User login
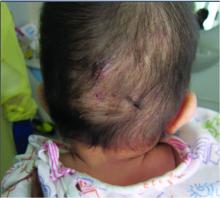
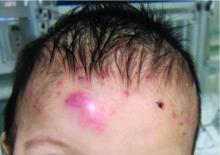
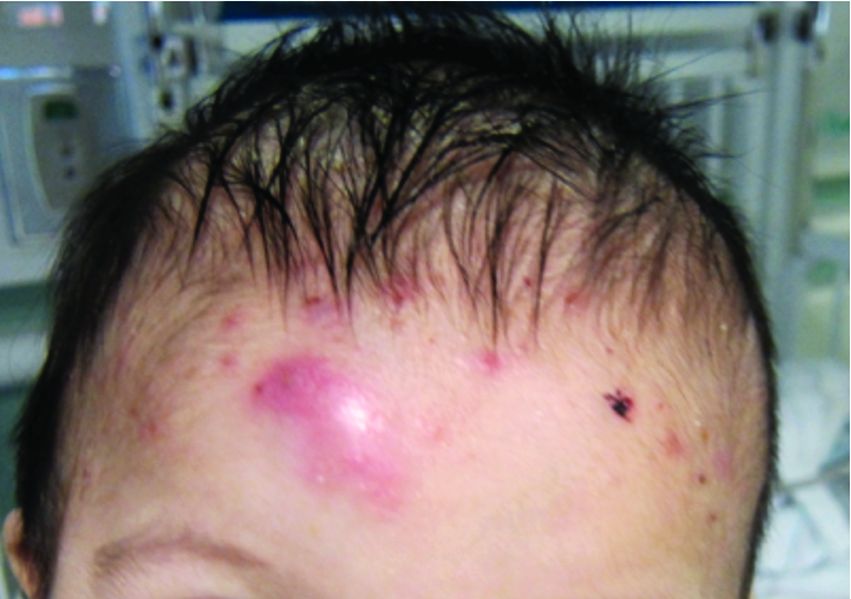
A 7-month-old male presents with pustules and inflamed papules on the scalp and extremities
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
A 7-month-old male is brought to the emergency department for evaluation of pustules and inflamed papules on the scalp and extremities for several weeks of duration. The parents report the lesions started about a month prior and he has already been treated with cephalexin, clindamycin, and sulfamethoxazole without any improvement. Cultures sent prior by the child's pediatrician did not reveal any fungus or bacteria. The parents report a low-grade fever for about 3 days.
He was born via natural vaginal delivery with no instrumentation or external monitoring. Mom had prenatal care. Besides the skin lesions, the baby has been healthy and growing well. He has no history of eczema or severe infections. He has not been hospitalized before.
On physical examination the baby was not febrile. On the scalp and forehead, he had diffusely distributed pustules, erythematous papules, and nodules. He also presented with scattered, fine, small, crusted 1-2-mm pink papules on the trunk and extremities. He had no adenopathy or hepatosplenomegaly.
At the emergency department, samples from one of the pustules were sent for bacterial, fungal, and atypical mycobacteria cultures. Laboratory test showed a normal blood count with associated eosinophilia (2.8 x 109 L), and normal liver and kidney function. A head ultrasound showed three ill-defined hypoechoic foci within the scalp.
The patient was admitted for treatment with broad-spectrum antibiotics and dermatology was consulted.
Perinatal HIV nearly eradicated in U.S.
, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
“This country has been really aggressive about counseling women who are pregnant and getting mothers in care,” Dr. Gandhi said.
The treatment method was discovered more than 30 years ago. Prior to the therapy and ensuing awareness campaigns to prevent transmission, mothers with HIV would typically pass the virus to their child in utero, during delivery, or while breastfeeding.
“There should be zero children born with HIV, given that we’ve had these drugs for so long,” Dr. Ghandi said.
Disparities persist
But challenges remain in some communities, where babies born to Black mothers are disproportionately affected by the disease, the new study found. “Racial and ethnic differences in perinatal HIV diagnoses persisted through the 10-year period,” the report’s authors concluded. “The highest rates of perinatal HIV diagnoses were seen among infants born to Black women.”
Although rates of perinatal HIV declined for babies born to Black mothers over the decade-long study, the diagnosis rate was above the goal of elimination at 3.1 for every 100,000 live births, according to the data.
Meanwhile, transmission rates hovered around 1%-2% for Latinx and Hispanic women and mothers who identified as “other races,” including Native American.
Despite the availability of medication, expectant mothers may face several hurdles to getting the daily treatment they need to prevent transmission to their fetus, according to Jennifer Jao, MD, MPH, a physician of infectious diseases at Lurie Children’s Hospital of Chicago.
They might have trouble securing health insurance or finding transportation to doctor’s appointments, or face other problems like lacking secure housing or food – all factors that prevent them from prioritizing the care.
“All of those things play into the mix,” Dr. Jao said. “We see over and over again that closing the gap means you’ve got to reach the women who are pregnant and who don’t have resources.”
Progress in ‘danger’
Experts said they’re not sure what the impact of the COVID-19 pandemic, accompanied by a recent uptick in sexually transmitted diseases, will be on rates of perinatal HIV. Some women were unable to access prenatal health care during the pandemic because they couldn’t access public transportation or childcare, the U.S. Government Accountability Office said in 2022.
Globally, a decline in rates of HIV and AIDS rates has slowed, prompting the World Health Organization to warn last year that progress on the disease is in danger. Researchers only included HIV rates in the United States through 2019, so the data are outdated, Dr. Gandhi noted.
“All of this put together means we don’t know where we are with perinatal transmission over the last 3 years,” she said.
In an accompanying editorial, coauthors Nahida Chakhtoura, MD, MsGH, and Bill Kapogiannis, MD, both with the National Institutes of Health, urge health care professionals to take an active role in eliminating these racial and ethnic disparities in an effort to – as the title of their editorial proclaims – achieve a “road to zero perinatal HIV transmission” in the United States.
“The more proactive we are in identifying and promptly addressing systematic deficiencies that exacerbate health inequities in cutting-edge research innovations and optimal clinical service provision,” they write, “the less reactive we will need to be when new transmissible infections appear at our doorstep.”
A version of this article first appeared on Medscape.com.
, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
“This country has been really aggressive about counseling women who are pregnant and getting mothers in care,” Dr. Gandhi said.
The treatment method was discovered more than 30 years ago. Prior to the therapy and ensuing awareness campaigns to prevent transmission, mothers with HIV would typically pass the virus to their child in utero, during delivery, or while breastfeeding.
“There should be zero children born with HIV, given that we’ve had these drugs for so long,” Dr. Ghandi said.
Disparities persist
But challenges remain in some communities, where babies born to Black mothers are disproportionately affected by the disease, the new study found. “Racial and ethnic differences in perinatal HIV diagnoses persisted through the 10-year period,” the report’s authors concluded. “The highest rates of perinatal HIV diagnoses were seen among infants born to Black women.”
Although rates of perinatal HIV declined for babies born to Black mothers over the decade-long study, the diagnosis rate was above the goal of elimination at 3.1 for every 100,000 live births, according to the data.
Meanwhile, transmission rates hovered around 1%-2% for Latinx and Hispanic women and mothers who identified as “other races,” including Native American.
Despite the availability of medication, expectant mothers may face several hurdles to getting the daily treatment they need to prevent transmission to their fetus, according to Jennifer Jao, MD, MPH, a physician of infectious diseases at Lurie Children’s Hospital of Chicago.
They might have trouble securing health insurance or finding transportation to doctor’s appointments, or face other problems like lacking secure housing or food – all factors that prevent them from prioritizing the care.
“All of those things play into the mix,” Dr. Jao said. “We see over and over again that closing the gap means you’ve got to reach the women who are pregnant and who don’t have resources.”
Progress in ‘danger’
Experts said they’re not sure what the impact of the COVID-19 pandemic, accompanied by a recent uptick in sexually transmitted diseases, will be on rates of perinatal HIV. Some women were unable to access prenatal health care during the pandemic because they couldn’t access public transportation or childcare, the U.S. Government Accountability Office said in 2022.
Globally, a decline in rates of HIV and AIDS rates has slowed, prompting the World Health Organization to warn last year that progress on the disease is in danger. Researchers only included HIV rates in the United States through 2019, so the data are outdated, Dr. Gandhi noted.
“All of this put together means we don’t know where we are with perinatal transmission over the last 3 years,” she said.
In an accompanying editorial, coauthors Nahida Chakhtoura, MD, MsGH, and Bill Kapogiannis, MD, both with the National Institutes of Health, urge health care professionals to take an active role in eliminating these racial and ethnic disparities in an effort to – as the title of their editorial proclaims – achieve a “road to zero perinatal HIV transmission” in the United States.
“The more proactive we are in identifying and promptly addressing systematic deficiencies that exacerbate health inequities in cutting-edge research innovations and optimal clinical service provision,” they write, “the less reactive we will need to be when new transmissible infections appear at our doorstep.”
A version of this article first appeared on Medscape.com.
, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
“This country has been really aggressive about counseling women who are pregnant and getting mothers in care,” Dr. Gandhi said.
The treatment method was discovered more than 30 years ago. Prior to the therapy and ensuing awareness campaigns to prevent transmission, mothers with HIV would typically pass the virus to their child in utero, during delivery, or while breastfeeding.
“There should be zero children born with HIV, given that we’ve had these drugs for so long,” Dr. Ghandi said.
Disparities persist
But challenges remain in some communities, where babies born to Black mothers are disproportionately affected by the disease, the new study found. “Racial and ethnic differences in perinatal HIV diagnoses persisted through the 10-year period,” the report’s authors concluded. “The highest rates of perinatal HIV diagnoses were seen among infants born to Black women.”
Although rates of perinatal HIV declined for babies born to Black mothers over the decade-long study, the diagnosis rate was above the goal of elimination at 3.1 for every 100,000 live births, according to the data.
Meanwhile, transmission rates hovered around 1%-2% for Latinx and Hispanic women and mothers who identified as “other races,” including Native American.
Despite the availability of medication, expectant mothers may face several hurdles to getting the daily treatment they need to prevent transmission to their fetus, according to Jennifer Jao, MD, MPH, a physician of infectious diseases at Lurie Children’s Hospital of Chicago.
They might have trouble securing health insurance or finding transportation to doctor’s appointments, or face other problems like lacking secure housing or food – all factors that prevent them from prioritizing the care.
“All of those things play into the mix,” Dr. Jao said. “We see over and over again that closing the gap means you’ve got to reach the women who are pregnant and who don’t have resources.”
Progress in ‘danger’
Experts said they’re not sure what the impact of the COVID-19 pandemic, accompanied by a recent uptick in sexually transmitted diseases, will be on rates of perinatal HIV. Some women were unable to access prenatal health care during the pandemic because they couldn’t access public transportation or childcare, the U.S. Government Accountability Office said in 2022.
Globally, a decline in rates of HIV and AIDS rates has slowed, prompting the World Health Organization to warn last year that progress on the disease is in danger. Researchers only included HIV rates in the United States through 2019, so the data are outdated, Dr. Gandhi noted.
“All of this put together means we don’t know where we are with perinatal transmission over the last 3 years,” she said.
In an accompanying editorial, coauthors Nahida Chakhtoura, MD, MsGH, and Bill Kapogiannis, MD, both with the National Institutes of Health, urge health care professionals to take an active role in eliminating these racial and ethnic disparities in an effort to – as the title of their editorial proclaims – achieve a “road to zero perinatal HIV transmission” in the United States.
“The more proactive we are in identifying and promptly addressing systematic deficiencies that exacerbate health inequities in cutting-edge research innovations and optimal clinical service provision,” they write, “the less reactive we will need to be when new transmissible infections appear at our doorstep.”
A version of this article first appeared on Medscape.com.
Progress, gaps as pediatricians expand mental health roles
but a review of electronic health records highlights areas for improvement in delivering the care.
The findings were published online in Pediatrics.
The researchers, led by Talia R. Lester, MD, with the division of developmental behavioral pediatrics in the quantitative science unit at Stanford (Calif.) University, identified 1,685 patients aged 6-18 years who had at least one visit with a diagnosis of anxiety and/or depression in a large primary care network in northern California and who were prescribed an SSRI by a network primary care pediatrician (PCP). The team randomly chose 110 patients and reviewed charts from the visit when the SSRI was first prescribed (medication visit); the immediately previous visit; and immediately subsequent visit.
Encouraging signs
The chart reviews showed some encouraging signs. For example, when pediatricians prescribe an SSRI, 82% are appropriately documenting rationales for starting the medication at the medication visit. However, they are not monitoring medication side effects systematically, according to the report. Of 69 patients with a visit after the medication visit, fewer than half (48%) had documentation of monitoring for side effects.
Three areas for improvement
The researchers identified three main shortfall areas and suggested improvements.
PCPs often referred patients for unspecified therapy at the medication visit; however, they rarely prescribed evidence-based therapies such as cognitive-behavioral therapy (CBT) (4% of patients). The authors suggested embedding a summary of evidence-based treatment into order sets.
Secondly, PCPs are not often using screening tools. The data show only 26% of patients had a documented depression- or anxiety-specific screening tool result at the medication visit. The authors recommend making the screening tools accessible through the EHR to increase use.
The researchers also found many patients didn’t have a follow-up visit after SSRI medication was prescribed. Even when they did, the range was so wide between the medication visit and the follow-up (7-365 days) that it’s clear pediatricians are taking inconsistent approaches to scheduling follow-up.
Half are seeing only their primary care pediatrician
About half of children and adolescents prescribed an SSRI by a pediatrician for mental health reasons were seeing only their primary care pediatrician, the data showed.
Eric M. Butter, PhD, chief of psychology at Nationwide Children’s Hospital and Ohio State University, Columbus, pointed out in an accompanying editorial that some of the news in pediatricians’ expanded role is particularly encouraging.
Pediatricians, he noted, are making medication decisions consistent with decisions a subspecialist would make.
Of cases in which a subspecialist became involved after a pediatrician initiated medication, subspecialists changed the medication for only two patients, which “is encouraging because it validates pediatricians’ decisions,” Dr. Butter said.
It’s important for pediatricians to understand key evidence-based programs that can work in combination with medications to achieve better results, Dr. Butter said. For example, CBT can help with depression “and break the cycle of avoidance that worsens symptoms of anxiety.”
He highlighted Interpersonal Therapy for Adolescents, a 12-session treatment that “can also address depression by improving patients’ personal relationships.”
“No primary care pediatrician will have the training or time to implement the many treatments that are available,” Dr. Butter wrote. “However, pediatricians can work to understand the key features of the evidence-based treatments referenced by Lester et al.”
Most concerning statistics
Dr. Butter said the most concerning shortcoming in the pediatricians’ health care delivery was lack of referral for evidence-based psychological treatments and low rates for referral to access supports from schools through programs such as the education 504 plan and Individualized Education Plans.
Dr. Lester’s team found that pediatricians recommended that patients receive support from such programs in only 8% of cases.
“The children’s mental health crisis requires all child-serving health care providers to do more. Improved care for anxiety and depression in pediatric primary care is needed and does not have to be overly burdensome to pediatricians,” Dr. Butter wrote.
The authors and Dr. Butter declared no relevant financial relationships.
but a review of electronic health records highlights areas for improvement in delivering the care.
The findings were published online in Pediatrics.
The researchers, led by Talia R. Lester, MD, with the division of developmental behavioral pediatrics in the quantitative science unit at Stanford (Calif.) University, identified 1,685 patients aged 6-18 years who had at least one visit with a diagnosis of anxiety and/or depression in a large primary care network in northern California and who were prescribed an SSRI by a network primary care pediatrician (PCP). The team randomly chose 110 patients and reviewed charts from the visit when the SSRI was first prescribed (medication visit); the immediately previous visit; and immediately subsequent visit.
Encouraging signs
The chart reviews showed some encouraging signs. For example, when pediatricians prescribe an SSRI, 82% are appropriately documenting rationales for starting the medication at the medication visit. However, they are not monitoring medication side effects systematically, according to the report. Of 69 patients with a visit after the medication visit, fewer than half (48%) had documentation of monitoring for side effects.
Three areas for improvement
The researchers identified three main shortfall areas and suggested improvements.
PCPs often referred patients for unspecified therapy at the medication visit; however, they rarely prescribed evidence-based therapies such as cognitive-behavioral therapy (CBT) (4% of patients). The authors suggested embedding a summary of evidence-based treatment into order sets.
Secondly, PCPs are not often using screening tools. The data show only 26% of patients had a documented depression- or anxiety-specific screening tool result at the medication visit. The authors recommend making the screening tools accessible through the EHR to increase use.
The researchers also found many patients didn’t have a follow-up visit after SSRI medication was prescribed. Even when they did, the range was so wide between the medication visit and the follow-up (7-365 days) that it’s clear pediatricians are taking inconsistent approaches to scheduling follow-up.
Half are seeing only their primary care pediatrician
About half of children and adolescents prescribed an SSRI by a pediatrician for mental health reasons were seeing only their primary care pediatrician, the data showed.
Eric M. Butter, PhD, chief of psychology at Nationwide Children’s Hospital and Ohio State University, Columbus, pointed out in an accompanying editorial that some of the news in pediatricians’ expanded role is particularly encouraging.
Pediatricians, he noted, are making medication decisions consistent with decisions a subspecialist would make.
Of cases in which a subspecialist became involved after a pediatrician initiated medication, subspecialists changed the medication for only two patients, which “is encouraging because it validates pediatricians’ decisions,” Dr. Butter said.
It’s important for pediatricians to understand key evidence-based programs that can work in combination with medications to achieve better results, Dr. Butter said. For example, CBT can help with depression “and break the cycle of avoidance that worsens symptoms of anxiety.”
He highlighted Interpersonal Therapy for Adolescents, a 12-session treatment that “can also address depression by improving patients’ personal relationships.”
“No primary care pediatrician will have the training or time to implement the many treatments that are available,” Dr. Butter wrote. “However, pediatricians can work to understand the key features of the evidence-based treatments referenced by Lester et al.”
Most concerning statistics
Dr. Butter said the most concerning shortcoming in the pediatricians’ health care delivery was lack of referral for evidence-based psychological treatments and low rates for referral to access supports from schools through programs such as the education 504 plan and Individualized Education Plans.
Dr. Lester’s team found that pediatricians recommended that patients receive support from such programs in only 8% of cases.
“The children’s mental health crisis requires all child-serving health care providers to do more. Improved care for anxiety and depression in pediatric primary care is needed and does not have to be overly burdensome to pediatricians,” Dr. Butter wrote.
The authors and Dr. Butter declared no relevant financial relationships.
but a review of electronic health records highlights areas for improvement in delivering the care.
The findings were published online in Pediatrics.
The researchers, led by Talia R. Lester, MD, with the division of developmental behavioral pediatrics in the quantitative science unit at Stanford (Calif.) University, identified 1,685 patients aged 6-18 years who had at least one visit with a diagnosis of anxiety and/or depression in a large primary care network in northern California and who were prescribed an SSRI by a network primary care pediatrician (PCP). The team randomly chose 110 patients and reviewed charts from the visit when the SSRI was first prescribed (medication visit); the immediately previous visit; and immediately subsequent visit.
Encouraging signs
The chart reviews showed some encouraging signs. For example, when pediatricians prescribe an SSRI, 82% are appropriately documenting rationales for starting the medication at the medication visit. However, they are not monitoring medication side effects systematically, according to the report. Of 69 patients with a visit after the medication visit, fewer than half (48%) had documentation of monitoring for side effects.
Three areas for improvement
The researchers identified three main shortfall areas and suggested improvements.
PCPs often referred patients for unspecified therapy at the medication visit; however, they rarely prescribed evidence-based therapies such as cognitive-behavioral therapy (CBT) (4% of patients). The authors suggested embedding a summary of evidence-based treatment into order sets.
Secondly, PCPs are not often using screening tools. The data show only 26% of patients had a documented depression- or anxiety-specific screening tool result at the medication visit. The authors recommend making the screening tools accessible through the EHR to increase use.
The researchers also found many patients didn’t have a follow-up visit after SSRI medication was prescribed. Even when they did, the range was so wide between the medication visit and the follow-up (7-365 days) that it’s clear pediatricians are taking inconsistent approaches to scheduling follow-up.
Half are seeing only their primary care pediatrician
About half of children and adolescents prescribed an SSRI by a pediatrician for mental health reasons were seeing only their primary care pediatrician, the data showed.
Eric M. Butter, PhD, chief of psychology at Nationwide Children’s Hospital and Ohio State University, Columbus, pointed out in an accompanying editorial that some of the news in pediatricians’ expanded role is particularly encouraging.
Pediatricians, he noted, are making medication decisions consistent with decisions a subspecialist would make.
Of cases in which a subspecialist became involved after a pediatrician initiated medication, subspecialists changed the medication for only two patients, which “is encouraging because it validates pediatricians’ decisions,” Dr. Butter said.
It’s important for pediatricians to understand key evidence-based programs that can work in combination with medications to achieve better results, Dr. Butter said. For example, CBT can help with depression “and break the cycle of avoidance that worsens symptoms of anxiety.”
He highlighted Interpersonal Therapy for Adolescents, a 12-session treatment that “can also address depression by improving patients’ personal relationships.”
“No primary care pediatrician will have the training or time to implement the many treatments that are available,” Dr. Butter wrote. “However, pediatricians can work to understand the key features of the evidence-based treatments referenced by Lester et al.”
Most concerning statistics
Dr. Butter said the most concerning shortcoming in the pediatricians’ health care delivery was lack of referral for evidence-based psychological treatments and low rates for referral to access supports from schools through programs such as the education 504 plan and Individualized Education Plans.
Dr. Lester’s team found that pediatricians recommended that patients receive support from such programs in only 8% of cases.
“The children’s mental health crisis requires all child-serving health care providers to do more. Improved care for anxiety and depression in pediatric primary care is needed and does not have to be overly burdensome to pediatricians,” Dr. Butter wrote.
The authors and Dr. Butter declared no relevant financial relationships.
FROM PEDIATRICS
What will vaping lead to? Emerging research shows damage, and addiction
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Jake Warn calls vaping “a toxic artificial love.”
Jake, of Winslow, Maine, was 16 years old when he began vaping. Unlike cigarettes, vaping can be odorless, and its smoke leaves no trace, which allowed him and his friends to use the devices in school bathrooms without fear of being caught.
He would use an entire cartridge containing the vape liquid, the equivalent of smoking one pack of tobacco cigarettes, within 1 school day. By the fall semester of his first year in college, Jake said his use had increased even more.
“It got pricey, so that’s when I really started to notice” the extent of his dependency, he said recently.
Vaping rates among teenagers in Maine doubled from 15.3% to 28.7% between 2017 and 2019, while Jake was in high school. In 2021, 11% of high schoolers across the nation said they regularly smoked e-cigarettes, and an estimated 28% have ever tried the devices, according to the Centers for Disease Control and Prevention.
The Food and Drug Administration classifies e-cigarettes as a tobacco product because many contain nicotine, which comes from tobacco. Like Jake, the habit is likely to carry into adulthood for many who start in their teenage years, experts say.
Electronic nicotine delivery systems (ENDS) such as vapes have been touted by their manufacturers and by some in the medical field as a healthier alternative to cigarettes and as a method to help smokers give up the habit.
But, that’s not how Jake – who had never used combustible cigarettes – picked up vaping, or how he sold the idea to his mother.
“It’s all organic and natural flavoring, it’s just flavored water,” Mary Lou Warn recalled her son saying to her. She researched the health effects of vaping but didn’t find much online. “I knew they were dangerous because you don’t put anything in your lungs that isn’t fresh air.”
A determined athlete in high school, Jake found that his asthma worsened as he transitioned to college, especially when he ran a track meet or during a soccer game.
Mrs. Warn noticed changes off the field, too.
“He was coughing constantly, he wasn’t sleeping well, he wasn’t eating well,” she said. “I knew the addiction was taking over.”
Vaping irritated Jake’s throat, and he would get nosebleeds that he couldn’t stop, she added.
Since Mrs. Warn first looked into the effects of e-cigarettes on respiratory health back in 2017, many studies have been conducted of the short-term health outcomes for first-time smokers who never used combustible tobacco products. Studies suggest that vaping may worsen bronchitis and asthma, raise blood pressure, interfere with brain development in young users, suppress the immune system, and increase the risk of developing a chronic lung disease (Am J Prev Med. 2020 Feb;58[2]:182-90). Studies of mice and cell cultures have found that the vapor or extracts from vapes damage the chemical structure of DNA.
Still, the limited number of long-term human studies has made it hard to know what the health outcomes of e-cigarette users will be in the future. Conclusive studies linking commercial cigarette use to deaths from heart disease and cancer didn’t emerge until the mid-1950s, decades after manufacturers began mass production and marketing in the early 20th century.
Years could pass before researchers gain a clearer understanding of the health implications of long-term e-cigarette use, according to Nigar Nargis, PhD, senior scientific director of tobacco control research at the American Cancer Society.
“There hasn’t been any such study to establish the direct link from ENDS to cancer, but it is understood that it [vaping] may promote the development of cancer and lung damage and inflammation,” Dr. Nargis said.
For decades, advocates built awareness of the harms of tobacco use, which led to a sharp decline in tobacco-related illnesses such as lung cancer. But Hilary Schneider, Maine’s director of government relations for the ACS Cancer Action Network, said she fears the uptick in the use of vapes – especially among those who never smoked or those who use both combustible cigarettes and e-cigarettes – may reverse declines in the rates of smoking-relating diseases.
Multiple studies suggest that inhaling chemicals found in e-cigarettes – including nicotine-carrying aerosols – can damage arteries and inflame and injure the lungs.
Vapes “basically have created a pediatric tobacco-use epidemic,” Ms. Schneider said. “What we’re seeing is unprecedented tobacco use rates, higher rates than we’ve seen in decades.”
One reason many young people start vaping is the attraction to flavors, which range from classic menthol to fruits and sweets. A handful of states have enacted bans or restrictions on the sale of flavored vapes.
“It’s new, and it’s just been marketed in a way that we’re really fighting the false narrative put out there by makers of these products that are trying to make them appealing to kids,” said Rachel Boykan, MD, clinical professor of pediatrics and attending physician at Stony Brook (N.Y.) Children’s Hospital.
The flavor Red Bull, in particular, hooked Jake. And though he wasn’t aware of it at the time, nicotine packed into the pods may have kept him from quitting: The average nicotine concentration in e-cigarettes more than doubled from 2013 to 2018, according to a study by the Truth Initiative and the CDC.
The immediate risks of nicotine on the developing brain are well documented. Studies suggest that nicotine – which is found in ENDS products – may affect adolescents’ ability to learn, remember, and maintain attention.
But many adolescents and young adults who use e-cigarettes say that vaping helps alleviate anxiety and keep them attentive, which adds to the complexity of their dependency, according to Dr. Boykan.
Nicotine “actually interrupts neural circuits, that it can be associated with more anxiety, depression, attention to learning, and susceptibility to other addictive substances,” she said. “That is enough to make it very scary.”
Jake also said a social environment in which so many of his friends vaped also made it difficult for him to quit.
“You’re hanging out with your friends at night, and all of them are using it, and you’re trying not to,” he said.
Jake eventually took a semester off from college for an unrelated surgery. He moved home, away from his vaping classmates. He eventually transferred to a different college and lived at home, where no one vaped and where he wasn’t allowed to smoke in the house, he said.
“He came home and we took him to a doctor, and they didn’t know quite how to handle kids and addiction to e-cigarettes,” Mrs. Warn said.
Not fully understanding the long-term health implications of e-cigarette use has precluded many clinicians from offering clear messaging on the risk of vaping to current and potential users.
“It’s taken pediatricians time to ask the right questions and recognize nicotine addiction” from vaping, said Dr. Boykan, who serves as chair of the Section on Nicotine and Tobacco Prevention and Treatment of the American Academy of Pediatrics. “It’s just hit us so fast.”
But once pediatricians do identify a nicotine dependency, it can be difficult to treat, Dr. Boykan said. Many pediatricians now recognize that e-cigarette addiction may occur in children as early as middle school.
“We don’t have a lot of evidence-based treatments for kids to recommend,” Dr. Boykan said.
Will vaping be a ‘phase?’
Aware of his vaping dependency and the possible risks to his long-term health, Jake, now 23, said he’s lessened his use, compared with his college days, but still struggles to kick the habit for good.
“I’d like to not be able to use all the time, not to feel the urge,” Jake said. “But I think over time it’ll just kind of phase out.”
But his mother said quitting may not be that simple.
“This will be a lifelong journey,” she said. “When I think of who he is, addiction is something he will always have. It’s a part of him now.”
Dr. Boykan, Ms. Schneider, and Dr. Nardis reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Teen girls are in crisis: A call to action resulting from 2021 CDC data
Case: “Where’s my mommy?”
A 13-year-old girl “D” appeared lifeless in her hospital bed, swallowed by tubes, gauze, and crisp white sheets. She seemed fragile next to the giant machines beeping all around her, as they churned and groaned to keep her alive. She was in the pediatric intensive care unit, a place she had only seen once or twice on TV. Her sleeping mother lay next to her in an uncomfortable-looking recliner chair, curled up in a ball. She abruptly woke up when I walked into the room, doing her best to wipe away 5 days’ worth of worry and sadness from her exhausted face. She saw “Child Psychiatrist” written on my hospital badge, desperately searching my face for answers or a sign of hope.
Her daughter – a straight-A middle school student who loved Taylor Swift and soccer – had overdosed on Tylenol after discovering that she did not make the cheerleading team. I reported that her daughter’s liver enzymes were finally trending down and that she would likely not require a liver transplant. She would survive. As tears welled up in this mother’s eyes, I heard a faint whisper from across the room. “Where’s my mommy?” D was awake and frantically searching the room for her mother, someone who could soothe her in this living nightmare. As the two embraced, I felt tears well up in my eyes as I couldn’t help but think of my own 3-year-old daughter at home. How could I protect her from the sadness and despair that this little girl was feeling? How can we collectively protect every little girl from wanting to end their life?
CDC data: Teen girls need help now
The latest biennial Centers for Disease Control and Prevention Youth Risk Behavior Survey, administered in the fall of 2021, resulted in alarming data showing that mental health has worsened for all adolescents, but especially for girls. The survey was administered to more than 17,000 students in 152 public and private schools throughout the United States, showing that “America’s teen girls are engulfed in a growing wave of sadness, violence, and trauma.”1 In particular, rates of sadness, suicidal ideation, suicide attempts, and mental health crisis ED visits among girls are the highest reported in a decade. Nearly 60% of girls felt persistent sadness or hopelessness during the past year, double the rate of boys. More than 25% of girls made a suicide plan; this percentage increased 60% over the past 10 years. Alarmingly, ED visits for suicide attempts for girls increased more than 50% in the past 2 years alone.
Even before the COVID-19 pandemic, experts were sounding the alarm on the growing rates of anxiety and depression in U.S. youth. The pandemic-driven isolation, lack of social connection, and missing of major milestones did not help the situation and only deepened the cracks in a faulty mental health care system. Further, civil unrest and social upheaval in the United States felt – and continues to feel – chaotic and unpredictable. For teens, the current cultural climate represents their not-too-distant future as adults, causing worry and anxiety.
In addition to securing their futures through performance in school and extracurricular activities, teenagers are forming their identities. Establishing a personal identity is a difficult task for all teens, though teenage girls face uniquely difficult challenges in our current society. In particular, teenage girls are expected to conform their behaviors to fit societal expectations that may clash with their desires and self-conceptualization. This conflict is further complicated by heightened beauty standards, online hate and competition, academic pressure, and self-doubt. CDC data show that girls experience sexual harassment and cyberbullying at roughly twice the rate of their male counterparts. Girls also experience higher levels of sexual violence and bullying. Alarmingly, 14% of girls reported being forced to have sex at some point in their lives. The sad truth is that, for every 10 teenage girls you know, at least one of them, and probably more, has likely been raped.
A call to action for providers
As providers, what can we do about these alarming statistics? It’s easy to become overwhelmed by data on a national level. However, regardless of our current clinical practice situation, we cannot lose sight of the humanity behind these numbers. Five extra minutes of truly listening to our patients, normalizing conversations about mental health, and looking for mental health warning signs (that is, increased isolation, declining function in school, maladaptive coping skills such as self-injurious behavior or substance use) can mean the difference between life and death.
As pediatric providers, formally screening for suicide risk is critical. Specifically, the American Academy of Pediatrics recommends that all youth aged 12 years or older be screened for suicide risk.2 In addition to asking families to reduce access to lethal means, it is important to utilize suicide-specific screeners to prevent suicide attempts and deaths in the pediatric community. Pediatric providers must feel prepared to counsel patients and families on suicide prevention and, if this skill set is underdeveloped, appropriate referrals and support must be provided.
At the same time, it is important to note the larger context. This national tragedy has been long-standing and further accelerated by the social isolation and stress of the pandemic. Madigan and colleagues recently showed that the lack of a social outlet resulting from COVID-19 caused an increase in screen time among all children.3 As a result, many teen girls turned to social media to recreate these social connections online.4 This dependence on social media for validation has contributed to increased rates of depression by intensifying unrealistic body standards, comparisons, and competition among peers.5 However, recent pediatric partnership programs have improved mental health access, reduced ED visits, and increased primary care physician’s comfort with managing mental health concerns.6 These programs are called Child Psychiatry Access Programs (CPAPs) and utilize a collaborative care model through which primary care clinicians consult with child and adolescent psychiatrists. CPAPs, while not the entire solution, offer a major step in the right direction toward tackling this mental health crisis in a sustainable, collaborative, and effective way.
As students return to in-person learning, connectedness at school is a powerful protective factor against depression and anxiety. We must infuse resources and support into our schools and teachers, as they stand on the front lines for our children. Specifically, bolstering schools with school counselors and appropriate mental health support staff will help rescue teachers from burnout while also explicitly identifying mental health care as a priority. Finally, modeling positive behavior for families and identifying safe adults at school can help at-risk youth feel more connected. To achieve meaningful improvement in children’s mental health, it is crucial to collaboratively remodel broken systems to ensure that all children are supported early, effectively, and equitably.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences, program director of the child and adolescent psychiatry fellowship, and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior in Los Angeles.
References
1. Centers for Disease Control and Prevention. YRBSS Data Summary & Trends. 2023 Feb 13. https://www.cdc.gov/healthyyouth/data/yrbs/yrbs_data_summary_and_trends.htm
2. American Academy of Pediatrics. Screening for Suicide Risk in Clinical Practice. 2023 Feb 22. https://www.aap.org/en/patient-care/blueprint-for-youth-suicide-prevention/strategies-for-clinical-settings-for-youth-suicide-prevention/screening-for-suicide-risk-in-clinical-practice/
3. Madigan S et al. JAMA Pediatr. 2022;176(12):1188-98. doi: 10.1001/JAMAPEDIATRICS.2022.4116
4. Pew Research Center. Teens, Social Media and Technology 2022. 2022 Aug 10. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
5. Hunt MG et al. J Social Clin Psychology. 2018;37(10):751-68. doi: 10.1521/JSCP.2018.37.10.751
6. Godoy L et al. J Pediatr Health Care. 2022 Dec 16. doi: 10.1016/j.pedhc.2022.11.009.
Case: “Where’s my mommy?”
A 13-year-old girl “D” appeared lifeless in her hospital bed, swallowed by tubes, gauze, and crisp white sheets. She seemed fragile next to the giant machines beeping all around her, as they churned and groaned to keep her alive. She was in the pediatric intensive care unit, a place she had only seen once or twice on TV. Her sleeping mother lay next to her in an uncomfortable-looking recliner chair, curled up in a ball. She abruptly woke up when I walked into the room, doing her best to wipe away 5 days’ worth of worry and sadness from her exhausted face. She saw “Child Psychiatrist” written on my hospital badge, desperately searching my face for answers or a sign of hope.
Her daughter – a straight-A middle school student who loved Taylor Swift and soccer – had overdosed on Tylenol after discovering that she did not make the cheerleading team. I reported that her daughter’s liver enzymes were finally trending down and that she would likely not require a liver transplant. She would survive. As tears welled up in this mother’s eyes, I heard a faint whisper from across the room. “Where’s my mommy?” D was awake and frantically searching the room for her mother, someone who could soothe her in this living nightmare. As the two embraced, I felt tears well up in my eyes as I couldn’t help but think of my own 3-year-old daughter at home. How could I protect her from the sadness and despair that this little girl was feeling? How can we collectively protect every little girl from wanting to end their life?
CDC data: Teen girls need help now
The latest biennial Centers for Disease Control and Prevention Youth Risk Behavior Survey, administered in the fall of 2021, resulted in alarming data showing that mental health has worsened for all adolescents, but especially for girls. The survey was administered to more than 17,000 students in 152 public and private schools throughout the United States, showing that “America’s teen girls are engulfed in a growing wave of sadness, violence, and trauma.”1 In particular, rates of sadness, suicidal ideation, suicide attempts, and mental health crisis ED visits among girls are the highest reported in a decade. Nearly 60% of girls felt persistent sadness or hopelessness during the past year, double the rate of boys. More than 25% of girls made a suicide plan; this percentage increased 60% over the past 10 years. Alarmingly, ED visits for suicide attempts for girls increased more than 50% in the past 2 years alone.
Even before the COVID-19 pandemic, experts were sounding the alarm on the growing rates of anxiety and depression in U.S. youth. The pandemic-driven isolation, lack of social connection, and missing of major milestones did not help the situation and only deepened the cracks in a faulty mental health care system. Further, civil unrest and social upheaval in the United States felt – and continues to feel – chaotic and unpredictable. For teens, the current cultural climate represents their not-too-distant future as adults, causing worry and anxiety.
In addition to securing their futures through performance in school and extracurricular activities, teenagers are forming their identities. Establishing a personal identity is a difficult task for all teens, though teenage girls face uniquely difficult challenges in our current society. In particular, teenage girls are expected to conform their behaviors to fit societal expectations that may clash with their desires and self-conceptualization. This conflict is further complicated by heightened beauty standards, online hate and competition, academic pressure, and self-doubt. CDC data show that girls experience sexual harassment and cyberbullying at roughly twice the rate of their male counterparts. Girls also experience higher levels of sexual violence and bullying. Alarmingly, 14% of girls reported being forced to have sex at some point in their lives. The sad truth is that, for every 10 teenage girls you know, at least one of them, and probably more, has likely been raped.
A call to action for providers
As providers, what can we do about these alarming statistics? It’s easy to become overwhelmed by data on a national level. However, regardless of our current clinical practice situation, we cannot lose sight of the humanity behind these numbers. Five extra minutes of truly listening to our patients, normalizing conversations about mental health, and looking for mental health warning signs (that is, increased isolation, declining function in school, maladaptive coping skills such as self-injurious behavior or substance use) can mean the difference between life and death.
As pediatric providers, formally screening for suicide risk is critical. Specifically, the American Academy of Pediatrics recommends that all youth aged 12 years or older be screened for suicide risk.2 In addition to asking families to reduce access to lethal means, it is important to utilize suicide-specific screeners to prevent suicide attempts and deaths in the pediatric community. Pediatric providers must feel prepared to counsel patients and families on suicide prevention and, if this skill set is underdeveloped, appropriate referrals and support must be provided.
At the same time, it is important to note the larger context. This national tragedy has been long-standing and further accelerated by the social isolation and stress of the pandemic. Madigan and colleagues recently showed that the lack of a social outlet resulting from COVID-19 caused an increase in screen time among all children.3 As a result, many teen girls turned to social media to recreate these social connections online.4 This dependence on social media for validation has contributed to increased rates of depression by intensifying unrealistic body standards, comparisons, and competition among peers.5 However, recent pediatric partnership programs have improved mental health access, reduced ED visits, and increased primary care physician’s comfort with managing mental health concerns.6 These programs are called Child Psychiatry Access Programs (CPAPs) and utilize a collaborative care model through which primary care clinicians consult with child and adolescent psychiatrists. CPAPs, while not the entire solution, offer a major step in the right direction toward tackling this mental health crisis in a sustainable, collaborative, and effective way.
As students return to in-person learning, connectedness at school is a powerful protective factor against depression and anxiety. We must infuse resources and support into our schools and teachers, as they stand on the front lines for our children. Specifically, bolstering schools with school counselors and appropriate mental health support staff will help rescue teachers from burnout while also explicitly identifying mental health care as a priority. Finally, modeling positive behavior for families and identifying safe adults at school can help at-risk youth feel more connected. To achieve meaningful improvement in children’s mental health, it is crucial to collaboratively remodel broken systems to ensure that all children are supported early, effectively, and equitably.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences, program director of the child and adolescent psychiatry fellowship, and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior in Los Angeles.
References
1. Centers for Disease Control and Prevention. YRBSS Data Summary & Trends. 2023 Feb 13. https://www.cdc.gov/healthyyouth/data/yrbs/yrbs_data_summary_and_trends.htm
2. American Academy of Pediatrics. Screening for Suicide Risk in Clinical Practice. 2023 Feb 22. https://www.aap.org/en/patient-care/blueprint-for-youth-suicide-prevention/strategies-for-clinical-settings-for-youth-suicide-prevention/screening-for-suicide-risk-in-clinical-practice/
3. Madigan S et al. JAMA Pediatr. 2022;176(12):1188-98. doi: 10.1001/JAMAPEDIATRICS.2022.4116
4. Pew Research Center. Teens, Social Media and Technology 2022. 2022 Aug 10. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
5. Hunt MG et al. J Social Clin Psychology. 2018;37(10):751-68. doi: 10.1521/JSCP.2018.37.10.751
6. Godoy L et al. J Pediatr Health Care. 2022 Dec 16. doi: 10.1016/j.pedhc.2022.11.009.
Case: “Where’s my mommy?”
A 13-year-old girl “D” appeared lifeless in her hospital bed, swallowed by tubes, gauze, and crisp white sheets. She seemed fragile next to the giant machines beeping all around her, as they churned and groaned to keep her alive. She was in the pediatric intensive care unit, a place she had only seen once or twice on TV. Her sleeping mother lay next to her in an uncomfortable-looking recliner chair, curled up in a ball. She abruptly woke up when I walked into the room, doing her best to wipe away 5 days’ worth of worry and sadness from her exhausted face. She saw “Child Psychiatrist” written on my hospital badge, desperately searching my face for answers or a sign of hope.
Her daughter – a straight-A middle school student who loved Taylor Swift and soccer – had overdosed on Tylenol after discovering that she did not make the cheerleading team. I reported that her daughter’s liver enzymes were finally trending down and that she would likely not require a liver transplant. She would survive. As tears welled up in this mother’s eyes, I heard a faint whisper from across the room. “Where’s my mommy?” D was awake and frantically searching the room for her mother, someone who could soothe her in this living nightmare. As the two embraced, I felt tears well up in my eyes as I couldn’t help but think of my own 3-year-old daughter at home. How could I protect her from the sadness and despair that this little girl was feeling? How can we collectively protect every little girl from wanting to end their life?
CDC data: Teen girls need help now
The latest biennial Centers for Disease Control and Prevention Youth Risk Behavior Survey, administered in the fall of 2021, resulted in alarming data showing that mental health has worsened for all adolescents, but especially for girls. The survey was administered to more than 17,000 students in 152 public and private schools throughout the United States, showing that “America’s teen girls are engulfed in a growing wave of sadness, violence, and trauma.”1 In particular, rates of sadness, suicidal ideation, suicide attempts, and mental health crisis ED visits among girls are the highest reported in a decade. Nearly 60% of girls felt persistent sadness or hopelessness during the past year, double the rate of boys. More than 25% of girls made a suicide plan; this percentage increased 60% over the past 10 years. Alarmingly, ED visits for suicide attempts for girls increased more than 50% in the past 2 years alone.
Even before the COVID-19 pandemic, experts were sounding the alarm on the growing rates of anxiety and depression in U.S. youth. The pandemic-driven isolation, lack of social connection, and missing of major milestones did not help the situation and only deepened the cracks in a faulty mental health care system. Further, civil unrest and social upheaval in the United States felt – and continues to feel – chaotic and unpredictable. For teens, the current cultural climate represents their not-too-distant future as adults, causing worry and anxiety.
In addition to securing their futures through performance in school and extracurricular activities, teenagers are forming their identities. Establishing a personal identity is a difficult task for all teens, though teenage girls face uniquely difficult challenges in our current society. In particular, teenage girls are expected to conform their behaviors to fit societal expectations that may clash with their desires and self-conceptualization. This conflict is further complicated by heightened beauty standards, online hate and competition, academic pressure, and self-doubt. CDC data show that girls experience sexual harassment and cyberbullying at roughly twice the rate of their male counterparts. Girls also experience higher levels of sexual violence and bullying. Alarmingly, 14% of girls reported being forced to have sex at some point in their lives. The sad truth is that, for every 10 teenage girls you know, at least one of them, and probably more, has likely been raped.
A call to action for providers
As providers, what can we do about these alarming statistics? It’s easy to become overwhelmed by data on a national level. However, regardless of our current clinical practice situation, we cannot lose sight of the humanity behind these numbers. Five extra minutes of truly listening to our patients, normalizing conversations about mental health, and looking for mental health warning signs (that is, increased isolation, declining function in school, maladaptive coping skills such as self-injurious behavior or substance use) can mean the difference between life and death.
As pediatric providers, formally screening for suicide risk is critical. Specifically, the American Academy of Pediatrics recommends that all youth aged 12 years or older be screened for suicide risk.2 In addition to asking families to reduce access to lethal means, it is important to utilize suicide-specific screeners to prevent suicide attempts and deaths in the pediatric community. Pediatric providers must feel prepared to counsel patients and families on suicide prevention and, if this skill set is underdeveloped, appropriate referrals and support must be provided.
At the same time, it is important to note the larger context. This national tragedy has been long-standing and further accelerated by the social isolation and stress of the pandemic. Madigan and colleagues recently showed that the lack of a social outlet resulting from COVID-19 caused an increase in screen time among all children.3 As a result, many teen girls turned to social media to recreate these social connections online.4 This dependence on social media for validation has contributed to increased rates of depression by intensifying unrealistic body standards, comparisons, and competition among peers.5 However, recent pediatric partnership programs have improved mental health access, reduced ED visits, and increased primary care physician’s comfort with managing mental health concerns.6 These programs are called Child Psychiatry Access Programs (CPAPs) and utilize a collaborative care model through which primary care clinicians consult with child and adolescent psychiatrists. CPAPs, while not the entire solution, offer a major step in the right direction toward tackling this mental health crisis in a sustainable, collaborative, and effective way.
As students return to in-person learning, connectedness at school is a powerful protective factor against depression and anxiety. We must infuse resources and support into our schools and teachers, as they stand on the front lines for our children. Specifically, bolstering schools with school counselors and appropriate mental health support staff will help rescue teachers from burnout while also explicitly identifying mental health care as a priority. Finally, modeling positive behavior for families and identifying safe adults at school can help at-risk youth feel more connected. To achieve meaningful improvement in children’s mental health, it is crucial to collaboratively remodel broken systems to ensure that all children are supported early, effectively, and equitably.
Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences, program director of the child and adolescent psychiatry fellowship, and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior in Los Angeles.
References
1. Centers for Disease Control and Prevention. YRBSS Data Summary & Trends. 2023 Feb 13. https://www.cdc.gov/healthyyouth/data/yrbs/yrbs_data_summary_and_trends.htm
2. American Academy of Pediatrics. Screening for Suicide Risk in Clinical Practice. 2023 Feb 22. https://www.aap.org/en/patient-care/blueprint-for-youth-suicide-prevention/strategies-for-clinical-settings-for-youth-suicide-prevention/screening-for-suicide-risk-in-clinical-practice/
3. Madigan S et al. JAMA Pediatr. 2022;176(12):1188-98. doi: 10.1001/JAMAPEDIATRICS.2022.4116
4. Pew Research Center. Teens, Social Media and Technology 2022. 2022 Aug 10. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
5. Hunt MG et al. J Social Clin Psychology. 2018;37(10):751-68. doi: 10.1521/JSCP.2018.37.10.751
6. Godoy L et al. J Pediatr Health Care. 2022 Dec 16. doi: 10.1016/j.pedhc.2022.11.009.
Answers sought for mental health challenges in pediatric rheumatology patients
NEW ORLEANS – Pediatric patients with rheumatologic diseases experience a particularly high prevalence of psychological distress and depression and anxiety symptoms, according to research presented at the Pediatric Rheumatology Symposium. Although this finding is not necessarily surprising, the extent to which depression and psychological distress impacts these young patients’ quality of life has led to greater research and innovation in seeking ways to identify, address, and treat depression and anxiety in children and adolescents with diseases such as juvenile idiopathic arthritis (JIA) or systemic lupus erythematosus (SLE).
Accordingly, other studies presented at the conference examined more efficient ways to screen adolescent patients for depression and assessed programs designed to improve symptoms. In fact, the American College of Rheumatology award for the top Quality, Health Services, and Education Research abstract at this year’s symposium went to Lauren Harper, MD, a pediatric rheumatology fellow at Nationwide Children’s Hospital, Columbus, whose research examined the effects of automating depression screening during check-in for adolescent patients with SLE. Her findings revealed that automation of screening increased detection of depression and suicidality, thereby increasing interventions and ultimately resulting in a reduction in depression prevalence.
“The key clinical takeaway is that mental health screening is really important – it affects our patients in so many different ways – and it’s very doable in your rheumatology clinic,” Dr. Harper said in an interview. “It’s also important because they’re coming to us very frequently, but they don’t see their PCP [primary care provider] very often, so we can’t leave screening to the PCPs.”
Two other studies assessed the effectiveness of a 6-week cognitive-behavioral intervention for youth called Treatment and Education Approach for Childhood-Onset Lupus (TEACH). One study found that remote delivery of TEACH resulted in improved mood symptoms and reduced fatigue, and another found the program particularly effective in improving mood for patients deemed “high risk” because of greater depression and fatigue symptoms.
The impact of growing mental health problems has been enormous both in the pediatric rheumatology population and society at large, Daria Sosna, MSc, a research coordinator at the University of Calgary (Alta.), said in an interview as she visited the research posters related to psychological stress and depression.
“We need to do something,” said Ms. Sosna, whose department is currently applying for funding to develop a research project to improve mental health outcomes in adolescents with lupus. “This population, specifically, has higher numbers than anyone else does because they have chronic illness” – and those issues need to be addressed.
High psychological stress levels
The study looking at psychological stress in pediatric rheumatology patients, led by Natalie Rosenwasser, MD, of Seattle Children’s Hospital, relied on cross-sectional data from patients enrolled in two Childhood Arthritis and Rheumatology Research Alliance sites, one in Utah and one in Seattle. The average age of the 71 patients who completed the surveys was 13, and the researchers reported the findings in two separate age groups: those aged 13-17, who completed the surveys themselves, and those aged 8-12, whose parents completed the surveys. Nearly all the patients (94.4%) had JIA, but one had lupus and three had juvenile dermatomyositis.
The participants completed the Patient-Reported Outcomes Measurement Information System (PROMIS) for psychological stress, physical stress, and depressive symptoms. They also filled out the National Institutes of Health–Toolbox Perceived Stress survey, the 9-item Patient Health Questionnaire (PHQ-9), the Screen for Child Anxiety Related Disorders (SCARED), a visual analog scale for COVID-related distress, and a questionnaire asking about how receptive they were to mental health screening. The researchers determined that a score 1 standard deviation above the mean on the PROMIS and NIH-Toolbox assessments qualified as a high level of psychological stress.
“There are data that suggest that psychological stress can be a precursor to depression and anxiety, which raises the concern that not every patient who’s experiencing mental health symptoms is going to be picked up on traditional measures that meet that clinical threshold, but they may really need interventions to protect their mental health,” presenter Erin Treemarcki, DO, an assistant professor of pediatric rheumatology at the University of Utah, Salt Lake City, said in an interview. “Not every patient may necessarily need referral to a mental health specialist, but there are still potential interventions that we can do in the clinical setting to address mental health, which in turn can improve outcomes, including medication compliance and knowing how patients are feeling.”
More than one-third of the patients (39%) reported a high level of psychological stress, and 43% had elevated physical stress. Broken down by age, 26% of the teens and 15% of the younger patients reported high levels of perceived stress. The PROMIS only identified increased depressive symptoms in 26% of the participants, whereas more than half (54%) had a positive PHQ-9 depression screen. Furthermore, half the patients had SCARED scores (50%) that likely indicated anxiety disorder. Only 6% of patients reported severe stress specifically related to the pandemic, but most reported mild distress from the pandemic.
“Psychological stress was highly correlated with physical stress, perceived stress, depressive symptoms [PROMIS and PHQ-9], and anxiety,” the authors reported (P < .05). The authors next plan to expand their assessment to a third CARRA site and then explore the interaction between psychological distress and sociodemographic factors.
“There’s such an increase in mental health disorders right now, and we’re overwhelmed in general,” Ms. Sosna said in an interview. “There have to be interventions that approach this. We can use pharmacological approaches, we can use CBT, we can use a lot of these things that are very well established, and they’re absolutely fantastic, but we don’t necessarily have the resources or capabilities to do that all the time.”
Benefits of automated depression screening
To reduce the likelihood of depression screenings falling through the cracks during visits, Dr. Harper’s study assessed the impact of automating screens in an adolescent population. In her presentation, she noted previous research finding that nearly half of youth with lupus (47%) had depression, compared with 24% of adults with lupus. Pediatric patients have nearly three times the odds of depression and more than five times the odds of suicidal ideation, she told attendees. These mood disorders are correlated with greater physical disability, higher cardiovascular risk, more disease activity, higher risk of premature death, and decreased educational attainment, medication compliance, and quality of life.
Despite recommendations for depression screening from the U.S. Preventive Services Task Force and the American Academy of Pediatrics, only 2% of pediatric rheumatology patients are routinely screened for depression with a validated instrument, and only 7% of those with depressive symptoms are screened, according to a 2016 study that Dr. Harper cited. Yet the same study found that nearly all pediatric rheumatologists (95%) supported routine depression screening every 6-12 months. Hence her team’s decision to test whether automating screening improved their screening rates.
Their population included lupus patients aged 12 and older seen at Nationwide Children’s Hospital between 2014 and 2022. Initially, patients completed the PHQ-9 on paper, which was then transcribed into the electronic health record. The process became automated and administered on an iPad at every visit in 2022. Positive screens – those endorsing suicidality or with a score of at least 10 – caused an alert to pop up for clinicians during their workflow so that they would talk to the mental health team about the patient’s needs.
A total of 149 patients completed 529 screenings during the study’s 8 years. Only 1 patient completed a PHQ-9 in 2014, which increased to just 17 patients in 2017. Automation resulted in 225 screens (P < .01). Subsequently, positive screens increased from 0% in 2014 to 25%-30% in 2018-2021, but then fell to 12% in 2022 (P < .01). The median PHQ-9 score was 3; overall scores decreased as screening increased.
The overall incidence of positive screens during the study period was 20% and prevalence was 38%, the authors reported. Of the 10 automated alerts triggered by positive screens, 90% resulted in a meeting with a psychologist or social worker, and 90% completed a suicide risk assessment. The intrusive alert for clinicians requires them to acknowledge the alert, agreeing to initiate a risk assessment, before they can enter data into the patient’s chart.
The study findings reveal “that you can successfully screen a high-risk population using an automated, seamless process, and you can alert providers without too much disruption to their typical clinic flow,” Dr. Harper told attendees. “And all of these processes have led to sustainability for routine depression screening in our lupus clinic.”
Dr. Harper’s team next plans to expand the automated screenings to populations with other diseases, to add an automated screening for anxiety, and to explore how PHQ-9 scores correlate with disease activity.
Treating patients’ mental health
Another two other abstracts at the symposium looked at another option, the 6-week cognitive-behavioral TEACH program. Deborah Levy, MD, MS, an associate professor of pediatrics at the University of Toronto and the clinical director of rheumatology at The Hospital for Sick Children, and colleagues assessed the program’s success when delivered remotely to adolescent patients with lupus. Pilot testing with TEACH had already shown improvements in fatigue and mood, Dr. Levy told attendees, but barriers to in-person delivery limited its utility even before the pandemic, so this study aimed to determine a remote version’s feasibility and effects, compared with treatment as usual.
The randomized, controlled trial, led by Natoshia Cunningham, PhD, from Michigan State University, Grand Rapids, included 57 participants, aged 12-22, from seven U.S. and Canadian rheumatology sites. All had been diagnosed with childhood-onset SLE by age 18 and had elevated symptoms in fatigue, pain, or depression. A PROMIS Fatigue T score of 60 or greater indicated elevated fatigue scores, whereas a high pain score was at least a 3/10 on a visual analog scale, and a high depression T score was at least a 60 but not higher than 80 on the Children’s Depression Inventory–2 or the Beck Depression Inventory–II (depending on the patient’s age).
Patients with other chronic medical conditions, developmental delays, or untreated major psychiatric illness were excluded from the study, as were patients who were receiving overlapping treatment, such as cognitive-behavioral therapy for pain or mood. Thirty patients were randomly assigned to receive treatment as usual while 27 patients were assigned to participate in the remote TEACH program.
Nearly all the patients (94%) were female, but they were racially diverse, with 42% White, 28% Asian, 19% Black, 19% Hispanic, and 4% multiracial. The patients were an average 16 years old and had been diagnosed for a median 5 years. Three of the intervention’s six modules involved the caregivers or, for older patients, their partners if desired. The communication strategies taught in the program were also tailored to patients’ ages.
“All of these strategies are educational, cognitive, behavioral, mindfulness strategies that target fatigue [and] pain, and they also developed web content for participants to use on their own,” Dr. Levy told attendees.
The researchers had complete postassessment data from 88% of participants, but they also reported some of the statements made during qualitative interviews about the program’s feasibility.
“I think it makes people more aware of themselves to become a better version of themselves, whether that’s in their normal life or in handling a lupus kind of life,” one participant said about the program’s benefits. Another appreciated the “alternative ways of thinking,” including “being more mindful of my thoughts and how those kind of aggravate my stress.”
The quantitative findings revealed a statistically significant reduction in depressive symptoms and fatigue for TEACH participants, compared with treatment as usual. Mood scores fell by an average 13.7 points in the TEACH group, compared with a drop of 2.4 points in the treatment as usual group (P < .001). Scores for fatigue fell 9.16 points in the TEACH group and 2.93 in the control group (P = .003). No statistically significant difference showed up in pain scores between the groups, although pain, medication adherence, and disease activity did improve slightly more in the TEACH group.
In addition to the significant improvements in mood and fatigue, therefore, “completion of TEACH may be associated with improved medication adherence and disease activity versus treatment as usual,” Dr. Levy said.
A much smaller study authored by some of the same researchers also assessed TEACH’s impact not in remote form but in terms of its value specifically for adolescent patients with SLE and elevated depression and fatigue scores. Comparison of 6 high-risk patients with 10 low-risk patients who underwent TEACH suggested that the program was especially effective for improving depression in high-risk patients since these patients had a statistically significantly greater improvement in mood. Fatigue, pain, anxiety, quality of life, and disease activity scores did not statistically differ between the groups.
Authors of the automated depression screening study reported no disclosures or outside funding. The study assessing psychological distress was funded by a CARRA–Arthritis Foundation grant, and the authors reported no disclosures. The remote TEACH study was funded by a CARRA–Arthritis Foundation grant, and all but one author reported no disclosures. One author had disclosures with Janssen, Roche, and Sobi. The high-risk TEACH study was also funded by a CARRA grant, and the authors had no disclosures.
NEW ORLEANS – Pediatric patients with rheumatologic diseases experience a particularly high prevalence of psychological distress and depression and anxiety symptoms, according to research presented at the Pediatric Rheumatology Symposium. Although this finding is not necessarily surprising, the extent to which depression and psychological distress impacts these young patients’ quality of life has led to greater research and innovation in seeking ways to identify, address, and treat depression and anxiety in children and adolescents with diseases such as juvenile idiopathic arthritis (JIA) or systemic lupus erythematosus (SLE).
Accordingly, other studies presented at the conference examined more efficient ways to screen adolescent patients for depression and assessed programs designed to improve symptoms. In fact, the American College of Rheumatology award for the top Quality, Health Services, and Education Research abstract at this year’s symposium went to Lauren Harper, MD, a pediatric rheumatology fellow at Nationwide Children’s Hospital, Columbus, whose research examined the effects of automating depression screening during check-in for adolescent patients with SLE. Her findings revealed that automation of screening increased detection of depression and suicidality, thereby increasing interventions and ultimately resulting in a reduction in depression prevalence.
“The key clinical takeaway is that mental health screening is really important – it affects our patients in so many different ways – and it’s very doable in your rheumatology clinic,” Dr. Harper said in an interview. “It’s also important because they’re coming to us very frequently, but they don’t see their PCP [primary care provider] very often, so we can’t leave screening to the PCPs.”
Two other studies assessed the effectiveness of a 6-week cognitive-behavioral intervention for youth called Treatment and Education Approach for Childhood-Onset Lupus (TEACH). One study found that remote delivery of TEACH resulted in improved mood symptoms and reduced fatigue, and another found the program particularly effective in improving mood for patients deemed “high risk” because of greater depression and fatigue symptoms.
The impact of growing mental health problems has been enormous both in the pediatric rheumatology population and society at large, Daria Sosna, MSc, a research coordinator at the University of Calgary (Alta.), said in an interview as she visited the research posters related to psychological stress and depression.
“We need to do something,” said Ms. Sosna, whose department is currently applying for funding to develop a research project to improve mental health outcomes in adolescents with lupus. “This population, specifically, has higher numbers than anyone else does because they have chronic illness” – and those issues need to be addressed.
High psychological stress levels
The study looking at psychological stress in pediatric rheumatology patients, led by Natalie Rosenwasser, MD, of Seattle Children’s Hospital, relied on cross-sectional data from patients enrolled in two Childhood Arthritis and Rheumatology Research Alliance sites, one in Utah and one in Seattle. The average age of the 71 patients who completed the surveys was 13, and the researchers reported the findings in two separate age groups: those aged 13-17, who completed the surveys themselves, and those aged 8-12, whose parents completed the surveys. Nearly all the patients (94.4%) had JIA, but one had lupus and three had juvenile dermatomyositis.
The participants completed the Patient-Reported Outcomes Measurement Information System (PROMIS) for psychological stress, physical stress, and depressive symptoms. They also filled out the National Institutes of Health–Toolbox Perceived Stress survey, the 9-item Patient Health Questionnaire (PHQ-9), the Screen for Child Anxiety Related Disorders (SCARED), a visual analog scale for COVID-related distress, and a questionnaire asking about how receptive they were to mental health screening. The researchers determined that a score 1 standard deviation above the mean on the PROMIS and NIH-Toolbox assessments qualified as a high level of psychological stress.
“There are data that suggest that psychological stress can be a precursor to depression and anxiety, which raises the concern that not every patient who’s experiencing mental health symptoms is going to be picked up on traditional measures that meet that clinical threshold, but they may really need interventions to protect their mental health,” presenter Erin Treemarcki, DO, an assistant professor of pediatric rheumatology at the University of Utah, Salt Lake City, said in an interview. “Not every patient may necessarily need referral to a mental health specialist, but there are still potential interventions that we can do in the clinical setting to address mental health, which in turn can improve outcomes, including medication compliance and knowing how patients are feeling.”
More than one-third of the patients (39%) reported a high level of psychological stress, and 43% had elevated physical stress. Broken down by age, 26% of the teens and 15% of the younger patients reported high levels of perceived stress. The PROMIS only identified increased depressive symptoms in 26% of the participants, whereas more than half (54%) had a positive PHQ-9 depression screen. Furthermore, half the patients had SCARED scores (50%) that likely indicated anxiety disorder. Only 6% of patients reported severe stress specifically related to the pandemic, but most reported mild distress from the pandemic.
“Psychological stress was highly correlated with physical stress, perceived stress, depressive symptoms [PROMIS and PHQ-9], and anxiety,” the authors reported (P < .05). The authors next plan to expand their assessment to a third CARRA site and then explore the interaction between psychological distress and sociodemographic factors.
“There’s such an increase in mental health disorders right now, and we’re overwhelmed in general,” Ms. Sosna said in an interview. “There have to be interventions that approach this. We can use pharmacological approaches, we can use CBT, we can use a lot of these things that are very well established, and they’re absolutely fantastic, but we don’t necessarily have the resources or capabilities to do that all the time.”
Benefits of automated depression screening
To reduce the likelihood of depression screenings falling through the cracks during visits, Dr. Harper’s study assessed the impact of automating screens in an adolescent population. In her presentation, she noted previous research finding that nearly half of youth with lupus (47%) had depression, compared with 24% of adults with lupus. Pediatric patients have nearly three times the odds of depression and more than five times the odds of suicidal ideation, she told attendees. These mood disorders are correlated with greater physical disability, higher cardiovascular risk, more disease activity, higher risk of premature death, and decreased educational attainment, medication compliance, and quality of life.
Despite recommendations for depression screening from the U.S. Preventive Services Task Force and the American Academy of Pediatrics, only 2% of pediatric rheumatology patients are routinely screened for depression with a validated instrument, and only 7% of those with depressive symptoms are screened, according to a 2016 study that Dr. Harper cited. Yet the same study found that nearly all pediatric rheumatologists (95%) supported routine depression screening every 6-12 months. Hence her team’s decision to test whether automating screening improved their screening rates.
Their population included lupus patients aged 12 and older seen at Nationwide Children’s Hospital between 2014 and 2022. Initially, patients completed the PHQ-9 on paper, which was then transcribed into the electronic health record. The process became automated and administered on an iPad at every visit in 2022. Positive screens – those endorsing suicidality or with a score of at least 10 – caused an alert to pop up for clinicians during their workflow so that they would talk to the mental health team about the patient’s needs.
A total of 149 patients completed 529 screenings during the study’s 8 years. Only 1 patient completed a PHQ-9 in 2014, which increased to just 17 patients in 2017. Automation resulted in 225 screens (P < .01). Subsequently, positive screens increased from 0% in 2014 to 25%-30% in 2018-2021, but then fell to 12% in 2022 (P < .01). The median PHQ-9 score was 3; overall scores decreased as screening increased.
The overall incidence of positive screens during the study period was 20% and prevalence was 38%, the authors reported. Of the 10 automated alerts triggered by positive screens, 90% resulted in a meeting with a psychologist or social worker, and 90% completed a suicide risk assessment. The intrusive alert for clinicians requires them to acknowledge the alert, agreeing to initiate a risk assessment, before they can enter data into the patient’s chart.
The study findings reveal “that you can successfully screen a high-risk population using an automated, seamless process, and you can alert providers without too much disruption to their typical clinic flow,” Dr. Harper told attendees. “And all of these processes have led to sustainability for routine depression screening in our lupus clinic.”
Dr. Harper’s team next plans to expand the automated screenings to populations with other diseases, to add an automated screening for anxiety, and to explore how PHQ-9 scores correlate with disease activity.
Treating patients’ mental health
Another two other abstracts at the symposium looked at another option, the 6-week cognitive-behavioral TEACH program. Deborah Levy, MD, MS, an associate professor of pediatrics at the University of Toronto and the clinical director of rheumatology at The Hospital for Sick Children, and colleagues assessed the program’s success when delivered remotely to adolescent patients with lupus. Pilot testing with TEACH had already shown improvements in fatigue and mood, Dr. Levy told attendees, but barriers to in-person delivery limited its utility even before the pandemic, so this study aimed to determine a remote version’s feasibility and effects, compared with treatment as usual.
The randomized, controlled trial, led by Natoshia Cunningham, PhD, from Michigan State University, Grand Rapids, included 57 participants, aged 12-22, from seven U.S. and Canadian rheumatology sites. All had been diagnosed with childhood-onset SLE by age 18 and had elevated symptoms in fatigue, pain, or depression. A PROMIS Fatigue T score of 60 or greater indicated elevated fatigue scores, whereas a high pain score was at least a 3/10 on a visual analog scale, and a high depression T score was at least a 60 but not higher than 80 on the Children’s Depression Inventory–2 or the Beck Depression Inventory–II (depending on the patient’s age).
Patients with other chronic medical conditions, developmental delays, or untreated major psychiatric illness were excluded from the study, as were patients who were receiving overlapping treatment, such as cognitive-behavioral therapy for pain or mood. Thirty patients were randomly assigned to receive treatment as usual while 27 patients were assigned to participate in the remote TEACH program.
Nearly all the patients (94%) were female, but they were racially diverse, with 42% White, 28% Asian, 19% Black, 19% Hispanic, and 4% multiracial. The patients were an average 16 years old and had been diagnosed for a median 5 years. Three of the intervention’s six modules involved the caregivers or, for older patients, their partners if desired. The communication strategies taught in the program were also tailored to patients’ ages.
“All of these strategies are educational, cognitive, behavioral, mindfulness strategies that target fatigue [and] pain, and they also developed web content for participants to use on their own,” Dr. Levy told attendees.
The researchers had complete postassessment data from 88% of participants, but they also reported some of the statements made during qualitative interviews about the program’s feasibility.
“I think it makes people more aware of themselves to become a better version of themselves, whether that’s in their normal life or in handling a lupus kind of life,” one participant said about the program’s benefits. Another appreciated the “alternative ways of thinking,” including “being more mindful of my thoughts and how those kind of aggravate my stress.”
The quantitative findings revealed a statistically significant reduction in depressive symptoms and fatigue for TEACH participants, compared with treatment as usual. Mood scores fell by an average 13.7 points in the TEACH group, compared with a drop of 2.4 points in the treatment as usual group (P < .001). Scores for fatigue fell 9.16 points in the TEACH group and 2.93 in the control group (P = .003). No statistically significant difference showed up in pain scores between the groups, although pain, medication adherence, and disease activity did improve slightly more in the TEACH group.
In addition to the significant improvements in mood and fatigue, therefore, “completion of TEACH may be associated with improved medication adherence and disease activity versus treatment as usual,” Dr. Levy said.
A much smaller study authored by some of the same researchers also assessed TEACH’s impact not in remote form but in terms of its value specifically for adolescent patients with SLE and elevated depression and fatigue scores. Comparison of 6 high-risk patients with 10 low-risk patients who underwent TEACH suggested that the program was especially effective for improving depression in high-risk patients since these patients had a statistically significantly greater improvement in mood. Fatigue, pain, anxiety, quality of life, and disease activity scores did not statistically differ between the groups.
Authors of the automated depression screening study reported no disclosures or outside funding. The study assessing psychological distress was funded by a CARRA–Arthritis Foundation grant, and the authors reported no disclosures. The remote TEACH study was funded by a CARRA–Arthritis Foundation grant, and all but one author reported no disclosures. One author had disclosures with Janssen, Roche, and Sobi. The high-risk TEACH study was also funded by a CARRA grant, and the authors had no disclosures.
NEW ORLEANS – Pediatric patients with rheumatologic diseases experience a particularly high prevalence of psychological distress and depression and anxiety symptoms, according to research presented at the Pediatric Rheumatology Symposium. Although this finding is not necessarily surprising, the extent to which depression and psychological distress impacts these young patients’ quality of life has led to greater research and innovation in seeking ways to identify, address, and treat depression and anxiety in children and adolescents with diseases such as juvenile idiopathic arthritis (JIA) or systemic lupus erythematosus (SLE).
Accordingly, other studies presented at the conference examined more efficient ways to screen adolescent patients for depression and assessed programs designed to improve symptoms. In fact, the American College of Rheumatology award for the top Quality, Health Services, and Education Research abstract at this year’s symposium went to Lauren Harper, MD, a pediatric rheumatology fellow at Nationwide Children’s Hospital, Columbus, whose research examined the effects of automating depression screening during check-in for adolescent patients with SLE. Her findings revealed that automation of screening increased detection of depression and suicidality, thereby increasing interventions and ultimately resulting in a reduction in depression prevalence.
“The key clinical takeaway is that mental health screening is really important – it affects our patients in so many different ways – and it’s very doable in your rheumatology clinic,” Dr. Harper said in an interview. “It’s also important because they’re coming to us very frequently, but they don’t see their PCP [primary care provider] very often, so we can’t leave screening to the PCPs.”
Two other studies assessed the effectiveness of a 6-week cognitive-behavioral intervention for youth called Treatment and Education Approach for Childhood-Onset Lupus (TEACH). One study found that remote delivery of TEACH resulted in improved mood symptoms and reduced fatigue, and another found the program particularly effective in improving mood for patients deemed “high risk” because of greater depression and fatigue symptoms.
The impact of growing mental health problems has been enormous both in the pediatric rheumatology population and society at large, Daria Sosna, MSc, a research coordinator at the University of Calgary (Alta.), said in an interview as she visited the research posters related to psychological stress and depression.
“We need to do something,” said Ms. Sosna, whose department is currently applying for funding to develop a research project to improve mental health outcomes in adolescents with lupus. “This population, specifically, has higher numbers than anyone else does because they have chronic illness” – and those issues need to be addressed.
High psychological stress levels
The study looking at psychological stress in pediatric rheumatology patients, led by Natalie Rosenwasser, MD, of Seattle Children’s Hospital, relied on cross-sectional data from patients enrolled in two Childhood Arthritis and Rheumatology Research Alliance sites, one in Utah and one in Seattle. The average age of the 71 patients who completed the surveys was 13, and the researchers reported the findings in two separate age groups: those aged 13-17, who completed the surveys themselves, and those aged 8-12, whose parents completed the surveys. Nearly all the patients (94.4%) had JIA, but one had lupus and three had juvenile dermatomyositis.
The participants completed the Patient-Reported Outcomes Measurement Information System (PROMIS) for psychological stress, physical stress, and depressive symptoms. They also filled out the National Institutes of Health–Toolbox Perceived Stress survey, the 9-item Patient Health Questionnaire (PHQ-9), the Screen for Child Anxiety Related Disorders (SCARED), a visual analog scale for COVID-related distress, and a questionnaire asking about how receptive they were to mental health screening. The researchers determined that a score 1 standard deviation above the mean on the PROMIS and NIH-Toolbox assessments qualified as a high level of psychological stress.
“There are data that suggest that psychological stress can be a precursor to depression and anxiety, which raises the concern that not every patient who’s experiencing mental health symptoms is going to be picked up on traditional measures that meet that clinical threshold, but they may really need interventions to protect their mental health,” presenter Erin Treemarcki, DO, an assistant professor of pediatric rheumatology at the University of Utah, Salt Lake City, said in an interview. “Not every patient may necessarily need referral to a mental health specialist, but there are still potential interventions that we can do in the clinical setting to address mental health, which in turn can improve outcomes, including medication compliance and knowing how patients are feeling.”
More than one-third of the patients (39%) reported a high level of psychological stress, and 43% had elevated physical stress. Broken down by age, 26% of the teens and 15% of the younger patients reported high levels of perceived stress. The PROMIS only identified increased depressive symptoms in 26% of the participants, whereas more than half (54%) had a positive PHQ-9 depression screen. Furthermore, half the patients had SCARED scores (50%) that likely indicated anxiety disorder. Only 6% of patients reported severe stress specifically related to the pandemic, but most reported mild distress from the pandemic.
“Psychological stress was highly correlated with physical stress, perceived stress, depressive symptoms [PROMIS and PHQ-9], and anxiety,” the authors reported (P < .05). The authors next plan to expand their assessment to a third CARRA site and then explore the interaction between psychological distress and sociodemographic factors.
“There’s such an increase in mental health disorders right now, and we’re overwhelmed in general,” Ms. Sosna said in an interview. “There have to be interventions that approach this. We can use pharmacological approaches, we can use CBT, we can use a lot of these things that are very well established, and they’re absolutely fantastic, but we don’t necessarily have the resources or capabilities to do that all the time.”
Benefits of automated depression screening
To reduce the likelihood of depression screenings falling through the cracks during visits, Dr. Harper’s study assessed the impact of automating screens in an adolescent population. In her presentation, she noted previous research finding that nearly half of youth with lupus (47%) had depression, compared with 24% of adults with lupus. Pediatric patients have nearly three times the odds of depression and more than five times the odds of suicidal ideation, she told attendees. These mood disorders are correlated with greater physical disability, higher cardiovascular risk, more disease activity, higher risk of premature death, and decreased educational attainment, medication compliance, and quality of life.
Despite recommendations for depression screening from the U.S. Preventive Services Task Force and the American Academy of Pediatrics, only 2% of pediatric rheumatology patients are routinely screened for depression with a validated instrument, and only 7% of those with depressive symptoms are screened, according to a 2016 study that Dr. Harper cited. Yet the same study found that nearly all pediatric rheumatologists (95%) supported routine depression screening every 6-12 months. Hence her team’s decision to test whether automating screening improved their screening rates.
Their population included lupus patients aged 12 and older seen at Nationwide Children’s Hospital between 2014 and 2022. Initially, patients completed the PHQ-9 on paper, which was then transcribed into the electronic health record. The process became automated and administered on an iPad at every visit in 2022. Positive screens – those endorsing suicidality or with a score of at least 10 – caused an alert to pop up for clinicians during their workflow so that they would talk to the mental health team about the patient’s needs.
A total of 149 patients completed 529 screenings during the study’s 8 years. Only 1 patient completed a PHQ-9 in 2014, which increased to just 17 patients in 2017. Automation resulted in 225 screens (P < .01). Subsequently, positive screens increased from 0% in 2014 to 25%-30% in 2018-2021, but then fell to 12% in 2022 (P < .01). The median PHQ-9 score was 3; overall scores decreased as screening increased.
The overall incidence of positive screens during the study period was 20% and prevalence was 38%, the authors reported. Of the 10 automated alerts triggered by positive screens, 90% resulted in a meeting with a psychologist or social worker, and 90% completed a suicide risk assessment. The intrusive alert for clinicians requires them to acknowledge the alert, agreeing to initiate a risk assessment, before they can enter data into the patient’s chart.
The study findings reveal “that you can successfully screen a high-risk population using an automated, seamless process, and you can alert providers without too much disruption to their typical clinic flow,” Dr. Harper told attendees. “And all of these processes have led to sustainability for routine depression screening in our lupus clinic.”
Dr. Harper’s team next plans to expand the automated screenings to populations with other diseases, to add an automated screening for anxiety, and to explore how PHQ-9 scores correlate with disease activity.
Treating patients’ mental health
Another two other abstracts at the symposium looked at another option, the 6-week cognitive-behavioral TEACH program. Deborah Levy, MD, MS, an associate professor of pediatrics at the University of Toronto and the clinical director of rheumatology at The Hospital for Sick Children, and colleagues assessed the program’s success when delivered remotely to adolescent patients with lupus. Pilot testing with TEACH had already shown improvements in fatigue and mood, Dr. Levy told attendees, but barriers to in-person delivery limited its utility even before the pandemic, so this study aimed to determine a remote version’s feasibility and effects, compared with treatment as usual.
The randomized, controlled trial, led by Natoshia Cunningham, PhD, from Michigan State University, Grand Rapids, included 57 participants, aged 12-22, from seven U.S. and Canadian rheumatology sites. All had been diagnosed with childhood-onset SLE by age 18 and had elevated symptoms in fatigue, pain, or depression. A PROMIS Fatigue T score of 60 or greater indicated elevated fatigue scores, whereas a high pain score was at least a 3/10 on a visual analog scale, and a high depression T score was at least a 60 but not higher than 80 on the Children’s Depression Inventory–2 or the Beck Depression Inventory–II (depending on the patient’s age).
Patients with other chronic medical conditions, developmental delays, or untreated major psychiatric illness were excluded from the study, as were patients who were receiving overlapping treatment, such as cognitive-behavioral therapy for pain or mood. Thirty patients were randomly assigned to receive treatment as usual while 27 patients were assigned to participate in the remote TEACH program.
Nearly all the patients (94%) were female, but they were racially diverse, with 42% White, 28% Asian, 19% Black, 19% Hispanic, and 4% multiracial. The patients were an average 16 years old and had been diagnosed for a median 5 years. Three of the intervention’s six modules involved the caregivers or, for older patients, their partners if desired. The communication strategies taught in the program were also tailored to patients’ ages.
“All of these strategies are educational, cognitive, behavioral, mindfulness strategies that target fatigue [and] pain, and they also developed web content for participants to use on their own,” Dr. Levy told attendees.
The researchers had complete postassessment data from 88% of participants, but they also reported some of the statements made during qualitative interviews about the program’s feasibility.
“I think it makes people more aware of themselves to become a better version of themselves, whether that’s in their normal life or in handling a lupus kind of life,” one participant said about the program’s benefits. Another appreciated the “alternative ways of thinking,” including “being more mindful of my thoughts and how those kind of aggravate my stress.”
The quantitative findings revealed a statistically significant reduction in depressive symptoms and fatigue for TEACH participants, compared with treatment as usual. Mood scores fell by an average 13.7 points in the TEACH group, compared with a drop of 2.4 points in the treatment as usual group (P < .001). Scores for fatigue fell 9.16 points in the TEACH group and 2.93 in the control group (P = .003). No statistically significant difference showed up in pain scores between the groups, although pain, medication adherence, and disease activity did improve slightly more in the TEACH group.
In addition to the significant improvements in mood and fatigue, therefore, “completion of TEACH may be associated with improved medication adherence and disease activity versus treatment as usual,” Dr. Levy said.
A much smaller study authored by some of the same researchers also assessed TEACH’s impact not in remote form but in terms of its value specifically for adolescent patients with SLE and elevated depression and fatigue scores. Comparison of 6 high-risk patients with 10 low-risk patients who underwent TEACH suggested that the program was especially effective for improving depression in high-risk patients since these patients had a statistically significantly greater improvement in mood. Fatigue, pain, anxiety, quality of life, and disease activity scores did not statistically differ between the groups.
Authors of the automated depression screening study reported no disclosures or outside funding. The study assessing psychological distress was funded by a CARRA–Arthritis Foundation grant, and the authors reported no disclosures. The remote TEACH study was funded by a CARRA–Arthritis Foundation grant, and all but one author reported no disclosures. One author had disclosures with Janssen, Roche, and Sobi. The high-risk TEACH study was also funded by a CARRA grant, and the authors had no disclosures.
AT PRSYM 2023
LGBTQ+ Youth Consult Questions remain over use of sex hormone therapy
“They Paused Puberty but Is There a Cost?”
“Bone Health: Puberty Blockers Not Fully Reversible.”
Headlines such as these from major national news outlets have begun to cast doubt on one of the medications used in treating gender-diverse adolescents and young adults. GnRH agonists, such as leuprorelin and triptorelin, were first approved by the Food and Drug Administration in the 1980s and have been used since then for a variety of medical indications. In the decades since, these medications have been successfully used with a generally favorable side effect profile.
GnRH agonists and puberty
In the treatment of precocious puberty, GnRH agonists are often started prior to the age of 7, depending on the age at which the affected patient begins showing signs of central puberty. These include breast development, scrotal enlargement, and so on. GnRH agonists typically are continued until age 10-12, depending on the patient and an informed discussion with the patient’s parents about optimal outcomes.1 Therefore, it is not uncommon to see these medications used for anywhere from 1 to 4 years, depending on the age at which precocious puberty started.
GnRH agonists are used in two populations of transgender individuals. The first group is those youths who have just started their natal, or biological, puberty. The medication is not started until the patient has biochemical or physical exam evidence that puberty has started. The medication is then continued until hormones are started. This is usually 2-3 years on average, depending on the age at which the medication was started. This is essentially comparable with cisgender youths who have taken these medications for precocious puberty. The second population of individuals who use GnRH agonists is transgender women who are also on estrogen therapy. In these women, the GnRH agonist is used for androgen (testosterone) suppression.
Concerns over bone health
One of the main concerns recently expressed about long-term use of GnRH agonists is their effect on bone density. Adolescence is a critical time for bone mineral density (BMD) accrual and this is driven by sex hormones. When GnRH agonists are used to delay puberty in transgender adolescents, this then delays the maturation of the adult skeleton until the GnRH agonist is stopped (and natal puberty resumes) or cross-sex hormones are started. In a recent multicenter study2 looking at baseline BMD of transgender youth at the time of GnRH agonist initiation, 30% of those assigned male at birth and 13% of those assigned female at birth had low bone mineral density for age (defined as a BMD z score of <–2). For those with low BMD, their physical activity scores were significantly lower than those with normal BMD. Thus, these adolescents require close follow-up, just like their cisgender peers.
There are currently no long-term data on the risk of developing fractures or osteoporosis in those individuals who were treated with GnRH agonists and then went on to start cross-sex hormone therapy. Some studies suggest that there is a risk that BMD does not recover after being on cross-sex hormones,3 while another study suggested that transgender men recover their BMD after being on testosterone.4 It is still unclear in that study why transgender women did not recover their BMD or why they were low at baseline. Interestingly, a 2012 study5 from Brazil showed that there was no difference in BMD for cisgender girls who had been off their GnRH agonist therapy for at least 3 years, as compared with their age-matched controls who had never been on GnRH agonist therapy. These conflicting data highlight the importance of long-term follow-up, as well as the need to include age-matched, cisgender control subjects, to better understand if there is truly a difference in transgender individuals or if today’s adolescents, in general, have low BMD.
Lingering questions
In summary, the use of GnRH agonists in transgender adolescents remains controversial because of the potential long-term effects on bone mineral density. However, this risk must be balanced against the risks of allowing natal puberty to progress in certain transgender individuals with the development of undesired secondary sex characteristics. More longitudinal studies are needed to better understand the long-term risks of osteoporosis and fractures in those who have undergone GnRH agonist therapy as part of their gender-affirming medical care, as well as any clinical interventions that might help mitigate this risk.
Dr. Cooper is assistant professor of pediatrics at UT Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Harrington J et al. Treatment of precocious puberty. UpToDate. www.uptodate.com/contents/treatment-of-precocious-puberty.
2. Lee JY et al. J Endocr Soc. 2020;4(9):bvaa065. doi: 10.1210/jendso/bvaa065.
3. Klink D et al. J Clin Endocrinol Metab. 2015;100(2):E270-5. doi: 10.1210/jc.2014-2439.
4. Schagen SEE et al. J Clin Endocrinol Metab. 2020;105(12):e4252-e4263. doi: 10.1210/clinem/dgaa604.
5. Alessandri SB et al. Clinics (Sao Paulo). 2012;67(6):591-6. doi: 10.6061/clinics/2012(06)08.
“They Paused Puberty but Is There a Cost?”
“Bone Health: Puberty Blockers Not Fully Reversible.”
Headlines such as these from major national news outlets have begun to cast doubt on one of the medications used in treating gender-diverse adolescents and young adults. GnRH agonists, such as leuprorelin and triptorelin, were first approved by the Food and Drug Administration in the 1980s and have been used since then for a variety of medical indications. In the decades since, these medications have been successfully used with a generally favorable side effect profile.
GnRH agonists and puberty
In the treatment of precocious puberty, GnRH agonists are often started prior to the age of 7, depending on the age at which the affected patient begins showing signs of central puberty. These include breast development, scrotal enlargement, and so on. GnRH agonists typically are continued until age 10-12, depending on the patient and an informed discussion with the patient’s parents about optimal outcomes.1 Therefore, it is not uncommon to see these medications used for anywhere from 1 to 4 years, depending on the age at which precocious puberty started.
GnRH agonists are used in two populations of transgender individuals. The first group is those youths who have just started their natal, or biological, puberty. The medication is not started until the patient has biochemical or physical exam evidence that puberty has started. The medication is then continued until hormones are started. This is usually 2-3 years on average, depending on the age at which the medication was started. This is essentially comparable with cisgender youths who have taken these medications for precocious puberty. The second population of individuals who use GnRH agonists is transgender women who are also on estrogen therapy. In these women, the GnRH agonist is used for androgen (testosterone) suppression.
Concerns over bone health
One of the main concerns recently expressed about long-term use of GnRH agonists is their effect on bone density. Adolescence is a critical time for bone mineral density (BMD) accrual and this is driven by sex hormones. When GnRH agonists are used to delay puberty in transgender adolescents, this then delays the maturation of the adult skeleton until the GnRH agonist is stopped (and natal puberty resumes) or cross-sex hormones are started. In a recent multicenter study2 looking at baseline BMD of transgender youth at the time of GnRH agonist initiation, 30% of those assigned male at birth and 13% of those assigned female at birth had low bone mineral density for age (defined as a BMD z score of <–2). For those with low BMD, their physical activity scores were significantly lower than those with normal BMD. Thus, these adolescents require close follow-up, just like their cisgender peers.
There are currently no long-term data on the risk of developing fractures or osteoporosis in those individuals who were treated with GnRH agonists and then went on to start cross-sex hormone therapy. Some studies suggest that there is a risk that BMD does not recover after being on cross-sex hormones,3 while another study suggested that transgender men recover their BMD after being on testosterone.4 It is still unclear in that study why transgender women did not recover their BMD or why they were low at baseline. Interestingly, a 2012 study5 from Brazil showed that there was no difference in BMD for cisgender girls who had been off their GnRH agonist therapy for at least 3 years, as compared with their age-matched controls who had never been on GnRH agonist therapy. These conflicting data highlight the importance of long-term follow-up, as well as the need to include age-matched, cisgender control subjects, to better understand if there is truly a difference in transgender individuals or if today’s adolescents, in general, have low BMD.
Lingering questions
In summary, the use of GnRH agonists in transgender adolescents remains controversial because of the potential long-term effects on bone mineral density. However, this risk must be balanced against the risks of allowing natal puberty to progress in certain transgender individuals with the development of undesired secondary sex characteristics. More longitudinal studies are needed to better understand the long-term risks of osteoporosis and fractures in those who have undergone GnRH agonist therapy as part of their gender-affirming medical care, as well as any clinical interventions that might help mitigate this risk.
Dr. Cooper is assistant professor of pediatrics at UT Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Harrington J et al. Treatment of precocious puberty. UpToDate. www.uptodate.com/contents/treatment-of-precocious-puberty.
2. Lee JY et al. J Endocr Soc. 2020;4(9):bvaa065. doi: 10.1210/jendso/bvaa065.
3. Klink D et al. J Clin Endocrinol Metab. 2015;100(2):E270-5. doi: 10.1210/jc.2014-2439.
4. Schagen SEE et al. J Clin Endocrinol Metab. 2020;105(12):e4252-e4263. doi: 10.1210/clinem/dgaa604.
5. Alessandri SB et al. Clinics (Sao Paulo). 2012;67(6):591-6. doi: 10.6061/clinics/2012(06)08.
“They Paused Puberty but Is There a Cost?”
“Bone Health: Puberty Blockers Not Fully Reversible.”
Headlines such as these from major national news outlets have begun to cast doubt on one of the medications used in treating gender-diverse adolescents and young adults. GnRH agonists, such as leuprorelin and triptorelin, were first approved by the Food and Drug Administration in the 1980s and have been used since then for a variety of medical indications. In the decades since, these medications have been successfully used with a generally favorable side effect profile.
GnRH agonists and puberty
In the treatment of precocious puberty, GnRH agonists are often started prior to the age of 7, depending on the age at which the affected patient begins showing signs of central puberty. These include breast development, scrotal enlargement, and so on. GnRH agonists typically are continued until age 10-12, depending on the patient and an informed discussion with the patient’s parents about optimal outcomes.1 Therefore, it is not uncommon to see these medications used for anywhere from 1 to 4 years, depending on the age at which precocious puberty started.
GnRH agonists are used in two populations of transgender individuals. The first group is those youths who have just started their natal, or biological, puberty. The medication is not started until the patient has biochemical or physical exam evidence that puberty has started. The medication is then continued until hormones are started. This is usually 2-3 years on average, depending on the age at which the medication was started. This is essentially comparable with cisgender youths who have taken these medications for precocious puberty. The second population of individuals who use GnRH agonists is transgender women who are also on estrogen therapy. In these women, the GnRH agonist is used for androgen (testosterone) suppression.
Concerns over bone health
One of the main concerns recently expressed about long-term use of GnRH agonists is their effect on bone density. Adolescence is a critical time for bone mineral density (BMD) accrual and this is driven by sex hormones. When GnRH agonists are used to delay puberty in transgender adolescents, this then delays the maturation of the adult skeleton until the GnRH agonist is stopped (and natal puberty resumes) or cross-sex hormones are started. In a recent multicenter study2 looking at baseline BMD of transgender youth at the time of GnRH agonist initiation, 30% of those assigned male at birth and 13% of those assigned female at birth had low bone mineral density for age (defined as a BMD z score of <–2). For those with low BMD, their physical activity scores were significantly lower than those with normal BMD. Thus, these adolescents require close follow-up, just like their cisgender peers.
There are currently no long-term data on the risk of developing fractures or osteoporosis in those individuals who were treated with GnRH agonists and then went on to start cross-sex hormone therapy. Some studies suggest that there is a risk that BMD does not recover after being on cross-sex hormones,3 while another study suggested that transgender men recover their BMD after being on testosterone.4 It is still unclear in that study why transgender women did not recover their BMD or why they were low at baseline. Interestingly, a 2012 study5 from Brazil showed that there was no difference in BMD for cisgender girls who had been off their GnRH agonist therapy for at least 3 years, as compared with their age-matched controls who had never been on GnRH agonist therapy. These conflicting data highlight the importance of long-term follow-up, as well as the need to include age-matched, cisgender control subjects, to better understand if there is truly a difference in transgender individuals or if today’s adolescents, in general, have low BMD.
Lingering questions
In summary, the use of GnRH agonists in transgender adolescents remains controversial because of the potential long-term effects on bone mineral density. However, this risk must be balanced against the risks of allowing natal puberty to progress in certain transgender individuals with the development of undesired secondary sex characteristics. More longitudinal studies are needed to better understand the long-term risks of osteoporosis and fractures in those who have undergone GnRH agonist therapy as part of their gender-affirming medical care, as well as any clinical interventions that might help mitigate this risk.
Dr. Cooper is assistant professor of pediatrics at UT Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Harrington J et al. Treatment of precocious puberty. UpToDate. www.uptodate.com/contents/treatment-of-precocious-puberty.
2. Lee JY et al. J Endocr Soc. 2020;4(9):bvaa065. doi: 10.1210/jendso/bvaa065.
3. Klink D et al. J Clin Endocrinol Metab. 2015;100(2):E270-5. doi: 10.1210/jc.2014-2439.
4. Schagen SEE et al. J Clin Endocrinol Metab. 2020;105(12):e4252-e4263. doi: 10.1210/clinem/dgaa604.
5. Alessandri SB et al. Clinics (Sao Paulo). 2012;67(6):591-6. doi: 10.6061/clinics/2012(06)08.
New COVID variant on WHO’s radar causing itchy eyes in children
A new COVID-19 variant that recently landed on the World Health Organization’s radar may cause previously unseen symptoms in children, according to a new report.
While the variant, called “Arcturus,” hasn’t yet made the Centers for Disease Control and Prevention’s watchlist, , according to The Times of India.
The new itchy eye symptom is in addition to a high fever and cough, Vipin M. Vashishtha, MD, said on Twitter, noting that pediatric COVID cases have picked up there for the first time in 6 months.
The country has also seen a rise in adenovirus cases among children with similar symptoms. COVID and adenovirus cannot be distinguished without testing, and many parents don’t want to have their children tested because the swabs are uncomfortable, The Times of India reported. One doctor told the newspaper that among every 10 children with COVID-like symptoms, 2 or 3 of them had tested positive on a COVID test taken at home.
Health officials in India are doing mock drills to check how prepared the country’s hospitals are as India sees cases rise, the BBC reported. India struggled during a COVID-19 surge in 2021, at which time sickened people were seen lying on sidewalks outside overflowing hospitals, and reports surfaced of a black market for private citizens to buy oxygen.
Arcturus (formally, Omicron subvariant XBB.1.16) made news recently as it landed on the WHO’s radar after surfacing in India. A WHO official called it “one to watch.” The Times of India reported that 234 new cases of XBB.1.16 were included in the country’s latest 5,676 new infections, meaning the subvariant accounts for 4% of new COVID cases.
A version of this article originally appeared on WebMD.com.
A new COVID-19 variant that recently landed on the World Health Organization’s radar may cause previously unseen symptoms in children, according to a new report.
While the variant, called “Arcturus,” hasn’t yet made the Centers for Disease Control and Prevention’s watchlist, , according to The Times of India.
The new itchy eye symptom is in addition to a high fever and cough, Vipin M. Vashishtha, MD, said on Twitter, noting that pediatric COVID cases have picked up there for the first time in 6 months.
The country has also seen a rise in adenovirus cases among children with similar symptoms. COVID and adenovirus cannot be distinguished without testing, and many parents don’t want to have their children tested because the swabs are uncomfortable, The Times of India reported. One doctor told the newspaper that among every 10 children with COVID-like symptoms, 2 or 3 of them had tested positive on a COVID test taken at home.
Health officials in India are doing mock drills to check how prepared the country’s hospitals are as India sees cases rise, the BBC reported. India struggled during a COVID-19 surge in 2021, at which time sickened people were seen lying on sidewalks outside overflowing hospitals, and reports surfaced of a black market for private citizens to buy oxygen.
Arcturus (formally, Omicron subvariant XBB.1.16) made news recently as it landed on the WHO’s radar after surfacing in India. A WHO official called it “one to watch.” The Times of India reported that 234 new cases of XBB.1.16 were included in the country’s latest 5,676 new infections, meaning the subvariant accounts for 4% of new COVID cases.
A version of this article originally appeared on WebMD.com.
A new COVID-19 variant that recently landed on the World Health Organization’s radar may cause previously unseen symptoms in children, according to a new report.
While the variant, called “Arcturus,” hasn’t yet made the Centers for Disease Control and Prevention’s watchlist, , according to The Times of India.
The new itchy eye symptom is in addition to a high fever and cough, Vipin M. Vashishtha, MD, said on Twitter, noting that pediatric COVID cases have picked up there for the first time in 6 months.
The country has also seen a rise in adenovirus cases among children with similar symptoms. COVID and adenovirus cannot be distinguished without testing, and many parents don’t want to have their children tested because the swabs are uncomfortable, The Times of India reported. One doctor told the newspaper that among every 10 children with COVID-like symptoms, 2 or 3 of them had tested positive on a COVID test taken at home.
Health officials in India are doing mock drills to check how prepared the country’s hospitals are as India sees cases rise, the BBC reported. India struggled during a COVID-19 surge in 2021, at which time sickened people were seen lying on sidewalks outside overflowing hospitals, and reports surfaced of a black market for private citizens to buy oxygen.
Arcturus (formally, Omicron subvariant XBB.1.16) made news recently as it landed on the WHO’s radar after surfacing in India. A WHO official called it “one to watch.” The Times of India reported that 234 new cases of XBB.1.16 were included in the country’s latest 5,676 new infections, meaning the subvariant accounts for 4% of new COVID cases.
A version of this article originally appeared on WebMD.com.
Parents of patients with rheumatic disease, MIS-C strongly hesitant of COVID vaccination
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023
Study offers dozens of reasons to cut sugar
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
FROM THE BMJ