User login
AI tool perfect in study of inflammatory diseases
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Artificial intelligence can distinguish overlapping inflammatory conditions with total accuracy, according to a new study presented at the annual meeting of the American College of Rheumatology.
Texas pediatricians faced a conundrum during the pandemic. Endemic typhus, a flea-borne tropical infection common to the region, is nearly indistinguishable from multisystem inflammatory syndrome in children (MIS-C), a rare condition set in motion by SARS-CoV-2 infection. Children with either ailment had seemingly identical symptoms: fever, rash, gastrointestinal issues, and in need of swift treatment. A diagnosis of endemic typhus can take 4-6 days to confirm.
Tiphanie Vogel, MD, PhD, a pediatric rheumatologist at Texas Children’s Hospital, Houston, and colleagues sought to create a tool to hasten diagnosis and, ideally, treatment. To do so, they incorporated machine learning and clinical factors available within the first 6 hours of the onset of symptoms.
The team analyzed 49 demographic, clinical, and laboratory measures from the medical records of 133 children with MIS-C and 87 with endemic typhus. Using deep learning, they narrowed the model to 30 essential features that became the backbone of AI-MET, a two-phase clinical-decision support system.
Phase 1 uses 17 clinical factors and can be performed on paper. If a patient’s score in phase 1 is not determinative, clinicians proceed to phase 2, which uses an additional 13 weighted factors and machine learning.
In testing, the two-part tool classified each of the 220 test patients perfectly. And it diagnosed a second group of 111 patients with MIS-C with 99% (110/111) accuracy.
Of note, “that first step classifies [a patient] correctly half of the time,” Dr. Vogel said, so the second, AI phase of the tool was necessary for only half of cases. Dr. Vogel said that’s a good sign; it means that the tool is useful in settings where AI may not always be feasible, like in a busy ED.
Melissa Mizesko, MD, a pediatric rheumatologist at Driscoll Children’s Hospital in Corpus Christi, Tex., said that the new tool could help clinicians streamline care. When cases of MIS-C peaked in Texas, clinicians often would start sick children on doxycycline and treat for MIS-C at the same time, then wait to see whether the antibiotic brought the fever down.
“This [new tool] is helpful if you live in a part of the country that has typhus,” said Jane Burns, MD, director of the Kawasaki Disease Research Center at the University of California, San Diego, who helped develop a similar AI-based tool to distinguish MIS-C from Kawasaki disease. But she encouraged the researchers to expand their testing to include other conditions. Although the AI model Dr. Vogel’s group developed can pinpoint MIS-C or endemic typhus, what if a child has neither condition? “It’s not often you’re dealing with a diagnosis between just two specific diseases,” Dr. Burns said.
Dr. Vogel is also interested in making AI-MET more efficient. “This go-round we prioritized perfect accuracy,” she said. But 30 clinical factors, with 17 of them recorded and calculated by hand, is a lot. “Could we still get this to be very accurate, maybe not perfect, with less inputs?”
In addition to refining AI-MET, which Texas Children’s eventually hopes to make available to other institutions, Dr. Vogel and associates are also considering other use cases for AI. Lupus is one option. “Maybe with machine learning we could identify clues at diagnosis that would help recommend targeted treatment,” she said
Dr. Vogel disclosed potential conflicts of interest with Moderna, Novartis, Pfizer, and SOBI. Dr. Burns and Dr. Mizesko disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM ACR 2023
CDC says child vaccination exemptions hit all-time high
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
– the highest exemption rate ever reported in the United States.
Of the 3% of children who got exemptions, 0.2% were for medical reasons and 2.8% for nonmedical reasons, the CDC report said. The overall exemption rate was 2.6% for the previous school year.
Though more children received exemptions, the overall national vaccination rate remained steady at 93% for children entering kindergarten for the 2022-2023 school year. Before the COVID-19 pandemic, the overall rate was 95%, the CDC said.
“The bad news is that it’s gone down since the pandemic and still hasn’t rebounded,” Sean O’Leary, MD, a University of Colorado pediatric infectious diseases specialist, told The Associated Press. “The good news is that the vast majority of parents are still vaccinating their kids according to the recommended schedule.”
The CDC report did not offer a specific reason for higher vaccine exemptions. But it did note that the increase could be caused by the COVID-19 pandemic and COVID vaccine hesitancy.
“There is a rising distrust in the health care system,” Amna Husain, MD, a pediatrician in private practice in North Carolina and a spokesperson for the American Academy of Pediatrics, told NBC News. Vaccine exemptions “have unfortunately trended upward with it.”
Exemption rates varied across the nation. The CDC said 40 states reported a rise in exemptions and that the exemption rate went over 5% in 10 states: Alaska, Arizona, Hawaii, Idaho, Michigan, Nevada, North Dakota, Oregon, Utah, and Wisconsin. Idaho had the highest exemption rate in 2022 with 12%.
While requirements vary from state to state, most states require students entering kindergarten to receive four vaccines: MMR, DTaP, polio, and chickenpox.
A version of this article first appeared on WebMD.com.
Mental health characteristics of refugee children
Since 1983, when I was a child and fled as a boat refugee from Vietnam with my mother, the international plight of displaced people has only worsened. From 1997 to 2022, the number of forcibly displaced people has more than tripled, growing from 34 million to more than 108 million.1
Displaced people are designated as refugees only when they cross international borders and meet the United Nations High Commissioner for Refugees’ (UNHCR) definition as “persons outside their countries of origin who are in need of international protection because of a serious threat to their life, physical integrity, or freedom in their country of origin as a result of persecution, armed conflict, violence, or serious public disorder.”2 There is a separate mandate by the United Nations for the aid of Palestinian refugees under the United Nations General Assembly’s United Nations Relief and Works Agency for Palestinian Refugees in the Near East (UNRWA).3 Of the displaced in 2022, more than 36 million were recognized as refugees under UNHCR and UNRWA mandates.1 Of these, almost 50% were children, at 17.5 million.4 To make matters worse, worldwide children represent less than one-third of the population.4 Since 2022, the increase in refugeeism is mostly driven by Ukraine and Syria, though also significantly Afghanistan, Venezuela, Sudan, Myanmar, Congo, Somalia, and Central African Republic.4 Refugeeism is a growing problem that disproportionately impacts children through sheer number, and one suspects, given their greater overall vulnerabilities compared with adults, physical and mental health consequences.
Traumas of refugees compared with non-refugee immigrants
In terms of mental health, refugees are distinct from non-refugee immigrants in that they likely experience more severe psychosocial adversities from greater poverty, greater risk of family separation, and uncertainty of the asylum process.5-8
From my own experience, this stems from the urgent nature of the refugee’s displacement, where they are often fleeing an immediate danger. My family had fled persecution from Communist forces and the social economic collapse that rendered Vietnam, for a time, one of the poorest in the world.9 Or, as my mother observed, “We had to leave because even doctors were starving.”
Refugees often have little preparation, have little legal protection since they are often criminalized, and are forced to endure dangerous conditions where they are vulnerable to smugglers and criminals who exploit their unprotected status. Once they arrive in their new country, they often do not have other family as social supports or resources. They themselves become the anchor for future legal and orderly immigration of their remaining family, given that they can extend their refugee status to those left behind.10 These non-refugee immigrants, unlike their refugee counterparts, are often flown to their new homes with more preparation, protecting them from dangerous conditions, and have the benefit of family who provide them with resources. As such, refugees tend to experience more traumatic life events than non-refugee immigrants. This was true in my family where those of us who initially escaped became the anchors to legally, and more safely, immigrate most of our family in Vietnam. We became their resources, likely making their acclimation smoother.
The mental health of refugee children and their caregivers
It is important to understand the stressors affecting the caregivers of children, since effective treatment of their mental health conditions can also benefit the children as well.11 In fact, among the greatest protective factors for refugee children is the presence of an adult caregiver, suggesting that the child’s mental health is dependent on the caregivers.
Those children who are separated show much worse mental health sequalae.12 As such, an understanding of the caregiver’s stressors is important. For example, when we were escaping Vietnam, my mom would protect me from our hardships by talking about our goals in America, minimizing our dangers by saying that we would be rewarded with things like a hamburger with its seemingly impossible amount of meat. Physically, my mother would always sleep with her arms around me and a knife hidden in order to ward off any attackers at night. When I was starving in the hull of a boat, having not eaten for days, my mother begged for food and gave me what she could get. And post-escape, my family focused on work and applied for aid for shelter and food, while encouraging us to invest in education, likely preventing involvement in criminal activities or gangs. Though overall, my family shielded me from the worst consequences, they also passed on their fears. One of my uncles had been killed by the police when he tried to escape, and so my family passed down a deep suspicion of authorities, whether they were the police or school principals. My mother had vivid memories of Communist re-education camps, which likely gave her a lasting fear that a Communist would find out our identities in America and re-capture us.
The mental health risk of refugees
Given that refugees tend to experience greater amounts of traumatic life events and a vast array of stressors sustained across years and even decades before, during, and after migration, it is no wonder they have much higher rates of mental health conditions, most predominantly PTSD and affective disorders.13,14 They are at particular risk of developing psychoses because they are more likely to experience a range of physical, psychological, and psychosocial problems associated with adversities such as violence, discrimination, economic stress, and social isolation.13 For example, the period leading up to my escape consisted of decades of prolonged war: the French-Indochina from 1945 to 1954, then the Vietnam War from 1955 to 1975) as well as the persecution and re-education camps afterward. What my family had to endure created a period of fear and loss into which I was born into in 1976. That year, my family had lost its fortune due to the Communist government seizing of our home and business, plunging us from a comfortable middle- to upper-class life to poverty. There was also widespread fear of systematic rape by the Communist victors. So my family endured great stress and the loss of a way of life leading up to our escape.
For the refugees, the escape itself is often a dangerous journey where, given its emergent nature, they are often exposed to the elements. We know about the current situation in Ukraine and Gaza, where children are fleeing from bombs and bullets. In my situation, we endured weeks of starvation crammed in the hull of boat as we forged through the Indian Ocean to the Philippines. One of my aunts, on a separate trip, perished because her boat had capsized, like so many others. Though impossible to verify, it has been estimated that up to 70% of Vietnamese refugees died during their escape.15 After the boat, my mother and I still had to brave Malaysian jungles and prisons, and then refugee camps for a year before we reached safety at an American Embassy in the Philippines. After we gained sponsorship to America, the traumas did not abate, but were only replaced by those of culture shock, poverty, and alienation. Taken by themselves, significant traumas exist in each phase of a refugee child’s escape, whether before, during, or after. These traumas are likely compounded since they are continuously layered and sustained across years, even decades. They affect not only the children, but their parents, and sometimes even a whole nation of people.
Summary
Refugee children and their families experience a variety of traumas, often sustained across years and even decades, because of armed conflict, persecution, or social upheavals. It is known that refugees are at greater risk for PTSD and affective and psychotic disorders, presumably due to increased traumatic life events before, during, and after their migration. The writer uses his own experience as a child refugee from Vietnam to elucidate the stressors evident in various phases of forced displacement.
Dr. Nguyen is a second year resident at UCSF Fresno Psychiatry Residency. He was a public high school English teacher for 15 years previously.
References
1. UNHCR. Global Trends. Forced displacement in 2016. Geneva, Switzerland: The UN Refugee Agency, 2022. https://www.unhcr.org/global-trends.
2. Office of the United Nations High Commissioner for Refugees. The refugee concept under international law. Global compact for safe, orderly and regular migration. https://www.unhcr.org/sites/
3. United Nations. (2023, November 11). The Question of Palestine. Un.org. https://www.un.org/unispal/document/un-general-assembly-renews-unrwa-mandate-press-release/
4. UNICEF. (2023, November 11). Child displacement. Data.unicef.org. https://data.unicef.org/topic/child-migration-and-displacement/displacement
5. Kinzie JD. Immigrants and refugees: The psychiatric perspective. Transcult Psychiatry. 2006 Dec;43(4):577-91. doi: 10.1177/1363461506070782.
6. Eaton W and Harrison G. Ethnic disadvantage and schizophrenia. Acta Psychiatr Scand Suppl. 2000:(407):38-43. doi: 10.1034/j.1600-0447.2000.00007.x.
7. Gilliver SC et al. Recent research on the mental health of immigrants to Sweden: a literature review. Eur J Public Health. 2014 Aug:24 Suppl 1:72-9. doi: 10.1093/eurpub/cku101.
8. Rapp MA et al. When local poverty is more important than your income: Mental health in minorities in inner cities. World Psychiatry. 2015 Jun;14(2):249-50. doi: 10.1002/wps.20221.
9. Cima, Ronald, ed. Vietnam: A Country Study. Washington: GPO for the Library of Congress, 1987.
10. United States Citizenship & Immigration Services (2023, November 12). Refugees. Uscis.gov. https://www.uscis.gov/humanitarian/refugees-and-asylum/refugees
11. Fazel M and Betancourt TS. (2018). Preventive mental health interventions for refugee children and adolescents in high-income settings. Lancet Child Adolesc Health. 2018 Feb;2(2):121-32. doi: 10.1016/S2352-4642(17)30147-5.
12. Fazel M et al. Mental health of displaced and refugee children resettled in high-income countries: risk and protective factors. Lancet. 2012 Jan 21;379(9812):266-82. doi: 10.1016/S0140-6736(11)60051-2.
13. Dapunt J et al. Refugees and psychosis: A review of the literature. Transl Psychiatry. 2017 Jun 13;7(6):e1149. doi: 10.1038/tp.2017.119.
14. Fazel M et al. Prevalence of serious mental disorder in 7,000 refugees resettled in western countries: a systematic review. Lancet. 2005 Apr;365(9467):1309-14. doi: 10.1016/S0140-6736(05)61027-6.
15. Rummel R. Statistics of Vietnamese Democide, in his Statistics of Democide. 1997. Table 6.1B,lines 730, 749-51.
Since 1983, when I was a child and fled as a boat refugee from Vietnam with my mother, the international plight of displaced people has only worsened. From 1997 to 2022, the number of forcibly displaced people has more than tripled, growing from 34 million to more than 108 million.1
Displaced people are designated as refugees only when they cross international borders and meet the United Nations High Commissioner for Refugees’ (UNHCR) definition as “persons outside their countries of origin who are in need of international protection because of a serious threat to their life, physical integrity, or freedom in their country of origin as a result of persecution, armed conflict, violence, or serious public disorder.”2 There is a separate mandate by the United Nations for the aid of Palestinian refugees under the United Nations General Assembly’s United Nations Relief and Works Agency for Palestinian Refugees in the Near East (UNRWA).3 Of the displaced in 2022, more than 36 million were recognized as refugees under UNHCR and UNRWA mandates.1 Of these, almost 50% were children, at 17.5 million.4 To make matters worse, worldwide children represent less than one-third of the population.4 Since 2022, the increase in refugeeism is mostly driven by Ukraine and Syria, though also significantly Afghanistan, Venezuela, Sudan, Myanmar, Congo, Somalia, and Central African Republic.4 Refugeeism is a growing problem that disproportionately impacts children through sheer number, and one suspects, given their greater overall vulnerabilities compared with adults, physical and mental health consequences.
Traumas of refugees compared with non-refugee immigrants
In terms of mental health, refugees are distinct from non-refugee immigrants in that they likely experience more severe psychosocial adversities from greater poverty, greater risk of family separation, and uncertainty of the asylum process.5-8
From my own experience, this stems from the urgent nature of the refugee’s displacement, where they are often fleeing an immediate danger. My family had fled persecution from Communist forces and the social economic collapse that rendered Vietnam, for a time, one of the poorest in the world.9 Or, as my mother observed, “We had to leave because even doctors were starving.”
Refugees often have little preparation, have little legal protection since they are often criminalized, and are forced to endure dangerous conditions where they are vulnerable to smugglers and criminals who exploit their unprotected status. Once they arrive in their new country, they often do not have other family as social supports or resources. They themselves become the anchor for future legal and orderly immigration of their remaining family, given that they can extend their refugee status to those left behind.10 These non-refugee immigrants, unlike their refugee counterparts, are often flown to their new homes with more preparation, protecting them from dangerous conditions, and have the benefit of family who provide them with resources. As such, refugees tend to experience more traumatic life events than non-refugee immigrants. This was true in my family where those of us who initially escaped became the anchors to legally, and more safely, immigrate most of our family in Vietnam. We became their resources, likely making their acclimation smoother.
The mental health of refugee children and their caregivers
It is important to understand the stressors affecting the caregivers of children, since effective treatment of their mental health conditions can also benefit the children as well.11 In fact, among the greatest protective factors for refugee children is the presence of an adult caregiver, suggesting that the child’s mental health is dependent on the caregivers.
Those children who are separated show much worse mental health sequalae.12 As such, an understanding of the caregiver’s stressors is important. For example, when we were escaping Vietnam, my mom would protect me from our hardships by talking about our goals in America, minimizing our dangers by saying that we would be rewarded with things like a hamburger with its seemingly impossible amount of meat. Physically, my mother would always sleep with her arms around me and a knife hidden in order to ward off any attackers at night. When I was starving in the hull of a boat, having not eaten for days, my mother begged for food and gave me what she could get. And post-escape, my family focused on work and applied for aid for shelter and food, while encouraging us to invest in education, likely preventing involvement in criminal activities or gangs. Though overall, my family shielded me from the worst consequences, they also passed on their fears. One of my uncles had been killed by the police when he tried to escape, and so my family passed down a deep suspicion of authorities, whether they were the police or school principals. My mother had vivid memories of Communist re-education camps, which likely gave her a lasting fear that a Communist would find out our identities in America and re-capture us.
The mental health risk of refugees
Given that refugees tend to experience greater amounts of traumatic life events and a vast array of stressors sustained across years and even decades before, during, and after migration, it is no wonder they have much higher rates of mental health conditions, most predominantly PTSD and affective disorders.13,14 They are at particular risk of developing psychoses because they are more likely to experience a range of physical, psychological, and psychosocial problems associated with adversities such as violence, discrimination, economic stress, and social isolation.13 For example, the period leading up to my escape consisted of decades of prolonged war: the French-Indochina from 1945 to 1954, then the Vietnam War from 1955 to 1975) as well as the persecution and re-education camps afterward. What my family had to endure created a period of fear and loss into which I was born into in 1976. That year, my family had lost its fortune due to the Communist government seizing of our home and business, plunging us from a comfortable middle- to upper-class life to poverty. There was also widespread fear of systematic rape by the Communist victors. So my family endured great stress and the loss of a way of life leading up to our escape.
For the refugees, the escape itself is often a dangerous journey where, given its emergent nature, they are often exposed to the elements. We know about the current situation in Ukraine and Gaza, where children are fleeing from bombs and bullets. In my situation, we endured weeks of starvation crammed in the hull of boat as we forged through the Indian Ocean to the Philippines. One of my aunts, on a separate trip, perished because her boat had capsized, like so many others. Though impossible to verify, it has been estimated that up to 70% of Vietnamese refugees died during their escape.15 After the boat, my mother and I still had to brave Malaysian jungles and prisons, and then refugee camps for a year before we reached safety at an American Embassy in the Philippines. After we gained sponsorship to America, the traumas did not abate, but were only replaced by those of culture shock, poverty, and alienation. Taken by themselves, significant traumas exist in each phase of a refugee child’s escape, whether before, during, or after. These traumas are likely compounded since they are continuously layered and sustained across years, even decades. They affect not only the children, but their parents, and sometimes even a whole nation of people.
Summary
Refugee children and their families experience a variety of traumas, often sustained across years and even decades, because of armed conflict, persecution, or social upheavals. It is known that refugees are at greater risk for PTSD and affective and psychotic disorders, presumably due to increased traumatic life events before, during, and after their migration. The writer uses his own experience as a child refugee from Vietnam to elucidate the stressors evident in various phases of forced displacement.
Dr. Nguyen is a second year resident at UCSF Fresno Psychiatry Residency. He was a public high school English teacher for 15 years previously.
References
1. UNHCR. Global Trends. Forced displacement in 2016. Geneva, Switzerland: The UN Refugee Agency, 2022. https://www.unhcr.org/global-trends.
2. Office of the United Nations High Commissioner for Refugees. The refugee concept under international law. Global compact for safe, orderly and regular migration. https://www.unhcr.org/sites/
3. United Nations. (2023, November 11). The Question of Palestine. Un.org. https://www.un.org/unispal/document/un-general-assembly-renews-unrwa-mandate-press-release/
4. UNICEF. (2023, November 11). Child displacement. Data.unicef.org. https://data.unicef.org/topic/child-migration-and-displacement/displacement
5. Kinzie JD. Immigrants and refugees: The psychiatric perspective. Transcult Psychiatry. 2006 Dec;43(4):577-91. doi: 10.1177/1363461506070782.
6. Eaton W and Harrison G. Ethnic disadvantage and schizophrenia. Acta Psychiatr Scand Suppl. 2000:(407):38-43. doi: 10.1034/j.1600-0447.2000.00007.x.
7. Gilliver SC et al. Recent research on the mental health of immigrants to Sweden: a literature review. Eur J Public Health. 2014 Aug:24 Suppl 1:72-9. doi: 10.1093/eurpub/cku101.
8. Rapp MA et al. When local poverty is more important than your income: Mental health in minorities in inner cities. World Psychiatry. 2015 Jun;14(2):249-50. doi: 10.1002/wps.20221.
9. Cima, Ronald, ed. Vietnam: A Country Study. Washington: GPO for the Library of Congress, 1987.
10. United States Citizenship & Immigration Services (2023, November 12). Refugees. Uscis.gov. https://www.uscis.gov/humanitarian/refugees-and-asylum/refugees
11. Fazel M and Betancourt TS. (2018). Preventive mental health interventions for refugee children and adolescents in high-income settings. Lancet Child Adolesc Health. 2018 Feb;2(2):121-32. doi: 10.1016/S2352-4642(17)30147-5.
12. Fazel M et al. Mental health of displaced and refugee children resettled in high-income countries: risk and protective factors. Lancet. 2012 Jan 21;379(9812):266-82. doi: 10.1016/S0140-6736(11)60051-2.
13. Dapunt J et al. Refugees and psychosis: A review of the literature. Transl Psychiatry. 2017 Jun 13;7(6):e1149. doi: 10.1038/tp.2017.119.
14. Fazel M et al. Prevalence of serious mental disorder in 7,000 refugees resettled in western countries: a systematic review. Lancet. 2005 Apr;365(9467):1309-14. doi: 10.1016/S0140-6736(05)61027-6.
15. Rummel R. Statistics of Vietnamese Democide, in his Statistics of Democide. 1997. Table 6.1B,lines 730, 749-51.
Since 1983, when I was a child and fled as a boat refugee from Vietnam with my mother, the international plight of displaced people has only worsened. From 1997 to 2022, the number of forcibly displaced people has more than tripled, growing from 34 million to more than 108 million.1
Displaced people are designated as refugees only when they cross international borders and meet the United Nations High Commissioner for Refugees’ (UNHCR) definition as “persons outside their countries of origin who are in need of international protection because of a serious threat to their life, physical integrity, or freedom in their country of origin as a result of persecution, armed conflict, violence, or serious public disorder.”2 There is a separate mandate by the United Nations for the aid of Palestinian refugees under the United Nations General Assembly’s United Nations Relief and Works Agency for Palestinian Refugees in the Near East (UNRWA).3 Of the displaced in 2022, more than 36 million were recognized as refugees under UNHCR and UNRWA mandates.1 Of these, almost 50% were children, at 17.5 million.4 To make matters worse, worldwide children represent less than one-third of the population.4 Since 2022, the increase in refugeeism is mostly driven by Ukraine and Syria, though also significantly Afghanistan, Venezuela, Sudan, Myanmar, Congo, Somalia, and Central African Republic.4 Refugeeism is a growing problem that disproportionately impacts children through sheer number, and one suspects, given their greater overall vulnerabilities compared with adults, physical and mental health consequences.
Traumas of refugees compared with non-refugee immigrants
In terms of mental health, refugees are distinct from non-refugee immigrants in that they likely experience more severe psychosocial adversities from greater poverty, greater risk of family separation, and uncertainty of the asylum process.5-8
From my own experience, this stems from the urgent nature of the refugee’s displacement, where they are often fleeing an immediate danger. My family had fled persecution from Communist forces and the social economic collapse that rendered Vietnam, for a time, one of the poorest in the world.9 Or, as my mother observed, “We had to leave because even doctors were starving.”
Refugees often have little preparation, have little legal protection since they are often criminalized, and are forced to endure dangerous conditions where they are vulnerable to smugglers and criminals who exploit their unprotected status. Once they arrive in their new country, they often do not have other family as social supports or resources. They themselves become the anchor for future legal and orderly immigration of their remaining family, given that they can extend their refugee status to those left behind.10 These non-refugee immigrants, unlike their refugee counterparts, are often flown to their new homes with more preparation, protecting them from dangerous conditions, and have the benefit of family who provide them with resources. As such, refugees tend to experience more traumatic life events than non-refugee immigrants. This was true in my family where those of us who initially escaped became the anchors to legally, and more safely, immigrate most of our family in Vietnam. We became their resources, likely making their acclimation smoother.
The mental health of refugee children and their caregivers
It is important to understand the stressors affecting the caregivers of children, since effective treatment of their mental health conditions can also benefit the children as well.11 In fact, among the greatest protective factors for refugee children is the presence of an adult caregiver, suggesting that the child’s mental health is dependent on the caregivers.
Those children who are separated show much worse mental health sequalae.12 As such, an understanding of the caregiver’s stressors is important. For example, when we were escaping Vietnam, my mom would protect me from our hardships by talking about our goals in America, minimizing our dangers by saying that we would be rewarded with things like a hamburger with its seemingly impossible amount of meat. Physically, my mother would always sleep with her arms around me and a knife hidden in order to ward off any attackers at night. When I was starving in the hull of a boat, having not eaten for days, my mother begged for food and gave me what she could get. And post-escape, my family focused on work and applied for aid for shelter and food, while encouraging us to invest in education, likely preventing involvement in criminal activities or gangs. Though overall, my family shielded me from the worst consequences, they also passed on their fears. One of my uncles had been killed by the police when he tried to escape, and so my family passed down a deep suspicion of authorities, whether they were the police or school principals. My mother had vivid memories of Communist re-education camps, which likely gave her a lasting fear that a Communist would find out our identities in America and re-capture us.
The mental health risk of refugees
Given that refugees tend to experience greater amounts of traumatic life events and a vast array of stressors sustained across years and even decades before, during, and after migration, it is no wonder they have much higher rates of mental health conditions, most predominantly PTSD and affective disorders.13,14 They are at particular risk of developing psychoses because they are more likely to experience a range of physical, psychological, and psychosocial problems associated with adversities such as violence, discrimination, economic stress, and social isolation.13 For example, the period leading up to my escape consisted of decades of prolonged war: the French-Indochina from 1945 to 1954, then the Vietnam War from 1955 to 1975) as well as the persecution and re-education camps afterward. What my family had to endure created a period of fear and loss into which I was born into in 1976. That year, my family had lost its fortune due to the Communist government seizing of our home and business, plunging us from a comfortable middle- to upper-class life to poverty. There was also widespread fear of systematic rape by the Communist victors. So my family endured great stress and the loss of a way of life leading up to our escape.
For the refugees, the escape itself is often a dangerous journey where, given its emergent nature, they are often exposed to the elements. We know about the current situation in Ukraine and Gaza, where children are fleeing from bombs and bullets. In my situation, we endured weeks of starvation crammed in the hull of boat as we forged through the Indian Ocean to the Philippines. One of my aunts, on a separate trip, perished because her boat had capsized, like so many others. Though impossible to verify, it has been estimated that up to 70% of Vietnamese refugees died during their escape.15 After the boat, my mother and I still had to brave Malaysian jungles and prisons, and then refugee camps for a year before we reached safety at an American Embassy in the Philippines. After we gained sponsorship to America, the traumas did not abate, but were only replaced by those of culture shock, poverty, and alienation. Taken by themselves, significant traumas exist in each phase of a refugee child’s escape, whether before, during, or after. These traumas are likely compounded since they are continuously layered and sustained across years, even decades. They affect not only the children, but their parents, and sometimes even a whole nation of people.
Summary
Refugee children and their families experience a variety of traumas, often sustained across years and even decades, because of armed conflict, persecution, or social upheavals. It is known that refugees are at greater risk for PTSD and affective and psychotic disorders, presumably due to increased traumatic life events before, during, and after their migration. The writer uses his own experience as a child refugee from Vietnam to elucidate the stressors evident in various phases of forced displacement.
Dr. Nguyen is a second year resident at UCSF Fresno Psychiatry Residency. He was a public high school English teacher for 15 years previously.
References
1. UNHCR. Global Trends. Forced displacement in 2016. Geneva, Switzerland: The UN Refugee Agency, 2022. https://www.unhcr.org/global-trends.
2. Office of the United Nations High Commissioner for Refugees. The refugee concept under international law. Global compact for safe, orderly and regular migration. https://www.unhcr.org/sites/
3. United Nations. (2023, November 11). The Question of Palestine. Un.org. https://www.un.org/unispal/document/un-general-assembly-renews-unrwa-mandate-press-release/
4. UNICEF. (2023, November 11). Child displacement. Data.unicef.org. https://data.unicef.org/topic/child-migration-and-displacement/displacement
5. Kinzie JD. Immigrants and refugees: The psychiatric perspective. Transcult Psychiatry. 2006 Dec;43(4):577-91. doi: 10.1177/1363461506070782.
6. Eaton W and Harrison G. Ethnic disadvantage and schizophrenia. Acta Psychiatr Scand Suppl. 2000:(407):38-43. doi: 10.1034/j.1600-0447.2000.00007.x.
7. Gilliver SC et al. Recent research on the mental health of immigrants to Sweden: a literature review. Eur J Public Health. 2014 Aug:24 Suppl 1:72-9. doi: 10.1093/eurpub/cku101.
8. Rapp MA et al. When local poverty is more important than your income: Mental health in minorities in inner cities. World Psychiatry. 2015 Jun;14(2):249-50. doi: 10.1002/wps.20221.
9. Cima, Ronald, ed. Vietnam: A Country Study. Washington: GPO for the Library of Congress, 1987.
10. United States Citizenship & Immigration Services (2023, November 12). Refugees. Uscis.gov. https://www.uscis.gov/humanitarian/refugees-and-asylum/refugees
11. Fazel M and Betancourt TS. (2018). Preventive mental health interventions for refugee children and adolescents in high-income settings. Lancet Child Adolesc Health. 2018 Feb;2(2):121-32. doi: 10.1016/S2352-4642(17)30147-5.
12. Fazel M et al. Mental health of displaced and refugee children resettled in high-income countries: risk and protective factors. Lancet. 2012 Jan 21;379(9812):266-82. doi: 10.1016/S0140-6736(11)60051-2.
13. Dapunt J et al. Refugees and psychosis: A review of the literature. Transl Psychiatry. 2017 Jun 13;7(6):e1149. doi: 10.1038/tp.2017.119.
14. Fazel M et al. Prevalence of serious mental disorder in 7,000 refugees resettled in western countries: a systematic review. Lancet. 2005 Apr;365(9467):1309-14. doi: 10.1016/S0140-6736(05)61027-6.
15. Rummel R. Statistics of Vietnamese Democide, in his Statistics of Democide. 1997. Table 6.1B,lines 730, 749-51.
CKD-EPI eGFR formula surpasses alternatives in young adults
PHILADELPHIA –
The two alternative formulas for calculating eGFR, the CKiD U25 (Chronic Kidney Disease in Children under 25) and the European Kidney Function Consortium equations, showed higher levels of bias that resulted in underestimates of kidney function, particularly in younger adults 18-25 years old and in those with higher eGFR values, Leslie A. Inker, MD, said at Kidney Week 2023, organized by the American Society of Nephrology.
However, for young adults with a history of childhood CKD and especially for those who continue under the care of pediatric clinicians even after they become young adults, use of the CKiD U25 equation, remains a reasonable option, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston. The CKiD U25 equation is intended for people aged 1-25 years and came out in late 2020.
Pediatric nephrologists use the CKiD U25 but the results of the 2021 CKD-EPI race-free equation is what U.S. clinical labs routinely report for people aged 18 or older. “Our findings support the current practice of pediatric nephrologists” who may opt to use the CKiD U25 even when a patient turns 18 years or older, Dr. Inker said in an interview.
But the new results also support the current practice of U.S. labs, which is to focus on calculating eGFR in anyone at least 18 years old using the 2021 equation developed by the CKD-EPI, work led by Dr. Inker. The new findings confirm routine use of the 2021 CKD-EPI equation in adults as young as 18 years old, especially when they’re having their eGFR calculated for the first time, she said.
Discontinuity changing from CKiD U25 to CKD-EPI is ‘not huge’
“It’s important to understand that the 2021 CKD-EPI equation works in young adults,” noted Josef Coresh, MD, PhD, professor of clinical epidemiology at Johns Hopkins University, Baltimore, who collaborated on both the current study and on developing the 2021 CKD-EPI equation.
The new data show that the discontinuity in eGFR produced by switching from the CKiD U25 formula to the 2021 CKD-EPI formula “is not huge, maybe about 5 mL/min per 1.73 m2 higher. People should focus on the new baseline and subsequent trends, not the modest difference between equations,” Dr. Coresh advised in an interview.
The study run by Dr. Inker and her associates used 1,491 people aged 18-40 years and enrolled in a cohort created by the CKD-EPI. They compared measured GFR levels in each subject with the estimates generated by the 2021 race-free CKD-EPI equation, the CKiD U25 equation, and a third equation developed by the EKFC and introduced in 2021.
Less bias with the 2021 CKD-EPI equation
The researchers compared the three eGFR equations with their respective measured GFR values by two metrics: bias, defined as the median difference between measured and estimated GFR; and the percentage of eGFR values that fell within 30% of the corresponding measured GFR value.
The results showed that bias was lowest using the 2021 CKD-EPI equation, with an overall median difference of 0.5 mL/min per 1.73 m2. This compared with median differences of 7.2 with the CKiD U25 equation and 4.9 mL/min per 1.73 m2 with the EKFC equation. The disparity in bias was greatest among those with eGFR values in the range of 60-90 mL/min per 1.73 m2 and was also greatest for those 18-25 years old.
The CKD-EPI equation results also showed the greatest consistency of bias across the entire 18- to 40-year-old range. Between-group differences were small for the percentage of eGFR values that fell within 30% of measured GFR, with all three equations scoring in the range of 88%-90%.
The study received no commercial funding. Dr. Inker is a consultant to Diamtrix and her department receives research funding from Chinook, Omeros, Reata, and Tricida. Dr. Coresh is a consultant to Healthy.io and SomaLogic and he has an ownership interest in Healthy.io.
PHILADELPHIA –
The two alternative formulas for calculating eGFR, the CKiD U25 (Chronic Kidney Disease in Children under 25) and the European Kidney Function Consortium equations, showed higher levels of bias that resulted in underestimates of kidney function, particularly in younger adults 18-25 years old and in those with higher eGFR values, Leslie A. Inker, MD, said at Kidney Week 2023, organized by the American Society of Nephrology.
However, for young adults with a history of childhood CKD and especially for those who continue under the care of pediatric clinicians even after they become young adults, use of the CKiD U25 equation, remains a reasonable option, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston. The CKiD U25 equation is intended for people aged 1-25 years and came out in late 2020.
Pediatric nephrologists use the CKiD U25 but the results of the 2021 CKD-EPI race-free equation is what U.S. clinical labs routinely report for people aged 18 or older. “Our findings support the current practice of pediatric nephrologists” who may opt to use the CKiD U25 even when a patient turns 18 years or older, Dr. Inker said in an interview.
But the new results also support the current practice of U.S. labs, which is to focus on calculating eGFR in anyone at least 18 years old using the 2021 equation developed by the CKD-EPI, work led by Dr. Inker. The new findings confirm routine use of the 2021 CKD-EPI equation in adults as young as 18 years old, especially when they’re having their eGFR calculated for the first time, she said.
Discontinuity changing from CKiD U25 to CKD-EPI is ‘not huge’
“It’s important to understand that the 2021 CKD-EPI equation works in young adults,” noted Josef Coresh, MD, PhD, professor of clinical epidemiology at Johns Hopkins University, Baltimore, who collaborated on both the current study and on developing the 2021 CKD-EPI equation.
The new data show that the discontinuity in eGFR produced by switching from the CKiD U25 formula to the 2021 CKD-EPI formula “is not huge, maybe about 5 mL/min per 1.73 m2 higher. People should focus on the new baseline and subsequent trends, not the modest difference between equations,” Dr. Coresh advised in an interview.
The study run by Dr. Inker and her associates used 1,491 people aged 18-40 years and enrolled in a cohort created by the CKD-EPI. They compared measured GFR levels in each subject with the estimates generated by the 2021 race-free CKD-EPI equation, the CKiD U25 equation, and a third equation developed by the EKFC and introduced in 2021.
Less bias with the 2021 CKD-EPI equation
The researchers compared the three eGFR equations with their respective measured GFR values by two metrics: bias, defined as the median difference between measured and estimated GFR; and the percentage of eGFR values that fell within 30% of the corresponding measured GFR value.
The results showed that bias was lowest using the 2021 CKD-EPI equation, with an overall median difference of 0.5 mL/min per 1.73 m2. This compared with median differences of 7.2 with the CKiD U25 equation and 4.9 mL/min per 1.73 m2 with the EKFC equation. The disparity in bias was greatest among those with eGFR values in the range of 60-90 mL/min per 1.73 m2 and was also greatest for those 18-25 years old.
The CKD-EPI equation results also showed the greatest consistency of bias across the entire 18- to 40-year-old range. Between-group differences were small for the percentage of eGFR values that fell within 30% of measured GFR, with all three equations scoring in the range of 88%-90%.
The study received no commercial funding. Dr. Inker is a consultant to Diamtrix and her department receives research funding from Chinook, Omeros, Reata, and Tricida. Dr. Coresh is a consultant to Healthy.io and SomaLogic and he has an ownership interest in Healthy.io.
PHILADELPHIA –
The two alternative formulas for calculating eGFR, the CKiD U25 (Chronic Kidney Disease in Children under 25) and the European Kidney Function Consortium equations, showed higher levels of bias that resulted in underestimates of kidney function, particularly in younger adults 18-25 years old and in those with higher eGFR values, Leslie A. Inker, MD, said at Kidney Week 2023, organized by the American Society of Nephrology.
However, for young adults with a history of childhood CKD and especially for those who continue under the care of pediatric clinicians even after they become young adults, use of the CKiD U25 equation, remains a reasonable option, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston. The CKiD U25 equation is intended for people aged 1-25 years and came out in late 2020.
Pediatric nephrologists use the CKiD U25 but the results of the 2021 CKD-EPI race-free equation is what U.S. clinical labs routinely report for people aged 18 or older. “Our findings support the current practice of pediatric nephrologists” who may opt to use the CKiD U25 even when a patient turns 18 years or older, Dr. Inker said in an interview.
But the new results also support the current practice of U.S. labs, which is to focus on calculating eGFR in anyone at least 18 years old using the 2021 equation developed by the CKD-EPI, work led by Dr. Inker. The new findings confirm routine use of the 2021 CKD-EPI equation in adults as young as 18 years old, especially when they’re having their eGFR calculated for the first time, she said.
Discontinuity changing from CKiD U25 to CKD-EPI is ‘not huge’
“It’s important to understand that the 2021 CKD-EPI equation works in young adults,” noted Josef Coresh, MD, PhD, professor of clinical epidemiology at Johns Hopkins University, Baltimore, who collaborated on both the current study and on developing the 2021 CKD-EPI equation.
The new data show that the discontinuity in eGFR produced by switching from the CKiD U25 formula to the 2021 CKD-EPI formula “is not huge, maybe about 5 mL/min per 1.73 m2 higher. People should focus on the new baseline and subsequent trends, not the modest difference between equations,” Dr. Coresh advised in an interview.
The study run by Dr. Inker and her associates used 1,491 people aged 18-40 years and enrolled in a cohort created by the CKD-EPI. They compared measured GFR levels in each subject with the estimates generated by the 2021 race-free CKD-EPI equation, the CKiD U25 equation, and a third equation developed by the EKFC and introduced in 2021.
Less bias with the 2021 CKD-EPI equation
The researchers compared the three eGFR equations with their respective measured GFR values by two metrics: bias, defined as the median difference between measured and estimated GFR; and the percentage of eGFR values that fell within 30% of the corresponding measured GFR value.
The results showed that bias was lowest using the 2021 CKD-EPI equation, with an overall median difference of 0.5 mL/min per 1.73 m2. This compared with median differences of 7.2 with the CKiD U25 equation and 4.9 mL/min per 1.73 m2 with the EKFC equation. The disparity in bias was greatest among those with eGFR values in the range of 60-90 mL/min per 1.73 m2 and was also greatest for those 18-25 years old.
The CKD-EPI equation results also showed the greatest consistency of bias across the entire 18- to 40-year-old range. Between-group differences were small for the percentage of eGFR values that fell within 30% of measured GFR, with all three equations scoring in the range of 88%-90%.
The study received no commercial funding. Dr. Inker is a consultant to Diamtrix and her department receives research funding from Chinook, Omeros, Reata, and Tricida. Dr. Coresh is a consultant to Healthy.io and SomaLogic and he has an ownership interest in Healthy.io.
AT KIDNEY WEEK 2023
Pregnancies with low anti-SSA/Ro autoantibody levels: Forgo fetal heart rhythm monitoring?
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Alpha-gal syndrome: Red meat is ‘just the beginning,’ expert says
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – allergist and immunologist Scott P. Commins, MD, PhD, told attendees at the annual meeting of the American College of Allergy, Asthma, and Immunology (ACAAI) annual meeting.
Dr. Commins, associate chief for allergy and immunology at the University of North Carolina at Chapel Hill, has made alpha-gal, a potentially fatal allergy, which, in the United States is tied to the bite of the Lone Star tick, his primary research focus.
Beyond red meat, “there are some people who are allergic to all things mammal,” he explained. Dairy products from mammals, medical devices made from mammalian products, vaccines and medicines that contain gelatin, and even commercial products such as perfumes and cosmetics may be behind an AGS reaction.
“The derived products from pigs and cows really find their way into a lot of our day-to-day products,” he said. “I try to keep an open mind about these exposures.”
Physicians should also be aware that “this can happen to kids,” said Dr. Commins. “It looks very similar to adults’ [AGS]. They can end up in the emergency department.”
He also had clinical advice about food challenges for AGS. He explained that there’s more alpha-gal in beef than in other red meats (including pork, venison, and lamb) with the exception of pork kidney. Pork kidney, he said, “has the most alpha-gal that we can find in the lab.”
Dr. Commins said he has stopped using beef for AGS food challenges and has switched to pork sausage patties with a high fat content microwaved in the clinic because they have less alpha-gal in general and he views them as safer.
Long delay in symptom onset
AGS symptoms typically take 2-6 hours to appear after eating red meat or being exposed to mammalian products, but Dr. Commins related a story about a patient he sent home who had very mild symptoms (some lower back itching) after he had spent the day at the clinic after a pork sausage food challenge for AGS.
The patient had returned home. Eight hours after the food challenge, his wife sent Dr. Commins a picture of her husband’s back, which was riddled with welts and was itching badly.
“I learned that if you’re going to do these food challenges, if there is a hint of symptoms at the clinic at 6 hours, keep them in the clinic, because it may really take that long to evolve,” Dr. Commins said.
One of the early signs he’s discovered is palmar erythema (redness and swelling of the hands).
Research has shown that AGS has been heavily concentrated in the Southeast, where Lone Star tick populations are clustered, but research has shown that from 2017 to 2022, it moved up the East Coast to the central United States and Upper Midwest.
“We are seeing increasing diagnoses of AGS in places that are not, perhaps, where we first thought this allergy existed,” said Dr. Commins. “Stay aware,” he cautioned.
The allergy is not exclusive to the United States, he noted. In Europe and Australia, for example, AGS is not thought to be tied to the Lone Star tick, which doesn’t inhabit those regions.
“It is a global phenomenon,” Dr. Commins said.
In August, the CDC alerted physicians to emerging cases of alpha-gal allergy after an article in Morbidity and Mortality Weekly Report indicated that health care providers have little knowledge about the allergy. Of the 1,500 health care providers surveyed, 42% had never heard of the syndrome, and another 35% were not confident in diagnosing or managing affected patients.
Matthew Lau, MD, an allergist with Kaiser Permanente in Honolulu who listened to Dr. Commins’ talk, told this news organization, “It’s important to raise awareness in primary care particularly, he said, as “allergists see only a fraction of the [AGS] patients.”
Allergists can help raise awareness
“Allergists have a role to alert the general community” and to drive more referrals, he said. That includes emergency departments, where physicians commonly see anaphylaxis.
Dr. Lau said he expects the incidence of AGS to increase, because global warming will likely lengthen warmer seasons and cause the geographic distribution to change.
Jay Lieberman, MD, a pediatric allergist at Le Bonheur Children’s Hospital in Memphis, Tenn., told this news organization, “There’s still a lot of confusion, and hearing from an expert like Dr. Commins helps tease out the not-obvious things about patients who are having more mild symptoms,” such as from allergy to dairy or medicines or vaccines that contain gelatin.
As a pediatric allergist, Dr. Lieberman said he sees less alpha-gal than his colleagues, but, he said, “On the adult side in Tennessee, it’s rampant.”
Dr. Commins, Dr. Lieberman, and Dr. Lau report no relevant financial relationships.
A version of this article appeared on Medscape.com.
A new standard for treatment of torus fractures of the wrist?
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
PRACTICE CHANGER
For uncomplicated pediatric torus fractures of the distal radius, consider definitive management with soft bandage immobilization until pain resolution, rather than rigid immobilization and clinical follow-up.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial with patient-oriented outcomes.1
Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
U.S. infant mortality rates rise for first time in 2 decades
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
The overall mortality rate and the rate for neonatal infants, those younger than 28 days old, rose by 3% from 2021 to 2022, says the Centers for Disease Control and Prevention’s National Center for Health Statistics. The mortality rate for infants older than 28 days rose by 4%.
Meanwhile, infant deaths caused by maternal complications rose by 8% and those caused by bacterial sepsis rose by 14%, the report says.
“We live in a country with significant resources, so the infant mortality rate and the increase are shockingly high,” wrote Sandy Chung, MD, of the American Academy of Pediatrics, to CNN. “As pediatricians who help children grow into healthy adults, any death of any child is one too many. The infant mortality rate in this country in unacceptable.”
Experts say the increase could be a sign of an underlying health care issue, an unusual occurrence, or partly related to the COVID-19 pandemic.
The infant mortality rate rose among mothers aged 25-29 years; for preterm babies; for boys; and in Georgia, Iowa, Missouri, and Texas. The rate declined in Nevada.
“Mortality rates increased significantly among infants of American Indian and Alaska Native non-Hispanic ... and White non-Hispanic women,” the report says.
“Mortality rates for infants of Black women did not increase by much, the report found, but Black infants experienced the highest overall rates of infant mortality: nearly 11 deaths per 1,000 births, or over double the mortality rate of White infants,” CNN wrote.
“We know that for people who live in or near poverty and for certain racial and ethnic groups there are significant challenges with getting access to a doctor or getting treatments,” Dr. Chung wrote. “This can lead to moms and babies showing up for care when they are sicker and more likely have serious outcomes, even death.”
A version of this article first appeared on WebMD.com.
Pediatric Primary Cutaneous Marginal Zone Lymphoma Treated With Doxycycline
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.
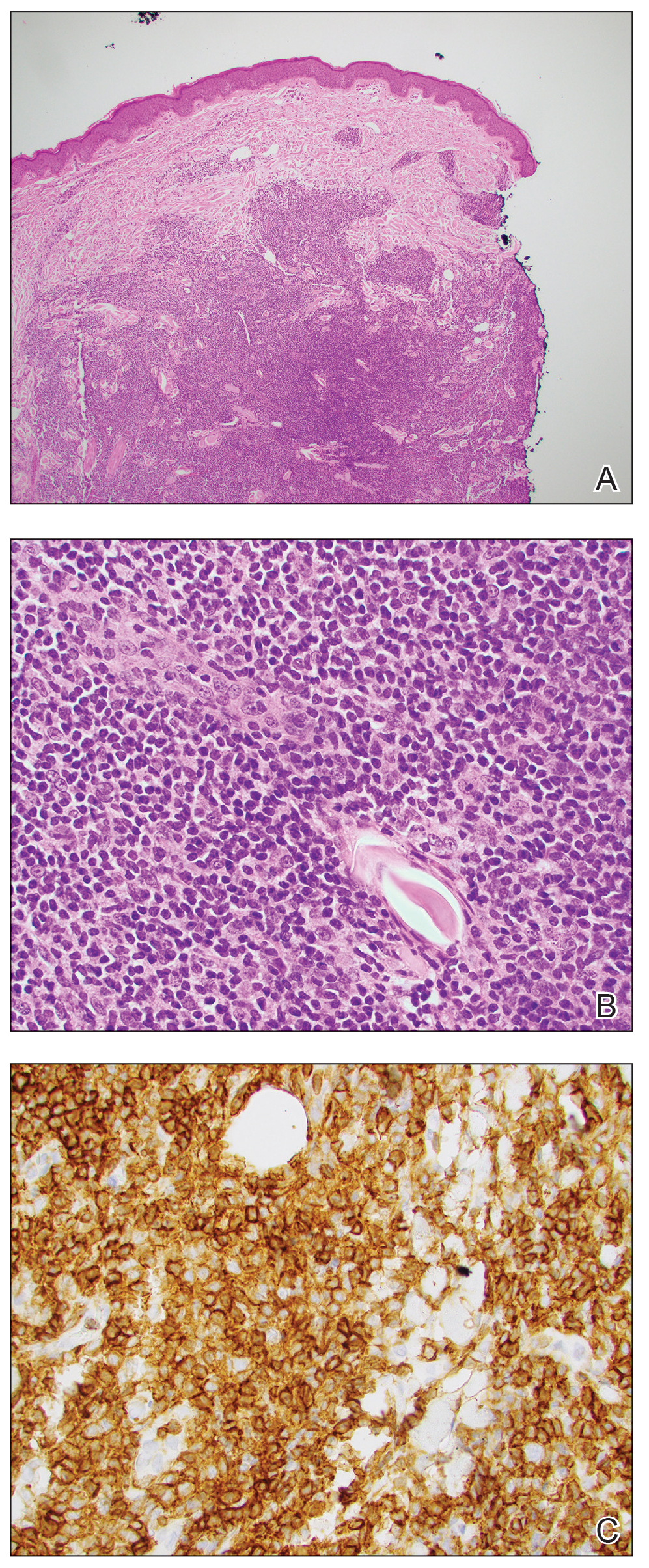
The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.
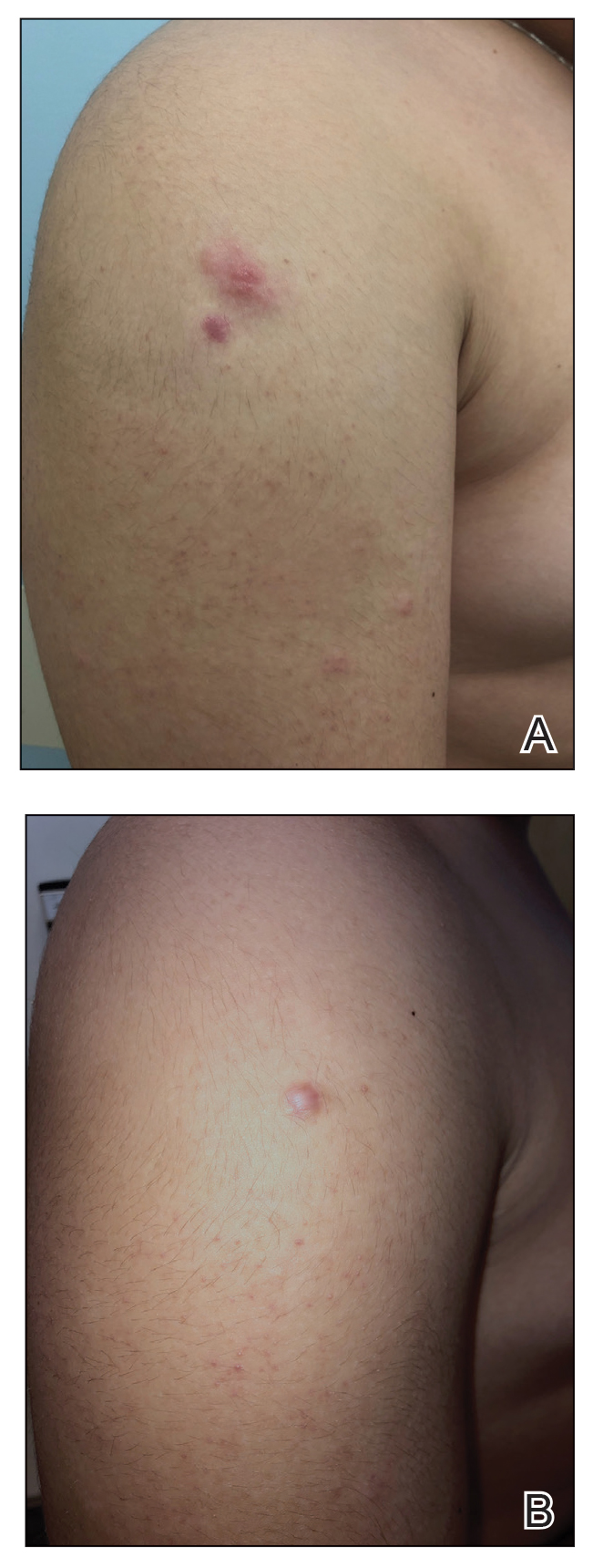
Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Practice Points
- When skin biopsy reveals marginal zone lymphoma, laboratory workup should include a complete blood cell count, chemistry, and serum lactate dehydrogenase levels. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed.
- For patients with multifocal skin lesions, positive emission tomography with computed tomography is utilized to exclude systemic disease and assess lymph node involvement.
- Treatments for primary cutaneous marginal zone lymphoma include excision, topical steroids, intralesional steroids, intralesional rituximab, radiation therapy, and antibiotics.
- Doxycycline can be considered as a treatment option for pediatric patients with widespread cutaneous involvement.
RNA therapeutics will ‘change everything’ in epilepsy
Epilepsy affects over 50 million people worldwide, making it one of the most common neurologic disorders. Though current antiseizure medications can control seizures in two-thirds of patients, drug-resistant epilepsy remains a major challenge for the remaining one-third, as does the lack of disease-modifying therapies.
But RNA-based therapeutics offer new hope, and experts predict they could fill these gaps and revolutionize epilepsy treatment.
“Current medicines for epilepsy are barely scraping the surface of what could be targeted. RNA therapeutics is going to change everything. It opens up entirely new targets – virtually anything in our genome becomes ‘druggable,’ ” said David Henshall, PhD, Royal College of Surgeons Ireland, Dublin.
Edward Kaye, MD, a pediatric neurologist and CEO of Stoke Therapeutics, agrees. “RNA therapeutics open up possibilities that could not have been imagined when I started my career,” he said in an interview.
Dr. Kaye said.
Thank COVID?
Henrik Klitgaard, PhD, and Sakari Kauppinen, PhD, scientific co-founders of NEUmiRNA Therapeutics, noted that the success of messenger RNA (mRNA) vaccines to counter the COVID-19 pandemic has fueled interest in exploring the potential of RNA-based therapies as a new modality in epilepsy with improved therapeutic properties.
Dr. Klitgaard and Dr. Kauppinen recently co-authored a “critical review” on RNA therapies for epilepsy published online in Epilepsia.
Unlike current antiseizure medications, which only target ion channels and receptors, RNA therapeutics can directly intervene at the genetic level.
RNA drugs can be targeted toward noncoding RNAs, such as microRNAs, or toward mRNA. Targeting noncoding RNAs shows promise in sporadic, nongenetic epilepsies, and targeting of mRNAs shows promise in childhood monogenic epilepsies.
Preclinical studies have highlighted the potential of RNA therapies for treatment of epilepsy.
“At NEUmiRNA Therapeutics, we have successfully designed potent and selective RNA drugs for a novel disease target that enable unprecedented elimination of the drug resistance and chronic epilepsy in a preclinical model mimicking temporal lobe epilepsy,” said Dr. Klitgaard.
“Interestingly,” he said, “these experiments also showed a disappearance of symptoms for epilepsy that outlasted drug exposure, suggesting significant disease-modifying properties with a curative potential for epilepsy.”
Hope for Dravet syndrome
Currently, there is significant interest in development of antisense oligonucleotides (ASOs), particularly for Dravet syndrome, a rare genetic epileptic encephalopathy that begins in infancy and gives rise to seizures that don’t respond well to seizure medications.
Stoke Therapeutics is developing antisense therapies aimed at correcting mutations in sodium channel genes, which cause up to 80% of cases of Dravet syndrome.
The company recently reported positive safety and efficacy data from patients treated with STK-001, a proprietary ASO, in the two ongoing phase 1/2a studies (MONARCH and ADMIRAL) and the SWALLOWTAIL open-label extension study.
“These new data suggest clinical benefit for patients 2-18 years of age treated with multiple doses of STK-001. The observed reductions in convulsive seizure frequency as well as substantial improvements in cognition and behavior support the potential for disease modification in a highly refractory patient population,” the company said in a news release.
Dr. Kaye noted that the company anticipates reporting additional data in the first quarter of 2024 and expects to provide an update on phase 3 planning in the first half of 2024.
“Twenty-five years ago, when I was caring for patients in my clinic, half of epilepsy was considered idiopathic because we didn’t know the cause,” Dr. Kaye commented.
“Since then, thanks to an understanding of the genetics and more widely available access to genetic testing, we can determine the root cause of most of them. Today, I believe we are on the verge of a fundamental shift in how we approach the treatment of Dravet syndrome and, hopefully, other genetic epilepsies,” said Dr. Kaye.
“We are now finally getting to the point that we not only know the causes, but we are in a position to develop medicines that target those causes. We have seen this happen in other diseases like cystic fibrosis, and the time has come for genetic epilepsies,” he added.
A promising future
Dr. Henshall said that the ability to target the cause rather than just the symptoms of epilepsy “offers the promise of disease-modifying and potentially curative medicines in the future.”
And what’s exciting is that the time frame of developing RNA medicines may be “radically” different than it is for traditional small-drug development, he noted.
Take, for example, a case reported recently in the New England Journal of Medicine.
Researchers identified a novel mutation in a child with neuronal ceroid lipofuscinosis 7 (a form of Batten’s disease), a rare and fatal neurodegenerative disease. Identification of the mutation was followed by the development and use (within 1 year) of a tailored RNA drug to treat the patient.
One downside perhaps is that current RNA drugs for epilepsy are delivered intrathecally, which is different from oral administration of small-molecule drugs.
However, Dr. Kauppinen from NEUmiRNA Therapeutics noted that “advances in intrathecal delivery technologies [and] the frequent use of this route of administration in other diseases and IT administration only being required two to three times per year will certainly facilitate use of RNA medicines.”
“This will also eliminate the issue of drug adherence by ensuring full patient compliance to treatment,” Dr. Kauppinen said.
The review article on RNA therapies in epilepsy had no commercial funding. Dr. Henshall holds a patent and has filed intellectual property related to microRNA targeting therapies for epilepsy and has received funding for microRNA research from NEUmiRNA Therapeutics. Dr. Klitgaard and Dr. Kauppinen are cofounders of NEUmiRNA Therapeutics. Dr. Kaye is CEO of Stoke Therapeutics.
A version of this article first appeared on Medscape.com.
Epilepsy affects over 50 million people worldwide, making it one of the most common neurologic disorders. Though current antiseizure medications can control seizures in two-thirds of patients, drug-resistant epilepsy remains a major challenge for the remaining one-third, as does the lack of disease-modifying therapies.
But RNA-based therapeutics offer new hope, and experts predict they could fill these gaps and revolutionize epilepsy treatment.
“Current medicines for epilepsy are barely scraping the surface of what could be targeted. RNA therapeutics is going to change everything. It opens up entirely new targets – virtually anything in our genome becomes ‘druggable,’ ” said David Henshall, PhD, Royal College of Surgeons Ireland, Dublin.
Edward Kaye, MD, a pediatric neurologist and CEO of Stoke Therapeutics, agrees. “RNA therapeutics open up possibilities that could not have been imagined when I started my career,” he said in an interview.
Dr. Kaye said.
Thank COVID?
Henrik Klitgaard, PhD, and Sakari Kauppinen, PhD, scientific co-founders of NEUmiRNA Therapeutics, noted that the success of messenger RNA (mRNA) vaccines to counter the COVID-19 pandemic has fueled interest in exploring the potential of RNA-based therapies as a new modality in epilepsy with improved therapeutic properties.
Dr. Klitgaard and Dr. Kauppinen recently co-authored a “critical review” on RNA therapies for epilepsy published online in Epilepsia.
Unlike current antiseizure medications, which only target ion channels and receptors, RNA therapeutics can directly intervene at the genetic level.
RNA drugs can be targeted toward noncoding RNAs, such as microRNAs, or toward mRNA. Targeting noncoding RNAs shows promise in sporadic, nongenetic epilepsies, and targeting of mRNAs shows promise in childhood monogenic epilepsies.
Preclinical studies have highlighted the potential of RNA therapies for treatment of epilepsy.
“At NEUmiRNA Therapeutics, we have successfully designed potent and selective RNA drugs for a novel disease target that enable unprecedented elimination of the drug resistance and chronic epilepsy in a preclinical model mimicking temporal lobe epilepsy,” said Dr. Klitgaard.
“Interestingly,” he said, “these experiments also showed a disappearance of symptoms for epilepsy that outlasted drug exposure, suggesting significant disease-modifying properties with a curative potential for epilepsy.”
Hope for Dravet syndrome
Currently, there is significant interest in development of antisense oligonucleotides (ASOs), particularly for Dravet syndrome, a rare genetic epileptic encephalopathy that begins in infancy and gives rise to seizures that don’t respond well to seizure medications.
Stoke Therapeutics is developing antisense therapies aimed at correcting mutations in sodium channel genes, which cause up to 80% of cases of Dravet syndrome.
The company recently reported positive safety and efficacy data from patients treated with STK-001, a proprietary ASO, in the two ongoing phase 1/2a studies (MONARCH and ADMIRAL) and the SWALLOWTAIL open-label extension study.
“These new data suggest clinical benefit for patients 2-18 years of age treated with multiple doses of STK-001. The observed reductions in convulsive seizure frequency as well as substantial improvements in cognition and behavior support the potential for disease modification in a highly refractory patient population,” the company said in a news release.
Dr. Kaye noted that the company anticipates reporting additional data in the first quarter of 2024 and expects to provide an update on phase 3 planning in the first half of 2024.
“Twenty-five years ago, when I was caring for patients in my clinic, half of epilepsy was considered idiopathic because we didn’t know the cause,” Dr. Kaye commented.
“Since then, thanks to an understanding of the genetics and more widely available access to genetic testing, we can determine the root cause of most of them. Today, I believe we are on the verge of a fundamental shift in how we approach the treatment of Dravet syndrome and, hopefully, other genetic epilepsies,” said Dr. Kaye.
“We are now finally getting to the point that we not only know the causes, but we are in a position to develop medicines that target those causes. We have seen this happen in other diseases like cystic fibrosis, and the time has come for genetic epilepsies,” he added.
A promising future
Dr. Henshall said that the ability to target the cause rather than just the symptoms of epilepsy “offers the promise of disease-modifying and potentially curative medicines in the future.”
And what’s exciting is that the time frame of developing RNA medicines may be “radically” different than it is for traditional small-drug development, he noted.
Take, for example, a case reported recently in the New England Journal of Medicine.
Researchers identified a novel mutation in a child with neuronal ceroid lipofuscinosis 7 (a form of Batten’s disease), a rare and fatal neurodegenerative disease. Identification of the mutation was followed by the development and use (within 1 year) of a tailored RNA drug to treat the patient.
One downside perhaps is that current RNA drugs for epilepsy are delivered intrathecally, which is different from oral administration of small-molecule drugs.
However, Dr. Kauppinen from NEUmiRNA Therapeutics noted that “advances in intrathecal delivery technologies [and] the frequent use of this route of administration in other diseases and IT administration only being required two to three times per year will certainly facilitate use of RNA medicines.”
“This will also eliminate the issue of drug adherence by ensuring full patient compliance to treatment,” Dr. Kauppinen said.
The review article on RNA therapies in epilepsy had no commercial funding. Dr. Henshall holds a patent and has filed intellectual property related to microRNA targeting therapies for epilepsy and has received funding for microRNA research from NEUmiRNA Therapeutics. Dr. Klitgaard and Dr. Kauppinen are cofounders of NEUmiRNA Therapeutics. Dr. Kaye is CEO of Stoke Therapeutics.
A version of this article first appeared on Medscape.com.
Epilepsy affects over 50 million people worldwide, making it one of the most common neurologic disorders. Though current antiseizure medications can control seizures in two-thirds of patients, drug-resistant epilepsy remains a major challenge for the remaining one-third, as does the lack of disease-modifying therapies.
But RNA-based therapeutics offer new hope, and experts predict they could fill these gaps and revolutionize epilepsy treatment.
“Current medicines for epilepsy are barely scraping the surface of what could be targeted. RNA therapeutics is going to change everything. It opens up entirely new targets – virtually anything in our genome becomes ‘druggable,’ ” said David Henshall, PhD, Royal College of Surgeons Ireland, Dublin.
Edward Kaye, MD, a pediatric neurologist and CEO of Stoke Therapeutics, agrees. “RNA therapeutics open up possibilities that could not have been imagined when I started my career,” he said in an interview.
Dr. Kaye said.
Thank COVID?
Henrik Klitgaard, PhD, and Sakari Kauppinen, PhD, scientific co-founders of NEUmiRNA Therapeutics, noted that the success of messenger RNA (mRNA) vaccines to counter the COVID-19 pandemic has fueled interest in exploring the potential of RNA-based therapies as a new modality in epilepsy with improved therapeutic properties.
Dr. Klitgaard and Dr. Kauppinen recently co-authored a “critical review” on RNA therapies for epilepsy published online in Epilepsia.
Unlike current antiseizure medications, which only target ion channels and receptors, RNA therapeutics can directly intervene at the genetic level.
RNA drugs can be targeted toward noncoding RNAs, such as microRNAs, or toward mRNA. Targeting noncoding RNAs shows promise in sporadic, nongenetic epilepsies, and targeting of mRNAs shows promise in childhood monogenic epilepsies.
Preclinical studies have highlighted the potential of RNA therapies for treatment of epilepsy.
“At NEUmiRNA Therapeutics, we have successfully designed potent and selective RNA drugs for a novel disease target that enable unprecedented elimination of the drug resistance and chronic epilepsy in a preclinical model mimicking temporal lobe epilepsy,” said Dr. Klitgaard.
“Interestingly,” he said, “these experiments also showed a disappearance of symptoms for epilepsy that outlasted drug exposure, suggesting significant disease-modifying properties with a curative potential for epilepsy.”
Hope for Dravet syndrome
Currently, there is significant interest in development of antisense oligonucleotides (ASOs), particularly for Dravet syndrome, a rare genetic epileptic encephalopathy that begins in infancy and gives rise to seizures that don’t respond well to seizure medications.
Stoke Therapeutics is developing antisense therapies aimed at correcting mutations in sodium channel genes, which cause up to 80% of cases of Dravet syndrome.
The company recently reported positive safety and efficacy data from patients treated with STK-001, a proprietary ASO, in the two ongoing phase 1/2a studies (MONARCH and ADMIRAL) and the SWALLOWTAIL open-label extension study.
“These new data suggest clinical benefit for patients 2-18 years of age treated with multiple doses of STK-001. The observed reductions in convulsive seizure frequency as well as substantial improvements in cognition and behavior support the potential for disease modification in a highly refractory patient population,” the company said in a news release.
Dr. Kaye noted that the company anticipates reporting additional data in the first quarter of 2024 and expects to provide an update on phase 3 planning in the first half of 2024.
“Twenty-five years ago, when I was caring for patients in my clinic, half of epilepsy was considered idiopathic because we didn’t know the cause,” Dr. Kaye commented.
“Since then, thanks to an understanding of the genetics and more widely available access to genetic testing, we can determine the root cause of most of them. Today, I believe we are on the verge of a fundamental shift in how we approach the treatment of Dravet syndrome and, hopefully, other genetic epilepsies,” said Dr. Kaye.
“We are now finally getting to the point that we not only know the causes, but we are in a position to develop medicines that target those causes. We have seen this happen in other diseases like cystic fibrosis, and the time has come for genetic epilepsies,” he added.
A promising future
Dr. Henshall said that the ability to target the cause rather than just the symptoms of epilepsy “offers the promise of disease-modifying and potentially curative medicines in the future.”
And what’s exciting is that the time frame of developing RNA medicines may be “radically” different than it is for traditional small-drug development, he noted.
Take, for example, a case reported recently in the New England Journal of Medicine.
Researchers identified a novel mutation in a child with neuronal ceroid lipofuscinosis 7 (a form of Batten’s disease), a rare and fatal neurodegenerative disease. Identification of the mutation was followed by the development and use (within 1 year) of a tailored RNA drug to treat the patient.
One downside perhaps is that current RNA drugs for epilepsy are delivered intrathecally, which is different from oral administration of small-molecule drugs.
However, Dr. Kauppinen from NEUmiRNA Therapeutics noted that “advances in intrathecal delivery technologies [and] the frequent use of this route of administration in other diseases and IT administration only being required two to three times per year will certainly facilitate use of RNA medicines.”
“This will also eliminate the issue of drug adherence by ensuring full patient compliance to treatment,” Dr. Kauppinen said.
The review article on RNA therapies in epilepsy had no commercial funding. Dr. Henshall holds a patent and has filed intellectual property related to microRNA targeting therapies for epilepsy and has received funding for microRNA research from NEUmiRNA Therapeutics. Dr. Klitgaard and Dr. Kauppinen are cofounders of NEUmiRNA Therapeutics. Dr. Kaye is CEO of Stoke Therapeutics.
A version of this article first appeared on Medscape.com.









