User login
Rapidly Growing Cutaneous Nodules on the Scalp
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
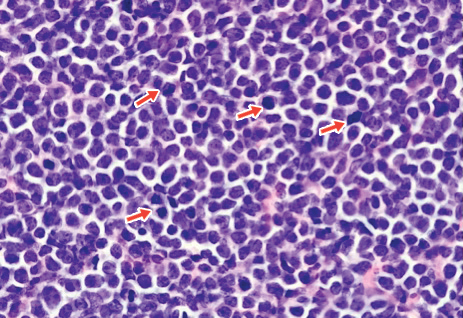
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.
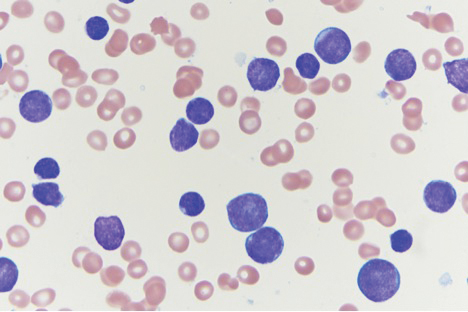
On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.
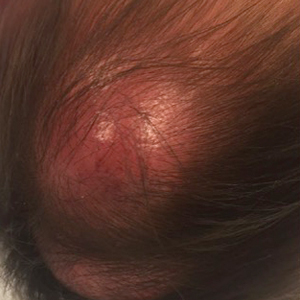
In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.

On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.

In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
The Diagnosis: B-Cell Acute Lymphoblastic Leukemia
A 4-mm punch biopsy of one of the scalp lesions showed a diffuse infiltrate of intermediately sized cells with variably mature chromatin and irregular nuclear contours, consistent with a neoplastic process. Numerous mitotic figures were present, indicating a high proliferation rate (Figure 1). At that time there was no evidence of systemic involvement. A repeat biopsy with concurrent bone marrow biopsy was scheduled 10 days after the patient's initial presentation for further classification. Laboratory studies at that time revealed leukocytosis with elevated neutrophils and lymphocytes as well as a high absolute blast count.

On immunohistochemical staining, the neoplastic cells were positive for CD45, which indicated the neoplasm was hematopoietic, as well as CD10 and the B-cell antigens PAX-5 and CD79a. The cells were negative for CD20, which also is a B-cell marker, but this marker is only expressed in approximately half of pediatric acute lymphoblastic leukemia (ALL) cases with B-cell precursor origin.1 Markers that typically are expressed in B-cell acute lymphoblastic leukemia (B-ALL)--CD34 and terminal deoxynucleotidyl transferase--were both negative. These results were somewhat contradictory, and the differential remained open to both B-ALL and mature B-cell lymphoma. A bone marrow biopsy showed approximately 65% blasts or leukemic cells (Figure 2). Flow cytometry showed the cells were positive for CD10, CD19, weak CD79a, and variable lambda surface antigen expression. The cells were negative for expression of CD20, CD34, terminal deoxynucleotidyl transferase, myeloid antigens, and CD3. Ultimately, the morphology and immunophenotype were most consistent with a diagnosis of B-ALL. Fluorescence in situ hybridization revealed mixed lineage leukemia, MLL, gene rearrangements.

In general, when considering the differential diagnosis of superficial nodules, 5 elements are helpful to consider: the number of nodules (single vs multiple); the location; and the presence or absence of tenderness, pigmentation or erythema, and firmness.2 Our patient had multiple nodules on the scalp, which were erythematous to slightly purple and firm. The differential diagnosis can be categorized into malignant; infectious; and benign inflammatory, vascular, and fibrous tumors.
Potential oncologic processes include leukemia cutis, lymphoblastic leukemia/lymphoma, Langerhans cell histiocytosis, and rhabdomyosarcoma. Initial laboratory test results were reassuring. Infectious processes in the differential include deep fungal infections such as coccidioidomycosis and nontuberculous mycobacterial infections. Coccidioidomycosis was the most likely to cause skin lesions or masses in our patient; however, it was considered less likely because the patient's family had not traveled or been exposed to an endemic area.3
Benign tumors in the differential include deep hemangioma, which was deemed less likely in our patient because most hemangiomas reach 80% of their maximum size by 5 months of age.4 Another possible benign tumor is infantile myofibromatosis, which is rare but is the most common fibrous tumor of infancy.5
Early-onset childhood sarcoidosis also has been shown to produce multiple nontender firm nodules.2 This process was considered unlikely in our patient because not only is the disease relatively rare in the pediatric population, but most reported childhood cases have occurred in patients aged 13 to 15 years.6 Additionally, no uveitis or arthritis was observed in this case.
Ultimately, histopathology and bone marrow biopsy were necessary to determine the diagnosis of B-ALL. Although uncommon, cutaneous involvement can be an early sign of ALL in children.7 Thus, neoplastic etiologies should be considered in the workup of cutaneous nodules in children, especially when these nodules are hard, rapidly growing, ulcerated, fixed, and/or vascular.8 Once the diagnosis is established, initial workup of ALL in children should include complete blood cell count with manual differential, prothrombin time, partial thromboplastin time, electrolytes, uric acid, and renal and liver function tests. Often, baseline viral titers such as cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus, hepatitis B virus, and varicella-zoster virus also are included. Patients are risk stratified to the appropriate level of treatment based on tumor immunophenotype, cytogenetic findings, patient age, white blood cell count at the time of diagnosis, and response to initial therapy. Treatment typically is comprised of a multidrug regimen divided into several phases--induction, consolidation, and maintenance--as well as therapy directed to the central nervous system. Treatment protocols usually take 2 to 3 years to complete.
Our patient was treated with 1 dose of intrathecal methotrexate before starting the Interfant-06 protocol with a 7-day methylprednisolone prophase. The patient's nodules shrank over time and were no longer present after 14 days of treatment.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
- Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982-3988.
- Whelan JP, Zembowicz A. Case records of the Massachusetts General Hospital. case 19-2006. a 22-month-old boy with the rapid growth of subcutaneous nodules. N Engl J Med. 2006;354:2697-2704.
- Malo J, Luraschi-Monjagatta C, Wolk DM, et al. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc. 2014;11:243-253.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Schurr P, Moulsdale W. Infantile myofibroma. Adv Neonatal Care. 2008;8:13-20.
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16.
- Millot F, Robert A, Bertrand Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children's Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100:60-64.
- Fogelson S, Dohil M. Papular and nodular skin lesions in children. Semin Plast Surg. 2006;20:180-191.
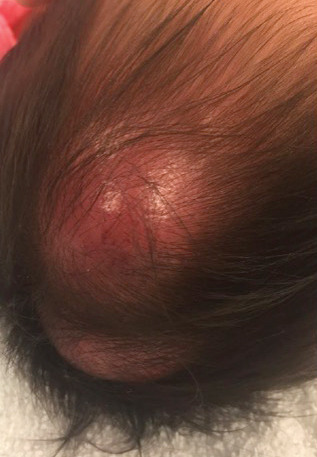
An 8-month-old infant girl presented with rapidly growing cutaneous nodules on the scalp of 1 month's duration. Her parents reported that she disliked lying flat but was otherwise growing and developing normally. Nondiagnostic ultrasonography of the head and brain had been performed as well as a skull radiograph, which found no evidence of lytic lesions. On physical examination, 3 erythematous to violaceous, subcutaneous, firm, fixed nodules were observed on the scalp. Notable cervical lymphadenopathy with several distinct, fixed, firm, subcutaneous nodules in the postauricular lymph chains also were noted. The patient had no pertinent medical history and was born via normal spontaneous vaginal delivery to healthy parents. The remainder of the physical examination and review of systems was negative.
Liraglutide indication expanded to include children
according to a news release from the agency. The approval comes after granting the application Priority Review, which means the FDA aimed to take action on it within 6 months instead of the usual 10 months seen with a Standard Review.
Liraglutide injection has been approved for treatment of adults with type 2 diabetes since 2010, but this is the first noninsulin treatment for pediatric patients since metformin received approval for this population in 2000.
“The expanded indication provides an additional treatment option at a time when an increasing number of children are being diagnosed with [type 2 diabetes],” said Lisa Yanoff, MD, acting director of the division of metabolism and endocrinology products in the agency’s Center for Drug Evaluation and Research.
The approval is based on efficacy and safety results from several placebo-controlled trials in adults, and one trial in 134 pediatric patients aged 10 years or older. In the latter trial, which ran for more than 26 weeks, hemoglobin A1c levels fell below 7% in approximately 64% of patients treated with liraglutide injection, compared with 37% of those who received placebo. Those results were seen regardless of whether patients concomitantly used insulin.
Although an increase in hypoglycemia risk has sometimes been seen in adult patients taking liraglutide injection with insulin or other drugs that raise insulin production, this heightened risk was seen in the pediatric patients regardless of whether they took other therapies for diabetes.
Liraglutide injection carries a Boxed Warning, the FDA’s strongest warning, for heightened risk of thyroid C-cell tumors. Therefore, the agency recommends against patients with history or family members with history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 using this treatment. Among its other warnings are those pertaining to patients with renal impairment or kidney failure, pancreatitis, and/or acute gallbladder disease.
The most common side effects are nausea, diarrhea, vomiting, decreased appetite, indigestion, and constipation. The full prescribing information can be found on the FDA website.
according to a news release from the agency. The approval comes after granting the application Priority Review, which means the FDA aimed to take action on it within 6 months instead of the usual 10 months seen with a Standard Review.
Liraglutide injection has been approved for treatment of adults with type 2 diabetes since 2010, but this is the first noninsulin treatment for pediatric patients since metformin received approval for this population in 2000.
“The expanded indication provides an additional treatment option at a time when an increasing number of children are being diagnosed with [type 2 diabetes],” said Lisa Yanoff, MD, acting director of the division of metabolism and endocrinology products in the agency’s Center for Drug Evaluation and Research.
The approval is based on efficacy and safety results from several placebo-controlled trials in adults, and one trial in 134 pediatric patients aged 10 years or older. In the latter trial, which ran for more than 26 weeks, hemoglobin A1c levels fell below 7% in approximately 64% of patients treated with liraglutide injection, compared with 37% of those who received placebo. Those results were seen regardless of whether patients concomitantly used insulin.
Although an increase in hypoglycemia risk has sometimes been seen in adult patients taking liraglutide injection with insulin or other drugs that raise insulin production, this heightened risk was seen in the pediatric patients regardless of whether they took other therapies for diabetes.
Liraglutide injection carries a Boxed Warning, the FDA’s strongest warning, for heightened risk of thyroid C-cell tumors. Therefore, the agency recommends against patients with history or family members with history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 using this treatment. Among its other warnings are those pertaining to patients with renal impairment or kidney failure, pancreatitis, and/or acute gallbladder disease.
The most common side effects are nausea, diarrhea, vomiting, decreased appetite, indigestion, and constipation. The full prescribing information can be found on the FDA website.
according to a news release from the agency. The approval comes after granting the application Priority Review, which means the FDA aimed to take action on it within 6 months instead of the usual 10 months seen with a Standard Review.
Liraglutide injection has been approved for treatment of adults with type 2 diabetes since 2010, but this is the first noninsulin treatment for pediatric patients since metformin received approval for this population in 2000.
“The expanded indication provides an additional treatment option at a time when an increasing number of children are being diagnosed with [type 2 diabetes],” said Lisa Yanoff, MD, acting director of the division of metabolism and endocrinology products in the agency’s Center for Drug Evaluation and Research.
The approval is based on efficacy and safety results from several placebo-controlled trials in adults, and one trial in 134 pediatric patients aged 10 years or older. In the latter trial, which ran for more than 26 weeks, hemoglobin A1c levels fell below 7% in approximately 64% of patients treated with liraglutide injection, compared with 37% of those who received placebo. Those results were seen regardless of whether patients concomitantly used insulin.
Although an increase in hypoglycemia risk has sometimes been seen in adult patients taking liraglutide injection with insulin or other drugs that raise insulin production, this heightened risk was seen in the pediatric patients regardless of whether they took other therapies for diabetes.
Liraglutide injection carries a Boxed Warning, the FDA’s strongest warning, for heightened risk of thyroid C-cell tumors. Therefore, the agency recommends against patients with history or family members with history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 using this treatment. Among its other warnings are those pertaining to patients with renal impairment or kidney failure, pancreatitis, and/or acute gallbladder disease.
The most common side effects are nausea, diarrhea, vomiting, decreased appetite, indigestion, and constipation. The full prescribing information can be found on the FDA website.
Obesity and overweight declined among lower-income kids
The combined rate of , according to a study in JAMA.
Liping Pan, MD, MPH, of the Centers for Disease Control and Prevention and colleagues used data from the WIC Participant and Program Characteristics survey from 2010, 2012, 2014, and 2016 for 12,403,629 children aged 2-4 years from 50 states, Washington, D.C., and 5 territories. In addition to a –3.2% change (95% confidence interval, –3.3% to –3.2%) in adjusted prevalence difference for the combined rate of obesity and overweight seen between 2010 and 2016, the researchers found the crude prevalence decreased from 32.5% to 29.1%. A decrease was also seen for obesity alone (crude prevalence, 15.9% to 13.9%; adjusted prevalence difference, –1.9%; 95% CI, –1.9% to –1.8%).
One of the limitations of the study is that the characteristics of enrolled children might differ from those of children not enrolled in this WIC program; however, the researchers noted that they accounted for many demographic characteristics in the trend analyses.
“Reasons for the declines in obesity among young children in WIC remain undetermined but may include WIC food package revisions and local, state, and national initiatives,” they wrote.
SOURCE: Pan L et al. JAMA. 2019 Jun 18;321(23):2364-6.
The combined rate of , according to a study in JAMA.
Liping Pan, MD, MPH, of the Centers for Disease Control and Prevention and colleagues used data from the WIC Participant and Program Characteristics survey from 2010, 2012, 2014, and 2016 for 12,403,629 children aged 2-4 years from 50 states, Washington, D.C., and 5 territories. In addition to a –3.2% change (95% confidence interval, –3.3% to –3.2%) in adjusted prevalence difference for the combined rate of obesity and overweight seen between 2010 and 2016, the researchers found the crude prevalence decreased from 32.5% to 29.1%. A decrease was also seen for obesity alone (crude prevalence, 15.9% to 13.9%; adjusted prevalence difference, –1.9%; 95% CI, –1.9% to –1.8%).
One of the limitations of the study is that the characteristics of enrolled children might differ from those of children not enrolled in this WIC program; however, the researchers noted that they accounted for many demographic characteristics in the trend analyses.
“Reasons for the declines in obesity among young children in WIC remain undetermined but may include WIC food package revisions and local, state, and national initiatives,” they wrote.
SOURCE: Pan L et al. JAMA. 2019 Jun 18;321(23):2364-6.
The combined rate of , according to a study in JAMA.
Liping Pan, MD, MPH, of the Centers for Disease Control and Prevention and colleagues used data from the WIC Participant and Program Characteristics survey from 2010, 2012, 2014, and 2016 for 12,403,629 children aged 2-4 years from 50 states, Washington, D.C., and 5 territories. In addition to a –3.2% change (95% confidence interval, –3.3% to –3.2%) in adjusted prevalence difference for the combined rate of obesity and overweight seen between 2010 and 2016, the researchers found the crude prevalence decreased from 32.5% to 29.1%. A decrease was also seen for obesity alone (crude prevalence, 15.9% to 13.9%; adjusted prevalence difference, –1.9%; 95% CI, –1.9% to –1.8%).
One of the limitations of the study is that the characteristics of enrolled children might differ from those of children not enrolled in this WIC program; however, the researchers noted that they accounted for many demographic characteristics in the trend analyses.
“Reasons for the declines in obesity among young children in WIC remain undetermined but may include WIC food package revisions and local, state, and national initiatives,” they wrote.
SOURCE: Pan L et al. JAMA. 2019 Jun 18;321(23):2364-6.
FROM JAMA
Suicide rates rise in U.S. adolescents and young adults
Suicides in teens and young adults reached 6,241 in 2017, the highest since 2000, according to data from a review of the Centers for Disease Control and Prevention Underlying Cause of Death database.
The suicide rate overall was 12 per 100,000 in 2017 for 15-19 year olds.
Although suicide rates have increased across all age groups in the United States since 2000, “adolescents are of particular concern, with increases in social media use, anxiety, depression, and self-inflicted injuries,” wrote Oren Miron of Harvard Medical School, Boston, and colleagues.
In a research letter published in JAMA, the researchers analyzed trends in teen and young adult suicides from 2000 to 2017. The combined suicide rate for males and females aged 15-19 years in 2000 was 8 per 100,000 with no significant changes until 2007, followed by an annual percentage change (APC) of 3% from 2007 to 2014 and 10% from 2014 to 2017.
When the data were broken out by gender, Of note, these young men showed a decreasing trend in APC of –2% from 2000 to 2007 before increasing.
Among females aged 15-19 years, no increase was noted until 2010, then researchers identified an APC of 8% from 2010 to 2017.
For ages 20-24 years, the combined suicide rate for males and females was 13 per 100,000 in 2000, which rose to 17 per 100,000 in 2017. The APC in the older group was 1% from 2000 to 2013 and 6% from 2013 to 2017. Increasing trends were observed for both males and females over the study period.
The study was limited by the potential inaccuracy in cause of death listed on death certificates, such as mistaking a suicide for an accidental overdose, and the increased suicide rate could reflect more accurate reporting, the researchers noted.
Nonetheless, the results support the need for more studies of contributing factors to teen and young adult suicides to help develop prevention strategies and analysis of factors that may have contributed to declines in suicide rates in the past, they said.
Coauthor Dr. Yu was supported by the Harvard Data Science Fellowship. The researchers had no relevant financial disclosures.
SOURCE: Miron O et al. JAMA. 2019 Jun 28;321:2362-4.
Suicides in teens and young adults reached 6,241 in 2017, the highest since 2000, according to data from a review of the Centers for Disease Control and Prevention Underlying Cause of Death database.
The suicide rate overall was 12 per 100,000 in 2017 for 15-19 year olds.
Although suicide rates have increased across all age groups in the United States since 2000, “adolescents are of particular concern, with increases in social media use, anxiety, depression, and self-inflicted injuries,” wrote Oren Miron of Harvard Medical School, Boston, and colleagues.
In a research letter published in JAMA, the researchers analyzed trends in teen and young adult suicides from 2000 to 2017. The combined suicide rate for males and females aged 15-19 years in 2000 was 8 per 100,000 with no significant changes until 2007, followed by an annual percentage change (APC) of 3% from 2007 to 2014 and 10% from 2014 to 2017.
When the data were broken out by gender, Of note, these young men showed a decreasing trend in APC of –2% from 2000 to 2007 before increasing.
Among females aged 15-19 years, no increase was noted until 2010, then researchers identified an APC of 8% from 2010 to 2017.
For ages 20-24 years, the combined suicide rate for males and females was 13 per 100,000 in 2000, which rose to 17 per 100,000 in 2017. The APC in the older group was 1% from 2000 to 2013 and 6% from 2013 to 2017. Increasing trends were observed for both males and females over the study period.
The study was limited by the potential inaccuracy in cause of death listed on death certificates, such as mistaking a suicide for an accidental overdose, and the increased suicide rate could reflect more accurate reporting, the researchers noted.
Nonetheless, the results support the need for more studies of contributing factors to teen and young adult suicides to help develop prevention strategies and analysis of factors that may have contributed to declines in suicide rates in the past, they said.
Coauthor Dr. Yu was supported by the Harvard Data Science Fellowship. The researchers had no relevant financial disclosures.
SOURCE: Miron O et al. JAMA. 2019 Jun 28;321:2362-4.
Suicides in teens and young adults reached 6,241 in 2017, the highest since 2000, according to data from a review of the Centers for Disease Control and Prevention Underlying Cause of Death database.
The suicide rate overall was 12 per 100,000 in 2017 for 15-19 year olds.
Although suicide rates have increased across all age groups in the United States since 2000, “adolescents are of particular concern, with increases in social media use, anxiety, depression, and self-inflicted injuries,” wrote Oren Miron of Harvard Medical School, Boston, and colleagues.
In a research letter published in JAMA, the researchers analyzed trends in teen and young adult suicides from 2000 to 2017. The combined suicide rate for males and females aged 15-19 years in 2000 was 8 per 100,000 with no significant changes until 2007, followed by an annual percentage change (APC) of 3% from 2007 to 2014 and 10% from 2014 to 2017.
When the data were broken out by gender, Of note, these young men showed a decreasing trend in APC of –2% from 2000 to 2007 before increasing.
Among females aged 15-19 years, no increase was noted until 2010, then researchers identified an APC of 8% from 2010 to 2017.
For ages 20-24 years, the combined suicide rate for males and females was 13 per 100,000 in 2000, which rose to 17 per 100,000 in 2017. The APC in the older group was 1% from 2000 to 2013 and 6% from 2013 to 2017. Increasing trends were observed for both males and females over the study period.
The study was limited by the potential inaccuracy in cause of death listed on death certificates, such as mistaking a suicide for an accidental overdose, and the increased suicide rate could reflect more accurate reporting, the researchers noted.
Nonetheless, the results support the need for more studies of contributing factors to teen and young adult suicides to help develop prevention strategies and analysis of factors that may have contributed to declines in suicide rates in the past, they said.
Coauthor Dr. Yu was supported by the Harvard Data Science Fellowship. The researchers had no relevant financial disclosures.
SOURCE: Miron O et al. JAMA. 2019 Jun 28;321:2362-4.
FROM JAMA
Key clinical point: Suicide rates in U.S. adolescents and young adults have increased since 2000.
Major finding: The combined suicide rate for males and females aged 15-19 years underwent an annual percentage change of 3% from 2007 to 2014 and 10% from 2014 to 2017.
Study details: The data come from the CDC Underlying Cause of Death database.
Disclosures: Coauthor Dr. Yu was supported by the Harvard Data Science Fellowship. The researchers had no relevant financial disclosures.
Source: Miron O et al. JAMA. 2019 Jun 28;321:2362-4.
Pediatric-onset MS may slow information processing in adulthood
independent of age or disease duration, according to a study published in JAMA Neurology.
Information-processing efficiency as measured by the Symbol Digit Modalities Test (SDMT) may decrease more rapidly in patients with pediatric-onset multiple sclerosis (MS).
“Children and adolescents who develop [MS] should be monitored closely for cognitive changes and helped to manage the potential challenges that early-onset multiple sclerosis poses for cognitive abilities later in life,” Kyla A. McKay, PhD, a researcher at Karolinska Institutet in Stockholm, and colleagues wrote.
Prior research has found that an SDMT score of 55 may be a point at which a person with MS is “employed but work challenged.” In the present study, patients with pediatric-onset MS reached this threshold at about age 34 years, whereas patients with adult-onset MS reached it at approximately 50 years. These findings suggest that the groups’ different cognitive outcomes “may be meaningful,” the researchers wrote.
Onset of MS before age 18 years occurs in 2%-10% of cases, but few studies have looked at cognitive outcomes of patients with pediatric-onset MS in adulthood. Cognitive impairment is common in patients with MS and may affect quality of life, social functioning, and employment.
To compare changes in cognitive function over time in adults with pediatric-onset MS versus adults with adult-onset MS, Dr. McKay and colleagues conducted a population-based, longitudinal cohort study using data from more than 5,700 patients in the Swedish Multiple Sclerosis Registry. The registry includes information from all neurology clinics in Sweden, and the researchers examined data collected between April 2006 and April 2018.
SDMT scores range from 0 to 120, and higher scores indicate greater information-processing efficiency.
The researchers classified patients with MS onset at younger than 18 years as pediatric-onset MS. The researchers excluded patients with fewer than two SDMT scores, patients younger than 18 years or older than 55 years at the time of testing, and patients with disease duration of 30 years or more.
The researchers included 5,704 patients, 300 of whom had pediatric-onset MS (5.3%). About 70% of the patients were female, and 98% had a relapsing-onset disease course. The pediatric-onset MS group had a younger median age at baseline than the adult-onset group did (26 years vs. 38 years). The patients had more than 46,000 SDMT scores, with an average baseline SDMT of 51; the median follow-up time was 3 years.
Patients with pediatric-onset MS had significantly lower SDMT scores (beta coefficient, –3.59), after adjusting for sex, age, disease duration, disease course, total number of SDMTs completed, oral or visual SDMT form, and exposure to disease-modifying therapy. Their scores also declined faster than those of patients with adult-onset MS (beta coefficient, –0.30; 95% confidence interval, –5.56 to –1.54), and they were more likely to ever have cognitive impairment (odds ratio, 1.44).
“At younger than 30 years, SDMT scores between the ... groups were comparable; but after 30 years of age the trajectories began to diverge,” Dr. McKay and associates wrote. At age 35 years, the mean SDMT score for patients with adult-onset MS was 61, whereas for patients with pediatric-onset MS it was 51. By age 40 years, the mean score was 58 for adult-onset MS versus 46 for pediatric-onset MS.
The study was supported by the Swedish Research Council and the Swedish Brain Foundation and by postdoctoral awards from the Canadian Institutes of Health Research to Dr. McKay and European Committee for Treatment and Research in Multiple Sclerosis to Dr. McKay. Coauthors reported receiving honoraria for speaking and serving on advisory boards for various pharmaceutical companies, as well as receiving research funding from agencies, foundations, and pharmaceutical companies.
SOURCE: McKay KA et al. JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546.
The study by McKay et al. indicates that onset of multiple sclerosis (MS) during childhood or adolescence has long-term effects, Lauren B. Krupp, MD, and Leigh E. Charvet, PhD, wrote in an accompanying editorial.
In early adulthood, patients with pediatric-onset MS initially may perform better on the Symbol Digit Modalities Test, compared with patients with adult-onset MS. “However, the two groups diverged by the time the [pediatric-onset MS] patients reached 30 years of age, with slower performance in the [pediatric-onset MS] group relative to the [adult-onset MS] group, a difference that persisted over time,” Dr. Krupp and Dr. Charvet wrote. The findings remained even when the researchers adjusted for disease duration.
“Despite initial resiliency and age-based advantages, the study’s findings suggest greater deleterious long-term consequences from developing MS during a period of ongoing brain development,” they wrote.
The effect of slowed cognitive processing on quality of life is unclear, however. “The key question for future research is whether those with [pediatric-onset MS] attain their anticipated educational and occupational achievements in a manner comparable to those with [adult-onset MS],” Dr. Krupp and Dr. Charvet concluded.
Dr. Krupp and Dr. Charvet are affiliated with the Multiple Sclerosis Comprehensive Care Center at New York University. These comments are adapted from an editorial accompanying the article by McKay et al. ( JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546 ). Dr. Krupp reported receiving grants from the National Multiple Sclerosis Society; grants and personal fees from Biogen; and personal fees from Novartis, Sanofi Aventis, Sanofi Genzyme, Shire Pharmaceuticals, Roche, and RedHill Biopharma outside the submitted work. Dr. Charvet reported receiving grants and personal fees from Biogen, grants from the National Multiple Sclerosis Society, and research funding from Novartis and Biogen outside the submitted work.
The study by McKay et al. indicates that onset of multiple sclerosis (MS) during childhood or adolescence has long-term effects, Lauren B. Krupp, MD, and Leigh E. Charvet, PhD, wrote in an accompanying editorial.
In early adulthood, patients with pediatric-onset MS initially may perform better on the Symbol Digit Modalities Test, compared with patients with adult-onset MS. “However, the two groups diverged by the time the [pediatric-onset MS] patients reached 30 years of age, with slower performance in the [pediatric-onset MS] group relative to the [adult-onset MS] group, a difference that persisted over time,” Dr. Krupp and Dr. Charvet wrote. The findings remained even when the researchers adjusted for disease duration.
“Despite initial resiliency and age-based advantages, the study’s findings suggest greater deleterious long-term consequences from developing MS during a period of ongoing brain development,” they wrote.
The effect of slowed cognitive processing on quality of life is unclear, however. “The key question for future research is whether those with [pediatric-onset MS] attain their anticipated educational and occupational achievements in a manner comparable to those with [adult-onset MS],” Dr. Krupp and Dr. Charvet concluded.
Dr. Krupp and Dr. Charvet are affiliated with the Multiple Sclerosis Comprehensive Care Center at New York University. These comments are adapted from an editorial accompanying the article by McKay et al. ( JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546 ). Dr. Krupp reported receiving grants from the National Multiple Sclerosis Society; grants and personal fees from Biogen; and personal fees from Novartis, Sanofi Aventis, Sanofi Genzyme, Shire Pharmaceuticals, Roche, and RedHill Biopharma outside the submitted work. Dr. Charvet reported receiving grants and personal fees from Biogen, grants from the National Multiple Sclerosis Society, and research funding from Novartis and Biogen outside the submitted work.
The study by McKay et al. indicates that onset of multiple sclerosis (MS) during childhood or adolescence has long-term effects, Lauren B. Krupp, MD, and Leigh E. Charvet, PhD, wrote in an accompanying editorial.
In early adulthood, patients with pediatric-onset MS initially may perform better on the Symbol Digit Modalities Test, compared with patients with adult-onset MS. “However, the two groups diverged by the time the [pediatric-onset MS] patients reached 30 years of age, with slower performance in the [pediatric-onset MS] group relative to the [adult-onset MS] group, a difference that persisted over time,” Dr. Krupp and Dr. Charvet wrote. The findings remained even when the researchers adjusted for disease duration.
“Despite initial resiliency and age-based advantages, the study’s findings suggest greater deleterious long-term consequences from developing MS during a period of ongoing brain development,” they wrote.
The effect of slowed cognitive processing on quality of life is unclear, however. “The key question for future research is whether those with [pediatric-onset MS] attain their anticipated educational and occupational achievements in a manner comparable to those with [adult-onset MS],” Dr. Krupp and Dr. Charvet concluded.
Dr. Krupp and Dr. Charvet are affiliated with the Multiple Sclerosis Comprehensive Care Center at New York University. These comments are adapted from an editorial accompanying the article by McKay et al. ( JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546 ). Dr. Krupp reported receiving grants from the National Multiple Sclerosis Society; grants and personal fees from Biogen; and personal fees from Novartis, Sanofi Aventis, Sanofi Genzyme, Shire Pharmaceuticals, Roche, and RedHill Biopharma outside the submitted work. Dr. Charvet reported receiving grants and personal fees from Biogen, grants from the National Multiple Sclerosis Society, and research funding from Novartis and Biogen outside the submitted work.
independent of age or disease duration, according to a study published in JAMA Neurology.
Information-processing efficiency as measured by the Symbol Digit Modalities Test (SDMT) may decrease more rapidly in patients with pediatric-onset multiple sclerosis (MS).
“Children and adolescents who develop [MS] should be monitored closely for cognitive changes and helped to manage the potential challenges that early-onset multiple sclerosis poses for cognitive abilities later in life,” Kyla A. McKay, PhD, a researcher at Karolinska Institutet in Stockholm, and colleagues wrote.
Prior research has found that an SDMT score of 55 may be a point at which a person with MS is “employed but work challenged.” In the present study, patients with pediatric-onset MS reached this threshold at about age 34 years, whereas patients with adult-onset MS reached it at approximately 50 years. These findings suggest that the groups’ different cognitive outcomes “may be meaningful,” the researchers wrote.
Onset of MS before age 18 years occurs in 2%-10% of cases, but few studies have looked at cognitive outcomes of patients with pediatric-onset MS in adulthood. Cognitive impairment is common in patients with MS and may affect quality of life, social functioning, and employment.
To compare changes in cognitive function over time in adults with pediatric-onset MS versus adults with adult-onset MS, Dr. McKay and colleagues conducted a population-based, longitudinal cohort study using data from more than 5,700 patients in the Swedish Multiple Sclerosis Registry. The registry includes information from all neurology clinics in Sweden, and the researchers examined data collected between April 2006 and April 2018.
SDMT scores range from 0 to 120, and higher scores indicate greater information-processing efficiency.
The researchers classified patients with MS onset at younger than 18 years as pediatric-onset MS. The researchers excluded patients with fewer than two SDMT scores, patients younger than 18 years or older than 55 years at the time of testing, and patients with disease duration of 30 years or more.
The researchers included 5,704 patients, 300 of whom had pediatric-onset MS (5.3%). About 70% of the patients were female, and 98% had a relapsing-onset disease course. The pediatric-onset MS group had a younger median age at baseline than the adult-onset group did (26 years vs. 38 years). The patients had more than 46,000 SDMT scores, with an average baseline SDMT of 51; the median follow-up time was 3 years.
Patients with pediatric-onset MS had significantly lower SDMT scores (beta coefficient, –3.59), after adjusting for sex, age, disease duration, disease course, total number of SDMTs completed, oral or visual SDMT form, and exposure to disease-modifying therapy. Their scores also declined faster than those of patients with adult-onset MS (beta coefficient, –0.30; 95% confidence interval, –5.56 to –1.54), and they were more likely to ever have cognitive impairment (odds ratio, 1.44).
“At younger than 30 years, SDMT scores between the ... groups were comparable; but after 30 years of age the trajectories began to diverge,” Dr. McKay and associates wrote. At age 35 years, the mean SDMT score for patients with adult-onset MS was 61, whereas for patients with pediatric-onset MS it was 51. By age 40 years, the mean score was 58 for adult-onset MS versus 46 for pediatric-onset MS.
The study was supported by the Swedish Research Council and the Swedish Brain Foundation and by postdoctoral awards from the Canadian Institutes of Health Research to Dr. McKay and European Committee for Treatment and Research in Multiple Sclerosis to Dr. McKay. Coauthors reported receiving honoraria for speaking and serving on advisory boards for various pharmaceutical companies, as well as receiving research funding from agencies, foundations, and pharmaceutical companies.
SOURCE: McKay KA et al. JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546.
independent of age or disease duration, according to a study published in JAMA Neurology.
Information-processing efficiency as measured by the Symbol Digit Modalities Test (SDMT) may decrease more rapidly in patients with pediatric-onset multiple sclerosis (MS).
“Children and adolescents who develop [MS] should be monitored closely for cognitive changes and helped to manage the potential challenges that early-onset multiple sclerosis poses for cognitive abilities later in life,” Kyla A. McKay, PhD, a researcher at Karolinska Institutet in Stockholm, and colleagues wrote.
Prior research has found that an SDMT score of 55 may be a point at which a person with MS is “employed but work challenged.” In the present study, patients with pediatric-onset MS reached this threshold at about age 34 years, whereas patients with adult-onset MS reached it at approximately 50 years. These findings suggest that the groups’ different cognitive outcomes “may be meaningful,” the researchers wrote.
Onset of MS before age 18 years occurs in 2%-10% of cases, but few studies have looked at cognitive outcomes of patients with pediatric-onset MS in adulthood. Cognitive impairment is common in patients with MS and may affect quality of life, social functioning, and employment.
To compare changes in cognitive function over time in adults with pediatric-onset MS versus adults with adult-onset MS, Dr. McKay and colleagues conducted a population-based, longitudinal cohort study using data from more than 5,700 patients in the Swedish Multiple Sclerosis Registry. The registry includes information from all neurology clinics in Sweden, and the researchers examined data collected between April 2006 and April 2018.
SDMT scores range from 0 to 120, and higher scores indicate greater information-processing efficiency.
The researchers classified patients with MS onset at younger than 18 years as pediatric-onset MS. The researchers excluded patients with fewer than two SDMT scores, patients younger than 18 years or older than 55 years at the time of testing, and patients with disease duration of 30 years or more.
The researchers included 5,704 patients, 300 of whom had pediatric-onset MS (5.3%). About 70% of the patients were female, and 98% had a relapsing-onset disease course. The pediatric-onset MS group had a younger median age at baseline than the adult-onset group did (26 years vs. 38 years). The patients had more than 46,000 SDMT scores, with an average baseline SDMT of 51; the median follow-up time was 3 years.
Patients with pediatric-onset MS had significantly lower SDMT scores (beta coefficient, –3.59), after adjusting for sex, age, disease duration, disease course, total number of SDMTs completed, oral or visual SDMT form, and exposure to disease-modifying therapy. Their scores also declined faster than those of patients with adult-onset MS (beta coefficient, –0.30; 95% confidence interval, –5.56 to –1.54), and they were more likely to ever have cognitive impairment (odds ratio, 1.44).
“At younger than 30 years, SDMT scores between the ... groups were comparable; but after 30 years of age the trajectories began to diverge,” Dr. McKay and associates wrote. At age 35 years, the mean SDMT score for patients with adult-onset MS was 61, whereas for patients with pediatric-onset MS it was 51. By age 40 years, the mean score was 58 for adult-onset MS versus 46 for pediatric-onset MS.
The study was supported by the Swedish Research Council and the Swedish Brain Foundation and by postdoctoral awards from the Canadian Institutes of Health Research to Dr. McKay and European Committee for Treatment and Research in Multiple Sclerosis to Dr. McKay. Coauthors reported receiving honoraria for speaking and serving on advisory boards for various pharmaceutical companies, as well as receiving research funding from agencies, foundations, and pharmaceutical companies.
SOURCE: McKay KA et al. JAMA Neurol. 2019 Jun 17. doi: 10.1001/jamaneurol.2019.1546.
FROM JAMA NEUROLOGY
U.S. travelers to Europe need up to date measles immunization
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
FROM PEDIATRICS
Booster vaccines found largely safe in children on immunosuppressive drugs
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
REPORTING FROM EULAR 2019 CONGRESS
Rapid assay distinguishes viral from bacterial infection
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
REPORTING FROM ESPID 2019
Key clinical point: A novel real-time single-gene–expression PCR test quickly distinguishes viral from bacterial infection in febrile children.
Major finding: The expression signature of the IFI44L gene rapidly distinguished bacterial from viral infection in febrile children with 91% sensitivity and 93% specificity.
Study details: This translational study included 25 febrile children with definite bacterial or viral infections and 10 healthy controls.
Disclosures: The presenter reported having no financial conflicts regarding her study, supported by institutional funding.
Get patients vaccinated: Avoid unwelcome international travel souvenirs
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.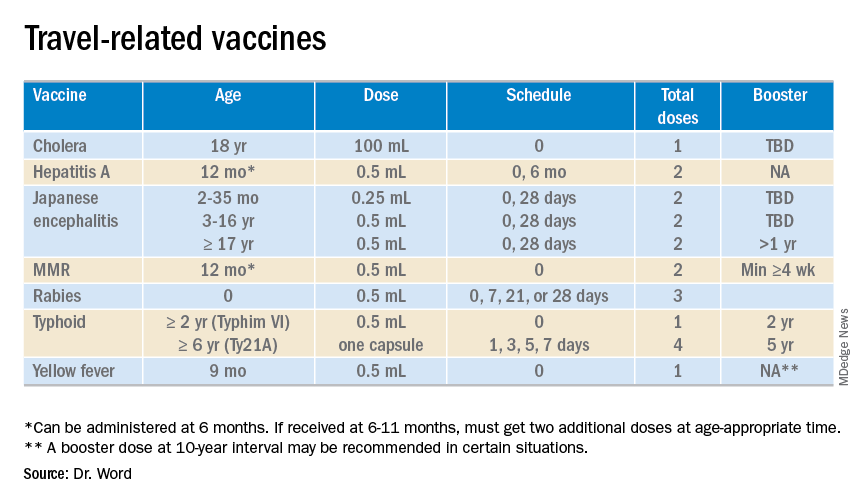
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Patients with CAPS still improving on long-term canakinumab
MADRID – An observational study that includes adults and children with cryopyrin-associated periodic syndromes and related diseases has provided real-world evidence that clinical improvement accrues on canakinumab (Ilaris) years after treatment was initiated, according to Norbert Blank, MD, of the division of rheumatology at the University of Heidelberg (Germany).
Summarizing data he presented at the European Congress of Rheumatology, Dr. Blank explained in an interview that the observational study has accrued more than 50 patients so far, with the goal of reaching 300 patients with cryopyrin-associated periodic syndromes and related rare diseases that have responded to anti–interleukin-1 therapy, such as Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and familial Mediterranean fever.
Most of the patients participating in the observational study, called RELIANCE, were already on canakinumab at the time of enrollment, often for several years. Yet in follow-up so far – which exceeds 1 year for some of the participants – improvement from the time of entry has been seen for some outcomes, such as activity level, according to Dr. Blank.
Canakinumab has been well tolerated with no new or unexpected adverse events emerging in the follow-up so far. Although these data remain limited, Dr. Blank considers them reassuring.
With detailed characterization of these rare diseases at baseline, observational studies like RELIANCE provide valuable real-world data about disease presentation, according to Dr. Blank. He believes that further follow-up will provide a rich source of information about disease course in response to anti-IL-1 therapy, which is being individualized according to response.
MADRID – An observational study that includes adults and children with cryopyrin-associated periodic syndromes and related diseases has provided real-world evidence that clinical improvement accrues on canakinumab (Ilaris) years after treatment was initiated, according to Norbert Blank, MD, of the division of rheumatology at the University of Heidelberg (Germany).
Summarizing data he presented at the European Congress of Rheumatology, Dr. Blank explained in an interview that the observational study has accrued more than 50 patients so far, with the goal of reaching 300 patients with cryopyrin-associated periodic syndromes and related rare diseases that have responded to anti–interleukin-1 therapy, such as Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and familial Mediterranean fever.
Most of the patients participating in the observational study, called RELIANCE, were already on canakinumab at the time of enrollment, often for several years. Yet in follow-up so far – which exceeds 1 year for some of the participants – improvement from the time of entry has been seen for some outcomes, such as activity level, according to Dr. Blank.
Canakinumab has been well tolerated with no new or unexpected adverse events emerging in the follow-up so far. Although these data remain limited, Dr. Blank considers them reassuring.
With detailed characterization of these rare diseases at baseline, observational studies like RELIANCE provide valuable real-world data about disease presentation, according to Dr. Blank. He believes that further follow-up will provide a rich source of information about disease course in response to anti-IL-1 therapy, which is being individualized according to response.
MADRID – An observational study that includes adults and children with cryopyrin-associated periodic syndromes and related diseases has provided real-world evidence that clinical improvement accrues on canakinumab (Ilaris) years after treatment was initiated, according to Norbert Blank, MD, of the division of rheumatology at the University of Heidelberg (Germany).
Summarizing data he presented at the European Congress of Rheumatology, Dr. Blank explained in an interview that the observational study has accrued more than 50 patients so far, with the goal of reaching 300 patients with cryopyrin-associated periodic syndromes and related rare diseases that have responded to anti–interleukin-1 therapy, such as Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and familial Mediterranean fever.
Most of the patients participating in the observational study, called RELIANCE, were already on canakinumab at the time of enrollment, often for several years. Yet in follow-up so far – which exceeds 1 year for some of the participants – improvement from the time of entry has been seen for some outcomes, such as activity level, according to Dr. Blank.
Canakinumab has been well tolerated with no new or unexpected adverse events emerging in the follow-up so far. Although these data remain limited, Dr. Blank considers them reassuring.
With detailed characterization of these rare diseases at baseline, observational studies like RELIANCE provide valuable real-world data about disease presentation, according to Dr. Blank. He believes that further follow-up will provide a rich source of information about disease course in response to anti-IL-1 therapy, which is being individualized according to response.
REPORTING FROM EULAR 2019 CONGRESS