the reformulated drug “meaningfully” reduced abuse via intranasal administration and intravenous injection, but not overall opioid abuse or overdose.
The reformulated OxyContin “was the first out of the gate,” and “has the greatest market penetration of any ADF” so “it gives us the greatest opportunity to measure change before and after reformulation,” said committee member Traci C. Green, PhD, MSc, professor and director of the Opioid Policy Research Collaborative at Brandeis University, Waltham, Mass.
The FDA approved the original formulation of OxyContin (oxycodone hydrochloride), a mu-receptor opioid agonist, in December 1995 for the management of pain requiring daily round-the-clock opioid treatment in cases where other treatments were inadequate. It approved an ADF version of the product in April 2010.
The updated formulation incorporates polyethylene oxide, an inactive polymer that makes the tablet harder and more crush resistant. The tablet turns into a gel or glue-like substance when wet.
At the request of the FDA, the company carried out four postmarketing studies, which the FDA also reviewed.
- A National Addictions Vigilance Intervention and Prevention Program study that included 66,897 assessments in patients undergoing evaluation for substance use or entering an opioid addiction program. Results showed a drop in up to 52% of self-reported past 30-day OxyContin injection and snorting versus comparators, including extended-release morphine and immediate-release hydrocodone.
- An analysis of 308,465 calls to U.S. poison centers showing a reduction of up to 28% for calls regarding intentional OxyContin-related exposures immediately following the drug’s reformulation. However, the FDA analysis concluded it is unclear whether the decline was attributable to the drug’s reformulation or co-occurring trends.
- A study of 63,528 individuals entering methadone clinics or treatment programs that showed a reduction of up to 27% in OxyContin abuse versus comparators. There was no information on route of abuse. Here, the FDA analysis determined the results were mixed and didn’t provide compelling evidence.
- A claims-based analysis of patients who were dispensed an opioid (297,836 OxyContin; 659,673 a comparator) that showed no evidence that the updated product affected the rate of fatal and nonfatal opioid overdoses.
During the meeting, committee members heard that opioid use in the United States peaked in 2012, with 260 million prescriptions dispensed, then declined by 41% by 2019. ADFs accounted for only 2% of prescriptions in 2019. They also heard that results of a wide variety of studies and surveys support the conclusion that misuse, abuse, and diversion of OxyContin decreased after it was reformulated.
Ultimately, the joint committee voted 20 to 7 (with 1 abstention) that the reformulated drug reduced nonoral abuse. Most members who voted in favor cited the NAVIPPRO study as a reason for their decision, but few found the strength of the evidence better than moderate.
Meeting chair Sonia Hernandez-Diaz, MD, professor of epidemiology, Harvard School of Public Health, Boston, noted the reduction in abuse may, in part, be a result of the overall reduction in opioid use.
Jon E. Zibbell, PhD, senior public health scientist, behavioral health research division, RTI International, Atlanta, who voted “no,” was disappointed there was not more data.
“We had a bunch of years for this and so many of us could have done some amazing studies” related to how abuse changed post reformulation, he said.
As for overall abuse deterrence, the committee believed the evidence was less compelling. Only two members voted that the reformulated version of the drug reduced overall abuse and only one member voted that the reformulated tablets reduced opioid overdose.
Members generally agreed that all of the studies had limitations, including retrospective designs, confounding, and potential misclassifications. Many noted the challenge of assessing abuse pre- and post reformulation given the evolving situation.
For instance, at the time the reformulated drug was launched, public health initiatives targeting opioid abuse were introduced, more treatment centers were opening, and there was a crackdown on “pill-mill” doctors.
In addition, prescribing and consumption habits were changing. Some doctors may have switched only “at-risk” patients to the reformulated opioid and there may have been “self-selection” among patients – with some potentially opting for another drug such as immediate-release oxycodone.
During the meeting, there was discussion about how to interpret a “meaningful” abuse reduction. However, there was no consensus of a percentage the reduction had to reach in order to be deemed meaningful.
Another issue discussed was the term “abuse deterrent,” which some members believed was stigmatizing and should be changed to crush resistant.
There was also concern that prescribers might consider the ADF a “safe” or less addictive opioid. Michael Sprintz, DO, clinical assistant professor, division of geriatric and palliative medicine, University of Texas Health Science Center, Houston, said ADFs might provide physicians with “a false sense of security.”
Dr. Sprintz, also founder of the Sprintz Center for Pain and Recovery, noted the importance of pain medicine physicians understanding addiction and addiction specialists understanding pain management.
Other committee members voiced concern that the reformulation results in patients switching from intravenous and intranasal abuse to oral abuse. Committed abusers can still swallow multiple pills.
Some members noted that reformulated OxyContin coincided with increased transition to heroin, which is relatively cheap and readily available. However, they recognized that proving causality is difficult.
The committee was reminded that the reformulated drug provides a significant barrier against, but doesn’t altogether eliminate, opioid abuse. With hot water and the right tools, the tablets can still be manipulated.
In addition, the reformulated drug will not solve the U.S. opioid epidemic, which requires a multifaceted approach. The opioid crisis, said Wilson Compton, MD, deputy director at the National Institute on Drug Abuse, has resulted in a “skyrocketing” of deaths linked to “tremendously potent” forms of fentanyl, emerging stimulant use issues, and the possible increase in drug overdoses linked to COVID-19.
A version of this article originally appeared on Medscape.com.
4-year-old girl • limited movement & diffuse pain in both arms • pronated hands • Dx?
THE CASE
A 4-year-old girl was triaged to the Pediatric Emergency Department (PED) Fast Track, complaining of pain and limited movement in both arms. For an unknown reason, she had attempted to lift a heavy, 3-person sofa several hours earlier.
Her prior medical history included left nursemaid elbow (NME) at both 15 months and 33 months of age. Neither event had a known mechanism of injury. In both episodes, it was noted in the medical record that the child was not using her arm, “was holding it funny,” and was complaining of pain. Each time, she presented about 24 hours after symptom onset.
During the physical exam in the PED, the patient showed no signs of acute distress. She held both arms close to her body, with a slight flexion at the elbows, and her hands were pronated. She could not pinpoint the location of her discomfort and described diffuse pain in her forearms, elbows, and upper arms. Examination revealed no localized pain or tenderness in her hands, wrists, or clavicles. Radial pulses were easily palpated, and capillary refill was less than 2 seconds. There was no swelling or bruising. The rest of her physical exam was normal.
DIAGNOSIS
The patient was given a diagnosis of self-inflicted bilateral
DISCUSSION
BNME is an uncommon diagnosis; a literature review of reported cases indicates none were self-inflicted.1-4 However, NME is a common injury and is easily reduced. The classic mechanism of injury for NME involves the elbow in extension, while the forearm is pronated, and a sudden brisk axial traction is applied. This combination of motions causes the annular ligament to slip over the head of the radius and become displaced downward into the radiohumeral joint, where it becomes entrapped. In this case, the patient apparently exerted enough longitudinal traction while trying to lift the couch to produce the injury.
NME occurs most commonly in the left arm of girls between the ages of 4 months and 7 years and peaks at around the age of 2 years.5 A 2014 study by Irie et al6 corroborated the findings on left-side predominance and increased incidence with age, noting that frequency of injury peaked at 6 months in those younger than 1 year of age and at 2 years for those 1 year or older. However, the researchers found no significant sex difference.6
NME is radiographically indistinguishable from a healthy elbow.7 To prevent unnecessary expense and radiation exposure in young children, prereduction radiographs should only be used to rule out the possibility of fracture or other injury.7 Krul et al8 recommend restricting x-ray use to cases with an unclear history or those that are due to trauma other than an arm pull.
Continue to: Methods of reduction
Methods of reduction. Once NME is diagnosed, there are 2 methods of reduction: hyper-pronation and supination-flexion. Reduction is best performed with the child sitting in the parent’s lap with the injured arm facing the examiner.
Success rates for both methods of NME reduction are statistically similar; however, first-attempt success rates are significantly higher with the hyper-pronation method than with supination-flexion.9 Furthermore, physicians have deemed the hyper-pronation method significantly easier to perform than supination-flexion.9 A Cochrane review by Krul et al10 concluded that the hyper-pronation method may result in lower failure rates than supination-flexion, but due to limited evidence, the researchers were unable to draw any conclusions on other outcomes, such as pain. Green et al11 noted that hyper-pronation is perceived by parents of children with NME as being less painful. For these reasons, hyper-pronation should be utilized as the first method of reduction, followed by supination-flexion if the former does not work.12
Additional management. In a limited study of 50 children with pulled-elbow injuries, ultrasound revealed that 78% had an intact yet interposed radial annular ligament and 22% had a tear in the radial annular ligament.13 The authors propose that if, after appropriate reduction methods are attempted, no pop is felt, or there is no prompt clinical improvement, and ultrasound is not available to assess the integrity of the annular ligament, the child should be placed in a splint for 7 days
Our patient returned to the PED 3 days later, complaining of pain and an inability to move her left arm after her older sibling pulled her by her outstretched arms. She was once again diagnosed with NME, the injury was reduced, and she was using the arm within minutes. She has not presented to either the PED or the pediatric clinic with a similar complaint since. Discarding outliers, NME recurrence rates fall within a range of 23.7% to 32.9%.14,15
THE TAKEAWAY
Pre-reduction x-rays are not warranted in cases of NME unless there is suspicion for fracture or another injury. The 2 reduction methods, hyper-pronation and supination-flexion, are easily mastered. Any reduction should be quick, easy, and as painless as possible. Hyper-pronation should be utilized first, as this maneuver seems to be the more successful and is perceived by parents as being less painful. However, it is always most helpful to be proficient in both methods. If, after appropriate attempts at reduction, the child has not regained the use of the arm, 7 days of splinting is recommended, along with an orthopedic referral.
CORRESPONDENCE
Robert N. Anderson, DNP, APRN, (F)NP-C, ENP-BC, Vanderbilt Health, 512 Autumn Springs Court, Suite 100 C, Franklin, TN 37067; [email protected]
1. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
2. Michaels MG. A case of bilateral nursemaid’s elbow. Pediatr Emerg Care. 1989;5:226-227.
3. Meiner EV, Sama AE, Lee DC, et al. Bilateral nursemaid’s elbow. Am J Emerg Med. 2004;6:502-503.
4. Wang YX, Zhang G, Song B, et al. Radial head subluxation in pediatric clinics and emergency departments in China. Chin J Traum. 2019;22:340-344.
5. Schunk JE. Radial head subluxation: epidemiology and treatment of 87 episodes. Ann Emerg Med. 1990;19:1019-1023.
6. Irie T, Sono T, Hayama Y, et al. Investigation on 2331 cases of pulled elbow over the last 10 years. Pediatr Rep. 2014;6:5090. doi: 10.4081/pr.2014.5090
7. Eismann EA, Cosco ED, Wall EJ. Absence of radiographic abnormalities in nursemaid’s elbows. J Pediatr Orthop. 2014;34:426-431.
8. Krul M, van der Wouden JC, Koes BW, et al. Nursemaid’s Elbow: its diagnostic clues and preferred means of reduction. J Fam Pract. 2010:59:E5-E7.
9. Bek D, Yildiz C, Köse O, et al. Pronation versus supination maneuvers for the reduction of ‘pulled elbow’: a randomized clinical trial. Eur J Emerg Med. 2009;16:135-138.
10. Krul M, van der Wouden JC, Kruithof EJ, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2017.
11. Green DA, Linares MYR, Garcia Peña BM, et al. Randomized comparison of pain perception during radial head subluxation reduction using supination-flexion of forced pronation. Pediatr Emerg Care. 2006;22:235-238.
12. García-Mata S, Hidalgo-Ovejero A. Efficacy of reduction maneuvers for “pulled elbow” in children: a prospective study of 115 cases. J Pediatr Orthop. 2014;34:432-436.
13. Diab HS, Hamed MMS, Allam Y. Obscure pathology of pulled elbow: dynamic high-resolution ultrasound-assisted classification. J Child Orthop. 2010;4:539-543.
14. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
15. Macias CG, Bothner J, Wiebe R. Comparison of supination/flexion to hyperpronation in the reduction of radial head subluxation. Pediatrics. 1998;102:E10. doi: 10.1542/peds.102.1.e10.
THE CASE
A 4-year-old girl was triaged to the Pediatric Emergency Department (PED) Fast Track, complaining of pain and limited movement in both arms. For an unknown reason, she had attempted to lift a heavy, 3-person sofa several hours earlier.
Her prior medical history included left nursemaid elbow (NME) at both 15 months and 33 months of age. Neither event had a known mechanism of injury. In both episodes, it was noted in the medical record that the child was not using her arm, “was holding it funny,” and was complaining of pain. Each time, she presented about 24 hours after symptom onset.
During the physical exam in the PED, the patient showed no signs of acute distress. She held both arms close to her body, with a slight flexion at the elbows, and her hands were pronated. She could not pinpoint the location of her discomfort and described diffuse pain in her forearms, elbows, and upper arms. Examination revealed no localized pain or tenderness in her hands, wrists, or clavicles. Radial pulses were easily palpated, and capillary refill was less than 2 seconds. There was no swelling or bruising. The rest of her physical exam was normal.
DIAGNOSIS
The patient was given a diagnosis of self-inflicted bilateral
DISCUSSION
BNME is an uncommon diagnosis; a literature review of reported cases indicates none were self-inflicted.1-4 However, NME is a common injury and is easily reduced. The classic mechanism of injury for NME involves the elbow in extension, while the forearm is pronated, and a sudden brisk axial traction is applied. This combination of motions causes the annular ligament to slip over the head of the radius and become displaced downward into the radiohumeral joint, where it becomes entrapped. In this case, the patient apparently exerted enough longitudinal traction while trying to lift the couch to produce the injury.
NME occurs most commonly in the left arm of girls between the ages of 4 months and 7 years and peaks at around the age of 2 years.5 A 2014 study by Irie et al6 corroborated the findings on left-side predominance and increased incidence with age, noting that frequency of injury peaked at 6 months in those younger than 1 year of age and at 2 years for those 1 year or older. However, the researchers found no significant sex difference.6
NME is radiographically indistinguishable from a healthy elbow.7 To prevent unnecessary expense and radiation exposure in young children, prereduction radiographs should only be used to rule out the possibility of fracture or other injury.7 Krul et al8 recommend restricting x-ray use to cases with an unclear history or those that are due to trauma other than an arm pull.
Continue to: Methods of reduction
Methods of reduction. Once NME is diagnosed, there are 2 methods of reduction: hyper-pronation and supination-flexion. Reduction is best performed with the child sitting in the parent’s lap with the injured arm facing the examiner.
Success rates for both methods of NME reduction are statistically similar; however, first-attempt success rates are significantly higher with the hyper-pronation method than with supination-flexion.9 Furthermore, physicians have deemed the hyper-pronation method significantly easier to perform than supination-flexion.9 A Cochrane review by Krul et al10 concluded that the hyper-pronation method may result in lower failure rates than supination-flexion, but due to limited evidence, the researchers were unable to draw any conclusions on other outcomes, such as pain. Green et al11 noted that hyper-pronation is perceived by parents of children with NME as being less painful. For these reasons, hyper-pronation should be utilized as the first method of reduction, followed by supination-flexion if the former does not work.12
Additional management. In a limited study of 50 children with pulled-elbow injuries, ultrasound revealed that 78% had an intact yet interposed radial annular ligament and 22% had a tear in the radial annular ligament.13 The authors propose that if, after appropriate reduction methods are attempted, no pop is felt, or there is no prompt clinical improvement, and ultrasound is not available to assess the integrity of the annular ligament, the child should be placed in a splint for 7 days
Our patient returned to the PED 3 days later, complaining of pain and an inability to move her left arm after her older sibling pulled her by her outstretched arms. She was once again diagnosed with NME, the injury was reduced, and she was using the arm within minutes. She has not presented to either the PED or the pediatric clinic with a similar complaint since. Discarding outliers, NME recurrence rates fall within a range of 23.7% to 32.9%.14,15
THE TAKEAWAY
Pre-reduction x-rays are not warranted in cases of NME unless there is suspicion for fracture or another injury. The 2 reduction methods, hyper-pronation and supination-flexion, are easily mastered. Any reduction should be quick, easy, and as painless as possible. Hyper-pronation should be utilized first, as this maneuver seems to be the more successful and is perceived by parents as being less painful. However, it is always most helpful to be proficient in both methods. If, after appropriate attempts at reduction, the child has not regained the use of the arm, 7 days of splinting is recommended, along with an orthopedic referral.
CORRESPONDENCE
Robert N. Anderson, DNP, APRN, (F)NP-C, ENP-BC, Vanderbilt Health, 512 Autumn Springs Court, Suite 100 C, Franklin, TN 37067; [email protected]
THE CASE
A 4-year-old girl was triaged to the Pediatric Emergency Department (PED) Fast Track, complaining of pain and limited movement in both arms. For an unknown reason, she had attempted to lift a heavy, 3-person sofa several hours earlier.
Her prior medical history included left nursemaid elbow (NME) at both 15 months and 33 months of age. Neither event had a known mechanism of injury. In both episodes, it was noted in the medical record that the child was not using her arm, “was holding it funny,” and was complaining of pain. Each time, she presented about 24 hours after symptom onset.
During the physical exam in the PED, the patient showed no signs of acute distress. She held both arms close to her body, with a slight flexion at the elbows, and her hands were pronated. She could not pinpoint the location of her discomfort and described diffuse pain in her forearms, elbows, and upper arms. Examination revealed no localized pain or tenderness in her hands, wrists, or clavicles. Radial pulses were easily palpated, and capillary refill was less than 2 seconds. There was no swelling or bruising. The rest of her physical exam was normal.
DIAGNOSIS
The patient was given a diagnosis of self-inflicted bilateral
DISCUSSION
BNME is an uncommon diagnosis; a literature review of reported cases indicates none were self-inflicted.1-4 However, NME is a common injury and is easily reduced. The classic mechanism of injury for NME involves the elbow in extension, while the forearm is pronated, and a sudden brisk axial traction is applied. This combination of motions causes the annular ligament to slip over the head of the radius and become displaced downward into the radiohumeral joint, where it becomes entrapped. In this case, the patient apparently exerted enough longitudinal traction while trying to lift the couch to produce the injury.
NME occurs most commonly in the left arm of girls between the ages of 4 months and 7 years and peaks at around the age of 2 years.5 A 2014 study by Irie et al6 corroborated the findings on left-side predominance and increased incidence with age, noting that frequency of injury peaked at 6 months in those younger than 1 year of age and at 2 years for those 1 year or older. However, the researchers found no significant sex difference.6
NME is radiographically indistinguishable from a healthy elbow.7 To prevent unnecessary expense and radiation exposure in young children, prereduction radiographs should only be used to rule out the possibility of fracture or other injury.7 Krul et al8 recommend restricting x-ray use to cases with an unclear history or those that are due to trauma other than an arm pull.
Continue to: Methods of reduction
Methods of reduction. Once NME is diagnosed, there are 2 methods of reduction: hyper-pronation and supination-flexion. Reduction is best performed with the child sitting in the parent’s lap with the injured arm facing the examiner.
Success rates for both methods of NME reduction are statistically similar; however, first-attempt success rates are significantly higher with the hyper-pronation method than with supination-flexion.9 Furthermore, physicians have deemed the hyper-pronation method significantly easier to perform than supination-flexion.9 A Cochrane review by Krul et al10 concluded that the hyper-pronation method may result in lower failure rates than supination-flexion, but due to limited evidence, the researchers were unable to draw any conclusions on other outcomes, such as pain. Green et al11 noted that hyper-pronation is perceived by parents of children with NME as being less painful. For these reasons, hyper-pronation should be utilized as the first method of reduction, followed by supination-flexion if the former does not work.12
Additional management. In a limited study of 50 children with pulled-elbow injuries, ultrasound revealed that 78% had an intact yet interposed radial annular ligament and 22% had a tear in the radial annular ligament.13 The authors propose that if, after appropriate reduction methods are attempted, no pop is felt, or there is no prompt clinical improvement, and ultrasound is not available to assess the integrity of the annular ligament, the child should be placed in a splint for 7 days
Our patient returned to the PED 3 days later, complaining of pain and an inability to move her left arm after her older sibling pulled her by her outstretched arms. She was once again diagnosed with NME, the injury was reduced, and she was using the arm within minutes. She has not presented to either the PED or the pediatric clinic with a similar complaint since. Discarding outliers, NME recurrence rates fall within a range of 23.7% to 32.9%.14,15
THE TAKEAWAY
Pre-reduction x-rays are not warranted in cases of NME unless there is suspicion for fracture or another injury. The 2 reduction methods, hyper-pronation and supination-flexion, are easily mastered. Any reduction should be quick, easy, and as painless as possible. Hyper-pronation should be utilized first, as this maneuver seems to be the more successful and is perceived by parents as being less painful. However, it is always most helpful to be proficient in both methods. If, after appropriate attempts at reduction, the child has not regained the use of the arm, 7 days of splinting is recommended, along with an orthopedic referral.
CORRESPONDENCE
Robert N. Anderson, DNP, APRN, (F)NP-C, ENP-BC, Vanderbilt Health, 512 Autumn Springs Court, Suite 100 C, Franklin, TN 37067; [email protected]
1. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
2. Michaels MG. A case of bilateral nursemaid’s elbow. Pediatr Emerg Care. 1989;5:226-227.
3. Meiner EV, Sama AE, Lee DC, et al. Bilateral nursemaid’s elbow. Am J Emerg Med. 2004;6:502-503.
4. Wang YX, Zhang G, Song B, et al. Radial head subluxation in pediatric clinics and emergency departments in China. Chin J Traum. 2019;22:340-344.
5. Schunk JE. Radial head subluxation: epidemiology and treatment of 87 episodes. Ann Emerg Med. 1990;19:1019-1023.
6. Irie T, Sono T, Hayama Y, et al. Investigation on 2331 cases of pulled elbow over the last 10 years. Pediatr Rep. 2014;6:5090. doi: 10.4081/pr.2014.5090
7. Eismann EA, Cosco ED, Wall EJ. Absence of radiographic abnormalities in nursemaid’s elbows. J Pediatr Orthop. 2014;34:426-431.
8. Krul M, van der Wouden JC, Koes BW, et al. Nursemaid’s Elbow: its diagnostic clues and preferred means of reduction. J Fam Pract. 2010:59:E5-E7.
9. Bek D, Yildiz C, Köse O, et al. Pronation versus supination maneuvers for the reduction of ‘pulled elbow’: a randomized clinical trial. Eur J Emerg Med. 2009;16:135-138.
10. Krul M, van der Wouden JC, Kruithof EJ, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2017.
11. Green DA, Linares MYR, Garcia Peña BM, et al. Randomized comparison of pain perception during radial head subluxation reduction using supination-flexion of forced pronation. Pediatr Emerg Care. 2006;22:235-238.
12. García-Mata S, Hidalgo-Ovejero A. Efficacy of reduction maneuvers for “pulled elbow” in children: a prospective study of 115 cases. J Pediatr Orthop. 2014;34:432-436.
13. Diab HS, Hamed MMS, Allam Y. Obscure pathology of pulled elbow: dynamic high-resolution ultrasound-assisted classification. J Child Orthop. 2010;4:539-543.
14. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
15. Macias CG, Bothner J, Wiebe R. Comparison of supination/flexion to hyperpronation in the reduction of radial head subluxation. Pediatrics. 1998;102:E10. doi: 10.1542/peds.102.1.e10.
1. Quan L, Marcuse EK. The epidemiology and treatment of radial head subluxation. Am J Dis Child. 1985;139:1194-1197.
2. Michaels MG. A case of bilateral nursemaid’s elbow. Pediatr Emerg Care. 1989;5:226-227.
3. Meiner EV, Sama AE, Lee DC, et al. Bilateral nursemaid’s elbow. Am J Emerg Med. 2004;6:502-503.
4. Wang YX, Zhang G, Song B, et al. Radial head subluxation in pediatric clinics and emergency departments in China. Chin J Traum. 2019;22:340-344.
5. Schunk JE. Radial head subluxation: epidemiology and treatment of 87 episodes. Ann Emerg Med. 1990;19:1019-1023.
6. Irie T, Sono T, Hayama Y, et al. Investigation on 2331 cases of pulled elbow over the last 10 years. Pediatr Rep. 2014;6:5090. doi: 10.4081/pr.2014.5090
7. Eismann EA, Cosco ED, Wall EJ. Absence of radiographic abnormalities in nursemaid’s elbows. J Pediatr Orthop. 2014;34:426-431.
8. Krul M, van der Wouden JC, Koes BW, et al. Nursemaid’s Elbow: its diagnostic clues and preferred means of reduction. J Fam Pract. 2010:59:E5-E7.
9. Bek D, Yildiz C, Köse O, et al. Pronation versus supination maneuvers for the reduction of ‘pulled elbow’: a randomized clinical trial. Eur J Emerg Med. 2009;16:135-138.
10. Krul M, van der Wouden JC, Kruithof EJ, et al. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database Syst Rev. 2017.
11. Green DA, Linares MYR, Garcia Peña BM, et al. Randomized comparison of pain perception during radial head subluxation reduction using supination-flexion of forced pronation. Pediatr Emerg Care. 2006;22:235-238.
12. García-Mata S, Hidalgo-Ovejero A. Efficacy of reduction maneuvers for “pulled elbow” in children: a prospective study of 115 cases. J Pediatr Orthop. 2014;34:432-436.
13. Diab HS, Hamed MMS, Allam Y. Obscure pathology of pulled elbow: dynamic high-resolution ultrasound-assisted classification. J Child Orthop. 2010;4:539-543.
14. Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150:164-166.
15. Macias CG, Bothner J, Wiebe R. Comparison of supination/flexion to hyperpronation in the reduction of radial head subluxation. Pediatrics. 1998;102:E10. doi: 10.1542/peds.102.1.e10.