User login
Allowed Publications
LayerRx Mapping ID
577
Slot System
Featured Buckets
Featured Buckets Admin
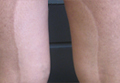
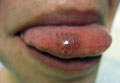
Futcher Lines: A Case Report in Pregnancy and Literature Review
Article Type
Changed
Display Headline
Futcher Lines: A Case Report in Pregnancy and Literature Review
Article PDF
Issue
Cutis - 92(2)
Publications
Topics
Page Number
100-101
Legacy Keywords
pigmentary demarcation lines, voight lines, futcher lines, pigmentation disorders, pigmentation treatment, skin pigmentation disorders
Article PDF
Article PDF
Issue
Cutis - 92(2)
Issue
Cutis - 92(2)
Page Number
100-101
Page Number
100-101
Publications
Publications
Topics
Article Type
Display Headline
Futcher Lines: A Case Report in Pregnancy and Literature Review
Display Headline
Futcher Lines: A Case Report in Pregnancy and Literature Review
Legacy Keywords
pigmentary demarcation lines, voight lines, futcher lines, pigmentation disorders, pigmentation treatment, skin pigmentation disorders
Legacy Keywords
pigmentary demarcation lines, voight lines, futcher lines, pigmentation disorders, pigmentation treatment, skin pigmentation disorders
Disallow All Ads
Alternative CME
Article PDF Media
Document
Addisonian Pigmentation and Vitamin B12 Deficiency: A Case Series and Review of the Literature
Article Type
Changed
Display Headline
Addisonian Pigmentation and Vitamin B12 Deficiency: A Case Series and Review of the Literature
Article PDF
Issue
Cutis - 92(2)
Publications
Topics
Page Number
94-99
Legacy Keywords
pigmentation disorders, skin of color, vitamin B12, addisonian pigmentation, anemia, oral ulcers, graying of hair, skin pigmentation treatment, mouth diseases
Article PDF
Article PDF
Issue
Cutis - 92(2)
Issue
Cutis - 92(2)
Page Number
94-99
Page Number
94-99
Publications
Publications
Topics
Article Type
Display Headline
Addisonian Pigmentation and Vitamin B12 Deficiency: A Case Series and Review of the Literature
Display Headline
Addisonian Pigmentation and Vitamin B12 Deficiency: A Case Series and Review of the Literature
Legacy Keywords
pigmentation disorders, skin of color, vitamin B12, addisonian pigmentation, anemia, oral ulcers, graying of hair, skin pigmentation treatment, mouth diseases
Legacy Keywords
pigmentation disorders, skin of color, vitamin B12, addisonian pigmentation, anemia, oral ulcers, graying of hair, skin pigmentation treatment, mouth diseases
Inside the Article
Test your knowledge on Addisonian pigmentation and vitamin B12 with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.
Disallow All Ads
Alternative CME
Article PDF Media
Document
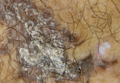
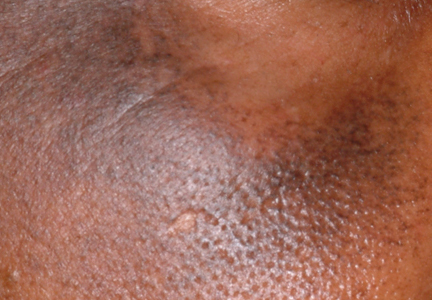
What Is Your Diagnosis? Axillary Granular Parakeratosis
Article Type
Changed
Display Headline
What Is Your Diagnosis? Axillary Granular Parakeratosis
Article PDF
Issue
Cutis - 92(2)
Publications
Topics
Page Number
61, 65-66
Legacy Keywords
papules, parakeratosis, skin disease, skin condition, hyperkeratosis, granular parakeratosis, epidermal reaction pattern
Sections
Article PDF
Article PDF
Issue
Cutis - 92(2)
Issue
Cutis - 92(2)
Page Number
61, 65-66
Page Number
61, 65-66
Publications
Publications
Topics
Article Type
Display Headline
What Is Your Diagnosis? Axillary Granular Parakeratosis
Display Headline
What Is Your Diagnosis? Axillary Granular Parakeratosis
Legacy Keywords
papules, parakeratosis, skin disease, skin condition, hyperkeratosis, granular parakeratosis, epidermal reaction pattern
Legacy Keywords
papules, parakeratosis, skin disease, skin condition, hyperkeratosis, granular parakeratosis, epidermal reaction pattern
Sections
Disallow All Ads
Alternative CME
Article PDF Media
Document
Psychological Screening for Patients With Vitiligo Prior to Depigmentation Therapy
Article Type
Changed
Display Headline
Psychological Screening for Patients With Vitiligo Prior to Depigmentation Therapy
Publications
Topics
Publications
Publications
Topics
Article Type
Display Headline
Psychological Screening for Patients With Vitiligo Prior to Depigmentation Therapy
Display Headline
Psychological Screening for Patients With Vitiligo Prior to Depigmentation Therapy
Disallow All Ads
Alternative CME
Consolidated Pubs: Do Not Show Source Publication Logo
Use ProPublica
Oculocutaneous Albinism
Article Type
Changed
Display Headline
Oculocutaneous Albinism
Article PDF
Issue
Cutis - 91(5)
Publications
Topics
Page Number
E1-E4
Legacy Keywords
oculocutaneous albinism, albino awareness initiatives, patient support
Sections
Article PDF
Article PDF
Issue
Cutis - 91(5)
Issue
Cutis - 91(5)
Page Number
E1-E4
Page Number
E1-E4
Publications
Publications
Topics
Article Type
Display Headline
Oculocutaneous Albinism
Display Headline
Oculocutaneous Albinism
Legacy Keywords
oculocutaneous albinism, albino awareness initiatives, patient support
Legacy Keywords
oculocutaneous albinism, albino awareness initiatives, patient support
Sections
Disallow All Ads
Alternative CME
Article PDF Media
Document
Treating Acne Scars in Patients With Fitzpatrick Skin Types IV to VI Using the 1450-nm Diode Laser
Article Type
Changed
Display Headline
Treating Acne Scars in Patients With Fitzpatrick Skin Types IV to VI Using the 1450-nm Diode Laser
Article PDF
Issue
Cutis - 92(1)
Publications
Topics
Page Number
49-53
Legacy Keywords
acne scarring, 1450-nm diode laser, postinflammatory hyperpigmentation, skin of color, acne scars in darker skin types, lasers and acne scars
Sections
Article PDF
Article PDF
Issue
Cutis - 92(1)
Issue
Cutis - 92(1)
Page Number
49-53
Page Number
49-53
Publications
Publications
Topics
Article Type
Display Headline
Treating Acne Scars in Patients With Fitzpatrick Skin Types IV to VI Using the 1450-nm Diode Laser
Display Headline
Treating Acne Scars in Patients With Fitzpatrick Skin Types IV to VI Using the 1450-nm Diode Laser
Legacy Keywords
acne scarring, 1450-nm diode laser, postinflammatory hyperpigmentation, skin of color, acne scars in darker skin types, lasers and acne scars
Legacy Keywords
acne scarring, 1450-nm diode laser, postinflammatory hyperpigmentation, skin of color, acne scars in darker skin types, lasers and acne scars
Sections
Disallow All Ads
Alternative CME
Article PDF Media
Document
Minocycline-Induced Hyperpigmentation Involving the Oral Mucosa After Short-term Minocycline Use
Article Type
Changed
Display Headline
Minocycline-Induced Hyperpigmentation Involving the Oral Mucosa After Short-term Minocycline Use
Article PDF
Issue
Cutis - 92(1)
Publications
Topics
Page Number
46-48
Legacy Keywords
side effects of minocycline, minocycline and oral hyperpigmentation, oral hyperpigmentation, MIH, minocycline induced hyperpigmentation patterns, hyperpigmentation of the oral mucosa
Article PDF
Article PDF
Issue
Cutis - 92(1)
Issue
Cutis - 92(1)
Page Number
46-48
Page Number
46-48
Publications
Publications
Topics
Article Type
Display Headline
Minocycline-Induced Hyperpigmentation Involving the Oral Mucosa After Short-term Minocycline Use
Display Headline
Minocycline-Induced Hyperpigmentation Involving the Oral Mucosa After Short-term Minocycline Use
Legacy Keywords
side effects of minocycline, minocycline and oral hyperpigmentation, oral hyperpigmentation, MIH, minocycline induced hyperpigmentation patterns, hyperpigmentation of the oral mucosa
Legacy Keywords
side effects of minocycline, minocycline and oral hyperpigmentation, oral hyperpigmentation, MIH, minocycline induced hyperpigmentation patterns, hyperpigmentation of the oral mucosa
Disallow All Ads
Alternative CME
Article PDF Media
Document
Skin-Lightening Agents: An Overview of Prescription, Office-Dispensed, and Over-the-counter Products
Article Type
Changed
Display Headline
Skin-Lightening Agents: An Overview of Prescription, Office-Dispensed, and Over-the-counter Products
Article PDF
Publications
Page Number
18-26
Legacy Keywords
periorbital rejuvenation, aesthetic procedures, eyes and facial aging, dermal fillers, cosmetic evaluation of the eyes
Article PDF
Article PDF
Page Number
18-26
Page Number
18-26
Publications
Publications
Article Type
Display Headline
Skin-Lightening Agents: An Overview of Prescription, Office-Dispensed, and Over-the-counter Products
Display Headline
Skin-Lightening Agents: An Overview of Prescription, Office-Dispensed, and Over-the-counter Products
Legacy Keywords
periorbital rejuvenation, aesthetic procedures, eyes and facial aging, dermal fillers, cosmetic evaluation of the eyes
Legacy Keywords
periorbital rejuvenation, aesthetic procedures, eyes and facial aging, dermal fillers, cosmetic evaluation of the eyes
Article Source
Citation Override
Originally published in Cosmetic Dermatology
PURLs Copyright
Inside the Article
Article PDF Media
Document
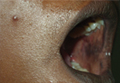
Pigmented Bowen Disease in a Black Patient: Novel Dermoscopic Findings [letter]
Article Type
Changed
Display Headline
Pigmented Bowen Disease in a Black Patient: Novel Dermoscopic Findings [letter]
Article PDF
Issue
Cutis - 91(5)
Publications
Page Number
258-259
Legacy Keywords
Bowen disease, melanoma misdiagnosis in Bowen disease, nonmelanoma skin cancer, pigmented lesions in skin of color, basal cell carcinomas
Sections
Article PDF
Article PDF
Issue
Cutis - 91(5)
Issue
Cutis - 91(5)
Page Number
258-259
Page Number
258-259
Publications
Publications
Article Type
Display Headline
Pigmented Bowen Disease in a Black Patient: Novel Dermoscopic Findings [letter]
Display Headline
Pigmented Bowen Disease in a Black Patient: Novel Dermoscopic Findings [letter]
Legacy Keywords
Bowen disease, melanoma misdiagnosis in Bowen disease, nonmelanoma skin cancer, pigmented lesions in skin of color, basal cell carcinomas
Legacy Keywords
Bowen disease, melanoma misdiagnosis in Bowen disease, nonmelanoma skin cancer, pigmented lesions in skin of color, basal cell carcinomas
Sections
Disallow All Ads
Alternative CME
Article PDF Media
Document
Vitiligo Patients Seeking Depigmentation Therapy: A Case Report and Guidelines for Psychological Screening
Article Type
Changed
Display Headline
Vitiligo Patients Seeking Depigmentation Therapy: A Case Report and Guidelines for Psychological Screening
Article PDF
Audio / Podcast
Issue
Cutis - 91(5)
Publications
Topics
Page Number
248-252
Legacy Keywords
obsessive compulsive disorder in dermatology, psychocutaneous medicine, pigmentation disorders, pigmentation treatment, psychological concerns and vitiligo, psychological screening prior to dermatology procedures
Audio / Podcast
Audio / Podcast
Article PDF
Article PDF
Issue
Cutis - 91(5)
Issue
Cutis - 91(5)
Page Number
248-252
Page Number
248-252
Publications
Publications
Topics
Article Type
Display Headline
Vitiligo Patients Seeking Depigmentation Therapy: A Case Report and Guidelines for Psychological Screening
Display Headline
Vitiligo Patients Seeking Depigmentation Therapy: A Case Report and Guidelines for Psychological Screening
Legacy Keywords
obsessive compulsive disorder in dermatology, psychocutaneous medicine, pigmentation disorders, pigmentation treatment, psychological concerns and vitiligo, psychological screening prior to dermatology procedures
Legacy Keywords
obsessive compulsive disorder in dermatology, psychocutaneous medicine, pigmentation disorders, pigmentation treatment, psychological concerns and vitiligo, psychological screening prior to dermatology procedures
Disallow All Ads
Alternative CME
Article PDF Media
Document