User login
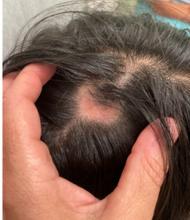
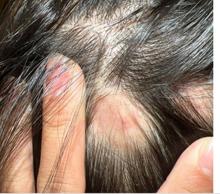
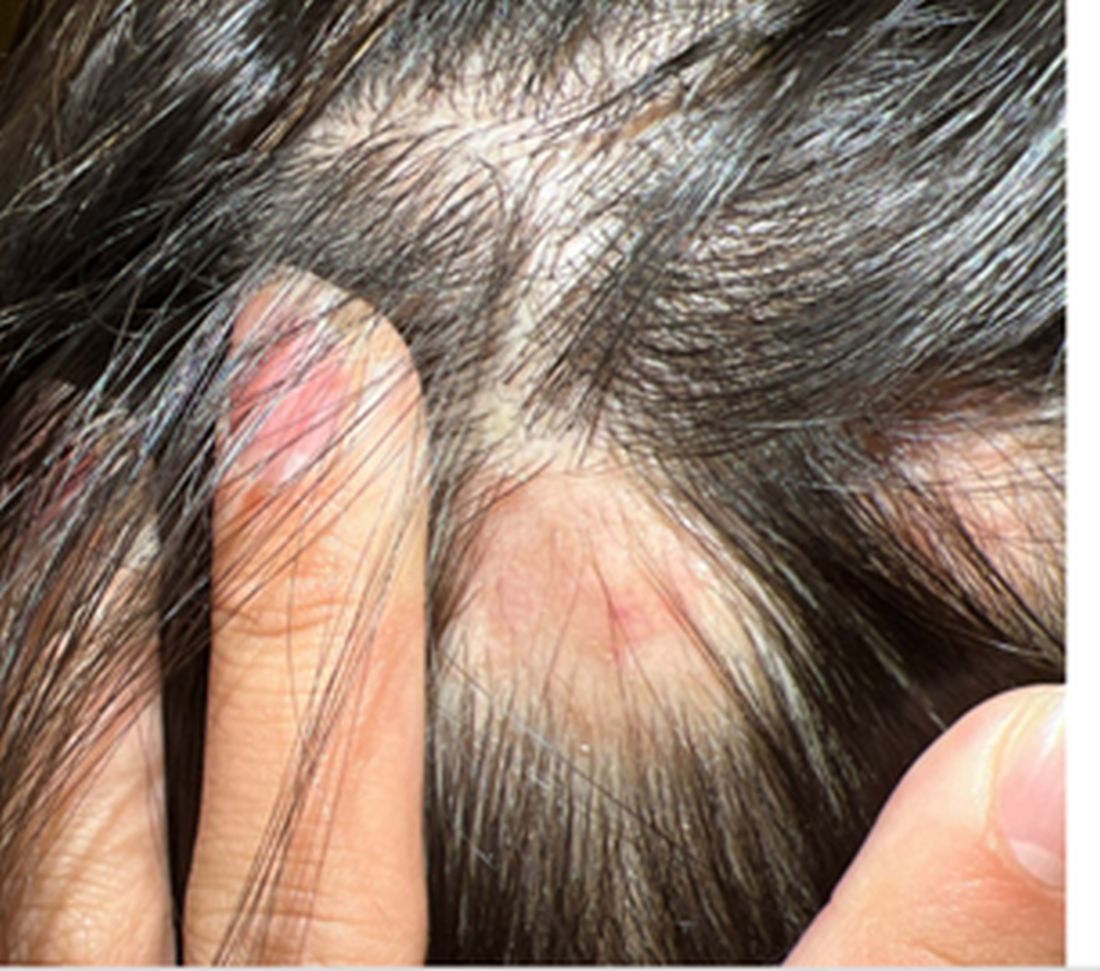
A 16-Year-Old Female Presents With Multiple Areas of Hair Loss on the Scalp
KOH analysis of the scales from the scalp areas revealed no fungal elements. Given the observed erythema and scaling, a punch biopsy was conducted. Histopathological examination of the biopsy sample displayed interface inflammation affecting both the infundibular and lower portions of hair follicles. The presence of folliculosebaceous units transitioning from intermediate to terminal size follicles was noted. A perifollicular, peri eccrine, superficial, and deep perivascular lymphoplasmacytic infiltrate was identified, alongside increased dermal mucin, findings consistent with a diagnosis of discoid lupus erythematosus.
Subsequent laboratory investigations were largely unremarkable, except for an elevated ANA titer (1:320, with a speckled pattern). The patient was initiated on a treatment regimen comprising intralesional triamcinolone and oral hydroxychloroquine (Plaquenil).
Discussion
It predominantly affects adults, yet pediatric cases account for 5%-7% of DLE diagnoses, with a significant predominance in females. Pediatric scalp DLE is particularly concerning due to its potential for causing scarring and permanent hair loss, which can significantly impact the psychological wellbeing of affected children.
The pathogenesis of DLE is multifactorial, involving genetic predispositions, environmental factors like UV light exposure, and immunological mechanisms leading to skin damage.
In children, DLE typically presents as well-demarcated, erythematous plaques with scale and follicular plugging, primarily affecting the scalp. Lesions may also exhibit changes in pigmentation, atrophy, and telangiectasia. The scalp involvement often leads to scarring alopecia, which can be distressing for pediatric patients. Unlike systemic lupus erythematosus (SLE), DLE is usually limited to the skin without systemic involvement. The progression of DLE to systemic lupus erythematosus in children has been previously described to be 22.2%. In a recent report of 201 pediatric cases of DLE, 12% of the cases progressed to systemic lupus erythematosus (SLE) and 14.5% had concurrent SLE. The onset of symptoms before the age of 10 years was the only statistically significant predictor for progression to SLE. Pruritus is a common symptom and may be correlated with disease activity.
The differential diagnosis for this patient encompassed a variety of conditions, including tinea capitis, alopecia areata, trichotillomania, and lichen planopilaris, each considered based on clinical presentation but ultimately excluded through clinical, microscopic, and biopsy findings.
Management strategies for pediatric scalp DLE aim at preventing disease progression, minimizing scarring, and addressing aesthetic concerns. These include the use of topical and intralesional corticosteroids, calcineurin inhibitors, and antimalarial agents like hydroxychloroquine, alongside stringent photoprotection to mitigate UV-triggered exacerbations.
Conclusion
The prognosis for pediatric scalp DLE can be favorable with timely and appropriate management, underscoring the importance of early diagnosis and intervention to prevent scarring and hair loss. However, ongoing surveillance is crucial for monitoring potential progression to systemic lupus erythematosus, albeit a low-risk transformation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. George PM and Tunnessen WW. Childhood discoid lupus erythematosus. Arch Dermatol. 1993;129(5):613-617.
2. Hawat T et al. Pediatric discoid lupus erythematosus: Short report. Dermatol Ther. 2022 Jan;35(1):e15170. doi: 10.1111/dth.15170.
3. Arkin LM et al. Practice-based differences in paediatric discoid lupus erythematosus. Br J Dermatol. 2019 Oct;181(4):805-810. doi: 10.1111/bjd.17780.
KOH analysis of the scales from the scalp areas revealed no fungal elements. Given the observed erythema and scaling, a punch biopsy was conducted. Histopathological examination of the biopsy sample displayed interface inflammation affecting both the infundibular and lower portions of hair follicles. The presence of folliculosebaceous units transitioning from intermediate to terminal size follicles was noted. A perifollicular, peri eccrine, superficial, and deep perivascular lymphoplasmacytic infiltrate was identified, alongside increased dermal mucin, findings consistent with a diagnosis of discoid lupus erythematosus.
Subsequent laboratory investigations were largely unremarkable, except for an elevated ANA titer (1:320, with a speckled pattern). The patient was initiated on a treatment regimen comprising intralesional triamcinolone and oral hydroxychloroquine (Plaquenil).
Discussion
It predominantly affects adults, yet pediatric cases account for 5%-7% of DLE diagnoses, with a significant predominance in females. Pediatric scalp DLE is particularly concerning due to its potential for causing scarring and permanent hair loss, which can significantly impact the psychological wellbeing of affected children.
The pathogenesis of DLE is multifactorial, involving genetic predispositions, environmental factors like UV light exposure, and immunological mechanisms leading to skin damage.
In children, DLE typically presents as well-demarcated, erythematous plaques with scale and follicular plugging, primarily affecting the scalp. Lesions may also exhibit changes in pigmentation, atrophy, and telangiectasia. The scalp involvement often leads to scarring alopecia, which can be distressing for pediatric patients. Unlike systemic lupus erythematosus (SLE), DLE is usually limited to the skin without systemic involvement. The progression of DLE to systemic lupus erythematosus in children has been previously described to be 22.2%. In a recent report of 201 pediatric cases of DLE, 12% of the cases progressed to systemic lupus erythematosus (SLE) and 14.5% had concurrent SLE. The onset of symptoms before the age of 10 years was the only statistically significant predictor for progression to SLE. Pruritus is a common symptom and may be correlated with disease activity.
The differential diagnosis for this patient encompassed a variety of conditions, including tinea capitis, alopecia areata, trichotillomania, and lichen planopilaris, each considered based on clinical presentation but ultimately excluded through clinical, microscopic, and biopsy findings.
Management strategies for pediatric scalp DLE aim at preventing disease progression, minimizing scarring, and addressing aesthetic concerns. These include the use of topical and intralesional corticosteroids, calcineurin inhibitors, and antimalarial agents like hydroxychloroquine, alongside stringent photoprotection to mitigate UV-triggered exacerbations.
Conclusion
The prognosis for pediatric scalp DLE can be favorable with timely and appropriate management, underscoring the importance of early diagnosis and intervention to prevent scarring and hair loss. However, ongoing surveillance is crucial for monitoring potential progression to systemic lupus erythematosus, albeit a low-risk transformation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. George PM and Tunnessen WW. Childhood discoid lupus erythematosus. Arch Dermatol. 1993;129(5):613-617.
2. Hawat T et al. Pediatric discoid lupus erythematosus: Short report. Dermatol Ther. 2022 Jan;35(1):e15170. doi: 10.1111/dth.15170.
3. Arkin LM et al. Practice-based differences in paediatric discoid lupus erythematosus. Br J Dermatol. 2019 Oct;181(4):805-810. doi: 10.1111/bjd.17780.
KOH analysis of the scales from the scalp areas revealed no fungal elements. Given the observed erythema and scaling, a punch biopsy was conducted. Histopathological examination of the biopsy sample displayed interface inflammation affecting both the infundibular and lower portions of hair follicles. The presence of folliculosebaceous units transitioning from intermediate to terminal size follicles was noted. A perifollicular, peri eccrine, superficial, and deep perivascular lymphoplasmacytic infiltrate was identified, alongside increased dermal mucin, findings consistent with a diagnosis of discoid lupus erythematosus.
Subsequent laboratory investigations were largely unremarkable, except for an elevated ANA titer (1:320, with a speckled pattern). The patient was initiated on a treatment regimen comprising intralesional triamcinolone and oral hydroxychloroquine (Plaquenil).
Discussion
It predominantly affects adults, yet pediatric cases account for 5%-7% of DLE diagnoses, with a significant predominance in females. Pediatric scalp DLE is particularly concerning due to its potential for causing scarring and permanent hair loss, which can significantly impact the psychological wellbeing of affected children.
The pathogenesis of DLE is multifactorial, involving genetic predispositions, environmental factors like UV light exposure, and immunological mechanisms leading to skin damage.
In children, DLE typically presents as well-demarcated, erythematous plaques with scale and follicular plugging, primarily affecting the scalp. Lesions may also exhibit changes in pigmentation, atrophy, and telangiectasia. The scalp involvement often leads to scarring alopecia, which can be distressing for pediatric patients. Unlike systemic lupus erythematosus (SLE), DLE is usually limited to the skin without systemic involvement. The progression of DLE to systemic lupus erythematosus in children has been previously described to be 22.2%. In a recent report of 201 pediatric cases of DLE, 12% of the cases progressed to systemic lupus erythematosus (SLE) and 14.5% had concurrent SLE. The onset of symptoms before the age of 10 years was the only statistically significant predictor for progression to SLE. Pruritus is a common symptom and may be correlated with disease activity.
The differential diagnosis for this patient encompassed a variety of conditions, including tinea capitis, alopecia areata, trichotillomania, and lichen planopilaris, each considered based on clinical presentation but ultimately excluded through clinical, microscopic, and biopsy findings.
Management strategies for pediatric scalp DLE aim at preventing disease progression, minimizing scarring, and addressing aesthetic concerns. These include the use of topical and intralesional corticosteroids, calcineurin inhibitors, and antimalarial agents like hydroxychloroquine, alongside stringent photoprotection to mitigate UV-triggered exacerbations.
Conclusion
The prognosis for pediatric scalp DLE can be favorable with timely and appropriate management, underscoring the importance of early diagnosis and intervention to prevent scarring and hair loss. However, ongoing surveillance is crucial for monitoring potential progression to systemic lupus erythematosus, albeit a low-risk transformation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. George PM and Tunnessen WW. Childhood discoid lupus erythematosus. Arch Dermatol. 1993;129(5):613-617.
2. Hawat T et al. Pediatric discoid lupus erythematosus: Short report. Dermatol Ther. 2022 Jan;35(1):e15170. doi: 10.1111/dth.15170.
3. Arkin LM et al. Practice-based differences in paediatric discoid lupus erythematosus. Br J Dermatol. 2019 Oct;181(4):805-810. doi: 10.1111/bjd.17780.
Upon physical examination, the patient exhibited several patches of alopecia with accompanying perifollicular scaling, crusting, and erythema on the affected areas. Examination of her face revealed comedones and papules.
Study Identifies Several Factors That Influence Longterm Antibiotic Prescribing for Acne
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
to follow them, according to the authors of a recently published study.
“This study explored why dermatologists still prescribe a good number of long-term antibiotics for people with acne,” the study’s senior author Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview. “And we found a lot of reasons.” The study was published online in JAMA Dermatology.
Using online surveys and semi-structured video interviews of 30 dermatologists, infectious disease physicians with expertise in antimicrobial stewardship, dermatology residents, and nonphysician clinicians, the investigators assessed respondents’ knowledge and attitudes regarding long-term antibiotics in acne. Salient themes impacting long-term antibiotic prescriptions included the following:
- A perceived dearth of evidence to justify changes in practice.
- Difficulties with iPLEDGE, the Risk Evaluation and Mitigation Strategy (REMS) for managing the teratogenic risks associated with isotretinoin, and with discussing oral contraceptives.
- “Navigating” discussions with about tapering-off of antibiotics.
- Challenging patient demands.
- A lack of effective tools for monitoring progress in antibiotic stewardship.
“It’s surprising there are so many barriers that make it difficult for dermatologists to stick with the guidelines even if they want to,” said Dr. Yeung, a coauthor of the recently released updated American Academy of Dermatology (AAD) acne management guidelines.
A dermatologist who wants to stop systemic antibiotics within 3 months may not know how to do so, he explained, or high demand for appointments may prevent timely follow-ups.
A major reason why dermatologists struggle to limit long-term antibiotic use is that there are very few substitutes that are perceived to work as well, said David J. Margolis, MD, PhD, who was not involved with the study and was asked to comment on the results. He is professor of epidemiology and dermatology at the University of Pennsylvania, Philadelphia.
“Part of the reason antibiotics are being used to treat acne is that they’re effective, and effective for severe disease,” he said. The alternatives, which are mostly topicals, said Dr. Margolis, do not work as well for moderate to severe disease or, with isotretinoin, involve time-consuming hurdles. Dr. Margolis said that he often hears such concerns from individual dermatologists. “But it’s helpful to see these in a well-organized, well-reported qualitative study.”
Infectious disease specialists surveyed considered limiting long-term antibiotic use as extremely important, while several dermatologists “argued that other specialties ‘underestimate the impact acne has on people’s lives,’ ” the authors wrote. Other respondents prioritized making the right choice for the patient at hand.
Although guidelines were never meant to be black and white, Dr. Yeung said, it is crucial to target the goal of tapering off after about 3-4 months — a cutoff with which guidelines from groups including the AAD, the Japanese Dermatological Association in guidelines from 2016, and 2017, respectively, and others concur.
He added, “Some folks believe that if the oral antibiotic is working, why stop? We need to develop evidence to show that reducing oral antibiotic use is important to our patients, not just to a theoretical problem of antibiotic resistance in society.” For example, in a study published in The Lancet in 2004, patients who used strictly topical regimens achieved efficacy similar to that of those who used only oral antibiotics.
In addition, some clinicians worried that limiting antibiotics could reduce patient satisfaction, spurring switches to other providers. However, he and the other authors of the JAMA Dermatology study noted that in a survey of patients with acne published in the Journal of Clinical and Aesthetic Dermatology in 2019, 76.9% said they would be “very or extremely likely” to use effective antibiotic-free treatments if offered.
Because most respondents were highly aware of the importance of antibiotic stewardship, Dr. Yeung said, additional passive education is not necessarily the answer. “It will take a concerted effort by our national societies to come up with resources and solutions for individual dermatologists to overcome some of these larger barriers.” Such solutions could range from training in communication and shared decision-making to implementing systems that provide individualized feedback to support antibiotic stewardship.
Many ongoing studies are examining antibiotic stewardship, Dr. Margolis said in the interview. However, he added, dermatologists’ idea of long-term use is 3 months, versus 1 month or less in other specialties. “Moreover, dermatology patients tend to be much healthier individuals and are rarely hospitalized, so there may be some issues comparing the ongoing studies to individuals with acne.” Future research will need to account for such differences, he said.
The study was funded by an American Acne & Rosacea Society Clinical Research Award. Dr. Yeung is associate editor of JAMA Dermatology. Dr. Margolis has received a National Institutes of Health grant to study doxycycline versus spironolactone in acne.
FROM JAMA DERMATOLOGY
Childhood Atopic Dermatitis Linked to IBD Risk
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but atopic manifestations are generally not associated with IBD.
METHODOLOGY:
- Studies examining the link between atopy and IBD have yielded inconsistent results. Many of these studies included adults, introducing recall bias, or relied on physician diagnoses that might have overlooked mild cases.
- Researchers analyzed prospectively collected data on 83,311 children from two cohort studies, ABIS (1997-1999) and MoBa (1999-2008), who were followed up from birth until 2021 or a diagnosis of IBD.
- Information on parents was collected prospectively via questionnaires on any atopy their children might have developed by the age of 3 years. Atopy included conditions such as AD, asthma, food allergy, or allergic rhinitis.
TAKEAWAY:
- A total of 301 participants were diagnosed with IBD over 1,174,756 person-years of follow-up. By the age of 3 years, 31,671 children (38%) were reported to have any atopic manifestation.
- Children with AD at the age of 3 years demonstrated a significantly higher risk for IBD (pooled adjusted hazard ratio [aHR], 1.46), Crohn’s disease (pooled aHR, 1.53), and ulcerative colitis (pooled aHR, 1.78).
- Any atopic manifestation by the age of 3 years was not associated with a subsequent risk for IBD, Crohn’s disease, or ulcerative colitis, nor were analyses focused on early-life food-related allergy, asthma, and allergic rhinitis.
IN PRACTICE:
According to the authors, these findings suggested potential shared underlying causes between AD and IBD, which could help identify individuals at risk, and “a deeper understanding could significantly benefit the development of novel treatment approaches capable of effectively addressing both conditions, consequently enhancing patient outcomes.”
SOURCE:
This study, led by Tereza Lerchova, MD, PhD, Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, was published online in The Journal of Pediatrics.
LIMITATIONS:
The findings of this study were mostly related to childhood-onset IBD instead of IBD in adult life. Lower participation in the MoBa study could limit generalizability to a broader population. In addition, there might have been lower participation from families without atopic manifestations.
DISCLOSURES:
The study was funded by the Swedish Society for Medical Research, Swedish Research Council, and ALF and supported by grants from the Swedish Child Diabetes Foundation, Swedish Council for Working Life and Social Research, Swedish Research Council, Medical Research Council of Southeast Sweden, JDRF Wallenberg Foundation, Linkoping University, and Joanna Cocozza Foundation. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Less Than 50% of Accelerated Approvals Show Clinical Benefit
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.
despite being on the US market for more than 5 years, according to a new study.
Under the program, drugs are approved for marketing if they show benefit in surrogate markers thought to indicate efficacy. Progression-free survival, tumor response, and duration of response are the most used surrogate markers for accelerated approvals of cancer drugs. These are based largely on imaging studies that show either a stop in growth in the case of progression-free survival or tumor shrinkage in the case of tumor response.
Following accelerated approvals, companies are then supposed to show actual clinical benefit in confirmatory trials.
The problem with relying on surrogate markers for drug approvals is that they don’t always correlate with longer survival or improved quality of life, said Edward Cliff, MBBS, who presented the findings at the American Association for Cancer Research 2024 annual meeting (abstract 918). The study was also published in JAMA to coincide with the meeting presentation.
In some cancers, these markers work well, but in others they don’t, said Dr. Cliff, a hematology trainee at Brigham and Women’s Hospital, Boston, when the work was conducted, and now a hematology fellow at the Peter MacCallum Cancer Centre in Melbourne, Australia.
To determine whether cancer drugs granted accelerated approval ultimately show an overall survival or quality of life benefit, researchers reviewed 46 cancer drugs granted accelerated approvals between 2013 and 2017. Twenty (43%) were granted full approval after demonstrating survival or quality-of-life benefits.
Nine, however, were converted to full approvals on the basis of surrogate markers. These include a full approval for pembrolizumab in previously treated recurrent or refractory head and neck squamous cell carcinoma and a full approval for nivolumab for refractory locally advanced or metastatic urothelial carcinoma, both based on tumor response rate and duration of response.
Of the remaining 17 drugs evaluated in the trial, 10 have been withdrawn and seven do not yet have confirmatory trial results.
The reliance on surrogate markers means that these drugs are used for treatment, covered by insurance, and added to guidelines — all without solid evidence of real-world clinical benefit, said Dr. Cliff.
However, the goal should not be to do away with the accelerated approval process, because it sometimes does deliver powerful agents to patients quickly. Instead, Dr. Cliff told this news organization, the system needs to be improved so that “we keep the speed while getting certainty around clinical benefits” with robust and timely confirmatory trials.
In the meantime, “clinicians should communicate with patients about any residual uncertainty of clinical benefit when they offer novel therapies,” Dr. Cliff explained. “It’s important for them to have the information.”
There has been some progress on the issue. In December 2022, the US Congress passed the Food and Drug Administration Omnibus Reform Act. Among other things, the Act requires companies to have confirmation trials underway as a condition for accelerated approval, and to provide regular reports on their progress. The Act also expedites the withdrawal process for drugs that don’t show a benefit.
The Act has been put to the test twice recently. In February, FDA used the expedited process to remove the multiple myeloma drug melphalan flufenamide from the market. Melphalan flufenamide hadn’t been sold in the US for quite some time, so the process wasn’t contentious.
In March, Regeneron announced that accelerated approval for the follicular and diffuse B cell lymphoma drug odronextamab has been delayed pending enrollment in a confirmatory trial.
“There have been some promising steps,” Dr. Cliff said, but much work needs to be done.
Study moderator Shivaani Kummar, MD, agreed, noting that “the data is showing that the confirmatory trials aren’t happening at the pace which they should.”
But the solution is not to curtail approvals; it’s to make sure that accelerated approval commitments are met, said Dr. Kummar.
Still, “as a practicing oncologist, I welcome the accelerated pathway,” Dr. Kummar, a medical oncologist/hematologist at Oregon Health & Science University, Portland, told this news organization. “I want the availability to my patients.”
Having drugs approved on the basis of surrogate markers doesn’t necessarily mean patients are getting ineffective therapies, Dr. Kummar noted. For instance, if an agent just shrinks the tumor, it can sometimes still be “a huge clinical benefit because it can take the symptoms away.”
As for prescribing drugs based on accelerated approvals, she said she tells her patients that trials have been promising, but we don’t know what the long-term effects are. She and her patient then make a decision together.
The study was funded by Arnold Ventures. Dr. Kummar reported support from several companies, including Bayer, Gilead, and others. Dr. Cliff had no disclosures.
A version of this article appeared on Medscape.com.
Analysis Finds Low Malignancy Rate in Pediatric Longitudinal Melanonychia
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- LM — a pigmented band in the nail plate caused by increased melanin deposition — occurs in children and adults, resulting from melanocytic activation or proliferation in response to infection, systemic disease, medication, trauma, and other factors.
- Clinical features of LM in children mimic red-flag signs of subungual melanoma in adults although rarely is subungual melanoma.
- A biopsy can confirm the diagnosis, but other considerations include the scar, cost and stress of a procedure, and possibly pain or deformity.
- The researchers conducted a systematic review and meta-analysis of the prevalence of clinical and dermoscopic features in 1391 pediatric patients with LM (diagnosed at a mean age of 5-13 years) from 24 studies published between 1996 and 2023.
TAKEAWAY:
- Of 731 lesions in which a diagnosis was provided, benign nail matrix nevus accounted for 86% of cases.
- Only eight cases of subungual melanoma in situ were diagnosed, with no cases of invasive melanoma identified.
- Most lesions occurred on the fingernails (76%), particularly in the first digits (45%), and the most frequent clinical features included dark-colored bands (70%), multicolored bands (48%), broad bandwidth (41%), and pseudo-Hutchinson sign (41%).
- During a median follow-up of 1-5.5 years, 30% of lesions continued to evolve with changes in width or color, while 23% remained stable and 20% underwent spontaneous regression.
IN PRACTICE:
“In the pivotal clinical decision of whether to biopsy a child with longitudinal melanonychia, perhaps with features that would require a prompt biopsy in an adult, this study provides data to support the option of clinical monitoring,” the authors wrote.
SOURCE:
The meta-analysis, led by Serena Yun-Chen Tsai, MD, in the Department of Dermatology, Massachusetts General Hospital, Boston, Massachusetts, was published online in Pediatric Dermatology.
LIMITATIONS:
Most studies were conducted in Asia, and data stratified by skin type were limited. Inconsistent reporting and missing critical features could affect data quality. Also, certain features displayed high heterogeneity.
DISCLOSURES:
This meta-analysis was supported by the Pediatric Dermatology Research Alliance Career Bridge Research Grant. One co-author disclosed relationships with UpToDate (author, reviewer), Skin Analytics (consultant), and DermTech (research materials).
A version of this article appeared on Medscape.com.
Tooth Enamel Disorder Is a Feature of Kindler EB
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- KEB or Kindler syndrome, a genetic skin-blistering disease associated with pathogenic variants in FERMT1, is the rarest type of EB. Early detection and preventive measures can minimize complications, such as gum disease and other oral health issues, that have been reported in patients with KEB.
- Amelogenesis imperfecta is a group of rare genetic developmental conditions characterized by tooth enamel defects and can be associated with hypersensitivity and eruption disturbances in teeth, as well as periodontal conditions.
- Researchers conducted a longitudinal study on 36 patients with KEB (age, 2 weeks to 70 years; 42% female) from two clinics in Germany and Chile from 2003 to 2023, with follow-up times of 1-24 years.
- The primary outcomes were presence of orofacial features, including amelogenesis imperfecta, intraoral wounds, and periodontal disease, and oral squamous cell carcinoma.
TAKEAWAY:
- All 11 patients with information on enamel structure in their records had pitted enamel anomalies (pitted amelogenesis imperfecta), with variable severity.
- Of patients whose enamel could not be analyzed, three had all teeth crowned in their 20s, suggesting enamel defects, and two had all teeth extracted in their teens or 20s, indicating severe periodontal disease.
- The most common orofacial features were periodontal disease (27 of 36 patients), intraoral lesions (16 of 22 patients), angular cheilitis (24 of 33 patients), and cheilitis (22 of 34 patients), gingival overgrowth (17 of 26 patients), microstomia (14 of 25 patients), and vestibular obliteration (8 of 16 patients).
- Oral squamous cell carcinoma was diagnosed at the site of chronic lip lesions in two patients, with lethal outcomes.
IN PRACTICE:
These findings highlight the extent and severity of oral manifestations in KEB, the authors concluded, adding that “oral care is mandatory” in patients with KEB.
SOURCE:
This report, led by Susanne Krämer, DDS, MSc, of Medical Faculty and Medical Center, University of Freiburg, Freiburg im Breisgau, Germany, was published online in JAMA Dermatology.
LIMITATIONS:
The small sample size and the retrospective nature of the study could limit its generalizability.
DISCLOSURES:
The authors did not disclose any source of funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Lead Has Not Gone Away — What Should Pediatric Clinicians Do?
following a 2023 outbreak of elevated levels of lead in children associated with consumption of contaminated applesauce.
Federal legislation in the 1970s eliminated lead from gasoline, paints, and other consumer products, and resulted in significantly reduced blood lead levels (BLLs) in children throughout the United States.
But recently published studies highlight persistent issues with lead in drinking water and consumer products, suggesting that the fight is not over.
It’s in the Water
In 2014 the city of Flint, Michigan, changed its water supply and high levels of lead were later found in the municipal water supply.
Effects of that crisis still plague the city today. An initial study found that elevated BLLs had doubled among children between 2013 and 2015.
Lead exposure in young children is associated with several negative outcomes, including decreased cognitive ability, brain volume, and social mobility, and increased anxiety/depression and impulsivity, and higher rates of criminal offenses later in life.
Many other water systems still contain lead pipes, despite a 1986 ban by the US Environmental Protection Agency on using them for installing or repairing public water systems. The mayor of Chicago announced a plan to start replacing lead service lines in 2020; however, 400,000 households are still served by these pipes, the most in the nation.
Benjamin Huynh, a native of Chicago, was curious about the impact of all those lead service lines. Now an assistant professor in the Department of Environmental Health and Engineering at Johns Hopkins University in Baltimore, Maryland, he and his colleagues researched how many children under the age of 6 years were exposed to contaminated water.
The results showed that lead contamination of water is widespread.
“We’re estimating that 68% of kids under the age of 6 in Chicago were exposed to lead-contaminated drinking water,” Mr. Huynh said.
He added that residents in predominantly Black and Latino neighborhoods had the highest risk for lead contamination in their water, but children living on these blocks were less likely to get tested, suggesting a need for more outreach to raise awareness.
Meanwhile, a little over one third of Chicago residents reported drinking bottled water as their main source of drinking water.
But even bottled water could contain lead. The US Food and Drug Administration (FDA) has set a limit for lead in bottled water to five parts per billion. The FDA threshold for taking action in public drinking water systems is 15 parts per billion. But the American Academy of Pediatrics states that no amount of lead in drinking water is considered safe for drinking.
Mr. Huynh also pointed out that not all home water filters remove lead. Only devices that meet National Sanitation Foundation 53 standards are certified for lead removal. Consumers should verify that the filter package specifically lists the device as certified for removing contaminant lead.
Lead-tainted Cinnamon
Last fall, the North Carolina Department of Health and Human Services identified several children with elevated levels of lead who had consumed WanaBana Apple Cinnamon Fruit Puree pouches.
An investigation by the FDA identified additional brands containing lead and issued a recall of applesauce pouches sold by retailers like Dollar Tree and Amazon.
According to the US Centers for Disease Control and Prevention, nearly 500 children were affected by the tainted applesauce. The FDA traced the source of the lead to cinnamon from a supplier in Ecuador.
An FDA spokesperson told this news organization the episode appears to have resulted from “economically motivated adulteration,” which occurs when a manufacturer leaves out or substitutes a valuable ingredient or part of a food. In the case of spices, lead may be added as a coloring agent or to increase the product weight.
“When we look at domestically made products from large, reputable companies, in general, they do a pretty good job of following safe product guidelines and regulations,” said Kevin Osterhoudt, MD, professor of pediatrics at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia. “But when we use third-party sellers and we import things from other countries that aren’t regulated as closely, we certainly take a lot more risk in the products that we receive.”
While the Food Safety Modernization Act of 2011 aimed to improve agency’s capacity to manage the ever-rising volume of food produced domestically and imported from overseas, the funding has stayed flat while the volume of inspections has increased. In the early 1990s, the number of shipments screened by the agency numbered in the thousands annually. Last year the FDA screened 15 million shipments from more than 200 countries, according to the agency.
Prompted by the finding of lead in applesauce, the FDA began a wider investigation into ground cinnamon by sampling the product from discount retail stores. It recalled an additional six brands of cinnamon sold in the United States containing lead.
Dr. Osterhoudt’s message to families who think their child might have been exposed to a contaminated product is to dispose of it as directed by FDA and CDC guidelines.
In Philadelphia, where Dr. Osterhoudt practices as an emergency room physician, baseline rates of childhood lead poisoning are already high, so he advises families to “do a larger inventory of all the source potential sources of lead in their life and to reduce all the exposures as low as possible.”
He also advises parents that a nutritious diet high in calcium and iron can protect their children from the deleterious effects of lead.
Current Standards for Lead Screening and Testing
Lead is ubiquitous. The common routes of exposure to humans include use of fossil fuels such as leaded gasoline, some types of industrial facilities, and past use of lead-based paint in homes. In addition to spices, lead has been found in a wide variety of products such as toys, jewelry, antiques, cosmetics, and dietary supplements imported from other countries.
Noah Buncher, DO, is a primary care pediatrician in South Philadelphia at Children’s Hospital of Pennsylvania and the former director of a lead clinic in Boston that provides care for children with lead poisoning. He follows guidelines from the American Academy of Pediatrics that define an elevated BLL as ≥ 3.5 µg/dL. The guidelines recommend screening children for lead exposures during well child visits starting at age 6 months up to 6 years and obtaining a BLL if risks for lead exposure are present.
Dr. Buncher starts with a basic environmental history that covers items like the age, condition, zip code of home, parental occupations, or hobbies that might result in exposing family members to lead, and if another child in the home has a history of elevated BLLs.
But a careful history for potential lead exposures can be time-consuming.
“There’s a lot to cover in a routine well child visit,” Dr. Buncher said. “We have maybe 15-20 minutes to cover a lot.”
Clinics also vary on whether lead screening questions are put into workflows in the electronic medical record. Although parents can complete a written questionnaire about possible lead exposures, they may have difficulty answering questions about the age of their home or not know whether their occupation is high risk.
Transportation to a clinic is often a barrier for families, and sometimes patients must travel to a separate lab to be tested for lead.
Dr. Buncher also pointed to the patchwork of local and state requirements that can lead to confusion among providers. Massachusetts, where he formerly practiced, has a universal requirement to test all children at ages 1, 2, and 3 years. But in Pennsylvania, screening laws vary from county to county.
“Pennsylvania should implement universal screening recommendations for all kids under 6 regardless of what county you live in,” Dr. Buncher said.
Protective Measures
Alan Woolf, MD, a professor of pediatrics at Harvard Medical School, Boston, Massachusetts, and director of the Pediatric Environmental Health Center at Boston Children’s Hospital, has a few ideas about how providers can step up their lead game, including partnering with their local health department.
The CDC funds Childhood Lead Poisoning Prevention Programs based in state and local health departments to work with clinicians to improve rates of blood lead testing, monitor the prevalence of lead in their jurisdictions, and ensure that a system of referral is available for treatment and lead remediation services in the home.
Dr. Woolf also suggested that clinicians refer patients under age 3 years with high BLLs to their local Early Intervention Program.
“They’ll assess their child’s development, their speech, their motor skills, their social skills, and if they qualify, it’s free,” Dr. Woolf said.
He cited research showing children with elevated lead levels who received early intervention services performed better in grade school than equally exposed children who did not access similar services.
Another key strategy for pediatric clinicians is to learn local or state regulations for testing children for lead and how to access lead surveillance data in their practice area. Children who reside in high-risk areas are automatic candidates for screening.
Dr. Woolf pointed out that big cities are not the only localities with lead in the drinking water. If families are drawing water from their own well, they should collect that water annually to have it tested for lead and microbes.
At the clinic-wide level, Dr. Woolf recommends the use of blood lead testing as a quality improvement measure. For example, Akron Children’s Hospital developed a quality improvement initiative using a clinical decision support tool to raise screening rates in their network of 30 clinics. One year after beginning the project, lead screenings during 12-month well visits increased from 71% to 96%.
“What we’re interested in as pediatric health professionals is eliminating all background sources of lead in a child’s environment,” Dr. Woolf said. “Whether that’s applesauce pouches, whether that’s lead-containing paint, lead in water, lead in spices, or lead in imported pottery or cookware — there are just a tremendous number of sources of lead that we can do something about.”
None of the subjects reported financial conflicts of interest.
A former pediatrician, Dr. Thomas is a freelance science writer living in Portland, Oregon.
A version of this article appeared on Medscape.com.
following a 2023 outbreak of elevated levels of lead in children associated with consumption of contaminated applesauce.
Federal legislation in the 1970s eliminated lead from gasoline, paints, and other consumer products, and resulted in significantly reduced blood lead levels (BLLs) in children throughout the United States.
But recently published studies highlight persistent issues with lead in drinking water and consumer products, suggesting that the fight is not over.
It’s in the Water
In 2014 the city of Flint, Michigan, changed its water supply and high levels of lead were later found in the municipal water supply.
Effects of that crisis still plague the city today. An initial study found that elevated BLLs had doubled among children between 2013 and 2015.
Lead exposure in young children is associated with several negative outcomes, including decreased cognitive ability, brain volume, and social mobility, and increased anxiety/depression and impulsivity, and higher rates of criminal offenses later in life.
Many other water systems still contain lead pipes, despite a 1986 ban by the US Environmental Protection Agency on using them for installing or repairing public water systems. The mayor of Chicago announced a plan to start replacing lead service lines in 2020; however, 400,000 households are still served by these pipes, the most in the nation.
Benjamin Huynh, a native of Chicago, was curious about the impact of all those lead service lines. Now an assistant professor in the Department of Environmental Health and Engineering at Johns Hopkins University in Baltimore, Maryland, he and his colleagues researched how many children under the age of 6 years were exposed to contaminated water.
The results showed that lead contamination of water is widespread.
“We’re estimating that 68% of kids under the age of 6 in Chicago were exposed to lead-contaminated drinking water,” Mr. Huynh said.
He added that residents in predominantly Black and Latino neighborhoods had the highest risk for lead contamination in their water, but children living on these blocks were less likely to get tested, suggesting a need for more outreach to raise awareness.
Meanwhile, a little over one third of Chicago residents reported drinking bottled water as their main source of drinking water.
But even bottled water could contain lead. The US Food and Drug Administration (FDA) has set a limit for lead in bottled water to five parts per billion. The FDA threshold for taking action in public drinking water systems is 15 parts per billion. But the American Academy of Pediatrics states that no amount of lead in drinking water is considered safe for drinking.
Mr. Huynh also pointed out that not all home water filters remove lead. Only devices that meet National Sanitation Foundation 53 standards are certified for lead removal. Consumers should verify that the filter package specifically lists the device as certified for removing contaminant lead.
Lead-tainted Cinnamon
Last fall, the North Carolina Department of Health and Human Services identified several children with elevated levels of lead who had consumed WanaBana Apple Cinnamon Fruit Puree pouches.
An investigation by the FDA identified additional brands containing lead and issued a recall of applesauce pouches sold by retailers like Dollar Tree and Amazon.
According to the US Centers for Disease Control and Prevention, nearly 500 children were affected by the tainted applesauce. The FDA traced the source of the lead to cinnamon from a supplier in Ecuador.
An FDA spokesperson told this news organization the episode appears to have resulted from “economically motivated adulteration,” which occurs when a manufacturer leaves out or substitutes a valuable ingredient or part of a food. In the case of spices, lead may be added as a coloring agent or to increase the product weight.
“When we look at domestically made products from large, reputable companies, in general, they do a pretty good job of following safe product guidelines and regulations,” said Kevin Osterhoudt, MD, professor of pediatrics at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia. “But when we use third-party sellers and we import things from other countries that aren’t regulated as closely, we certainly take a lot more risk in the products that we receive.”
While the Food Safety Modernization Act of 2011 aimed to improve agency’s capacity to manage the ever-rising volume of food produced domestically and imported from overseas, the funding has stayed flat while the volume of inspections has increased. In the early 1990s, the number of shipments screened by the agency numbered in the thousands annually. Last year the FDA screened 15 million shipments from more than 200 countries, according to the agency.
Prompted by the finding of lead in applesauce, the FDA began a wider investigation into ground cinnamon by sampling the product from discount retail stores. It recalled an additional six brands of cinnamon sold in the United States containing lead.
Dr. Osterhoudt’s message to families who think their child might have been exposed to a contaminated product is to dispose of it as directed by FDA and CDC guidelines.
In Philadelphia, where Dr. Osterhoudt practices as an emergency room physician, baseline rates of childhood lead poisoning are already high, so he advises families to “do a larger inventory of all the source potential sources of lead in their life and to reduce all the exposures as low as possible.”
He also advises parents that a nutritious diet high in calcium and iron can protect their children from the deleterious effects of lead.
Current Standards for Lead Screening and Testing
Lead is ubiquitous. The common routes of exposure to humans include use of fossil fuels such as leaded gasoline, some types of industrial facilities, and past use of lead-based paint in homes. In addition to spices, lead has been found in a wide variety of products such as toys, jewelry, antiques, cosmetics, and dietary supplements imported from other countries.
Noah Buncher, DO, is a primary care pediatrician in South Philadelphia at Children’s Hospital of Pennsylvania and the former director of a lead clinic in Boston that provides care for children with lead poisoning. He follows guidelines from the American Academy of Pediatrics that define an elevated BLL as ≥ 3.5 µg/dL. The guidelines recommend screening children for lead exposures during well child visits starting at age 6 months up to 6 years and obtaining a BLL if risks for lead exposure are present.
Dr. Buncher starts with a basic environmental history that covers items like the age, condition, zip code of home, parental occupations, or hobbies that might result in exposing family members to lead, and if another child in the home has a history of elevated BLLs.
But a careful history for potential lead exposures can be time-consuming.
“There’s a lot to cover in a routine well child visit,” Dr. Buncher said. “We have maybe 15-20 minutes to cover a lot.”
Clinics also vary on whether lead screening questions are put into workflows in the electronic medical record. Although parents can complete a written questionnaire about possible lead exposures, they may have difficulty answering questions about the age of their home or not know whether their occupation is high risk.
Transportation to a clinic is often a barrier for families, and sometimes patients must travel to a separate lab to be tested for lead.
Dr. Buncher also pointed to the patchwork of local and state requirements that can lead to confusion among providers. Massachusetts, where he formerly practiced, has a universal requirement to test all children at ages 1, 2, and 3 years. But in Pennsylvania, screening laws vary from county to county.
“Pennsylvania should implement universal screening recommendations for all kids under 6 regardless of what county you live in,” Dr. Buncher said.
Protective Measures
Alan Woolf, MD, a professor of pediatrics at Harvard Medical School, Boston, Massachusetts, and director of the Pediatric Environmental Health Center at Boston Children’s Hospital, has a few ideas about how providers can step up their lead game, including partnering with their local health department.
The CDC funds Childhood Lead Poisoning Prevention Programs based in state and local health departments to work with clinicians to improve rates of blood lead testing, monitor the prevalence of lead in their jurisdictions, and ensure that a system of referral is available for treatment and lead remediation services in the home.
Dr. Woolf also suggested that clinicians refer patients under age 3 years with high BLLs to their local Early Intervention Program.
“They’ll assess their child’s development, their speech, their motor skills, their social skills, and if they qualify, it’s free,” Dr. Woolf said.
He cited research showing children with elevated lead levels who received early intervention services performed better in grade school than equally exposed children who did not access similar services.
Another key strategy for pediatric clinicians is to learn local or state regulations for testing children for lead and how to access lead surveillance data in their practice area. Children who reside in high-risk areas are automatic candidates for screening.
Dr. Woolf pointed out that big cities are not the only localities with lead in the drinking water. If families are drawing water from their own well, they should collect that water annually to have it tested for lead and microbes.
At the clinic-wide level, Dr. Woolf recommends the use of blood lead testing as a quality improvement measure. For example, Akron Children’s Hospital developed a quality improvement initiative using a clinical decision support tool to raise screening rates in their network of 30 clinics. One year after beginning the project, lead screenings during 12-month well visits increased from 71% to 96%.
“What we’re interested in as pediatric health professionals is eliminating all background sources of lead in a child’s environment,” Dr. Woolf said. “Whether that’s applesauce pouches, whether that’s lead-containing paint, lead in water, lead in spices, or lead in imported pottery or cookware — there are just a tremendous number of sources of lead that we can do something about.”
None of the subjects reported financial conflicts of interest.
A former pediatrician, Dr. Thomas is a freelance science writer living in Portland, Oregon.
A version of this article appeared on Medscape.com.
following a 2023 outbreak of elevated levels of lead in children associated with consumption of contaminated applesauce.
Federal legislation in the 1970s eliminated lead from gasoline, paints, and other consumer products, and resulted in significantly reduced blood lead levels (BLLs) in children throughout the United States.
But recently published studies highlight persistent issues with lead in drinking water and consumer products, suggesting that the fight is not over.
It’s in the Water
In 2014 the city of Flint, Michigan, changed its water supply and high levels of lead were later found in the municipal water supply.
Effects of that crisis still plague the city today. An initial study found that elevated BLLs had doubled among children between 2013 and 2015.
Lead exposure in young children is associated with several negative outcomes, including decreased cognitive ability, brain volume, and social mobility, and increased anxiety/depression and impulsivity, and higher rates of criminal offenses later in life.
Many other water systems still contain lead pipes, despite a 1986 ban by the US Environmental Protection Agency on using them for installing or repairing public water systems. The mayor of Chicago announced a plan to start replacing lead service lines in 2020; however, 400,000 households are still served by these pipes, the most in the nation.
Benjamin Huynh, a native of Chicago, was curious about the impact of all those lead service lines. Now an assistant professor in the Department of Environmental Health and Engineering at Johns Hopkins University in Baltimore, Maryland, he and his colleagues researched how many children under the age of 6 years were exposed to contaminated water.
The results showed that lead contamination of water is widespread.
“We’re estimating that 68% of kids under the age of 6 in Chicago were exposed to lead-contaminated drinking water,” Mr. Huynh said.
He added that residents in predominantly Black and Latino neighborhoods had the highest risk for lead contamination in their water, but children living on these blocks were less likely to get tested, suggesting a need for more outreach to raise awareness.
Meanwhile, a little over one third of Chicago residents reported drinking bottled water as their main source of drinking water.
But even bottled water could contain lead. The US Food and Drug Administration (FDA) has set a limit for lead in bottled water to five parts per billion. The FDA threshold for taking action in public drinking water systems is 15 parts per billion. But the American Academy of Pediatrics states that no amount of lead in drinking water is considered safe for drinking.
Mr. Huynh also pointed out that not all home water filters remove lead. Only devices that meet National Sanitation Foundation 53 standards are certified for lead removal. Consumers should verify that the filter package specifically lists the device as certified for removing contaminant lead.
Lead-tainted Cinnamon
Last fall, the North Carolina Department of Health and Human Services identified several children with elevated levels of lead who had consumed WanaBana Apple Cinnamon Fruit Puree pouches.
An investigation by the FDA identified additional brands containing lead and issued a recall of applesauce pouches sold by retailers like Dollar Tree and Amazon.
According to the US Centers for Disease Control and Prevention, nearly 500 children were affected by the tainted applesauce. The FDA traced the source of the lead to cinnamon from a supplier in Ecuador.
An FDA spokesperson told this news organization the episode appears to have resulted from “economically motivated adulteration,” which occurs when a manufacturer leaves out or substitutes a valuable ingredient or part of a food. In the case of spices, lead may be added as a coloring agent or to increase the product weight.
“When we look at domestically made products from large, reputable companies, in general, they do a pretty good job of following safe product guidelines and regulations,” said Kevin Osterhoudt, MD, professor of pediatrics at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia. “But when we use third-party sellers and we import things from other countries that aren’t regulated as closely, we certainly take a lot more risk in the products that we receive.”
While the Food Safety Modernization Act of 2011 aimed to improve agency’s capacity to manage the ever-rising volume of food produced domestically and imported from overseas, the funding has stayed flat while the volume of inspections has increased. In the early 1990s, the number of shipments screened by the agency numbered in the thousands annually. Last year the FDA screened 15 million shipments from more than 200 countries, according to the agency.
Prompted by the finding of lead in applesauce, the FDA began a wider investigation into ground cinnamon by sampling the product from discount retail stores. It recalled an additional six brands of cinnamon sold in the United States containing lead.
Dr. Osterhoudt’s message to families who think their child might have been exposed to a contaminated product is to dispose of it as directed by FDA and CDC guidelines.
In Philadelphia, where Dr. Osterhoudt practices as an emergency room physician, baseline rates of childhood lead poisoning are already high, so he advises families to “do a larger inventory of all the source potential sources of lead in their life and to reduce all the exposures as low as possible.”
He also advises parents that a nutritious diet high in calcium and iron can protect their children from the deleterious effects of lead.
Current Standards for Lead Screening and Testing
Lead is ubiquitous. The common routes of exposure to humans include use of fossil fuels such as leaded gasoline, some types of industrial facilities, and past use of lead-based paint in homes. In addition to spices, lead has been found in a wide variety of products such as toys, jewelry, antiques, cosmetics, and dietary supplements imported from other countries.
Noah Buncher, DO, is a primary care pediatrician in South Philadelphia at Children’s Hospital of Pennsylvania and the former director of a lead clinic in Boston that provides care for children with lead poisoning. He follows guidelines from the American Academy of Pediatrics that define an elevated BLL as ≥ 3.5 µg/dL. The guidelines recommend screening children for lead exposures during well child visits starting at age 6 months up to 6 years and obtaining a BLL if risks for lead exposure are present.
Dr. Buncher starts with a basic environmental history that covers items like the age, condition, zip code of home, parental occupations, or hobbies that might result in exposing family members to lead, and if another child in the home has a history of elevated BLLs.
But a careful history for potential lead exposures can be time-consuming.
“There’s a lot to cover in a routine well child visit,” Dr. Buncher said. “We have maybe 15-20 minutes to cover a lot.”
Clinics also vary on whether lead screening questions are put into workflows in the electronic medical record. Although parents can complete a written questionnaire about possible lead exposures, they may have difficulty answering questions about the age of their home or not know whether their occupation is high risk.
Transportation to a clinic is often a barrier for families, and sometimes patients must travel to a separate lab to be tested for lead.
Dr. Buncher also pointed to the patchwork of local and state requirements that can lead to confusion among providers. Massachusetts, where he formerly practiced, has a universal requirement to test all children at ages 1, 2, and 3 years. But in Pennsylvania, screening laws vary from county to county.
“Pennsylvania should implement universal screening recommendations for all kids under 6 regardless of what county you live in,” Dr. Buncher said.
Protective Measures
Alan Woolf, MD, a professor of pediatrics at Harvard Medical School, Boston, Massachusetts, and director of the Pediatric Environmental Health Center at Boston Children’s Hospital, has a few ideas about how providers can step up their lead game, including partnering with their local health department.
The CDC funds Childhood Lead Poisoning Prevention Programs based in state and local health departments to work with clinicians to improve rates of blood lead testing, monitor the prevalence of lead in their jurisdictions, and ensure that a system of referral is available for treatment and lead remediation services in the home.
Dr. Woolf also suggested that clinicians refer patients under age 3 years with high BLLs to their local Early Intervention Program.
“They’ll assess their child’s development, their speech, their motor skills, their social skills, and if they qualify, it’s free,” Dr. Woolf said.
He cited research showing children with elevated lead levels who received early intervention services performed better in grade school than equally exposed children who did not access similar services.
Another key strategy for pediatric clinicians is to learn local or state regulations for testing children for lead and how to access lead surveillance data in their practice area. Children who reside in high-risk areas are automatic candidates for screening.
Dr. Woolf pointed out that big cities are not the only localities with lead in the drinking water. If families are drawing water from their own well, they should collect that water annually to have it tested for lead and microbes.
At the clinic-wide level, Dr. Woolf recommends the use of blood lead testing as a quality improvement measure. For example, Akron Children’s Hospital developed a quality improvement initiative using a clinical decision support tool to raise screening rates in their network of 30 clinics. One year after beginning the project, lead screenings during 12-month well visits increased from 71% to 96%.
“What we’re interested in as pediatric health professionals is eliminating all background sources of lead in a child’s environment,” Dr. Woolf said. “Whether that’s applesauce pouches, whether that’s lead-containing paint, lead in water, lead in spices, or lead in imported pottery or cookware — there are just a tremendous number of sources of lead that we can do something about.”
None of the subjects reported financial conflicts of interest.
A former pediatrician, Dr. Thomas is a freelance science writer living in Portland, Oregon.
A version of this article appeared on Medscape.com.
Virtual Reality Brings Relief to Hospitalized Patients With Cancer
suggests a new randomized controlled trial.
While both interventions brought some pain relief, VR therapy yielded greater, longer-lasting comfort, reported lead author Hunter Groninger, MD, of MedStar Health Research Institute, Hyattsville, Maryland, and colleagues.
“Investigators have explored immersive VR interventions in cancer populations for a variety of indications including anxiety, depression, fatigue, and procedure‐associated pain, particularly among patients with pediatric cancer and adult breast cancer,” the investigators wrote in Cancer. “Nevertheless, despite growing evidence supporting the efficacy of VR‐delivered interventions for analgesia, few data address its role to mitigate cancer‐related pain specifically.”
To address this knowledge gap, Dr. Groninger and colleagues enrolled 128 adult hospitalized patients with cancer of any kind, all of whom had moderate to severe pain (self-reported score at least 4 out of 10) within the past 24 hours.
Study Methods and Results
Patients were randomized to receive either 10 minutes of immersive VR distraction therapy or 10 minutes of two-dimensional guided imagery distraction therapy.
“[The VR therapy] provides noncompetitive experiences in which the user can move around and explore natural environments (e.g., beachscape, forest) from standing, seated, or fixed positions, including within a hospital bed or chair,” the investigators wrote. “We provided over‐the‐ear headphones to assure high sound quality for the experience in the virtual natural environment.”
The two-dimensional intervention, delivered via electronic tablet, featured a meditation with images of natural landscapes and instrumental background music.
“We chose this active control because it is readily available and reflects content similar to relaxation‐focused television channels that are increasingly common in hospital settings,” the investigators noted.
Compared with this more common approach, patients who received VR therapy had significantly greater immediate reduction in pain (mean change in pain score, –1.4 vs –0.7; P = .03). Twenty-four hours later, improvements in the VR group generally persisted, while pain level in the two-dimensional group returned almost to baseline (P = .004). In addition, patients in the VR group reported significantly greater improvements in general distress and pain bothersomeness.
“VR therapies may modulate the pain experience by reducing the level of attention paid to noxious stimuli, thereby suppressing transmission of painful sensations via pain processing pathways to the cerebral cortex, particularly with more active VR experiences compared to passive experiences,” the investigators wrote.
Downsides to Using VR
Although VR brought more benefit, participants in the VR group more often reported difficulty using the intervention compared with those who interacted with an electronic tablet.
Plus, one VR user described mild dizziness that resolved with pharmacologic intervention. Still, approximately 9 out of 10 participants in each group reported willingness to try the intervention again.
Future VR Research
“Virtual reality is a rapidly evolving technology with a wealth of potential patient‐facing applications,” the investigators wrote. “Future studies should explore repeated use, optimal dosing, and impact on VR therapy on opioid analgesic requirements as well as usability testing, VR content preferences and facilitators of analgesia, and barriers and facilitators to use in acute care settings.”
This study was supported by the American Cancer Society. The investigators disclosed no conflicts of interest.
suggests a new randomized controlled trial.
While both interventions brought some pain relief, VR therapy yielded greater, longer-lasting comfort, reported lead author Hunter Groninger, MD, of MedStar Health Research Institute, Hyattsville, Maryland, and colleagues.
“Investigators have explored immersive VR interventions in cancer populations for a variety of indications including anxiety, depression, fatigue, and procedure‐associated pain, particularly among patients with pediatric cancer and adult breast cancer,” the investigators wrote in Cancer. “Nevertheless, despite growing evidence supporting the efficacy of VR‐delivered interventions for analgesia, few data address its role to mitigate cancer‐related pain specifically.”
To address this knowledge gap, Dr. Groninger and colleagues enrolled 128 adult hospitalized patients with cancer of any kind, all of whom had moderate to severe pain (self-reported score at least 4 out of 10) within the past 24 hours.
Study Methods and Results
Patients were randomized to receive either 10 minutes of immersive VR distraction therapy or 10 minutes of two-dimensional guided imagery distraction therapy.
“[The VR therapy] provides noncompetitive experiences in which the user can move around and explore natural environments (e.g., beachscape, forest) from standing, seated, or fixed positions, including within a hospital bed or chair,” the investigators wrote. “We provided over‐the‐ear headphones to assure high sound quality for the experience in the virtual natural environment.”
The two-dimensional intervention, delivered via electronic tablet, featured a meditation with images of natural landscapes and instrumental background music.
“We chose this active control because it is readily available and reflects content similar to relaxation‐focused television channels that are increasingly common in hospital settings,” the investigators noted.
Compared with this more common approach, patients who received VR therapy had significantly greater immediate reduction in pain (mean change in pain score, –1.4 vs –0.7; P = .03). Twenty-four hours later, improvements in the VR group generally persisted, while pain level in the two-dimensional group returned almost to baseline (P = .004). In addition, patients in the VR group reported significantly greater improvements in general distress and pain bothersomeness.
“VR therapies may modulate the pain experience by reducing the level of attention paid to noxious stimuli, thereby suppressing transmission of painful sensations via pain processing pathways to the cerebral cortex, particularly with more active VR experiences compared to passive experiences,” the investigators wrote.
Downsides to Using VR
Although VR brought more benefit, participants in the VR group more often reported difficulty using the intervention compared with those who interacted with an electronic tablet.
Plus, one VR user described mild dizziness that resolved with pharmacologic intervention. Still, approximately 9 out of 10 participants in each group reported willingness to try the intervention again.
Future VR Research
“Virtual reality is a rapidly evolving technology with a wealth of potential patient‐facing applications,” the investigators wrote. “Future studies should explore repeated use, optimal dosing, and impact on VR therapy on opioid analgesic requirements as well as usability testing, VR content preferences and facilitators of analgesia, and barriers and facilitators to use in acute care settings.”
This study was supported by the American Cancer Society. The investigators disclosed no conflicts of interest.
suggests a new randomized controlled trial.
While both interventions brought some pain relief, VR therapy yielded greater, longer-lasting comfort, reported lead author Hunter Groninger, MD, of MedStar Health Research Institute, Hyattsville, Maryland, and colleagues.
“Investigators have explored immersive VR interventions in cancer populations for a variety of indications including anxiety, depression, fatigue, and procedure‐associated pain, particularly among patients with pediatric cancer and adult breast cancer,” the investigators wrote in Cancer. “Nevertheless, despite growing evidence supporting the efficacy of VR‐delivered interventions for analgesia, few data address its role to mitigate cancer‐related pain specifically.”
To address this knowledge gap, Dr. Groninger and colleagues enrolled 128 adult hospitalized patients with cancer of any kind, all of whom had moderate to severe pain (self-reported score at least 4 out of 10) within the past 24 hours.
Study Methods and Results
Patients were randomized to receive either 10 minutes of immersive VR distraction therapy or 10 minutes of two-dimensional guided imagery distraction therapy.
“[The VR therapy] provides noncompetitive experiences in which the user can move around and explore natural environments (e.g., beachscape, forest) from standing, seated, or fixed positions, including within a hospital bed or chair,” the investigators wrote. “We provided over‐the‐ear headphones to assure high sound quality for the experience in the virtual natural environment.”
The two-dimensional intervention, delivered via electronic tablet, featured a meditation with images of natural landscapes and instrumental background music.
“We chose this active control because it is readily available and reflects content similar to relaxation‐focused television channels that are increasingly common in hospital settings,” the investigators noted.
Compared with this more common approach, patients who received VR therapy had significantly greater immediate reduction in pain (mean change in pain score, –1.4 vs –0.7; P = .03). Twenty-four hours later, improvements in the VR group generally persisted, while pain level in the two-dimensional group returned almost to baseline (P = .004). In addition, patients in the VR group reported significantly greater improvements in general distress and pain bothersomeness.
“VR therapies may modulate the pain experience by reducing the level of attention paid to noxious stimuli, thereby suppressing transmission of painful sensations via pain processing pathways to the cerebral cortex, particularly with more active VR experiences compared to passive experiences,” the investigators wrote.
Downsides to Using VR
Although VR brought more benefit, participants in the VR group more often reported difficulty using the intervention compared with those who interacted with an electronic tablet.
Plus, one VR user described mild dizziness that resolved with pharmacologic intervention. Still, approximately 9 out of 10 participants in each group reported willingness to try the intervention again.
Future VR Research
“Virtual reality is a rapidly evolving technology with a wealth of potential patient‐facing applications,” the investigators wrote. “Future studies should explore repeated use, optimal dosing, and impact on VR therapy on opioid analgesic requirements as well as usability testing, VR content preferences and facilitators of analgesia, and barriers and facilitators to use in acute care settings.”
This study was supported by the American Cancer Society. The investigators disclosed no conflicts of interest.
FROM CANCER
Nontraditional Risk Factors Play an Outsized Role in Young Adult Stroke Risk
, new research showed.
The findings may offer insight into the increased incidence of stroke in adults under age 45, which has more than doubled in the past 20 years in high-income countries, while incidence in those over 45 has decreased.
Investigators believe the findings are important because most conventional prevention efforts focus on traditional risk factors.
“The younger they are at the time of stroke, the more likely their stroke is due to a nontraditional risk factor,” lead author Michelle Leppert, MD, an assistant professor of neurology at the University of Colorado School of Medicine, Aurora, Colorado, said in a news release.
The findings were published online in Circulation: Cardiovascular Quality and Outcomes.
Traditional Versus Nontraditional
The researchers retrospectively analyzed 2618 stroke cases (52% female; 73% ischemic stroke) that resulted in an inpatient admission and 7827 controls, all aged 18-55 years. Data came from the Colorado All Payer Claims Database between January 2012 and April 2019. Controls were matched by age, sex, and insurance type.
Traditional risk factors were defined as being a well-established risk factor for stroke that is routinely noted during stroke prevention screenings in older adults, including hypertension, diabetes, hyperlipidemia, sleep apnea, cardiovascular disease, alcohol, substance use disorder, and obesity.
Nontraditional risk factors were defined as those that are rarely cited as a cause of stroke in older adults, including migraines, malignancy, HIV, hepatitis, thrombophilia, autoimmune disease, vasculitis, sickle cell disease, heart valve disease, renal failure, and hormonal risk factors in women, such as oral contraceptives, pregnancy, or puerperium.
Overall, traditional risk factors were more common in stroke cases, with nontraditional factors playing a smaller role. However, among adults aged 18-34 years, more strokes were associated with nontraditional than traditional risk factors in men (31% vs 25%, respectively) and in women (43% vs 33%, respectively).
Migraine, the most common nontraditional risk factor for stroke in this younger age group, was found in 20% of men (odds ratio [OR], 3.9) and 35% of women (OR, 3.3).
Other notable nontraditional risk factors included heart valve disease in both men and women (OR, 3.1 and OR, 4.2, respectively); renal failure in men (OR, 8.9); and autoimmune diseases in women (OR, 8.8).
An Underestimate?
The contribution of nontraditional risk factors declined with age. After the age of 44, they were no longer significant. Hypertension was the most important traditional risk factor and increased in contribution with age.
“There have been many studies demonstrating the association between migraines and strokes, but to our knowledge, this study may be the first to demonstrate just how much stroke risk may be attributable to migraines,” Dr. Leppert said.
Overall, women had significantly more risk factors for stroke than men. Among controls, 52% and 34% of women had at least one traditional and nontraditional risk factors, respectively, compared with 48% and 22% in men.
The total contribution of nontraditional risk factors was likely an underestimate because some such factors, including the autoimmune disorder antiphospholipid syndrome and patent foramen ovale, “lacked reliable administrative algorithms” and could not be assessed in this study, the researchers noted.
Further research on how nontraditional risk factors affect strokes could lead to better prevention.
“We need to better understand the underlying mechanisms of these nontraditional risk factors to develop targeted interventions,” Dr. Leppert said.
The study was funded by the National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award. Dr. Leppert reports receiving an American Heart Association Career Development Grant. Other disclosures are included in the original article.
A version of this article appeared on Medscape.com.
, new research showed.
The findings may offer insight into the increased incidence of stroke in adults under age 45, which has more than doubled in the past 20 years in high-income countries, while incidence in those over 45 has decreased.
Investigators believe the findings are important because most conventional prevention efforts focus on traditional risk factors.
“The younger they are at the time of stroke, the more likely their stroke is due to a nontraditional risk factor,” lead author Michelle Leppert, MD, an assistant professor of neurology at the University of Colorado School of Medicine, Aurora, Colorado, said in a news release.
The findings were published online in Circulation: Cardiovascular Quality and Outcomes.
Traditional Versus Nontraditional
The researchers retrospectively analyzed 2618 stroke cases (52% female; 73% ischemic stroke) that resulted in an inpatient admission and 7827 controls, all aged 18-55 years. Data came from the Colorado All Payer Claims Database between January 2012 and April 2019. Controls were matched by age, sex, and insurance type.
Traditional risk factors were defined as being a well-established risk factor for stroke that is routinely noted during stroke prevention screenings in older adults, including hypertension, diabetes, hyperlipidemia, sleep apnea, cardiovascular disease, alcohol, substance use disorder, and obesity.
Nontraditional risk factors were defined as those that are rarely cited as a cause of stroke in older adults, including migraines, malignancy, HIV, hepatitis, thrombophilia, autoimmune disease, vasculitis, sickle cell disease, heart valve disease, renal failure, and hormonal risk factors in women, such as oral contraceptives, pregnancy, or puerperium.
Overall, traditional risk factors were more common in stroke cases, with nontraditional factors playing a smaller role. However, among adults aged 18-34 years, more strokes were associated with nontraditional than traditional risk factors in men (31% vs 25%, respectively) and in women (43% vs 33%, respectively).
Migraine, the most common nontraditional risk factor for stroke in this younger age group, was found in 20% of men (odds ratio [OR], 3.9) and 35% of women (OR, 3.3).
Other notable nontraditional risk factors included heart valve disease in both men and women (OR, 3.1 and OR, 4.2, respectively); renal failure in men (OR, 8.9); and autoimmune diseases in women (OR, 8.8).
An Underestimate?
The contribution of nontraditional risk factors declined with age. After the age of 44, they were no longer significant. Hypertension was the most important traditional risk factor and increased in contribution with age.
“There have been many studies demonstrating the association between migraines and strokes, but to our knowledge, this study may be the first to demonstrate just how much stroke risk may be attributable to migraines,” Dr. Leppert said.
Overall, women had significantly more risk factors for stroke than men. Among controls, 52% and 34% of women had at least one traditional and nontraditional risk factors, respectively, compared with 48% and 22% in men.
The total contribution of nontraditional risk factors was likely an underestimate because some such factors, including the autoimmune disorder antiphospholipid syndrome and patent foramen ovale, “lacked reliable administrative algorithms” and could not be assessed in this study, the researchers noted.
Further research on how nontraditional risk factors affect strokes could lead to better prevention.
“We need to better understand the underlying mechanisms of these nontraditional risk factors to develop targeted interventions,” Dr. Leppert said.
The study was funded by the National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award. Dr. Leppert reports receiving an American Heart Association Career Development Grant. Other disclosures are included in the original article.
A version of this article appeared on Medscape.com.
, new research showed.
The findings may offer insight into the increased incidence of stroke in adults under age 45, which has more than doubled in the past 20 years in high-income countries, while incidence in those over 45 has decreased.
Investigators believe the findings are important because most conventional prevention efforts focus on traditional risk factors.
“The younger they are at the time of stroke, the more likely their stroke is due to a nontraditional risk factor,” lead author Michelle Leppert, MD, an assistant professor of neurology at the University of Colorado School of Medicine, Aurora, Colorado, said in a news release.
The findings were published online in Circulation: Cardiovascular Quality and Outcomes.
Traditional Versus Nontraditional
The researchers retrospectively analyzed 2618 stroke cases (52% female; 73% ischemic stroke) that resulted in an inpatient admission and 7827 controls, all aged 18-55 years. Data came from the Colorado All Payer Claims Database between January 2012 and April 2019. Controls were matched by age, sex, and insurance type.
Traditional risk factors were defined as being a well-established risk factor for stroke that is routinely noted during stroke prevention screenings in older adults, including hypertension, diabetes, hyperlipidemia, sleep apnea, cardiovascular disease, alcohol, substance use disorder, and obesity.
Nontraditional risk factors were defined as those that are rarely cited as a cause of stroke in older adults, including migraines, malignancy, HIV, hepatitis, thrombophilia, autoimmune disease, vasculitis, sickle cell disease, heart valve disease, renal failure, and hormonal risk factors in women, such as oral contraceptives, pregnancy, or puerperium.
Overall, traditional risk factors were more common in stroke cases, with nontraditional factors playing a smaller role. However, among adults aged 18-34 years, more strokes were associated with nontraditional than traditional risk factors in men (31% vs 25%, respectively) and in women (43% vs 33%, respectively).
Migraine, the most common nontraditional risk factor for stroke in this younger age group, was found in 20% of men (odds ratio [OR], 3.9) and 35% of women (OR, 3.3).
Other notable nontraditional risk factors included heart valve disease in both men and women (OR, 3.1 and OR, 4.2, respectively); renal failure in men (OR, 8.9); and autoimmune diseases in women (OR, 8.8).
An Underestimate?
The contribution of nontraditional risk factors declined with age. After the age of 44, they were no longer significant. Hypertension was the most important traditional risk factor and increased in contribution with age.
“There have been many studies demonstrating the association between migraines and strokes, but to our knowledge, this study may be the first to demonstrate just how much stroke risk may be attributable to migraines,” Dr. Leppert said.
Overall, women had significantly more risk factors for stroke than men. Among controls, 52% and 34% of women had at least one traditional and nontraditional risk factors, respectively, compared with 48% and 22% in men.
The total contribution of nontraditional risk factors was likely an underestimate because some such factors, including the autoimmune disorder antiphospholipid syndrome and patent foramen ovale, “lacked reliable administrative algorithms” and could not be assessed in this study, the researchers noted.
Further research on how nontraditional risk factors affect strokes could lead to better prevention.
“We need to better understand the underlying mechanisms of these nontraditional risk factors to develop targeted interventions,” Dr. Leppert said.
The study was funded by the National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award. Dr. Leppert reports receiving an American Heart Association Career Development Grant. Other disclosures are included in the original article.
A version of this article appeared on Medscape.com.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES
Should Opioids Be Used for Chronic Cancer Pain?
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
from legal concerns to threats of violence, say the authors of new research.
These findings suggest that evidence-based, systematic guidance is needed to steer opioid usage in cancer survivorship, wrote lead author Hailey W. Bulls, PhD, of the University of Pittsburgh, and colleagues.
“Prescription opioids are considered the standard of care to treat moderate to severe cancer pain during active treatment, yet guidance in the posttreatment survivorship phase is much less clear,” the investigators wrote. “Existing clinical resources recognize that opioid prescribing in survivorship is complex and nuanced and that the relative benefits and risks in this population are not fully understood.”
Who Should Manage Chronic Cancer Pain?
Despite the knowledge gap, survivors are typically excluded from long-term opioid use studies, leaving providers in a largely data-free zone. Simultaneously, patients who had been receiving focused care during their cancer treatment find themselves with an ill-defined health care team.
“Without a clear transition of care, survivors may seek pain management services from a variety of specialties, including oncologists, palliative care clinicians, primary care clinicians, and pain management specialists,” the investigators wrote. “However, many clinicians may view pain management to be outside of their skill set and may not be well equipped to handle opioid continuation or deprescribing [or] to manage the potential consequences of long‐term opioid use like side effects, misuse, and/or opioid use disorder.”
What Factors Guide Opioid Prescribing Practices for Chronic Cancer Pain?
To learn more about prescribing practices in this setting, Dr. Bulls and colleagues conducted qualitative interviews with 20 providers representing four specialties: oncology (n = 5), palliative care (n = 8), primary care (n = 5), and pain management (n = 2). Eighteen of these participants were physicians and two were advanced practice providers. Average time in clinical practice was about 16 years.
These interviews yielded three themes.
First, no “medical home” exists for chronic pain management in cancer survivors.
“Although clinicians generally agreed that minimizing the role of opioids in chronic pain management in cancer survivors was desirable, they described a lack of common treatment protocols to guide pain management in survivorship,” the investigators wrote.
Second, the interviews revealed that prescribing strategies are partly driven by peer pressure, sometimes leading to tension between providers and feelings of self-doubt.
“I feel like there’s been this weird judgment thing that’s happened [to] the prescribers,” one primary care provider said during the interview. “Because, when I trained … pain was a vital sign, and we were supposed to treat pain, and now I feel like we’re all being judged for that.”
The third theme revolved around fear of consequences resulting from prescribing practices, including fears of violent repercussions.
“You may not know, but pain specialists have been shot in this country for [refusing to prescribe opioids],” one pain management specialist said during the interview. “There’s been a number of shootings of pain specialists who would not prescribe opioids. So, I mean, there’s real issues of violence.”
Meanwhile, a palliative care provider described legal pressure from the opposite direction:
“I think there’s a lot of fear of litigiousness … and loss of licenses. That sort of makes them pressure us into not prescribing opioids or sticking with a certain number per day that might not be therapeutic for a patient.”
Reflecting on these themes, the investigators identified “a fundamental uncertainty in survivorship pain management.”
What Strategies Might Improve Opioid Prescribing Practices for Chronic Cancer Pain?
After sharing their attitudes about prescribing opioids for chronic cancer pain, the clinicians were asked for suggestions to improve the situation.
They offered four main suggestions: create relevant guidelines, increase education and access to pain management options for clinicians, increase interdisciplinary communication across medical subspecialties, and promote multidisciplinary care in the survivorship setting.
Dr. Bulls and colleagues supported these strategies in their concluding remarks and called for more research.
This study was supported by the National Institute of Drug Abuse, the National Institutes of Health, the National Center for Advancing Translational Sciences, and the National Cancer Institute. The investigators disclosed relationships with Arcadia Health Solutions and Biomotivate.
FROM CANCER