User login
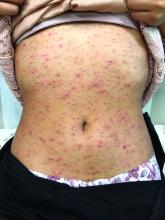
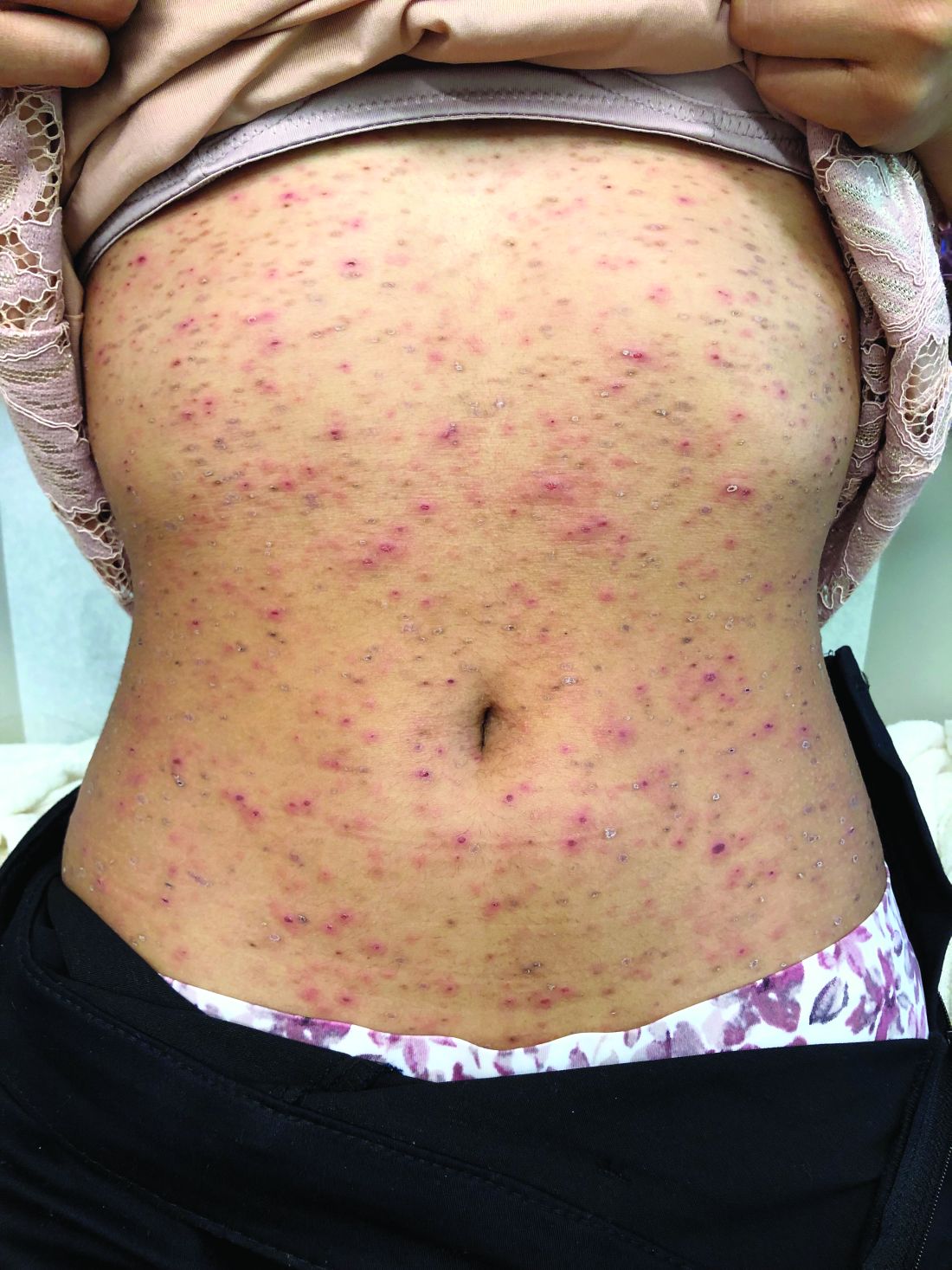
Pruritic, pink to violaceous, scaly papules
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A healthy 14-year-old female was referred urgently by her pediatrician to our pediatric dermatology clinic for evaluation of a rash. The rash had been present for 4 weeks on her torso and proximal extremities, and had been spreading. She had been very itchy. She denied any fevers, chills, joint pain, oral or genital lesions.
She was visiting some family members in Washington State during the summer. The rash started 1 month after this visit.
The adolescent had been treated with acyclovir, trimethoprim/sulfamethoxazole, and intramuscular triamcinolone without improvement. She had been taking children's multivitamins occasionally. Her vaccinations were up-to-date. She denied any history of varicella or herpes infection. Her mom has a history of cold sores. The teen is not sexually active.
On physical examination, the girl was not in acute distress. Her vital signs were stable. She was not febrile. She had pink, scaly, and hyperpigmented papules and plaques, some of which were crusted with violaceous centers on the trunk and proximal extremities. There were no lesions on the mouth, palms, or soles. She had no lymphadenopathy or hepatosplenomegaly.
Gender bias and pediatric hospital medicine
Where do we go from here?
Autumn is a busy time for pediatric hospitalists, with this autumn being particularly eventful as the first American Board of Pediatrics (ABP) certifying exam for Pediatric Hospital Medicine (PHM) will be offered on Nov. 12, 2019.
More than 1,600 med/peds and pediatric hospitalists applied to be eligible for the 2019 exam, 71% of whom were women. At least 3.9% of those applicants were denied eligibility for the 2019 exam.1 These denials resulted in discussions on the American Academy of Pediatrics Section on Hospital Medicine (AAP SOHM) email listserv related to unintentional gender bias.
PHM was first recognized as a subspecialty by the American Board of Medical Specialties in December 2015.2 Since that time, the ABP’s PHM sub-board developed eligibility criteria for practicing pediatric and med/peds hospitalists to apply for the exam. The sub-board identified three paths: a training pathway for applicants who had completed a 2-year PHM fellowship, a practice pathway for those satisfying ABP criteria for clinical activity in PHM, and a combined pathway for applicants who had completed PHM fellowships lasting less than 2 years.
Based on these pathways, 1,627 applicants applied for eligibility for the first PHM board certification exam.1 However, many concerns arose with the practice pathway eligibility criteria.
The PHM practice pathway initially included the following eligibility criteria:
• General pediatrics board certification.
• PHM practice “look back” period ends on or before June 30 of the exam year and starts 4 years earlier.
• More than 0.5 FTE professional PHM-related activities (patient-care, research, administration), defined as more than 900 hours/year every year for the preceding 4 years.
• More than 0.25 FTE direct patient care of hospitalized children, defined as more than 450 hours/year every year for the preceding 4 years.
• Practice covers the full range of hospitalized children with regard to age, diagnoses, and complexity.
• Practice interruptions cannot exceed 3 months in the preceding 4 years, or 6 months in the preceding 5 years.
• Practice experience and hours were acquired in the United States and Canada.1,3
The start date and practice interruptions criteria in the practice pathway posed hurdles for many female applicants. Many women voiced concerns about feeling disadvantaged when applying for the PHM certifying exam and some of these women shared their concerns on the AAP SOHM email listserv. In response to these concerns, the PHM community called for increased transparency from the ABP related to denials, specifically related to unintentional gender bias against women applying for the exam.
David Skey, MD, and Jamee Walters, MD, pediatric hospitalists at Arnold Palmer Medical Center in Orlando, heard these concerns and decided to draft a petition with the help of legal counsel. The petition “demand[ed] immediate action,” and “request[ed] a formal response from the ABP regarding the practice pathway criteria.” The petition also stated that there was insufficient data to determine if the practice pathway “disadvantages women.” The petition asked the ABP to “facilitate a timely analysis to determine if gender bias” was present, or to perform an internal analysis and “release the findings publicly.”4
The petition was shared with the PHM community via the AAP SOHM listserv on July 29, 2019. Dr. Walters stated she was pleased by the response she and Dr. Skey received from the PHM community, on and off the AAP SOHM listserv. The petition was submitted to the ABP on Aug. 6, 2019, with 1,479 signatures.
On Aug. 29, 2019, the ABP’s response was shared on the AAP SOHM email listserv1 and was later published in the Journal of Hospital Medicine as a Special Announcement.5 In its response, the ABP stated that the gender bias allegation was “not supported by the facts” as there was “no significant difference between the percentage of women and men who were denied” eligibility.”5 In addressing the gender bias allegations and clarifying the practice pathway eligibility, the ABP removed the practice interruption criteria and modified the practice pathway criteria as follows:
• General pediatrics board certification.
• PHM practice started on or before July 2015 (for board eligibility in 2019).
• Professional PHM-related activities (patient-care, research, administration), defined as more than 900-1000 hours/year every year for the preceding 4 years.
• Direct patient care of hospitalized children, defined as more than 450-500 hours/year every year for the preceding 4 years.
• Practice covers the full range of hospitalized children with regard to age, diagnoses, and complexity.
• Practice experience and hours were acquired in the United States and Canada.1
Following the release of the ABP’s response, many members of the PHM community remain concerned about the ABP’s revised criteria. Arti Desai, MD, pediatric hospitalist at Seattle Children’s and senior author on a “Perspectives in Hospital Medicine” in the Journal of Hospital Medicine,6 was appreciative that the ABP chose to remove the practice interruptions criterion. However, she and her colleagues remain concerned about lingering gender bias in the ABP’s practice pathway eligibility criteria surrounding the “start date” criterion. The authors state that this criterion differentially affects women, as women may take time off during or after residency for maternity or family leave. Dr. Desai states that this criterion alone can affect a woman’s chance for being eligible for the practice pathway.
Other members of the PHM community also expressed concerns about the ABP’s response to the PHM petition. Beth C. Natt, MD, pediatric hospitalist and director of pediatric hospital medicine regional programs at Connecticut Children’s in Hartford, felt that the population may have been self-selected, as the ABP’s data were limited to individuals who applied for exam eligibility. She was concerned that the data excluded pediatric hospitalists who chose not to apply because of uncertainty about meeting eligibility criteria. Klint Schwenk, MD, pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., stated that he wished the ABP had addressed the number of pediatric hospitalists who elected not to apply based on fear of ineligibility before concluding that there was no bias. He likened the ABP’s response to that of study authors omitting selection bias when discussing the limitations of their study.
Courtney Edgar-Zarate, MD, med/peds hospitalist and associate program director of the internal medicine/pediatrics residency at the University of Arkansas, expressed concerns that the ABP’s stringent clinical patient care hours criterion may unintentionally result in ineligibility for many mid-career or senior med/peds hospitalists. Dr. Edgar-Zarate also voiced concerns that graduating med/peds residents were electing not to pursue careers in hospital medicine because they would be required to complete a PHM fellowship to become a pediatric hospitalist, when a similar fellowship is not required to practice adult hospital medicine.
The Society of Hospital Medicine shared its position in regard to the ABP’s response in a Special Announcement in the Journal of Hospital Medicine.7 In it, SHM’s pediatric leaders recognized physicians for the excellent care they provide to hospitalized children. They stated that SHM would continue to support all hospitalists, independent of board eligibility status, and would continue to offer these hospitalists the merit-based Fellow designation. SHM’s pediatric leaders also proposed future directions for the ABP, including a Focused Practice Pathway in Hospital Medicine (FPHM), such as what the American Board of Internal Medicine and the American Board of Family Medicine have adopted for board recertification in internal medicine and family medicine. This maintenance of certification program that allows physicians primarily practicing in inpatient settings to focus their continuing education on inpatient practice, and is not a subspecialty.7
Dr. Edgar-Zarate fully supports the future directions for pediatric hospitalists outlined in SHM’s Special Announcement. She hopes that the ABP will support the FPHM. She feels the FPHM will encourage more med/peds physicians to practice med/peds hospital medicine. L. Nell Hodo, MD, a family medicine–trained pediatric hospitalist at Icahn School of Medicine at Mount Sinai in New York, joins Dr. Edgar-Zarate in supporting an FPHM for PHM, and feels that it will open the door for hospitalists who are ineligible for the practice pathway to be able to focus their recertification on the inpatient setting.
Dr. Hodo and Dr. Desai hope that rather than excluding those who are not PHM board eligible/certified, institutions and professional organizations will consider all qualifications when hiring, mentoring, and promoting physicians who care for hospitalized children. Dr. Natt, Dr. Schwenk, Dr. Edgar-Zarate, and Dr. Hodo appreciate that SHM is leading the way, and will continue to allow all hospitalists who care for children to receive Fellow designation.
Dr. Kumar is clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of The Hospitalist.
References
1. The American Board of Pediatrics. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. https://www.abp.org/sites/abp/files/phm-petition-response.pdf. Published 2019.
2. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). doi: 10.1542/peds.2016-1823.
3. The American Board of Pediatrics. Pediatric Hospital Medicine Certification. https://www.abp.org/content/pediatric-hospital-medicine-certification. Published 2019.
4. Skey D. Pediatric Hospitalists, It’s time to take a stand on the PHM Boards Application Process! Five Dog Development, LLC.
5. Nichols DG, Woods SZ. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019 Oct;14(10):586-8. doi: 10.12788/jhm.3322.
6. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019 Oct;14(10):630-2. doi: 10.12788/jhm.3331.
7. Chang WW et al. Society of Hospital Medicine position on the American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019 Oct;14(10):589-90. doi: 10.12788/jhm.3326.
Where do we go from here?
Where do we go from here?
Autumn is a busy time for pediatric hospitalists, with this autumn being particularly eventful as the first American Board of Pediatrics (ABP) certifying exam for Pediatric Hospital Medicine (PHM) will be offered on Nov. 12, 2019.
More than 1,600 med/peds and pediatric hospitalists applied to be eligible for the 2019 exam, 71% of whom were women. At least 3.9% of those applicants were denied eligibility for the 2019 exam.1 These denials resulted in discussions on the American Academy of Pediatrics Section on Hospital Medicine (AAP SOHM) email listserv related to unintentional gender bias.
PHM was first recognized as a subspecialty by the American Board of Medical Specialties in December 2015.2 Since that time, the ABP’s PHM sub-board developed eligibility criteria for practicing pediatric and med/peds hospitalists to apply for the exam. The sub-board identified three paths: a training pathway for applicants who had completed a 2-year PHM fellowship, a practice pathway for those satisfying ABP criteria for clinical activity in PHM, and a combined pathway for applicants who had completed PHM fellowships lasting less than 2 years.
Based on these pathways, 1,627 applicants applied for eligibility for the first PHM board certification exam.1 However, many concerns arose with the practice pathway eligibility criteria.
The PHM practice pathway initially included the following eligibility criteria:
• General pediatrics board certification.
• PHM practice “look back” period ends on or before June 30 of the exam year and starts 4 years earlier.
• More than 0.5 FTE professional PHM-related activities (patient-care, research, administration), defined as more than 900 hours/year every year for the preceding 4 years.
• More than 0.25 FTE direct patient care of hospitalized children, defined as more than 450 hours/year every year for the preceding 4 years.
• Practice covers the full range of hospitalized children with regard to age, diagnoses, and complexity.
• Practice interruptions cannot exceed 3 months in the preceding 4 years, or 6 months in the preceding 5 years.
• Practice experience and hours were acquired in the United States and Canada.1,3
The start date and practice interruptions criteria in the practice pathway posed hurdles for many female applicants. Many women voiced concerns about feeling disadvantaged when applying for the PHM certifying exam and some of these women shared their concerns on the AAP SOHM email listserv. In response to these concerns, the PHM community called for increased transparency from the ABP related to denials, specifically related to unintentional gender bias against women applying for the exam.
David Skey, MD, and Jamee Walters, MD, pediatric hospitalists at Arnold Palmer Medical Center in Orlando, heard these concerns and decided to draft a petition with the help of legal counsel. The petition “demand[ed] immediate action,” and “request[ed] a formal response from the ABP regarding the practice pathway criteria.” The petition also stated that there was insufficient data to determine if the practice pathway “disadvantages women.” The petition asked the ABP to “facilitate a timely analysis to determine if gender bias” was present, or to perform an internal analysis and “release the findings publicly.”4
The petition was shared with the PHM community via the AAP SOHM listserv on July 29, 2019. Dr. Walters stated she was pleased by the response she and Dr. Skey received from the PHM community, on and off the AAP SOHM listserv. The petition was submitted to the ABP on Aug. 6, 2019, with 1,479 signatures.
On Aug. 29, 2019, the ABP’s response was shared on the AAP SOHM email listserv1 and was later published in the Journal of Hospital Medicine as a Special Announcement.5 In its response, the ABP stated that the gender bias allegation was “not supported by the facts” as there was “no significant difference between the percentage of women and men who were denied” eligibility.”5 In addressing the gender bias allegations and clarifying the practice pathway eligibility, the ABP removed the practice interruption criteria and modified the practice pathway criteria as follows:
• General pediatrics board certification.
• PHM practice started on or before July 2015 (for board eligibility in 2019).
• Professional PHM-related activities (patient-care, research, administration), defined as more than 900-1000 hours/year every year for the preceding 4 years.
• Direct patient care of hospitalized children, defined as more than 450-500 hours/year every year for the preceding 4 years.
• Practice covers the full range of hospitalized children with regard to age, diagnoses, and complexity.
• Practice experience and hours were acquired in the United States and Canada.1
Following the release of the ABP’s response, many members of the PHM community remain concerned about the ABP’s revised criteria. Arti Desai, MD, pediatric hospitalist at Seattle Children’s and senior author on a “Perspectives in Hospital Medicine” in the Journal of Hospital Medicine,6 was appreciative that the ABP chose to remove the practice interruptions criterion. However, she and her colleagues remain concerned about lingering gender bias in the ABP’s practice pathway eligibility criteria surrounding the “start date” criterion. The authors state that this criterion differentially affects women, as women may take time off during or after residency for maternity or family leave. Dr. Desai states that this criterion alone can affect a woman’s chance for being eligible for the practice pathway.
Other members of the PHM community also expressed concerns about the ABP’s response to the PHM petition. Beth C. Natt, MD, pediatric hospitalist and director of pediatric hospital medicine regional programs at Connecticut Children’s in Hartford, felt that the population may have been self-selected, as the ABP’s data were limited to individuals who applied for exam eligibility. She was concerned that the data excluded pediatric hospitalists who chose not to apply because of uncertainty about meeting eligibility criteria. Klint Schwenk, MD, pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., stated that he wished the ABP had addressed the number of pediatric hospitalists who elected not to apply based on fear of ineligibility before concluding that there was no bias. He likened the ABP’s response to that of study authors omitting selection bias when discussing the limitations of their study.
Courtney Edgar-Zarate, MD, med/peds hospitalist and associate program director of the internal medicine/pediatrics residency at the University of Arkansas, expressed concerns that the ABP’s stringent clinical patient care hours criterion may unintentionally result in ineligibility for many mid-career or senior med/peds hospitalists. Dr. Edgar-Zarate also voiced concerns that graduating med/peds residents were electing not to pursue careers in hospital medicine because they would be required to complete a PHM fellowship to become a pediatric hospitalist, when a similar fellowship is not required to practice adult hospital medicine.
The Society of Hospital Medicine shared its position in regard to the ABP’s response in a Special Announcement in the Journal of Hospital Medicine.7 In it, SHM’s pediatric leaders recognized physicians for the excellent care they provide to hospitalized children. They stated that SHM would continue to support all hospitalists, independent of board eligibility status, and would continue to offer these hospitalists the merit-based Fellow designation. SHM’s pediatric leaders also proposed future directions for the ABP, including a Focused Practice Pathway in Hospital Medicine (FPHM), such as what the American Board of Internal Medicine and the American Board of Family Medicine have adopted for board recertification in internal medicine and family medicine. This maintenance of certification program that allows physicians primarily practicing in inpatient settings to focus their continuing education on inpatient practice, and is not a subspecialty.7
Dr. Edgar-Zarate fully supports the future directions for pediatric hospitalists outlined in SHM’s Special Announcement. She hopes that the ABP will support the FPHM. She feels the FPHM will encourage more med/peds physicians to practice med/peds hospital medicine. L. Nell Hodo, MD, a family medicine–trained pediatric hospitalist at Icahn School of Medicine at Mount Sinai in New York, joins Dr. Edgar-Zarate in supporting an FPHM for PHM, and feels that it will open the door for hospitalists who are ineligible for the practice pathway to be able to focus their recertification on the inpatient setting.
Dr. Hodo and Dr. Desai hope that rather than excluding those who are not PHM board eligible/certified, institutions and professional organizations will consider all qualifications when hiring, mentoring, and promoting physicians who care for hospitalized children. Dr. Natt, Dr. Schwenk, Dr. Edgar-Zarate, and Dr. Hodo appreciate that SHM is leading the way, and will continue to allow all hospitalists who care for children to receive Fellow designation.
Dr. Kumar is clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of The Hospitalist.
References
1. The American Board of Pediatrics. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. https://www.abp.org/sites/abp/files/phm-petition-response.pdf. Published 2019.
2. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). doi: 10.1542/peds.2016-1823.
3. The American Board of Pediatrics. Pediatric Hospital Medicine Certification. https://www.abp.org/content/pediatric-hospital-medicine-certification. Published 2019.
4. Skey D. Pediatric Hospitalists, It’s time to take a stand on the PHM Boards Application Process! Five Dog Development, LLC.
5. Nichols DG, Woods SZ. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019 Oct;14(10):586-8. doi: 10.12788/jhm.3322.
6. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019 Oct;14(10):630-2. doi: 10.12788/jhm.3331.
7. Chang WW et al. Society of Hospital Medicine position on the American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019 Oct;14(10):589-90. doi: 10.12788/jhm.3326.
Autumn is a busy time for pediatric hospitalists, with this autumn being particularly eventful as the first American Board of Pediatrics (ABP) certifying exam for Pediatric Hospital Medicine (PHM) will be offered on Nov. 12, 2019.
More than 1,600 med/peds and pediatric hospitalists applied to be eligible for the 2019 exam, 71% of whom were women. At least 3.9% of those applicants were denied eligibility for the 2019 exam.1 These denials resulted in discussions on the American Academy of Pediatrics Section on Hospital Medicine (AAP SOHM) email listserv related to unintentional gender bias.
PHM was first recognized as a subspecialty by the American Board of Medical Specialties in December 2015.2 Since that time, the ABP’s PHM sub-board developed eligibility criteria for practicing pediatric and med/peds hospitalists to apply for the exam. The sub-board identified three paths: a training pathway for applicants who had completed a 2-year PHM fellowship, a practice pathway for those satisfying ABP criteria for clinical activity in PHM, and a combined pathway for applicants who had completed PHM fellowships lasting less than 2 years.
Based on these pathways, 1,627 applicants applied for eligibility for the first PHM board certification exam.1 However, many concerns arose with the practice pathway eligibility criteria.
The PHM practice pathway initially included the following eligibility criteria:
• General pediatrics board certification.
• PHM practice “look back” period ends on or before June 30 of the exam year and starts 4 years earlier.
• More than 0.5 FTE professional PHM-related activities (patient-care, research, administration), defined as more than 900 hours/year every year for the preceding 4 years.
• More than 0.25 FTE direct patient care of hospitalized children, defined as more than 450 hours/year every year for the preceding 4 years.
• Practice covers the full range of hospitalized children with regard to age, diagnoses, and complexity.
• Practice interruptions cannot exceed 3 months in the preceding 4 years, or 6 months in the preceding 5 years.
• Practice experience and hours were acquired in the United States and Canada.1,3
The start date and practice interruptions criteria in the practice pathway posed hurdles for many female applicants. Many women voiced concerns about feeling disadvantaged when applying for the PHM certifying exam and some of these women shared their concerns on the AAP SOHM email listserv. In response to these concerns, the PHM community called for increased transparency from the ABP related to denials, specifically related to unintentional gender bias against women applying for the exam.
David Skey, MD, and Jamee Walters, MD, pediatric hospitalists at Arnold Palmer Medical Center in Orlando, heard these concerns and decided to draft a petition with the help of legal counsel. The petition “demand[ed] immediate action,” and “request[ed] a formal response from the ABP regarding the practice pathway criteria.” The petition also stated that there was insufficient data to determine if the practice pathway “disadvantages women.” The petition asked the ABP to “facilitate a timely analysis to determine if gender bias” was present, or to perform an internal analysis and “release the findings publicly.”4
The petition was shared with the PHM community via the AAP SOHM listserv on July 29, 2019. Dr. Walters stated she was pleased by the response she and Dr. Skey received from the PHM community, on and off the AAP SOHM listserv. The petition was submitted to the ABP on Aug. 6, 2019, with 1,479 signatures.
On Aug. 29, 2019, the ABP’s response was shared on the AAP SOHM email listserv1 and was later published in the Journal of Hospital Medicine as a Special Announcement.5 In its response, the ABP stated that the gender bias allegation was “not supported by the facts” as there was “no significant difference between the percentage of women and men who were denied” eligibility.”5 In addressing the gender bias allegations and clarifying the practice pathway eligibility, the ABP removed the practice interruption criteria and modified the practice pathway criteria as follows:
• General pediatrics board certification.
• PHM practice started on or before July 2015 (for board eligibility in 2019).
• Professional PHM-related activities (patient-care, research, administration), defined as more than 900-1000 hours/year every year for the preceding 4 years.
• Direct patient care of hospitalized children, defined as more than 450-500 hours/year every year for the preceding 4 years.
• Practice covers the full range of hospitalized children with regard to age, diagnoses, and complexity.
• Practice experience and hours were acquired in the United States and Canada.1
Following the release of the ABP’s response, many members of the PHM community remain concerned about the ABP’s revised criteria. Arti Desai, MD, pediatric hospitalist at Seattle Children’s and senior author on a “Perspectives in Hospital Medicine” in the Journal of Hospital Medicine,6 was appreciative that the ABP chose to remove the practice interruptions criterion. However, she and her colleagues remain concerned about lingering gender bias in the ABP’s practice pathway eligibility criteria surrounding the “start date” criterion. The authors state that this criterion differentially affects women, as women may take time off during or after residency for maternity or family leave. Dr. Desai states that this criterion alone can affect a woman’s chance for being eligible for the practice pathway.
Other members of the PHM community also expressed concerns about the ABP’s response to the PHM petition. Beth C. Natt, MD, pediatric hospitalist and director of pediatric hospital medicine regional programs at Connecticut Children’s in Hartford, felt that the population may have been self-selected, as the ABP’s data were limited to individuals who applied for exam eligibility. She was concerned that the data excluded pediatric hospitalists who chose not to apply because of uncertainty about meeting eligibility criteria. Klint Schwenk, MD, pediatric hospitalist at Norton Children’s Hospital in Louisville, Ky., stated that he wished the ABP had addressed the number of pediatric hospitalists who elected not to apply based on fear of ineligibility before concluding that there was no bias. He likened the ABP’s response to that of study authors omitting selection bias when discussing the limitations of their study.
Courtney Edgar-Zarate, MD, med/peds hospitalist and associate program director of the internal medicine/pediatrics residency at the University of Arkansas, expressed concerns that the ABP’s stringent clinical patient care hours criterion may unintentionally result in ineligibility for many mid-career or senior med/peds hospitalists. Dr. Edgar-Zarate also voiced concerns that graduating med/peds residents were electing not to pursue careers in hospital medicine because they would be required to complete a PHM fellowship to become a pediatric hospitalist, when a similar fellowship is not required to practice adult hospital medicine.
The Society of Hospital Medicine shared its position in regard to the ABP’s response in a Special Announcement in the Journal of Hospital Medicine.7 In it, SHM’s pediatric leaders recognized physicians for the excellent care they provide to hospitalized children. They stated that SHM would continue to support all hospitalists, independent of board eligibility status, and would continue to offer these hospitalists the merit-based Fellow designation. SHM’s pediatric leaders also proposed future directions for the ABP, including a Focused Practice Pathway in Hospital Medicine (FPHM), such as what the American Board of Internal Medicine and the American Board of Family Medicine have adopted for board recertification in internal medicine and family medicine. This maintenance of certification program that allows physicians primarily practicing in inpatient settings to focus their continuing education on inpatient practice, and is not a subspecialty.7
Dr. Edgar-Zarate fully supports the future directions for pediatric hospitalists outlined in SHM’s Special Announcement. She hopes that the ABP will support the FPHM. She feels the FPHM will encourage more med/peds physicians to practice med/peds hospital medicine. L. Nell Hodo, MD, a family medicine–trained pediatric hospitalist at Icahn School of Medicine at Mount Sinai in New York, joins Dr. Edgar-Zarate in supporting an FPHM for PHM, and feels that it will open the door for hospitalists who are ineligible for the practice pathway to be able to focus their recertification on the inpatient setting.
Dr. Hodo and Dr. Desai hope that rather than excluding those who are not PHM board eligible/certified, institutions and professional organizations will consider all qualifications when hiring, mentoring, and promoting physicians who care for hospitalized children. Dr. Natt, Dr. Schwenk, Dr. Edgar-Zarate, and Dr. Hodo appreciate that SHM is leading the way, and will continue to allow all hospitalists who care for children to receive Fellow designation.
Dr. Kumar is clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine at Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of The Hospitalist.
References
1. The American Board of Pediatrics. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. https://www.abp.org/sites/abp/files/phm-petition-response.pdf. Published 2019.
2. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). doi: 10.1542/peds.2016-1823.
3. The American Board of Pediatrics. Pediatric Hospital Medicine Certification. https://www.abp.org/content/pediatric-hospital-medicine-certification. Published 2019.
4. Skey D. Pediatric Hospitalists, It’s time to take a stand on the PHM Boards Application Process! Five Dog Development, LLC.
5. Nichols DG, Woods SZ. The American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019 Oct;14(10):586-8. doi: 10.12788/jhm.3322.
6. Gold JM et al. Collective action and effective dialogue to address gender bias in medicine. J Hosp Med. 2019 Oct;14(10):630-2. doi: 10.12788/jhm.3331.
7. Chang WW et al. Society of Hospital Medicine position on the American Board of Pediatrics response to the Pediatric Hospital Medicine petition. J Hosp Med. 2019 Oct;14(10):589-90. doi: 10.12788/jhm.3326.
Pediatric stroke thrombectomy study sheds light on off-label procedure
based on data from a retrospective, multicenter study of 73 patients.
Children with high scores on the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) or large-vessel occlusion in the anterior or posterior circulation are at increased risk for morbidity and mortality, but the safety of thrombectomy in children has not been well studied. Several randomized trials have showed its safety and efficacy in adults, wrote Peter Sporns, MD, of the Institute of Clinical Radiology at Universitätsklinikum Muenster (Germany), and colleagues.
In a study published in JAMA Neurology, Dr. Sporns and coauthors reviewed data from pediatric patients aged younger than 18 years who underwent endovascular recanalization between Jan. 1, 2000, and Dec. 31, 2018, at 25 stroke centers in Europe and 2 in the United States.
The primary outcome was change in the PedNIHSS scores and the endovascular recanalization involved “a combination of techniques using distal thrombaspiration and/or clot retrievers,” the researchers wrote.
Neurologic outcomes improved from a median PedNIHSS score of 14.0 at hospital admission to 4.0 at day 7. The average time from stroke onset to hospital admission was 3 hours, and the median time from stroke onset to recanalization was 4 hours.
“The rapidity of recanalization across the large number of centers in the Save ChildS study is a commendable achievement, establishing feasibility for acute pediatric stroke treatment within the short window of time for embolectomy at centers prepared for this event,” wrote Christine Fox, MD, of the University of California, San Francisco, and Nomazulu Dlamini, MBBS, PhD, of the Hospital for Sick Children, Toronto, in an accompanying editorial.
In addition, the median modified Rankin Scale score was 1.0 at discharge and at 6 and 24 months, and the median Pediatric Stroke Outcome Measure score was 1.0 at discharge and 0.5 at 6 and 24 months.
The median age of the patients was 11 years, and approximately half were boys (51%). A total of 63 children (86%) were treated for anterior circulation occlusion, and 10 (14%) were treated for posterior circulation occlusion; (22%) received concomitant intravenous thrombolysis.
Transient vasospasm was the only observed periprocedural complication, seen in four patients, and all cases resolved without clinical sequelae. One patient with a history of a heart anomaly died of cardiac arrest after recanalization. No vascular complications were reported, and the proportion of symptomatic intracerebral hemorrhage events was 1.37 per 100 observations, compared with 2.79 in a meta-analysis of adult studies.
The main limitation of the study was its retrospective design, as well as the absence of a control group, the researchers noted. However, the results “may support clinicians’ practice of off-label thrombectomy in childhood stroke in the absence of high-level evidence.”
The editorial authors emphasized that safety concerns remain despite the relatively low level of complications observed in the current study. “The safety of thrombectomy in children with suspected focal cerebral arteriopathy or bilateral arteriopathies is a particular concern because of the potential to further injure an acutely inflamed or chronically diseased vessel,” they wrote.
“We should be cautious about the interpretation of long-term outcome measures in the Save ChildS study,” Dr. Fox and Dr. Dlamini added, noting that additional multicenter studies are needed “to advance our knowledge of pediatric stroke and inform best practices.”
Dr. Sporns had no financial conflicts to disclose; several coauthors disclosed relationships with multiple pharmaceutical companies. Dr. Fox and Dr. Dlamini had no financial conflicts to disclose.
SOURCES: Sporns P et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3403; Fox C, Dlamini N. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3412.
based on data from a retrospective, multicenter study of 73 patients.
Children with high scores on the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) or large-vessel occlusion in the anterior or posterior circulation are at increased risk for morbidity and mortality, but the safety of thrombectomy in children has not been well studied. Several randomized trials have showed its safety and efficacy in adults, wrote Peter Sporns, MD, of the Institute of Clinical Radiology at Universitätsklinikum Muenster (Germany), and colleagues.
In a study published in JAMA Neurology, Dr. Sporns and coauthors reviewed data from pediatric patients aged younger than 18 years who underwent endovascular recanalization between Jan. 1, 2000, and Dec. 31, 2018, at 25 stroke centers in Europe and 2 in the United States.
The primary outcome was change in the PedNIHSS scores and the endovascular recanalization involved “a combination of techniques using distal thrombaspiration and/or clot retrievers,” the researchers wrote.
Neurologic outcomes improved from a median PedNIHSS score of 14.0 at hospital admission to 4.0 at day 7. The average time from stroke onset to hospital admission was 3 hours, and the median time from stroke onset to recanalization was 4 hours.
“The rapidity of recanalization across the large number of centers in the Save ChildS study is a commendable achievement, establishing feasibility for acute pediatric stroke treatment within the short window of time for embolectomy at centers prepared for this event,” wrote Christine Fox, MD, of the University of California, San Francisco, and Nomazulu Dlamini, MBBS, PhD, of the Hospital for Sick Children, Toronto, in an accompanying editorial.
In addition, the median modified Rankin Scale score was 1.0 at discharge and at 6 and 24 months, and the median Pediatric Stroke Outcome Measure score was 1.0 at discharge and 0.5 at 6 and 24 months.
The median age of the patients was 11 years, and approximately half were boys (51%). A total of 63 children (86%) were treated for anterior circulation occlusion, and 10 (14%) were treated for posterior circulation occlusion; (22%) received concomitant intravenous thrombolysis.
Transient vasospasm was the only observed periprocedural complication, seen in four patients, and all cases resolved without clinical sequelae. One patient with a history of a heart anomaly died of cardiac arrest after recanalization. No vascular complications were reported, and the proportion of symptomatic intracerebral hemorrhage events was 1.37 per 100 observations, compared with 2.79 in a meta-analysis of adult studies.
The main limitation of the study was its retrospective design, as well as the absence of a control group, the researchers noted. However, the results “may support clinicians’ practice of off-label thrombectomy in childhood stroke in the absence of high-level evidence.”
The editorial authors emphasized that safety concerns remain despite the relatively low level of complications observed in the current study. “The safety of thrombectomy in children with suspected focal cerebral arteriopathy or bilateral arteriopathies is a particular concern because of the potential to further injure an acutely inflamed or chronically diseased vessel,” they wrote.
“We should be cautious about the interpretation of long-term outcome measures in the Save ChildS study,” Dr. Fox and Dr. Dlamini added, noting that additional multicenter studies are needed “to advance our knowledge of pediatric stroke and inform best practices.”
Dr. Sporns had no financial conflicts to disclose; several coauthors disclosed relationships with multiple pharmaceutical companies. Dr. Fox and Dr. Dlamini had no financial conflicts to disclose.
SOURCES: Sporns P et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3403; Fox C, Dlamini N. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3412.
based on data from a retrospective, multicenter study of 73 patients.
Children with high scores on the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) or large-vessel occlusion in the anterior or posterior circulation are at increased risk for morbidity and mortality, but the safety of thrombectomy in children has not been well studied. Several randomized trials have showed its safety and efficacy in adults, wrote Peter Sporns, MD, of the Institute of Clinical Radiology at Universitätsklinikum Muenster (Germany), and colleagues.
In a study published in JAMA Neurology, Dr. Sporns and coauthors reviewed data from pediatric patients aged younger than 18 years who underwent endovascular recanalization between Jan. 1, 2000, and Dec. 31, 2018, at 25 stroke centers in Europe and 2 in the United States.
The primary outcome was change in the PedNIHSS scores and the endovascular recanalization involved “a combination of techniques using distal thrombaspiration and/or clot retrievers,” the researchers wrote.
Neurologic outcomes improved from a median PedNIHSS score of 14.0 at hospital admission to 4.0 at day 7. The average time from stroke onset to hospital admission was 3 hours, and the median time from stroke onset to recanalization was 4 hours.
“The rapidity of recanalization across the large number of centers in the Save ChildS study is a commendable achievement, establishing feasibility for acute pediatric stroke treatment within the short window of time for embolectomy at centers prepared for this event,” wrote Christine Fox, MD, of the University of California, San Francisco, and Nomazulu Dlamini, MBBS, PhD, of the Hospital for Sick Children, Toronto, in an accompanying editorial.
In addition, the median modified Rankin Scale score was 1.0 at discharge and at 6 and 24 months, and the median Pediatric Stroke Outcome Measure score was 1.0 at discharge and 0.5 at 6 and 24 months.
The median age of the patients was 11 years, and approximately half were boys (51%). A total of 63 children (86%) were treated for anterior circulation occlusion, and 10 (14%) were treated for posterior circulation occlusion; (22%) received concomitant intravenous thrombolysis.
Transient vasospasm was the only observed periprocedural complication, seen in four patients, and all cases resolved without clinical sequelae. One patient with a history of a heart anomaly died of cardiac arrest after recanalization. No vascular complications were reported, and the proportion of symptomatic intracerebral hemorrhage events was 1.37 per 100 observations, compared with 2.79 in a meta-analysis of adult studies.
The main limitation of the study was its retrospective design, as well as the absence of a control group, the researchers noted. However, the results “may support clinicians’ practice of off-label thrombectomy in childhood stroke in the absence of high-level evidence.”
The editorial authors emphasized that safety concerns remain despite the relatively low level of complications observed in the current study. “The safety of thrombectomy in children with suspected focal cerebral arteriopathy or bilateral arteriopathies is a particular concern because of the potential to further injure an acutely inflamed or chronically diseased vessel,” they wrote.
“We should be cautious about the interpretation of long-term outcome measures in the Save ChildS study,” Dr. Fox and Dr. Dlamini added, noting that additional multicenter studies are needed “to advance our knowledge of pediatric stroke and inform best practices.”
Dr. Sporns had no financial conflicts to disclose; several coauthors disclosed relationships with multiple pharmaceutical companies. Dr. Fox and Dr. Dlamini had no financial conflicts to disclose.
SOURCES: Sporns P et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3403; Fox C, Dlamini N. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3412.
FROM JAMA NEUROLOGY
Suicidality risk high in transgender youth, varies by gender identity subtype
, new research suggests.
The study, which included more than 2,000 adolescents and was published in the October 14 issue of Pediatrics, provides new insights into suicide risk in gender identity subgroups, according to the investigators.
“Limited measures of gender identity may have led to inaccurate estimates of suicidality among transgender females in previous studies,” wrote Brian C. Thoma, PhD, and his colleagues at the University of Pittsburgh. The researchers noted that transgender females and nonbinary adolescents assigned male at birth are frequently combined in studies.
“However, our results indicate transgender females have higher risk for suicidal ideation and attempt compared with cisgender adolescents, whereas nonbinary adolescents assigned male at birth do not,” they wrote. “It is possible that estimates of suicidality that aggregate all transgender adolescents assigned male at birth into one group underestimate rates of suicidality among transgender females.”
The study, which analyzed results from a cross-sectional online survey from July to October 2018, was comprised of 2,020 adolescents, including 1,134 transgender adolescents.
The researchers divided respondents into seven categories: Cisgender males, cisgender females, transgender males, transgender females, nonbinary adolescents assigned female at birth, nonbinary adolescents assigned male at birth, and questioning gender identity. They then assessed non-suicidal self-injury (NSSI) and lifetime suicidality.
Compared to cisgender youth, transgender adolescents overall were more likely to report all outcomes: passive death wish (odds ratio [OR]=2.60), suicidal ideation (OR=2.20), suicide plan (OR=1.82), suicide attempt (OR=1.65), attempt requiring medical care (OR=2.01), and NSSI (OR=2.88).
Using cisgender males as reference after adjustment for all demographics, “cisgender females, transgender males, and nonbinary adolescents assigned female at birth had higher odds of each suicidality outcome” (OR= 1.49-5.85; OR=2.72-12.12; OR=1.84-8.59, respectively), the authors reported. “Transgender females had higher odds of each outcome [OR=2.73-6.30] except for suicide attempt requiring medical care. Nonbinary adolescents assigned male at birth had higher odds of suicide attempt requiring medical care [OR=10.13] and NSSI [OR=3.79]. Adolescents questioning their gender identity had higher odds of all outcomes [OR=3.23-7.59] except for suicide attempt.”
When compared to cisgender females as reference, however, only transgender males and transgender females had higher odds of suicidal ideation and attempts.
The overall findings were unsurprising since the higher rates of suicidality among transgender youth have already been documented, but the classification of participants was interesting, Gerald Montano, DO, an assistant professor of pediatrics at the University of Pittsburgh School of Medicine, said in an interview. Dr Montano was not involved in the study.
“It’s always been a challenge because, in the past, they always lumped transgender youth along with lesbian, gay, and bisexual youth,” Dr Montano said. This study is one of the few to go into more detail in considering participants’ gender identity, which was wise given that suicidal risk may differ accordingly.
The biggest take-home message of this study is the importance of screening for suicidality after informing adolescent patients of the limits of confidentiality, Dr Montano said.
“I think it’s very important for the physician to be aware of the reasons for those thoughts of suicide,” he continued. “A lot of it has to do with their gender identity and from discrimination and stigma from the general population.”
The research was funded by the University of Pittsburgh Central Research Development Fund and the National Institutes of Health. The authors reported no conflicts of interest.
SOURCE: Thoma BC et al, Pediatrics, October 14, 2019. DOI: 10.1542/peds.2019-1183
, new research suggests.
The study, which included more than 2,000 adolescents and was published in the October 14 issue of Pediatrics, provides new insights into suicide risk in gender identity subgroups, according to the investigators.
“Limited measures of gender identity may have led to inaccurate estimates of suicidality among transgender females in previous studies,” wrote Brian C. Thoma, PhD, and his colleagues at the University of Pittsburgh. The researchers noted that transgender females and nonbinary adolescents assigned male at birth are frequently combined in studies.
“However, our results indicate transgender females have higher risk for suicidal ideation and attempt compared with cisgender adolescents, whereas nonbinary adolescents assigned male at birth do not,” they wrote. “It is possible that estimates of suicidality that aggregate all transgender adolescents assigned male at birth into one group underestimate rates of suicidality among transgender females.”
The study, which analyzed results from a cross-sectional online survey from July to October 2018, was comprised of 2,020 adolescents, including 1,134 transgender adolescents.
The researchers divided respondents into seven categories: Cisgender males, cisgender females, transgender males, transgender females, nonbinary adolescents assigned female at birth, nonbinary adolescents assigned male at birth, and questioning gender identity. They then assessed non-suicidal self-injury (NSSI) and lifetime suicidality.
Compared to cisgender youth, transgender adolescents overall were more likely to report all outcomes: passive death wish (odds ratio [OR]=2.60), suicidal ideation (OR=2.20), suicide plan (OR=1.82), suicide attempt (OR=1.65), attempt requiring medical care (OR=2.01), and NSSI (OR=2.88).
Using cisgender males as reference after adjustment for all demographics, “cisgender females, transgender males, and nonbinary adolescents assigned female at birth had higher odds of each suicidality outcome” (OR= 1.49-5.85; OR=2.72-12.12; OR=1.84-8.59, respectively), the authors reported. “Transgender females had higher odds of each outcome [OR=2.73-6.30] except for suicide attempt requiring medical care. Nonbinary adolescents assigned male at birth had higher odds of suicide attempt requiring medical care [OR=10.13] and NSSI [OR=3.79]. Adolescents questioning their gender identity had higher odds of all outcomes [OR=3.23-7.59] except for suicide attempt.”
When compared to cisgender females as reference, however, only transgender males and transgender females had higher odds of suicidal ideation and attempts.
The overall findings were unsurprising since the higher rates of suicidality among transgender youth have already been documented, but the classification of participants was interesting, Gerald Montano, DO, an assistant professor of pediatrics at the University of Pittsburgh School of Medicine, said in an interview. Dr Montano was not involved in the study.
“It’s always been a challenge because, in the past, they always lumped transgender youth along with lesbian, gay, and bisexual youth,” Dr Montano said. This study is one of the few to go into more detail in considering participants’ gender identity, which was wise given that suicidal risk may differ accordingly.
The biggest take-home message of this study is the importance of screening for suicidality after informing adolescent patients of the limits of confidentiality, Dr Montano said.
“I think it’s very important for the physician to be aware of the reasons for those thoughts of suicide,” he continued. “A lot of it has to do with their gender identity and from discrimination and stigma from the general population.”
The research was funded by the University of Pittsburgh Central Research Development Fund and the National Institutes of Health. The authors reported no conflicts of interest.
SOURCE: Thoma BC et al, Pediatrics, October 14, 2019. DOI: 10.1542/peds.2019-1183
, new research suggests.
The study, which included more than 2,000 adolescents and was published in the October 14 issue of Pediatrics, provides new insights into suicide risk in gender identity subgroups, according to the investigators.
“Limited measures of gender identity may have led to inaccurate estimates of suicidality among transgender females in previous studies,” wrote Brian C. Thoma, PhD, and his colleagues at the University of Pittsburgh. The researchers noted that transgender females and nonbinary adolescents assigned male at birth are frequently combined in studies.
“However, our results indicate transgender females have higher risk for suicidal ideation and attempt compared with cisgender adolescents, whereas nonbinary adolescents assigned male at birth do not,” they wrote. “It is possible that estimates of suicidality that aggregate all transgender adolescents assigned male at birth into one group underestimate rates of suicidality among transgender females.”
The study, which analyzed results from a cross-sectional online survey from July to October 2018, was comprised of 2,020 adolescents, including 1,134 transgender adolescents.
The researchers divided respondents into seven categories: Cisgender males, cisgender females, transgender males, transgender females, nonbinary adolescents assigned female at birth, nonbinary adolescents assigned male at birth, and questioning gender identity. They then assessed non-suicidal self-injury (NSSI) and lifetime suicidality.
Compared to cisgender youth, transgender adolescents overall were more likely to report all outcomes: passive death wish (odds ratio [OR]=2.60), suicidal ideation (OR=2.20), suicide plan (OR=1.82), suicide attempt (OR=1.65), attempt requiring medical care (OR=2.01), and NSSI (OR=2.88).
Using cisgender males as reference after adjustment for all demographics, “cisgender females, transgender males, and nonbinary adolescents assigned female at birth had higher odds of each suicidality outcome” (OR= 1.49-5.85; OR=2.72-12.12; OR=1.84-8.59, respectively), the authors reported. “Transgender females had higher odds of each outcome [OR=2.73-6.30] except for suicide attempt requiring medical care. Nonbinary adolescents assigned male at birth had higher odds of suicide attempt requiring medical care [OR=10.13] and NSSI [OR=3.79]. Adolescents questioning their gender identity had higher odds of all outcomes [OR=3.23-7.59] except for suicide attempt.”
When compared to cisgender females as reference, however, only transgender males and transgender females had higher odds of suicidal ideation and attempts.
The overall findings were unsurprising since the higher rates of suicidality among transgender youth have already been documented, but the classification of participants was interesting, Gerald Montano, DO, an assistant professor of pediatrics at the University of Pittsburgh School of Medicine, said in an interview. Dr Montano was not involved in the study.
“It’s always been a challenge because, in the past, they always lumped transgender youth along with lesbian, gay, and bisexual youth,” Dr Montano said. This study is one of the few to go into more detail in considering participants’ gender identity, which was wise given that suicidal risk may differ accordingly.
The biggest take-home message of this study is the importance of screening for suicidality after informing adolescent patients of the limits of confidentiality, Dr Montano said.
“I think it’s very important for the physician to be aware of the reasons for those thoughts of suicide,” he continued. “A lot of it has to do with their gender identity and from discrimination and stigma from the general population.”
The research was funded by the University of Pittsburgh Central Research Development Fund and the National Institutes of Health. The authors reported no conflicts of interest.
SOURCE: Thoma BC et al, Pediatrics, October 14, 2019. DOI: 10.1542/peds.2019-1183
FROM PEDIATRICS
Key clinical point: Transgender adolescents should be screened for suicidality.
Major finding: Transgender youth as well as nonbinary adolescents assigned female at birth are at markedly high risk for suicidal ideation and attempt.
Study details: The findings are based on a cross-sectional survey of 2,020 U.S. adolescents, including 1,134 transgender or gender-diverse adolescents.
Disclosures: The research was funded by the University of Pittsburgh Central Research Development Fund and the National Institutes of Health. The authors reported no conflicts of interest.
Source: Thoma BC et al. Pediatrics. October 14, 2019. DOI: 10.1542/peds.2019-1183
Suicide attempts up in black U.S. teens
New research presents a complex picture of self-reported suicidal behavior in U.S. teenagers over the last few decades. Rates of suicidal ideation and plans dipped overall from 1991 to 2017, but the rate of suicide attempts grew slightly in black adolescents.
White young people “have historically had higher rates of suicide attempts...compared with their black counterparts; however, this study provides some evidence to the contrary,” wrote the authors of the report, which appears in the November issue of Pediatrics.
The investigators, led by Michael A. Lindsey, PhD, executive director of the McSilver Institute for Poverty Policy and Research at New York University, New York, note that suicide is the second leading cause of death in the United States in those aged 12-17. (Accidents rank first.) According to recent research, black children younger than 12 are at higher risk for death by suicide, compared with whites.
For the new study, researchers analyzed data from the Youth Risk Behavior Survey, which is conducted every 2 years among high school students in all 50 states and the District of Columbia. They examined data from 198,540 teens (mean age=16, 51% male; 49% female).
During the study period, the weighted overall prevalences rates of suicidal ideation, planning, attempt, and injury due to attempt were 19%, 15%, 8%, and 3%, respectively. “Our findings reveal that over that span of time, almost 1 in 5 adolescents are thinking about suicide... and > 1 in 10 has a suicide plan,” the researchers wrote.
Rates of suicidal ideation and planning fell overall, and among females, the rate of suicide attempts fell significantly (odds ratio [OR]=0.98). But self-reported suicide attempts grew significantly among black teens (OR=1.02), and injuries due to suicide attempts grew among black males (OR=1.04).
The findings are “troubling because attempts are the most prominent risk factor associated with suicide death,” the study authors wrote. “Findings regarding the rising rates of suicide attempts in black youth may be related to the documented disparities in mental health treatment and common social etiologic factors disproportionately experienced by black youth.”
In an accompanying commentary, psychiatrist Benjamin N. Shain, MD, PhD, of the University of Chicago, noted a seemingly “counterintuitive” fact: Black teens still have lower rates of suicide than whites teens “despite the greater, long-standing difficulties encountered by black adolescents, including disparities in mental health treatment and disproportionately higher stressors, racial discrimination, and childhood abuse and neglect, as well as other adverse experiences, such as poverty.”
It’s not clear why the reported suicide rate is lower in black adolescents than their white counterparts, Dr. Shain wrote, but misclassification and “undercount as a result of violence with suicidal intent, for example, ‘suicide by cop’” may play a role. Additionally, protective factors may have kept suicide rates down. “External attributional orientation (eg, blaming others or ‘the system’ for difficulties) among blacks may have buffered this group from internalizing blame related to psychological stressors,” he wrote.
Among black adolescents, the growing rate of suicidal behavior is concerning and may be due to a weakening of the hypothesized protective mechanism. Perhaps, Dr. Shain wrote, they now are blaming themselves more for difficulties encountered, “thus leading to an increase in suicide risk factors, particularly depression.” He stressed that, regardless of the reasons for the increase in suicide and suicide attempts, prevention and intervention efforts remain critical.
No study funding is reported, and authors report no relevant disclosures. Dr. Shain reports no relevant disclosures.
SOURCE: Lindsey MA et al, Pediatrics. 2019;144(5): e20191187, DOI:10.1542/peds.2019-1187.
New research presents a complex picture of self-reported suicidal behavior in U.S. teenagers over the last few decades. Rates of suicidal ideation and plans dipped overall from 1991 to 2017, but the rate of suicide attempts grew slightly in black adolescents.
White young people “have historically had higher rates of suicide attempts...compared with their black counterparts; however, this study provides some evidence to the contrary,” wrote the authors of the report, which appears in the November issue of Pediatrics.
The investigators, led by Michael A. Lindsey, PhD, executive director of the McSilver Institute for Poverty Policy and Research at New York University, New York, note that suicide is the second leading cause of death in the United States in those aged 12-17. (Accidents rank first.) According to recent research, black children younger than 12 are at higher risk for death by suicide, compared with whites.
For the new study, researchers analyzed data from the Youth Risk Behavior Survey, which is conducted every 2 years among high school students in all 50 states and the District of Columbia. They examined data from 198,540 teens (mean age=16, 51% male; 49% female).
During the study period, the weighted overall prevalences rates of suicidal ideation, planning, attempt, and injury due to attempt were 19%, 15%, 8%, and 3%, respectively. “Our findings reveal that over that span of time, almost 1 in 5 adolescents are thinking about suicide... and > 1 in 10 has a suicide plan,” the researchers wrote.
Rates of suicidal ideation and planning fell overall, and among females, the rate of suicide attempts fell significantly (odds ratio [OR]=0.98). But self-reported suicide attempts grew significantly among black teens (OR=1.02), and injuries due to suicide attempts grew among black males (OR=1.04).
The findings are “troubling because attempts are the most prominent risk factor associated with suicide death,” the study authors wrote. “Findings regarding the rising rates of suicide attempts in black youth may be related to the documented disparities in mental health treatment and common social etiologic factors disproportionately experienced by black youth.”
In an accompanying commentary, psychiatrist Benjamin N. Shain, MD, PhD, of the University of Chicago, noted a seemingly “counterintuitive” fact: Black teens still have lower rates of suicide than whites teens “despite the greater, long-standing difficulties encountered by black adolescents, including disparities in mental health treatment and disproportionately higher stressors, racial discrimination, and childhood abuse and neglect, as well as other adverse experiences, such as poverty.”
It’s not clear why the reported suicide rate is lower in black adolescents than their white counterparts, Dr. Shain wrote, but misclassification and “undercount as a result of violence with suicidal intent, for example, ‘suicide by cop’” may play a role. Additionally, protective factors may have kept suicide rates down. “External attributional orientation (eg, blaming others or ‘the system’ for difficulties) among blacks may have buffered this group from internalizing blame related to psychological stressors,” he wrote.
Among black adolescents, the growing rate of suicidal behavior is concerning and may be due to a weakening of the hypothesized protective mechanism. Perhaps, Dr. Shain wrote, they now are blaming themselves more for difficulties encountered, “thus leading to an increase in suicide risk factors, particularly depression.” He stressed that, regardless of the reasons for the increase in suicide and suicide attempts, prevention and intervention efforts remain critical.
No study funding is reported, and authors report no relevant disclosures. Dr. Shain reports no relevant disclosures.
SOURCE: Lindsey MA et al, Pediatrics. 2019;144(5): e20191187, DOI:10.1542/peds.2019-1187.
New research presents a complex picture of self-reported suicidal behavior in U.S. teenagers over the last few decades. Rates of suicidal ideation and plans dipped overall from 1991 to 2017, but the rate of suicide attempts grew slightly in black adolescents.
White young people “have historically had higher rates of suicide attempts...compared with their black counterparts; however, this study provides some evidence to the contrary,” wrote the authors of the report, which appears in the November issue of Pediatrics.
The investigators, led by Michael A. Lindsey, PhD, executive director of the McSilver Institute for Poverty Policy and Research at New York University, New York, note that suicide is the second leading cause of death in the United States in those aged 12-17. (Accidents rank first.) According to recent research, black children younger than 12 are at higher risk for death by suicide, compared with whites.
For the new study, researchers analyzed data from the Youth Risk Behavior Survey, which is conducted every 2 years among high school students in all 50 states and the District of Columbia. They examined data from 198,540 teens (mean age=16, 51% male; 49% female).
During the study period, the weighted overall prevalences rates of suicidal ideation, planning, attempt, and injury due to attempt were 19%, 15%, 8%, and 3%, respectively. “Our findings reveal that over that span of time, almost 1 in 5 adolescents are thinking about suicide... and > 1 in 10 has a suicide plan,” the researchers wrote.
Rates of suicidal ideation and planning fell overall, and among females, the rate of suicide attempts fell significantly (odds ratio [OR]=0.98). But self-reported suicide attempts grew significantly among black teens (OR=1.02), and injuries due to suicide attempts grew among black males (OR=1.04).
The findings are “troubling because attempts are the most prominent risk factor associated with suicide death,” the study authors wrote. “Findings regarding the rising rates of suicide attempts in black youth may be related to the documented disparities in mental health treatment and common social etiologic factors disproportionately experienced by black youth.”
In an accompanying commentary, psychiatrist Benjamin N. Shain, MD, PhD, of the University of Chicago, noted a seemingly “counterintuitive” fact: Black teens still have lower rates of suicide than whites teens “despite the greater, long-standing difficulties encountered by black adolescents, including disparities in mental health treatment and disproportionately higher stressors, racial discrimination, and childhood abuse and neglect, as well as other adverse experiences, such as poverty.”
It’s not clear why the reported suicide rate is lower in black adolescents than their white counterparts, Dr. Shain wrote, but misclassification and “undercount as a result of violence with suicidal intent, for example, ‘suicide by cop’” may play a role. Additionally, protective factors may have kept suicide rates down. “External attributional orientation (eg, blaming others or ‘the system’ for difficulties) among blacks may have buffered this group from internalizing blame related to psychological stressors,” he wrote.
Among black adolescents, the growing rate of suicidal behavior is concerning and may be due to a weakening of the hypothesized protective mechanism. Perhaps, Dr. Shain wrote, they now are blaming themselves more for difficulties encountered, “thus leading to an increase in suicide risk factors, particularly depression.” He stressed that, regardless of the reasons for the increase in suicide and suicide attempts, prevention and intervention efforts remain critical.
No study funding is reported, and authors report no relevant disclosures. Dr. Shain reports no relevant disclosures.
SOURCE: Lindsey MA et al, Pediatrics. 2019;144(5): e20191187, DOI:10.1542/peds.2019-1187.
FROM PEDIATRICS
Key clinical point: Overall self-reported suicidal behavior is down in U.S. teens, but attempts are up in black adolescents.
Major finding: Self-reported suicide attempts grew significantly among black teens (OR=1.02).
Study details: Retrospective analysis of 1991-2017 surveys of 198,540 U.S. teens (mean age=16, 51% male; 49% female).
Disclosures: No study funding is reported, and the study authors report no relevant disclosures.
Source: Lindsey MA et al, Pediatrics. 2019;144(5): e20191187,https://doi.org/10.1542/peds.2019-1187.
Influenza: U.S. activity was low this summer
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.
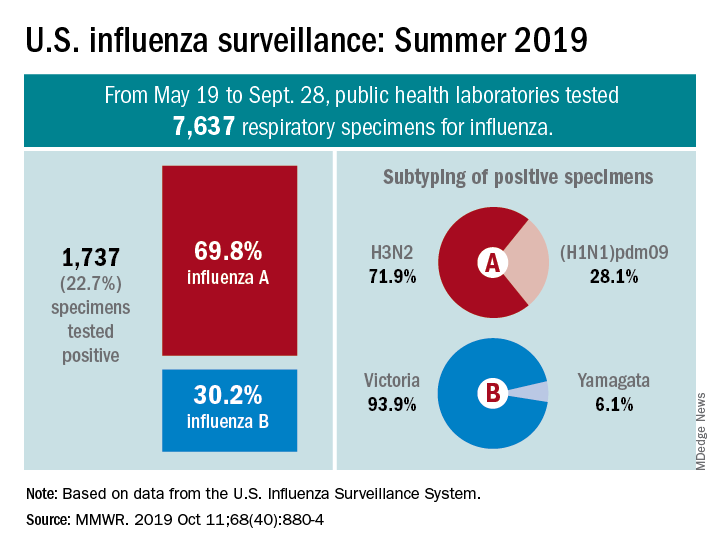
From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.

From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.

From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
FROM MMWR
Tape strips useful to identify biomarkers in skin of young children with atopic dermatitis
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
FROM JAMA DERMATOLOGY
Much work to be done in optimizing treatment for transgender children
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASBMR 2019
Newly described lung disorder strikes children with systemic juvenile idiopathic arthritis
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.
Congenital heart disease in children linked to increased autism risk
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
FROM PEDIATRICS
Key clinical point: Children born with congenital heart disease have higher odds of developing autism, especially with certain forms of CHD, such as atrial and ventricular septal defects.
Major finding: After sensitivity analysis, children with congenital heart disease had increased odds of autism, compared with controls (odds ratio, 1.32; 95% confidence interval, 1.10-1.59).
Study details: A case-control study of children enrolled in the U.S. Military Health System from 2001 to 2013.
Disclosures: The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
Source: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.