User login
Recently Immunized Febrile Infants Have Low Infection Risk
TOPLINE:
METHODOLOGY:
- Researchers evaluated 508 infants aged 6-12 weeks who presented with a fever of 38 °C or greater at two US military academic emergency departments (EDs) over a span of 4 years.
- The infants were categorized as “recently immunized” if they had received immunizations within 72 hours before ED presentation and “not recently immunized” if they had not. Among the 508 infants, 114 were immunized recently.
- The primary outcome was the prevalence of a serious bacterial infection (SBI), categorized into IBI and non-IBI on the basis of culture and radiography findings.
TAKEAWAY:
- The prevalence of SBI was 3.5% in the recently immunized febrile infants and 13.7% in not recently immunized febrile infants.
- Among the recently immunized infants, the prevalence of SBI was lower in those immunized within the first 24 hours than those immunized more than 24 hours before ED presentation (2% vs 14.3%, respectively).
- Almost all identified SBI cases were of urinary tract infection (UTI), with the only non-UTI case being pneumonia in an infant who exhibited respiratory symptoms within 24 hours of receiving immunization.
IN PRACTICE:
Physicians should discuss the possibilities of a less invasive approach for evaluating recently immunized febrile infants. The study findings support the general recommendation to obtain a urinalysis for all recently immunized infants over 60 days presenting with fever, including those presenting less than 24 hours post immunization.
SOURCE:
This study, led by Kyla Casey, MD, Department of Emergency Medicine, Naval Medical Center San Diego, was published online in The American Journal of Emergency Medicine.
LIMITATIONS:
The small sample size and retrospective design might have resulted in an overestimation of outcomes like IBIs within 24 hours after immunization. As the study was conducted in a specific clinical setting with febrile infants from military medical centers, the findings may have limited generalizability. Moreover, the inclusion of premature infants without age correction for prematurity could have impacted the prevalence of IBIs. Factors like missing vaccination history, healthcare referral patterns, and immunization practices in the military system may have introduced bias.
DISCLOSURE:
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The authors had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers evaluated 508 infants aged 6-12 weeks who presented with a fever of 38 °C or greater at two US military academic emergency departments (EDs) over a span of 4 years.
- The infants were categorized as “recently immunized” if they had received immunizations within 72 hours before ED presentation and “not recently immunized” if they had not. Among the 508 infants, 114 were immunized recently.
- The primary outcome was the prevalence of a serious bacterial infection (SBI), categorized into IBI and non-IBI on the basis of culture and radiography findings.
TAKEAWAY:
- The prevalence of SBI was 3.5% in the recently immunized febrile infants and 13.7% in not recently immunized febrile infants.
- Among the recently immunized infants, the prevalence of SBI was lower in those immunized within the first 24 hours than those immunized more than 24 hours before ED presentation (2% vs 14.3%, respectively).
- Almost all identified SBI cases were of urinary tract infection (UTI), with the only non-UTI case being pneumonia in an infant who exhibited respiratory symptoms within 24 hours of receiving immunization.
IN PRACTICE:
Physicians should discuss the possibilities of a less invasive approach for evaluating recently immunized febrile infants. The study findings support the general recommendation to obtain a urinalysis for all recently immunized infants over 60 days presenting with fever, including those presenting less than 24 hours post immunization.
SOURCE:
This study, led by Kyla Casey, MD, Department of Emergency Medicine, Naval Medical Center San Diego, was published online in The American Journal of Emergency Medicine.
LIMITATIONS:
The small sample size and retrospective design might have resulted in an overestimation of outcomes like IBIs within 24 hours after immunization. As the study was conducted in a specific clinical setting with febrile infants from military medical centers, the findings may have limited generalizability. Moreover, the inclusion of premature infants without age correction for prematurity could have impacted the prevalence of IBIs. Factors like missing vaccination history, healthcare referral patterns, and immunization practices in the military system may have introduced bias.
DISCLOSURE:
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The authors had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers evaluated 508 infants aged 6-12 weeks who presented with a fever of 38 °C or greater at two US military academic emergency departments (EDs) over a span of 4 years.
- The infants were categorized as “recently immunized” if they had received immunizations within 72 hours before ED presentation and “not recently immunized” if they had not. Among the 508 infants, 114 were immunized recently.
- The primary outcome was the prevalence of a serious bacterial infection (SBI), categorized into IBI and non-IBI on the basis of culture and radiography findings.
TAKEAWAY:
- The prevalence of SBI was 3.5% in the recently immunized febrile infants and 13.7% in not recently immunized febrile infants.
- Among the recently immunized infants, the prevalence of SBI was lower in those immunized within the first 24 hours than those immunized more than 24 hours before ED presentation (2% vs 14.3%, respectively).
- Almost all identified SBI cases were of urinary tract infection (UTI), with the only non-UTI case being pneumonia in an infant who exhibited respiratory symptoms within 24 hours of receiving immunization.
IN PRACTICE:
Physicians should discuss the possibilities of a less invasive approach for evaluating recently immunized febrile infants. The study findings support the general recommendation to obtain a urinalysis for all recently immunized infants over 60 days presenting with fever, including those presenting less than 24 hours post immunization.
SOURCE:
This study, led by Kyla Casey, MD, Department of Emergency Medicine, Naval Medical Center San Diego, was published online in The American Journal of Emergency Medicine.
LIMITATIONS:
The small sample size and retrospective design might have resulted in an overestimation of outcomes like IBIs within 24 hours after immunization. As the study was conducted in a specific clinical setting with febrile infants from military medical centers, the findings may have limited generalizability. Moreover, the inclusion of premature infants without age correction for prematurity could have impacted the prevalence of IBIs. Factors like missing vaccination history, healthcare referral patterns, and immunization practices in the military system may have introduced bias.
DISCLOSURE:
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors. The authors had no conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
Children With ASD May Have Earlier Onset of Suicidal Thoughts, Behaviors
, according to a research letter in JAMA Pediatrics.
Suicide rates among all US children ages 10-14 years tripled between 2007 and 2021, becoming the second leading cause of death for this age bracket. Between 2018 and 2021, 315 suicides were reported among US children ages 5 to 11 years.
People with ASD show increased rates of STB, although prevalence estimates vary by study, which led the authors to study the issue.
Lead author Benjamin Joffe Schindel, MD, MPH, a fellow in neurodevelopmental medicine at the Kennedy Krieger Institute in Columbia, Maryland, and colleagues, analyzed responses from 968 caregivers of children ages 8-25 with ASD.
They found the following reported lifetime STB incidence:
- 392 (40.5%) reported wanting to die
- 187 (19.3%) reported wanting to end their own lives
- 72 (7.4%) reported having a suicide plan
Among those answering affirmatively to each of the above questions regarding STB, onset at 8 years or younger was reported in 142 (36.2%); 66 (35.3%); and 13 (18.1%) of the children, respectively. Included in the findings was one suicide attempt by cutting in an 8-year-old child.
Dr. Schindel said though there is no direct comparison with age of these thoughts among the general population, a previous study in 2013 showed that through age 10 prevalence of suicide ideation is very low (< 1%), then increases slowly through age 12 and then more rapidly until age 17.
Disturbing Findings
“The unexpectedly high frequency of STBs among children with ASD who were 8 years or younger is particularly disturbing given the lack of validated suicide risk screening tools and interventions for this age group,” the authors wrote. They added that early start of STB in children with ASD is important as this population has been underrepresented in suicide research and prevention efforts.
The average child age in this study was 13.4; 84.8% were White; and 81% were male. More than half of the children (54.8%) were taking medications for emotional, behavioral, or mood-related issues.
Data were collected from May to October 2017 from responses to the Mental Health and Suicidal Behaviors Questionnaire, an online caregiver-answered survey. The survey was created and distributed by the Interactive Autism Network (IAN), an international autism registry, from 2006 to 2019 with approximately 55, 000 participating families.
Thoughts Come at a ‘Shockingly Young Age’
Suzanne Rybczynski, MD, chief medical officer at East Tennessee Children’s Hospital in Knoxville, who was not part of the research, said the study was small but will help get the message out that “kids start thinking about suicide, especially kids with autism,” at a “shockingly young age.”
The results demonstrate the great demand for studying thoughts and behaviors especially in younger children and in children with neurodiversity — autism or other neurodevelopmental disabilities.
Studying children with ASD in relation to suicidal thoughts is difficult, Dr. Rybczynski said, because the way they think about death and how much is understood about the finality of suicide has not been well studied. It’s also uncertain how well the children understood the questions in this study, she added.
This retrospective study also asked for responses from caregivers who may remember or interpret a child’s thoughts and words differently from the child’s true intent, Dr. Rybczynski said.
“We need more studies like this asking questions to kids directly,” she said, so researchers can figure what children think it means to die.
Current Screening Recommendations
Current recommendations from the American Academy of Pediatrics (AAP) are to screen children universally for suicide risk at age 12 using a validated tool and if there are behavioral health concerns, screen as needed from ages 8 to 12.
This study suggests that screening needs to start earlier, Dr. Rybczynski said. “But we also need to know that we’re asking the right questions” and whether questions might be different for children with different abilities.
Children who are less verbal are often not included in screening. Screening studies often specifically exclude children with neurodisabilities, she explained. Getting these youngsters involved and making appropriate screening available “would be lifesaving,” she said.
“There are no validated (screening) tools down to age 8, which is not to say that some organizations don’t use them, but they’re not validated,” she said.
Dr. Rybczynski pointed out that most of the children were White and male and future work investigating these thoughts in girls and other racial/ethnic groups with ASD will be important as well. In addition, it will be important to revisit the issue post-pandemic with the rise in mental health issues with COVID-19.
Identifying children struggling with thoughts of suicide is the key to preventing tragedy, Dr. Rybczynski said, adding, “All those deaths are avoidable.”
Various study coauthors disclosed ties to the Simons Foundation, the Patient-Centered Outcomes Research Institute, the US Social Security Administration, American Foundation for Suicide Prevention, and Sarepta. No other disclosures were reported. Dr. Rybczynski, who provided commentary on the study, has no relevant financial relationships.
, according to a research letter in JAMA Pediatrics.
Suicide rates among all US children ages 10-14 years tripled between 2007 and 2021, becoming the second leading cause of death for this age bracket. Between 2018 and 2021, 315 suicides were reported among US children ages 5 to 11 years.
People with ASD show increased rates of STB, although prevalence estimates vary by study, which led the authors to study the issue.
Lead author Benjamin Joffe Schindel, MD, MPH, a fellow in neurodevelopmental medicine at the Kennedy Krieger Institute in Columbia, Maryland, and colleagues, analyzed responses from 968 caregivers of children ages 8-25 with ASD.
They found the following reported lifetime STB incidence:
- 392 (40.5%) reported wanting to die
- 187 (19.3%) reported wanting to end their own lives
- 72 (7.4%) reported having a suicide plan
Among those answering affirmatively to each of the above questions regarding STB, onset at 8 years or younger was reported in 142 (36.2%); 66 (35.3%); and 13 (18.1%) of the children, respectively. Included in the findings was one suicide attempt by cutting in an 8-year-old child.
Dr. Schindel said though there is no direct comparison with age of these thoughts among the general population, a previous study in 2013 showed that through age 10 prevalence of suicide ideation is very low (< 1%), then increases slowly through age 12 and then more rapidly until age 17.
Disturbing Findings
“The unexpectedly high frequency of STBs among children with ASD who were 8 years or younger is particularly disturbing given the lack of validated suicide risk screening tools and interventions for this age group,” the authors wrote. They added that early start of STB in children with ASD is important as this population has been underrepresented in suicide research and prevention efforts.
The average child age in this study was 13.4; 84.8% were White; and 81% were male. More than half of the children (54.8%) were taking medications for emotional, behavioral, or mood-related issues.
Data were collected from May to October 2017 from responses to the Mental Health and Suicidal Behaviors Questionnaire, an online caregiver-answered survey. The survey was created and distributed by the Interactive Autism Network (IAN), an international autism registry, from 2006 to 2019 with approximately 55, 000 participating families.
Thoughts Come at a ‘Shockingly Young Age’
Suzanne Rybczynski, MD, chief medical officer at East Tennessee Children’s Hospital in Knoxville, who was not part of the research, said the study was small but will help get the message out that “kids start thinking about suicide, especially kids with autism,” at a “shockingly young age.”
The results demonstrate the great demand for studying thoughts and behaviors especially in younger children and in children with neurodiversity — autism or other neurodevelopmental disabilities.
Studying children with ASD in relation to suicidal thoughts is difficult, Dr. Rybczynski said, because the way they think about death and how much is understood about the finality of suicide has not been well studied. It’s also uncertain how well the children understood the questions in this study, she added.
This retrospective study also asked for responses from caregivers who may remember or interpret a child’s thoughts and words differently from the child’s true intent, Dr. Rybczynski said.
“We need more studies like this asking questions to kids directly,” she said, so researchers can figure what children think it means to die.
Current Screening Recommendations
Current recommendations from the American Academy of Pediatrics (AAP) are to screen children universally for suicide risk at age 12 using a validated tool and if there are behavioral health concerns, screen as needed from ages 8 to 12.
This study suggests that screening needs to start earlier, Dr. Rybczynski said. “But we also need to know that we’re asking the right questions” and whether questions might be different for children with different abilities.
Children who are less verbal are often not included in screening. Screening studies often specifically exclude children with neurodisabilities, she explained. Getting these youngsters involved and making appropriate screening available “would be lifesaving,” she said.
“There are no validated (screening) tools down to age 8, which is not to say that some organizations don’t use them, but they’re not validated,” she said.
Dr. Rybczynski pointed out that most of the children were White and male and future work investigating these thoughts in girls and other racial/ethnic groups with ASD will be important as well. In addition, it will be important to revisit the issue post-pandemic with the rise in mental health issues with COVID-19.
Identifying children struggling with thoughts of suicide is the key to preventing tragedy, Dr. Rybczynski said, adding, “All those deaths are avoidable.”
Various study coauthors disclosed ties to the Simons Foundation, the Patient-Centered Outcomes Research Institute, the US Social Security Administration, American Foundation for Suicide Prevention, and Sarepta. No other disclosures were reported. Dr. Rybczynski, who provided commentary on the study, has no relevant financial relationships.
, according to a research letter in JAMA Pediatrics.
Suicide rates among all US children ages 10-14 years tripled between 2007 and 2021, becoming the second leading cause of death for this age bracket. Between 2018 and 2021, 315 suicides were reported among US children ages 5 to 11 years.
People with ASD show increased rates of STB, although prevalence estimates vary by study, which led the authors to study the issue.
Lead author Benjamin Joffe Schindel, MD, MPH, a fellow in neurodevelopmental medicine at the Kennedy Krieger Institute in Columbia, Maryland, and colleagues, analyzed responses from 968 caregivers of children ages 8-25 with ASD.
They found the following reported lifetime STB incidence:
- 392 (40.5%) reported wanting to die
- 187 (19.3%) reported wanting to end their own lives
- 72 (7.4%) reported having a suicide plan
Among those answering affirmatively to each of the above questions regarding STB, onset at 8 years or younger was reported in 142 (36.2%); 66 (35.3%); and 13 (18.1%) of the children, respectively. Included in the findings was one suicide attempt by cutting in an 8-year-old child.
Dr. Schindel said though there is no direct comparison with age of these thoughts among the general population, a previous study in 2013 showed that through age 10 prevalence of suicide ideation is very low (< 1%), then increases slowly through age 12 and then more rapidly until age 17.
Disturbing Findings
“The unexpectedly high frequency of STBs among children with ASD who were 8 years or younger is particularly disturbing given the lack of validated suicide risk screening tools and interventions for this age group,” the authors wrote. They added that early start of STB in children with ASD is important as this population has been underrepresented in suicide research and prevention efforts.
The average child age in this study was 13.4; 84.8% were White; and 81% were male. More than half of the children (54.8%) were taking medications for emotional, behavioral, or mood-related issues.
Data were collected from May to October 2017 from responses to the Mental Health and Suicidal Behaviors Questionnaire, an online caregiver-answered survey. The survey was created and distributed by the Interactive Autism Network (IAN), an international autism registry, from 2006 to 2019 with approximately 55, 000 participating families.
Thoughts Come at a ‘Shockingly Young Age’
Suzanne Rybczynski, MD, chief medical officer at East Tennessee Children’s Hospital in Knoxville, who was not part of the research, said the study was small but will help get the message out that “kids start thinking about suicide, especially kids with autism,” at a “shockingly young age.”
The results demonstrate the great demand for studying thoughts and behaviors especially in younger children and in children with neurodiversity — autism or other neurodevelopmental disabilities.
Studying children with ASD in relation to suicidal thoughts is difficult, Dr. Rybczynski said, because the way they think about death and how much is understood about the finality of suicide has not been well studied. It’s also uncertain how well the children understood the questions in this study, she added.
This retrospective study also asked for responses from caregivers who may remember or interpret a child’s thoughts and words differently from the child’s true intent, Dr. Rybczynski said.
“We need more studies like this asking questions to kids directly,” she said, so researchers can figure what children think it means to die.
Current Screening Recommendations
Current recommendations from the American Academy of Pediatrics (AAP) are to screen children universally for suicide risk at age 12 using a validated tool and if there are behavioral health concerns, screen as needed from ages 8 to 12.
This study suggests that screening needs to start earlier, Dr. Rybczynski said. “But we also need to know that we’re asking the right questions” and whether questions might be different for children with different abilities.
Children who are less verbal are often not included in screening. Screening studies often specifically exclude children with neurodisabilities, she explained. Getting these youngsters involved and making appropriate screening available “would be lifesaving,” she said.
“There are no validated (screening) tools down to age 8, which is not to say that some organizations don’t use them, but they’re not validated,” she said.
Dr. Rybczynski pointed out that most of the children were White and male and future work investigating these thoughts in girls and other racial/ethnic groups with ASD will be important as well. In addition, it will be important to revisit the issue post-pandemic with the rise in mental health issues with COVID-19.
Identifying children struggling with thoughts of suicide is the key to preventing tragedy, Dr. Rybczynski said, adding, “All those deaths are avoidable.”
Various study coauthors disclosed ties to the Simons Foundation, the Patient-Centered Outcomes Research Institute, the US Social Security Administration, American Foundation for Suicide Prevention, and Sarepta. No other disclosures were reported. Dr. Rybczynski, who provided commentary on the study, has no relevant financial relationships.
FROM JAMA PEDIATRICS
The Rise of Positive Psychiatry (and How Pediatrics Can Join the Effort)
Psychiatry, like all medical disciplines, changes over time. For many decades, psychiatrists were primarily psychotherapists. As medications slowly became available, these became a second tool for treatment — so much so that by the 21st century many, if not most, psychiatrists saw themselves primarily as psychopharmacologists and diagnosticians who were skilled at identifying various forms of mental illness and using medications in the hopes of inducing a clinically meaningful “response” in symptoms. While still belonging to the umbrella category of a mental health professional, more and more psychiatrists trained and practiced as mental illness professionals.
Slowly, however, there have been stirrings within the field by many who have found the identity of the psychiatrist as a “prescriber” to be too narrow, and the current “med check” model of treatment too confining. This change was partly inspired by our colleagues in clinical psychology who were challenged in the 1990s by then American Psychological Association President Martin Seligman, PhD, to develop knowledge and expertise not only in alleviating mental suffering but also in promoting true mental well-being, a construct that still was often vaguely defined. One framework of well-being that was advanced at the time was the PERMA model, representing the five well-being dimensions of Positive emotions, Engagement, Relationships, Meaning, and Accomplishment.1
While there have always been those in psychiatry who have advocated for a broad emphasis that incorporates the full spectrum of mental health, there has been a surge of interest in the past 10-15 years, urging a focus on well-being and the tools that can help a person achieve it. This trend has variably been referred to as positive psychiatry, lifestyle psychiatry, and other terms.2 As one might expect, child and adolescent psychiatry has been particularly fertile ground for such principles, and models such as the Vermont Family Based Approach have expanded the concept beyond the individual to the family and even community.3
It is important to note here that embracing the concept of well-being in treatment does not in any way require one to abandon the idea that genetic or environmental factors can lead to negative outcomes in brain development, nor does it mandate that one leaves behind important treatment modalities such as traditional psychotherapy and medication treatment. Further, this approach should not be confused with some “wellness” activities that offer quick fixes and lack scientific rigor. Positive psychiatry does, however, offer a third pathway to advance positive emotional behavioral growth, namely through health promotion activities ranging from exercise to good nutrition to positive parenting in ways that have been shown to benefit both those who are already doing fairly well as well as those who are actively struggling with significant psychiatric disorders.4
Primary care clinicians already have extensive familiarity talking about these kinds of health promoting activities with families. That said, it’s been my observation from many years of doing consultations and reviewing notes that these conversations happen almost exclusively during well-check visits and can get forgotten when a child presents with emotional behavioral challenges.
So how can the primary care clinician who is interested in more fully incorporating the burgeoning science on well-being work these principles into routine practice? Here are three suggestions.
Ask Some New Questions
It’s difficult to treat things that aren’t assessed. To best incorporate true mental health within one’s work with families, it can be very helpful to expand the regular questions one asks to include those that address some of the PERMA and health promotion areas described above. Some examples could include the following:
- Hopes. What would a perfect life look like for you when you’re older?
- Connection. Is there anything that you just love doing, so much so that time sometimes just seems to go away?
- Strengths. What are you good at? What good things would your friends say about you?
- Parenting. What are you most proud of as a parent, and where are your biggest challenges?
- Nutrition. What does a typical school day breakfast look like for you?
- Screens. Do you have any restrictions related to what you do on screens?
- Sleep. Tell me about your typical bedtime routine.
Add Some New Interventions
Counseling and medications can be powerful ways to bring improvement in a child’s life, but thinking about health promotion opens up a whole new avenue for intervention. This domain includes areas like physical activity, nutrition, sleep practices, parenting, participation in music and the arts, practicing kindness towards others, and mindfulness, among others.
For someone newly diagnosed with ADHD, for example, consider expanding your treatment plan to include not only medications but also specific guidance to exercise more, limit screen usage, practice good bedtime routines, eat a real breakfast, and reduce the helicopter parenting. Monitor these areas over time.
Another example relates to common sleep problems. Before making that melatonin recommendation, ask yourself if you understand what is happening in that child’s environment at night. Are they allowed to play video games until 2 a.m.? Are they taking naps during the day because they have nothing to do? Are they downing caffeinated drinks with dinner? Does the child get zero physical activity outside of the PE class? Maybe you still will need the melatonin, but perhaps other areas need to be addressed first.
Find Some New Colleagues
While it can be challenging sometimes to find anyone in mental health who sees new patients, there is value is finding out the approach and methodology that psychiatric clinicians and therapists apply in their practice. Working collaboratively with those who value a well-being orientation and who can work productively with the whole family to increase health promotion can yield benefits for a patient’s long-term physical and mental health.
The renewed interest and attention on well-being and health promotion activities that can optimize brain growth are a welcome and overdue development in mental health treatment. Pediatricians and other primary care clinicians can be a critical part of this growing initiative by gaining knowledge about youth well-being, applying this knowledge in day-to-day practice, and working collaboratively with those who share a similar perspective.
Dr. Rettew is a child & adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Oregon. He is on the psychiatry faculty at Oregon Health & Science University. You can follow him on Facebook and X @PediPsych. His latest book is Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.
References
1. Seligman, MEP. Flourish: a visionary new understanding of happiness and well-being. New York: Simon & Schuster; 2011.
2. Jeste DV, Palmer BW. (Eds.). Positive psychiatry: a clinical handbook. Washington DC: American Psychiatric Publishing; 2015. doi: 10.1176/appi.books.9781615370818.
3. Hudziak J, Ivanova MY. The Vermont family based approach: Family based health promotion, illness prevention, and intervention. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78. doi: 10.1016/j.chc.2015.11.002.
4. Rettew DC. Incorporating positive psychiatry with children and adolescents. Current Psychiatry. 2022 November;21(11):12-16,45. doi: 10.12788/cp.0303.
Psychiatry, like all medical disciplines, changes over time. For many decades, psychiatrists were primarily psychotherapists. As medications slowly became available, these became a second tool for treatment — so much so that by the 21st century many, if not most, psychiatrists saw themselves primarily as psychopharmacologists and diagnosticians who were skilled at identifying various forms of mental illness and using medications in the hopes of inducing a clinically meaningful “response” in symptoms. While still belonging to the umbrella category of a mental health professional, more and more psychiatrists trained and practiced as mental illness professionals.
Slowly, however, there have been stirrings within the field by many who have found the identity of the psychiatrist as a “prescriber” to be too narrow, and the current “med check” model of treatment too confining. This change was partly inspired by our colleagues in clinical psychology who were challenged in the 1990s by then American Psychological Association President Martin Seligman, PhD, to develop knowledge and expertise not only in alleviating mental suffering but also in promoting true mental well-being, a construct that still was often vaguely defined. One framework of well-being that was advanced at the time was the PERMA model, representing the five well-being dimensions of Positive emotions, Engagement, Relationships, Meaning, and Accomplishment.1
While there have always been those in psychiatry who have advocated for a broad emphasis that incorporates the full spectrum of mental health, there has been a surge of interest in the past 10-15 years, urging a focus on well-being and the tools that can help a person achieve it. This trend has variably been referred to as positive psychiatry, lifestyle psychiatry, and other terms.2 As one might expect, child and adolescent psychiatry has been particularly fertile ground for such principles, and models such as the Vermont Family Based Approach have expanded the concept beyond the individual to the family and even community.3
It is important to note here that embracing the concept of well-being in treatment does not in any way require one to abandon the idea that genetic or environmental factors can lead to negative outcomes in brain development, nor does it mandate that one leaves behind important treatment modalities such as traditional psychotherapy and medication treatment. Further, this approach should not be confused with some “wellness” activities that offer quick fixes and lack scientific rigor. Positive psychiatry does, however, offer a third pathway to advance positive emotional behavioral growth, namely through health promotion activities ranging from exercise to good nutrition to positive parenting in ways that have been shown to benefit both those who are already doing fairly well as well as those who are actively struggling with significant psychiatric disorders.4
Primary care clinicians already have extensive familiarity talking about these kinds of health promoting activities with families. That said, it’s been my observation from many years of doing consultations and reviewing notes that these conversations happen almost exclusively during well-check visits and can get forgotten when a child presents with emotional behavioral challenges.
So how can the primary care clinician who is interested in more fully incorporating the burgeoning science on well-being work these principles into routine practice? Here are three suggestions.
Ask Some New Questions
It’s difficult to treat things that aren’t assessed. To best incorporate true mental health within one’s work with families, it can be very helpful to expand the regular questions one asks to include those that address some of the PERMA and health promotion areas described above. Some examples could include the following:
- Hopes. What would a perfect life look like for you when you’re older?
- Connection. Is there anything that you just love doing, so much so that time sometimes just seems to go away?
- Strengths. What are you good at? What good things would your friends say about you?
- Parenting. What are you most proud of as a parent, and where are your biggest challenges?
- Nutrition. What does a typical school day breakfast look like for you?
- Screens. Do you have any restrictions related to what you do on screens?
- Sleep. Tell me about your typical bedtime routine.
Add Some New Interventions
Counseling and medications can be powerful ways to bring improvement in a child’s life, but thinking about health promotion opens up a whole new avenue for intervention. This domain includes areas like physical activity, nutrition, sleep practices, parenting, participation in music and the arts, practicing kindness towards others, and mindfulness, among others.
For someone newly diagnosed with ADHD, for example, consider expanding your treatment plan to include not only medications but also specific guidance to exercise more, limit screen usage, practice good bedtime routines, eat a real breakfast, and reduce the helicopter parenting. Monitor these areas over time.
Another example relates to common sleep problems. Before making that melatonin recommendation, ask yourself if you understand what is happening in that child’s environment at night. Are they allowed to play video games until 2 a.m.? Are they taking naps during the day because they have nothing to do? Are they downing caffeinated drinks with dinner? Does the child get zero physical activity outside of the PE class? Maybe you still will need the melatonin, but perhaps other areas need to be addressed first.
Find Some New Colleagues
While it can be challenging sometimes to find anyone in mental health who sees new patients, there is value is finding out the approach and methodology that psychiatric clinicians and therapists apply in their practice. Working collaboratively with those who value a well-being orientation and who can work productively with the whole family to increase health promotion can yield benefits for a patient’s long-term physical and mental health.
The renewed interest and attention on well-being and health promotion activities that can optimize brain growth are a welcome and overdue development in mental health treatment. Pediatricians and other primary care clinicians can be a critical part of this growing initiative by gaining knowledge about youth well-being, applying this knowledge in day-to-day practice, and working collaboratively with those who share a similar perspective.
Dr. Rettew is a child & adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Oregon. He is on the psychiatry faculty at Oregon Health & Science University. You can follow him on Facebook and X @PediPsych. His latest book is Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.
References
1. Seligman, MEP. Flourish: a visionary new understanding of happiness and well-being. New York: Simon & Schuster; 2011.
2. Jeste DV, Palmer BW. (Eds.). Positive psychiatry: a clinical handbook. Washington DC: American Psychiatric Publishing; 2015. doi: 10.1176/appi.books.9781615370818.
3. Hudziak J, Ivanova MY. The Vermont family based approach: Family based health promotion, illness prevention, and intervention. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78. doi: 10.1016/j.chc.2015.11.002.
4. Rettew DC. Incorporating positive psychiatry with children and adolescents. Current Psychiatry. 2022 November;21(11):12-16,45. doi: 10.12788/cp.0303.
Psychiatry, like all medical disciplines, changes over time. For many decades, psychiatrists were primarily psychotherapists. As medications slowly became available, these became a second tool for treatment — so much so that by the 21st century many, if not most, psychiatrists saw themselves primarily as psychopharmacologists and diagnosticians who were skilled at identifying various forms of mental illness and using medications in the hopes of inducing a clinically meaningful “response” in symptoms. While still belonging to the umbrella category of a mental health professional, more and more psychiatrists trained and practiced as mental illness professionals.
Slowly, however, there have been stirrings within the field by many who have found the identity of the psychiatrist as a “prescriber” to be too narrow, and the current “med check” model of treatment too confining. This change was partly inspired by our colleagues in clinical psychology who were challenged in the 1990s by then American Psychological Association President Martin Seligman, PhD, to develop knowledge and expertise not only in alleviating mental suffering but also in promoting true mental well-being, a construct that still was often vaguely defined. One framework of well-being that was advanced at the time was the PERMA model, representing the five well-being dimensions of Positive emotions, Engagement, Relationships, Meaning, and Accomplishment.1
While there have always been those in psychiatry who have advocated for a broad emphasis that incorporates the full spectrum of mental health, there has been a surge of interest in the past 10-15 years, urging a focus on well-being and the tools that can help a person achieve it. This trend has variably been referred to as positive psychiatry, lifestyle psychiatry, and other terms.2 As one might expect, child and adolescent psychiatry has been particularly fertile ground for such principles, and models such as the Vermont Family Based Approach have expanded the concept beyond the individual to the family and even community.3
It is important to note here that embracing the concept of well-being in treatment does not in any way require one to abandon the idea that genetic or environmental factors can lead to negative outcomes in brain development, nor does it mandate that one leaves behind important treatment modalities such as traditional psychotherapy and medication treatment. Further, this approach should not be confused with some “wellness” activities that offer quick fixes and lack scientific rigor. Positive psychiatry does, however, offer a third pathway to advance positive emotional behavioral growth, namely through health promotion activities ranging from exercise to good nutrition to positive parenting in ways that have been shown to benefit both those who are already doing fairly well as well as those who are actively struggling with significant psychiatric disorders.4
Primary care clinicians already have extensive familiarity talking about these kinds of health promoting activities with families. That said, it’s been my observation from many years of doing consultations and reviewing notes that these conversations happen almost exclusively during well-check visits and can get forgotten when a child presents with emotional behavioral challenges.
So how can the primary care clinician who is interested in more fully incorporating the burgeoning science on well-being work these principles into routine practice? Here are three suggestions.
Ask Some New Questions
It’s difficult to treat things that aren’t assessed. To best incorporate true mental health within one’s work with families, it can be very helpful to expand the regular questions one asks to include those that address some of the PERMA and health promotion areas described above. Some examples could include the following:
- Hopes. What would a perfect life look like for you when you’re older?
- Connection. Is there anything that you just love doing, so much so that time sometimes just seems to go away?
- Strengths. What are you good at? What good things would your friends say about you?
- Parenting. What are you most proud of as a parent, and where are your biggest challenges?
- Nutrition. What does a typical school day breakfast look like for you?
- Screens. Do you have any restrictions related to what you do on screens?
- Sleep. Tell me about your typical bedtime routine.
Add Some New Interventions
Counseling and medications can be powerful ways to bring improvement in a child’s life, but thinking about health promotion opens up a whole new avenue for intervention. This domain includes areas like physical activity, nutrition, sleep practices, parenting, participation in music and the arts, practicing kindness towards others, and mindfulness, among others.
For someone newly diagnosed with ADHD, for example, consider expanding your treatment plan to include not only medications but also specific guidance to exercise more, limit screen usage, practice good bedtime routines, eat a real breakfast, and reduce the helicopter parenting. Monitor these areas over time.
Another example relates to common sleep problems. Before making that melatonin recommendation, ask yourself if you understand what is happening in that child’s environment at night. Are they allowed to play video games until 2 a.m.? Are they taking naps during the day because they have nothing to do? Are they downing caffeinated drinks with dinner? Does the child get zero physical activity outside of the PE class? Maybe you still will need the melatonin, but perhaps other areas need to be addressed first.
Find Some New Colleagues
While it can be challenging sometimes to find anyone in mental health who sees new patients, there is value is finding out the approach and methodology that psychiatric clinicians and therapists apply in their practice. Working collaboratively with those who value a well-being orientation and who can work productively with the whole family to increase health promotion can yield benefits for a patient’s long-term physical and mental health.
The renewed interest and attention on well-being and health promotion activities that can optimize brain growth are a welcome and overdue development in mental health treatment. Pediatricians and other primary care clinicians can be a critical part of this growing initiative by gaining knowledge about youth well-being, applying this knowledge in day-to-day practice, and working collaboratively with those who share a similar perspective.
Dr. Rettew is a child & adolescent psychiatrist and medical director of Lane County Behavioral Health in Eugene, Oregon. He is on the psychiatry faculty at Oregon Health & Science University. You can follow him on Facebook and X @PediPsych. His latest book is Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.
References
1. Seligman, MEP. Flourish: a visionary new understanding of happiness and well-being. New York: Simon & Schuster; 2011.
2. Jeste DV, Palmer BW. (Eds.). Positive psychiatry: a clinical handbook. Washington DC: American Psychiatric Publishing; 2015. doi: 10.1176/appi.books.9781615370818.
3. Hudziak J, Ivanova MY. The Vermont family based approach: Family based health promotion, illness prevention, and intervention. Child Adolesc Psychiatr Clin N Am. 2016 Apr;25(2):167-78. doi: 10.1016/j.chc.2015.11.002.
4. Rettew DC. Incorporating positive psychiatry with children and adolescents. Current Psychiatry. 2022 November;21(11):12-16,45. doi: 10.12788/cp.0303.
Worldwide Uptick in Invasive Group A Streptococcus Disease Post Pandemic — What Should We Know?
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.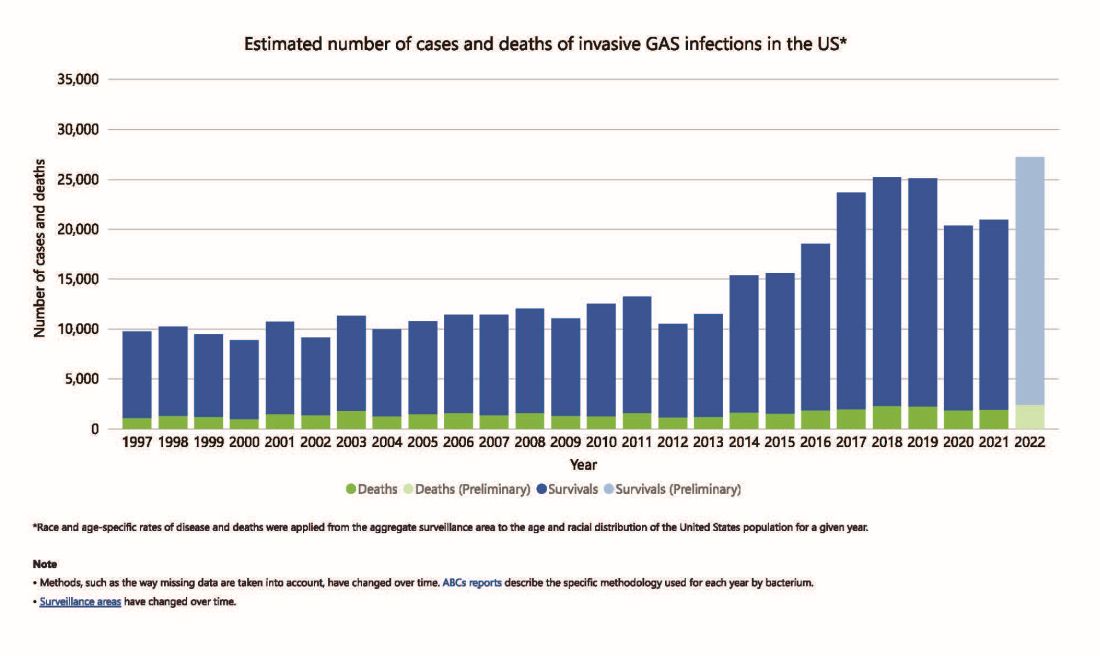
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 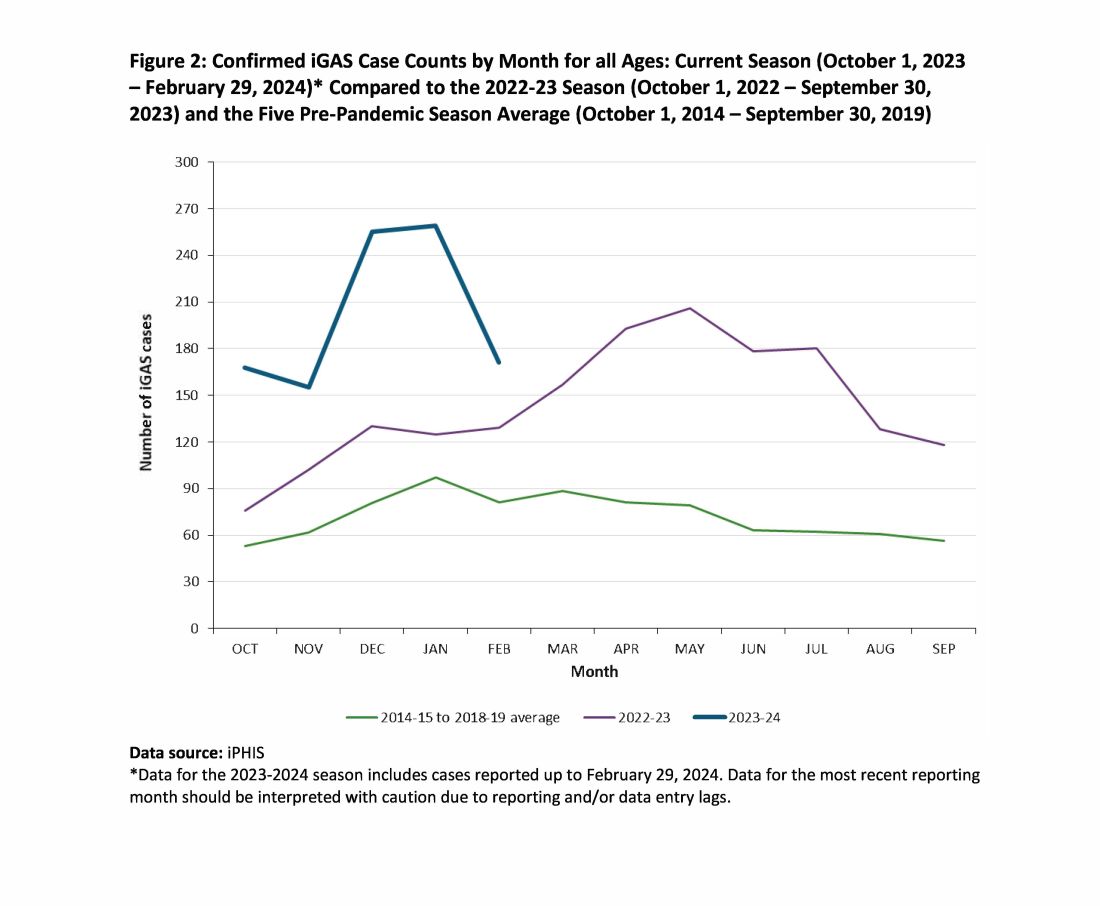
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4 
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Durable Tocilizumab Responses Seen in Trial Extensions of Polyarticular and Systemic JIA Subtypes
TOPLINE:
Subcutaneous tocilizumab provides durable disease control rates in patients with polyarticular and systemic juvenile idiopathic arthritis (pJIA and sJIA, respectively).
METHODOLOGY:
- This long-term extension (LTE) study included 44 patients with pJIA and 38 patients with sJIA, according to the International League of Associations for Rheumatology criteria, from two 52-week phase 1b trials (NCT01904292 and NCT01904279).
- In the core trials, the dosing frequency of subcutaneous tocilizumab was determined by weight: Every 3 weeks for those < 30 kg in pJIA and every 2 weeks for those ≥ 30 kg; in sJIA, initially every 10 days for those < 30 kg, transitioning to every 2 weeks, and weekly for those ≥ 30 kg.
- Patients who had adequate disease control with subcutaneous tocilizumab, comparable with the use of intravenous tocilizumab in the core trials, continued to receive subcutaneous tocilizumab.
- The study outcome was the change in Juvenile Arthritis Disease Activity Score on 71 joints (JADAS-71, range 0-101).
TAKEAWAY:
- Disease control remained stable in both groups, with sustained improvements in median JADAS-71 scores in pJIA (−0.2 with lower frequency dosing to −0.5 with higher frequency) and sJIA (−0.1 at both dosing frequencies).
- In the pJIA group, 90% and 53% of patients weighing < 30 kg and ≥ 30 kg achieved inactive disease, respectively, whereas in the sJIA group, the respective rates were 91% and 92%.
- A total of five of 15 patients with pJIA weighing ≥ 30 kg who received subcutaneous tocilizumab every 2 weeks achieved clinical remission, whereas in other groups, the clinical remission rates ranged from 74% to 92%.
- Six patients with pJIA reported seven serious adverse events (SAEs), while five patients with sJIA experienced six SAEs. Five patients with pJIA and one patient with sJIA reported serious infections.
IN PRACTICE:
The authors concluded that subcutaneous tocilizumab treatment provided long-term disease control in patients with pJIA or sJIA, with a safety profile consistent with past studies of tocilizumab.
SOURCE:
The study was led by Hermine I. Brunner, MD, director of the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center. It was published online in Rheumatology (Oxford).
LIMITATIONS:
The open-label design and lack of a control group limited the analysis. Only a few patients continued the treatment for 5 years.
DISCLOSURES:
This work was supported by F. Hoffmann-La Roche Ltd. Eight authors reported receiving honoraria and consulting or speaker fees from various pharma sources. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Subcutaneous tocilizumab provides durable disease control rates in patients with polyarticular and systemic juvenile idiopathic arthritis (pJIA and sJIA, respectively).
METHODOLOGY:
- This long-term extension (LTE) study included 44 patients with pJIA and 38 patients with sJIA, according to the International League of Associations for Rheumatology criteria, from two 52-week phase 1b trials (NCT01904292 and NCT01904279).
- In the core trials, the dosing frequency of subcutaneous tocilizumab was determined by weight: Every 3 weeks for those < 30 kg in pJIA and every 2 weeks for those ≥ 30 kg; in sJIA, initially every 10 days for those < 30 kg, transitioning to every 2 weeks, and weekly for those ≥ 30 kg.
- Patients who had adequate disease control with subcutaneous tocilizumab, comparable with the use of intravenous tocilizumab in the core trials, continued to receive subcutaneous tocilizumab.
- The study outcome was the change in Juvenile Arthritis Disease Activity Score on 71 joints (JADAS-71, range 0-101).
TAKEAWAY:
- Disease control remained stable in both groups, with sustained improvements in median JADAS-71 scores in pJIA (−0.2 with lower frequency dosing to −0.5 with higher frequency) and sJIA (−0.1 at both dosing frequencies).
- In the pJIA group, 90% and 53% of patients weighing < 30 kg and ≥ 30 kg achieved inactive disease, respectively, whereas in the sJIA group, the respective rates were 91% and 92%.
- A total of five of 15 patients with pJIA weighing ≥ 30 kg who received subcutaneous tocilizumab every 2 weeks achieved clinical remission, whereas in other groups, the clinical remission rates ranged from 74% to 92%.
- Six patients with pJIA reported seven serious adverse events (SAEs), while five patients with sJIA experienced six SAEs. Five patients with pJIA and one patient with sJIA reported serious infections.
IN PRACTICE:
The authors concluded that subcutaneous tocilizumab treatment provided long-term disease control in patients with pJIA or sJIA, with a safety profile consistent with past studies of tocilizumab.
SOURCE:
The study was led by Hermine I. Brunner, MD, director of the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center. It was published online in Rheumatology (Oxford).
LIMITATIONS:
The open-label design and lack of a control group limited the analysis. Only a few patients continued the treatment for 5 years.
DISCLOSURES:
This work was supported by F. Hoffmann-La Roche Ltd. Eight authors reported receiving honoraria and consulting or speaker fees from various pharma sources. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Subcutaneous tocilizumab provides durable disease control rates in patients with polyarticular and systemic juvenile idiopathic arthritis (pJIA and sJIA, respectively).
METHODOLOGY:
- This long-term extension (LTE) study included 44 patients with pJIA and 38 patients with sJIA, according to the International League of Associations for Rheumatology criteria, from two 52-week phase 1b trials (NCT01904292 and NCT01904279).
- In the core trials, the dosing frequency of subcutaneous tocilizumab was determined by weight: Every 3 weeks for those < 30 kg in pJIA and every 2 weeks for those ≥ 30 kg; in sJIA, initially every 10 days for those < 30 kg, transitioning to every 2 weeks, and weekly for those ≥ 30 kg.
- Patients who had adequate disease control with subcutaneous tocilizumab, comparable with the use of intravenous tocilizumab in the core trials, continued to receive subcutaneous tocilizumab.
- The study outcome was the change in Juvenile Arthritis Disease Activity Score on 71 joints (JADAS-71, range 0-101).
TAKEAWAY:
- Disease control remained stable in both groups, with sustained improvements in median JADAS-71 scores in pJIA (−0.2 with lower frequency dosing to −0.5 with higher frequency) and sJIA (−0.1 at both dosing frequencies).
- In the pJIA group, 90% and 53% of patients weighing < 30 kg and ≥ 30 kg achieved inactive disease, respectively, whereas in the sJIA group, the respective rates were 91% and 92%.
- A total of five of 15 patients with pJIA weighing ≥ 30 kg who received subcutaneous tocilizumab every 2 weeks achieved clinical remission, whereas in other groups, the clinical remission rates ranged from 74% to 92%.
- Six patients with pJIA reported seven serious adverse events (SAEs), while five patients with sJIA experienced six SAEs. Five patients with pJIA and one patient with sJIA reported serious infections.
IN PRACTICE:
The authors concluded that subcutaneous tocilizumab treatment provided long-term disease control in patients with pJIA or sJIA, with a safety profile consistent with past studies of tocilizumab.
SOURCE:
The study was led by Hermine I. Brunner, MD, director of the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center. It was published online in Rheumatology (Oxford).
LIMITATIONS:
The open-label design and lack of a control group limited the analysis. Only a few patients continued the treatment for 5 years.
DISCLOSURES:
This work was supported by F. Hoffmann-La Roche Ltd. Eight authors reported receiving honoraria and consulting or speaker fees from various pharma sources. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Progressively Worsening Scaly Patches and Plaques in an Infant
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
A 5-month-old male with moderately brown skin that rarely burns and tans profusely presented to the emergency department with a worsening red rash of more than 4 months’ duration. The patient had diffuse erythroderma and eczematous patches and plaques covering 95% of the total body surface area, including lichenified plaques on the arms and elbows, with no signs of infection. He initially presented for his 1-month appointment at the pediatric clinic with scaly patches and plaques on the face and trunk as well as diffuse xerosis. He was prescribed daily oatmeal baths and topical Minerin (Major Pharmaceuticals)—containing water, petrolatum, mineral oil, mineral wax, lanolin alcohol, methylchloroisothiazolinone, and methylisothiazolinone—to be applied to the whole body twice daily. At the patient’s 2-month well visit, symptoms persisted. The patient’s pediatrician increased application of Minerin to 2 to 3 times daily, and hydrocortisone cream 2.5% application 2 to 3 times daily was added.
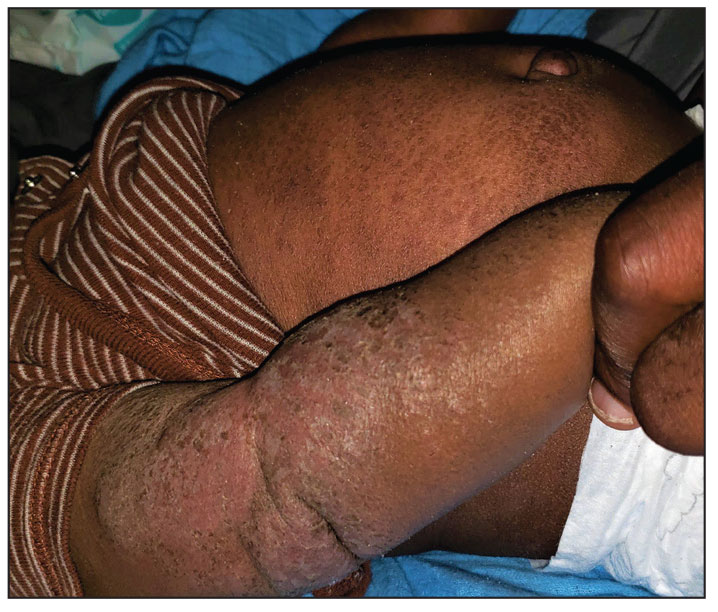
New Trial Deepens Debate Over Late-Preterm Steroids
The early cancellation of a trial in southern India suggests that the use of antenatal steroids to prevent respiratory complications after late-preterm birth — a recommended practice in the United States — may not be effective in the developing world.
As reported in Obstetrics & Gynecology, researchers led by Hilda Yenuberi, MD, of Christian Medical College, Vellore, Tamil Nadu, India, stopped the randomized, triple-blinded, placebo-controlled CLAP (Corticosteroids in Late Pregnancy) study at 70% enrollment. An interim analysis found no benefit from prescribing betamethasone vs placebo to women at risk of late-preterm delivery between 34 and 36 and 6/7 weeks of gestation (primary outcome of respiratory distress: 4.9% vs 4.8%, respectively, relative risk [RR], 1.03; 95% CI, 0.57-1.84; number needed to treat = 786).
“These findings may suggest differing efficacy of antenatal corticosteroids in developing countries compared with developed countries ... that should be considered when late-preterm antenatal corticosteroids are administered,” the researchers wrote.
The use of steroids in patients at risk of delivery before 34 weeks is widely accepted as a way to prevent neonatal respiratory distress, a common and potentially deadly condition in premature infants whose lungs are not fully developed. However, there’s debate over steroid treatment in women who are expected to deliver later than 34 weeks but still preterm.
As the study notes, “the American College of Obstetricians and Gynecologists recommends a single course of betamethasone for pregnant individuals at risk of delivering between 34 and 36 6/7 weeks of gestation on the basis of the ALPS (Antenatal Late Preterm Steroid) trial.”
But other randomized trials have reached different conclusions, and steroids are not without risks. Studies have linked prenatal steroids to neurosensory disorders in babies, meaning they’re more likely to need hearing aids and eyeglasses, said Kellie Murphy, MD, MSc, professor of obstetrics and gynecology, University of Toronto, Toronto, Ontario, Canada, in an interview. Dr. Murphy, who was not involved in the new trial, added that there are links between steroids and greater likelihood of poorer performance in school,
For the new study, conducted from 2020 to 2022 at Christian Medical College and Hospital in Vellore, India, researchers randomly assigned 423 patients to betamethasone (410 in the interim analysis; average age, 26.8 years) and 424 to placebo (415 in the interim analysis; average age, 26.2 years).
The average age of participants was 26.8 years. All were between 34 and 36 6/7 weeks of gestation and expected to give birth within the next week. A quarter of participants delivered at term, which the authors wrote “may have influenced the primary outcome.” The total number of neonates was 883, including 58 twin pregnancies.
There was no significant difference in respiratory distress between groups, “defined as need for oxygen or continuous positive airway pressure or mechanical ventilation for at least 2 hours in the first 72 hours of life.” There also were no significant differences in maternal outcomes such as chorioamnionitis or length of hospitalization or neonatal secondary outcomes such as transient tachypnea of the newborn, respiratory distress syndrome, necrotizing enterocolitis, sepsis, hyperbilirubinemia, stillbirth, and early neonatal death.
Serious adverse events occurred in four neonates but none were linked to the intervention.
The study doesn’t discuss cost, but a 2019 report suggests that use of betamethasone to prevent neonatal respiratory distress is cost-effective.
“Our findings are contradictory to those of a systematic review, the major contributor of which was the ALPS trial,” the authors of the new study reported. “The primary outcome of the ALPS trial, the composite of neonatal treatment in the first 72 hours, was significantly less in the group who received betamethasone (11.6%), compared with the placebo group (14.4%; relative risk [RR], 0.80; 95% CI, 0.66-0.97).”
The study authors, who didn’t respond to requests for comment, noted that their trial included twin pregnancies and patients with gestational diabetes; the ALPS trial did not.
Perinatologist Cynthia Gyamfi-Bannerman, MD, MS, chair and professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California,San Diego, and principal investigator of the ALPS study, said in an interview that the inclusion of twins in the new trial is “a fundamental flaw.”
“Because antenatal corticosteroids have not been shown to be useful in twins at any gestational age, it is not surprising that including twins likely moved the findings to the null in this study,” she said. “Twins were purposefully excluded from the ALPS trial for this reason.”
According to the new study, “the primary outcome among singleton neonates occurred in 4.8% (18/374) who received betamethasone and 5.1% (20/393) who received placebo (RR, 0.94; 95% CI, 0.51-1.75)
What should clinicians take from the study findings? In an accompanying commentary, Blair J. Wylie, MD, MPH, of Columbia University Medical Center, New York, NY, and Syed Asad Ali, MBBS, MPH, of Aga Khan University, Karachi, Pakistan, wrote that, “in settings similar to the US-based ALPS trial, the practice of administering a course of late-preterm antenatal corticosteroids should be continued, as espoused by our professional organizations.”
However, the new study suggests that “research in high-resource environments may not be generalizable to low-resource settings,” they write.
Neonatologist Elizabeth Asztalos, MD, MSc, an associate scientist with Sunnybrook Health Sciences Center in Toronto, Canada, said in an interview that she doesn’t worry about pregnant mothers not getting steroids later than 34 weeks. “We have tools in our armamentarium in the NICU setting to help babies if they need it,” said Dr. Asztalos, who didn’t take part in the new trial. “We can put them on CPAP if they have wet lung. If they have an element of respiratory distress, we can give them surfactants. These bigger babies have more ability to recover from all this compared to a baby who was born at 24, 25, 26 weeks.”
For her part, the University of Toronto’s Dr. Murphy said decision-making about late-preterm steroids is complicated. “You don’t want to miss the opportunity to give to provide benefits for the patients” via steroids, she said. “But on the flip side, it’s a double-edged sword. It’s not easy. It’s not straightforward.”
In the big picture, she said, “people need to be really clear why they’re giving an intervention and what they hope to achieve.”
Christian Medical College supported the study. The authors, Dr. Murphy, Dr. Asztalos, and commentary co-author Dr. Ali have no disclosures. Dr. Gyamfi-Bannerman discloses being principal investigator of the ALPS trial. Commentary co-author Dr. Wylie serves on the ultrasound quality assurance committee of a trial discussed in the commentary.
The early cancellation of a trial in southern India suggests that the use of antenatal steroids to prevent respiratory complications after late-preterm birth — a recommended practice in the United States — may not be effective in the developing world.
As reported in Obstetrics & Gynecology, researchers led by Hilda Yenuberi, MD, of Christian Medical College, Vellore, Tamil Nadu, India, stopped the randomized, triple-blinded, placebo-controlled CLAP (Corticosteroids in Late Pregnancy) study at 70% enrollment. An interim analysis found no benefit from prescribing betamethasone vs placebo to women at risk of late-preterm delivery between 34 and 36 and 6/7 weeks of gestation (primary outcome of respiratory distress: 4.9% vs 4.8%, respectively, relative risk [RR], 1.03; 95% CI, 0.57-1.84; number needed to treat = 786).
“These findings may suggest differing efficacy of antenatal corticosteroids in developing countries compared with developed countries ... that should be considered when late-preterm antenatal corticosteroids are administered,” the researchers wrote.
The use of steroids in patients at risk of delivery before 34 weeks is widely accepted as a way to prevent neonatal respiratory distress, a common and potentially deadly condition in premature infants whose lungs are not fully developed. However, there’s debate over steroid treatment in women who are expected to deliver later than 34 weeks but still preterm.
As the study notes, “the American College of Obstetricians and Gynecologists recommends a single course of betamethasone for pregnant individuals at risk of delivering between 34 and 36 6/7 weeks of gestation on the basis of the ALPS (Antenatal Late Preterm Steroid) trial.”
But other randomized trials have reached different conclusions, and steroids are not without risks. Studies have linked prenatal steroids to neurosensory disorders in babies, meaning they’re more likely to need hearing aids and eyeglasses, said Kellie Murphy, MD, MSc, professor of obstetrics and gynecology, University of Toronto, Toronto, Ontario, Canada, in an interview. Dr. Murphy, who was not involved in the new trial, added that there are links between steroids and greater likelihood of poorer performance in school,
For the new study, conducted from 2020 to 2022 at Christian Medical College and Hospital in Vellore, India, researchers randomly assigned 423 patients to betamethasone (410 in the interim analysis; average age, 26.8 years) and 424 to placebo (415 in the interim analysis; average age, 26.2 years).
The average age of participants was 26.8 years. All were between 34 and 36 6/7 weeks of gestation and expected to give birth within the next week. A quarter of participants delivered at term, which the authors wrote “may have influenced the primary outcome.” The total number of neonates was 883, including 58 twin pregnancies.
There was no significant difference in respiratory distress between groups, “defined as need for oxygen or continuous positive airway pressure or mechanical ventilation for at least 2 hours in the first 72 hours of life.” There also were no significant differences in maternal outcomes such as chorioamnionitis or length of hospitalization or neonatal secondary outcomes such as transient tachypnea of the newborn, respiratory distress syndrome, necrotizing enterocolitis, sepsis, hyperbilirubinemia, stillbirth, and early neonatal death.
Serious adverse events occurred in four neonates but none were linked to the intervention.
The study doesn’t discuss cost, but a 2019 report suggests that use of betamethasone to prevent neonatal respiratory distress is cost-effective.
“Our findings are contradictory to those of a systematic review, the major contributor of which was the ALPS trial,” the authors of the new study reported. “The primary outcome of the ALPS trial, the composite of neonatal treatment in the first 72 hours, was significantly less in the group who received betamethasone (11.6%), compared with the placebo group (14.4%; relative risk [RR], 0.80; 95% CI, 0.66-0.97).”
The study authors, who didn’t respond to requests for comment, noted that their trial included twin pregnancies and patients with gestational diabetes; the ALPS trial did not.
Perinatologist Cynthia Gyamfi-Bannerman, MD, MS, chair and professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California,San Diego, and principal investigator of the ALPS study, said in an interview that the inclusion of twins in the new trial is “a fundamental flaw.”
“Because antenatal corticosteroids have not been shown to be useful in twins at any gestational age, it is not surprising that including twins likely moved the findings to the null in this study,” she said. “Twins were purposefully excluded from the ALPS trial for this reason.”
According to the new study, “the primary outcome among singleton neonates occurred in 4.8% (18/374) who received betamethasone and 5.1% (20/393) who received placebo (RR, 0.94; 95% CI, 0.51-1.75)
What should clinicians take from the study findings? In an accompanying commentary, Blair J. Wylie, MD, MPH, of Columbia University Medical Center, New York, NY, and Syed Asad Ali, MBBS, MPH, of Aga Khan University, Karachi, Pakistan, wrote that, “in settings similar to the US-based ALPS trial, the practice of administering a course of late-preterm antenatal corticosteroids should be continued, as espoused by our professional organizations.”
However, the new study suggests that “research in high-resource environments may not be generalizable to low-resource settings,” they write.
Neonatologist Elizabeth Asztalos, MD, MSc, an associate scientist with Sunnybrook Health Sciences Center in Toronto, Canada, said in an interview that she doesn’t worry about pregnant mothers not getting steroids later than 34 weeks. “We have tools in our armamentarium in the NICU setting to help babies if they need it,” said Dr. Asztalos, who didn’t take part in the new trial. “We can put them on CPAP if they have wet lung. If they have an element of respiratory distress, we can give them surfactants. These bigger babies have more ability to recover from all this compared to a baby who was born at 24, 25, 26 weeks.”
For her part, the University of Toronto’s Dr. Murphy said decision-making about late-preterm steroids is complicated. “You don’t want to miss the opportunity to give to provide benefits for the patients” via steroids, she said. “But on the flip side, it’s a double-edged sword. It’s not easy. It’s not straightforward.”
In the big picture, she said, “people need to be really clear why they’re giving an intervention and what they hope to achieve.”
Christian Medical College supported the study. The authors, Dr. Murphy, Dr. Asztalos, and commentary co-author Dr. Ali have no disclosures. Dr. Gyamfi-Bannerman discloses being principal investigator of the ALPS trial. Commentary co-author Dr. Wylie serves on the ultrasound quality assurance committee of a trial discussed in the commentary.
The early cancellation of a trial in southern India suggests that the use of antenatal steroids to prevent respiratory complications after late-preterm birth — a recommended practice in the United States — may not be effective in the developing world.
As reported in Obstetrics & Gynecology, researchers led by Hilda Yenuberi, MD, of Christian Medical College, Vellore, Tamil Nadu, India, stopped the randomized, triple-blinded, placebo-controlled CLAP (Corticosteroids in Late Pregnancy) study at 70% enrollment. An interim analysis found no benefit from prescribing betamethasone vs placebo to women at risk of late-preterm delivery between 34 and 36 and 6/7 weeks of gestation (primary outcome of respiratory distress: 4.9% vs 4.8%, respectively, relative risk [RR], 1.03; 95% CI, 0.57-1.84; number needed to treat = 786).
“These findings may suggest differing efficacy of antenatal corticosteroids in developing countries compared with developed countries ... that should be considered when late-preterm antenatal corticosteroids are administered,” the researchers wrote.
The use of steroids in patients at risk of delivery before 34 weeks is widely accepted as a way to prevent neonatal respiratory distress, a common and potentially deadly condition in premature infants whose lungs are not fully developed. However, there’s debate over steroid treatment in women who are expected to deliver later than 34 weeks but still preterm.
As the study notes, “the American College of Obstetricians and Gynecologists recommends a single course of betamethasone for pregnant individuals at risk of delivering between 34 and 36 6/7 weeks of gestation on the basis of the ALPS (Antenatal Late Preterm Steroid) trial.”
But other randomized trials have reached different conclusions, and steroids are not without risks. Studies have linked prenatal steroids to neurosensory disorders in babies, meaning they’re more likely to need hearing aids and eyeglasses, said Kellie Murphy, MD, MSc, professor of obstetrics and gynecology, University of Toronto, Toronto, Ontario, Canada, in an interview. Dr. Murphy, who was not involved in the new trial, added that there are links between steroids and greater likelihood of poorer performance in school,
For the new study, conducted from 2020 to 2022 at Christian Medical College and Hospital in Vellore, India, researchers randomly assigned 423 patients to betamethasone (410 in the interim analysis; average age, 26.8 years) and 424 to placebo (415 in the interim analysis; average age, 26.2 years).
The average age of participants was 26.8 years. All were between 34 and 36 6/7 weeks of gestation and expected to give birth within the next week. A quarter of participants delivered at term, which the authors wrote “may have influenced the primary outcome.” The total number of neonates was 883, including 58 twin pregnancies.
There was no significant difference in respiratory distress between groups, “defined as need for oxygen or continuous positive airway pressure or mechanical ventilation for at least 2 hours in the first 72 hours of life.” There also were no significant differences in maternal outcomes such as chorioamnionitis or length of hospitalization or neonatal secondary outcomes such as transient tachypnea of the newborn, respiratory distress syndrome, necrotizing enterocolitis, sepsis, hyperbilirubinemia, stillbirth, and early neonatal death.
Serious adverse events occurred in four neonates but none were linked to the intervention.
The study doesn’t discuss cost, but a 2019 report suggests that use of betamethasone to prevent neonatal respiratory distress is cost-effective.
“Our findings are contradictory to those of a systematic review, the major contributor of which was the ALPS trial,” the authors of the new study reported. “The primary outcome of the ALPS trial, the composite of neonatal treatment in the first 72 hours, was significantly less in the group who received betamethasone (11.6%), compared with the placebo group (14.4%; relative risk [RR], 0.80; 95% CI, 0.66-0.97).”
The study authors, who didn’t respond to requests for comment, noted that their trial included twin pregnancies and patients with gestational diabetes; the ALPS trial did not.
Perinatologist Cynthia Gyamfi-Bannerman, MD, MS, chair and professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California,San Diego, and principal investigator of the ALPS study, said in an interview that the inclusion of twins in the new trial is “a fundamental flaw.”
“Because antenatal corticosteroids have not been shown to be useful in twins at any gestational age, it is not surprising that including twins likely moved the findings to the null in this study,” she said. “Twins were purposefully excluded from the ALPS trial for this reason.”
According to the new study, “the primary outcome among singleton neonates occurred in 4.8% (18/374) who received betamethasone and 5.1% (20/393) who received placebo (RR, 0.94; 95% CI, 0.51-1.75)
What should clinicians take from the study findings? In an accompanying commentary, Blair J. Wylie, MD, MPH, of Columbia University Medical Center, New York, NY, and Syed Asad Ali, MBBS, MPH, of Aga Khan University, Karachi, Pakistan, wrote that, “in settings similar to the US-based ALPS trial, the practice of administering a course of late-preterm antenatal corticosteroids should be continued, as espoused by our professional organizations.”
However, the new study suggests that “research in high-resource environments may not be generalizable to low-resource settings,” they write.
Neonatologist Elizabeth Asztalos, MD, MSc, an associate scientist with Sunnybrook Health Sciences Center in Toronto, Canada, said in an interview that she doesn’t worry about pregnant mothers not getting steroids later than 34 weeks. “We have tools in our armamentarium in the NICU setting to help babies if they need it,” said Dr. Asztalos, who didn’t take part in the new trial. “We can put them on CPAP if they have wet lung. If they have an element of respiratory distress, we can give them surfactants. These bigger babies have more ability to recover from all this compared to a baby who was born at 24, 25, 26 weeks.”
For her part, the University of Toronto’s Dr. Murphy said decision-making about late-preterm steroids is complicated. “You don’t want to miss the opportunity to give to provide benefits for the patients” via steroids, she said. “But on the flip side, it’s a double-edged sword. It’s not easy. It’s not straightforward.”
In the big picture, she said, “people need to be really clear why they’re giving an intervention and what they hope to achieve.”
Christian Medical College supported the study. The authors, Dr. Murphy, Dr. Asztalos, and commentary co-author Dr. Ali have no disclosures. Dr. Gyamfi-Bannerman discloses being principal investigator of the ALPS trial. Commentary co-author Dr. Wylie serves on the ultrasound quality assurance committee of a trial discussed in the commentary.
FROM OBSTETRICS & GYNECOLOGY
No Routine Cancer Screening Option? New MCED Tests May Help
Analyses presented during a session at the American Association for Cancer Research annual meeting, revealed that three new MCED tests — CanScan, MERCURY, and OncoSeek — could detect a range of cancers and recognize the tissue of origin with high accuracy. One — OncoSeek — could also provide an affordable cancer screening option for individuals living in lower-income countries.
The need for these noninvasive liquid biopsy tests that can accurately identify multiple cancer types with a single blood draw, especially cancers without routine screening strategies, is pressing. “We know that the current cancer standard of care screening will identify less than 50% of all cancers, while more than 50% of all cancer deaths occur in types of cancer with no recommended screening,” said co-moderator Marie E. Wood, MD, of the University of Colorado Anschutz Medical Campus, in Aurora, Colorado.
That being said, “the clinical utility of multicancer detection tests has not been established and we’re concerned about issues of overdiagnosis and overtreatment,” she noted.
The Early Data
One new MCED test called CanScan, developed by Geneseeq Technology, uses plasma cell-free DNA fragment patterns to detect cancer signals as well as identify the tissue of origin across 13 cancer types.
Overall, the CanScan test covers cancer types that contribute to two thirds of new cancer cases and 74% of morality globally, said presenter Shanshan Yang, of Geneseeq Research Institute, in Nanjing, China.
However, only five of these cancer types have screening recommendations issued by the US Preventive Services Task Force (USPSTF), Dr. Yang added.
The interim data comes from an ongoing large-scale prospective study evaluating the MCED test in a cohort of asymptomatic individuals between ages 45 and 75 years with an average risk for cancer and no cancer-related symptoms on enrollment.
Patients at baseline had their blood collected for the CanScan test and subsequently received annual routine physical exams once a year for 3 consecutive years, with an additional 2 years of follow-up.
The analysis included 3724 participants with analyzable samples at the data cutoff in September 2023. Among the 3724 participants, 29 had confirmed cancer diagnoses. Among these cases, 14 patients had their cancer confirmed through USPSTF recommended screening and 15 were detected through outside of standard USPSTF screening, such as a thyroid ultrasound, Dr. Yang explained.
Almost 90% of the cancers (26 of 29) were detected in the stage I or II, and eight (27.5%) were not one of the test’s 13 targeted cancer types.
The CanScan test had a sensitivity of 55.2%, identifying 16 of 29 of the patients with cancer, including 10 of 21 individuals with stage I (47.6%), and two of three with stage II (66.7%).
The test had a high specificity of 97.9%, meaning out of 100 people screened, only two had false negative findings.
Among the 15 patients who had their cancer detected outside of USPSTF screening recommendations, eight (53.3%) were found using a CanScan test, including patients with liver and endometrial cancers.
Compared with a positive predictive value of (PPV) of 1.6% with screening or physical exam methods alone, the CanScan test had a PPV of 17.4%, Dr. Yang reported.
“The MCED test holds significant potential for early cancer screening in asymptomatic populations,” Dr. Yang and colleagues concluded.
Another new MCED test called MERCURY, also developed by Geneseeq Technology and presented during the session, used a similar method to detect cancer signals and predict the tissue of origin across 13 cancer types.
The researchers initially validated the test using 3076 patients with cancer and 3477 healthy controls with a target specificity of 99%. In this group, researchers reported a sensitivity of 0.865 and a specificity of 0.989.
The team then performed an independent validation analysis with 1465 participants, 732 with cancer and 733 with no cancer, and confirmed a high sensitivity and specificity of 0.874 and 0.978, respectively. The sensitivity increased incrementally by cancer stage — 0.768 for stage I, 0.840 for stage II, 0.923 for stage III, and 0.971 for stage IV.
The test identified the tissue of origin with high accuracy, the researchers noted, but cautioned that the test needs “to be further validated in a prospective cohort study.”
MCED in Low-Income Settings
The session also featured findings on a new affordable MCED test called OncoSeek, which could provide greater access to cancer testing in low- and middle-income countries.
The OncoSeek algorithm identifies the presence of cancer using seven protein tumor markers alongside clinical information, such as gender and age. Like other tests, the test also predicts the possible tissue of origin.
The test can be run on clinical protein assay instruments that are already widely available, such as Roche cobas analyzer, Mao Mao, MD, PhD, the founder and CEO of SeekIn, of Shenzhen, China, told this news organization.
This “feature makes the test accessible worldwide, even in low- and middle-income countries,” he said. “These instruments are fully-automated and part of today’s clinical practice. Therefore, the test does not require additional infrastructure building and lab personal training.”
Another notable advantage: the OncoSeek test only costs about $20, compared with other MCED tests, which can cost anywhere from $200 to $1000.
To validate the technology in a large, diverse cohort, Dr. Mao and colleagues enrolled approximately 10,000 participants, including 2003 cancer cases and 7888 non-cancer cases.
Peripheral blood was collected from each participant and analyzed using a panel of the seven protein tumor markers — AFP, CA125, CA15-3, CA19-9, CA72-4, CEA, and CYFRA 21-1.
To reduce the risk for false positive findings, the team designed the OncoSeek algorithm to achieve a specificity of 93%. Dr. Mao and colleagues found a sensitivity of 51.7%, resulting in an overall accuracy of 84.6%.
The performance was consistent in additional validation cohorts in Brazil, China, and the United States, with sensitivities ranging from 39.0% to 77.6% for detecting nine common cancer types, including breast, colorectal, liver, lung, lymphoma, esophagus, ovary, pancreas, and stomach. The sensitivity for pancreatic cancer was at the high end of 77.6%.
The test could predict the tissue of origin in about two thirds of cases.
Given its low cost, OncoSeek represents an affordable and accessible option for cancer screening, the authors concluded.
Overall, “I think MCEDs have the potential to enhance cancer screening,” Dr. Wood told this news organization.
Still, questions remain about the optimal use of these tests, such as whether they are best for average-risk or higher risk populations, and how to integrate them into standard screening, she said.
Dr. Wood also cautioned that the studies presented in the session represent early data, and it is likely that the numbers, such as sensitivity and specificity, will change with further prospective analyses.
And ultimately, these tests should complement, not replace, standard screening. “A negative testing should not be taken as a sign to avoid standard screening,” Dr. Wood said.
Dr. Yang is an employee of Geneseeq Technology, Inc., and Dr. Mao is an employee of SeekIn. Dr. Wood had no disclosures to report.
A version of this article appeared on Medscape.com.
Analyses presented during a session at the American Association for Cancer Research annual meeting, revealed that three new MCED tests — CanScan, MERCURY, and OncoSeek — could detect a range of cancers and recognize the tissue of origin with high accuracy. One — OncoSeek — could also provide an affordable cancer screening option for individuals living in lower-income countries.
The need for these noninvasive liquid biopsy tests that can accurately identify multiple cancer types with a single blood draw, especially cancers without routine screening strategies, is pressing. “We know that the current cancer standard of care screening will identify less than 50% of all cancers, while more than 50% of all cancer deaths occur in types of cancer with no recommended screening,” said co-moderator Marie E. Wood, MD, of the University of Colorado Anschutz Medical Campus, in Aurora, Colorado.
That being said, “the clinical utility of multicancer detection tests has not been established and we’re concerned about issues of overdiagnosis and overtreatment,” she noted.
The Early Data
One new MCED test called CanScan, developed by Geneseeq Technology, uses plasma cell-free DNA fragment patterns to detect cancer signals as well as identify the tissue of origin across 13 cancer types.
Overall, the CanScan test covers cancer types that contribute to two thirds of new cancer cases and 74% of morality globally, said presenter Shanshan Yang, of Geneseeq Research Institute, in Nanjing, China.
However, only five of these cancer types have screening recommendations issued by the US Preventive Services Task Force (USPSTF), Dr. Yang added.
The interim data comes from an ongoing large-scale prospective study evaluating the MCED test in a cohort of asymptomatic individuals between ages 45 and 75 years with an average risk for cancer and no cancer-related symptoms on enrollment.
Patients at baseline had their blood collected for the CanScan test and subsequently received annual routine physical exams once a year for 3 consecutive years, with an additional 2 years of follow-up.
The analysis included 3724 participants with analyzable samples at the data cutoff in September 2023. Among the 3724 participants, 29 had confirmed cancer diagnoses. Among these cases, 14 patients had their cancer confirmed through USPSTF recommended screening and 15 were detected through outside of standard USPSTF screening, such as a thyroid ultrasound, Dr. Yang explained.
Almost 90% of the cancers (26 of 29) were detected in the stage I or II, and eight (27.5%) were not one of the test’s 13 targeted cancer types.
The CanScan test had a sensitivity of 55.2%, identifying 16 of 29 of the patients with cancer, including 10 of 21 individuals with stage I (47.6%), and two of three with stage II (66.7%).
The test had a high specificity of 97.9%, meaning out of 100 people screened, only two had false negative findings.
Among the 15 patients who had their cancer detected outside of USPSTF screening recommendations, eight (53.3%) were found using a CanScan test, including patients with liver and endometrial cancers.
Compared with a positive predictive value of (PPV) of 1.6% with screening or physical exam methods alone, the CanScan test had a PPV of 17.4%, Dr. Yang reported.
“The MCED test holds significant potential for early cancer screening in asymptomatic populations,” Dr. Yang and colleagues concluded.
Another new MCED test called MERCURY, also developed by Geneseeq Technology and presented during the session, used a similar method to detect cancer signals and predict the tissue of origin across 13 cancer types.
The researchers initially validated the test using 3076 patients with cancer and 3477 healthy controls with a target specificity of 99%. In this group, researchers reported a sensitivity of 0.865 and a specificity of 0.989.
The team then performed an independent validation analysis with 1465 participants, 732 with cancer and 733 with no cancer, and confirmed a high sensitivity and specificity of 0.874 and 0.978, respectively. The sensitivity increased incrementally by cancer stage — 0.768 for stage I, 0.840 for stage II, 0.923 for stage III, and 0.971 for stage IV.
The test identified the tissue of origin with high accuracy, the researchers noted, but cautioned that the test needs “to be further validated in a prospective cohort study.”
MCED in Low-Income Settings
The session also featured findings on a new affordable MCED test called OncoSeek, which could provide greater access to cancer testing in low- and middle-income countries.
The OncoSeek algorithm identifies the presence of cancer using seven protein tumor markers alongside clinical information, such as gender and age. Like other tests, the test also predicts the possible tissue of origin.
The test can be run on clinical protein assay instruments that are already widely available, such as Roche cobas analyzer, Mao Mao, MD, PhD, the founder and CEO of SeekIn, of Shenzhen, China, told this news organization.
This “feature makes the test accessible worldwide, even in low- and middle-income countries,” he said. “These instruments are fully-automated and part of today’s clinical practice. Therefore, the test does not require additional infrastructure building and lab personal training.”
Another notable advantage: the OncoSeek test only costs about $20, compared with other MCED tests, which can cost anywhere from $200 to $1000.
To validate the technology in a large, diverse cohort, Dr. Mao and colleagues enrolled approximately 10,000 participants, including 2003 cancer cases and 7888 non-cancer cases.
Peripheral blood was collected from each participant and analyzed using a panel of the seven protein tumor markers — AFP, CA125, CA15-3, CA19-9, CA72-4, CEA, and CYFRA 21-1.
To reduce the risk for false positive findings, the team designed the OncoSeek algorithm to achieve a specificity of 93%. Dr. Mao and colleagues found a sensitivity of 51.7%, resulting in an overall accuracy of 84.6%.
The performance was consistent in additional validation cohorts in Brazil, China, and the United States, with sensitivities ranging from 39.0% to 77.6% for detecting nine common cancer types, including breast, colorectal, liver, lung, lymphoma, esophagus, ovary, pancreas, and stomach. The sensitivity for pancreatic cancer was at the high end of 77.6%.
The test could predict the tissue of origin in about two thirds of cases.
Given its low cost, OncoSeek represents an affordable and accessible option for cancer screening, the authors concluded.
Overall, “I think MCEDs have the potential to enhance cancer screening,” Dr. Wood told this news organization.
Still, questions remain about the optimal use of these tests, such as whether they are best for average-risk or higher risk populations, and how to integrate them into standard screening, she said.
Dr. Wood also cautioned that the studies presented in the session represent early data, and it is likely that the numbers, such as sensitivity and specificity, will change with further prospective analyses.
And ultimately, these tests should complement, not replace, standard screening. “A negative testing should not be taken as a sign to avoid standard screening,” Dr. Wood said.
Dr. Yang is an employee of Geneseeq Technology, Inc., and Dr. Mao is an employee of SeekIn. Dr. Wood had no disclosures to report.
A version of this article appeared on Medscape.com.
Analyses presented during a session at the American Association for Cancer Research annual meeting, revealed that three new MCED tests — CanScan, MERCURY, and OncoSeek — could detect a range of cancers and recognize the tissue of origin with high accuracy. One — OncoSeek — could also provide an affordable cancer screening option for individuals living in lower-income countries.
The need for these noninvasive liquid biopsy tests that can accurately identify multiple cancer types with a single blood draw, especially cancers without routine screening strategies, is pressing. “We know that the current cancer standard of care screening will identify less than 50% of all cancers, while more than 50% of all cancer deaths occur in types of cancer with no recommended screening,” said co-moderator Marie E. Wood, MD, of the University of Colorado Anschutz Medical Campus, in Aurora, Colorado.
That being said, “the clinical utility of multicancer detection tests has not been established and we’re concerned about issues of overdiagnosis and overtreatment,” she noted.
The Early Data
One new MCED test called CanScan, developed by Geneseeq Technology, uses plasma cell-free DNA fragment patterns to detect cancer signals as well as identify the tissue of origin across 13 cancer types.
Overall, the CanScan test covers cancer types that contribute to two thirds of new cancer cases and 74% of morality globally, said presenter Shanshan Yang, of Geneseeq Research Institute, in Nanjing, China.
However, only five of these cancer types have screening recommendations issued by the US Preventive Services Task Force (USPSTF), Dr. Yang added.
The interim data comes from an ongoing large-scale prospective study evaluating the MCED test in a cohort of asymptomatic individuals between ages 45 and 75 years with an average risk for cancer and no cancer-related symptoms on enrollment.
Patients at baseline had their blood collected for the CanScan test and subsequently received annual routine physical exams once a year for 3 consecutive years, with an additional 2 years of follow-up.
The analysis included 3724 participants with analyzable samples at the data cutoff in September 2023. Among the 3724 participants, 29 had confirmed cancer diagnoses. Among these cases, 14 patients had their cancer confirmed through USPSTF recommended screening and 15 were detected through outside of standard USPSTF screening, such as a thyroid ultrasound, Dr. Yang explained.
Almost 90% of the cancers (26 of 29) were detected in the stage I or II, and eight (27.5%) were not one of the test’s 13 targeted cancer types.
The CanScan test had a sensitivity of 55.2%, identifying 16 of 29 of the patients with cancer, including 10 of 21 individuals with stage I (47.6%), and two of three with stage II (66.7%).
The test had a high specificity of 97.9%, meaning out of 100 people screened, only two had false negative findings.
Among the 15 patients who had their cancer detected outside of USPSTF screening recommendations, eight (53.3%) were found using a CanScan test, including patients with liver and endometrial cancers.
Compared with a positive predictive value of (PPV) of 1.6% with screening or physical exam methods alone, the CanScan test had a PPV of 17.4%, Dr. Yang reported.
“The MCED test holds significant potential for early cancer screening in asymptomatic populations,” Dr. Yang and colleagues concluded.
Another new MCED test called MERCURY, also developed by Geneseeq Technology and presented during the session, used a similar method to detect cancer signals and predict the tissue of origin across 13 cancer types.
The researchers initially validated the test using 3076 patients with cancer and 3477 healthy controls with a target specificity of 99%. In this group, researchers reported a sensitivity of 0.865 and a specificity of 0.989.
The team then performed an independent validation analysis with 1465 participants, 732 with cancer and 733 with no cancer, and confirmed a high sensitivity and specificity of 0.874 and 0.978, respectively. The sensitivity increased incrementally by cancer stage — 0.768 for stage I, 0.840 for stage II, 0.923 for stage III, and 0.971 for stage IV.
The test identified the tissue of origin with high accuracy, the researchers noted, but cautioned that the test needs “to be further validated in a prospective cohort study.”
MCED in Low-Income Settings
The session also featured findings on a new affordable MCED test called OncoSeek, which could provide greater access to cancer testing in low- and middle-income countries.
The OncoSeek algorithm identifies the presence of cancer using seven protein tumor markers alongside clinical information, such as gender and age. Like other tests, the test also predicts the possible tissue of origin.
The test can be run on clinical protein assay instruments that are already widely available, such as Roche cobas analyzer, Mao Mao, MD, PhD, the founder and CEO of SeekIn, of Shenzhen, China, told this news organization.
This “feature makes the test accessible worldwide, even in low- and middle-income countries,” he said. “These instruments are fully-automated and part of today’s clinical practice. Therefore, the test does not require additional infrastructure building and lab personal training.”
Another notable advantage: the OncoSeek test only costs about $20, compared with other MCED tests, which can cost anywhere from $200 to $1000.
To validate the technology in a large, diverse cohort, Dr. Mao and colleagues enrolled approximately 10,000 participants, including 2003 cancer cases and 7888 non-cancer cases.
Peripheral blood was collected from each participant and analyzed using a panel of the seven protein tumor markers — AFP, CA125, CA15-3, CA19-9, CA72-4, CEA, and CYFRA 21-1.
To reduce the risk for false positive findings, the team designed the OncoSeek algorithm to achieve a specificity of 93%. Dr. Mao and colleagues found a sensitivity of 51.7%, resulting in an overall accuracy of 84.6%.
The performance was consistent in additional validation cohorts in Brazil, China, and the United States, with sensitivities ranging from 39.0% to 77.6% for detecting nine common cancer types, including breast, colorectal, liver, lung, lymphoma, esophagus, ovary, pancreas, and stomach. The sensitivity for pancreatic cancer was at the high end of 77.6%.
The test could predict the tissue of origin in about two thirds of cases.
Given its low cost, OncoSeek represents an affordable and accessible option for cancer screening, the authors concluded.
Overall, “I think MCEDs have the potential to enhance cancer screening,” Dr. Wood told this news organization.
Still, questions remain about the optimal use of these tests, such as whether they are best for average-risk or higher risk populations, and how to integrate them into standard screening, she said.
Dr. Wood also cautioned that the studies presented in the session represent early data, and it is likely that the numbers, such as sensitivity and specificity, will change with further prospective analyses.
And ultimately, these tests should complement, not replace, standard screening. “A negative testing should not be taken as a sign to avoid standard screening,” Dr. Wood said.
Dr. Yang is an employee of Geneseeq Technology, Inc., and Dr. Mao is an employee of SeekIn. Dr. Wood had no disclosures to report.
A version of this article appeared on Medscape.com.
Safety Risks Persist with Out-of-Hospital Births
Safety concerns persist for out-of-hospital births in the United States with multiple potential risk factors and few safety requirements, according to a paper published in the American Journal of Obstetrics and Gynecology.
In 2022, the Centers for Disease Control and Prevention (CDC) reported the highest number of planned home births in 30 years. The numbers rose 12% from 2020 to 2021, the latest period for which complete data are available. Home births rose from 45,646 (1.26% of births) in 2020 to 51,642 (1.41% of births).
Amos Grünebaum, MD, and Frank A. Chervenak, MD, with Northwell Health, and the Department of Obstetrics and Gynecology, Lenox Hill Hospital, Zucker School of Medicine in New Hyde Park, New York, reviewed the latest safety data surrounding community births in the United States along with well-known perinatal risks and safety requirements for safe out-of-hospital births.
“Most planned home births continue to have one or more risk factors that are associated with an increase in adverse pregnancy outcomes,” they wrote.
Birth Certificate Data Analyzed
The researchers used the CDC birth certificate database and analyzed deliveries between 2016 and 2022 regarding the incidence of perinatal risks in community births. The risks included were prior cesarean, first baby, mother older than 35 years, twins, breech presentation, gestational age of less than 37 weeks or more than 41 weeks, newborn weight over 4,000 grams, adequacy of prenatal care, grand multiparity (5 or more prior pregnancies), and a prepregnancy body mass index of at least 35.
The incidence of perinatal risks for out-of-hospital births ranged individually from 0.2% to 28.54% among birthing center births and 0.32% to 24.4% for planned home births.
“The ACOG committee opinion on home births states that for every 1000 home births, 3.9 babies will die,” the authors noted, or about twice the risk of hospital births. The deaths are “potentially avoidable with easy access to an operating room,” they wrote.
Among the safety concerns for perinatal morbidity and mortality in community births, the authors cited the lack of:
- Appropriate patient selection for out-of-hospital births through standardized guidelines.
- Availability of a Certified Nurse Midwife, a Certified Midwife, or midwife whose education and licensure meet International Confederation of Midwives’ (ICM) Global Standards for Midwifery Education.
- Providers practicing obstetrics within an integrated and regulated health system with ready access and availability of board-certified obstetricians to provide consultation for qualified midwives.
- Standardized guidelines on when transport to a hospital is necessary.
“While prerequisites for a safe out-of-hospital delivery may be in place in other high-income countries, these prerequisites have not been actualized in the United States,” the authors wrote.
Incorporating Patient Preferences Into Delivery Models
Yalda Afshar, MD, PhD, maternal-fetal medicine subspecialist and a physician-scientist at UCLA Health in California, said obstetricians are responsible for offering the most evidence-based care to pregnant people.
“What this birth certificate data demonstrates,” she said, “is a tendency among birthing people to opt for out-of-hospital births, despite documented risks to both the pregnant person and the neonate. This underscores the need to persist in educating on risk stratification, risk reduction, and safe birthing practices, while also fostering innovation. Innovation should stem from our commitment to incorporate the preferences of pregnant people into our healthcare delivery model.”
Dr. Afshar, who was not part of the study, said clinicians should develop innovative ways to effectively meet the needs of pregnant patients while ensuring their safety and well-being.
“Ideally, we would establish safe environments within hospital systems and centers that emulate home-like birthing experiences, thereby mitigating risks for these families,” she said.
Though not explicitly stated in the data, she added, it is crucial to emphasize the need for continuous risk assessment throughout pregnancy and childbirth, “with a paramount focus on the safety of the pregnant individual.”
The authors and Dr. Afshar have no relevant financial disclosures.
Safety concerns persist for out-of-hospital births in the United States with multiple potential risk factors and few safety requirements, according to a paper published in the American Journal of Obstetrics and Gynecology.
In 2022, the Centers for Disease Control and Prevention (CDC) reported the highest number of planned home births in 30 years. The numbers rose 12% from 2020 to 2021, the latest period for which complete data are available. Home births rose from 45,646 (1.26% of births) in 2020 to 51,642 (1.41% of births).
Amos Grünebaum, MD, and Frank A. Chervenak, MD, with Northwell Health, and the Department of Obstetrics and Gynecology, Lenox Hill Hospital, Zucker School of Medicine in New Hyde Park, New York, reviewed the latest safety data surrounding community births in the United States along with well-known perinatal risks and safety requirements for safe out-of-hospital births.
“Most planned home births continue to have one or more risk factors that are associated with an increase in adverse pregnancy outcomes,” they wrote.
Birth Certificate Data Analyzed
The researchers used the CDC birth certificate database and analyzed deliveries between 2016 and 2022 regarding the incidence of perinatal risks in community births. The risks included were prior cesarean, first baby, mother older than 35 years, twins, breech presentation, gestational age of less than 37 weeks or more than 41 weeks, newborn weight over 4,000 grams, adequacy of prenatal care, grand multiparity (5 or more prior pregnancies), and a prepregnancy body mass index of at least 35.
The incidence of perinatal risks for out-of-hospital births ranged individually from 0.2% to 28.54% among birthing center births and 0.32% to 24.4% for planned home births.
“The ACOG committee opinion on home births states that for every 1000 home births, 3.9 babies will die,” the authors noted, or about twice the risk of hospital births. The deaths are “potentially avoidable with easy access to an operating room,” they wrote.
Among the safety concerns for perinatal morbidity and mortality in community births, the authors cited the lack of:
- Appropriate patient selection for out-of-hospital births through standardized guidelines.
- Availability of a Certified Nurse Midwife, a Certified Midwife, or midwife whose education and licensure meet International Confederation of Midwives’ (ICM) Global Standards for Midwifery Education.
- Providers practicing obstetrics within an integrated and regulated health system with ready access and availability of board-certified obstetricians to provide consultation for qualified midwives.
- Standardized guidelines on when transport to a hospital is necessary.
“While prerequisites for a safe out-of-hospital delivery may be in place in other high-income countries, these prerequisites have not been actualized in the United States,” the authors wrote.
Incorporating Patient Preferences Into Delivery Models
Yalda Afshar, MD, PhD, maternal-fetal medicine subspecialist and a physician-scientist at UCLA Health in California, said obstetricians are responsible for offering the most evidence-based care to pregnant people.
“What this birth certificate data demonstrates,” she said, “is a tendency among birthing people to opt for out-of-hospital births, despite documented risks to both the pregnant person and the neonate. This underscores the need to persist in educating on risk stratification, risk reduction, and safe birthing practices, while also fostering innovation. Innovation should stem from our commitment to incorporate the preferences of pregnant people into our healthcare delivery model.”
Dr. Afshar, who was not part of the study, said clinicians should develop innovative ways to effectively meet the needs of pregnant patients while ensuring their safety and well-being.
“Ideally, we would establish safe environments within hospital systems and centers that emulate home-like birthing experiences, thereby mitigating risks for these families,” she said.
Though not explicitly stated in the data, she added, it is crucial to emphasize the need for continuous risk assessment throughout pregnancy and childbirth, “with a paramount focus on the safety of the pregnant individual.”
The authors and Dr. Afshar have no relevant financial disclosures.
Safety concerns persist for out-of-hospital births in the United States with multiple potential risk factors and few safety requirements, according to a paper published in the American Journal of Obstetrics and Gynecology.
In 2022, the Centers for Disease Control and Prevention (CDC) reported the highest number of planned home births in 30 years. The numbers rose 12% from 2020 to 2021, the latest period for which complete data are available. Home births rose from 45,646 (1.26% of births) in 2020 to 51,642 (1.41% of births).
Amos Grünebaum, MD, and Frank A. Chervenak, MD, with Northwell Health, and the Department of Obstetrics and Gynecology, Lenox Hill Hospital, Zucker School of Medicine in New Hyde Park, New York, reviewed the latest safety data surrounding community births in the United States along with well-known perinatal risks and safety requirements for safe out-of-hospital births.
“Most planned home births continue to have one or more risk factors that are associated with an increase in adverse pregnancy outcomes,” they wrote.
Birth Certificate Data Analyzed
The researchers used the CDC birth certificate database and analyzed deliveries between 2016 and 2022 regarding the incidence of perinatal risks in community births. The risks included were prior cesarean, first baby, mother older than 35 years, twins, breech presentation, gestational age of less than 37 weeks or more than 41 weeks, newborn weight over 4,000 grams, adequacy of prenatal care, grand multiparity (5 or more prior pregnancies), and a prepregnancy body mass index of at least 35.
The incidence of perinatal risks for out-of-hospital births ranged individually from 0.2% to 28.54% among birthing center births and 0.32% to 24.4% for planned home births.
“The ACOG committee opinion on home births states that for every 1000 home births, 3.9 babies will die,” the authors noted, or about twice the risk of hospital births. The deaths are “potentially avoidable with easy access to an operating room,” they wrote.
Among the safety concerns for perinatal morbidity and mortality in community births, the authors cited the lack of:
- Appropriate patient selection for out-of-hospital births through standardized guidelines.
- Availability of a Certified Nurse Midwife, a Certified Midwife, or midwife whose education and licensure meet International Confederation of Midwives’ (ICM) Global Standards for Midwifery Education.
- Providers practicing obstetrics within an integrated and regulated health system with ready access and availability of board-certified obstetricians to provide consultation for qualified midwives.
- Standardized guidelines on when transport to a hospital is necessary.
“While prerequisites for a safe out-of-hospital delivery may be in place in other high-income countries, these prerequisites have not been actualized in the United States,” the authors wrote.
Incorporating Patient Preferences Into Delivery Models
Yalda Afshar, MD, PhD, maternal-fetal medicine subspecialist and a physician-scientist at UCLA Health in California, said obstetricians are responsible for offering the most evidence-based care to pregnant people.
“What this birth certificate data demonstrates,” she said, “is a tendency among birthing people to opt for out-of-hospital births, despite documented risks to both the pregnant person and the neonate. This underscores the need to persist in educating on risk stratification, risk reduction, and safe birthing practices, while also fostering innovation. Innovation should stem from our commitment to incorporate the preferences of pregnant people into our healthcare delivery model.”
Dr. Afshar, who was not part of the study, said clinicians should develop innovative ways to effectively meet the needs of pregnant patients while ensuring their safety and well-being.
“Ideally, we would establish safe environments within hospital systems and centers that emulate home-like birthing experiences, thereby mitigating risks for these families,” she said.
Though not explicitly stated in the data, she added, it is crucial to emphasize the need for continuous risk assessment throughout pregnancy and childbirth, “with a paramount focus on the safety of the pregnant individual.”
The authors and Dr. Afshar have no relevant financial disclosures.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Oncologists Voice Ethical Concerns Over AI in Cancer Care
TOPLINE:
Most respondents, for instance, said patients should not be expected to understand how AI tools work, but many also felt patients could make treatment decisions based on AI-generated recommendations. Most oncologists also felt responsible for protecting patients from biased AI, but few were confident that they could do so.
METHODOLOGY:
- The US Food and Drug Administration (FDA) has for use in various medical specialties over the past few decades, and increasingly, AI tools are being integrated into cancer care.
- However, the uptake of these tools in oncology has raised ethical questions and concerns, including challenges with AI bias, error, or misuse, as well as issues explaining how an AI model reached a result.
- In the current study, researchers asked 204 oncologists from 37 states for their views on the ethical implications of using AI for cancer care.
- Among the survey respondents, 64% were men and 63% were non-Hispanic White; 29% were from academic practices, 47% had received some education on AI use in healthcare, and 45% were familiar with clinical decision models.
- The researchers assessed respondents’ answers to various questions, including whether to provide informed consent for AI use and how oncologists would approach a scenario where the AI model and the oncologist recommended a different treatment regimen.
TAKEAWAY:
- Overall, 81% of oncologists supported having patient consent to use an AI model during treatment decisions, and 85% felt that oncologists needed to be able to explain an AI-based clinical decision model to use it in the clinic; however, only 23% felt that patients also needed to be able to explain an AI model.
- When an AI decision model recommended a different treatment regimen than the treating oncologist, the most common response (36.8%) was to present both options to the patient and let the patient decide. Oncologists from academic settings were about 2.5 times more likely than those from other settings to let the patient decide. About 34% of respondents said they would present both options but recommend the oncologist’s regimen, whereas about 22% said they would present both but recommend the AI’s regimen. A small percentage would only present the oncologist’s regimen (5%) or the AI’s regimen (about 2.5%).
- About three of four respondents (76.5%) agreed that oncologists should protect patients from biased AI tools; however, only about one of four (27.9%) felt confident they could identify biased AI models.
- Most oncologists (91%) felt that AI developers were responsible for the medico-legal problems associated with AI use; less than half (47%) said oncologists or hospitals (43%) shared this responsibility.
IN PRACTICE:
“Together, these data characterize barriers that may impede the ethical adoption of AI into cancer care. The findings suggest that the implementation of AI in oncology must include rigorous assessments of its effect on care decisions, as well as decisional responsibility when problems related to AI use arise,” the authors concluded.
SOURCE:
The study, with first author Andrew Hantel, MD, from Dana-Farber Cancer Institute, Boston, was published last month in JAMA Network Open.
LIMITATIONS:
The study had a moderate sample size and response rate, although demographics of participating oncologists appear to be nationally representative. The cross-sectional study design limited the generalizability of the findings over time as AI is integrated into cancer care.
DISCLOSURES:
The study was funded by the National Cancer Institute, the Dana-Farber McGraw/Patterson Research Fund, and the Mark Foundation Emerging Leader Award. Dr. Hantel reported receiving personal fees from AbbVie, AstraZeneca, the American Journal of Managed Care, Genentech, and GSK.
A version of this article appeared on Medscape.com.
TOPLINE:
Most respondents, for instance, said patients should not be expected to understand how AI tools work, but many also felt patients could make treatment decisions based on AI-generated recommendations. Most oncologists also felt responsible for protecting patients from biased AI, but few were confident that they could do so.
METHODOLOGY:
- The US Food and Drug Administration (FDA) has for use in various medical specialties over the past few decades, and increasingly, AI tools are being integrated into cancer care.
- However, the uptake of these tools in oncology has raised ethical questions and concerns, including challenges with AI bias, error, or misuse, as well as issues explaining how an AI model reached a result.
- In the current study, researchers asked 204 oncologists from 37 states for their views on the ethical implications of using AI for cancer care.
- Among the survey respondents, 64% were men and 63% were non-Hispanic White; 29% were from academic practices, 47% had received some education on AI use in healthcare, and 45% were familiar with clinical decision models.
- The researchers assessed respondents’ answers to various questions, including whether to provide informed consent for AI use and how oncologists would approach a scenario where the AI model and the oncologist recommended a different treatment regimen.
TAKEAWAY:
- Overall, 81% of oncologists supported having patient consent to use an AI model during treatment decisions, and 85% felt that oncologists needed to be able to explain an AI-based clinical decision model to use it in the clinic; however, only 23% felt that patients also needed to be able to explain an AI model.
- When an AI decision model recommended a different treatment regimen than the treating oncologist, the most common response (36.8%) was to present both options to the patient and let the patient decide. Oncologists from academic settings were about 2.5 times more likely than those from other settings to let the patient decide. About 34% of respondents said they would present both options but recommend the oncologist’s regimen, whereas about 22% said they would present both but recommend the AI’s regimen. A small percentage would only present the oncologist’s regimen (5%) or the AI’s regimen (about 2.5%).
- About three of four respondents (76.5%) agreed that oncologists should protect patients from biased AI tools; however, only about one of four (27.9%) felt confident they could identify biased AI models.
- Most oncologists (91%) felt that AI developers were responsible for the medico-legal problems associated with AI use; less than half (47%) said oncologists or hospitals (43%) shared this responsibility.
IN PRACTICE:
“Together, these data characterize barriers that may impede the ethical adoption of AI into cancer care. The findings suggest that the implementation of AI in oncology must include rigorous assessments of its effect on care decisions, as well as decisional responsibility when problems related to AI use arise,” the authors concluded.
SOURCE:
The study, with first author Andrew Hantel, MD, from Dana-Farber Cancer Institute, Boston, was published last month in JAMA Network Open.
LIMITATIONS:
The study had a moderate sample size and response rate, although demographics of participating oncologists appear to be nationally representative. The cross-sectional study design limited the generalizability of the findings over time as AI is integrated into cancer care.
DISCLOSURES:
The study was funded by the National Cancer Institute, the Dana-Farber McGraw/Patterson Research Fund, and the Mark Foundation Emerging Leader Award. Dr. Hantel reported receiving personal fees from AbbVie, AstraZeneca, the American Journal of Managed Care, Genentech, and GSK.
A version of this article appeared on Medscape.com.
TOPLINE:
Most respondents, for instance, said patients should not be expected to understand how AI tools work, but many also felt patients could make treatment decisions based on AI-generated recommendations. Most oncologists also felt responsible for protecting patients from biased AI, but few were confident that they could do so.
METHODOLOGY:
- The US Food and Drug Administration (FDA) has for use in various medical specialties over the past few decades, and increasingly, AI tools are being integrated into cancer care.
- However, the uptake of these tools in oncology has raised ethical questions and concerns, including challenges with AI bias, error, or misuse, as well as issues explaining how an AI model reached a result.
- In the current study, researchers asked 204 oncologists from 37 states for their views on the ethical implications of using AI for cancer care.
- Among the survey respondents, 64% were men and 63% were non-Hispanic White; 29% were from academic practices, 47% had received some education on AI use in healthcare, and 45% were familiar with clinical decision models.
- The researchers assessed respondents’ answers to various questions, including whether to provide informed consent for AI use and how oncologists would approach a scenario where the AI model and the oncologist recommended a different treatment regimen.
TAKEAWAY:
- Overall, 81% of oncologists supported having patient consent to use an AI model during treatment decisions, and 85% felt that oncologists needed to be able to explain an AI-based clinical decision model to use it in the clinic; however, only 23% felt that patients also needed to be able to explain an AI model.
- When an AI decision model recommended a different treatment regimen than the treating oncologist, the most common response (36.8%) was to present both options to the patient and let the patient decide. Oncologists from academic settings were about 2.5 times more likely than those from other settings to let the patient decide. About 34% of respondents said they would present both options but recommend the oncologist’s regimen, whereas about 22% said they would present both but recommend the AI’s regimen. A small percentage would only present the oncologist’s regimen (5%) or the AI’s regimen (about 2.5%).
- About three of four respondents (76.5%) agreed that oncologists should protect patients from biased AI tools; however, only about one of four (27.9%) felt confident they could identify biased AI models.
- Most oncologists (91%) felt that AI developers were responsible for the medico-legal problems associated with AI use; less than half (47%) said oncologists or hospitals (43%) shared this responsibility.
IN PRACTICE:
“Together, these data characterize barriers that may impede the ethical adoption of AI into cancer care. The findings suggest that the implementation of AI in oncology must include rigorous assessments of its effect on care decisions, as well as decisional responsibility when problems related to AI use arise,” the authors concluded.
SOURCE:
The study, with first author Andrew Hantel, MD, from Dana-Farber Cancer Institute, Boston, was published last month in JAMA Network Open.
LIMITATIONS:
The study had a moderate sample size and response rate, although demographics of participating oncologists appear to be nationally representative. The cross-sectional study design limited the generalizability of the findings over time as AI is integrated into cancer care.
DISCLOSURES:
The study was funded by the National Cancer Institute, the Dana-Farber McGraw/Patterson Research Fund, and the Mark Foundation Emerging Leader Award. Dr. Hantel reported receiving personal fees from AbbVie, AstraZeneca, the American Journal of Managed Care, Genentech, and GSK.
A version of this article appeared on Medscape.com.